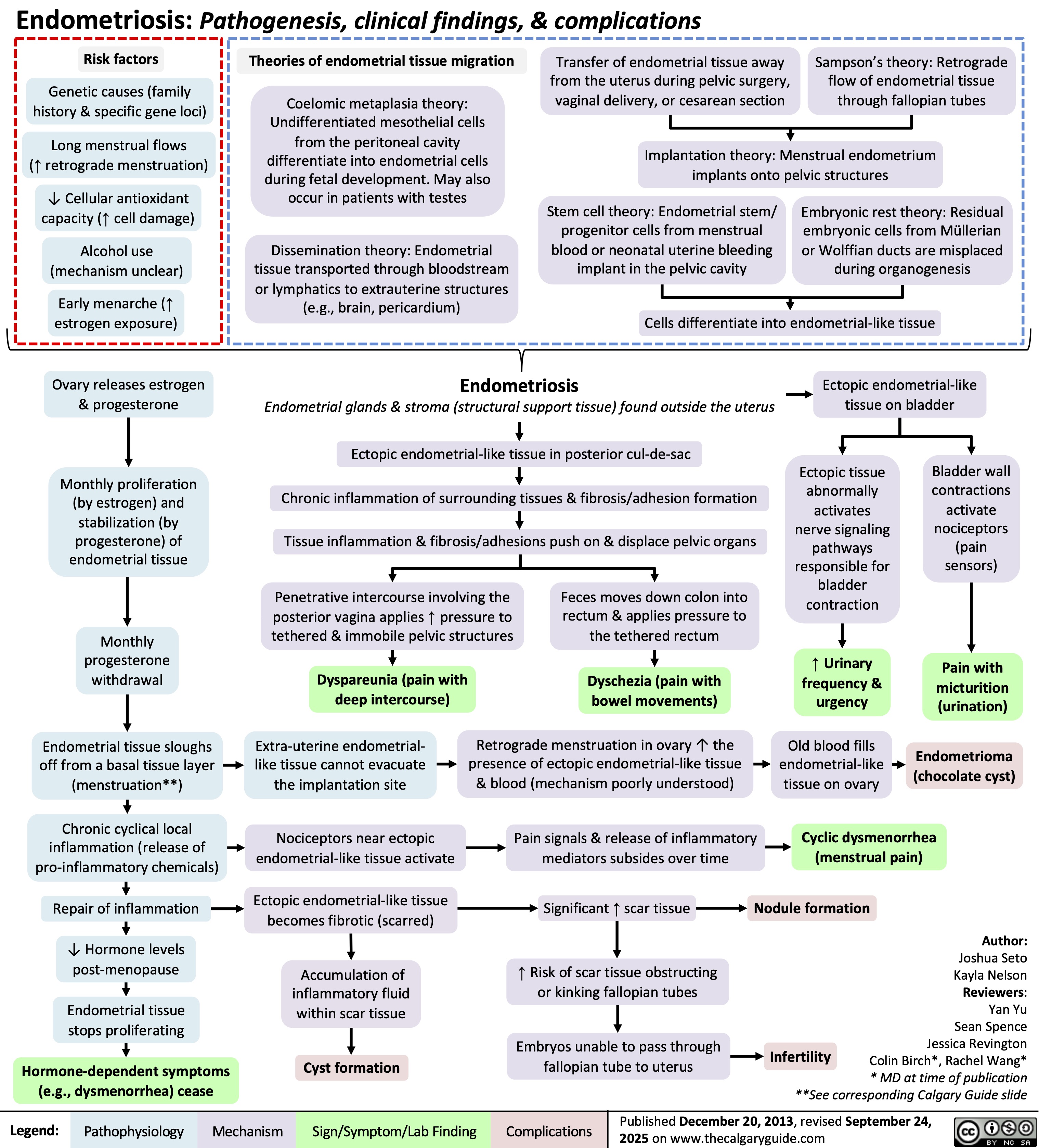
SEARCH RESULTS FOR: Endometriosis
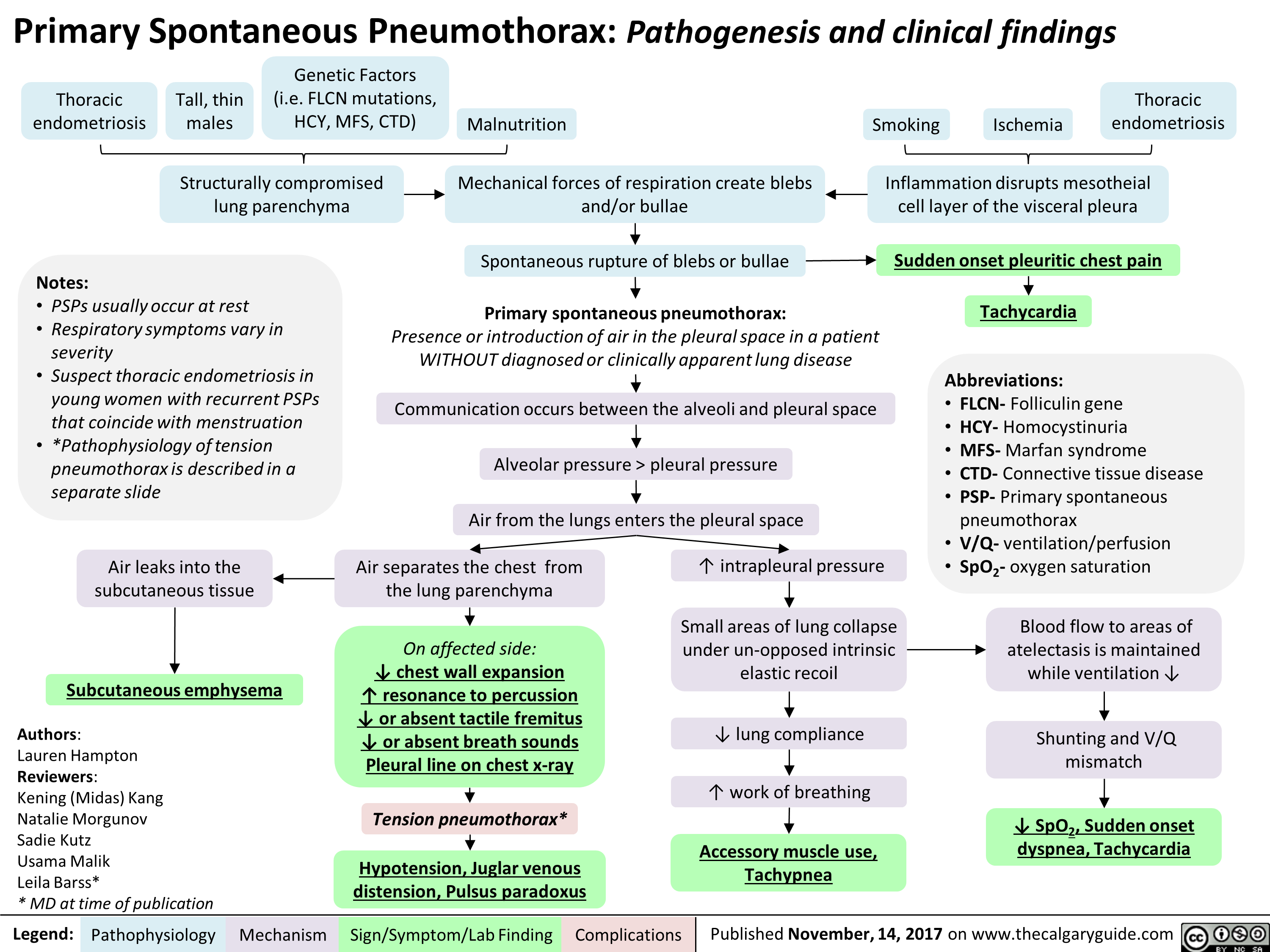
Primary Spontaneous Pneumothorax: Pathogenesis and clinical findings
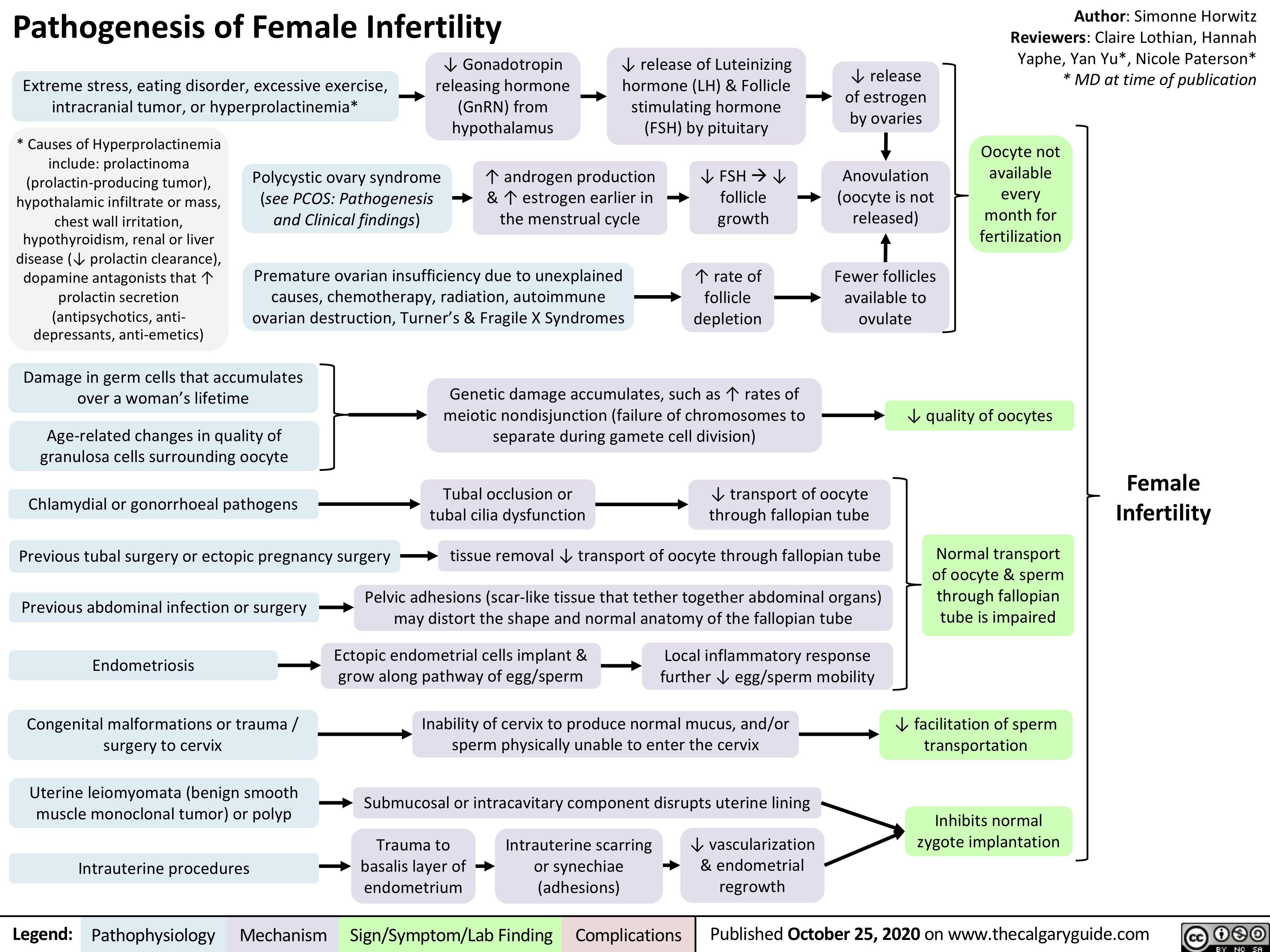
Pathogenesis-of-Female-Infertility
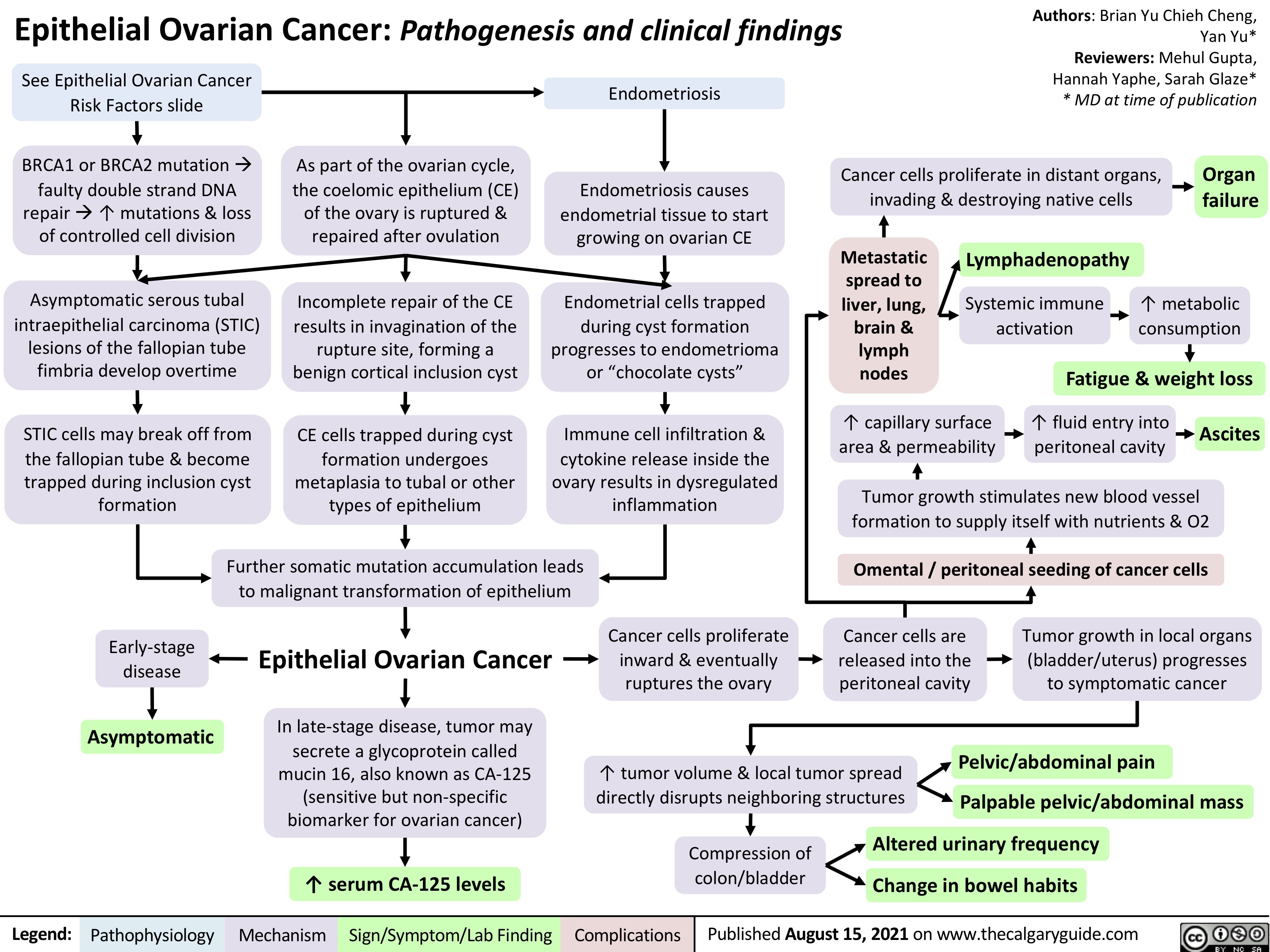
epithelial-ovarian-cancer-pathogenesis-and-clinical-findings
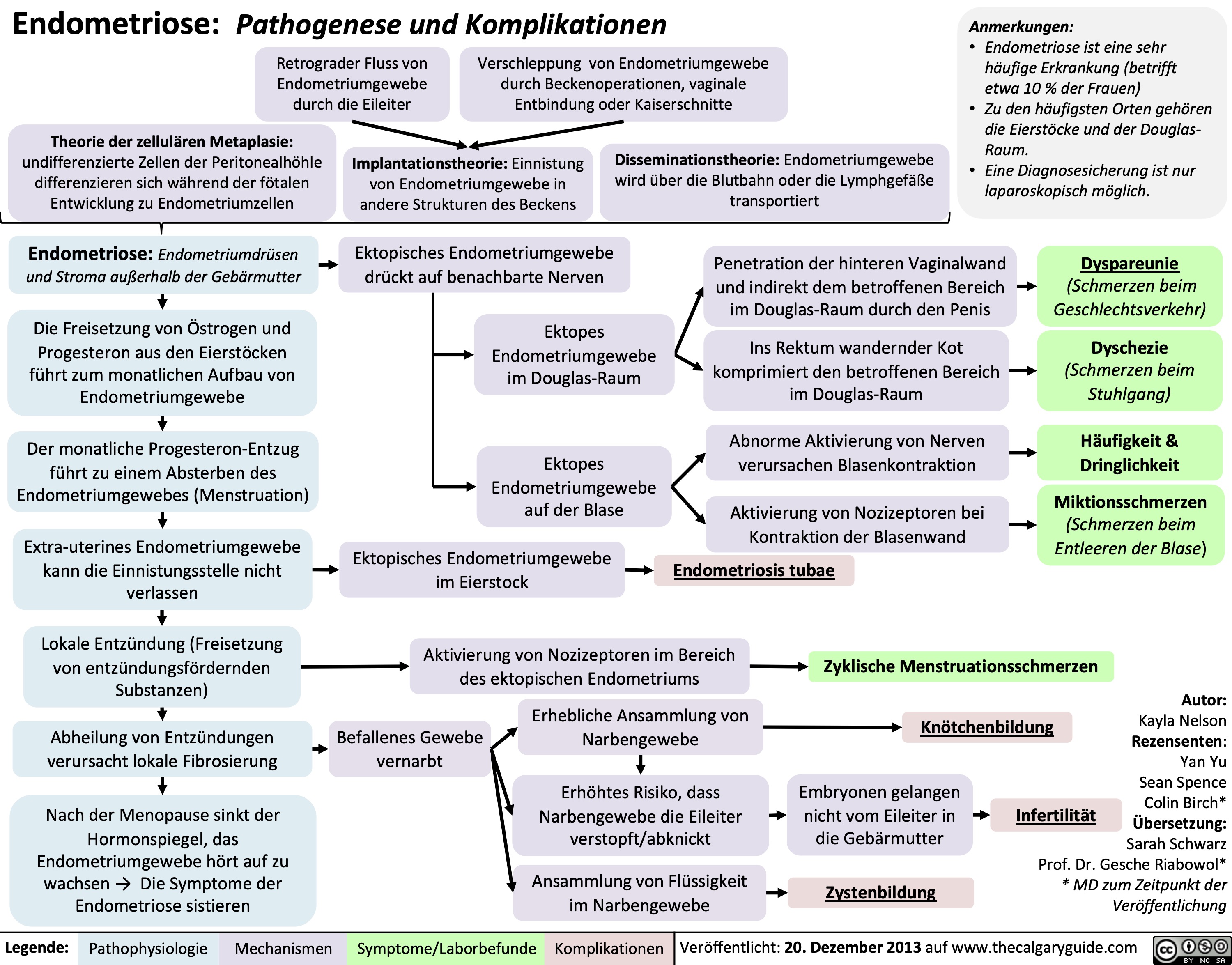
Ectopic Pregnancy
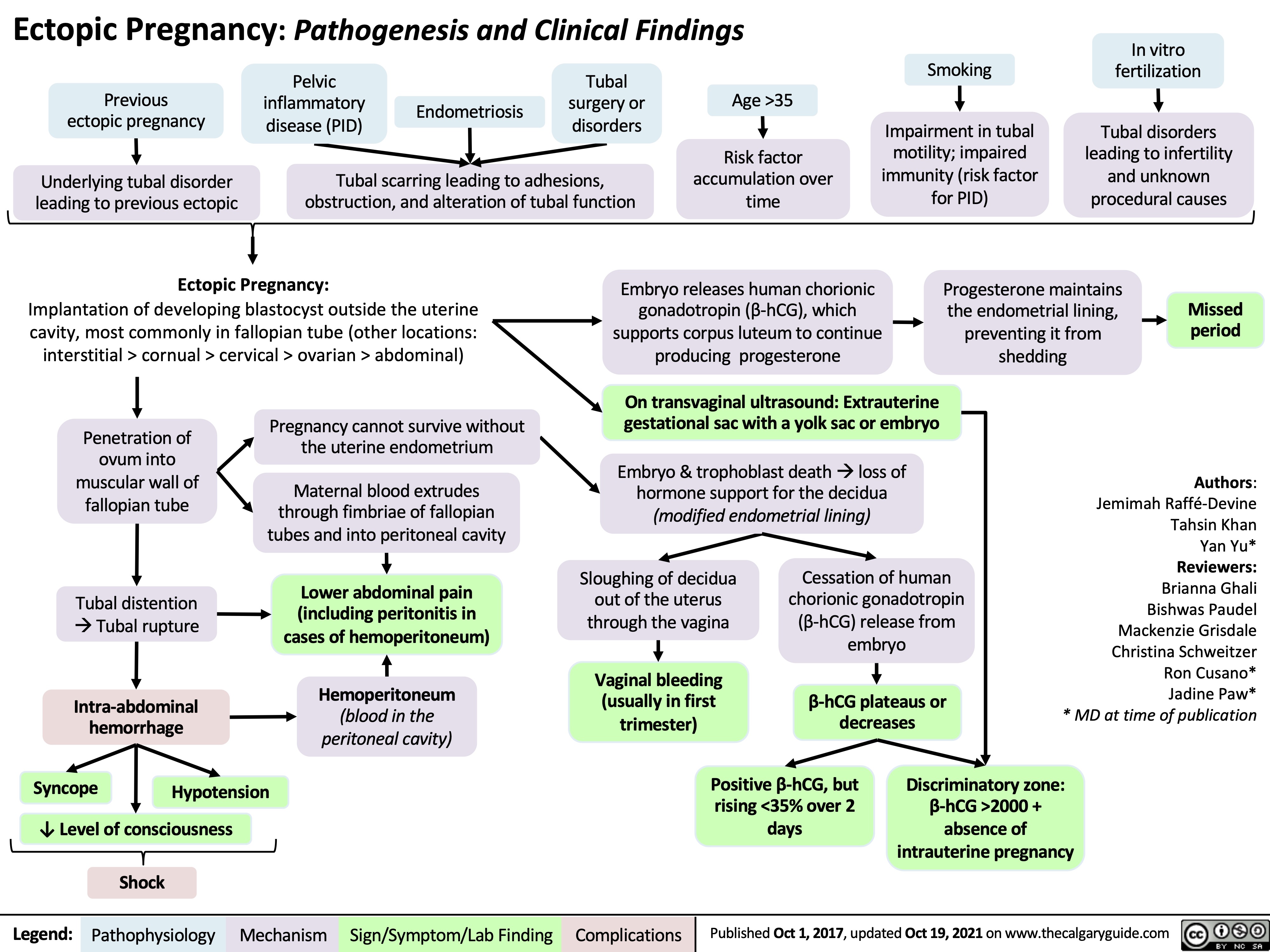
epithelial-ovarian-cancer-subtypes-molecular-alterations-risk-factors
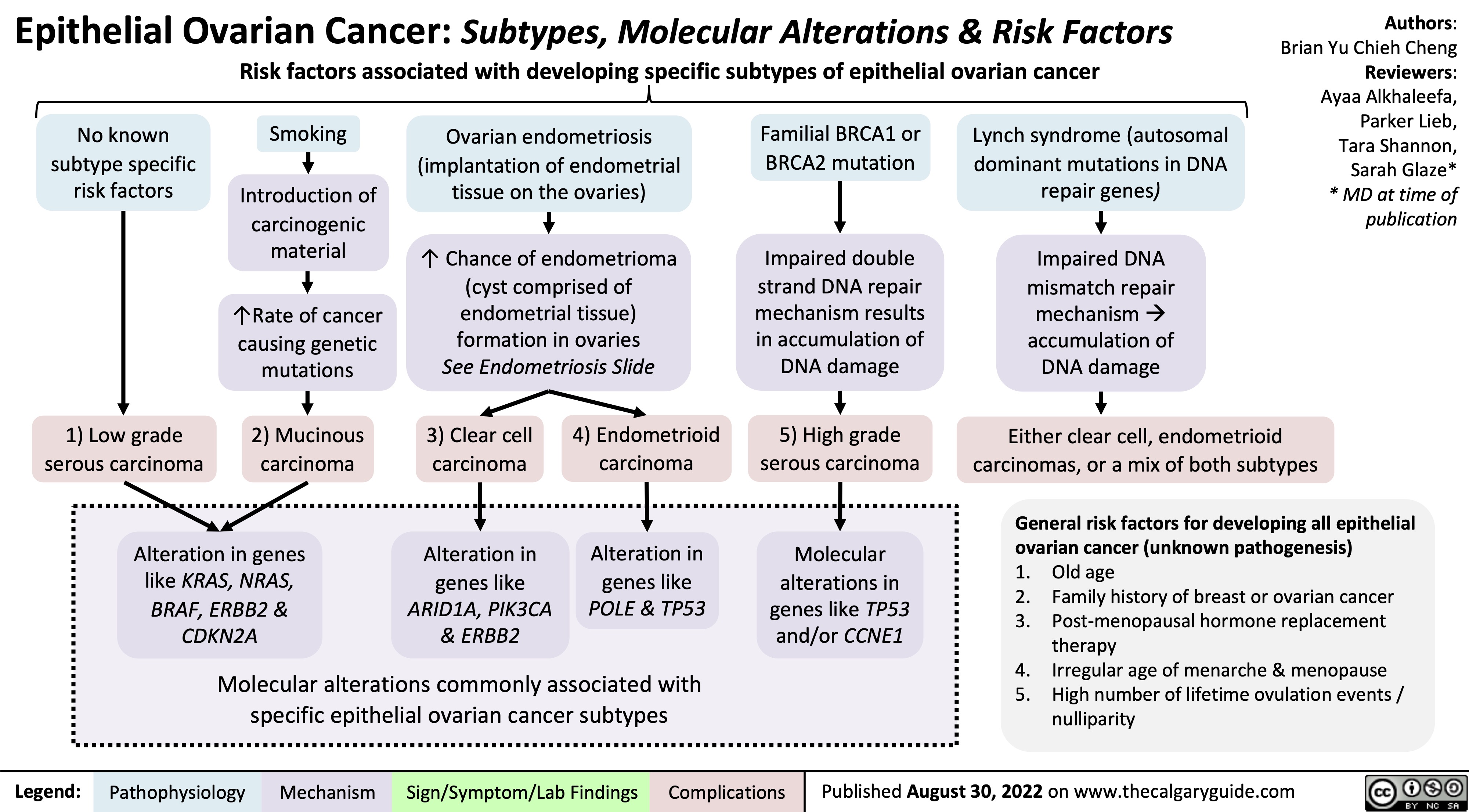
Endometriose Pathogenese und Komplikationen
Endometriosis