SEARCH RESULTS FOR: 2023
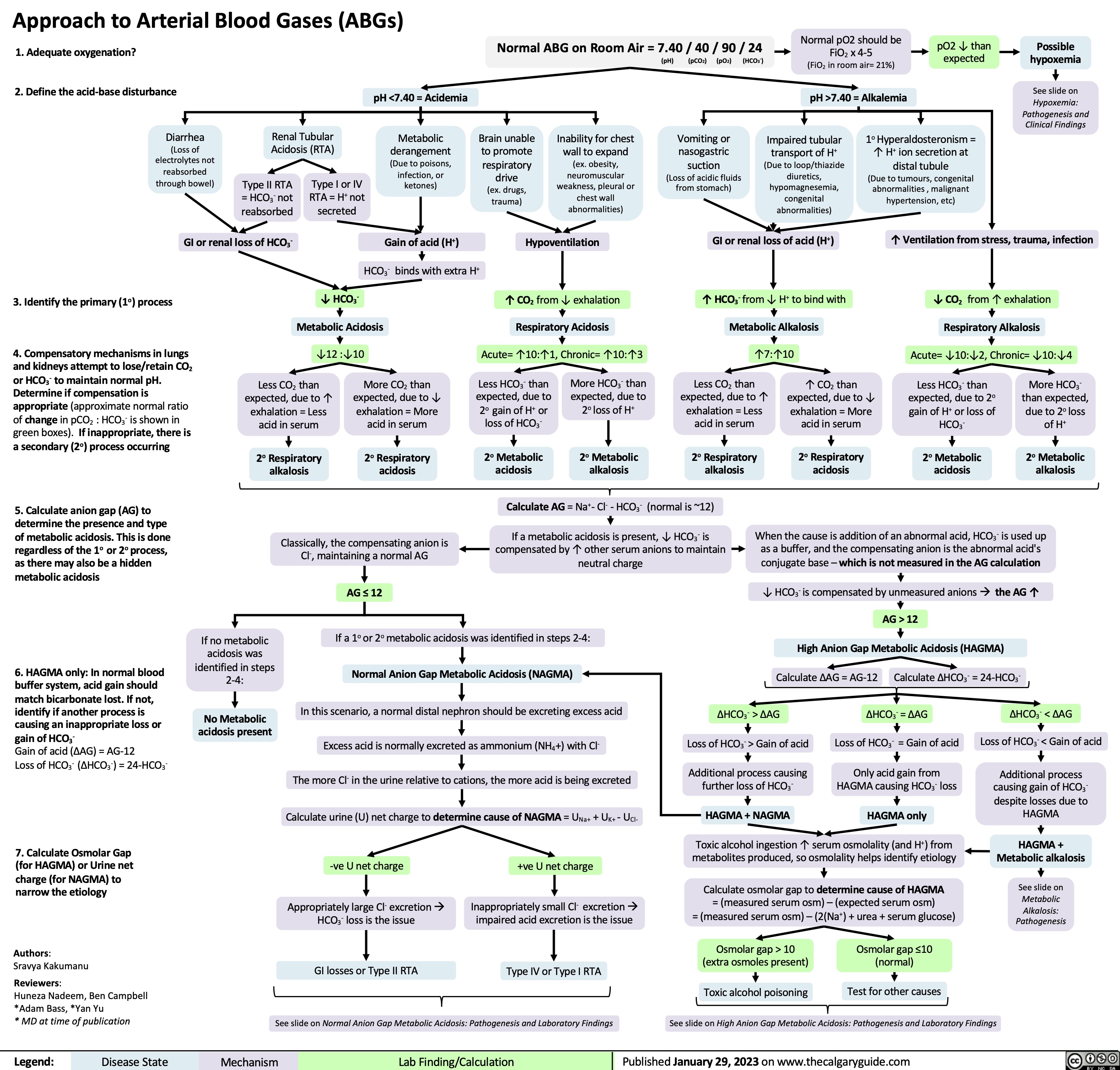
Approach to Arterial Blood Gases ABGs
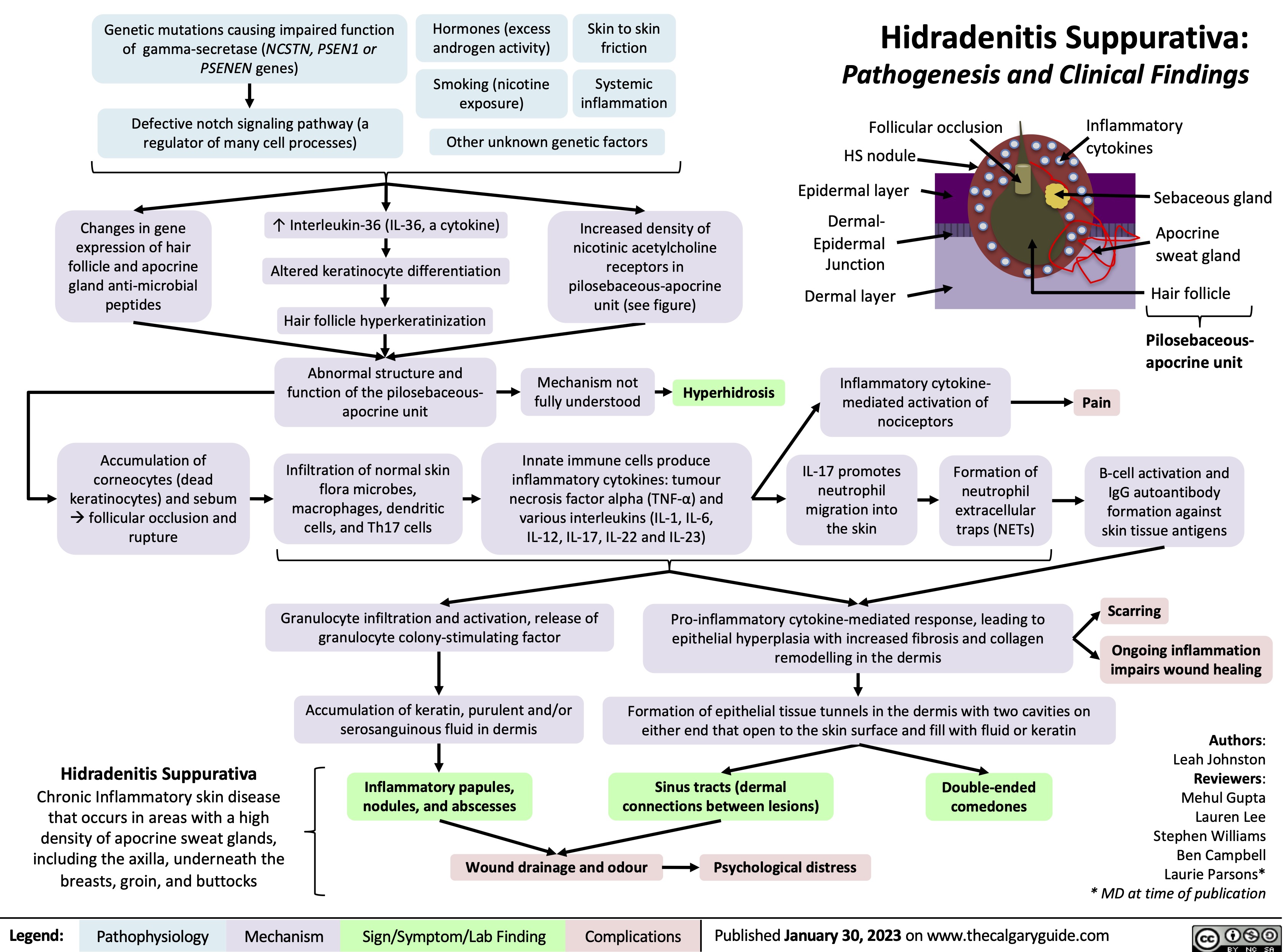
Hidradenitis Suppurativa
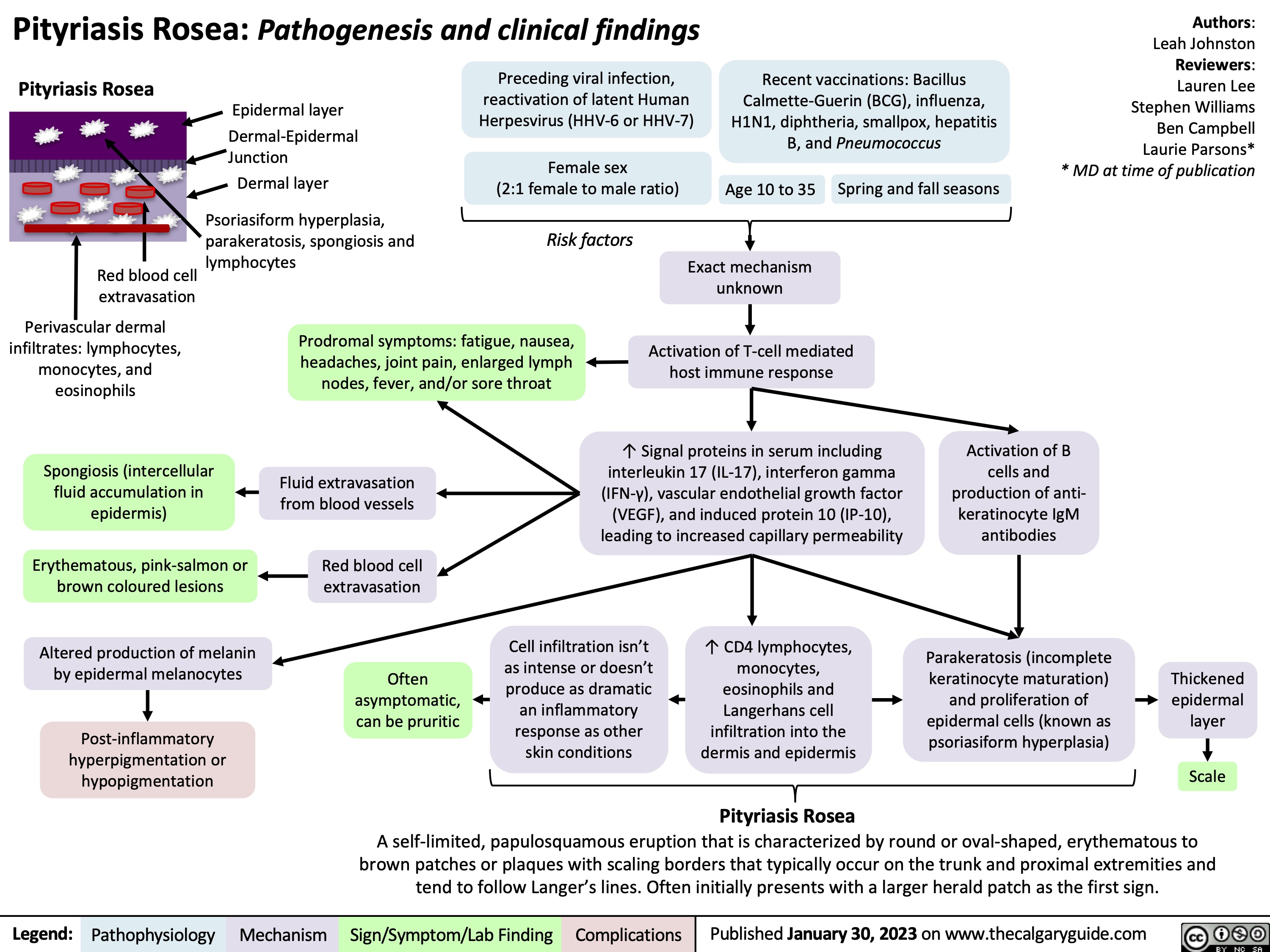
Pityriasis Rosea
Inhalational Injury
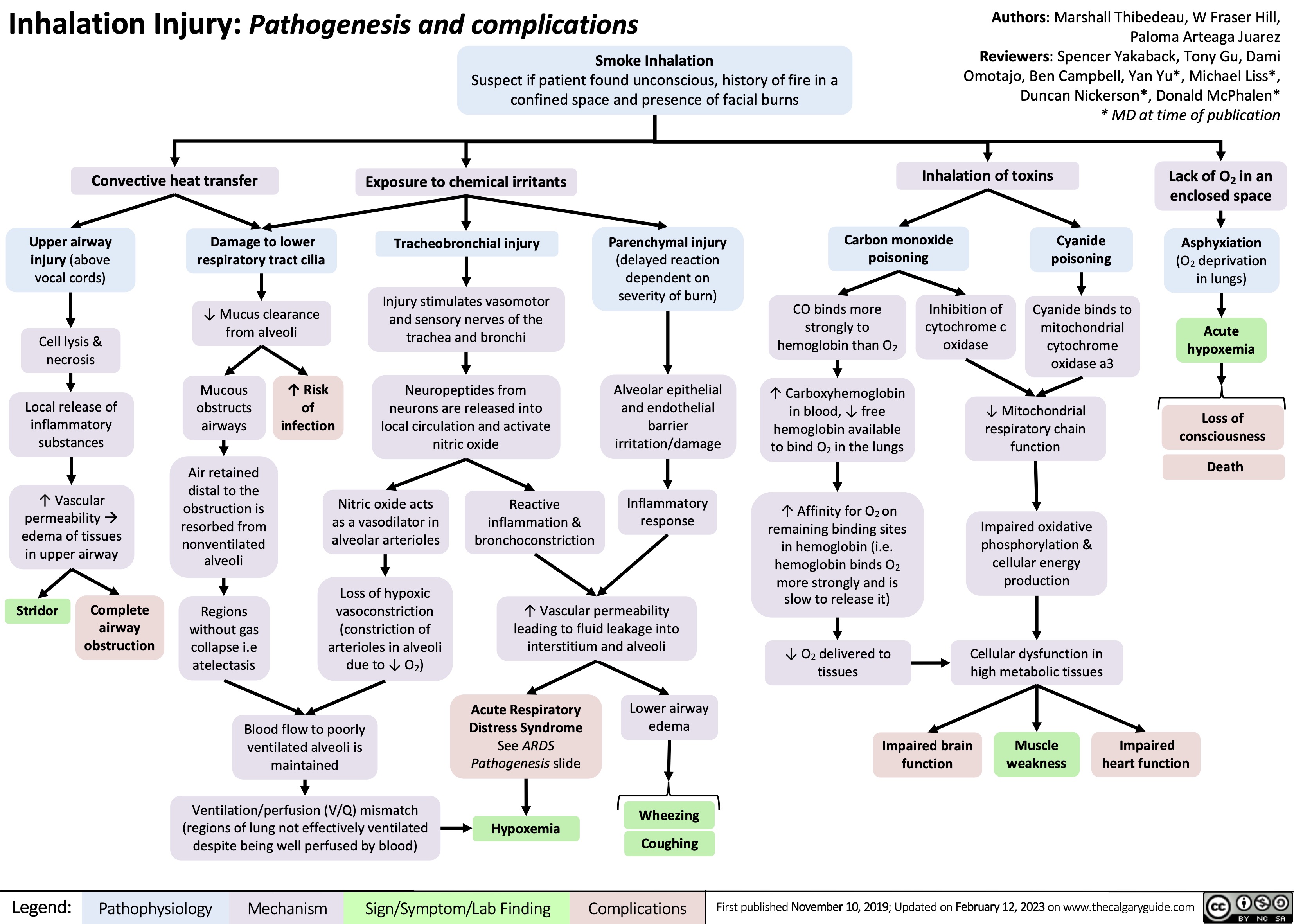
Large Bowel Obstruction: Findings on Abdominal X-ray
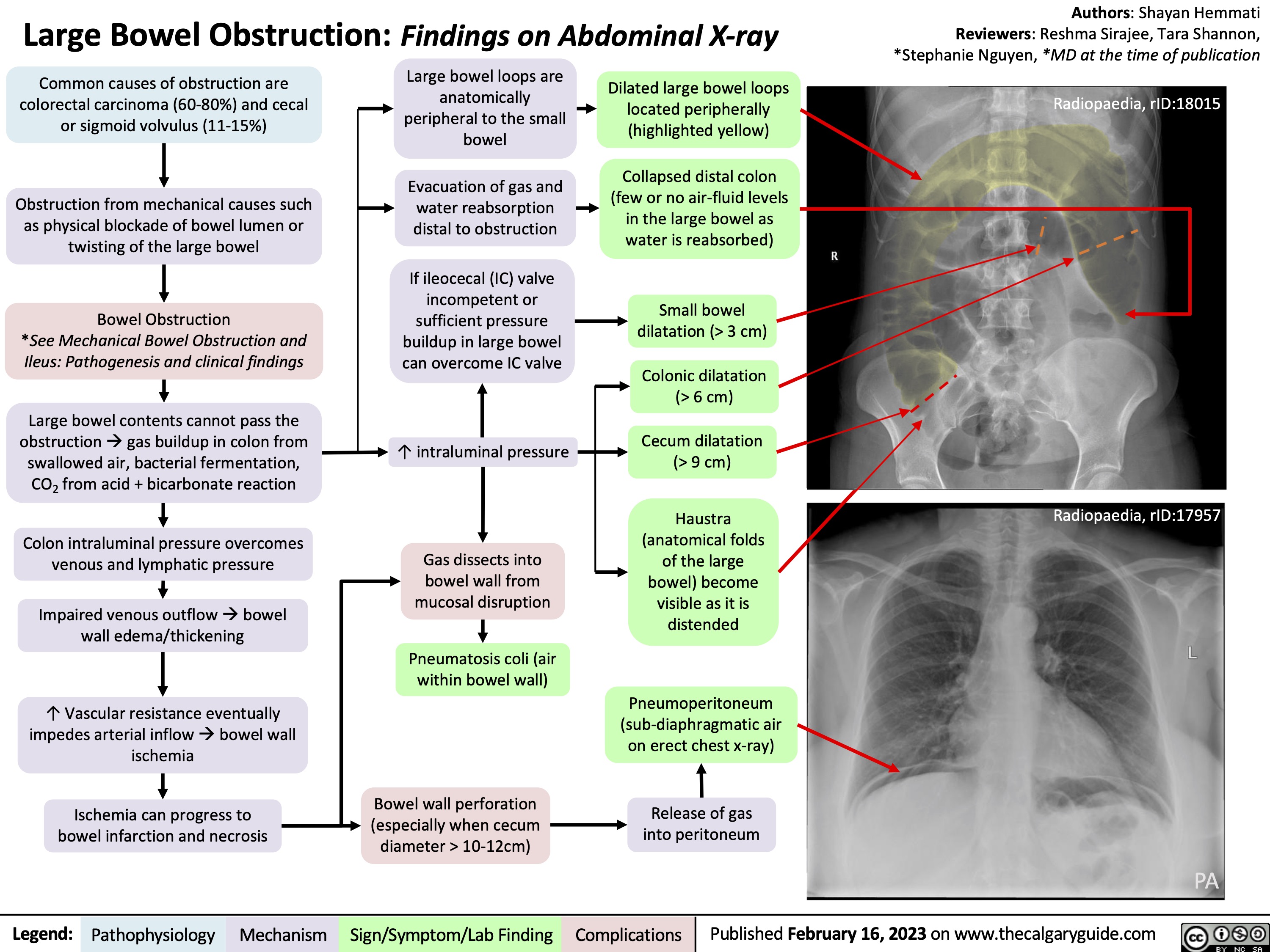
Ischemic Stroke Impairment by Localization
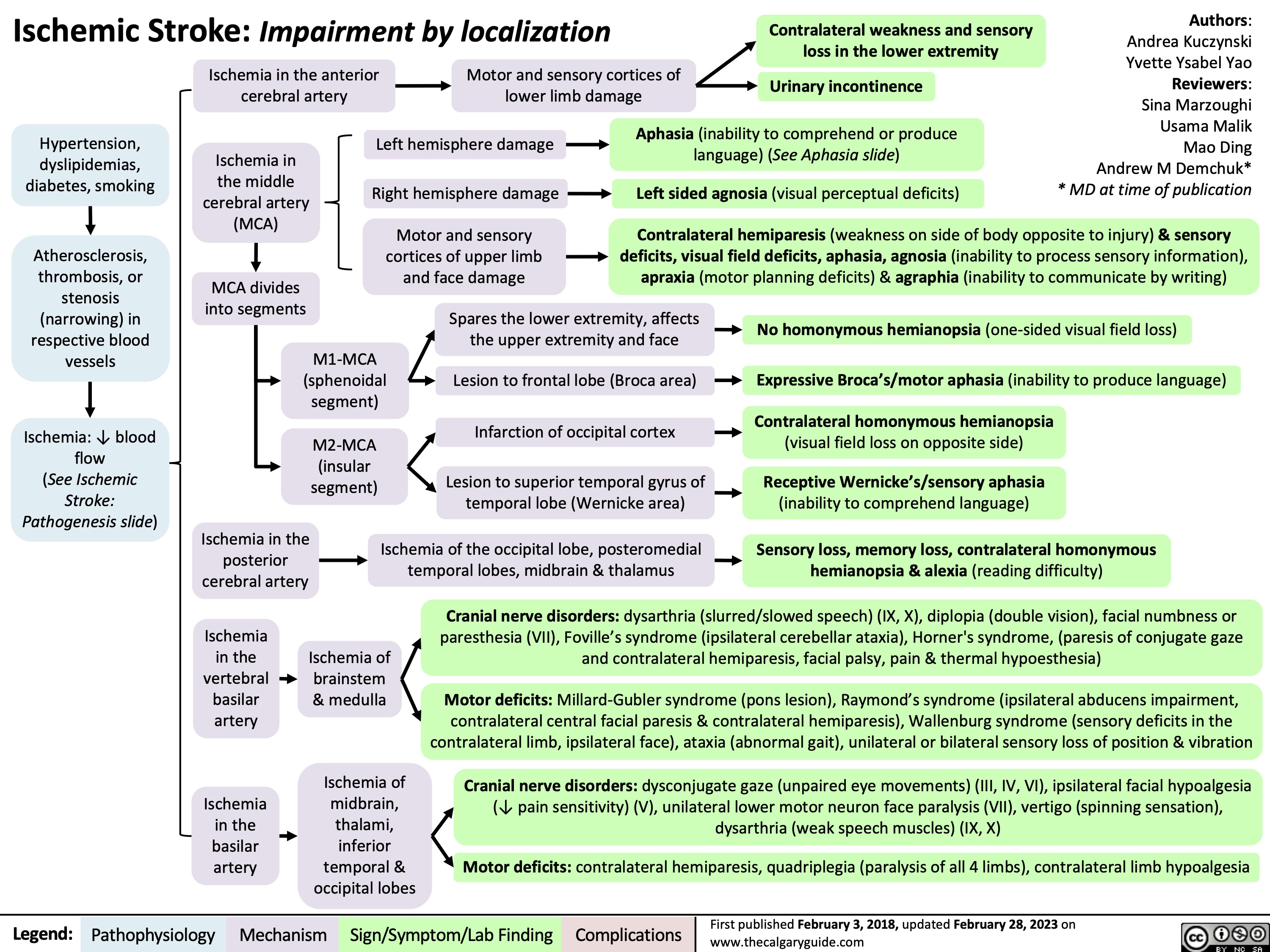
Hemorrhagic Stroke
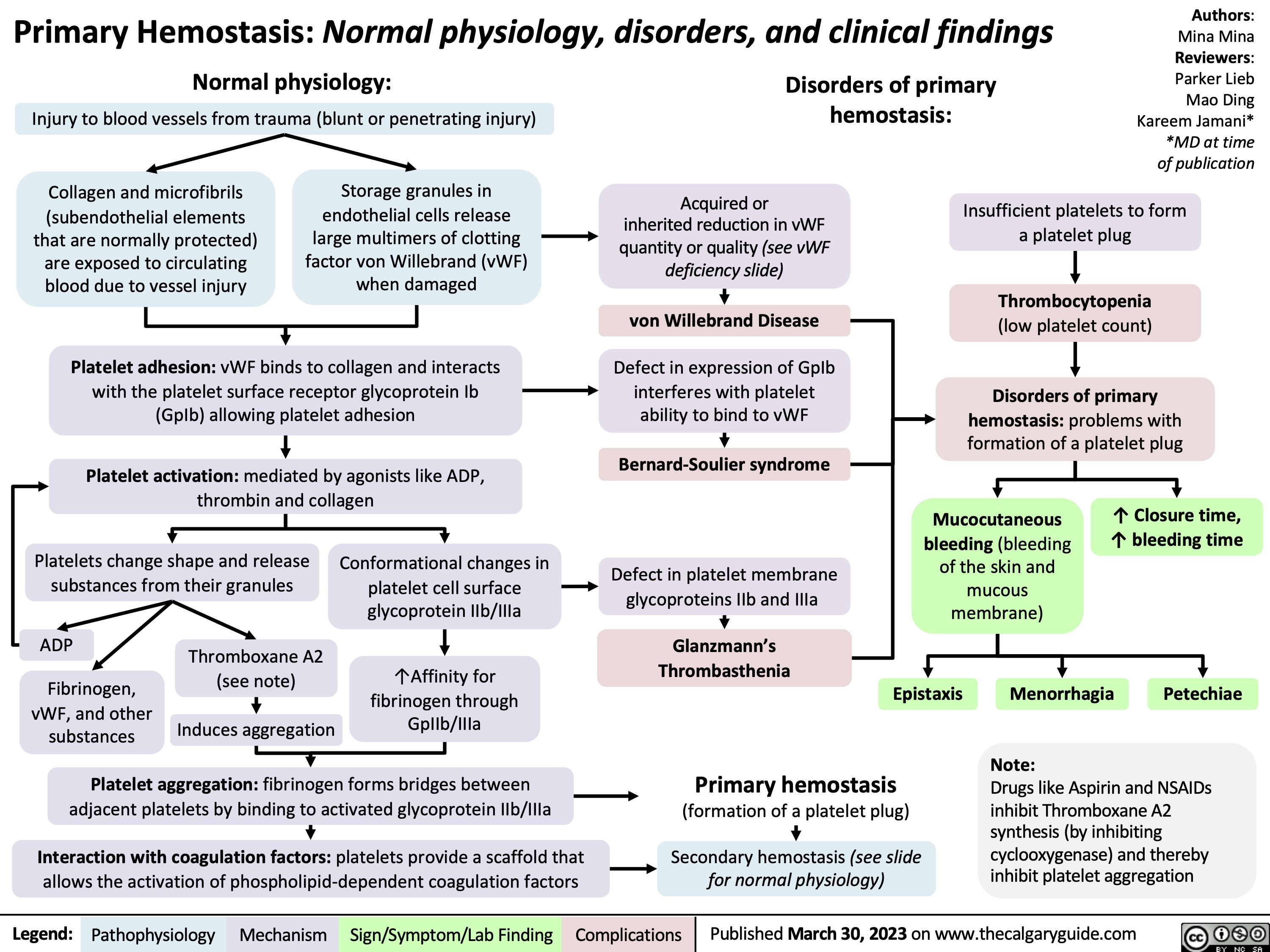
Myelodysplastic Syndrome Pathogenesis and clinical findings
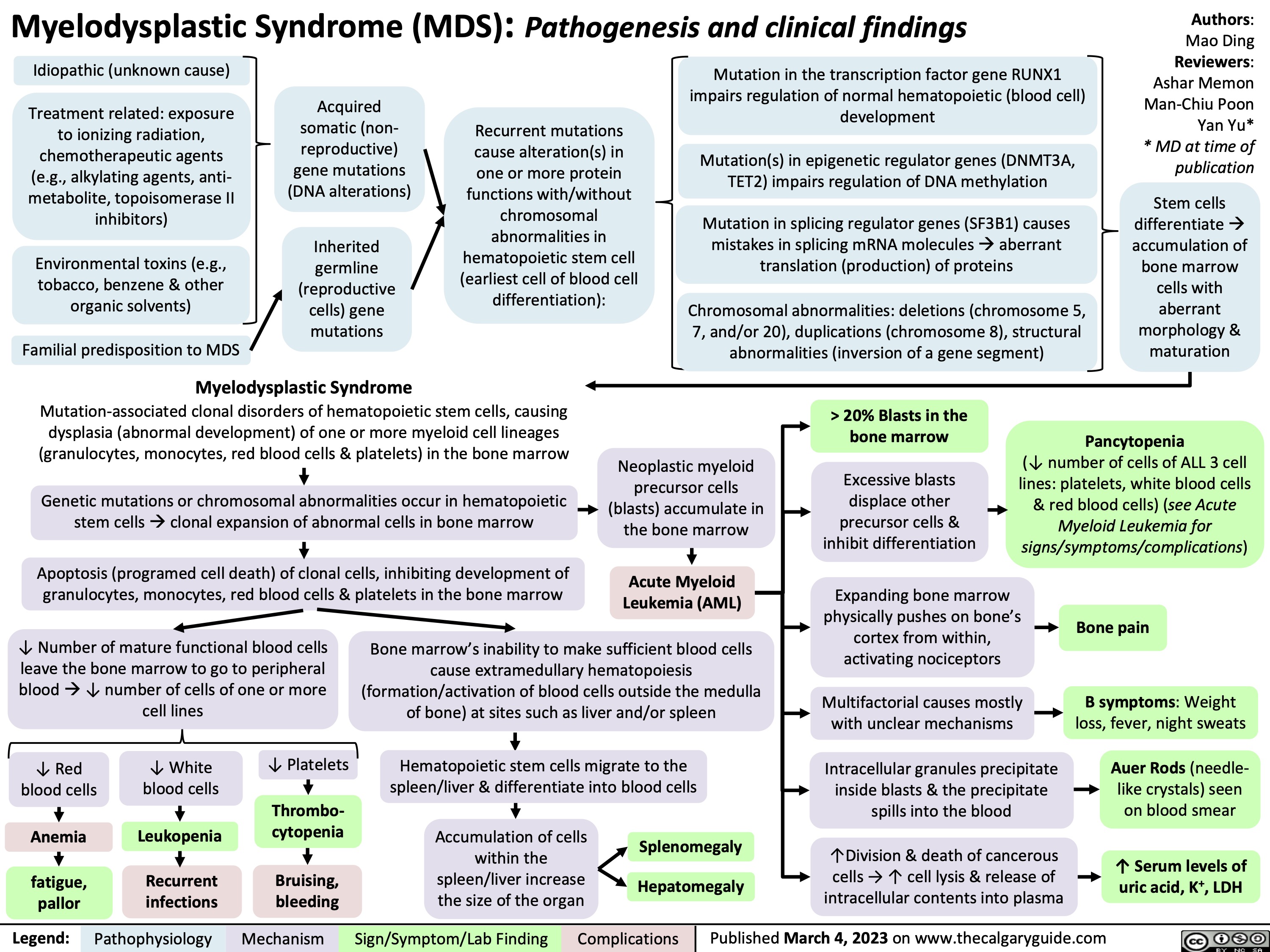
Central Retinal Vein Occlusion
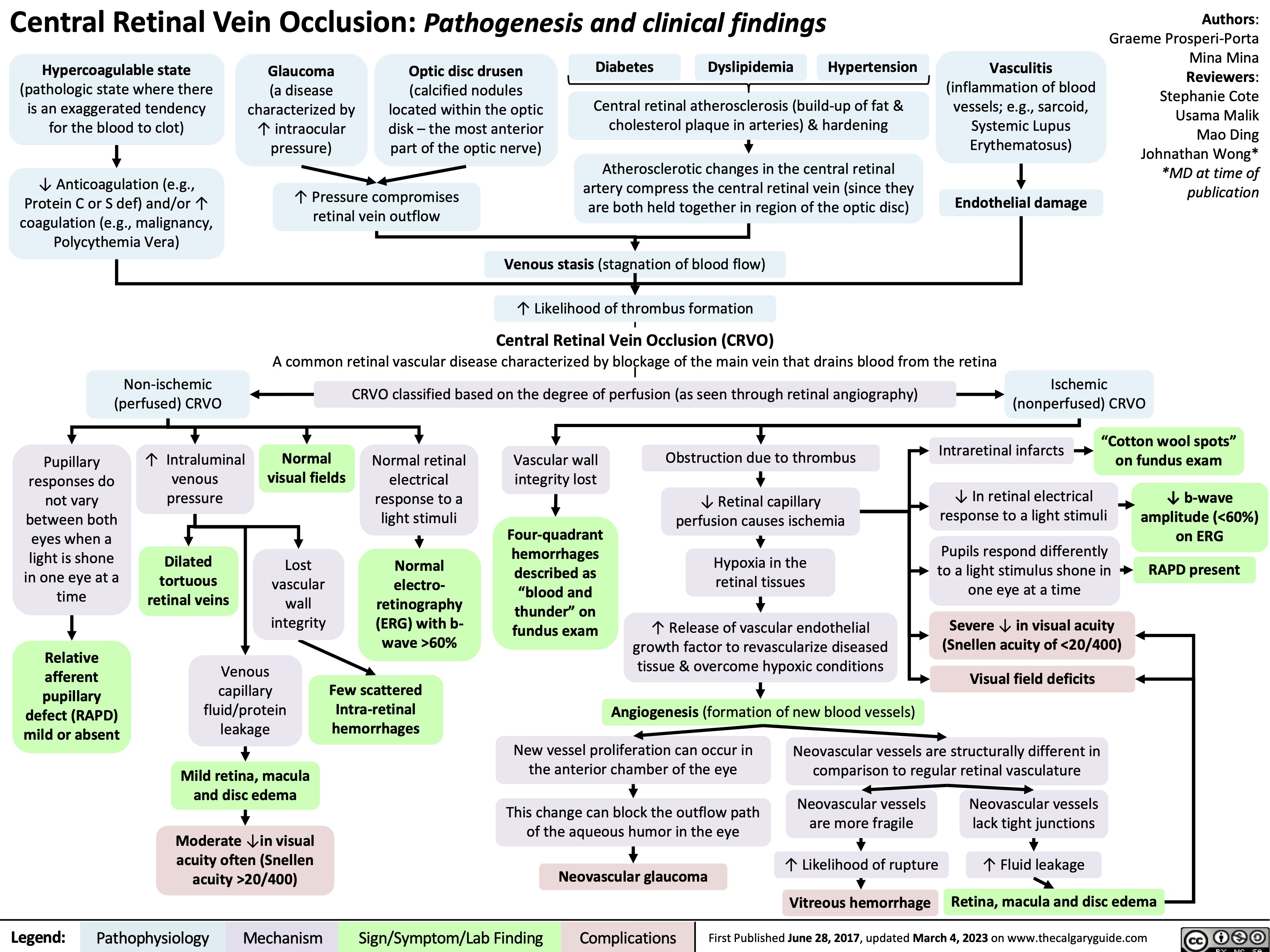
Skin Grafts Transplant physiology and clinical findings
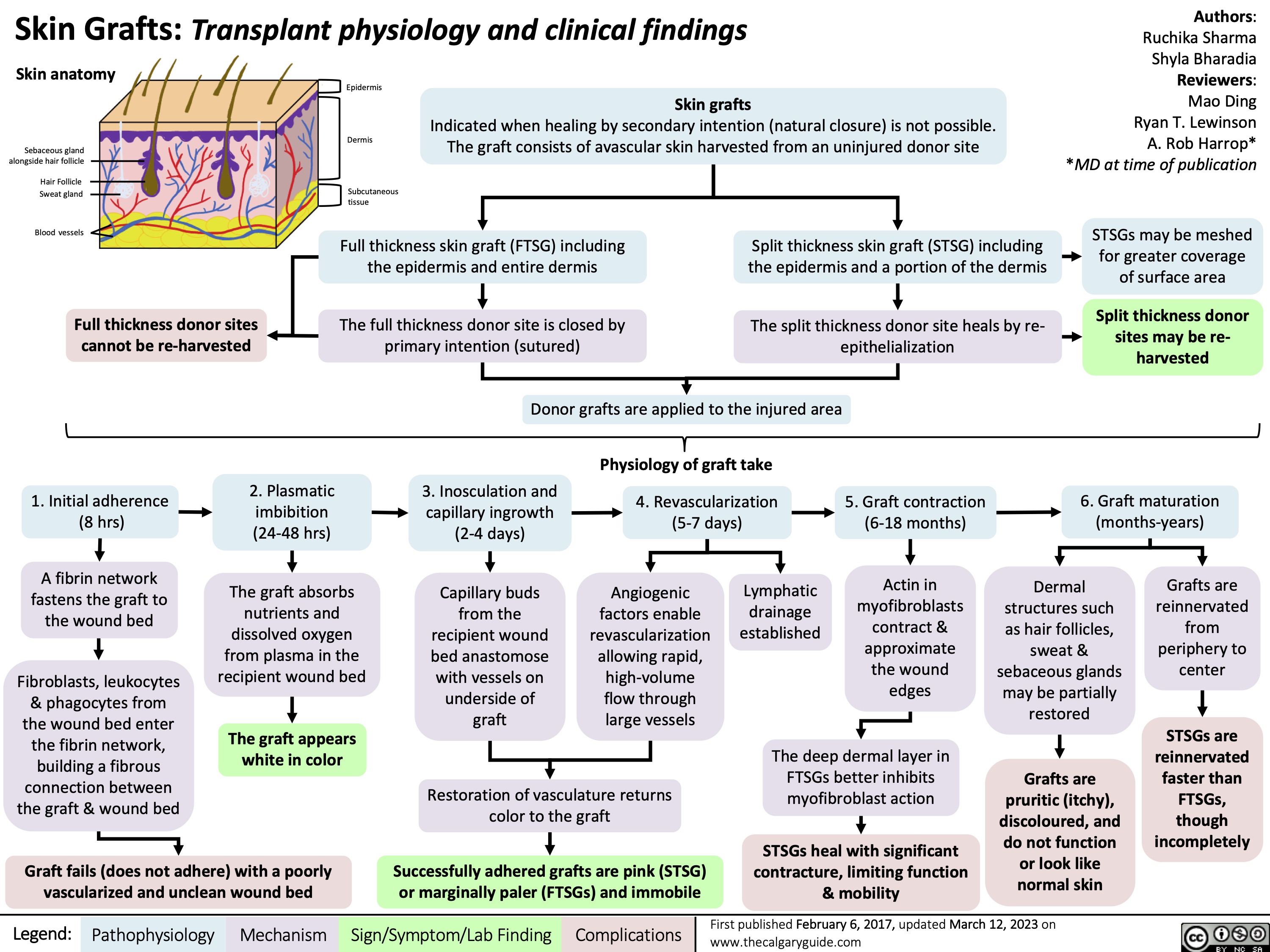
Primary Hemostasis
Concussion
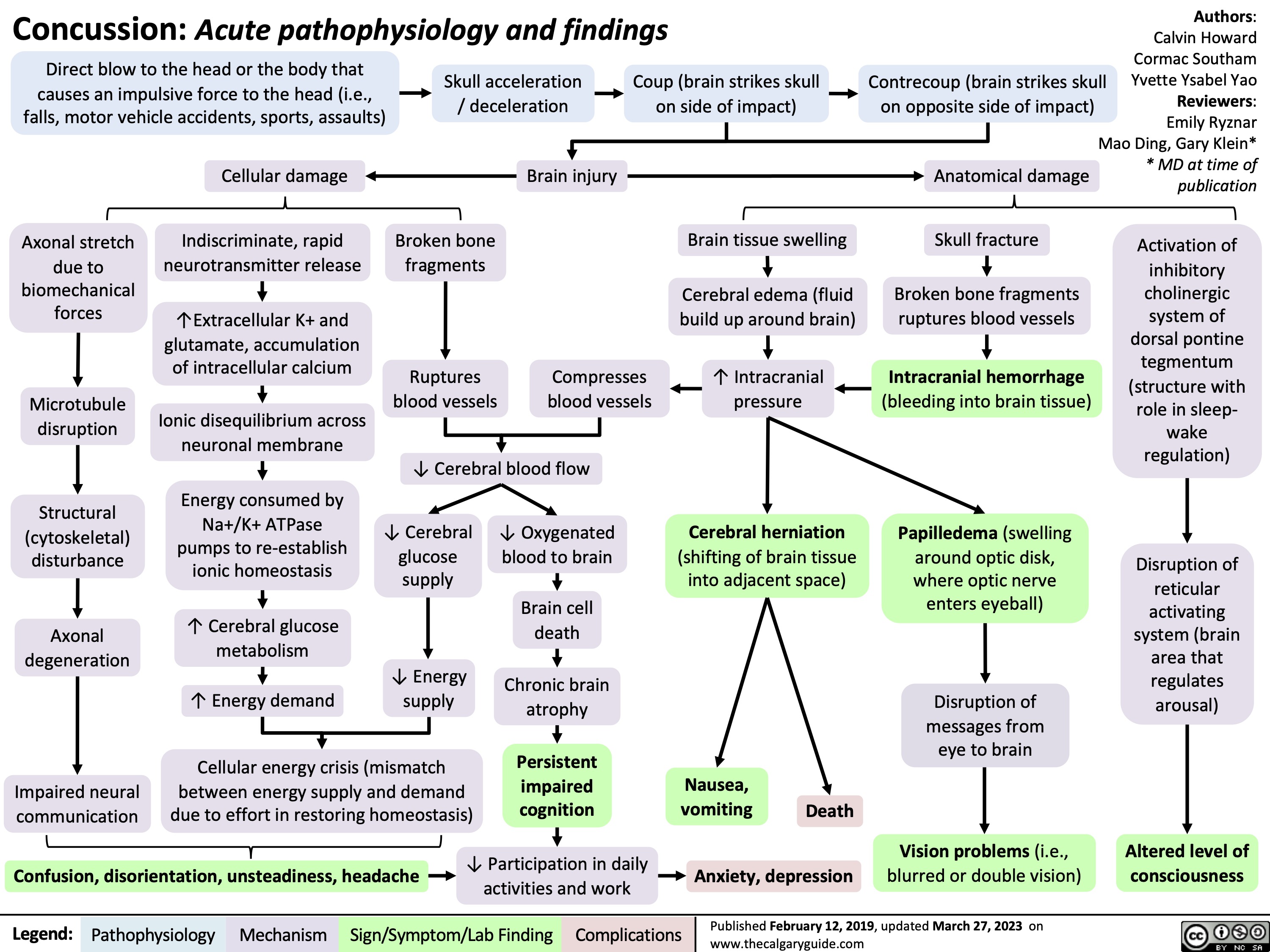
Pyogenic Brain Abscess on MRI
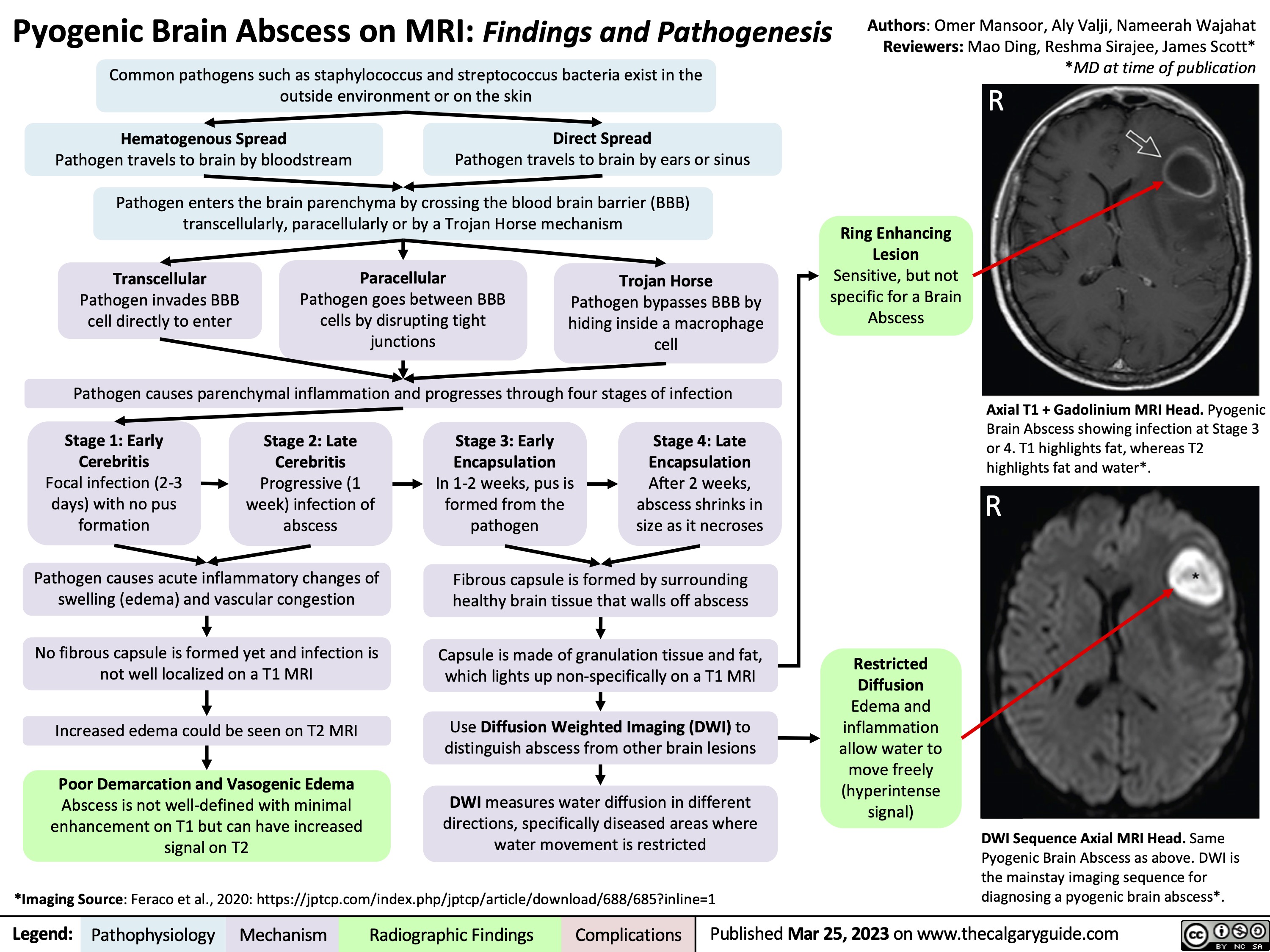
Carpal Tunnel Syndrome
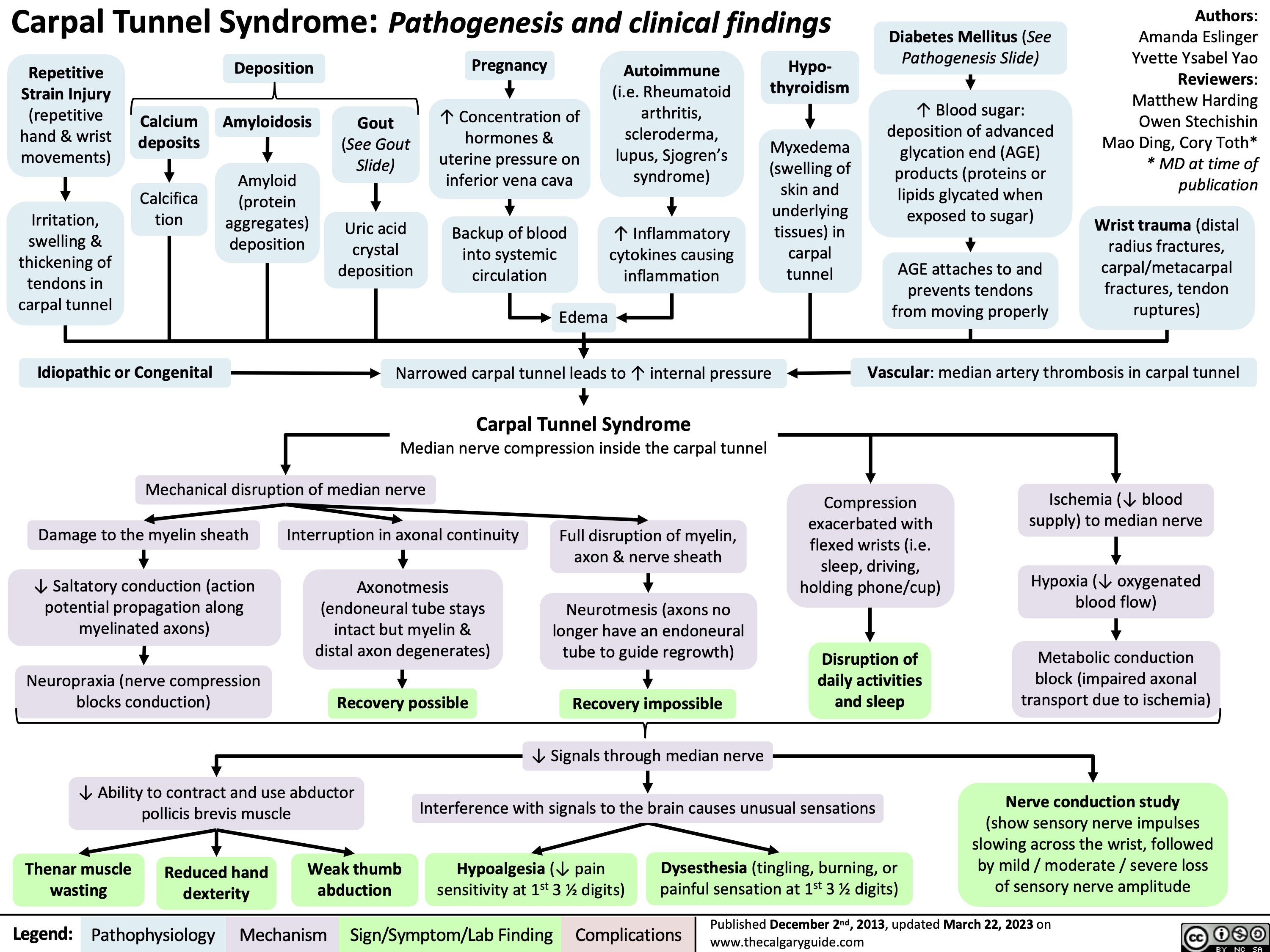
Complications of Prematurity
Acute Pulmonary Embolism on CTPA
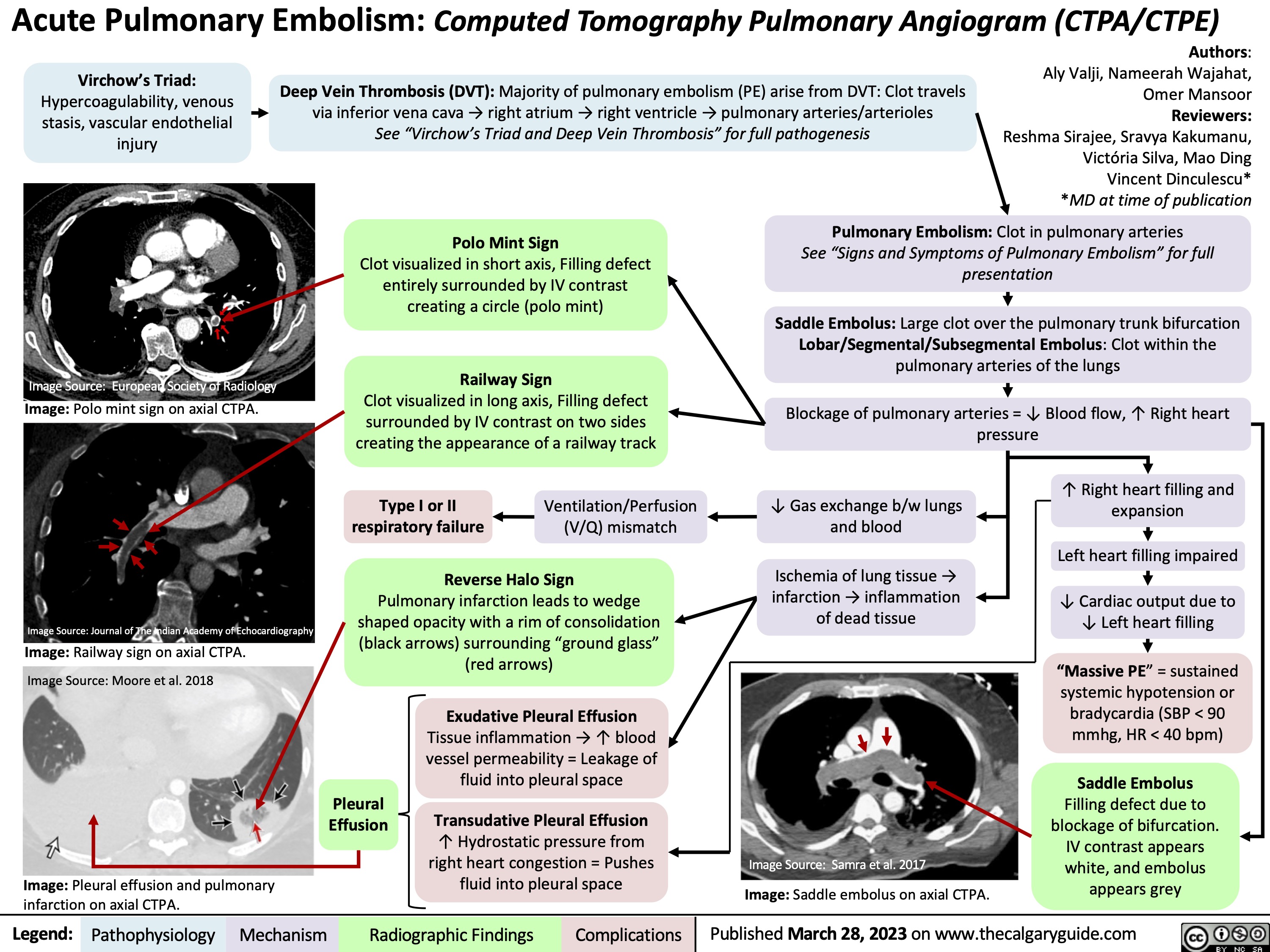
Sarcoidosis pathogenesis and CXR findings
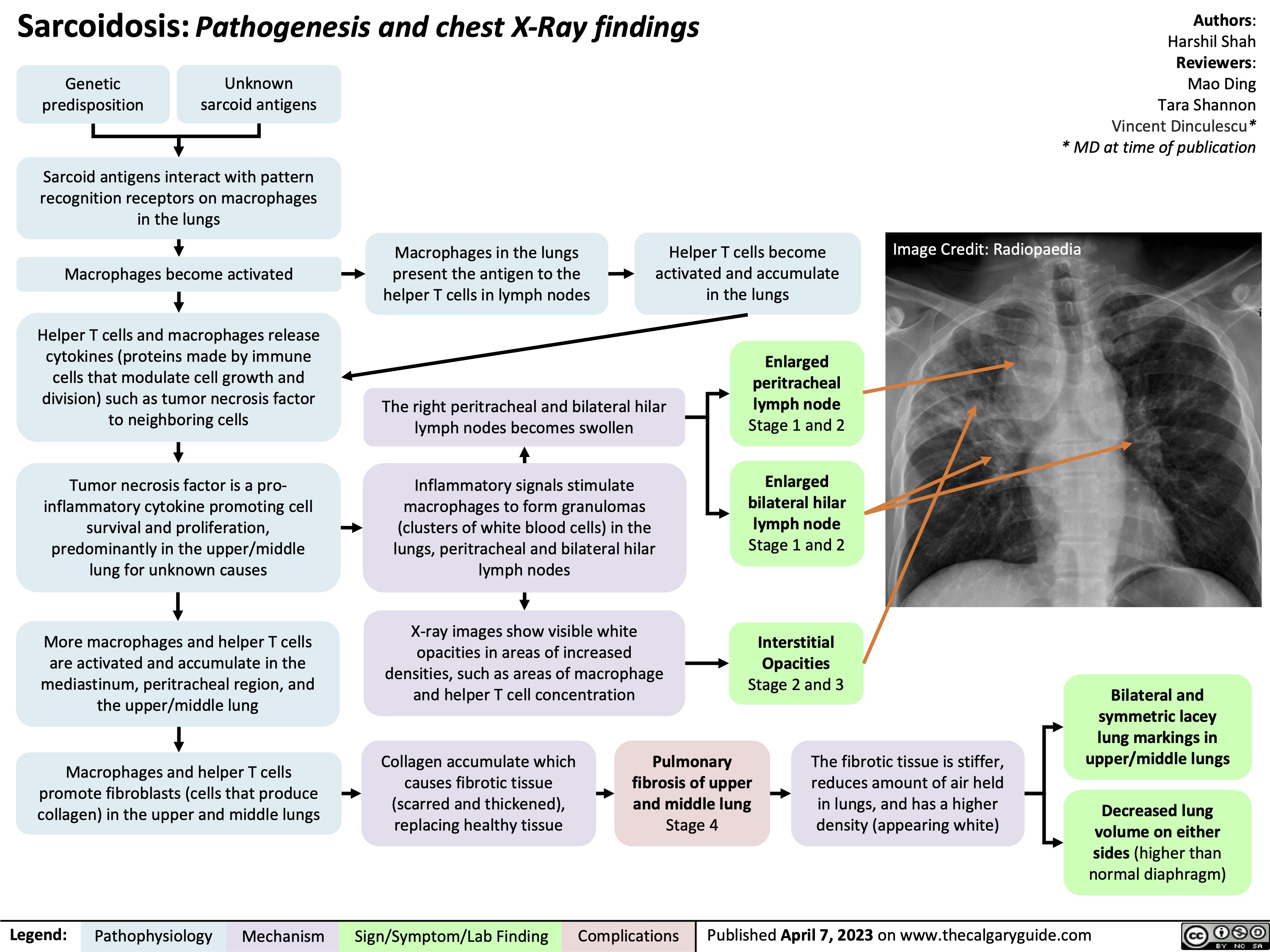
Coronary Artery Bypass Graft CABG Indications
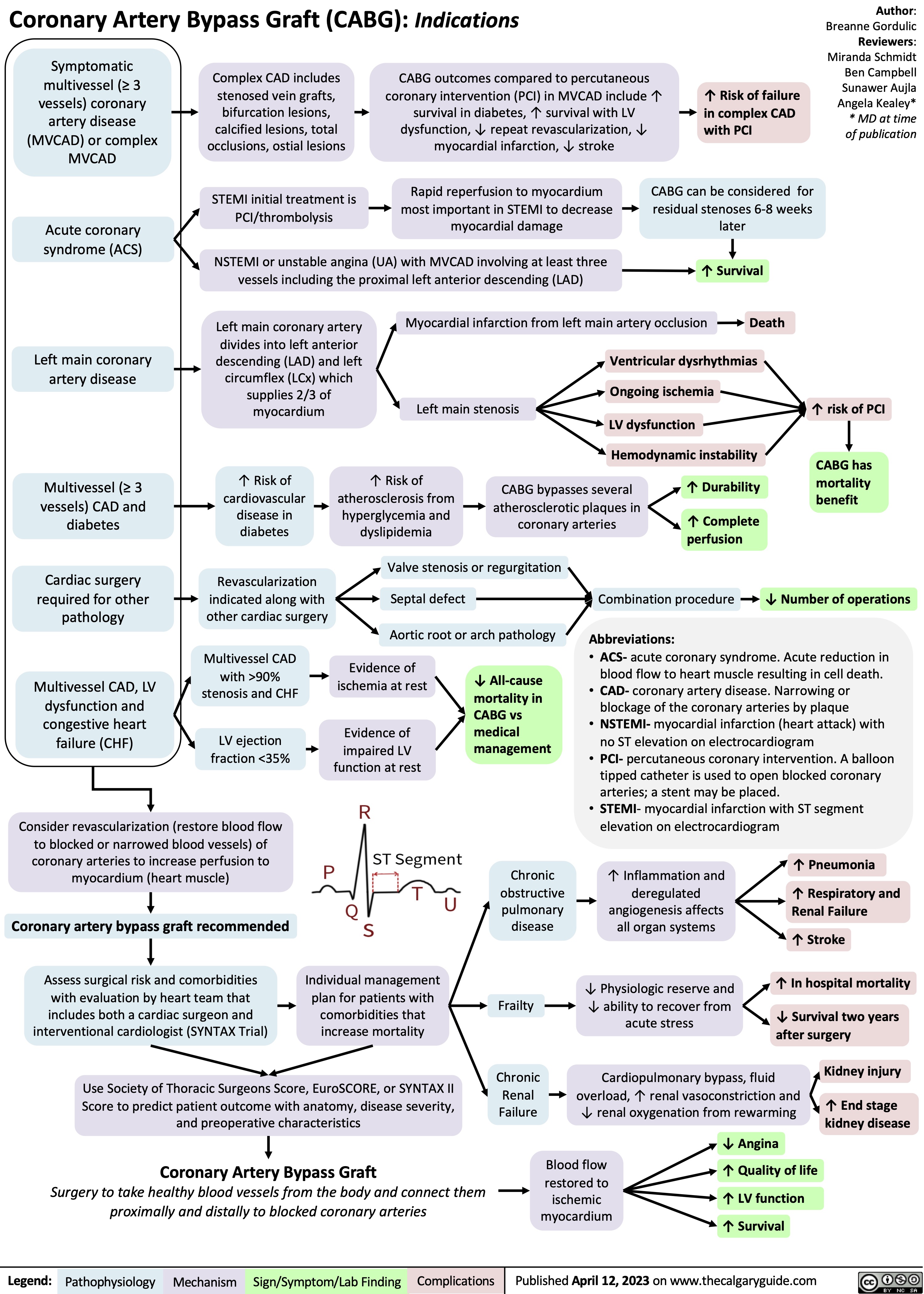
Benzodiazepine Mechanism of Action
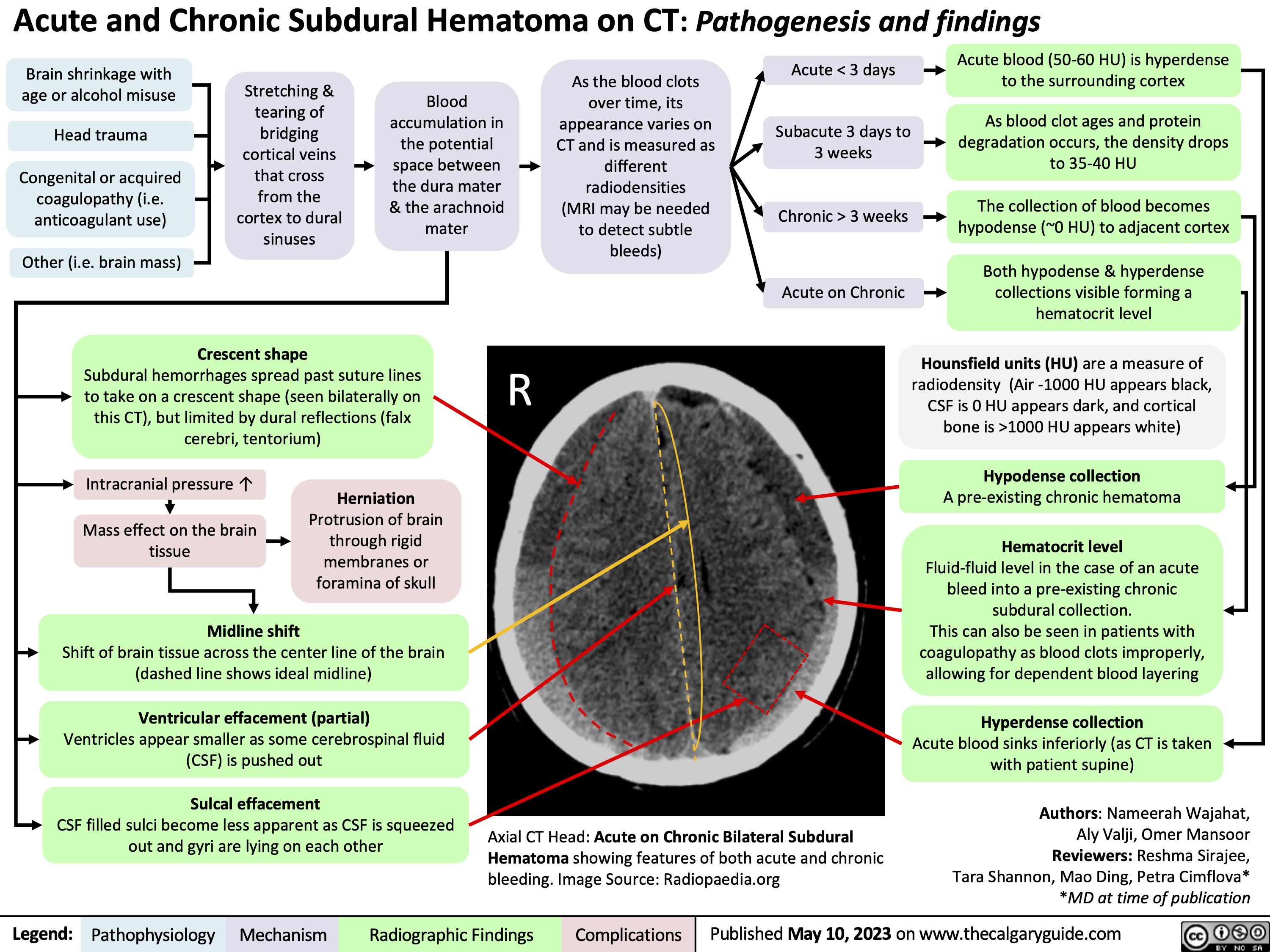
Subdural Hematoma on CT
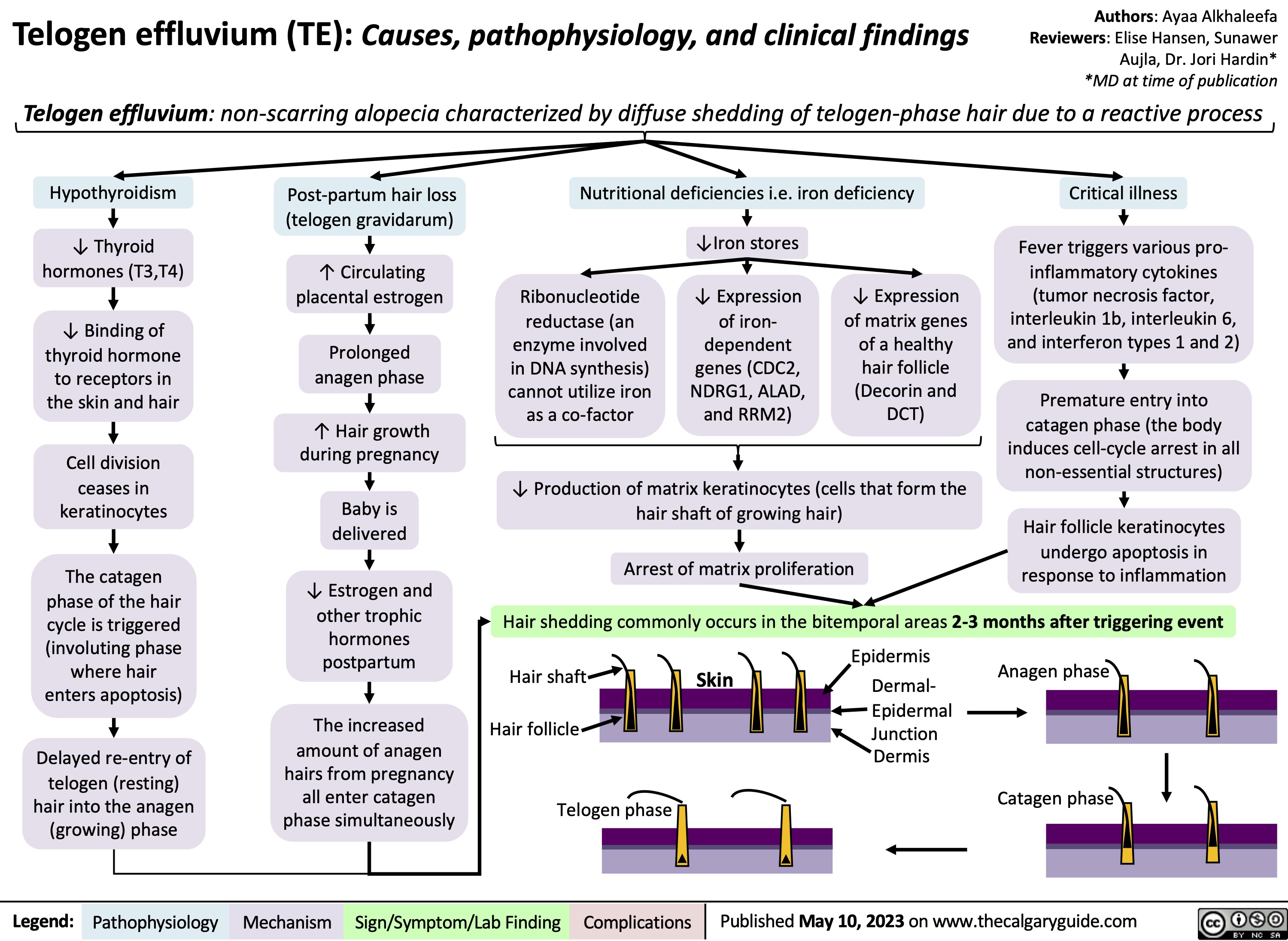
Telogen Effluvium
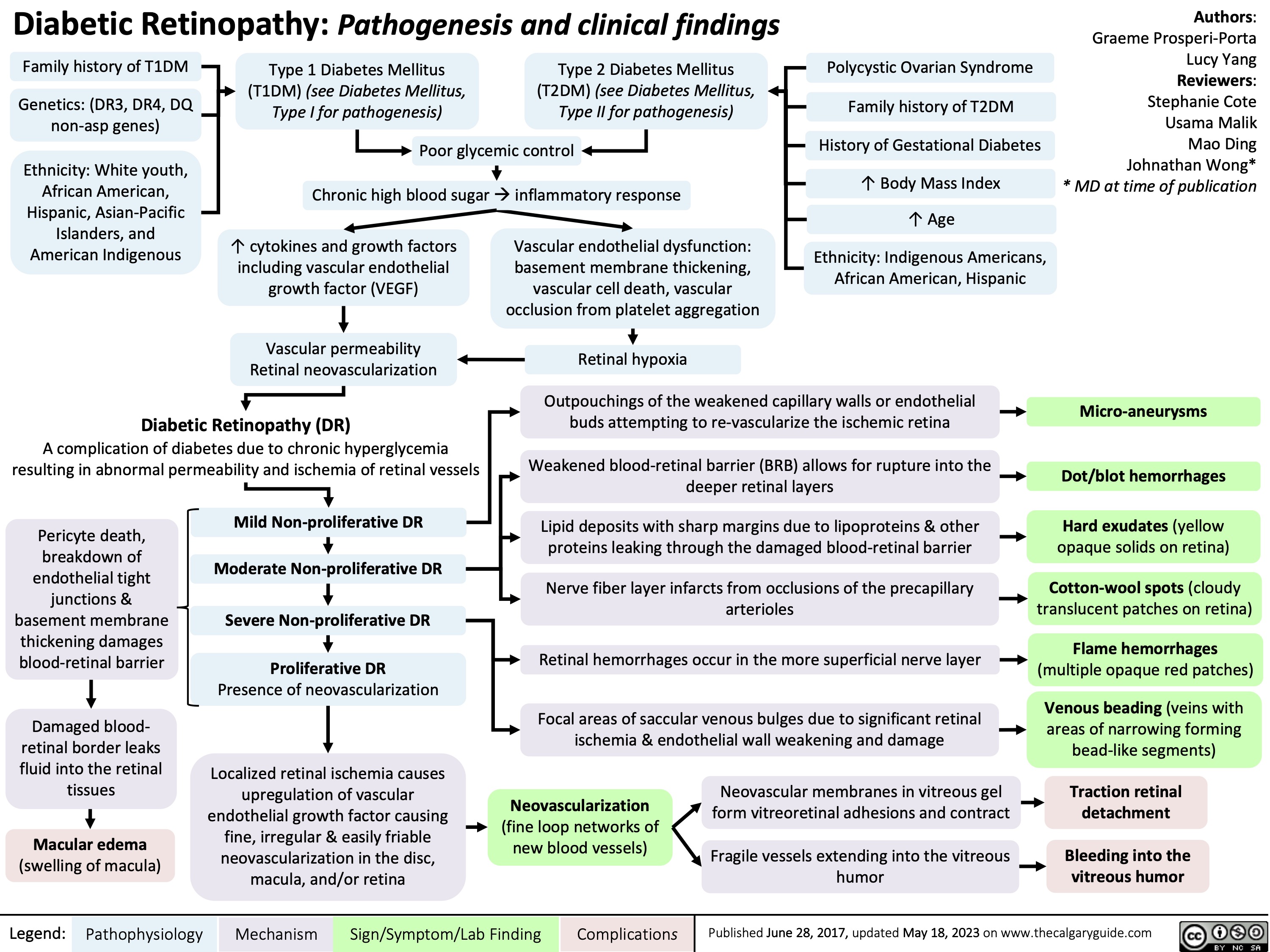
Diabetic Retinopathy
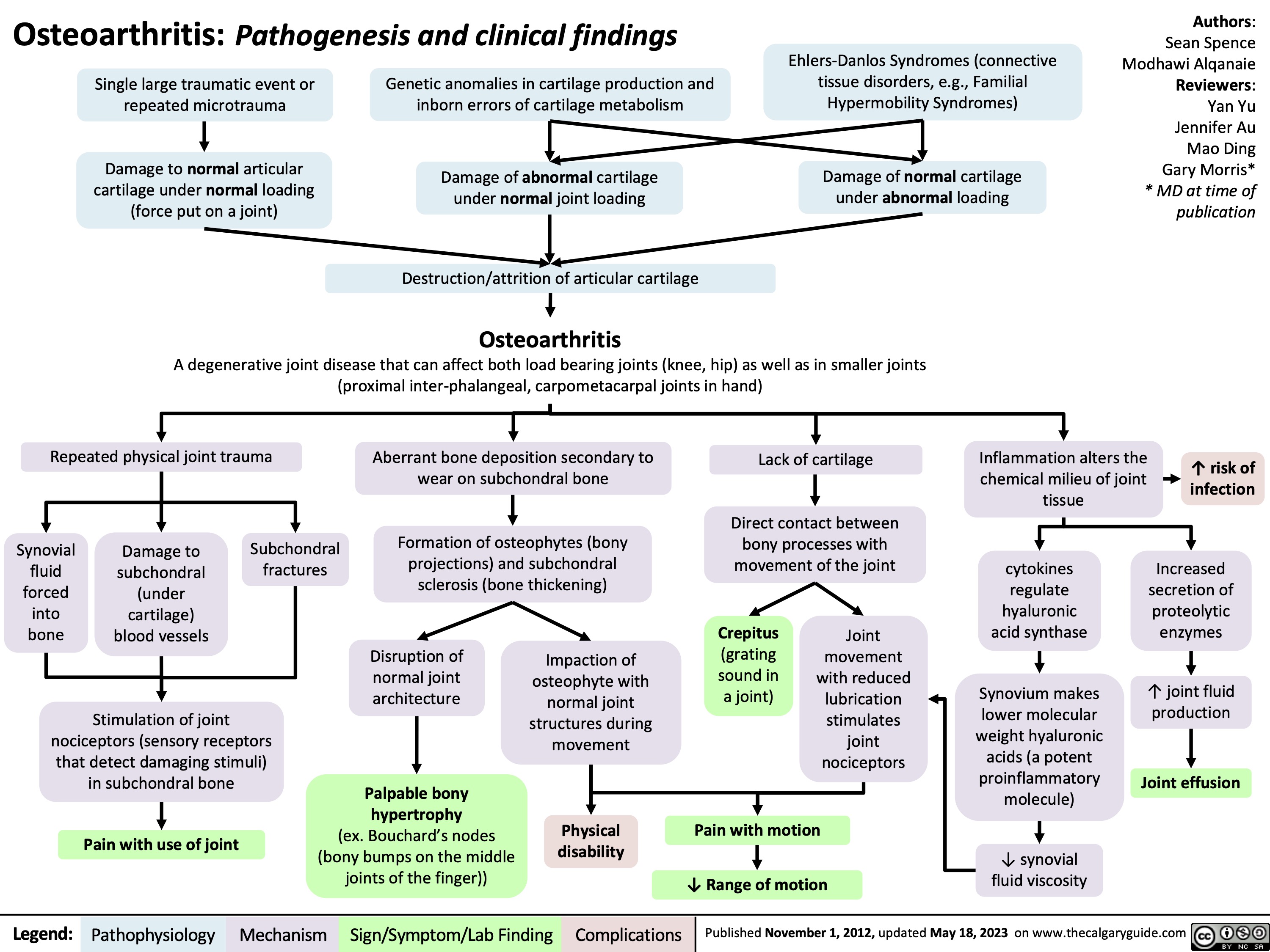
OA Clinical findings
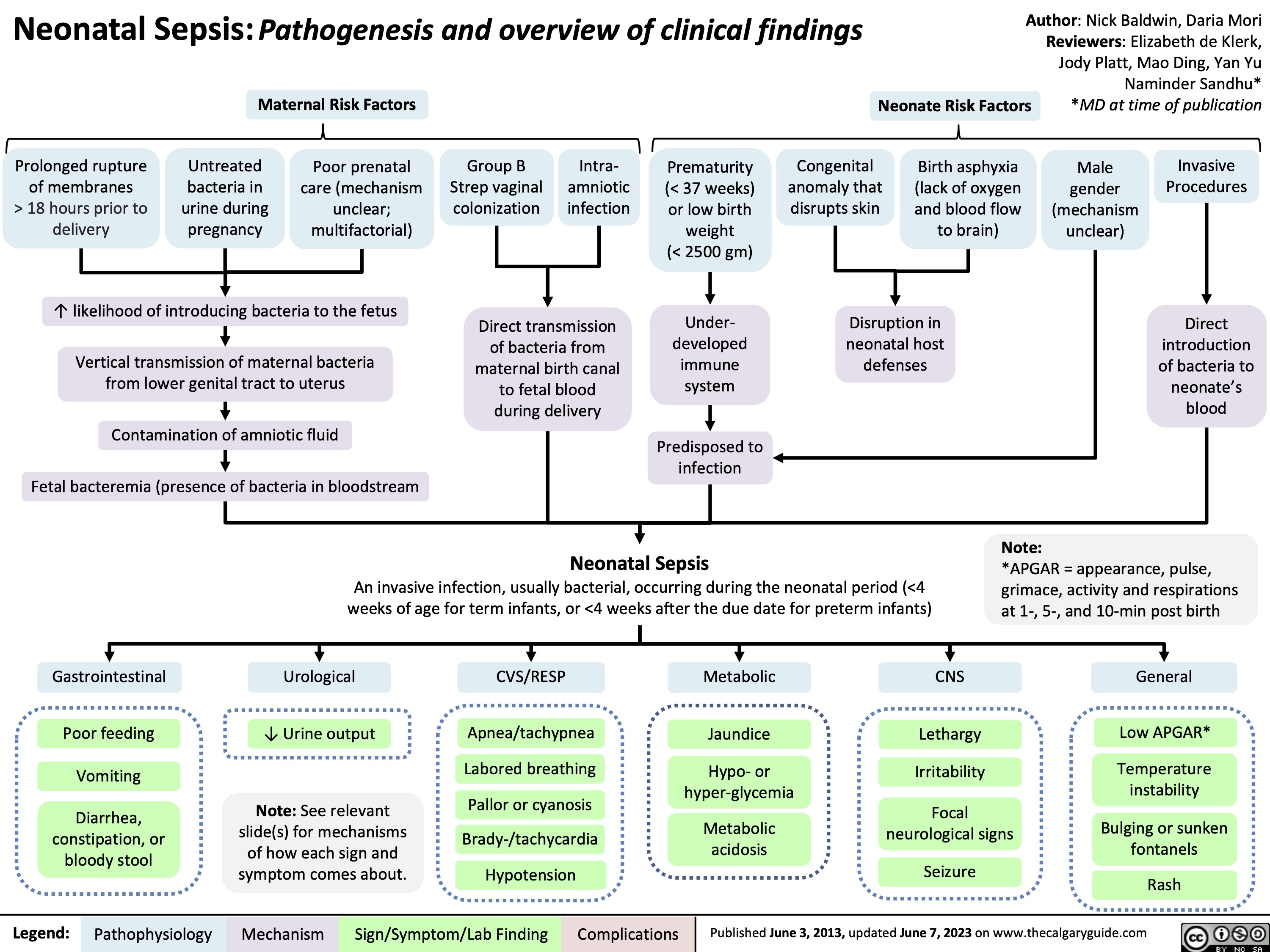
Digestion and Absorption of Macromolecules
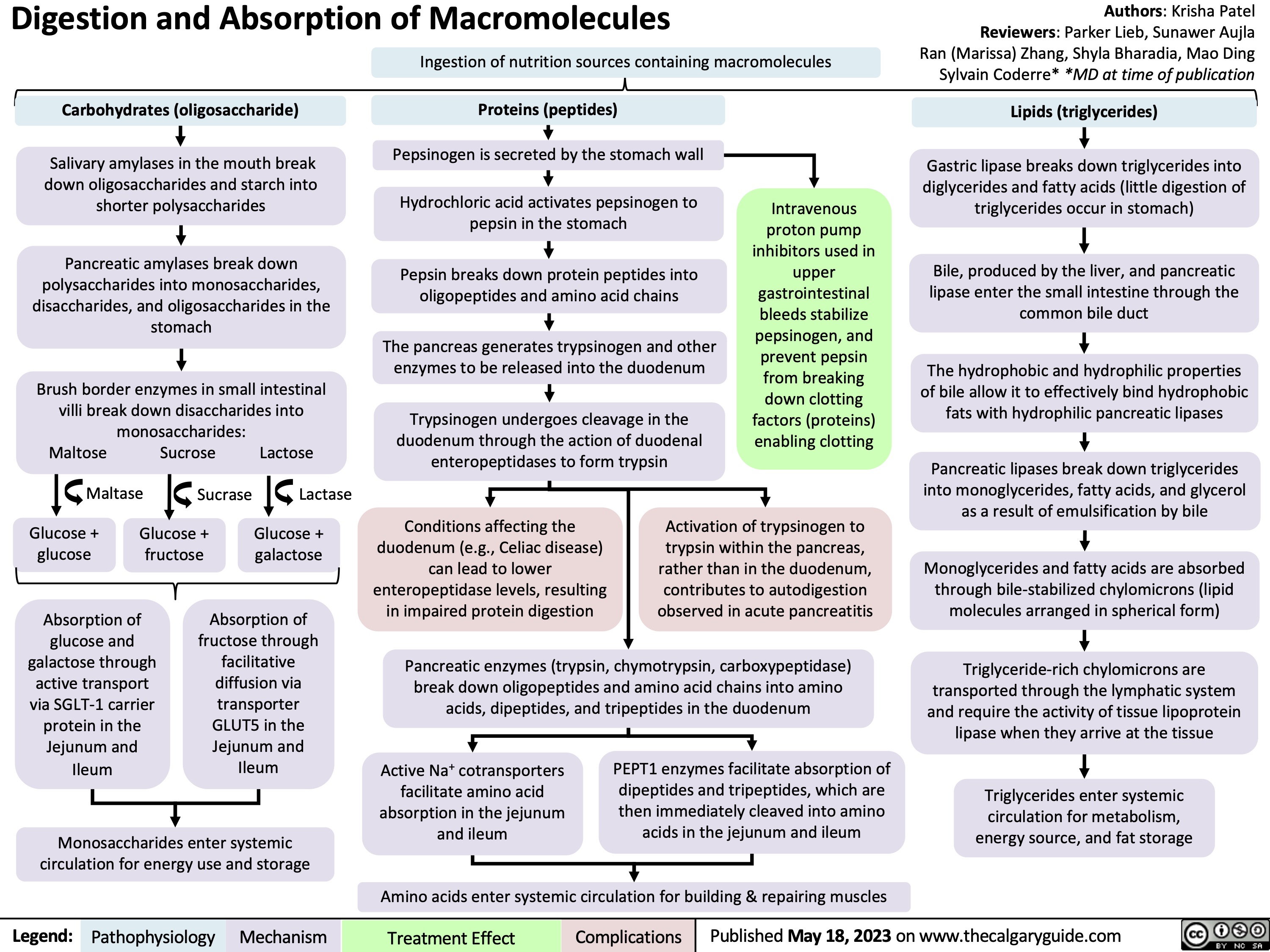
Physiology of Anti-diuretic hormone
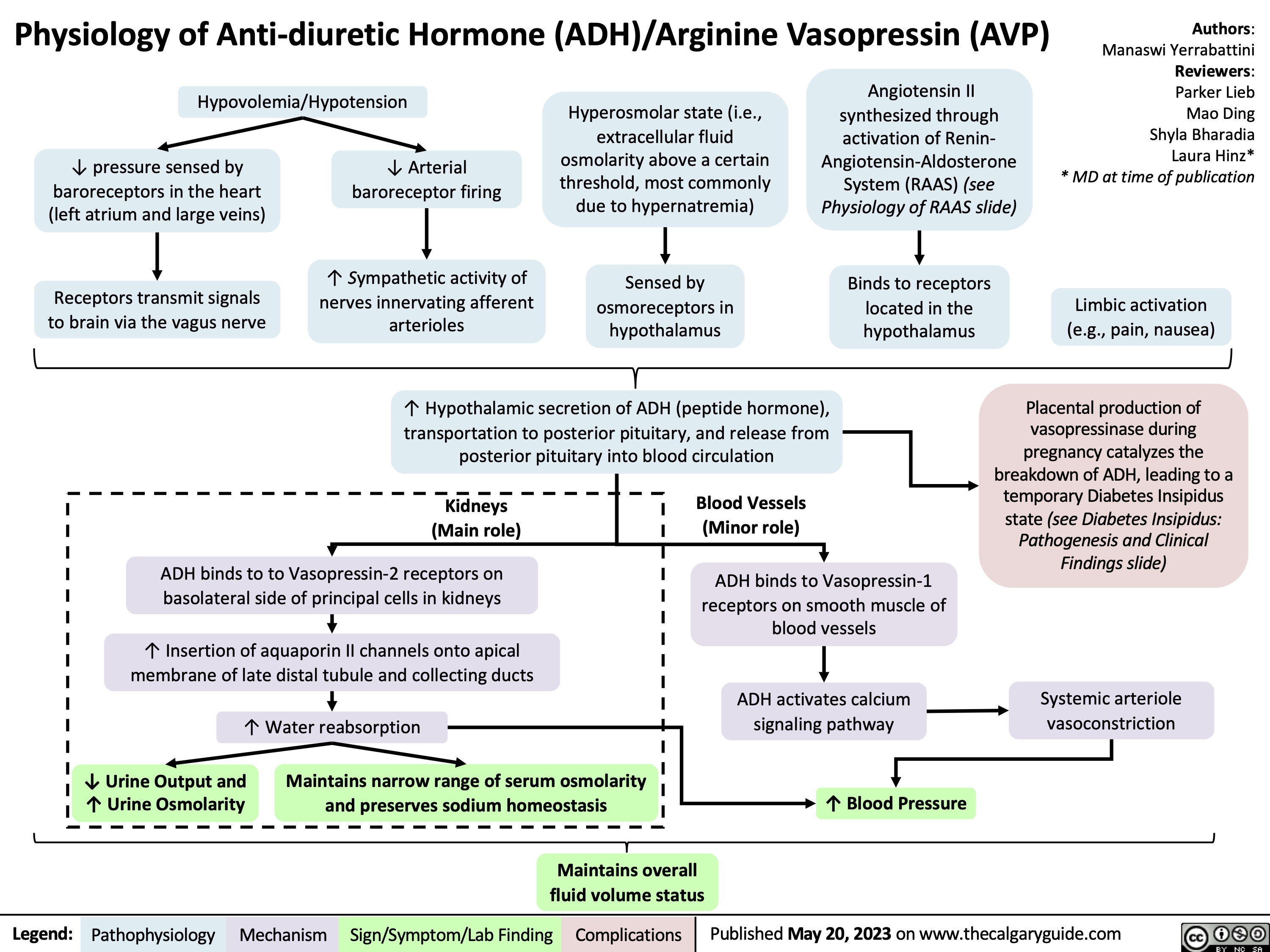
Acute Laryngitis
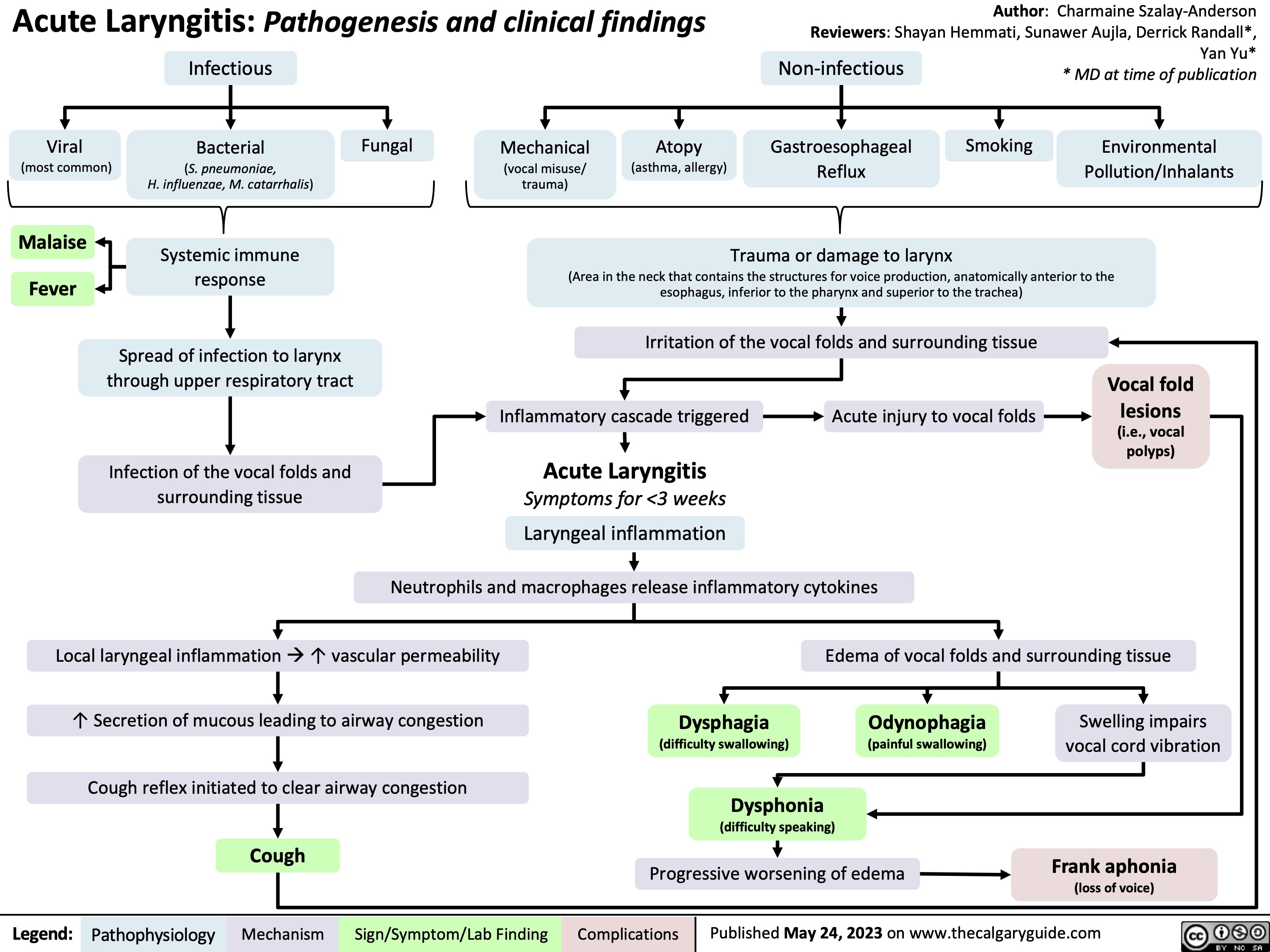
Neonatal Sepsis
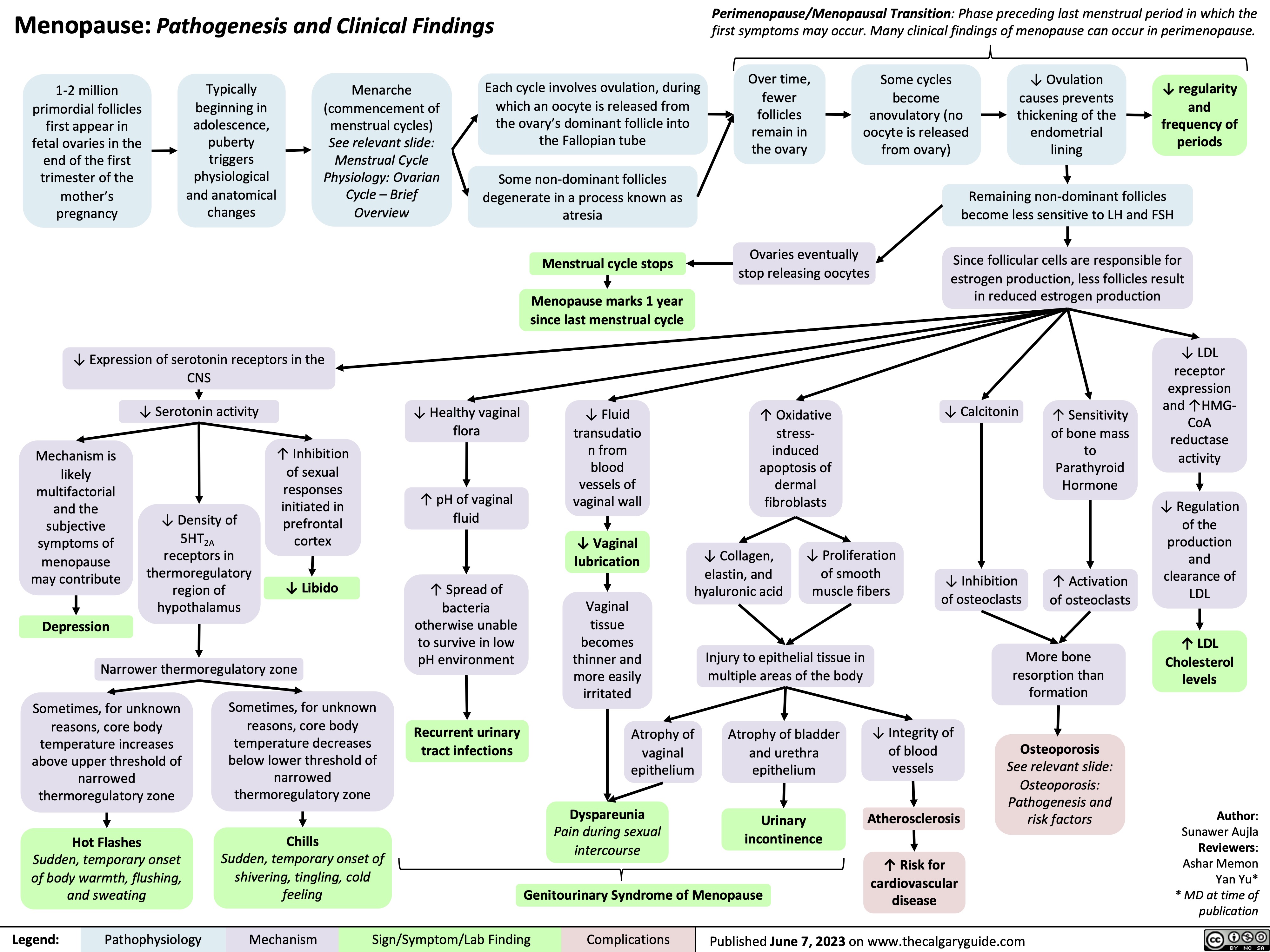
Menopause
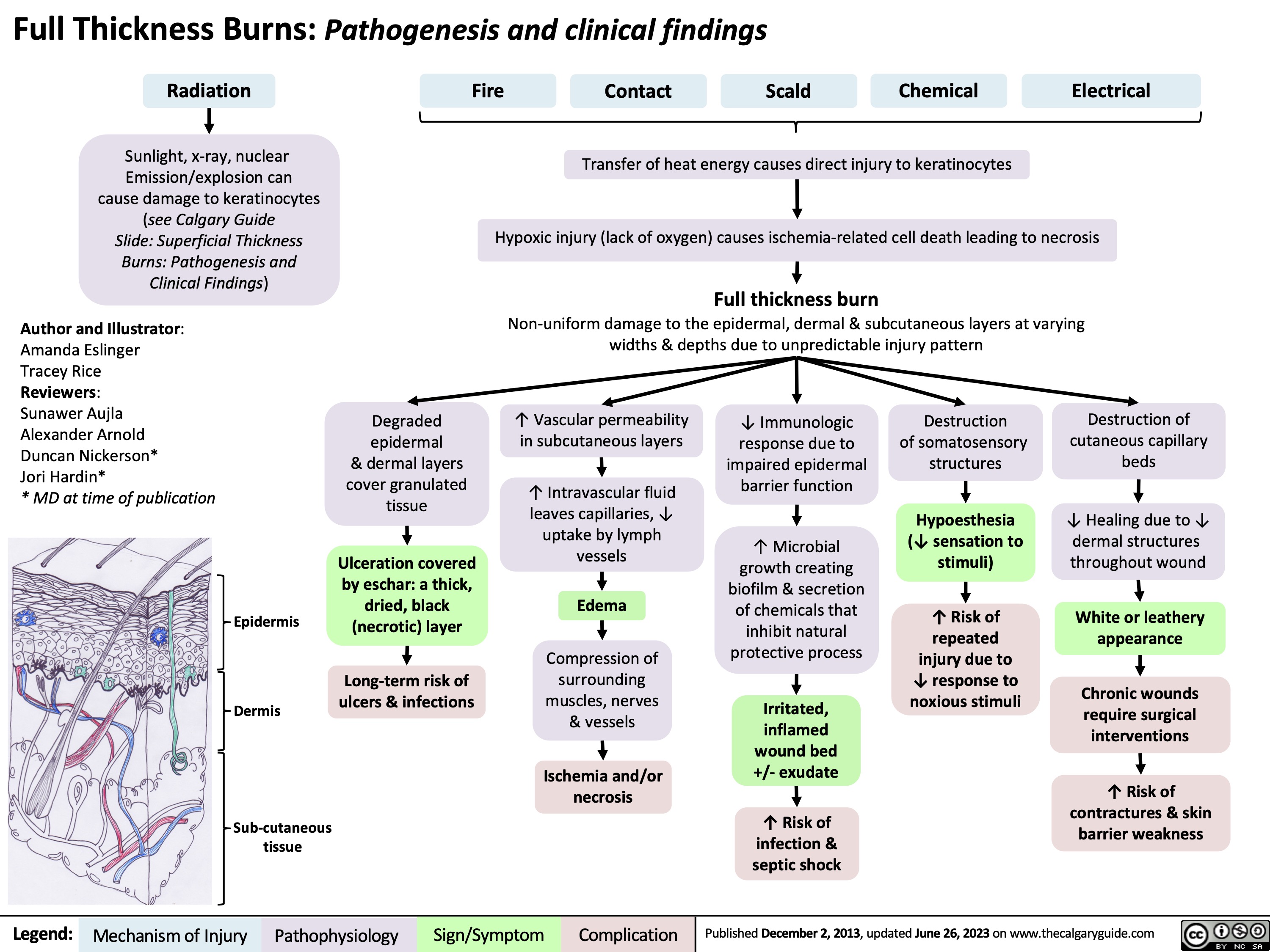
Burns - Full Thickness - Pathogenesis and Clinical Findings 2023
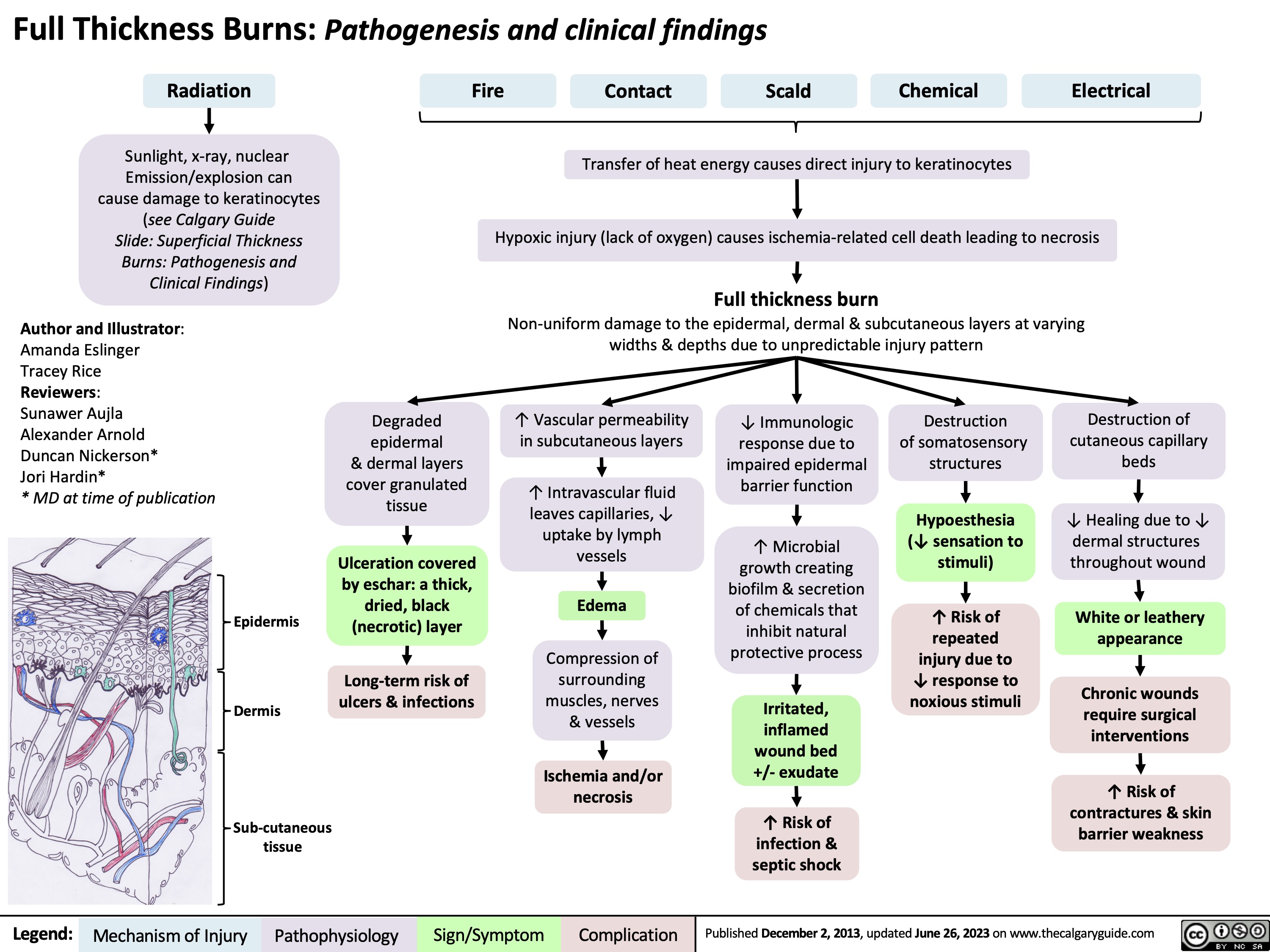
Burns - Full Thickness - Pathogenesis and Clinical Findings
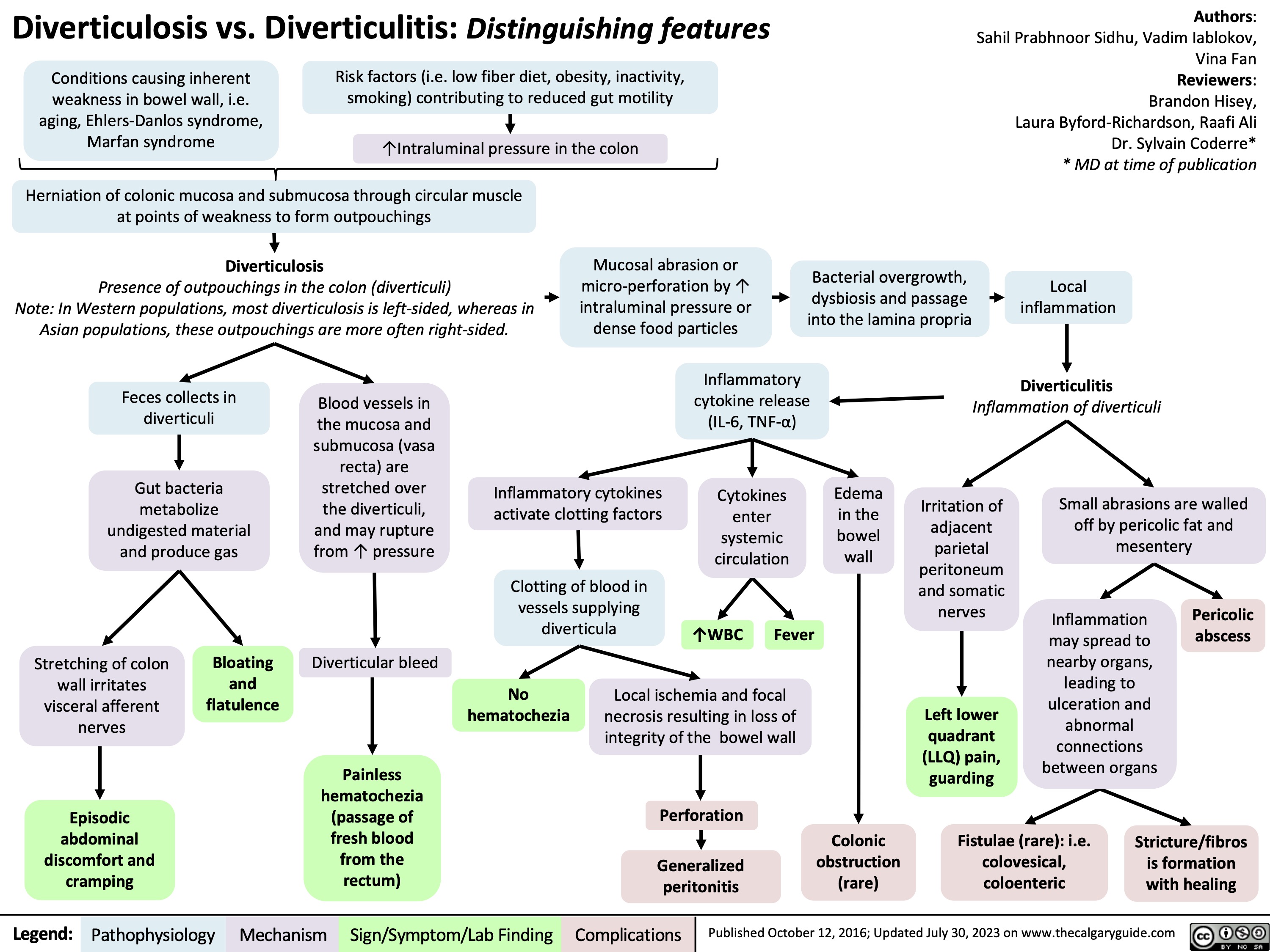
diverticulosis-vs-diverticulitis-distinguishing-features
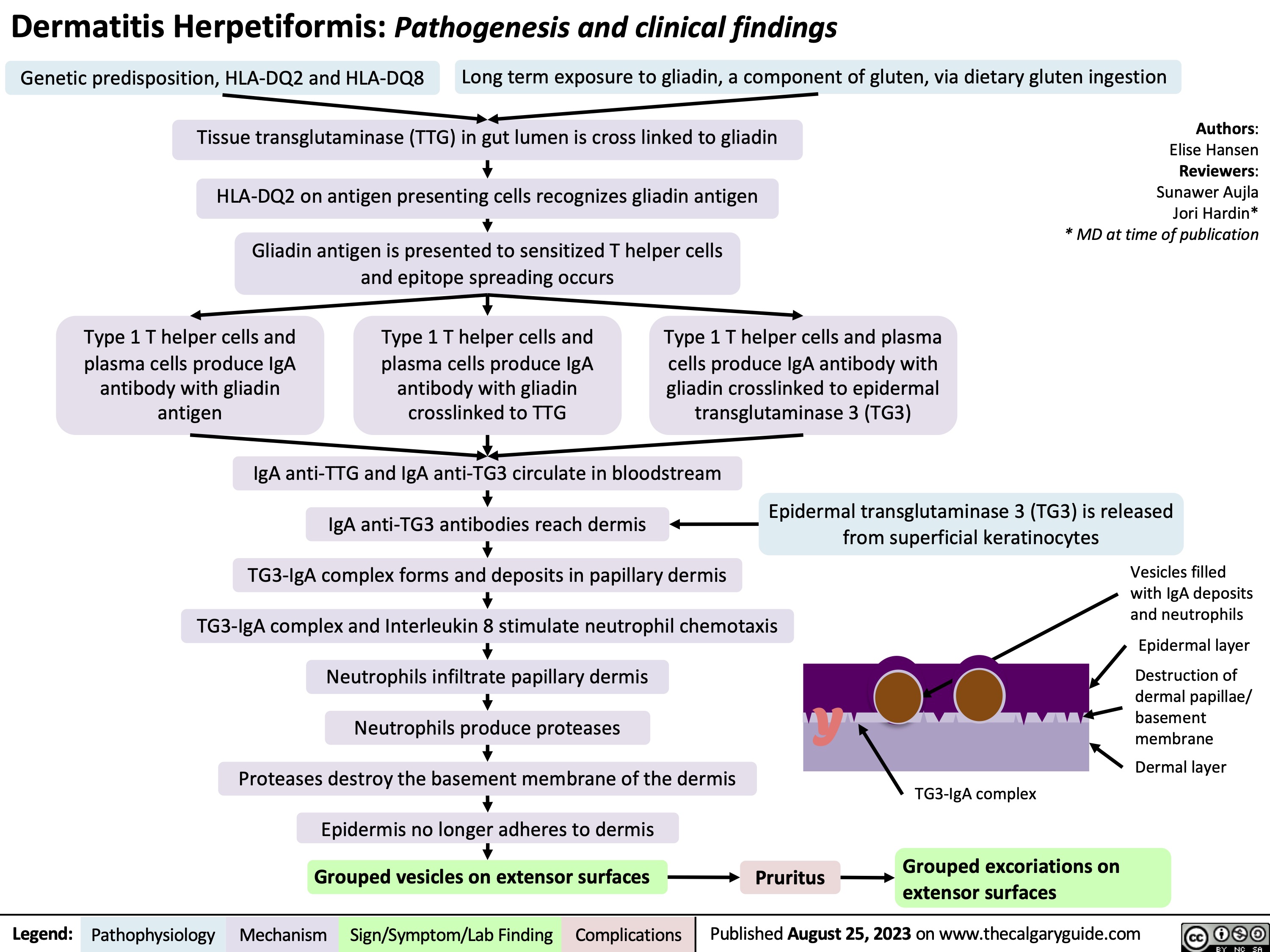
Knee Osteoarthritis
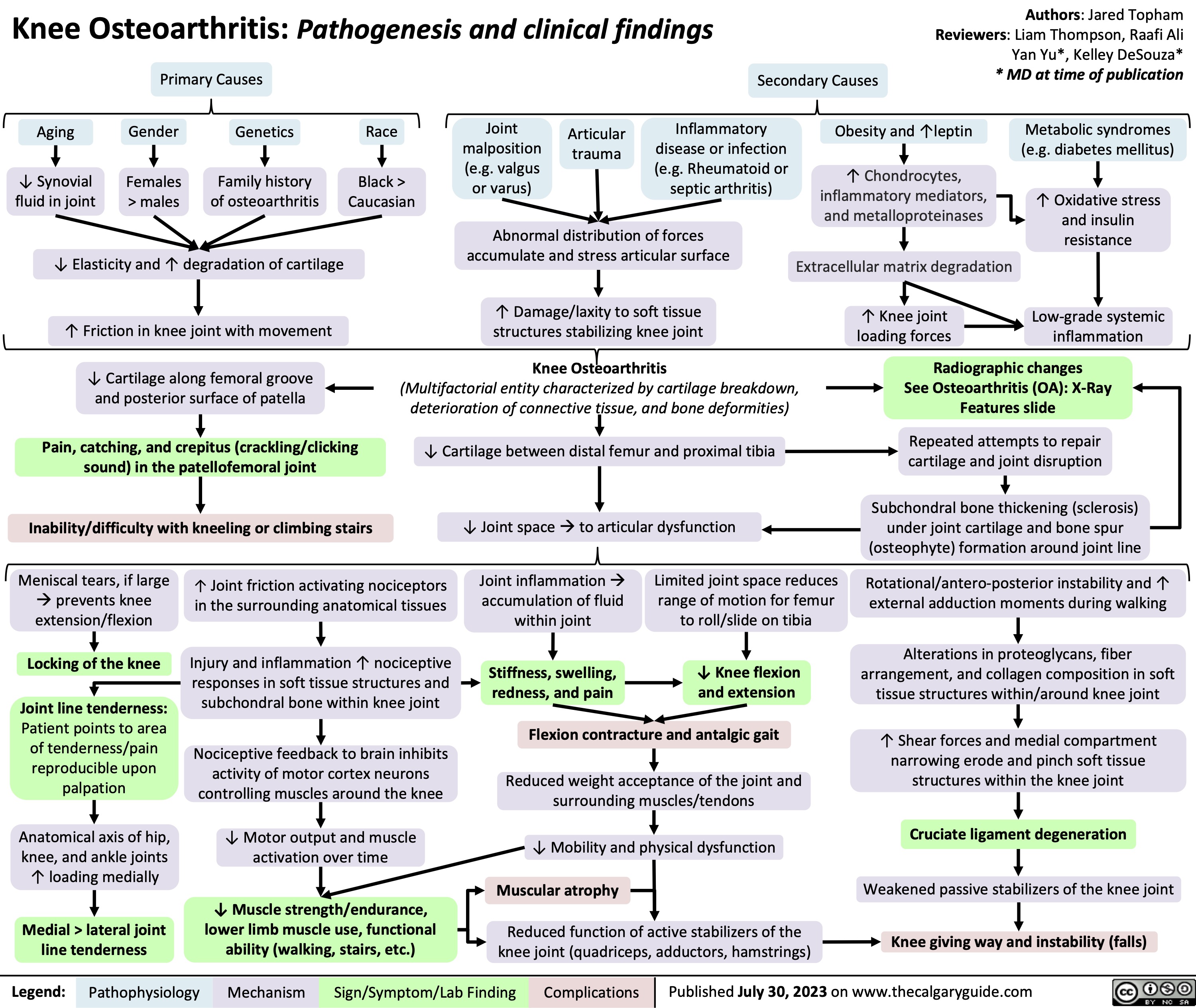
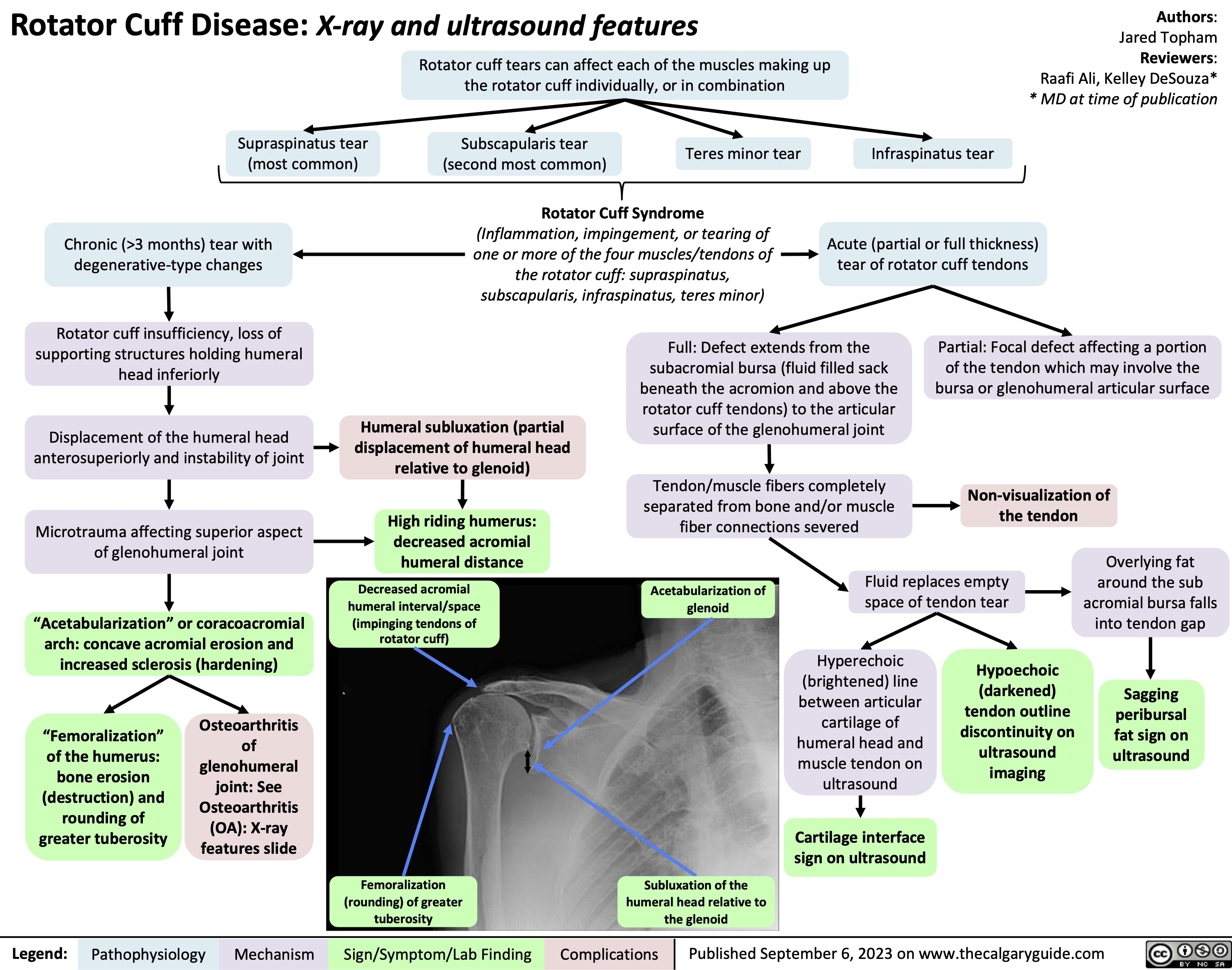
Rotator Cuff Disease
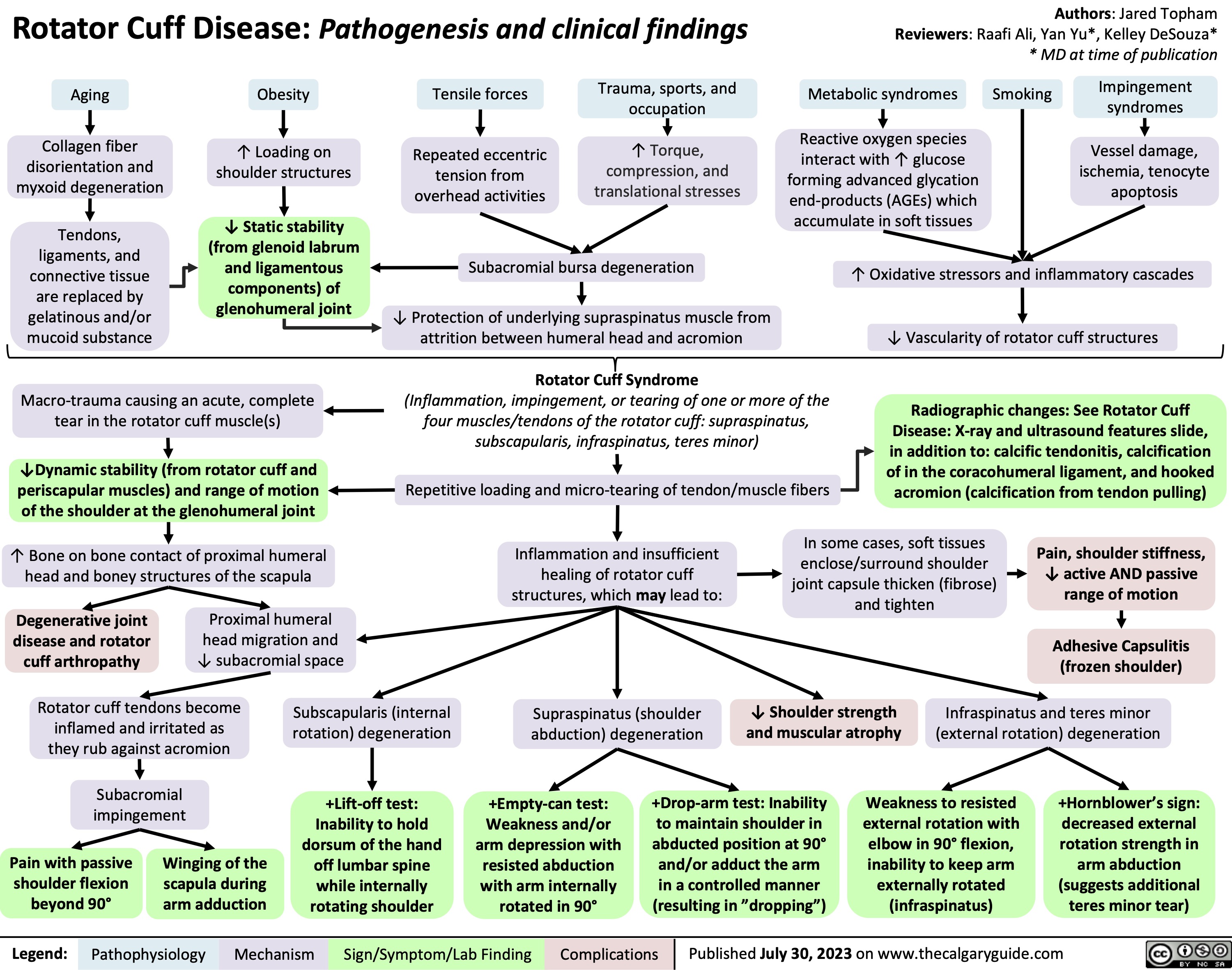
Keratosis pilaris
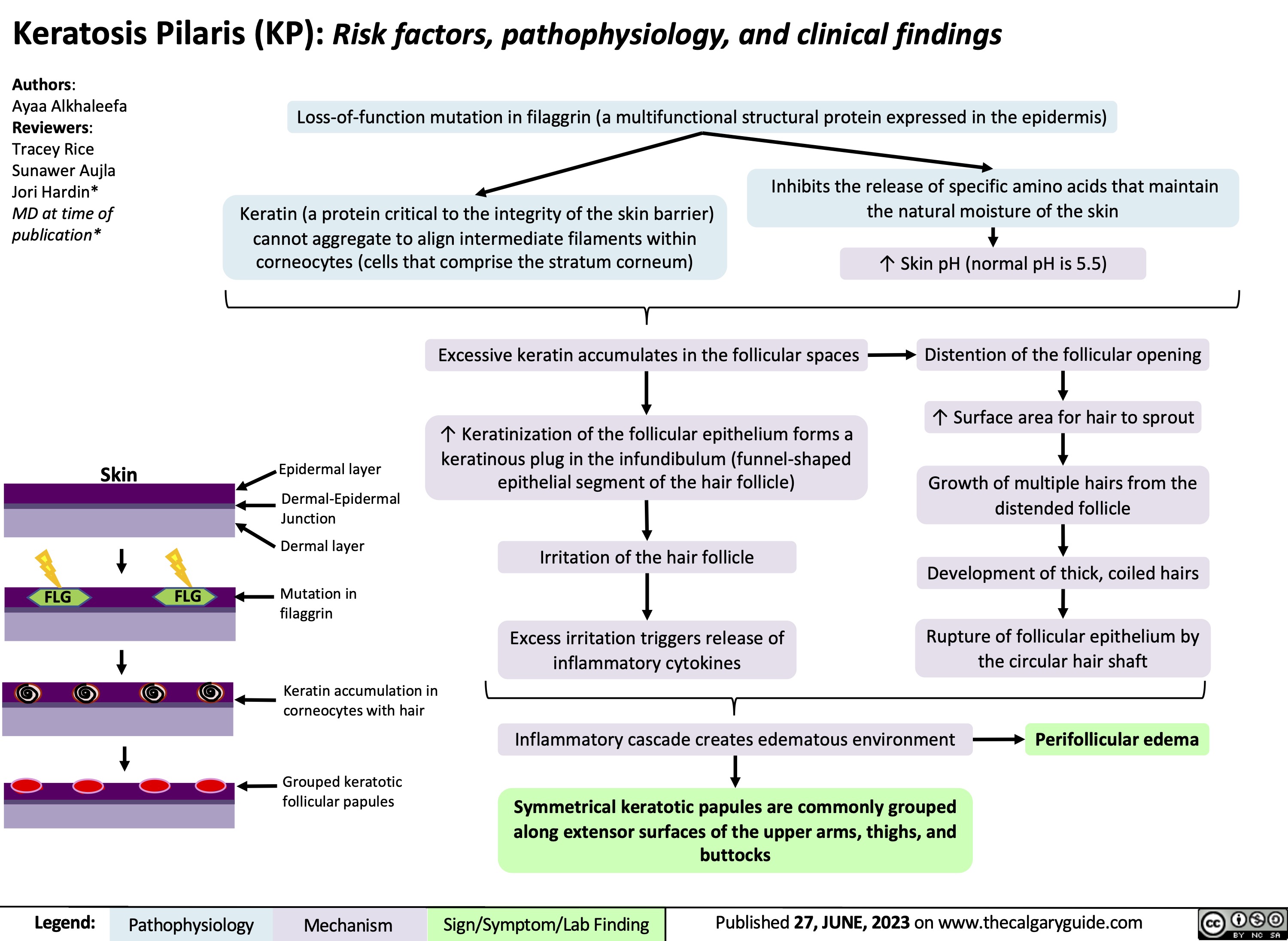
Dermatitis herpetiformis
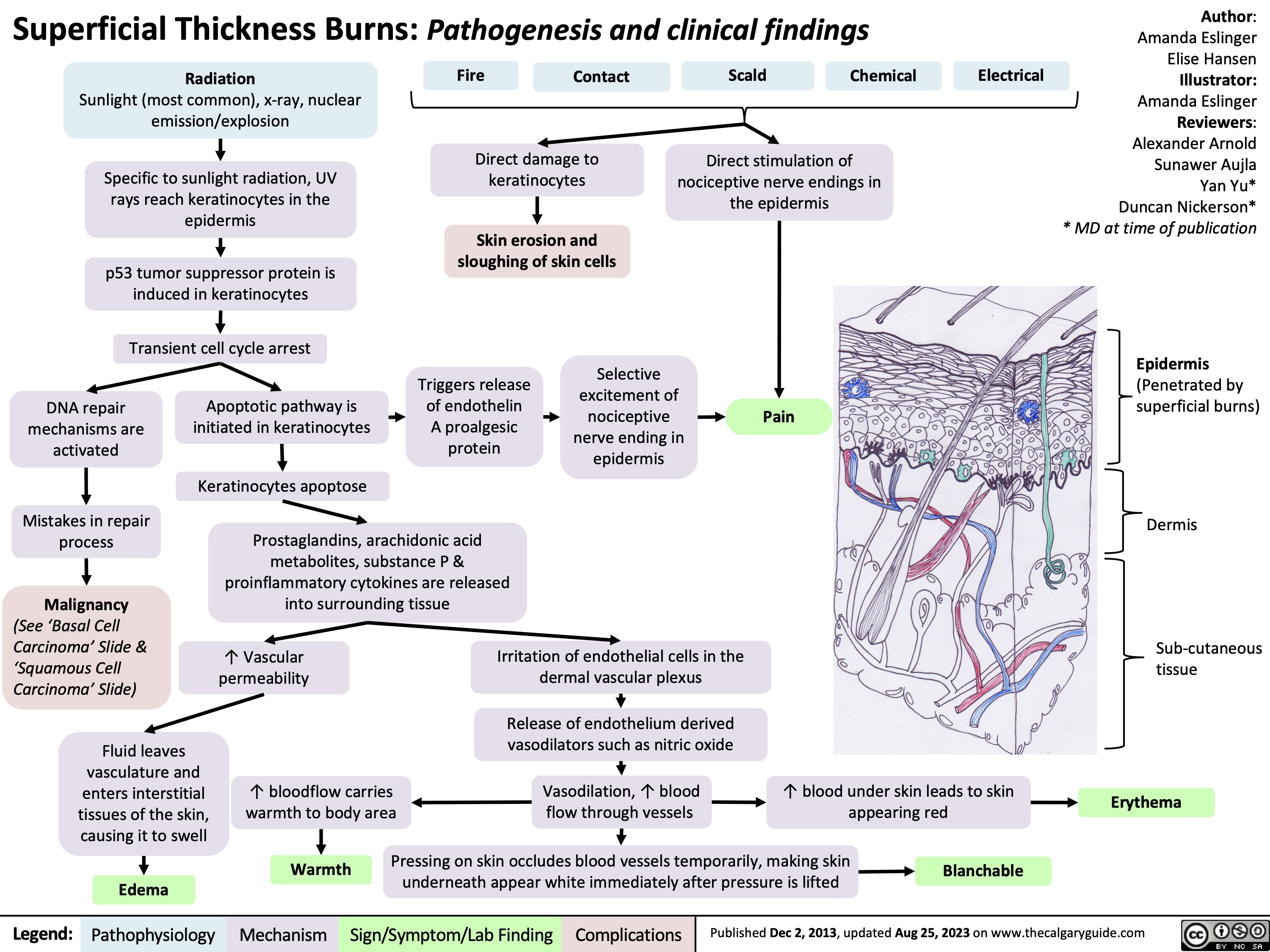
Superficial thickness burns
Rotator Cuff Disease Xray and Ultrasound Features
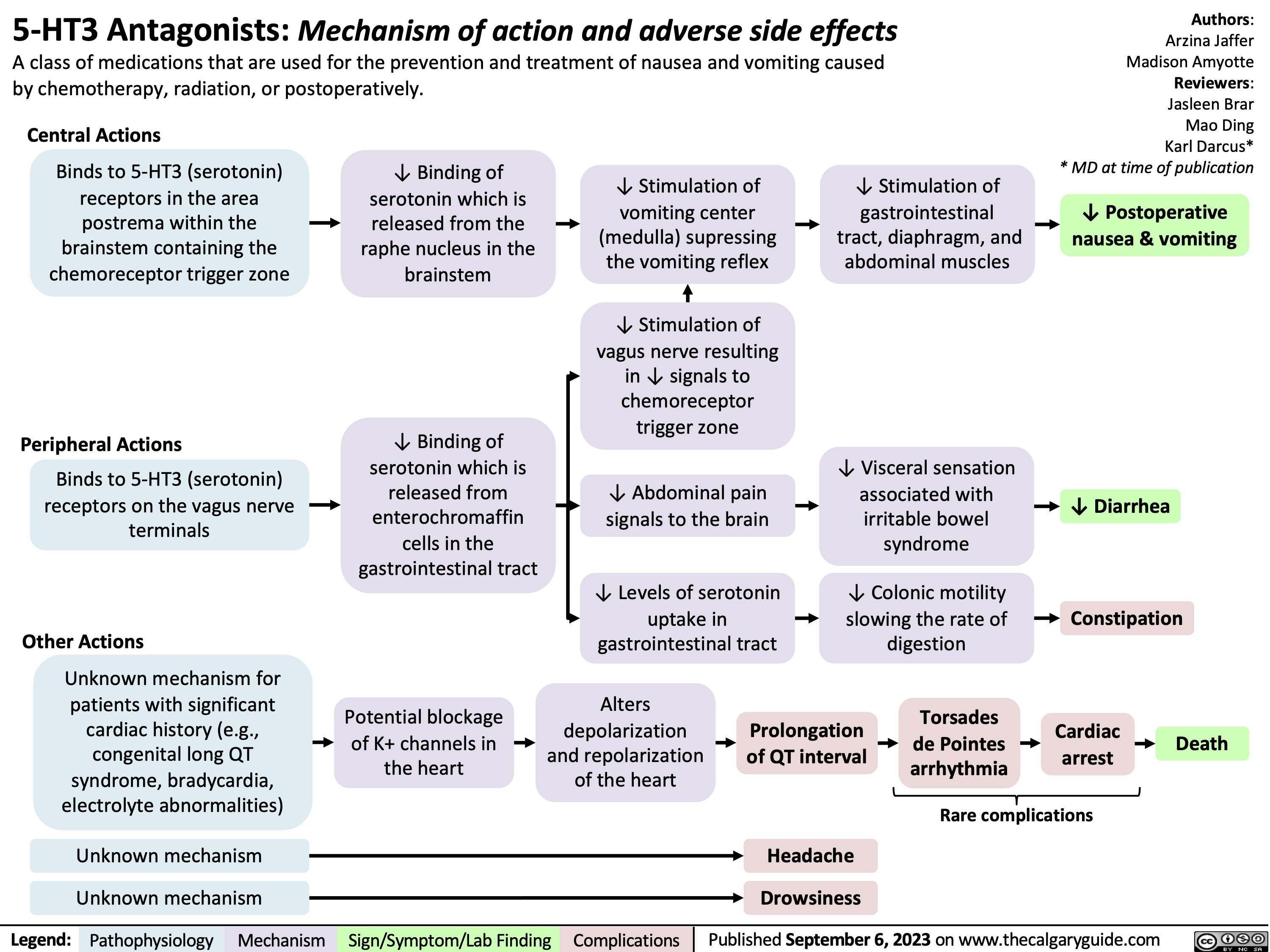
5HT3 Antagonists
Strabismus
![Strabismus: Pathogenesis and clinical findings
Microvascular dysfunction, trauma, or compression of oculomotor nerve
Oculomotor
nerve palsy: Dysfunction of the nerve innervating the superior rectus (elevation), inferior rectus (depression), medial rectus (adduction), and inferior oblique muscles (excyclotorsion)
Thyroid eye disease (see Thyroid Eye Disease slide for pathogenesis)
Inflammatory processes
Thickening & fibrosis of extraocular muscles, most commonly the inferior rectus muscle (functions to rotate the eye and depress the gaze)
Brown syndrome (congenital)
Anomalous
interaction between the trochlea and superior oblique muscle tendon
Restriction of normal movement of the superior oblique tendon through the trochlea
Trochlear palsy (Dysfunction of the trochlear nerve (CN IV))
Weakness of the superior oblique muscle innervated by CN IV (responsible for depression of the gaze and incyclotorsion & rotation of the eye)
Near reflex: convergence (eyes adduct), accommodati on (thickening of the lens) & miosis (constriction of the pupil)
Excessive accommodation in hyperopic (farsighted) eyes
Over-activation of near reflex
Accommodative esotropia
Aneurysm, infection, iatrogenic injury to cranial nerve (CN) VI
Abducens palsy: ocular motor paralysis
Failure of CN VI to develop normally in utero
Duane syndrome: congenital malformation of CN VI
Congenital fibrosis of the extraocular muscles (CFEOM)
Restrictive global paralysis of the extraocular muscles that control the movements of the eye
Phenotype CFEOM2
Phenotype CFEOM1 & 3
Dysfunction of the abducens
nerve (CN VI: innervates the ipsilateral lateral rectus muscle which abducts the eye [turns it laterally])
Orbital fracture (fracture of the orbital floor)
Intraorbital contents (inferior rectus muscle and/or surrounding tissue) herniate through the fractured site & are entrapped
Idiopathic infantile esotropia
Intermittent exotropia
Unclear process
Exotropia: affected eye is rotated laterally
Esotropia: affected eye is rotated medially
Hypotropia: affected eye is rotated downward compared to non-affected eye
Hypertropia: affected eye is rotated upward compared to non-affected eye
Horizontal Strabismus
Two different images are received by the eye that cannot be fused together Visual cortex suppresses the input from one eye in order to avoid having diplopia
Amblyopia/lazy eye: visual cortex diminishes neural inputs from the corresponding cortical areas of affected eye
↓ Spatial awareness
Vertical Strabismus
Binocular diplopia: double vision when both eyes are open, and absent when either eye is closed
Atypical alignment of the eye
Psychosocial consequences: negative impact to mental health due to social bias or abuse, social anxiety, and difficulties with self-image
Authors: Mina Mina Lucy Yang Reviewers: Mao Ding William Stell* * MD at time of publication
↓ Visual acuity
↓ Oculomotor control
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published September 6, 2023 on www.thecalgaryguide.com
Strabismus: Pathogenesis and clinical findings
Microvascular dysfunction, trauma, or compression of oculomotor nerve
Oculomotor
nerve palsy: Dysfunction of the nerve innervating the superior rectus (elevation), inferior rectus (depression), medial rectus (adduction), and inferior oblique muscles (excyclotorsion)
Thyroid eye disease (see Thyroid Eye Disease slide for pathogenesis)
Inflammatory processes
Thickening & fibrosis of extraocular muscles, most commonly the inferior rectus muscle (functions to rotate the eye and depress the gaze)
Brown syndrome (congenital)
Anomalous
interaction between the trochlea and superior oblique muscle tendon
Restriction of normal movement of the superior oblique tendon through the trochlea
Trochlear palsy (Dysfunction of the trochlear nerve (CN IV))
Weakness of the superior oblique muscle innervated by CN IV (responsible for depression of the gaze and incyclotorsion & rotation of the eye)
Near reflex: convergence (eyes adduct), accommodati on (thickening of the lens) & miosis (constriction of the pupil)
Excessive accommodation in hyperopic (farsighted) eyes
Over-activation of near reflex
Accommodative esotropia
Aneurysm, infection, iatrogenic injury to cranial nerve (CN) VI
Abducens palsy: ocular motor paralysis
Failure of CN VI to develop normally in utero
Duane syndrome: congenital malformation of CN VI
Congenital fibrosis of the extraocular muscles (CFEOM)
Restrictive global paralysis of the extraocular muscles that control the movements of the eye
Phenotype CFEOM2
Phenotype CFEOM1 & 3
Dysfunction of the abducens
nerve (CN VI: innervates the ipsilateral lateral rectus muscle which abducts the eye [turns it laterally])
Orbital fracture (fracture of the orbital floor)
Intraorbital contents (inferior rectus muscle and/or surrounding tissue) herniate through the fractured site & are entrapped
Idiopathic infantile esotropia
Intermittent exotropia
Unclear process
Exotropia: affected eye is rotated laterally
Esotropia: affected eye is rotated medially
Hypotropia: affected eye is rotated downward compared to non-affected eye
Hypertropia: affected eye is rotated upward compared to non-affected eye
Horizontal Strabismus
Two different images are received by the eye that cannot be fused together Visual cortex suppresses the input from one eye in order to avoid having diplopia
Amblyopia/lazy eye: visual cortex diminishes neural inputs from the corresponding cortical areas of affected eye
↓ Spatial awareness
Vertical Strabismus
Binocular diplopia: double vision when both eyes are open, and absent when either eye is closed
Atypical alignment of the eye
Psychosocial consequences: negative impact to mental health due to social bias or abuse, social anxiety, and difficulties with self-image
Authors: Mina Mina Lucy Yang Reviewers: Mao Ding William Stell* * MD at time of publication
↓ Visual acuity
↓ Oculomotor control
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published September 6, 2023 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2023/09/Strabismus.jpg)
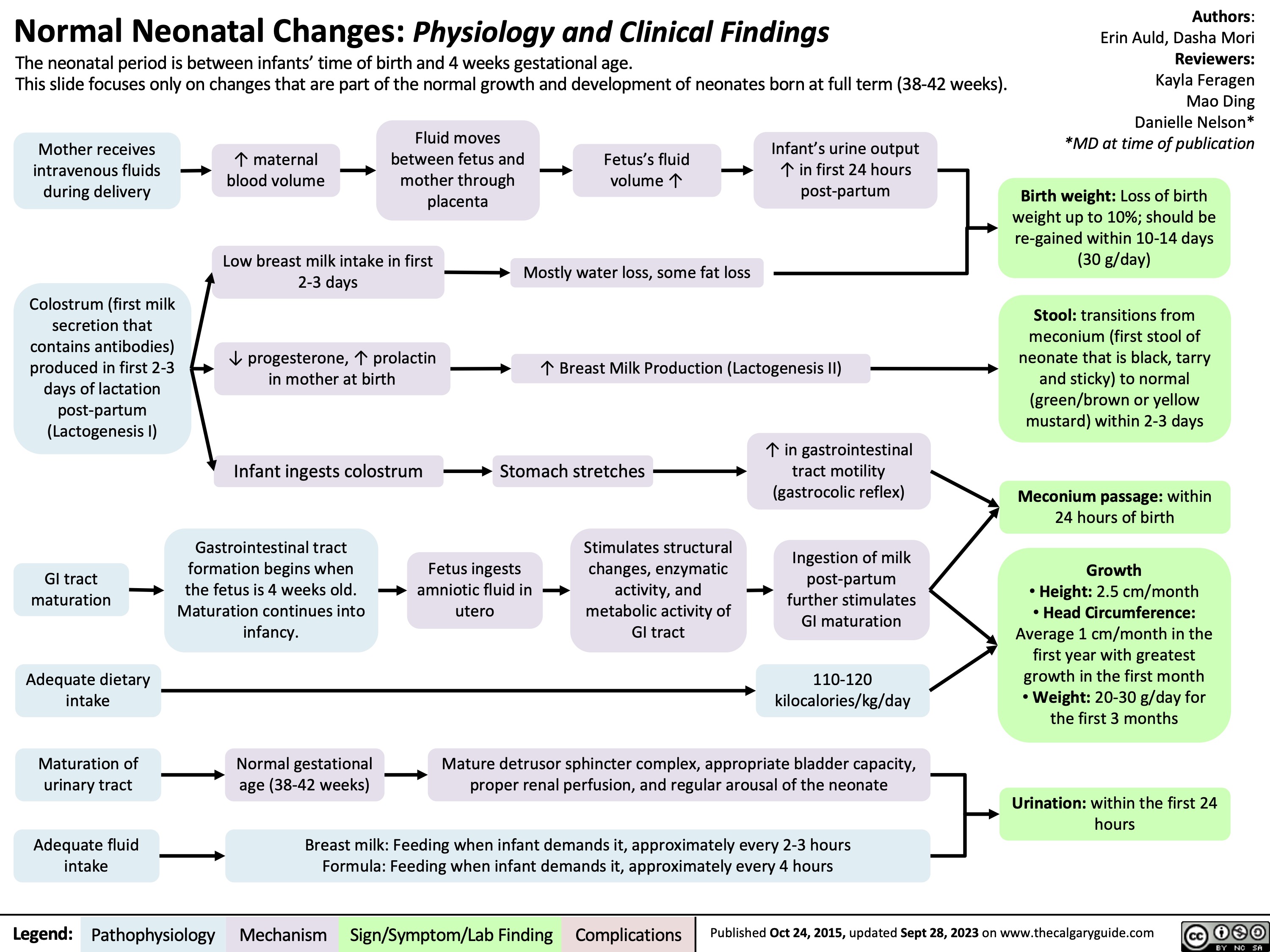
normal neonatal changes pathogenesis and clinical findings
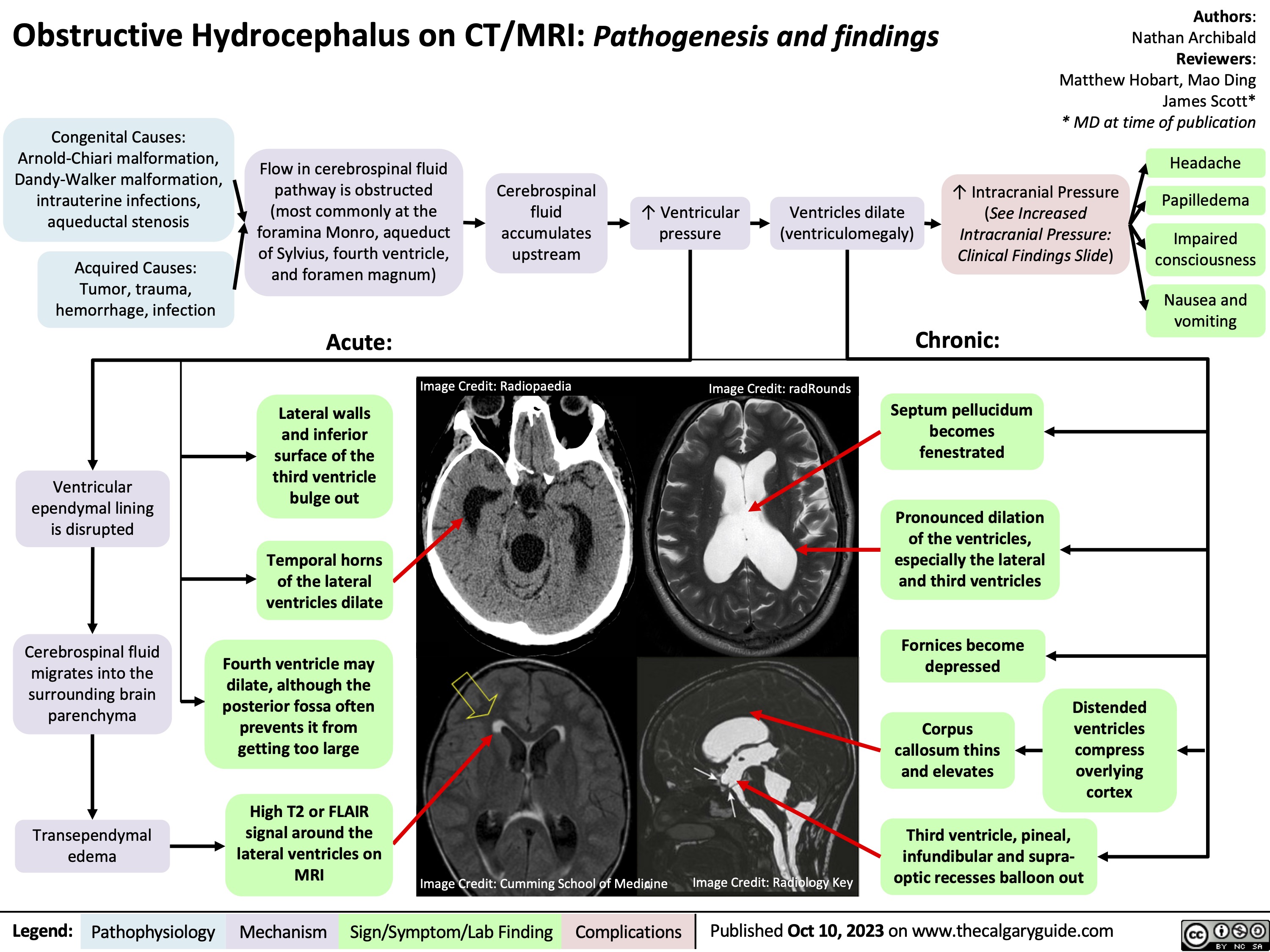
Obstructive Hydrocephalus on CT MRI Pathogenesis and findings
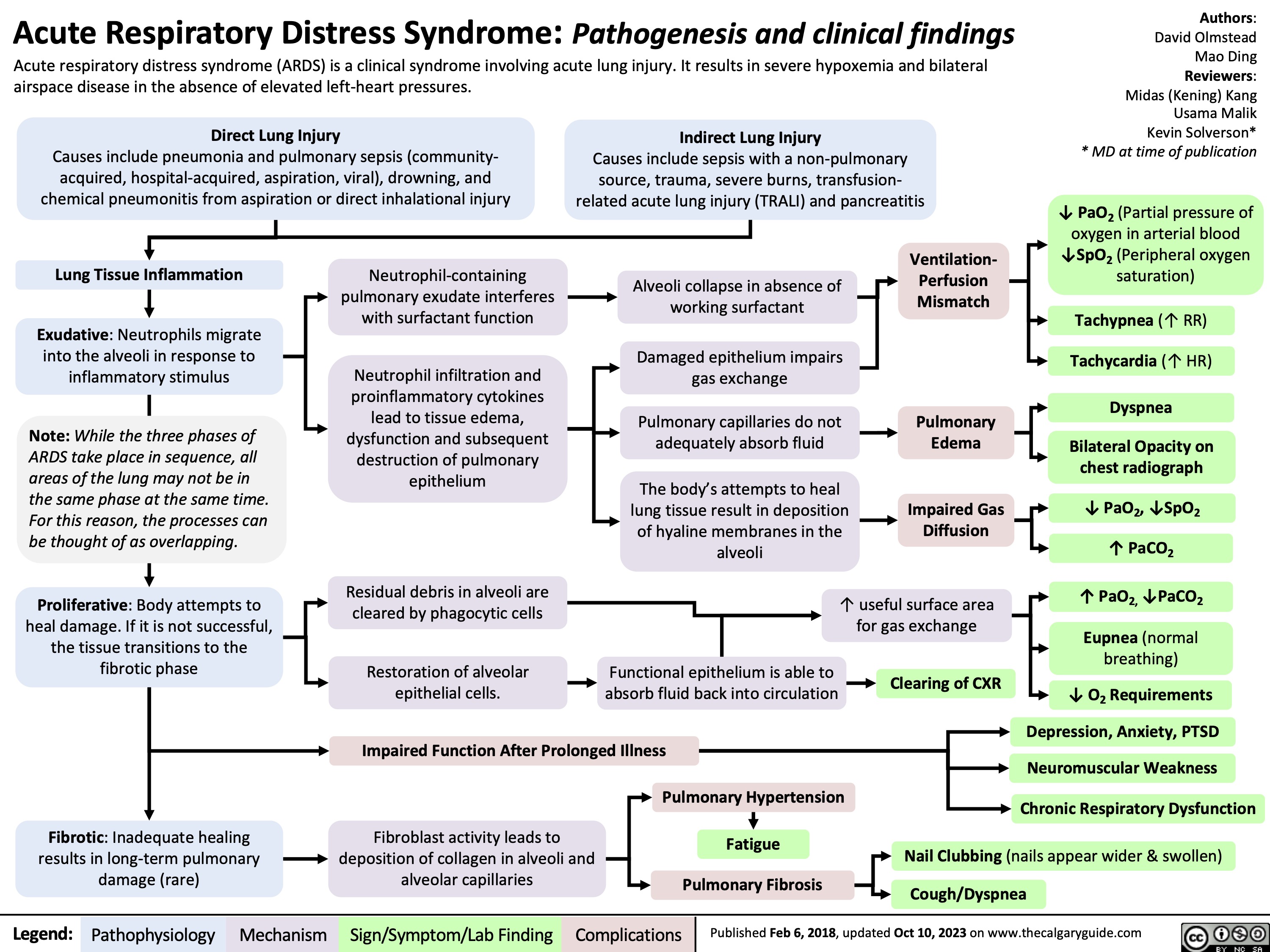
Acute Respiratory Distress Syndrome
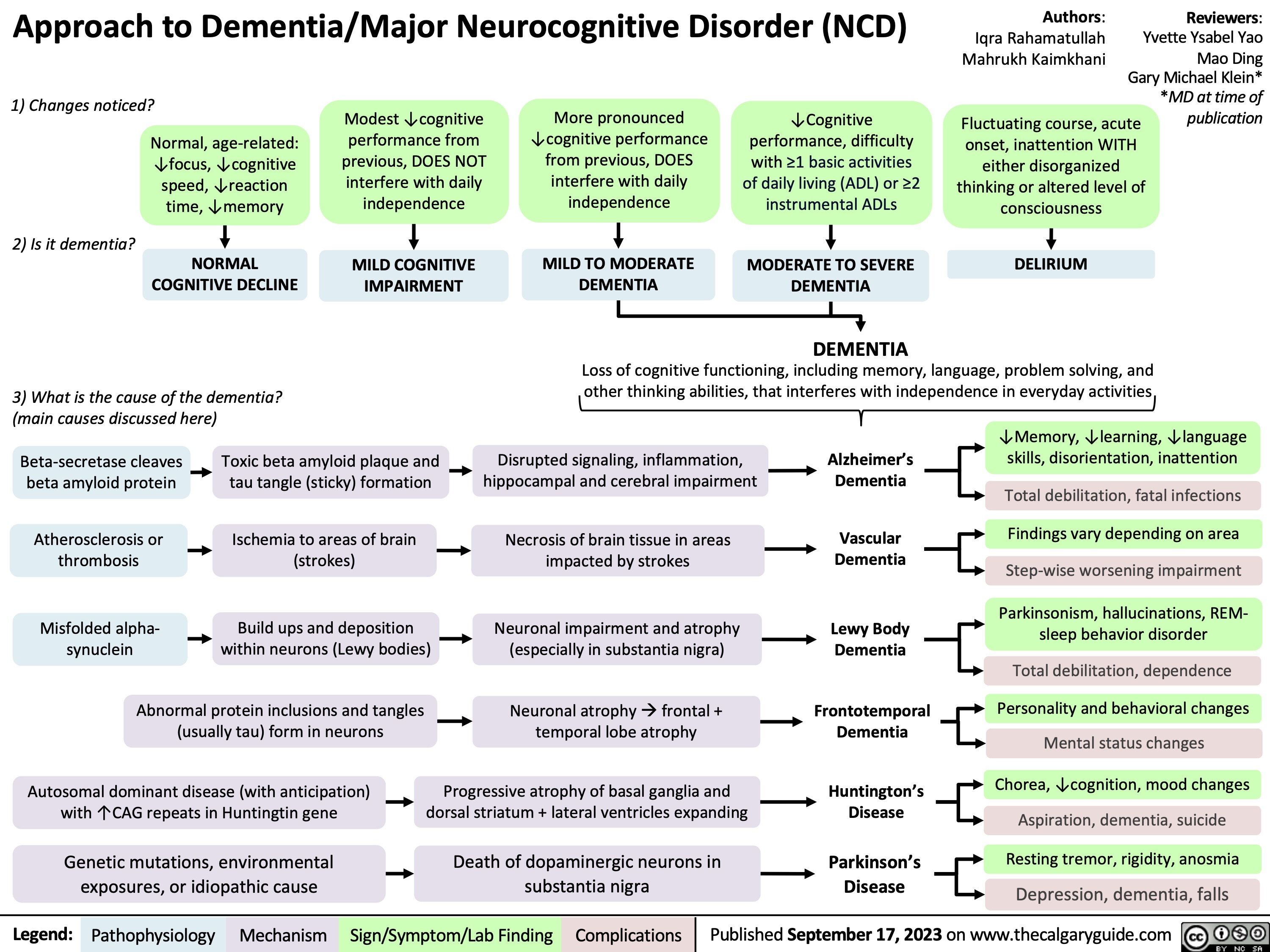
Approach To Dementia
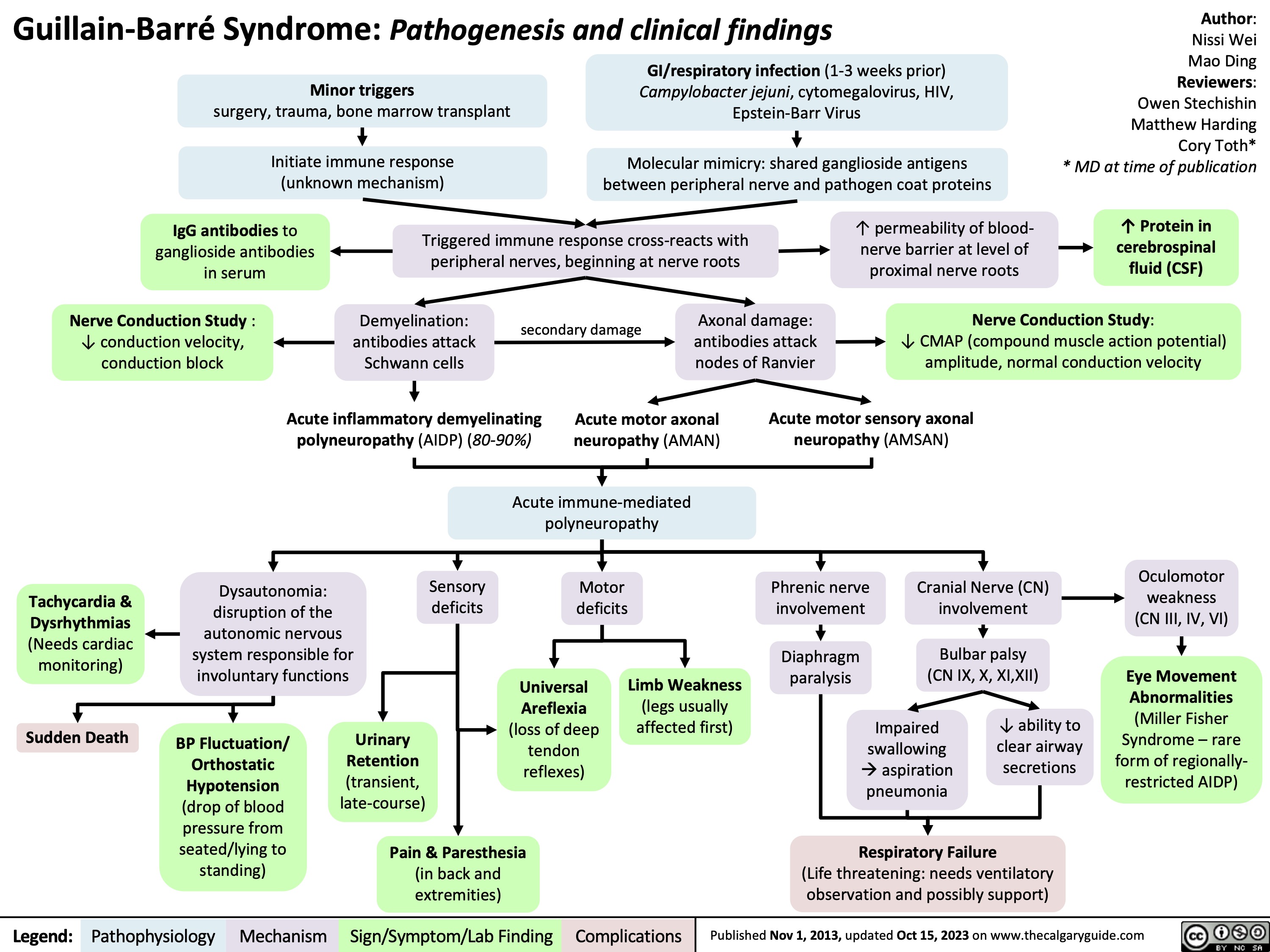
Guillain-Barre Syndrome
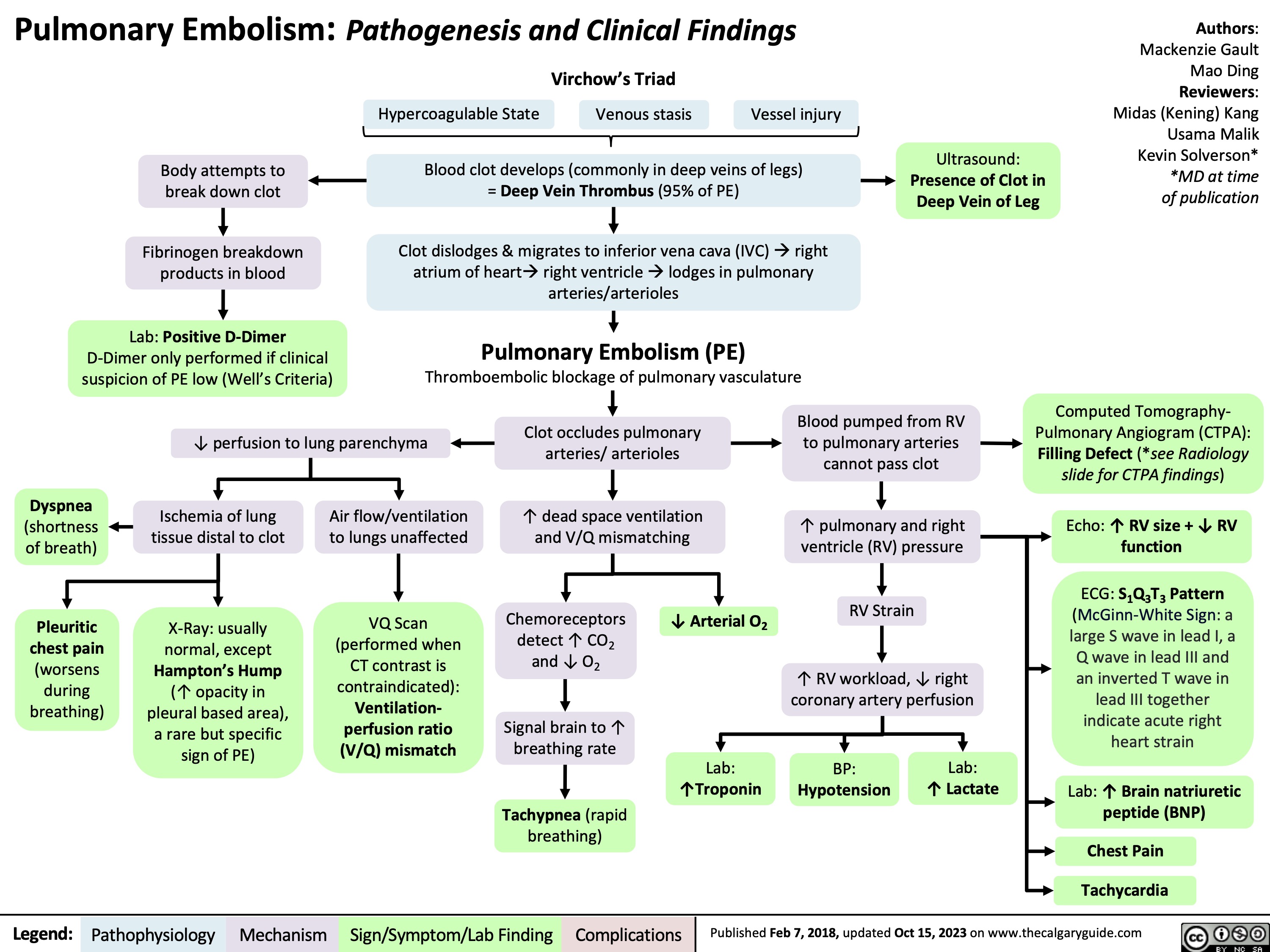
Pulmonary Embolism Pathogenesis and Clinical Findings
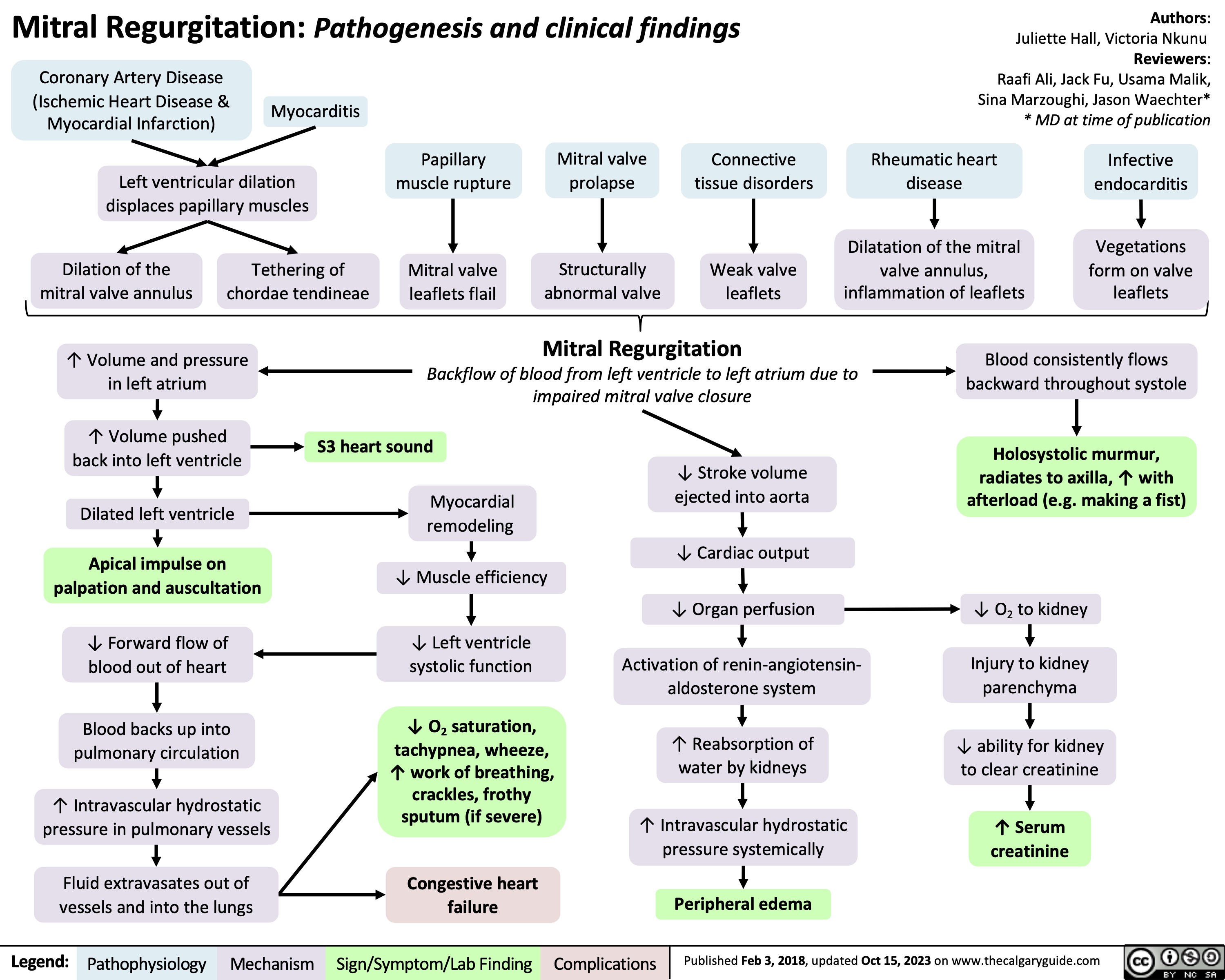
Mitral Regurgitation Pathogenesis and clinical findings
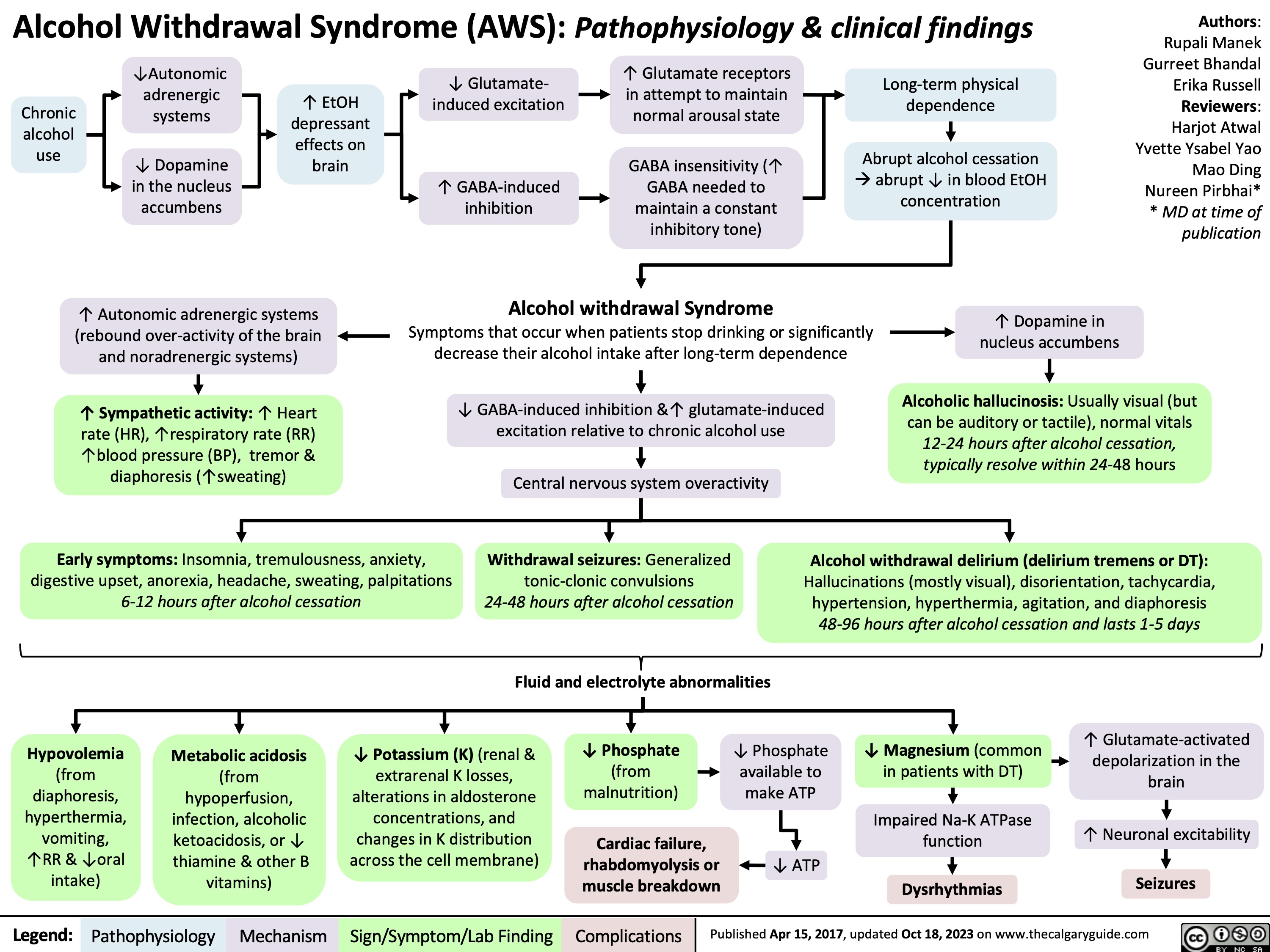
Alcohol Withdrawal Syndrome
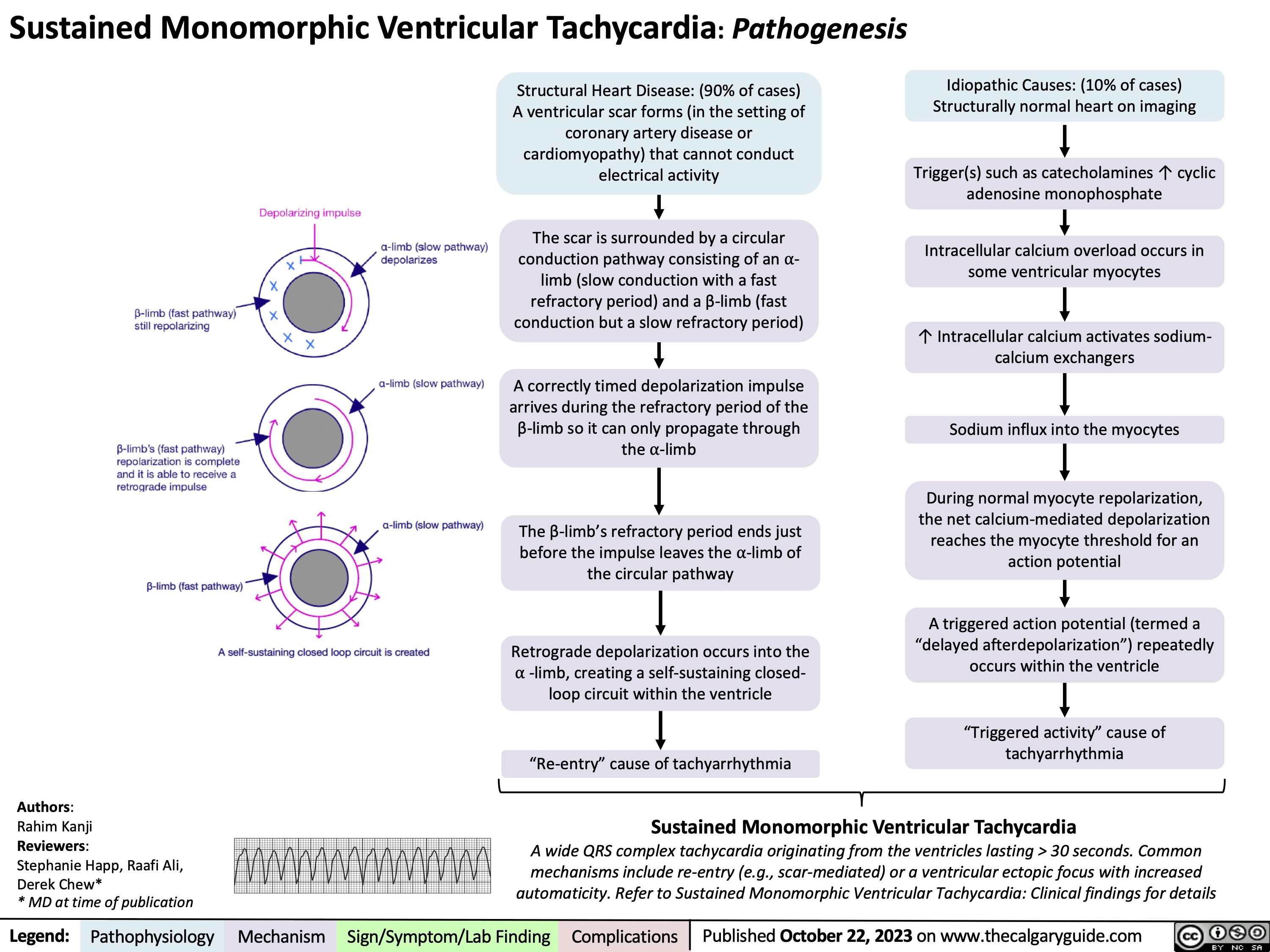
Sustained Monomorphic Ventricular Tachycardia Pathogenesis
Sustained Monomorphic Ventricular Tachycardia Clinical findings
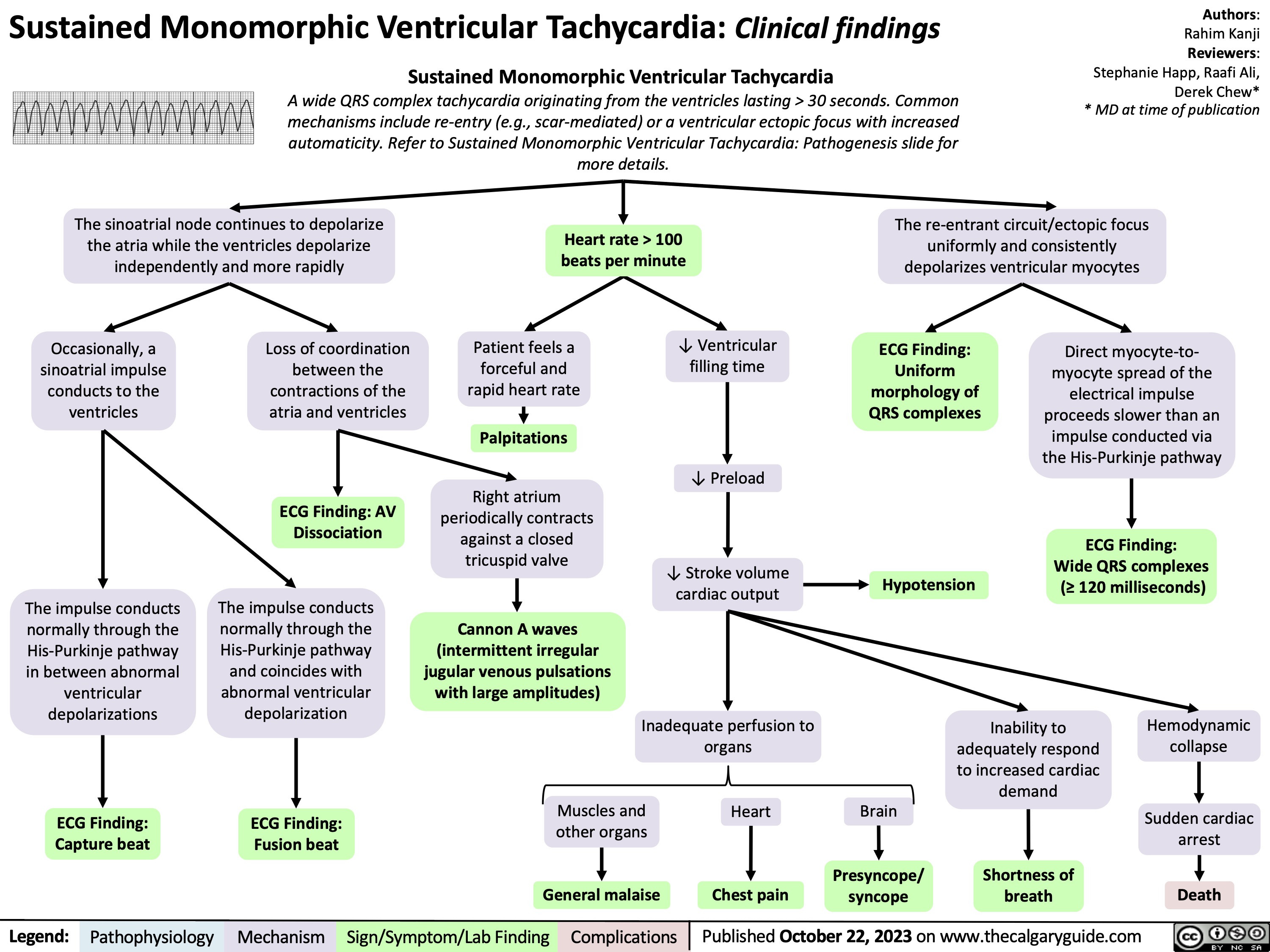
Statins Mechanisms and Side Effects
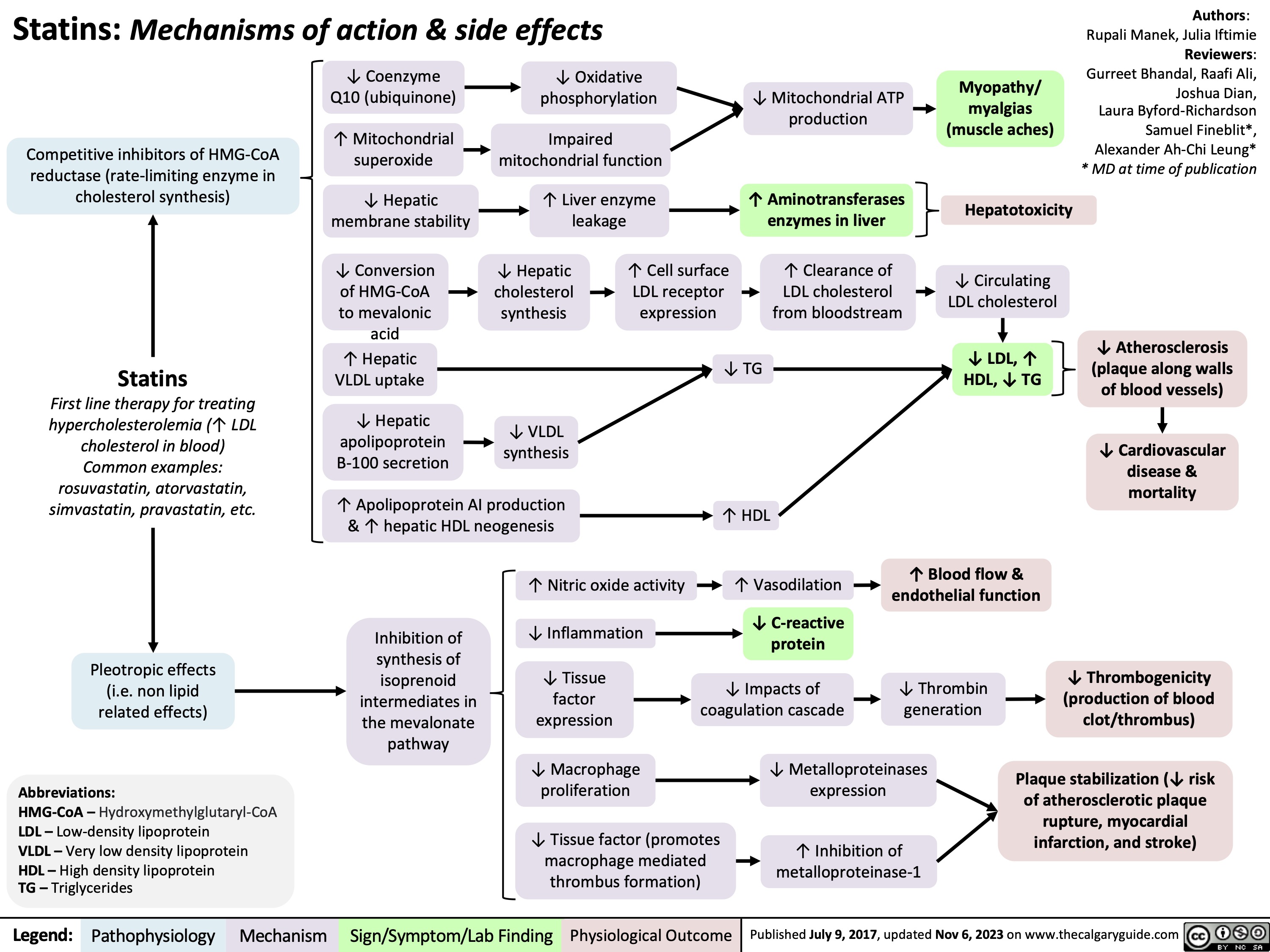
Pharmacotherapy for Dyslipidemia Overview
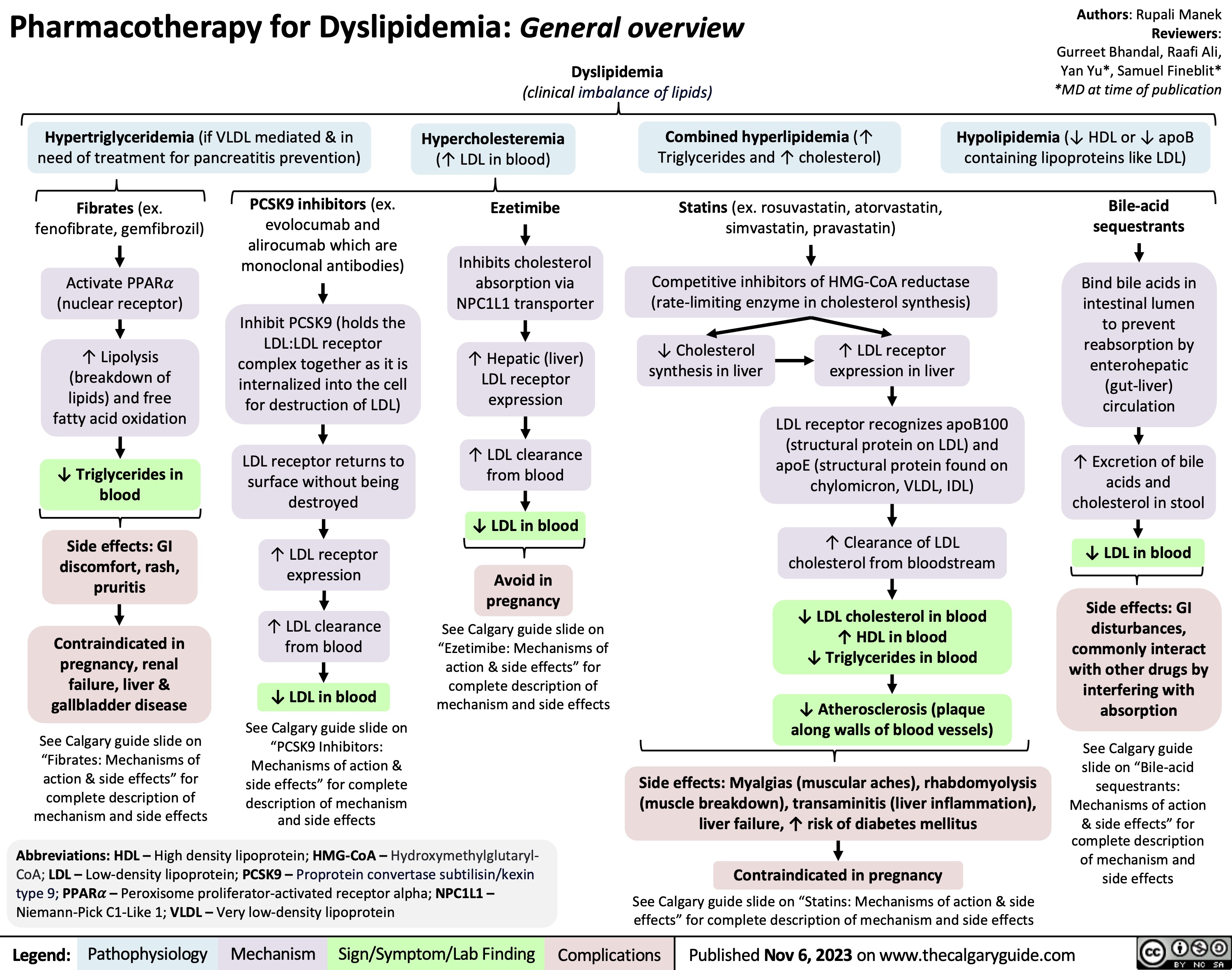
Anesthetic Considerations for Obese Patients
Overview of burns
Death Cardiovascular Respiratory and Neurologic Mechanisms
Turner Syndrome Pathogenesis and Clinical Findings
Non-Alcoholic Fatty Liver Disease
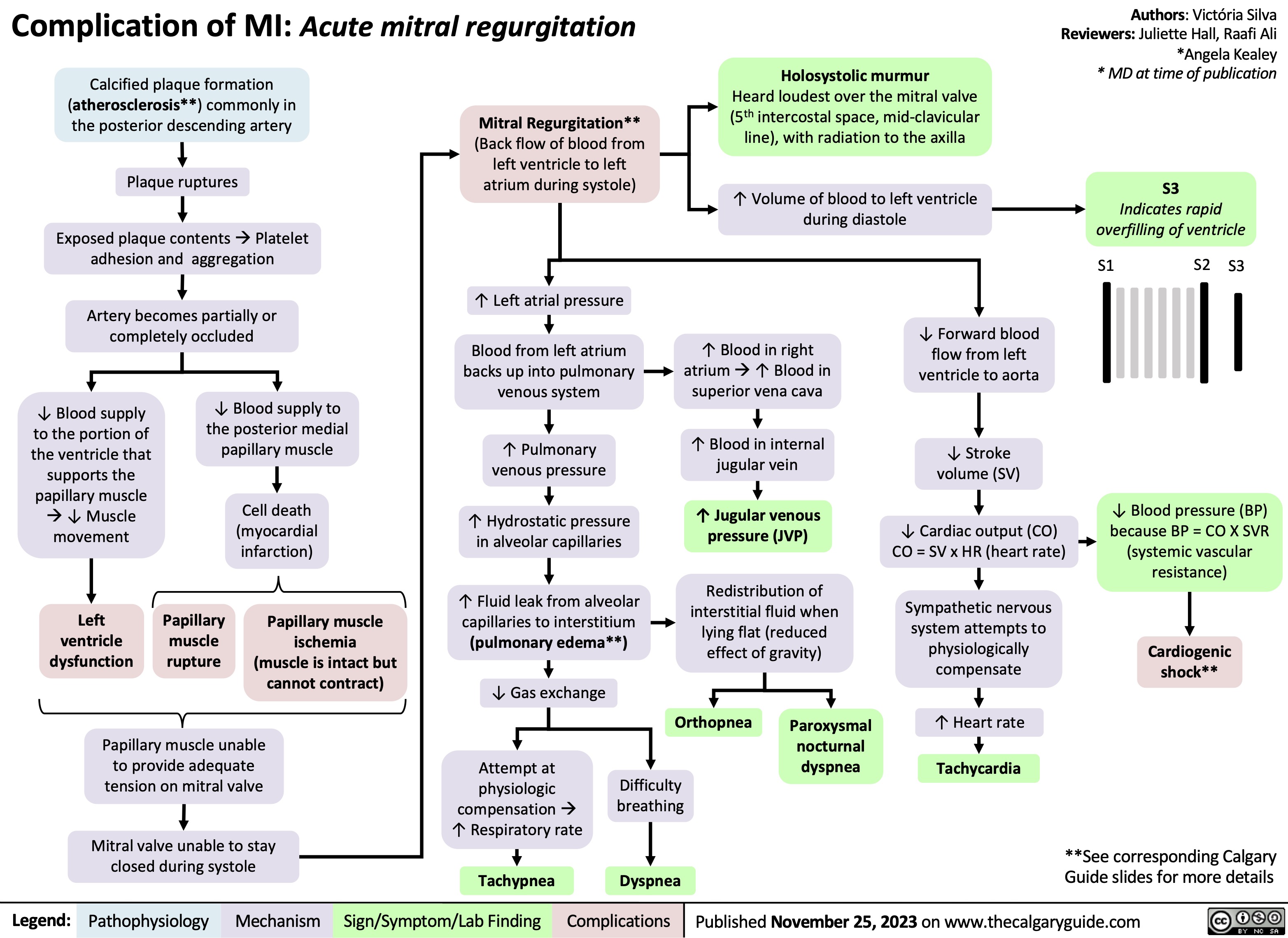
Complication of MI - Acute Mitral Regurgitation
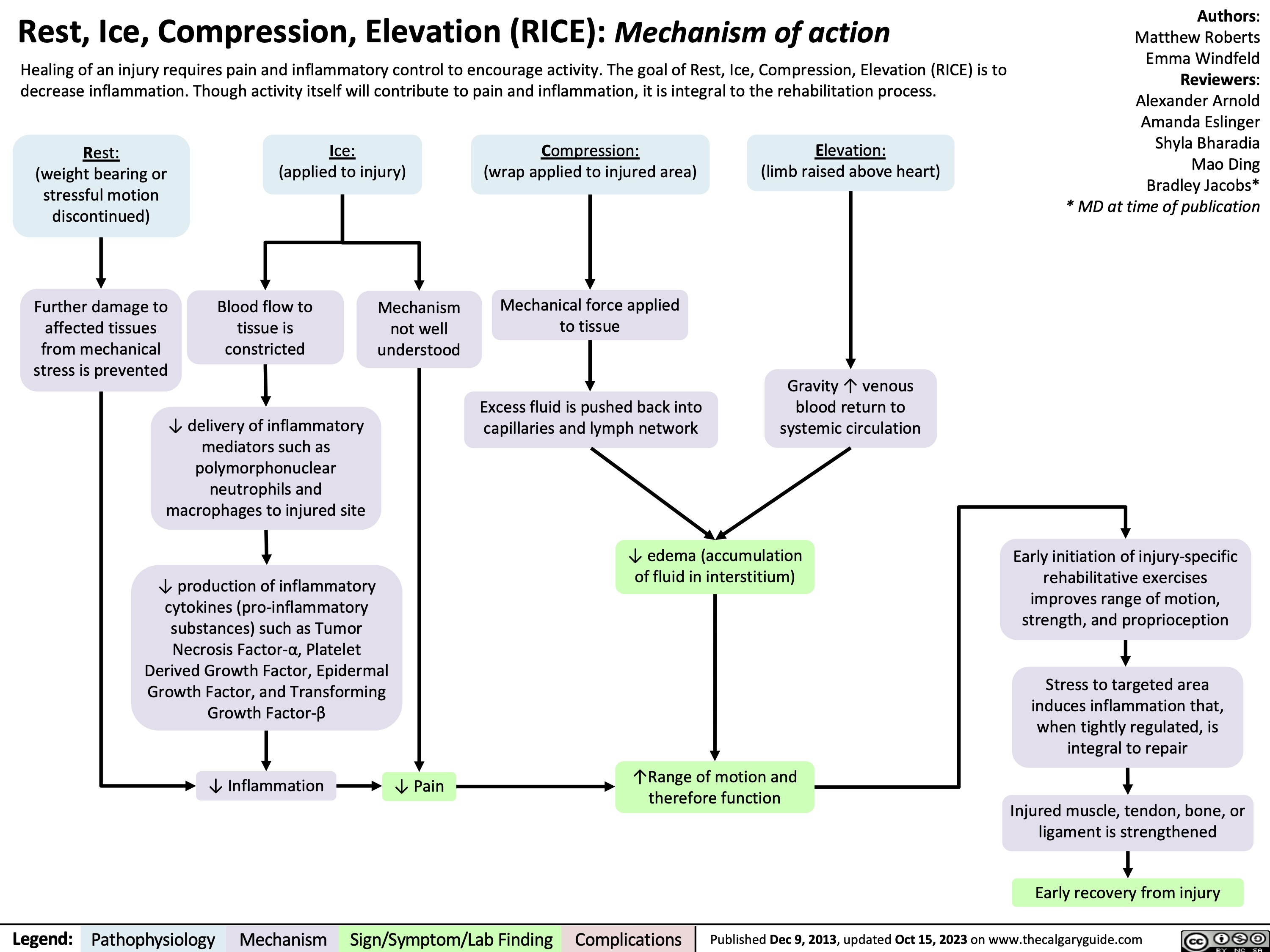
RICE mechanism of action
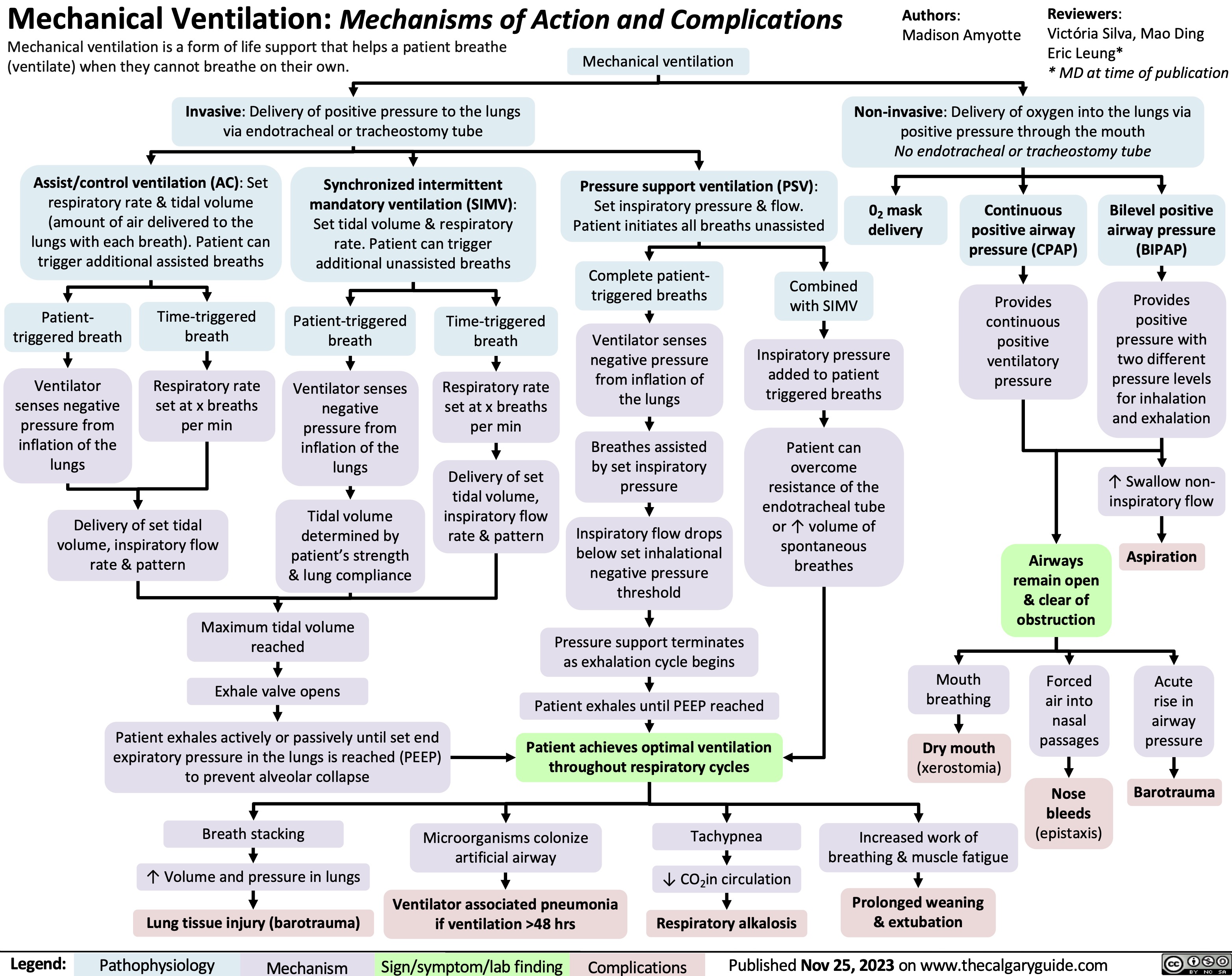
Mechanical Ventilation mechanisms of action and complications
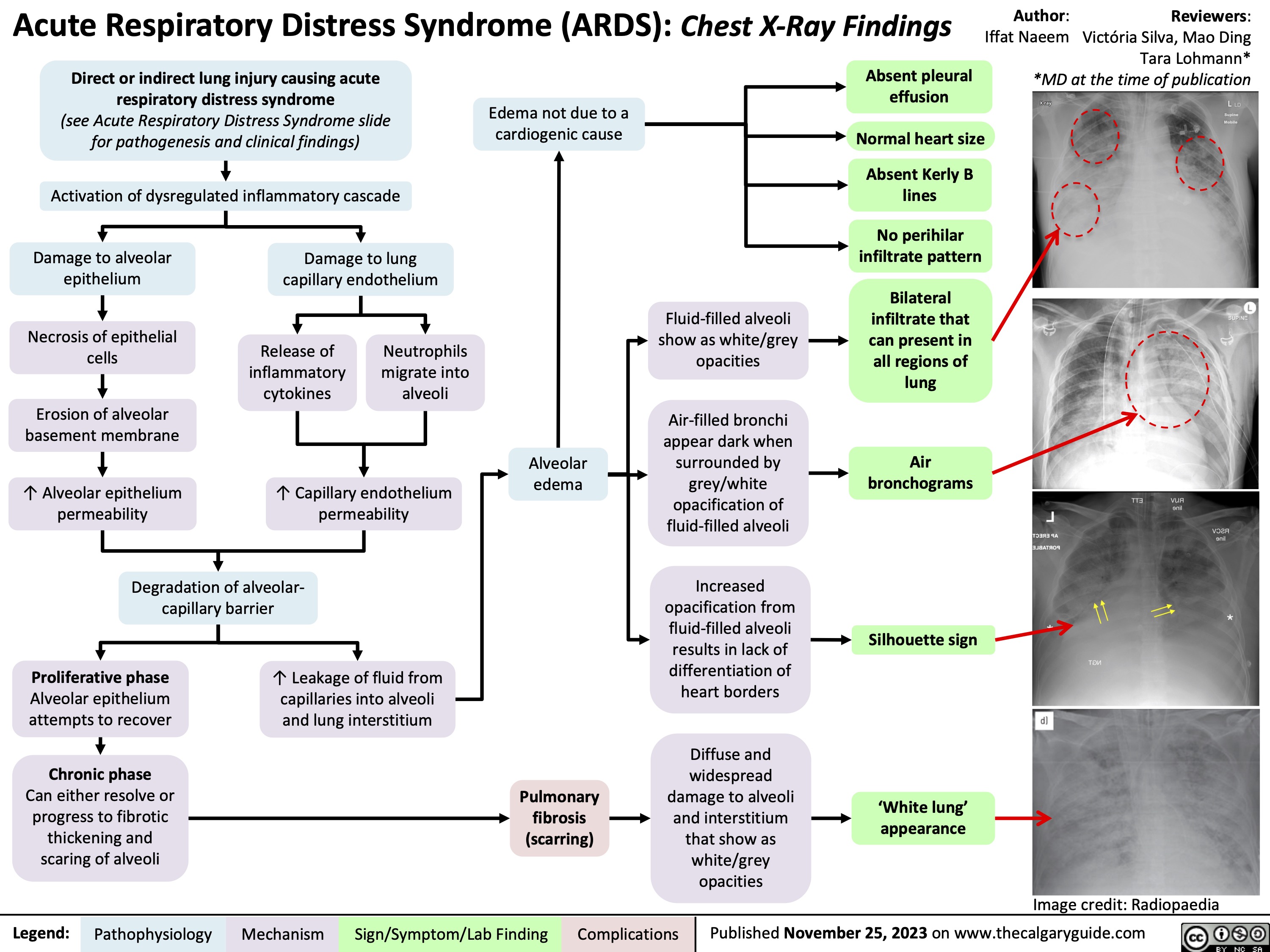
Acute Respiratory Distress Syndrome ARDS CXR findings
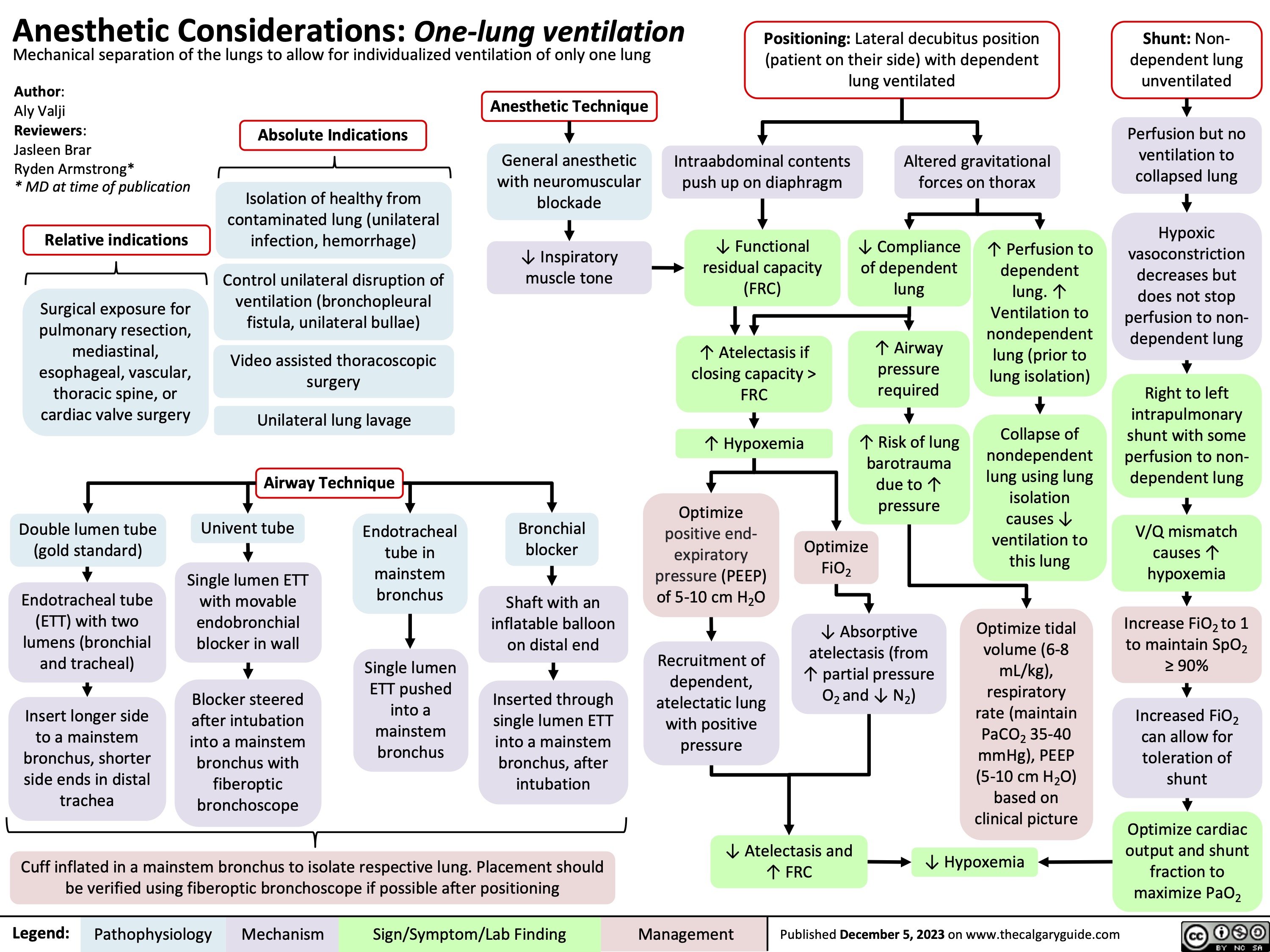
Anesthetic Considerations One Lung Ventilation
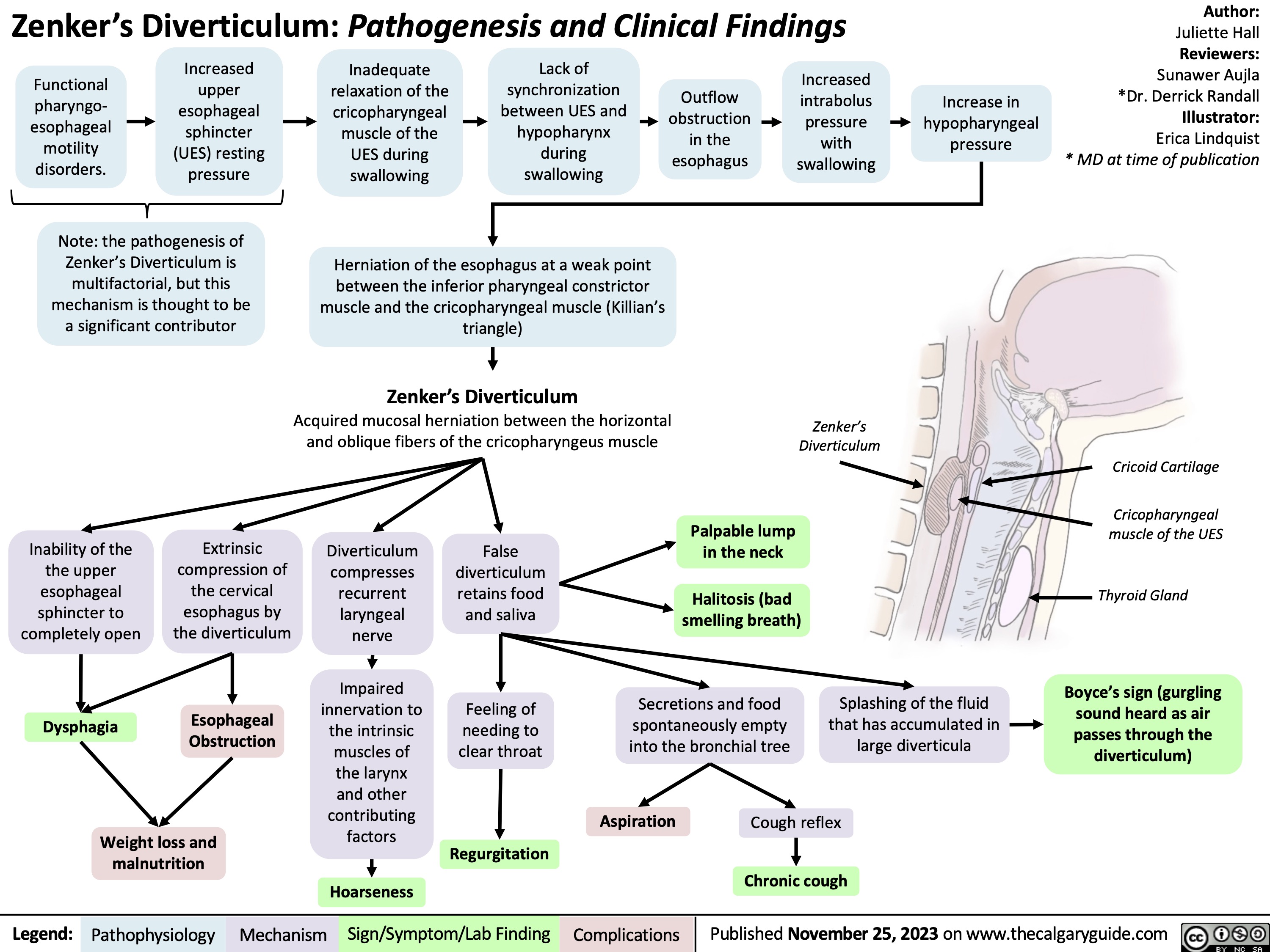
Zenkers Diverticulum Pathogenesis and Clinical Findings
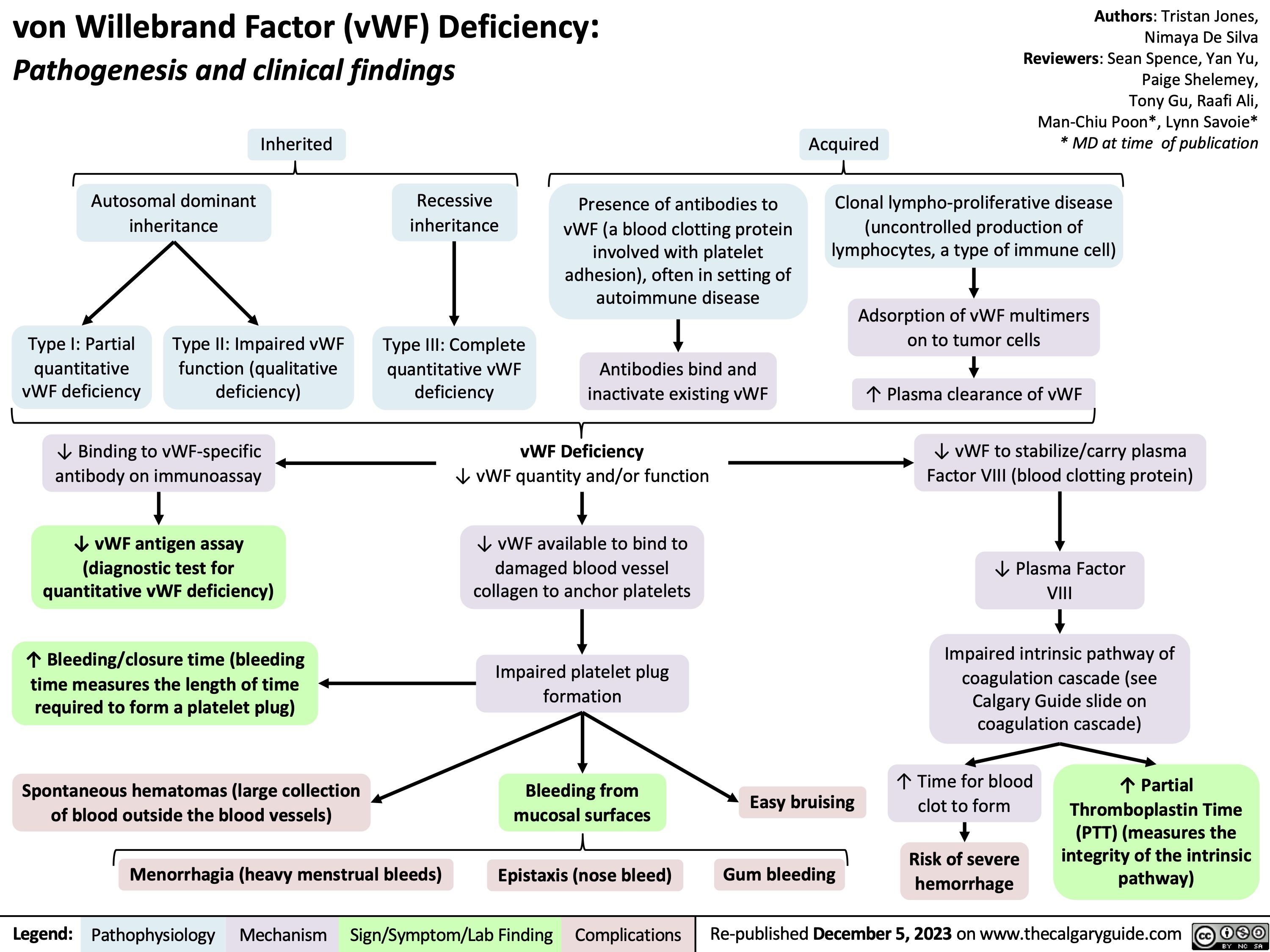
vWF Deficiency
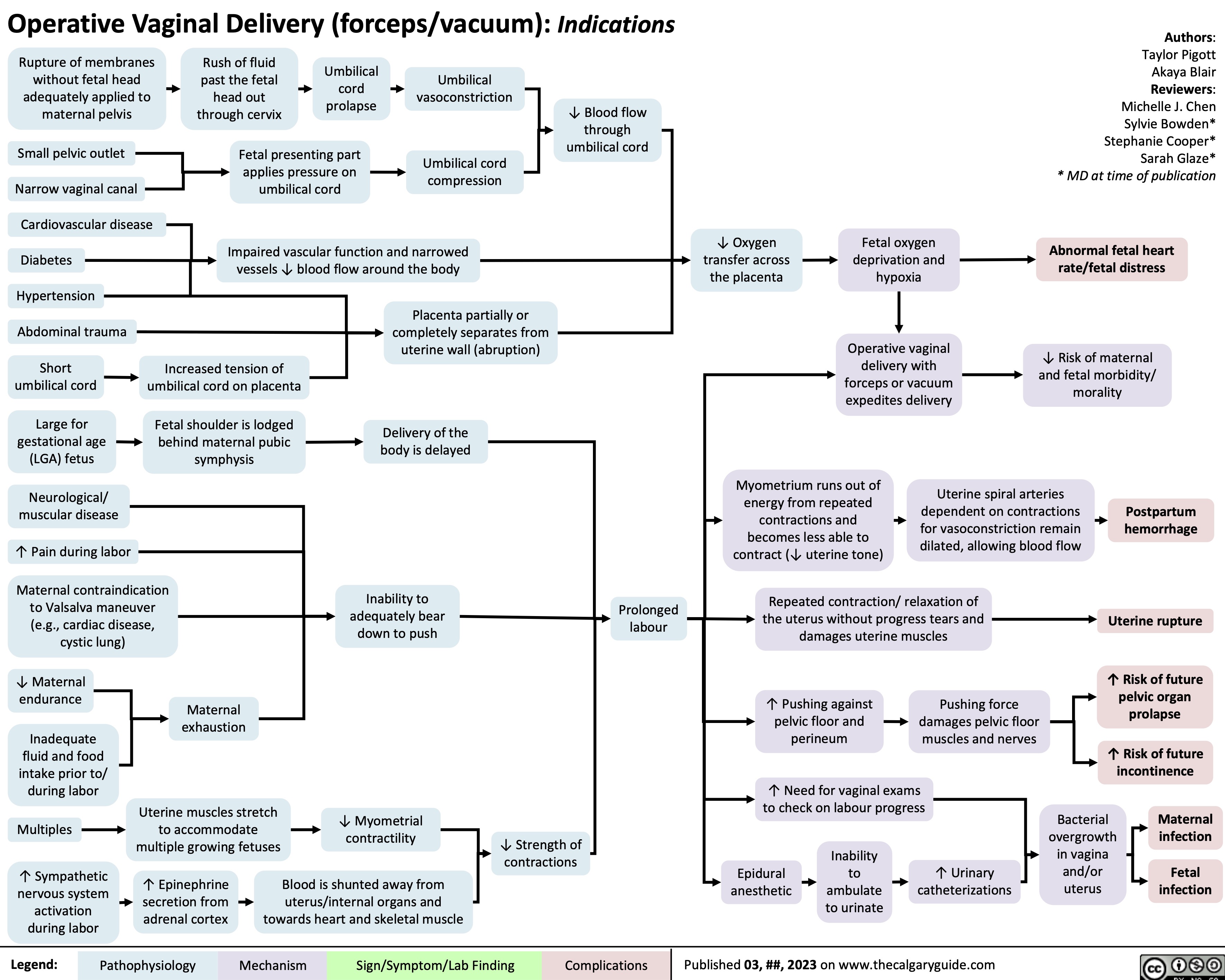
Operative Vaginal Delivery Indications
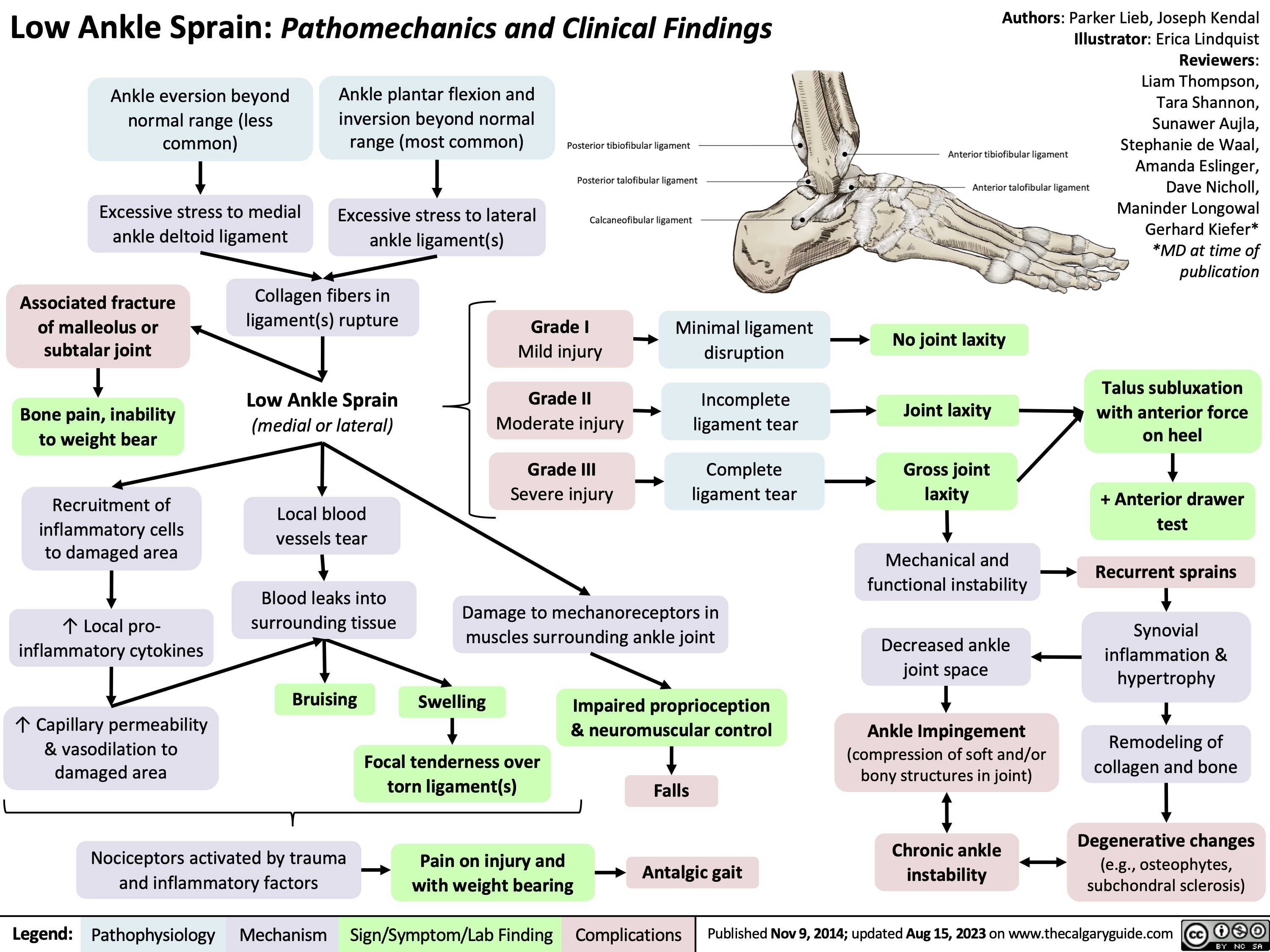
Low Ankle Sprain