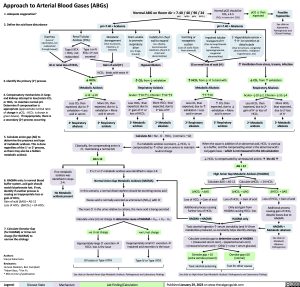
Approach to Arterial Blood Gases (ABGs)
Normal pO2 should be FiO2 x 4-5
(FiO2 in room air= 21%)
pH >7.40 = Alkalemia
1. Adequate oxygenation?
2. Define the acid-base disturbance
Diarrhea (Loss of electrolytes not reabsorbed through bowel)
Normal ABG on Room Air = 7.40 / 40 / 90 / 24
pO2 ↓ than expected
Possible hypoxemia
See slide on
Hypoxemia: Pathogenesis and Clinical Findings
(pH)
(Loss of acidic fluids from stomach)
(HCO3-)
(pCO2)
(pO2)
Renal Tubular Acidosis (RTA)
pH <7.40 = Acidemia
Metabolic derangement (Due to poisons, infection, or ketones)
Gain of acid (H+)
Brain unable to promote respiratory drive
(ex. drugs, trauma)
Inability for chest wall to expand (ex. obesity, neuromuscular weakness, pleural or chest wall abnormalities)
Vomiting or
nasogastric
suction
Impaired tubular transport of H+
(Due to loop/thiazide diuretics, hypomagnesemia, congenital abnormalities)
1o Hyperaldosteronism =
↑ H+ ion secretion at
distal tubule
(Due to tumours, congenital abnormalities , malignant hypertension, etc)
Type II RTA = HCO3- not reabsorbed
Type I or IV RTA = H+ not secreted
GI or renal loss of HCO3-
Hypoventilation
↑ CO2 from ↓ exhalation Respiratory Acidosis Acute= ↑10:↑1, Chronic= ↑10:↑3
GI or renal loss of acid (H+)
↑ HCO3- from ↓ H+ to bind with Metabolic Alkalosis ↑7:↑10
↑ Ventilation from stress, trauma, infection
↓ CO2 from ↑ exhalation Respiratory Alkalosis Acute= ↓10:↓2, Chronic= ↓10:↓4
3. Identify the primary (1o) process
4. Compensatory mechanisms in lungs and kidneys attempt to lose/retain CO2 or HCO3- to maintain normal pH. Determine if compensation is appropriate (approximate normal ratio of change in pCO2 : HCO3- is shown in green boxes). If inappropriate, there is a secondary (2o) process occurring
HCO3- binds with extra H+
↓ HCO3- Metabolic Acidosis ↓12 :↓10
Less CO2 than expected, due to ↑ exhalation = Less acid in serum
2o Respiratory alkalosis
More CO2 than expected, due to ↓ exhalation = More acid in serum
2o Respiratory acidosis
Less HCO3- than expected, due to 2o gain of H+ or loss of HCO3-
2o Metabolic acidosis
More HCO3- than expected, due to 2o loss of H+
2o Metabolic alkalosis
Less CO2 than expected, due to ↑ exhalation = Less acid in serum
2o Respiratory alkalosis
↑ CO2 than expected, due to ↓ exhalation = More acid in serum
2o Respiratory acidosis
Less HCO3- than expected, due to 2o gain of H+ or loss of HCO3-
2o Metabolic acidosis
More HCO3- than expected, due to 2o loss of H+
2o Metabolic alkalosis
5. Calculate anion gap (AG) to determine the presence and type of metabolic acidosis. This is done regardlessofthe1o or2oprocess, as there may also be a hidden metabolic acidosis
6. HAGMA only: In normal blood buffer system, acid gain should match bicarbonate lost. If not, identify if another process is causing an inappropriate loss or gain of HCO3-
Gain of acid (∆AG) = AG-12 LossofHCO3- (∆HCO3-)=24-HCO3-
7. Calculate Osmolar Gap (for HAGMA) or Urine net charge (for NAGMA) to narrow the etiology
Authors:
Sravya Kakumanu
Reviewers:
Huneza Nadeem, Ben Campbell *Adam Bass, *Yan Yu
* MD at time of publication
(normal is ~12)
Calculate AG = Na+- Cl- - HCO3-
If a metabolic acidosis is present, ↓ HCO3- is
compensatedby↑otherserumanionstomaintain neutral charge
If no metabolic acidosis was identified in steps 2-4:
No Metabolic acidosis present
Classically, the compensating anion is Cl-,maintaininganormalAG
AG ≤ 12
If a 1o or 2o metabolic acidosis was identified in steps 2-4:
Normal Anion Gap Metabolic Acidosis (NAGMA)
In this scenario, a normal distal nephron should be excreting excess acid
Excess acid is normally excreted as ammonium (NH +) with Cl- 4
The more Cl- in the urine relative to cations, the more acid is being excreted Calculate urine (U) net charge to determine cause of NAGMA = UNa+ + UK+ - UCl-
When the cause is addition of an abnormal acid, HCO3- is used up asabuffer,andthecompensatinganionistheabnormalacid's conjugate base – which is not measured in the AG calculation
↓ HCO3- is compensated by unmeasured anionsà the AG ↑ AG>12
High Anion Gap Metabolic Acidosis (HAGMA)
Calculate ∆AG = AG-12
Calculate ∆HCO3- = 24-HCO3-
∆HCO3- > ∆AG
Loss of HCO3- > Gain of acid
Additional process causing further loss of HCO3-
HAGMA + NAGMA
∆HCO3- = ∆AG
Loss of HCO3- = Gain of acid
Only acid gain from HAGMA causing HCO3- loss
HAGMA only
∆HCO3- < ∆AG
Loss of HCO3- < Gain of acid
Additional process causing gain of HCO3- despite losses due to HAGMA
HAGMA + Metabolic alkalosis
See slide on
Metabolic Alkalosis: Pathogenesis
-ve U net charge Appropriately large Cl- excretionà
Toxic alcohol ingestion ↑ serum osmolality (and H+) from metabolites produced, so osmolality helps identify etiology
Calculate osmolar gap to determine cause of HAGMA
= (measured serum osm) – (expected serum osm)
= (measured serum osm) – (2(Na+) + urea + serum glucose)
+ve U net charge
Inappropriately small Cl- excretionà impaired acid excretion is the issue
Type IV or Type I RTA See slide on Normal Anion Gap Metabolic Acidosis: Pathogenesis and Laboratory Findings
HCO3
GI losses or Type II RTA
-
loss is the issue
Osmolar gap > 10 (extra osmoles present)
Toxic alcohol poisoning
Osmolar gap ≤10 (normal)
Test for other causes
See slide on High Anion Gap Metabolic Acidosis: Pathogenesis and Laboratory Findings
Legend:
Disease State
Mechanism
Lab Finding/Calculation
Published January 29, 2023 on www.thecalgaryguide.com
Foundations
Systems
Other Languages
Physiology Respirology Approach to Arterial Blood Gases (ABGs) Approach to Arterial Blood Gases ABGs