SEARCH RESULTS FOR: renal
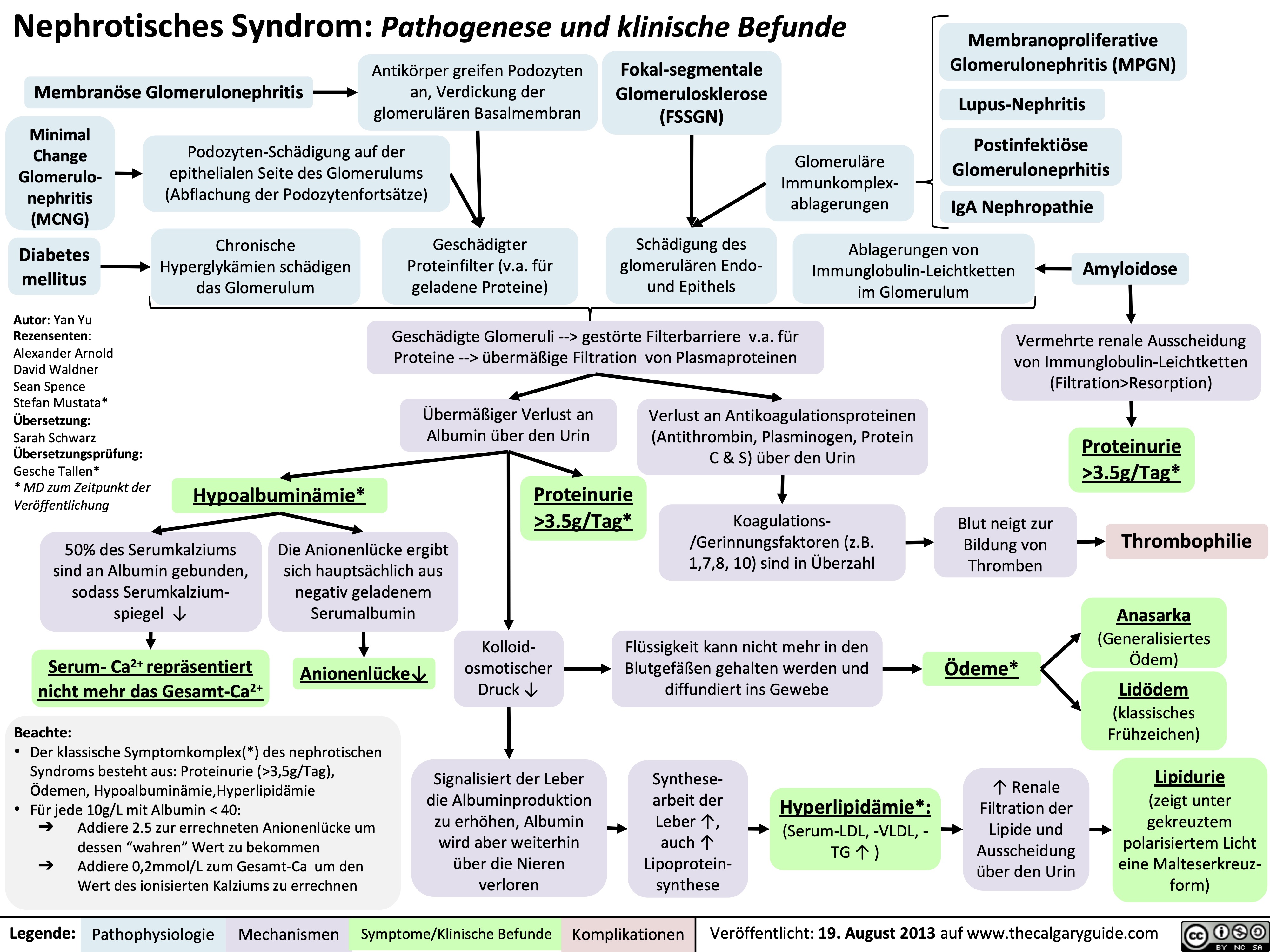
Nephrotic Syndrome: Pathogenesis and Clinical Findings
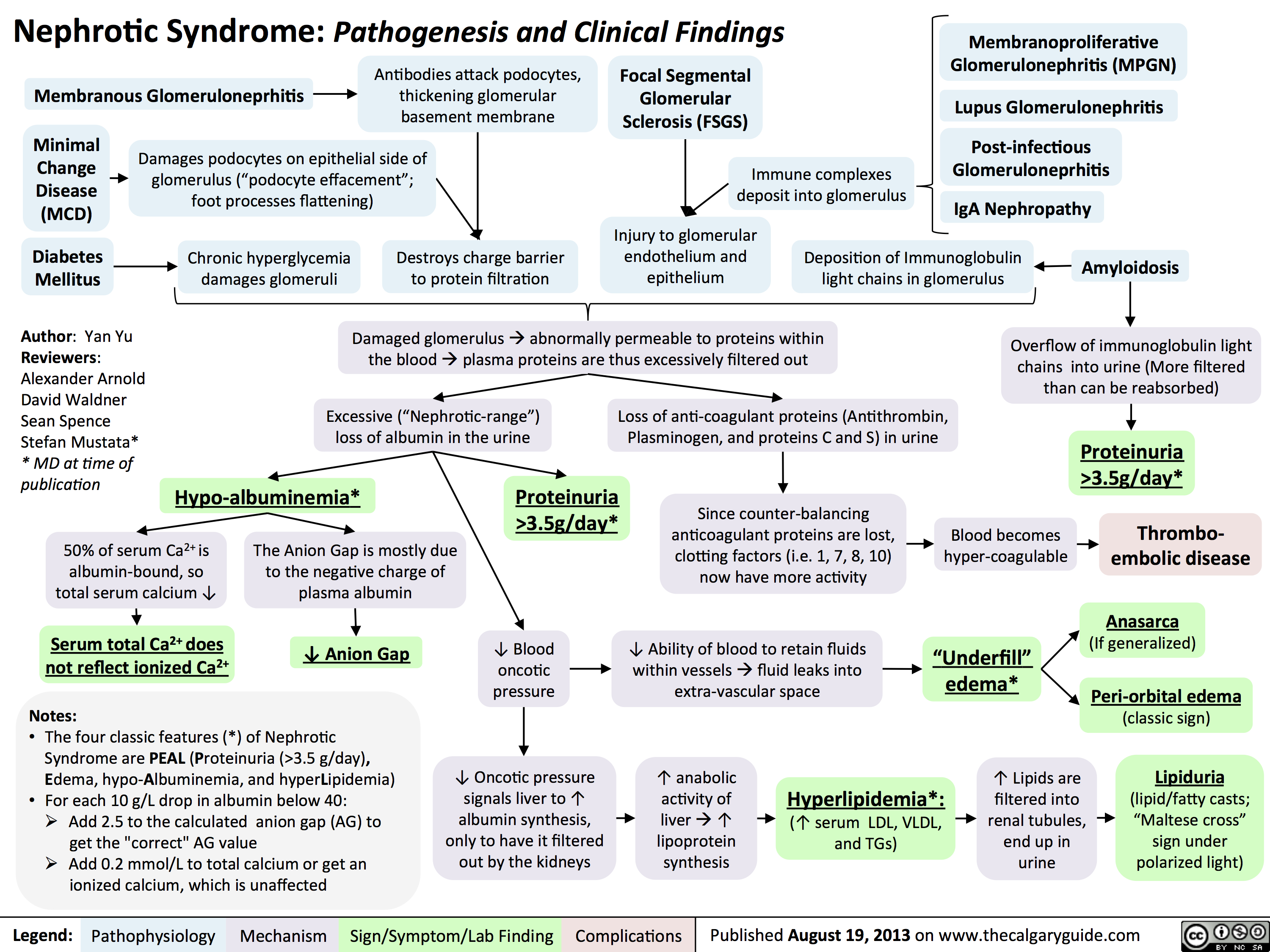 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
Upper Urinary Tract infection (UUTI): Pathogenesis and Clinical Findings
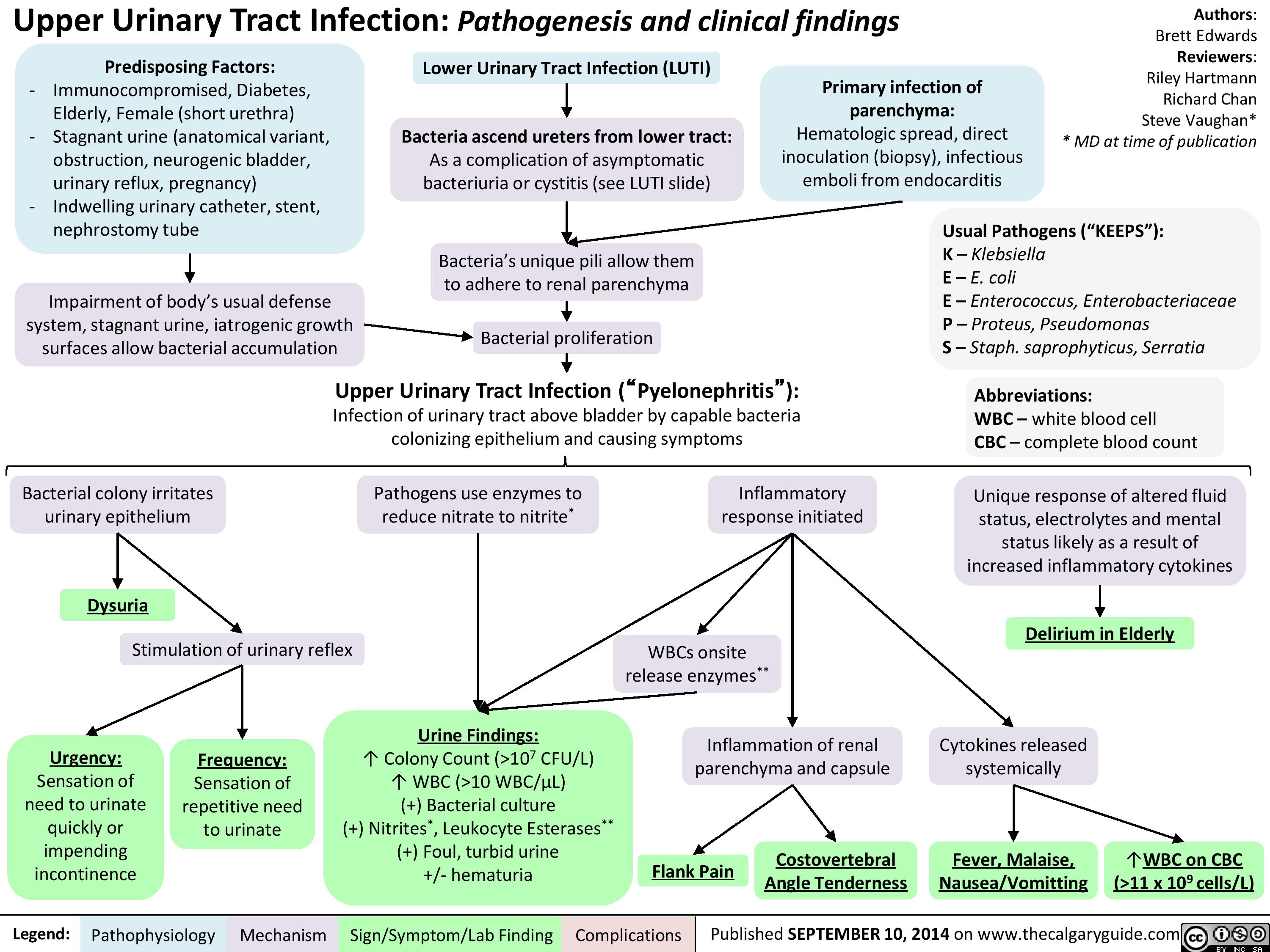
Central Adrenal Insufficiency - Pathogenesis and Clinical Findings
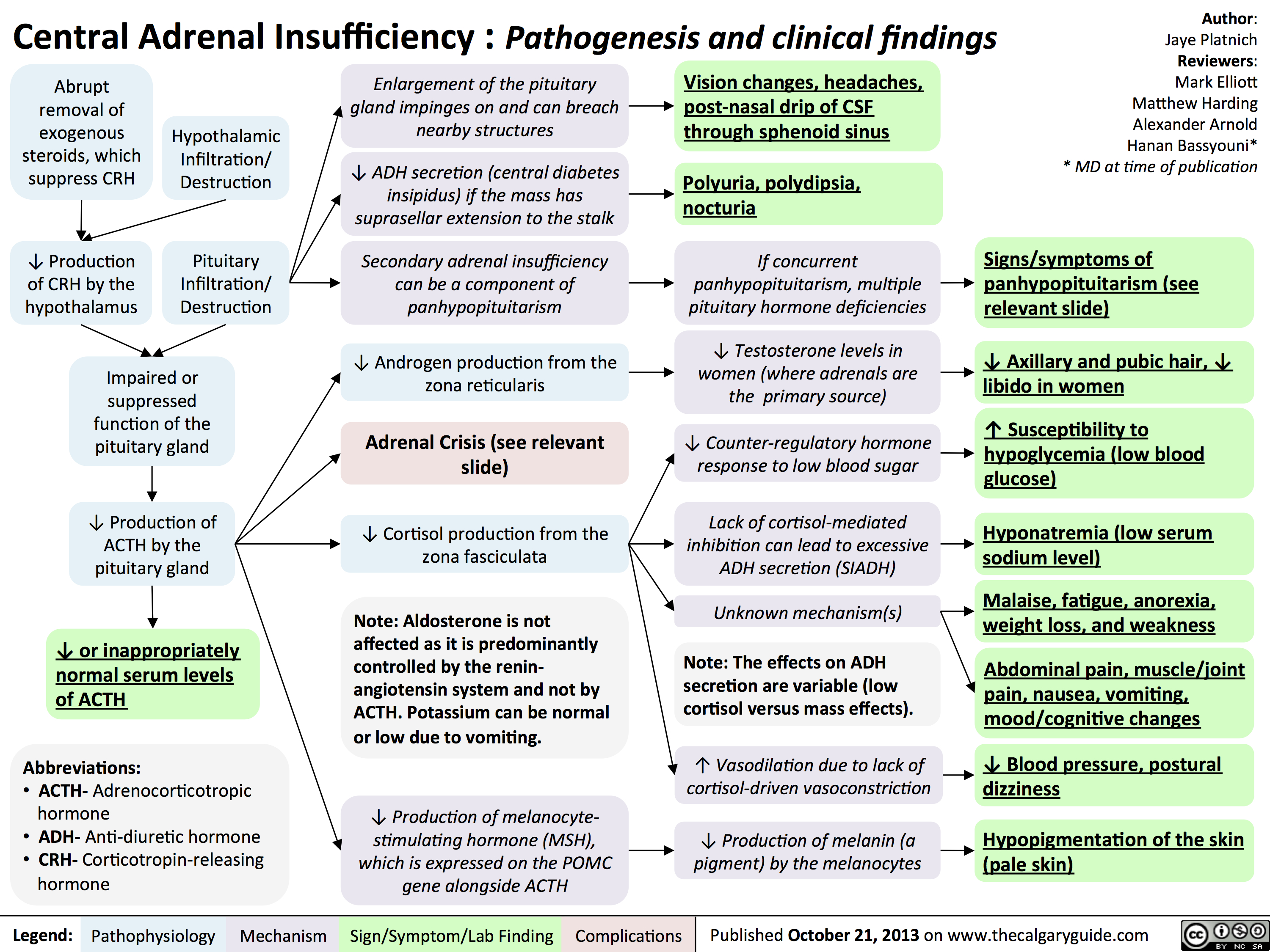
Hypercalcemia - Clinical Findings
![Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hypercalcemia-Clinical-Findings.jpg)
Pathogenesis of Anxiety Disorders

Pre-Renal Acute Kidney Injury Pathogenesis
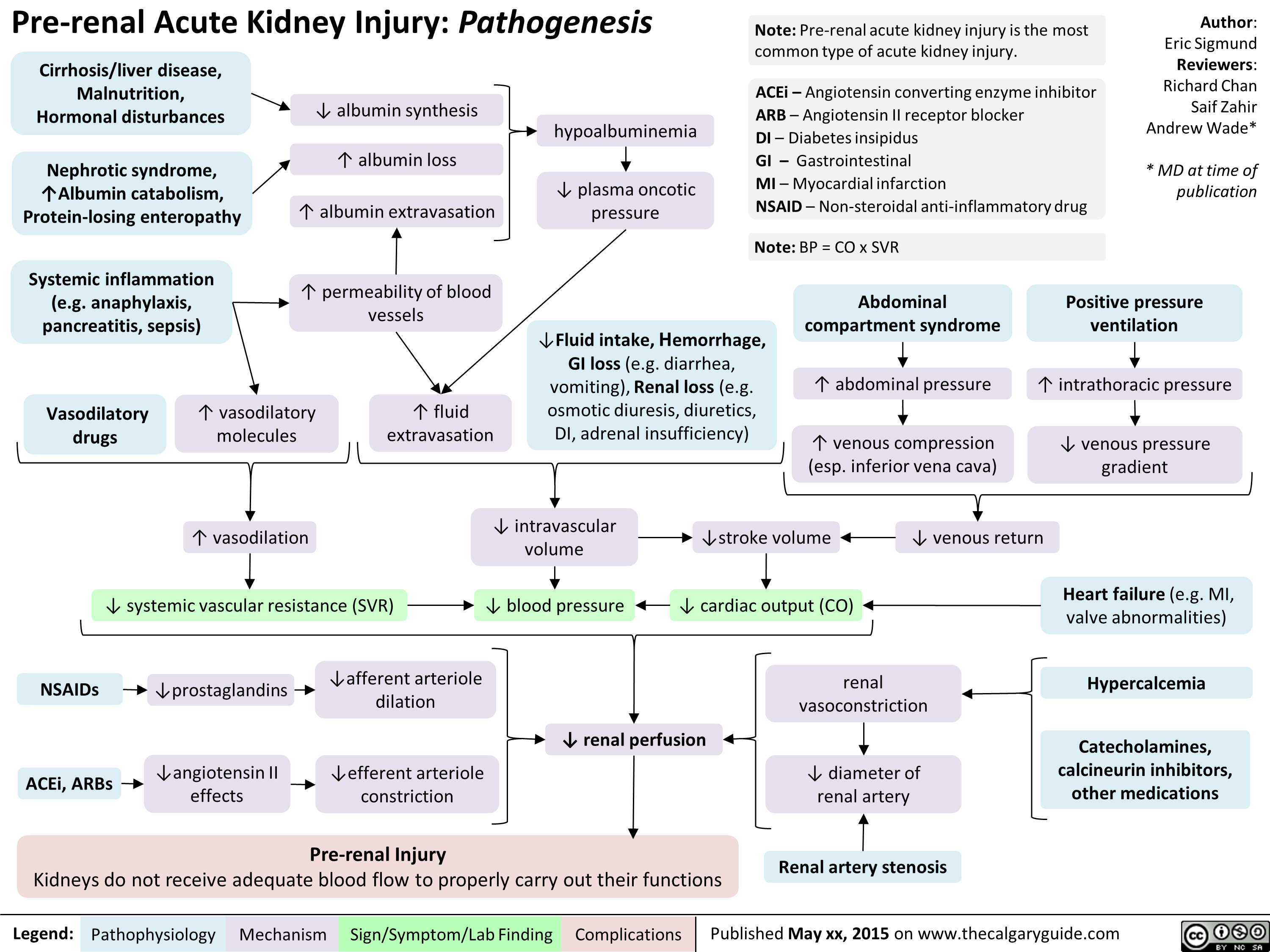
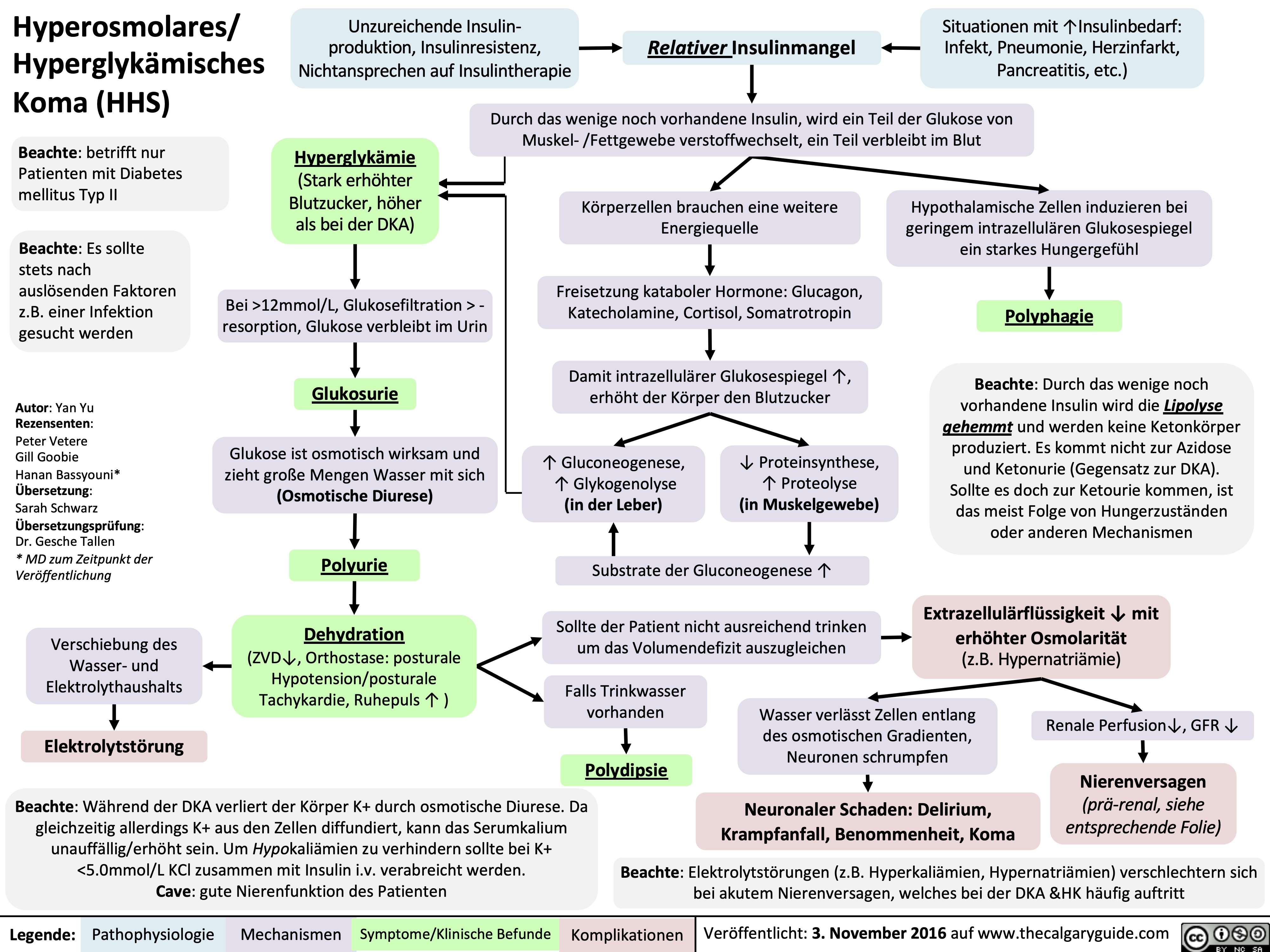
Hyperosmolar Hyperglycemic State
![Hyperosmolar Hyperglycemic State (HHS)
Note: HHS is only seen in Type II DM patients!
Note: In patients with either DKA or HHS, always look for an underlying cause (i.e. an infection)
Author: Yan Yu Reviewers:
Peter Vetere
Gill Goobie
Hanan Bassyouni* * MD at time of publication
Alters total body water & ion osmosis
Inadequate insulin production, insulin resistance, non- adherence to insulin Tx
Relative Insulin deficit
Stresses that ↑ Insulin demand: infections, pneumonia, MI, pancreatitis, etc)
Hyperglycemia
(Very high blood [glucose], higher than in DKA)
When blood [glucose] > 12mmol/L, glucose filtration > reabsorption, ↑ urine [glucose]
Glucosuria
Glucose in filtrate promotes osmotic diuresis: large- volume urine output
Polyuria
Dehydration
(↓ JVP, orthostasis: postural hypotension/ postural tachycardia, ↑ resting HR)
Some insulin still present, but not enoughsome glucose is utilized by muscle/fat cells, some remain in the blood
Cells not “starved”, but still need more energy
↑ release of Catabolic hormones: Glucagon, Epinephrine, Cortisol, GH
Body tries to ↑ blood [glucose], to hopefully ↑ cell glucose absorption
Hypothalamic cells sense low intra-cellular glucose, triggering feelings of hunger
Polyphagia
Note: the presence of some insulin directly inhibits lipolysis; thus, in HHS there is no ketone body production, and no subsequent metabolic acidosis and ketouria (unlike in DKA). If ketones are detected in an HHS patient it’s likely secondary to starvation or other mechanisms.
↓ ECF volume, ↑ ECF osmolarity (i.e. hypernatremia)
↑ Gluconeogenesis ↑ Glycogenolysis (in liver)
↓ Protein synthesis, ↑ proteolysis
(in muscle)
↑ Gluconeogenic substrates for liver If the patient doesn’t drink enough
water to replenish lost blood volume If pt is alert and
Electrolyte imbalance
water is accessible
Water osmotically leaves neurons, shrinking them
Neural damage: delirium, lethargy, seizure, stupor, coma
↓ renal perfusion, ↓ GFR
Renal Failure
(pre-renal cause; see relevant slides)
Polydipsia Note: in HHS, body K+ is lost via osmotic diuresis. But diffusion of K+ out of cells
may cause serum [K+] to be falsely normal/elevated. To prevent hypokalemia, give IV KCl along with IV insulin as soon as serum K+ <5.0mmol/L. But ensure patient has good renal function/urine output first, to avoid iatrogenic hyperkalemia!
Note: Electrolyte imbalances (i.e. hyperkalemia, hypernatremia) are worsened by the acute renal failure commonly coexisting with DKA/HHS
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published November 3, 2016 on www.thecalgaryguide.com
Hyperosmolar Hyperglycemic State (HHS)
Note: HHS is only seen in Type II DM patients!
Note: In patients with either DKA or HHS, always look for an underlying cause (i.e. an infection)
Author: Yan Yu Reviewers:
Peter Vetere
Gill Goobie
Hanan Bassyouni* * MD at time of publication
Alters total body water & ion osmosis
Inadequate insulin production, insulin resistance, non- adherence to insulin Tx
Relative Insulin deficit
Stresses that ↑ Insulin demand: infections, pneumonia, MI, pancreatitis, etc)
Hyperglycemia
(Very high blood [glucose], higher than in DKA)
When blood [glucose] > 12mmol/L, glucose filtration > reabsorption, ↑ urine [glucose]
Glucosuria
Glucose in filtrate promotes osmotic diuresis: large- volume urine output
Polyuria
Dehydration
(↓ JVP, orthostasis: postural hypotension/ postural tachycardia, ↑ resting HR)
Some insulin still present, but not enoughsome glucose is utilized by muscle/fat cells, some remain in the blood
Cells not “starved”, but still need more energy
↑ release of Catabolic hormones: Glucagon, Epinephrine, Cortisol, GH
Body tries to ↑ blood [glucose], to hopefully ↑ cell glucose absorption
Hypothalamic cells sense low intra-cellular glucose, triggering feelings of hunger
Polyphagia
Note: the presence of some insulin directly inhibits lipolysis; thus, in HHS there is no ketone body production, and no subsequent metabolic acidosis and ketouria (unlike in DKA). If ketones are detected in an HHS patient it’s likely secondary to starvation or other mechanisms.
↓ ECF volume, ↑ ECF osmolarity (i.e. hypernatremia)
↑ Gluconeogenesis ↑ Glycogenolysis (in liver)
↓ Protein synthesis, ↑ proteolysis
(in muscle)
↑ Gluconeogenic substrates for liver If the patient doesn’t drink enough
water to replenish lost blood volume If pt is alert and
Electrolyte imbalance
water is accessible
Water osmotically leaves neurons, shrinking them
Neural damage: delirium, lethargy, seizure, stupor, coma
↓ renal perfusion, ↓ GFR
Renal Failure
(pre-renal cause; see relevant slides)
Polydipsia Note: in HHS, body K+ is lost via osmotic diuresis. But diffusion of K+ out of cells
may cause serum [K+] to be falsely normal/elevated. To prevent hypokalemia, give IV KCl along with IV insulin as soon as serum K+ <5.0mmol/L. But ensure patient has good renal function/urine output first, to avoid iatrogenic hyperkalemia!
Note: Electrolyte imbalances (i.e. hyperkalemia, hypernatremia) are worsened by the acute renal failure commonly coexisting with DKA/HHS
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published November 3, 2016 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hyperosmolar-Hyperglycemic-State-HHS.jpg)
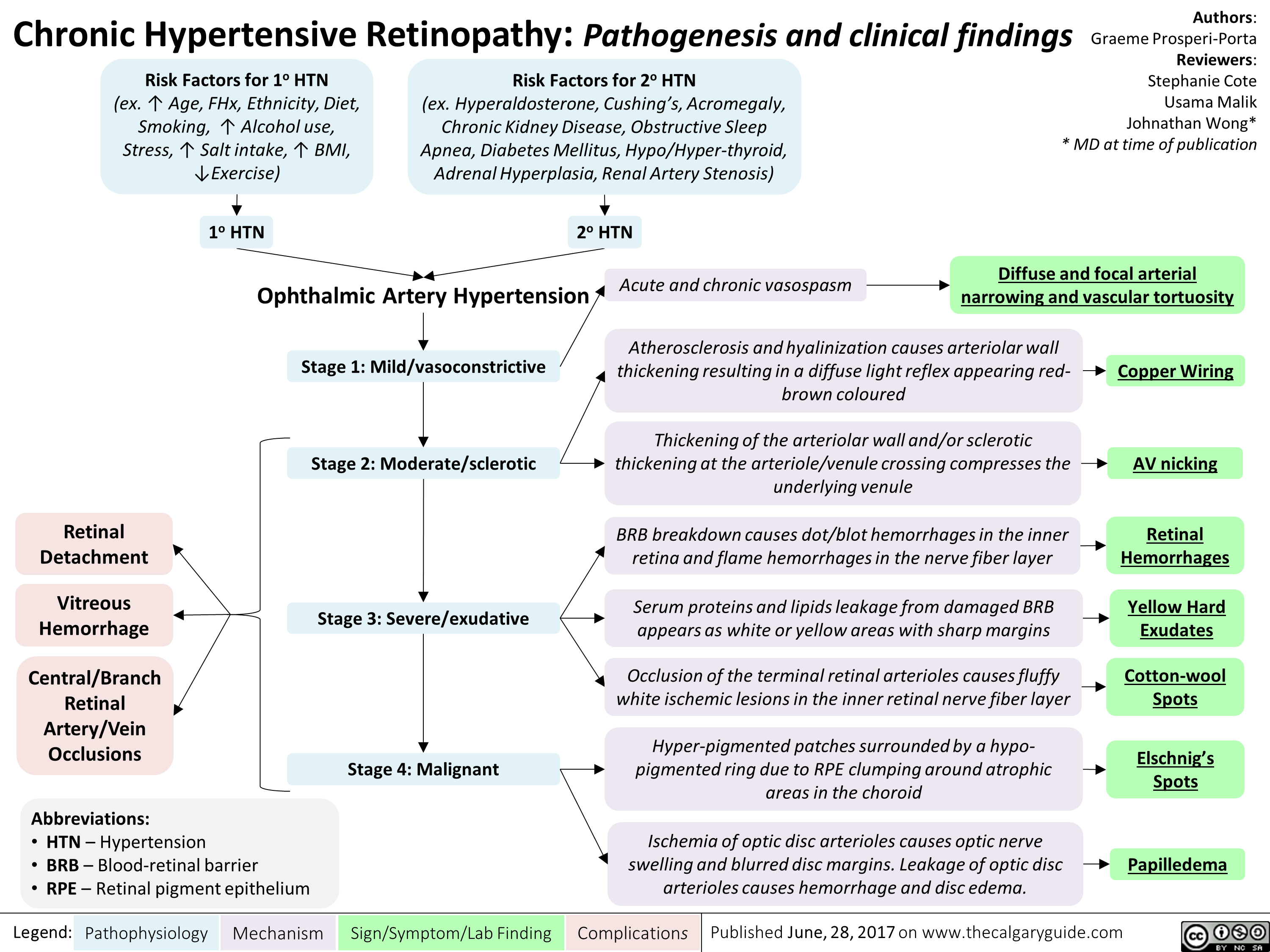
chronic-hypertensive-retinopathy-pathogenesis-and-clinical-findings
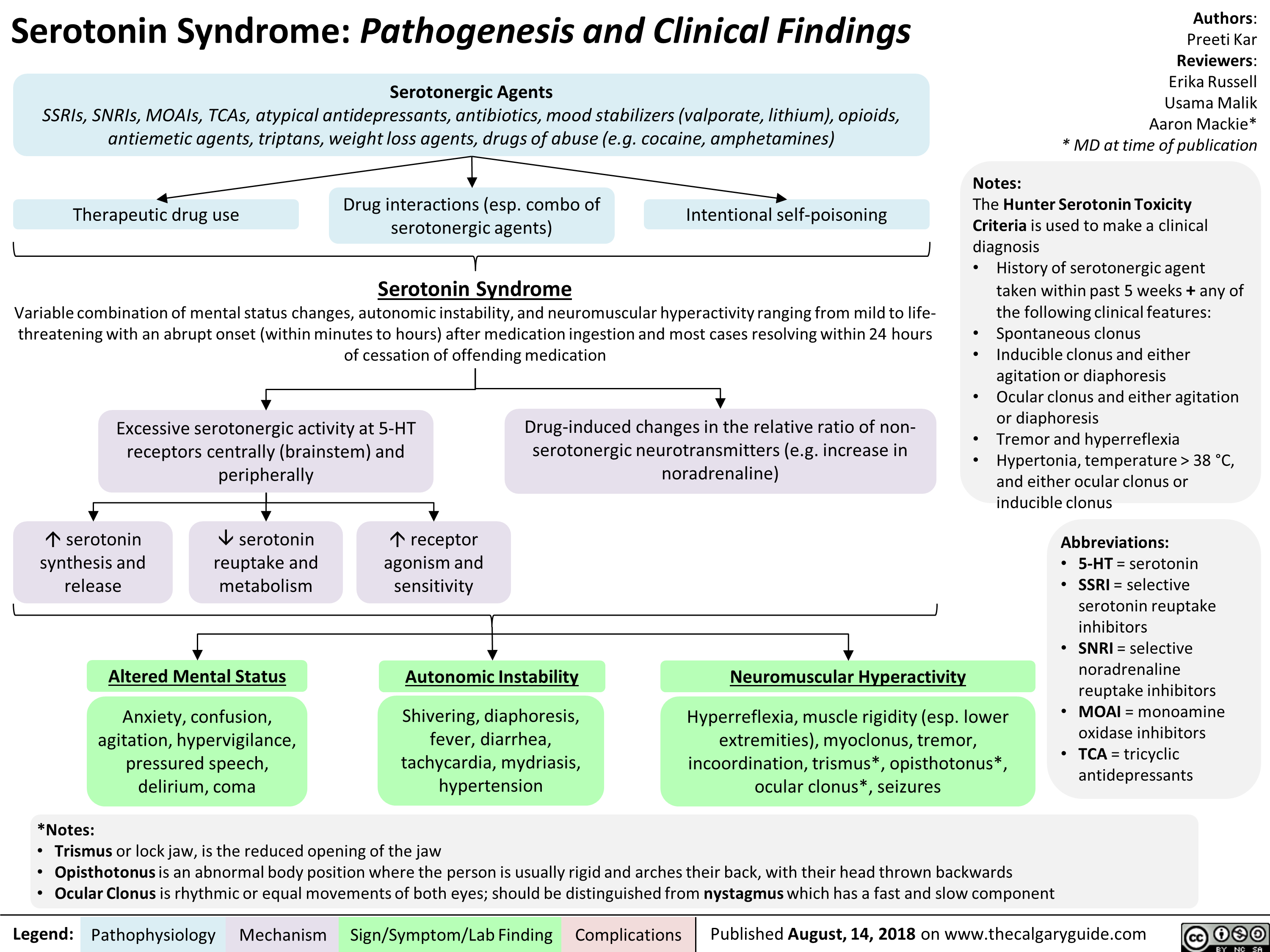
Serotonin Syndrome Pathogenesis and Clinical Findings
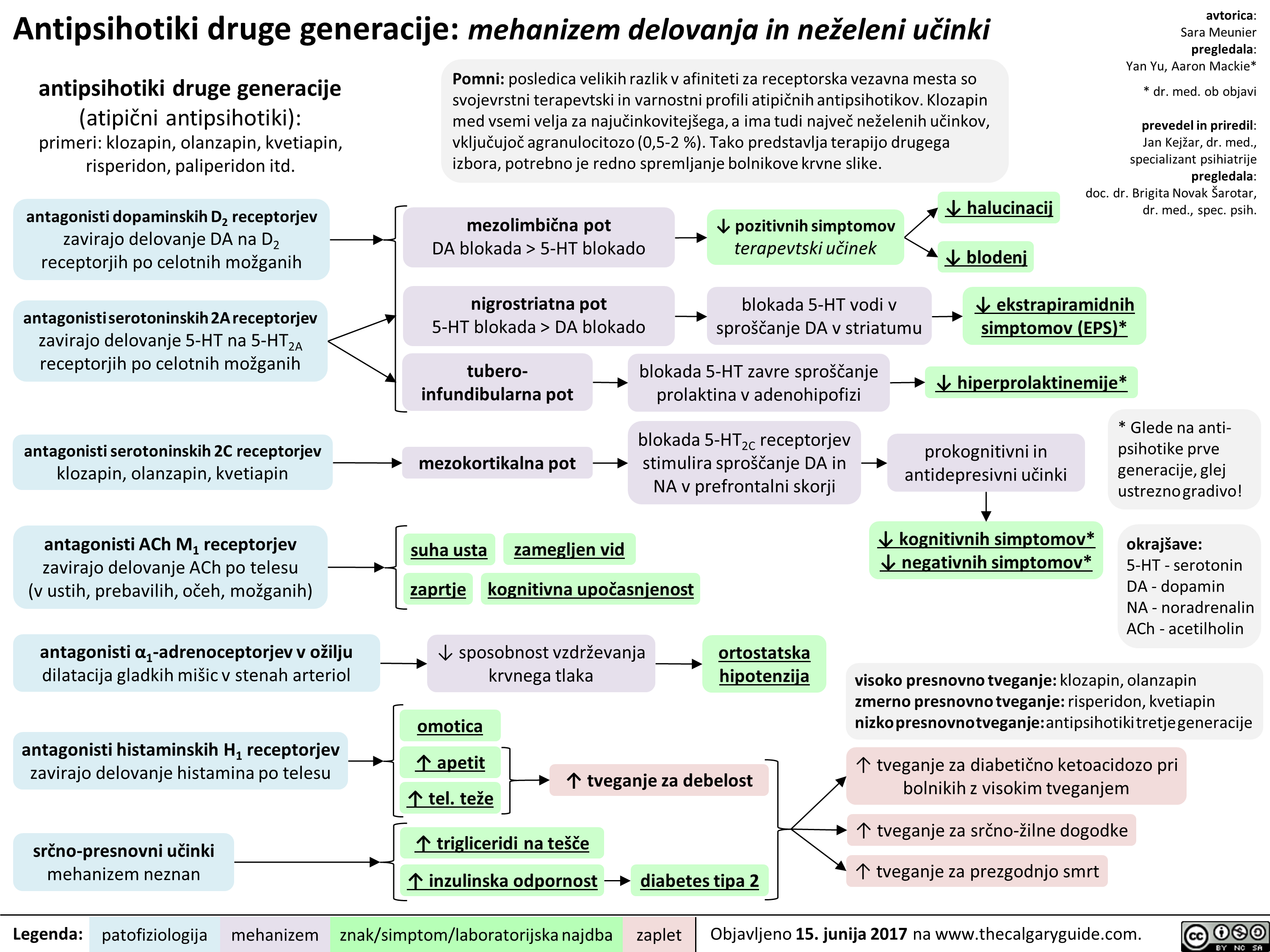
2nd gen antipsychotics (Slovenian translation) - FINAL VERSION
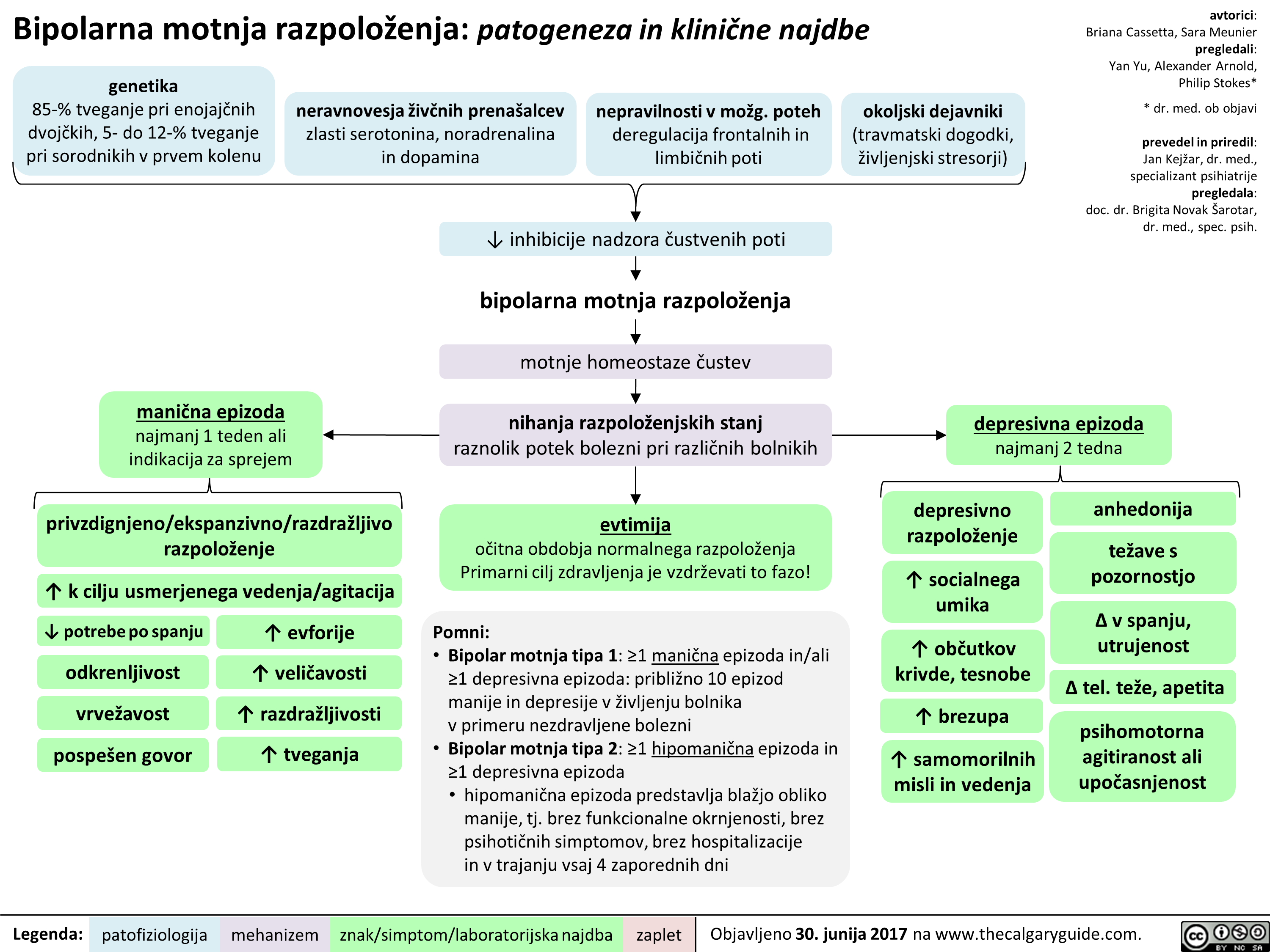
BMR (Slovenian translation) - FINAL VERSION
Bupropion (Slovenian translation) - FINAL VERSION
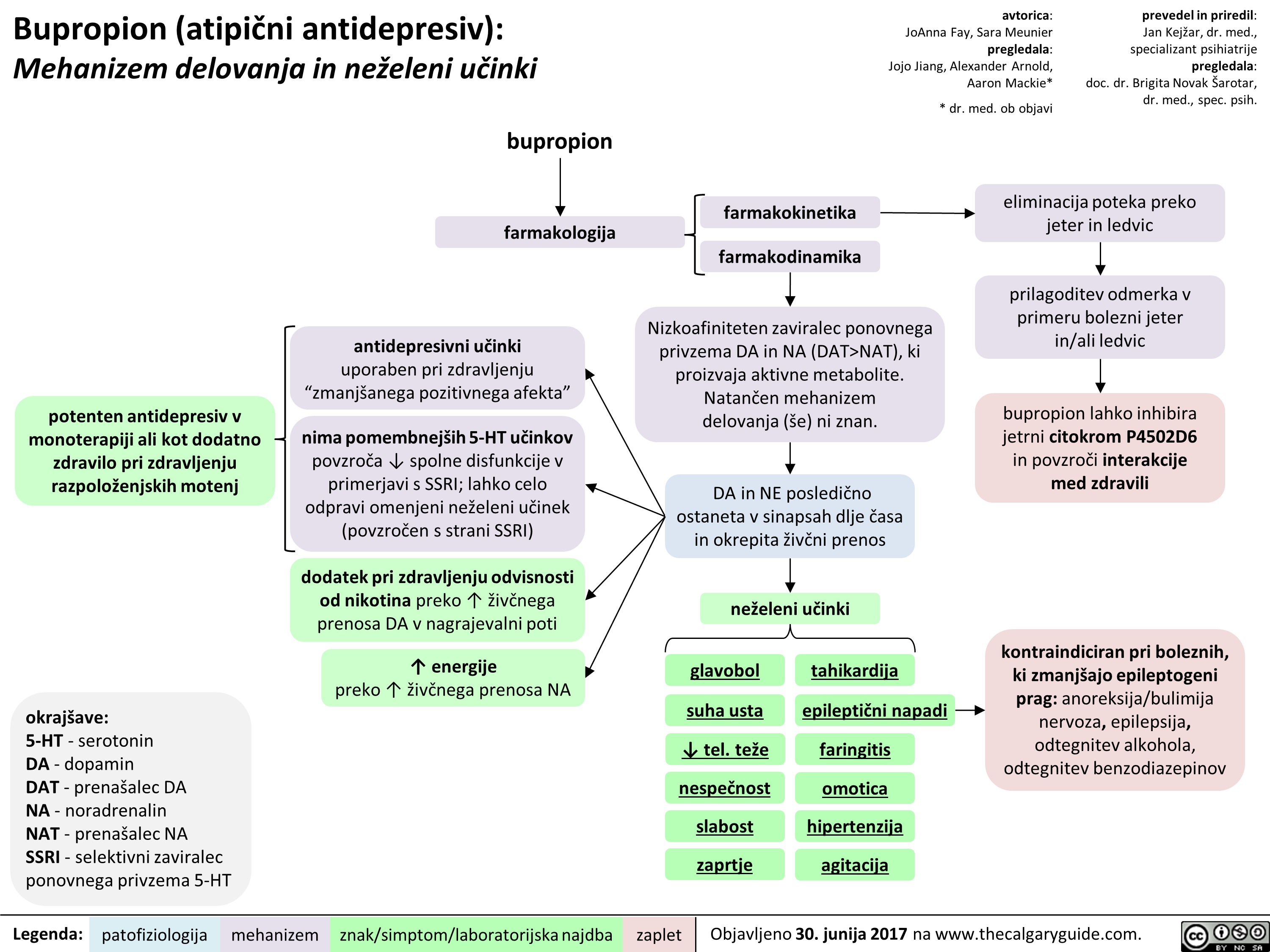 NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" title="Bupropion (atipiEni antidepresiv): Mehanizem delovanja in neieleni utinki
potenten antidepresiv v monoterapiji all kot dodatno zdravilo pri zdravljenju razpoloienjskih motenj
okrajgave: 5-HT - serotonin DA - dopamin DAT - prenagalec DA NA - noradrenalin NAT - prenagalec NA SSRI - selektivni zaviralec ponovnega privzema 5-HT
Legenda:
bupropion
farmakologija
antidepresivni udnki uporaben pri zdravljenju "zmaniganega pozitivnega afekta"
nima pomembnelgih 5-HT udnkov povzraa spolne disfunkcije v primerjavi s SSRI; lahko celo odpravi omenjeni neieleni udnek (povzraen s strani SSRI)
dodatek pri zdravljenju odvisnosti od nikotina preko T iive'nega prenosa DA v nagrajevalni poti
energije preko T 2ivbega prenosa NA
patofiziologija mehanizem
farmakokinetika
farmakodinamika
avtorica: JoAnna Fay, Sara Meunier pregledala: Jojo Jiang, Alexander Arnold, Aaron Mackie*
* dr. med. ob objavi
Nizkoafiniteten zaviralec ponovnega privzema DA in NA (DAT>NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" />
NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" title="Bupropion (atipiEni antidepresiv): Mehanizem delovanja in neieleni utinki
potenten antidepresiv v monoterapiji all kot dodatno zdravilo pri zdravljenju razpoloienjskih motenj
okrajgave: 5-HT - serotonin DA - dopamin DAT - prenagalec DA NA - noradrenalin NAT - prenagalec NA SSRI - selektivni zaviralec ponovnega privzema 5-HT
Legenda:
bupropion
farmakologija
antidepresivni udnki uporaben pri zdravljenju "zmaniganega pozitivnega afekta"
nima pomembnelgih 5-HT udnkov povzraa spolne disfunkcije v primerjavi s SSRI; lahko celo odpravi omenjeni neieleni udnek (povzraen s strani SSRI)
dodatek pri zdravljenju odvisnosti od nikotina preko T iive'nega prenosa DA v nagrajevalni poti
energije preko T 2ivbega prenosa NA
patofiziologija mehanizem
farmakokinetika
farmakodinamika
avtorica: JoAnna Fay, Sara Meunier pregledala: Jojo Jiang, Alexander Arnold, Aaron Mackie*
* dr. med. ob objavi
Nizkoafiniteten zaviralec ponovnega privzema DA in NA (DAT>NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" />
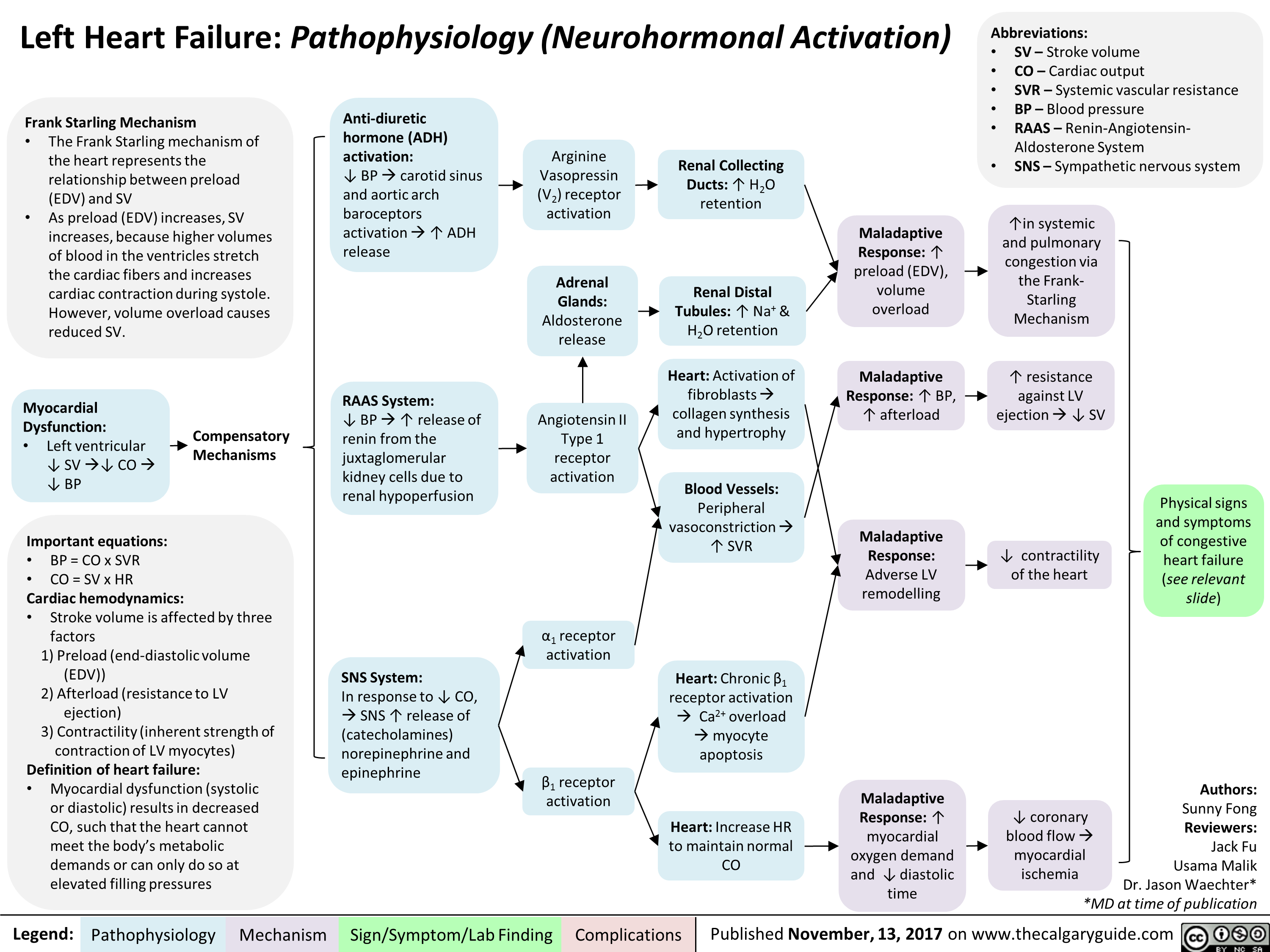
Left Heart Failure: Pathophysiology (Neurohormonal Activation)
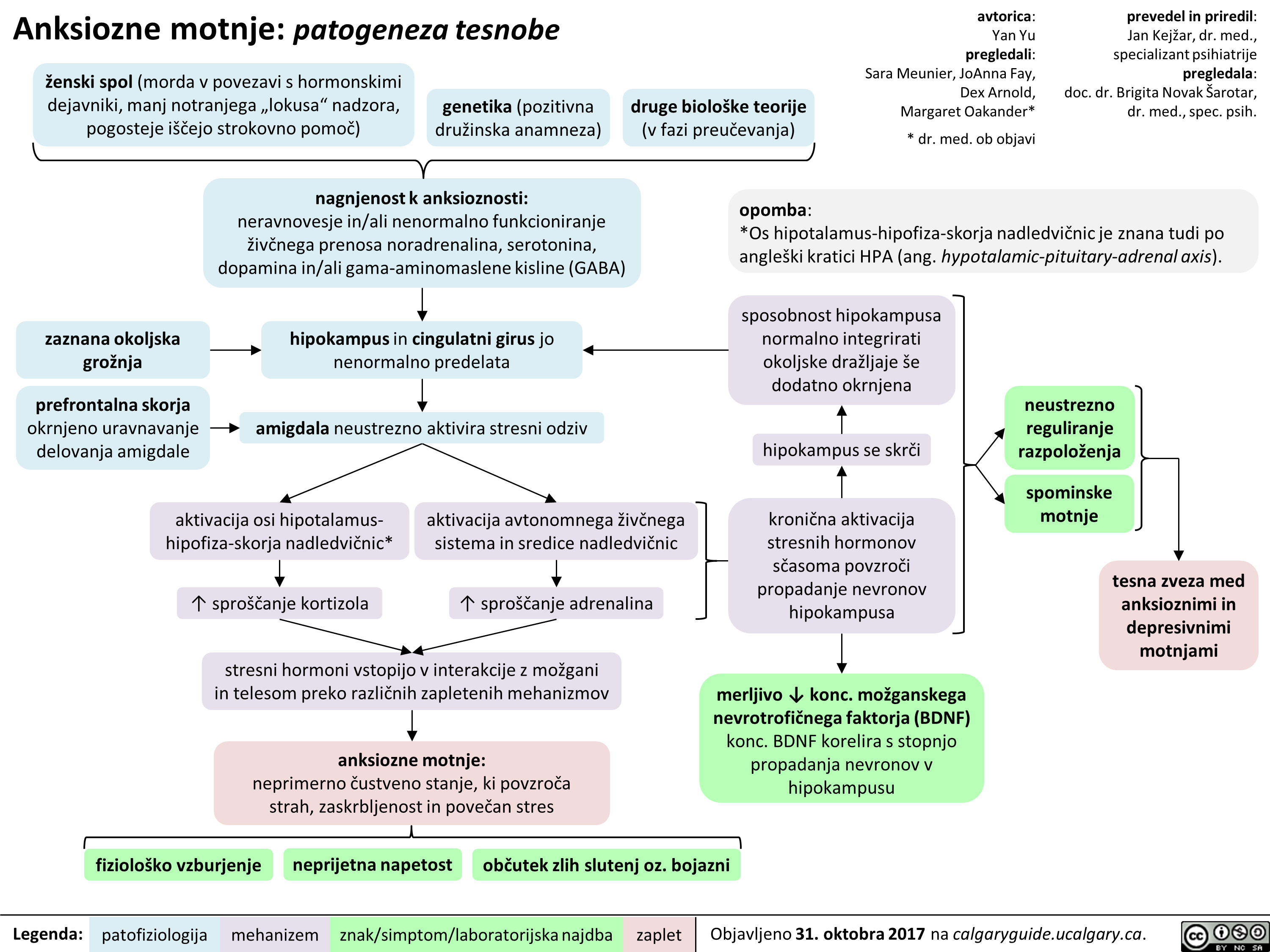
Anksiozne motnje: patogeneza tesnobe
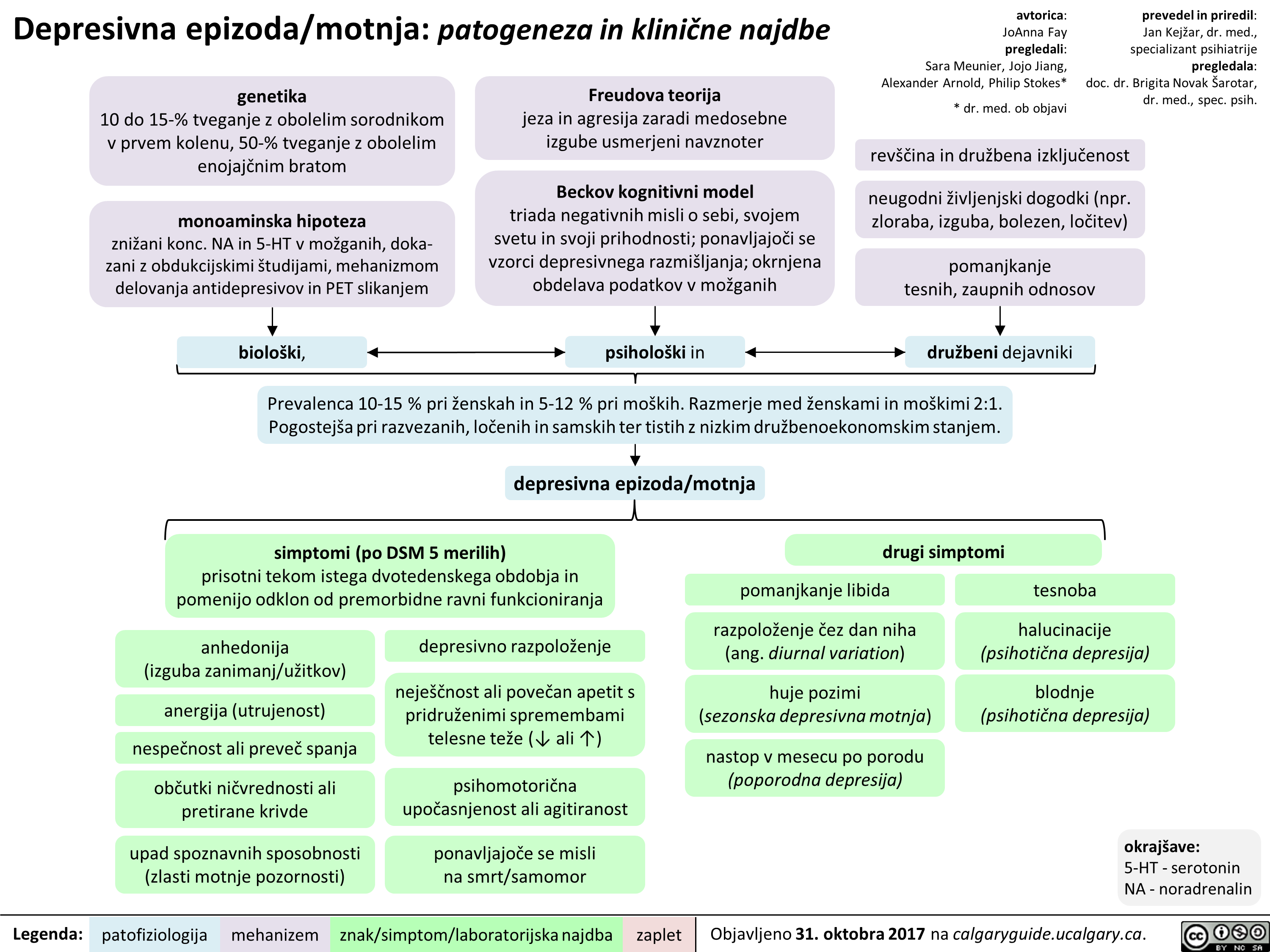
Depresivna epizoda/motnja: patogeneza in klinične najdbe
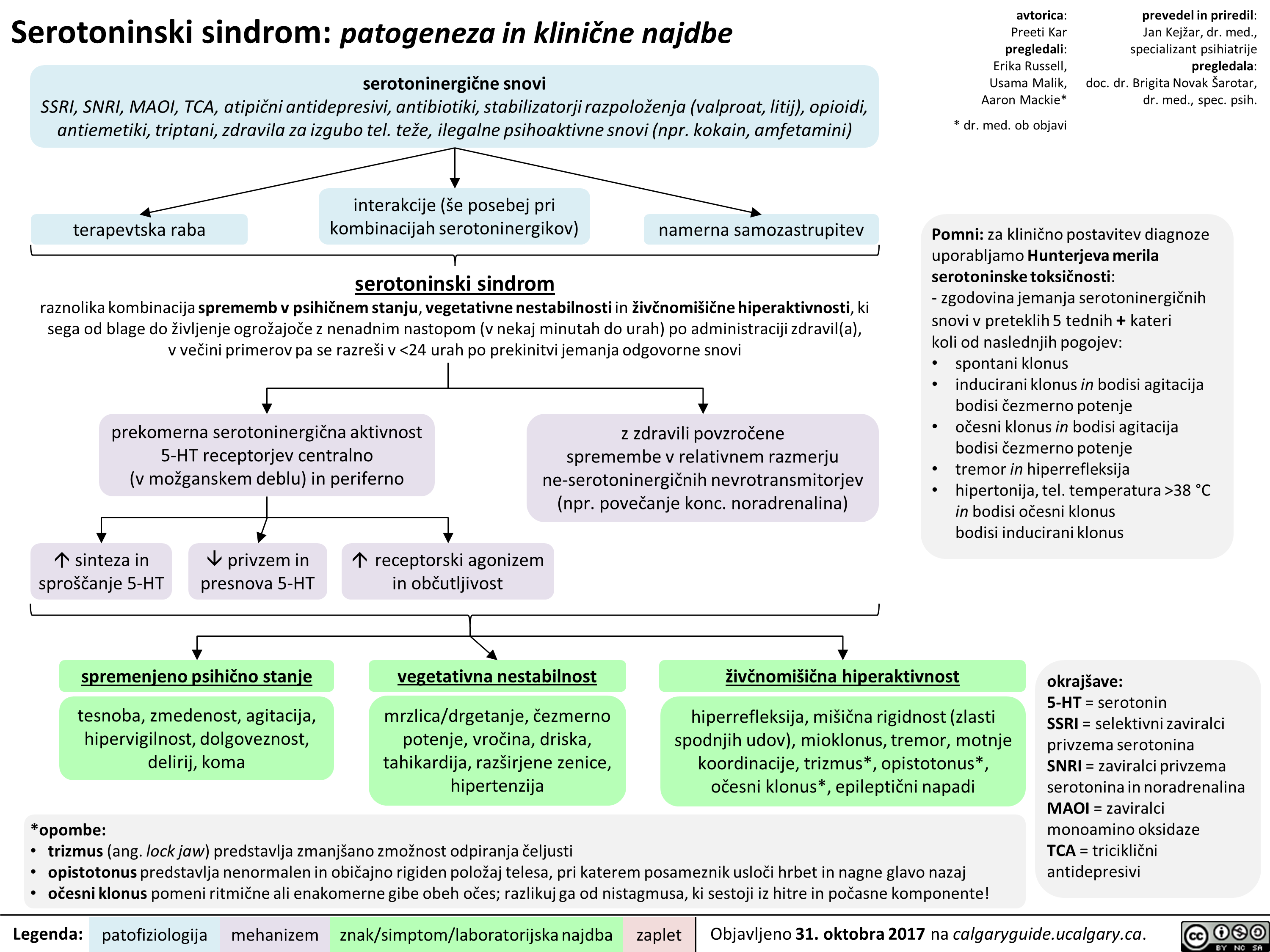
Serotoninski sindrom: patogeneza in klinične najdbe
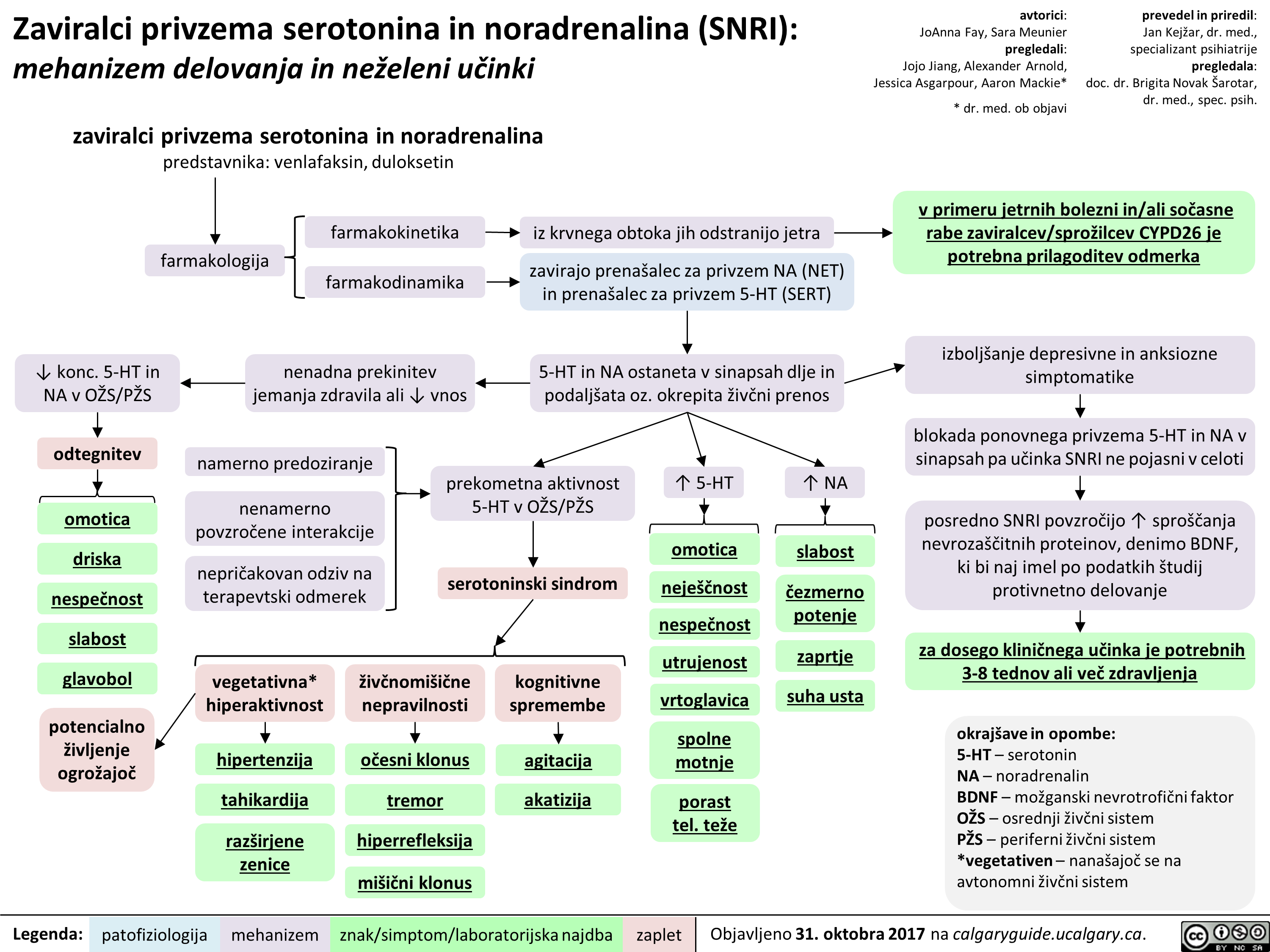
Zaviralci privzema serotonina in noradrenalina (SNRI): mehanizem delovanja in neželeni učinki
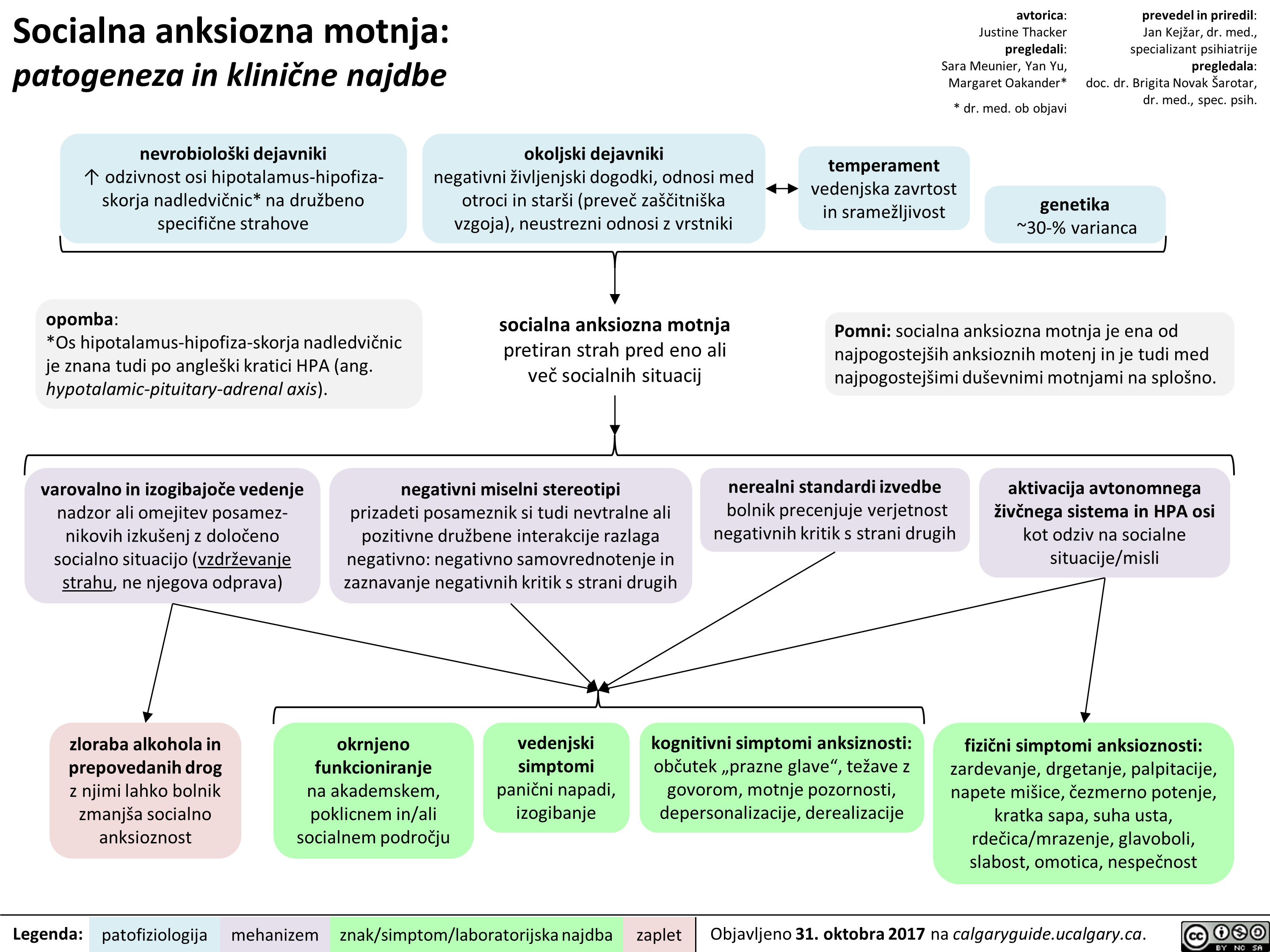
Socialna anksiozna motnja: patogeneza in klinične najdbe
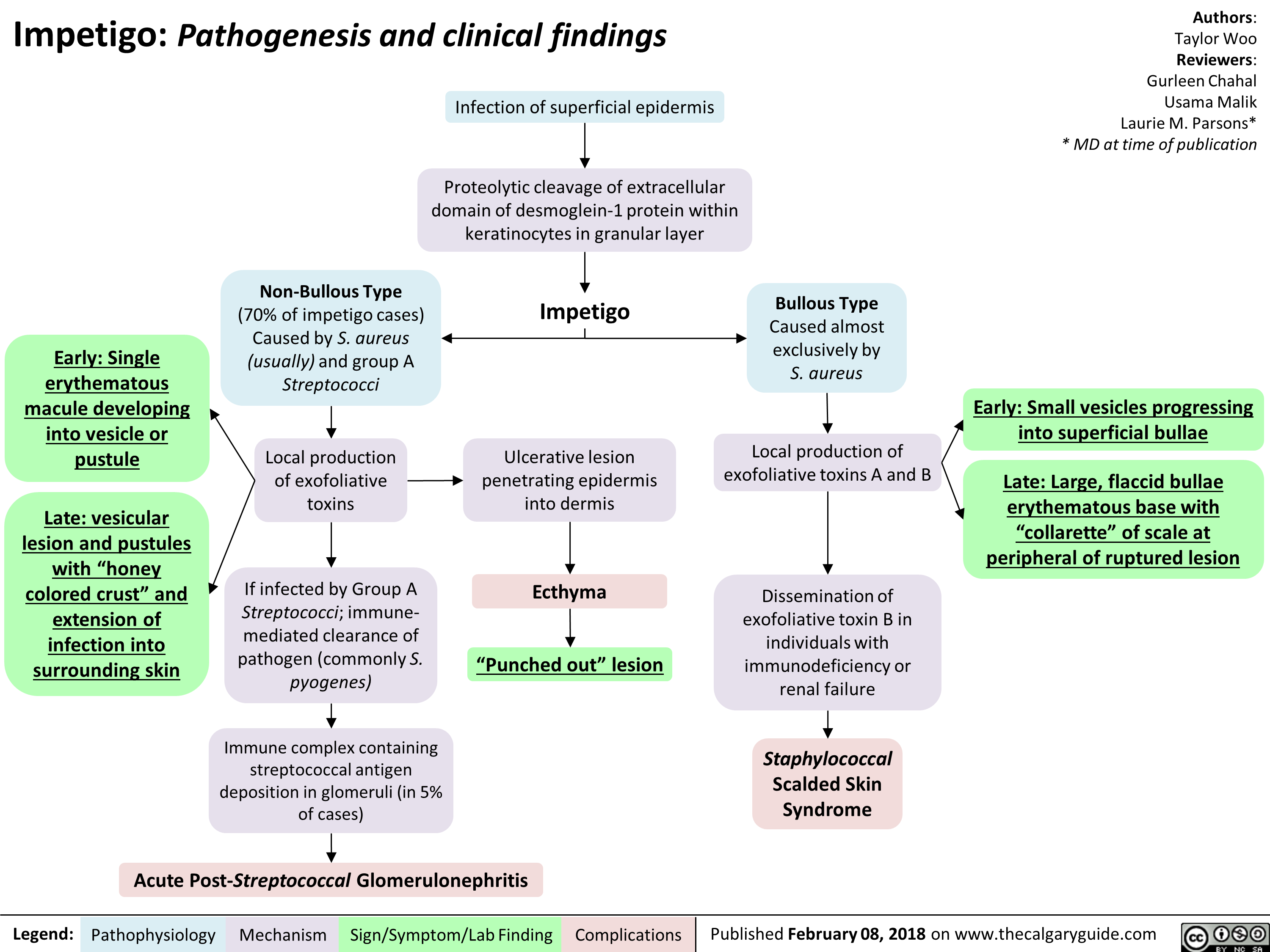
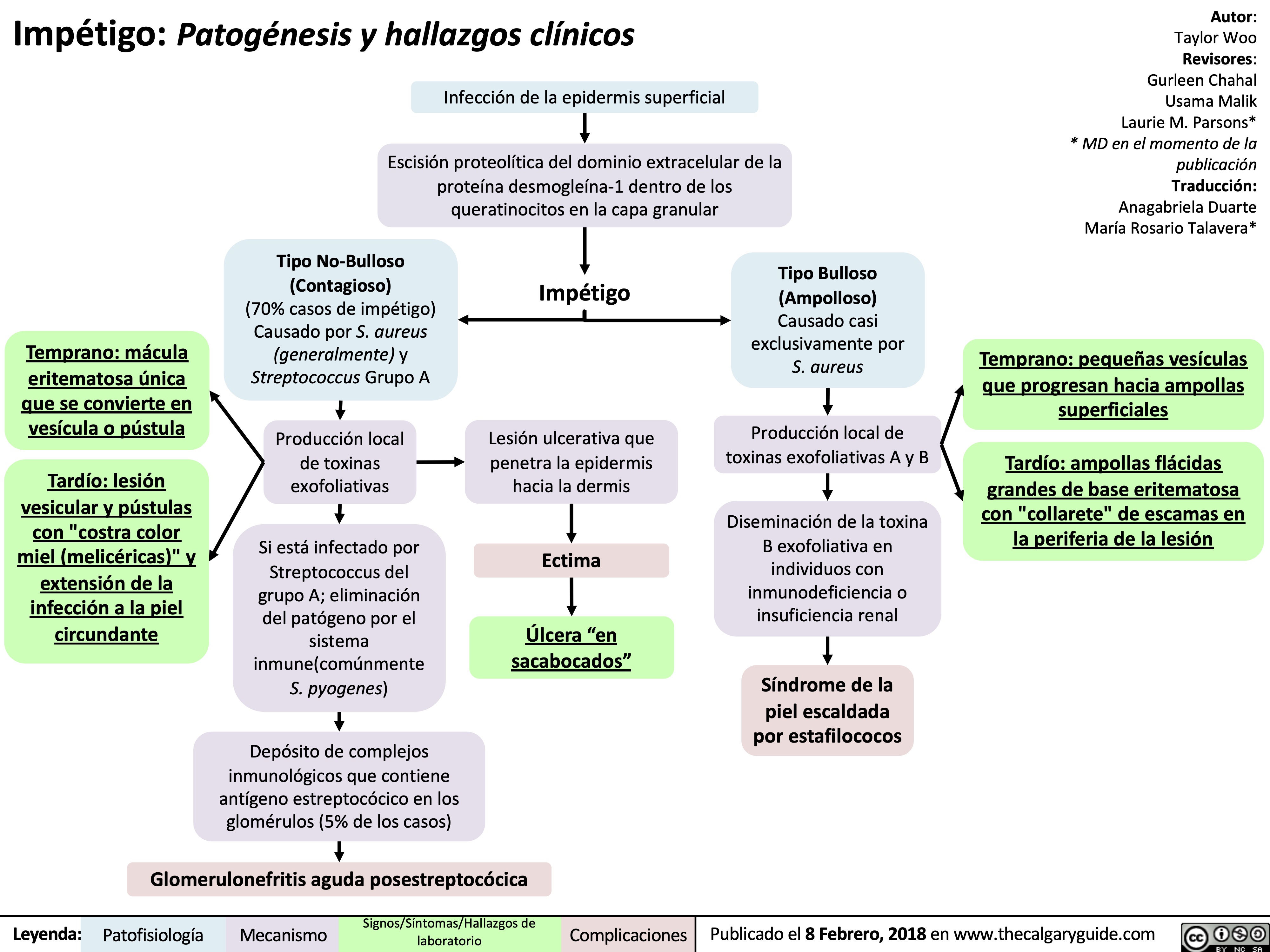
Impetigo Pathogenesis and clinical findings
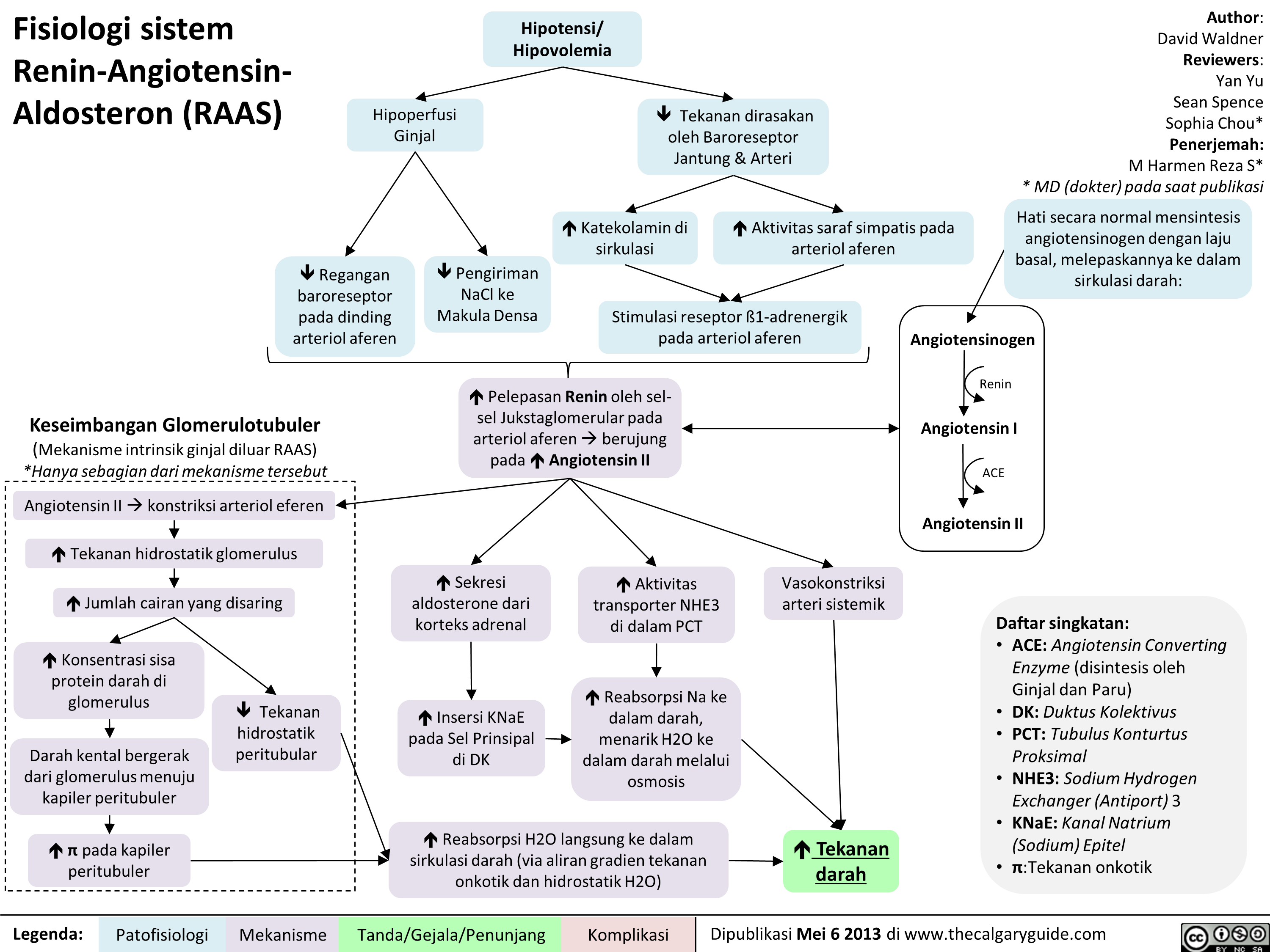
Fisiologi sistem Renin-Angiotensin-Aldosteron (RAAS)
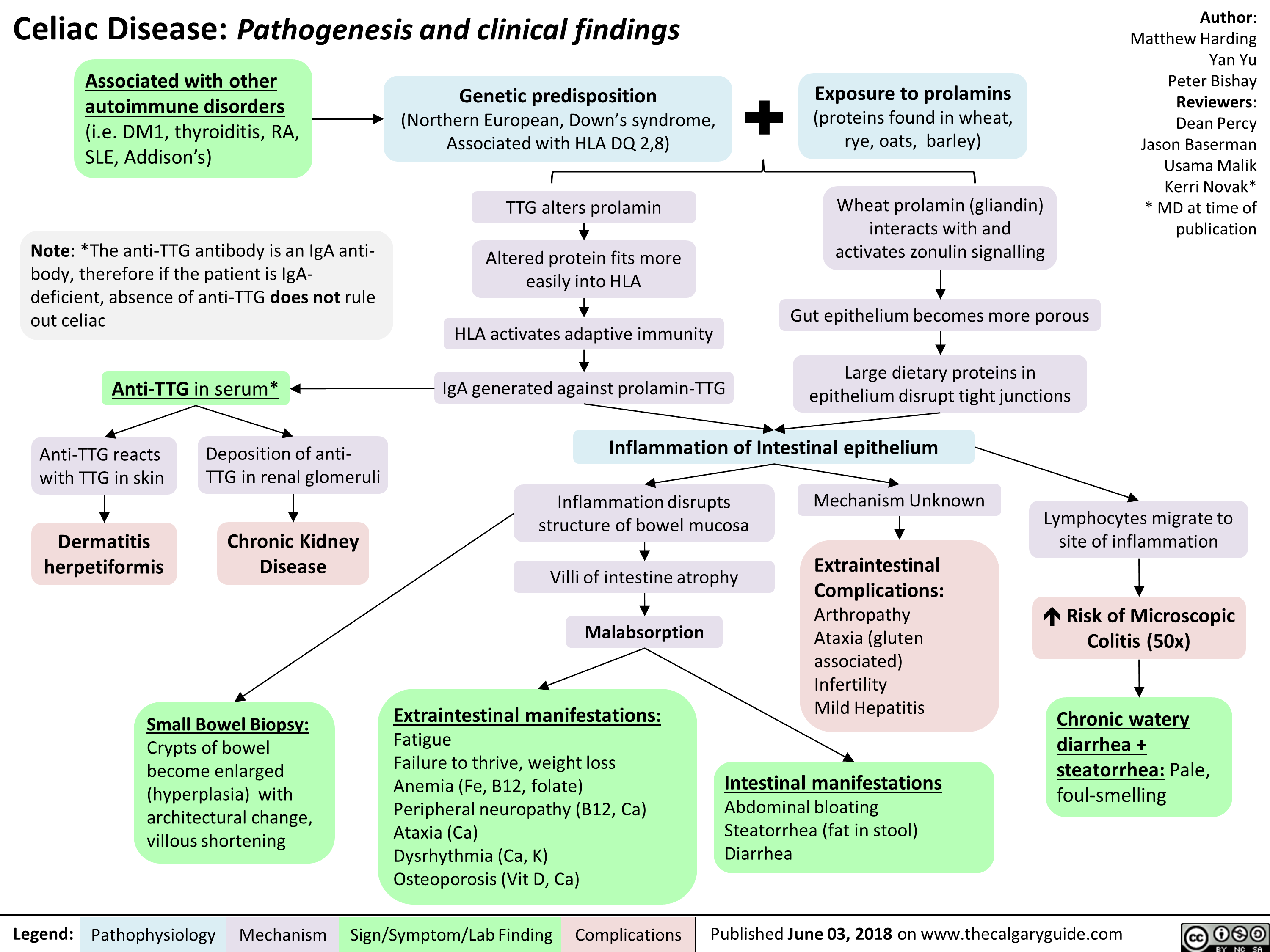
Celiac Disease: Pathogenesis and clinical findings
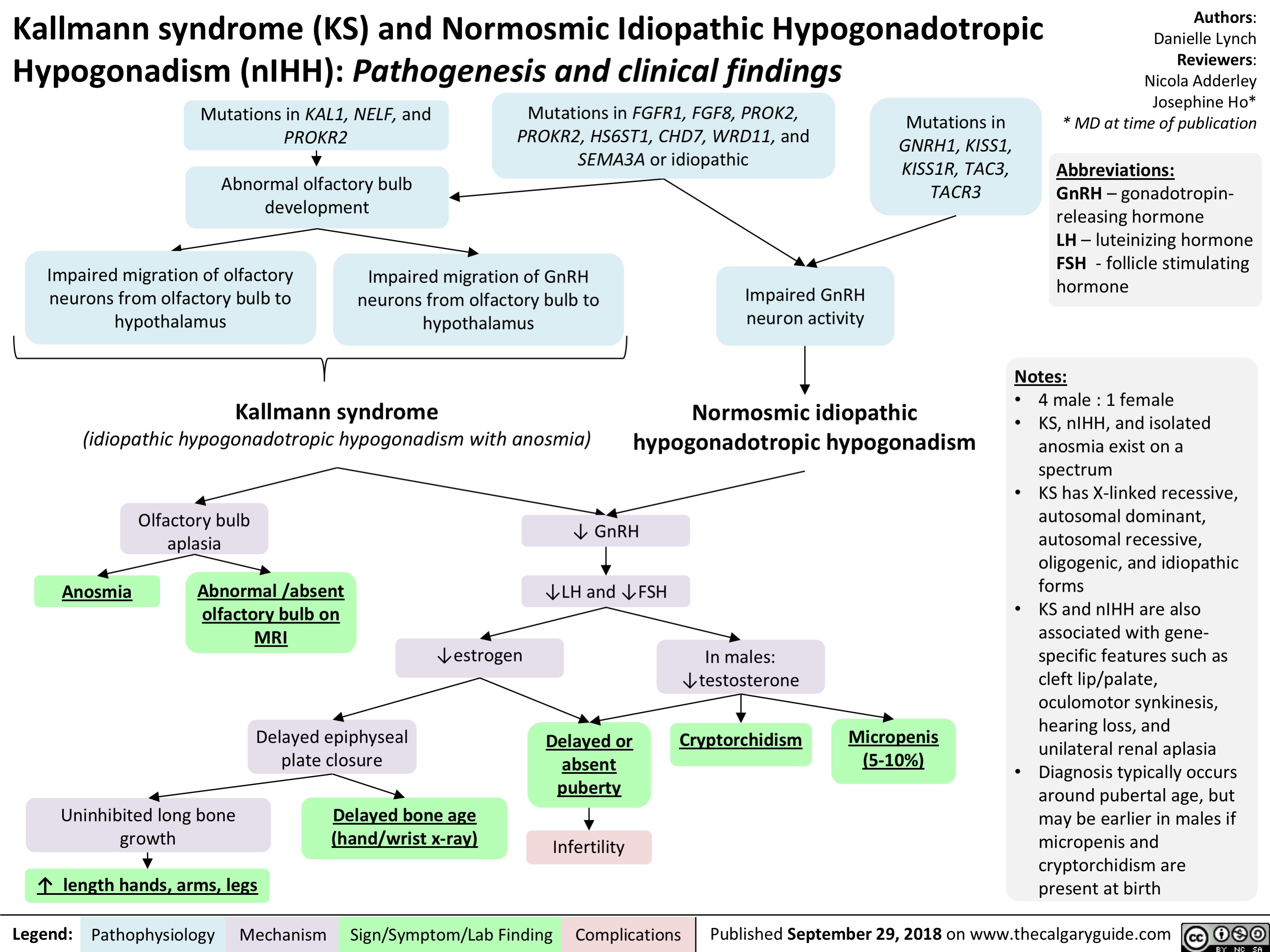
Kallmann Syndrome and Normosmotic Idiopathic Hypogonadotropism: Pathogenesis and Clinical Findings
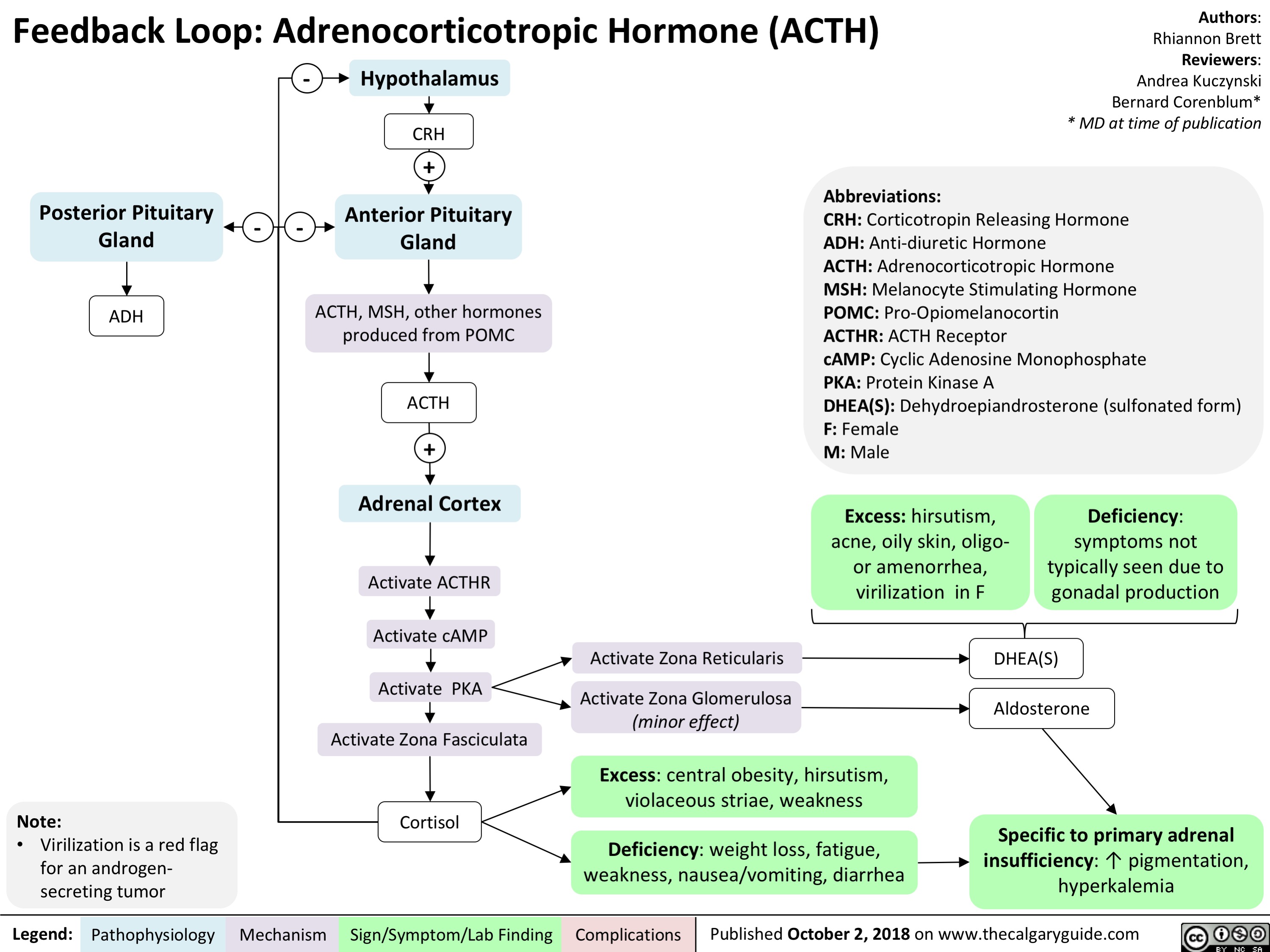
Feedback Loop: Adrenocorticotropic Hormone (ACTH)
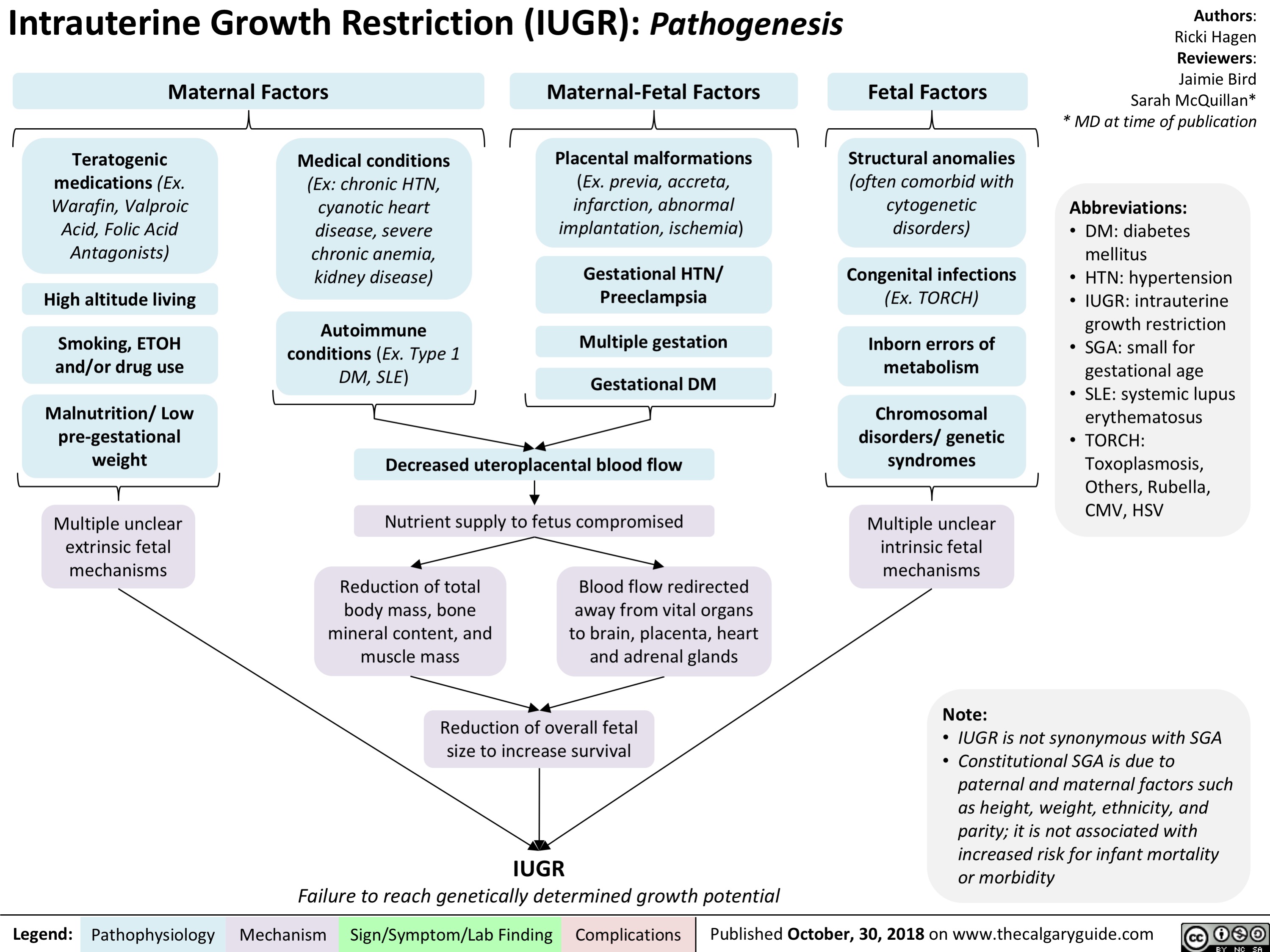
intrauterine-growth-restriction-iugr-pathogenesis
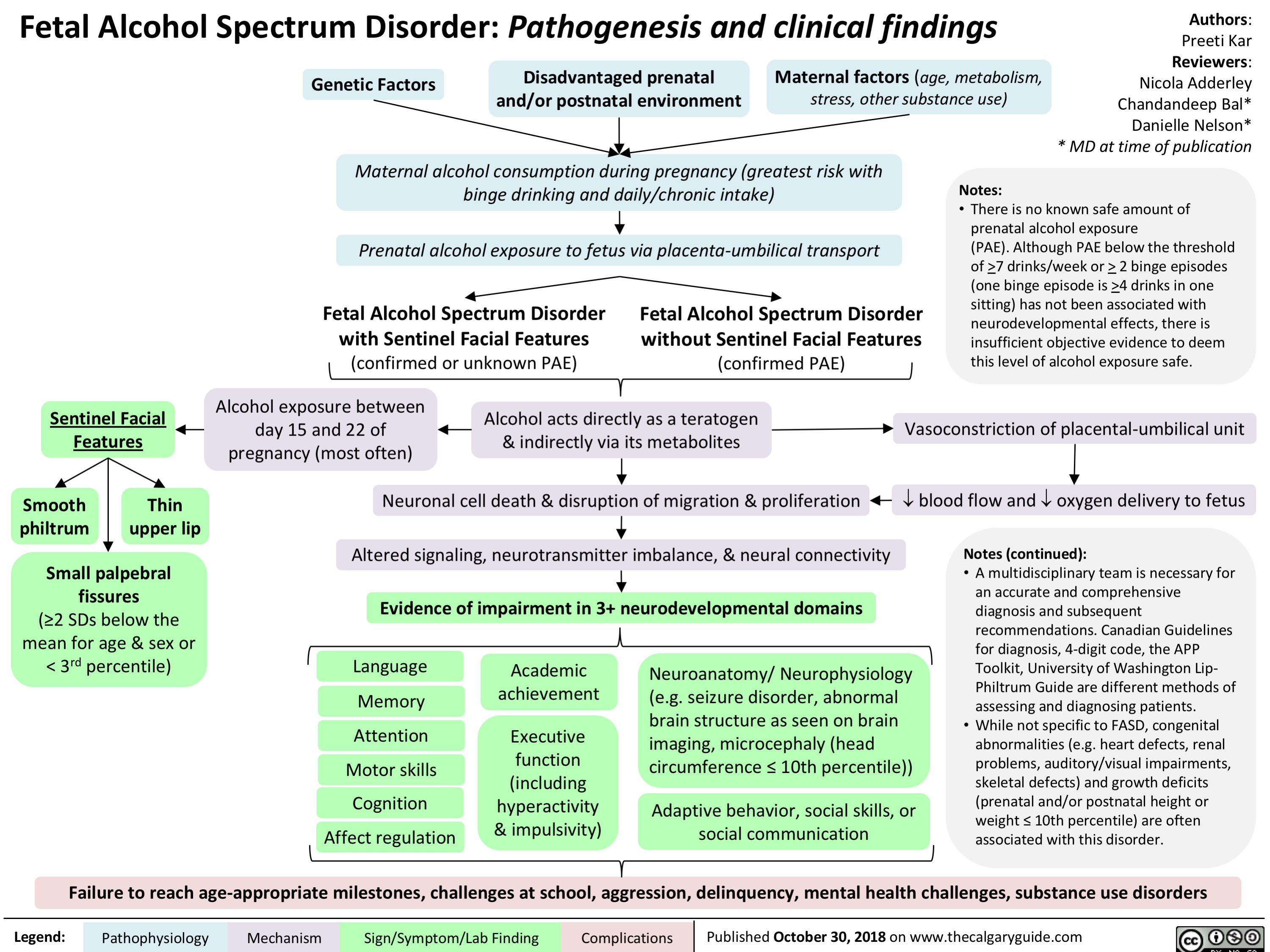
fetal-alcohol-spectrum-disorder-pathogenesis-and-clinical-findings
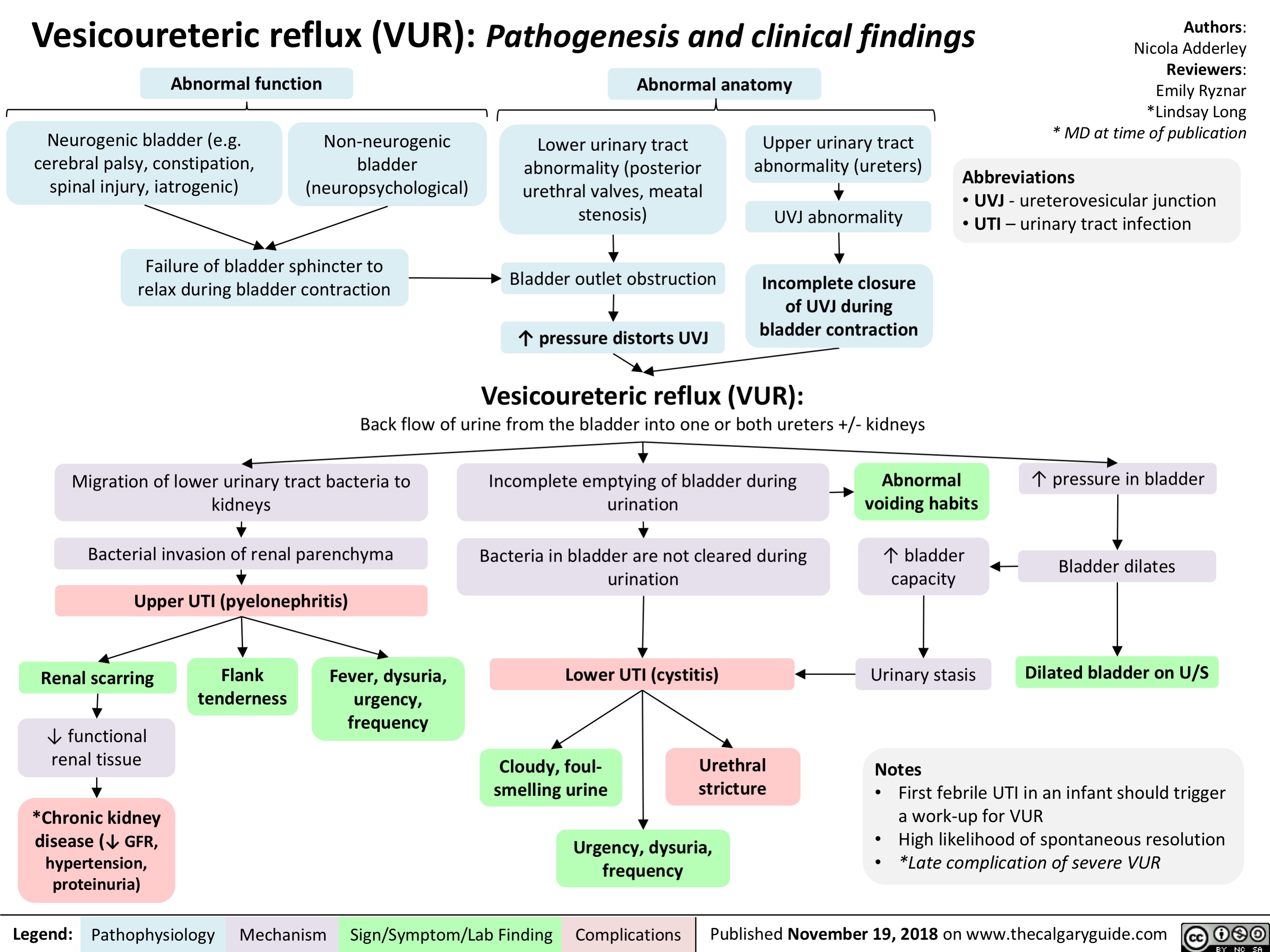
Vesicoureteric reflux (VUR): Pathogenesis and clinical findings
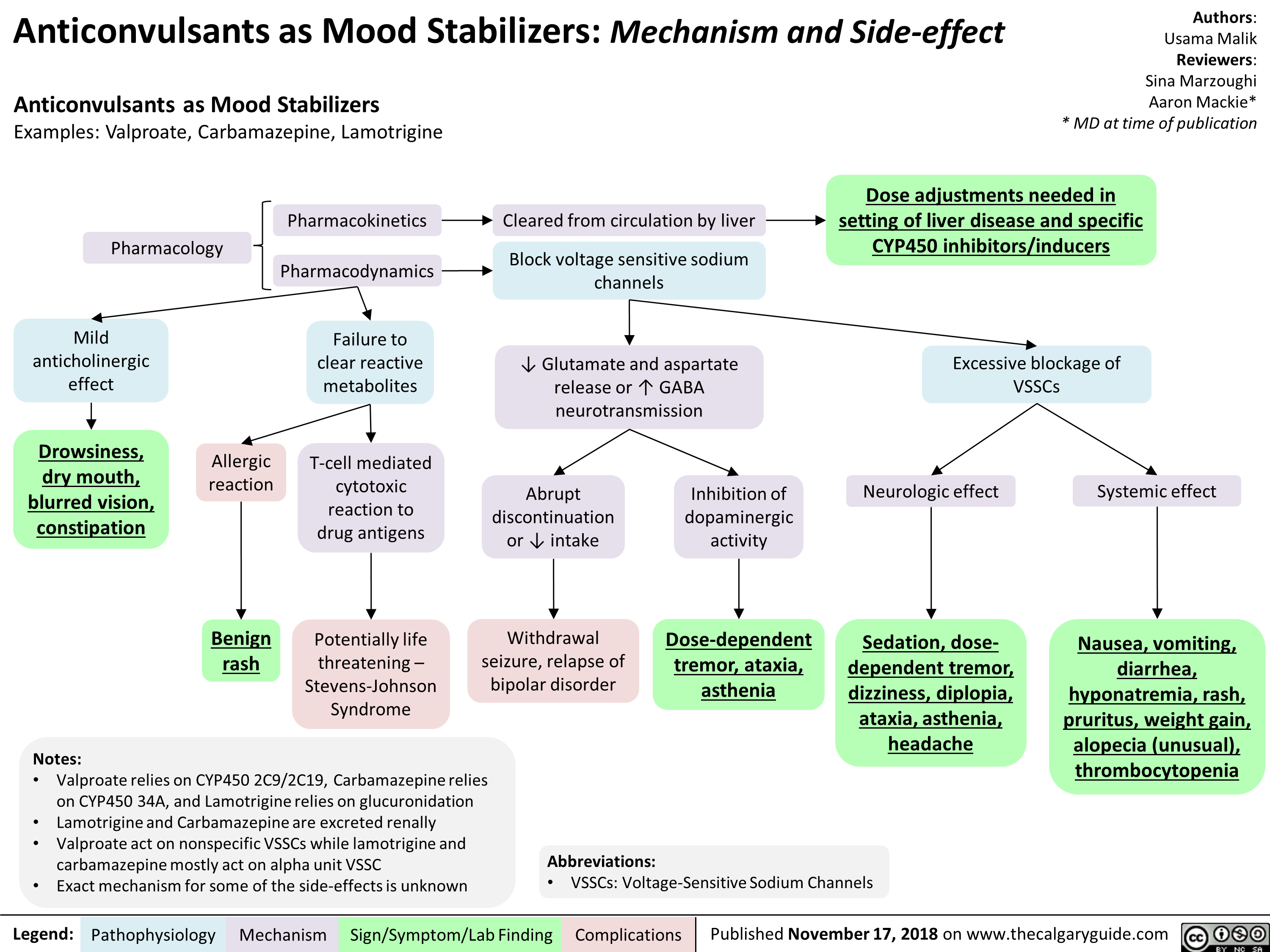
Anticonvulsants as Mood Stabilizers: Mechanism and Side-effect
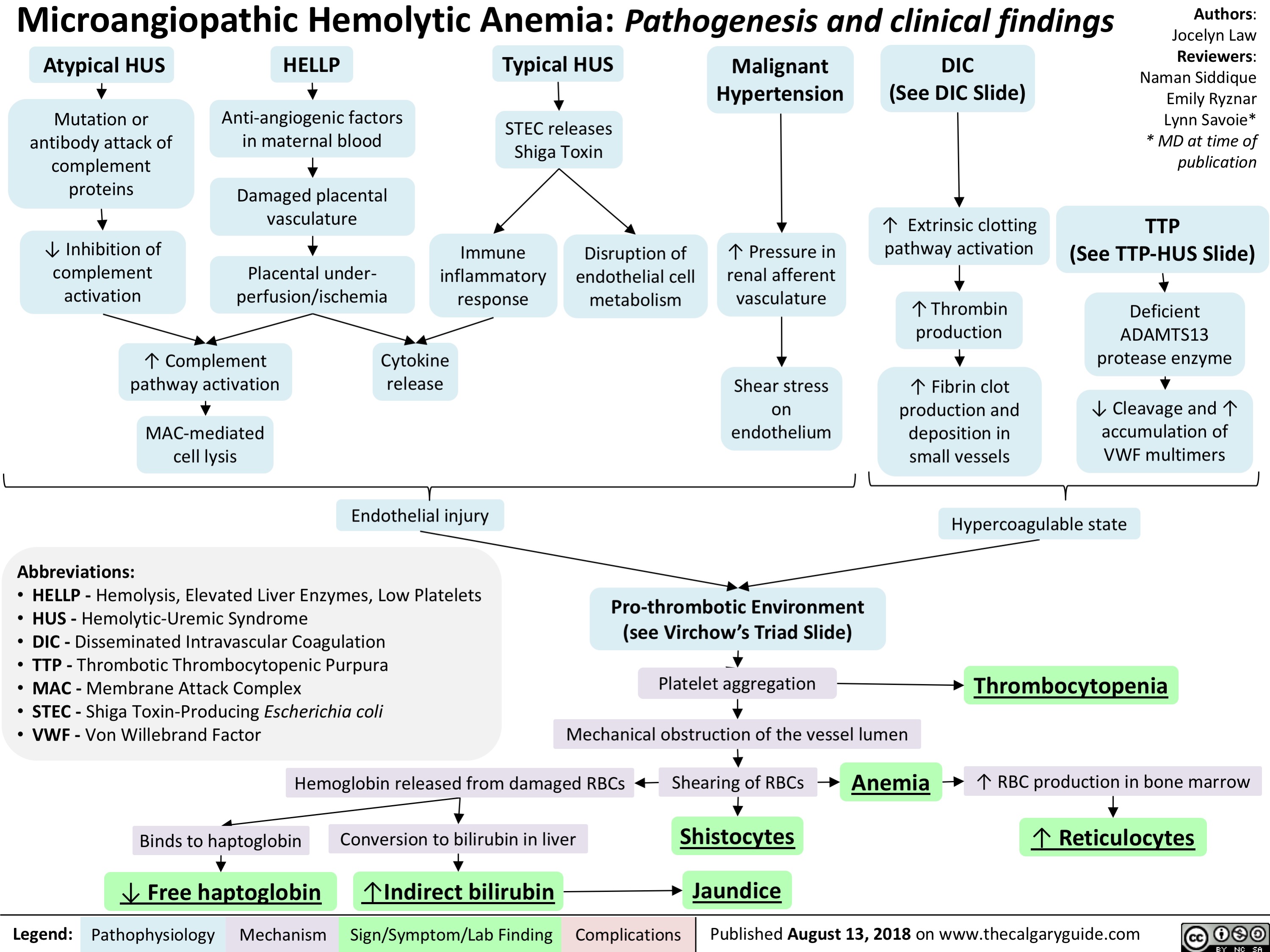
Microangiopathic Hemolytic Anemia: Pathogenesis and clinical findings
Hypernatremia Physiology
![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)
Hyponatremia- Physiology
![Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hyponatremia-Physiology-.jpg)
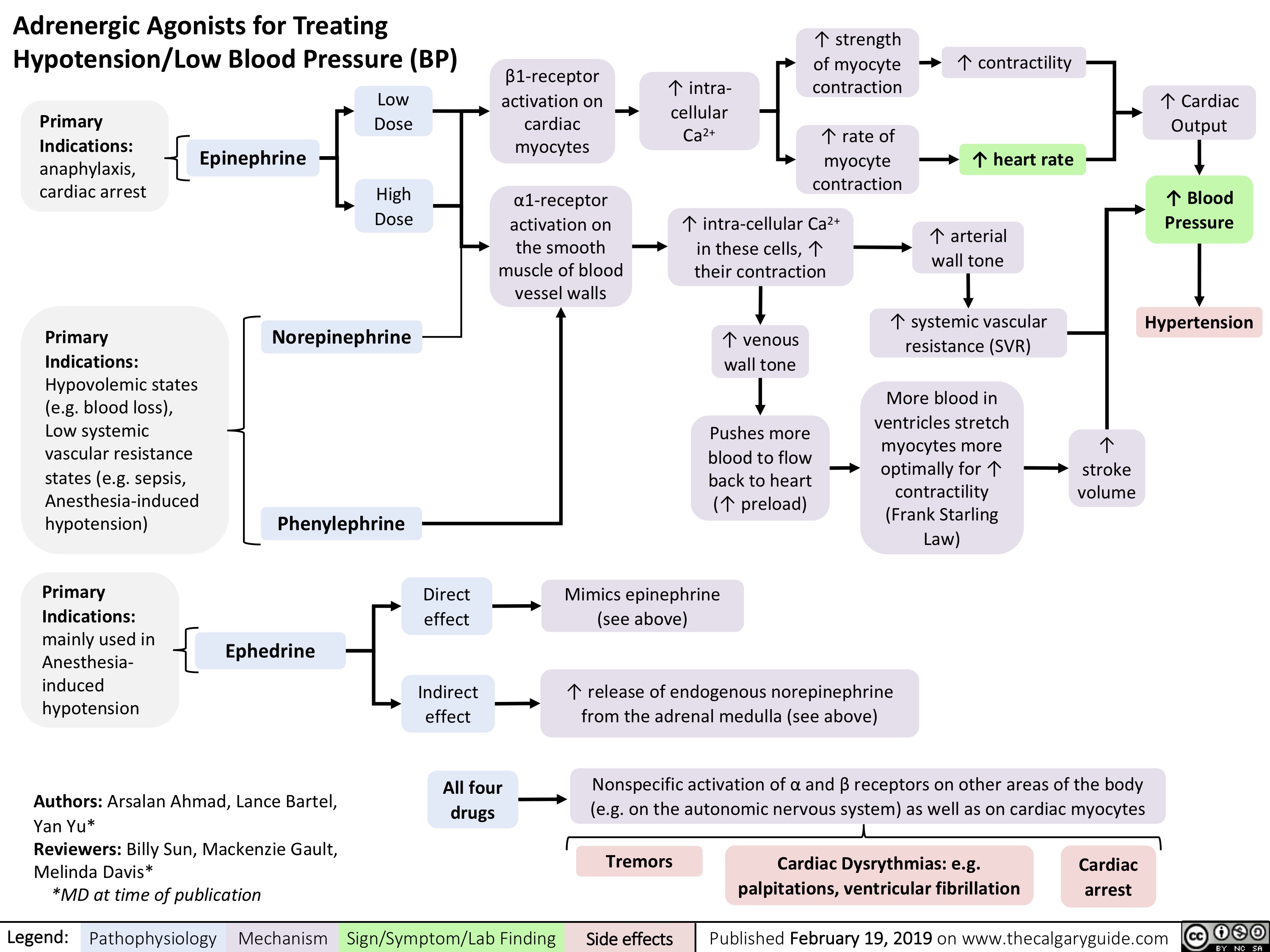
adrenergic-agonists-for-treating-hypotensionlow-blood-pressure
Hyperkalemia- Physiology
![Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hyperkalemia-Physiology-.jpg)
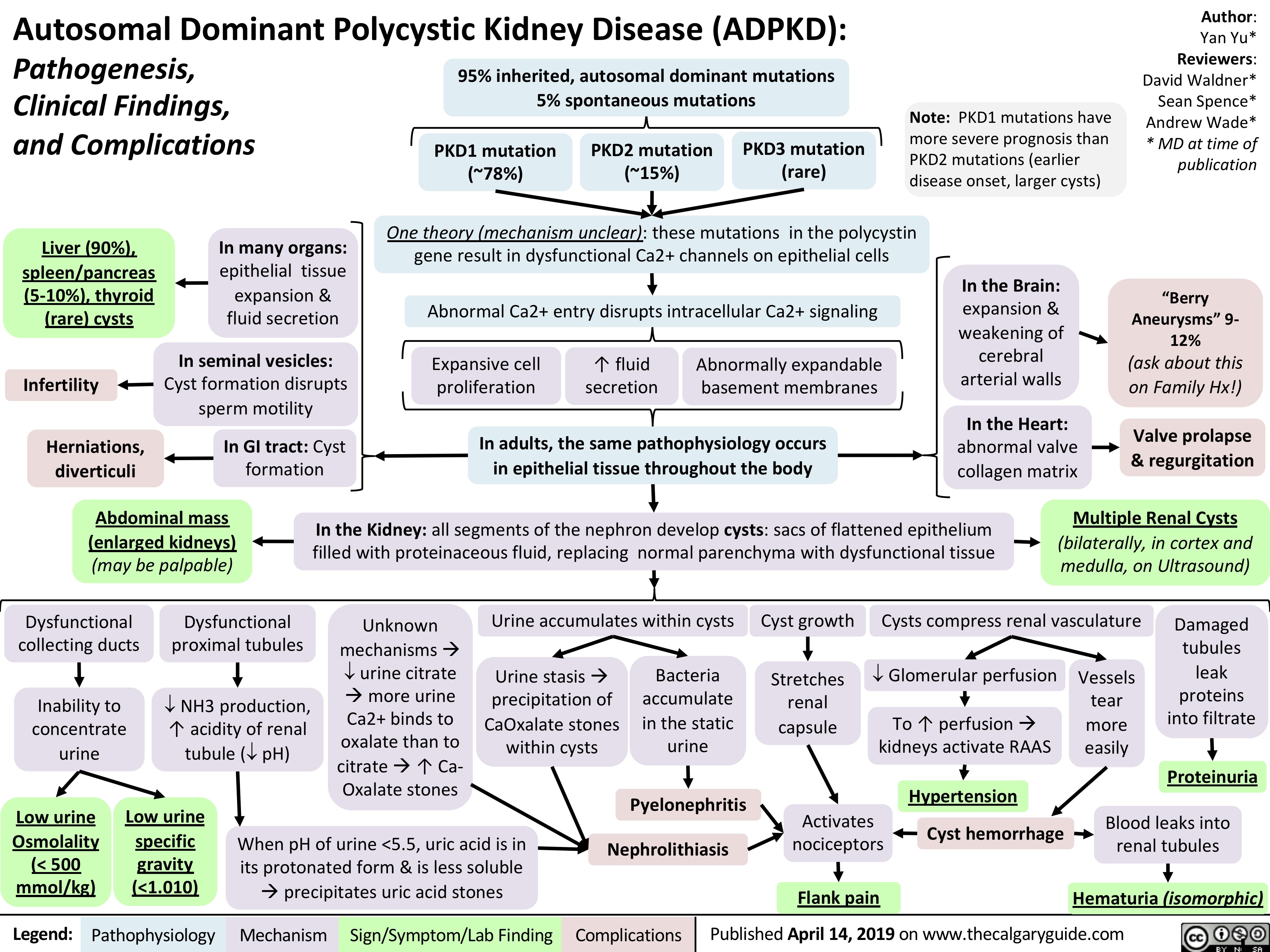
autosomal-dominant-polycystic-kidney-disease-adpkd
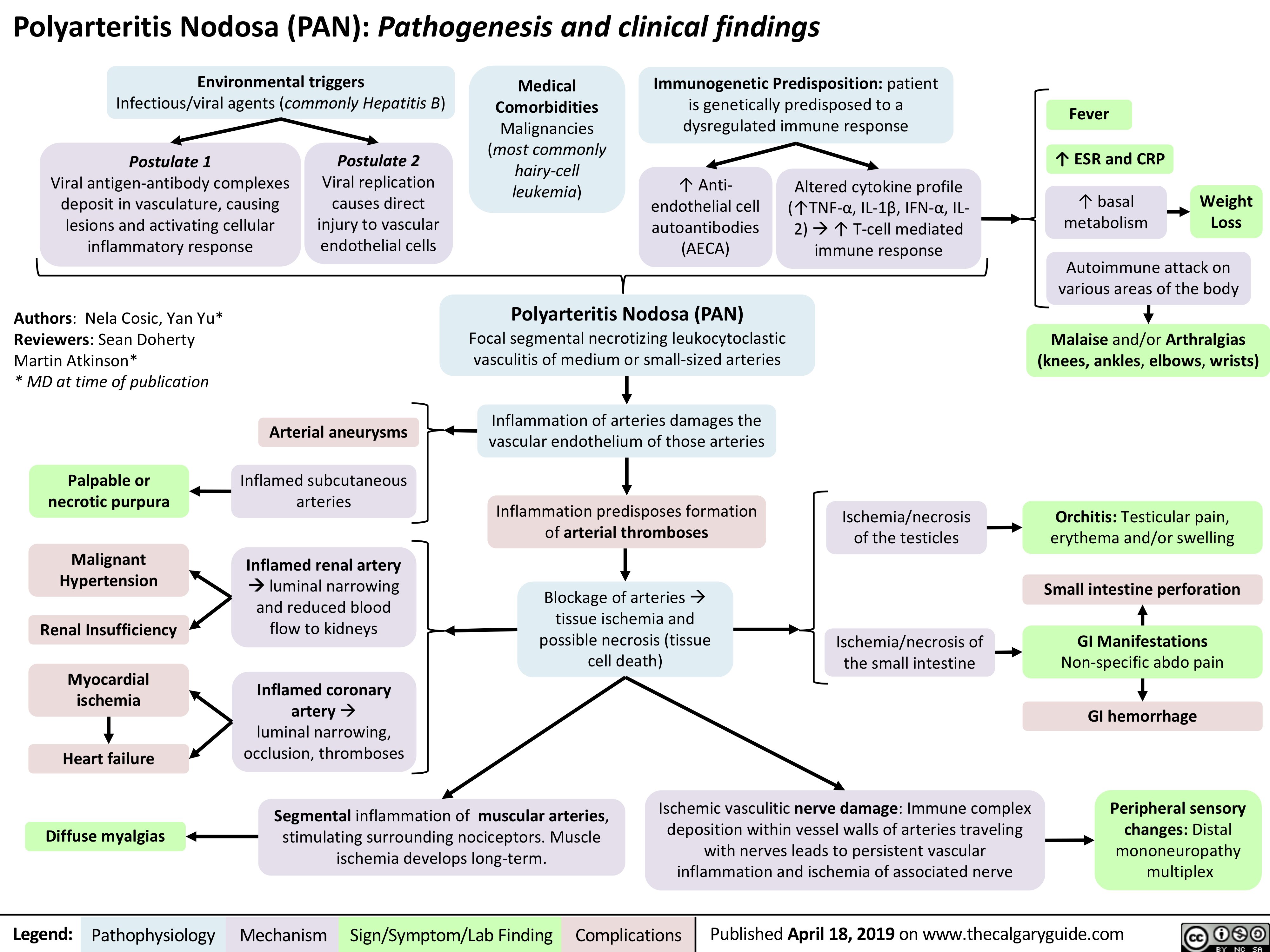
Polyarteritis Nodosa (PAN): Pathogenesis and Clinical Findings
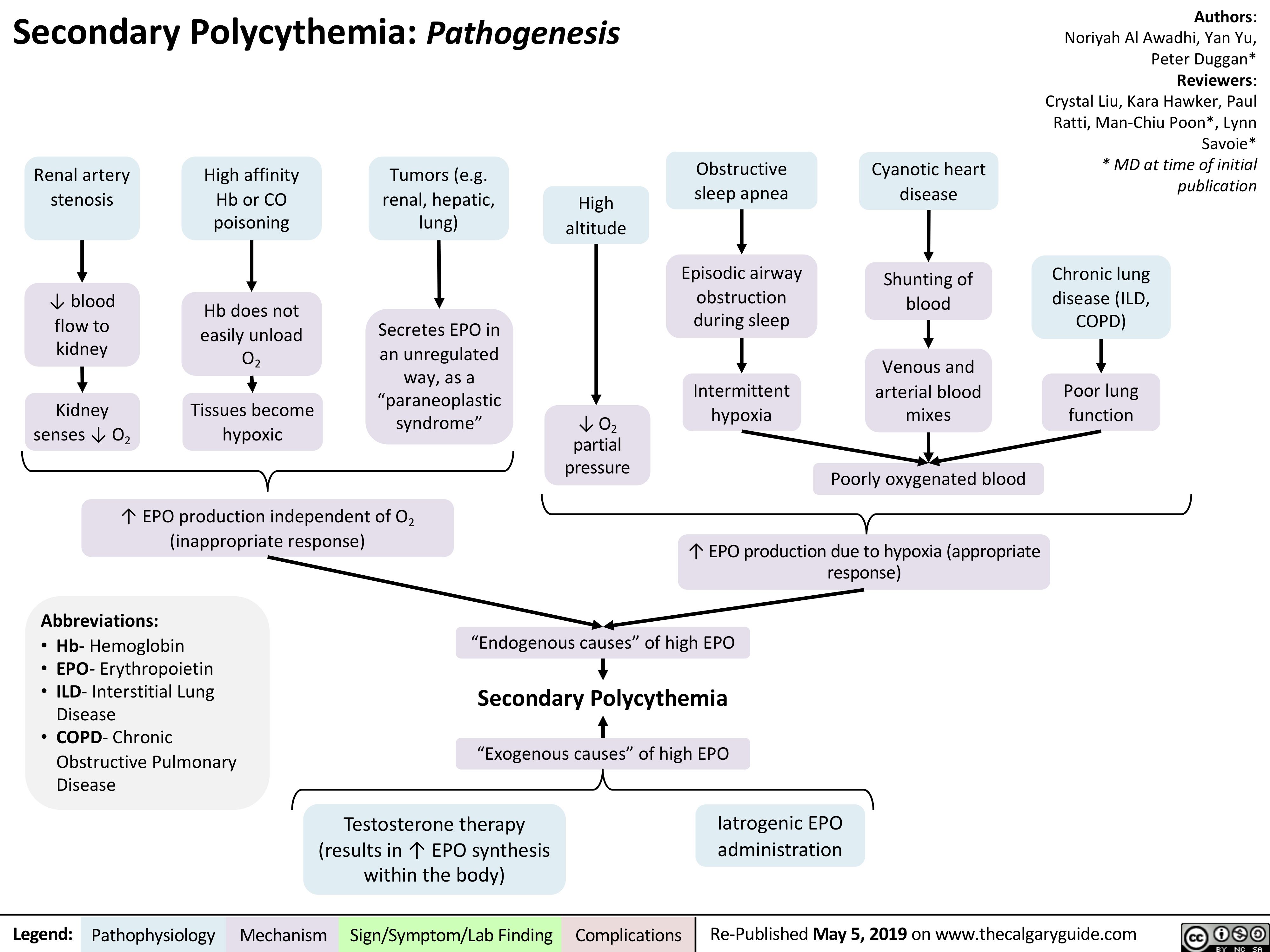
Secondary Polycythemia
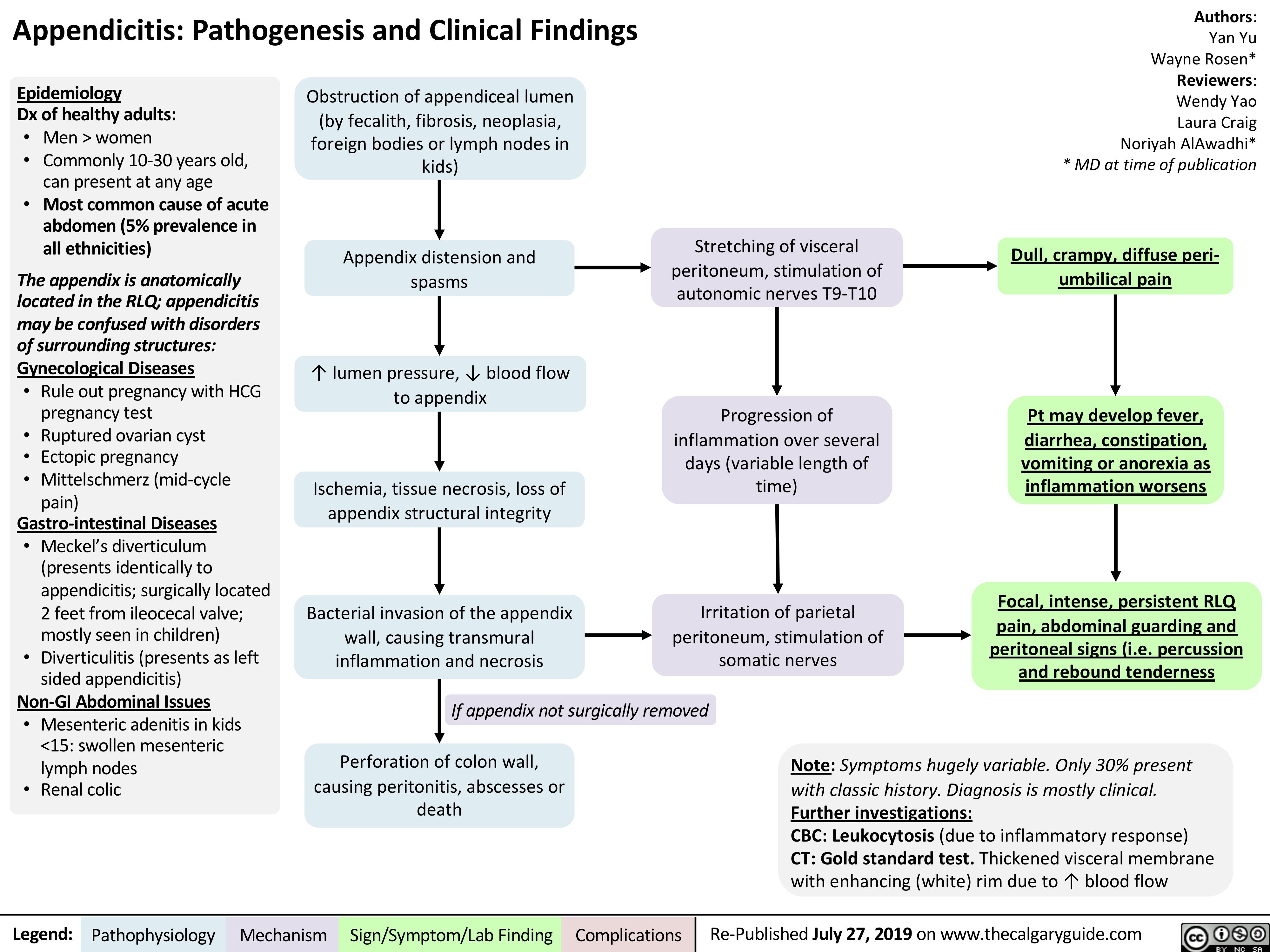
Appendicitis
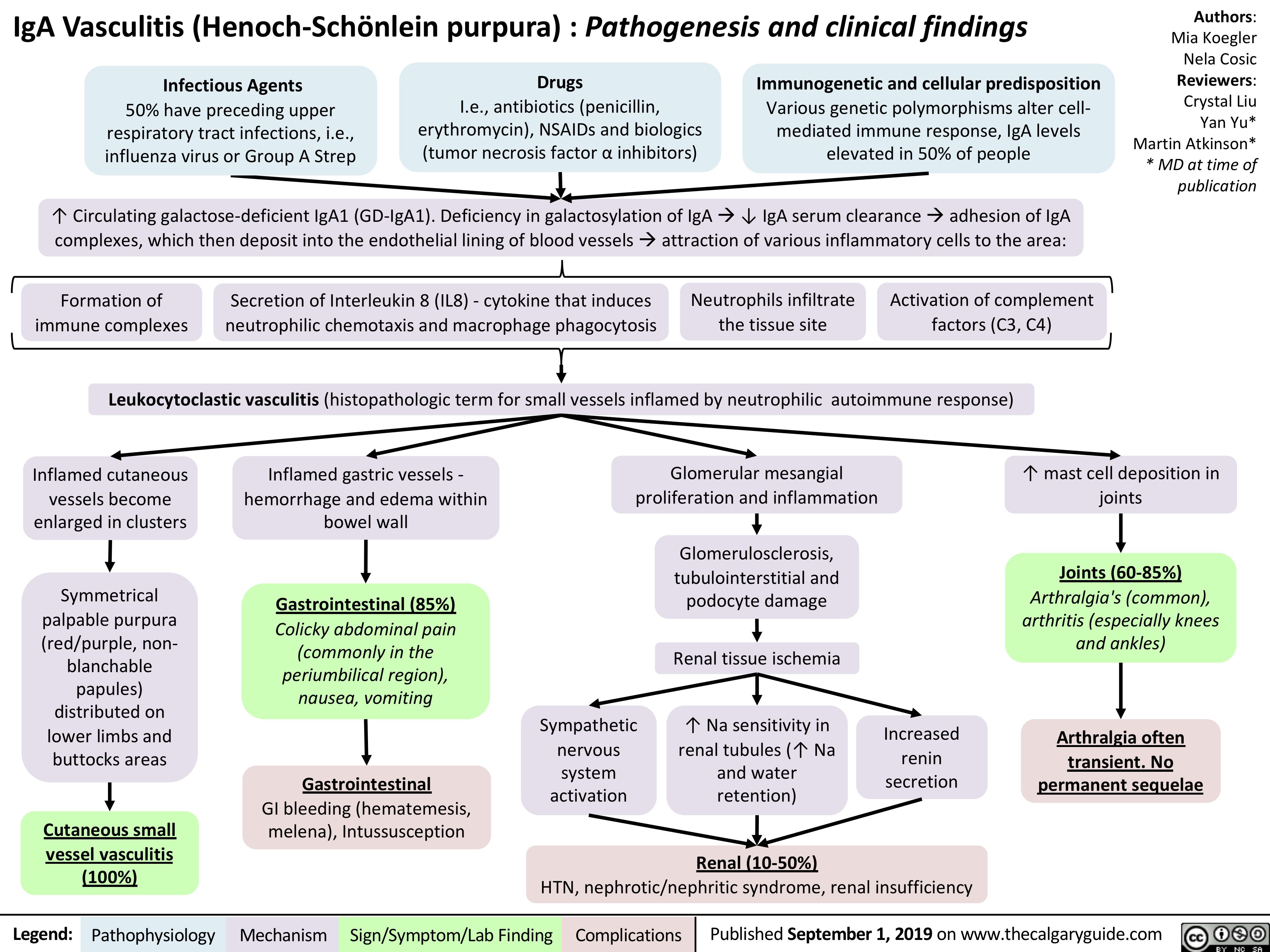
iga-vasculitis-henoch-scholein-purpura-pathogenesis-and-clinical-findings
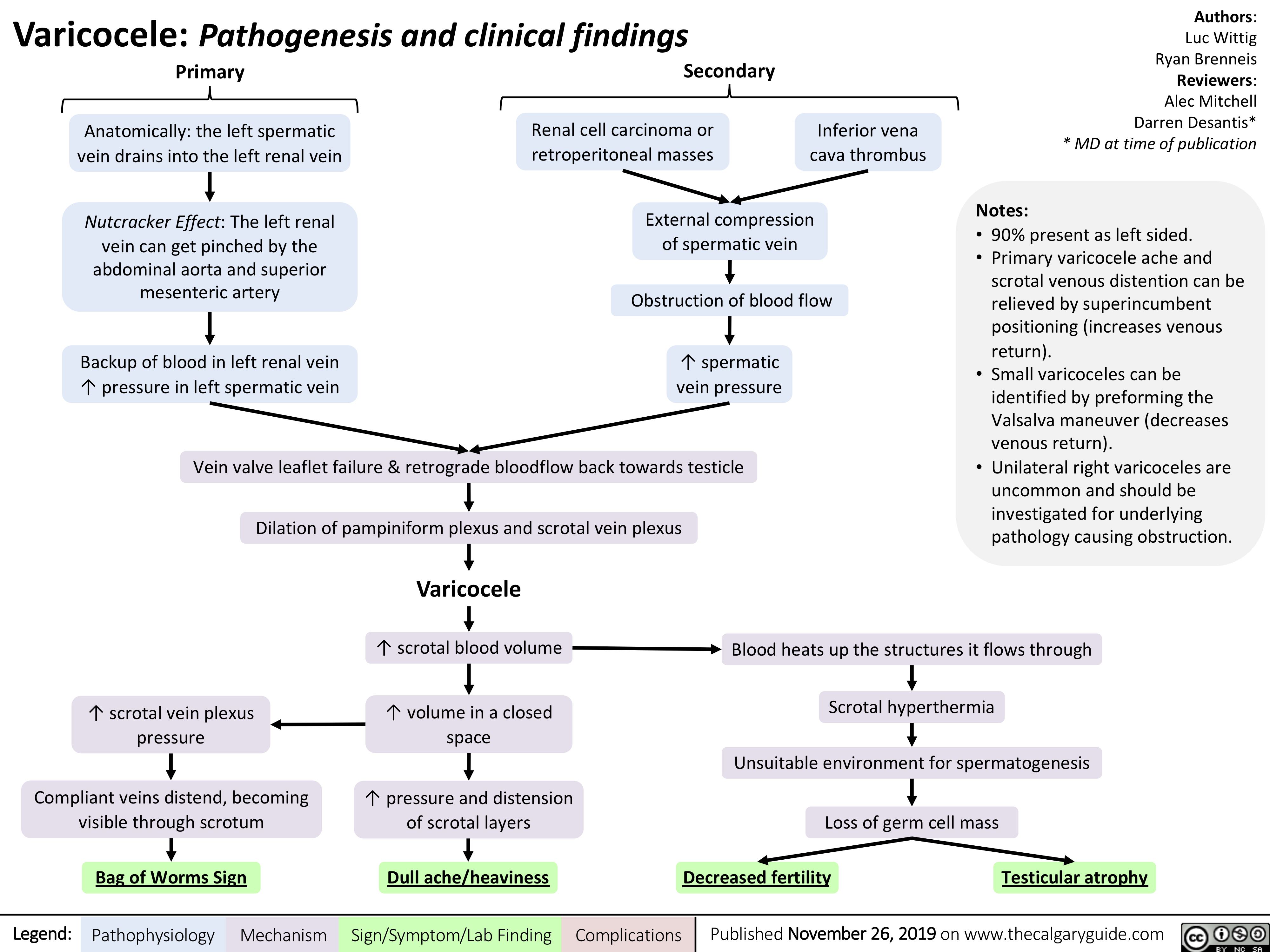
Varicocele
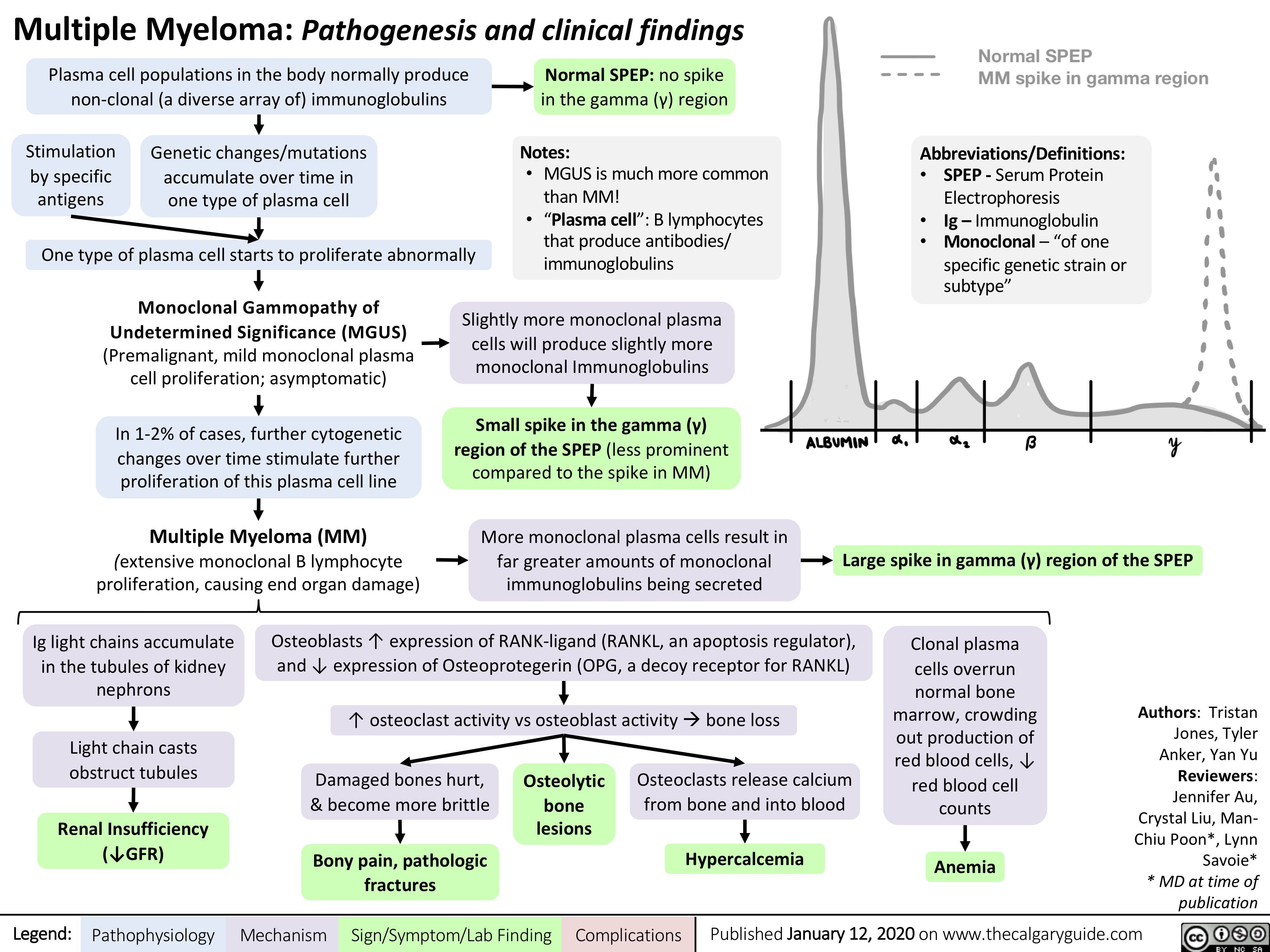
Multiple-Myeloma
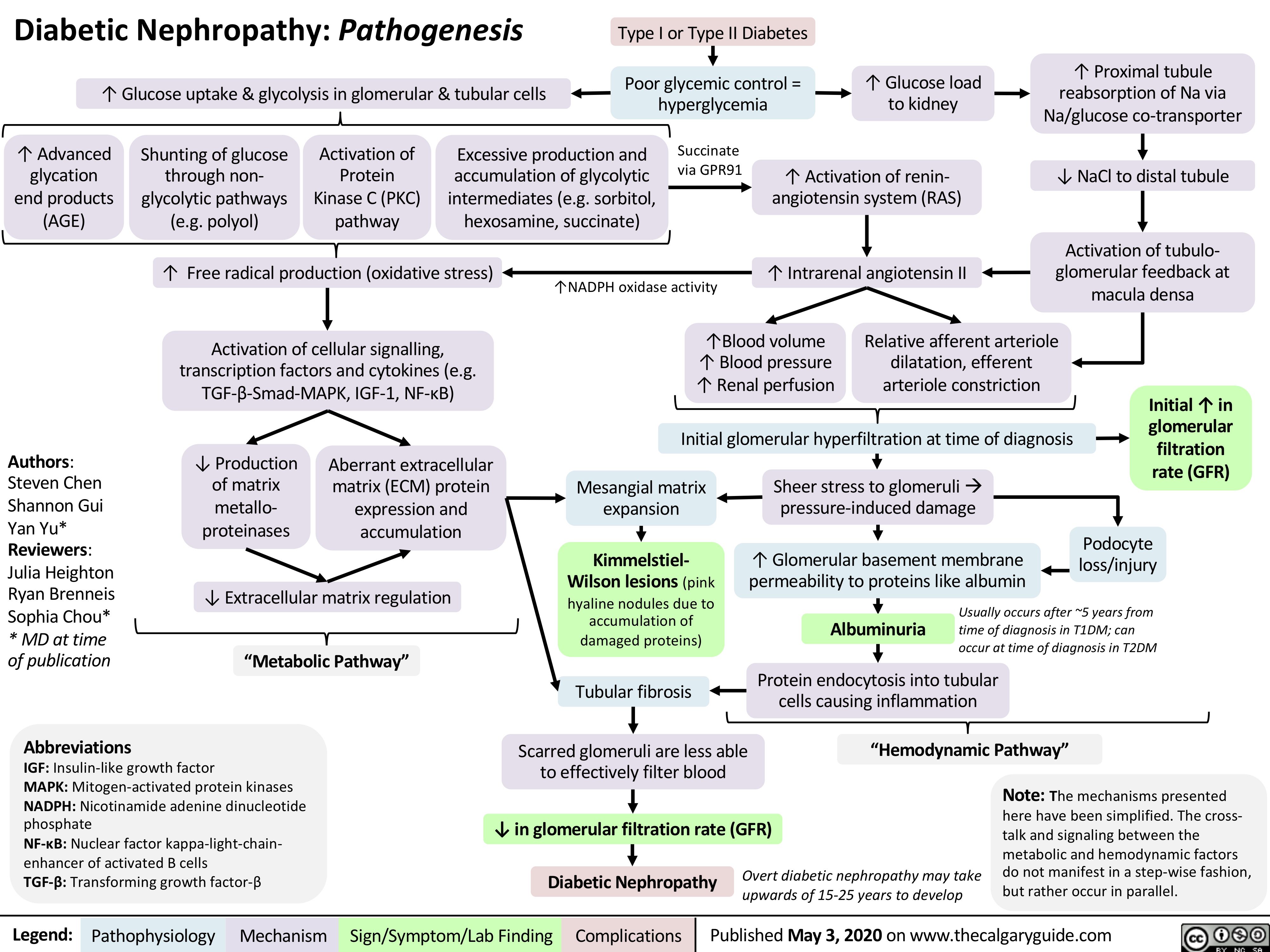
Diabetic-Nephropathy
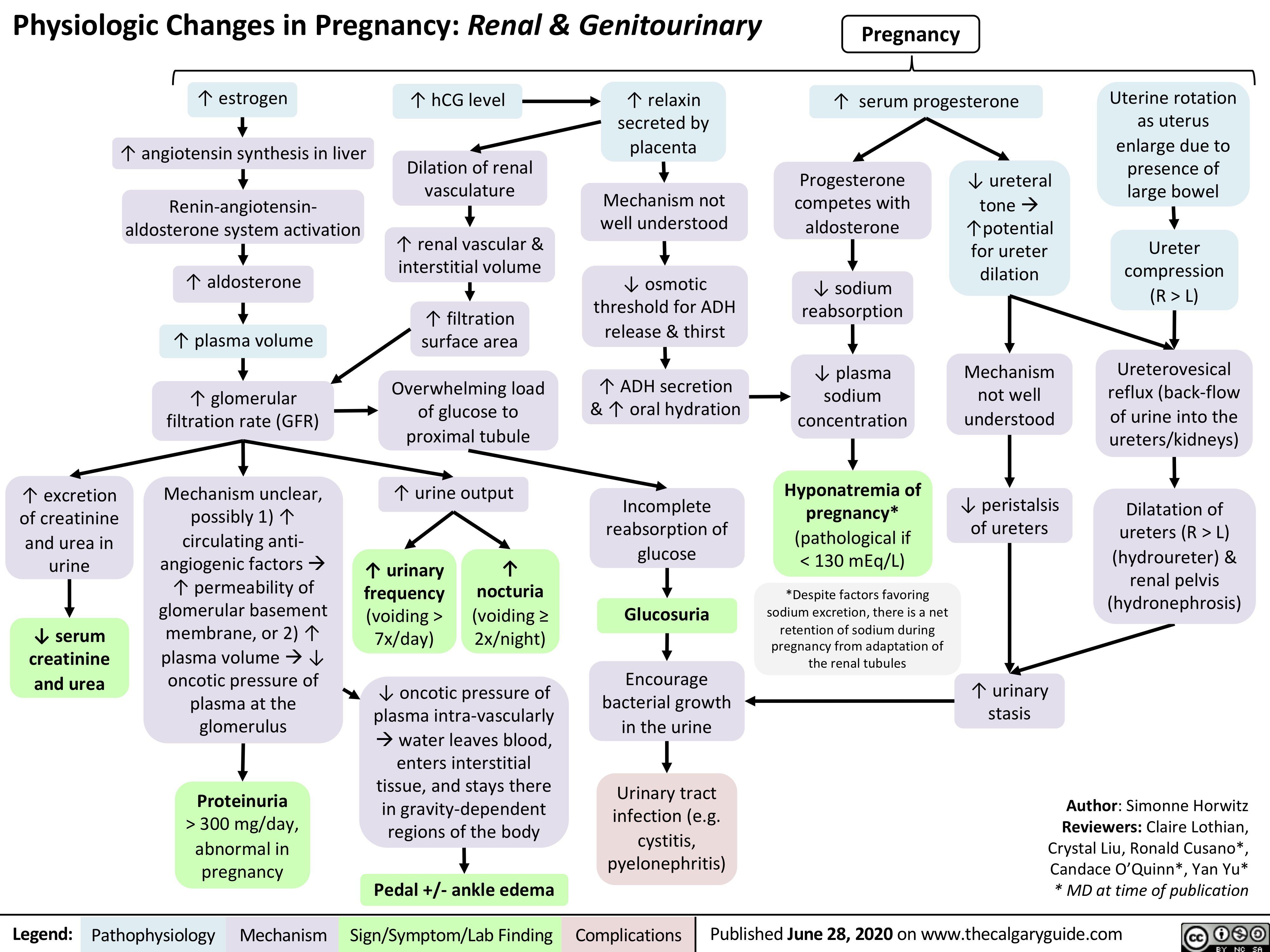
GU-changes-in-pregnancy
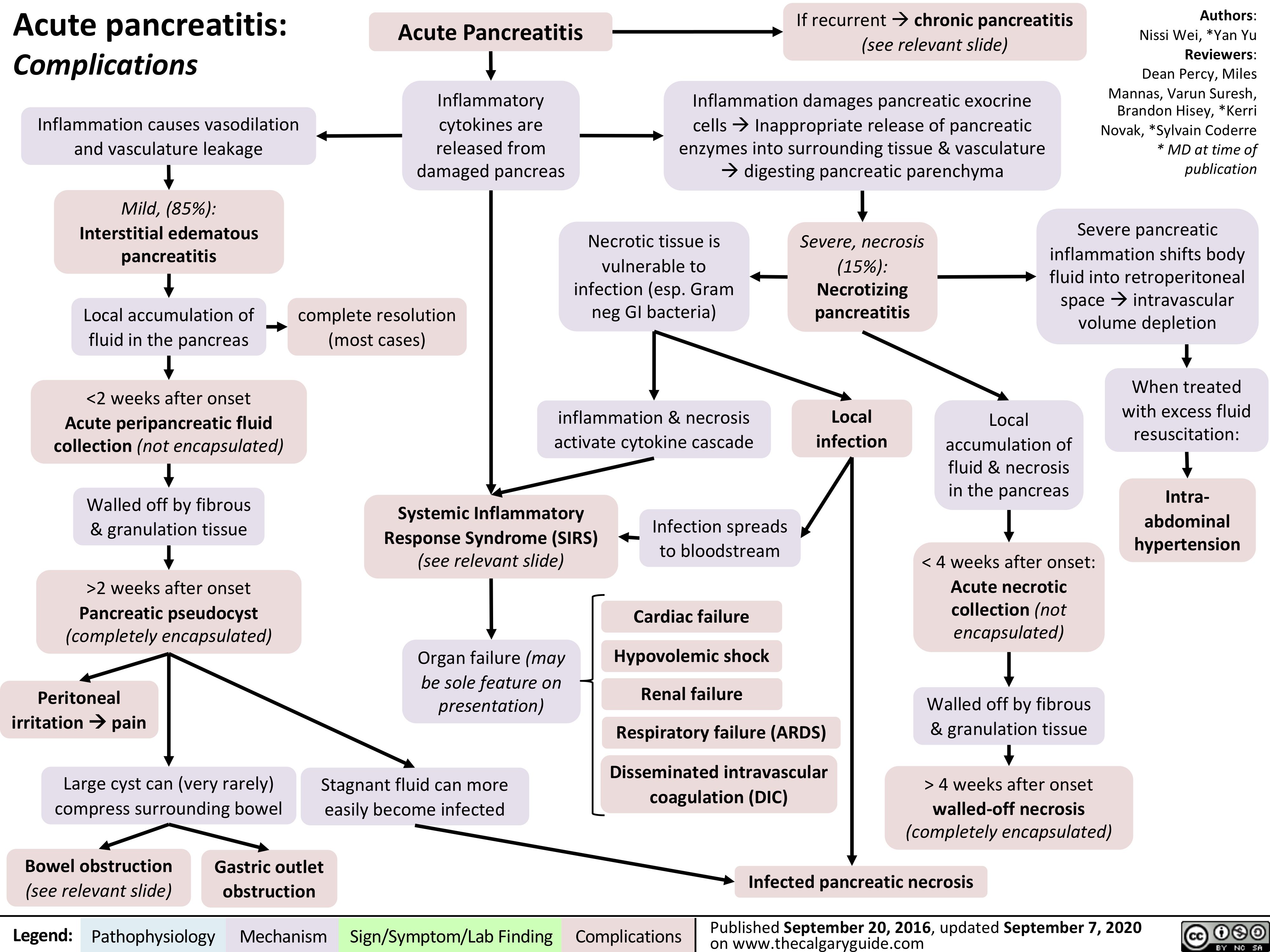
acute-pancreatitis-complications
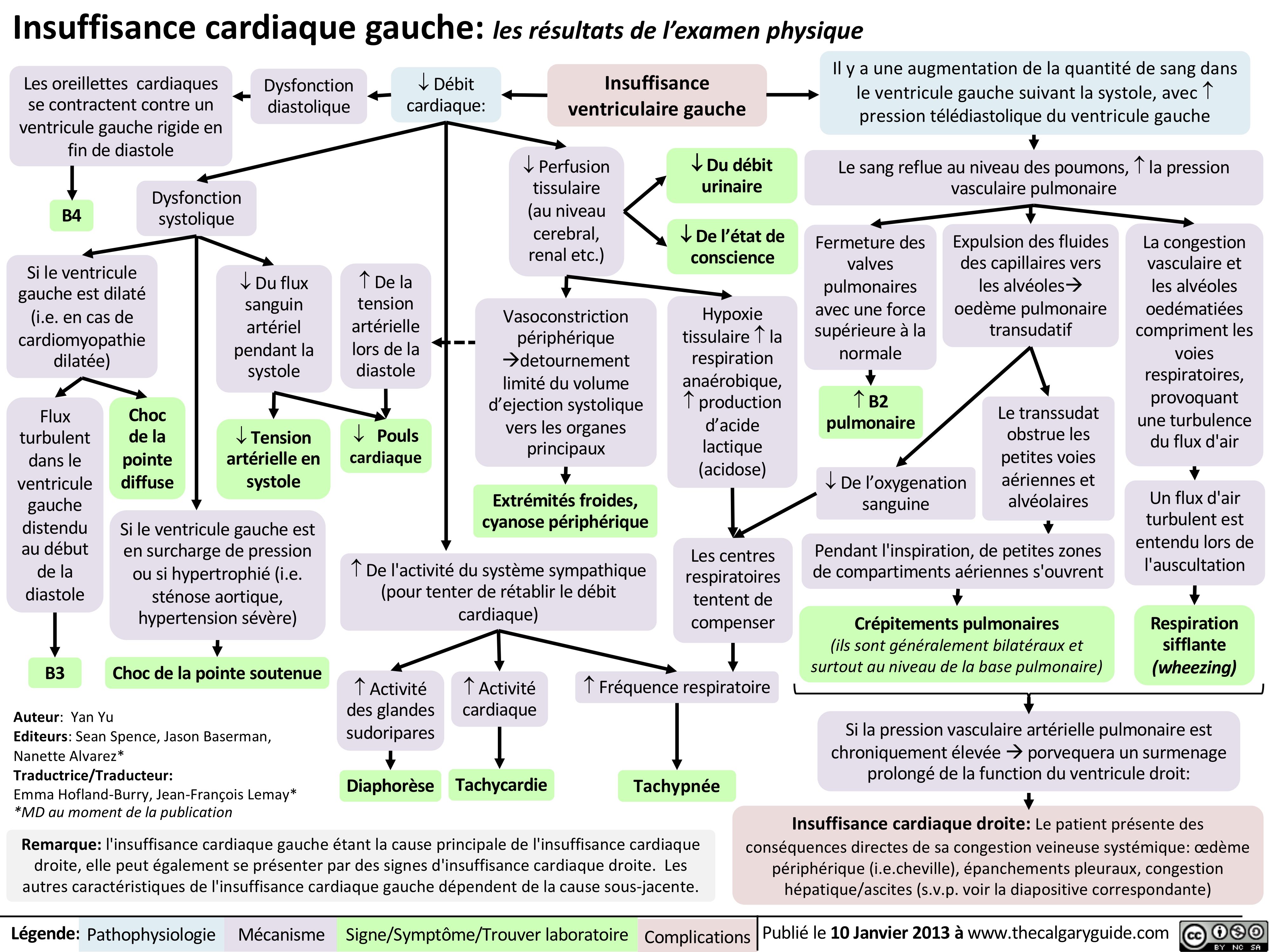
insuffisance-cardiaque-gauche-les-resultats-de-lexamen-physique
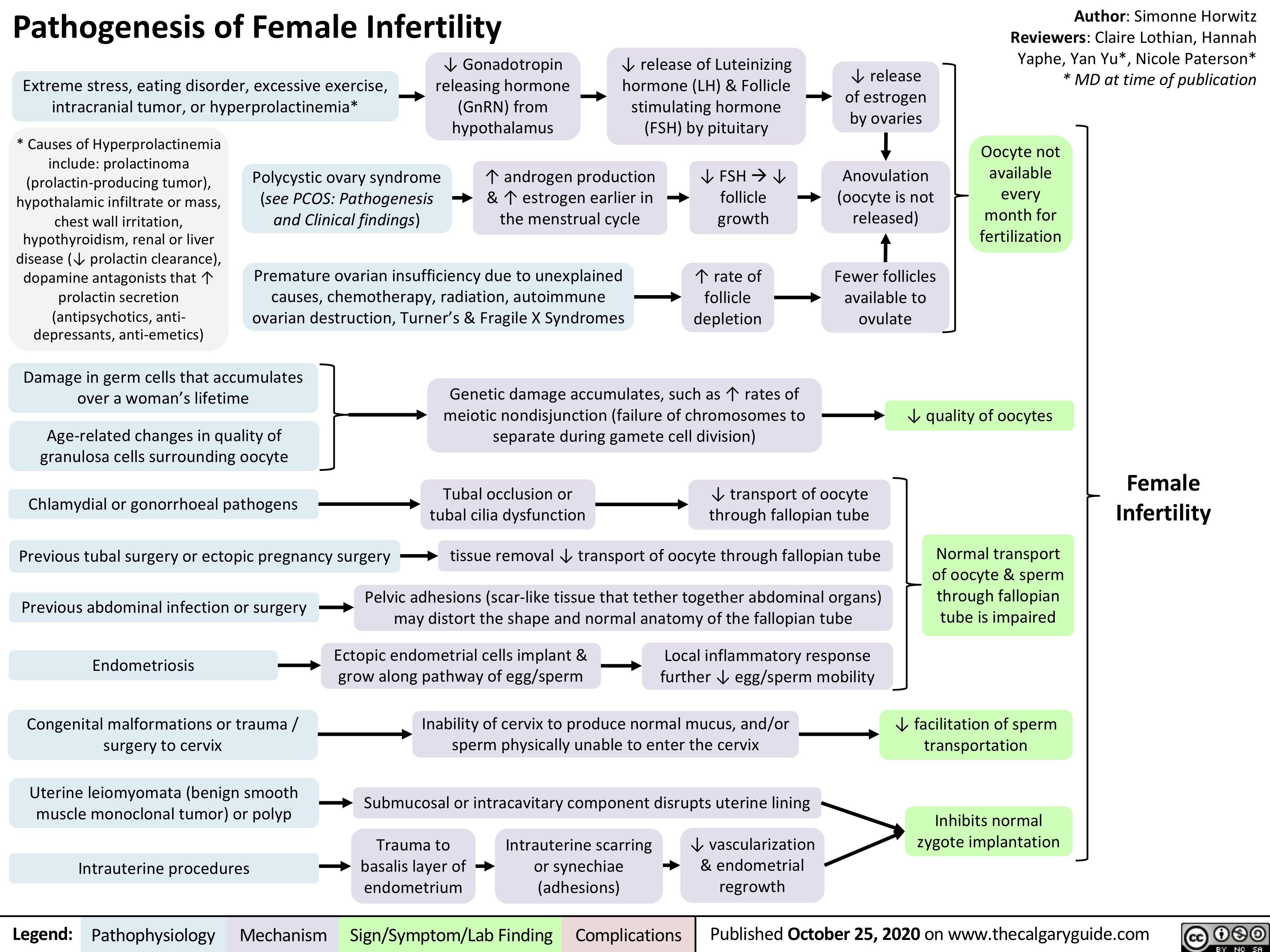
Pathogenesis-of-Female-Infertility
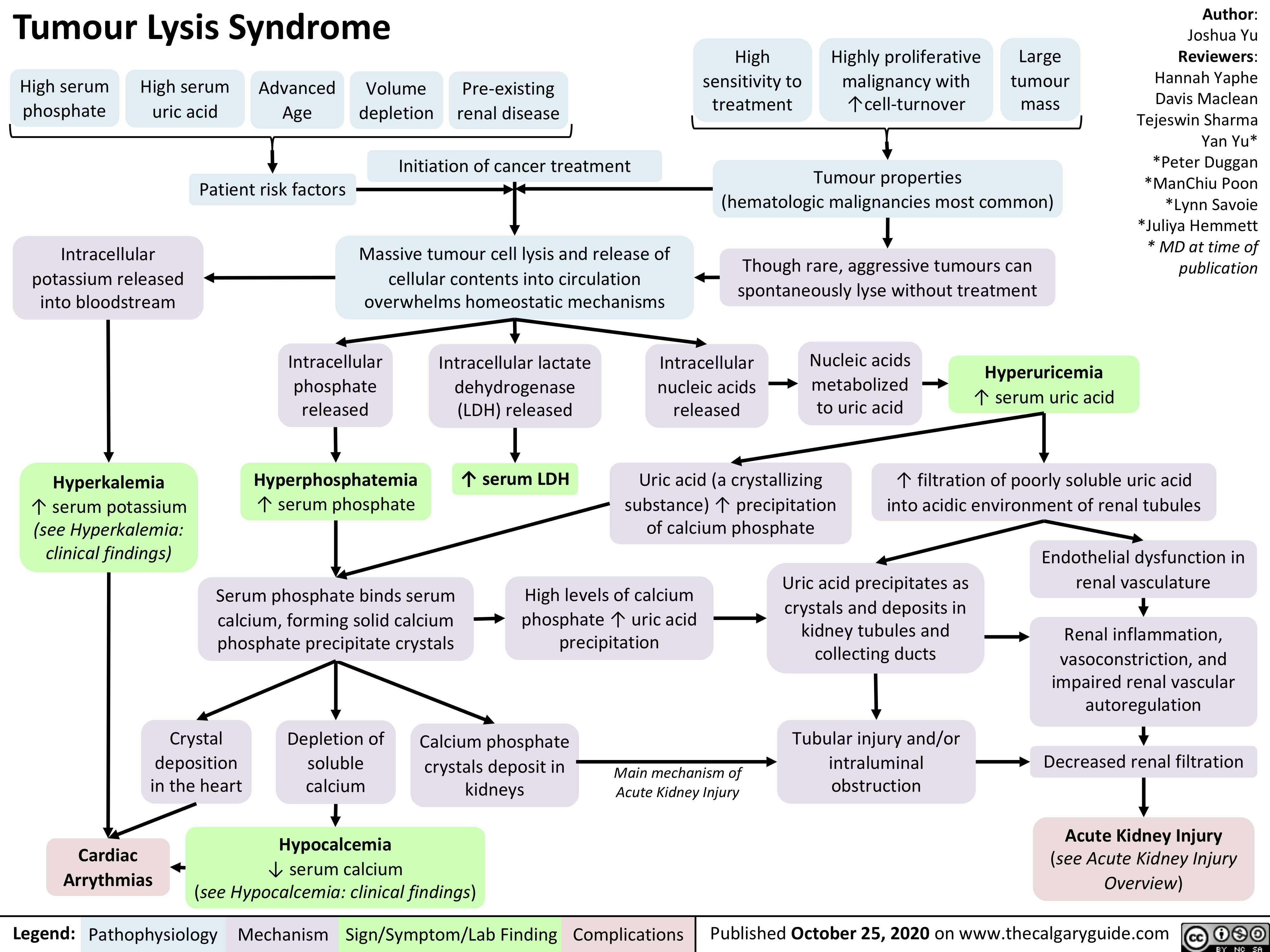
Tumour-Lysis-Syndrome
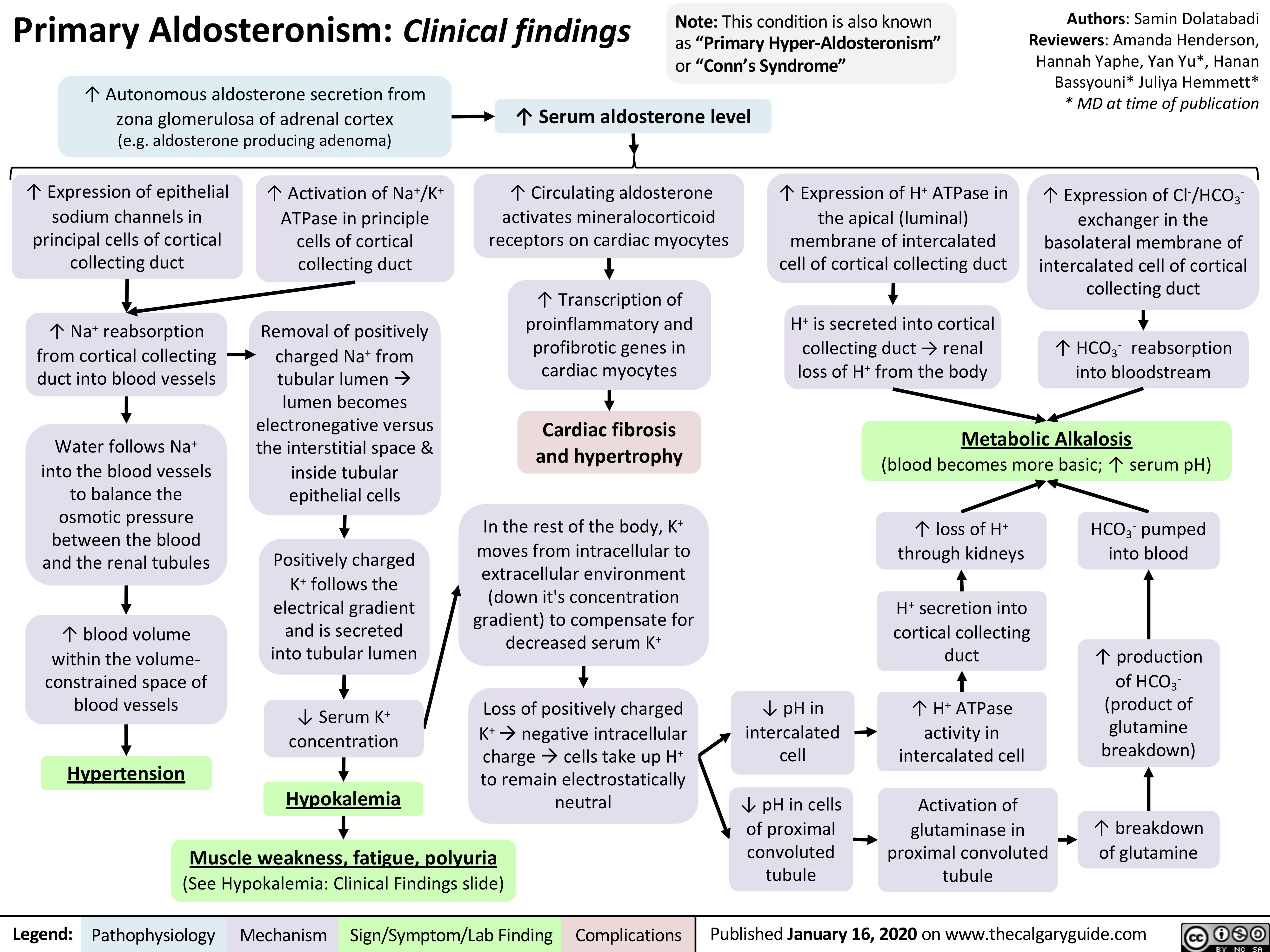
Primary-Aldosteronism
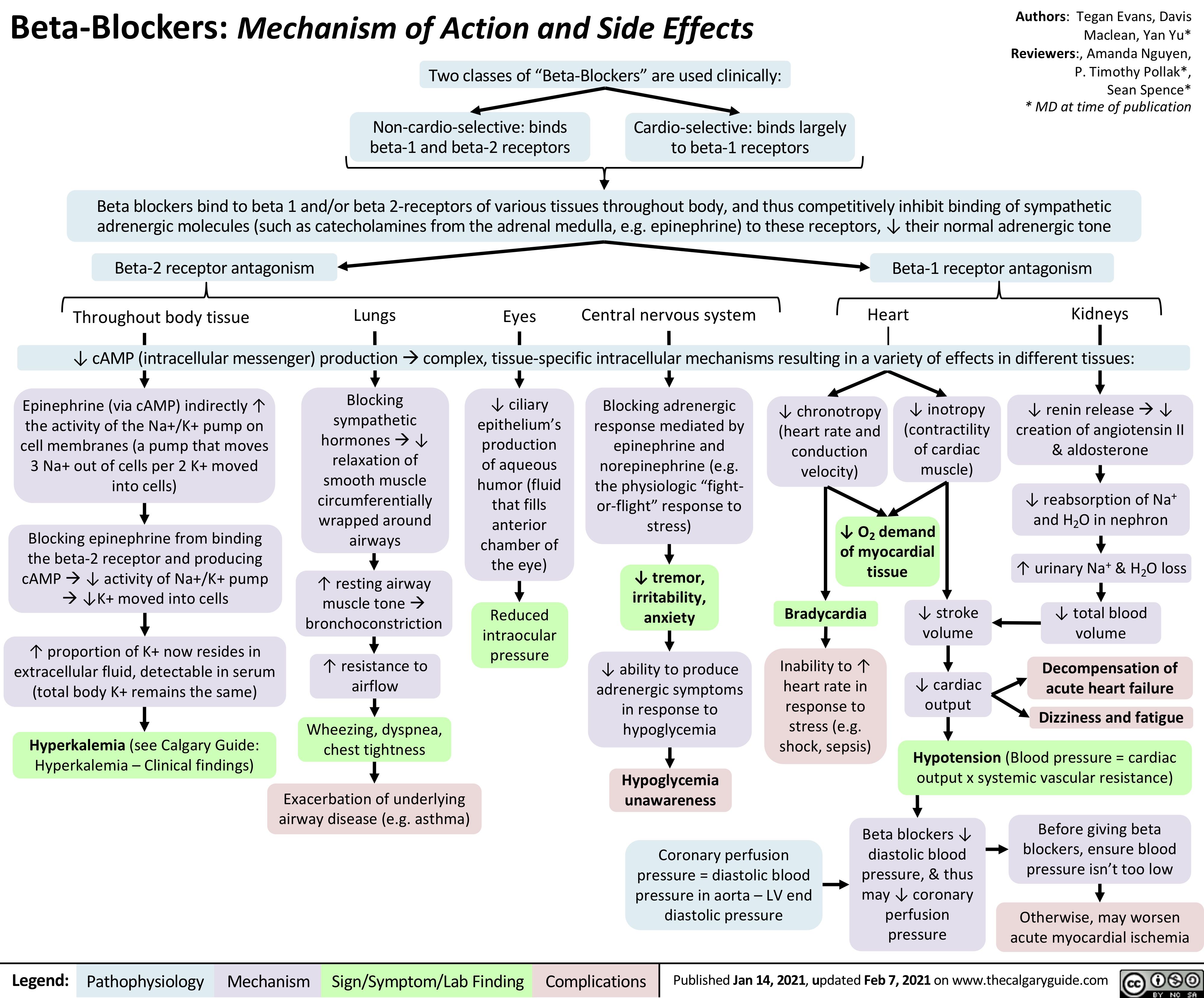
Beta-Blockers-Mechanism-of-Action-and-Side-Effects
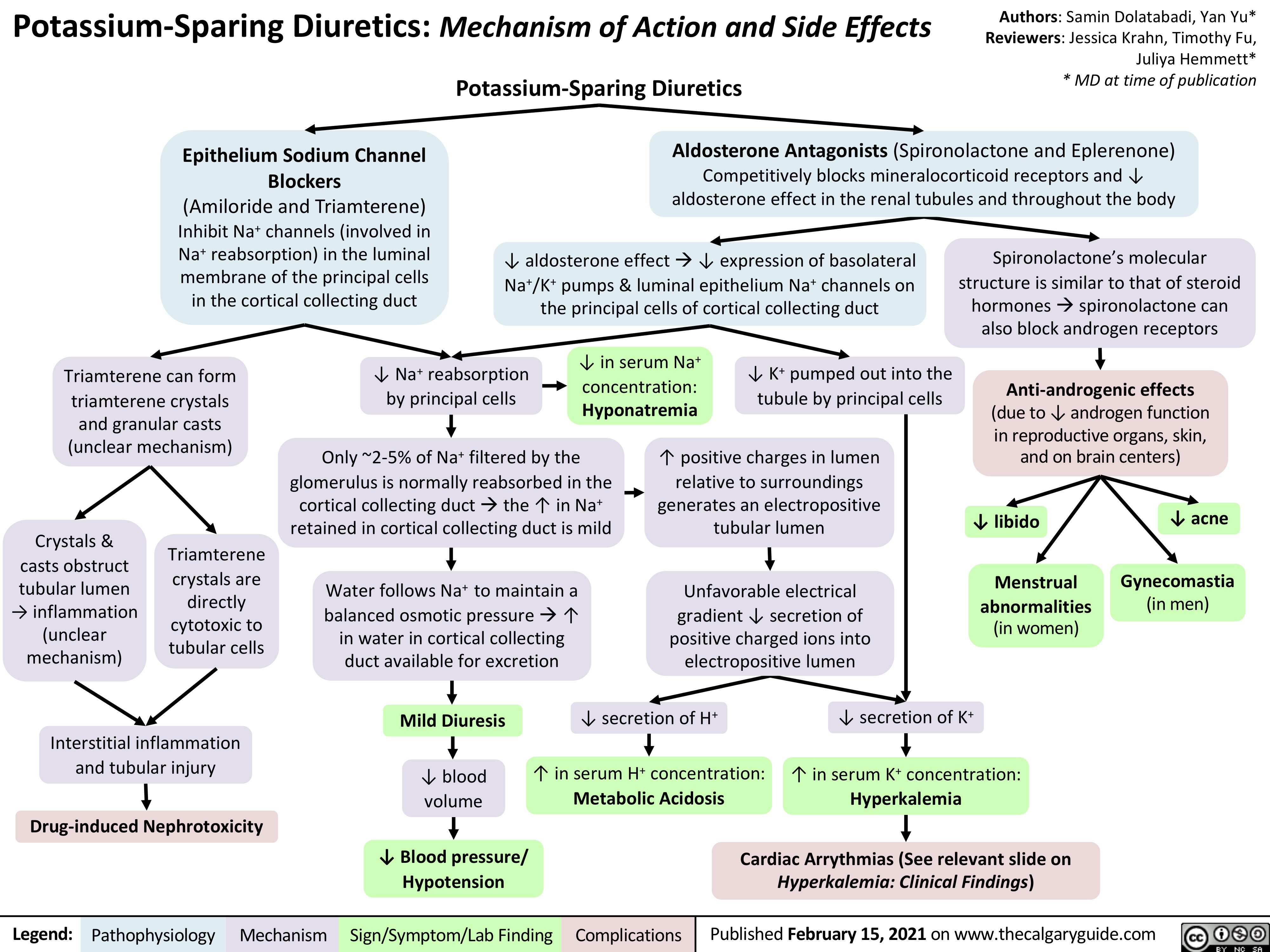
Potassium-Sparing-Diuretics-Mechanism-of-Action-and-Side-Effects
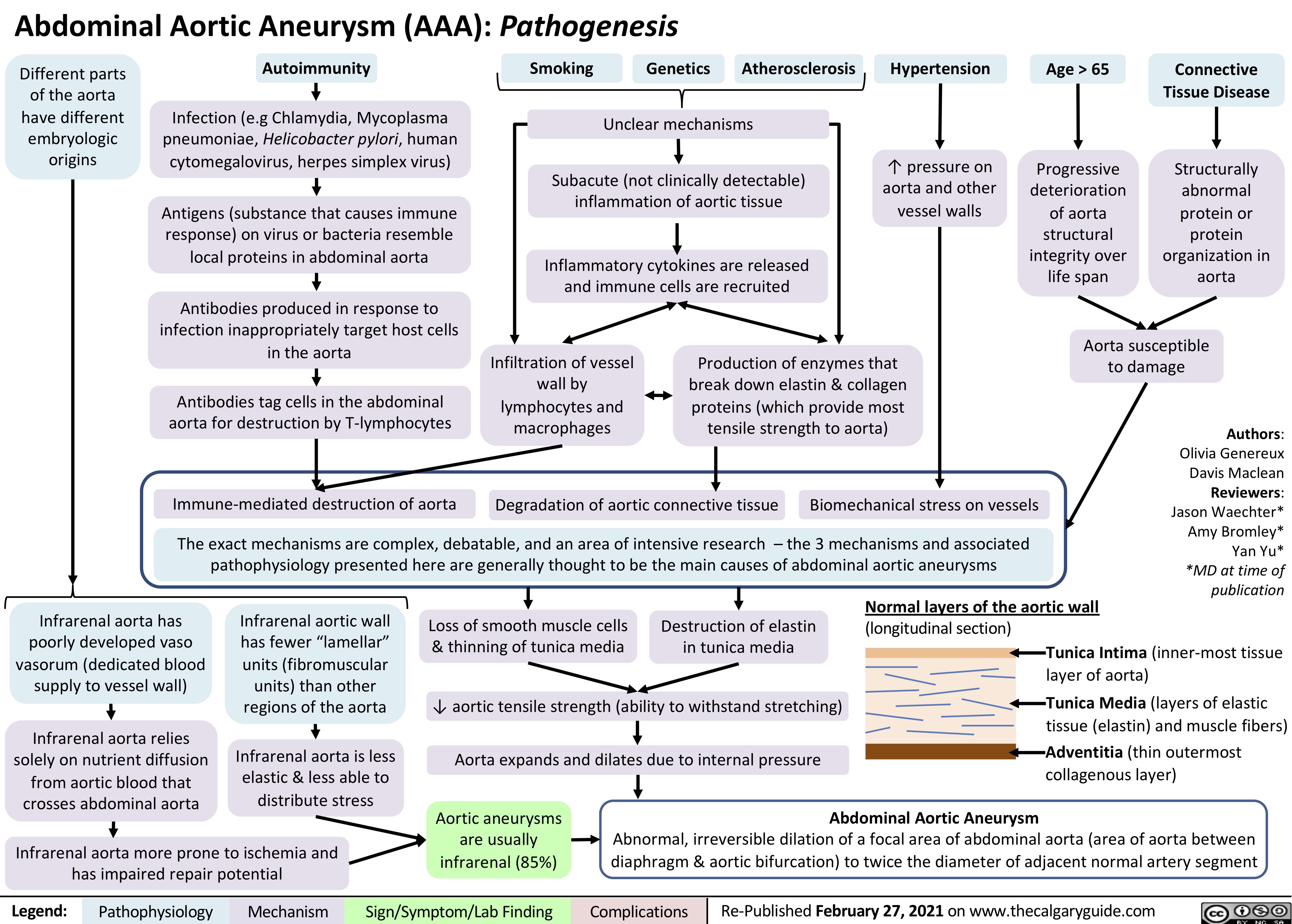
AAA-Pathogenesis
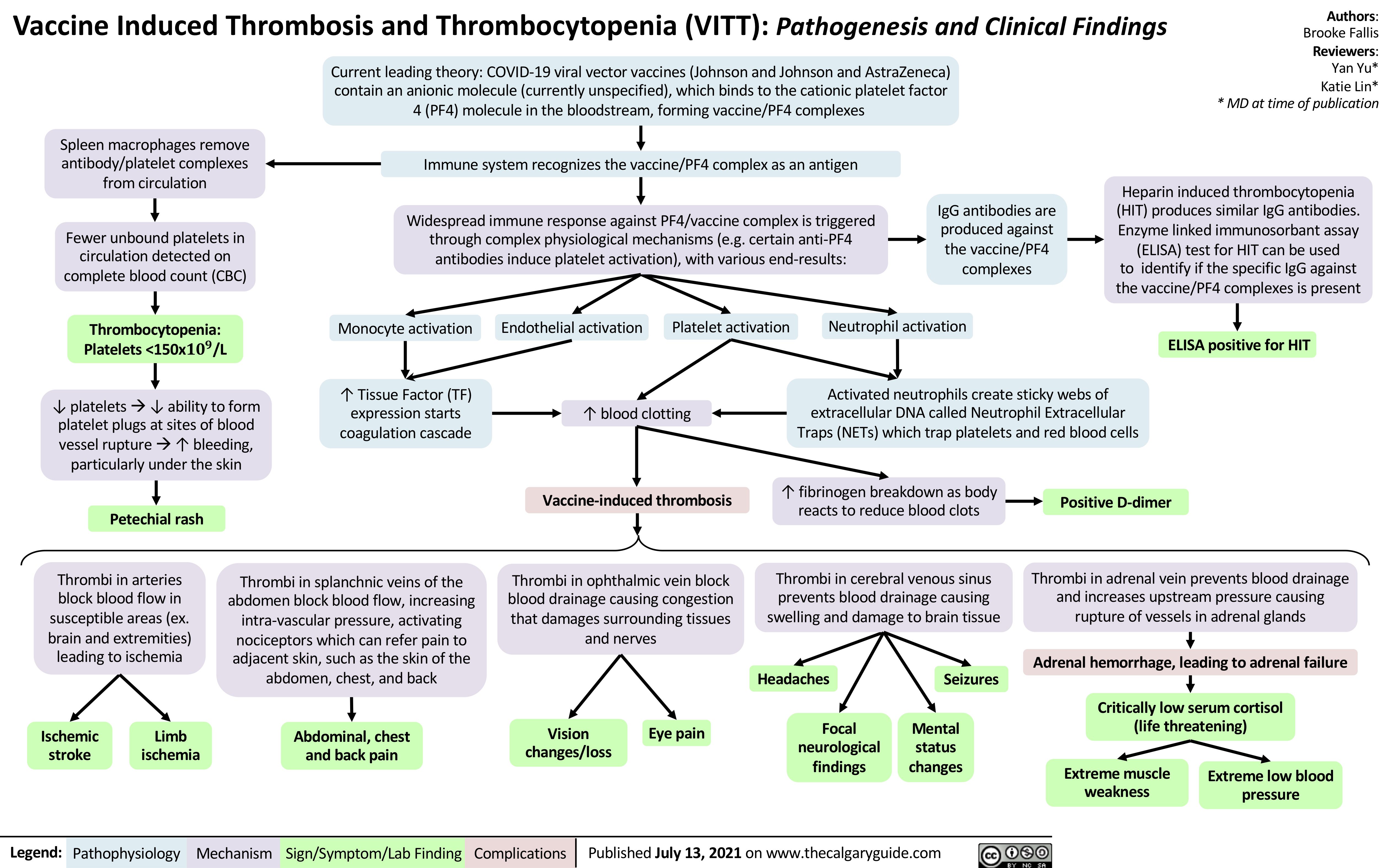
VITT
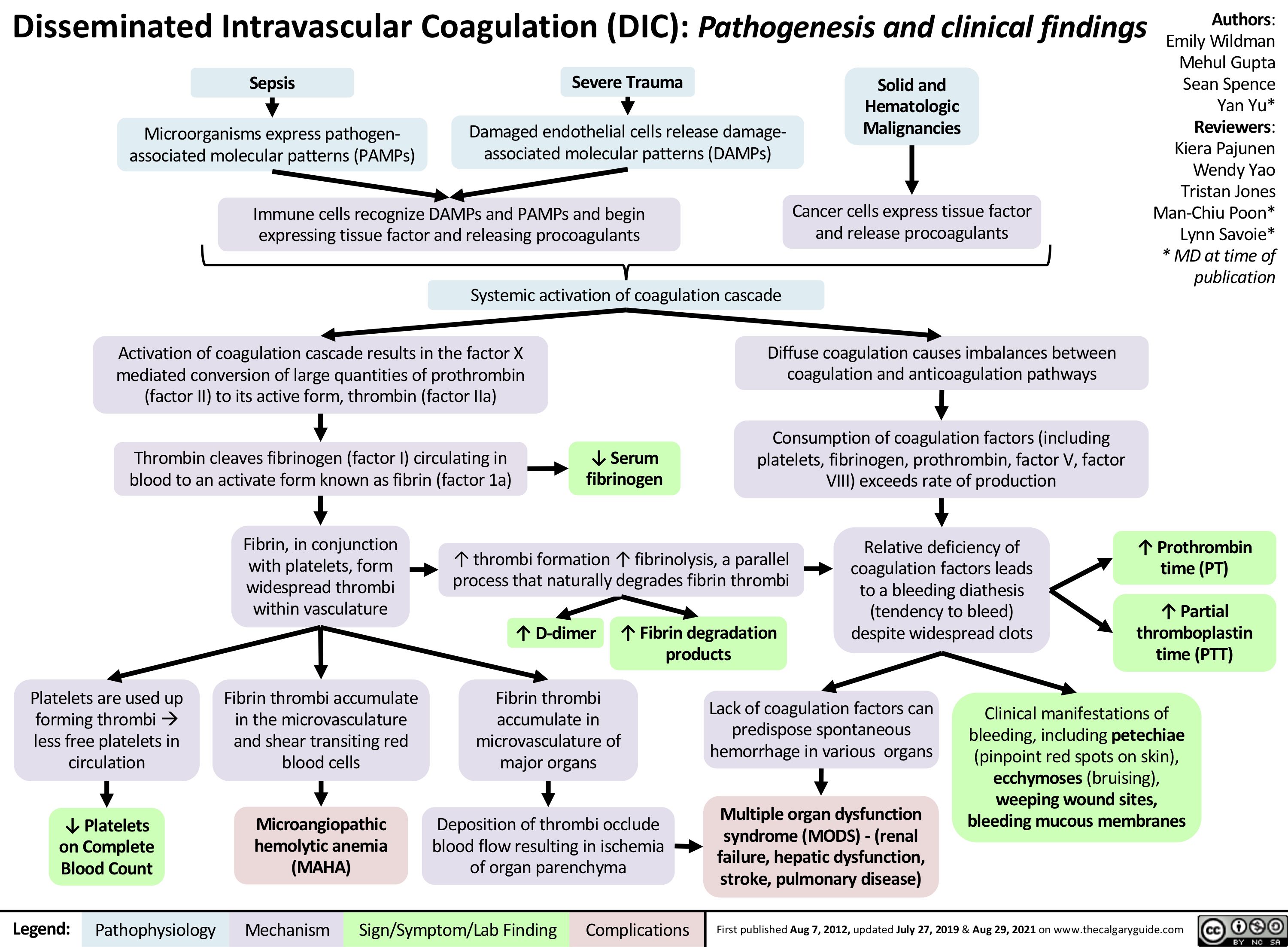
disseminated-intravascular-coagulation
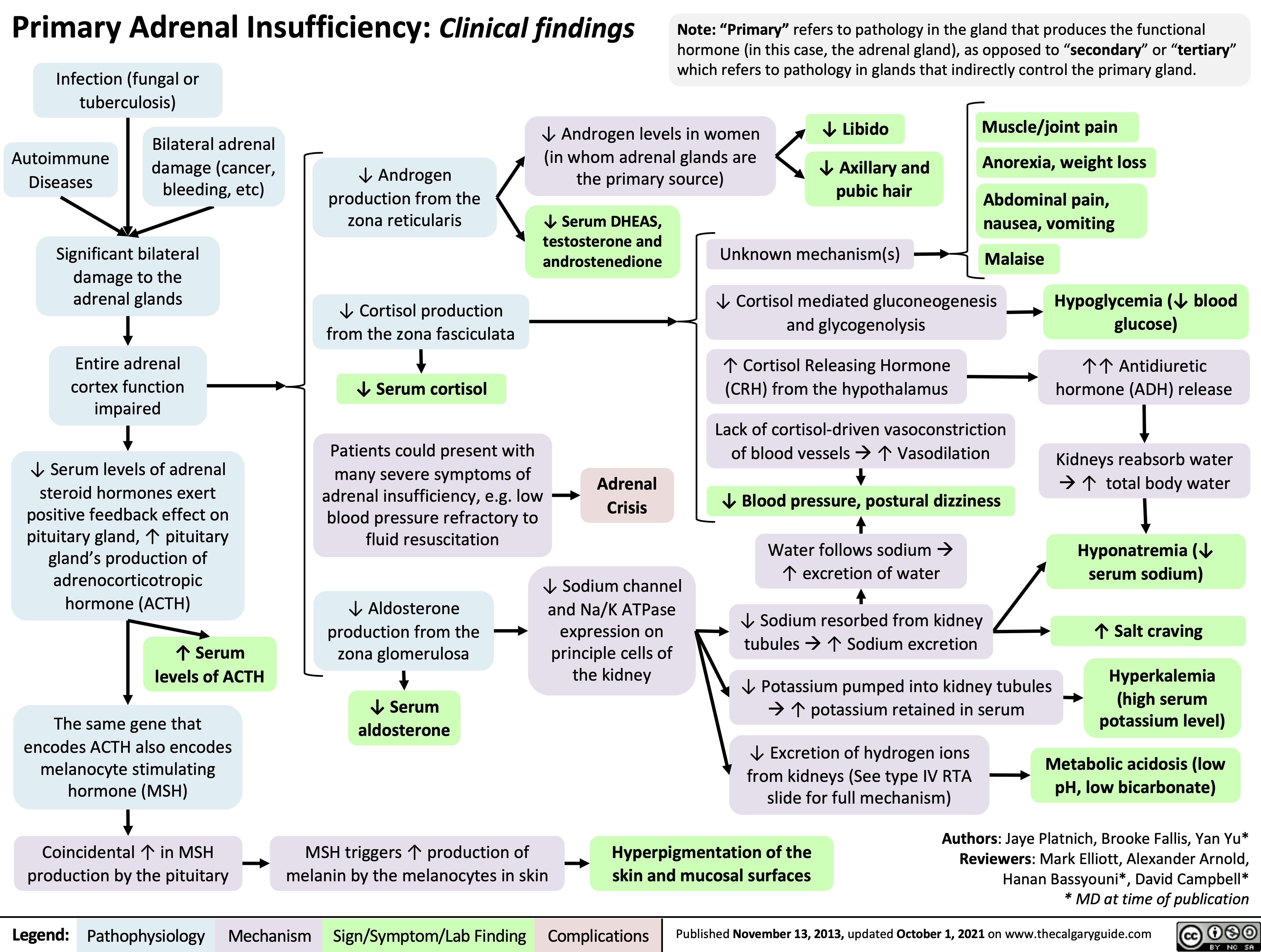
Primary-Adrenal-Insufficiency
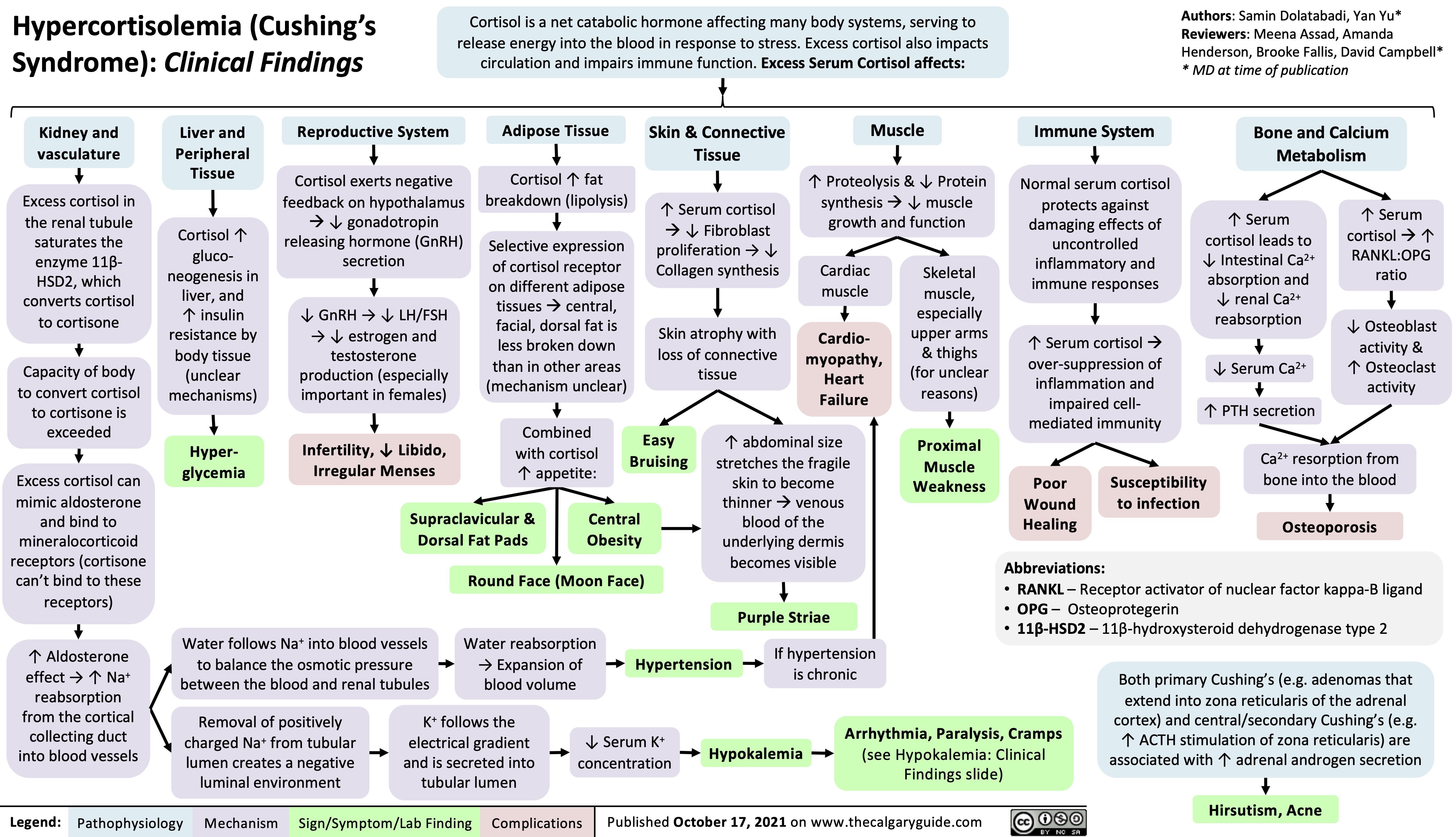
Hypercortisolemia
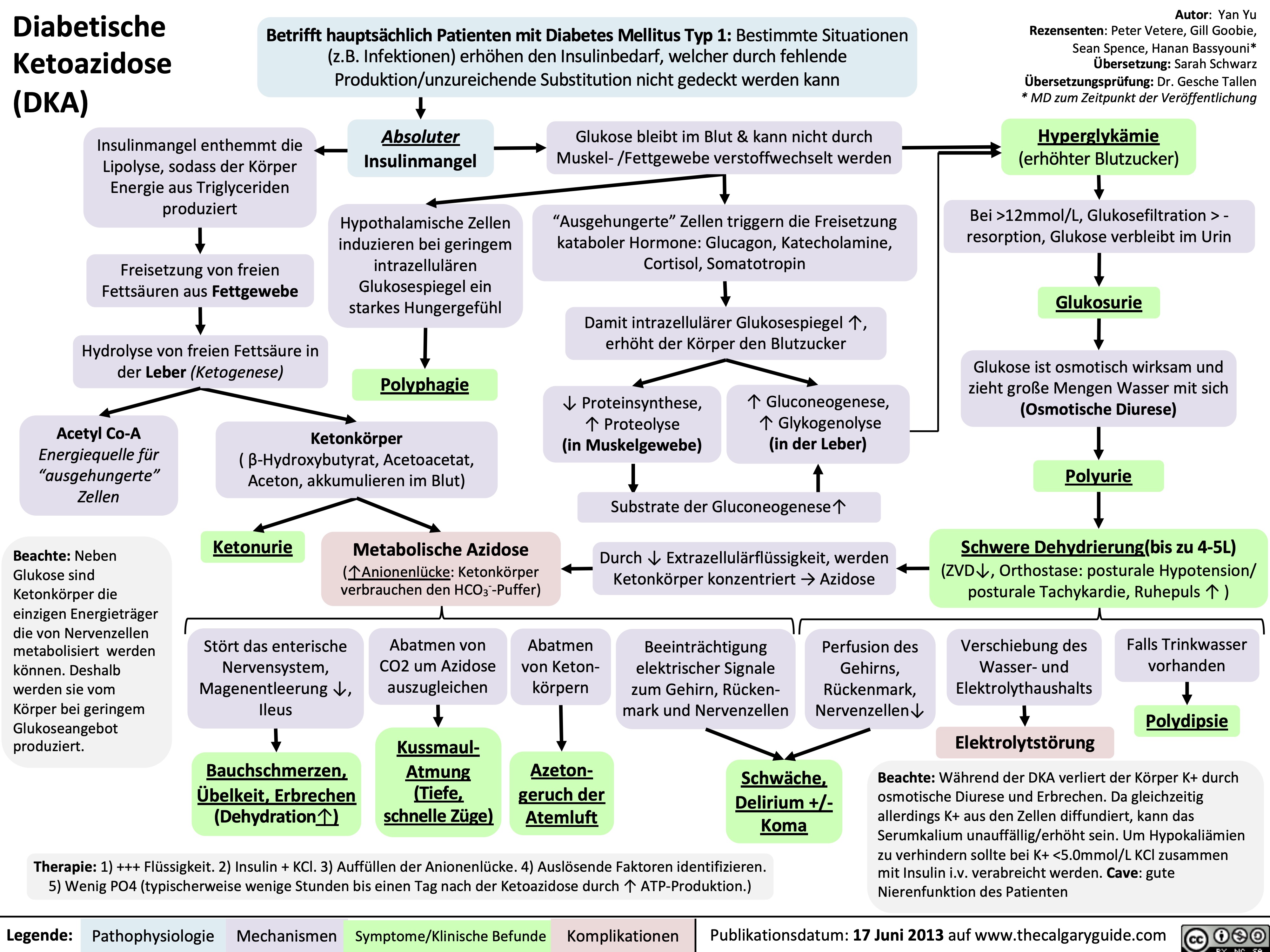
Diabetische Ketoazidose (DKA)
Hyperosmolares/ Hyperglykämisches Koma (HHS)
Nephrotisches Syndrom: Pathogenese und klinische Befunde
impetigo-patogenesis-y-hallazgos-clinicos
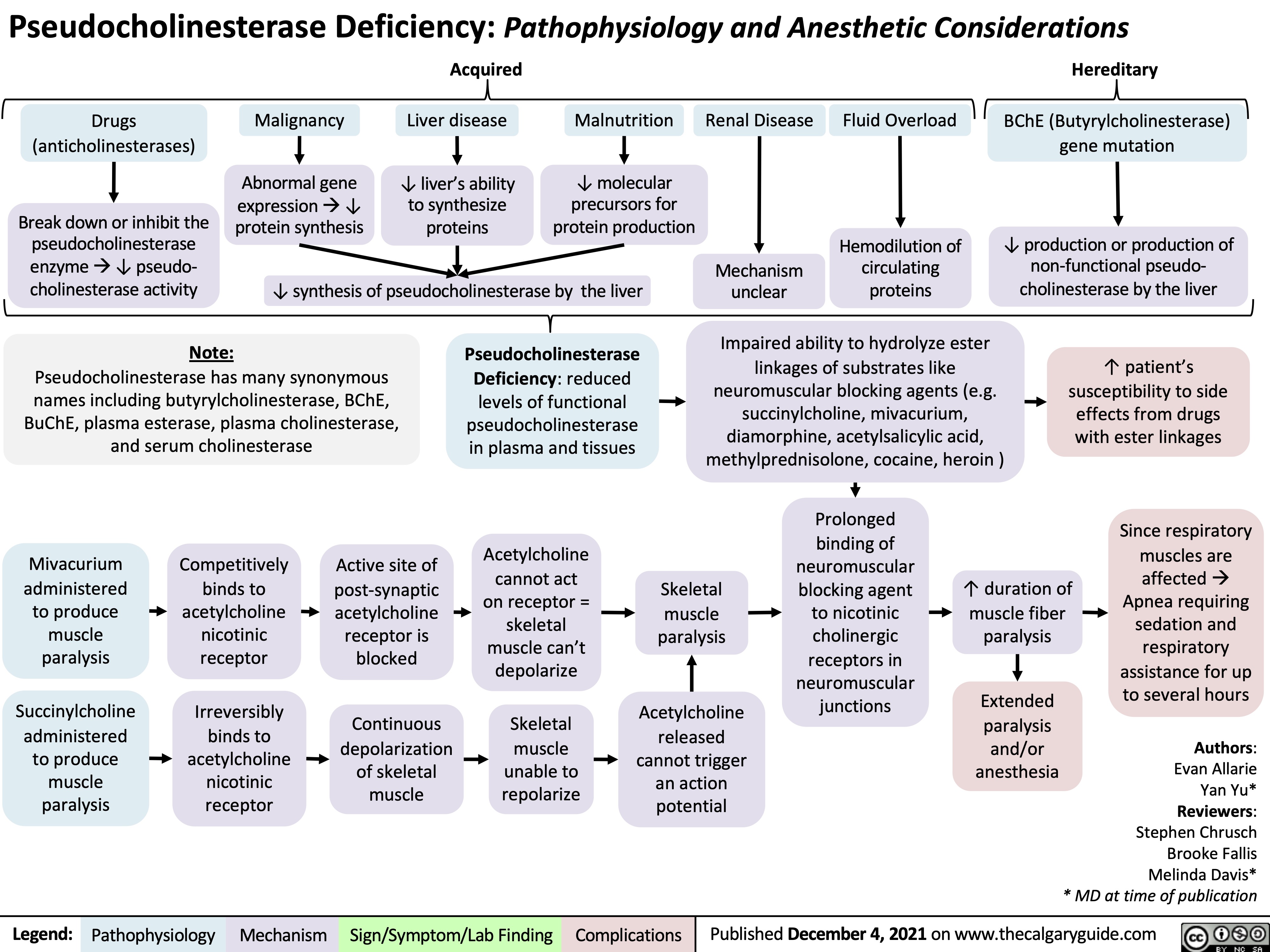
Psuedocholinesterase Deficiency
Primary Aldosteronism Pathogenesis
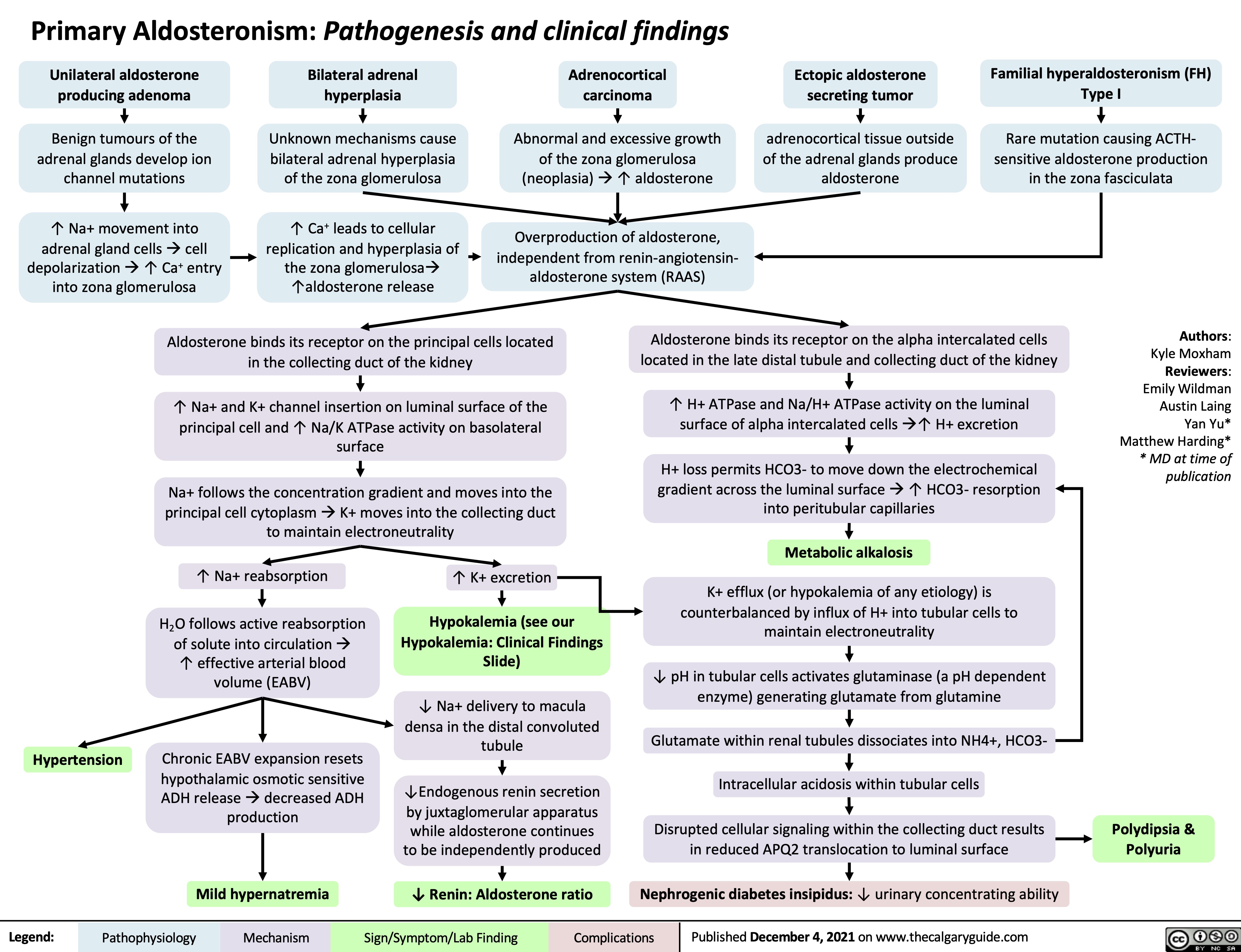
Normal anion gap metabolic acidosis
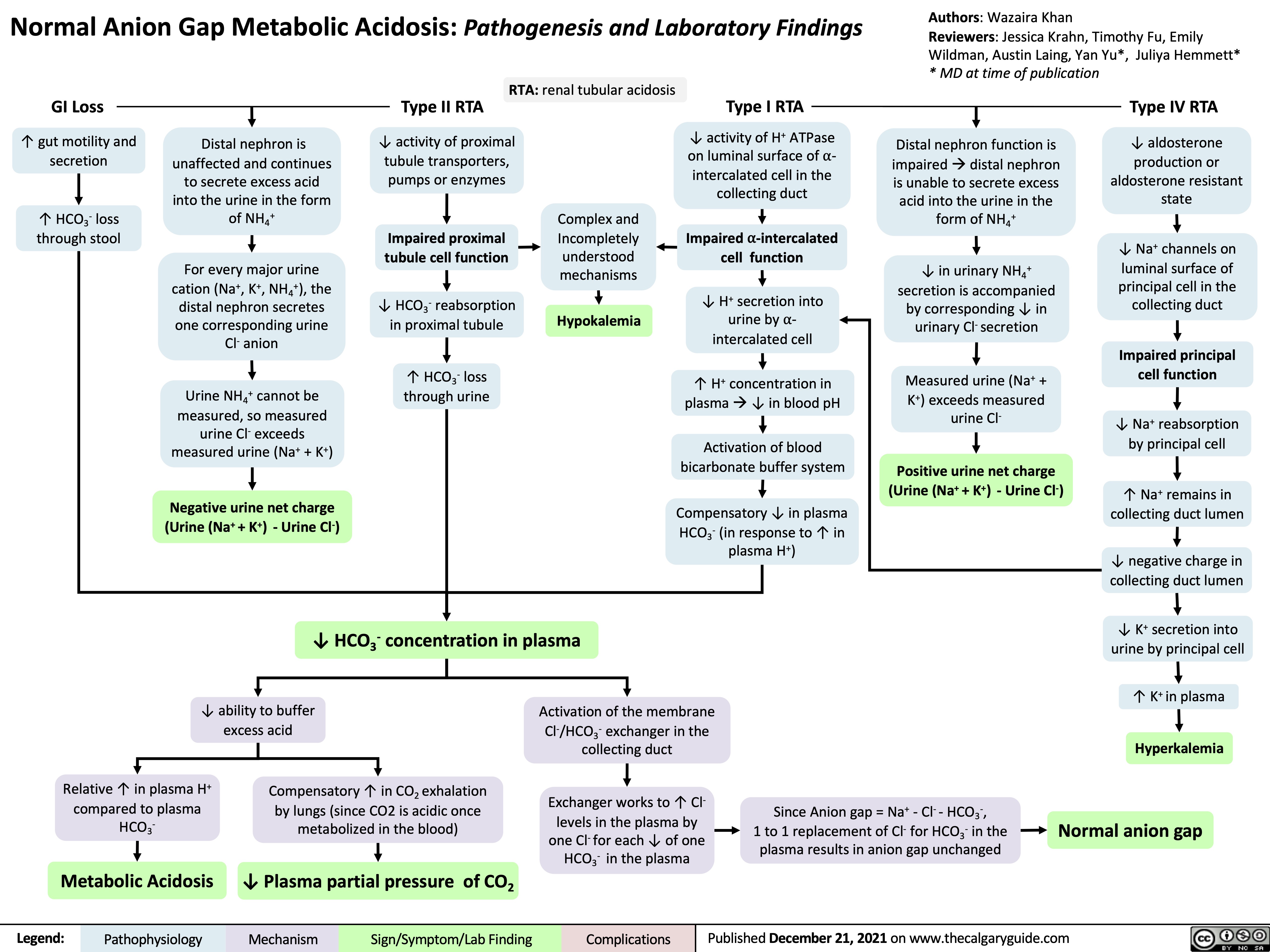
Renal Artery Stenosis
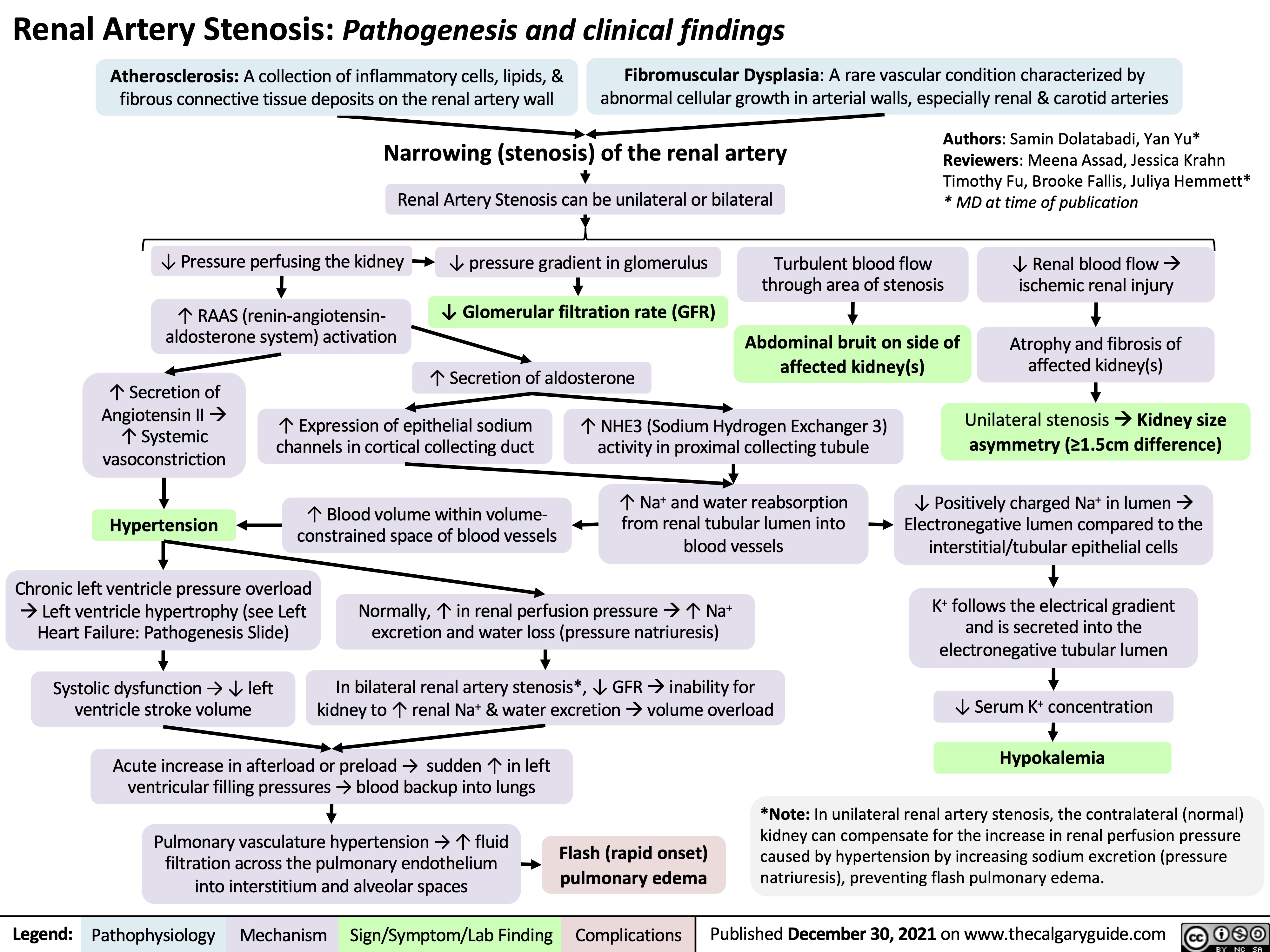
Membranous Nephropathy
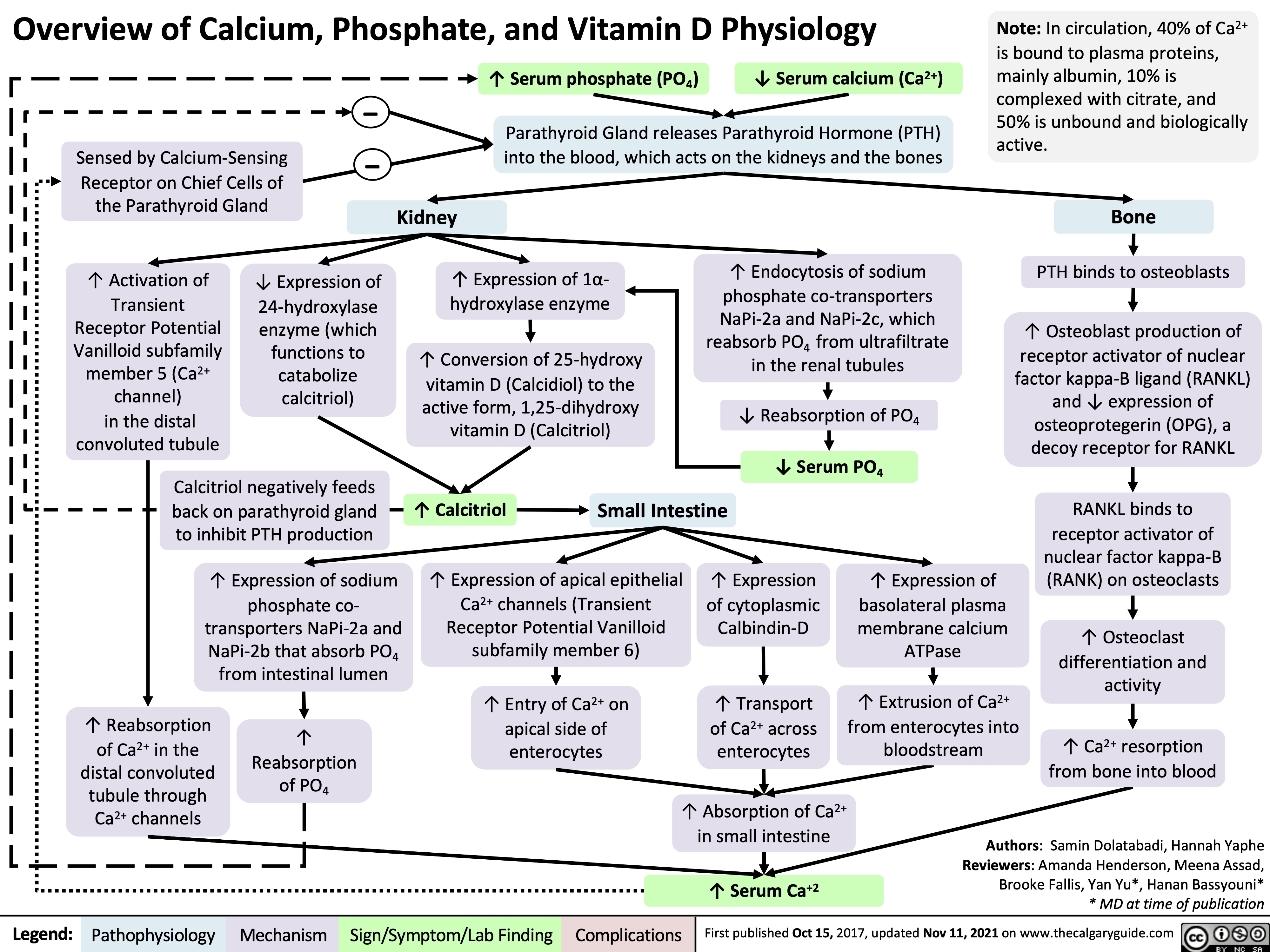
Overview of Calcium Phosphate Vitamin D Physiology
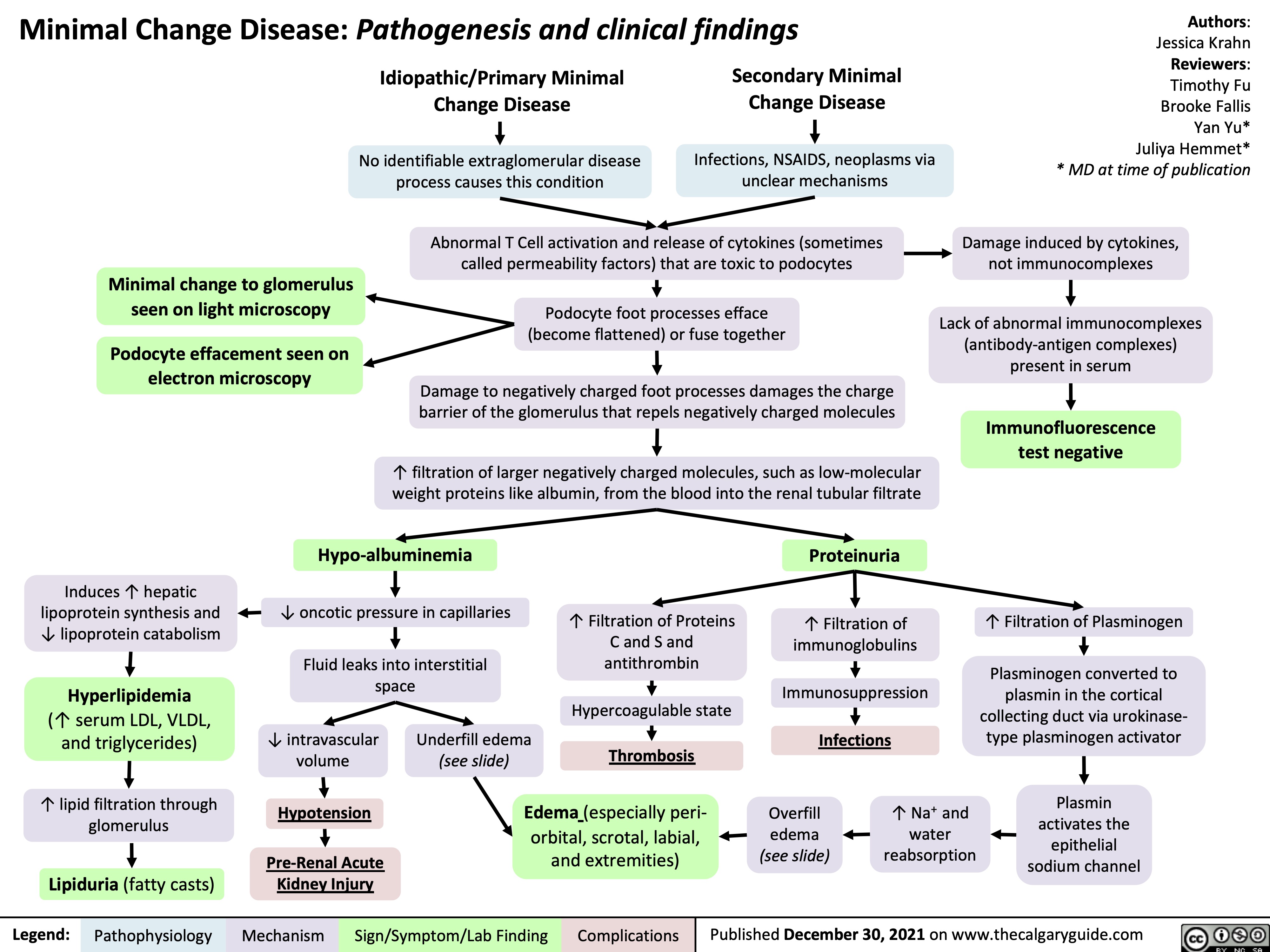
Minimal Change Disease
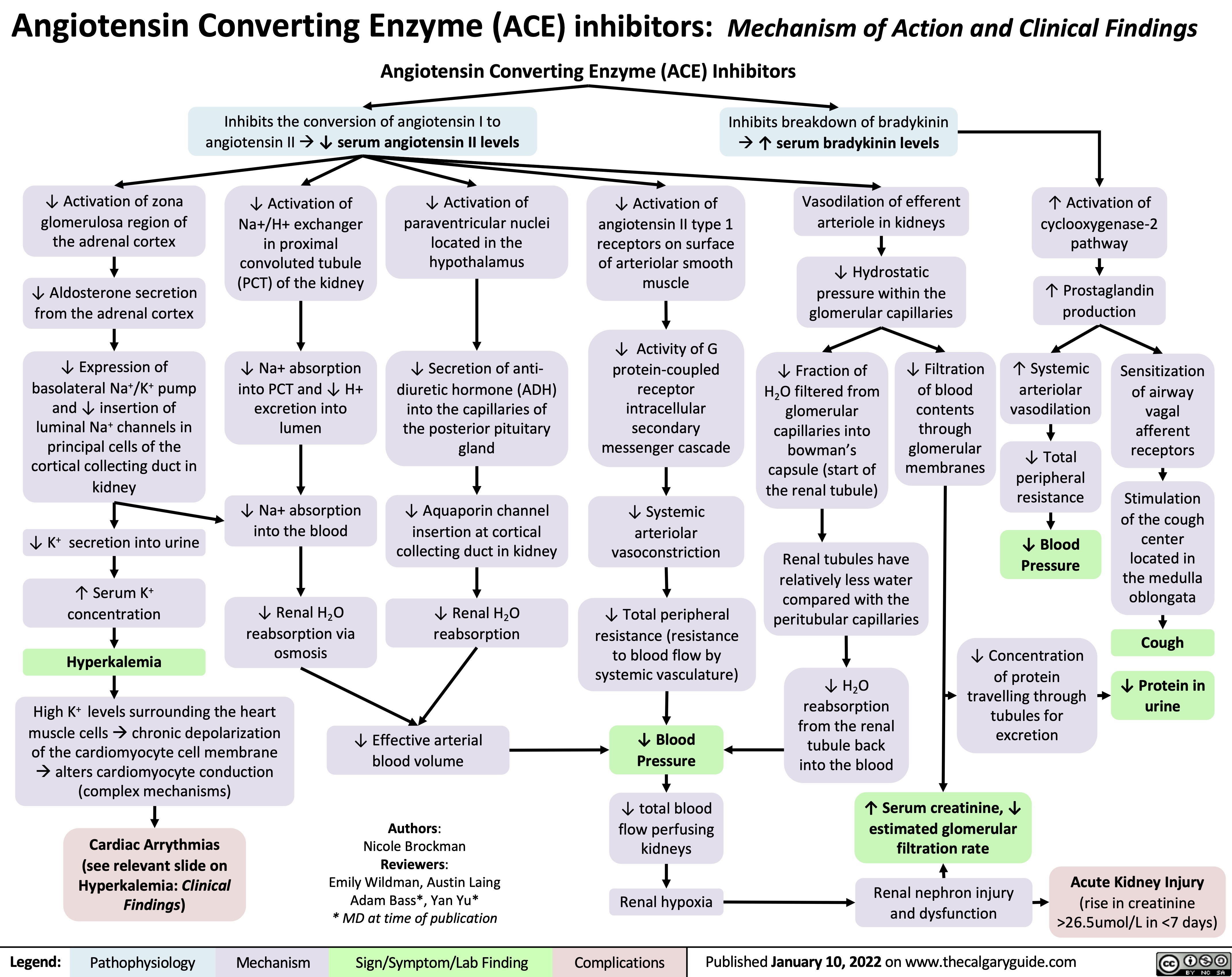
ACE inhibitors
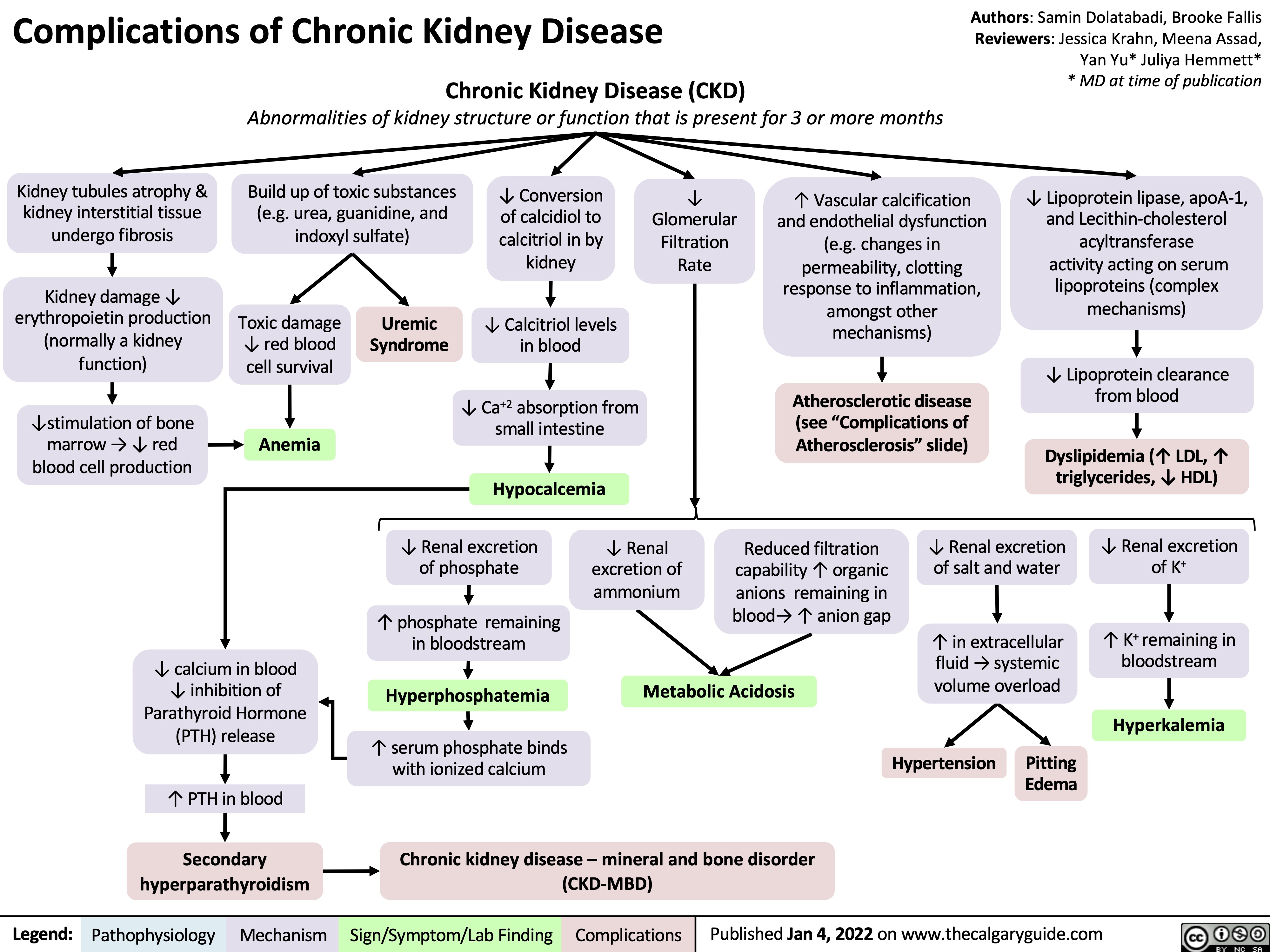
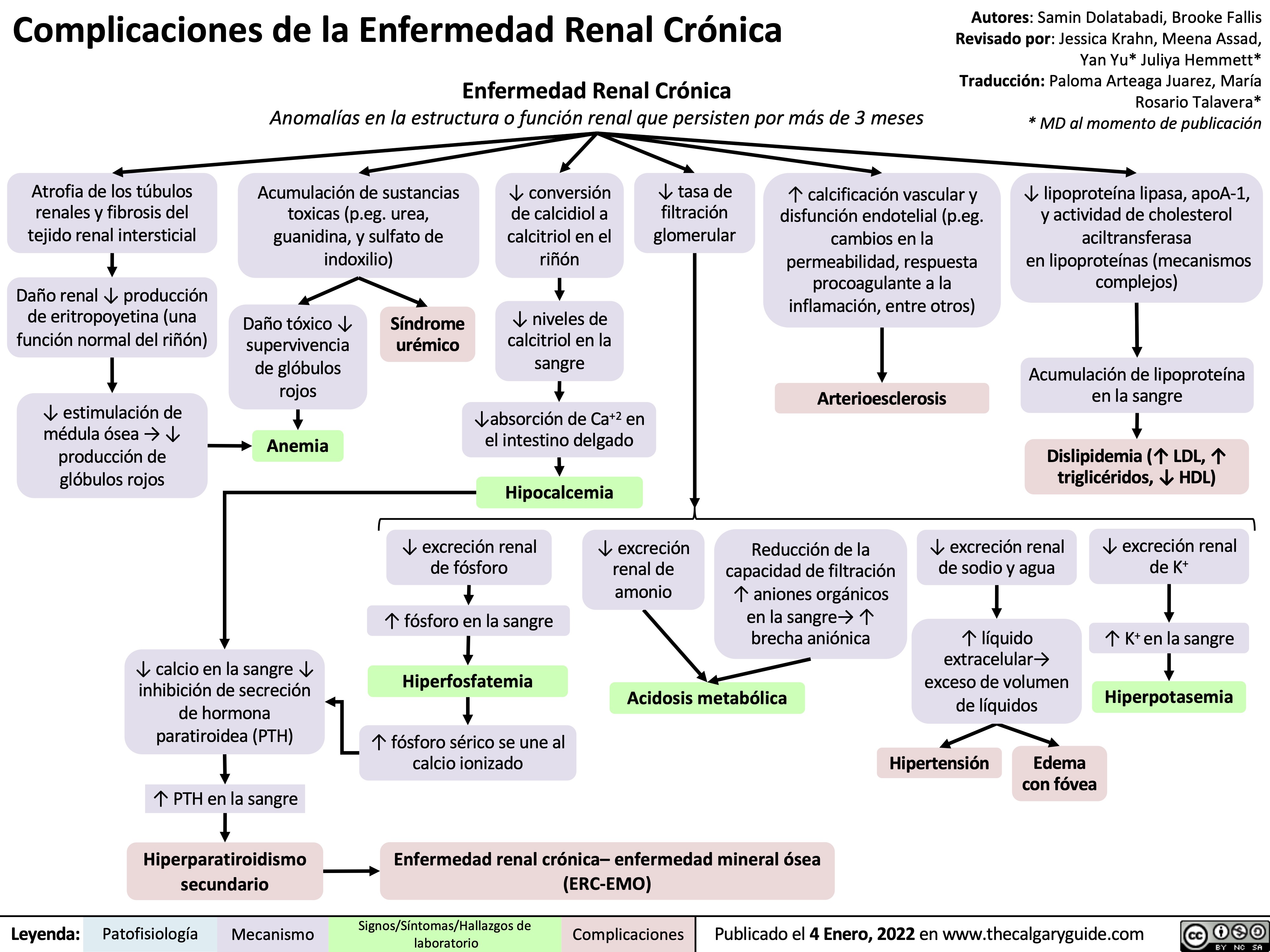
complications-of-chronic-kidney-disease-ckd
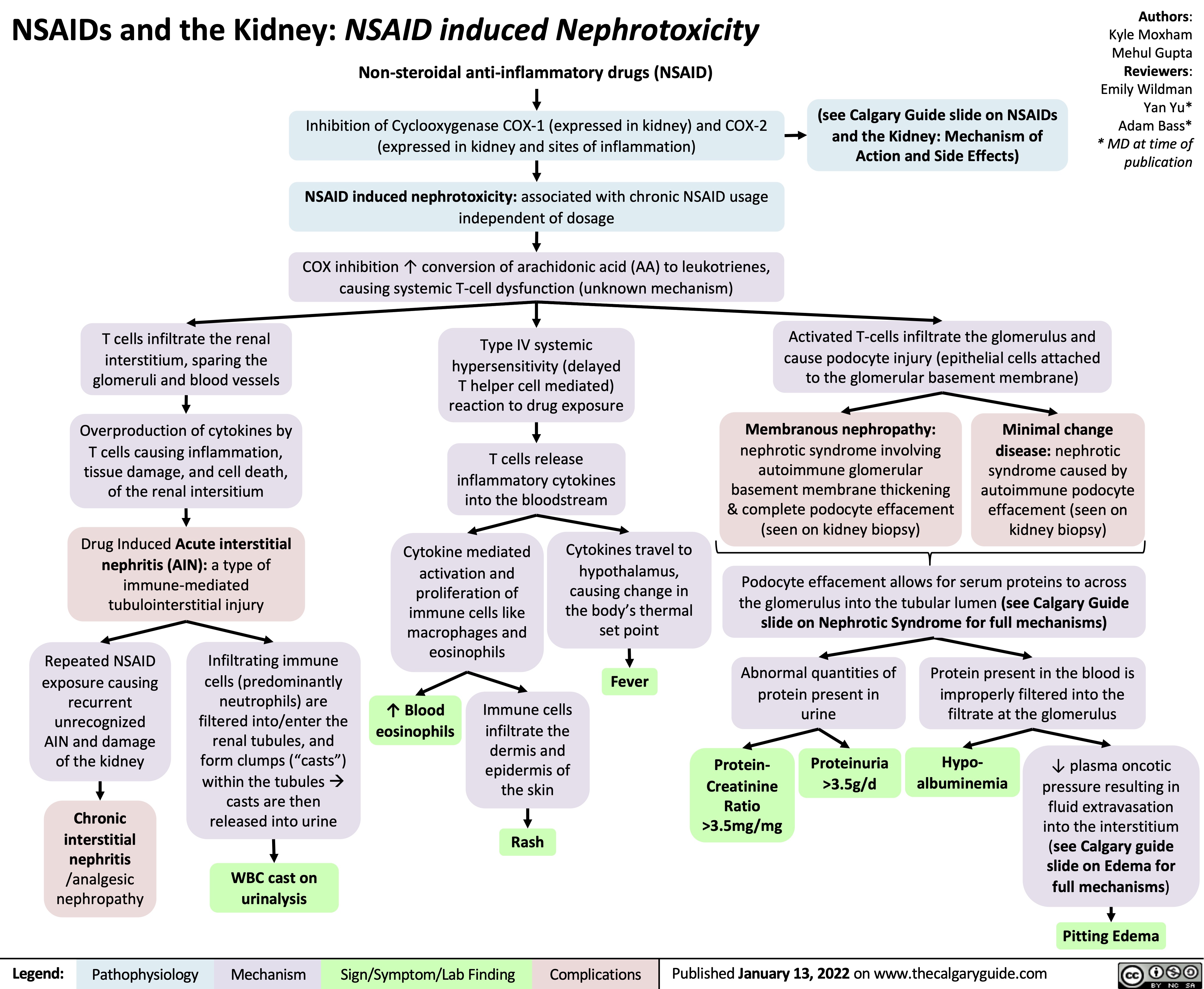
NSAIDs and the Kidney Nephrotoxicity
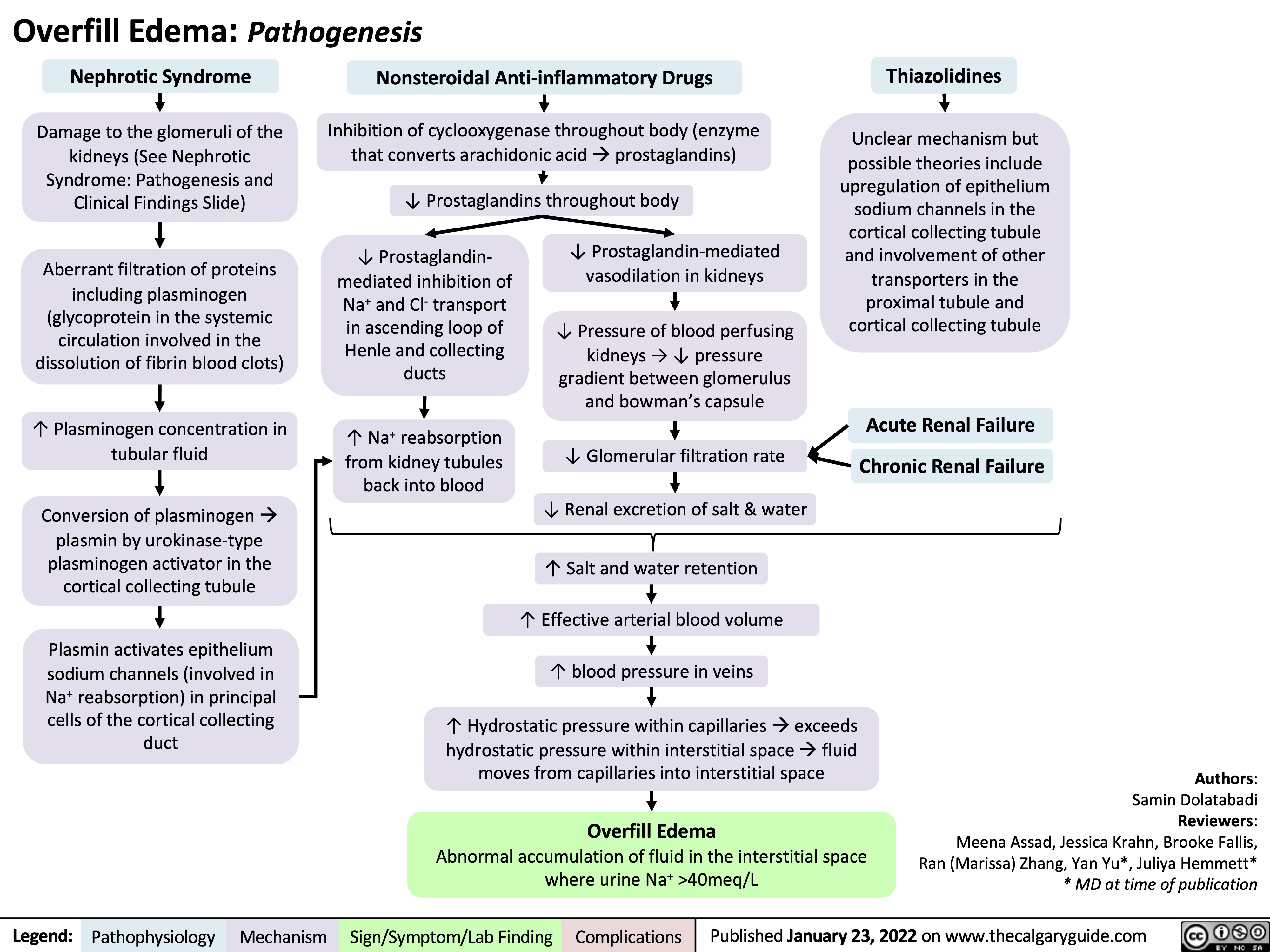
Overfill Edema Pathogenesis
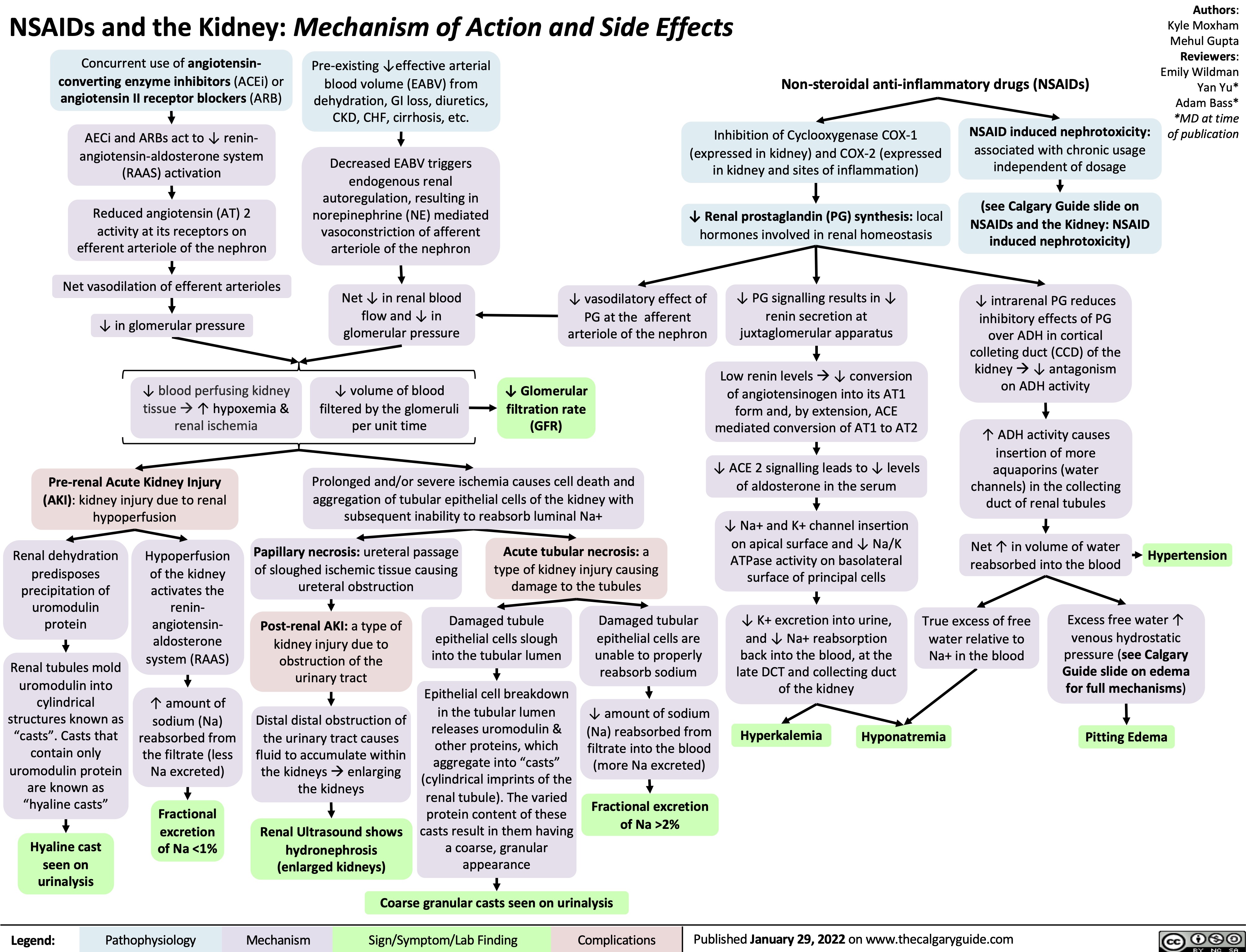
NSAIDs and the Kidney mechanism of action and side effects
Hypokalemia Physiology
![Hypokalemia: Physiology
Authors: Samin Dolatabadi, Ran (Marissa) Zhang, Mannat Dhillon Reviewers: Meena Assad, Yan Yu*, Juliya Hemmett*
Beta-2 receptor stimulation
(e.g. Salbutamol)
↑ Red blood cell production
↑ Na+/K+ ATPase activity in skeletal muscle cells (moves K+ into the cell & Na+ out of cell)
↑ K+ entry into skeletal muscle cells
* MD at time of publication
Abbreviations:
• EABV – Effective Arterial
Blood Volume
• ENaC – Epithelial Sodium
Channel
• HCL – Hydrochloric acid • HCO3- –Bicarbonate ion
↑ K+ uptake by new red blood cells
↑ Intracellular shift of K+ into cells
Refeeding Syndrome Exogenous insulin
↓ K+ dietary intake
(rare cause in isolation)
↑ Insulin in response to carbohydrate load
↑ Na+/K+ ATPase activity in skeletal muscle & hepatic cells
↑ K+ entry into skeletal muscle & hepatic cells
↓ K+ availability for gastrointestinal absorption
Hypokalemia (Serum [K+] < 3.5 mmol/L)
↑ Renal K+ secretion
K+ follows the electrical gradient into tubular lumen
↑ Electronegativity of tubular lumen
↑ Na+ reabsorptionin principal cellsà Cl- left behind in tubular lumen of kidneys
Gastric acid depletionà ↓HCl
Loss of H+àShift in bicarbonate buffer system to ↑ plasma HCO3-
Plasma HCO3- above reabsorptive capacity of the proximal tubule
↑ HCO3- in the distal tubular lumen of kidneys
Vomiting
Diarrhea Laxatives
Renin secreting tumour Hyperaldosteronism Renal artery stenosis Loop and Thiazide
diuretics
Bartter’s and Gittelman’s syndrome
Liddle syndrome
Extracellular fluid volume depletion
↓ EABV ↑ Renin secretion
↓ Afferent arteriole pressure perfusing kidneys
Renin-Angiotensin- Aldosterone System (RAAS) activationà ↑ Aldosterone release from the adrenal cortex
↑ Expression of ENaC (Na+ reabsorption) in principal cells of the cortical collectingduct)
+ ↑Na &
water excretion in kidneys
↓ EABV
Genetic condition leading to inability to degrade ENaC channels in principal cells of the cortical collecting duct
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019, updated Jan 23, 2022 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi Reviewers: Meena Assad Dr. Juliya Hemmett* * MD at time of publication
TTKG > 4 with N/↑ EABV in hypokalemia is inappropriate and a principal cell problem.
β2 Stimulation (e.g., Salbutamol)
↑ Na+/K+ ATPase activity
↑ K+ entry into cell
↑ RBC Production
↑ Cell production
↑ K+ uptake by new cells
↓ Extracellular ↑ Insulin in response to H+
carbohydrate load
↑ Na+/H+ antiporter activity (movement of H+ out of cell and Na+ into cell)
↑ Intracellular Na+
↑ Na+/K+ ATPase activity ↑ K+ entry into cell
↑ Intracellular Shift of K+
Notes:
Refeeding Syndrome
Insulin
Alkalemia
•
Abbreviations:
• CCD – Cortical Collecting Duct
• EABV – Effective Arterial Blood Volume
• RAAS – Renin-Angiotensin-Aldosterone System • TTKG – Trans-tubular Potassium Gradient
• ENaC – Epithelial Sodium Channel
↓ K+ Intake (Rare cause in isolation)
↓ K+ availability
Diarrhea, Vomiting, Laxatives
↑ Gastrointestinal loss of K+
Polyuria
↑ Renal loss of K+ (TTKG < 4 as principal cell is working appropriately but small amount of K+ is lost per urination)
Hypokalemia (Serum [K+] < 3.5 mmol/L)
Liddle Syndrome Hyperaldosteronism Renin Secreting Tumour
Renal artery stenosis
Loop and Thiazide Diuretics
Bartter’s and Gittelman’s Syndrome
Genetic condition leading to inability to degrade ENaC channels ↑ Renin
↑ Renal K+ secretion
K+ follows the electrical gradient
Electronegative lumen
↓ Pressure perfusing the kidney
RAAS activation RAAS activation
↑ Aldosterone
↑ Na+ and water excretion
↓ EABV
↑ Expression of ENaC in principal cells of CCD
↑ Na+ reabsorption
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi, Ran (Marissa) Zhang, Mannat Dhillon Reviewers: Meena Assad, Yan Yu*, Juliya Hemmett*
Beta-2 receptor stimulation
(e.g. Salbutamol)
↑ Red blood cell production
↑ Na+/K+ ATPase activity in skeletal muscle cells (moves K+ into the cell & Na+ out of cell)
↑ K+ entry into skeletal muscle cells
* MD at time of publication
Abbreviations:
• EABV – Effective Arterial
Blood Volume
• ENaC – Epithelial Sodium
Channel
• HCL – Hydrochloric acid • HCO3- –Bicarbonate ion
↑ K+ uptake by new red blood cells
↑ Intracellular shift of K+ into cells
Refeeding Syndrome Exogenous insulin
↓ K+ dietary intake
(rare cause in isolation)
↑ Insulin in response to carbohydrate load
↑ Na+/K+ ATPase activity in skeletal muscle & hepatic cells
↑ K+ entry into skeletal muscle & hepatic cells
↓ K+ availability for gastrointestinal absorption
Hypokalemia (Serum [K+] < 3.5 mmol/L)
↑ Renal K+ secretion
K+ follows the electrical gradient into tubular lumen
↑ Electronegativity of tubular lumen
↑ Na+ reabsorptionin principal cellsà Cl- left behind in tubular lumen of kidneys
Gastric acid depletionà ↓HCl
Loss of H+àShift in bicarbonate buffer system to ↑ plasma HCO3-
Plasma HCO3- above reabsorptive capacity of the proximal tubule
↑ HCO3- in the distal tubular lumen of kidneys
Vomiting
Diarrhea Laxatives
Renin secreting tumour Hyperaldosteronism Renal artery stenosis Loop and Thiazide
diuretics
Bartter’s and Gittelman’s syndrome
Liddle syndrome
Extracellular fluid volume depletion
↓ EABV ↑ Renin secretion
↓ Afferent arteriole pressure perfusing kidneys
Renin-Angiotensin- Aldosterone System (RAAS) activationà ↑ Aldosterone release from the adrenal cortex
↑ Expression of ENaC (Na+ reabsorption) in principal cells of the cortical collectingduct)
+ ↑Na &
water excretion in kidneys
↓ EABV
Genetic condition leading to inability to degrade ENaC channels in principal cells of the cortical collecting duct
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019, updated Jan 23, 2022 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi Reviewers: Meena Assad Dr. Juliya Hemmett* * MD at time of publication
TTKG > 4 with N/↑ EABV in hypokalemia is inappropriate and a principal cell problem.
β2 Stimulation (e.g., Salbutamol)
↑ Na+/K+ ATPase activity
↑ K+ entry into cell
↑ RBC Production
↑ Cell production
↑ K+ uptake by new cells
↓ Extracellular ↑ Insulin in response to H+
carbohydrate load
↑ Na+/H+ antiporter activity (movement of H+ out of cell and Na+ into cell)
↑ Intracellular Na+
↑ Na+/K+ ATPase activity ↑ K+ entry into cell
↑ Intracellular Shift of K+
Notes:
Refeeding Syndrome
Insulin
Alkalemia
•
Abbreviations:
• CCD – Cortical Collecting Duct
• EABV – Effective Arterial Blood Volume
• RAAS – Renin-Angiotensin-Aldosterone System • TTKG – Trans-tubular Potassium Gradient
• ENaC – Epithelial Sodium Channel
↓ K+ Intake (Rare cause in isolation)
↓ K+ availability
Diarrhea, Vomiting, Laxatives
↑ Gastrointestinal loss of K+
Polyuria
↑ Renal loss of K+ (TTKG < 4 as principal cell is working appropriately but small amount of K+ is lost per urination)
Hypokalemia (Serum [K+] < 3.5 mmol/L)
Liddle Syndrome Hyperaldosteronism Renin Secreting Tumour
Renal artery stenosis
Loop and Thiazide Diuretics
Bartter’s and Gittelman’s Syndrome
Genetic condition leading to inability to degrade ENaC channels ↑ Renin
↑ Renal K+ secretion
K+ follows the electrical gradient
Electronegative lumen
↓ Pressure perfusing the kidney
RAAS activation RAAS activation
↑ Aldosterone
↑ Na+ and water excretion
↓ EABV
↑ Expression of ENaC in principal cells of CCD
↑ Na+ reabsorption
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hypokalemia-Physiology.jpg)
metabolic-alkalosis-pathogenesis
![Metabolic Alkalosis: Pathogenesis
Authors: Wazaira Khan Reviewers: Jessica Krahn, Emily Wildman, Austin Laing, Huneza Nadeem, Ran (Marissa) Zhang, Adam Bass* * MD at time of publication
Primary hyperaldosteronism E.g., aldosterone- secreting mass, adrenal hyperplasia
Secondary hyperaldosteronism E.g., renin-secreting mass, renal artery stenosis
Pseudo- hypoaldosteronism E.g., Liddle’s Syndrome
Unregulated aldosterone production in adrenal cortex
Excess aldosterone suppresses renin production
Unregulated renin production by juxtaglomerular cells
Sustained ↑ in mineralocorticoid (aldosterone) activity
Insertion of epithelial sodium channels on principal cells in collecting duct
↑ Water retention by the kidney
↑ Na+ reabsorption by principal cells
↑ EABV
• ↑ Jugular venous pressure • Hypertension
• Urine Na+ > 40 mEq/L
Low renin, high aldosterone state
Activation of renin- angiotensin-aldosterone system (RAAS)
Release of aldosterone from adrenal cortex
High renin, high aldosterone state
↑ Negative charge in collecting duct lumen
K+ leaks into collecting duct lumen by principal cell to maintain electroneutrality
Hypokalemia
Intracellular K+ leaks out of any cell in the body to compensate for low serum K+ levels
Extracellular H+ enters cell to maintain electroneutrality
Intracellular acidosis
Activation of compensatory acid secreting mechanisms in kidney
Impaired tubular function
E.g., loop/thiazide diuretics, Bartter’s/ Gitelman’s Syndrome
Upper GI Loss
E.g., vomiting, nasogastric suction
Unregulated epithelial sodium channel activity in collecting duct mimicking aldosterone function
↑ in Na+ and water retention à RAAS inhibition
↓ Na+ and Cl- reabsorption in thick ascending limb or distal convoluted tubule
Low renin, low aldosterone state
↑ Na+, Cl- and water secretion through kidney
RAAS activation
See “Physiology of RAAS” slide
↓ EABV • • •
Temporary ↑ in mineralocorticoid (aldosterone) activity
↓ Jugular venous pressure Orthostatic hypotension Dry mucous membranes Urine Na+ < 20 mEq/L
•
Loss of fluid through GI tract
↓ HCl delivery to small intestine
↑ NH + secretion and 4
↑ HCO3- reabsorption in proximal tubule
↑ H+ secretion in cortical collecting duct
Loss of gastric contents, including HCl
↓ HCO3- secretion by pancreas, liver and intestines to neutralize HCl
Loss of intrinsic acid to neutralize HCO3- ↑ Plasma [HCO3-]
Metabolic Alkalosis
Arterial blood gas pH > 7.40 Plasma [HCO3-] > 24 mEq/L
Effective Arterial Blood Volume (EABV): component of arterial blood volume that is effectively perfusing organs
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published May 31, 2022 on www.thecalgaryguide.com
Metabolic Alkalosis: Pathogenesis
Authors: Wazaira Khan Reviewers: Jessica Krahn, Emily Wildman, Austin Laing, Huneza Nadeem, Ran (Marissa) Zhang, Adam Bass* * MD at time of publication
Primary hyperaldosteronism E.g., aldosterone- secreting mass, adrenal hyperplasia
Secondary hyperaldosteronism E.g., renin-secreting mass, renal artery stenosis
Pseudo- hypoaldosteronism E.g., Liddle’s Syndrome
Unregulated aldosterone production in adrenal cortex
Excess aldosterone suppresses renin production
Unregulated renin production by juxtaglomerular cells
Sustained ↑ in mineralocorticoid (aldosterone) activity
Insertion of epithelial sodium channels on principal cells in collecting duct
↑ Water retention by the kidney
↑ Na+ reabsorption by principal cells
↑ EABV
• ↑ Jugular venous pressure • Hypertension
• Urine Na+ > 40 mEq/L
Low renin, high aldosterone state
Activation of renin- angiotensin-aldosterone system (RAAS)
Release of aldosterone from adrenal cortex
High renin, high aldosterone state
↑ Negative charge in collecting duct lumen
K+ leaks into collecting duct lumen by principal cell to maintain electroneutrality
Hypokalemia
Intracellular K+ leaks out of any cell in the body to compensate for low serum K+ levels
Extracellular H+ enters cell to maintain electroneutrality
Intracellular acidosis
Activation of compensatory acid secreting mechanisms in kidney
Impaired tubular function
E.g., loop/thiazide diuretics, Bartter’s/ Gitelman’s Syndrome
Upper GI Loss
E.g., vomiting, nasogastric suction
Unregulated epithelial sodium channel activity in collecting duct mimicking aldosterone function
↑ in Na+ and water retention à RAAS inhibition
↓ Na+ and Cl- reabsorption in thick ascending limb or distal convoluted tubule
Low renin, low aldosterone state
↑ Na+, Cl- and water secretion through kidney
RAAS activation
See “Physiology of RAAS” slide
↓ EABV • • •
Temporary ↑ in mineralocorticoid (aldosterone) activity
↓ Jugular venous pressure Orthostatic hypotension Dry mucous membranes Urine Na+ < 20 mEq/L
•
Loss of fluid through GI tract
↓ HCl delivery to small intestine
↑ NH + secretion and 4
↑ HCO3- reabsorption in proximal tubule
↑ H+ secretion in cortical collecting duct
Loss of gastric contents, including HCl
↓ HCO3- secretion by pancreas, liver and intestines to neutralize HCl
Loss of intrinsic acid to neutralize HCO3- ↑ Plasma [HCO3-]
Metabolic Alkalosis
Arterial blood gas pH > 7.40 Plasma [HCO3-] > 24 mEq/L
Effective Arterial Blood Volume (EABV): component of arterial blood volume that is effectively perfusing organs
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published May 31, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/05/Metabolic-Alkalosis-1.jpg)
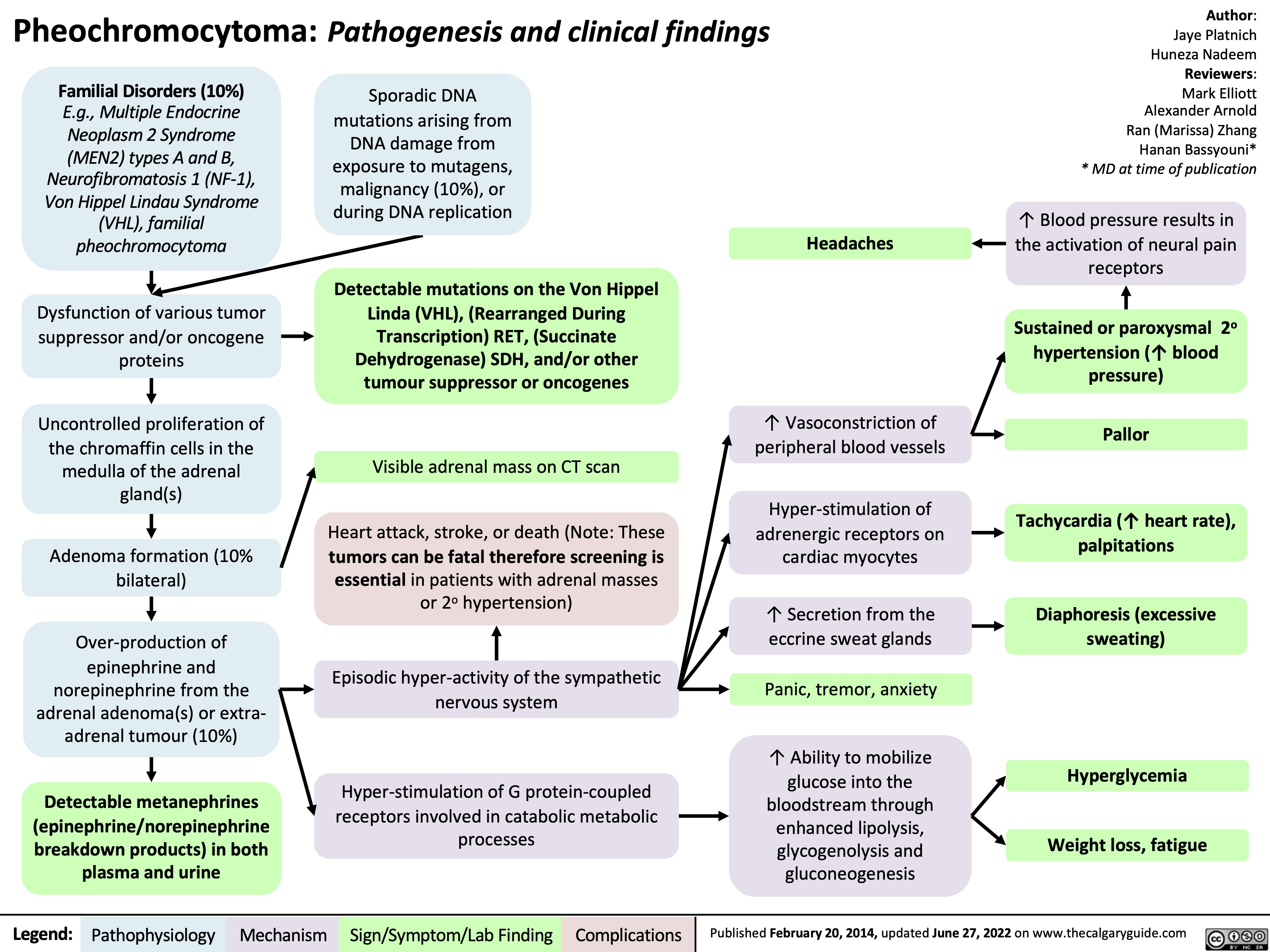
pheochromocytoma-pathogenesis-and-clinical-findings
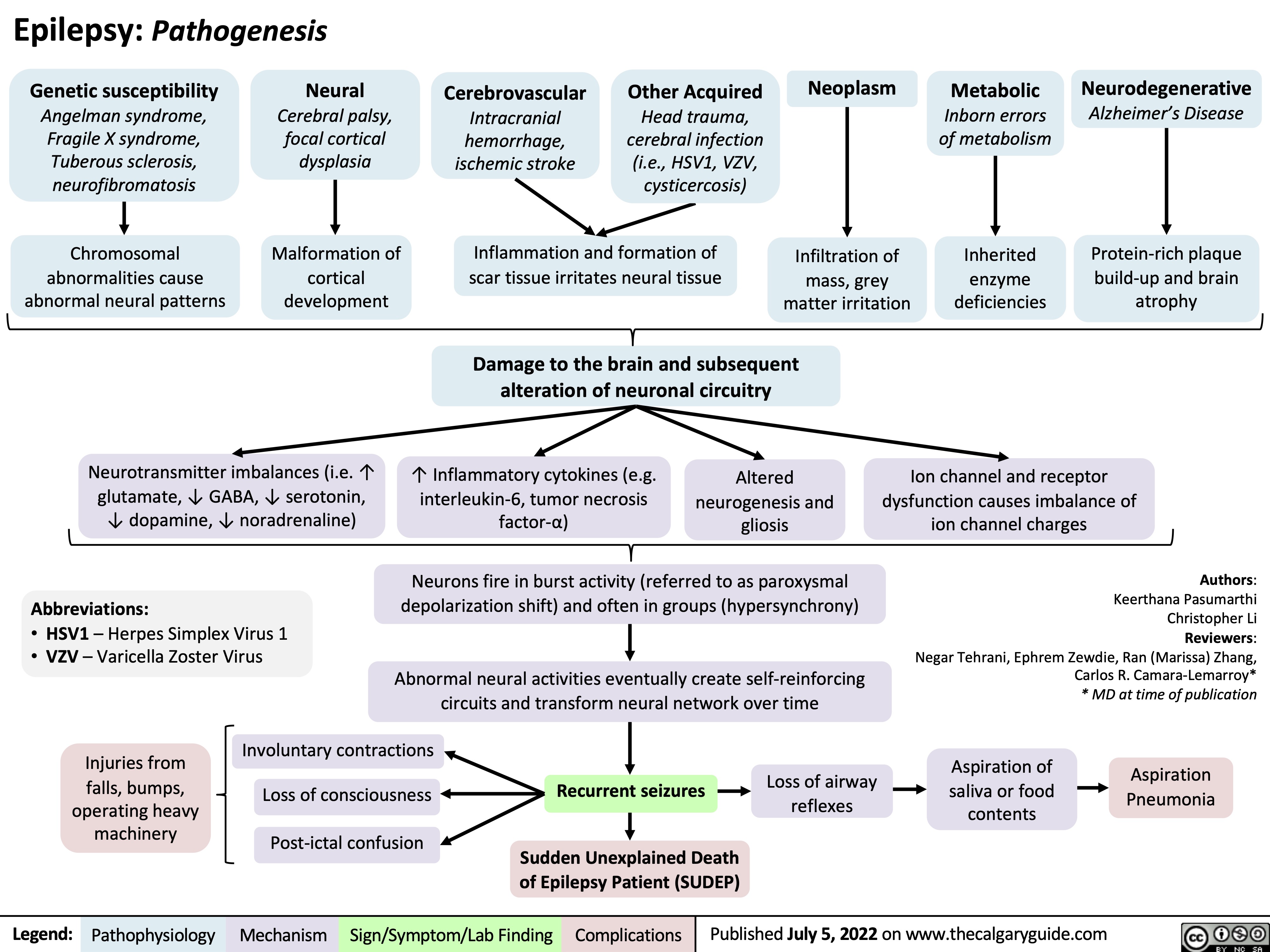
Epilepsy Pathogenesis
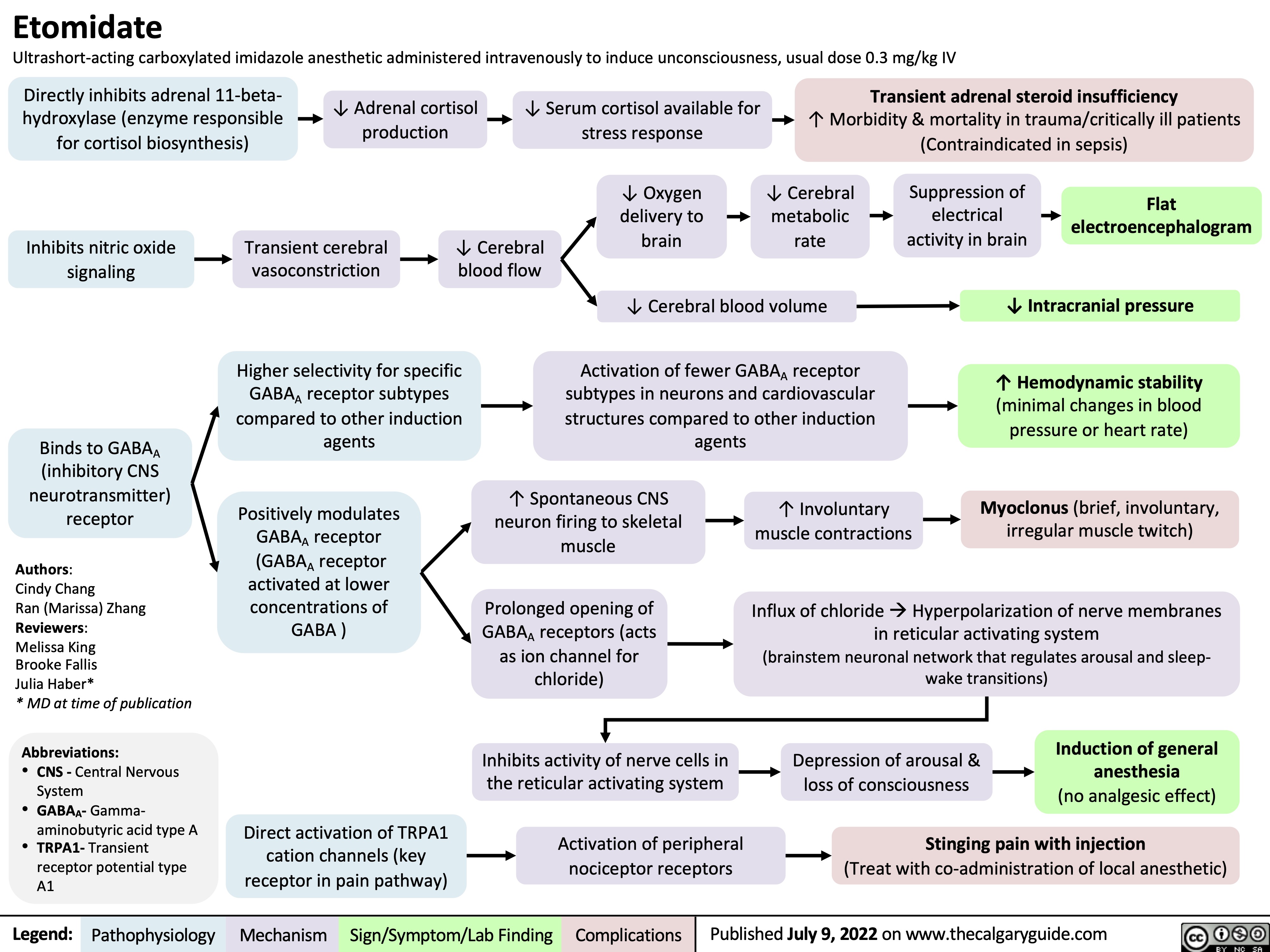
etomidate
complicaciones-de-la-enfermedad-renal-cronica
hyperkalemia-↓-renal-excretion-pathophysiology
![Hyperkalemia (↓ Renal Excretion): Pathophysiology
Non-steroidal anti- inflammatory drugs (NSAIDs)
Inhibition of prostaglandins which promote renin secretion (see NSAIDs and the Kidney: Mechanism of Action and Side Effects slide)
Diabetic Nephropathy
Acute (AIN)and chronic (CIN) interstitial nephritis
Immune-mediated damage of the kidney tubule and interstitium
Damage to distal tubule leads to aldosterone resistance at principal cell
Aldosterone cannot ↑ EnaC insertion on principal cell of CCD
Epithelial sodium channel (ENaC) blockers
Acute kidney injury and chronic kidney disease
↓ Effective arterial blood volume (volume of blood effectively perfusing tissue)
↓ Oxygen perfusion to renal tissue causing renal ischemia
Autonomic neuropathy ↓ sympathetic drive to produce renin
Chronic juxtaglomerular cell damage ↓ synthesis of renin
Blockage of ENaC on the principal cells of the CCD
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is either not formed (ACEi), or blocked at its receptor (ARBs)
Angiotensin II cannot stimulate the release of aldosterone from the adrenal cortex
Adrenal Insufficiency
Adrenal gland cannot produce sufficient amounts of aldosterone
↓ Renin secretion by the afferent arteriole prevents RAAS activation
↓ Aldosterone release from adrenal cortex
↓ ENaC (Na+ reabsorption channel) expression on principal cells of the cortical collecting duct (CCD)
Damage to kidney causes renal impairment and ↓ glomerular filtration rate
↓ Glomerular filtrate production means ↓ tubular flow rate
↓ Na+ delivery to the distal tubule
↓ Na+ reabsorption by ENaCs at the principal cell in CCD
↓ Na+ and water reabsorption by ENaCs in CCD ↓ Effective arterial blood volume (EABV)
Activates renin-angiotensin-aldosterone system (RAAS) leads to ↑ renin secretion in the afferent arteriole (see Physiology of the renin-angiotensin-aldosterone system (RAAS) slide)
↑ Na+ and water loss in tubular lumen
↑ Positive charge in tubular lumen
↓ Electronegativity gradient in tubular lumen
of CCD
↓ K+ excretion by the principal cell in the CCD as there is less of a electronegative gradient
↑ Accumulation of K+ in blood Hyperkalemia
Serum [K+] > 5.1 mmol/L
See Hyperkalemia: Clinical Findings slide
In the case of diabetic nephropathy and NSAIDS ↓ EABV does not stimulate RAAS and therefore aldosterone production
↓ Renin
↓ Aldosterone
↑ Renin secretion activates an ↑ in aldosterone production but aldosterone action at the principal cell is blocked because of ENaCs or resistance in AIN and CIN
↑ Renin
↑ Aldosterone
In the case of ACEi, ARBs, or adrenal insufficiency ↑ renin secretion does not lead to ↑ aldosterone
↑ Renin
↓ Aldosterone
Note: as described in the above flow chart, measuring serum renin and aldosterone levels can be used to help diagnose the cause of hyperkalemia.
Authors: Mannat Dhillon, Joshua Low, Emily Wildman, Huneza Nadeem Reviewers: Andrea Kuczynski, Marissa (Ran) Zhang, Adam Bass*, Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Aug 2, 2022 on www.thecalgaryguide.com
Hyperkalemia (↓ Renal Excretion): Pathophysiology
Non-steroidal anti- inflammatory drugs (NSAIDs)
Inhibition of prostaglandins which promote renin secretion (see NSAIDs and the Kidney: Mechanism of Action and Side Effects slide)
Diabetic Nephropathy
Acute (AIN)and chronic (CIN) interstitial nephritis
Immune-mediated damage of the kidney tubule and interstitium
Damage to distal tubule leads to aldosterone resistance at principal cell
Aldosterone cannot ↑ EnaC insertion on principal cell of CCD
Epithelial sodium channel (ENaC) blockers
Acute kidney injury and chronic kidney disease
↓ Effective arterial blood volume (volume of blood effectively perfusing tissue)
↓ Oxygen perfusion to renal tissue causing renal ischemia
Autonomic neuropathy ↓ sympathetic drive to produce renin
Chronic juxtaglomerular cell damage ↓ synthesis of renin
Blockage of ENaC on the principal cells of the CCD
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is either not formed (ACEi), or blocked at its receptor (ARBs)
Angiotensin II cannot stimulate the release of aldosterone from the adrenal cortex
Adrenal Insufficiency
Adrenal gland cannot produce sufficient amounts of aldosterone
↓ Renin secretion by the afferent arteriole prevents RAAS activation
↓ Aldosterone release from adrenal cortex
↓ ENaC (Na+ reabsorption channel) expression on principal cells of the cortical collecting duct (CCD)
Damage to kidney causes renal impairment and ↓ glomerular filtration rate
↓ Glomerular filtrate production means ↓ tubular flow rate
↓ Na+ delivery to the distal tubule
↓ Na+ reabsorption by ENaCs at the principal cell in CCD
↓ Na+ and water reabsorption by ENaCs in CCD ↓ Effective arterial blood volume (EABV)
Activates renin-angiotensin-aldosterone system (RAAS) leads to ↑ renin secretion in the afferent arteriole (see Physiology of the renin-angiotensin-aldosterone system (RAAS) slide)
↑ Na+ and water loss in tubular lumen
↑ Positive charge in tubular lumen
↓ Electronegativity gradient in tubular lumen
of CCD
↓ K+ excretion by the principal cell in the CCD as there is less of a electronegative gradient
↑ Accumulation of K+ in blood Hyperkalemia
Serum [K+] > 5.1 mmol/L
See Hyperkalemia: Clinical Findings slide
In the case of diabetic nephropathy and NSAIDS ↓ EABV does not stimulate RAAS and therefore aldosterone production
↓ Renin
↓ Aldosterone
↑ Renin secretion activates an ↑ in aldosterone production but aldosterone action at the principal cell is blocked because of ENaCs or resistance in AIN and CIN
↑ Renin
↑ Aldosterone
In the case of ACEi, ARBs, or adrenal insufficiency ↑ renin secretion does not lead to ↑ aldosterone
↑ Renin
↓ Aldosterone
Note: as described in the above flow chart, measuring serum renin and aldosterone levels can be used to help diagnose the cause of hyperkalemia.
Authors: Mannat Dhillon, Joshua Low, Emily Wildman, Huneza Nadeem Reviewers: Andrea Kuczynski, Marissa (Ran) Zhang, Adam Bass*, Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Aug 2, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/08/Hyperkalemia-renal-excretion.jpg)
presentation-of-sah
![Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com
Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/SAH-Clinical-Findings-2022.jpg)
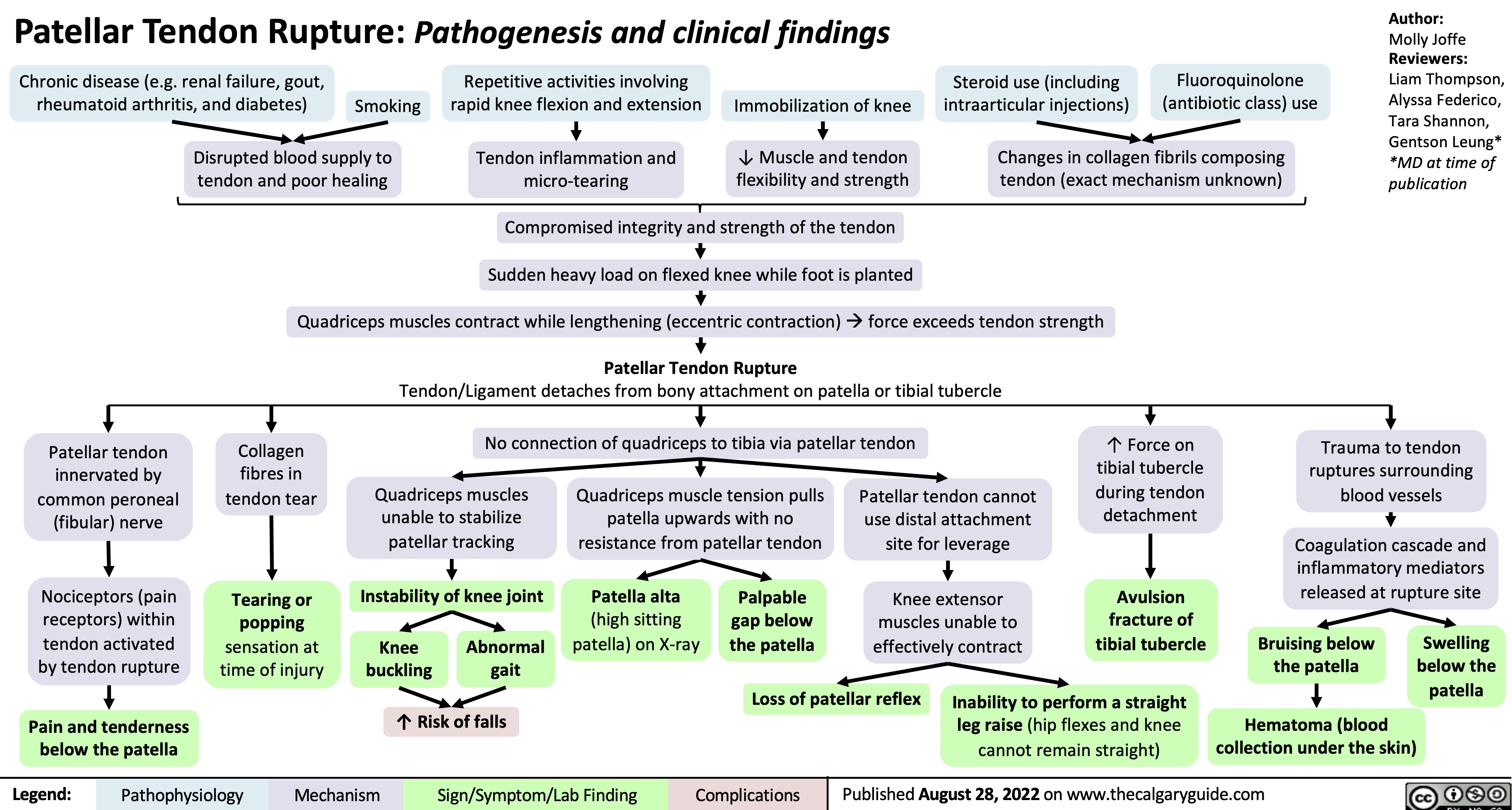
patellar-tendon-rupture-pathogenesis-and-clinical-findings
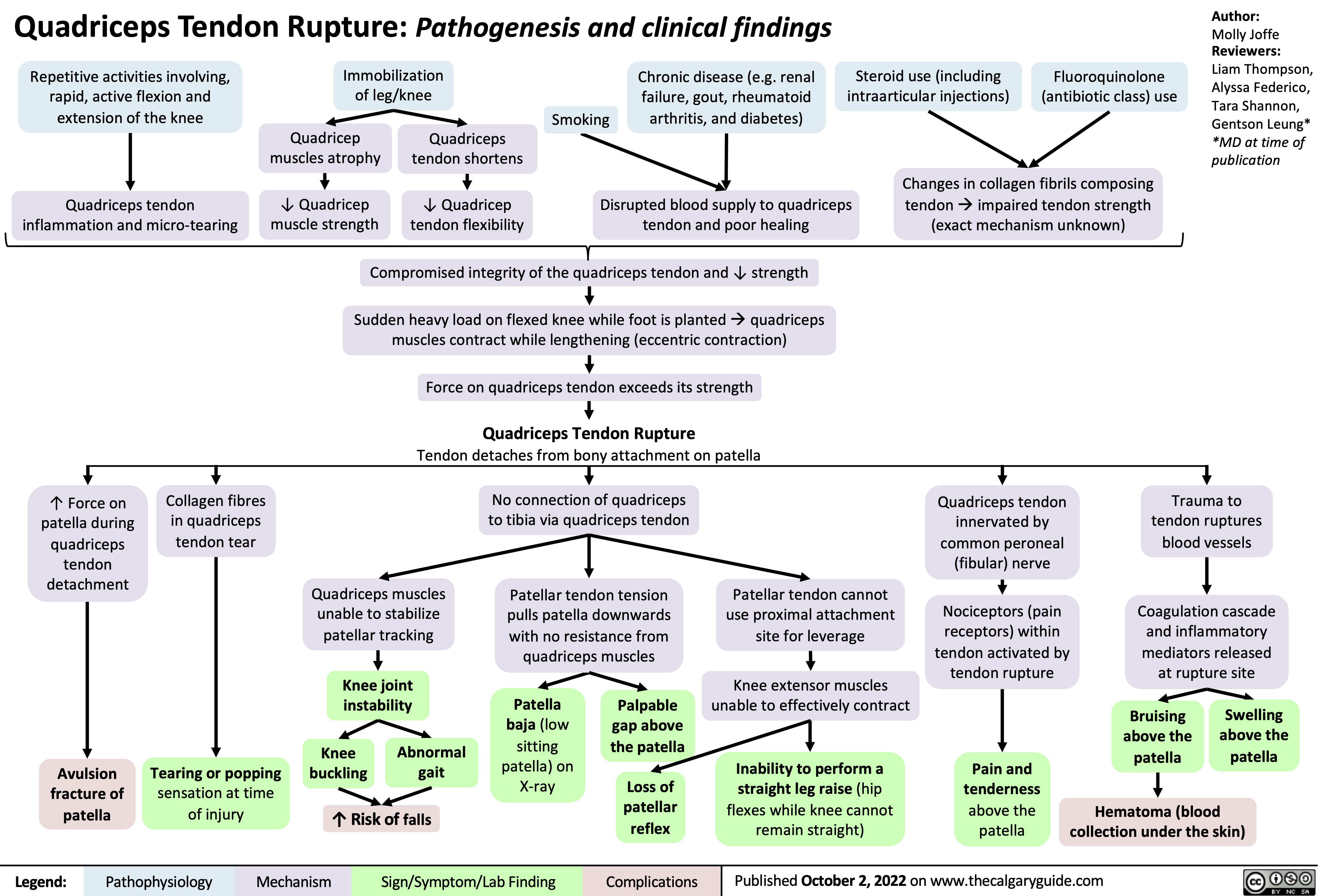
quadriceps-tendon-rupture-pathogenesis-and-clinical-findings
unstable-angina-pathogenesis-and-clinical-findings
![Unstable Angina/Unstable Angina Pectoris: Pathogenesis and clinical findings Primary cause:
Secondary causes:
Coronary artery vasospasm - primary or drug induced (Ex: cocaine, triptans)
Coagulopathy
(Ex: antiphospholipid antibody syndrome)
Vasculitic syndromes (Ex: Takayasu arteritis)
Authors: Marisa Vigna Ryan Wilkie Yan Yu* Reviewers: Julena Foglia Davis Maclean Mehul Gupta Andrew Grant* * MD at time of publication
Atherosclerosis
Fatty plaque accumulates inside the intimal walls of arteries Coronary arterial atherosclerotic plaque rupture or erosion
Plaque disruption exposes subendothelial components of damaged vessel wall to platelets, initiating the coagulation cascade and platelet adhesion
Aggregation of platelets results in the formation of a thrombus Thrombus partially occludes blood flow through a coronary artery âmyocardial blood supply
Congenital anomalies (Ex: myocardial bridge, anomalous coronary)
Spontaneous coronary artery dissection
Increased blood viscosity (Ex: polycythemia, thrombocytopenia)
Factors thatámyocardial (cardiac muscle) oxygen demand (Ex: tachycardia, hypotension, hypertension, anemia, exertion, stress)
Coronary embolism (Ex: A. Fib, endocarditis, prosthetic valve thrombus)
áheart rate, contractility, and/or wall tension ámyocardial oxygen demand
Myocardial ischemia due to imbalance between blood supply and oxygen demand (insufficient blood/oxygen supply)
Unstable Angina/Unstable Angina Pectoris
Can be new onset angina; typically progressive in frequency, severity, or duration; can occur at rest
Subtotal occlusion of a coronary arteryà
reduced, but continued, myocardial blood supply
Maintained perfusion means cardiomyocytes are still alive and thus do not leak troponin into bloodstream
Normal serum troponin
Diaphoresis
(sweating)
Since bloodflow occurs from epicardium to endocardium, myocardial ischemia is more
pronounced in the subendocardium (region furthest away from heart’s external surface)
Sufficient blood flow is maintained in regions superficial to the subendocardium, resulting in non-transmural (partial thickness) heart wall ischemia
Non-inferior wall ischemia triggers a predominantáin sympathetic nervous system activity, given the proximity of cardiac sympathetic nerve innervation
Ischemiaâ cardiomyocyte resting membrane potential andâ action potential duration
Voltage gradient between normal and subendocardial ischemic zones creates injury currents, shifting the ST- vector on ECG
ECG: ST depression
and/or T wave inversion
Cardiac sensory nerve fibres mix with somatic sensory nerve
fibres and enter the spinal cord via the T1-T4 nerve roots
Brain perceives increased cardiac sensory nerve signaling as nerve pain coming from the skin of T1-T4 dermatomes (“Referred Pain”)
Myocardial ischemia causes hypoxic stress on cardiomyocytesàâaerobic (requiring oxygen) metabolism,áanaerobic (not requiring oxygen) metabolism
áanerobic respirationálactic acid production,á[H+], andâcellular pH which impairs cardiomyocyte function
Cardiomyocyte dysfunction impairs myocardial relaxation in diastole and/orâ left ventricular contractility in systole
âleft ventricular cardiac output àbackup of blood in the left ventricle, atrium, and pulmonary vasculature
ápulmonary capillary pressures pushes fluid out of the capillaries into the alveoli in the lungs
Fluid filled alveoliâgas exchange andâ oxygenation, triggering harder and faster breathing in order to compensate
Dyspnea
Activation of sweat glands via acetylcholine release
Hormones bind to cardiac β1 receptors
Tachycardia
(áheart rate)
Epinephrine/ Norepinephrine hormone release from the adrenal medulla
Hormones bind to arterial smooth muscle α1 receptors ávascular tone (vasoconstriction)
Hypertension
The Vagus nerve sits in close physical proximity to the inferior wall of the heart àinferior wall ischemia triggers involuntary Vagus nerve activation
Since the Vagus nerve coordinates parasympathetic activity,áVagus nerve activity leads to a variety of parasympathetic nervous system responses:
Retrosternal discomfort: May present as pain, heaviness, tightness, aching, pressure, burning or squeezing
Pain radiation to T1-T4 dermatomes:
Left shoulder and arm, lower jaw, neck, abdomen, upper back
Syncope
(fainting)
Bradycardia
Nausea Hypotension
(âheart rate)
(âblood pressure)
(áblood pressure)
(shortness of breath)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Findings
Complications
Published Oct 18, 2015, updated Aug 29, 2021 on www.thecalgaryguide.com
Unstable Angina/Unstable Angina Pectoris: Pathogenesis and clinical findings Primary cause:
Secondary causes:
Coronary artery vasospasm - primary or drug induced (Ex: cocaine, triptans)
Coagulopathy
(Ex: antiphospholipid antibody syndrome)
Vasculitic syndromes (Ex: Takayasu arteritis)
Authors: Marisa Vigna Ryan Wilkie Yan Yu* Reviewers: Julena Foglia Davis Maclean Mehul Gupta Andrew Grant* * MD at time of publication
Atherosclerosis
Fatty plaque accumulates inside the intimal walls of arteries Coronary arterial atherosclerotic plaque rupture or erosion
Plaque disruption exposes subendothelial components of damaged vessel wall to platelets, initiating the coagulation cascade and platelet adhesion
Aggregation of platelets results in the formation of a thrombus Thrombus partially occludes blood flow through a coronary artery âmyocardial blood supply
Congenital anomalies (Ex: myocardial bridge, anomalous coronary)
Spontaneous coronary artery dissection
Increased blood viscosity (Ex: polycythemia, thrombocytopenia)
Factors thatámyocardial (cardiac muscle) oxygen demand (Ex: tachycardia, hypotension, hypertension, anemia, exertion, stress)
Coronary embolism (Ex: A. Fib, endocarditis, prosthetic valve thrombus)
áheart rate, contractility, and/or wall tension ámyocardial oxygen demand
Myocardial ischemia due to imbalance between blood supply and oxygen demand (insufficient blood/oxygen supply)
Unstable Angina/Unstable Angina Pectoris
Can be new onset angina; typically progressive in frequency, severity, or duration; can occur at rest
Subtotal occlusion of a coronary arteryà
reduced, but continued, myocardial blood supply
Maintained perfusion means cardiomyocytes are still alive and thus do not leak troponin into bloodstream
Normal serum troponin
Diaphoresis
(sweating)
Since bloodflow occurs from epicardium to endocardium, myocardial ischemia is more
pronounced in the subendocardium (region furthest away from heart’s external surface)
Sufficient blood flow is maintained in regions superficial to the subendocardium, resulting in non-transmural (partial thickness) heart wall ischemia
Non-inferior wall ischemia triggers a predominantáin sympathetic nervous system activity, given the proximity of cardiac sympathetic nerve innervation
Ischemiaâ cardiomyocyte resting membrane potential andâ action potential duration
Voltage gradient between normal and subendocardial ischemic zones creates injury currents, shifting the ST- vector on ECG
ECG: ST depression
and/or T wave inversion
Cardiac sensory nerve fibres mix with somatic sensory nerve
fibres and enter the spinal cord via the T1-T4 nerve roots
Brain perceives increased cardiac sensory nerve signaling as nerve pain coming from the skin of T1-T4 dermatomes (“Referred Pain”)
Myocardial ischemia causes hypoxic stress on cardiomyocytesàâaerobic (requiring oxygen) metabolism,áanaerobic (not requiring oxygen) metabolism
áanerobic respirationálactic acid production,á[H+], andâcellular pH which impairs cardiomyocyte function
Cardiomyocyte dysfunction impairs myocardial relaxation in diastole and/orâ left ventricular contractility in systole
âleft ventricular cardiac output àbackup of blood in the left ventricle, atrium, and pulmonary vasculature
ápulmonary capillary pressures pushes fluid out of the capillaries into the alveoli in the lungs
Fluid filled alveoliâgas exchange andâ oxygenation, triggering harder and faster breathing in order to compensate
Dyspnea
Activation of sweat glands via acetylcholine release
Hormones bind to cardiac β1 receptors
Tachycardia
(áheart rate)
Epinephrine/ Norepinephrine hormone release from the adrenal medulla
Hormones bind to arterial smooth muscle α1 receptors ávascular tone (vasoconstriction)
Hypertension
The Vagus nerve sits in close physical proximity to the inferior wall of the heart àinferior wall ischemia triggers involuntary Vagus nerve activation
Since the Vagus nerve coordinates parasympathetic activity,áVagus nerve activity leads to a variety of parasympathetic nervous system responses:
Retrosternal discomfort: May present as pain, heaviness, tightness, aching, pressure, burning or squeezing
Pain radiation to T1-T4 dermatomes:
Left shoulder and arm, lower jaw, neck, abdomen, upper back
Syncope
(fainting)
Bradycardia
Nausea Hypotension
(âheart rate)
(âblood pressure)
(áblood pressure)
(shortness of breath)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Findings
Complications
Published Oct 18, 2015, updated Aug 29, 2021 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/10/Unstable-Angina-2021.jpg)
type-ii-proximal-renal-tubular-acidosis-pathogenesis-and-laboratory-findings
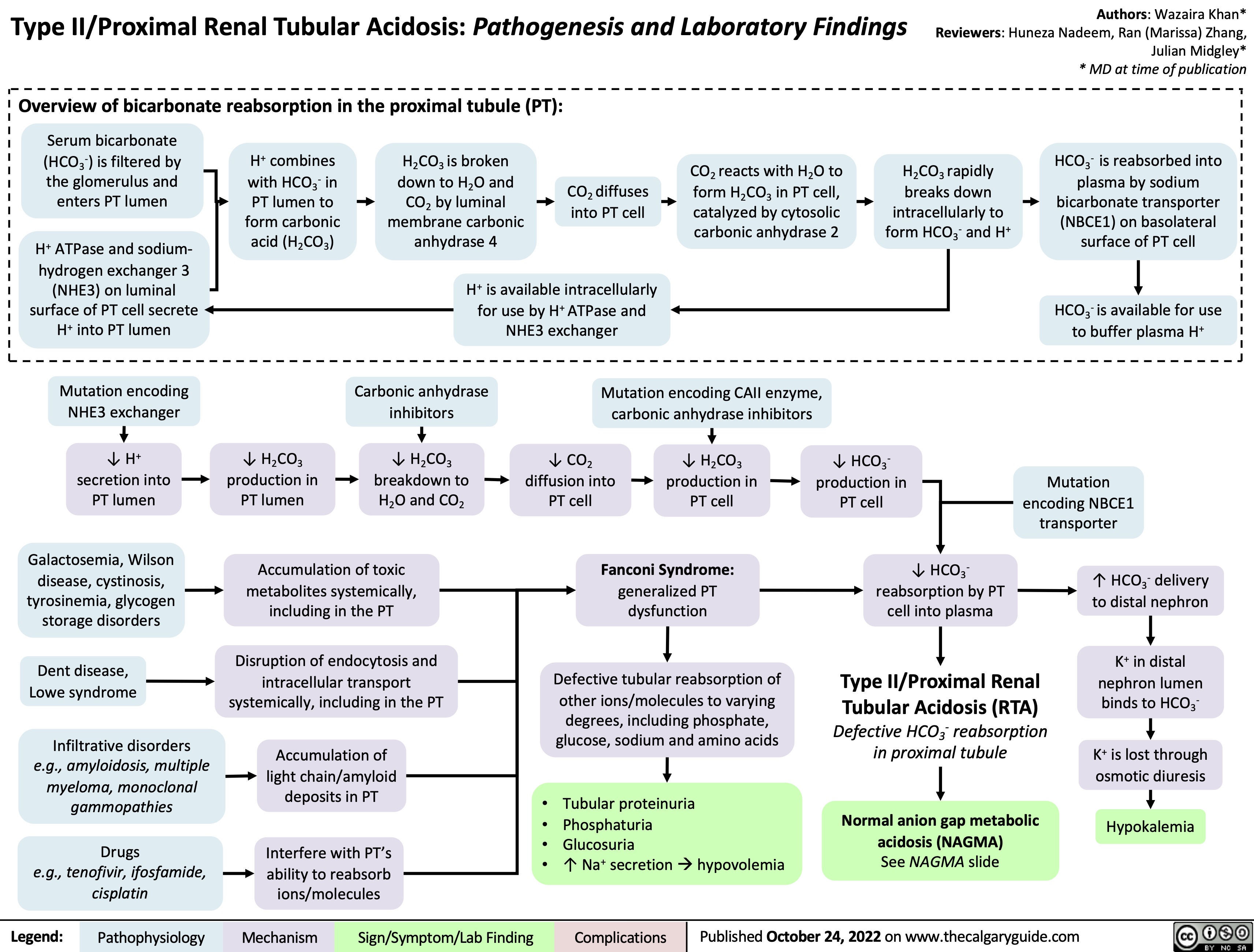
Granulomatosis with Polyangiitis Pathogenesis
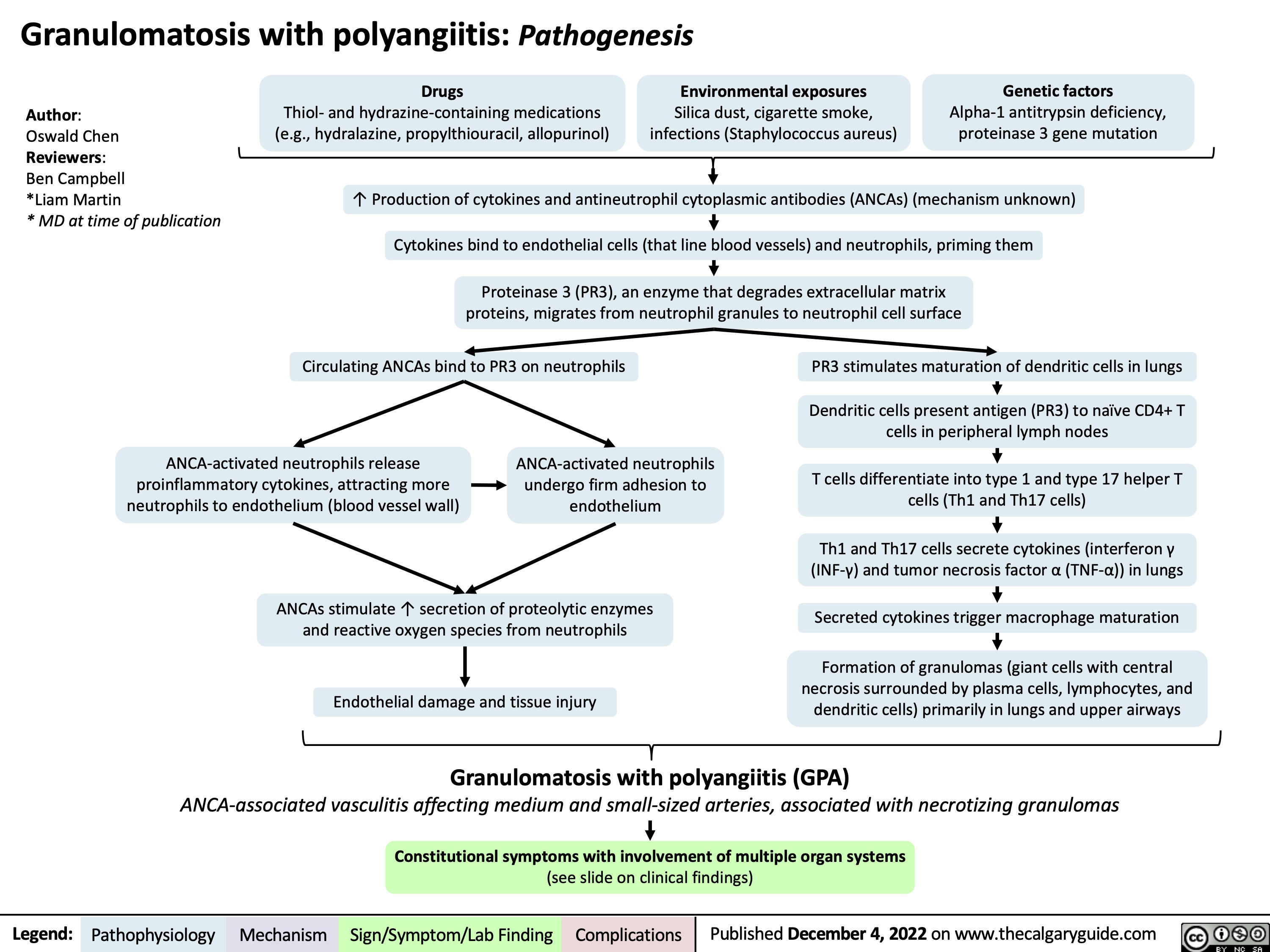
Granulomatosis with polyangiitis: Clinical findings
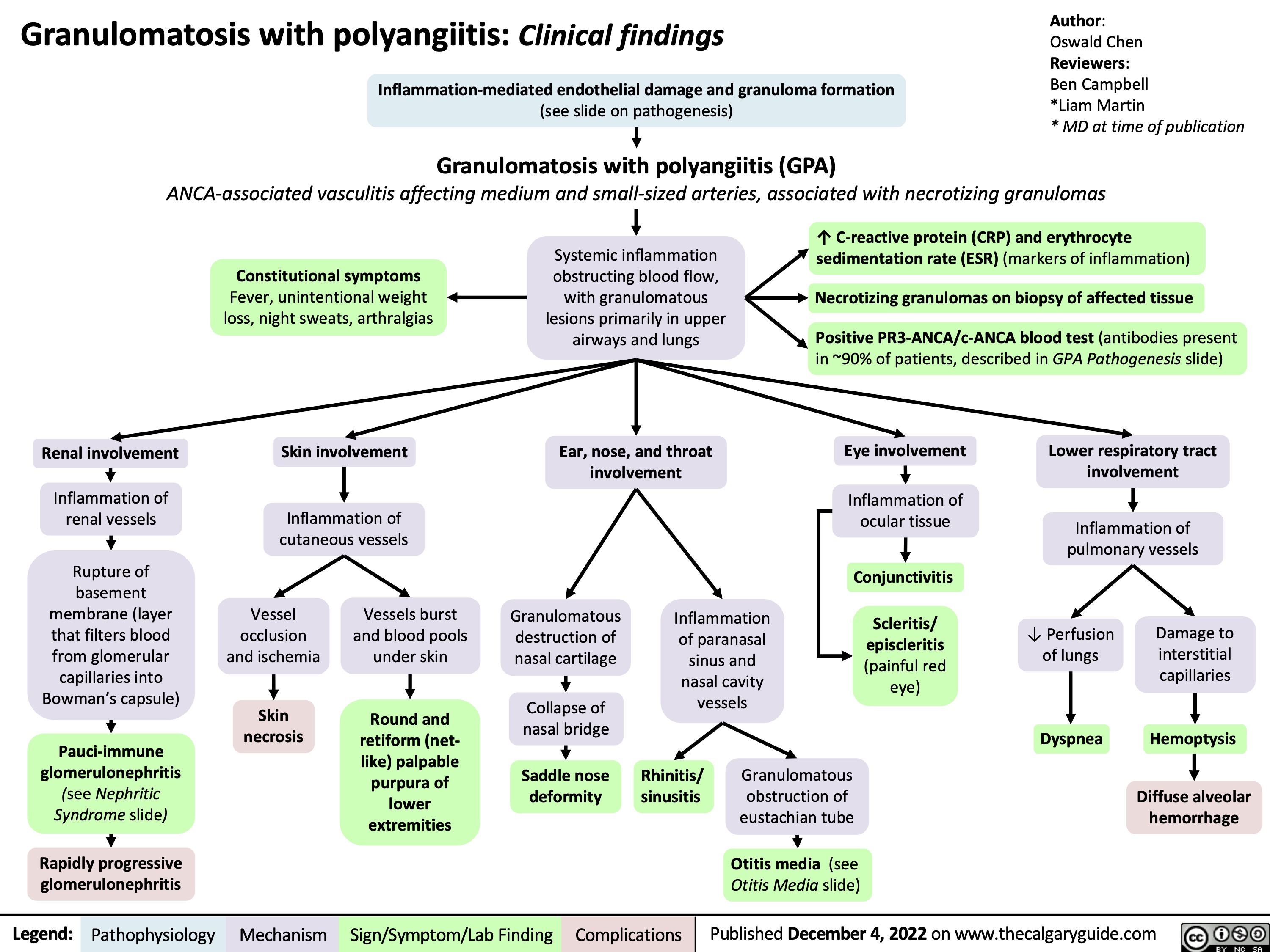
hypovolemic-shock
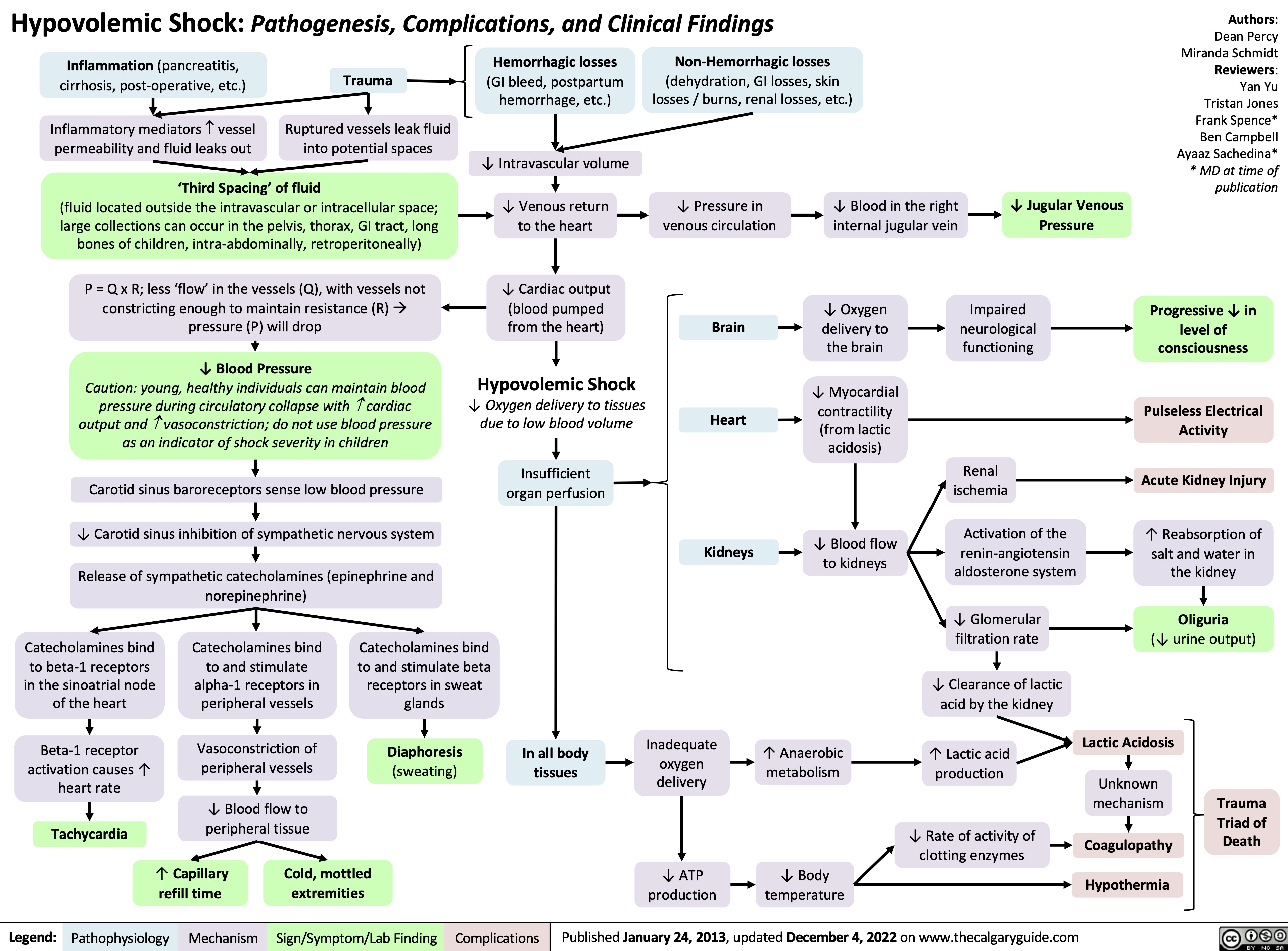
Approach to Arterial Blood Gases ABGs
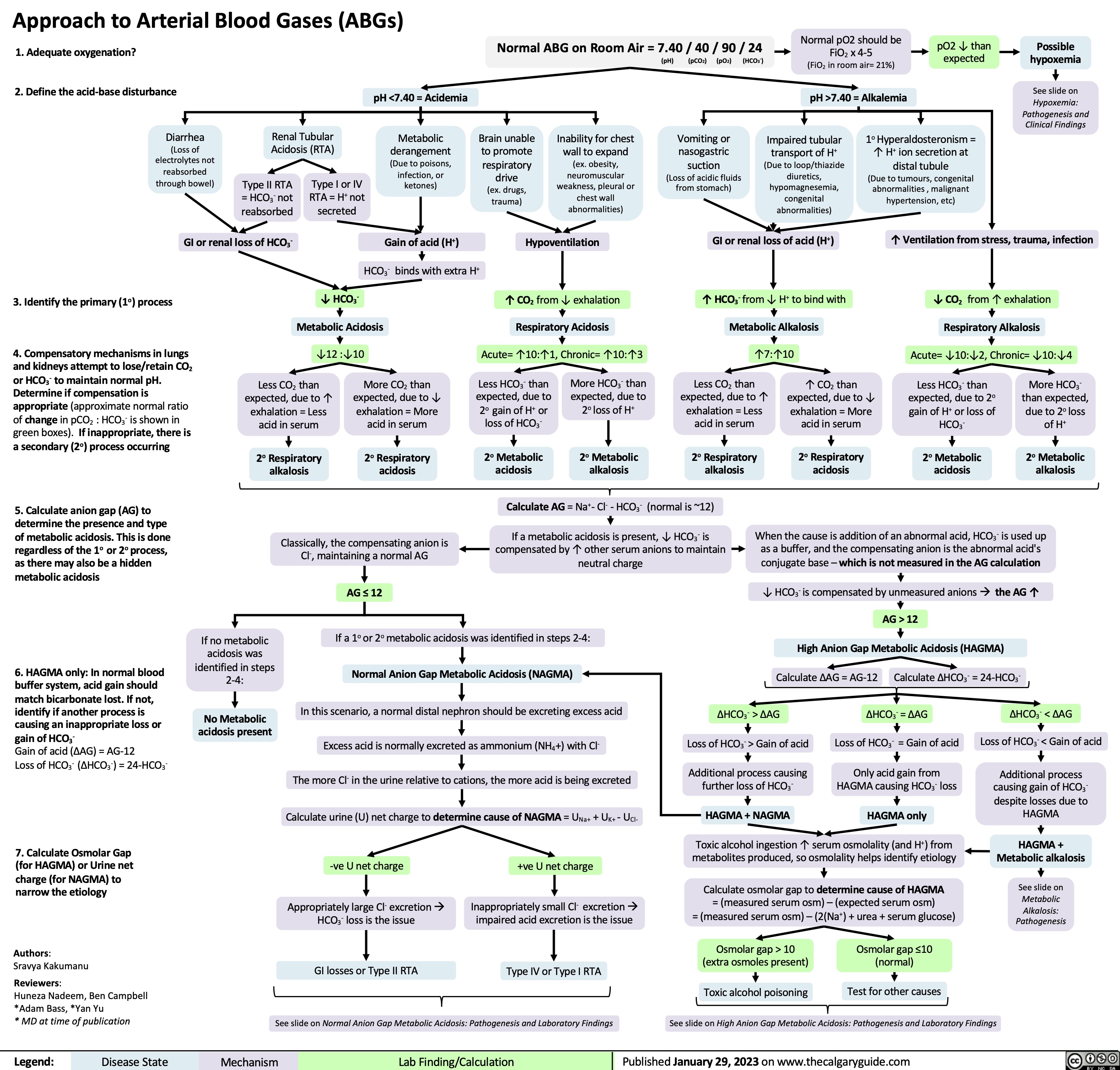
Coronary Artery Bypass Graft CABG Indications
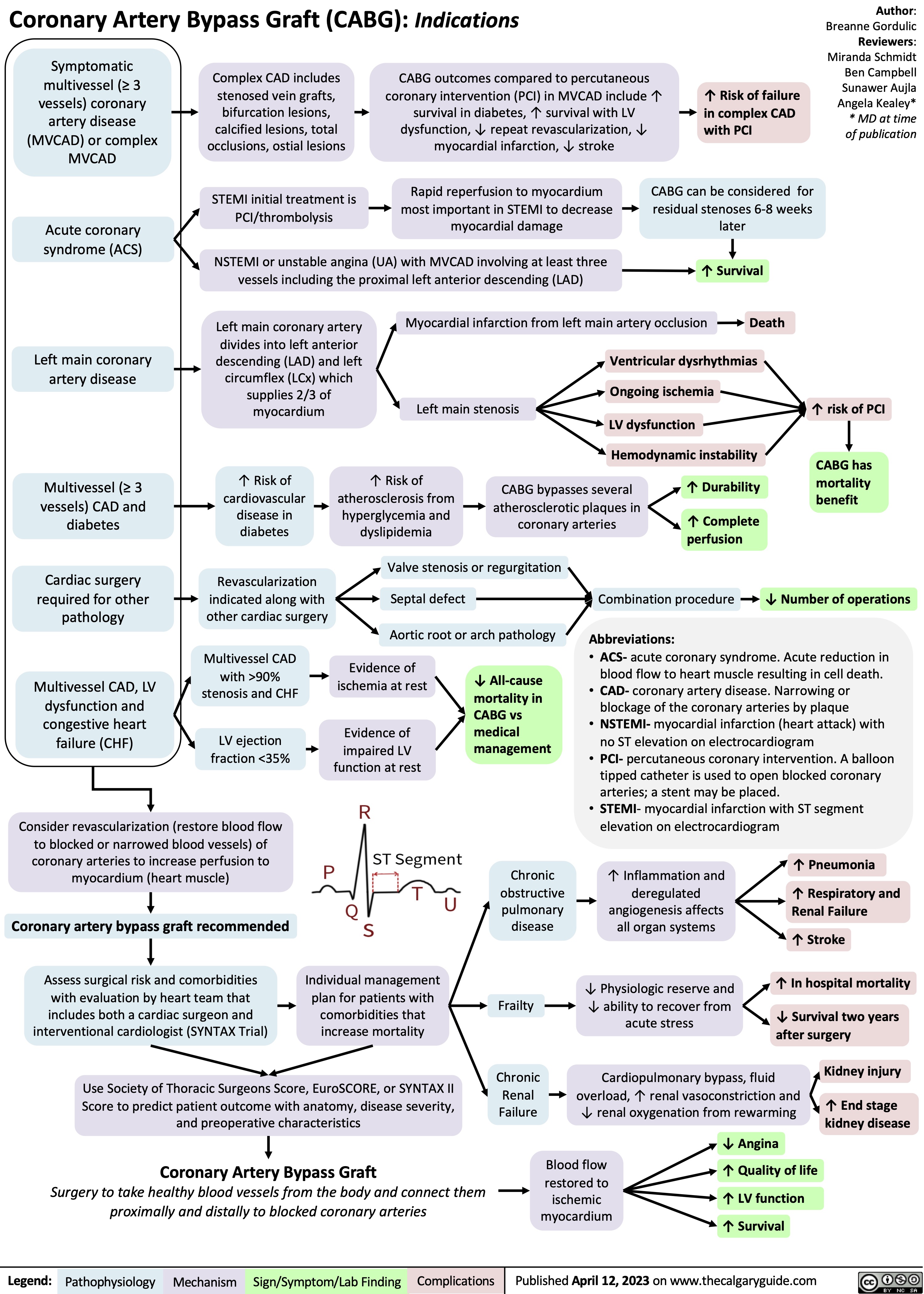
normal neonatal changes pathogenesis and clinical findings
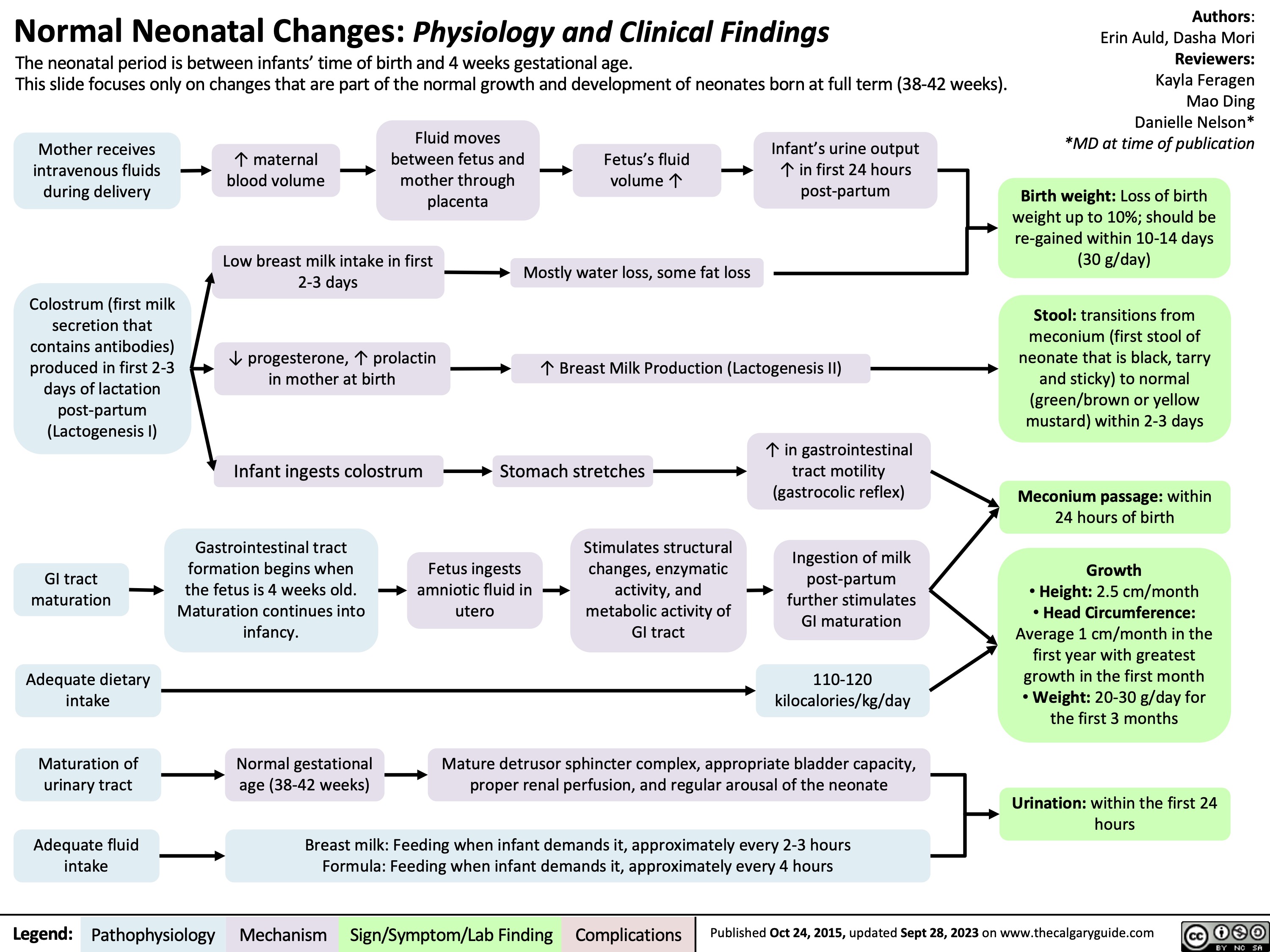
Alcohol Withdrawal Syndrome
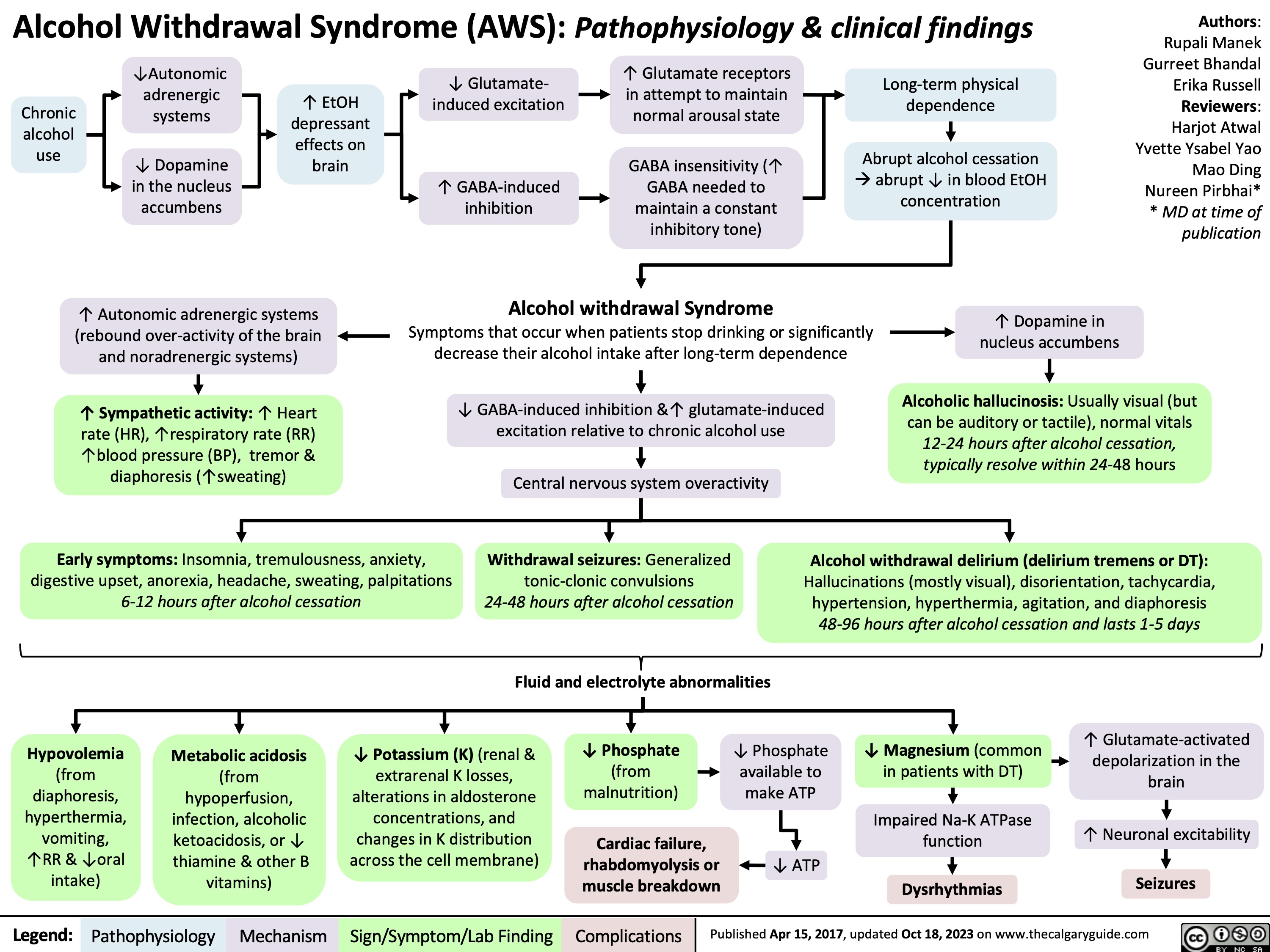
Pharmacotherapy for Dyslipidemia Overview
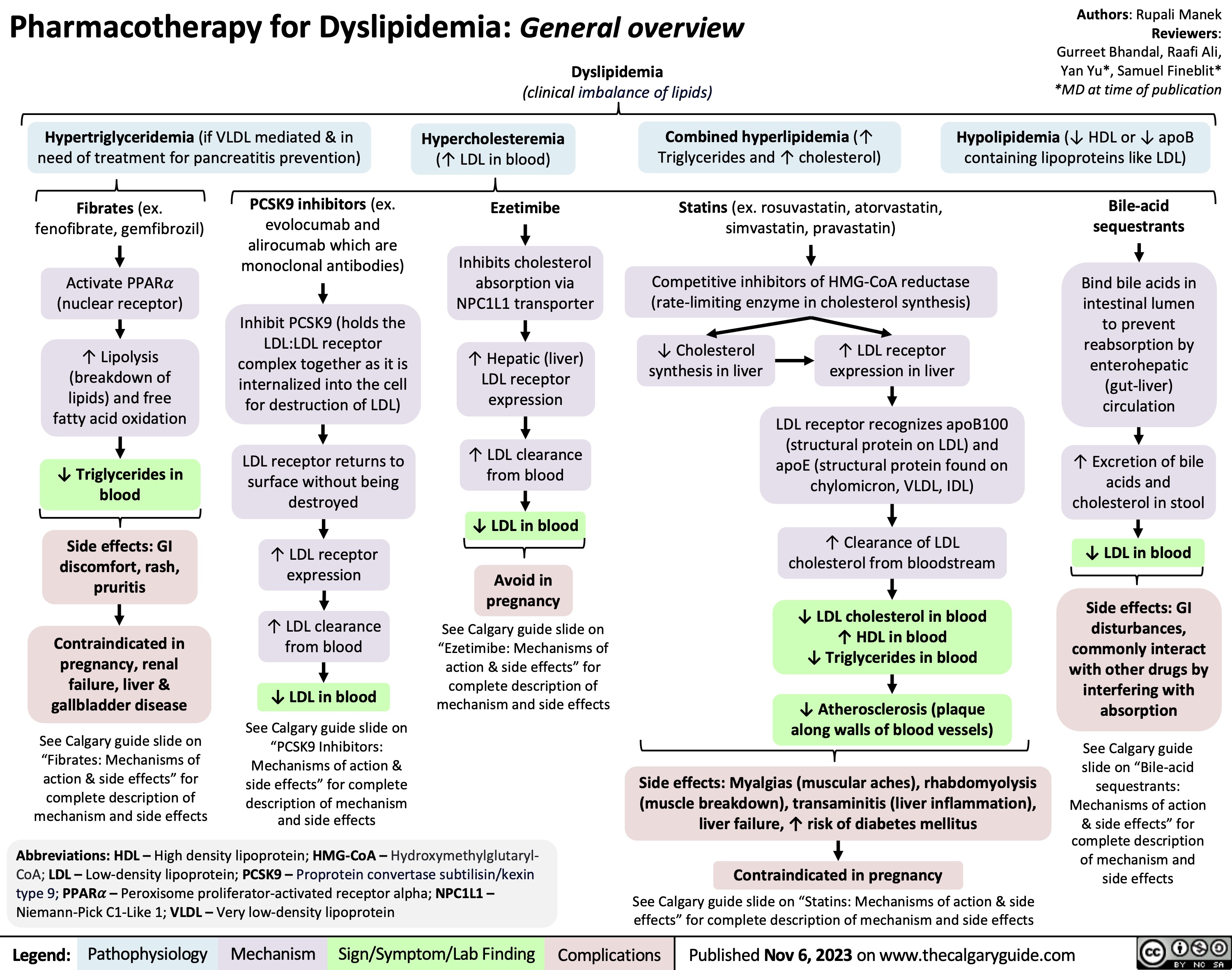
Death Cardiovascular Respiratory and Neurologic Mechanisms
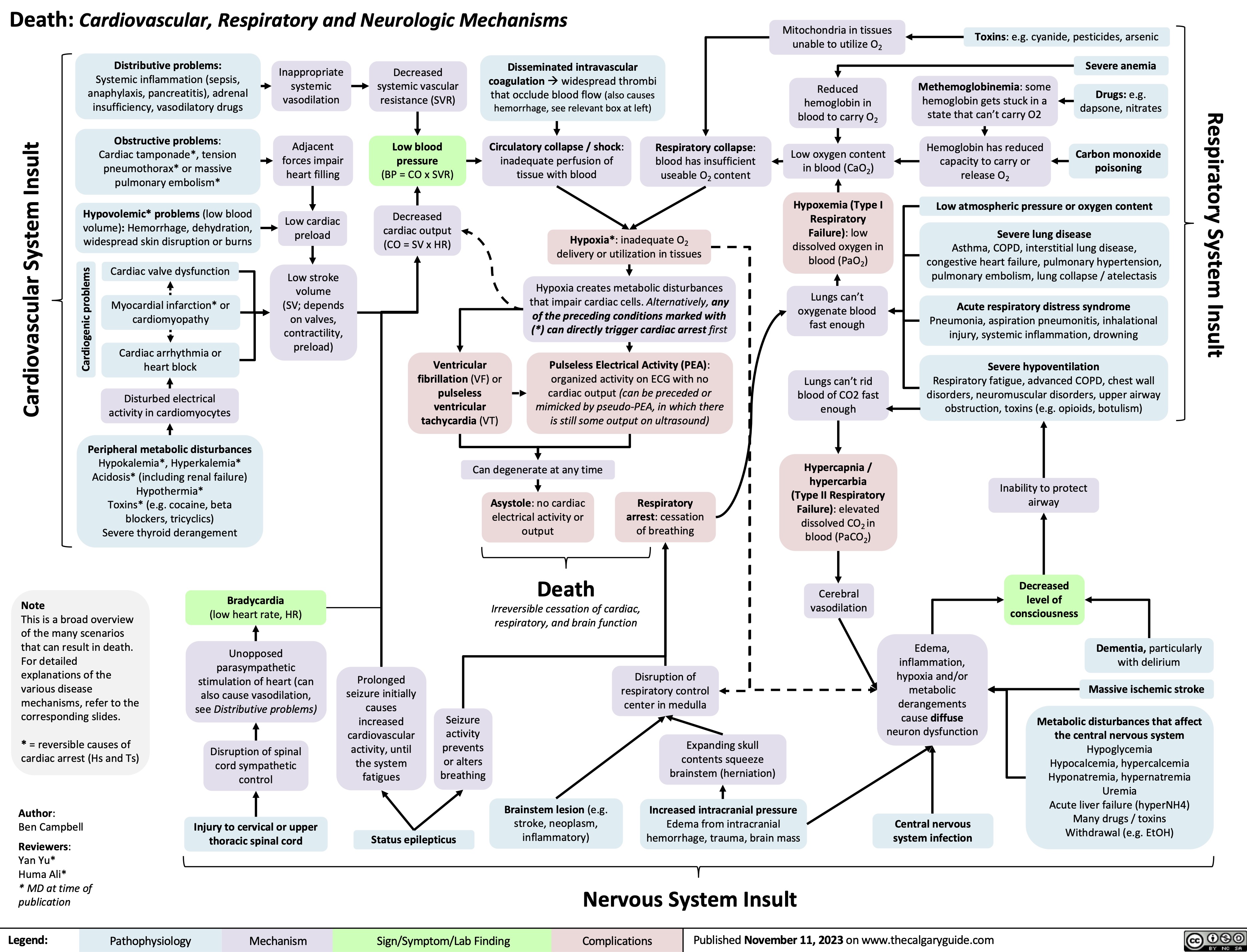
Turner Syndrome Pathogenesis and Clinical Findings
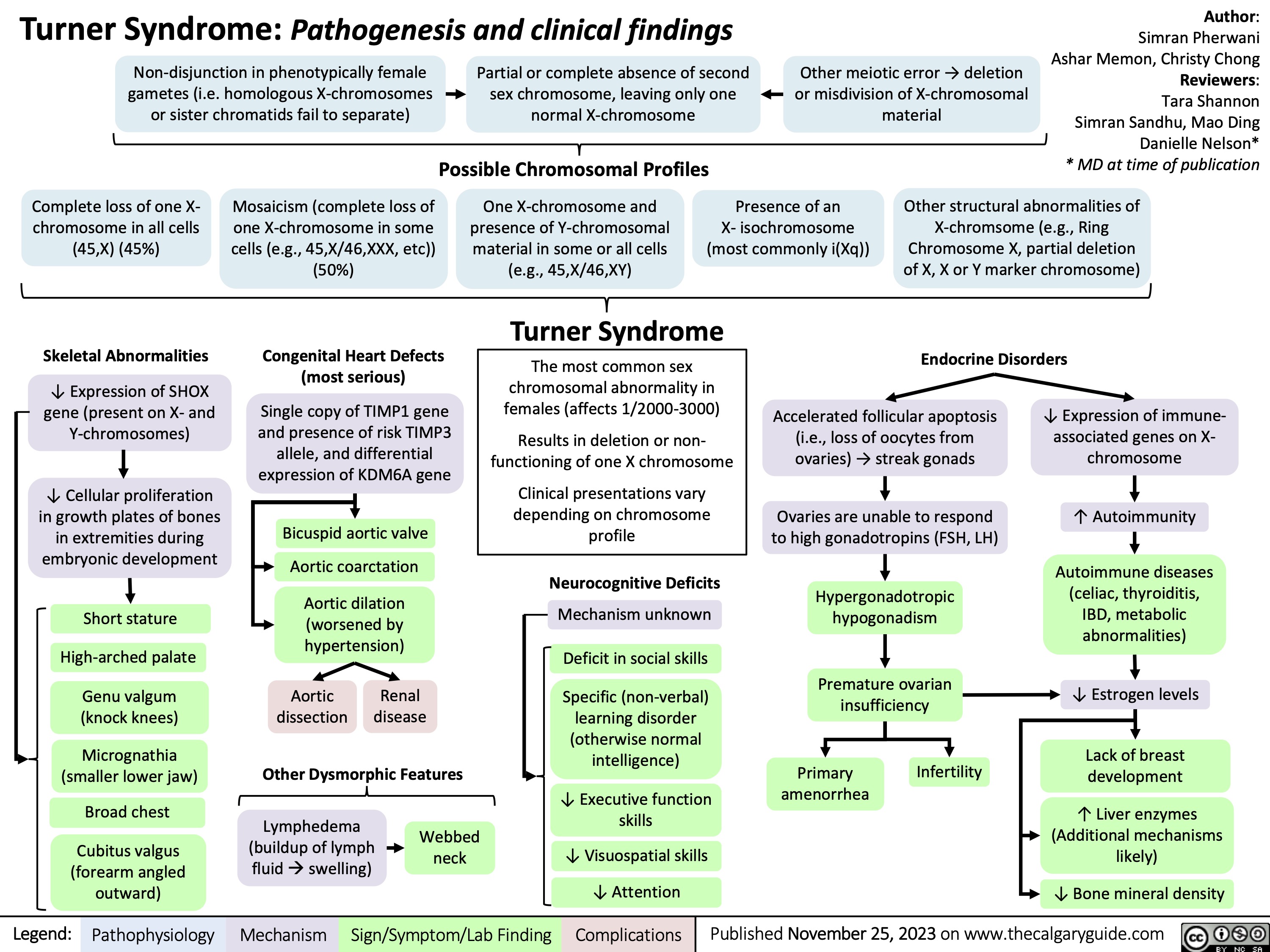
Sugammadex
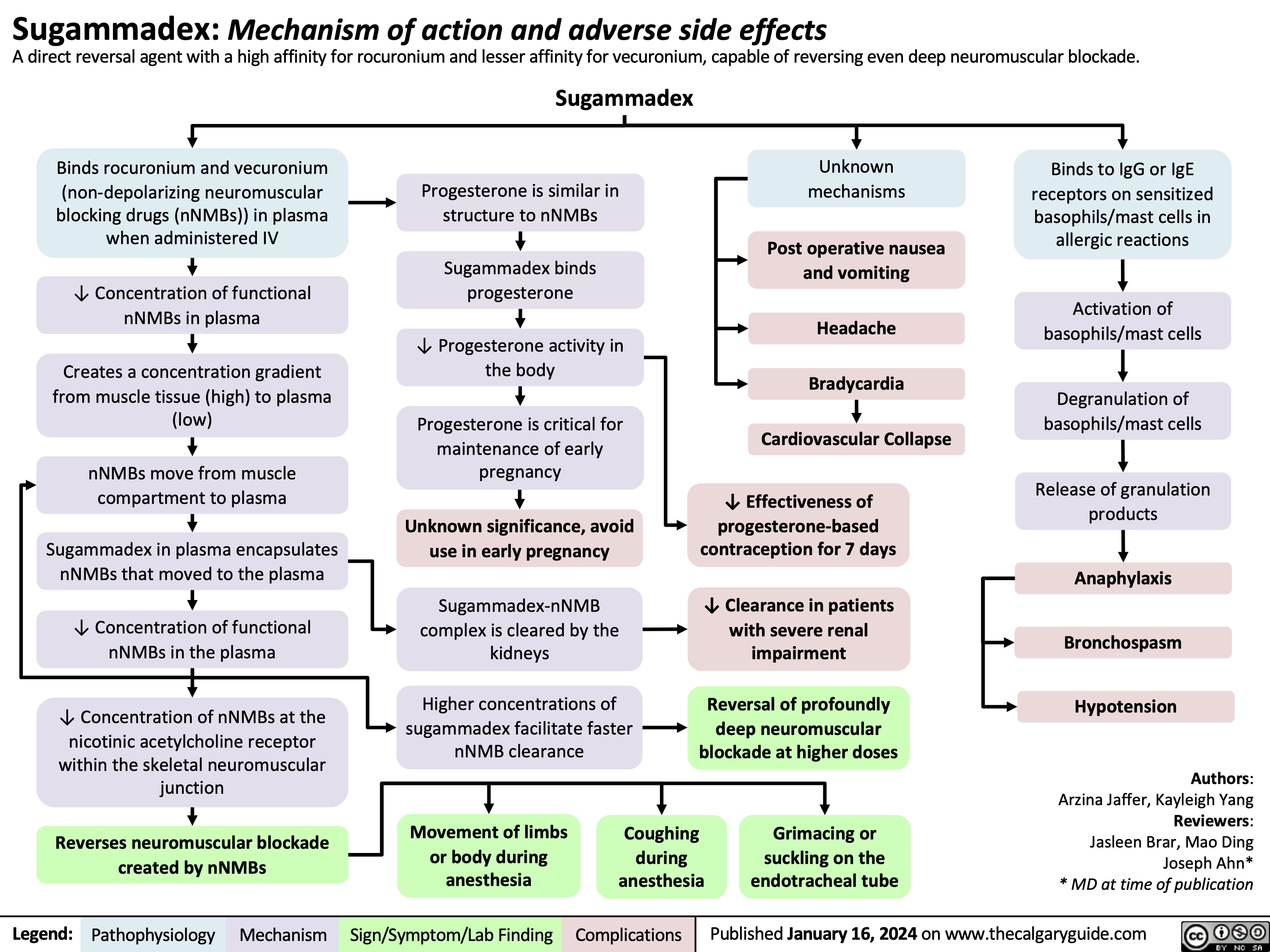
Bacterial Infections from Transfusion
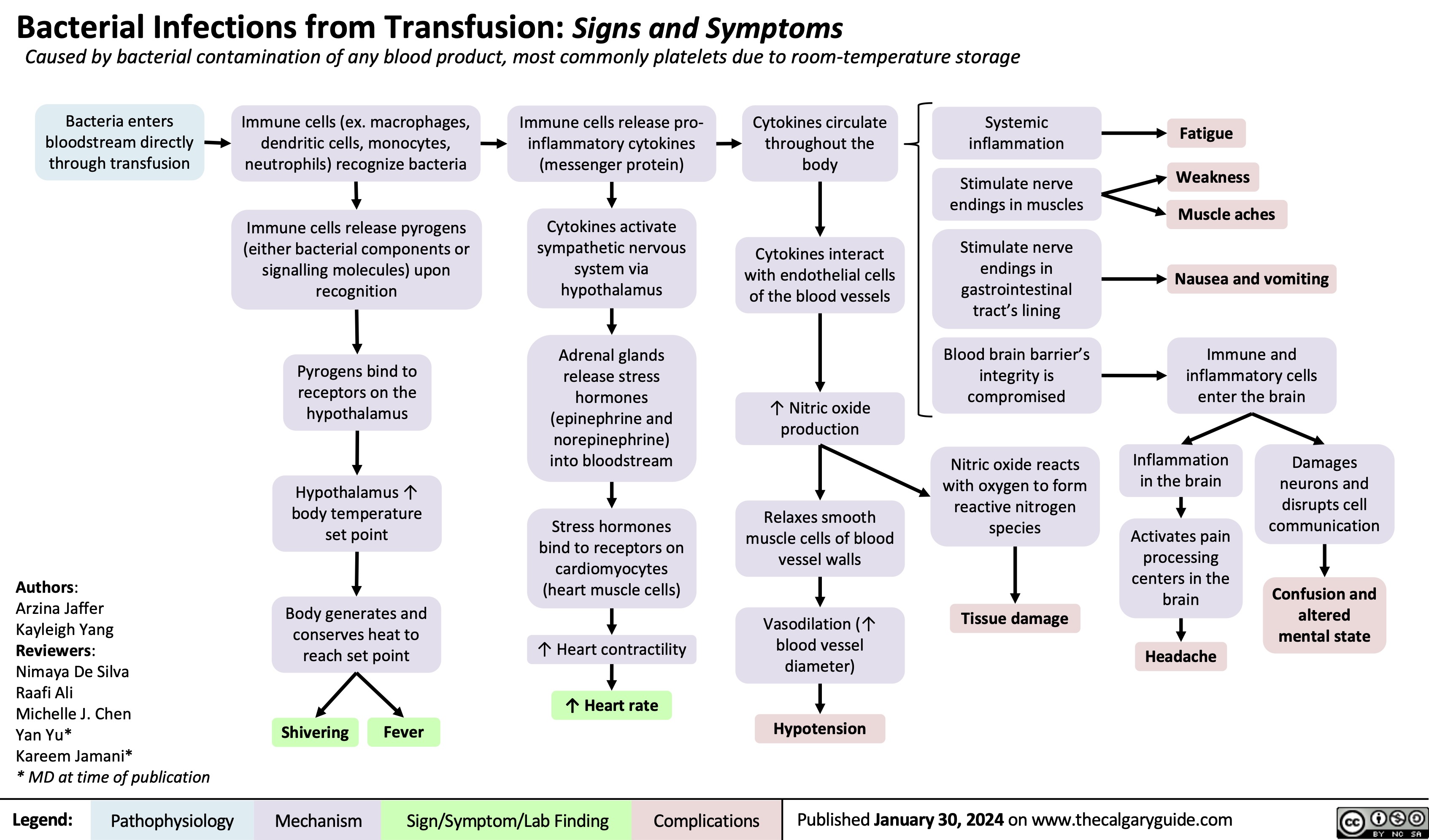
Carbonic Anhydrase Inhibitor Diuretics
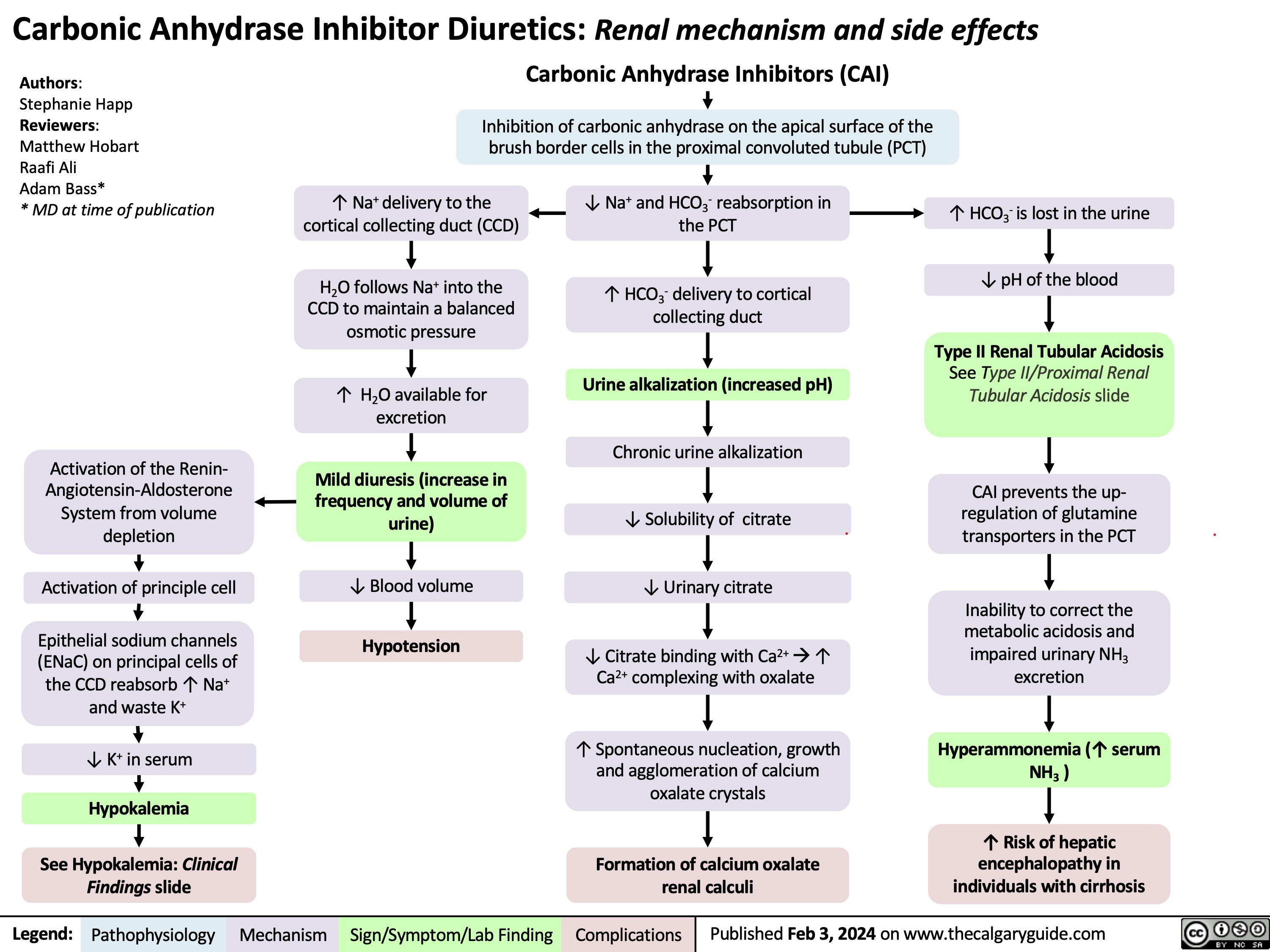
Stable Angina
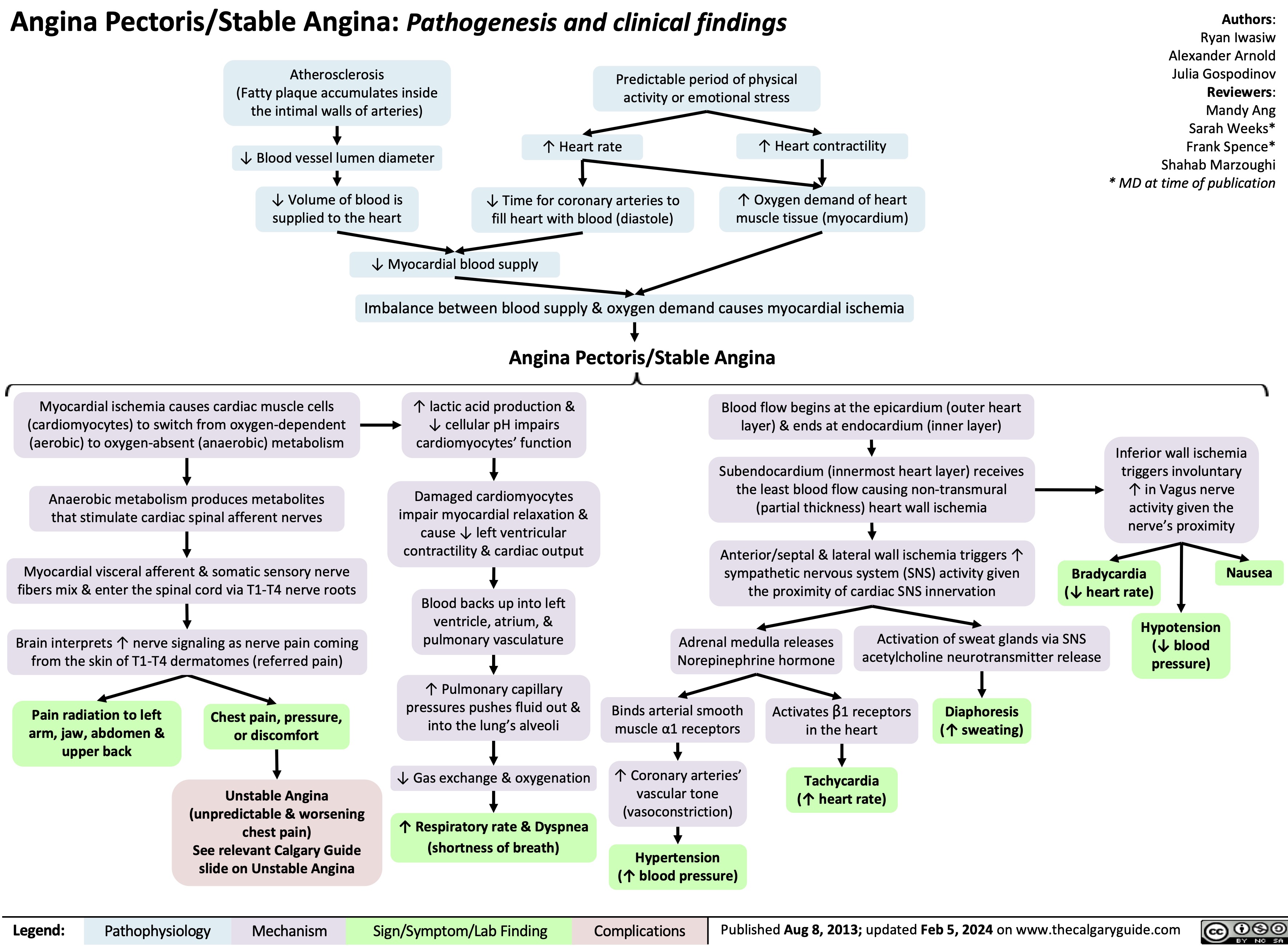
Gestational Diabetes Risk factors and pathogenesis
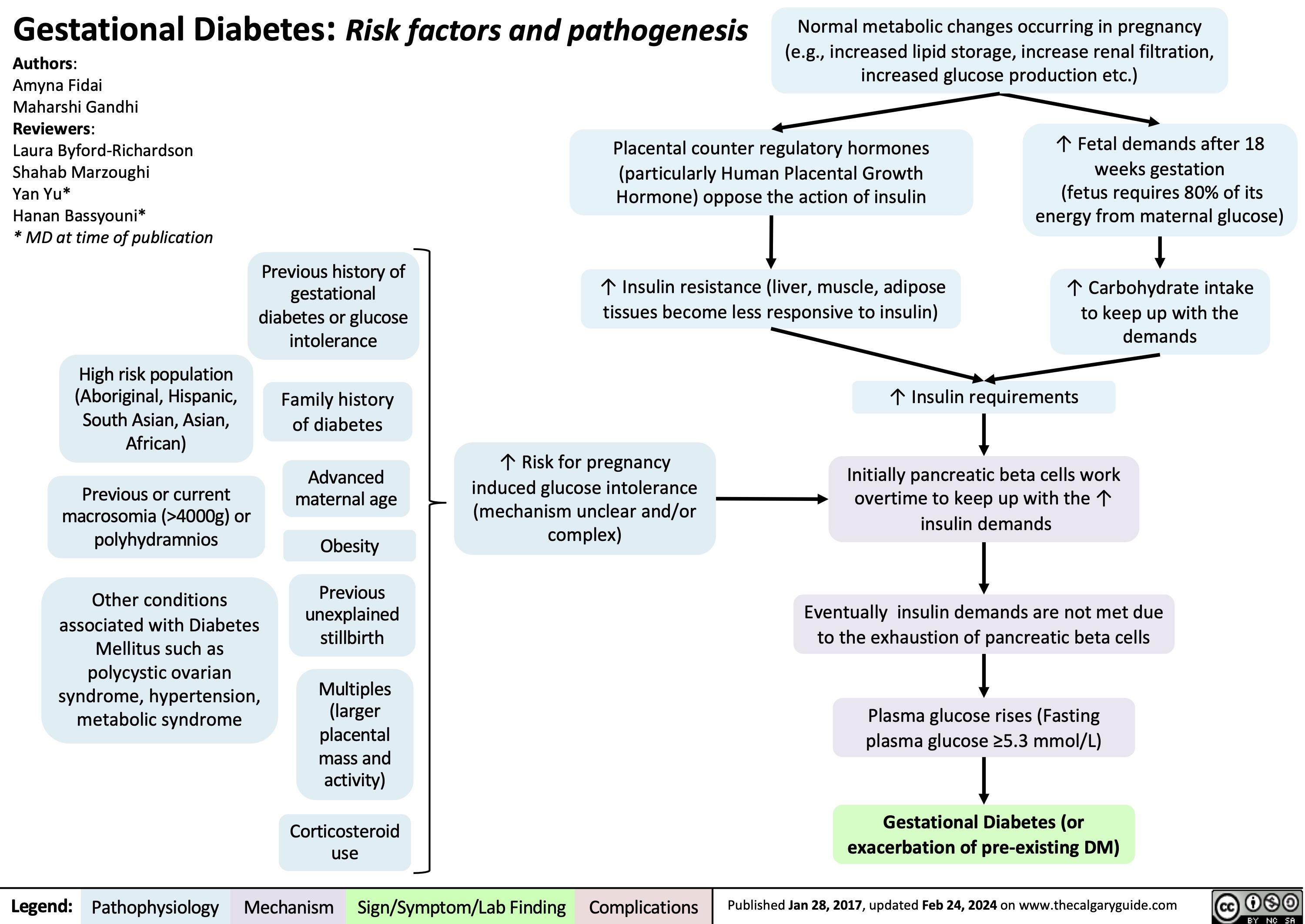
Infectious Small Bowel Diarrhea
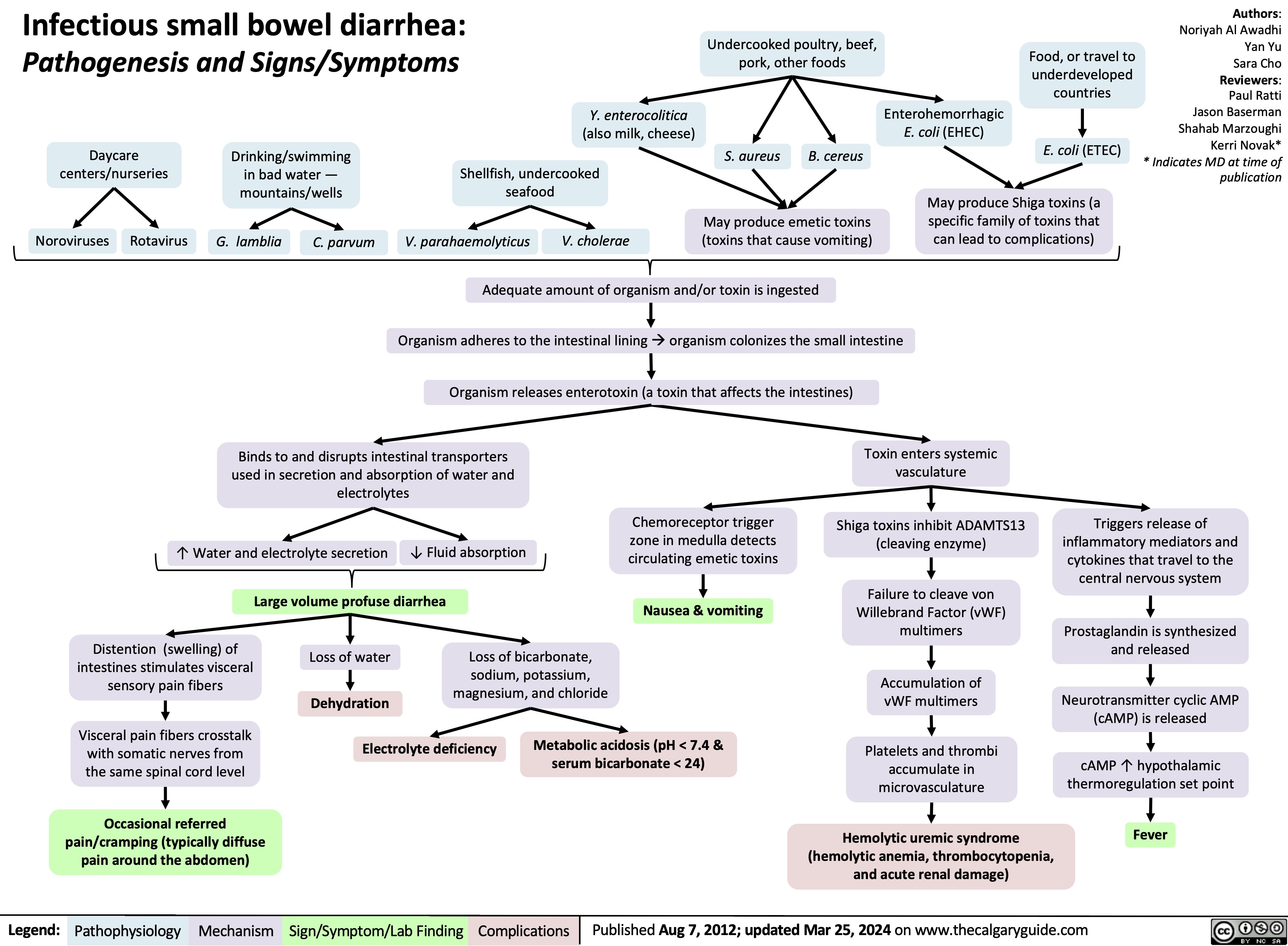
Post-Renal Acute Kidney Injury AKI
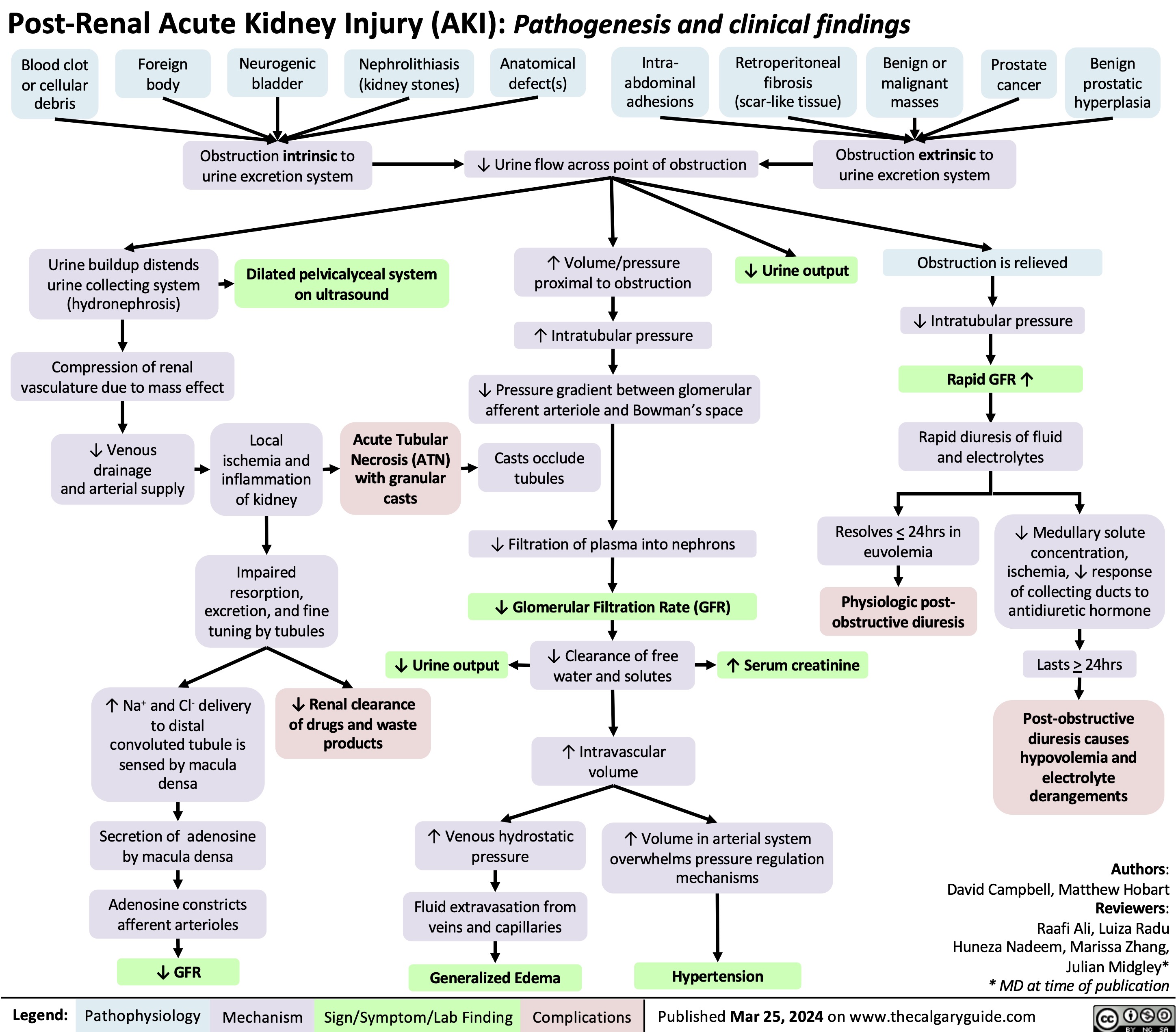
Operative Vaginal Delivery Indications
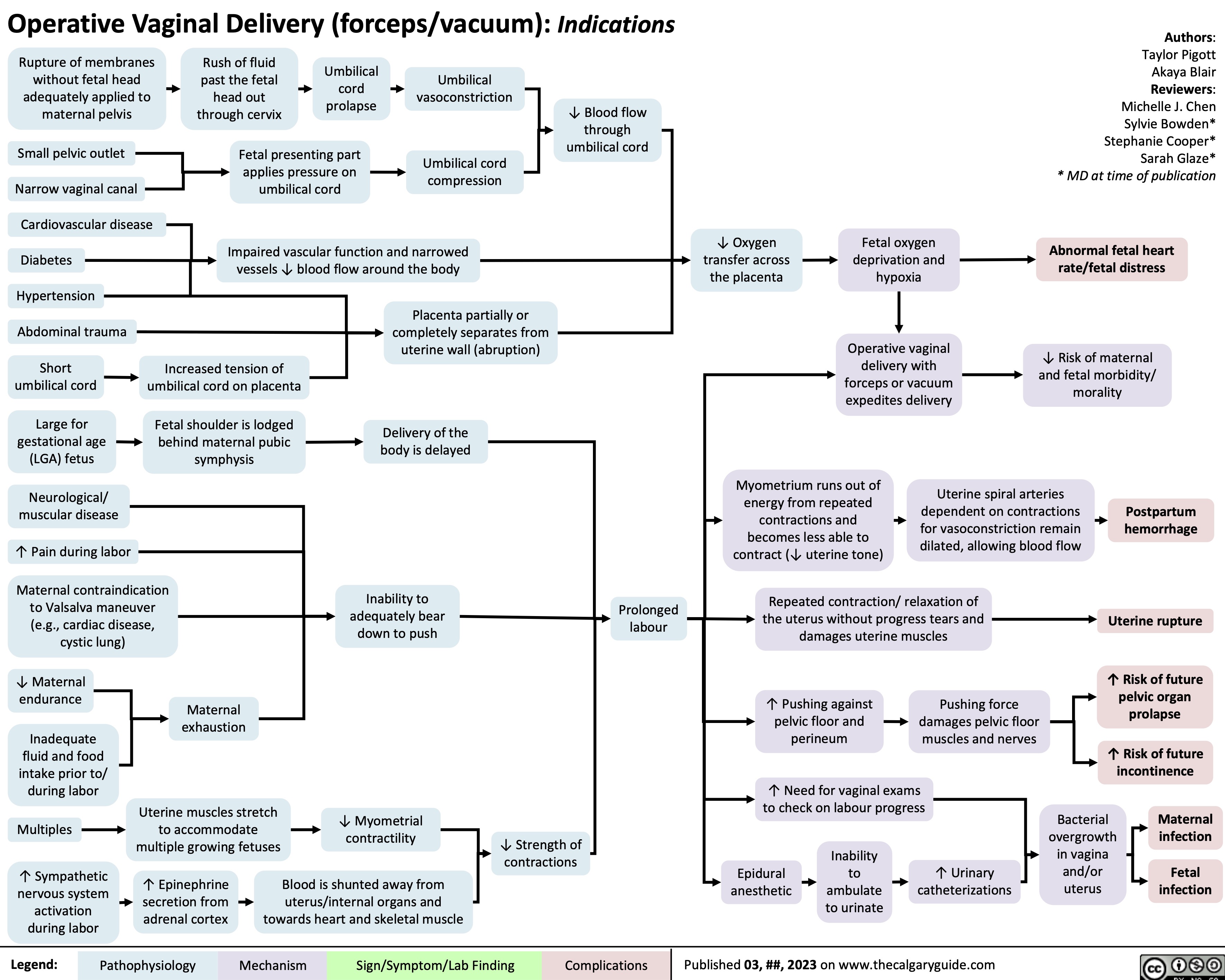
Sickle Cell Disease Pathogenesis Clinical Findings and Complications
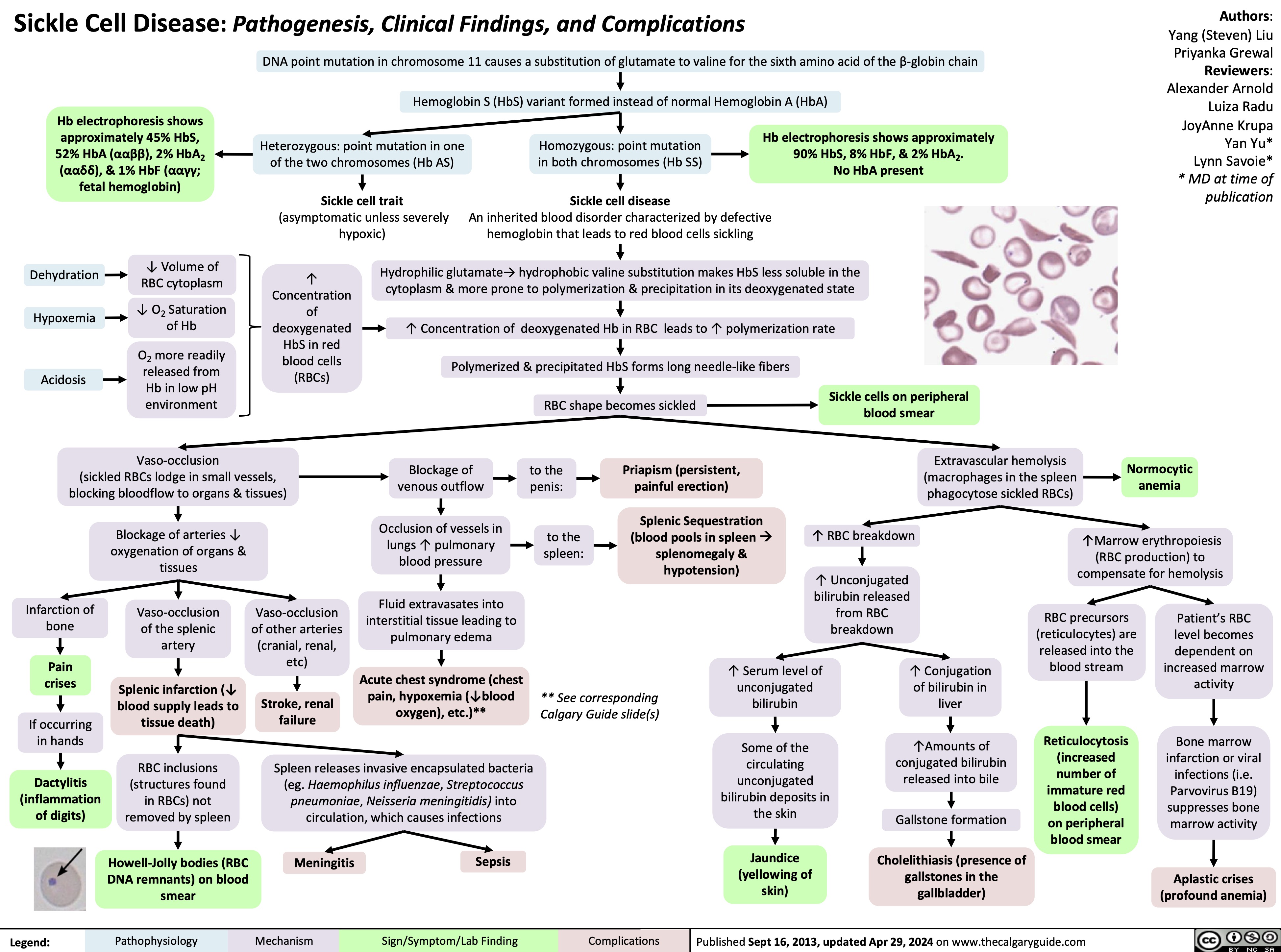
Gynecomastia
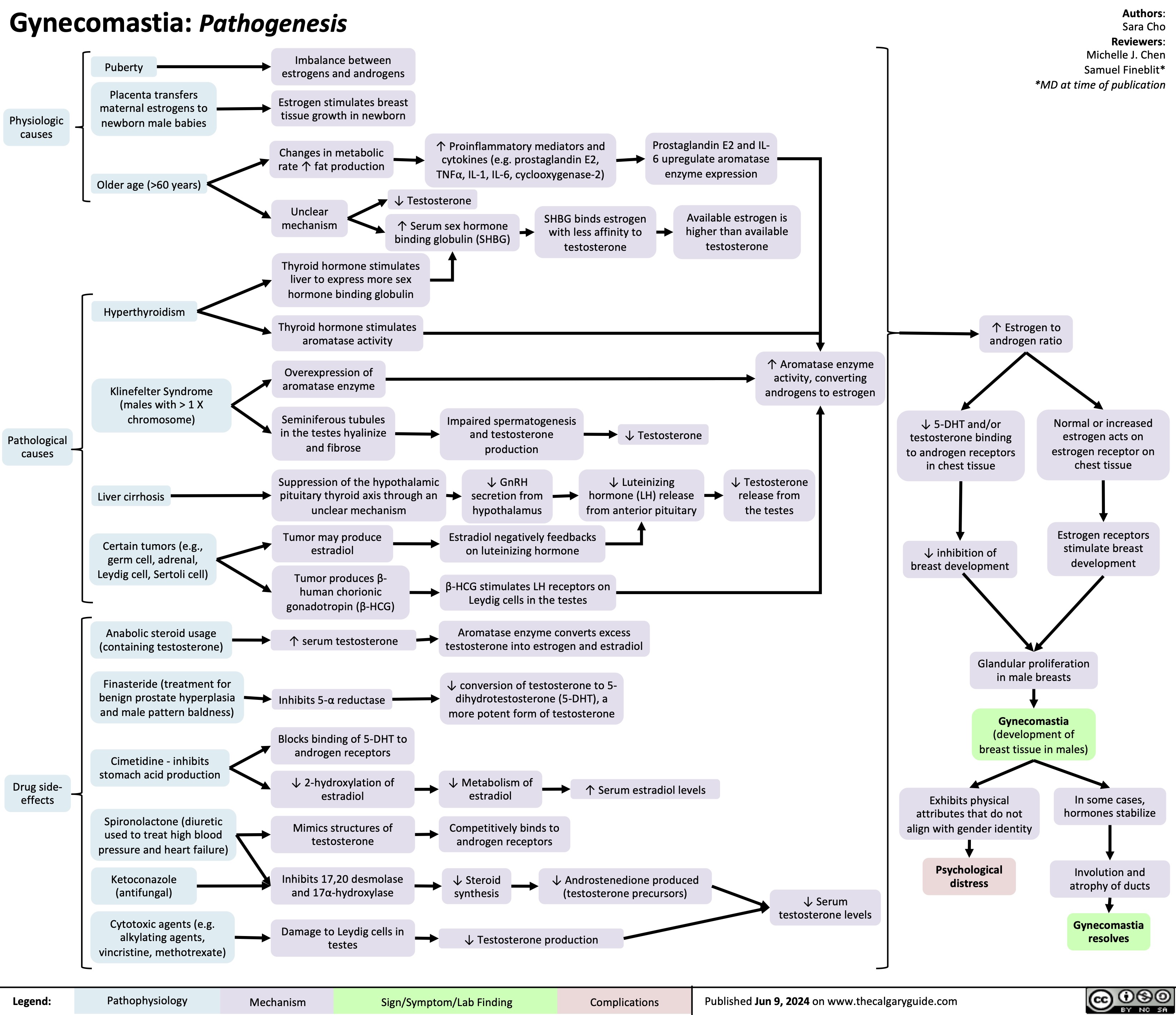
Anesthetic Considerations in Pregnancy
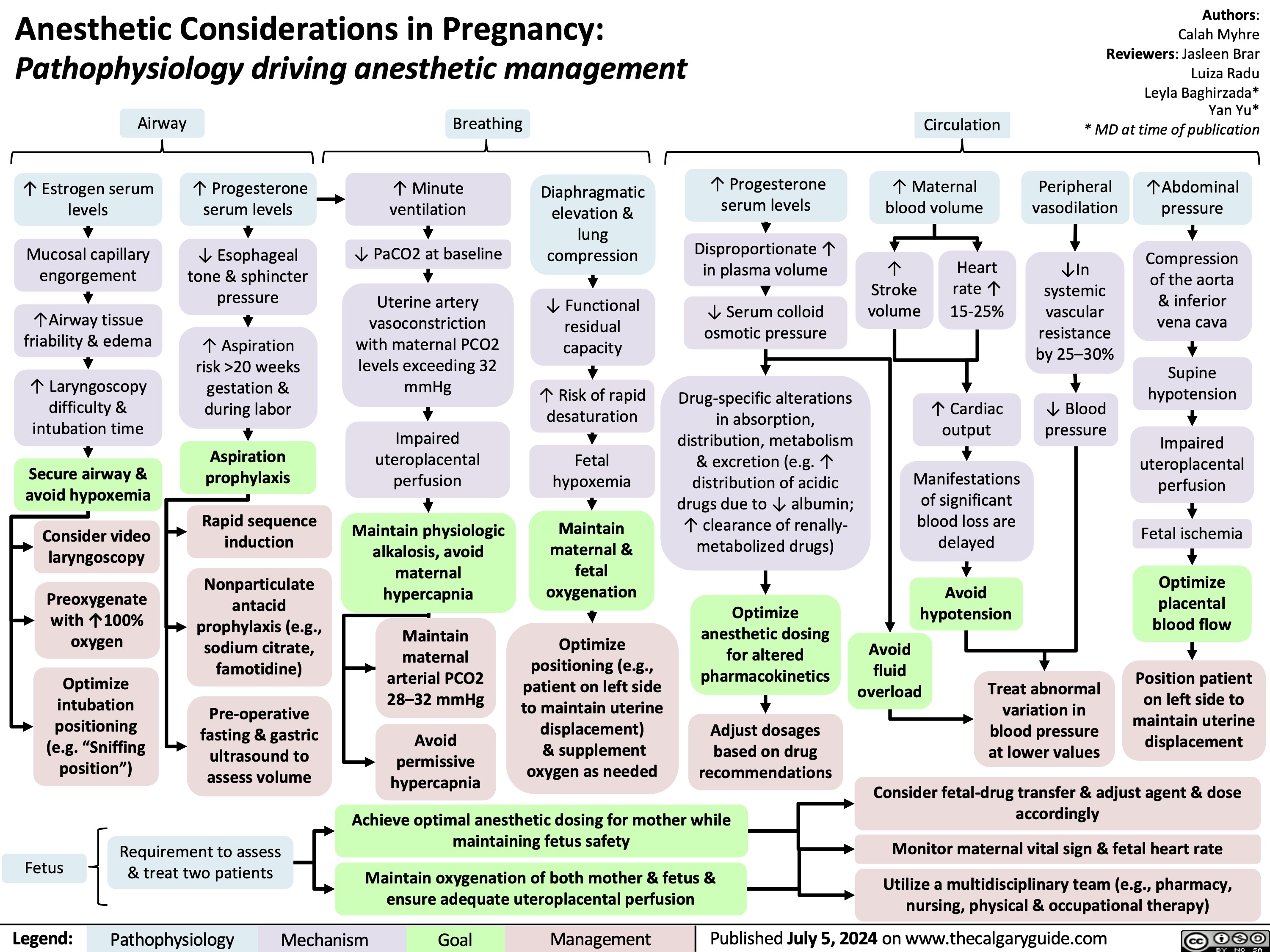
Diabetes Mellitus Pathophysiology Behind Lab Findings
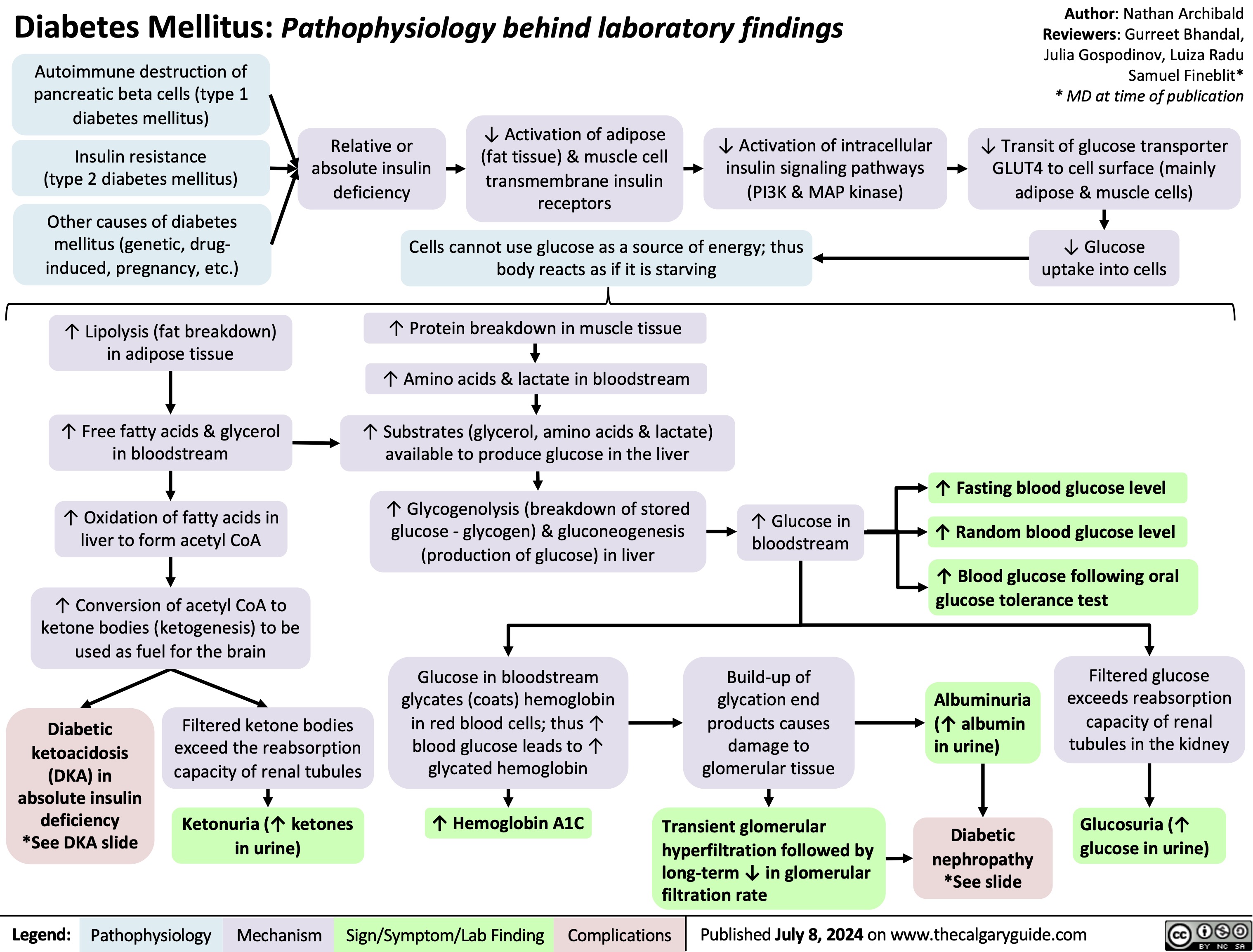
Neonatal Hypoglycemia Pathogenesis
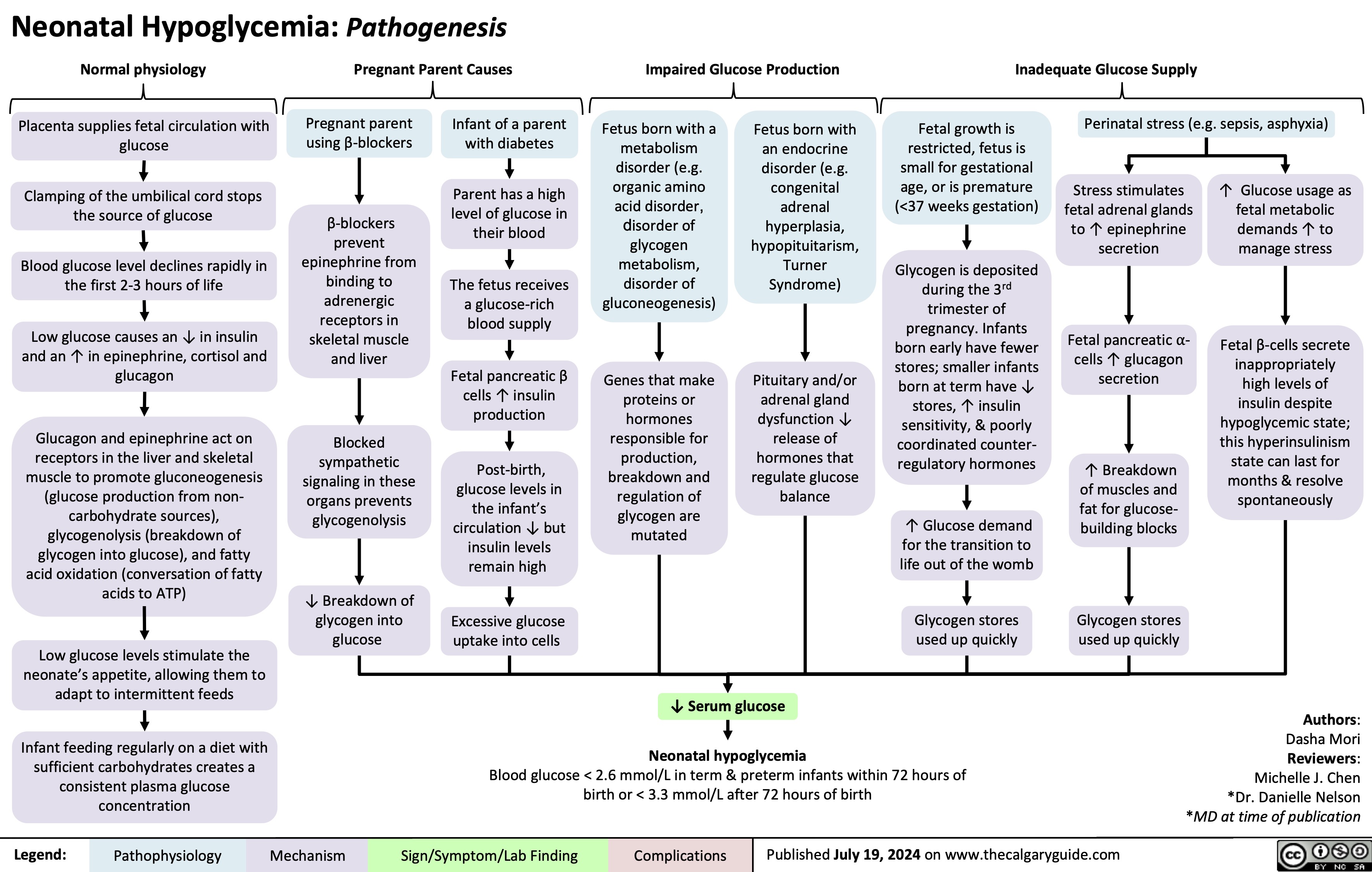
Generalized Anxiety Disorder GAD
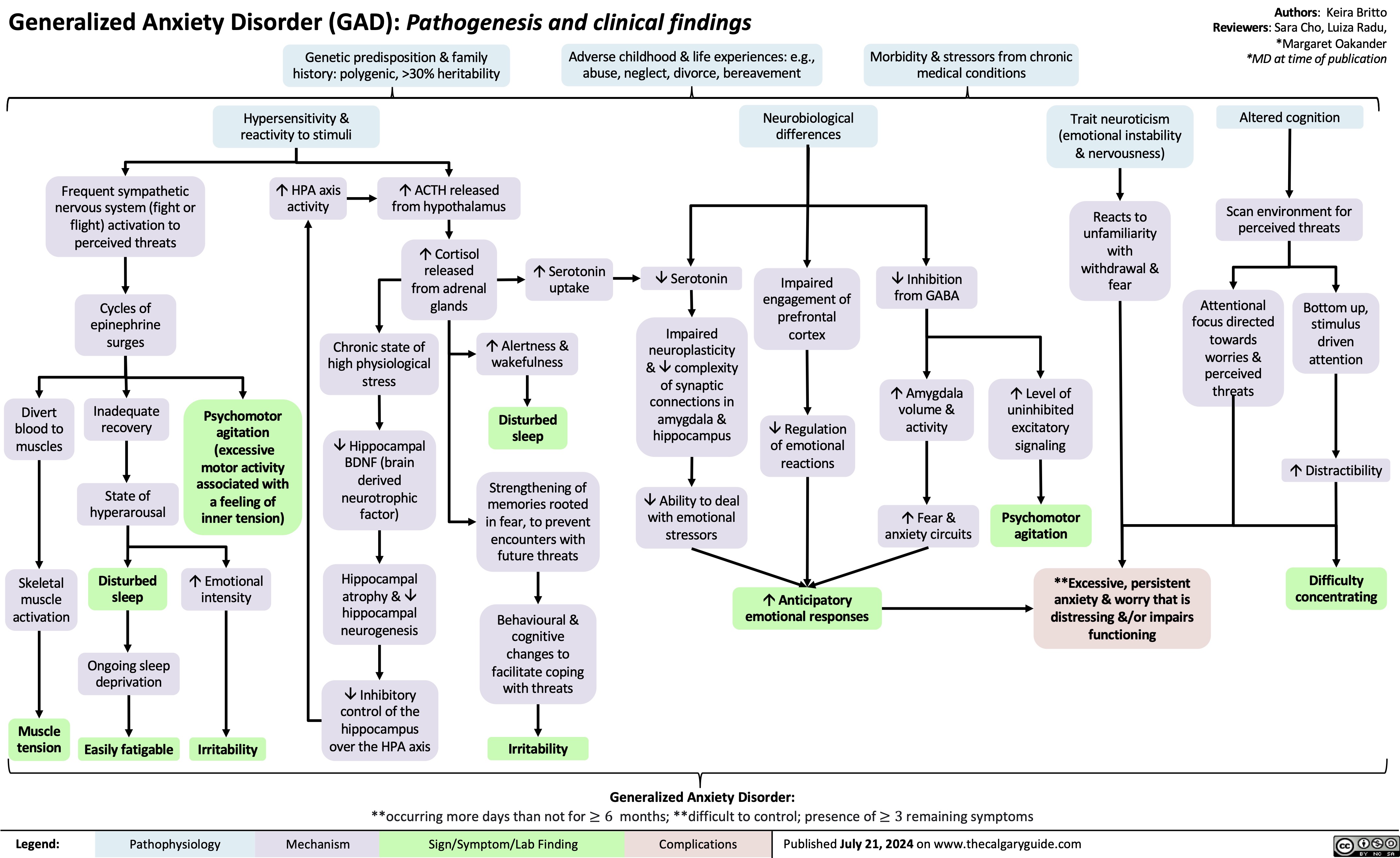
Underfill Edema Pathogenesis
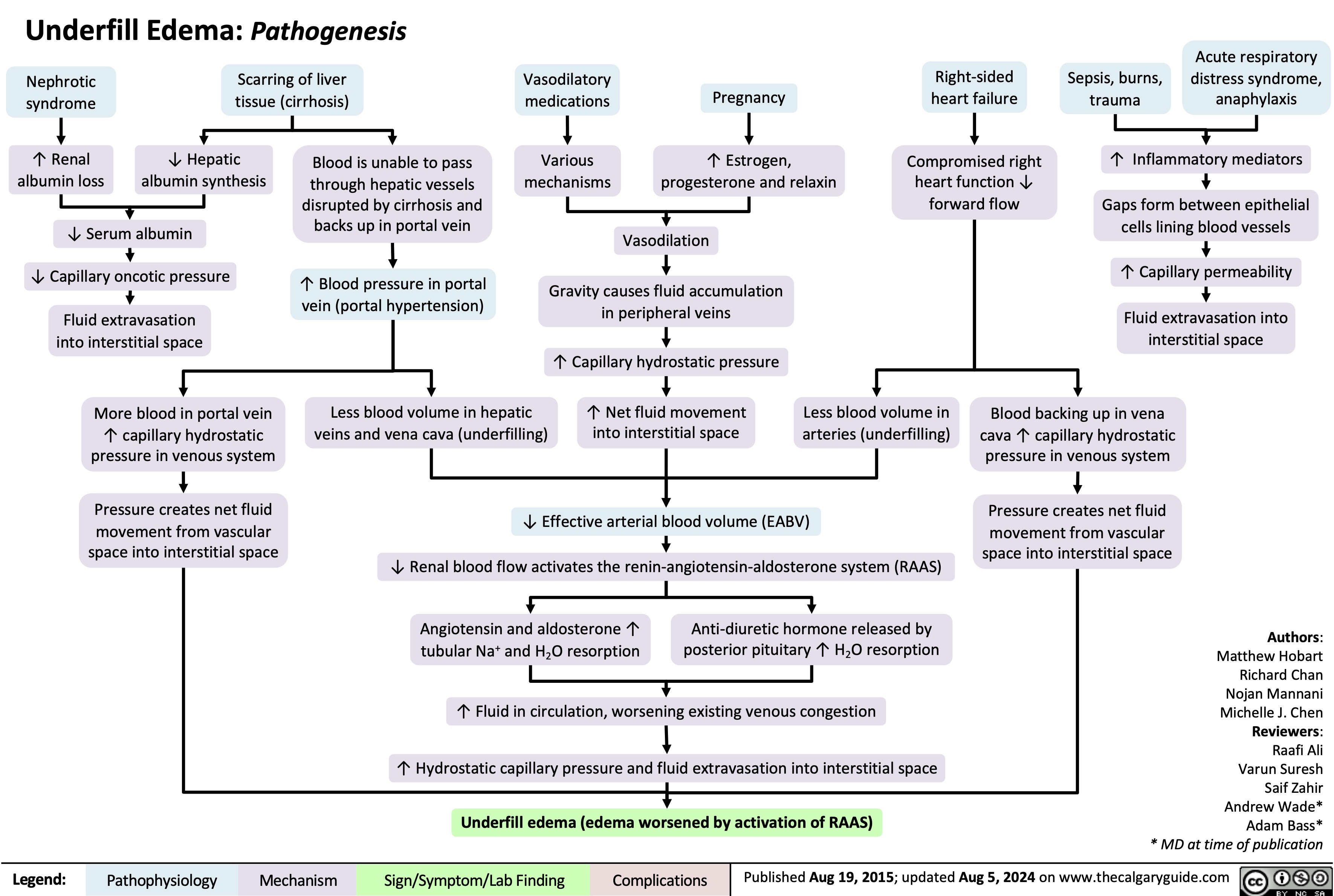
IgA Nephropathy
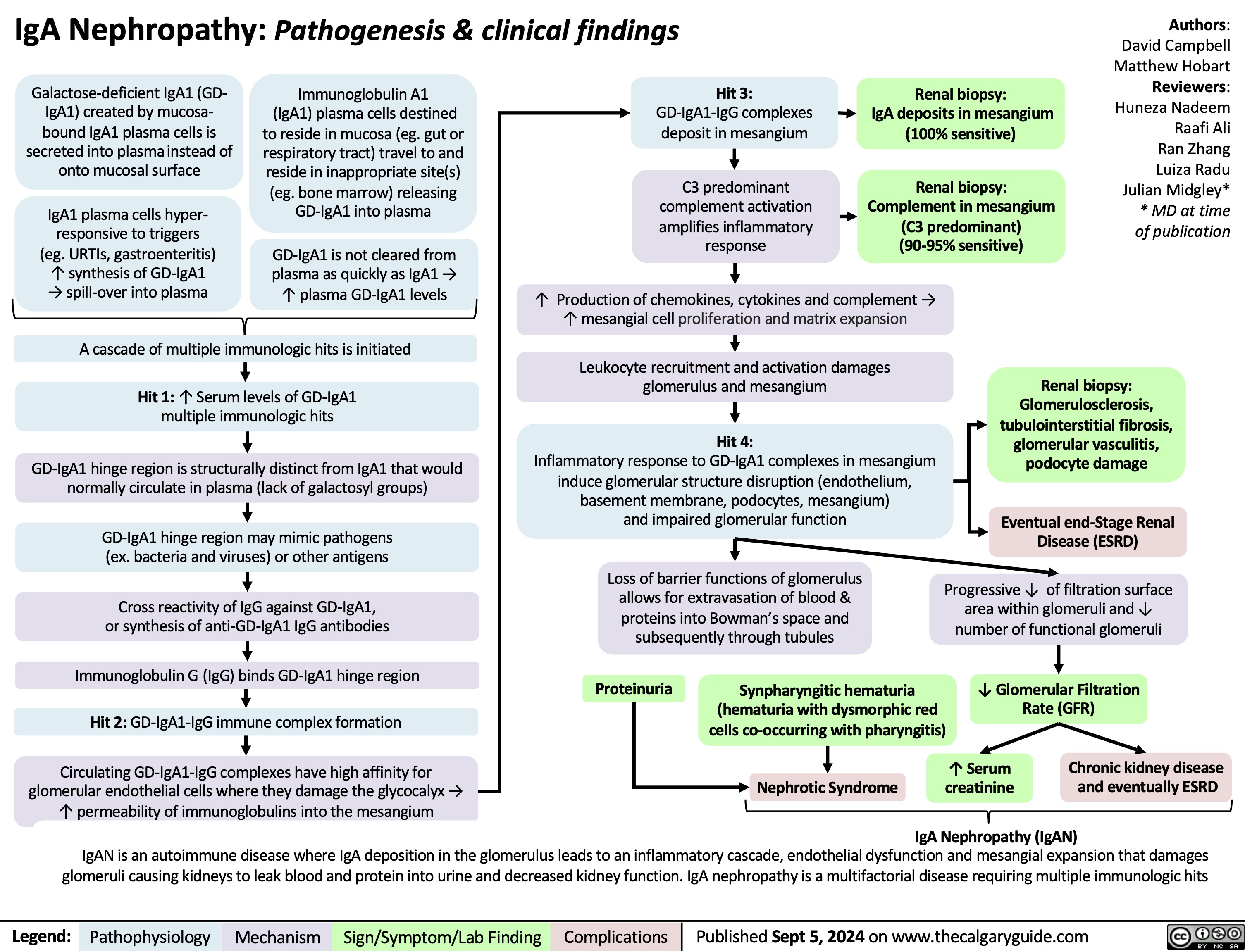
Major Depressive Disorder 2024
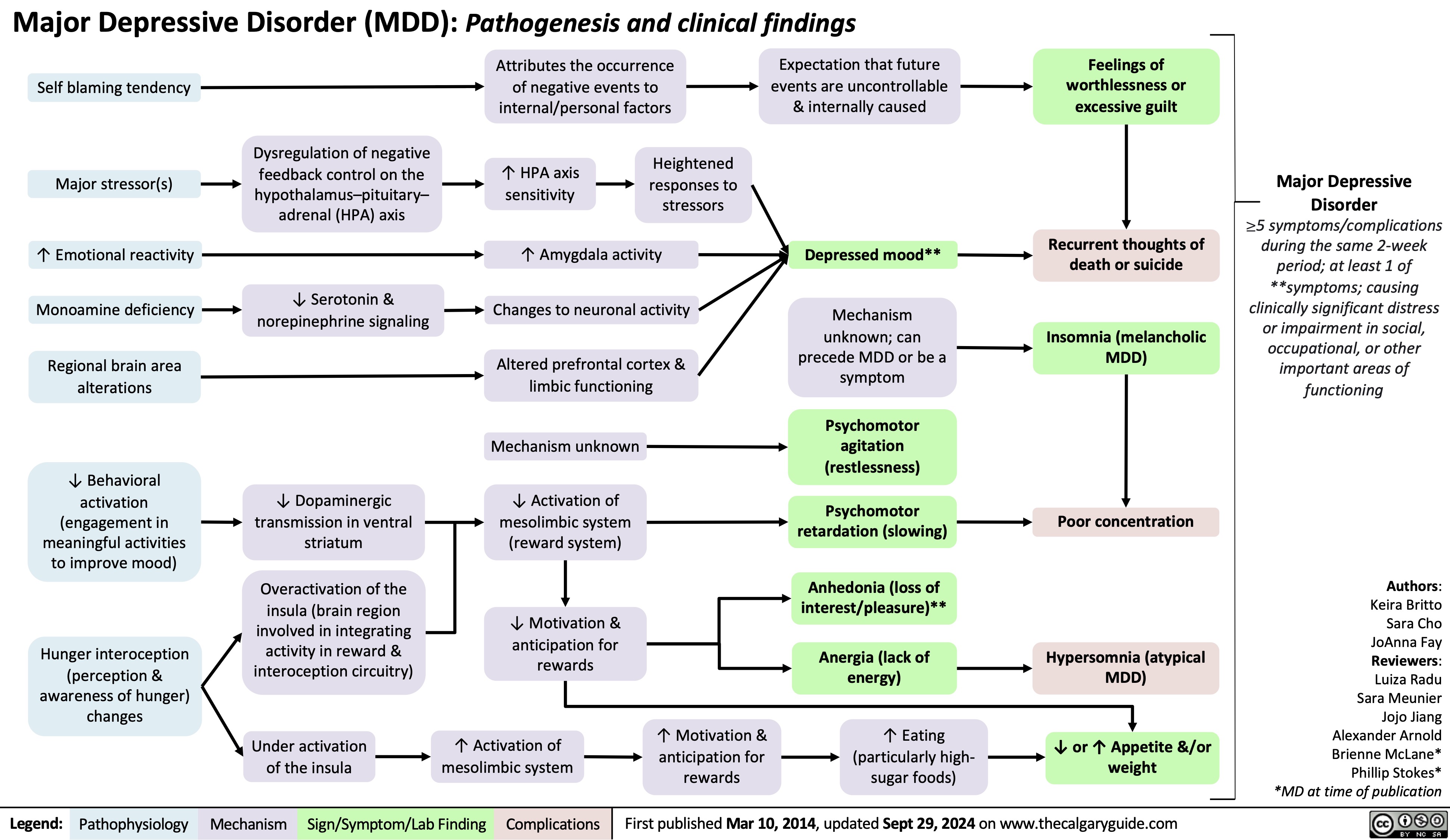
Hypomagnesemia
![Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com
Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/10/Hypomagnesia.jpg)
Precocious Puberty
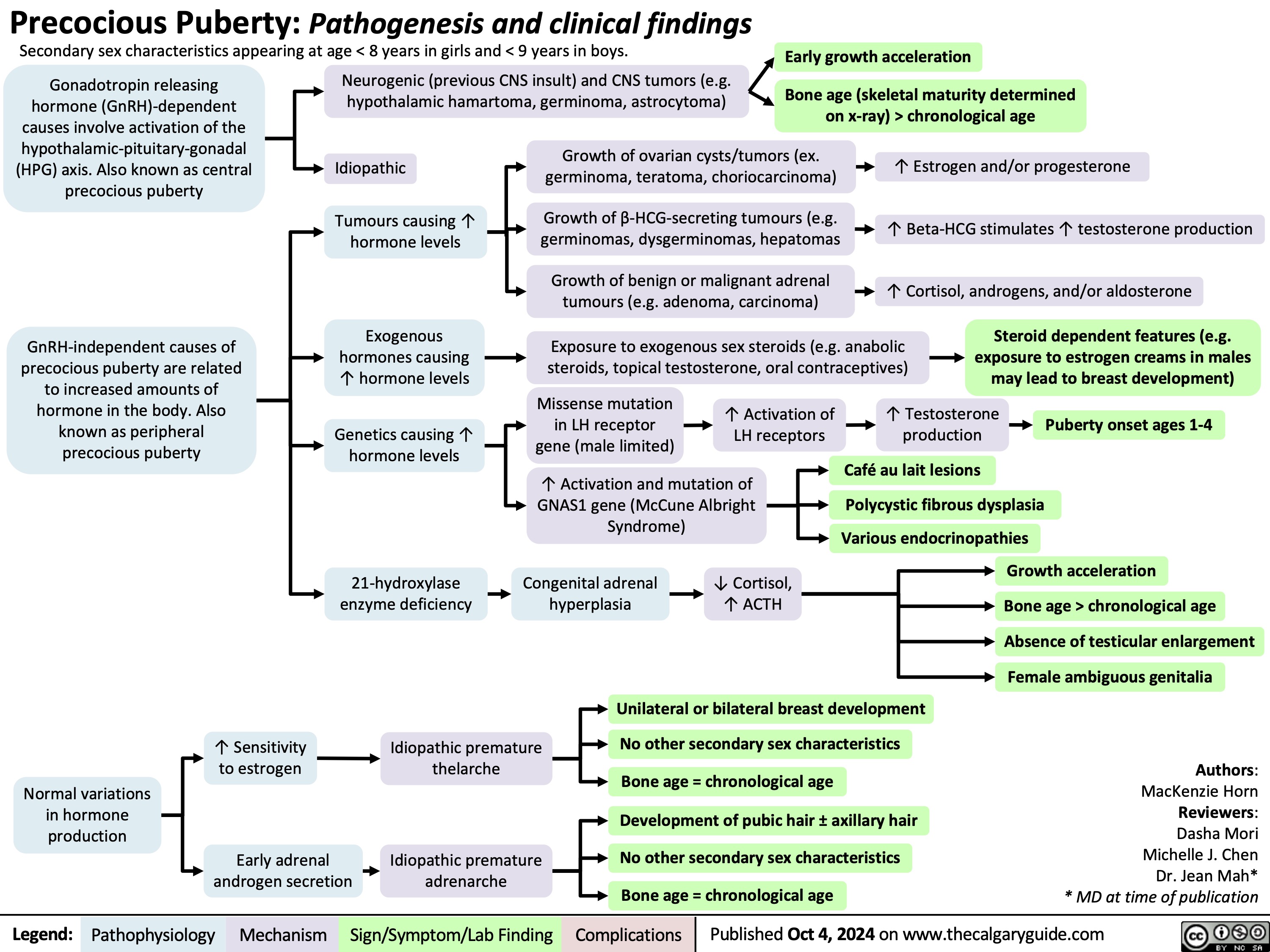
Bone Remodeling Physiology
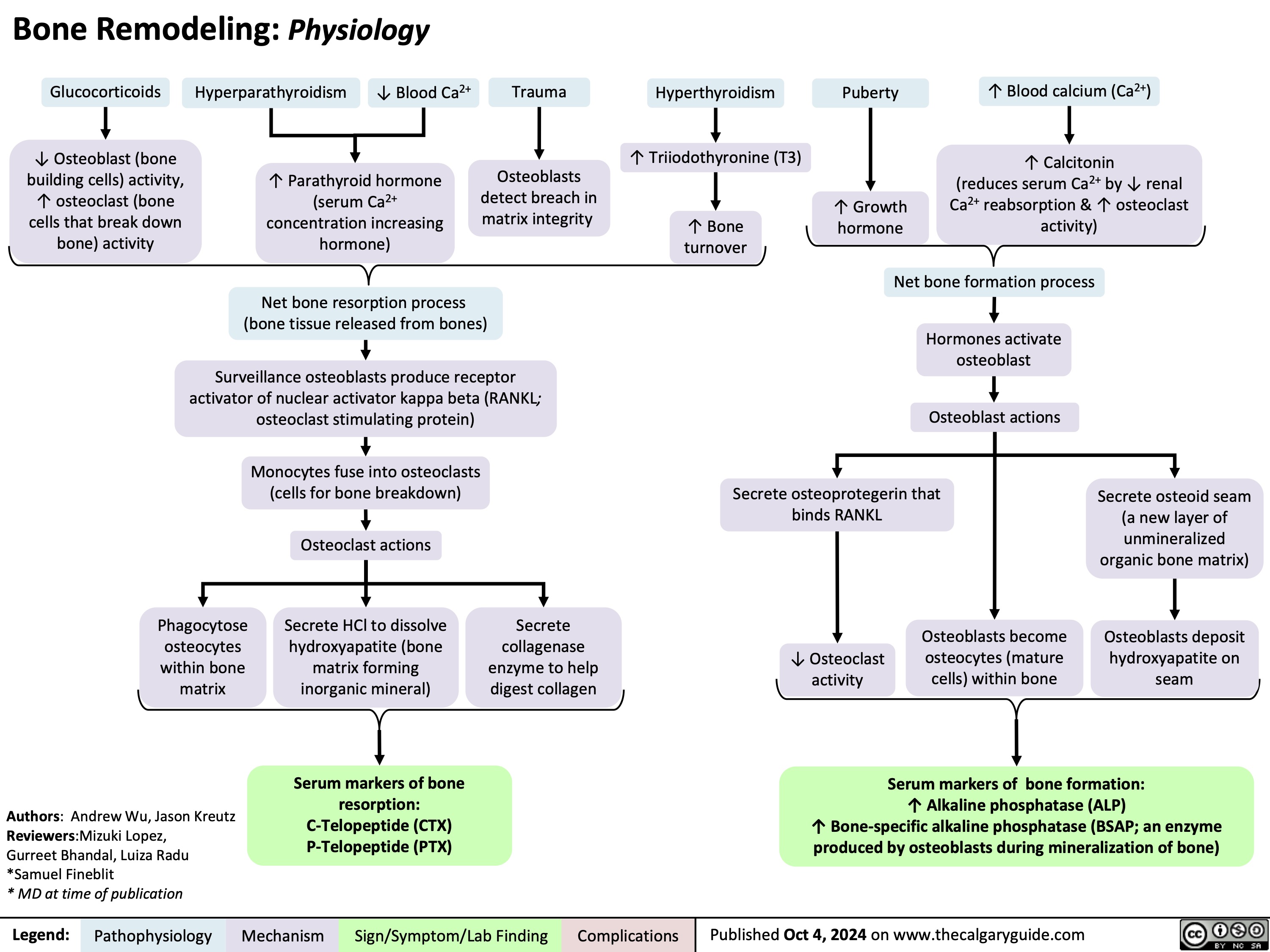
Newborn Disorders of Sexual Development
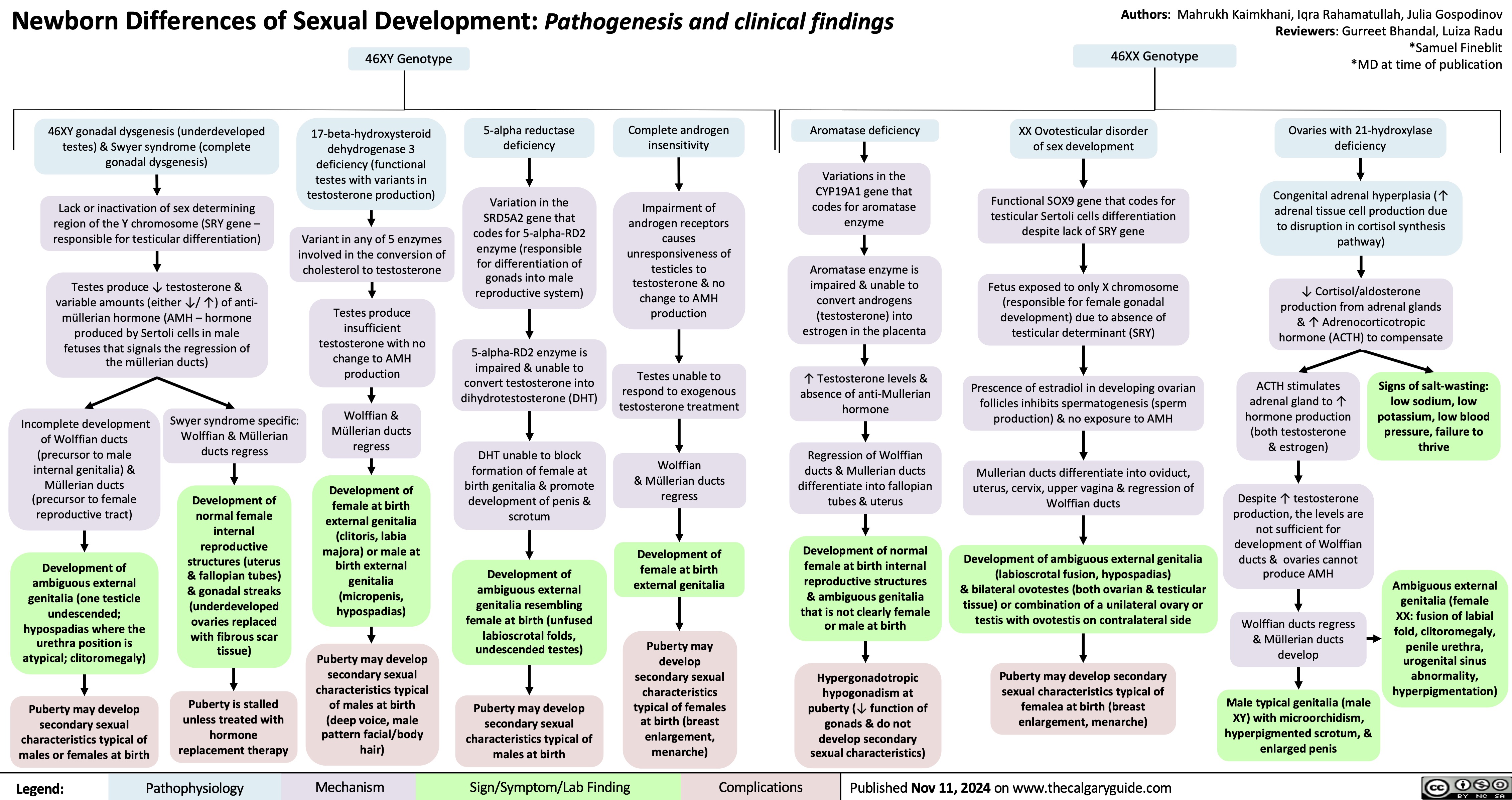
Suboxone & Methadone
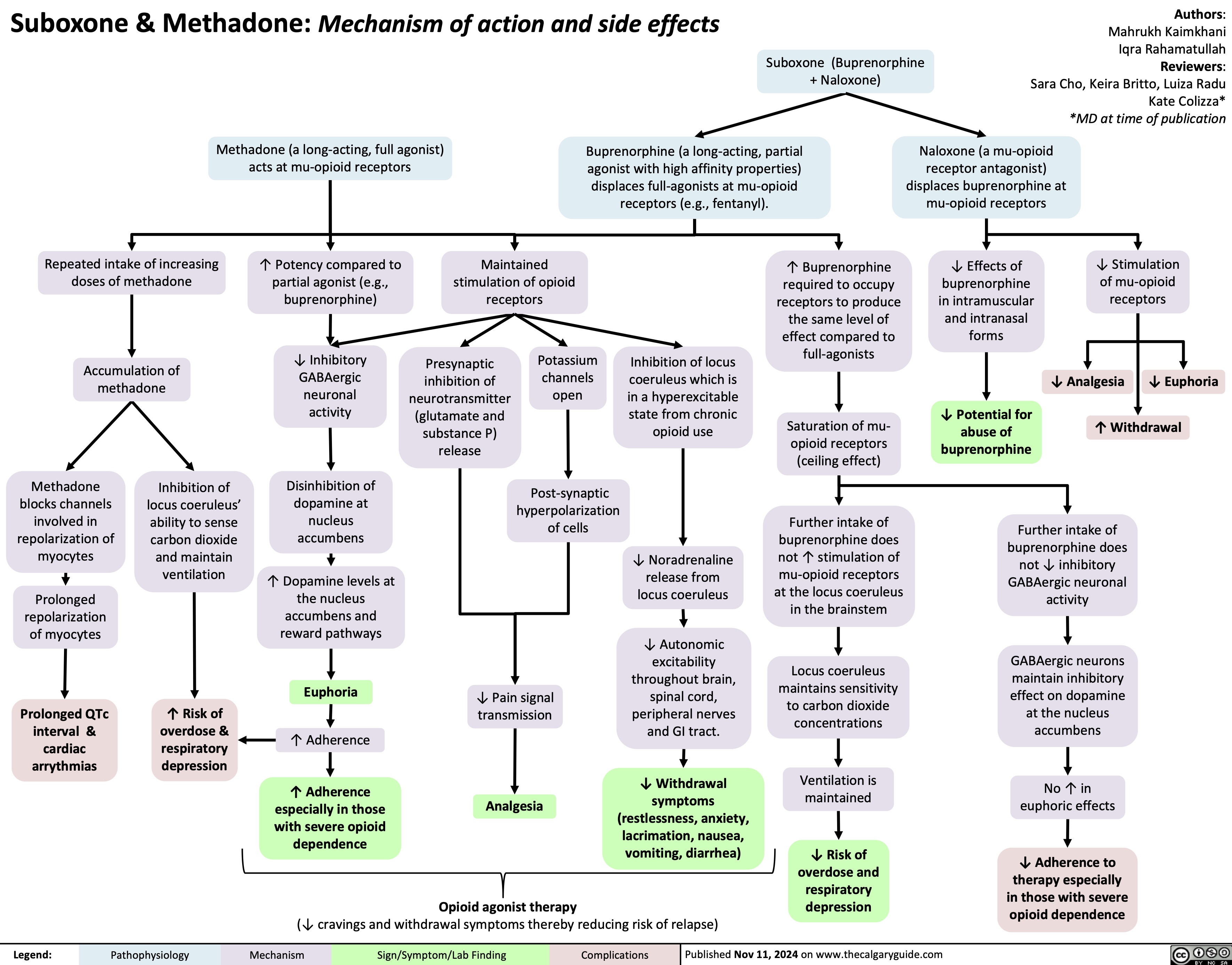
Cannabis Use Disorder
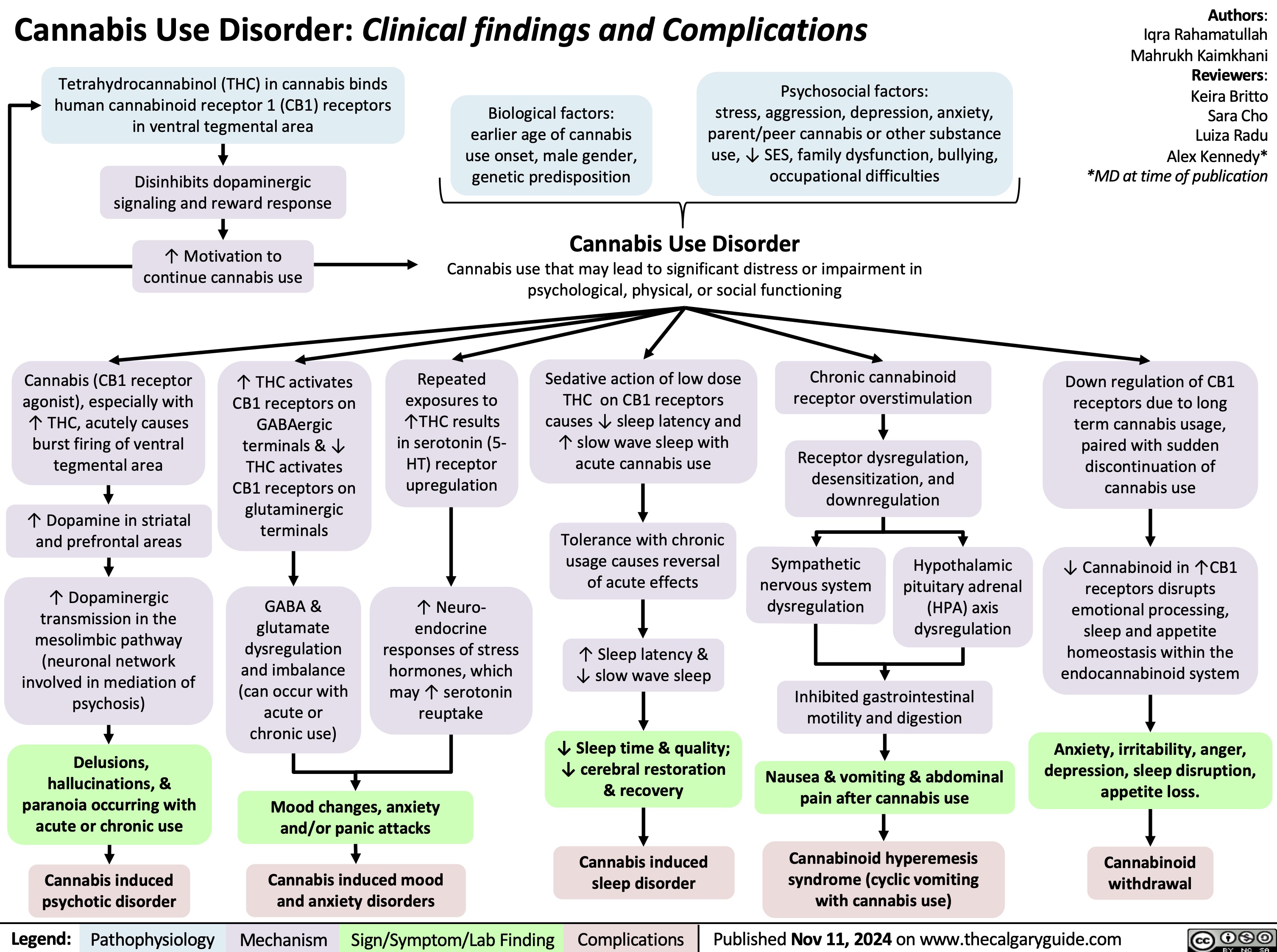
Renal manifestations of SLE
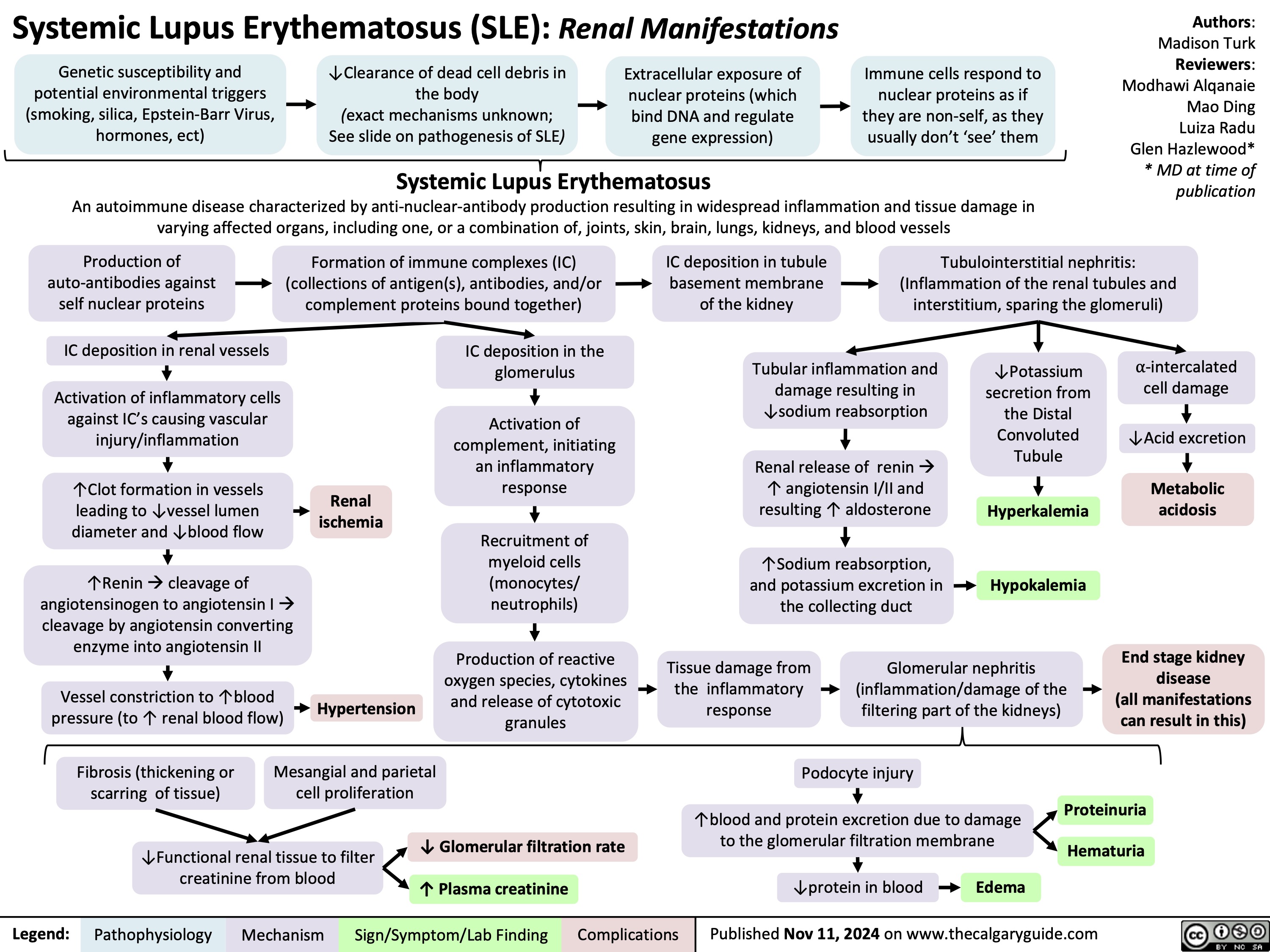
Rapid sequence induction and intubation
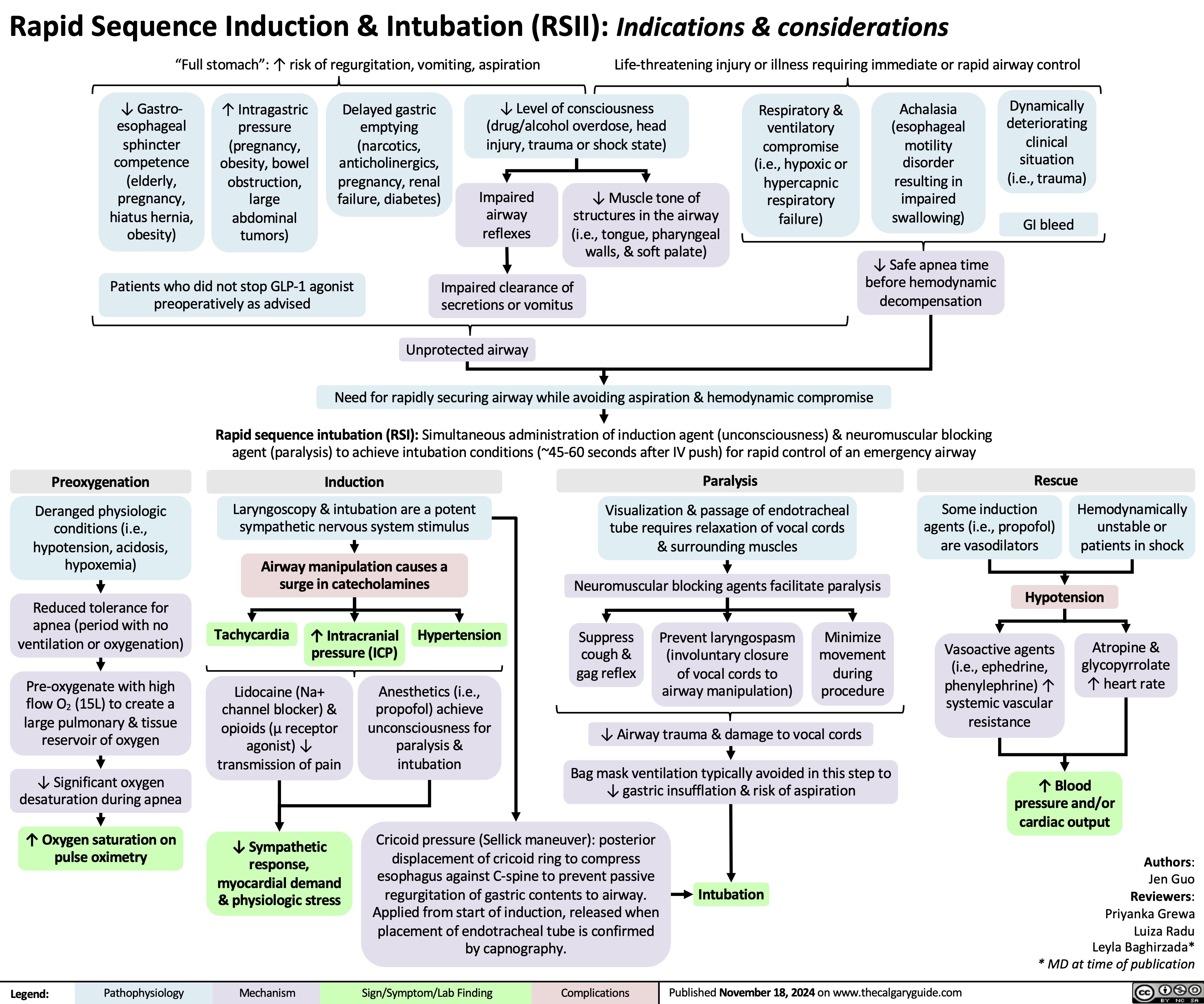
Calcium Channel Blockers
![Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com
Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/11/Calcium-Channel-Blockers.jpg)
Pre-Eclampsia Pathogenesis
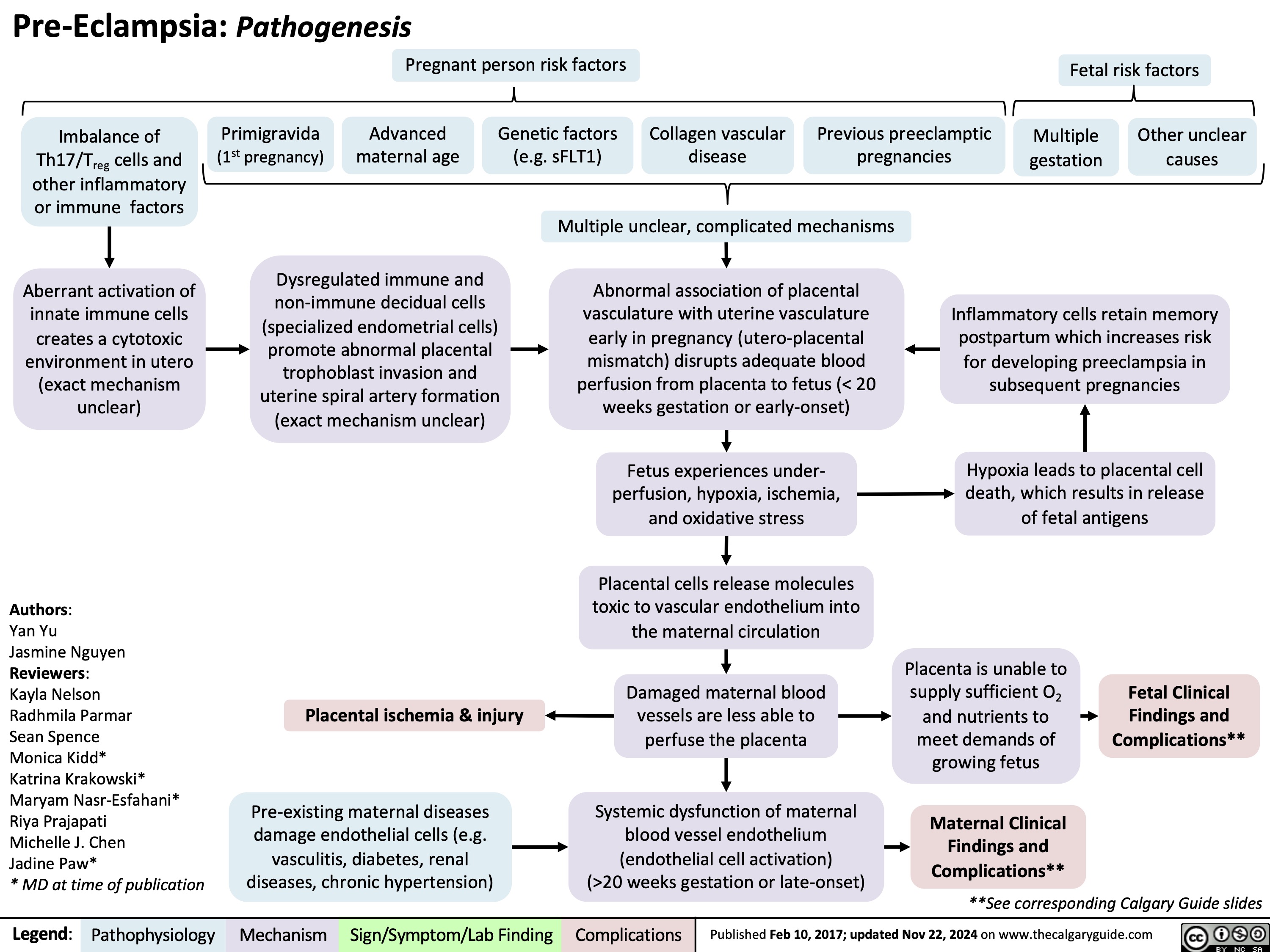
Loop diuretics
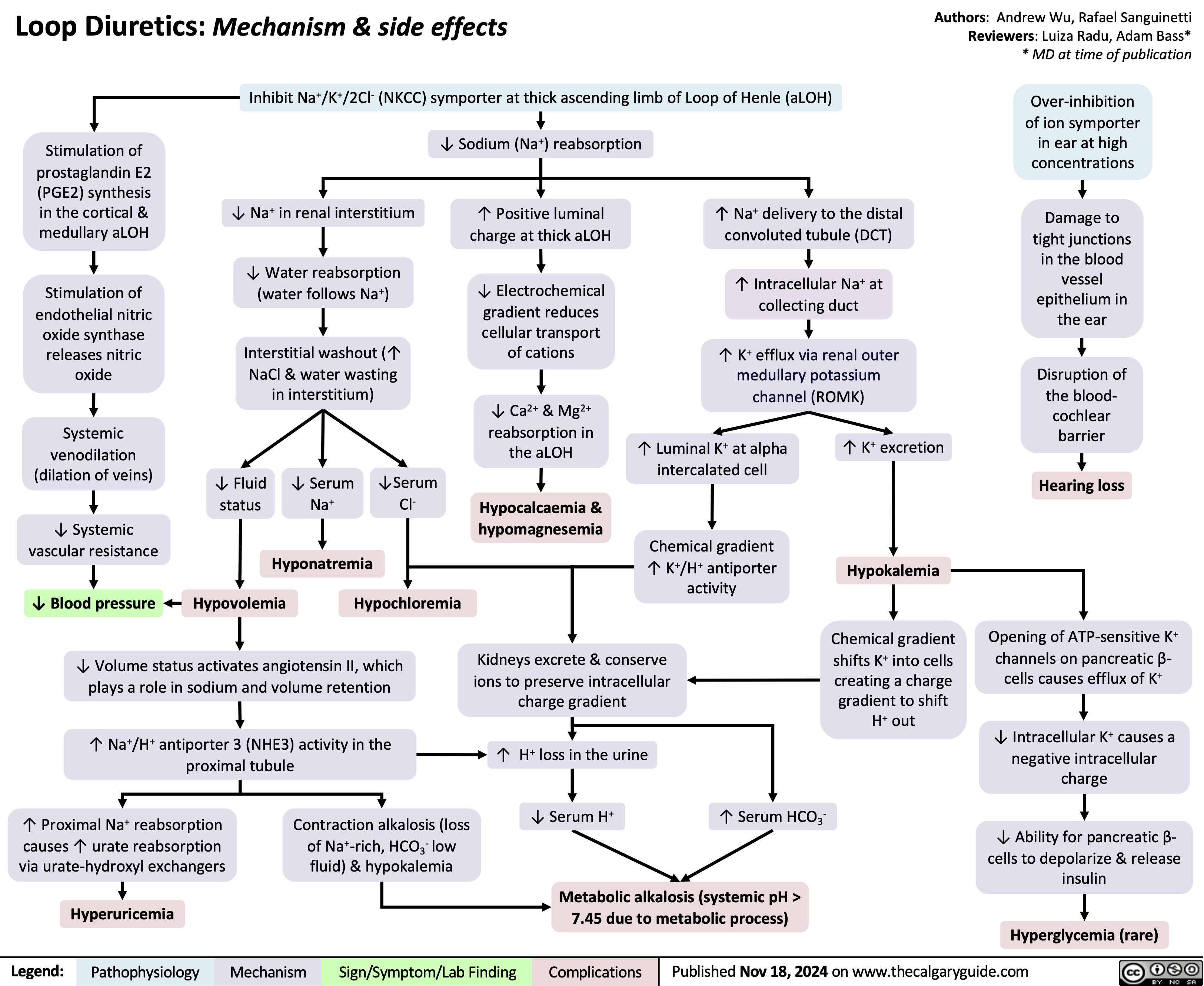
Hypocalcemia Pathogenesis
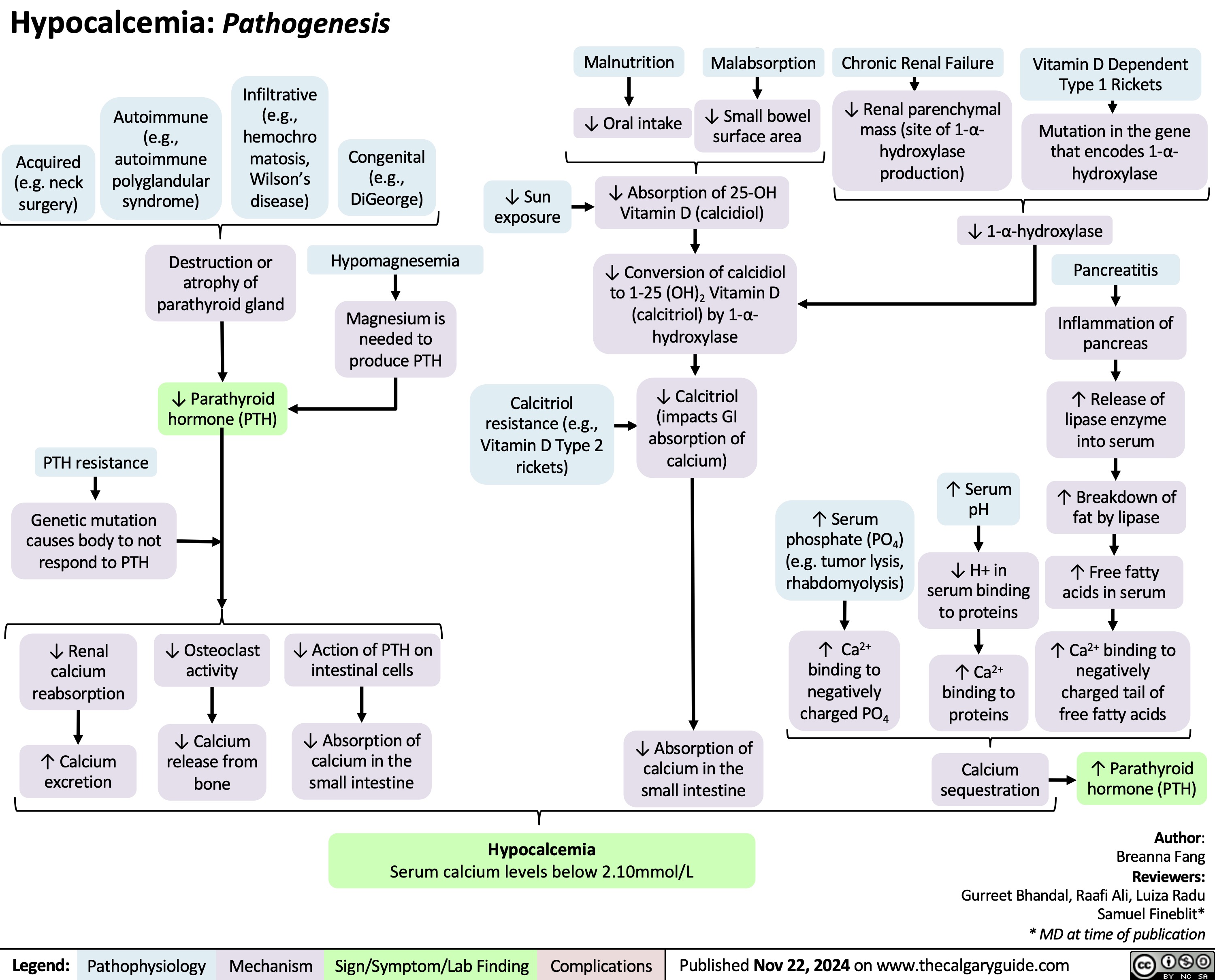
Anesthetic Considerations Laparoscopic Abdominal Surgery
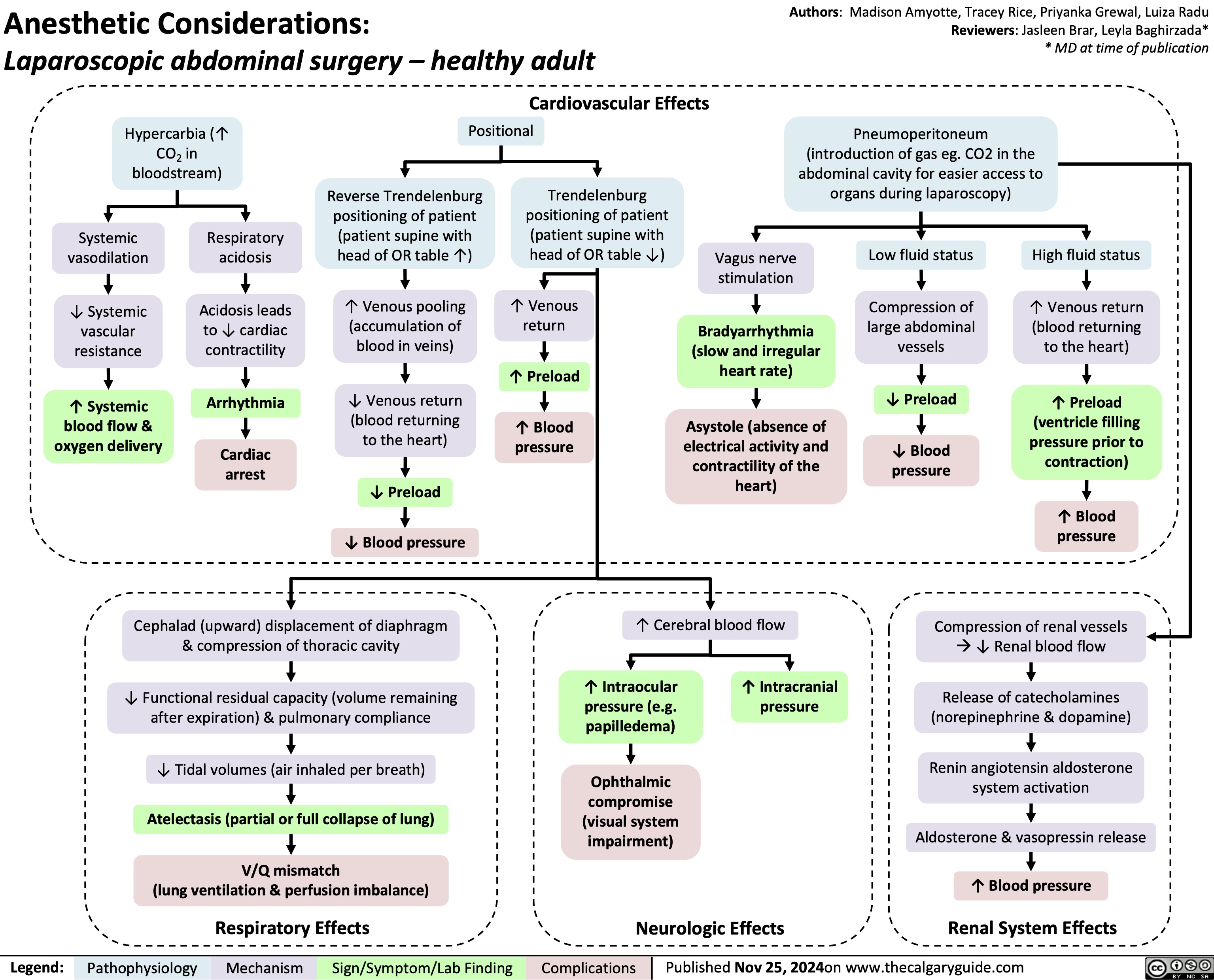
Ewing Sarcoma
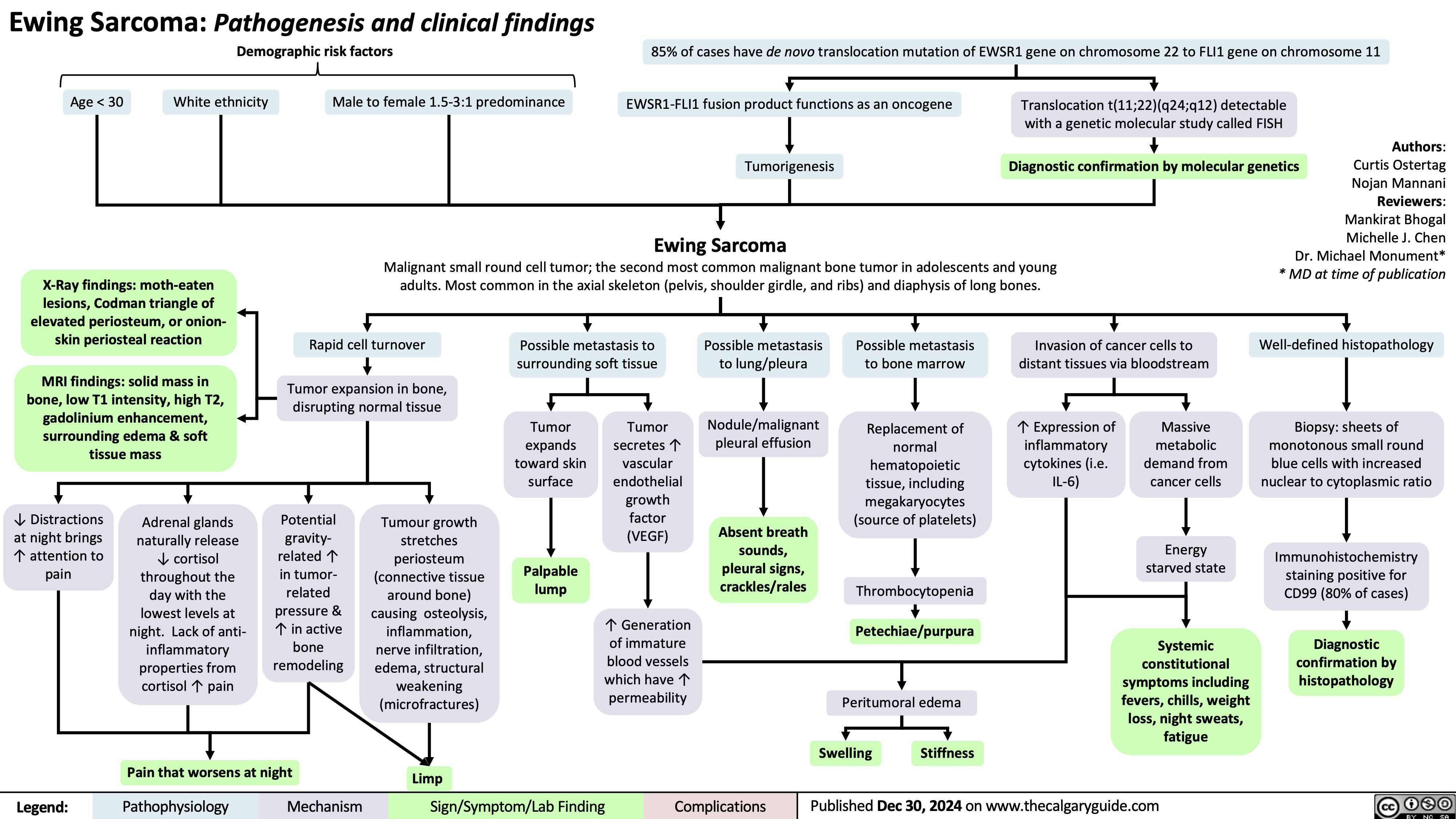
Pituitary Tumour Classification and Clinical Outcomes
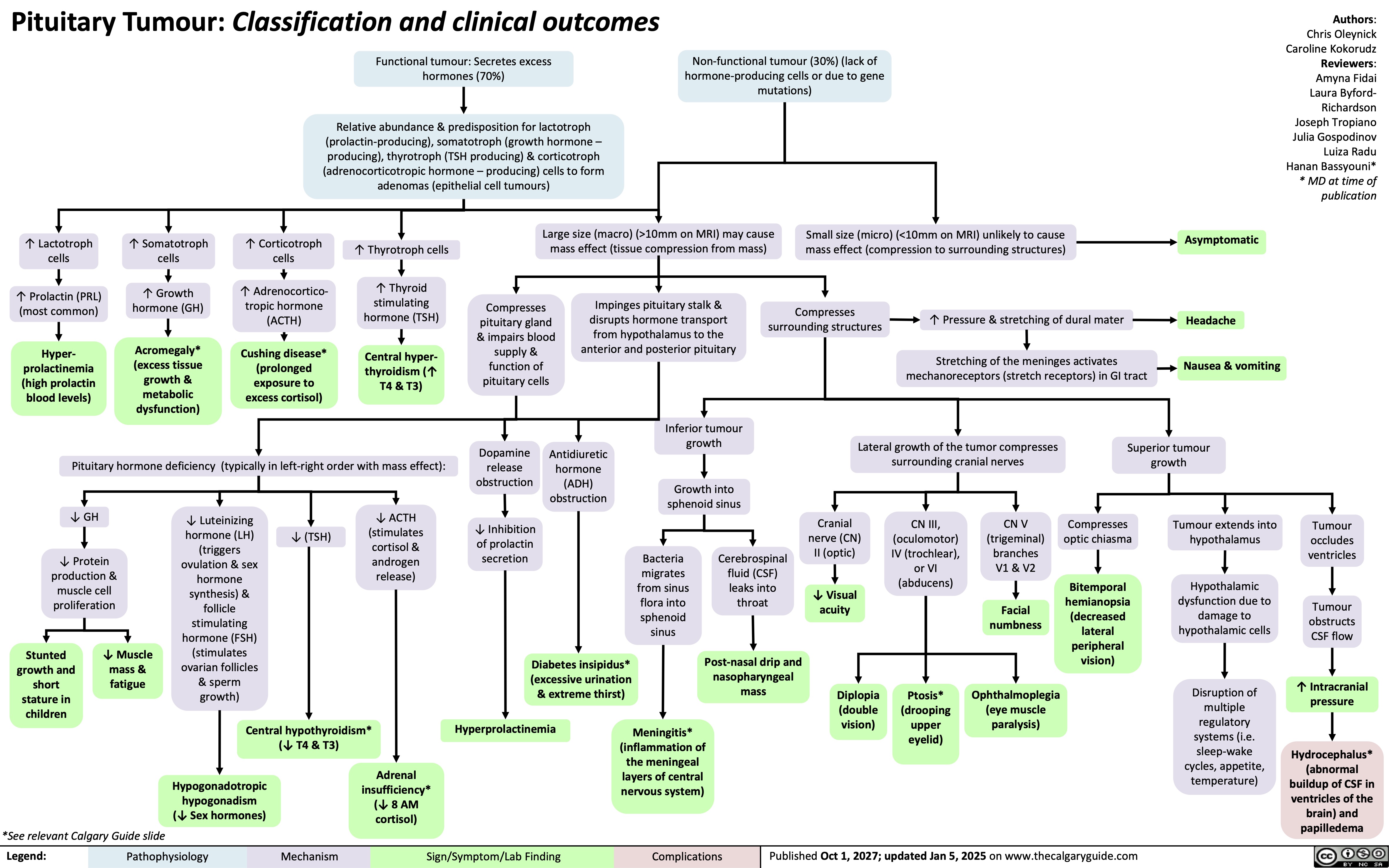
Insuficiencia Renal Aguda Post-Renal
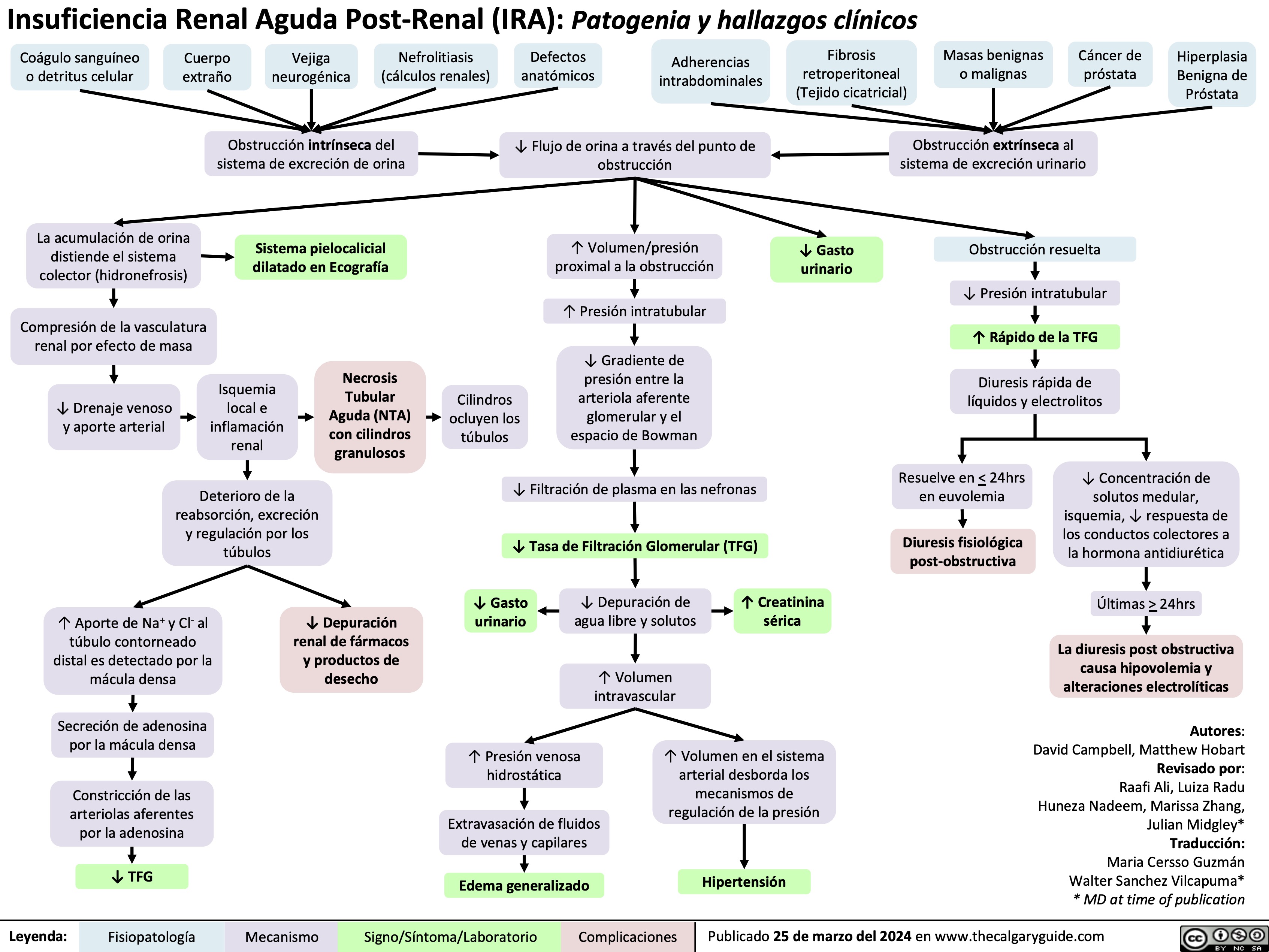
Central Precocious Puberty
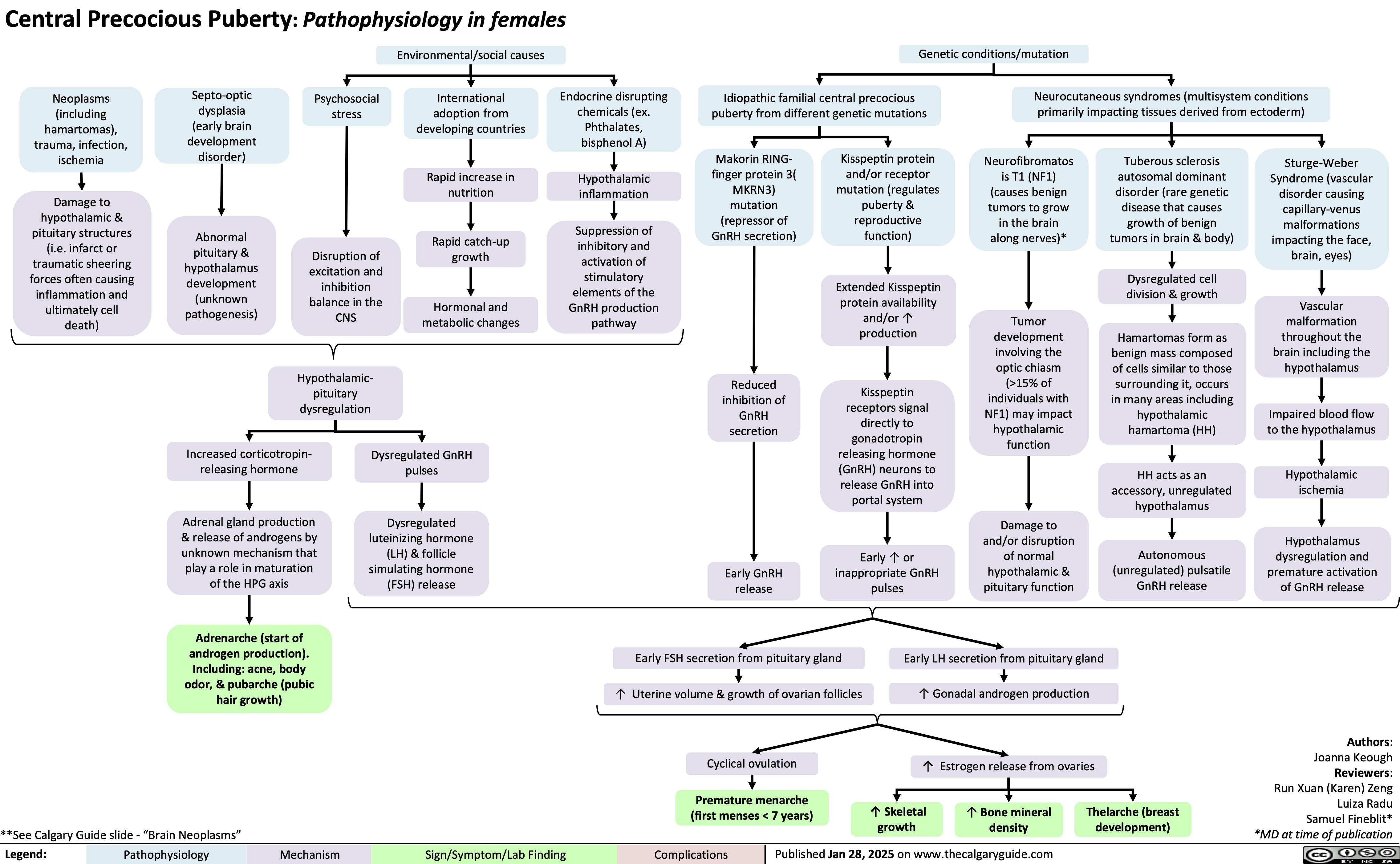
PROP1-Related Combined Pituitary Hormone Deficiency
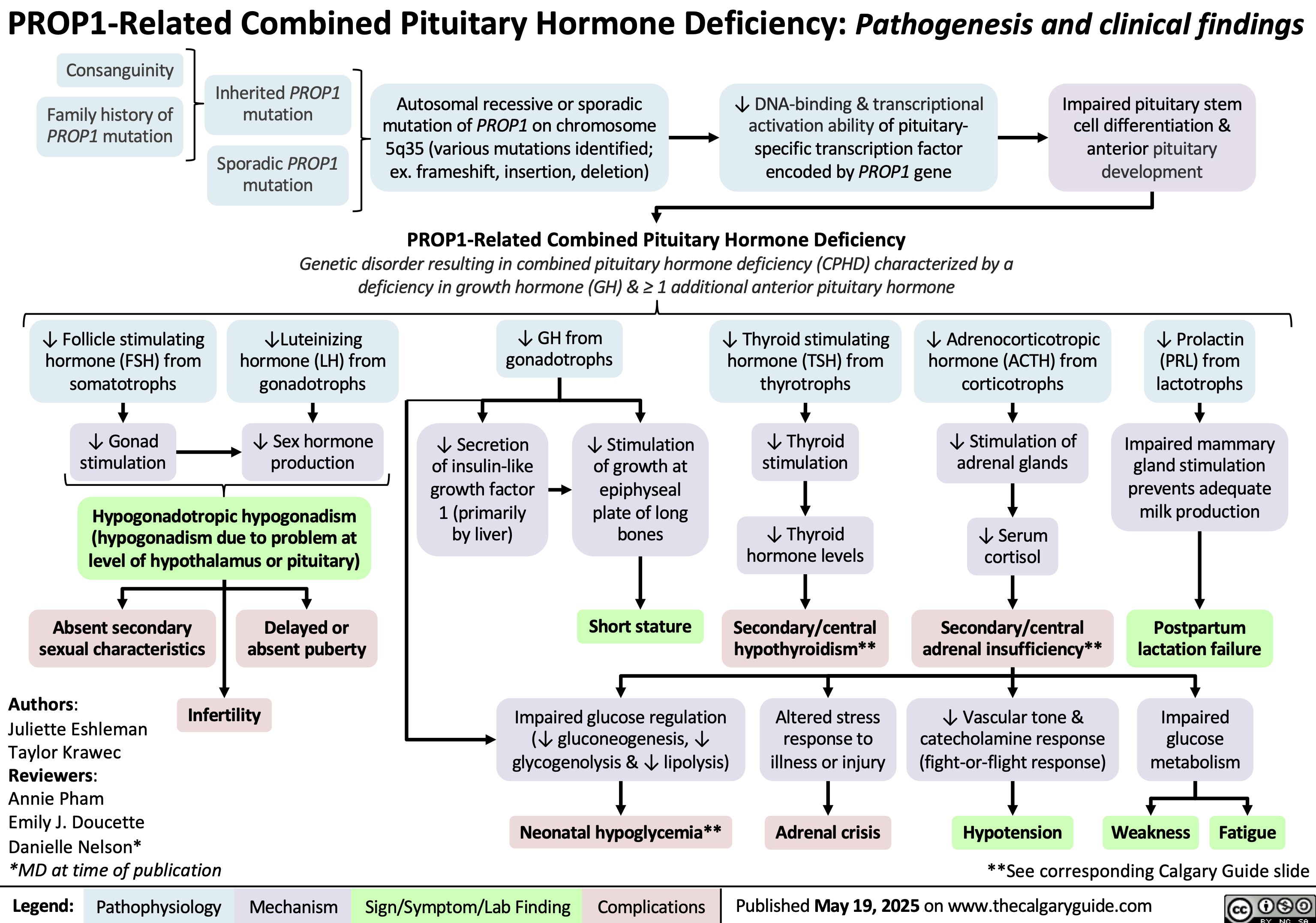
Tetralogy of Fallot
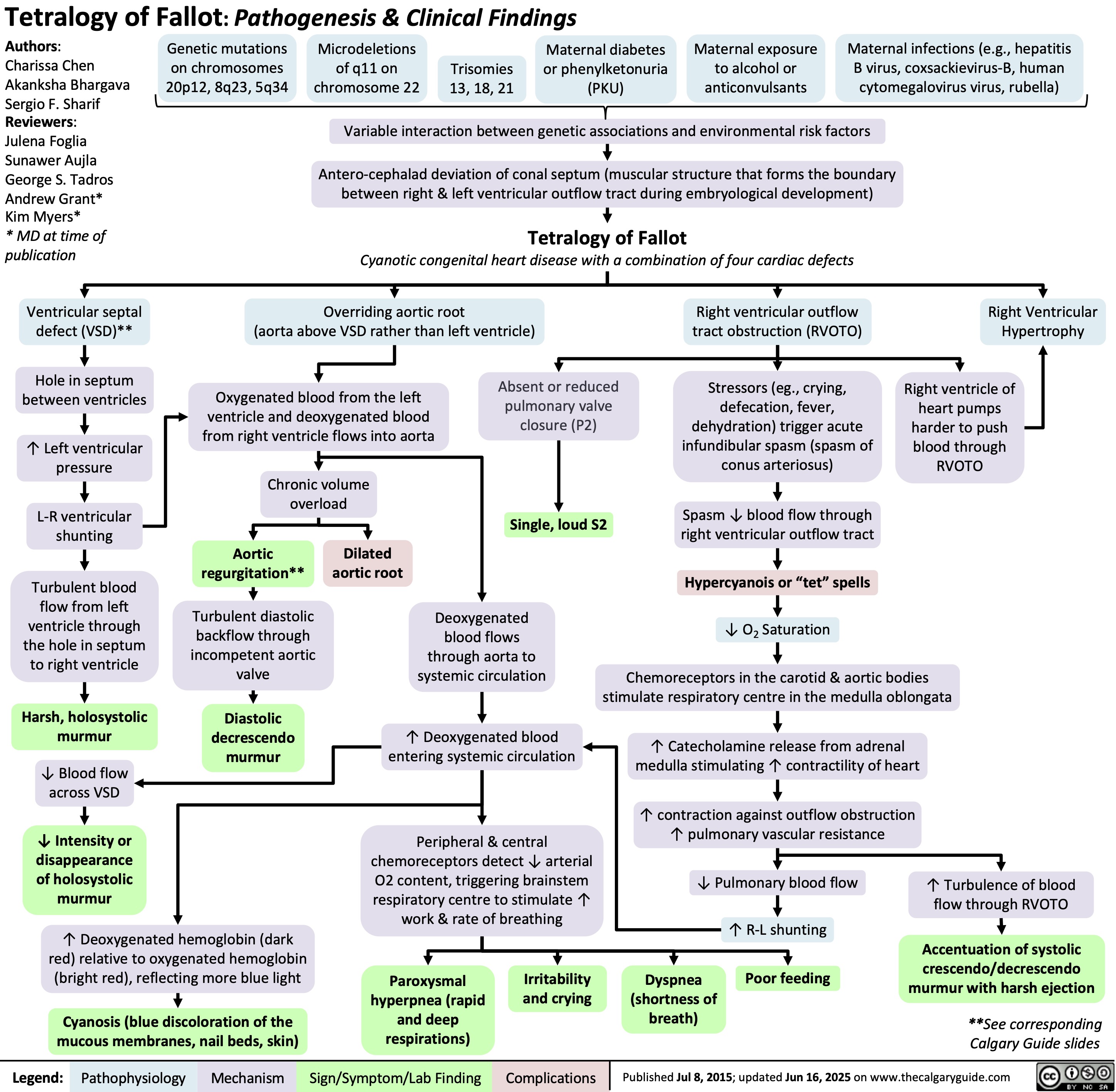
Obesity Hypoventilation Syndrome
![Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/06/Obesity-Hypoventilation-Syndrome.jpg)
Neuroanatomy and Physiology of Fear
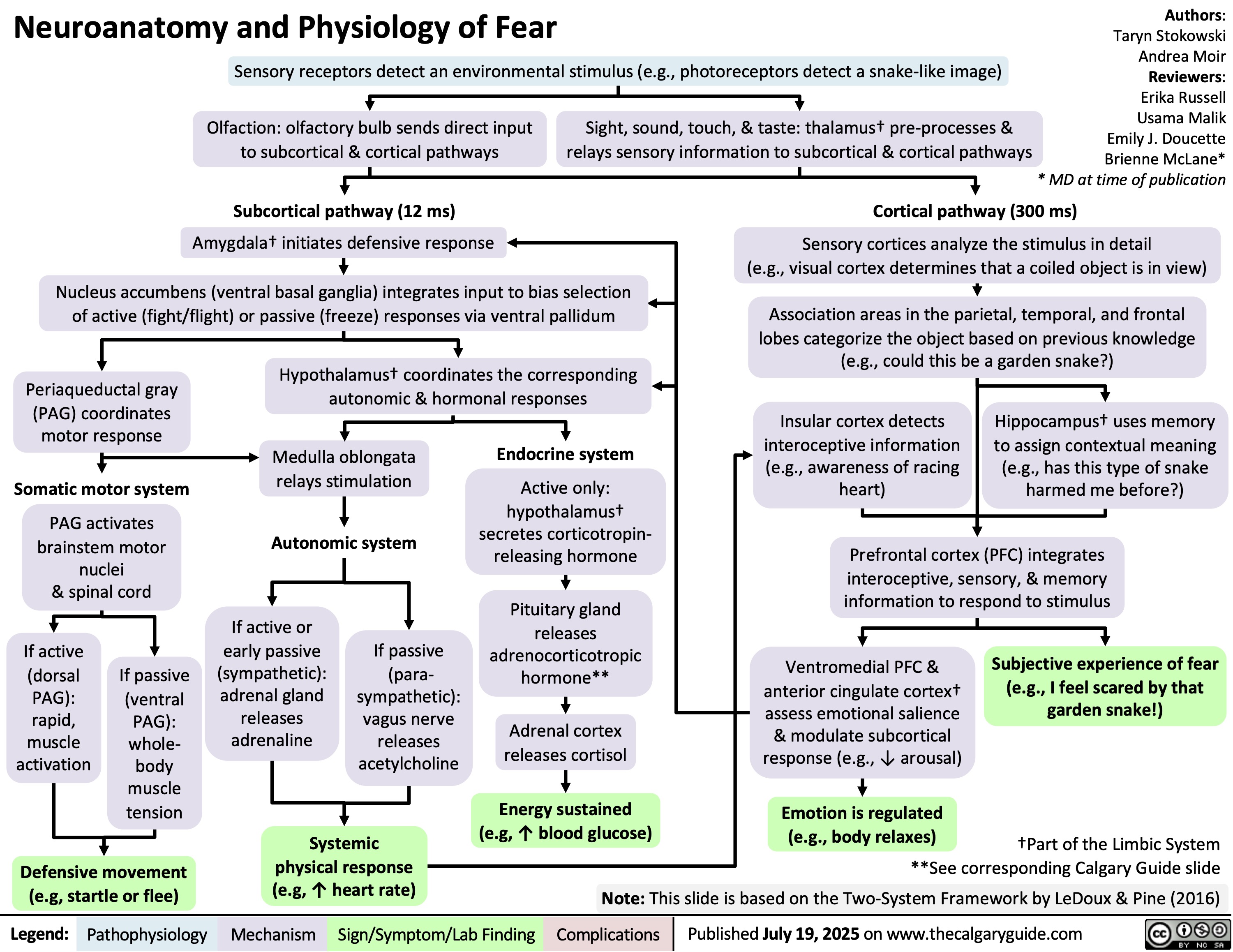
Hypocalcemia Physiology
![Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/08/Hypocalcemia-Pathogenesis.jpg)
Malignant Renal Mass
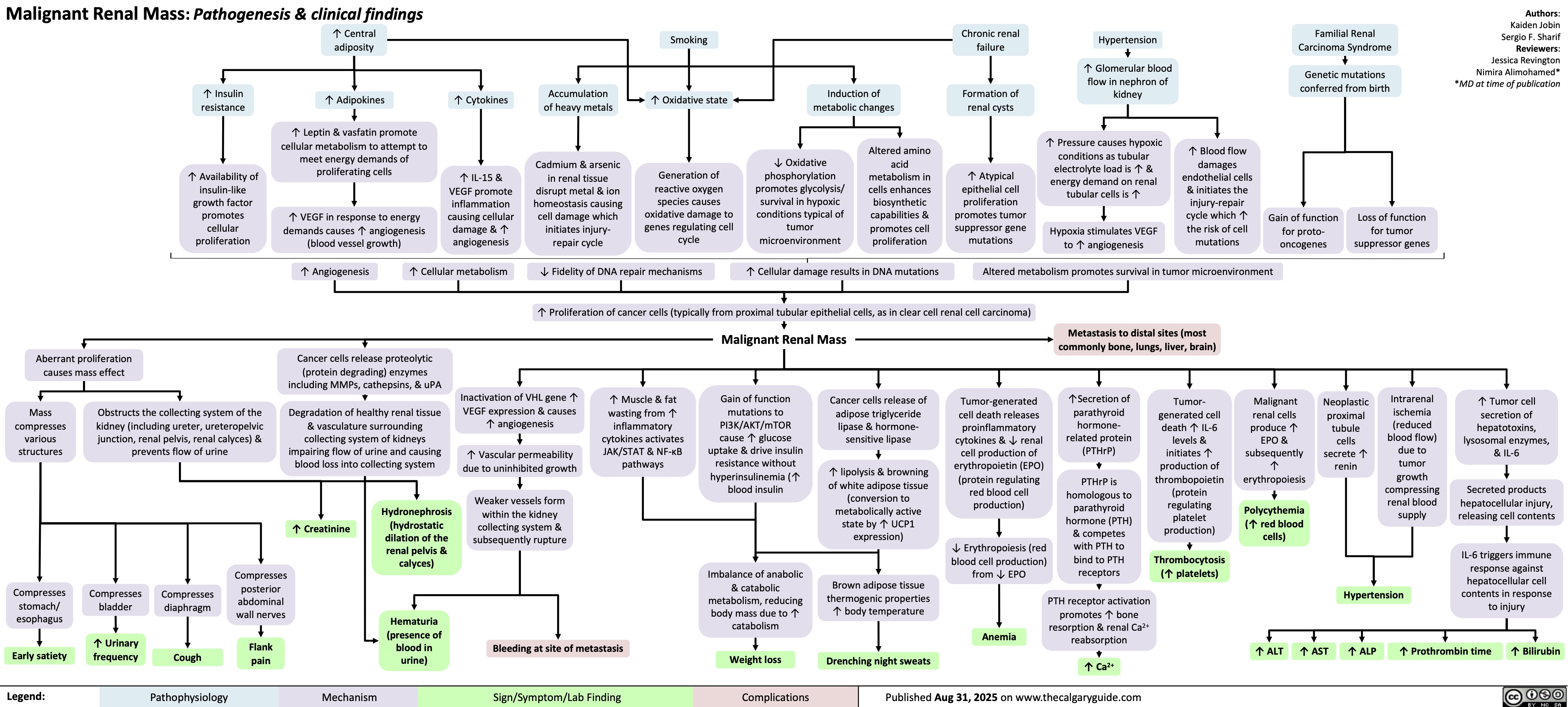
Obstructive Shock
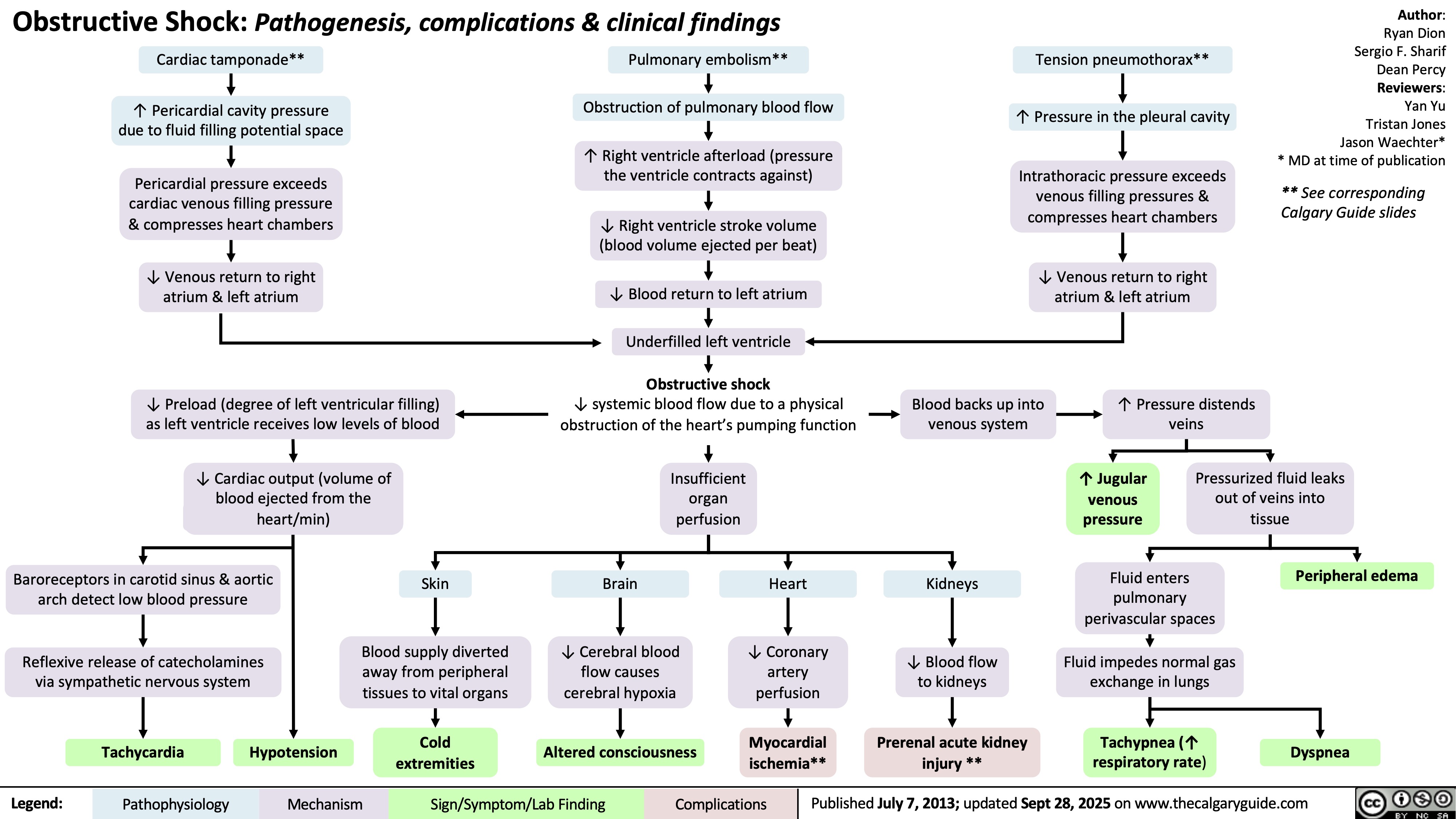
GLP-1 Receptor Agonists
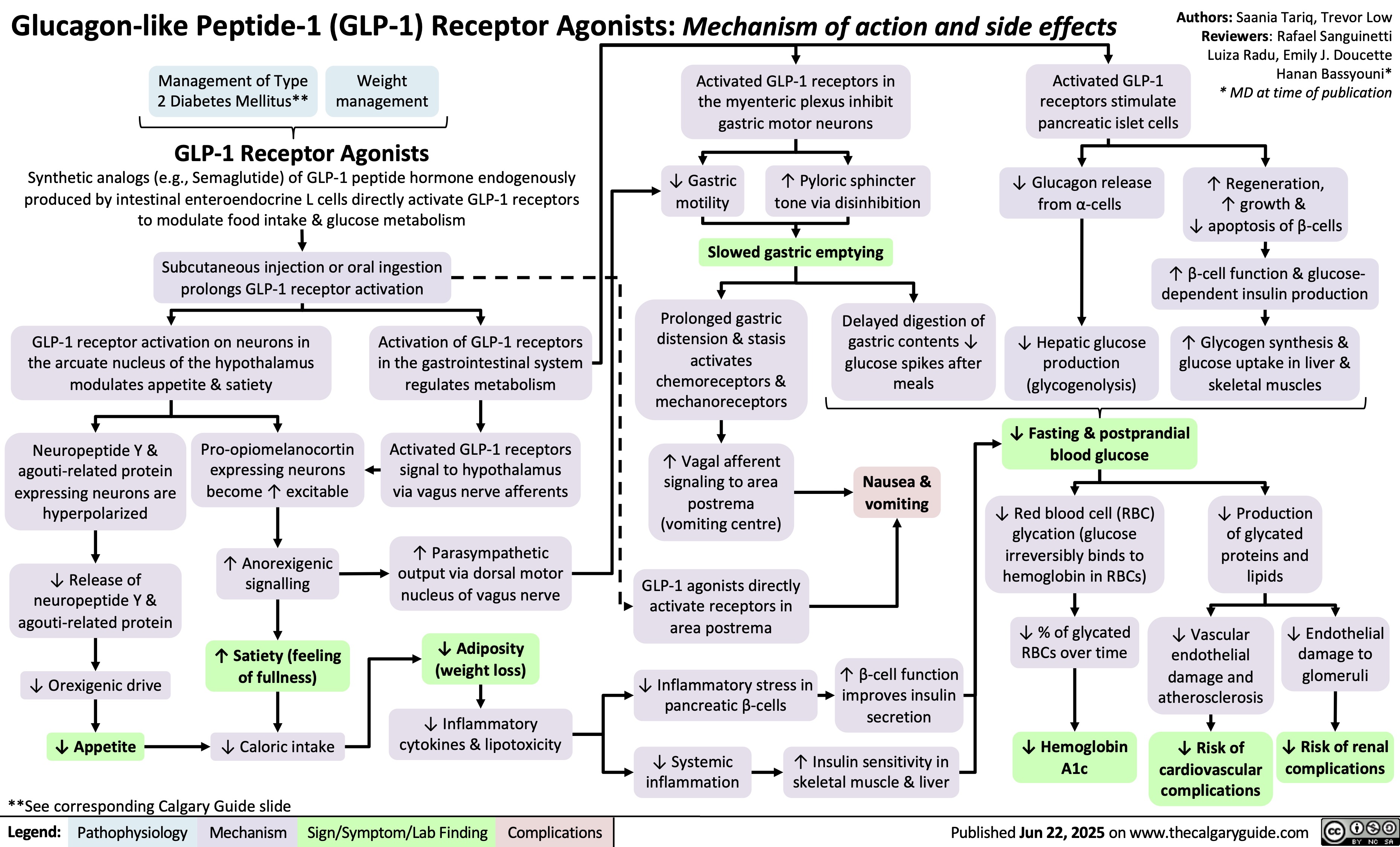
Rickets and Osteomalacia Pathogenesis and Clinical Findings
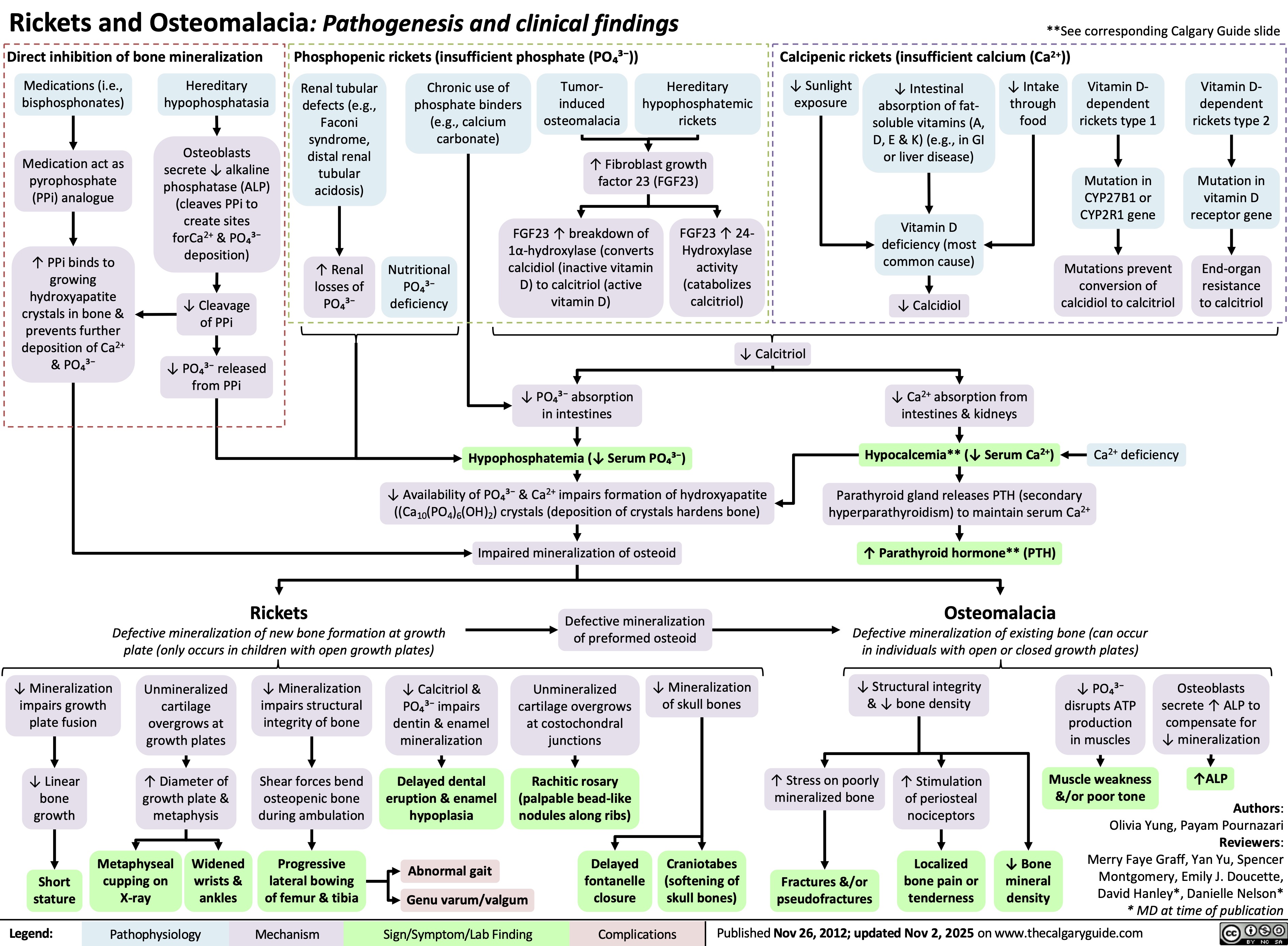
Acute Tubular Necrosis
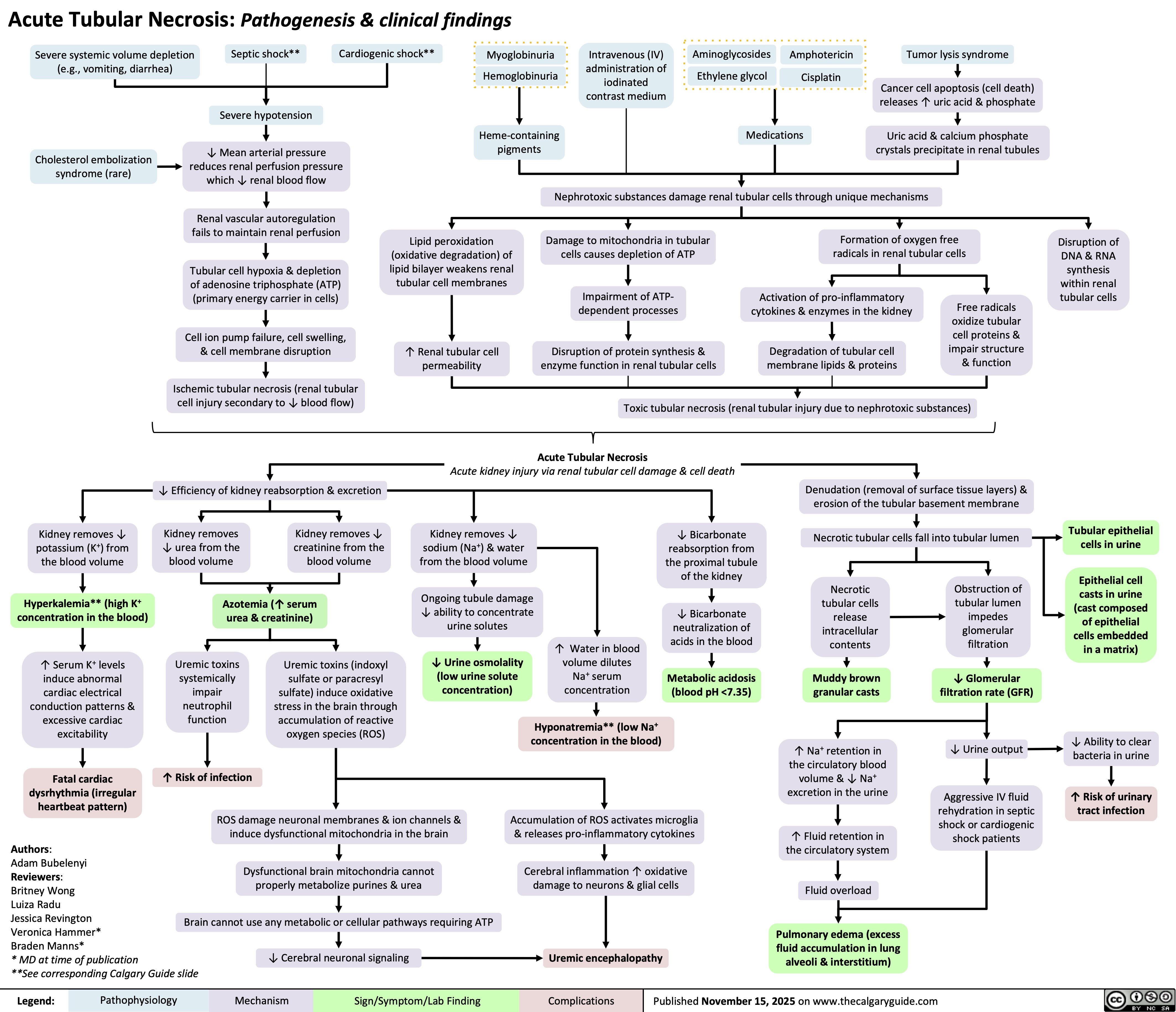
Infection-Related Glomerulonephritis
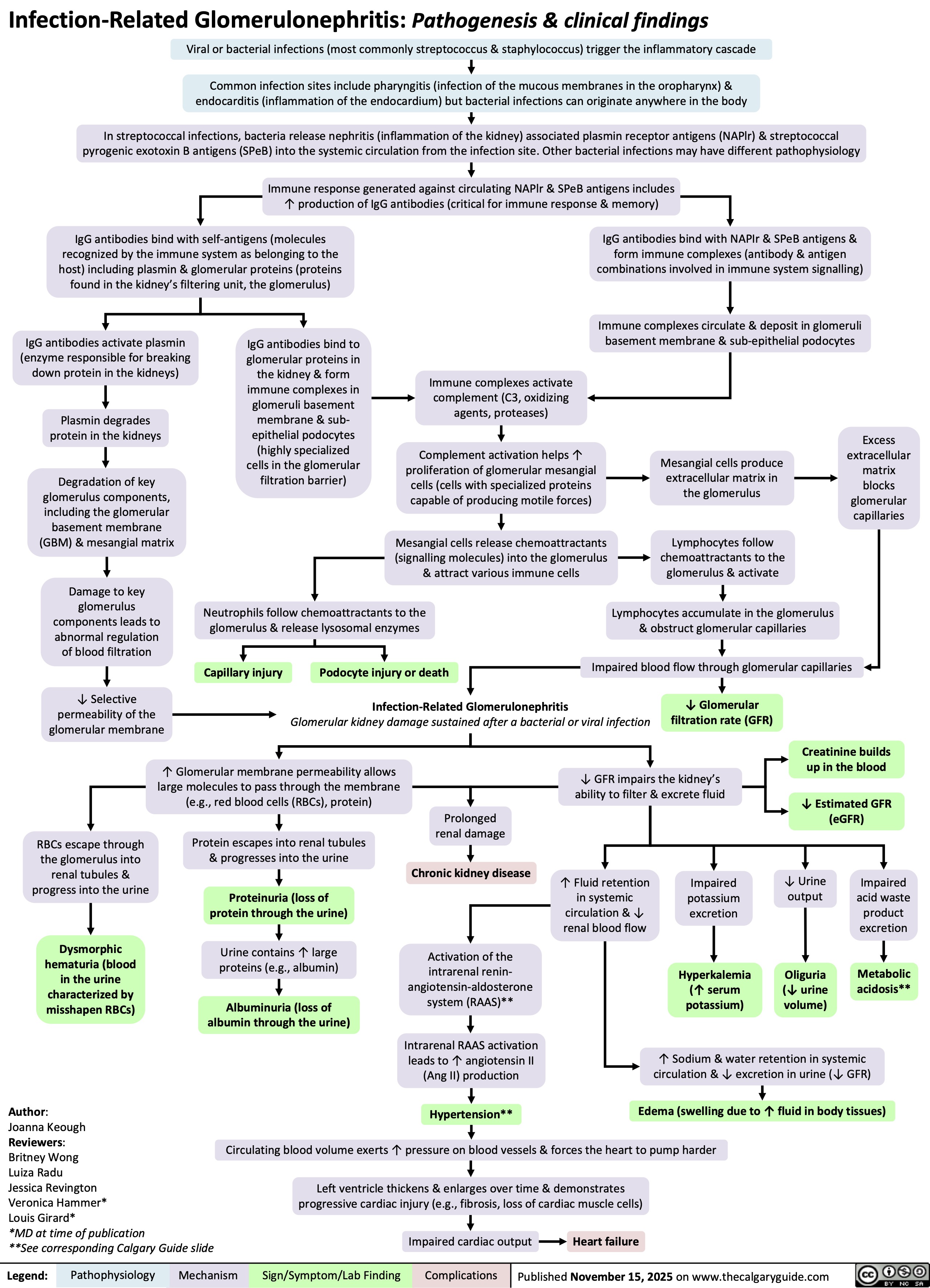
Pyelonephritis
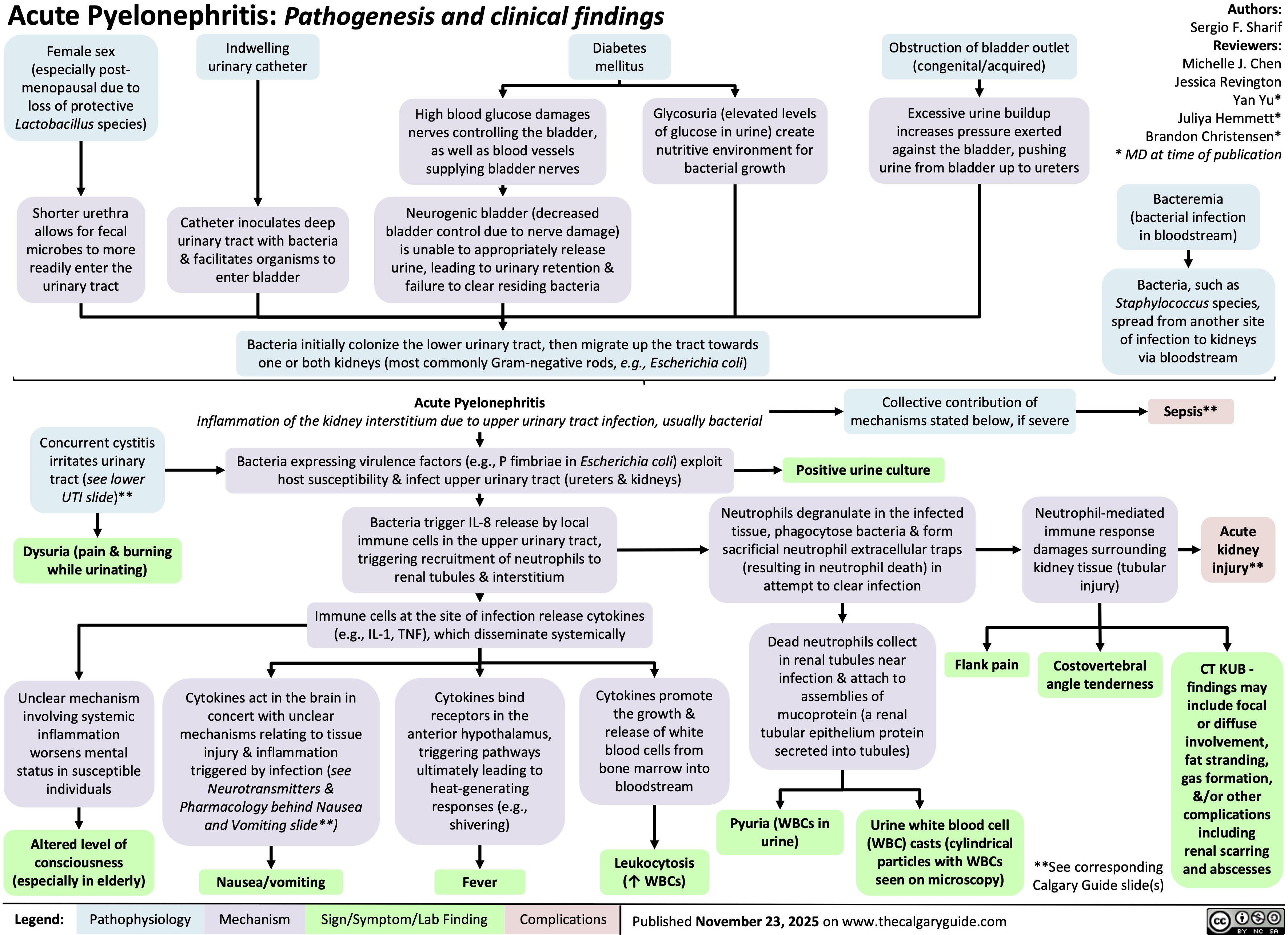
Hypothyroidism
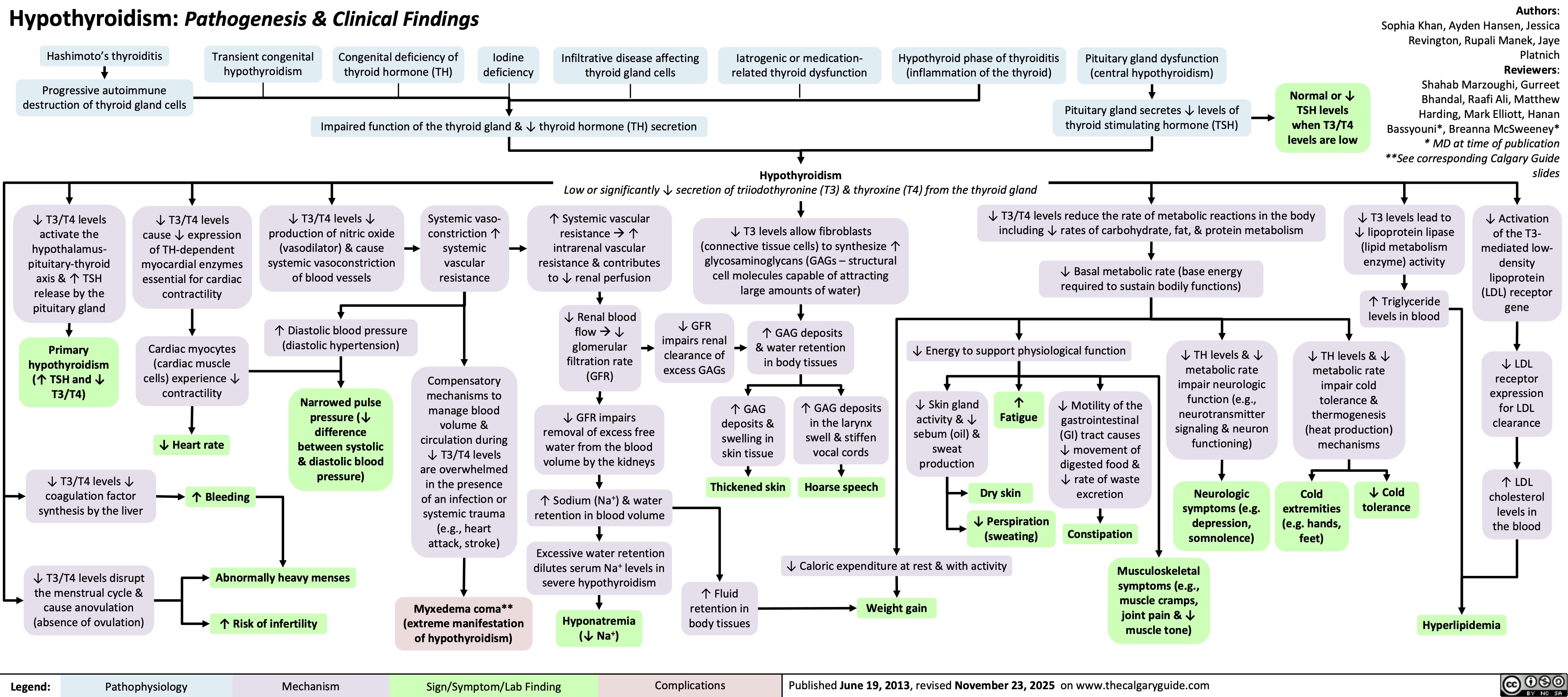
Diverticulosis and Angiodysplasia
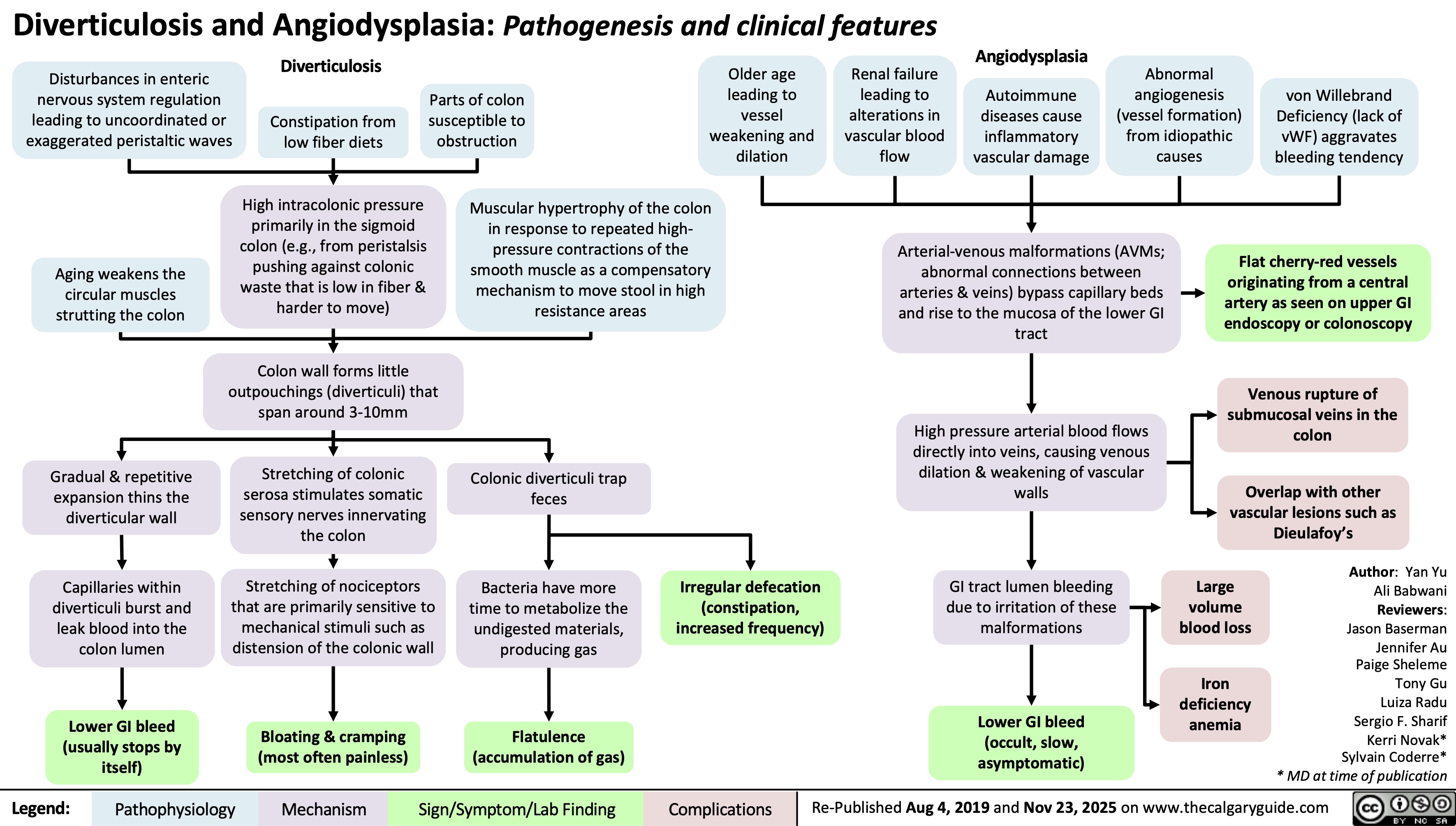
Vancomycin
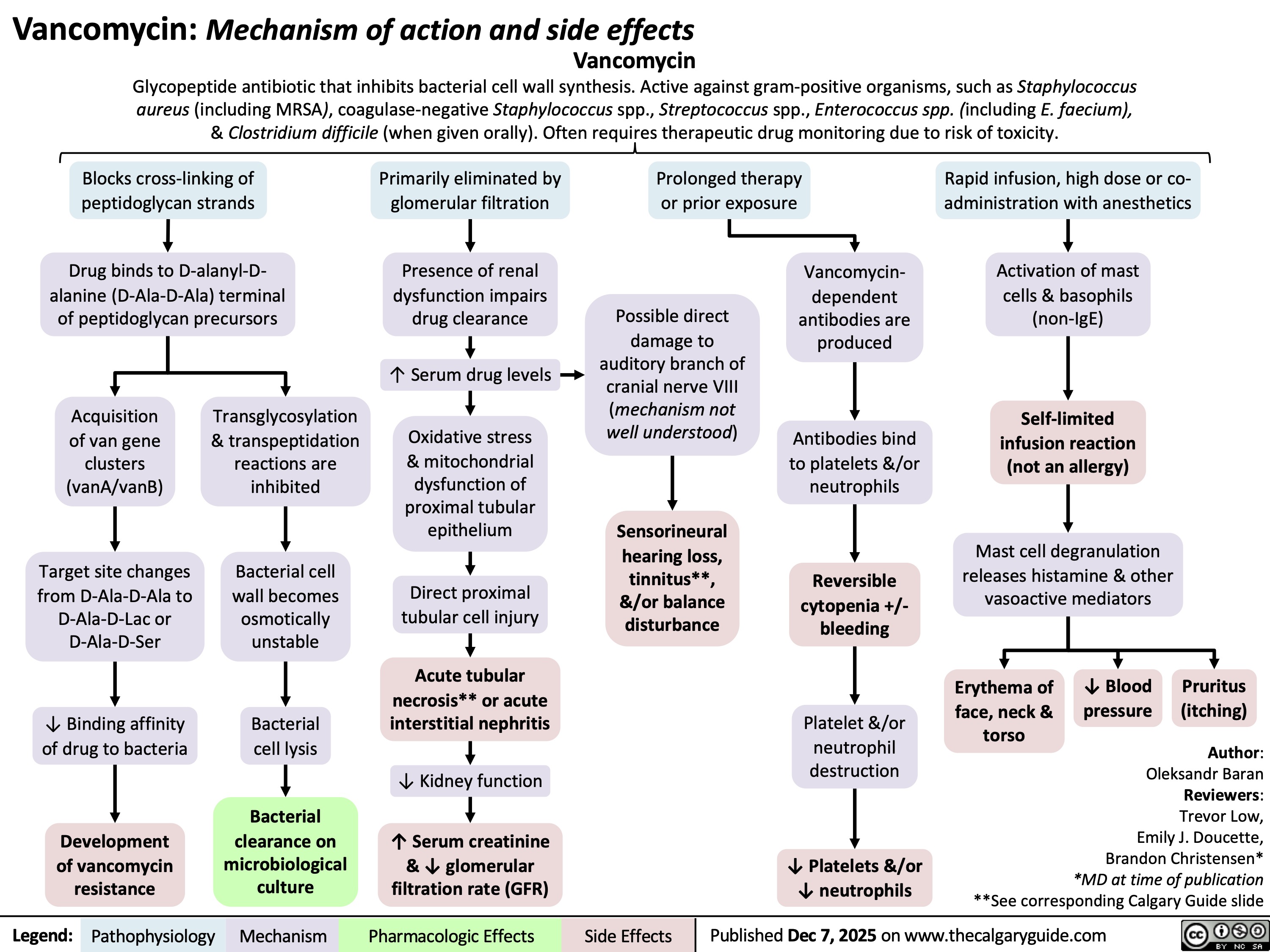
Diphtheria
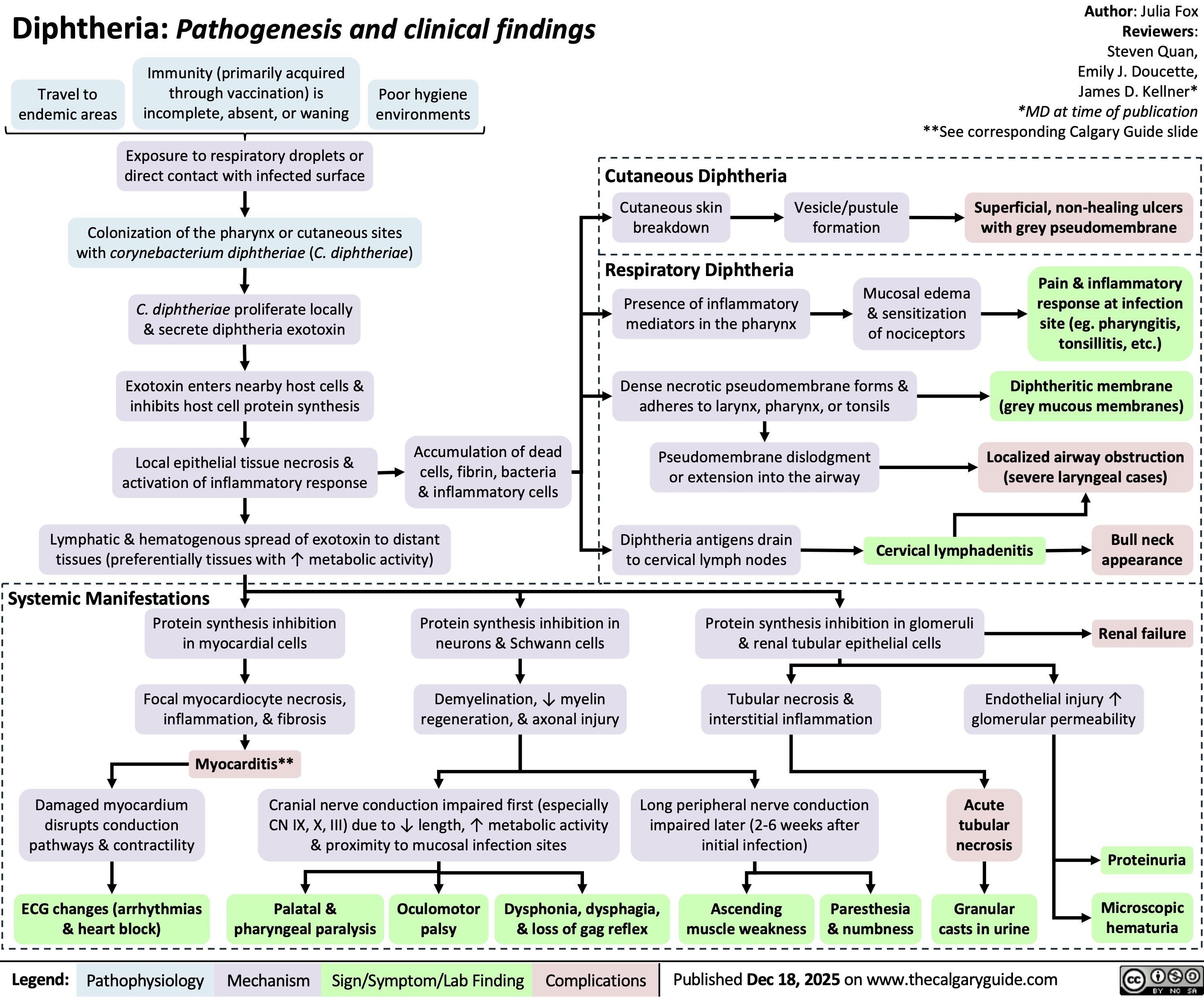
Necrosis versus Apoptosis
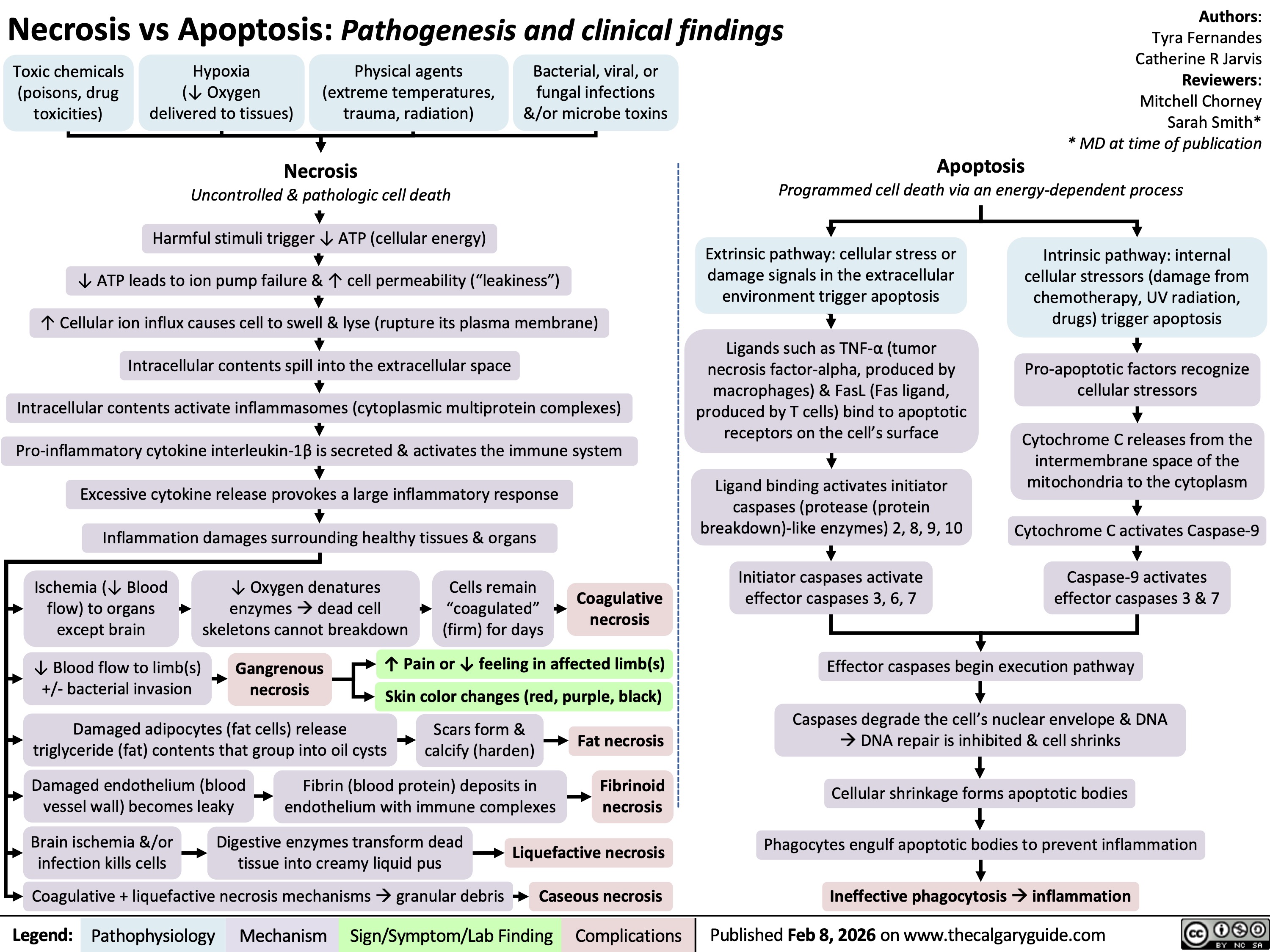
 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />


![Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hypercalcemia-Clinical-Findings.jpg)


![Hyperosmolar Hyperglycemic State (HHS)
Note: HHS is only seen in Type II DM patients!
Note: In patients with either DKA or HHS, always look for an underlying cause (i.e. an infection)
Author: Yan Yu Reviewers:
Peter Vetere
Gill Goobie
Hanan Bassyouni* * MD at time of publication
Alters total body water & ion osmosis
Inadequate insulin production, insulin resistance, non- adherence to insulin Tx
Relative Insulin deficit
Stresses that ↑ Insulin demand: infections, pneumonia, MI, pancreatitis, etc)
Hyperglycemia
(Very high blood [glucose], higher than in DKA)
When blood [glucose] > 12mmol/L, glucose filtration > reabsorption, ↑ urine [glucose]
Glucosuria
Glucose in filtrate promotes osmotic diuresis: large- volume urine output
Polyuria
Dehydration
(↓ JVP, orthostasis: postural hypotension/ postural tachycardia, ↑ resting HR)
Some insulin still present, but not enoughsome glucose is utilized by muscle/fat cells, some remain in the blood
Cells not “starved”, but still need more energy
↑ release of Catabolic hormones: Glucagon, Epinephrine, Cortisol, GH
Body tries to ↑ blood [glucose], to hopefully ↑ cell glucose absorption
Hypothalamic cells sense low intra-cellular glucose, triggering feelings of hunger
Polyphagia
Note: the presence of some insulin directly inhibits lipolysis; thus, in HHS there is no ketone body production, and no subsequent metabolic acidosis and ketouria (unlike in DKA). If ketones are detected in an HHS patient it’s likely secondary to starvation or other mechanisms.
↓ ECF volume, ↑ ECF osmolarity (i.e. hypernatremia)
↑ Gluconeogenesis ↑ Glycogenolysis (in liver)
↓ Protein synthesis, ↑ proteolysis
(in muscle)
↑ Gluconeogenic substrates for liver If the patient doesn’t drink enough
water to replenish lost blood volume If pt is alert and
Electrolyte imbalance
water is accessible
Water osmotically leaves neurons, shrinking them
Neural damage: delirium, lethargy, seizure, stupor, coma
↓ renal perfusion, ↓ GFR
Renal Failure
(pre-renal cause; see relevant slides)
Polydipsia Note: in HHS, body K+ is lost via osmotic diuresis. But diffusion of K+ out of cells
may cause serum [K+] to be falsely normal/elevated. To prevent hypokalemia, give IV KCl along with IV insulin as soon as serum K+ <5.0mmol/L. But ensure patient has good renal function/urine output first, to avoid iatrogenic hyperkalemia!
Note: Electrolyte imbalances (i.e. hyperkalemia, hypernatremia) are worsened by the acute renal failure commonly coexisting with DKA/HHS
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published November 3, 2016 on www.thecalgaryguide.com
Hyperosmolar Hyperglycemic State (HHS)
Note: HHS is only seen in Type II DM patients!
Note: In patients with either DKA or HHS, always look for an underlying cause (i.e. an infection)
Author: Yan Yu Reviewers:
Peter Vetere
Gill Goobie
Hanan Bassyouni* * MD at time of publication
Alters total body water & ion osmosis
Inadequate insulin production, insulin resistance, non- adherence to insulin Tx
Relative Insulin deficit
Stresses that ↑ Insulin demand: infections, pneumonia, MI, pancreatitis, etc)
Hyperglycemia
(Very high blood [glucose], higher than in DKA)
When blood [glucose] > 12mmol/L, glucose filtration > reabsorption, ↑ urine [glucose]
Glucosuria
Glucose in filtrate promotes osmotic diuresis: large- volume urine output
Polyuria
Dehydration
(↓ JVP, orthostasis: postural hypotension/ postural tachycardia, ↑ resting HR)
Some insulin still present, but not enoughsome glucose is utilized by muscle/fat cells, some remain in the blood
Cells not “starved”, but still need more energy
↑ release of Catabolic hormones: Glucagon, Epinephrine, Cortisol, GH
Body tries to ↑ blood [glucose], to hopefully ↑ cell glucose absorption
Hypothalamic cells sense low intra-cellular glucose, triggering feelings of hunger
Polyphagia
Note: the presence of some insulin directly inhibits lipolysis; thus, in HHS there is no ketone body production, and no subsequent metabolic acidosis and ketouria (unlike in DKA). If ketones are detected in an HHS patient it’s likely secondary to starvation or other mechanisms.
↓ ECF volume, ↑ ECF osmolarity (i.e. hypernatremia)
↑ Gluconeogenesis ↑ Glycogenolysis (in liver)
↓ Protein synthesis, ↑ proteolysis
(in muscle)
↑ Gluconeogenic substrates for liver If the patient doesn’t drink enough
water to replenish lost blood volume If pt is alert and
Electrolyte imbalance
water is accessible
Water osmotically leaves neurons, shrinking them
Neural damage: delirium, lethargy, seizure, stupor, coma
↓ renal perfusion, ↓ GFR
Renal Failure
(pre-renal cause; see relevant slides)
Polydipsia Note: in HHS, body K+ is lost via osmotic diuresis. But diffusion of K+ out of cells
may cause serum [K+] to be falsely normal/elevated. To prevent hypokalemia, give IV KCl along with IV insulin as soon as serum K+ <5.0mmol/L. But ensure patient has good renal function/urine output first, to avoid iatrogenic hyperkalemia!
Note: Electrolyte imbalances (i.e. hyperkalemia, hypernatremia) are worsened by the acute renal failure commonly coexisting with DKA/HHS
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published November 3, 2016 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hyperosmolar-Hyperglycemic-State-HHS.jpg)




 NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" title="Bupropion (atipiEni antidepresiv): Mehanizem delovanja in neieleni utinki
potenten antidepresiv v monoterapiji all kot dodatno zdravilo pri zdravljenju razpoloienjskih motenj
okrajgave: 5-HT - serotonin DA - dopamin DAT - prenagalec DA NA - noradrenalin NAT - prenagalec NA SSRI - selektivni zaviralec ponovnega privzema 5-HT
Legenda:
bupropion
farmakologija
antidepresivni udnki uporaben pri zdravljenju "zmaniganega pozitivnega afekta"
nima pomembnelgih 5-HT udnkov povzraa spolne disfunkcije v primerjavi s SSRI; lahko celo odpravi omenjeni neieleni udnek (povzraen s strani SSRI)
dodatek pri zdravljenju odvisnosti od nikotina preko T iive'nega prenosa DA v nagrajevalni poti
energije preko T 2ivbega prenosa NA
patofiziologija mehanizem
farmakokinetika
farmakodinamika
avtorica: JoAnna Fay, Sara Meunier pregledala: Jojo Jiang, Alexander Arnold, Aaron Mackie*
* dr. med. ob objavi
Nizkoafiniteten zaviralec ponovnega privzema DA in NA (DAT>NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" />
NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" title="Bupropion (atipiEni antidepresiv): Mehanizem delovanja in neieleni utinki
potenten antidepresiv v monoterapiji all kot dodatno zdravilo pri zdravljenju razpoloienjskih motenj
okrajgave: 5-HT - serotonin DA - dopamin DAT - prenagalec DA NA - noradrenalin NAT - prenagalec NA SSRI - selektivni zaviralec ponovnega privzema 5-HT
Legenda:
bupropion
farmakologija
antidepresivni udnki uporaben pri zdravljenju "zmaniganega pozitivnega afekta"
nima pomembnelgih 5-HT udnkov povzraa spolne disfunkcije v primerjavi s SSRI; lahko celo odpravi omenjeni neieleni udnek (povzraen s strani SSRI)
dodatek pri zdravljenju odvisnosti od nikotina preko T iive'nega prenosa DA v nagrajevalni poti
energije preko T 2ivbega prenosa NA
patofiziologija mehanizem
farmakokinetika
farmakodinamika
avtorica: JoAnna Fay, Sara Meunier pregledala: Jojo Jiang, Alexander Arnold, Aaron Mackie*
* dr. med. ob objavi
Nizkoafiniteten zaviralec ponovnega privzema DA in NA (DAT>NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" />















![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)
![Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hyponatremia-Physiology-.jpg)

![Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hyperkalemia-Physiology-.jpg)





































![Hypokalemia: Physiology
Authors: Samin Dolatabadi, Ran (Marissa) Zhang, Mannat Dhillon Reviewers: Meena Assad, Yan Yu*, Juliya Hemmett*
Beta-2 receptor stimulation
(e.g. Salbutamol)
↑ Red blood cell production
↑ Na+/K+ ATPase activity in skeletal muscle cells (moves K+ into the cell & Na+ out of cell)
↑ K+ entry into skeletal muscle cells
* MD at time of publication
Abbreviations:
• EABV – Effective Arterial
Blood Volume
• ENaC – Epithelial Sodium
Channel
• HCL – Hydrochloric acid • HCO3- –Bicarbonate ion
↑ K+ uptake by new red blood cells
↑ Intracellular shift of K+ into cells
Refeeding Syndrome Exogenous insulin
↓ K+ dietary intake
(rare cause in isolation)
↑ Insulin in response to carbohydrate load
↑ Na+/K+ ATPase activity in skeletal muscle & hepatic cells
↑ K+ entry into skeletal muscle & hepatic cells
↓ K+ availability for gastrointestinal absorption
Hypokalemia (Serum [K+] < 3.5 mmol/L)
↑ Renal K+ secretion
K+ follows the electrical gradient into tubular lumen
↑ Electronegativity of tubular lumen
↑ Na+ reabsorptionin principal cellsà Cl- left behind in tubular lumen of kidneys
Gastric acid depletionà ↓HCl
Loss of H+àShift in bicarbonate buffer system to ↑ plasma HCO3-
Plasma HCO3- above reabsorptive capacity of the proximal tubule
↑ HCO3- in the distal tubular lumen of kidneys
Vomiting
Diarrhea Laxatives
Renin secreting tumour Hyperaldosteronism Renal artery stenosis Loop and Thiazide
diuretics
Bartter’s and Gittelman’s syndrome
Liddle syndrome
Extracellular fluid volume depletion
↓ EABV ↑ Renin secretion
↓ Afferent arteriole pressure perfusing kidneys
Renin-Angiotensin- Aldosterone System (RAAS) activationà ↑ Aldosterone release from the adrenal cortex
↑ Expression of ENaC (Na+ reabsorption) in principal cells of the cortical collectingduct)
+ ↑Na &
water excretion in kidneys
↓ EABV
Genetic condition leading to inability to degrade ENaC channels in principal cells of the cortical collecting duct
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019, updated Jan 23, 2022 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi Reviewers: Meena Assad Dr. Juliya Hemmett* * MD at time of publication
TTKG > 4 with N/↑ EABV in hypokalemia is inappropriate and a principal cell problem.
β2 Stimulation (e.g., Salbutamol)
↑ Na+/K+ ATPase activity
↑ K+ entry into cell
↑ RBC Production
↑ Cell production
↑ K+ uptake by new cells
↓ Extracellular ↑ Insulin in response to H+
carbohydrate load
↑ Na+/H+ antiporter activity (movement of H+ out of cell and Na+ into cell)
↑ Intracellular Na+
↑ Na+/K+ ATPase activity ↑ K+ entry into cell
↑ Intracellular Shift of K+
Notes:
Refeeding Syndrome
Insulin
Alkalemia
•
Abbreviations:
• CCD – Cortical Collecting Duct
• EABV – Effective Arterial Blood Volume
• RAAS – Renin-Angiotensin-Aldosterone System • TTKG – Trans-tubular Potassium Gradient
• ENaC – Epithelial Sodium Channel
↓ K+ Intake (Rare cause in isolation)
↓ K+ availability
Diarrhea, Vomiting, Laxatives
↑ Gastrointestinal loss of K+
Polyuria
↑ Renal loss of K+ (TTKG < 4 as principal cell is working appropriately but small amount of K+ is lost per urination)
Hypokalemia (Serum [K+] < 3.5 mmol/L)
Liddle Syndrome Hyperaldosteronism Renin Secreting Tumour
Renal artery stenosis
Loop and Thiazide Diuretics
Bartter’s and Gittelman’s Syndrome
Genetic condition leading to inability to degrade ENaC channels ↑ Renin
↑ Renal K+ secretion
K+ follows the electrical gradient
Electronegative lumen
↓ Pressure perfusing the kidney
RAAS activation RAAS activation
↑ Aldosterone
↑ Na+ and water excretion
↓ EABV
↑ Expression of ENaC in principal cells of CCD
↑ Na+ reabsorption
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi, Ran (Marissa) Zhang, Mannat Dhillon Reviewers: Meena Assad, Yan Yu*, Juliya Hemmett*
Beta-2 receptor stimulation
(e.g. Salbutamol)
↑ Red blood cell production
↑ Na+/K+ ATPase activity in skeletal muscle cells (moves K+ into the cell & Na+ out of cell)
↑ K+ entry into skeletal muscle cells
* MD at time of publication
Abbreviations:
• EABV – Effective Arterial
Blood Volume
• ENaC – Epithelial Sodium
Channel
• HCL – Hydrochloric acid • HCO3- –Bicarbonate ion
↑ K+ uptake by new red blood cells
↑ Intracellular shift of K+ into cells
Refeeding Syndrome Exogenous insulin
↓ K+ dietary intake
(rare cause in isolation)
↑ Insulin in response to carbohydrate load
↑ Na+/K+ ATPase activity in skeletal muscle & hepatic cells
↑ K+ entry into skeletal muscle & hepatic cells
↓ K+ availability for gastrointestinal absorption
Hypokalemia (Serum [K+] < 3.5 mmol/L)
↑ Renal K+ secretion
K+ follows the electrical gradient into tubular lumen
↑ Electronegativity of tubular lumen
↑ Na+ reabsorptionin principal cellsà Cl- left behind in tubular lumen of kidneys
Gastric acid depletionà ↓HCl
Loss of H+àShift in bicarbonate buffer system to ↑ plasma HCO3-
Plasma HCO3- above reabsorptive capacity of the proximal tubule
↑ HCO3- in the distal tubular lumen of kidneys
Vomiting
Diarrhea Laxatives
Renin secreting tumour Hyperaldosteronism Renal artery stenosis Loop and Thiazide
diuretics
Bartter’s and Gittelman’s syndrome
Liddle syndrome
Extracellular fluid volume depletion
↓ EABV ↑ Renin secretion
↓ Afferent arteriole pressure perfusing kidneys
Renin-Angiotensin- Aldosterone System (RAAS) activationà ↑ Aldosterone release from the adrenal cortex
↑ Expression of ENaC (Na+ reabsorption) in principal cells of the cortical collectingduct)
+ ↑Na &
water excretion in kidneys
↓ EABV
Genetic condition leading to inability to degrade ENaC channels in principal cells of the cortical collecting duct
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019, updated Jan 23, 2022 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi Reviewers: Meena Assad Dr. Juliya Hemmett* * MD at time of publication
TTKG > 4 with N/↑ EABV in hypokalemia is inappropriate and a principal cell problem.
β2 Stimulation (e.g., Salbutamol)
↑ Na+/K+ ATPase activity
↑ K+ entry into cell
↑ RBC Production
↑ Cell production
↑ K+ uptake by new cells
↓ Extracellular ↑ Insulin in response to H+
carbohydrate load
↑ Na+/H+ antiporter activity (movement of H+ out of cell and Na+ into cell)
↑ Intracellular Na+
↑ Na+/K+ ATPase activity ↑ K+ entry into cell
↑ Intracellular Shift of K+
Notes:
Refeeding Syndrome
Insulin
Alkalemia
•
Abbreviations:
• CCD – Cortical Collecting Duct
• EABV – Effective Arterial Blood Volume
• RAAS – Renin-Angiotensin-Aldosterone System • TTKG – Trans-tubular Potassium Gradient
• ENaC – Epithelial Sodium Channel
↓ K+ Intake (Rare cause in isolation)
↓ K+ availability
Diarrhea, Vomiting, Laxatives
↑ Gastrointestinal loss of K+
Polyuria
↑ Renal loss of K+ (TTKG < 4 as principal cell is working appropriately but small amount of K+ is lost per urination)
Hypokalemia (Serum [K+] < 3.5 mmol/L)
Liddle Syndrome Hyperaldosteronism Renin Secreting Tumour
Renal artery stenosis
Loop and Thiazide Diuretics
Bartter’s and Gittelman’s Syndrome
Genetic condition leading to inability to degrade ENaC channels ↑ Renin
↑ Renal K+ secretion
K+ follows the electrical gradient
Electronegative lumen
↓ Pressure perfusing the kidney
RAAS activation RAAS activation
↑ Aldosterone
↑ Na+ and water excretion
↓ EABV
↑ Expression of ENaC in principal cells of CCD
↑ Na+ reabsorption
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hypokalemia-Physiology.jpg)
![Metabolic Alkalosis: Pathogenesis
Authors: Wazaira Khan Reviewers: Jessica Krahn, Emily Wildman, Austin Laing, Huneza Nadeem, Ran (Marissa) Zhang, Adam Bass* * MD at time of publication
Primary hyperaldosteronism E.g., aldosterone- secreting mass, adrenal hyperplasia
Secondary hyperaldosteronism E.g., renin-secreting mass, renal artery stenosis
Pseudo- hypoaldosteronism E.g., Liddle’s Syndrome
Unregulated aldosterone production in adrenal cortex
Excess aldosterone suppresses renin production
Unregulated renin production by juxtaglomerular cells
Sustained ↑ in mineralocorticoid (aldosterone) activity
Insertion of epithelial sodium channels on principal cells in collecting duct
↑ Water retention by the kidney
↑ Na+ reabsorption by principal cells
↑ EABV
• ↑ Jugular venous pressure • Hypertension
• Urine Na+ > 40 mEq/L
Low renin, high aldosterone state
Activation of renin- angiotensin-aldosterone system (RAAS)
Release of aldosterone from adrenal cortex
High renin, high aldosterone state
↑ Negative charge in collecting duct lumen
K+ leaks into collecting duct lumen by principal cell to maintain electroneutrality
Hypokalemia
Intracellular K+ leaks out of any cell in the body to compensate for low serum K+ levels
Extracellular H+ enters cell to maintain electroneutrality
Intracellular acidosis
Activation of compensatory acid secreting mechanisms in kidney
Impaired tubular function
E.g., loop/thiazide diuretics, Bartter’s/ Gitelman’s Syndrome
Upper GI Loss
E.g., vomiting, nasogastric suction
Unregulated epithelial sodium channel activity in collecting duct mimicking aldosterone function
↑ in Na+ and water retention à RAAS inhibition
↓ Na+ and Cl- reabsorption in thick ascending limb or distal convoluted tubule
Low renin, low aldosterone state
↑ Na+, Cl- and water secretion through kidney
RAAS activation
See “Physiology of RAAS” slide
↓ EABV • • •
Temporary ↑ in mineralocorticoid (aldosterone) activity
↓ Jugular venous pressure Orthostatic hypotension Dry mucous membranes Urine Na+ < 20 mEq/L
•
Loss of fluid through GI tract
↓ HCl delivery to small intestine
↑ NH + secretion and 4
↑ HCO3- reabsorption in proximal tubule
↑ H+ secretion in cortical collecting duct
Loss of gastric contents, including HCl
↓ HCO3- secretion by pancreas, liver and intestines to neutralize HCl
Loss of intrinsic acid to neutralize HCO3- ↑ Plasma [HCO3-]
Metabolic Alkalosis
Arterial blood gas pH > 7.40 Plasma [HCO3-] > 24 mEq/L
Effective Arterial Blood Volume (EABV): component of arterial blood volume that is effectively perfusing organs
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published May 31, 2022 on www.thecalgaryguide.com
Metabolic Alkalosis: Pathogenesis
Authors: Wazaira Khan Reviewers: Jessica Krahn, Emily Wildman, Austin Laing, Huneza Nadeem, Ran (Marissa) Zhang, Adam Bass* * MD at time of publication
Primary hyperaldosteronism E.g., aldosterone- secreting mass, adrenal hyperplasia
Secondary hyperaldosteronism E.g., renin-secreting mass, renal artery stenosis
Pseudo- hypoaldosteronism E.g., Liddle’s Syndrome
Unregulated aldosterone production in adrenal cortex
Excess aldosterone suppresses renin production
Unregulated renin production by juxtaglomerular cells
Sustained ↑ in mineralocorticoid (aldosterone) activity
Insertion of epithelial sodium channels on principal cells in collecting duct
↑ Water retention by the kidney
↑ Na+ reabsorption by principal cells
↑ EABV
• ↑ Jugular venous pressure • Hypertension
• Urine Na+ > 40 mEq/L
Low renin, high aldosterone state
Activation of renin- angiotensin-aldosterone system (RAAS)
Release of aldosterone from adrenal cortex
High renin, high aldosterone state
↑ Negative charge in collecting duct lumen
K+ leaks into collecting duct lumen by principal cell to maintain electroneutrality
Hypokalemia
Intracellular K+ leaks out of any cell in the body to compensate for low serum K+ levels
Extracellular H+ enters cell to maintain electroneutrality
Intracellular acidosis
Activation of compensatory acid secreting mechanisms in kidney
Impaired tubular function
E.g., loop/thiazide diuretics, Bartter’s/ Gitelman’s Syndrome
Upper GI Loss
E.g., vomiting, nasogastric suction
Unregulated epithelial sodium channel activity in collecting duct mimicking aldosterone function
↑ in Na+ and water retention à RAAS inhibition
↓ Na+ and Cl- reabsorption in thick ascending limb or distal convoluted tubule
Low renin, low aldosterone state
↑ Na+, Cl- and water secretion through kidney
RAAS activation
See “Physiology of RAAS” slide
↓ EABV • • •
Temporary ↑ in mineralocorticoid (aldosterone) activity
↓ Jugular venous pressure Orthostatic hypotension Dry mucous membranes Urine Na+ < 20 mEq/L
•
Loss of fluid through GI tract
↓ HCl delivery to small intestine
↑ NH + secretion and 4
↑ HCO3- reabsorption in proximal tubule
↑ H+ secretion in cortical collecting duct
Loss of gastric contents, including HCl
↓ HCO3- secretion by pancreas, liver and intestines to neutralize HCl
Loss of intrinsic acid to neutralize HCO3- ↑ Plasma [HCO3-]
Metabolic Alkalosis
Arterial blood gas pH > 7.40 Plasma [HCO3-] > 24 mEq/L
Effective Arterial Blood Volume (EABV): component of arterial blood volume that is effectively perfusing organs
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published May 31, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/05/Metabolic-Alkalosis-1.jpg)




![Hyperkalemia (↓ Renal Excretion): Pathophysiology
Non-steroidal anti- inflammatory drugs (NSAIDs)
Inhibition of prostaglandins which promote renin secretion (see NSAIDs and the Kidney: Mechanism of Action and Side Effects slide)
Diabetic Nephropathy
Acute (AIN)and chronic (CIN) interstitial nephritis
Immune-mediated damage of the kidney tubule and interstitium
Damage to distal tubule leads to aldosterone resistance at principal cell
Aldosterone cannot ↑ EnaC insertion on principal cell of CCD
Epithelial sodium channel (ENaC) blockers
Acute kidney injury and chronic kidney disease
↓ Effective arterial blood volume (volume of blood effectively perfusing tissue)
↓ Oxygen perfusion to renal tissue causing renal ischemia
Autonomic neuropathy ↓ sympathetic drive to produce renin
Chronic juxtaglomerular cell damage ↓ synthesis of renin
Blockage of ENaC on the principal cells of the CCD
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is either not formed (ACEi), or blocked at its receptor (ARBs)
Angiotensin II cannot stimulate the release of aldosterone from the adrenal cortex
Adrenal Insufficiency
Adrenal gland cannot produce sufficient amounts of aldosterone
↓ Renin secretion by the afferent arteriole prevents RAAS activation
↓ Aldosterone release from adrenal cortex
↓ ENaC (Na+ reabsorption channel) expression on principal cells of the cortical collecting duct (CCD)
Damage to kidney causes renal impairment and ↓ glomerular filtration rate
↓ Glomerular filtrate production means ↓ tubular flow rate
↓ Na+ delivery to the distal tubule
↓ Na+ reabsorption by ENaCs at the principal cell in CCD
↓ Na+ and water reabsorption by ENaCs in CCD ↓ Effective arterial blood volume (EABV)
Activates renin-angiotensin-aldosterone system (RAAS) leads to ↑ renin secretion in the afferent arteriole (see Physiology of the renin-angiotensin-aldosterone system (RAAS) slide)
↑ Na+ and water loss in tubular lumen
↑ Positive charge in tubular lumen
↓ Electronegativity gradient in tubular lumen
of CCD
↓ K+ excretion by the principal cell in the CCD as there is less of a electronegative gradient
↑ Accumulation of K+ in blood Hyperkalemia
Serum [K+] > 5.1 mmol/L
See Hyperkalemia: Clinical Findings slide
In the case of diabetic nephropathy and NSAIDS ↓ EABV does not stimulate RAAS and therefore aldosterone production
↓ Renin
↓ Aldosterone
↑ Renin secretion activates an ↑ in aldosterone production but aldosterone action at the principal cell is blocked because of ENaCs or resistance in AIN and CIN
↑ Renin
↑ Aldosterone
In the case of ACEi, ARBs, or adrenal insufficiency ↑ renin secretion does not lead to ↑ aldosterone
↑ Renin
↓ Aldosterone
Note: as described in the above flow chart, measuring serum renin and aldosterone levels can be used to help diagnose the cause of hyperkalemia.
Authors: Mannat Dhillon, Joshua Low, Emily Wildman, Huneza Nadeem Reviewers: Andrea Kuczynski, Marissa (Ran) Zhang, Adam Bass*, Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Aug 2, 2022 on www.thecalgaryguide.com
Hyperkalemia (↓ Renal Excretion): Pathophysiology
Non-steroidal anti- inflammatory drugs (NSAIDs)
Inhibition of prostaglandins which promote renin secretion (see NSAIDs and the Kidney: Mechanism of Action and Side Effects slide)
Diabetic Nephropathy
Acute (AIN)and chronic (CIN) interstitial nephritis
Immune-mediated damage of the kidney tubule and interstitium
Damage to distal tubule leads to aldosterone resistance at principal cell
Aldosterone cannot ↑ EnaC insertion on principal cell of CCD
Epithelial sodium channel (ENaC) blockers
Acute kidney injury and chronic kidney disease
↓ Effective arterial blood volume (volume of blood effectively perfusing tissue)
↓ Oxygen perfusion to renal tissue causing renal ischemia
Autonomic neuropathy ↓ sympathetic drive to produce renin
Chronic juxtaglomerular cell damage ↓ synthesis of renin
Blockage of ENaC on the principal cells of the CCD
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is either not formed (ACEi), or blocked at its receptor (ARBs)
Angiotensin II cannot stimulate the release of aldosterone from the adrenal cortex
Adrenal Insufficiency
Adrenal gland cannot produce sufficient amounts of aldosterone
↓ Renin secretion by the afferent arteriole prevents RAAS activation
↓ Aldosterone release from adrenal cortex
↓ ENaC (Na+ reabsorption channel) expression on principal cells of the cortical collecting duct (CCD)
Damage to kidney causes renal impairment and ↓ glomerular filtration rate
↓ Glomerular filtrate production means ↓ tubular flow rate
↓ Na+ delivery to the distal tubule
↓ Na+ reabsorption by ENaCs at the principal cell in CCD
↓ Na+ and water reabsorption by ENaCs in CCD ↓ Effective arterial blood volume (EABV)
Activates renin-angiotensin-aldosterone system (RAAS) leads to ↑ renin secretion in the afferent arteriole (see Physiology of the renin-angiotensin-aldosterone system (RAAS) slide)
↑ Na+ and water loss in tubular lumen
↑ Positive charge in tubular lumen
↓ Electronegativity gradient in tubular lumen
of CCD
↓ K+ excretion by the principal cell in the CCD as there is less of a electronegative gradient
↑ Accumulation of K+ in blood Hyperkalemia
Serum [K+] > 5.1 mmol/L
See Hyperkalemia: Clinical Findings slide
In the case of diabetic nephropathy and NSAIDS ↓ EABV does not stimulate RAAS and therefore aldosterone production
↓ Renin
↓ Aldosterone
↑ Renin secretion activates an ↑ in aldosterone production but aldosterone action at the principal cell is blocked because of ENaCs or resistance in AIN and CIN
↑ Renin
↑ Aldosterone
In the case of ACEi, ARBs, or adrenal insufficiency ↑ renin secretion does not lead to ↑ aldosterone
↑ Renin
↓ Aldosterone
Note: as described in the above flow chart, measuring serum renin and aldosterone levels can be used to help diagnose the cause of hyperkalemia.
Authors: Mannat Dhillon, Joshua Low, Emily Wildman, Huneza Nadeem Reviewers: Andrea Kuczynski, Marissa (Ran) Zhang, Adam Bass*, Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Aug 2, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/08/Hyperkalemia-renal-excretion.jpg)
![Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com
Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/SAH-Clinical-Findings-2022.jpg)


![Unstable Angina/Unstable Angina Pectoris: Pathogenesis and clinical findings Primary cause:
Secondary causes:
Coronary artery vasospasm - primary or drug induced (Ex: cocaine, triptans)
Coagulopathy
(Ex: antiphospholipid antibody syndrome)
Vasculitic syndromes (Ex: Takayasu arteritis)
Authors: Marisa Vigna Ryan Wilkie Yan Yu* Reviewers: Julena Foglia Davis Maclean Mehul Gupta Andrew Grant* * MD at time of publication
Atherosclerosis
Fatty plaque accumulates inside the intimal walls of arteries Coronary arterial atherosclerotic plaque rupture or erosion
Plaque disruption exposes subendothelial components of damaged vessel wall to platelets, initiating the coagulation cascade and platelet adhesion
Aggregation of platelets results in the formation of a thrombus Thrombus partially occludes blood flow through a coronary artery âmyocardial blood supply
Congenital anomalies (Ex: myocardial bridge, anomalous coronary)
Spontaneous coronary artery dissection
Increased blood viscosity (Ex: polycythemia, thrombocytopenia)
Factors thatámyocardial (cardiac muscle) oxygen demand (Ex: tachycardia, hypotension, hypertension, anemia, exertion, stress)
Coronary embolism (Ex: A. Fib, endocarditis, prosthetic valve thrombus)
áheart rate, contractility, and/or wall tension ámyocardial oxygen demand
Myocardial ischemia due to imbalance between blood supply and oxygen demand (insufficient blood/oxygen supply)
Unstable Angina/Unstable Angina Pectoris
Can be new onset angina; typically progressive in frequency, severity, or duration; can occur at rest
Subtotal occlusion of a coronary arteryà
reduced, but continued, myocardial blood supply
Maintained perfusion means cardiomyocytes are still alive and thus do not leak troponin into bloodstream
Normal serum troponin
Diaphoresis
(sweating)
Since bloodflow occurs from epicardium to endocardium, myocardial ischemia is more
pronounced in the subendocardium (region furthest away from heart’s external surface)
Sufficient blood flow is maintained in regions superficial to the subendocardium, resulting in non-transmural (partial thickness) heart wall ischemia
Non-inferior wall ischemia triggers a predominantáin sympathetic nervous system activity, given the proximity of cardiac sympathetic nerve innervation
Ischemiaâ cardiomyocyte resting membrane potential andâ action potential duration
Voltage gradient between normal and subendocardial ischemic zones creates injury currents, shifting the ST- vector on ECG
ECG: ST depression
and/or T wave inversion
Cardiac sensory nerve fibres mix with somatic sensory nerve
fibres and enter the spinal cord via the T1-T4 nerve roots
Brain perceives increased cardiac sensory nerve signaling as nerve pain coming from the skin of T1-T4 dermatomes (“Referred Pain”)
Myocardial ischemia causes hypoxic stress on cardiomyocytesàâaerobic (requiring oxygen) metabolism,áanaerobic (not requiring oxygen) metabolism
áanerobic respirationálactic acid production,á[H+], andâcellular pH which impairs cardiomyocyte function
Cardiomyocyte dysfunction impairs myocardial relaxation in diastole and/orâ left ventricular contractility in systole
âleft ventricular cardiac output àbackup of blood in the left ventricle, atrium, and pulmonary vasculature
ápulmonary capillary pressures pushes fluid out of the capillaries into the alveoli in the lungs
Fluid filled alveoliâgas exchange andâ oxygenation, triggering harder and faster breathing in order to compensate
Dyspnea
Activation of sweat glands via acetylcholine release
Hormones bind to cardiac β1 receptors
Tachycardia
(áheart rate)
Epinephrine/ Norepinephrine hormone release from the adrenal medulla
Hormones bind to arterial smooth muscle α1 receptors ávascular tone (vasoconstriction)
Hypertension
The Vagus nerve sits in close physical proximity to the inferior wall of the heart àinferior wall ischemia triggers involuntary Vagus nerve activation
Since the Vagus nerve coordinates parasympathetic activity,áVagus nerve activity leads to a variety of parasympathetic nervous system responses:
Retrosternal discomfort: May present as pain, heaviness, tightness, aching, pressure, burning or squeezing
Pain radiation to T1-T4 dermatomes:
Left shoulder and arm, lower jaw, neck, abdomen, upper back
Syncope
(fainting)
Bradycardia
Nausea Hypotension
(âheart rate)
(âblood pressure)
(áblood pressure)
(shortness of breath)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Findings
Complications
Published Oct 18, 2015, updated Aug 29, 2021 on www.thecalgaryguide.com
Unstable Angina/Unstable Angina Pectoris: Pathogenesis and clinical findings Primary cause:
Secondary causes:
Coronary artery vasospasm - primary or drug induced (Ex: cocaine, triptans)
Coagulopathy
(Ex: antiphospholipid antibody syndrome)
Vasculitic syndromes (Ex: Takayasu arteritis)
Authors: Marisa Vigna Ryan Wilkie Yan Yu* Reviewers: Julena Foglia Davis Maclean Mehul Gupta Andrew Grant* * MD at time of publication
Atherosclerosis
Fatty plaque accumulates inside the intimal walls of arteries Coronary arterial atherosclerotic plaque rupture or erosion
Plaque disruption exposes subendothelial components of damaged vessel wall to platelets, initiating the coagulation cascade and platelet adhesion
Aggregation of platelets results in the formation of a thrombus Thrombus partially occludes blood flow through a coronary artery âmyocardial blood supply
Congenital anomalies (Ex: myocardial bridge, anomalous coronary)
Spontaneous coronary artery dissection
Increased blood viscosity (Ex: polycythemia, thrombocytopenia)
Factors thatámyocardial (cardiac muscle) oxygen demand (Ex: tachycardia, hypotension, hypertension, anemia, exertion, stress)
Coronary embolism (Ex: A. Fib, endocarditis, prosthetic valve thrombus)
áheart rate, contractility, and/or wall tension ámyocardial oxygen demand
Myocardial ischemia due to imbalance between blood supply and oxygen demand (insufficient blood/oxygen supply)
Unstable Angina/Unstable Angina Pectoris
Can be new onset angina; typically progressive in frequency, severity, or duration; can occur at rest
Subtotal occlusion of a coronary arteryà
reduced, but continued, myocardial blood supply
Maintained perfusion means cardiomyocytes are still alive and thus do not leak troponin into bloodstream
Normal serum troponin
Diaphoresis
(sweating)
Since bloodflow occurs from epicardium to endocardium, myocardial ischemia is more
pronounced in the subendocardium (region furthest away from heart’s external surface)
Sufficient blood flow is maintained in regions superficial to the subendocardium, resulting in non-transmural (partial thickness) heart wall ischemia
Non-inferior wall ischemia triggers a predominantáin sympathetic nervous system activity, given the proximity of cardiac sympathetic nerve innervation
Ischemiaâ cardiomyocyte resting membrane potential andâ action potential duration
Voltage gradient between normal and subendocardial ischemic zones creates injury currents, shifting the ST- vector on ECG
ECG: ST depression
and/or T wave inversion
Cardiac sensory nerve fibres mix with somatic sensory nerve
fibres and enter the spinal cord via the T1-T4 nerve roots
Brain perceives increased cardiac sensory nerve signaling as nerve pain coming from the skin of T1-T4 dermatomes (“Referred Pain”)
Myocardial ischemia causes hypoxic stress on cardiomyocytesàâaerobic (requiring oxygen) metabolism,áanaerobic (not requiring oxygen) metabolism
áanerobic respirationálactic acid production,á[H+], andâcellular pH which impairs cardiomyocyte function
Cardiomyocyte dysfunction impairs myocardial relaxation in diastole and/orâ left ventricular contractility in systole
âleft ventricular cardiac output àbackup of blood in the left ventricle, atrium, and pulmonary vasculature
ápulmonary capillary pressures pushes fluid out of the capillaries into the alveoli in the lungs
Fluid filled alveoliâgas exchange andâ oxygenation, triggering harder and faster breathing in order to compensate
Dyspnea
Activation of sweat glands via acetylcholine release
Hormones bind to cardiac β1 receptors
Tachycardia
(áheart rate)
Epinephrine/ Norepinephrine hormone release from the adrenal medulla
Hormones bind to arterial smooth muscle α1 receptors ávascular tone (vasoconstriction)
Hypertension
The Vagus nerve sits in close physical proximity to the inferior wall of the heart àinferior wall ischemia triggers involuntary Vagus nerve activation
Since the Vagus nerve coordinates parasympathetic activity,áVagus nerve activity leads to a variety of parasympathetic nervous system responses:
Retrosternal discomfort: May present as pain, heaviness, tightness, aching, pressure, burning or squeezing
Pain radiation to T1-T4 dermatomes:
Left shoulder and arm, lower jaw, neck, abdomen, upper back
Syncope
(fainting)
Bradycardia
Nausea Hypotension
(âheart rate)
(âblood pressure)
(áblood pressure)
(shortness of breath)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Findings
Complications
Published Oct 18, 2015, updated Aug 29, 2021 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/10/Unstable-Angina-2021.jpg)




























![Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com
Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/10/Hypomagnesia.jpg)







![Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com
Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/11/Calcium-Channel-Blockers.jpg)










![Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/06/Obesity-Hypoventilation-Syndrome.jpg)

![Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/08/Hypocalcemia-Pathogenesis.jpg)












