SEARCH RESULTS FOR: stroke
Myocardial Infarction: Findings on History
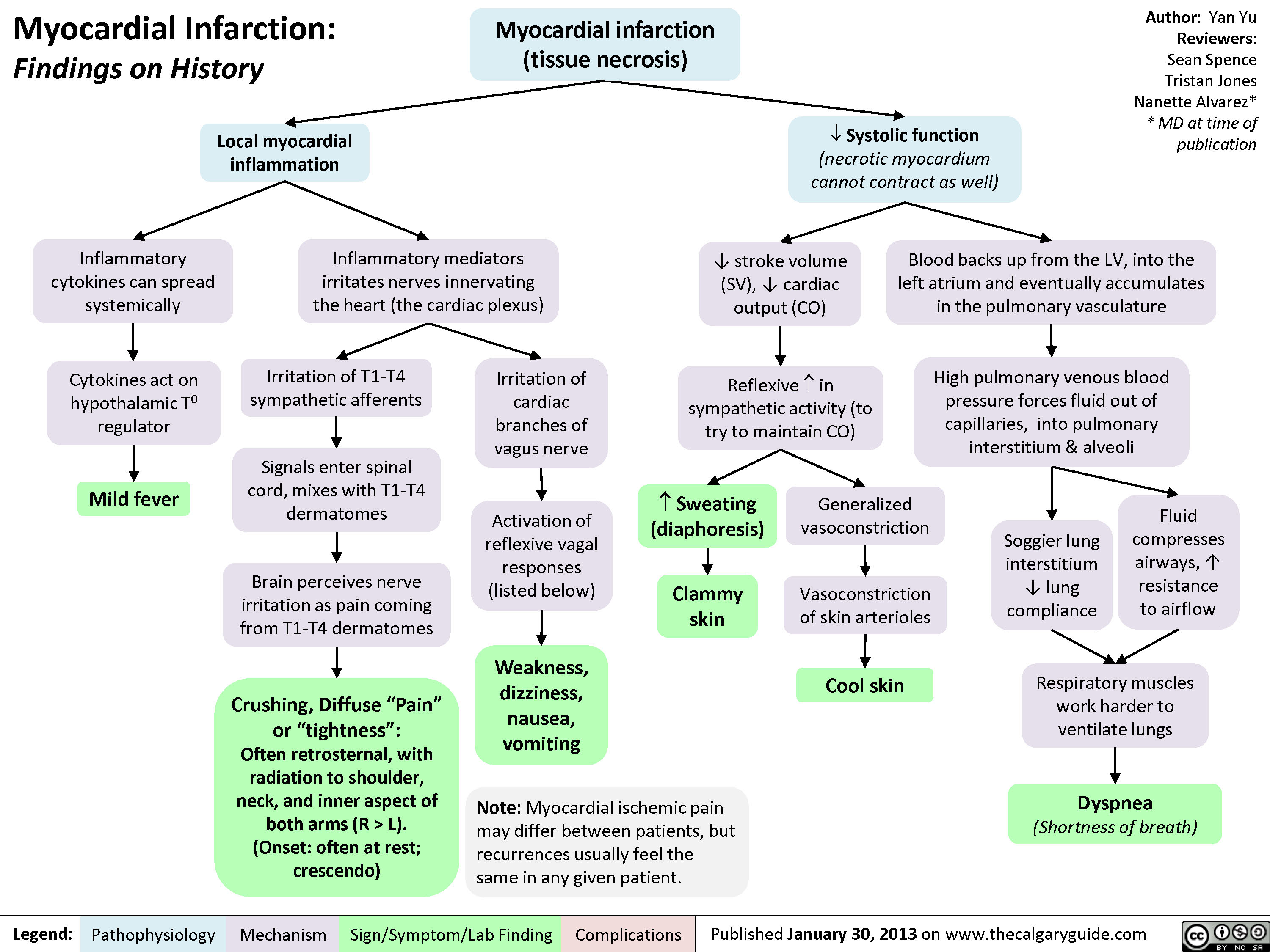 L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" title="Yu Yan - MI Findings on History - FINAL.pptx -
Myocardial Infarction: Findings on HistoryLegend:Published January 30, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsAuthor: Yan YuReviewers:Sean SpenceTristan JonesNanette Alvarez** MD at time of publication Systolic function(necrotic myocardium cannot contract as well)Reflexive ? in sympathetic activity (to try to maintain CO)Clammy skin? stroke volume (SV), ? cardiac output (CO)Myocardial infarction (tissue necrosis)Note: Myocardial ischemic pain may differ between patients, but recurrences usually feel the same in any given patient.Generalized vasoconstrictionVasoconstriction of skin arteriolesCool skinLocal myocardial inflammationIrritation of T1-T4 sympathetic afferentsIrritation of cardiac branches of vagus nerveSignals enter spinal cord, mixes with T1-T4 dermatomesCrushing, Diffuse "Pain" or "tightness": Often retrosternal, with radiation to shoulder, neck, and inner aspect of both arms (R > L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" />
L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" title="Yu Yan - MI Findings on History - FINAL.pptx -
Myocardial Infarction: Findings on HistoryLegend:Published January 30, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsAuthor: Yan YuReviewers:Sean SpenceTristan JonesNanette Alvarez** MD at time of publication Systolic function(necrotic myocardium cannot contract as well)Reflexive ? in sympathetic activity (to try to maintain CO)Clammy skin? stroke volume (SV), ? cardiac output (CO)Myocardial infarction (tissue necrosis)Note: Myocardial ischemic pain may differ between patients, but recurrences usually feel the same in any given patient.Generalized vasoconstrictionVasoconstriction of skin arteriolesCool skinLocal myocardial inflammationIrritation of T1-T4 sympathetic afferentsIrritation of cardiac branches of vagus nerveSignals enter spinal cord, mixes with T1-T4 dermatomesCrushing, Diffuse "Pain" or "tightness": Often retrosternal, with radiation to shoulder, neck, and inner aspect of both arms (R > L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" />
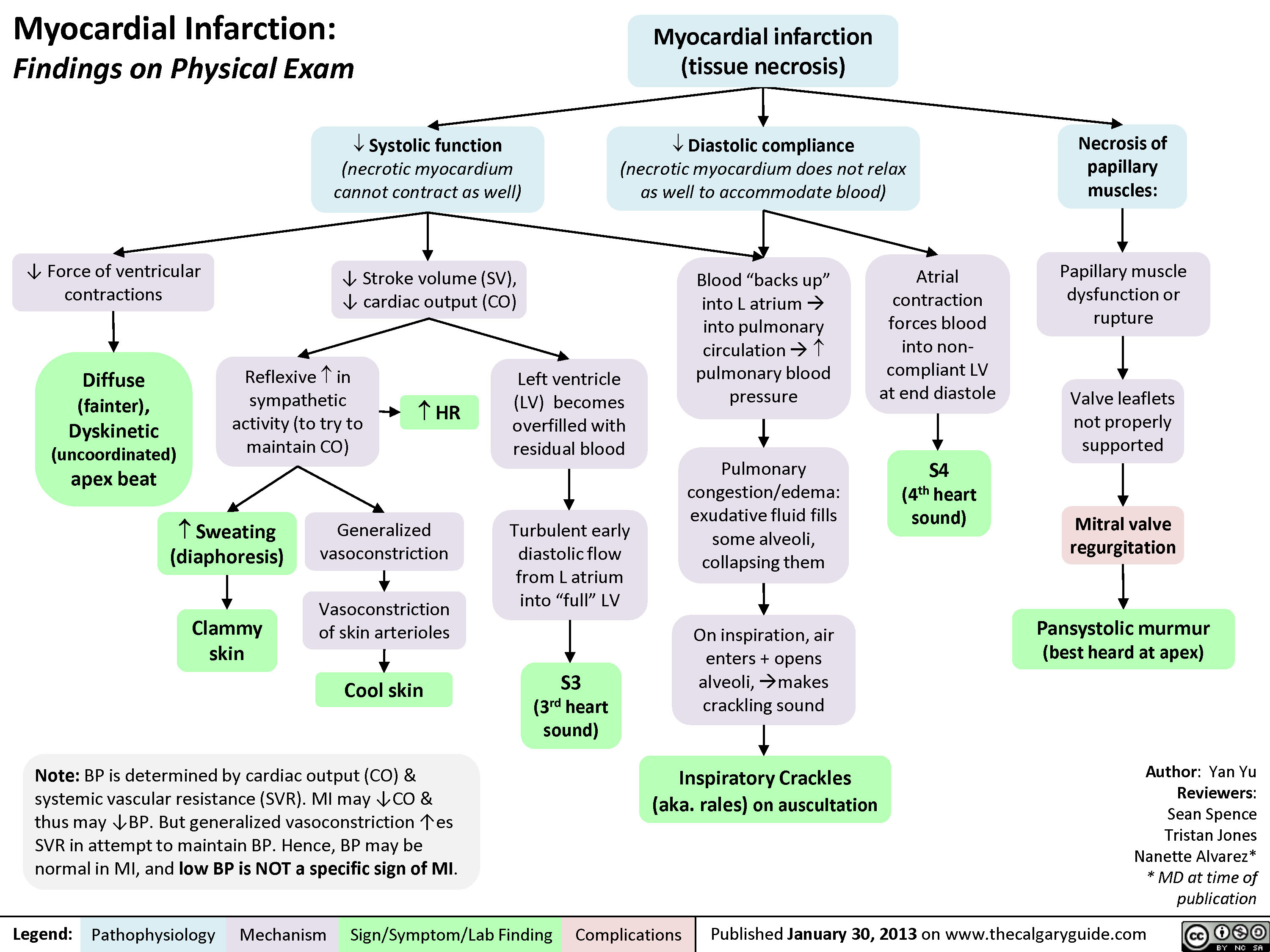
myocardial-infarction-findings-on-physical-exam
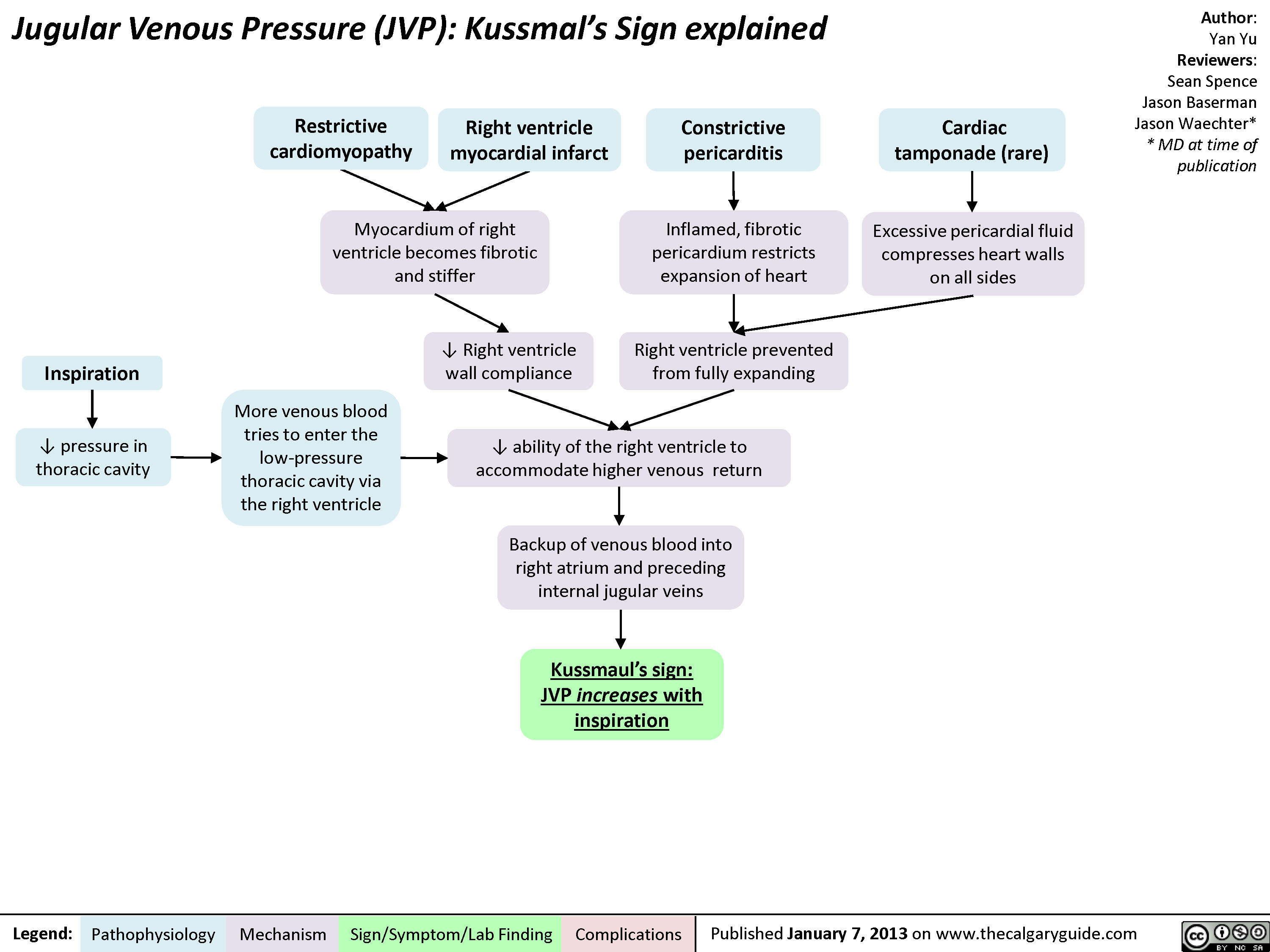
jvp-kussmals-sign-explained
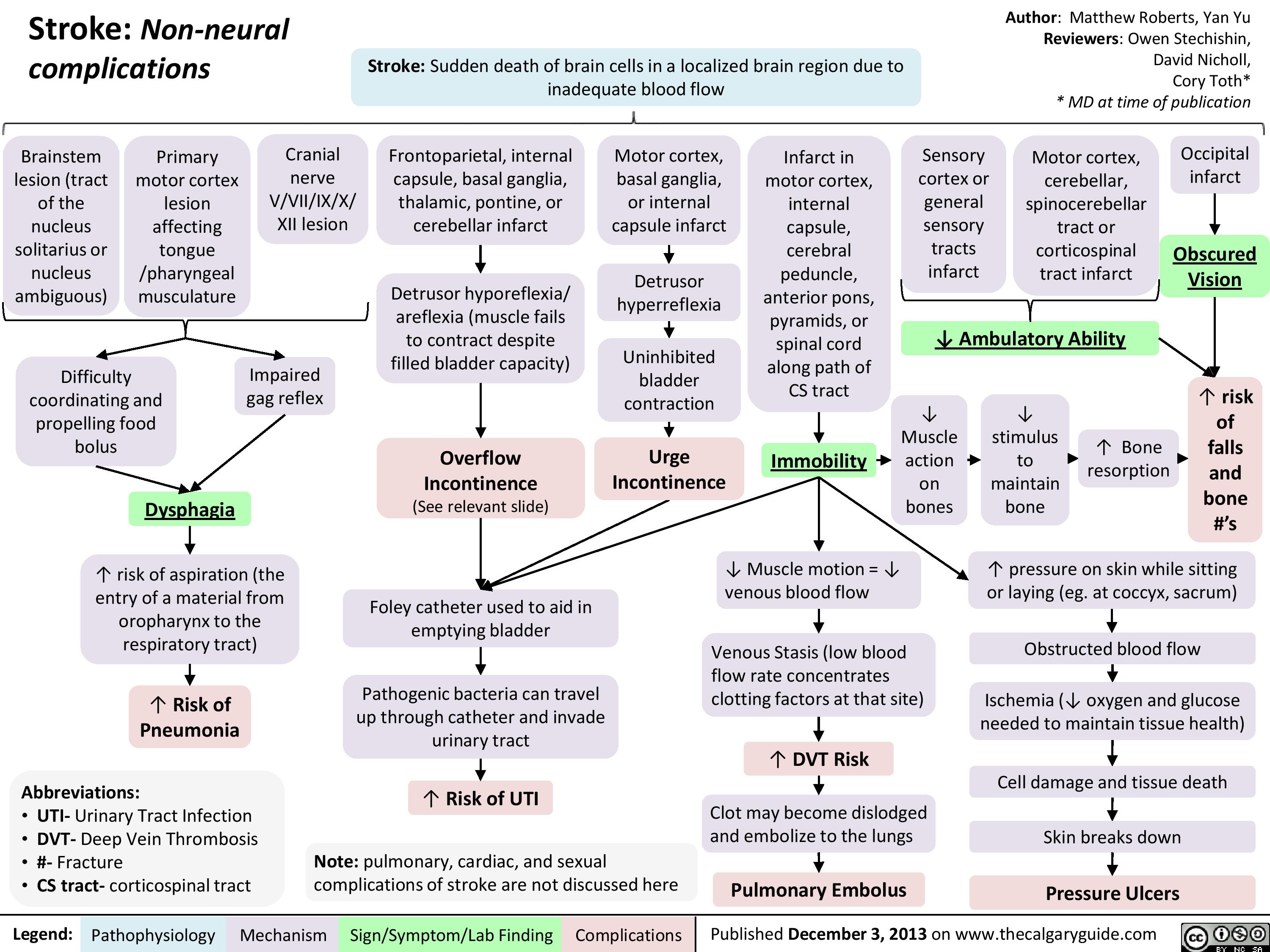
Non Neural Complications of Stroke
Stroke - Pathogenesis
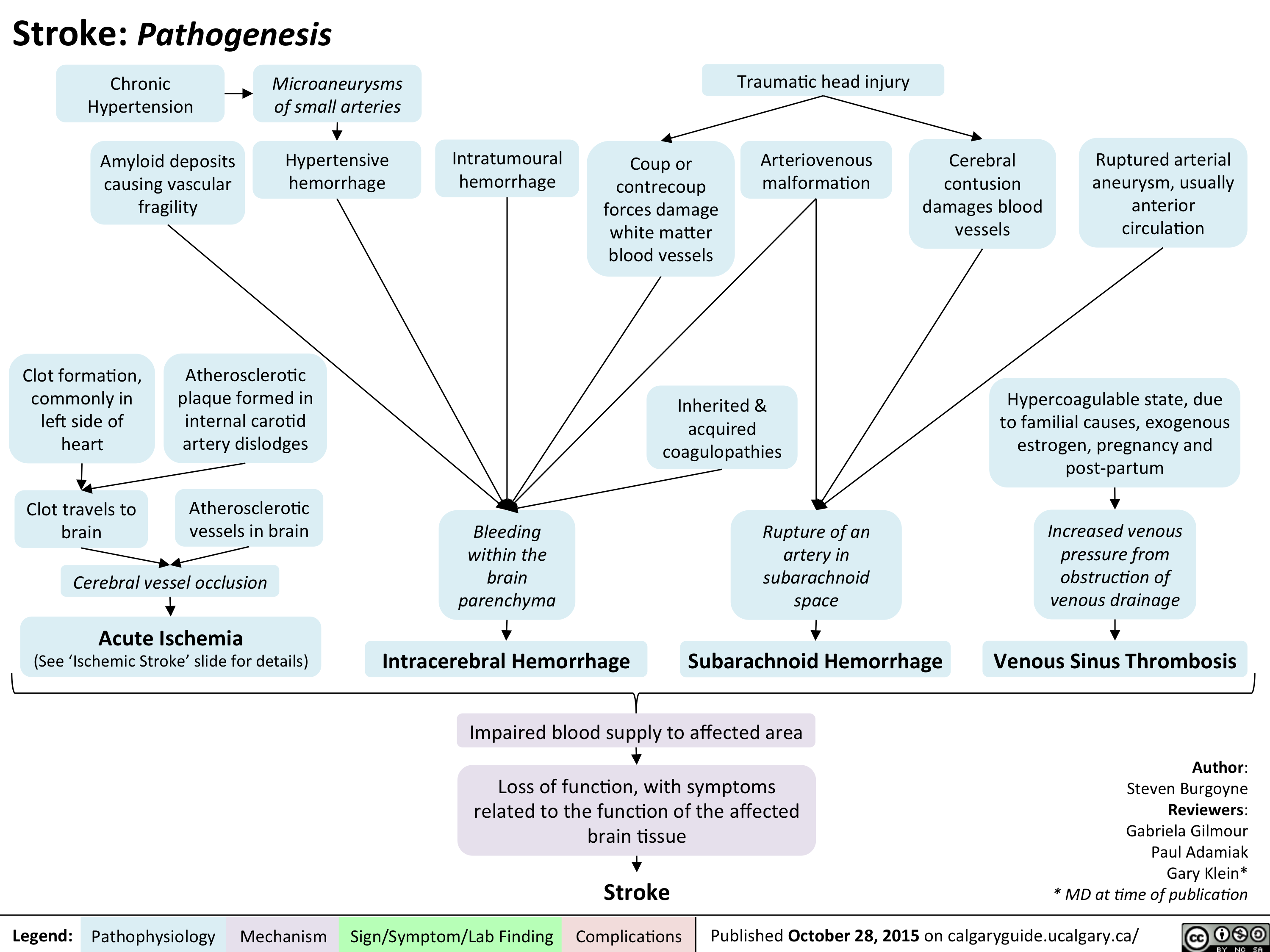
Left Heart Failure: Pathophysiology (Neurohormonal Activation)
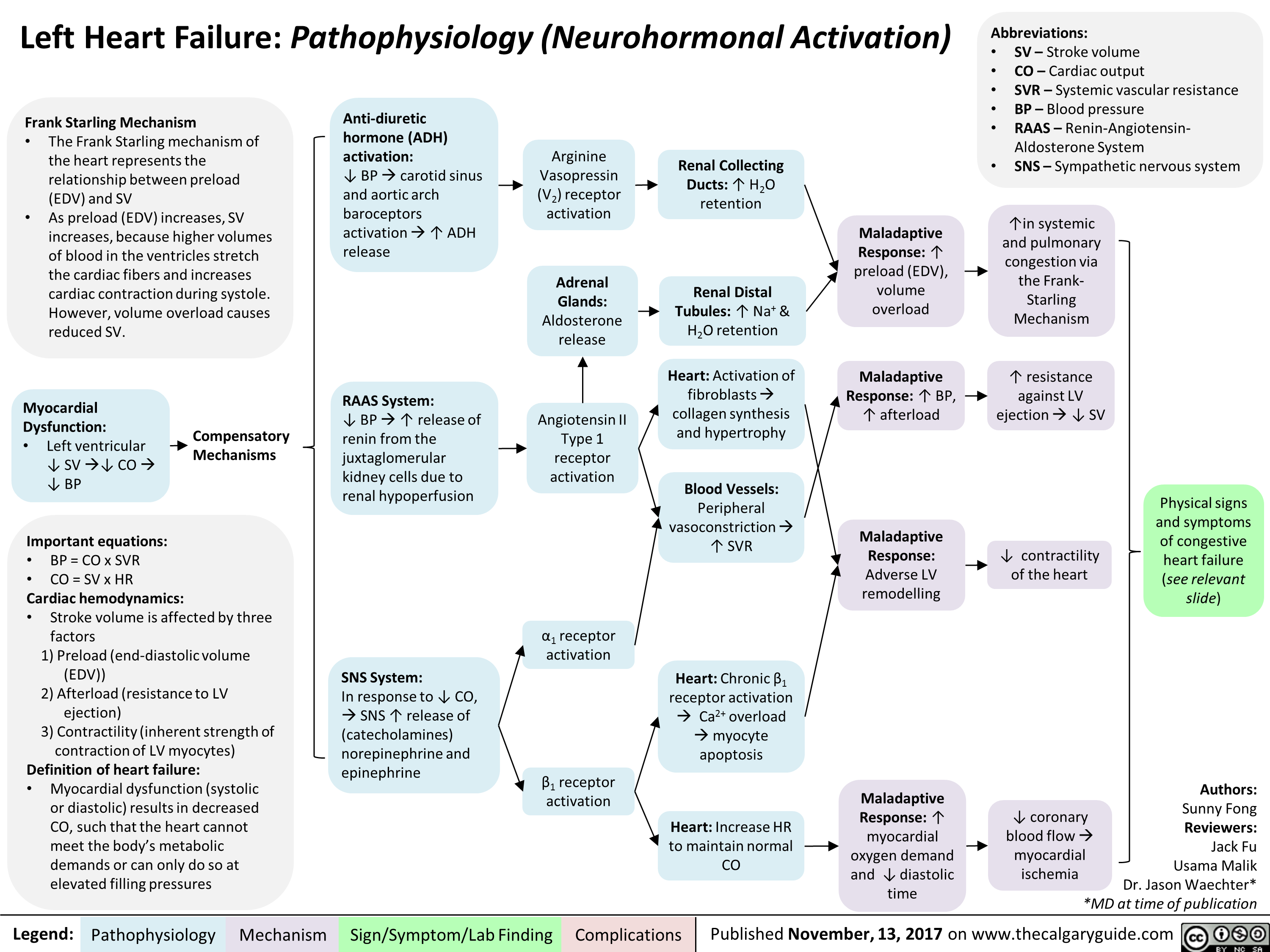
Benzodiazepine (BZD) withdrawal: clinical findings and complications
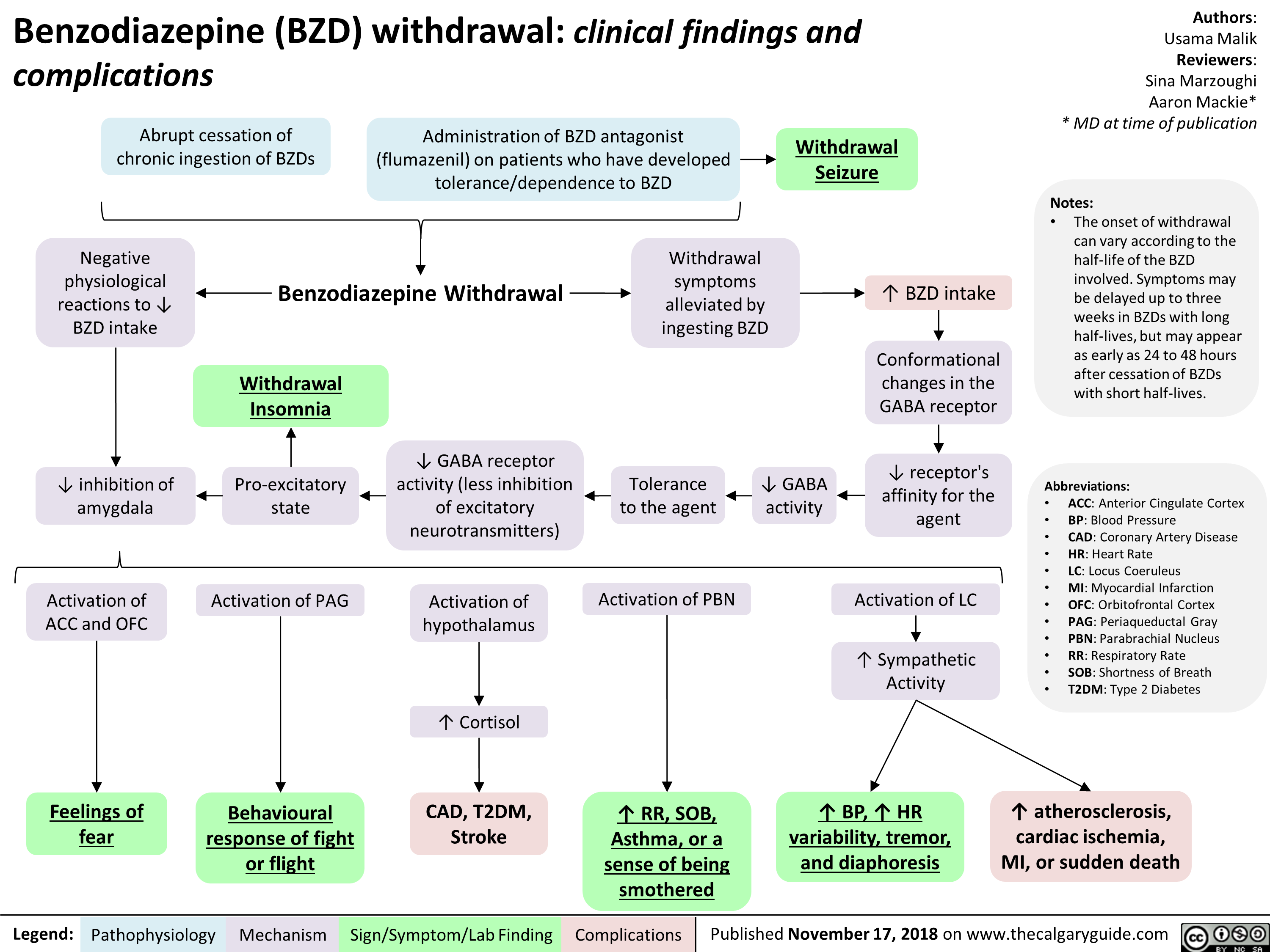
Arterial Insufficiency- Signs and symptoms
Reactive Neutrophilia- Pathogenesis and Clinical Findings
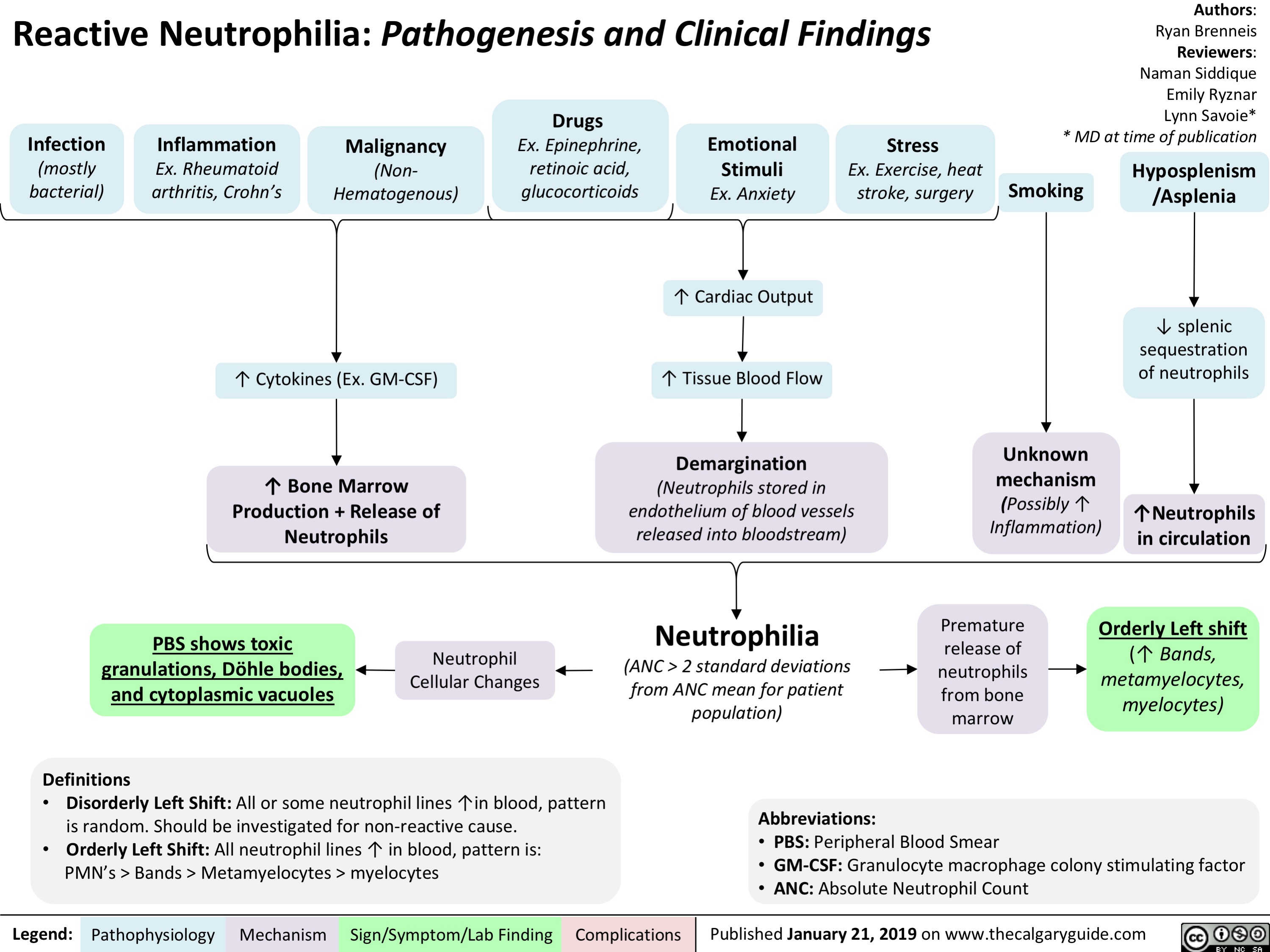
adrenergic-agonists-for-treating-hypotensionlow-blood-pressure
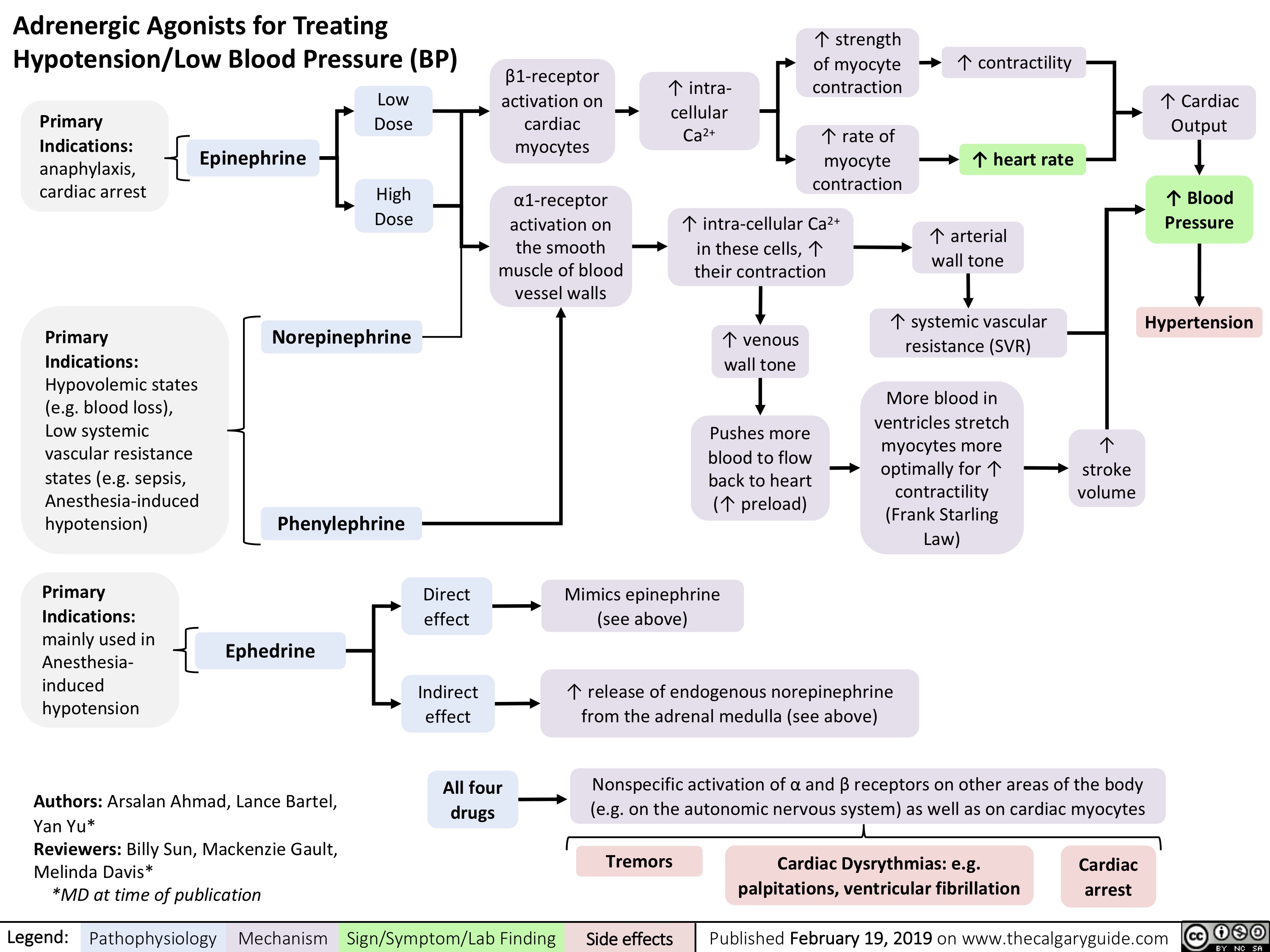
virchows-triad-and-deep-vein-thrombosis-dvt
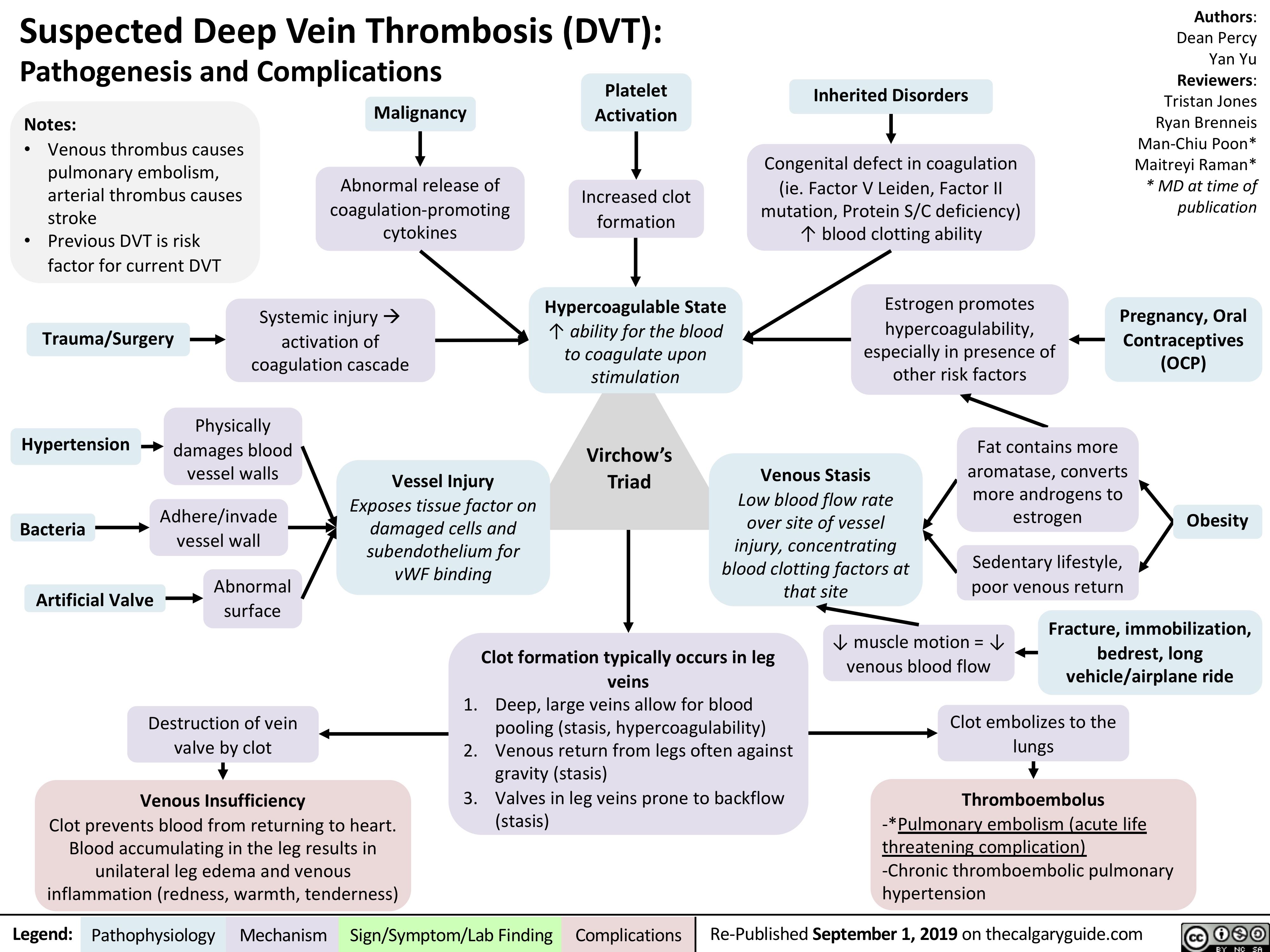
Aphasia
vomiting-pathogenesis
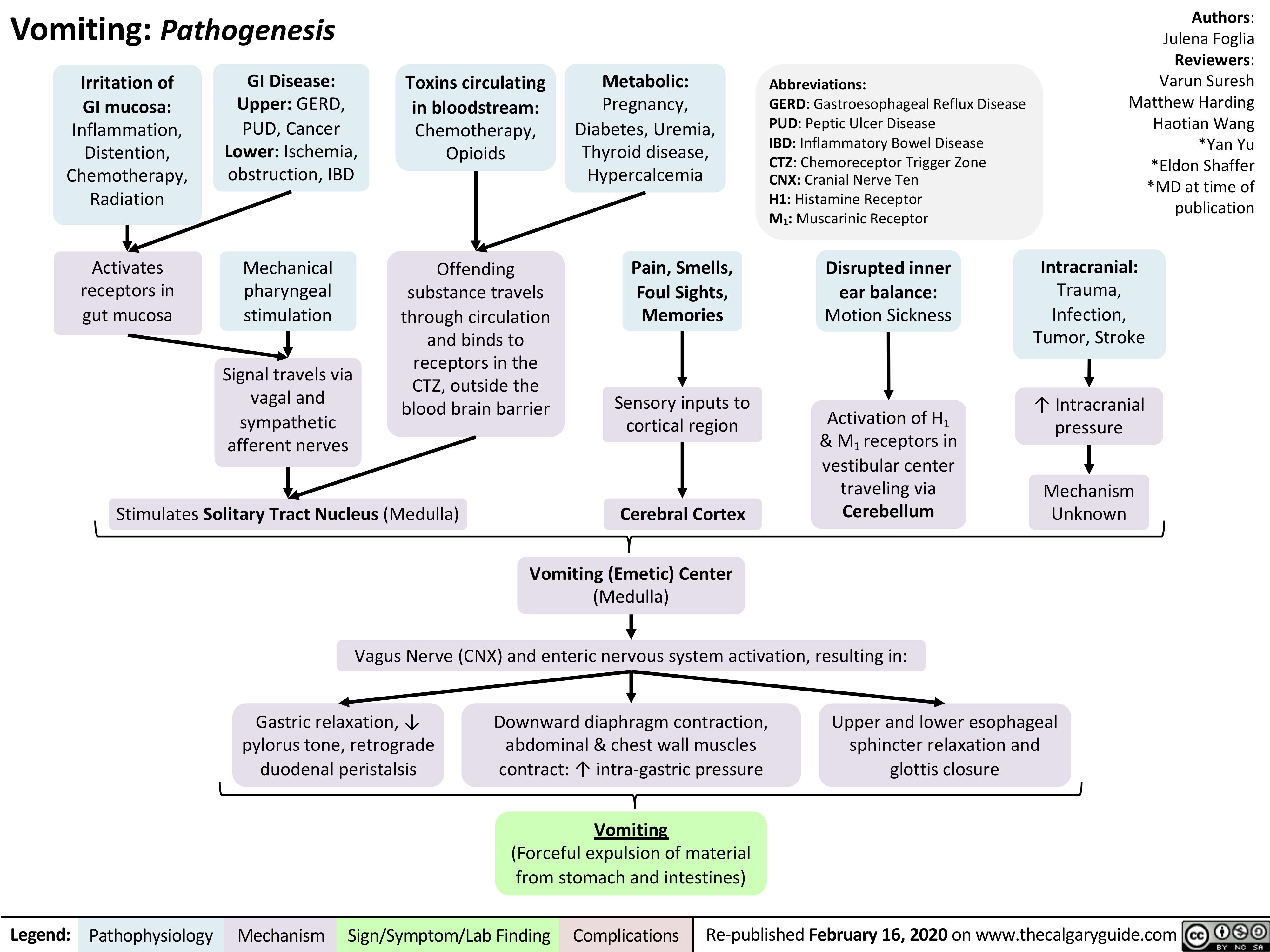
Marfan-Syndrome
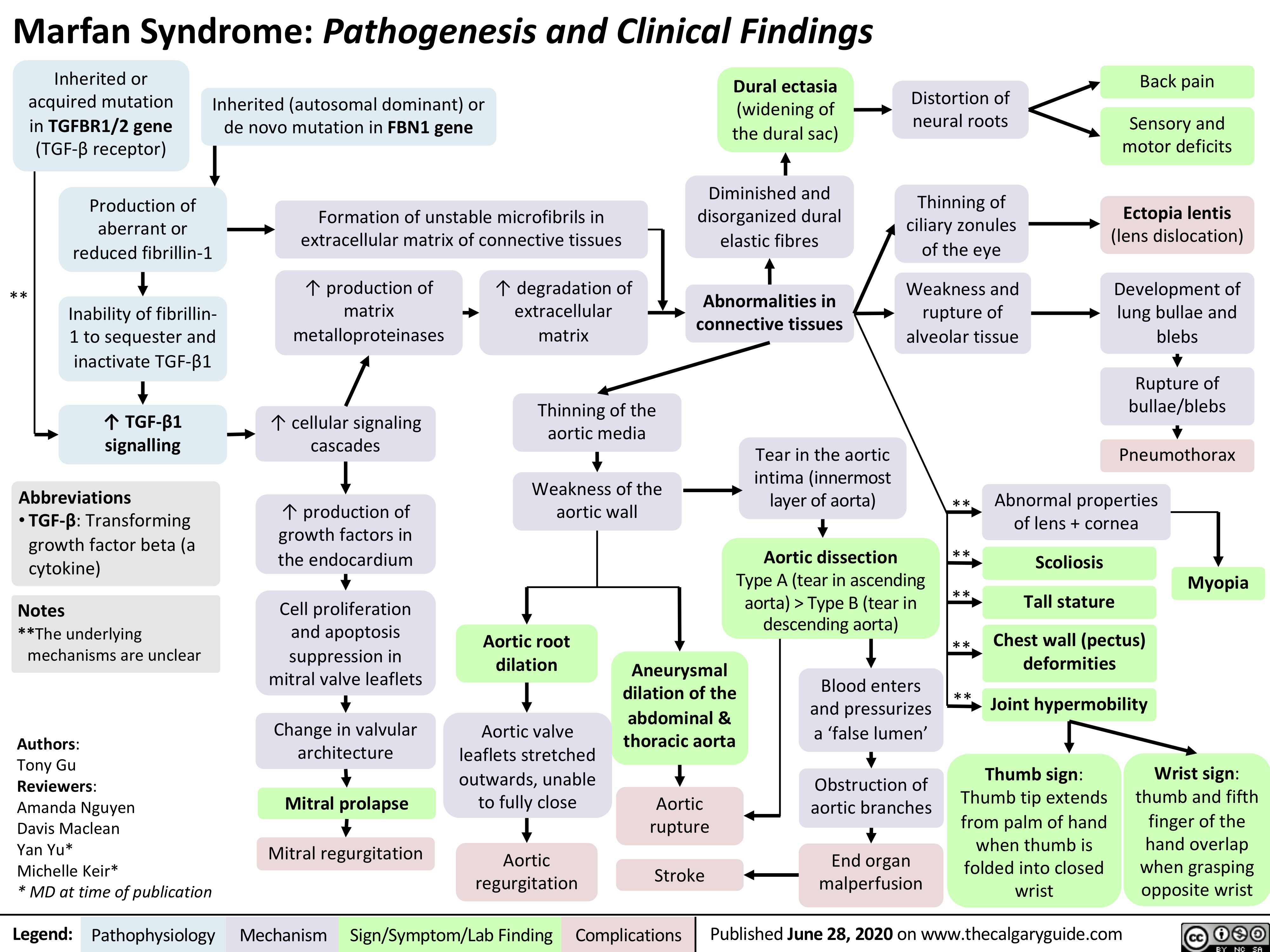
Hereditary Hemorrhagic Telangiectasia (Osler-Weber-Rendu disease)
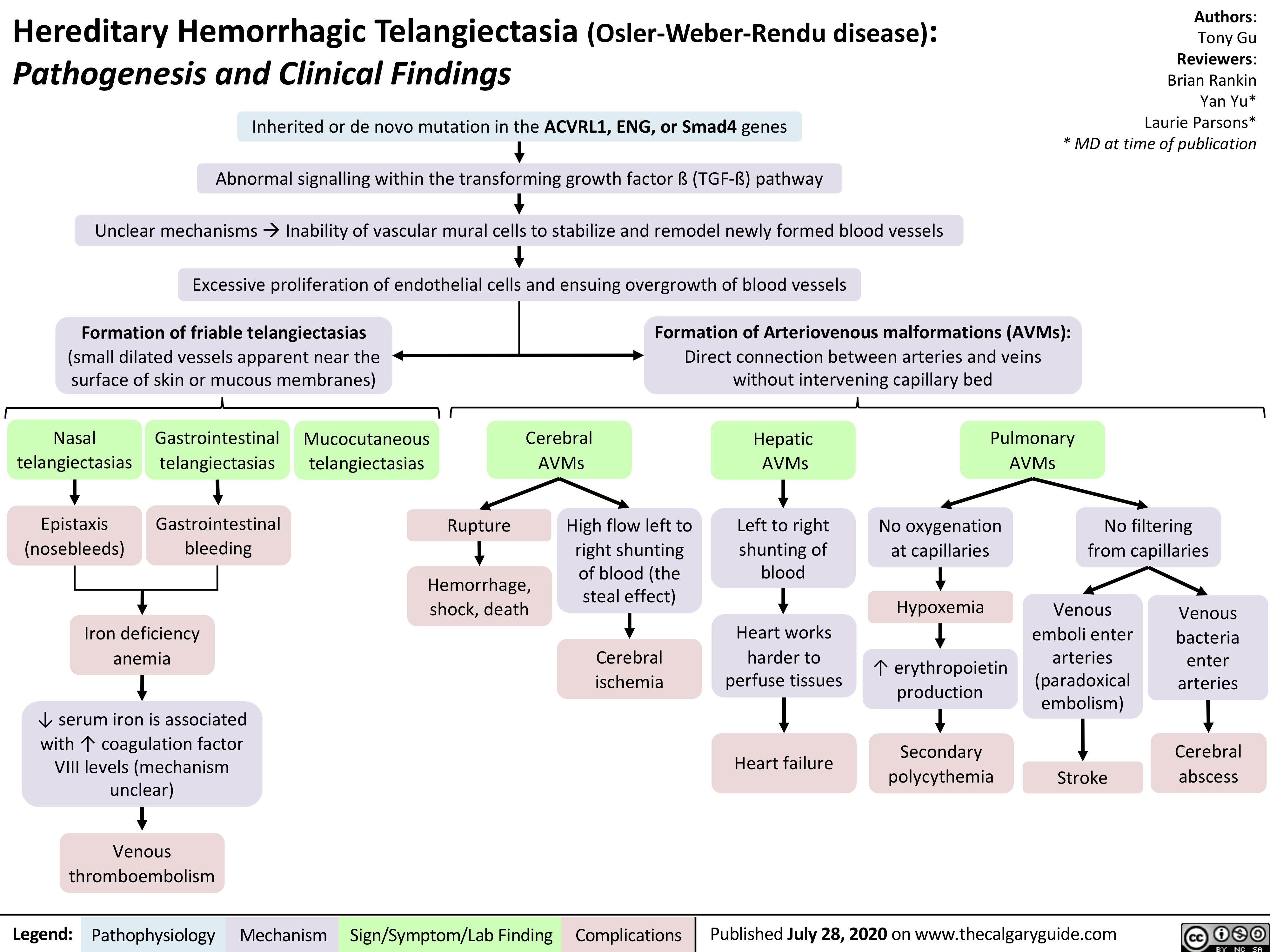
Anesthetic-Considerations-Aortic-Stenosis
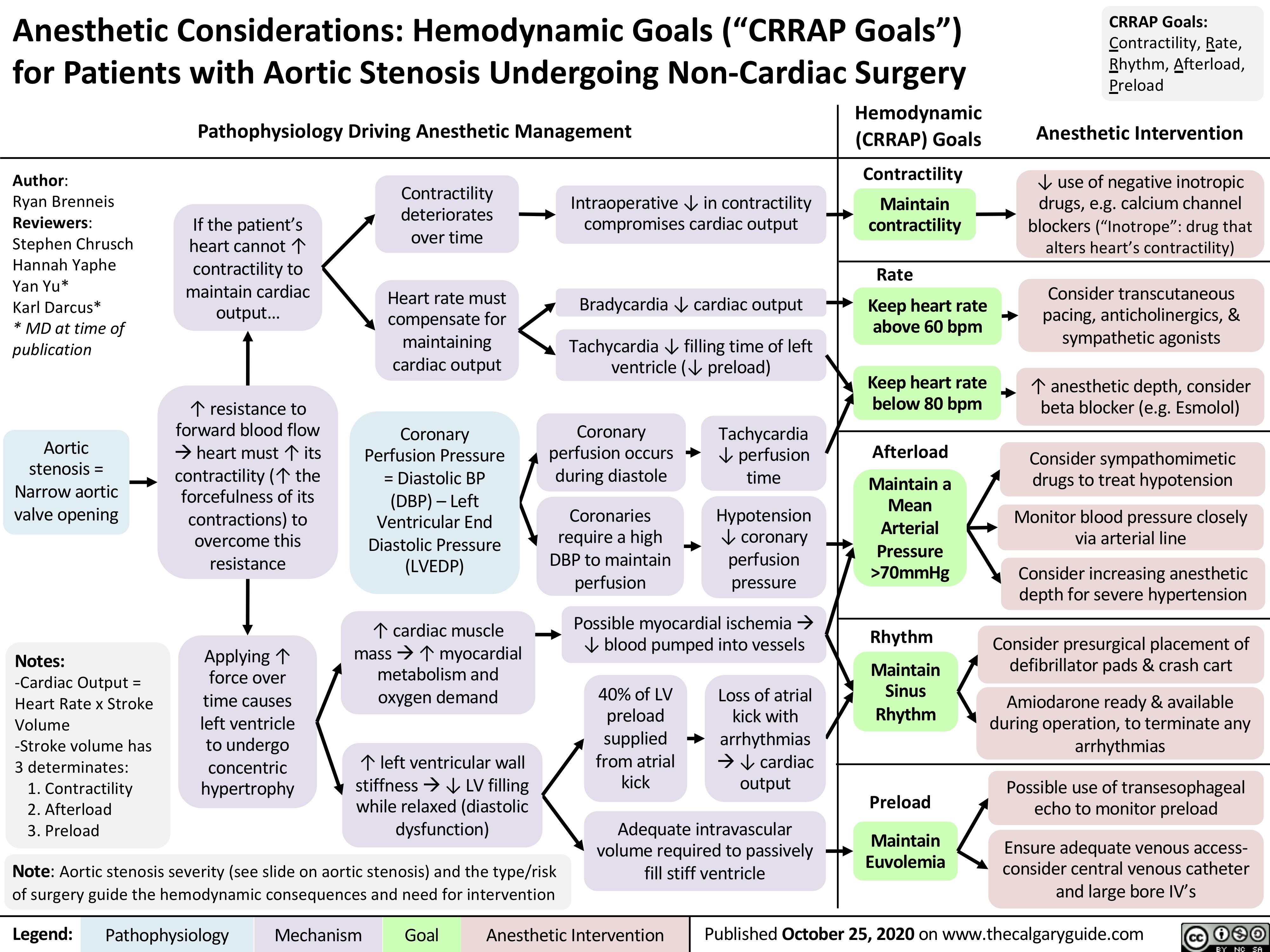
Beta-Blockers-Mechanism-of-Action-and-Side-Effects
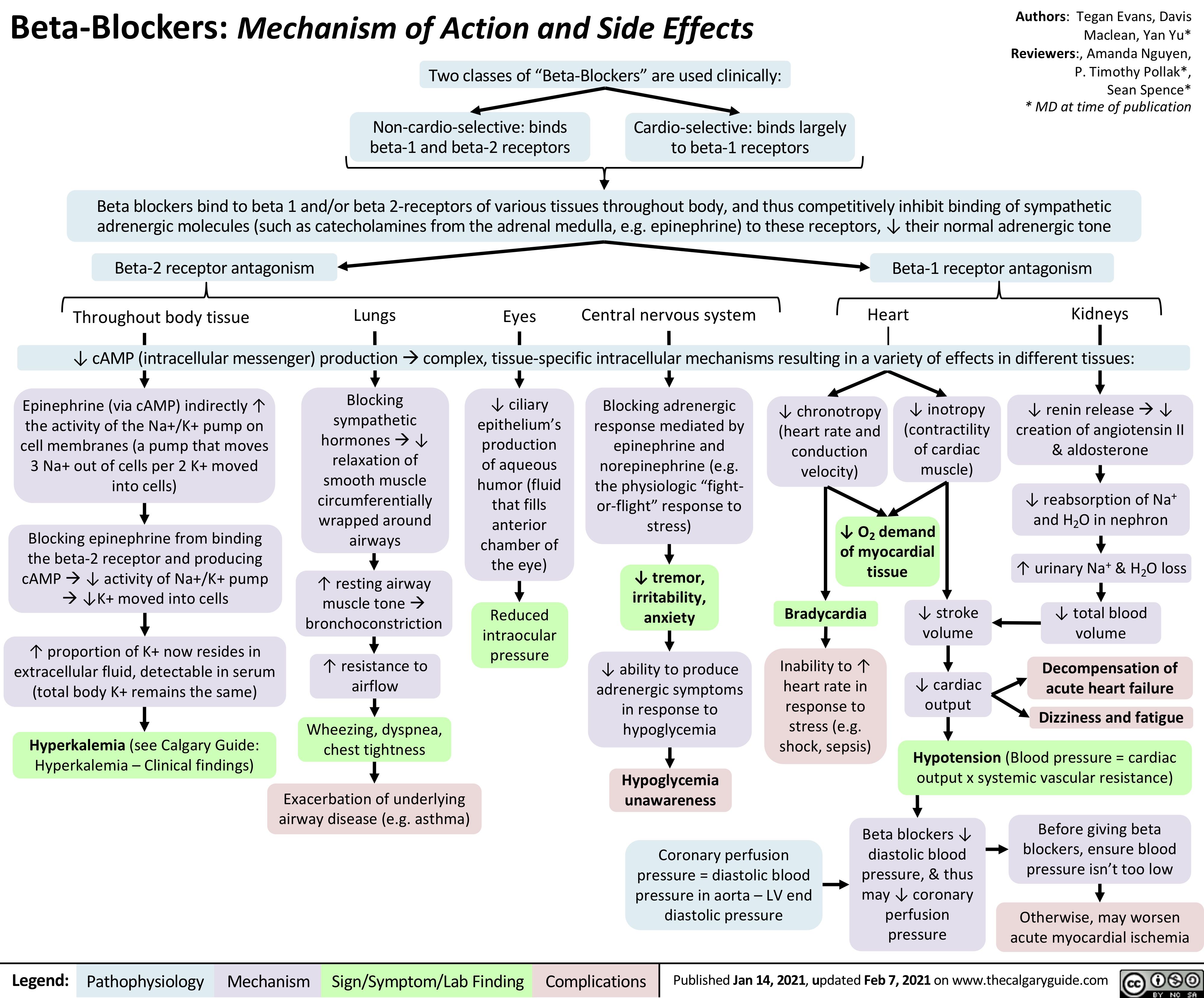
acute-mca-territory-ischemic-stroke-findings-on-non-contrast-ct
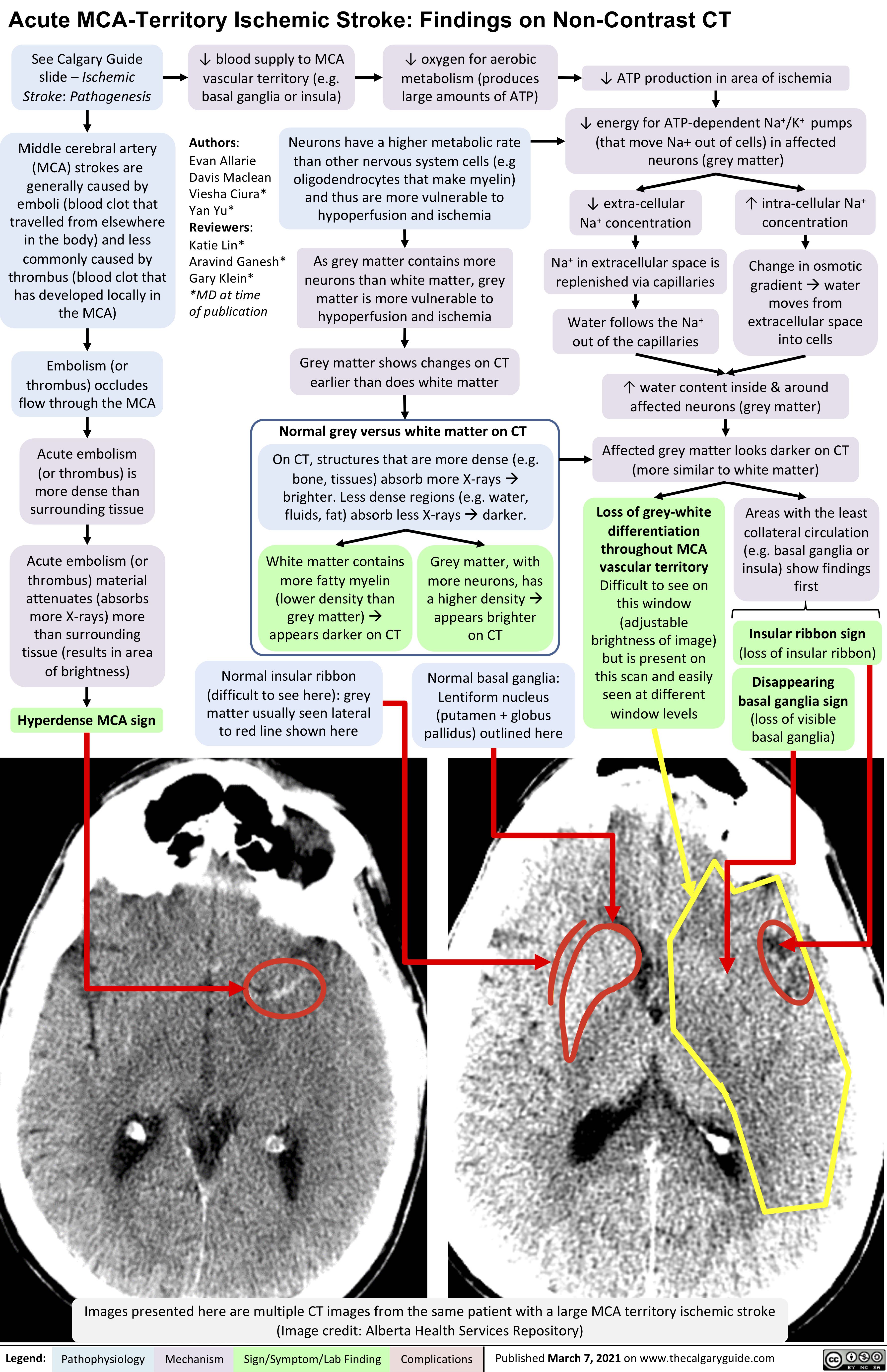
VITT
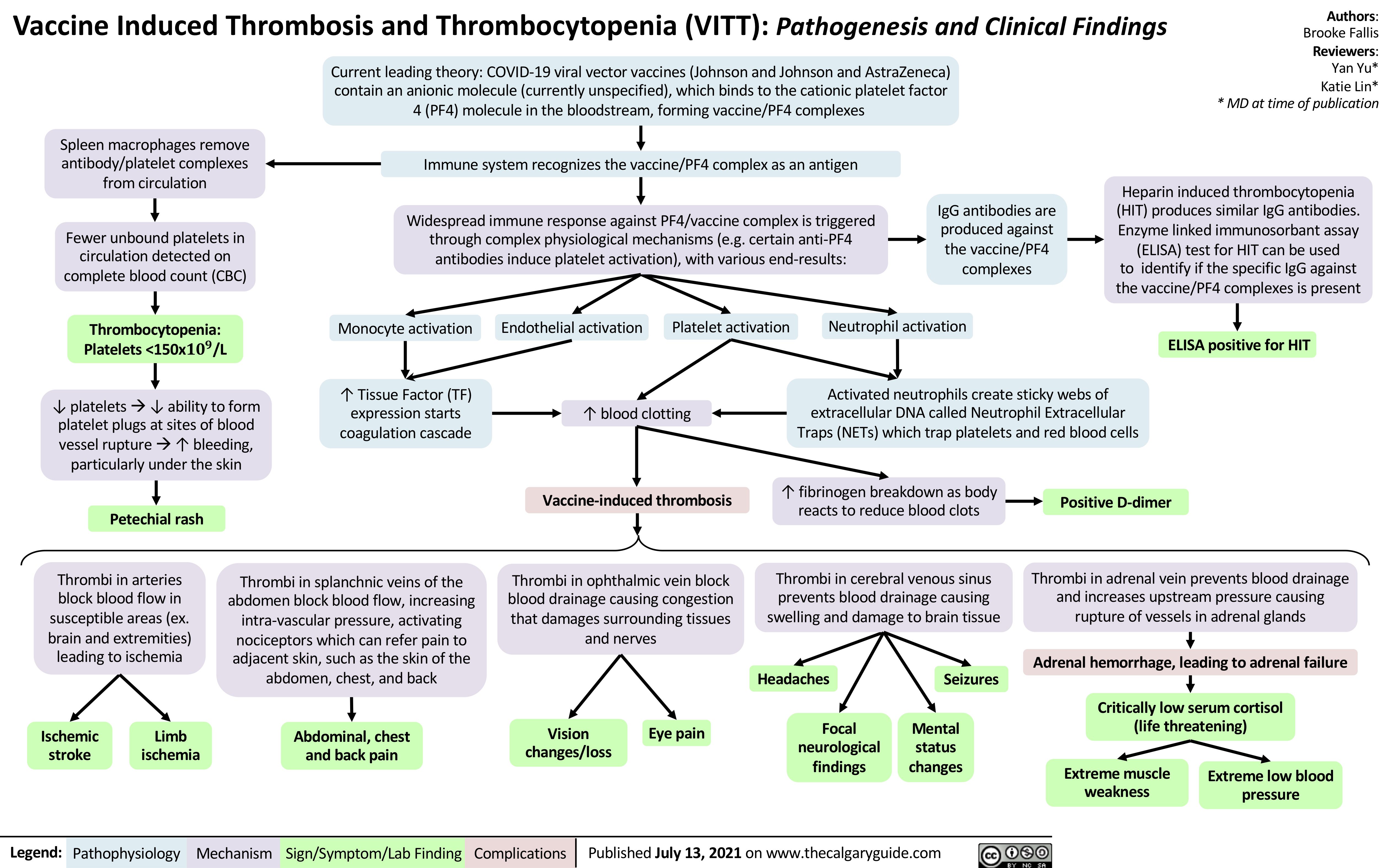
disseminated-intravascular-coagulation
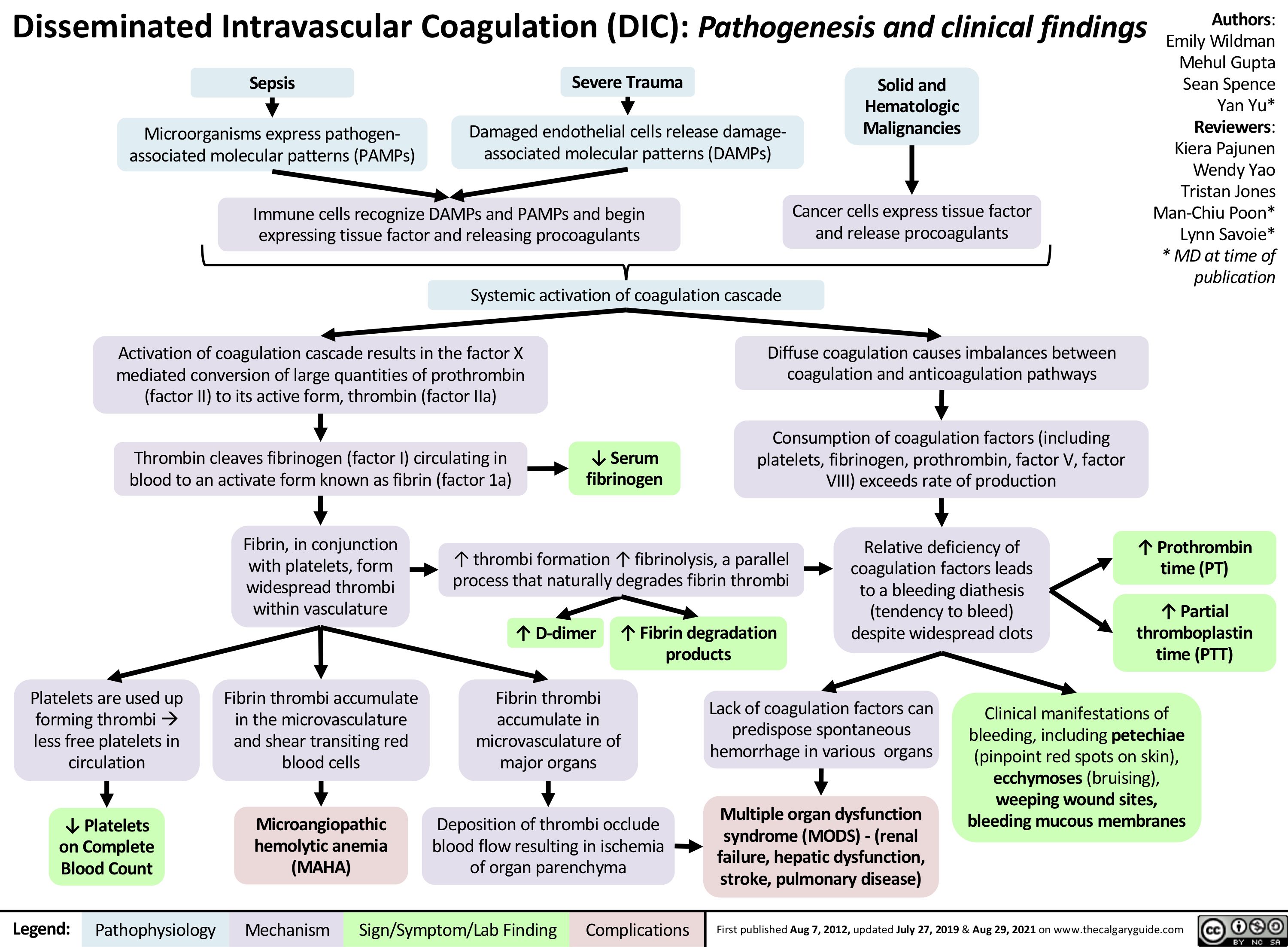
chronic-hypertension-complications
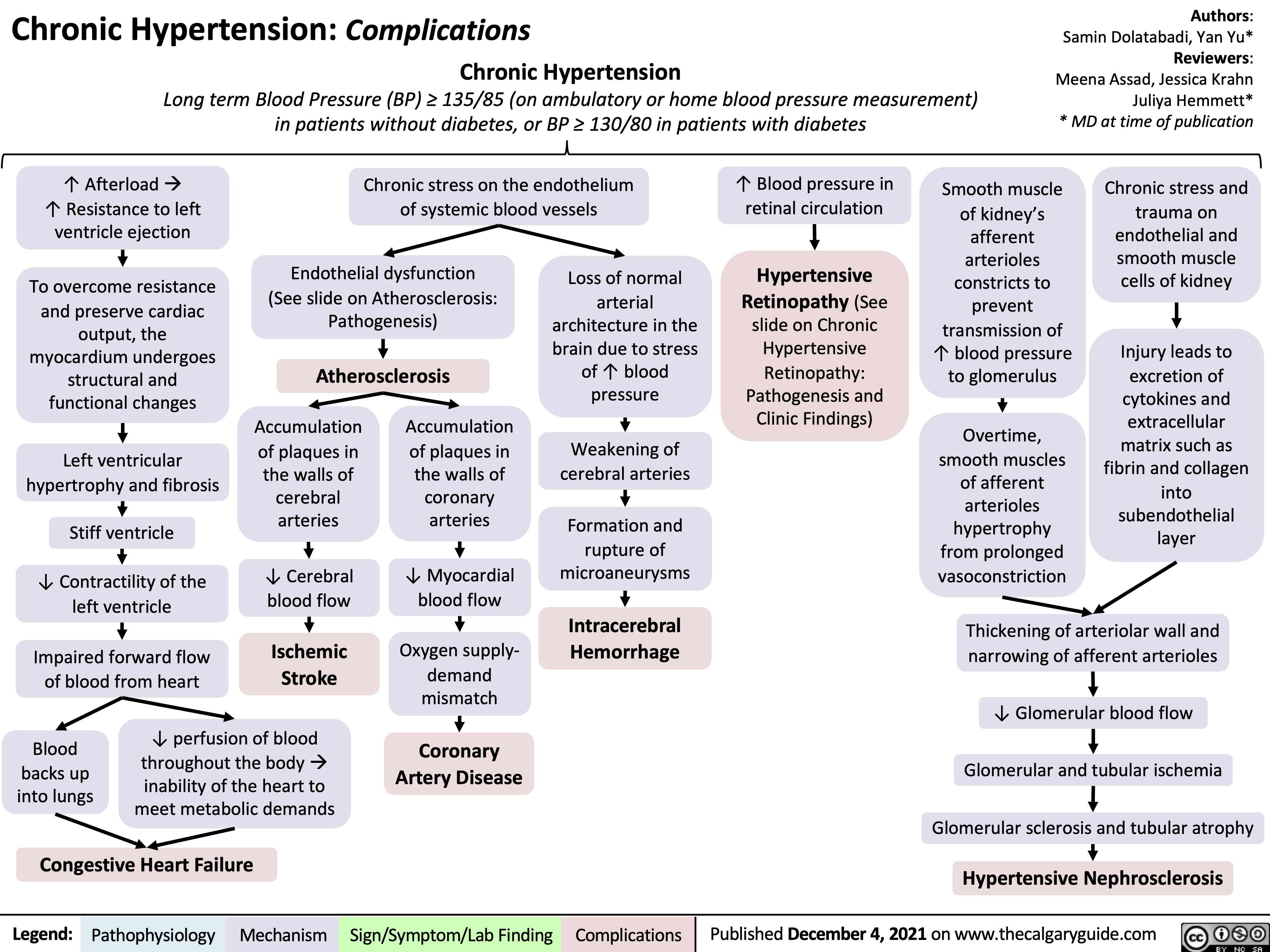
Syndrome of Inappropriate Anti-Diuretic Hormone SIADH Pathogenesis and Clinical Findings
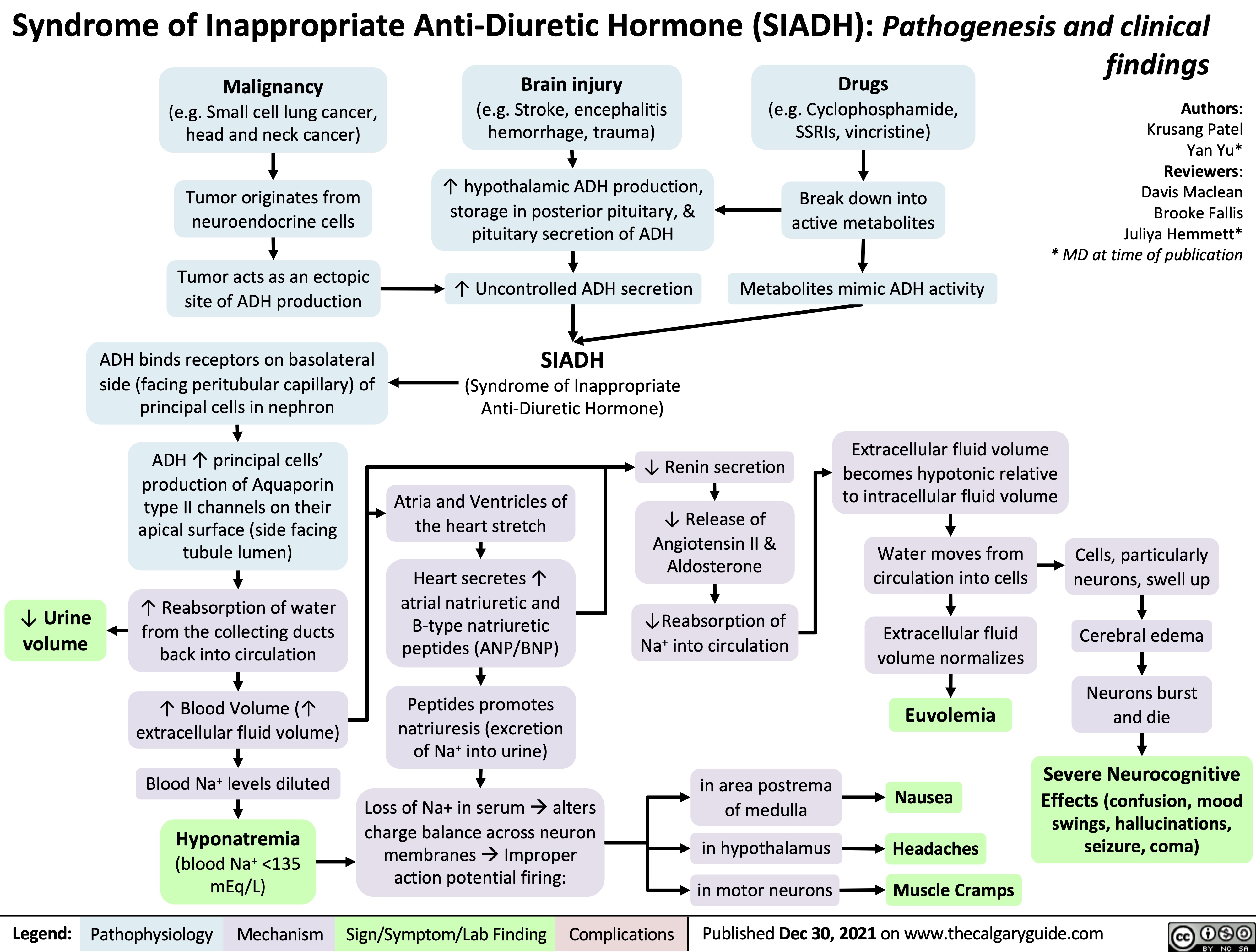
Renal Artery Stenosis
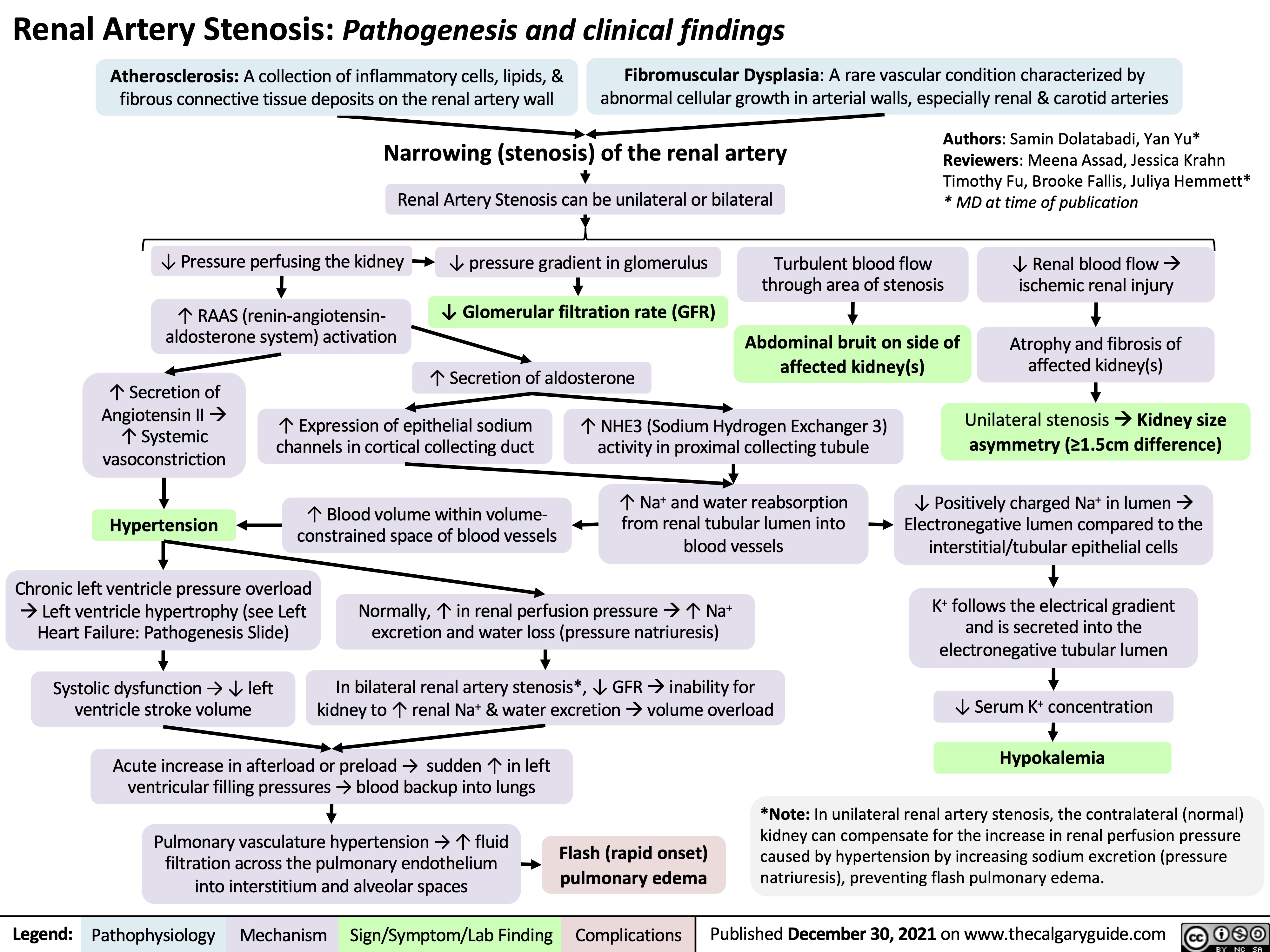
obstructive-sleep-apnea-pathogenesis-and-clinical-findings
![Obstructive Sleep Apnea: Pathogenesis and clinical findings
Vascular Factors: During recumbent sleep, more bodily fluids enter the head and neck area (compared to when the patient is standing/sitting)
↑ volume of head/neck tissue surrounding the upper airwayà possible airway obstruction
Authors: Ciara Hanly Austin Laing Alexander Arnold Reviewers: Steven Liu Amogh Agrawal Yonglin Mai (麦泳琳) Naushad Hirani* Yan Yu* *MD at time of publication
Neuromuscular Factors: Sleep onset and/or the sleeping state reduces the drive of respiratory muscles to breathe
↓ Upper airway neuromuscular activityà↓ upper airway caliber, ↑ upper airway resistance, ↑ upper airway collapsibility during sleep
Structural Factors: Obesity, tonsillar or adenoid hypertrophy, macroglossia, ↑ neck circumference, craniofacial abnormalities
Excess pressure on upper airway, or deformity to that area, ↑ risk of upper airway collapse
Polysomnography
Absence of airflow but persistent ventilatory effort
Hypopnea or Apnea
Paradoxical breathing Chest wall draws in and abdomen expands during inspiration
Ventilatory effort persists against closed airway
No air entry due to collapsed upper airway
↑ Negative intrathoracic pressure
↑ Venous return to right atrium
Stretching of right atrial myocardium à secretion of atrial natriuretic peptide (ANP)
ANP inhibits epithelial Na+ channels (ENaC) in the collecting ducts of the kidney from reabsorbing Na+ à Na+ excretion
↑ Na+ excretionà↑ water excretion
Nocturia
Complete or partial upper airway obstruction during sleep
↑ PCO2 & ̄ PO2
in the lungsà ̄ diffusion gradient of CO2 & O2 between lungs & arteries
↑ PaCO2,, ̄ PaO2
Respiratory acidosis (↑ [H+] in blood)àactivation of vascular endothelial voltage gated K+ channels
Cerebral blood vessel dilation to provide adequate O2 to brain
Morning Headaches
Activation of central (medulla oblongata) & peripheral (carotid body) chemoreceptors
↑ Respiratory drive à ↑ activation of respiratory muscles (ventilatory effort )
Transient arousal from sleep
↑ sympathetic nervous system activityà arterial vasoconstriction
↑ systemic vascular resistance
Systemic Hypertension
↑ intraluminal pressure within blood vesselsàadaptive vascular endothelial and smooth muscle changes
Artery walls thicken, harden and lose elasticityà ̄ perfusion to end organs (such as the brain)
Ischemic stroke
Hypoxia during the day and night
↑ pulmonary vascular resistance
Pulmonary Hypertension
Right heart pumps against higher pulmonary pressure àcardiomyocytes undergo concentric hypertrophy over time
Cor Pulmonale
(Right heart failure due to pulmonary hypertension, separate from left heart failure)
Respiratory muscles overcome upper airway obstructionà airway patency restored
Sleep fragmentation
̄ Daytime cognitive performance and attentiveness
↑ Risk of motor vehicle accidents
Daytime Sleepiness
Eg. Epworth Sleepiness Scale >10
Abbreviations:
PCO2: partial pressure of carbon dioxide PO2: partial pressure of oxygen PaCO2: partial pressure of carbon dioxide in arteries PaO2: partial pressure of oxygen in arteries
Ventilatory response overcompensatesà breathe out more CO2 than is required for homeostasisà ̄ PaCO2
̄ respiratory driveà ̄ ventilatory effort
Resuscitative Gasping
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 19, 2013, updated May 31, 2022 on www.thecalgaryguide.com
阻塞性睡眠呼吸暂停:发病机制及临床表现
作者:Ciara Hanly, Austin Laing, Alexander Arnold 审稿人: Steven Liu, Amogh Agrawal, Naushad Hirani*,Yan Yu* 译者: Zesheng Ye(叶泽生) 翻译审稿人: Yonglin Mai(麦泳琳) *发表时担任临床医生
神经肌肉因素: 睡眠状态下, 患者无法通过 适当增加上气道肌张力来维持气道通畅
上气道神经肌肉活动 ̄à上气道直径 ̄, 上气道 阻力↑, 睡眠时上气道塌陷
结构(解剖)因素: 肥胖、扁桃体或腺样体 肥大, 舌体肥大, 颈围增大, 颅面部畸形
上气道压力过大或上气道畸形, 上气道塌陷 的风险 ↑
血管因素: 仰卧位睡觉引起 夜间嘴侧液体移位
周围组织与压力 ↑à上气道阻塞
多导睡眠描记术
没有气流,但持
续通气
呼吸浅慢或 呼吸暂停
反常呼吸 吸气时胸壁凹陷, 腹部膨隆
持续通气以抵抗气道 闭合
上气道塌陷导致空气进
入气道受阻
腹膜腔负压↑ 静脉血回流右心室阻力↑
右心房心肌细胞拉伸 à心房利钠肽分泌 (ANP)
ANP抑制肾集合管的上 皮Na+通道(ENaC)对 Na+重吸收à Na+排出
Na+排出量↑ à 水排出量 ↑
睡眠时全部
或部分上呼
吸道阻塞
肺内PO2 ̄ 且 PCO2↑ à CO2 及 O2在肺和动脉 间的扩散梯度 ̄
↑ PaCO2, ̄ PaO2
呼吸性酸中毒 (血液中 [H+] ↑) à激活血管内皮电压
门控 K+
脑血管扩张为大 晨间头痛 脑提供足够的 O2
激活中央(延髓)和外周(颈动脉体)的化学感受器 呼吸驱动↑à呼吸肌活动 (呼吸做功 )↑
短暂的睡眠唤醒
通道 交感神经系统活动↑
全天缺氧 肺血管阻力↑
肺动脉高压
右心泵血以抵抗肺 动脉高压à 随着时 间推移,心肌向心 性肥大
肺心病(区别于左
心衰,右心衰是肺
动脉高压所致)
呼吸肌克服上气道阻力à 气道 明显恢复
睡眠过程不连续
白天的认知功能
及注意力 ̄
机动车辆事故风险↑
白天嗜睡
à 动脉收缩 全身血管阻力↑
高血压
血管内压力↑ à 血 管内皮和平滑肌发生 适应性改变
动脉壁增厚、硬化、失 去弹性à器官血液灌 注量 ̄ (如脑部)
缩写: PCO2:二氧化碳分压 PO2:氧分压 PaCO2:动脉二氧化 碳分压 PaO2:动脉血氧分压
通气过度 à呼出CO2 ↑ à PaCO2 ̄
呼吸驱动 ̄à 呼吸做功 ̄
复苏性鼾音
夜尿症
如:伊普沃斯嗜睡评分
>10
缺血性卒中
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年8月19日发表 www.thecalgaryguide.com, 2022年5月31日更新
Obstructive Sleep Apnea: Pathogenesis and clinical findings
Vascular Factors: During recumbent sleep, more bodily fluids enter the head and neck area (compared to when the patient is standing/sitting)
↑ volume of head/neck tissue surrounding the upper airwayà possible airway obstruction
Authors: Ciara Hanly Austin Laing Alexander Arnold Reviewers: Steven Liu Amogh Agrawal Yonglin Mai (麦泳琳) Naushad Hirani* Yan Yu* *MD at time of publication
Neuromuscular Factors: Sleep onset and/or the sleeping state reduces the drive of respiratory muscles to breathe
↓ Upper airway neuromuscular activityà↓ upper airway caliber, ↑ upper airway resistance, ↑ upper airway collapsibility during sleep
Structural Factors: Obesity, tonsillar or adenoid hypertrophy, macroglossia, ↑ neck circumference, craniofacial abnormalities
Excess pressure on upper airway, or deformity to that area, ↑ risk of upper airway collapse
Polysomnography
Absence of airflow but persistent ventilatory effort
Hypopnea or Apnea
Paradoxical breathing Chest wall draws in and abdomen expands during inspiration
Ventilatory effort persists against closed airway
No air entry due to collapsed upper airway
↑ Negative intrathoracic pressure
↑ Venous return to right atrium
Stretching of right atrial myocardium à secretion of atrial natriuretic peptide (ANP)
ANP inhibits epithelial Na+ channels (ENaC) in the collecting ducts of the kidney from reabsorbing Na+ à Na+ excretion
↑ Na+ excretionà↑ water excretion
Nocturia
Complete or partial upper airway obstruction during sleep
↑ PCO2 & ̄ PO2
in the lungsà ̄ diffusion gradient of CO2 & O2 between lungs & arteries
↑ PaCO2,, ̄ PaO2
Respiratory acidosis (↑ [H+] in blood)àactivation of vascular endothelial voltage gated K+ channels
Cerebral blood vessel dilation to provide adequate O2 to brain
Morning Headaches
Activation of central (medulla oblongata) & peripheral (carotid body) chemoreceptors
↑ Respiratory drive à ↑ activation of respiratory muscles (ventilatory effort )
Transient arousal from sleep
↑ sympathetic nervous system activityà arterial vasoconstriction
↑ systemic vascular resistance
Systemic Hypertension
↑ intraluminal pressure within blood vesselsàadaptive vascular endothelial and smooth muscle changes
Artery walls thicken, harden and lose elasticityà ̄ perfusion to end organs (such as the brain)
Ischemic stroke
Hypoxia during the day and night
↑ pulmonary vascular resistance
Pulmonary Hypertension
Right heart pumps against higher pulmonary pressure àcardiomyocytes undergo concentric hypertrophy over time
Cor Pulmonale
(Right heart failure due to pulmonary hypertension, separate from left heart failure)
Respiratory muscles overcome upper airway obstructionà airway patency restored
Sleep fragmentation
̄ Daytime cognitive performance and attentiveness
↑ Risk of motor vehicle accidents
Daytime Sleepiness
Eg. Epworth Sleepiness Scale >10
Abbreviations:
PCO2: partial pressure of carbon dioxide PO2: partial pressure of oxygen PaCO2: partial pressure of carbon dioxide in arteries PaO2: partial pressure of oxygen in arteries
Ventilatory response overcompensatesà breathe out more CO2 than is required for homeostasisà ̄ PaCO2
̄ respiratory driveà ̄ ventilatory effort
Resuscitative Gasping
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 19, 2013, updated May 31, 2022 on www.thecalgaryguide.com
阻塞性睡眠呼吸暂停:发病机制及临床表现
作者:Ciara Hanly, Austin Laing, Alexander Arnold 审稿人: Steven Liu, Amogh Agrawal, Naushad Hirani*,Yan Yu* 译者: Zesheng Ye(叶泽生) 翻译审稿人: Yonglin Mai(麦泳琳) *发表时担任临床医生
神经肌肉因素: 睡眠状态下, 患者无法通过 适当增加上气道肌张力来维持气道通畅
上气道神经肌肉活动 ̄à上气道直径 ̄, 上气道 阻力↑, 睡眠时上气道塌陷
结构(解剖)因素: 肥胖、扁桃体或腺样体 肥大, 舌体肥大, 颈围增大, 颅面部畸形
上气道压力过大或上气道畸形, 上气道塌陷 的风险 ↑
血管因素: 仰卧位睡觉引起 夜间嘴侧液体移位
周围组织与压力 ↑à上气道阻塞
多导睡眠描记术
没有气流,但持
续通气
呼吸浅慢或 呼吸暂停
反常呼吸 吸气时胸壁凹陷, 腹部膨隆
持续通气以抵抗气道 闭合
上气道塌陷导致空气进
入气道受阻
腹膜腔负压↑ 静脉血回流右心室阻力↑
右心房心肌细胞拉伸 à心房利钠肽分泌 (ANP)
ANP抑制肾集合管的上 皮Na+通道(ENaC)对 Na+重吸收à Na+排出
Na+排出量↑ à 水排出量 ↑
睡眠时全部
或部分上呼
吸道阻塞
肺内PO2 ̄ 且 PCO2↑ à CO2 及 O2在肺和动脉 间的扩散梯度 ̄
↑ PaCO2, ̄ PaO2
呼吸性酸中毒 (血液中 [H+] ↑) à激活血管内皮电压
门控 K+
脑血管扩张为大 晨间头痛 脑提供足够的 O2
激活中央(延髓)和外周(颈动脉体)的化学感受器 呼吸驱动↑à呼吸肌活动 (呼吸做功 )↑
短暂的睡眠唤醒
通道 交感神经系统活动↑
全天缺氧 肺血管阻力↑
肺动脉高压
右心泵血以抵抗肺 动脉高压à 随着时 间推移,心肌向心 性肥大
肺心病(区别于左
心衰,右心衰是肺
动脉高压所致)
呼吸肌克服上气道阻力à 气道 明显恢复
睡眠过程不连续
白天的认知功能
及注意力 ̄
机动车辆事故风险↑
白天嗜睡
à 动脉收缩 全身血管阻力↑
高血压
血管内压力↑ à 血 管内皮和平滑肌发生 适应性改变
动脉壁增厚、硬化、失 去弹性à器官血液灌 注量 ̄ (如脑部)
缩写: PCO2:二氧化碳分压 PO2:氧分压 PaCO2:动脉二氧化 碳分压 PaO2:动脉血氧分压
通气过度 à呼出CO2 ↑ à PaCO2 ̄
呼吸驱动 ̄à 呼吸做功 ̄
复苏性鼾音
夜尿症
如:伊普沃斯嗜睡评分
>10
缺血性卒中
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年8月19日发表 www.thecalgaryguide.com, 2022年5月31日更新](https://calgaryguide.ucalgary.ca/wp-content/uploads/2014/09/OSA-2021-1.jpg)
Status-Epilepticus
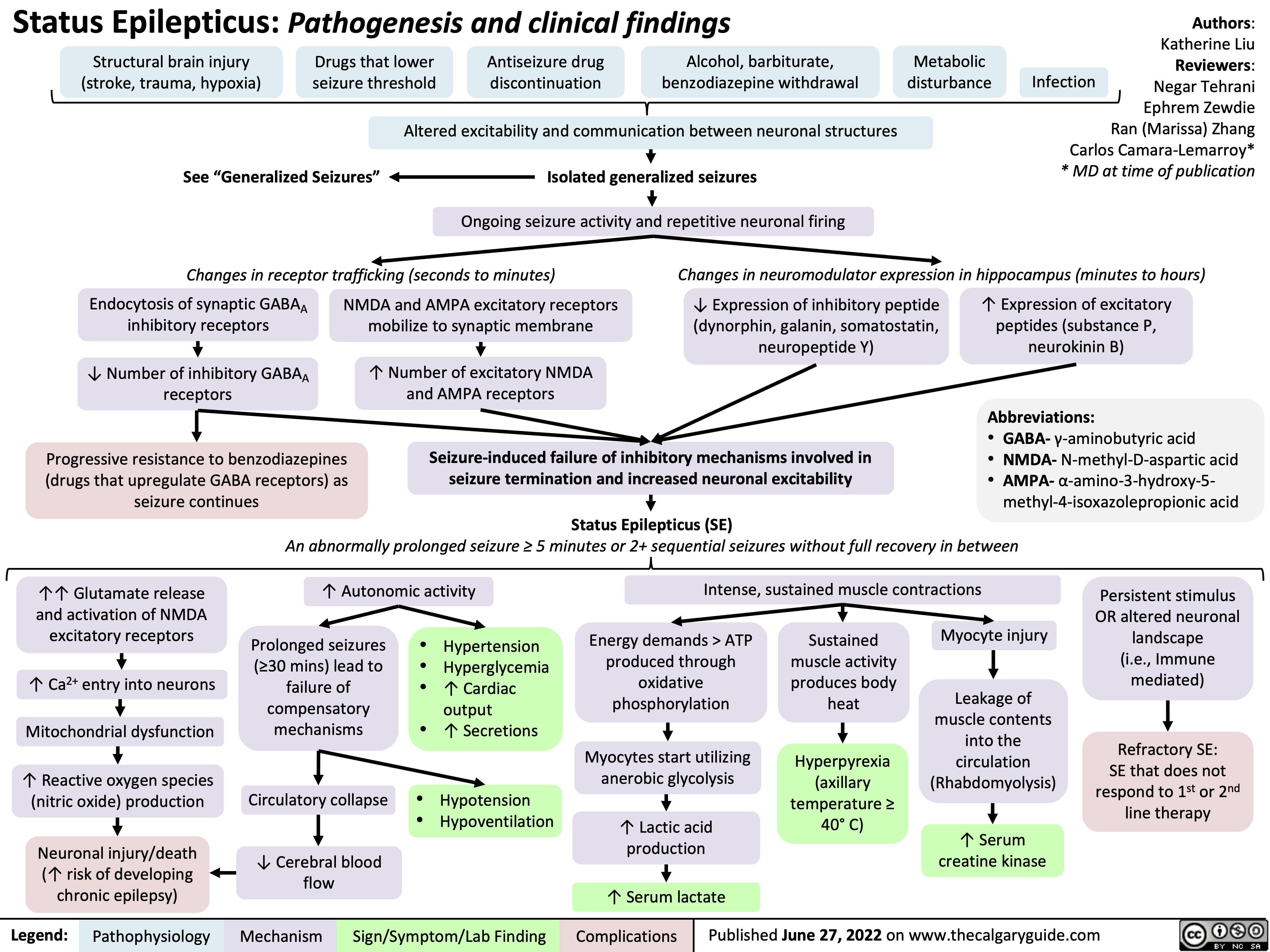
pheochromocytoma-pathogenesis-and-clinical-findings
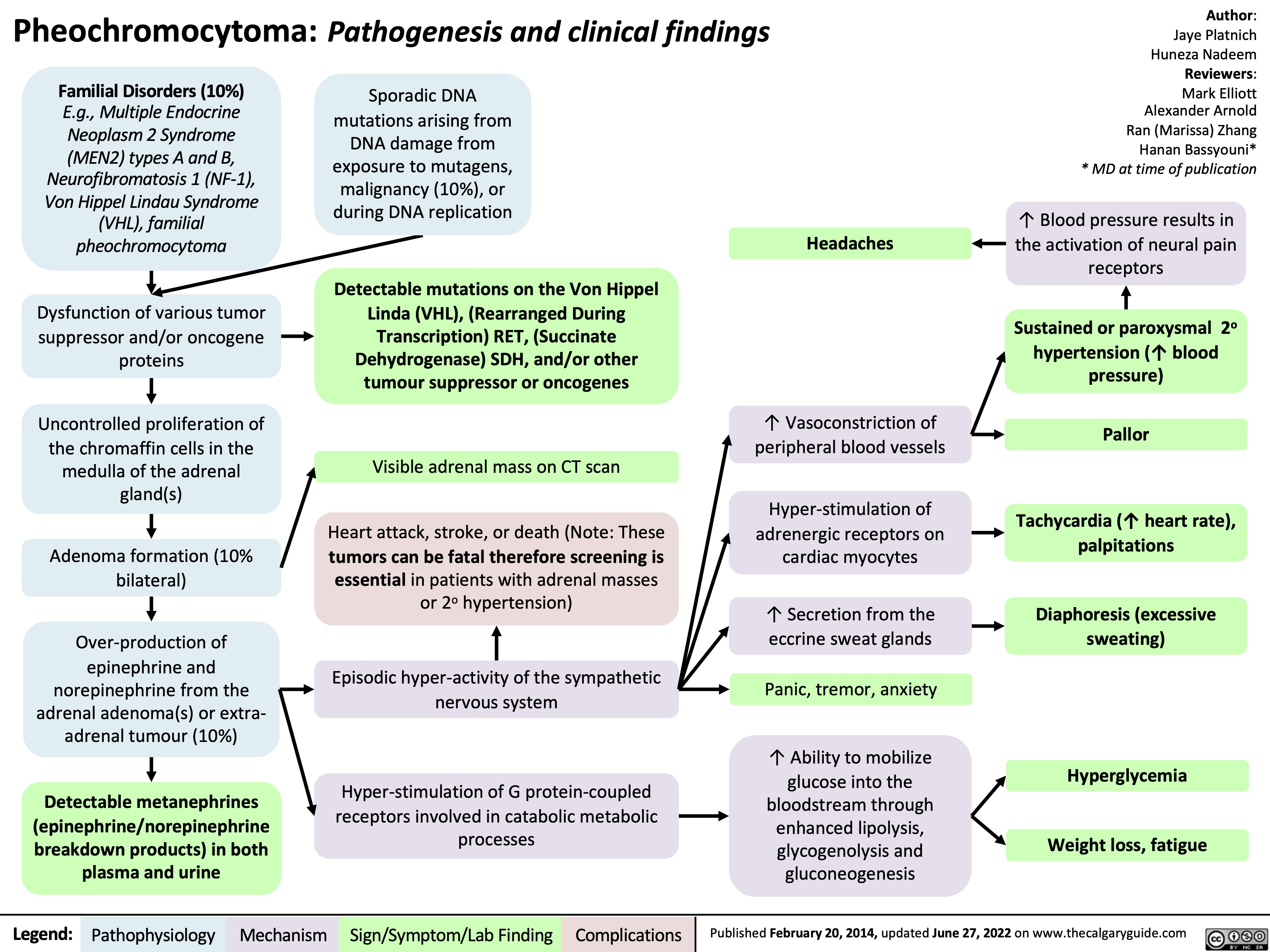
Epilepsy Pathogenesis
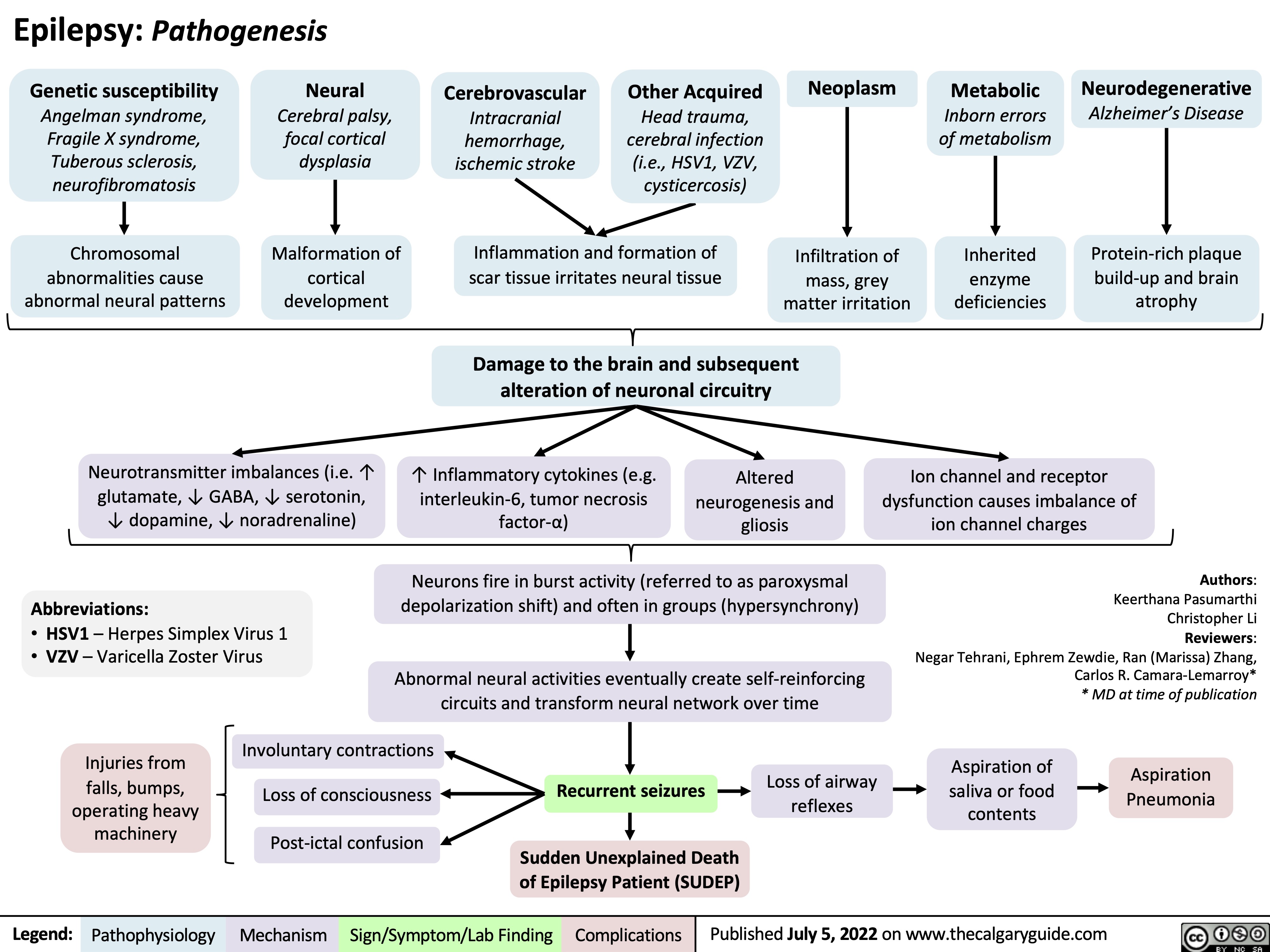
burn-shock-pathogenesis-complications-and-clinical-findings
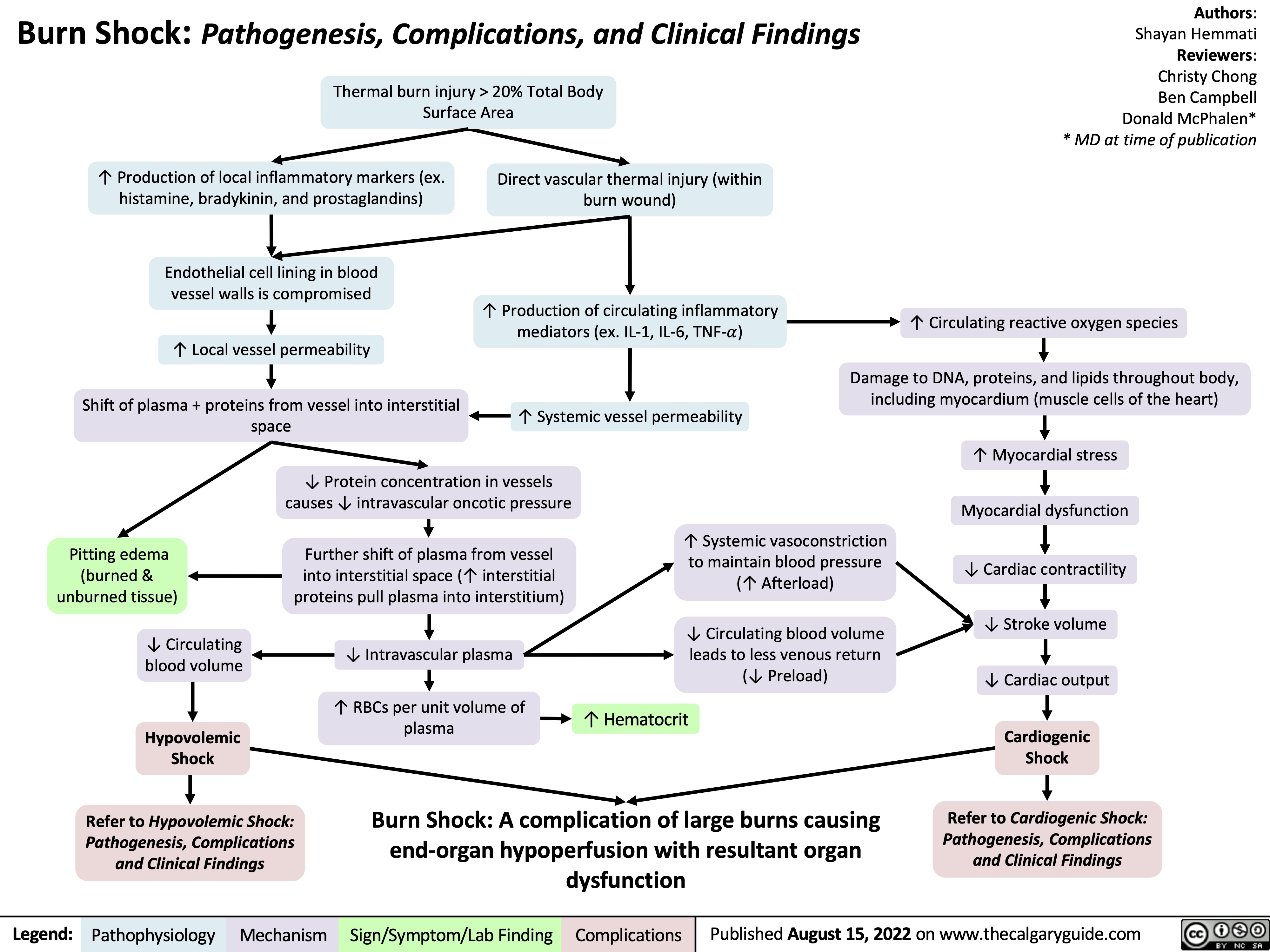
Ischemic Stroke: Pathogenesis
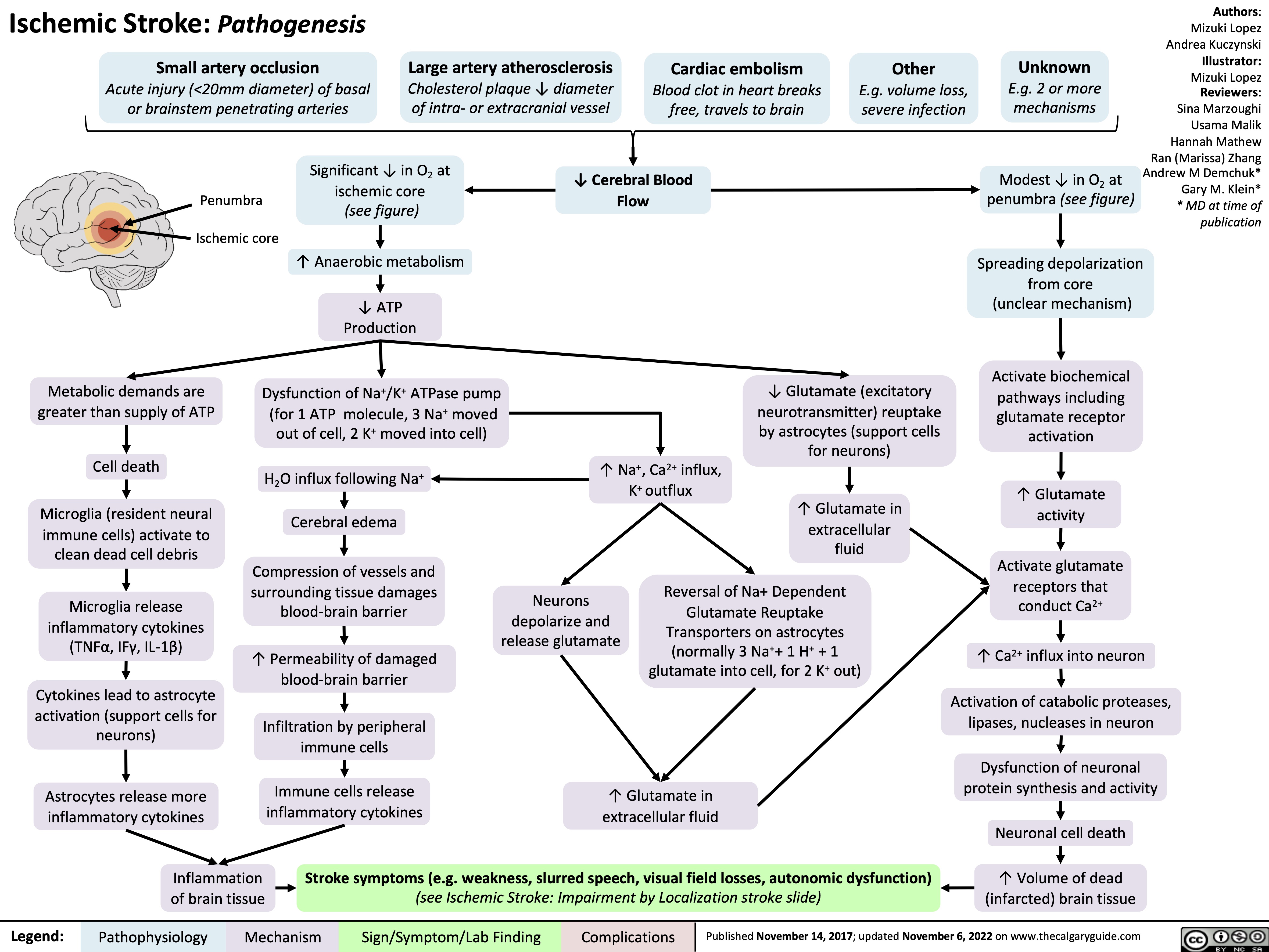
Ischemic Stroke Impairment by Localization
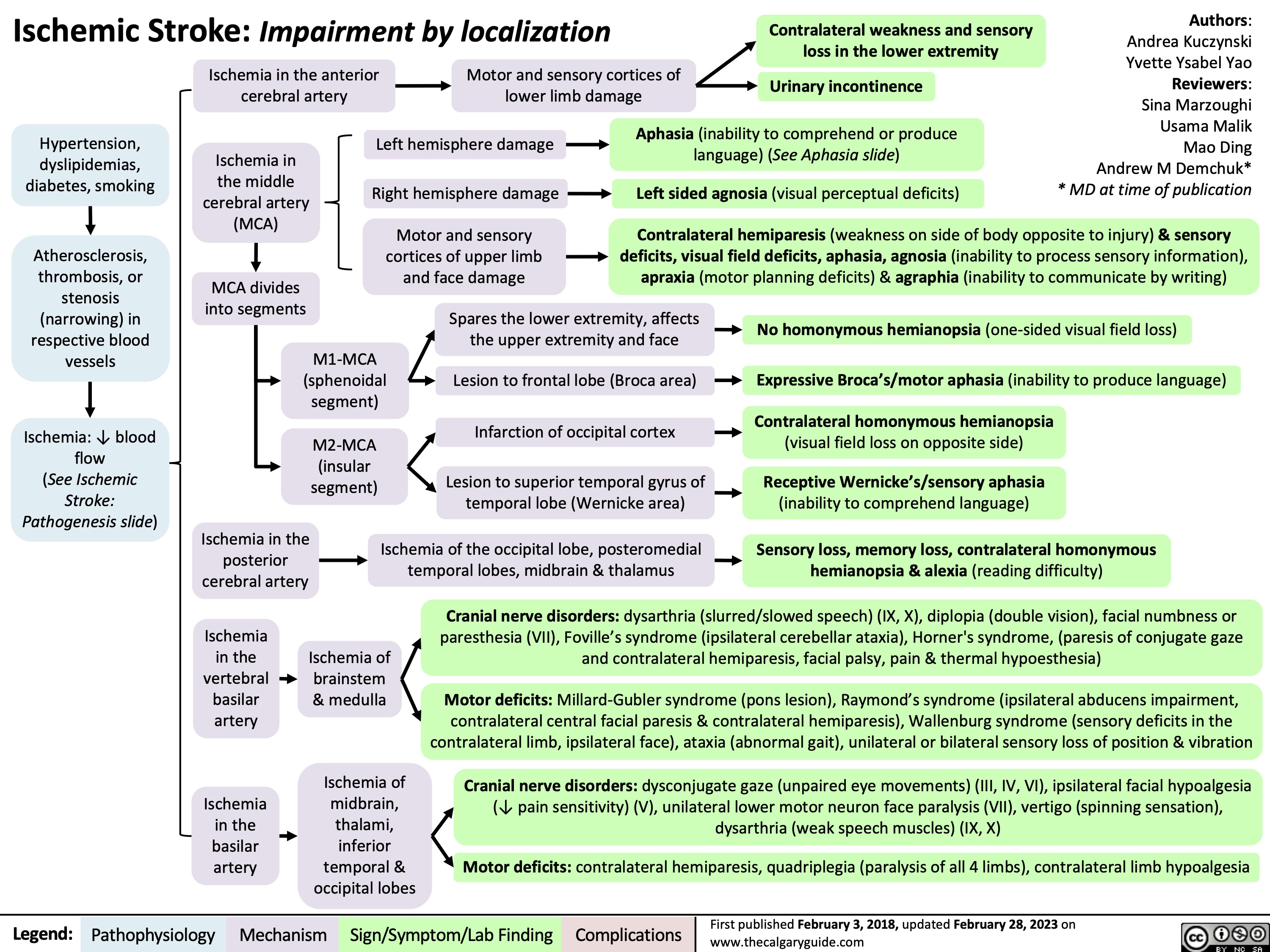
Hemorrhagic Stroke
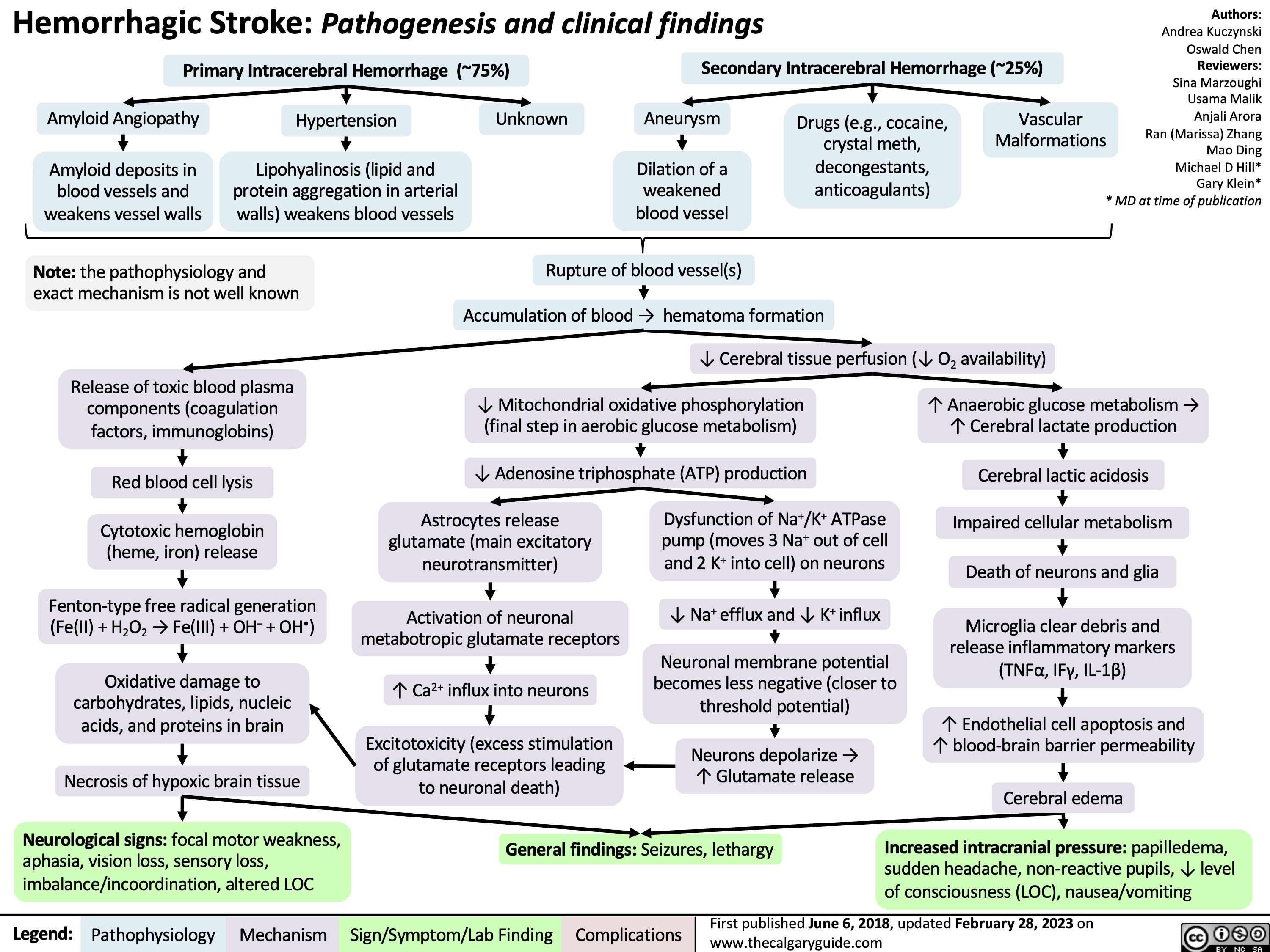
Coronary Artery Bypass Graft CABG Indications
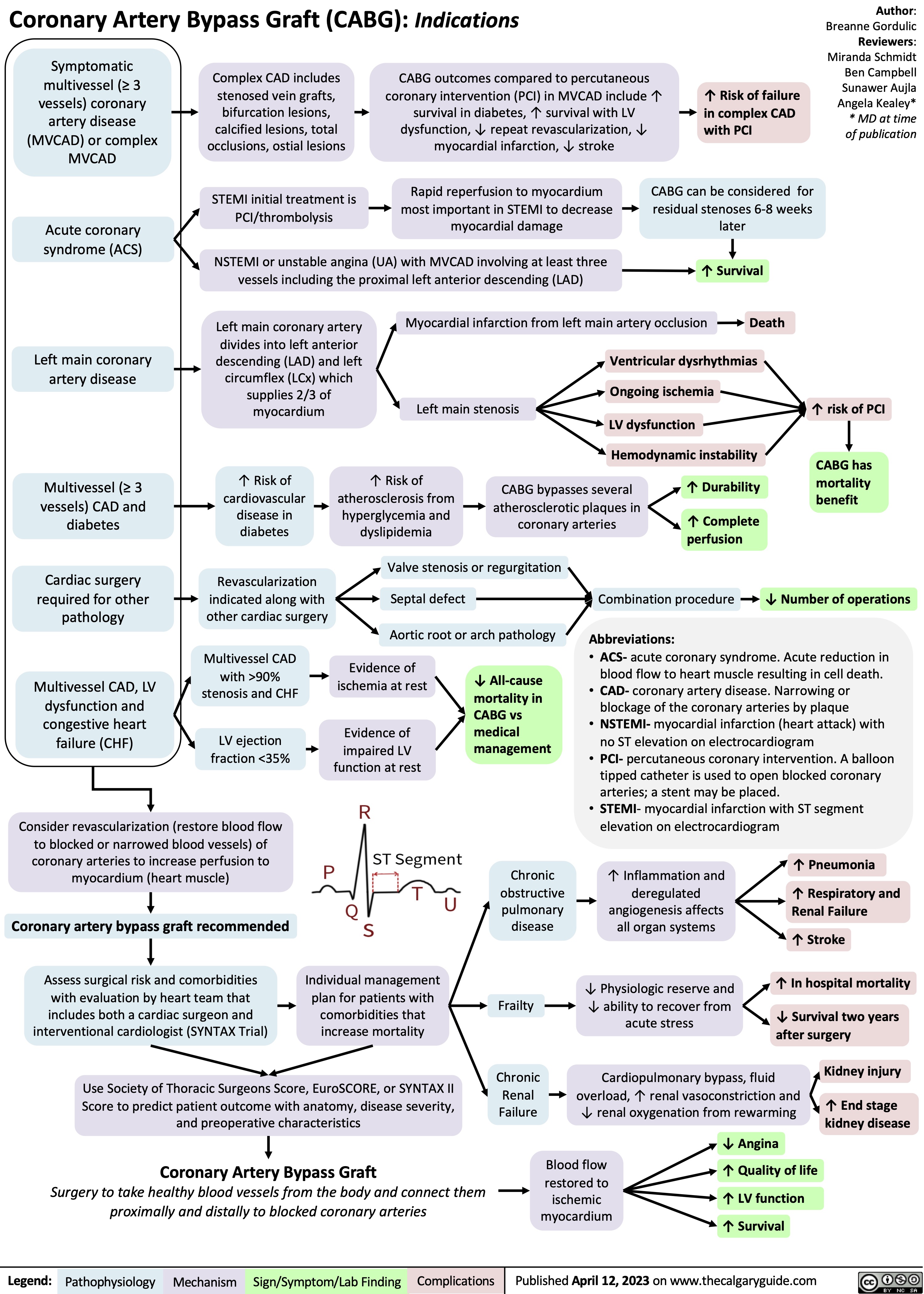
Approach To Dementia
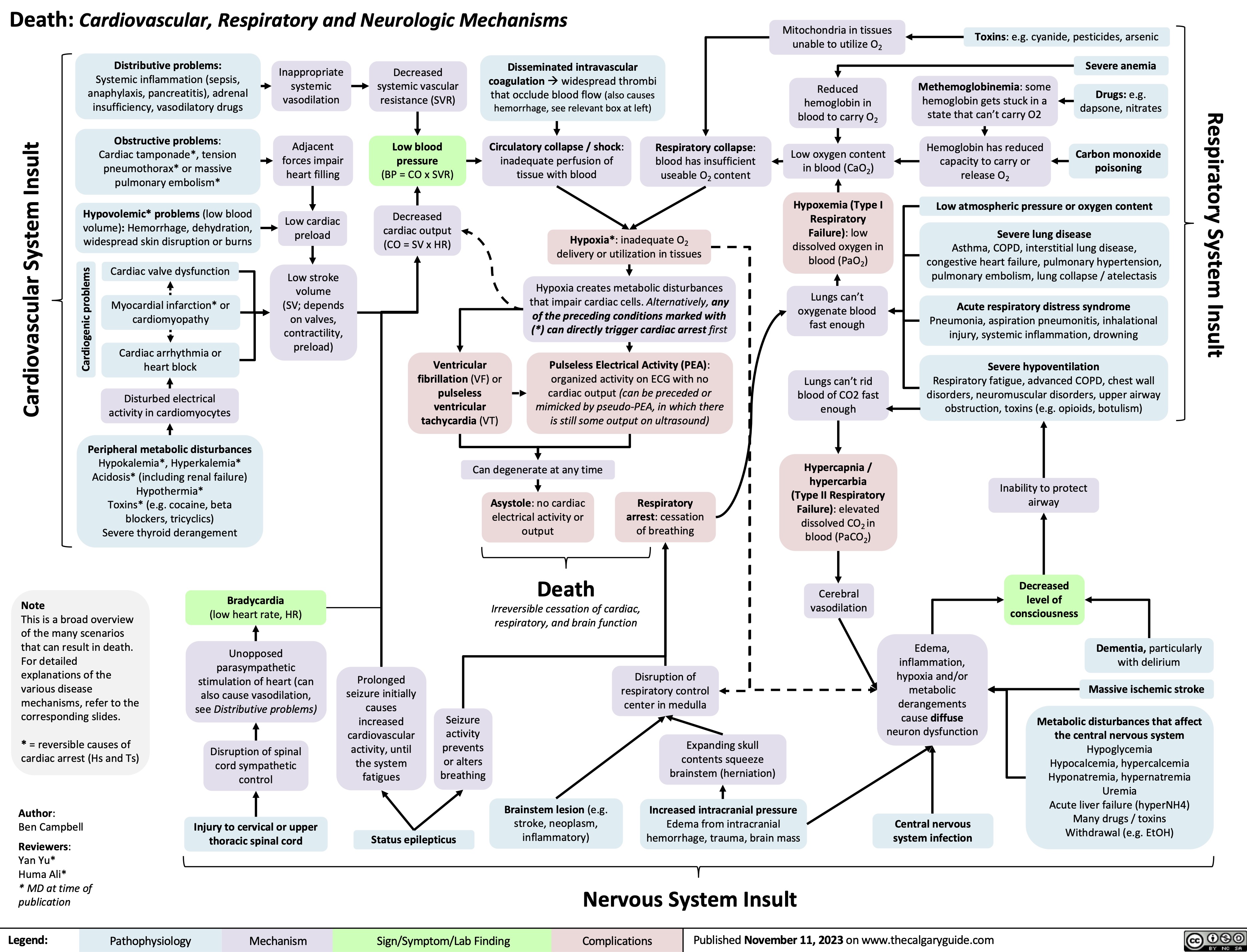
Mitral Regurgitation Pathogenesis and clinical findings
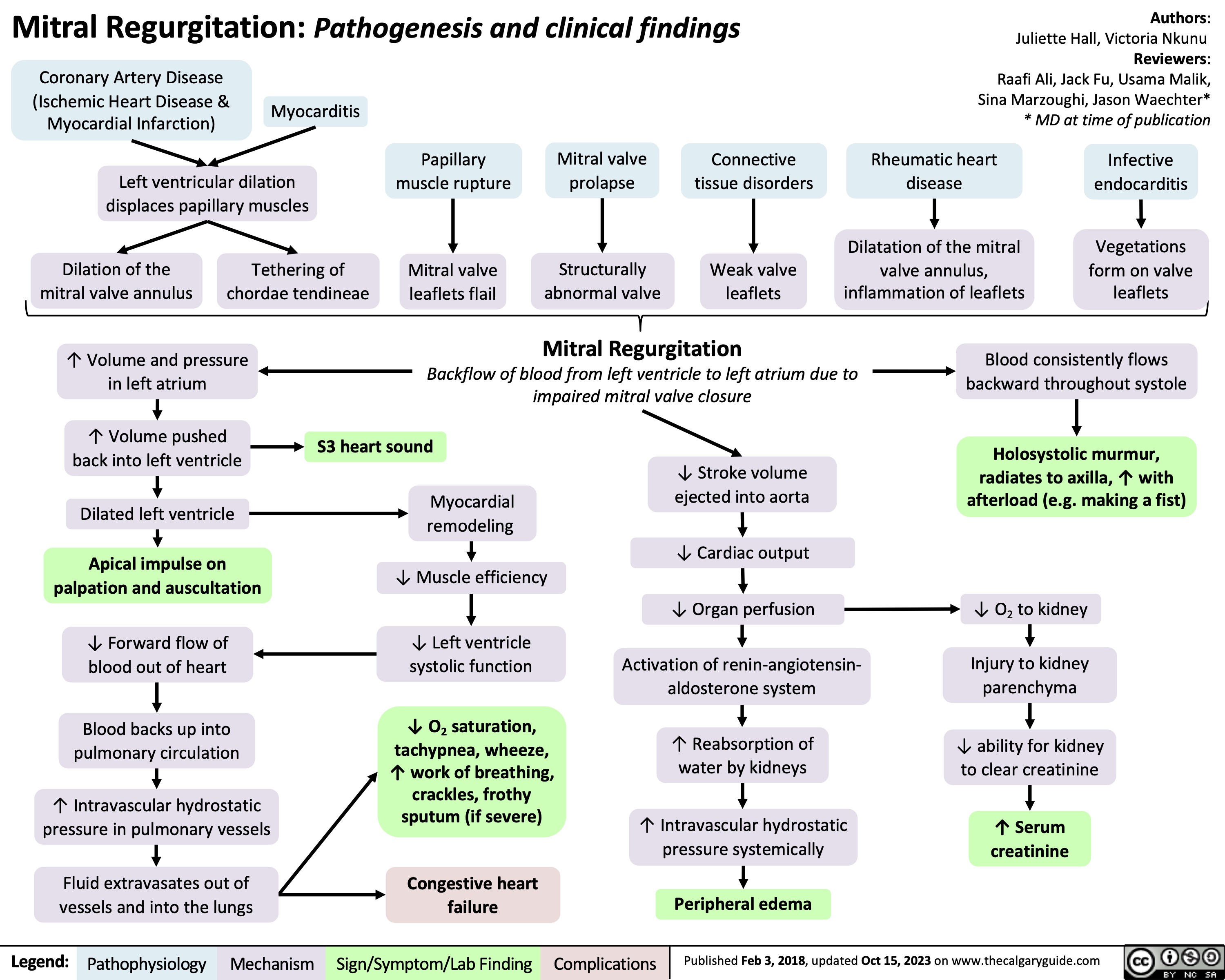
Sustained Monomorphic Ventricular Tachycardia Clinical findings
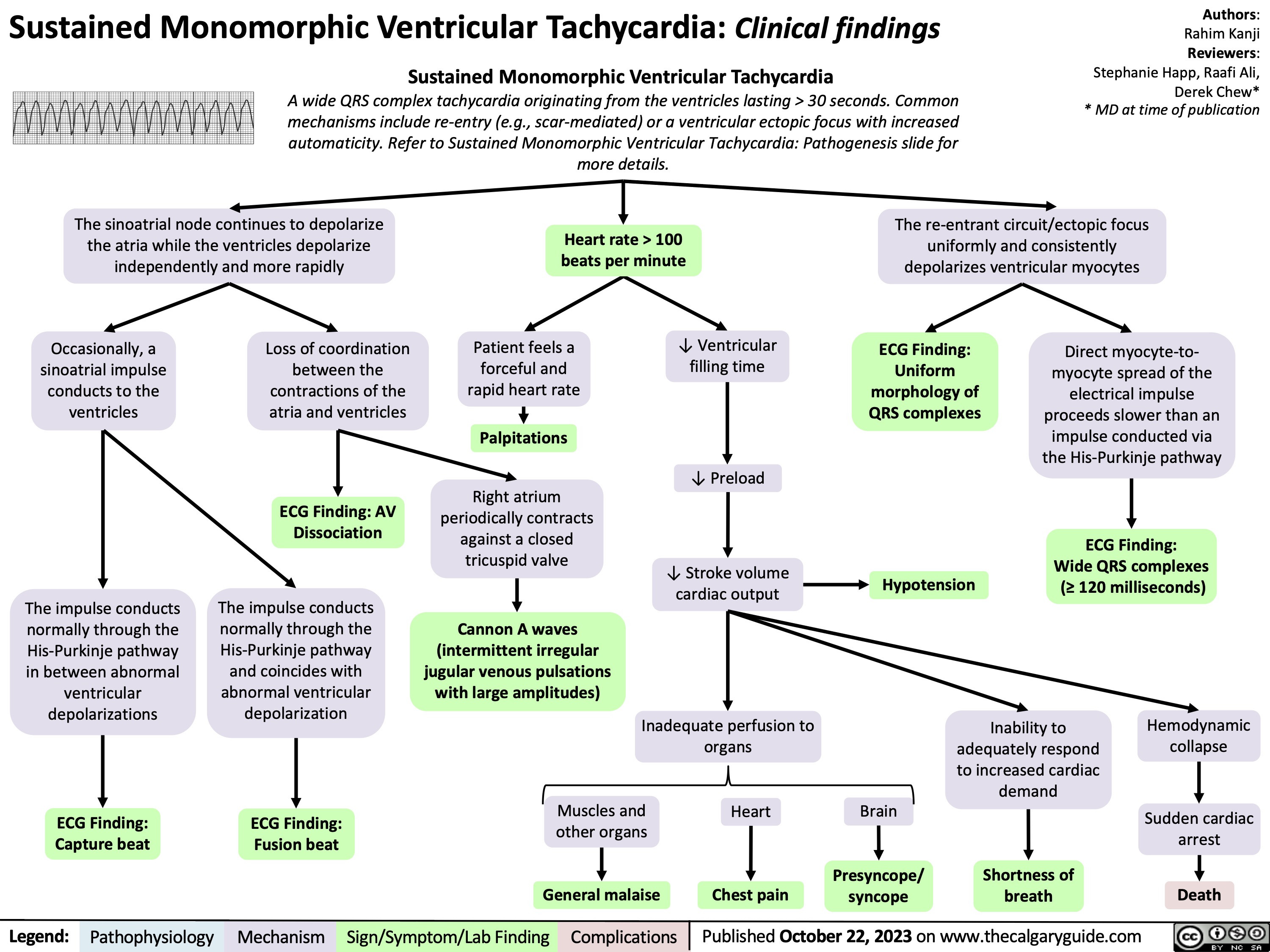
Statins Mechanisms and Side Effects
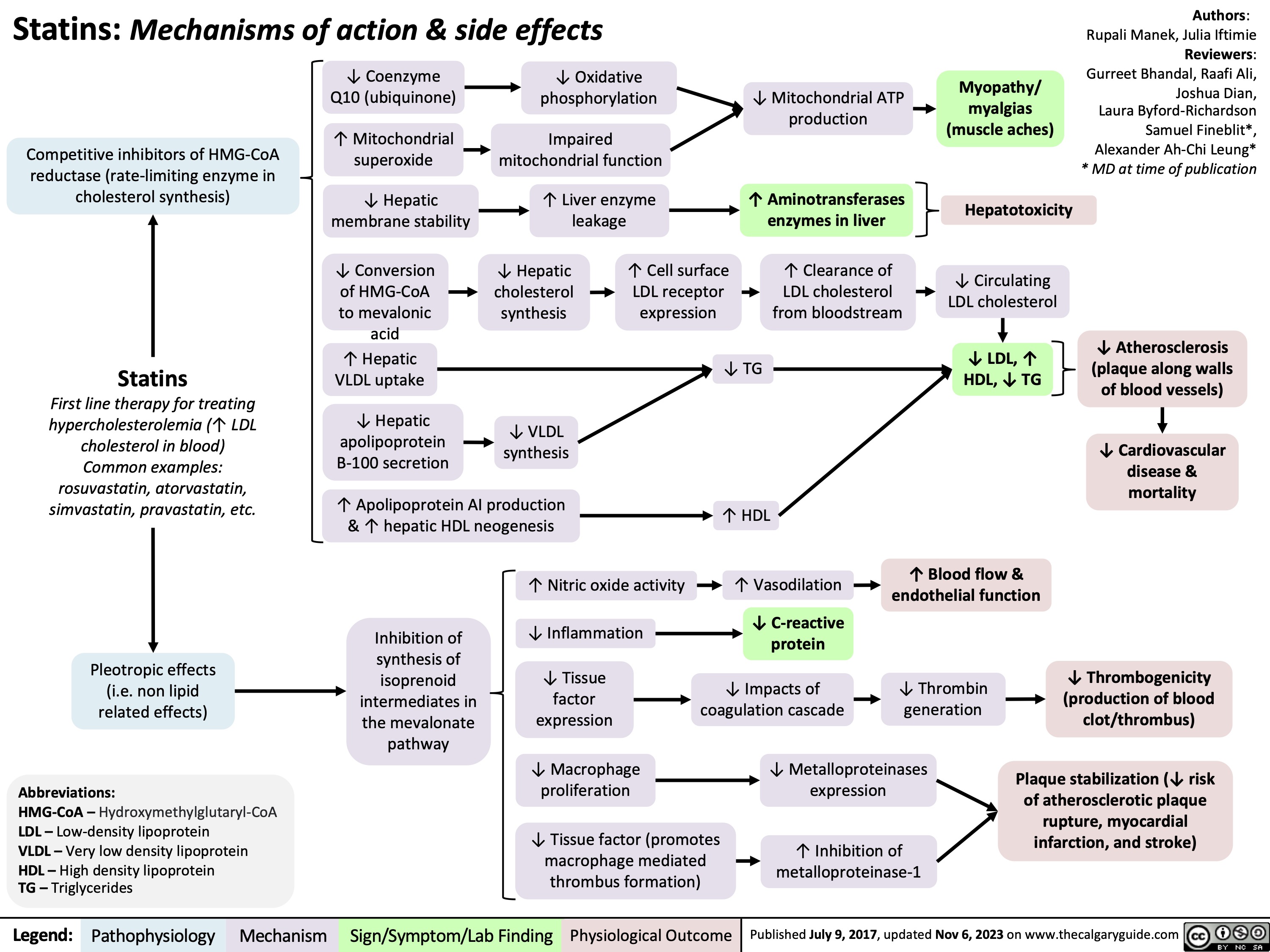
Death Cardiovascular Respiratory and Neurologic Mechanisms
Complication of MI - Acute Mitral Regurgitation
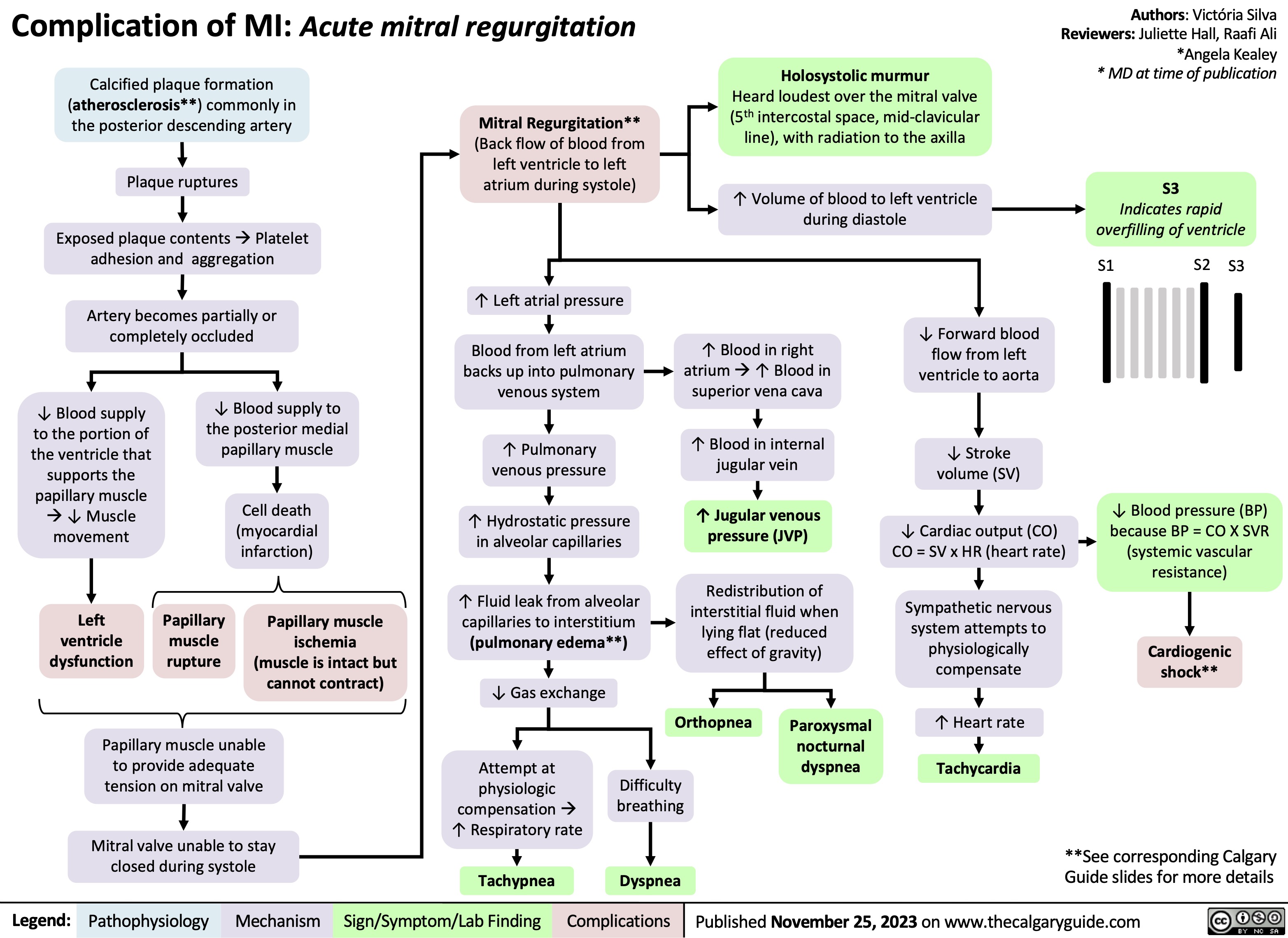
Aspiration Pneumonia
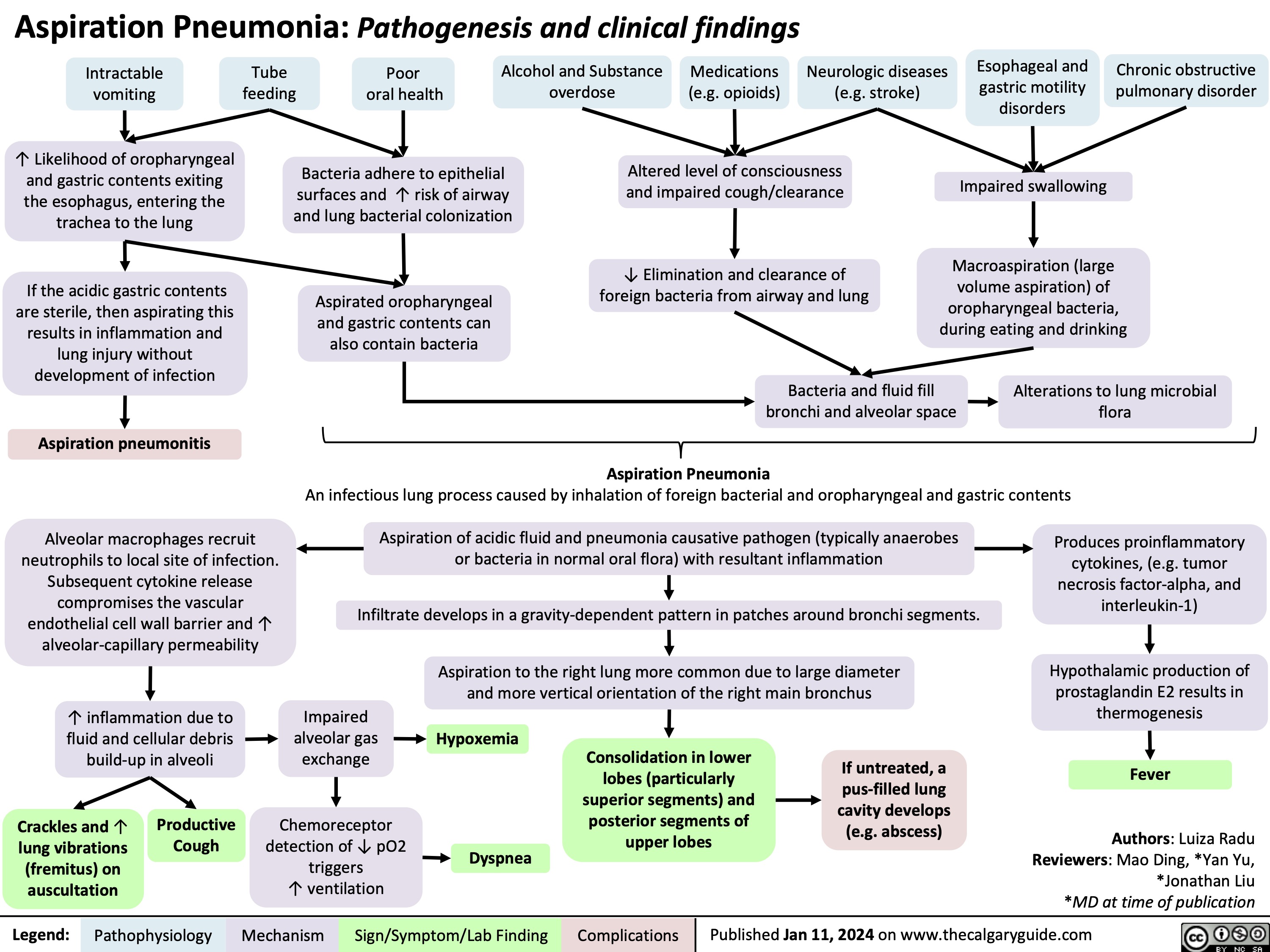
Cranial Nerve IV Palsy
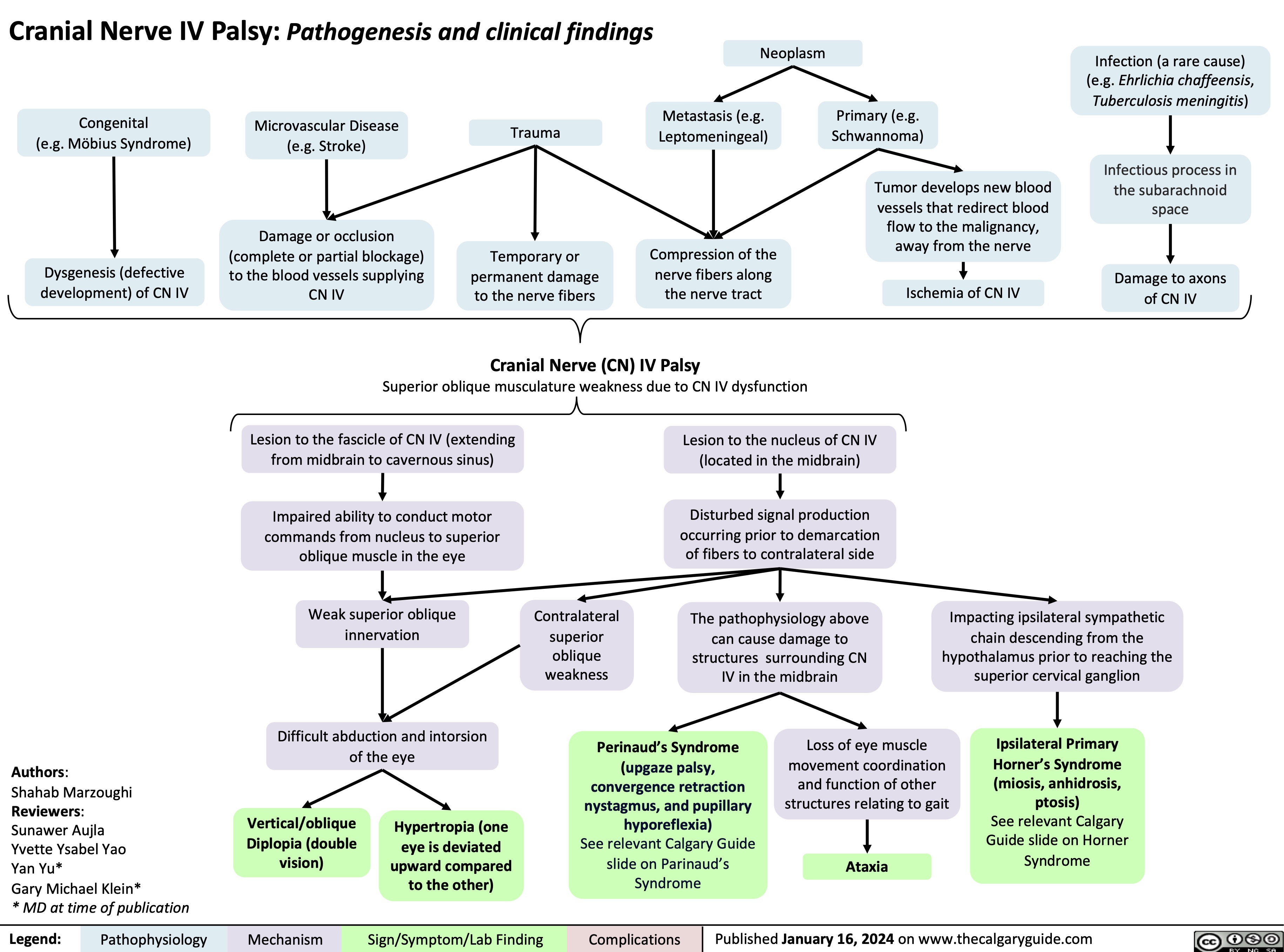
Eisenmenger Syndrome
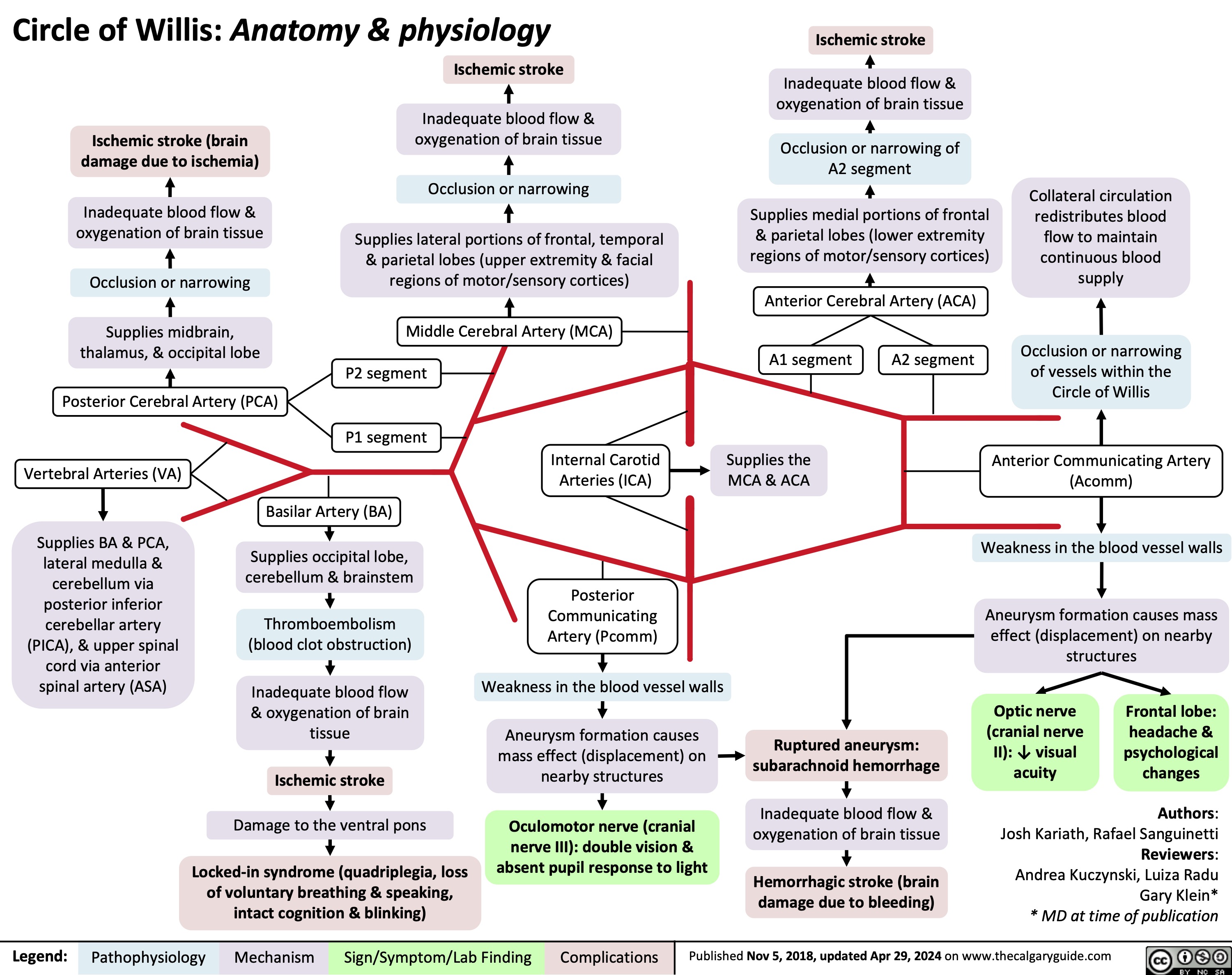
Circle of Willis Anatomy and Physiology
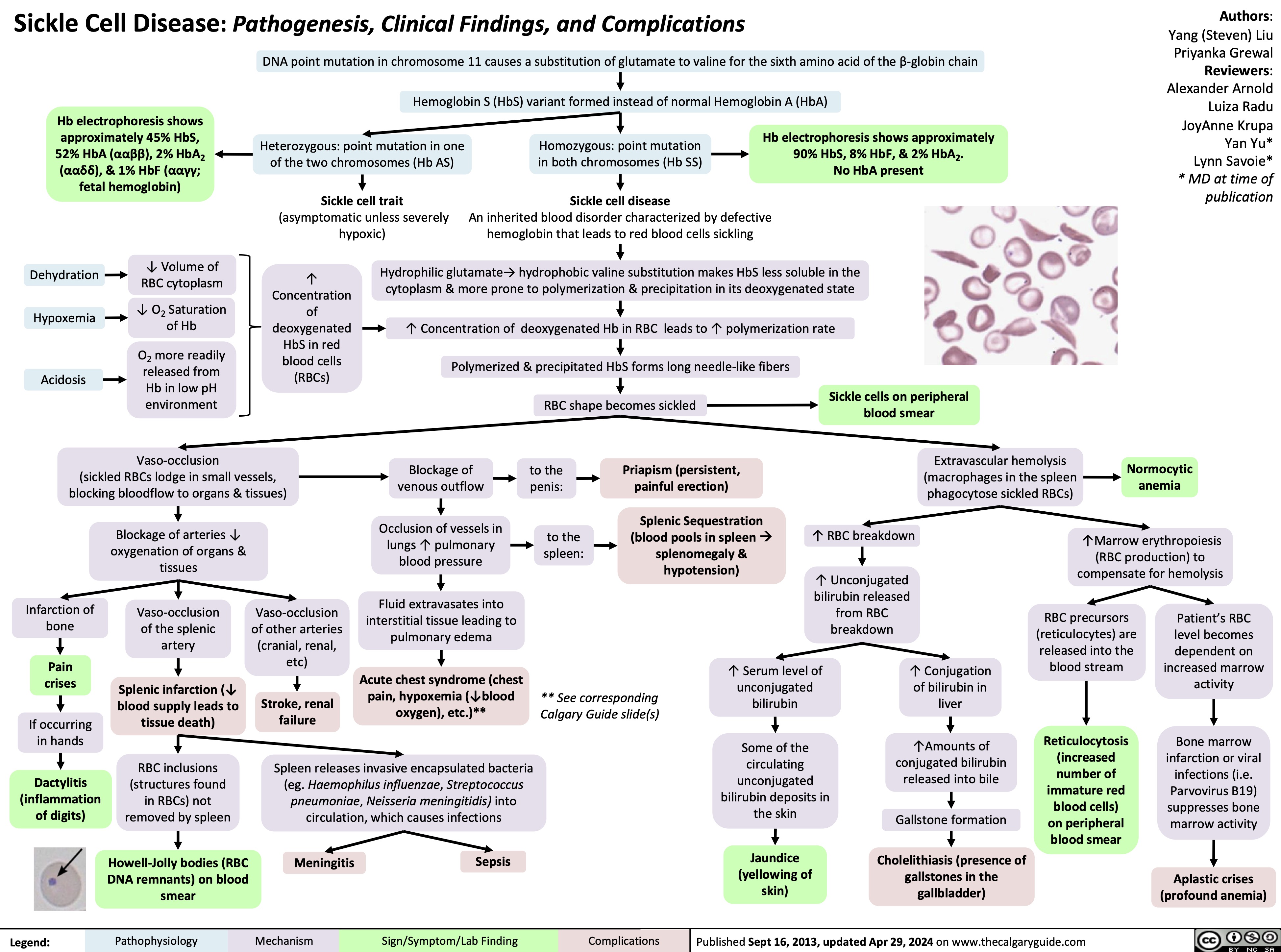
Sickle Cell Disease Pathogenesis Clinical Findings and Complications
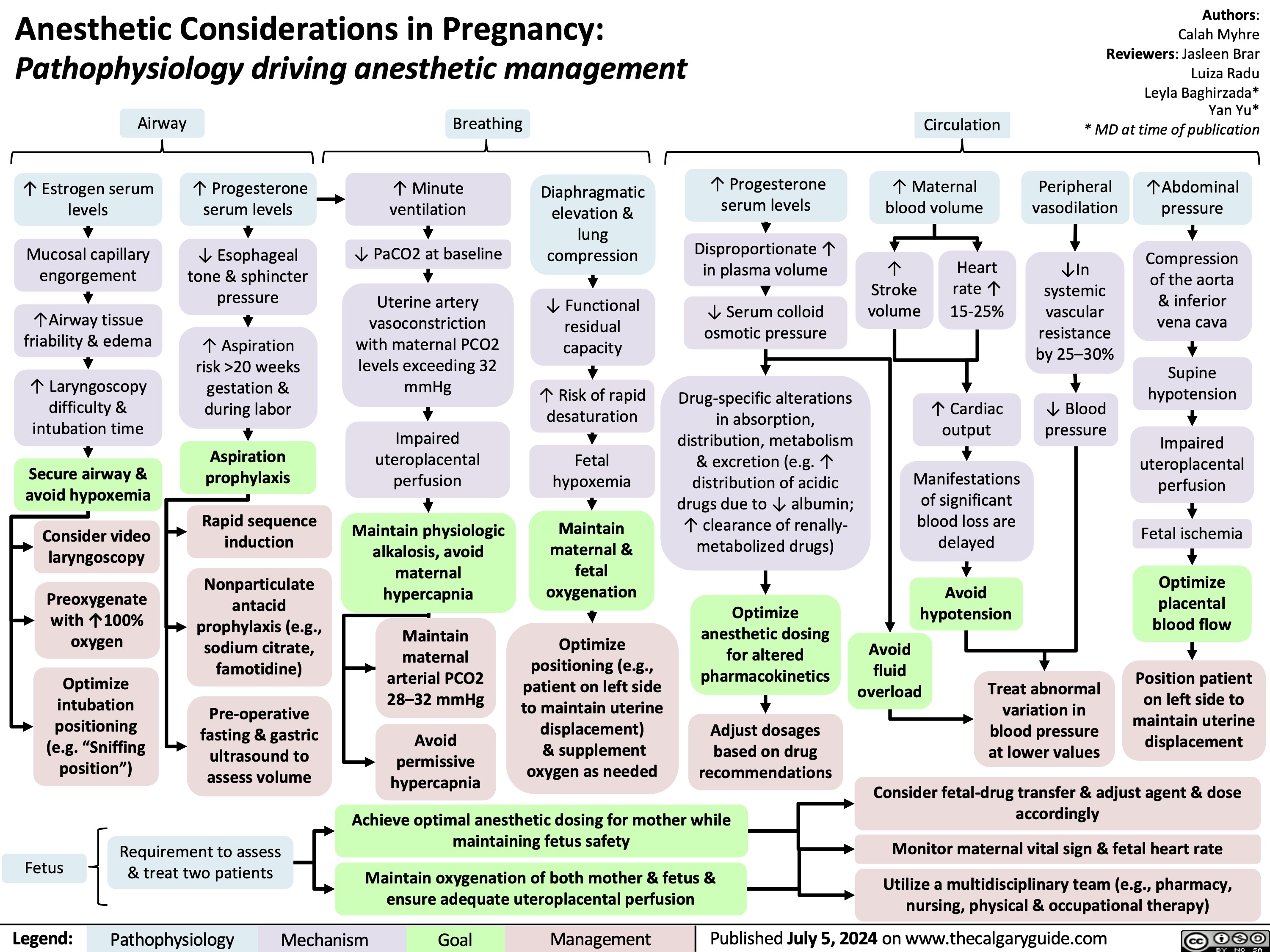
Anesthetic Considerations in Pregnancy
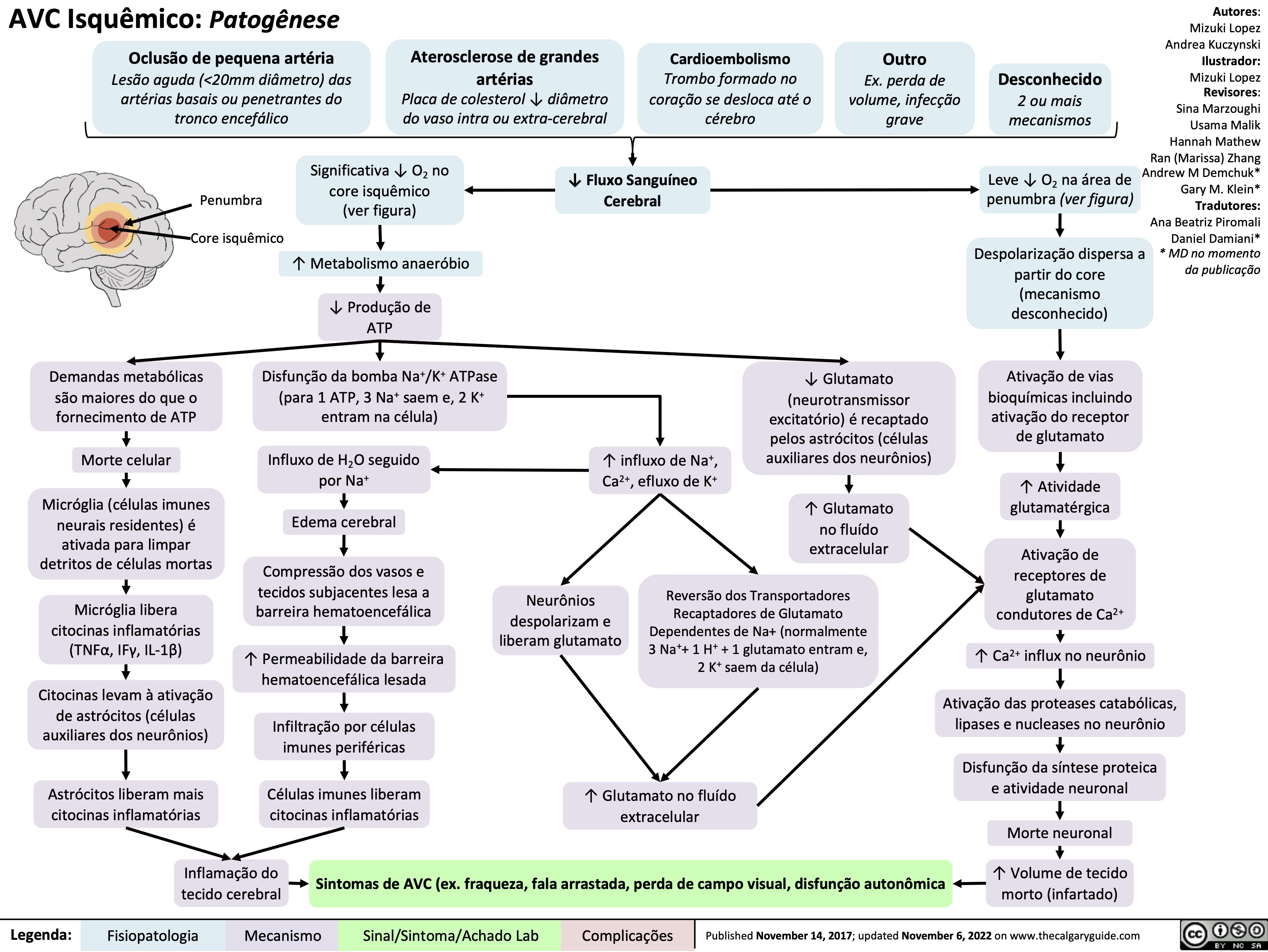
AVC Isquemico Patogenese
Epilepsy in Older Adults
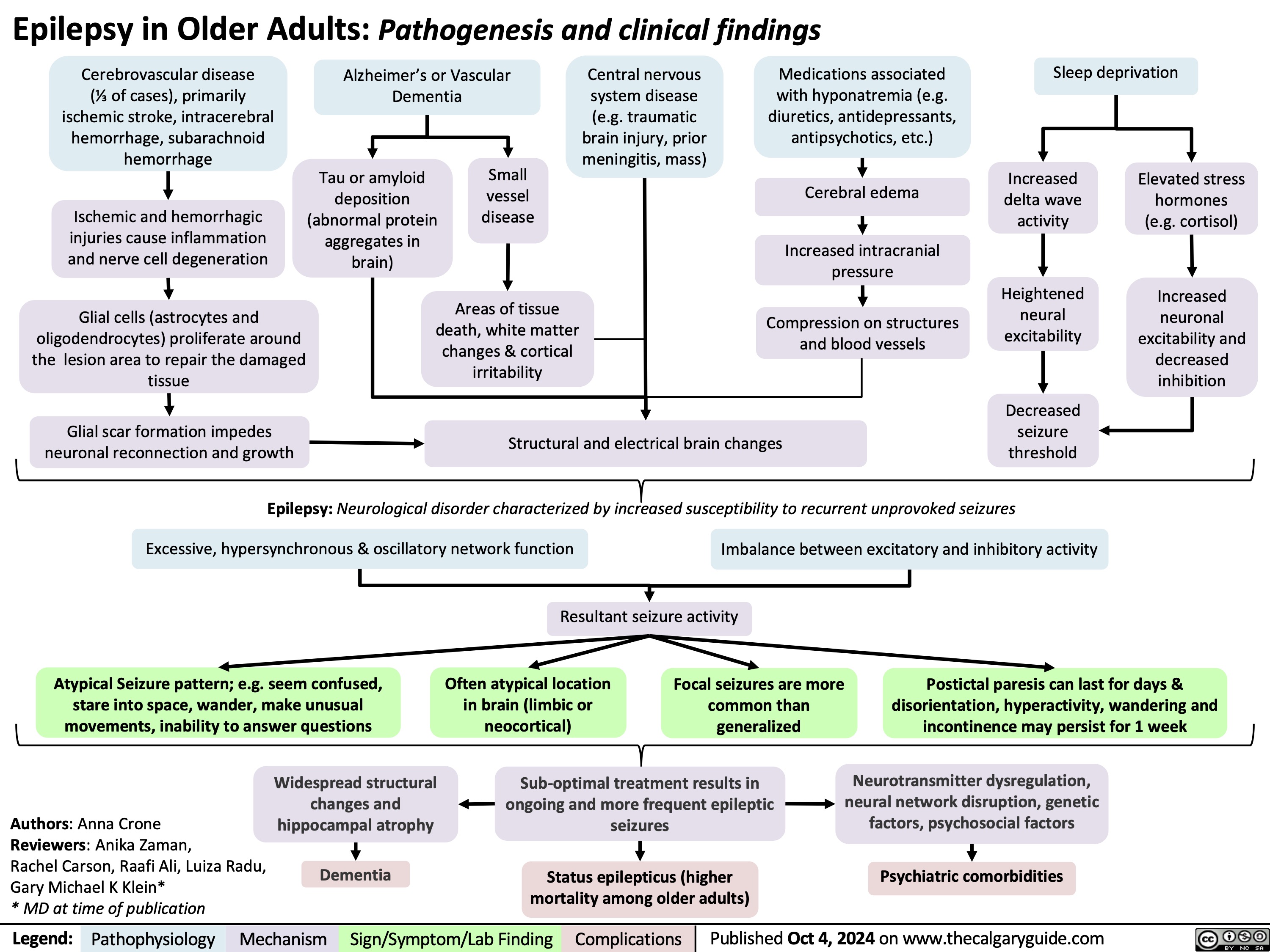
Pulsus Paradoxus
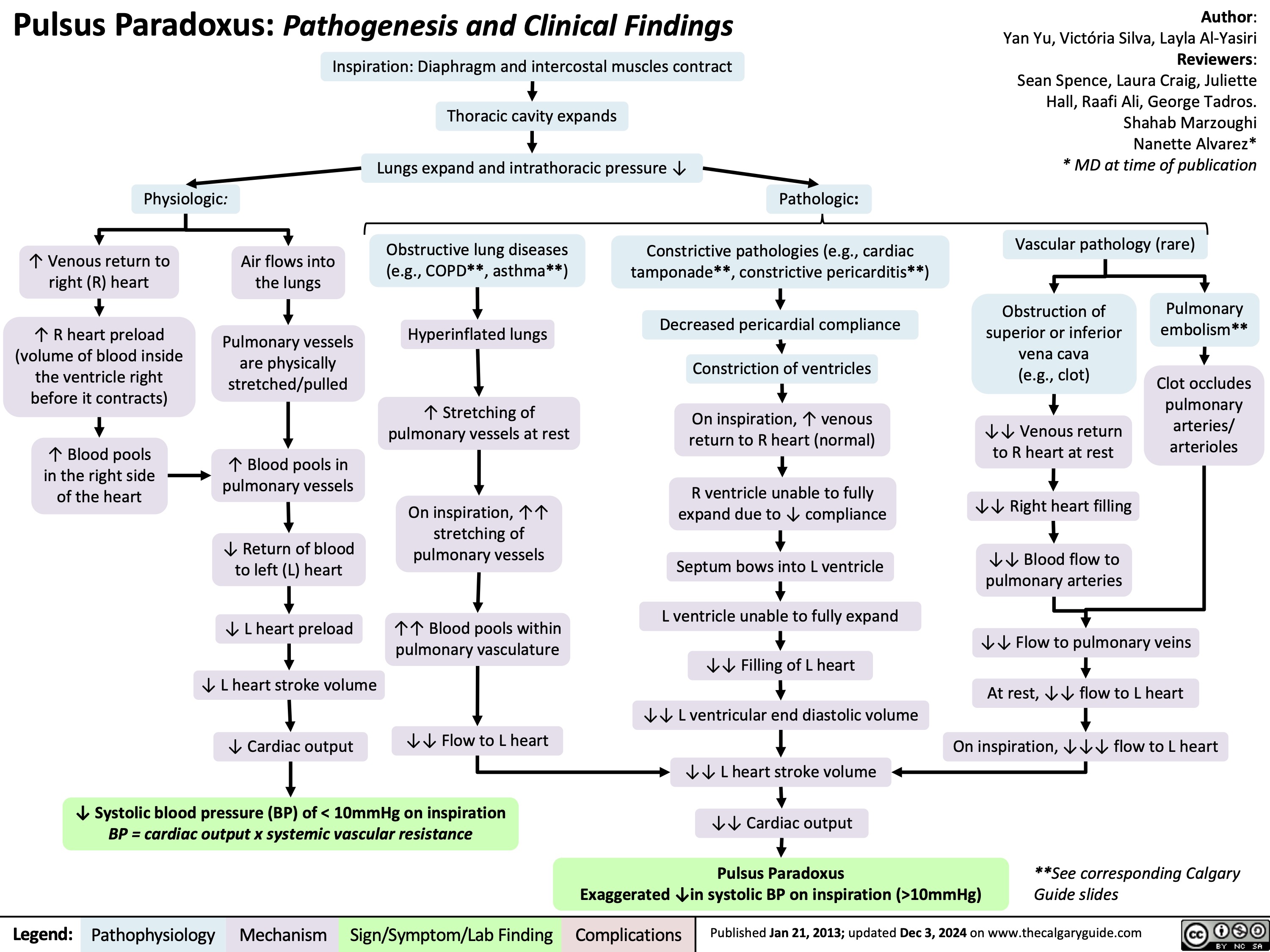
Polycythemia Vera Complications
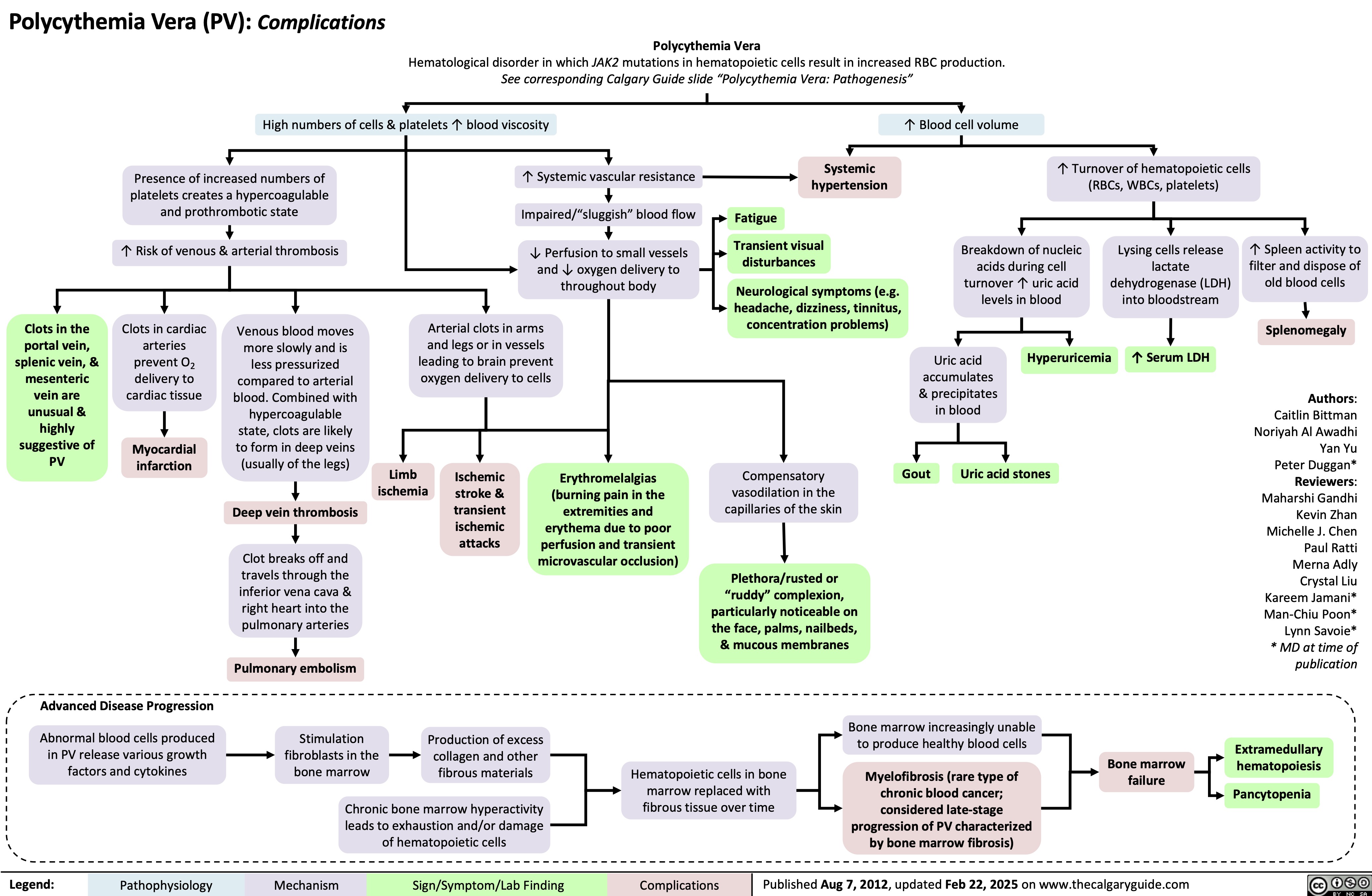
Polymyalgia Rheumatica
Heparin-Induced Thrombocytopenia
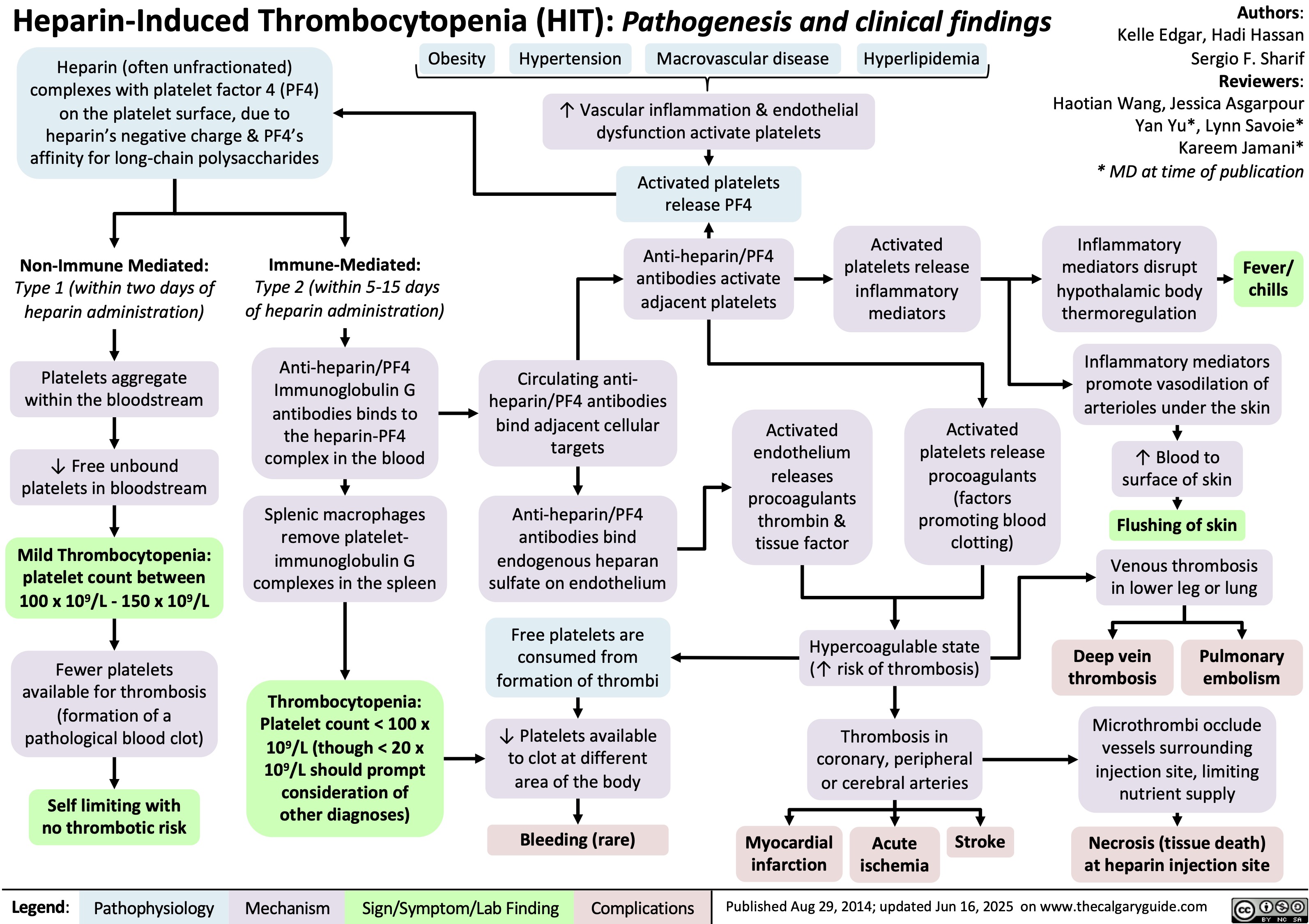
Volatile Gases
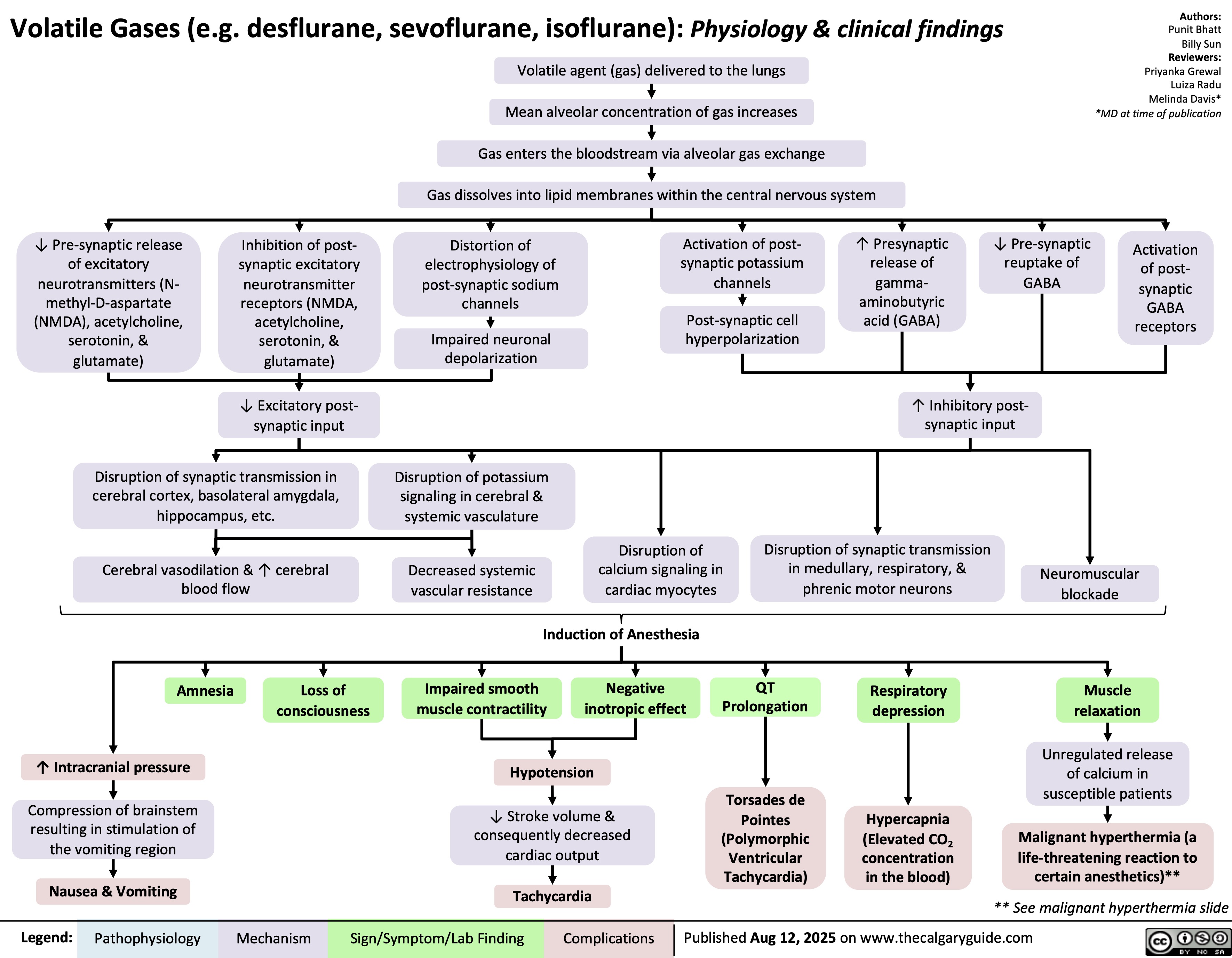
Obstructive Shock
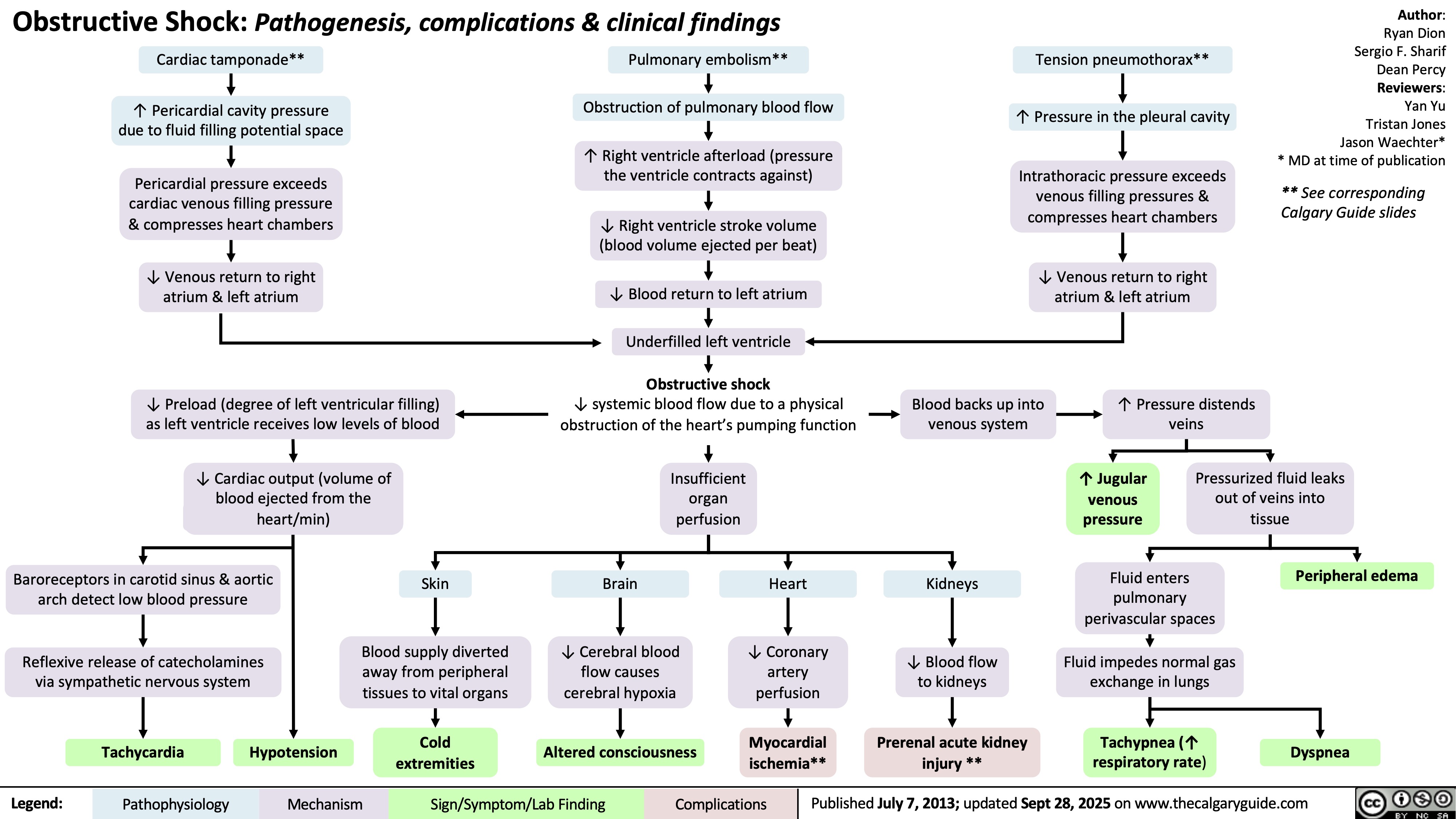
Sturge-Weber Syndrome
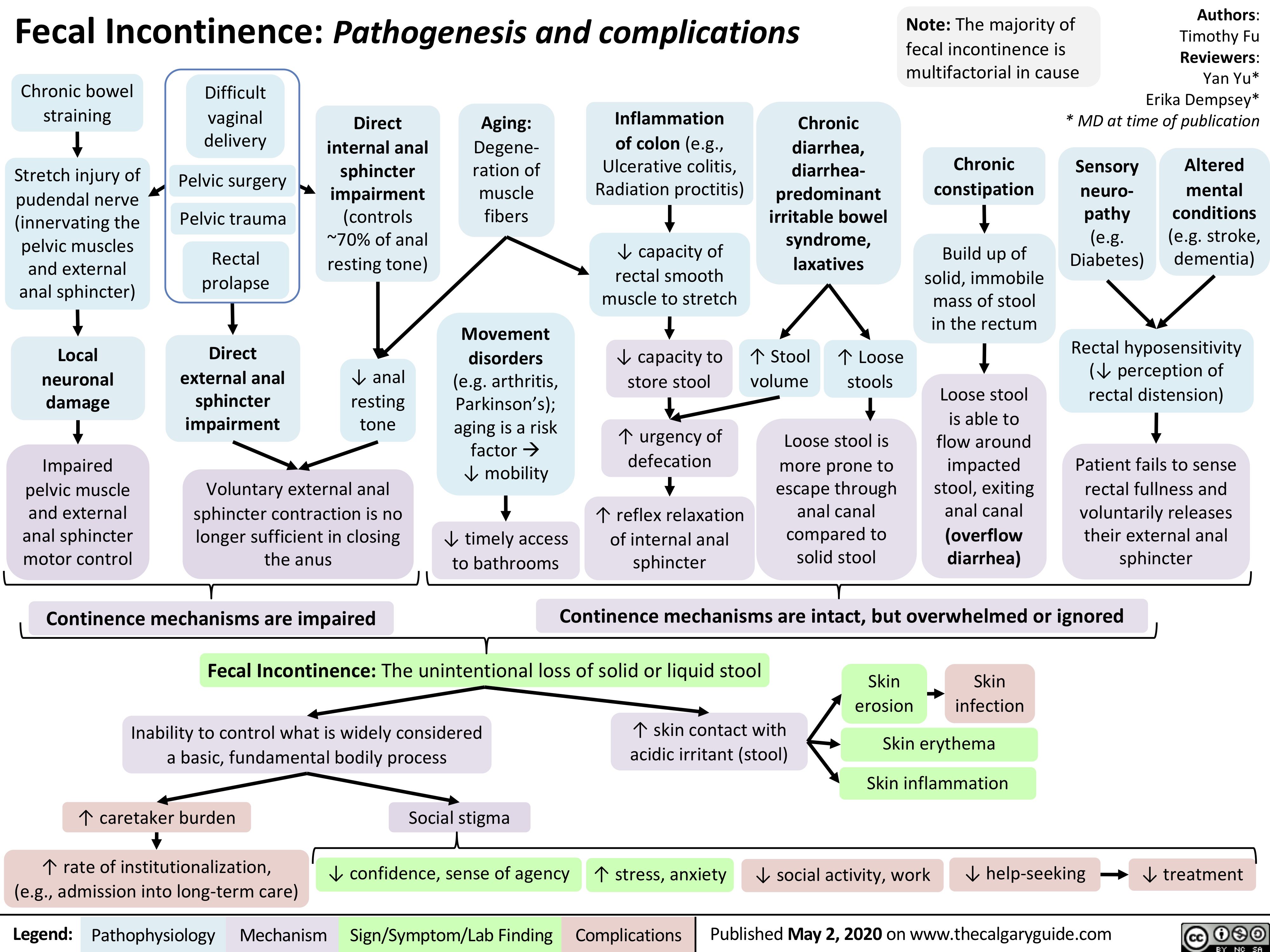
Fecal Incontinence
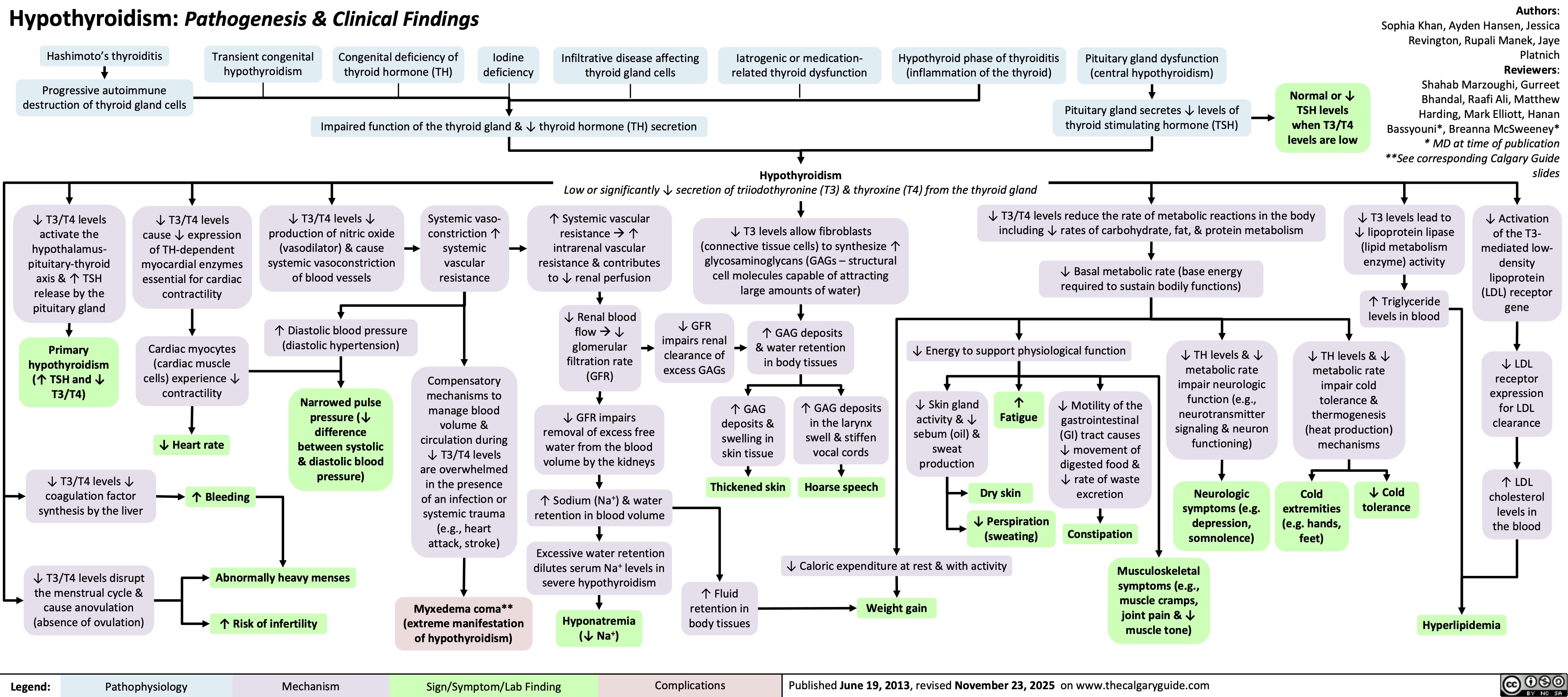
Hypothyroidism
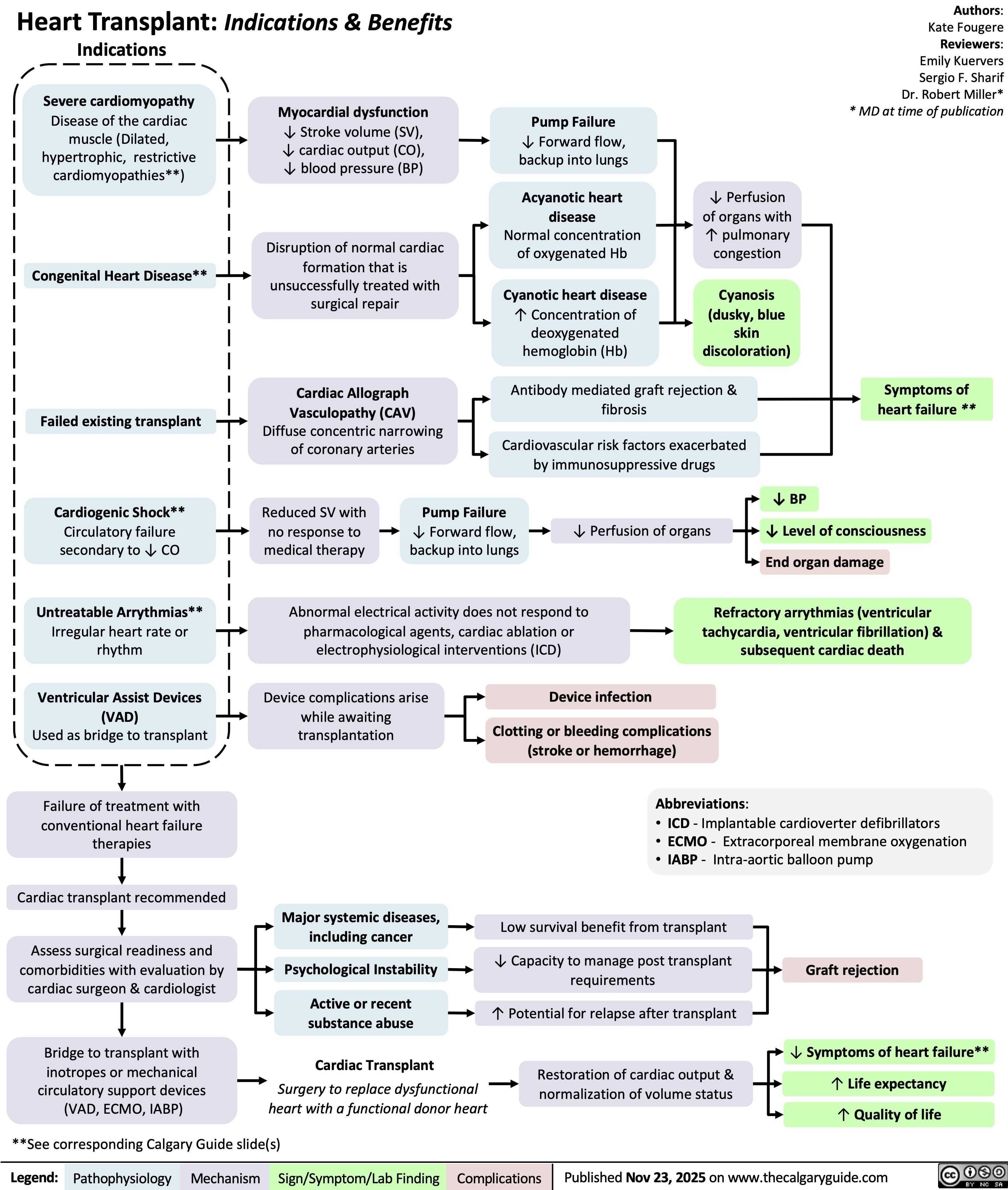
Heart Transplant Indications and Benefits
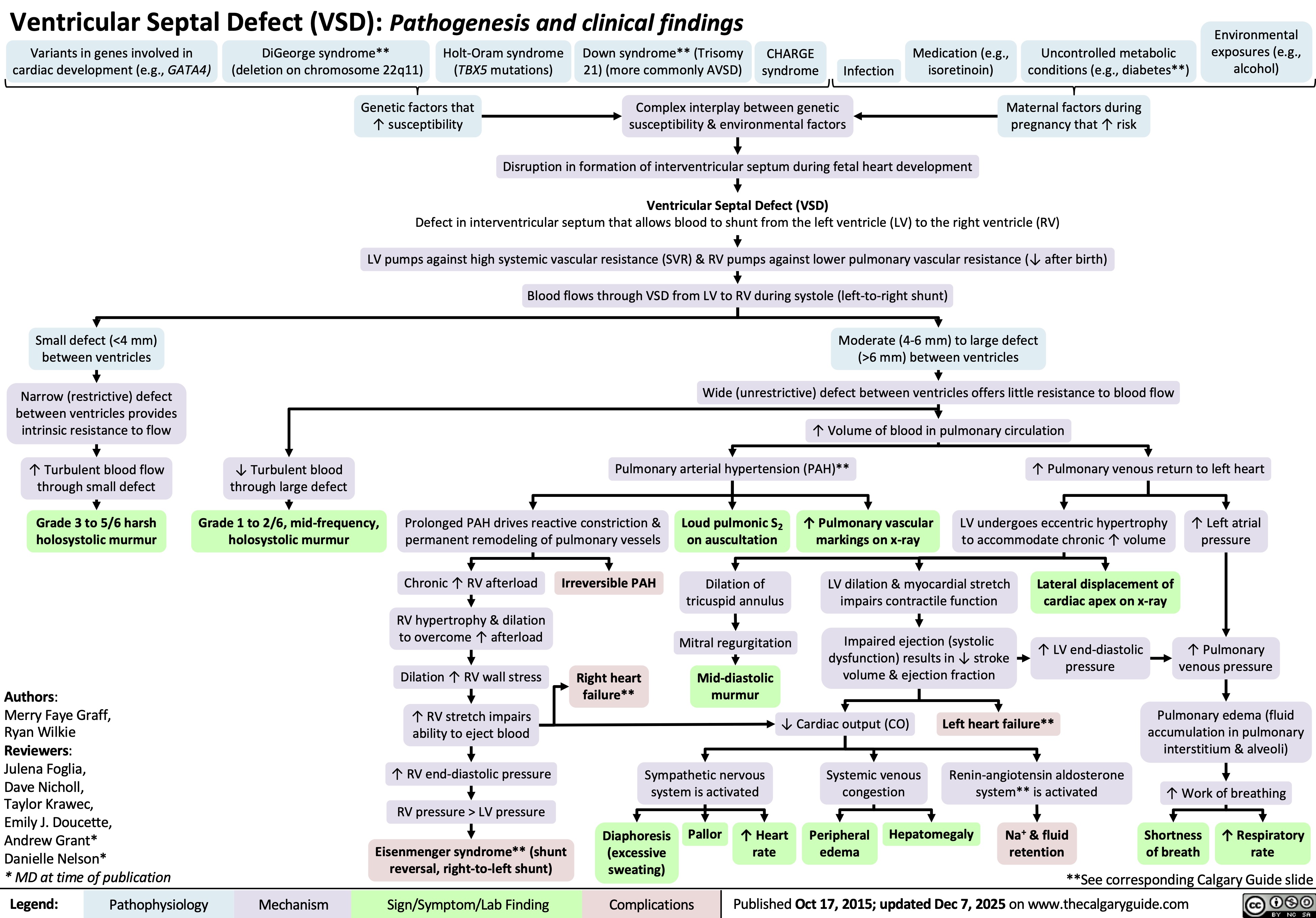
Ventricular Septal Defect
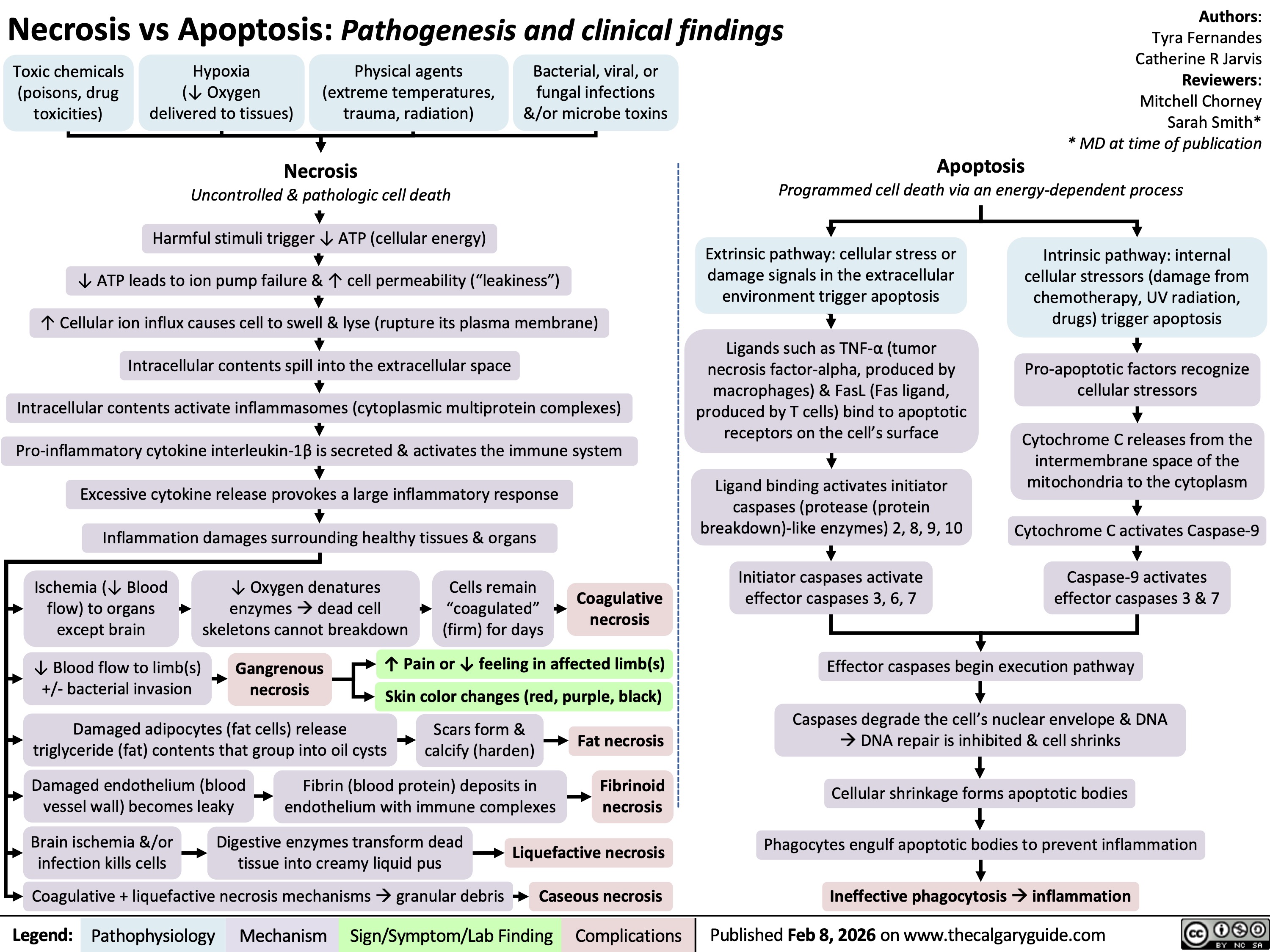
Necrosis versus Apoptosis
 L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" title="Yu Yan - MI Findings on History - FINAL.pptx -
Myocardial Infarction: Findings on HistoryLegend:Published January 30, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsAuthor: Yan YuReviewers:Sean SpenceTristan JonesNanette Alvarez** MD at time of publication Systolic function(necrotic myocardium cannot contract as well)Reflexive ? in sympathetic activity (to try to maintain CO)Clammy skin? stroke volume (SV), ? cardiac output (CO)Myocardial infarction (tissue necrosis)Note: Myocardial ischemic pain may differ between patients, but recurrences usually feel the same in any given patient.Generalized vasoconstrictionVasoconstriction of skin arteriolesCool skinLocal myocardial inflammationIrritation of T1-T4 sympathetic afferentsIrritation of cardiac branches of vagus nerveSignals enter spinal cord, mixes with T1-T4 dermatomesCrushing, Diffuse "Pain" or "tightness": Often retrosternal, with radiation to shoulder, neck, and inner aspect of both arms (R > L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" />
L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" title="Yu Yan - MI Findings on History - FINAL.pptx -
Myocardial Infarction: Findings on HistoryLegend:Published January 30, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsAuthor: Yan YuReviewers:Sean SpenceTristan JonesNanette Alvarez** MD at time of publication Systolic function(necrotic myocardium cannot contract as well)Reflexive ? in sympathetic activity (to try to maintain CO)Clammy skin? stroke volume (SV), ? cardiac output (CO)Myocardial infarction (tissue necrosis)Note: Myocardial ischemic pain may differ between patients, but recurrences usually feel the same in any given patient.Generalized vasoconstrictionVasoconstriction of skin arteriolesCool skinLocal myocardial inflammationIrritation of T1-T4 sympathetic afferentsIrritation of cardiac branches of vagus nerveSignals enter spinal cord, mixes with T1-T4 dermatomesCrushing, Diffuse "Pain" or "tightness": Often retrosternal, with radiation to shoulder, neck, and inner aspect of both arms (R > L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" />






















![Obstructive Sleep Apnea: Pathogenesis and clinical findings
Vascular Factors: During recumbent sleep, more bodily fluids enter the head and neck area (compared to when the patient is standing/sitting)
↑ volume of head/neck tissue surrounding the upper airwayà possible airway obstruction
Authors: Ciara Hanly Austin Laing Alexander Arnold Reviewers: Steven Liu Amogh Agrawal Yonglin Mai (麦泳琳) Naushad Hirani* Yan Yu* *MD at time of publication
Neuromuscular Factors: Sleep onset and/or the sleeping state reduces the drive of respiratory muscles to breathe
↓ Upper airway neuromuscular activityà↓ upper airway caliber, ↑ upper airway resistance, ↑ upper airway collapsibility during sleep
Structural Factors: Obesity, tonsillar or adenoid hypertrophy, macroglossia, ↑ neck circumference, craniofacial abnormalities
Excess pressure on upper airway, or deformity to that area, ↑ risk of upper airway collapse
Polysomnography
Absence of airflow but persistent ventilatory effort
Hypopnea or Apnea
Paradoxical breathing Chest wall draws in and abdomen expands during inspiration
Ventilatory effort persists against closed airway
No air entry due to collapsed upper airway
↑ Negative intrathoracic pressure
↑ Venous return to right atrium
Stretching of right atrial myocardium à secretion of atrial natriuretic peptide (ANP)
ANP inhibits epithelial Na+ channels (ENaC) in the collecting ducts of the kidney from reabsorbing Na+ à Na+ excretion
↑ Na+ excretionà↑ water excretion
Nocturia
Complete or partial upper airway obstruction during sleep
↑ PCO2 & ̄ PO2
in the lungsà ̄ diffusion gradient of CO2 & O2 between lungs & arteries
↑ PaCO2,, ̄ PaO2
Respiratory acidosis (↑ [H+] in blood)àactivation of vascular endothelial voltage gated K+ channels
Cerebral blood vessel dilation to provide adequate O2 to brain
Morning Headaches
Activation of central (medulla oblongata) & peripheral (carotid body) chemoreceptors
↑ Respiratory drive à ↑ activation of respiratory muscles (ventilatory effort )
Transient arousal from sleep
↑ sympathetic nervous system activityà arterial vasoconstriction
↑ systemic vascular resistance
Systemic Hypertension
↑ intraluminal pressure within blood vesselsàadaptive vascular endothelial and smooth muscle changes
Artery walls thicken, harden and lose elasticityà ̄ perfusion to end organs (such as the brain)
Ischemic stroke
Hypoxia during the day and night
↑ pulmonary vascular resistance
Pulmonary Hypertension
Right heart pumps against higher pulmonary pressure àcardiomyocytes undergo concentric hypertrophy over time
Cor Pulmonale
(Right heart failure due to pulmonary hypertension, separate from left heart failure)
Respiratory muscles overcome upper airway obstructionà airway patency restored
Sleep fragmentation
̄ Daytime cognitive performance and attentiveness
↑ Risk of motor vehicle accidents
Daytime Sleepiness
Eg. Epworth Sleepiness Scale >10
Abbreviations:
PCO2: partial pressure of carbon dioxide PO2: partial pressure of oxygen PaCO2: partial pressure of carbon dioxide in arteries PaO2: partial pressure of oxygen in arteries
Ventilatory response overcompensatesà breathe out more CO2 than is required for homeostasisà ̄ PaCO2
̄ respiratory driveà ̄ ventilatory effort
Resuscitative Gasping
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 19, 2013, updated May 31, 2022 on www.thecalgaryguide.com
阻塞性睡眠呼吸暂停:发病机制及临床表现
作者:Ciara Hanly, Austin Laing, Alexander Arnold 审稿人: Steven Liu, Amogh Agrawal, Naushad Hirani*,Yan Yu* 译者: Zesheng Ye(叶泽生) 翻译审稿人: Yonglin Mai(麦泳琳) *发表时担任临床医生
神经肌肉因素: 睡眠状态下, 患者无法通过 适当增加上气道肌张力来维持气道通畅
上气道神经肌肉活动 ̄à上气道直径 ̄, 上气道 阻力↑, 睡眠时上气道塌陷
结构(解剖)因素: 肥胖、扁桃体或腺样体 肥大, 舌体肥大, 颈围增大, 颅面部畸形
上气道压力过大或上气道畸形, 上气道塌陷 的风险 ↑
血管因素: 仰卧位睡觉引起 夜间嘴侧液体移位
周围组织与压力 ↑à上气道阻塞
多导睡眠描记术
没有气流,但持
续通气
呼吸浅慢或 呼吸暂停
反常呼吸 吸气时胸壁凹陷, 腹部膨隆
持续通气以抵抗气道 闭合
上气道塌陷导致空气进
入气道受阻
腹膜腔负压↑ 静脉血回流右心室阻力↑
右心房心肌细胞拉伸 à心房利钠肽分泌 (ANP)
ANP抑制肾集合管的上 皮Na+通道(ENaC)对 Na+重吸收à Na+排出
Na+排出量↑ à 水排出量 ↑
睡眠时全部
或部分上呼
吸道阻塞
肺内PO2 ̄ 且 PCO2↑ à CO2 及 O2在肺和动脉 间的扩散梯度 ̄
↑ PaCO2, ̄ PaO2
呼吸性酸中毒 (血液中 [H+] ↑) à激活血管内皮电压
门控 K+
脑血管扩张为大 晨间头痛 脑提供足够的 O2
激活中央(延髓)和外周(颈动脉体)的化学感受器 呼吸驱动↑à呼吸肌活动 (呼吸做功 )↑
短暂的睡眠唤醒
通道 交感神经系统活动↑
全天缺氧 肺血管阻力↑
肺动脉高压
右心泵血以抵抗肺 动脉高压à 随着时 间推移,心肌向心 性肥大
肺心病(区别于左
心衰,右心衰是肺
动脉高压所致)
呼吸肌克服上气道阻力à 气道 明显恢复
睡眠过程不连续
白天的认知功能
及注意力 ̄
机动车辆事故风险↑
白天嗜睡
à 动脉收缩 全身血管阻力↑
高血压
血管内压力↑ à 血 管内皮和平滑肌发生 适应性改变
动脉壁增厚、硬化、失 去弹性à器官血液灌 注量 ̄ (如脑部)
缩写: PCO2:二氧化碳分压 PO2:氧分压 PaCO2:动脉二氧化 碳分压 PaO2:动脉血氧分压
通气过度 à呼出CO2 ↑ à PaCO2 ̄
呼吸驱动 ̄à 呼吸做功 ̄
复苏性鼾音
夜尿症
如:伊普沃斯嗜睡评分
>10
缺血性卒中
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年8月19日发表 www.thecalgaryguide.com, 2022年5月31日更新
Obstructive Sleep Apnea: Pathogenesis and clinical findings
Vascular Factors: During recumbent sleep, more bodily fluids enter the head and neck area (compared to when the patient is standing/sitting)
↑ volume of head/neck tissue surrounding the upper airwayà possible airway obstruction
Authors: Ciara Hanly Austin Laing Alexander Arnold Reviewers: Steven Liu Amogh Agrawal Yonglin Mai (麦泳琳) Naushad Hirani* Yan Yu* *MD at time of publication
Neuromuscular Factors: Sleep onset and/or the sleeping state reduces the drive of respiratory muscles to breathe
↓ Upper airway neuromuscular activityà↓ upper airway caliber, ↑ upper airway resistance, ↑ upper airway collapsibility during sleep
Structural Factors: Obesity, tonsillar or adenoid hypertrophy, macroglossia, ↑ neck circumference, craniofacial abnormalities
Excess pressure on upper airway, or deformity to that area, ↑ risk of upper airway collapse
Polysomnography
Absence of airflow but persistent ventilatory effort
Hypopnea or Apnea
Paradoxical breathing Chest wall draws in and abdomen expands during inspiration
Ventilatory effort persists against closed airway
No air entry due to collapsed upper airway
↑ Negative intrathoracic pressure
↑ Venous return to right atrium
Stretching of right atrial myocardium à secretion of atrial natriuretic peptide (ANP)
ANP inhibits epithelial Na+ channels (ENaC) in the collecting ducts of the kidney from reabsorbing Na+ à Na+ excretion
↑ Na+ excretionà↑ water excretion
Nocturia
Complete or partial upper airway obstruction during sleep
↑ PCO2 & ̄ PO2
in the lungsà ̄ diffusion gradient of CO2 & O2 between lungs & arteries
↑ PaCO2,, ̄ PaO2
Respiratory acidosis (↑ [H+] in blood)àactivation of vascular endothelial voltage gated K+ channels
Cerebral blood vessel dilation to provide adequate O2 to brain
Morning Headaches
Activation of central (medulla oblongata) & peripheral (carotid body) chemoreceptors
↑ Respiratory drive à ↑ activation of respiratory muscles (ventilatory effort )
Transient arousal from sleep
↑ sympathetic nervous system activityà arterial vasoconstriction
↑ systemic vascular resistance
Systemic Hypertension
↑ intraluminal pressure within blood vesselsàadaptive vascular endothelial and smooth muscle changes
Artery walls thicken, harden and lose elasticityà ̄ perfusion to end organs (such as the brain)
Ischemic stroke
Hypoxia during the day and night
↑ pulmonary vascular resistance
Pulmonary Hypertension
Right heart pumps against higher pulmonary pressure àcardiomyocytes undergo concentric hypertrophy over time
Cor Pulmonale
(Right heart failure due to pulmonary hypertension, separate from left heart failure)
Respiratory muscles overcome upper airway obstructionà airway patency restored
Sleep fragmentation
̄ Daytime cognitive performance and attentiveness
↑ Risk of motor vehicle accidents
Daytime Sleepiness
Eg. Epworth Sleepiness Scale >10
Abbreviations:
PCO2: partial pressure of carbon dioxide PO2: partial pressure of oxygen PaCO2: partial pressure of carbon dioxide in arteries PaO2: partial pressure of oxygen in arteries
Ventilatory response overcompensatesà breathe out more CO2 than is required for homeostasisà ̄ PaCO2
̄ respiratory driveà ̄ ventilatory effort
Resuscitative Gasping
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 19, 2013, updated May 31, 2022 on www.thecalgaryguide.com
阻塞性睡眠呼吸暂停:发病机制及临床表现
作者:Ciara Hanly, Austin Laing, Alexander Arnold 审稿人: Steven Liu, Amogh Agrawal, Naushad Hirani*,Yan Yu* 译者: Zesheng Ye(叶泽生) 翻译审稿人: Yonglin Mai(麦泳琳) *发表时担任临床医生
神经肌肉因素: 睡眠状态下, 患者无法通过 适当增加上气道肌张力来维持气道通畅
上气道神经肌肉活动 ̄à上气道直径 ̄, 上气道 阻力↑, 睡眠时上气道塌陷
结构(解剖)因素: 肥胖、扁桃体或腺样体 肥大, 舌体肥大, 颈围增大, 颅面部畸形
上气道压力过大或上气道畸形, 上气道塌陷 的风险 ↑
血管因素: 仰卧位睡觉引起 夜间嘴侧液体移位
周围组织与压力 ↑à上气道阻塞
多导睡眠描记术
没有气流,但持
续通气
呼吸浅慢或 呼吸暂停
反常呼吸 吸气时胸壁凹陷, 腹部膨隆
持续通气以抵抗气道 闭合
上气道塌陷导致空气进
入气道受阻
腹膜腔负压↑ 静脉血回流右心室阻力↑
右心房心肌细胞拉伸 à心房利钠肽分泌 (ANP)
ANP抑制肾集合管的上 皮Na+通道(ENaC)对 Na+重吸收à Na+排出
Na+排出量↑ à 水排出量 ↑
睡眠时全部
或部分上呼
吸道阻塞
肺内PO2 ̄ 且 PCO2↑ à CO2 及 O2在肺和动脉 间的扩散梯度 ̄
↑ PaCO2, ̄ PaO2
呼吸性酸中毒 (血液中 [H+] ↑) à激活血管内皮电压
门控 K+
脑血管扩张为大 晨间头痛 脑提供足够的 O2
激活中央(延髓)和外周(颈动脉体)的化学感受器 呼吸驱动↑à呼吸肌活动 (呼吸做功 )↑
短暂的睡眠唤醒
通道 交感神经系统活动↑
全天缺氧 肺血管阻力↑
肺动脉高压
右心泵血以抵抗肺 动脉高压à 随着时 间推移,心肌向心 性肥大
肺心病(区别于左
心衰,右心衰是肺
动脉高压所致)
呼吸肌克服上气道阻力à 气道 明显恢复
睡眠过程不连续
白天的认知功能
及注意力 ̄
机动车辆事故风险↑
白天嗜睡
à 动脉收缩 全身血管阻力↑
高血压
血管内压力↑ à 血 管内皮和平滑肌发生 适应性改变
动脉壁增厚、硬化、失 去弹性à器官血液灌 注量 ̄ (如脑部)
缩写: PCO2:二氧化碳分压 PO2:氧分压 PaCO2:动脉二氧化 碳分压 PaO2:动脉血氧分压
通气过度 à呼出CO2 ↑ à PaCO2 ̄
呼吸驱动 ̄à 呼吸做功 ̄
复苏性鼾音
夜尿症
如:伊普沃斯嗜睡评分
>10
缺血性卒中
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年8月19日发表 www.thecalgaryguide.com, 2022年5月31日更新](https://calgaryguide.ucalgary.ca/wp-content/uploads/2014/09/OSA-2021-1.jpg)


































