SEARCH RESULTS FOR: Cirrhosis
Clinical Findings of Androgen Deficiency
![Yu, Yan - Androgen Deficiency - FINAL.pptx
Hypogonadism in Males:Clinical Findings of Androgen Deficiency? secretion volume from seminal vesicle and prostateAuthor: Yan YuReviewers:Peter VetereGillian GoobieHanan Bassyouni** MD at time of publicationLegend:Published June 18, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplications? effect of testosterone on the brain? Libido(sensitive, but less specific)? [testosterone] : [estrogen] ratio at the male breast? ejaculate volume(a sensitive and specific sign)Gynecomastia (palpable breast tissue, not fat, directly under nipple)Fatigue,low mood, irrtabilityHot flashes, sweats(Can be nocturnal; occur only when hypogonadism is severe)Vasomotor neural response of unknown causeFewer spontaneous erections (i.e. in the morning)Lack of androgens (i.e. testosterone, DHT) in men past the age of pubertyIn advanced stages of the disease, after years of hypogonadism:(thus, less commonly seen)Low Bone Mass Density (BMD)Less testosterone to be converted into estrogen in bone? muscle bulk and strengthSmall, soft testicles(<4cm long on orchidometer)Lack of hormones to stimulate and maintain testicular hyperplasia/growthLoss of androgenic hair (on face, midline, and pubic area)Vertebral fracture (height loss), or other fragility fracturesIf sexual development is incomplete from puberty:Note: These clinical findings apply to many disorders, including:-Andropause-Hypopituitarism (suspect if other hormone abnormalities & Sx of mass lesion like visual field loss, diplopia, and headache exist)-Testicular Failure (if Hx of chemo, radiation, excess alcohol, and chronic liver disease)-Klinefelter's (if assoc. tall and eunuchoid stature, breast enlargement and cognitive deficiency - XXY)-Kallman's (if assoc. anosmia, and tall/eunuchoid stature)-Drugs (e.g. ketoconazole, anabolic steroids, spironolactone, digoxin, marijuana)Testosterone's inhibitory effect on estrogen is not enough to prevent breast growthDeficiency in testosterone during puberty delays fusion of epiphysesTall, eunuchoid statureNote: any disease involving an increase in aromatase activity (hyperthyroidism, cirrhosis, HCG-secreting tumors) will also cause relative estrogen excess & subsequent gynecomastia.
111 kB / 272 words Yu, Yan - Androgen Deficiency - FINAL.pptx
Hypogonadism in Males:Clinical Findings of Androgen Deficiency? secretion volume from seminal vesicle and prostateAuthor: Yan YuReviewers:Peter VetereGillian GoobieHanan Bassyouni** MD at time of publicationLegend:Published June 18, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplications? effect of testosterone on the brain? Libido(sensitive, but less specific)? [testosterone] : [estrogen] ratio at the male breast? ejaculate volume(a sensitive and specific sign)Gynecomastia (palpable breast tissue, not fat, directly under nipple)Fatigue,low mood, irrtabilityHot flashes, sweats(Can be nocturnal; occur only when hypogonadism is severe)Vasomotor neural response of unknown causeFewer spontaneous erections (i.e. in the morning)Lack of androgens (i.e. testosterone, DHT) in men past the age of pubertyIn advanced stages of the disease, after years of hypogonadism:(thus, less commonly seen)Low Bone Mass Density (BMD)Less testosterone to be converted into estrogen in bone? muscle bulk and strengthSmall, soft testicles(<4cm long on orchidometer)Lack of hormones to stimulate and maintain testicular hyperplasia/growthLoss of androgenic hair (on face, midline, and pubic area)Vertebral fracture (height loss), or other fragility fracturesIf sexual development is incomplete from puberty:Note: These clinical findings apply to many disorders, including:-Andropause-Hypopituitarism (suspect if other hormone abnormalities & Sx of mass lesion like visual field loss, diplopia, and headache exist)-Testicular Failure (if Hx of chemo, radiation, excess alcohol, and chronic liver disease)-Klinefelter's (if assoc. tall and eunuchoid stature, breast enlargement and cognitive deficiency - XXY)-Kallman's (if assoc. anosmia, and tall/eunuchoid stature)-Drugs (e.g. ketoconazole, anabolic steroids, spironolactone, digoxin, marijuana)Testosterone's inhibitory effect on estrogen is not enough to prevent breast growthDeficiency in testosterone during puberty delays fusion of epiphysesTall, eunuchoid statureNote: any disease involving an increase in aromatase activity (hyperthyroidism, cirrhosis, HCG-secreting tumors) will also cause relative estrogen excess & subsequent gynecomastia.
111 kB / 272 words](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Clinical-Findings-of-Androgen-Deficiency.jpg)
cirrhosis
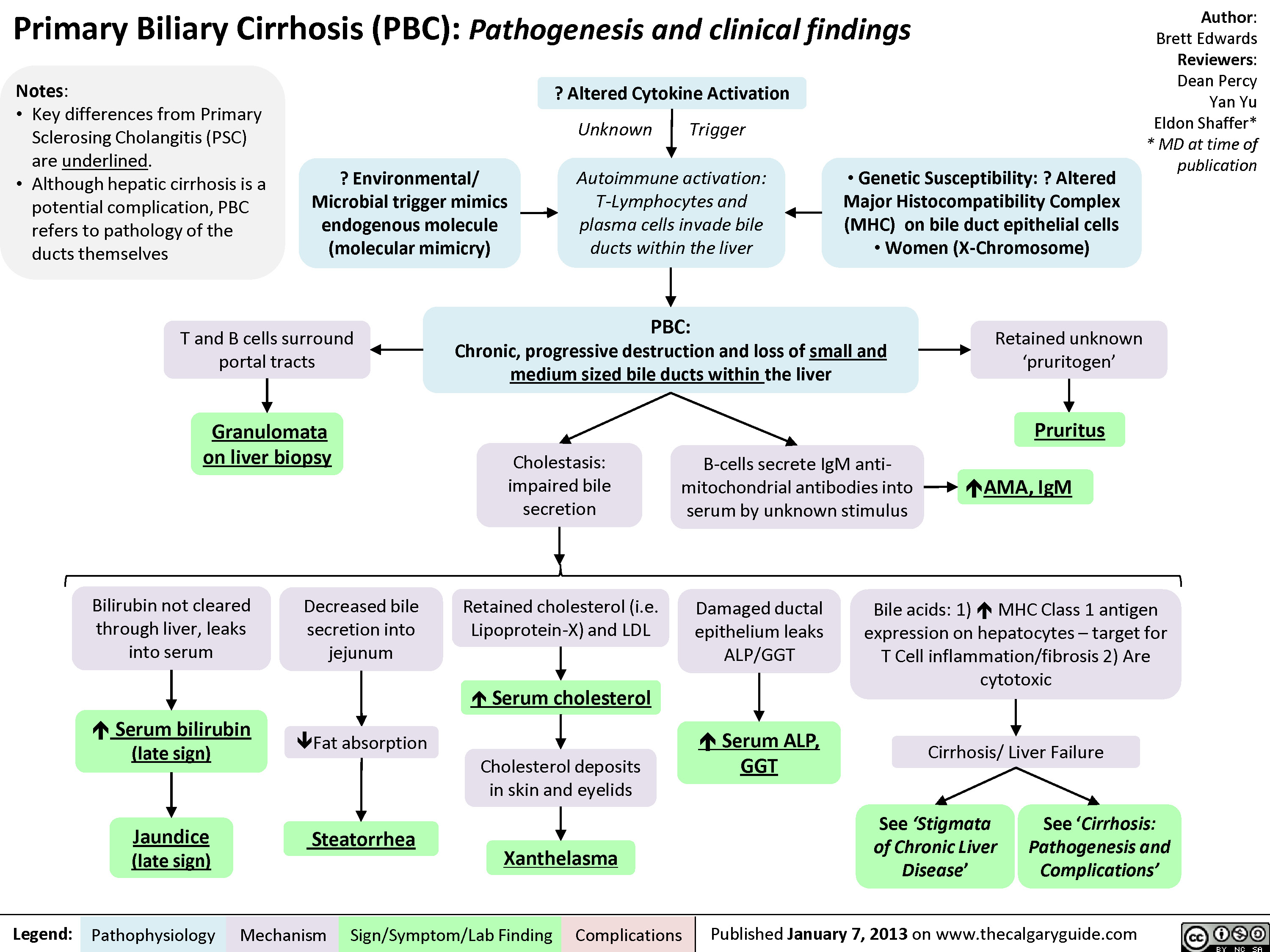
Primary Biliary Cirrhosis (PBC)
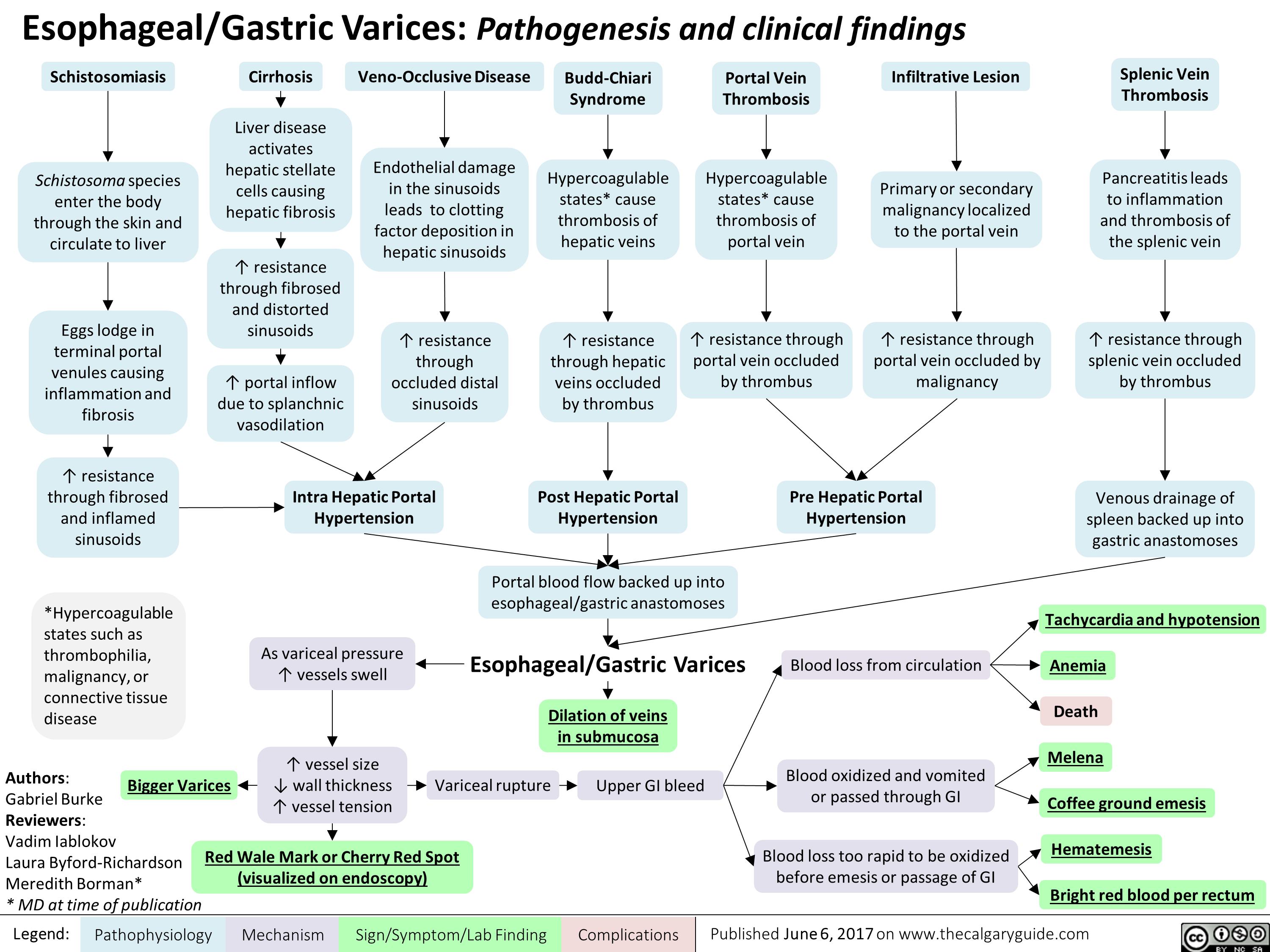
esophageal-gastric-varices
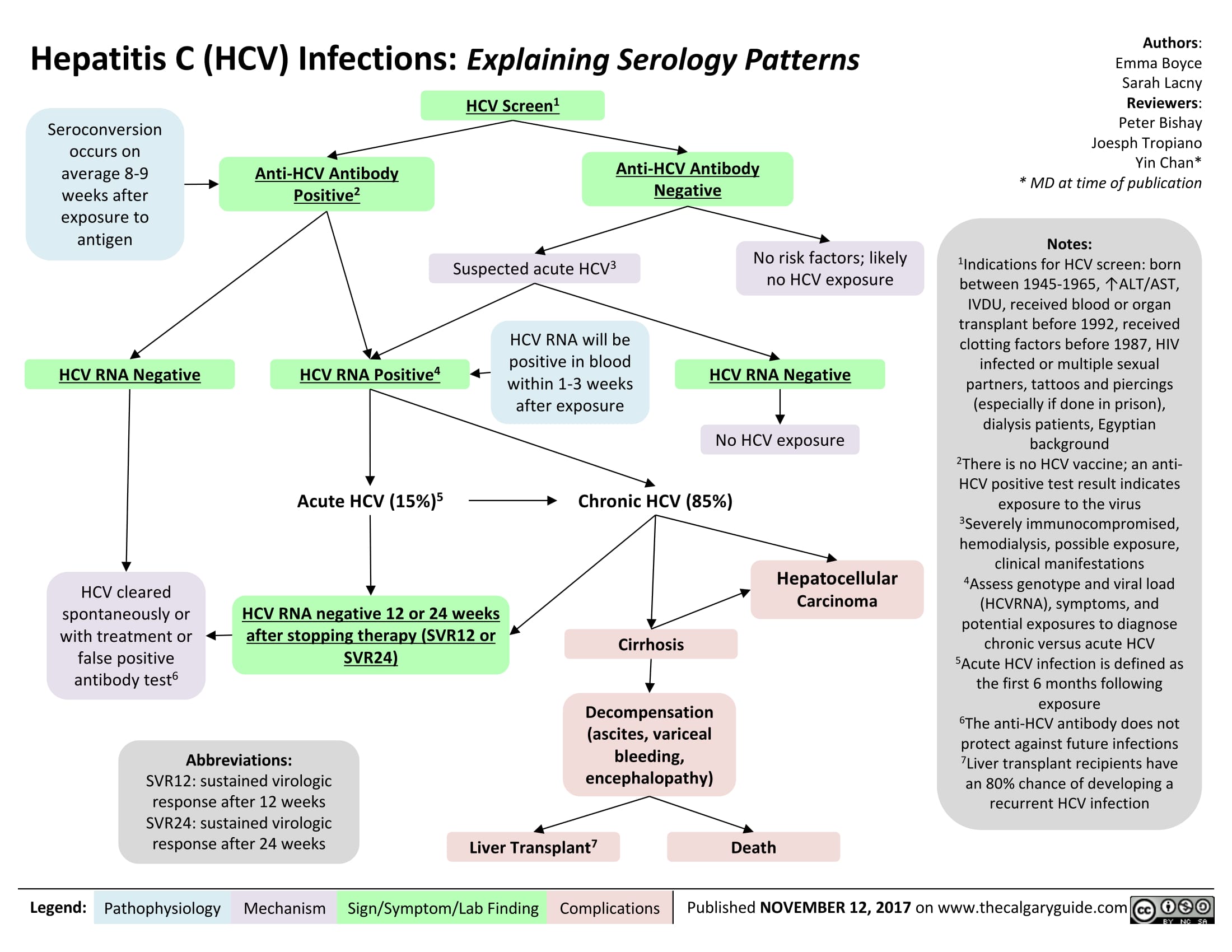
Hepatitis C (HCV) Infections: Explaining Serology Patterns
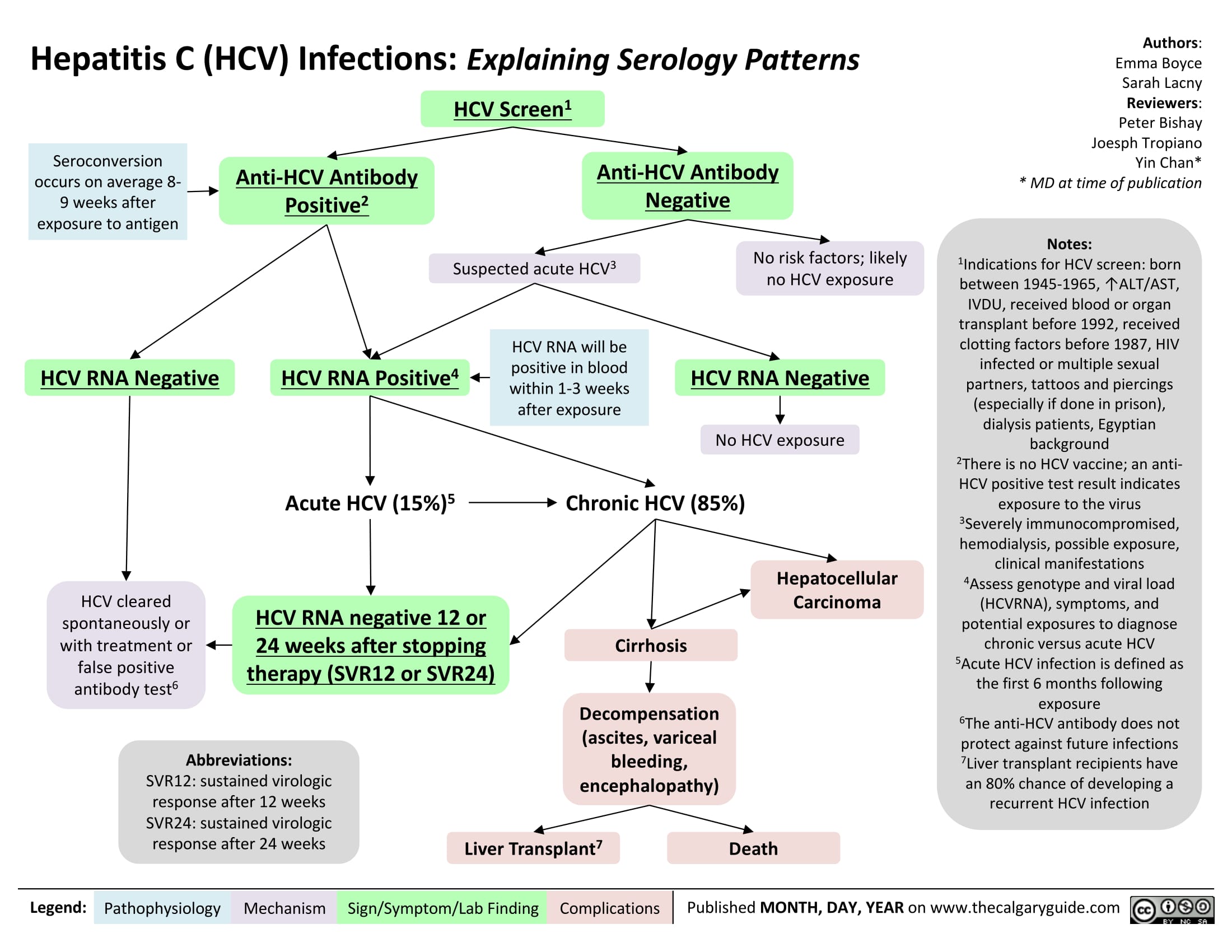
Hepatitis C (HCV) Infection: Explaining Serology Patterns
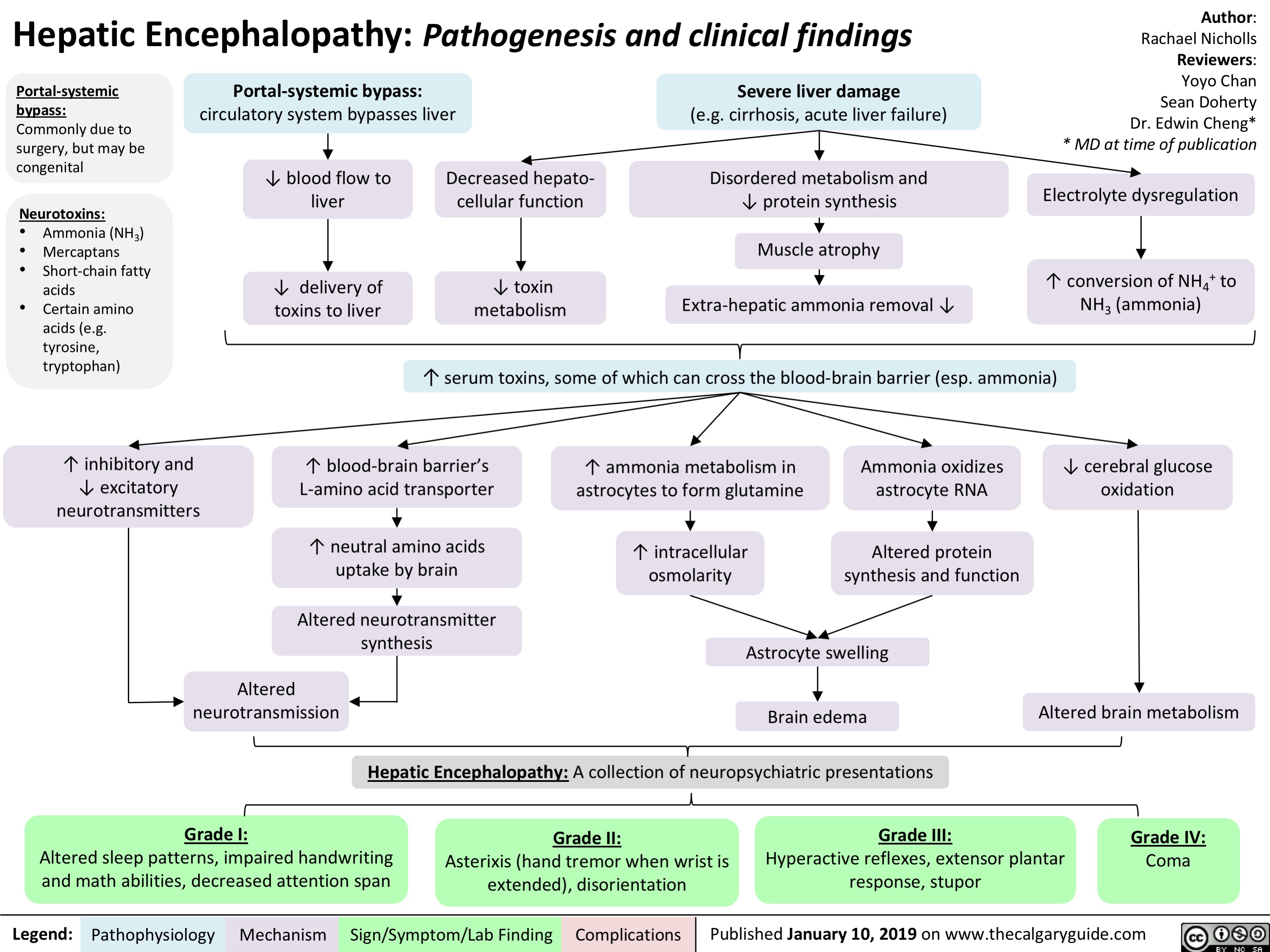
Hepatic Encephalopathy: Pathogenesis and Clinical Findings
Hyponatremia- Physiology
![Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hyponatremia-Physiology-.jpg)
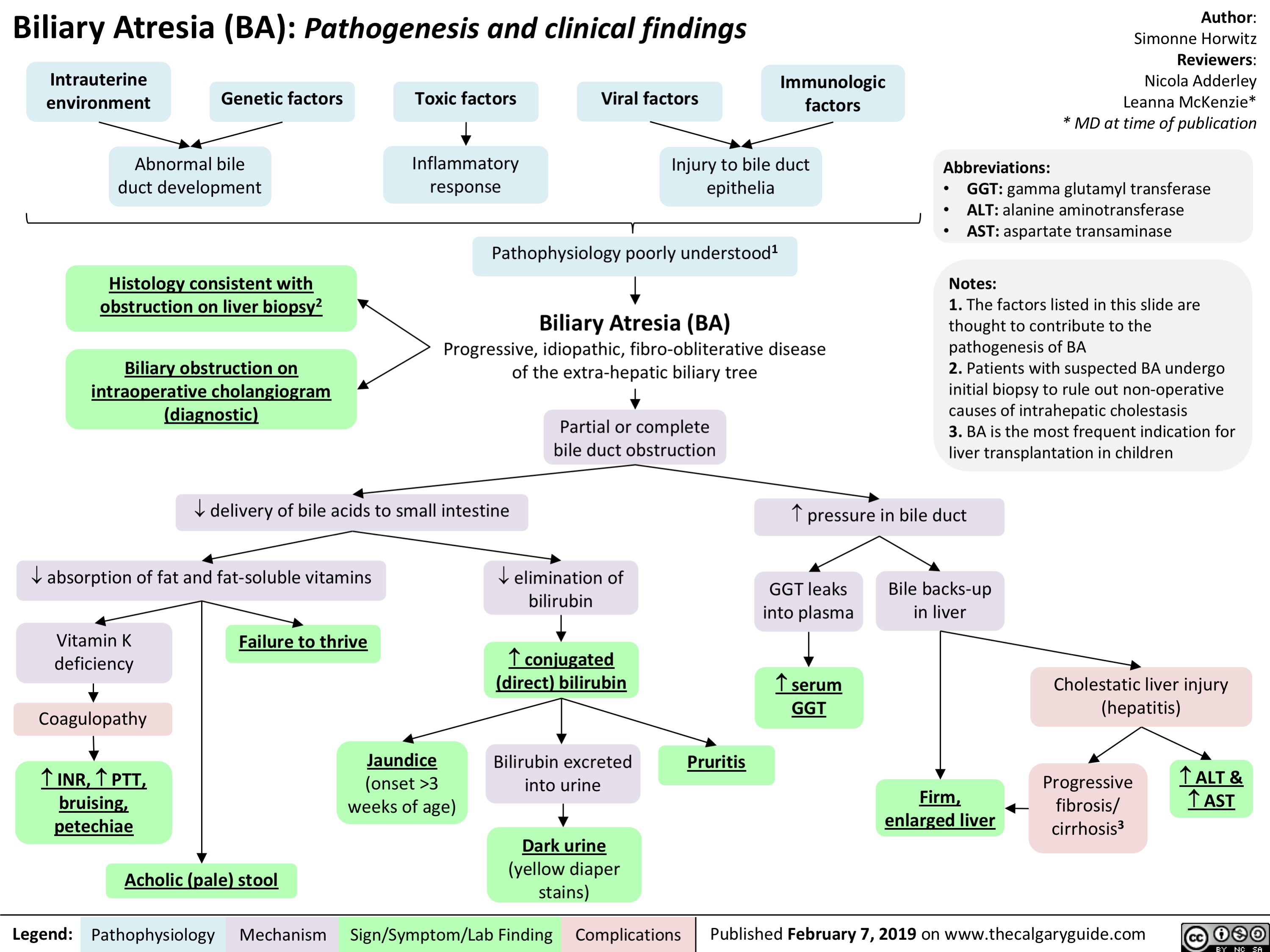
Biliary Atresia (BA)- Pathogenesis and clinical findings
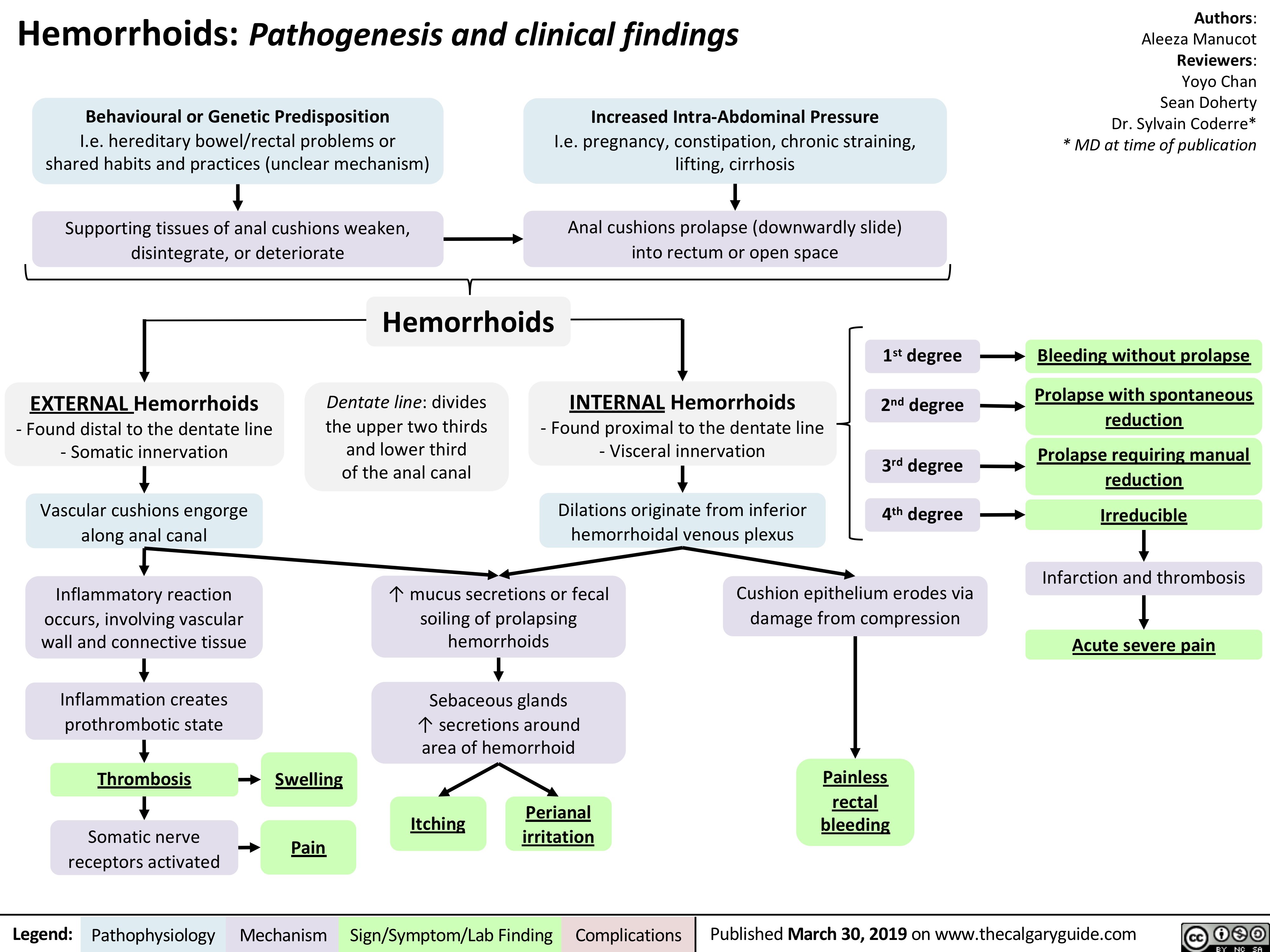
Hemorrhoids - Pathogenesis and Clinical Findings
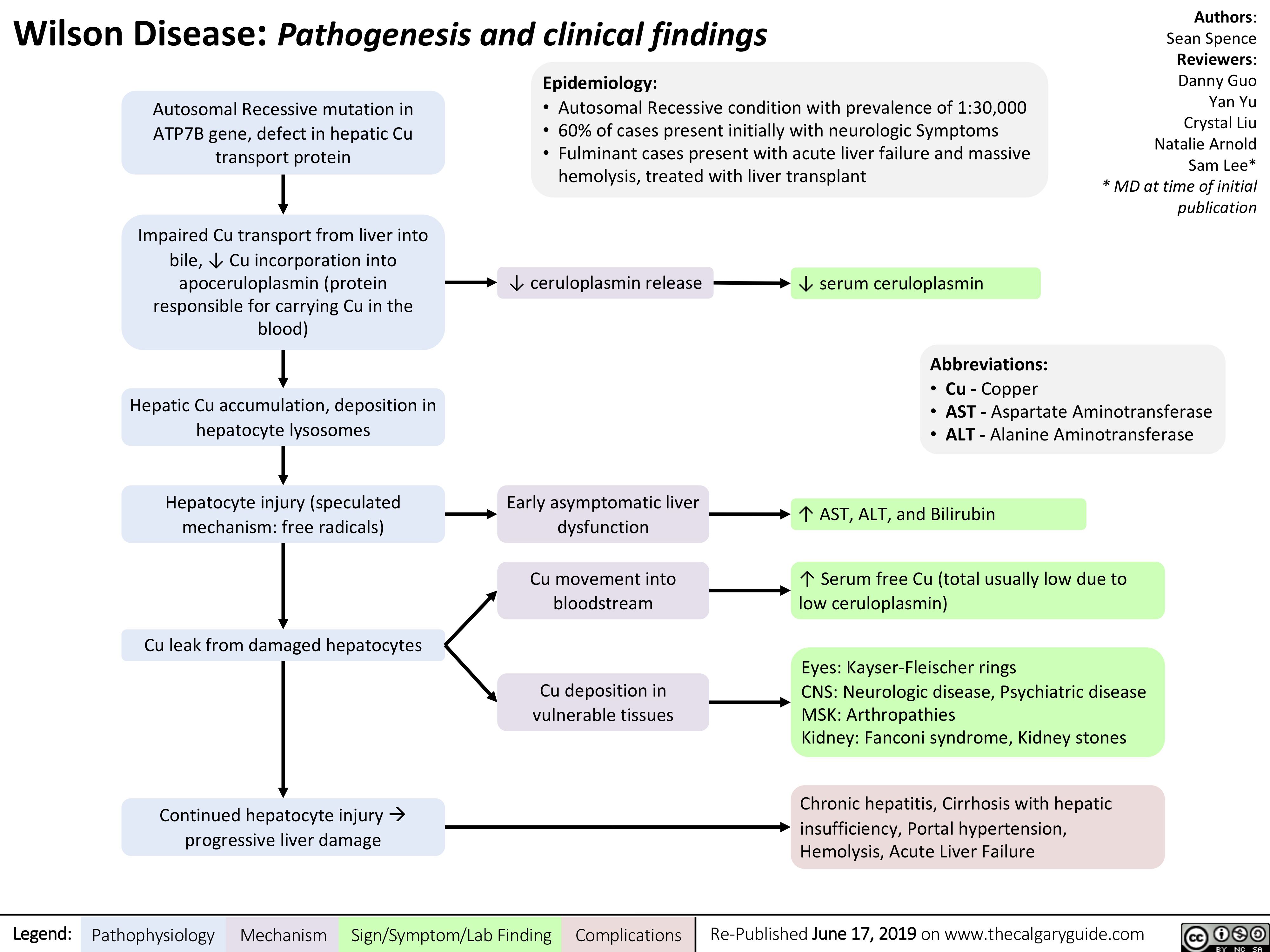
Wilson's Disease
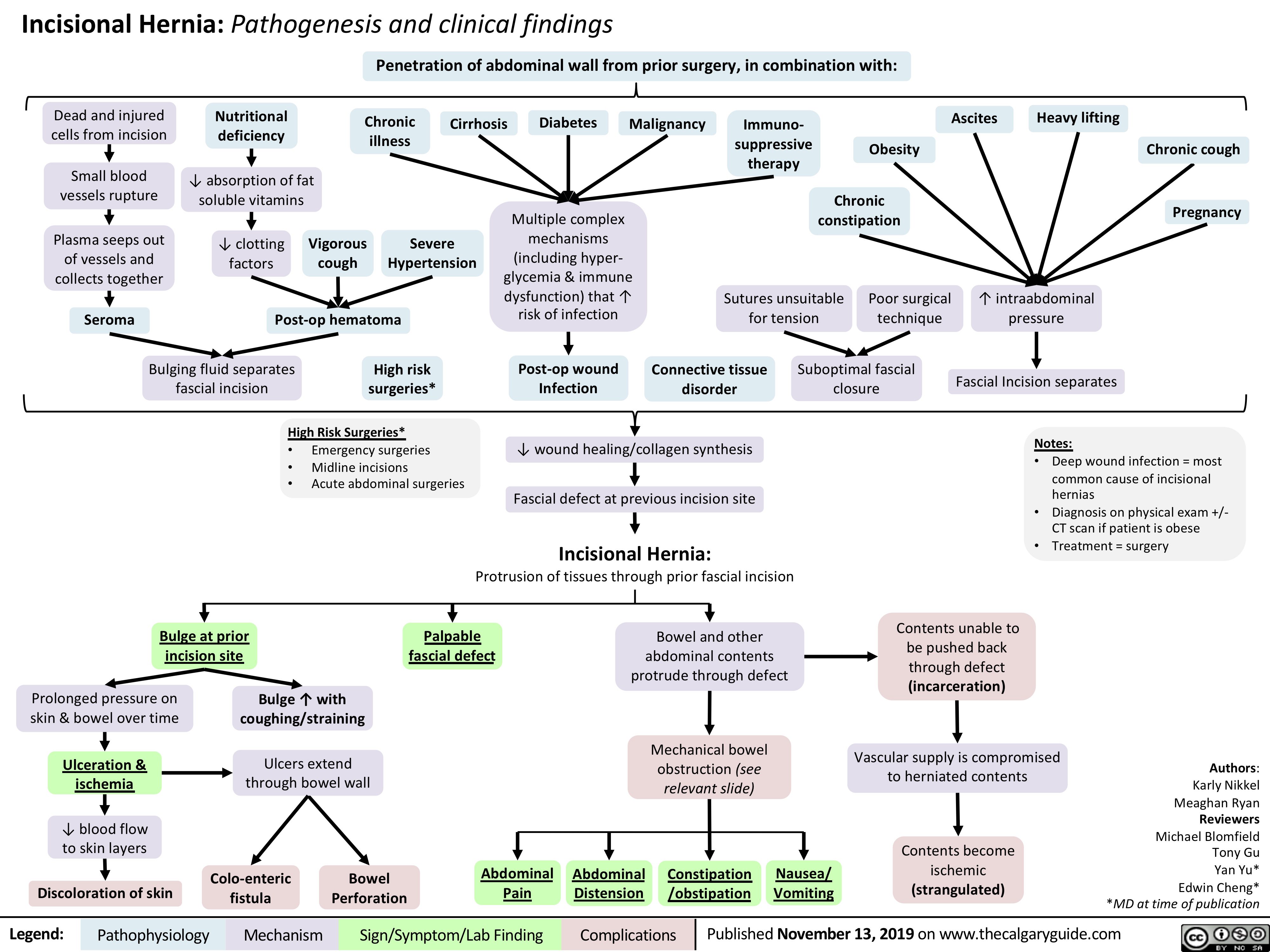
Incisional-Hernia
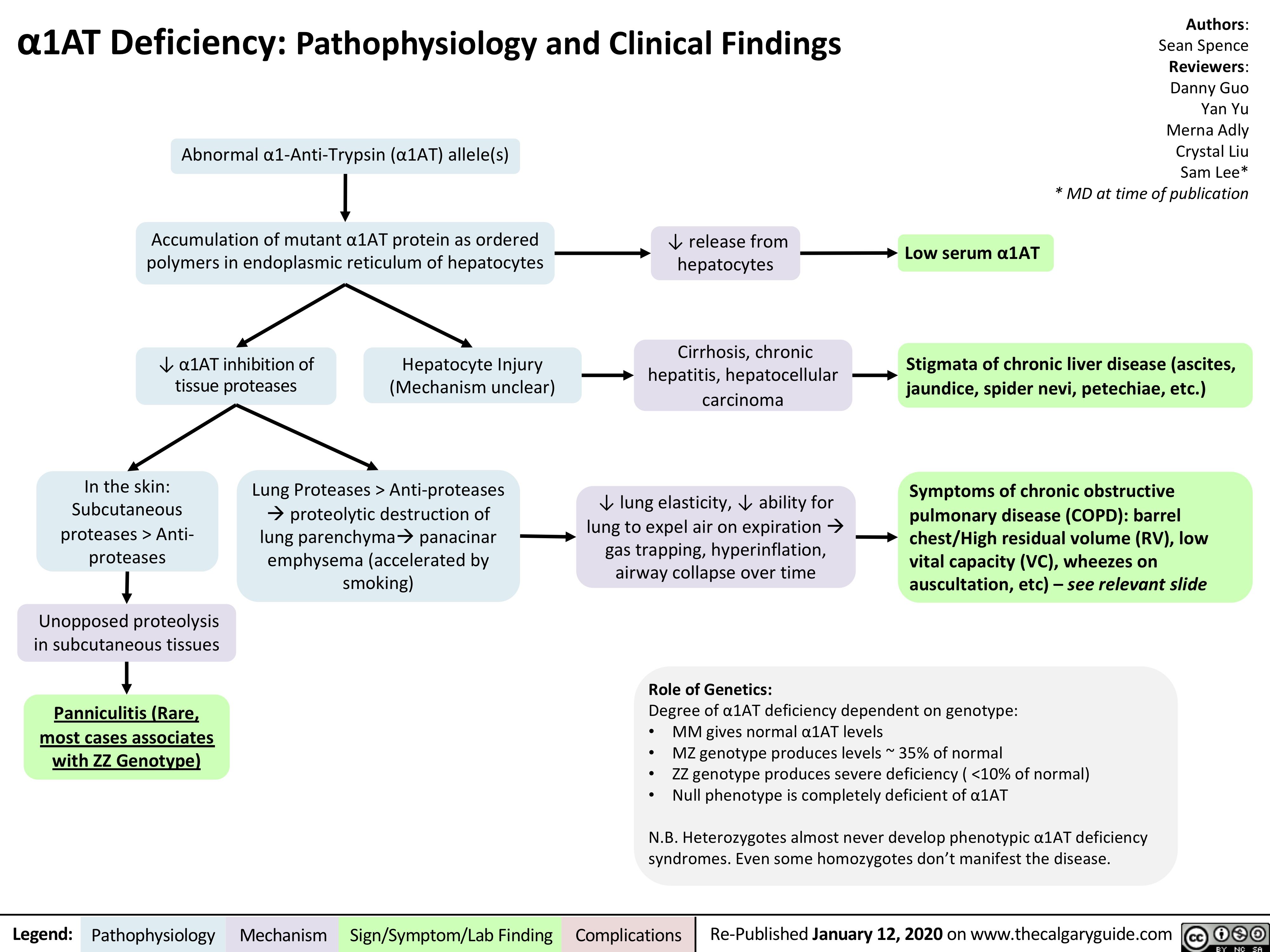
A1AT-Deficiency
Viral-Hepatitis
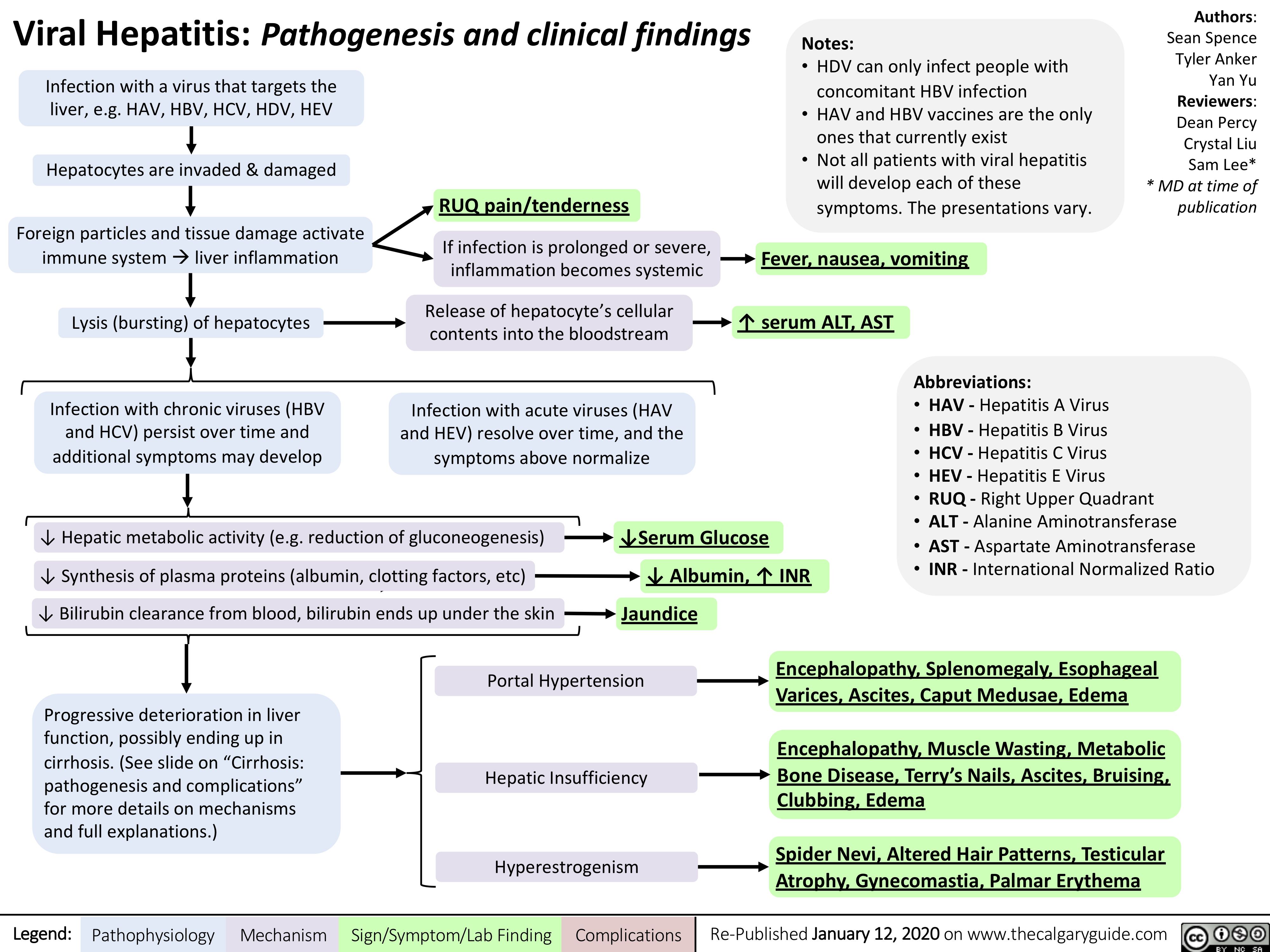
leberzirrhose-pathogenese-und-komplikationen
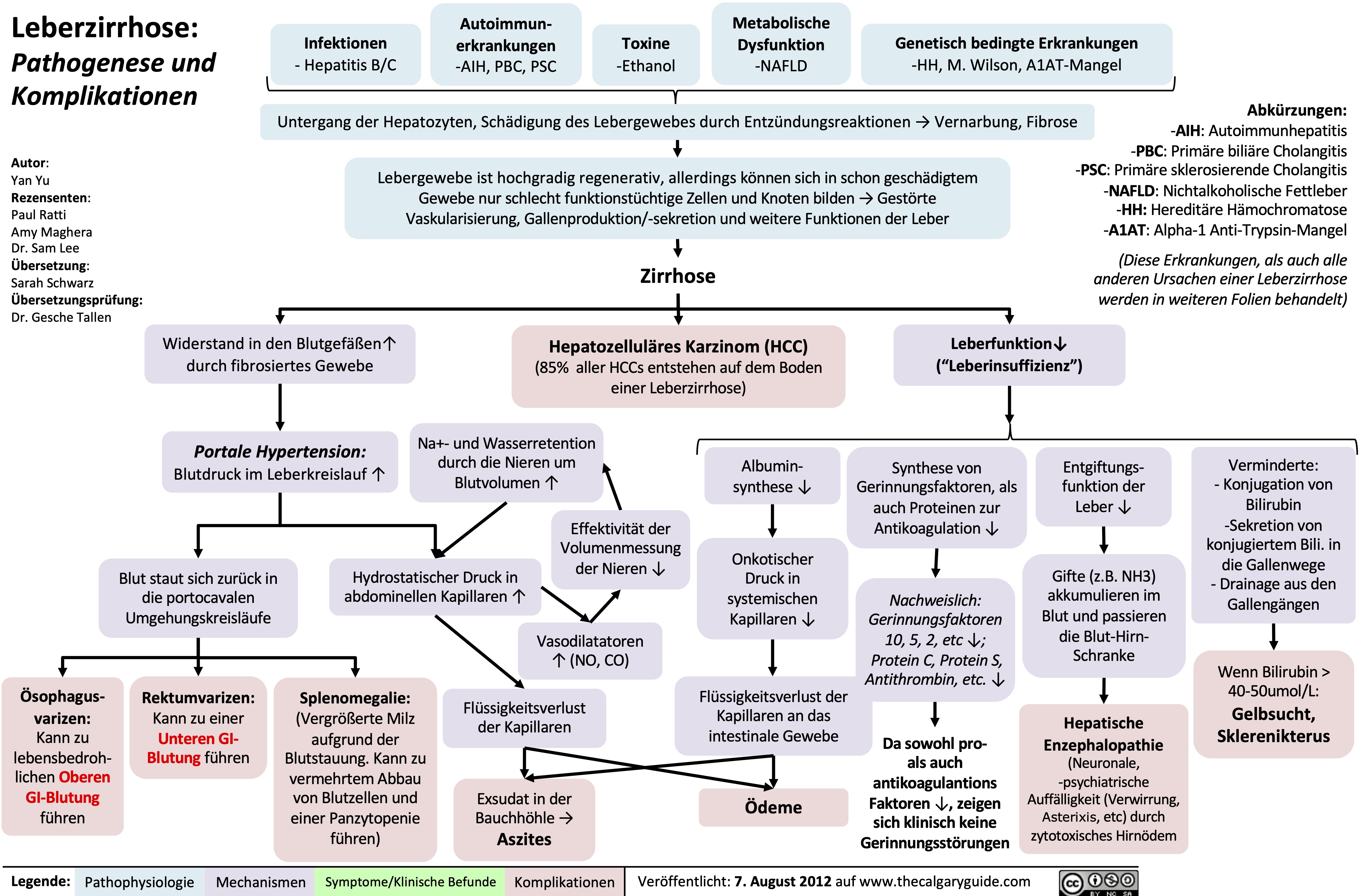
NSAIDs and the Kidney mechanism of action and side effects
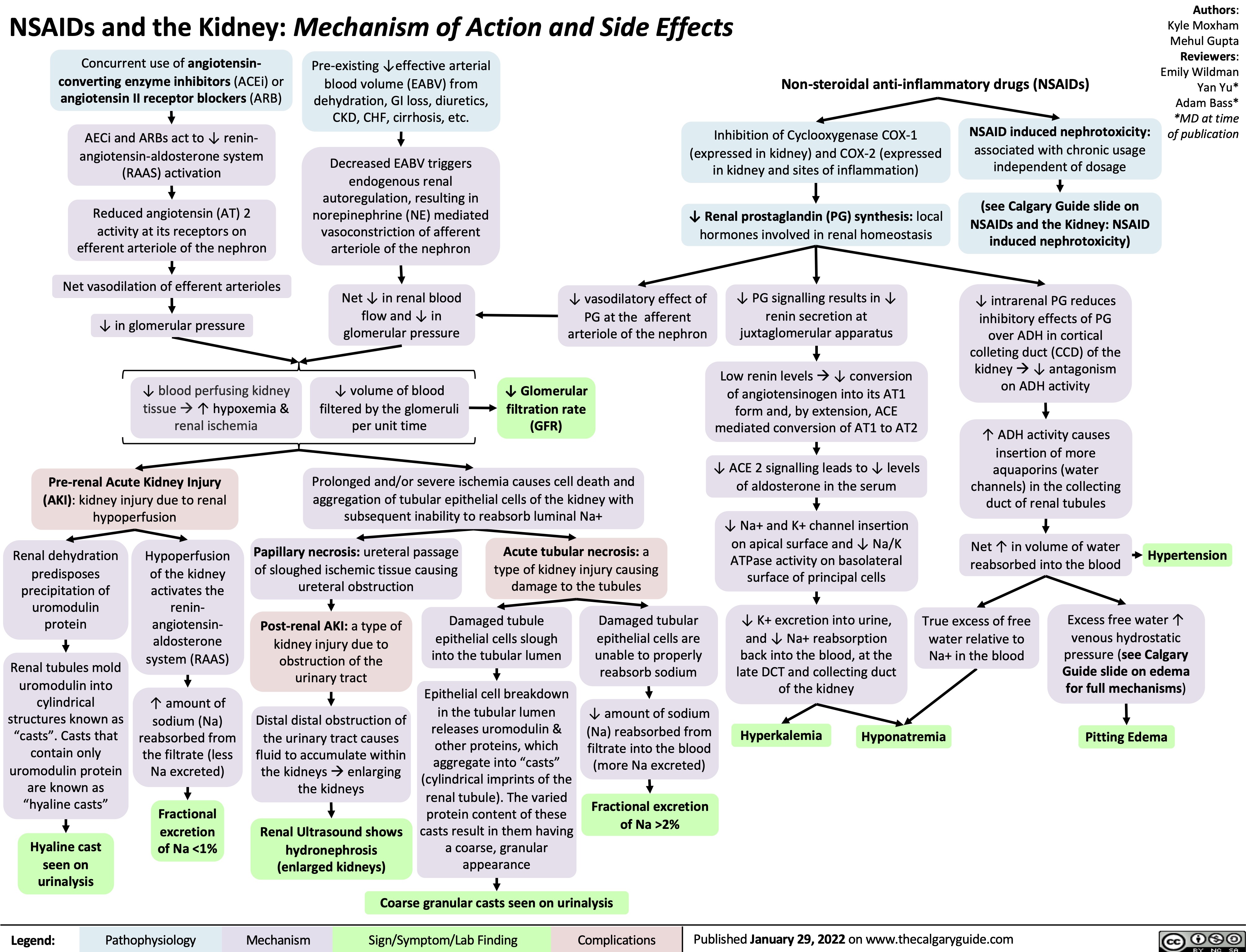
clostridium-difficile-infection-pathogenesis-and-clinical-findings
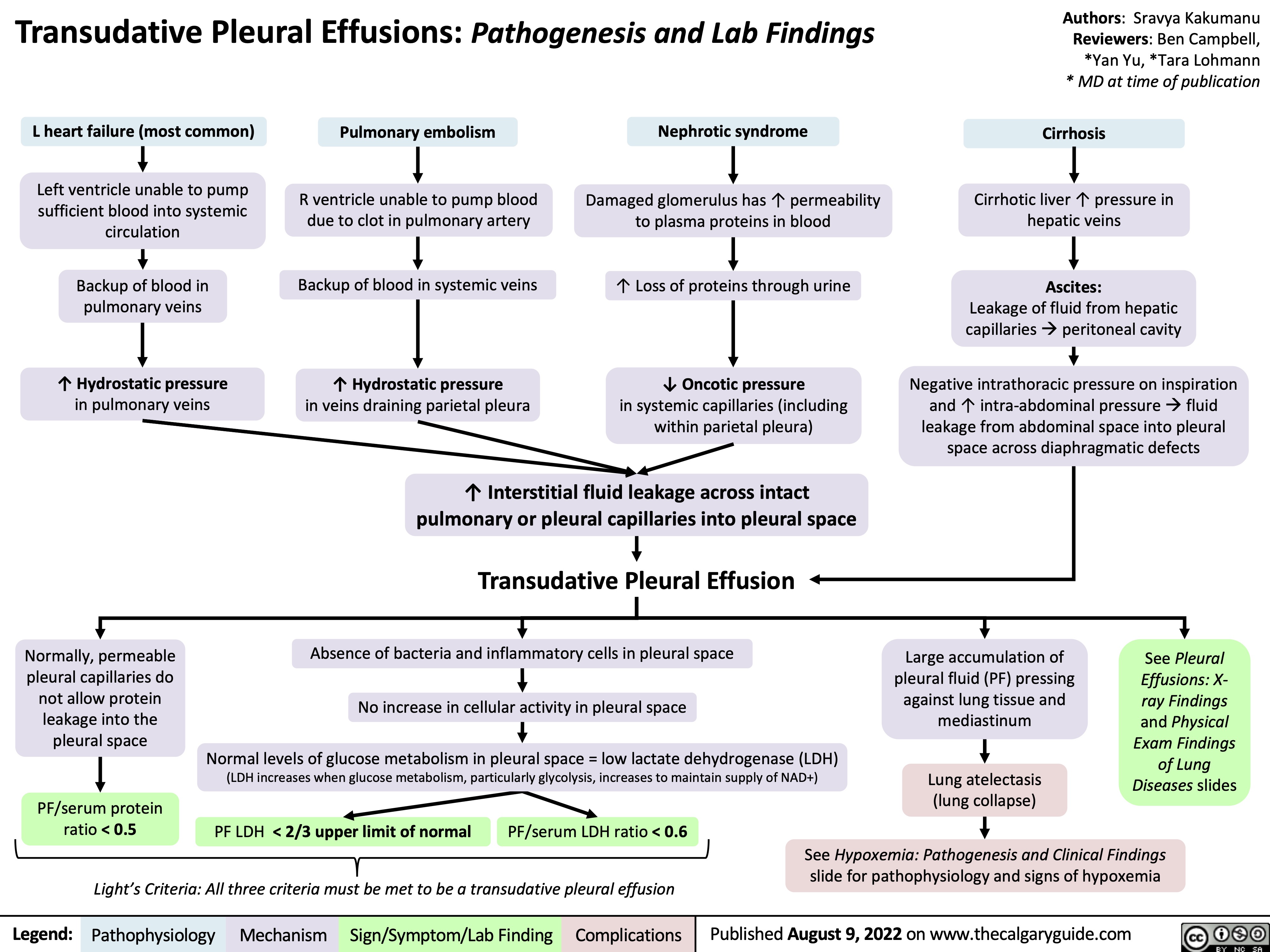
transudative-pleural-effusions-pathogenesis-and-lab-findings
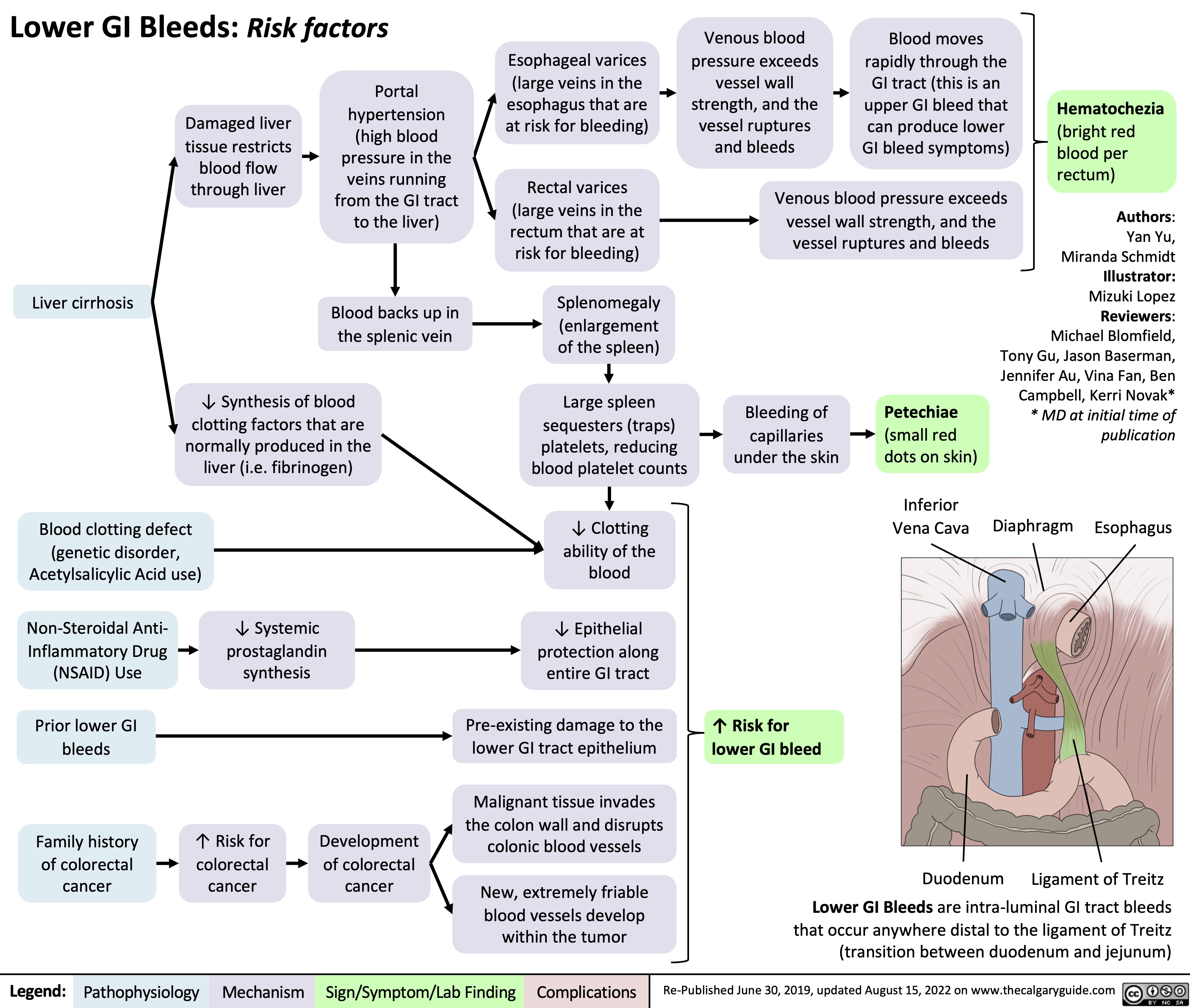
lower-gi-bleed-risk-factors
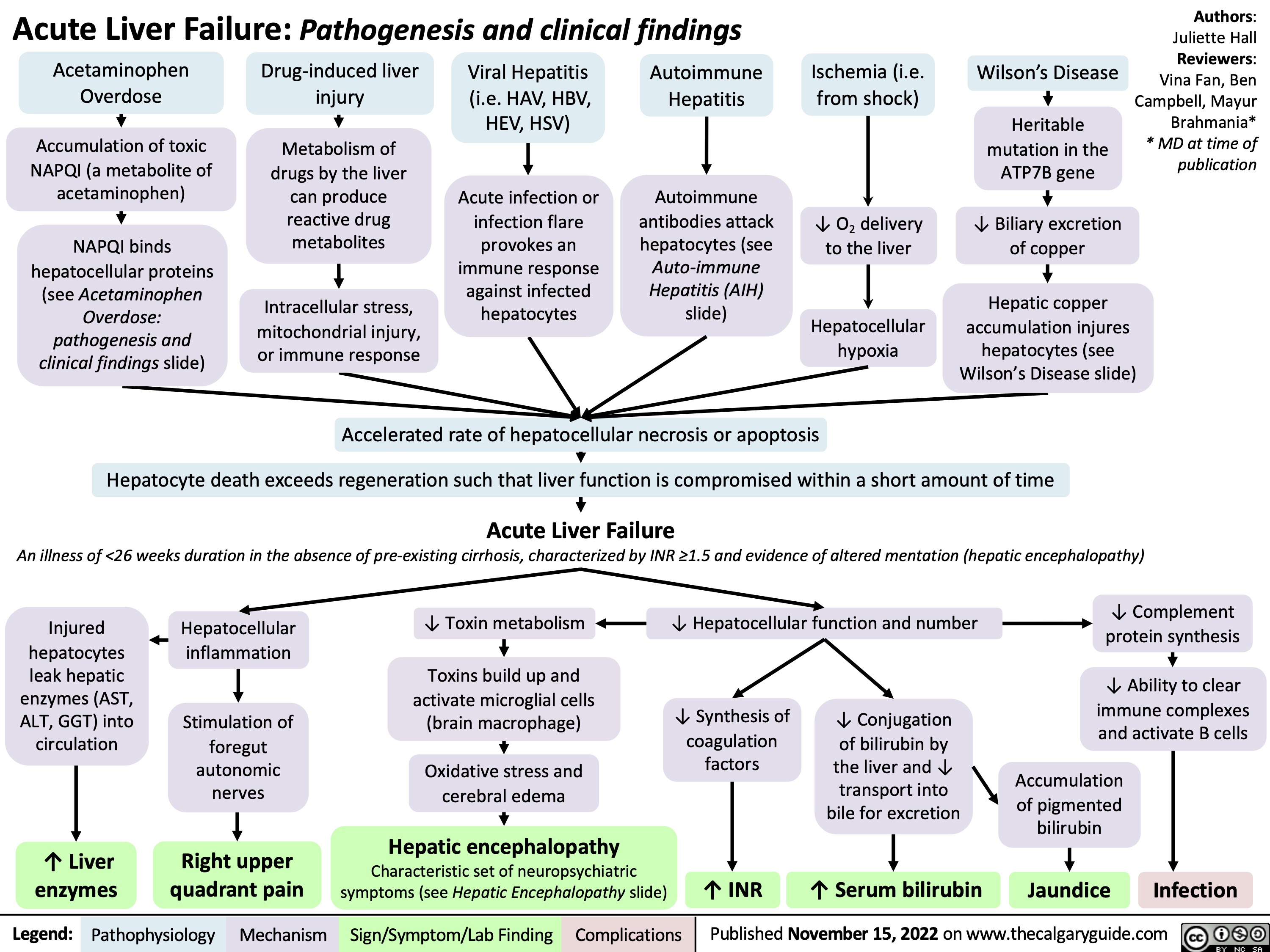
Acute Liver Failure: Pathogenesis and clinical findings
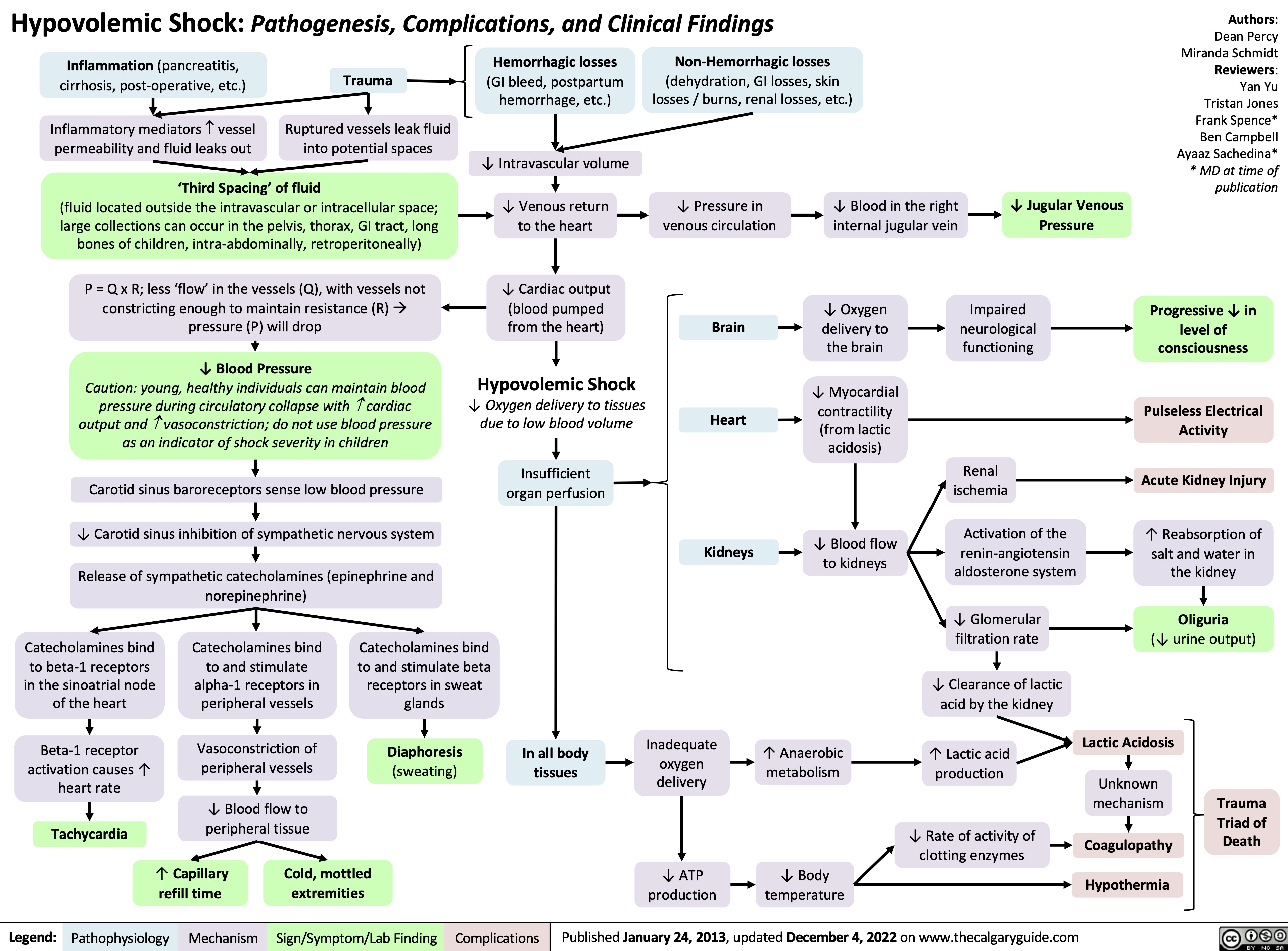
hypovolemic-shock
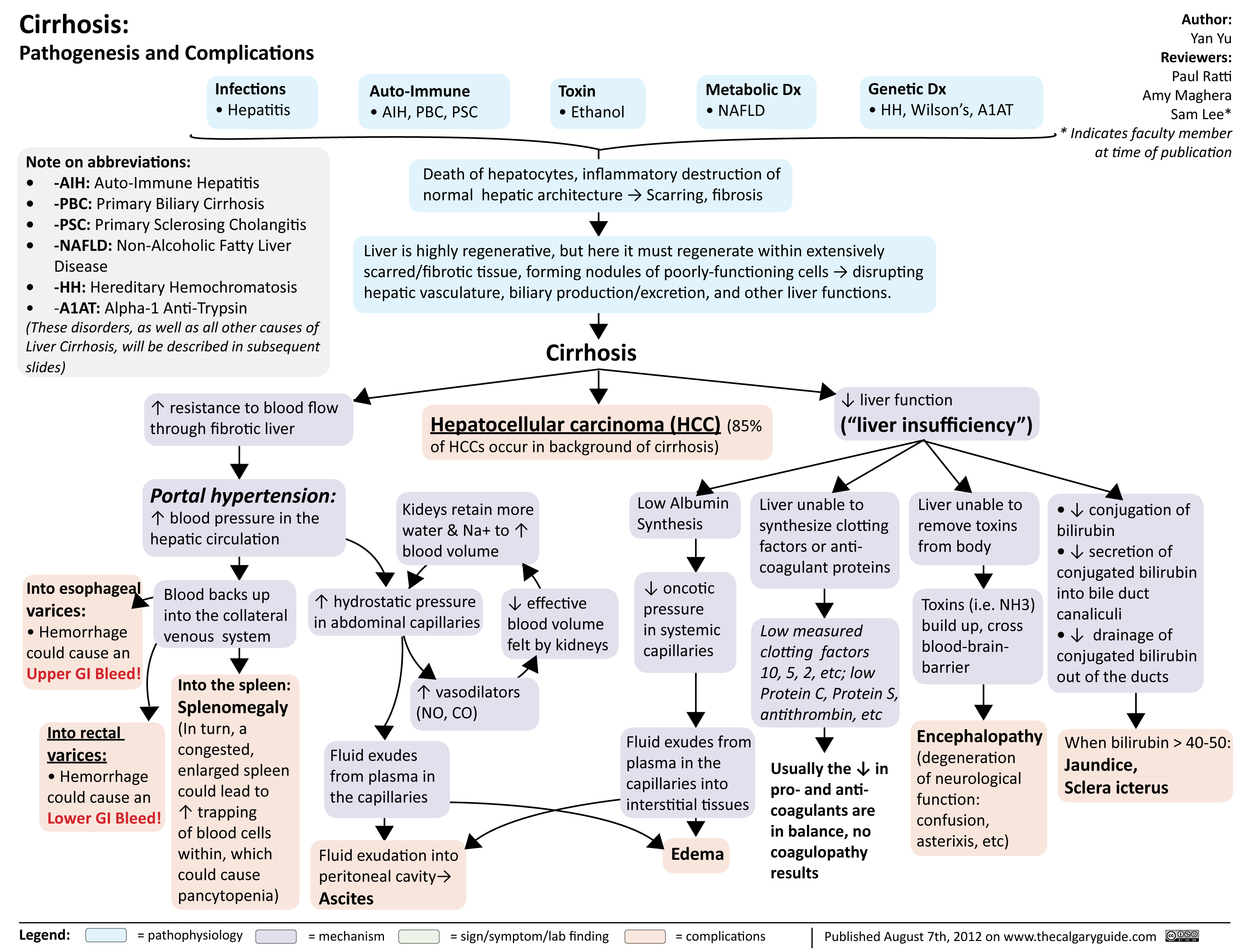
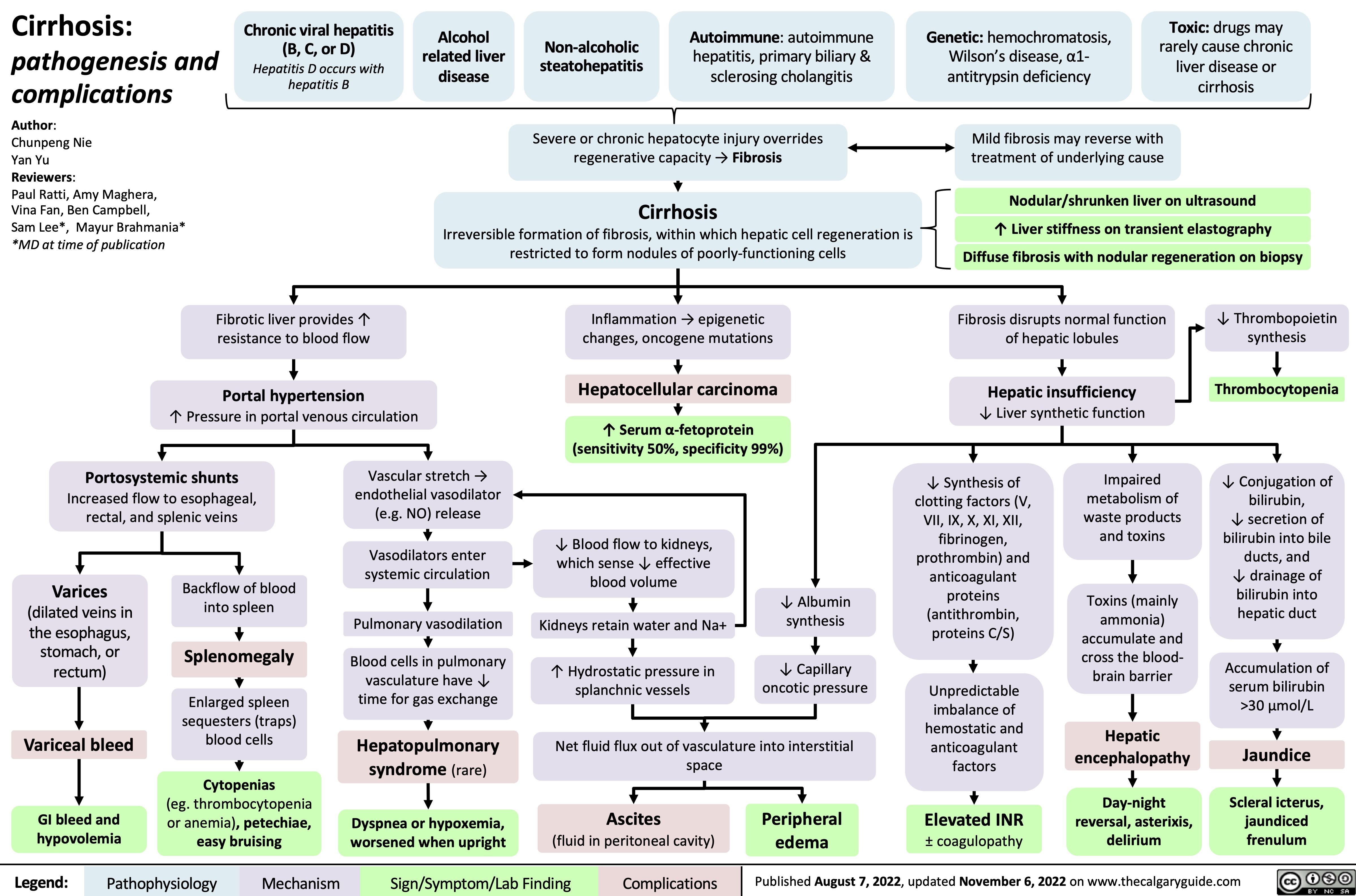
Cirrhosis
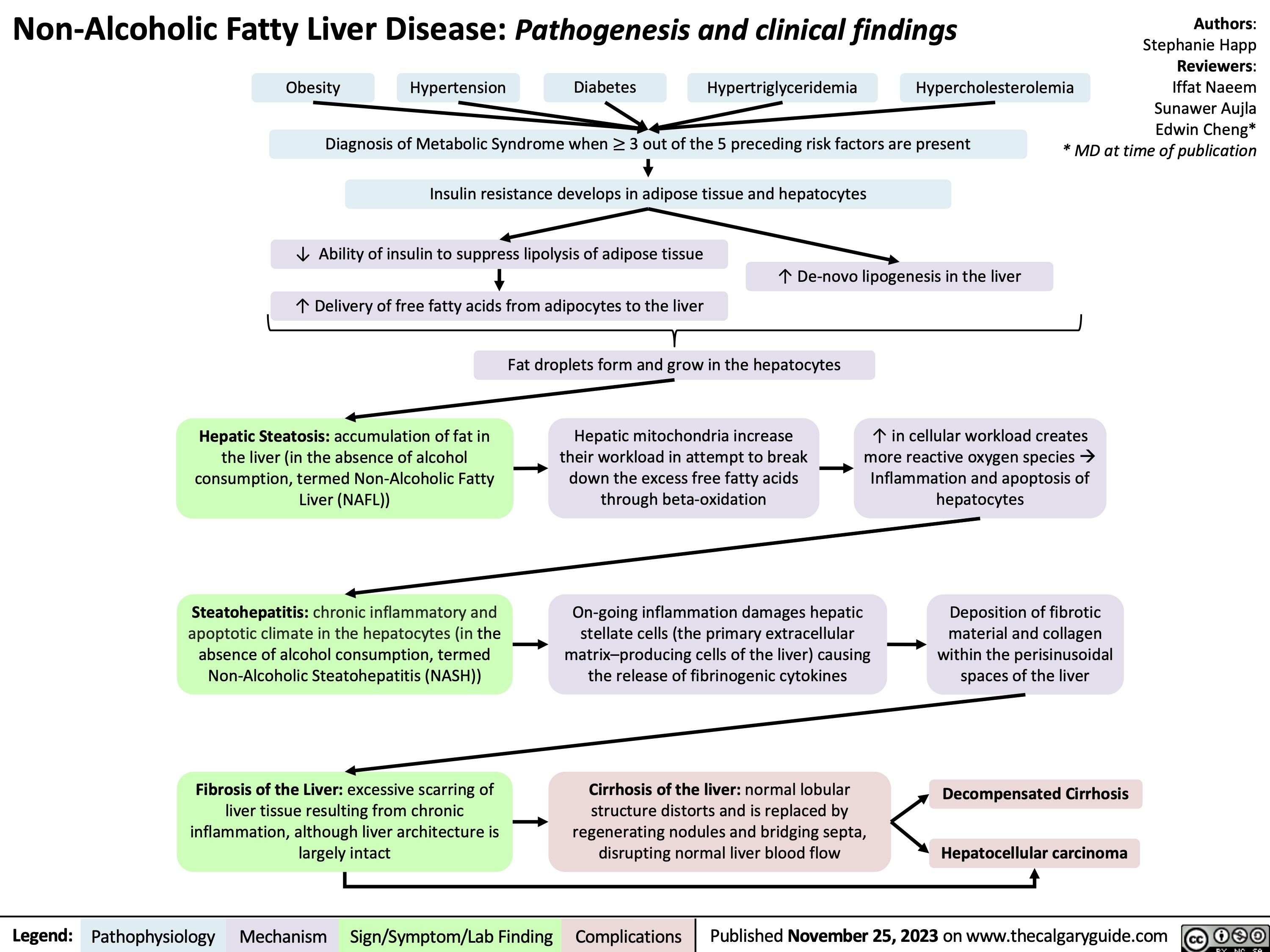
Non-Alcoholic Fatty Liver Disease
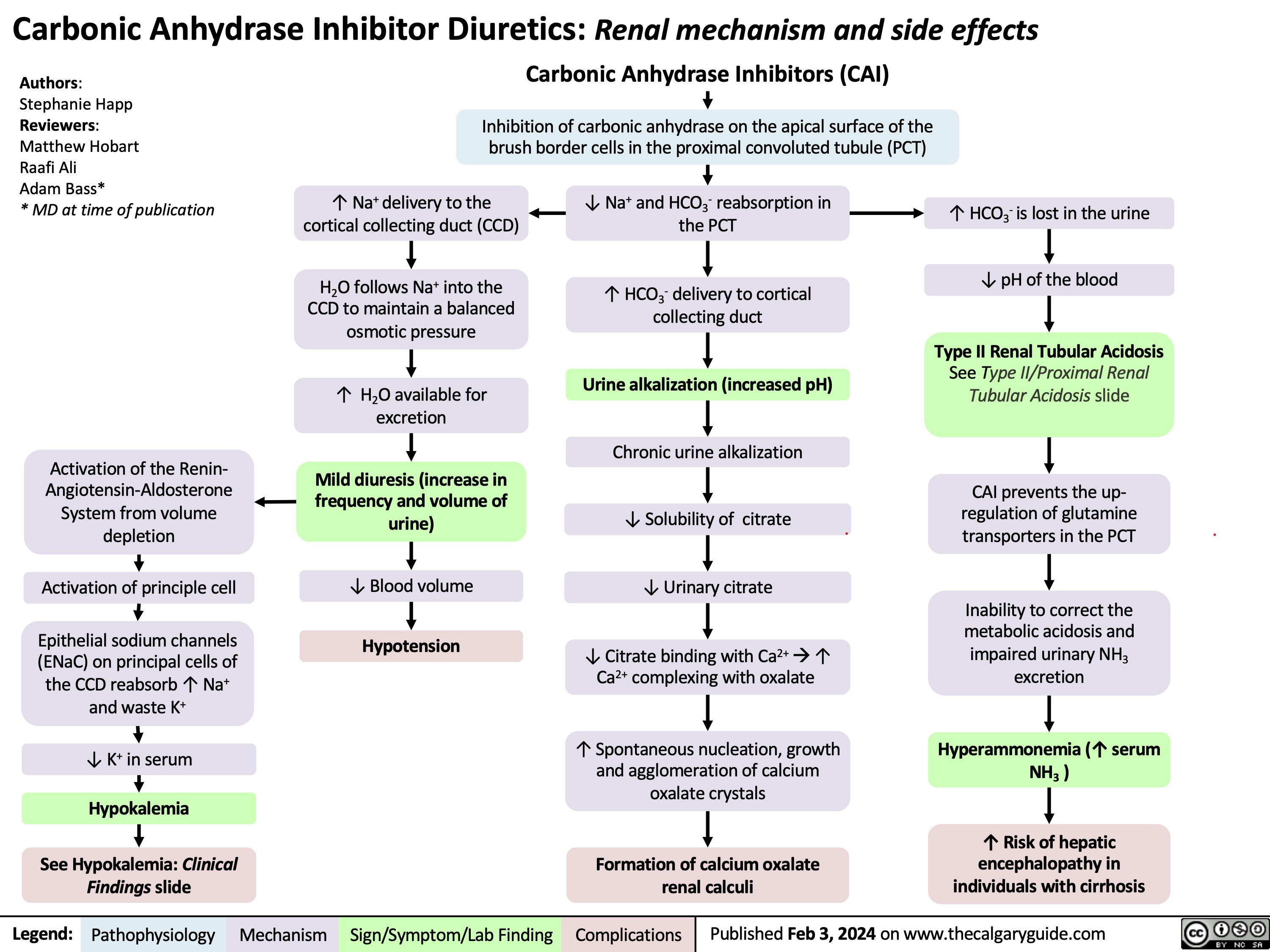
Carbonic Anhydrase Inhibitor Diuretics
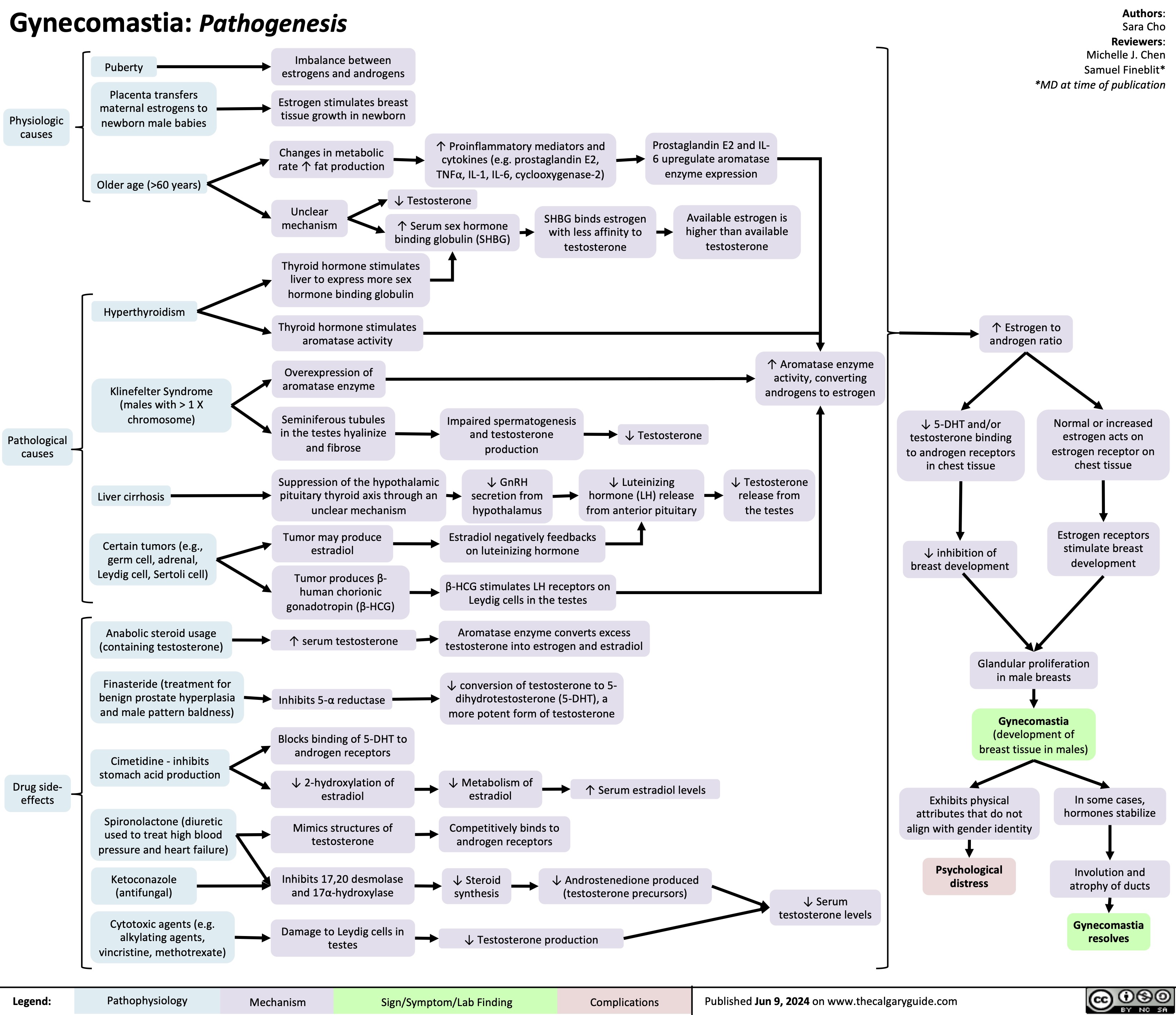
Gynecomastia
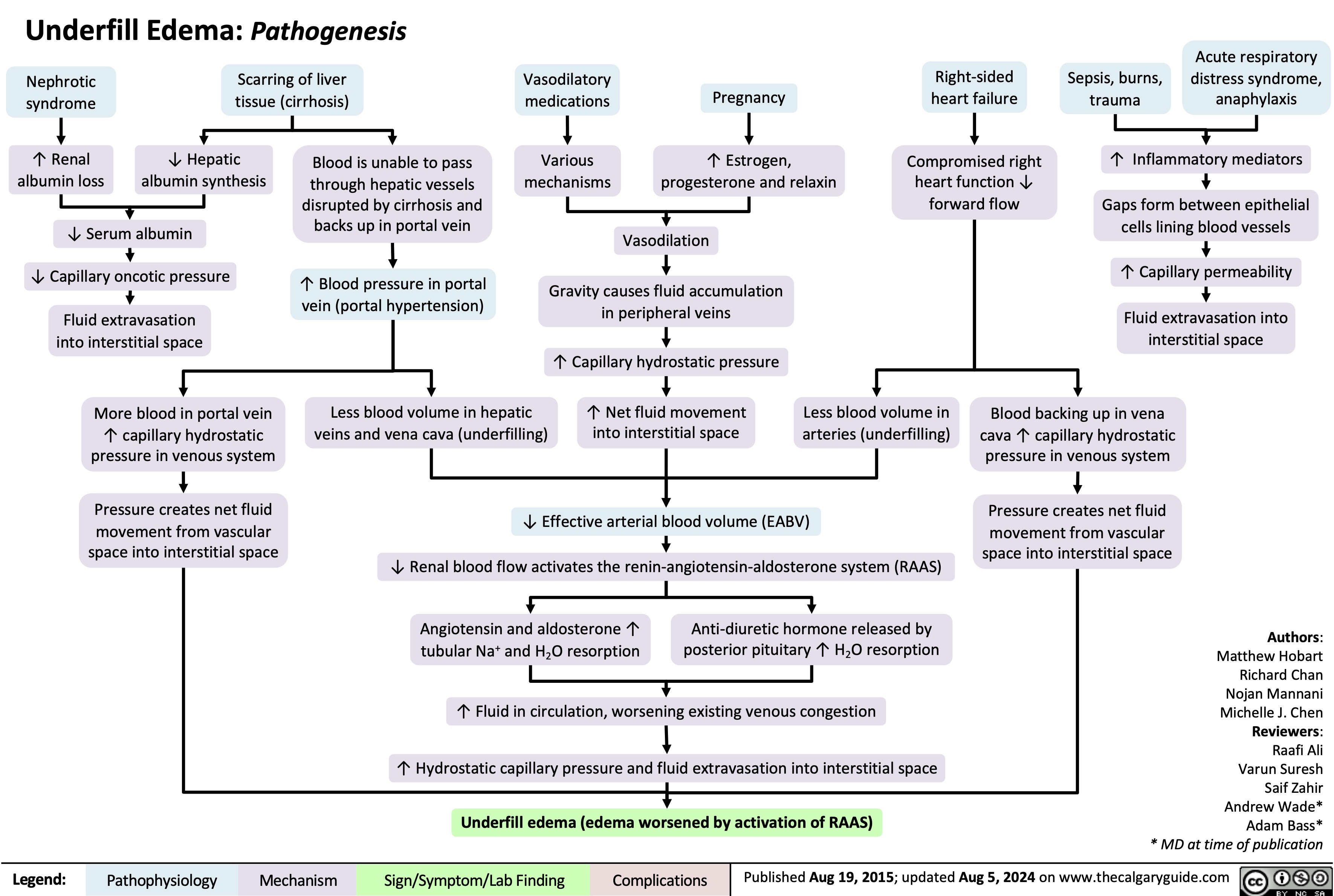
Underfill Edema Pathogenesis
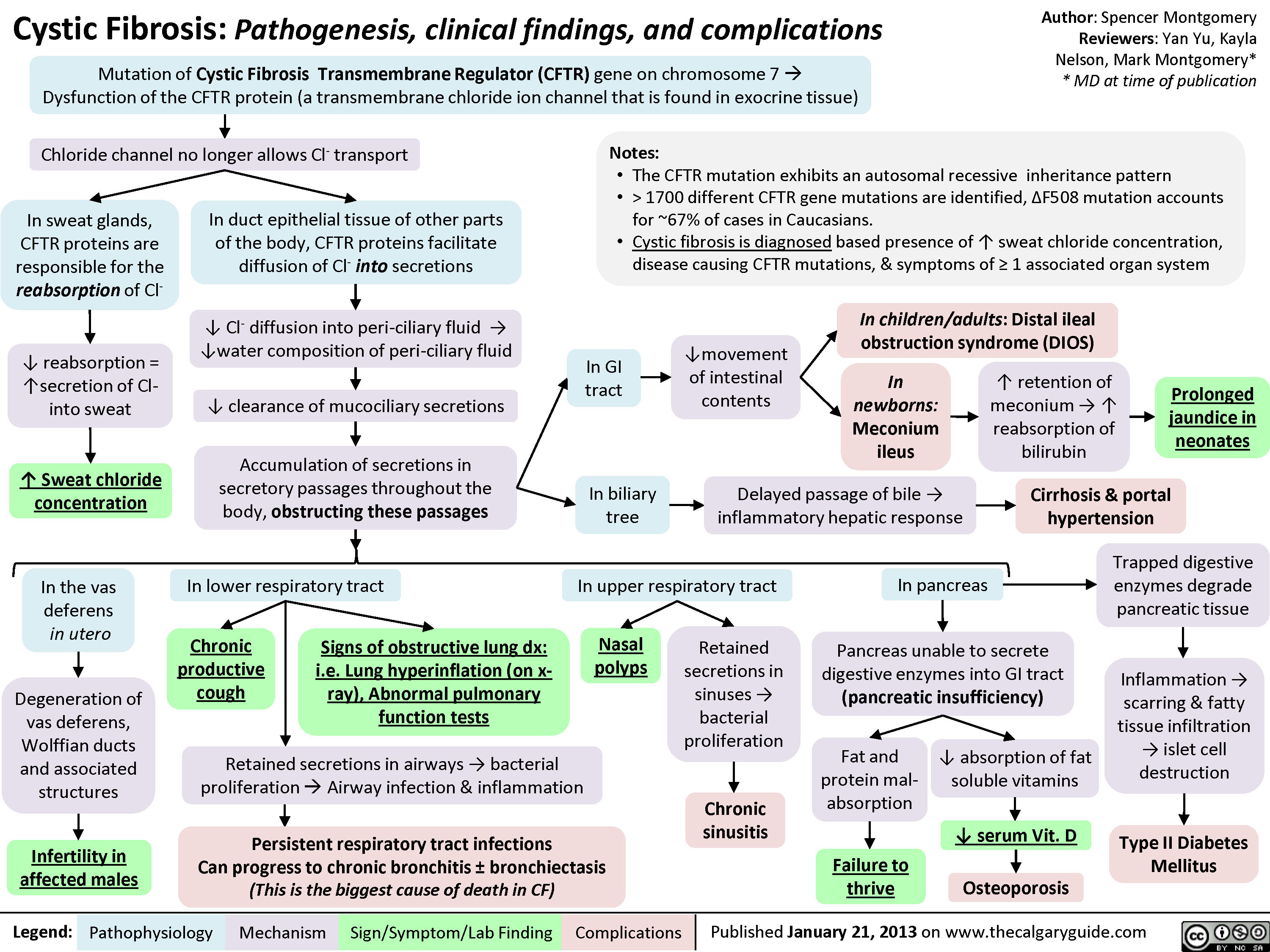
Cystic Fibrosis
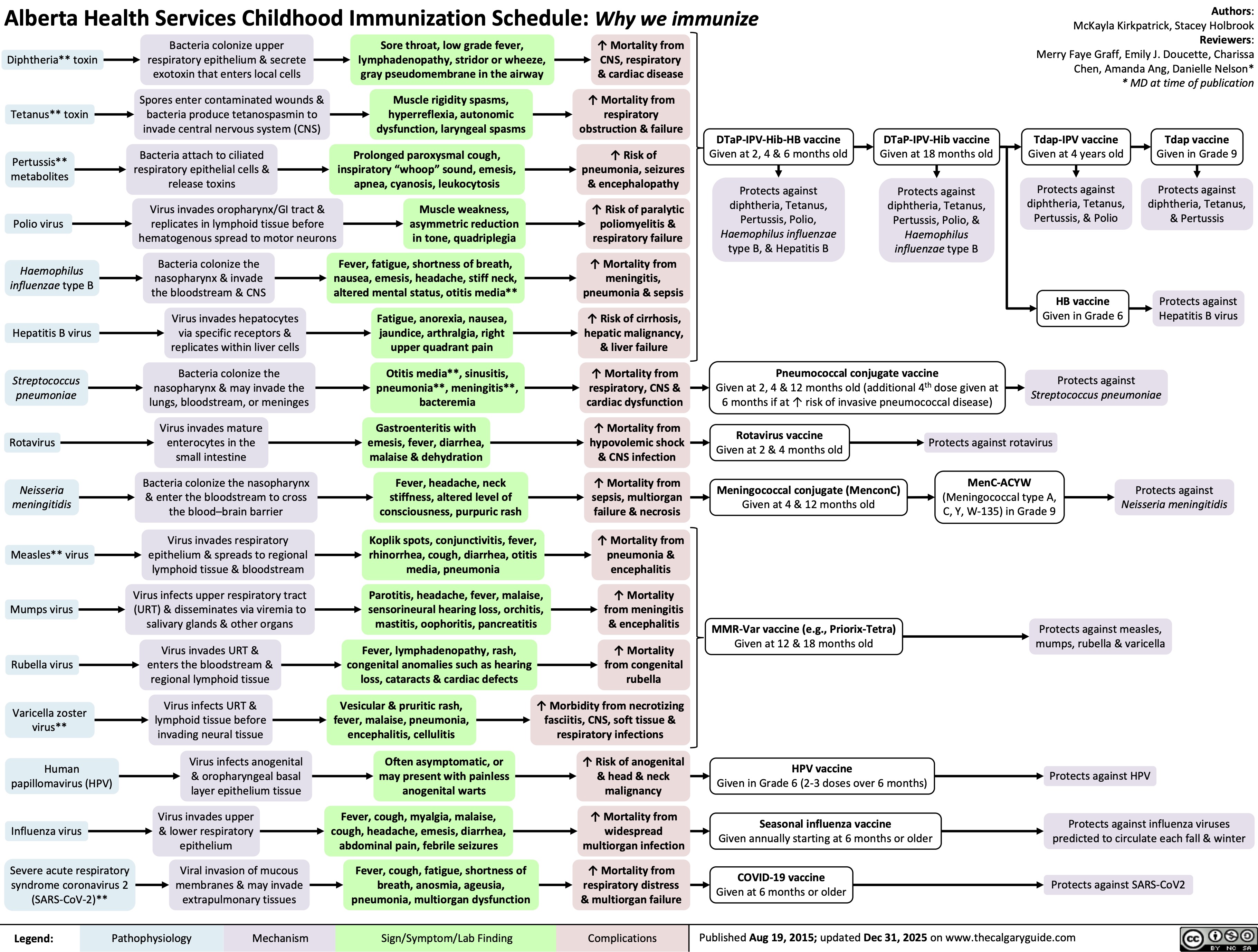
Childhood Immunization Schedule