SEARCH RESULTS FOR: Ptosis
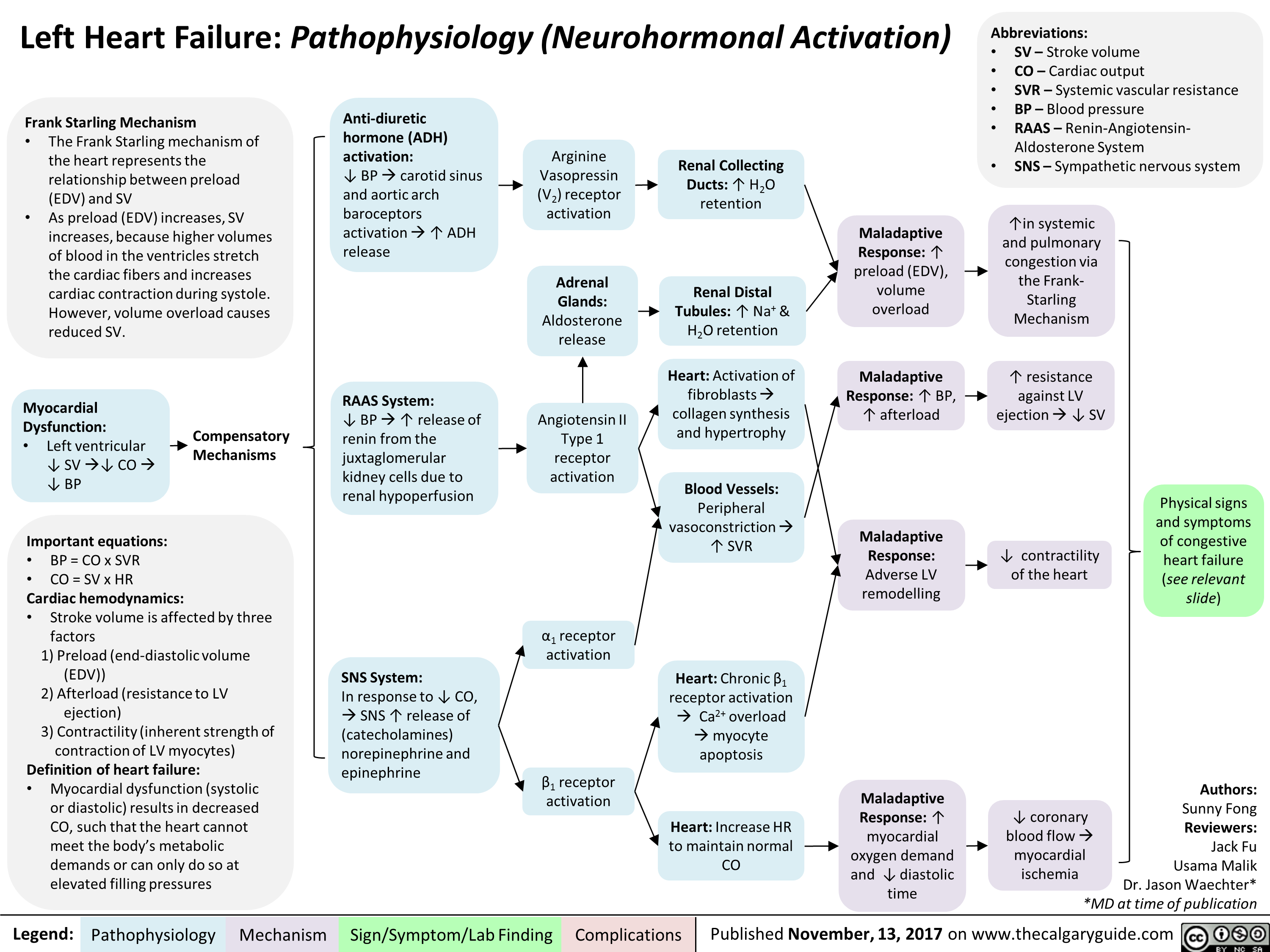
Left Heart Failure: Pathophysiology (Neurohormonal Activation)
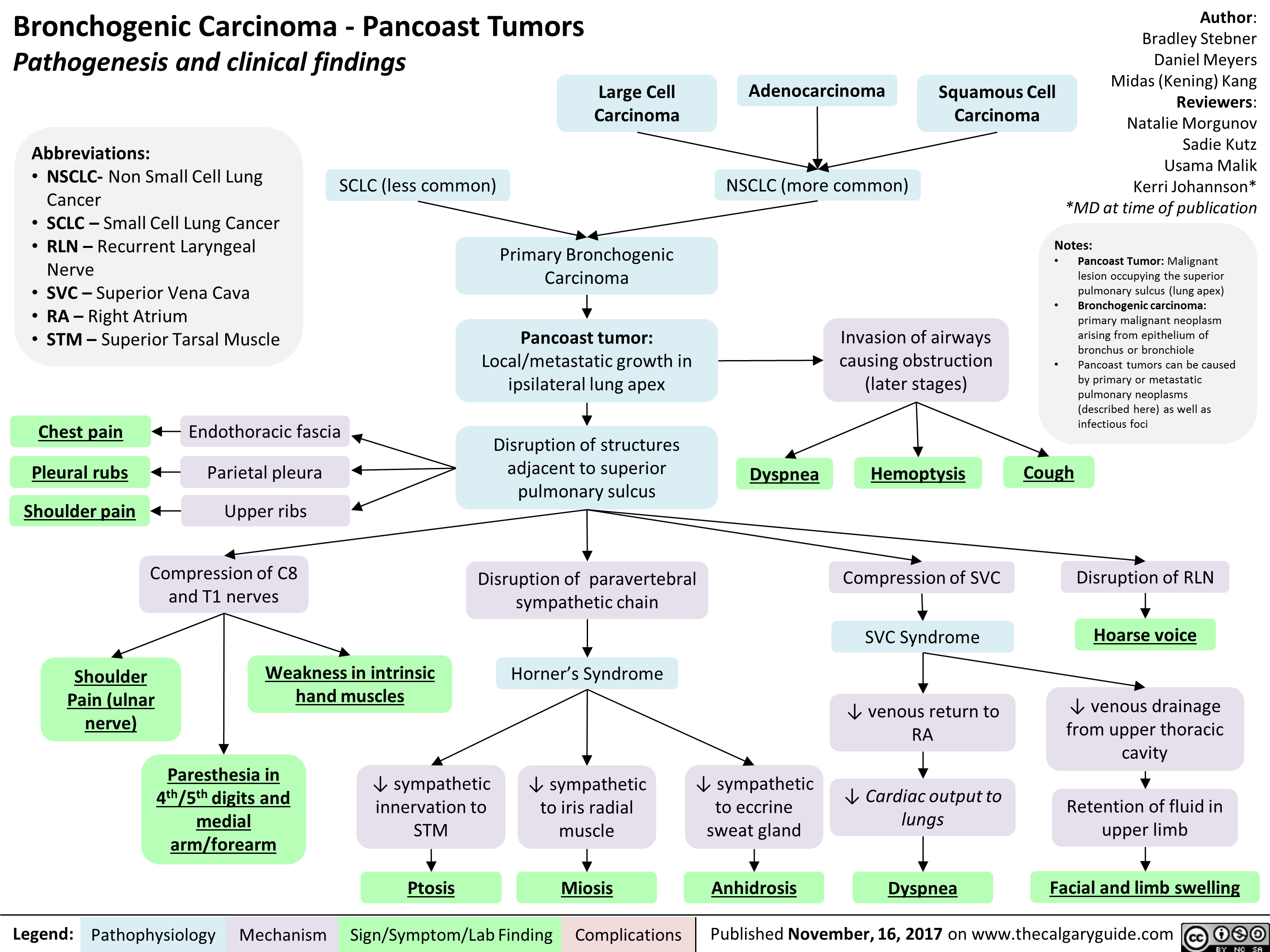
Bronchogenic Carcinoma - Pancoast Tumors Pathogenesis and clinical findings
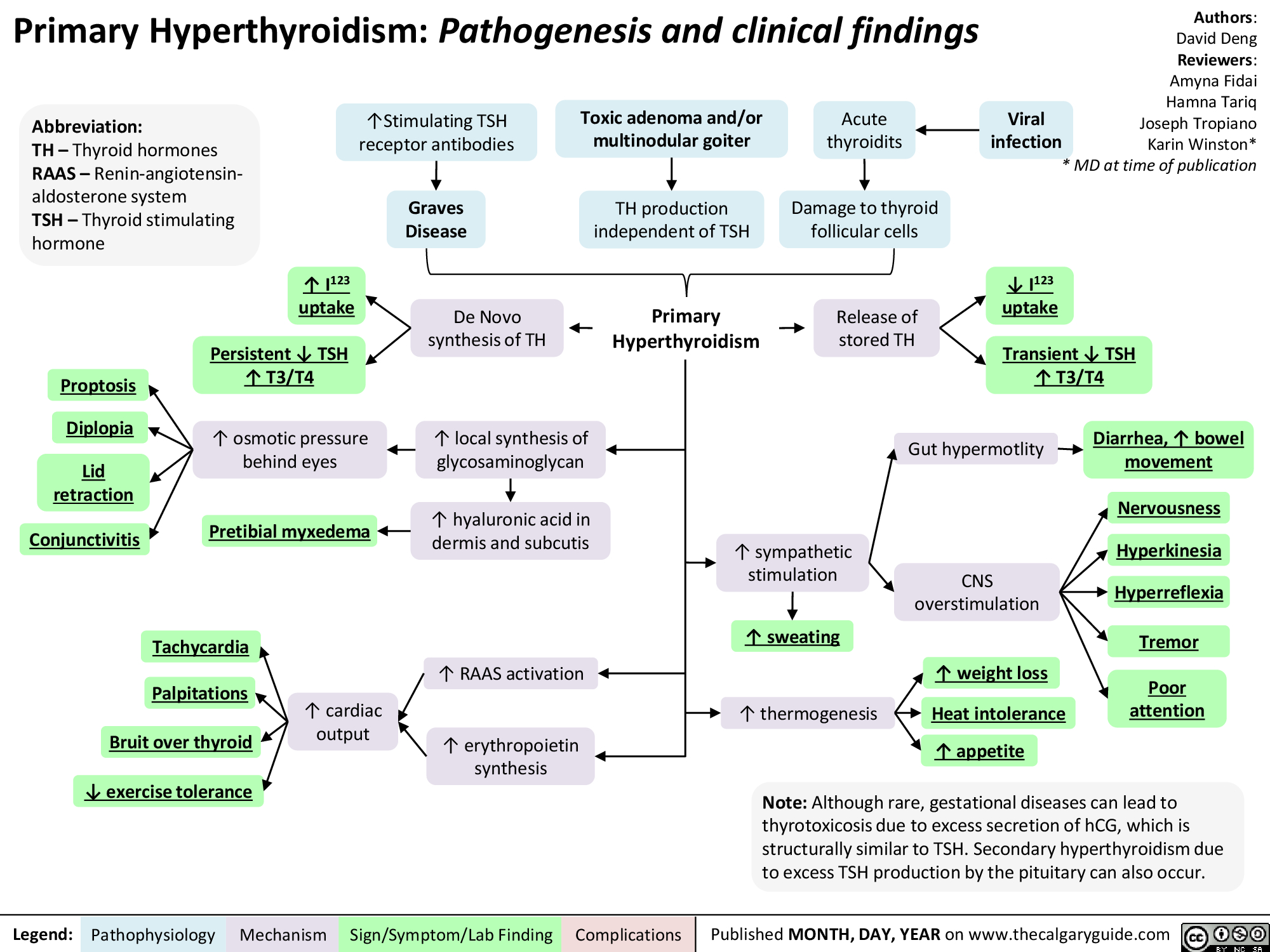
Hyperthyroidism
Orbital Cellulitis: Pathogenesis and clinical findings
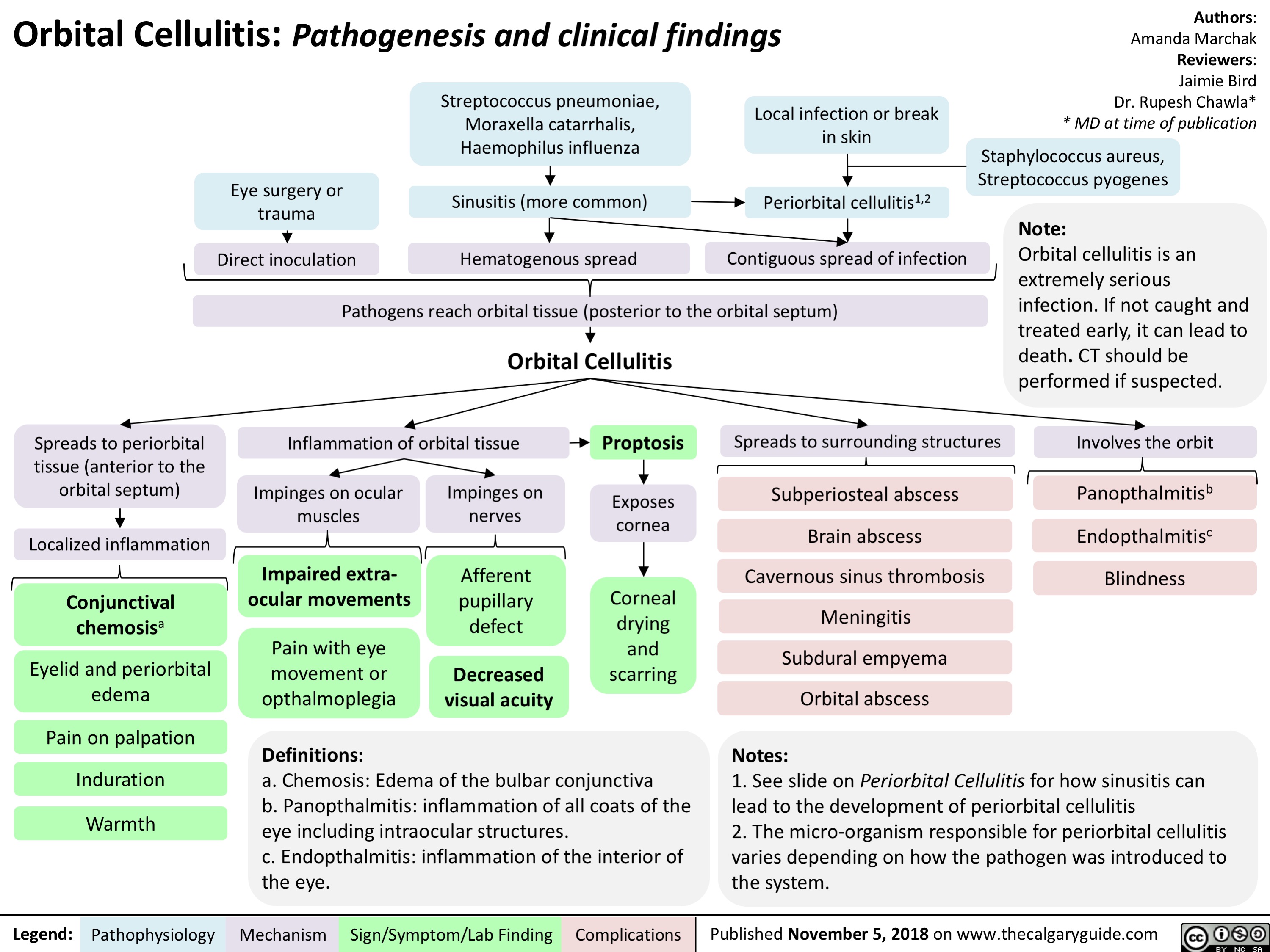
Primary Myelofibrosis pathogenesis and clinical findings
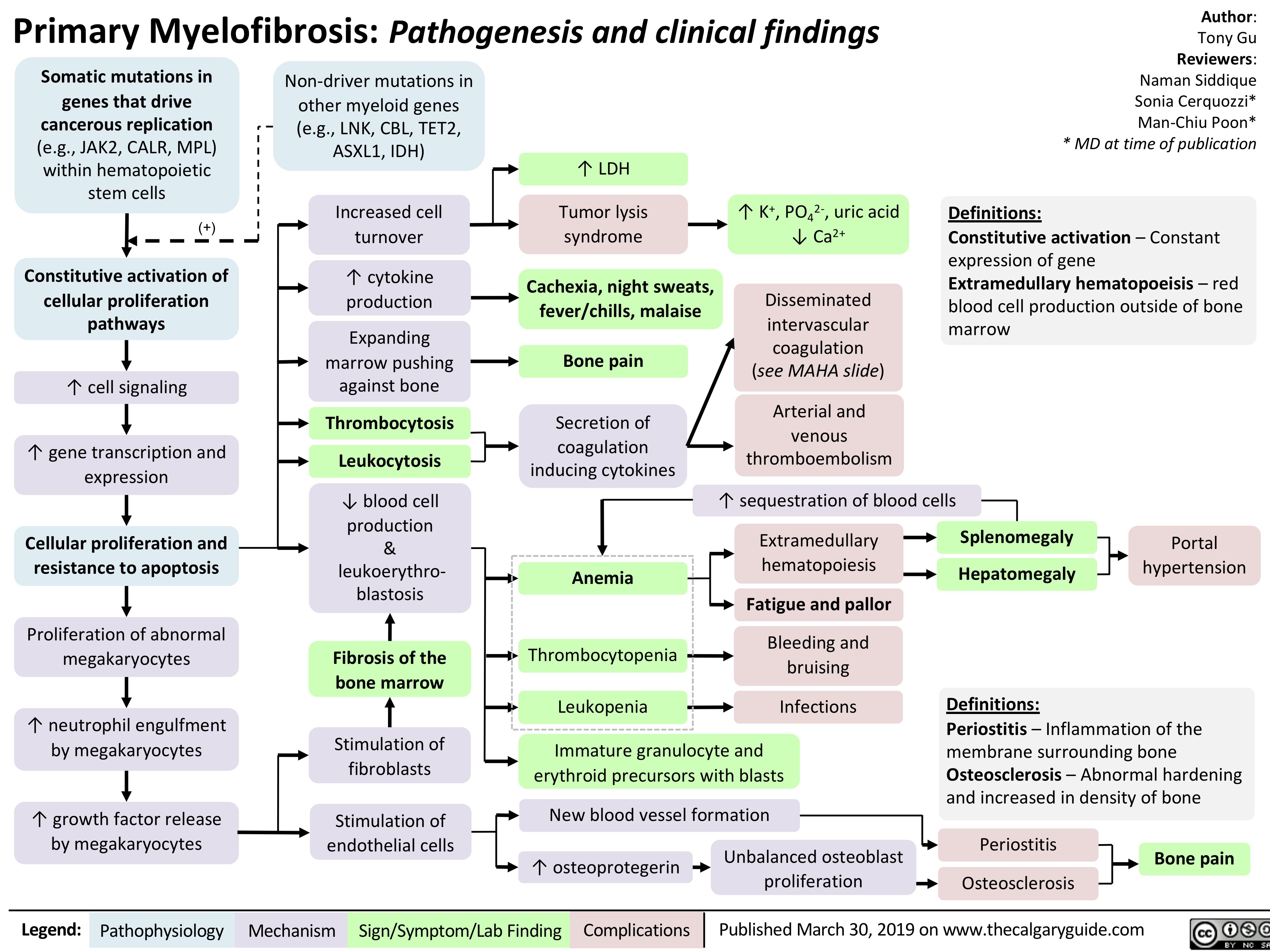
Crohn's Disease
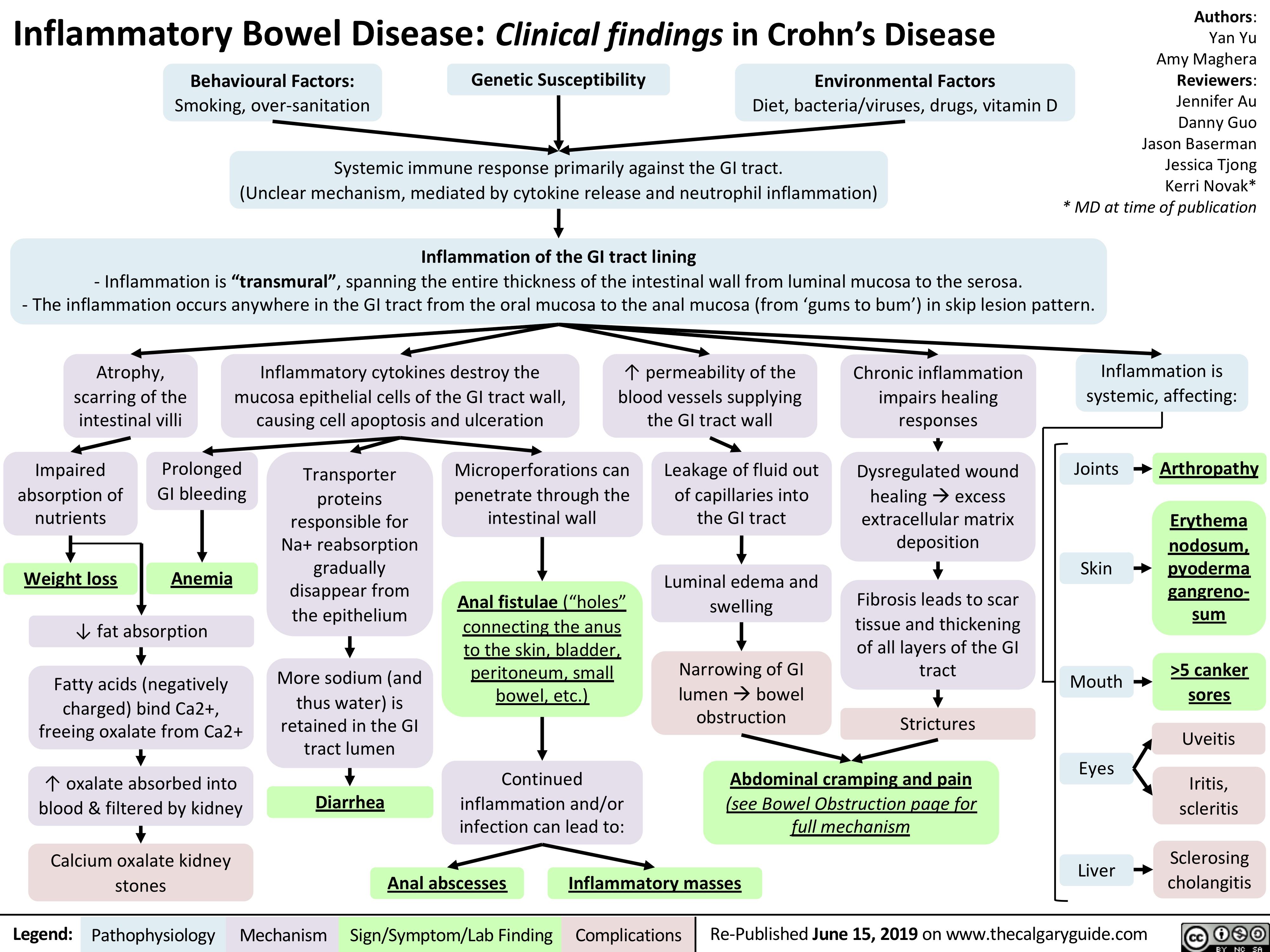
Ulcerative Colitis
Ataxia Telangiectasia Pathogenesis and Clinical Findings
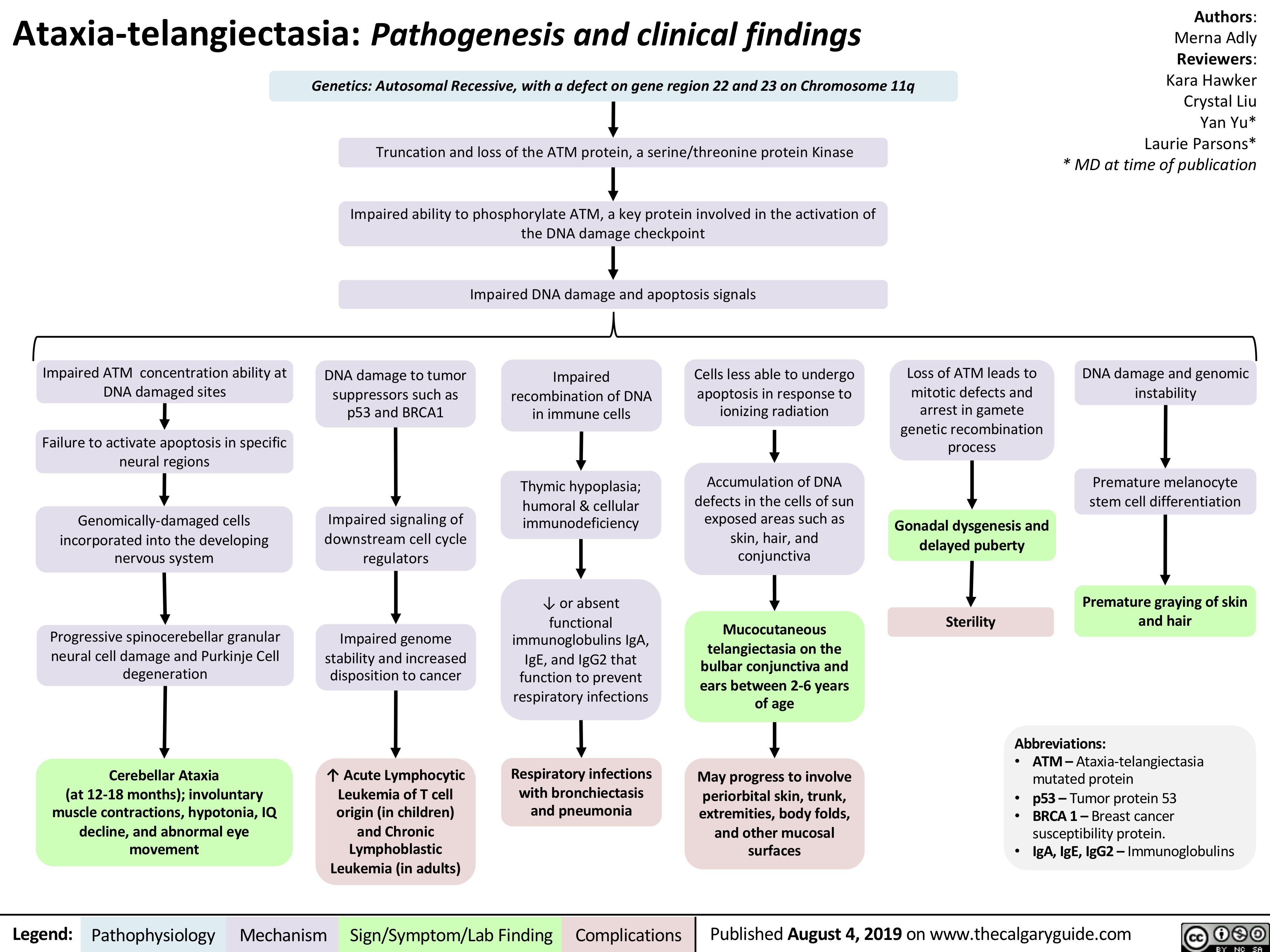
Multiple-Myeloma
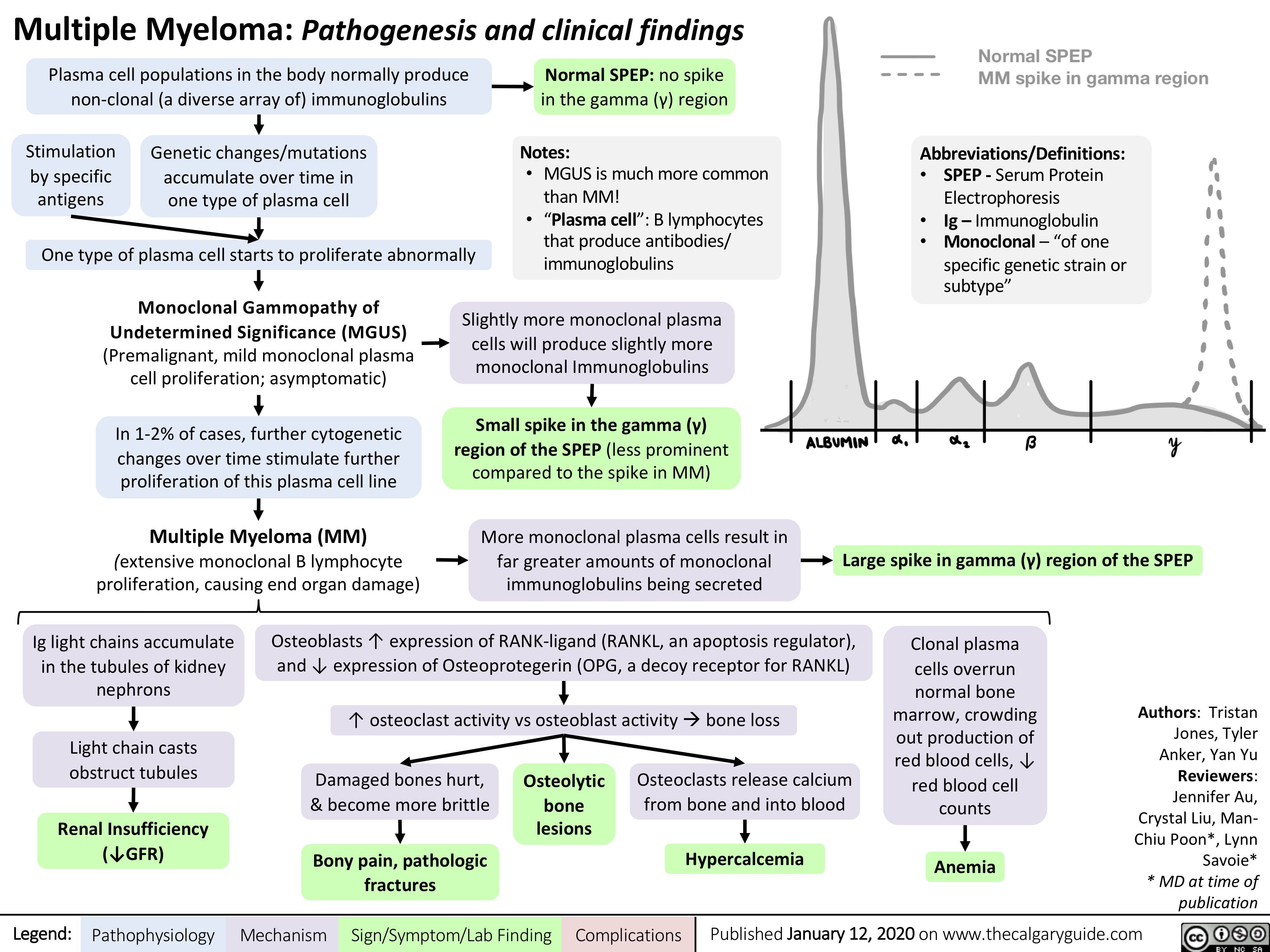
Pathophysiology-Behind-the-Leukemias
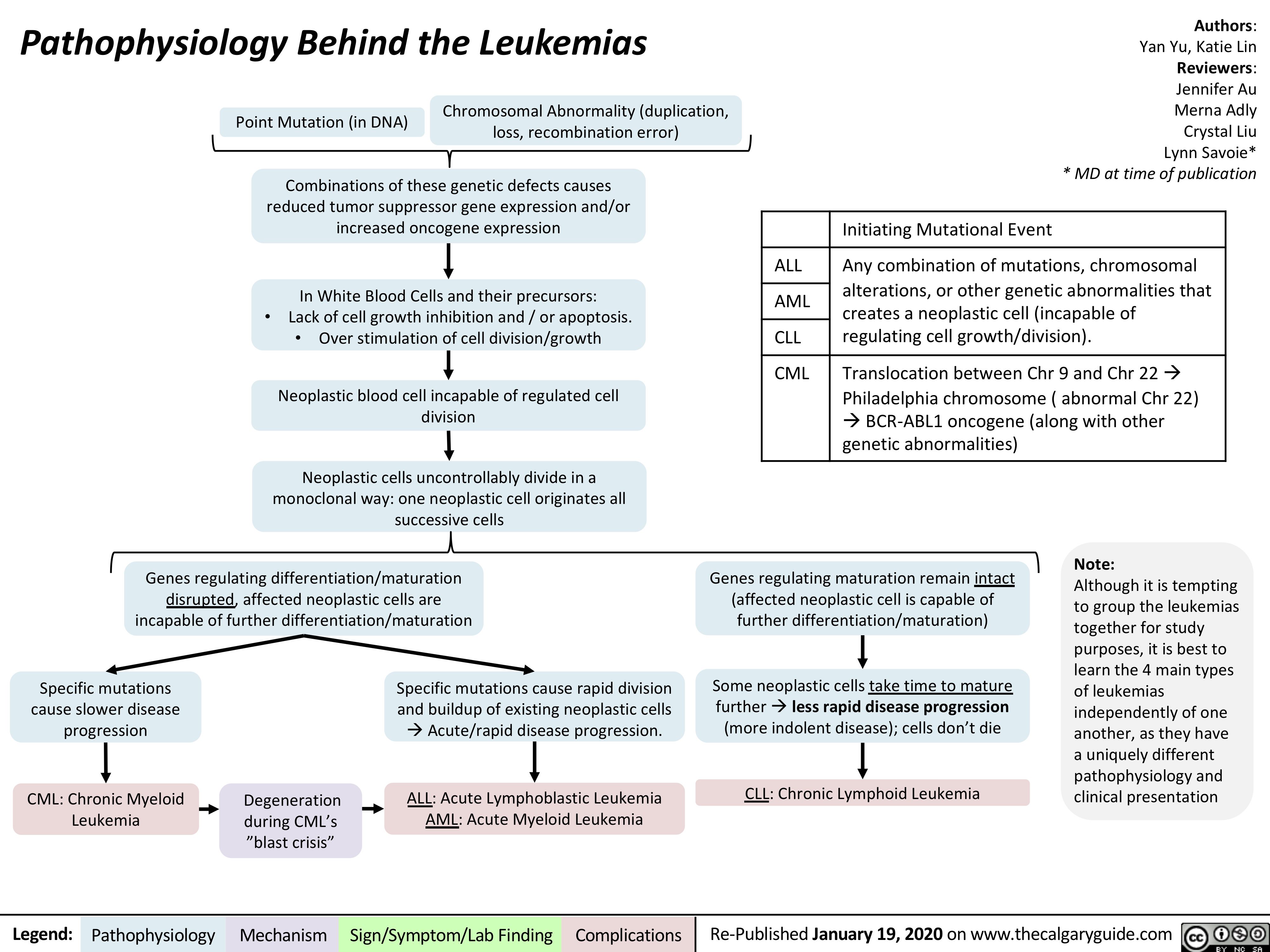
Marfan-Syndrome
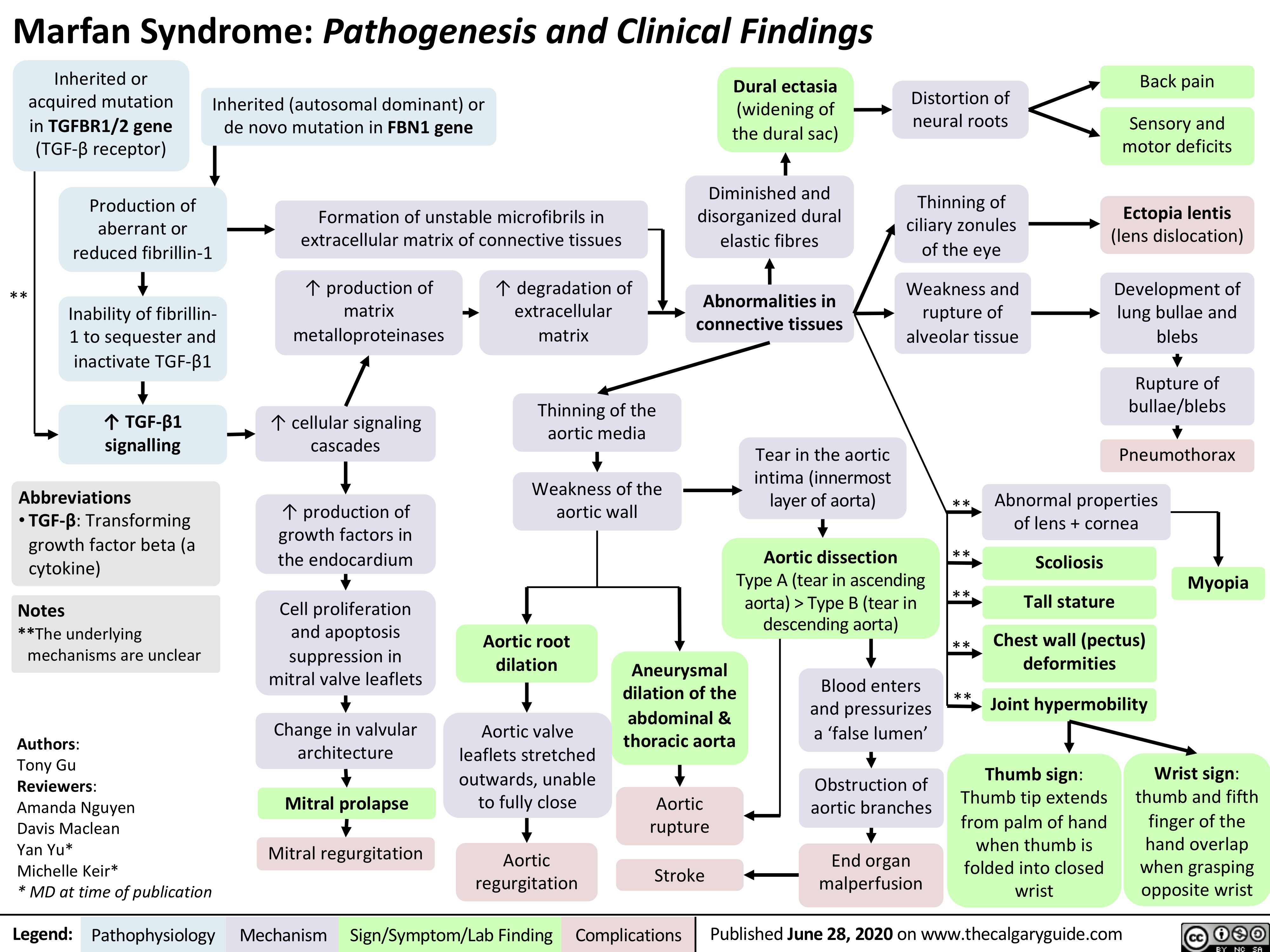
Avascular-necrosis-of-the-femoral-head
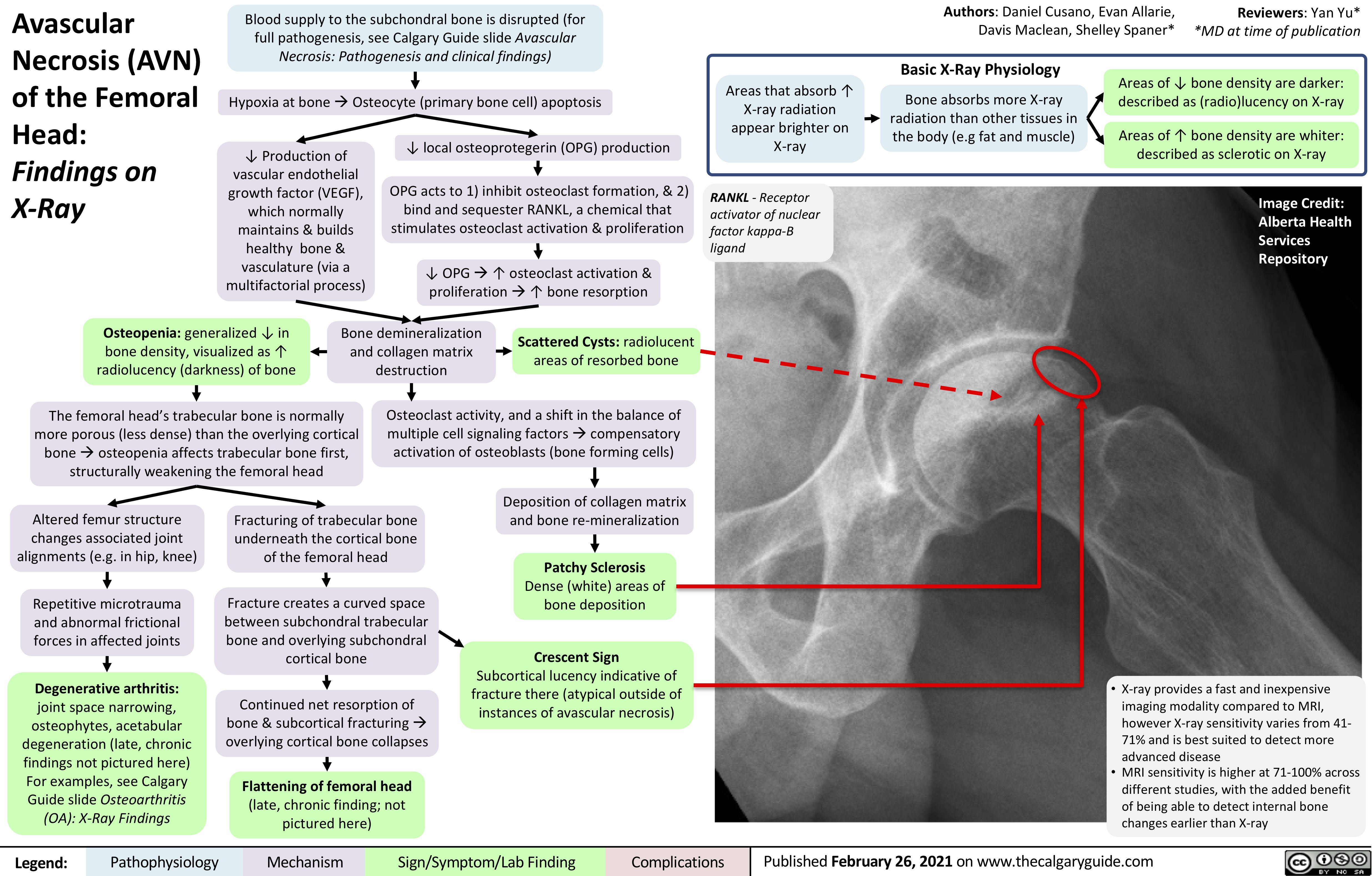
Lambert-Eaton-Myasthenic-Syndrome-Pathogenesis-and-Clinical-Findings
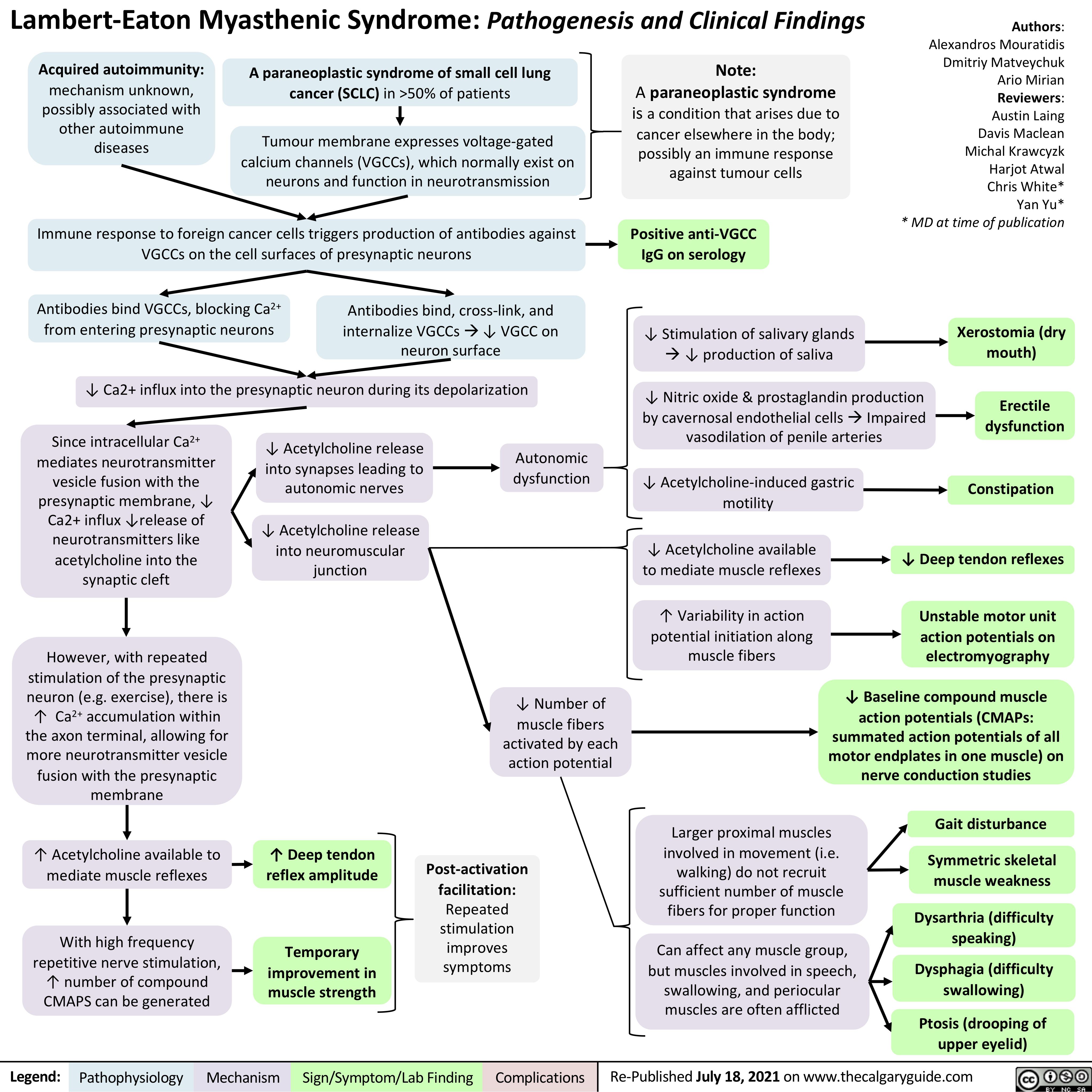
Corneal-Abrasion
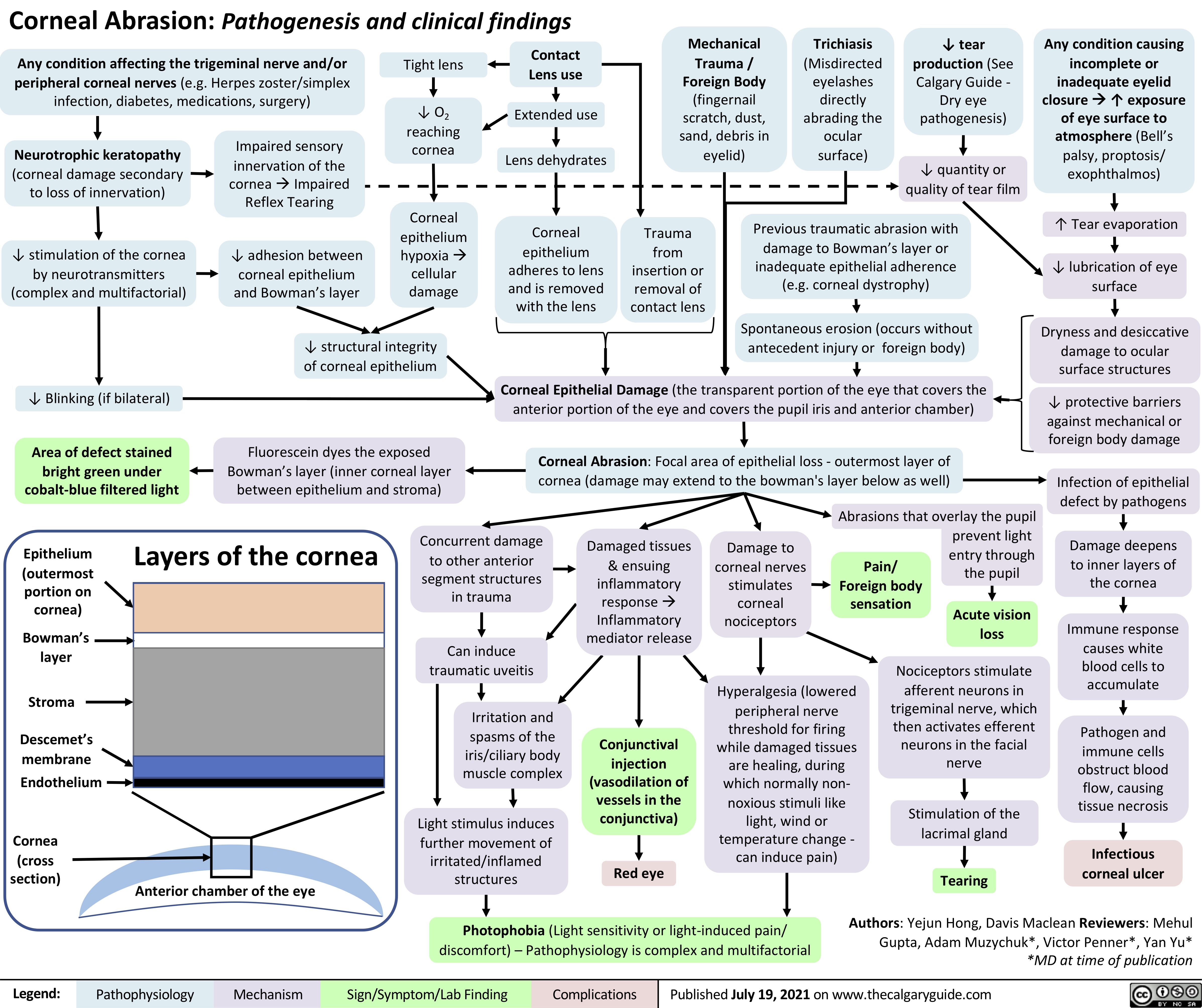
Dry-Eye-Syndrome-Pathogenesis
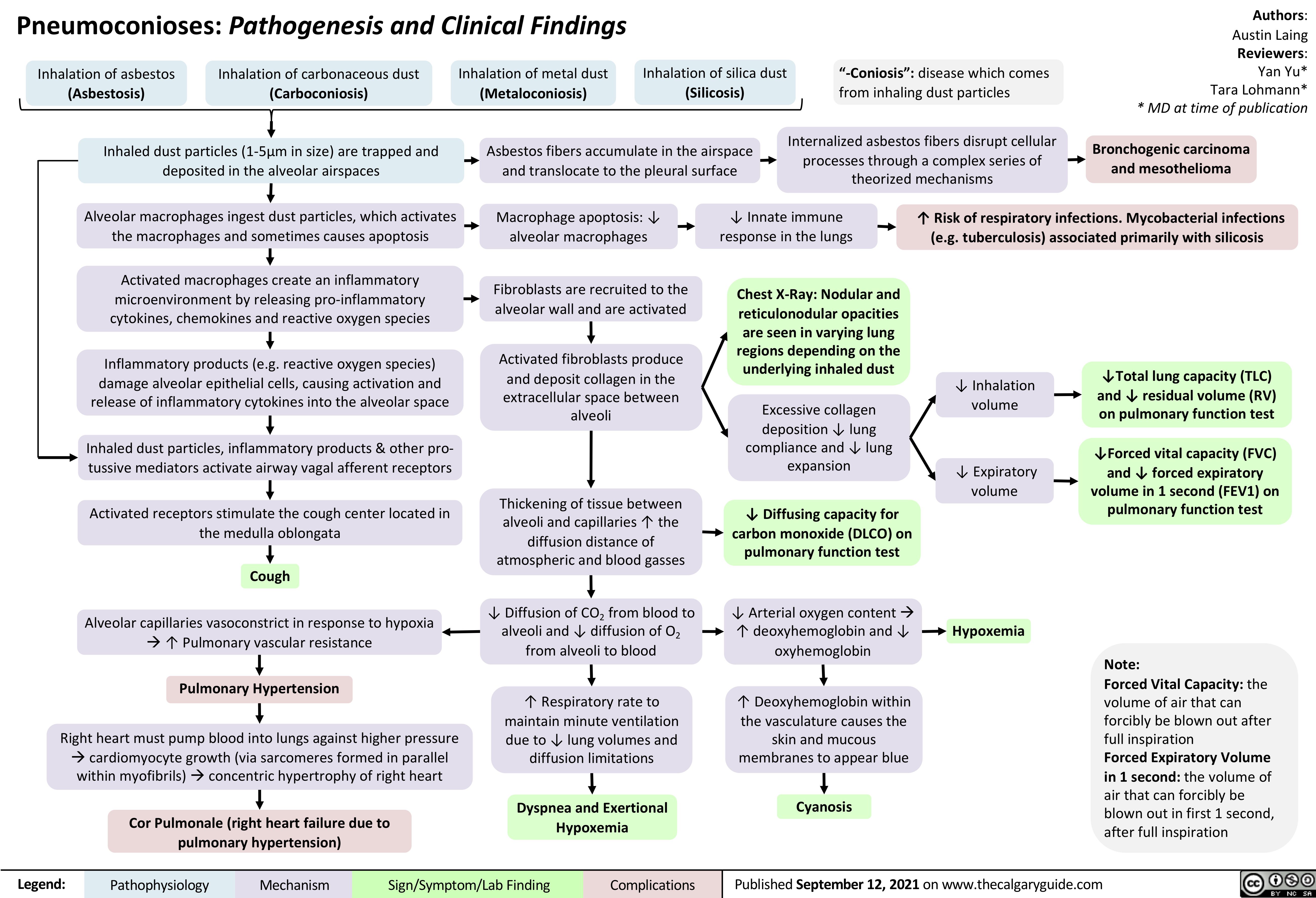
Pneumoconioses
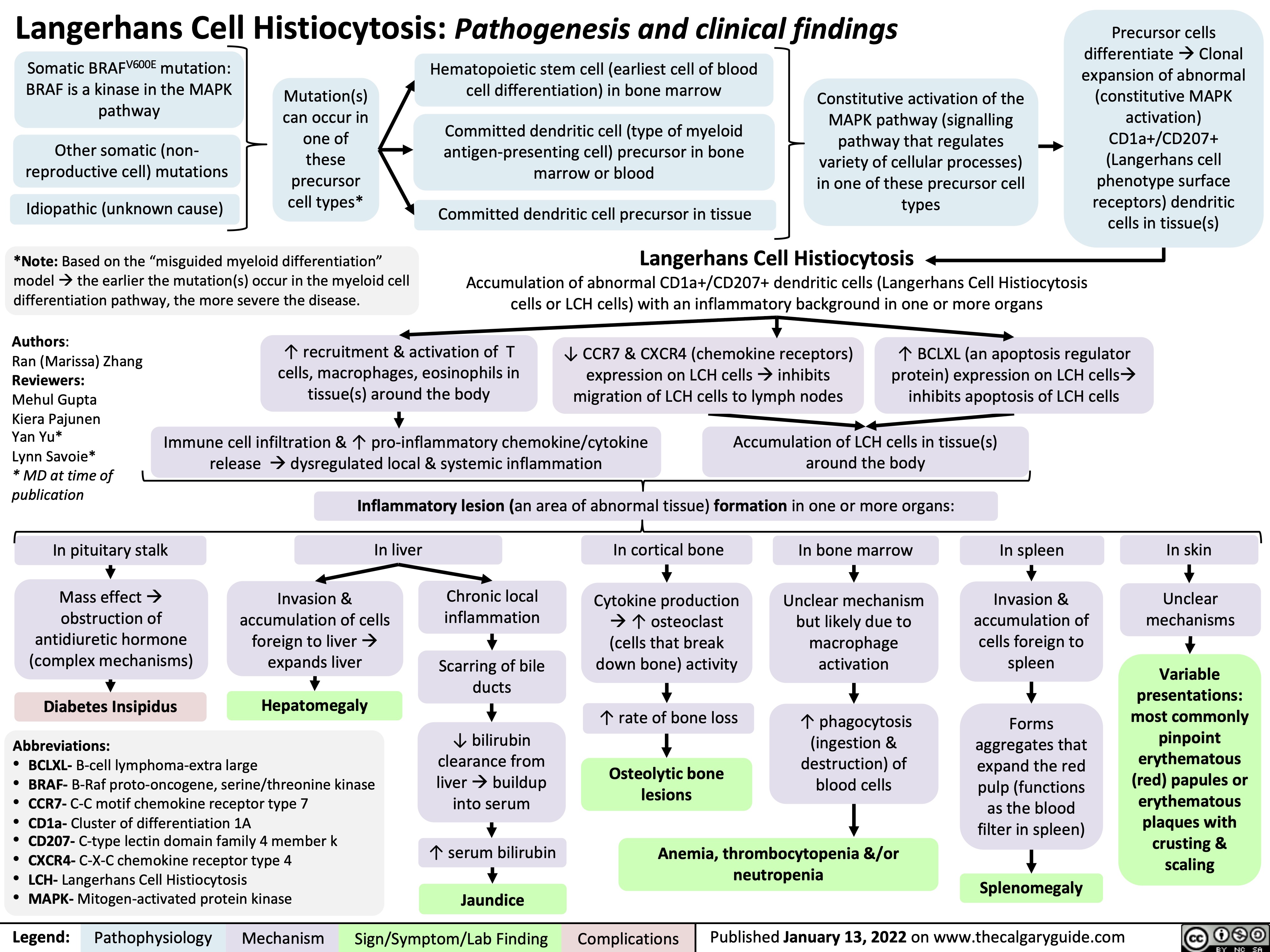
Langerhans Cell Histiocytosis
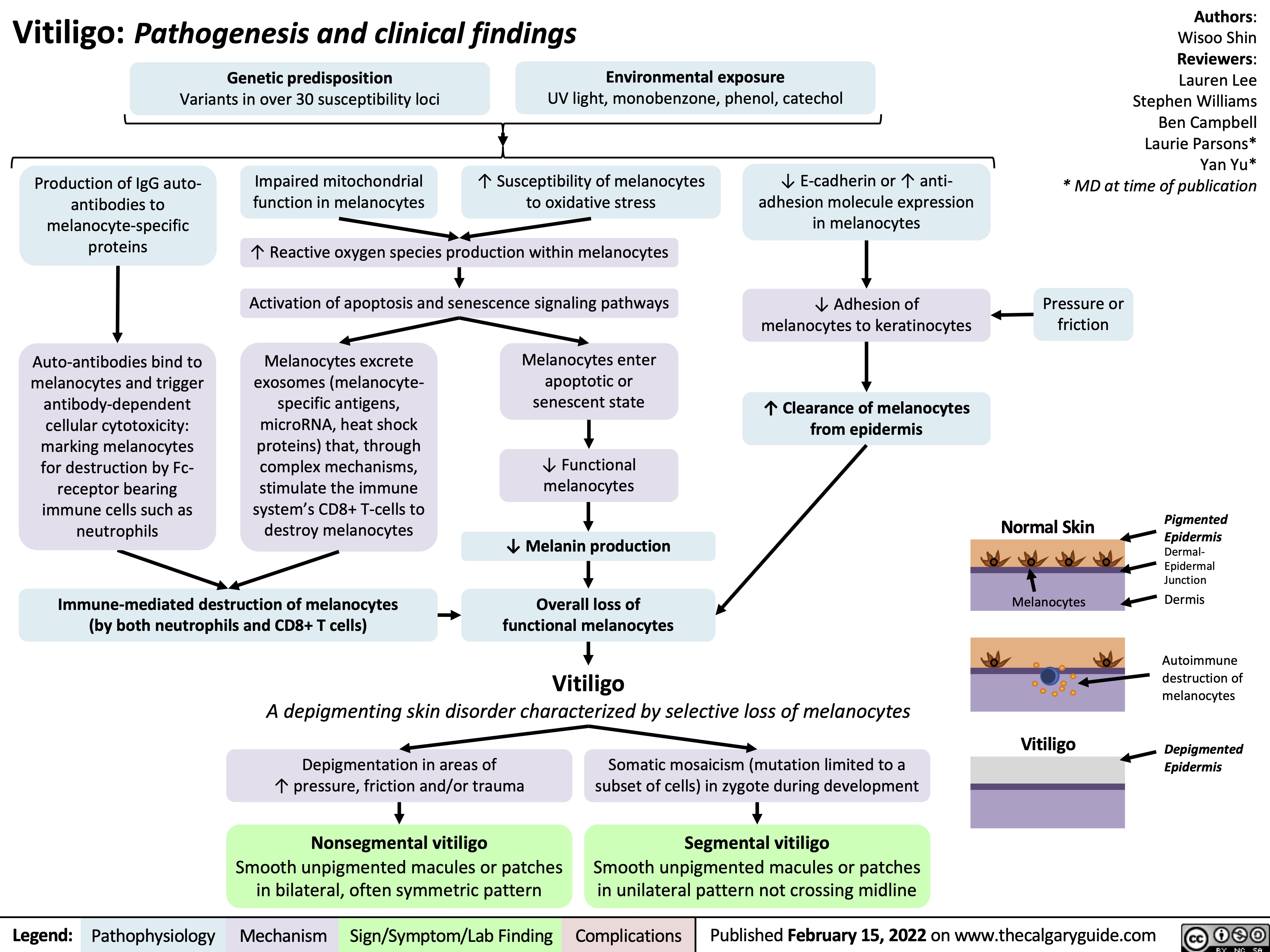
Vitiligo Pathogenesis and Clinical Findings
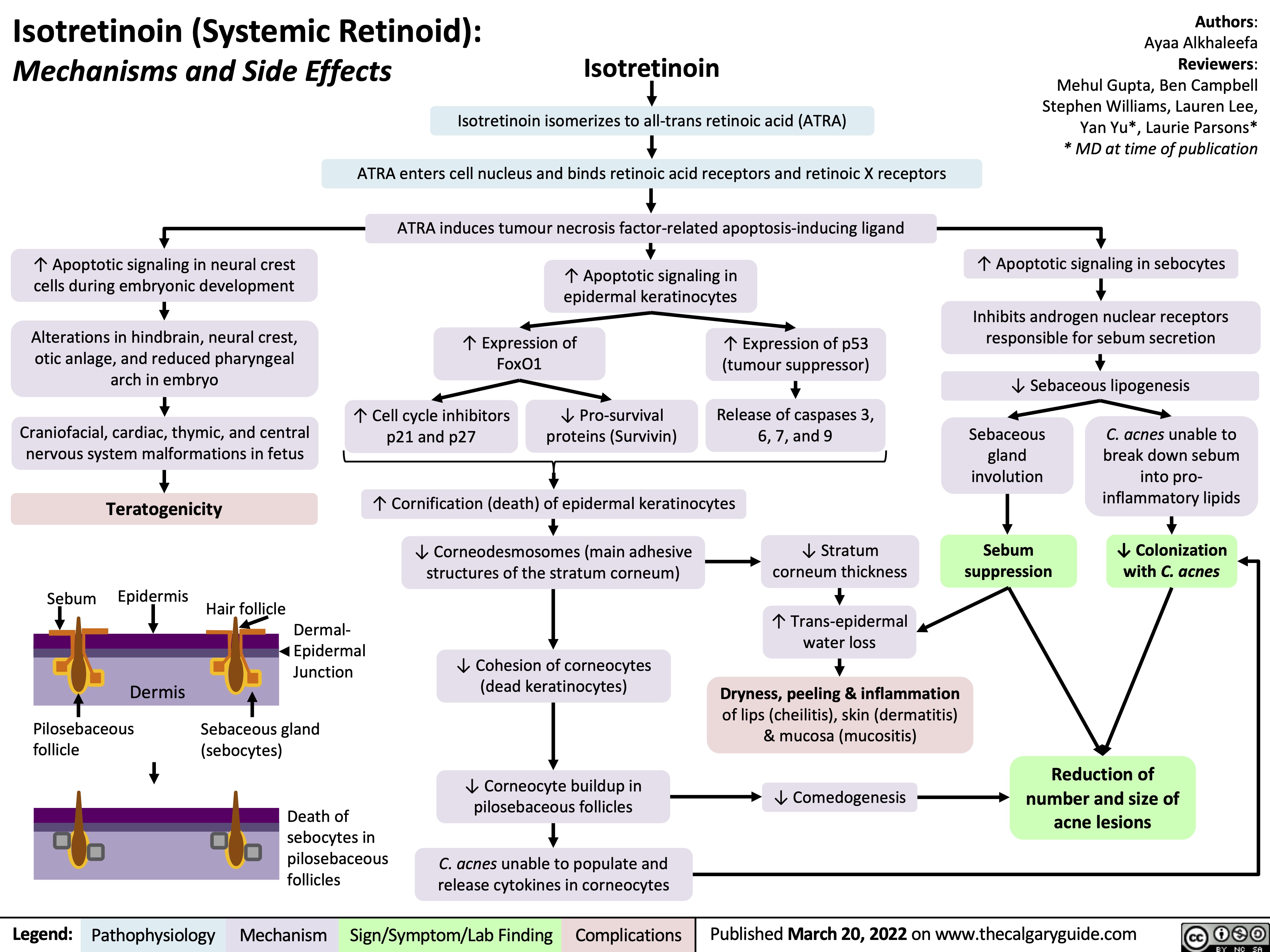
isotretinoin-systemic-retinoid-mechanisms-and-side-effects
presentation-of-sah
![Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com
Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/SAH-Clinical-Findings-2022.jpg)
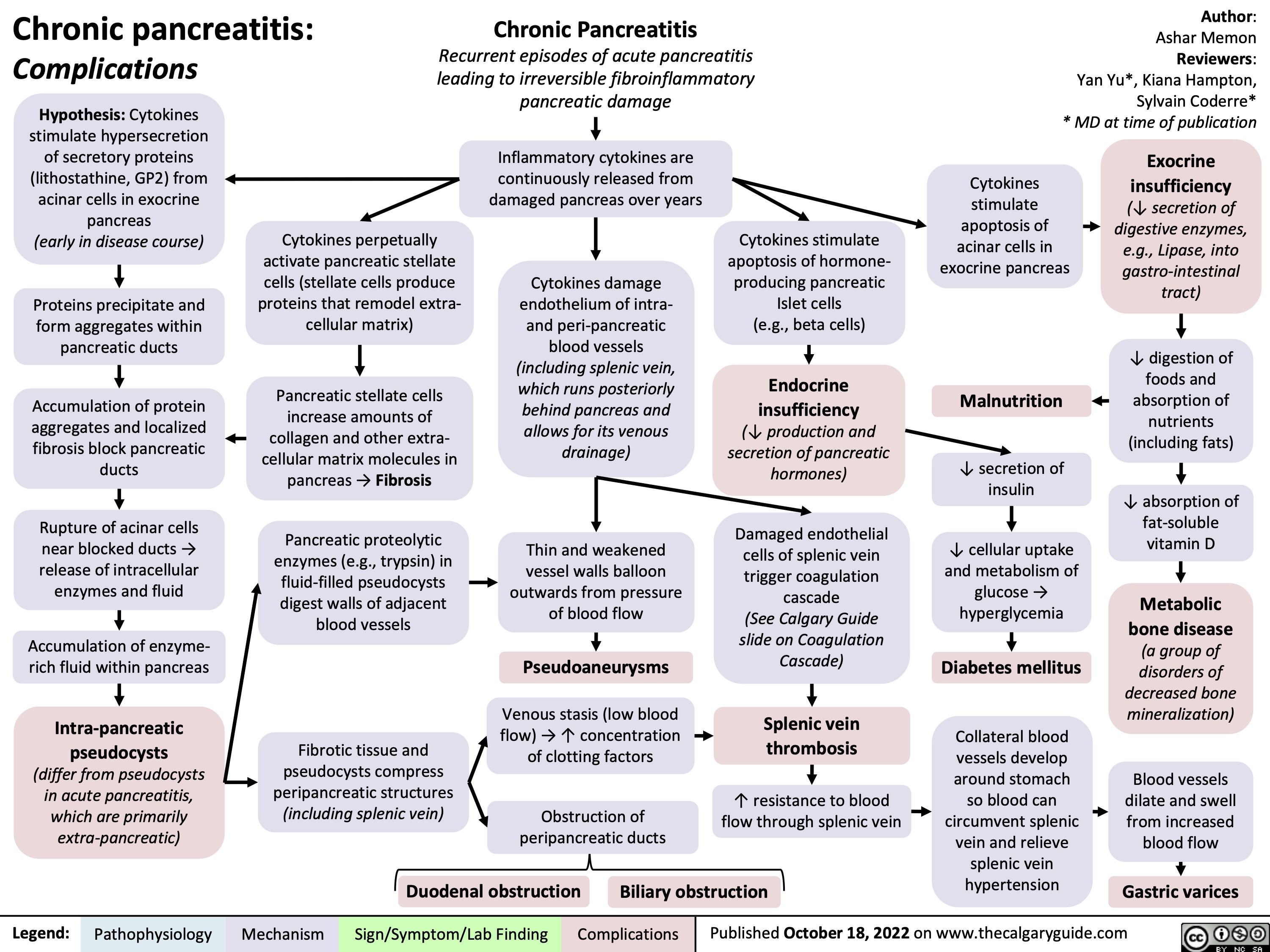
chronic-pancreatitis-complications
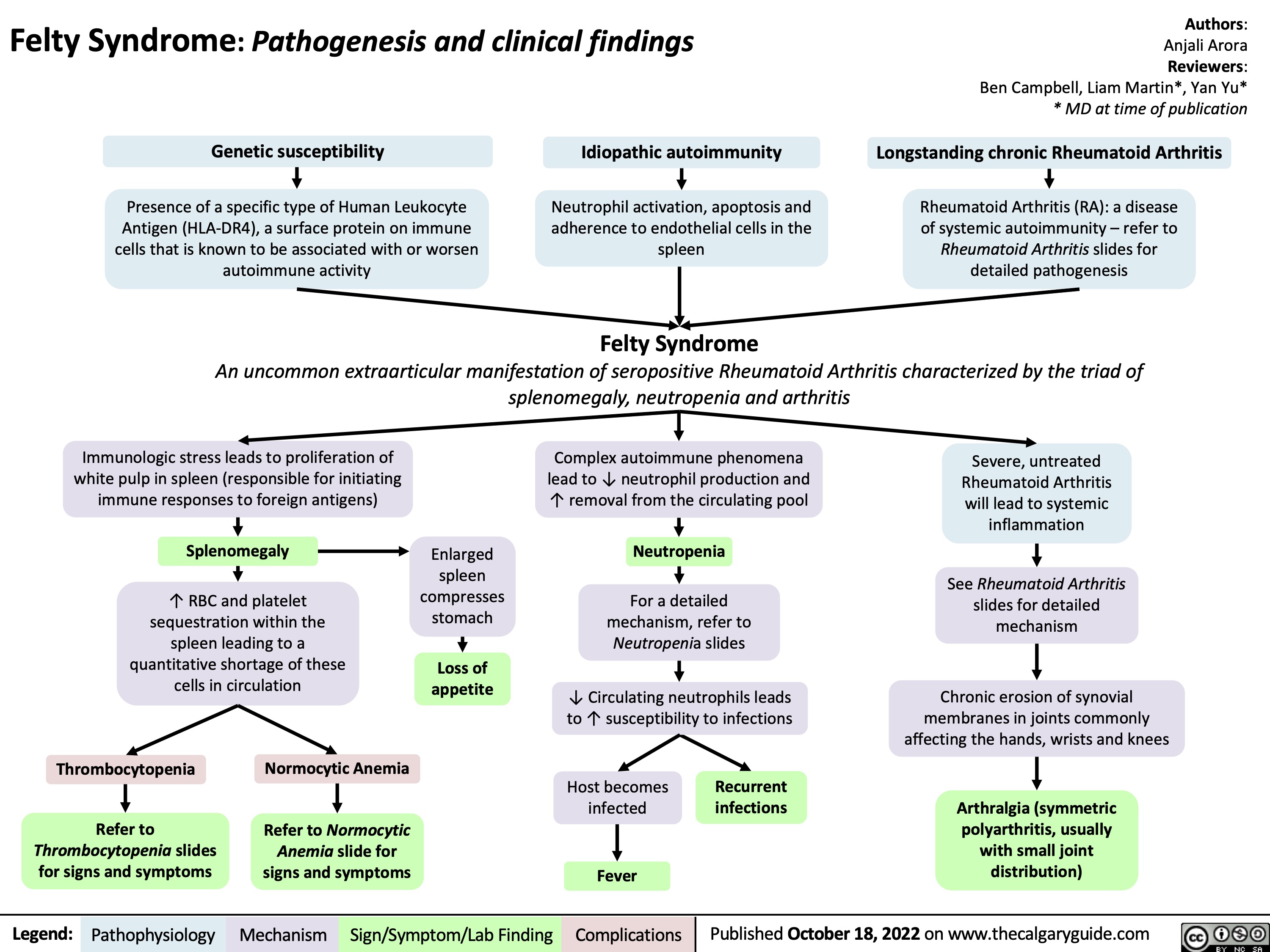
felty-syndrome
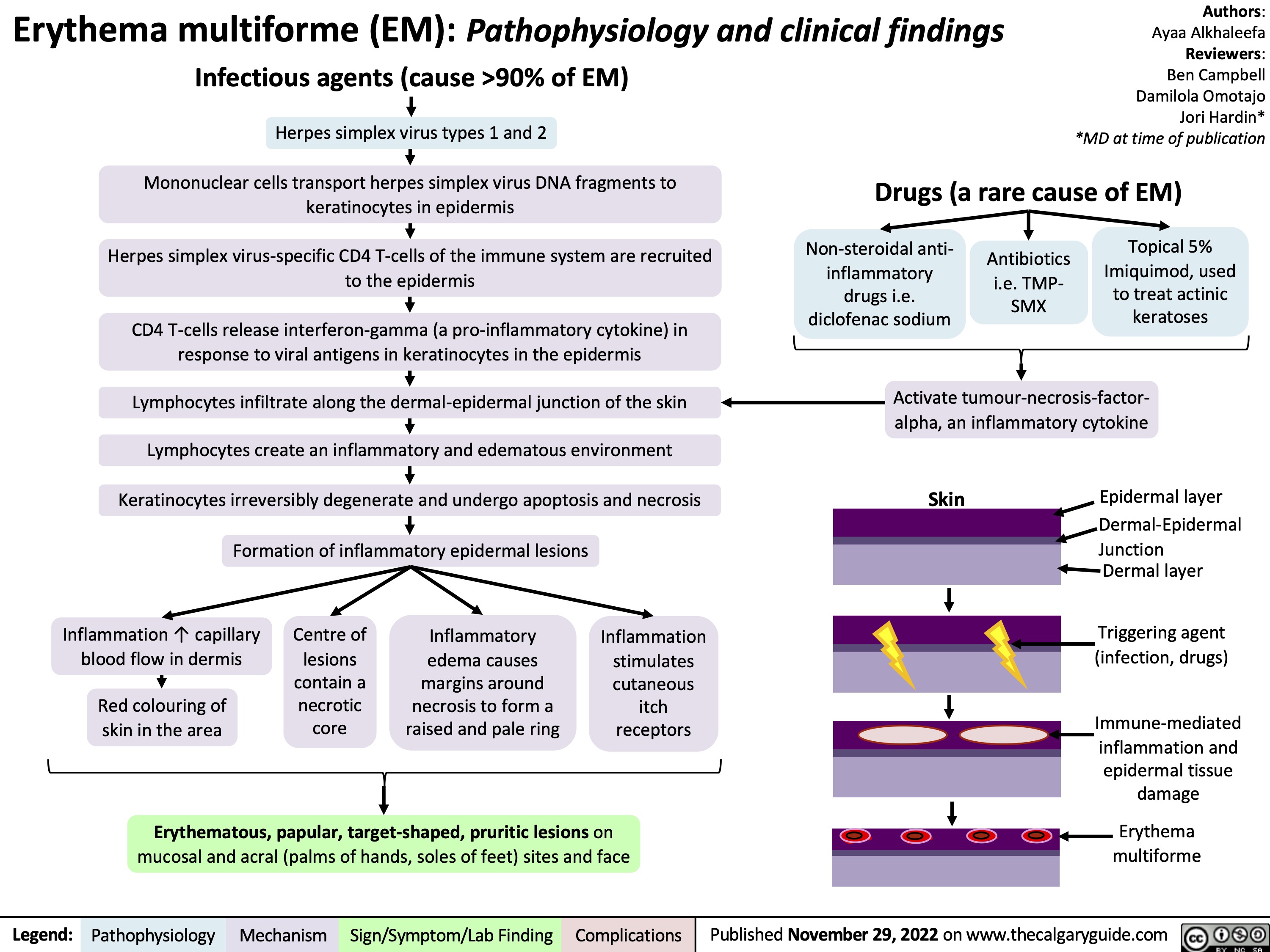
Erythema multiforme (EM): Pathophysiology and clinical findings
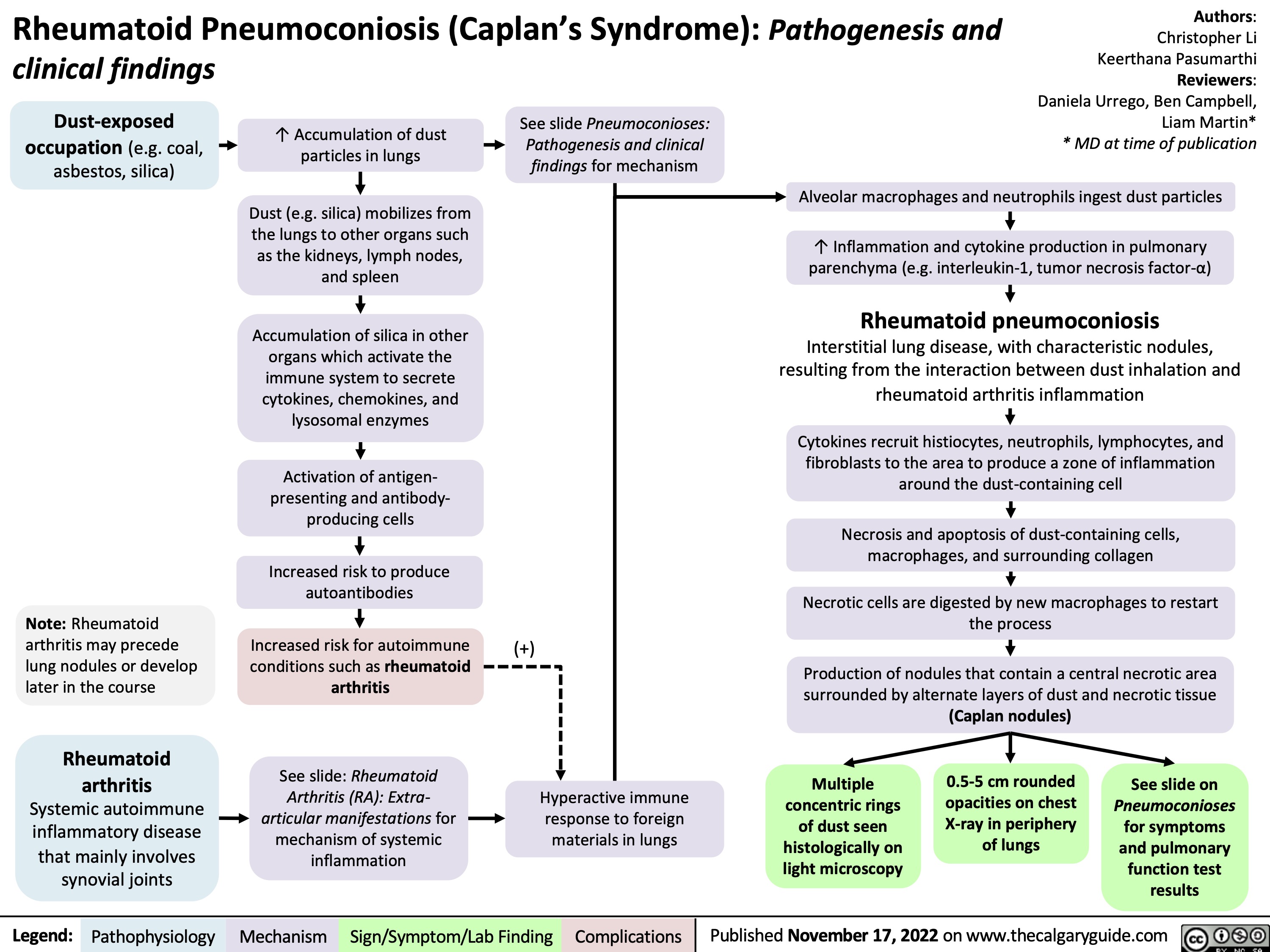
rheumatoid-pneumoconiosis-caplans-syndrome-pathogenesis-and-clinical-findings
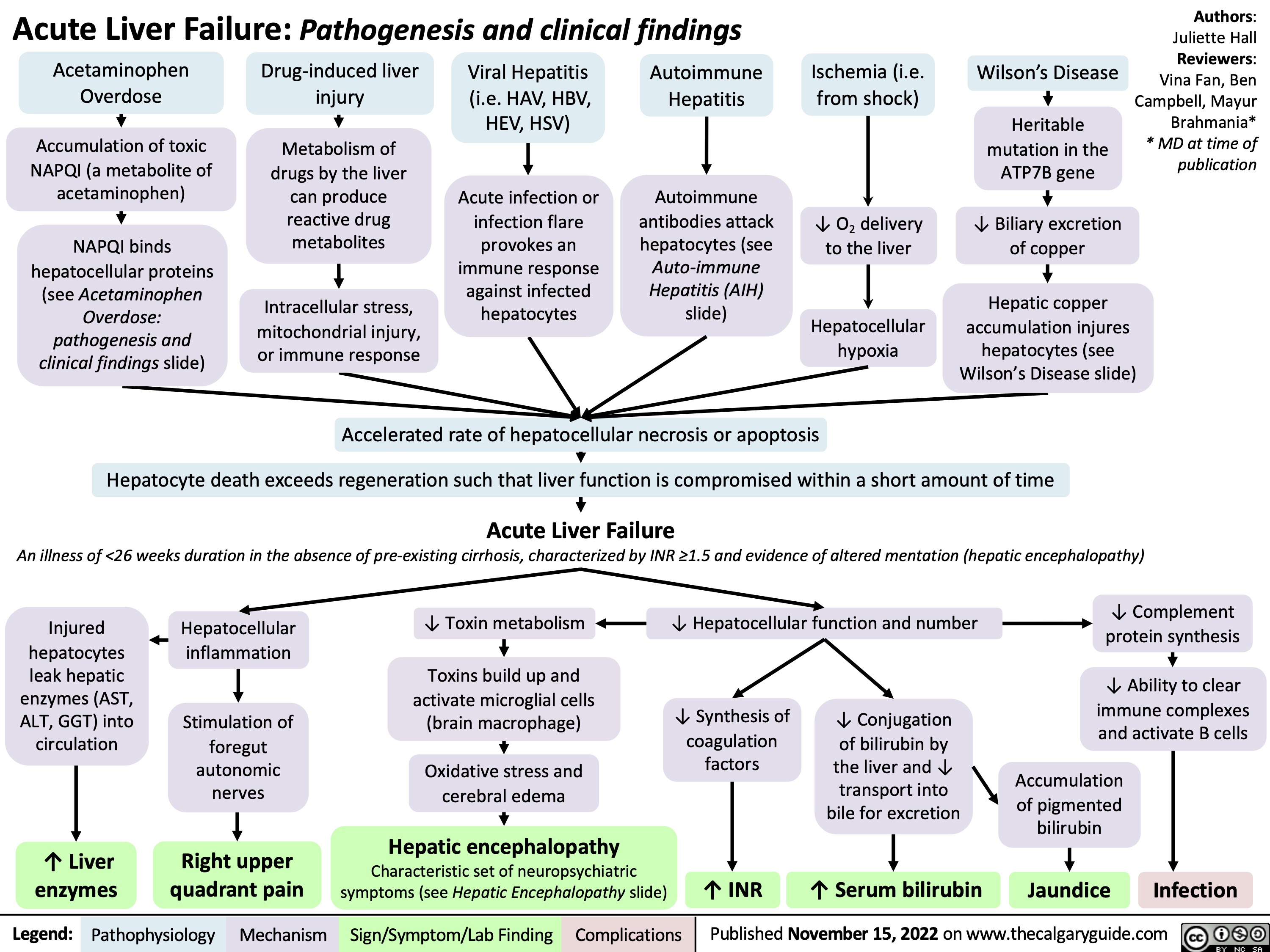
Acute Liver Failure: Pathogenesis and clinical findings
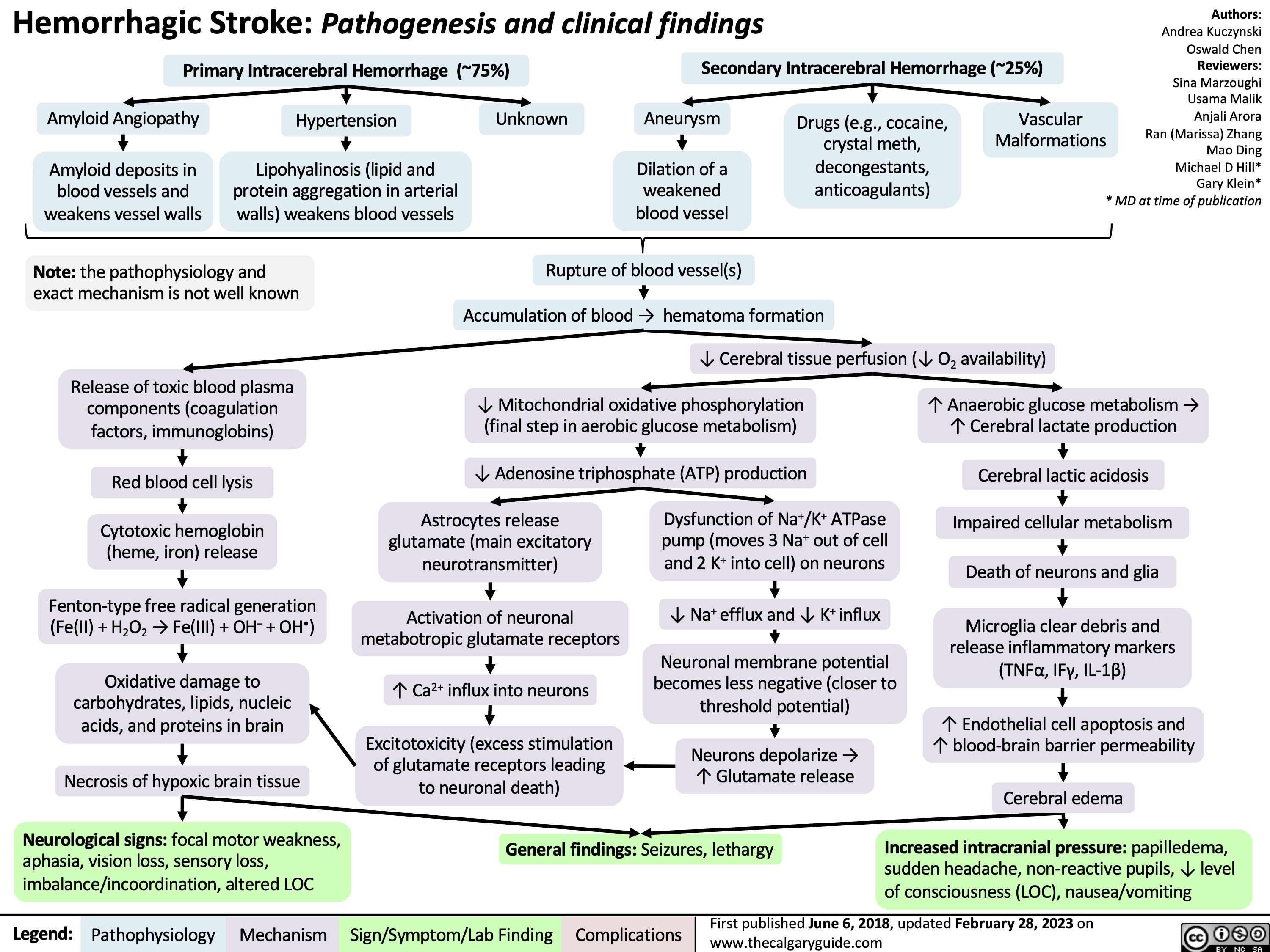
Hemorrhagic Stroke
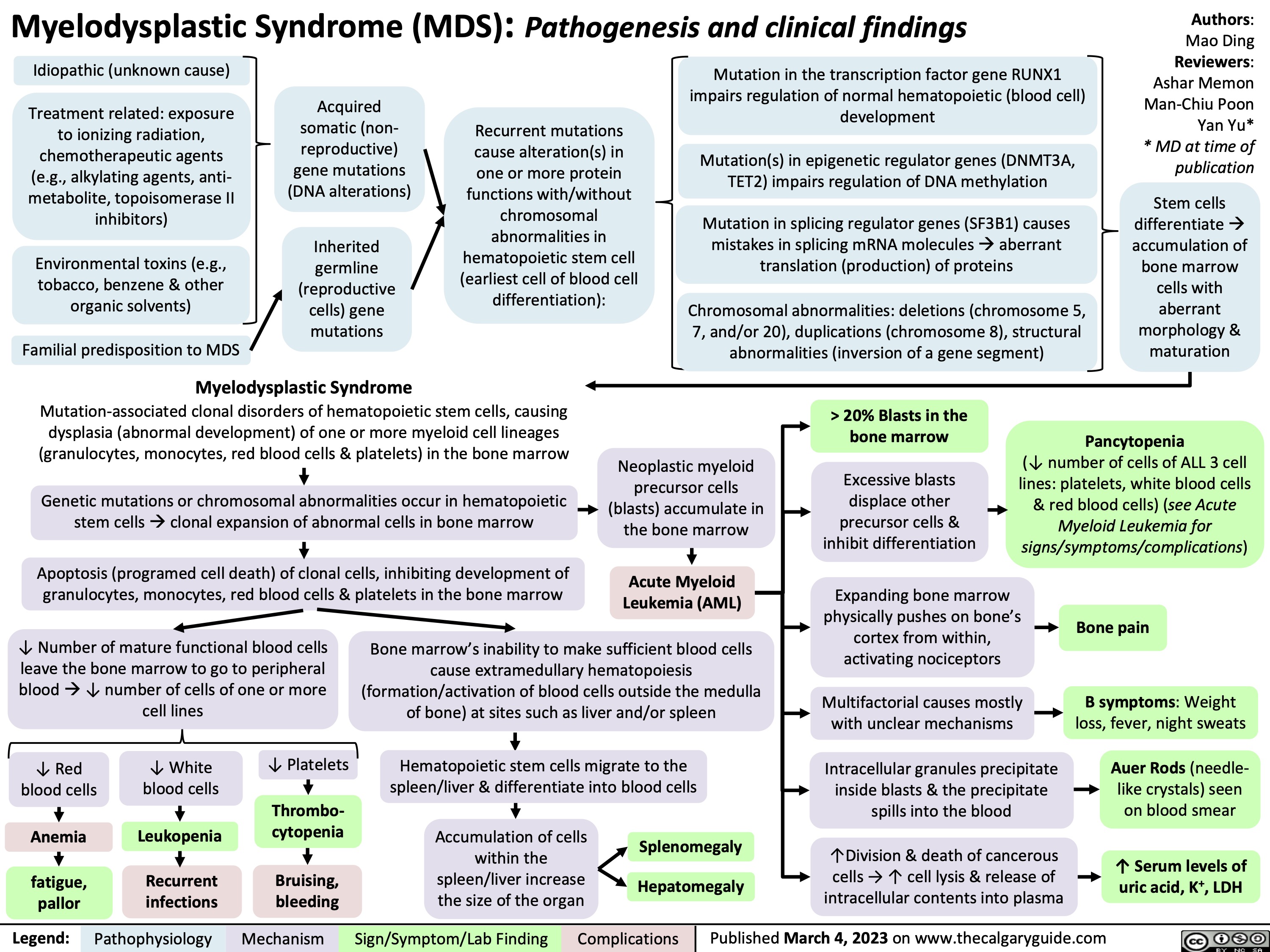
Myelodysplastic Syndrome Pathogenesis and clinical findings
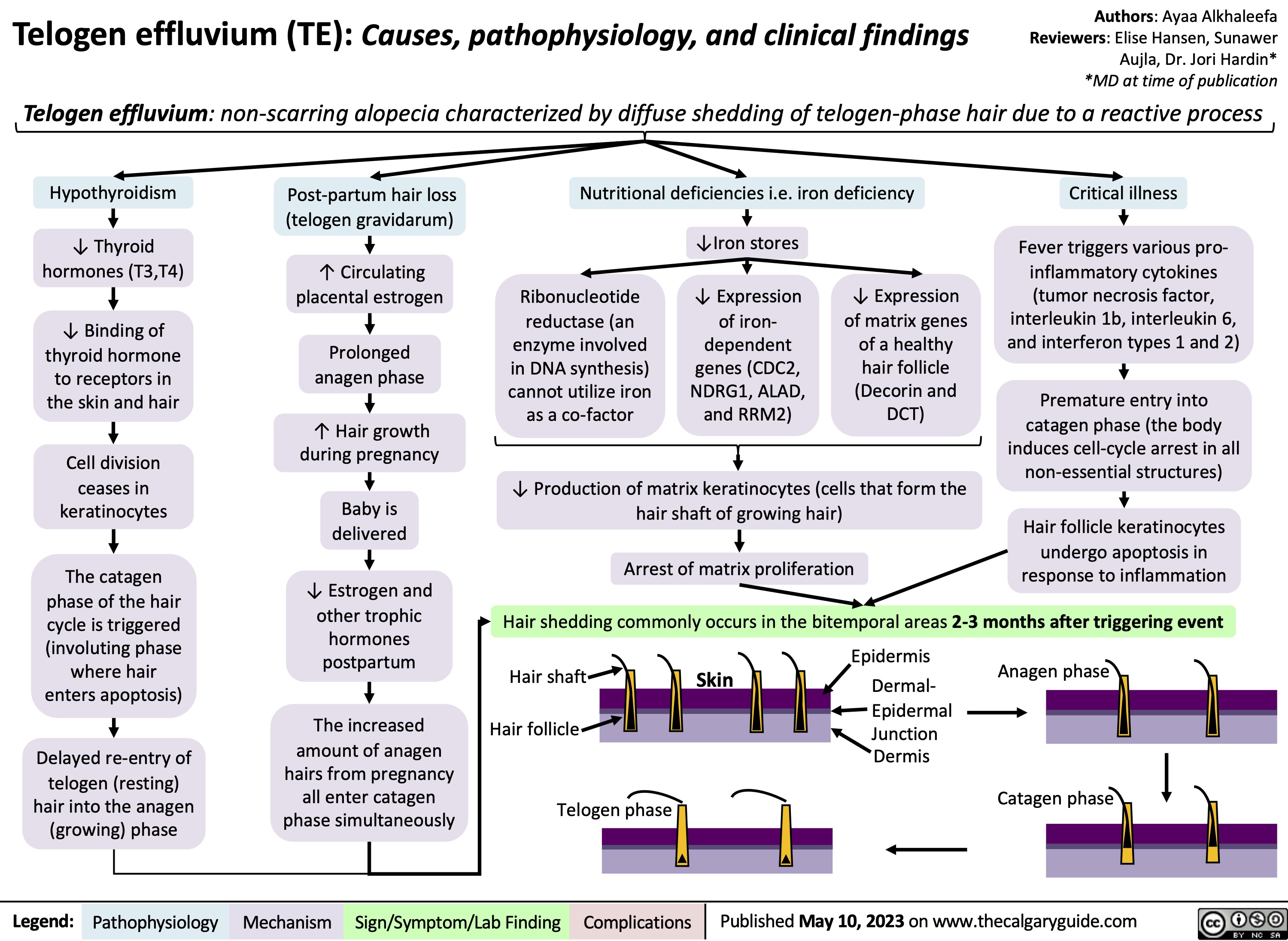
Telogen Effluvium
Menopause
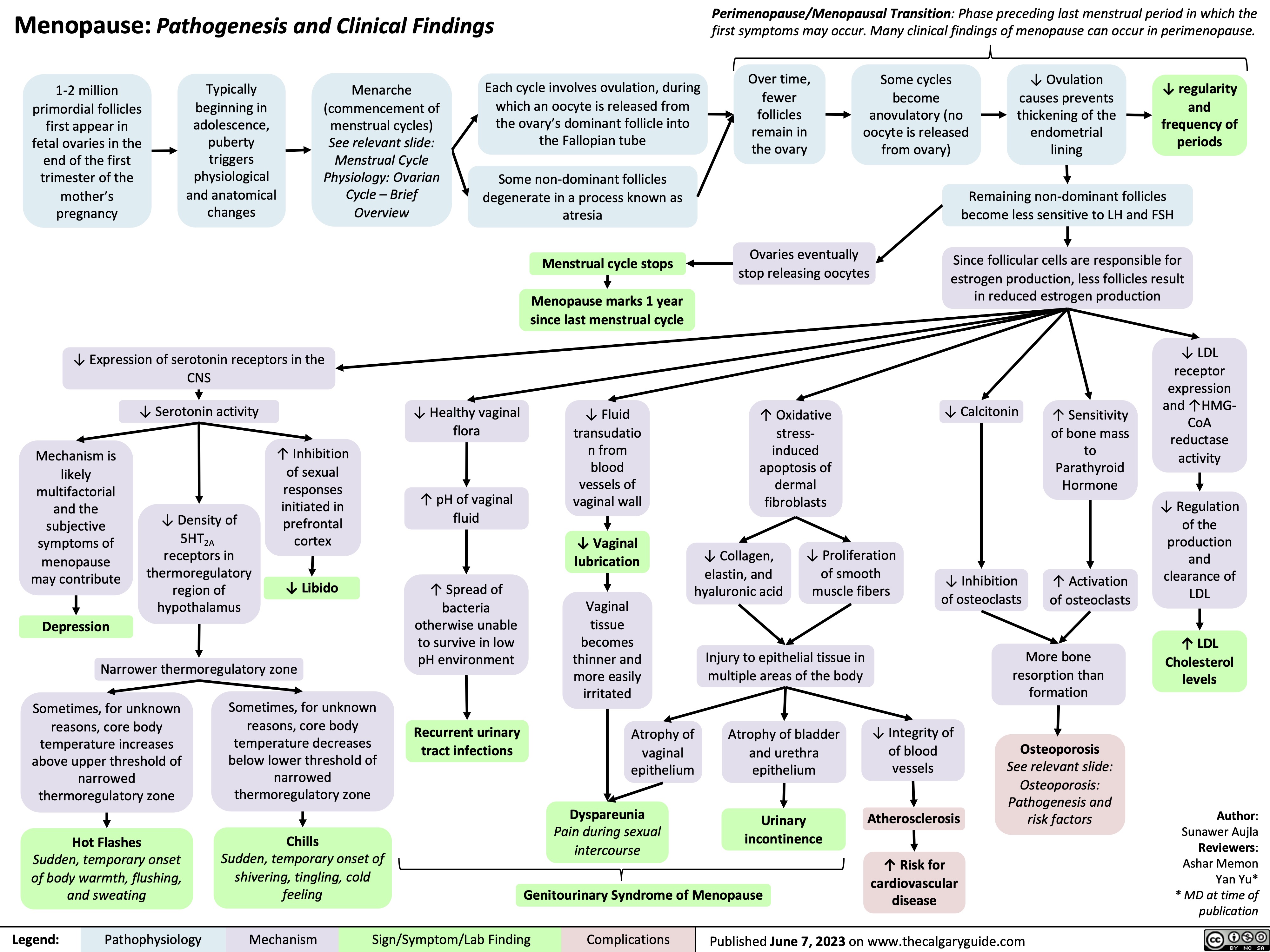
Rotator Cuff Disease
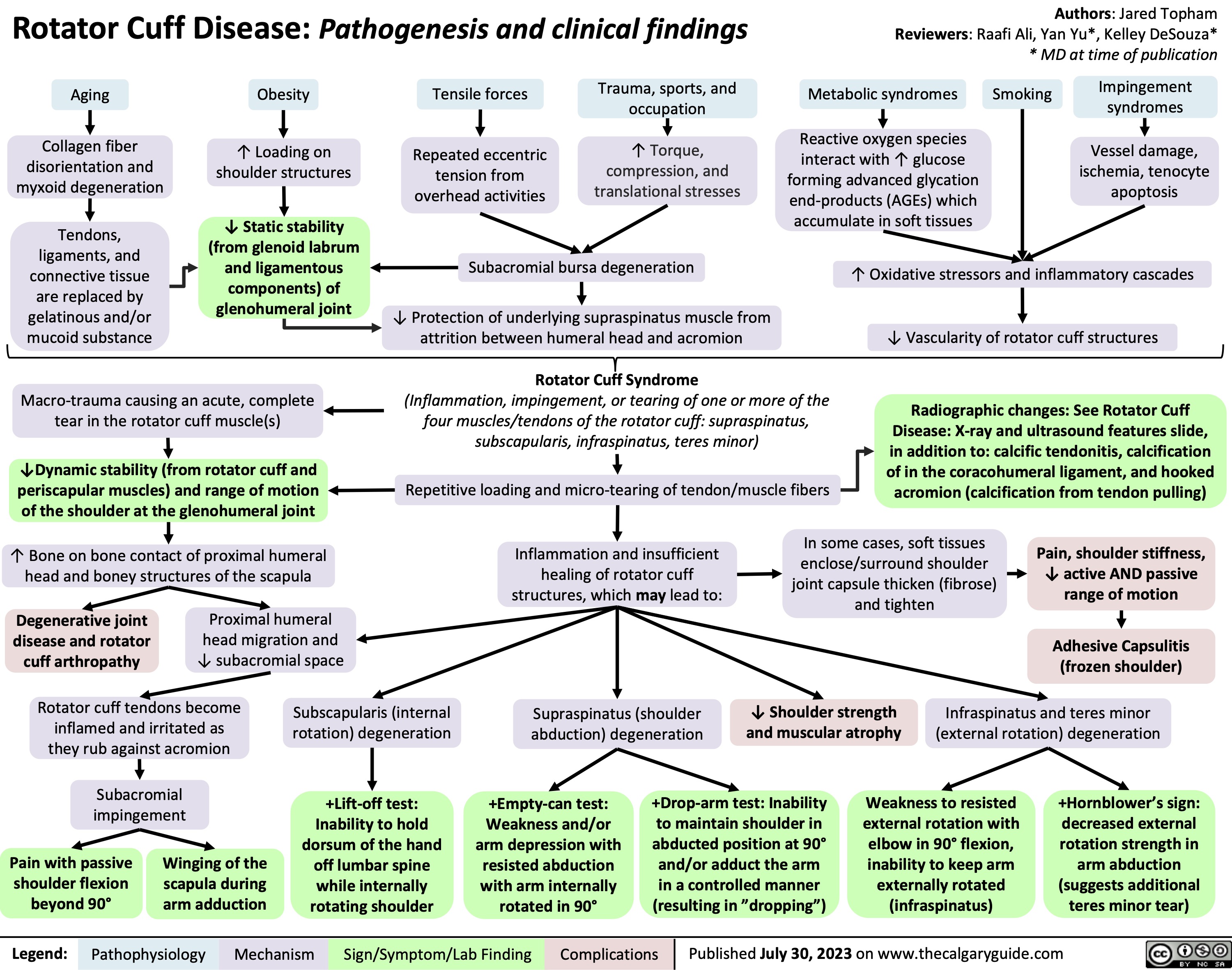
Turner Syndrome Pathogenesis and Clinical Findings
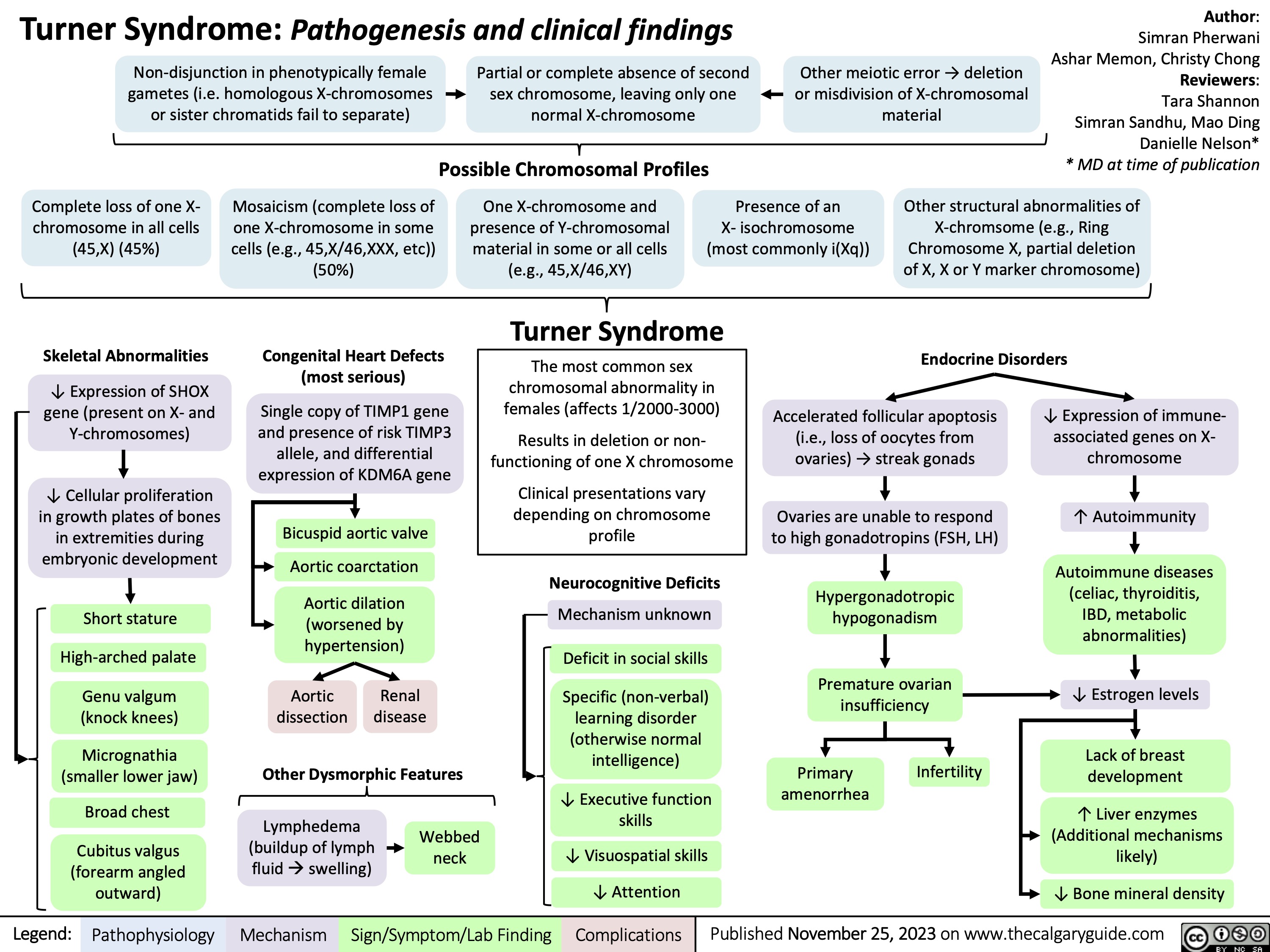
Non-Alcoholic Fatty Liver Disease
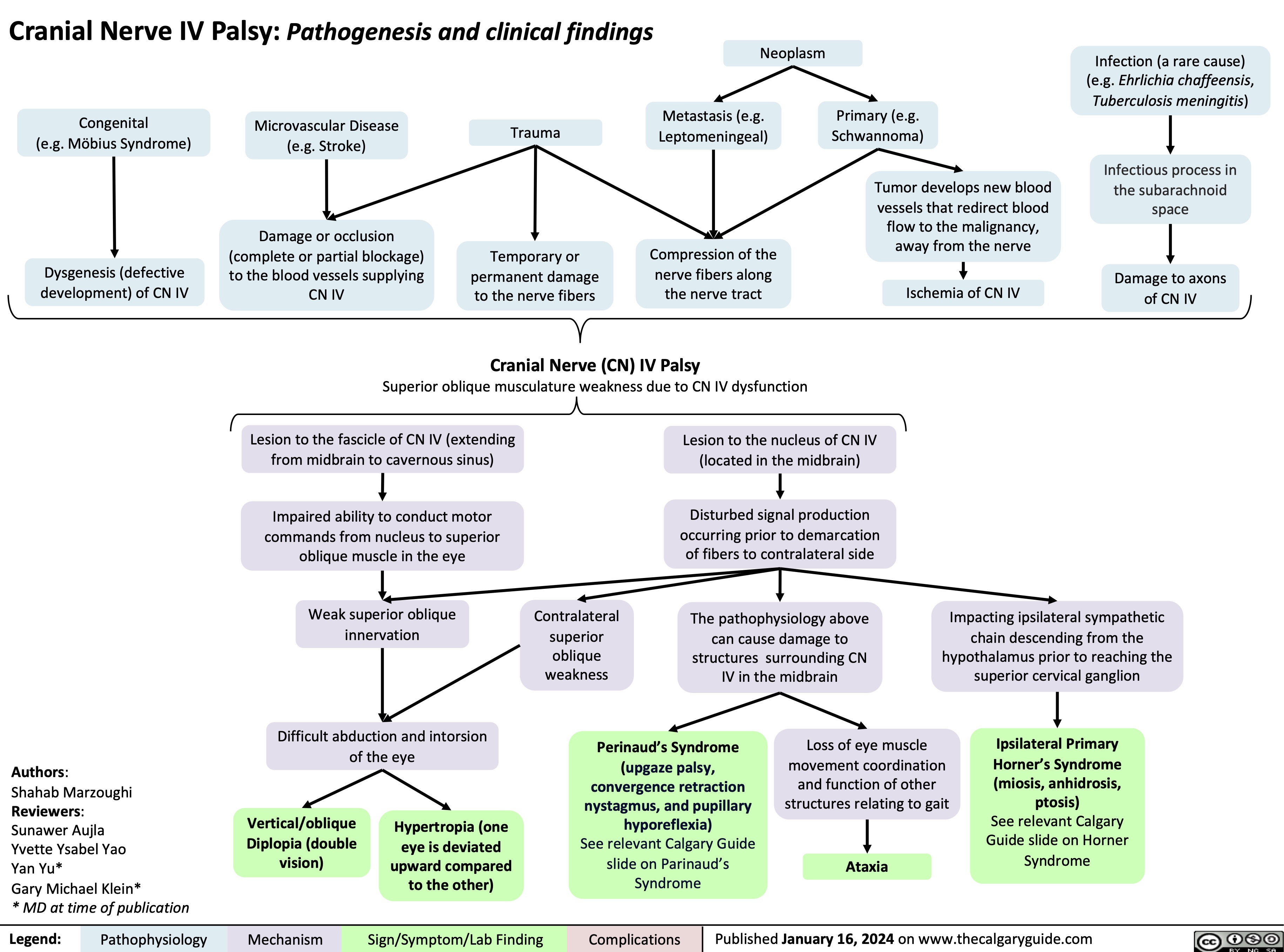
Cranial Nerve IV Palsy
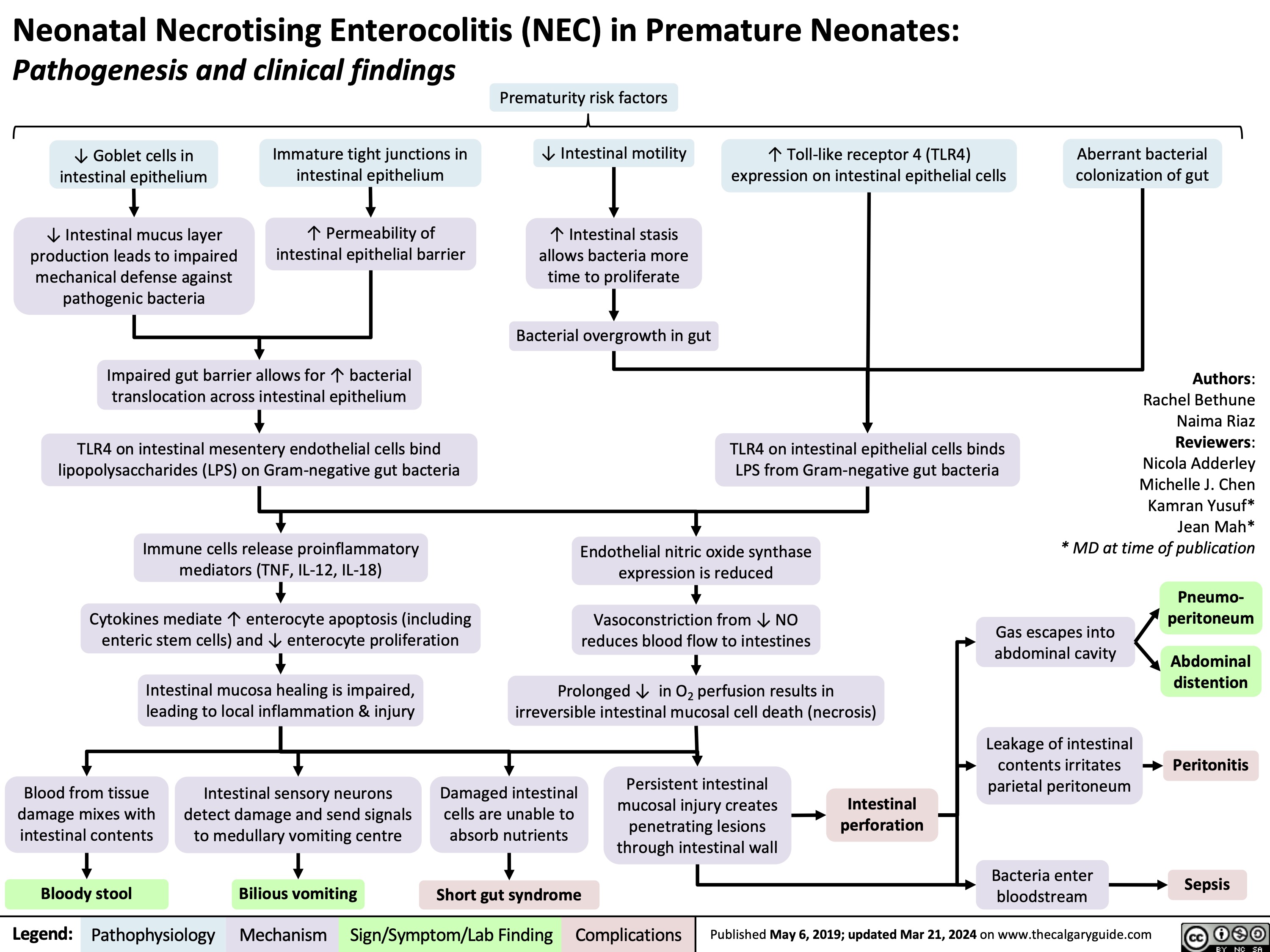
Neonatal Necrotising Enterocolitis in Premature Neonates
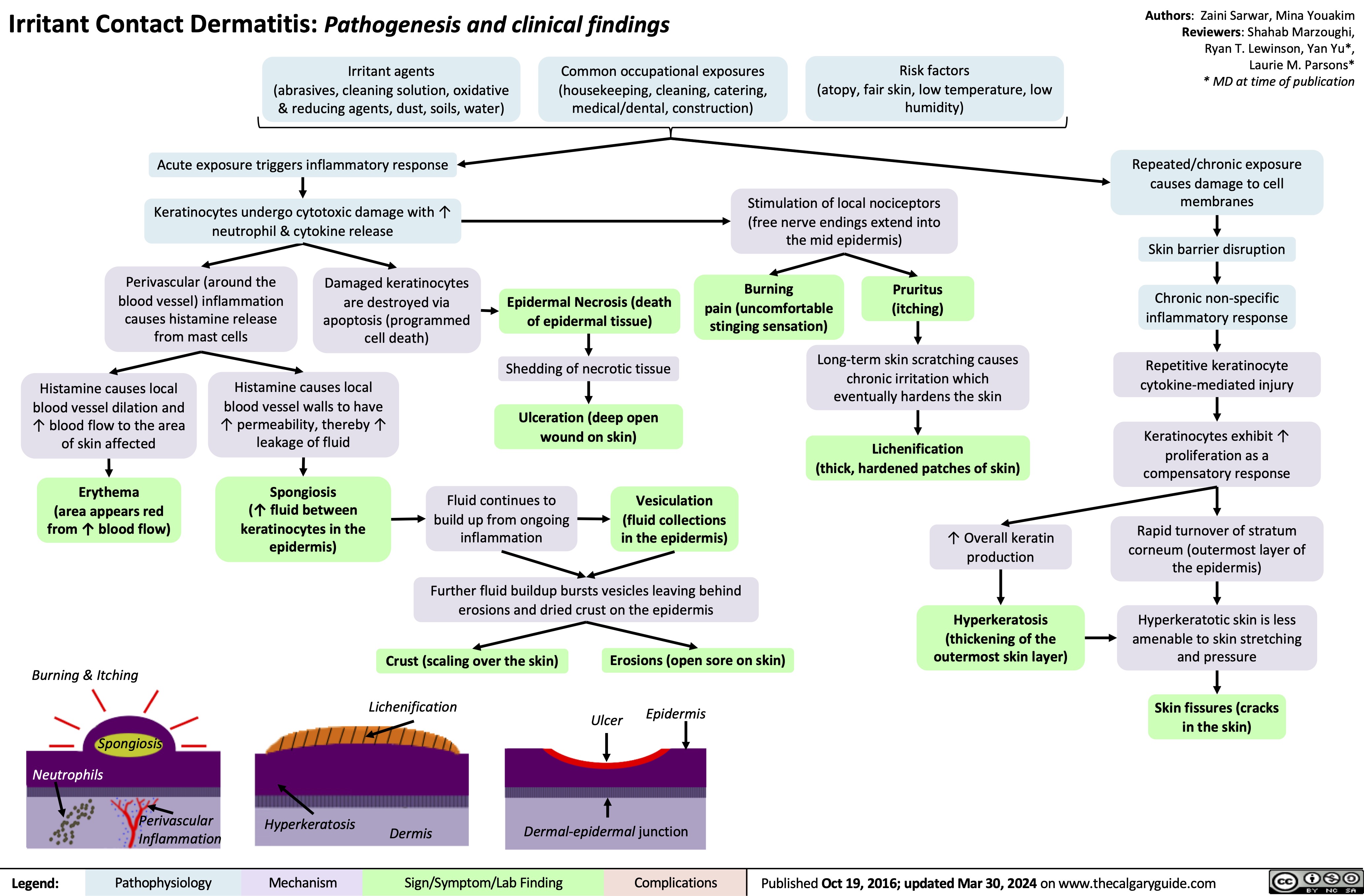
Irritant Contact Dermatitis Pathogenesis and Clinical Findings
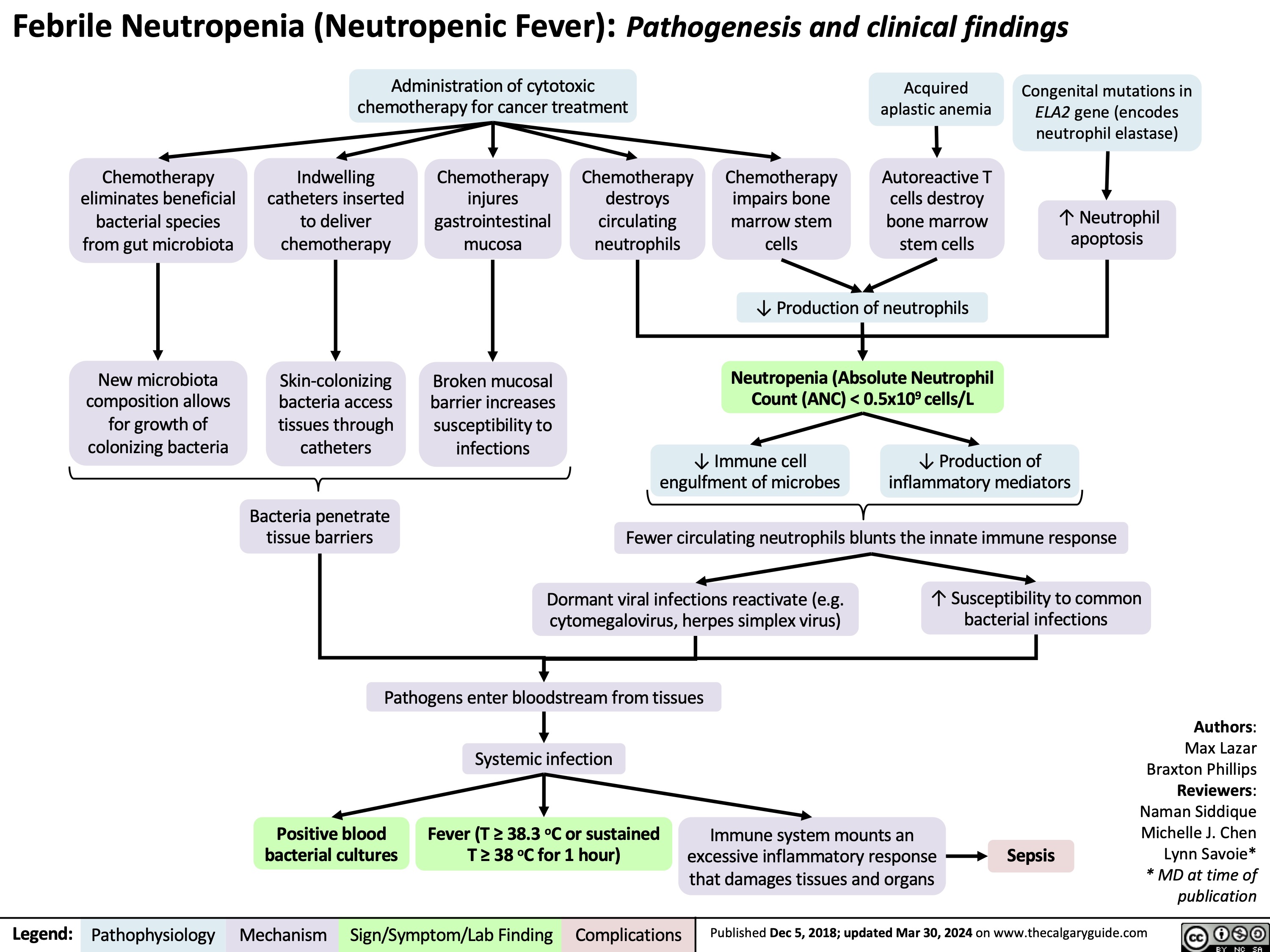
Febrile Neutropenia Pathogenesis and clinical findings
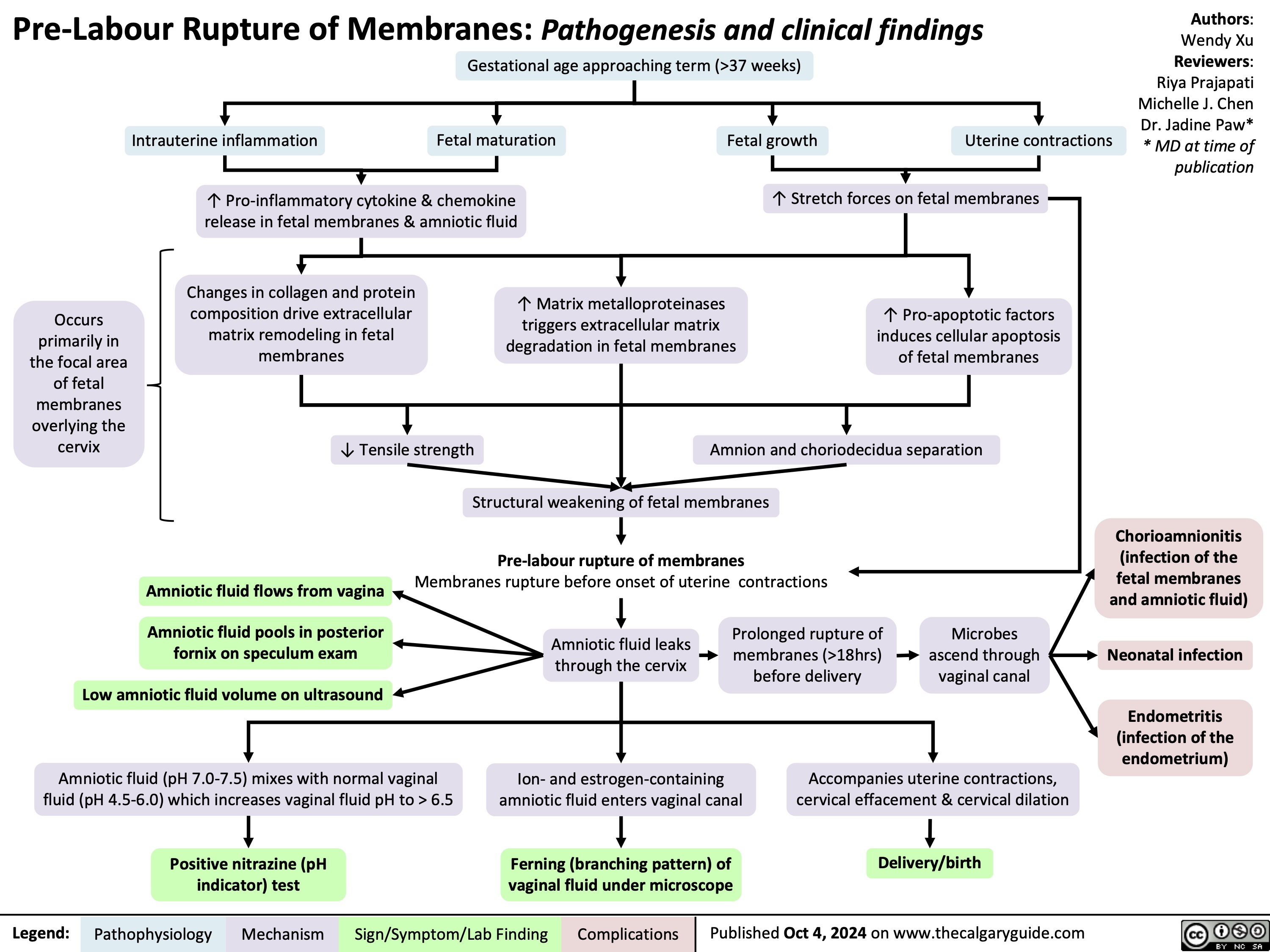
Spontaneous Rupture of Membranes
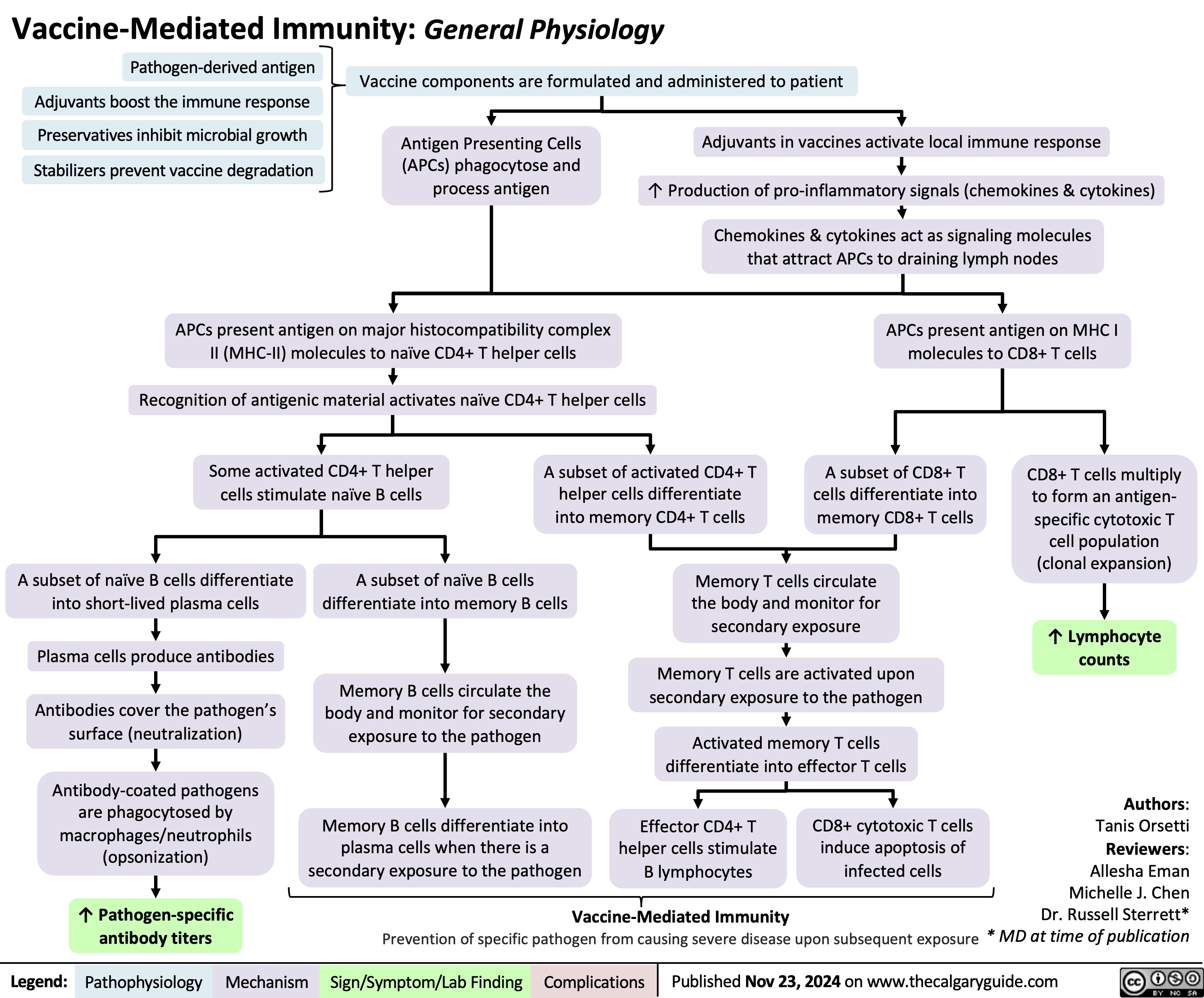
Vaccine-Mediated Immunity General Physiology
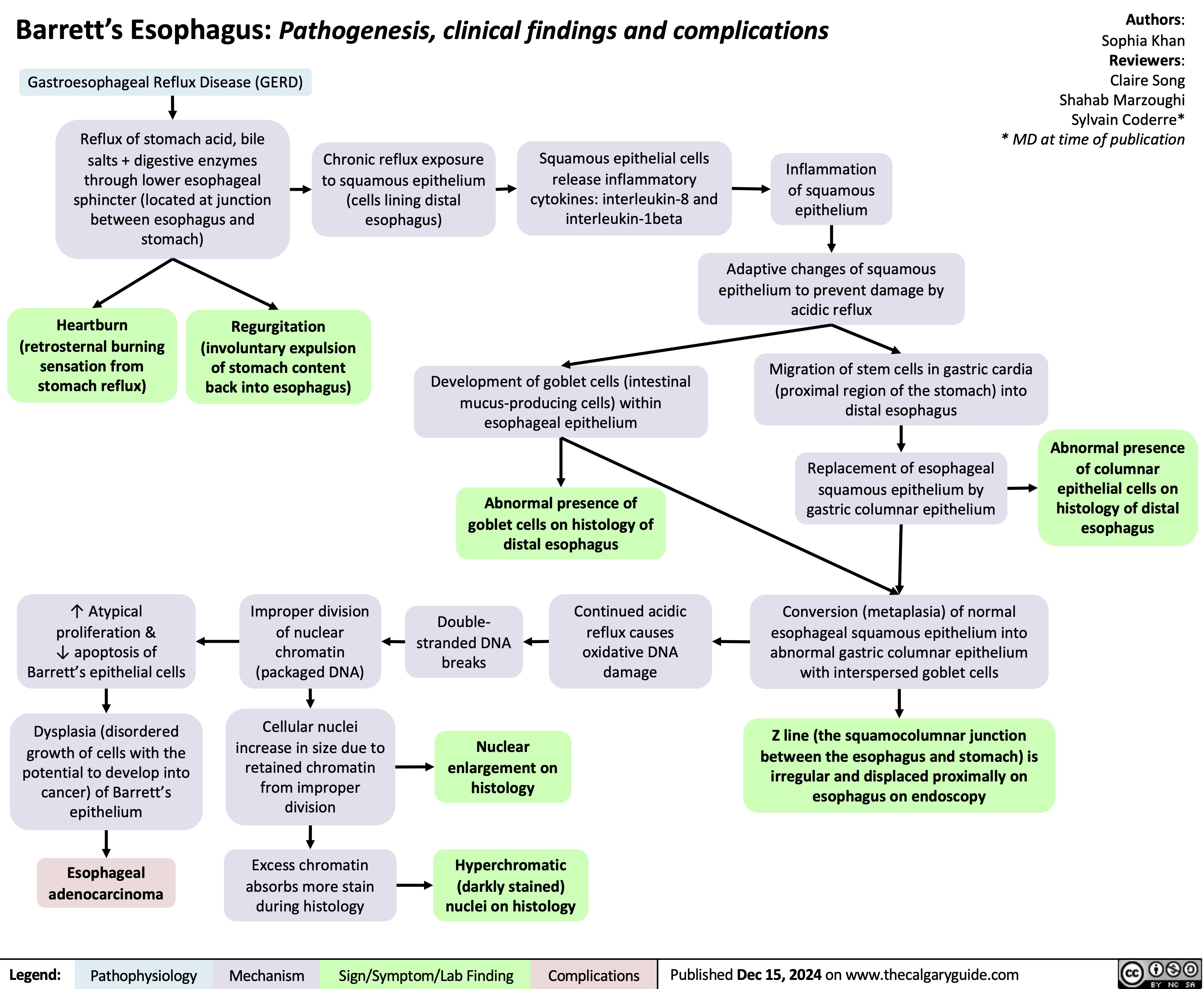
Barretts Esophagus
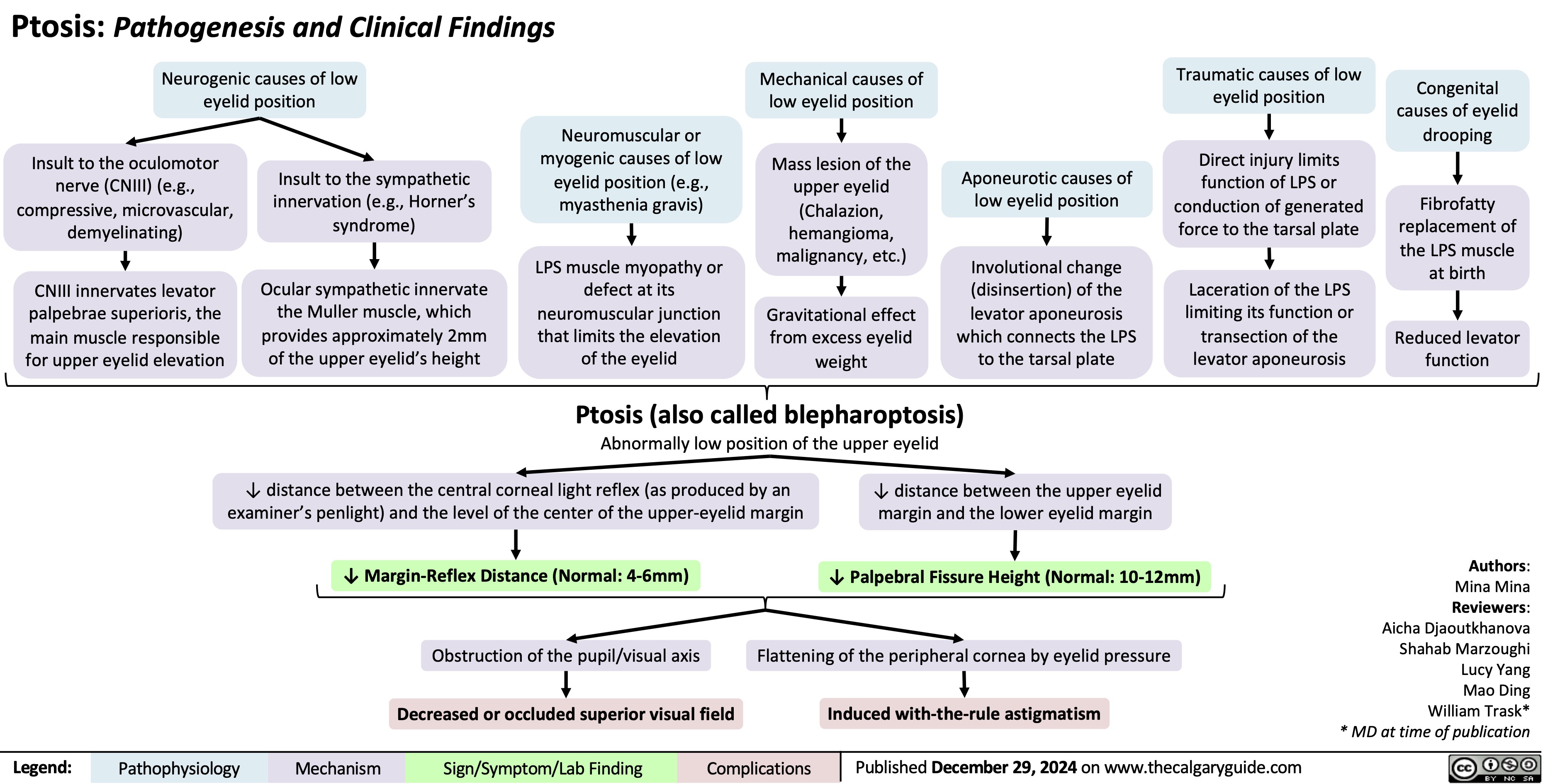
Ptosis
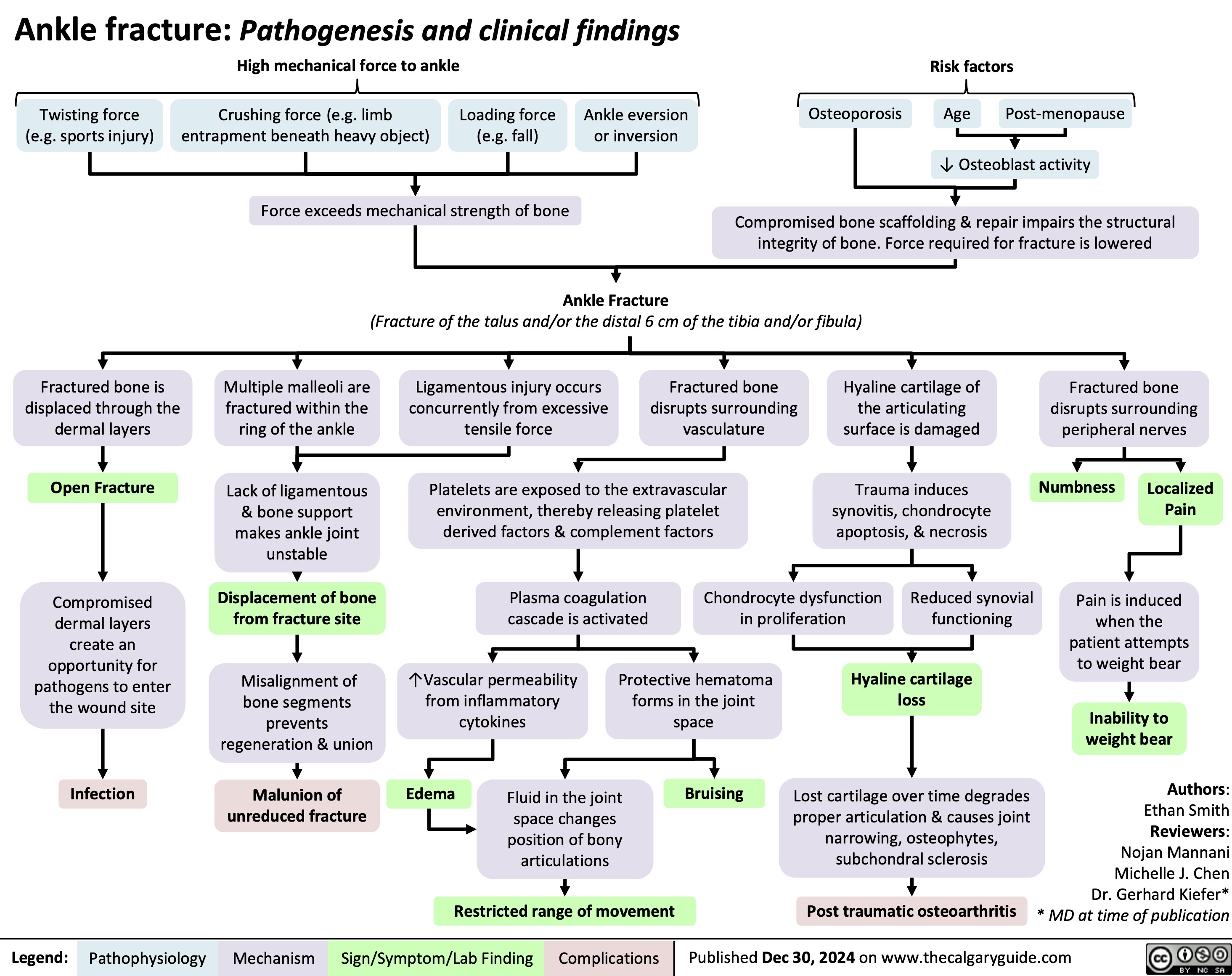
Ankle Fracture
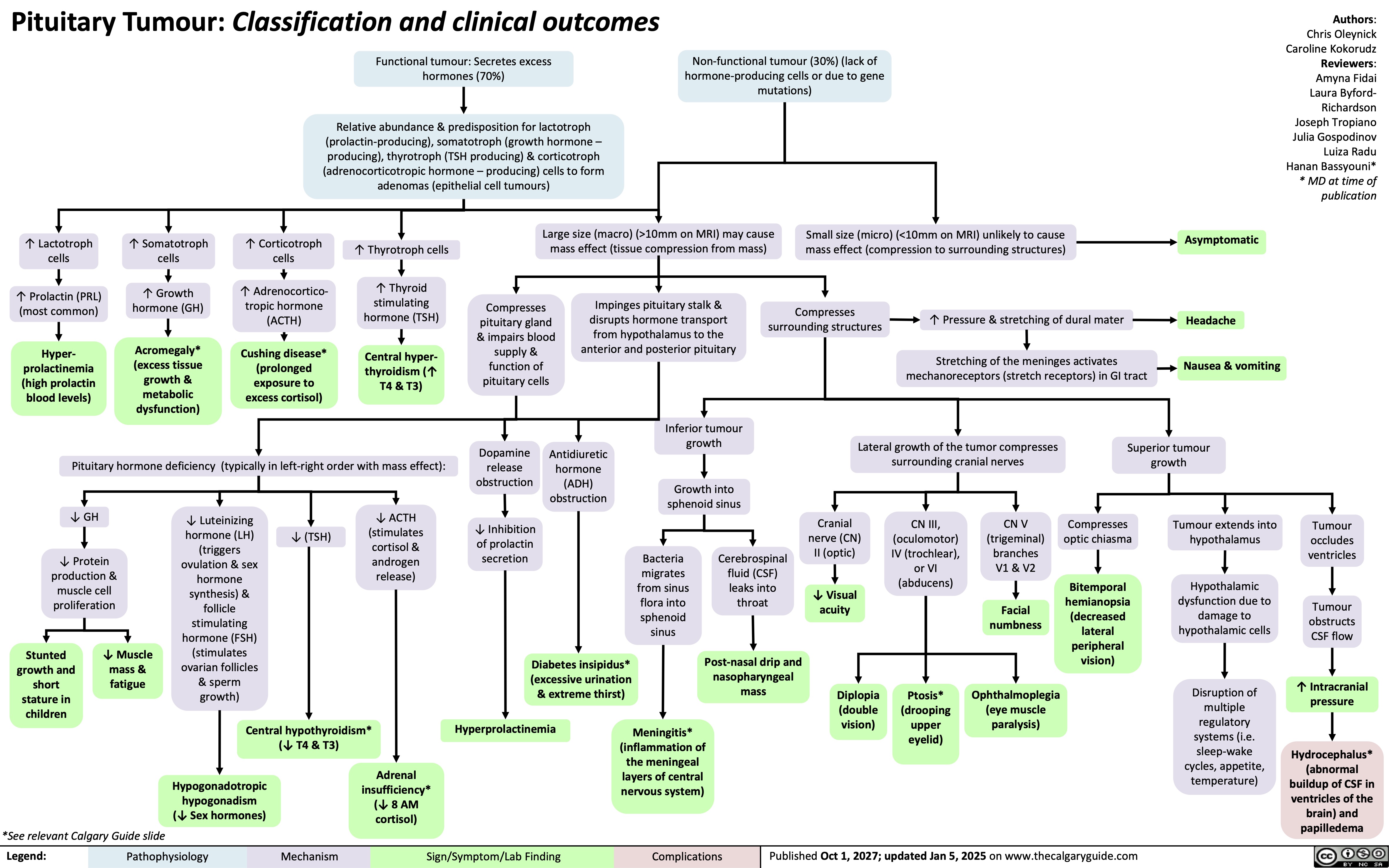
Pituitary Tumour Classification and Clinical Outcomes
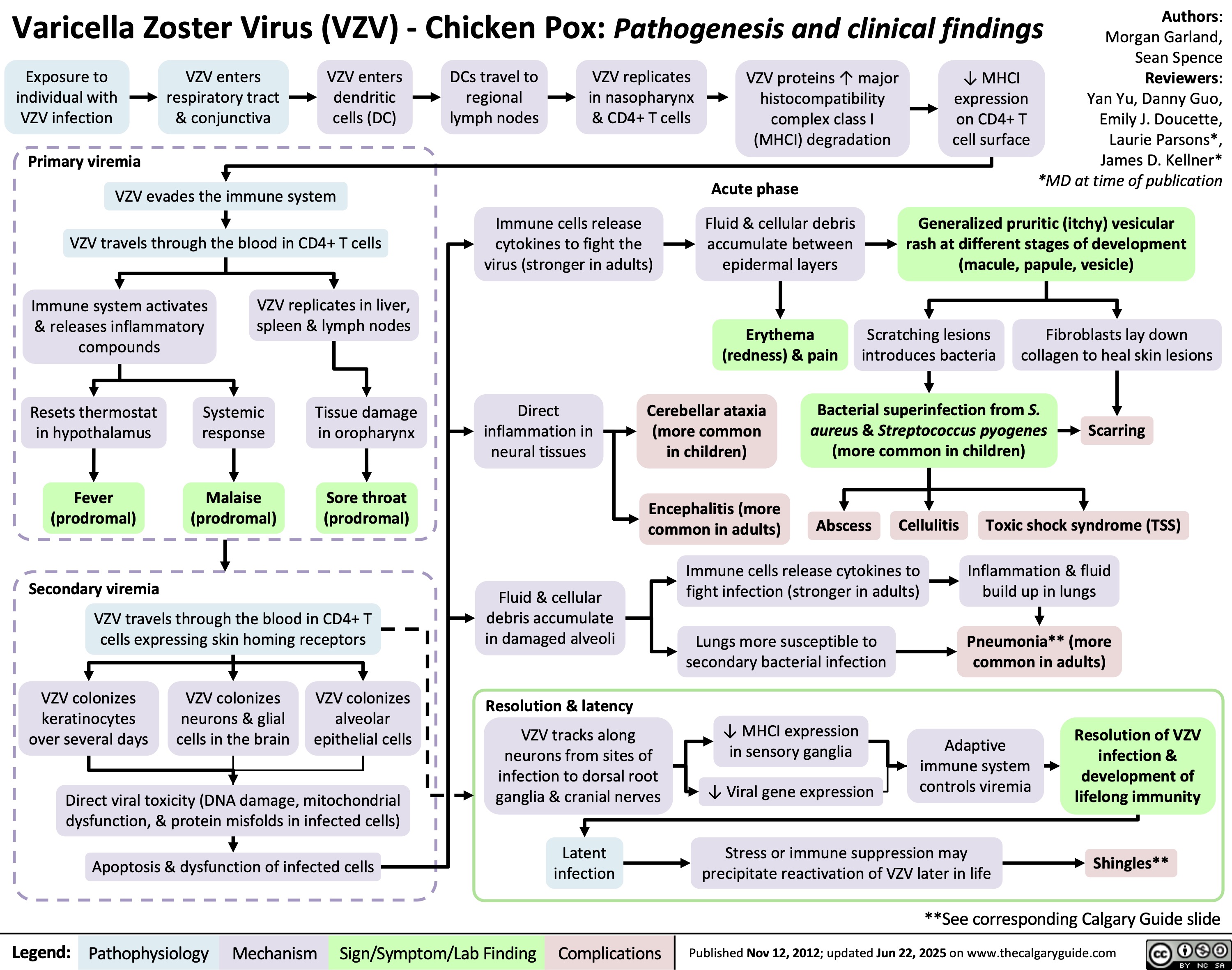
Varicella Zoster Virus
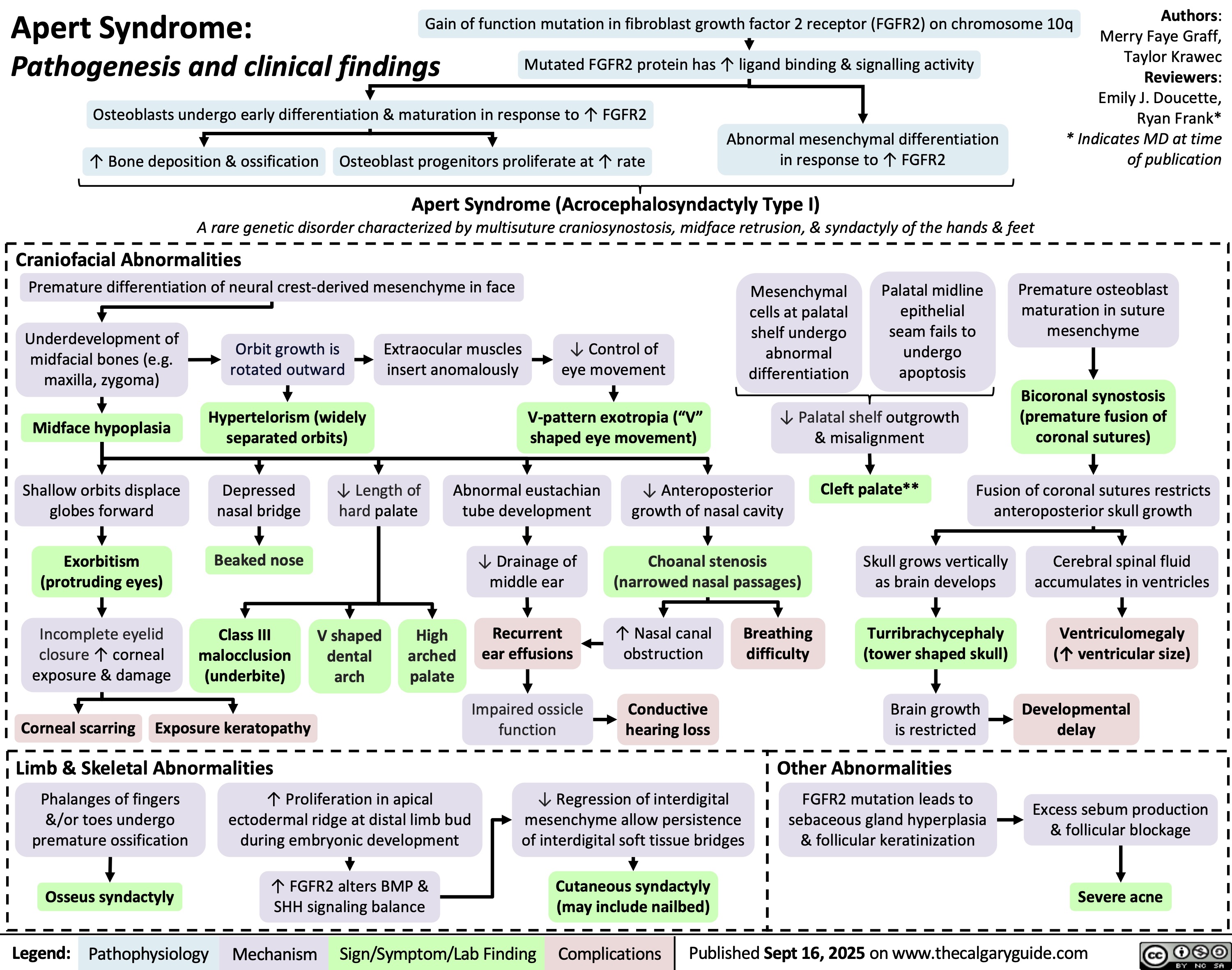
Apert Syndrome
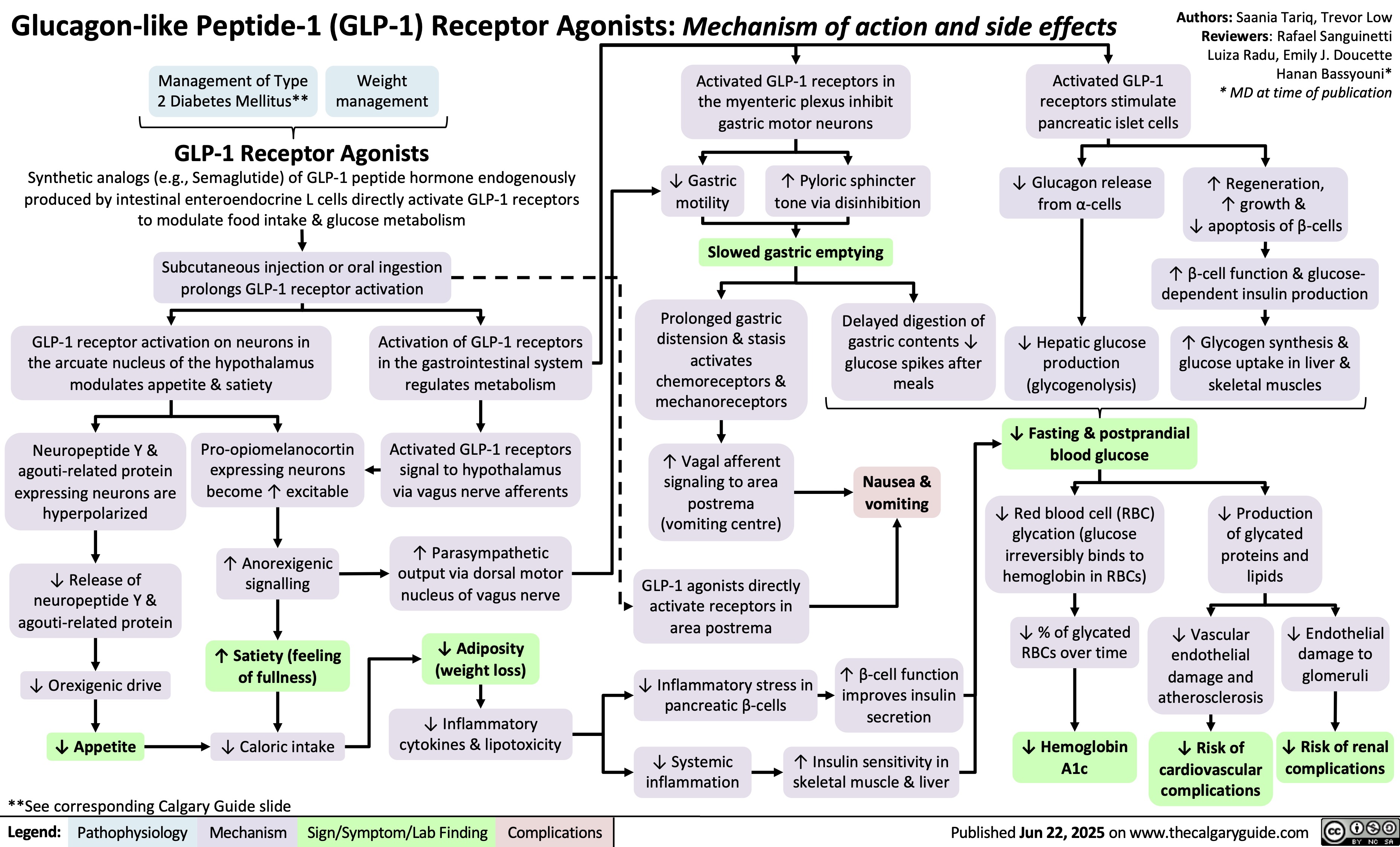
GLP-1 Receptor Agonists
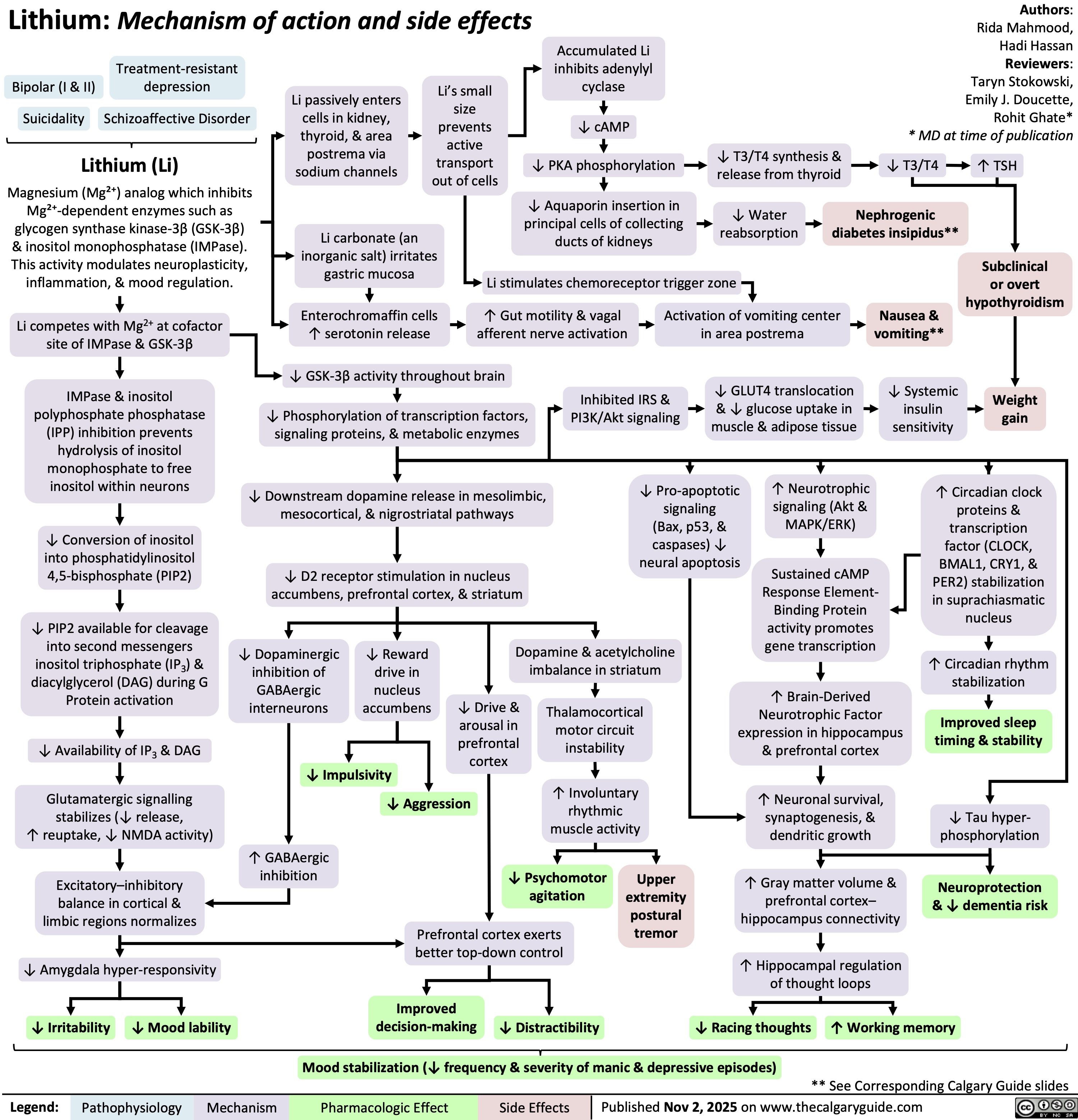
Lithium
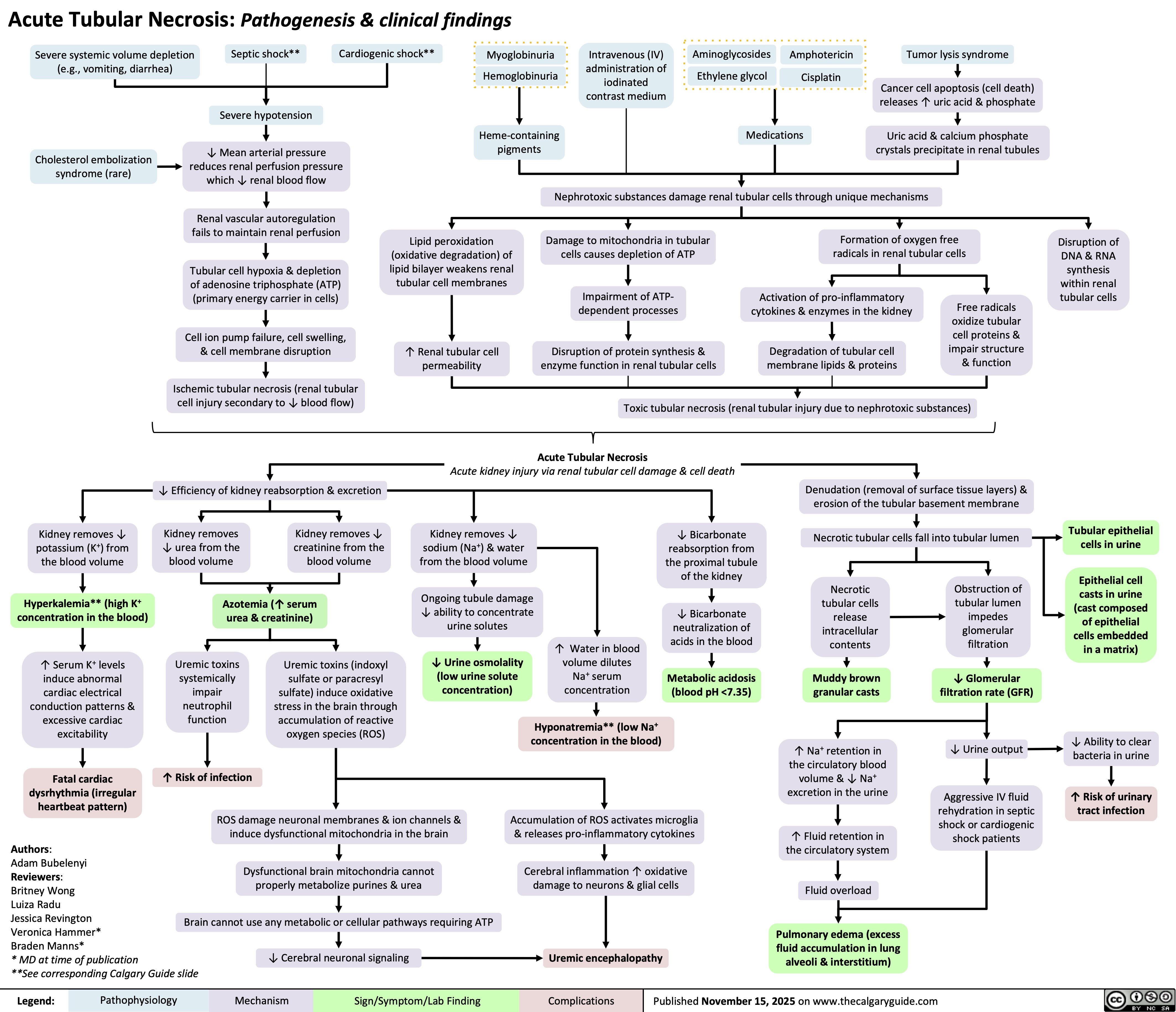
Acute Tubular Necrosis
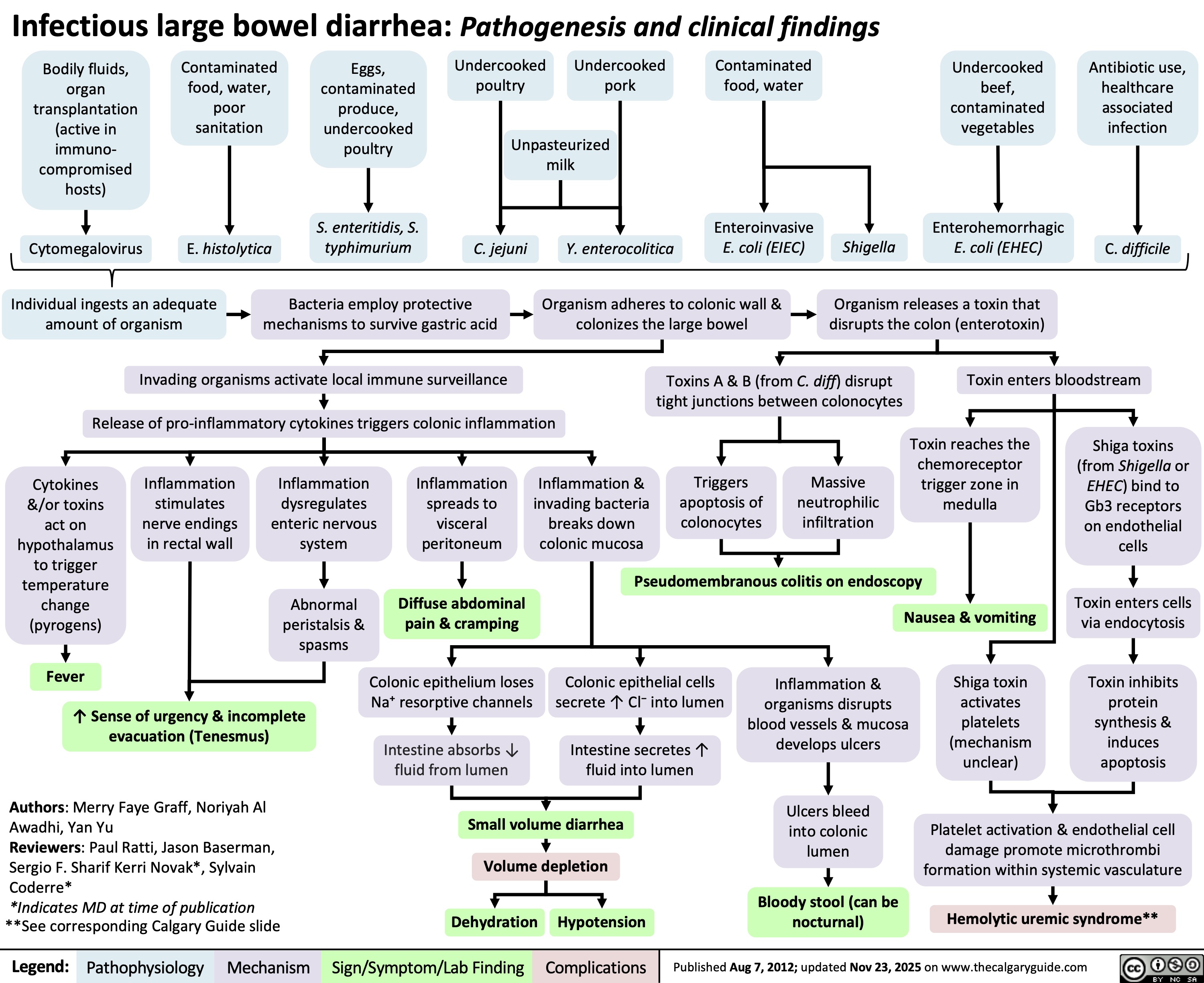
Infectious Large Bowel Diarrhea
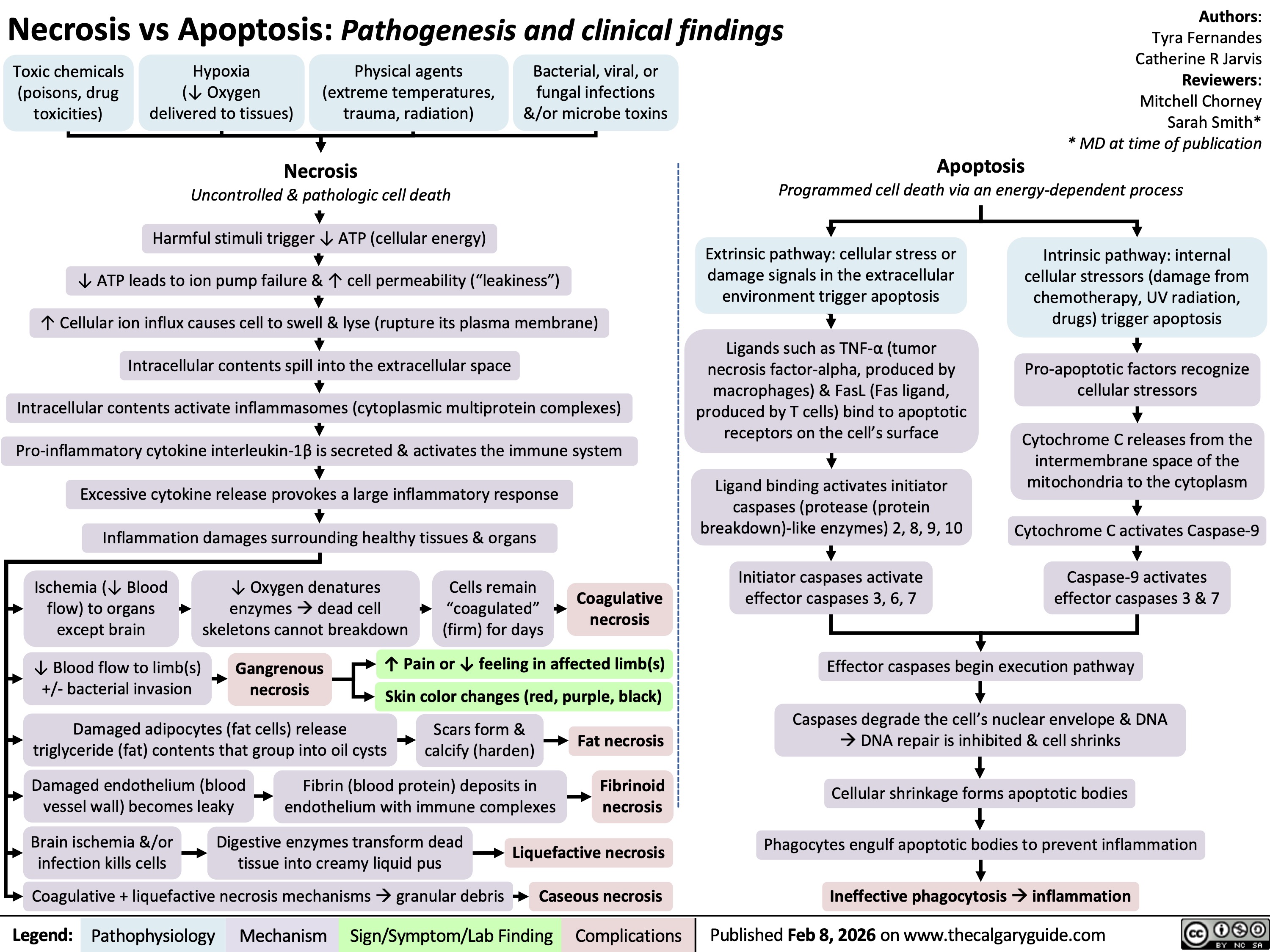
Necrosis versus Apoptosis