Local Anesthetics (Lidocaine, Bupivacaine, Ropivacaine, Procaine, Cocaine): Side Effects and Complications
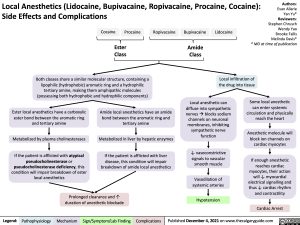
Cocaine
Procaine
Ropivacaine
Bupivacaine
Amide Class
Lidocaine
Authors: Evan Allarie Yan Yu* Reviewers: Stephen Chrusch Wendy Yao Brooke Fallis Melinda Davis* * MD at time of publication
Ester Class
Both classes share a similar molecular structure, containing a lipophilic (hydrophobic) aromatic ring and a hydrophilic tertiary amine, making them amphipathic molecules (possessing both hydrophobic and hydrophilic components)
Local infiltration of the drug into tissue
Ester local anesthetics have a carboxylic ester bond between the aromatic ring and tertiary amine
Metabolized by plasma cholinesterases If the patient is afflicted with atypical
pseudocholinesterase or pseudocholinesterase deficiency, this condition will impair breakdown of ester local anesthetics
Amide local anesthetics have an amide bond between the aromatic ring and tertiary amine
Metabolized in liver by hepatic enzymes
If the patient is afflicted with liver disease, this condition will impair breakdown of amide local anesthetics
Local anesthetic can diffuse into sympathetic nervesàblocks sodium channels on neuronal membranes, inhibiting sympathetic nerve function
↓ vasoconstrictive signals to vascular smooth muscle
Vasodilation of systemic arteries
Hypotension
Some local anesthetic can enter systemic circulation and physically reach the heart
Anesthetic molecule will block ion channels on cardiac myocytes
If enough anesthetic reaches cardiac myocytes, their action will ↓ myocardial electrical signalling and thus ↓ cardiac rhythm and contractility
Prolonged clearance and ↑ duration of anesthetic blockade
Cardiac Arrest
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published December 4, 2021 on www.thecalgaryguide.com
Foundations
Systems
Other Languages
Anesthesia Complications of Anesthetic Drugs Local Anesthetics (Lidocaine, Bupivacaine, Ropivacaine, Procaine, Cocaine): Side Effects and Complications local-anesthetics-lidocaine-bupivacaine-ropivacaine-procaine-cocaine-side-effects-and-complications