SEARCH RESULTS FOR: diabetes
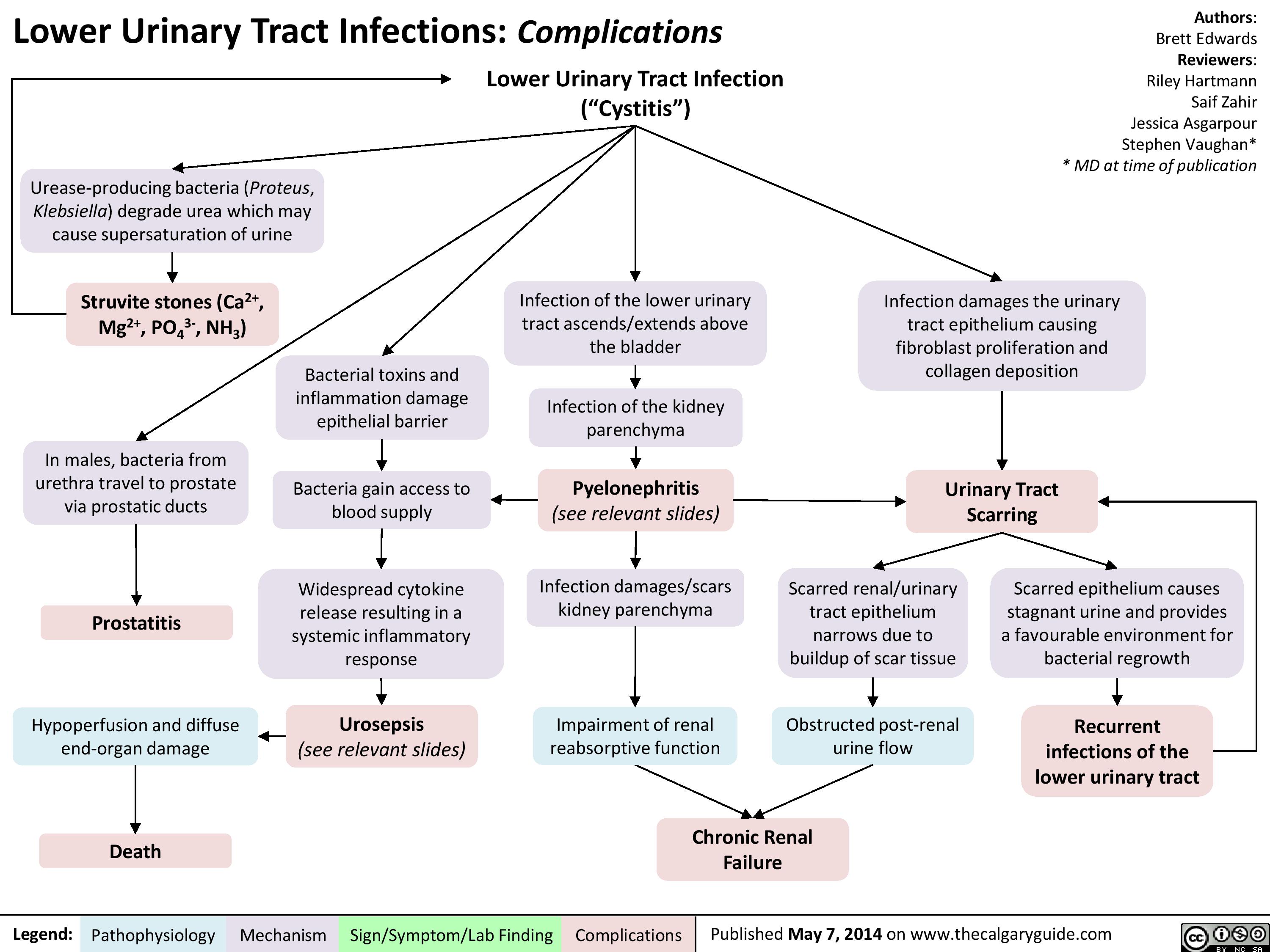
lower-urinary-tract-infections-complications
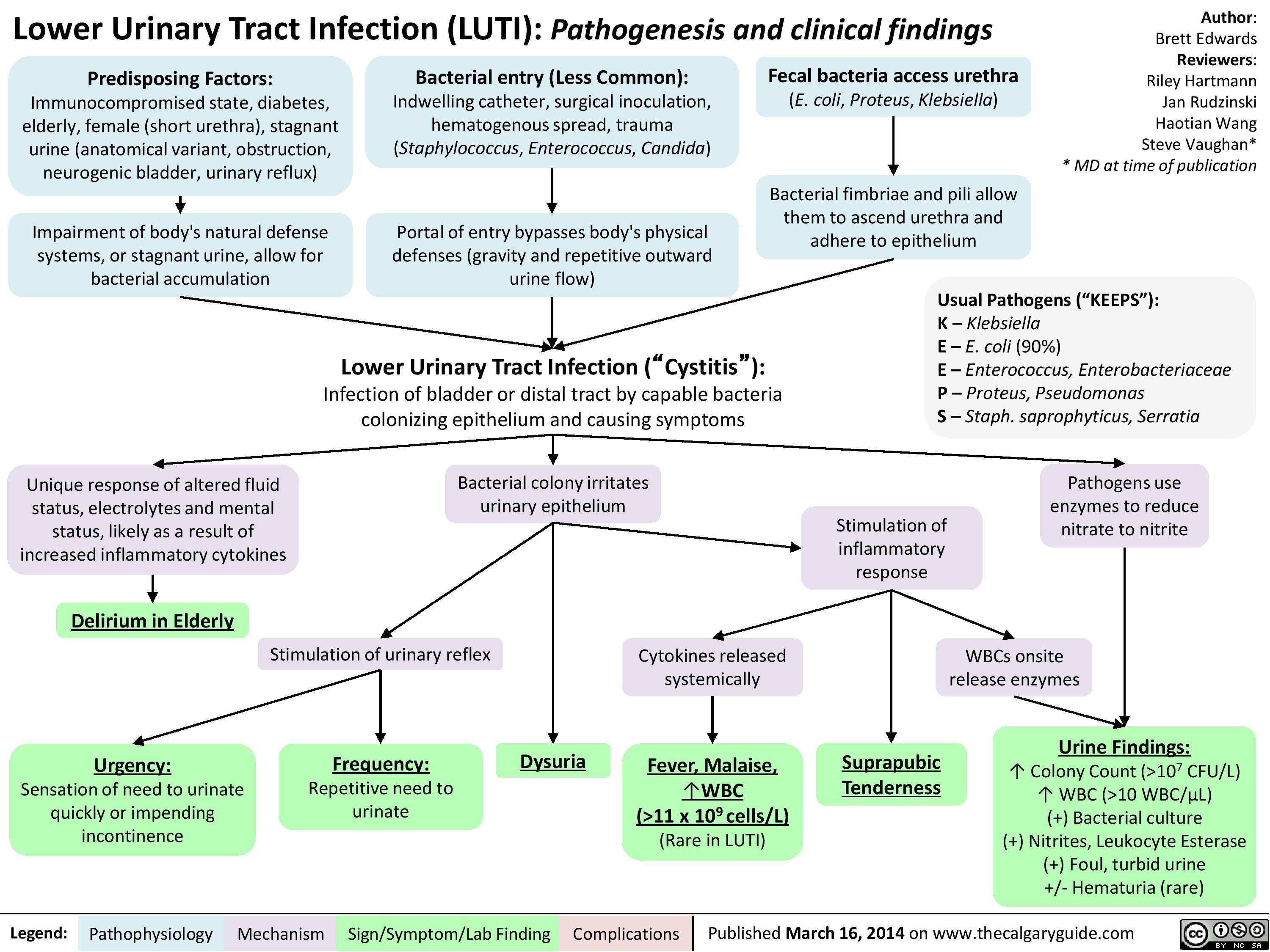
lower-urinary-tract-infection-pathogenesis-and-clinical-findings
Hypokalemia: Clinical Findings
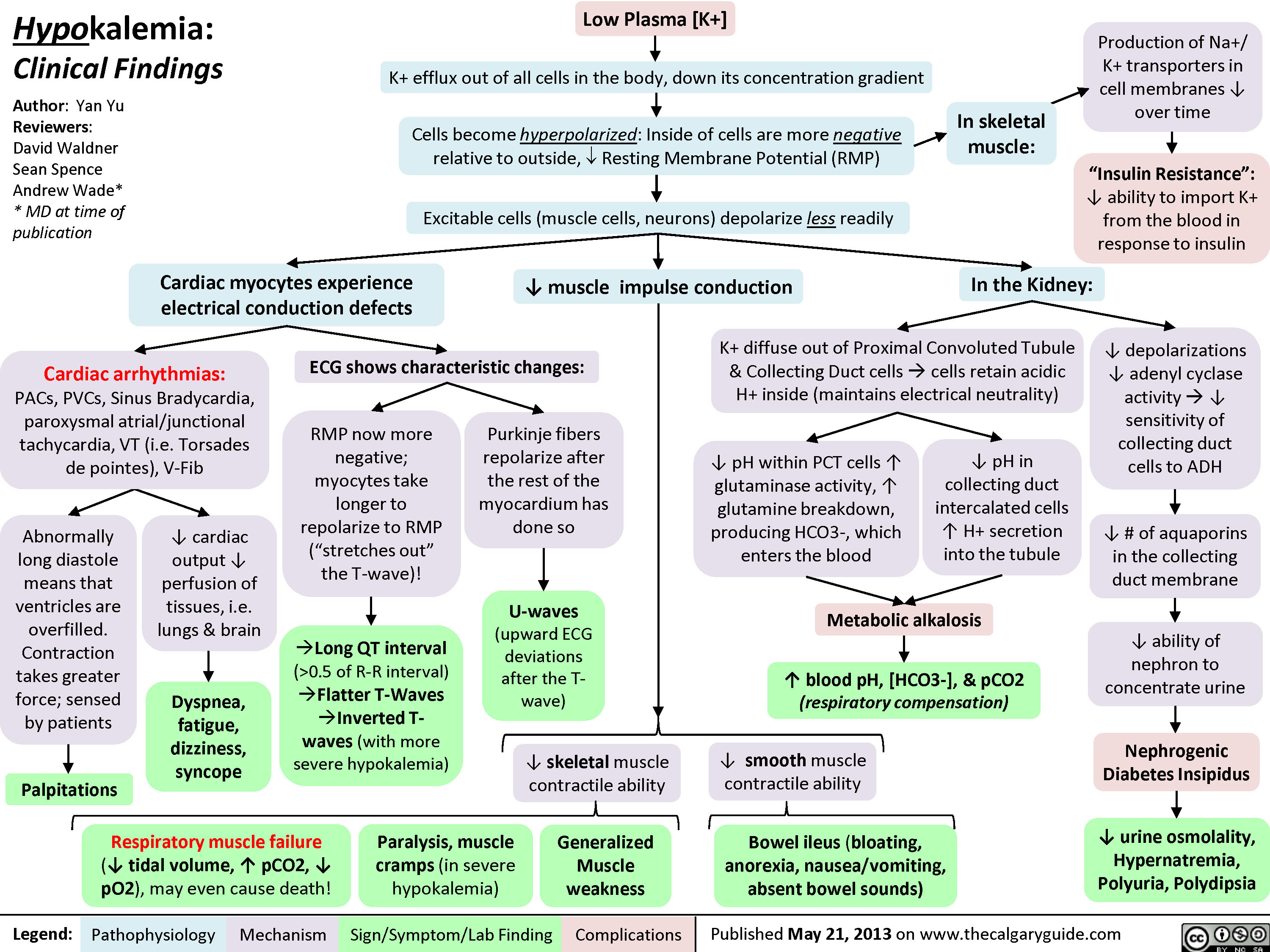 0.5 of R-R interval)?Flatter T-Waves ?Inverted T-waves (with more severe hypokalemia)Purkinje fibers repolarize after the rest of the myocardium has done soU-waves (upward ECG deviations after the T-wave)Cells become hyperpolarized: Inside of cells are more negative relative to outside, ? Resting Membrane Potential (RMP)In the Kidney:Generalized Muscle weaknessK+ diffuse out of Proximal Convoluted Tubule & Collecting Duct cells ? cells retain acidic H+ inside (maintains electrical neutrality)? pH within PCT cells ? glutaminase activity, ? glutamine breakdown, producing HCO3-, which enters the blood? blood pH, [HCO3-], & pCO2 (respiratory compensation)Low Plasma [K+]Abnormally long diastole means that ventricles are overfilled. Contraction takes greater force; sensed by patientsDyspnea, fatigue, dizziness, syncope? cardiac output ? perfusion of tissues, i.e. lungs & brainCardiac arrhythmias: PACs, PVCs, Sinus Bradycardia, paroxysmal atrial/junctional tachycardia, VT (i.e. Torsades de pointes), V-Fib? smooth muscle contractile abilityBowel ileus (bloating, anorexia, nausea/vomiting, absent bowel sounds)? pH in collecting duct intercalated cells ? H+ secretion into the tubuleMetabolic alkalosisParalysis, muscle cramps (in severe hypokalemia)Respiratory muscle failure (? tidal volume, ? pCO2, ? pO2), may even cause death!? depolarizations ? adenyl cyclase activity ? ? sensitivity of collecting duct cells to ADH? ability of nephron to concentrate urineNephrogenic Diabetes Insipidus? urine osmolality, Hypernatremia, Polyuria, Polydipsia? # of aquaporins in the collecting duct membrane"Insulin Resistance": ? ability to import K+ from the blood in response to insulinIn skeletal muscle:
117 kB / 307 word" title="Yu, Yan - Hypokalemia clinical findings - FINAL.pptx
Production of Na+/ K+ transporters in cell membranes ? over timeHypokalemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceAndrew Wade** MD at time of publicationLegend:Published May 21, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsPalpitationsExcitable cells (muscle cells, neurons) depolarize less readilyK+ efflux out of all cells in the body, down its concentration gradientCardiac myocytes experience electrical conduction defects? muscle impulse conductionECG shows characteristic changes:? skeletal muscle contractile abilityRMP now more negative; myocytes take longer to repolarize to RMP("stretches out" the T-wave)! Long QT interval (>0.5 of R-R interval)?Flatter T-Waves ?Inverted T-waves (with more severe hypokalemia)Purkinje fibers repolarize after the rest of the myocardium has done soU-waves (upward ECG deviations after the T-wave)Cells become hyperpolarized: Inside of cells are more negative relative to outside, ? Resting Membrane Potential (RMP)In the Kidney:Generalized Muscle weaknessK+ diffuse out of Proximal Convoluted Tubule & Collecting Duct cells ? cells retain acidic H+ inside (maintains electrical neutrality)? pH within PCT cells ? glutaminase activity, ? glutamine breakdown, producing HCO3-, which enters the blood? blood pH, [HCO3-], & pCO2 (respiratory compensation)Low Plasma [K+]Abnormally long diastole means that ventricles are overfilled. Contraction takes greater force; sensed by patientsDyspnea, fatigue, dizziness, syncope? cardiac output ? perfusion of tissues, i.e. lungs & brainCardiac arrhythmias: PACs, PVCs, Sinus Bradycardia, paroxysmal atrial/junctional tachycardia, VT (i.e. Torsades de pointes), V-Fib? smooth muscle contractile abilityBowel ileus (bloating, anorexia, nausea/vomiting, absent bowel sounds)? pH in collecting duct intercalated cells ? H+ secretion into the tubuleMetabolic alkalosisParalysis, muscle cramps (in severe hypokalemia)Respiratory muscle failure (? tidal volume, ? pCO2, ? pO2), may even cause death!? depolarizations ? adenyl cyclase activity ? ? sensitivity of collecting duct cells to ADH? ability of nephron to concentrate urineNephrogenic Diabetes Insipidus? urine osmolality, Hypernatremia, Polyuria, Polydipsia? # of aquaporins in the collecting duct membrane"Insulin Resistance": ? ability to import K+ from the blood in response to insulinIn skeletal muscle:
117 kB / 307 word" />
0.5 of R-R interval)?Flatter T-Waves ?Inverted T-waves (with more severe hypokalemia)Purkinje fibers repolarize after the rest of the myocardium has done soU-waves (upward ECG deviations after the T-wave)Cells become hyperpolarized: Inside of cells are more negative relative to outside, ? Resting Membrane Potential (RMP)In the Kidney:Generalized Muscle weaknessK+ diffuse out of Proximal Convoluted Tubule & Collecting Duct cells ? cells retain acidic H+ inside (maintains electrical neutrality)? pH within PCT cells ? glutaminase activity, ? glutamine breakdown, producing HCO3-, which enters the blood? blood pH, [HCO3-], & pCO2 (respiratory compensation)Low Plasma [K+]Abnormally long diastole means that ventricles are overfilled. Contraction takes greater force; sensed by patientsDyspnea, fatigue, dizziness, syncope? cardiac output ? perfusion of tissues, i.e. lungs & brainCardiac arrhythmias: PACs, PVCs, Sinus Bradycardia, paroxysmal atrial/junctional tachycardia, VT (i.e. Torsades de pointes), V-Fib? smooth muscle contractile abilityBowel ileus (bloating, anorexia, nausea/vomiting, absent bowel sounds)? pH in collecting duct intercalated cells ? H+ secretion into the tubuleMetabolic alkalosisParalysis, muscle cramps (in severe hypokalemia)Respiratory muscle failure (? tidal volume, ? pCO2, ? pO2), may even cause death!? depolarizations ? adenyl cyclase activity ? ? sensitivity of collecting duct cells to ADH? ability of nephron to concentrate urineNephrogenic Diabetes Insipidus? urine osmolality, Hypernatremia, Polyuria, Polydipsia? # of aquaporins in the collecting duct membrane"Insulin Resistance": ? ability to import K+ from the blood in response to insulinIn skeletal muscle:
117 kB / 307 word" title="Yu, Yan - Hypokalemia clinical findings - FINAL.pptx
Production of Na+/ K+ transporters in cell membranes ? over timeHypokalemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceAndrew Wade** MD at time of publicationLegend:Published May 21, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsPalpitationsExcitable cells (muscle cells, neurons) depolarize less readilyK+ efflux out of all cells in the body, down its concentration gradientCardiac myocytes experience electrical conduction defects? muscle impulse conductionECG shows characteristic changes:? skeletal muscle contractile abilityRMP now more negative; myocytes take longer to repolarize to RMP("stretches out" the T-wave)! Long QT interval (>0.5 of R-R interval)?Flatter T-Waves ?Inverted T-waves (with more severe hypokalemia)Purkinje fibers repolarize after the rest of the myocardium has done soU-waves (upward ECG deviations after the T-wave)Cells become hyperpolarized: Inside of cells are more negative relative to outside, ? Resting Membrane Potential (RMP)In the Kidney:Generalized Muscle weaknessK+ diffuse out of Proximal Convoluted Tubule & Collecting Duct cells ? cells retain acidic H+ inside (maintains electrical neutrality)? pH within PCT cells ? glutaminase activity, ? glutamine breakdown, producing HCO3-, which enters the blood? blood pH, [HCO3-], & pCO2 (respiratory compensation)Low Plasma [K+]Abnormally long diastole means that ventricles are overfilled. Contraction takes greater force; sensed by patientsDyspnea, fatigue, dizziness, syncope? cardiac output ? perfusion of tissues, i.e. lungs & brainCardiac arrhythmias: PACs, PVCs, Sinus Bradycardia, paroxysmal atrial/junctional tachycardia, VT (i.e. Torsades de pointes), V-Fib? smooth muscle contractile abilityBowel ileus (bloating, anorexia, nausea/vomiting, absent bowel sounds)? pH in collecting duct intercalated cells ? H+ secretion into the tubuleMetabolic alkalosisParalysis, muscle cramps (in severe hypokalemia)Respiratory muscle failure (? tidal volume, ? pCO2, ? pO2), may even cause death!? depolarizations ? adenyl cyclase activity ? ? sensitivity of collecting duct cells to ADH? ability of nephron to concentrate urineNephrogenic Diabetes Insipidus? urine osmolality, Hypernatremia, Polyuria, Polydipsia? # of aquaporins in the collecting duct membrane"Insulin Resistance": ? ability to import K+ from the blood in response to insulinIn skeletal muscle:
117 kB / 307 word" />
Nephrotic Syndrome: Pathogenesis and Clinical Findings
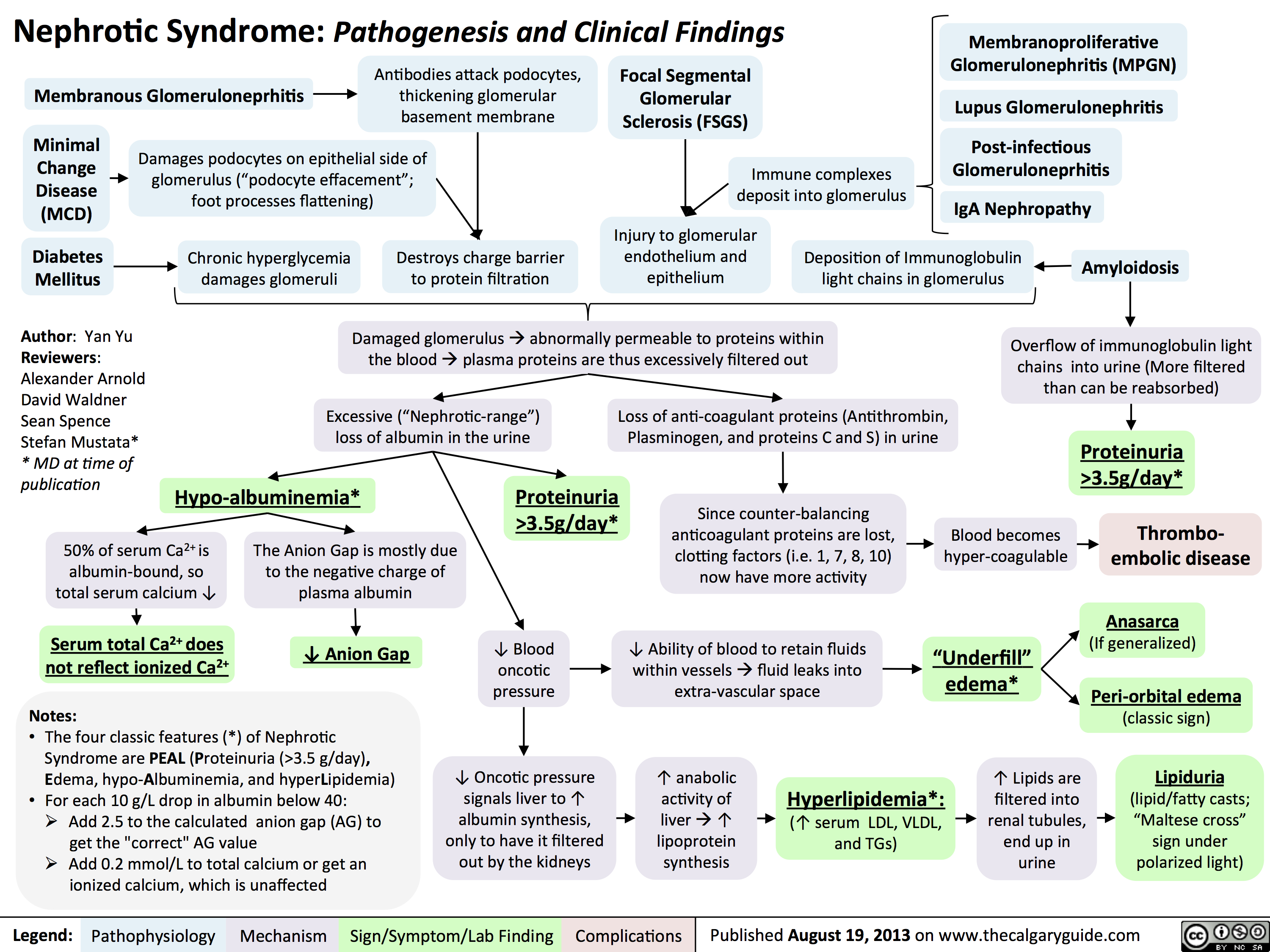 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
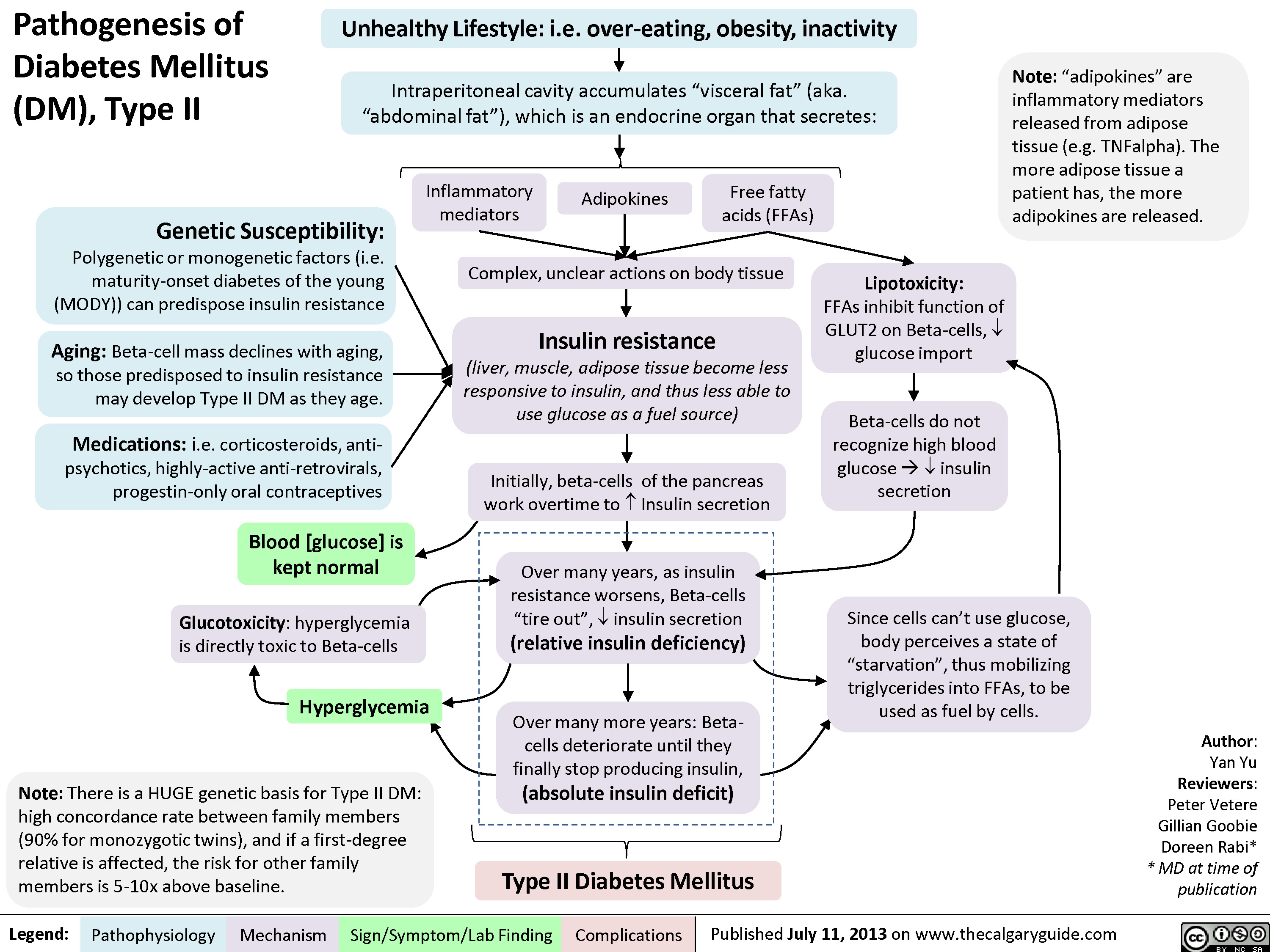
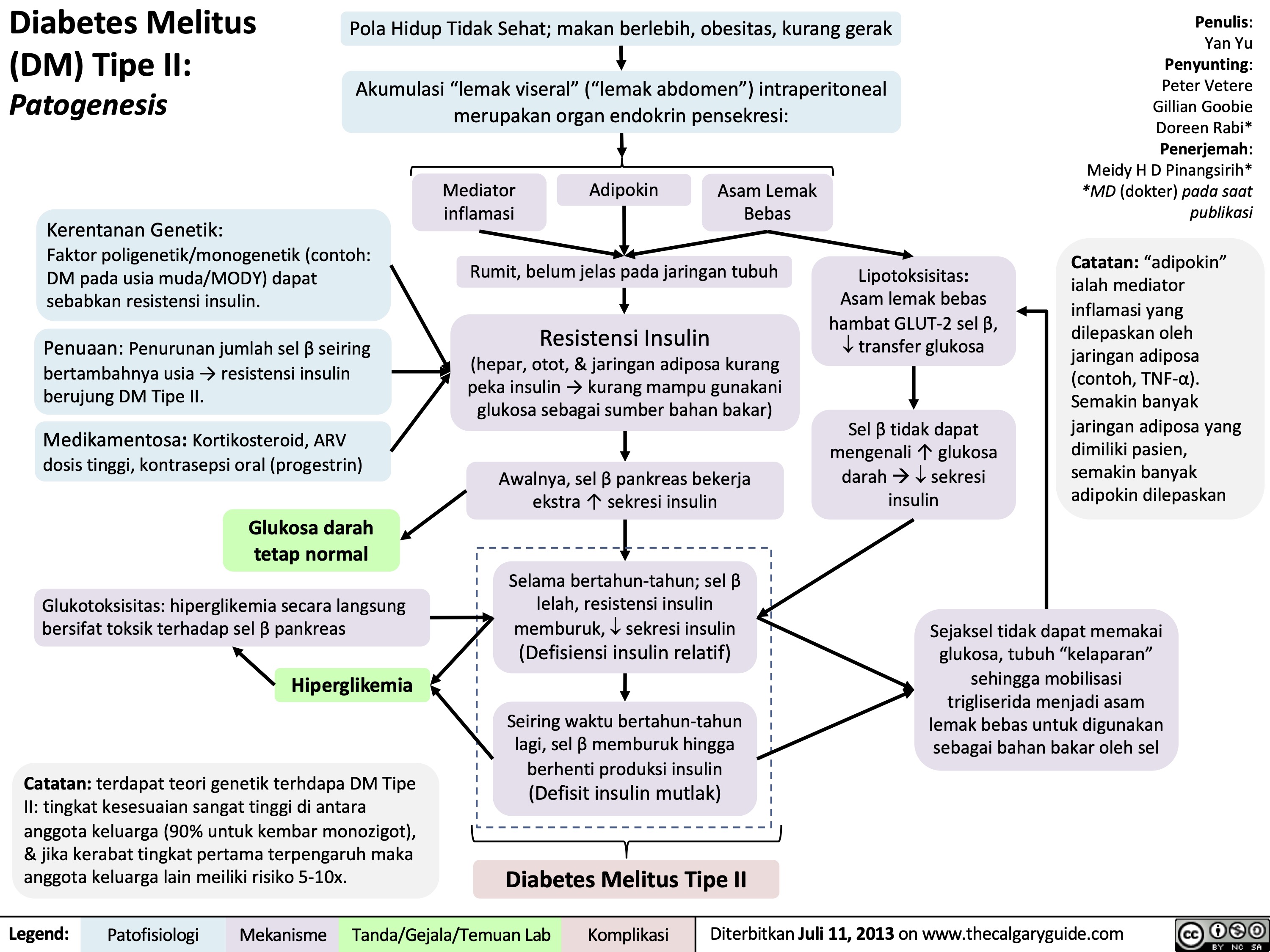
Pathogenesis of Diabetes mellitus DM), Type II
Diabetic Hypoglycemia
![Yu, Yan - Diabetic Hypoglycemia - Clinical Findings - FINAL.pptx
? Epinephrine(Released within seconds as [glucose] falls further) Growth hormone, ? Cortisol (if hypoglycemia persists for minutes)Glucagon should ? when [glucose] falls. But here, glucagon release is inhibited by 1) diabetic auto-immune destruction of Alpha cells & 2) the high insulin.43210Plasma Glucose concentration (mmol/L)Liver should ? glycogenolysis & gluconeogenesisPeripheral vaso-constrictionPlasma [glucose] stays lowActivation of sympathetic (adrenergic) receptors across body, triggering Neurogenic symptomsPlasma [glucose] ?Excess subcutaneous insulin or insulin-secretagogue ?? [insulin] in the bloodOver time: [insulin] in the DM patient depends only on how much was injected or how much secretagogue was consumed; not on the body's physiological state.[Insulin] stays high in excessively-treated DM patientsPlasma [glucose] normally ?, but...High insulin transports plasma glucose into cells!In pts with existing diabetic autonomic neuropathy, epi-nephrine secretion will already be ?Brain does not get enough glucose, ? neuron function ? Neuroglycopenic symptomsTx: glucose intake![Glucose] returns to normalIf no glucose intake:Hypoglycemia-unawareness: No autonomic Sx felt so hypoglycemia not treated early ? pts present later on with more severe hypoglycemia and neuroglycopenic sxBrain cells kept chronically euglycemic due to GLUT1 receptor over-expression (despite rest of body being hypoglycemic)With many hypoglycemic events over time:Brain feels no need to ? glucose, so it ? autonomic epinephrine secretion!This is the normal sequence of hormone responses to ?ing plasma glucose levels.But this normal hormonal response will be blunted over time if there is 1) continued hypoglycemia dampening the sympathetic nervous system, and 2) long-standing diabetic neuropathy! (To be explained later in this flow chart)Abbreviations: [ ] = concentrationTx = TreatmentDM = Diabetes mellitusDiabetic Hypoglycemia: Pathogenesis and Clinical FindingsConfusionCan't concentrateWeaknessSlurred speech? coordination (staggering, etc)SeizuresComa, deathAdrenergic symptoms (epinephrine-mediated):Anxiety, irritability, trembling, pallor (skin vasoconstriction), palpitations, ? systolic BP, tachycardia Cholinergic symptoms(Acetylcholine-mediated):Sweating, hunger, tingling, blurry visionNote: In pts w/out DM, endogenous insulin secretion normally stops when blood [glucose] drops to <4mmol/LAuthor: Yan YuReviewers: Peter Vetere, Gillian Goobie, Hanan Bassyouni** MD at time of publicationLegend:Published June 14, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsMany hypoglycemic events over time blunt epinephrine secretion further.Hypoglycemia unawareness can be reversedIf pt stays hypoglycemia-free for >6 weeks, brain restores its ability to detect low glucose levels? peripheral glucose delivery and uptake (saving more glucose for the brain)Lack of glucagon effect reinforces hypoglycemia
124 kB / 361 words Yu, Yan - Diabetic Hypoglycemia - Clinical Findings - FINAL.pptx
? Epinephrine(Released within seconds as [glucose] falls further) Growth hormone, ? Cortisol (if hypoglycemia persists for minutes)Glucagon should ? when [glucose] falls. But here, glucagon release is inhibited by 1) diabetic auto-immune destruction of Alpha cells & 2) the high insulin.43210Plasma Glucose concentration (mmol/L)Liver should ? glycogenolysis & gluconeogenesisPeripheral vaso-constrictionPlasma [glucose] stays lowActivation of sympathetic (adrenergic) receptors across body, triggering Neurogenic symptomsPlasma [glucose] ?Excess subcutaneous insulin or insulin-secretagogue ?? [insulin] in the bloodOver time: [insulin] in the DM patient depends only on how much was injected or how much secretagogue was consumed; not on the body's physiological state.[Insulin] stays high in excessively-treated DM patientsPlasma [glucose] normally ?, but...High insulin transports plasma glucose into cells!In pts with existing diabetic autonomic neuropathy, epi-nephrine secretion will already be ?Brain does not get enough glucose, ? neuron function ? Neuroglycopenic symptomsTx: glucose intake![Glucose] returns to normalIf no glucose intake:Hypoglycemia-unawareness: No autonomic Sx felt so hypoglycemia not treated early ? pts present later on with more severe hypoglycemia and neuroglycopenic sxBrain cells kept chronically euglycemic due to GLUT1 receptor over-expression (despite rest of body being hypoglycemic)With many hypoglycemic events over time:Brain feels no need to ? glucose, so it ? autonomic epinephrine secretion!This is the normal sequence of hormone responses to ?ing plasma glucose levels.But this normal hormonal response will be blunted over time if there is 1) continued hypoglycemia dampening the sympathetic nervous system, and 2) long-standing diabetic neuropathy! (To be explained later in this flow chart)Abbreviations: [ ] = concentrationTx = TreatmentDM = Diabetes mellitusDiabetic Hypoglycemia: Pathogenesis and Clinical FindingsConfusionCan't concentrateWeaknessSlurred speech? coordination (staggering, etc)SeizuresComa, deathAdrenergic symptoms (epinephrine-mediated):Anxiety, irritability, trembling, pallor (skin vasoconstriction), palpitations, ? systolic BP, tachycardia Cholinergic symptoms(Acetylcholine-mediated):Sweating, hunger, tingling, blurry visionNote: In pts w/out DM, endogenous insulin secretion normally stops when blood [glucose] drops to <4mmol/LAuthor: Yan YuReviewers: Peter Vetere, Gillian Goobie, Hanan Bassyouni** MD at time of publicationLegend:Published June 14, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsMany hypoglycemic events over time blunt epinephrine secretion further.Hypoglycemia unawareness can be reversedIf pt stays hypoglycemia-free for >6 weeks, brain restores its ability to detect low glucose levels? peripheral glucose delivery and uptake (saving more glucose for the brain)Lack of glucagon effect reinforces hypoglycemia
124 kB / 361 words](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Diabetic-Hypoglycemia-Clinical-Findings.jpg)
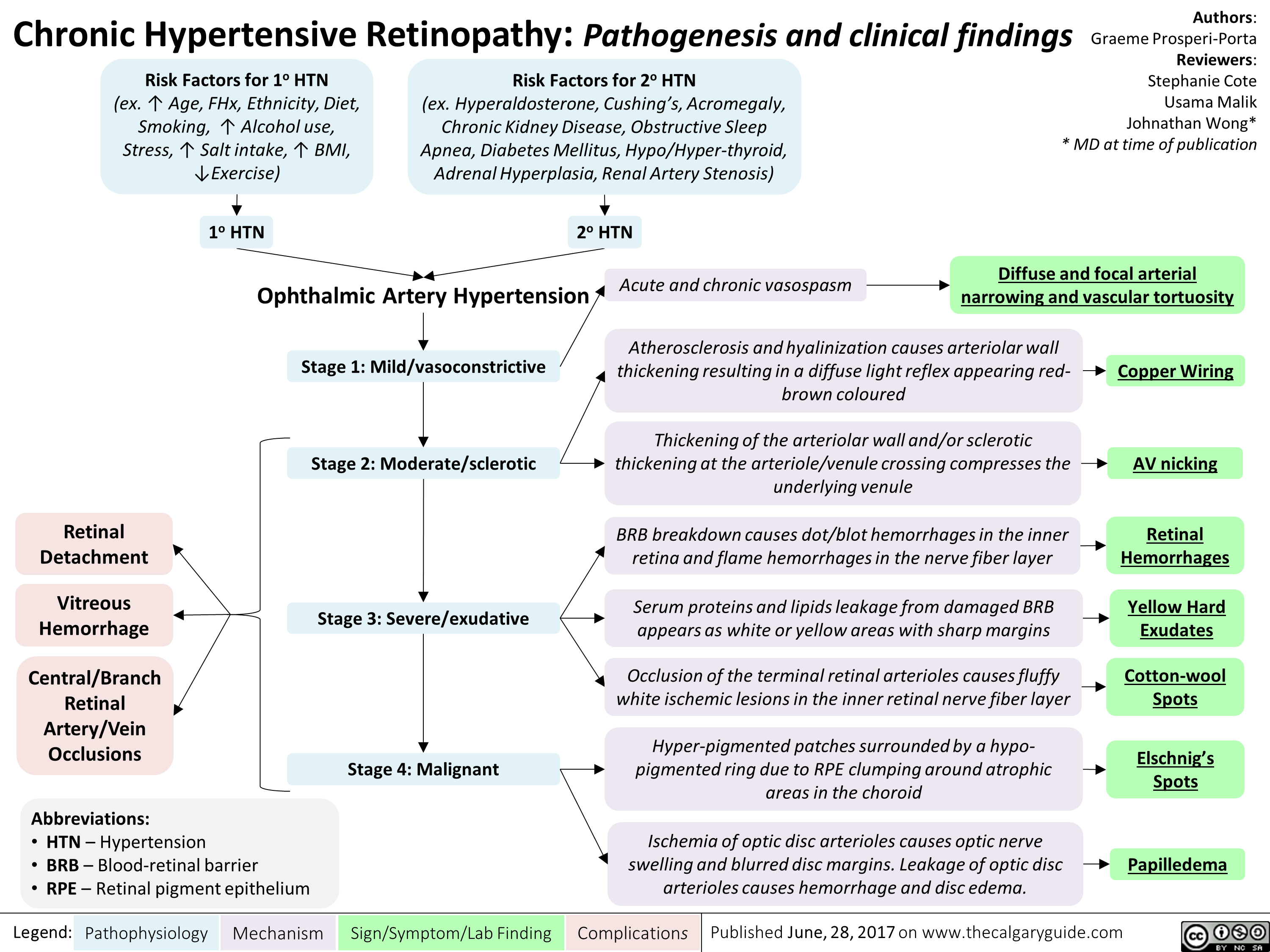
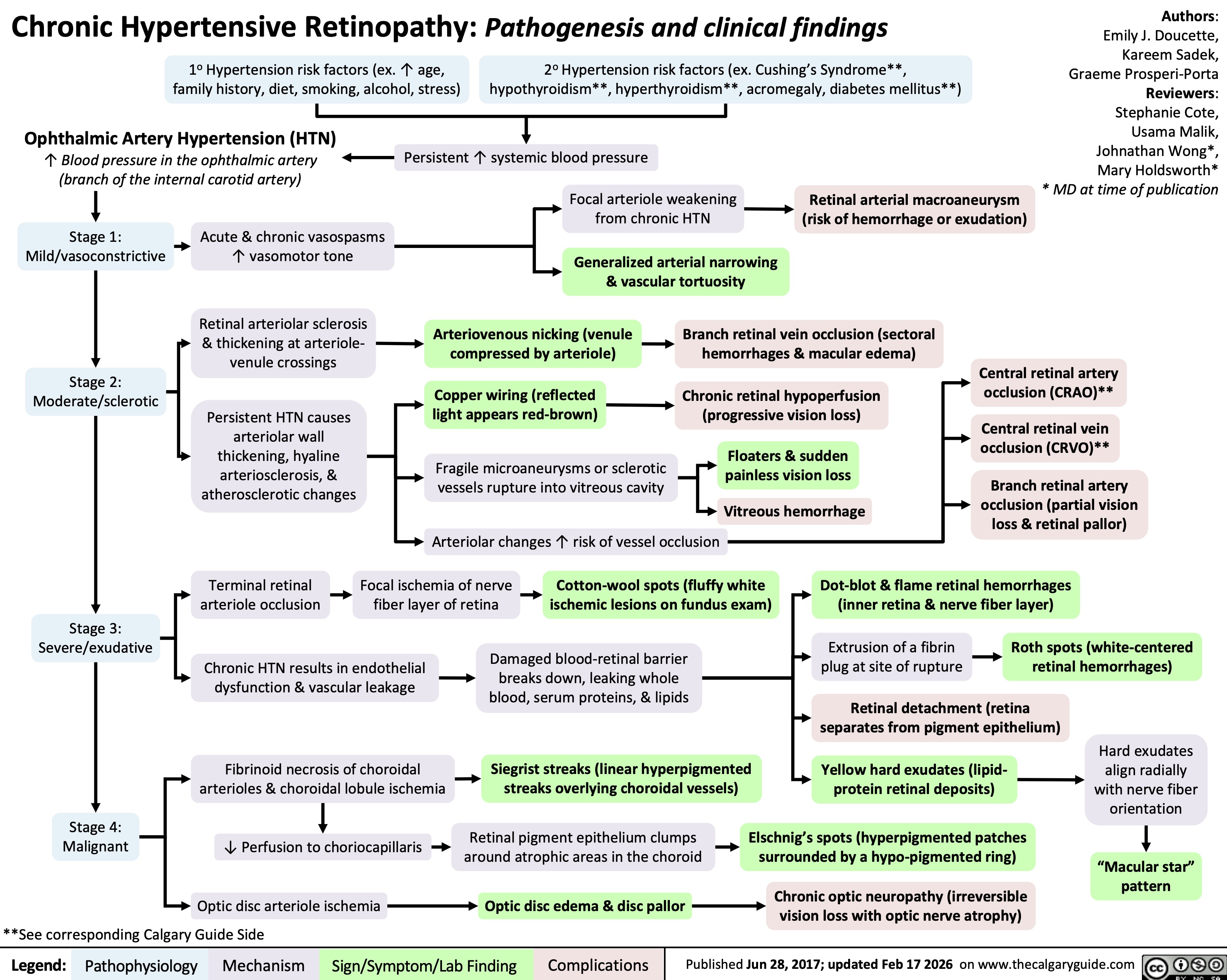
chronic-hypertensive-retinopathy-pathogenesis-and-clinical-findings
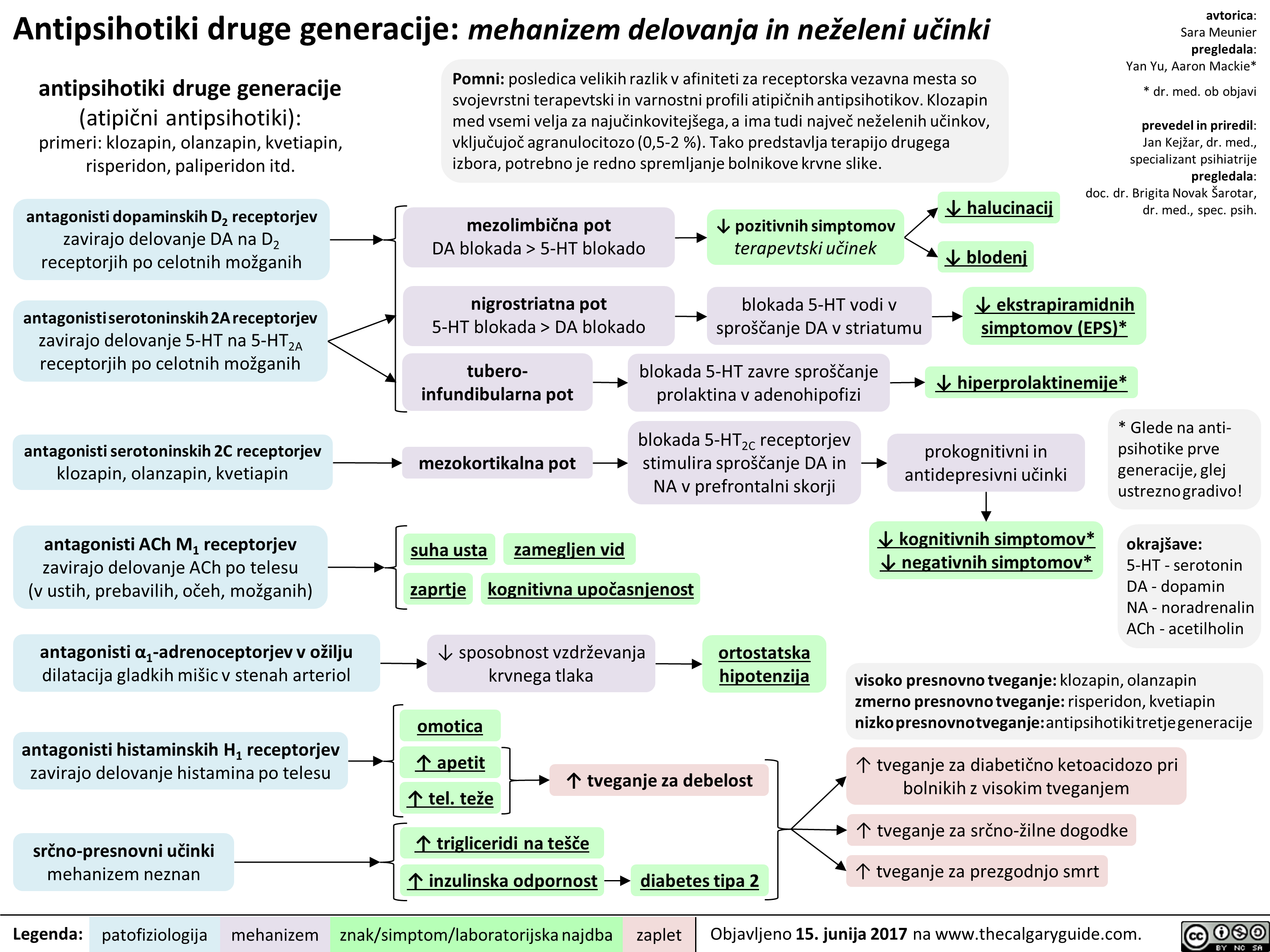
2nd gen antipsychotics (Slovenian translation) - FINAL VERSION
Pituitary Mass Effects
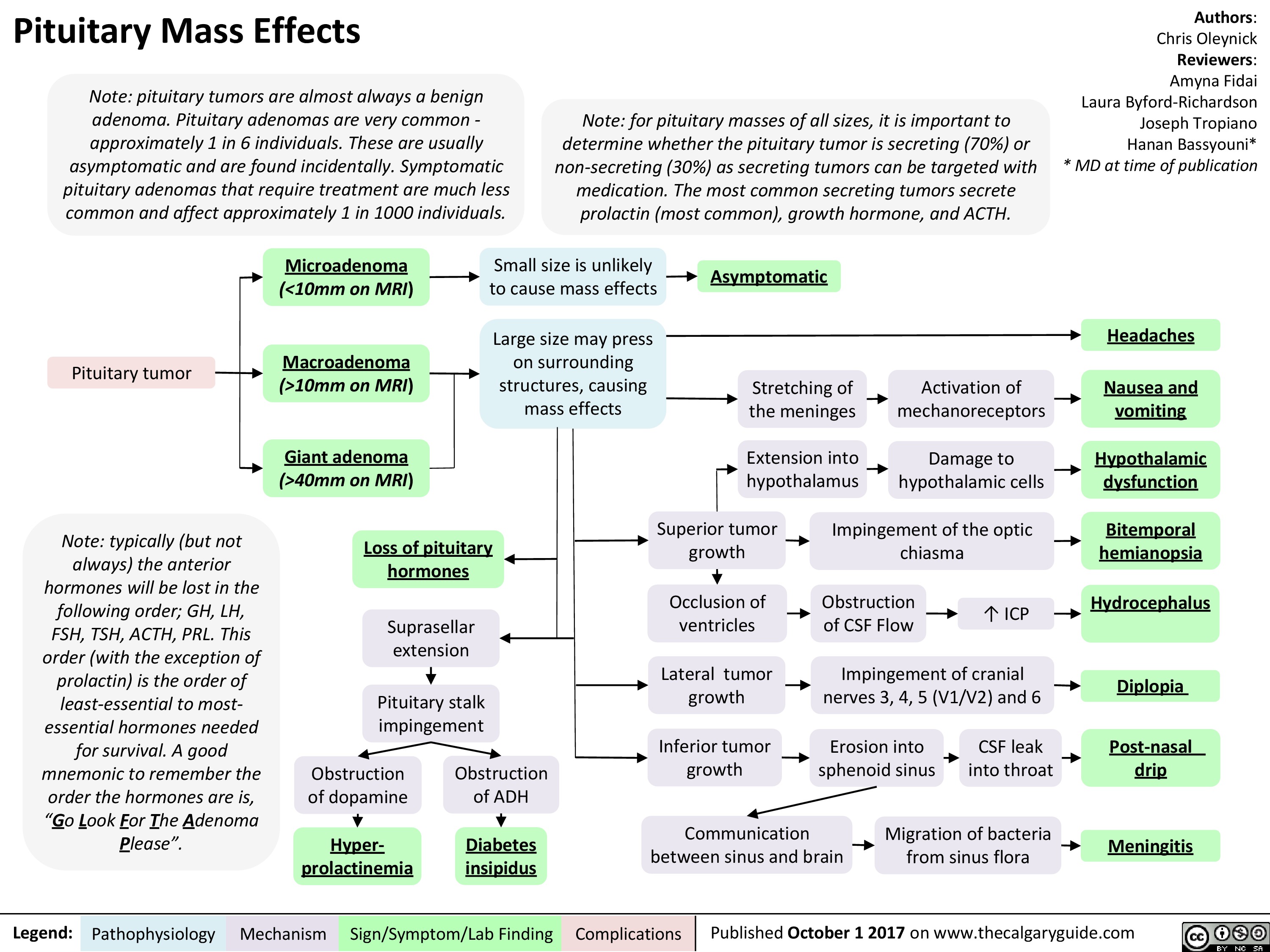 10mm on MRI) vomiting Giant adenoma Extension into hypothalamus —1■• Damage to hypothalamic cells Hypothalamic (>40mm on MRI) dysfunction Obstruction of dopamine Superior tumor growth Impingement of the optic chiasma Bitemporal Loss of pituitary hemianopsia hormones ICP Suprasellar extension Occlusion of ventricles Obstruction of CSF Flow Hydrocephalus Lateral tumor growth Impingement of cranial nerves 3, 4, 5 (V1/V2) and 6 4 Pituitary stalk impingement Diplopia Inferior tumor growth Erosion into sphenoid sinus CSF leak into throat Post-nasal Obstruction of ADH drip Communication between sinus and brain Migration of bacteria from sinus flora Hyper-Diabetes Meningitis prolactinemia insipidus
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Complications
Published October 1 2017 on www.thecalgaryguide.com
" title="Pituitary Mass Effects
Note: pituitary tumors are almost always a benign adenoma. Pituitary adenomas are very common -approximately 1 in 6 individuals. These are usually asymptomatic and are found incidentally. Symptomatic pituitary adenomas that require treatment are much less common and affect approximately 1 in 1000 individuals.
Pituitary tumor
Note: typically (but not always) the anterior hormones will be lost in the following order; GH, LH, FSH, TSH, ACTH, PRL. This order (with the exception of prolactin) is the order of least-essential to most-essential hormones needed for survival. A good mnemonic to remember the order the hormones are is, "Go Look For The Adenoma Please".
Legend:
Note: for pituitary masses of all sizes, it is important to determine whether the pituitary tumor is secreting (70%) or non-secreting (30%) as secreting tumors can be targeted with medication. The most common secreting tumors secrete prolactin (most common), growth hormone, and ACTH.
Authors: Chris Oleynick Reviewers: Amyna Fidai Laura Byford-Richardson Joseph Tropiano Hanan Bassyouni* * MD at time of publication
Microadenoma Small size is unlikely to cause mass effects (<10mm on MRI) Asymptomatic Macroadenoma Large size may press on surrounding structures, causing mass effects Headaches Stretching of the meninges Activation of mechanoreceptors Nausea and (>10mm on MRI) vomiting Giant adenoma Extension into hypothalamus —1■• Damage to hypothalamic cells Hypothalamic (>40mm on MRI) dysfunction Obstruction of dopamine Superior tumor growth Impingement of the optic chiasma Bitemporal Loss of pituitary hemianopsia hormones ICP Suprasellar extension Occlusion of ventricles Obstruction of CSF Flow Hydrocephalus Lateral tumor growth Impingement of cranial nerves 3, 4, 5 (V1/V2) and 6 4 Pituitary stalk impingement Diplopia Inferior tumor growth Erosion into sphenoid sinus CSF leak into throat Post-nasal Obstruction of ADH drip Communication between sinus and brain Migration of bacteria from sinus flora Hyper-Diabetes Meningitis prolactinemia insipidus
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Complications
Published October 1 2017 on www.thecalgaryguide.com
" />
10mm on MRI) vomiting Giant adenoma Extension into hypothalamus —1■• Damage to hypothalamic cells Hypothalamic (>40mm on MRI) dysfunction Obstruction of dopamine Superior tumor growth Impingement of the optic chiasma Bitemporal Loss of pituitary hemianopsia hormones ICP Suprasellar extension Occlusion of ventricles Obstruction of CSF Flow Hydrocephalus Lateral tumor growth Impingement of cranial nerves 3, 4, 5 (V1/V2) and 6 4 Pituitary stalk impingement Diplopia Inferior tumor growth Erosion into sphenoid sinus CSF leak into throat Post-nasal Obstruction of ADH drip Communication between sinus and brain Migration of bacteria from sinus flora Hyper-Diabetes Meningitis prolactinemia insipidus
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Complications
Published October 1 2017 on www.thecalgaryguide.com
" title="Pituitary Mass Effects
Note: pituitary tumors are almost always a benign adenoma. Pituitary adenomas are very common -approximately 1 in 6 individuals. These are usually asymptomatic and are found incidentally. Symptomatic pituitary adenomas that require treatment are much less common and affect approximately 1 in 1000 individuals.
Pituitary tumor
Note: typically (but not always) the anterior hormones will be lost in the following order; GH, LH, FSH, TSH, ACTH, PRL. This order (with the exception of prolactin) is the order of least-essential to most-essential hormones needed for survival. A good mnemonic to remember the order the hormones are is, "Go Look For The Adenoma Please".
Legend:
Note: for pituitary masses of all sizes, it is important to determine whether the pituitary tumor is secreting (70%) or non-secreting (30%) as secreting tumors can be targeted with medication. The most common secreting tumors secrete prolactin (most common), growth hormone, and ACTH.
Authors: Chris Oleynick Reviewers: Amyna Fidai Laura Byford-Richardson Joseph Tropiano Hanan Bassyouni* * MD at time of publication
Microadenoma Small size is unlikely to cause mass effects (<10mm on MRI) Asymptomatic Macroadenoma Large size may press on surrounding structures, causing mass effects Headaches Stretching of the meninges Activation of mechanoreceptors Nausea and (>10mm on MRI) vomiting Giant adenoma Extension into hypothalamus —1■• Damage to hypothalamic cells Hypothalamic (>40mm on MRI) dysfunction Obstruction of dopamine Superior tumor growth Impingement of the optic chiasma Bitemporal Loss of pituitary hemianopsia hormones ICP Suprasellar extension Occlusion of ventricles Obstruction of CSF Flow Hydrocephalus Lateral tumor growth Impingement of cranial nerves 3, 4, 5 (V1/V2) and 6 4 Pituitary stalk impingement Diplopia Inferior tumor growth Erosion into sphenoid sinus CSF leak into throat Post-nasal Obstruction of ADH drip Communication between sinus and brain Migration of bacteria from sinus flora Hyper-Diabetes Meningitis prolactinemia insipidus
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Complications
Published October 1 2017 on www.thecalgaryguide.com
" />
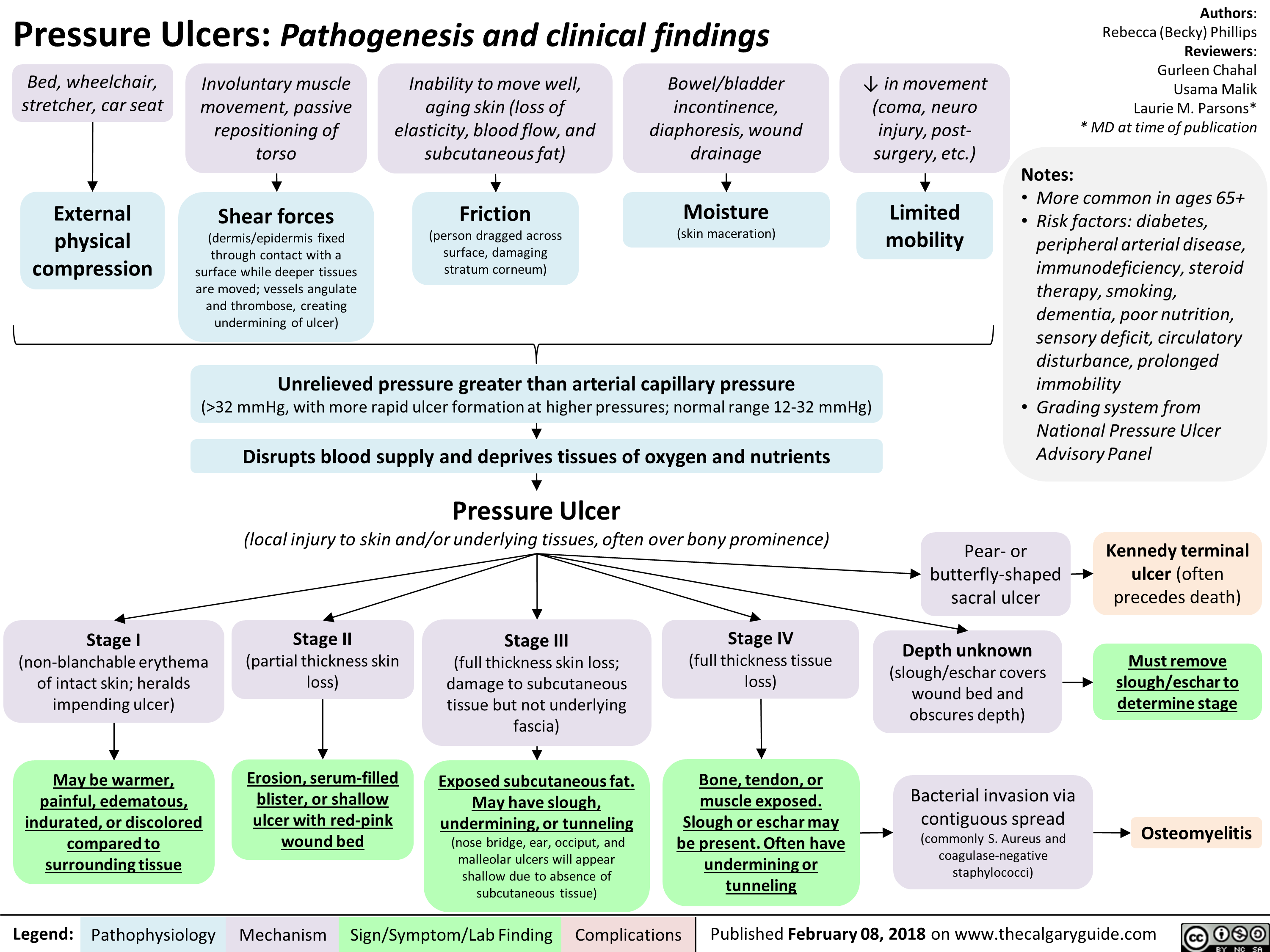
Pressure Ulcers Pathogenesis and clinical findings
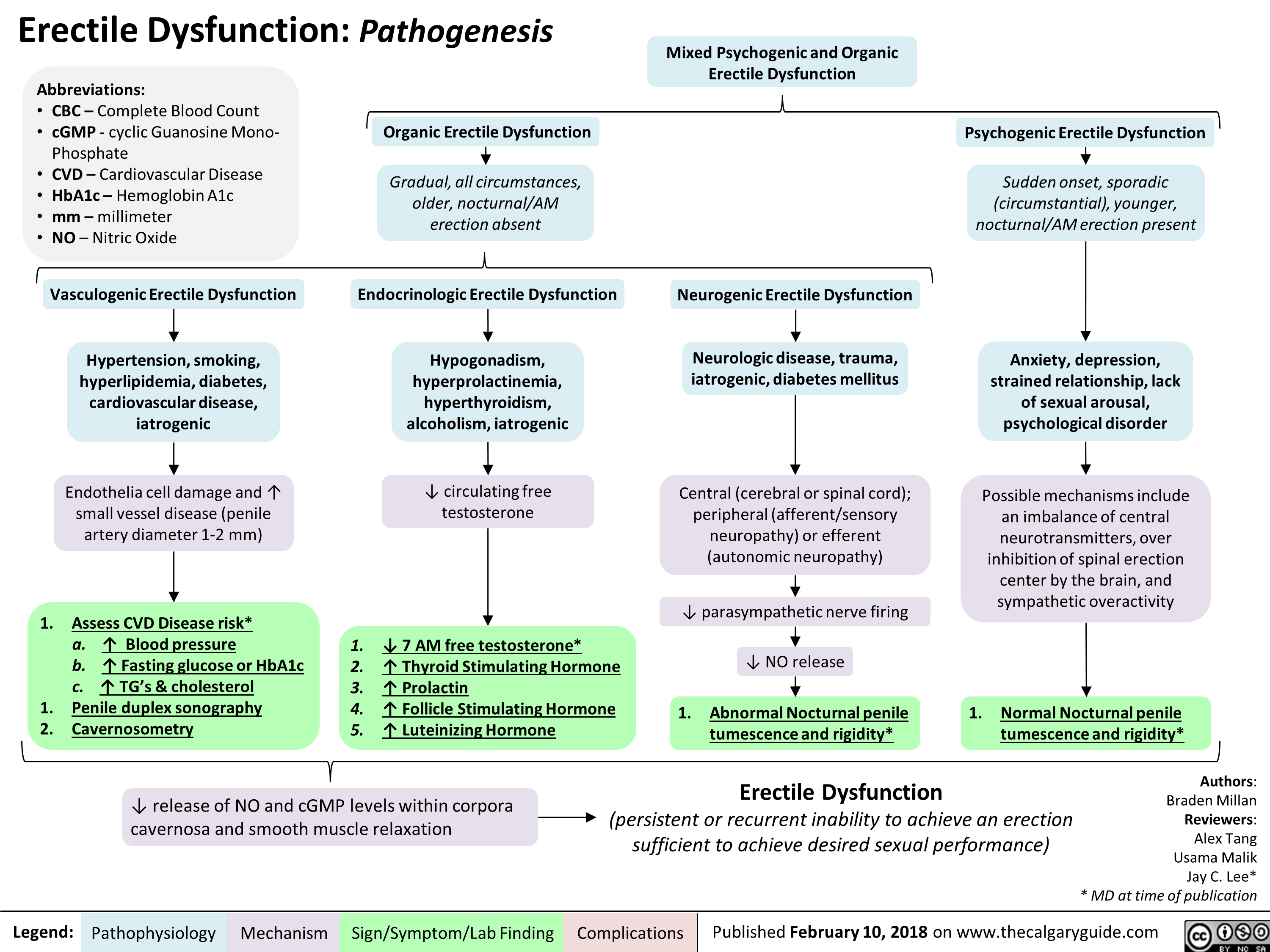
Erectile Dysfunction: Pathogenesis
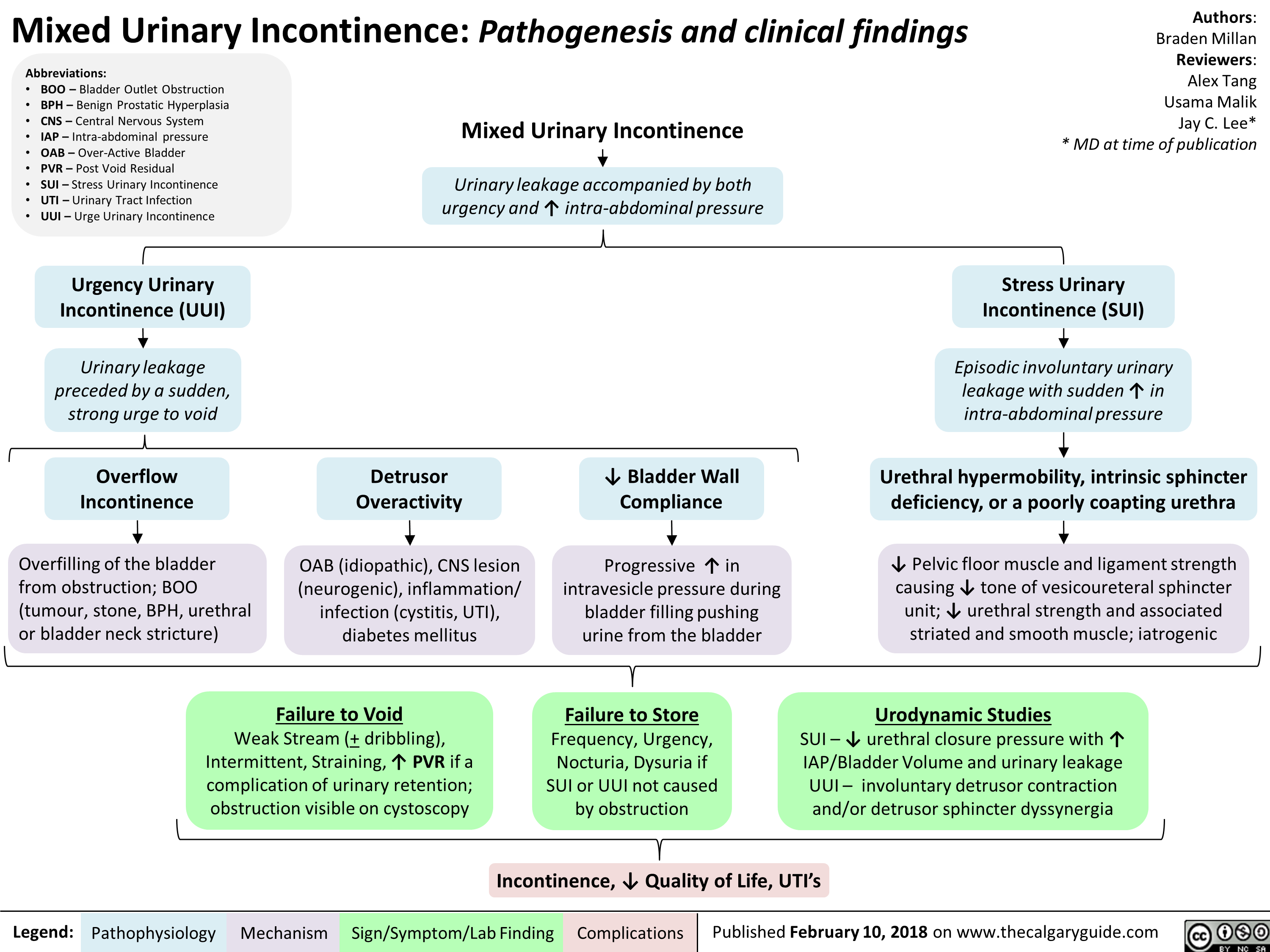
Mixed Urinary Incontinence Pathogenesis and clinical findings
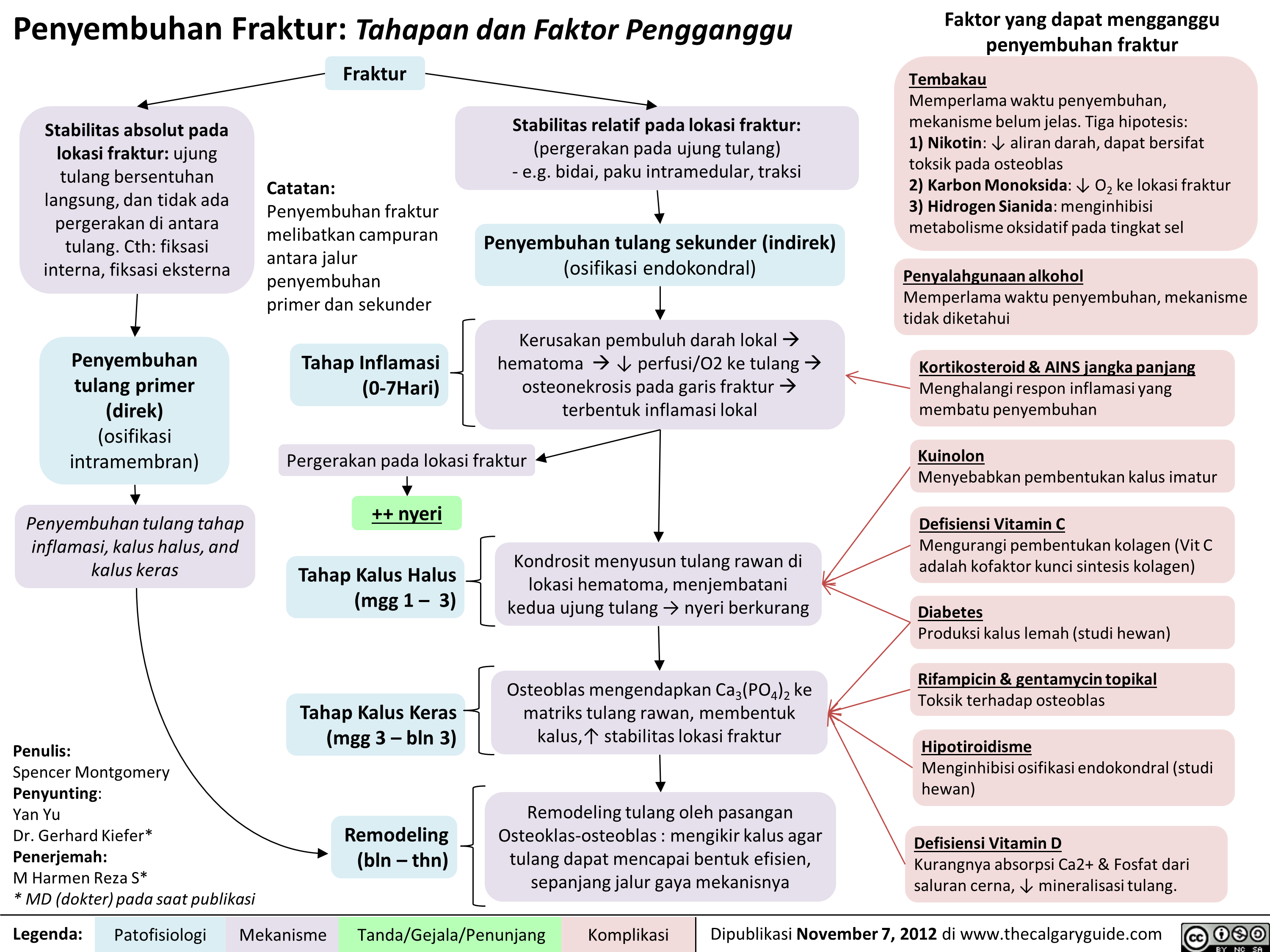
Penyembuhan Fraktur: Tahapan dan Faktor Pengganggu
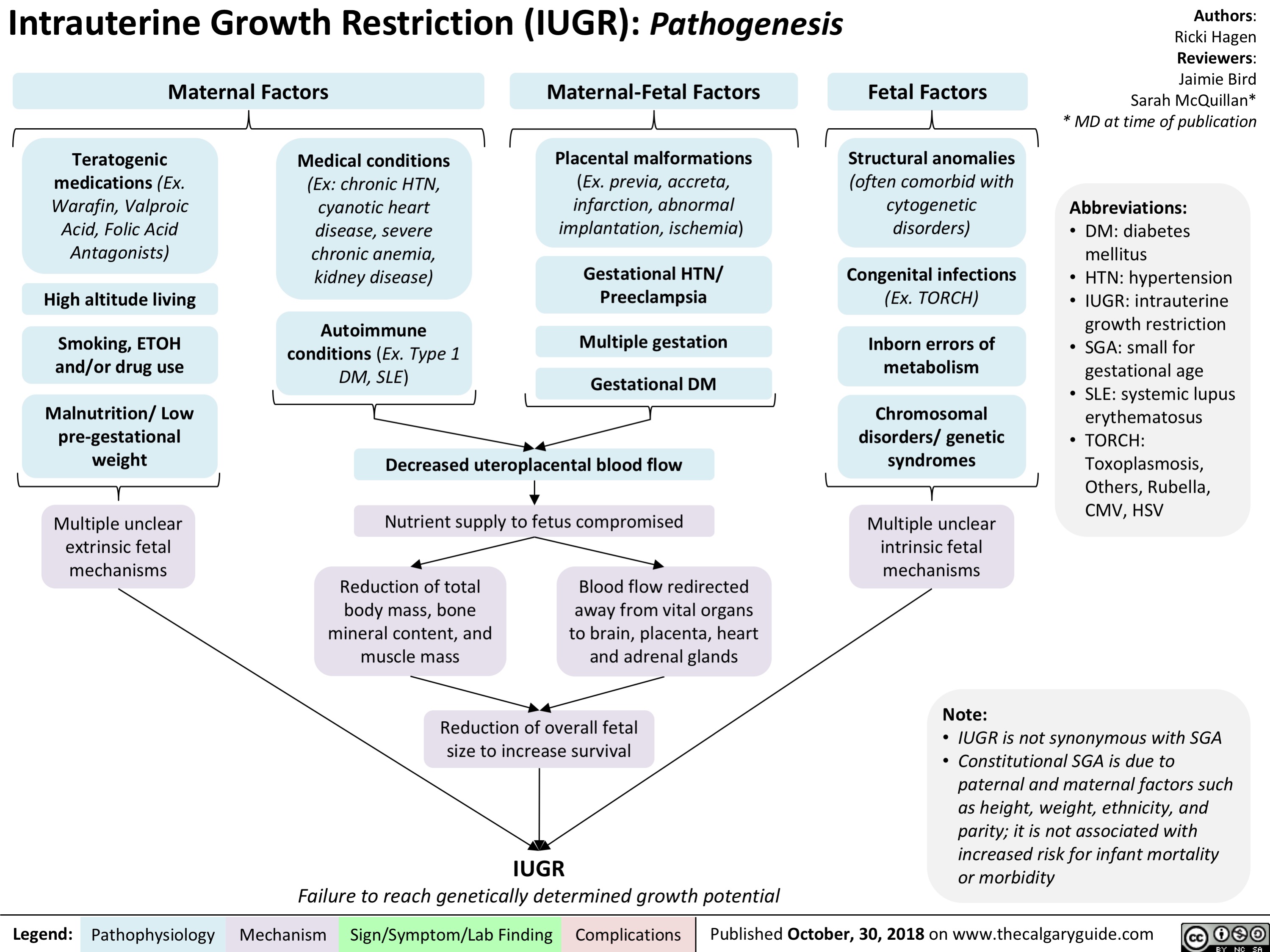
intrauterine-growth-restriction-iugr-pathogenesis
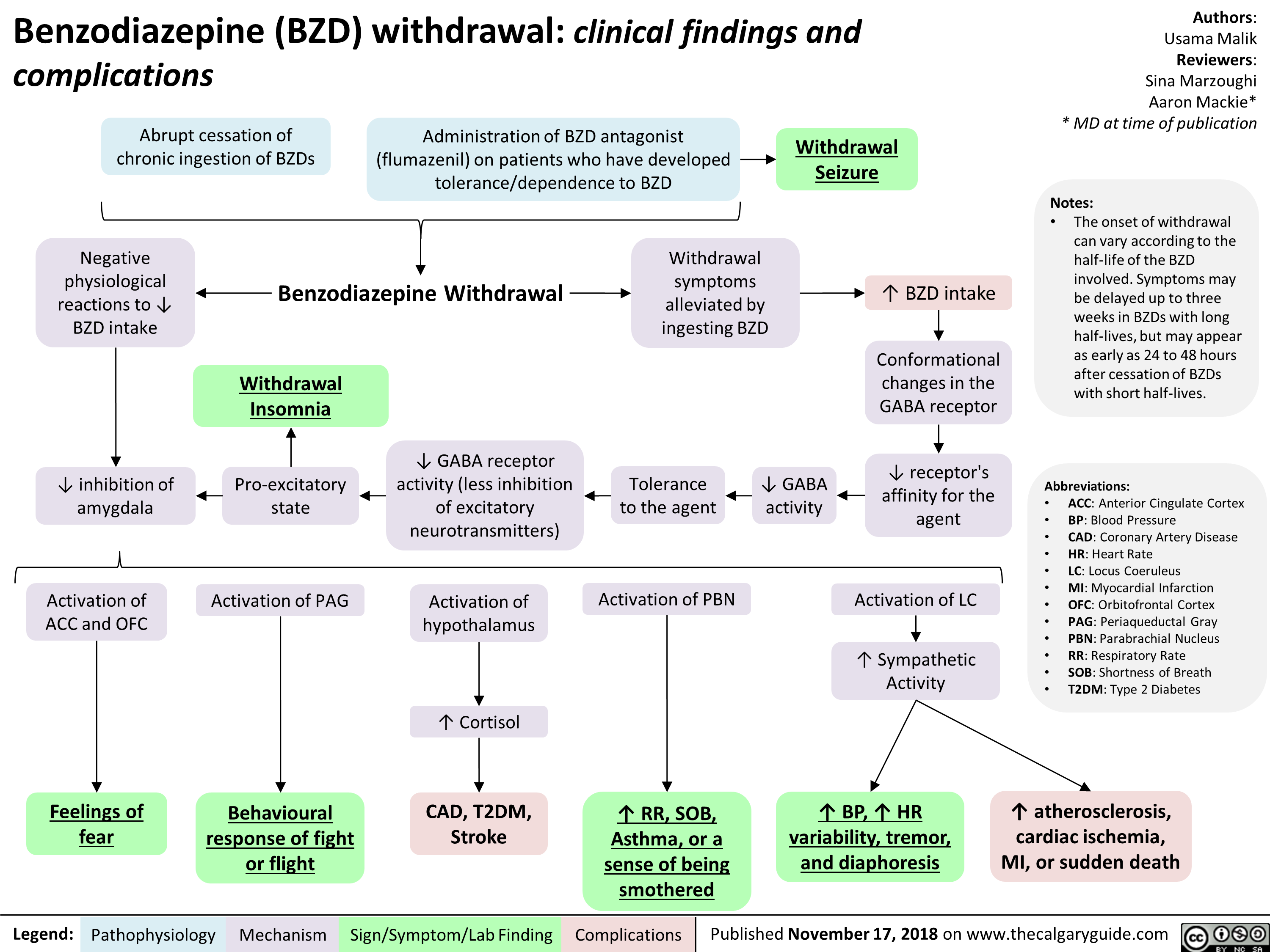
Benzodiazepine (BZD) withdrawal: clinical findings and complications
Hypernatremia Physiology
![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)
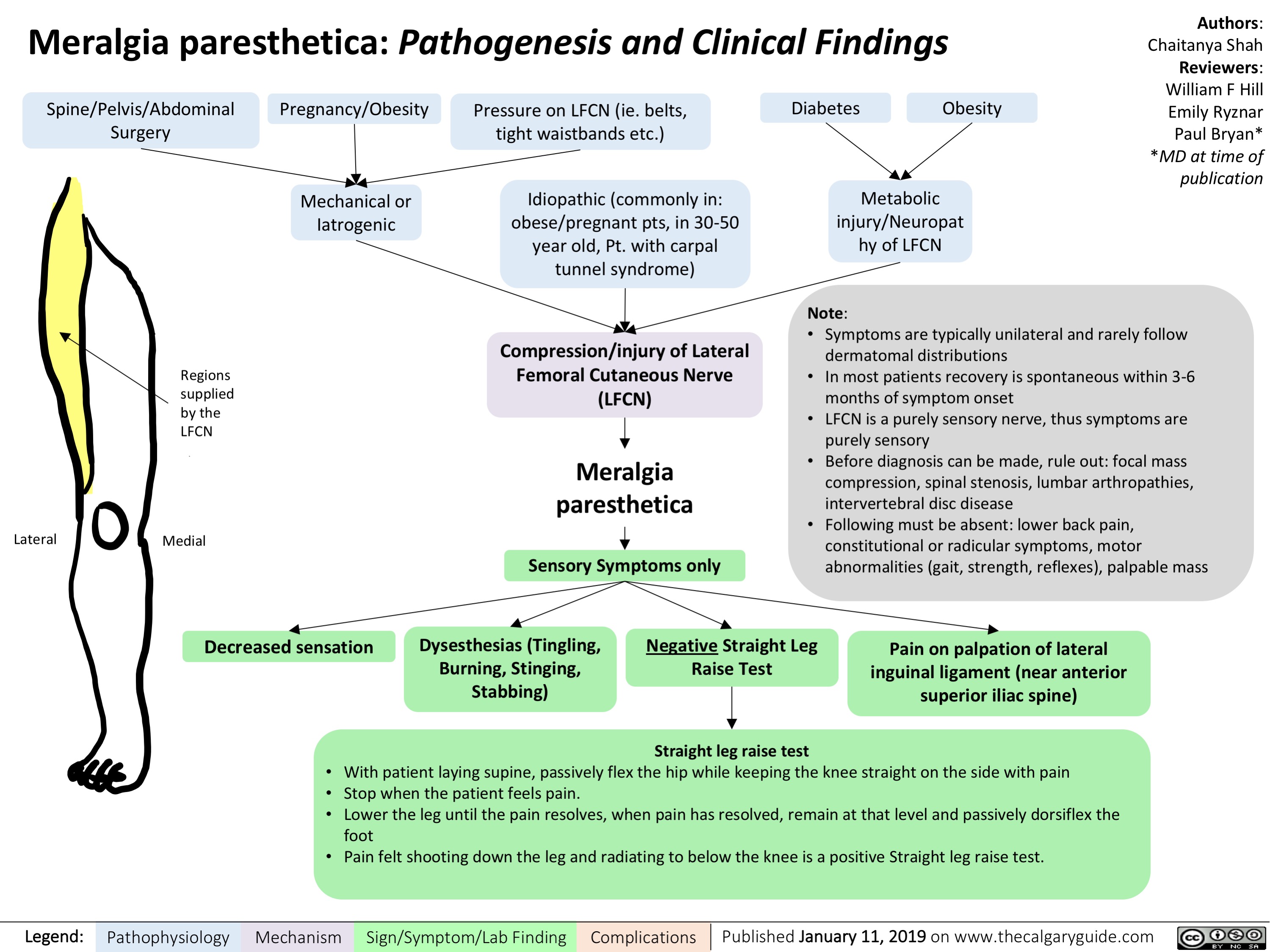
Meralgia paresthetica- Pathogenesis and Clinical Findings
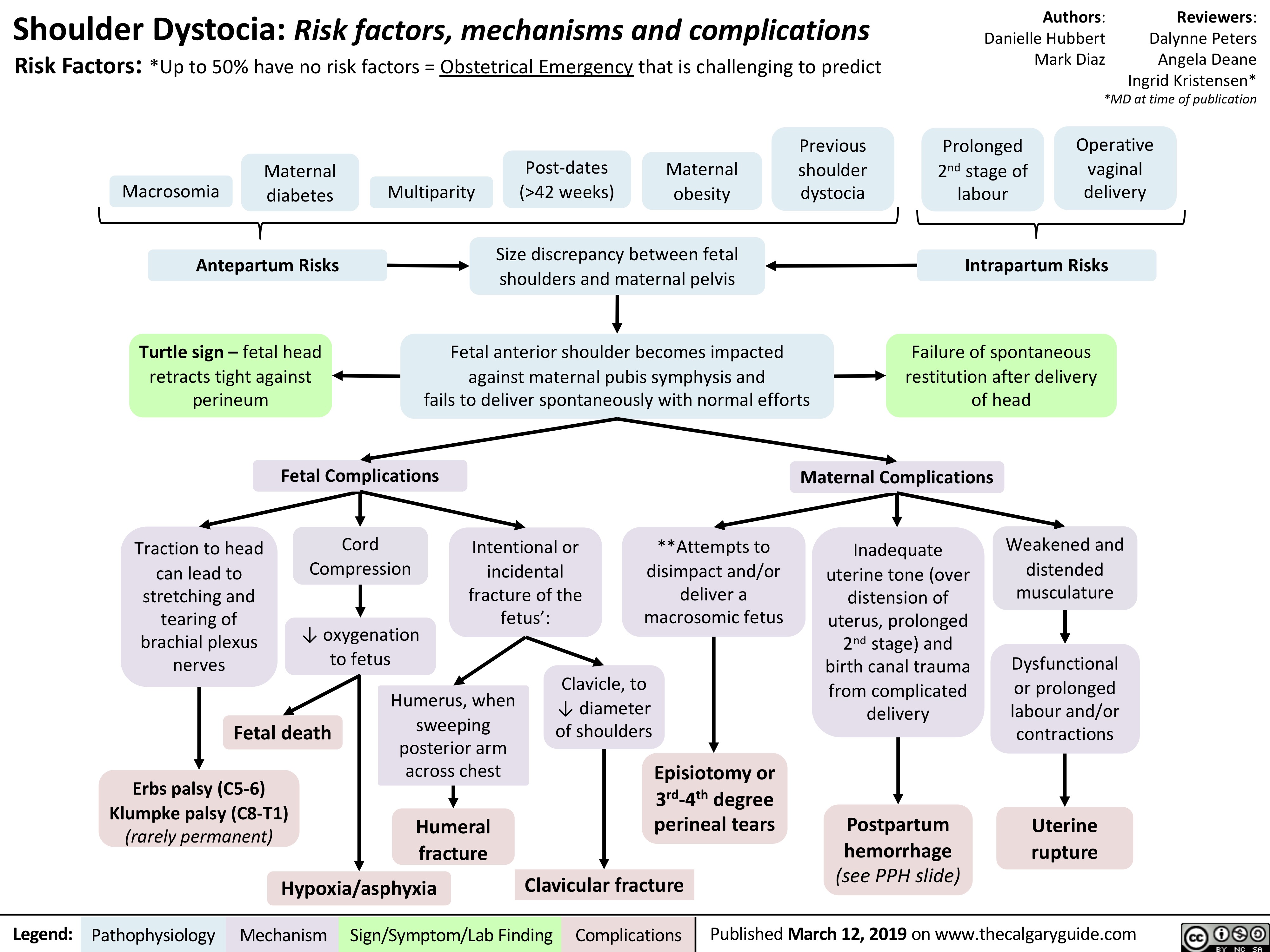
Shoulder Dystocia: Complications
Small Bowel Infarction
acanthosis-nigricans-pathogenesis-and-clinical-findings
Macrosomia-Fetal-Complications
Incisional-Hernia
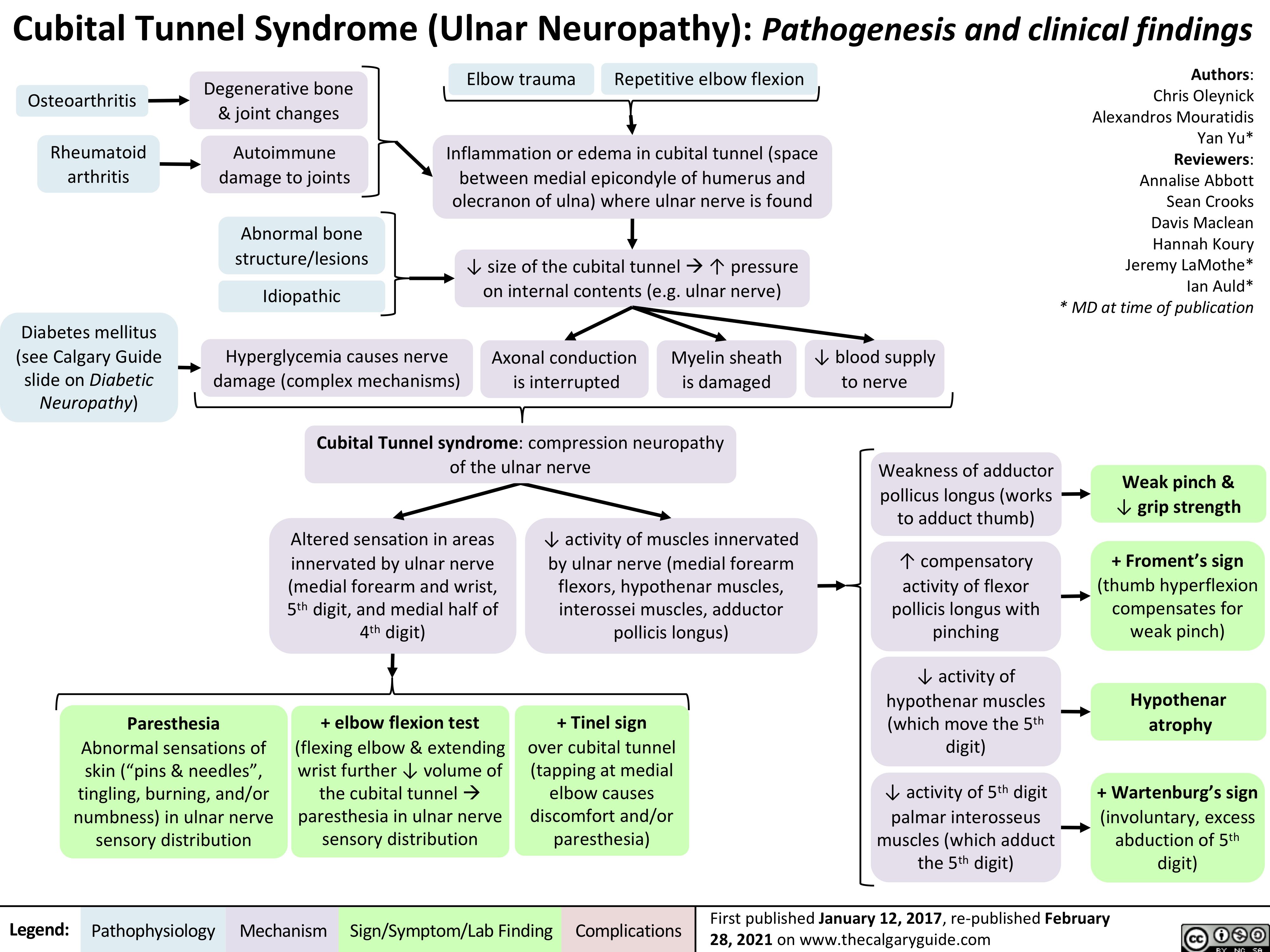
vomiting-pathogenesis
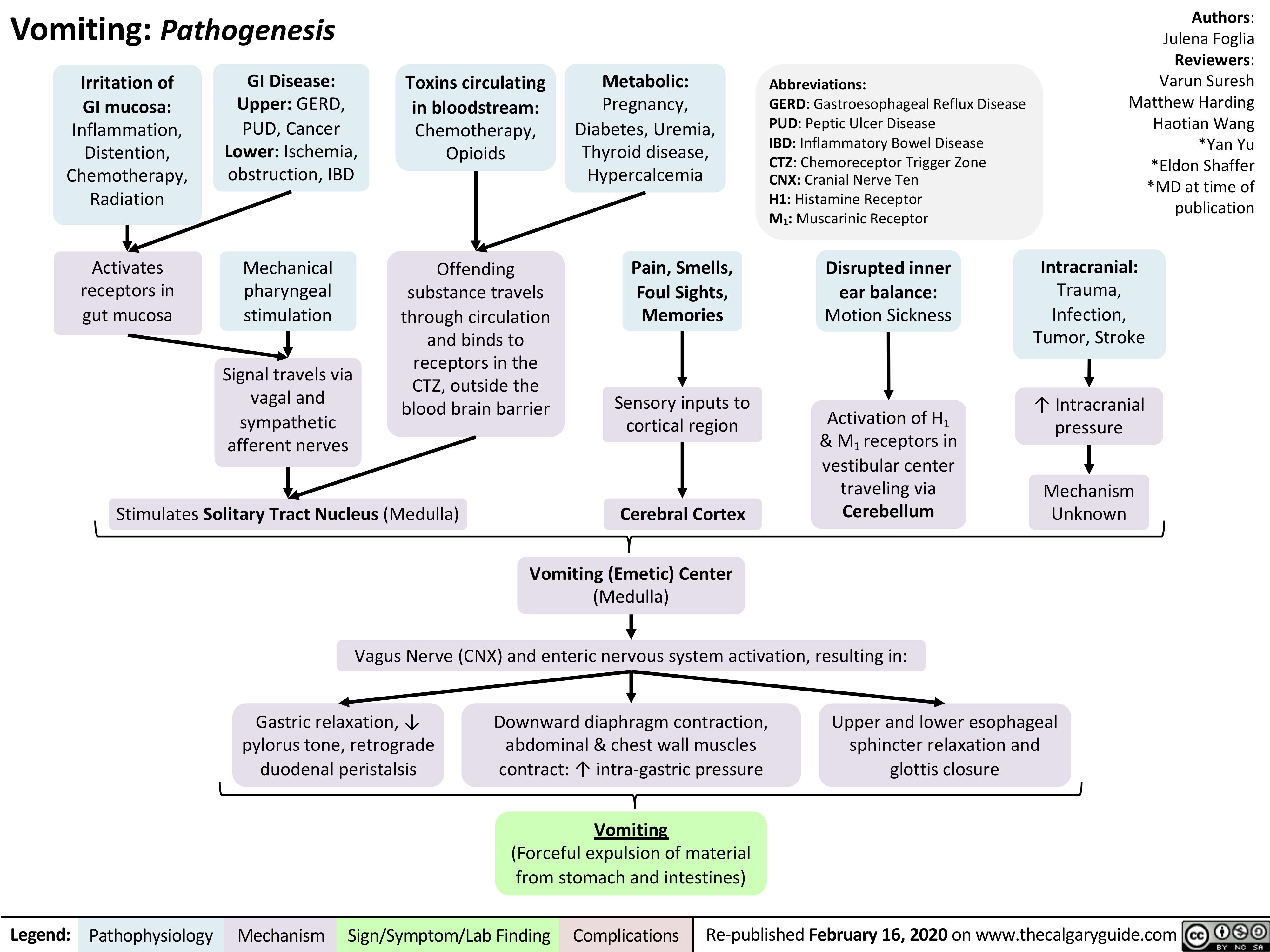
Diabetic-Nephropathy
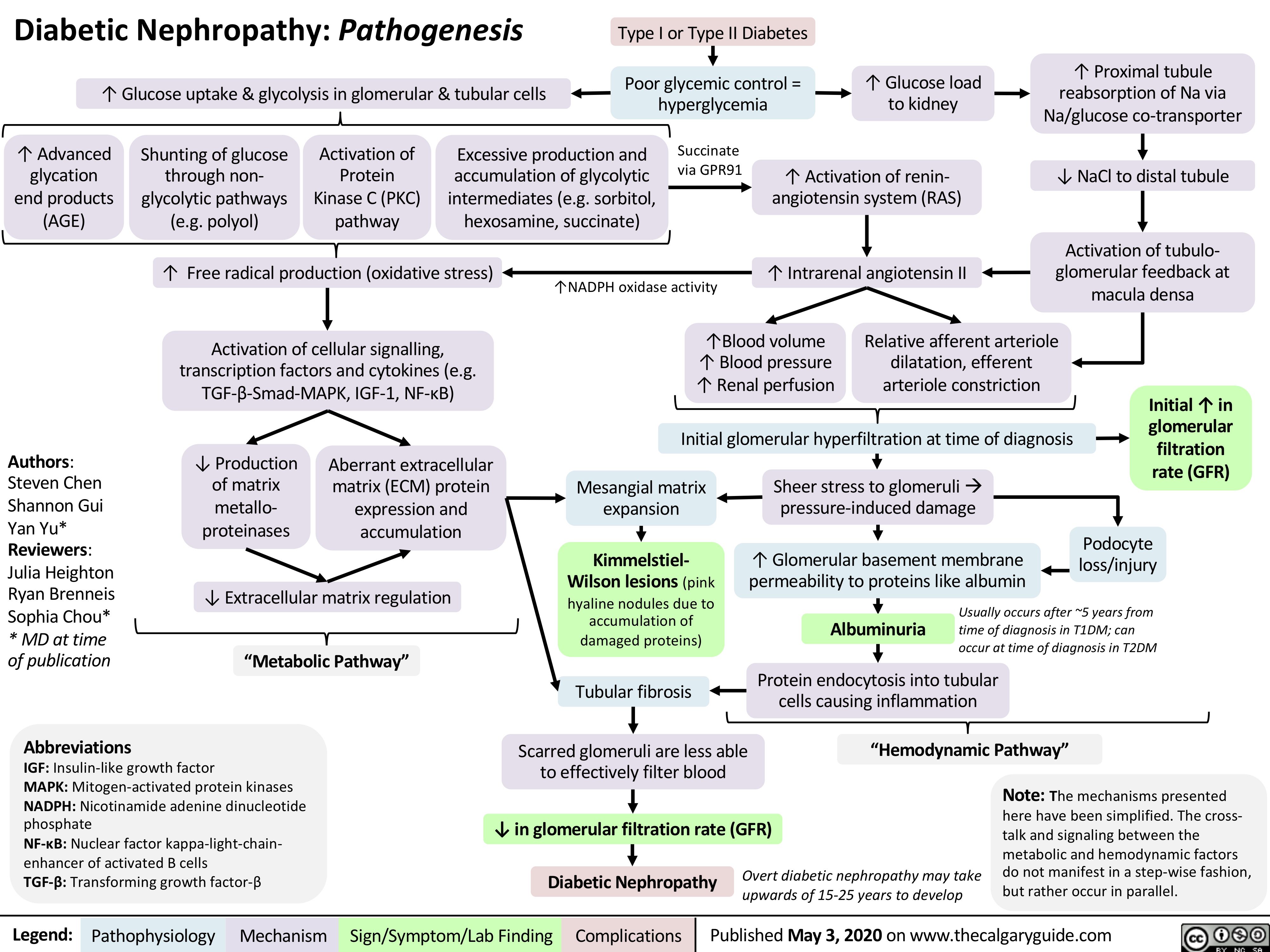
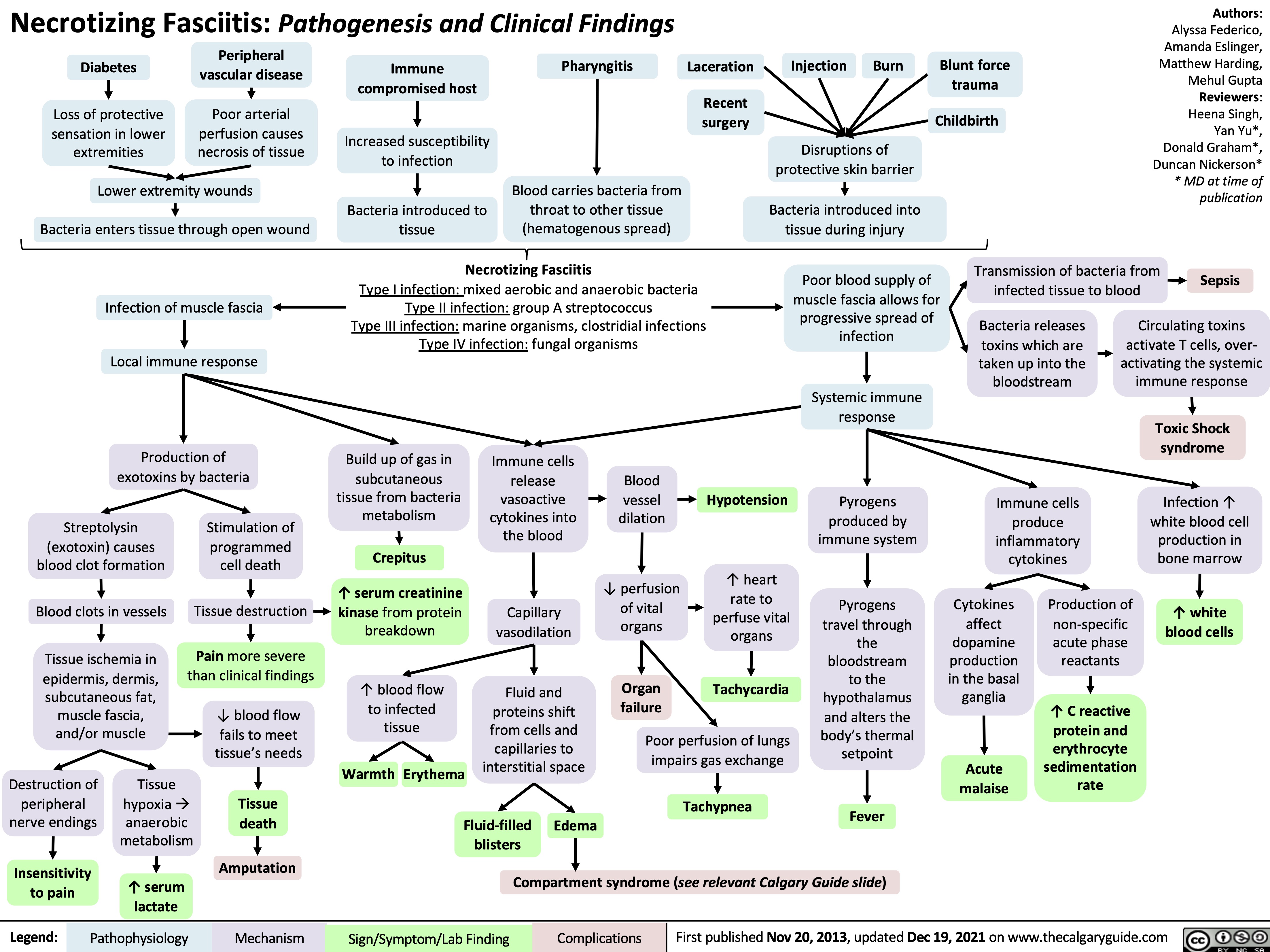
Cellulitis
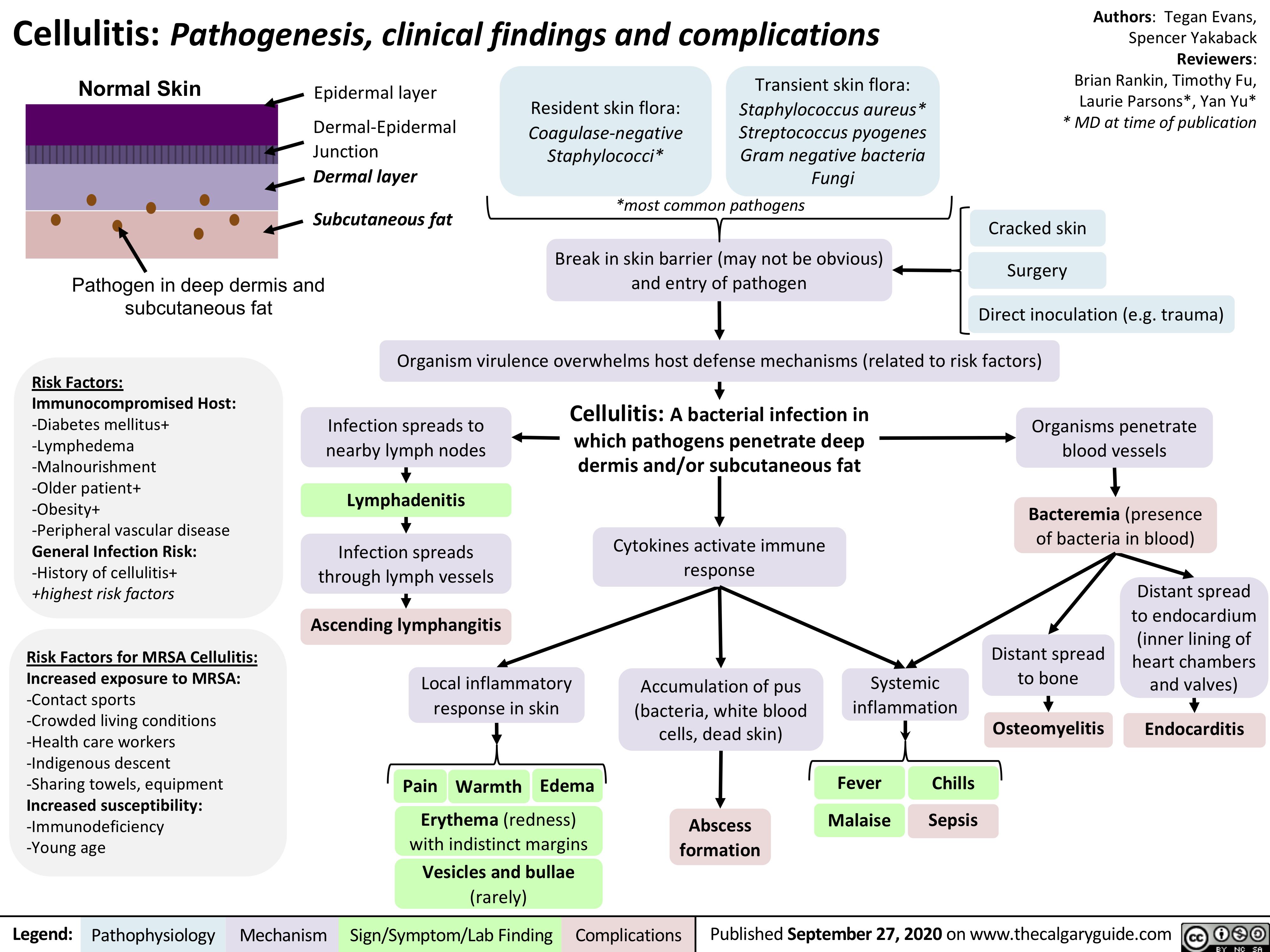
Cubital-Tunnel-Syndrome-Ulnar-Neuropathy
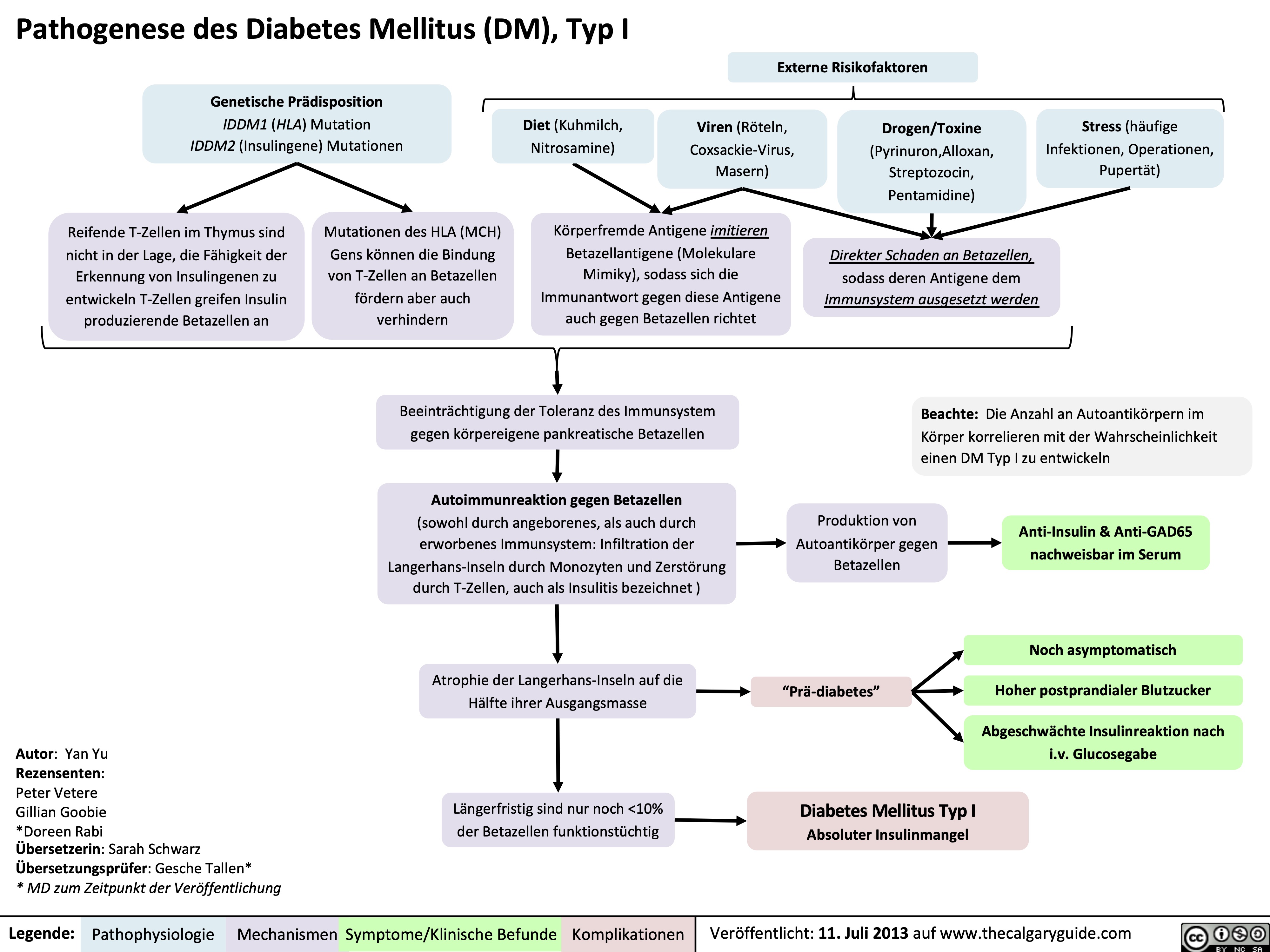
Corneal-Abrasion
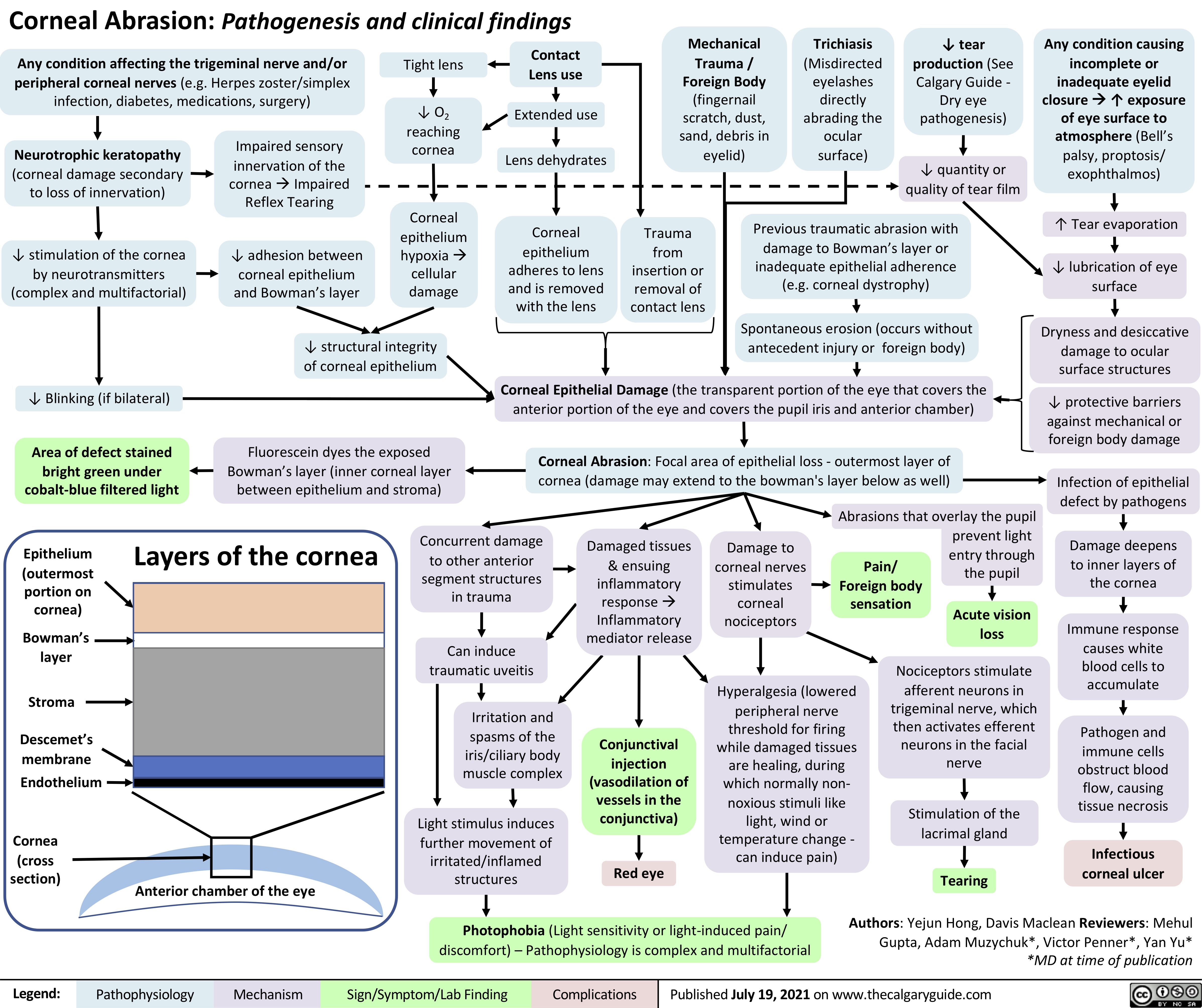
Fat-Embolism-Syndrome
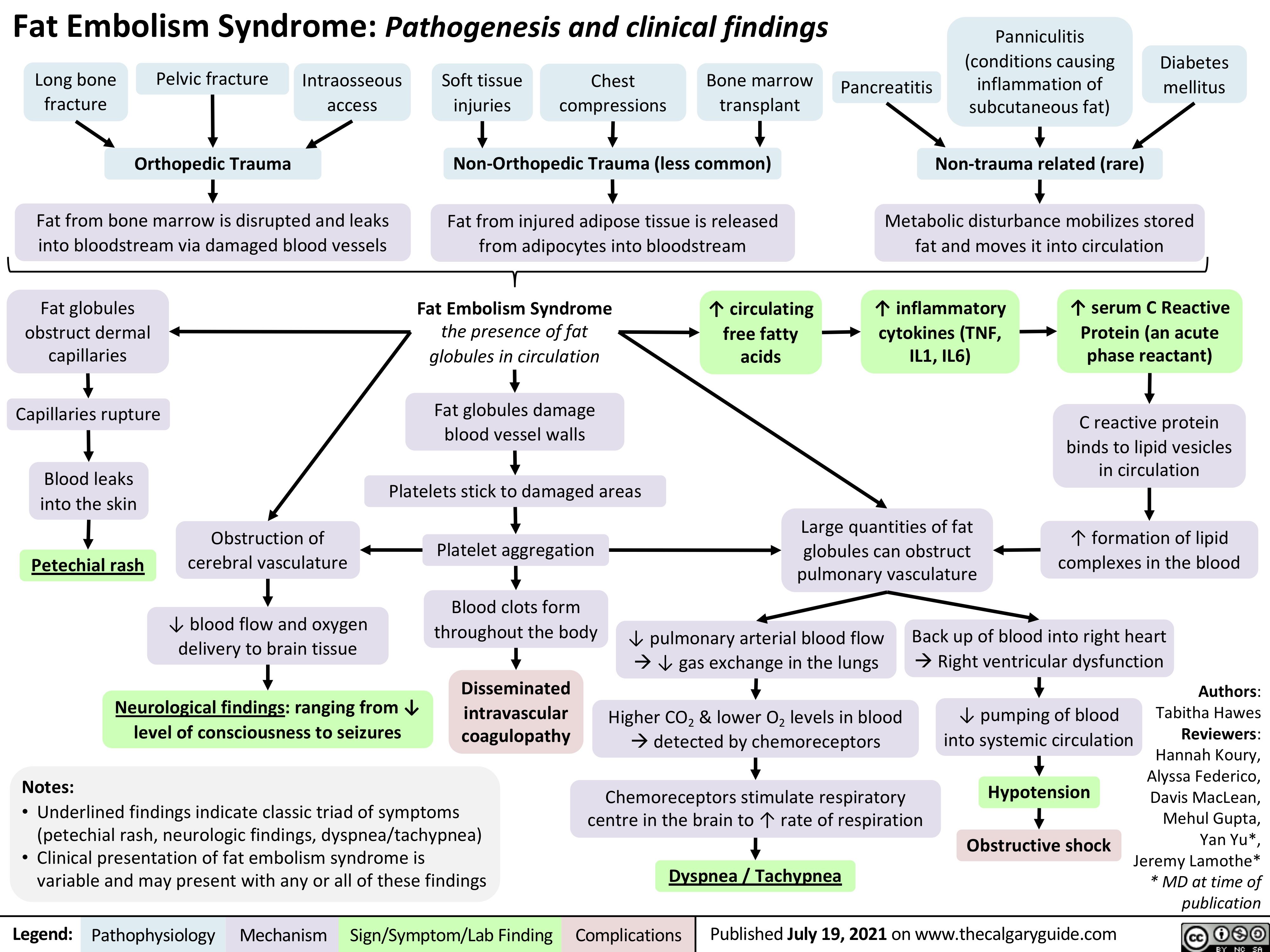
Pathogenese des Diabetes Mellitus (DM), Typ II
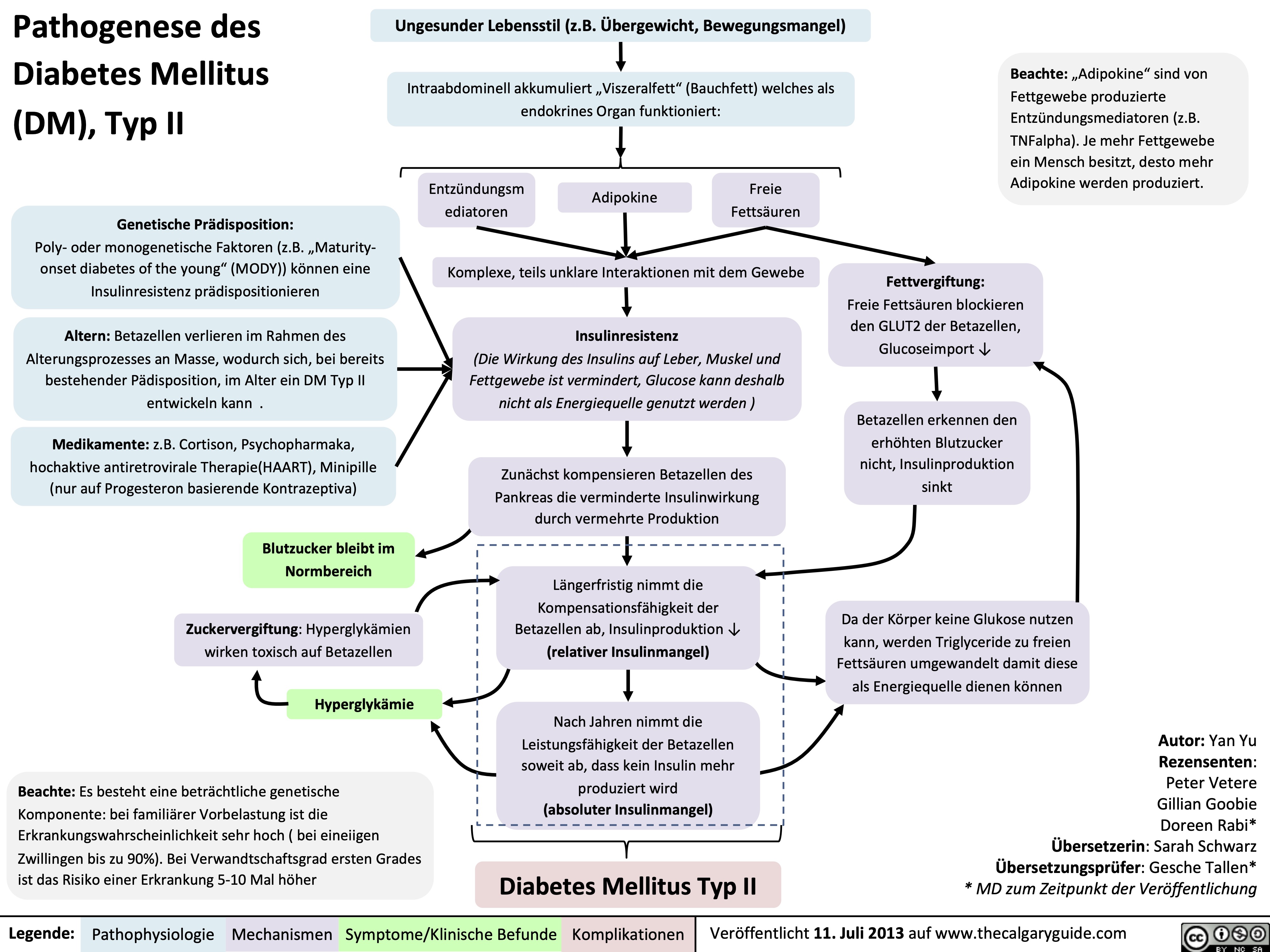
Pathogenese des Diabetes Mellitus (DM), Typ I
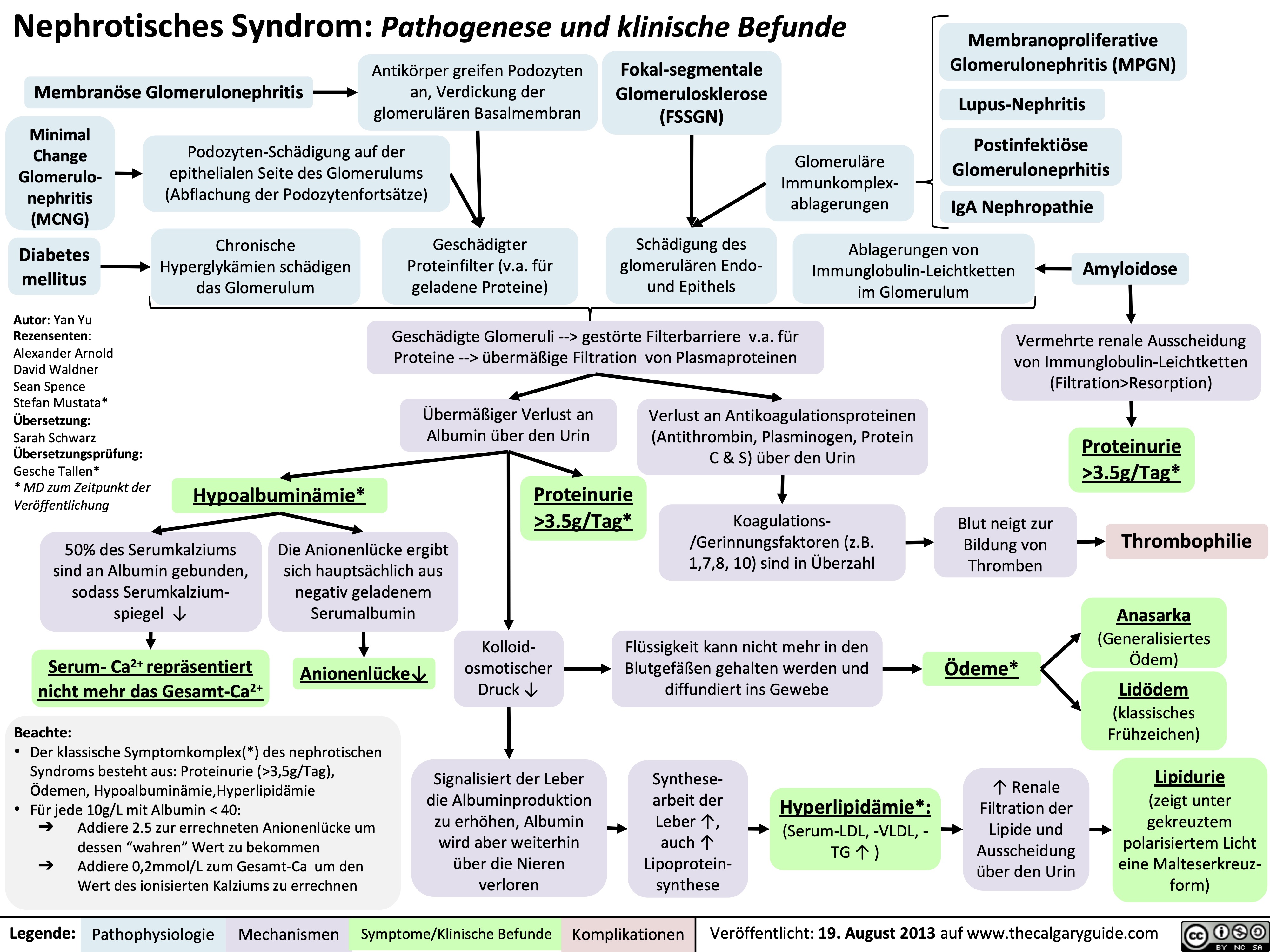
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
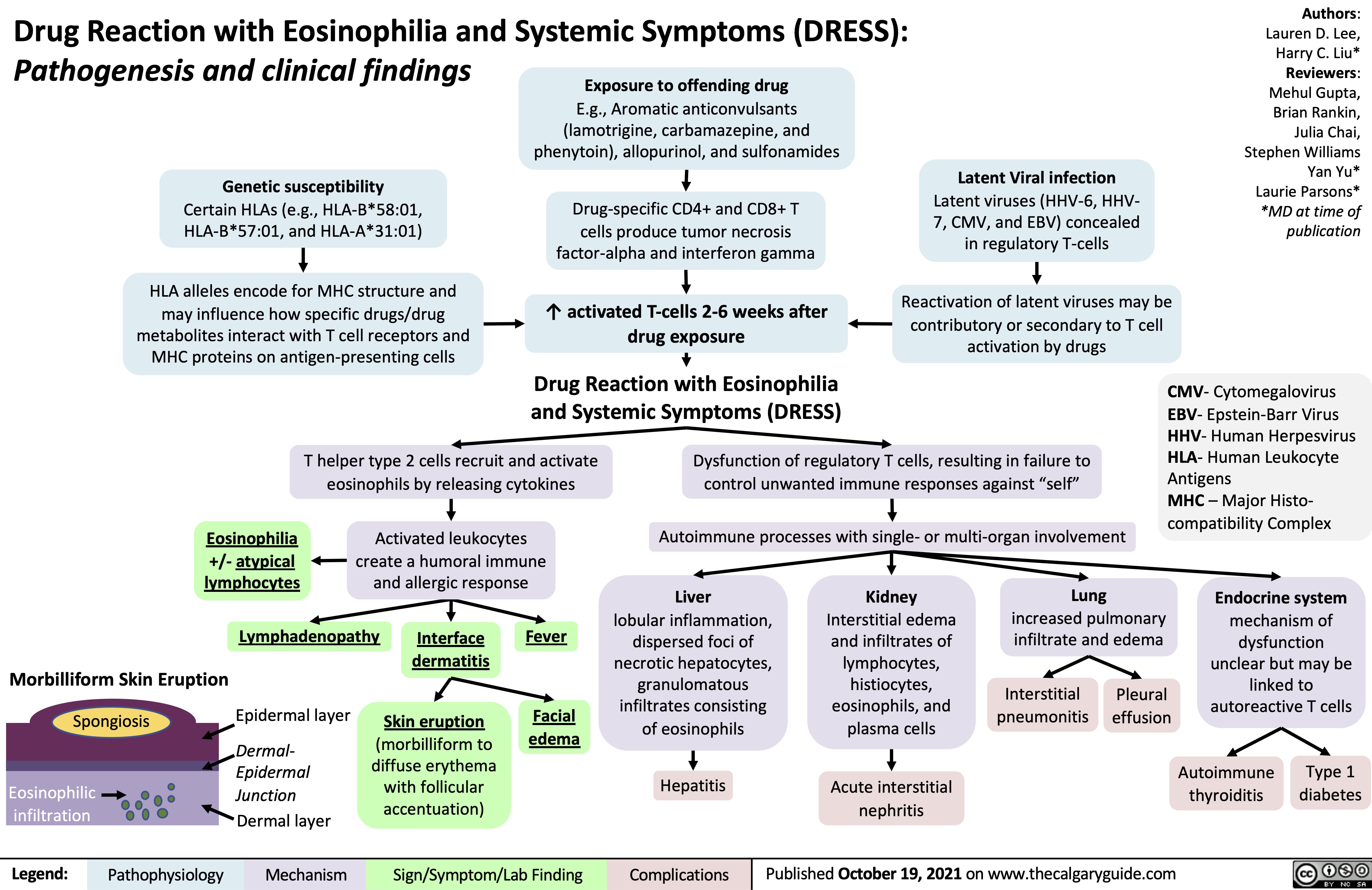
Diabetische Ketoazidose (DKA)
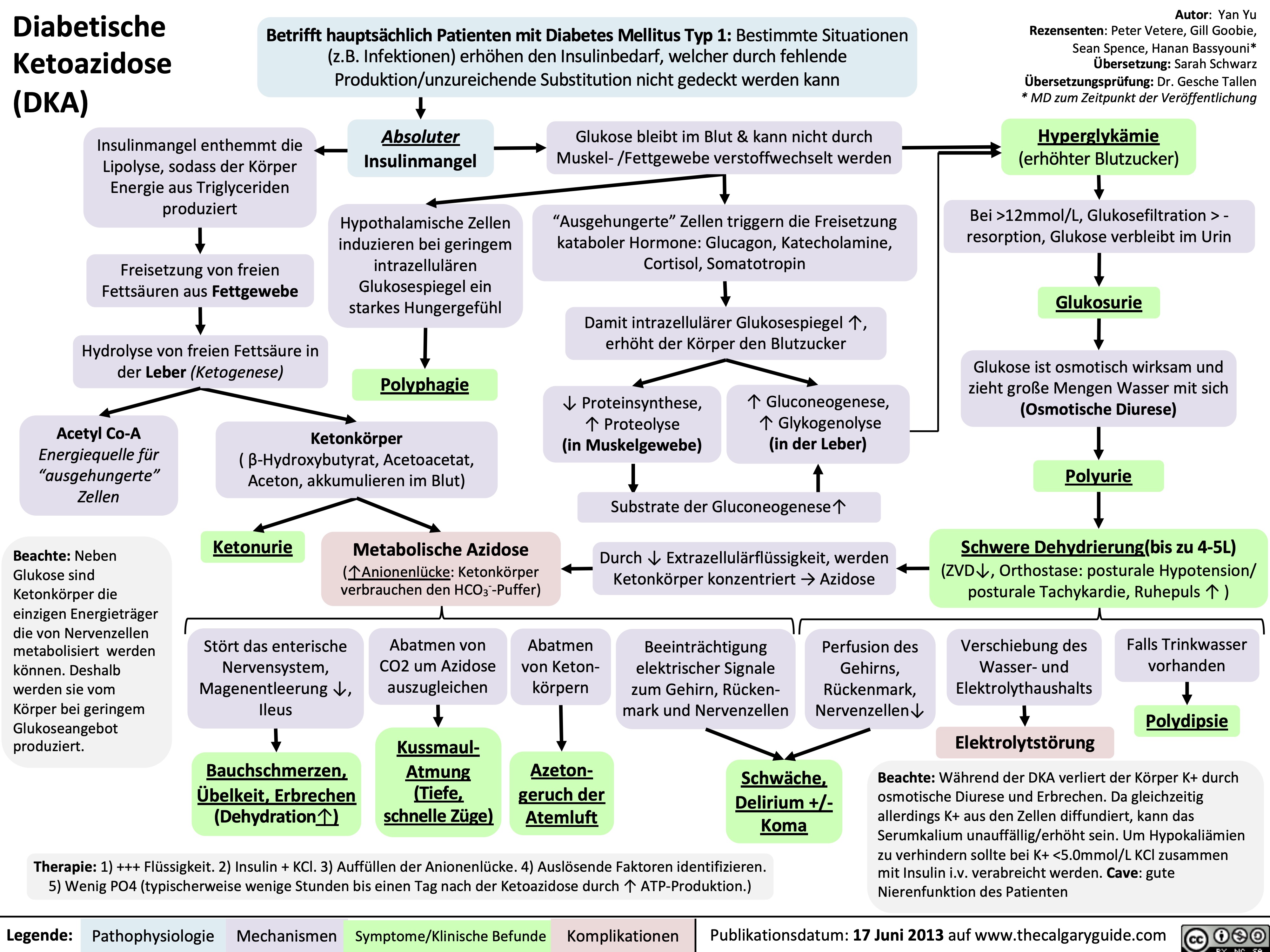
Hyperosmolares/ Hyperglykämisches Koma (HHS)
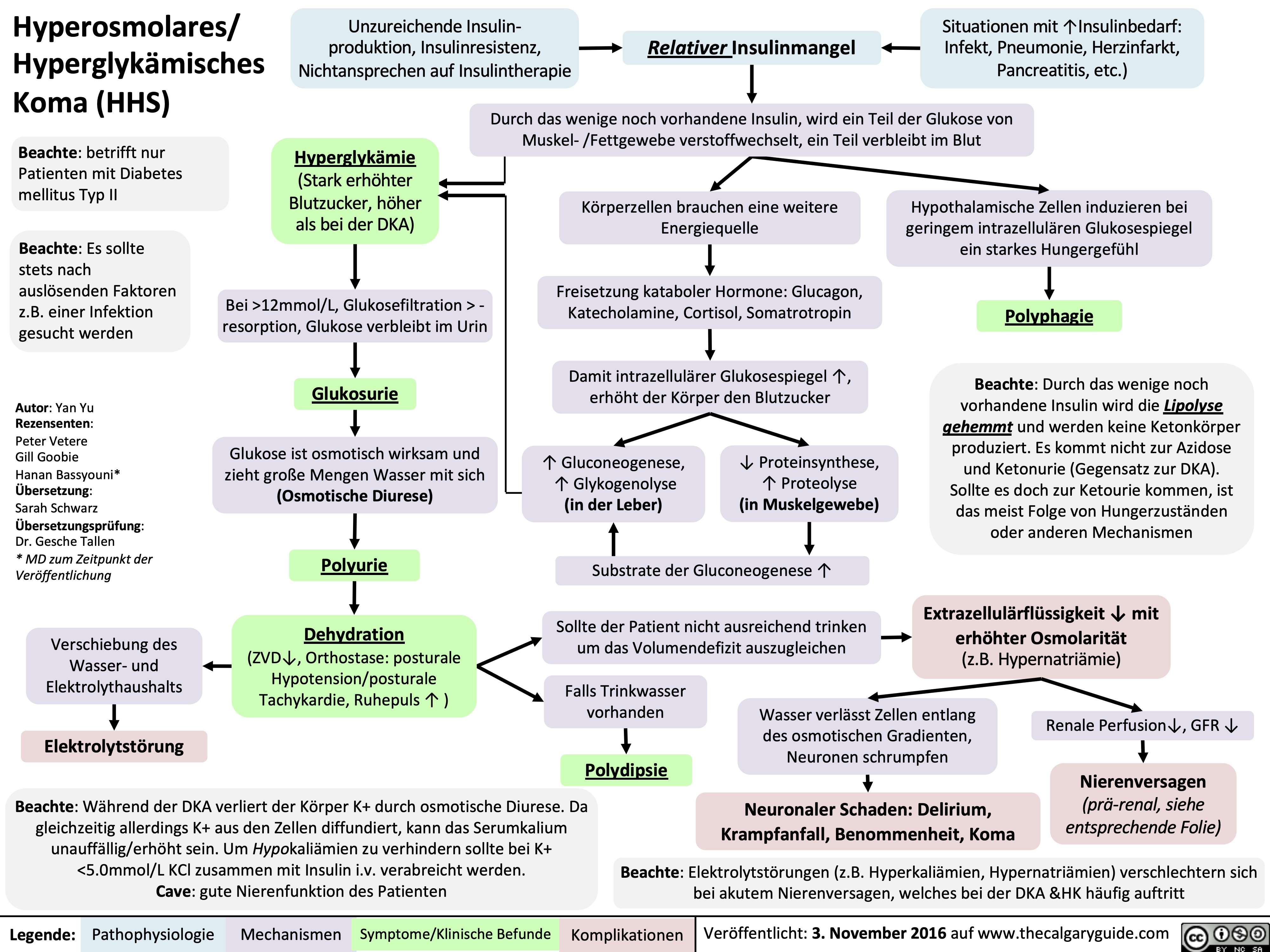
Nephrotisches Syndrom: Pathogenese und klinische Befunde
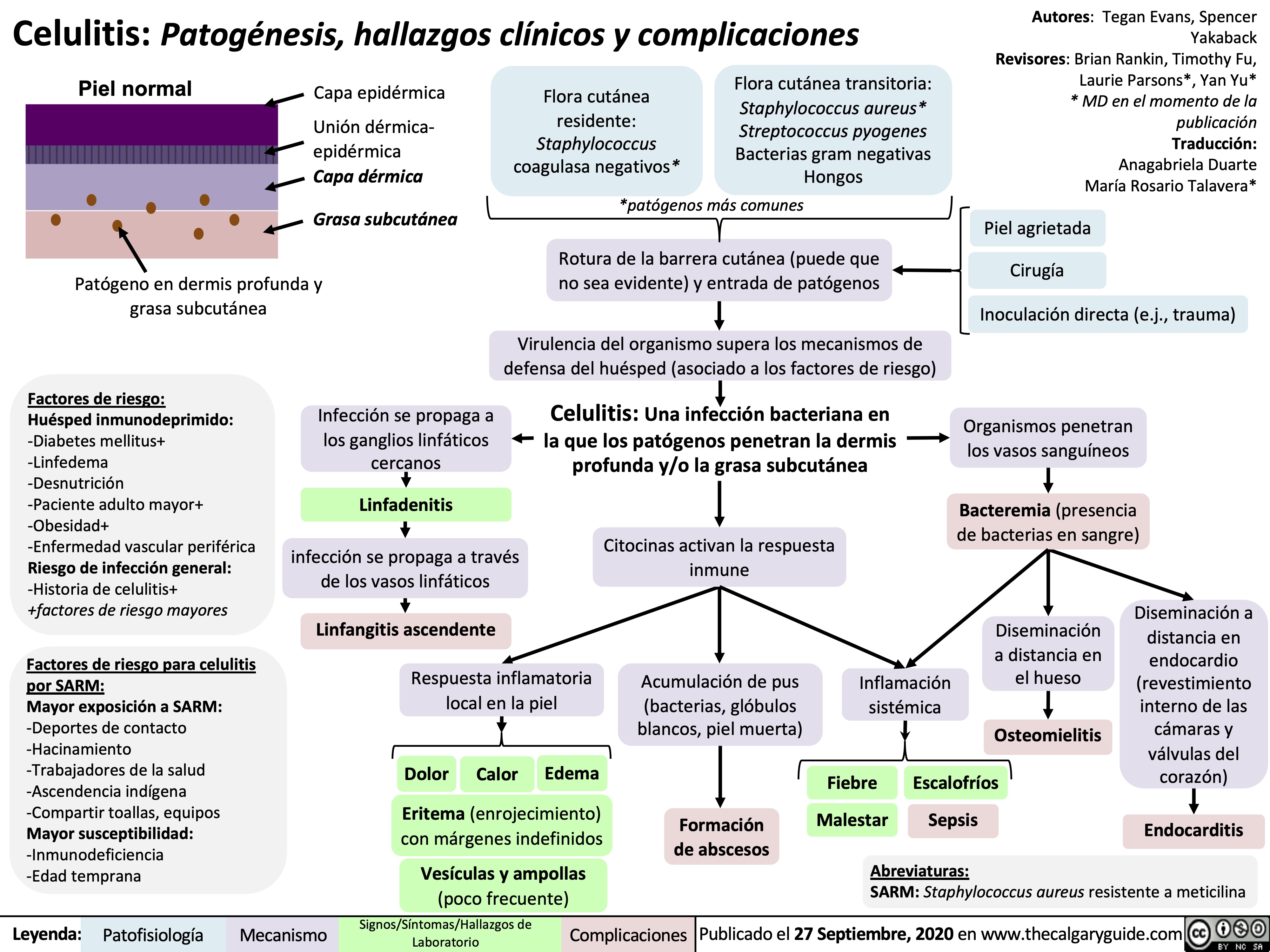
Celulitis
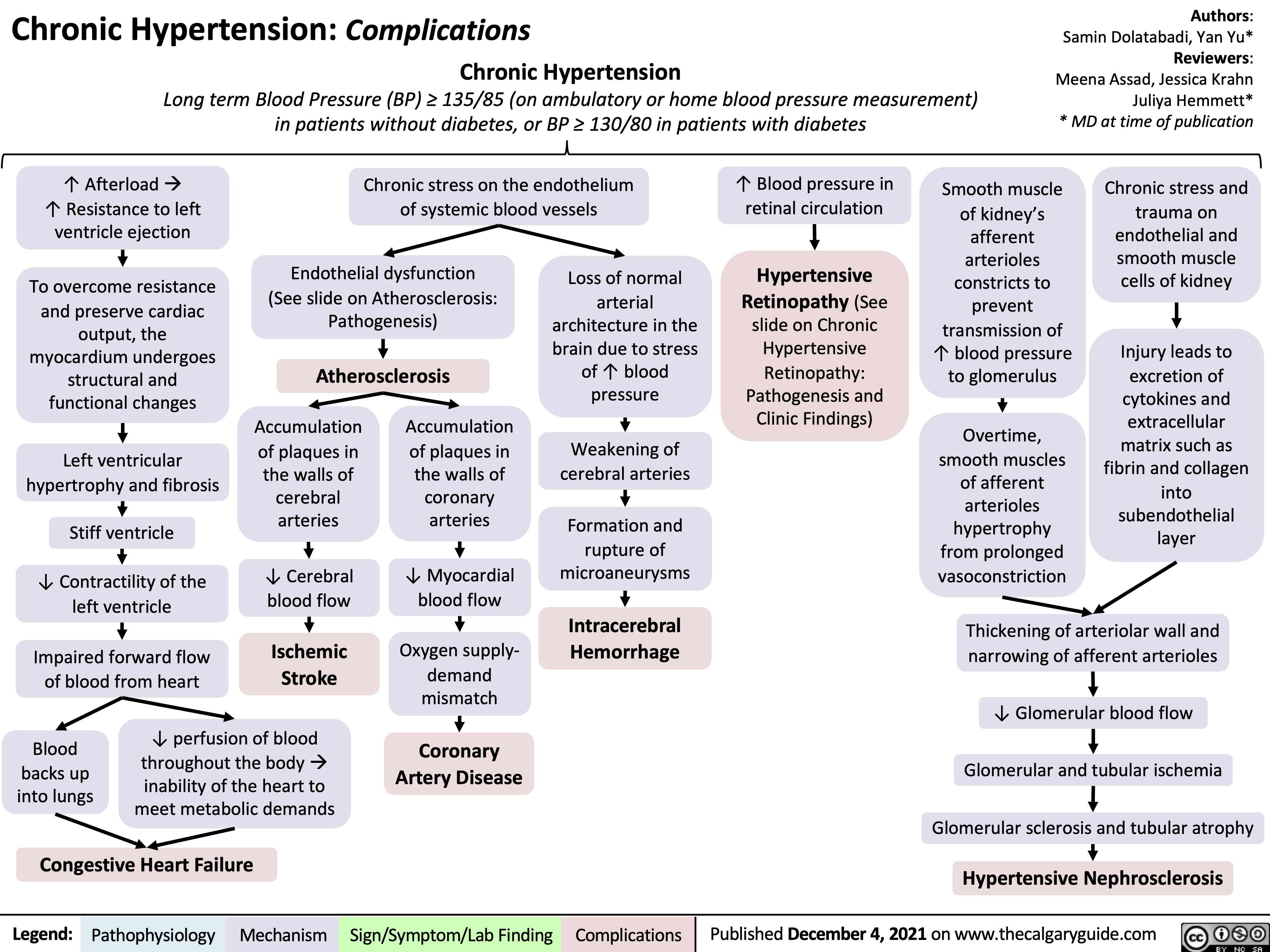
chronic-hypertension-complications
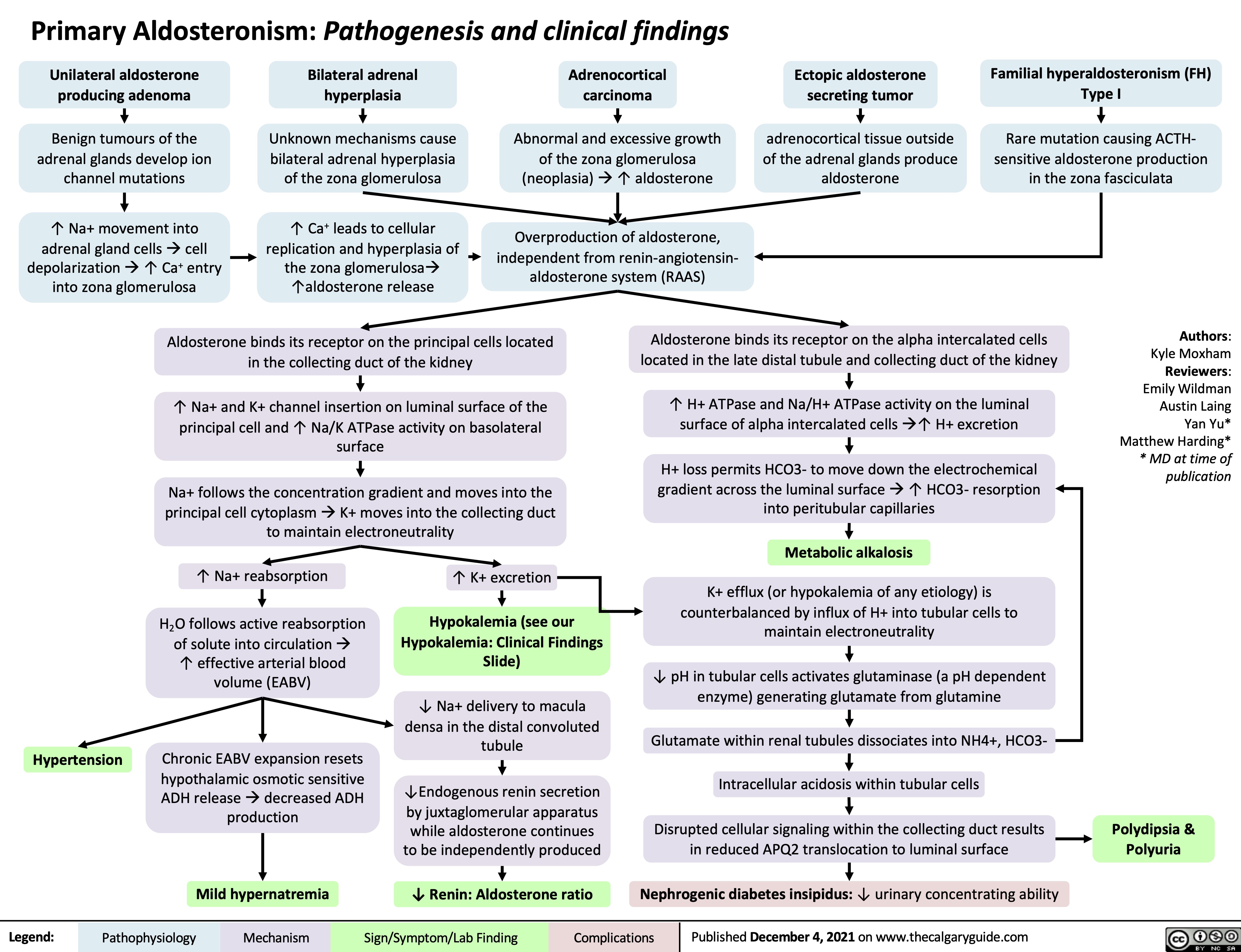
Primary Aldosteronism Pathogenesis
necrotizing fasciitis
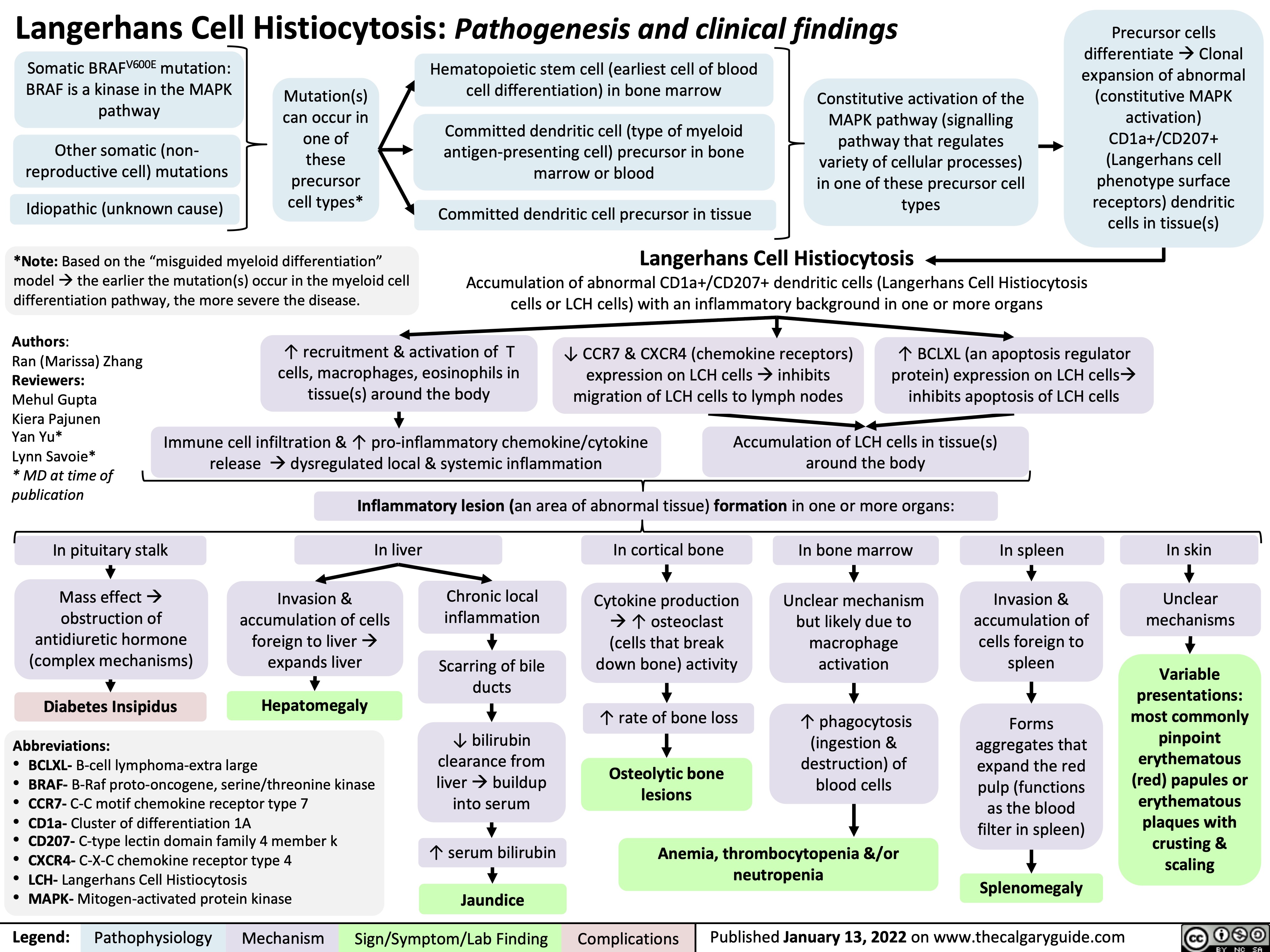
Langerhans Cell Histiocytosis
hyperkalemia-pathophysiology-intracellular-shift-and-intake
![Hyperkalemia (intracellular shift and ↑ intake): Pathophysiology
↑ K+ dietary intake (rarely causative)
β2 receptor inhibition (i.e. beta blockers)
Digoxin
α1 receptor stimulation (i.e. epinephrine, norepinephrine)
Insulin deficiency or resistance (i.e. diabetes mellitus)
↓ Stimulation of NHE1 (moves 1 Na+ into cell, 1 H+ out of cell) throughout body
Normal Anion- Gap Metabolic Acidosis (NAGMA)
Excess serum H+ results in ↓ NHE1 activity (See NAGMA: Pathogenesis and Laboratory Findings slide)
↑ Serum osmolarity (i.e. hyperglycemia)
Osmotic movement of water from cells to serum
Cell lysis (i.e. tumour lysis syndrome, rhabdomyolysis, hemolytic anemia)
↑ K+ release from lysed cells into the serum
Na+/K+ ATPase (moves 3K+ into cell, 2 Na+ out) activity inhibited on cells throughout body
↓ Activity of Na+/K+ ATPase on cells throughout the body
↓ Amount of K+ entering cells
↓ NHE1 activity prevents Na+ from entering the cell
Lack of high intracellular [Na+] needed to drive the Na+/K+ ATPase on cells
Loss of water from cells ↑ intracellular [K+]
K+ moves down concentration gradient from cell into serum
↑ K+ available for absorption
Since K+ is dissolved in water, some K+ is carried by water as water osmotically moves through water channels into the serum (phenomenon known as “solvent drag”)
See Hyperkalemia: Clinical Findings slide
Authors:
Mannat Dhillon, Joshua Low, Emily Wildman Reviewers:
Huneza Nadeem, Marissa (Ran) Zhang, Andrea Kuczynski, Yan Yu*, Kevin McLaughlin*, Adam Bass*
* MD at time of publication
↑ K+ in serum
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 17, 2022 on www.thecalgaryguide.com
Hyperkalemia (intracellular shift and ↑ intake): Pathophysiology
↑ K+ dietary intake (rarely causative)
β2 receptor inhibition (i.e. beta blockers)
Digoxin
α1 receptor stimulation (i.e. epinephrine, norepinephrine)
Insulin deficiency or resistance (i.e. diabetes mellitus)
↓ Stimulation of NHE1 (moves 1 Na+ into cell, 1 H+ out of cell) throughout body
Normal Anion- Gap Metabolic Acidosis (NAGMA)
Excess serum H+ results in ↓ NHE1 activity (See NAGMA: Pathogenesis and Laboratory Findings slide)
↑ Serum osmolarity (i.e. hyperglycemia)
Osmotic movement of water from cells to serum
Cell lysis (i.e. tumour lysis syndrome, rhabdomyolysis, hemolytic anemia)
↑ K+ release from lysed cells into the serum
Na+/K+ ATPase (moves 3K+ into cell, 2 Na+ out) activity inhibited on cells throughout body
↓ Activity of Na+/K+ ATPase on cells throughout the body
↓ Amount of K+ entering cells
↓ NHE1 activity prevents Na+ from entering the cell
Lack of high intracellular [Na+] needed to drive the Na+/K+ ATPase on cells
Loss of water from cells ↑ intracellular [K+]
K+ moves down concentration gradient from cell into serum
↑ K+ available for absorption
Since K+ is dissolved in water, some K+ is carried by water as water osmotically moves through water channels into the serum (phenomenon known as “solvent drag”)
See Hyperkalemia: Clinical Findings slide
Authors:
Mannat Dhillon, Joshua Low, Emily Wildman Reviewers:
Huneza Nadeem, Marissa (Ran) Zhang, Andrea Kuczynski, Yan Yu*, Kevin McLaughlin*, Adam Bass*
* MD at time of publication
↑ K+ in serum
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 17, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/07/Hyperkalemia-shift-1.jpg)
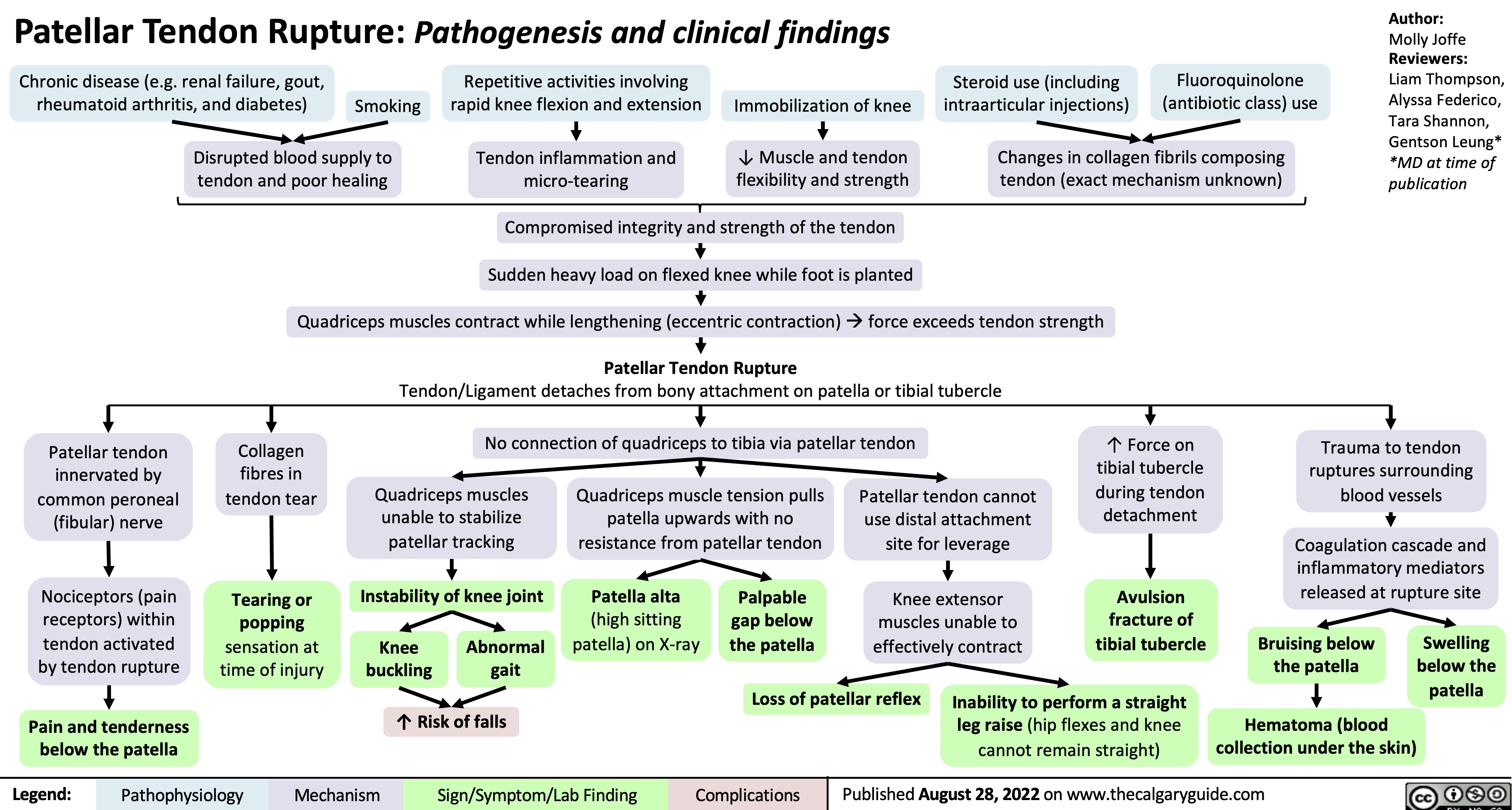
patellar-tendon-rupture-pathogenesis-and-clinical-findings
diabetes-insipidus-pathogenesis-and-clinical-findings
![Diabetes Insipidus: Pathogenesis and clinical findings
Hereditary
Autoimmune/ Idiopathic
Auto-antibodies destroy neurons that release antidiuretic hormone (ADH)
Mass Effect/ Tumor Invasion
Mass pressing on hypothalamus or pituitary
Electrolyte Imbalance
(mechanism unclear)
Hereditary
Lithium (Li)
(mechanism unclear)
Li enters principal cells of collecting ducts via ENaCs
Li inhibits GSK3β, reducing adenylyl cyclase activity
↓ cAMP- dependent phosphorylation of aquaporin-2
↑ Serum [Ca2+]
Activation of
CaSR in thick ascending limb of Loop of Henle
↓ NaCl reabsorption in thick ascending limb
↓ Generation of medullary osmotic gradient
↓ Serum [K+]
↑ Degradation of aquaporin-2 channels in collecting duct
↓ Aquaporin- 2 channels transporting water across apical membrane of collecting duct
Mutation of AVPR2 gene on X chromosome
Antidiuretic hormone (ADH) receptor cannot reach basolateral surface of principal cells of collecting duct
Mutation of aquaporin-2 gene on chromosome 12
↓ Fusion of aquaporins with apical membrane of collecting duct
Mutation of WFS1 gene on chromosome 4 (Wolfram syndrome)
↓ Processing of antidiuretic hormone (ADH) precursors and ↓ADH-releasing neurons
Surgery/ Trauma
Injury to hypothalamus or pituitary stalk
Mutation of PCSK1 gene on chromosome 5
Deficiency in PC1/3 (encoded by PCSK1)
↓ Processing of ADH by PC1/3
Aquaporin dysfunction
↓ Kidney response to ADH, which mediates reabsorption of water down its osmotic gradient through aquaporins
↓ Production of ADH by hypothalamus or ↓ secretion from ADH-releasing neurons in posterior pituitary (depending on location of lesion)
Central Diabetes Insipidus
Nephrogenic Diabetes Insipidus
Abbreviations:
AVPR2: arginine vasopressin receptor 2 CaSR: calcium-sensing receptor
ENaC: epithelial sodium channel
GSK3β: glycogen synthase kinase type 3 beta PC1/3: proprotein convertase
Diabetes Insipidus
Decreased ability of kidneys to concentrate urine
↓ Reabsorption of water from collecting duct into vasculature
Author:
Oswald Chen
Reviewers:
Huneza Nadeem,
Ran (Marissa) Zhang,
Yan Yu*
Sam Fineblit*
* MD at time of publication
Urine becomes more dilute
↓ Urine osmolality
↑ Urine output
↓ Blood volume
Blood becomes more concentrated
Occurs during late sleep period
Nocturia
Polyuria
(>3 L/day)
↑ Serum osmolality
Activation of hypothalamic osmoreceptors
Hypernatremia
(Serum [Na+] >145 mEq/L)
Polydipsia
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published September 25, 2022 on www.thecalgaryguide.com
Diabetes Insipidus: Pathogenesis and clinical findings
Hereditary
Autoimmune/ Idiopathic
Auto-antibodies destroy neurons that release antidiuretic hormone (ADH)
Mass Effect/ Tumor Invasion
Mass pressing on hypothalamus or pituitary
Electrolyte Imbalance
(mechanism unclear)
Hereditary
Lithium (Li)
(mechanism unclear)
Li enters principal cells of collecting ducts via ENaCs
Li inhibits GSK3β, reducing adenylyl cyclase activity
↓ cAMP- dependent phosphorylation of aquaporin-2
↑ Serum [Ca2+]
Activation of
CaSR in thick ascending limb of Loop of Henle
↓ NaCl reabsorption in thick ascending limb
↓ Generation of medullary osmotic gradient
↓ Serum [K+]
↑ Degradation of aquaporin-2 channels in collecting duct
↓ Aquaporin- 2 channels transporting water across apical membrane of collecting duct
Mutation of AVPR2 gene on X chromosome
Antidiuretic hormone (ADH) receptor cannot reach basolateral surface of principal cells of collecting duct
Mutation of aquaporin-2 gene on chromosome 12
↓ Fusion of aquaporins with apical membrane of collecting duct
Mutation of WFS1 gene on chromosome 4 (Wolfram syndrome)
↓ Processing of antidiuretic hormone (ADH) precursors and ↓ADH-releasing neurons
Surgery/ Trauma
Injury to hypothalamus or pituitary stalk
Mutation of PCSK1 gene on chromosome 5
Deficiency in PC1/3 (encoded by PCSK1)
↓ Processing of ADH by PC1/3
Aquaporin dysfunction
↓ Kidney response to ADH, which mediates reabsorption of water down its osmotic gradient through aquaporins
↓ Production of ADH by hypothalamus or ↓ secretion from ADH-releasing neurons in posterior pituitary (depending on location of lesion)
Central Diabetes Insipidus
Nephrogenic Diabetes Insipidus
Abbreviations:
AVPR2: arginine vasopressin receptor 2 CaSR: calcium-sensing receptor
ENaC: epithelial sodium channel
GSK3β: glycogen synthase kinase type 3 beta PC1/3: proprotein convertase
Diabetes Insipidus
Decreased ability of kidneys to concentrate urine
↓ Reabsorption of water from collecting duct into vasculature
Author:
Oswald Chen
Reviewers:
Huneza Nadeem,
Ran (Marissa) Zhang,
Yan Yu*
Sam Fineblit*
* MD at time of publication
Urine becomes more dilute
↓ Urine osmolality
↑ Urine output
↓ Blood volume
Blood becomes more concentrated
Occurs during late sleep period
Nocturia
Polyuria
(>3 L/day)
↑ Serum osmolality
Activation of hypothalamic osmoreceptors
Hypernatremia
(Serum [Na+] >145 mEq/L)
Polydipsia
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published September 25, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/09/Diabetes-Insipidus.jpg)
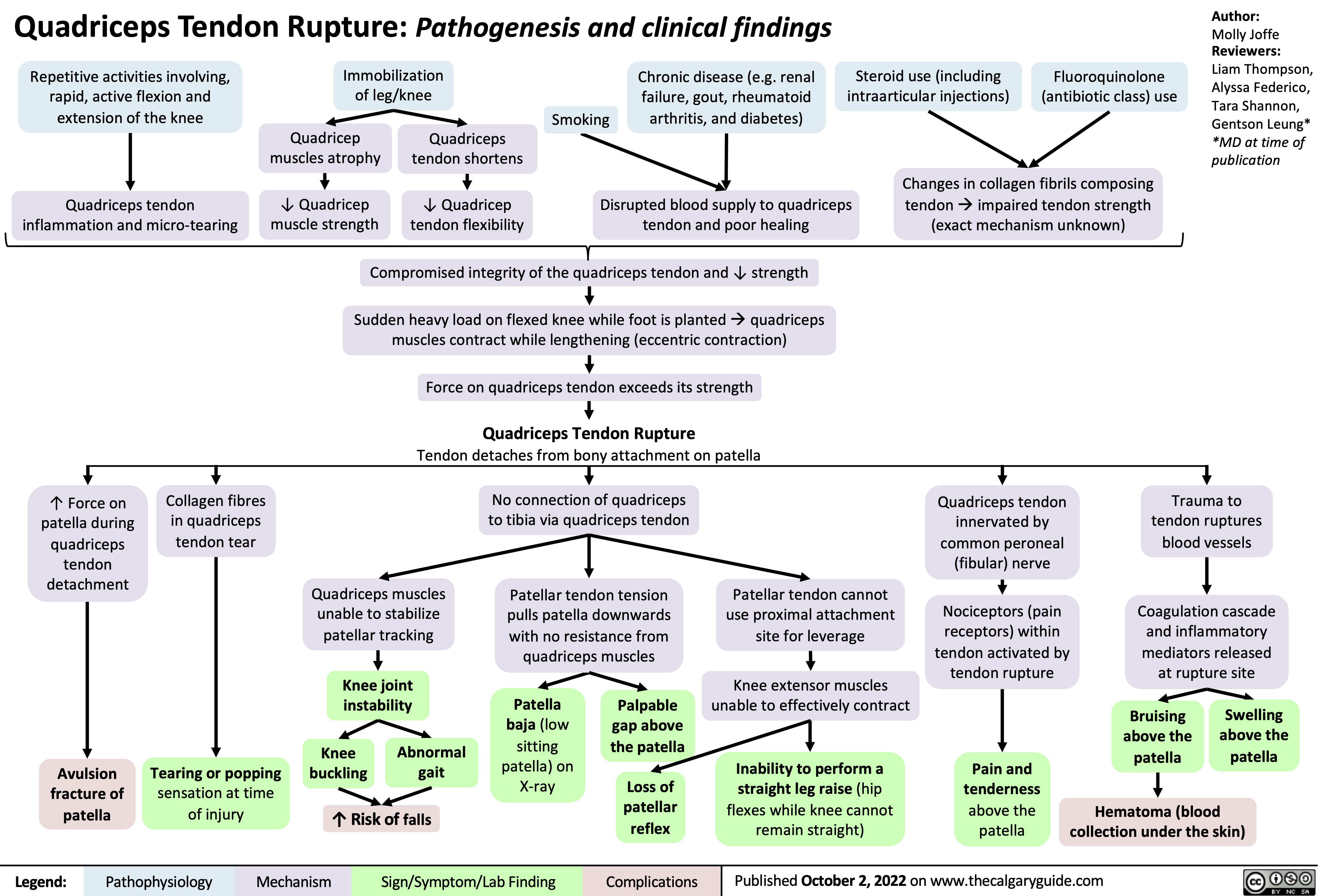
quadriceps-tendon-rupture-pathogenesis-and-clinical-findings
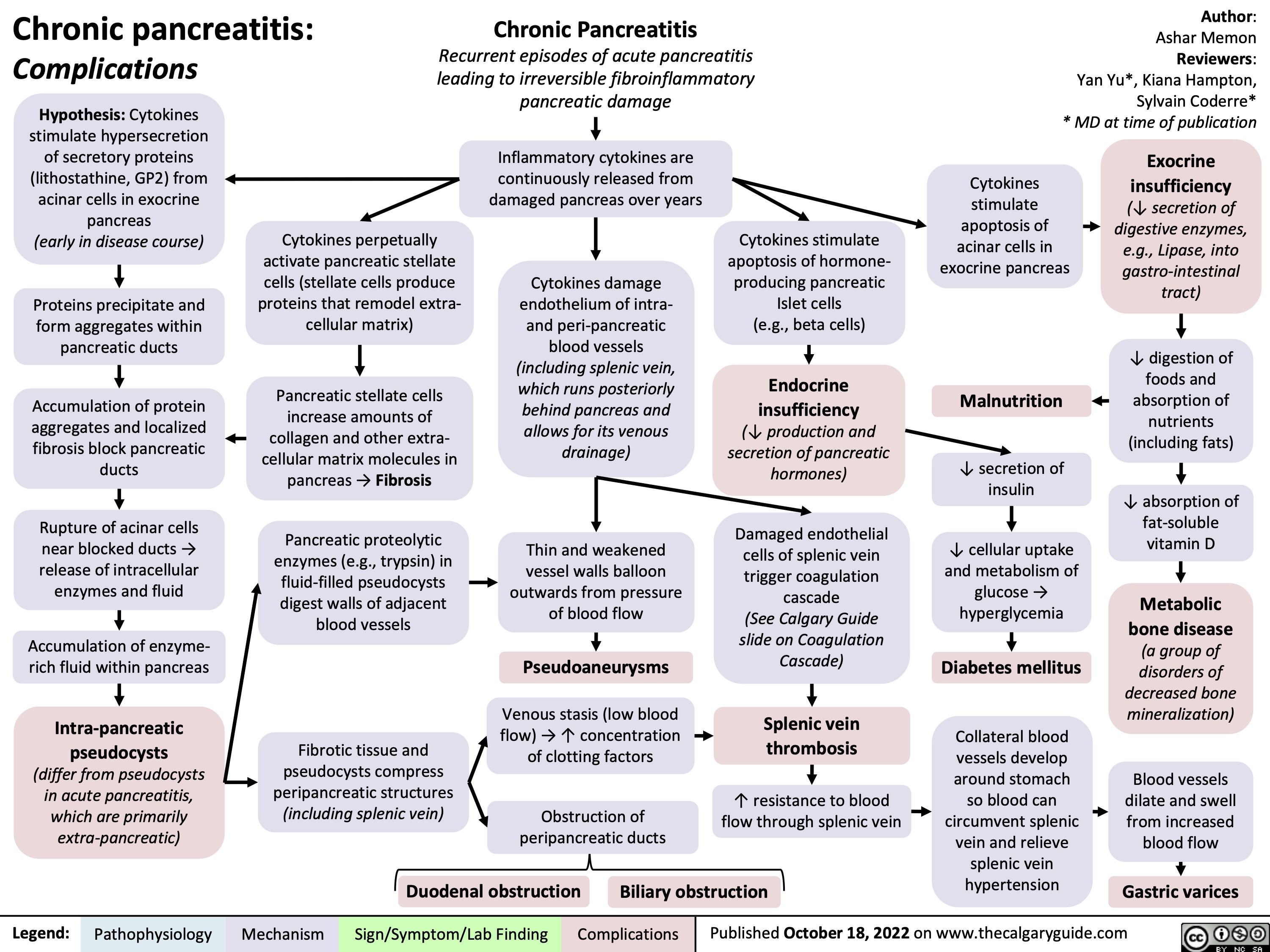
chronic-pancreatitis-complications
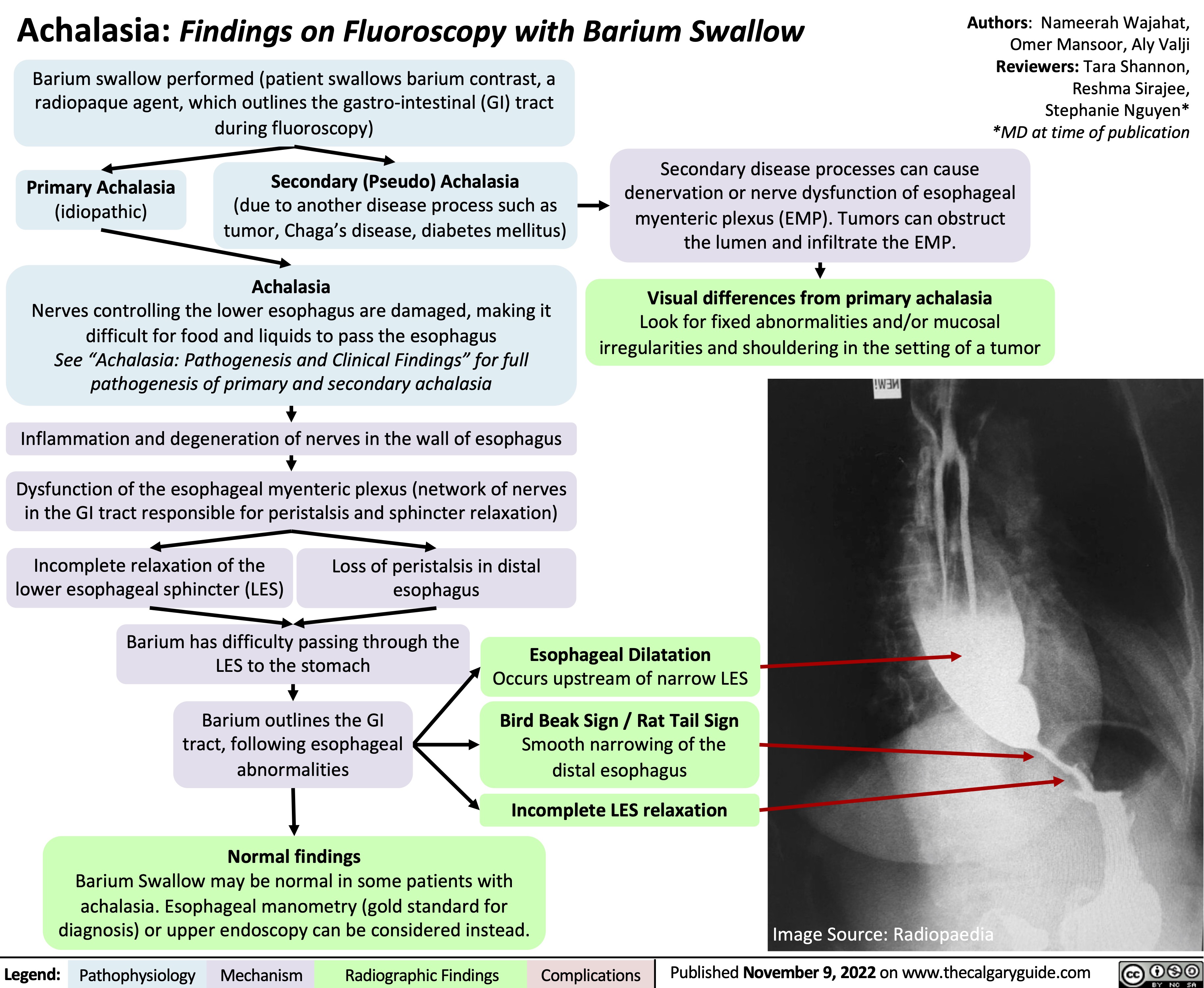
Achalasia: Findings on Fluoroscopy with Barium Swallow
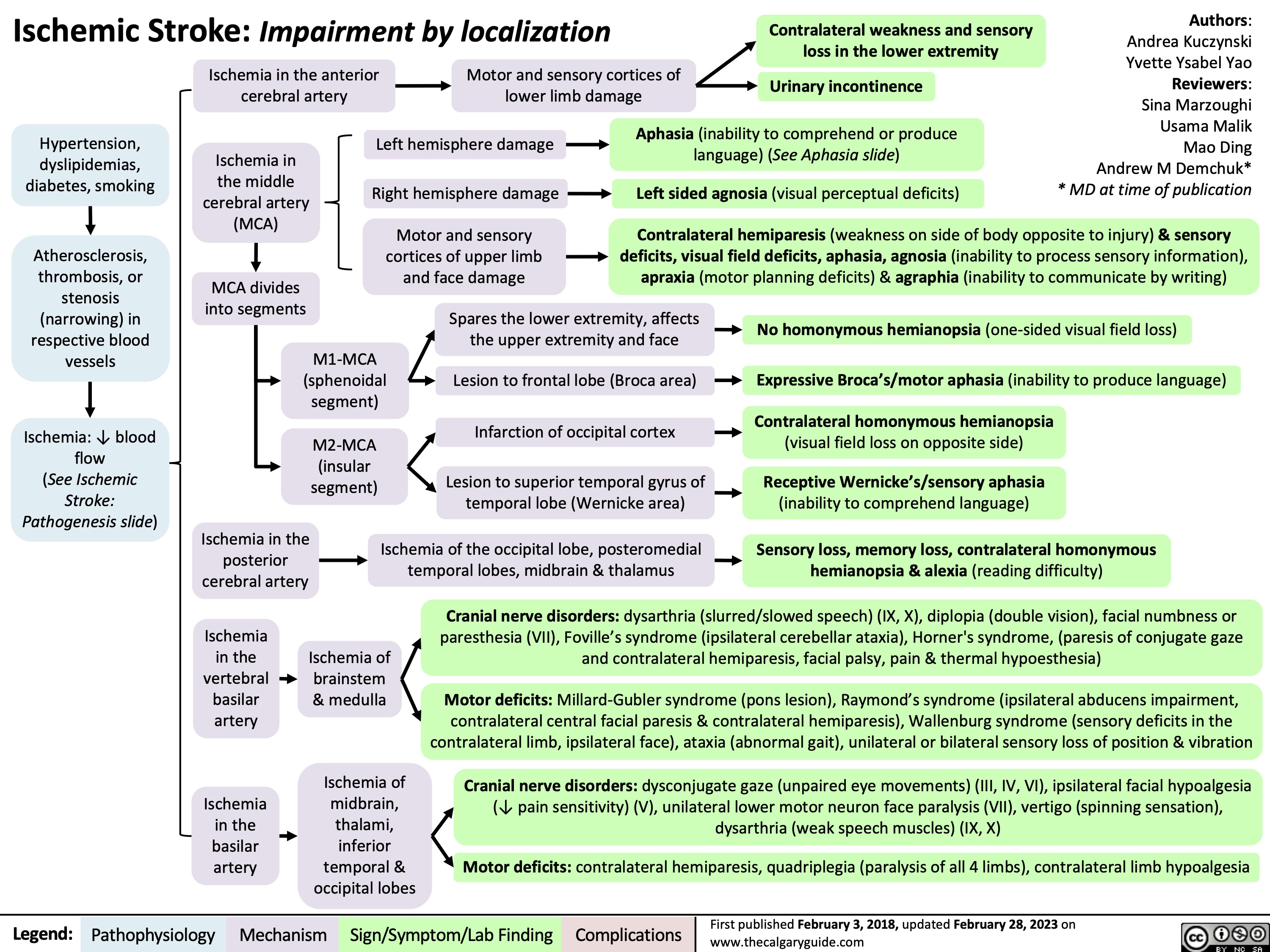
Ischemic Stroke Impairment by Localization
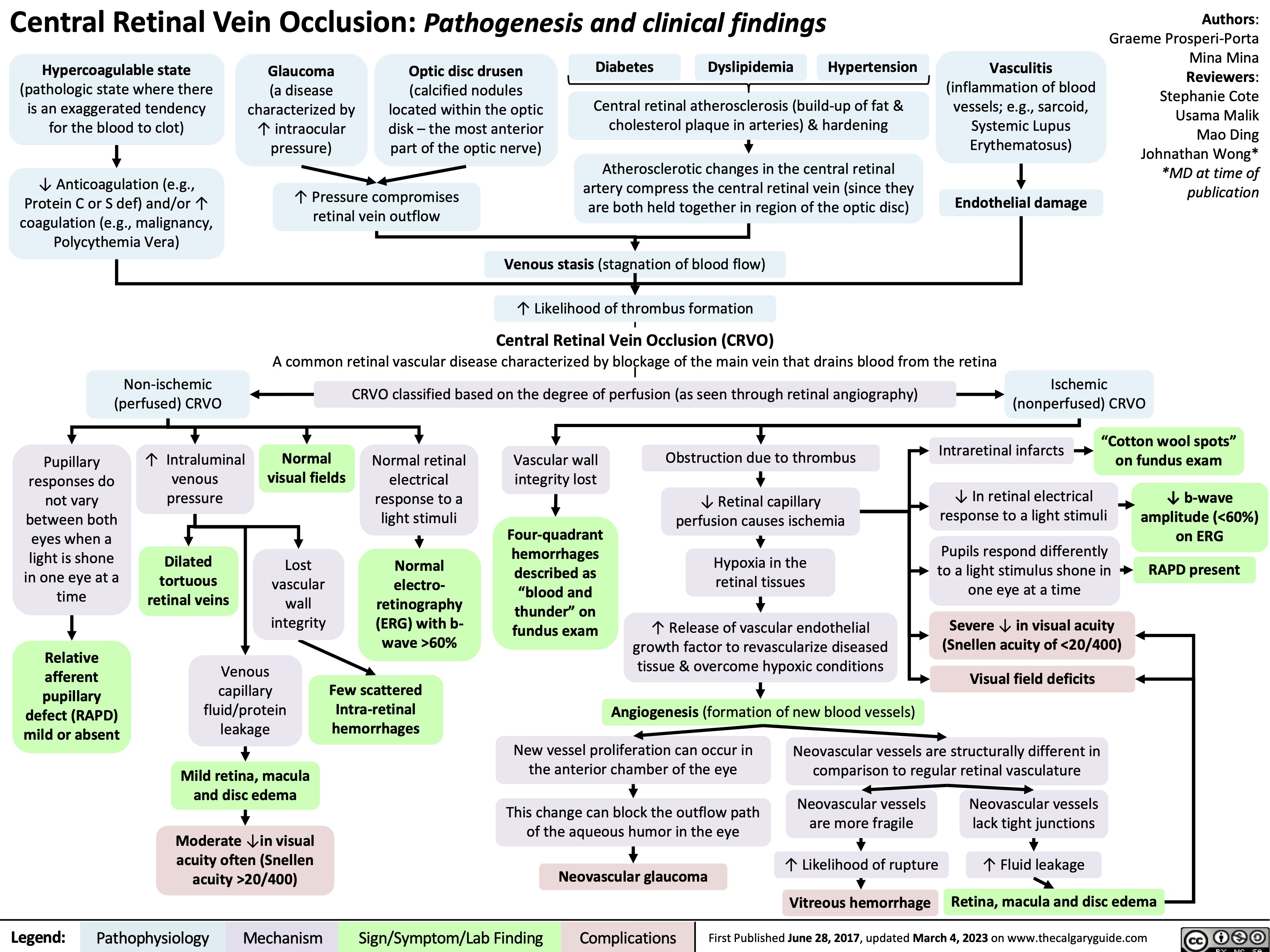
Central Retinal Vein Occlusion
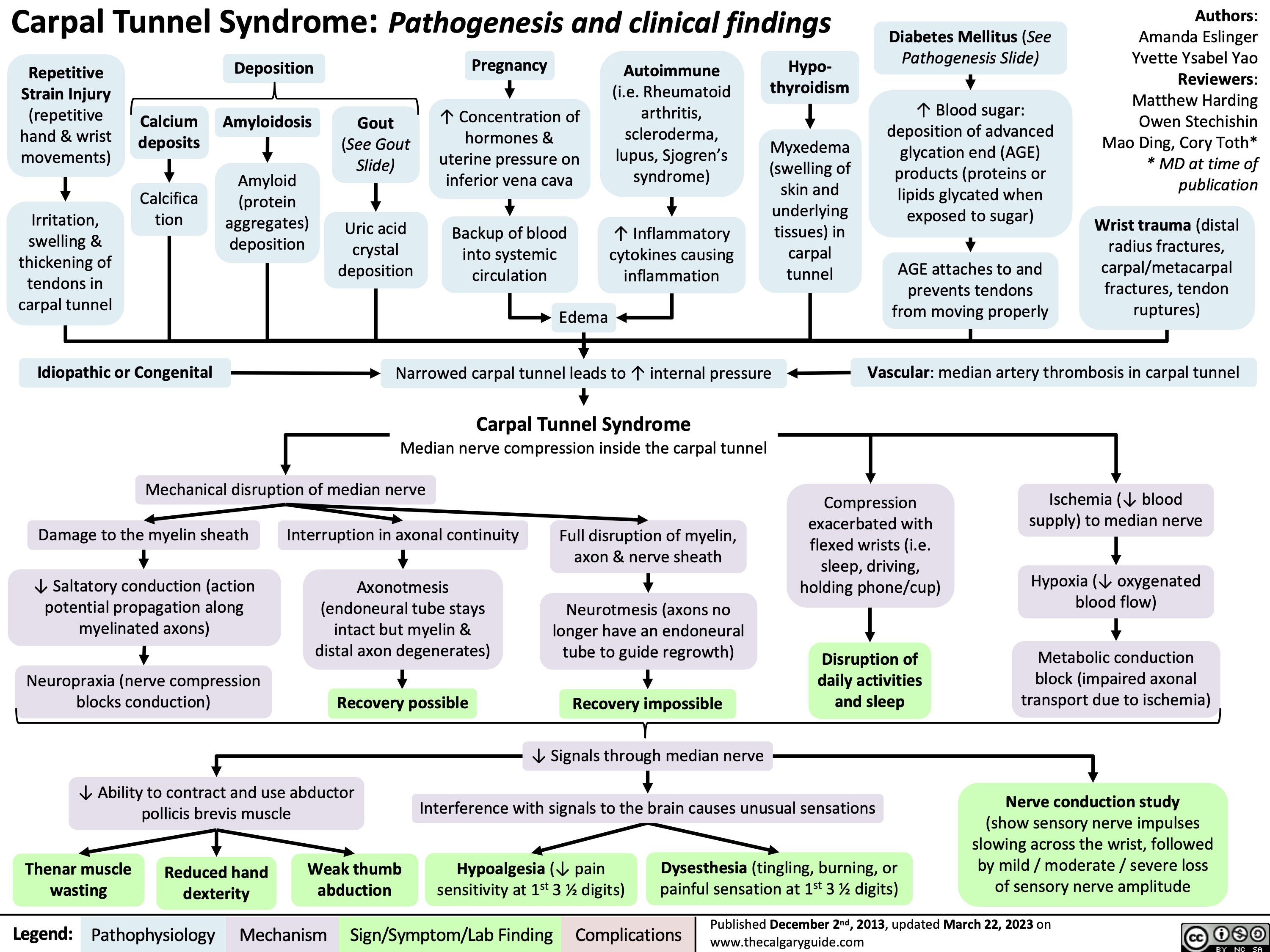
Carpal Tunnel Syndrome
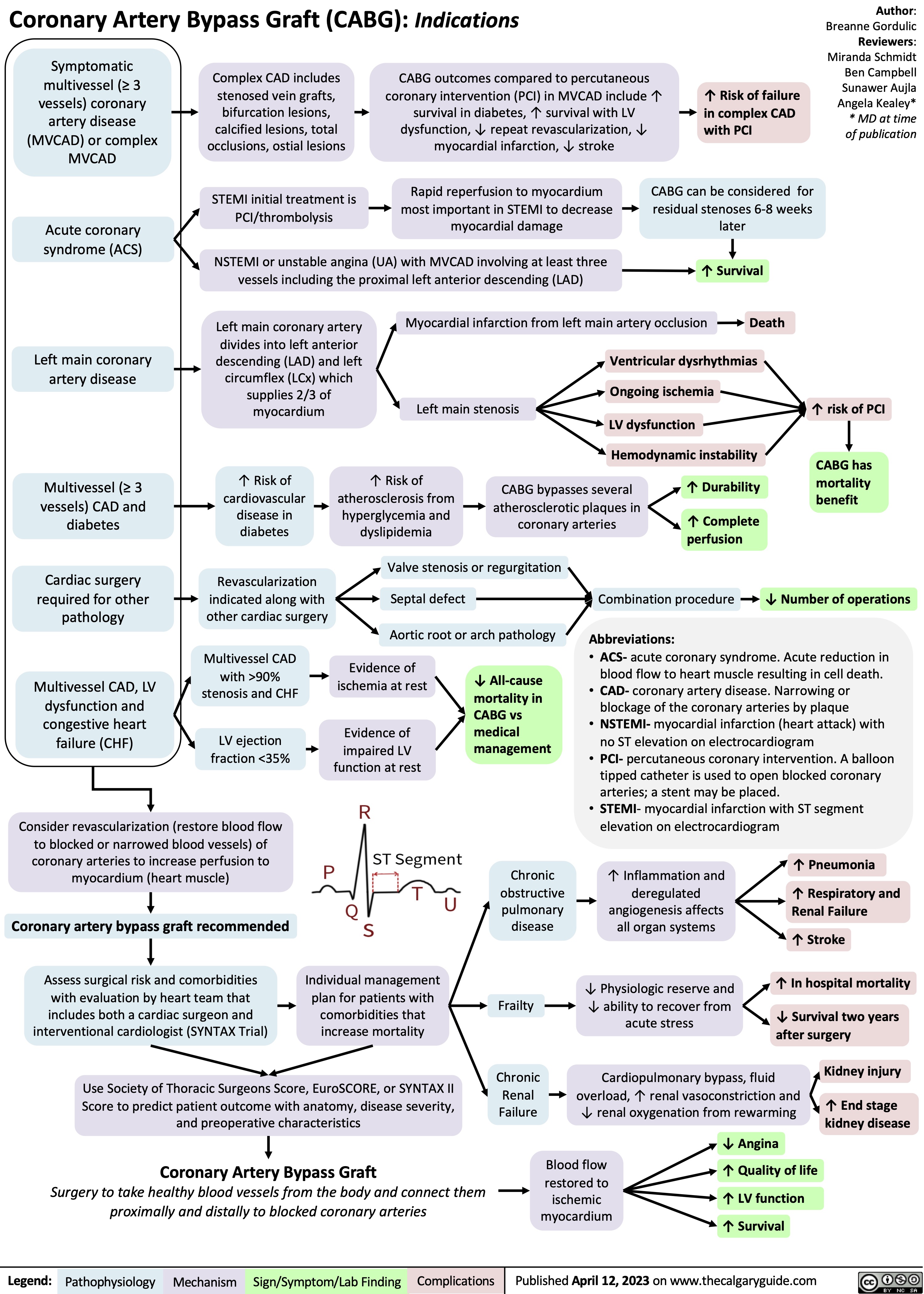
Coronary Artery Bypass Graft CABG Indications
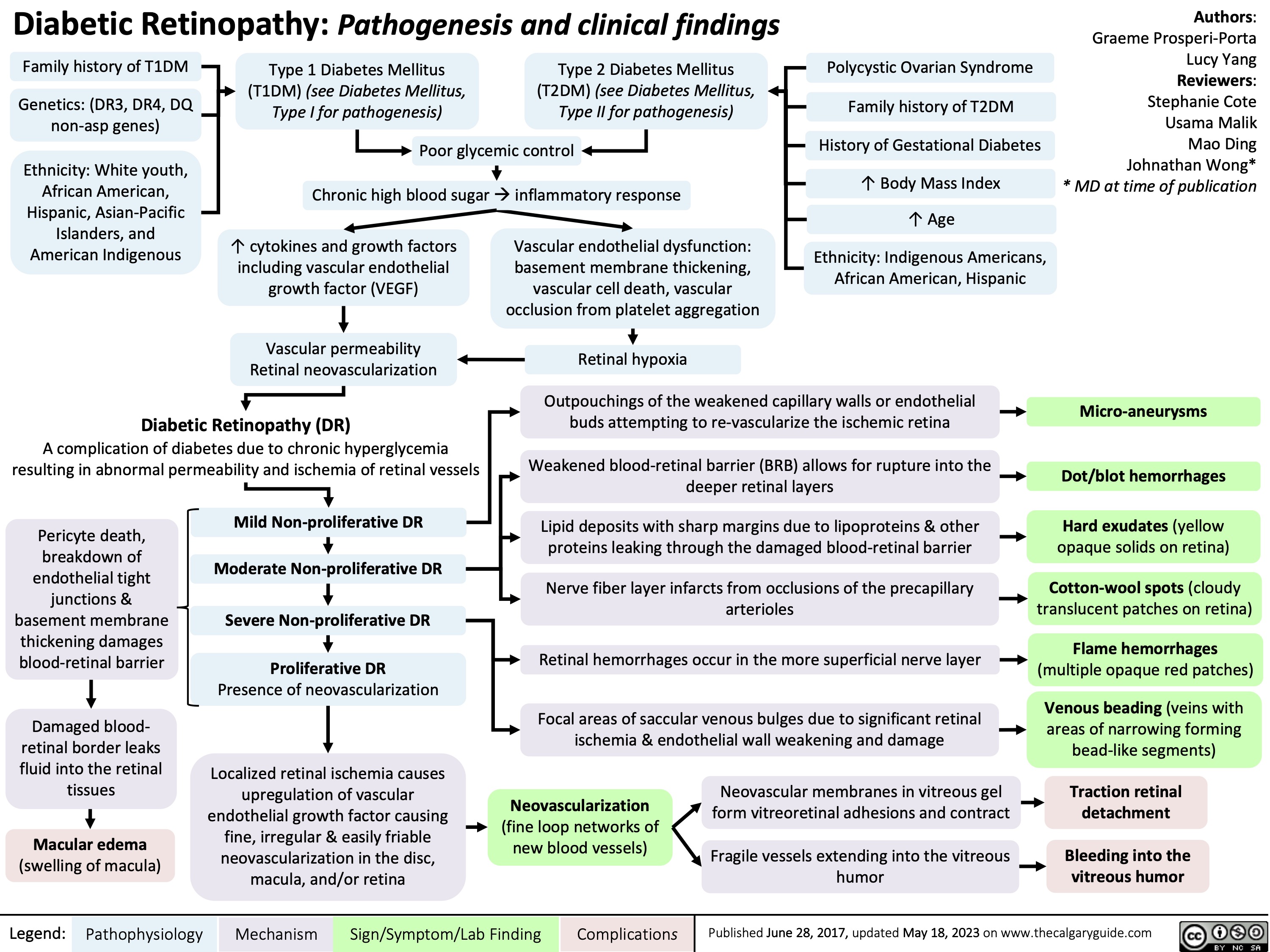
Diabetic Retinopathy
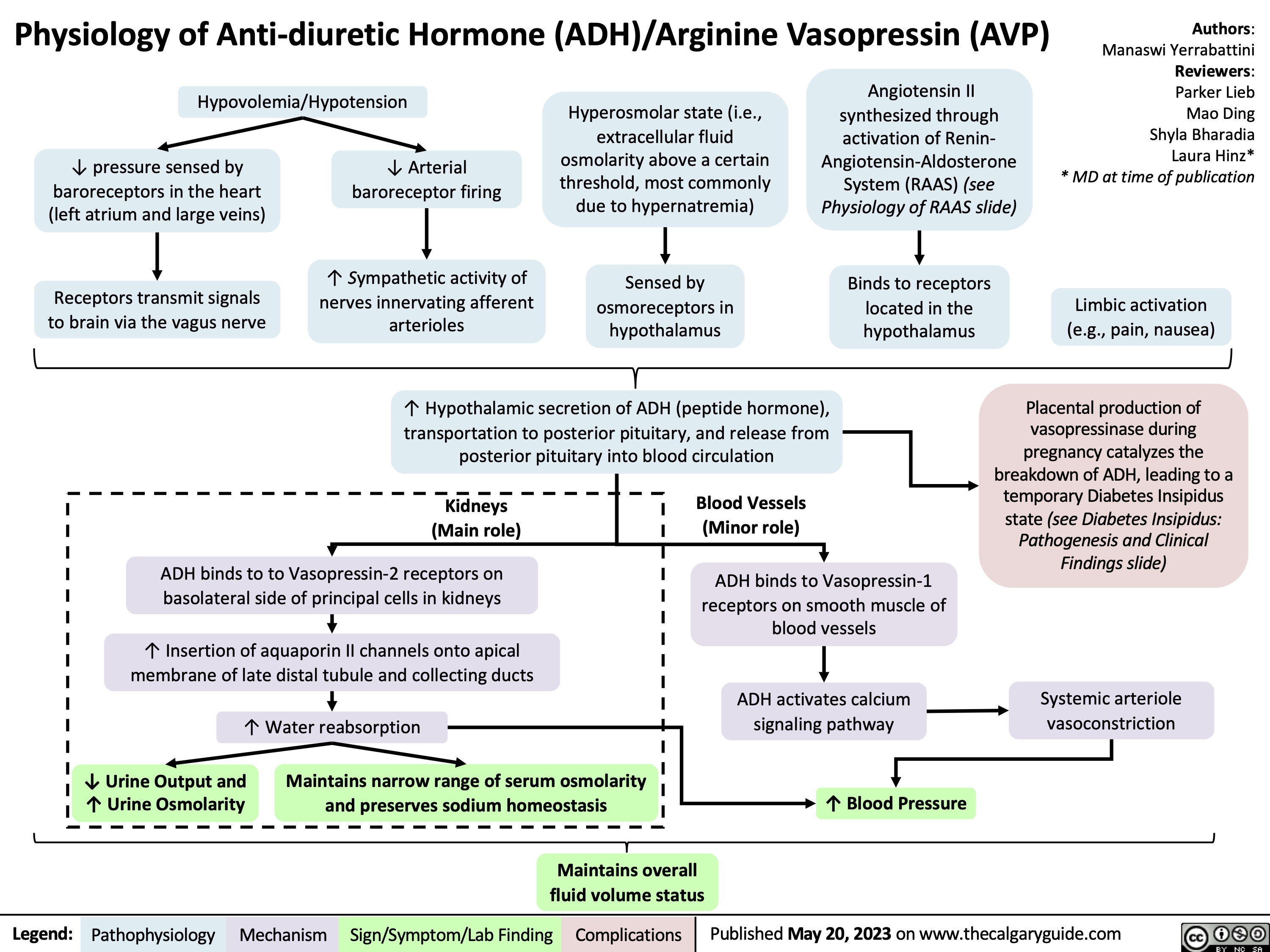
Physiology of Anti-diuretic hormone
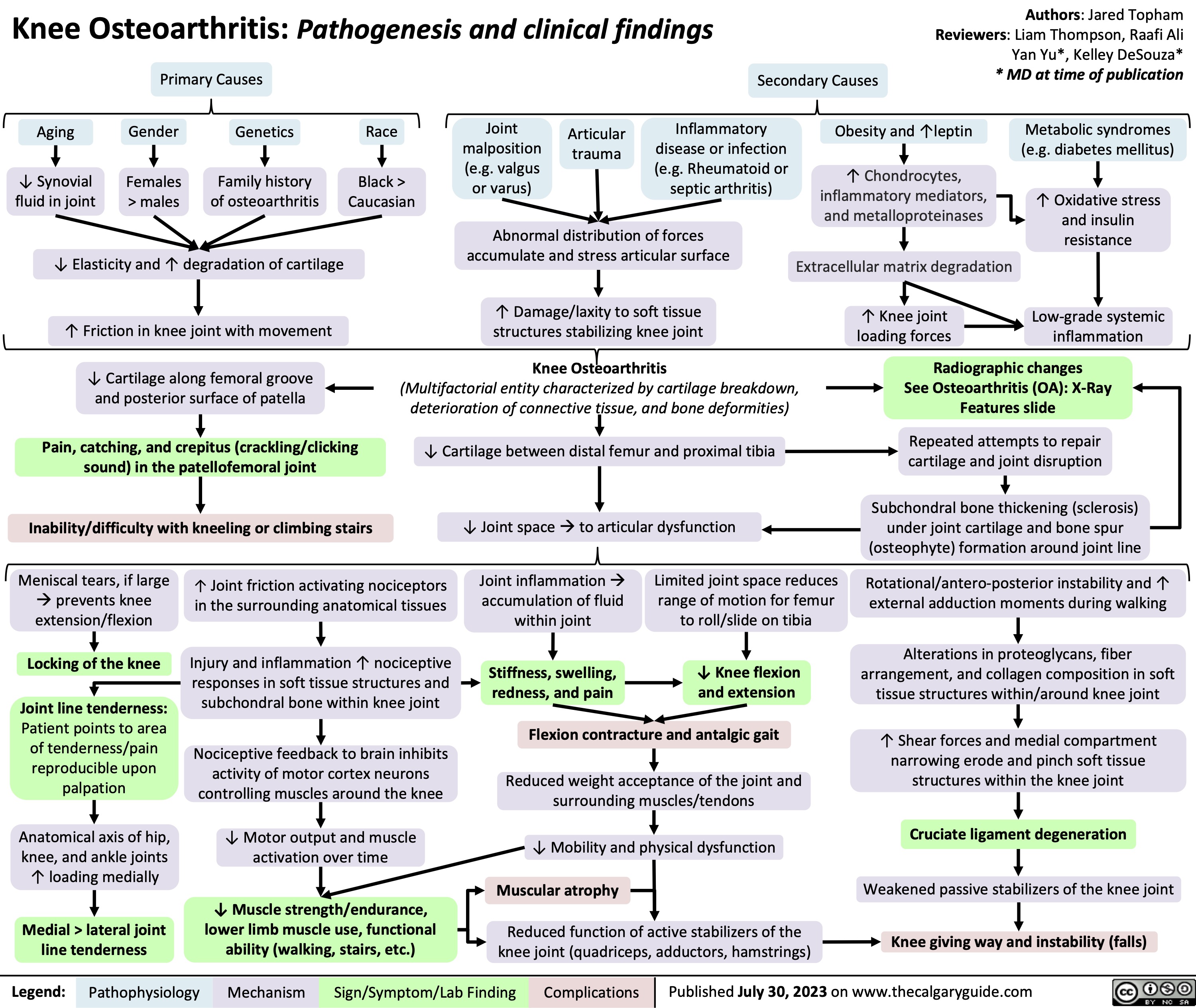
Knee Osteoarthritis
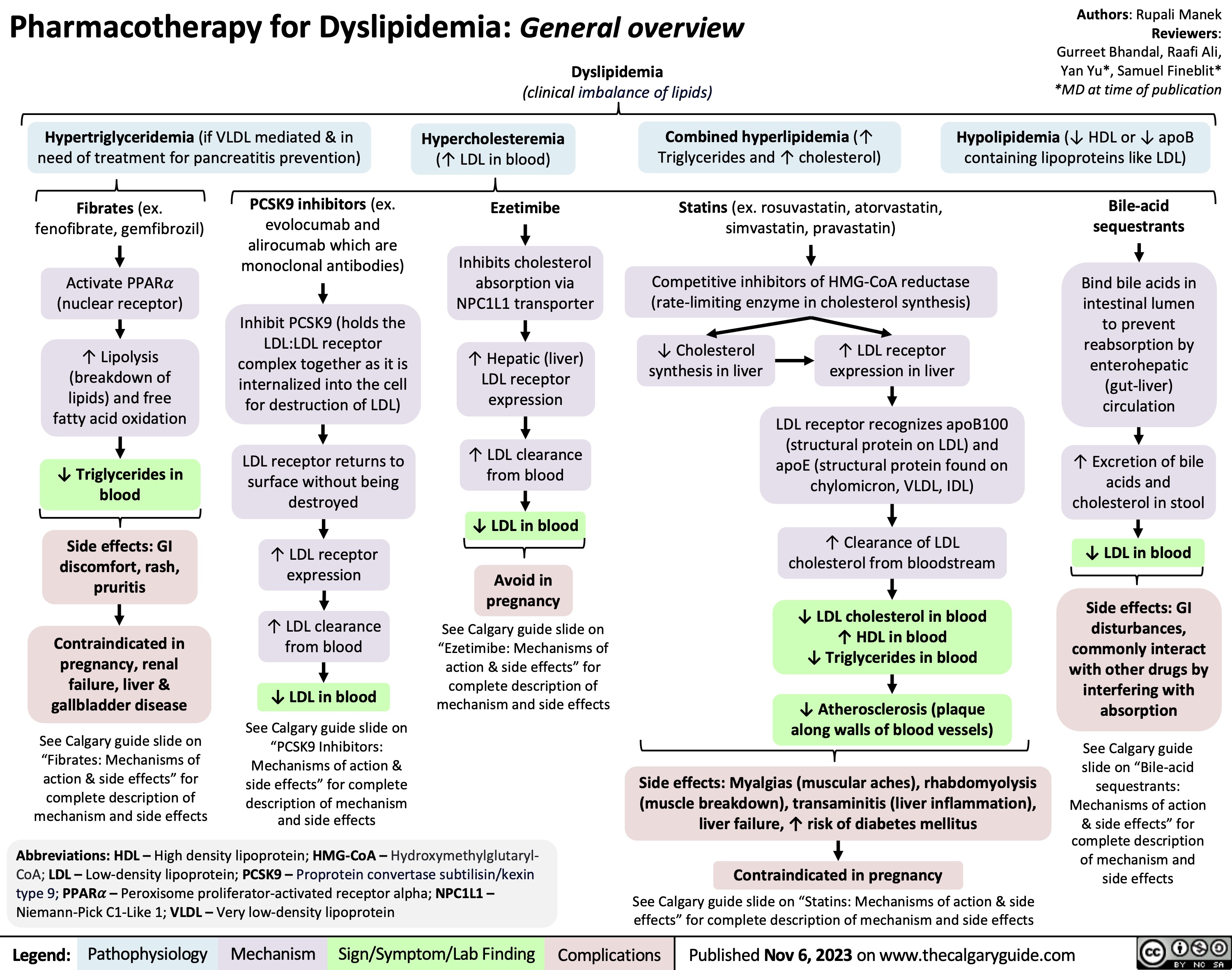
Pharmacotherapy for Dyslipidemia Overview
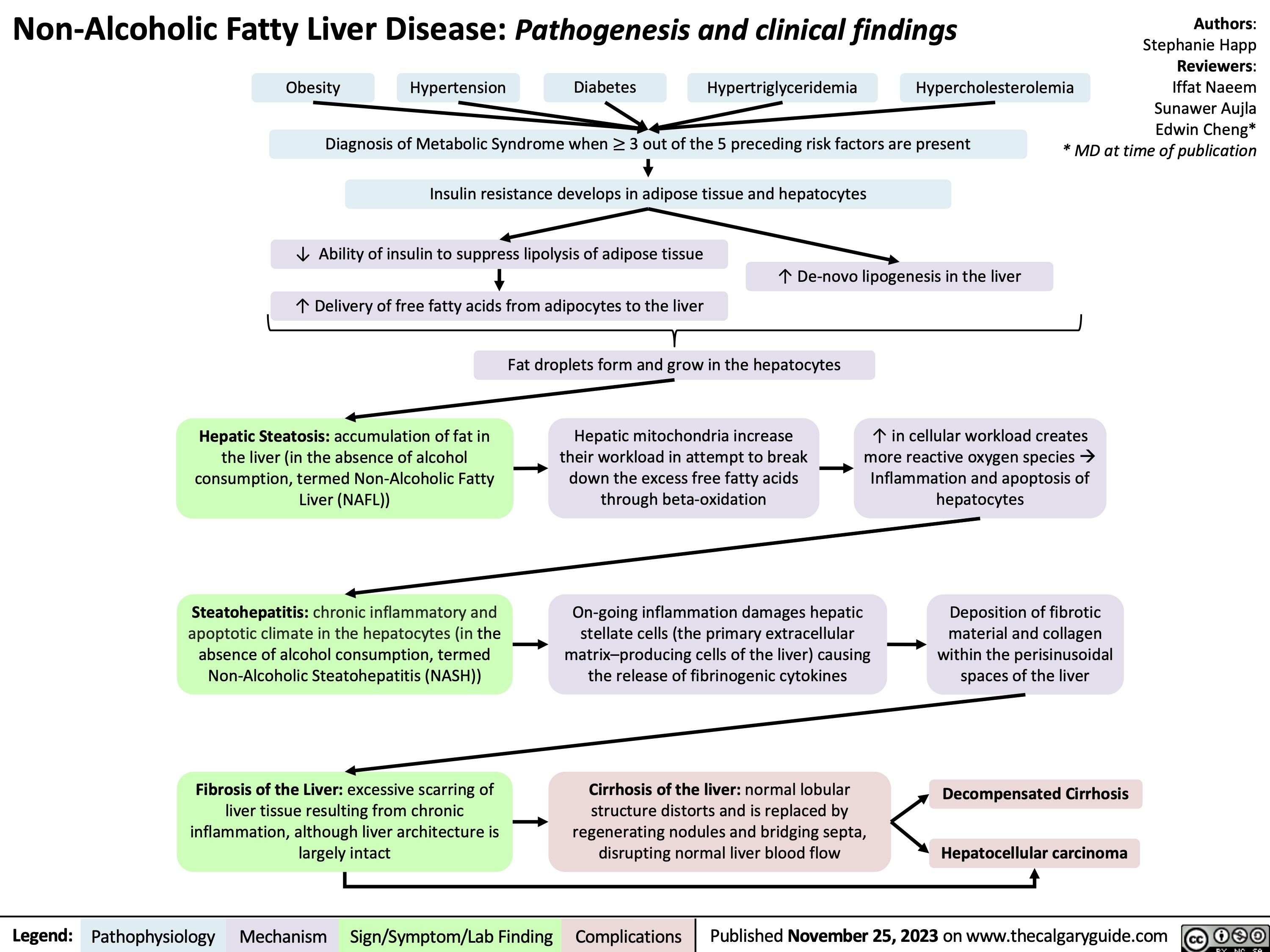
Non-Alcoholic Fatty Liver Disease
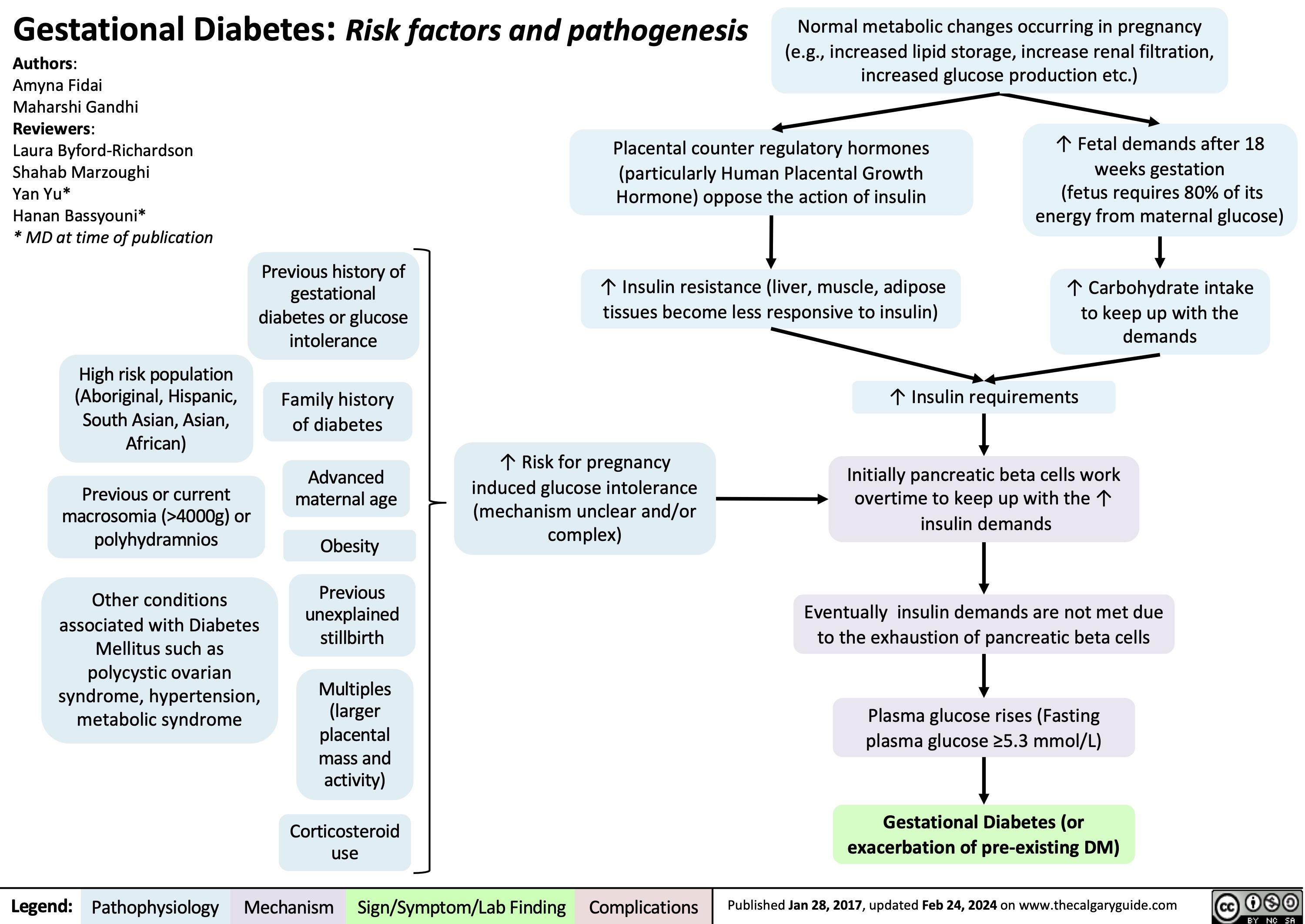
Gestational Diabetes Risk factors and pathogenesis
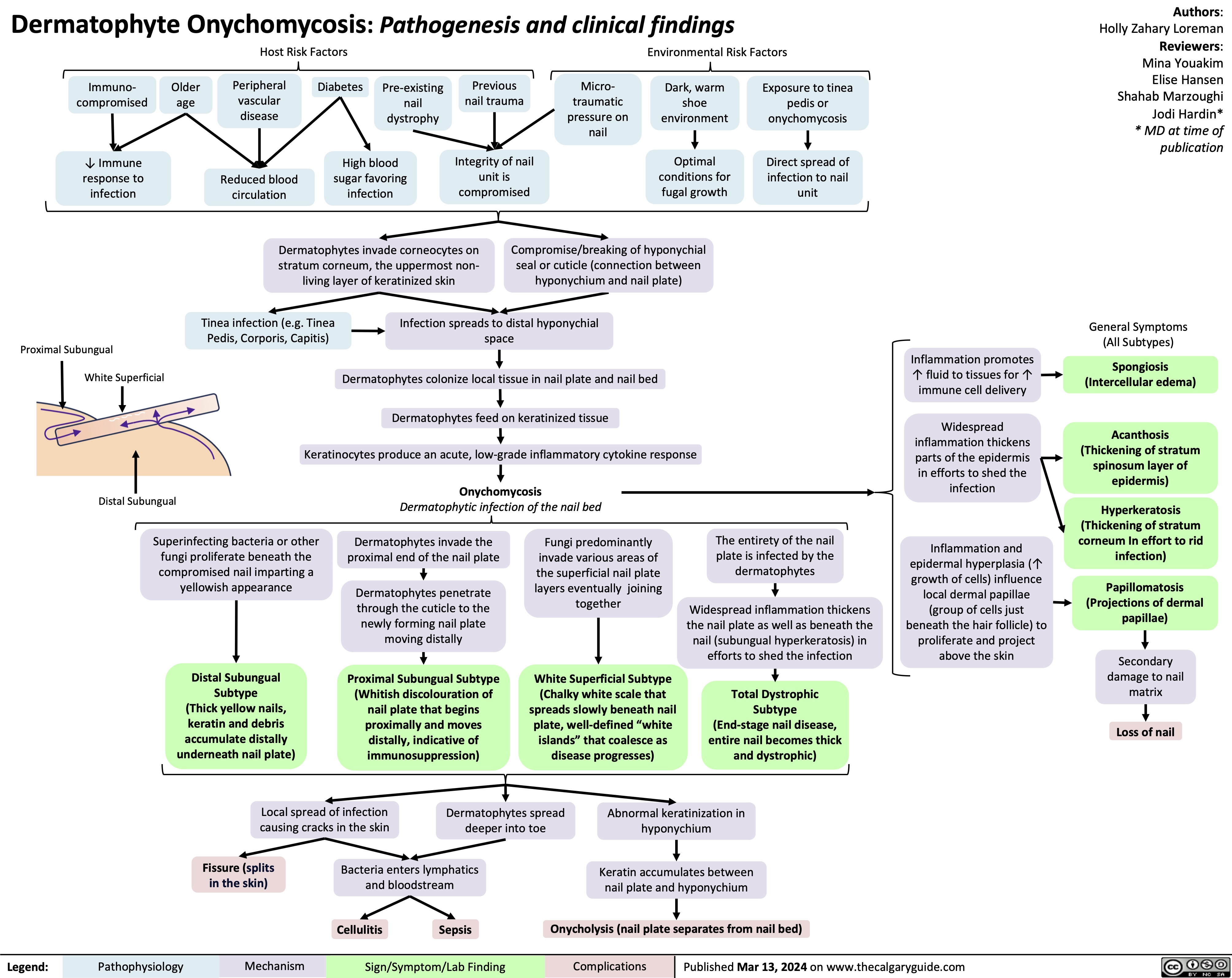
Onychomycosis
Macrosomia Pathogenesis and Complications
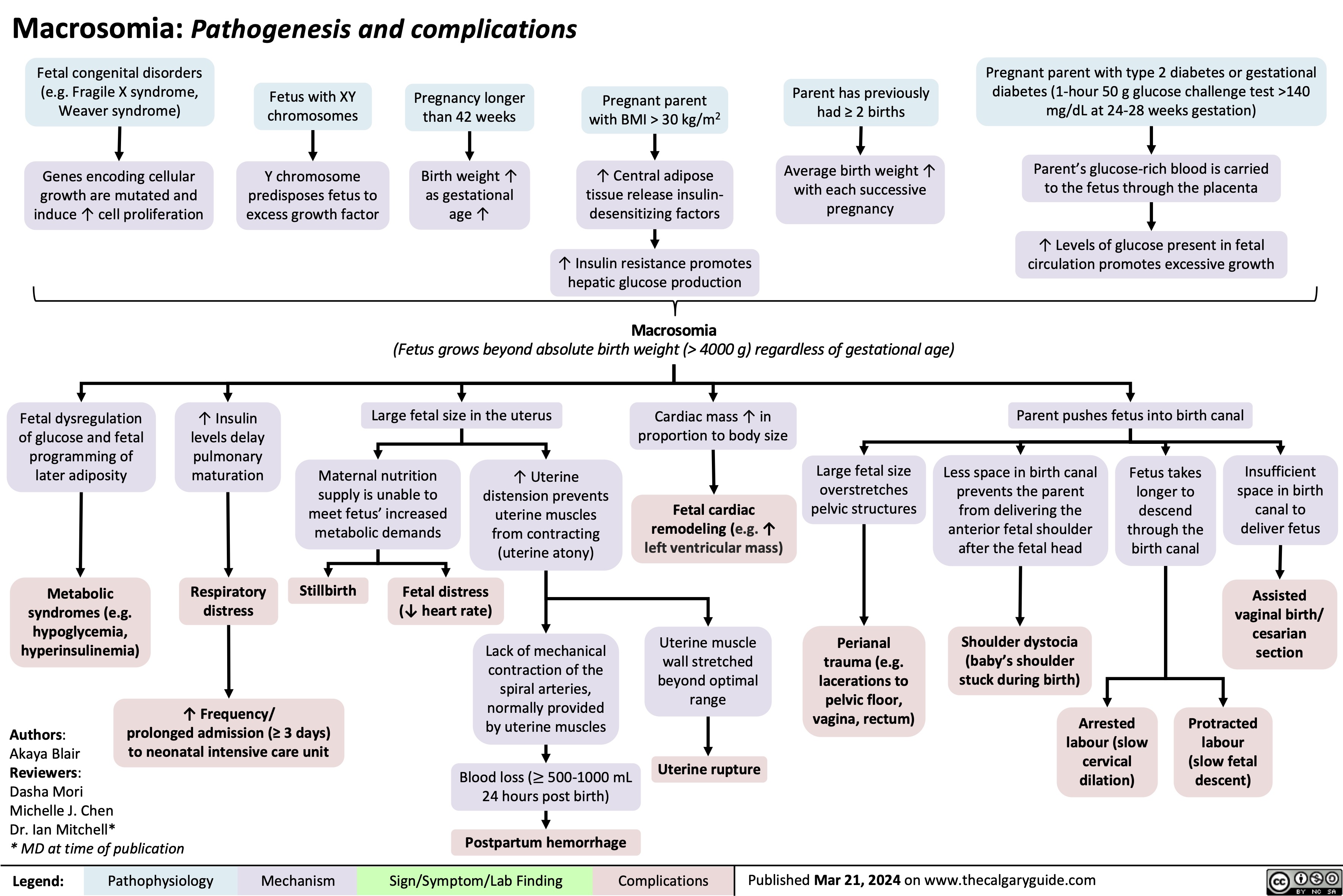
Operative Vaginal Delivery Indications
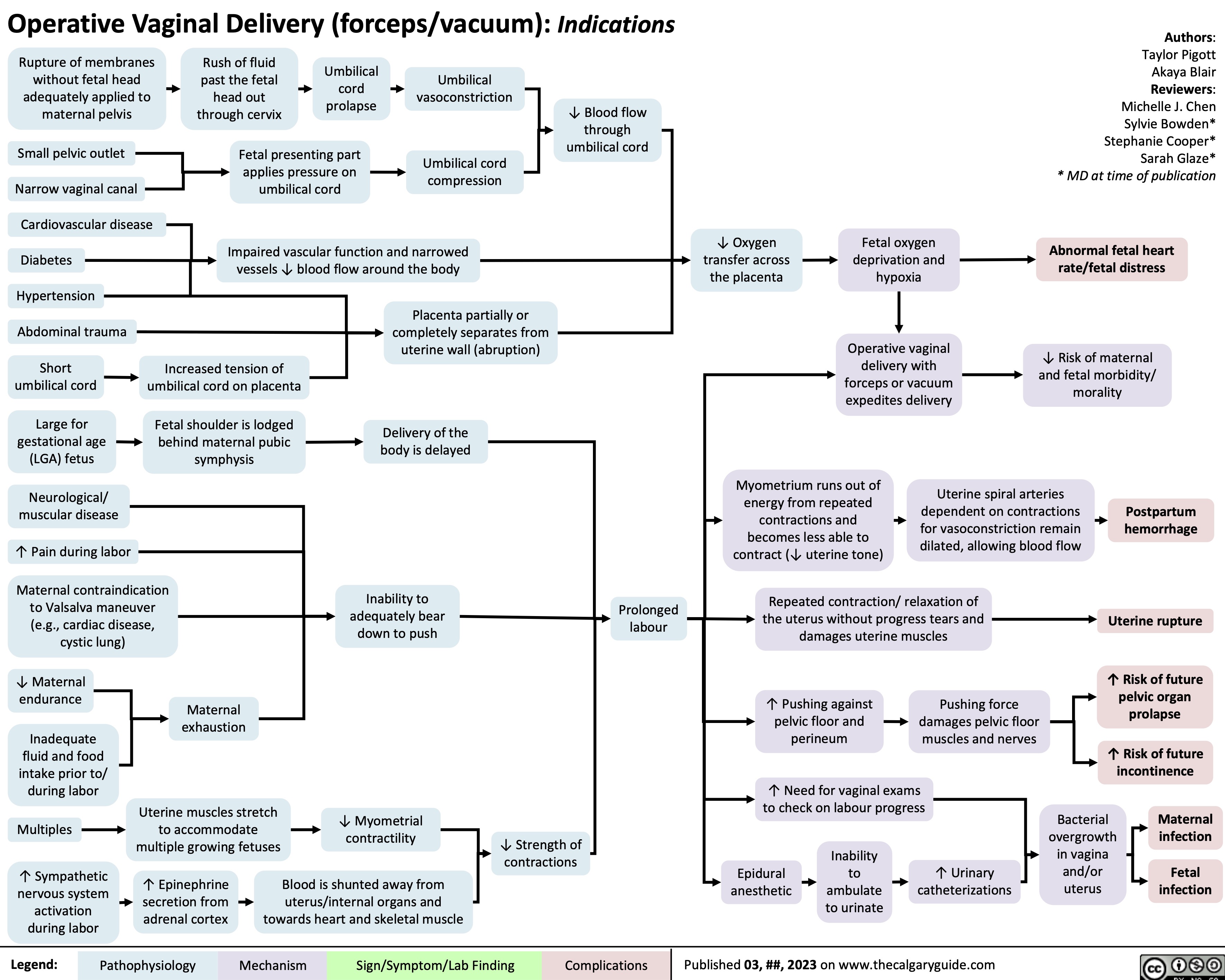
Diabetes Mellitus Pathophysiology Behind Lab Findings
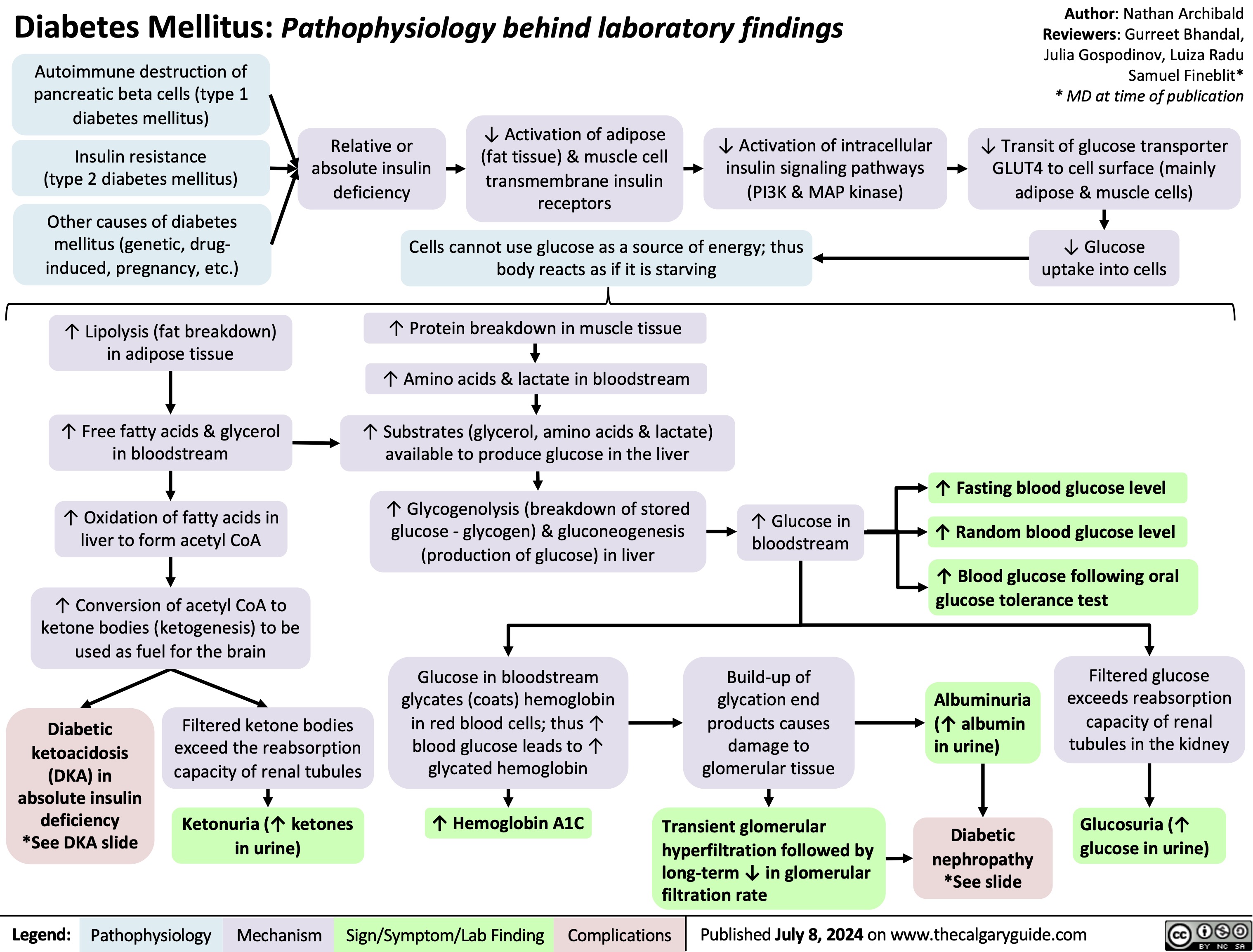
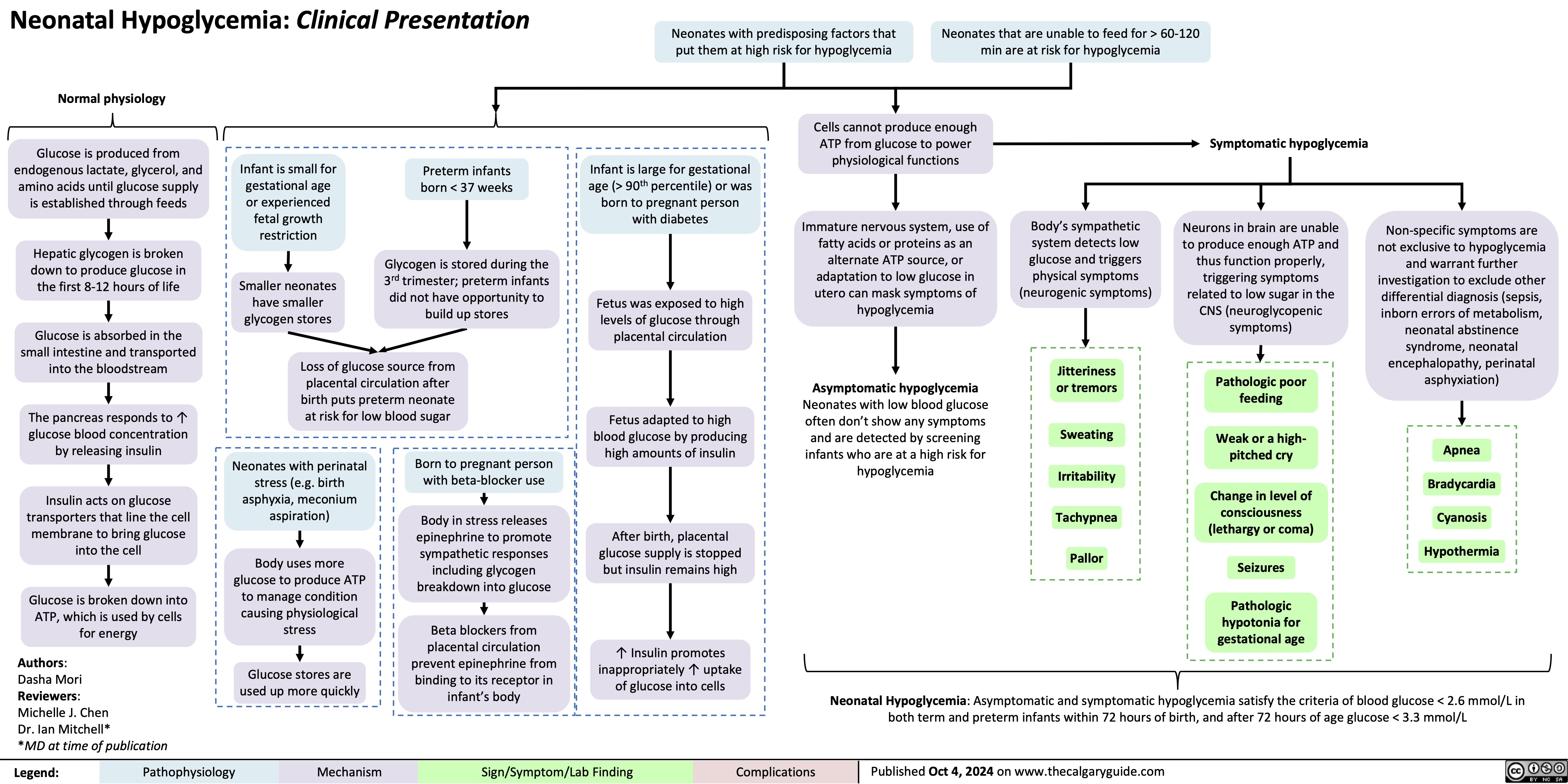
Neonatal Hypoglycemia Pathogenesis
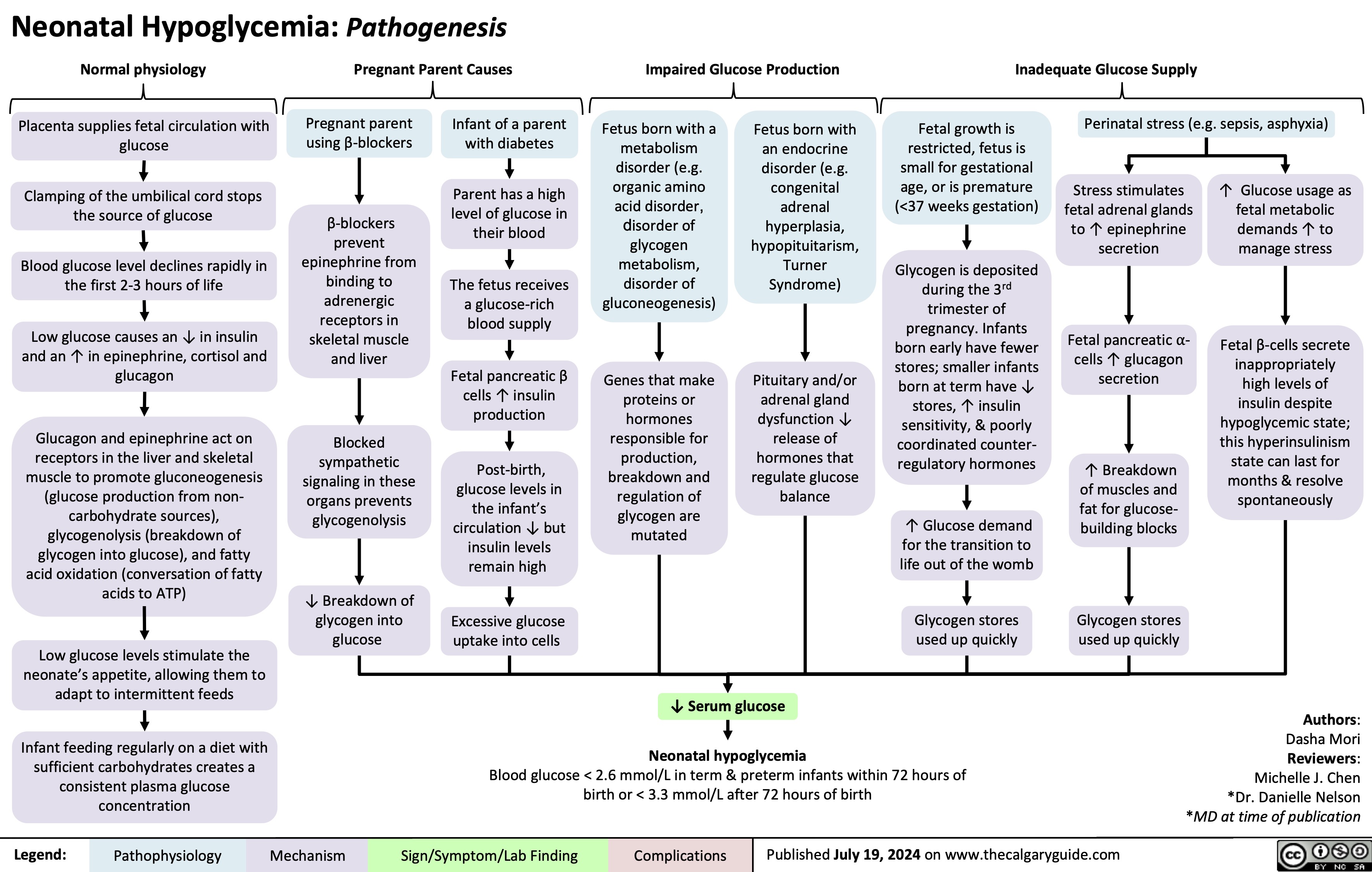
Diabete Gestazionale fattori di rischio e patogenesi
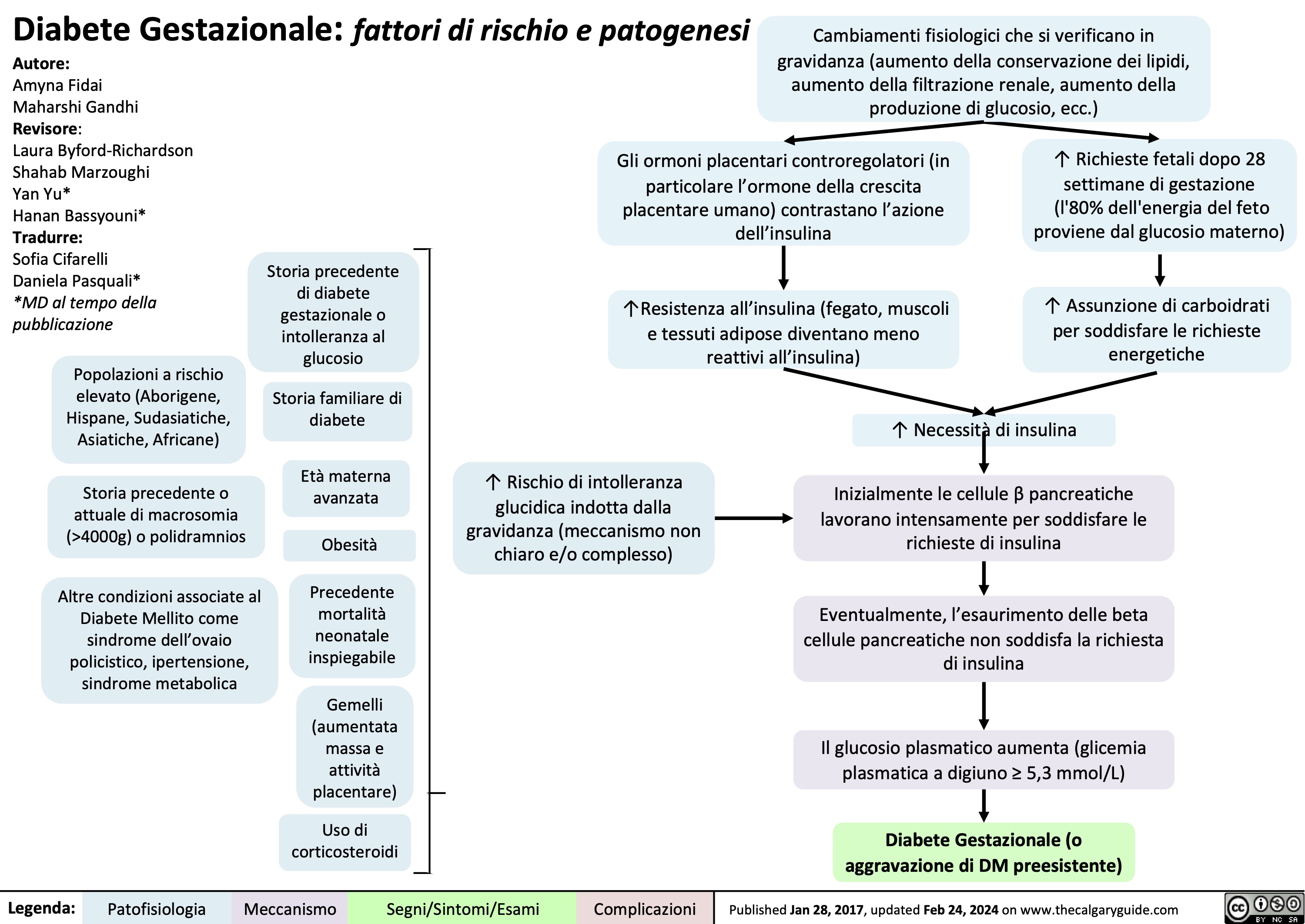
Obesity Pathogenesis
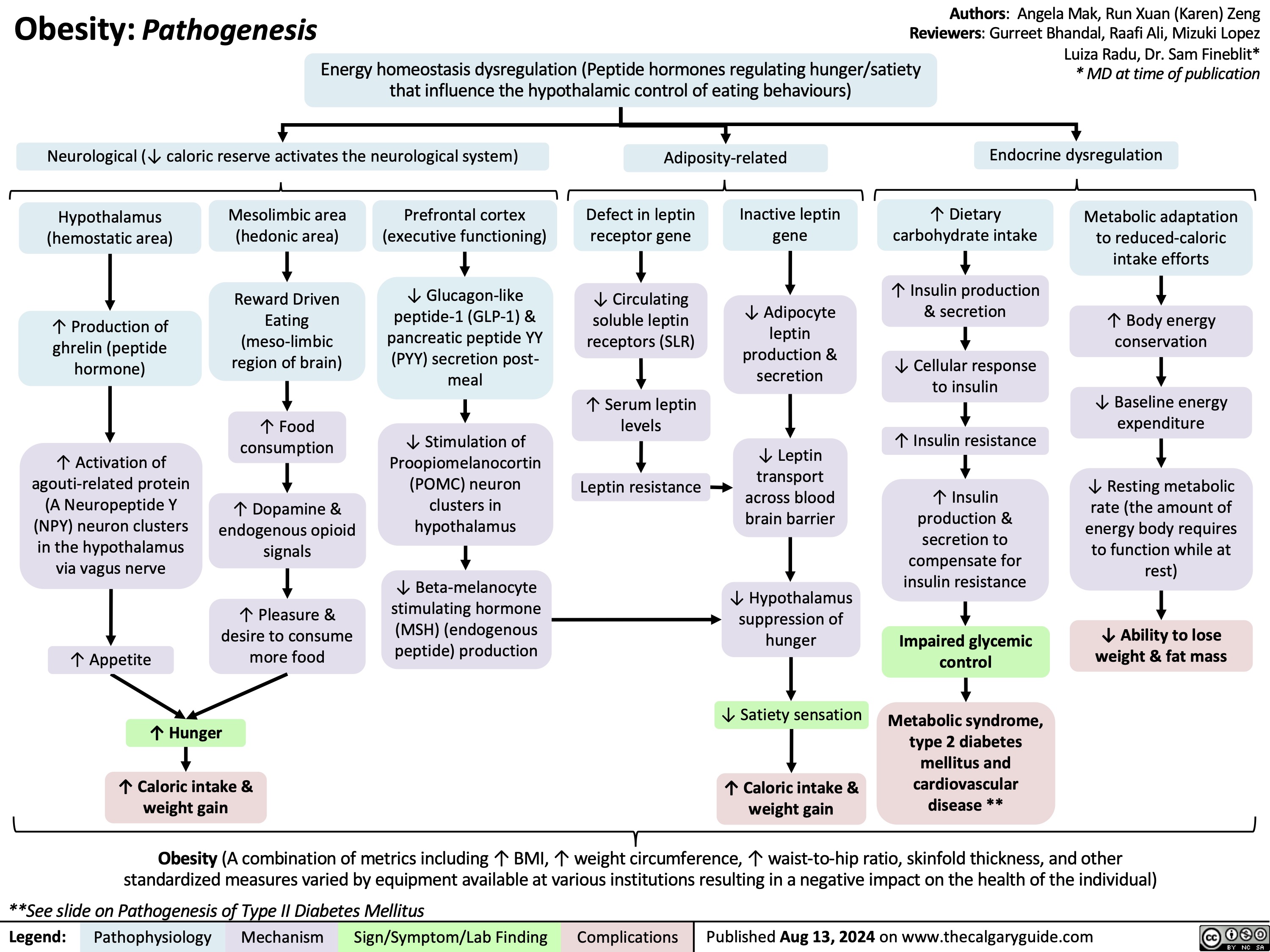
Transient Tachypnea of the Newborn
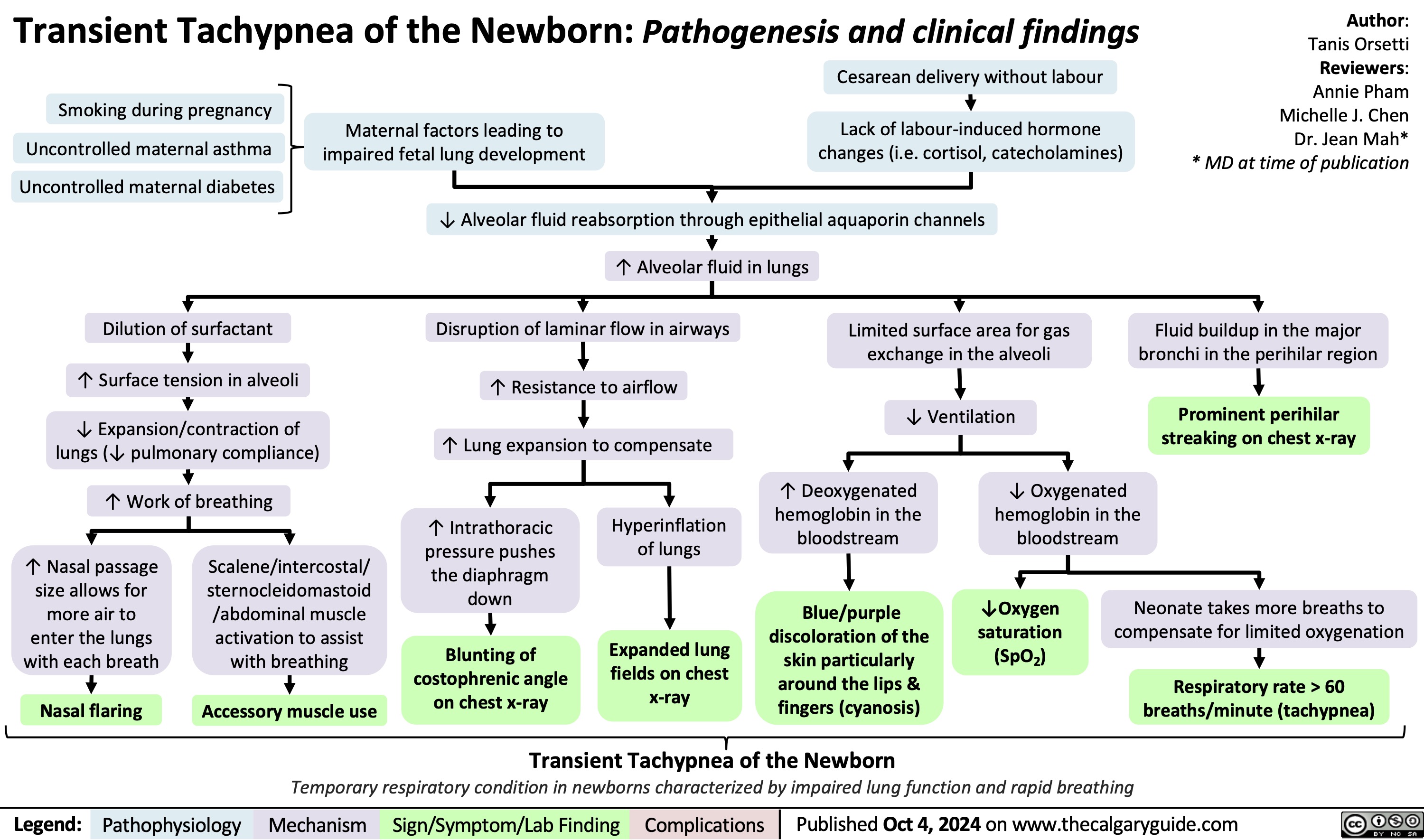
Neonatal Hypoglycemia Clinical Presentation
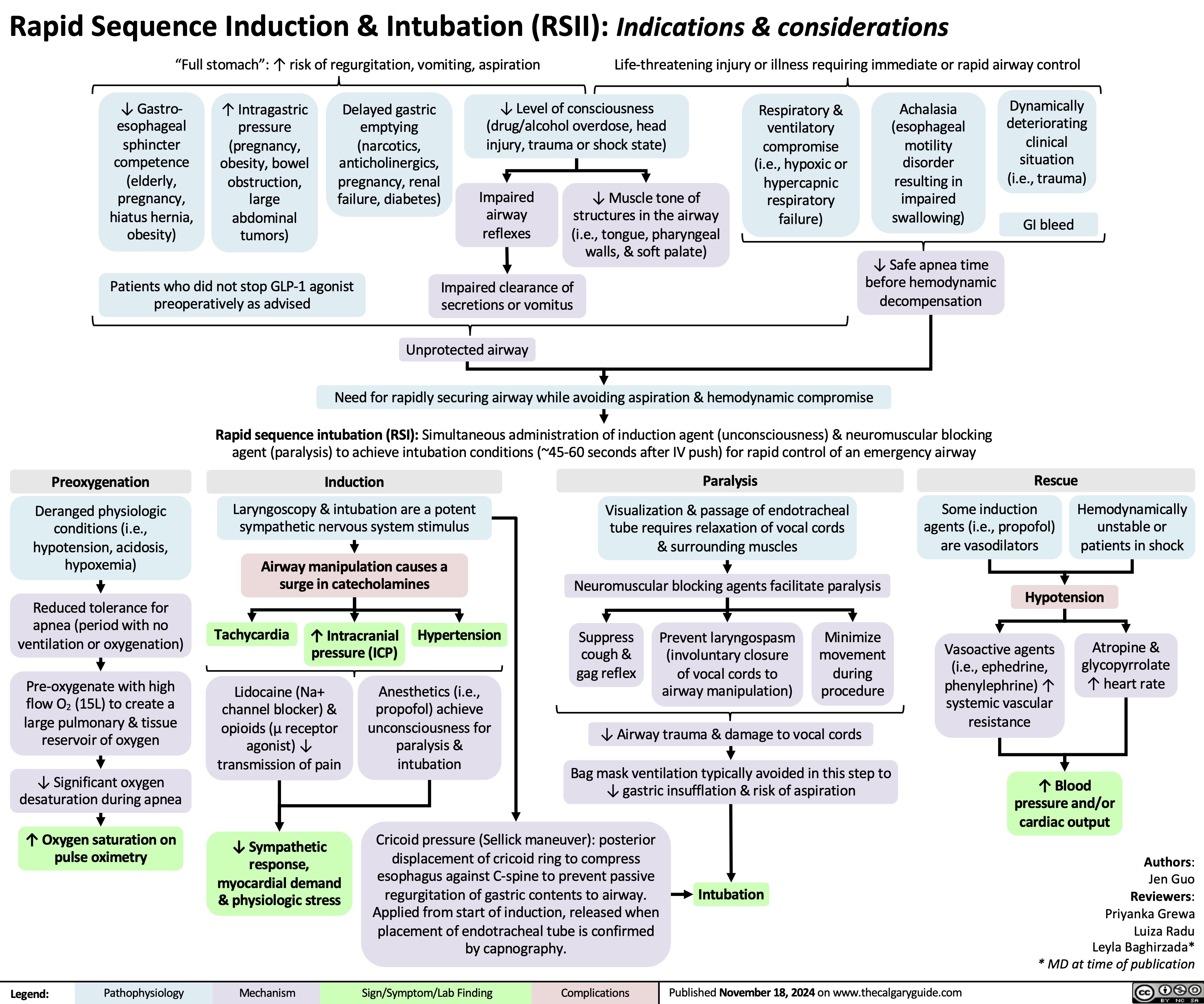
Rapid sequence induction and intubation
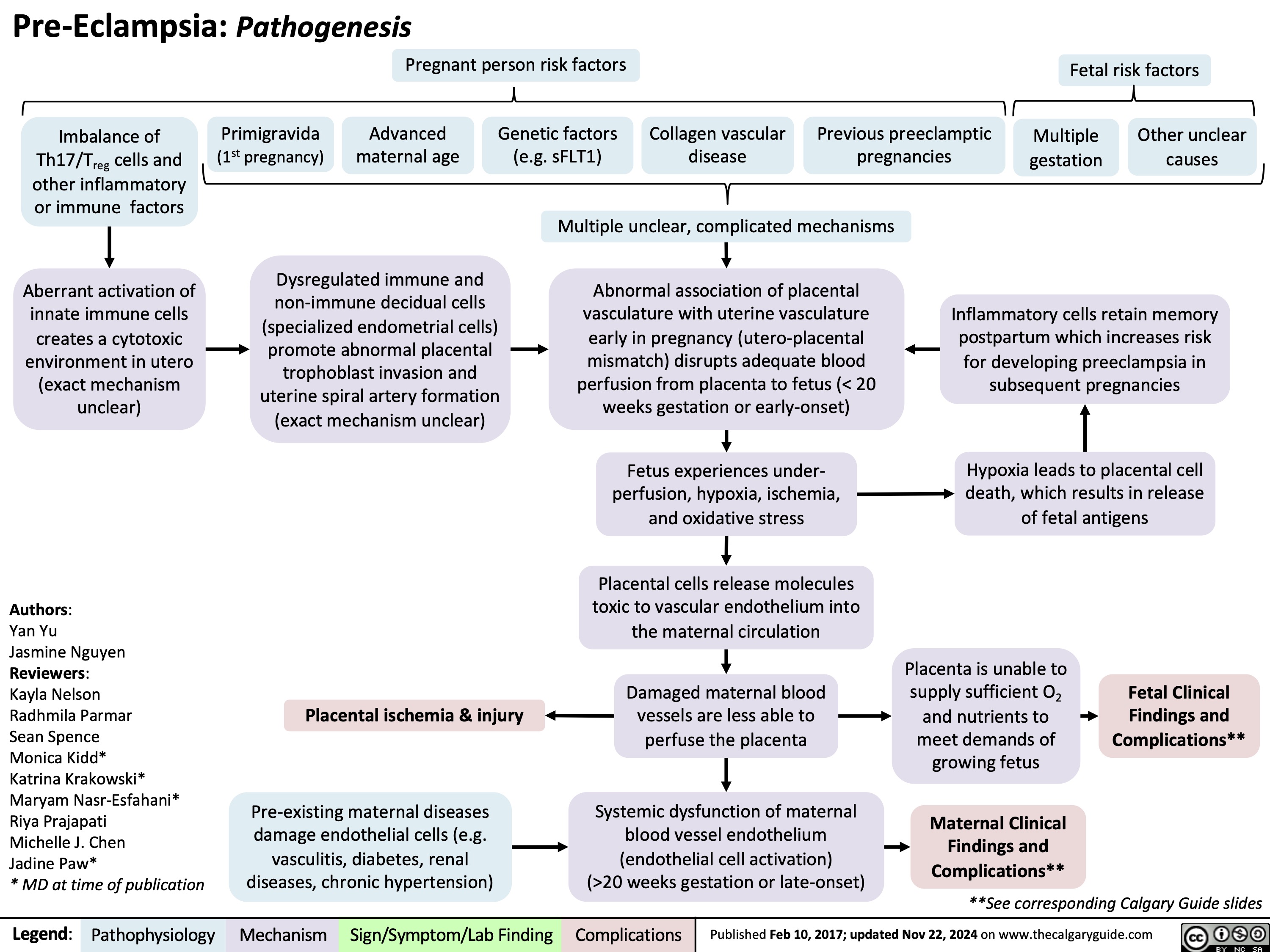
Pre-Eclampsia Pathogenesis
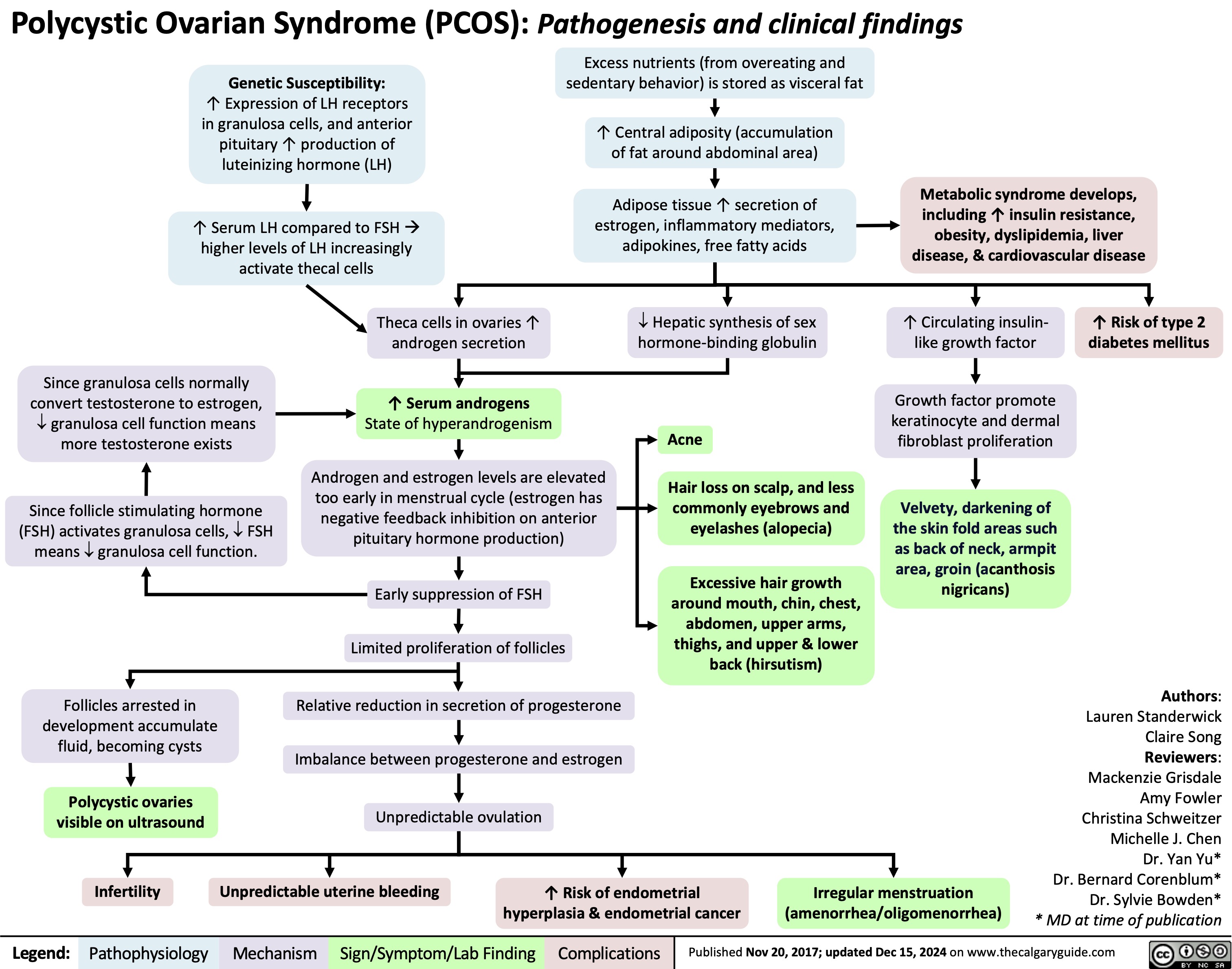
Polycystic Ovarian Syndrome
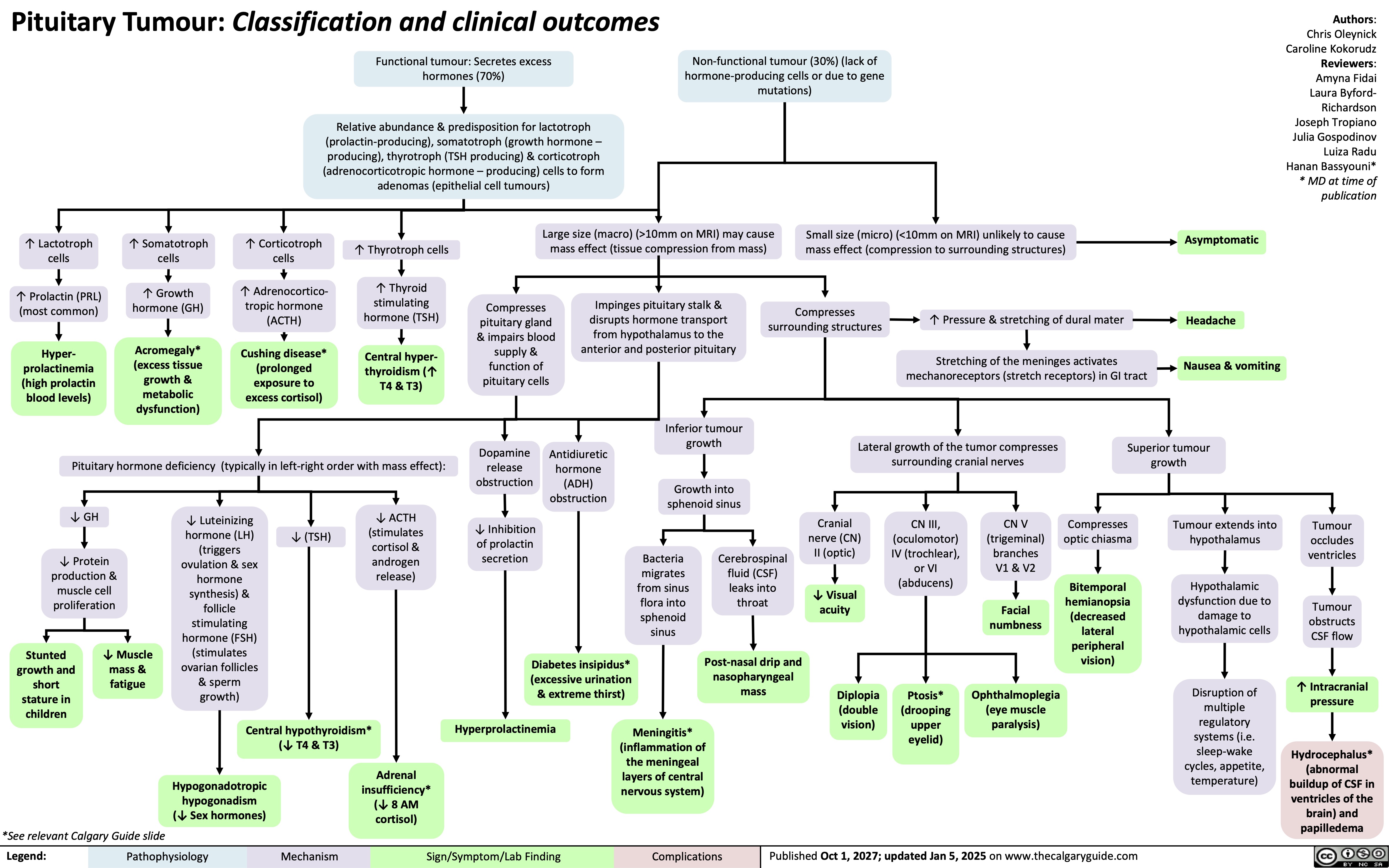
Pituitary Tumour Classification and Clinical Outcomes
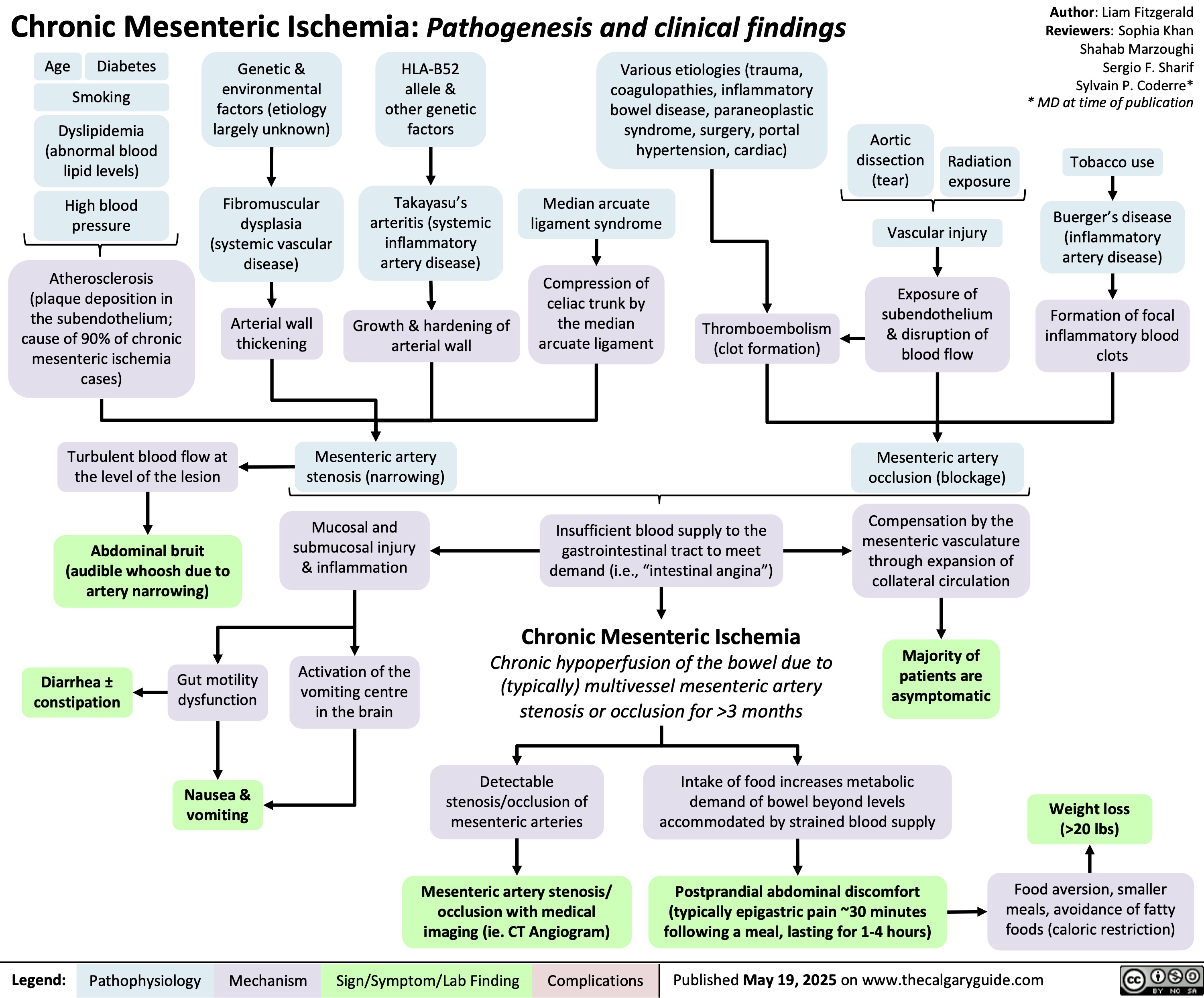
Chronic Mesenteric Ischemia
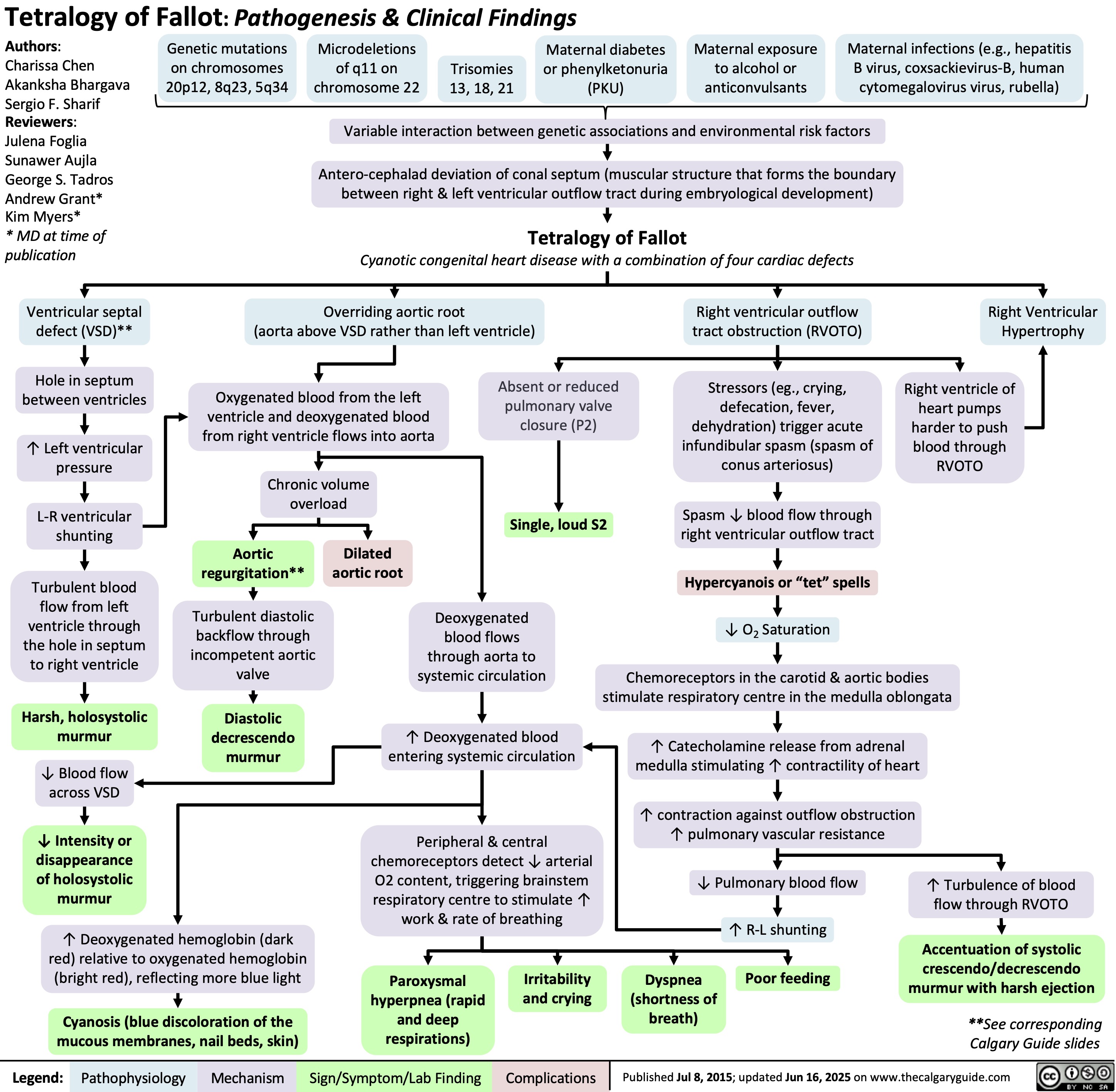
Tetralogy of Fallot
Obesity Hypoventilation Syndrome
![Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/06/Obesity-Hypoventilation-Syndrome.jpg)
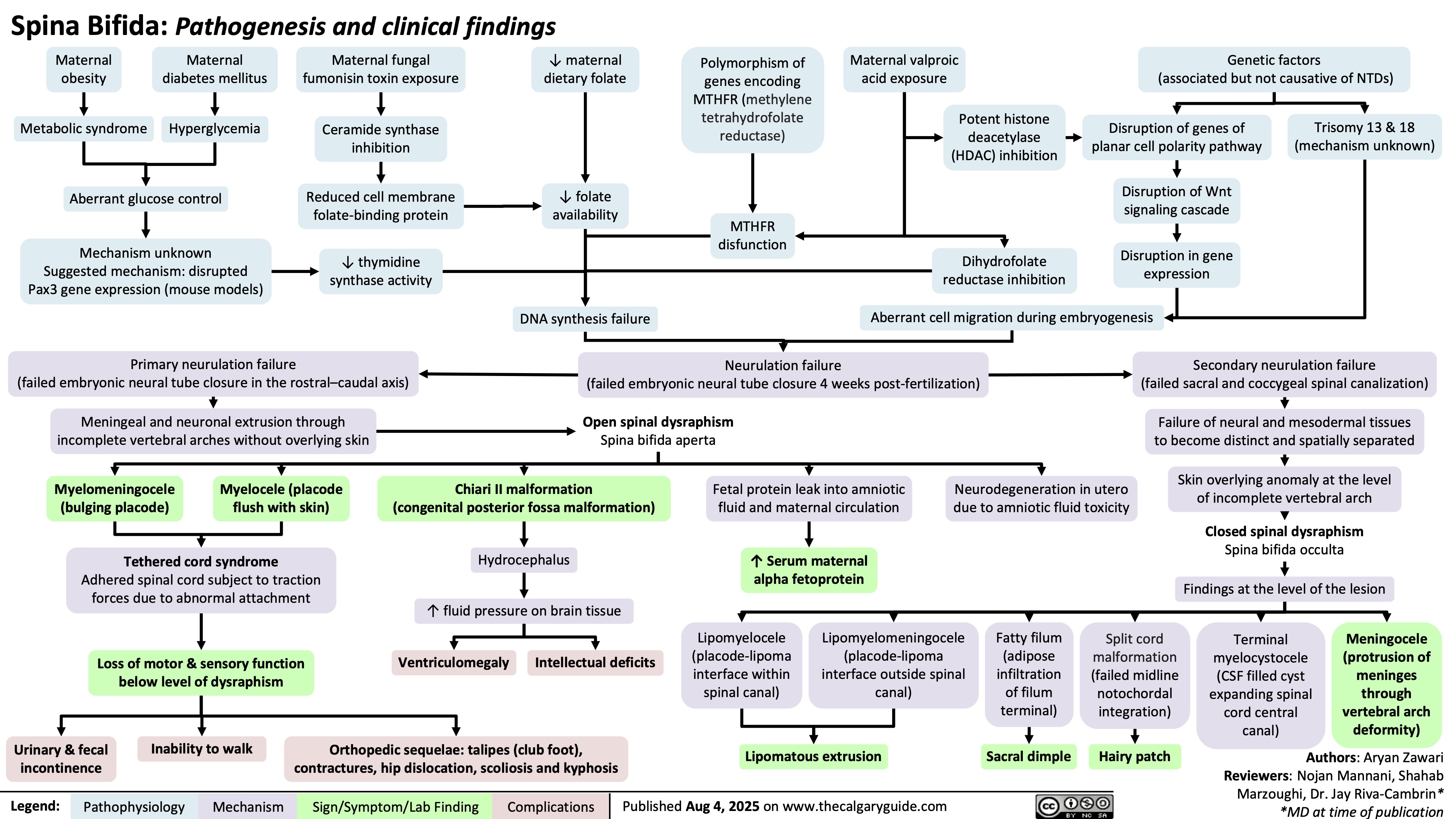
Spina Bifida
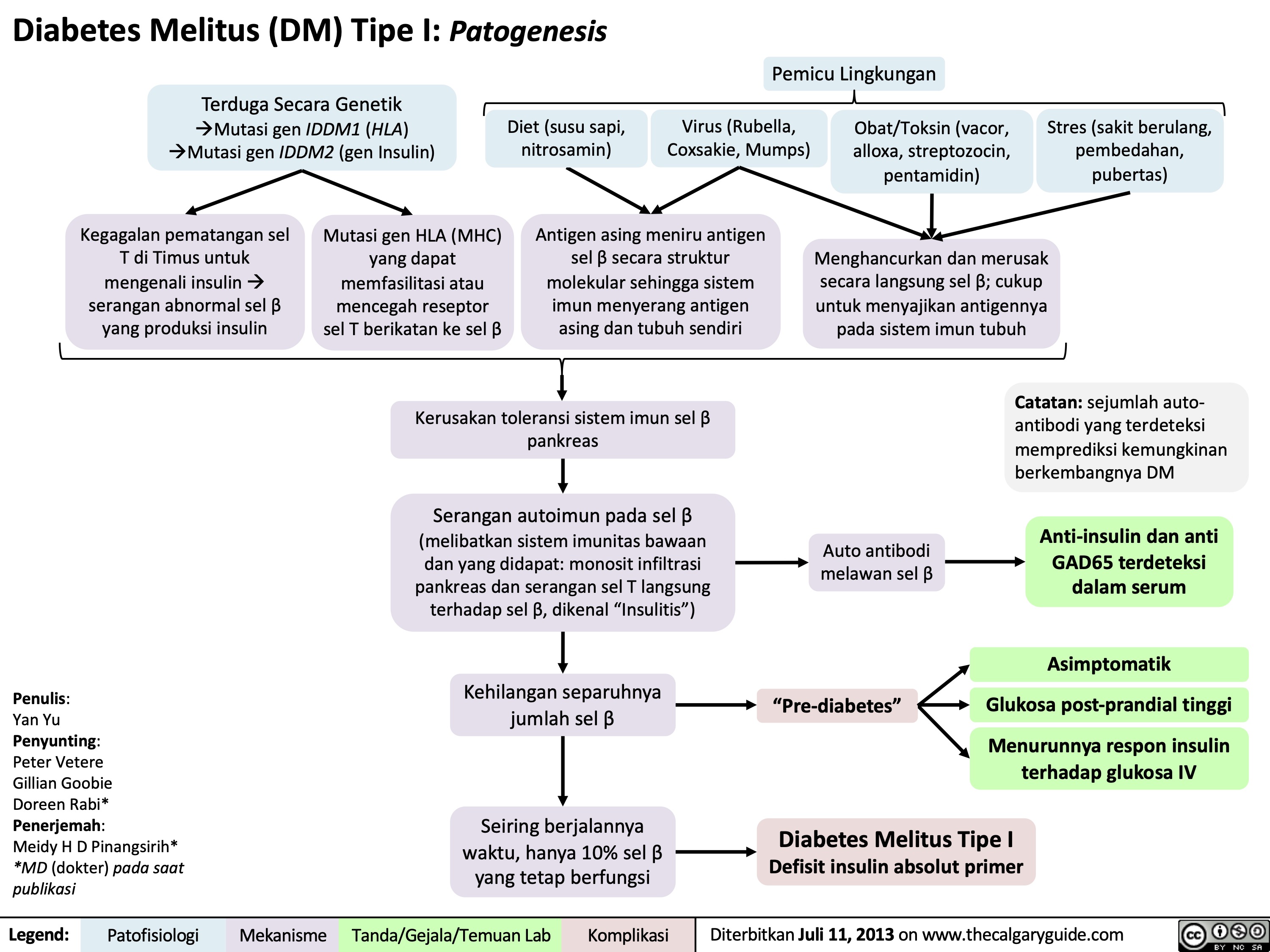
Diabetes Melitus Tipe I Patogenesis
Diabetes Melitus Tipe II Patogenesis
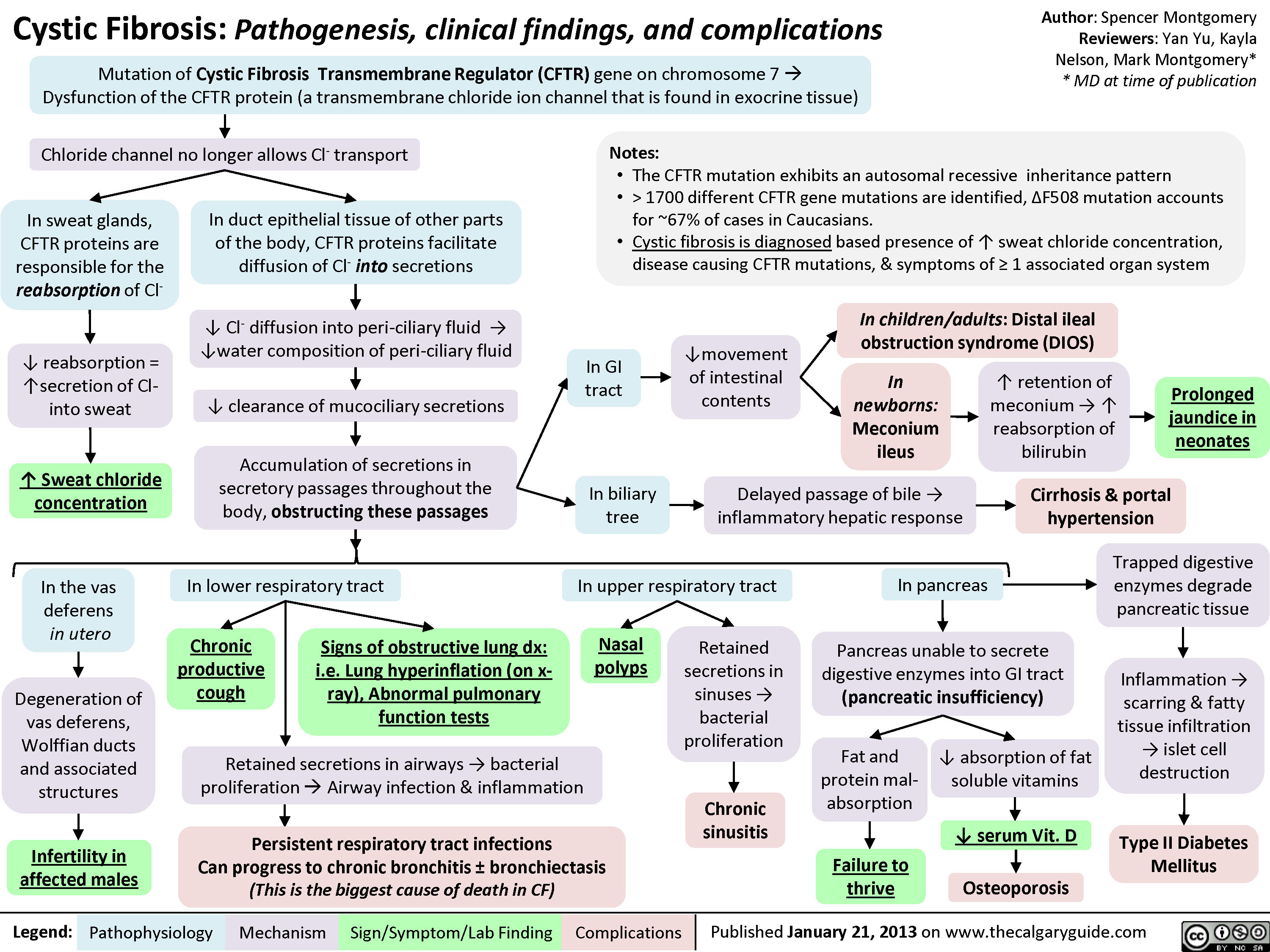
Cystic Fibrosis
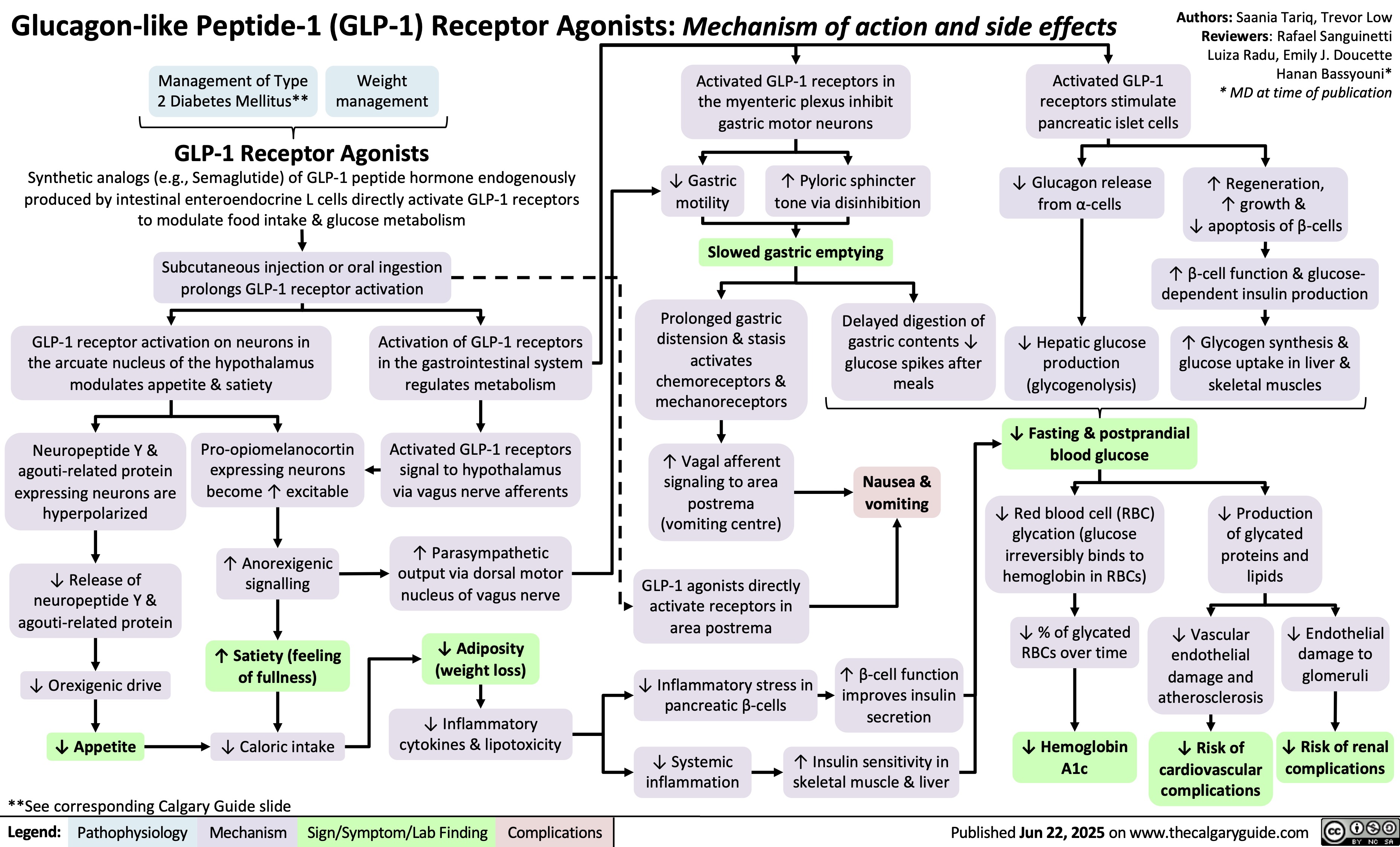
GLP-1 Receptor Agonists
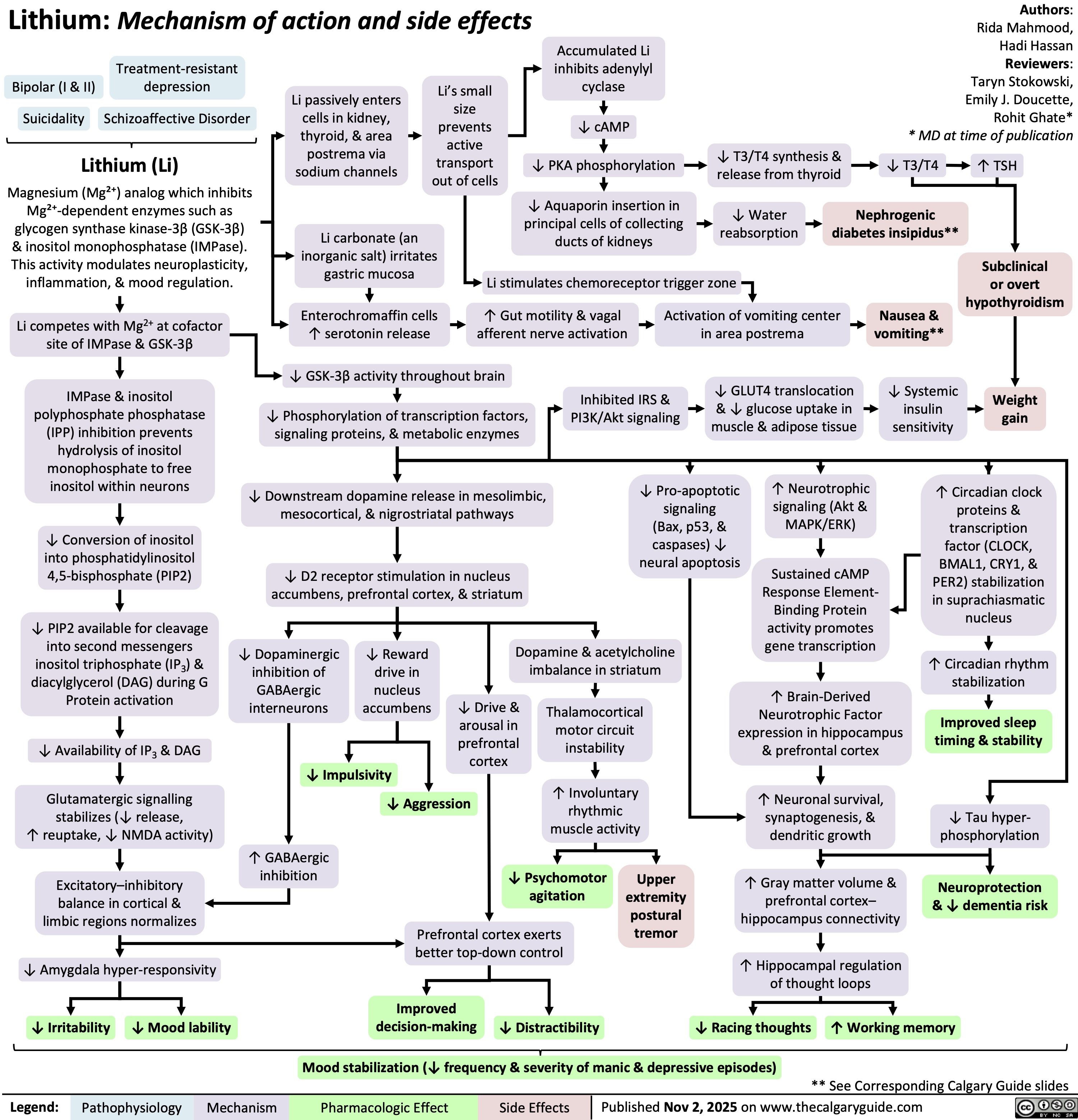
Lithium
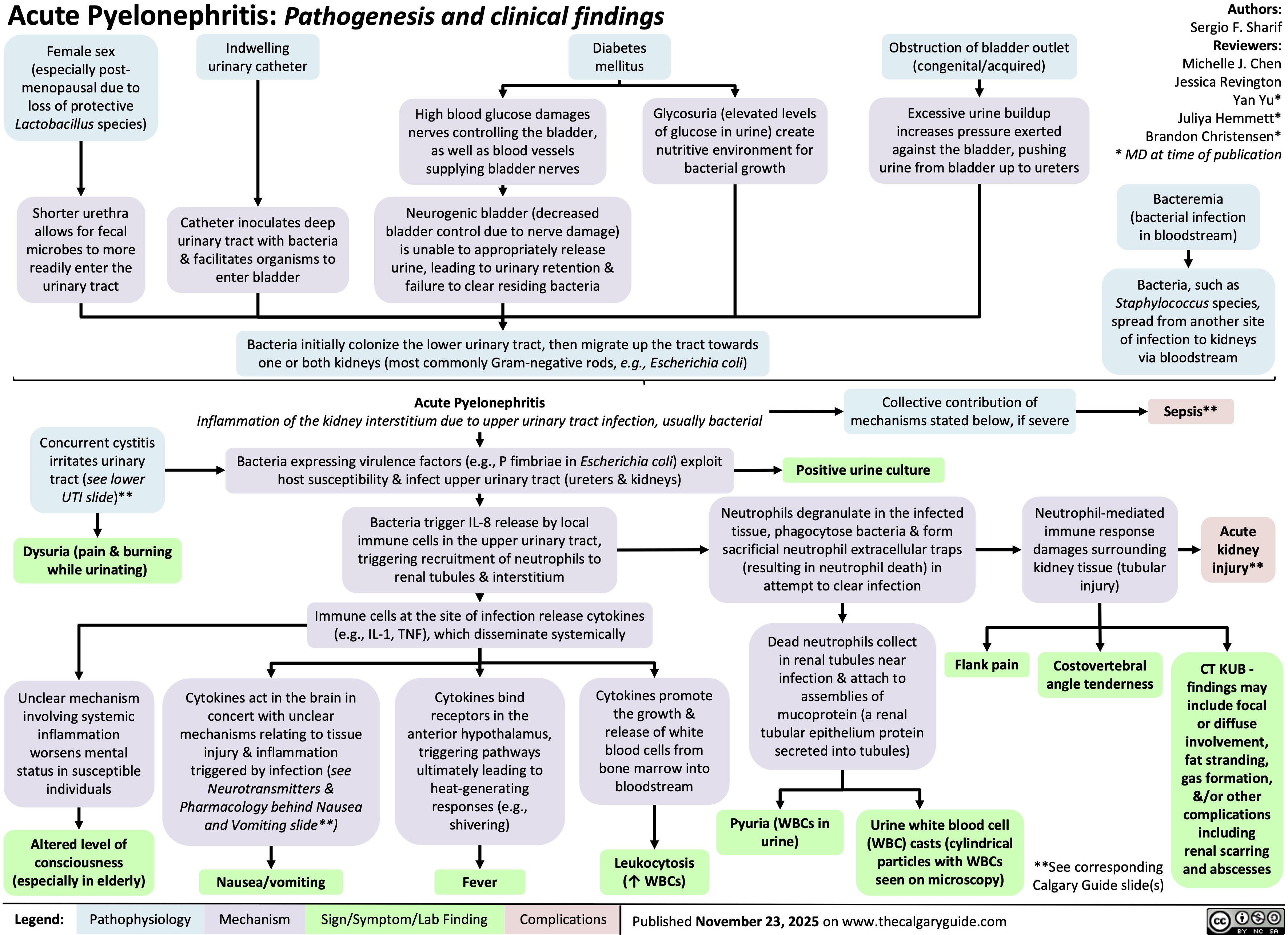
Pyelonephritis
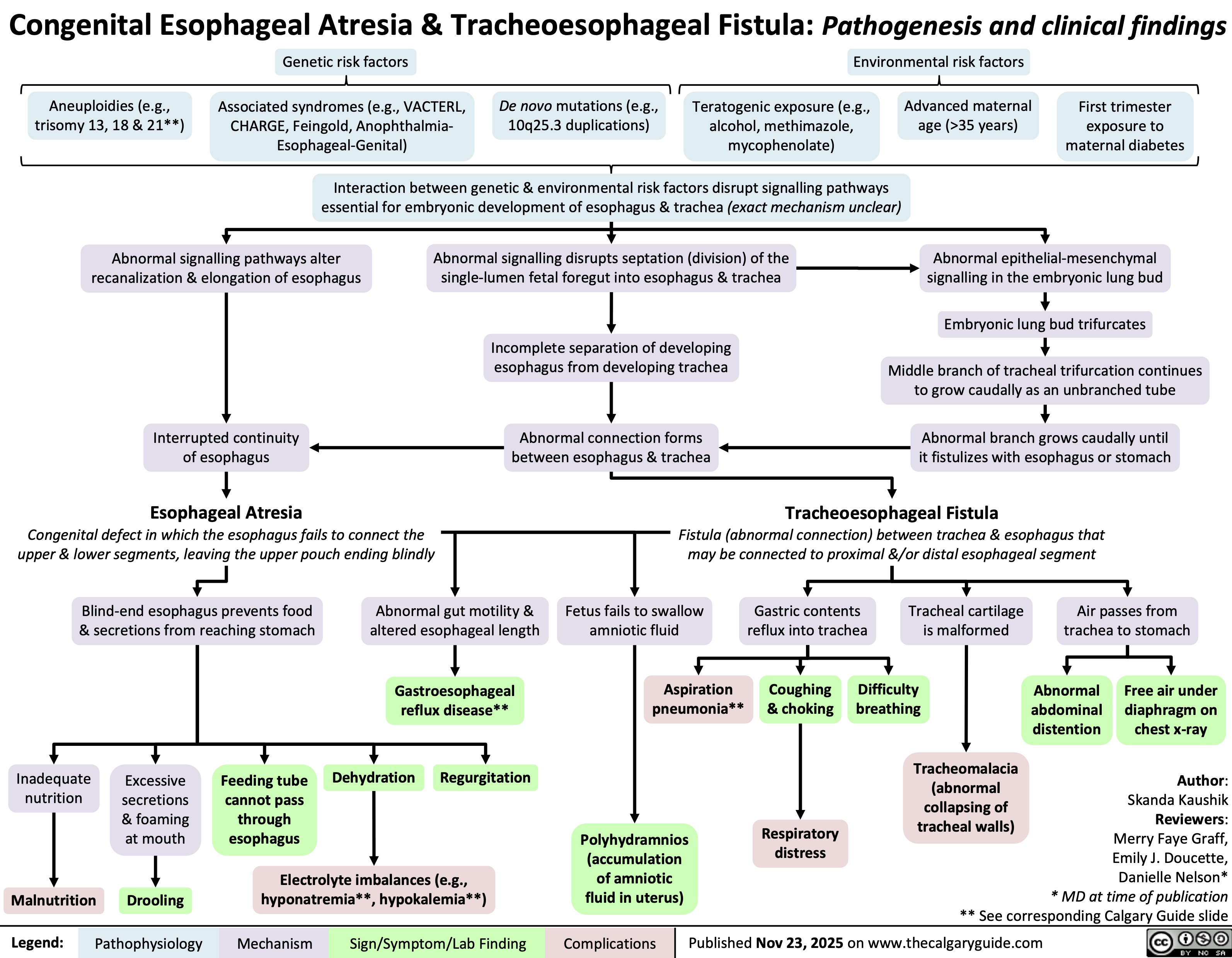
Esophageal Atresia & Tracheoesophageal Fistula
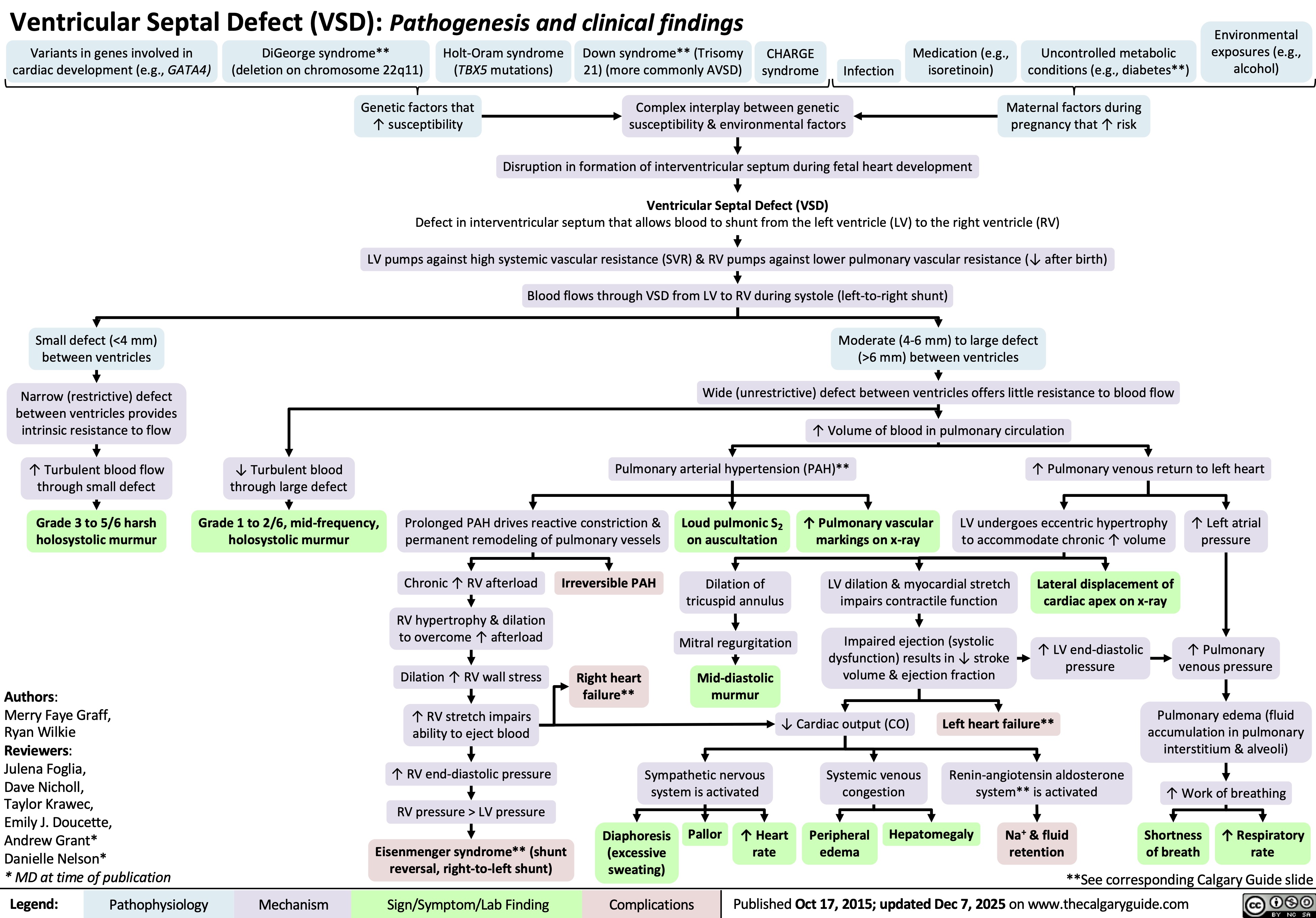
Ventricular Septal Defect
Chronic Hypertensive Retinopathy
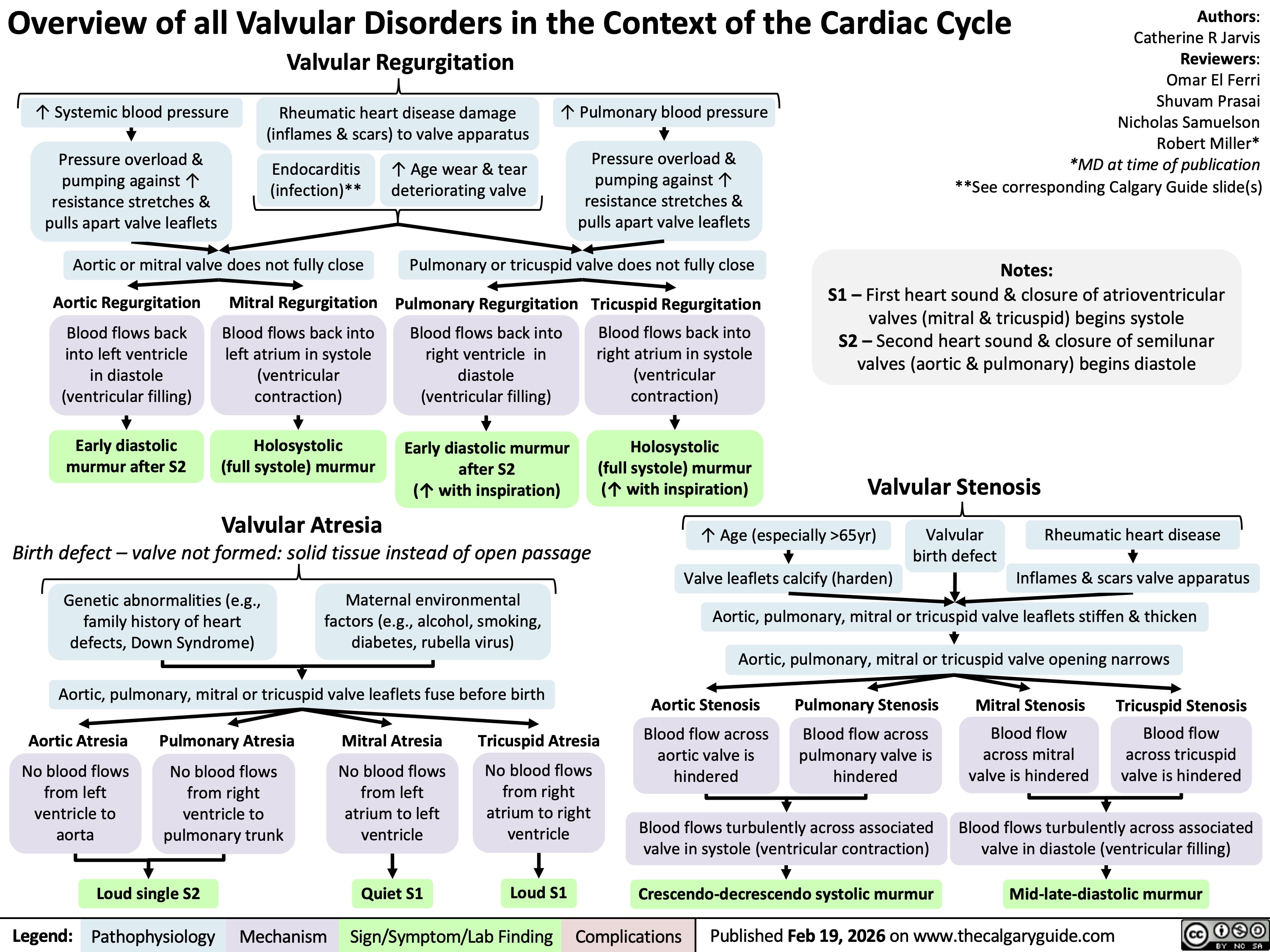
Overview of Valvular Disorders


 0.5 of R-R interval)?Flatter T-Waves ?Inverted T-waves (with more severe hypokalemia)Purkinje fibers repolarize after the rest of the myocardium has done soU-waves (upward ECG deviations after the T-wave)Cells become hyperpolarized: Inside of cells are more negative relative to outside, ? Resting Membrane Potential (RMP)In the Kidney:Generalized Muscle weaknessK+ diffuse out of Proximal Convoluted Tubule & Collecting Duct cells ? cells retain acidic H+ inside (maintains electrical neutrality)? pH within PCT cells ? glutaminase activity, ? glutamine breakdown, producing HCO3-, which enters the blood? blood pH, [HCO3-], & pCO2 (respiratory compensation)Low Plasma [K+]Abnormally long diastole means that ventricles are overfilled. Contraction takes greater force; sensed by patientsDyspnea, fatigue, dizziness, syncope? cardiac output ? perfusion of tissues, i.e. lungs & brainCardiac arrhythmias: PACs, PVCs, Sinus Bradycardia, paroxysmal atrial/junctional tachycardia, VT (i.e. Torsades de pointes), V-Fib? smooth muscle contractile abilityBowel ileus (bloating, anorexia, nausea/vomiting, absent bowel sounds)? pH in collecting duct intercalated cells ? H+ secretion into the tubuleMetabolic alkalosisParalysis, muscle cramps (in severe hypokalemia)Respiratory muscle failure (? tidal volume, ? pCO2, ? pO2), may even cause death!? depolarizations ? adenyl cyclase activity ? ? sensitivity of collecting duct cells to ADH? ability of nephron to concentrate urineNephrogenic Diabetes Insipidus? urine osmolality, Hypernatremia, Polyuria, Polydipsia? # of aquaporins in the collecting duct membrane"Insulin Resistance": ? ability to import K+ from the blood in response to insulinIn skeletal muscle:
117 kB / 307 word" title="Yu, Yan - Hypokalemia clinical findings - FINAL.pptx
Production of Na+/ K+ transporters in cell membranes ? over timeHypokalemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceAndrew Wade** MD at time of publicationLegend:Published May 21, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsPalpitationsExcitable cells (muscle cells, neurons) depolarize less readilyK+ efflux out of all cells in the body, down its concentration gradientCardiac myocytes experience electrical conduction defects? muscle impulse conductionECG shows characteristic changes:? skeletal muscle contractile abilityRMP now more negative; myocytes take longer to repolarize to RMP("stretches out" the T-wave)! Long QT interval (>0.5 of R-R interval)?Flatter T-Waves ?Inverted T-waves (with more severe hypokalemia)Purkinje fibers repolarize after the rest of the myocardium has done soU-waves (upward ECG deviations after the T-wave)Cells become hyperpolarized: Inside of cells are more negative relative to outside, ? Resting Membrane Potential (RMP)In the Kidney:Generalized Muscle weaknessK+ diffuse out of Proximal Convoluted Tubule & Collecting Duct cells ? cells retain acidic H+ inside (maintains electrical neutrality)? pH within PCT cells ? glutaminase activity, ? glutamine breakdown, producing HCO3-, which enters the blood? blood pH, [HCO3-], & pCO2 (respiratory compensation)Low Plasma [K+]Abnormally long diastole means that ventricles are overfilled. Contraction takes greater force; sensed by patientsDyspnea, fatigue, dizziness, syncope? cardiac output ? perfusion of tissues, i.e. lungs & brainCardiac arrhythmias: PACs, PVCs, Sinus Bradycardia, paroxysmal atrial/junctional tachycardia, VT (i.e. Torsades de pointes), V-Fib? smooth muscle contractile abilityBowel ileus (bloating, anorexia, nausea/vomiting, absent bowel sounds)? pH in collecting duct intercalated cells ? H+ secretion into the tubuleMetabolic alkalosisParalysis, muscle cramps (in severe hypokalemia)Respiratory muscle failure (? tidal volume, ? pCO2, ? pO2), may even cause death!? depolarizations ? adenyl cyclase activity ? ? sensitivity of collecting duct cells to ADH? ability of nephron to concentrate urineNephrogenic Diabetes Insipidus? urine osmolality, Hypernatremia, Polyuria, Polydipsia? # of aquaporins in the collecting duct membrane"Insulin Resistance": ? ability to import K+ from the blood in response to insulinIn skeletal muscle:
117 kB / 307 word" />
0.5 of R-R interval)?Flatter T-Waves ?Inverted T-waves (with more severe hypokalemia)Purkinje fibers repolarize after the rest of the myocardium has done soU-waves (upward ECG deviations after the T-wave)Cells become hyperpolarized: Inside of cells are more negative relative to outside, ? Resting Membrane Potential (RMP)In the Kidney:Generalized Muscle weaknessK+ diffuse out of Proximal Convoluted Tubule & Collecting Duct cells ? cells retain acidic H+ inside (maintains electrical neutrality)? pH within PCT cells ? glutaminase activity, ? glutamine breakdown, producing HCO3-, which enters the blood? blood pH, [HCO3-], & pCO2 (respiratory compensation)Low Plasma [K+]Abnormally long diastole means that ventricles are overfilled. Contraction takes greater force; sensed by patientsDyspnea, fatigue, dizziness, syncope? cardiac output ? perfusion of tissues, i.e. lungs & brainCardiac arrhythmias: PACs, PVCs, Sinus Bradycardia, paroxysmal atrial/junctional tachycardia, VT (i.e. Torsades de pointes), V-Fib? smooth muscle contractile abilityBowel ileus (bloating, anorexia, nausea/vomiting, absent bowel sounds)? pH in collecting duct intercalated cells ? H+ secretion into the tubuleMetabolic alkalosisParalysis, muscle cramps (in severe hypokalemia)Respiratory muscle failure (? tidal volume, ? pCO2, ? pO2), may even cause death!? depolarizations ? adenyl cyclase activity ? ? sensitivity of collecting duct cells to ADH? ability of nephron to concentrate urineNephrogenic Diabetes Insipidus? urine osmolality, Hypernatremia, Polyuria, Polydipsia? # of aquaporins in the collecting duct membrane"Insulin Resistance": ? ability to import K+ from the blood in response to insulinIn skeletal muscle:
117 kB / 307 word" title="Yu, Yan - Hypokalemia clinical findings - FINAL.pptx
Production of Na+/ K+ transporters in cell membranes ? over timeHypokalemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceAndrew Wade** MD at time of publicationLegend:Published May 21, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsPalpitationsExcitable cells (muscle cells, neurons) depolarize less readilyK+ efflux out of all cells in the body, down its concentration gradientCardiac myocytes experience electrical conduction defects? muscle impulse conductionECG shows characteristic changes:? skeletal muscle contractile abilityRMP now more negative; myocytes take longer to repolarize to RMP("stretches out" the T-wave)! Long QT interval (>0.5 of R-R interval)?Flatter T-Waves ?Inverted T-waves (with more severe hypokalemia)Purkinje fibers repolarize after the rest of the myocardium has done soU-waves (upward ECG deviations after the T-wave)Cells become hyperpolarized: Inside of cells are more negative relative to outside, ? Resting Membrane Potential (RMP)In the Kidney:Generalized Muscle weaknessK+ diffuse out of Proximal Convoluted Tubule & Collecting Duct cells ? cells retain acidic H+ inside (maintains electrical neutrality)? pH within PCT cells ? glutaminase activity, ? glutamine breakdown, producing HCO3-, which enters the blood? blood pH, [HCO3-], & pCO2 (respiratory compensation)Low Plasma [K+]Abnormally long diastole means that ventricles are overfilled. Contraction takes greater force; sensed by patientsDyspnea, fatigue, dizziness, syncope? cardiac output ? perfusion of tissues, i.e. lungs & brainCardiac arrhythmias: PACs, PVCs, Sinus Bradycardia, paroxysmal atrial/junctional tachycardia, VT (i.e. Torsades de pointes), V-Fib? smooth muscle contractile abilityBowel ileus (bloating, anorexia, nausea/vomiting, absent bowel sounds)? pH in collecting duct intercalated cells ? H+ secretion into the tubuleMetabolic alkalosisParalysis, muscle cramps (in severe hypokalemia)Respiratory muscle failure (? tidal volume, ? pCO2, ? pO2), may even cause death!? depolarizations ? adenyl cyclase activity ? ? sensitivity of collecting duct cells to ADH? ability of nephron to concentrate urineNephrogenic Diabetes Insipidus? urine osmolality, Hypernatremia, Polyuria, Polydipsia? # of aquaporins in the collecting duct membrane"Insulin Resistance": ? ability to import K+ from the blood in response to insulinIn skeletal muscle:
117 kB / 307 word" />
 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />

![Yu, Yan - Diabetic Hypoglycemia - Clinical Findings - FINAL.pptx
? Epinephrine(Released within seconds as [glucose] falls further) Growth hormone, ? Cortisol (if hypoglycemia persists for minutes)Glucagon should ? when [glucose] falls. But here, glucagon release is inhibited by 1) diabetic auto-immune destruction of Alpha cells & 2) the high insulin.43210Plasma Glucose concentration (mmol/L)Liver should ? glycogenolysis & gluconeogenesisPeripheral vaso-constrictionPlasma [glucose] stays lowActivation of sympathetic (adrenergic) receptors across body, triggering Neurogenic symptomsPlasma [glucose] ?Excess subcutaneous insulin or insulin-secretagogue ?? [insulin] in the bloodOver time: [insulin] in the DM patient depends only on how much was injected or how much secretagogue was consumed; not on the body's physiological state.[Insulin] stays high in excessively-treated DM patientsPlasma [glucose] normally ?, but...High insulin transports plasma glucose into cells!In pts with existing diabetic autonomic neuropathy, epi-nephrine secretion will already be ?Brain does not get enough glucose, ? neuron function ? Neuroglycopenic symptomsTx: glucose intake![Glucose] returns to normalIf no glucose intake:Hypoglycemia-unawareness: No autonomic Sx felt so hypoglycemia not treated early ? pts present later on with more severe hypoglycemia and neuroglycopenic sxBrain cells kept chronically euglycemic due to GLUT1 receptor over-expression (despite rest of body being hypoglycemic)With many hypoglycemic events over time:Brain feels no need to ? glucose, so it ? autonomic epinephrine secretion!This is the normal sequence of hormone responses to ?ing plasma glucose levels.But this normal hormonal response will be blunted over time if there is 1) continued hypoglycemia dampening the sympathetic nervous system, and 2) long-standing diabetic neuropathy! (To be explained later in this flow chart)Abbreviations: [ ] = concentrationTx = TreatmentDM = Diabetes mellitusDiabetic Hypoglycemia: Pathogenesis and Clinical FindingsConfusionCan't concentrateWeaknessSlurred speech? coordination (staggering, etc)SeizuresComa, deathAdrenergic symptoms (epinephrine-mediated):Anxiety, irritability, trembling, pallor (skin vasoconstriction), palpitations, ? systolic BP, tachycardia Cholinergic symptoms(Acetylcholine-mediated):Sweating, hunger, tingling, blurry visionNote: In pts w/out DM, endogenous insulin secretion normally stops when blood [glucose] drops to <4mmol/LAuthor: Yan YuReviewers: Peter Vetere, Gillian Goobie, Hanan Bassyouni** MD at time of publicationLegend:Published June 14, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsMany hypoglycemic events over time blunt epinephrine secretion further.Hypoglycemia unawareness can be reversedIf pt stays hypoglycemia-free for >6 weeks, brain restores its ability to detect low glucose levels? peripheral glucose delivery and uptake (saving more glucose for the brain)Lack of glucagon effect reinforces hypoglycemia
124 kB / 361 words Yu, Yan - Diabetic Hypoglycemia - Clinical Findings - FINAL.pptx
? Epinephrine(Released within seconds as [glucose] falls further) Growth hormone, ? Cortisol (if hypoglycemia persists for minutes)Glucagon should ? when [glucose] falls. But here, glucagon release is inhibited by 1) diabetic auto-immune destruction of Alpha cells & 2) the high insulin.43210Plasma Glucose concentration (mmol/L)Liver should ? glycogenolysis & gluconeogenesisPeripheral vaso-constrictionPlasma [glucose] stays lowActivation of sympathetic (adrenergic) receptors across body, triggering Neurogenic symptomsPlasma [glucose] ?Excess subcutaneous insulin or insulin-secretagogue ?? [insulin] in the bloodOver time: [insulin] in the DM patient depends only on how much was injected or how much secretagogue was consumed; not on the body's physiological state.[Insulin] stays high in excessively-treated DM patientsPlasma [glucose] normally ?, but...High insulin transports plasma glucose into cells!In pts with existing diabetic autonomic neuropathy, epi-nephrine secretion will already be ?Brain does not get enough glucose, ? neuron function ? Neuroglycopenic symptomsTx: glucose intake![Glucose] returns to normalIf no glucose intake:Hypoglycemia-unawareness: No autonomic Sx felt so hypoglycemia not treated early ? pts present later on with more severe hypoglycemia and neuroglycopenic sxBrain cells kept chronically euglycemic due to GLUT1 receptor over-expression (despite rest of body being hypoglycemic)With many hypoglycemic events over time:Brain feels no need to ? glucose, so it ? autonomic epinephrine secretion!This is the normal sequence of hormone responses to ?ing plasma glucose levels.But this normal hormonal response will be blunted over time if there is 1) continued hypoglycemia dampening the sympathetic nervous system, and 2) long-standing diabetic neuropathy! (To be explained later in this flow chart)Abbreviations: [ ] = concentrationTx = TreatmentDM = Diabetes mellitusDiabetic Hypoglycemia: Pathogenesis and Clinical FindingsConfusionCan't concentrateWeaknessSlurred speech? coordination (staggering, etc)SeizuresComa, deathAdrenergic symptoms (epinephrine-mediated):Anxiety, irritability, trembling, pallor (skin vasoconstriction), palpitations, ? systolic BP, tachycardia Cholinergic symptoms(Acetylcholine-mediated):Sweating, hunger, tingling, blurry visionNote: In pts w/out DM, endogenous insulin secretion normally stops when blood [glucose] drops to <4mmol/LAuthor: Yan YuReviewers: Peter Vetere, Gillian Goobie, Hanan Bassyouni** MD at time of publicationLegend:Published June 14, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsMany hypoglycemic events over time blunt epinephrine secretion further.Hypoglycemia unawareness can be reversedIf pt stays hypoglycemia-free for >6 weeks, brain restores its ability to detect low glucose levels? peripheral glucose delivery and uptake (saving more glucose for the brain)Lack of glucagon effect reinforces hypoglycemia
124 kB / 361 words](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Diabetic-Hypoglycemia-Clinical-Findings.jpg)


 10mm on MRI) vomiting Giant adenoma Extension into hypothalamus —1■• Damage to hypothalamic cells Hypothalamic (>40mm on MRI) dysfunction Obstruction of dopamine Superior tumor growth Impingement of the optic chiasma Bitemporal Loss of pituitary hemianopsia hormones ICP Suprasellar extension Occlusion of ventricles Obstruction of CSF Flow Hydrocephalus Lateral tumor growth Impingement of cranial nerves 3, 4, 5 (V1/V2) and 6 4 Pituitary stalk impingement Diplopia Inferior tumor growth Erosion into sphenoid sinus CSF leak into throat Post-nasal Obstruction of ADH drip Communication between sinus and brain Migration of bacteria from sinus flora Hyper-Diabetes Meningitis prolactinemia insipidus
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Complications
Published October 1 2017 on www.thecalgaryguide.com
" title="Pituitary Mass Effects
Note: pituitary tumors are almost always a benign adenoma. Pituitary adenomas are very common -approximately 1 in 6 individuals. These are usually asymptomatic and are found incidentally. Symptomatic pituitary adenomas that require treatment are much less common and affect approximately 1 in 1000 individuals.
Pituitary tumor
Note: typically (but not always) the anterior hormones will be lost in the following order; GH, LH, FSH, TSH, ACTH, PRL. This order (with the exception of prolactin) is the order of least-essential to most-essential hormones needed for survival. A good mnemonic to remember the order the hormones are is, "Go Look For The Adenoma Please".
Legend:
Note: for pituitary masses of all sizes, it is important to determine whether the pituitary tumor is secreting (70%) or non-secreting (30%) as secreting tumors can be targeted with medication. The most common secreting tumors secrete prolactin (most common), growth hormone, and ACTH.
Authors: Chris Oleynick Reviewers: Amyna Fidai Laura Byford-Richardson Joseph Tropiano Hanan Bassyouni* * MD at time of publication
Microadenoma Small size is unlikely to cause mass effects (<10mm on MRI) Asymptomatic Macroadenoma Large size may press on surrounding structures, causing mass effects Headaches Stretching of the meninges Activation of mechanoreceptors Nausea and (>10mm on MRI) vomiting Giant adenoma Extension into hypothalamus —1■• Damage to hypothalamic cells Hypothalamic (>40mm on MRI) dysfunction Obstruction of dopamine Superior tumor growth Impingement of the optic chiasma Bitemporal Loss of pituitary hemianopsia hormones ICP Suprasellar extension Occlusion of ventricles Obstruction of CSF Flow Hydrocephalus Lateral tumor growth Impingement of cranial nerves 3, 4, 5 (V1/V2) and 6 4 Pituitary stalk impingement Diplopia Inferior tumor growth Erosion into sphenoid sinus CSF leak into throat Post-nasal Obstruction of ADH drip Communication between sinus and brain Migration of bacteria from sinus flora Hyper-Diabetes Meningitis prolactinemia insipidus
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Complications
Published October 1 2017 on www.thecalgaryguide.com
" />
10mm on MRI) vomiting Giant adenoma Extension into hypothalamus —1■• Damage to hypothalamic cells Hypothalamic (>40mm on MRI) dysfunction Obstruction of dopamine Superior tumor growth Impingement of the optic chiasma Bitemporal Loss of pituitary hemianopsia hormones ICP Suprasellar extension Occlusion of ventricles Obstruction of CSF Flow Hydrocephalus Lateral tumor growth Impingement of cranial nerves 3, 4, 5 (V1/V2) and 6 4 Pituitary stalk impingement Diplopia Inferior tumor growth Erosion into sphenoid sinus CSF leak into throat Post-nasal Obstruction of ADH drip Communication between sinus and brain Migration of bacteria from sinus flora Hyper-Diabetes Meningitis prolactinemia insipidus
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Complications
Published October 1 2017 on www.thecalgaryguide.com
" title="Pituitary Mass Effects
Note: pituitary tumors are almost always a benign adenoma. Pituitary adenomas are very common -approximately 1 in 6 individuals. These are usually asymptomatic and are found incidentally. Symptomatic pituitary adenomas that require treatment are much less common and affect approximately 1 in 1000 individuals.
Pituitary tumor
Note: typically (but not always) the anterior hormones will be lost in the following order; GH, LH, FSH, TSH, ACTH, PRL. This order (with the exception of prolactin) is the order of least-essential to most-essential hormones needed for survival. A good mnemonic to remember the order the hormones are is, "Go Look For The Adenoma Please".
Legend:
Note: for pituitary masses of all sizes, it is important to determine whether the pituitary tumor is secreting (70%) or non-secreting (30%) as secreting tumors can be targeted with medication. The most common secreting tumors secrete prolactin (most common), growth hormone, and ACTH.
Authors: Chris Oleynick Reviewers: Amyna Fidai Laura Byford-Richardson Joseph Tropiano Hanan Bassyouni* * MD at time of publication
Microadenoma Small size is unlikely to cause mass effects (<10mm on MRI) Asymptomatic Macroadenoma Large size may press on surrounding structures, causing mass effects Headaches Stretching of the meninges Activation of mechanoreceptors Nausea and (>10mm on MRI) vomiting Giant adenoma Extension into hypothalamus —1■• Damage to hypothalamic cells Hypothalamic (>40mm on MRI) dysfunction Obstruction of dopamine Superior tumor growth Impingement of the optic chiasma Bitemporal Loss of pituitary hemianopsia hormones ICP Suprasellar extension Occlusion of ventricles Obstruction of CSF Flow Hydrocephalus Lateral tumor growth Impingement of cranial nerves 3, 4, 5 (V1/V2) and 6 4 Pituitary stalk impingement Diplopia Inferior tumor growth Erosion into sphenoid sinus CSF leak into throat Post-nasal Obstruction of ADH drip Communication between sinus and brain Migration of bacteria from sinus flora Hyper-Diabetes Meningitis prolactinemia insipidus
Pathophysiology Mechanism
Sign/Symptom/Lab Finding
Complications
Published October 1 2017 on www.thecalgaryguide.com
" />






![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)























![Hyperkalemia (intracellular shift and ↑ intake): Pathophysiology
↑ K+ dietary intake (rarely causative)
β2 receptor inhibition (i.e. beta blockers)
Digoxin
α1 receptor stimulation (i.e. epinephrine, norepinephrine)
Insulin deficiency or resistance (i.e. diabetes mellitus)
↓ Stimulation of NHE1 (moves 1 Na+ into cell, 1 H+ out of cell) throughout body
Normal Anion- Gap Metabolic Acidosis (NAGMA)
Excess serum H+ results in ↓ NHE1 activity (See NAGMA: Pathogenesis and Laboratory Findings slide)
↑ Serum osmolarity (i.e. hyperglycemia)
Osmotic movement of water from cells to serum
Cell lysis (i.e. tumour lysis syndrome, rhabdomyolysis, hemolytic anemia)
↑ K+ release from lysed cells into the serum
Na+/K+ ATPase (moves 3K+ into cell, 2 Na+ out) activity inhibited on cells throughout body
↓ Activity of Na+/K+ ATPase on cells throughout the body
↓ Amount of K+ entering cells
↓ NHE1 activity prevents Na+ from entering the cell
Lack of high intracellular [Na+] needed to drive the Na+/K+ ATPase on cells
Loss of water from cells ↑ intracellular [K+]
K+ moves down concentration gradient from cell into serum
↑ K+ available for absorption
Since K+ is dissolved in water, some K+ is carried by water as water osmotically moves through water channels into the serum (phenomenon known as “solvent drag”)
See Hyperkalemia: Clinical Findings slide
Authors:
Mannat Dhillon, Joshua Low, Emily Wildman Reviewers:
Huneza Nadeem, Marissa (Ran) Zhang, Andrea Kuczynski, Yan Yu*, Kevin McLaughlin*, Adam Bass*
* MD at time of publication
↑ K+ in serum
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 17, 2022 on www.thecalgaryguide.com
Hyperkalemia (intracellular shift and ↑ intake): Pathophysiology
↑ K+ dietary intake (rarely causative)
β2 receptor inhibition (i.e. beta blockers)
Digoxin
α1 receptor stimulation (i.e. epinephrine, norepinephrine)
Insulin deficiency or resistance (i.e. diabetes mellitus)
↓ Stimulation of NHE1 (moves 1 Na+ into cell, 1 H+ out of cell) throughout body
Normal Anion- Gap Metabolic Acidosis (NAGMA)
Excess serum H+ results in ↓ NHE1 activity (See NAGMA: Pathogenesis and Laboratory Findings slide)
↑ Serum osmolarity (i.e. hyperglycemia)
Osmotic movement of water from cells to serum
Cell lysis (i.e. tumour lysis syndrome, rhabdomyolysis, hemolytic anemia)
↑ K+ release from lysed cells into the serum
Na+/K+ ATPase (moves 3K+ into cell, 2 Na+ out) activity inhibited on cells throughout body
↓ Activity of Na+/K+ ATPase on cells throughout the body
↓ Amount of K+ entering cells
↓ NHE1 activity prevents Na+ from entering the cell
Lack of high intracellular [Na+] needed to drive the Na+/K+ ATPase on cells
Loss of water from cells ↑ intracellular [K+]
K+ moves down concentration gradient from cell into serum
↑ K+ available for absorption
Since K+ is dissolved in water, some K+ is carried by water as water osmotically moves through water channels into the serum (phenomenon known as “solvent drag”)
See Hyperkalemia: Clinical Findings slide
Authors:
Mannat Dhillon, Joshua Low, Emily Wildman Reviewers:
Huneza Nadeem, Marissa (Ran) Zhang, Andrea Kuczynski, Yan Yu*, Kevin McLaughlin*, Adam Bass*
* MD at time of publication
↑ K+ in serum
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 17, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/07/Hyperkalemia-shift-1.jpg)

![Diabetes Insipidus: Pathogenesis and clinical findings
Hereditary
Autoimmune/ Idiopathic
Auto-antibodies destroy neurons that release antidiuretic hormone (ADH)
Mass Effect/ Tumor Invasion
Mass pressing on hypothalamus or pituitary
Electrolyte Imbalance
(mechanism unclear)
Hereditary
Lithium (Li)
(mechanism unclear)
Li enters principal cells of collecting ducts via ENaCs
Li inhibits GSK3β, reducing adenylyl cyclase activity
↓ cAMP- dependent phosphorylation of aquaporin-2
↑ Serum [Ca2+]
Activation of
CaSR in thick ascending limb of Loop of Henle
↓ NaCl reabsorption in thick ascending limb
↓ Generation of medullary osmotic gradient
↓ Serum [K+]
↑ Degradation of aquaporin-2 channels in collecting duct
↓ Aquaporin- 2 channels transporting water across apical membrane of collecting duct
Mutation of AVPR2 gene on X chromosome
Antidiuretic hormone (ADH) receptor cannot reach basolateral surface of principal cells of collecting duct
Mutation of aquaporin-2 gene on chromosome 12
↓ Fusion of aquaporins with apical membrane of collecting duct
Mutation of WFS1 gene on chromosome 4 (Wolfram syndrome)
↓ Processing of antidiuretic hormone (ADH) precursors and ↓ADH-releasing neurons
Surgery/ Trauma
Injury to hypothalamus or pituitary stalk
Mutation of PCSK1 gene on chromosome 5
Deficiency in PC1/3 (encoded by PCSK1)
↓ Processing of ADH by PC1/3
Aquaporin dysfunction
↓ Kidney response to ADH, which mediates reabsorption of water down its osmotic gradient through aquaporins
↓ Production of ADH by hypothalamus or ↓ secretion from ADH-releasing neurons in posterior pituitary (depending on location of lesion)
Central Diabetes Insipidus
Nephrogenic Diabetes Insipidus
Abbreviations:
AVPR2: arginine vasopressin receptor 2 CaSR: calcium-sensing receptor
ENaC: epithelial sodium channel
GSK3β: glycogen synthase kinase type 3 beta PC1/3: proprotein convertase
Diabetes Insipidus
Decreased ability of kidneys to concentrate urine
↓ Reabsorption of water from collecting duct into vasculature
Author:
Oswald Chen
Reviewers:
Huneza Nadeem,
Ran (Marissa) Zhang,
Yan Yu*
Sam Fineblit*
* MD at time of publication
Urine becomes more dilute
↓ Urine osmolality
↑ Urine output
↓ Blood volume
Blood becomes more concentrated
Occurs during late sleep period
Nocturia
Polyuria
(>3 L/day)
↑ Serum osmolality
Activation of hypothalamic osmoreceptors
Hypernatremia
(Serum [Na+] >145 mEq/L)
Polydipsia
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published September 25, 2022 on www.thecalgaryguide.com
Diabetes Insipidus: Pathogenesis and clinical findings
Hereditary
Autoimmune/ Idiopathic
Auto-antibodies destroy neurons that release antidiuretic hormone (ADH)
Mass Effect/ Tumor Invasion
Mass pressing on hypothalamus or pituitary
Electrolyte Imbalance
(mechanism unclear)
Hereditary
Lithium (Li)
(mechanism unclear)
Li enters principal cells of collecting ducts via ENaCs
Li inhibits GSK3β, reducing adenylyl cyclase activity
↓ cAMP- dependent phosphorylation of aquaporin-2
↑ Serum [Ca2+]
Activation of
CaSR in thick ascending limb of Loop of Henle
↓ NaCl reabsorption in thick ascending limb
↓ Generation of medullary osmotic gradient
↓ Serum [K+]
↑ Degradation of aquaporin-2 channels in collecting duct
↓ Aquaporin- 2 channels transporting water across apical membrane of collecting duct
Mutation of AVPR2 gene on X chromosome
Antidiuretic hormone (ADH) receptor cannot reach basolateral surface of principal cells of collecting duct
Mutation of aquaporin-2 gene on chromosome 12
↓ Fusion of aquaporins with apical membrane of collecting duct
Mutation of WFS1 gene on chromosome 4 (Wolfram syndrome)
↓ Processing of antidiuretic hormone (ADH) precursors and ↓ADH-releasing neurons
Surgery/ Trauma
Injury to hypothalamus or pituitary stalk
Mutation of PCSK1 gene on chromosome 5
Deficiency in PC1/3 (encoded by PCSK1)
↓ Processing of ADH by PC1/3
Aquaporin dysfunction
↓ Kidney response to ADH, which mediates reabsorption of water down its osmotic gradient through aquaporins
↓ Production of ADH by hypothalamus or ↓ secretion from ADH-releasing neurons in posterior pituitary (depending on location of lesion)
Central Diabetes Insipidus
Nephrogenic Diabetes Insipidus
Abbreviations:
AVPR2: arginine vasopressin receptor 2 CaSR: calcium-sensing receptor
ENaC: epithelial sodium channel
GSK3β: glycogen synthase kinase type 3 beta PC1/3: proprotein convertase
Diabetes Insipidus
Decreased ability of kidneys to concentrate urine
↓ Reabsorption of water from collecting duct into vasculature
Author:
Oswald Chen
Reviewers:
Huneza Nadeem,
Ran (Marissa) Zhang,
Yan Yu*
Sam Fineblit*
* MD at time of publication
Urine becomes more dilute
↓ Urine osmolality
↑ Urine output
↓ Blood volume
Blood becomes more concentrated
Occurs during late sleep period
Nocturia
Polyuria
(>3 L/day)
↑ Serum osmolality
Activation of hypothalamic osmoreceptors
Hypernatremia
(Serum [Na+] >145 mEq/L)
Polydipsia
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published September 25, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/09/Diabetes-Insipidus.jpg)




























![Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/06/Obesity-Hypoventilation-Syndrome.jpg)











