SEARCH RESULTS FOR: sepsis
Bronchopulmonary Dysplasia (BPD)- Pathogenesis and clinical findings
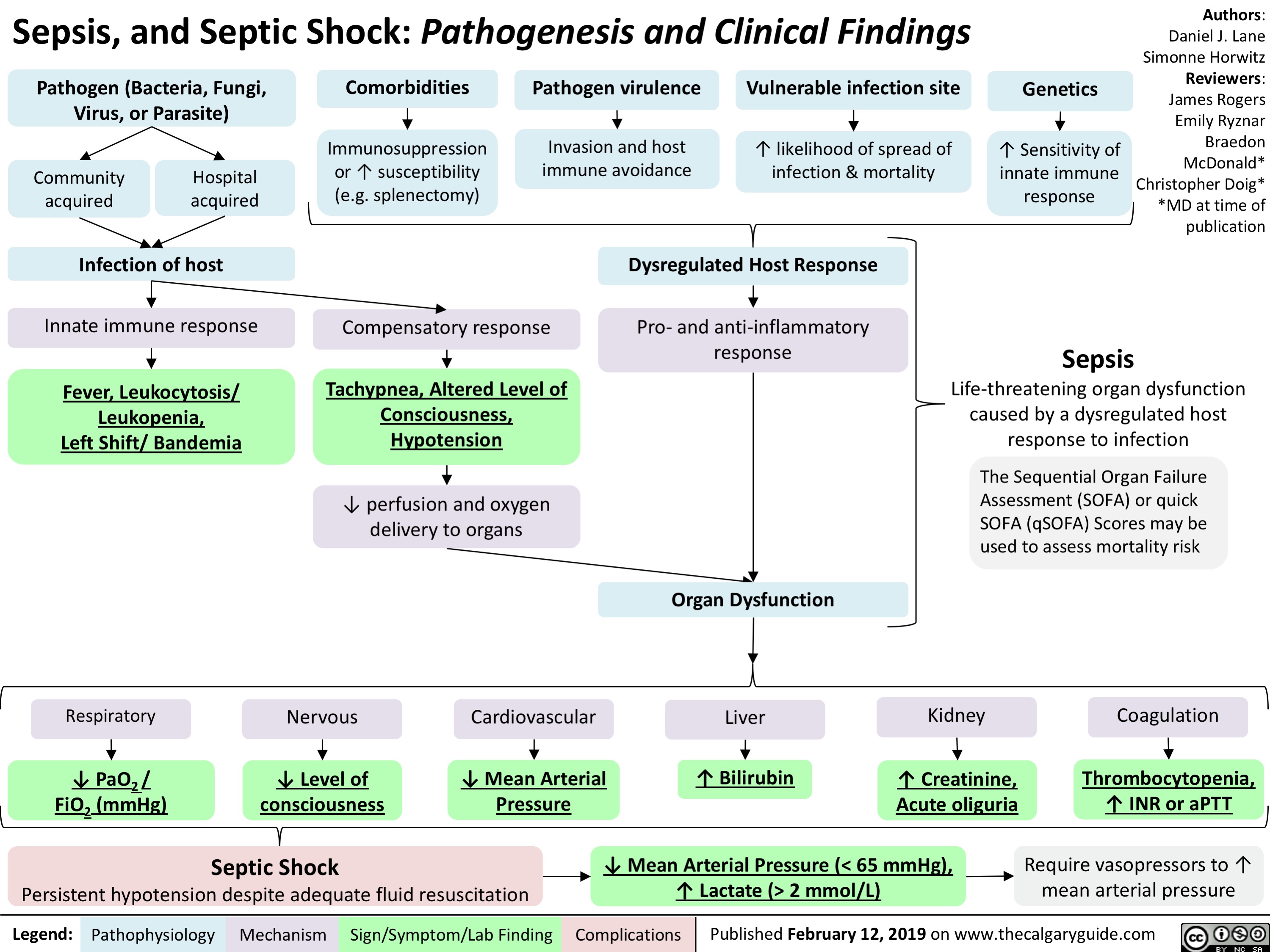
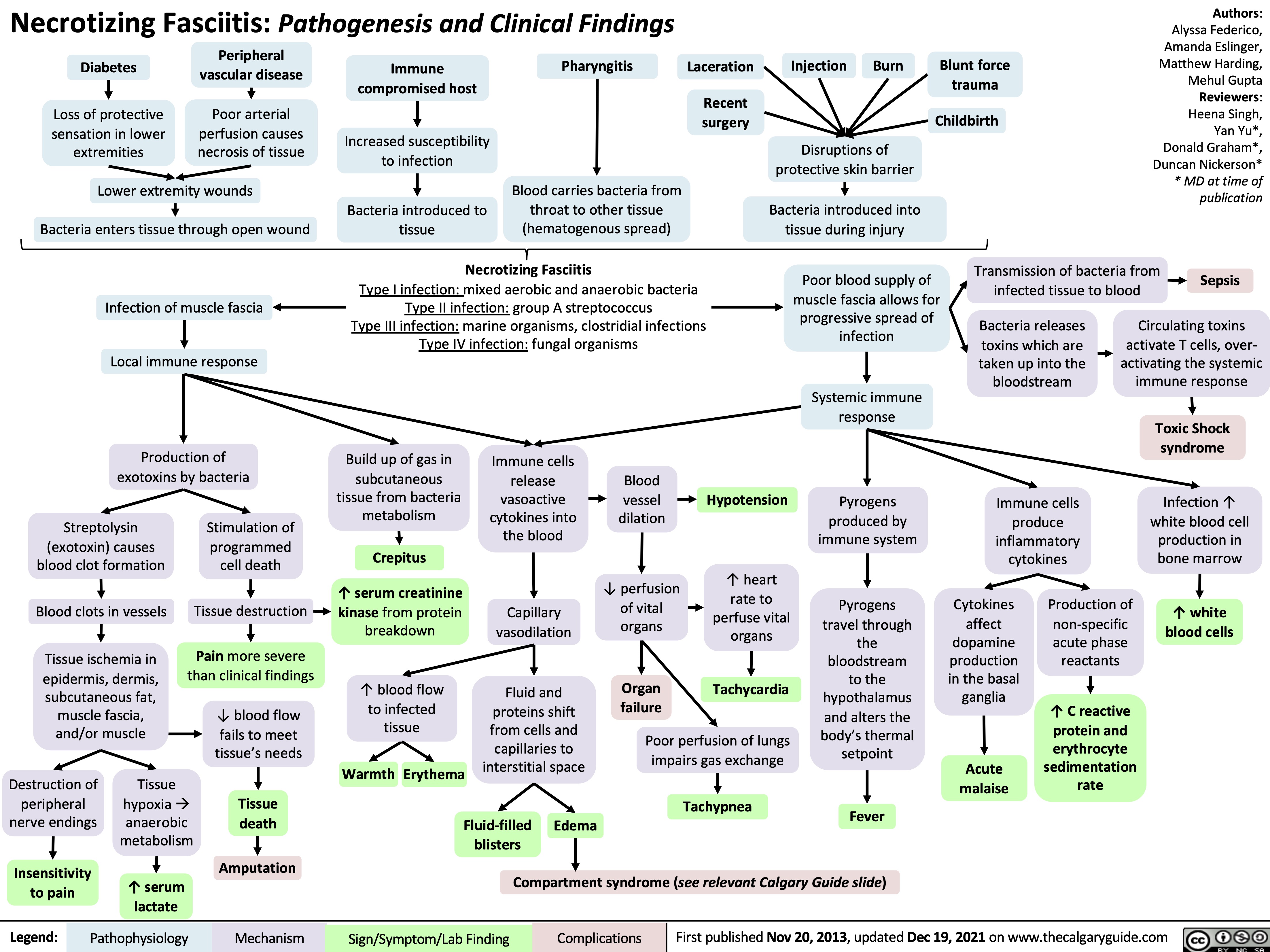
Sepsis, and Septic Shock- Pathogenesis and Clinical Findings
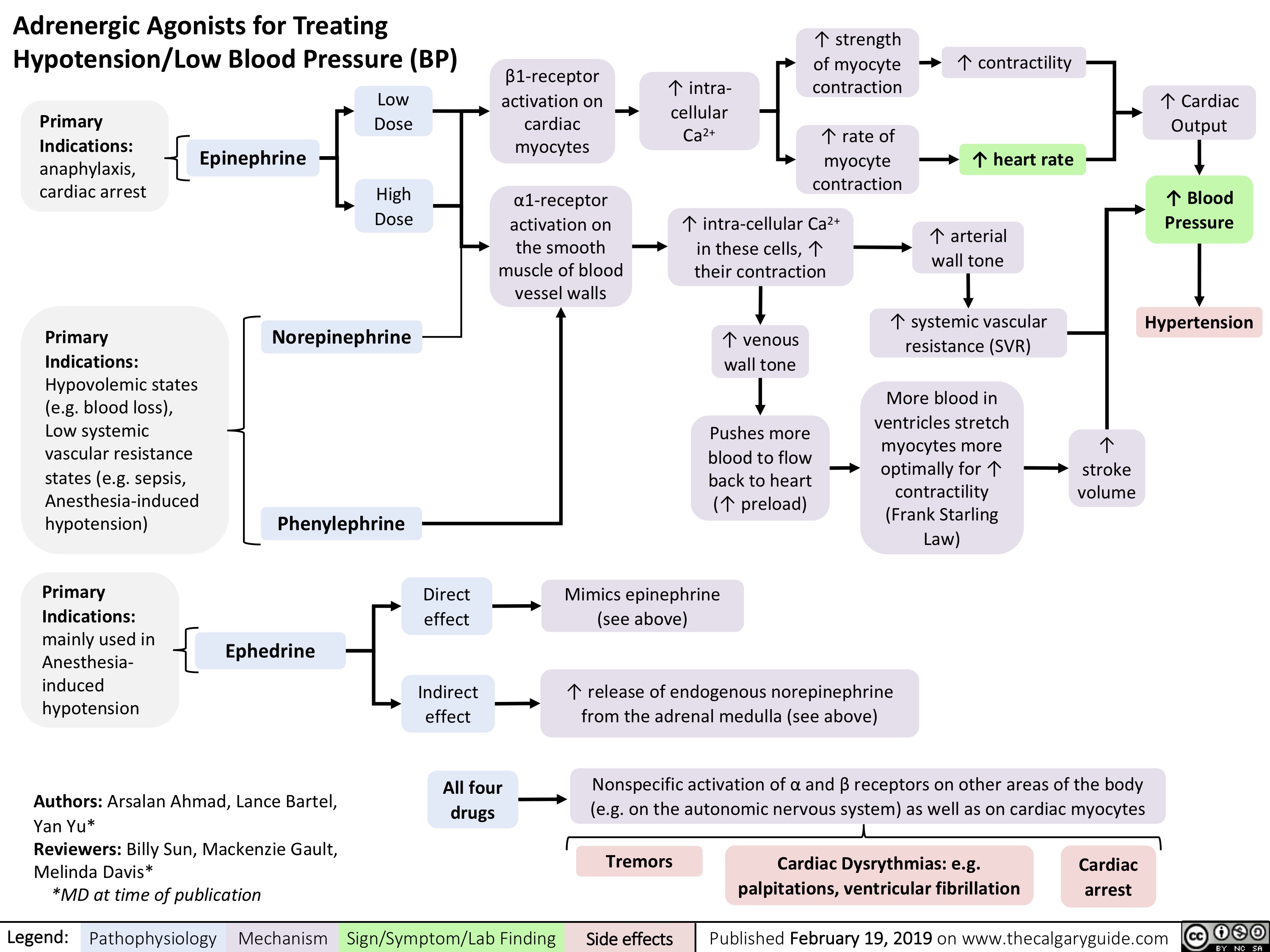
adrenergic-agonists-for-treating-hypotensionlow-blood-pressure
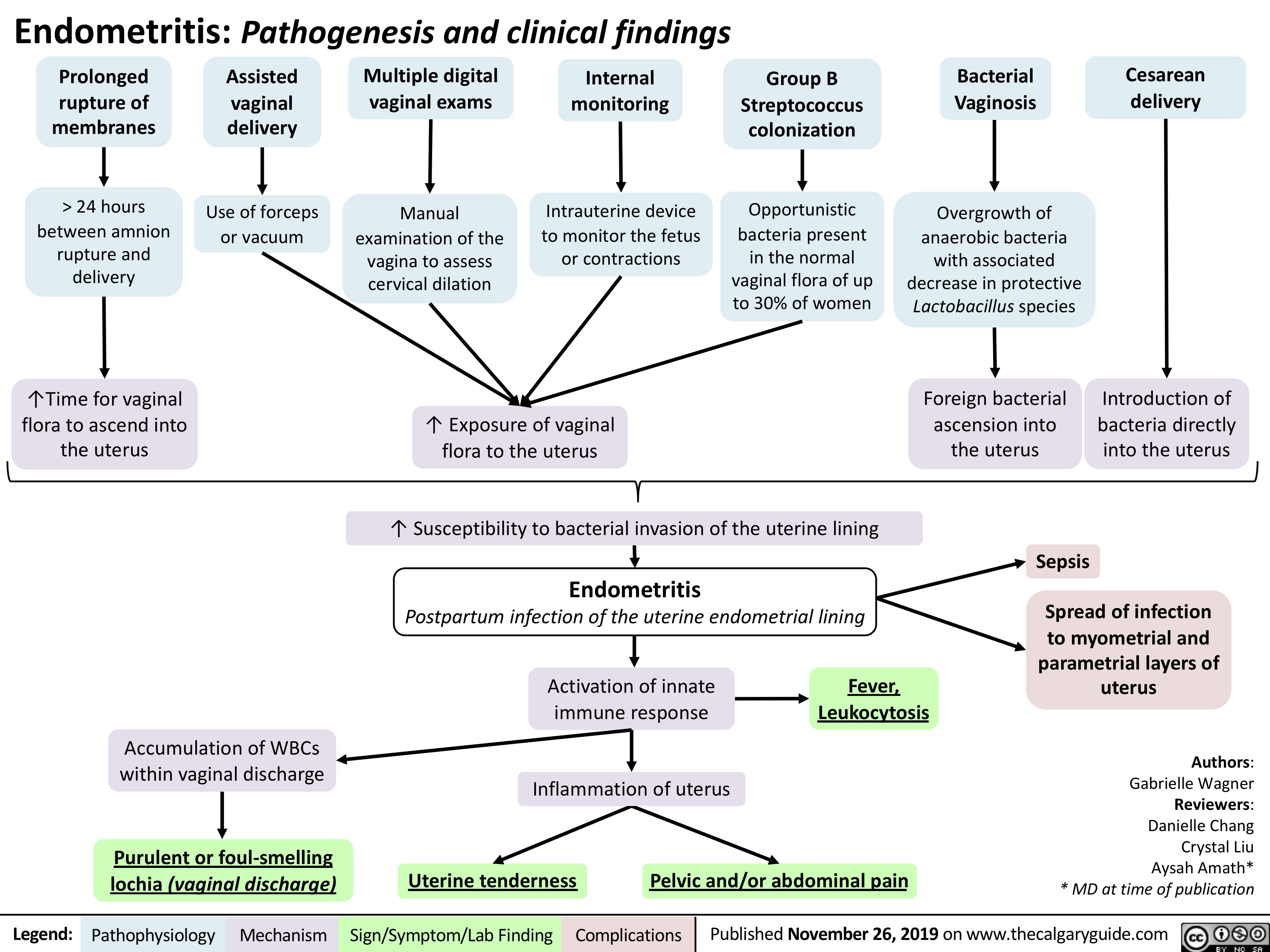
Endometritis
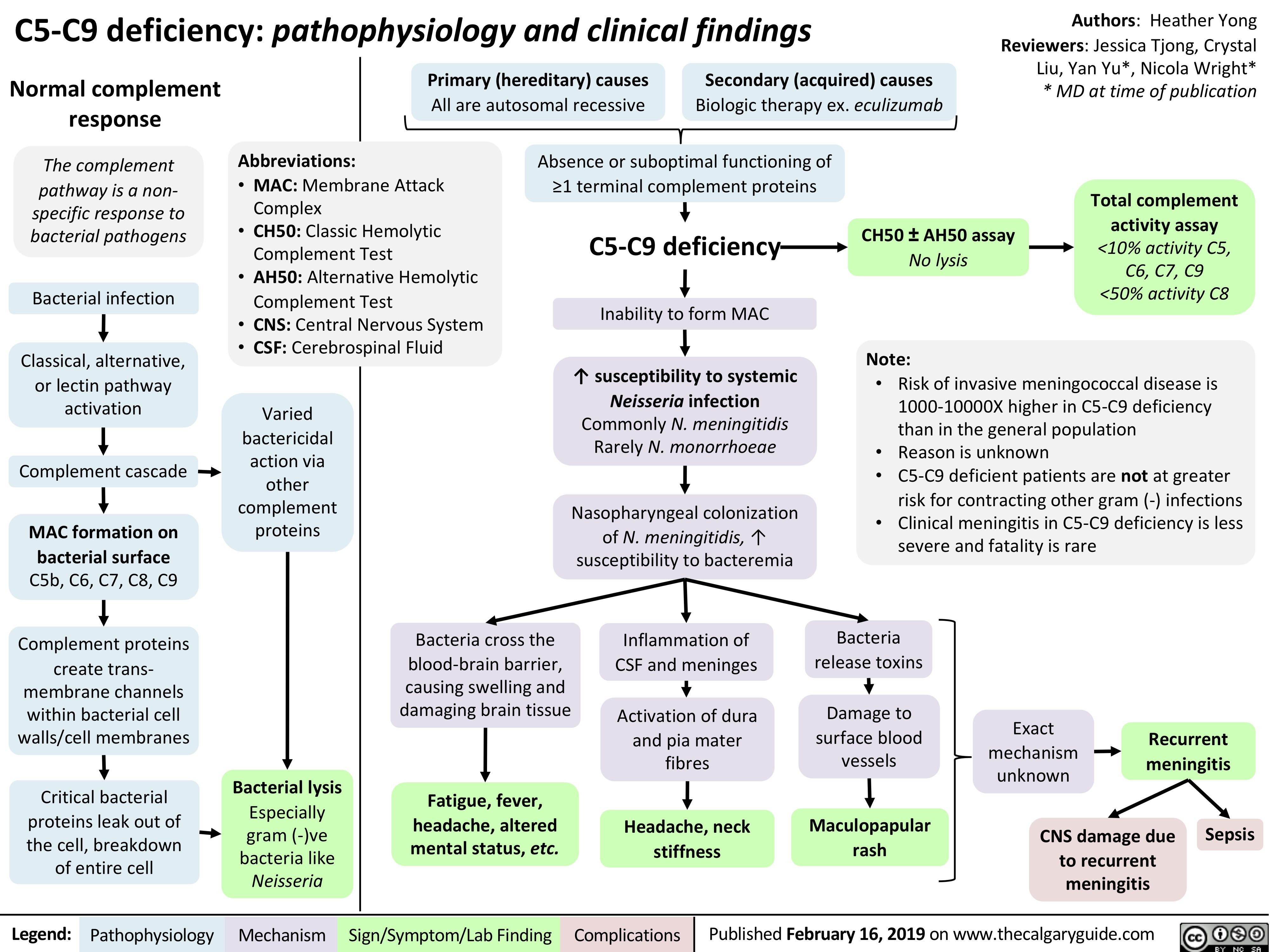
C5-C9-deficiency
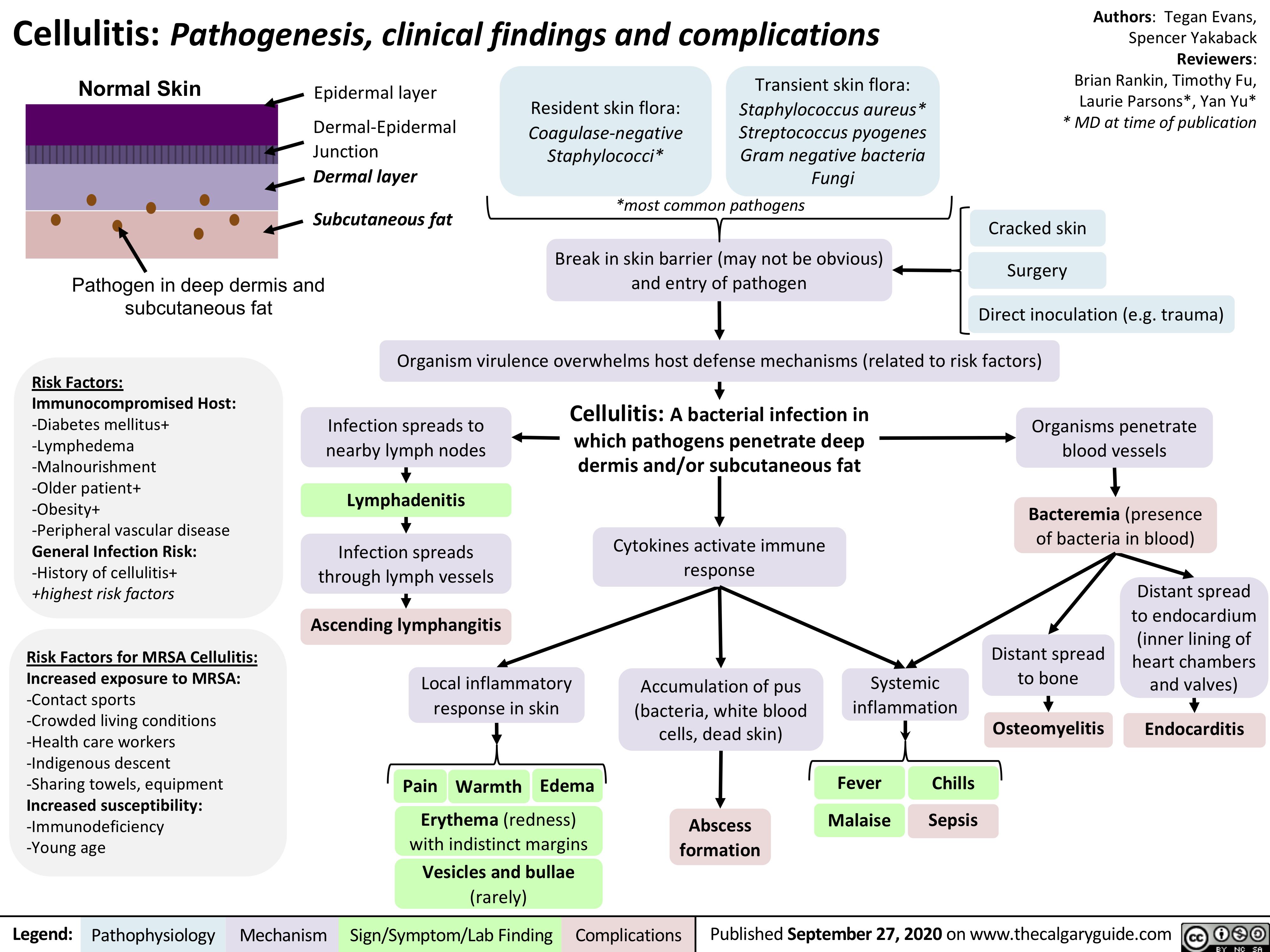
Cellulitis
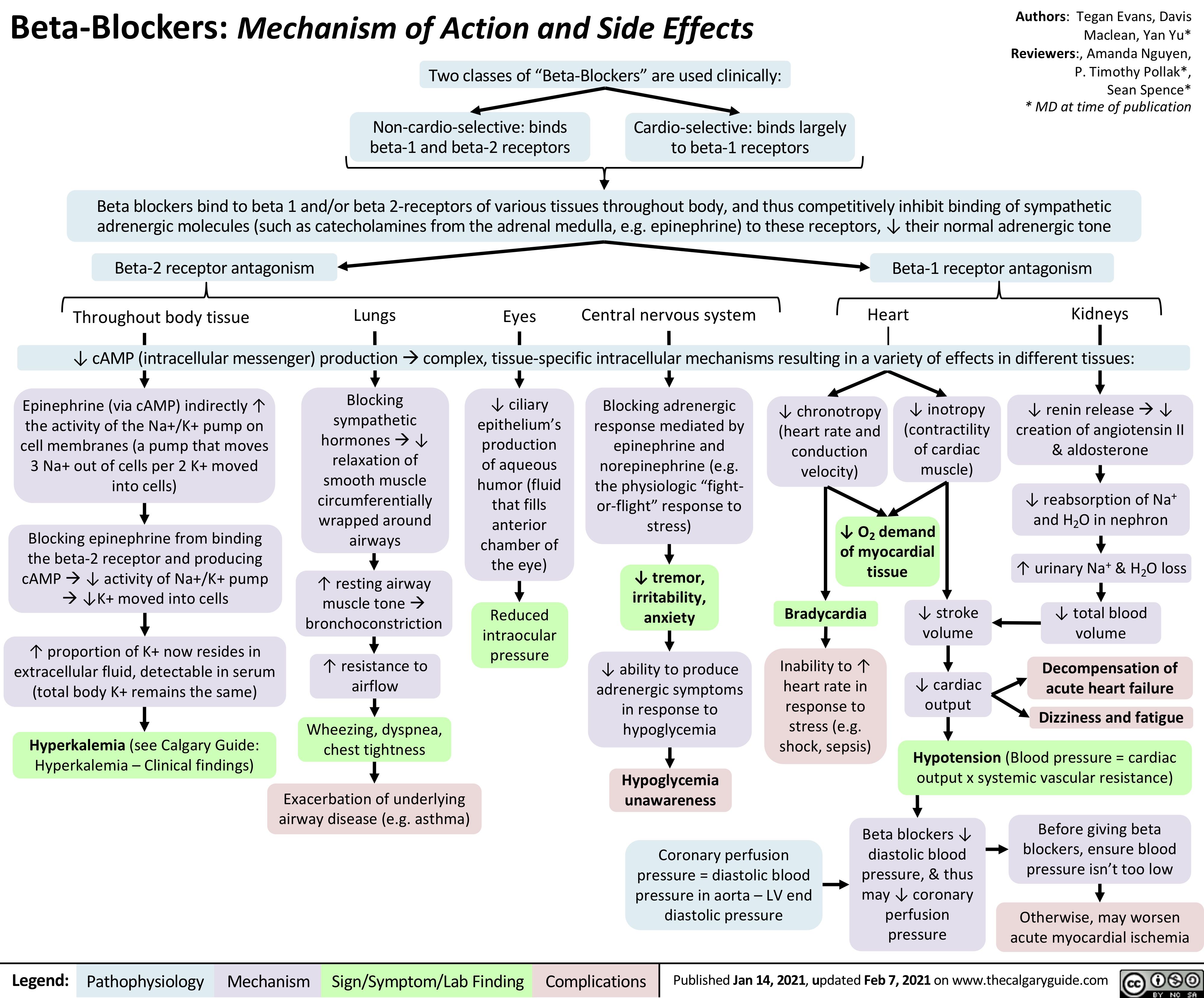
Beta-Blockers-Mechanism-of-Action-and-Side-Effects
disseminated-intravascular-coagulation
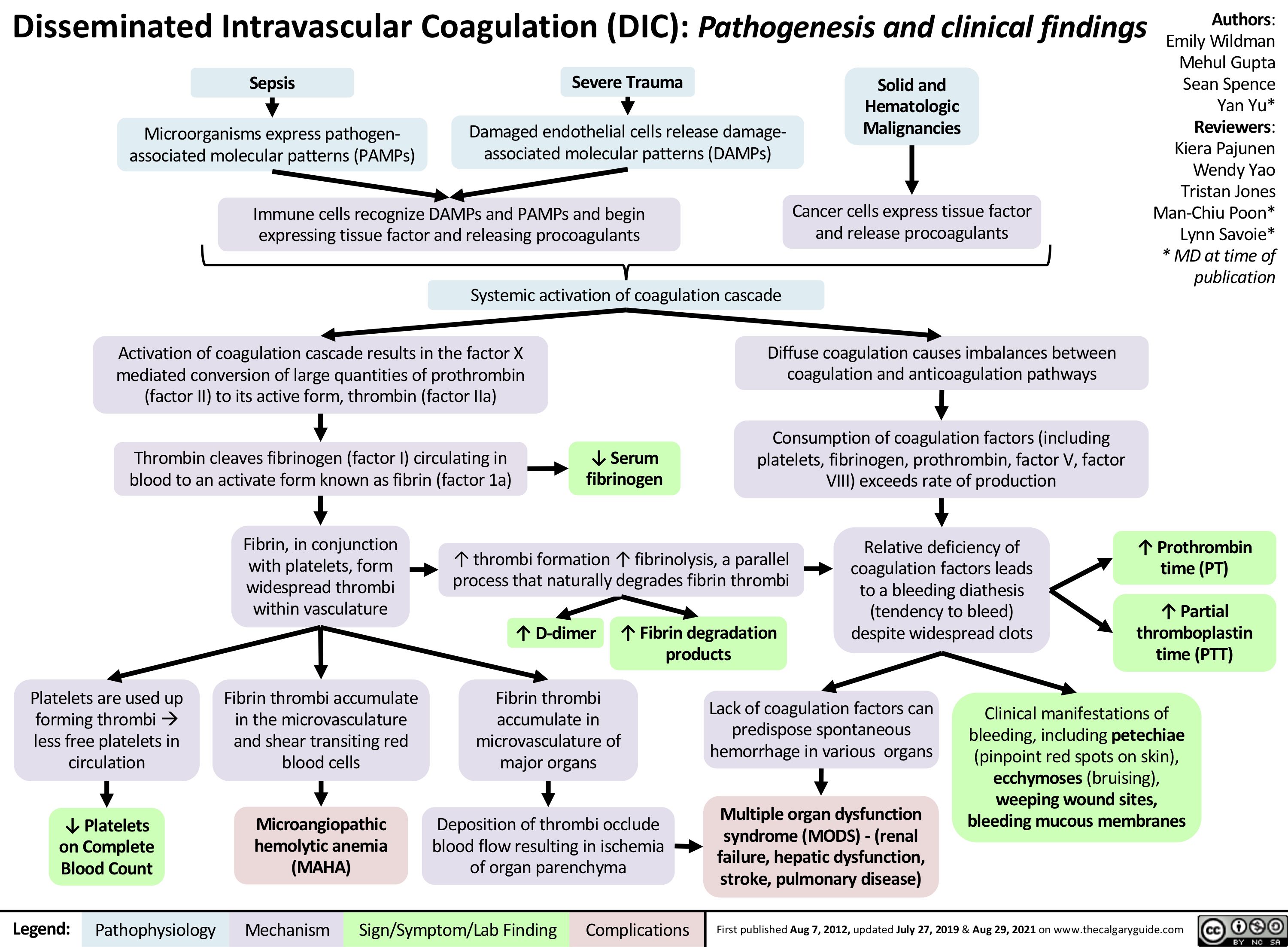
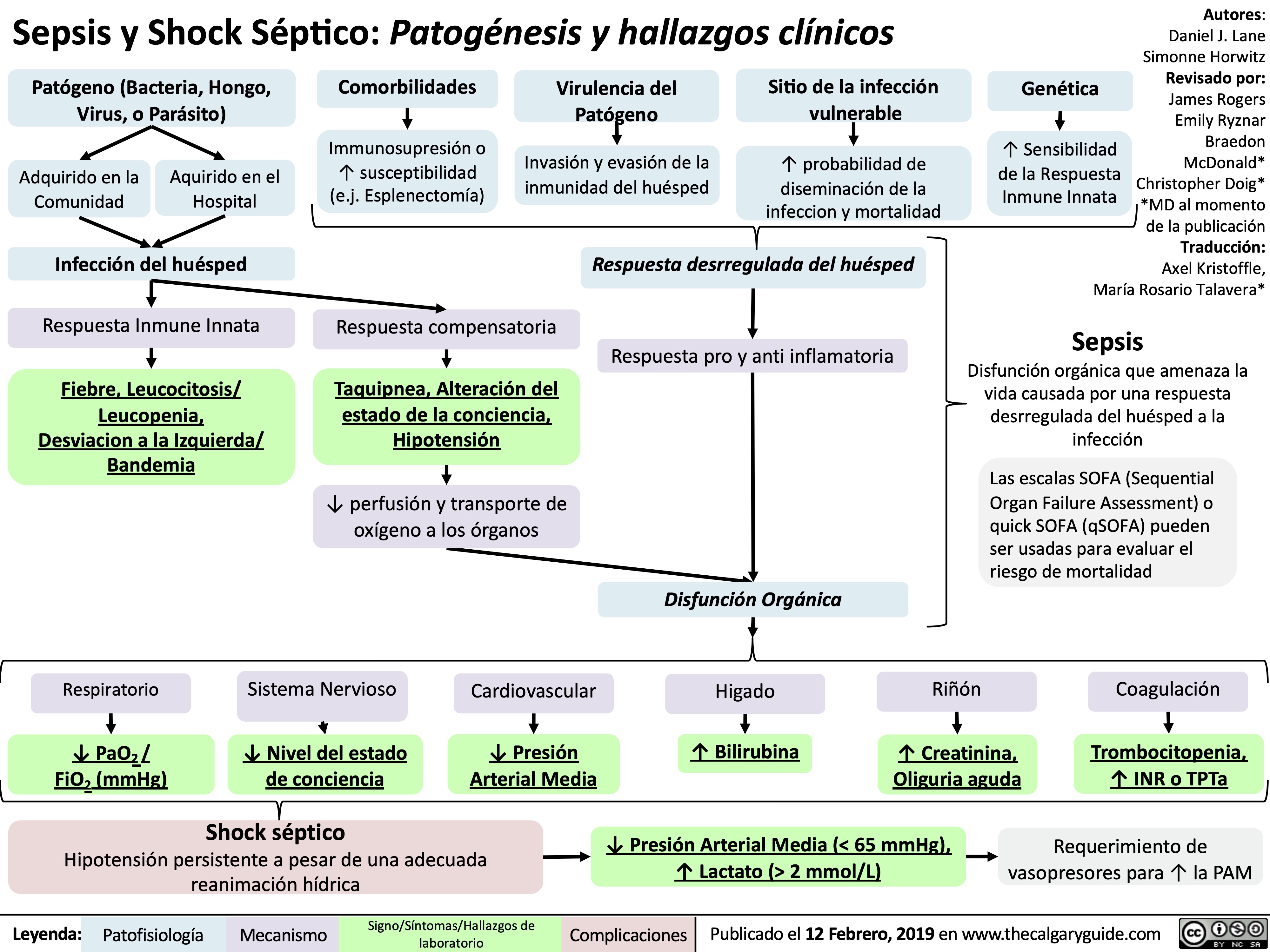
Pneumonie: Pathogenese und klinische Befunde
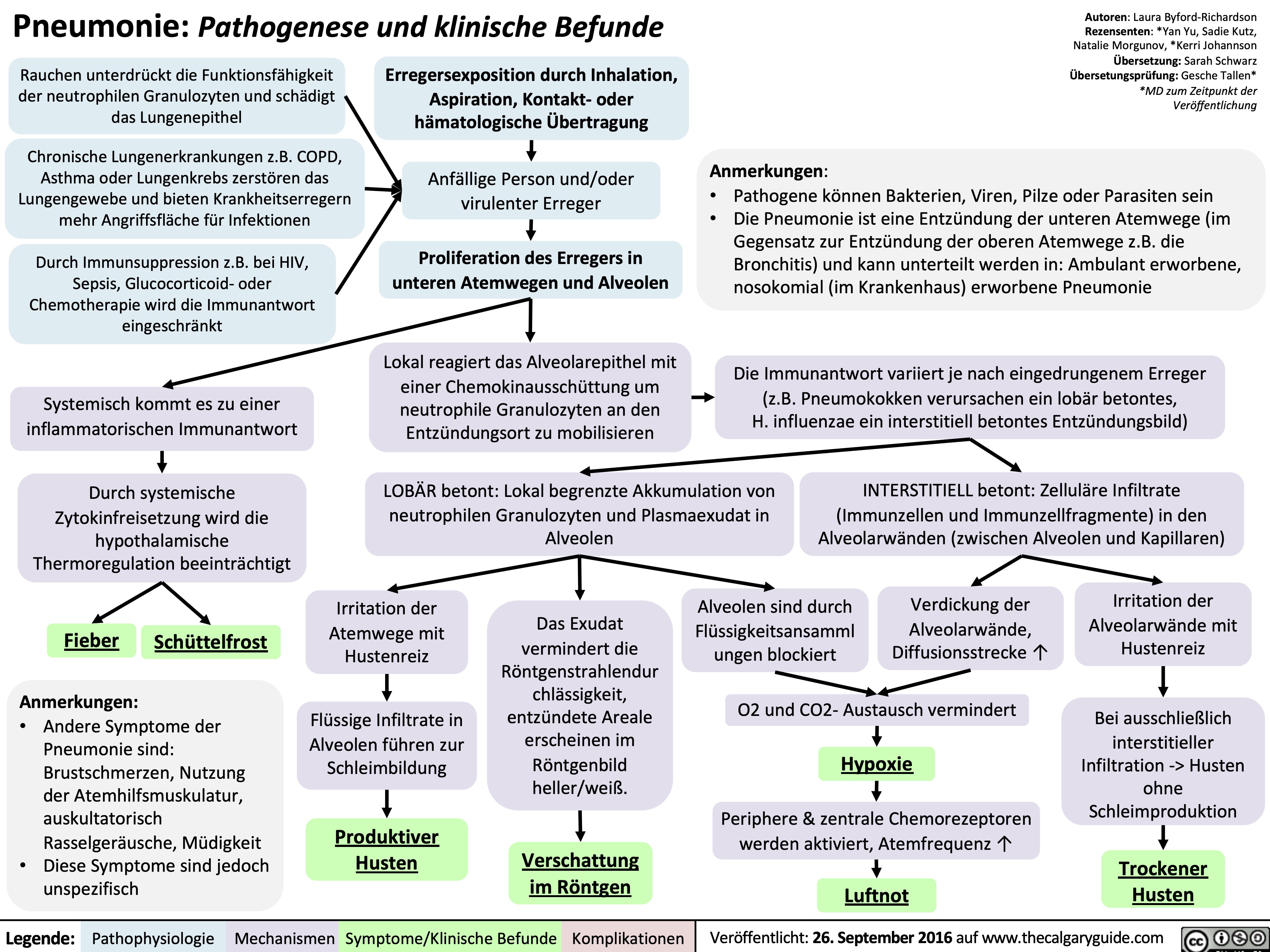
Celulitis
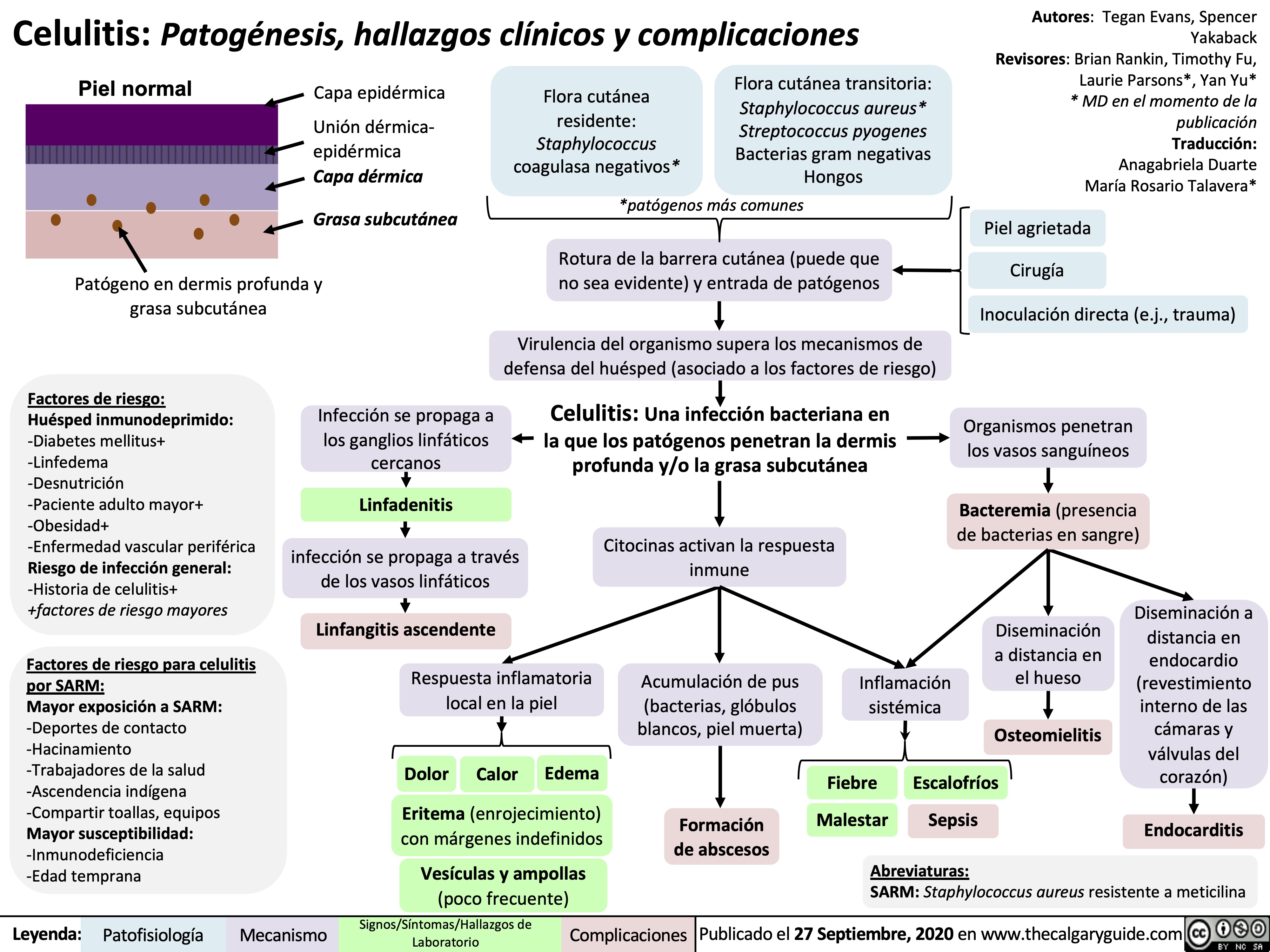
mechanical-bowel-obstruction-and-ileus-pathogenesis-and-clinical-findings
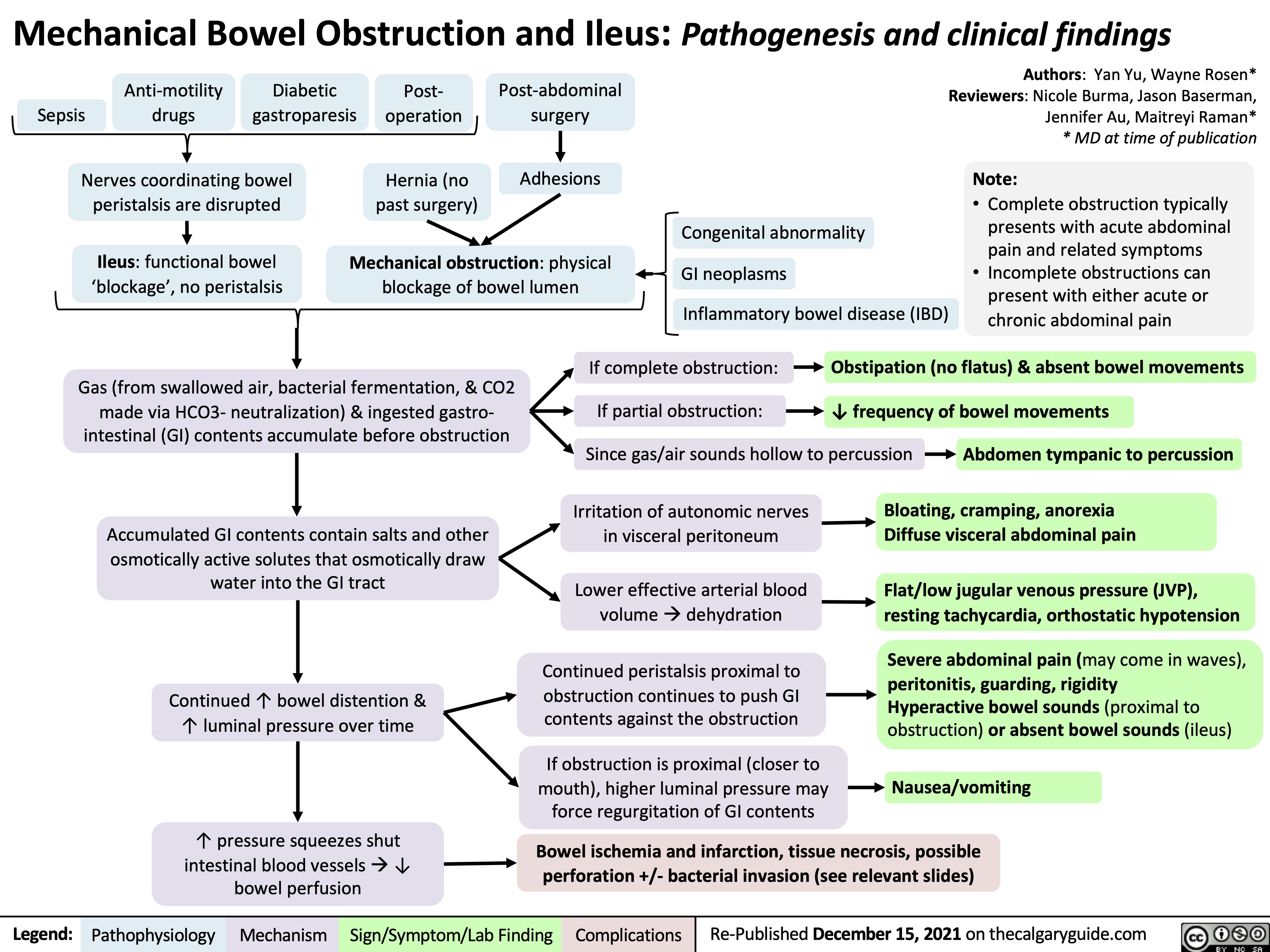
necrotizing fasciitis
sepsis-y-shock-septico-patogenesis-y-hallazgos-clinicos
adult-pneumonia-pathogenesis-and-clinical-findings
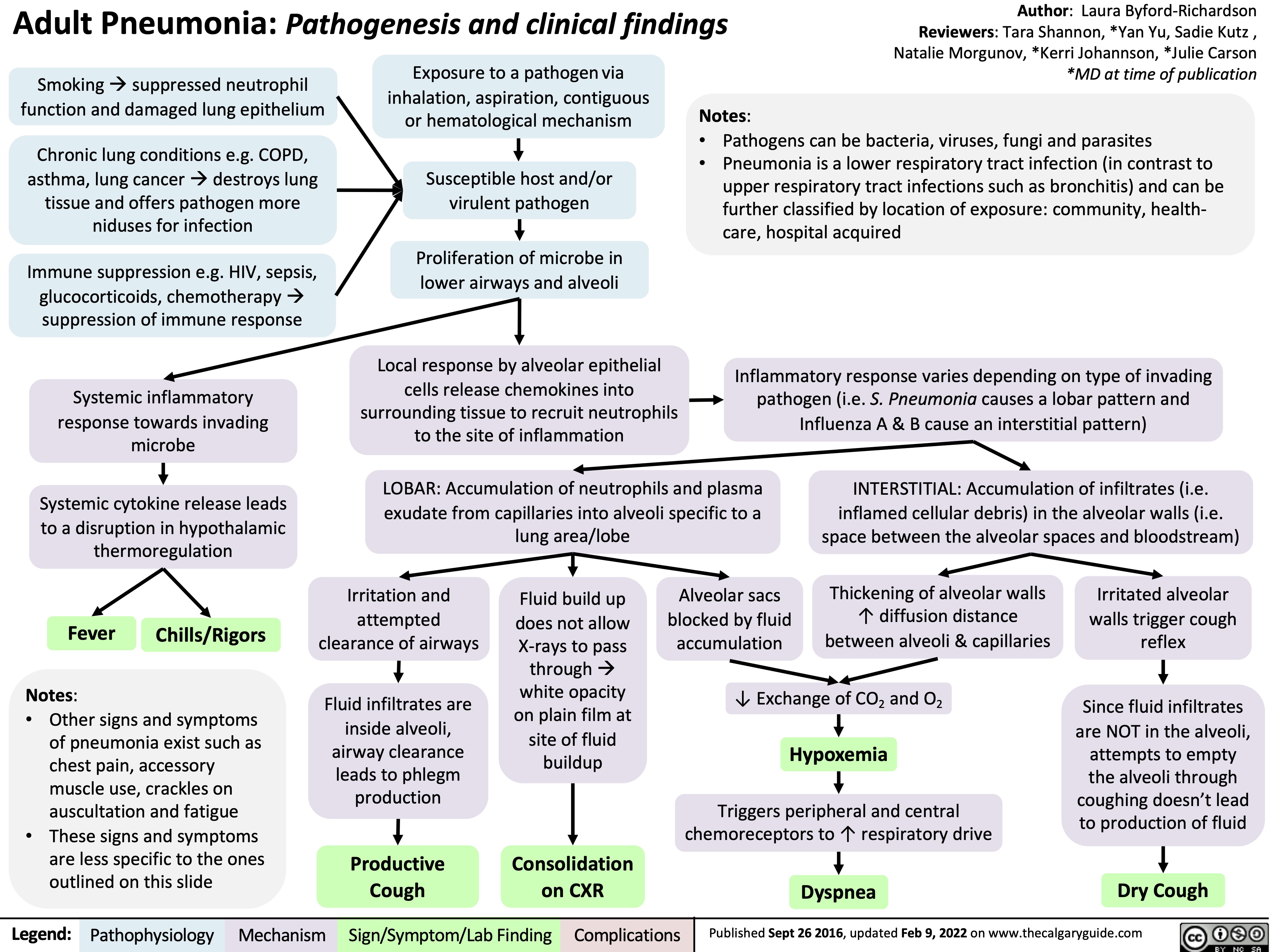
etomidate
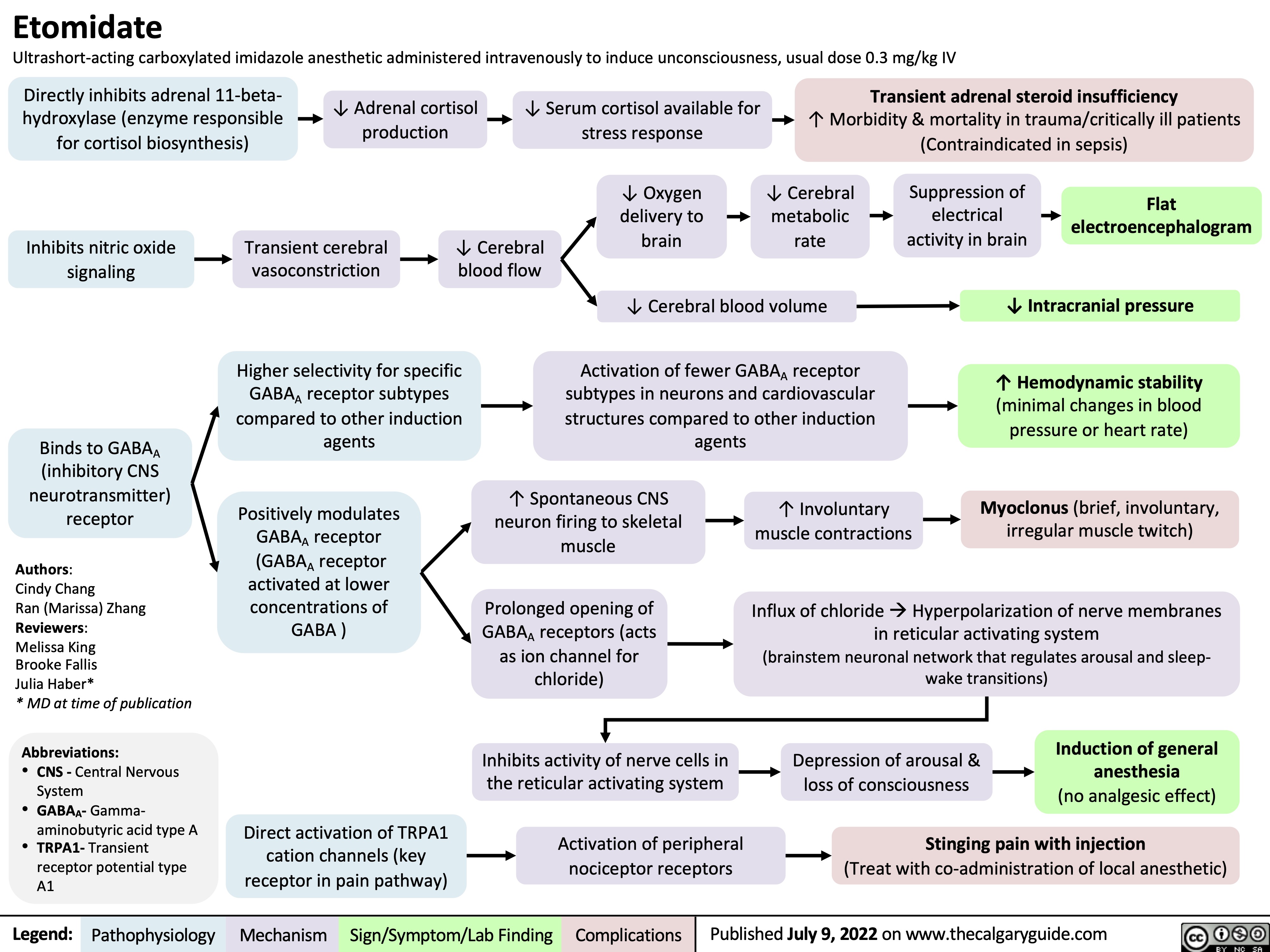
ventilator-associated-pneumonia-pathogenesis-and-clinical-findings
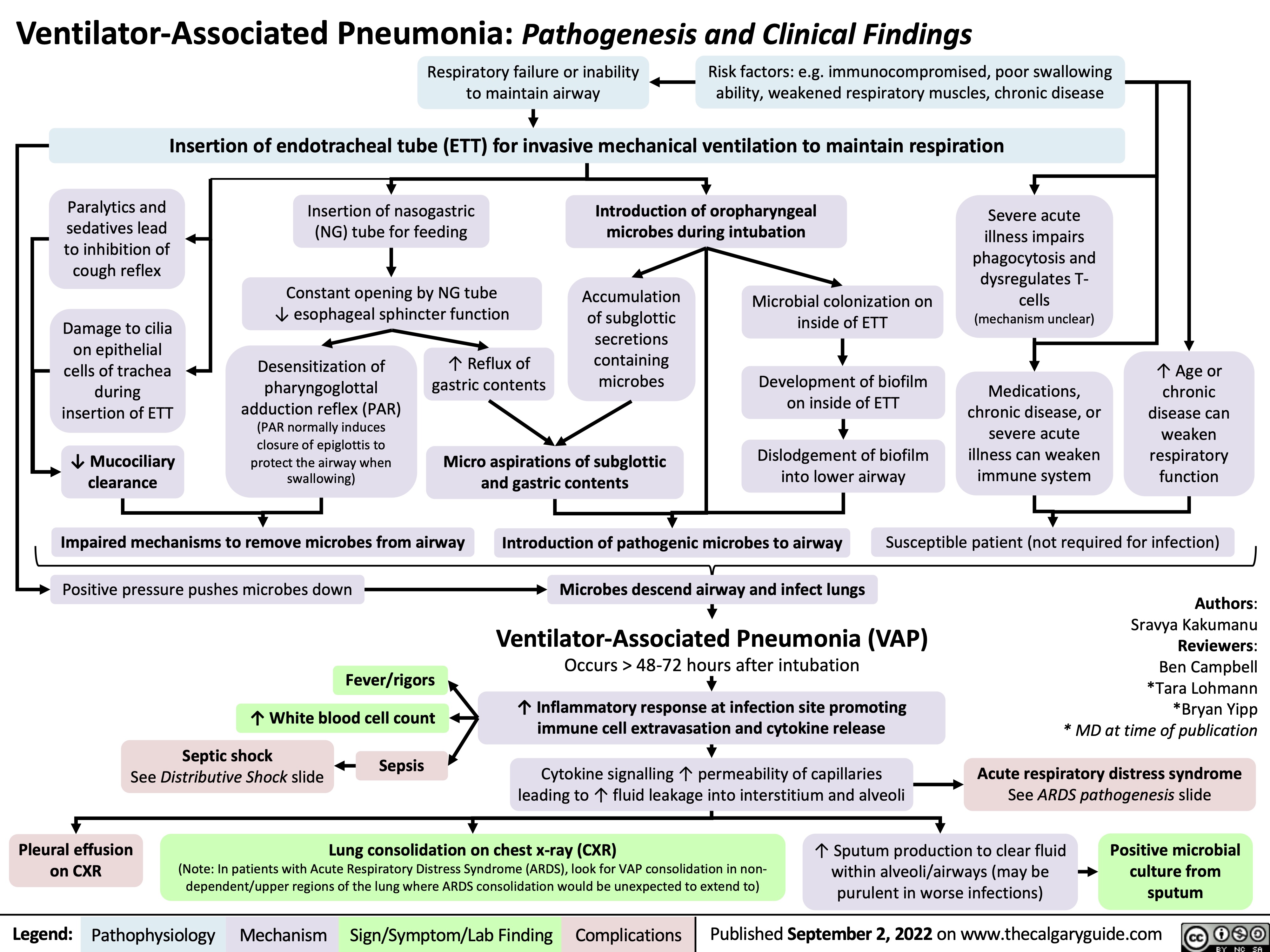
Neonatal Sepsis
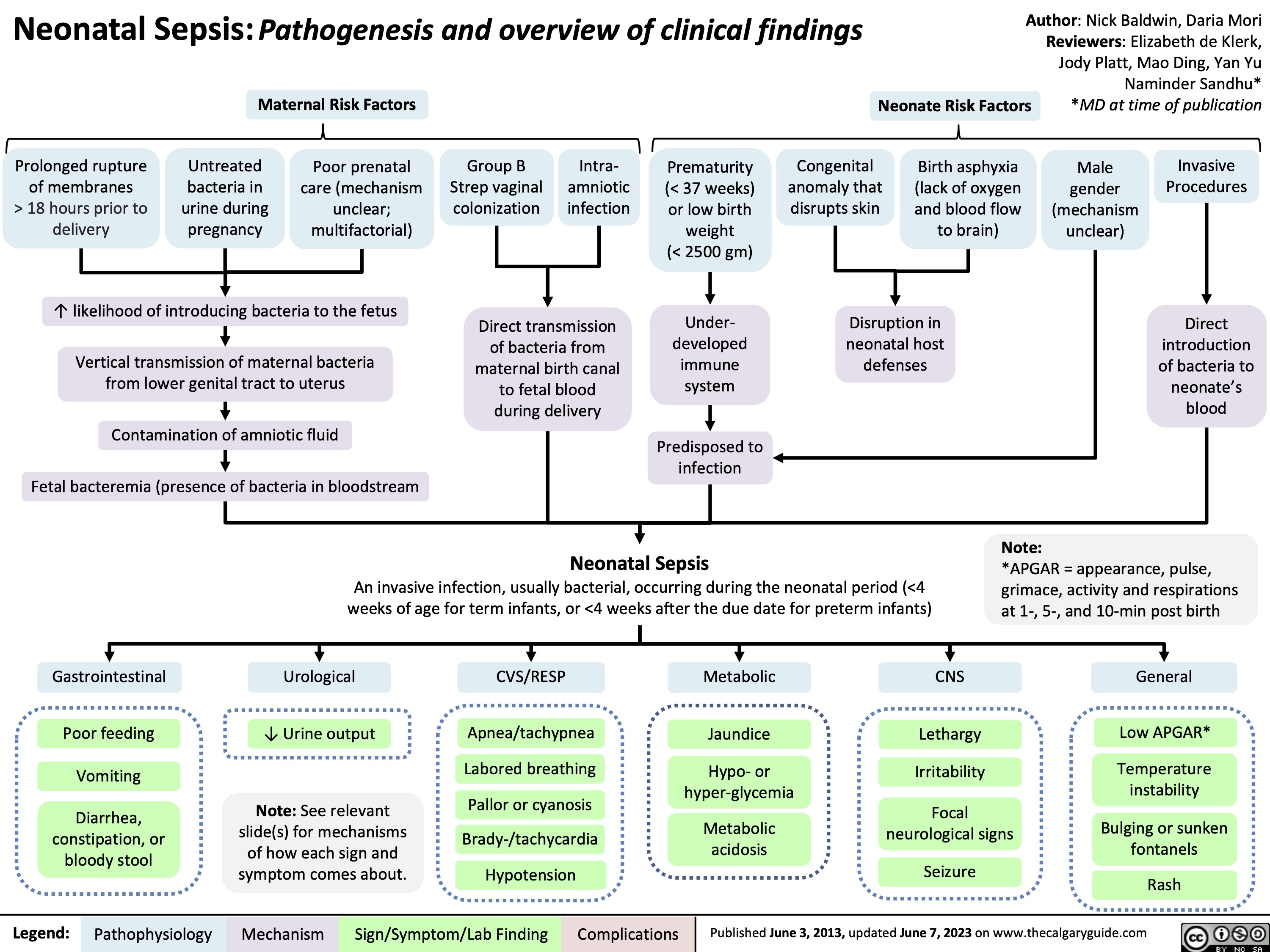
Acute Respiratory Distress Syndrome
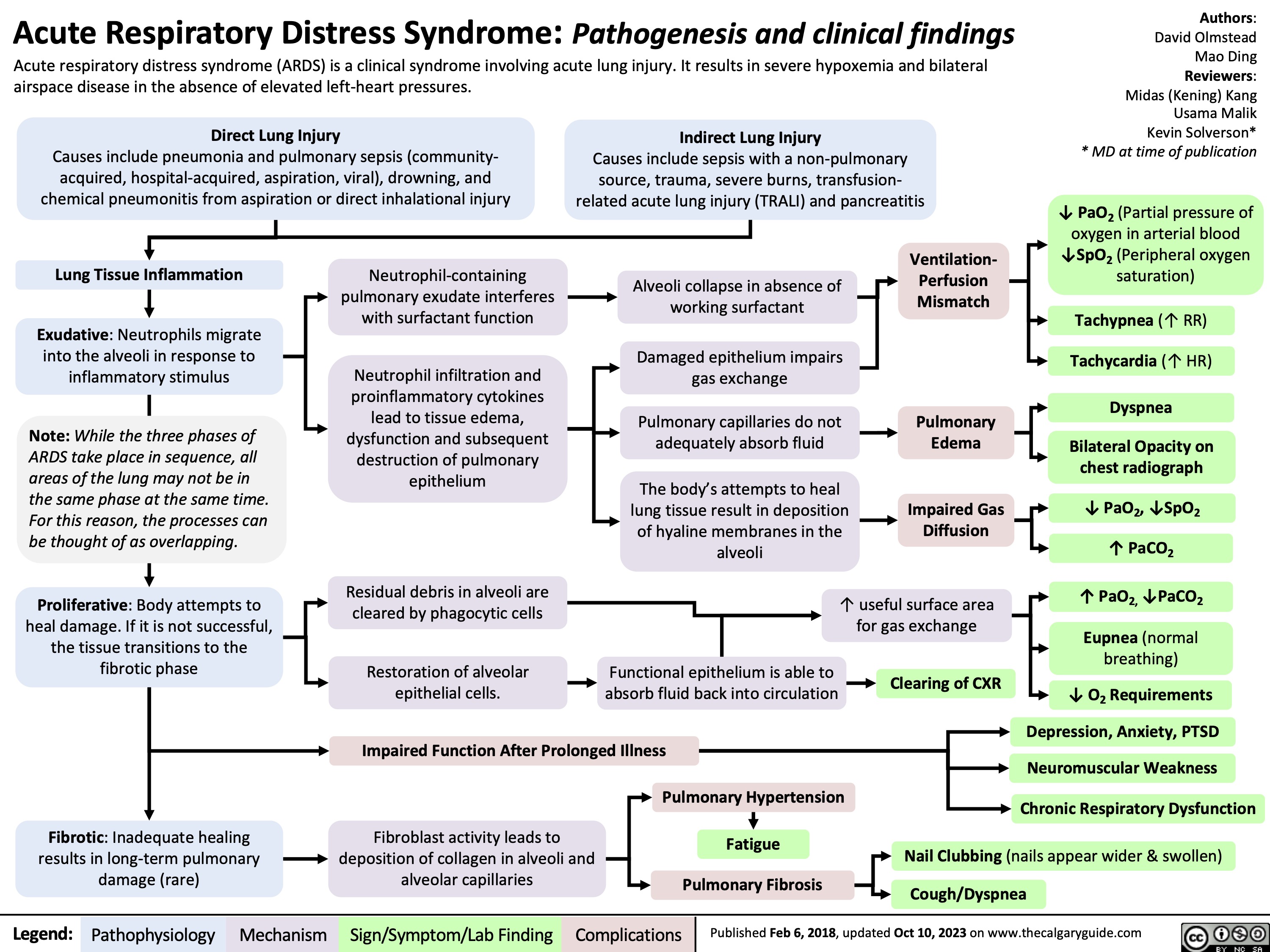
Death Cardiovascular Respiratory and Neurologic Mechanisms
Onychomycosis
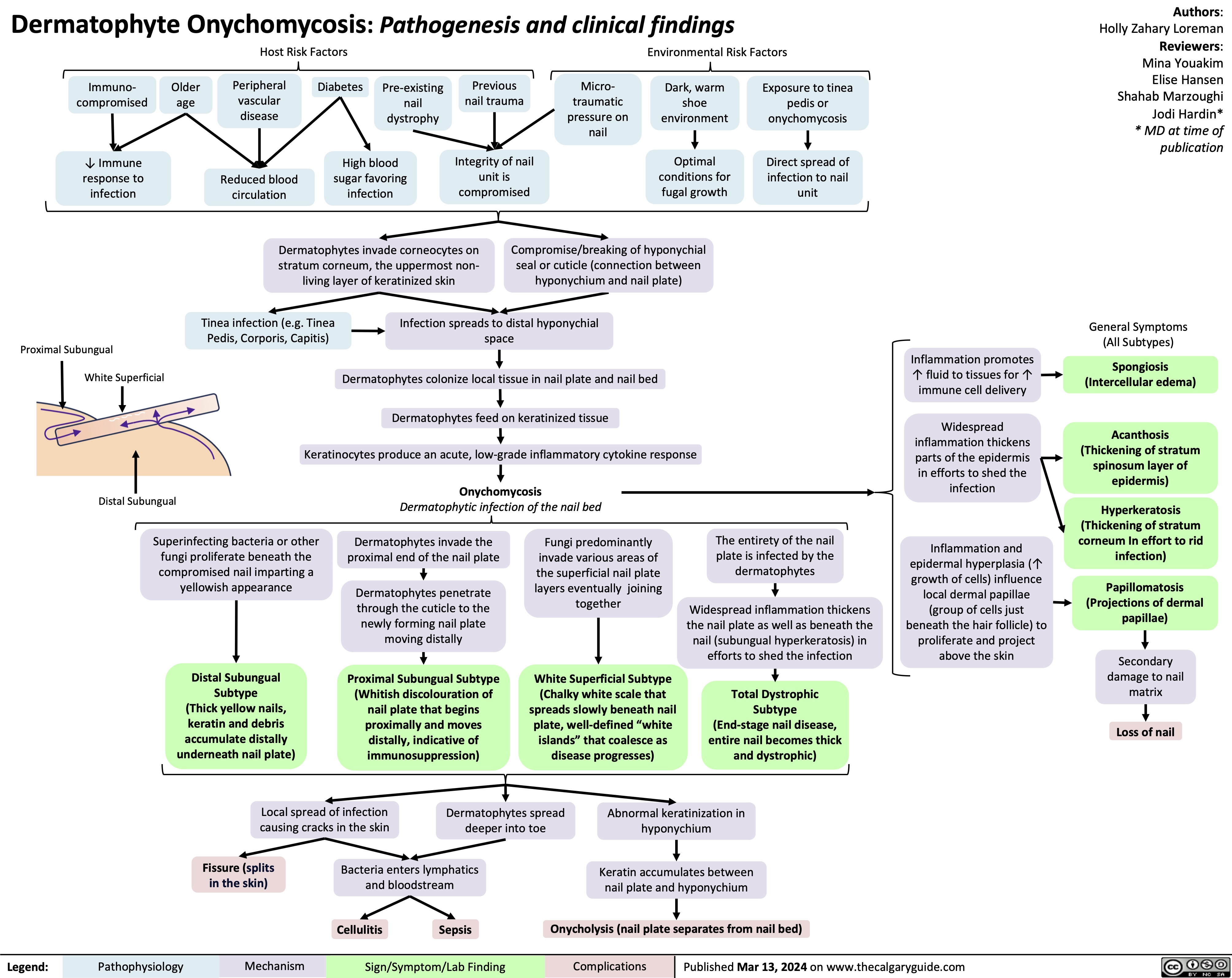
Neonatal Necrotising Enterocolitis in Premature Neonates
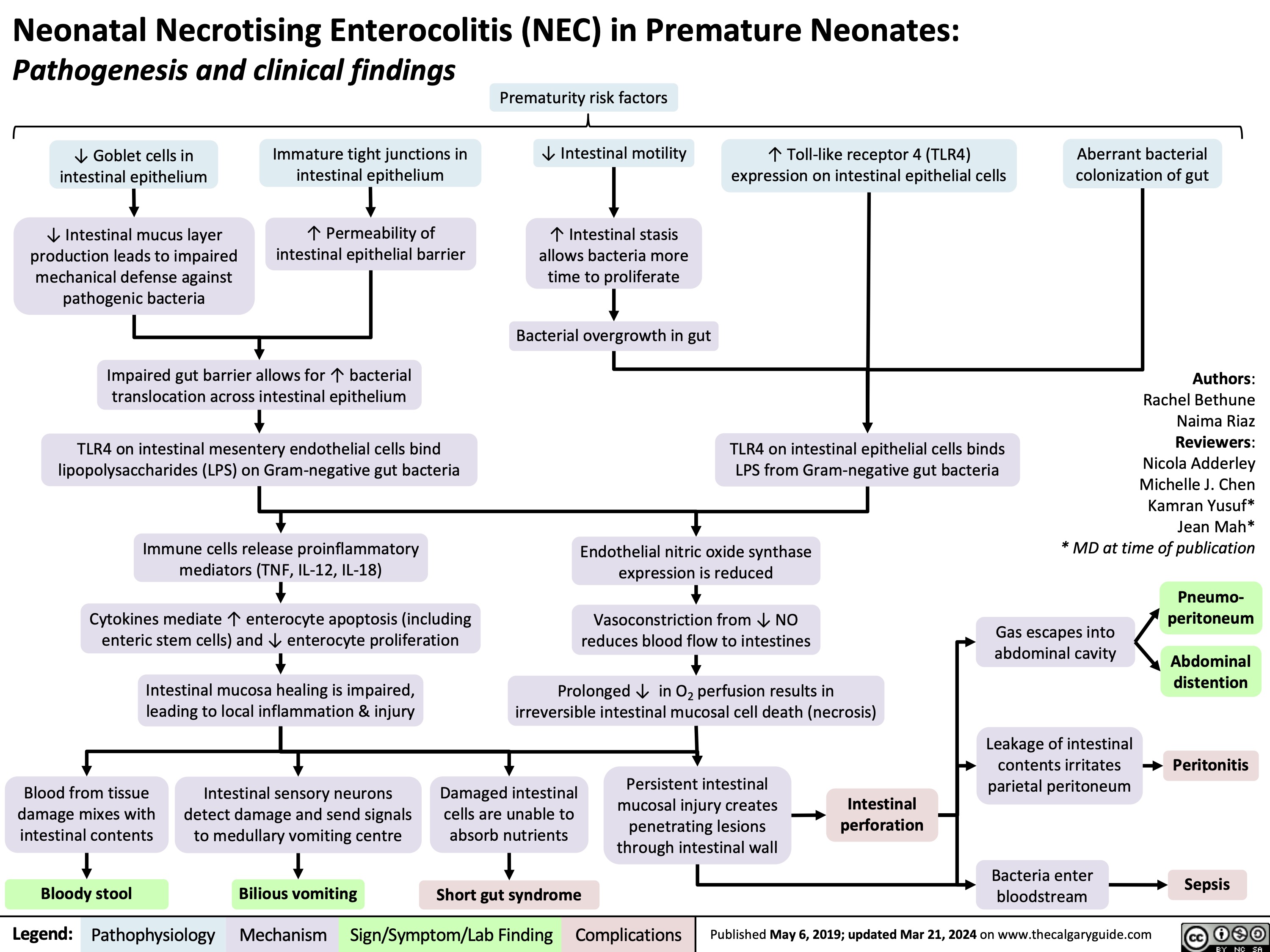
Febrile Neutropenia Pathogenesis and clinical findings
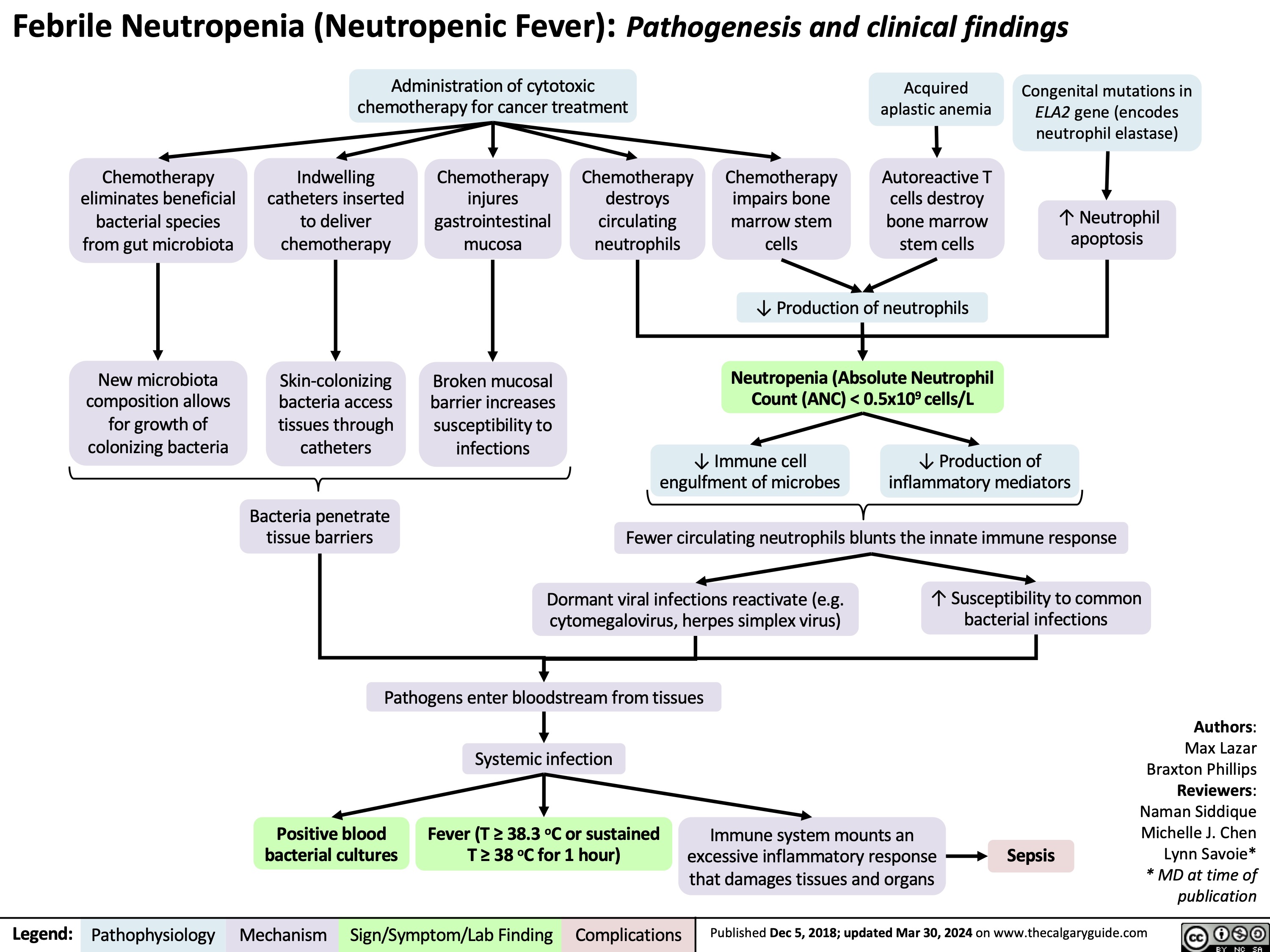
Acute Otitis Media Complications
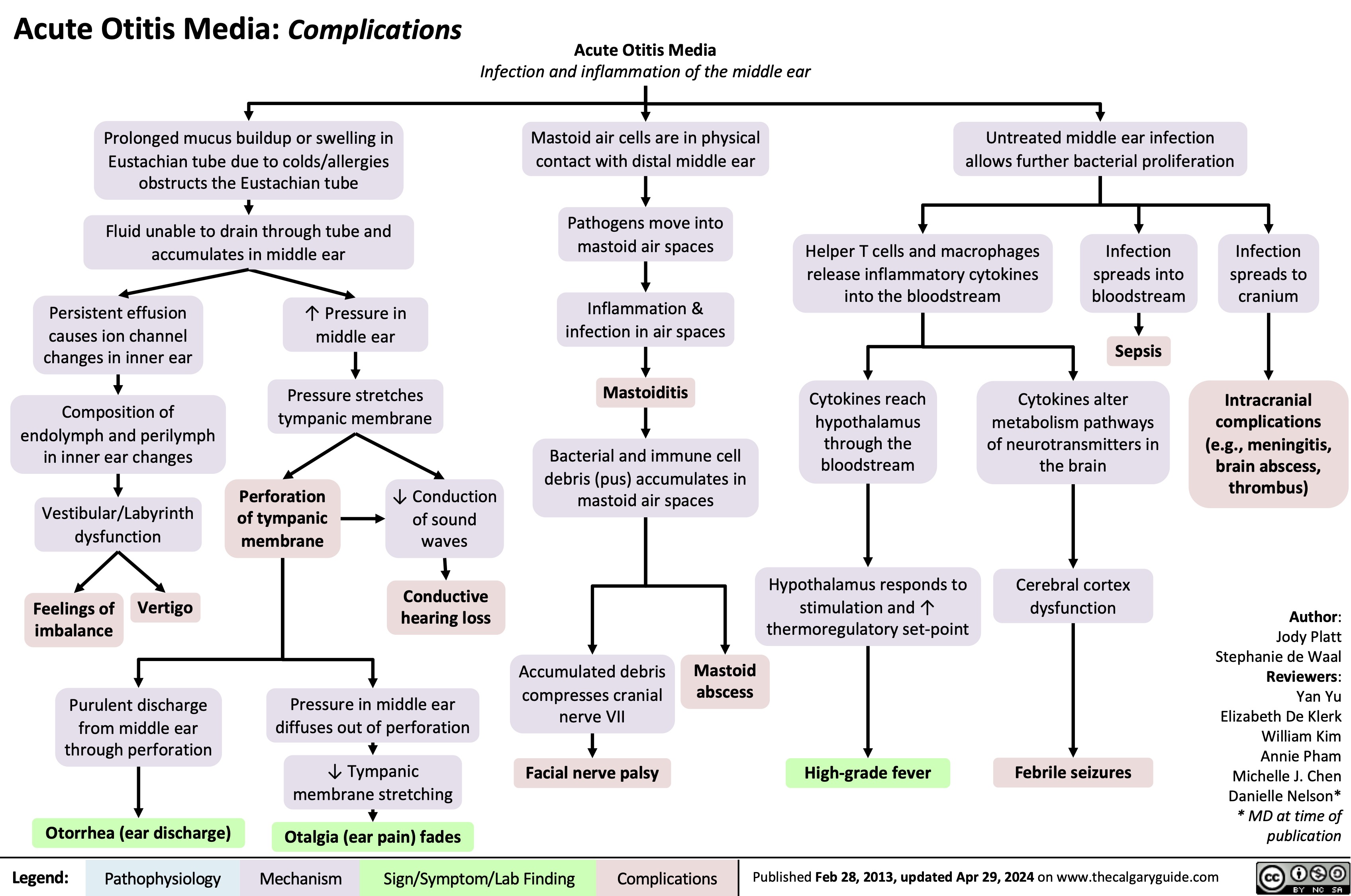
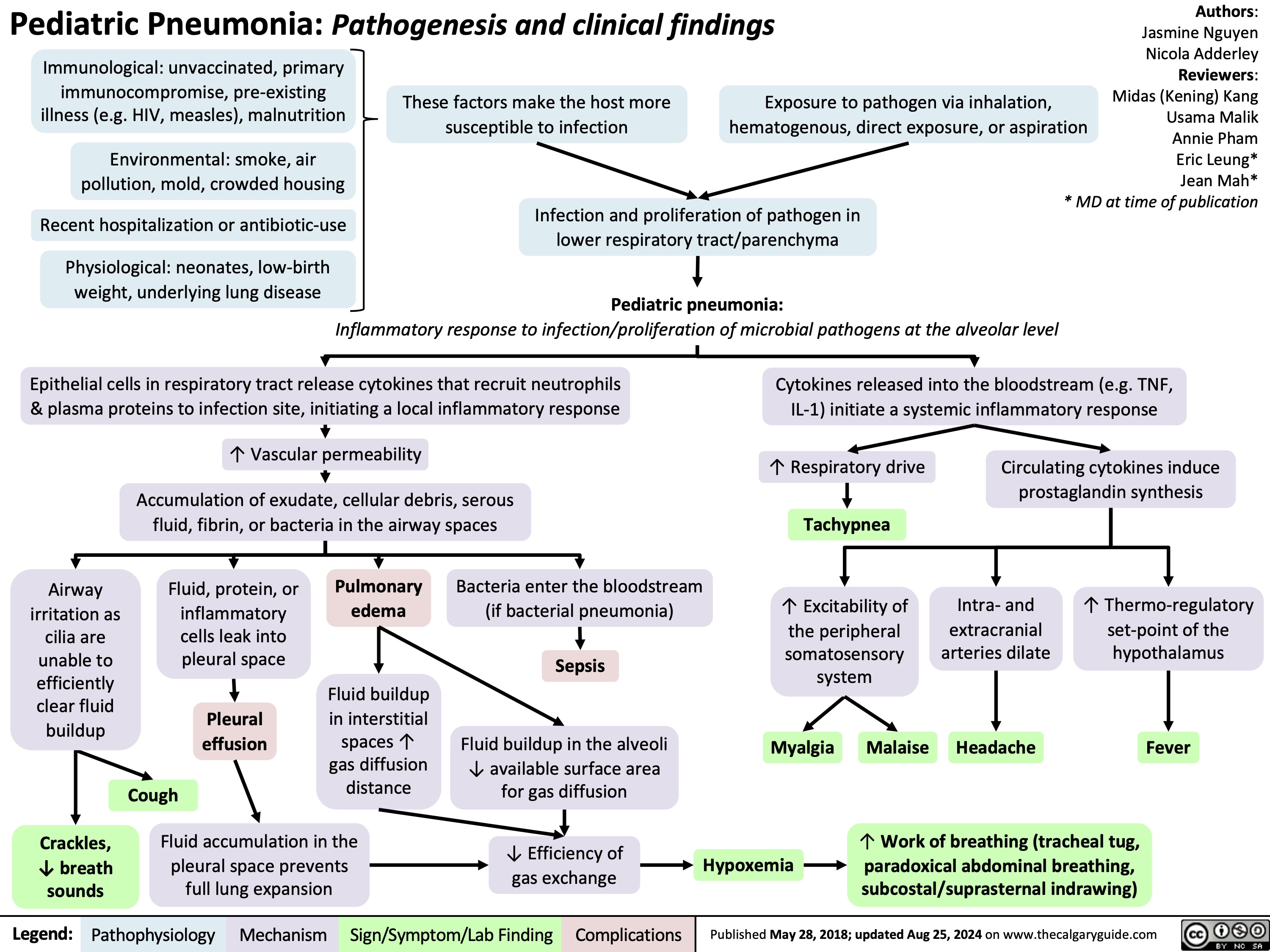
Sickle Cell Disease Pathogenesis Clinical Findings and Complications
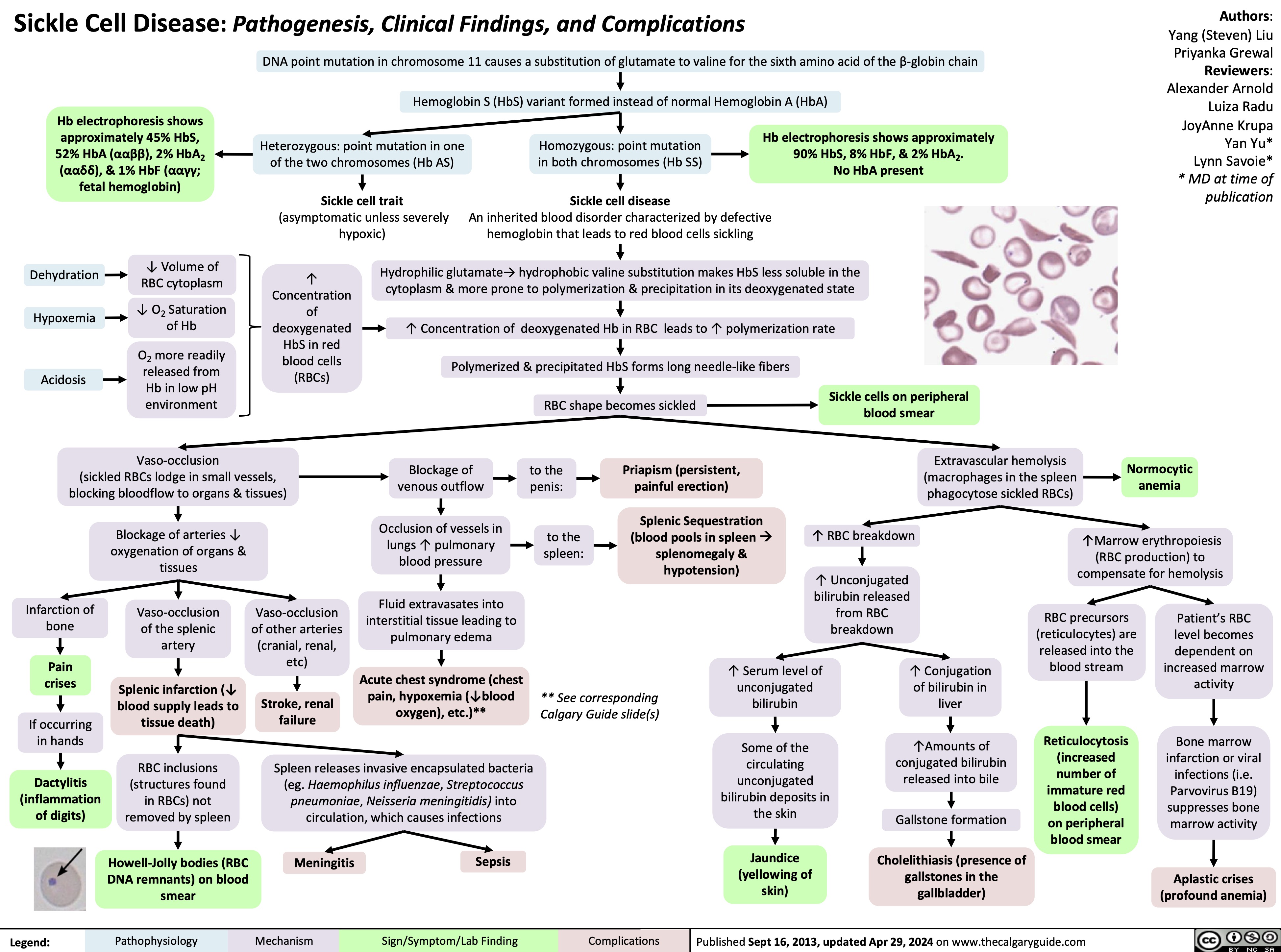
Neonatal Hypoglycemia Pathogenesis
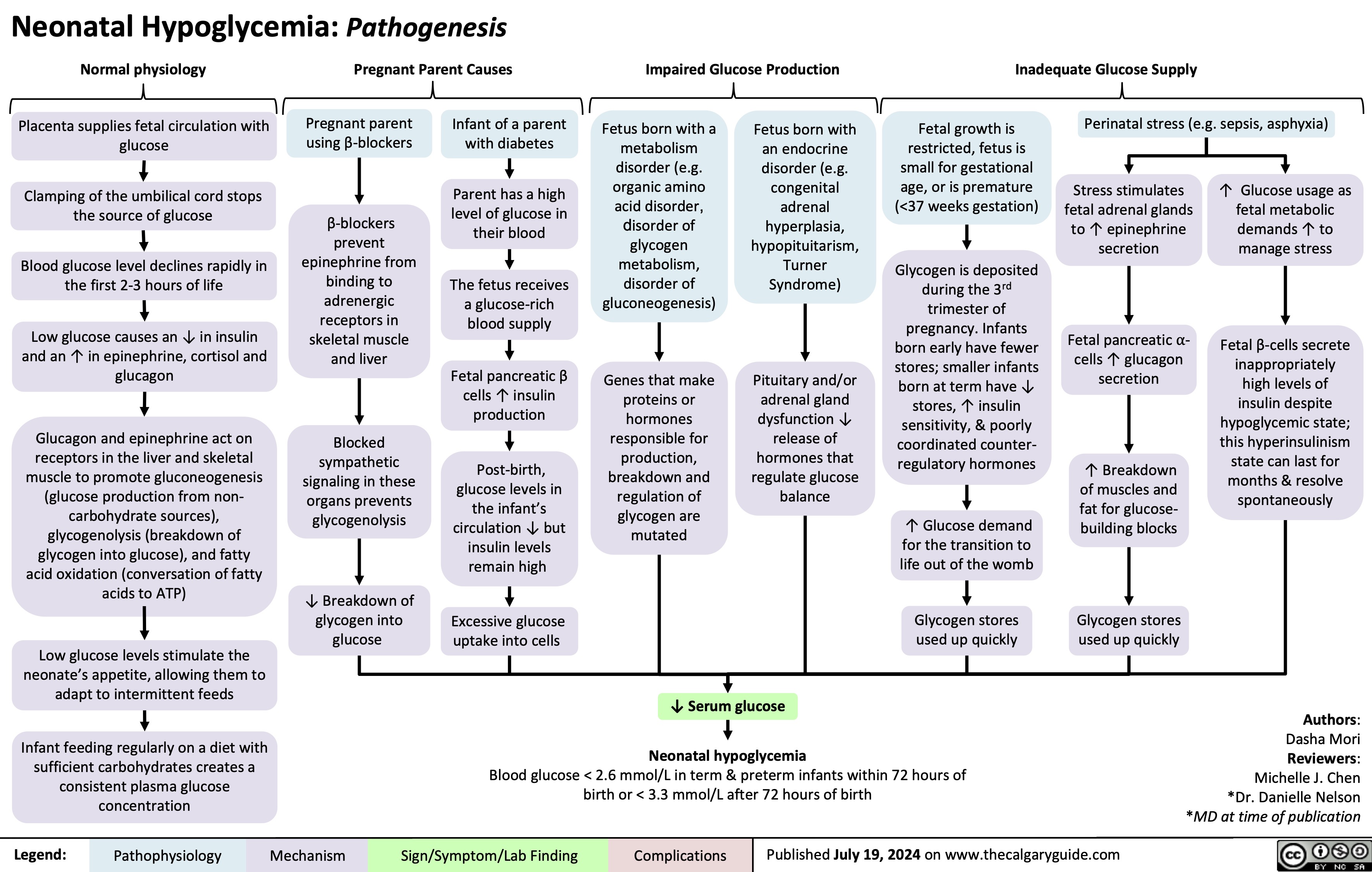
Underfill Edema Pathogenesis
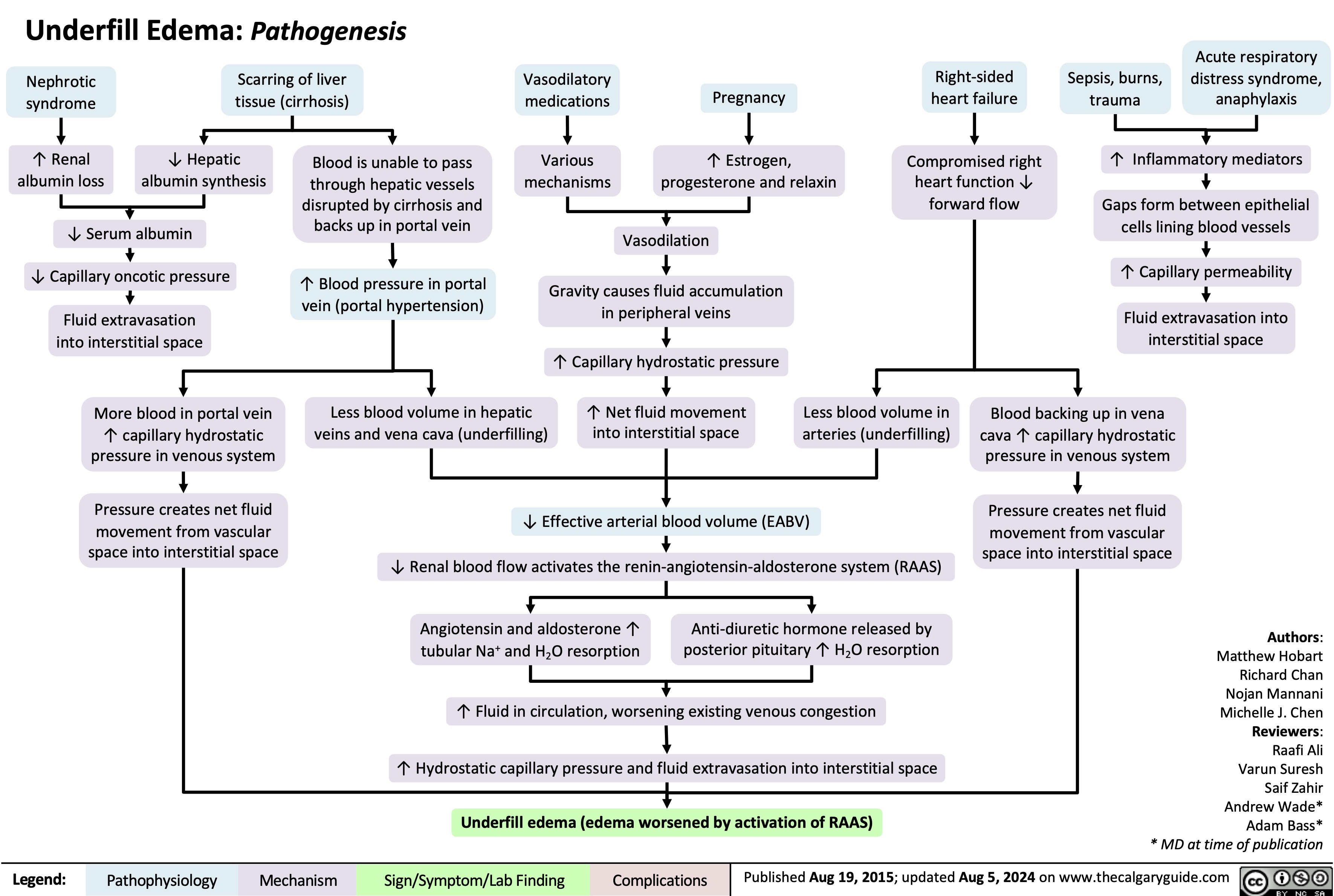
Pediatric Pneumonia Pathogenesis and Clinical Findings
Neonatal Hypoglycemia Clinical Presentation
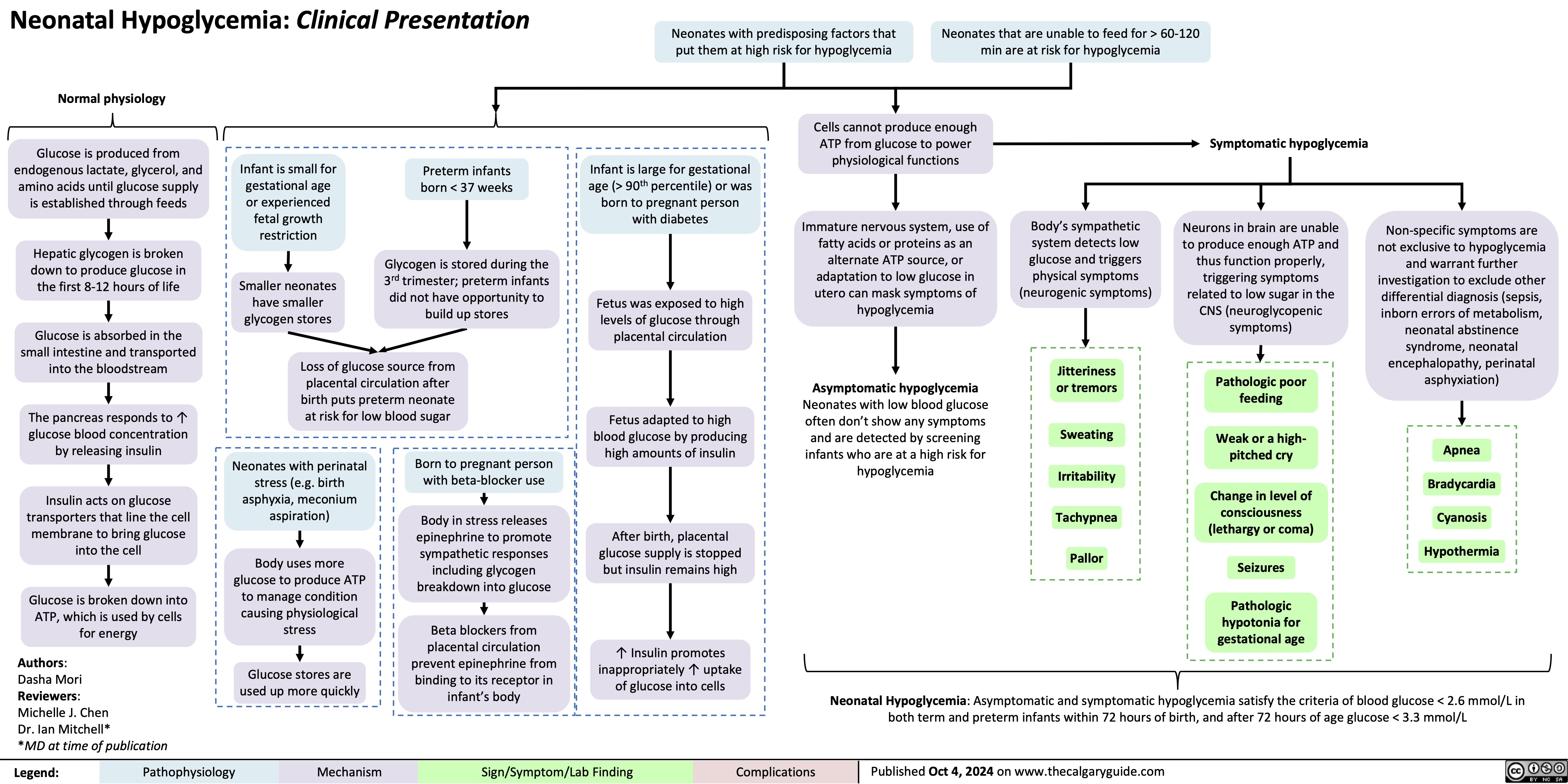
Tonsillitis Pathogenesis and clinical findings
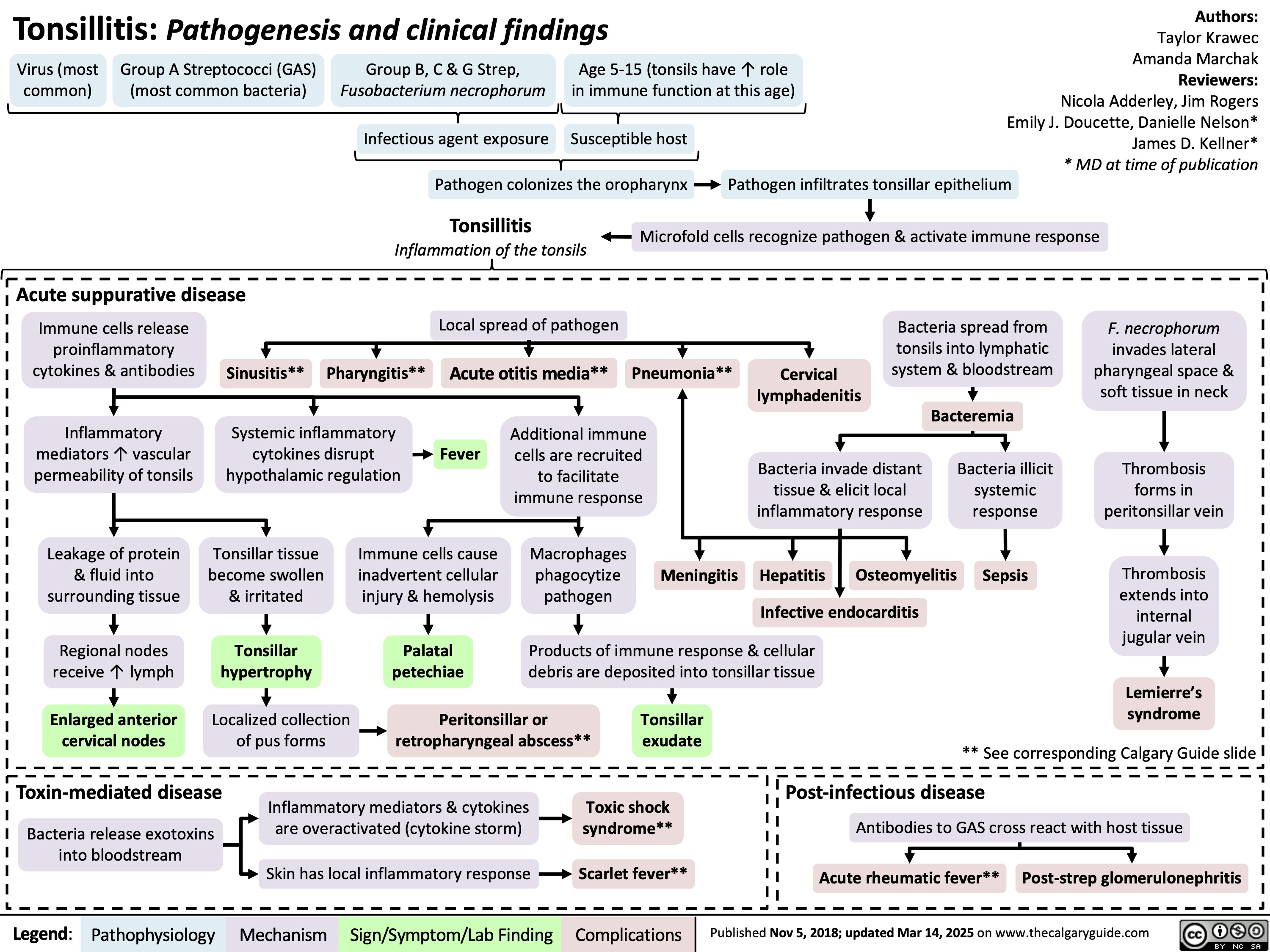
Meckels Diverticulum
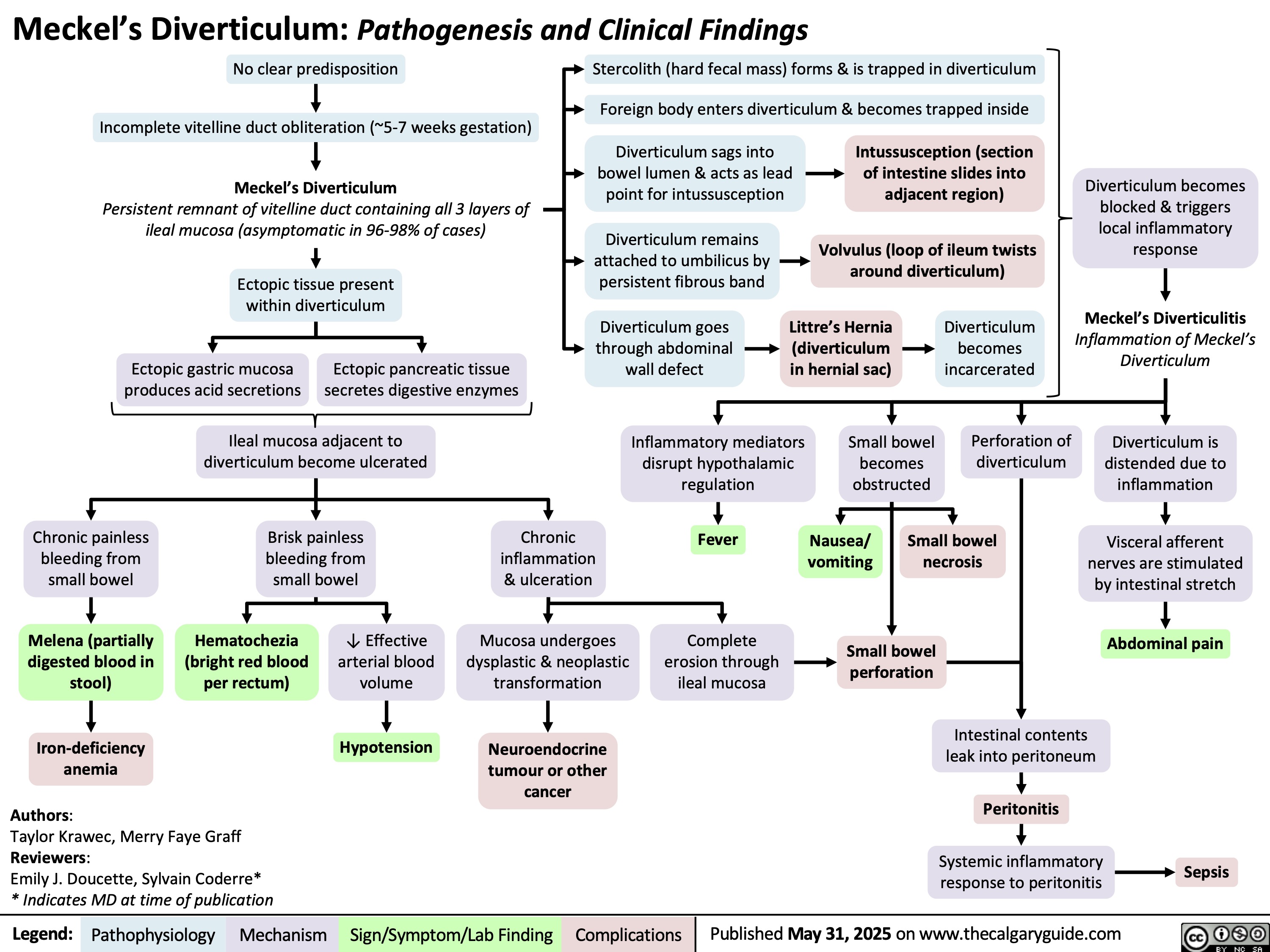
Unconjugated Hyperbilirubinemia
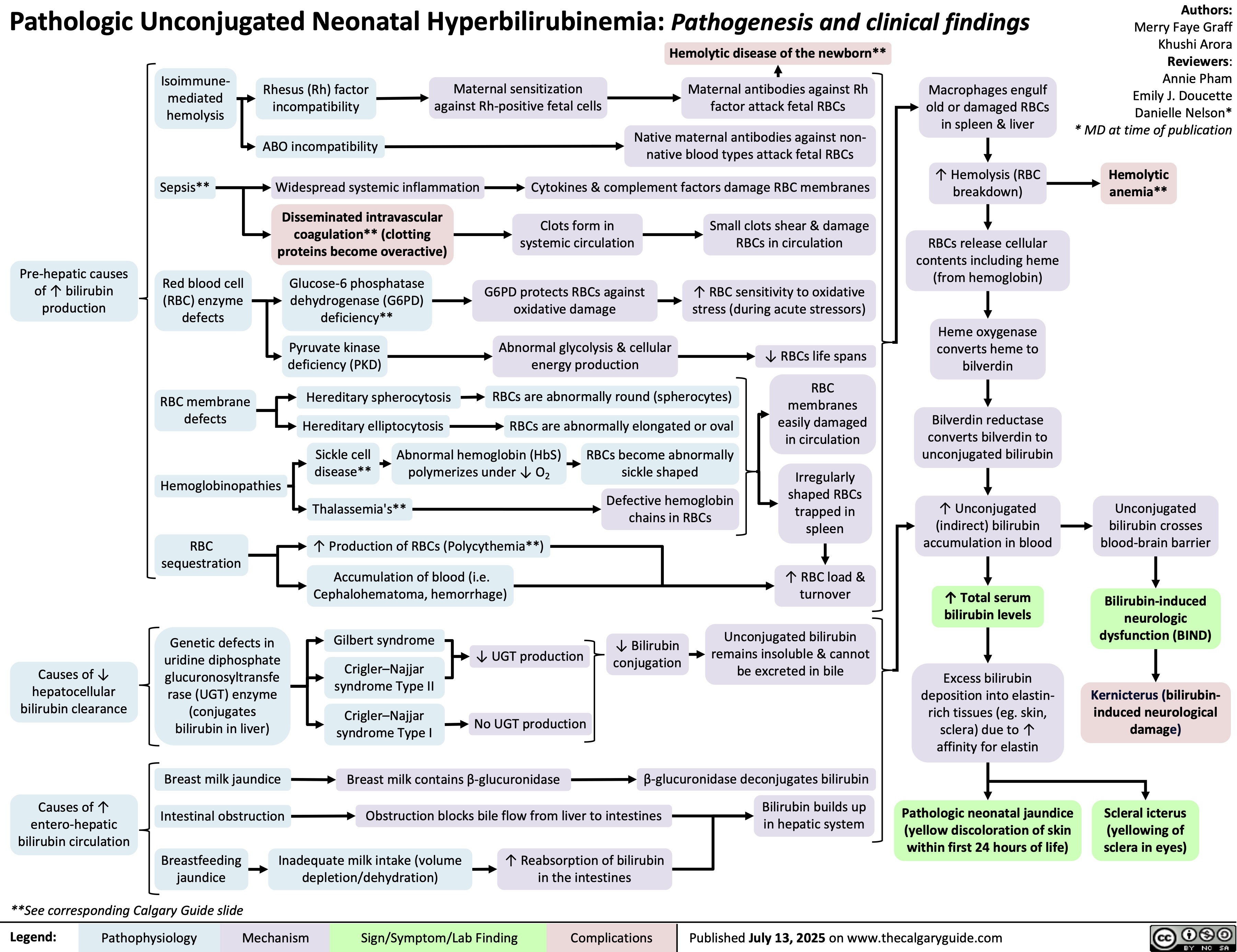
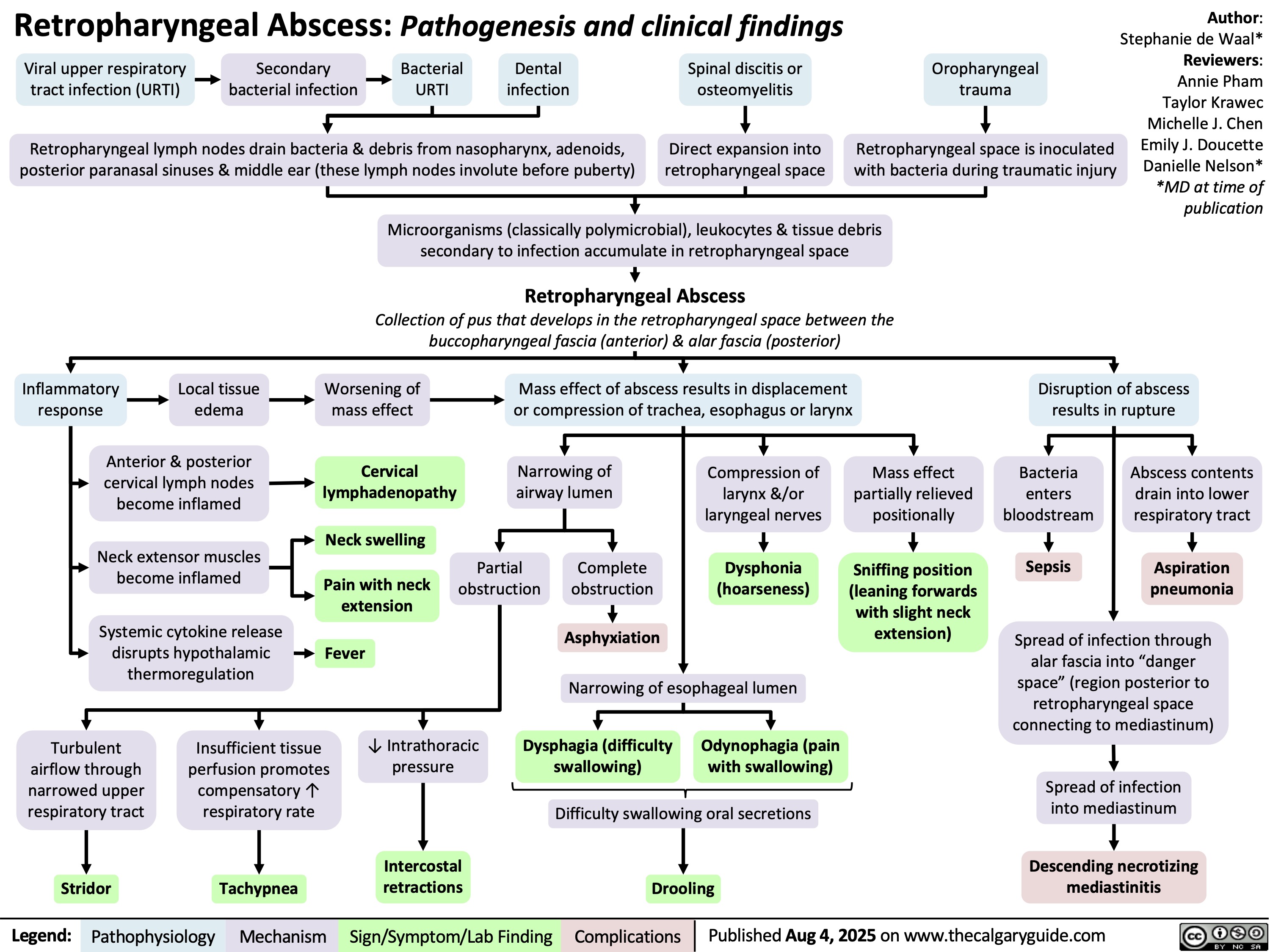
Retropharyngeal Abscess
Hypocalcemia Physiology
![Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia Hypocalcemia: Physiology
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Authors:
Serra Thai,
Ryan Dion
Reviewers:
Jessica Hammal,
Michelle J. Chen,
Emily J. Doucette,
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland hypofunction
from surgical removal,
autoimmune disease, or congenital
disease (e.g. DiGeorge Syndrome)
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Systemic
inflammation
↑ Calcium
sequestration into
cells (mechanism of
action unknown)
↓ Liver function &
albumin synthesis
↓ Or inappropriately normal
parathyroid hormone (PTH)
in circulation
↓ PTH receptor (PTHR) sensitivity
↓ Albumin-bound
calcium in blood
↓ PTHR signaling in kidneys
↓ PTHR signaling in
osteoblastic lineage
Vitamin D deficiency** (e.g.
cells within bones
↓ intake, malabsorption)
False hypocalcemia (↓
total serum calcium with
normal Ca2+ levels)
Acute pancreatitis**
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
Chronic
kidney
disease
(CKD)**
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity
in kidneys
↑ Claudin14 (tight
junction membrane
proteins) expression in
thick ascending limb (TAL)
of the loop of Henle
↓ Transcription of
calcium
transporter genes
(TRPV5, calbindin
D28K, NCX) in
distal convoluted
tubule
↓ Nuclear factor
kappa B ligand
(RANKL) expression
& binding to
receptors on
osteoclast
precursor cells
Pancreatic enzymes are
prematurely activated
↓ Sodium
reabsorption
triggers
electrochemical
gradient
changes
↓ Glomerular
filtration due
to ↓ kidney
function
↓ Activated
vitamin D
(calcitriol)
synthesis
Claudin14 binds cation
channels composed of
Claudin16 & 19 in TAL
tight junctions
Lipase released from
pancreatic
autodigestion breaks
down peripancreatic fat
↓ Renal phosphate
filtration from
blood
↓ Binding of vitamin
D regulated calcium
transporters in
duodenum & jejunum
Binding blocks
paracellular Ca & Mg
transport from tubule
into vasculature
through tight junctions
↓ Probability
of calcium
transport
channels
opening
↓ Osteoclast
differentiation
(responsible
for breaking
down bone)
Free fatty acids bind
ionized Ca2+ to form
insoluble calcium
soaps (saponification)
↓ Circulating ionized
calcium in blood
↑ Phosphate-calcium
crystal formation
↓ Gastrointestinal
↓ Renal reabsorption
↓ Bone resorption
of calcium
of calcium
reabsorption of calcium Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Published Aug 23, 2025 on www.thecalgaryguide.com
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
Emily J. Doucette
Hanan Bassyouni*
* MD at time of
publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Chronic kidney
disease (CKD)
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression
in proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate-calcium
complex formation
**See corresponding Calgary Guide slide
Legend: Pathophysiology Mechanism
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis** or severe illness
Impaired Mg-dependent
generation of cyclic
adenosine monophosphate
↑ Inflammation
↓ PTH receptor (PTHR) sensitivity
↑ Calcium
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to
active form) activity in
kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
↓ Synthesis of activated
vitamin D (calcitriol)
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
↓ Binding of vitamin D
in TAL tight junctions
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca & Mg transport from
tubule into vasculature
through tight junctions
between cells
↓ Probability
of calcium
transport
channels
opening
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression & binding to
receptors on osteoclast
precursor cells
↓ Osteoclast
differentiation
(responsible for
breaking down bone)
↓ Bone resorption
↓ Albumin-
bound calcium
with normal free
(biologically
active) Ca levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Sign/Symptom/Lab Finding Complications
Hypomagnesemia*
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↓ PTH receptor (PTHR) sensitivity
Hypocalcemia: Physiology
Authors:
Serra Thai
Ryan Dion
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
↓ Parathyroid hormone (PTH) in circulation
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
*See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
** See corresponding Calgary Guide slide: “Hypoca;cemia: Clinical Findings”
Legend: ↑ Inflammation
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free
(biologically
active) Ca
levels
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Complications
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Hypocalcemia: Physiology
Authors:
Serra Thai
Reviewers:
Jessica Hammal
Michelle J. Chen
* MD at time of publication
Parathyroid gland dysfunction
from surgical removal,
autoimmune disease, or
congenital disease (e.g. DiGeorge)
Hypomagnesemia**
Pseudohypoparathyroidism
(genetic resistance to PTH)
Sepsis or severe illness
Impaired magnesium-
dependent generation
of cyclic adenosine
monophosphate
↑ Inflammation
↓ Parathyroid hormone (PTH) in circulation
↓ PTH receptor (PTHR) sensitivity
↑ Ca
sequestration
into cells
(unknown
mechanism of
action)
↓ Liver
function
↓ Synthesis
of albumin
↓ PTHR signaling in kidneys
Vitamin D deficiency (e.g.
↓ intake, malabsorption)
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ 1-⍺ hydroxylase
enzyme (converts
inactive vitamin D to its
active form) activity in
the kidneys
↑ Claudin14 (tight
junction membrane
protein) proteins
are expressed in
the thick ascending
loop of Henle
↓ Transcription of
calcium transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted tubule
Chronic kidney
disease
↓ Sodium
reabsorption
↓ Synthesis of activated
vitamin D (calcitriol)
↓ Renal
blood flow
Electrochemical
gradient changes
Claudin14 binds to
cation channels
composed of
Claudin16 & 19 found
in TAL tight junctions
↓ Glomerular
filtration
↓ Phosphate
excretion
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the duodenum
& jejunum
Binding blocks paracellular
Ca (and Mg) transport
from the tubule into
vasculature through tight
junctions between cells
↓ Probability
of calcium
transport
channels
opening
↑ Phosphate-calcium
complex formation
↓ Gastrointestinal
reabsorption of Ca
↓ Renal reabsorption of calcium
↓ PTHR signaling in
osteoblastic lineage
cells in bones
↓ Nuclear factor kappa
B ligand (RANKL)
expression and binding
to its receptors on
osteoclast precursor
cells
↓ Differentiation of
osteoclasts which are
responsible for
breaking down
(resorbing) bone
↓ Bone resorption
↓ Albumin-
bound calcium
with normal
free Ca levels
due to
homeostatic
mechanisms
False
hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2+]
<2.1mmol/L+)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
**See corresponding Calgary Guide slide: “Hypomagnesemia: Physiology”
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D
deficiency:
↓intake,
malabsorption
Pseudohypoparathyroidism
Sepsis/severe illness
↓PTHR
activation in
kidneys
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
↓ Sodium-
hydrogen
exchanger 3
(NHE3) activity &
expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate calcium
complex formation
**See corresponding Calgary Guide slide(s)
Legend: ↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of vitamin
D regulated calcium
transporters (TRPV6,
calbindin9k, PMCa2B,
NCX2) in the
duodenum & jejunum
↓ Gastrointestinal
reabsorption of
calcium
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate ↑ Inflammation
↓ Parathyroid
hormone (PTH)
↑ Claudin14 (tight
junction membrane
protein) activity in
the thick ascending
loop of Henle
↑ Binding of
claudin14 to
claudin16
Inhibition of claudin
16 & 19 from
forming cation
permeable pores in
the tight junctions
↑ Calcium
sequestration
↓ Liver
function
into cells
(mechanism
of action
↓ Synthesis
of albumin
↓ PTH receptor
remains
(PTHR) sensitivity ↓ Albumin-
unknown)
bound calcium
with normal
↓ PTHR
activation in
bones
free Ca levels
due to
homeostatic
mechanisms
↓ Transcription
of calcium
transporter
genes (TRPV5,
calbindin D28K,
NCX) in the distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Osteoblast
stimulation
↓ Nuclear factor
kappa b ligand
(RANKL) secretion
↓ Nuclear factor
kappa b receptor
(RANK) binding on
osteoclast
precursors cells
↓Osteoclast
production
↓ Bone
resorption
False
Hypocalcemia
Acute pancreatitis
↓ Lipase secretion
from pancreas
↑ Undigested fats in
small intestine
Free fatty acids
percipitate calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Complications
Hypocalcemia: Physiology
Hypomagnesemia**
Authors:
Serra Thai
Pseudohypoparathyroidism
Reviewers:
Jessica Hammal
* MD at time of publication
Vitamin D
deficiency: ↓
intake,
malabsorption
↓ 1-alpha
hydroxylase
enzyme activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D regulated
calcium transporters
(TRPV6, calbindin9k,
PMCa2B, NCX2) in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
CKD
↓ Renal
blood flow
↓ Glomerular
filtration
**See corresponding Calgary Guide slide(s)
Legend: Sepsis/severe illness
Impaired magnesium
dependent generation of cyclic
adenosine monophosphate ↑ Inflammation
↓ Signaling proteins
↓ Parathyroid
hormone (PTH)
↓ PTH receptor
(PTHR) sensitivity
↑ Calcium
sequestration
into cells
(mechanism
of action
remains
unknown)
Pathophysiology Mechanism
↓PTHR1 activation
in kidneys
↓ Activation of G-
coupled protein
signaling pathways
↑ Expression of
sodium-phosphate
co-transporters
(NaPiIIa & NaPIIc)
in proximal tubule
↓ Expression &
phosphorylation of
transient receptor
potential vanilloid
(TRPV5, calcium
transporter channel) in
distal convoluted tubule
↓ PTHR1 activation
in bones
↓ Osteoblast
↑ Claudin14 (tight
stimulation
junction membrane
protein) activity in
the thick ascending
loop of Henle
↓ Nuclear
factor kappa b
ligand (RANKL)
secretion
↑ Binding of
claudin14 to
claudin16
↓ Nuclear
factor kappa b
receptor
Inhibition of
(RANK) binding
on osteoclast
↓ Phosphate
excretion
Electrochemical
↓ Amount of
claudin 16 & 19
gradient
TRPV5 & ↓
from forming
precursors cells
changes
probability of
cation-permeable
channels
pores in the tight
↑ Phosphate
opening
junctions
calcium
↓Osteoclast
production
complex
formation
↓ Paracellular
transport of
calcium ↓ Renal reabsorption
of calcium
↓ Bone
resorption
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
↓ Liver
function
↓ Synthesis
of albumin
↓ Albumin-
bound calcium
with normal
free calcium
levels due to
homeostatic
mechanisms
False
Hypocalcemia
Acute
pancreatitis
↓ Lipase
secretion
from pancreas
↑ Undigested
fats in small
intestine
Free fatty acids
percipitate
calcium
Hypocalcemia**
(serum [Ca2
<2.1mmol/L+])
Sign/Symptom/Lab Finding Complications
Hypocalcemia: Physiology
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
Vitamin D
deficiency:
↓intake,
malabsorption
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of
calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Authors:
Serra Thai
Reviewers:
Jessica Hammal
* MD at time of publication
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+]
<2.1mmol/L)
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Vitamin D
deficiency:
↓intake,
malabsorption
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Hypomagnesemia**
Impaired magnesium
dependent generation
of cyclic adenosine
monophosphate
↓ Parathyroid
hormone (PTH)
↓ PTH
sensitivity
↓ Enzyme 1-alpha
hydroxylase activity
↓ Synthesis of
activated vitamin
D (calcitriol)
↓ Binding of
vitamin D
regulated calcium
transporters in
the duodenum &
jejunum
↓ Gastrointestinal
reabsorption of calcium
Legend: ↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
Inhibition of
claudin 16 & 19
from forming
cation permeable
pores in the tight
junctions
↓ Transcription
of calcium
transporter
genes in the
distal
convoluted
tubule
↓ Probability
of calcium
transport
channels
opening
↓ Renal reabsorption
of calcium
↓ Sodium-hydrogen
exchanger 3 (NHE3)
activity & expression in
proximal tubule
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate excretion
↑ Phosphate calcium
complex formation
Pathophysiology Mechanism
Sign/Symptom/Lab Finding Complications
↑ Inflammation
Sepsis/severe illness
↑ Calcium
sequestration
into cells
↓ Liver
synthesis of
albumin
↓ Albumin-
calcium
binding
False
Hypocalcemia
Acute pancreatitis
↓ Lipase
secretion from
pancreas
↑ Levels of
undigested
fats in small
intestine
Free fatty
acids
percipitate
calcium
↓ PTH receptor
activation in
bones
↓ Osteoblast
stimulation
↓ RANKL
ligand
secretion
↓ RAANK
receptor
binding
↓Osteoclast
production
↓ Bone
resorption
Hypocalcemia
(serum [Ca2+] <2.1mmol/L)
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
Hypocalcemia: Physiology
Chronic kidney
disease
↓ Renal
blood flow
↓ Glomerular
filtration
Parathyroid gland
dysfunction: removal,
autoimmune,
congenital (DiGeorge)
Vitamin D deficiency:
↓intake, malabsorption
Sepsis/severe illness
↓ NHE3 activity and
expression in
proximal tubule
↓PTH receptor
activation in
kidneys
↑ Claudin14
activity in the
thick ascending
loop of Henle
↓ Parathyroid
hormone (PTH)
↓ Transcription of
calcium transporter
genes (TRPV5, calbindin
D28K, NCX) in the distal
convoluted tubule
↓ Enzyme 1-alpha
hydroxylase activity
↓ PTH receptor
activation in
bones
↓ osteoblast
stimulation
↓ RANKL ligand
secretion
↑ Inflammation ↓ PTH sensitivity
↓ Glomerular
filtration
↓ Liver
synthesis of
serum albumin
False
Hypocalcemia
↑ Calcium
sequestration
into cells**
↓ Lipase secretion
from pancreas
Acute pancreatitis
Legend: Pathophysiology Mechanism
Sign/Symptom/Lab Finding ↓ Albumin-
bound calcium
Current mechanism
is unknown
↑ levels of undigested
fats in small intestine
Complications
Hypomagnesemia Multiple blood
transfusions
↓ Sodium
reabsorption
Electrochemical
gradient changes
↓ Phosphate
excretion
↑ Phosphate
calcium complex
formation
Inhibits claudin16 and 19
from forming cation
permeable pores in the
tight junctions
↓Probability of
calcium transport
channels opening
↓ Synthesis of activated
vitamin D (calcitriol)
↓ RAANK
receptor
binding
↓ Calcium
reabsorption
↓ Binding of vitamin D
regulated calcium
transporters (TRPV6,
calbindin9K, PMCa2B,
NCX2) in the duodenum
and jejunum
↓osteoclast
production
↓ Bone
resorption
↓ Serum calcium
Hypocalcemia
<2.1 mmol/L
↑ Precipitation of calcium
by free fatty acids
Authors:
Name Name
Name Name*
Reviewers:
Name Name
Name Name*
* MD at time of publication
Published MONTH, DAY, YEAR on www.thecalgaryguide.com
https://journals.physiology.org/doi/full/10.1152/physrev.00003.2004?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-
2003&rfr_id=ori%3Arid%3Acrossref.org – Calcium Absoprtion across Epithelia
https://academic.oup.com/endo/article-abstract/137/1/13/2498579 - PTH and Calcium Signaling Pathways
https://www.pnas.org/doi/10.1073/pnas.1616733114 - Information on Claudin 14
https://onlinelibrary-wiley-com.ezproxy.lib.ucalgary.ca/doi/full/10.1111/apha.13959 - effects of PTH on renal calcium and
phosphate handling
https://www.ncbi.nlm.nih.gov/books/NBK430912/ - Hypocalcemia overview + causes + pathophys
https://www.orthobullets.com/basic-science/9010/bone-signaling-and-rankl - information about bone signaling
https://www.sciencedirect.com/science/article/abs/pii/S0889852921000682?via%3Dihub – Calcium homeostasis article
https://pubmed.ncbi.nlm.nih.gov/3012979/#:~:text=Abstract,intestine%2C%20require%20the%20parathyroid%20hormone.
vitamin D metabolism and function
–
https://pmc.ncbi.nlm.nih.gov/articles/PMC2669834/#:~:text=Calcium%20is%20actively%20absorbed%20from,for%20proper
%20mineralization%20of%20bone.
– more vitamin D metabolism and specific receptors
https://jidc.org/index.php/journal/article/view/32903236/2331 - sepsis + hypocalcemia](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/08/Hypocalcemia-Pathogenesis.jpg)
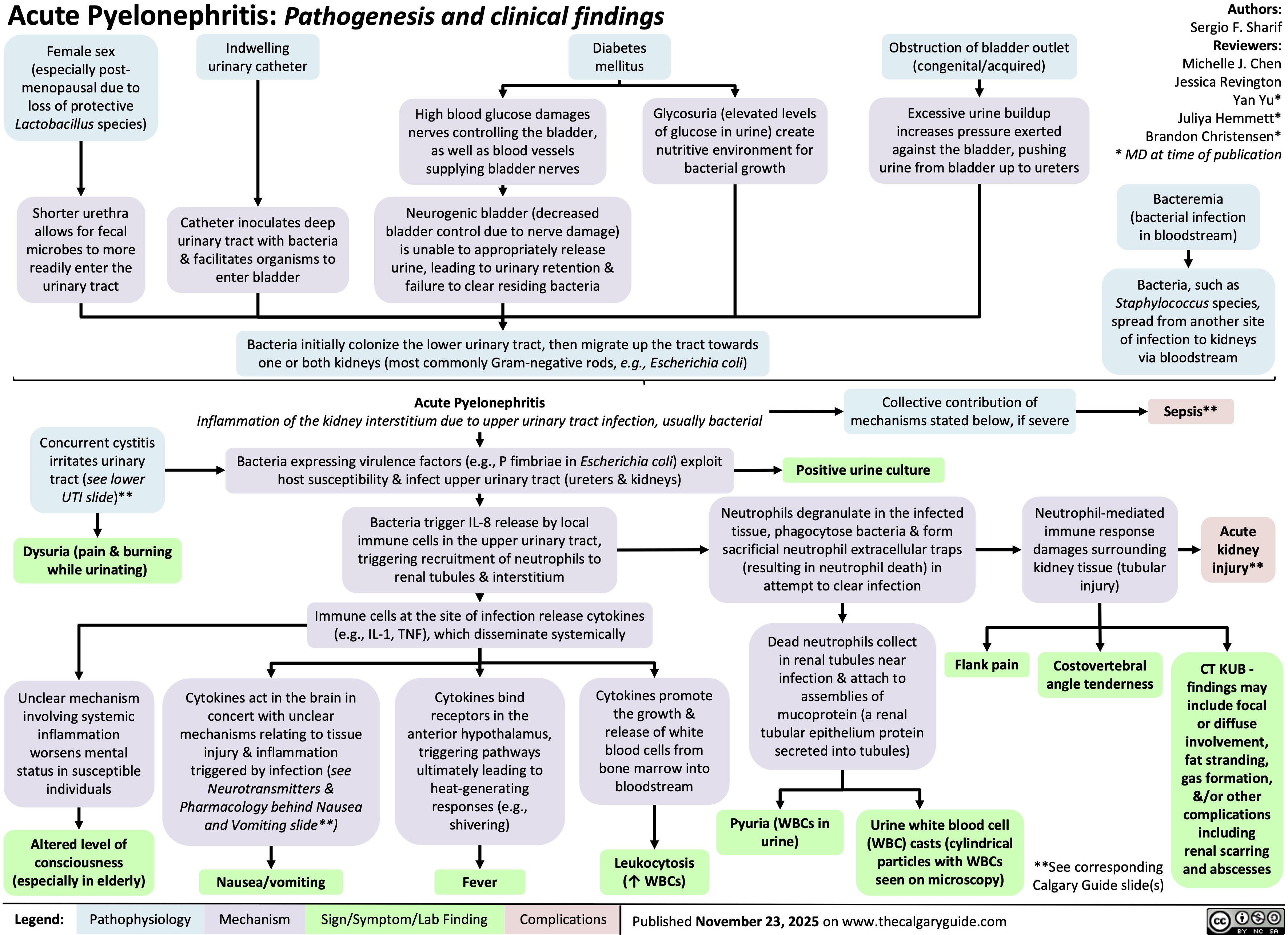
Pyelonephritis
Childhood Immunization Schedule