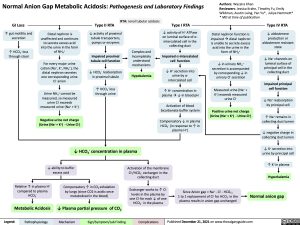
Normal Anion Gap Metabolic Acidosis: Pathogenesis and Laboratory Findings
Authors: Wazaira Khan
Reviewers: Jessica Krahn, Timothy Fu, Emily Wildman, Austin Laing, Yan Yu*, Juliya Hemmett* * MD at time of publication
GI Loss
↑ gut motility and secretion
↑ HCO3- loss through stool
Distal nephron is unaffected and continues to secrete excess acid into the urine in the form of NH4+
For every major urine cation (Na+, K+, NH4+), the distal nephron secretes one corresponding urine Cl- anion
Urine NH4+ cannot be measured, so measured
urine Cl- exceeds measured urine (Na+ + K+)
Negative urine net charge (Urine (Na+ + K+) – Urine Cl-)
Type II RTA RTA: renal tubular acidosis ↓ activity of proximal
Type I RTA
↓ activity of H+ ATPase on luminal surface of ⍺- intercalated cell in the collecting duct
Impaired ⍺-intercalated cell function
↓ H+ secretion into urine by ⍺- intercalated cell
↑ H+ concentration in plasmaà↓ in blood pH
Activation of blood bicarbonate buffer system
Compensatory ↓ in plasma HCO3- (in response to ↑ in plasma H+)
Type IV RTA
↓ aldosterone production or aldosterone resistant state
↓ Na+ channels on luminal surface of principal cell in the collecting duct
Impaired principal cell function
↓ Na+ reabsorption by principal cell
↑ Na+ remains in collecting duct lumen
↓ negative charge in collecting duct lumen
↓ K+ secretion into urine by principal cell
↑ K+ in plasma
Hyperkalemia
Normal anion gap
tubule transporters, pumps or enzymes
Impaired proximal tubule cell function
reabsorption
Complex and Incompletely understood mechanisms
Hypokalemia
Distal nephron function is impairedàdistal nephron is unable to secrete excess acid into the urine in the form of NH4+
↓ in urinary NH + 4
secretion is accompanied by corresponding ↓ in urinary Cl- secretion
Measured urine (Na+ + K+) exceeds measured urine Cl-
Positive urine net charge (Urine (Na+ + K+) – Urine Cl-)
↓ HCO3
–
in proximal tubule
↑ HCO3- loss through urine
↓ HCO3- concentration in plasma
Relative ↑ in plasma H+ compared to plasma HCO3-
Compensatory ↑ in CO2 exhalation by lungs (since CO2 is acidic once metabolized in the blood)
Since Anion gap = Na+ – Cl- – HCO3-,
1 to 1 replacement of Cl- for HCO – in the 3
plasma results in anion gap unchanged
↓ ability to buffer excess acid
Activation of the membrane Cl-/HCO3- exchanger in the collecting duct
Exchanger works to ↑ Cl- levels in the plasma by
one Cl- for each ↓ of one HCO3- in the plasma
Metabolic Acidosis
↓ Plasma partial pressure of CO2
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published December 21, 2021 on www.thecalgaryguide.com
Foundations
Systems
Other Languages
Nephrology Acid-Base Disturbances Normal Anion Gap Metabolic Acidosis: Pathogenesis and Laboratory Findings Normal anion gap metabolic acidosis