SEARCH RESULTS FOR: Inflammation
systemic-lupus-erythematosus-gastrointestinal-manifestations

esophageal-gastric-varices
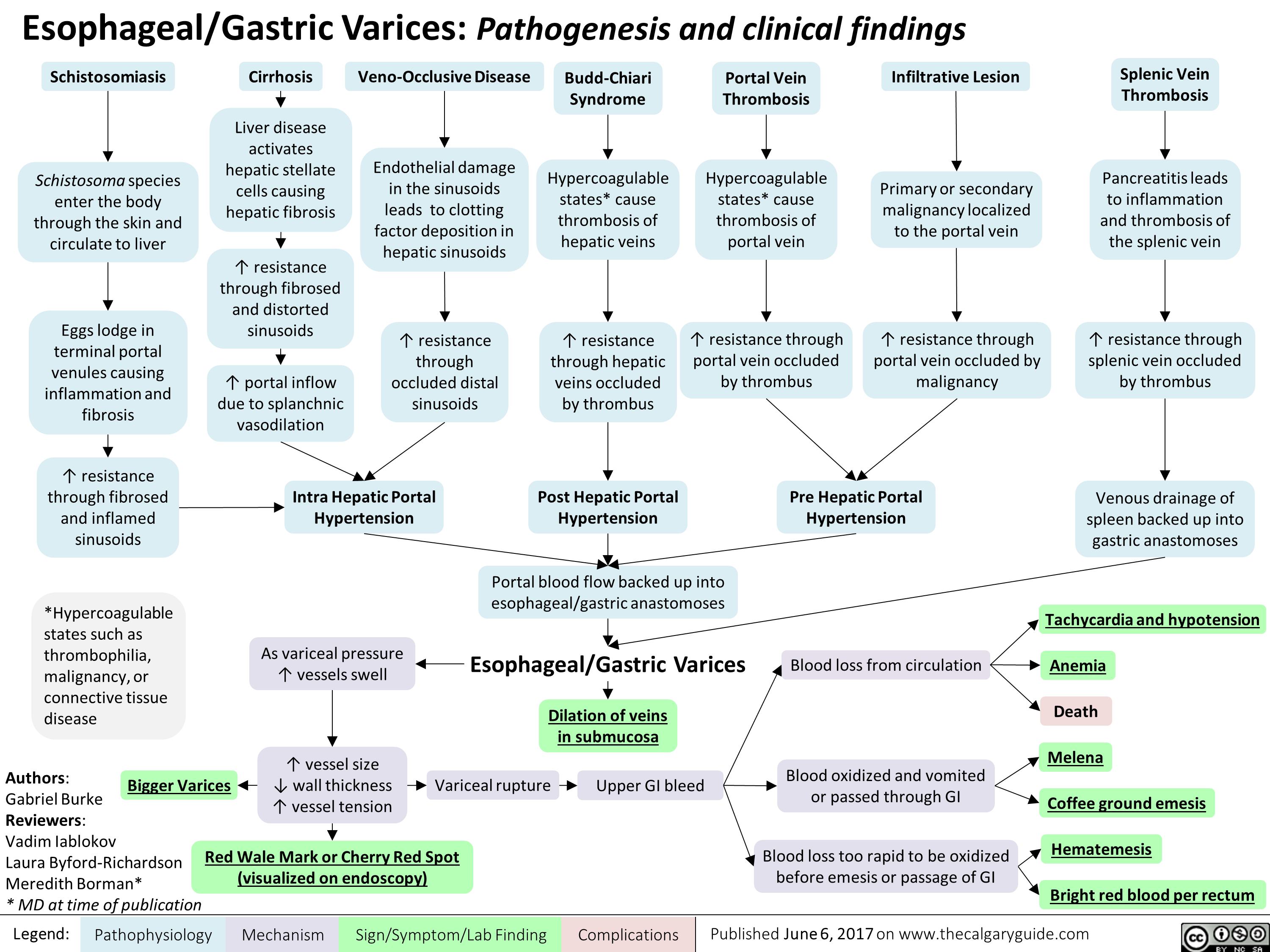
central-retinal-artery-occlusion-pathogenesis-and-clinical-findings
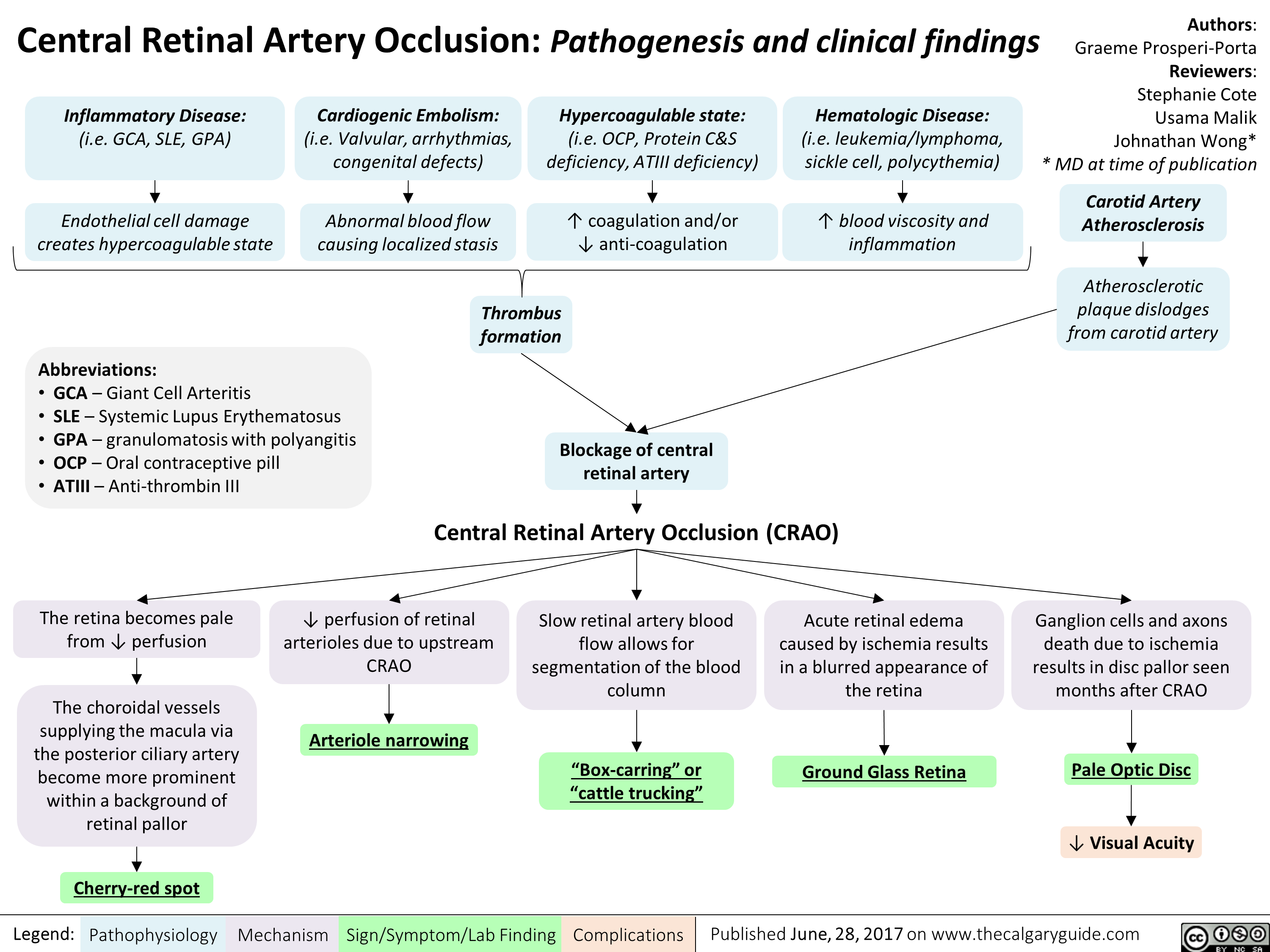
infectious-esophagitis-pathogenesis-and-clinical-findings
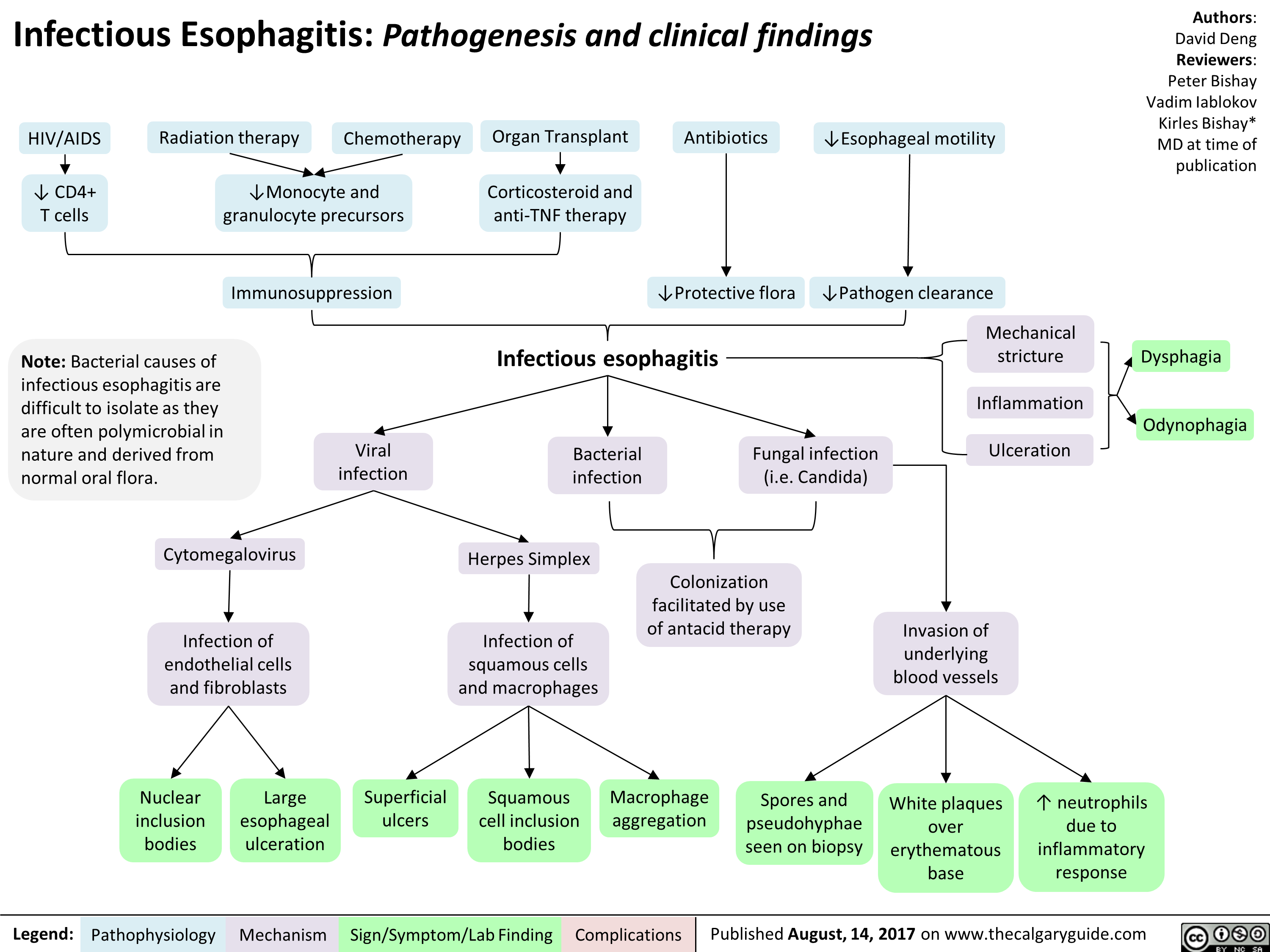
Primary Spontaneous Pneumothorax: Pathogenesis and clinical findings
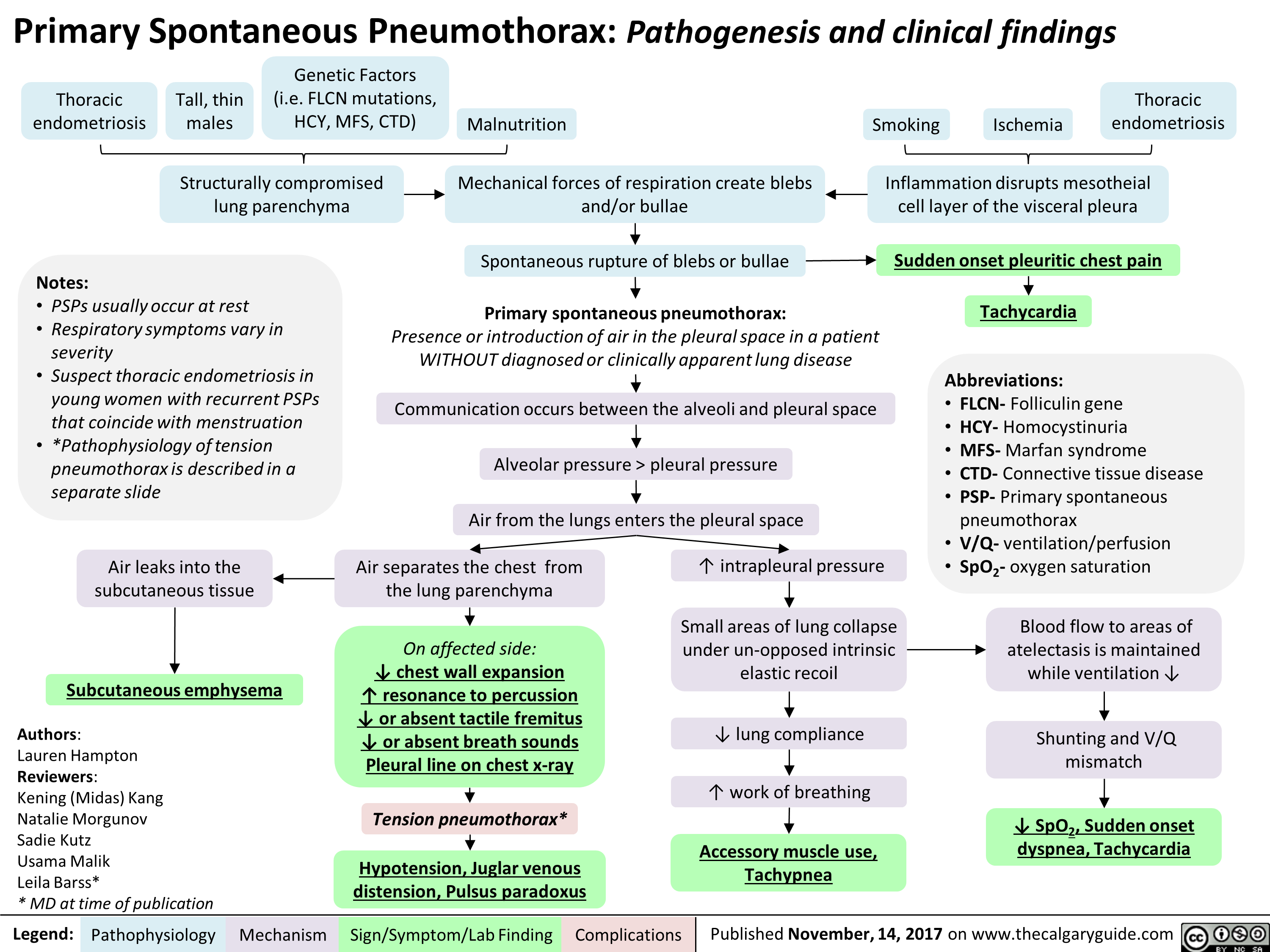
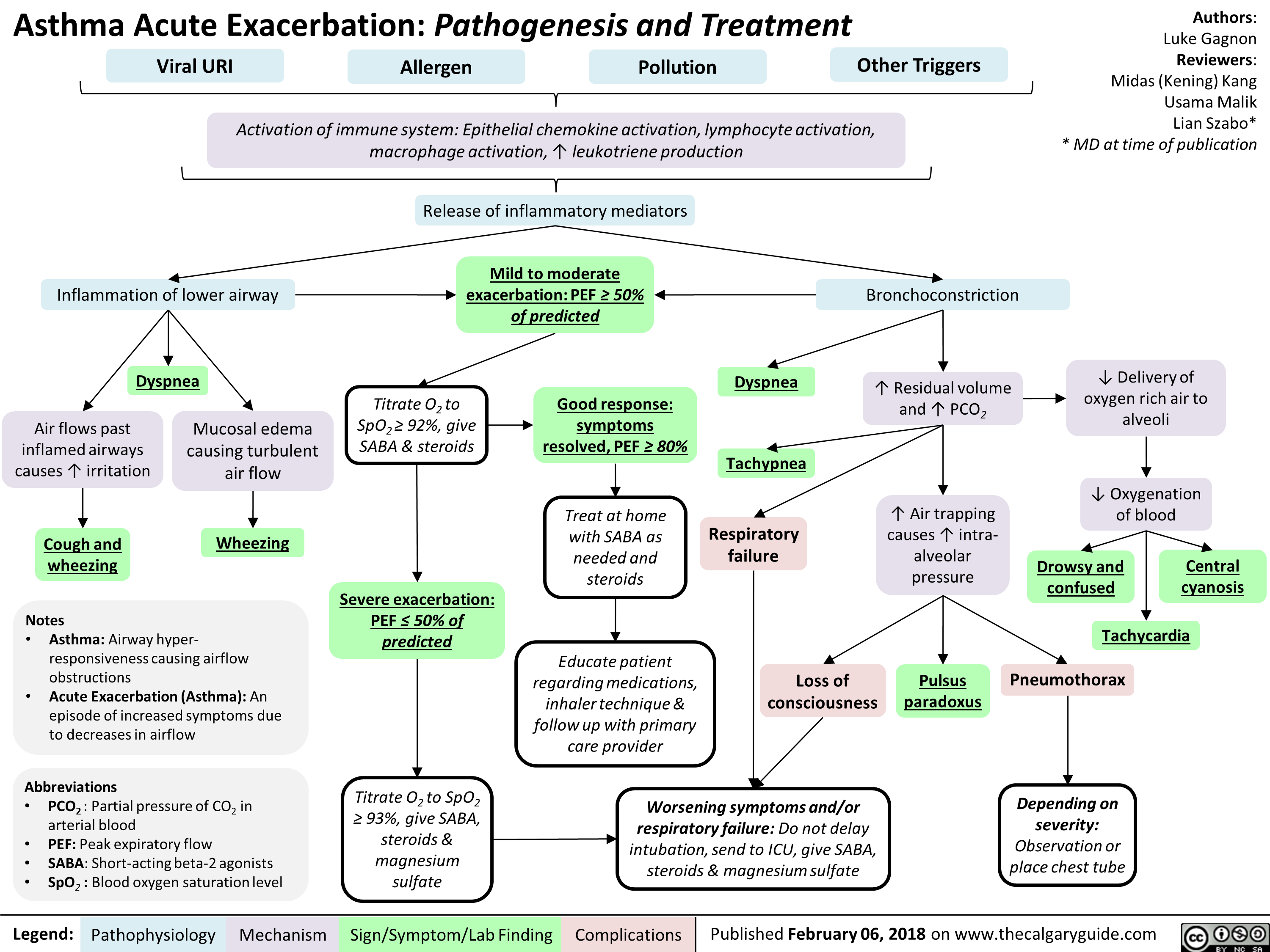
Asthma Acute Exacerbation: Pathogenesis and Treatment
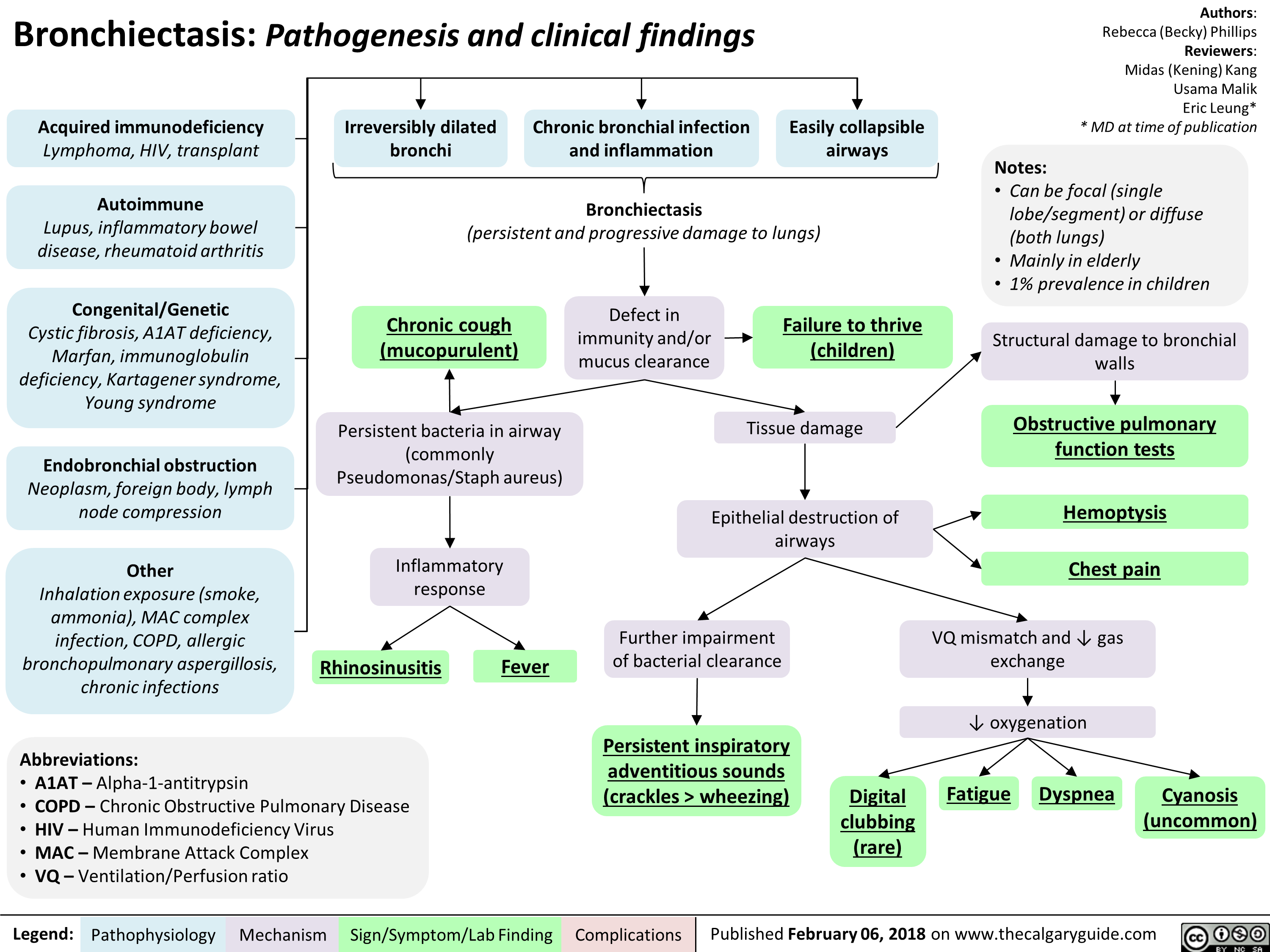
Bronchiectasis Pathogenesis and clinical findings
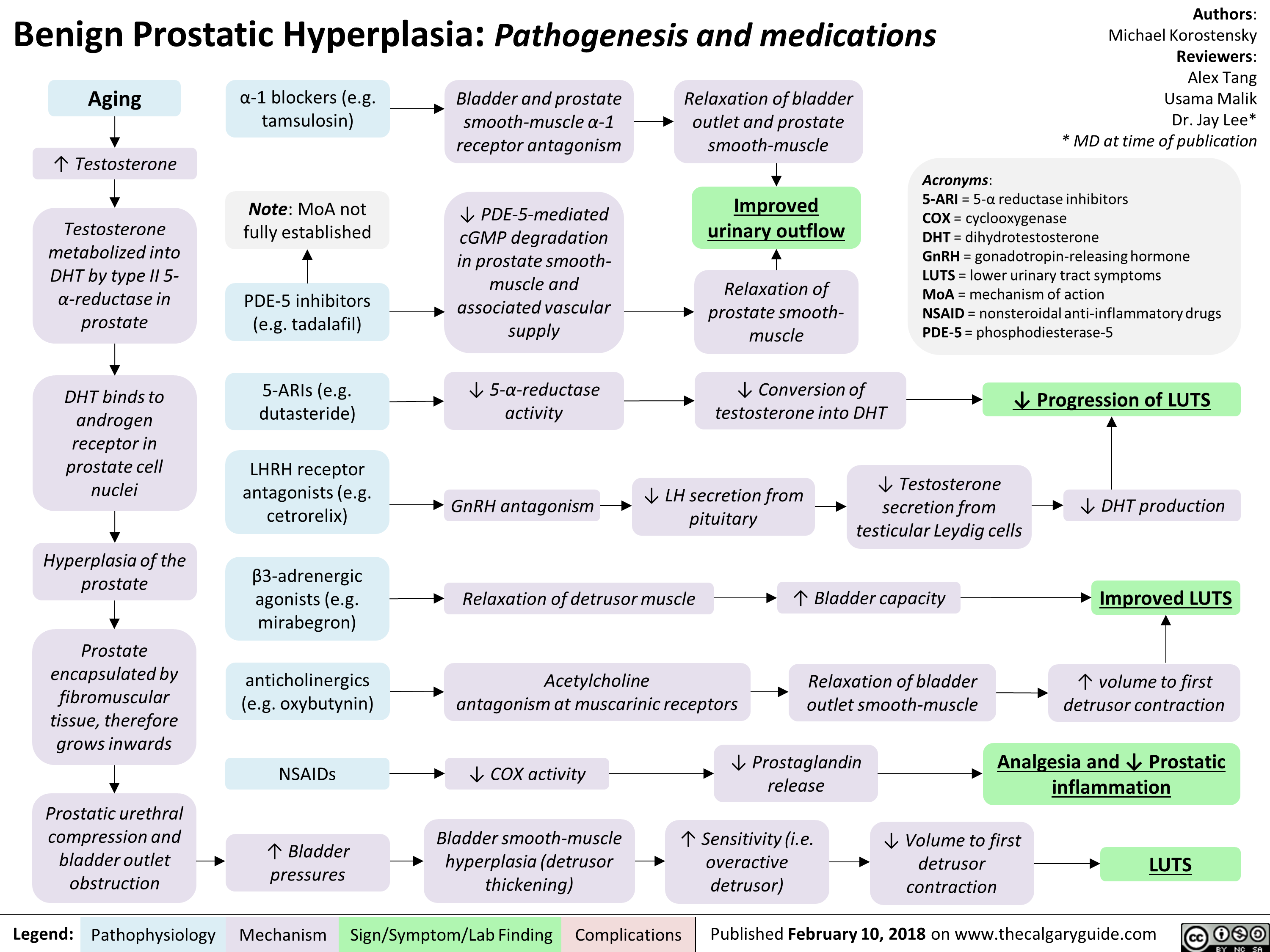
Benign Prostatic Hyperplasia: Pathogenesis and medications
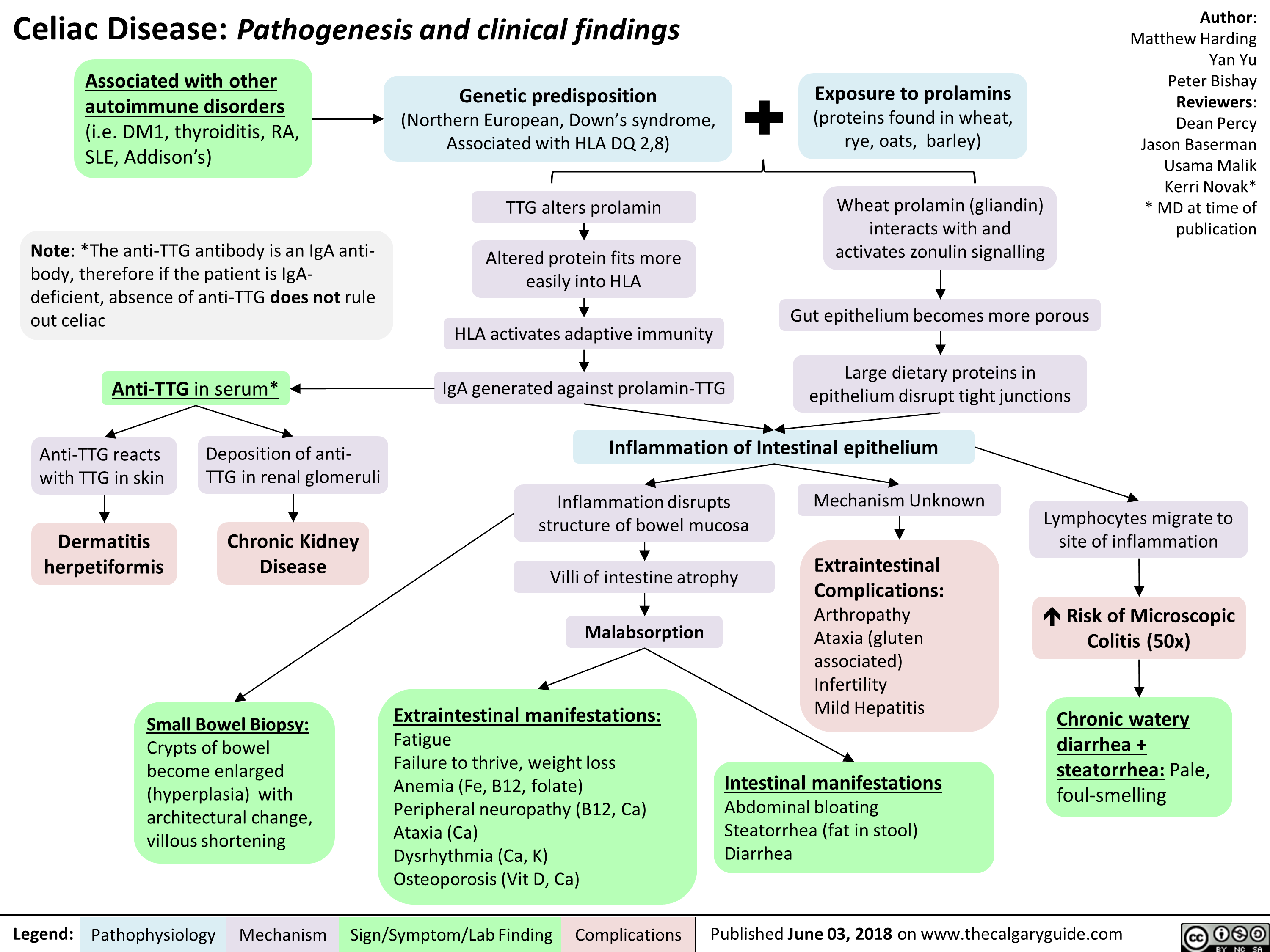
Celiac Disease: Pathogenesis and clinical findings
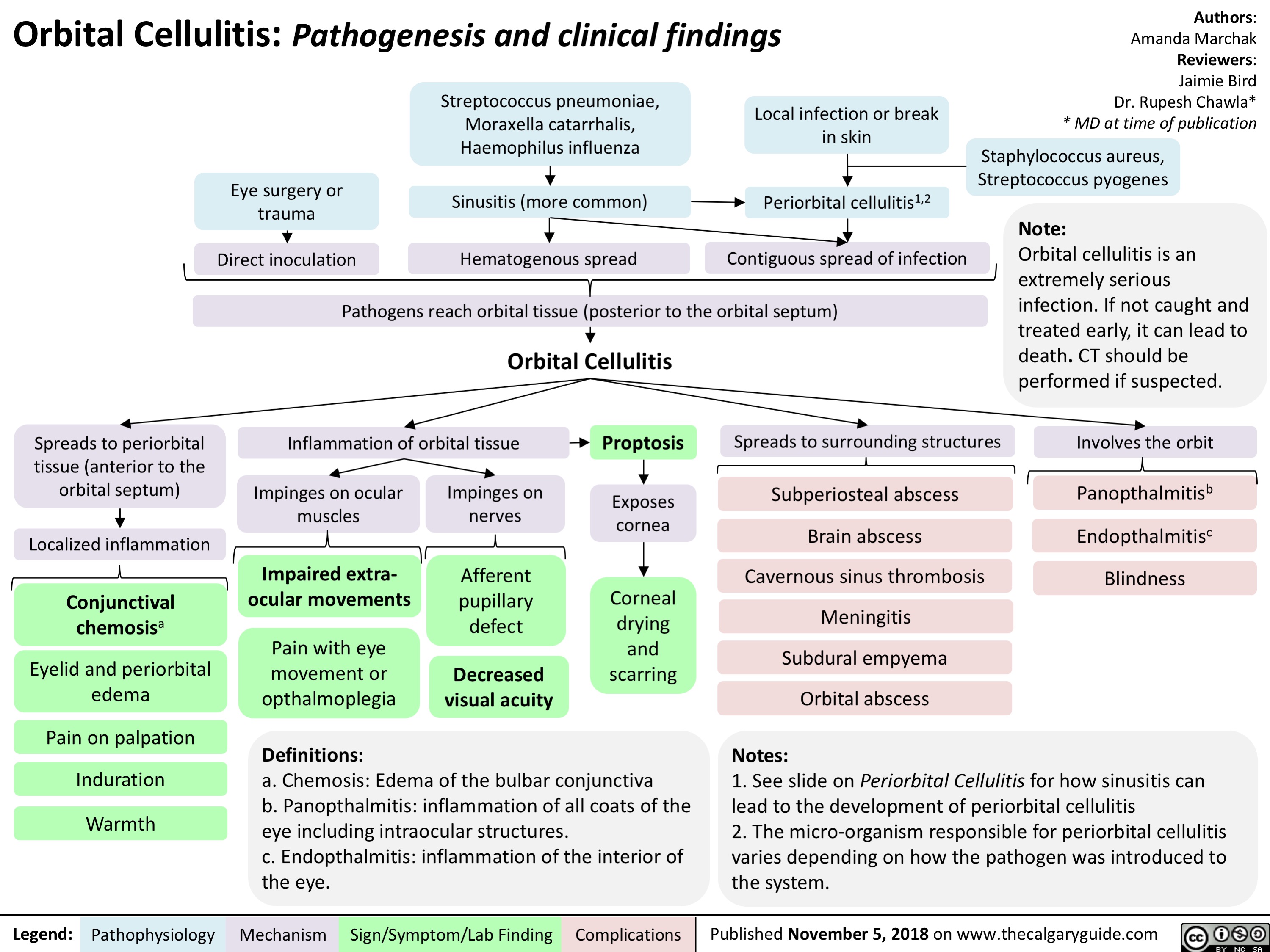
Orbital Cellulitis: Pathogenesis and clinical findings
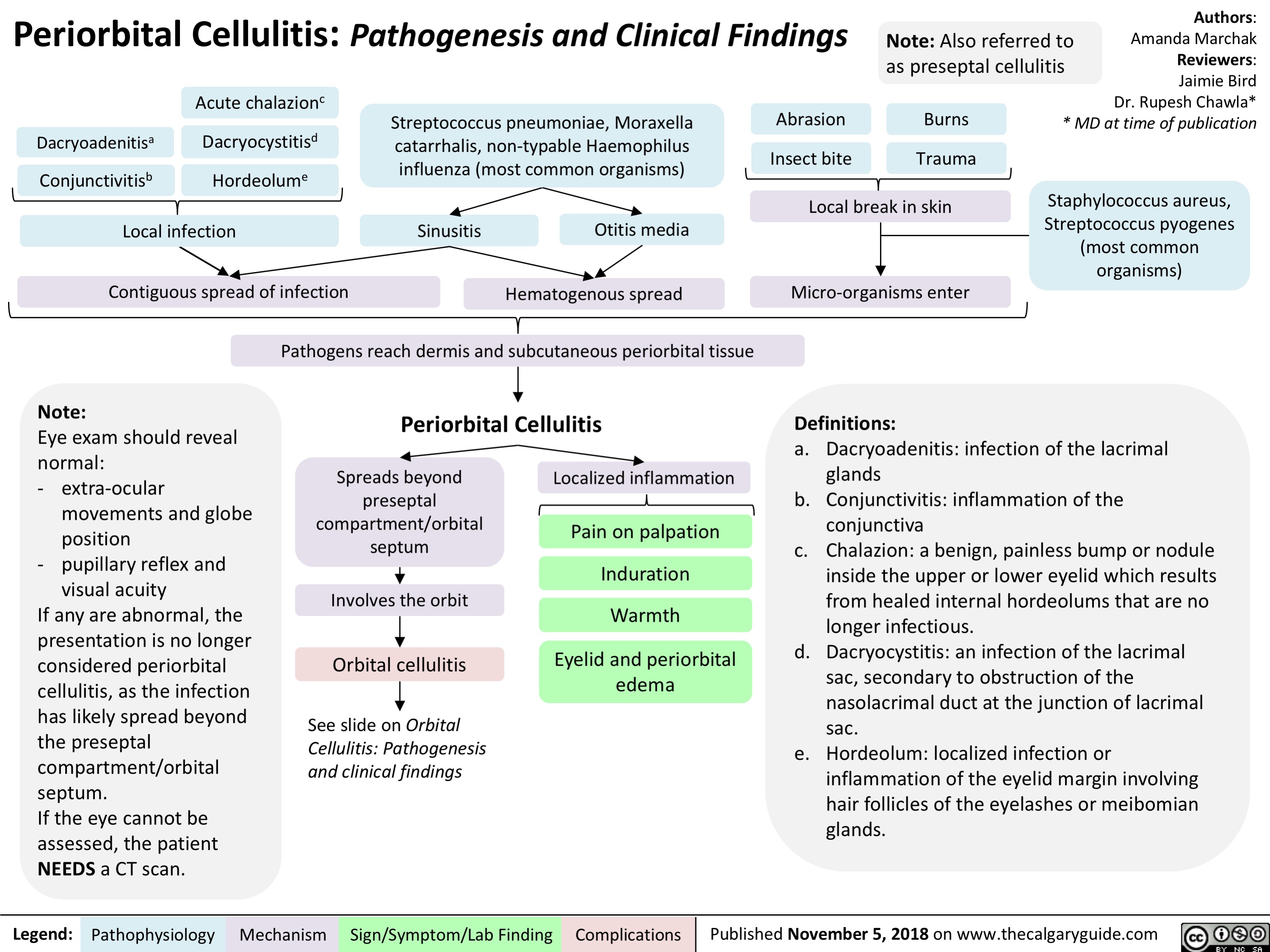
Periorbital Cellulitis: Pathogenesis and Clinical Findings
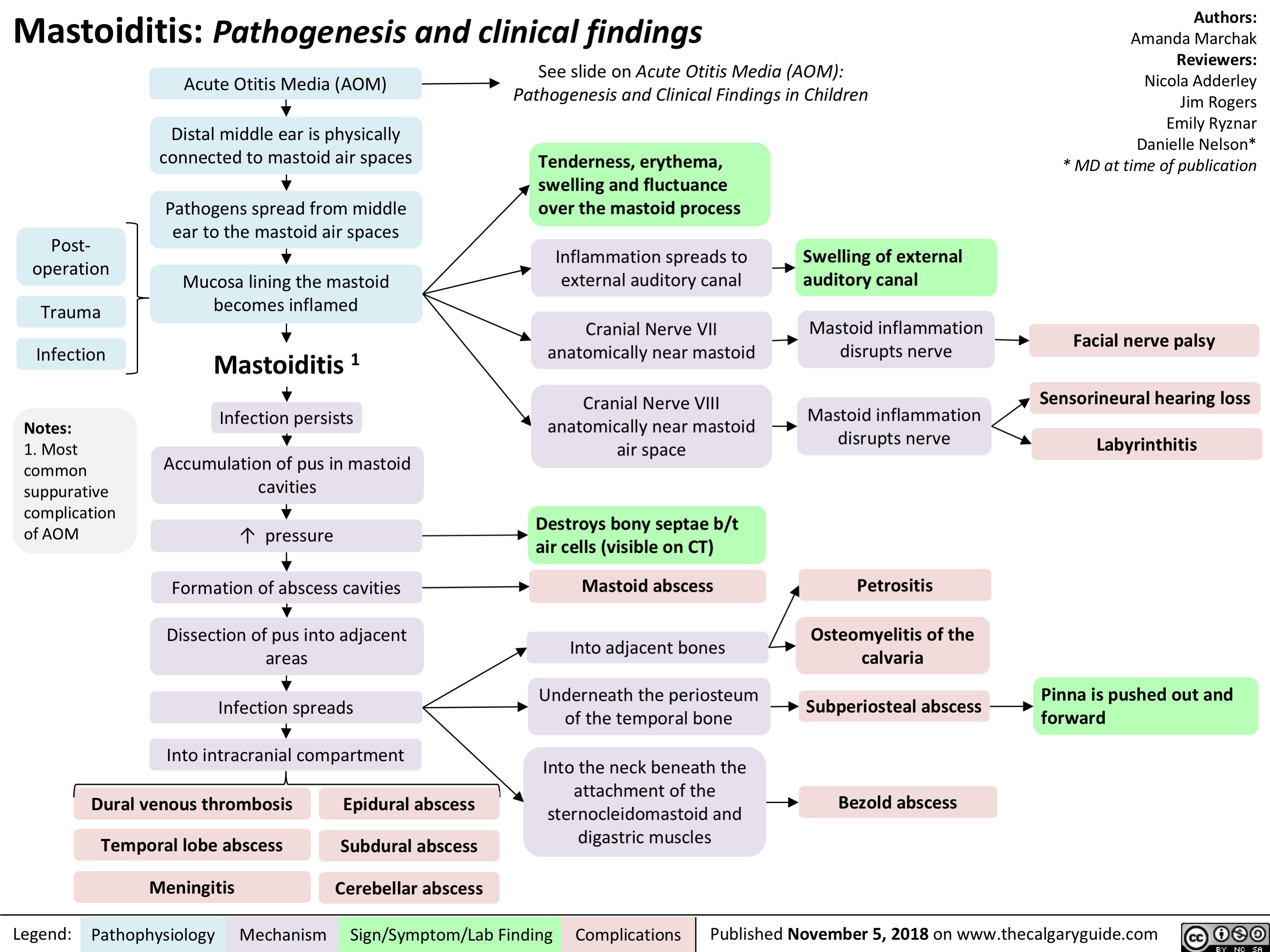
Mastoiditis: Pathogenesis and clinical findings
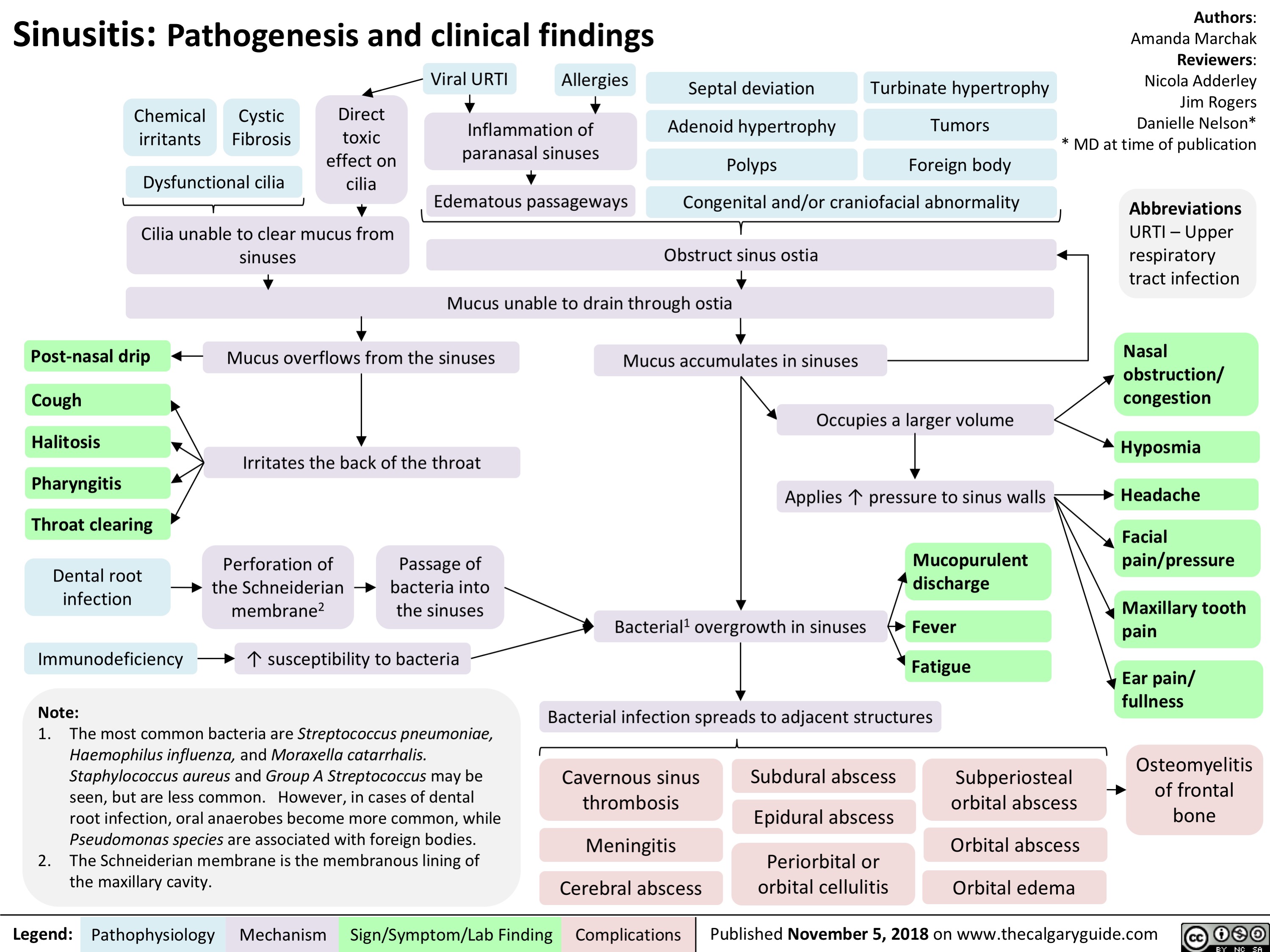
Sinusitis: Pathogenesis and clinical findings
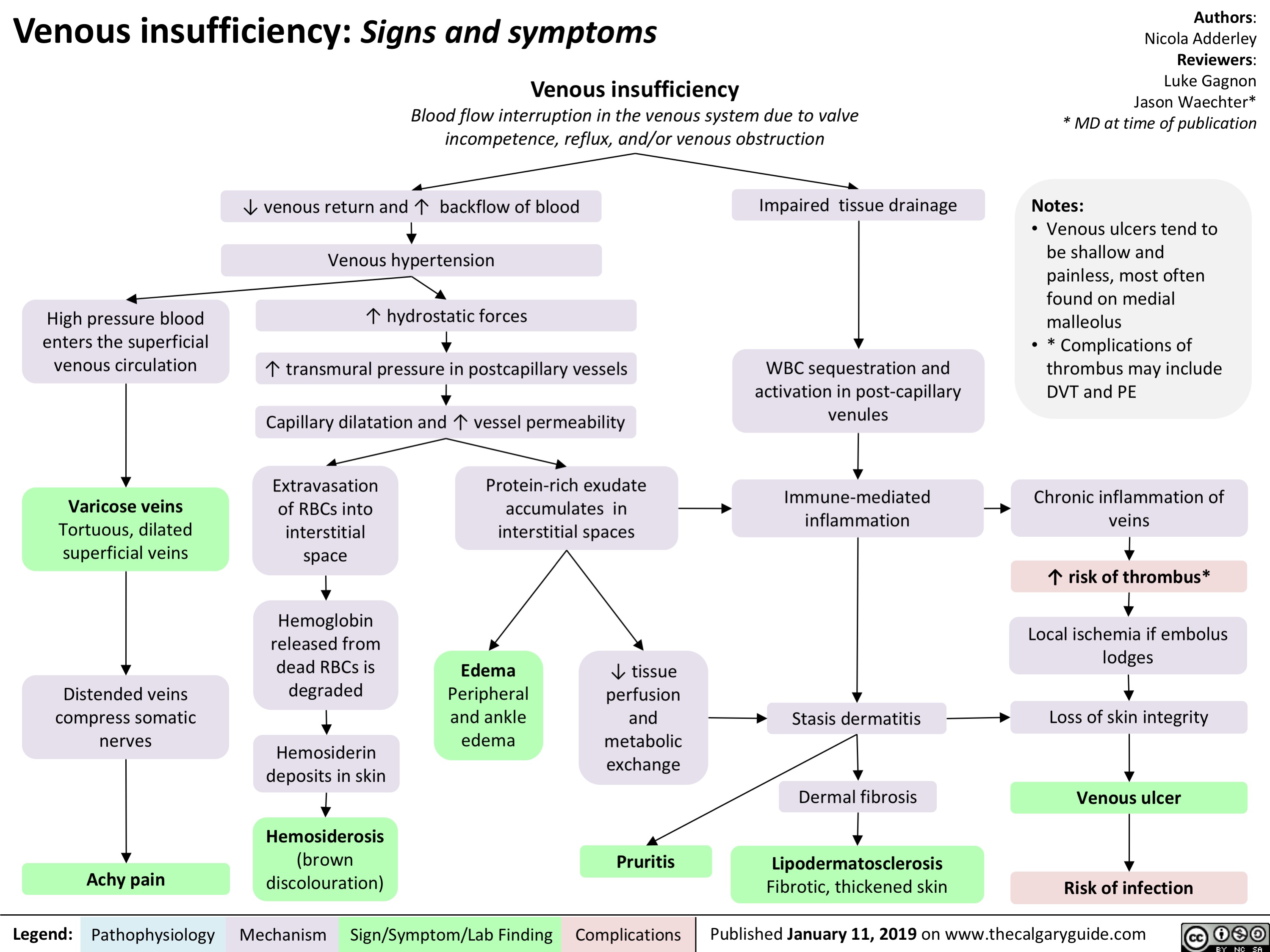
Venous insufficiency- Signs and symptoms
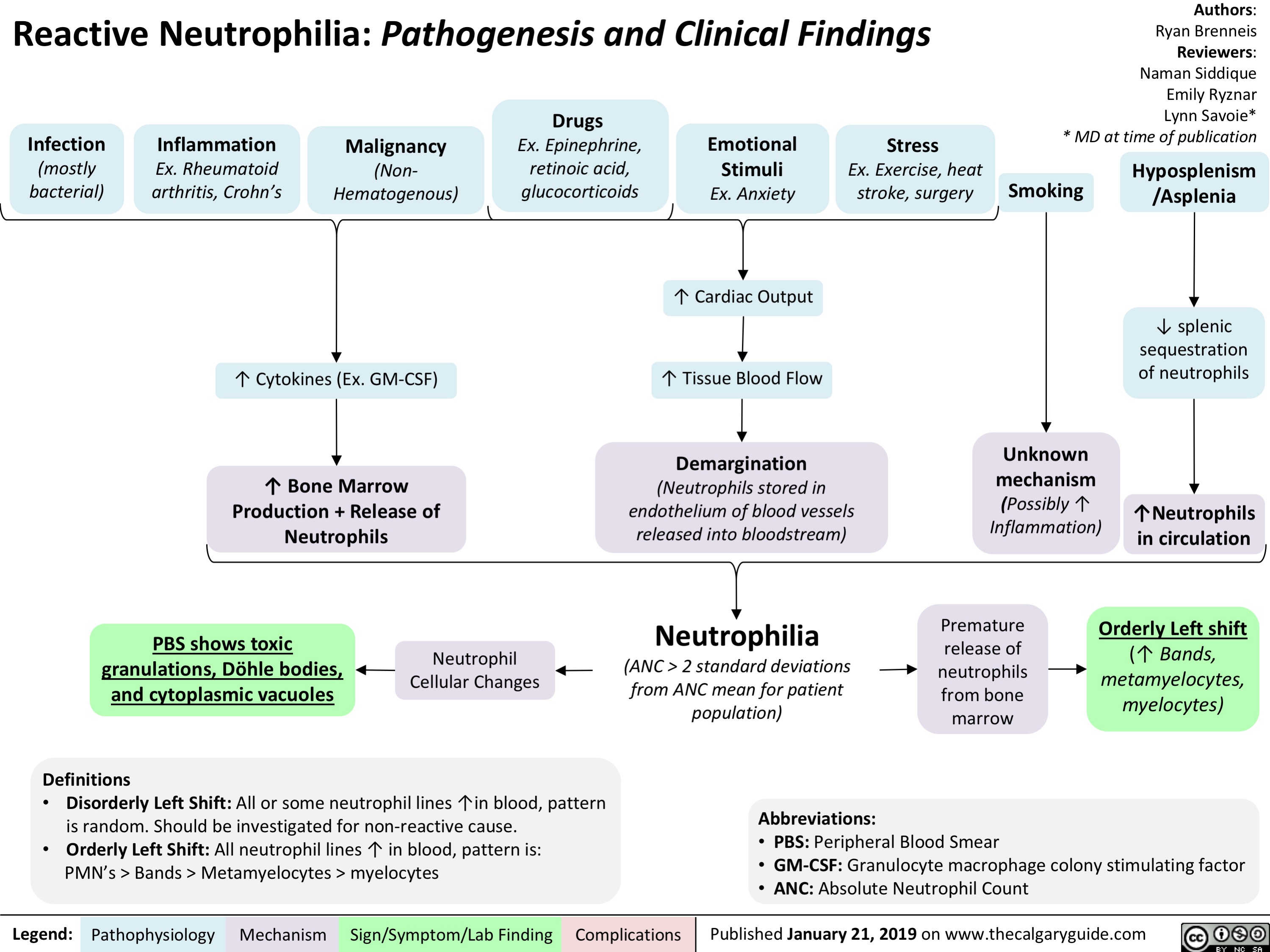
Reactive Neutrophilia- Pathogenesis and Clinical Findings
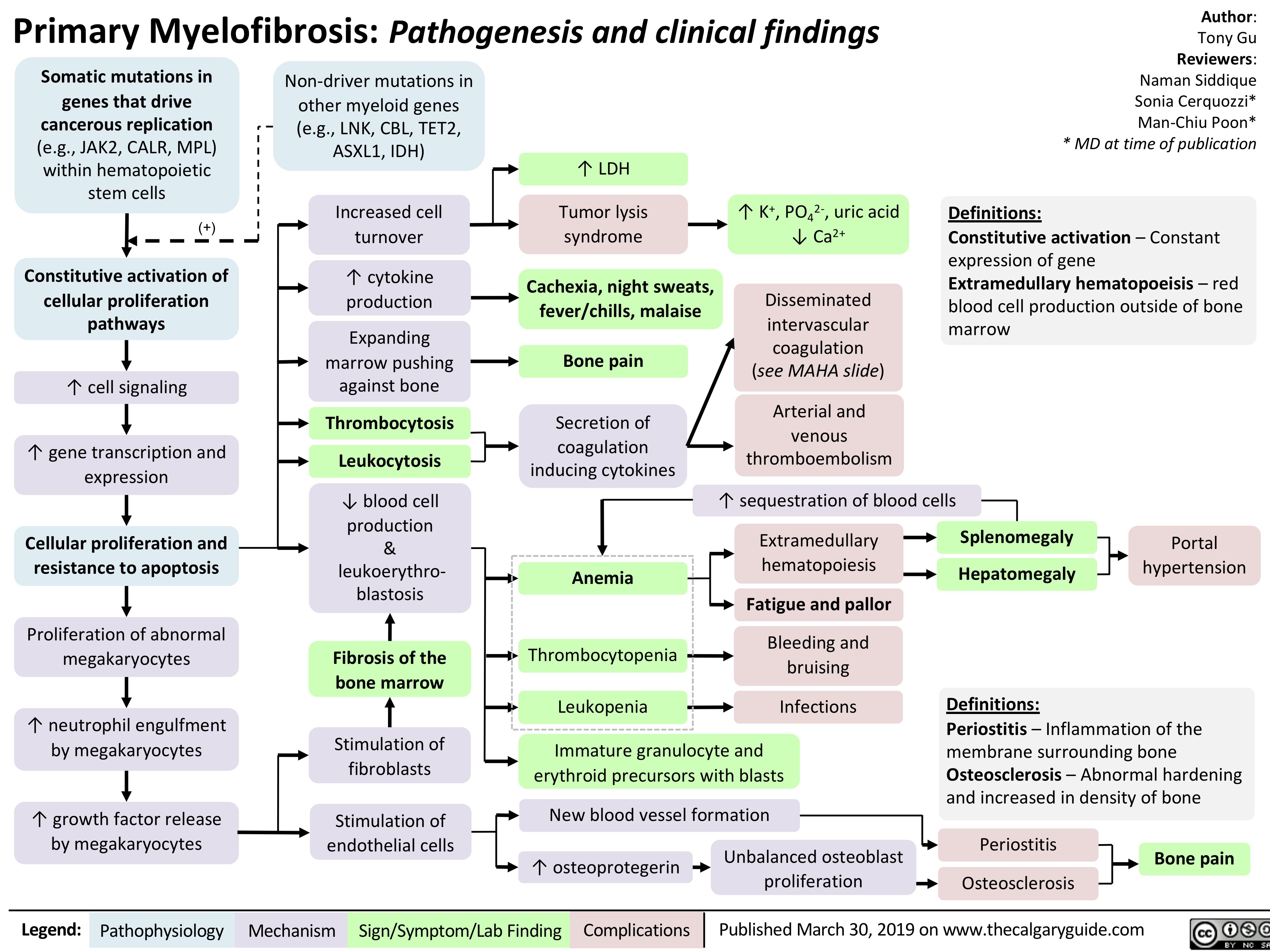
Primary Myelofibrosis pathogenesis and clinical findings
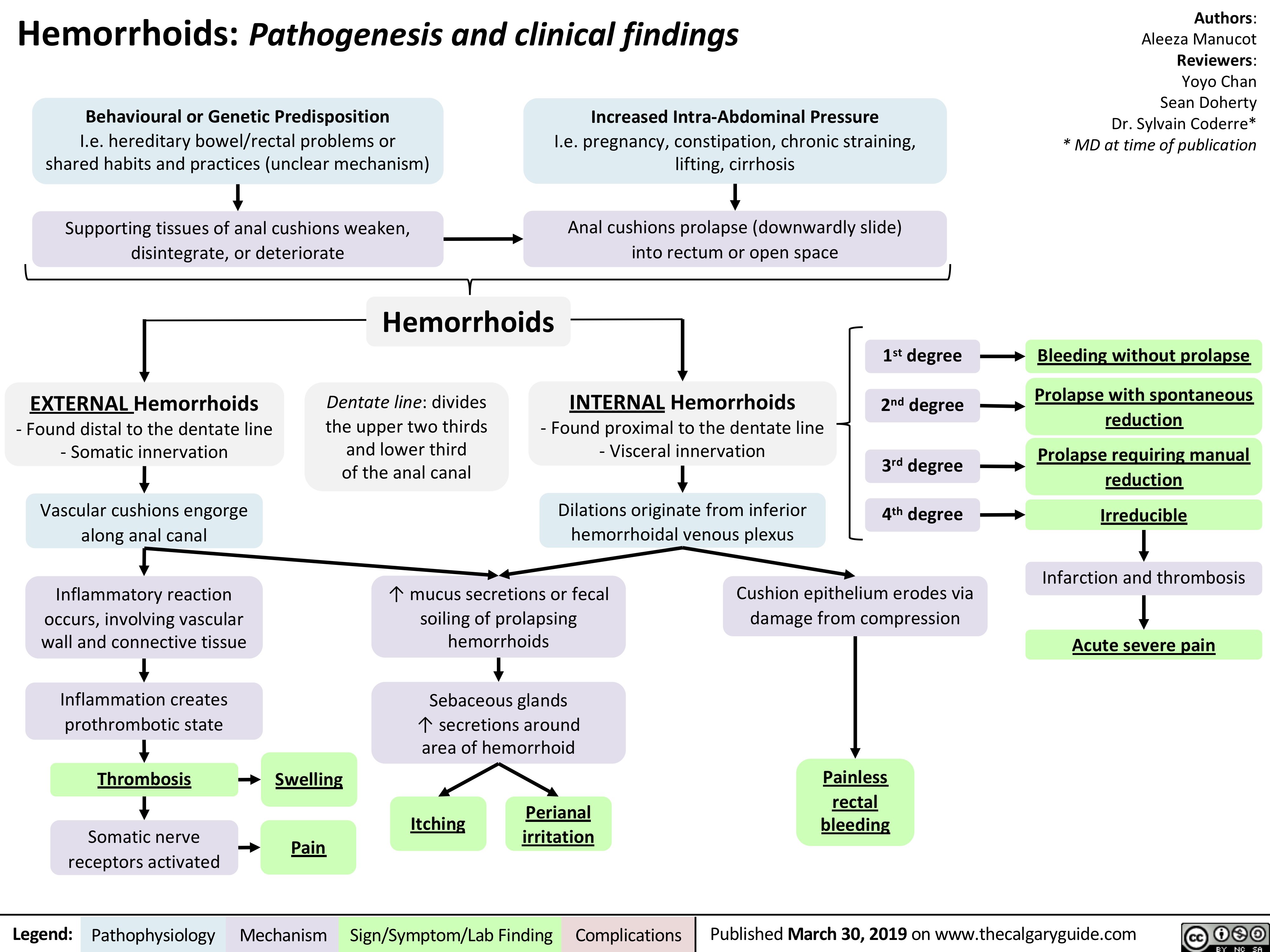
Hemorrhoids - Pathogenesis and Clinical Findings
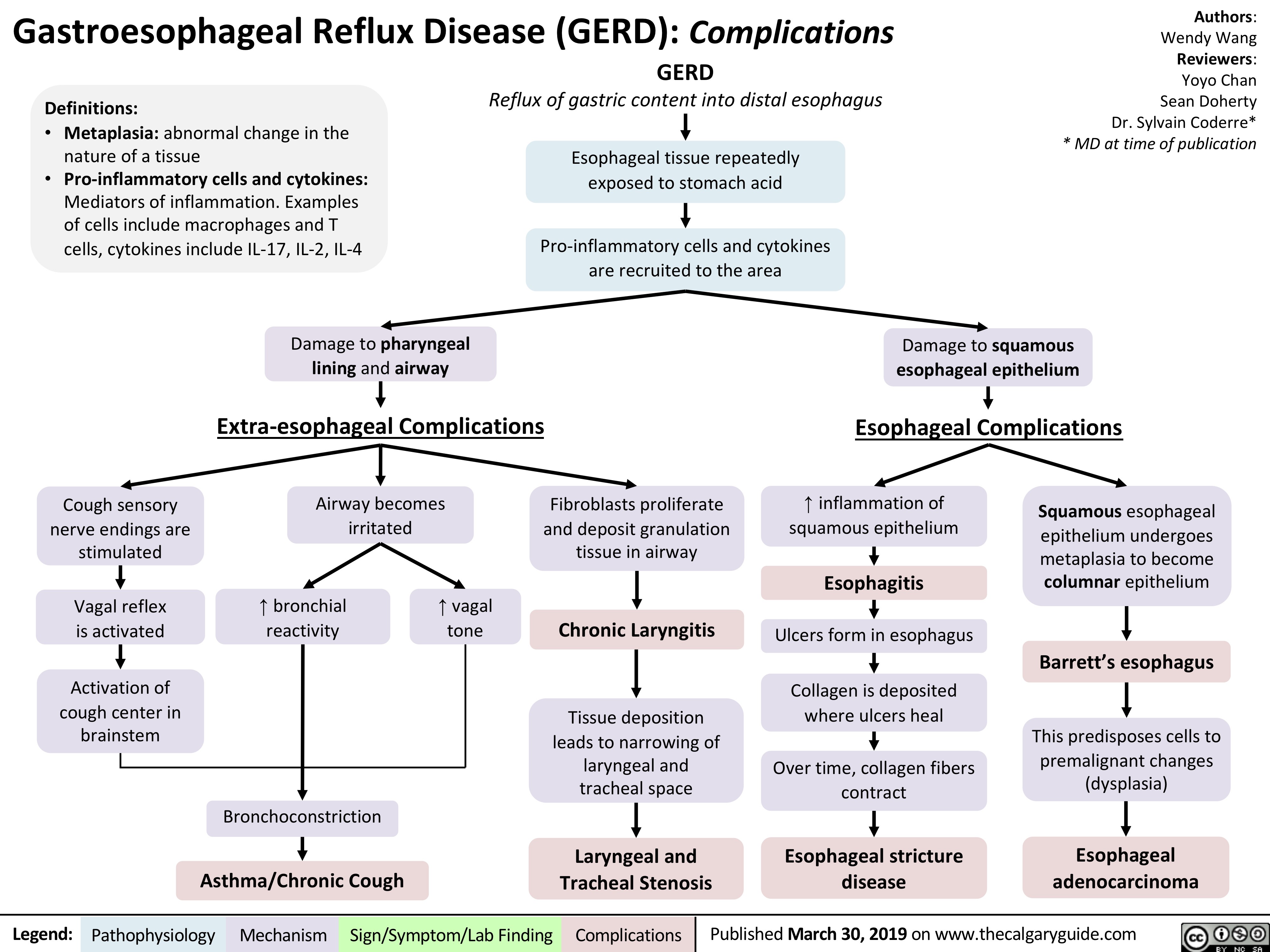
gastroesophageal-reflux-disease-gerd-complications
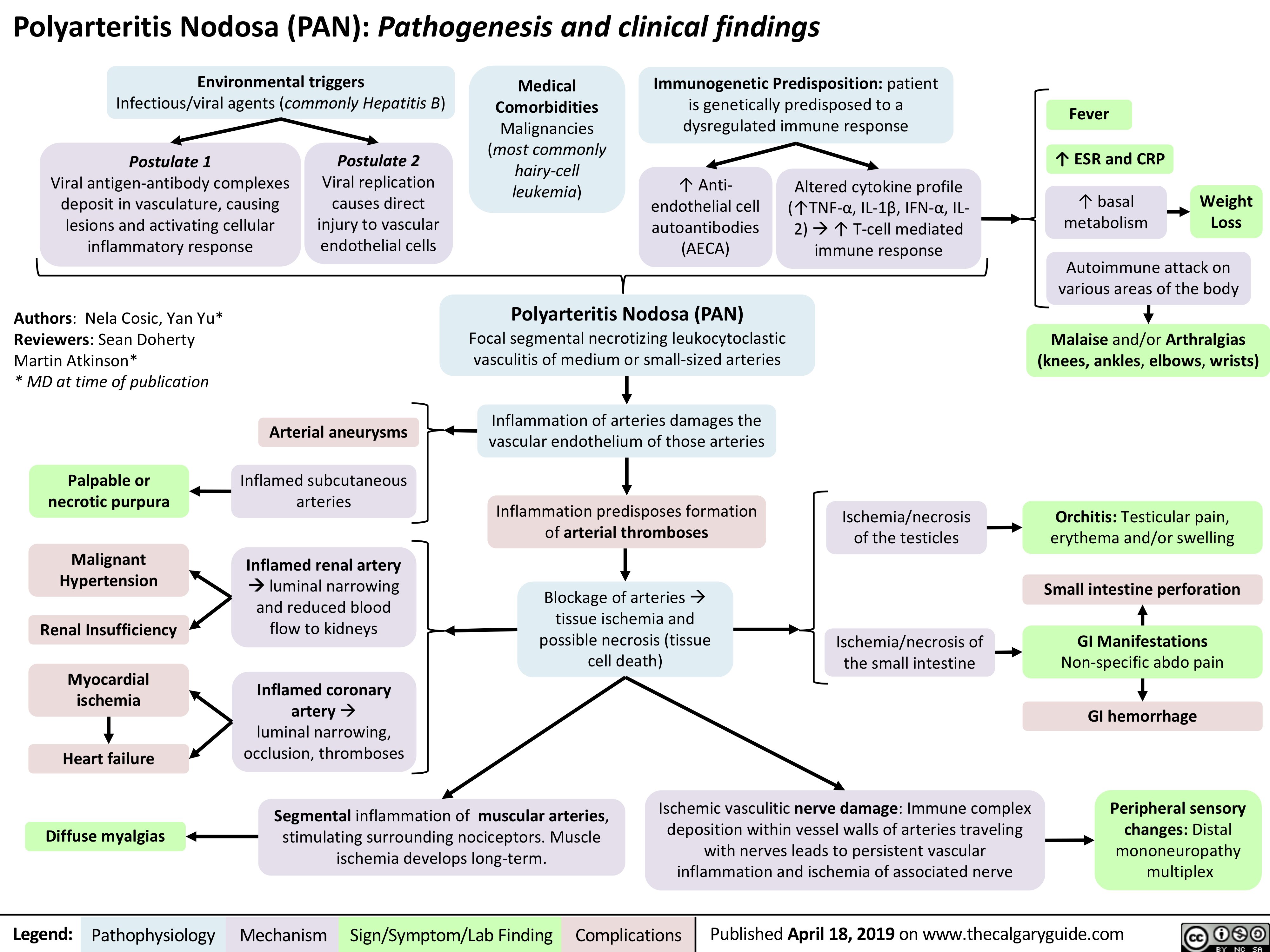
Polyarteritis Nodosa (PAN): Pathogenesis and Clinical Findings
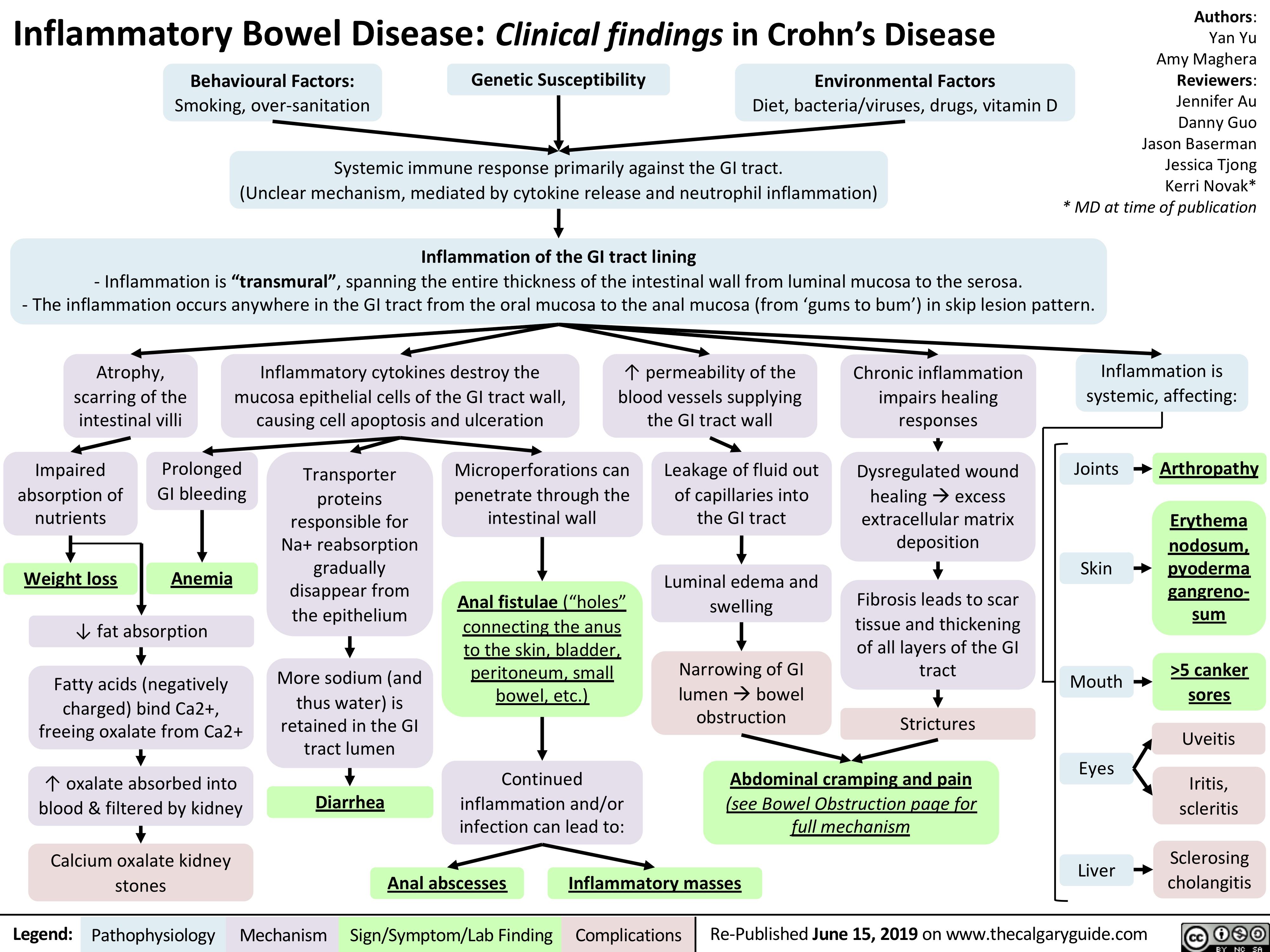
Crohn's Disease
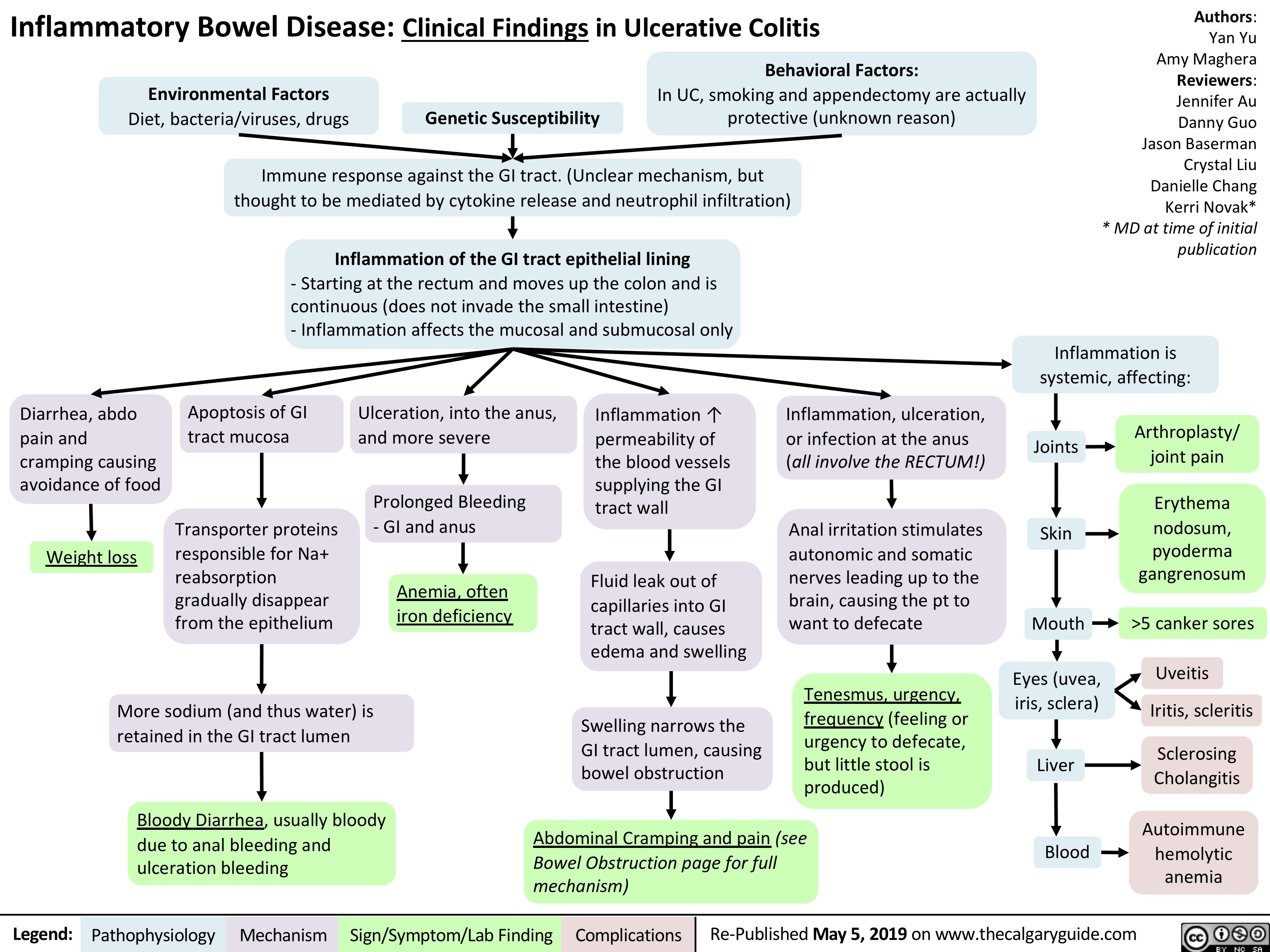
Ulcerative Colitis
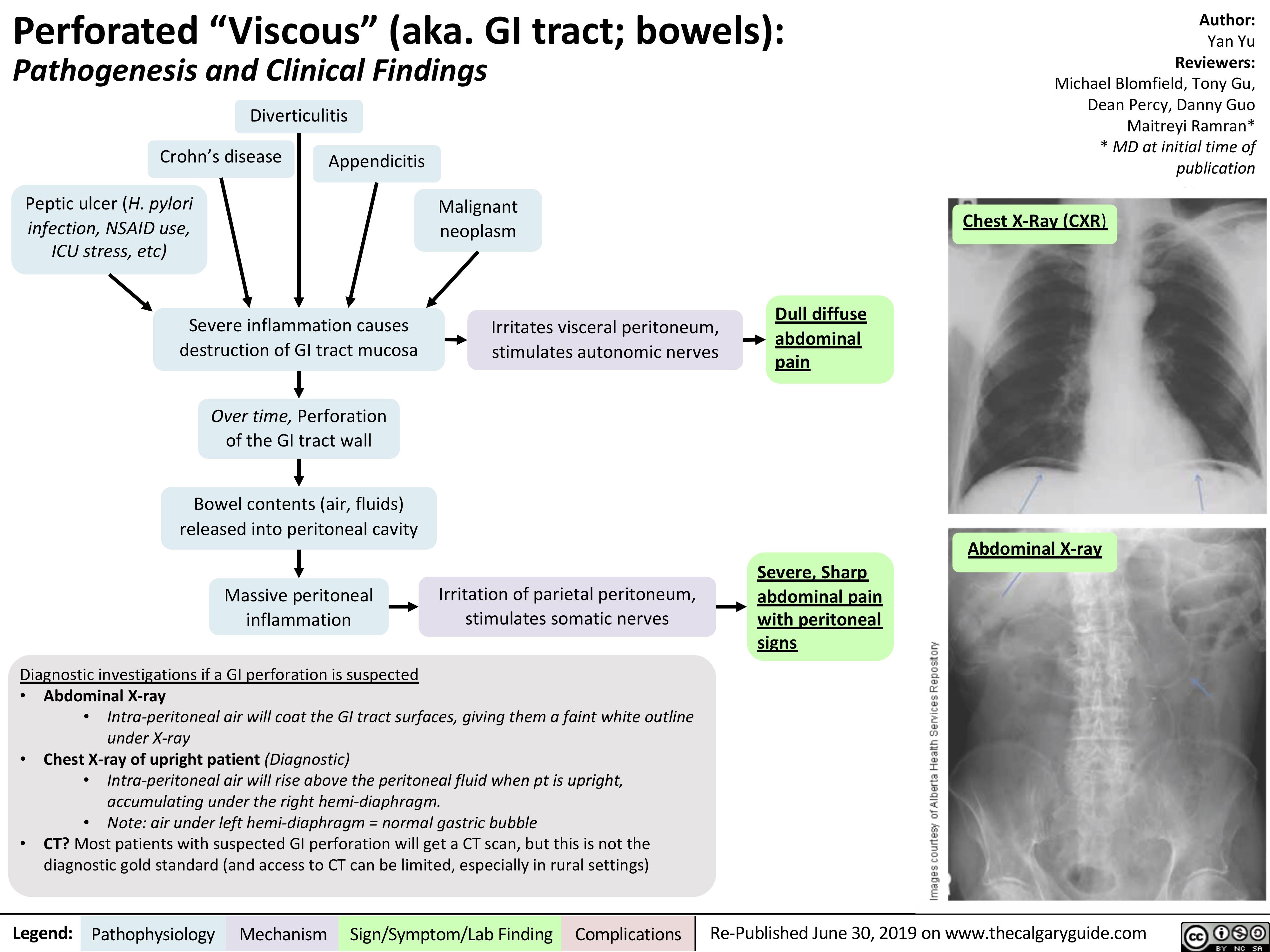
perforated-viscous
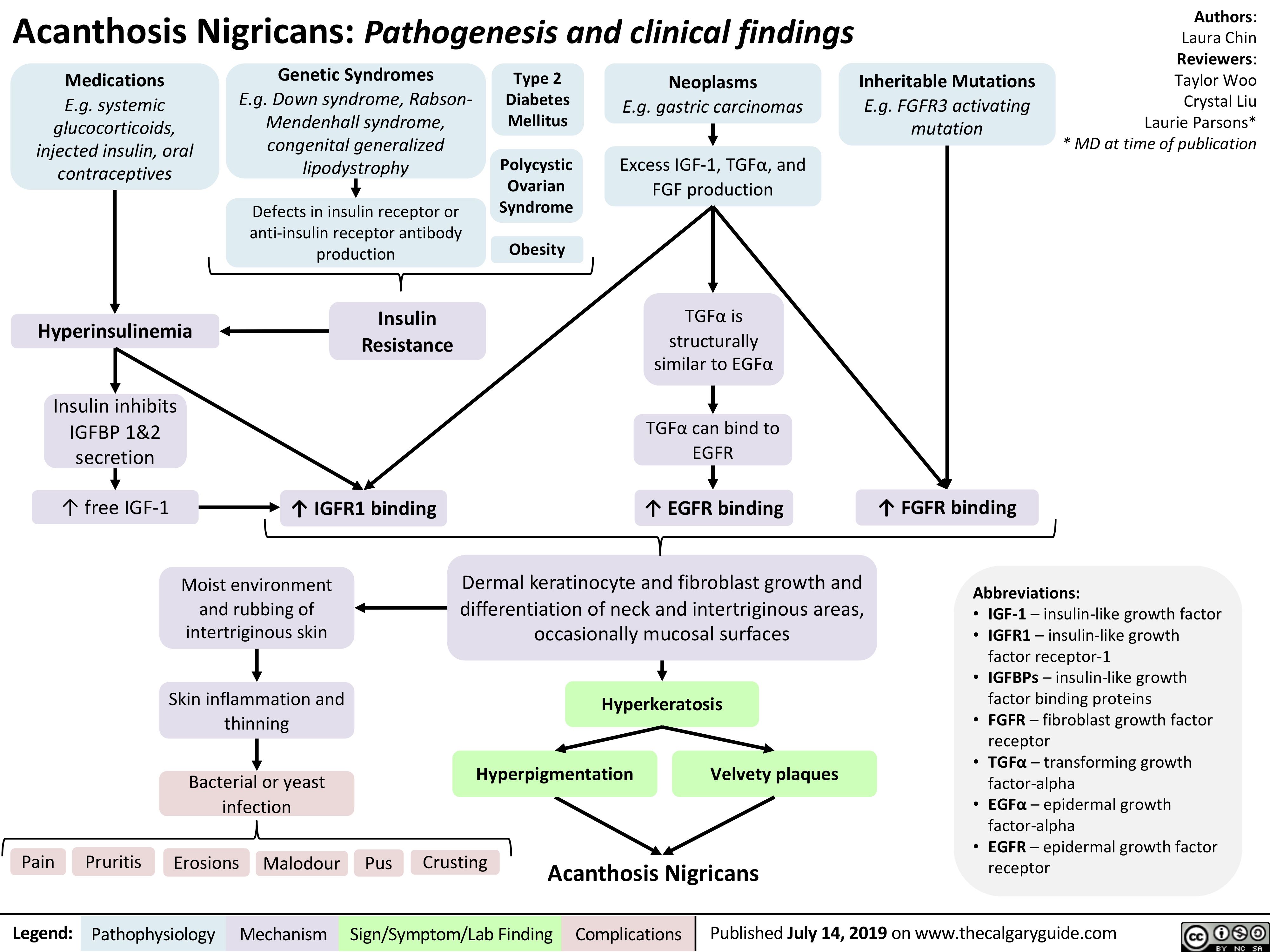
acanthosis-nigricans-pathogenesis-and-clinical-findings
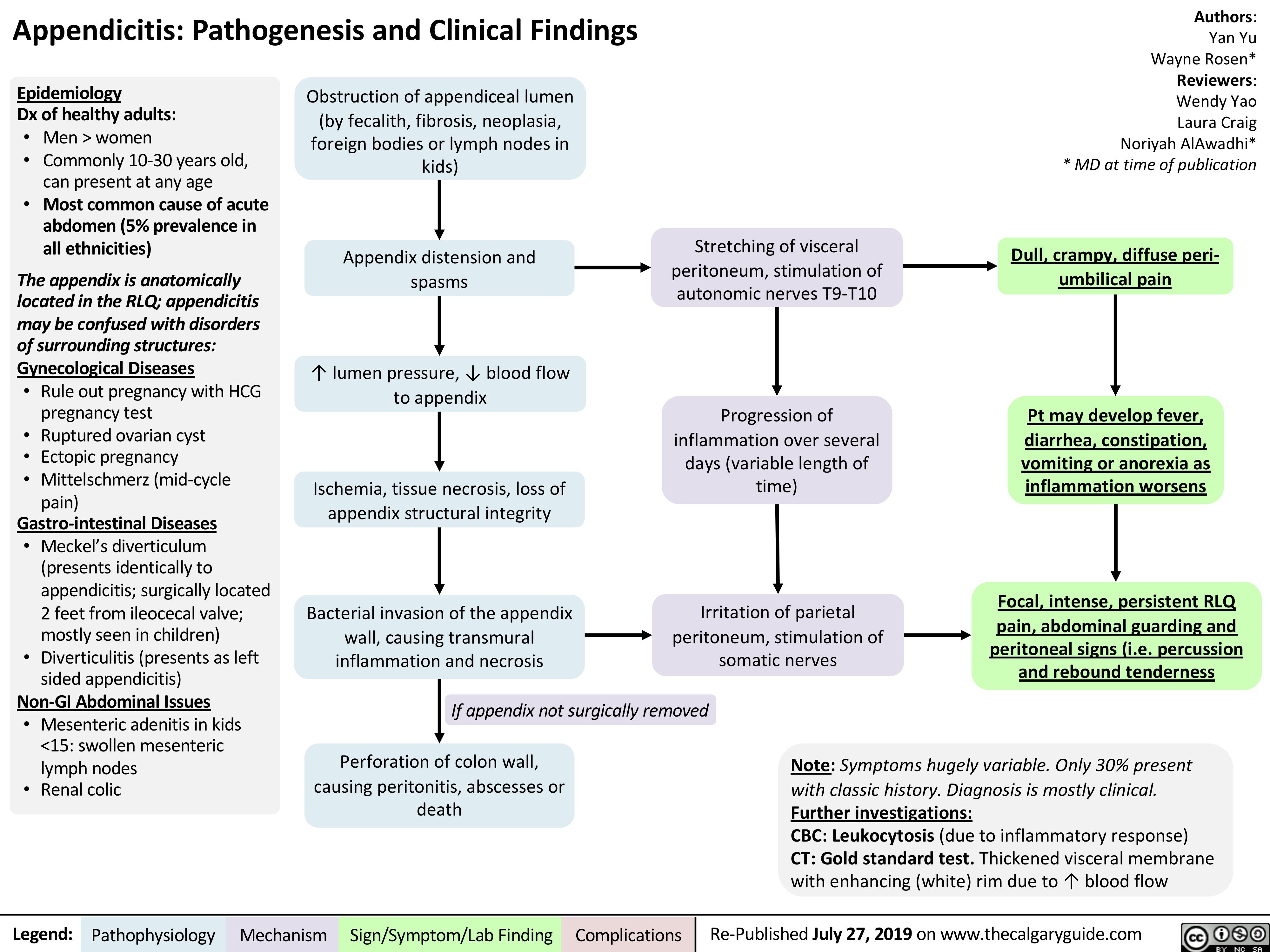
Appendicitis
Acute GI Related Abdominal Pain

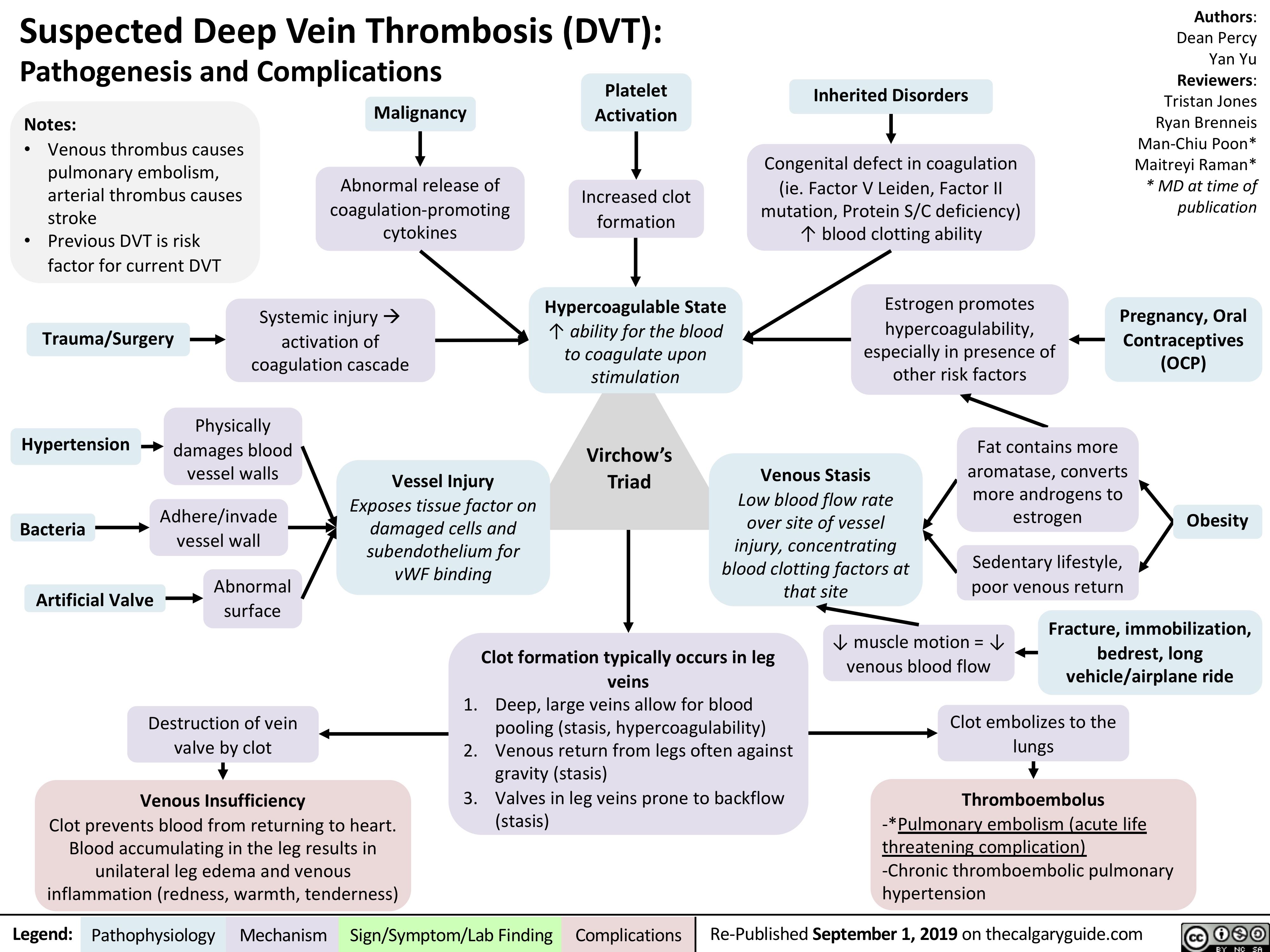
virchows-triad-and-deep-vein-thrombosis-dvt
Acute-Pancreatitis
![Acute Pancreatitis: Pathogenesis and Clinical Findings
Authors: Yan Yu Reviewers: Laura Craig Noriyah AlAwadhi Ryan Brenneis Maitreyi Raman* * MD at time of publication
Associated signs due to intra- abdominal hemorrhage from an unknown mechanism (classically associated with pancreatitis, but happens in <1% of cases):
Note:
It is not enough to just diagnose “acute pancreatitis”. Full management requires determining underlying etiology with further work-up.
Alcohol
↑ Toxic metabolites within pancreas and Spincter of Oddi Spasms
Gallstones
Migration to common bile duct blocks Sphincter of Oddi
Hypertriglyceridemia
Unknown
mechanism (rare)
Idiopathic
Further investigations:
CBC: Cell counts elevated, due to sever hypovolemia
Serum [Lipase]: Gold Standard Diagnostic Test; rupture of pancreatic cells releases lipase into circulation
Pancreatic secretions back up, ↑ pressure within pancreas
Hypercalcemia (Rare; Ca2+ depositions in bile ducts block outflow of pancreatic secretions)
Since pancreas is retroperitoneal, somatic
nerves in the parietal peritoneum are directly stimulated
Inflammation triggers cytokine release
Inflamed pancreas irritates adjacent intestines, causing ileus
Inflamed, more permeable blood vessels leak fluid into pancreas
• •
Cullen’s sign (bruising in peri-umbilical region) Grey-Turner’s sign (bruises along both flanks)
Sudden, severe epigastric pain (with peritoneal signs), radiates to the center of the back
Fever, nausea/vomiting
(general signs of inflammation)
Diminished bowel sounds Profound dehydration
(flat JVP, hypotension, tachycardia, oliguria) – may happen, not always
1. Pressure compresses pancreatic blood vessels, causing tissue ischemia.
2. Activation of inactive proteases (zymogens) digesting pancreatic tissue
Necrosis (death) of pancreatic cells
Inflammation self- perpetuates
Massive systemic inflammatory response
2 main complications, usually detected on CT;
may happen, but not always
1. Pancreatic pseudocyst (enlargement of the
pancreas due to fluid accumulation)
2. Pancreatic necrosis/abscesses (death of a part of the pancreas)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-published September 1, 2019 on thecalgaryguide.com
Acute Pancreatitis: Pathogenesis and Clinical Findings
Authors: Yan Yu Reviewers: Laura Craig Noriyah AlAwadhi Ryan Brenneis Maitreyi Raman* * MD at time of publication
Associated signs due to intra- abdominal hemorrhage from an unknown mechanism (classically associated with pancreatitis, but happens in <1% of cases):
Note:
It is not enough to just diagnose “acute pancreatitis”. Full management requires determining underlying etiology with further work-up.
Alcohol
↑ Toxic metabolites within pancreas and Spincter of Oddi Spasms
Gallstones
Migration to common bile duct blocks Sphincter of Oddi
Hypertriglyceridemia
Unknown
mechanism (rare)
Idiopathic
Further investigations:
CBC: Cell counts elevated, due to sever hypovolemia
Serum [Lipase]: Gold Standard Diagnostic Test; rupture of pancreatic cells releases lipase into circulation
Pancreatic secretions back up, ↑ pressure within pancreas
Hypercalcemia (Rare; Ca2+ depositions in bile ducts block outflow of pancreatic secretions)
Since pancreas is retroperitoneal, somatic
nerves in the parietal peritoneum are directly stimulated
Inflammation triggers cytokine release
Inflamed pancreas irritates adjacent intestines, causing ileus
Inflamed, more permeable blood vessels leak fluid into pancreas
• •
Cullen’s sign (bruising in peri-umbilical region) Grey-Turner’s sign (bruises along both flanks)
Sudden, severe epigastric pain (with peritoneal signs), radiates to the center of the back
Fever, nausea/vomiting
(general signs of inflammation)
Diminished bowel sounds Profound dehydration
(flat JVP, hypotension, tachycardia, oliguria) – may happen, not always
1. Pressure compresses pancreatic blood vessels, causing tissue ischemia.
2. Activation of inactive proteases (zymogens) digesting pancreatic tissue
Necrosis (death) of pancreatic cells
Inflammation self- perpetuates
Massive systemic inflammatory response
2 main complications, usually detected on CT;
may happen, but not always
1. Pancreatic pseudocyst (enlargement of the
pancreas due to fluid accumulation)
2. Pancreatic necrosis/abscesses (death of a part of the pancreas)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-published September 1, 2019 on thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/09/Acute-Pancreatitis.jpg)
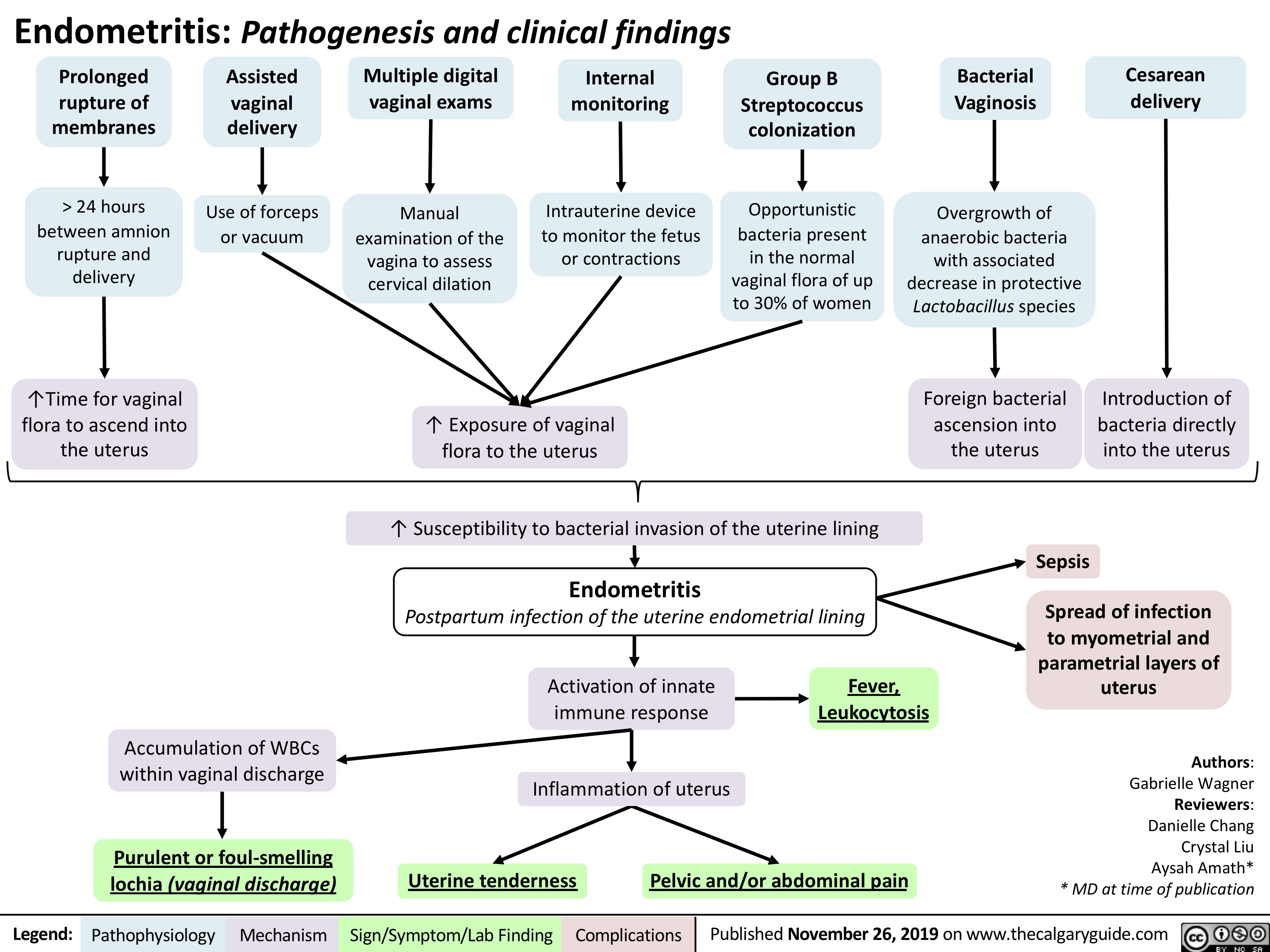
Endometritis
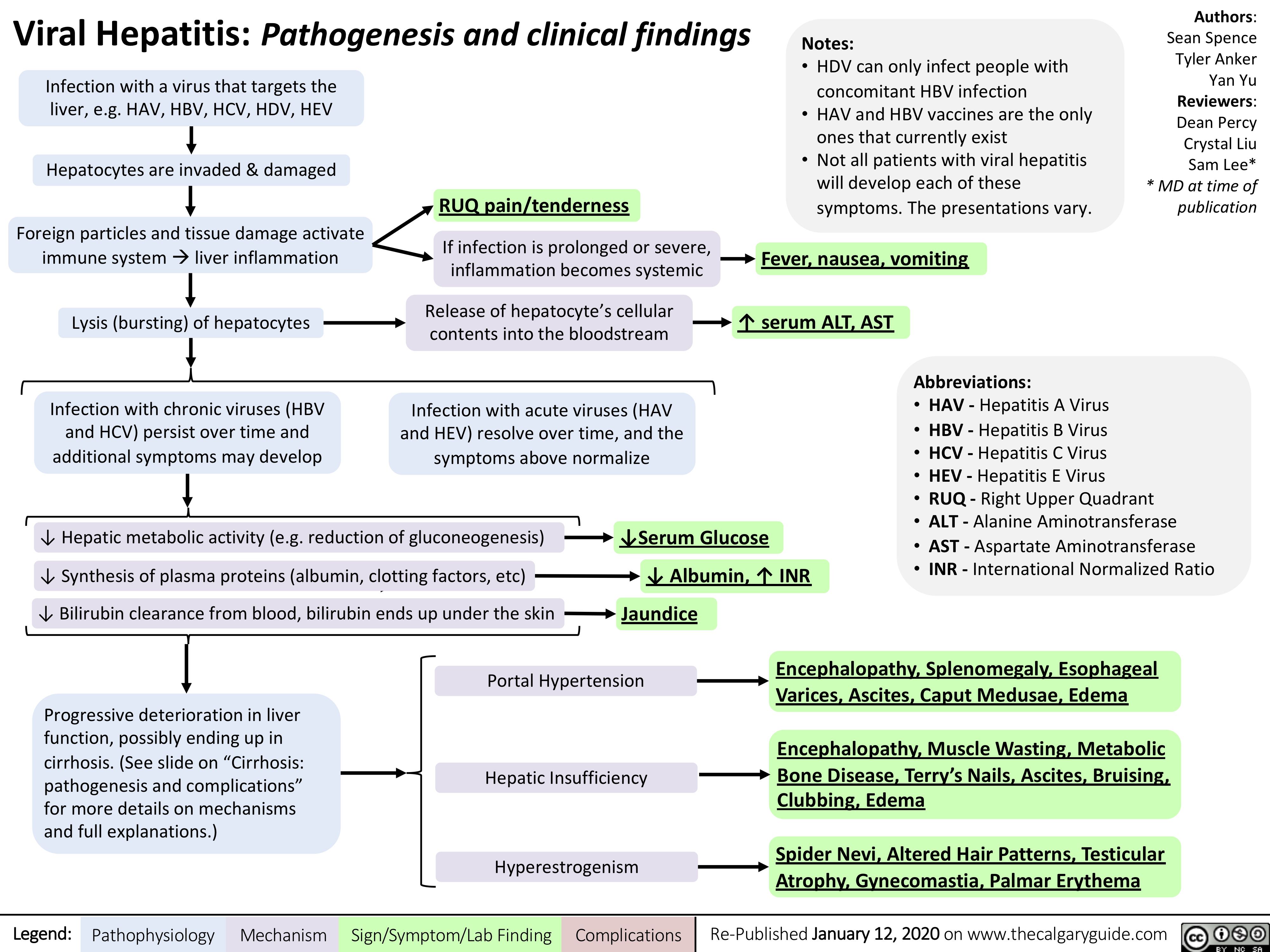
Viral-Hepatitis
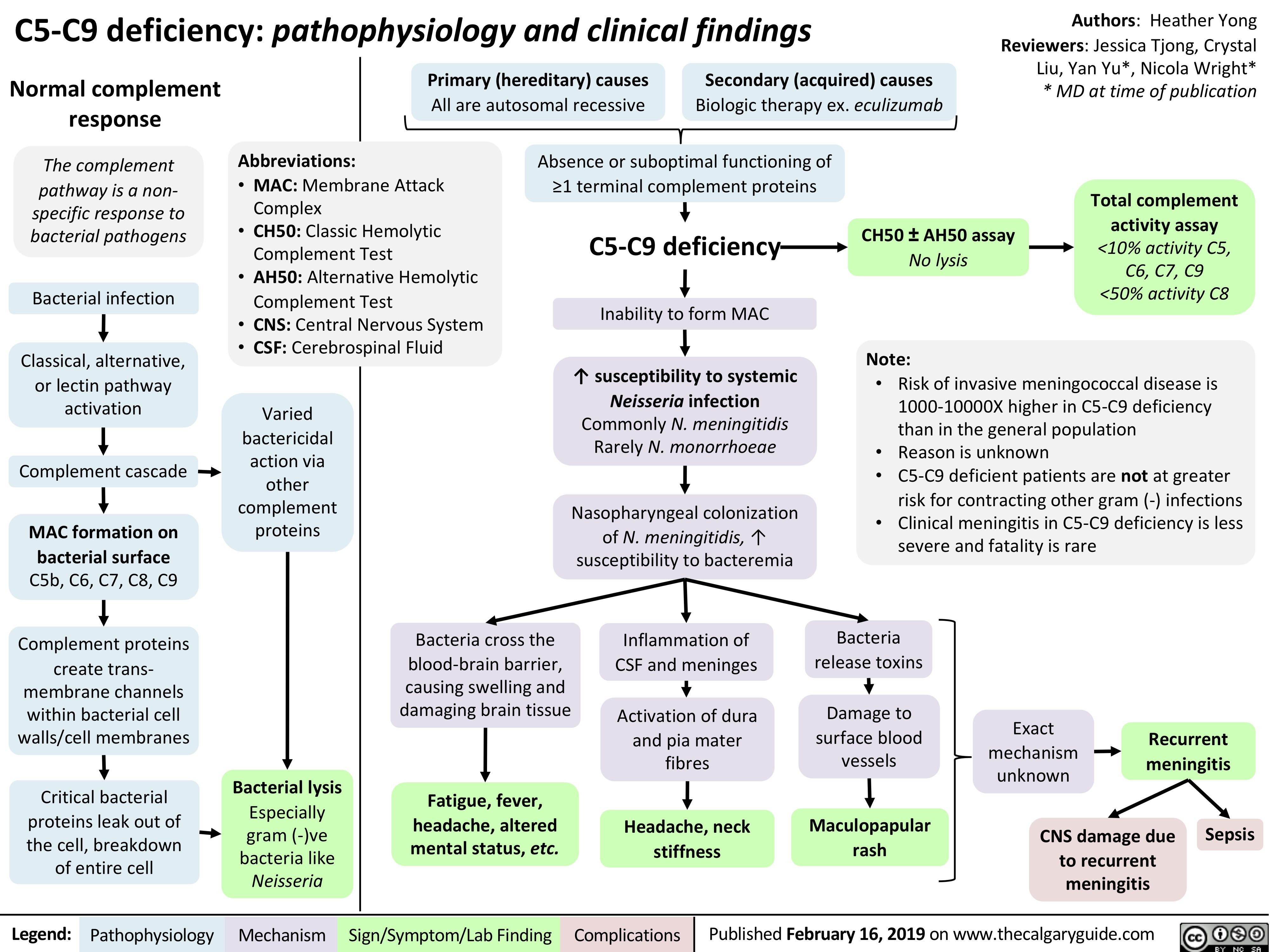
C5-C9-deficiency
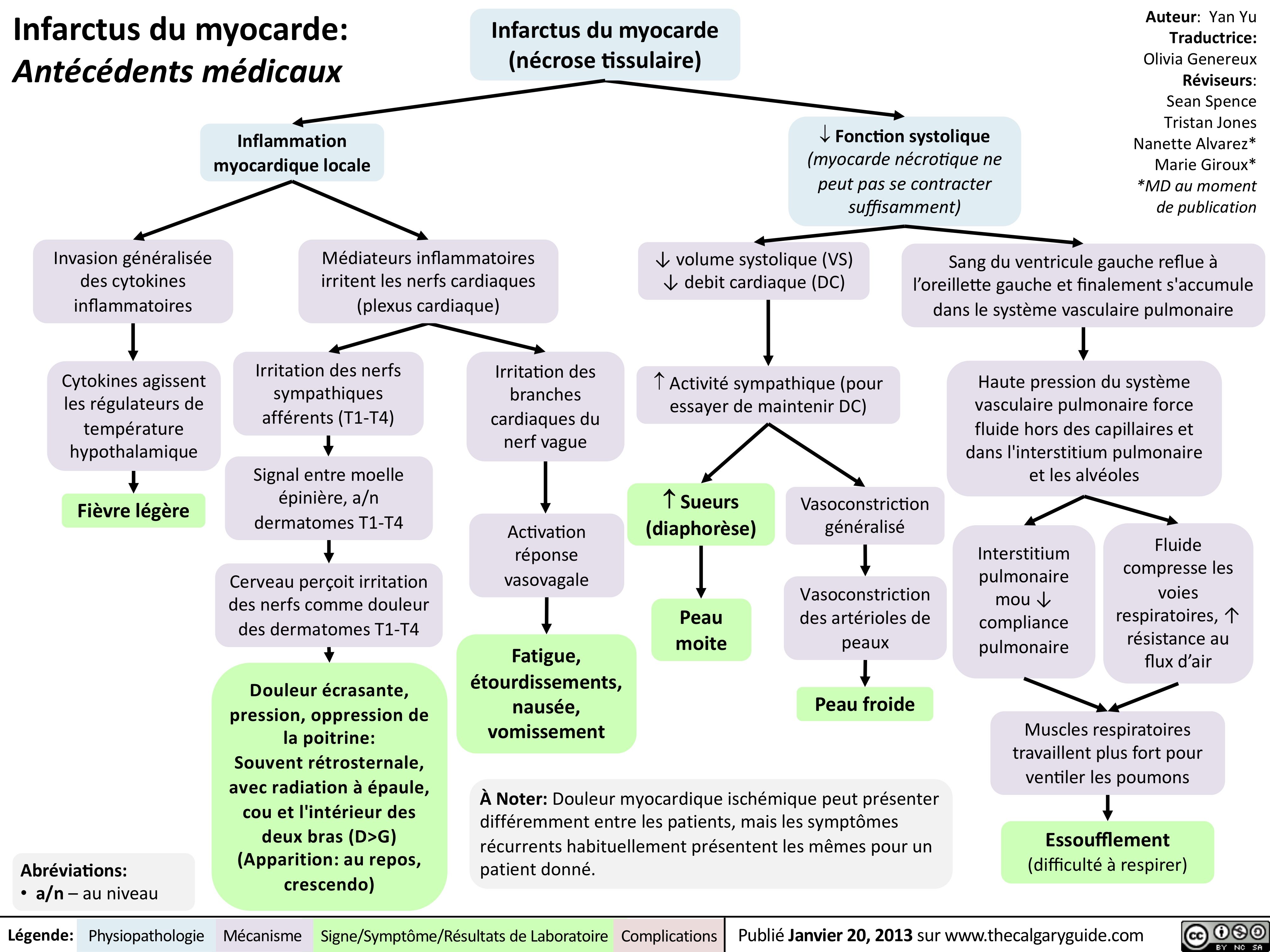
Infarctus du myocarde: Antécédents médicaux
acute-pancreatitis-complications
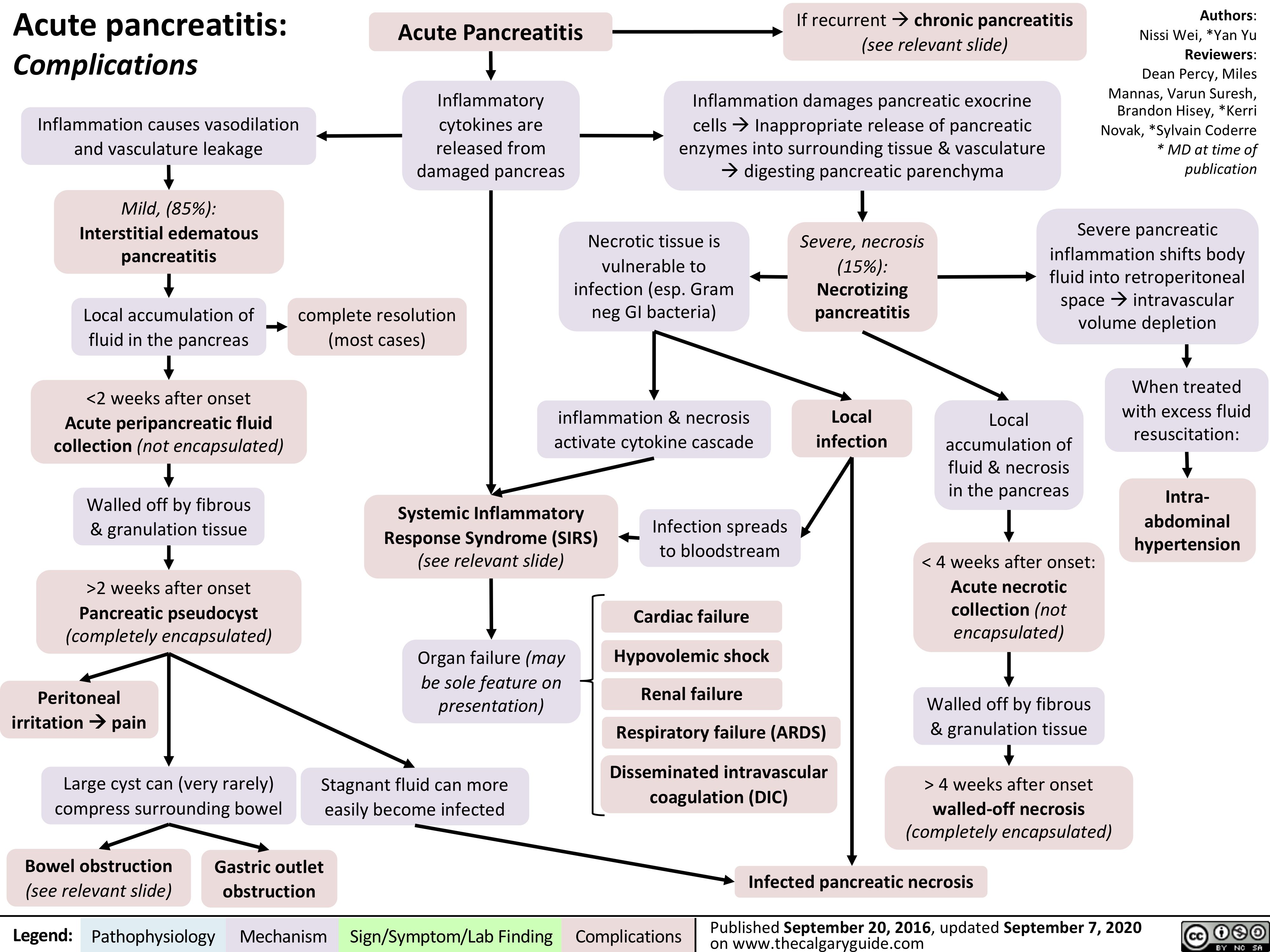
Multiple-Sclerosis-on-Brain-MRI
Bronchiolitis-updated
Potassium-Sparing-Diuretics-Mechanism-of-Action-and-Side-Effects
AAA-Pathogenesis
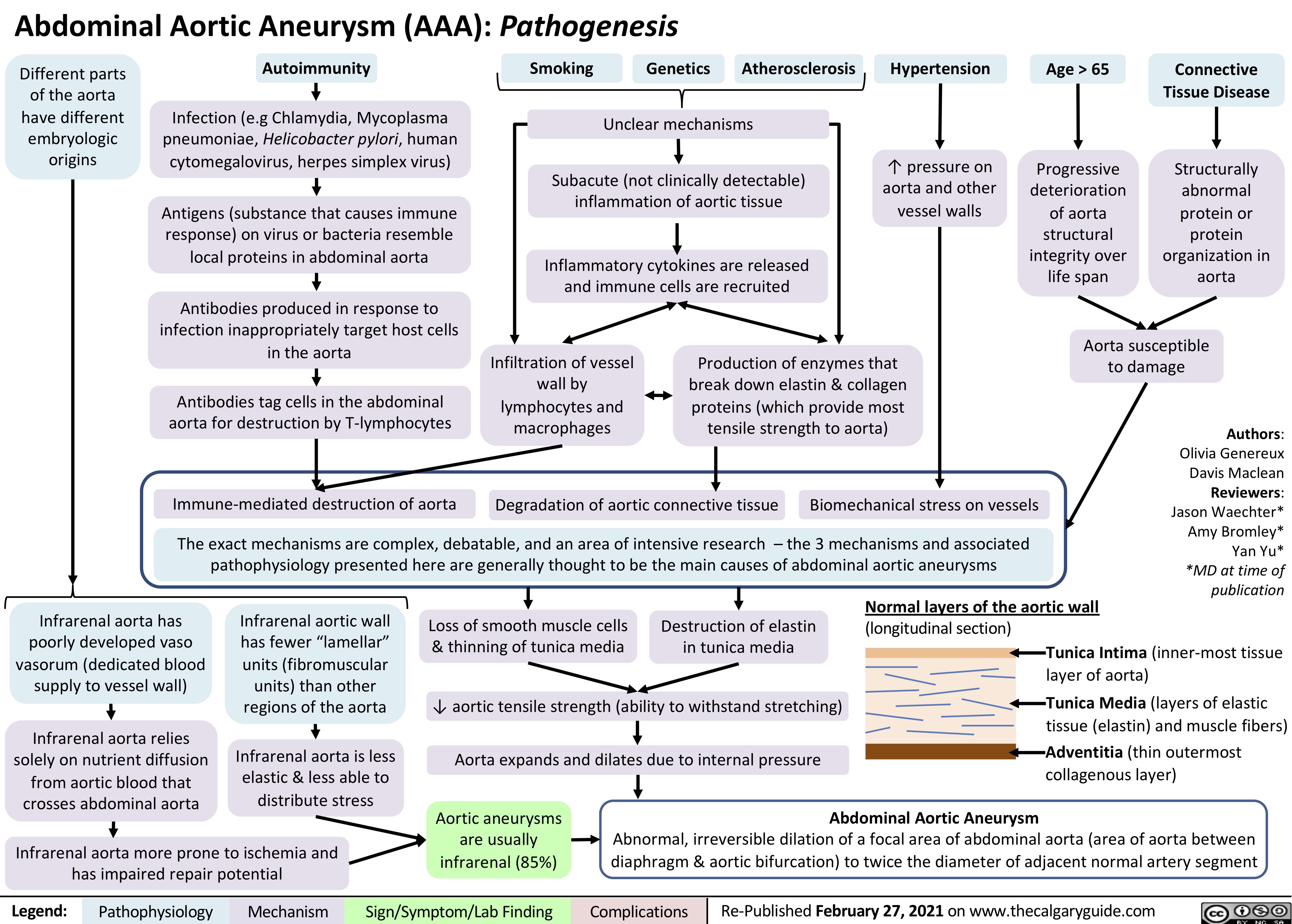
Cubital-Tunnel-Syndrome-Ulnar-Neuropathy
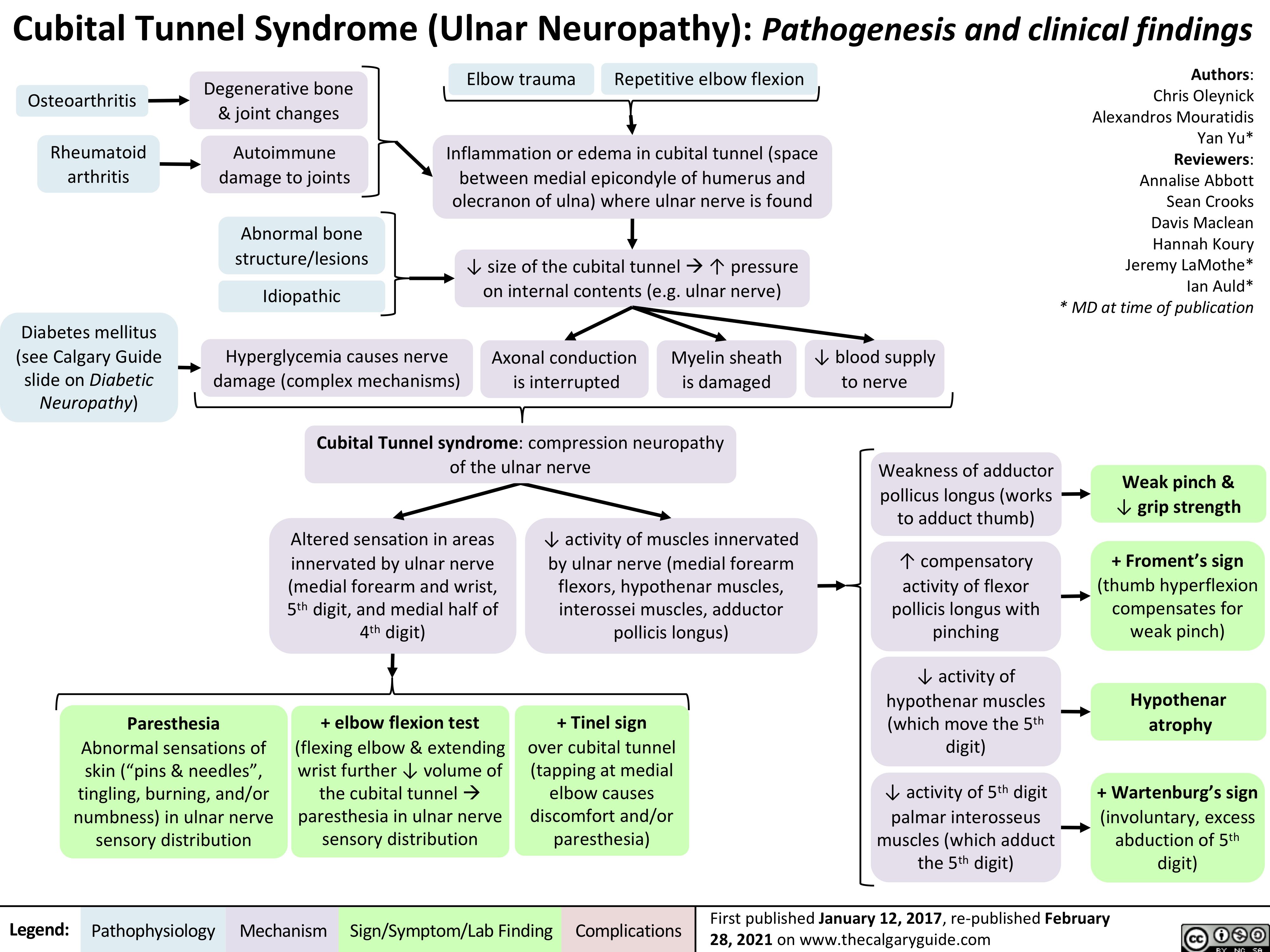
Achilles-Tendon-Rupture
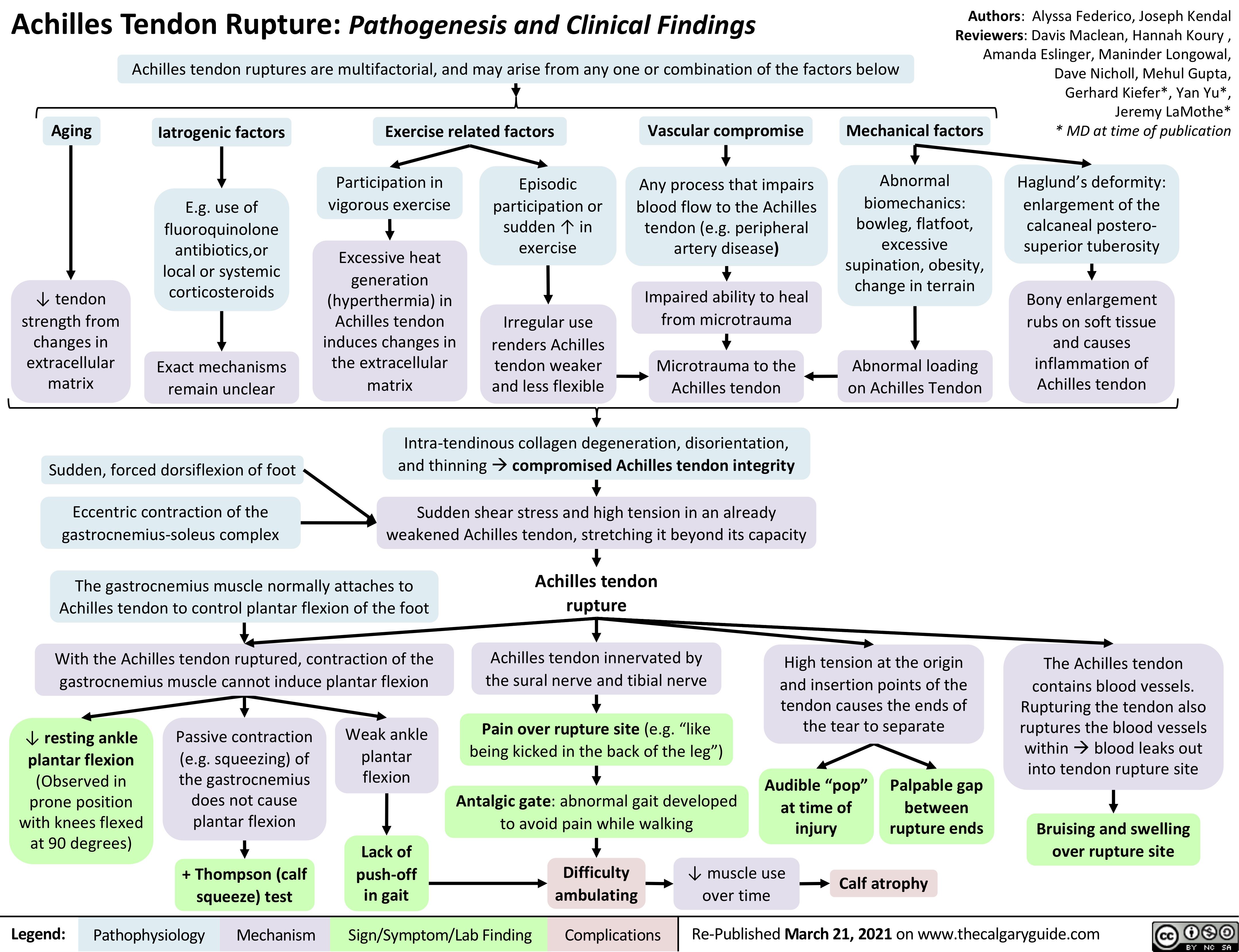
Thyroïdite
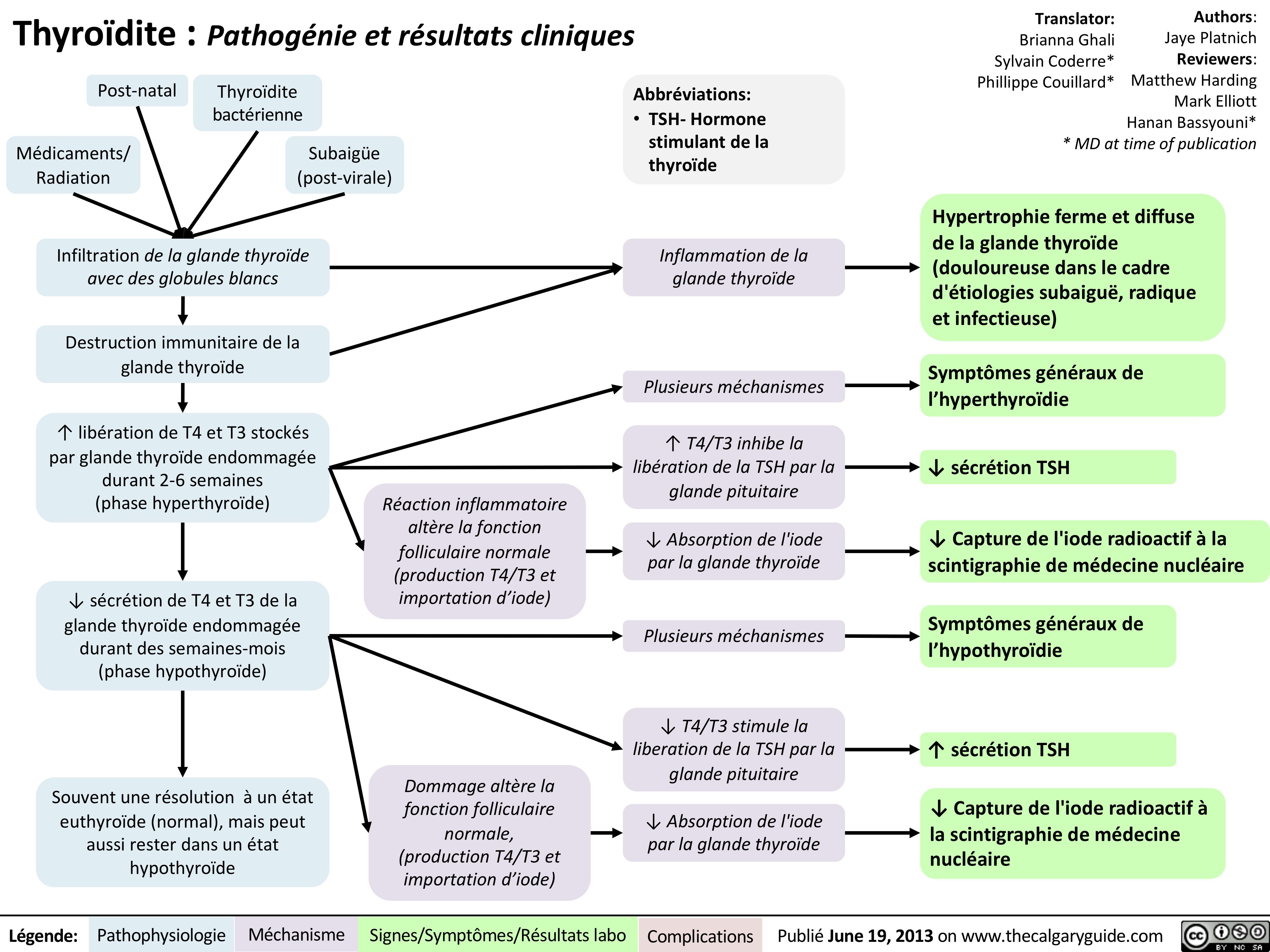
Fat-Embolism-Syndrome
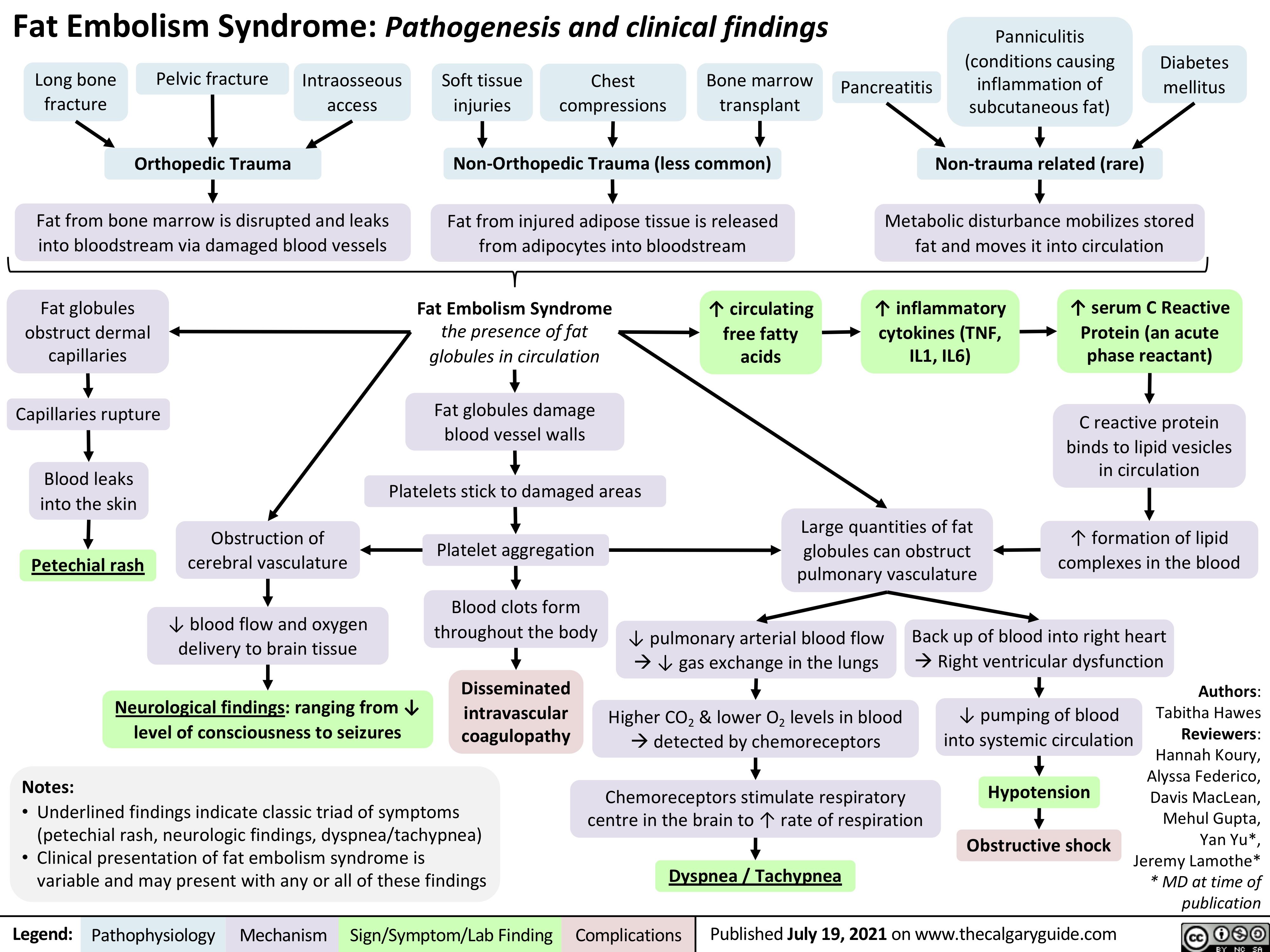
Dry-Eye-Syndrome-Clinical-Findings
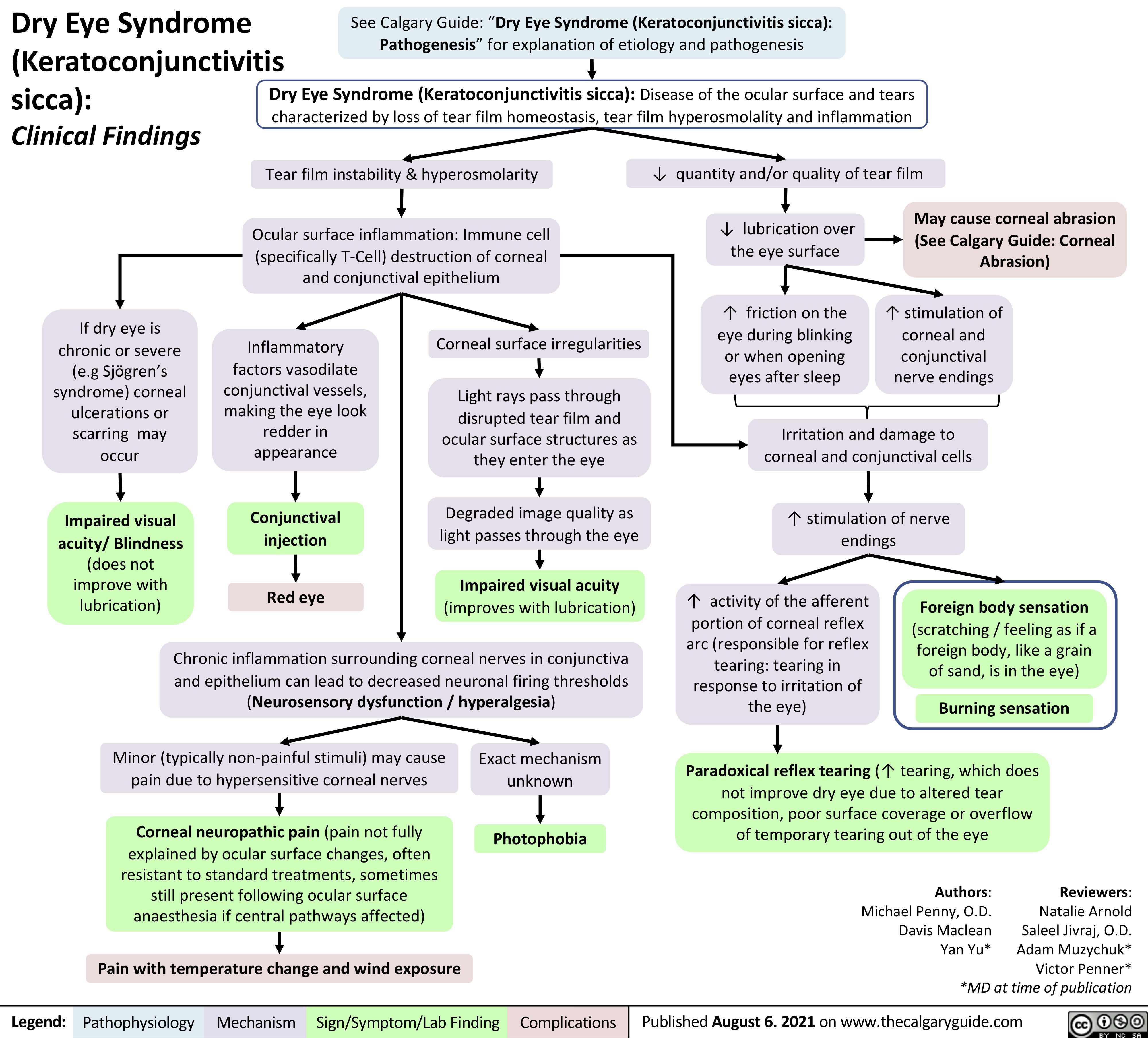
COPD Acute Exacerbations
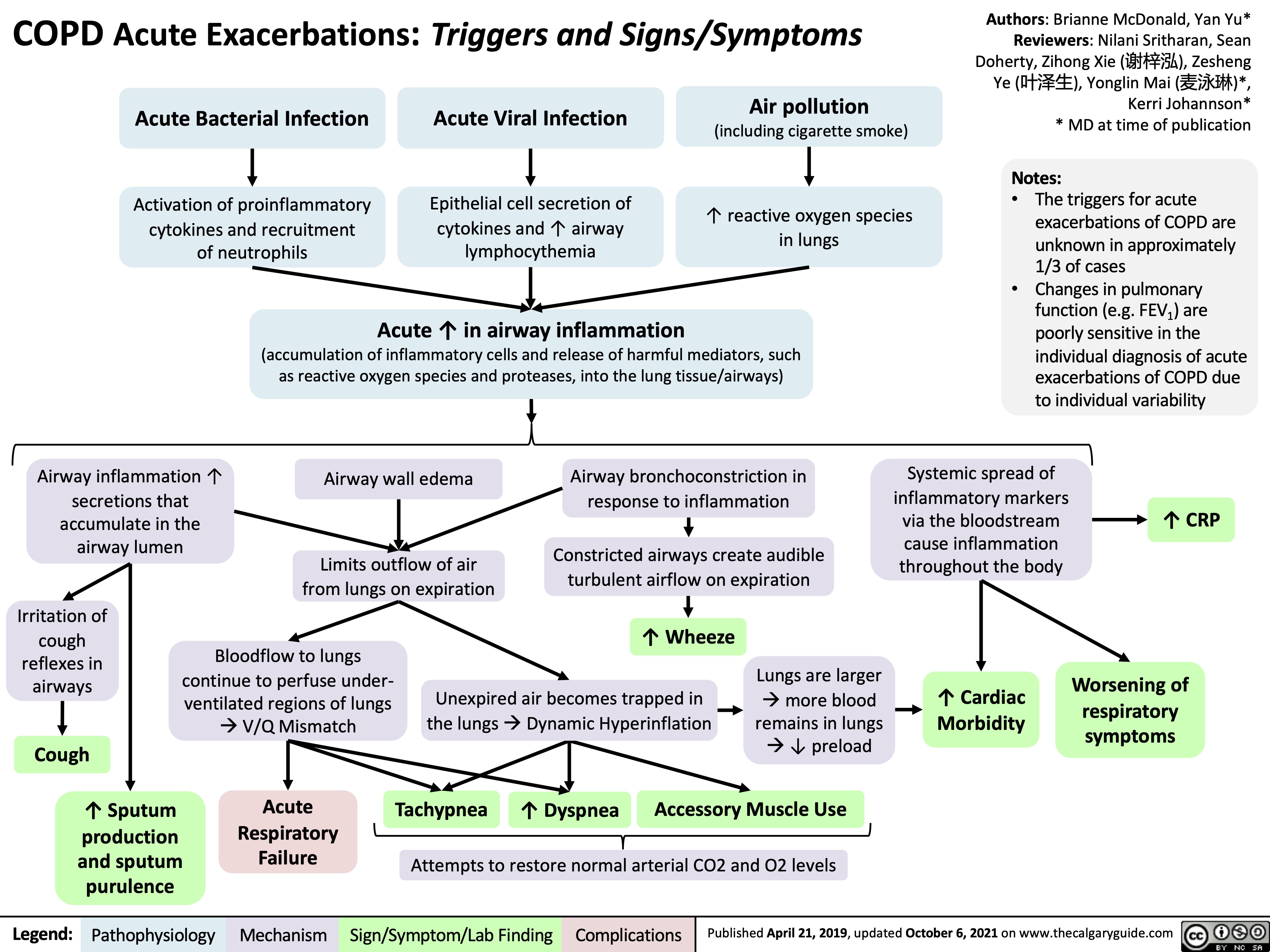
Hypercortisolemia
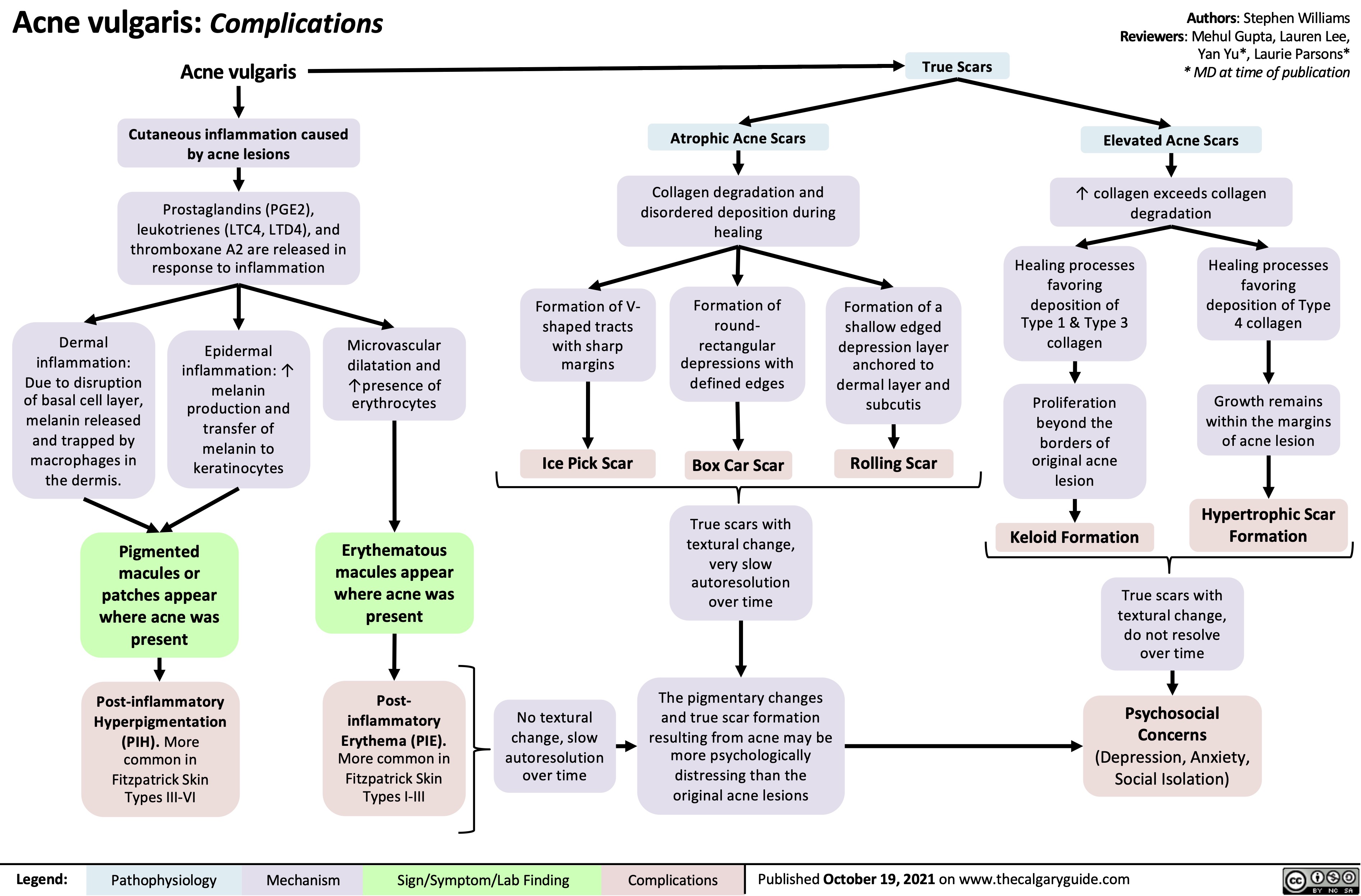
Acne Vulgaris Complications
Vestibular Neuritis

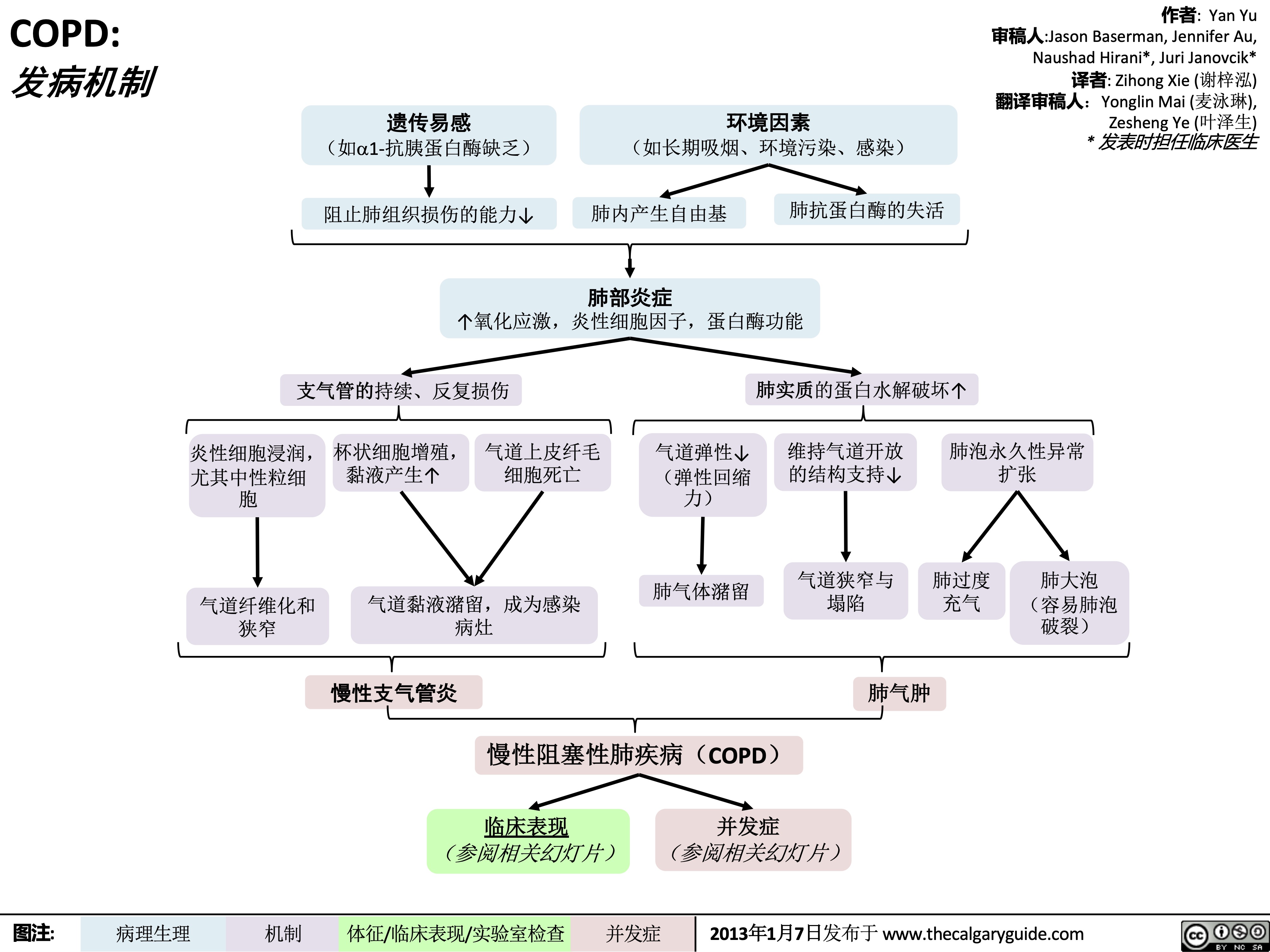
COPD-发病机制
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
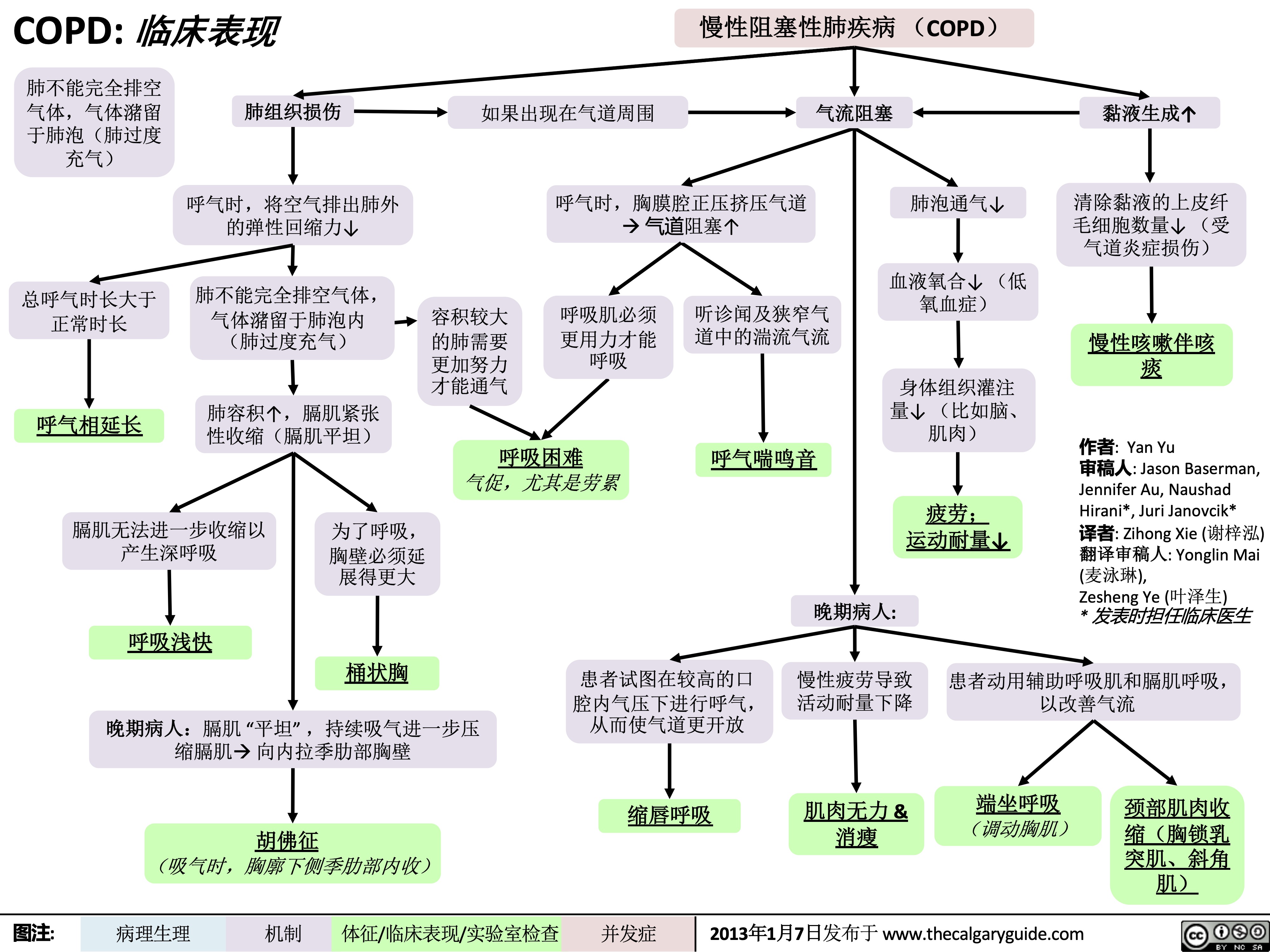
COPD-临床表现
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
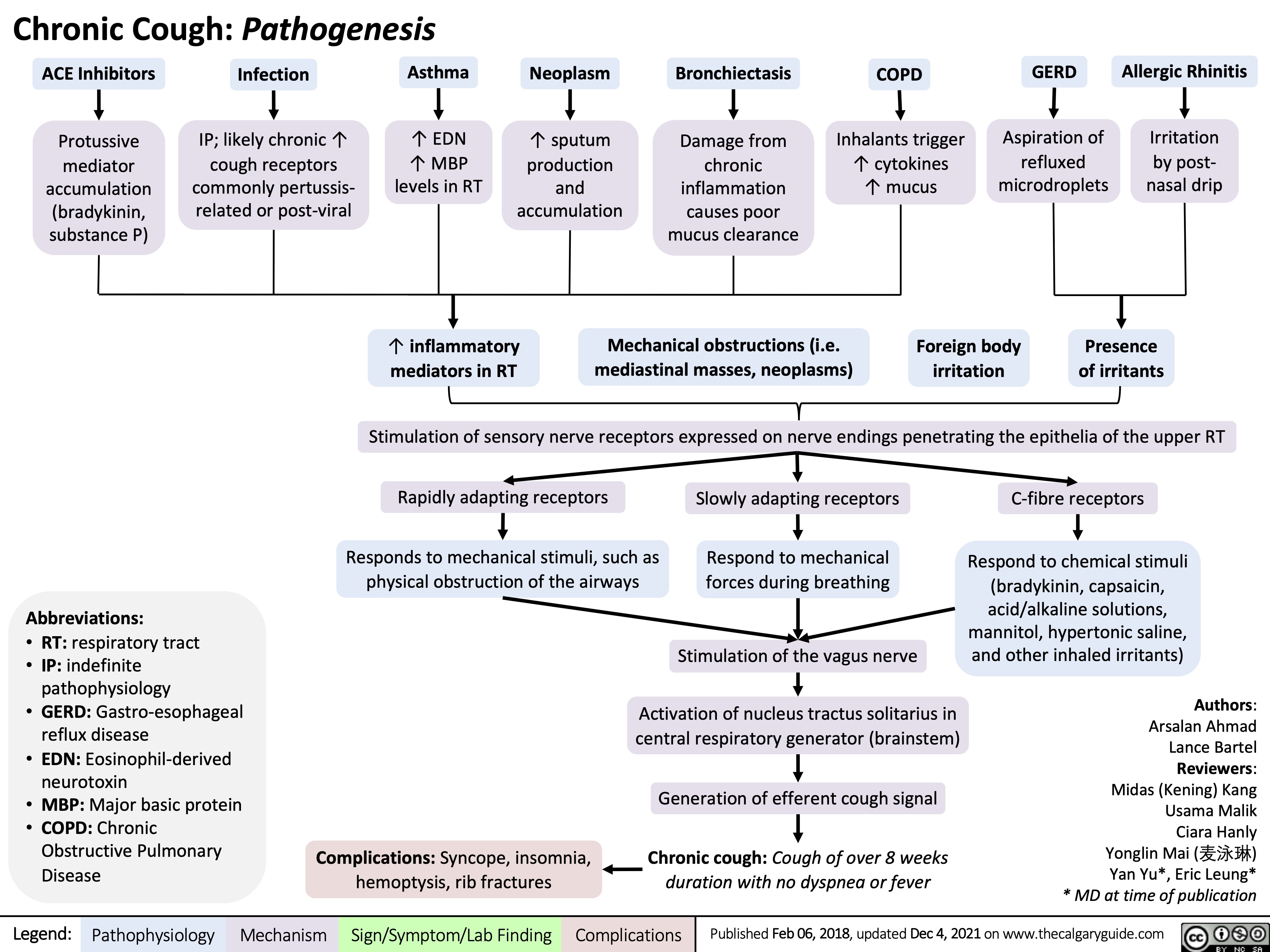
Chronic Cough Pathogenesis_2021
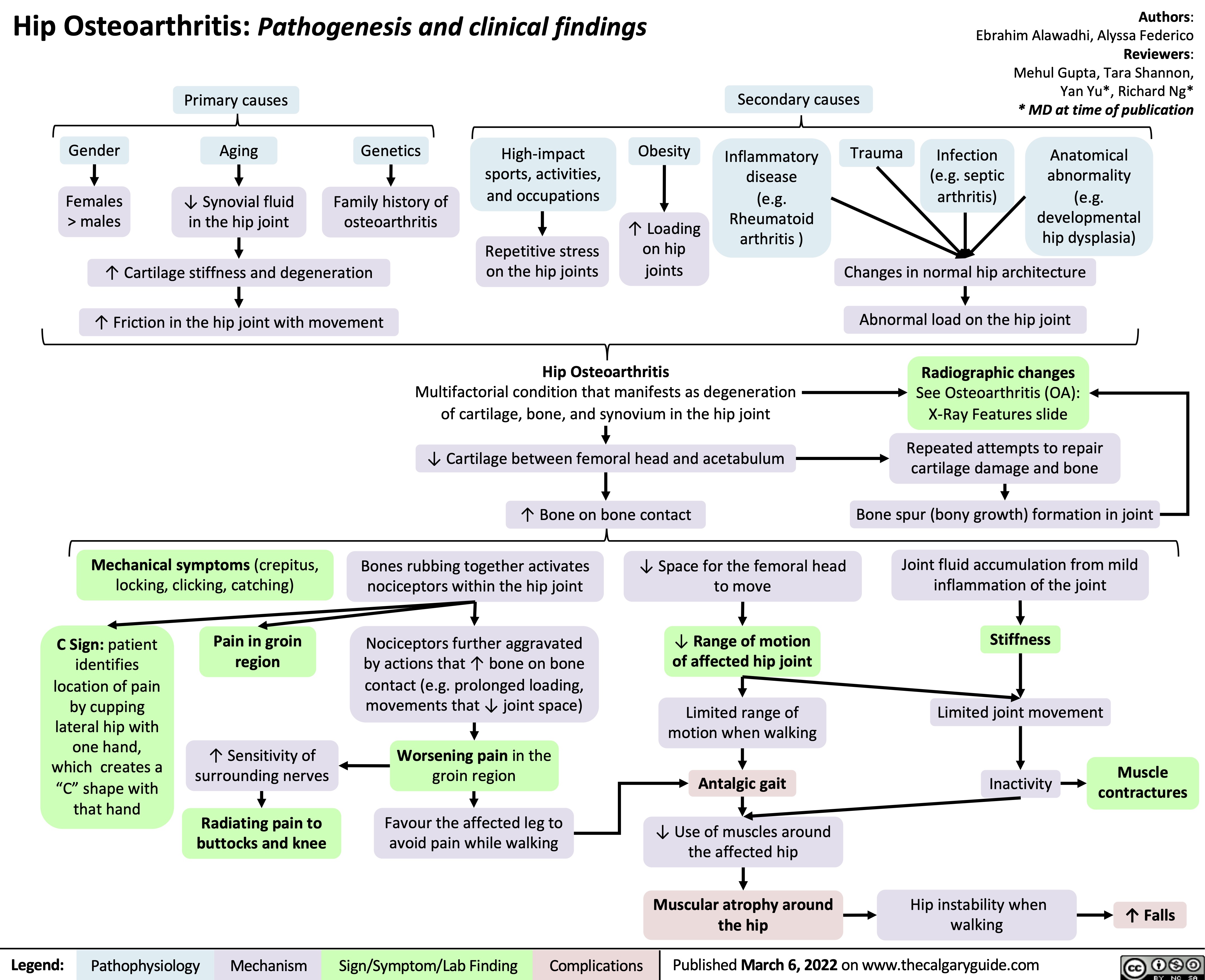
hip-osteoarthritis-pathogenesis-and-clinical-findings
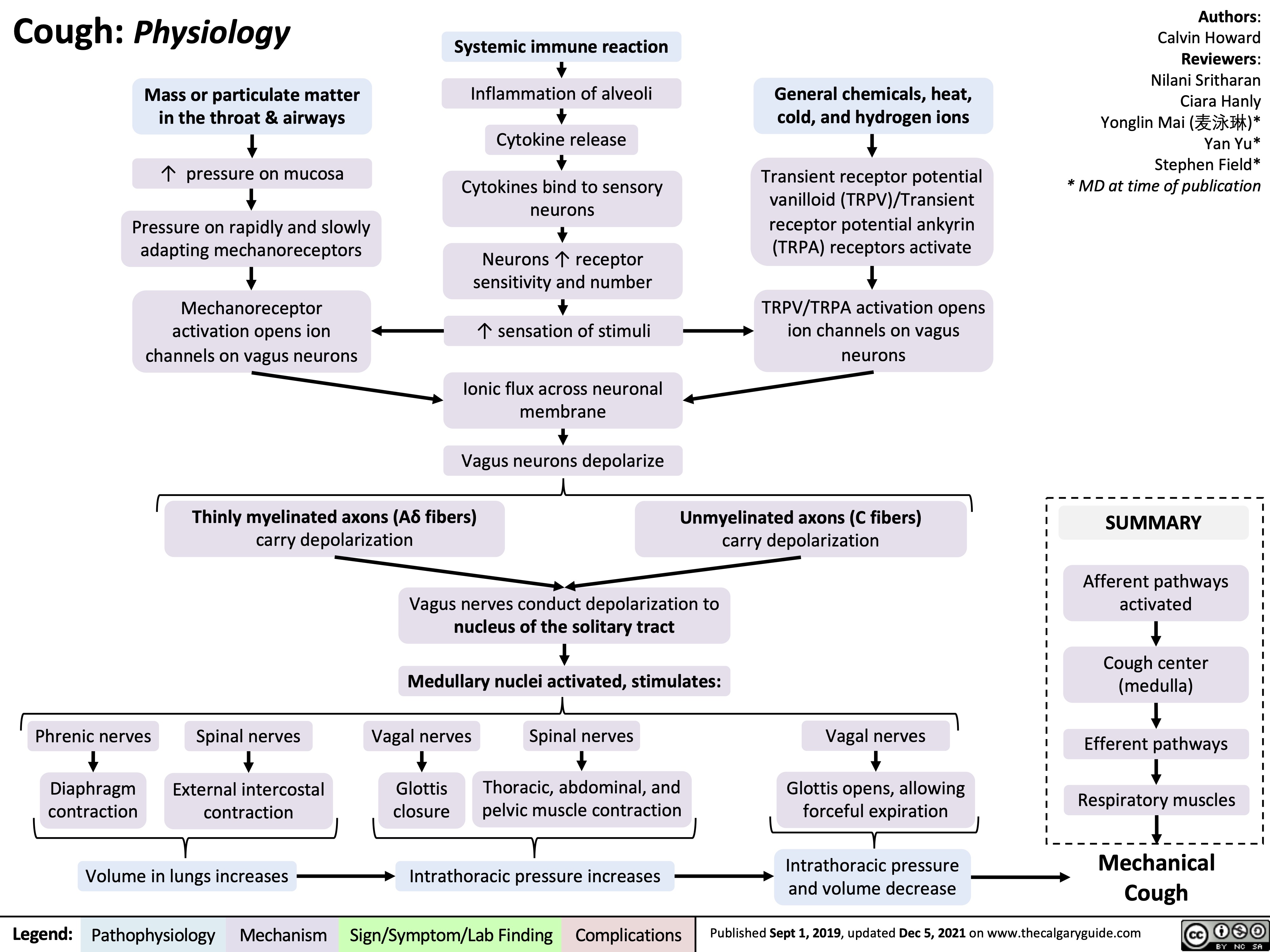
cough-physiology
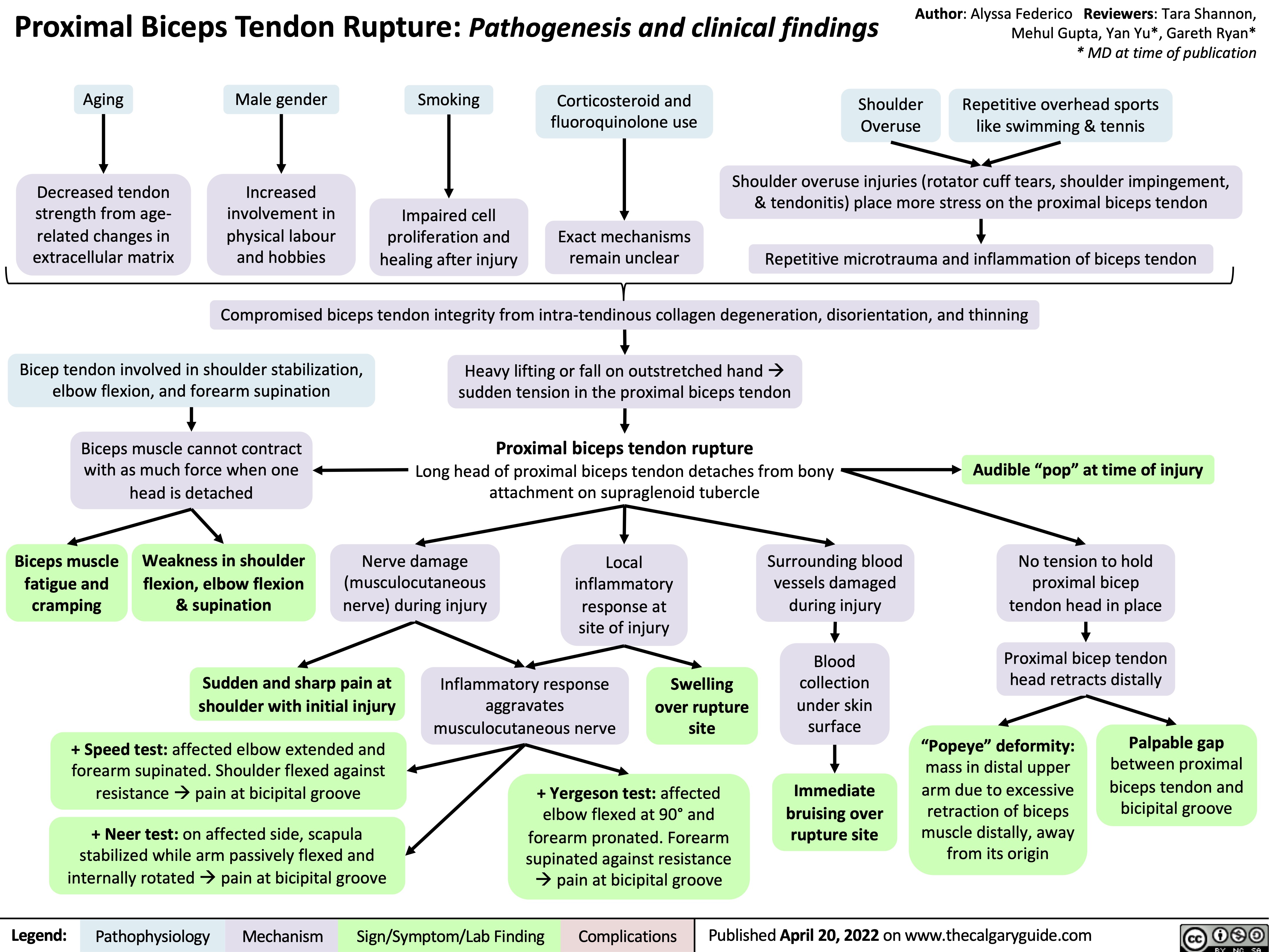
proximal-biceps-tendon-rupture
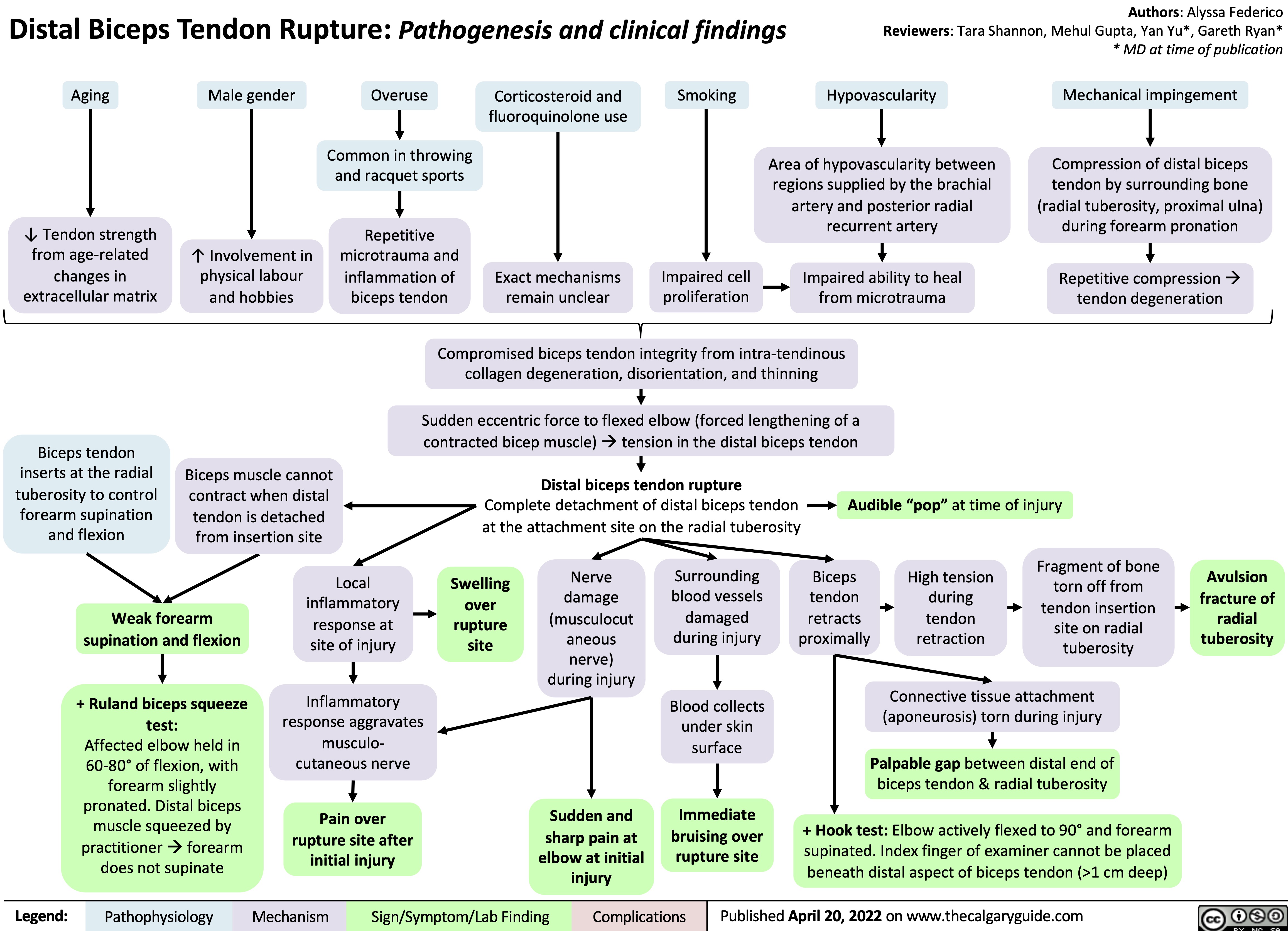
distal-biceps-tendon-rupture
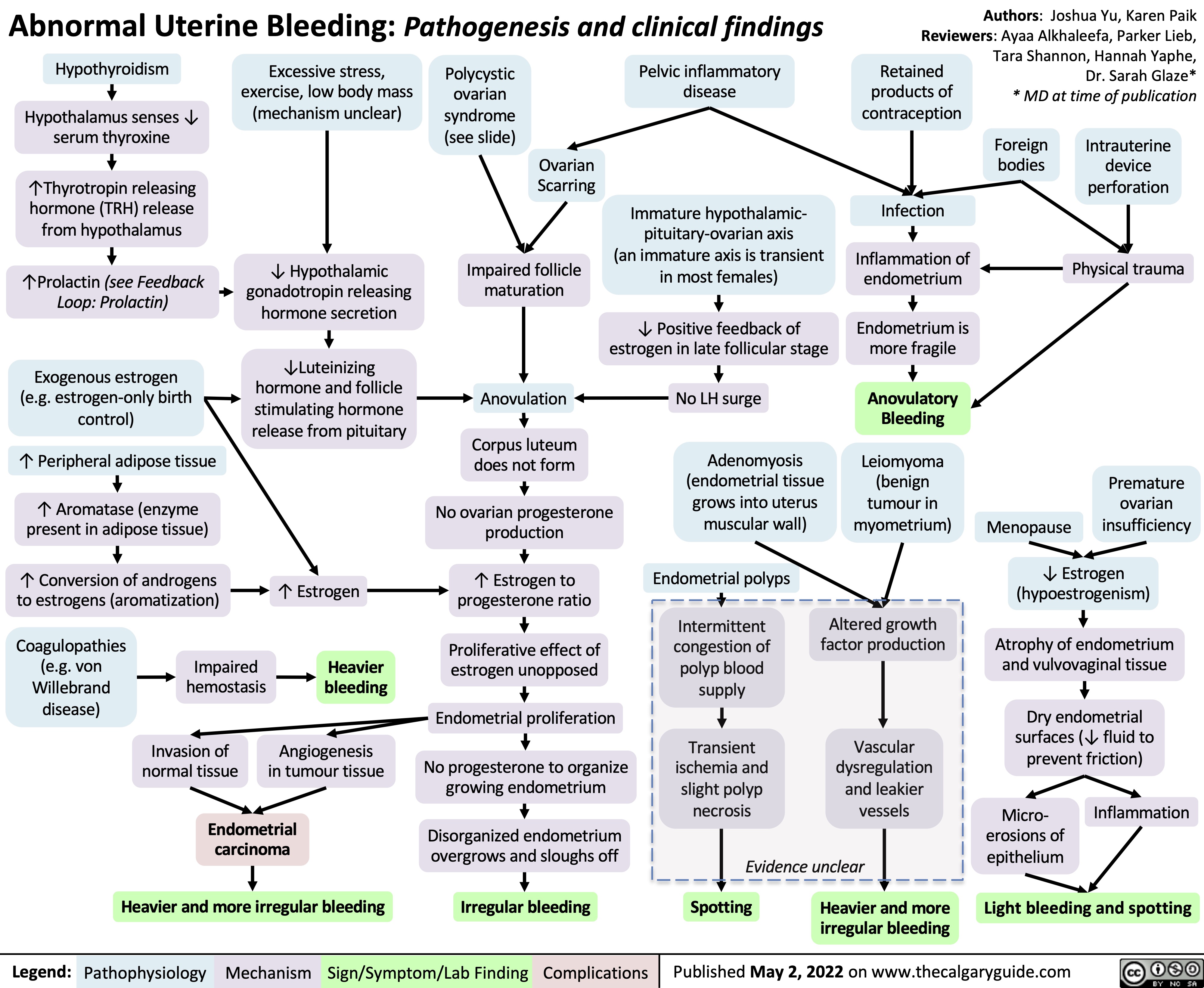
abnormal-uterine-bleeding-aub-pathogenesis-and-clinical-findings
rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
![rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
Psoriatic Arthritis: Pathogenesis and clinical findings
Auteur:
Payam Pournazari
Rédacteurs:
Yan Yu Scott Rapske Liam Martin* Traducteurs: Dianne Ganeswaran Stephen Williams Sylvain Coderre* * MD au moment de publication
Note:
L’arthrite rhumatoïde peut survenir avant ou après le psoriasis (la pathogenèse est la même).
Augmentation de l’activité ostéoclastique
Érosion de l’os sous- chondral mais formation de nouvelle osseuse ailleurs dans l’articulation
Radiographie: la périostite; les syndesmophytes, érosions, déformation du crayon en godets (les articulations IP), ankylose des articulations IP
HLA B27, Cw6 et d’autres sous- types
Antécédents familiaux positifs
Activation des cellules T (mécanisme inconnu)
Infiltration du tissu synovial de l’articulation par les cellules T et B, cellules tueuses naturelles et les macrophages
Production augmentée des molécules inflammatoires (les facteurs de nécrose tumorale [TNFs], les interleukines, etc.) qui agissent de façon systémique (dans tout le corps)
Dans les tendons et le tissu conjonctif
Inflammation des enthèses et du tissu conjonctif provoque le gonflement
Dans la peau
Réaction immunitaire détruisant les structures de la peau et des ongles (Voir diapositive Psoriasis)
Le psoriasis en plaques sur la peau, dépressions punctiformes, onychorrhexie, taches d’huile, onycholyse, décoloration et hyperkératose
Angiogenèse et vascularite dans les vaisseaux sanguins de la membrane synoviale
Dans les articulations
Les cytokines inflammatoires stimulent les nocicepteurs locaux
1. Oligoarthrites - asymétriques
2. Arthrite des articulations IPD
3. Polyarthrite rhumatoïde -
symétrique
4. Atteinte axiale (raison
inconnue, probablement en raison de « biomécanique », « innervation », et
« vascularisation ».
5. Arthrite mutilante (grave et destructrice)
L’enthésite
(Douleur/sensibilité à l’insertion du ligament dans l’os)
La dactylite (Inflammation de tout le doigt – les tissus mous et les articulations sont enflammés
Légende:
Physiopathologie
Mécanisme
Signe/Symptôme/Résultats de Laboratoire
Complications
Publié 10 November 2012 sur www.thecalgaryguide.com
rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
Psoriatic Arthritis: Pathogenesis and clinical findings
Auteur:
Payam Pournazari
Rédacteurs:
Yan Yu Scott Rapske Liam Martin* Traducteurs: Dianne Ganeswaran Stephen Williams Sylvain Coderre* * MD au moment de publication
Note:
L’arthrite rhumatoïde peut survenir avant ou après le psoriasis (la pathogenèse est la même).
Augmentation de l’activité ostéoclastique
Érosion de l’os sous- chondral mais formation de nouvelle osseuse ailleurs dans l’articulation
Radiographie: la périostite; les syndesmophytes, érosions, déformation du crayon en godets (les articulations IP), ankylose des articulations IP
HLA B27, Cw6 et d’autres sous- types
Antécédents familiaux positifs
Activation des cellules T (mécanisme inconnu)
Infiltration du tissu synovial de l’articulation par les cellules T et B, cellules tueuses naturelles et les macrophages
Production augmentée des molécules inflammatoires (les facteurs de nécrose tumorale [TNFs], les interleukines, etc.) qui agissent de façon systémique (dans tout le corps)
Dans les tendons et le tissu conjonctif
Inflammation des enthèses et du tissu conjonctif provoque le gonflement
Dans la peau
Réaction immunitaire détruisant les structures de la peau et des ongles (Voir diapositive Psoriasis)
Le psoriasis en plaques sur la peau, dépressions punctiformes, onychorrhexie, taches d’huile, onycholyse, décoloration et hyperkératose
Angiogenèse et vascularite dans les vaisseaux sanguins de la membrane synoviale
Dans les articulations
Les cytokines inflammatoires stimulent les nocicepteurs locaux
1. Oligoarthrites - asymétriques
2. Arthrite des articulations IPD
3. Polyarthrite rhumatoïde -
symétrique
4. Atteinte axiale (raison
inconnue, probablement en raison de « biomécanique », « innervation », et
« vascularisation ».
5. Arthrite mutilante (grave et destructrice)
L’enthésite
(Douleur/sensibilité à l’insertion du ligament dans l’os)
La dactylite (Inflammation de tout le doigt – les tissus mous et les articulations sont enflammés
Légende:
Physiopathologie
Mécanisme
Signe/Symptôme/Résultats de Laboratoire
Complications
Publié 10 November 2012 sur www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/05/Psoriatic-Arthritis-PsA-Pathclinical.jpg)
acute-cholecystitis
![Acute Cholecystitis: Pathogenesis and clinical findings
Gallstone blocks the cystic duct, backing up bile into the gallbladder
Gallstones causing physical trauma to gallbladder wall
Irritation of adjacent diaphragm, stimulates phrenic nerve (C3-C5)
Activates stretch receptors of visceral peritoneum, stimulates foregut autonomic nerves (T5-T8)
Inflammatory mediator (i.e. prostaglandin) release by gallbladder and systemic inflammatory response
Thickened gallbladder wall on ultrasound (gold standard test)
On inspiration, the diaphragm pushes the gallbladder downward
Irritation of parietal peritoneum, stimulates somatic nerves
↑ Permeability of vessels with systemic inflammation, which leak
fluid from the blood into the interstitial space
Radiating pain to the back and right shoulder
Dull, diffuse abdominal pain referred to the epigastric region
Fever, nausea/vomiting, tachycardia
Positive Murphy’s sign (pain upon palpation of right upper quadrant [RUQ] on inspiration)
Persistent RUQ pain, abdominal guarding and peritoneal signs
Dehydration
Authors: Yan Yu, Vina Fan Reviewers: Dean Percy, Mirna Matta, Crystal Liu, Ben Campbell Maitreyi Raman* * MD at time of publication
Inflammation self-perpetuates
Irritation of inner gallbladder wall/mucosa
↑ Gallbladder lumen pressure
Intraluminal pressure exceeds arterial pressure
↓ Blood flow to gallbladder
Gallbladder ischemia
Local inflammation, loss of gallbladder mucosal integrity
Bacterial invasionàtransmural inflammation of gallbladder
Without treatment, prolonged ischemia and inflammation of the gallbladder
Gallbladder gangrene (20%)
Gallbladder perforation (20%)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 4 2019, updated May 16 2022 on www.thecalgaryguide.com
Acute Cholecystitis: Pathogenesis and clinical findings
Gallstone blocks the cystic duct, backing up bile into the gallbladder
Gallstones causing physical trauma to gallbladder wall
Irritation of adjacent diaphragm, stimulates phrenic nerve (C3-C5)
Activates stretch receptors of visceral peritoneum, stimulates foregut autonomic nerves (T5-T8)
Inflammatory mediator (i.e. prostaglandin) release by gallbladder and systemic inflammatory response
Thickened gallbladder wall on ultrasound (gold standard test)
On inspiration, the diaphragm pushes the gallbladder downward
Irritation of parietal peritoneum, stimulates somatic nerves
↑ Permeability of vessels with systemic inflammation, which leak
fluid from the blood into the interstitial space
Radiating pain to the back and right shoulder
Dull, diffuse abdominal pain referred to the epigastric region
Fever, nausea/vomiting, tachycardia
Positive Murphy’s sign (pain upon palpation of right upper quadrant [RUQ] on inspiration)
Persistent RUQ pain, abdominal guarding and peritoneal signs
Dehydration
Authors: Yan Yu, Vina Fan Reviewers: Dean Percy, Mirna Matta, Crystal Liu, Ben Campbell Maitreyi Raman* * MD at time of publication
Inflammation self-perpetuates
Irritation of inner gallbladder wall/mucosa
↑ Gallbladder lumen pressure
Intraluminal pressure exceeds arterial pressure
↓ Blood flow to gallbladder
Gallbladder ischemia
Local inflammation, loss of gallbladder mucosal integrity
Bacterial invasionàtransmural inflammation of gallbladder
Without treatment, prolonged ischemia and inflammation of the gallbladder
Gallbladder gangrene (20%)
Gallbladder perforation (20%)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 4 2019, updated May 16 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Acute-Cholecystitis-2022.jpg)
clostridium-difficile-infection-pathogenesis-and-clinical-findings
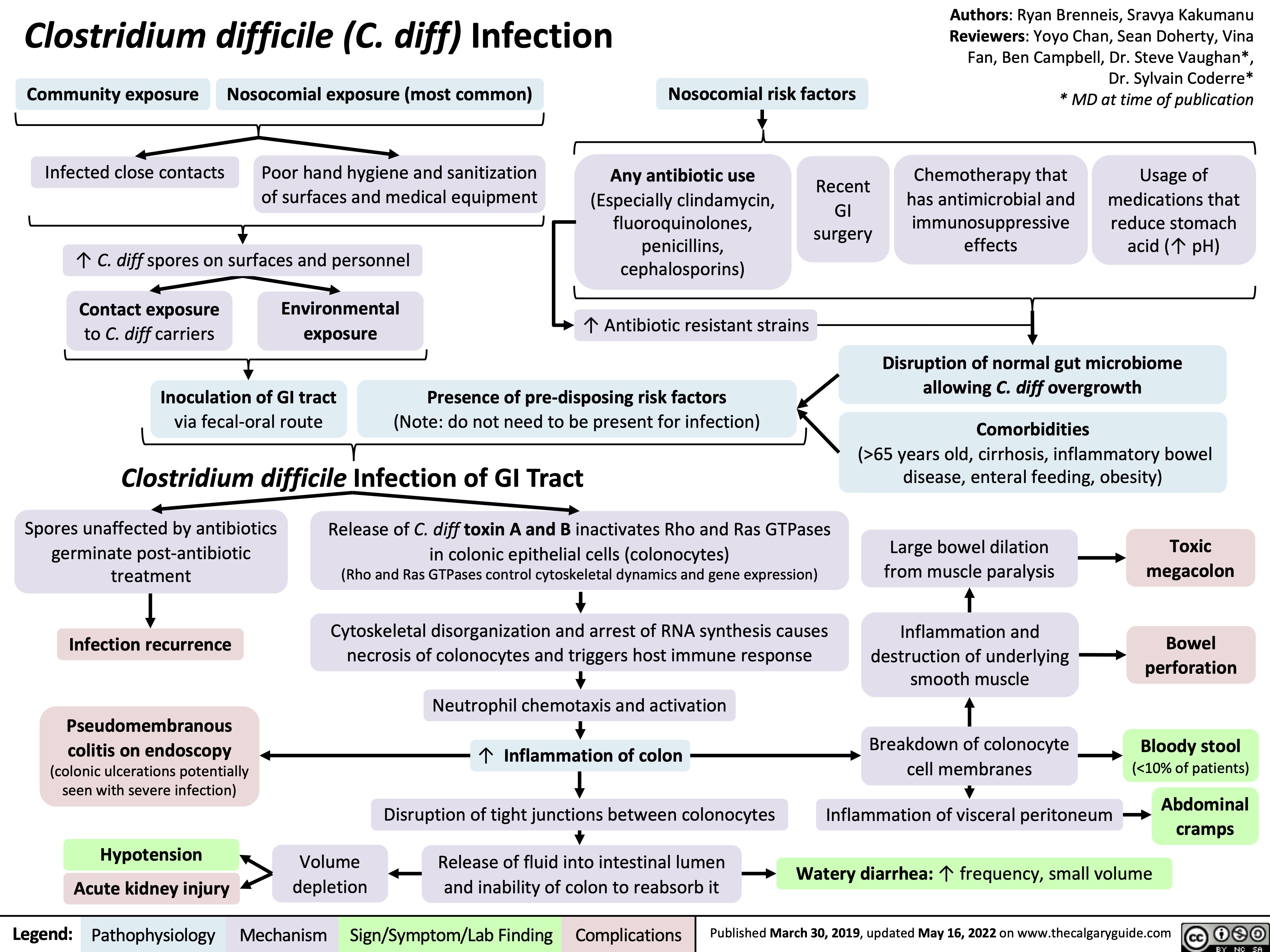
constatations-classiques-de-sclerose-en-plaques-sep-sur-irm-cerebrale
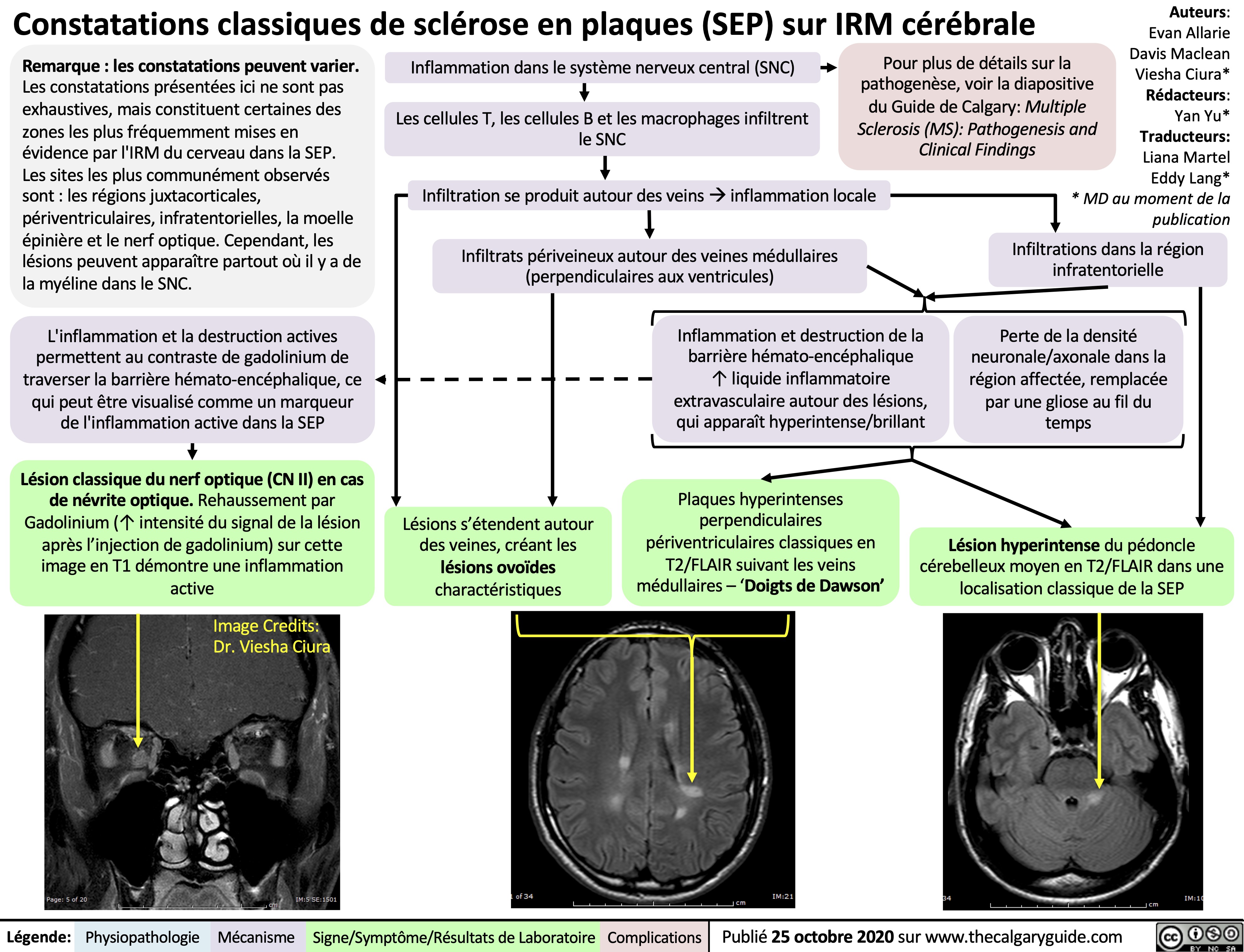
pleural-effusions-pathogenesis-and-anterior-posterior-chest-x-ray-findings
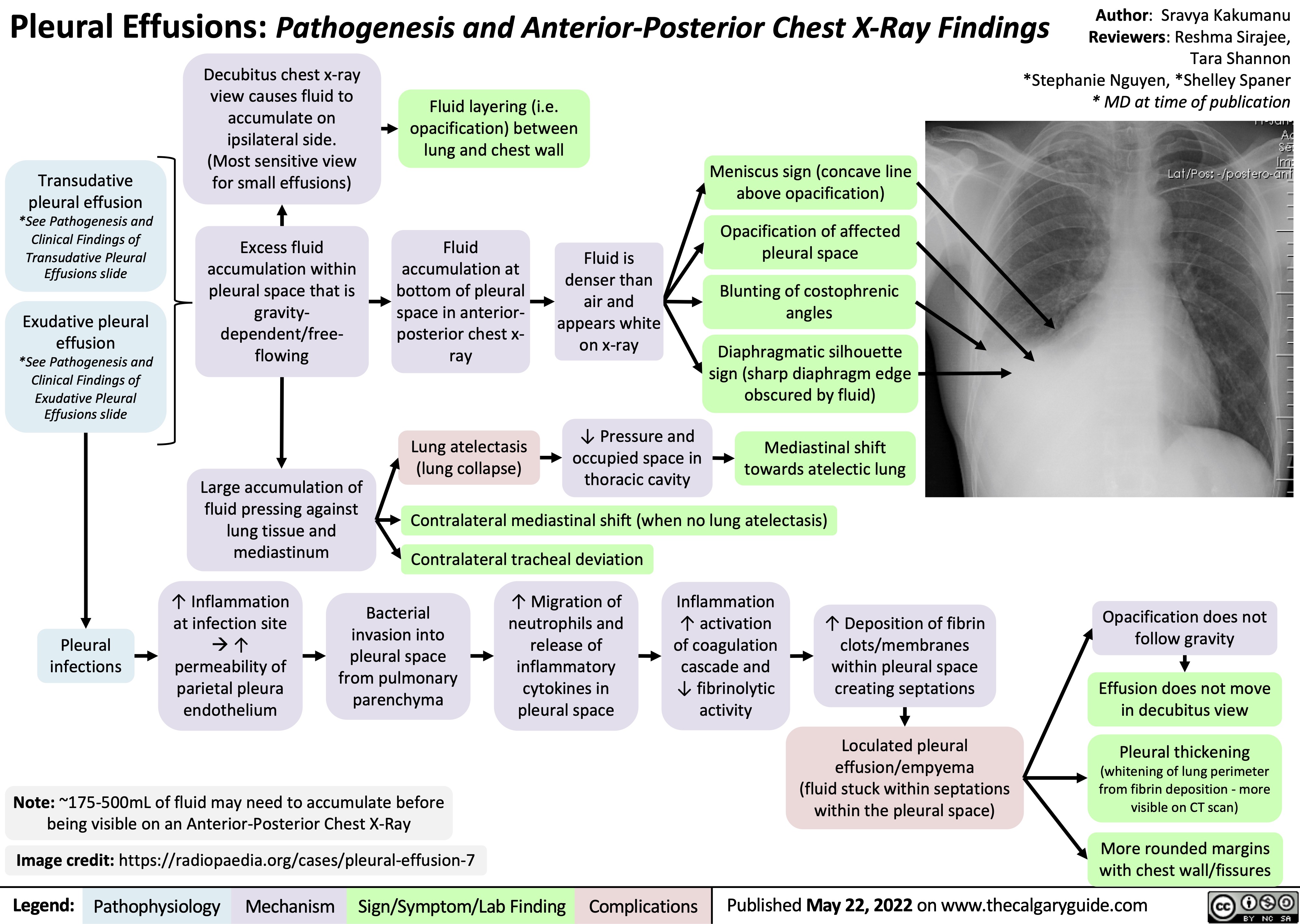
gout-pathogenesis-of-x-ray-findings
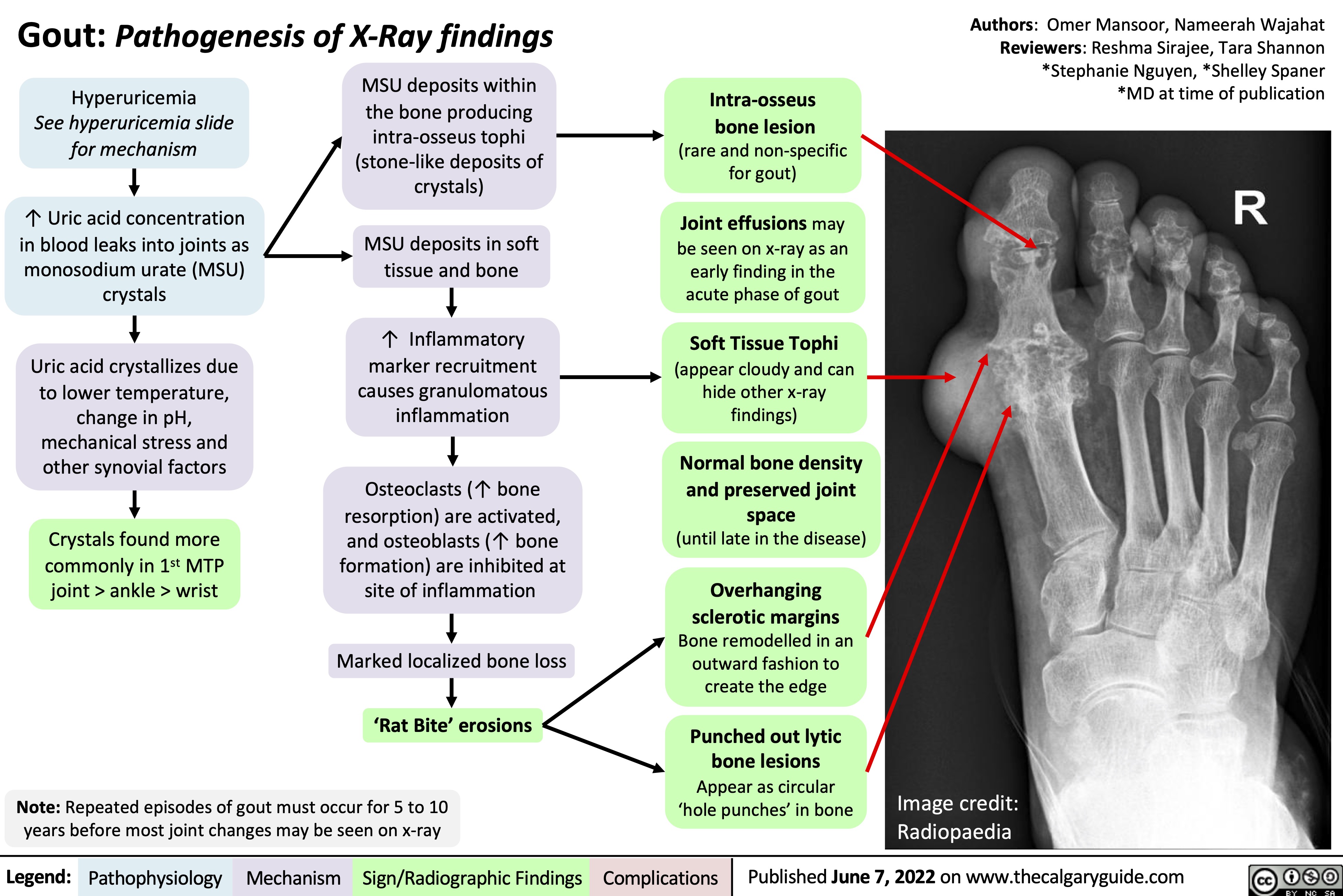
Epilepsy Pathogenesis
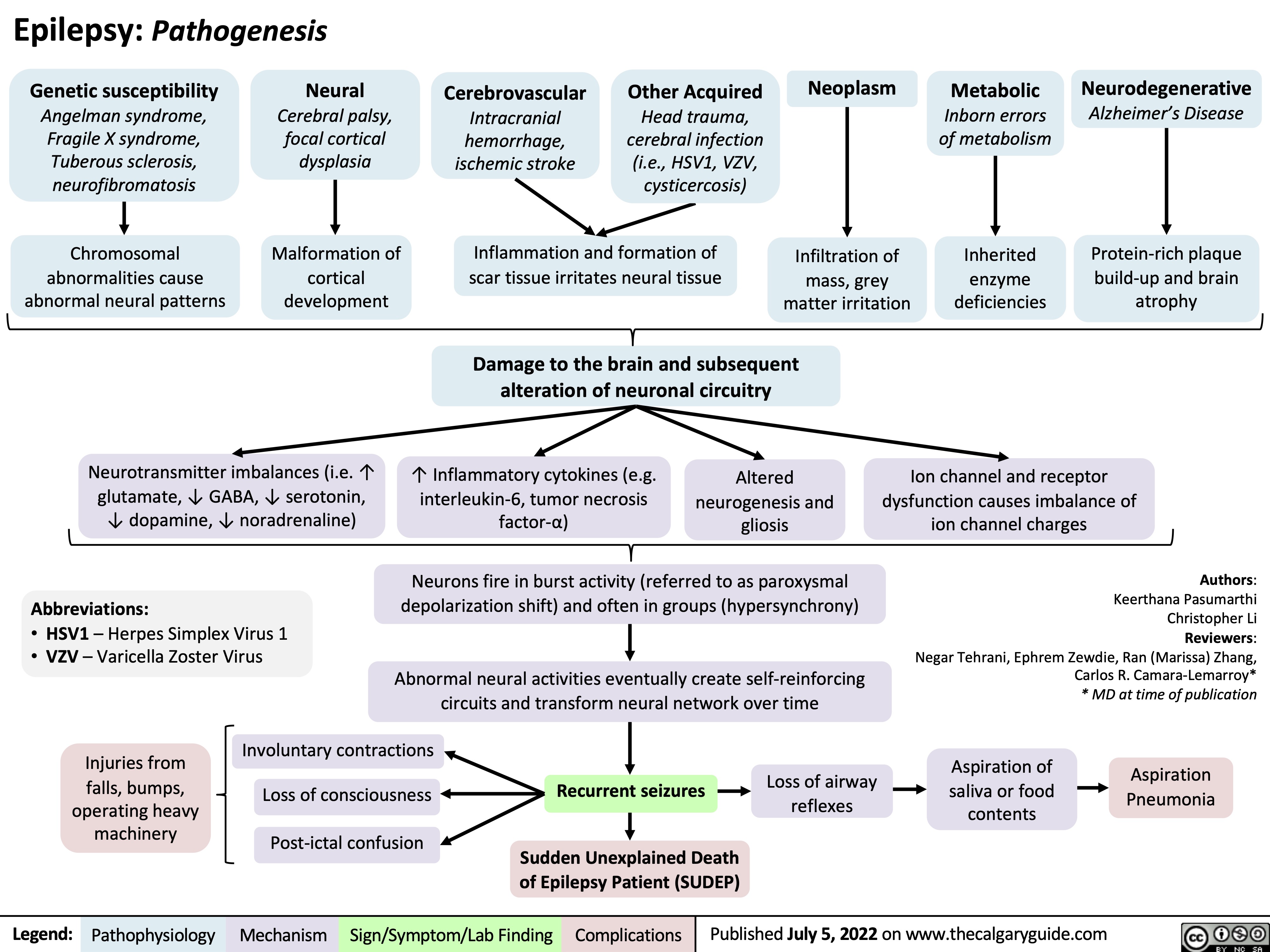
exudative-pleural-effusions-pathogenesis-and-lab-findings
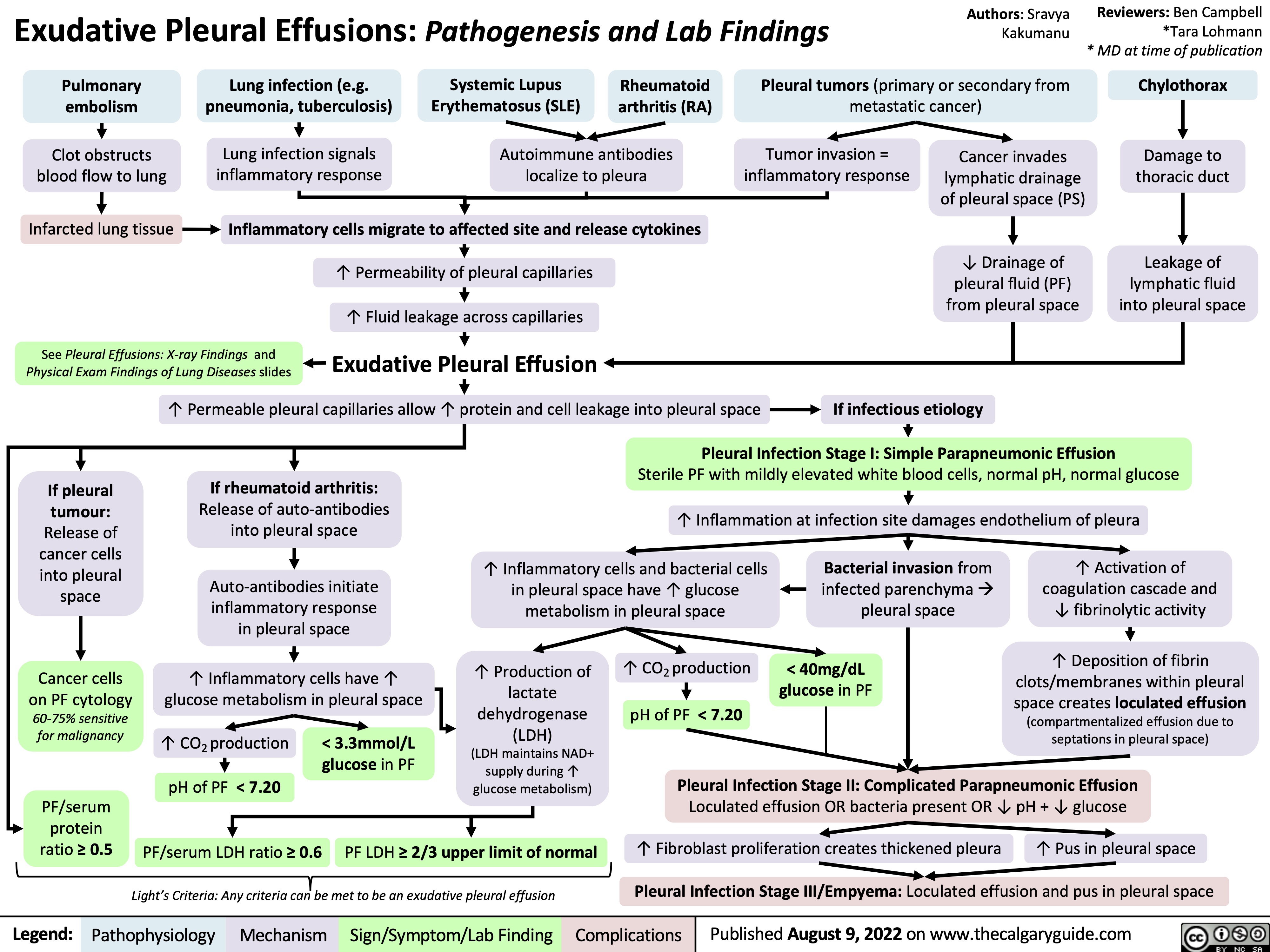
thrombose-veineuse-profonde-soupconne-tvp-pathogenese-et-complications
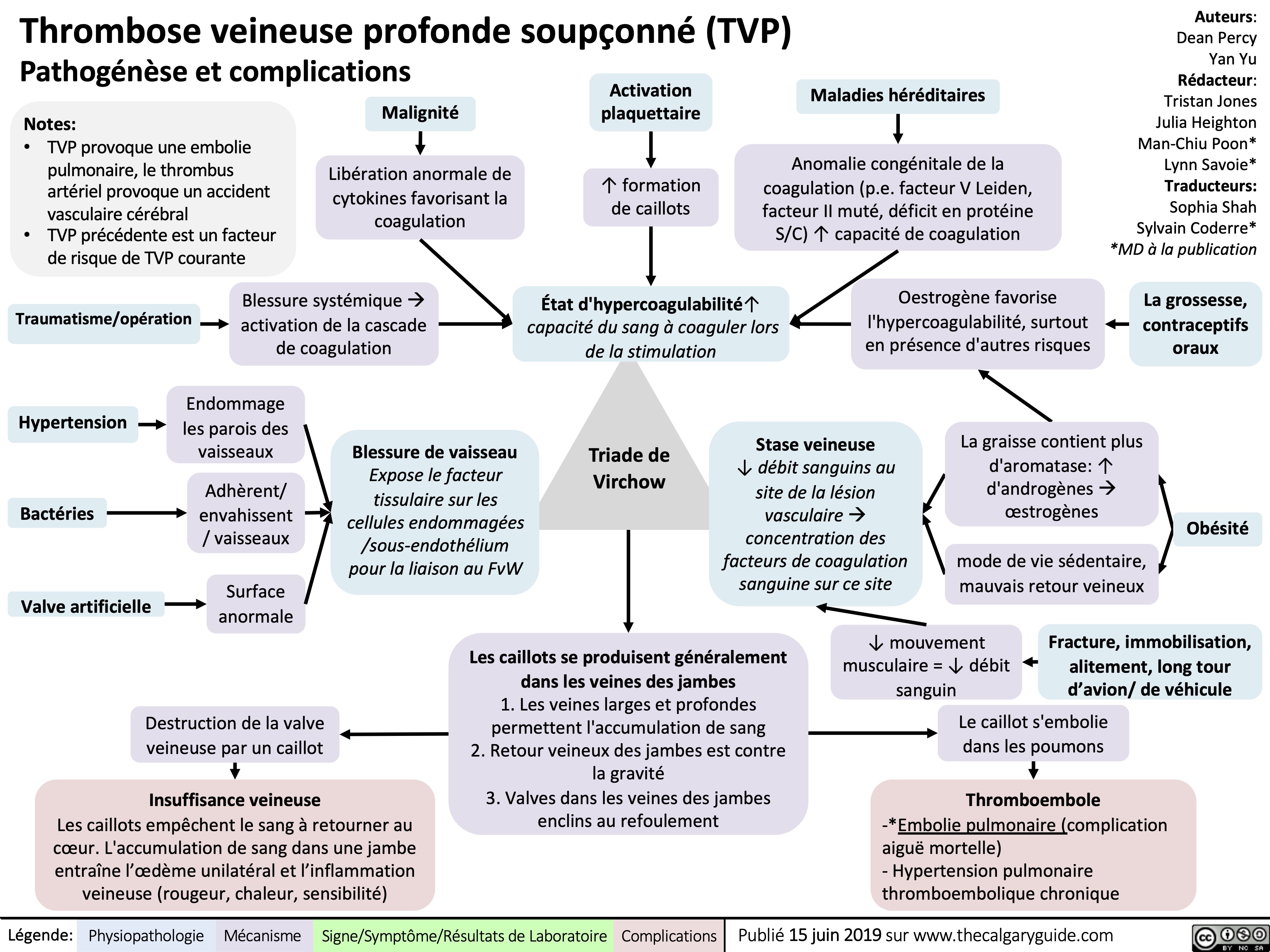
patellar-tendon-rupture-pathogenesis-and-clinical-findings
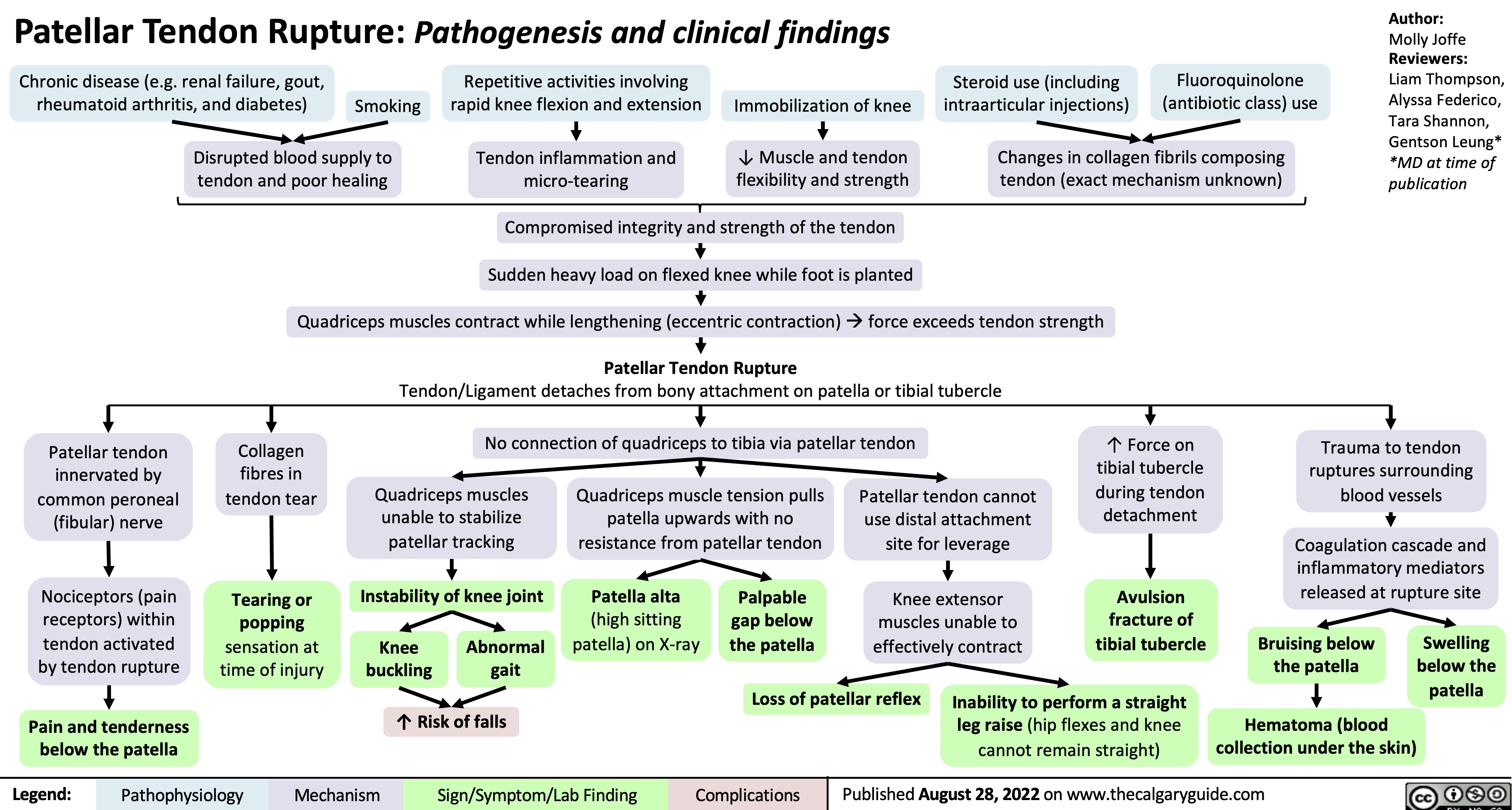
ascending-cholangitis-pathogenesis-clinical-findings
acute-lower-gi-bleeds-pathogenesis-and-clinical-findings
quadriceps-tendon-rupture-pathogenesis-and-clinical-findings
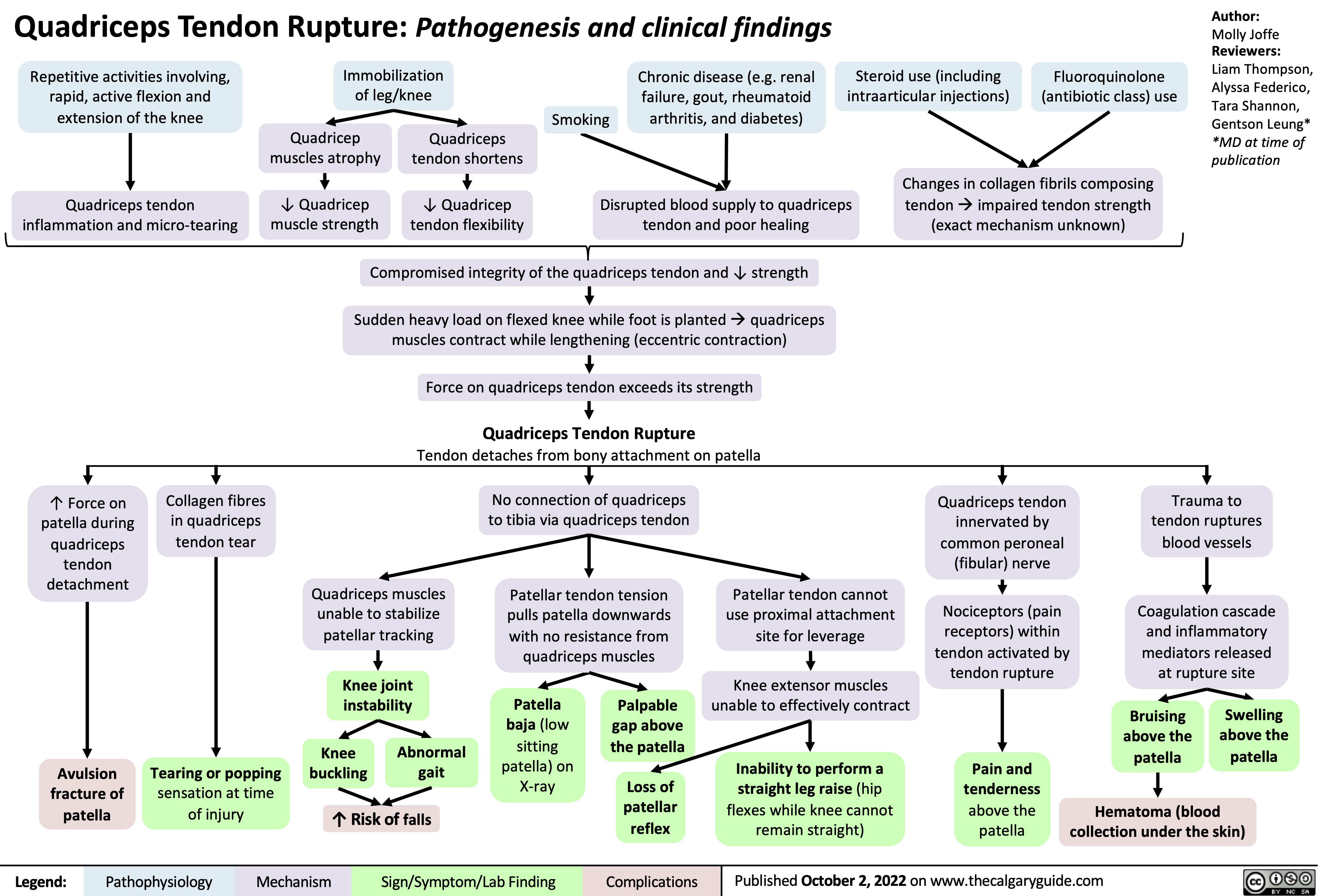
microscopic-colitis-pathogenesis-and-clinical-findings
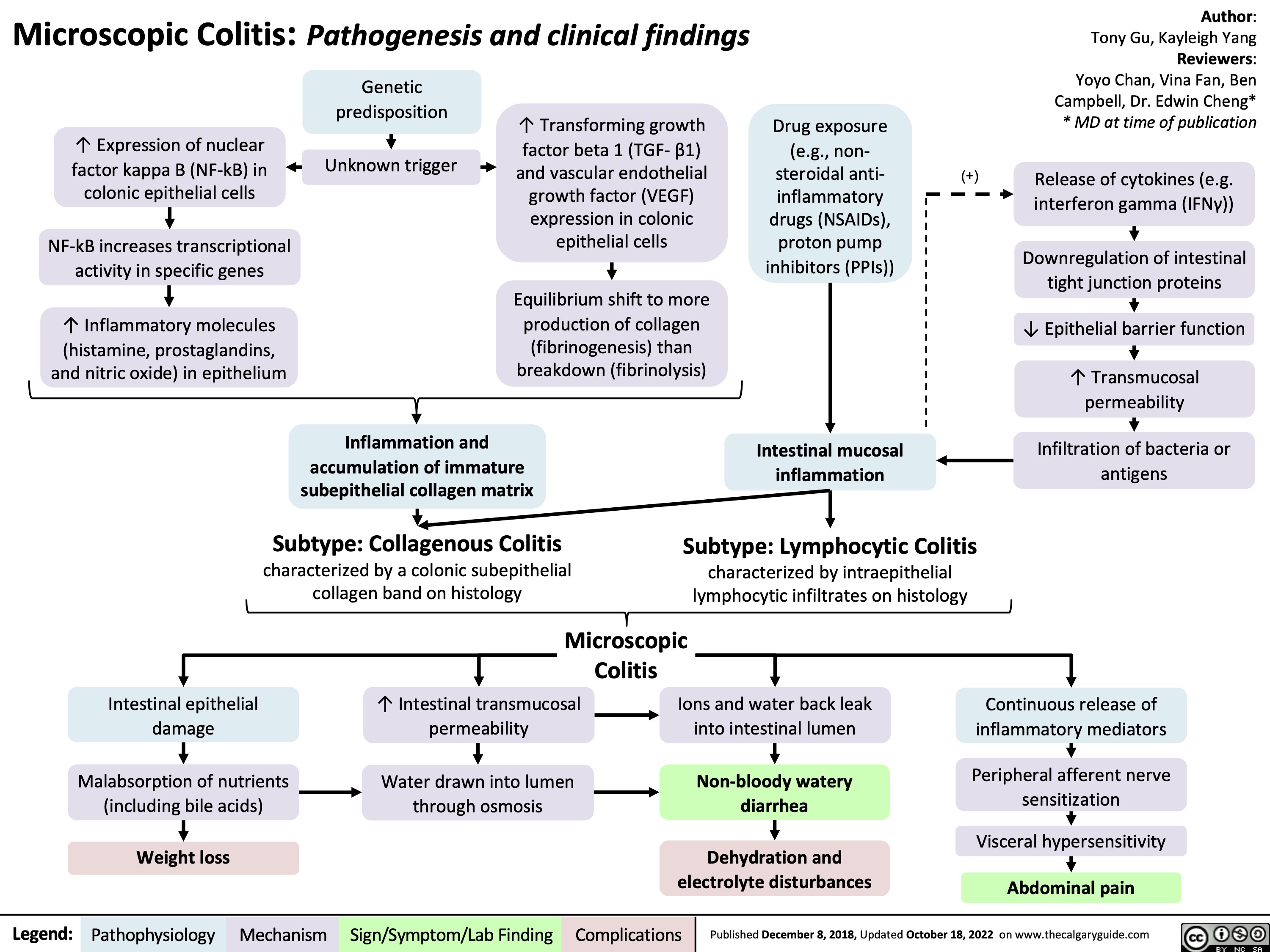
copd-findings-on-posterior-anterior-and-lateral-chest-x-ray-findings
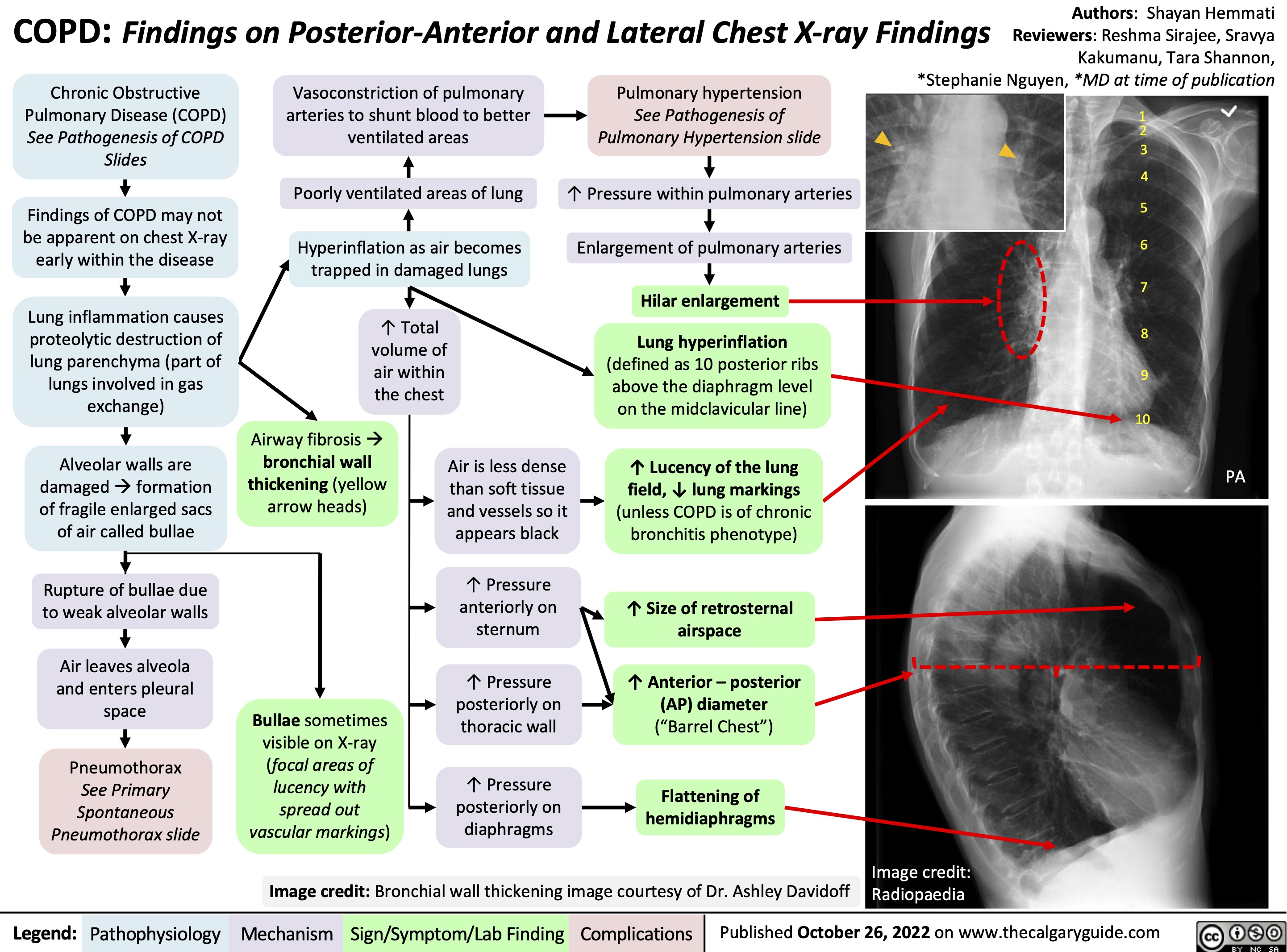
Granulomatosis with Polyangiitis Pathogenesis
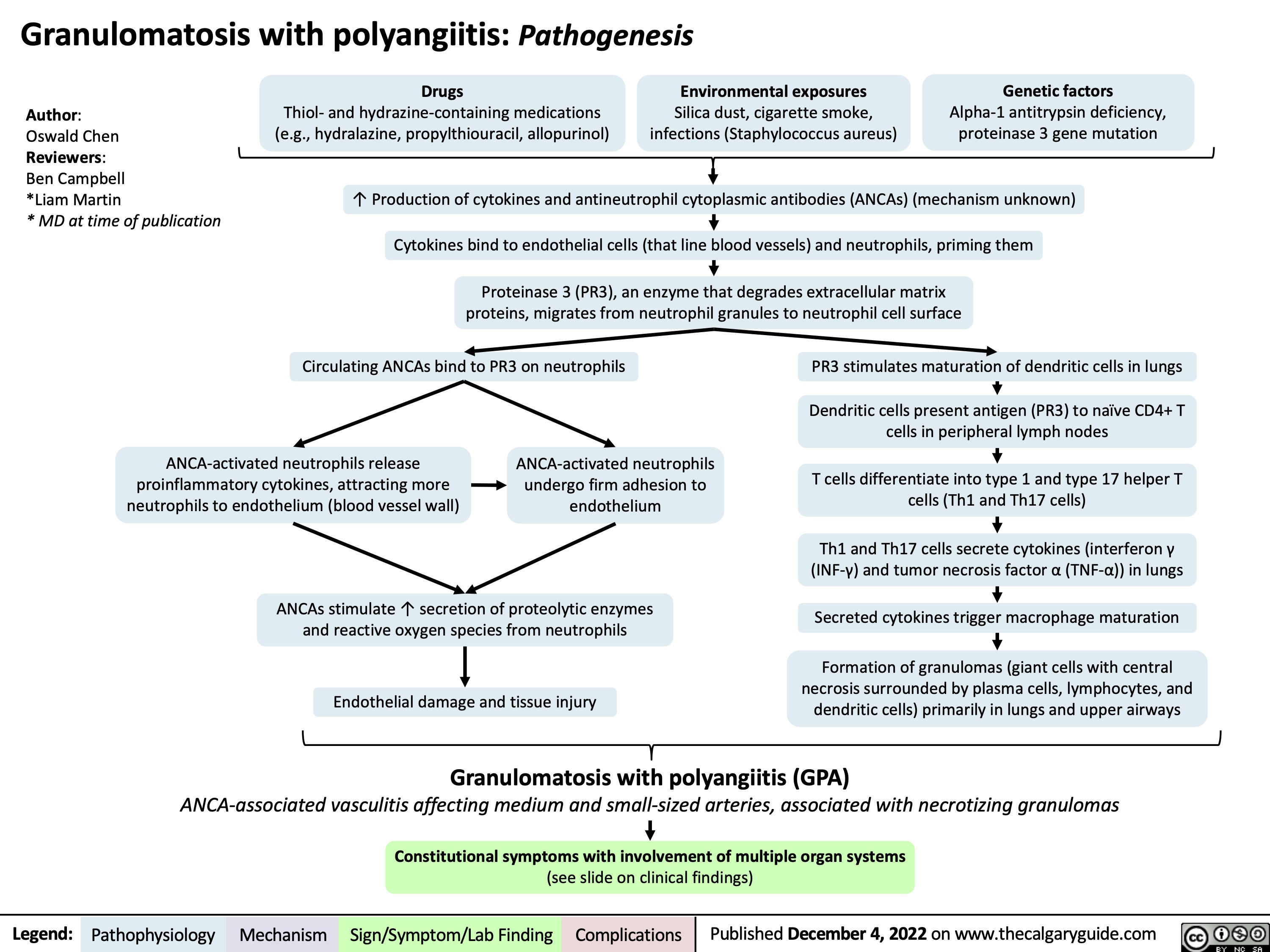
Granulomatosis with polyangiitis: Clinical findings
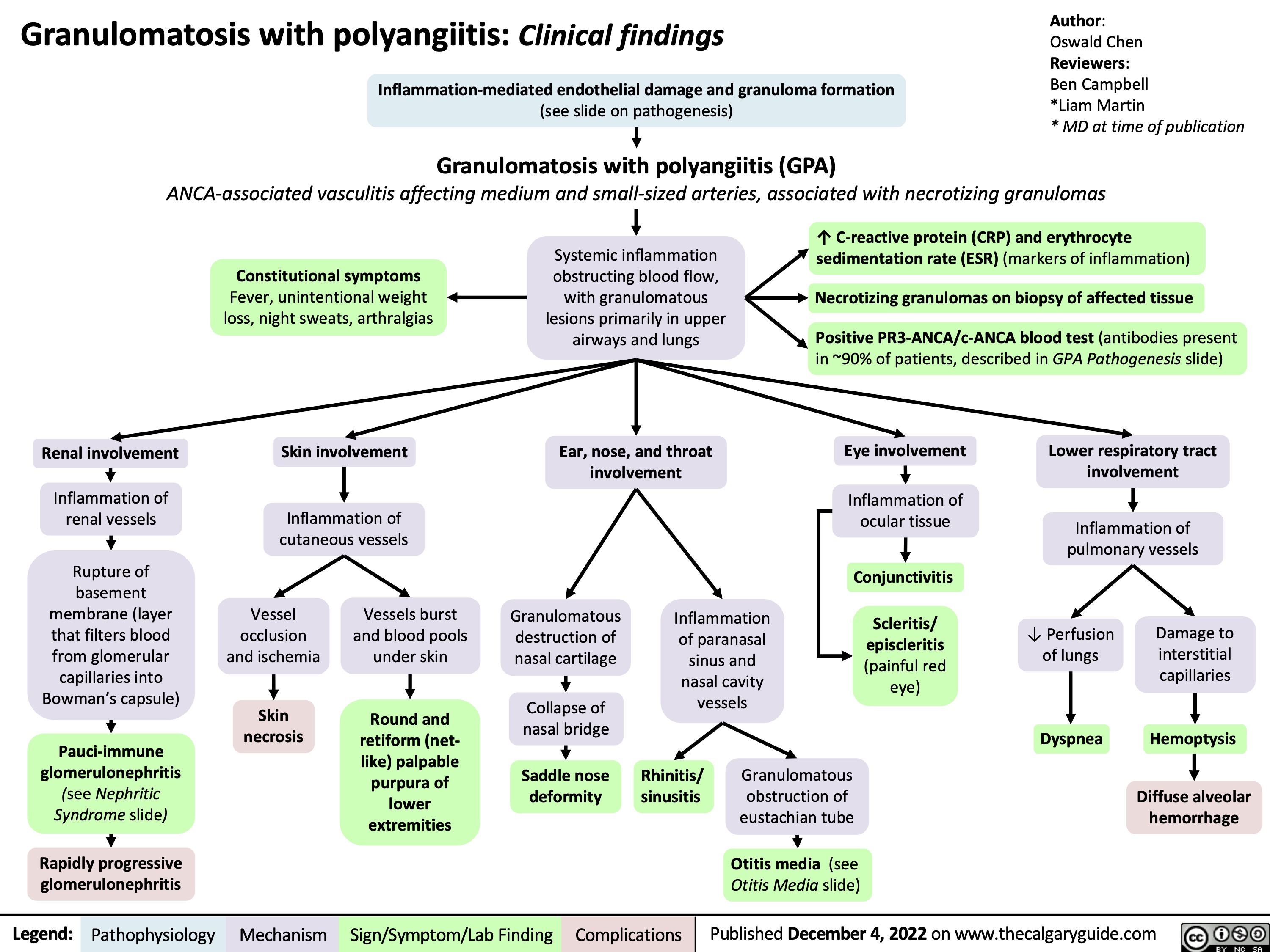
Erythema multiforme (EM): Pathophysiology and clinical findings
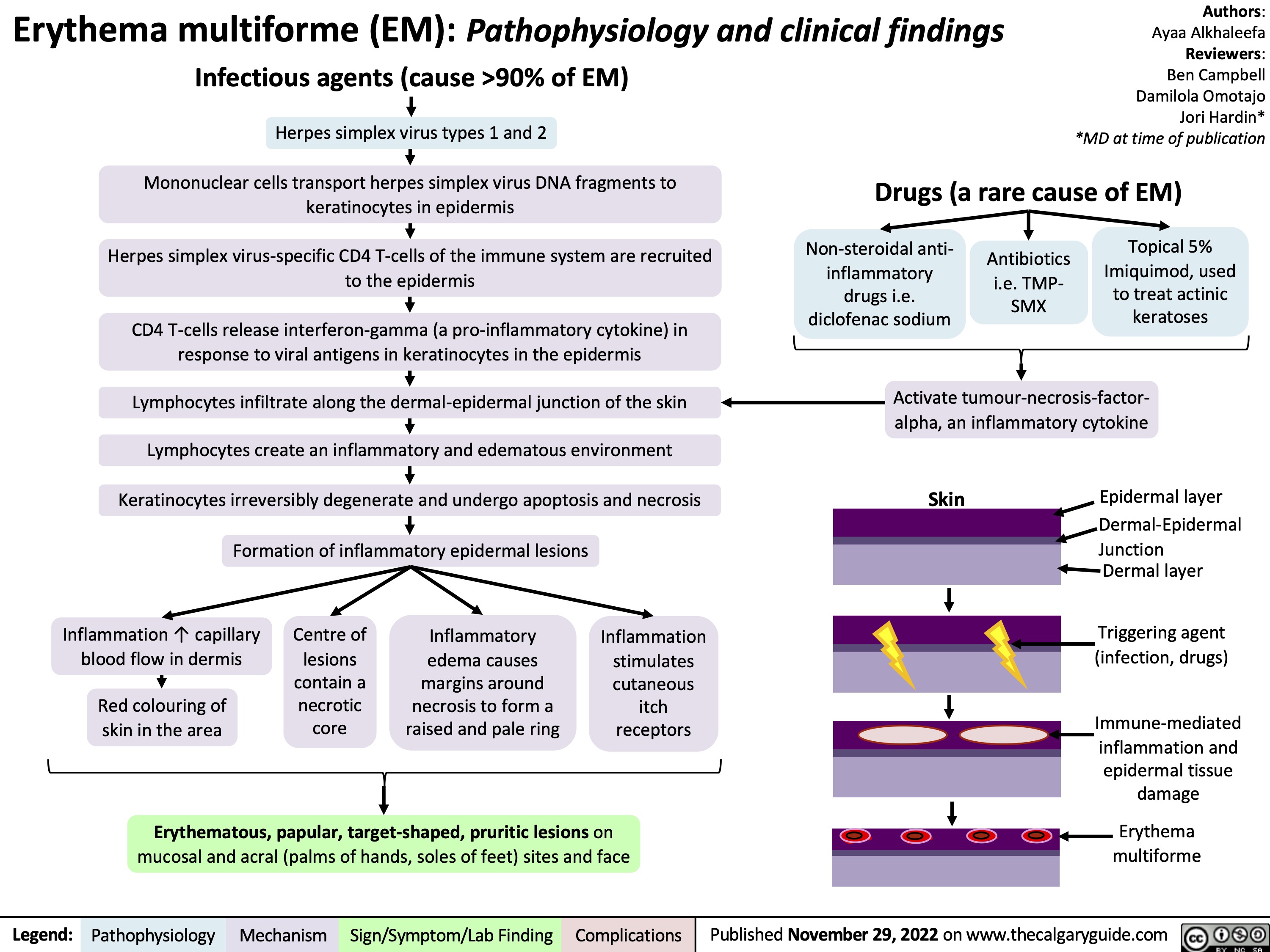
rheumatoid-pneumoconiosis-caplans-syndrome-pathogenesis-and-clinical-findings
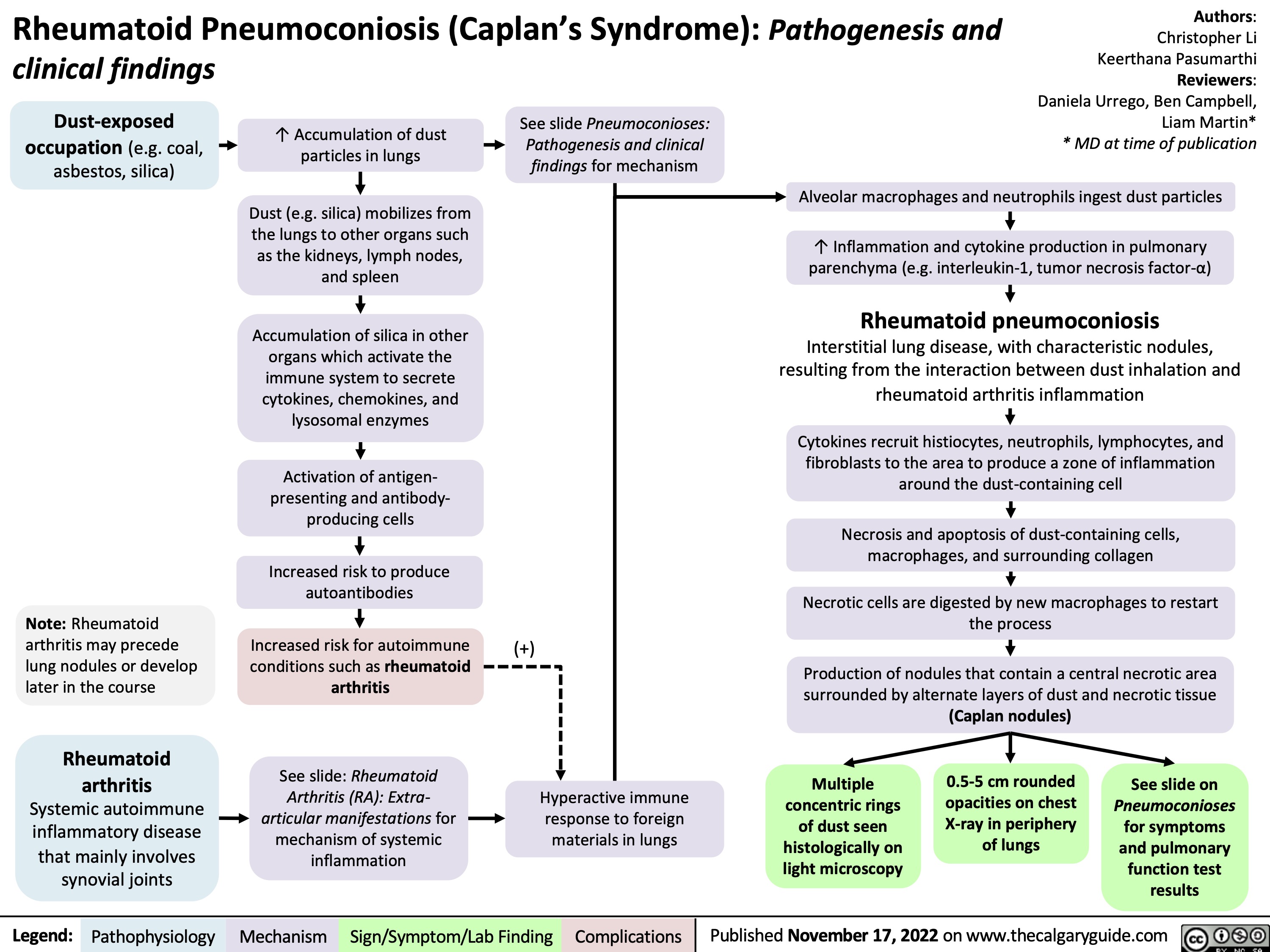
Acute and Chronic Gastritis: Pathogenesis and clinical findings
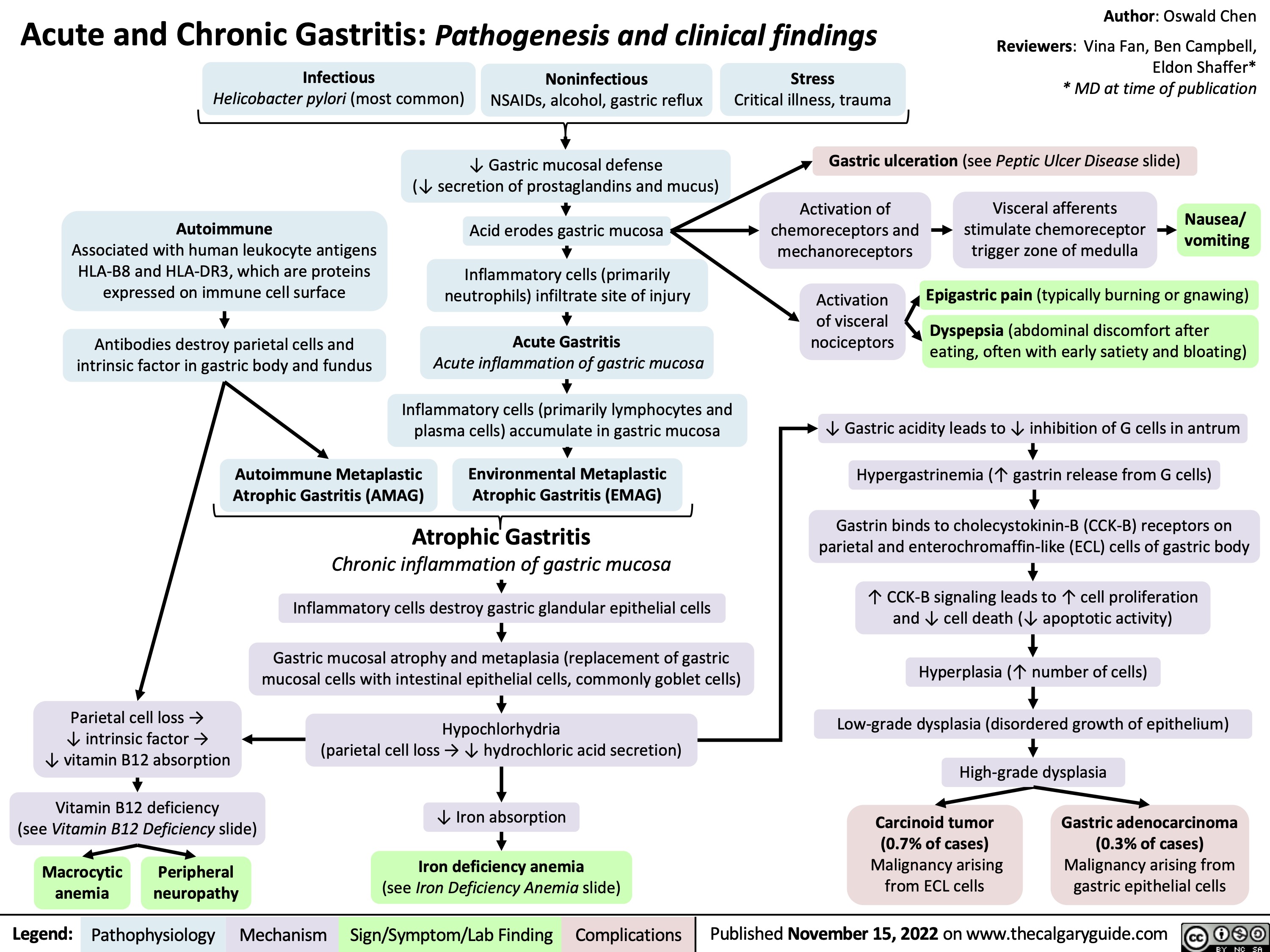
Achalasia: Findings on Fluoroscopy with Barium Swallow
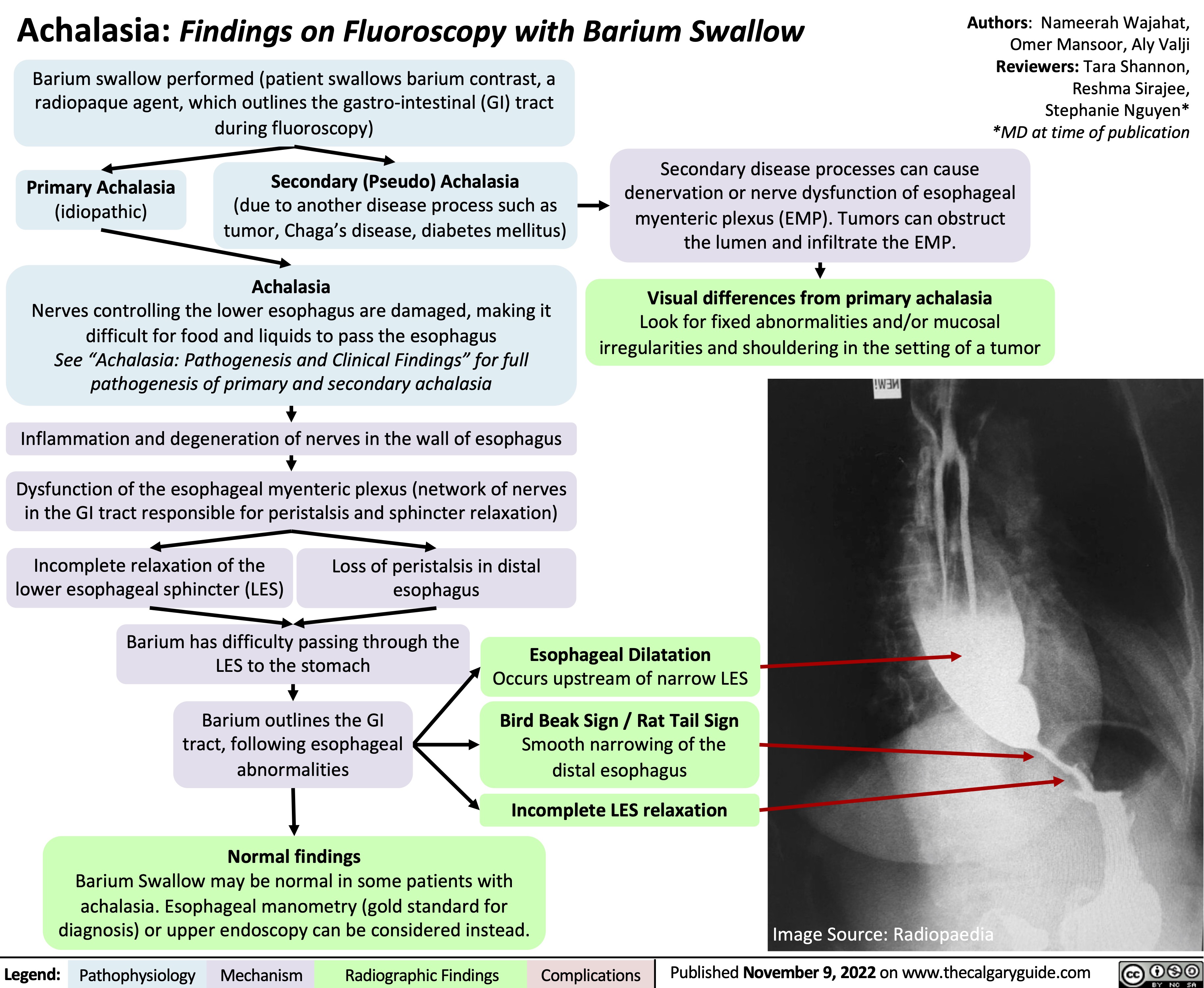
popliteal-bakers-cyst-pathogenesis-and-clinical-findings
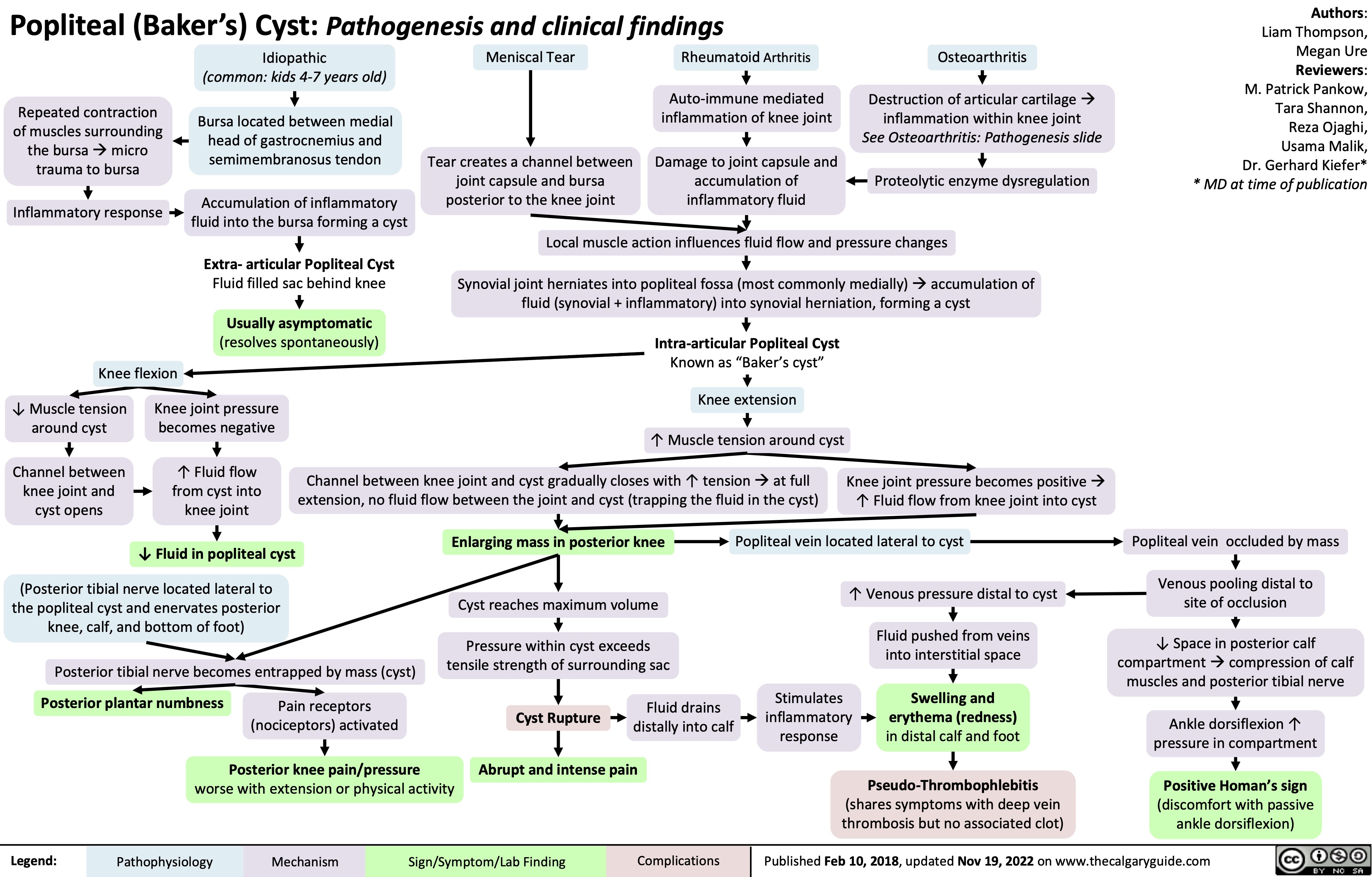
hypovolemic-shock
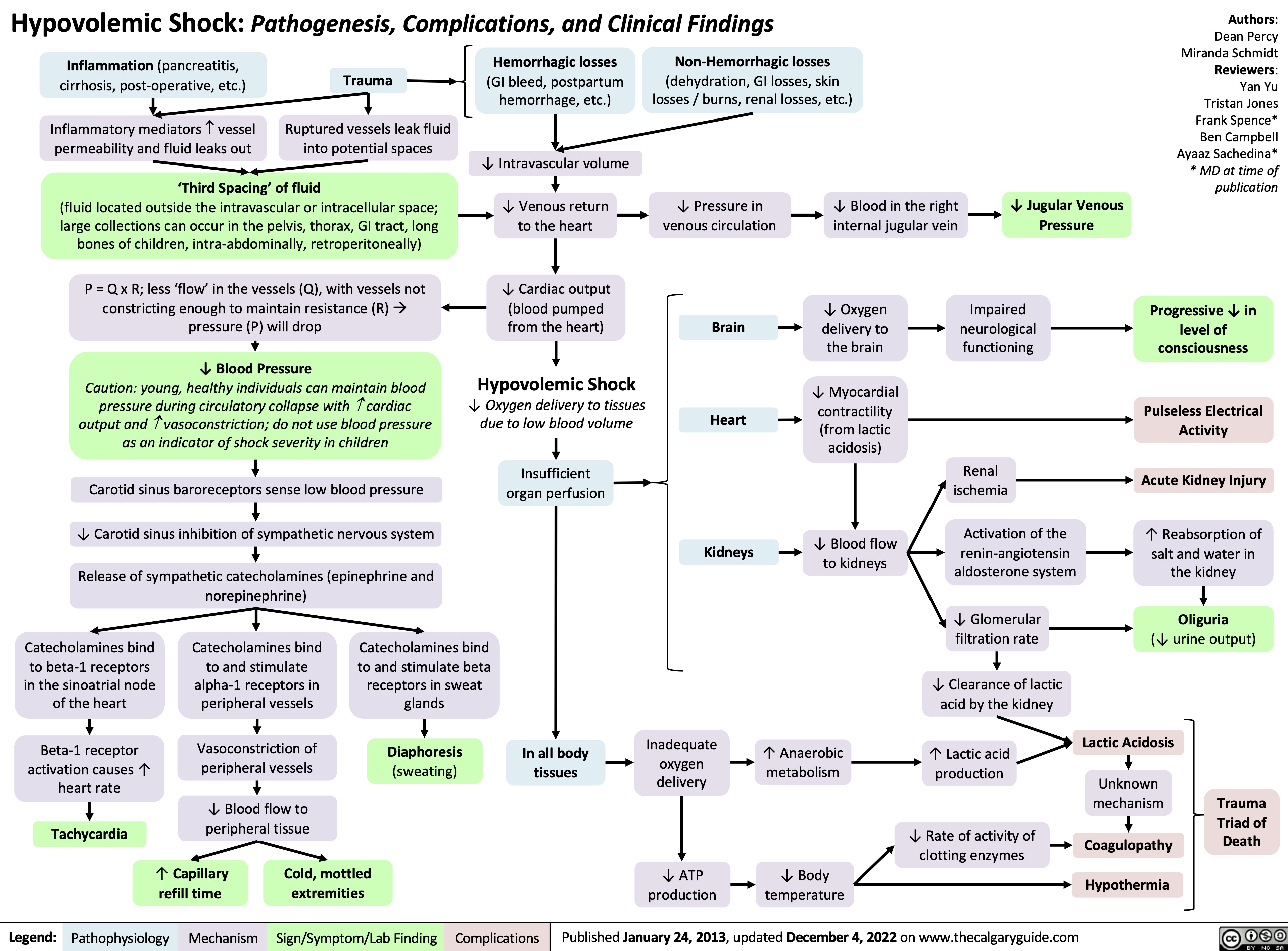
Ischemic Stroke: Pathogenesis
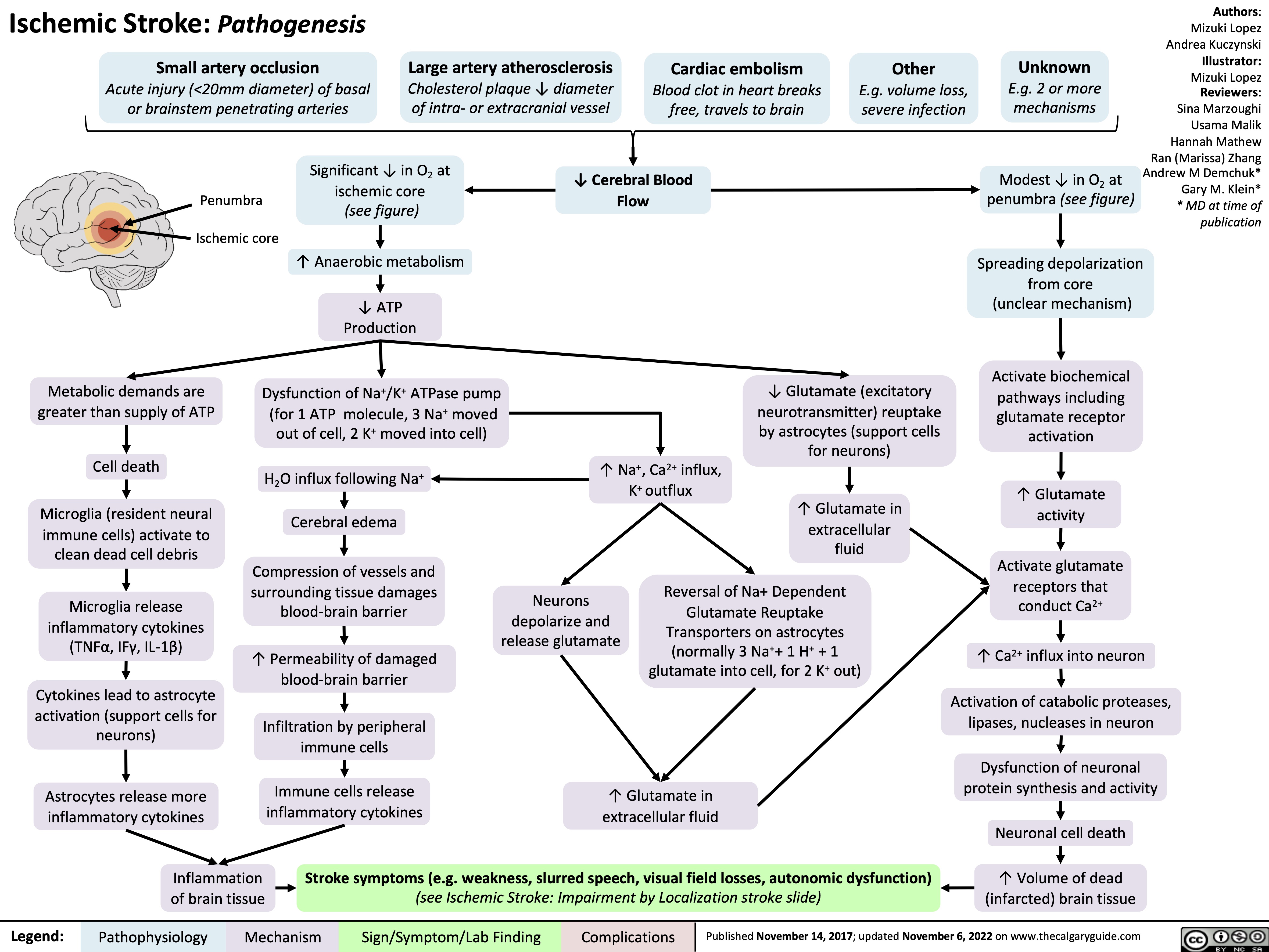
Cirrhosis
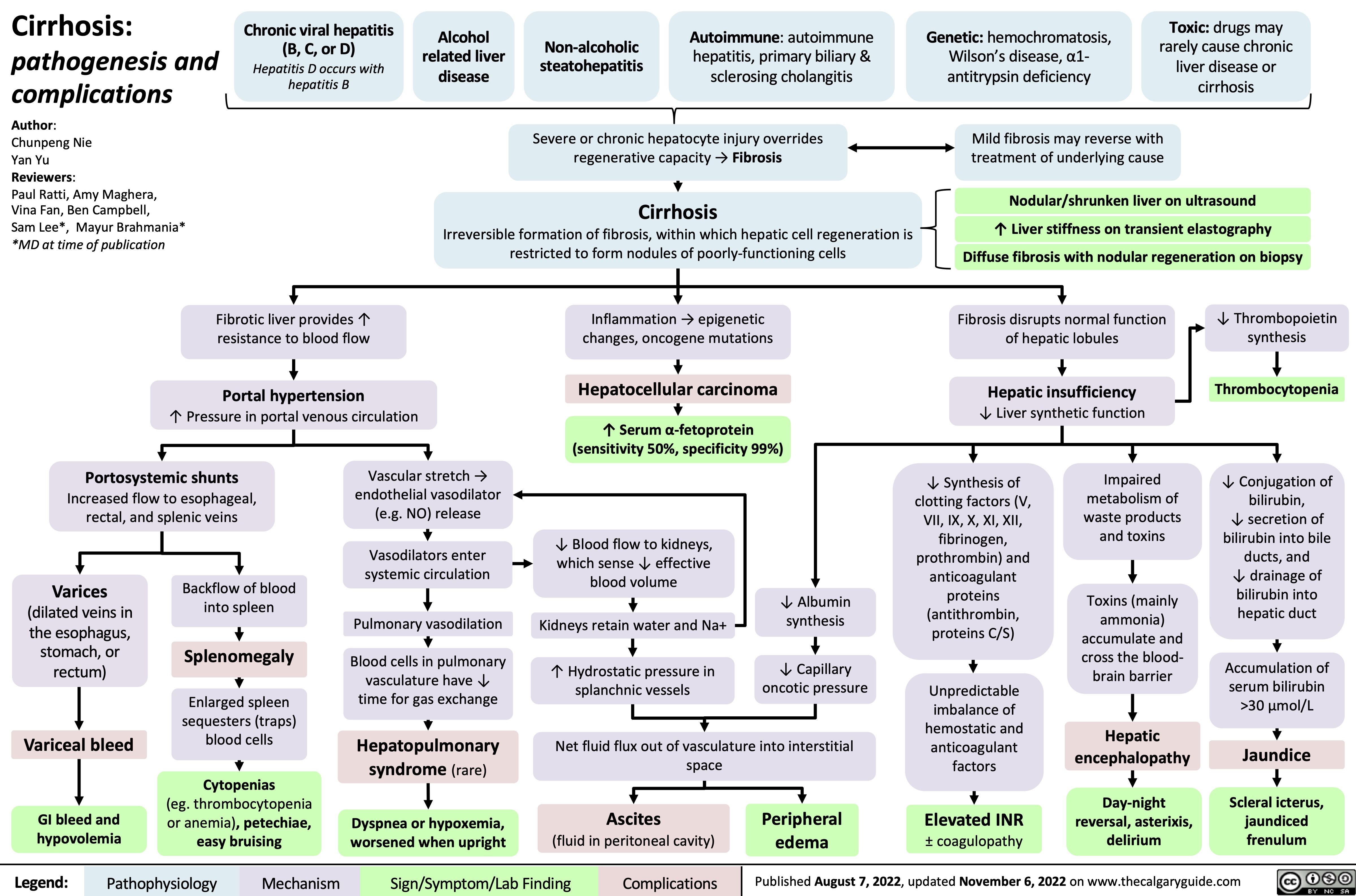
Hidradenitis Suppurativa
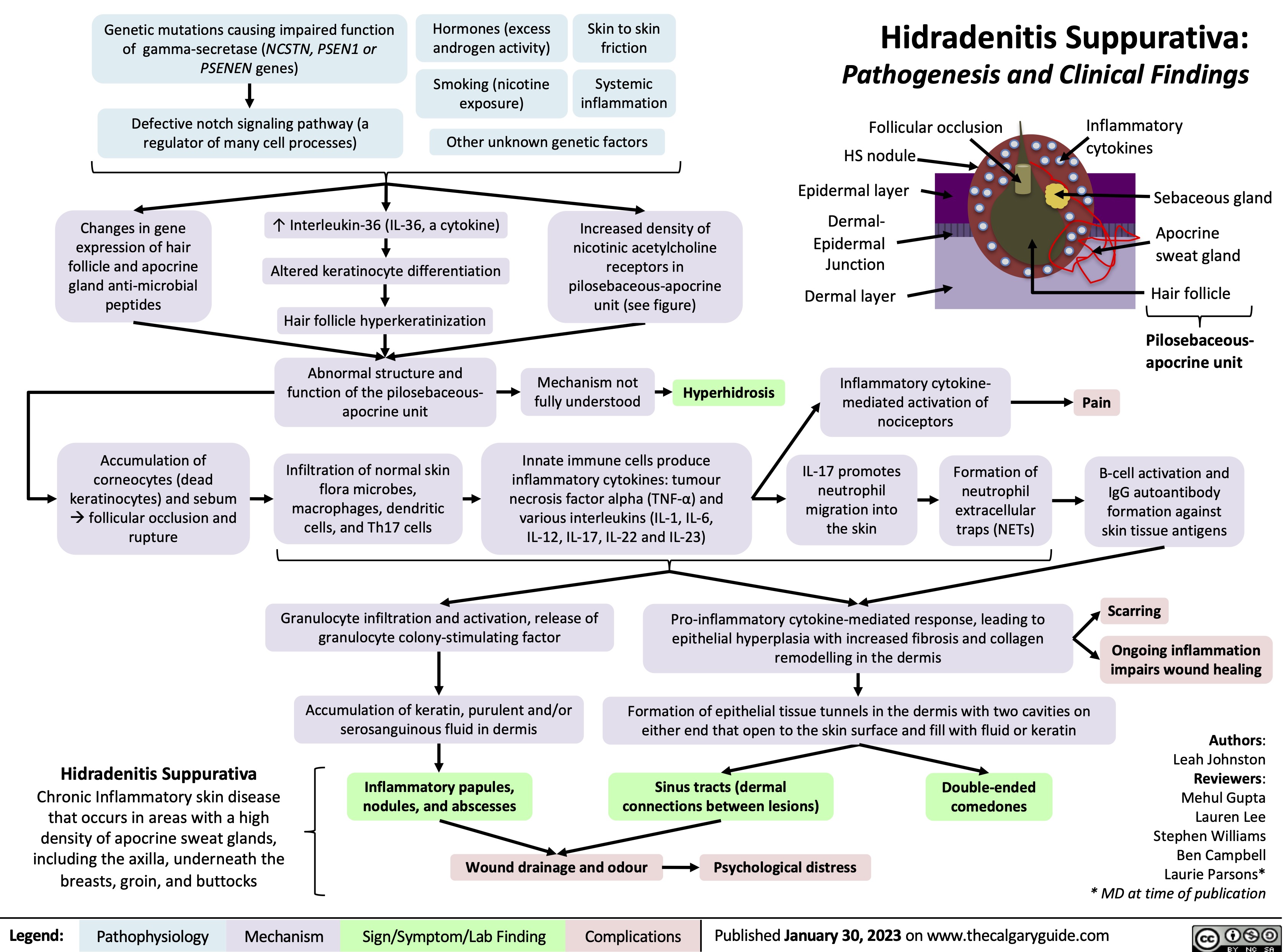
Inhalational Injury
Central Retinal Vein Occlusion
Pyogenic Brain Abscess on MRI
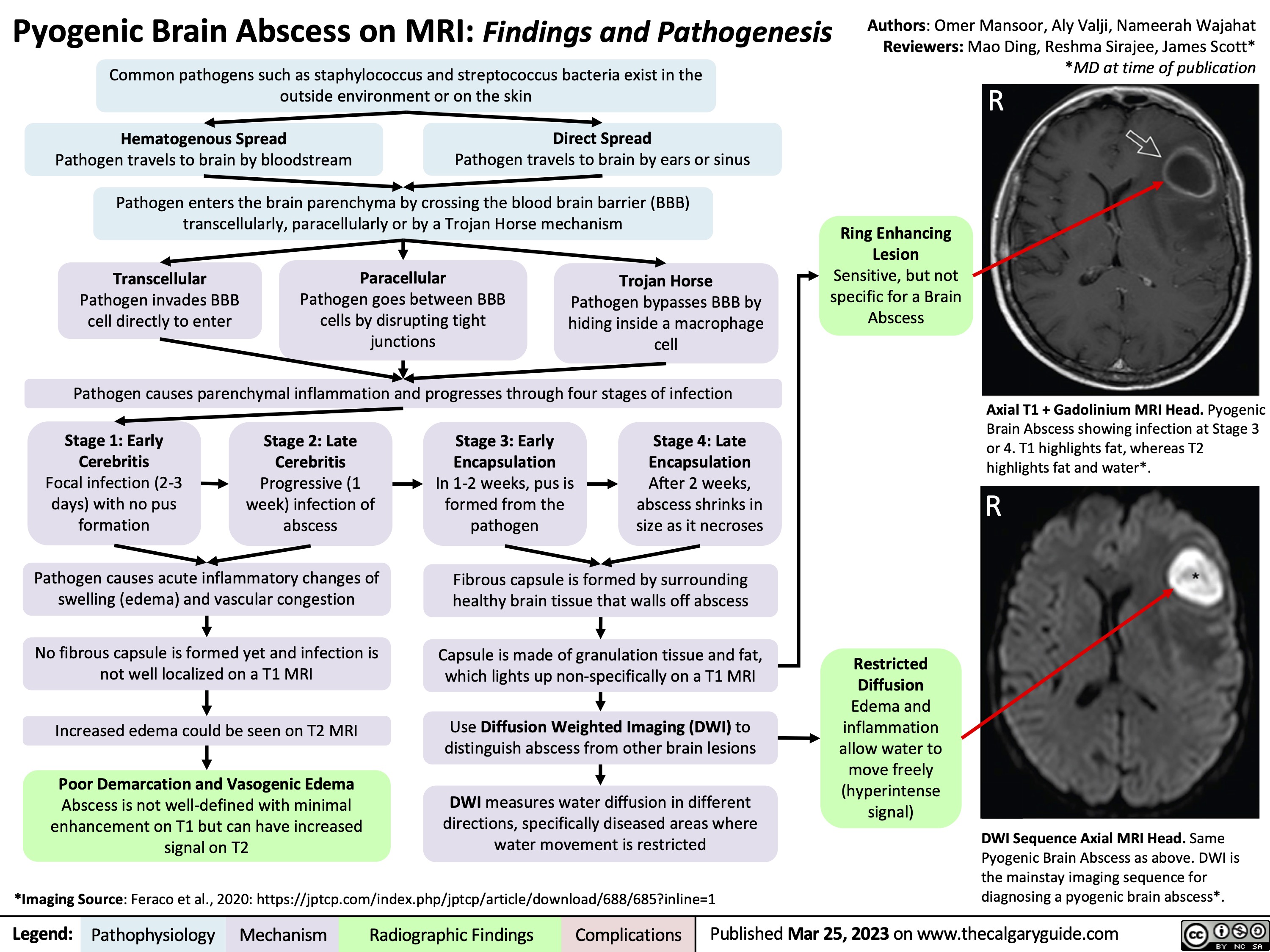
Acute Pulmonary Embolism on CTPA
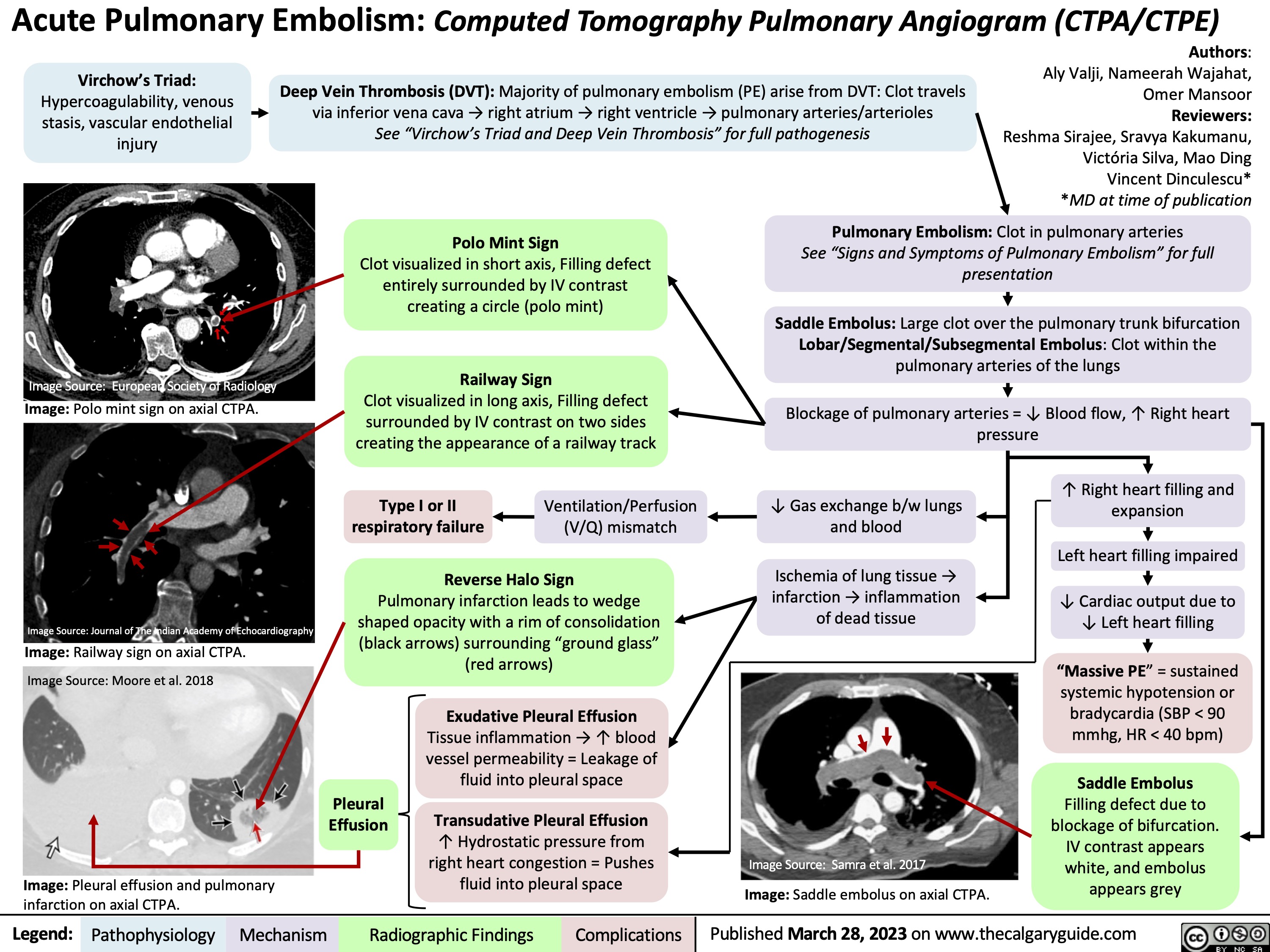
Coronary Artery Bypass Graft CABG Indications
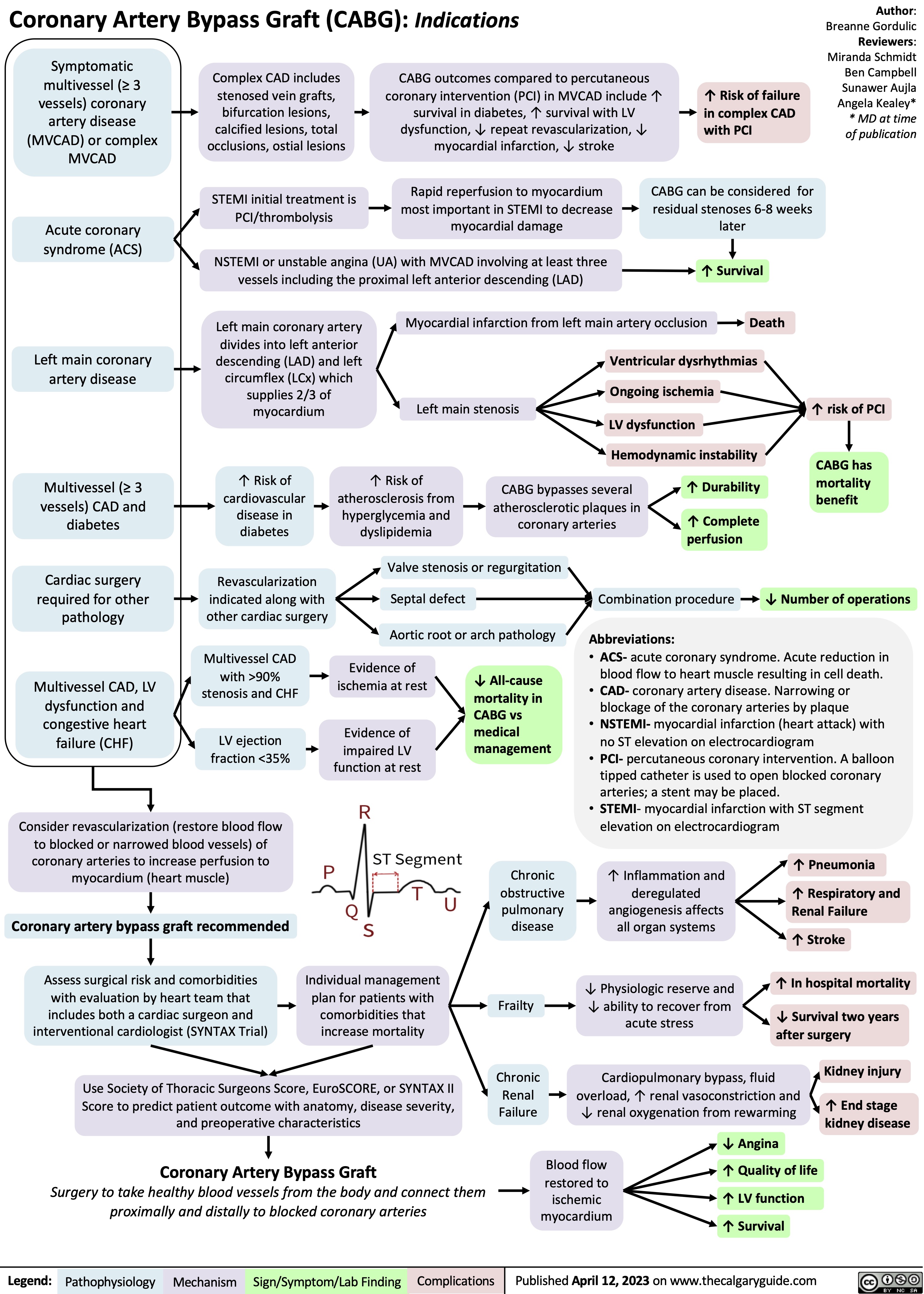
OA Clinical findings
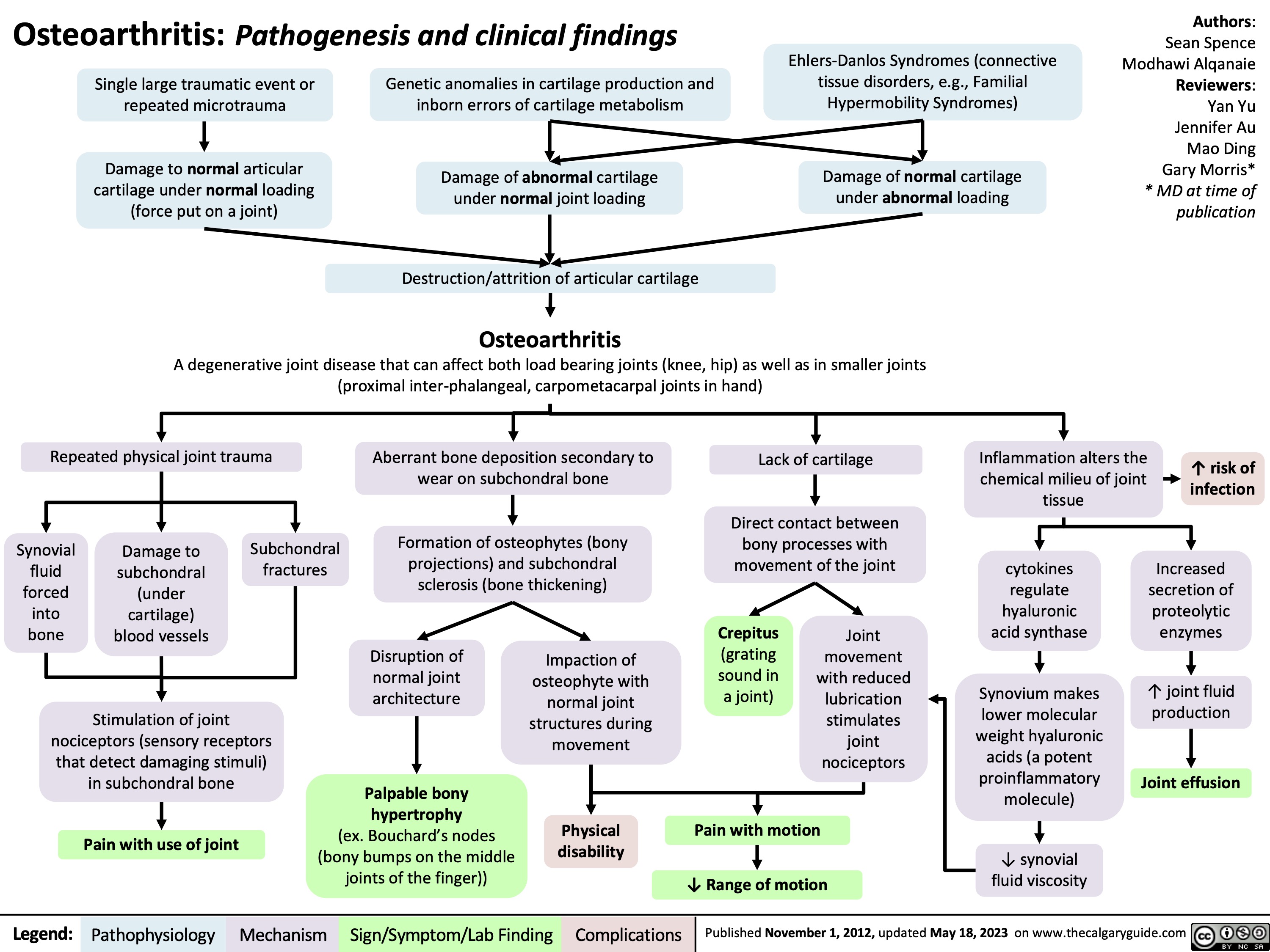
diverticulosis-vs-diverticulitis-distinguishing-features
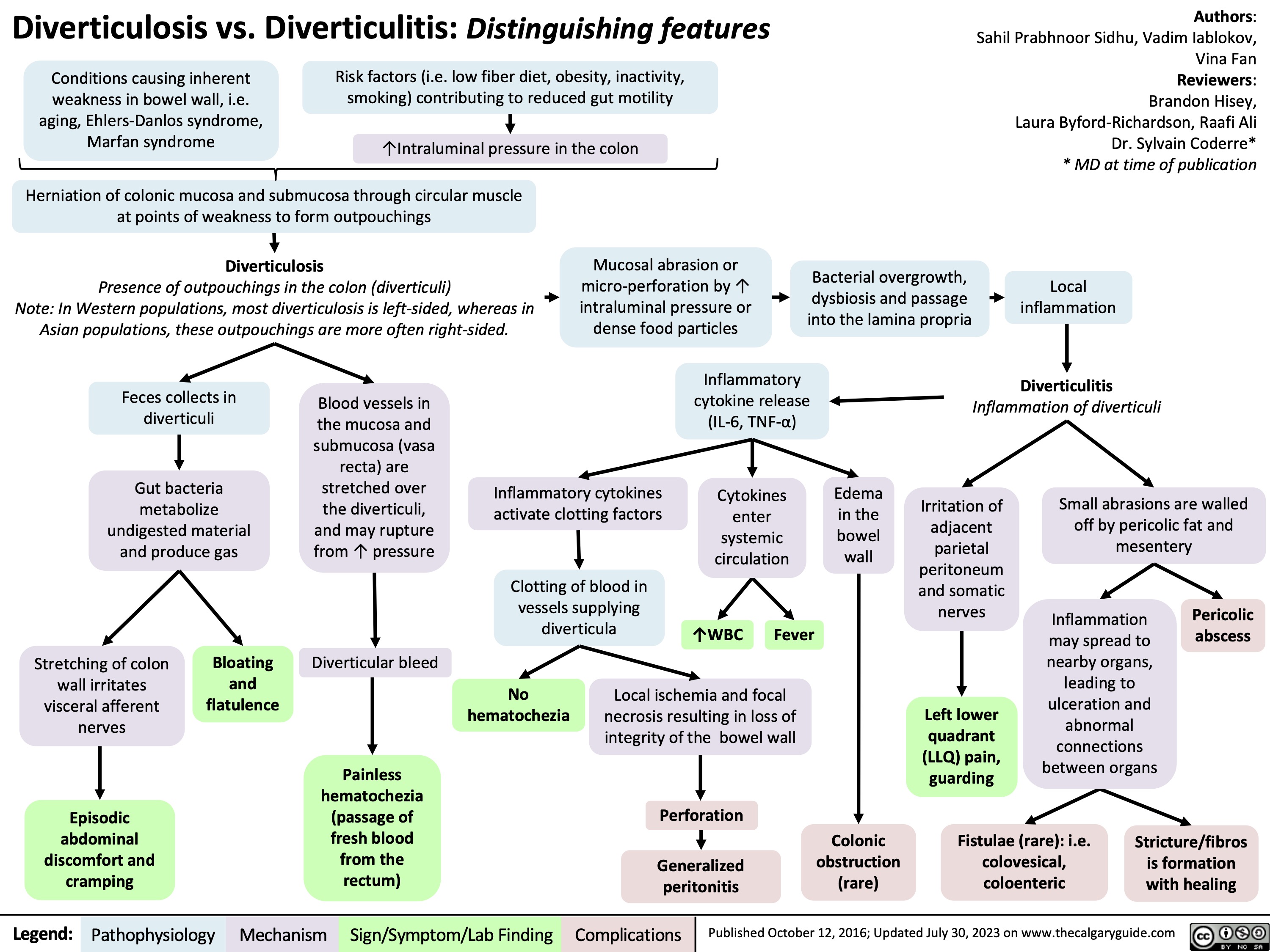
Knee Osteoarthritis
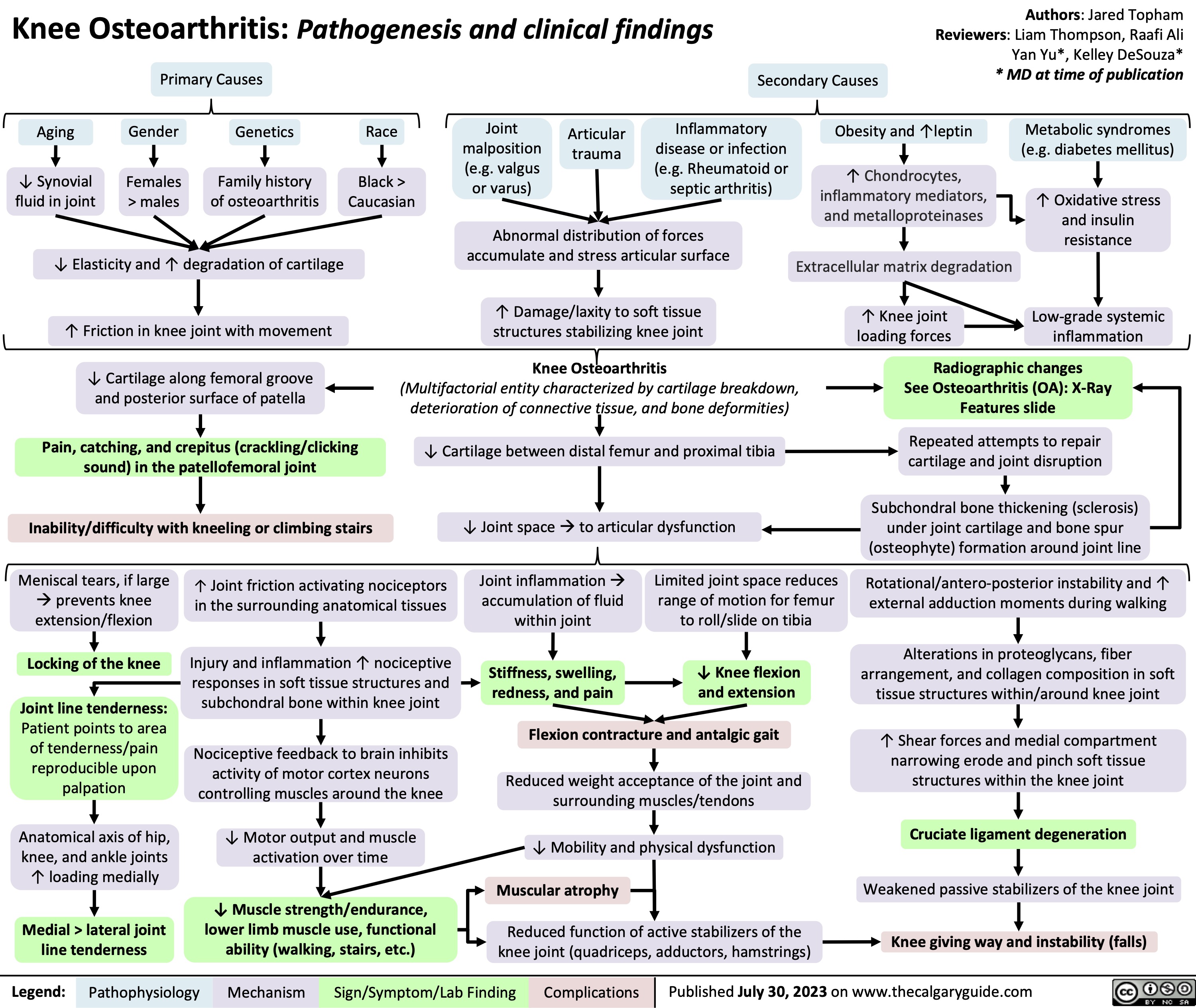
Rotator Cuff Disease
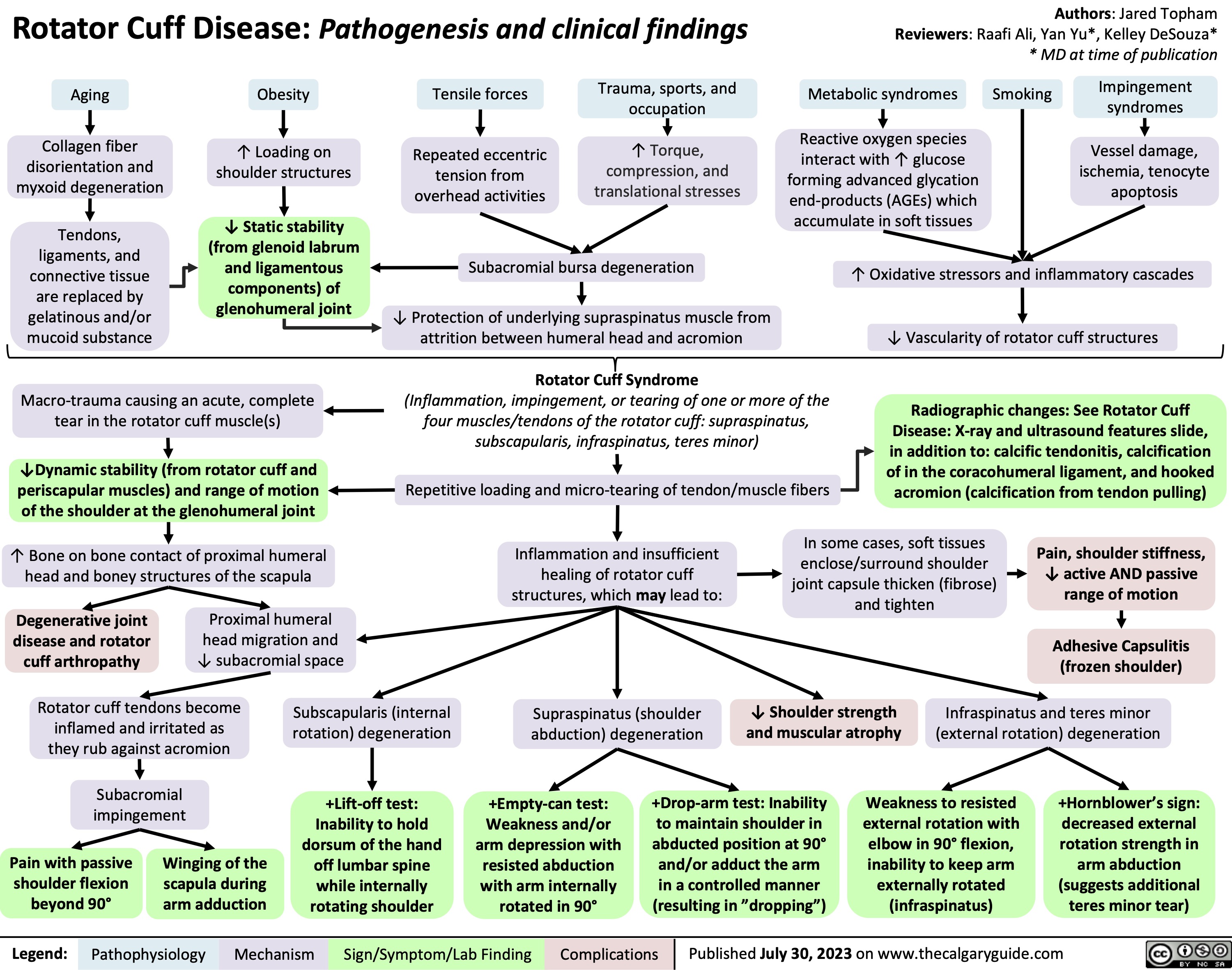
Mitral Regurgitation Pathogenesis and clinical findings
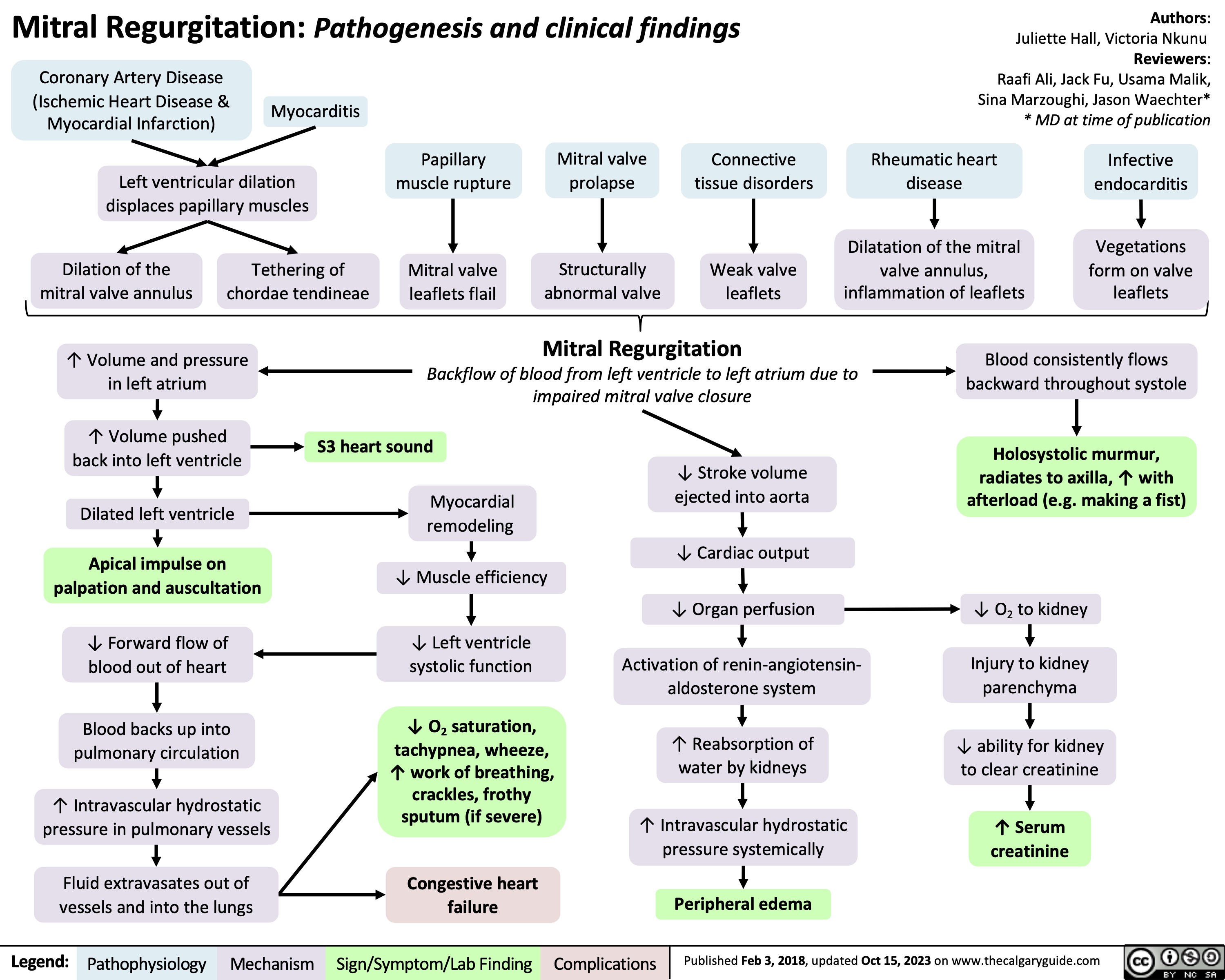
Death Cardiovascular Respiratory and Neurologic Mechanisms
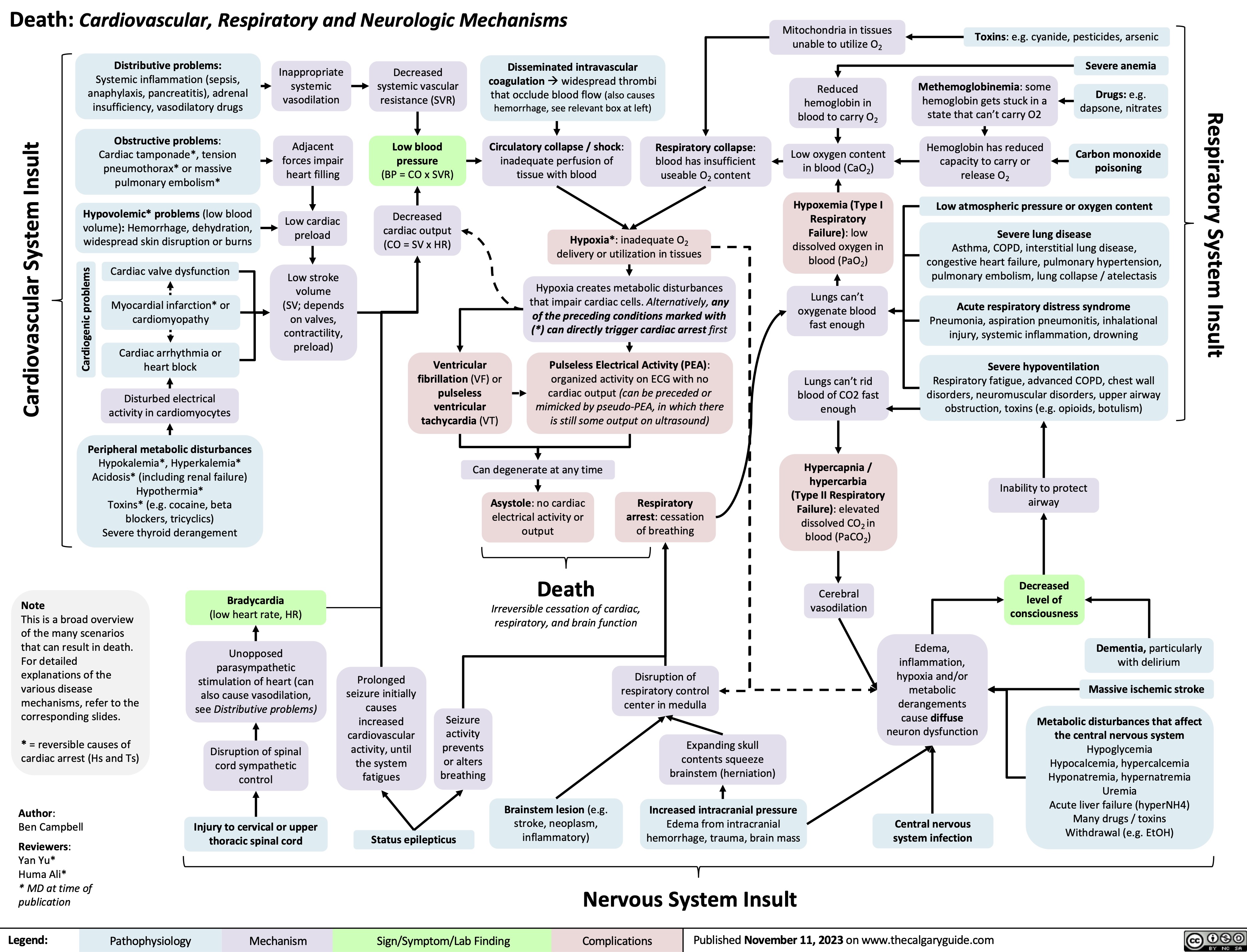
Non-Alcoholic Fatty Liver Disease
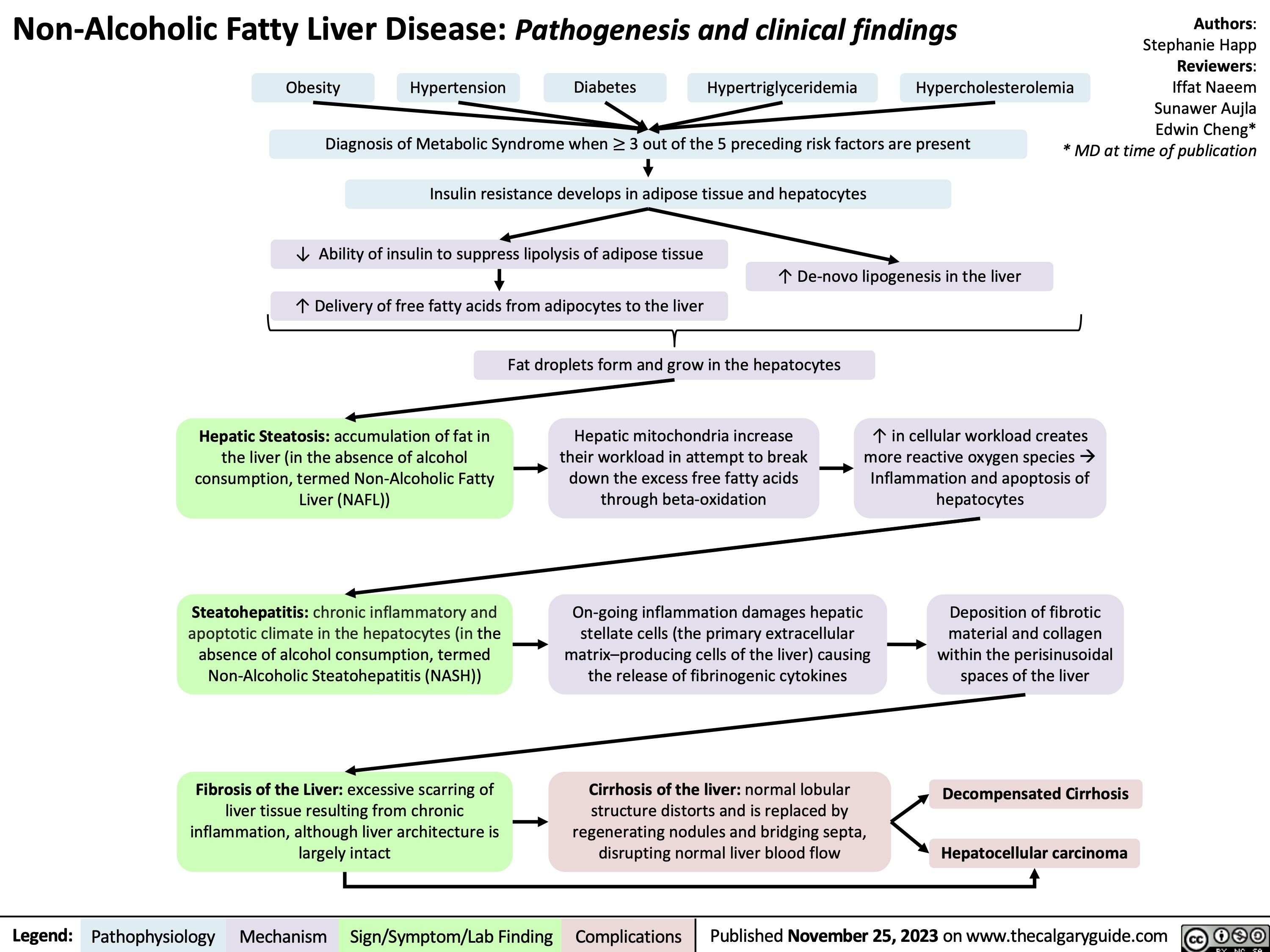
RICE mechanism of action
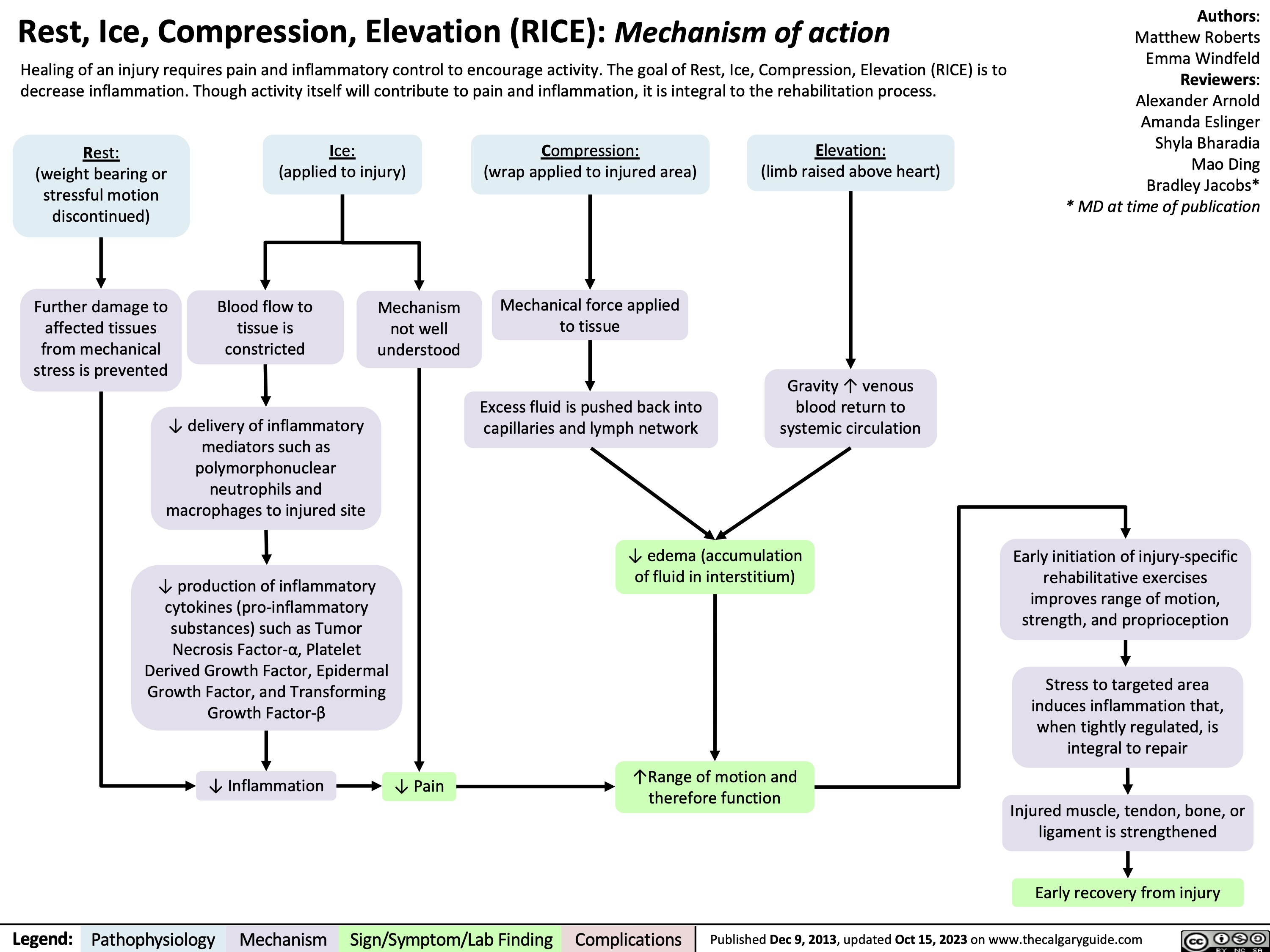
Aspiration Pneumonia
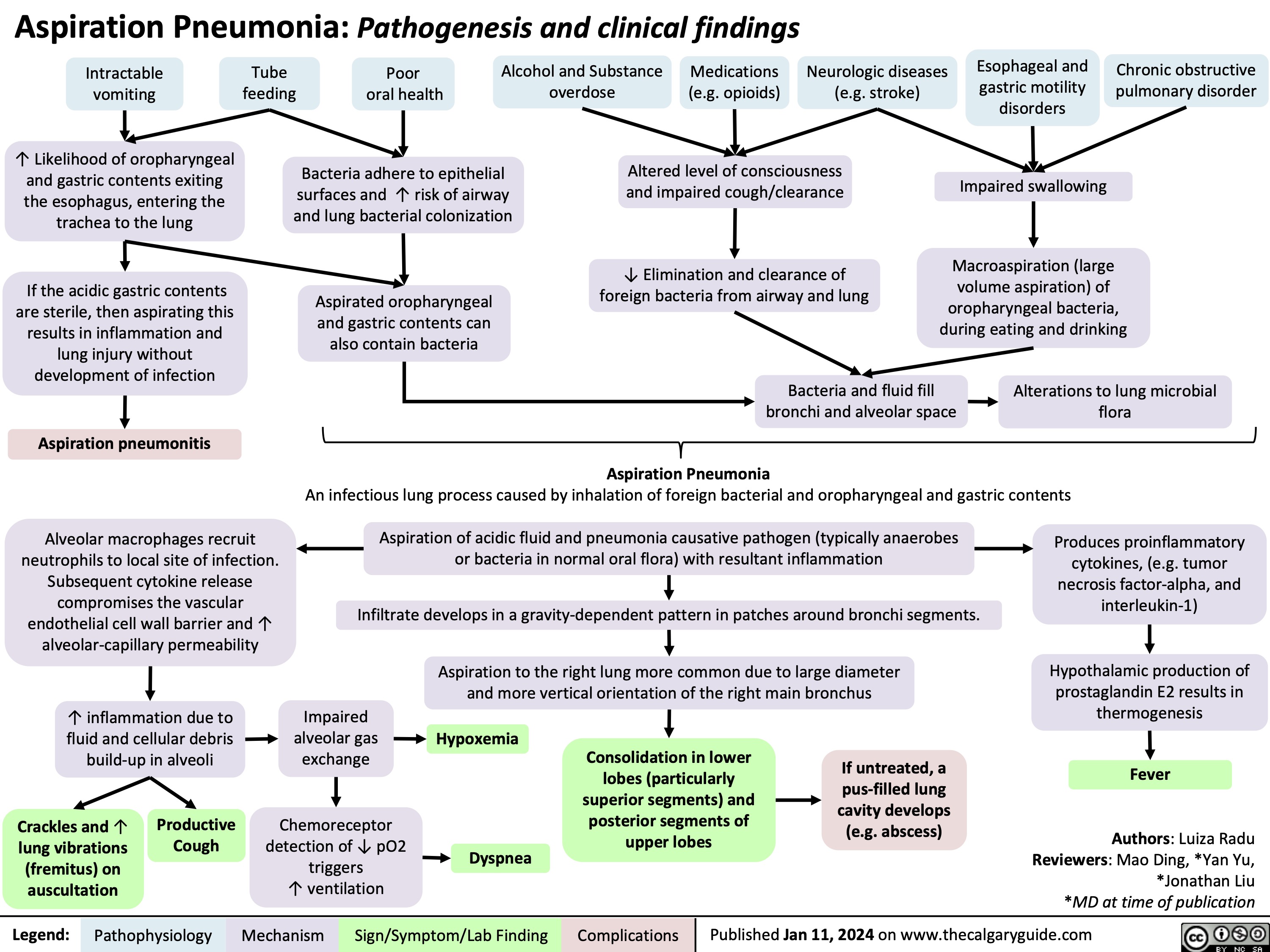
Shoulder Impingement Syndrome
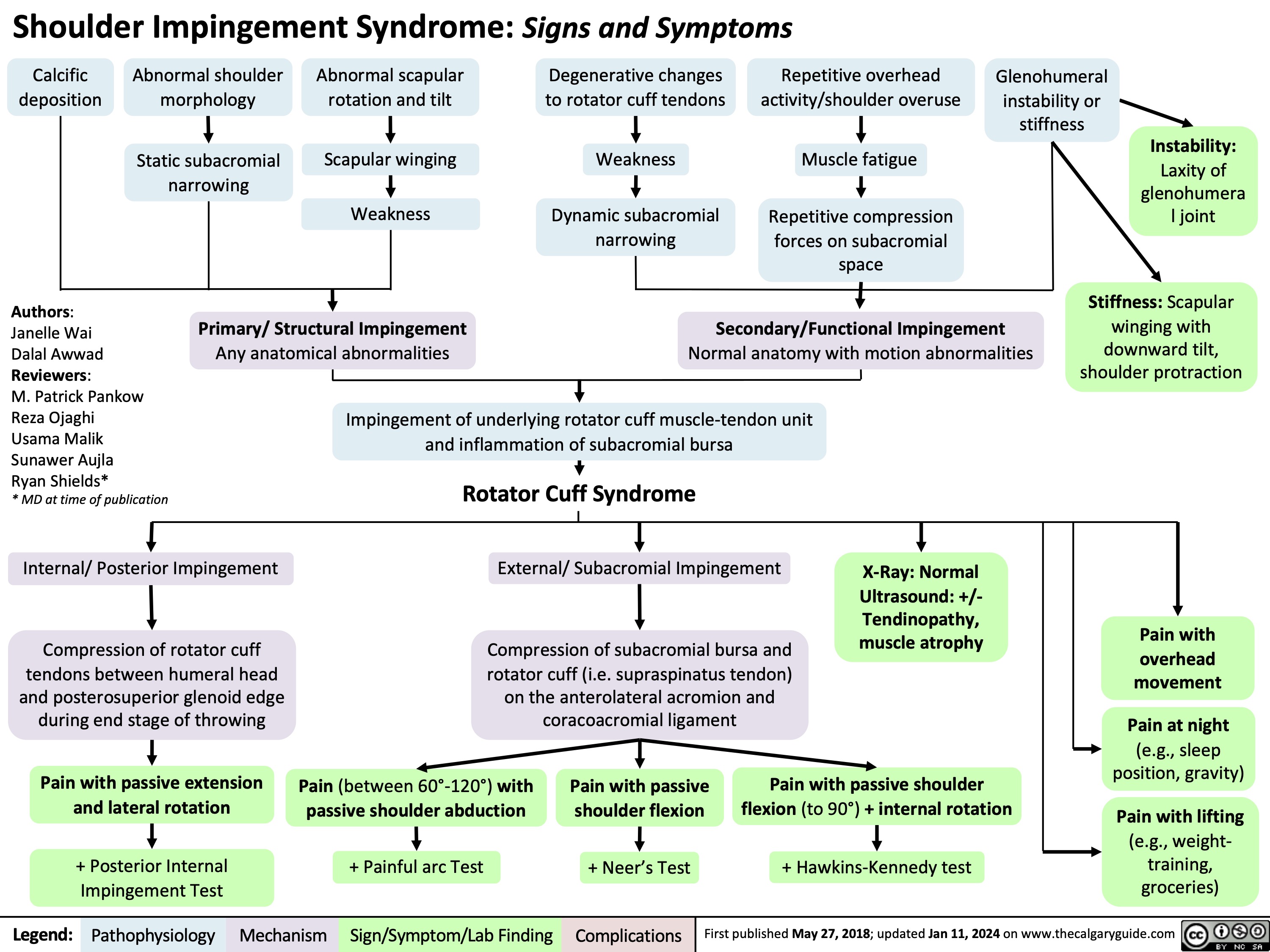
Arachnoid Cysts MRI Findings
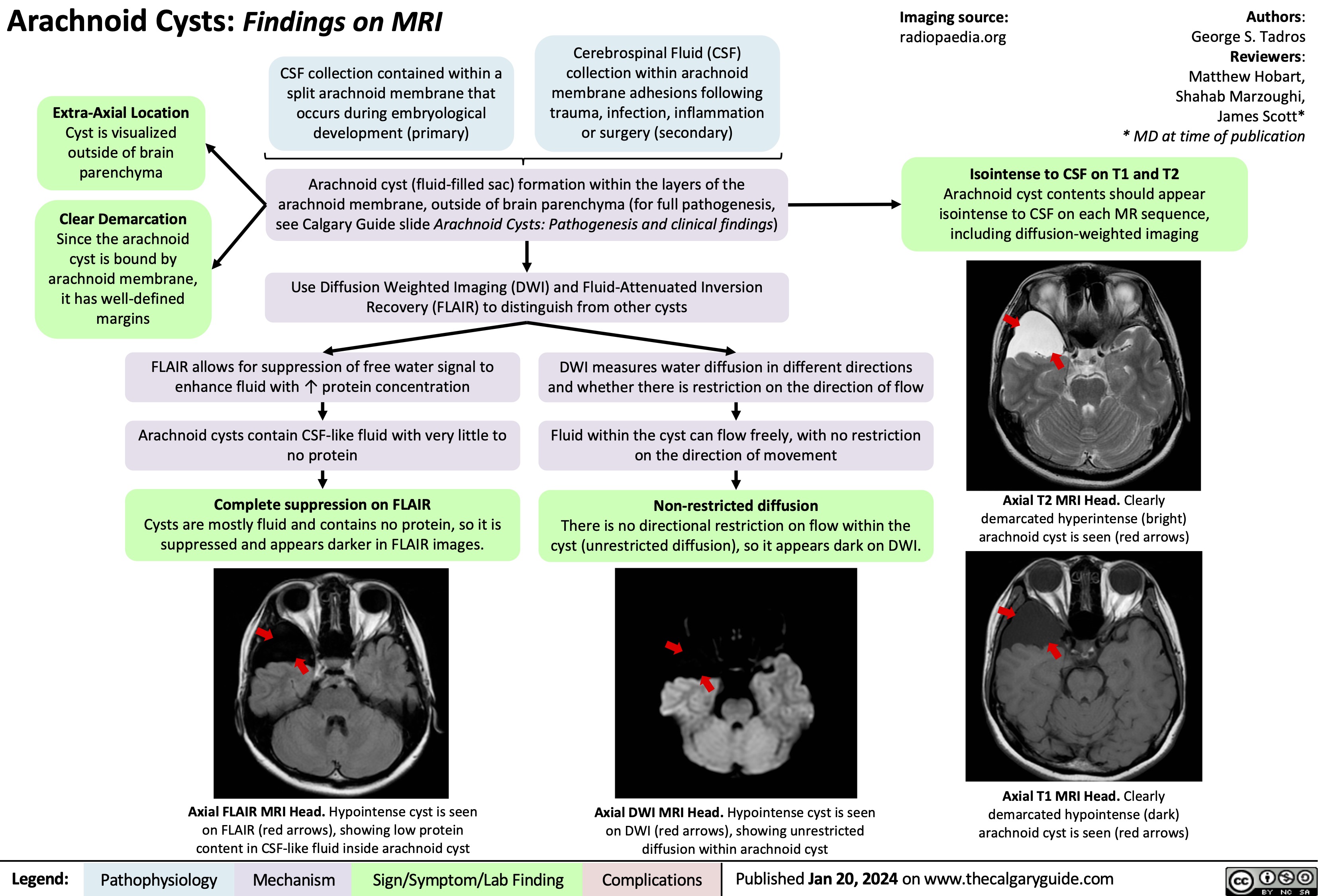
Arachnoid Cysts Pathogenesis and clinical findings
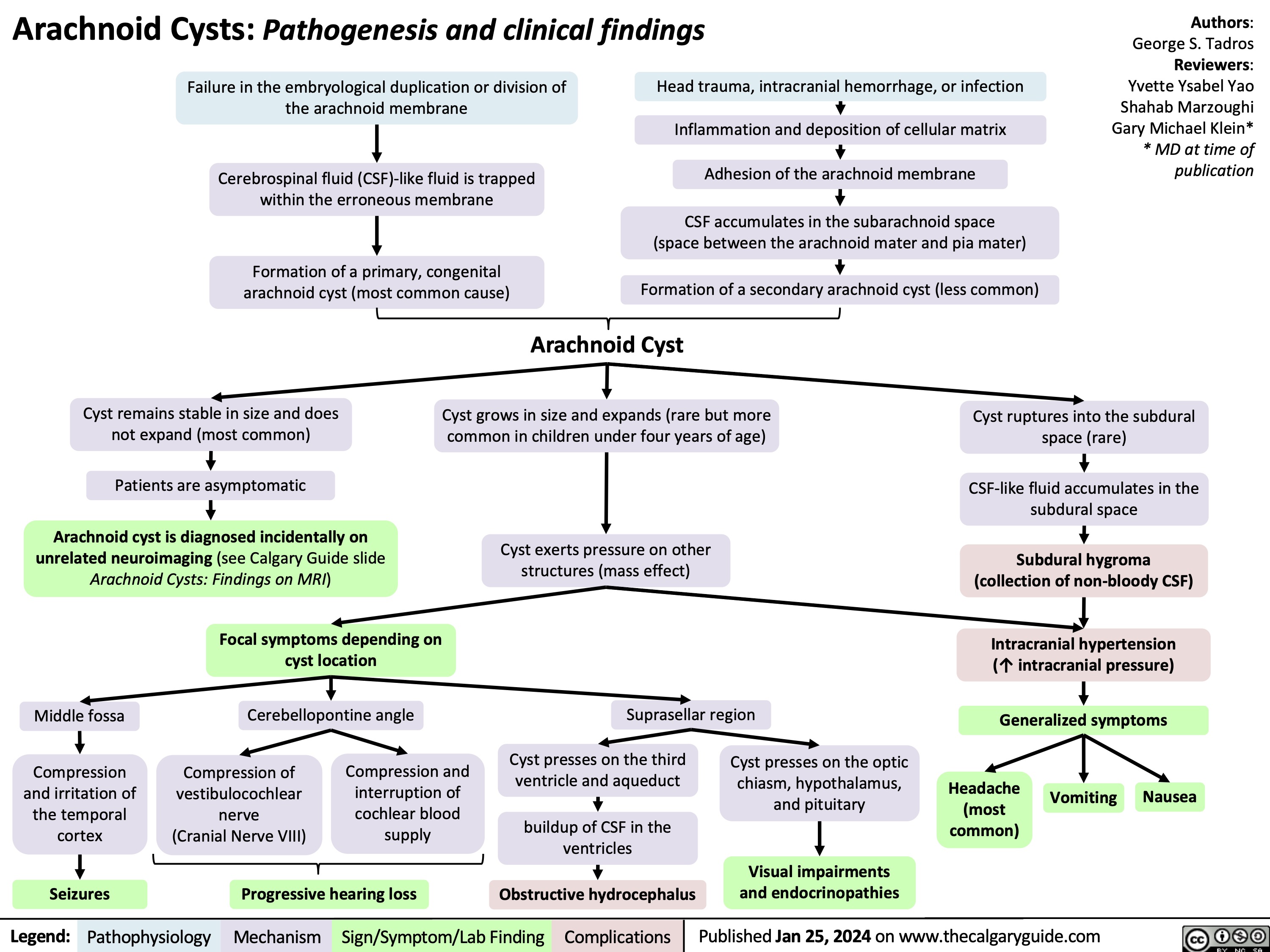
Avascular Necrosis AVN of the Femoral Head Findings on MRI
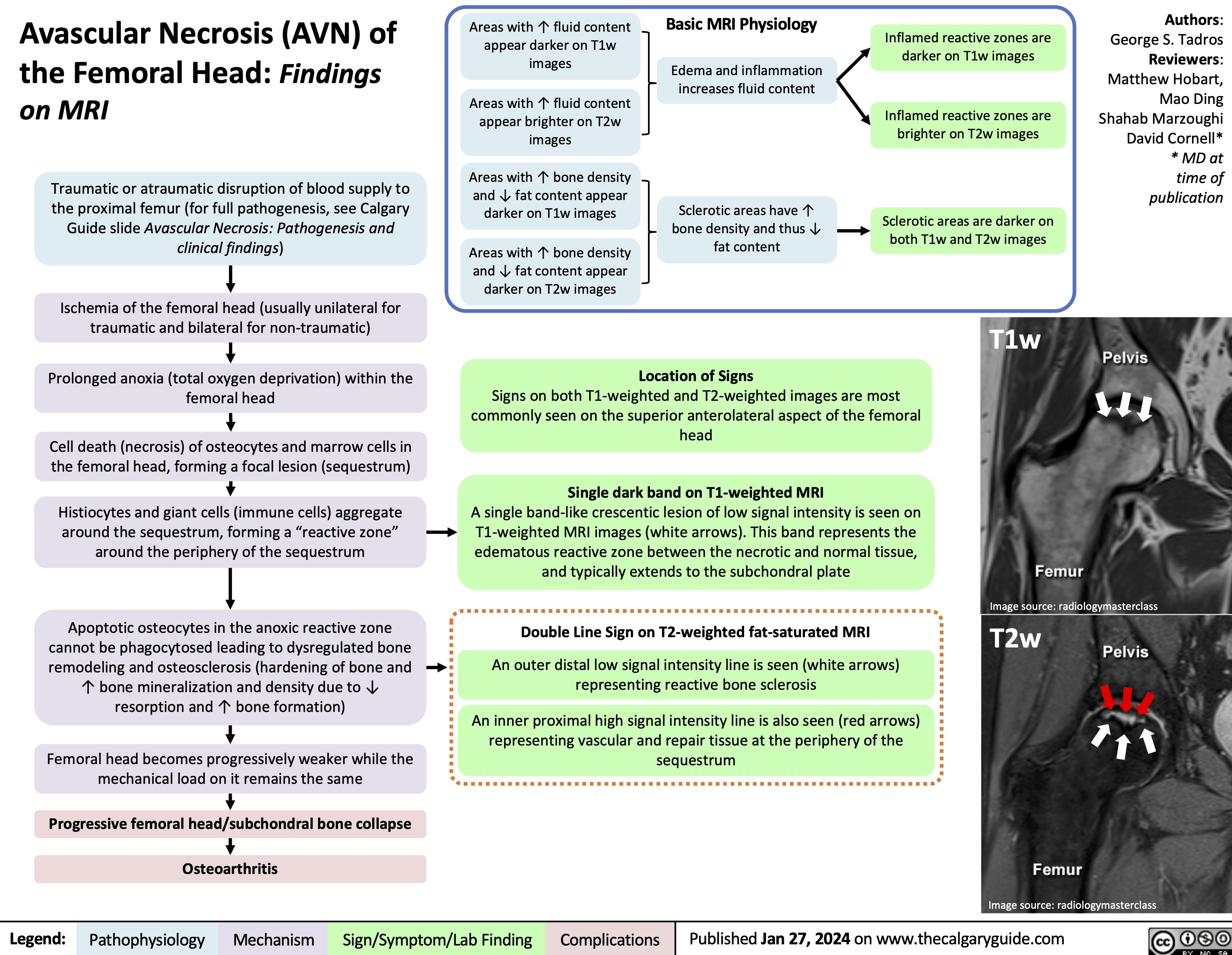
Bacterial Infections from Transfusion
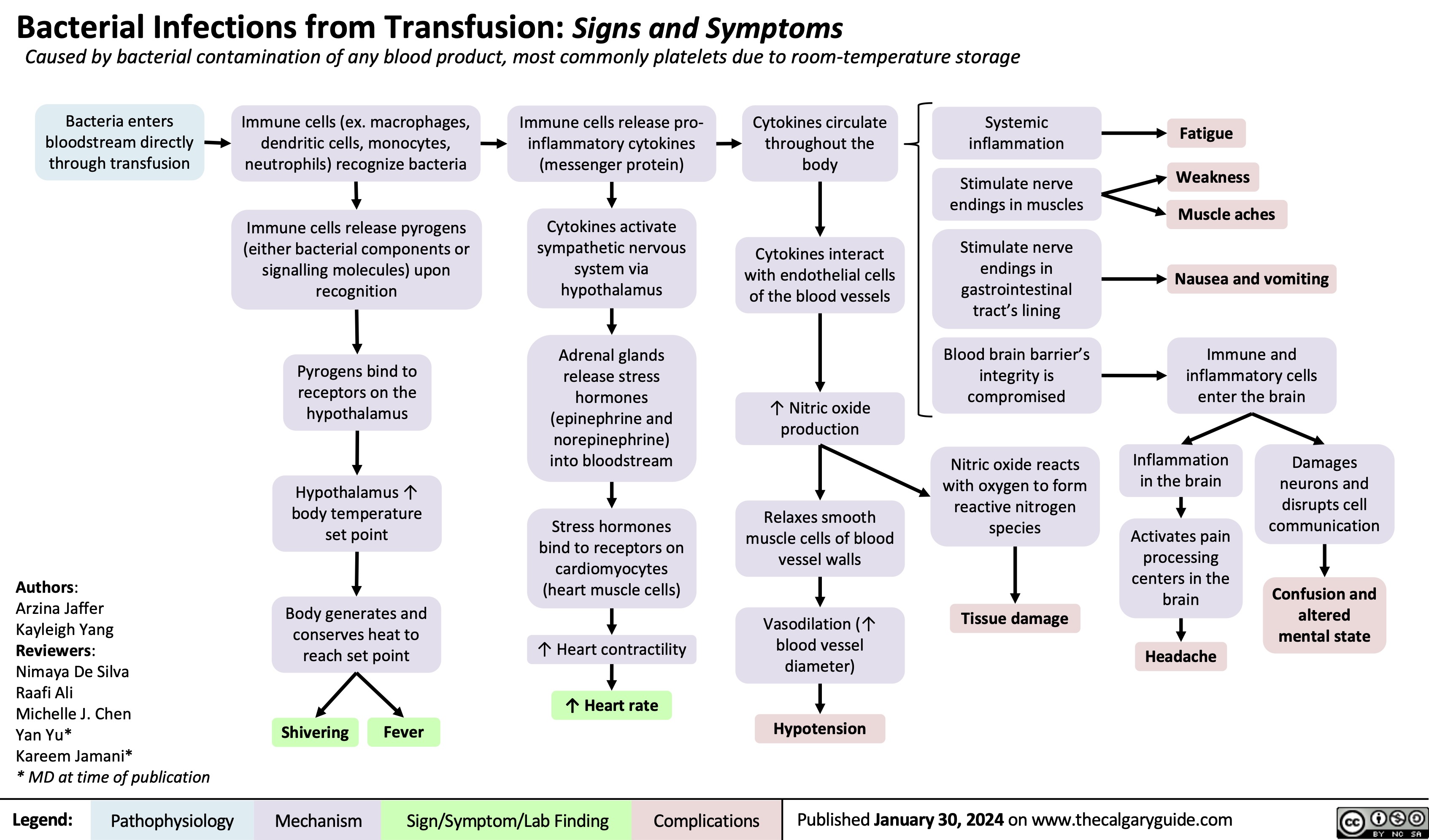
Acute Otitis Externa Complications
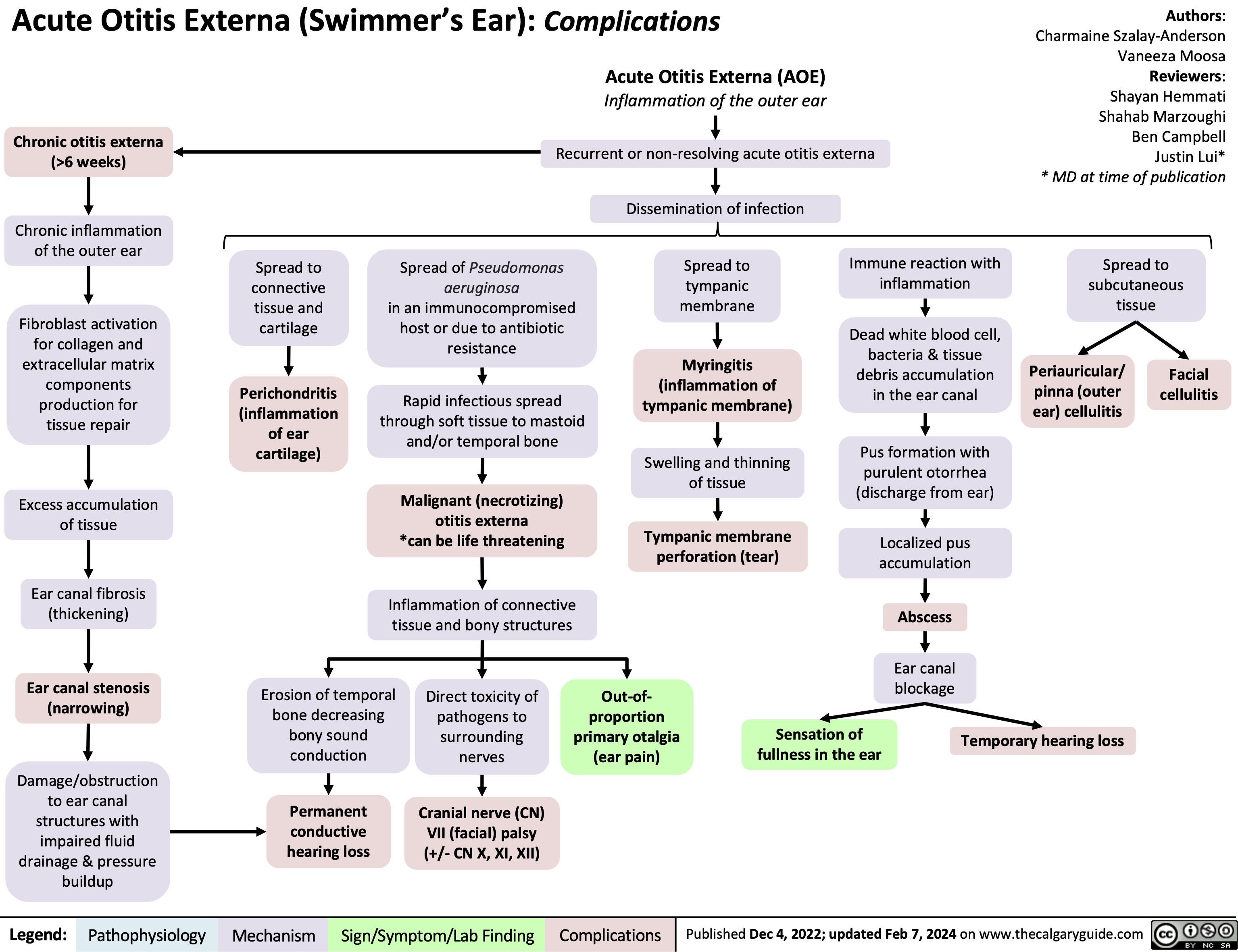
Infective endocarditis
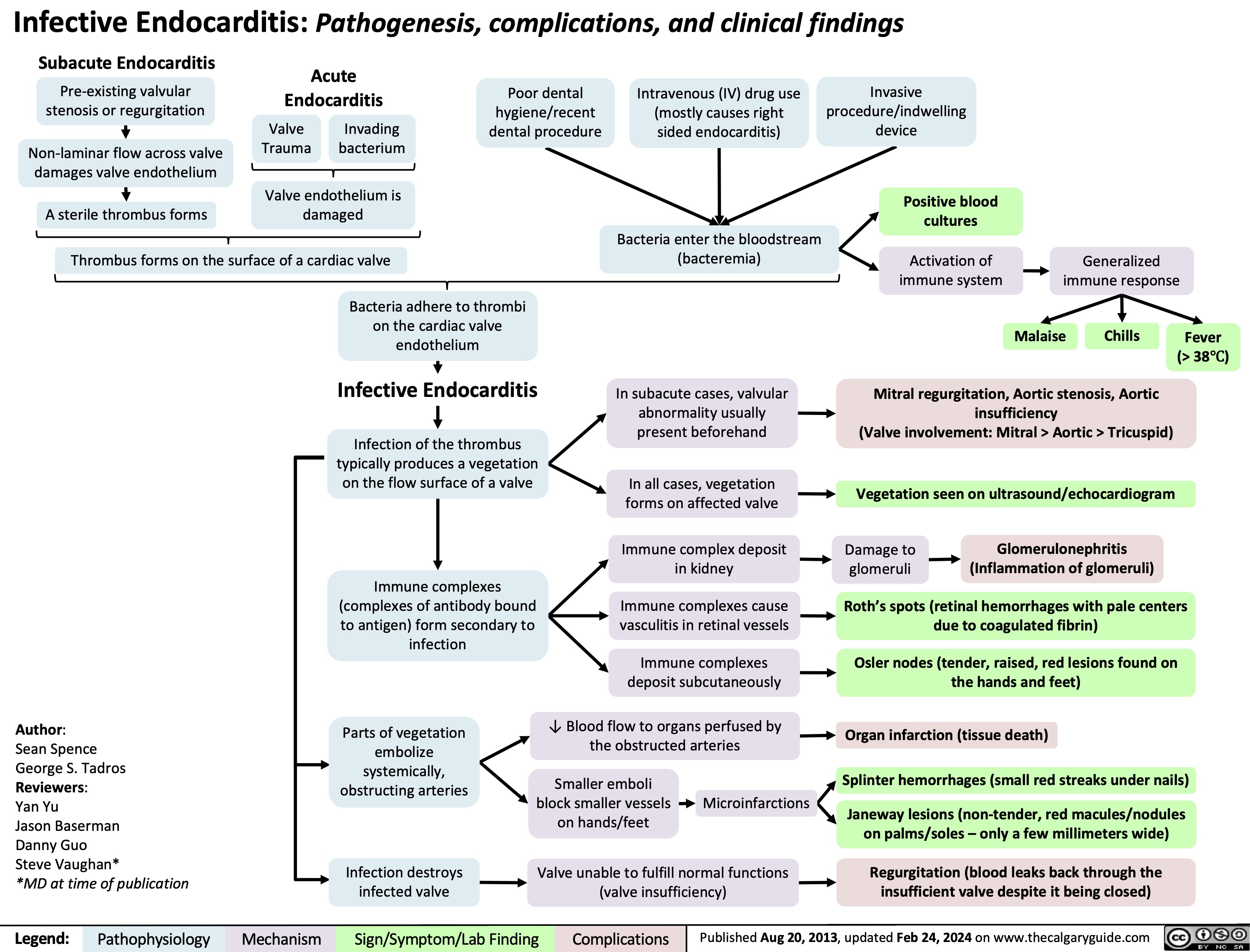
Onychomycosis
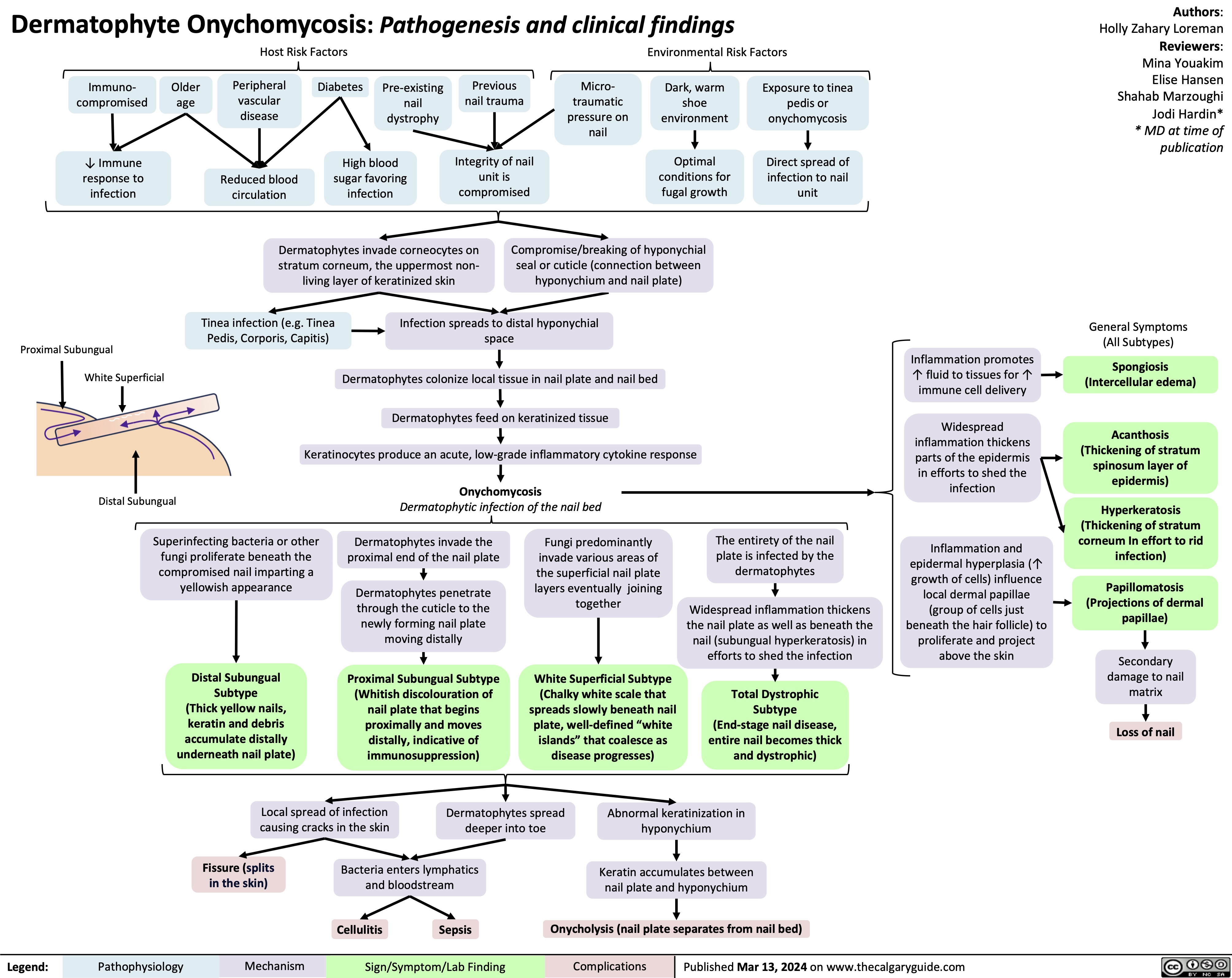
Neonatal Necrotising Enterocolitis in Premature Neonates
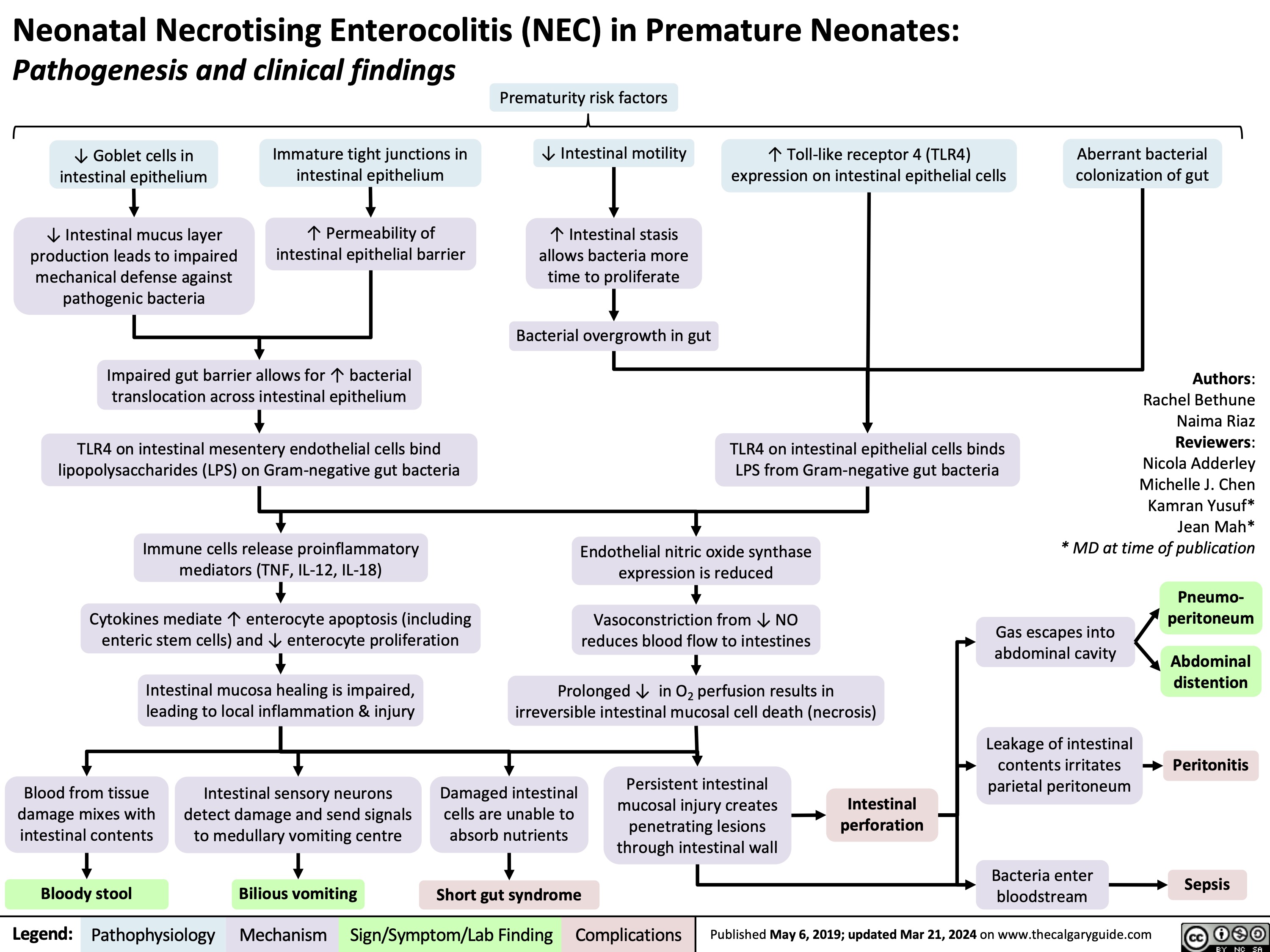
Lichen Sclerosus
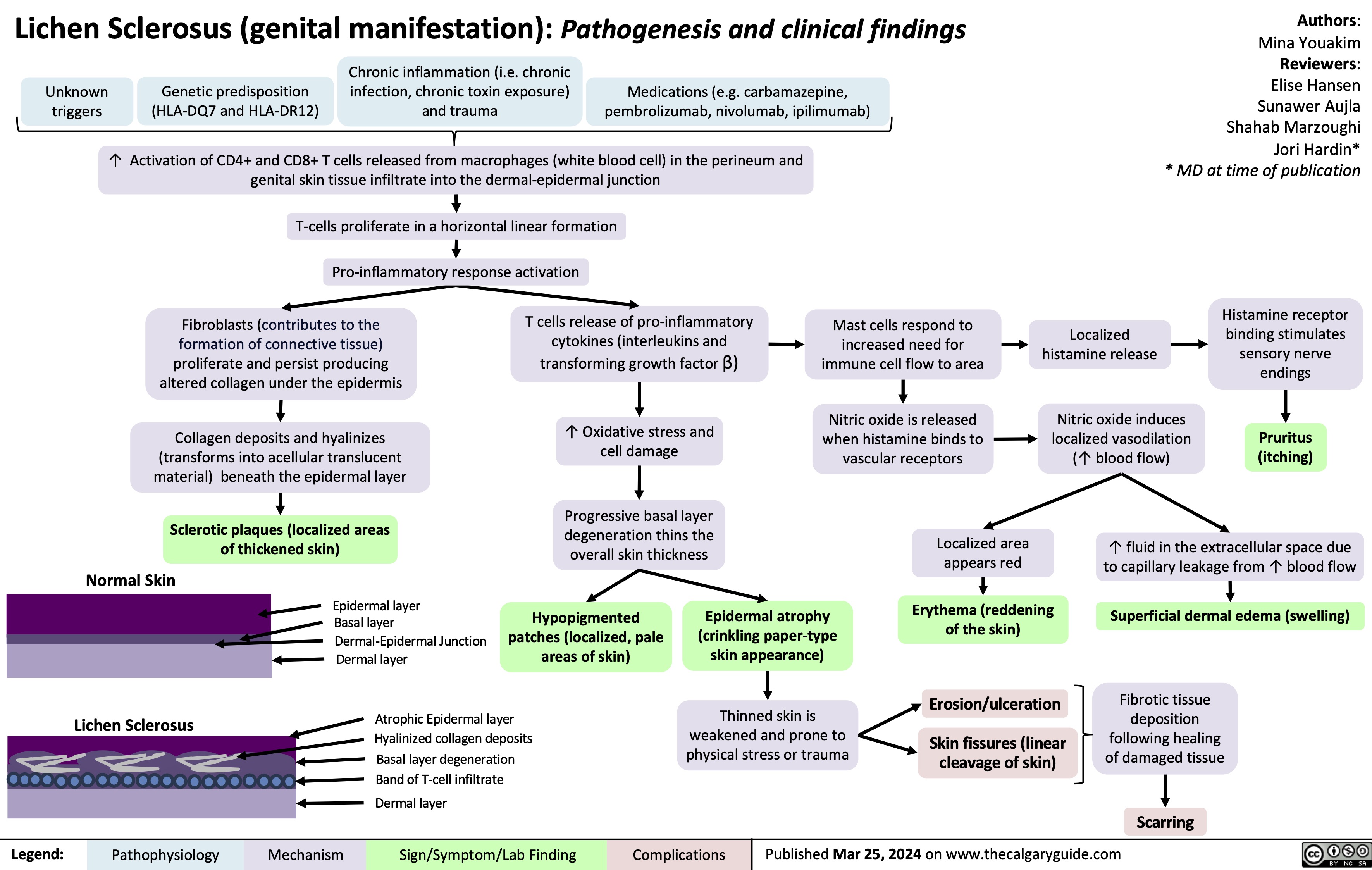
Post-Renal Acute Kidney Injury AKI
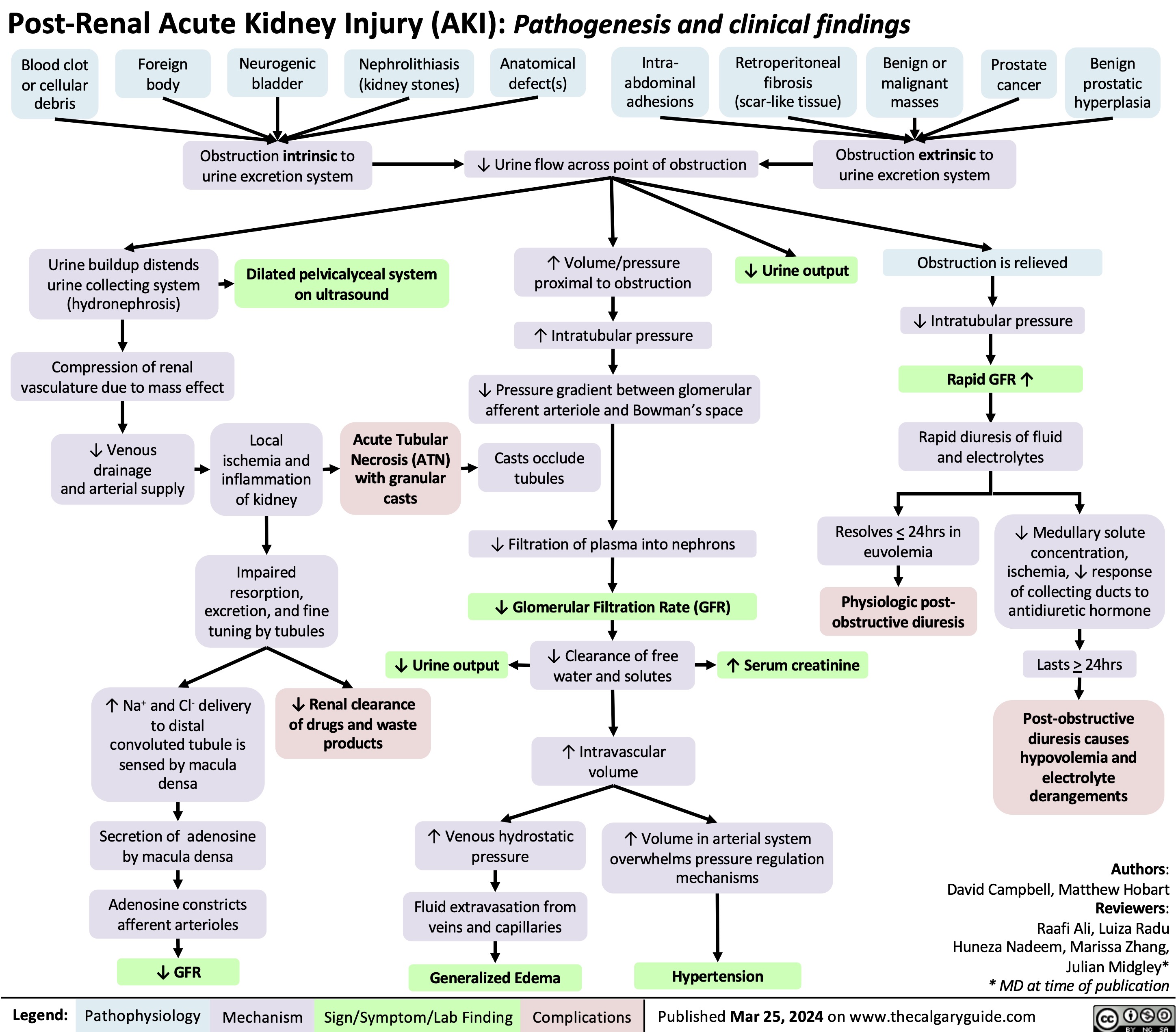
Irritant Contact Dermatitis Pathogenesis and Clinical Findings
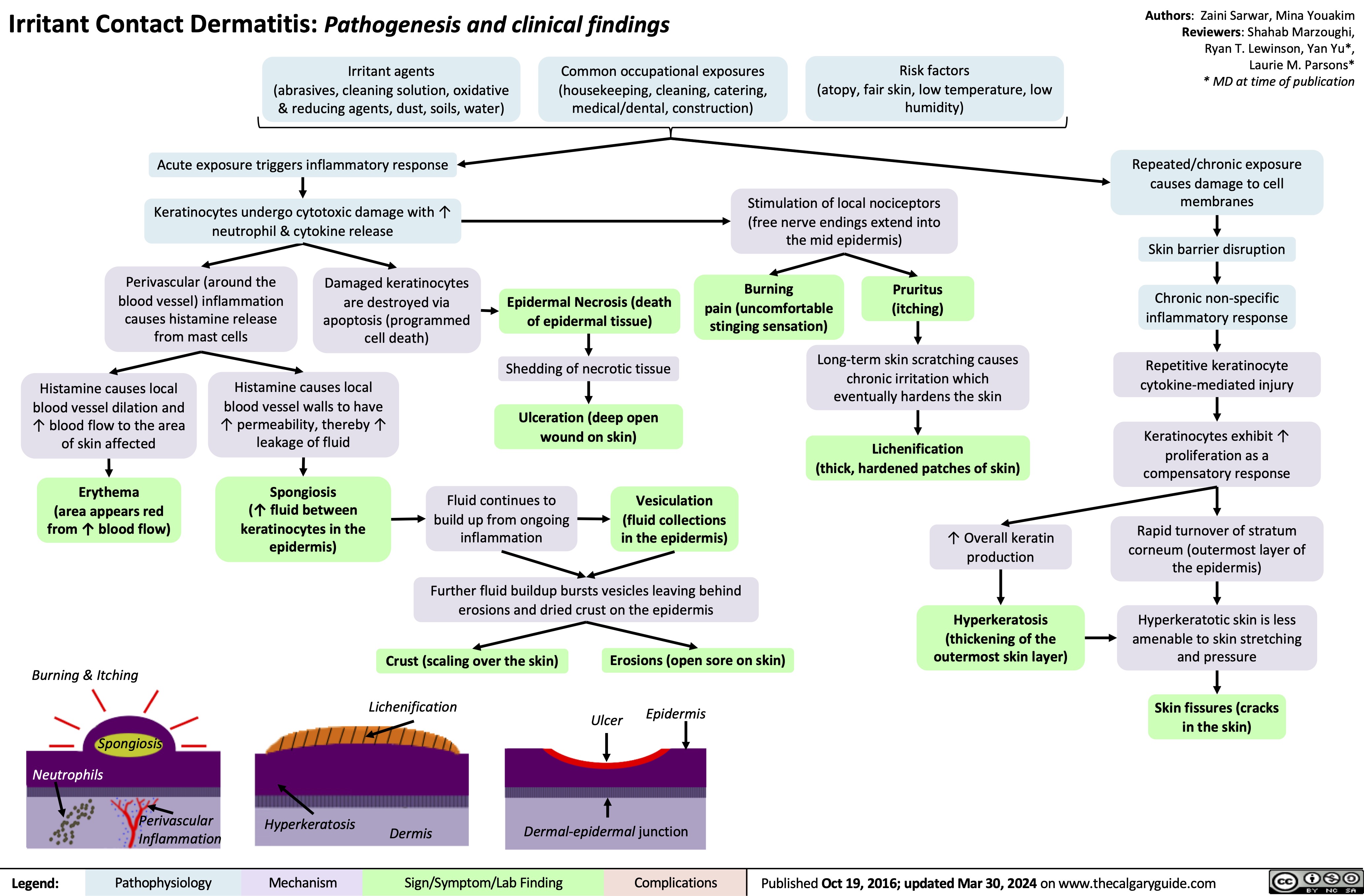
Deep Partial Thickness Burns Pathogenesis and Clinical Findings
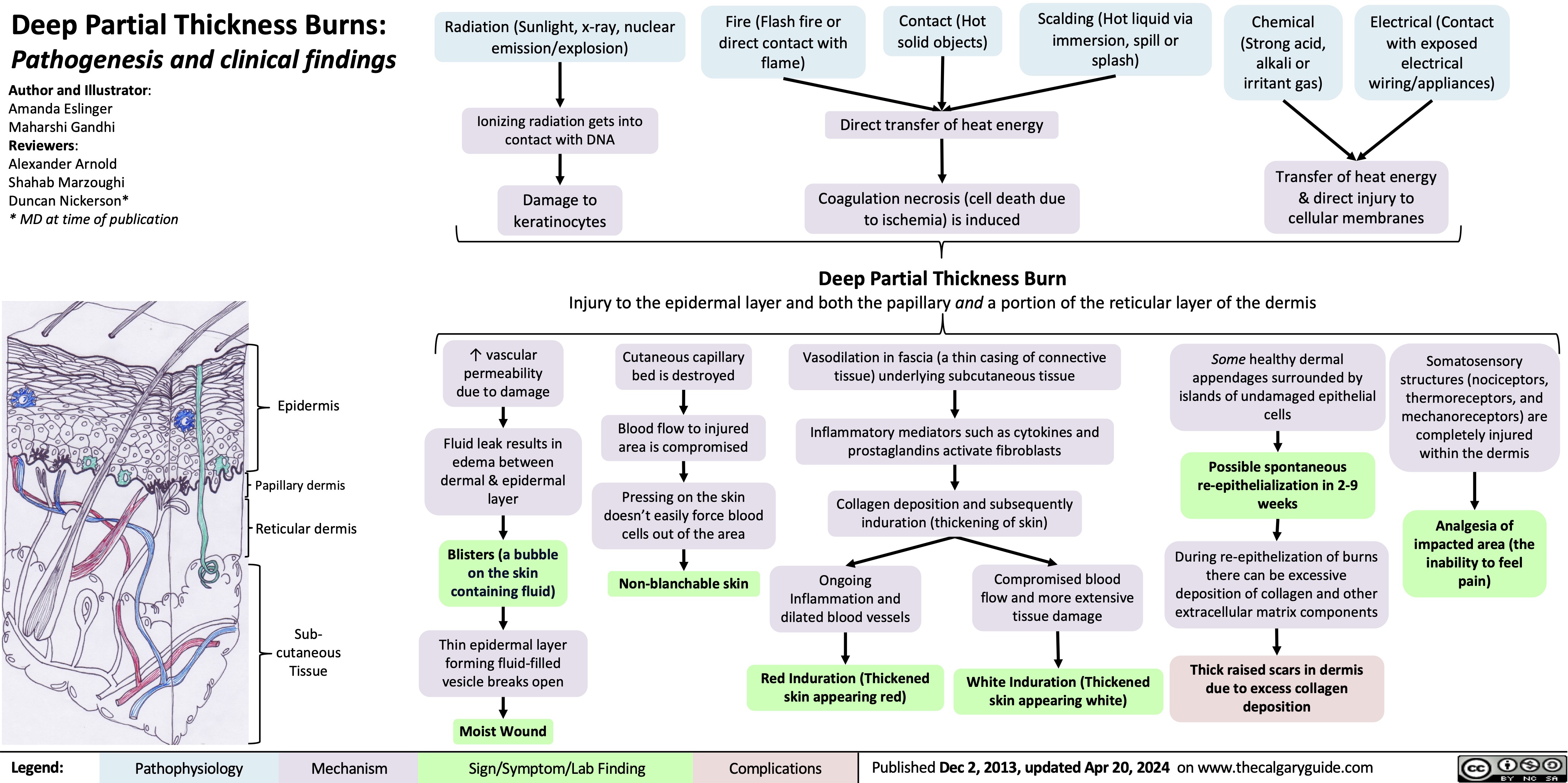
Costochondritis
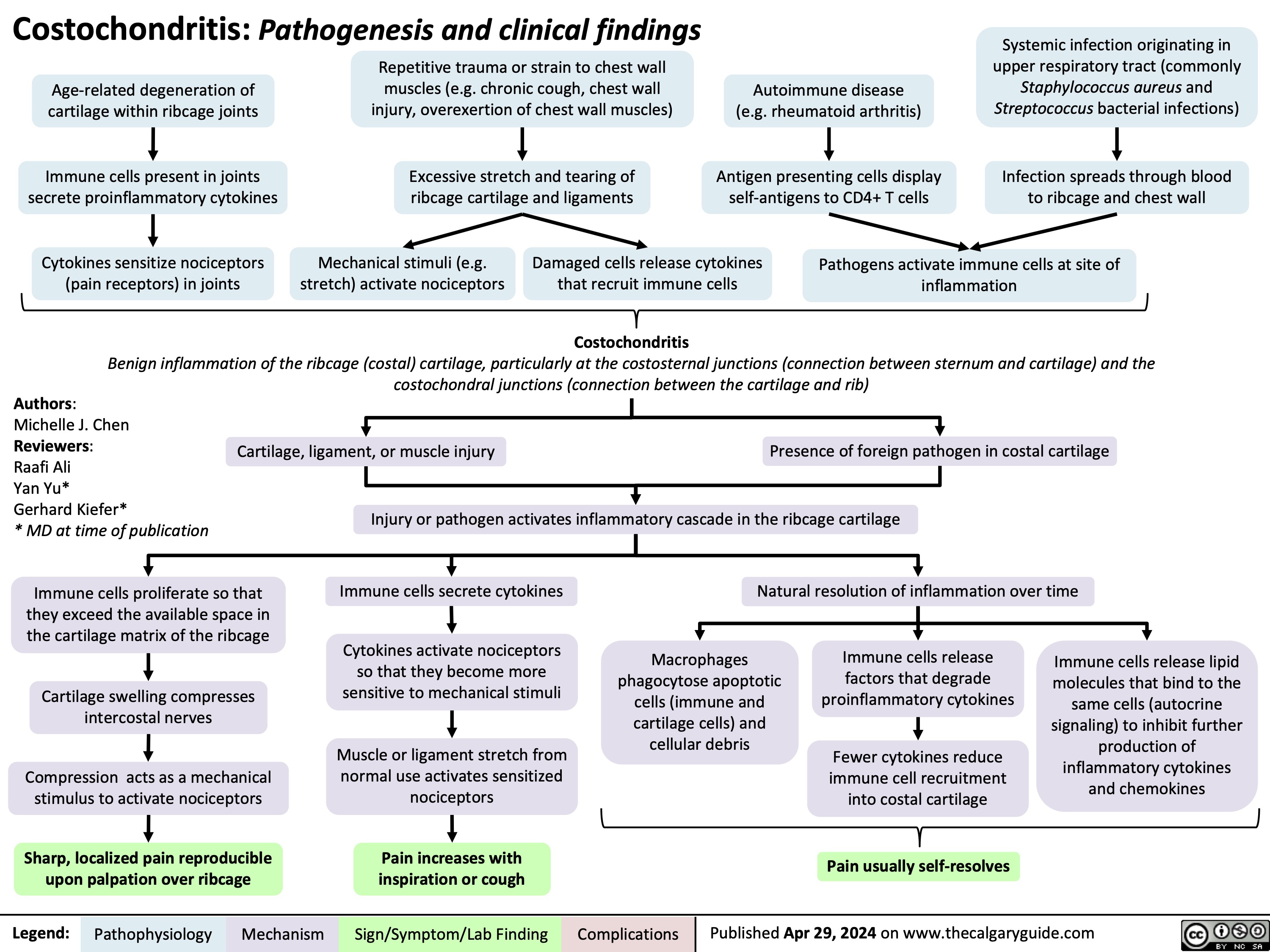
Acute Otitis Media Complications
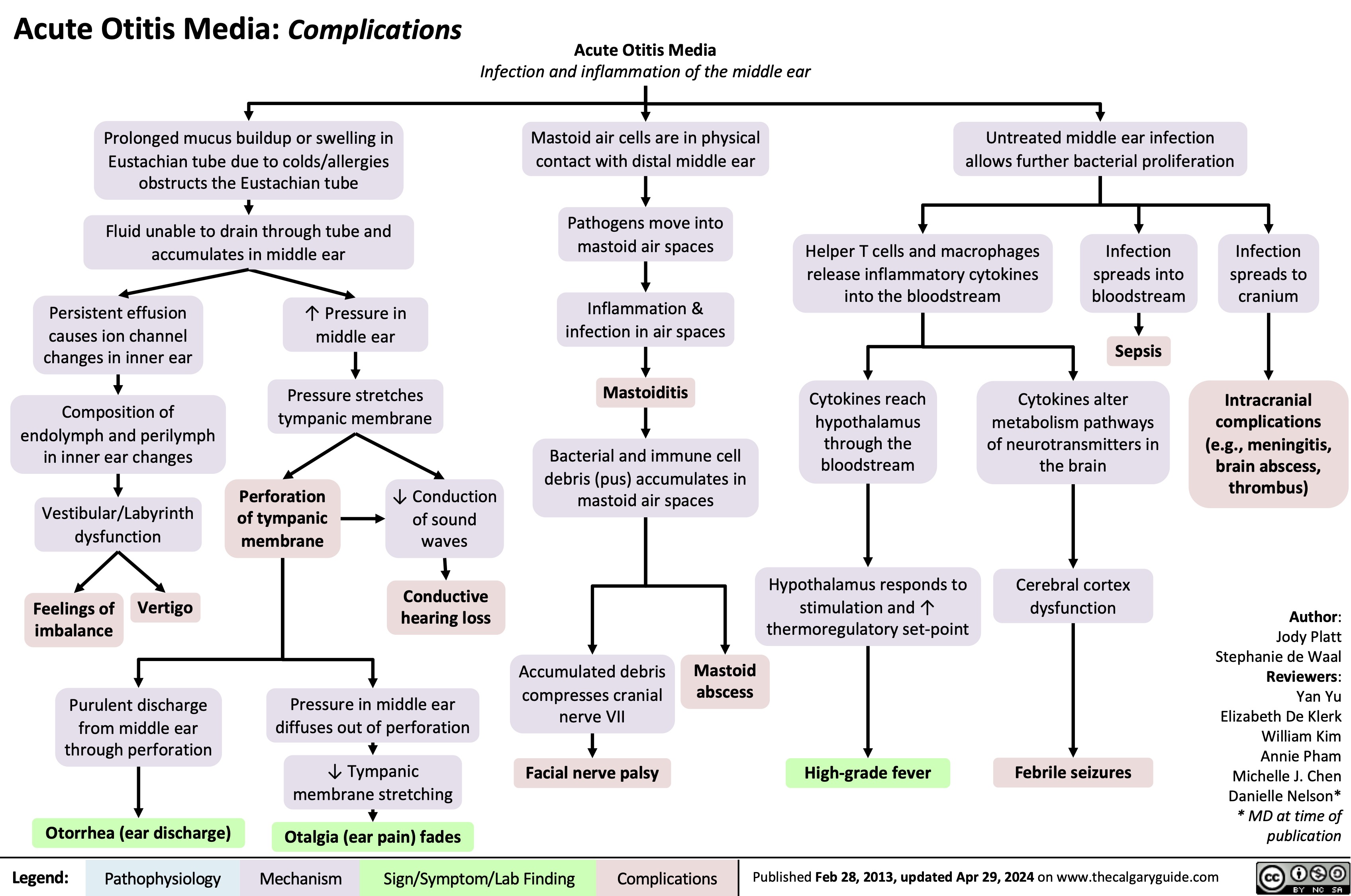
Sickle Cell Disease Pathogenesis Clinical Findings and Complications
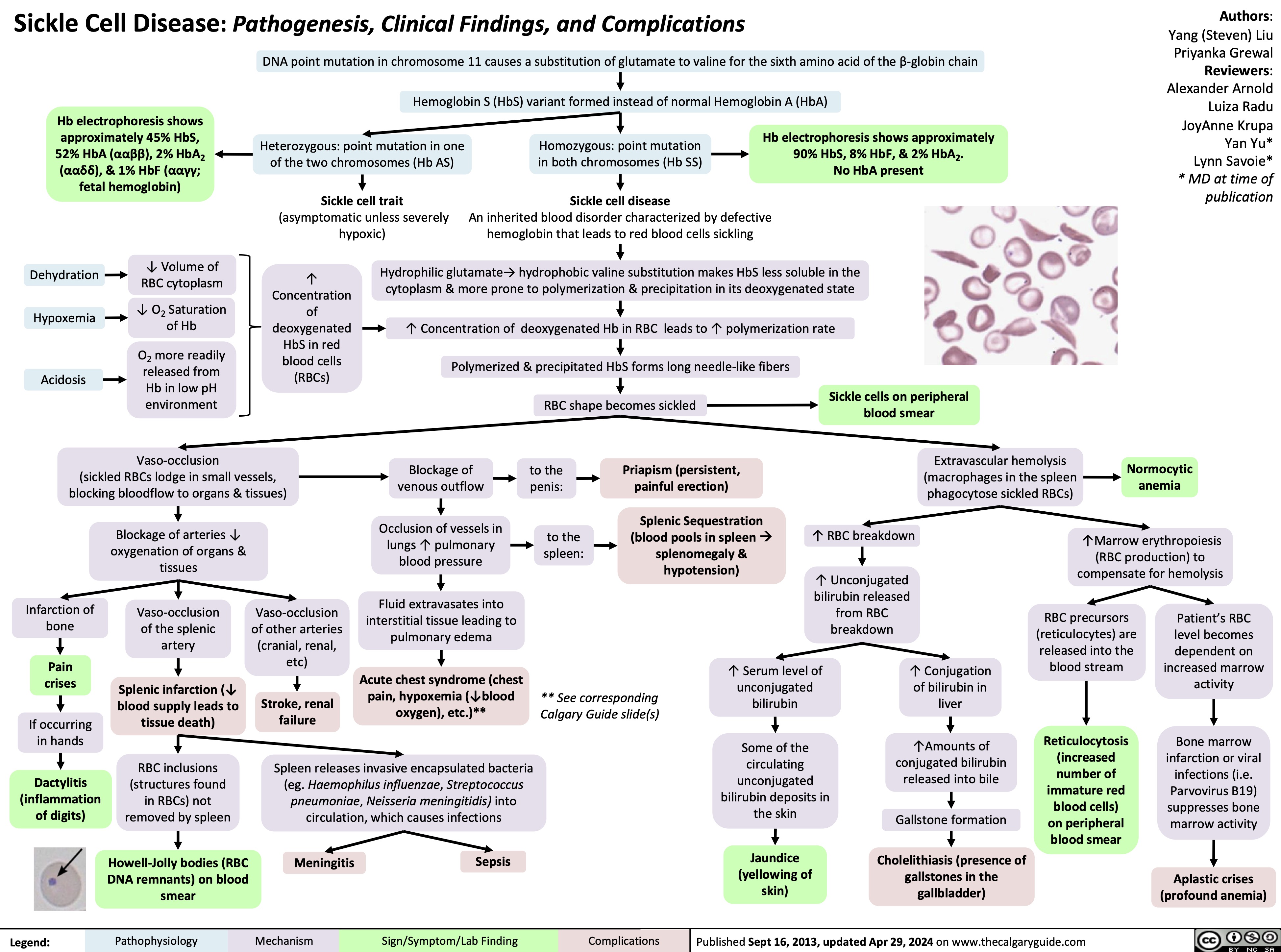
Wiskott-Aldrich Syndrome
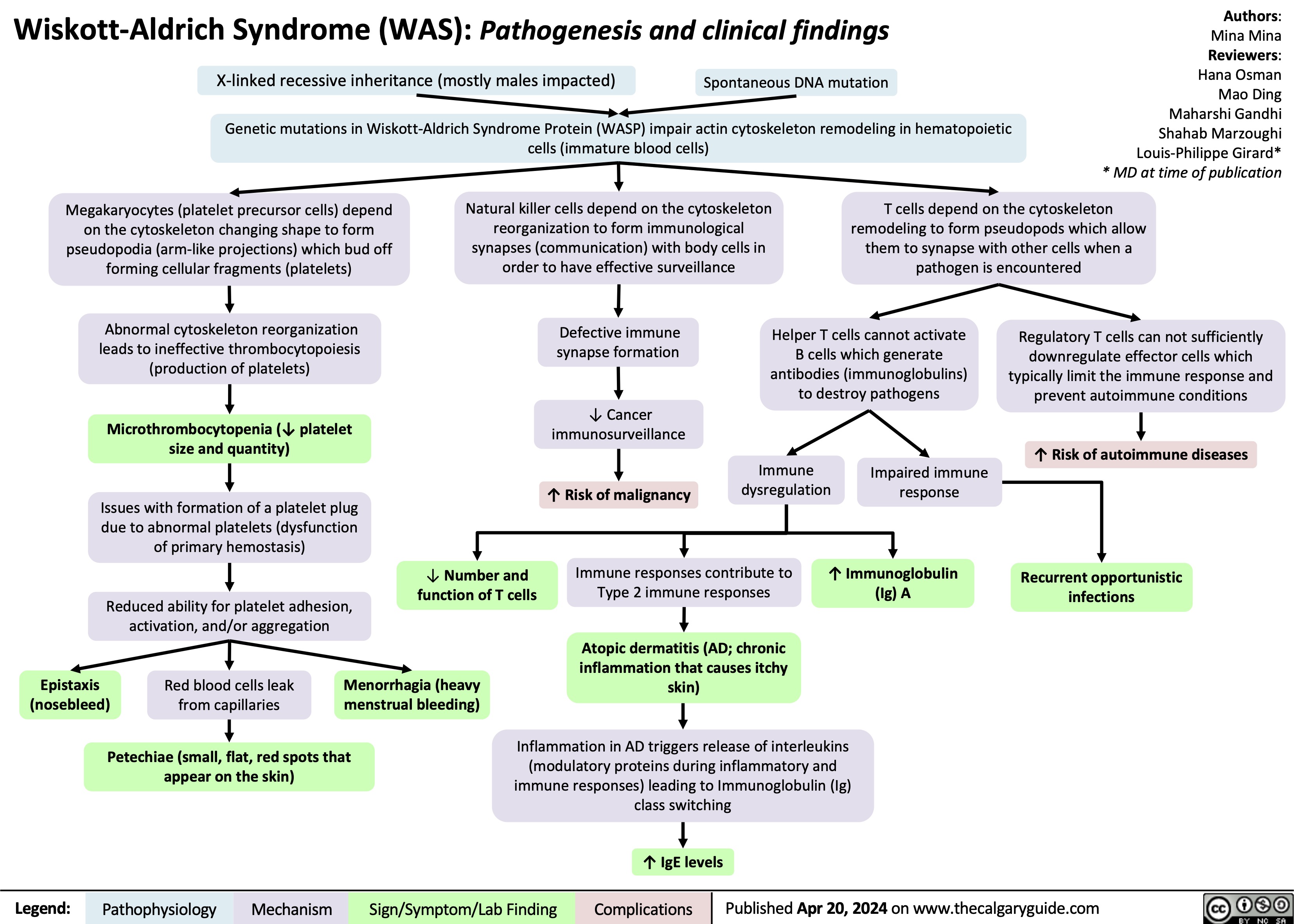
Scabies pathogenesis and clinical findings
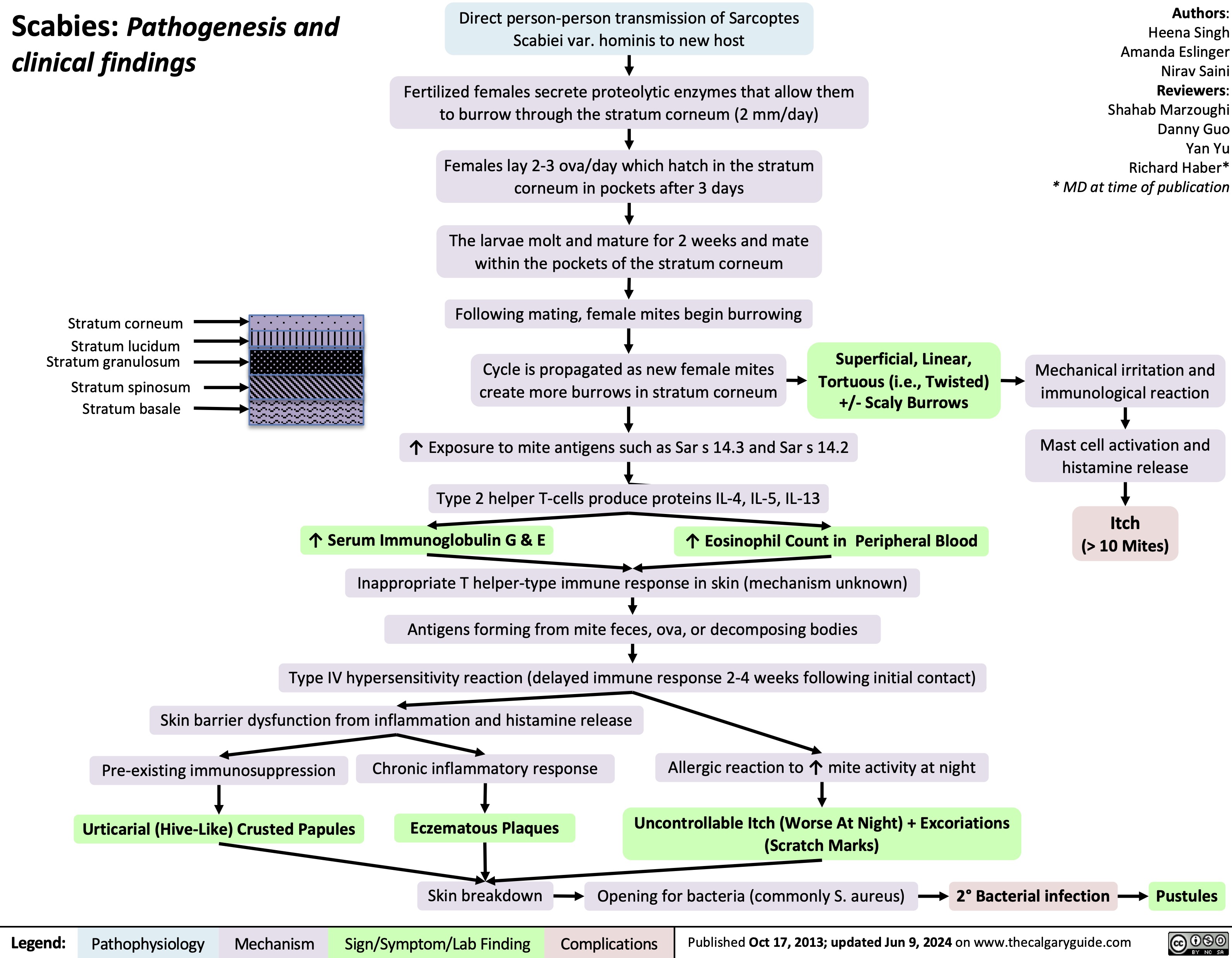
Posterior Cruciate Ligament PCL Injury Pathogenesis and Clinical Findings
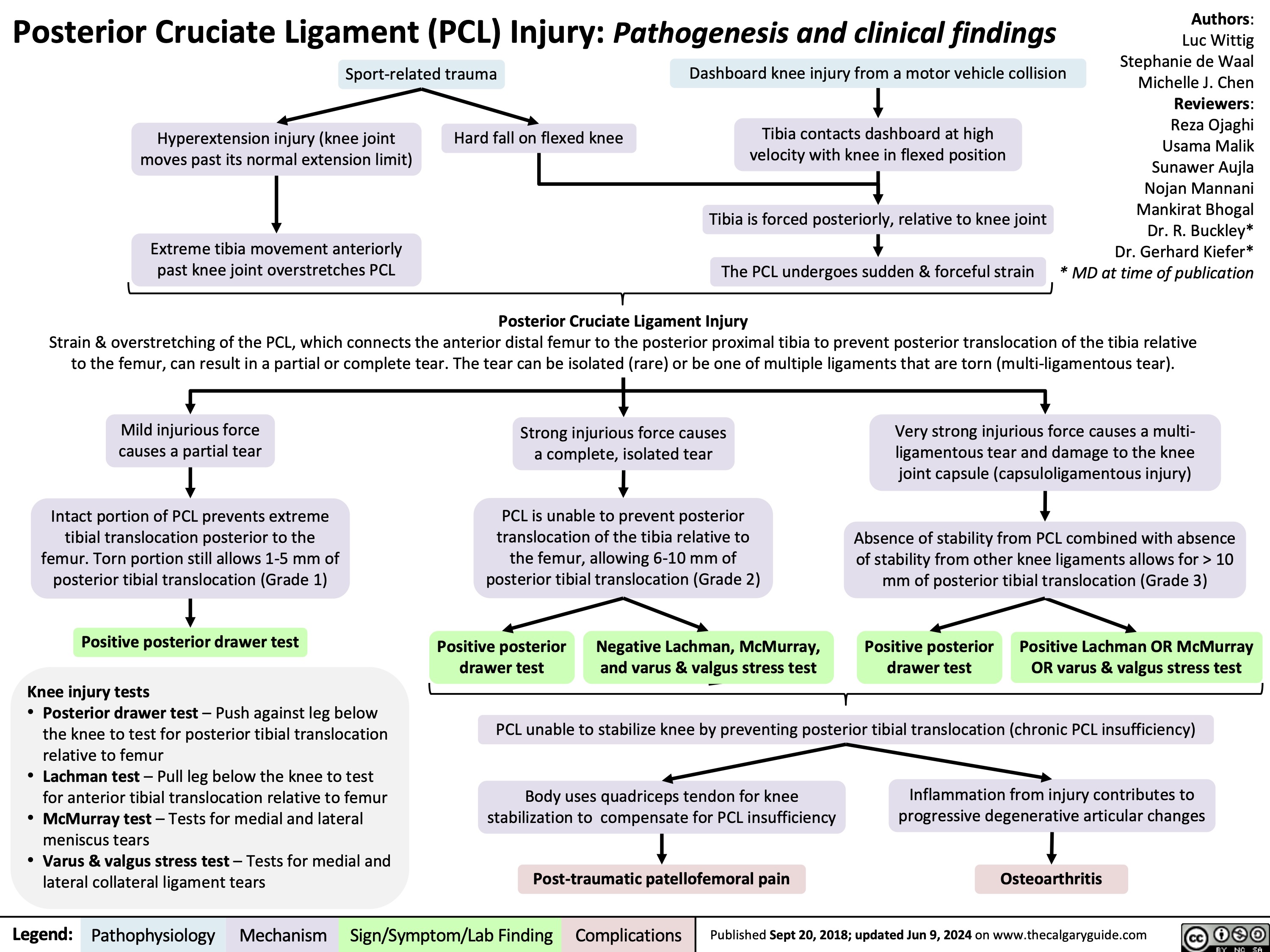
Migraines and auras pathogenesis and clinical findings
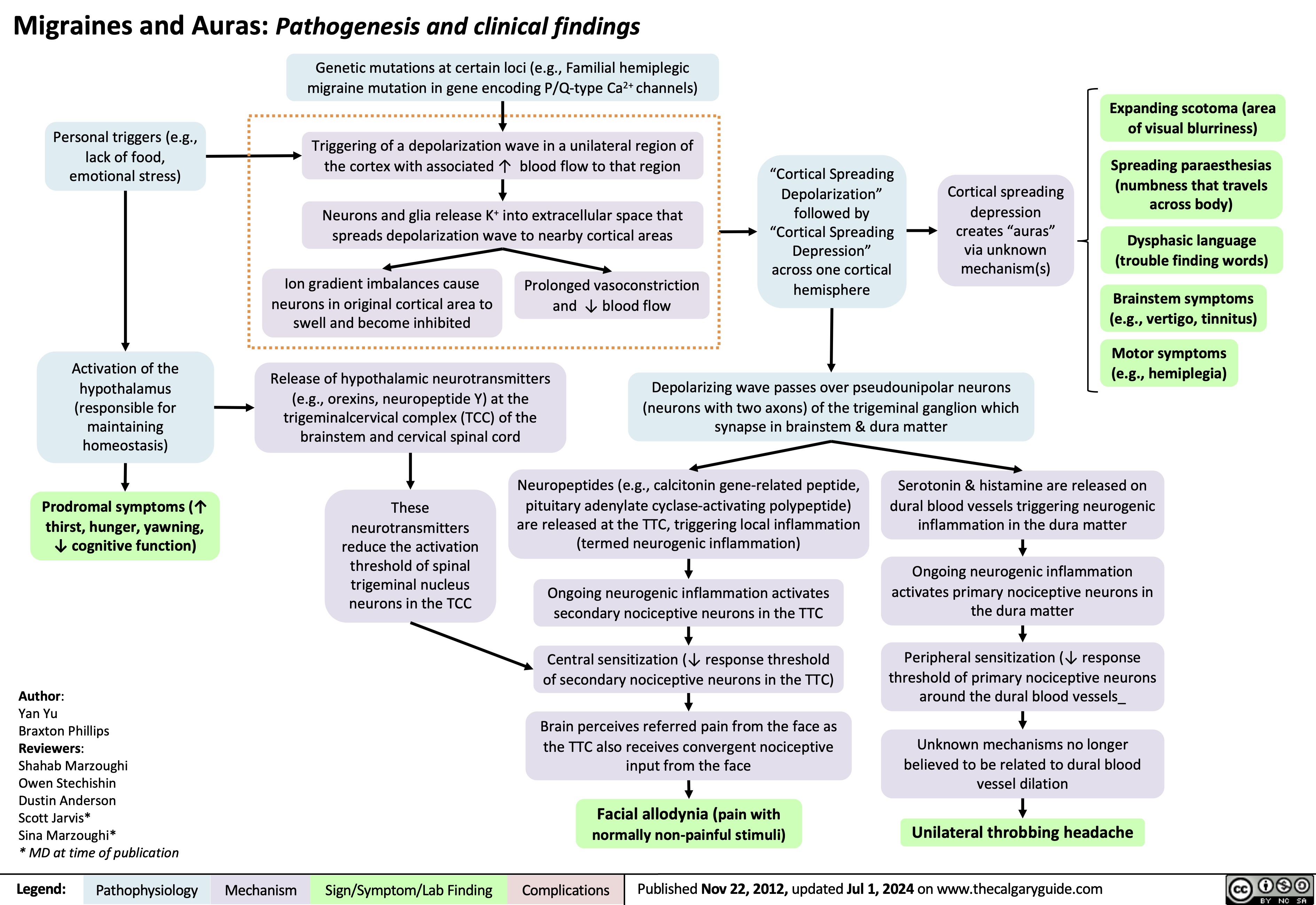
Legg Calve Perthes Disease
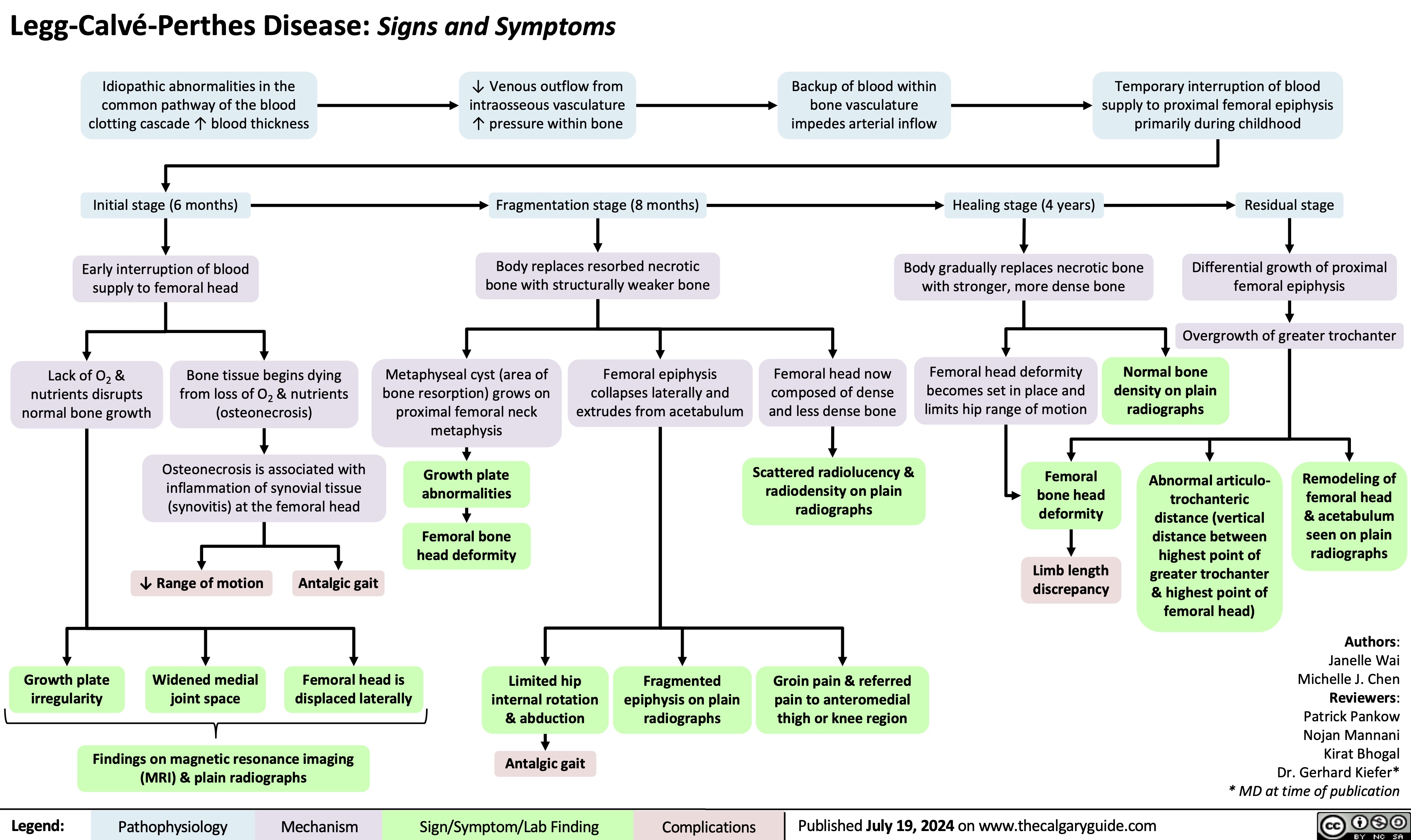
Acute Otitis Media Pathogenesis and Clinical Findings in Children
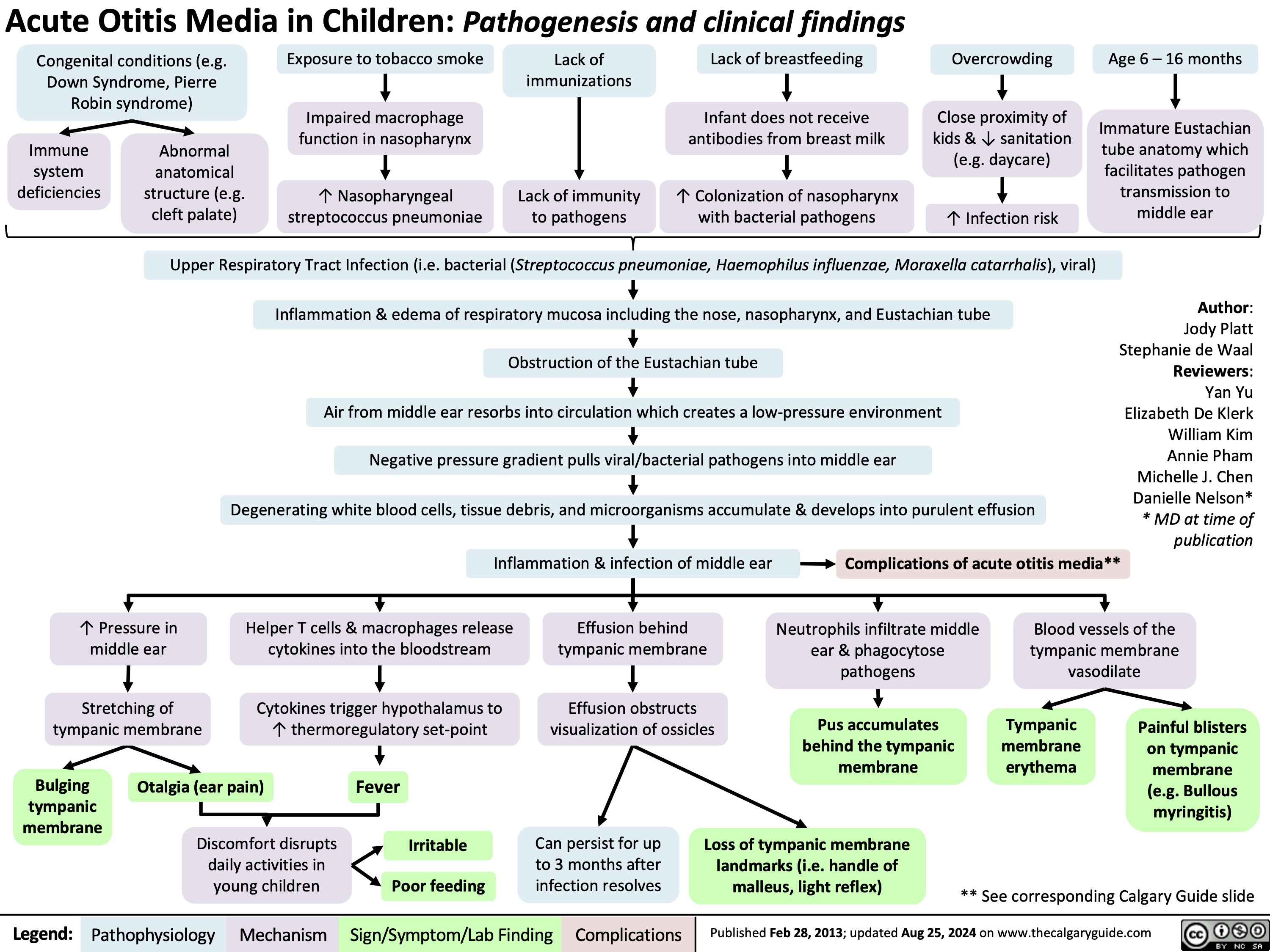
Low Ankle Sprain
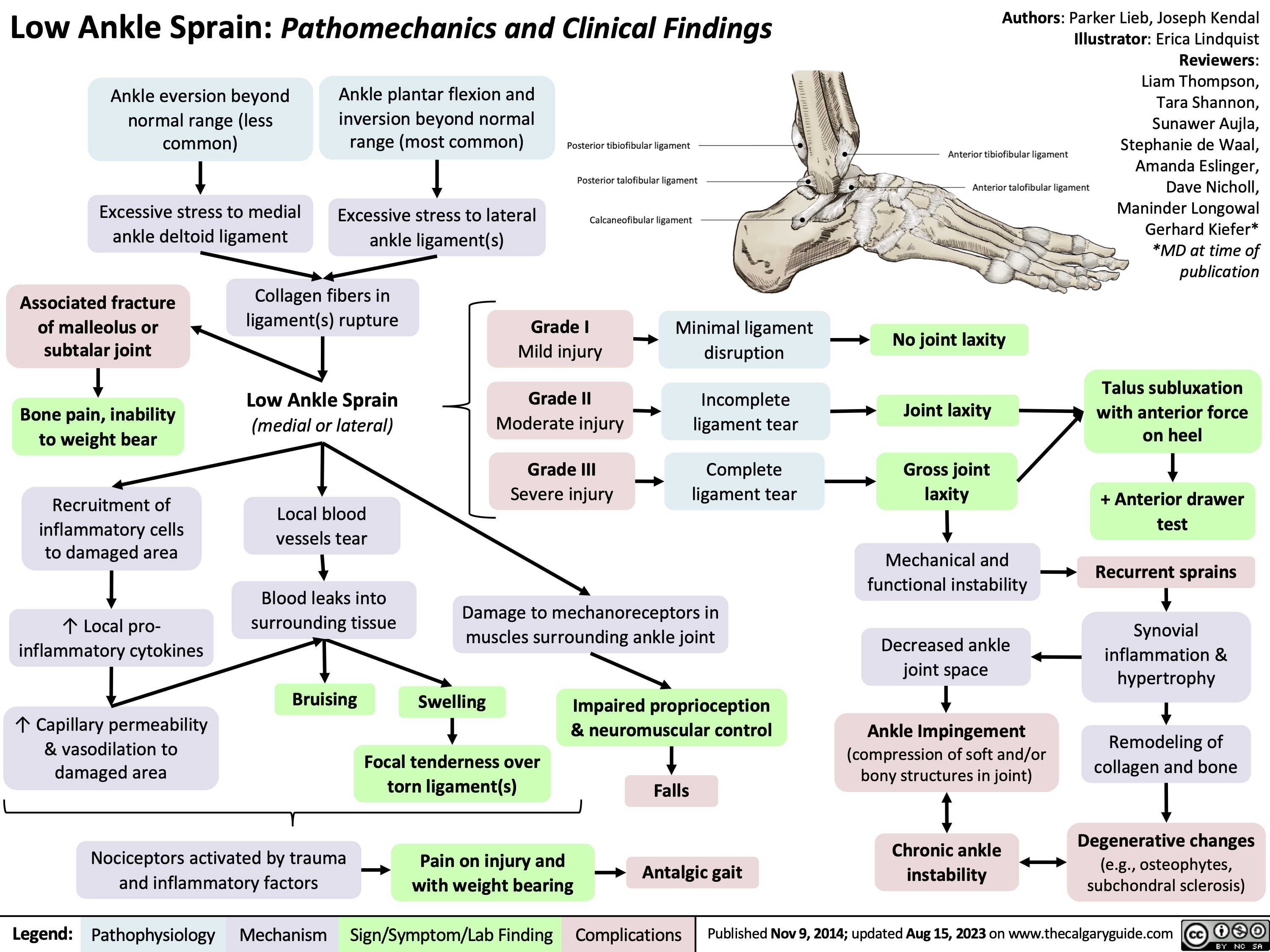
Hypomagnesemia
![Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com
Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/10/Hypomagnesia.jpg)
Acute Diverticulitis
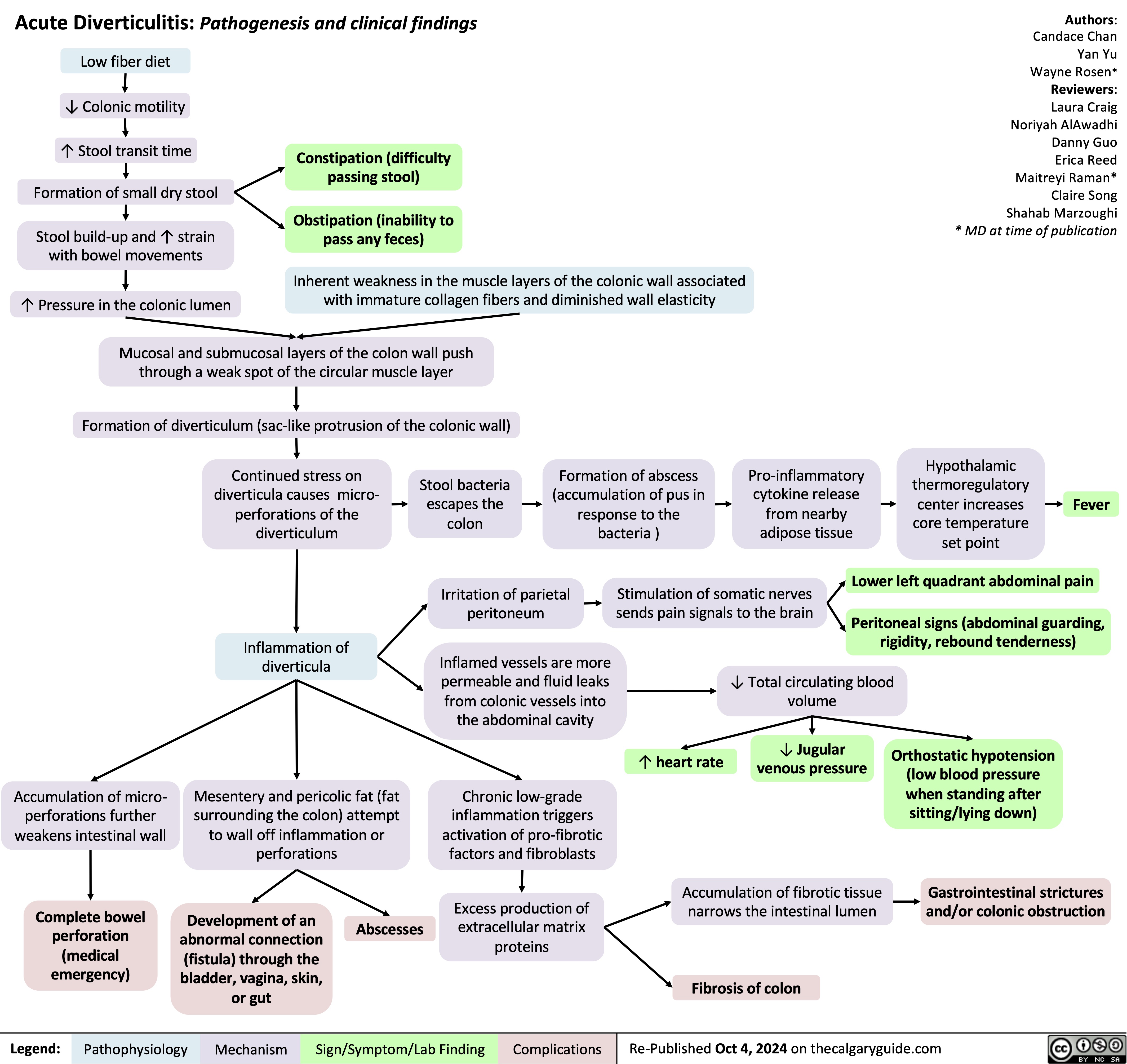
Epilepsy in Older Adults
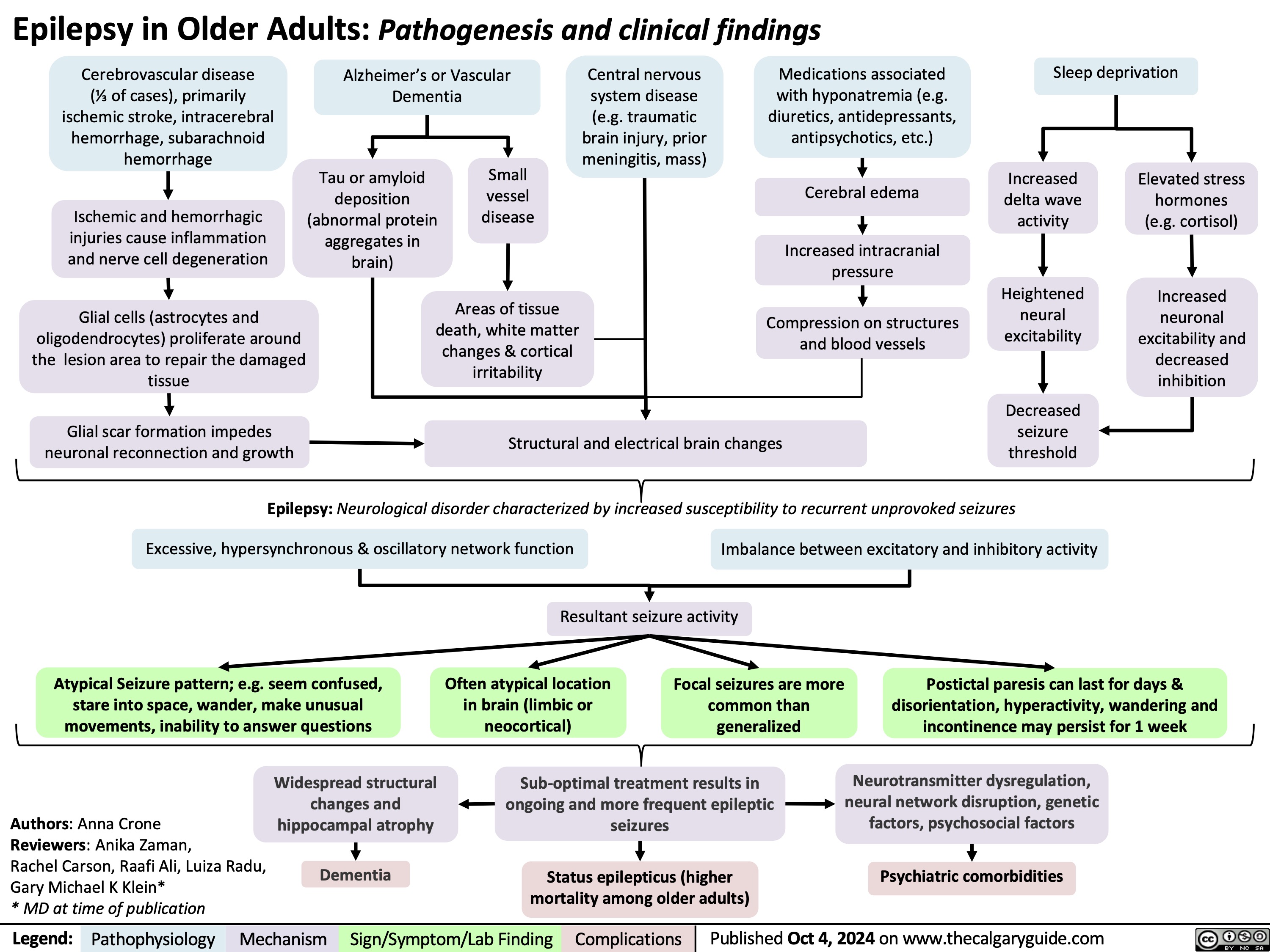
Cholesteatoma of middle ear
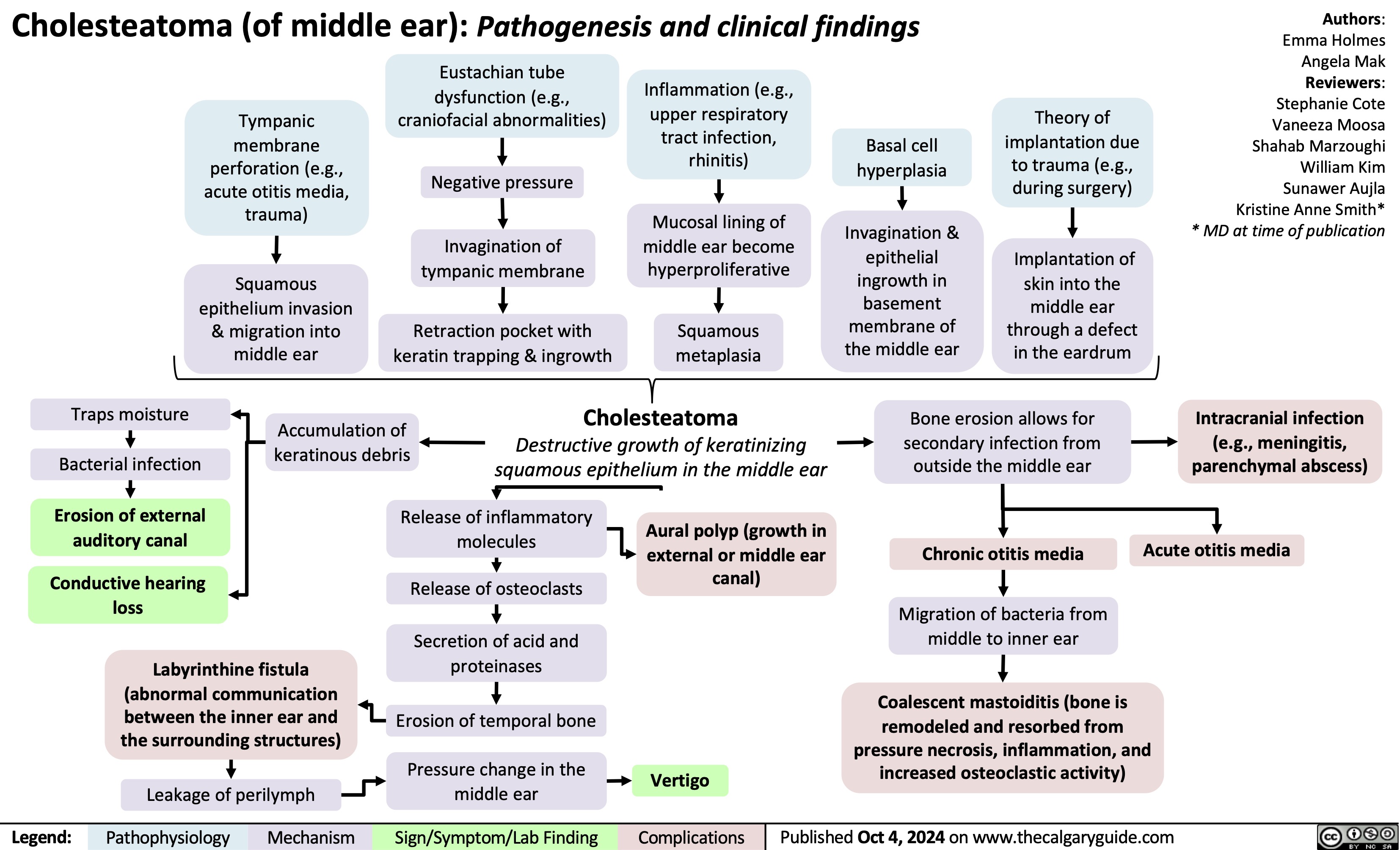
Complex Regional Pain Syndrome
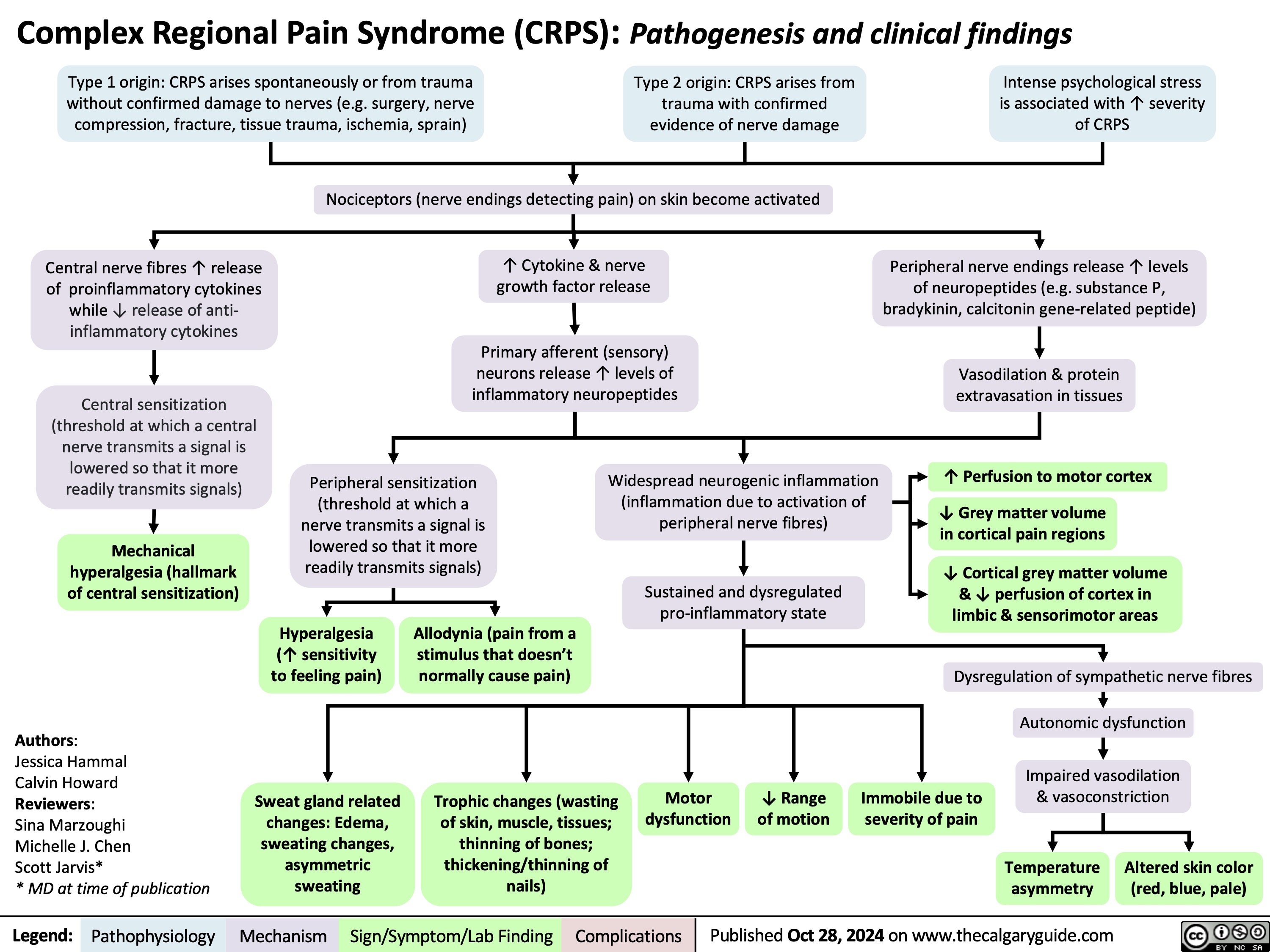
Renal manifestations of SLE
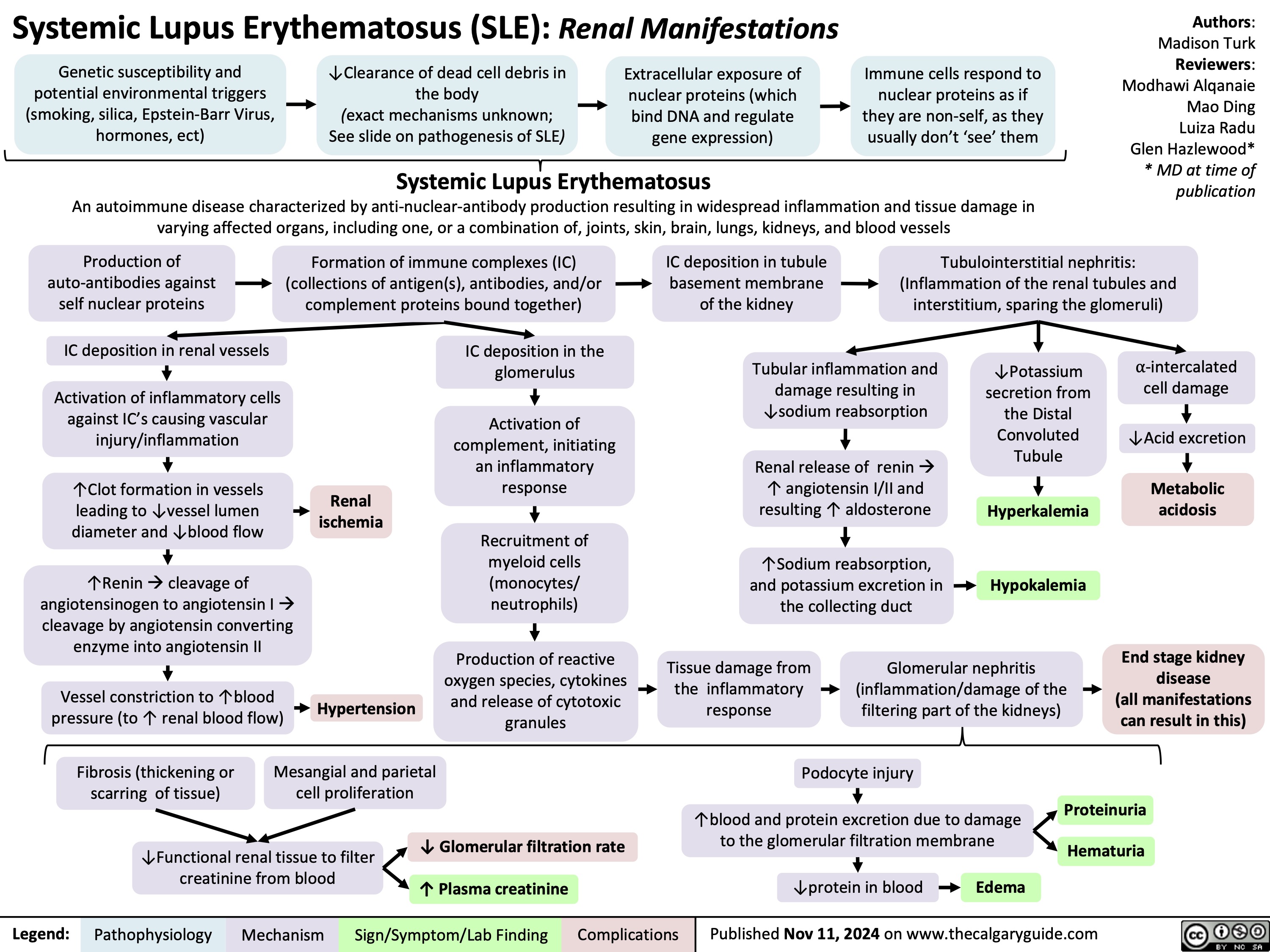
Diabetic Polyneuropathy
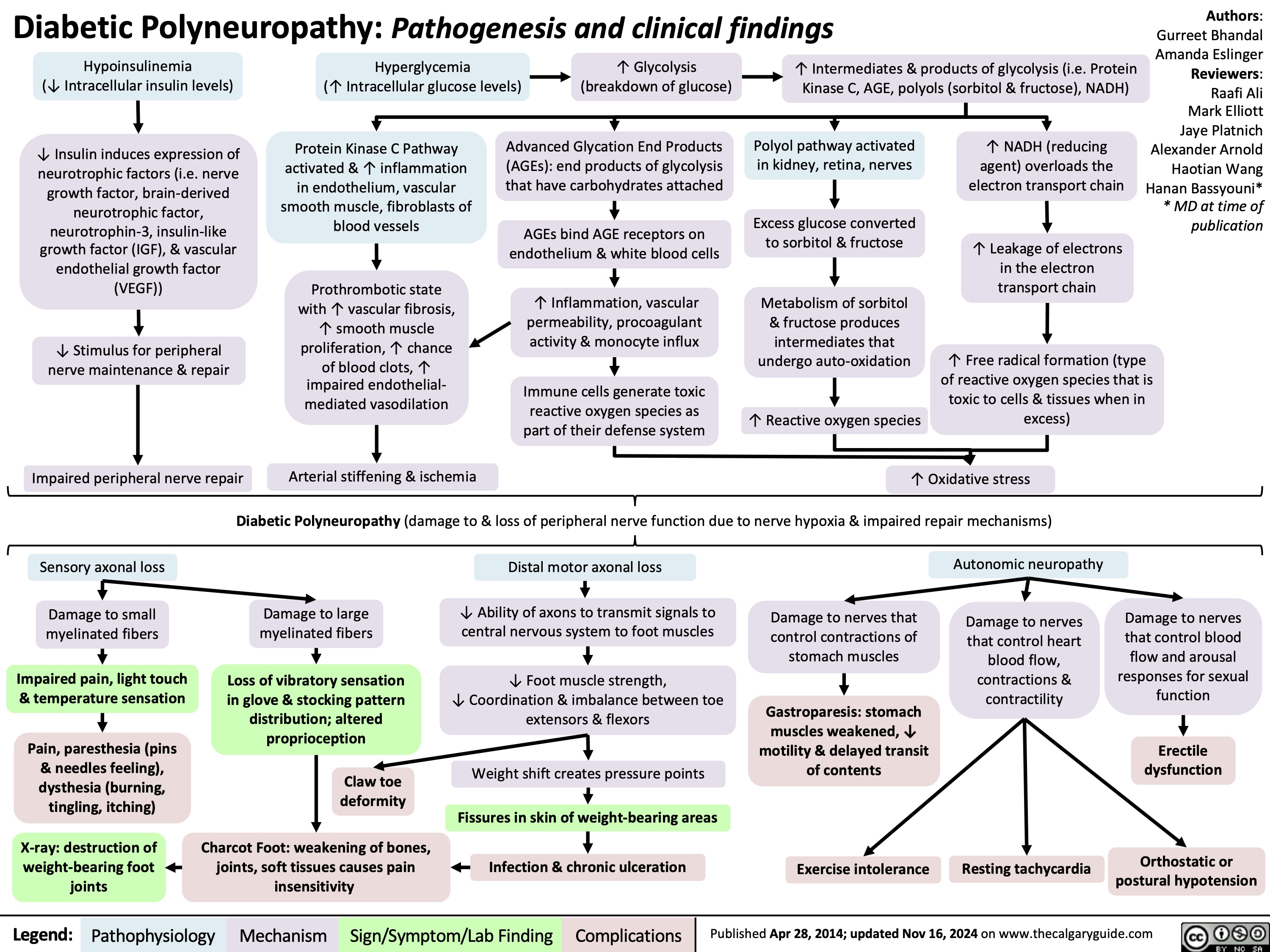
Open Fractures
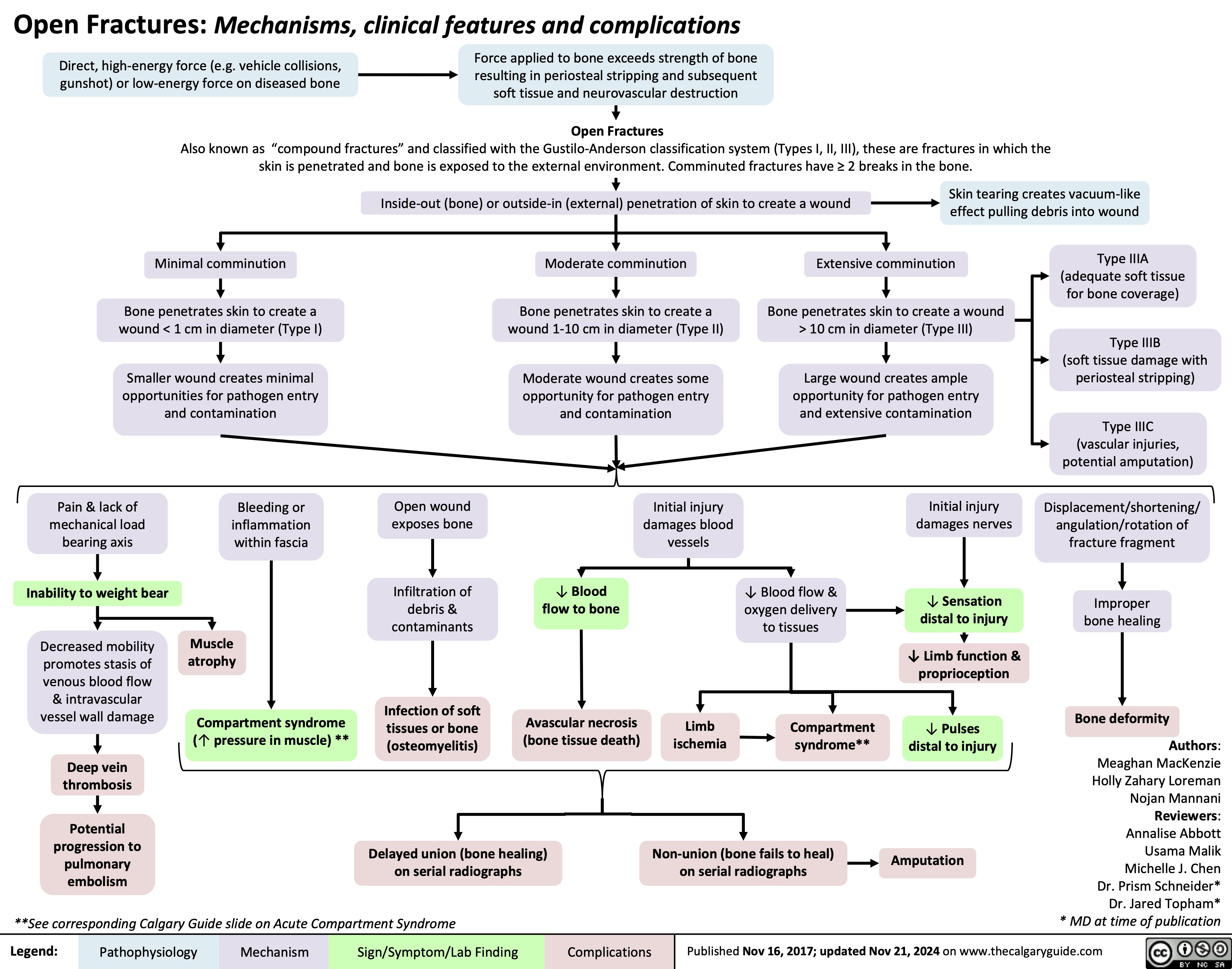
Statins Mechanisms and Side Effects
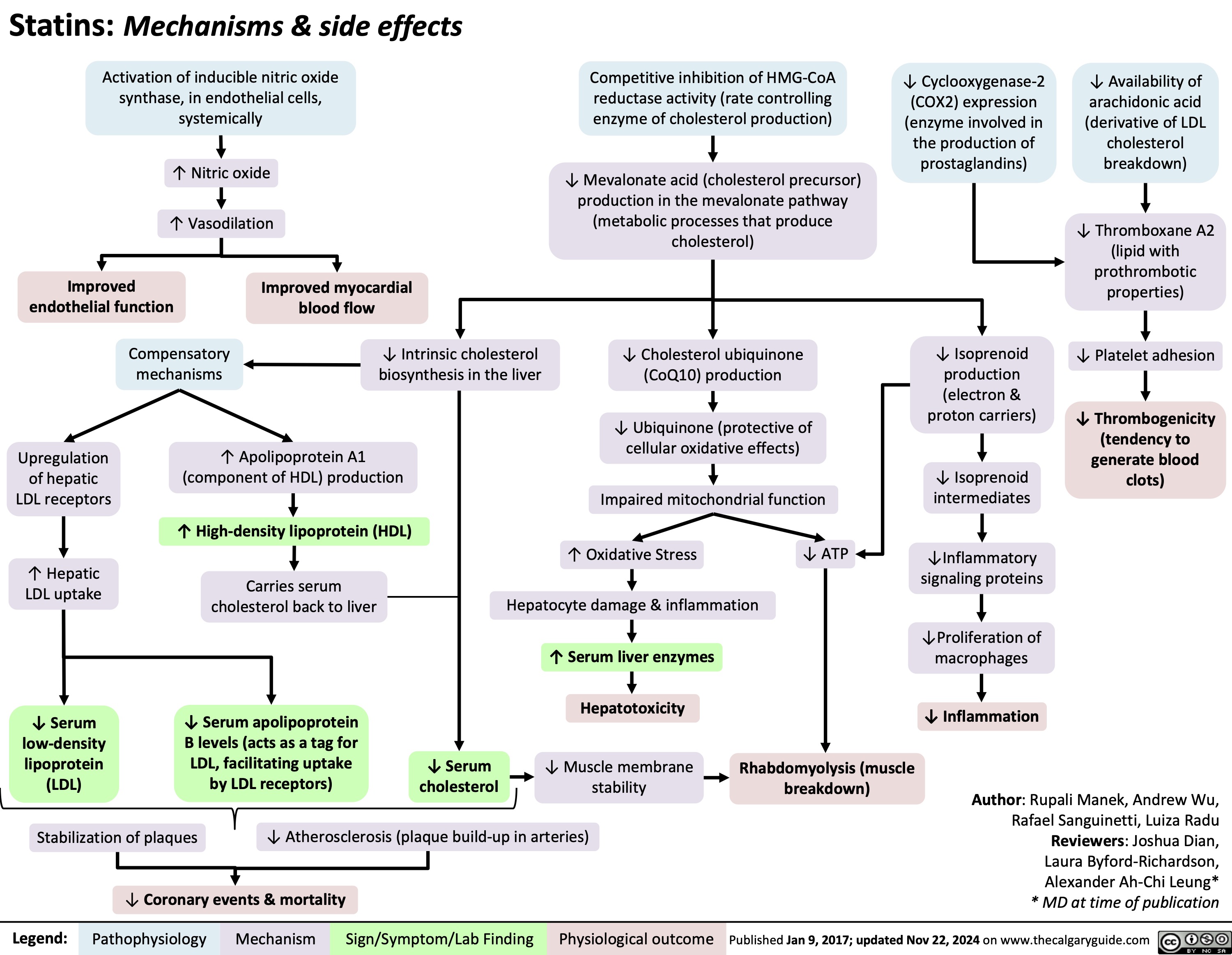
Hypocalcemia Pathogenesis
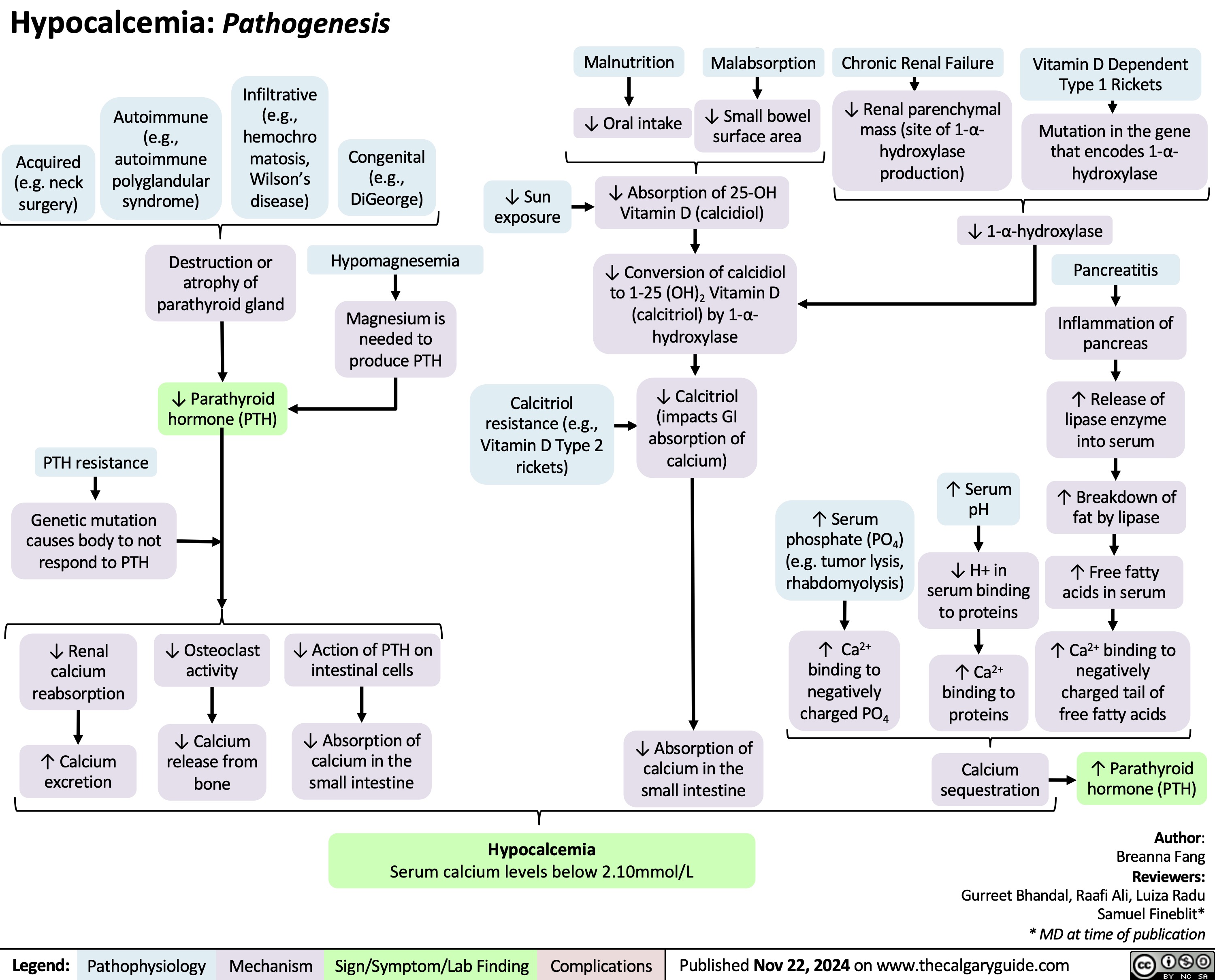
Acquired Inguinal Hernias
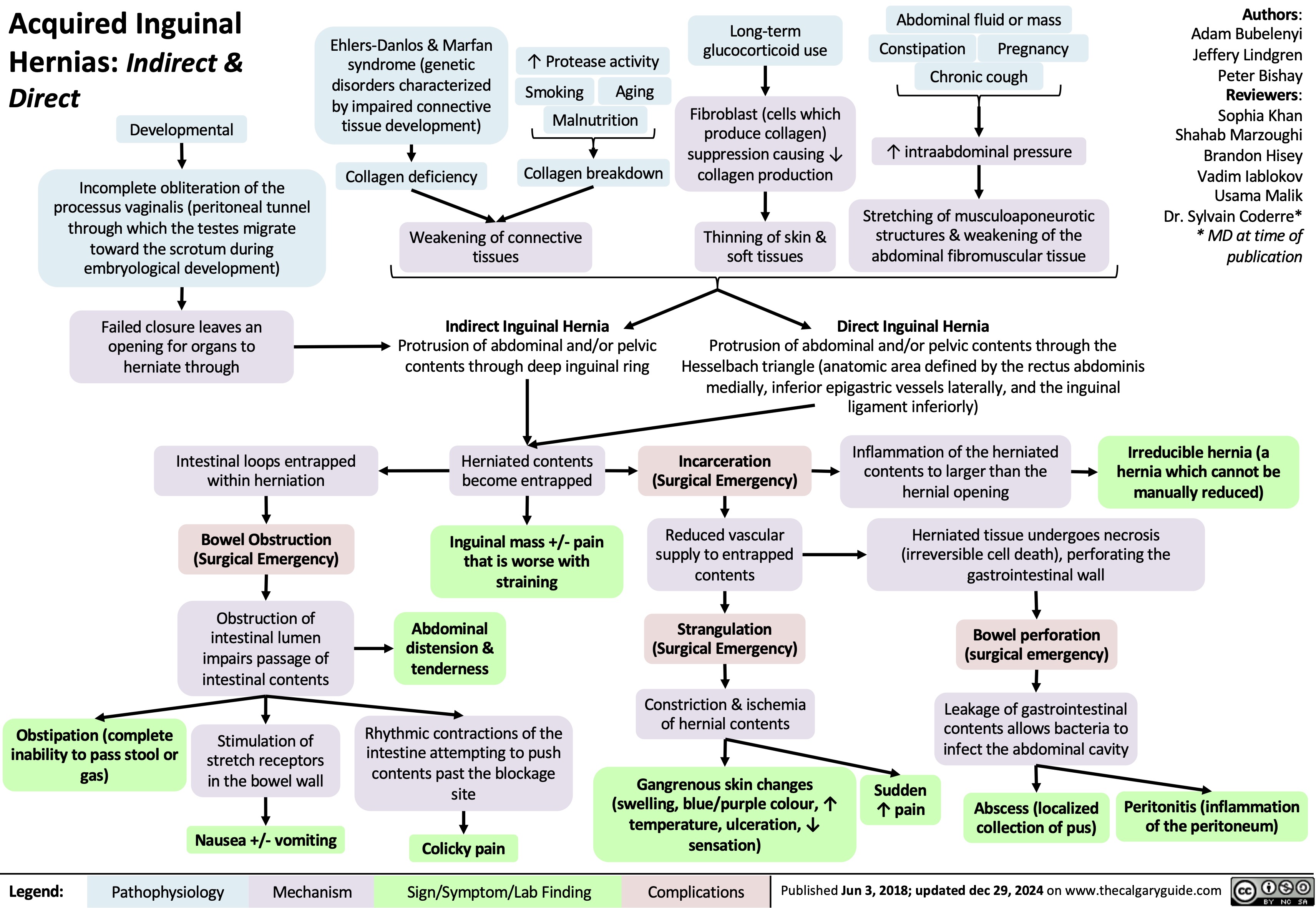
Barretts Esophagus
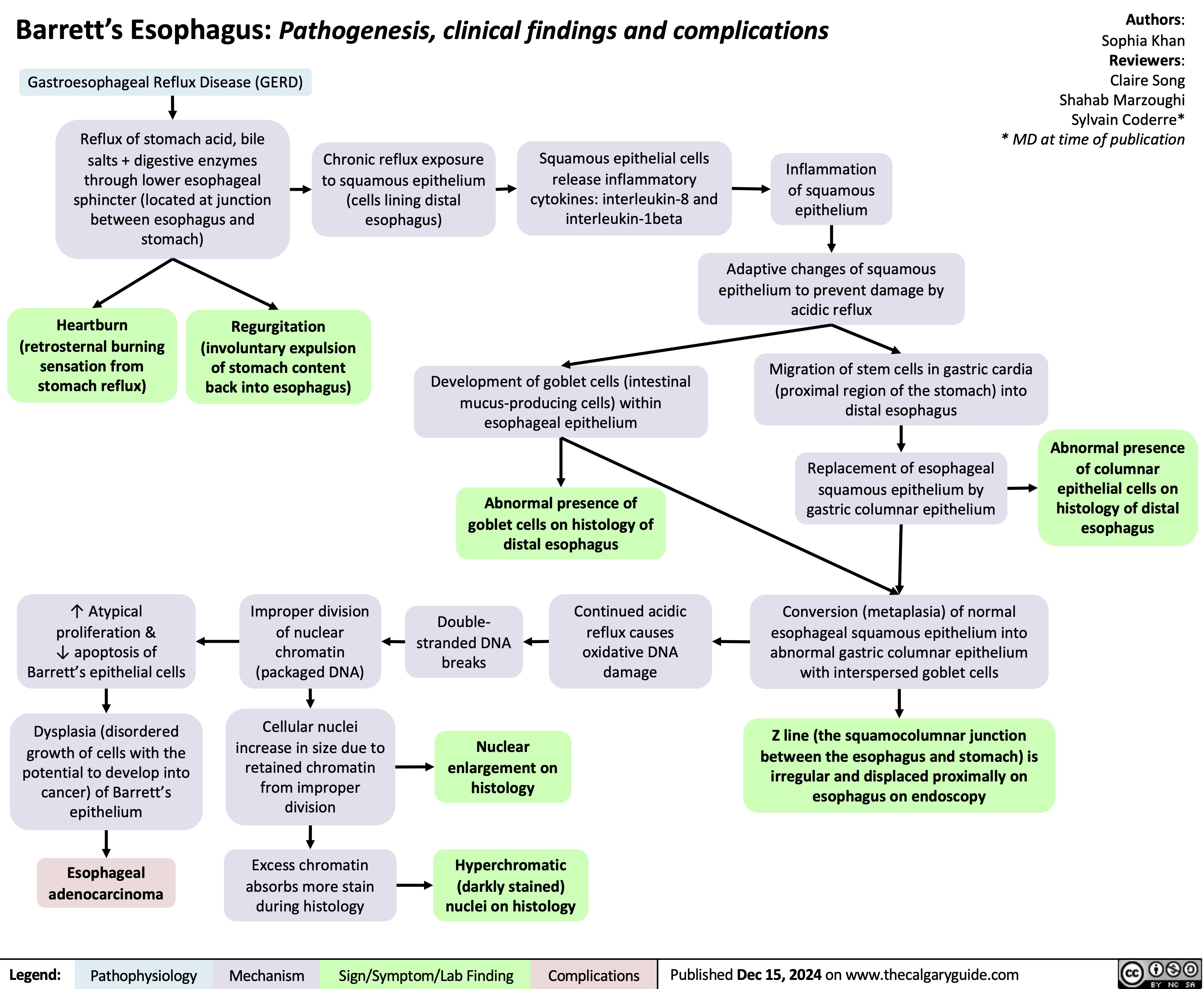
Malignant Esophagitis
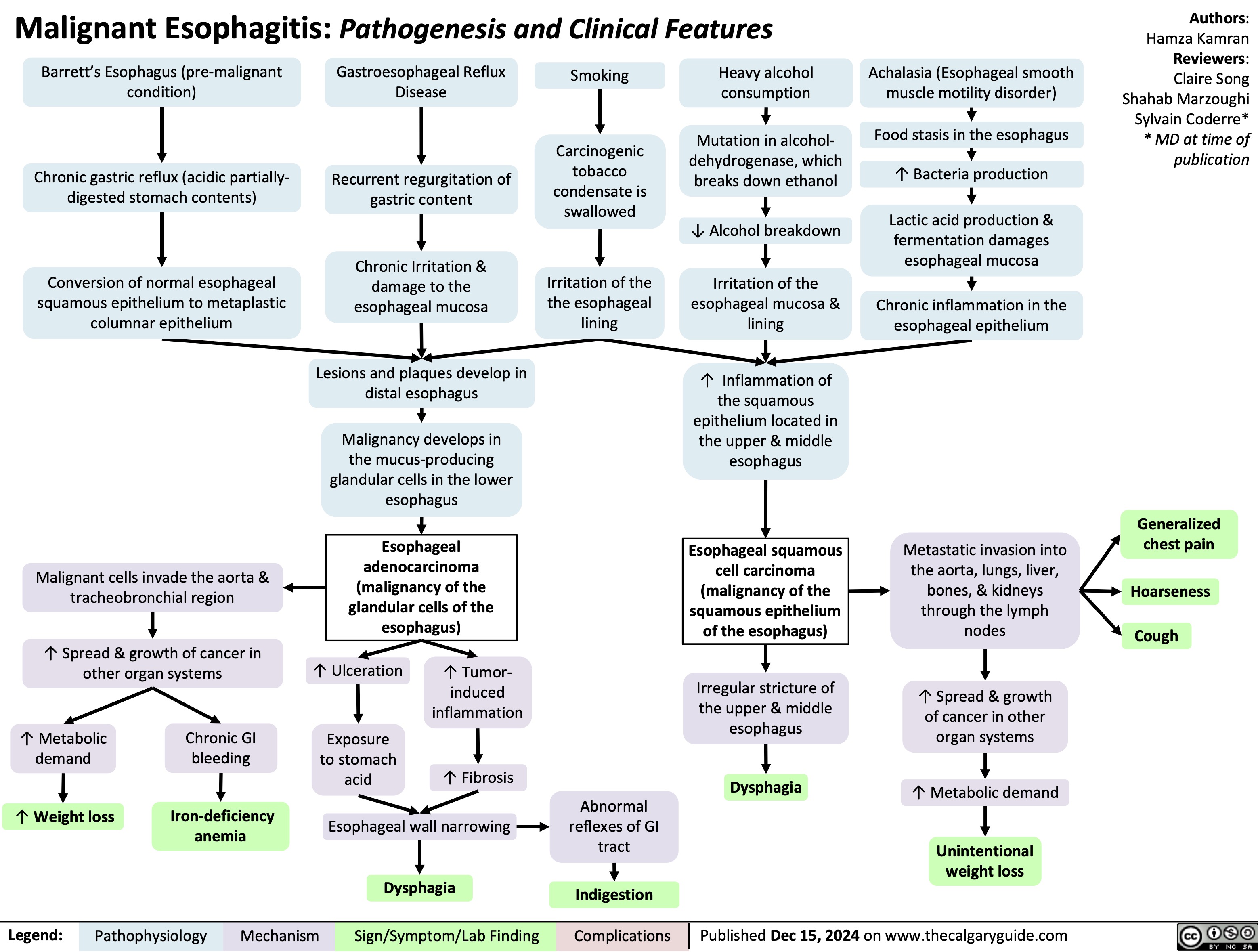
Pituitary Tumour Classification and Clinical Outcomes
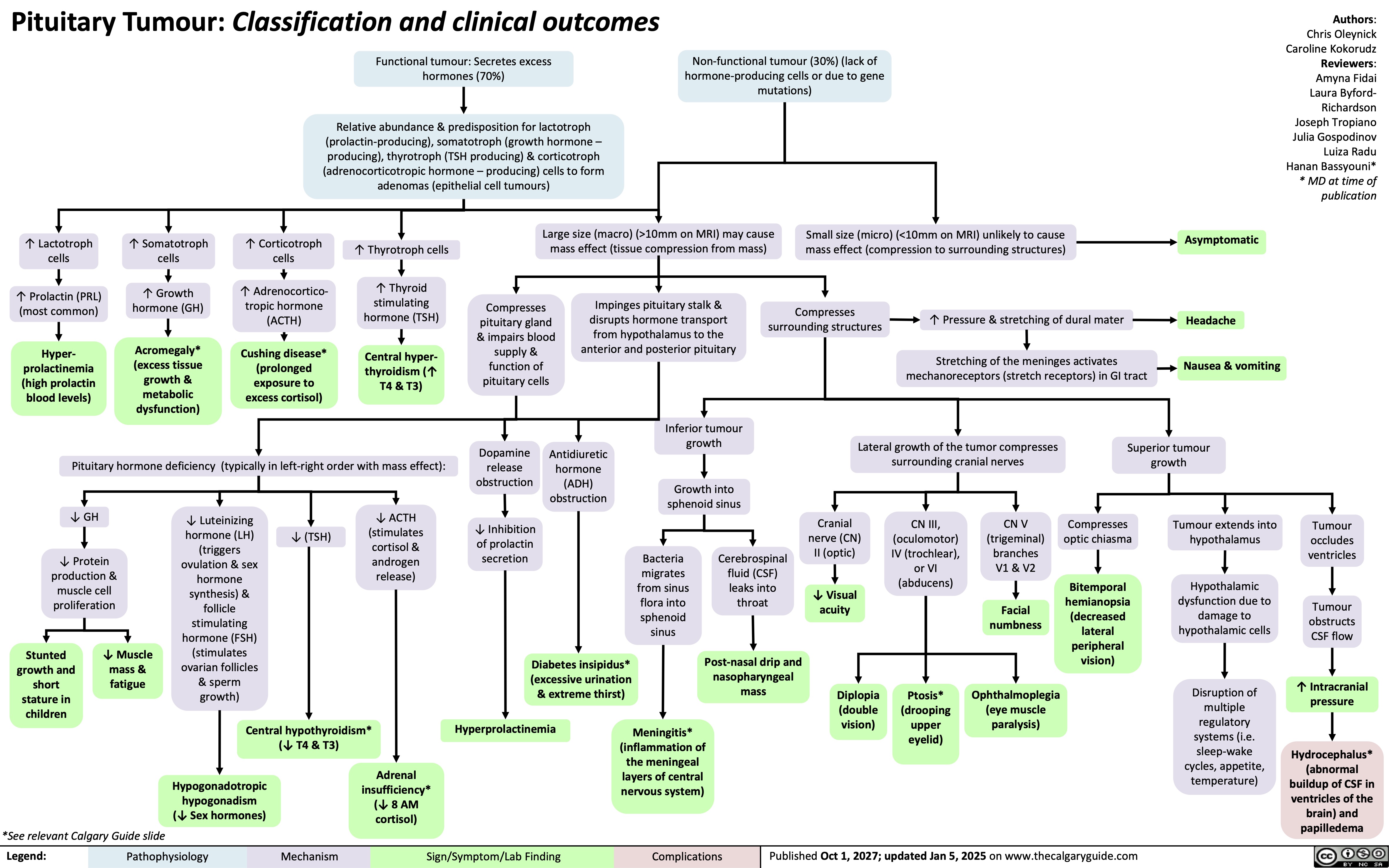
Central Precocious Puberty
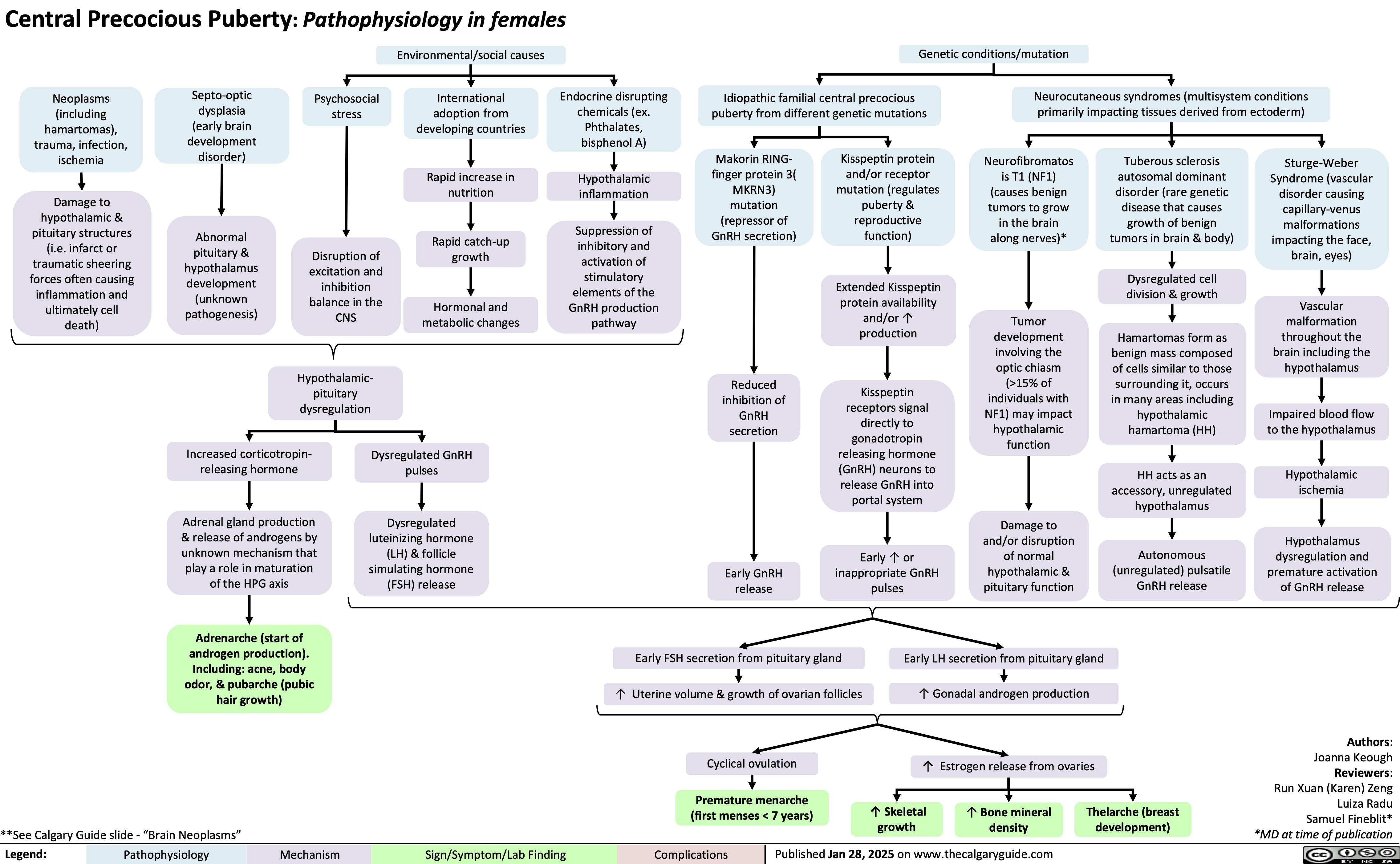
Osgood Schlatter Disease
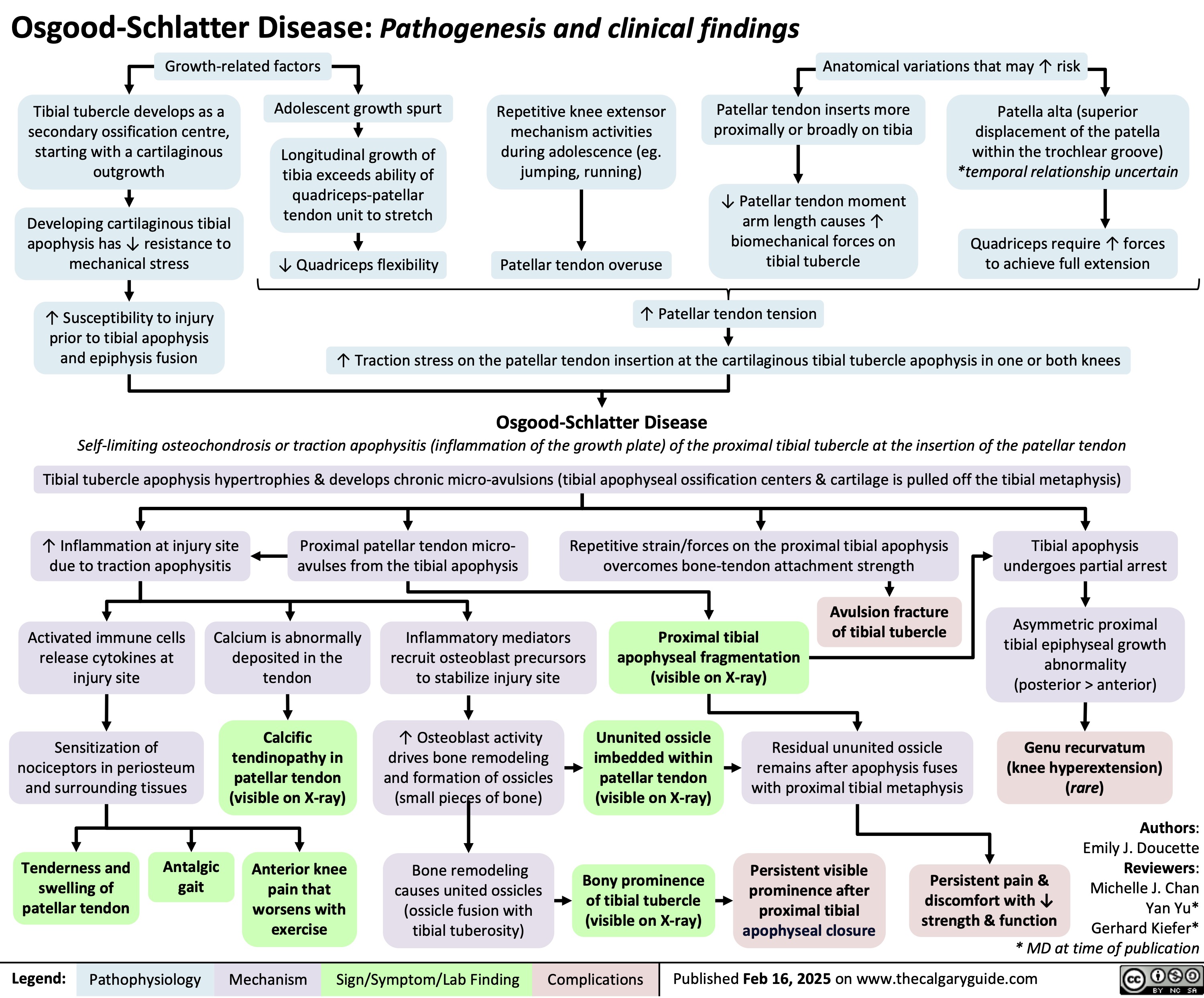
Tonsillitis Pathogenesis and clinical findings
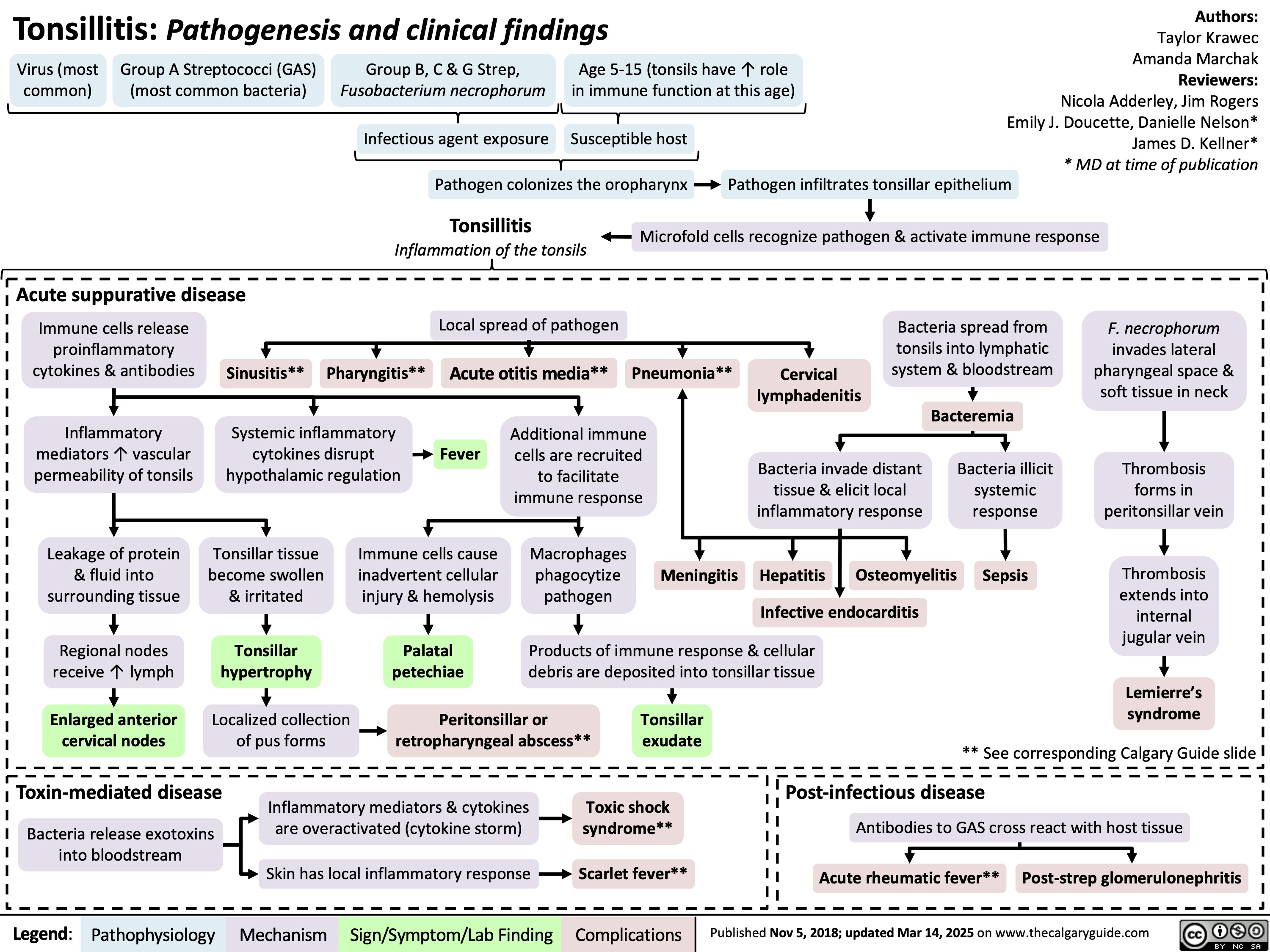
Acute Infectious Mononucleosis
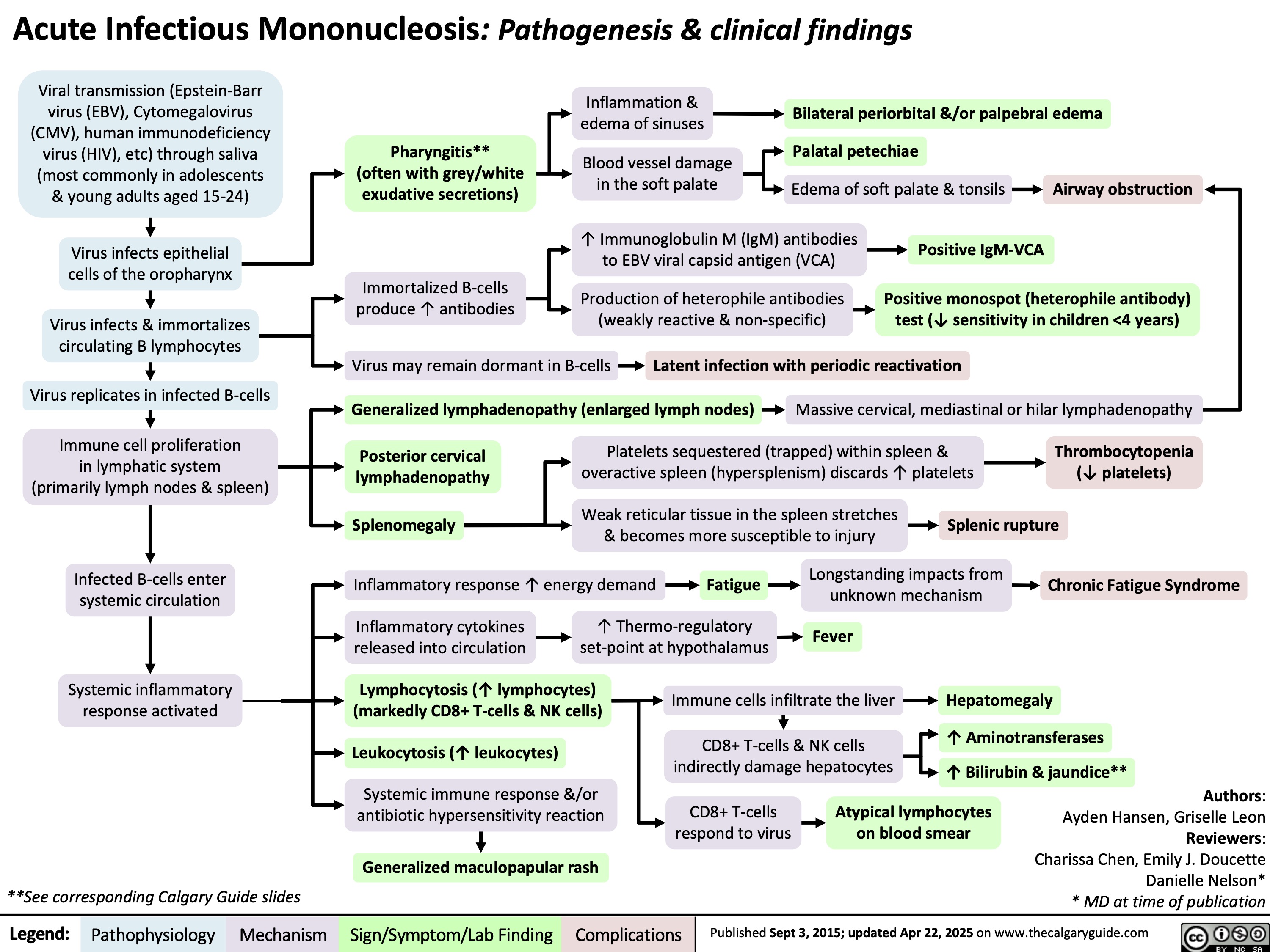
Trachoma
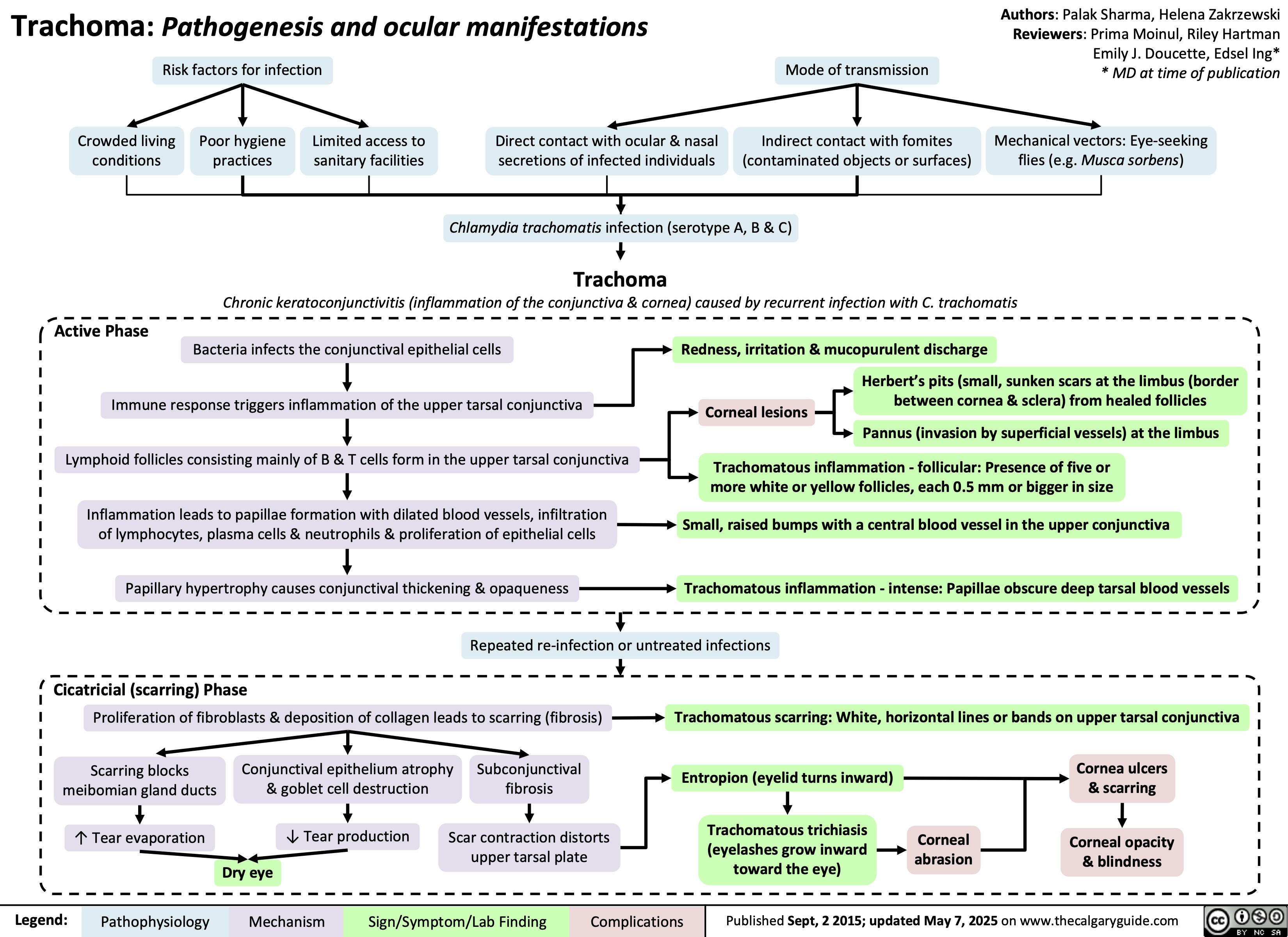
Meckels Diverticulum
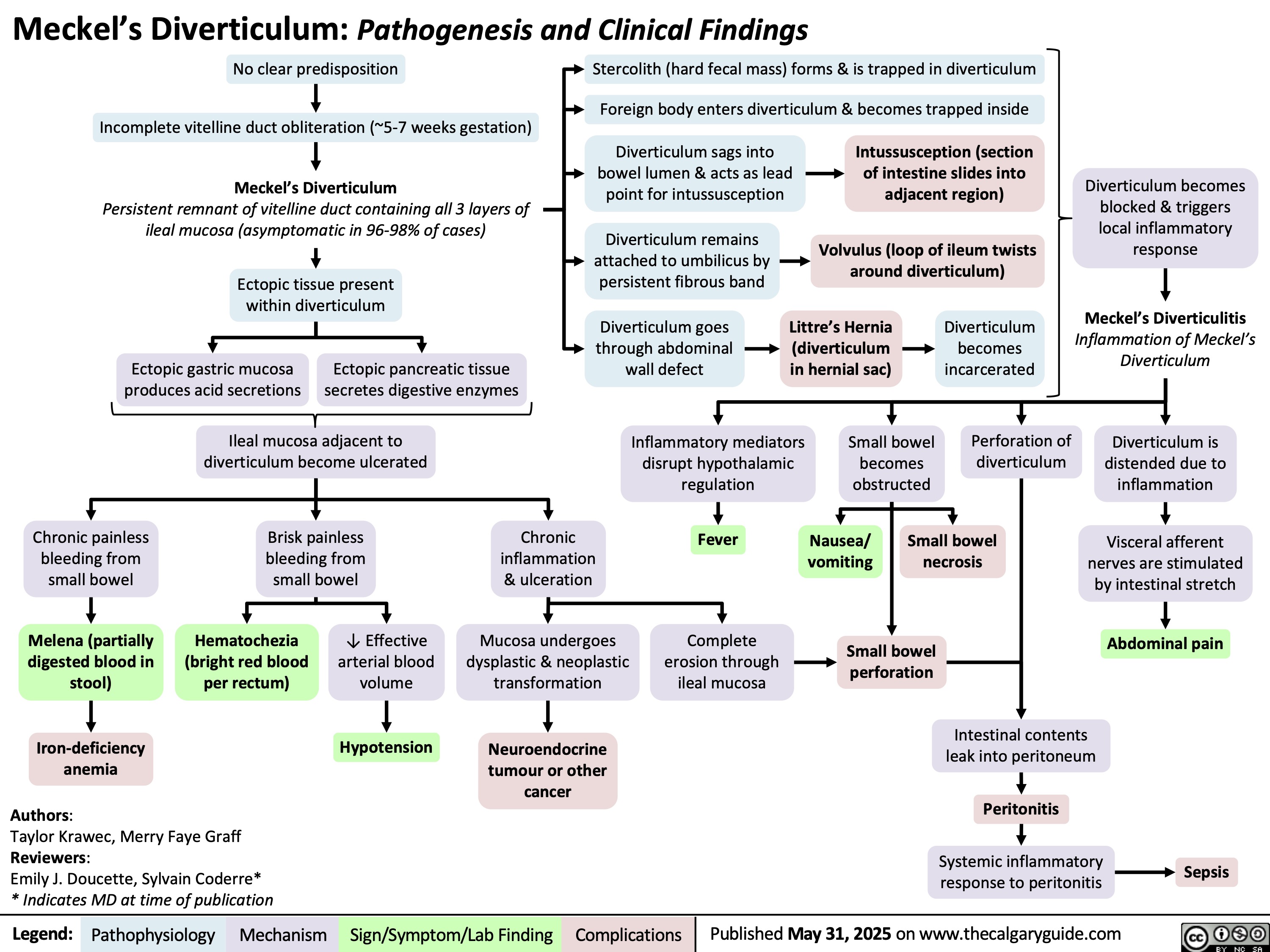
Heparin-Induced Thrombocytopenia
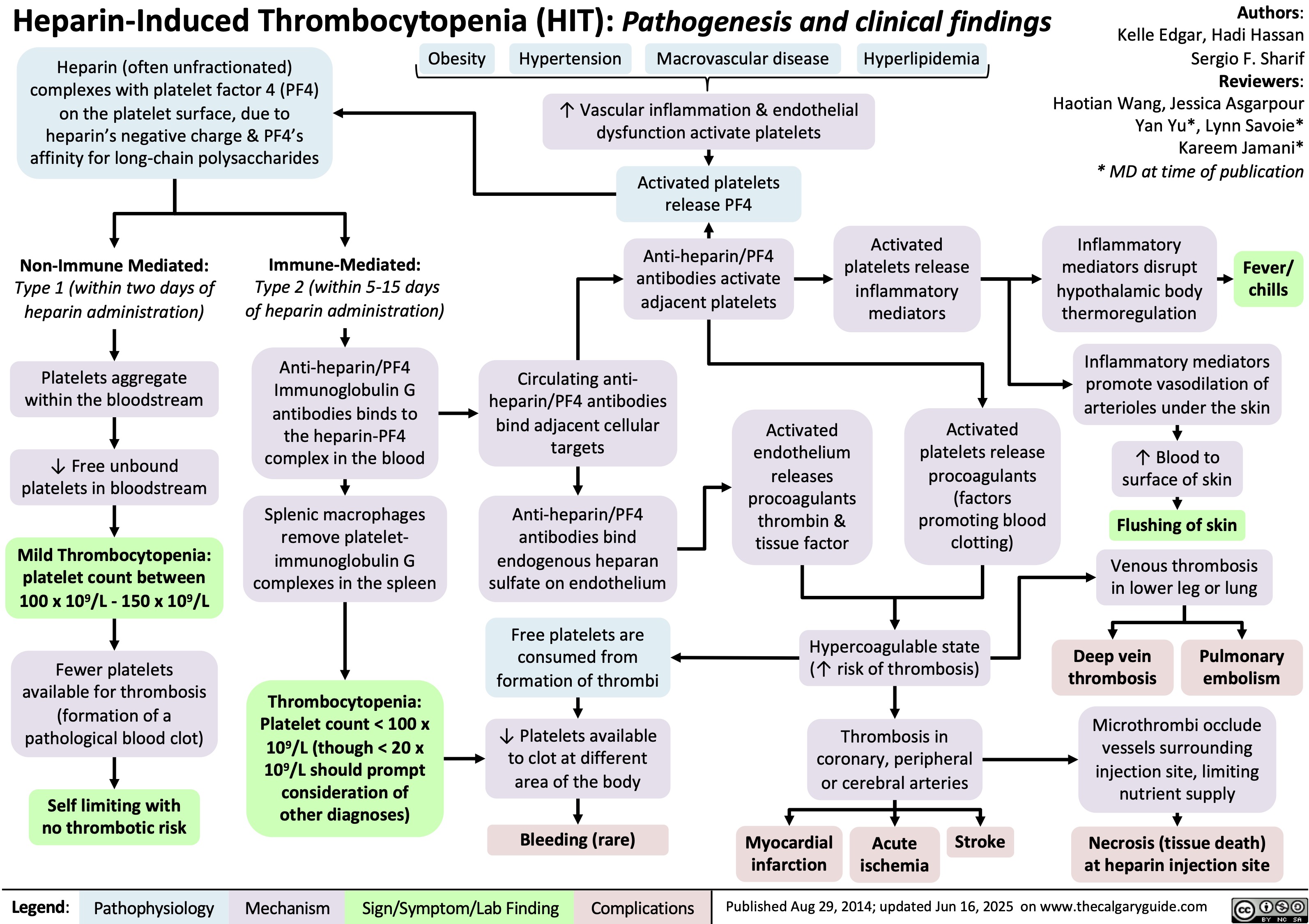
Varicella Zoster Virus
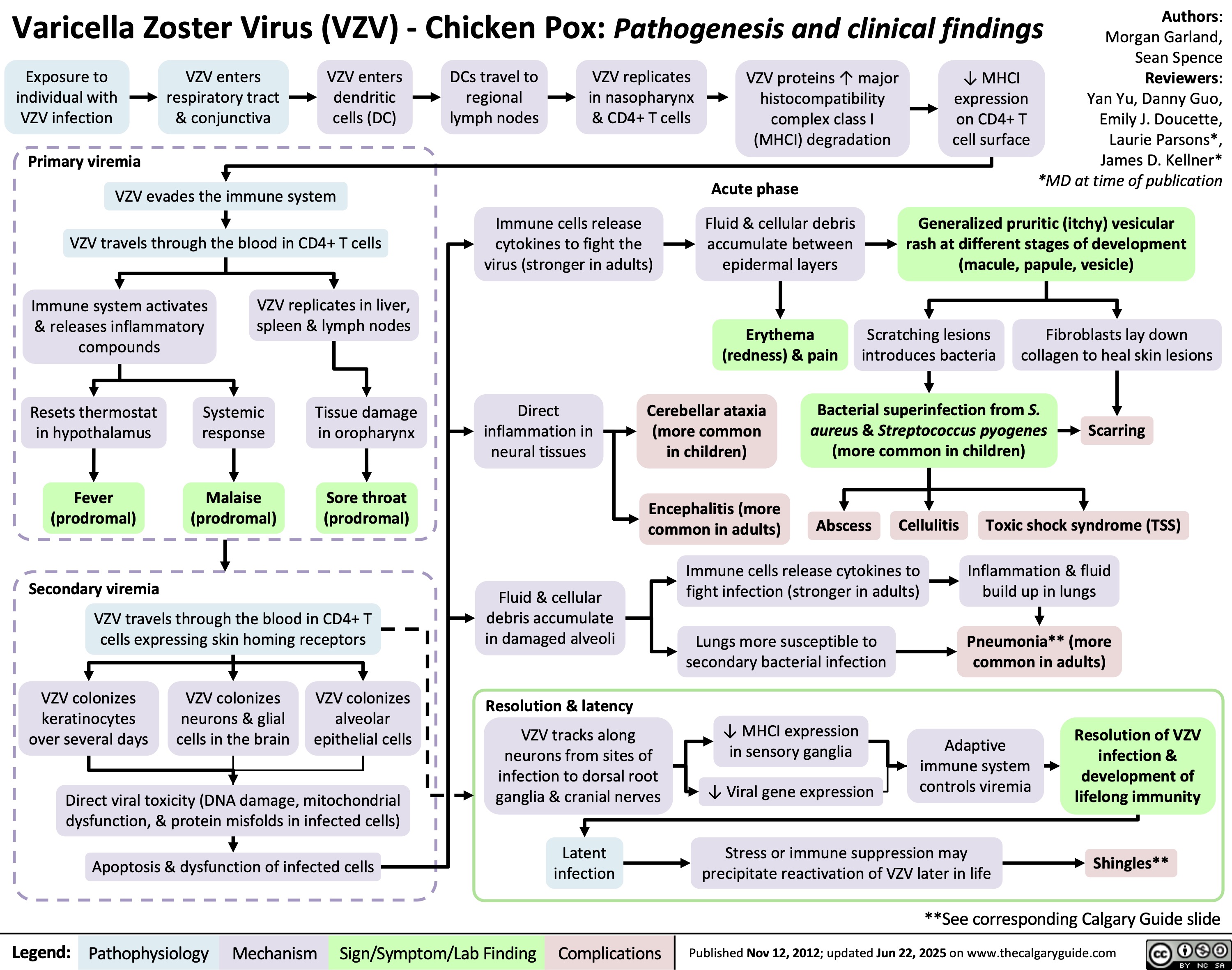
Unconjugated Hyperbilirubinemia
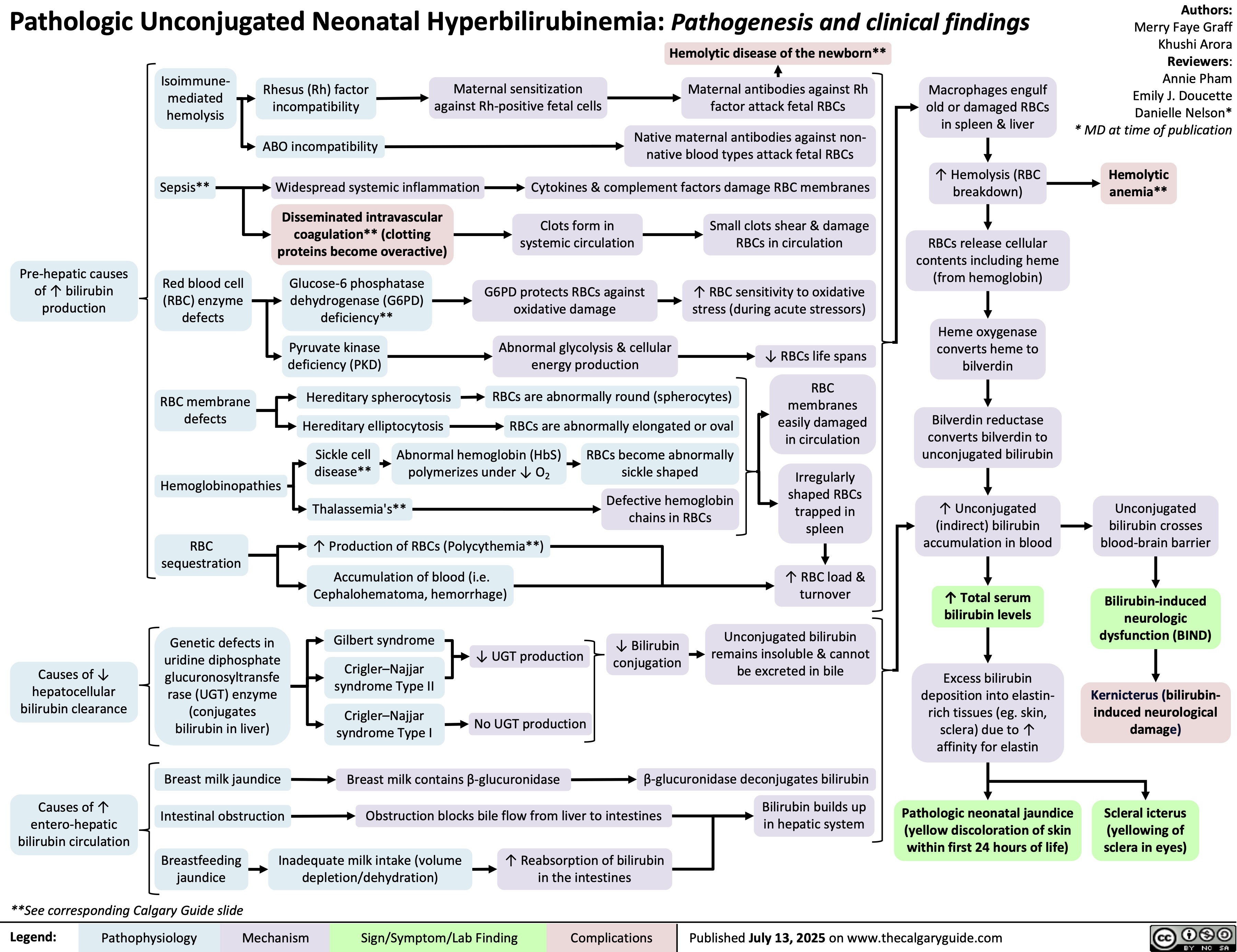
Lupus Muco-cutaneous Manifestations
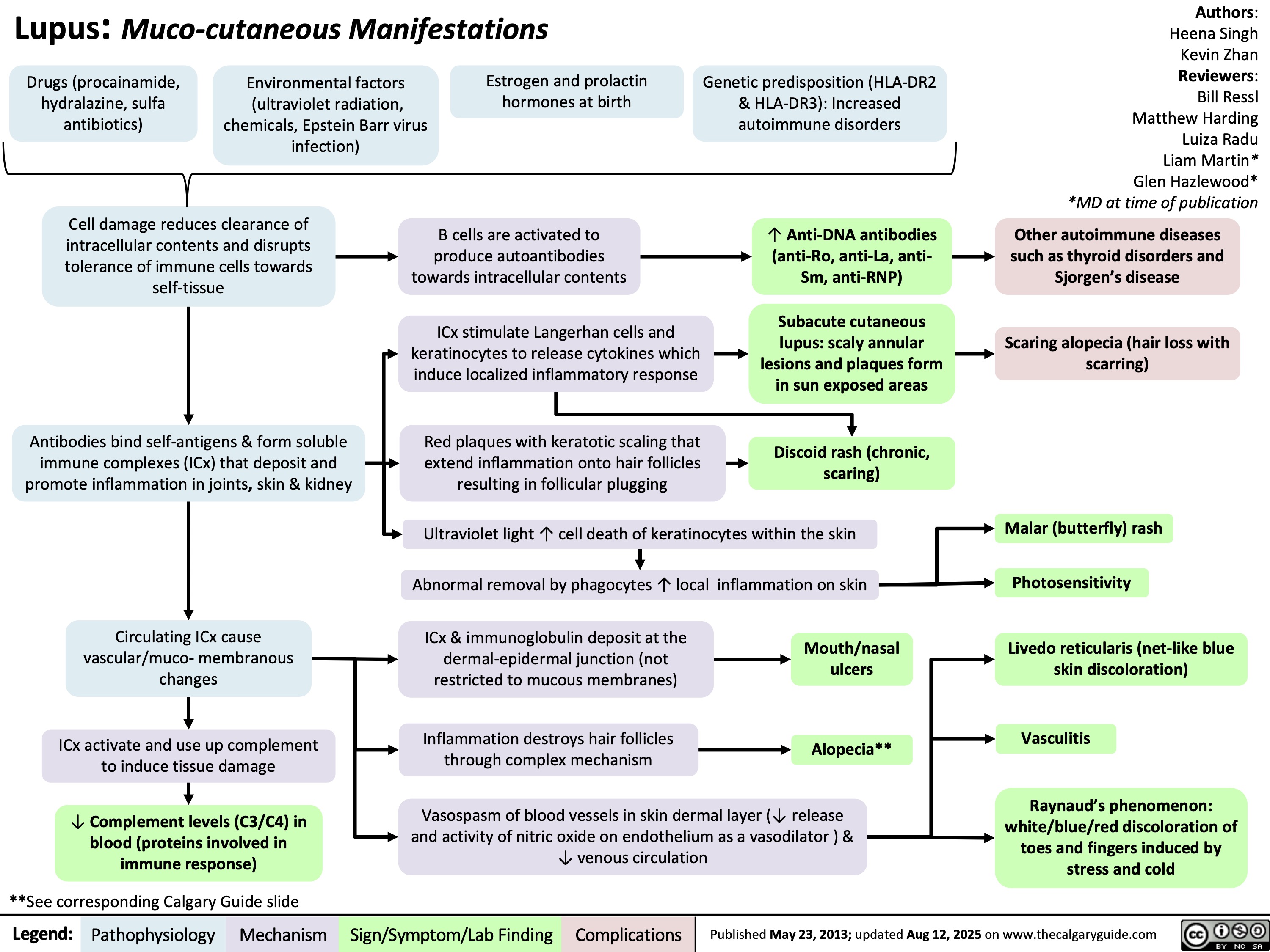
Chronic Thromboembolic Pulmonary Hypertension CTEPH Pathogenesis
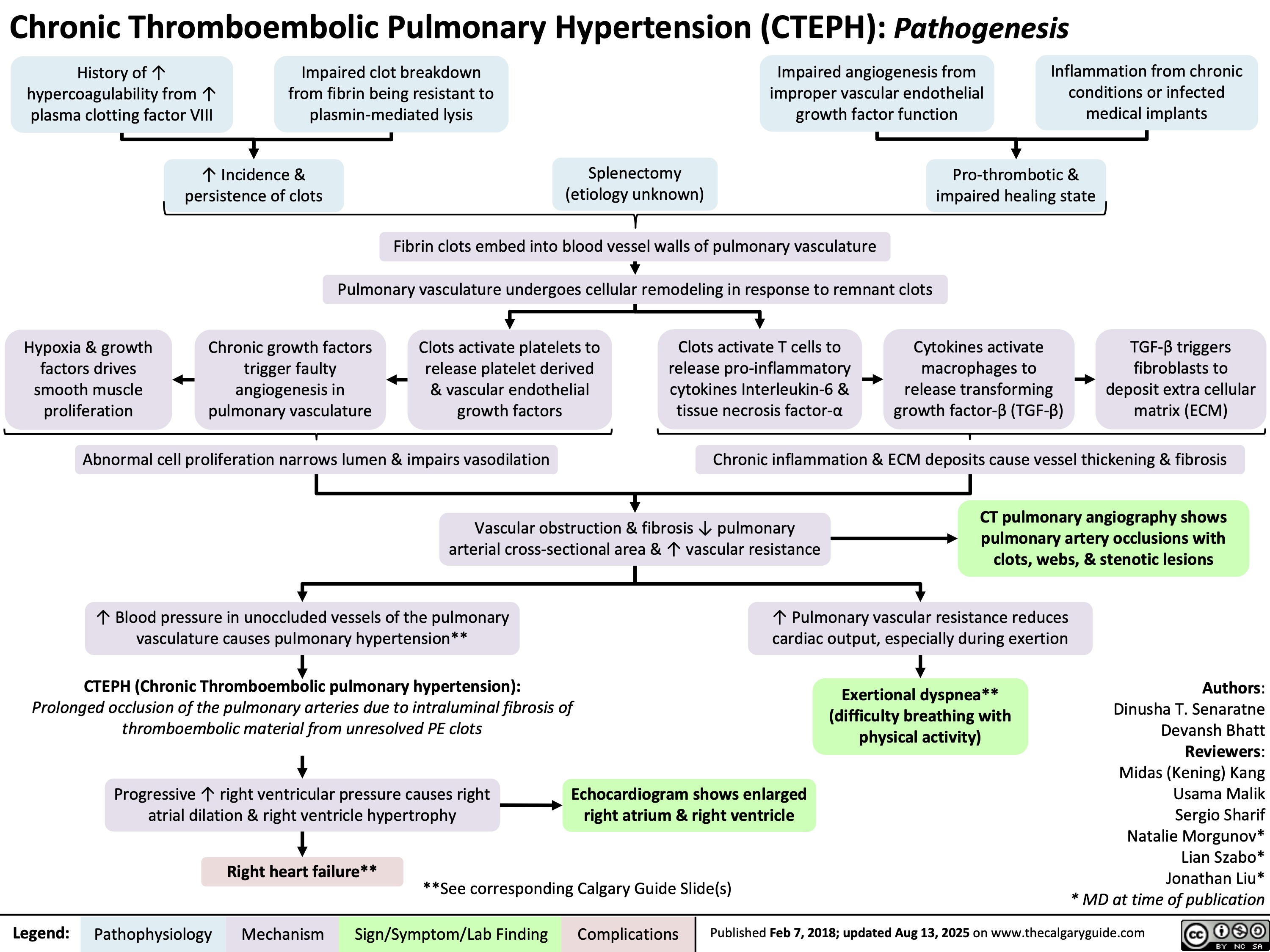
Cystic Fibrosis
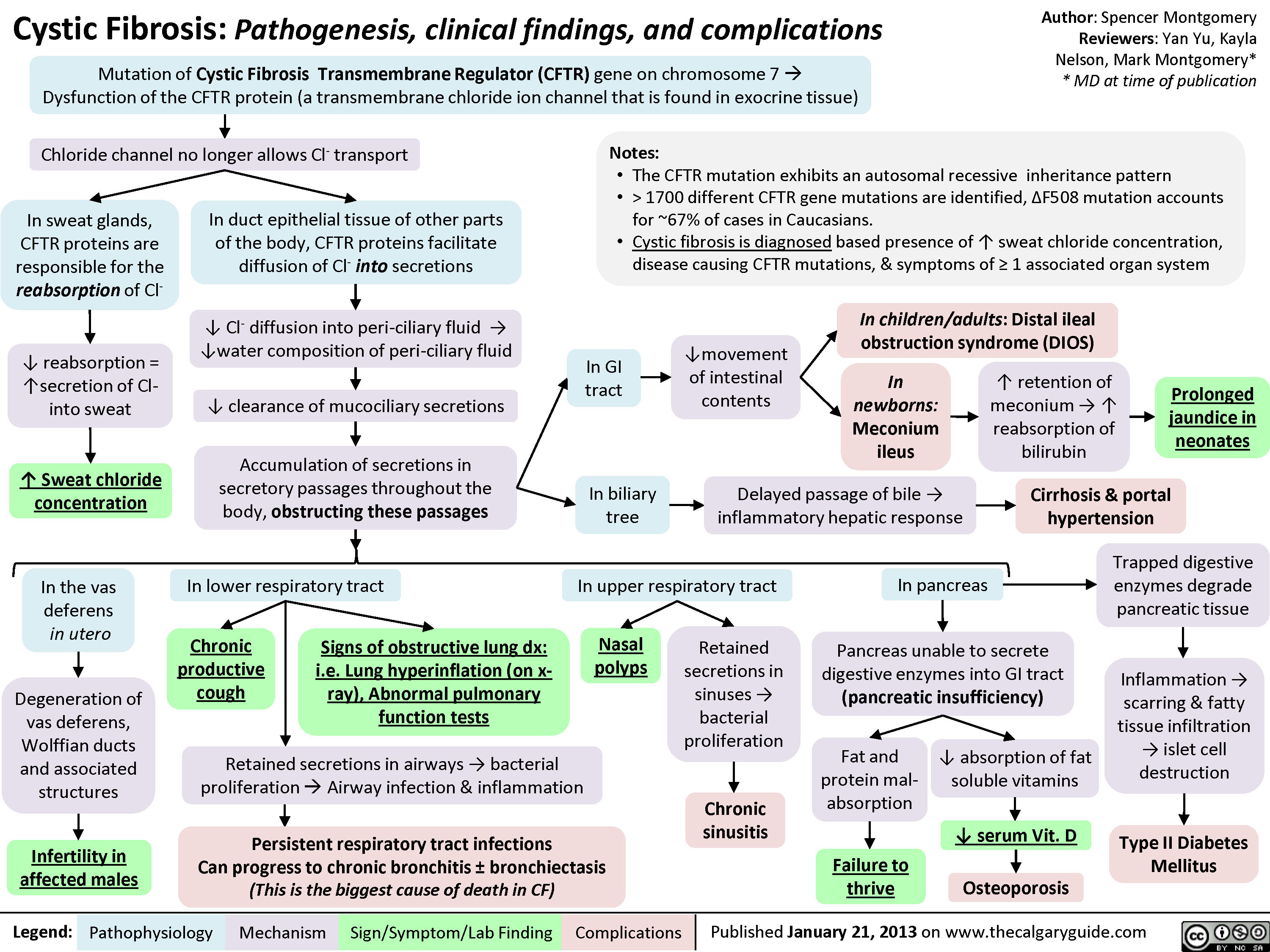
Hypocalcemia Physiology
![Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/08/Hypocalcemia-Pathogenesis.jpg)
Endometriosis
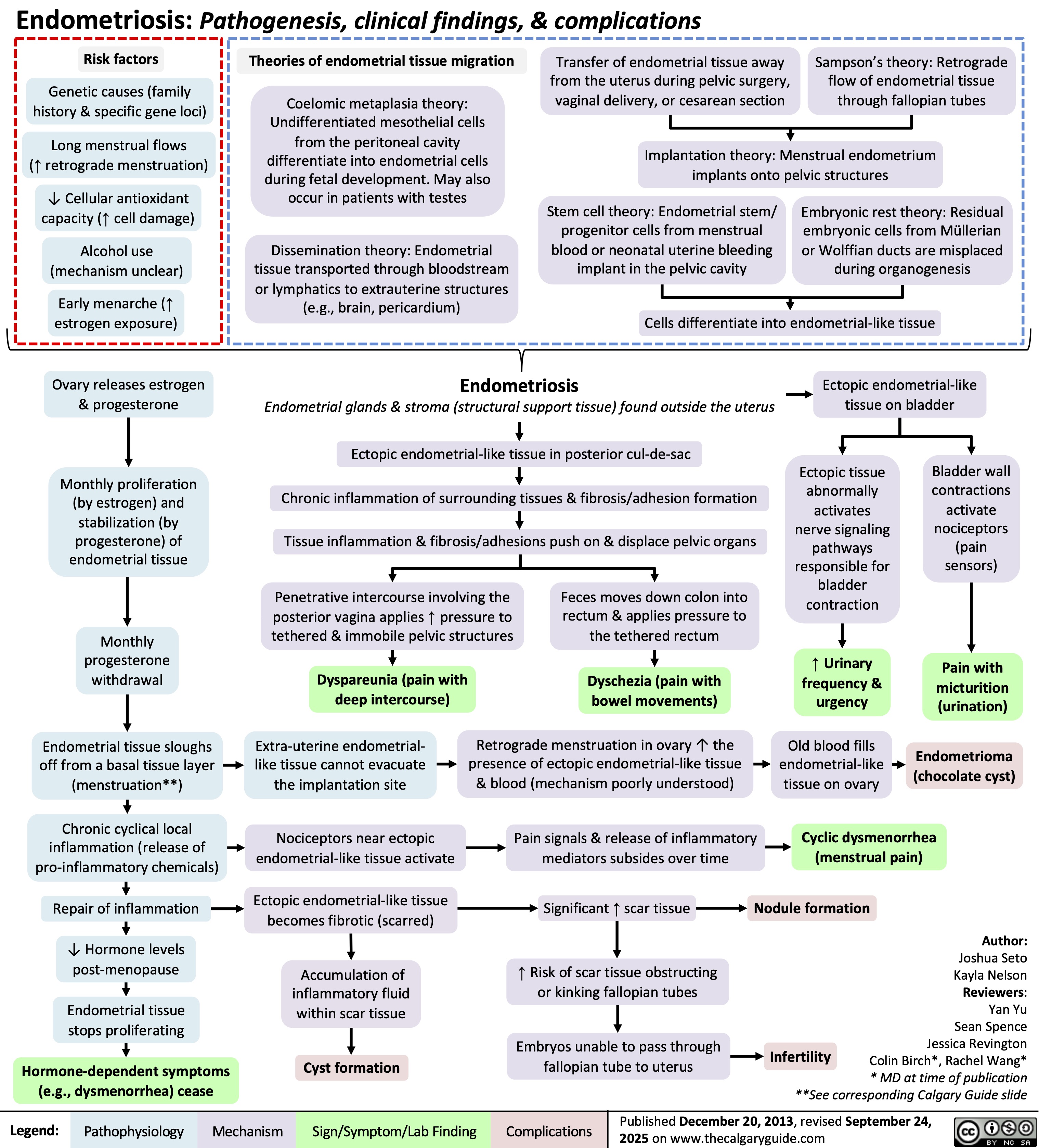
Hydrocephalus in pediatrics
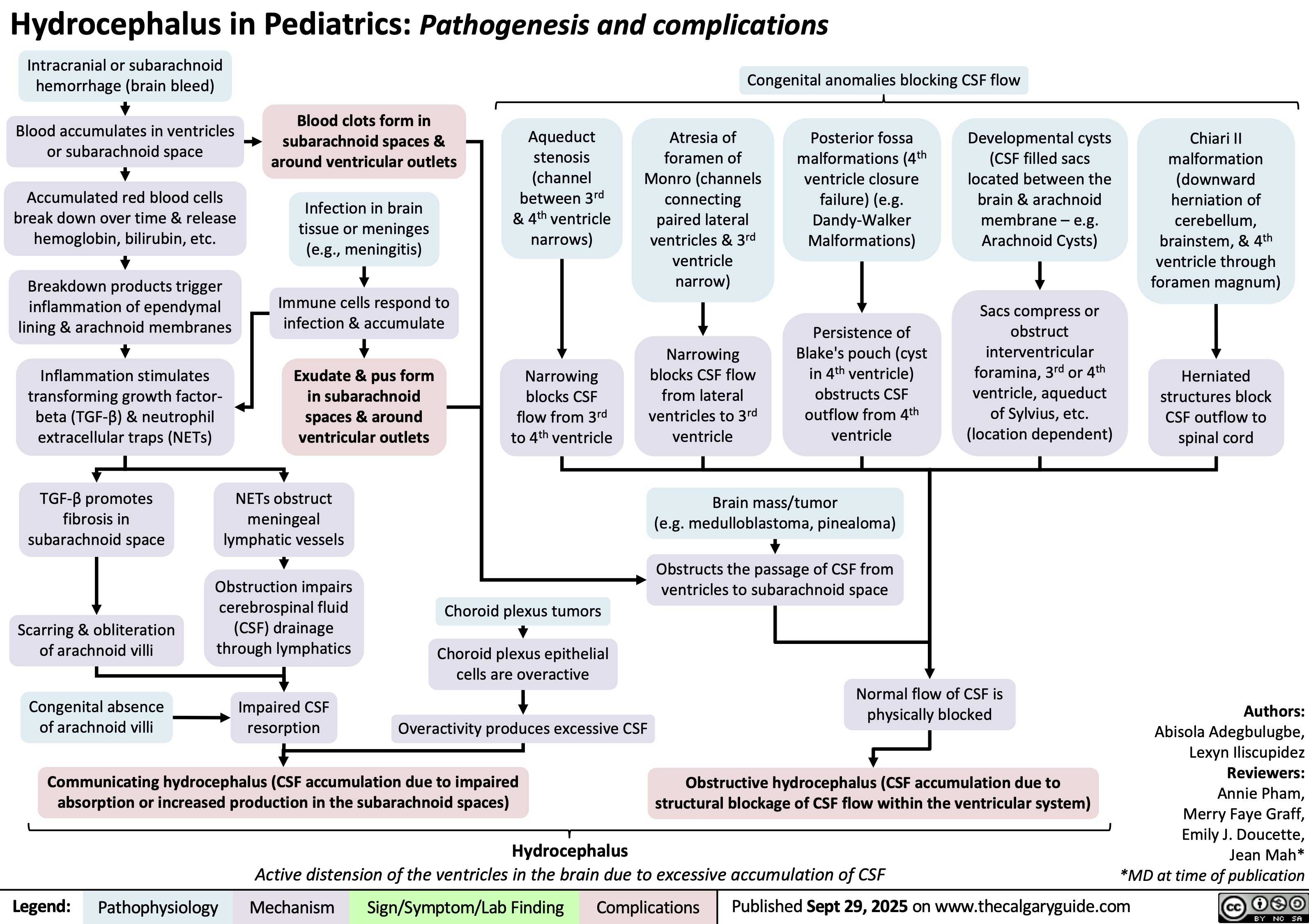
Reactive infectious mucocutaneous eruption
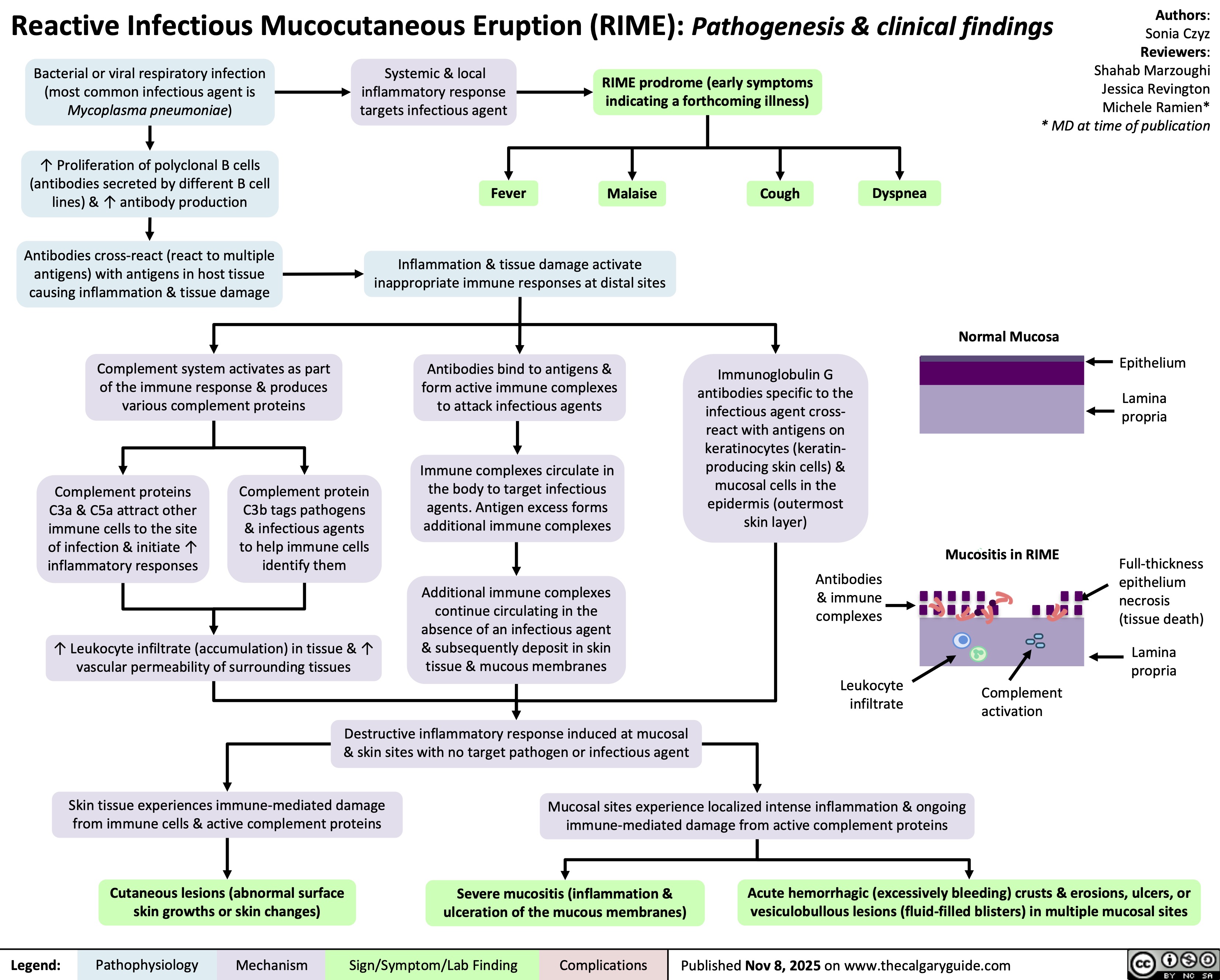
Acute Tubular Necrosis
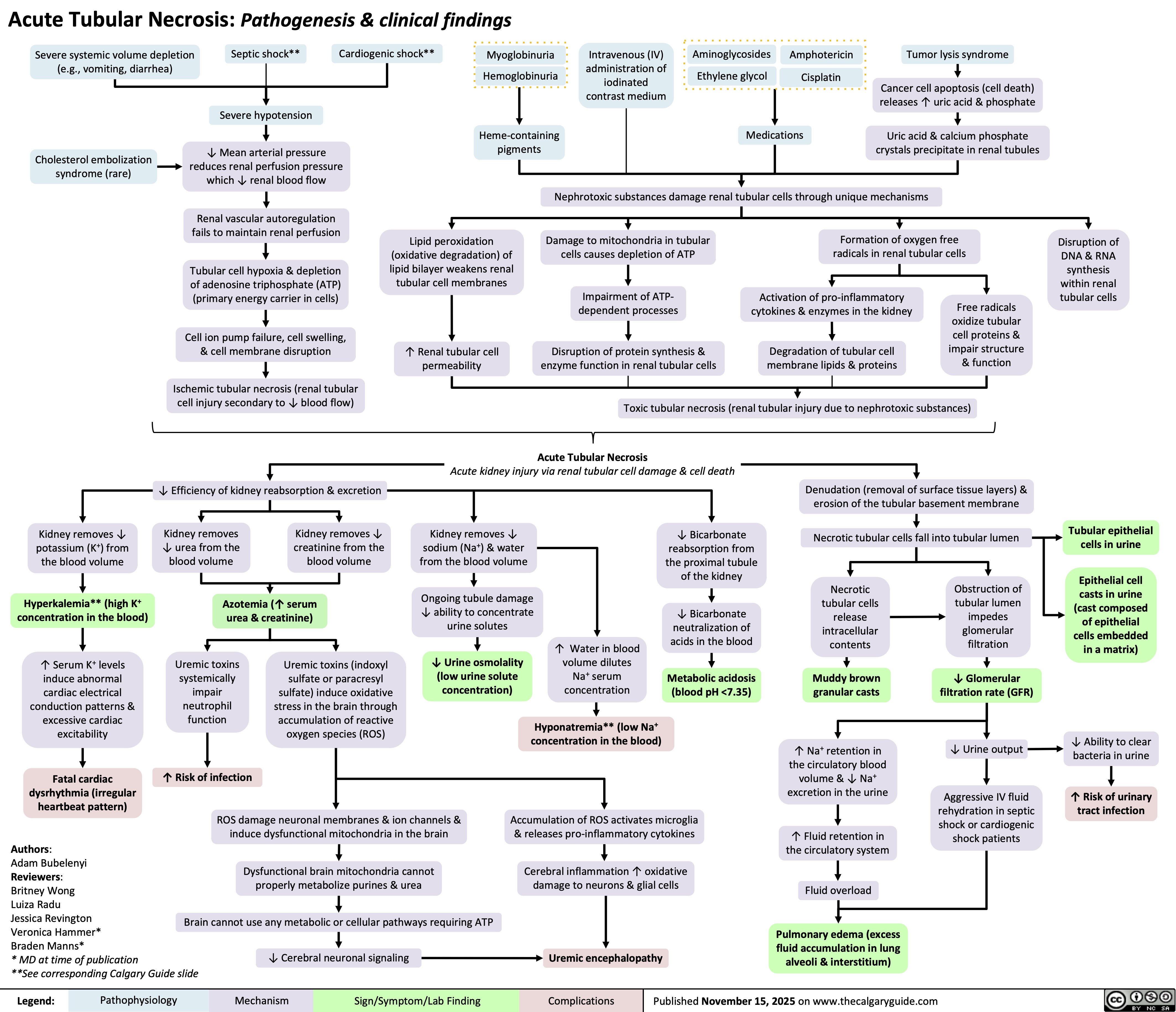
Infection-Related Glomerulonephritis
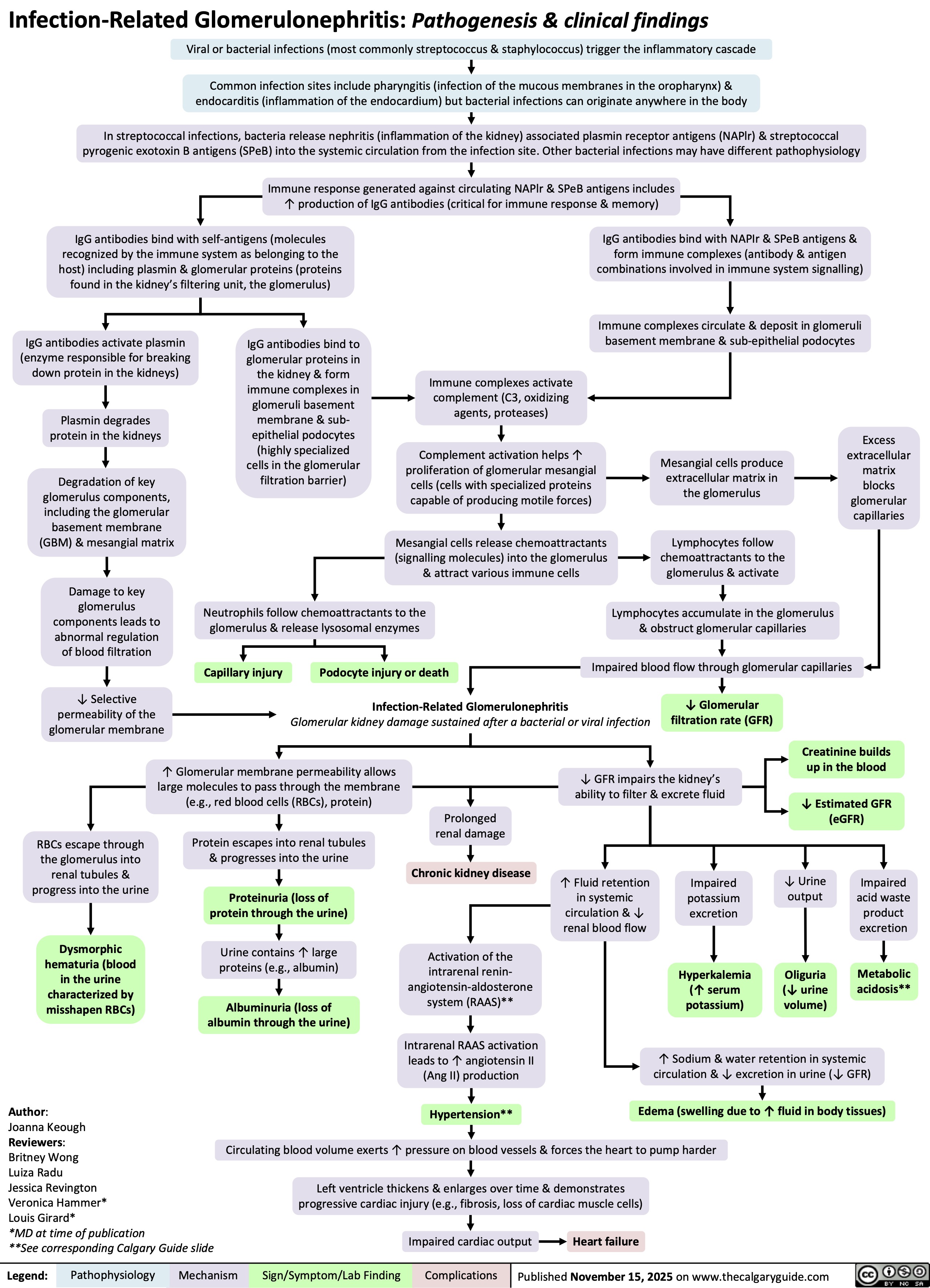
Pyelonephritis
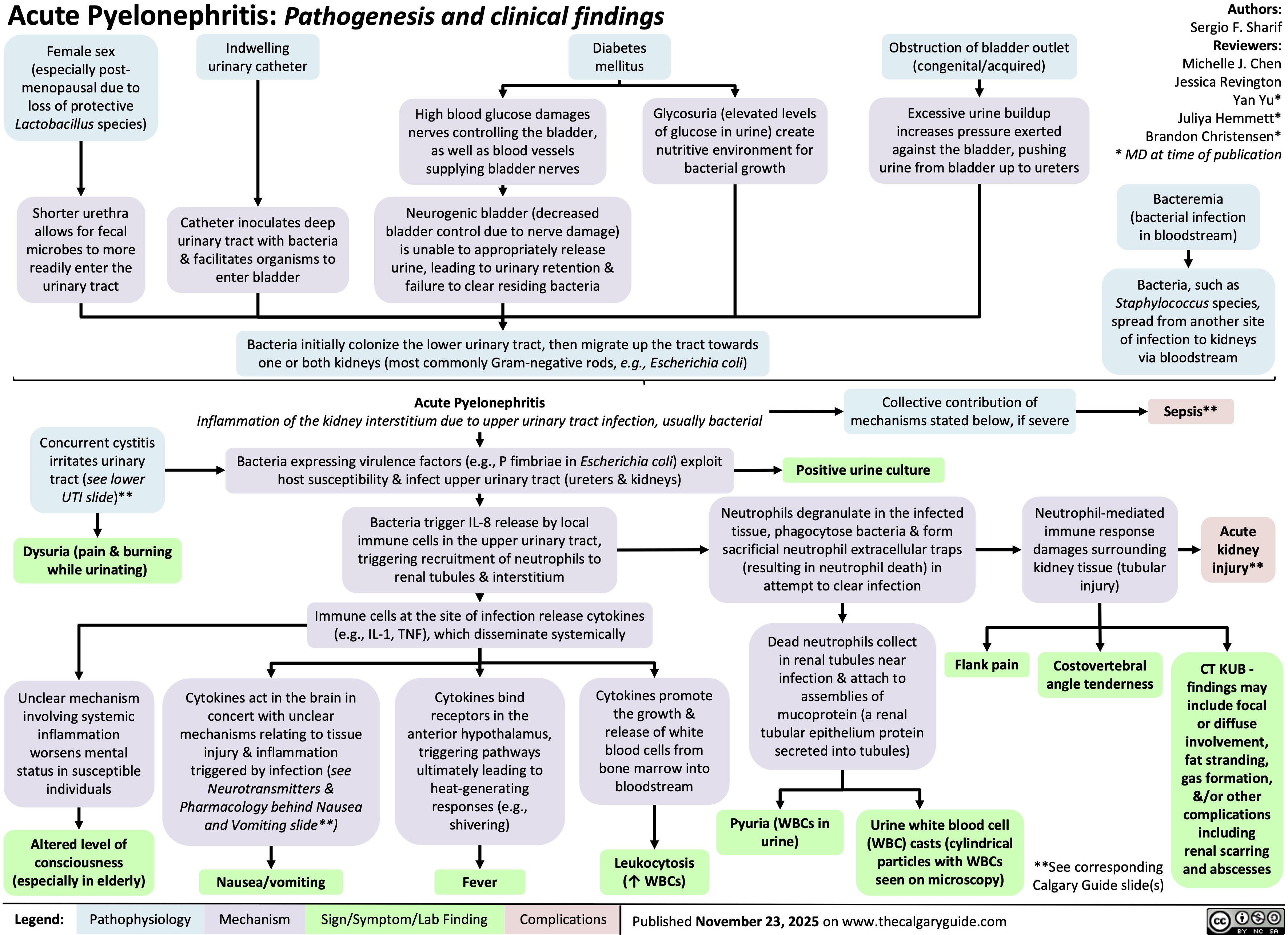
Hypothyroidism
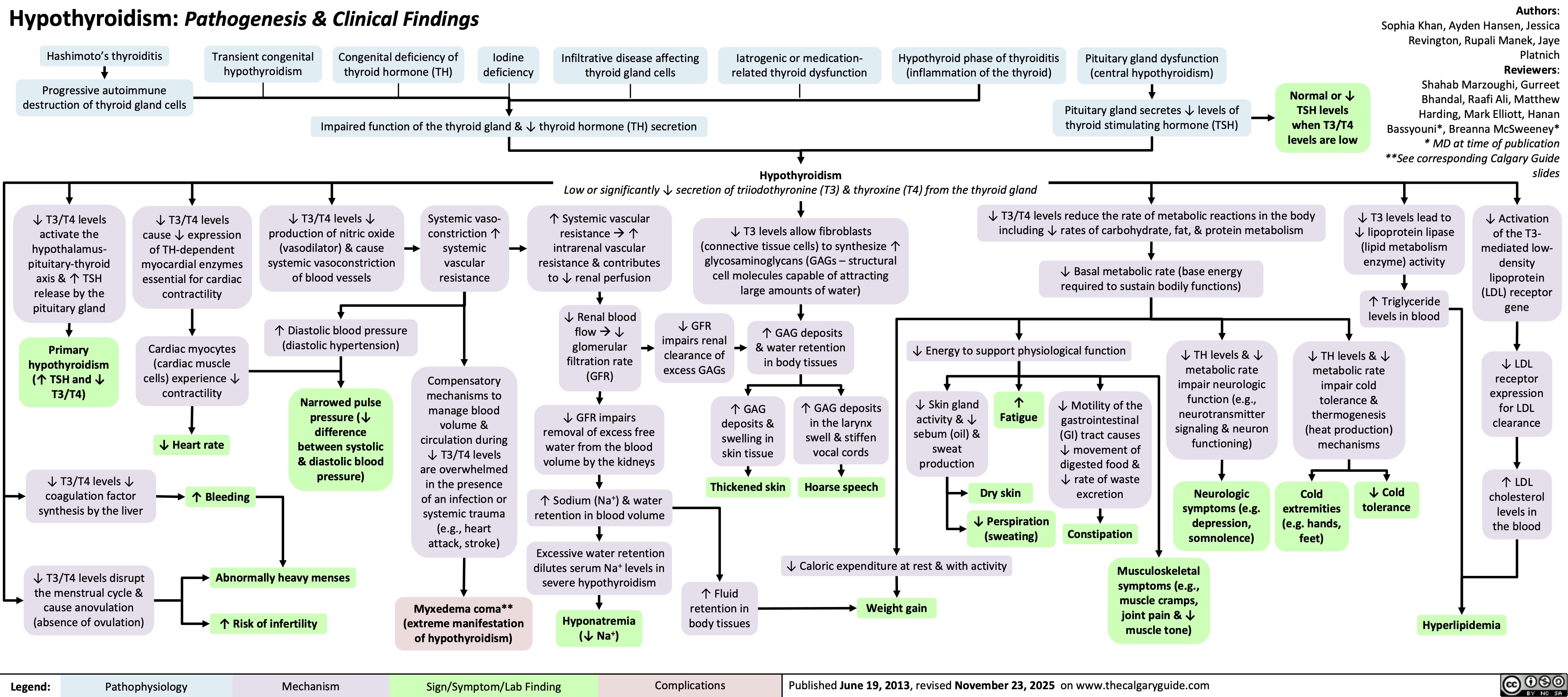
Infectious Large Bowel Diarrhea
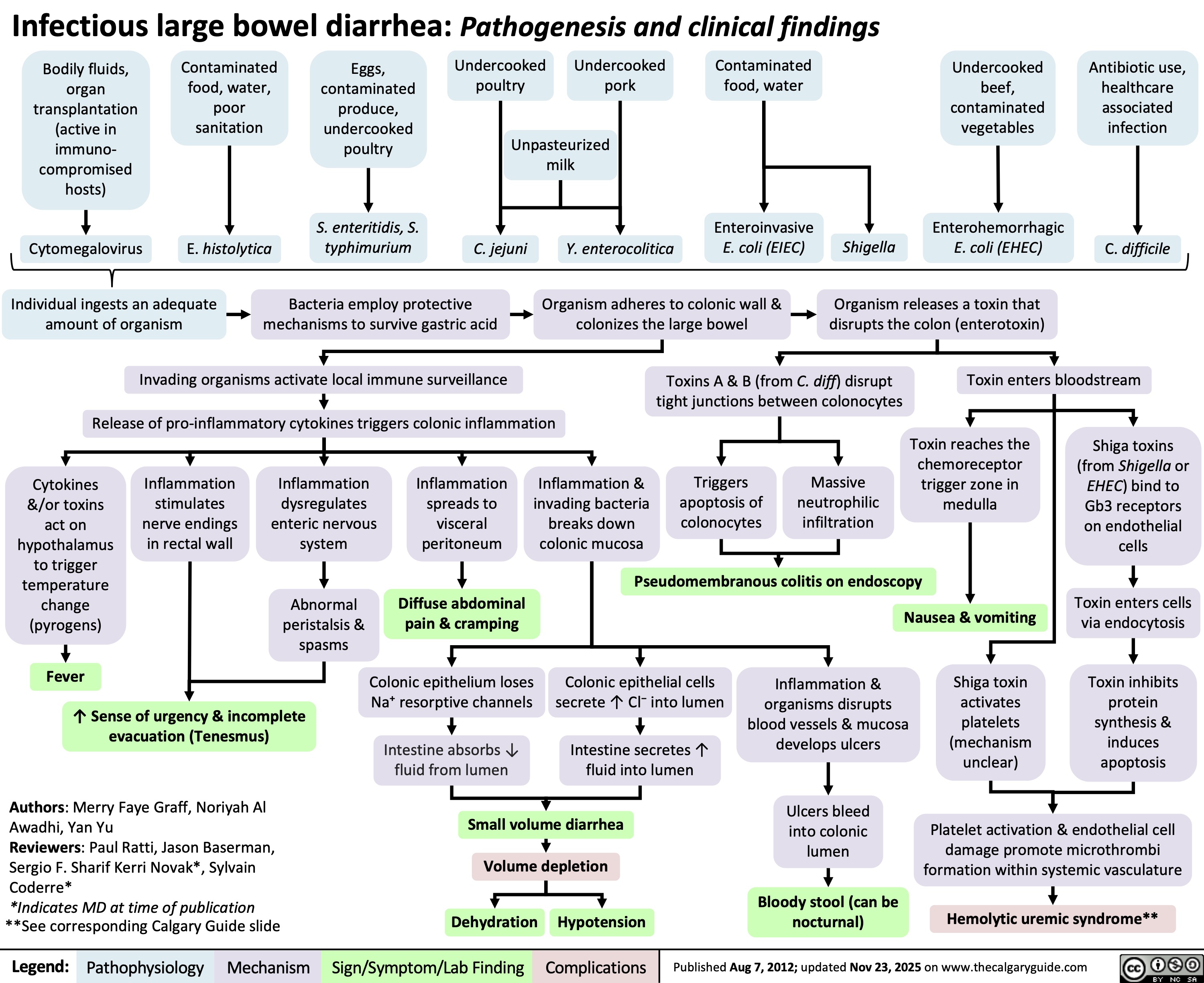
Kawasaki Disease



























![Acute Pancreatitis: Pathogenesis and Clinical Findings
Authors: Yan Yu Reviewers: Laura Craig Noriyah AlAwadhi Ryan Brenneis Maitreyi Raman* * MD at time of publication
Associated signs due to intra- abdominal hemorrhage from an unknown mechanism (classically associated with pancreatitis, but happens in <1% of cases):
Note:
It is not enough to just diagnose “acute pancreatitis”. Full management requires determining underlying etiology with further work-up.
Alcohol
↑ Toxic metabolites within pancreas and Spincter of Oddi Spasms
Gallstones
Migration to common bile duct blocks Sphincter of Oddi
Hypertriglyceridemia
Unknown
mechanism (rare)
Idiopathic
Further investigations:
CBC: Cell counts elevated, due to sever hypovolemia
Serum [Lipase]: Gold Standard Diagnostic Test; rupture of pancreatic cells releases lipase into circulation
Pancreatic secretions back up, ↑ pressure within pancreas
Hypercalcemia (Rare; Ca2+ depositions in bile ducts block outflow of pancreatic secretions)
Since pancreas is retroperitoneal, somatic
nerves in the parietal peritoneum are directly stimulated
Inflammation triggers cytokine release
Inflamed pancreas irritates adjacent intestines, causing ileus
Inflamed, more permeable blood vessels leak fluid into pancreas
• •
Cullen’s sign (bruising in peri-umbilical region) Grey-Turner’s sign (bruises along both flanks)
Sudden, severe epigastric pain (with peritoneal signs), radiates to the center of the back
Fever, nausea/vomiting
(general signs of inflammation)
Diminished bowel sounds Profound dehydration
(flat JVP, hypotension, tachycardia, oliguria) – may happen, not always
1. Pressure compresses pancreatic blood vessels, causing tissue ischemia.
2. Activation of inactive proteases (zymogens) digesting pancreatic tissue
Necrosis (death) of pancreatic cells
Inflammation self- perpetuates
Massive systemic inflammatory response
2 main complications, usually detected on CT;
may happen, but not always
1. Pancreatic pseudocyst (enlargement of the
pancreas due to fluid accumulation)
2. Pancreatic necrosis/abscesses (death of a part of the pancreas)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-published September 1, 2019 on thecalgaryguide.com
Acute Pancreatitis: Pathogenesis and Clinical Findings
Authors: Yan Yu Reviewers: Laura Craig Noriyah AlAwadhi Ryan Brenneis Maitreyi Raman* * MD at time of publication
Associated signs due to intra- abdominal hemorrhage from an unknown mechanism (classically associated with pancreatitis, but happens in <1% of cases):
Note:
It is not enough to just diagnose “acute pancreatitis”. Full management requires determining underlying etiology with further work-up.
Alcohol
↑ Toxic metabolites within pancreas and Spincter of Oddi Spasms
Gallstones
Migration to common bile duct blocks Sphincter of Oddi
Hypertriglyceridemia
Unknown
mechanism (rare)
Idiopathic
Further investigations:
CBC: Cell counts elevated, due to sever hypovolemia
Serum [Lipase]: Gold Standard Diagnostic Test; rupture of pancreatic cells releases lipase into circulation
Pancreatic secretions back up, ↑ pressure within pancreas
Hypercalcemia (Rare; Ca2+ depositions in bile ducts block outflow of pancreatic secretions)
Since pancreas is retroperitoneal, somatic
nerves in the parietal peritoneum are directly stimulated
Inflammation triggers cytokine release
Inflamed pancreas irritates adjacent intestines, causing ileus
Inflamed, more permeable blood vessels leak fluid into pancreas
• •
Cullen’s sign (bruising in peri-umbilical region) Grey-Turner’s sign (bruises along both flanks)
Sudden, severe epigastric pain (with peritoneal signs), radiates to the center of the back
Fever, nausea/vomiting
(general signs of inflammation)
Diminished bowel sounds Profound dehydration
(flat JVP, hypotension, tachycardia, oliguria) – may happen, not always
1. Pressure compresses pancreatic blood vessels, causing tissue ischemia.
2. Activation of inactive proteases (zymogens) digesting pancreatic tissue
Necrosis (death) of pancreatic cells
Inflammation self- perpetuates
Massive systemic inflammatory response
2 main complications, usually detected on CT;
may happen, but not always
1. Pancreatic pseudocyst (enlargement of the
pancreas due to fluid accumulation)
2. Pancreatic necrosis/abscesses (death of a part of the pancreas)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-published September 1, 2019 on thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/09/Acute-Pancreatitis.jpg)


















 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />






![rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
Psoriatic Arthritis: Pathogenesis and clinical findings
Auteur:
Payam Pournazari
Rédacteurs:
Yan Yu Scott Rapske Liam Martin* Traducteurs: Dianne Ganeswaran Stephen Williams Sylvain Coderre* * MD au moment de publication
Note:
L’arthrite rhumatoïde peut survenir avant ou après le psoriasis (la pathogenèse est la même).
Augmentation de l’activité ostéoclastique
Érosion de l’os sous- chondral mais formation de nouvelle osseuse ailleurs dans l’articulation
Radiographie: la périostite; les syndesmophytes, érosions, déformation du crayon en godets (les articulations IP), ankylose des articulations IP
HLA B27, Cw6 et d’autres sous- types
Antécédents familiaux positifs
Activation des cellules T (mécanisme inconnu)
Infiltration du tissu synovial de l’articulation par les cellules T et B, cellules tueuses naturelles et les macrophages
Production augmentée des molécules inflammatoires (les facteurs de nécrose tumorale [TNFs], les interleukines, etc.) qui agissent de façon systémique (dans tout le corps)
Dans les tendons et le tissu conjonctif
Inflammation des enthèses et du tissu conjonctif provoque le gonflement
Dans la peau
Réaction immunitaire détruisant les structures de la peau et des ongles (Voir diapositive Psoriasis)
Le psoriasis en plaques sur la peau, dépressions punctiformes, onychorrhexie, taches d’huile, onycholyse, décoloration et hyperkératose
Angiogenèse et vascularite dans les vaisseaux sanguins de la membrane synoviale
Dans les articulations
Les cytokines inflammatoires stimulent les nocicepteurs locaux
1. Oligoarthrites - asymétriques
2. Arthrite des articulations IPD
3. Polyarthrite rhumatoïde -
symétrique
4. Atteinte axiale (raison
inconnue, probablement en raison de « biomécanique », « innervation », et
« vascularisation ».
5. Arthrite mutilante (grave et destructrice)
L’enthésite
(Douleur/sensibilité à l’insertion du ligament dans l’os)
La dactylite (Inflammation de tout le doigt – les tissus mous et les articulations sont enflammés
Légende:
Physiopathologie
Mécanisme
Signe/Symptôme/Résultats de Laboratoire
Complications
Publié 10 November 2012 sur www.thecalgaryguide.com
rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
Psoriatic Arthritis: Pathogenesis and clinical findings
Auteur:
Payam Pournazari
Rédacteurs:
Yan Yu Scott Rapske Liam Martin* Traducteurs: Dianne Ganeswaran Stephen Williams Sylvain Coderre* * MD au moment de publication
Note:
L’arthrite rhumatoïde peut survenir avant ou après le psoriasis (la pathogenèse est la même).
Augmentation de l’activité ostéoclastique
Érosion de l’os sous- chondral mais formation de nouvelle osseuse ailleurs dans l’articulation
Radiographie: la périostite; les syndesmophytes, érosions, déformation du crayon en godets (les articulations IP), ankylose des articulations IP
HLA B27, Cw6 et d’autres sous- types
Antécédents familiaux positifs
Activation des cellules T (mécanisme inconnu)
Infiltration du tissu synovial de l’articulation par les cellules T et B, cellules tueuses naturelles et les macrophages
Production augmentée des molécules inflammatoires (les facteurs de nécrose tumorale [TNFs], les interleukines, etc.) qui agissent de façon systémique (dans tout le corps)
Dans les tendons et le tissu conjonctif
Inflammation des enthèses et du tissu conjonctif provoque le gonflement
Dans la peau
Réaction immunitaire détruisant les structures de la peau et des ongles (Voir diapositive Psoriasis)
Le psoriasis en plaques sur la peau, dépressions punctiformes, onychorrhexie, taches d’huile, onycholyse, décoloration et hyperkératose
Angiogenèse et vascularite dans les vaisseaux sanguins de la membrane synoviale
Dans les articulations
Les cytokines inflammatoires stimulent les nocicepteurs locaux
1. Oligoarthrites - asymétriques
2. Arthrite des articulations IPD
3. Polyarthrite rhumatoïde -
symétrique
4. Atteinte axiale (raison
inconnue, probablement en raison de « biomécanique », « innervation », et
« vascularisation ».
5. Arthrite mutilante (grave et destructrice)
L’enthésite
(Douleur/sensibilité à l’insertion du ligament dans l’os)
La dactylite (Inflammation de tout le doigt – les tissus mous et les articulations sont enflammés
Légende:
Physiopathologie
Mécanisme
Signe/Symptôme/Résultats de Laboratoire
Complications
Publié 10 November 2012 sur www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/05/Psoriatic-Arthritis-PsA-Pathclinical.jpg)
![Acute Cholecystitis: Pathogenesis and clinical findings
Gallstone blocks the cystic duct, backing up bile into the gallbladder
Gallstones causing physical trauma to gallbladder wall
Irritation of adjacent diaphragm, stimulates phrenic nerve (C3-C5)
Activates stretch receptors of visceral peritoneum, stimulates foregut autonomic nerves (T5-T8)
Inflammatory mediator (i.e. prostaglandin) release by gallbladder and systemic inflammatory response
Thickened gallbladder wall on ultrasound (gold standard test)
On inspiration, the diaphragm pushes the gallbladder downward
Irritation of parietal peritoneum, stimulates somatic nerves
↑ Permeability of vessels with systemic inflammation, which leak
fluid from the blood into the interstitial space
Radiating pain to the back and right shoulder
Dull, diffuse abdominal pain referred to the epigastric region
Fever, nausea/vomiting, tachycardia
Positive Murphy’s sign (pain upon palpation of right upper quadrant [RUQ] on inspiration)
Persistent RUQ pain, abdominal guarding and peritoneal signs
Dehydration
Authors: Yan Yu, Vina Fan Reviewers: Dean Percy, Mirna Matta, Crystal Liu, Ben Campbell Maitreyi Raman* * MD at time of publication
Inflammation self-perpetuates
Irritation of inner gallbladder wall/mucosa
↑ Gallbladder lumen pressure
Intraluminal pressure exceeds arterial pressure
↓ Blood flow to gallbladder
Gallbladder ischemia
Local inflammation, loss of gallbladder mucosal integrity
Bacterial invasionàtransmural inflammation of gallbladder
Without treatment, prolonged ischemia and inflammation of the gallbladder
Gallbladder gangrene (20%)
Gallbladder perforation (20%)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 4 2019, updated May 16 2022 on www.thecalgaryguide.com
Acute Cholecystitis: Pathogenesis and clinical findings
Gallstone blocks the cystic duct, backing up bile into the gallbladder
Gallstones causing physical trauma to gallbladder wall
Irritation of adjacent diaphragm, stimulates phrenic nerve (C3-C5)
Activates stretch receptors of visceral peritoneum, stimulates foregut autonomic nerves (T5-T8)
Inflammatory mediator (i.e. prostaglandin) release by gallbladder and systemic inflammatory response
Thickened gallbladder wall on ultrasound (gold standard test)
On inspiration, the diaphragm pushes the gallbladder downward
Irritation of parietal peritoneum, stimulates somatic nerves
↑ Permeability of vessels with systemic inflammation, which leak
fluid from the blood into the interstitial space
Radiating pain to the back and right shoulder
Dull, diffuse abdominal pain referred to the epigastric region
Fever, nausea/vomiting, tachycardia
Positive Murphy’s sign (pain upon palpation of right upper quadrant [RUQ] on inspiration)
Persistent RUQ pain, abdominal guarding and peritoneal signs
Dehydration
Authors: Yan Yu, Vina Fan Reviewers: Dean Percy, Mirna Matta, Crystal Liu, Ben Campbell Maitreyi Raman* * MD at time of publication
Inflammation self-perpetuates
Irritation of inner gallbladder wall/mucosa
↑ Gallbladder lumen pressure
Intraluminal pressure exceeds arterial pressure
↓ Blood flow to gallbladder
Gallbladder ischemia
Local inflammation, loss of gallbladder mucosal integrity
Bacterial invasionàtransmural inflammation of gallbladder
Without treatment, prolonged ischemia and inflammation of the gallbladder
Gallbladder gangrene (20%)
Gallbladder perforation (20%)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 4 2019, updated May 16 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Acute-Cholecystitis-2022.jpg)





























































![Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com
Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/10/Hypomagnesia.jpg)

























![Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/08/Hypocalcemia-Pathogenesis.jpg)









