SEARCH RESULTS FOR: Hyperthyroidism
Clinical Findings of Androgen Deficiency
![Yu, Yan - Androgen Deficiency - FINAL.pptx
Hypogonadism in Males:Clinical Findings of Androgen Deficiency? secretion volume from seminal vesicle and prostateAuthor: Yan YuReviewers:Peter VetereGillian GoobieHanan Bassyouni** MD at time of publicationLegend:Published June 18, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplications? effect of testosterone on the brain? Libido(sensitive, but less specific)? [testosterone] : [estrogen] ratio at the male breast? ejaculate volume(a sensitive and specific sign)Gynecomastia (palpable breast tissue, not fat, directly under nipple)Fatigue,low mood, irrtabilityHot flashes, sweats(Can be nocturnal; occur only when hypogonadism is severe)Vasomotor neural response of unknown causeFewer spontaneous erections (i.e. in the morning)Lack of androgens (i.e. testosterone, DHT) in men past the age of pubertyIn advanced stages of the disease, after years of hypogonadism:(thus, less commonly seen)Low Bone Mass Density (BMD)Less testosterone to be converted into estrogen in bone? muscle bulk and strengthSmall, soft testicles(<4cm long on orchidometer)Lack of hormones to stimulate and maintain testicular hyperplasia/growthLoss of androgenic hair (on face, midline, and pubic area)Vertebral fracture (height loss), or other fragility fracturesIf sexual development is incomplete from puberty:Note: These clinical findings apply to many disorders, including:-Andropause-Hypopituitarism (suspect if other hormone abnormalities & Sx of mass lesion like visual field loss, diplopia, and headache exist)-Testicular Failure (if Hx of chemo, radiation, excess alcohol, and chronic liver disease)-Klinefelter's (if assoc. tall and eunuchoid stature, breast enlargement and cognitive deficiency - XXY)-Kallman's (if assoc. anosmia, and tall/eunuchoid stature)-Drugs (e.g. ketoconazole, anabolic steroids, spironolactone, digoxin, marijuana)Testosterone's inhibitory effect on estrogen is not enough to prevent breast growthDeficiency in testosterone during puberty delays fusion of epiphysesTall, eunuchoid statureNote: any disease involving an increase in aromatase activity (hyperthyroidism, cirrhosis, HCG-secreting tumors) will also cause relative estrogen excess & subsequent gynecomastia.
111 kB / 272 words Yu, Yan - Androgen Deficiency - FINAL.pptx
Hypogonadism in Males:Clinical Findings of Androgen Deficiency? secretion volume from seminal vesicle and prostateAuthor: Yan YuReviewers:Peter VetereGillian GoobieHanan Bassyouni** MD at time of publicationLegend:Published June 18, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplications? effect of testosterone on the brain? Libido(sensitive, but less specific)? [testosterone] : [estrogen] ratio at the male breast? ejaculate volume(a sensitive and specific sign)Gynecomastia (palpable breast tissue, not fat, directly under nipple)Fatigue,low mood, irrtabilityHot flashes, sweats(Can be nocturnal; occur only when hypogonadism is severe)Vasomotor neural response of unknown causeFewer spontaneous erections (i.e. in the morning)Lack of androgens (i.e. testosterone, DHT) in men past the age of pubertyIn advanced stages of the disease, after years of hypogonadism:(thus, less commonly seen)Low Bone Mass Density (BMD)Less testosterone to be converted into estrogen in bone? muscle bulk and strengthSmall, soft testicles(<4cm long on orchidometer)Lack of hormones to stimulate and maintain testicular hyperplasia/growthLoss of androgenic hair (on face, midline, and pubic area)Vertebral fracture (height loss), or other fragility fracturesIf sexual development is incomplete from puberty:Note: These clinical findings apply to many disorders, including:-Andropause-Hypopituitarism (suspect if other hormone abnormalities & Sx of mass lesion like visual field loss, diplopia, and headache exist)-Testicular Failure (if Hx of chemo, radiation, excess alcohol, and chronic liver disease)-Klinefelter's (if assoc. tall and eunuchoid stature, breast enlargement and cognitive deficiency - XXY)-Kallman's (if assoc. anosmia, and tall/eunuchoid stature)-Drugs (e.g. ketoconazole, anabolic steroids, spironolactone, digoxin, marijuana)Testosterone's inhibitory effect on estrogen is not enough to prevent breast growthDeficiency in testosterone during puberty delays fusion of epiphysesTall, eunuchoid statureNote: any disease involving an increase in aromatase activity (hyperthyroidism, cirrhosis, HCG-secreting tumors) will also cause relative estrogen excess & subsequent gynecomastia.
111 kB / 272 words](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Clinical-Findings-of-Androgen-Deficiency.jpg)
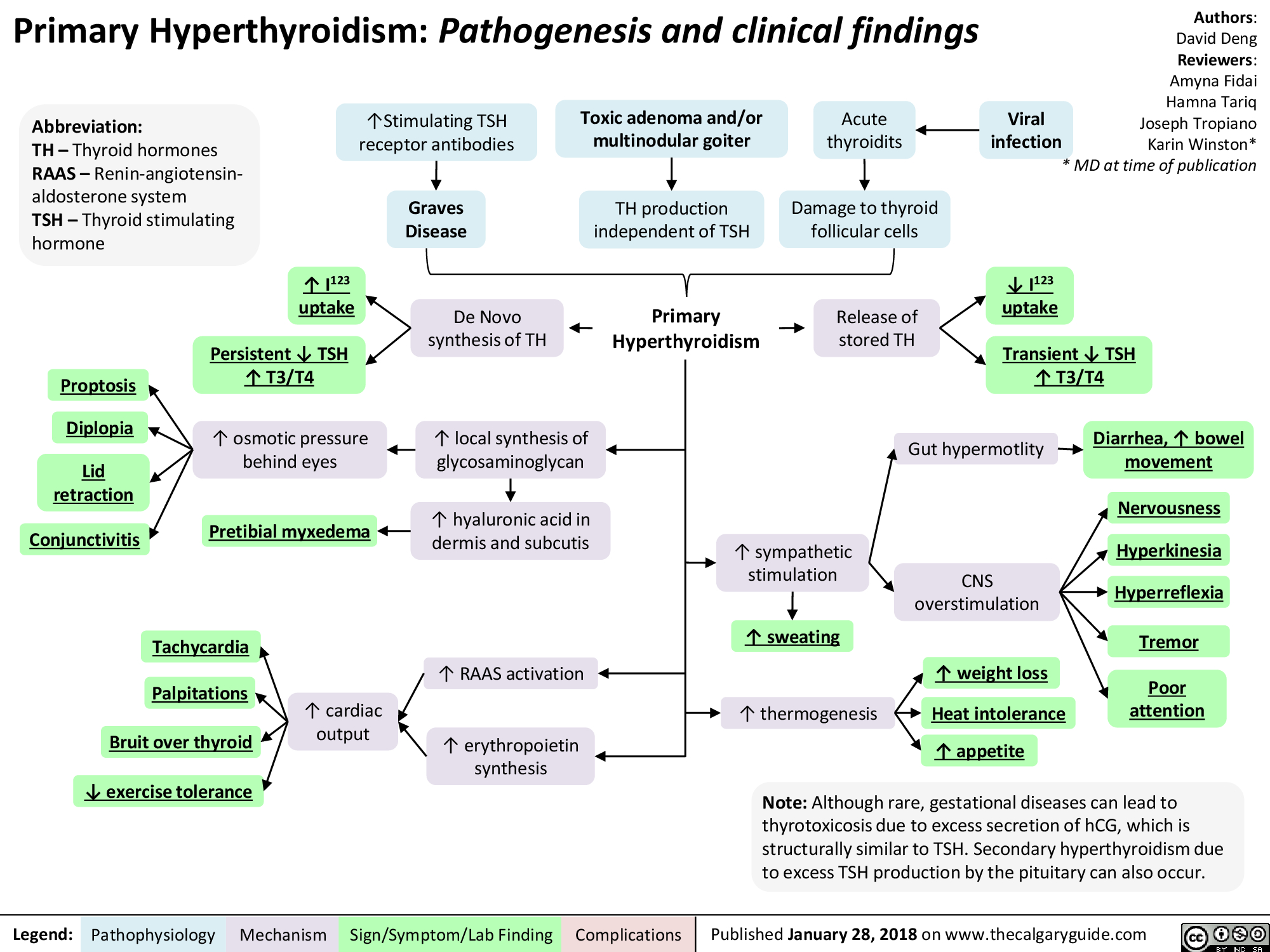
Hyperthyroidism
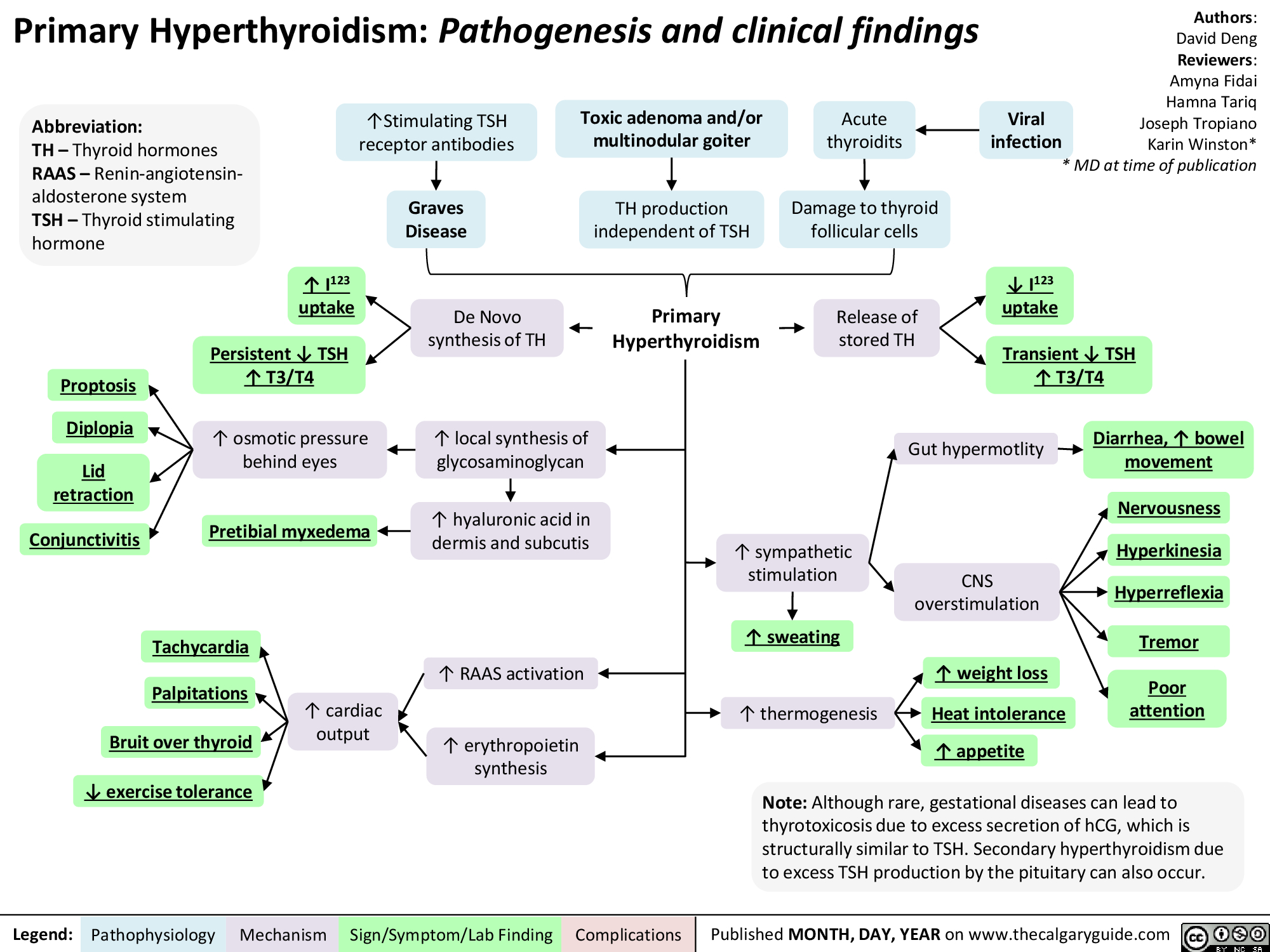
Hyperthyroidism
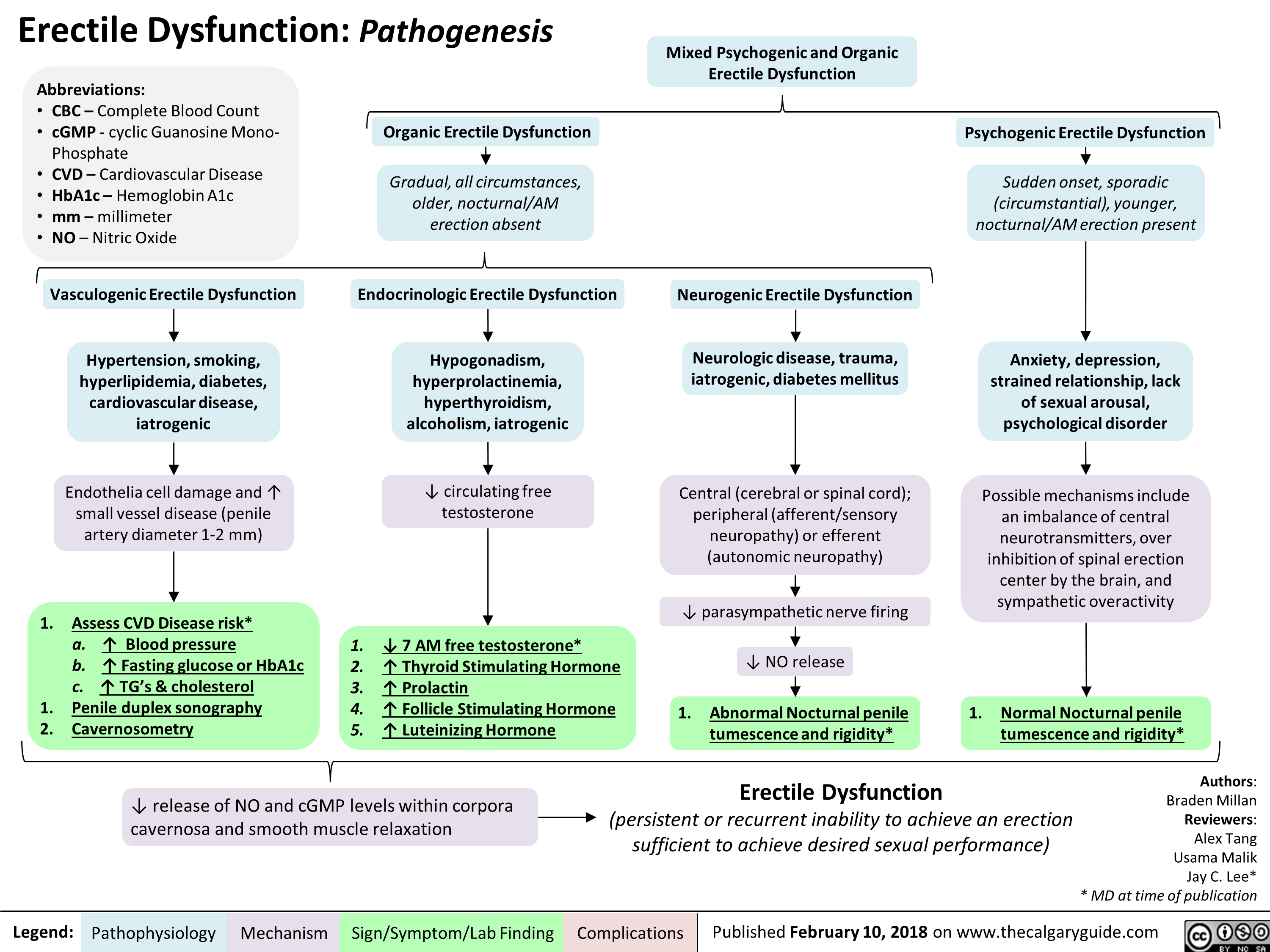
Erectile Dysfunction: Pathogenesis
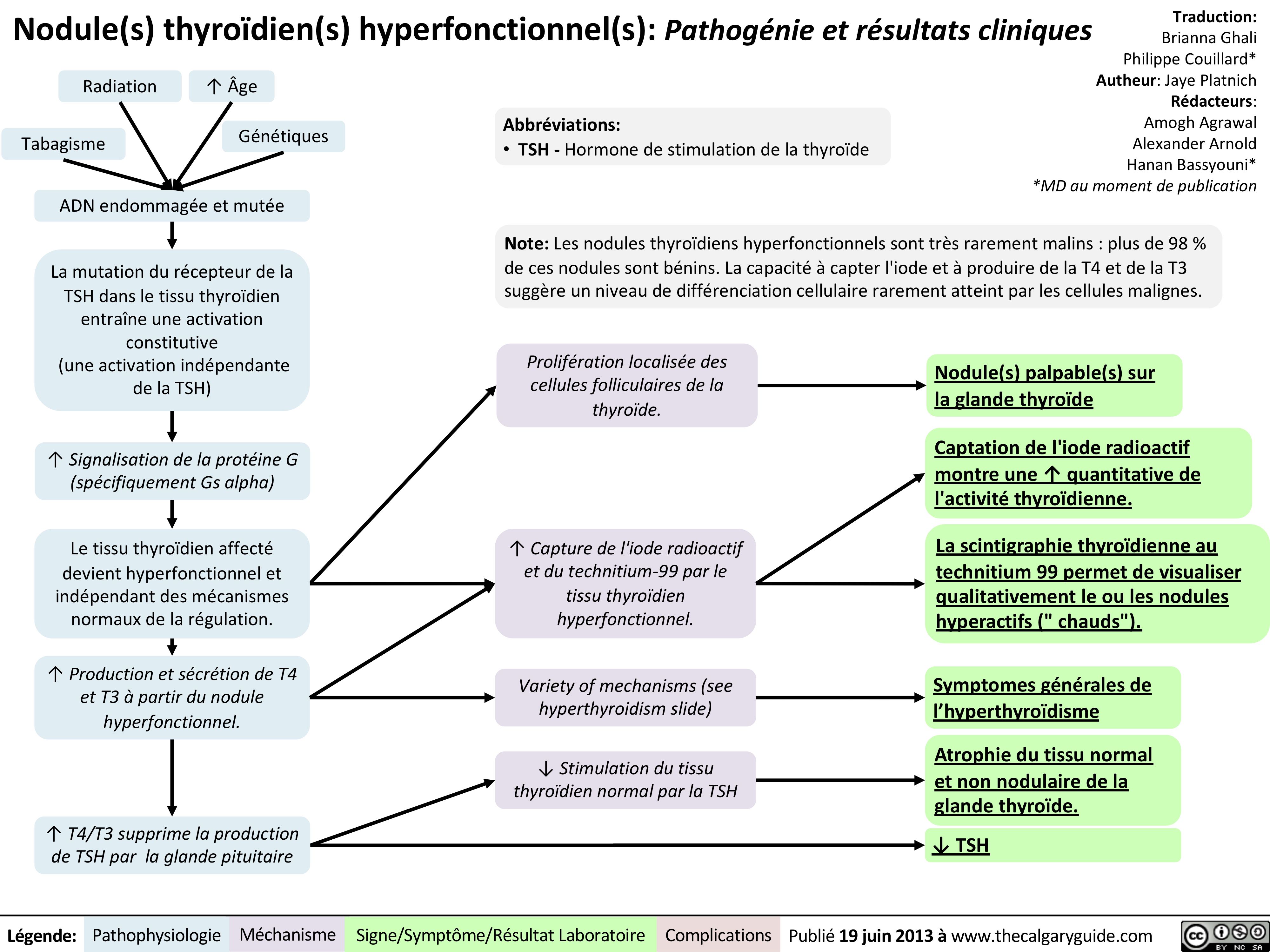
Nodule-thyroïdien
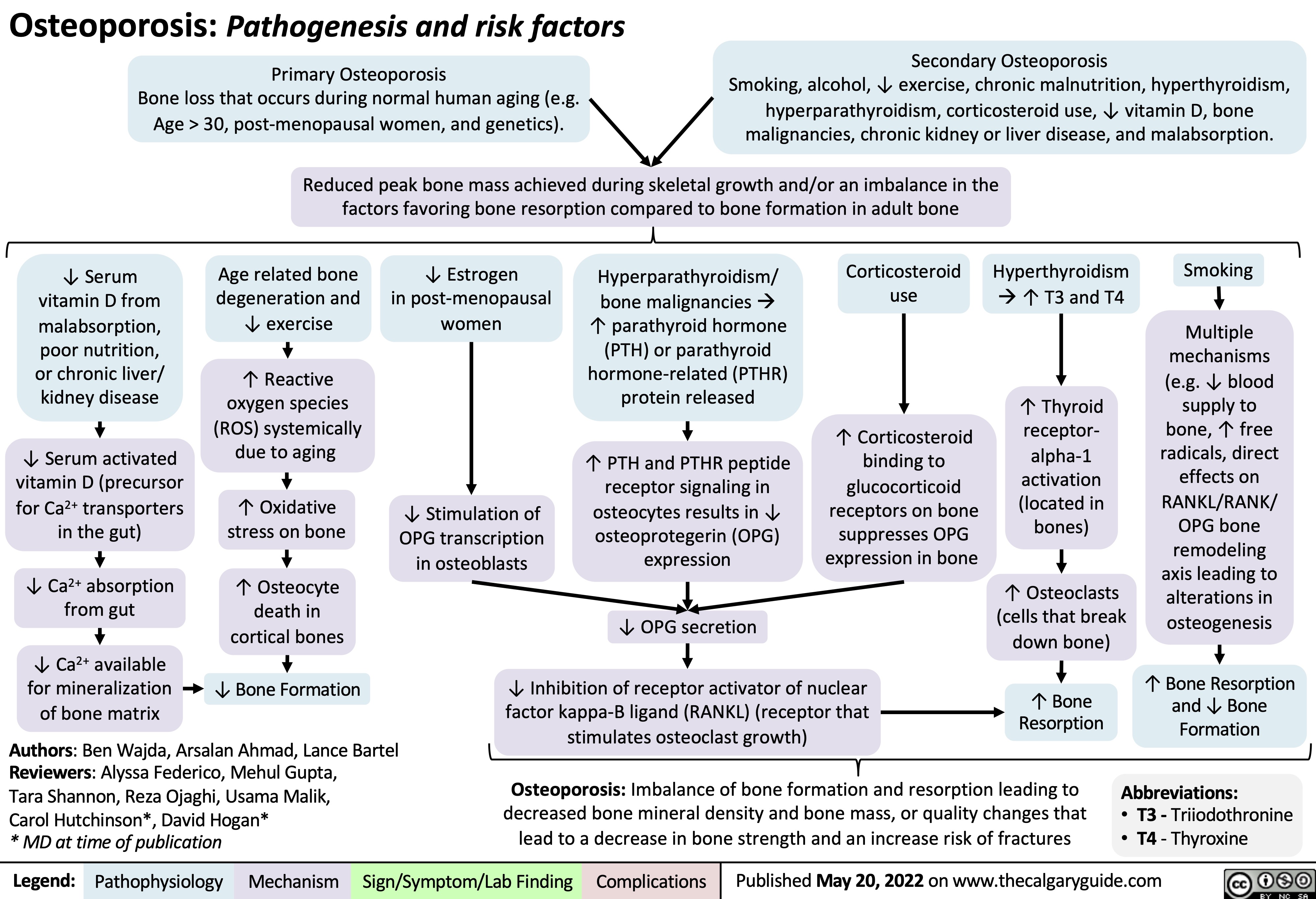
Osteoporosis Pathogenesis and risk factors
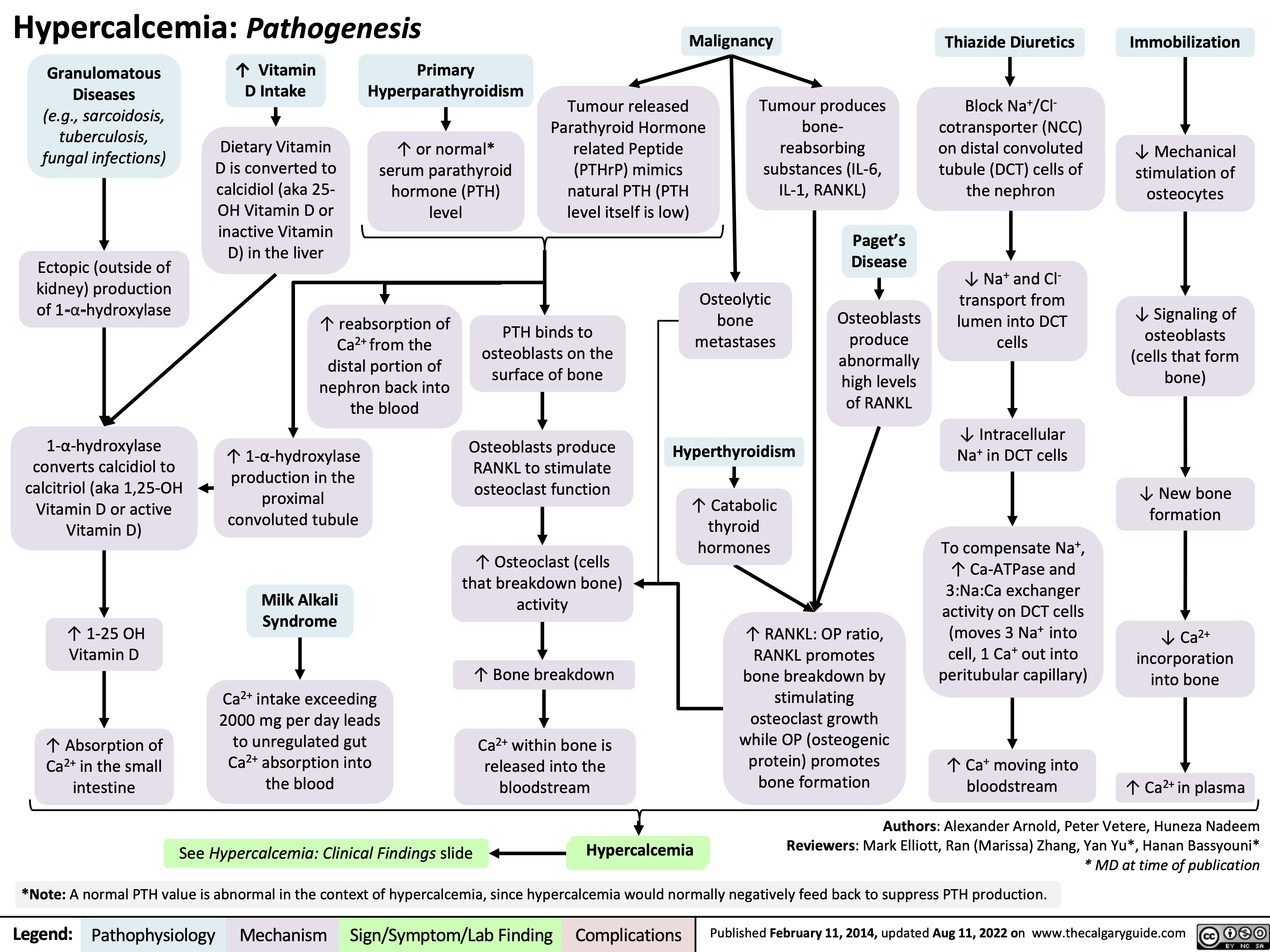
hypercalcemia-pathogenesis
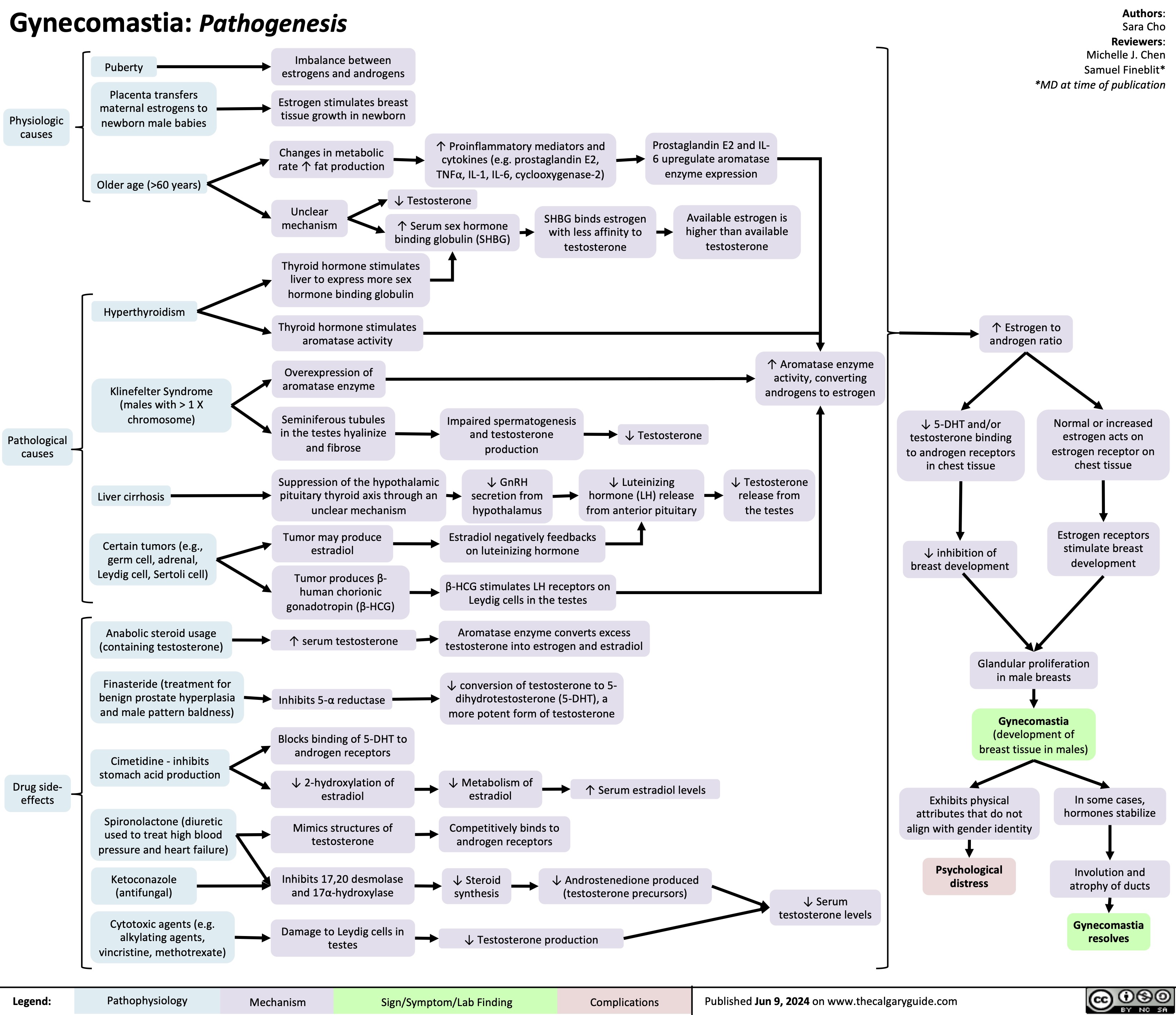
Gynecomastia
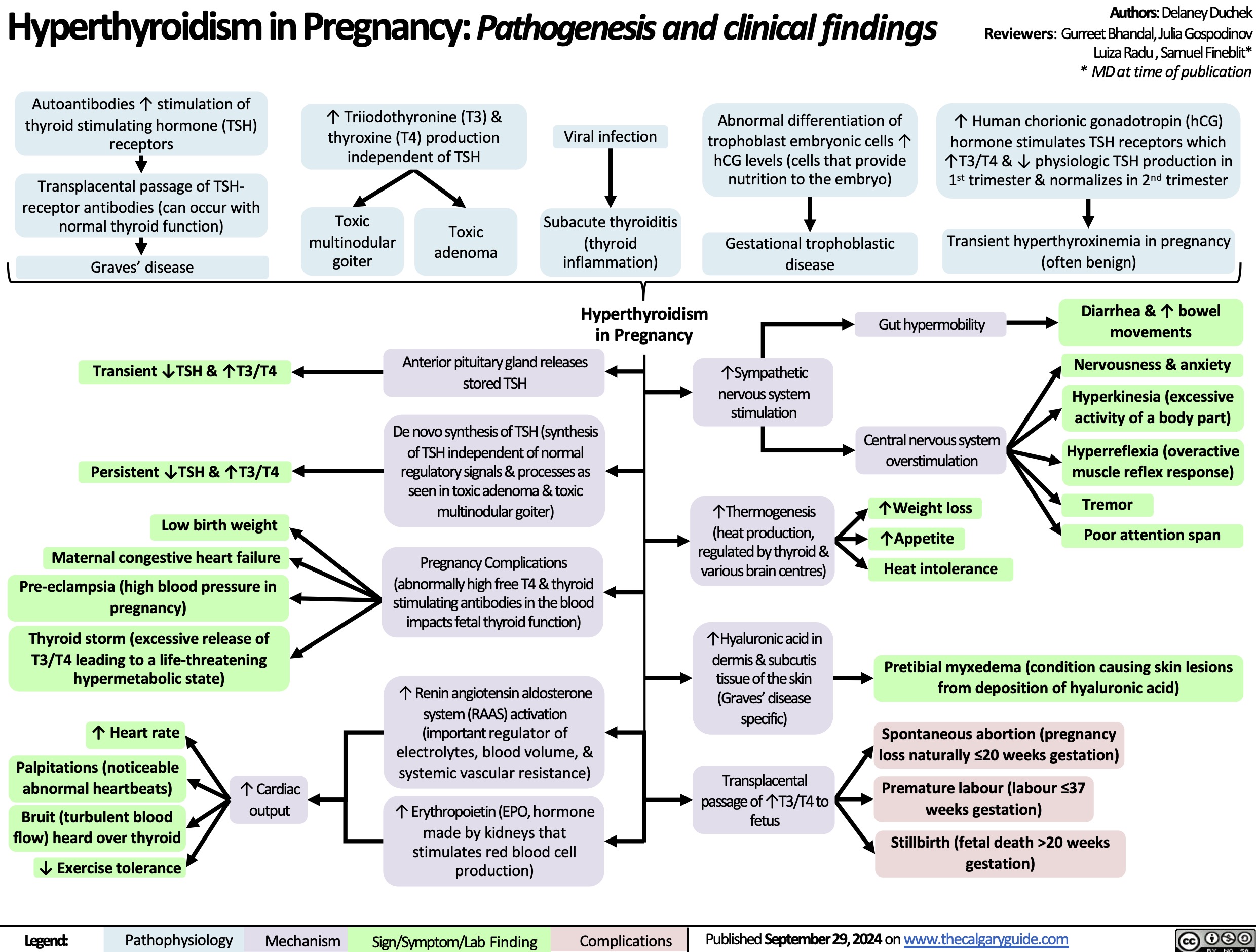
Hyperthyroidism in Pregnancy
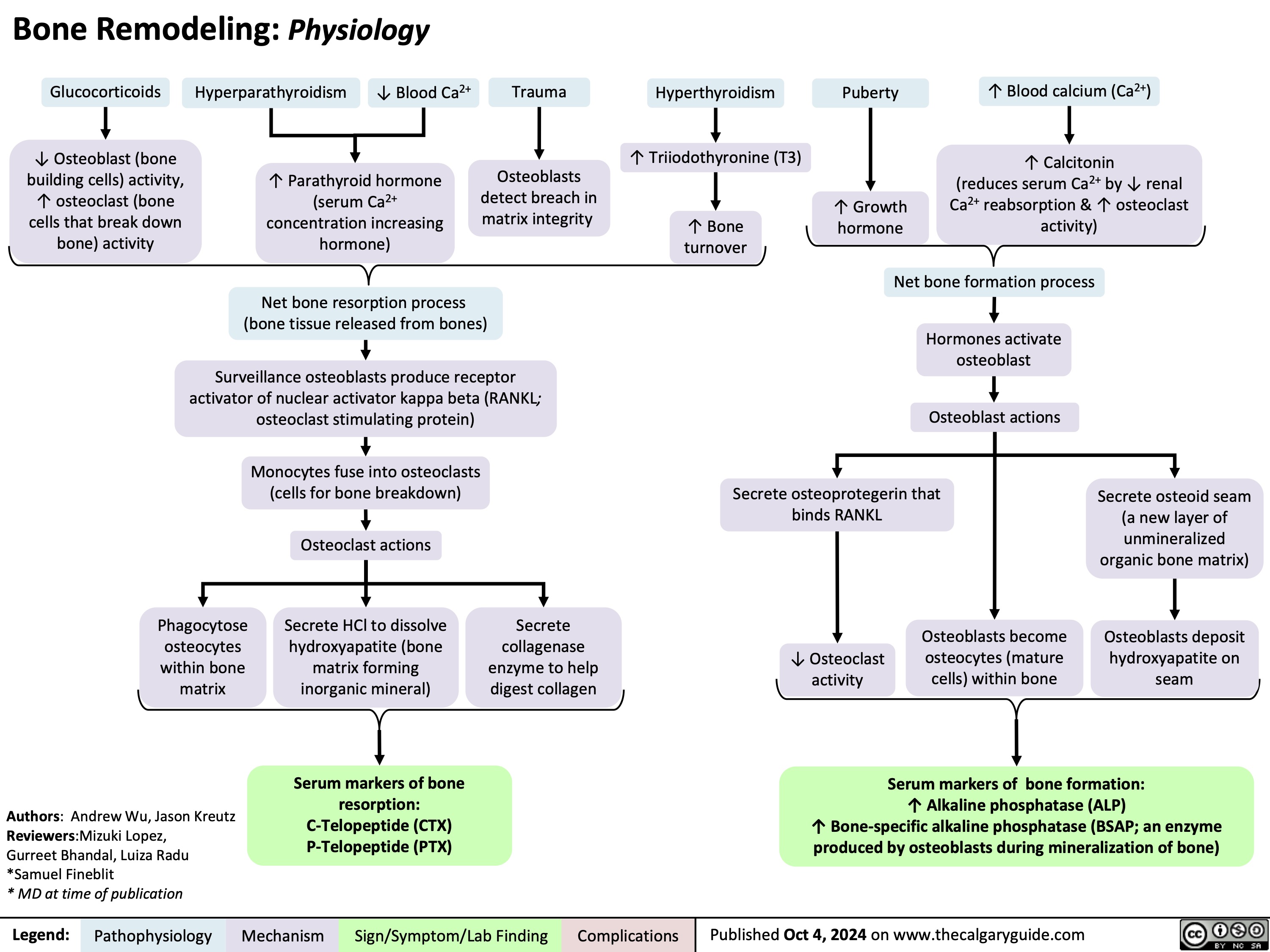
Bone Remodeling Physiology
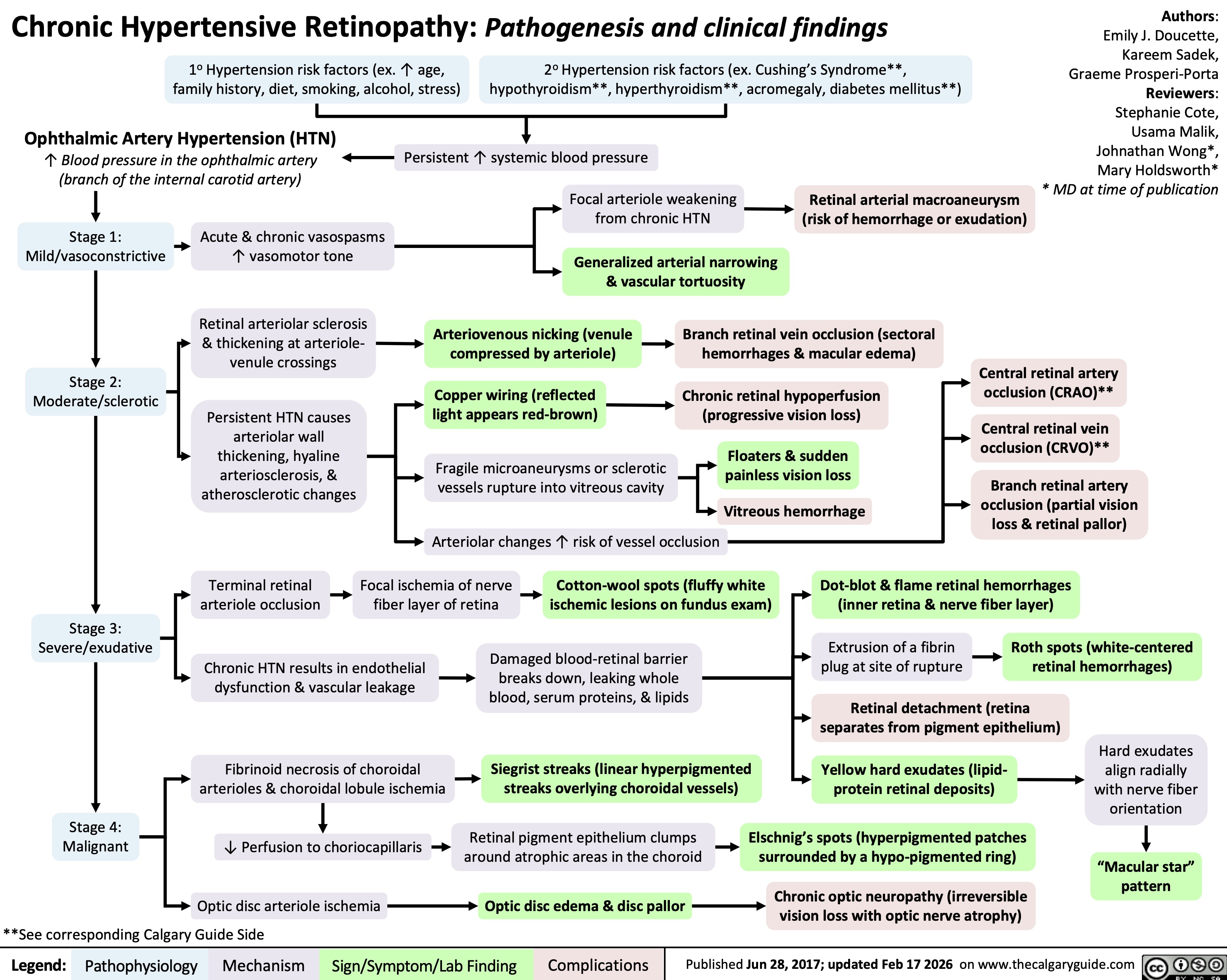
Chronic Hypertensive Retinopathy