SEARCH RESULTS FOR: 2024
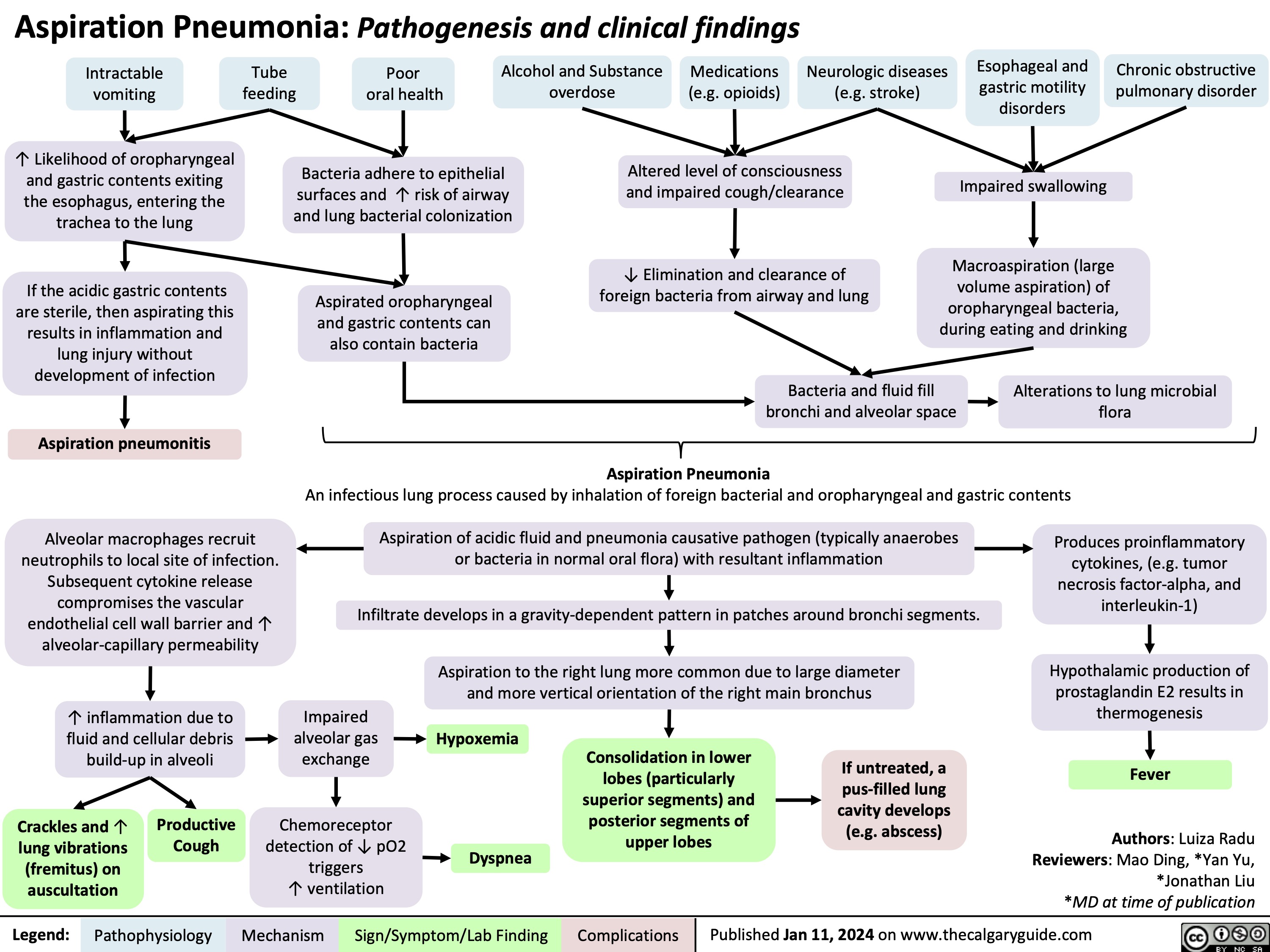
Aspiration Pneumonia
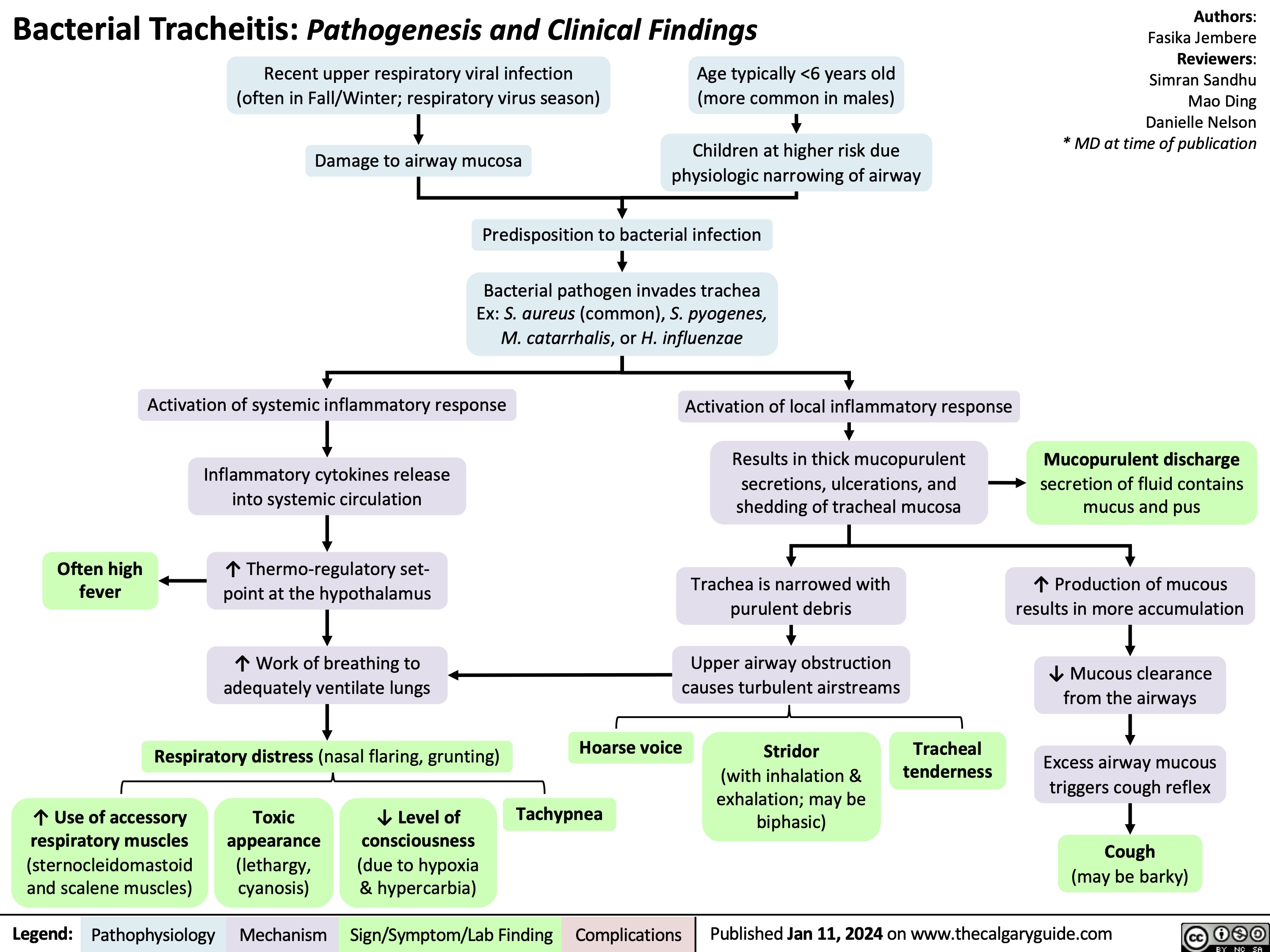
Bacterial Tracheitis
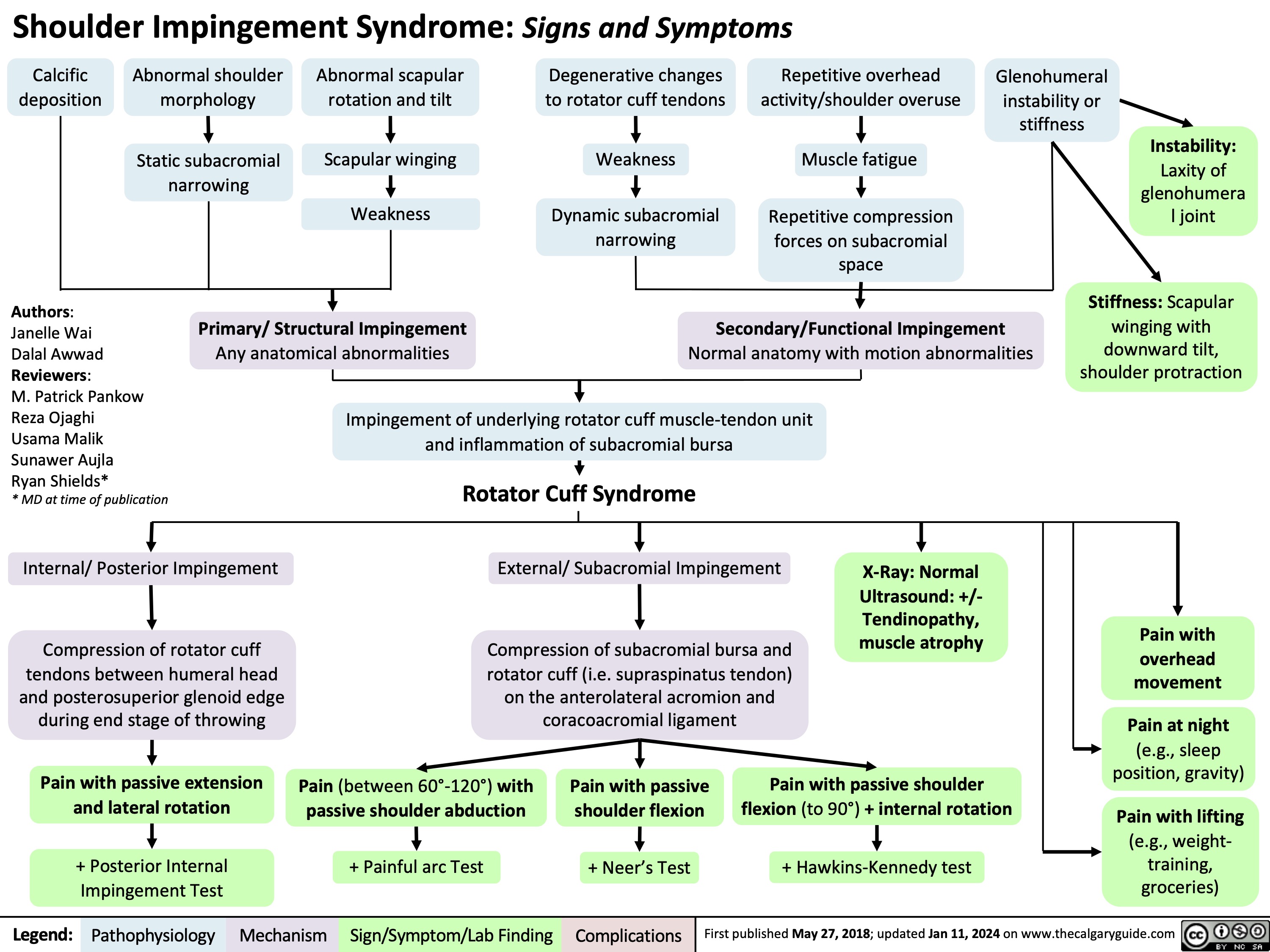
Shoulder Impingement Syndrome
Cranial Nerve IV Palsy
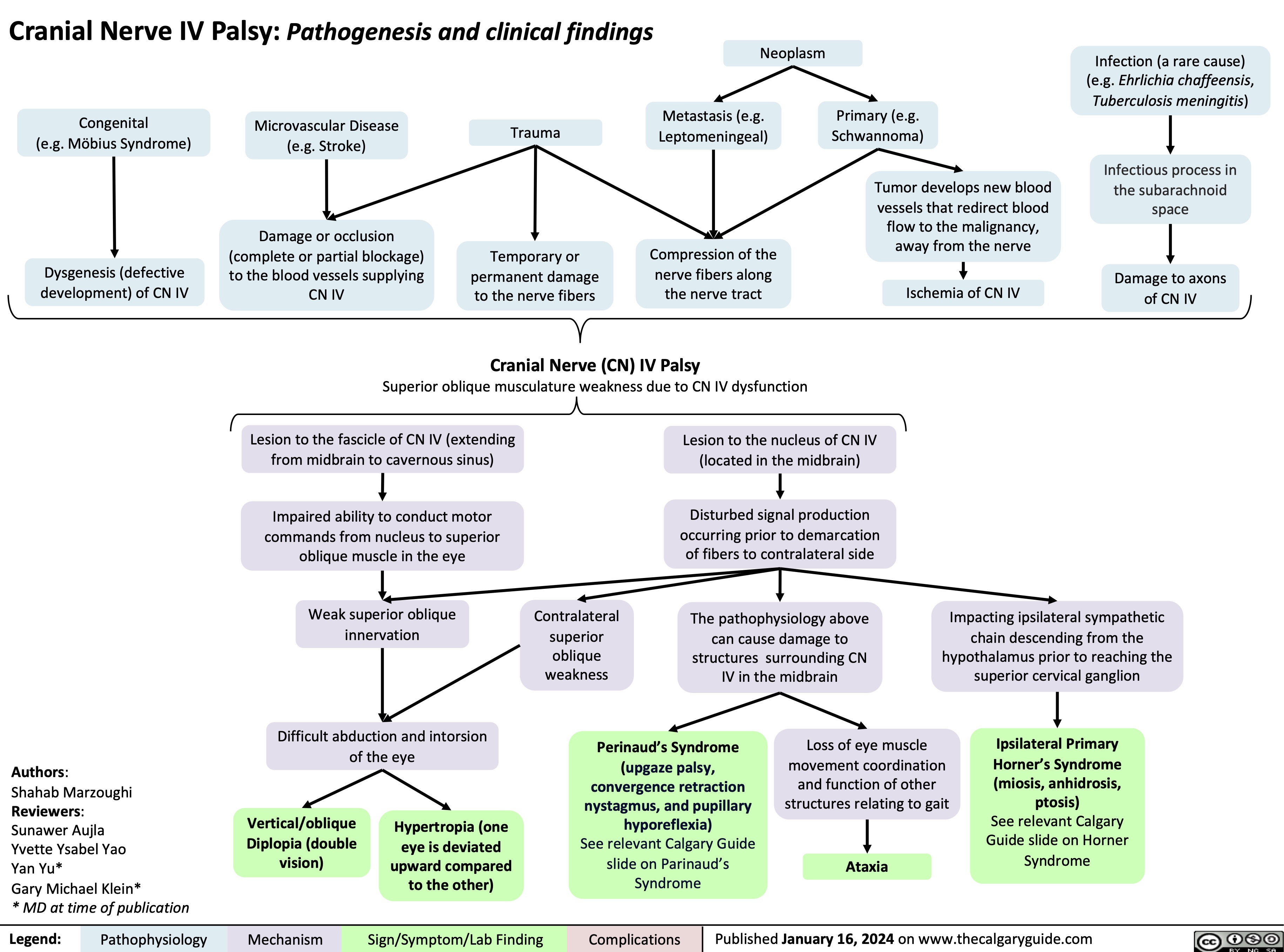
Sugammadex
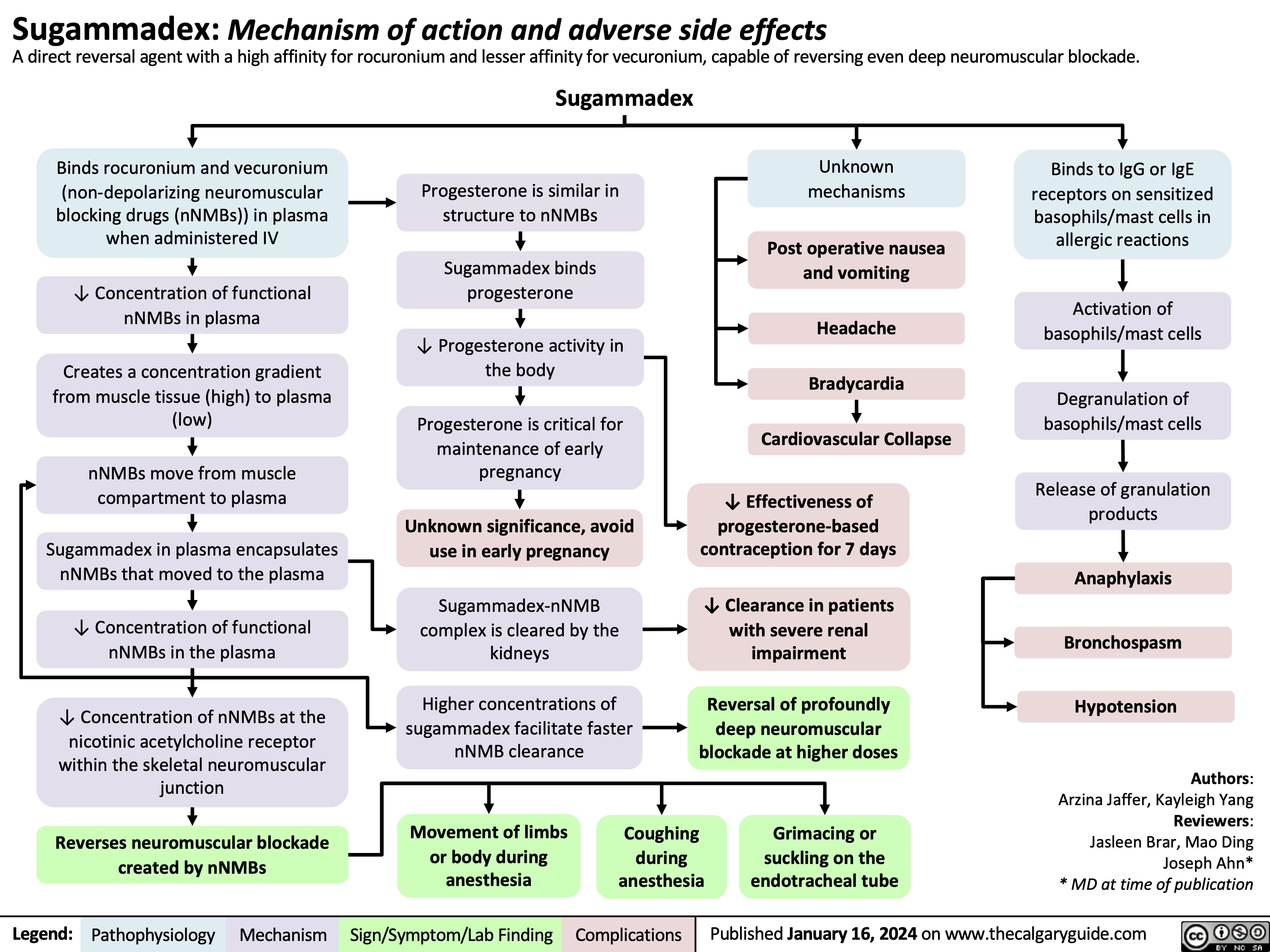
Arachnoid Cysts MRI Findings
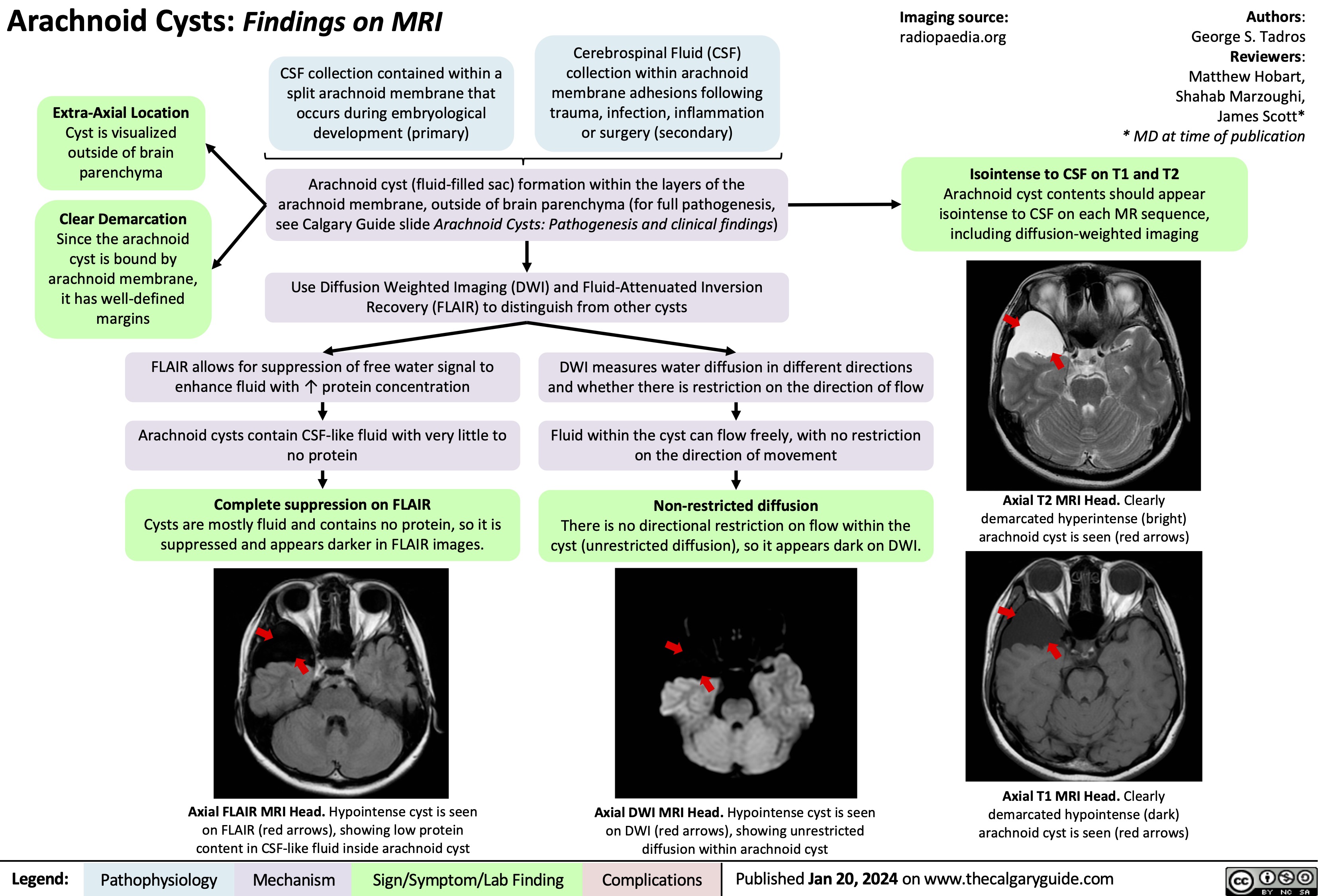
Arachnoid Cysts Pathogenesis and clinical findings
Avascular Necrosis AVN of the Femoral Head Findings on MRI
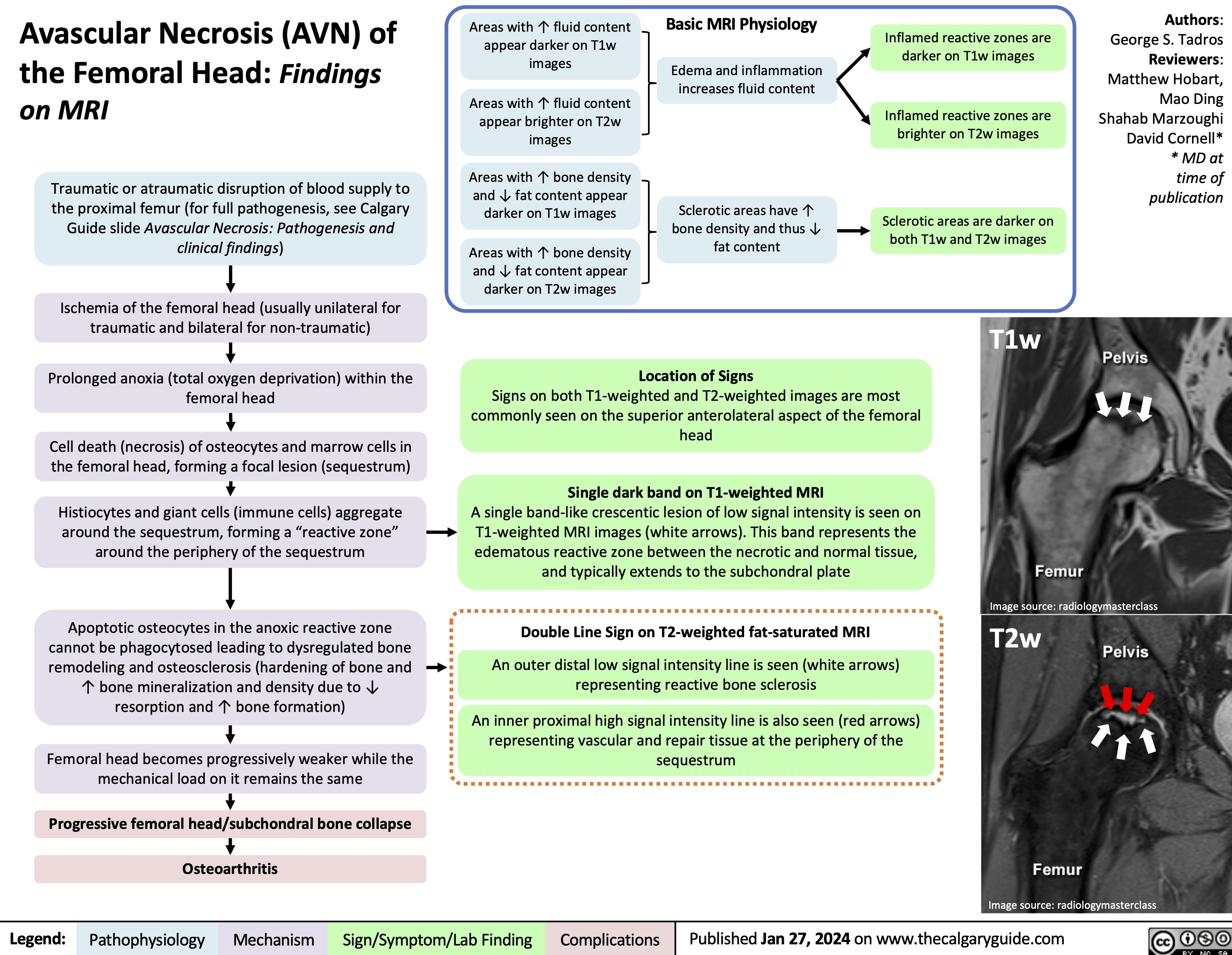
Bacterial Infections from Transfusion
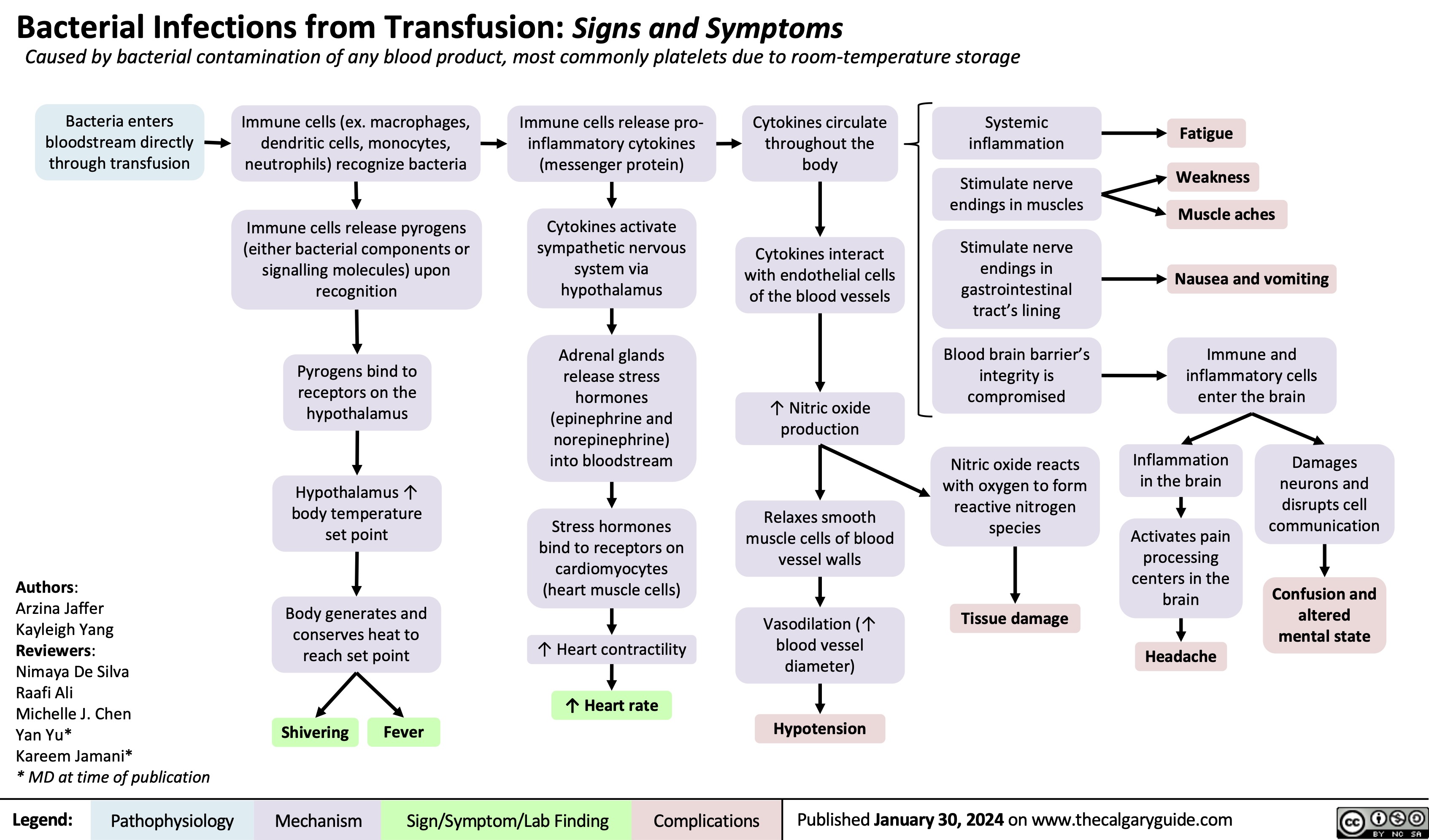
Acute Wound Healing
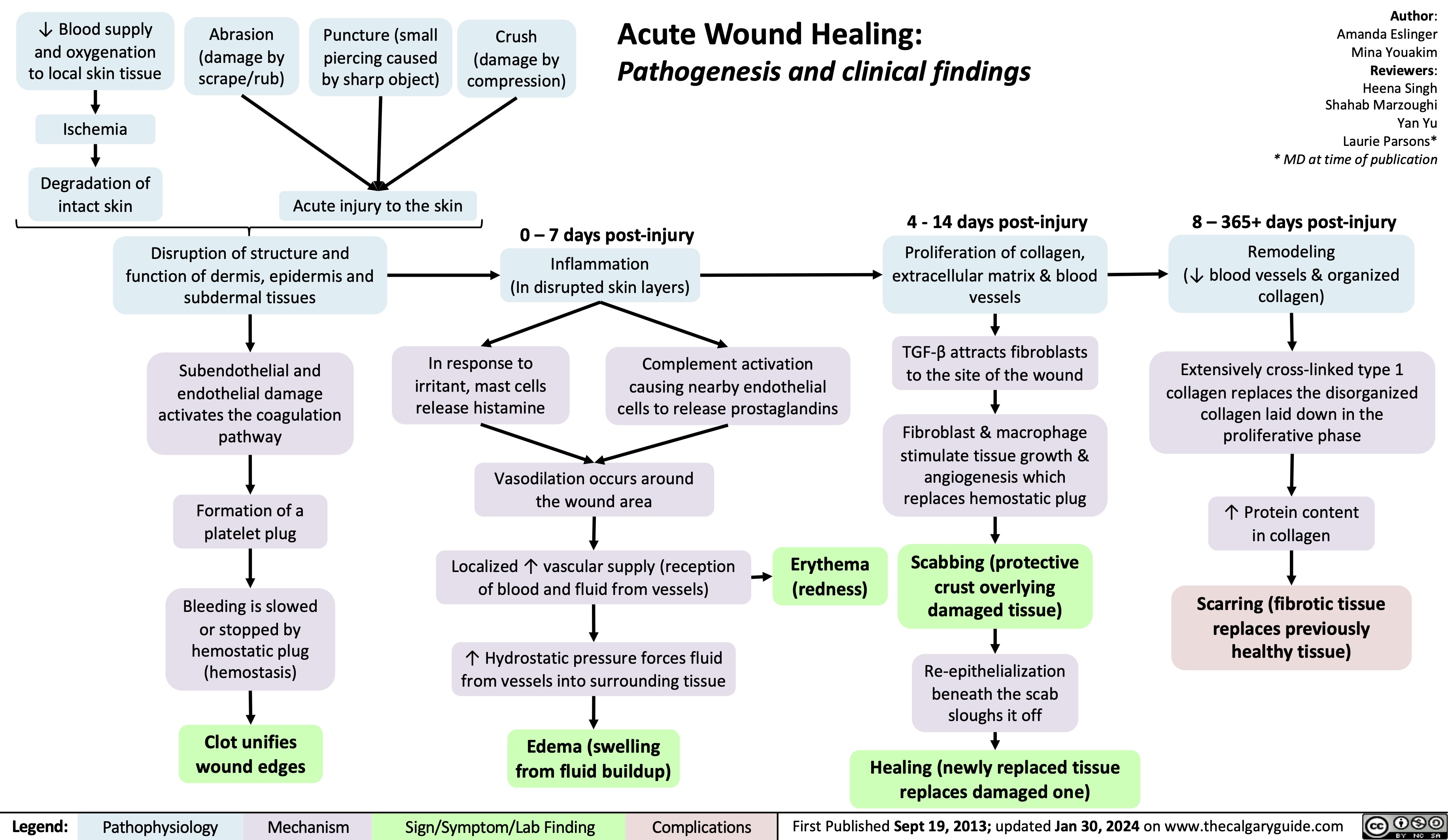
Carbonic Anhydrase Inhibitor Diuretics
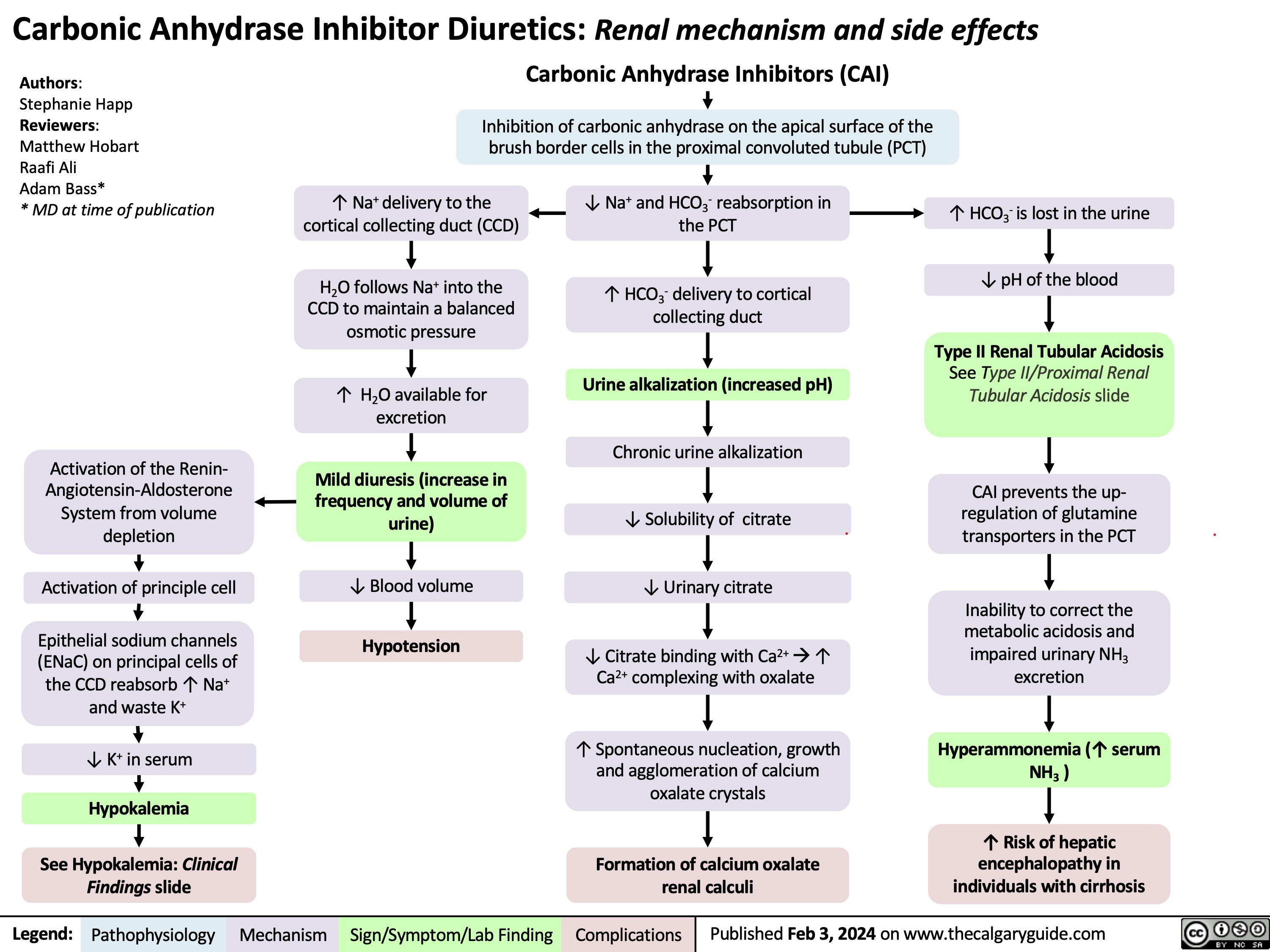
Epiglottitis
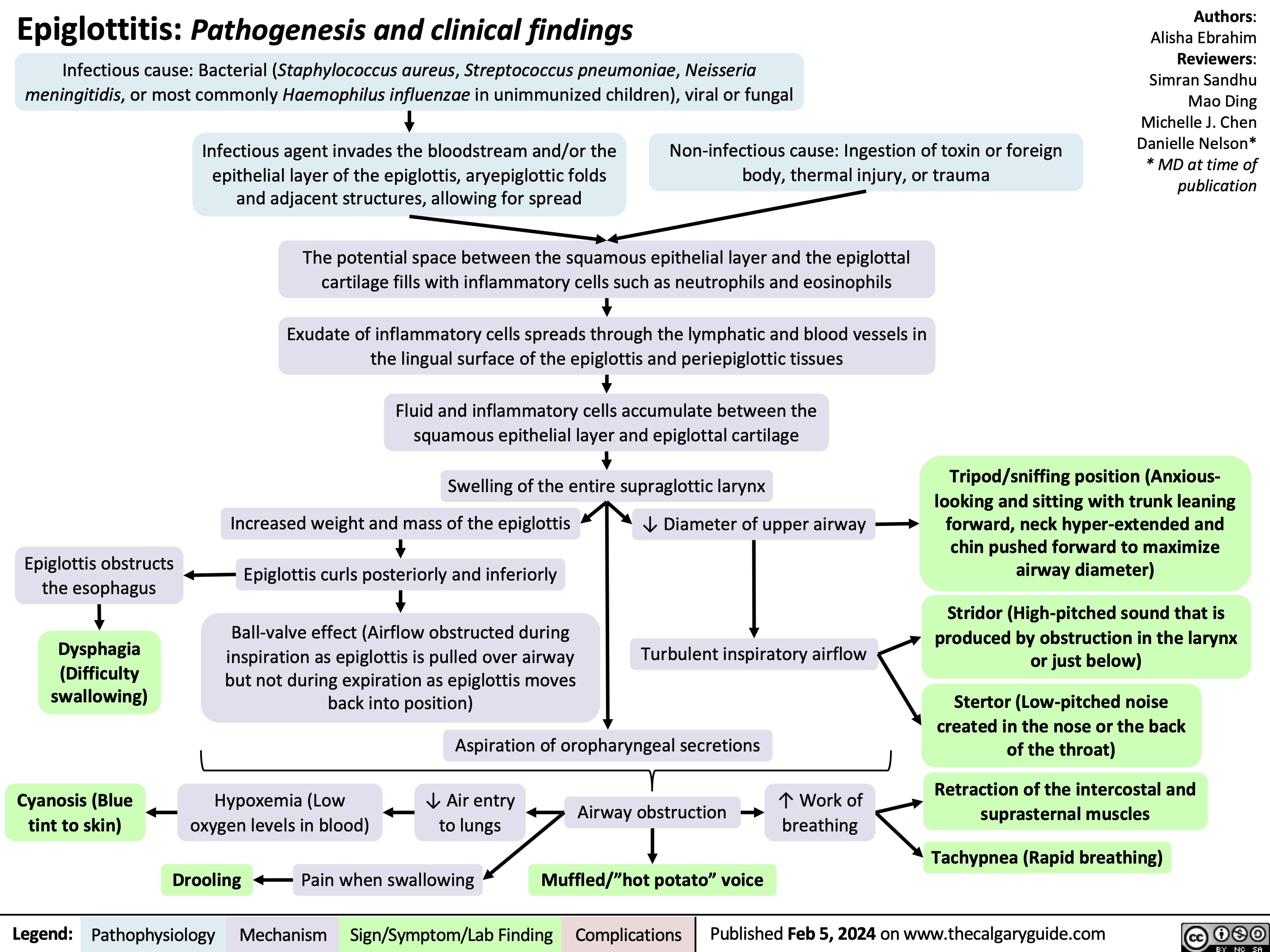
Stable Angina
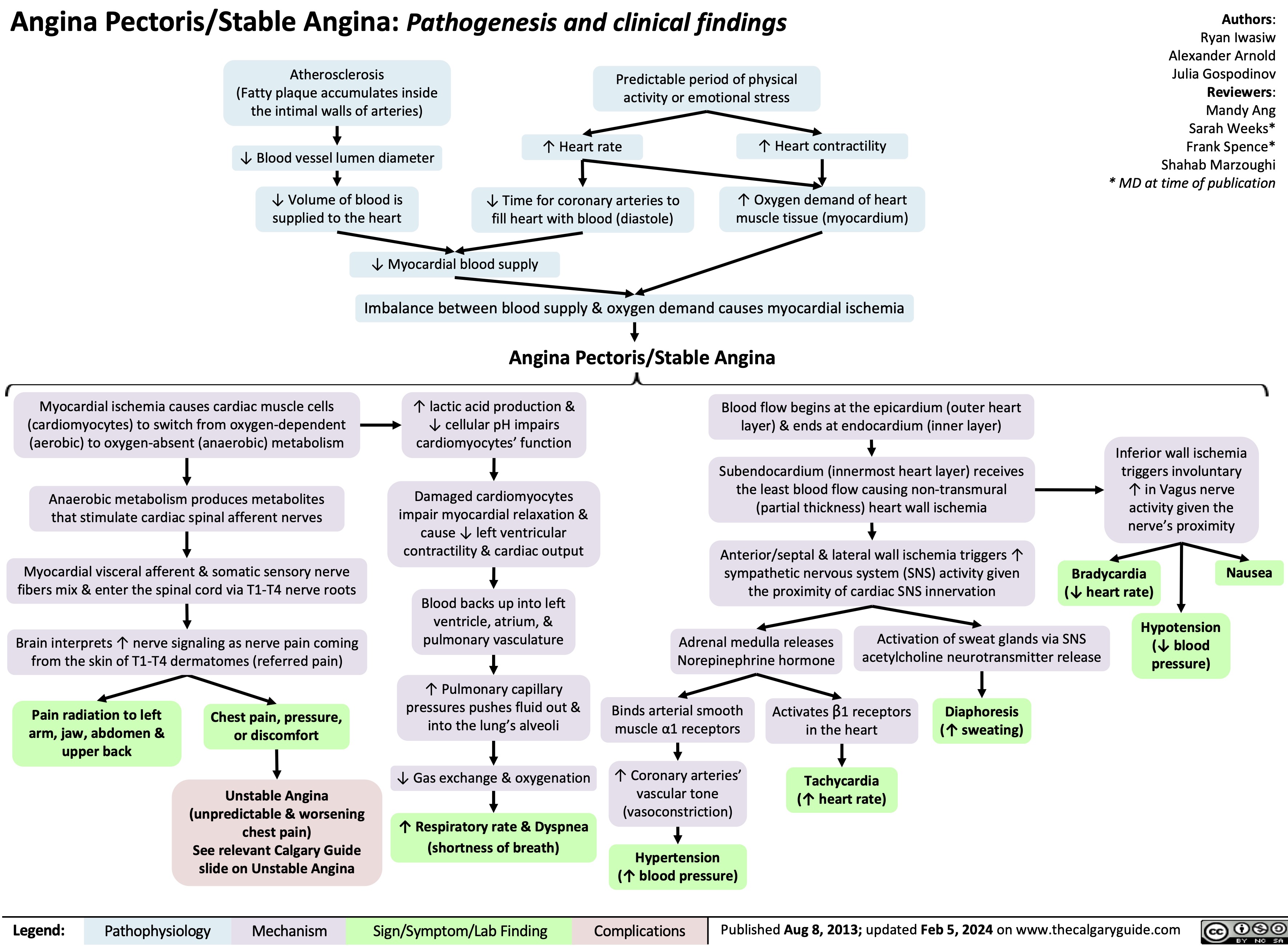
Acute Otitis Externa Complications
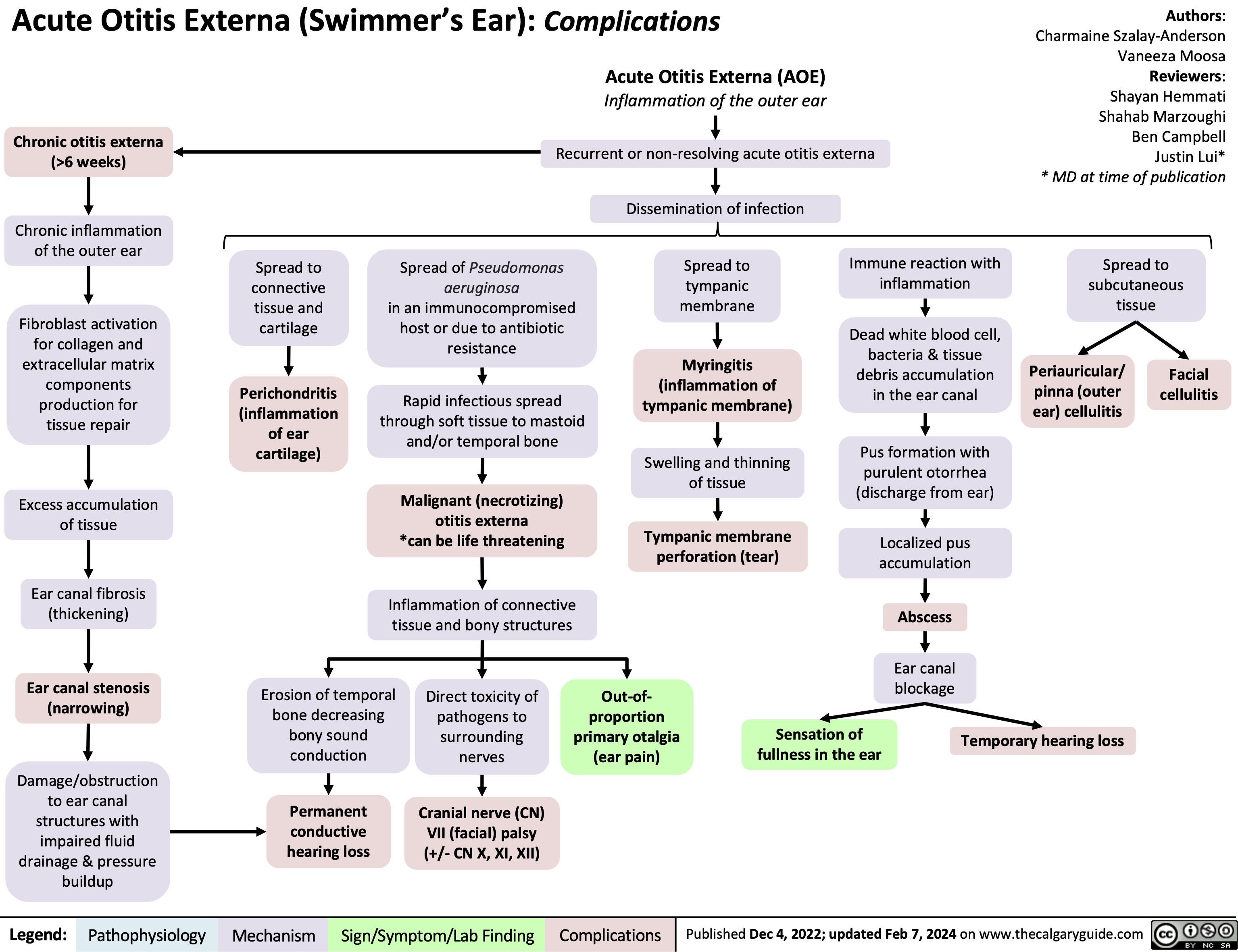
Chancroid
Dantrolene
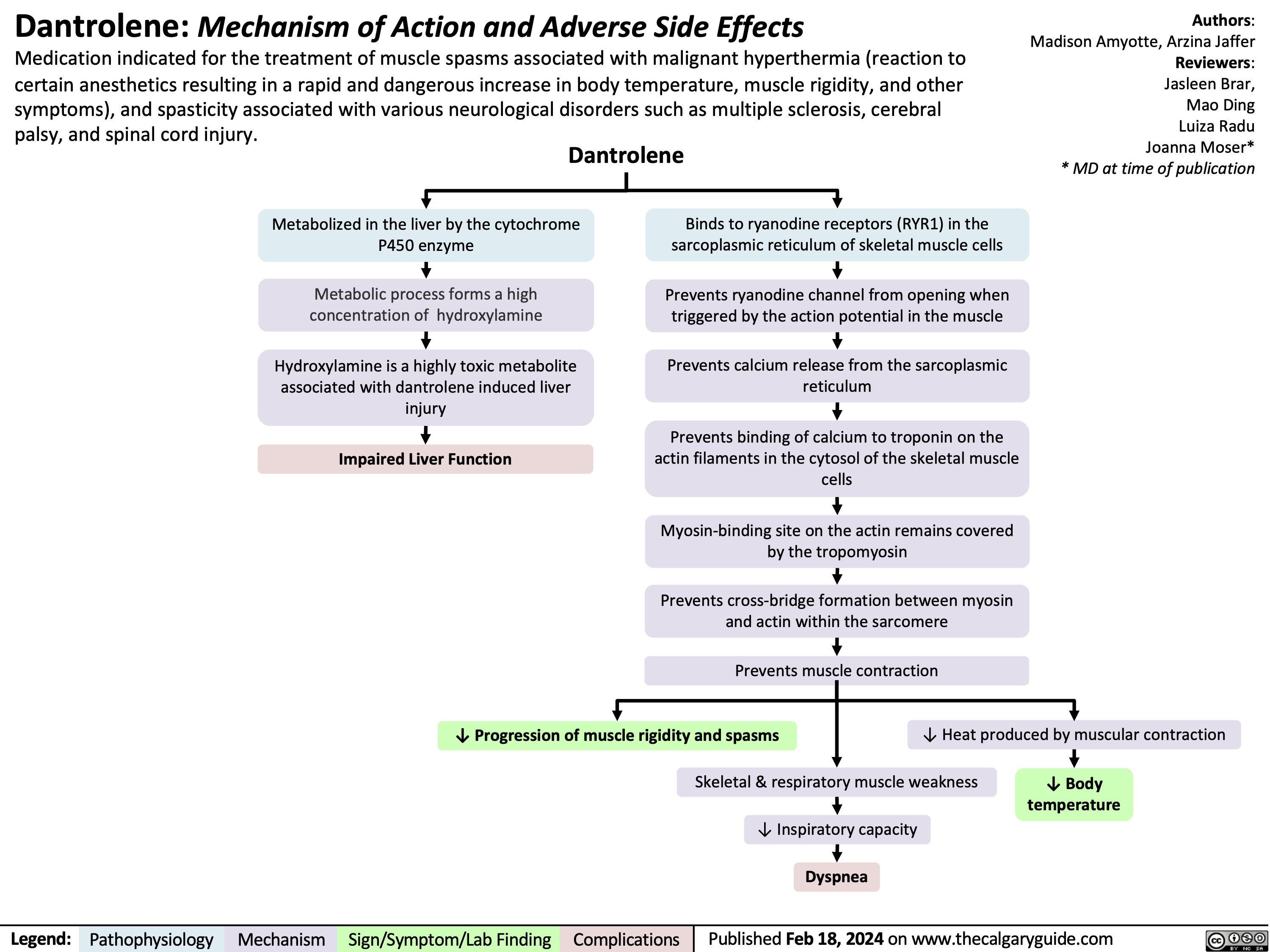
Infective endocarditis
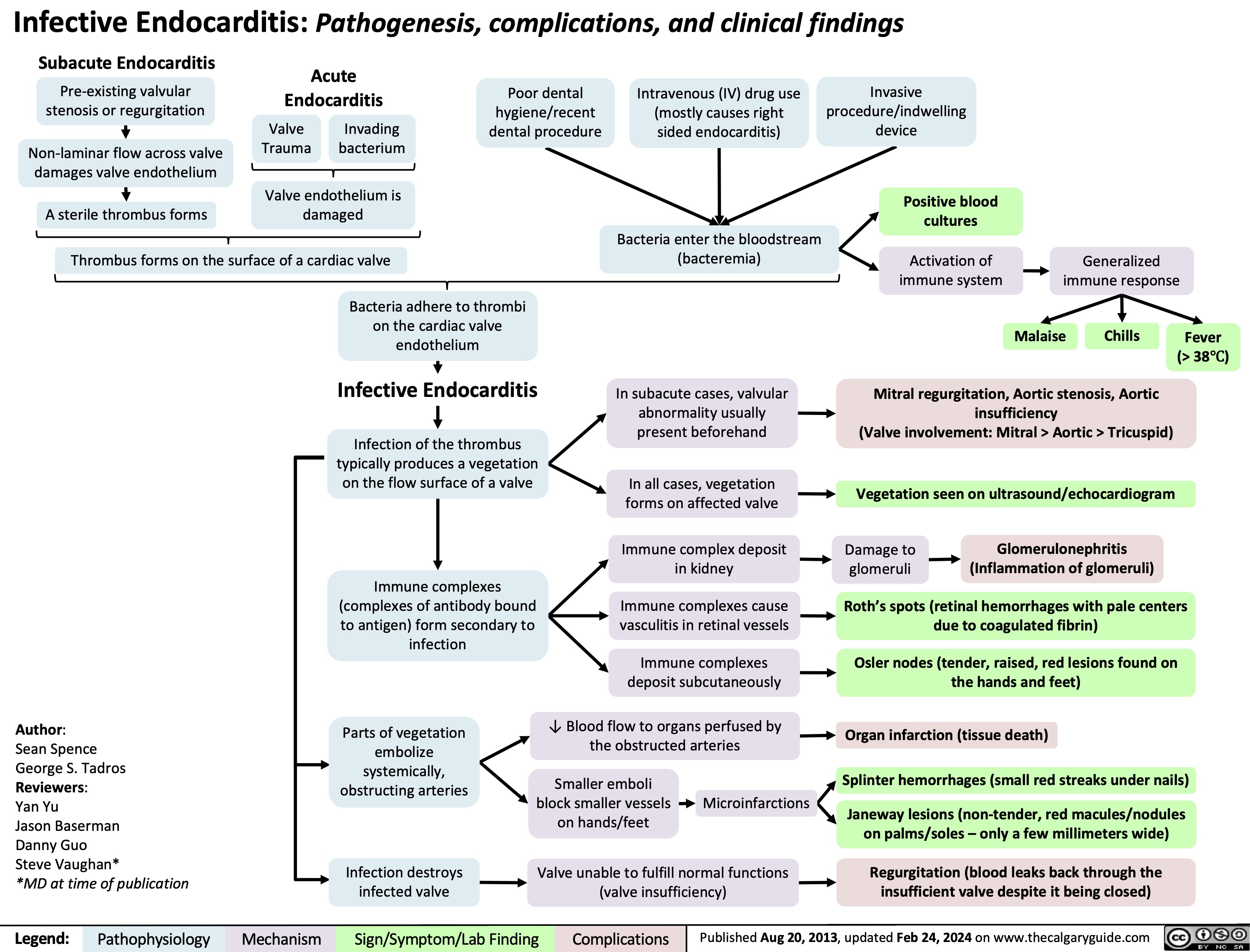
Gestational Diabetes Risk factors and pathogenesis
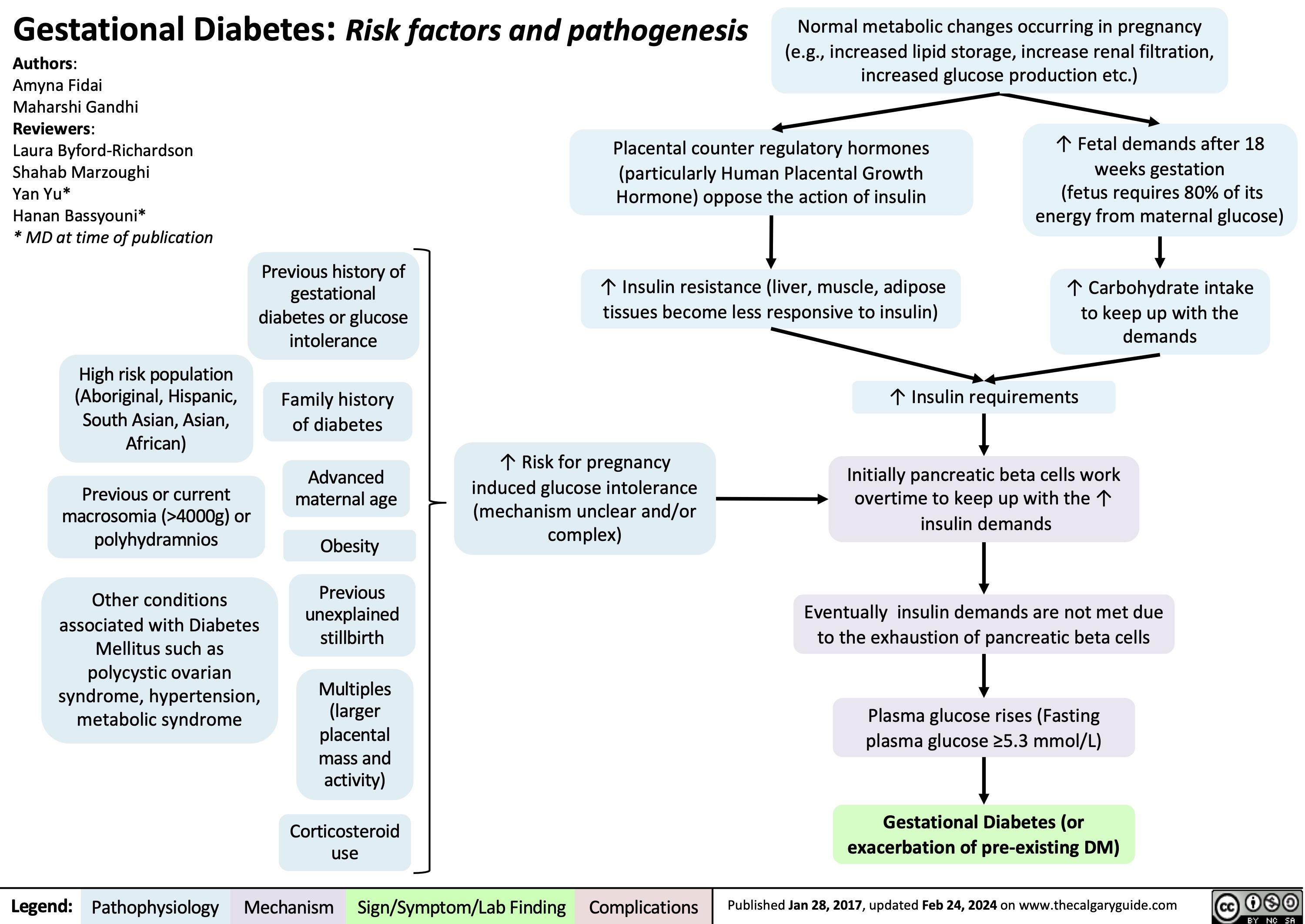
Eisenmenger Syndrome
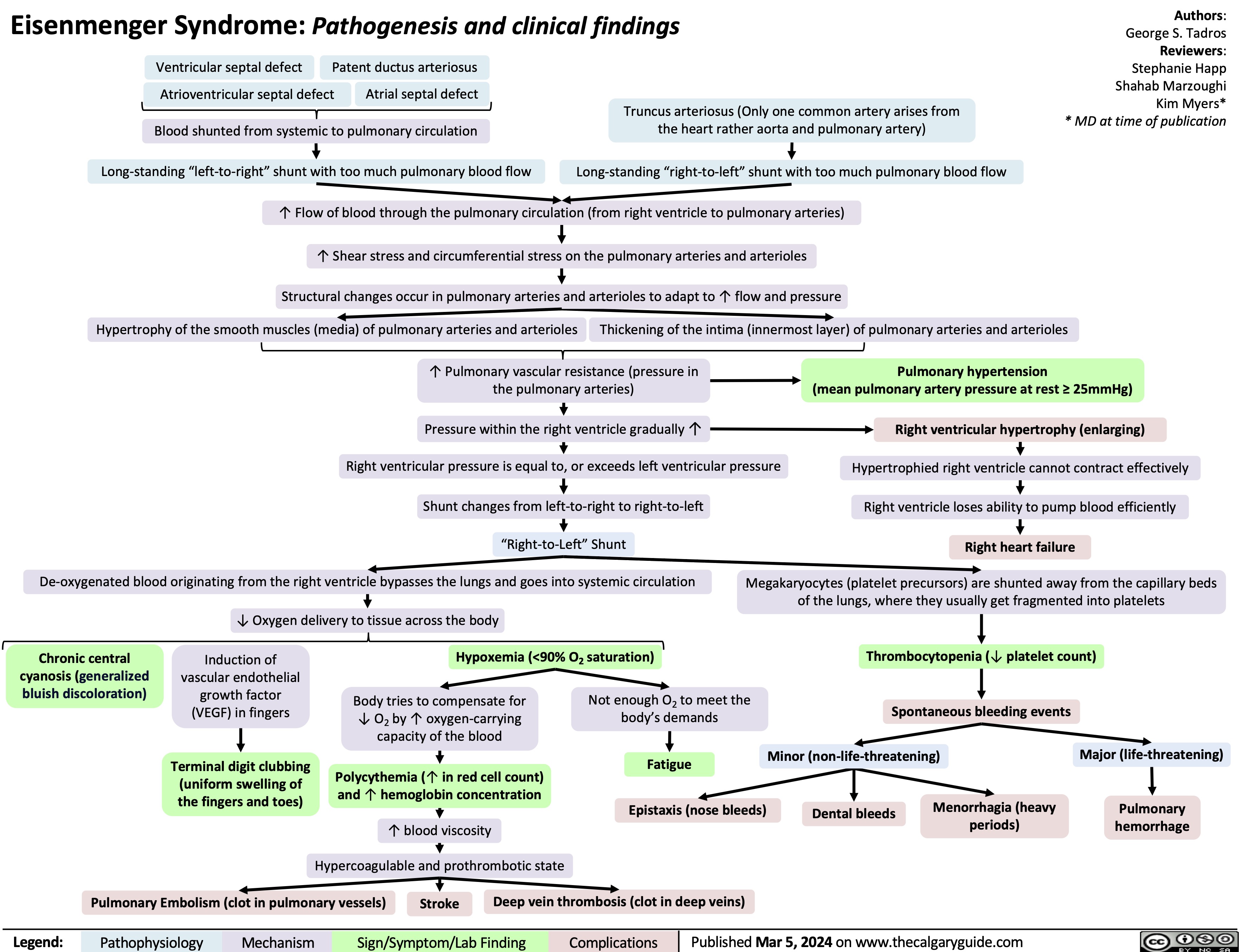
Pagets Disease pathogenesis and clinical findings
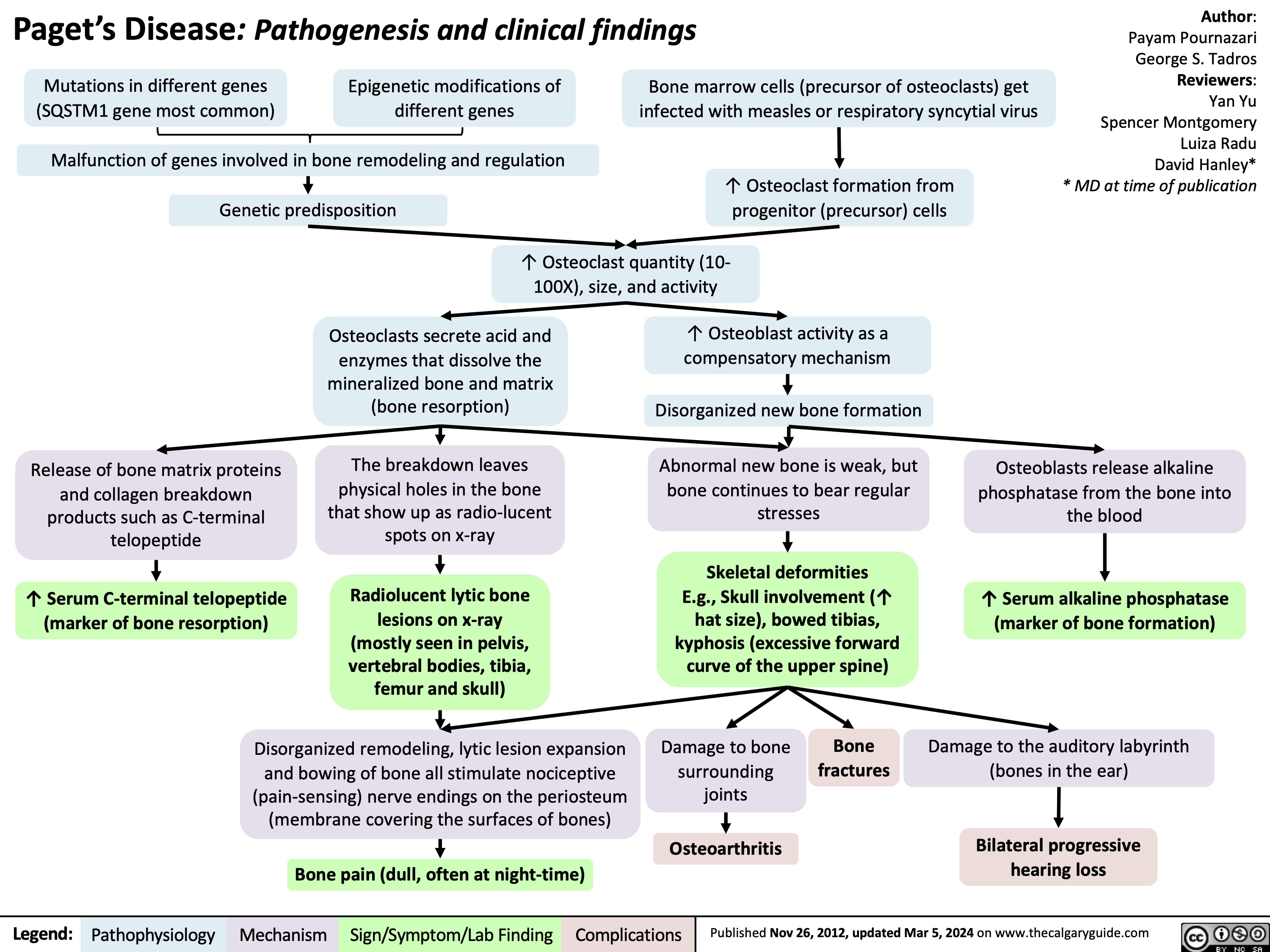
Onychomycosis
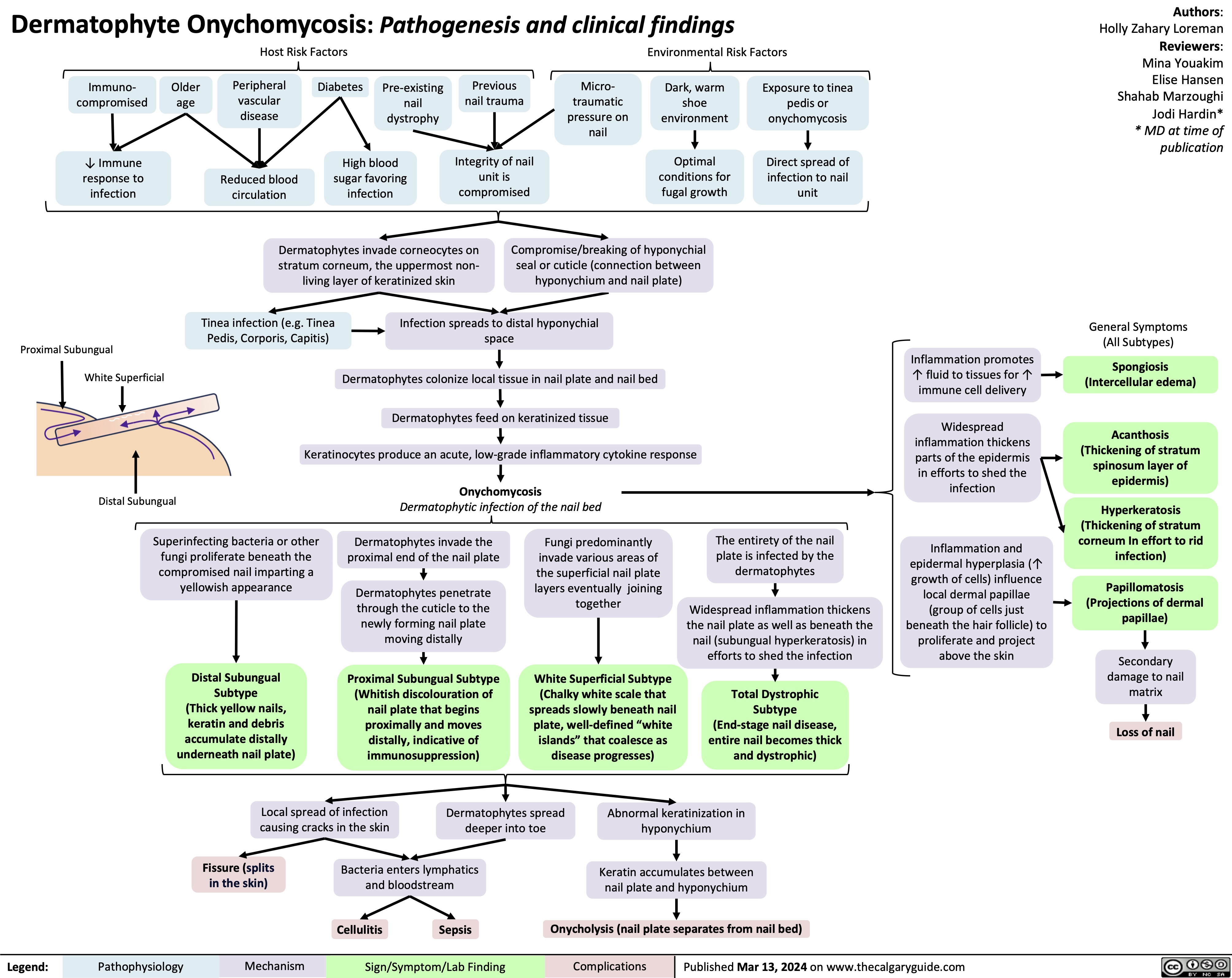
MI Complication Ventricular Wall Rupture
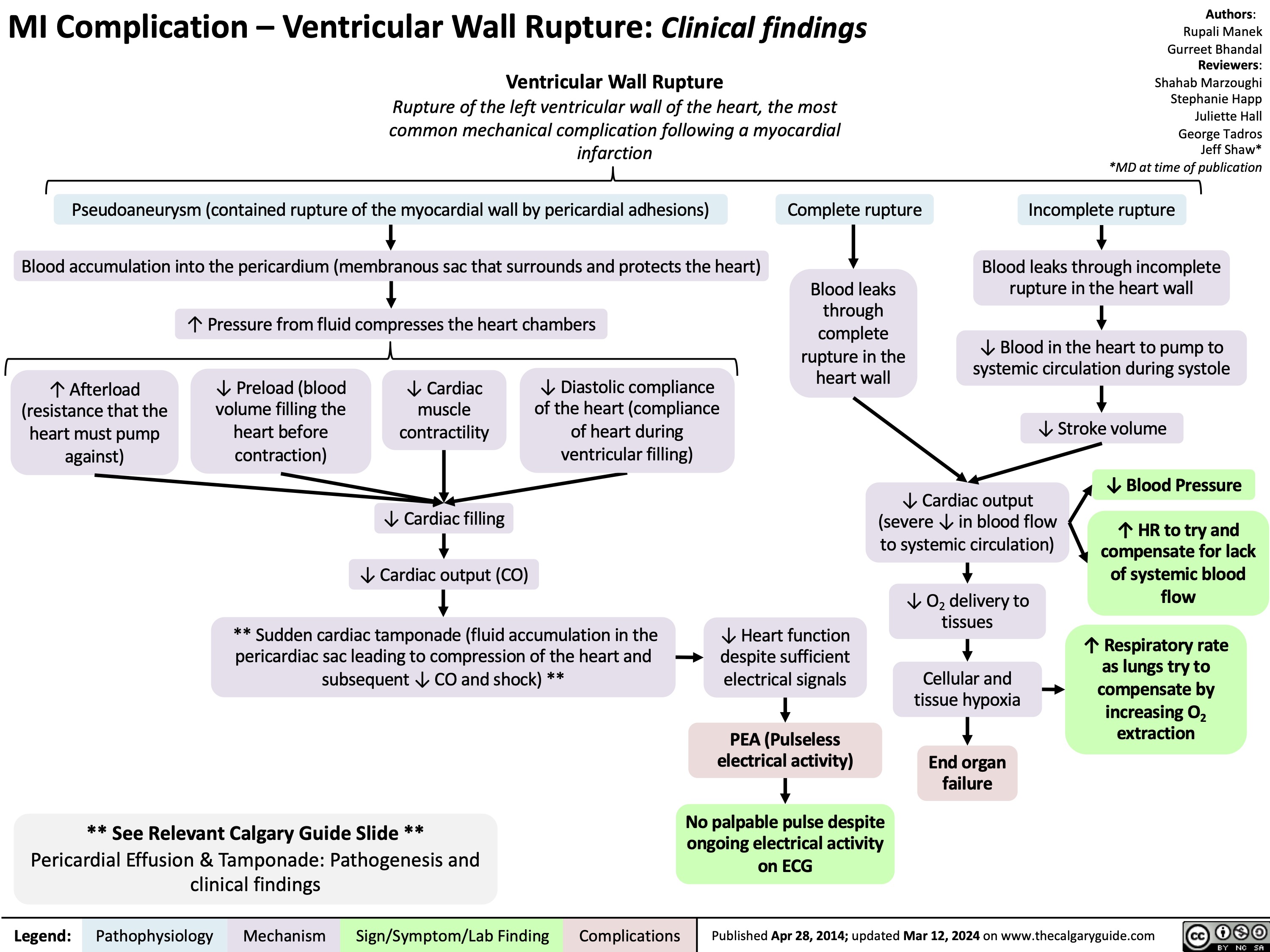
Atrial Septal Defect Pathogenesis and Clinical Findings
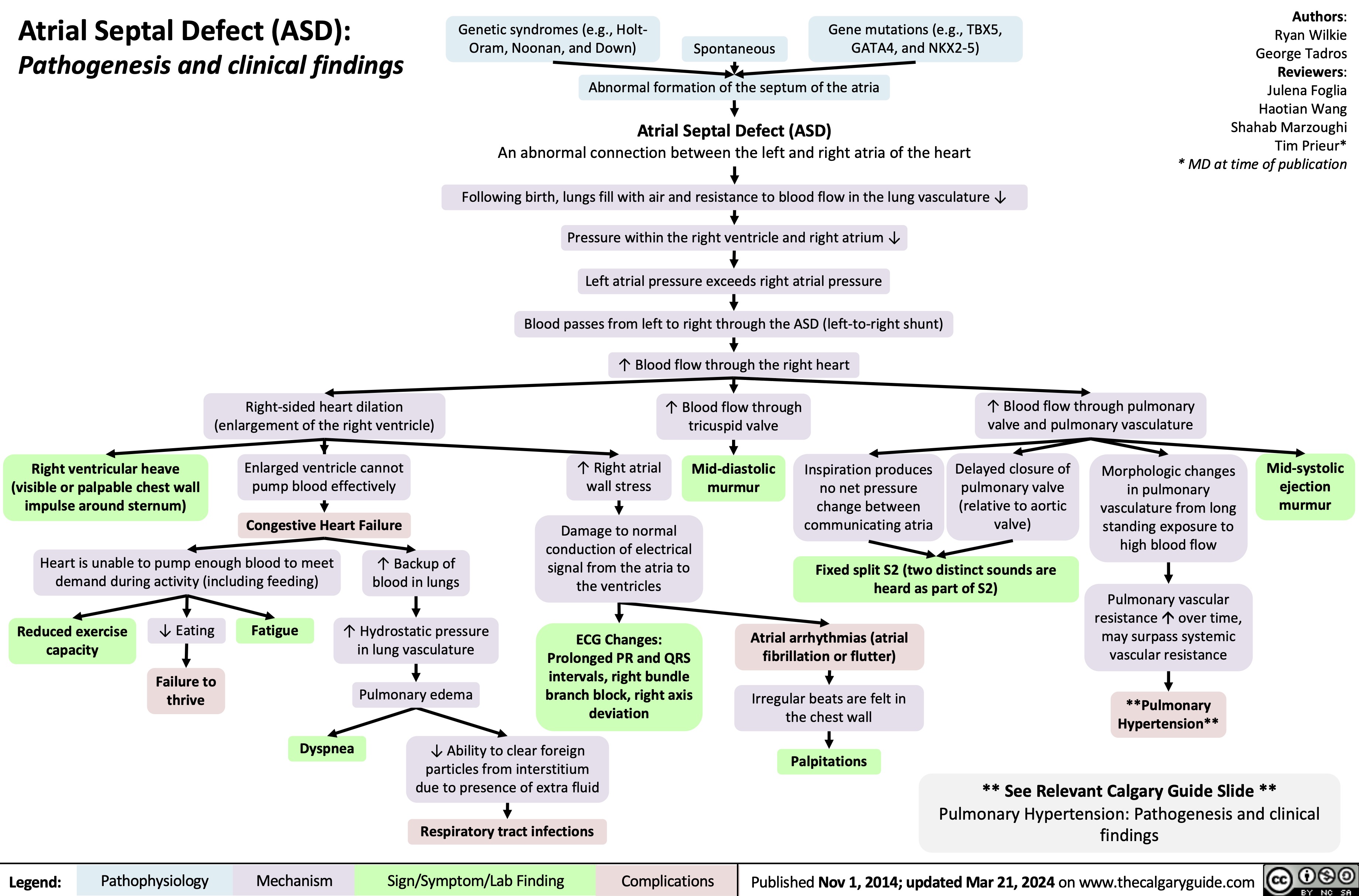
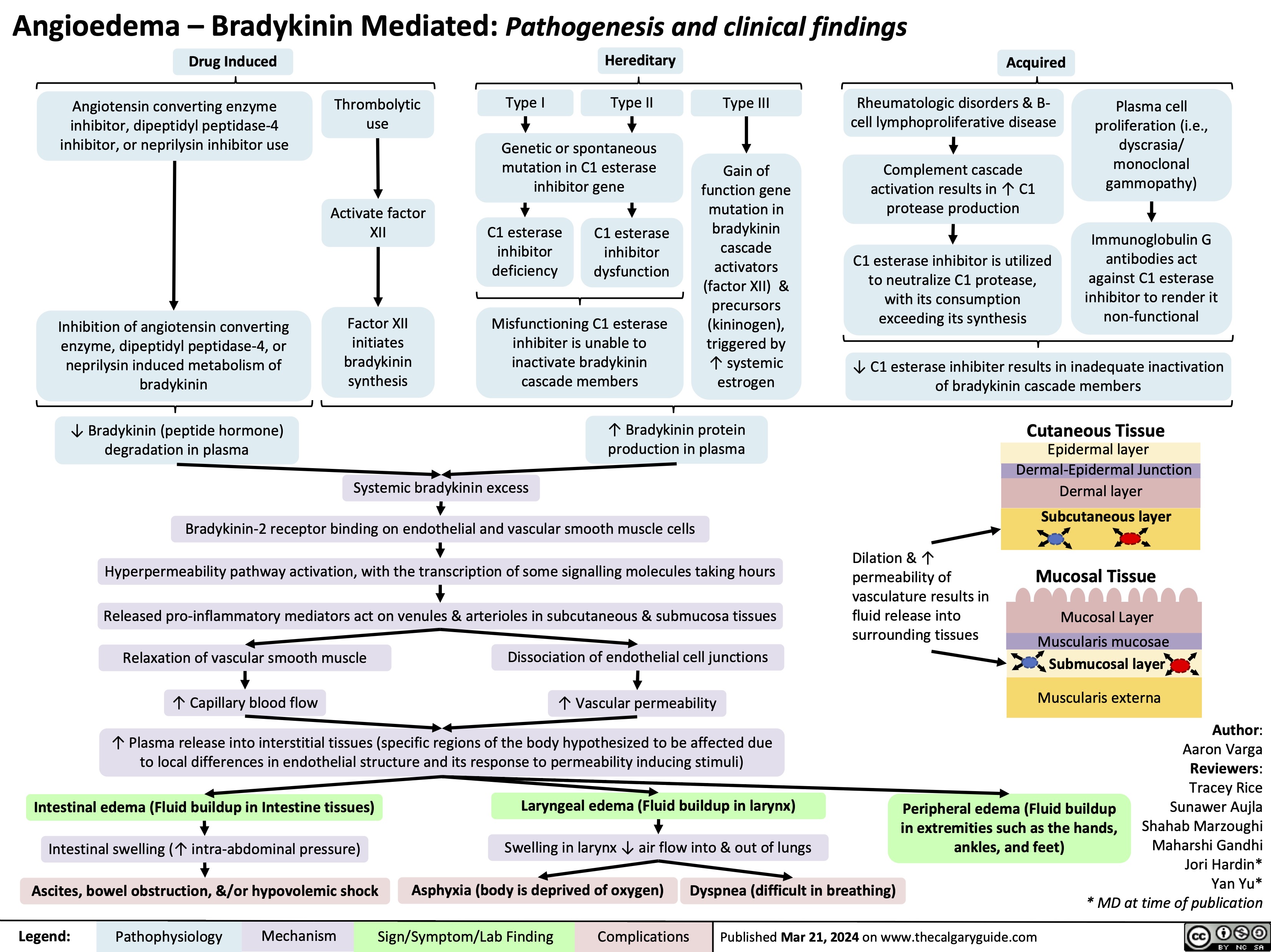
Angioedema Bradykinin Mediated
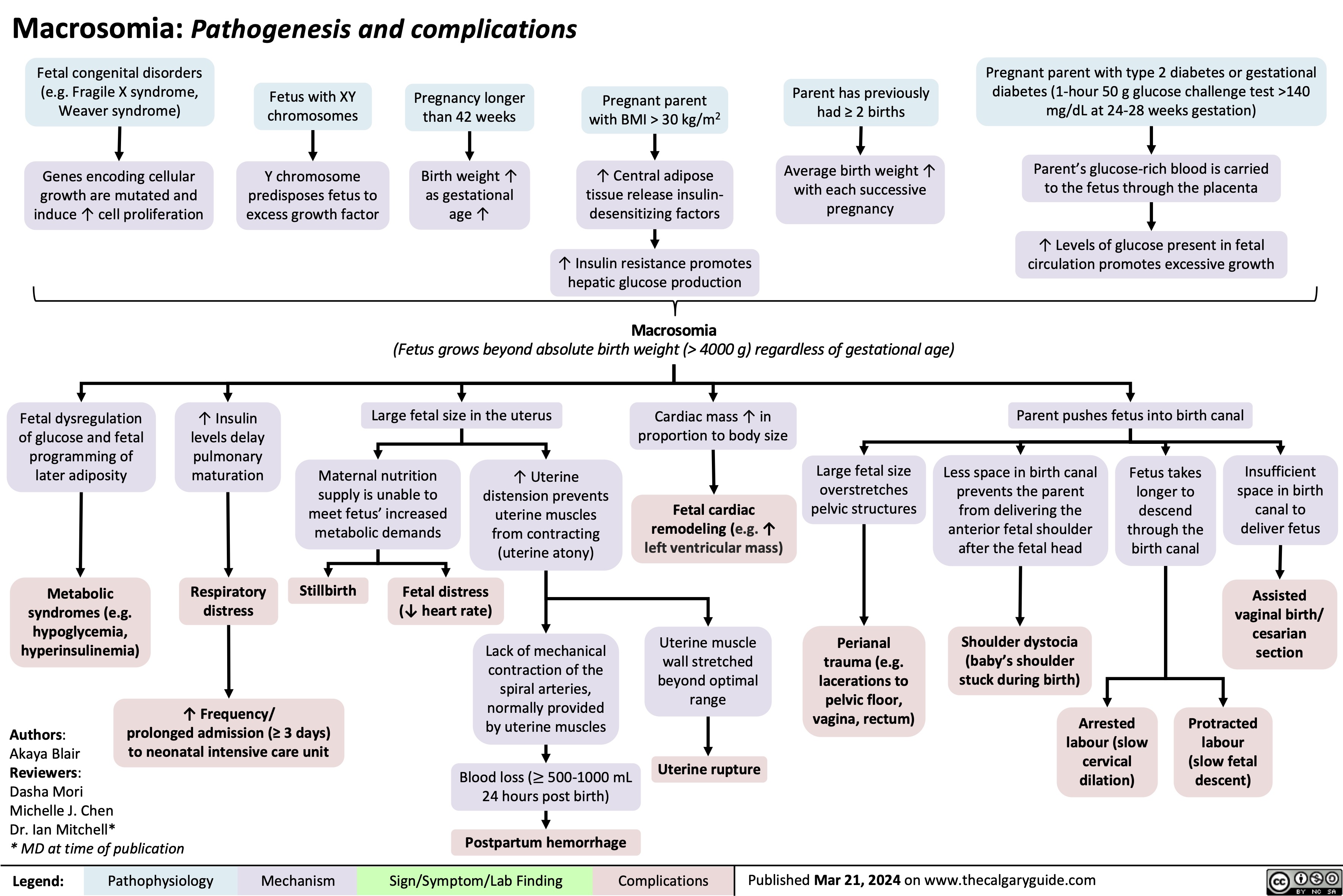
Macrosomia Pathogenesis and Complications
Apnea of Prematurity
![Apnea of Prematurity: Pathogenesis, Signs & Symptoms, and Complications Physiologic immaturity from birth at < 37 weeks gestation
Authors: Akaya Blair Reviewers:
Dasha Mori Michelle J. Chen Danielle Nelson* * MD at time of publication
↓ Synaptic connection & poor myelination
Fetal brain areas responsible for breathing are poorly developed Immature neurologic respiratory function
Immature mechanical respiratory function
Poor hypopharyngeal muscle tone (soft upper airway helps with size and compliance of airway)
Nasal obstruction (e.g. anatomic and/or iatrogenic [suctioning, NG tubes])
Neonate is reliant on nose breathing
Airway is unable to remain open (patent)
Laryngeal/tracheal abnormalities (e.g. tracheomalacia, laryngeal edema, tracheal stenosis)
Anatomical narrowing leading to ↑ airway resistance
↑ Risk of mechanical airway obstruction
Disruption of central respiratory drive
↓ Sensitivity to increased CO2 in the ventral medulla oblongata
Disruption of peripheral respiratory reflex pathways
↓ Sensitivity to CO2 levels in peripheral carotid bodies and aortic bodies
Large head size forces neck into flexion when laying supine
Immature airway sensitive to collapse when in flexion
↑ Hypotonia (decreased muscle tone) in REM sleep
↓ Signaling to brainstem
Brainstem unable to mount appropriate ventilatory response to insufficient oxygen
Upper airway collapse
Apnea of prematurity
Respiratory pauses >20 sec or pauses <20 sec with bradycardia (<100 beats per minute), central cyanosis, and/or oxygen saturation <85% in neonates born at <37 weeks gestation and with no underlying disorders causing apnea. Most apneas in apnea of prematurity are central or mixed.
↓ Breathing rate
Bradycardia (<100 bpm)
↓ Oxygen to brain
Poor neurodevelopmental outcomes (e.g. cognitive function, brain adaptive potential and plasticity)
Hypoxemia (↓blood oxygen levels where SpO2 <85%)
↓ Oxygen and hemoglobin to mucous membranes (e.g. lips) & fingers and toes (periphery)
Central & peripheral cyanosis (bluish discoloration)
↓ Oxygen to retina
Abnormal growth of blood vessels in eyes
Retinopathy of prematurity (changes in visual acuity and possible blindness)
Death/impairment in cell function from lack of oxygen
↑ Risk of infant mortality
Imbalanced oxygen intake and CO2 output in lungs
Body transiently ↑ HR to unsuccessfully try to compensate for ↓ tissue oxygenation
Respiratory failure
Respiratory rate >60 ↓ Heart rate
↓ Blood pressure
Head bobbing Abdominal breathing
Skin mottling
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Mar 21, 2024 on www.thecalgaryguide.com
Apnea of Prematurity: Pathogenesis, Signs & Symptoms, and Complications Physiologic immaturity from birth at < 37 weeks gestation
Authors: Akaya Blair Reviewers:
Dasha Mori Michelle J. Chen Danielle Nelson* * MD at time of publication
↓ Synaptic connection & poor myelination
Fetal brain areas responsible for breathing are poorly developed Immature neurologic respiratory function
Immature mechanical respiratory function
Poor hypopharyngeal muscle tone (soft upper airway helps with size and compliance of airway)
Nasal obstruction (e.g. anatomic and/or iatrogenic [suctioning, NG tubes])
Neonate is reliant on nose breathing
Airway is unable to remain open (patent)
Laryngeal/tracheal abnormalities (e.g. tracheomalacia, laryngeal edema, tracheal stenosis)
Anatomical narrowing leading to ↑ airway resistance
↑ Risk of mechanical airway obstruction
Disruption of central respiratory drive
↓ Sensitivity to increased CO2 in the ventral medulla oblongata
Disruption of peripheral respiratory reflex pathways
↓ Sensitivity to CO2 levels in peripheral carotid bodies and aortic bodies
Large head size forces neck into flexion when laying supine
Immature airway sensitive to collapse when in flexion
↑ Hypotonia (decreased muscle tone) in REM sleep
↓ Signaling to brainstem
Brainstem unable to mount appropriate ventilatory response to insufficient oxygen
Upper airway collapse
Apnea of prematurity
Respiratory pauses >20 sec or pauses <20 sec with bradycardia (<100 beats per minute), central cyanosis, and/or oxygen saturation <85% in neonates born at <37 weeks gestation and with no underlying disorders causing apnea. Most apneas in apnea of prematurity are central or mixed.
↓ Breathing rate
Bradycardia (<100 bpm)
↓ Oxygen to brain
Poor neurodevelopmental outcomes (e.g. cognitive function, brain adaptive potential and plasticity)
Hypoxemia (↓blood oxygen levels where SpO2 <85%)
↓ Oxygen and hemoglobin to mucous membranes (e.g. lips) & fingers and toes (periphery)
Central & peripheral cyanosis (bluish discoloration)
↓ Oxygen to retina
Abnormal growth of blood vessels in eyes
Retinopathy of prematurity (changes in visual acuity and possible blindness)
Death/impairment in cell function from lack of oxygen
↑ Risk of infant mortality
Imbalanced oxygen intake and CO2 output in lungs
Body transiently ↑ HR to unsuccessfully try to compensate for ↓ tissue oxygenation
Respiratory failure
Respiratory rate >60 ↓ Heart rate
↓ Blood pressure
Head bobbing Abdominal breathing
Skin mottling
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Mar 21, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/03/Apnea-of-Prematurity.jpg)
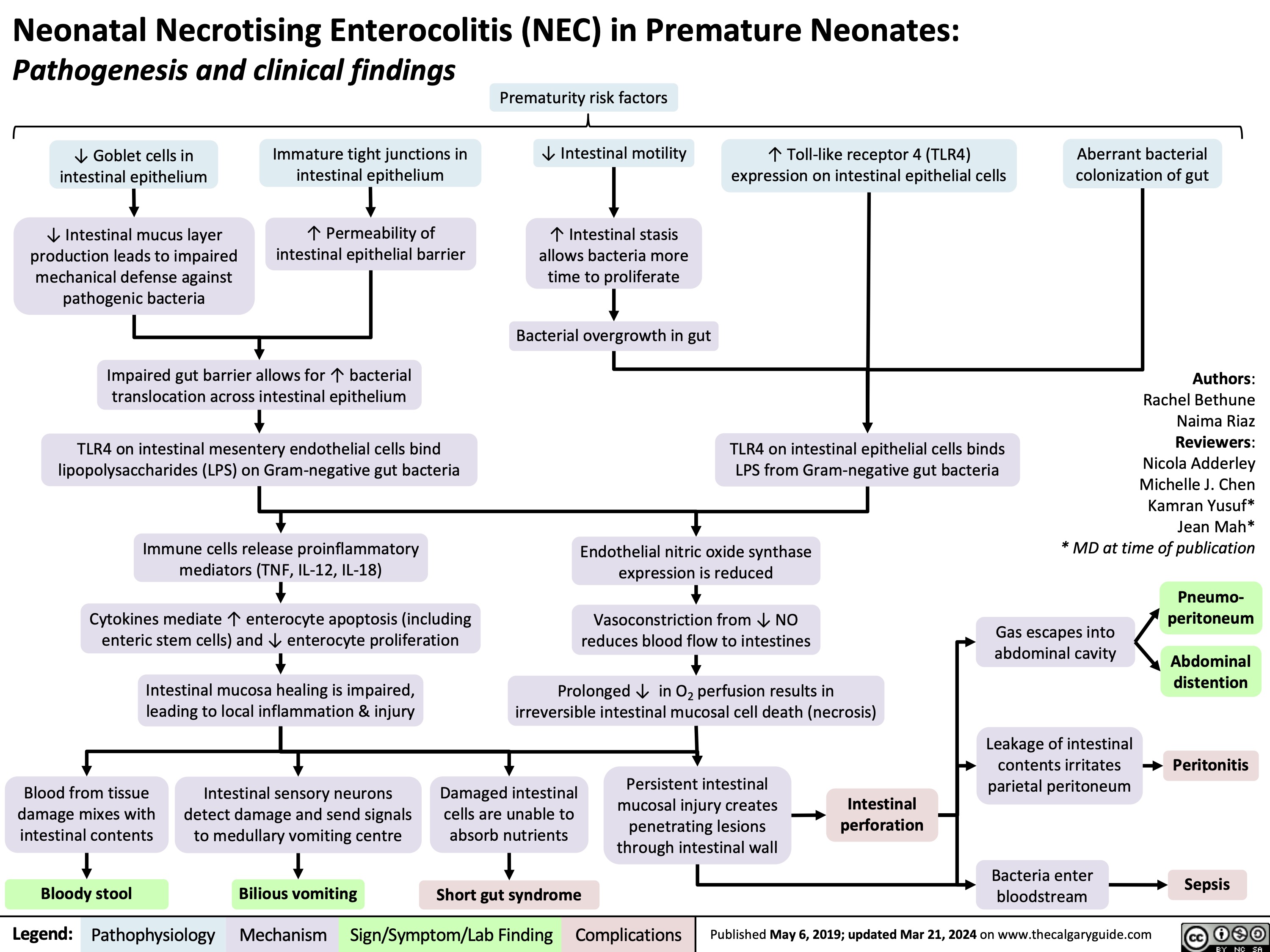
Neonatal Necrotising Enterocolitis in Premature Neonates
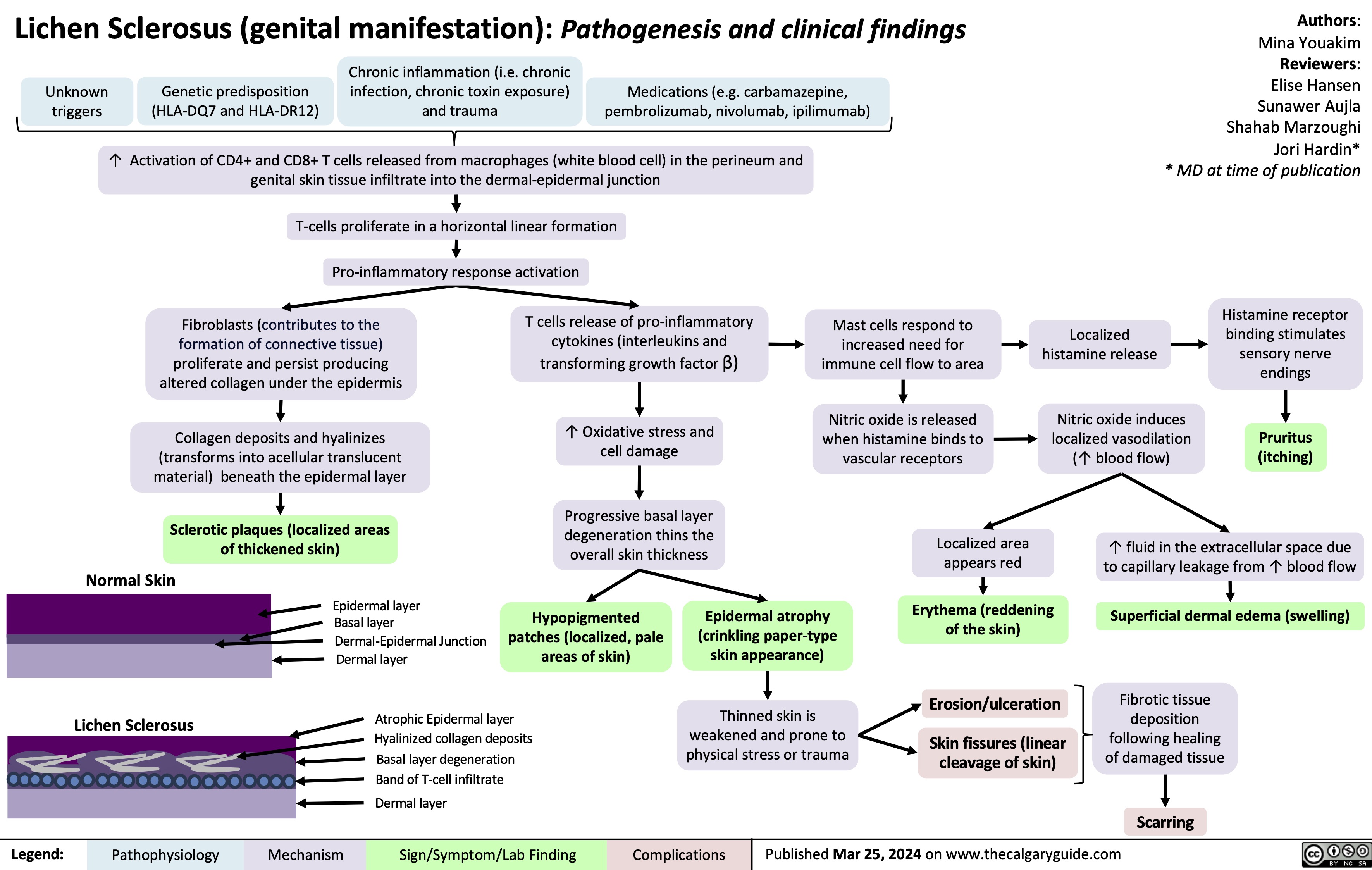
Lichen Sclerosus
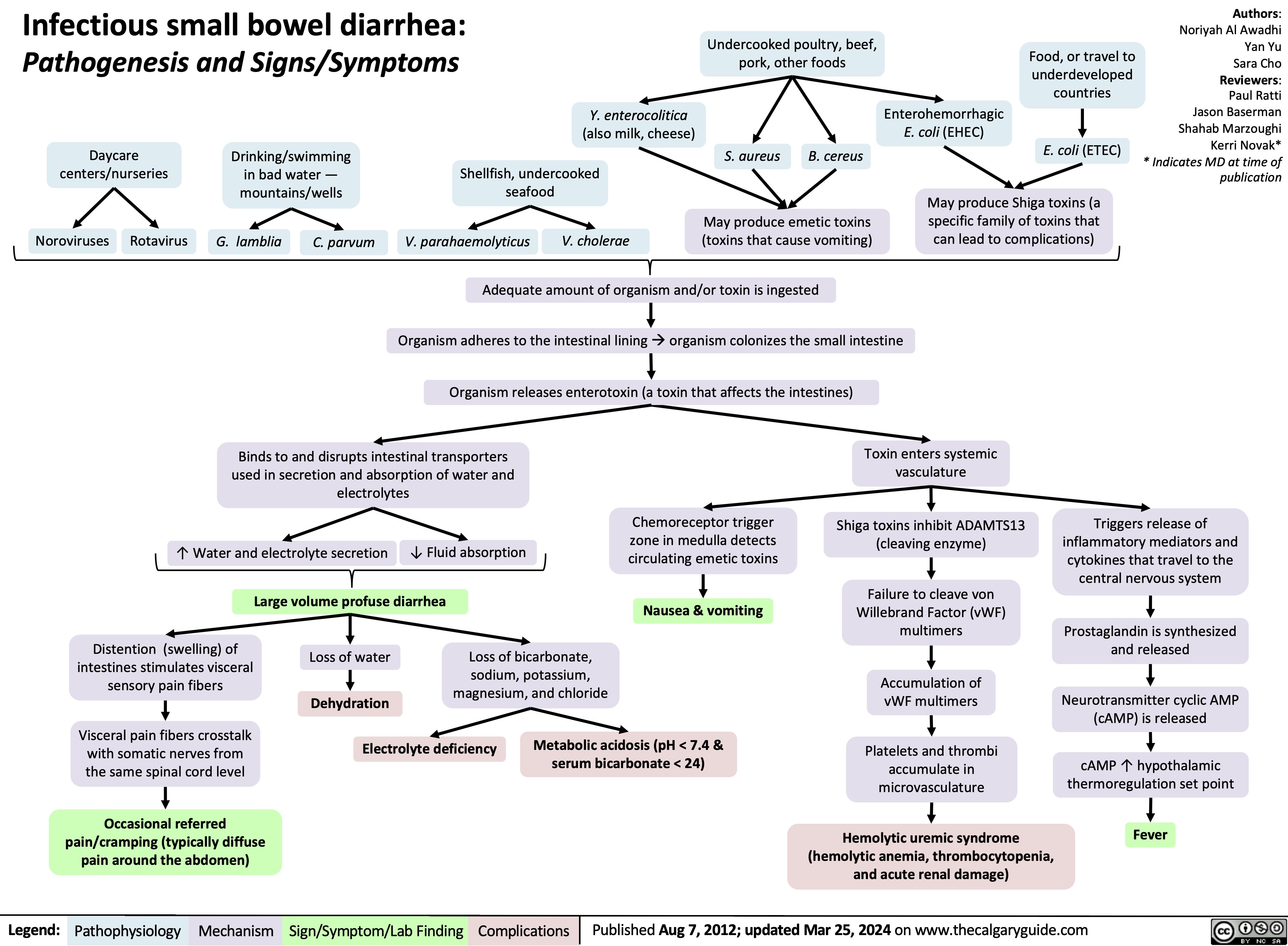
Infectious Small Bowel Diarrhea
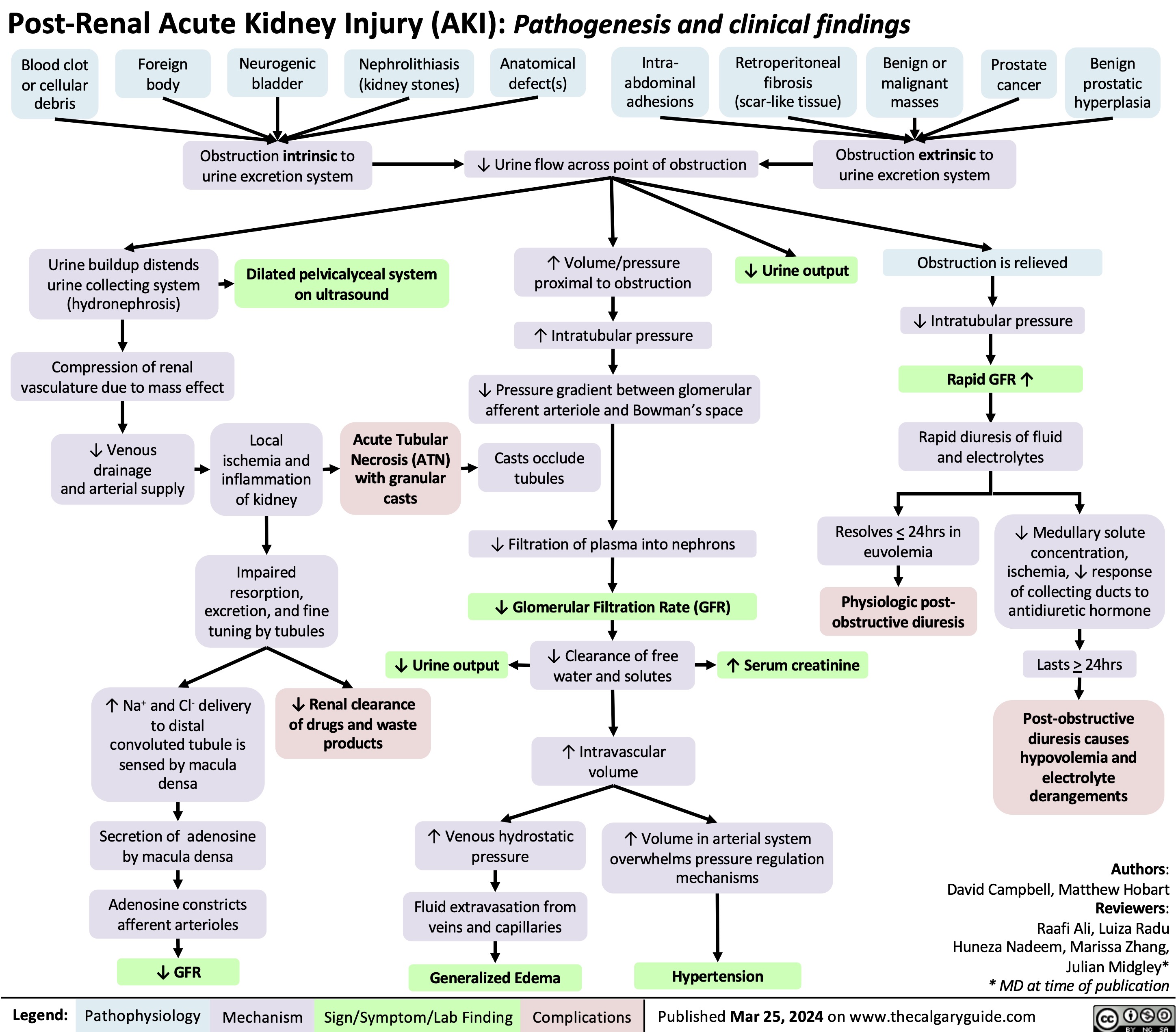
Post-Renal Acute Kidney Injury AKI
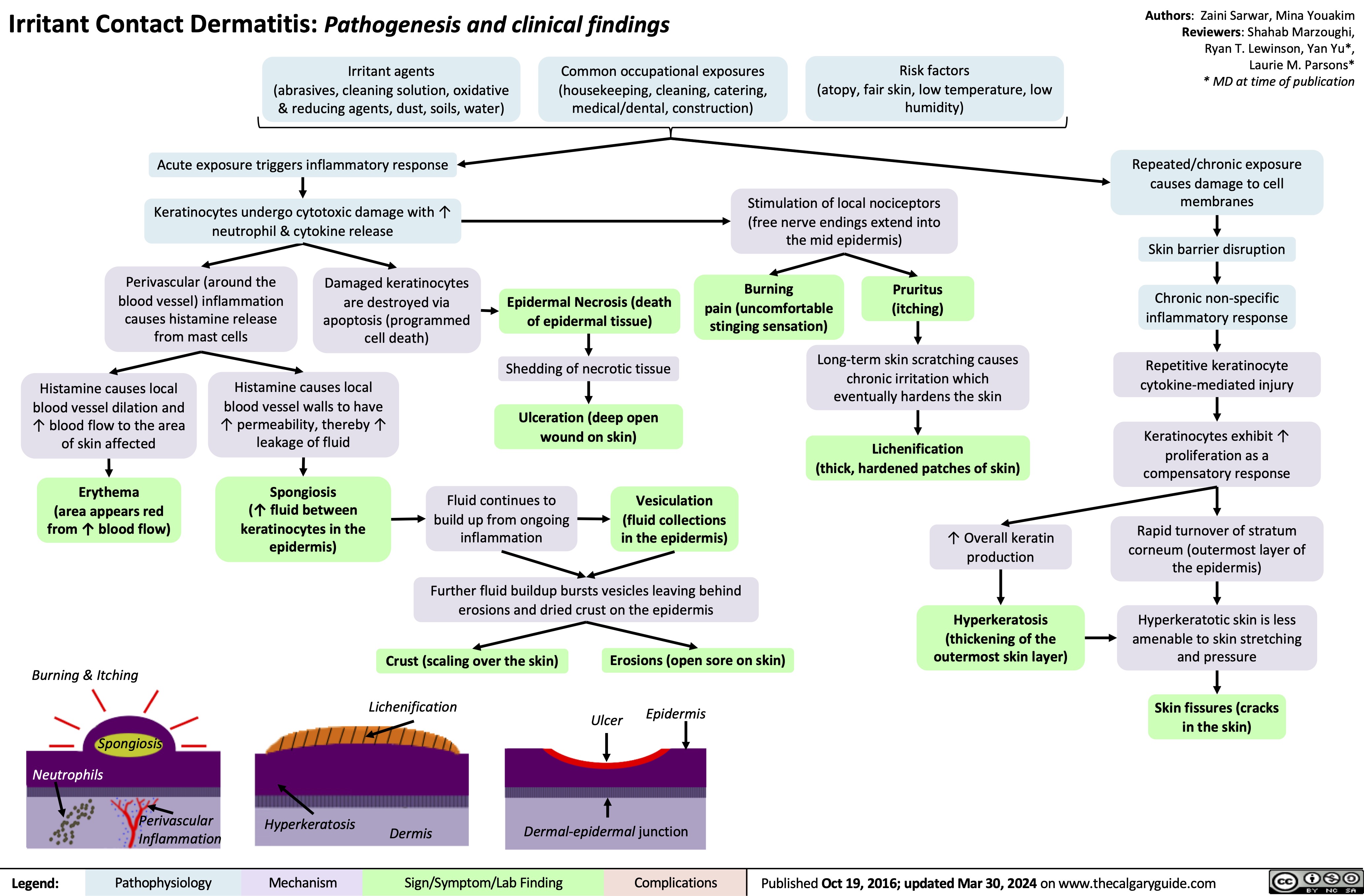
Irritant Contact Dermatitis Pathogenesis and Clinical Findings
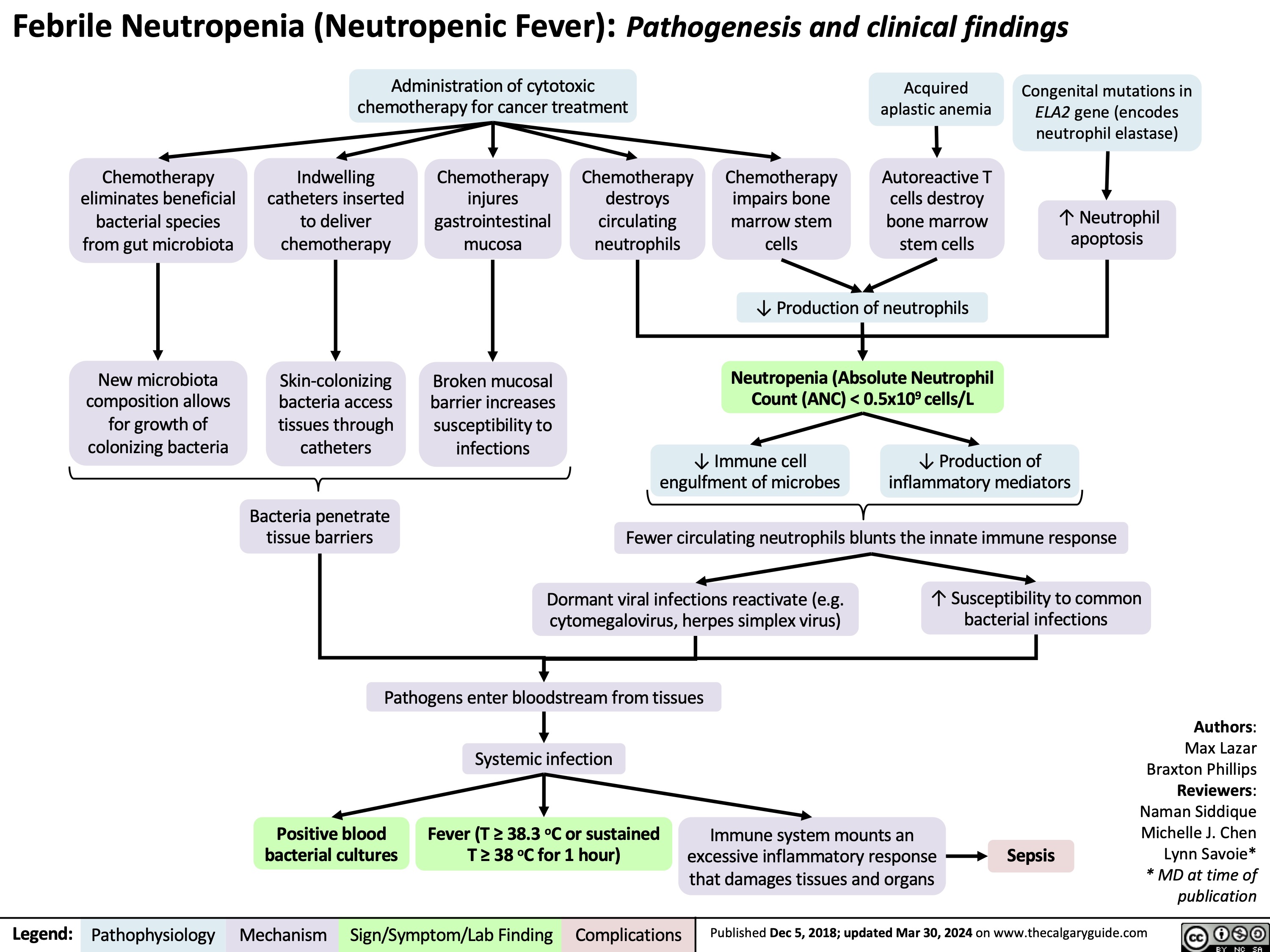
Febrile Neutropenia Pathogenesis and clinical findings
Overview of Ischemic Heart Disease
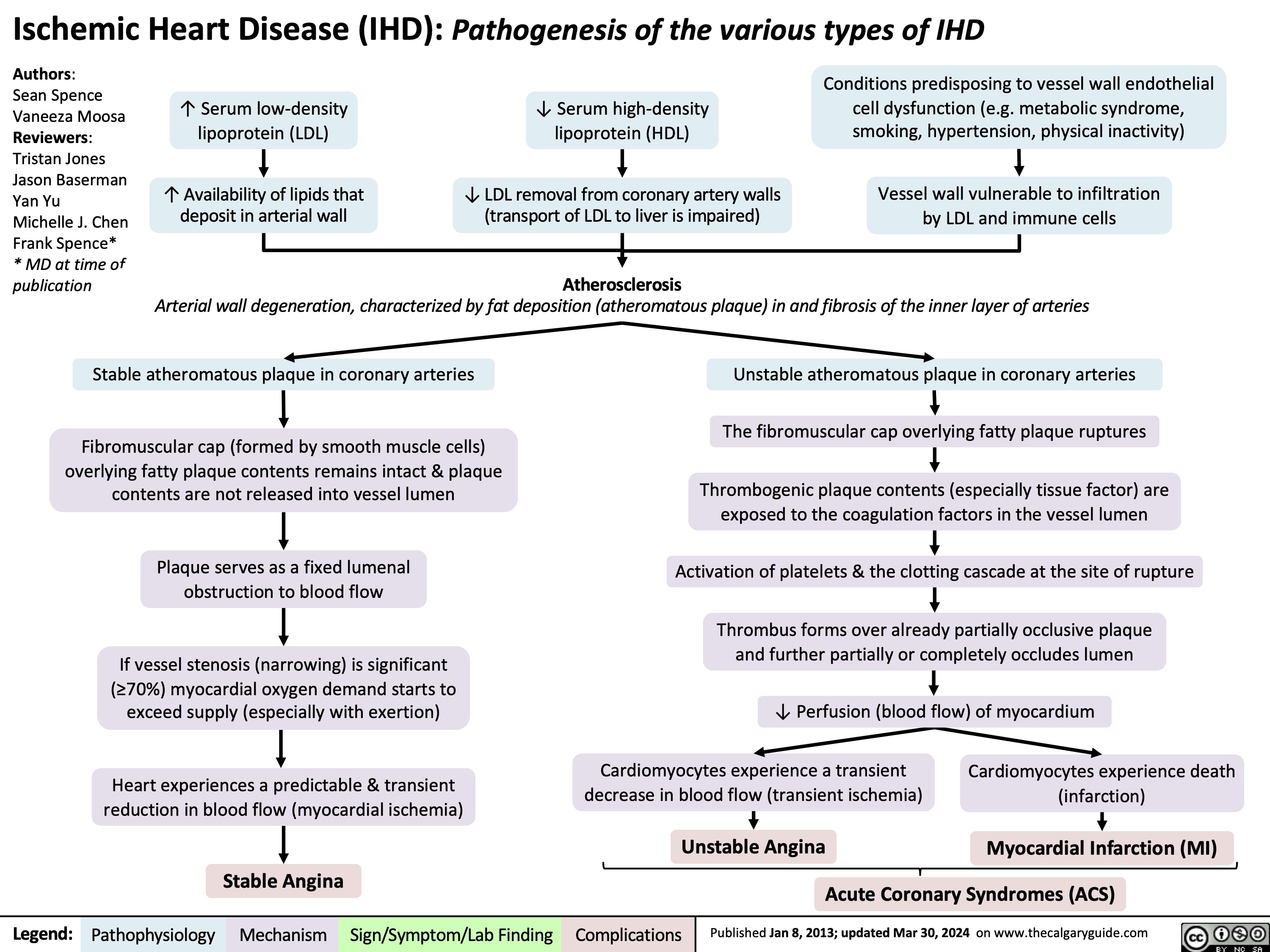
Bullous Pemphigoid
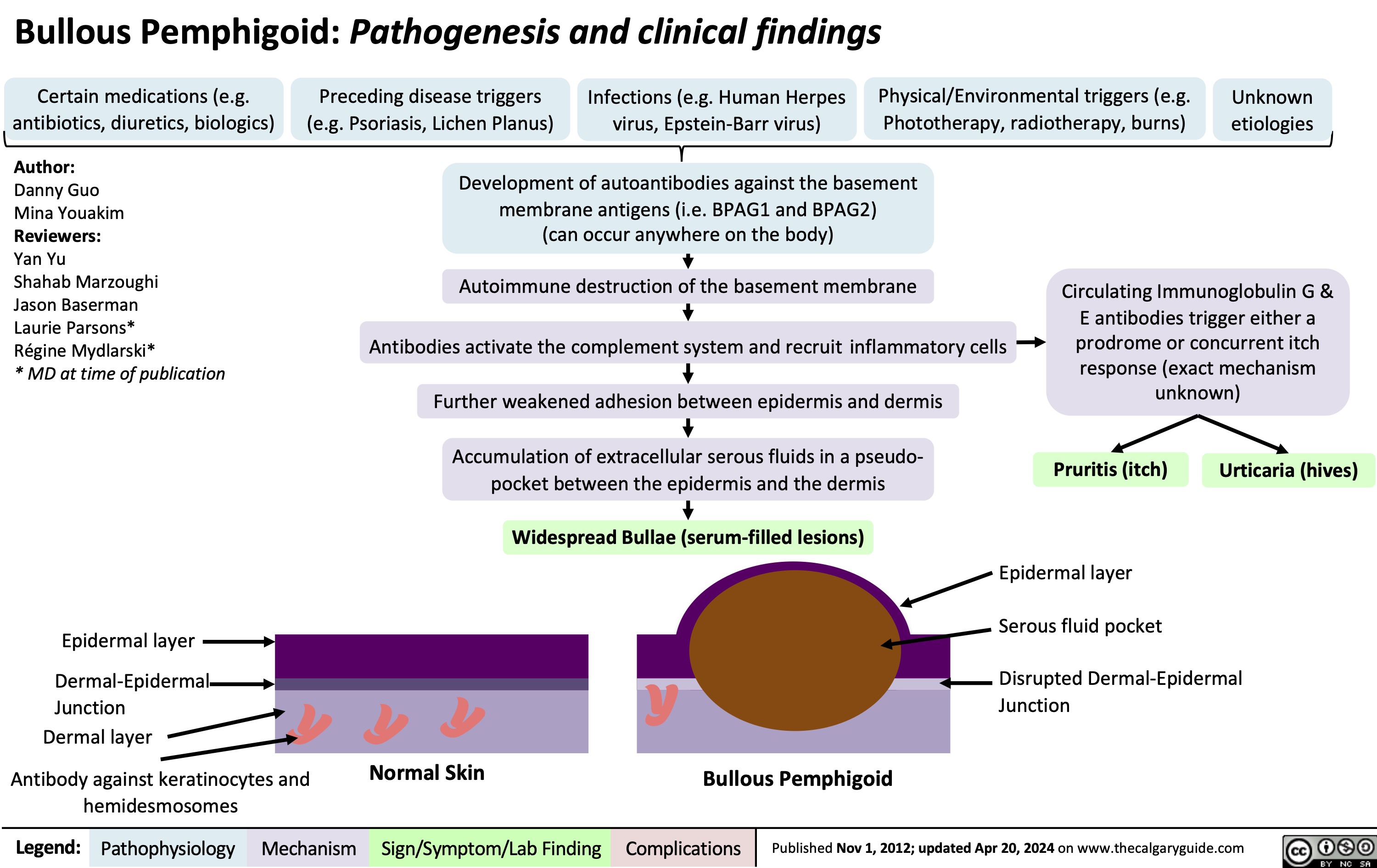
Deep Partial Thickness Burns Pathogenesis and Clinical Findings
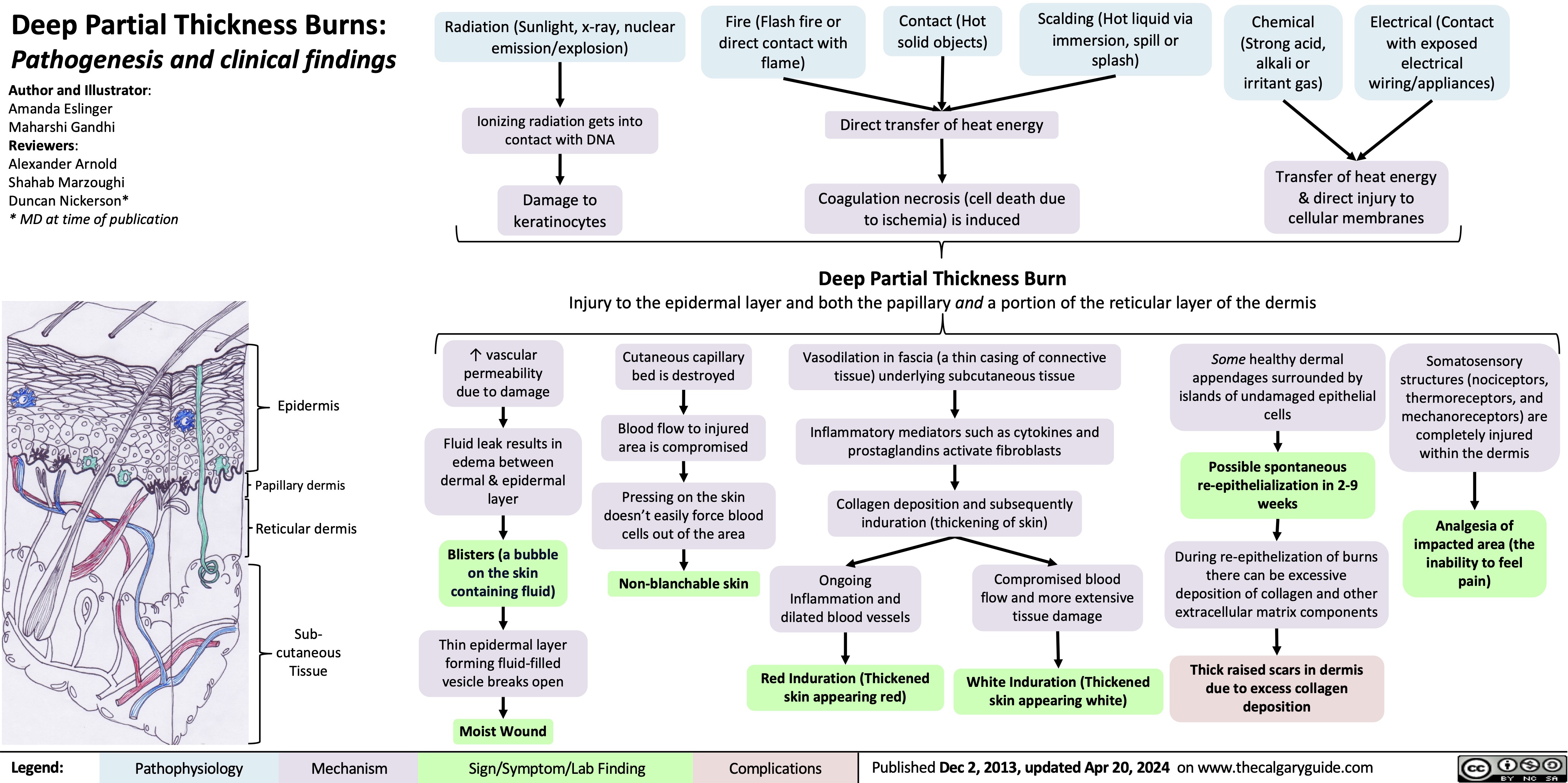
Fetal Complications of Labour and Vaginal Delivery
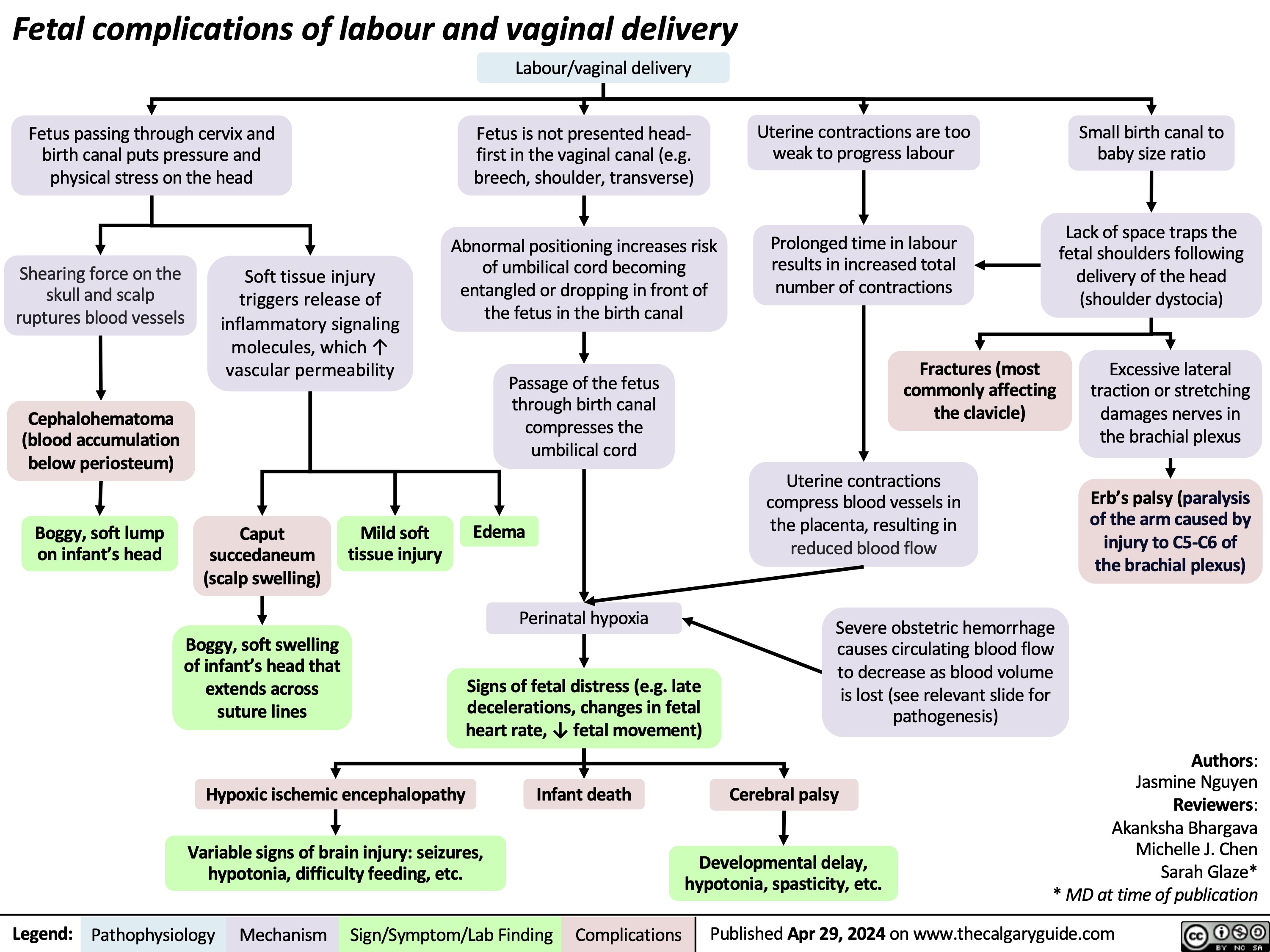
Costochondritis
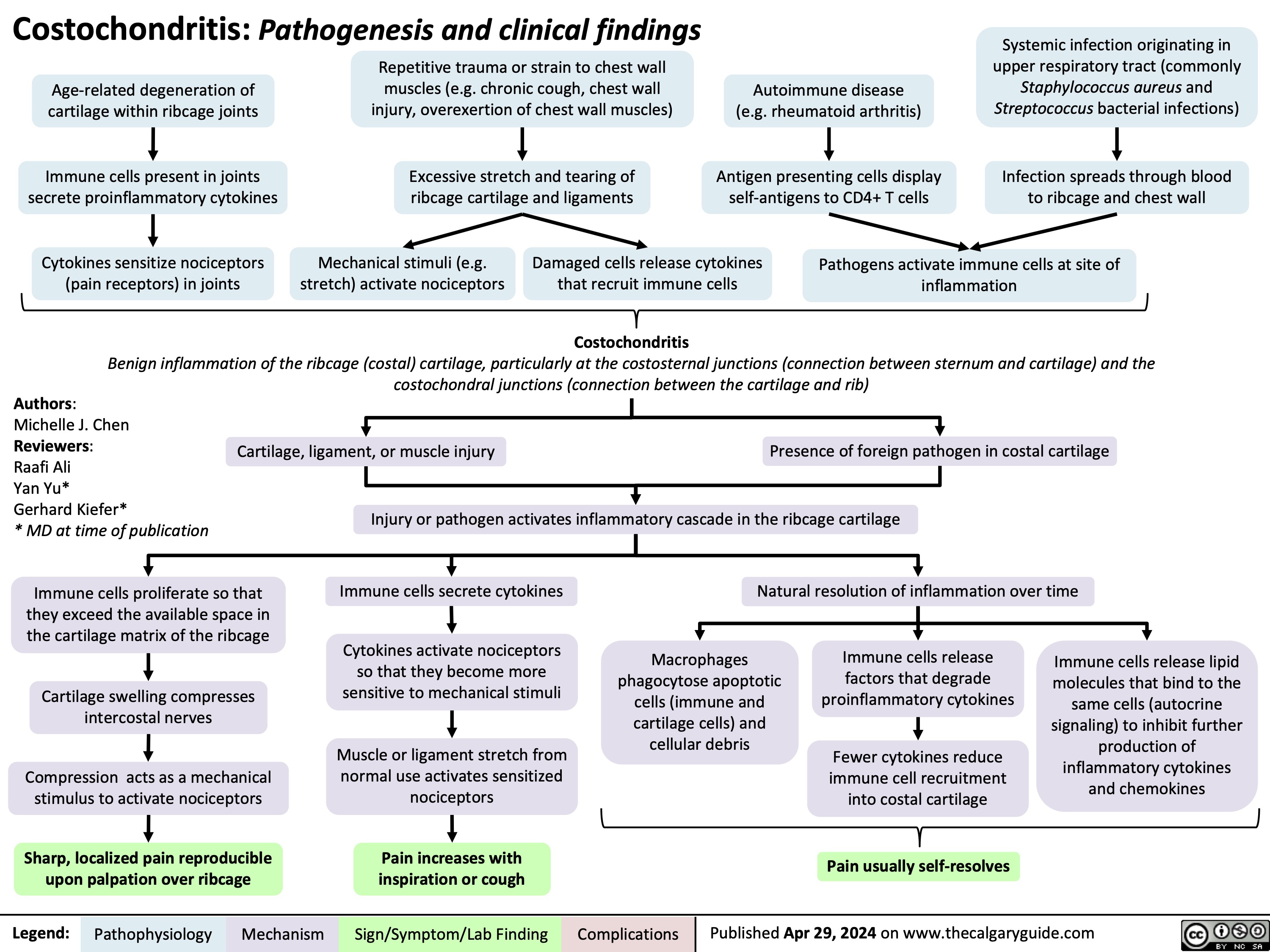
Acute Otitis Media Complications
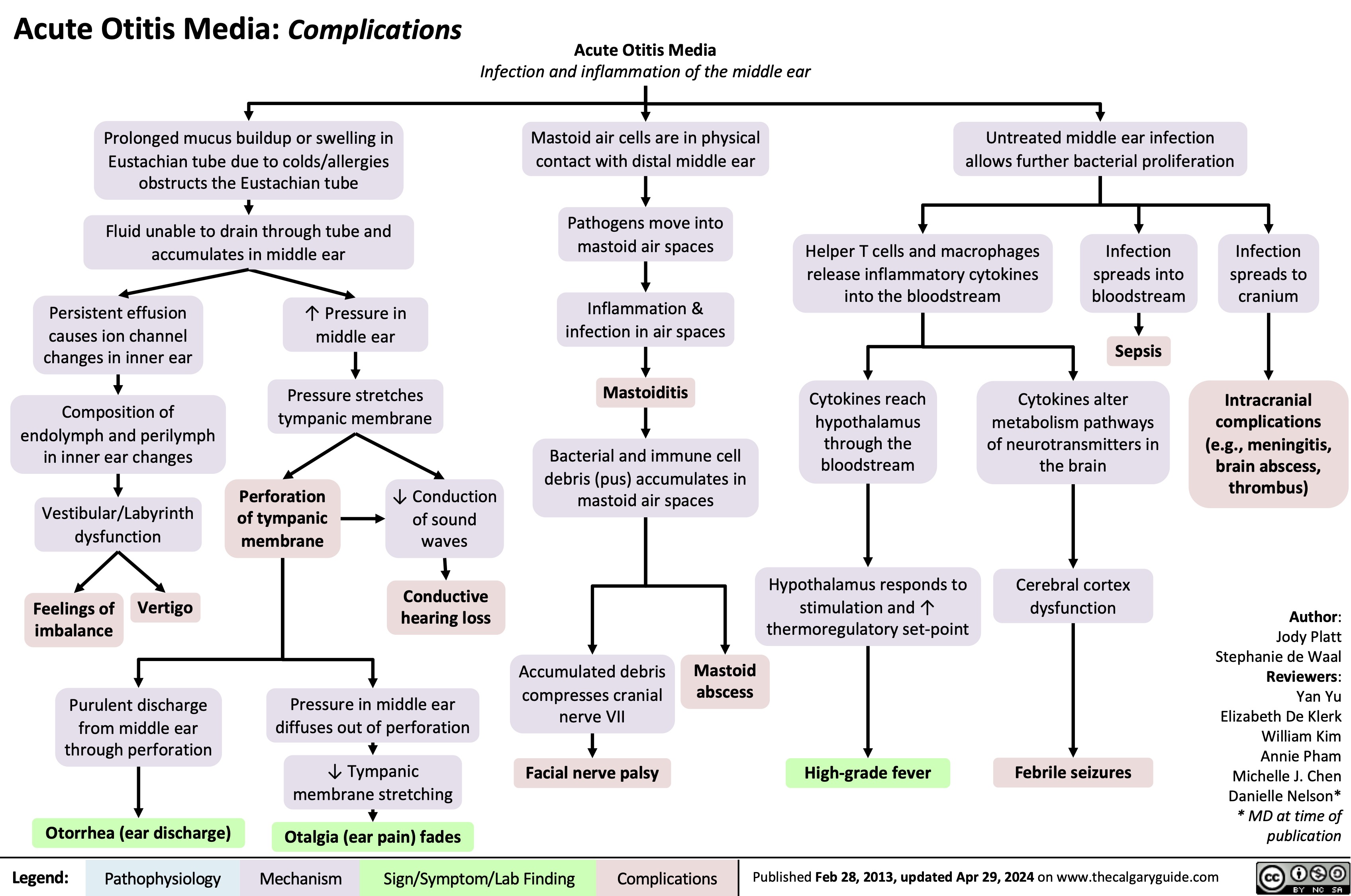
Circle of Willis Anatomy and Physiology
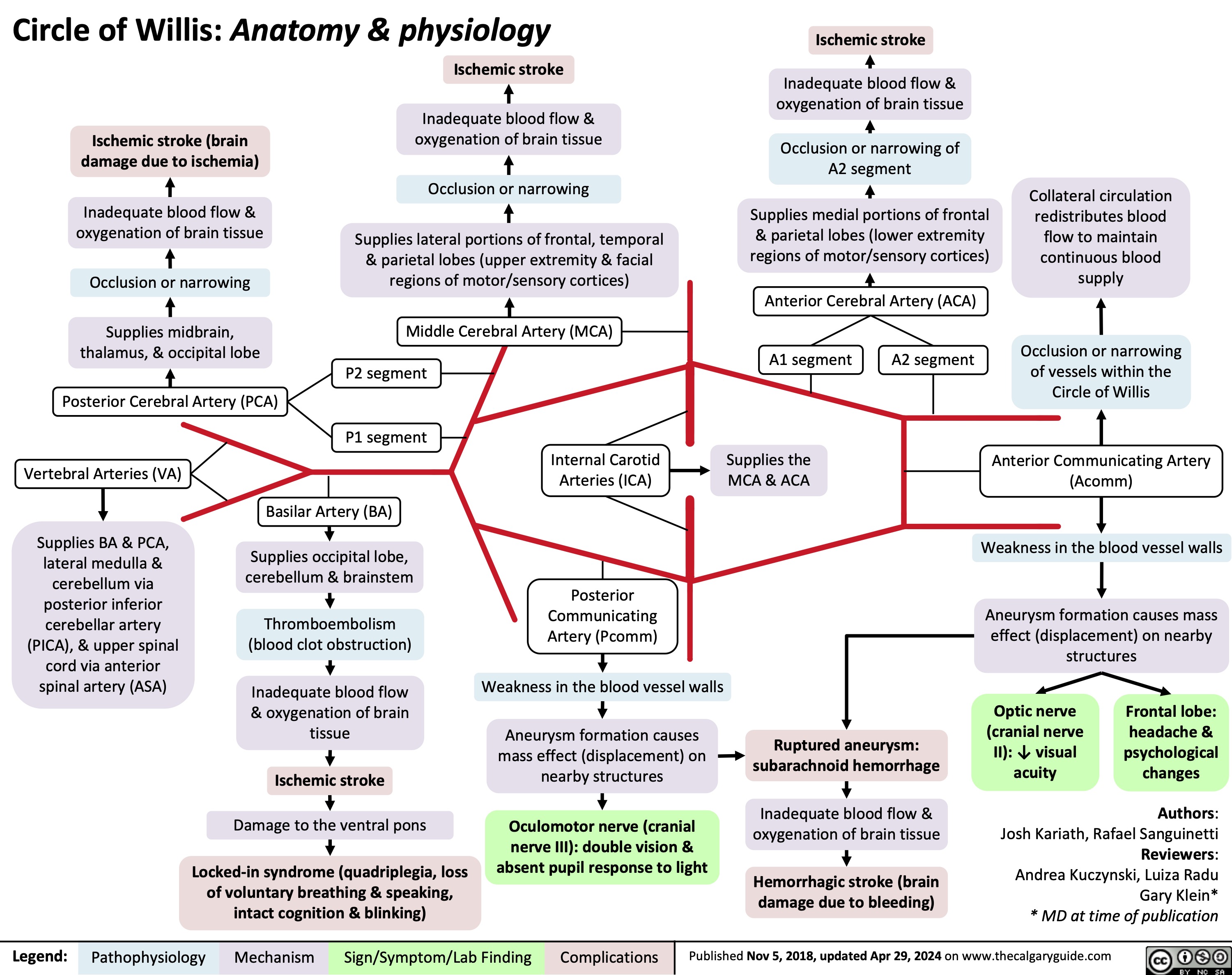
Sickle Cell Disease Pathogenesis Clinical Findings and Complications
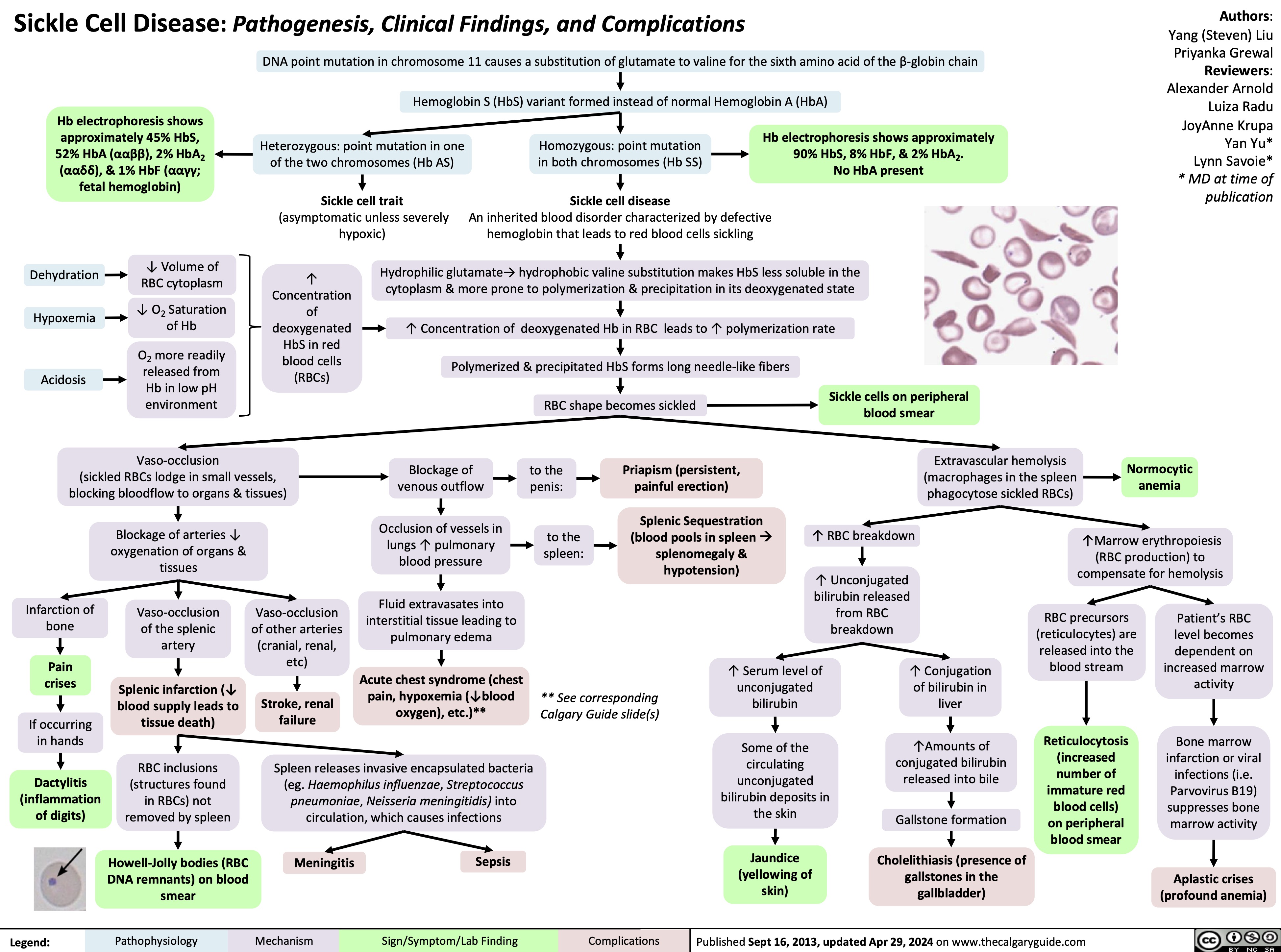
Wiskott-Aldrich Syndrome
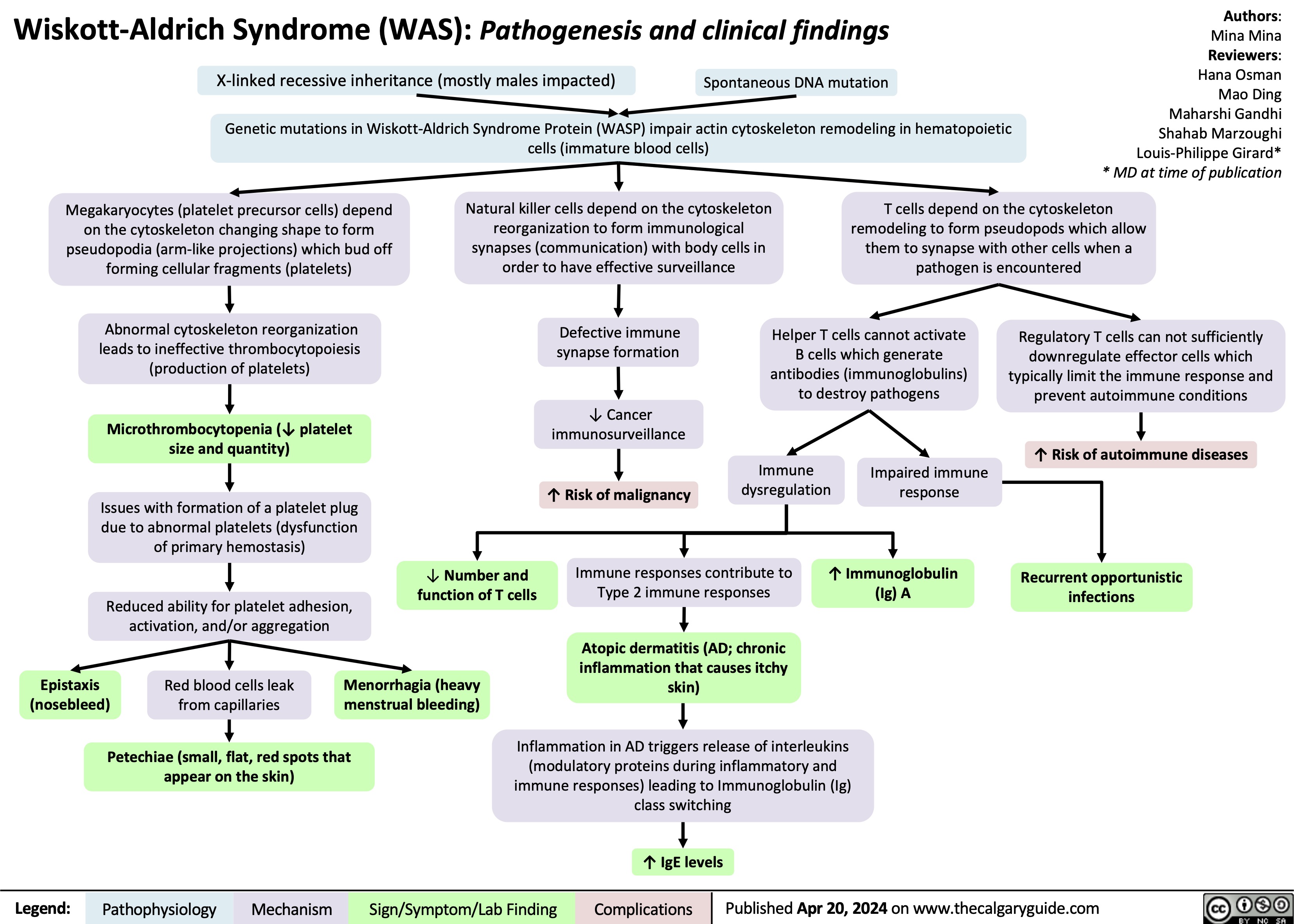
Scabies pathogenesis and clinical findings
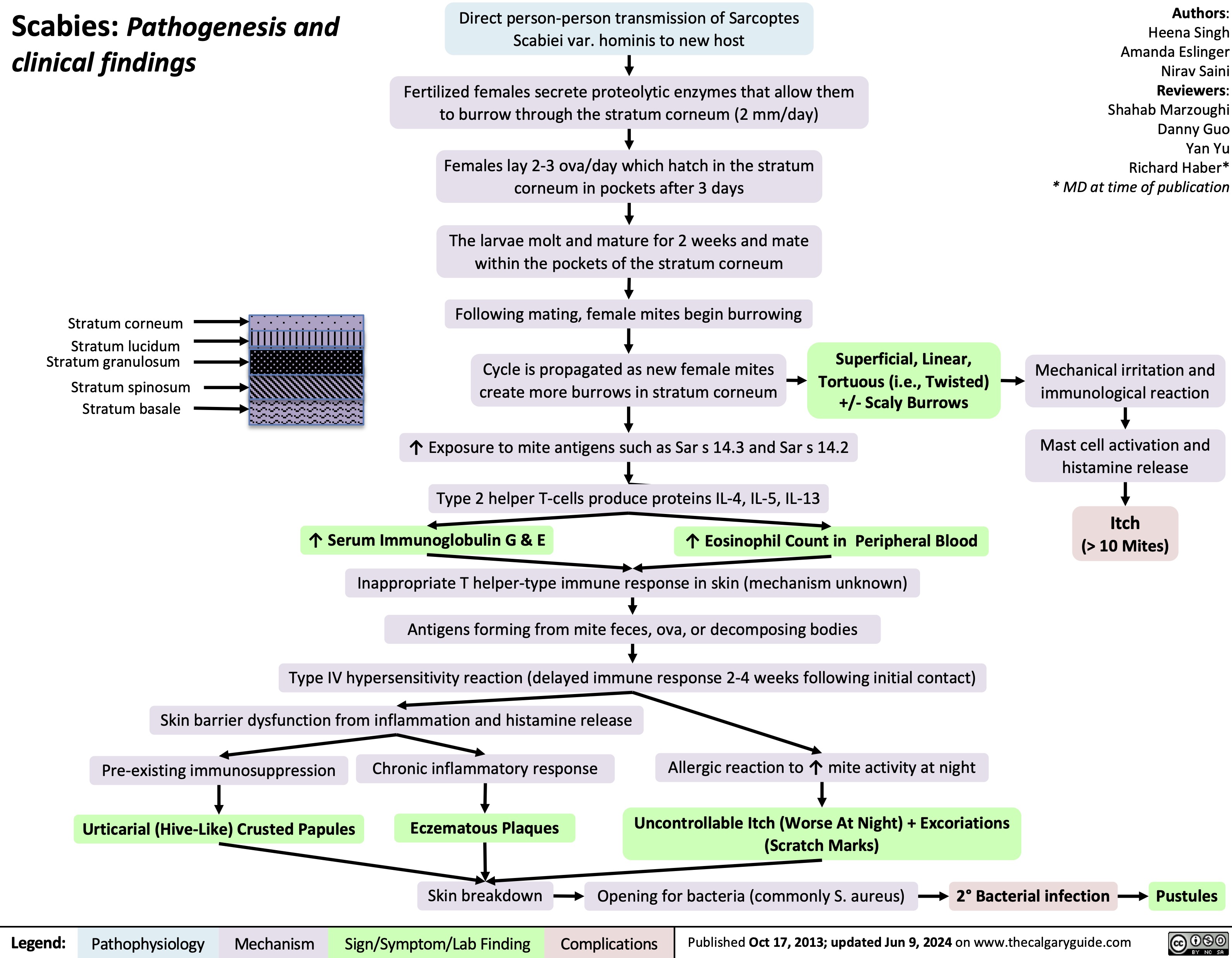
Gynecomastia
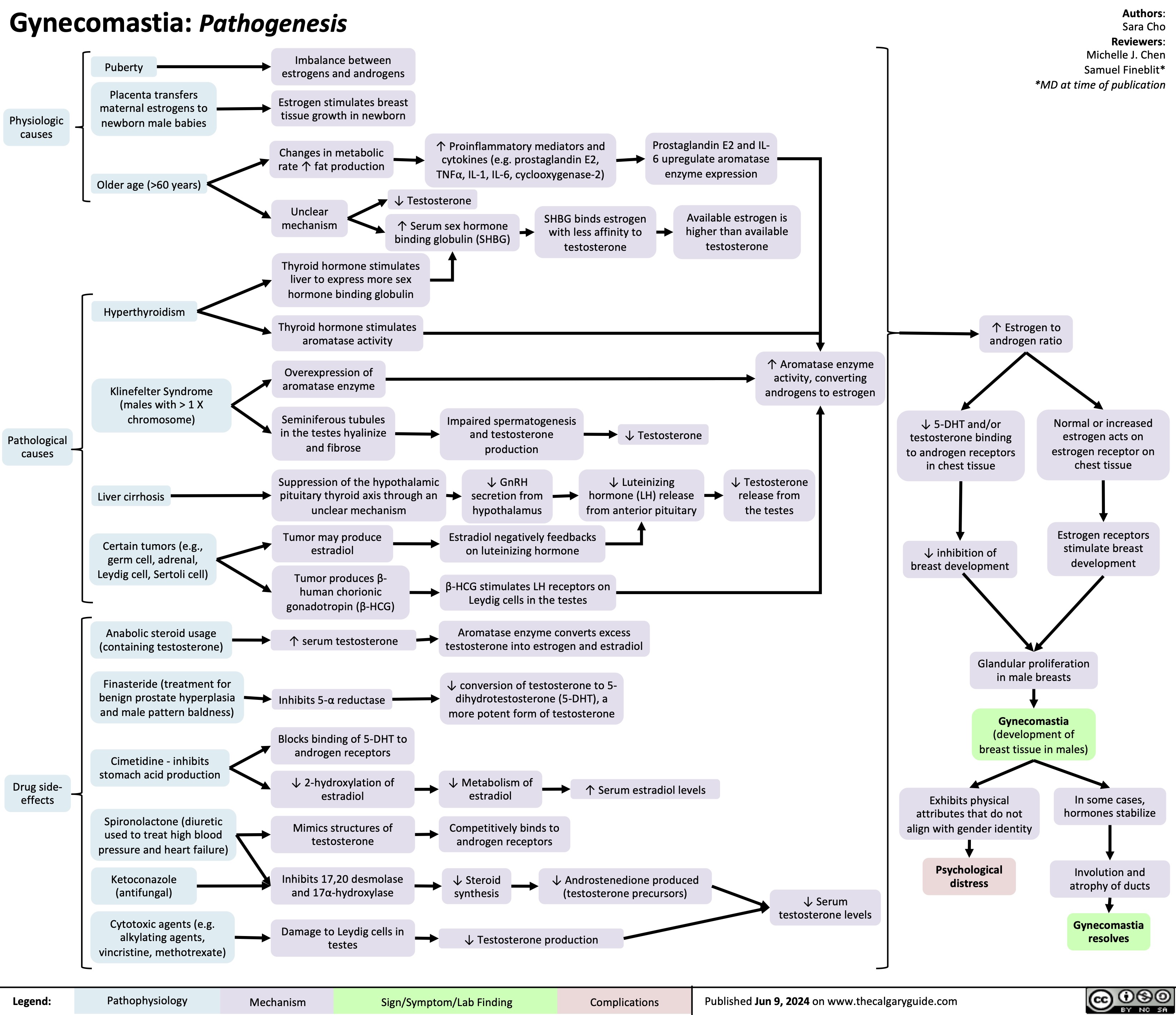
Posterior Cruciate Ligament PCL Injury Pathogenesis and Clinical Findings
Metastatic Bone Lesions
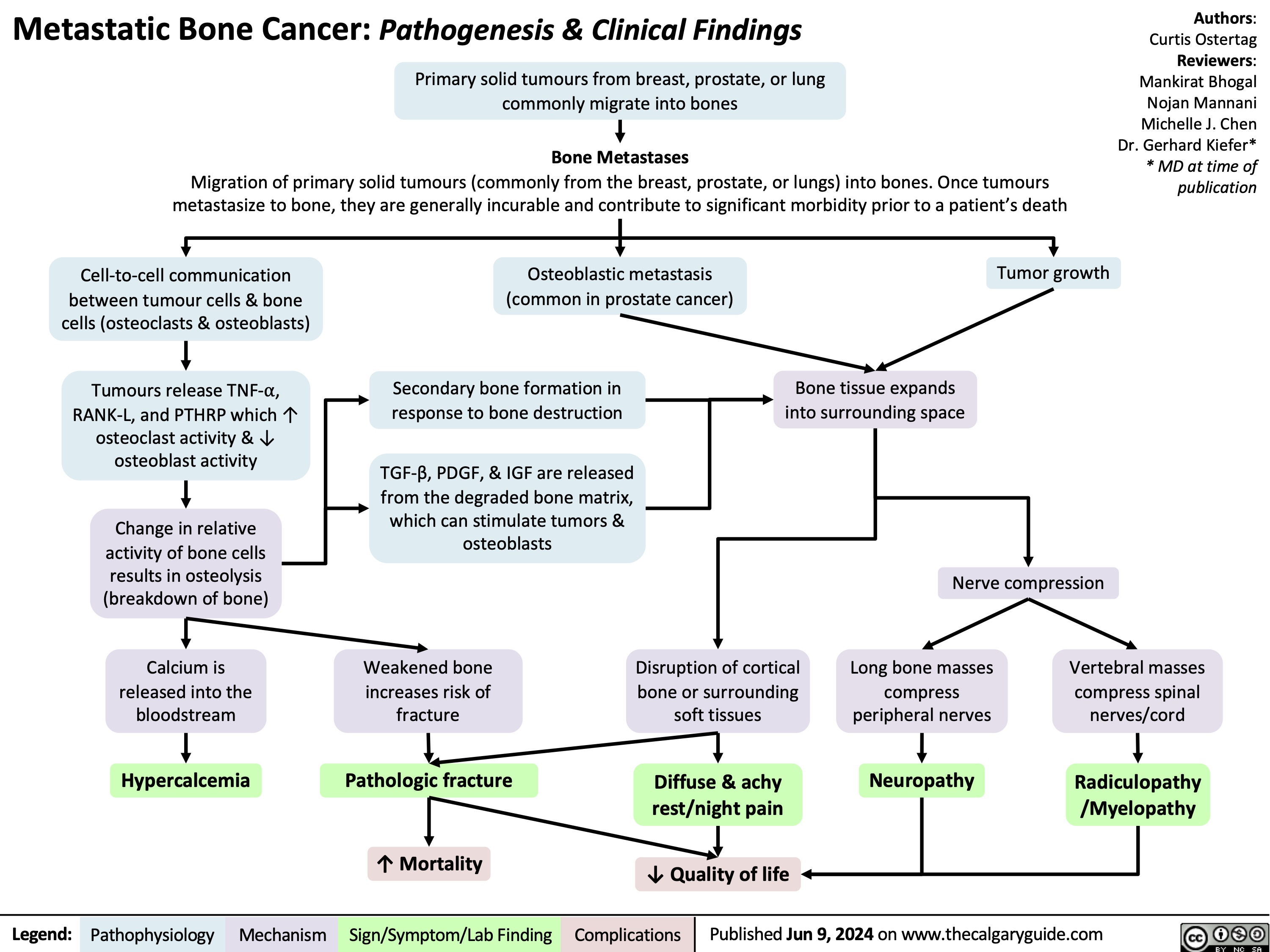
Migraines and auras pathogenesis and clinical findings
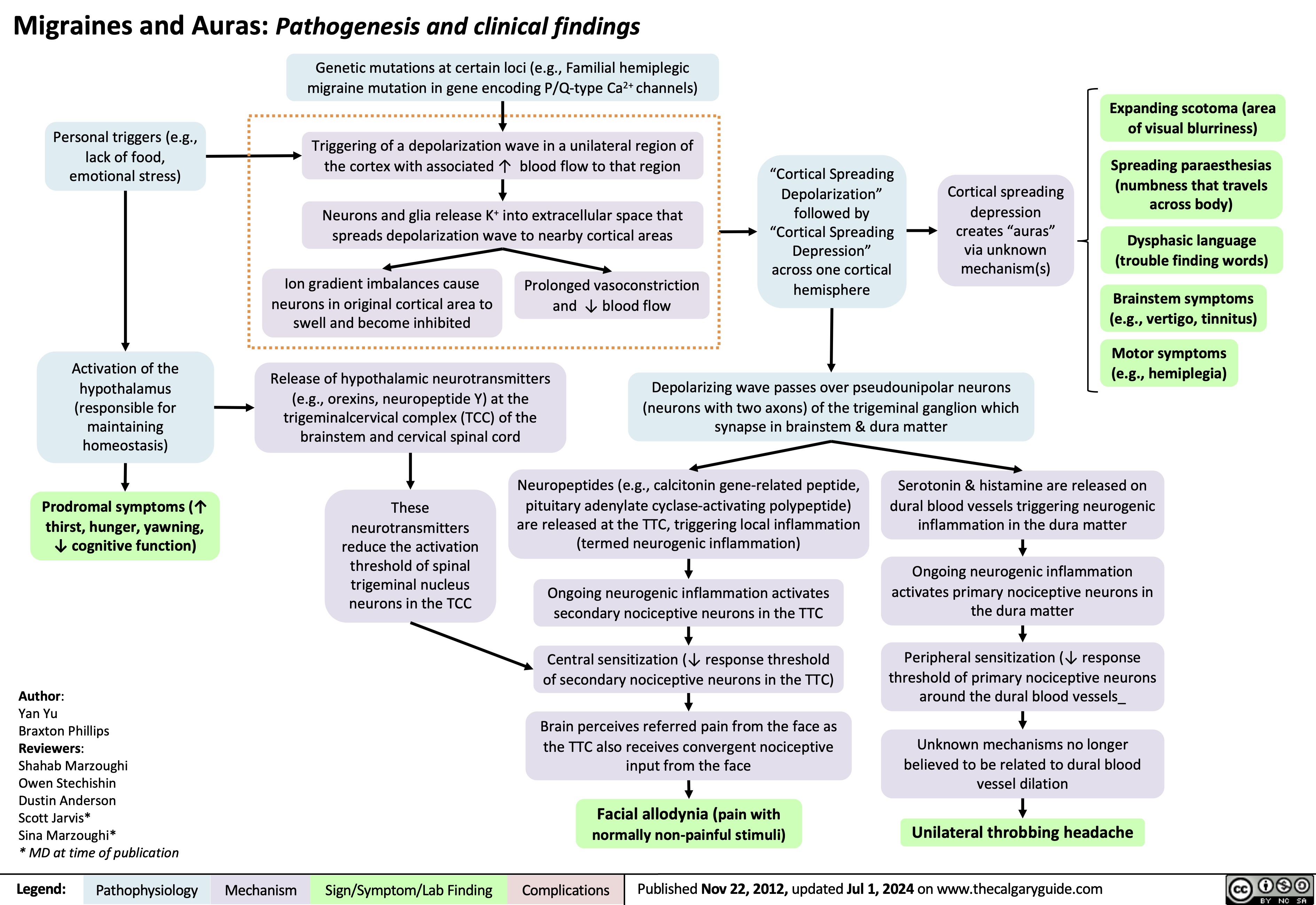
Anesthetic Considerations in Pregnancy
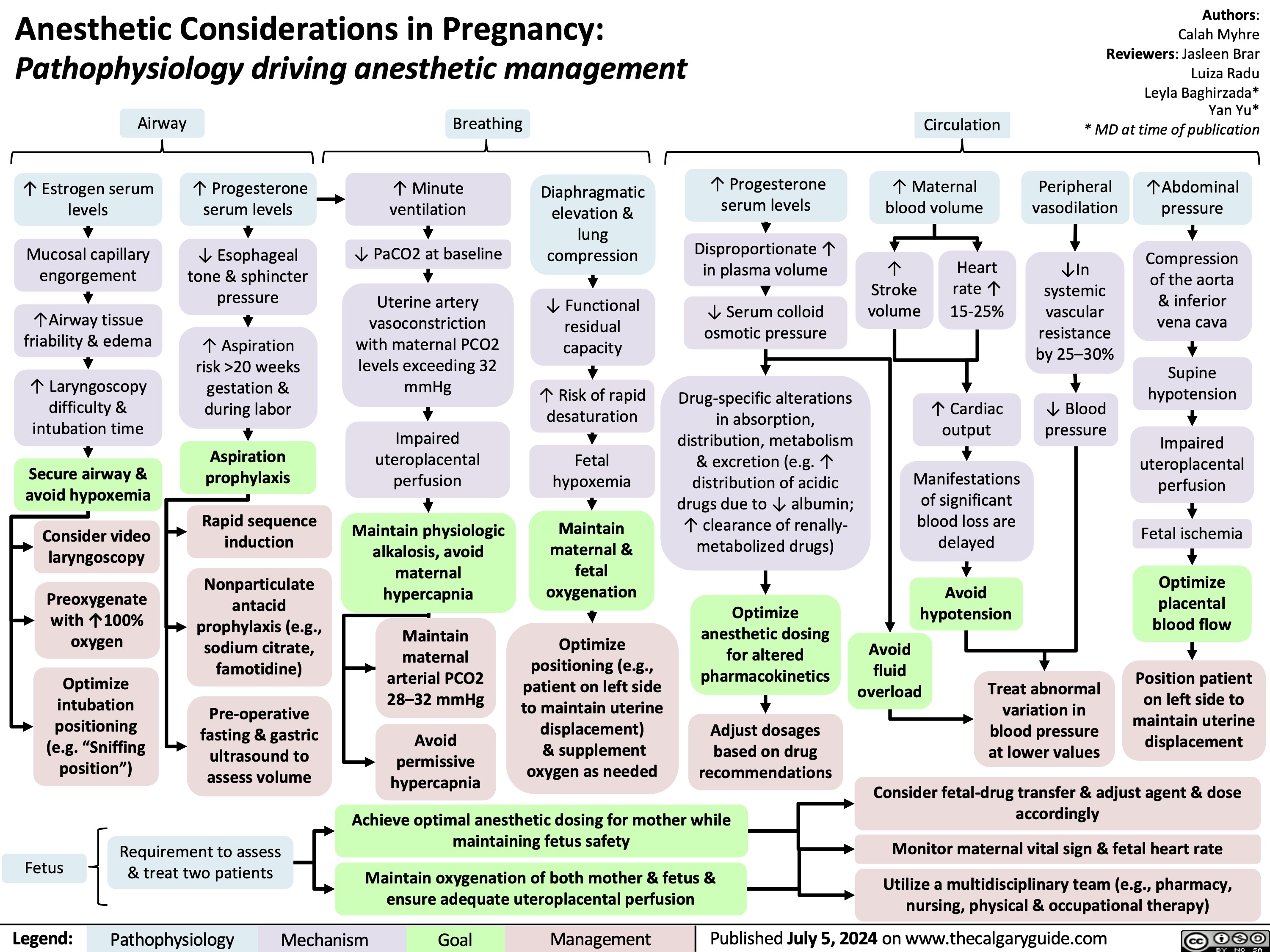
Diabetes Mellitus Pathophysiology Behind Lab Findings
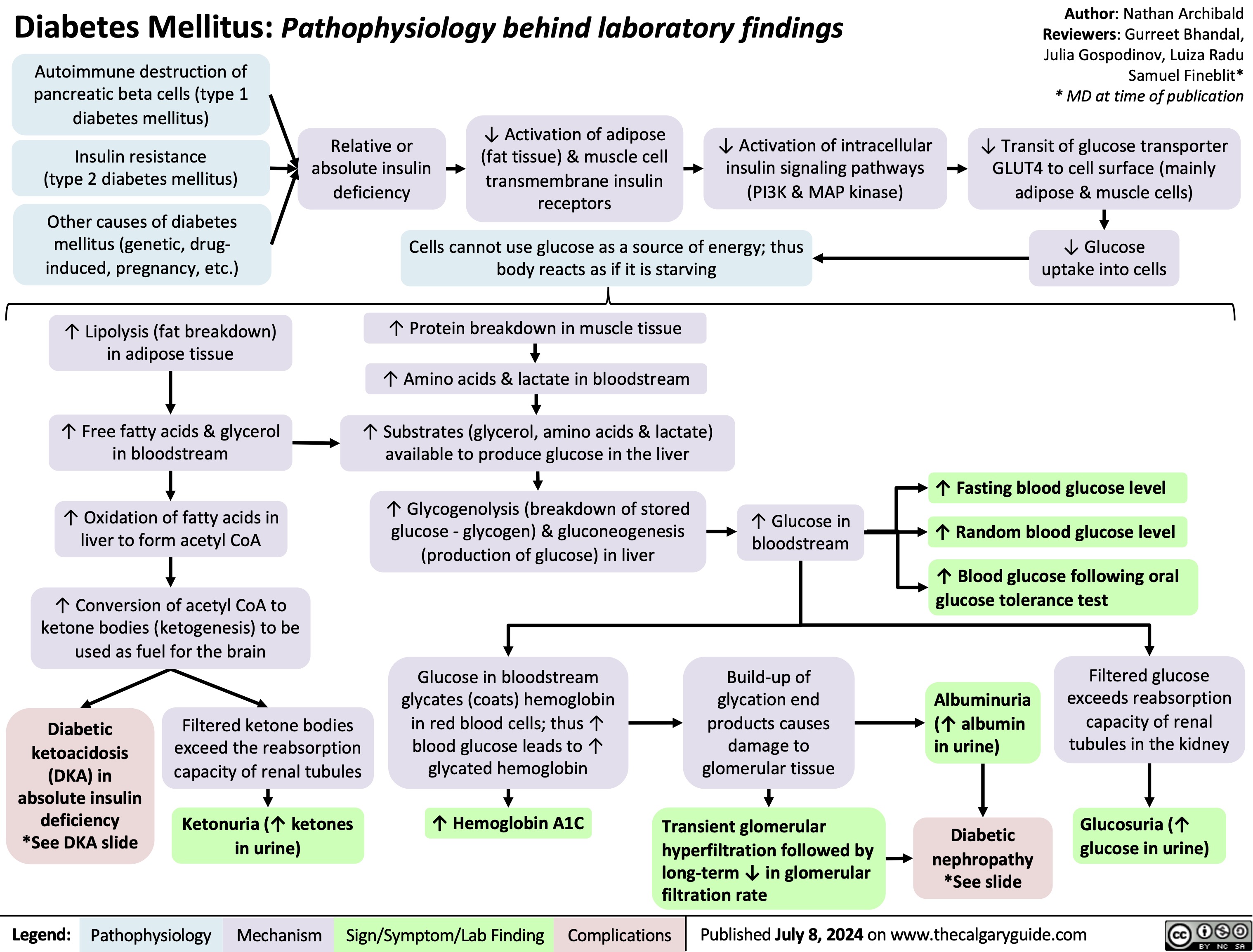
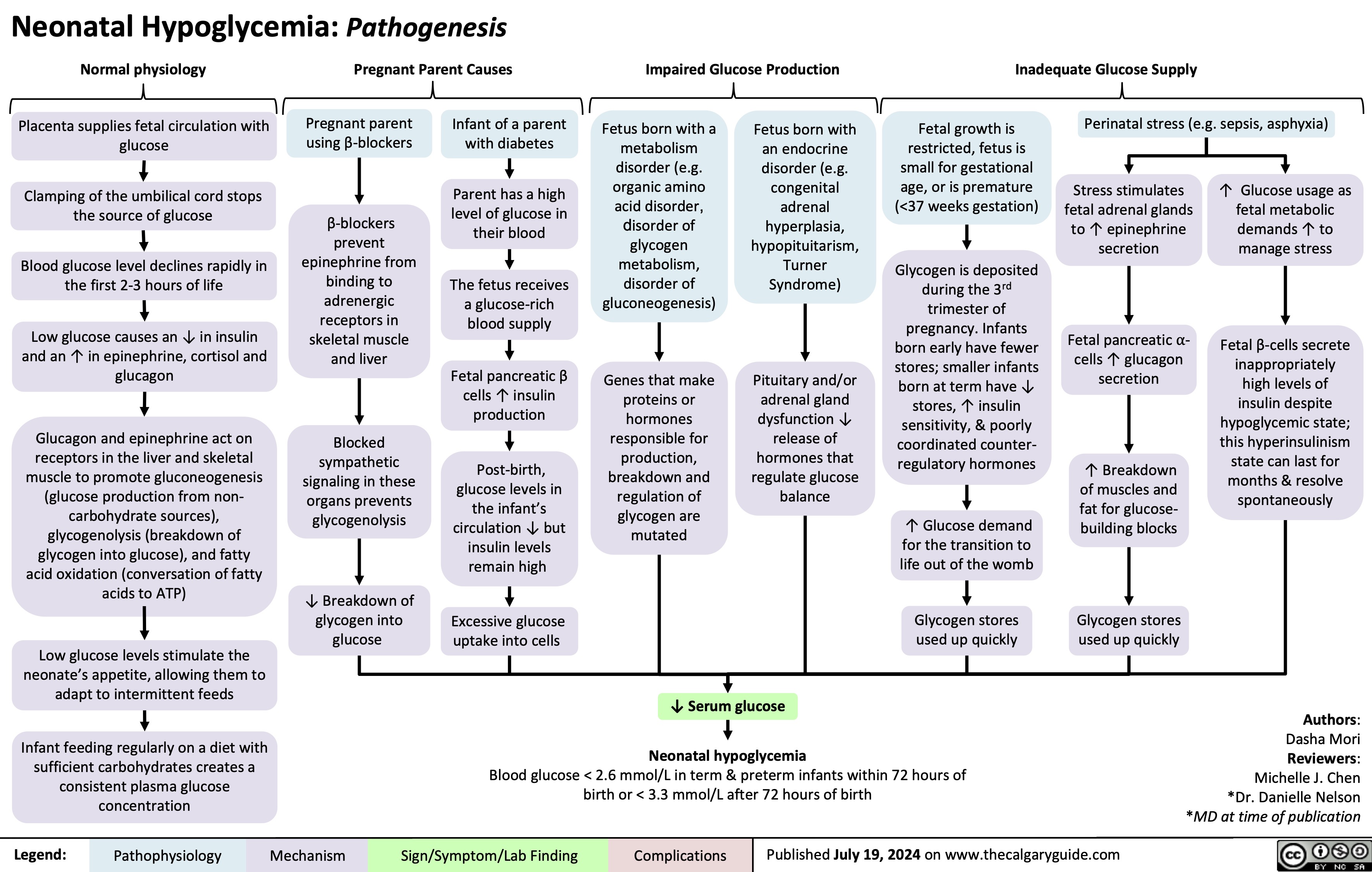
Neonatal Hypoglycemia Pathogenesis
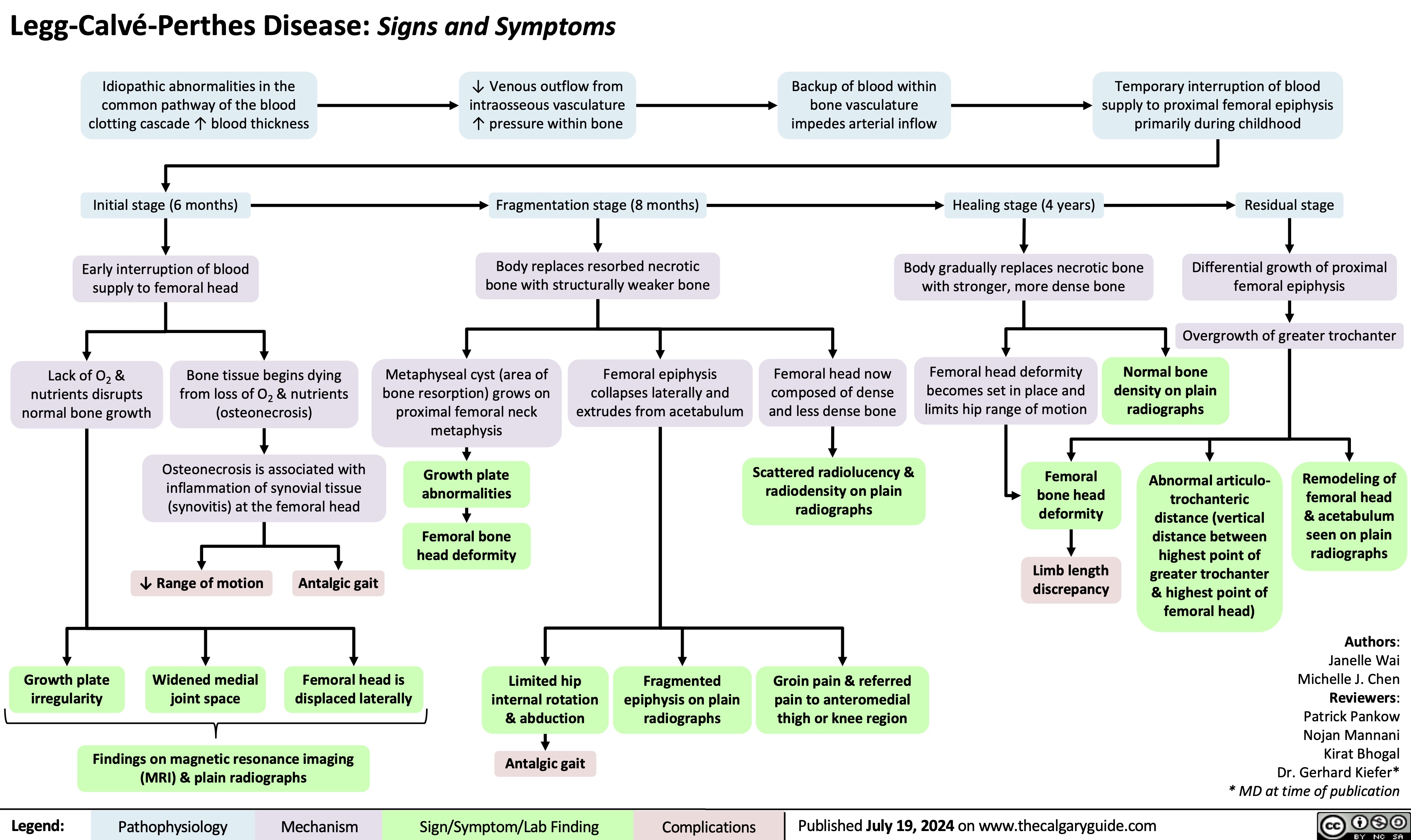
Legg Calve Perthes Disease
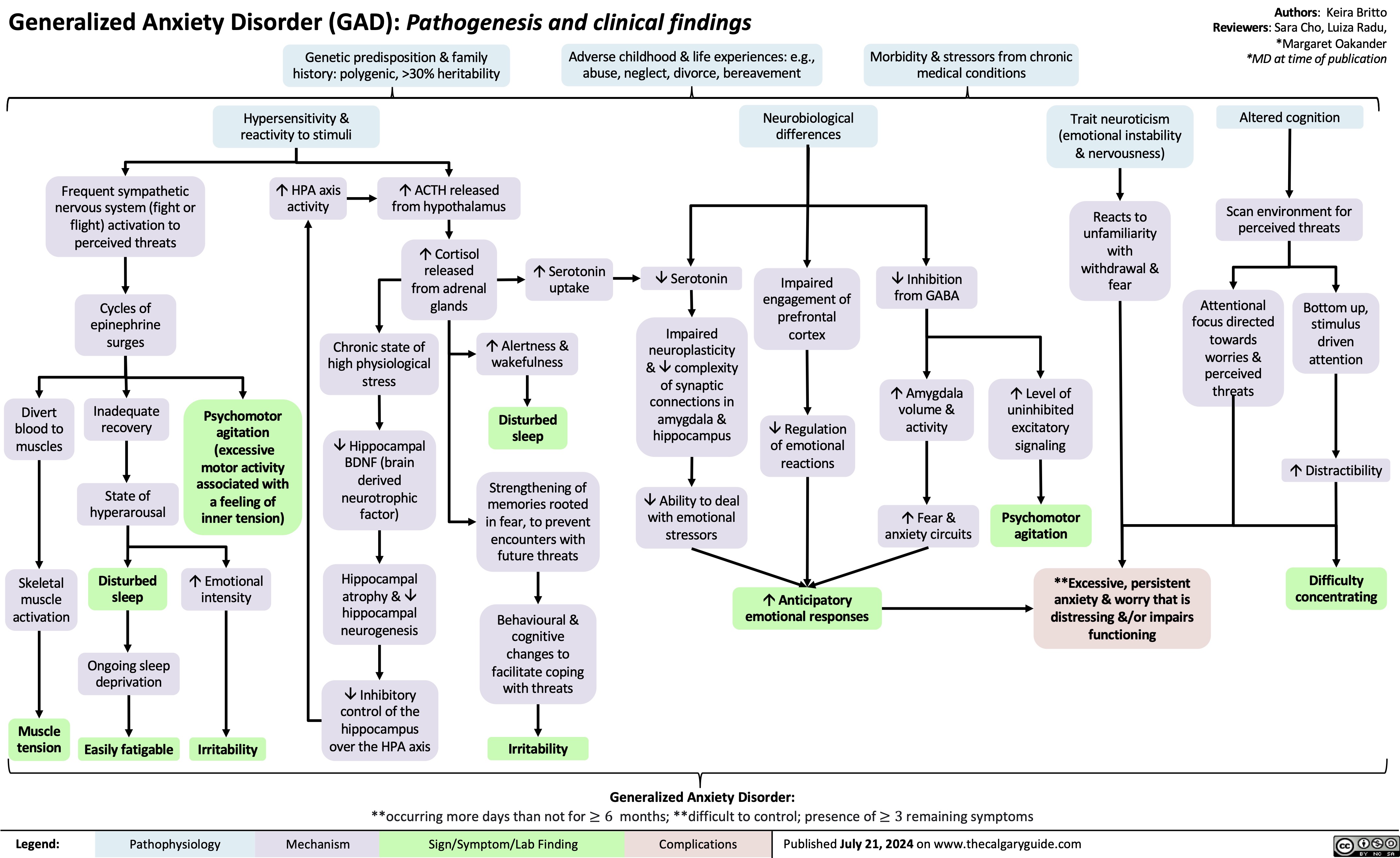
Generalized Anxiety Disorder GAD
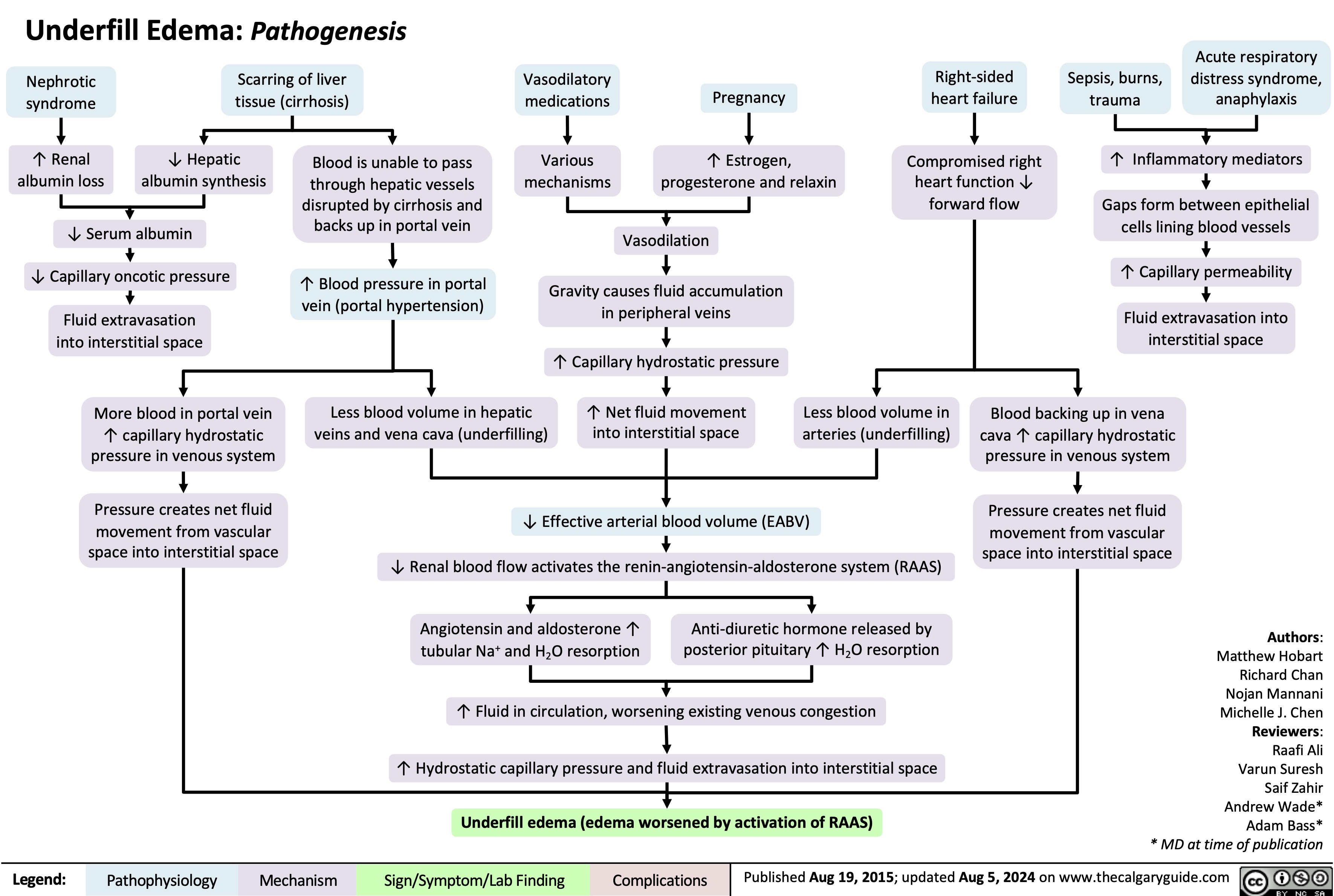
Underfill Edema Pathogenesis
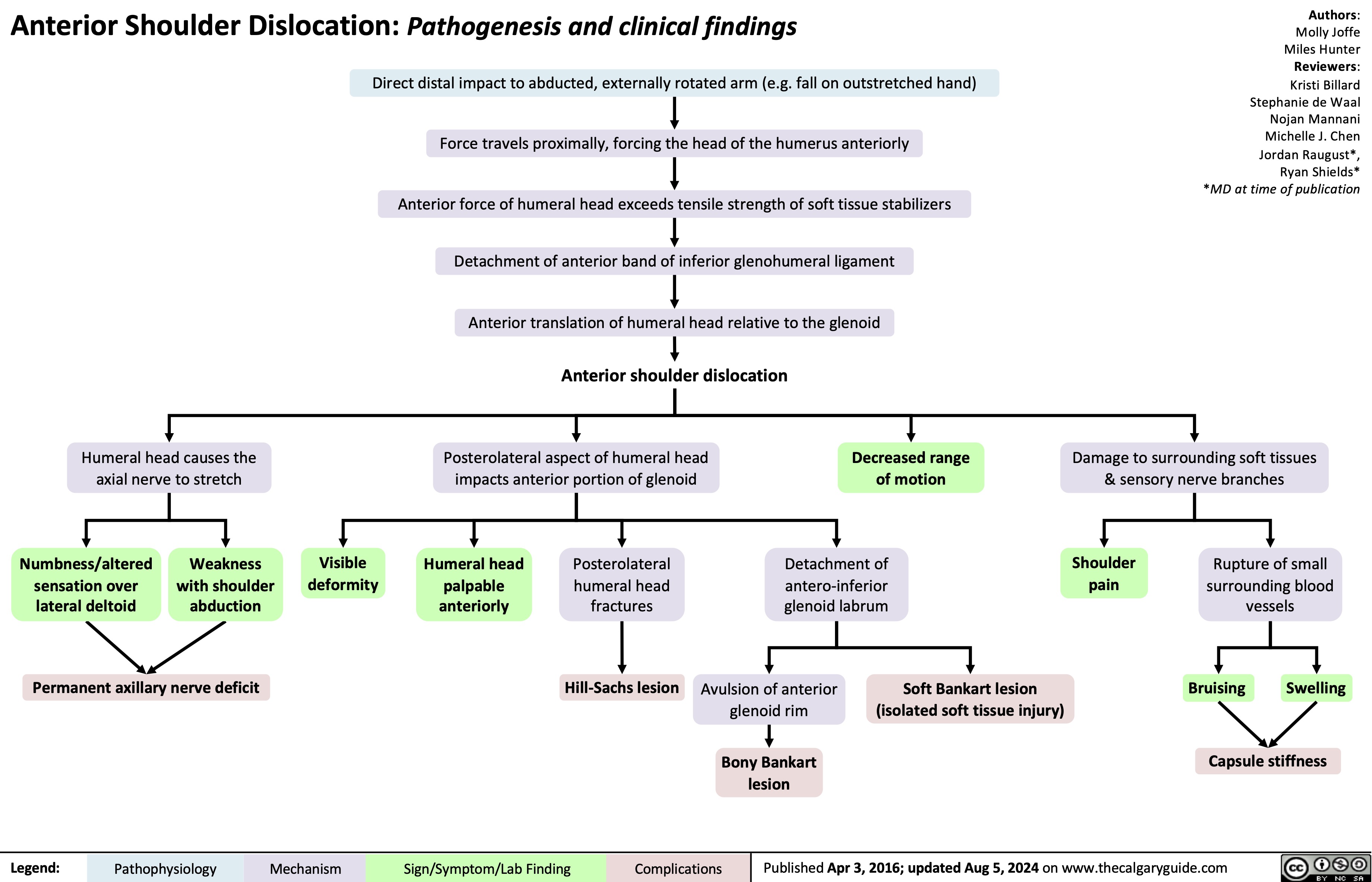
Anterior Shoulder Dislocation Pathogenesis and clinical findings
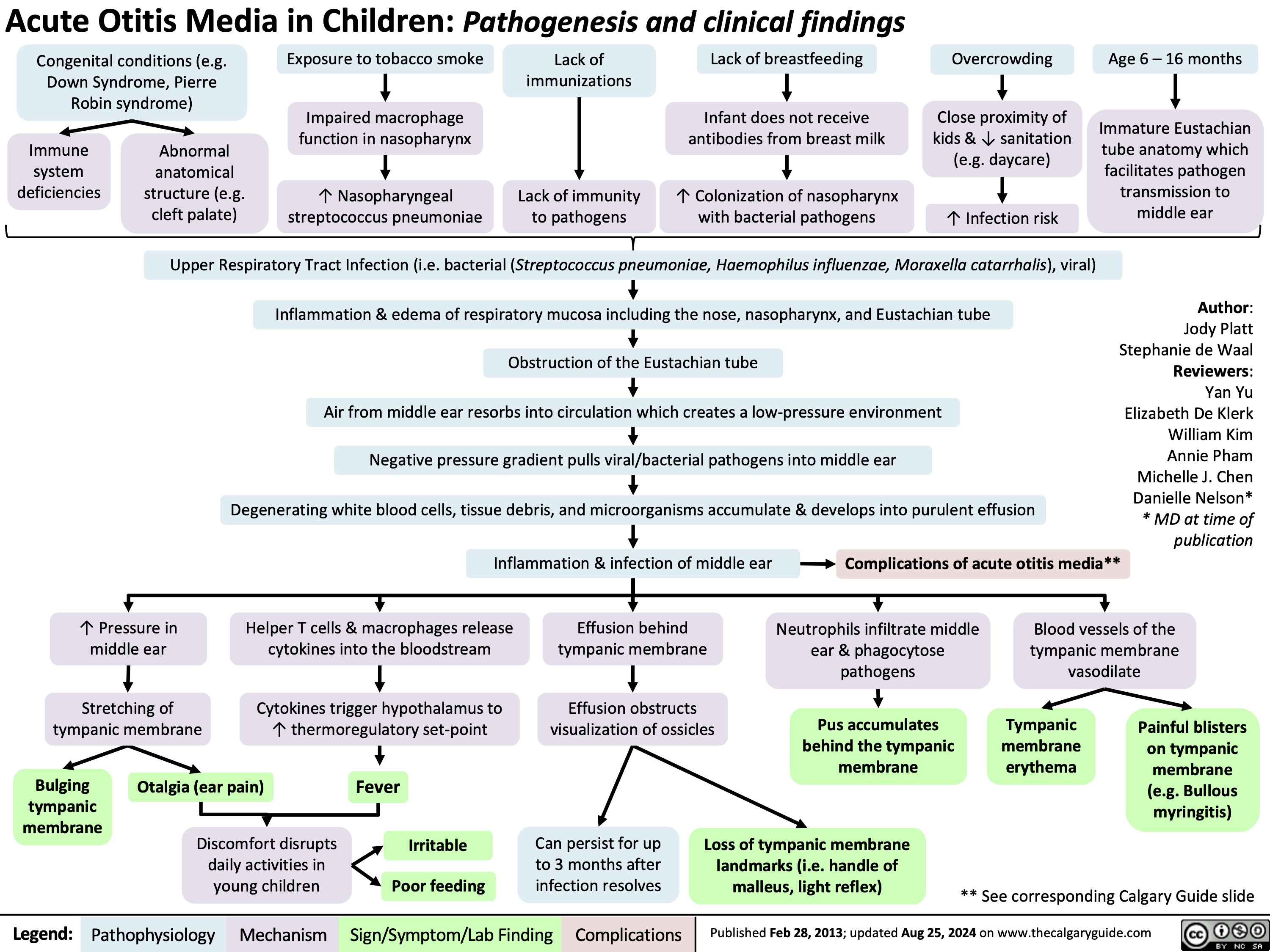
Acute Otitis Media Pathogenesis and Clinical Findings in Children
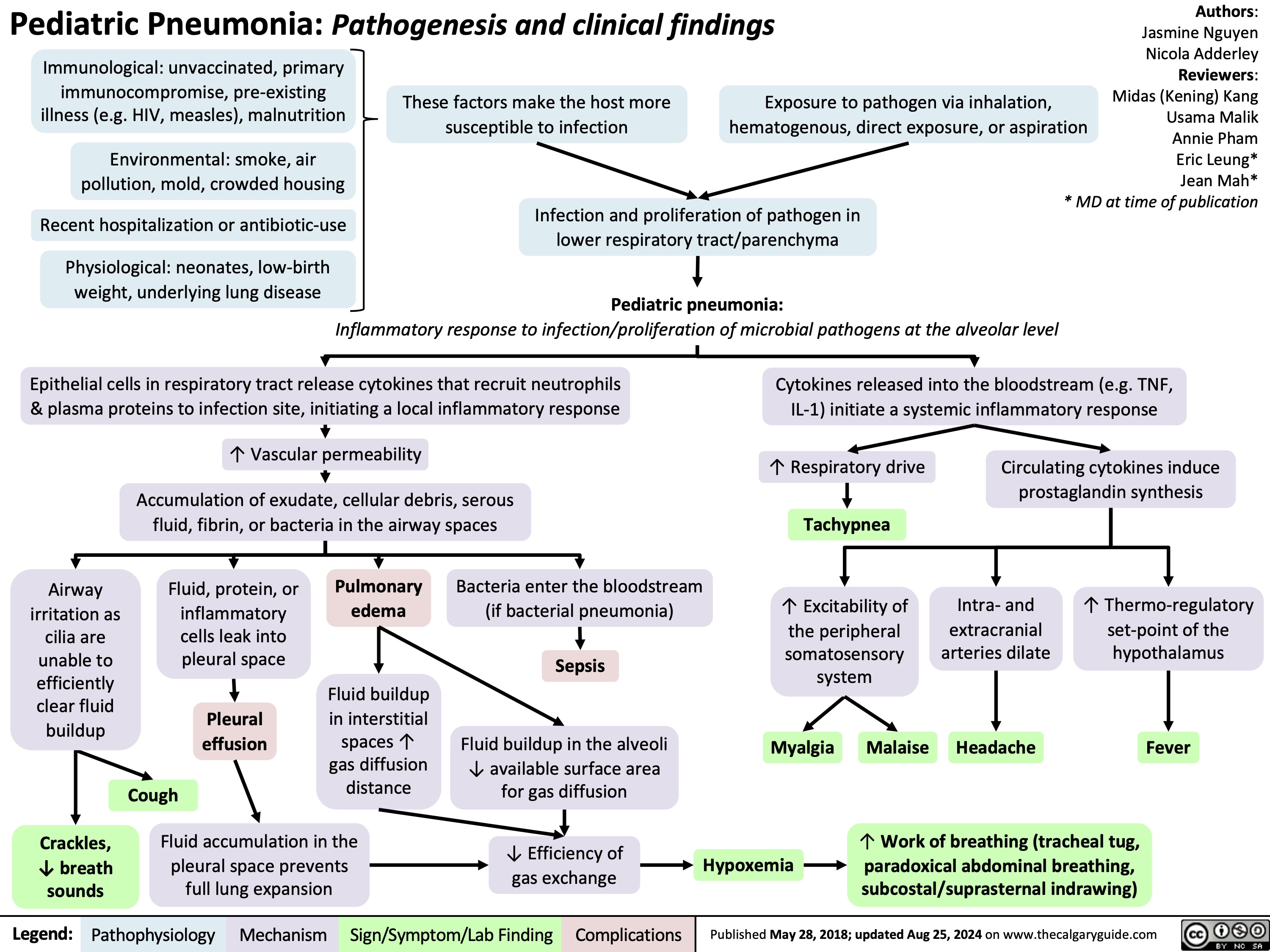
Pediatric Pneumonia Pathogenesis and Clinical Findings
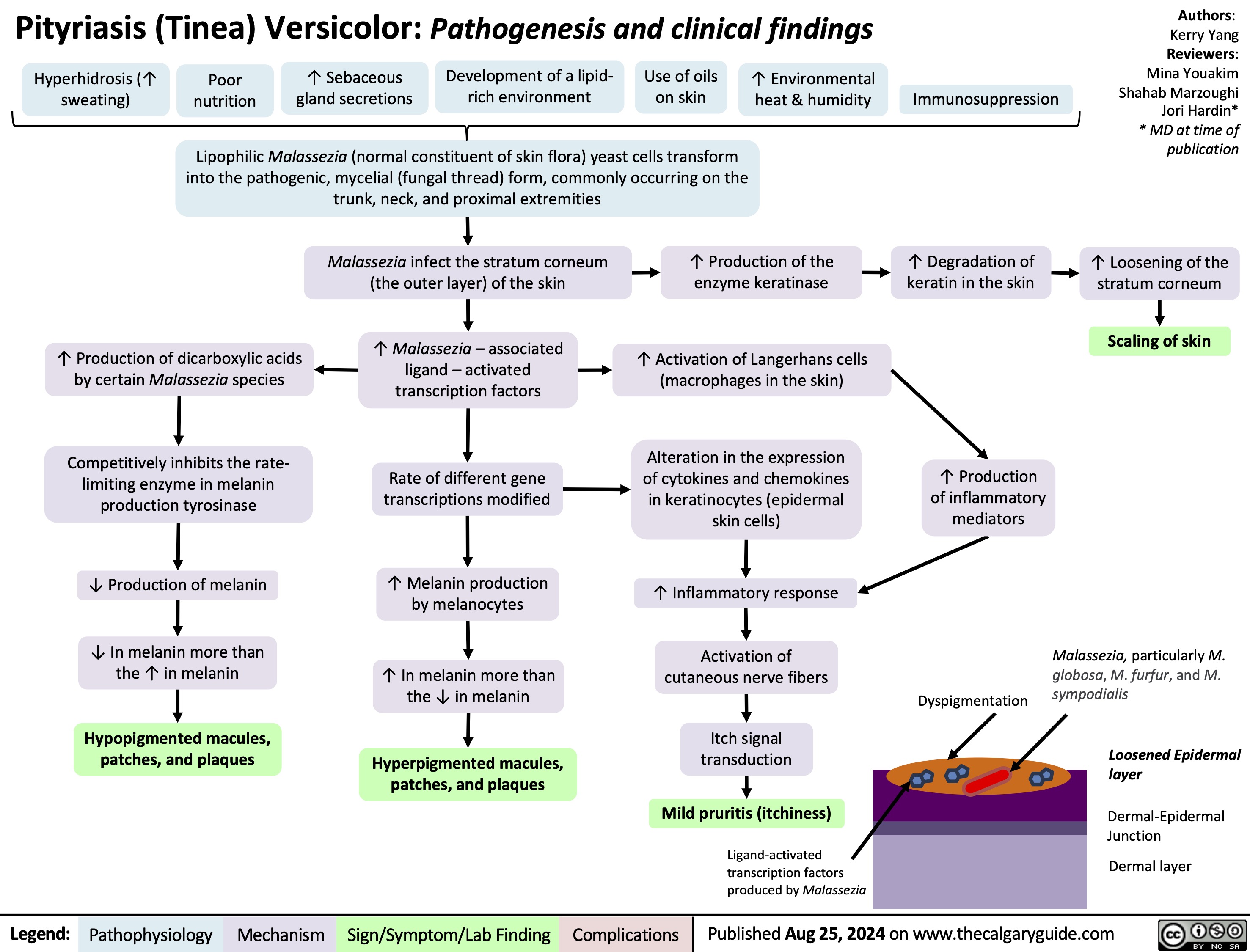
Pityriasis Tinea Versicolor
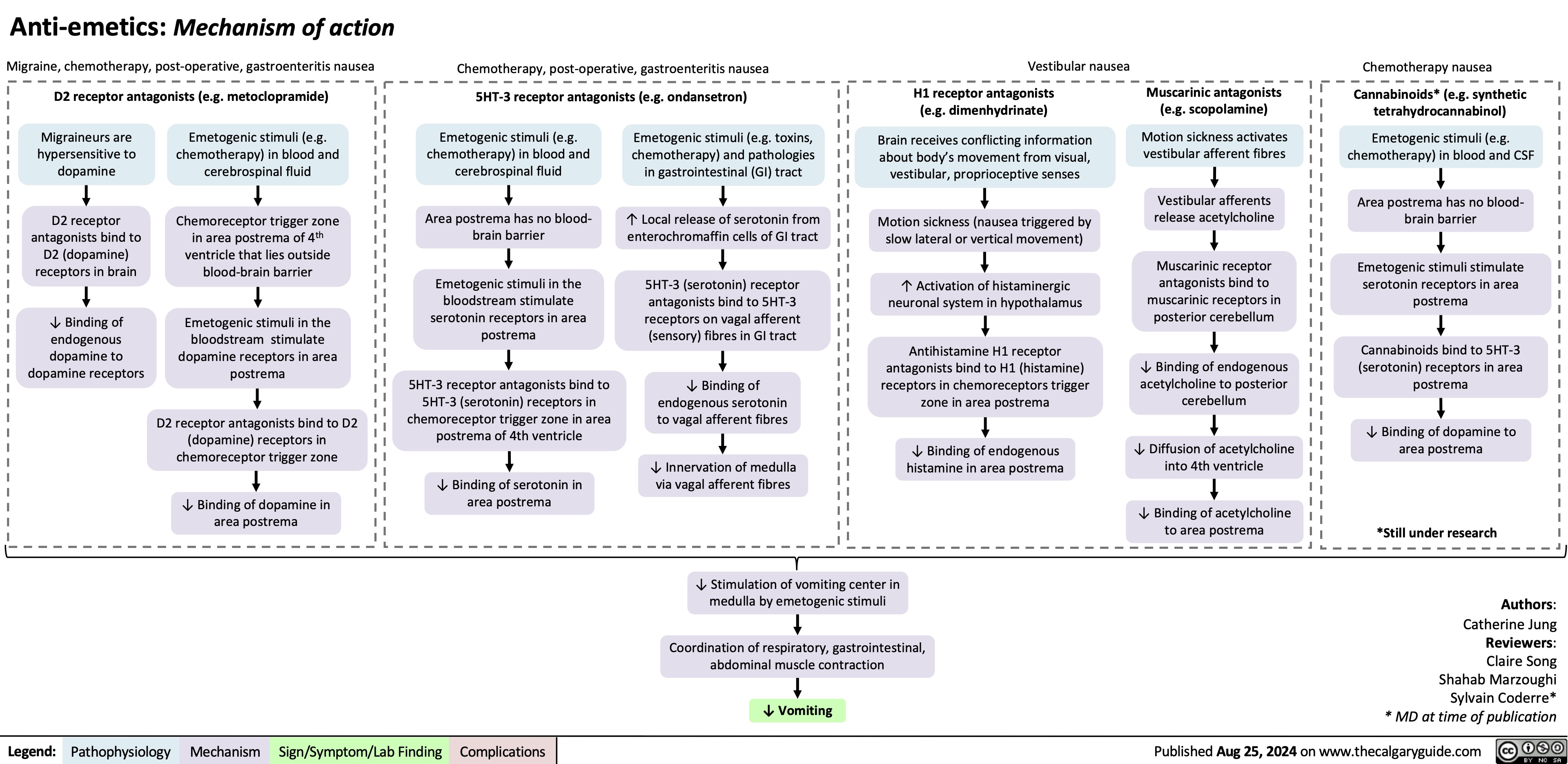
Anti-Emetics Mechanism of Action
Massive Transfusion Protocol
![Massive Transfusion Protocol: Considerations and rationale
Massive transfusion protocol (MTP) is a tool used by clinicians when there is a need to rapidly administer a large amount of blood products, including packed red blood cells (pRBCs), fresh frozen plasma (FFP), and platelets. Complications of MTP are commonly referred to as “The Lethal Triad” referring to hypothermia, acidosis and coagulopathy.
Authors: Kayleigh Yang Arzina Jaffer
Reviewers: Jasleen Brar,
Luiza Radu, Karl Darcus*
* MD at time of publication
Intervention
Indications Initial Response Pathophysiology Transfusion Targets
≥ 3 pRBCs unit transfusion requirement in 1 hour
Shock index (heart rate/systolic blood pressure) > 1
Blood volume loss >50% in ≤3 hours
ABC Score ≥ 3 of: 1. Penetrating mechanism of injury 2. Systolic blood pressure < 90 mmHg 3. Heart rate > 120 beats per minute 4. Evidence of hemoperitoneum or hemopericardium on ultrasound (positive FAST U/S exam)
RABT Score ≥ 2 of: 1. Penetrating mechanism of injury 2. Shock index > 1 3. Positive FAST U/S 4. Known or suspected pelvic fracture
Call for help
Activate institution's MTP protocol
Send for STAT type and screen
Establish large-bore intravenous access
Fluid resuscitation
Collect and send STAT bloodwork including hemoglobin, platelet, INR, fibrinogen, electrolytes, creatinine and arterial blood gas (ABG).
Citrate present in blood products to avoid clotting during storage
Stored pRBCs break down and release potassium due to time mediated degeneration
Temporary accumulation of citrate in patient's blood with rapid use of blood products
Citrate chelates calcium
Less negative cell membrane resting potential
Anaerobic metabolism
Promotes hypocalcaemia
Changes in membrane excitability
Lactic acid buildup
Coagulopathy
(see coagulation cascade slide)
Cardiac dysrhythmias (peaked T-waves, atrial block, “sine wave”, asystolic EKG changes)
Metabolic acidosis
End organ damage
Continued blood loss
Volume overload
Avoid hypocalcemia
Avoid hyperkalemia
pH 7.35-7.45
Bleeding source control
Hemoglobin >70-90
Platelets >50 INR <1.5 Fibrinogen >1.5
Avoid dilutional coagulopathy (clotting factor dilution)
Mean Arterial Pressure (MAP) >60mmHg
Temperature >35.0°C
Slow (over 5-10 minutes) IV calcium administration
Inhaled beta agonists
Insulin/Dextrose
EKG monitoring
Sodium bicarbonate
Increase minute ventilation
Fastest control method to prevent further blood loss (i.e., packing wounds)
Early tranexamic acid administration
Administer pRBCs, FFP, and platelets in a 1:1:1 ratio (fibrinogen replacement indicated if <1.5 despite FFP)
Minimize crystalloid use
Administer crystalloids in a 3:1 ratio to estimated blood loss until blood products available
Administer vasopressors to meet target, do not overshoot
Temperature monitoring Fluid warming
↑ [Potassium] in pRBCs solution
Administration of pRBCs ↑ potassium in patient's blood
Blood loss
↓ Hemoglobin
Tissue hypoperfusion
Tissue hypoxia
↑ Diluent volume
↓ Concentration of clotting factors
Tissue death
↓ Coagulation ability
↑ Transfusion requirements
Early fluid resuscitation
Rapid transfusion of cooled or room-temperature blood products/fluids
↑ Blood pressure
Development of hypothermia
↑ Bleeding and clot dislodgement potential
↓ Enzyme activity in the coagulation cascade
↓ Coagulation ability
Legend:
Pathophysiology
Mechanism
Targets
Intervention
Published Sept 5, 2024 on www.thecalgaryguide.com
Massive Transfusion Protocol: Considerations and rationale
Massive transfusion protocol (MTP) is a tool used by clinicians when there is a need to rapidly administer a large amount of blood products, including packed red blood cells (pRBCs), fresh frozen plasma (FFP), and platelets. Complications of MTP are commonly referred to as “The Lethal Triad” referring to hypothermia, acidosis and coagulopathy.
Authors: Kayleigh Yang Arzina Jaffer
Reviewers: Jasleen Brar,
Luiza Radu, Karl Darcus*
* MD at time of publication
Intervention
Indications Initial Response Pathophysiology Transfusion Targets
≥ 3 pRBCs unit transfusion requirement in 1 hour
Shock index (heart rate/systolic blood pressure) > 1
Blood volume loss >50% in ≤3 hours
ABC Score ≥ 3 of: 1. Penetrating mechanism of injury 2. Systolic blood pressure < 90 mmHg 3. Heart rate > 120 beats per minute 4. Evidence of hemoperitoneum or hemopericardium on ultrasound (positive FAST U/S exam)
RABT Score ≥ 2 of: 1. Penetrating mechanism of injury 2. Shock index > 1 3. Positive FAST U/S 4. Known or suspected pelvic fracture
Call for help
Activate institution's MTP protocol
Send for STAT type and screen
Establish large-bore intravenous access
Fluid resuscitation
Collect and send STAT bloodwork including hemoglobin, platelet, INR, fibrinogen, electrolytes, creatinine and arterial blood gas (ABG).
Citrate present in blood products to avoid clotting during storage
Stored pRBCs break down and release potassium due to time mediated degeneration
Temporary accumulation of citrate in patient's blood with rapid use of blood products
Citrate chelates calcium
Less negative cell membrane resting potential
Anaerobic metabolism
Promotes hypocalcaemia
Changes in membrane excitability
Lactic acid buildup
Coagulopathy
(see coagulation cascade slide)
Cardiac dysrhythmias (peaked T-waves, atrial block, “sine wave”, asystolic EKG changes)
Metabolic acidosis
End organ damage
Continued blood loss
Volume overload
Avoid hypocalcemia
Avoid hyperkalemia
pH 7.35-7.45
Bleeding source control
Hemoglobin >70-90
Platelets >50 INR <1.5 Fibrinogen >1.5
Avoid dilutional coagulopathy (clotting factor dilution)
Mean Arterial Pressure (MAP) >60mmHg
Temperature >35.0°C
Slow (over 5-10 minutes) IV calcium administration
Inhaled beta agonists
Insulin/Dextrose
EKG monitoring
Sodium bicarbonate
Increase minute ventilation
Fastest control method to prevent further blood loss (i.e., packing wounds)
Early tranexamic acid administration
Administer pRBCs, FFP, and platelets in a 1:1:1 ratio (fibrinogen replacement indicated if <1.5 despite FFP)
Minimize crystalloid use
Administer crystalloids in a 3:1 ratio to estimated blood loss until blood products available
Administer vasopressors to meet target, do not overshoot
Temperature monitoring Fluid warming
↑ [Potassium] in pRBCs solution
Administration of pRBCs ↑ potassium in patient's blood
Blood loss
↓ Hemoglobin
Tissue hypoperfusion
Tissue hypoxia
↑ Diluent volume
↓ Concentration of clotting factors
Tissue death
↓ Coagulation ability
↑ Transfusion requirements
Early fluid resuscitation
Rapid transfusion of cooled or room-temperature blood products/fluids
↑ Blood pressure
Development of hypothermia
↑ Bleeding and clot dislodgement potential
↓ Enzyme activity in the coagulation cascade
↓ Coagulation ability
Legend:
Pathophysiology
Mechanism
Targets
Intervention
Published Sept 5, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/09/Massive-Transfusion-Protocol.jpg)
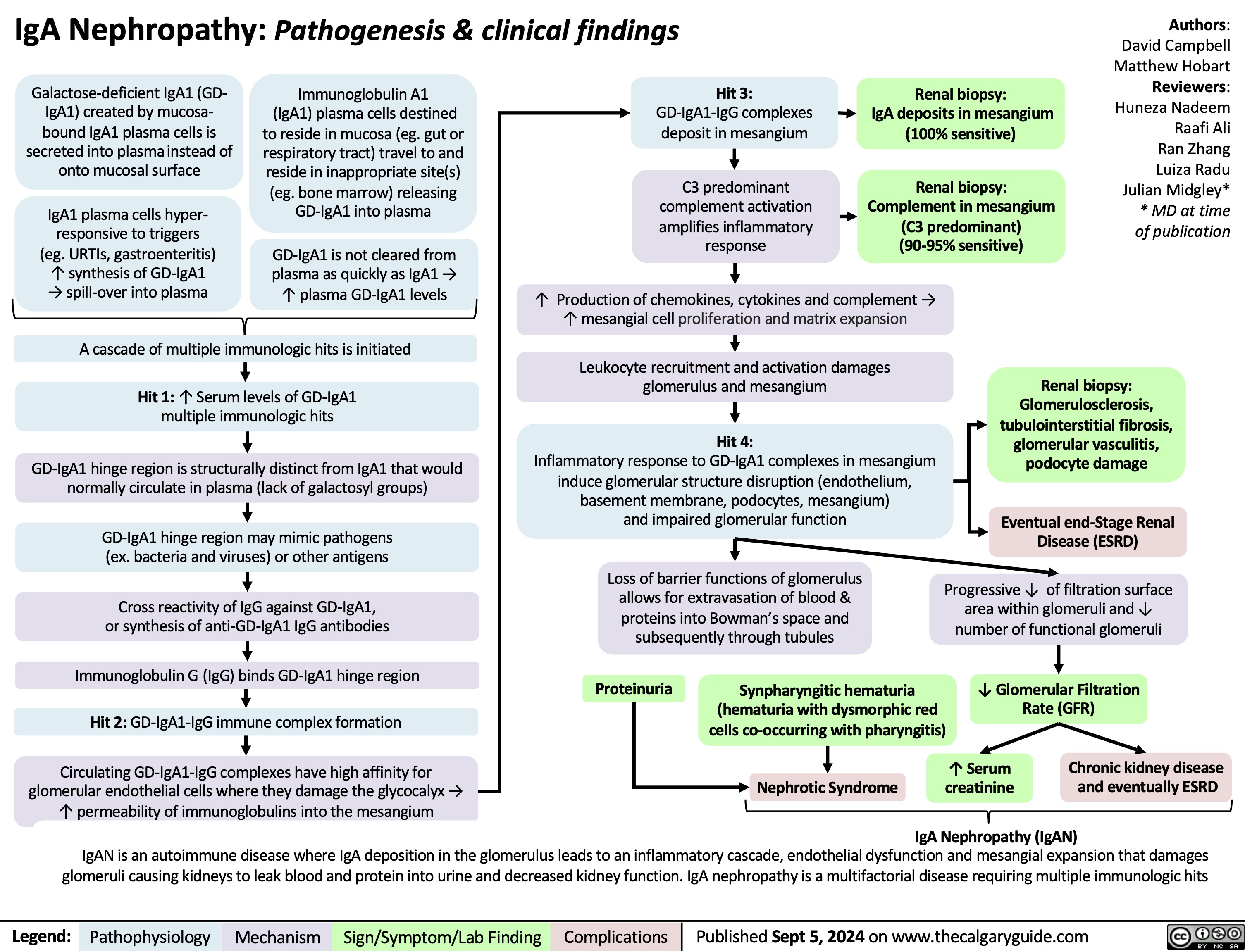
IgA Nephropathy
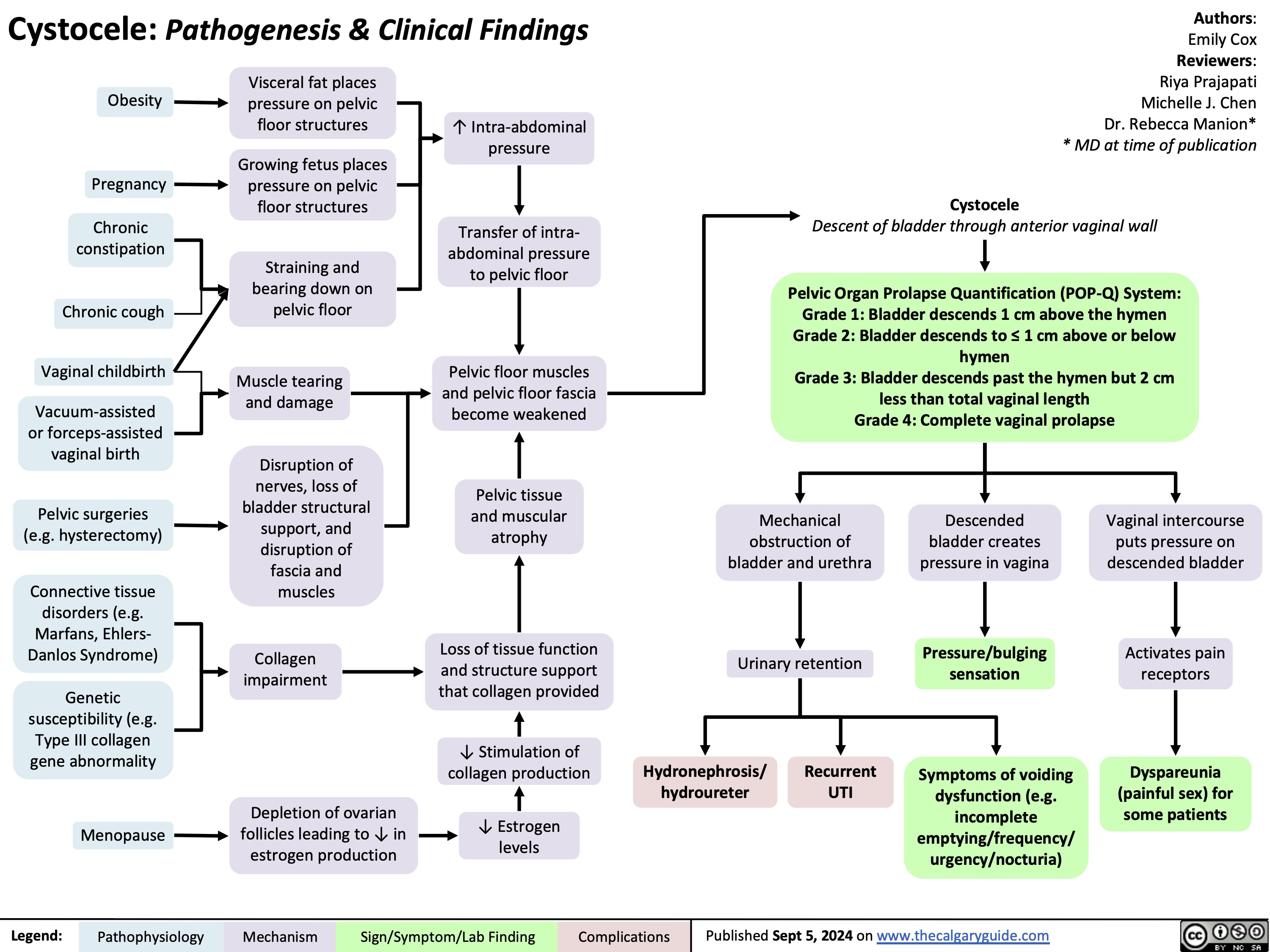
Cystocele
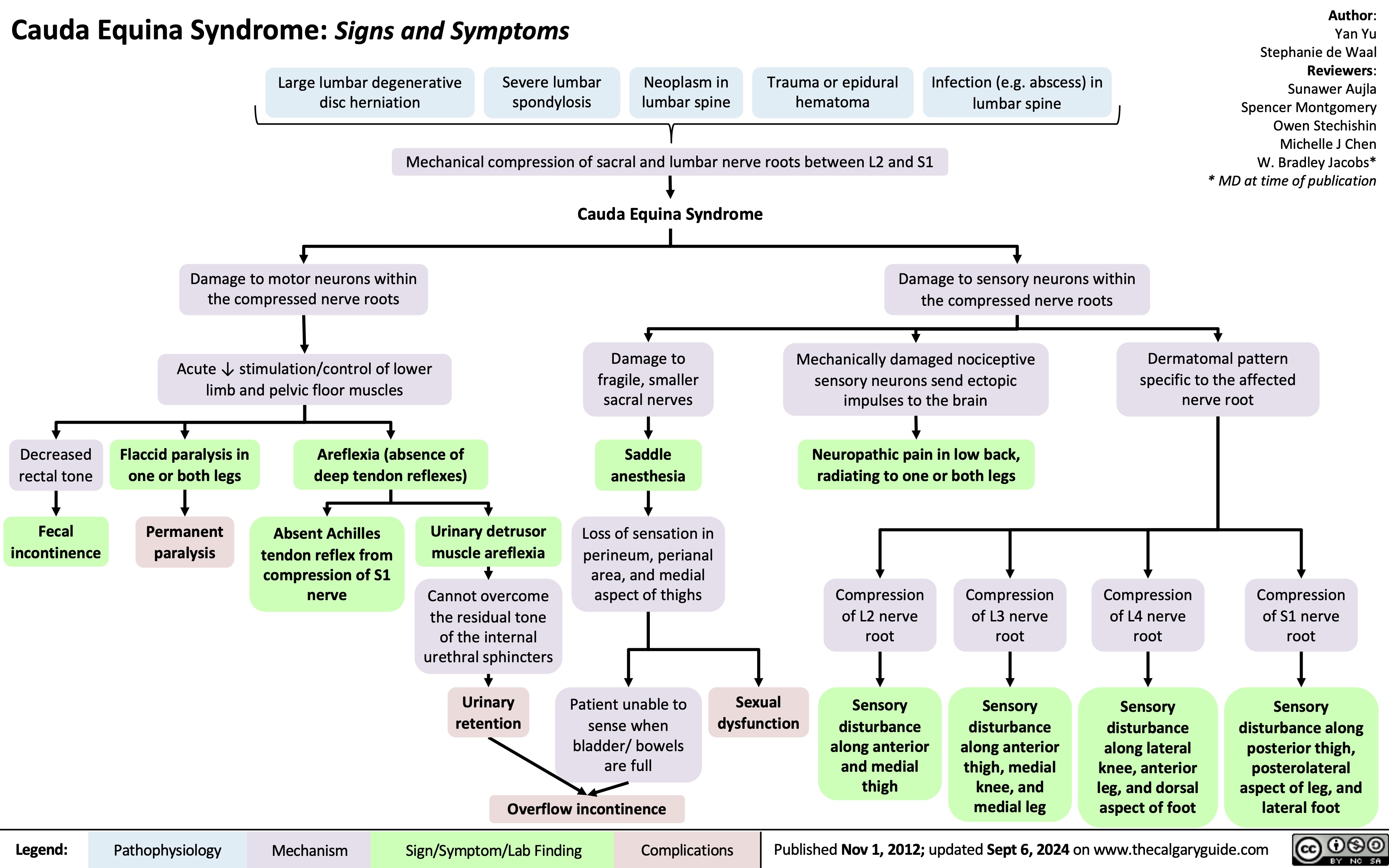
Cauda Equina Syndrome
Diffuse Axonal Injury

S3 Pathogenesis
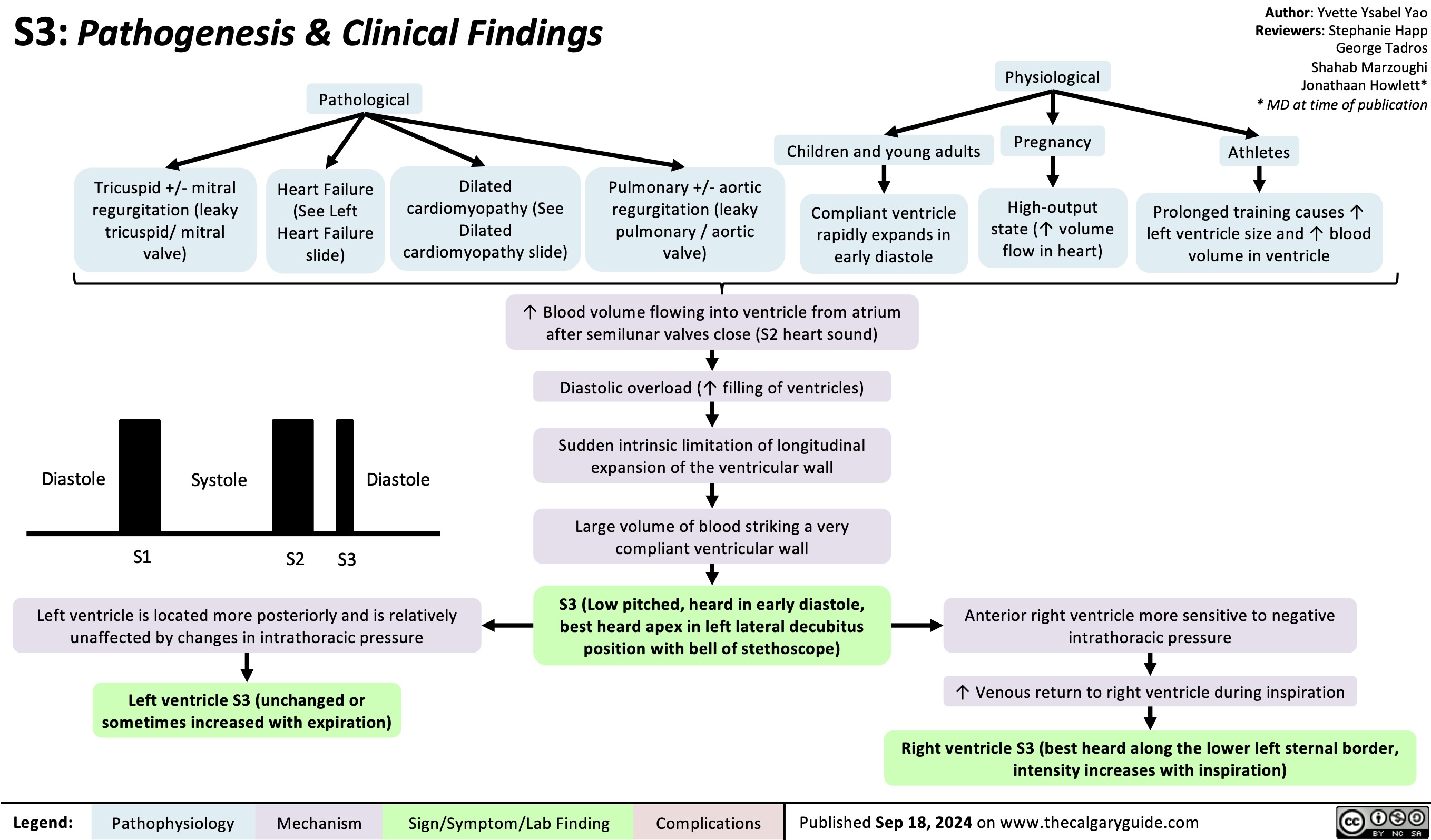
Malignant Hyperthermia
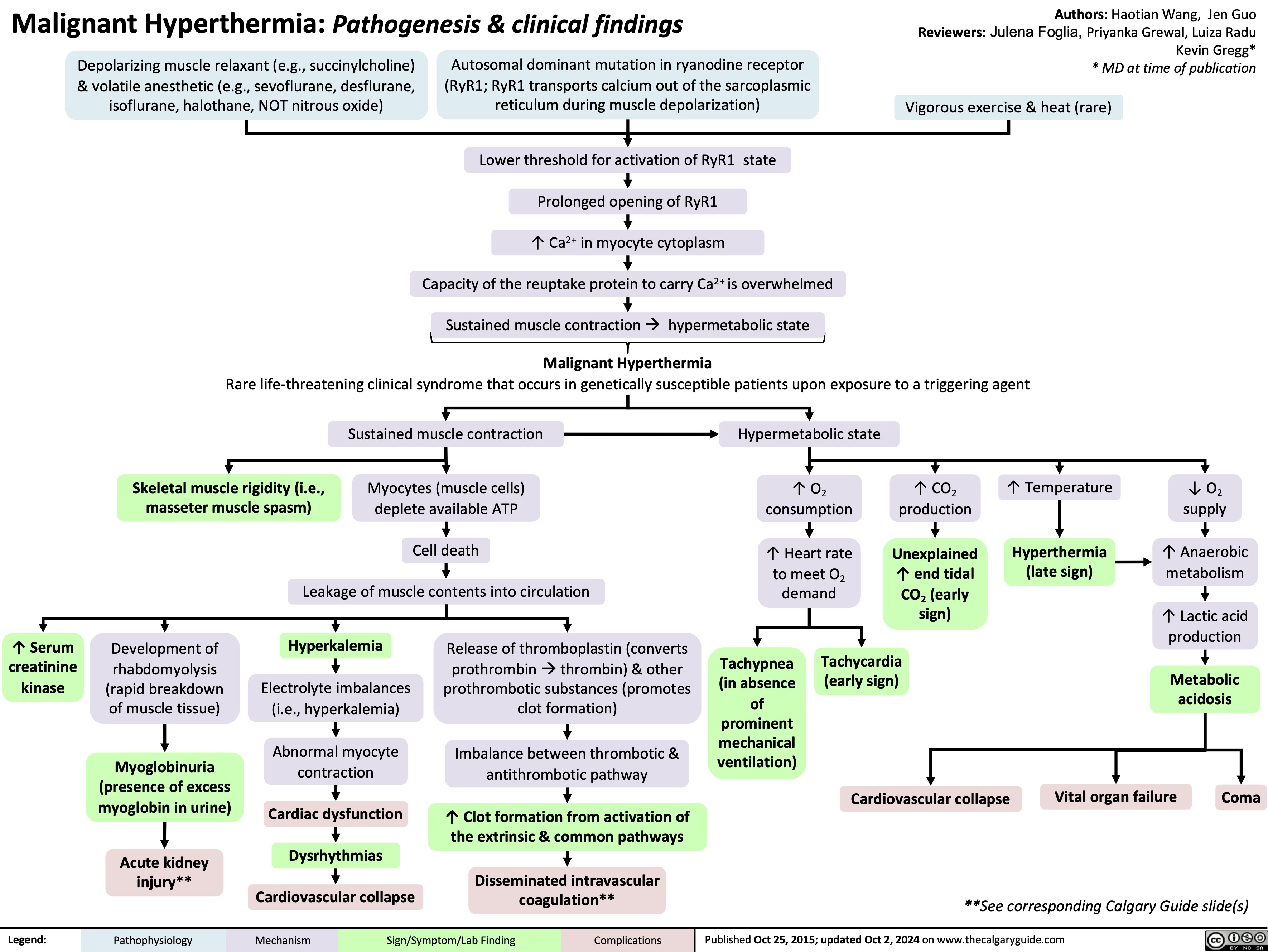
Obesity Pathogenesis
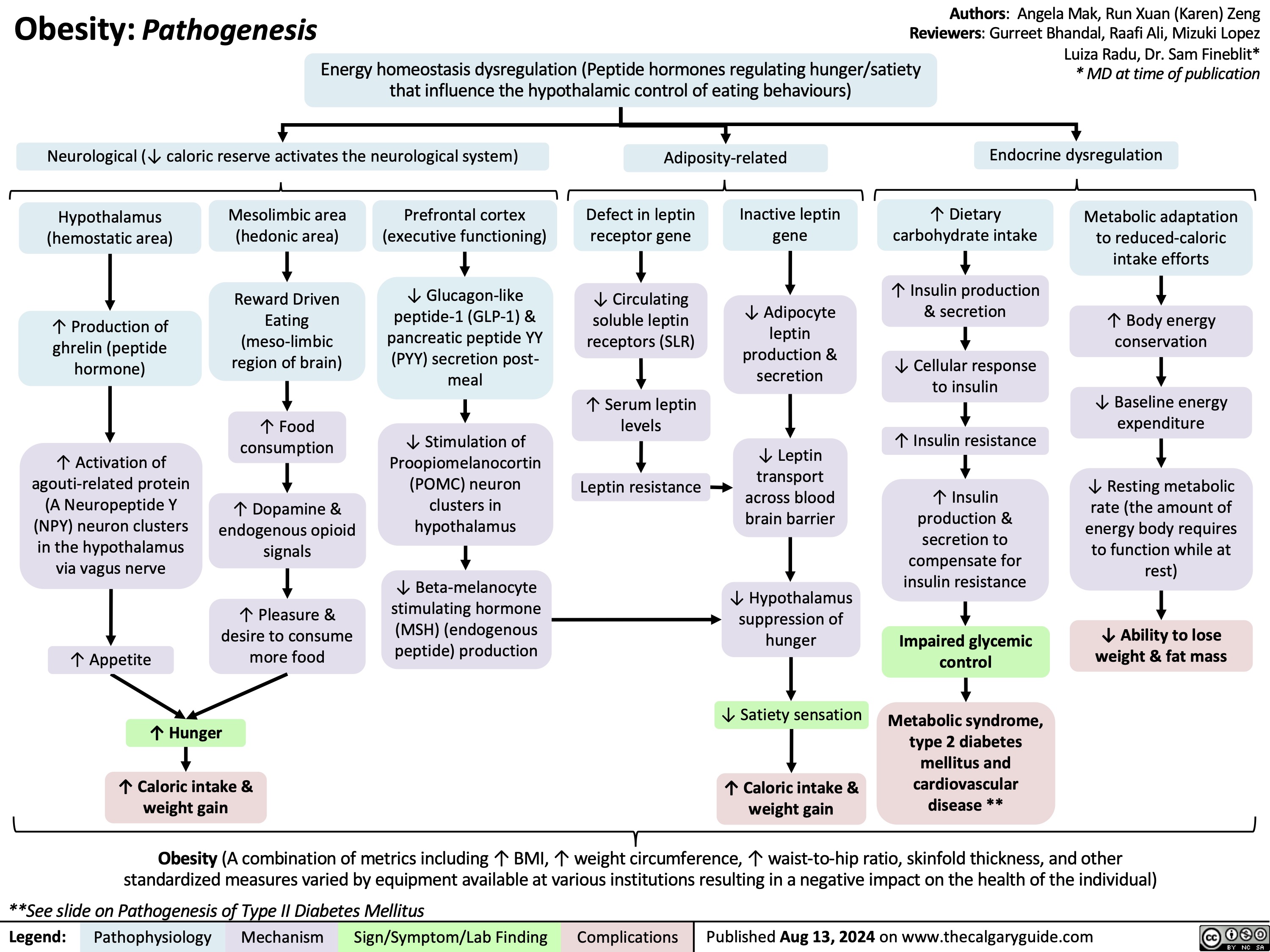
Hyperthyroidism in Pregnancy
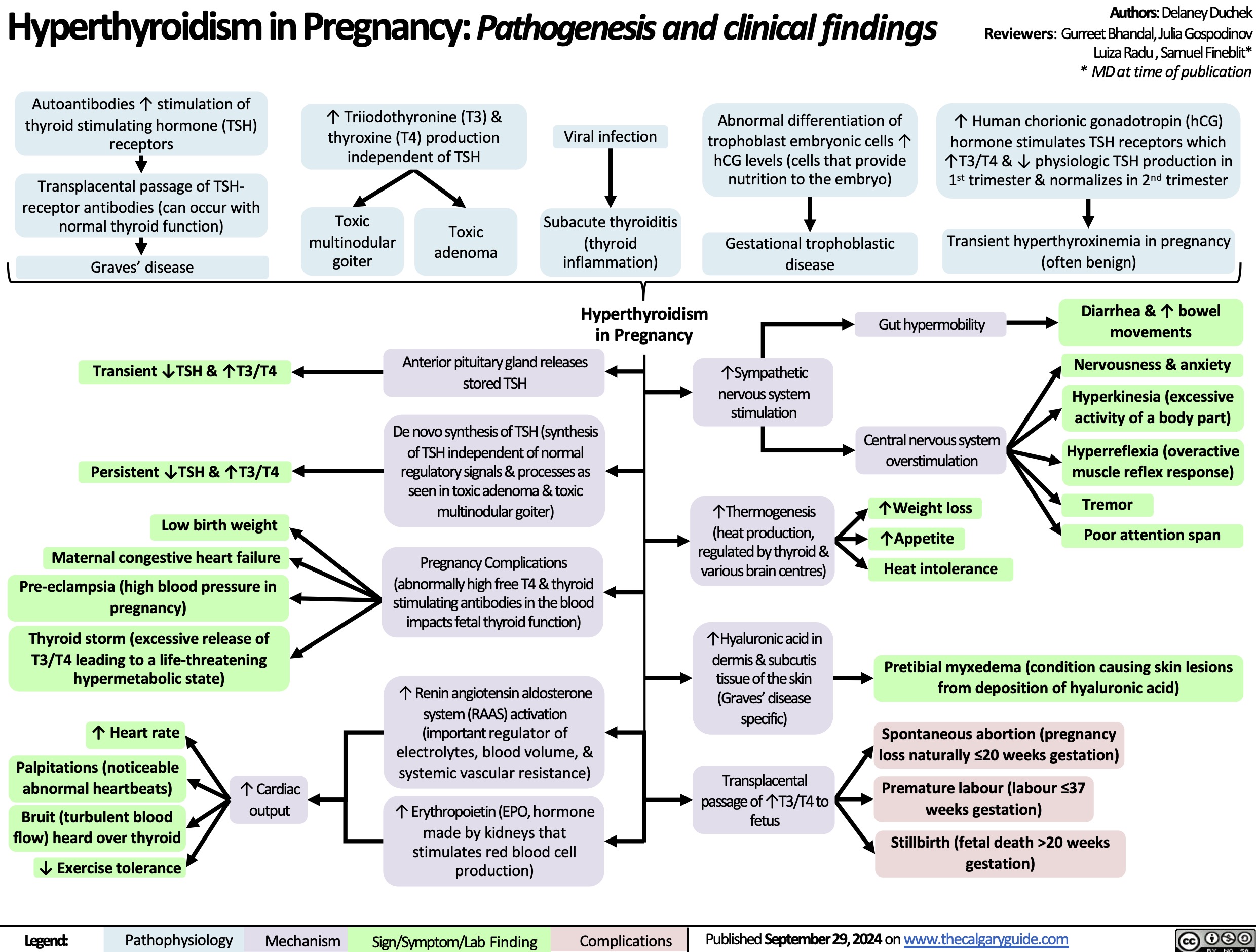
Secondary hypoglycemia Insulin Mediated
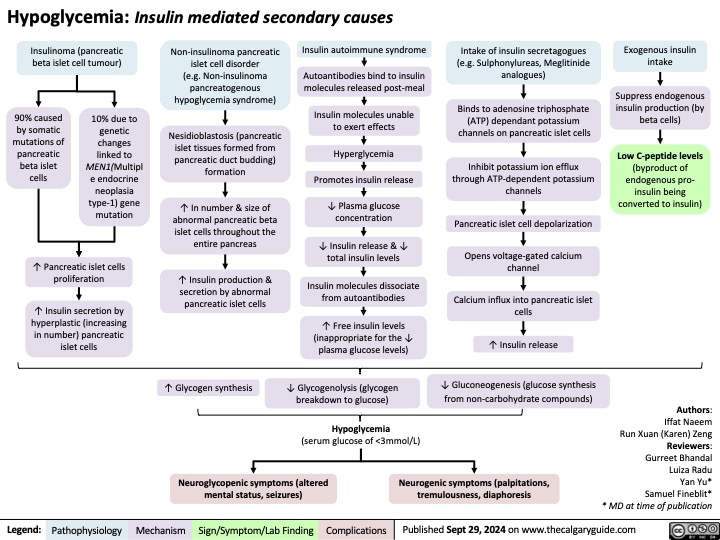
Major Depressive Disorder 2024
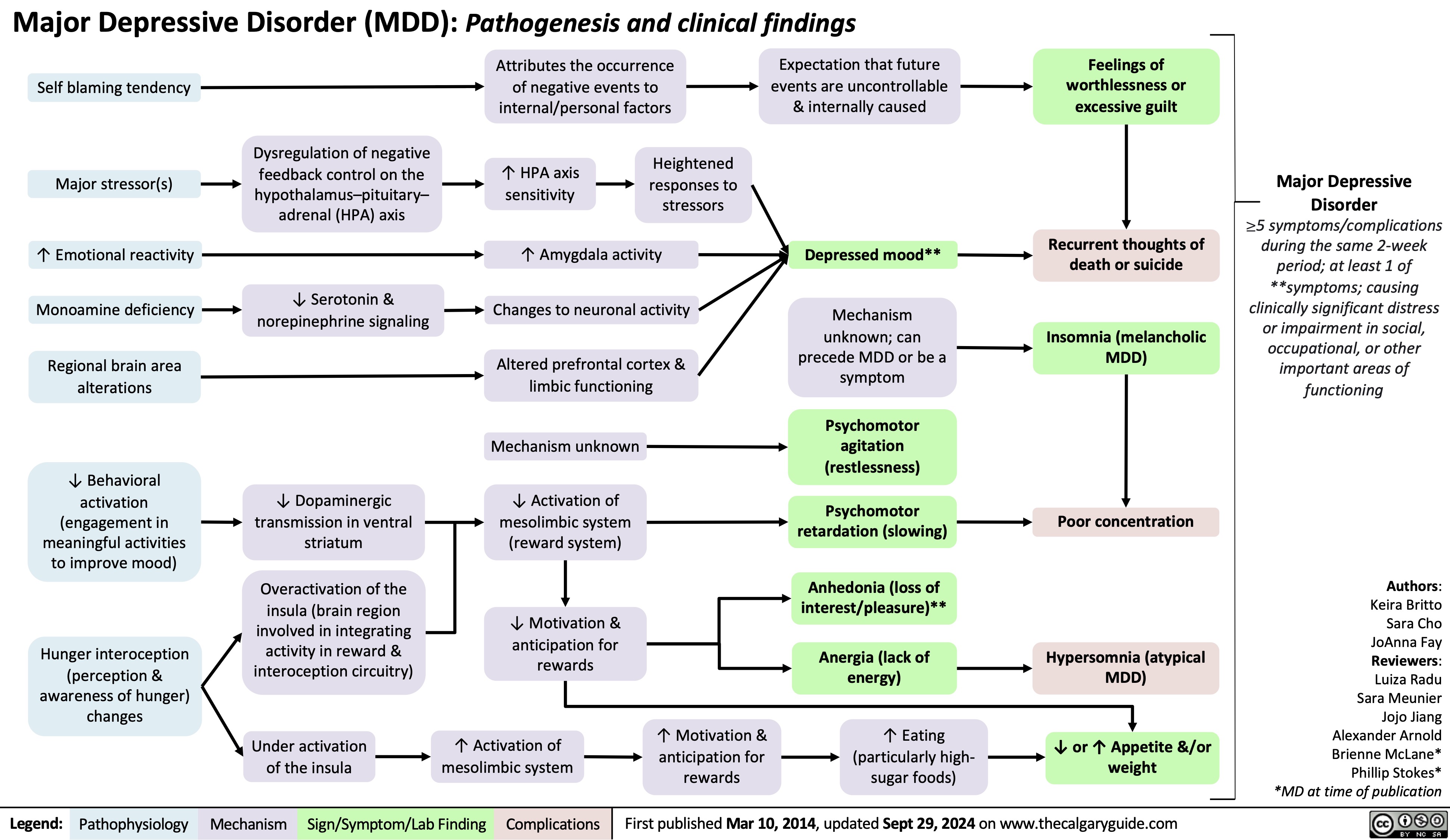
Hypomagnesemia
![Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com
Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/10/Hypomagnesia.jpg)
Transient Tachypnea of the Newborn
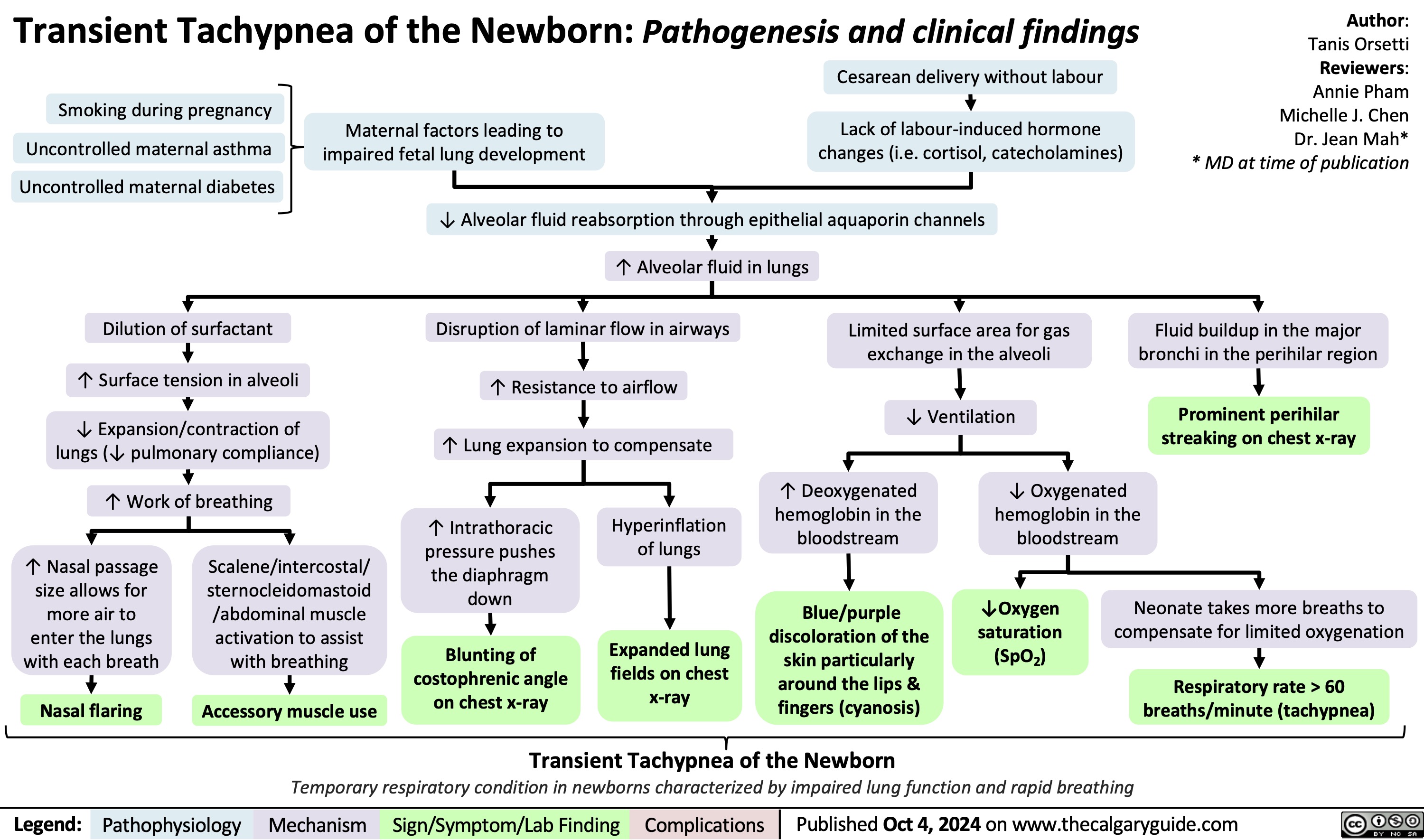
Neonatal Hypoglycemia Clinical Presentation
Spontaneous Rupture of Membranes
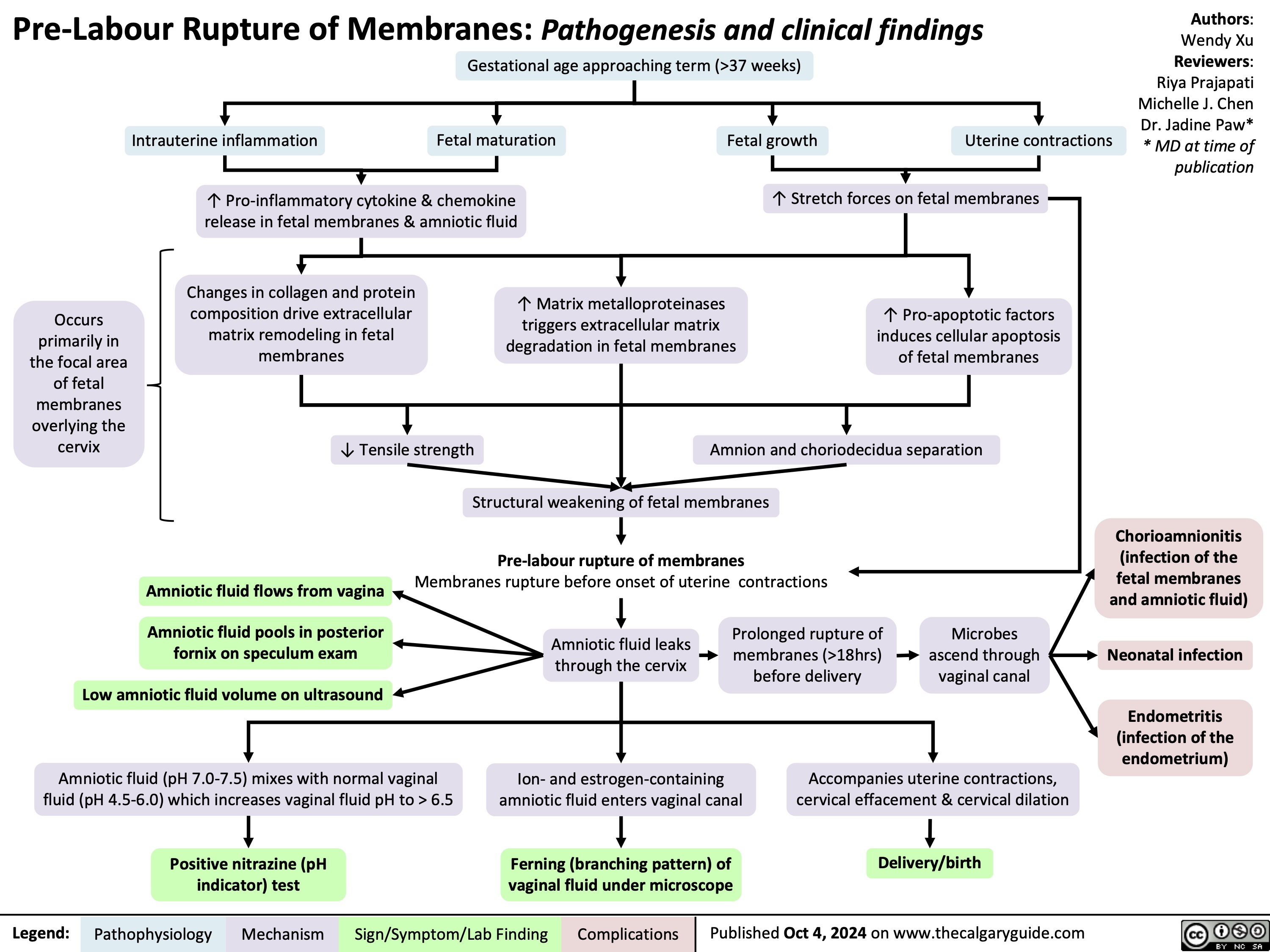
Precocious Puberty
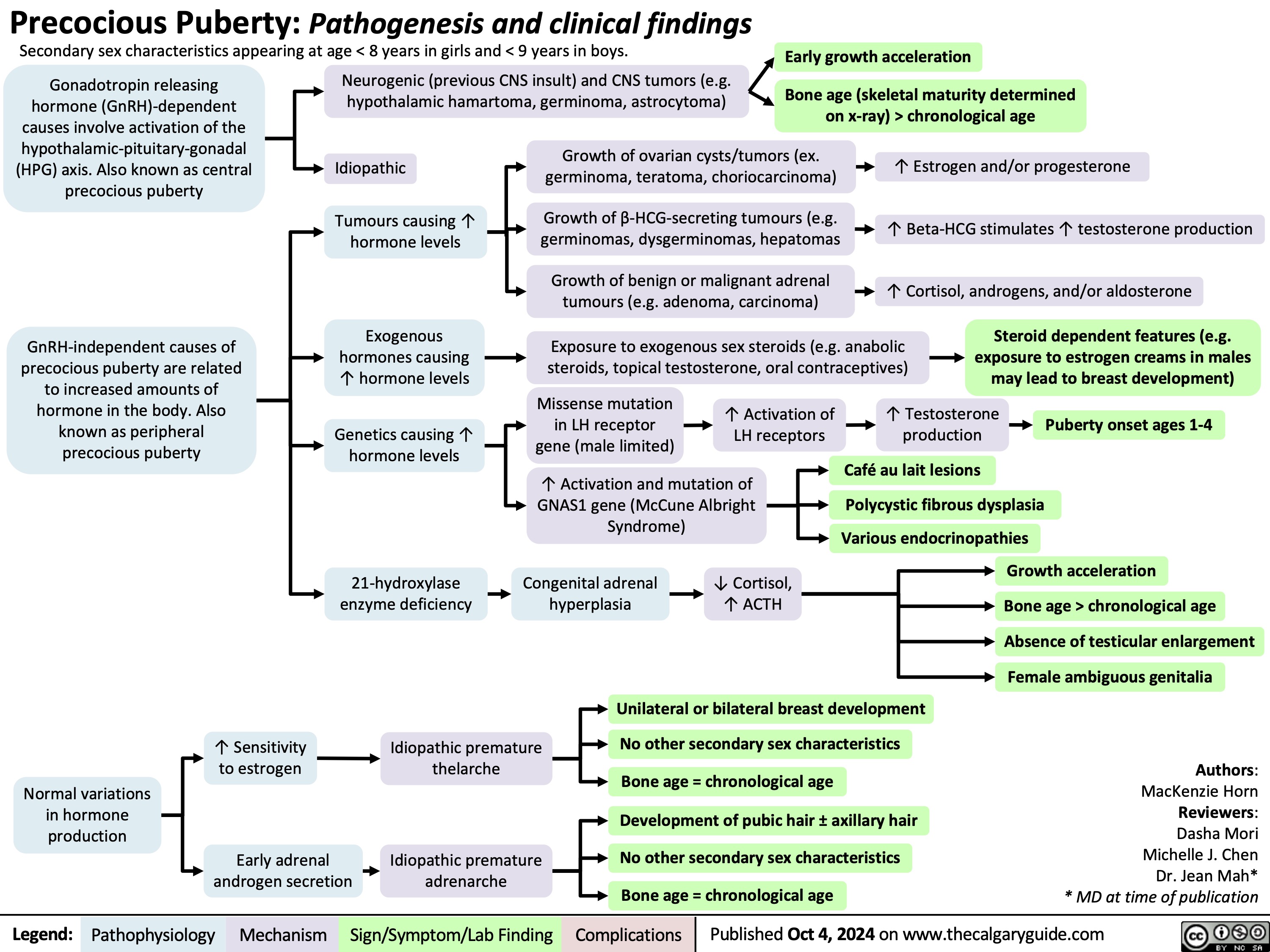
Acute Diverticulitis
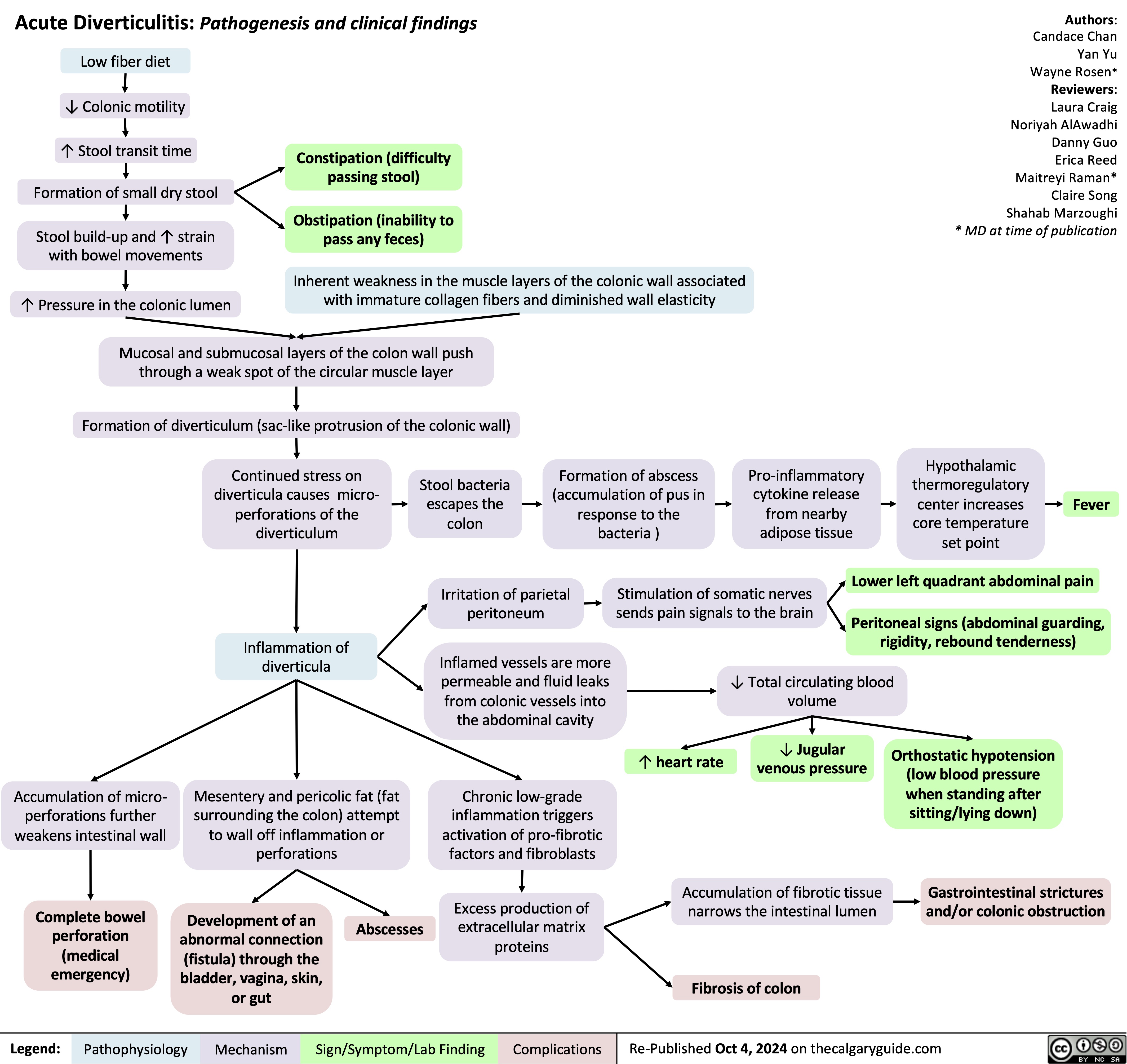
Tuberous Sclerosis Complex Dermatologic Manifestations
Chronic Subdural Hematoma
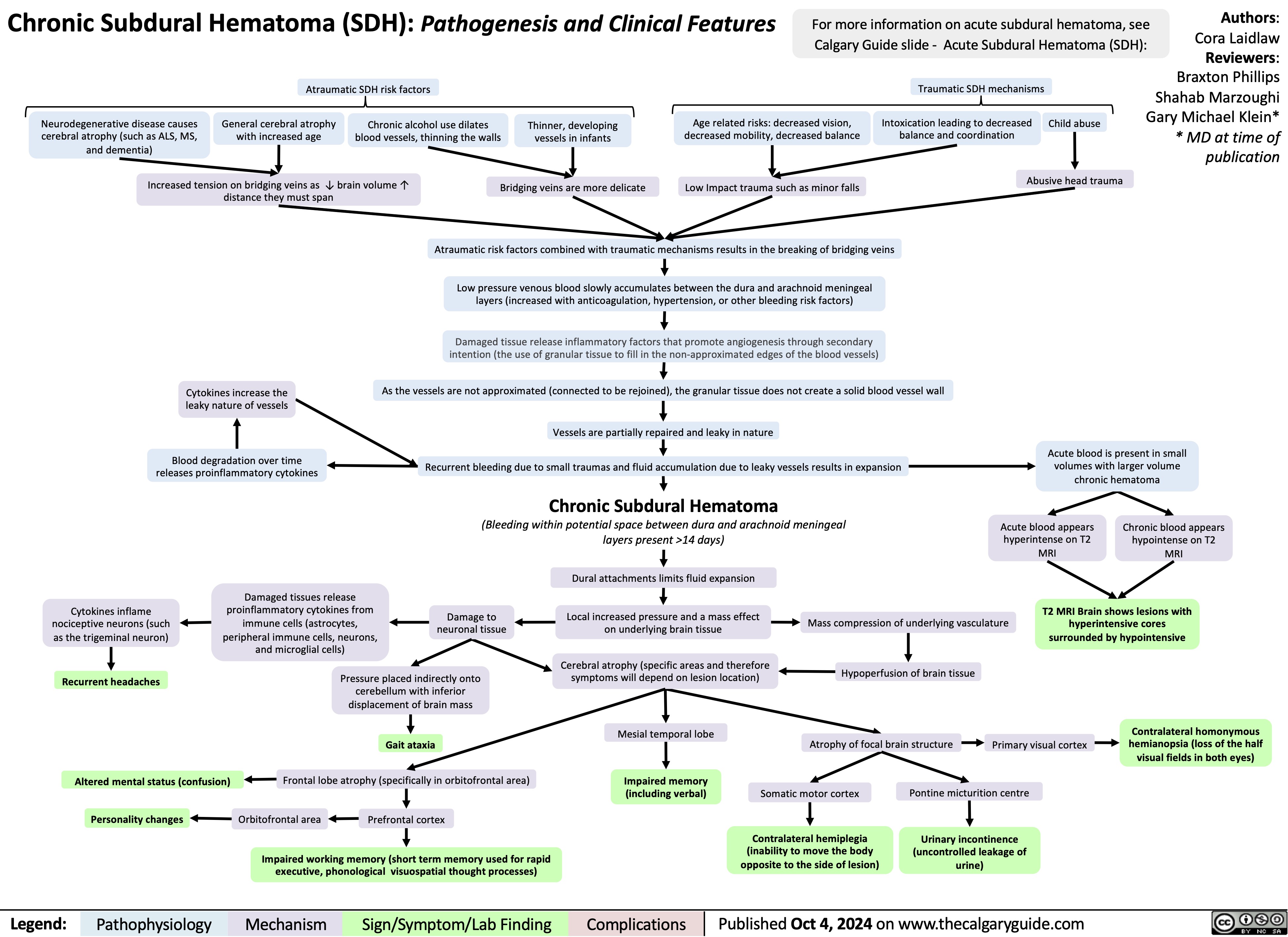
Epilepsy in Older Adults
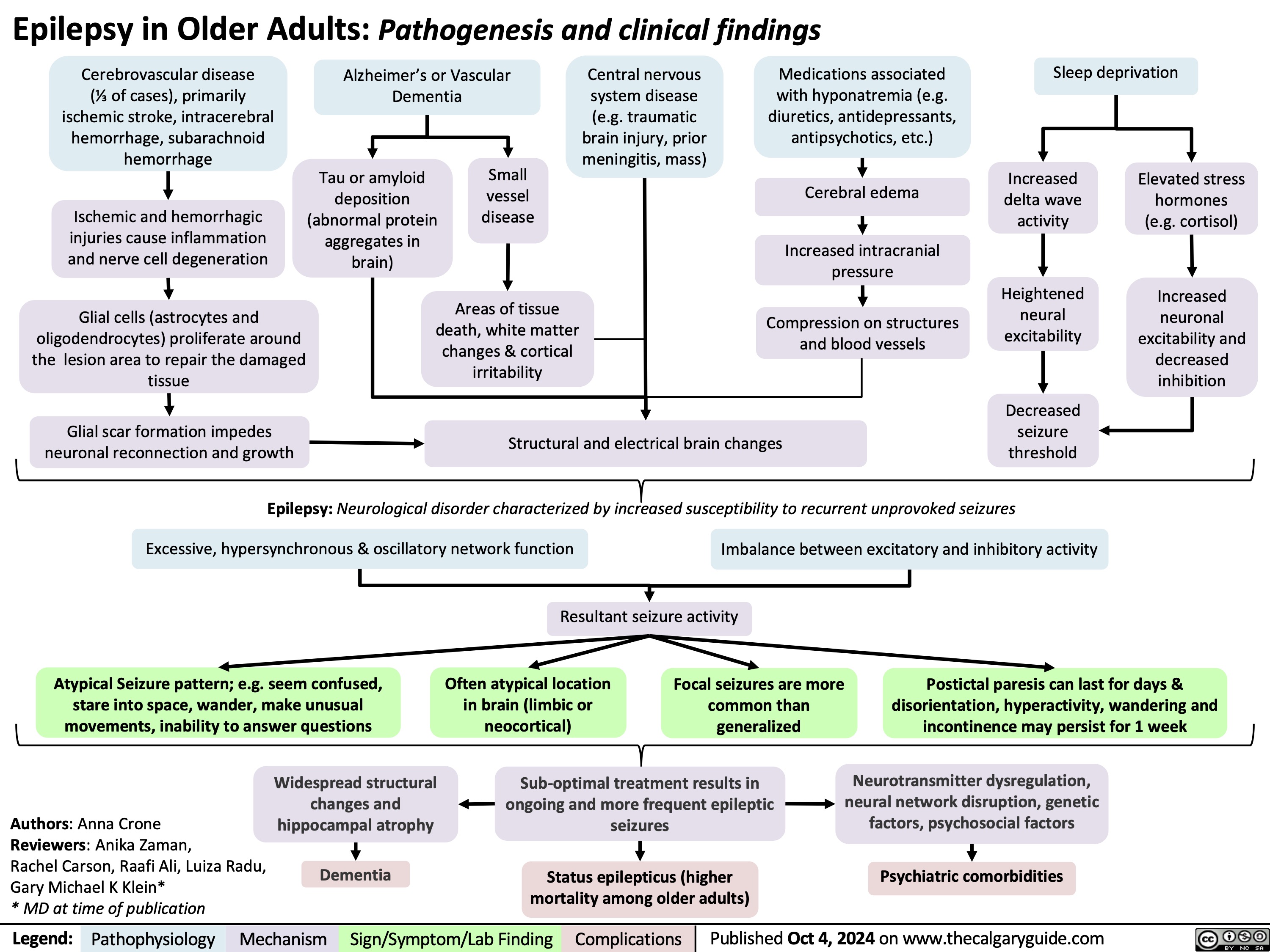
Cholesteatoma of middle ear
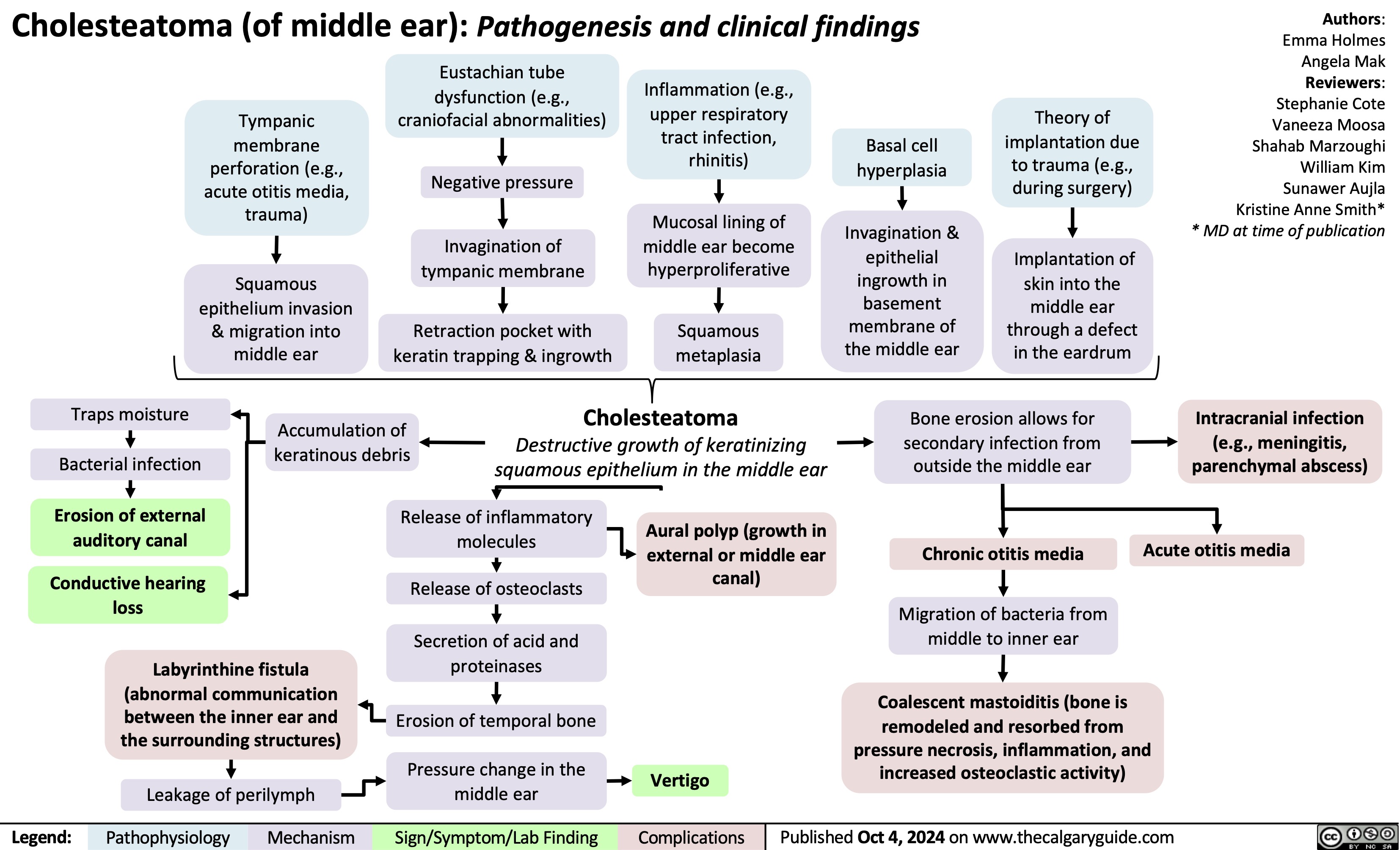
Acute Subdural Hematoma
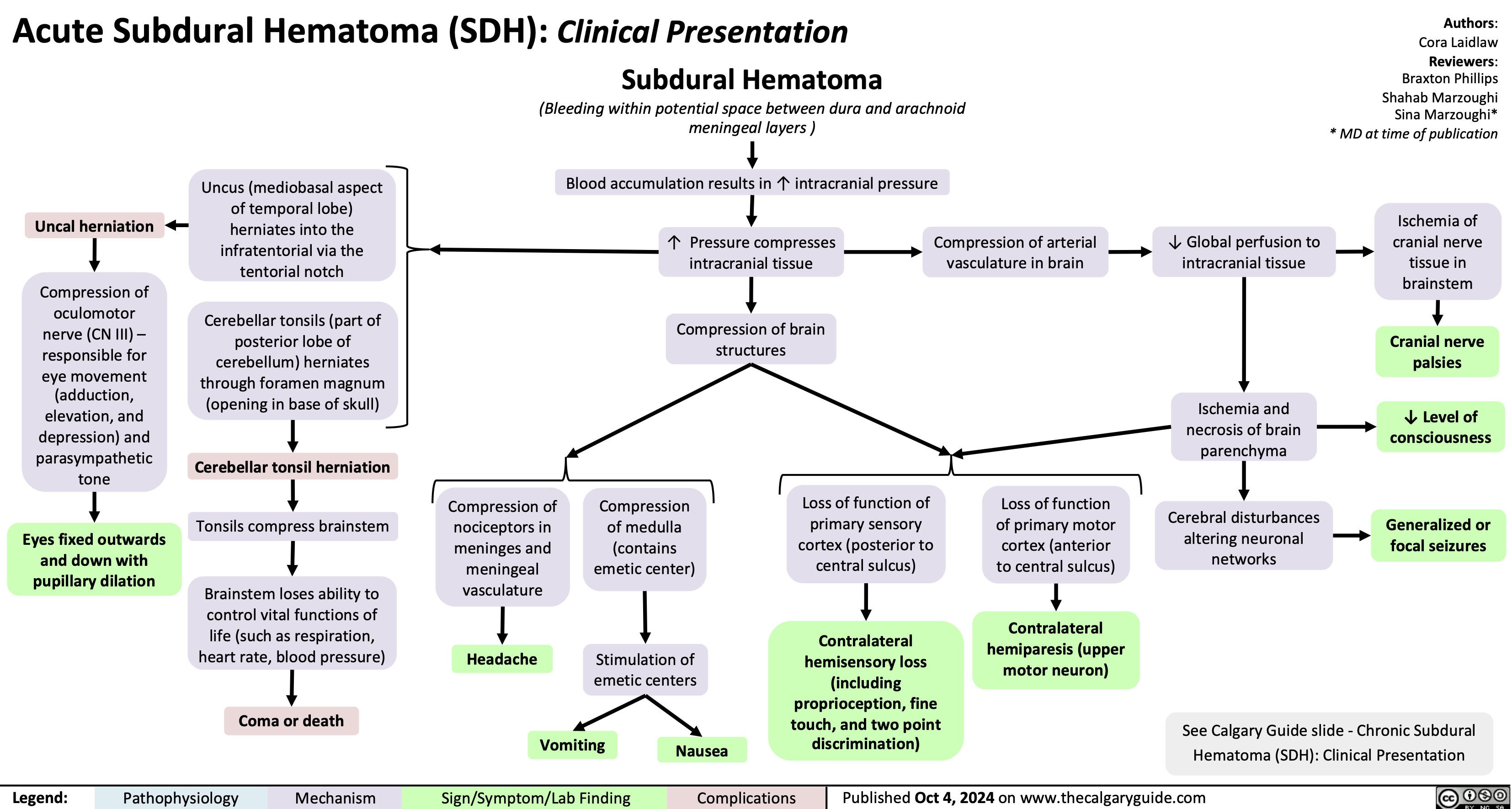
Primary Combined Hyperlipidemia
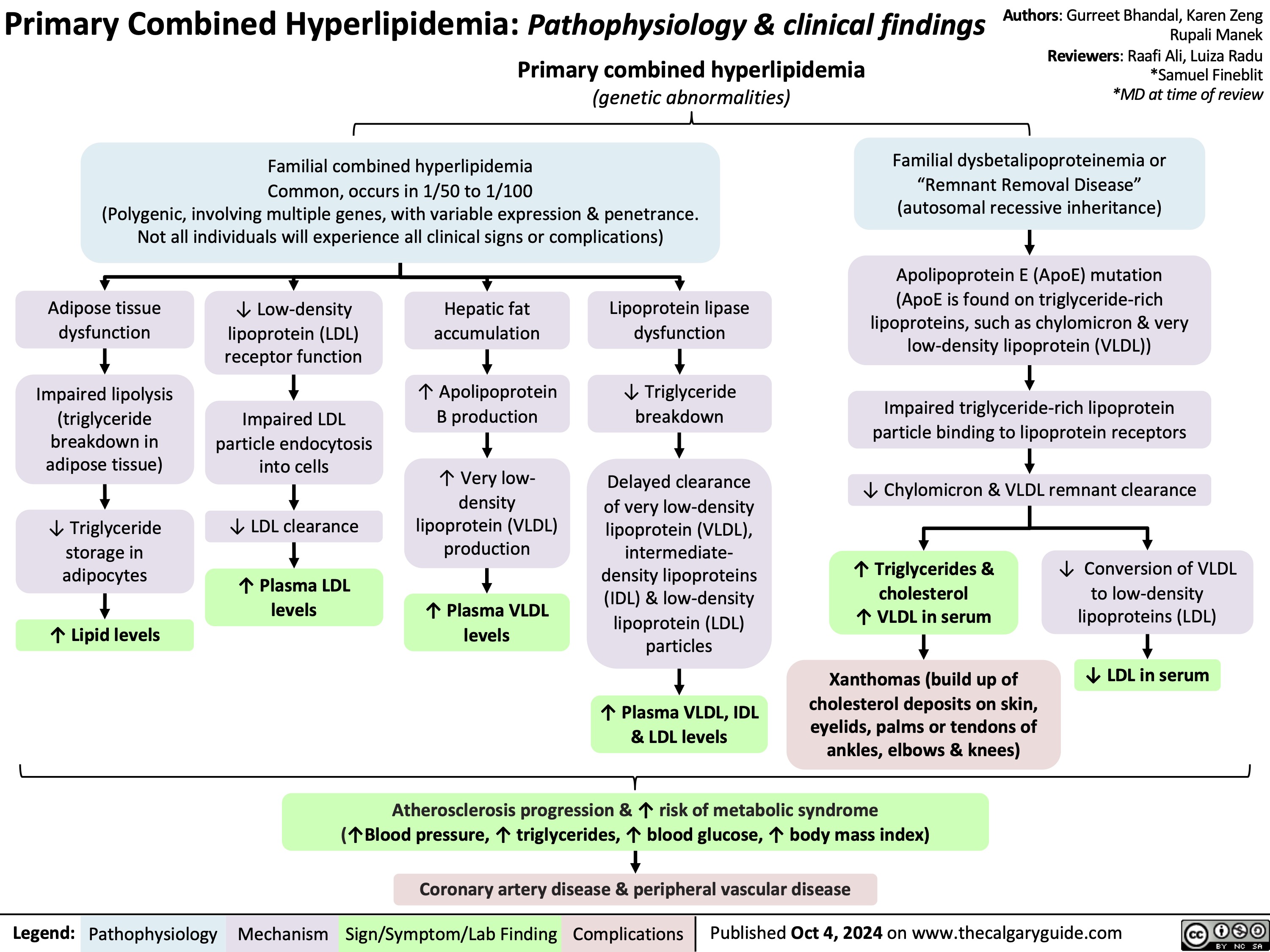
Bone Remodeling Physiology
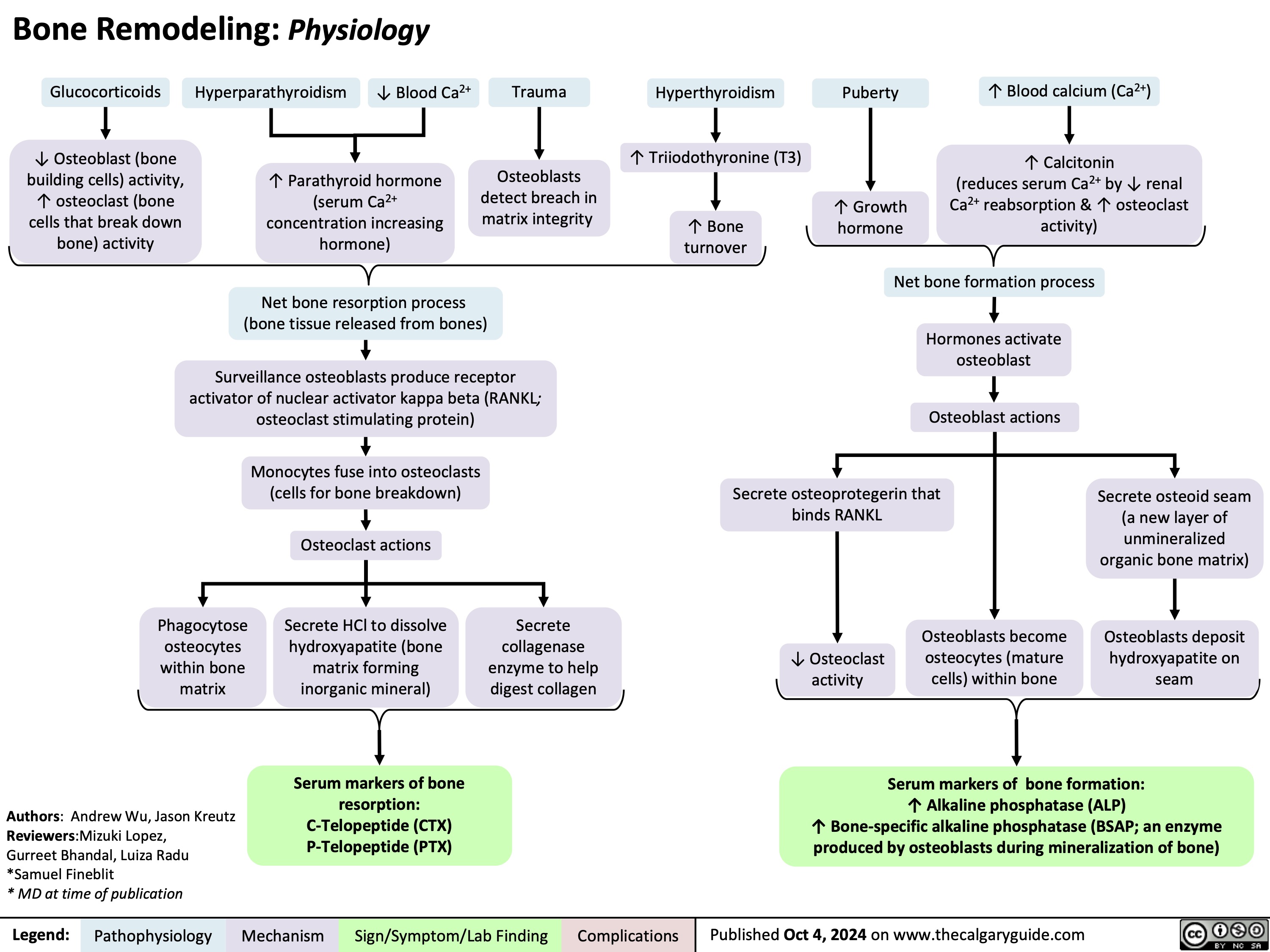
Persistent Truncus Arteriosus
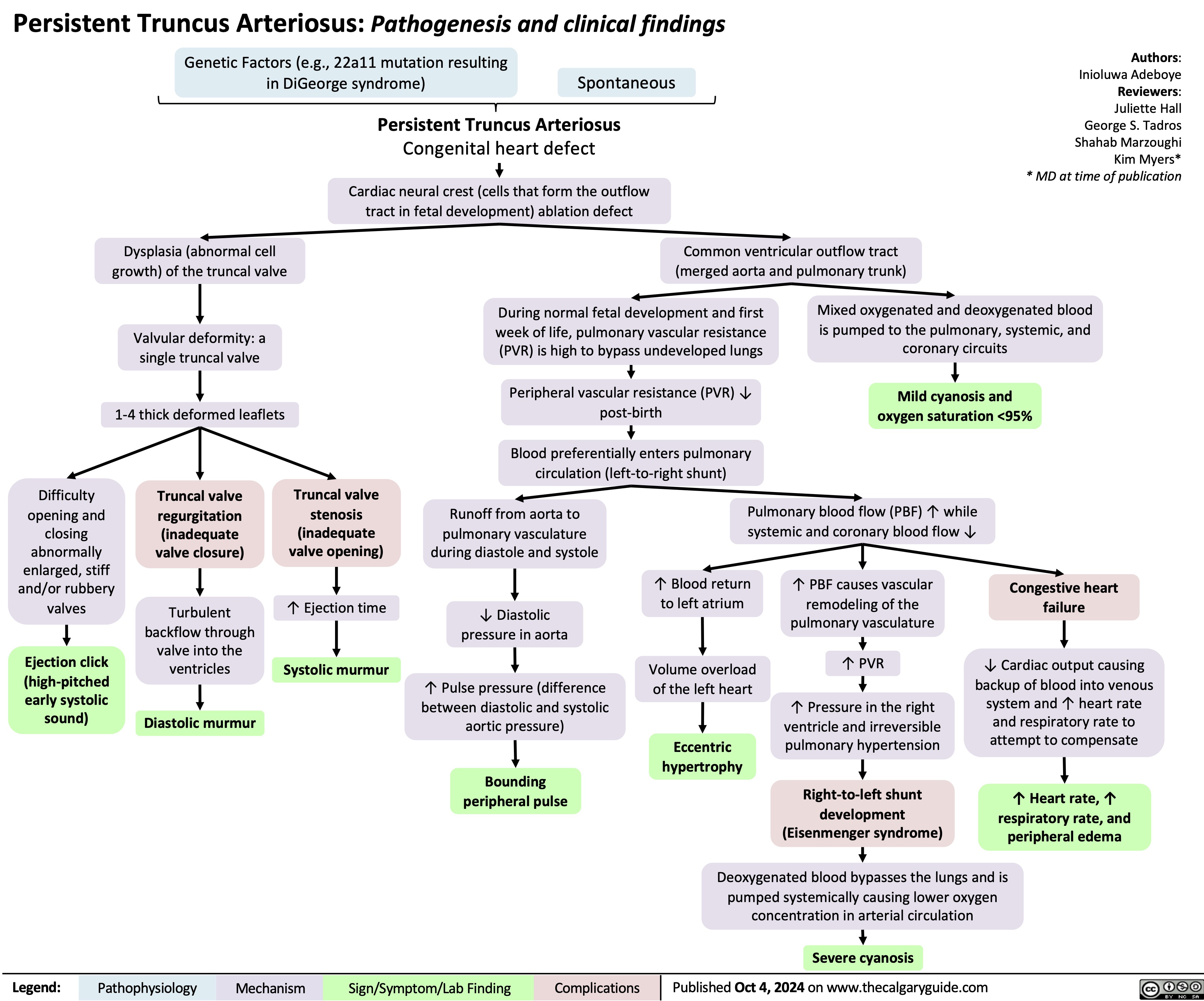
Thyroid Eye Disease
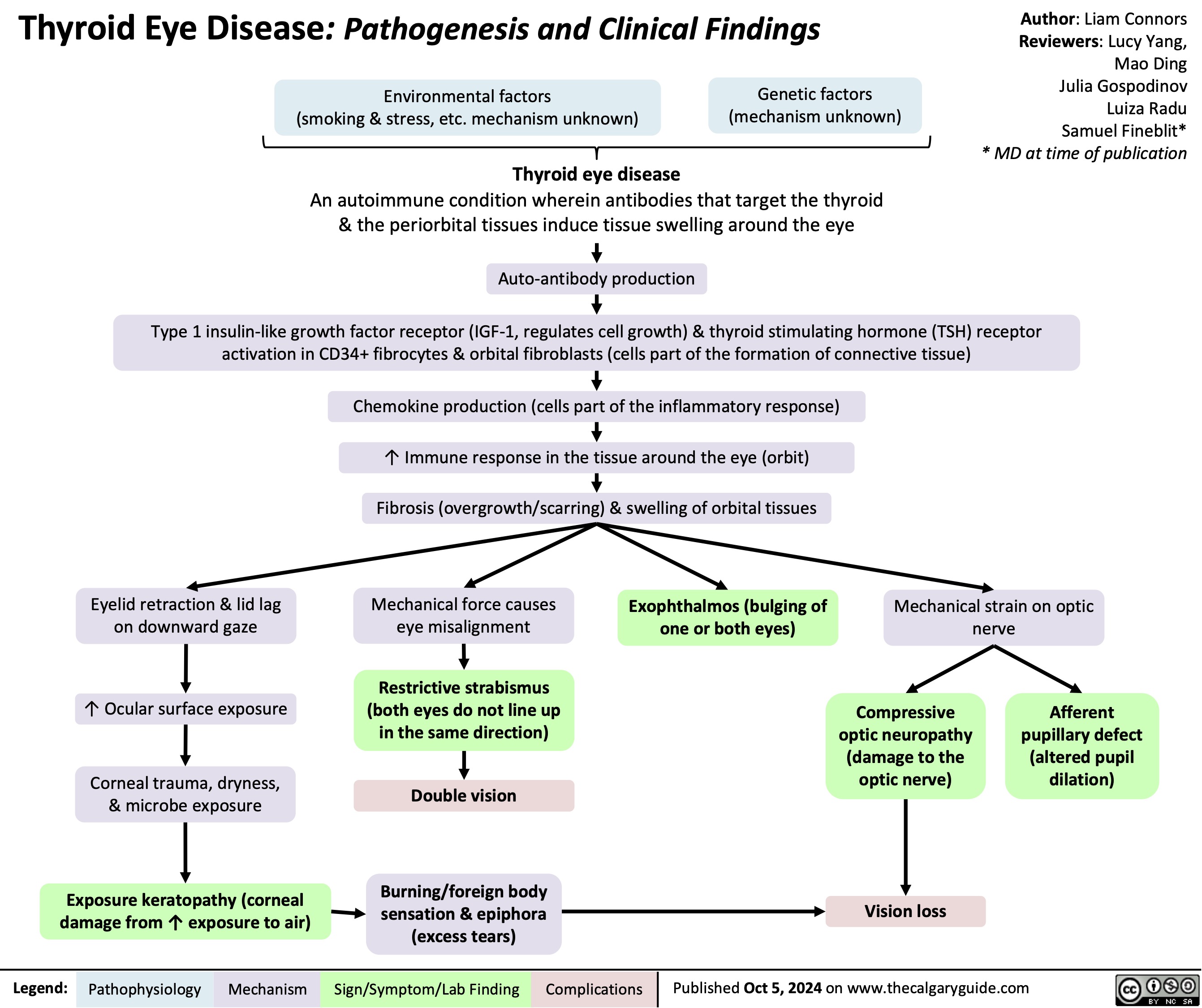
Subtrochanteric Femur Fracture
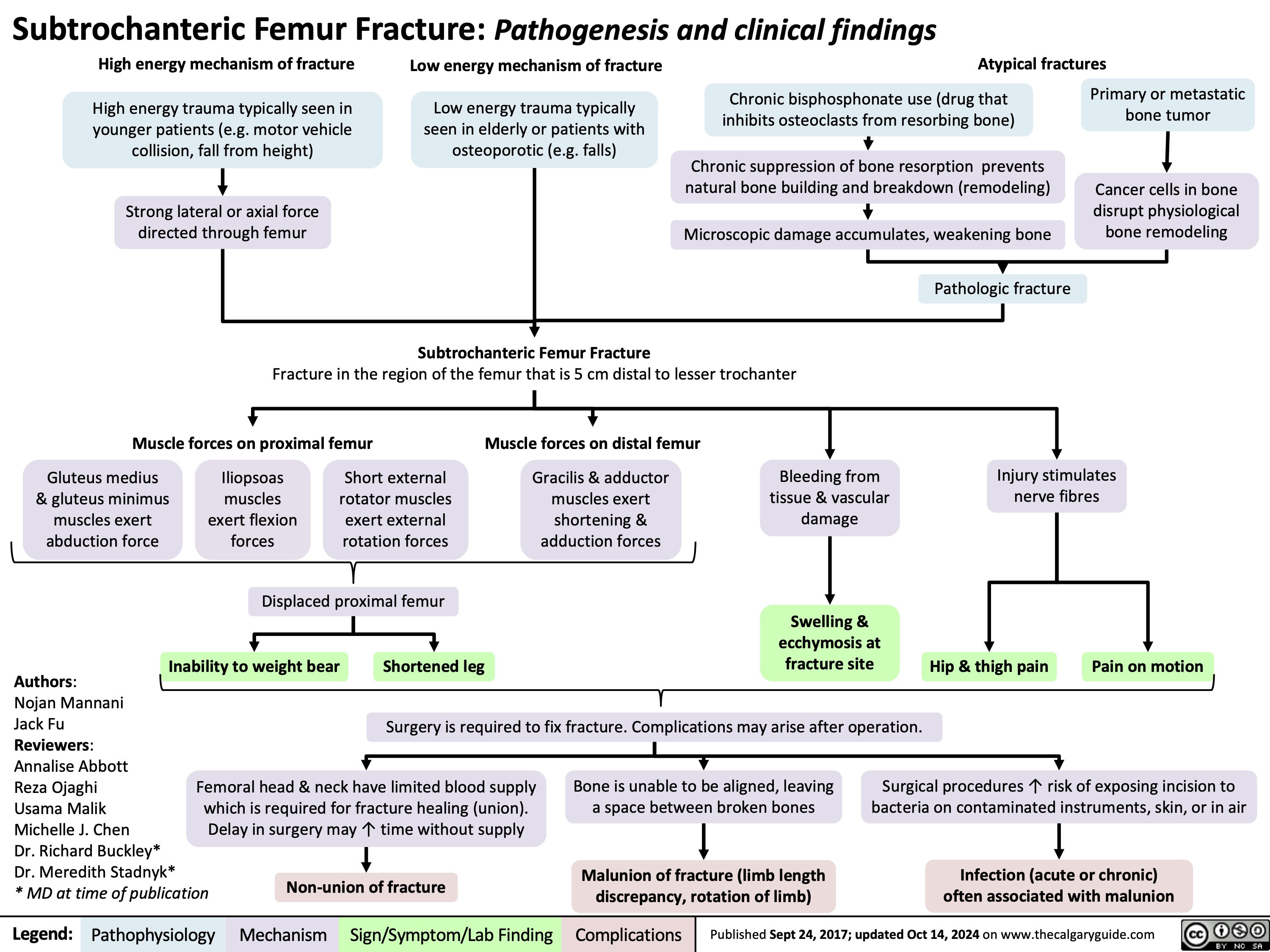
Complex Regional Pain Syndrome
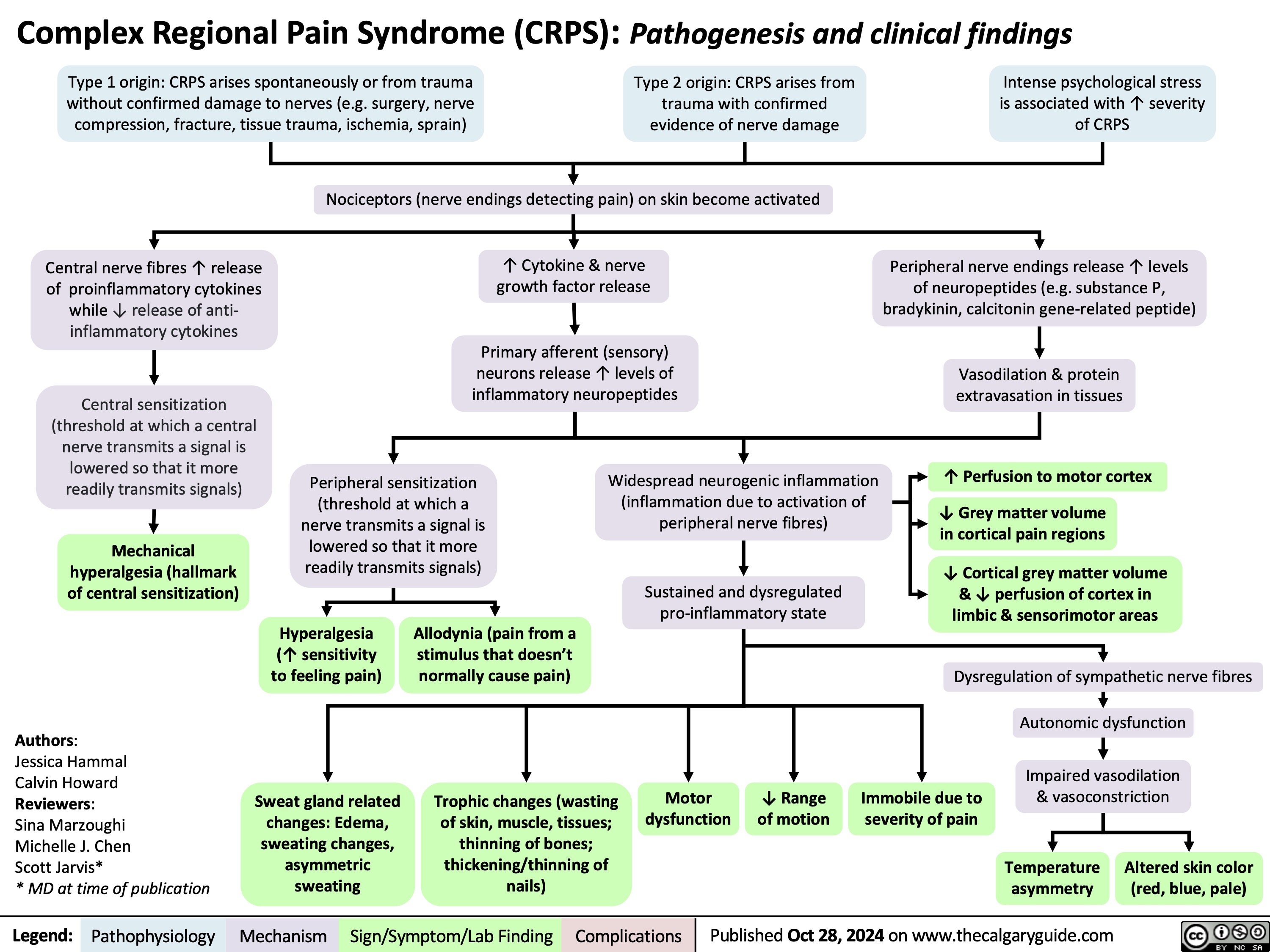
Newborn Disorders of Sexual Development
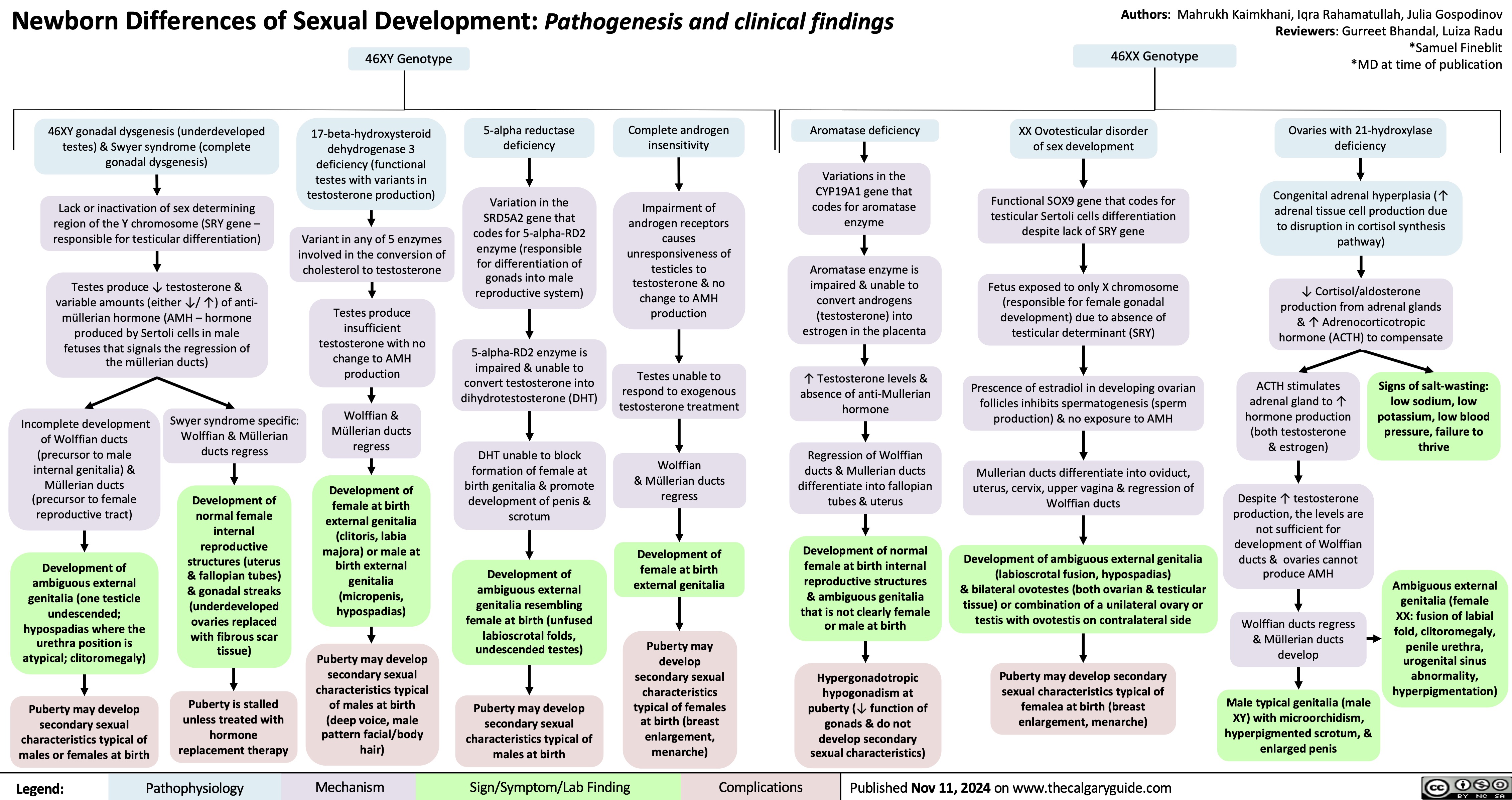
Primary Hypercholesterolemia
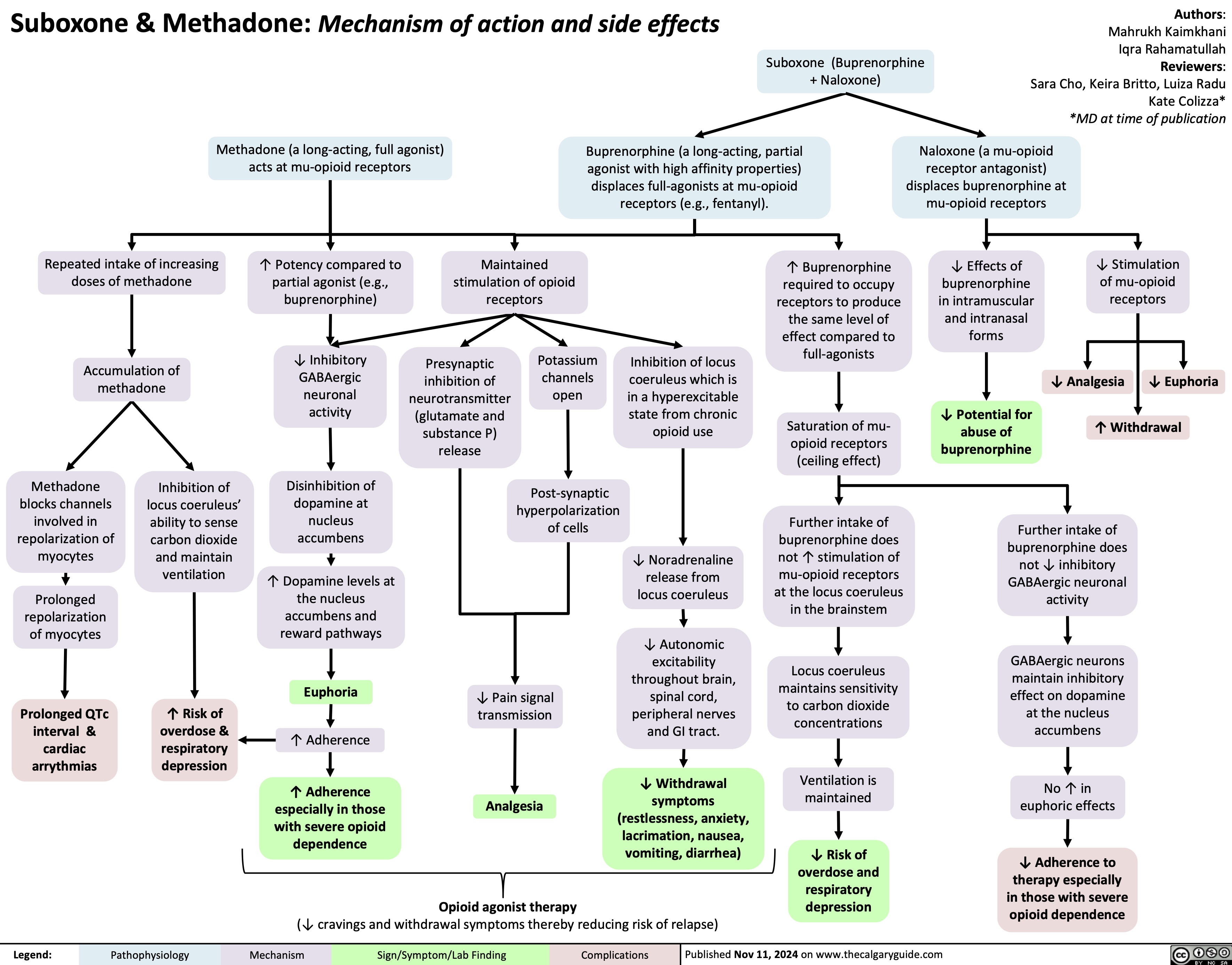
Suboxone & Methadone
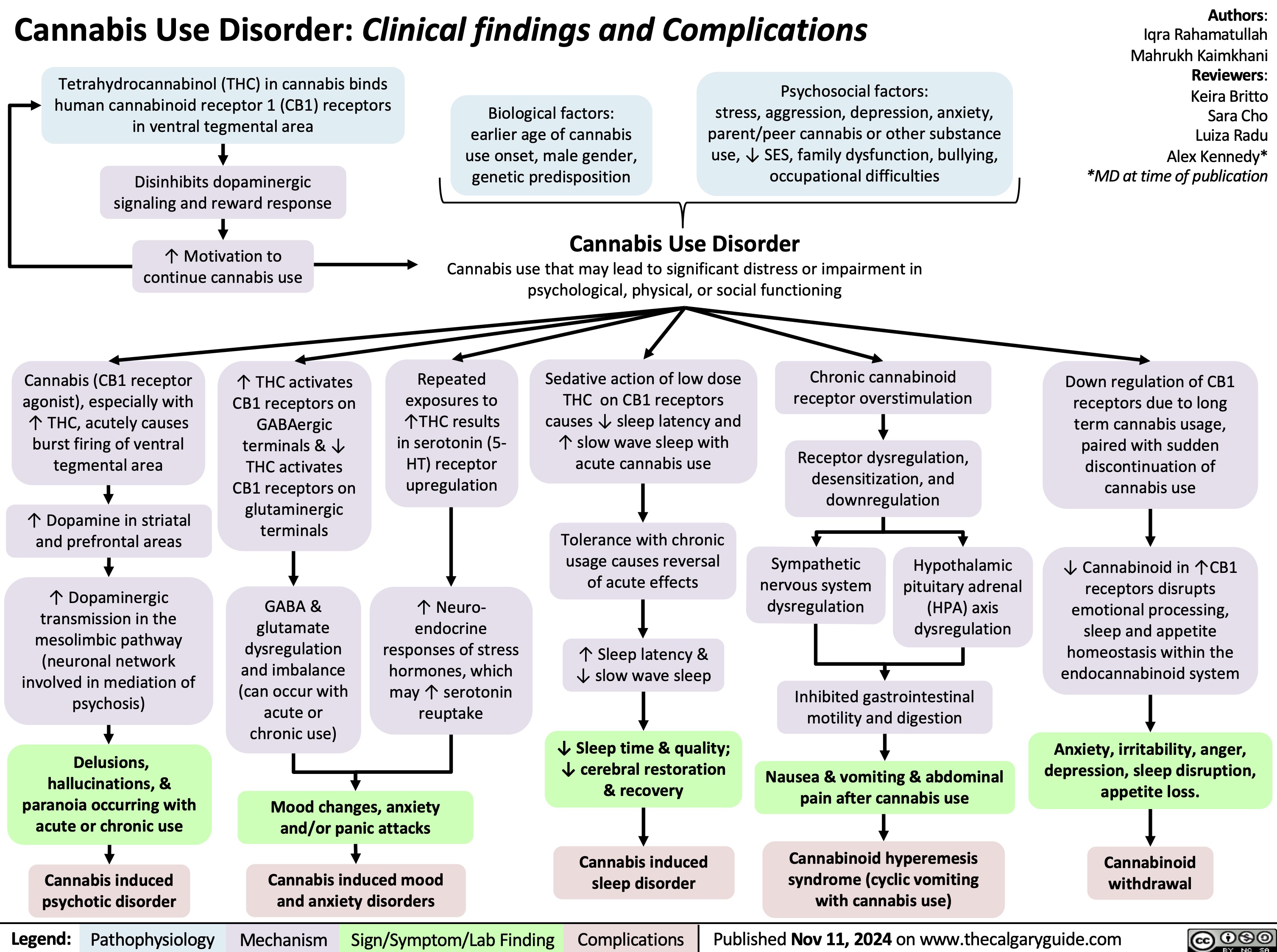
Cannabis Use Disorder
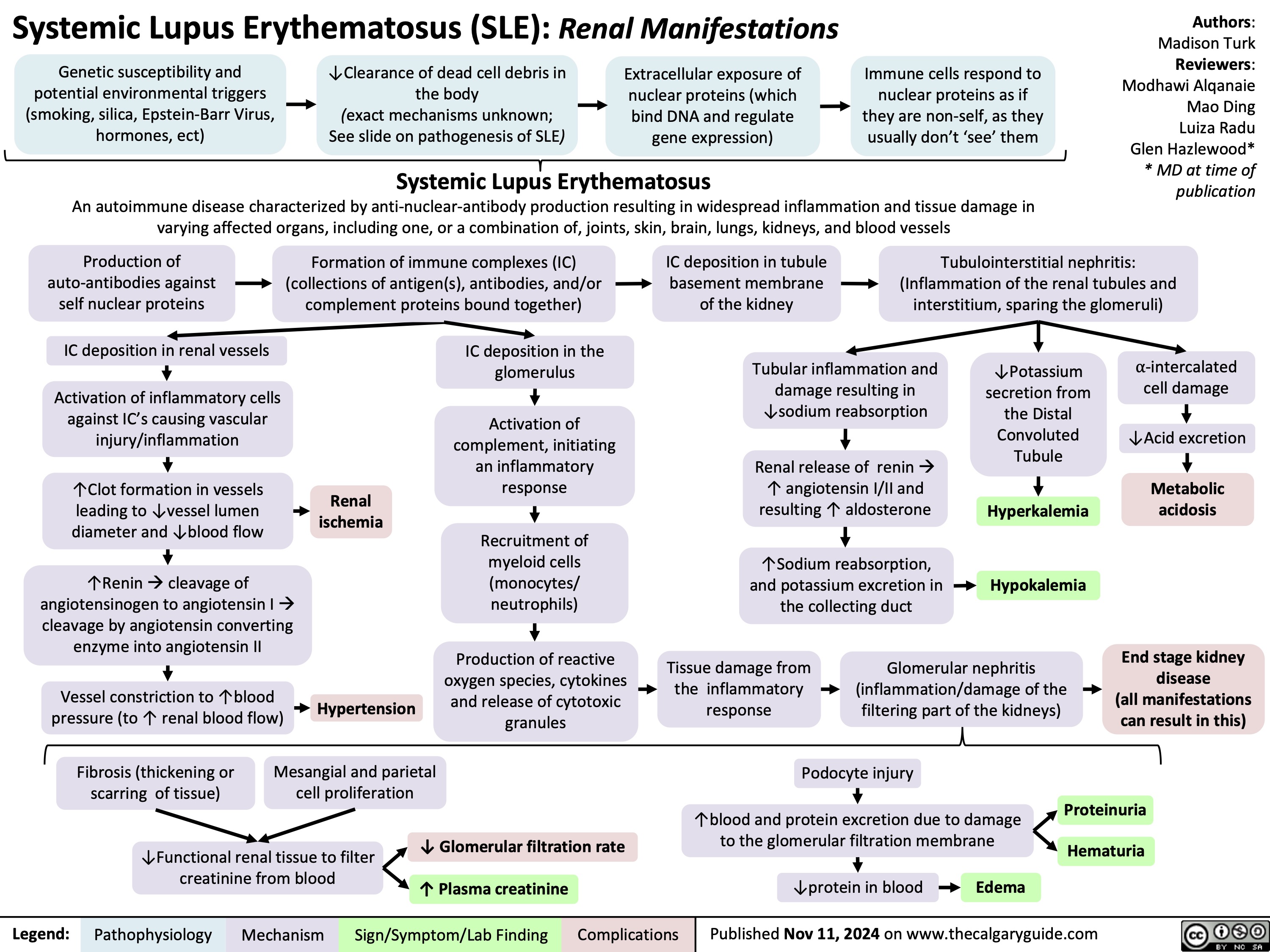
Renal manifestations of SLE
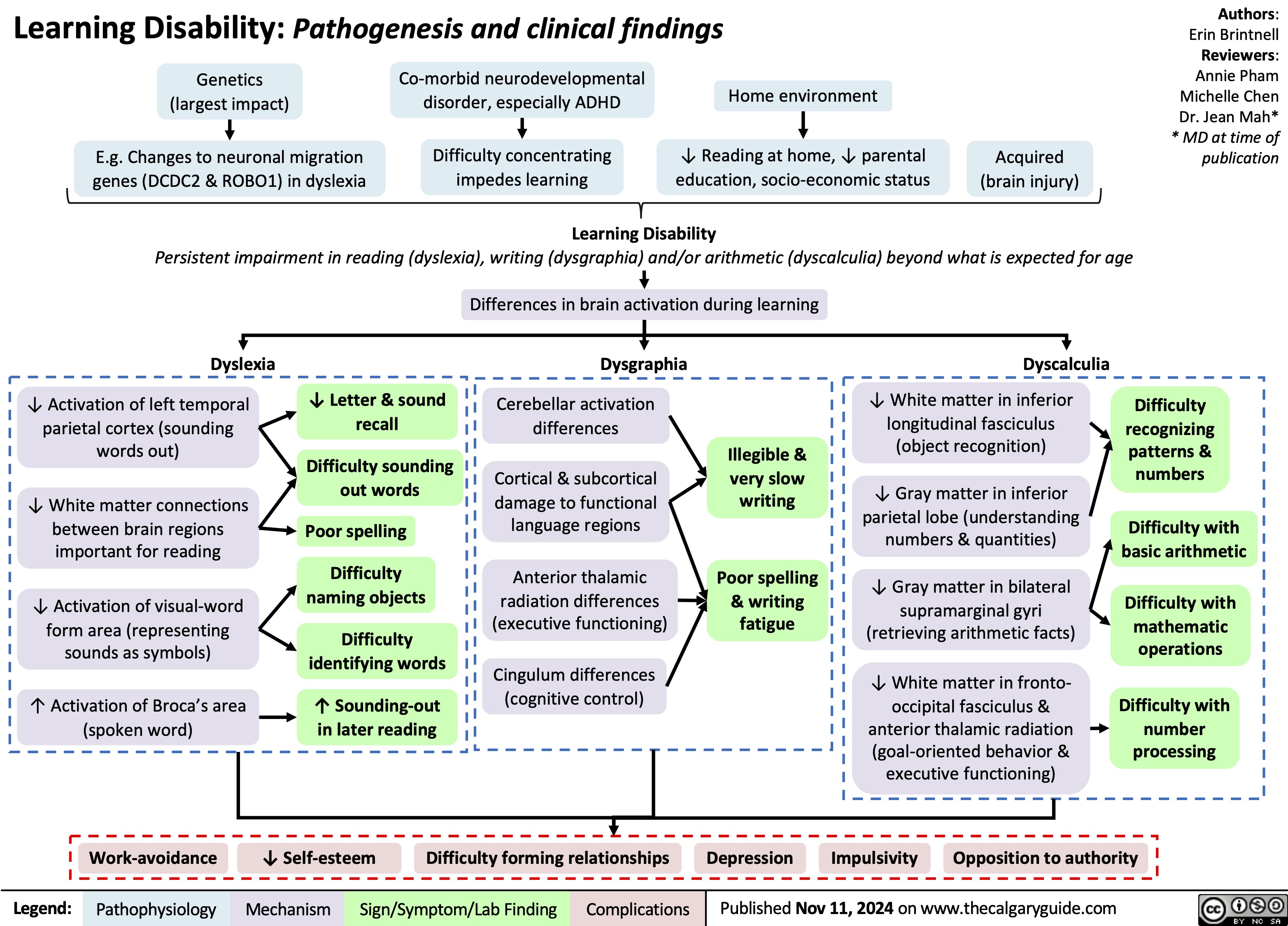
Learning Disability
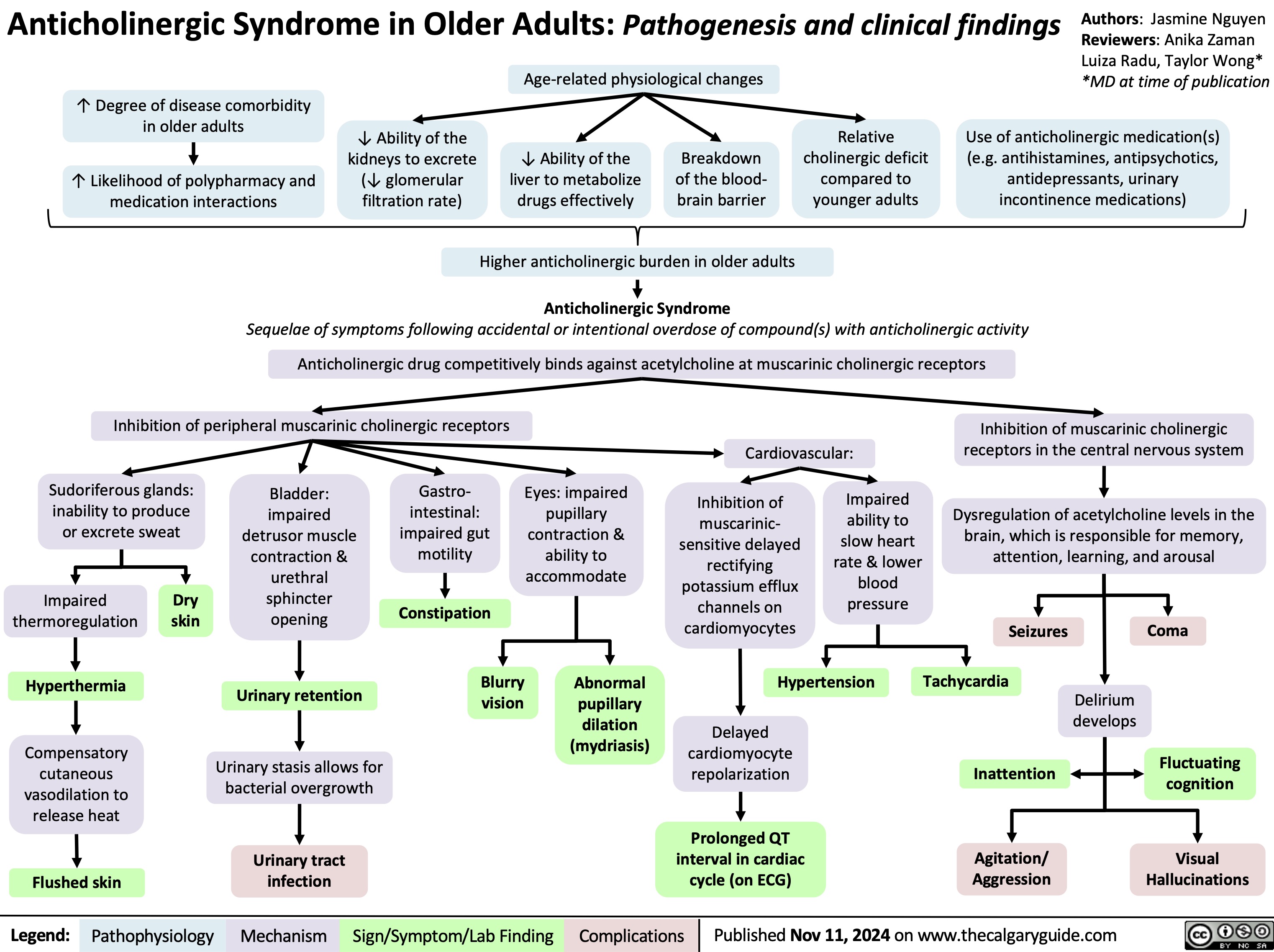
Anticholingeric Syndrome in Older Adults
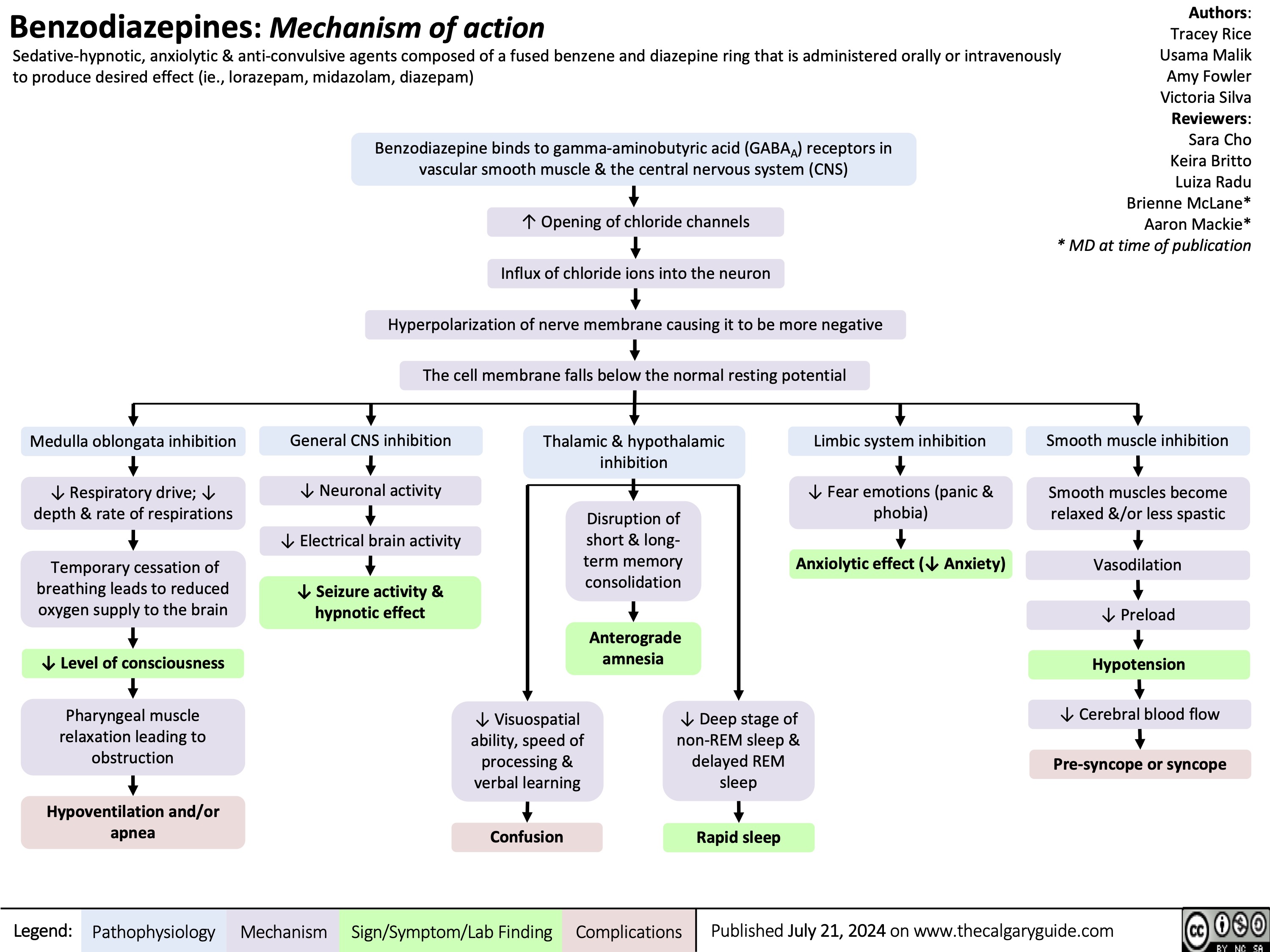
Benzodiazepine mechanism of action
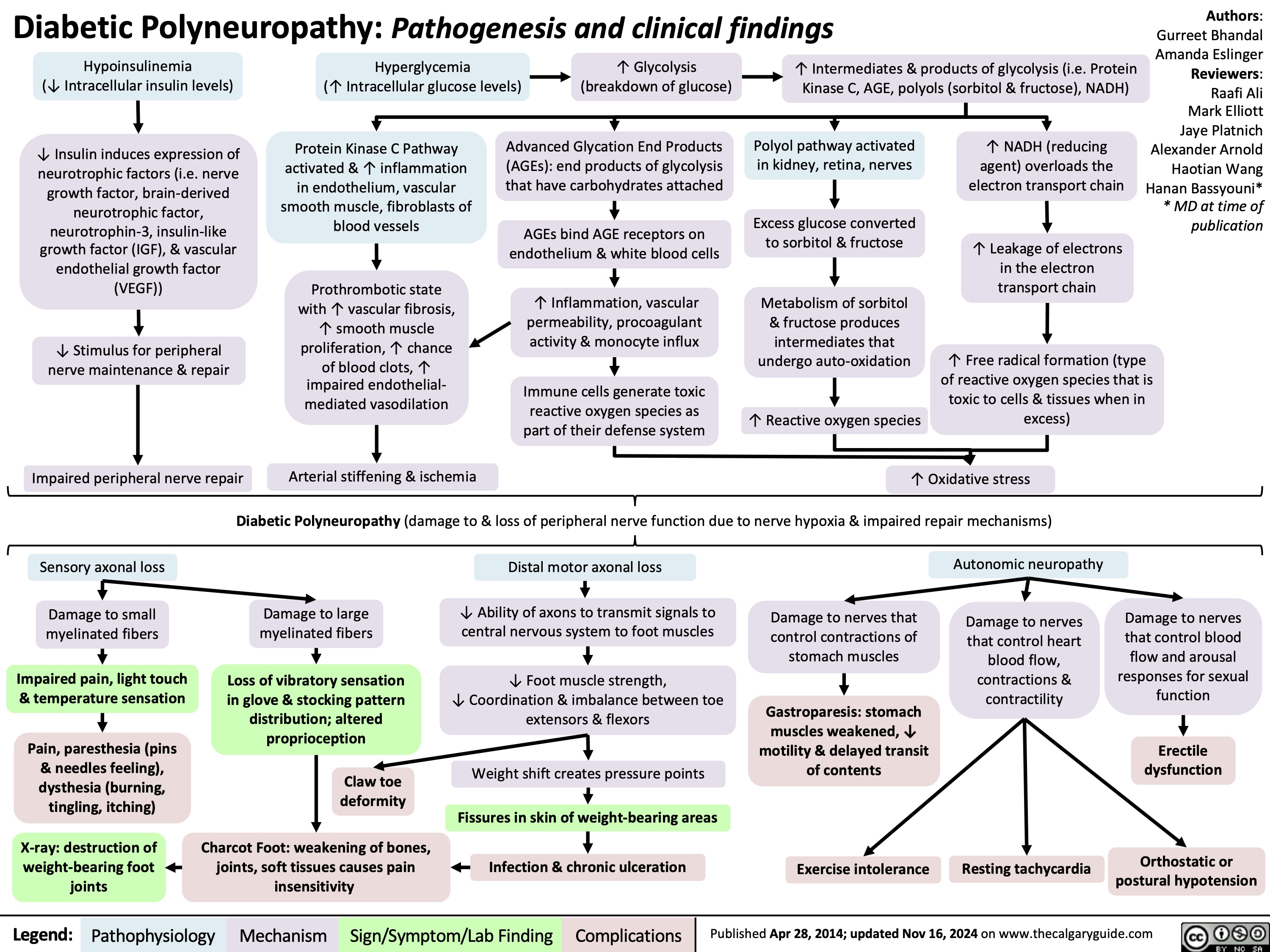
Diabetic Polyneuropathy
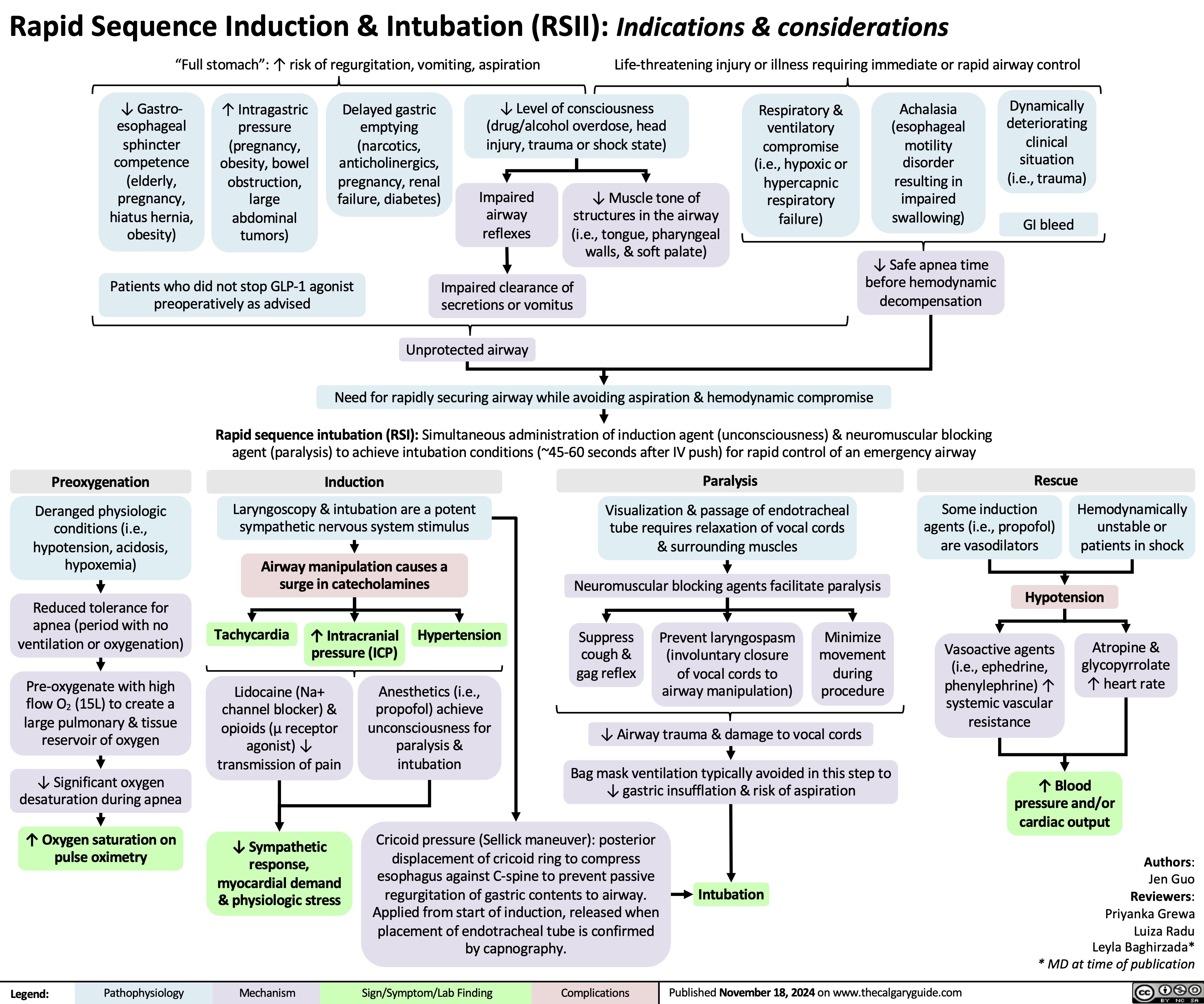
Rapid sequence induction and intubation
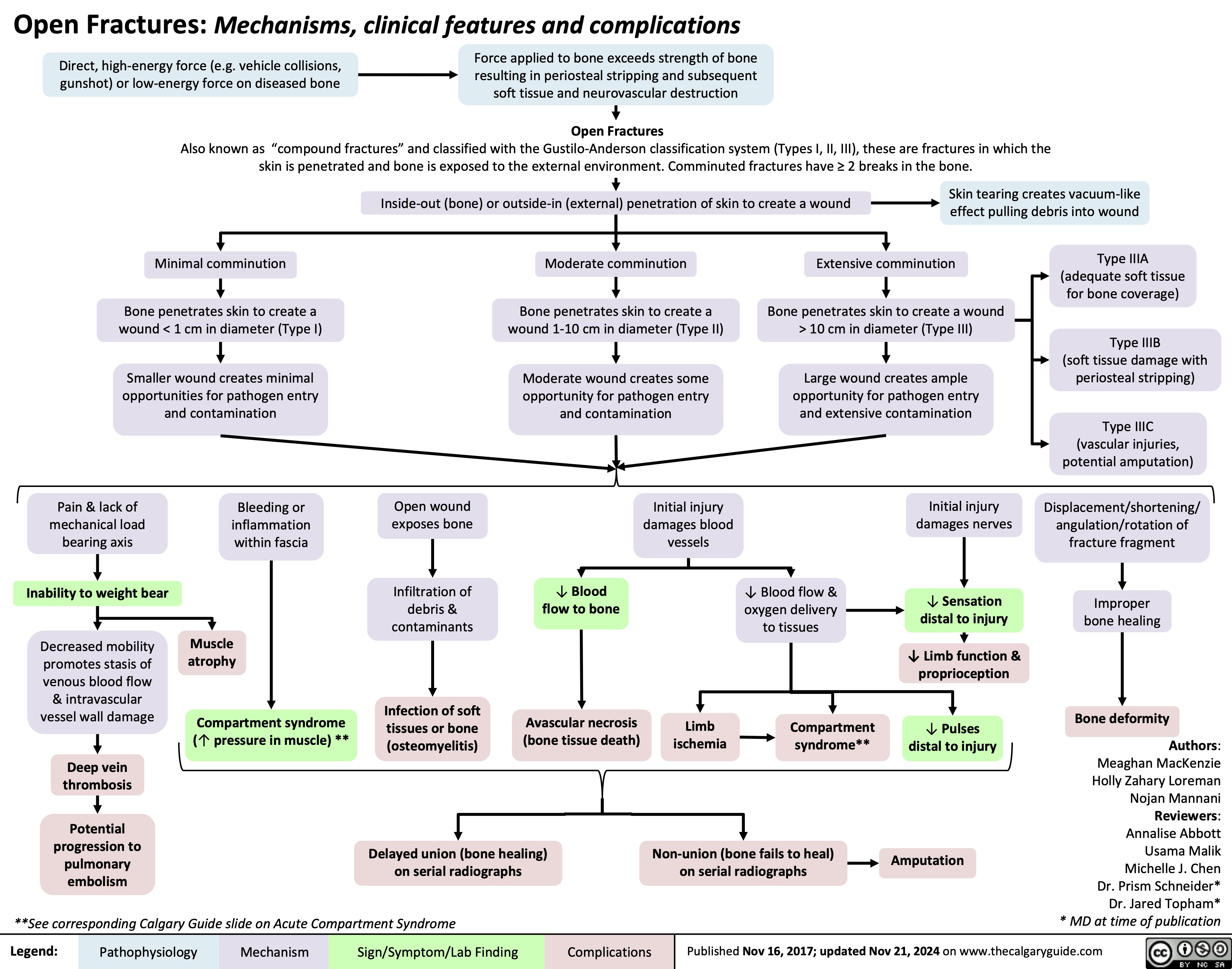
Open Fractures
Calcium Channel Blockers
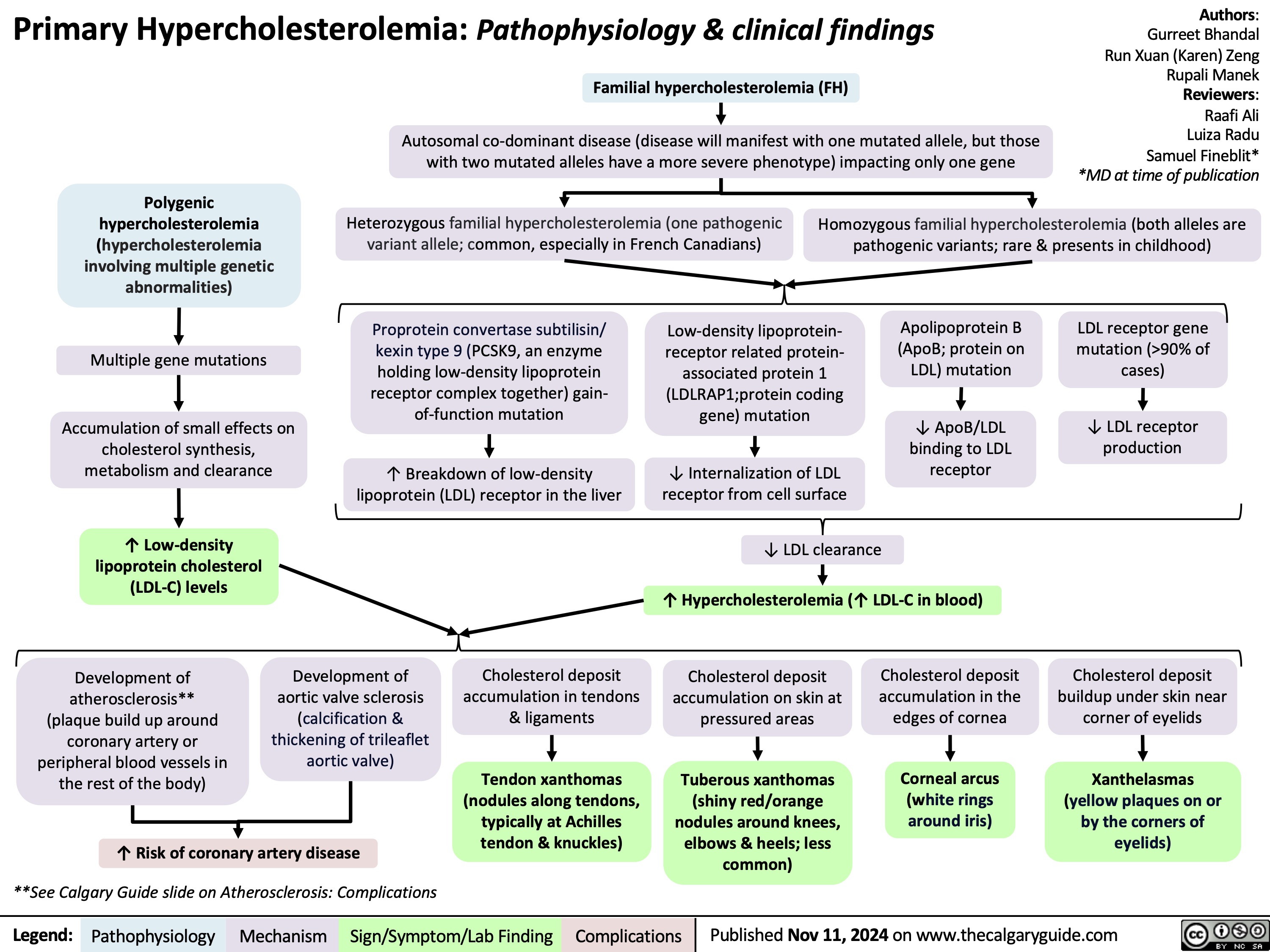
![Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com
Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/11/Calcium-Channel-Blockers.jpg)
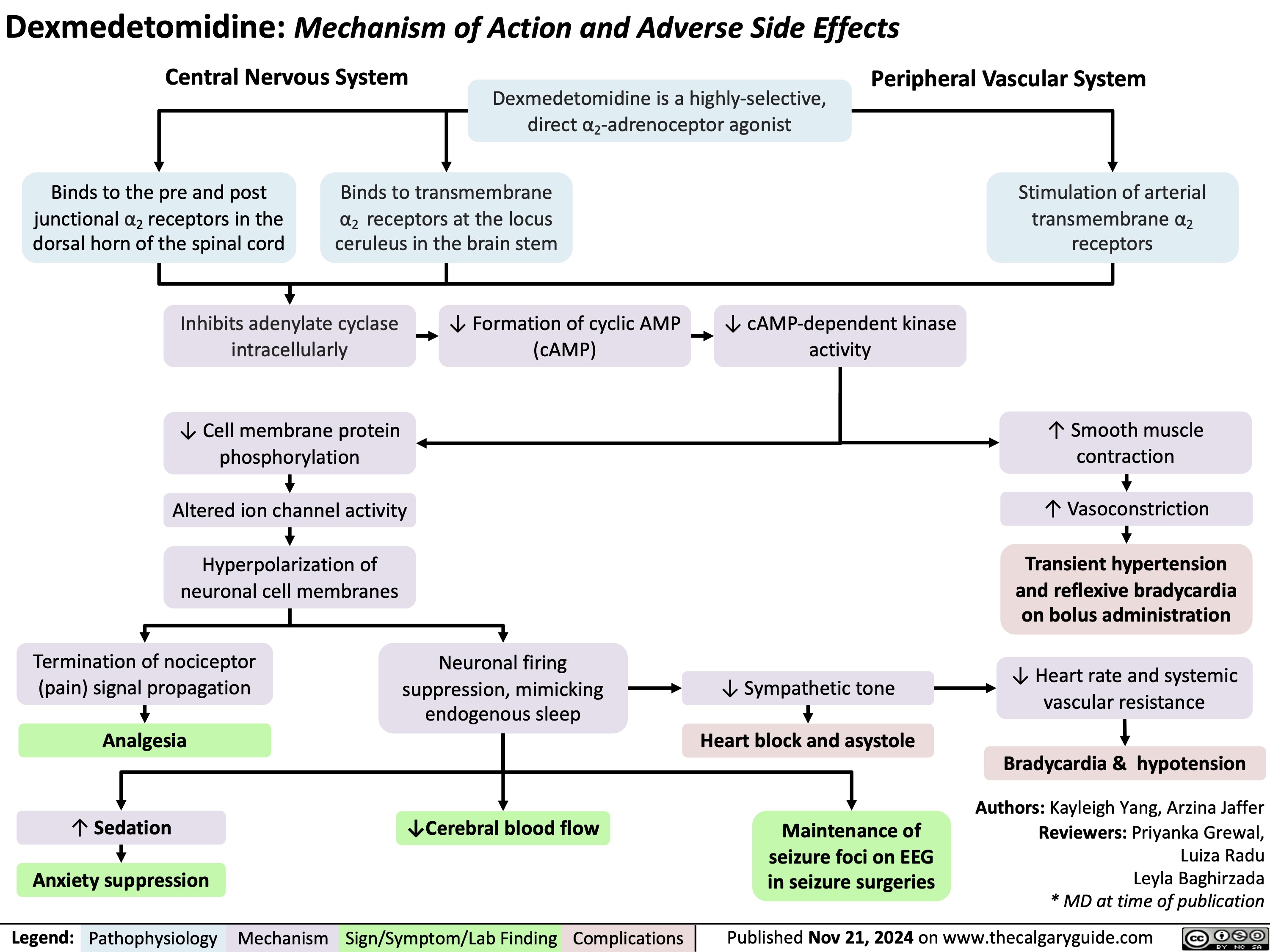
Dexmedetomidine
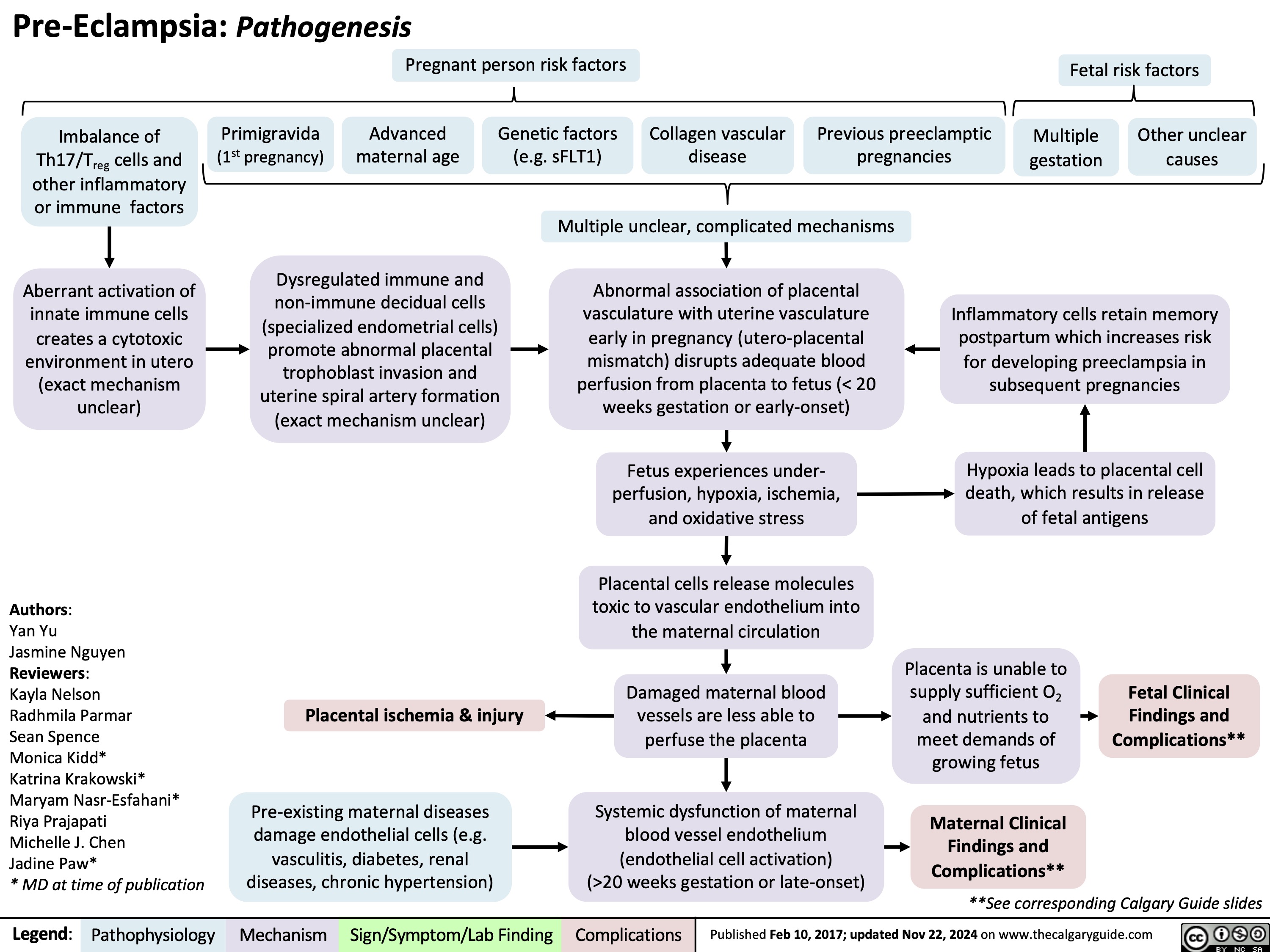
Pre-Eclampsia Pathogenesis
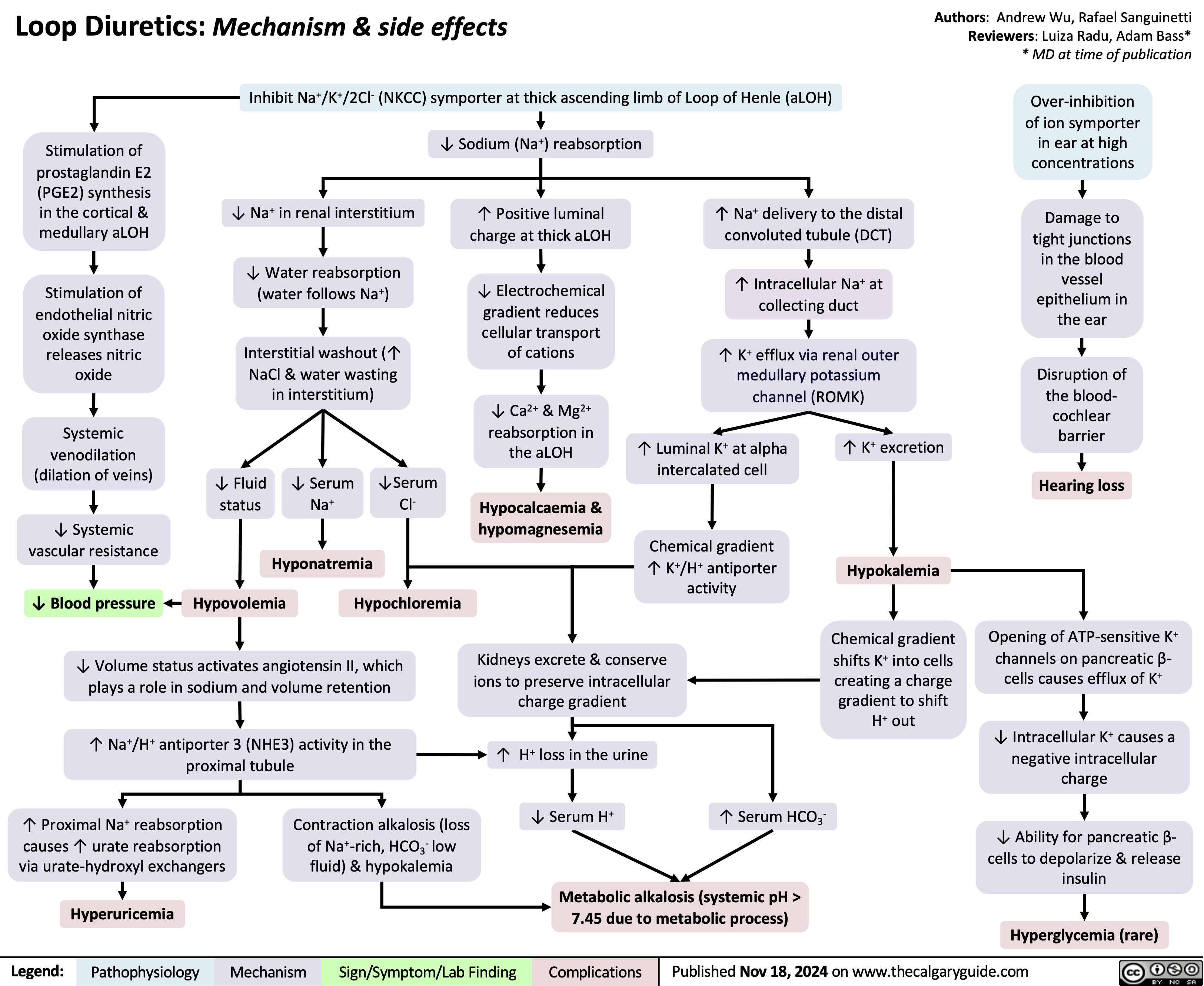
Loop diuretics
Statins Mechanisms and Side Effects
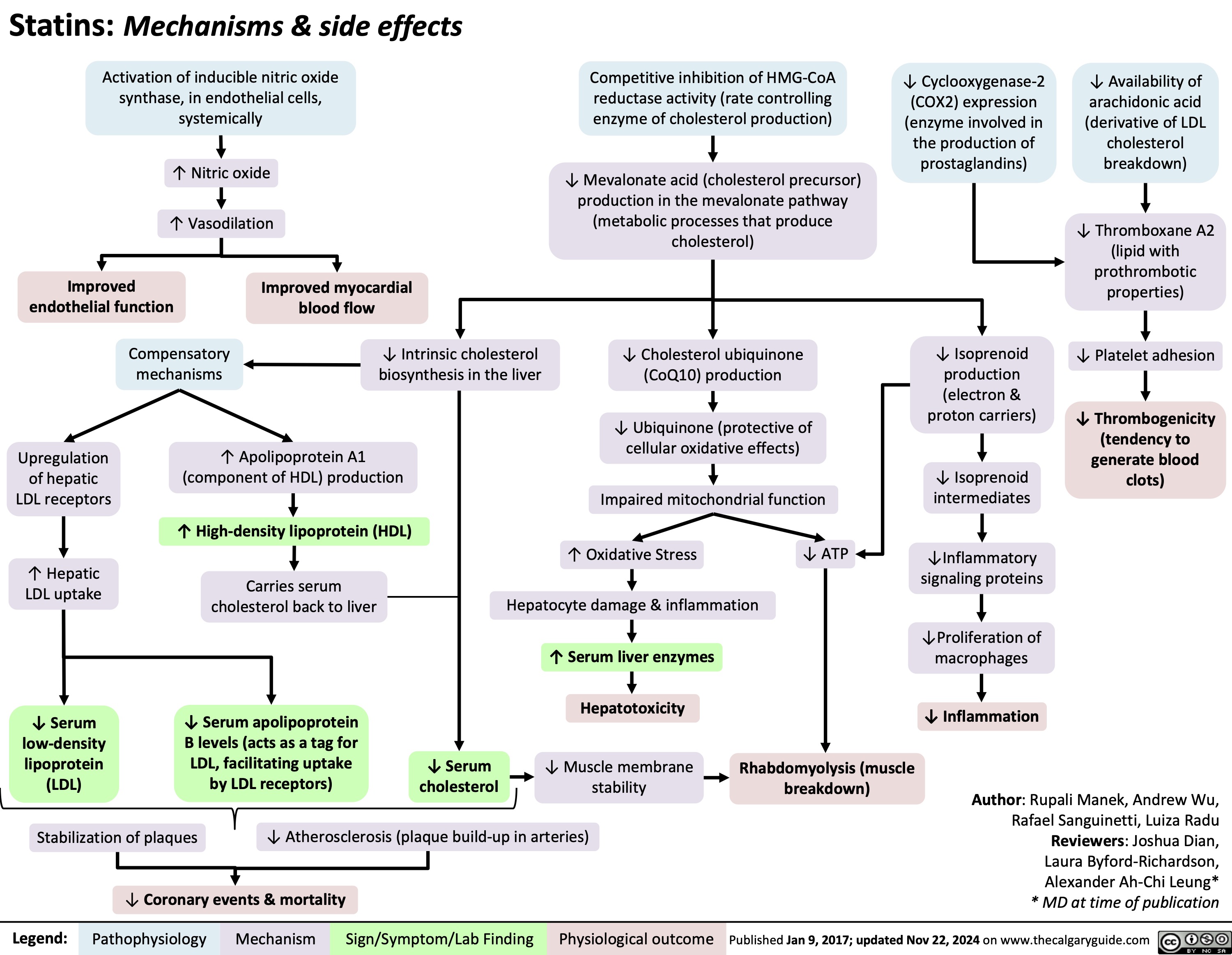
Fibromylagia
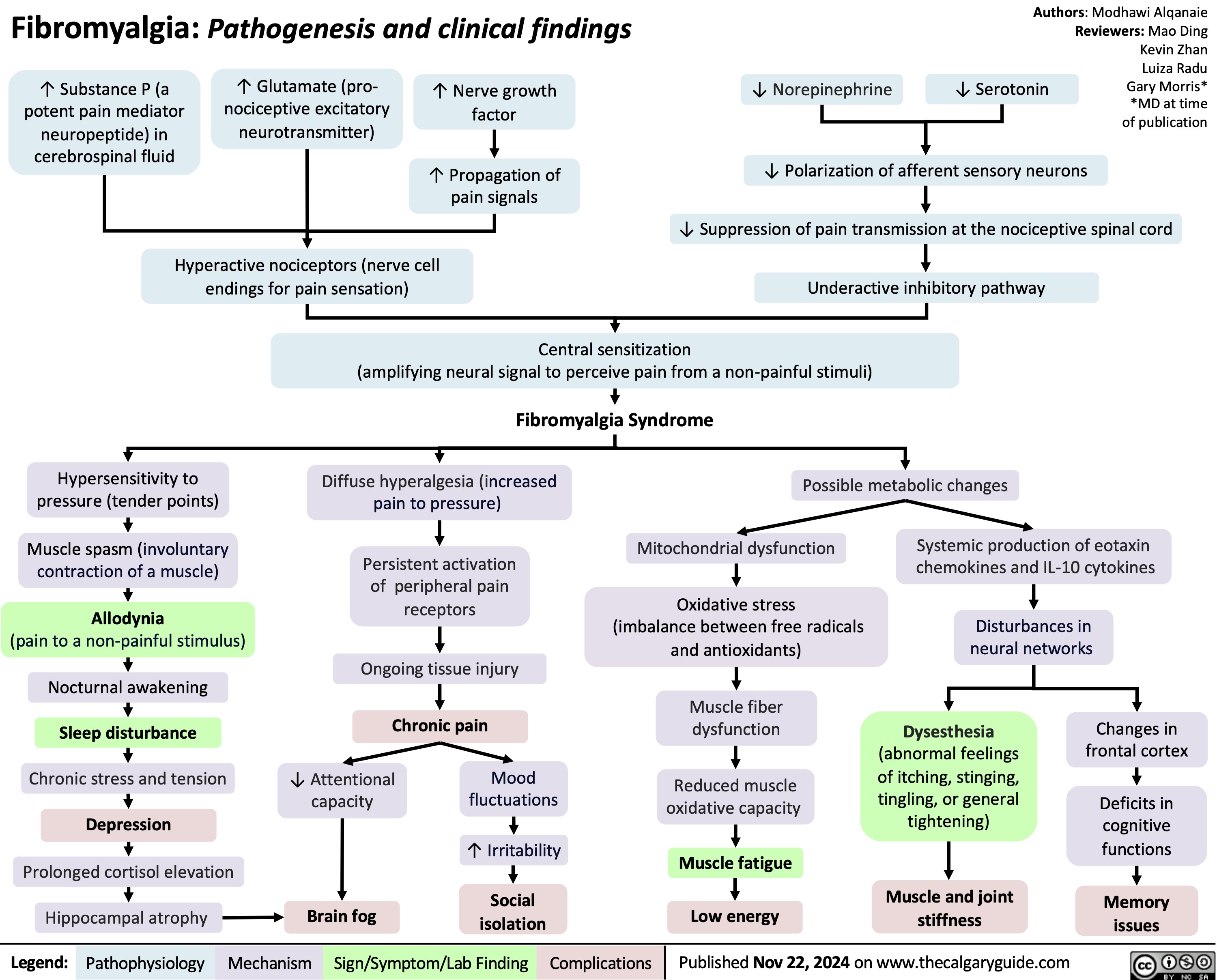
Hypocalcemia Pathogenesis
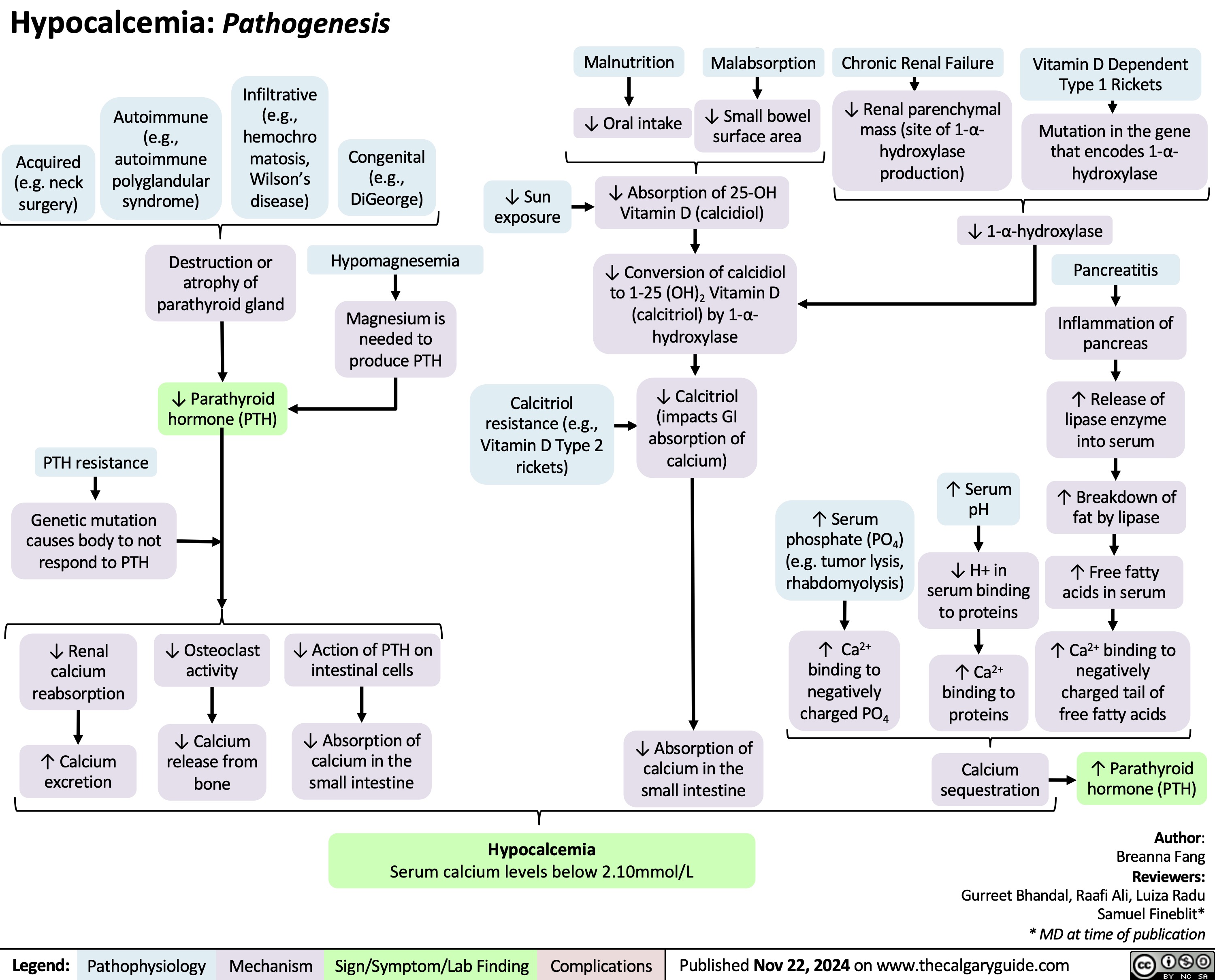
Acute Compartment Syndrome
Pelvic Ring Fractures
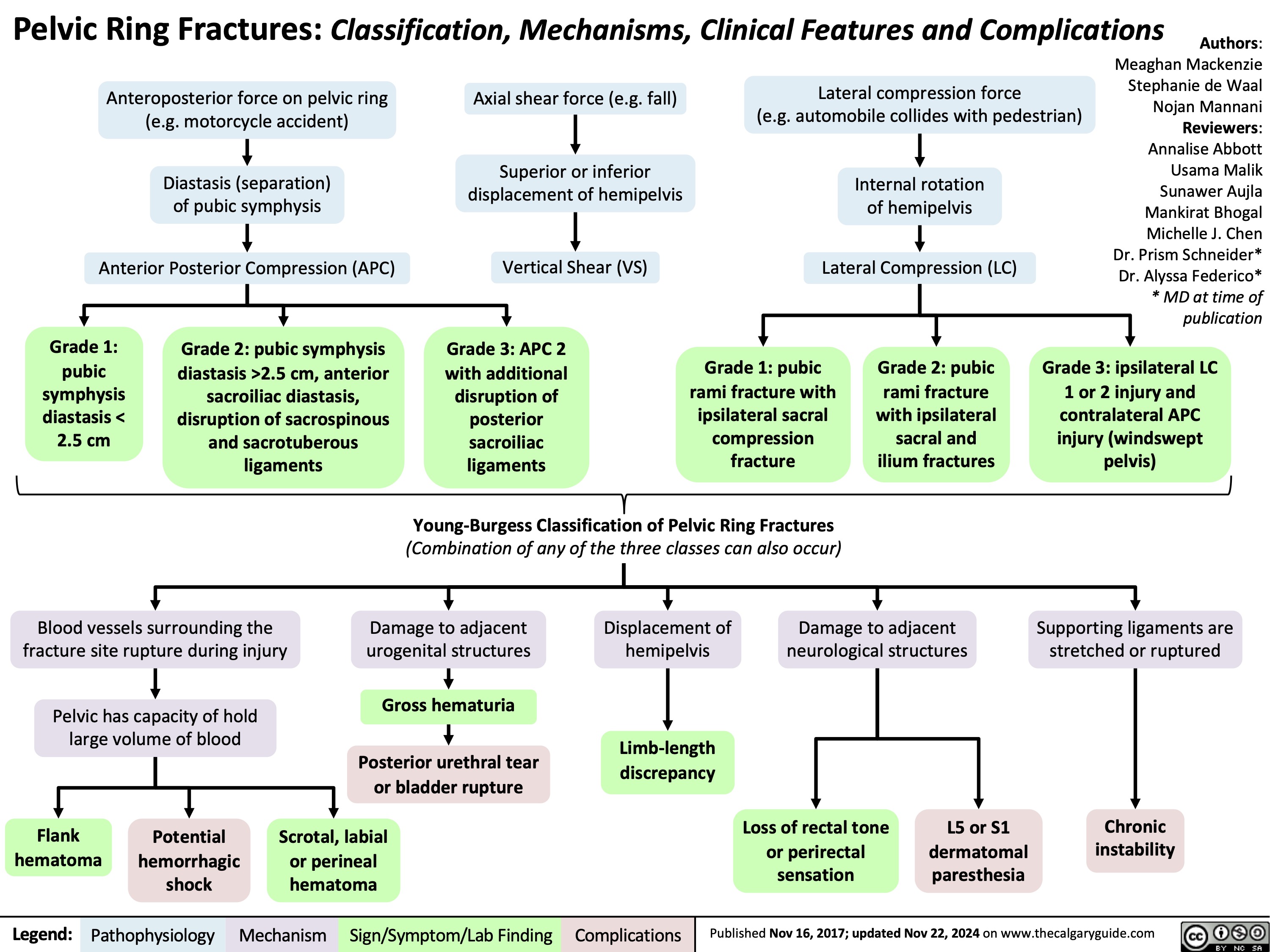
Vaccine-Mediated Immunity General Physiology
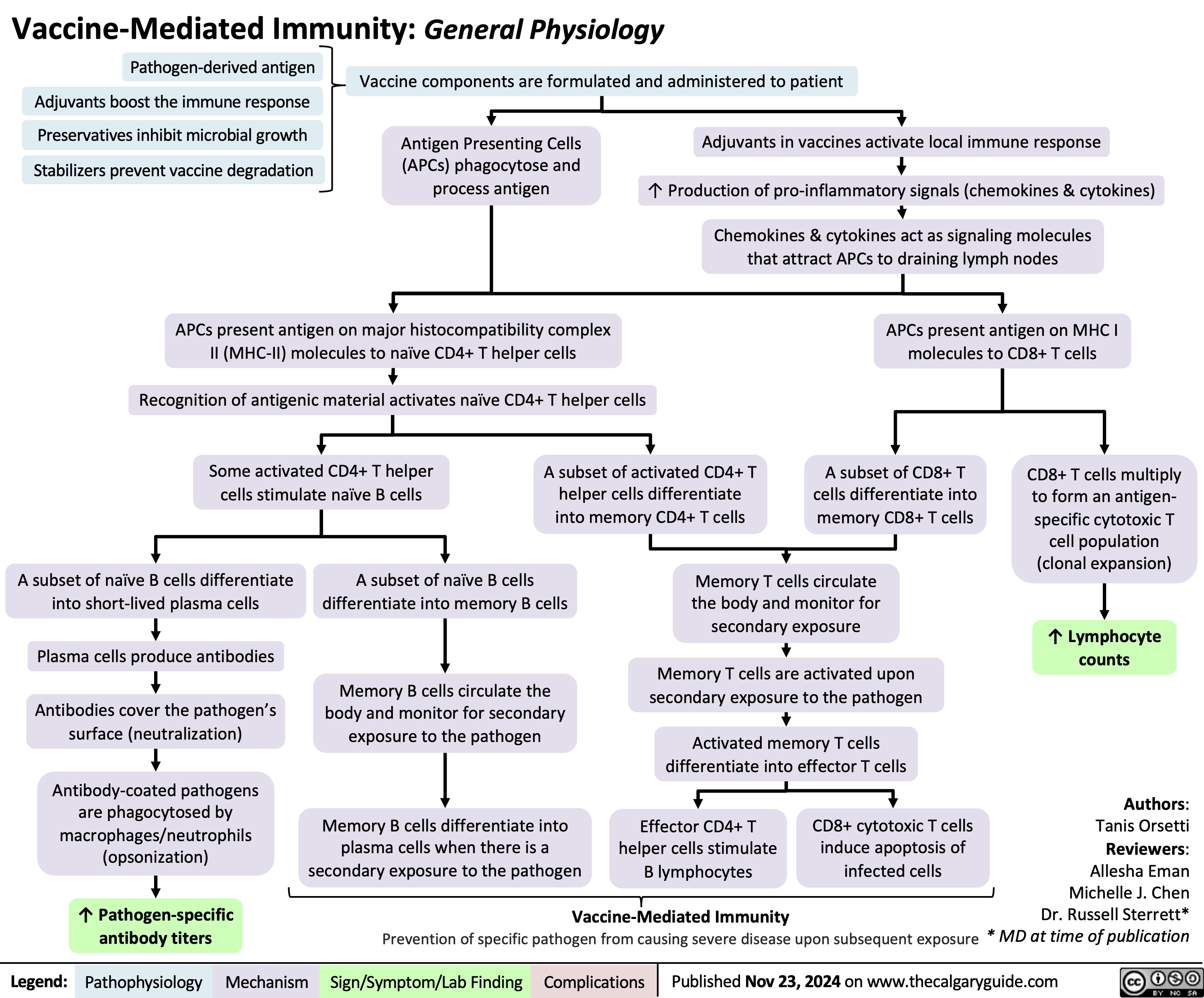
Anesthetic Considerations Laparoscopic Abdominal Surgery
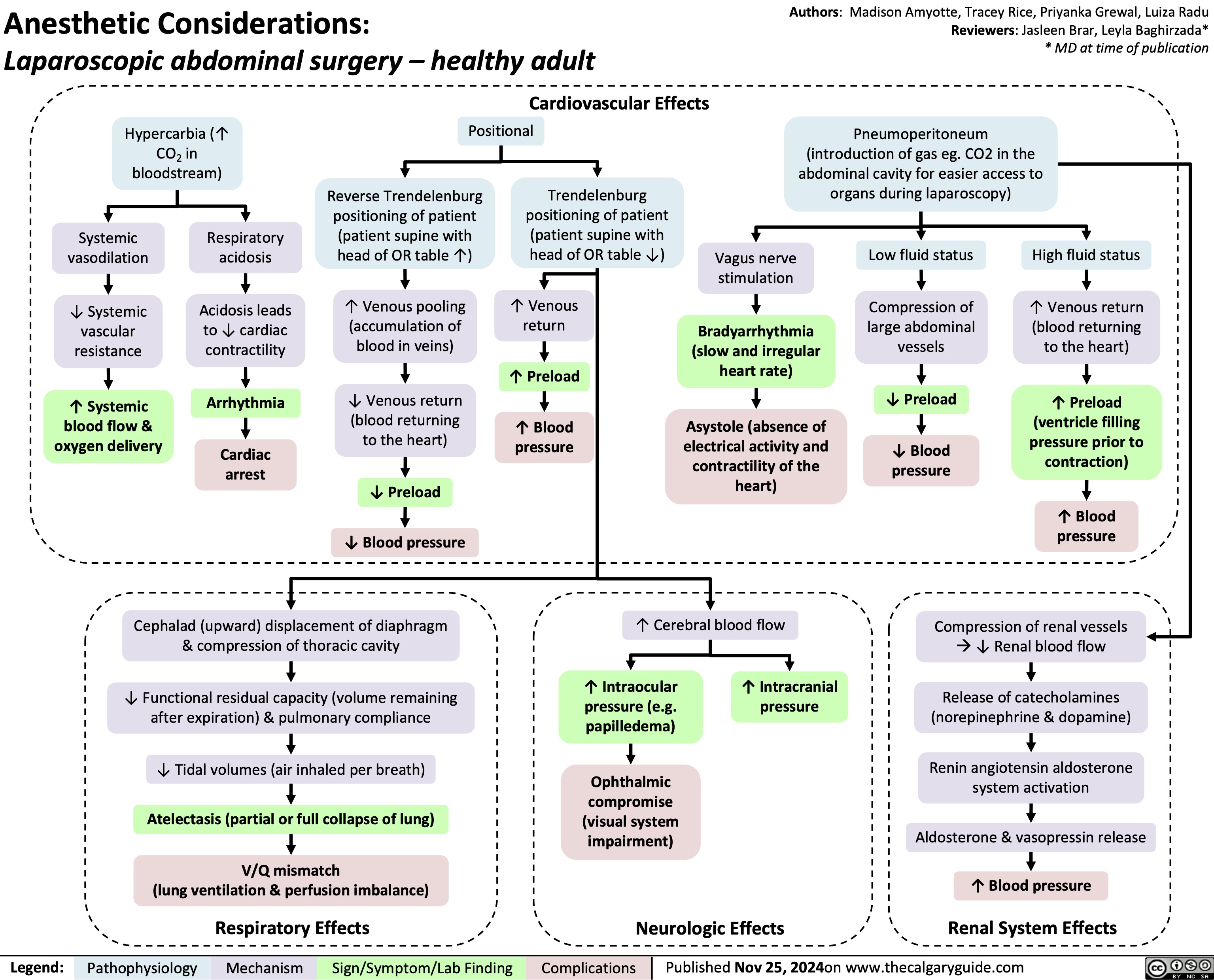
Chronic Inflammatory Demyelinating Polyneuropathy
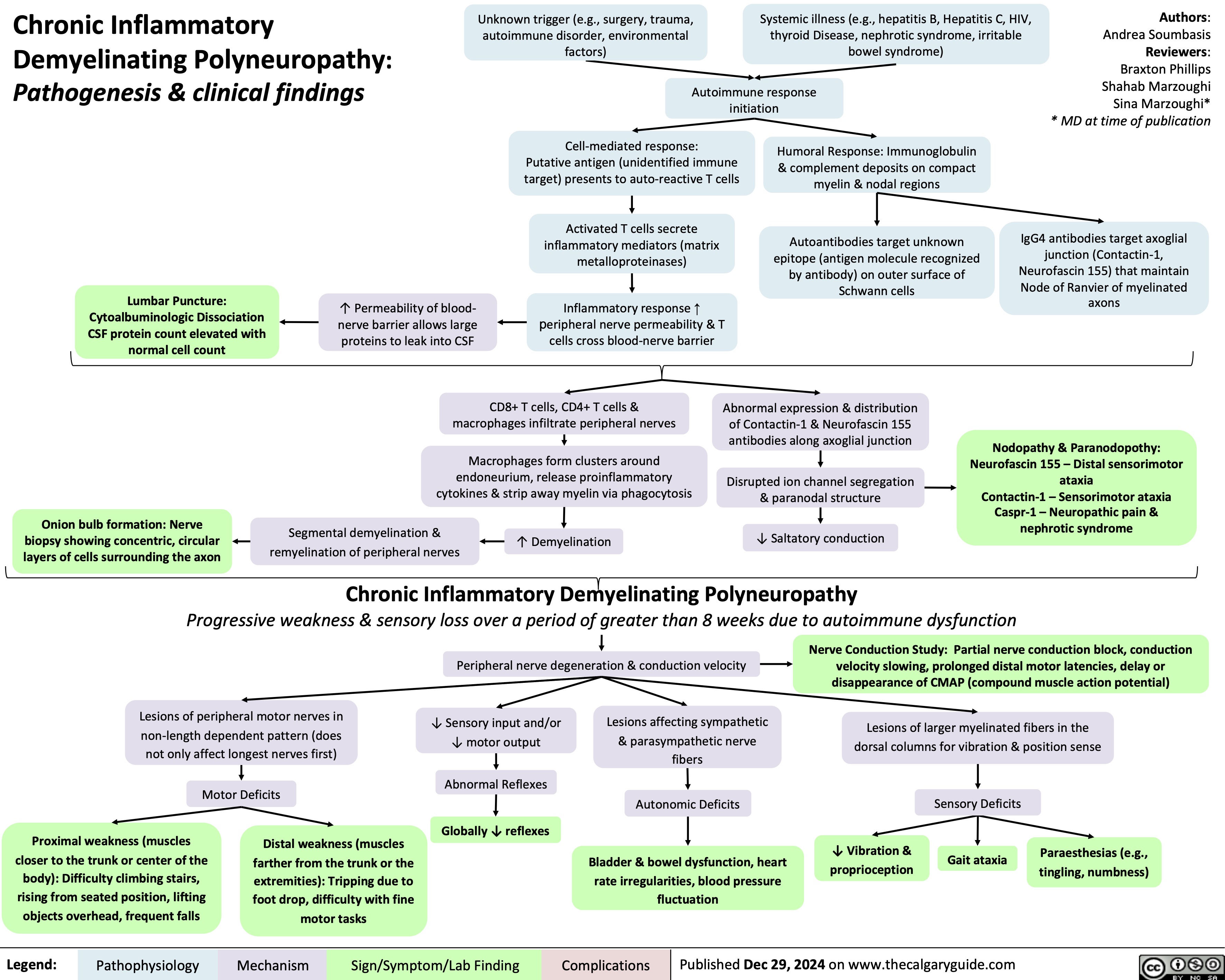
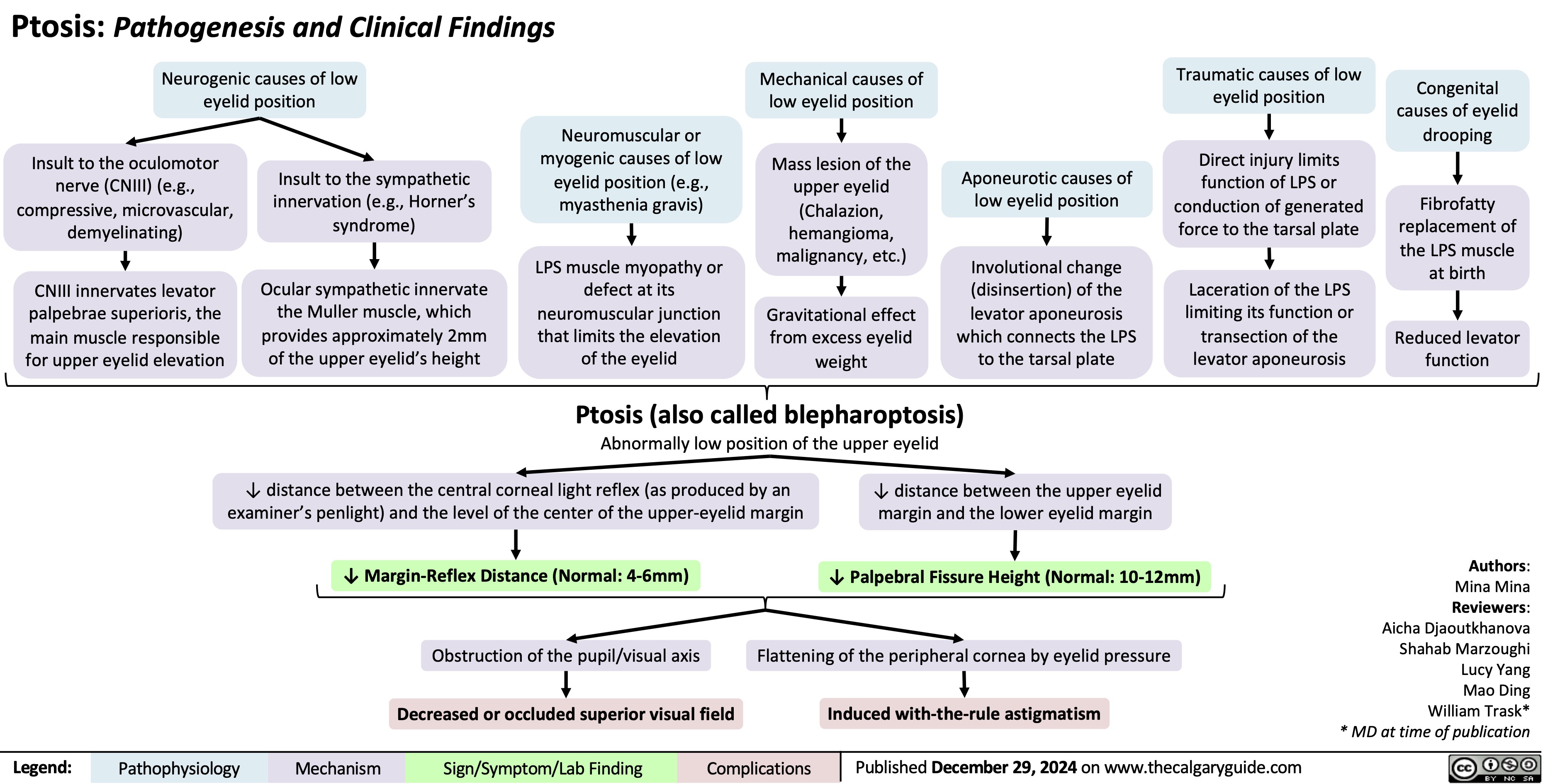
Acquired Inguinal Hernias
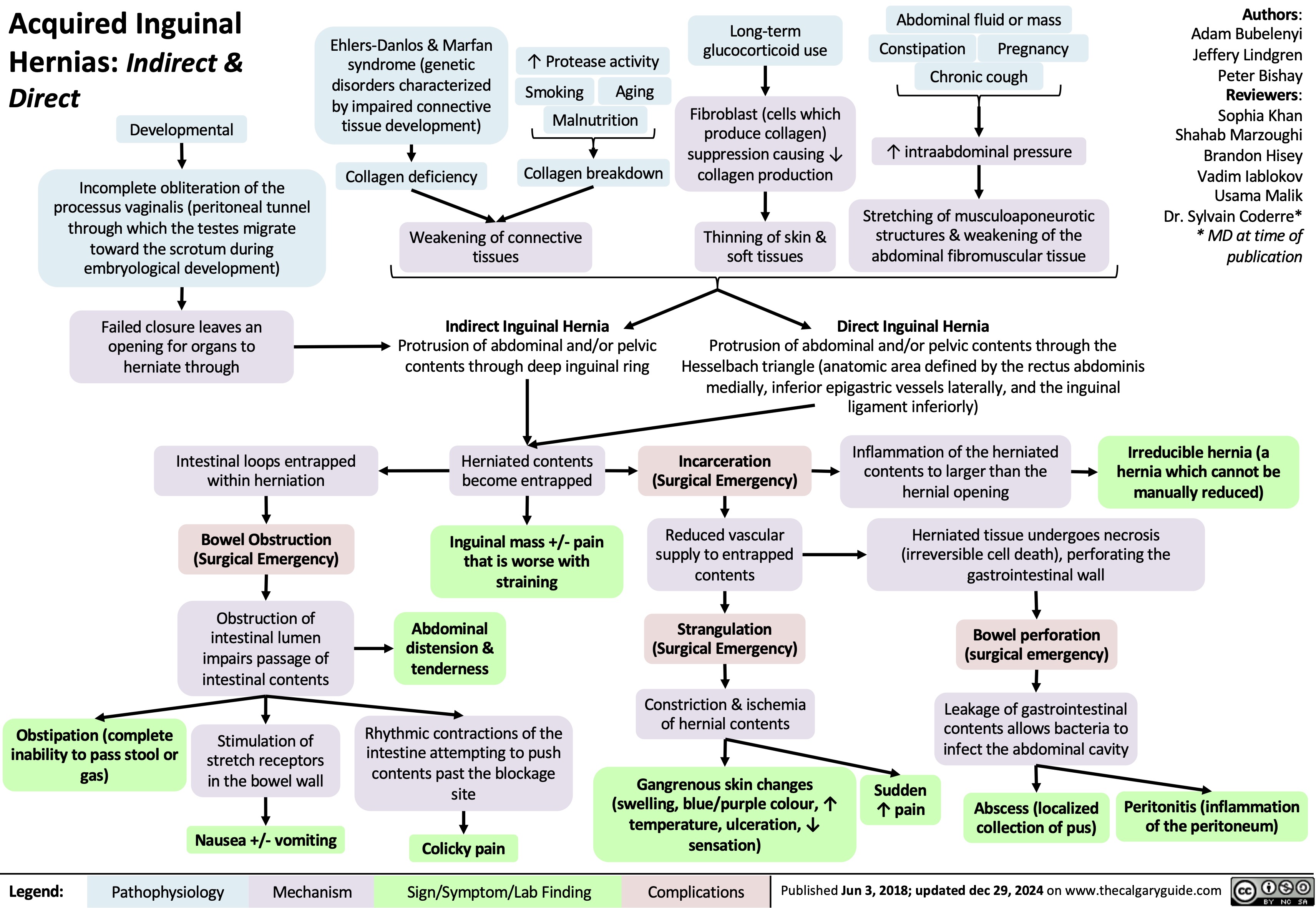
Polycystic Ovarian Syndrome
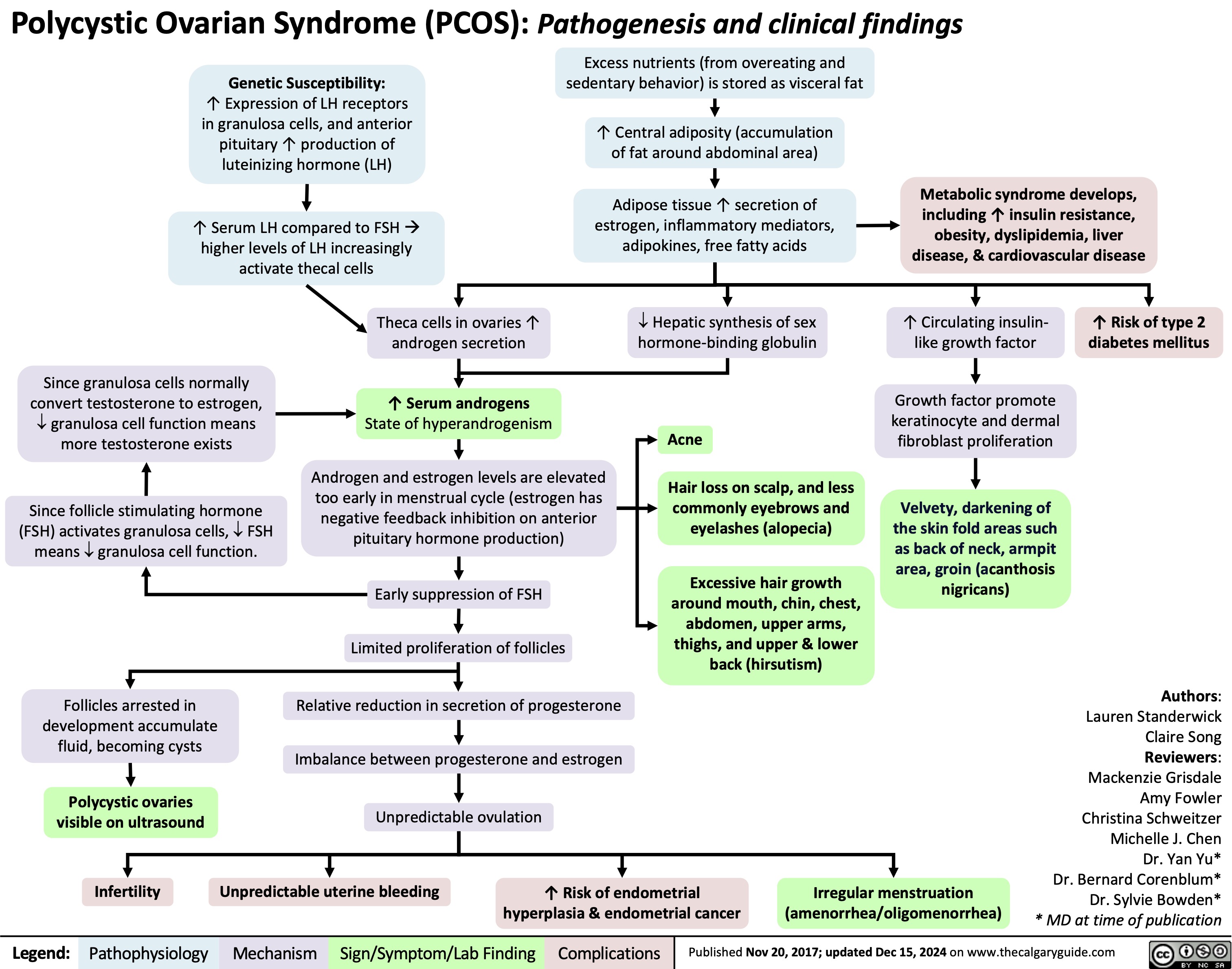
Barretts Esophagus
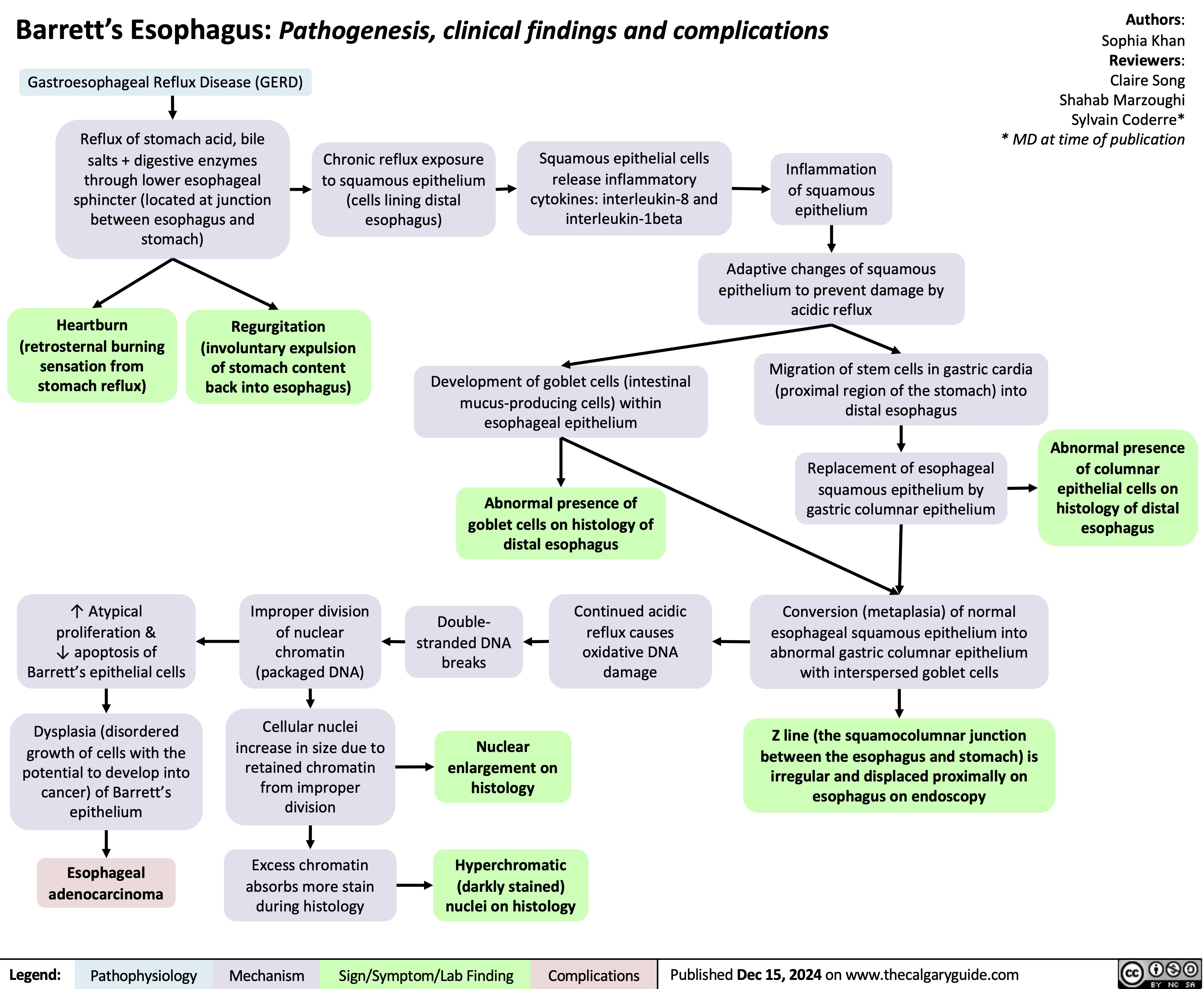
Ptosis
Malignant Esophagitis
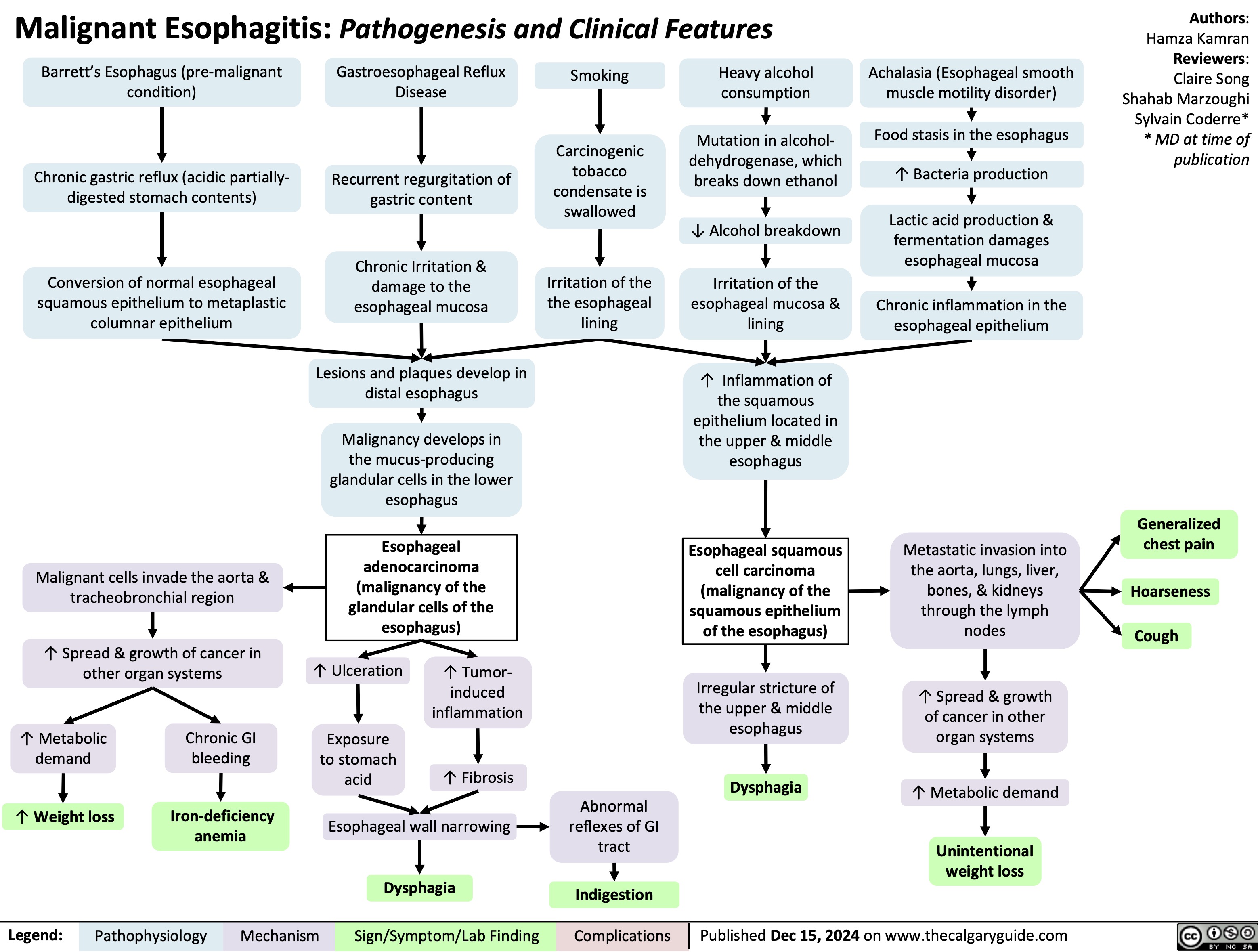
Pulsus Paradoxus
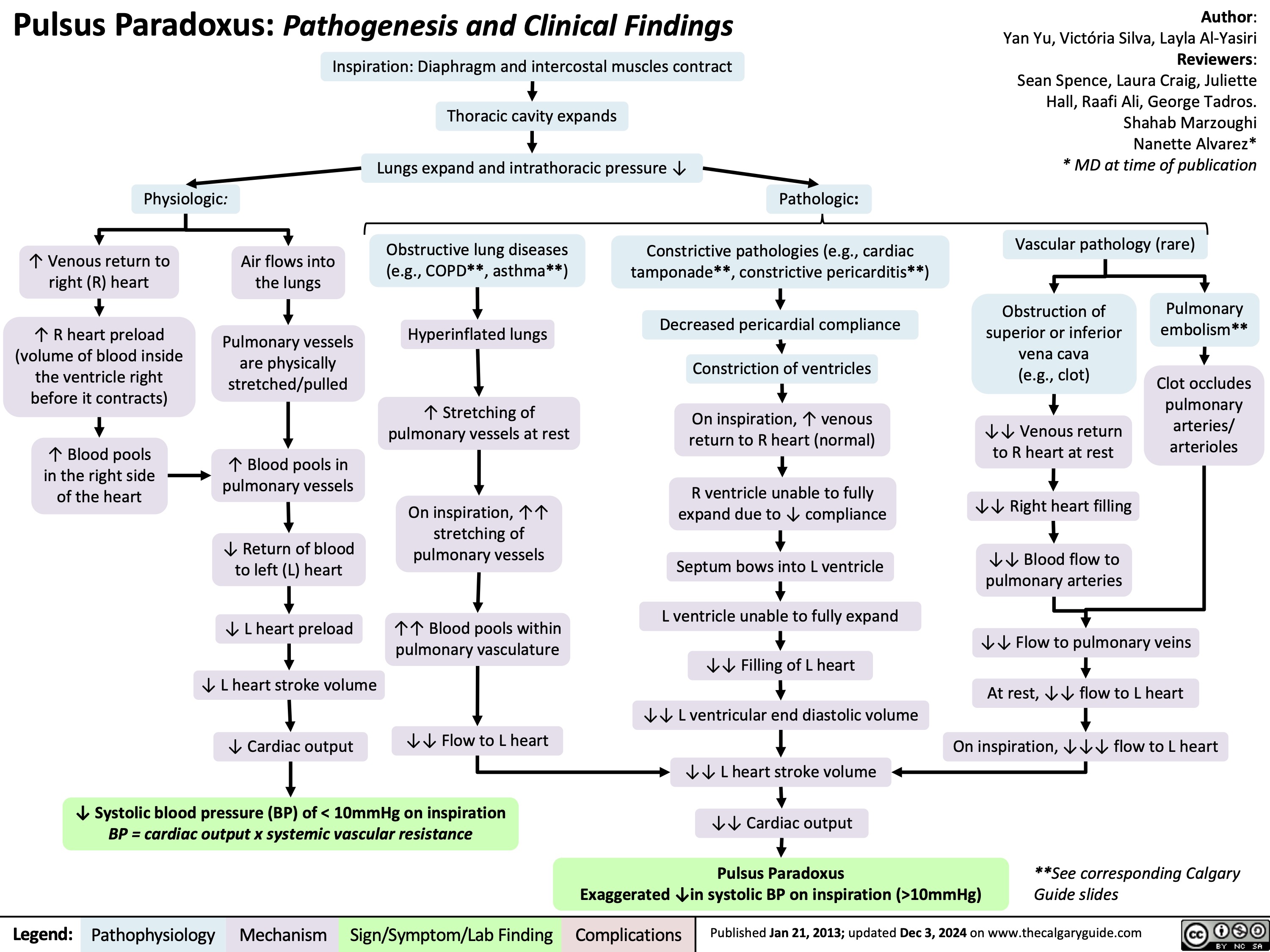
Constipation in Children
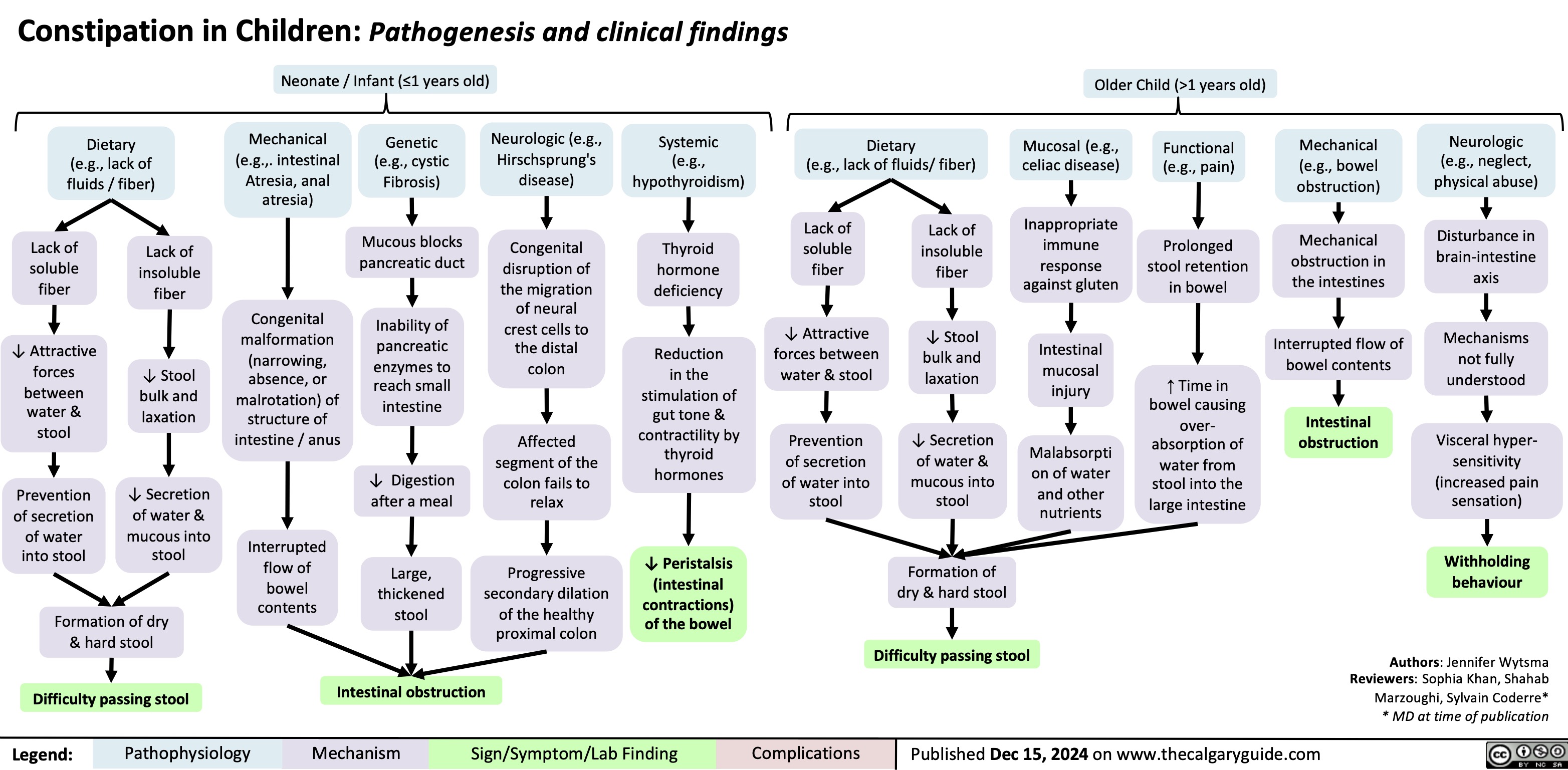
Avascular Necrosis of the Scaphoid
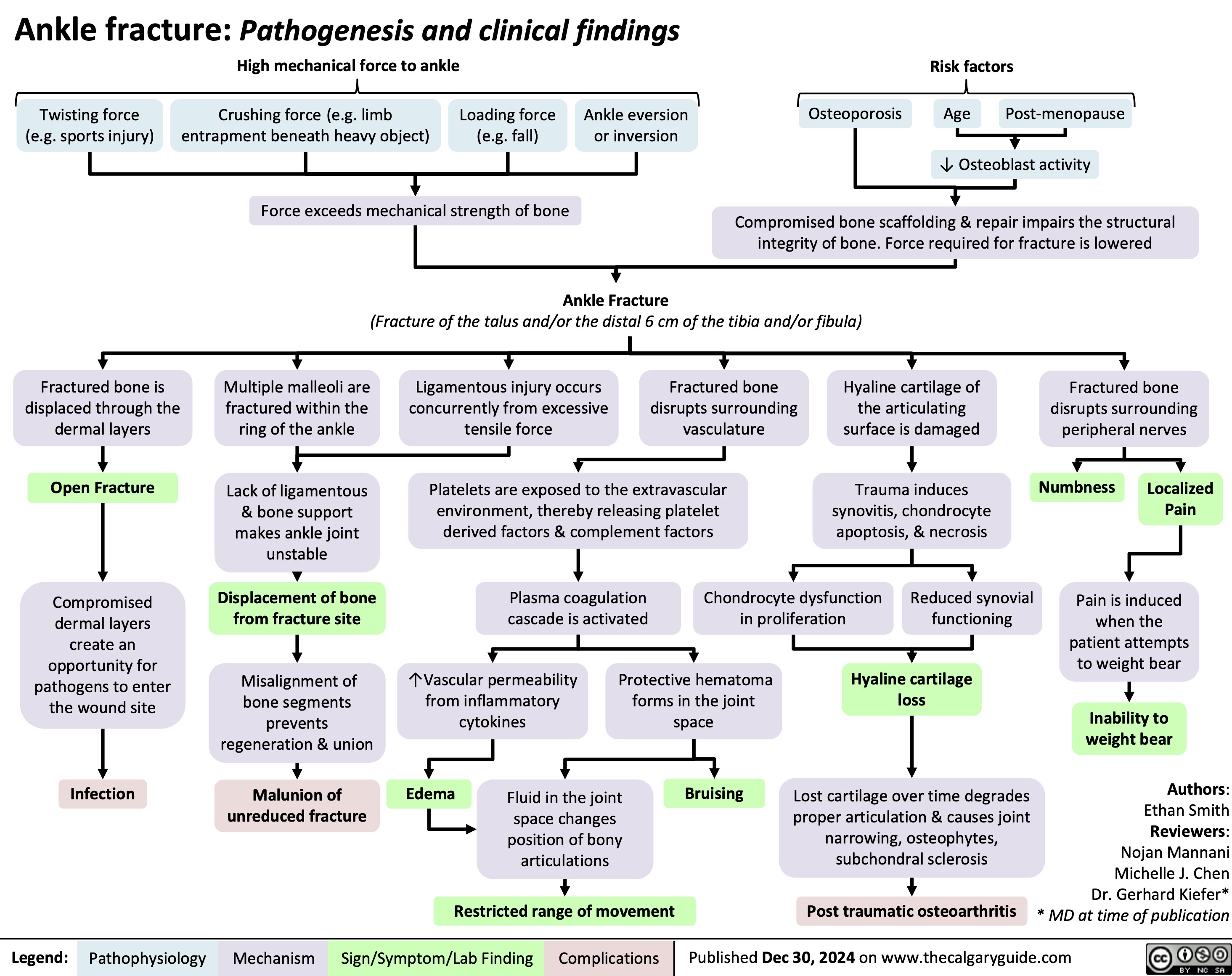
Ankle Fracture
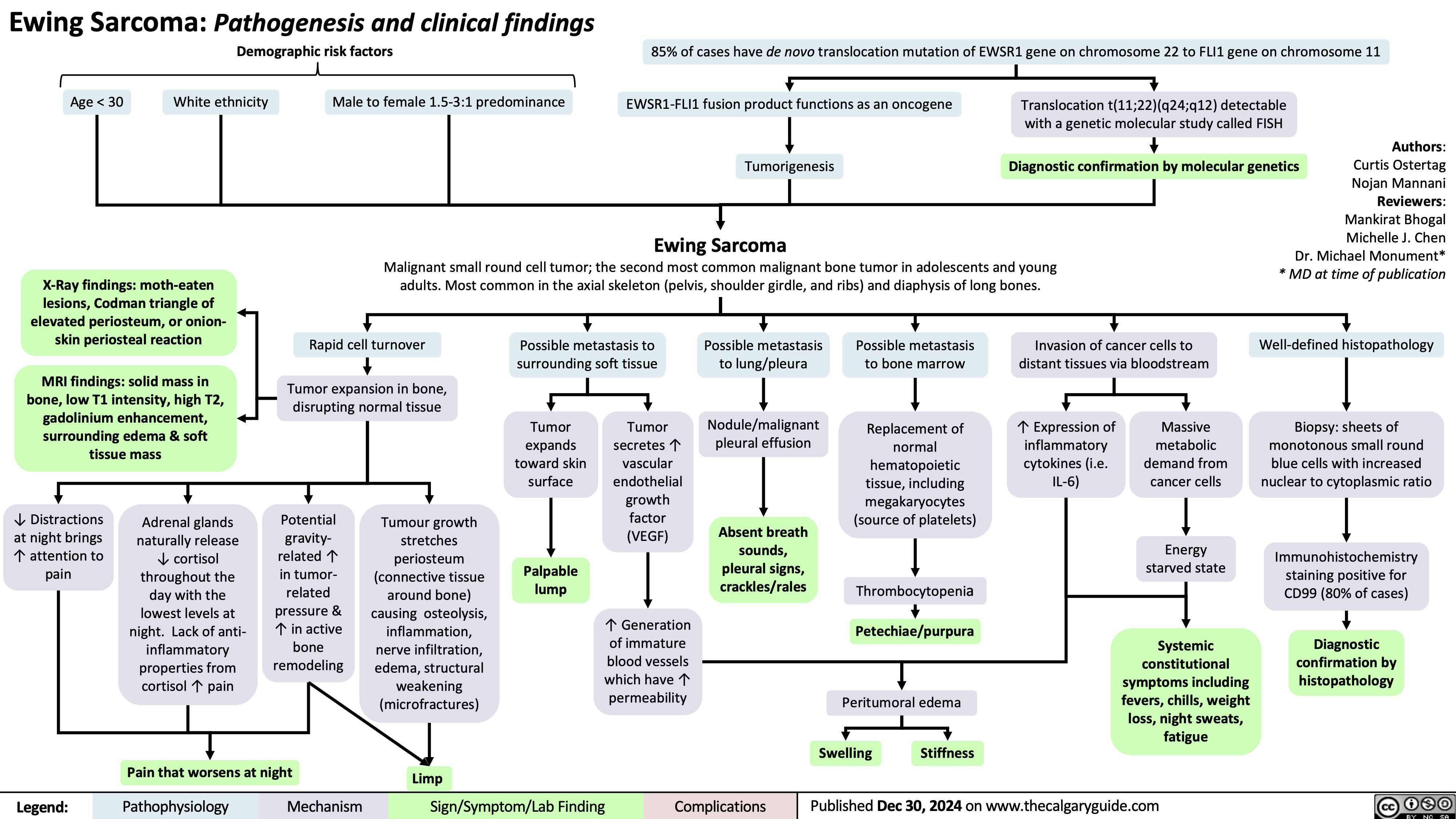
Ewing Sarcoma