SEARCH RESULTS FOR: Cellulitis
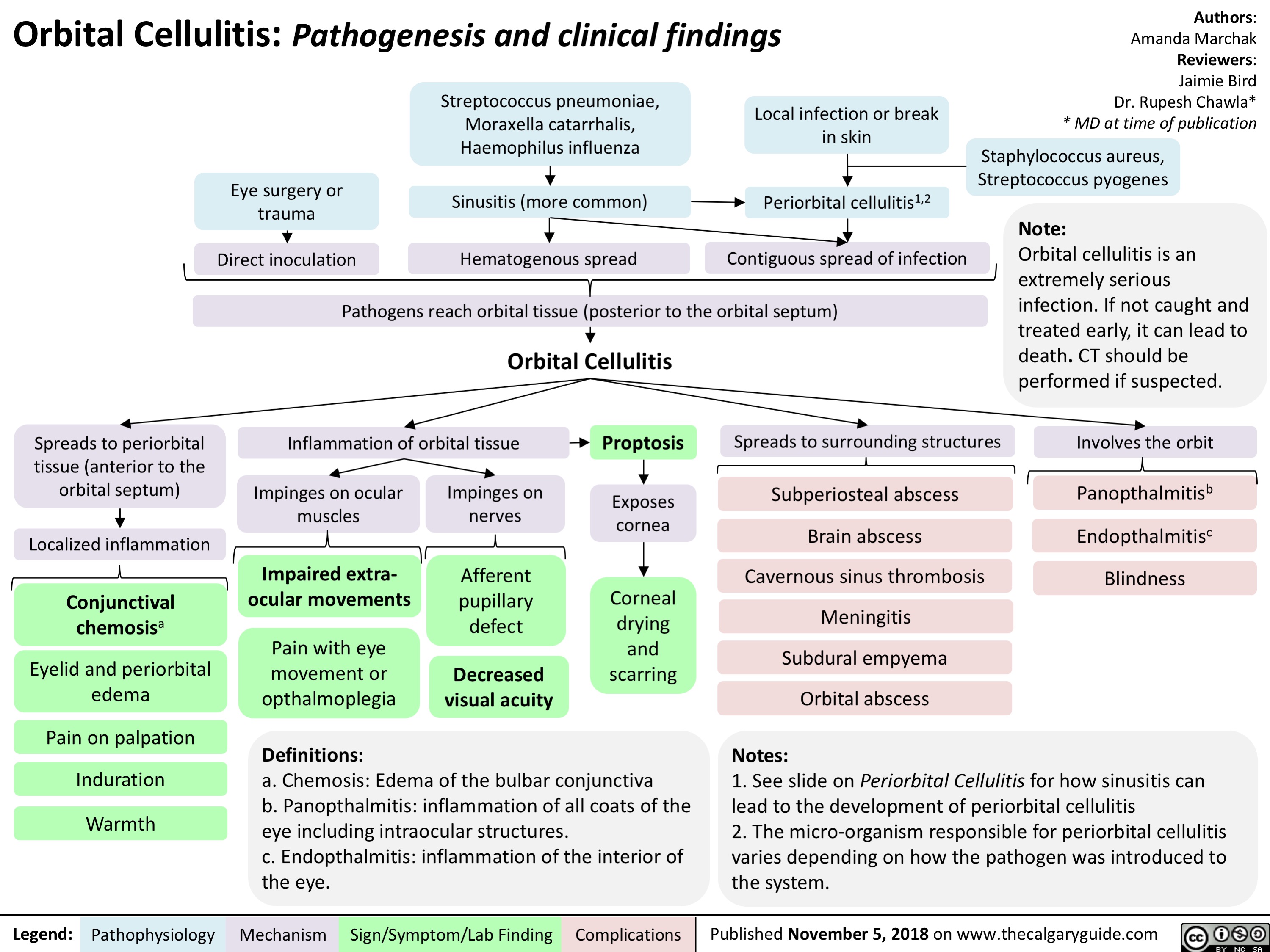
Orbital Cellulitis: Pathogenesis and clinical findings
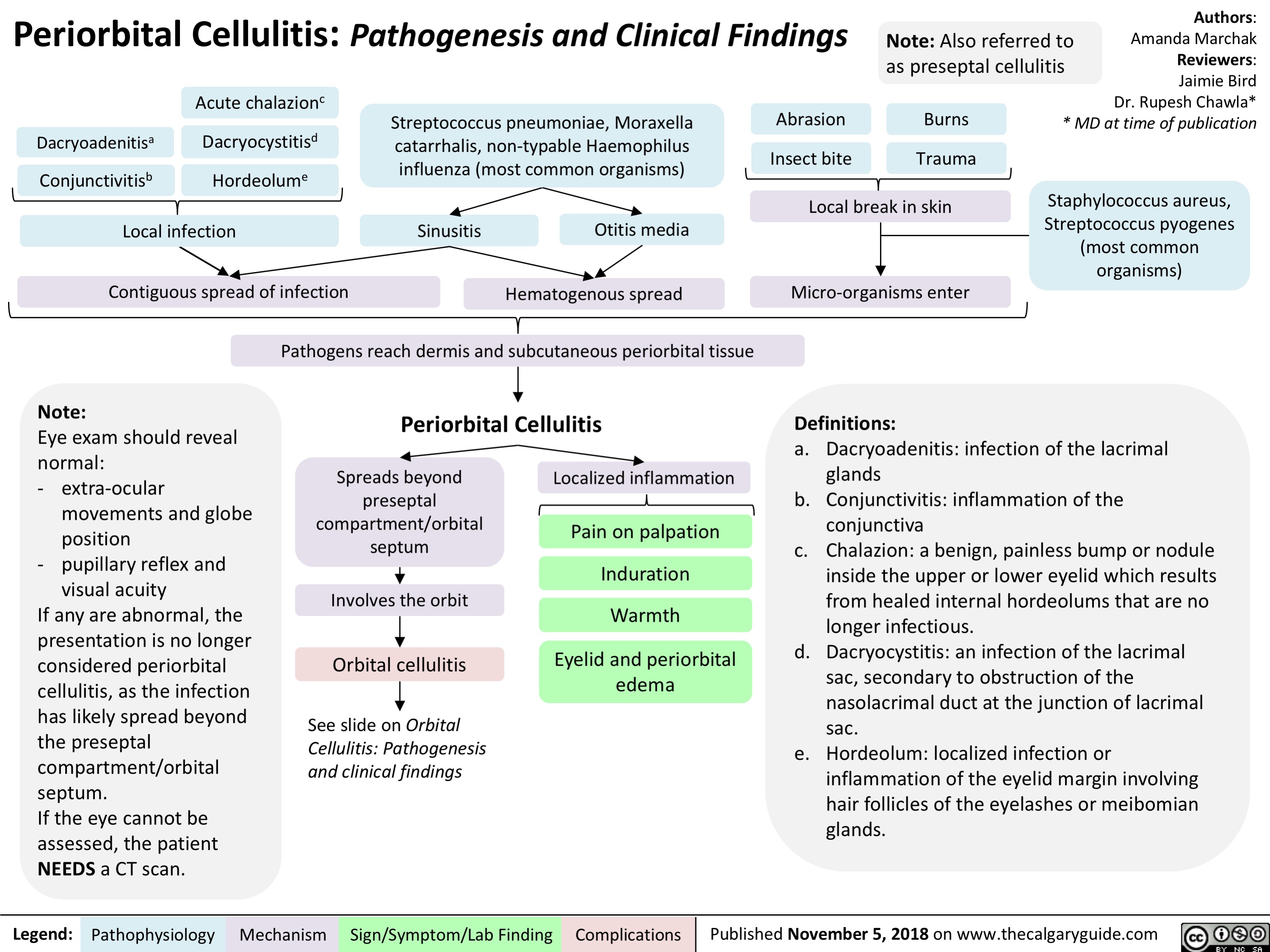
Periorbital Cellulitis: Pathogenesis and Clinical Findings
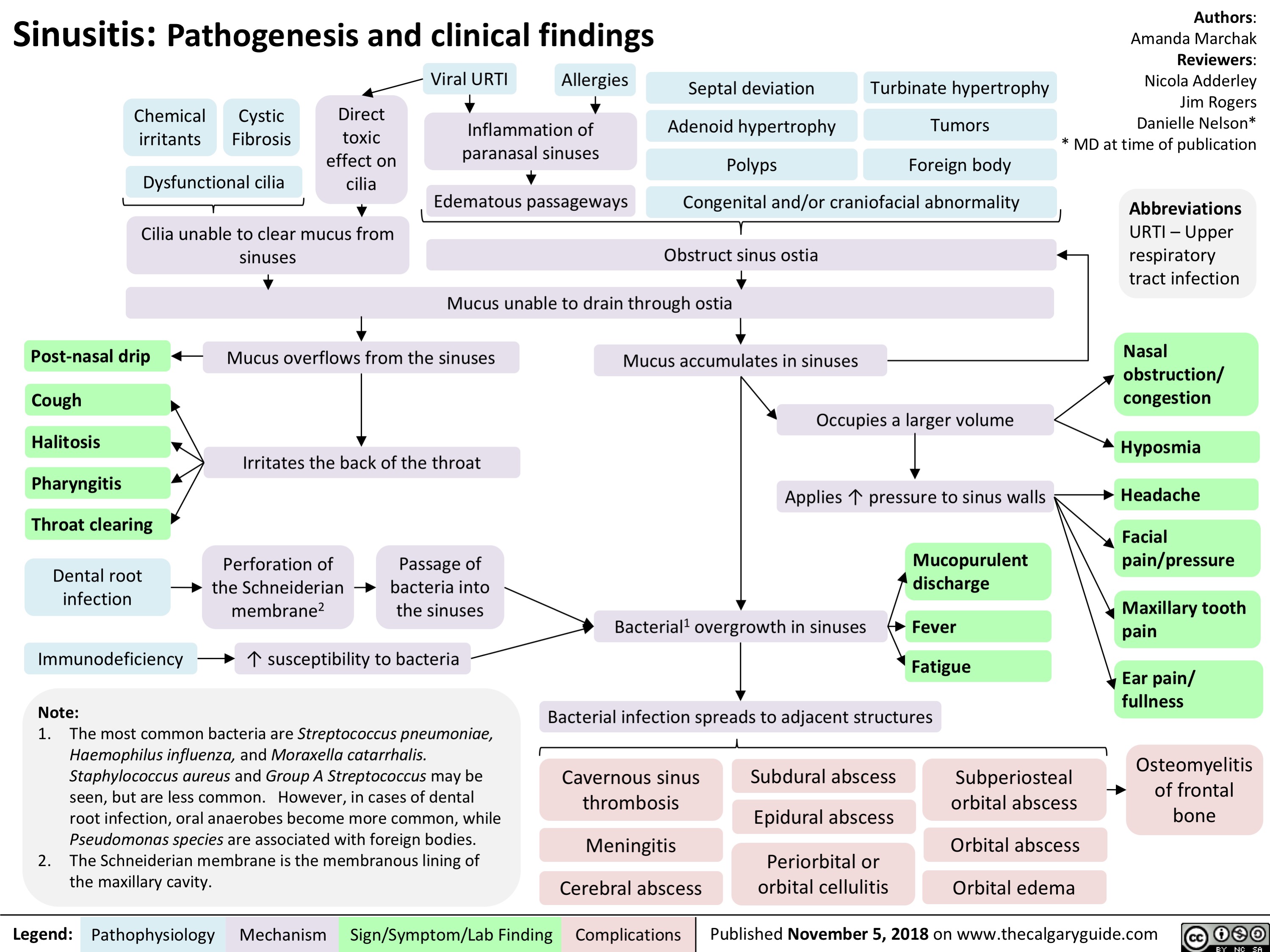
Sinusitis: Pathogenesis and clinical findings
Cellulitis
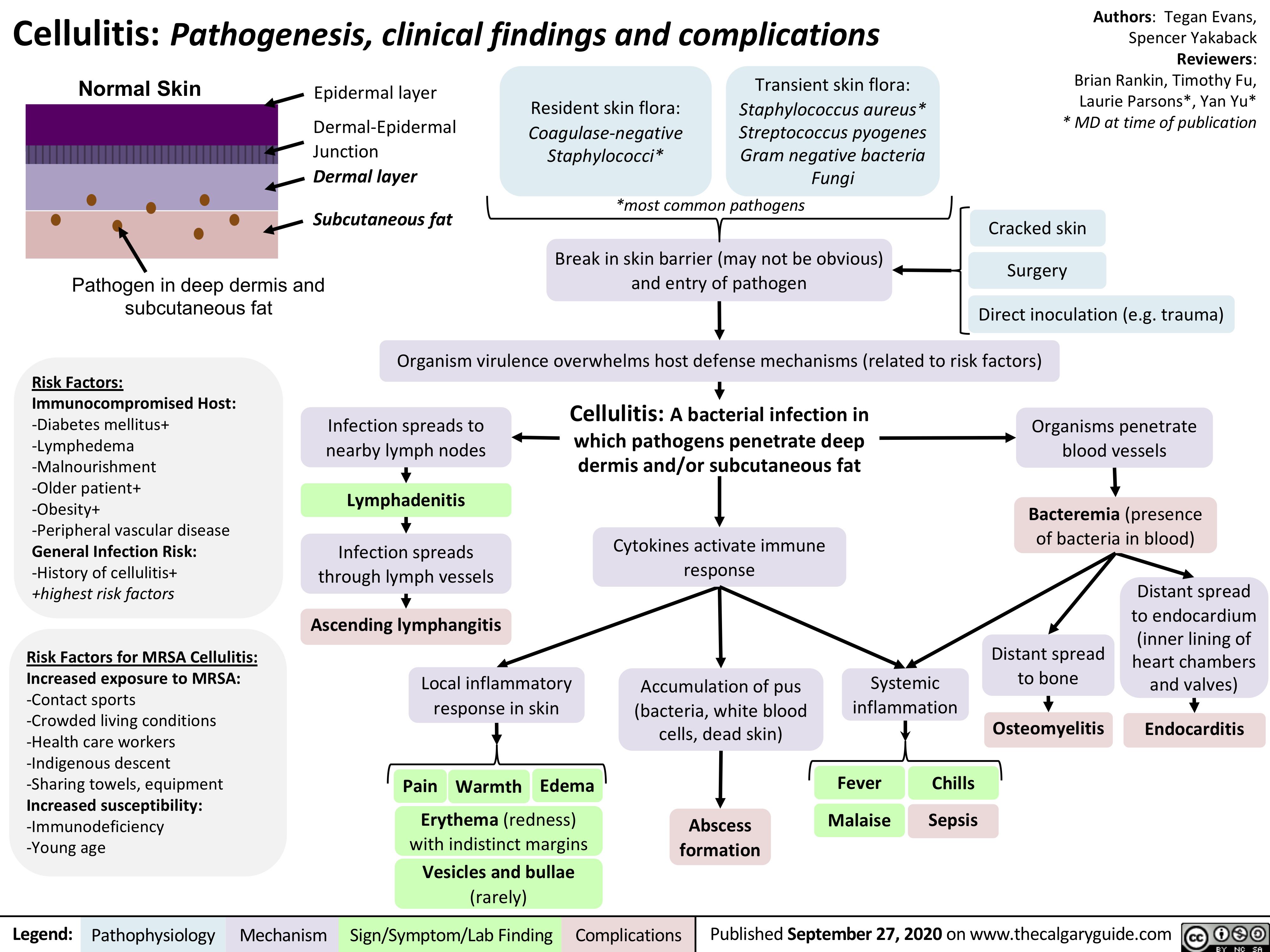
Celulitis
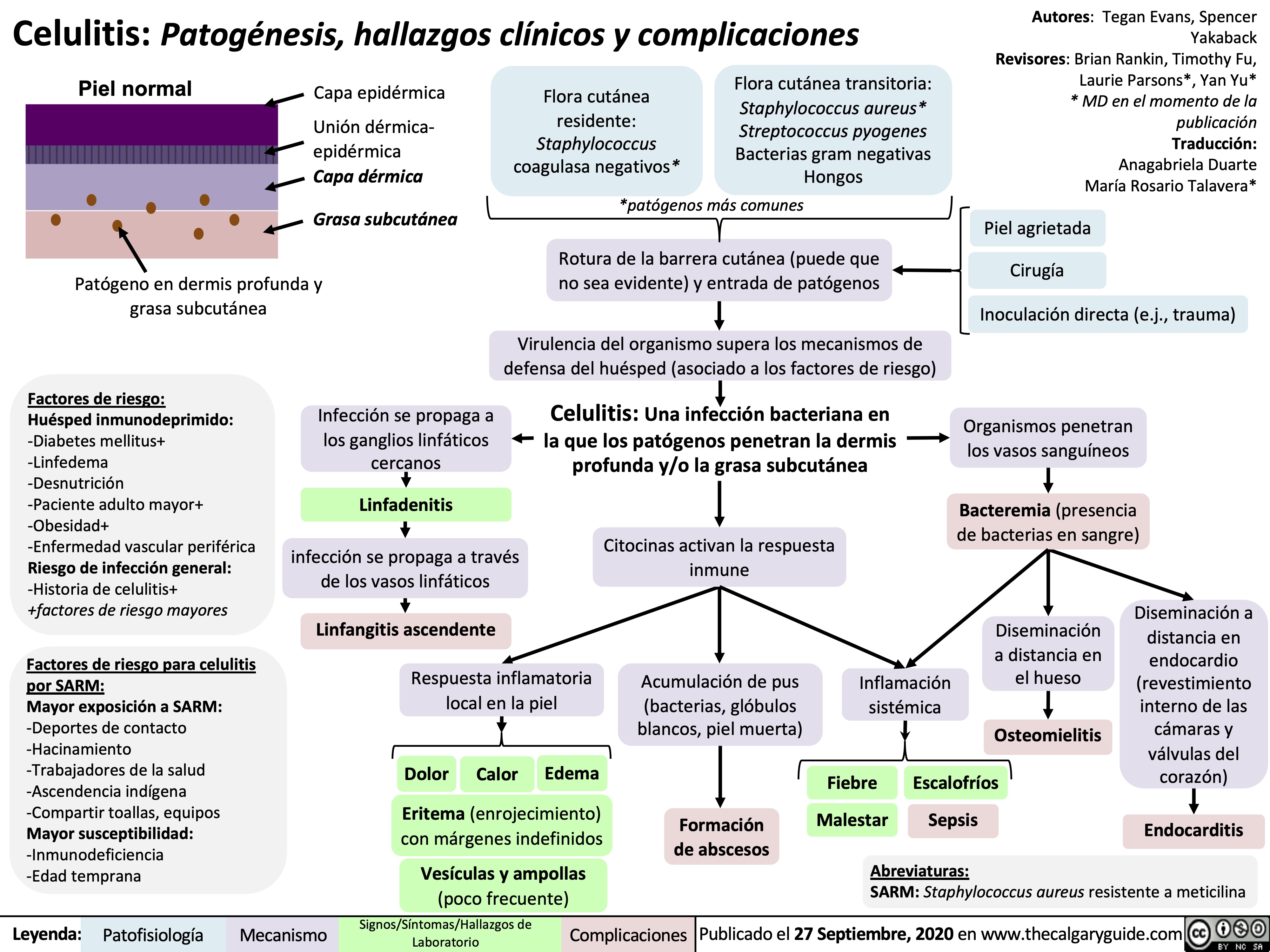
postpartum-puerperal-fever-pathogenesis-and-complications
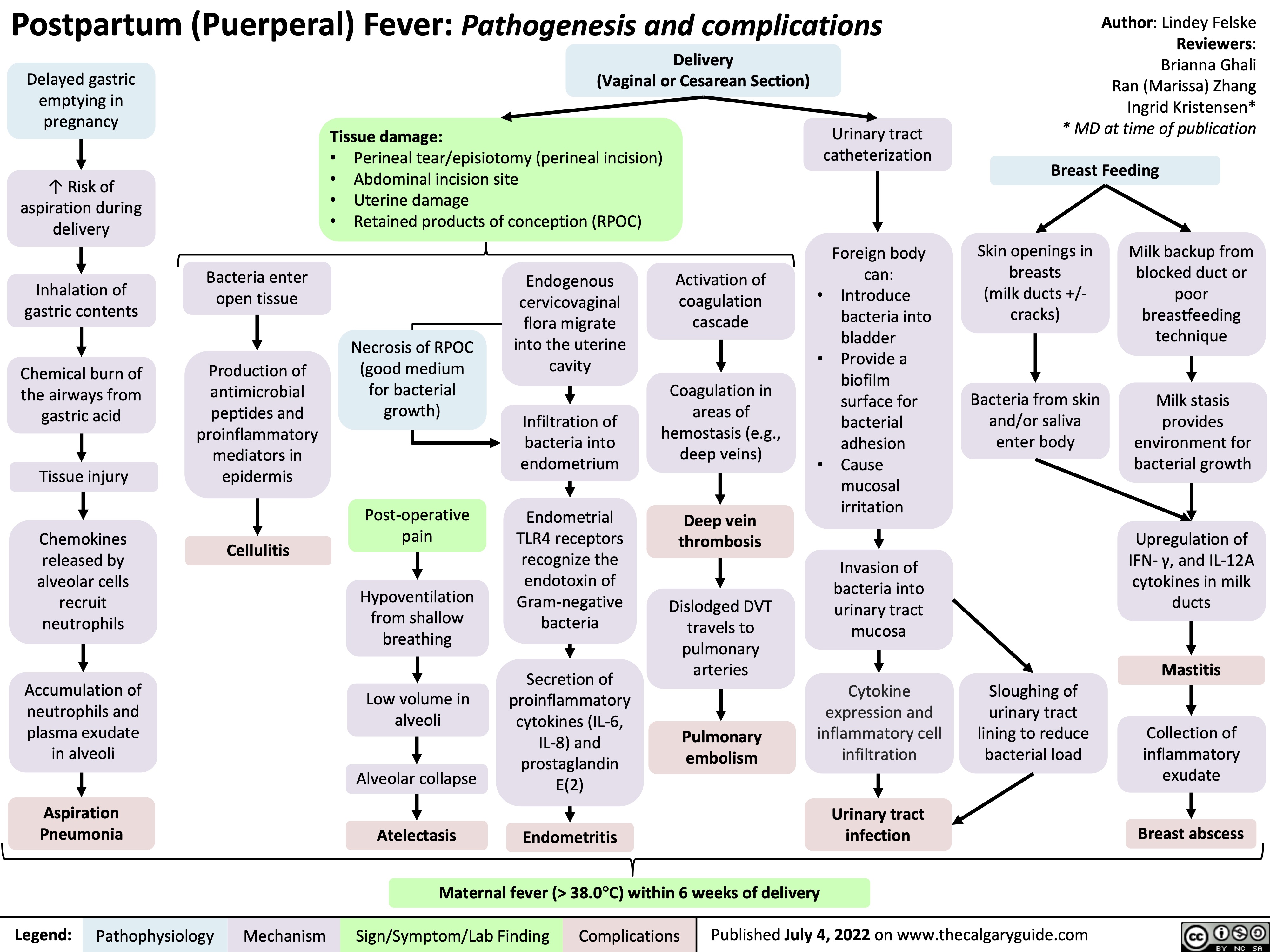
Acute Otitis Externa Complications
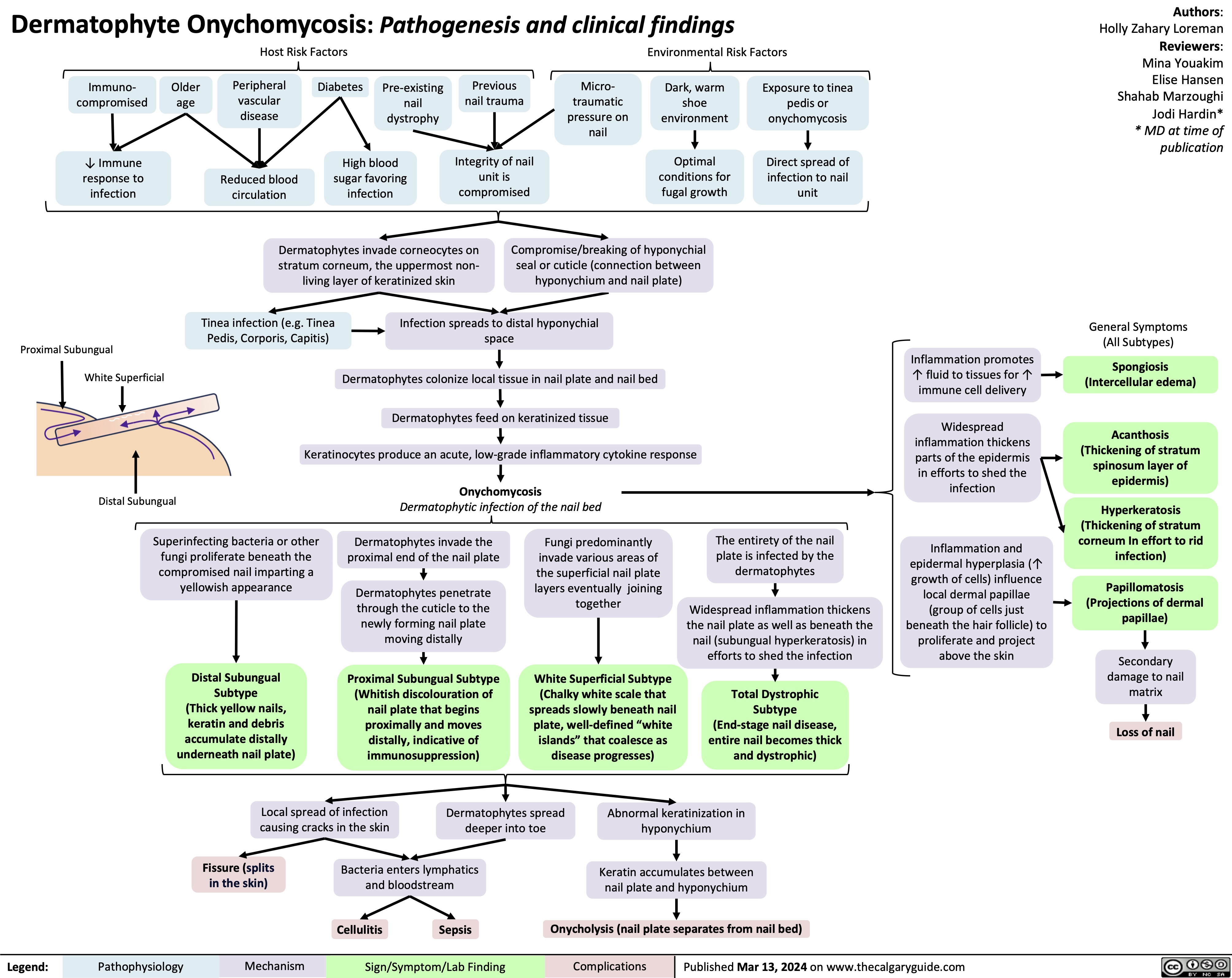
Onychomycosis
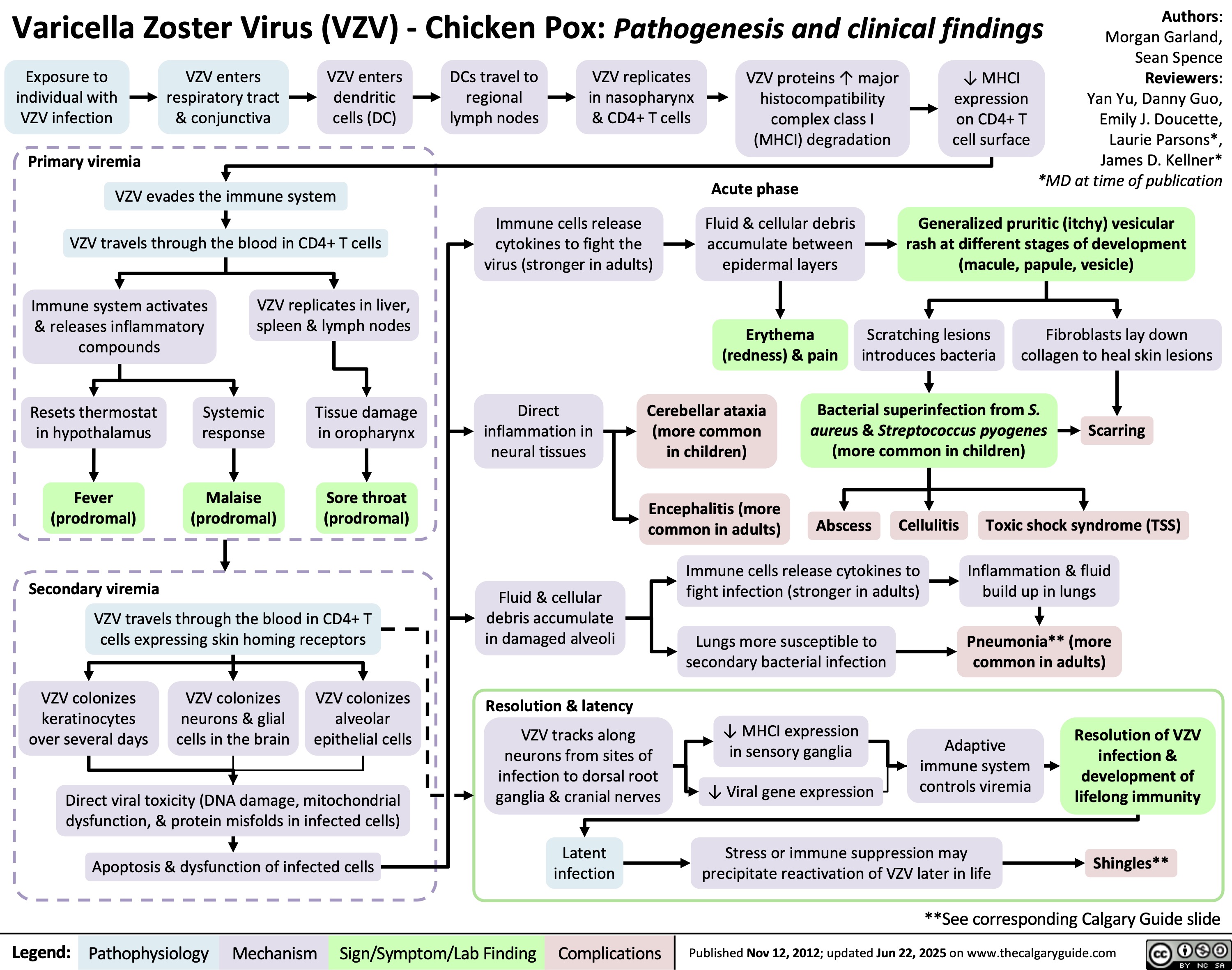
Varicella Zoster Virus
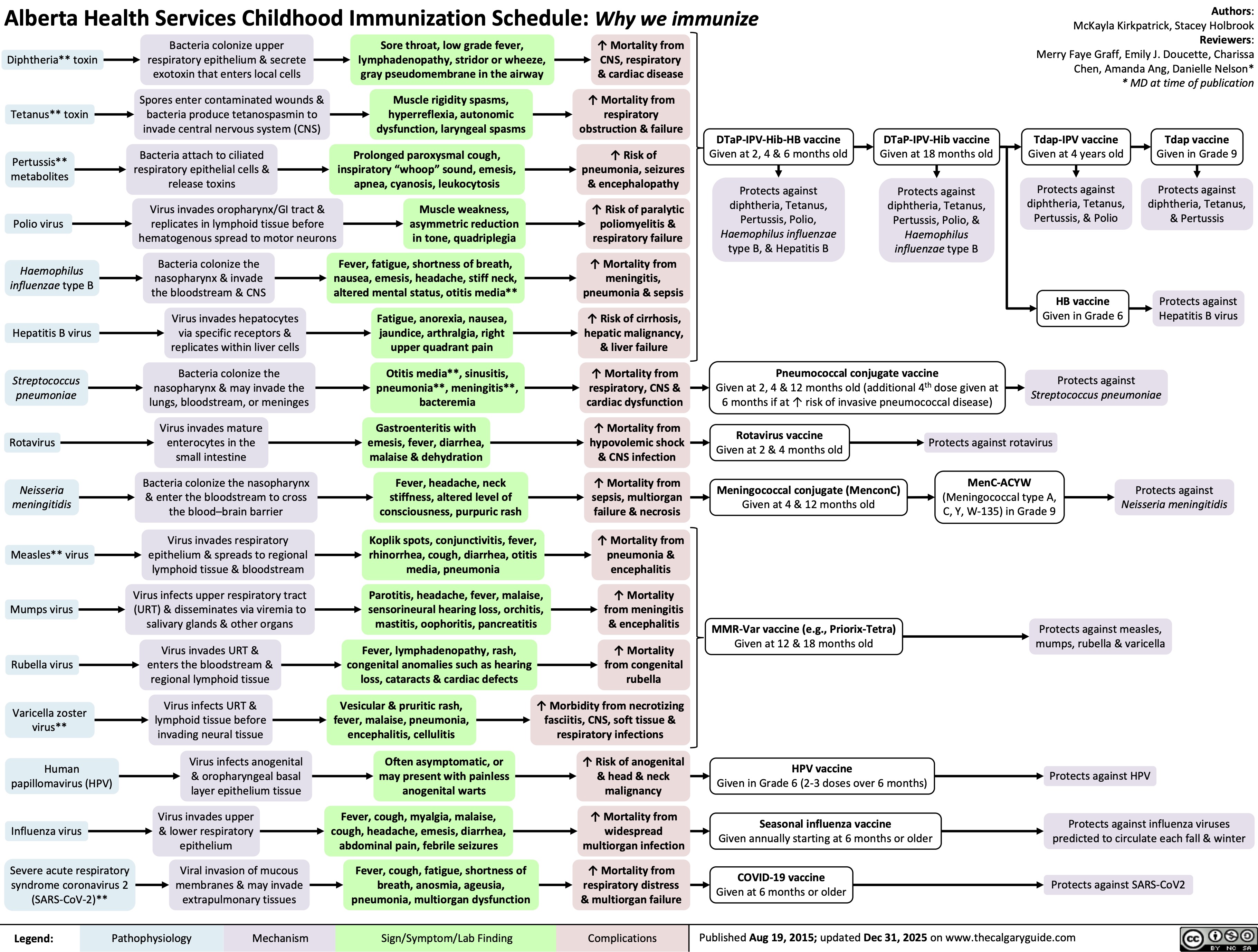
Childhood Immunization Schedule