Myelodysplastic Syndrome (MDS): Pathogenesis and clinical findings
Authors: Mao Ding Reviewers: Ashar Memon Man-Chiu Poon Yan Yu* * MD at time of publication
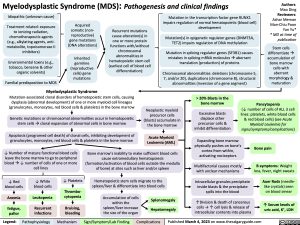
Stem cells differentiateà accumulation of bone marrow cells with aberrant morphology & maturation
Idiopathic (unknown cause)
Treatment related: exposure to ionizing radiation, chemotherapeutic agents (e.g., alkylating agents, anti- metabolite, topoisomerase II inhibitors)
Environmental toxins (e.g., tobacco, benzene & other organic solvents)
Familial predisposition to MDS
Acquired somatic (non- reproductive) gene mutations (DNA alterations)
Inherited
germline (reproductive cells) gene mutations
Recurrent mutations cause alteration(s) in one or more protein functions with/without chromosomal abnormalities in hematopoietic stem cell (earliest cell of blood cell differentiation):
Mutation in the transcription factor gene RUNX1 impairs regulation of normal hematopoietic (blood cell) development
Mutation(s) in epigenetic regulator genes (DNMT3A, TET2) impairs regulation of DNA methylation
Mutation in splicing regulator genes (SF3B1) causes mistakes in splicing mRNA moleculesàaberrant translation (production) of proteins
Chromosomal abnormalities: deletions (chromosome 5, 7, and/or 20), duplications (chromosome 8), structural abnormalities (inversion of a gene segment)
Myelodysplastic Syndrome
Mutation-associated clonal disorders of hematopoietic stem cells, causing dysplasia (abnormal development) of one or more myeloid cell lineages (granulocytes, monocytes, red blood cells & platelets) in the bone marrow
Genetic mutations or chromosomal abnormalities occur in hematopoietic stem cellsàclonal expansion of abnormal cells in bone marrow
Apoptosis (programed cell death) of clonal cells, inhibiting development of granulocytes, monocytes, red blood cells & platelets in the bone marrow
Neoplastic myeloid precursor cells (blasts) accumulate in the bone marrow
Acute Myeloid Leukemia (AML)
> 20% Blasts in the bone marrow
Excessive blasts displace other precursor cells & inhibit differentiation
Pancytopenia
(↓ number of cells of ALL 3 cell lines: platelets, white blood cells & red blood cells) (see Acute Myeloid Leukemia for signs/symptoms/complications)
↓ Number of mature functional blood cells leave the bone marrow to go to peripheral bloodà↓ number of cells of one or more cell lines
Bone marrow’s inability to make sufficient blood cells cause extramedullary hematopoiesis (formation/activation of blood cells outside the medulla of bone) at sites such as liver and/or spleen
Hematopoietic stem cells migrate to the spleen/liver & differentiate into blood cells
Expanding bone marrow physically pushes on bone’s cortex from within, activating nociceptors
Multifactorial causes mostly with unclear mechanisms
Intracellular granules precipitate inside blasts & the precipitate spills into the blood
↑Division & death of cancerous cells → ↑ cell lysis & release of intracellular contents into plasma
Bone pain
B symptoms: Weight
loss, fever, night sweats
Auer Rods (needle- like crystals) seen on blood smear
↑ Serum levels of uric acid, K+, LDH
↓ Red blood cells
Anemia
fatigue, pallor
↓ White blood cells
Leukopenia
Recurrent infections
↓ Platelets
Thrombo- cytopenia
Bruising, bleeding
Accumulation of cells within the spleen/liver increase the size of the organ
Splenomegaly Hepatomegaly
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 4, 2023 on www.thecalgaryguide.com
Foundations
Systems
Other Languages
Hematology Blood Cell Malignancies Myelodysplastic Syndrome: Pathogenesis and Clinical Findings Myelodysplastic Syndrome Pathogenesis and clinical findings