SEARCH RESULTS FOR: Menopause
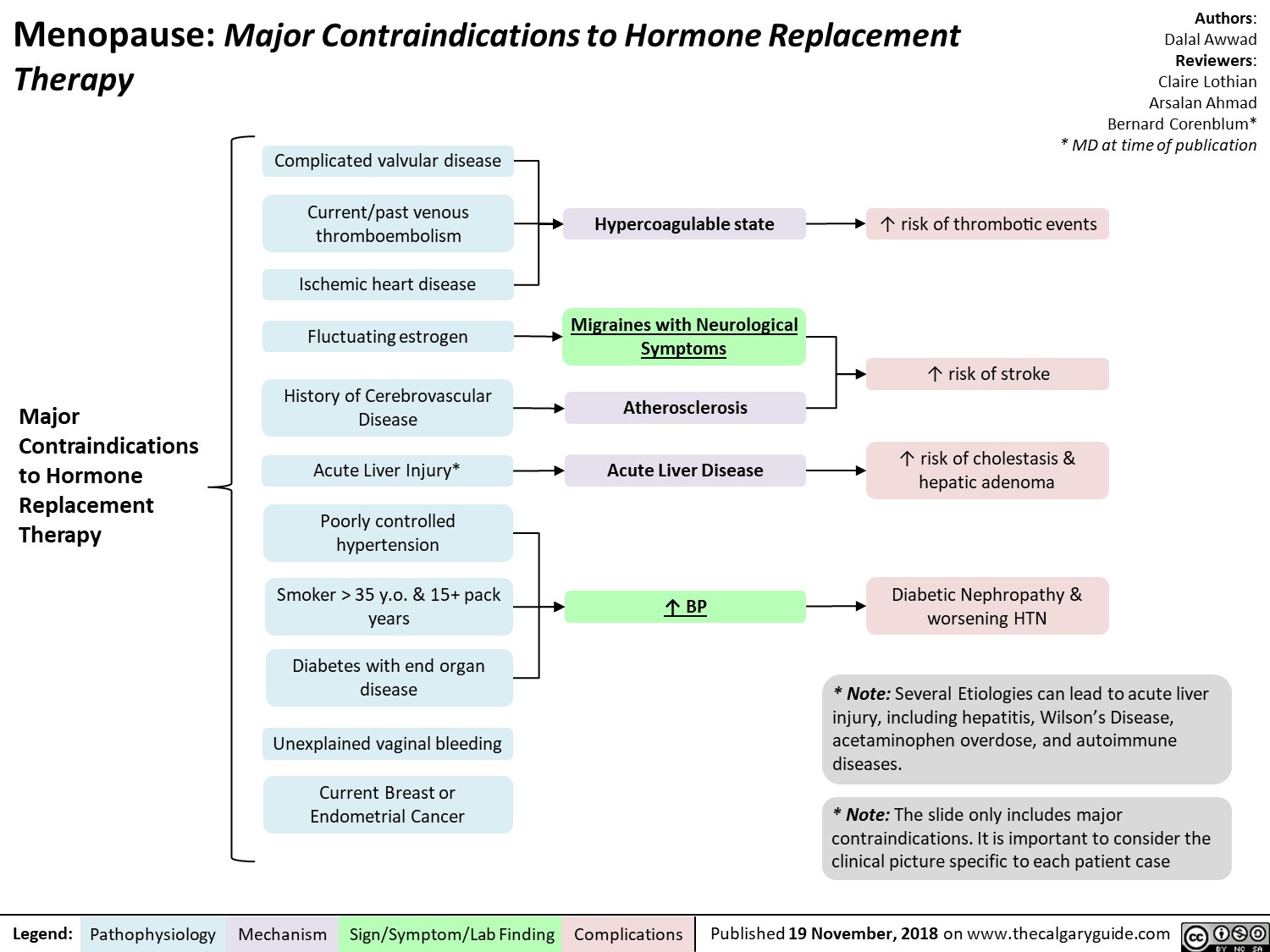
Menopause contraindications to hormone replacement therapy
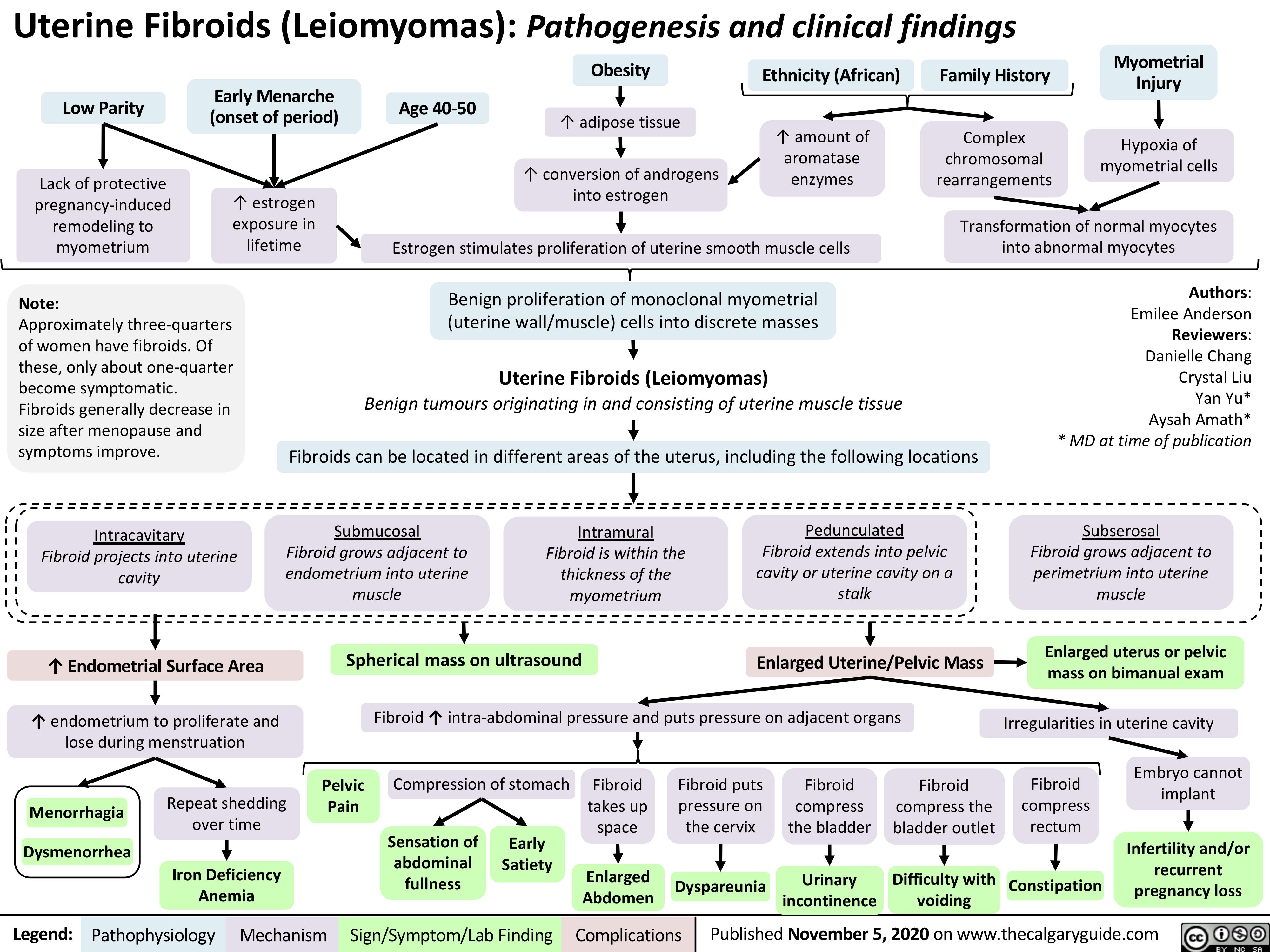
Uterine-Fibroids
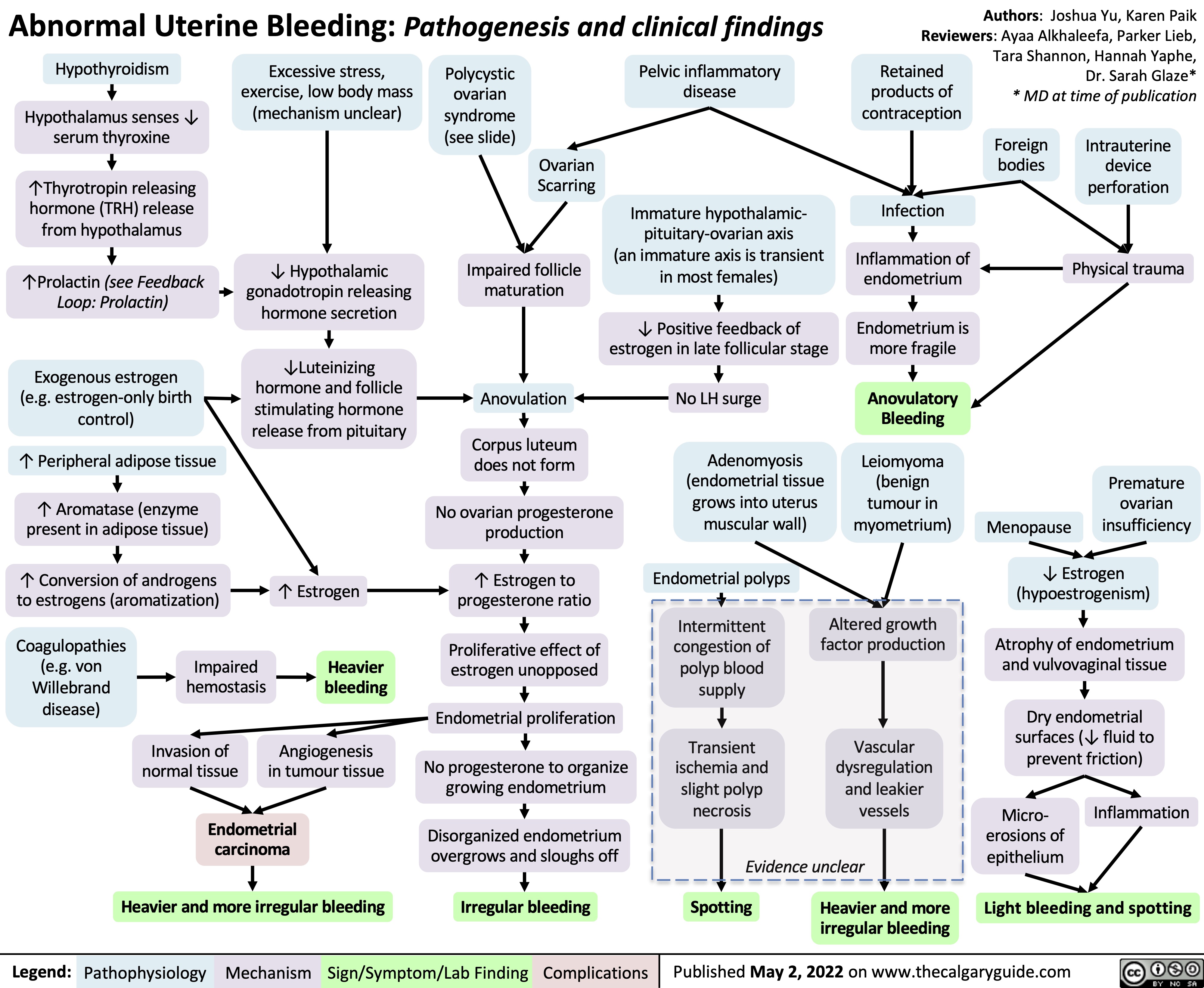
abnormal-uterine-bleeding-aub-pathogenesis-and-clinical-findings
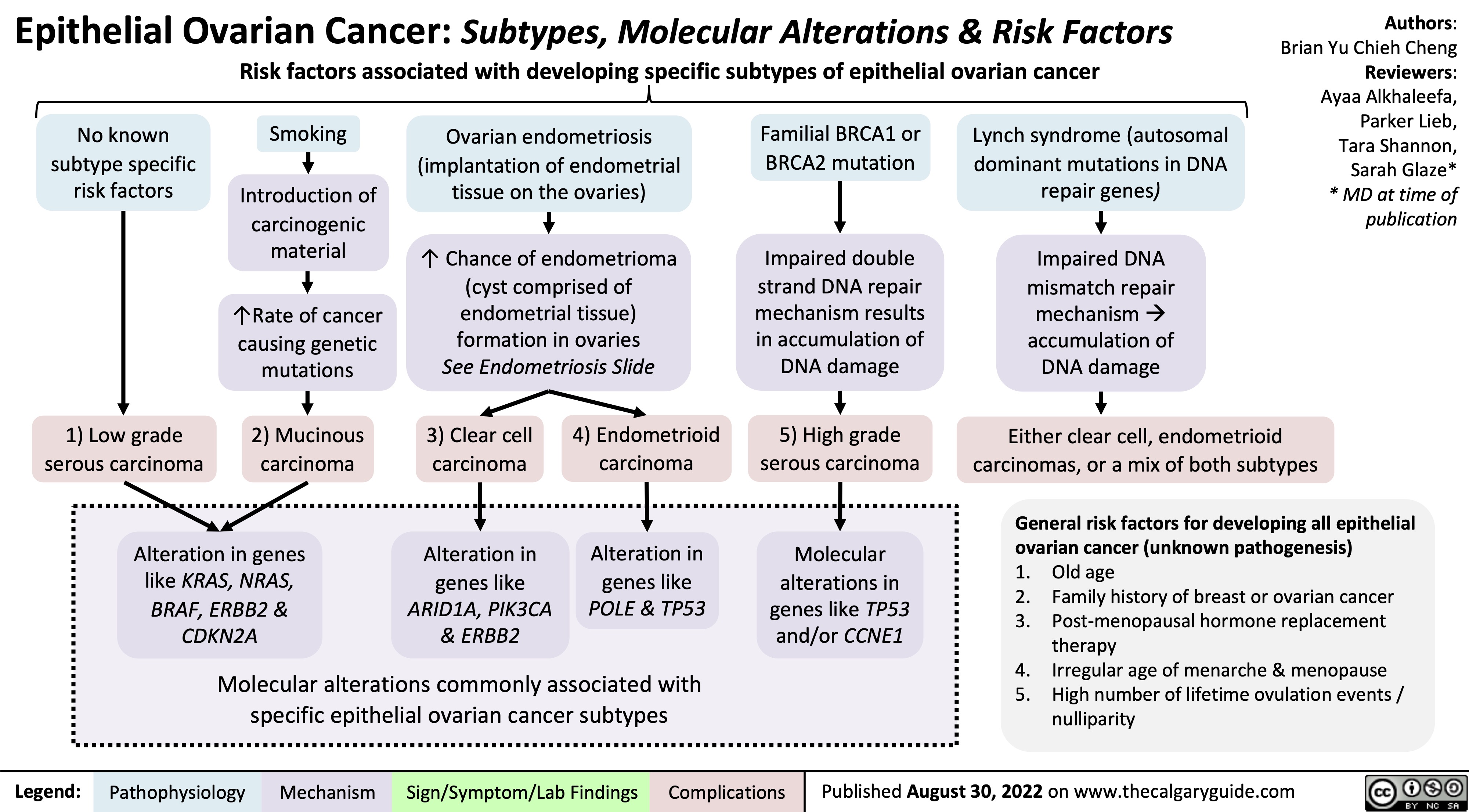
epithelial-ovarian-cancer-subtypes-molecular-alterations-risk-factors
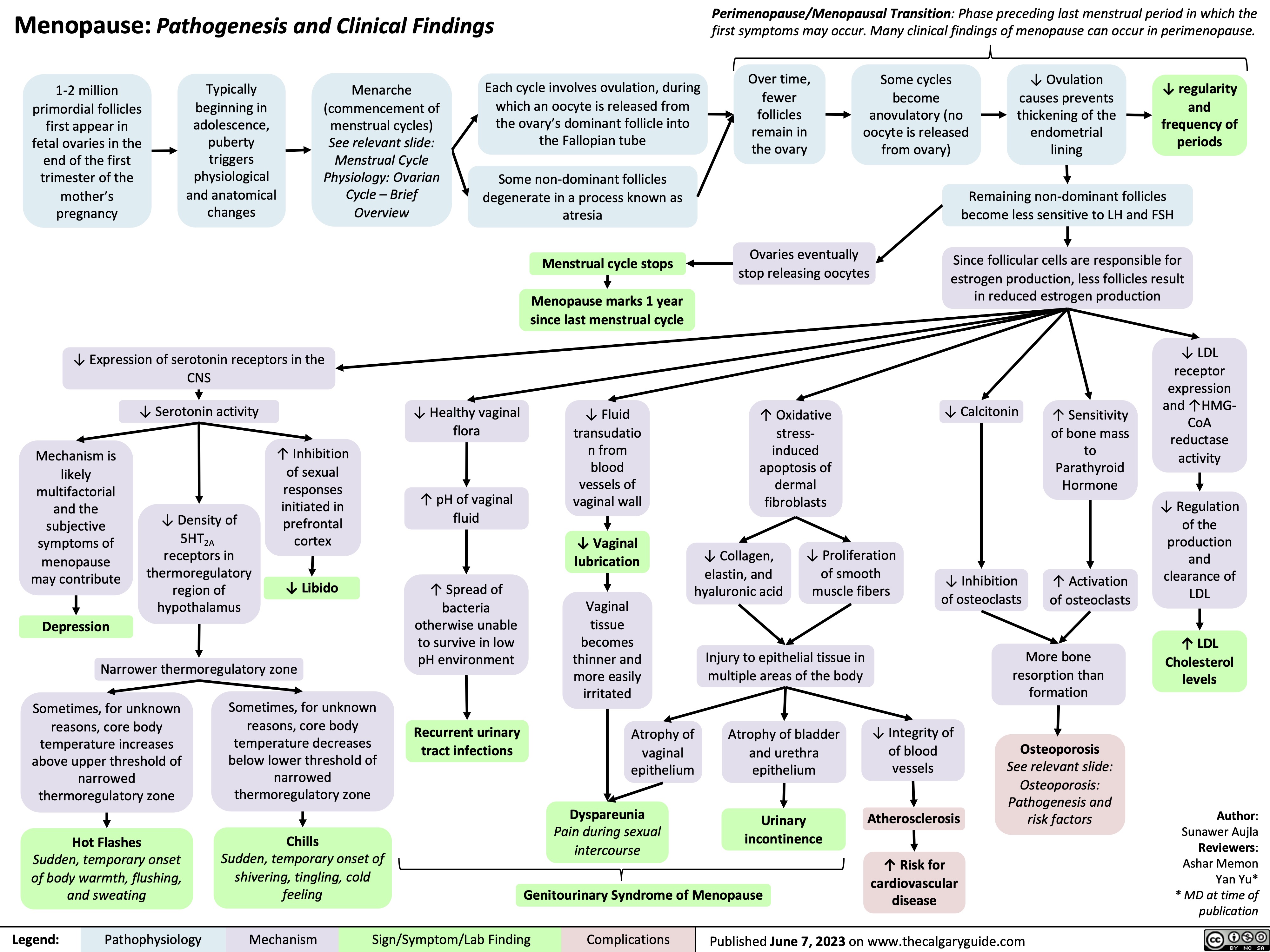
Menopause
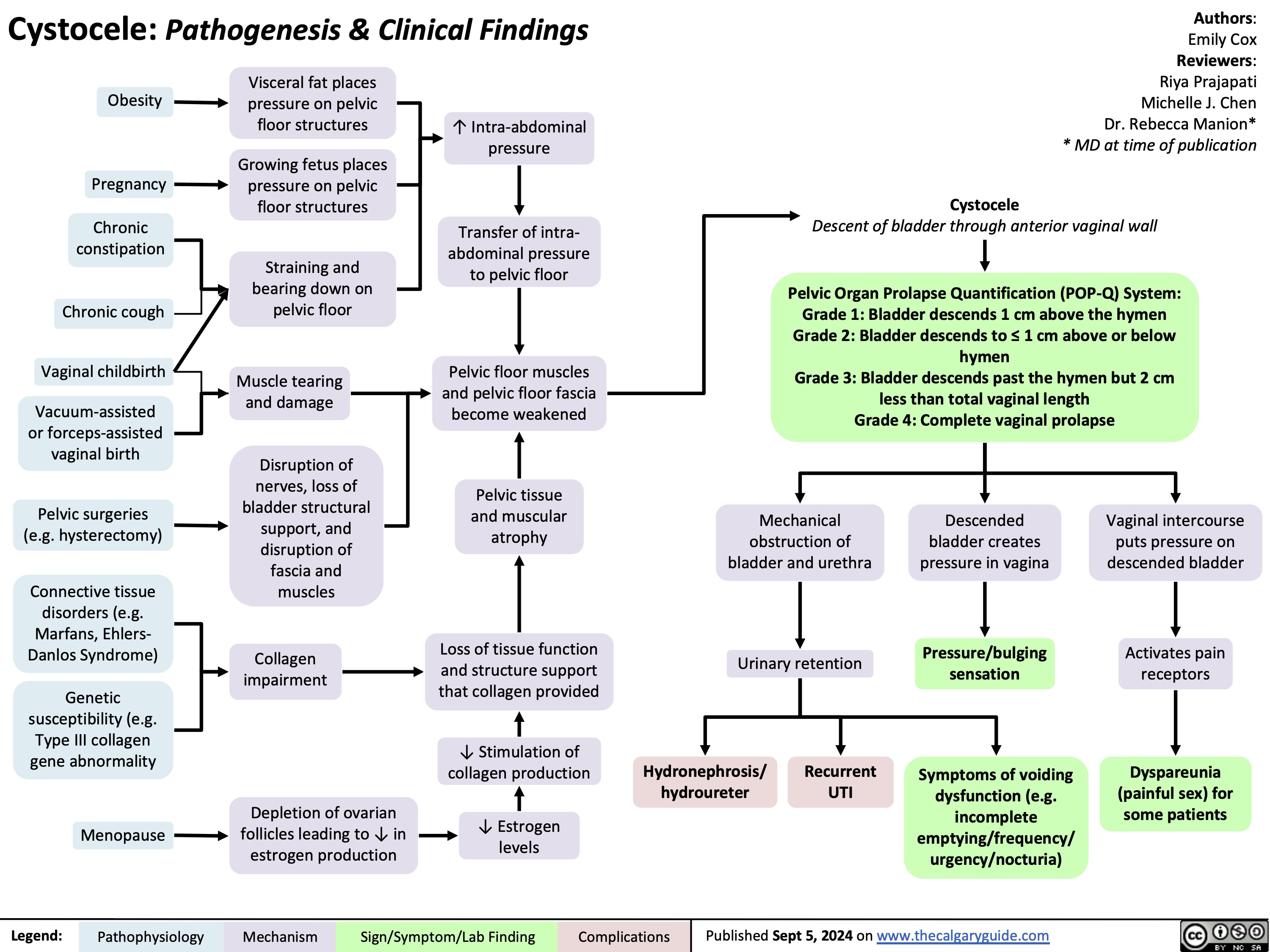
Cystocele
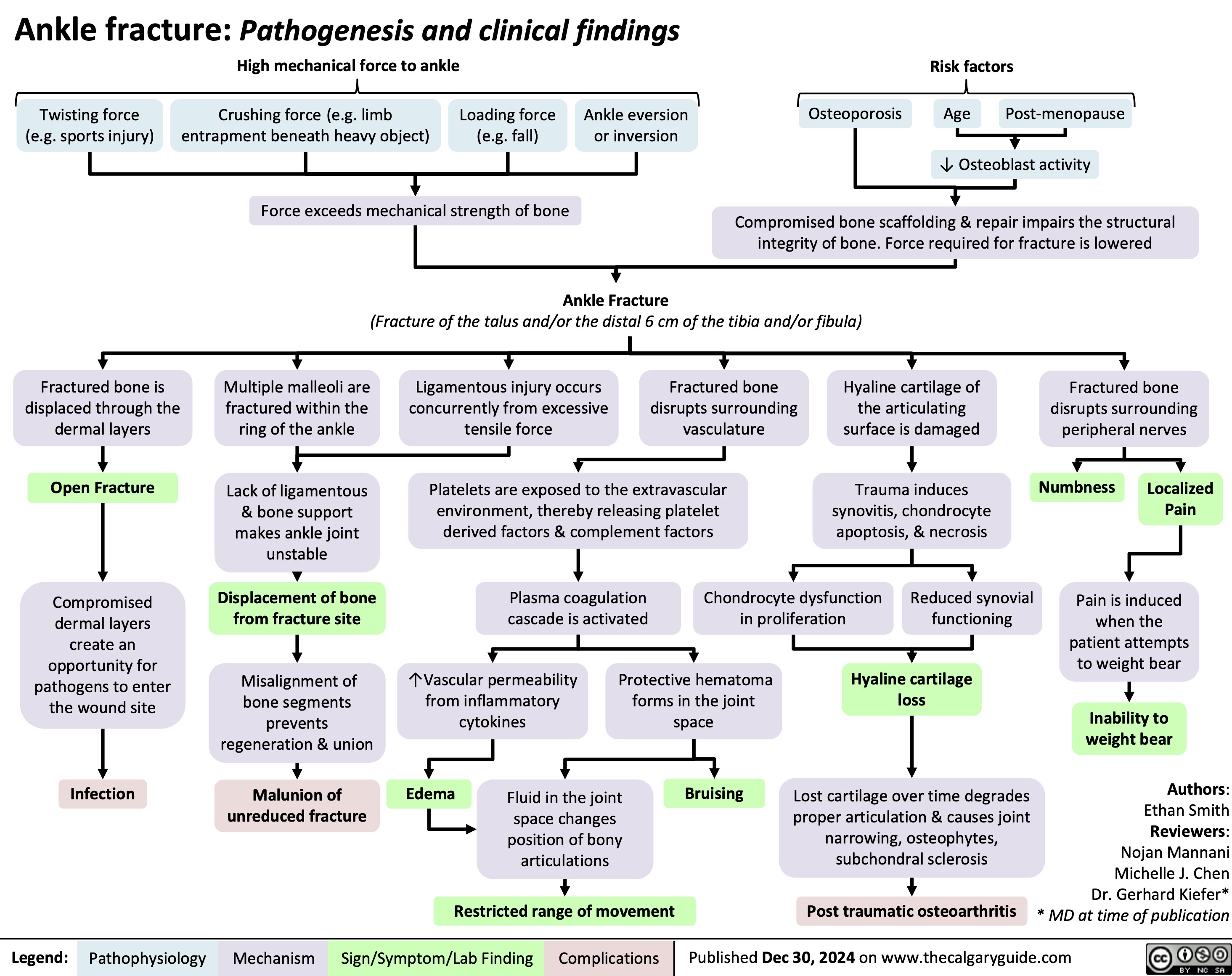
Ankle Fracture
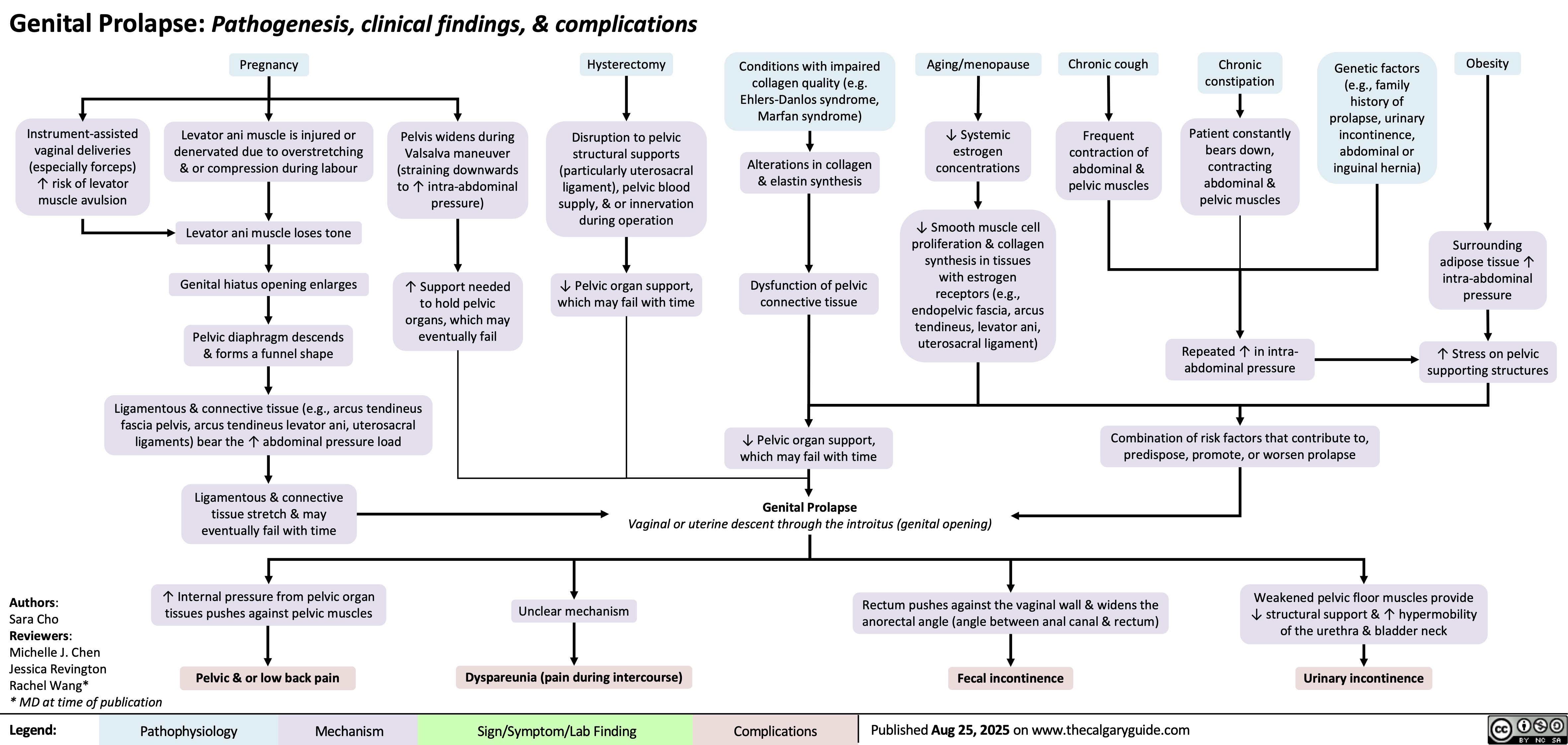
Genital Prolapse
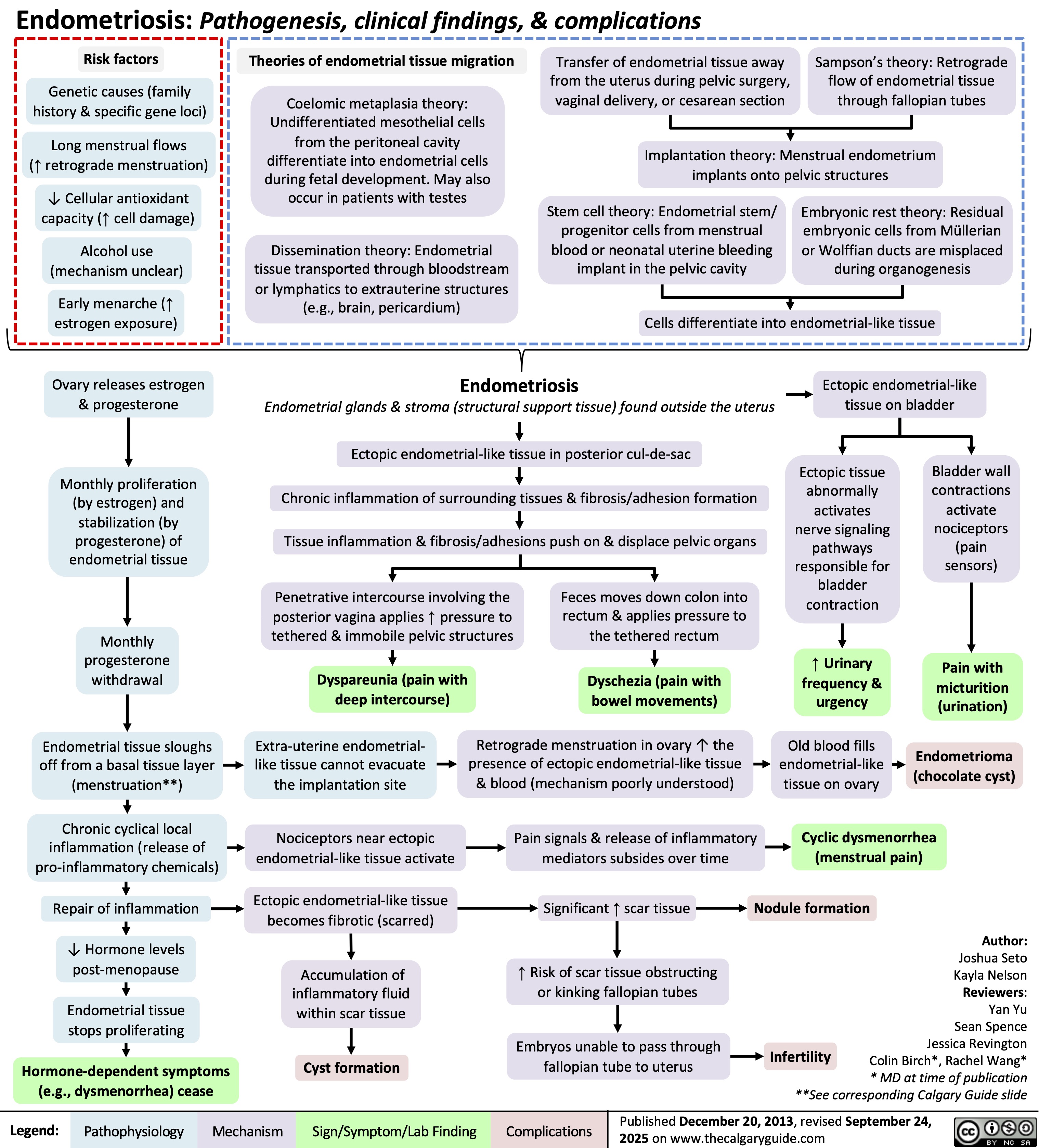
Endometriosis