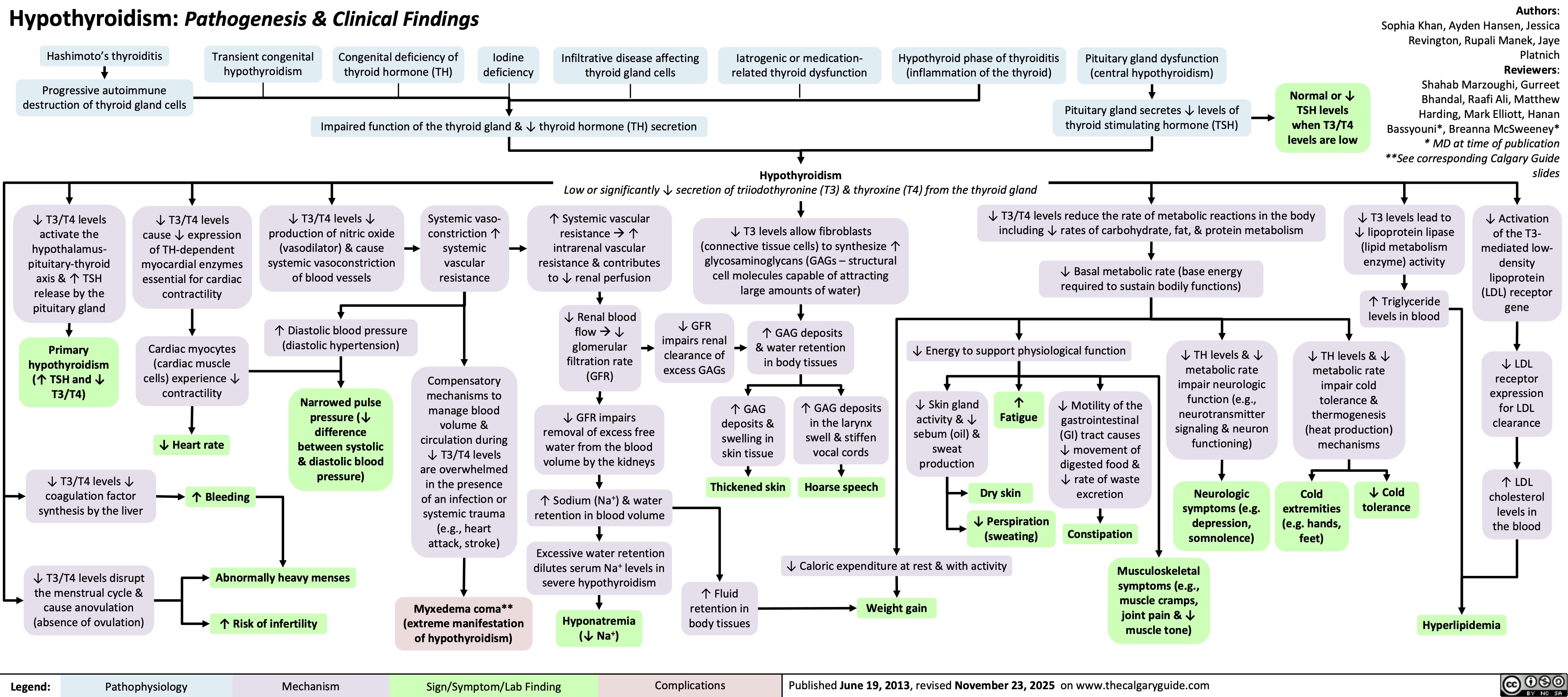
SEARCH RESULTS FOR: Hypothyroidism
Hyponatremia- Physiology
![Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hyponatremia-Physiology-.jpg)
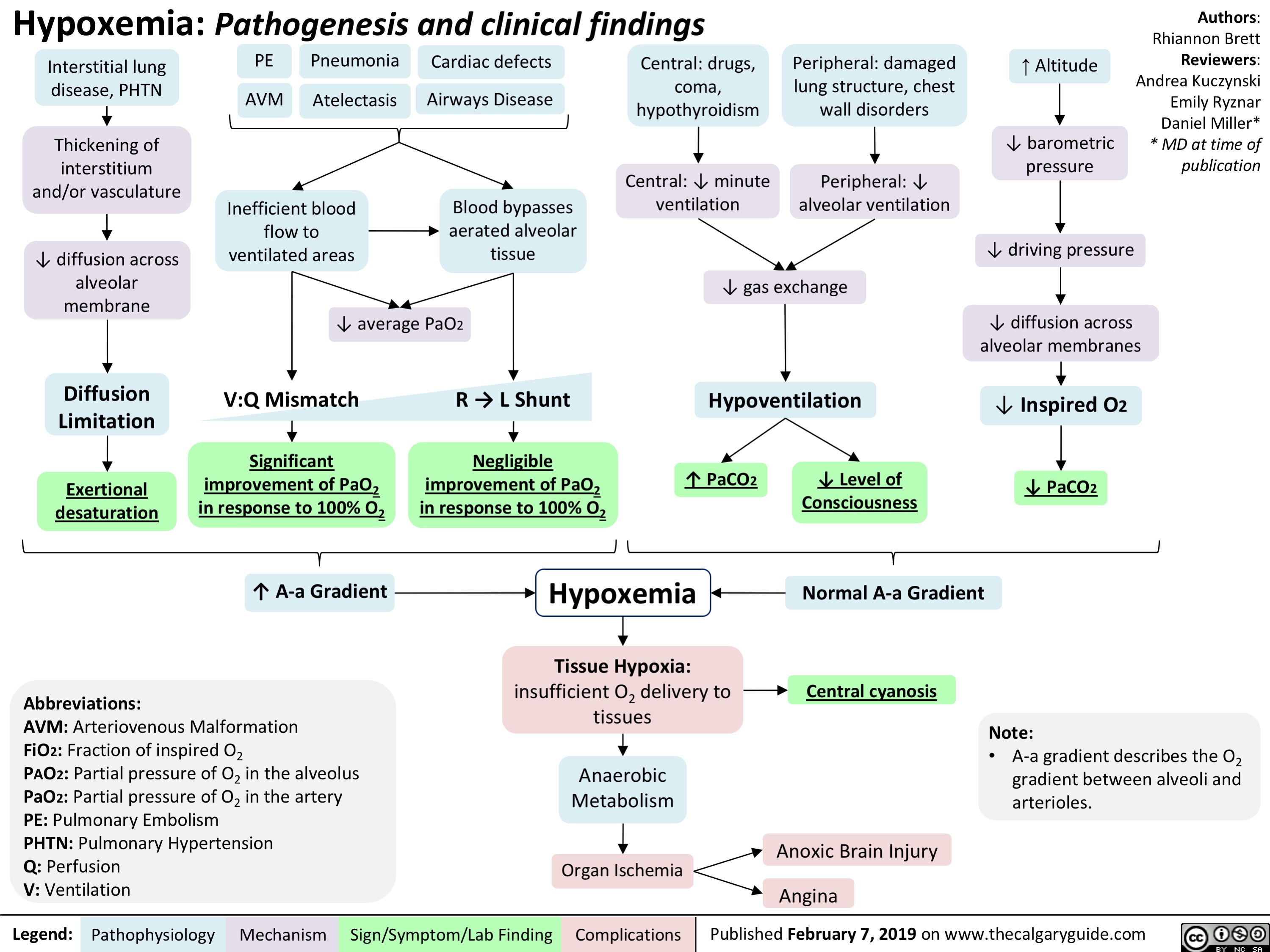
Hypoxemia- Pathogenesis and clinical findings
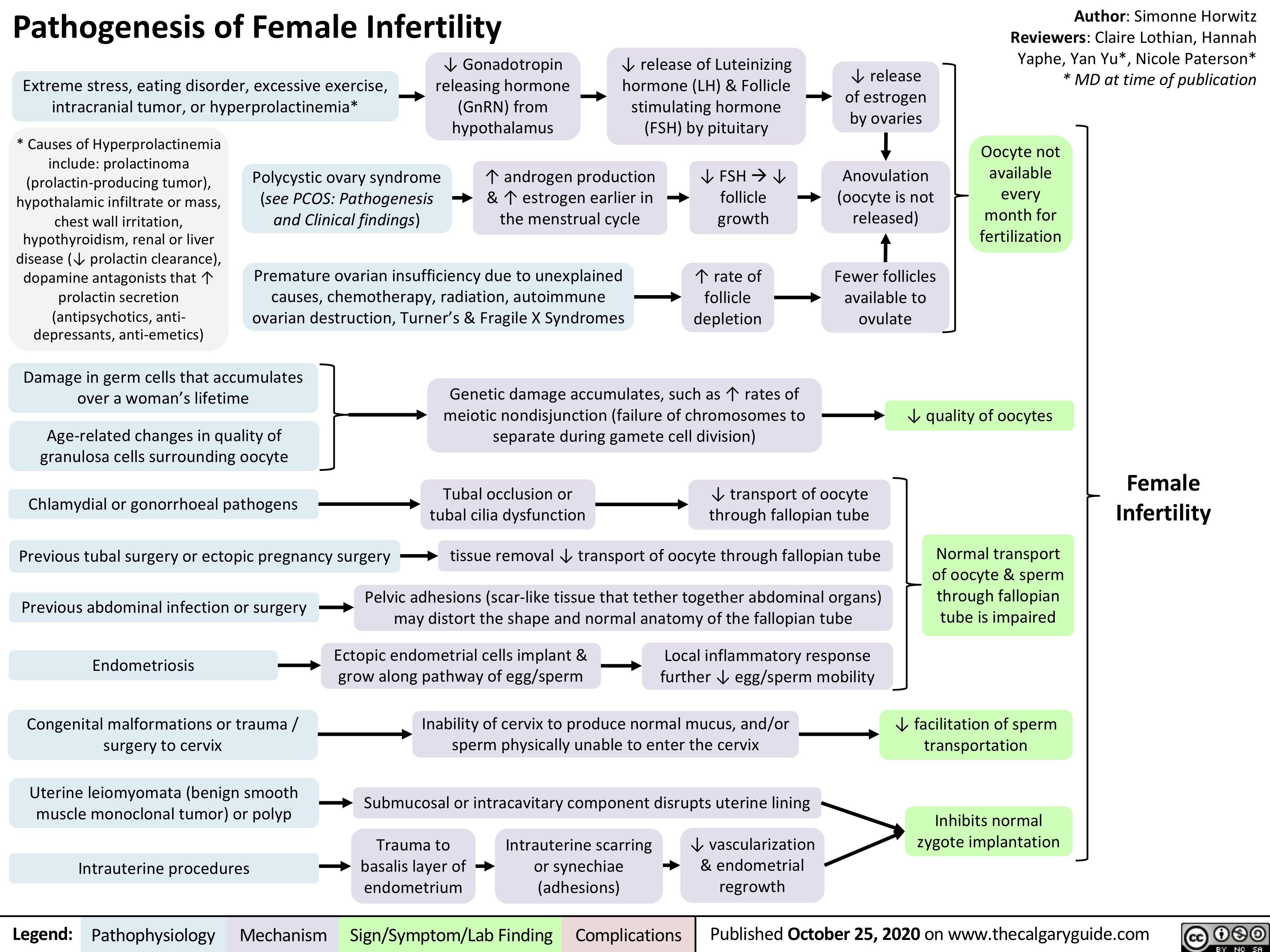
Pathogenesis-of-Female-Infertility
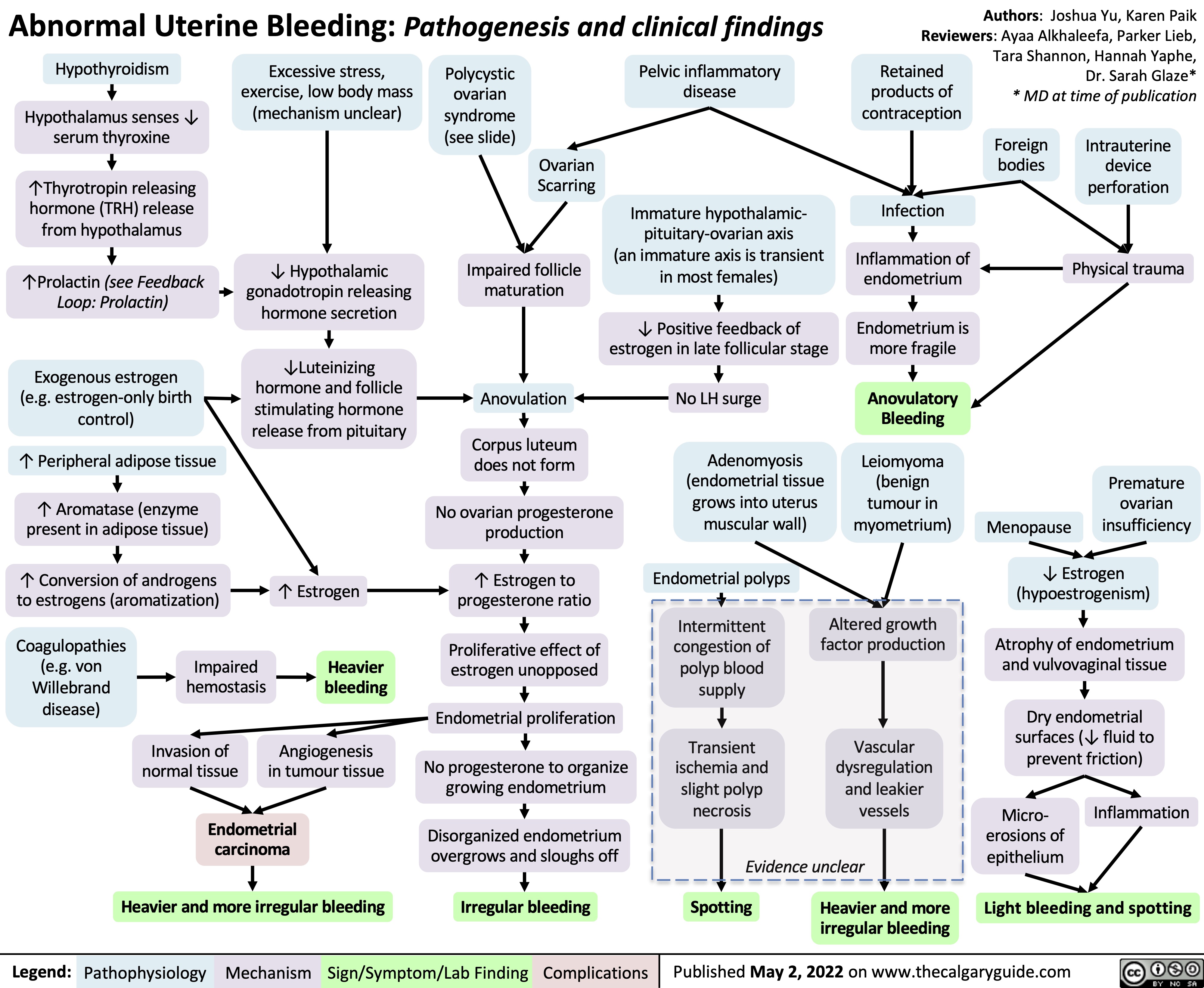
abnormal-uterine-bleeding-aub-pathogenesis-and-clinical-findings
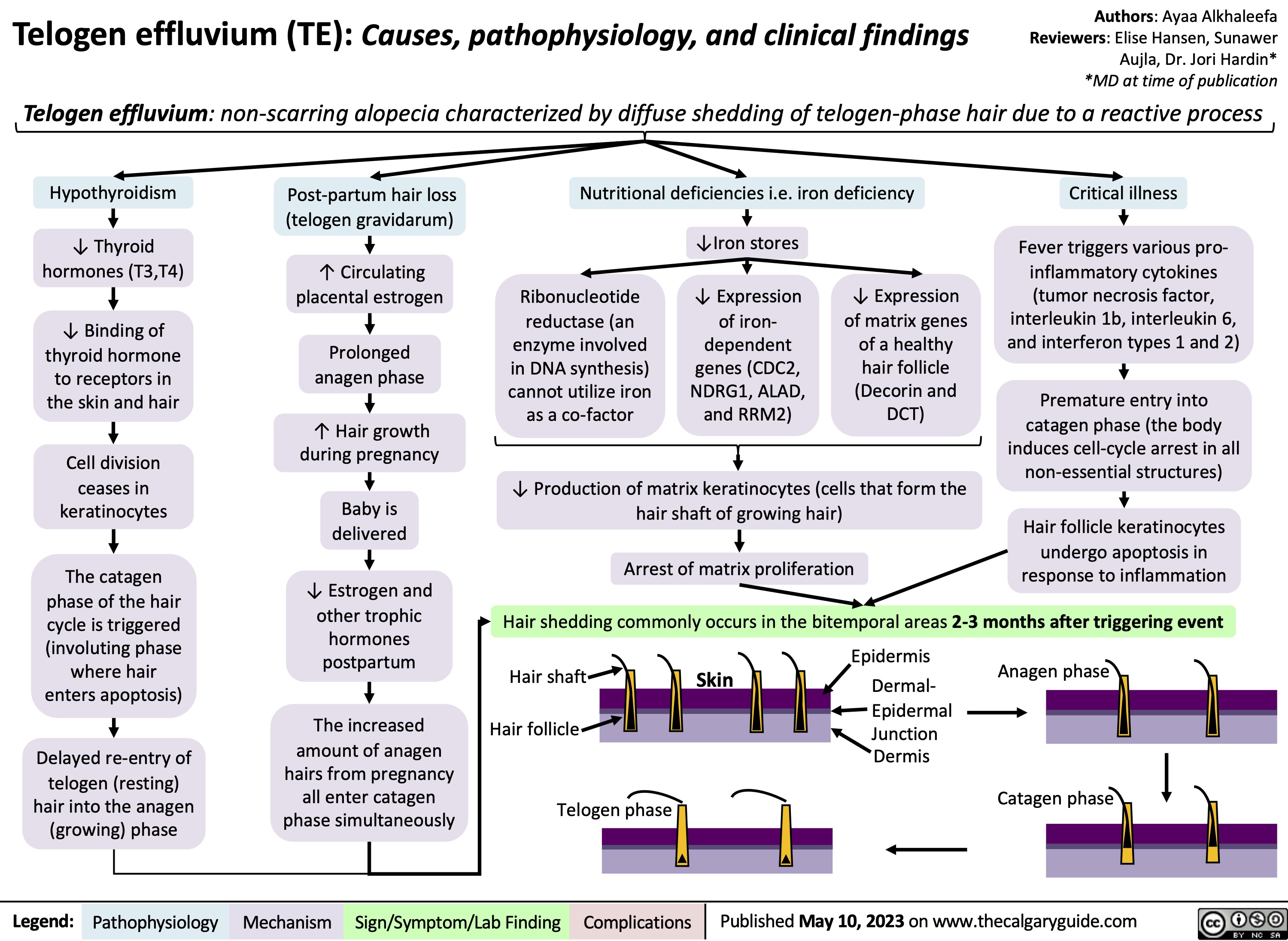
Telogen Effluvium
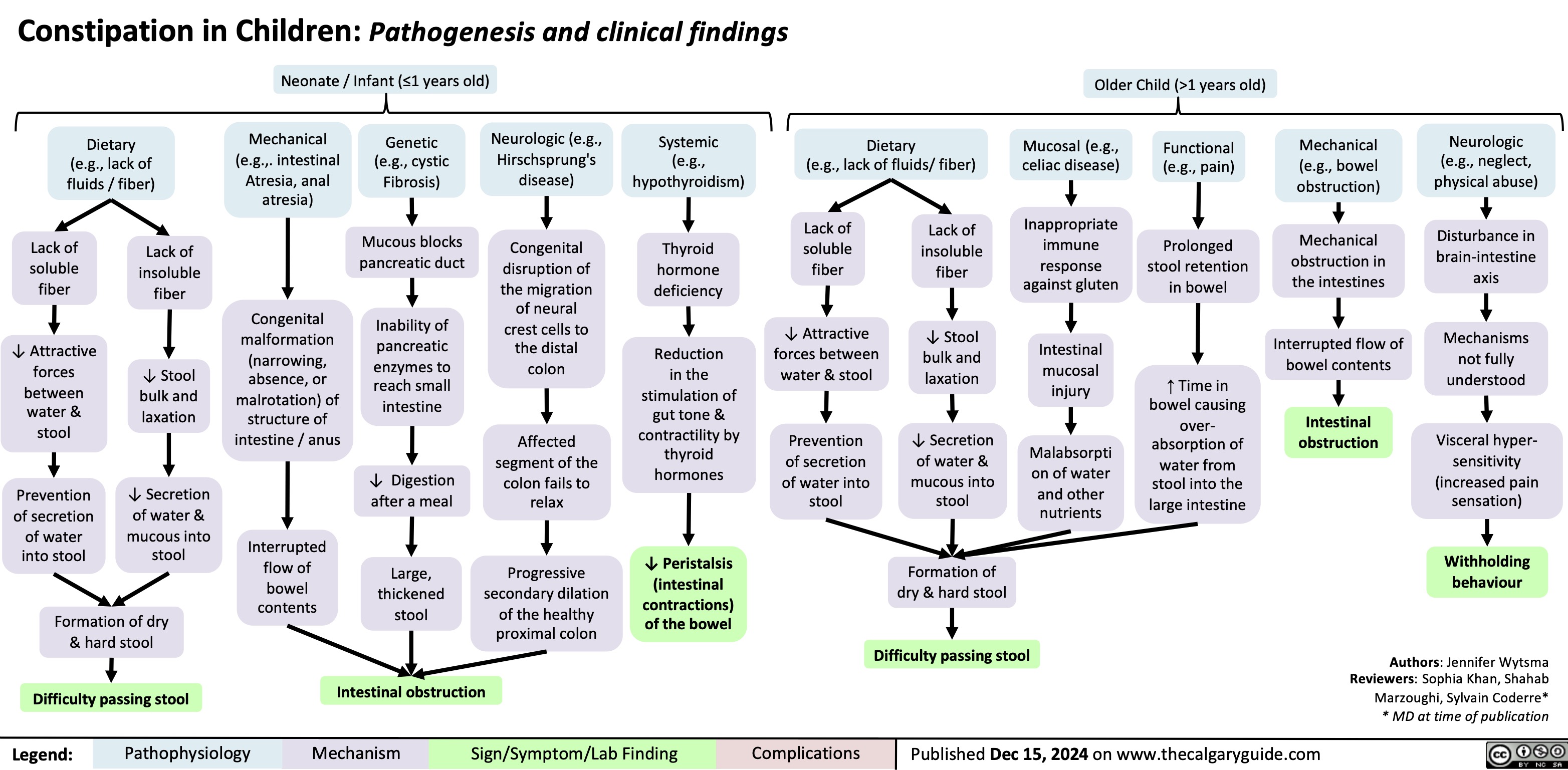
Constipation in Children
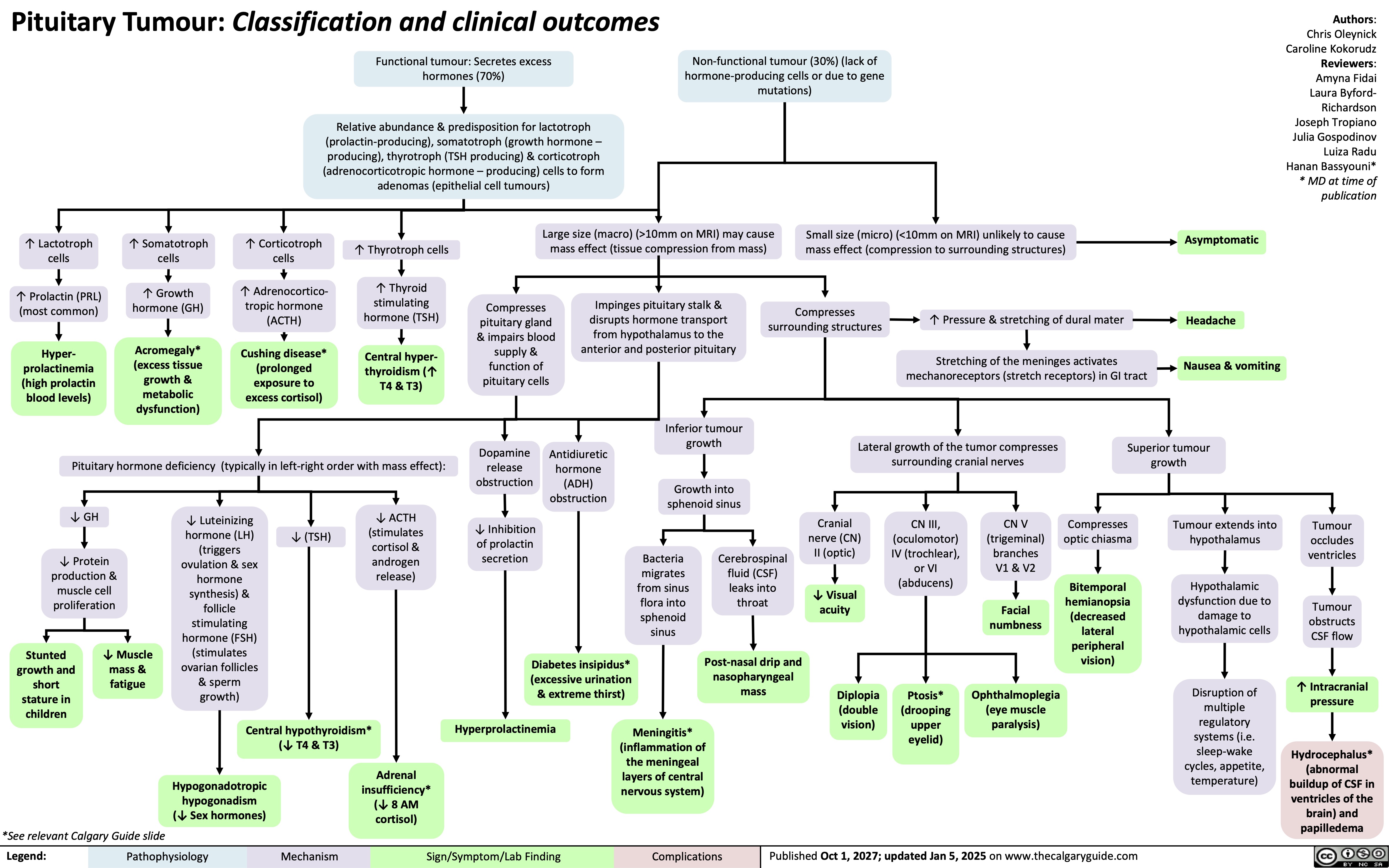
Pituitary Tumour Classification and Clinical Outcomes
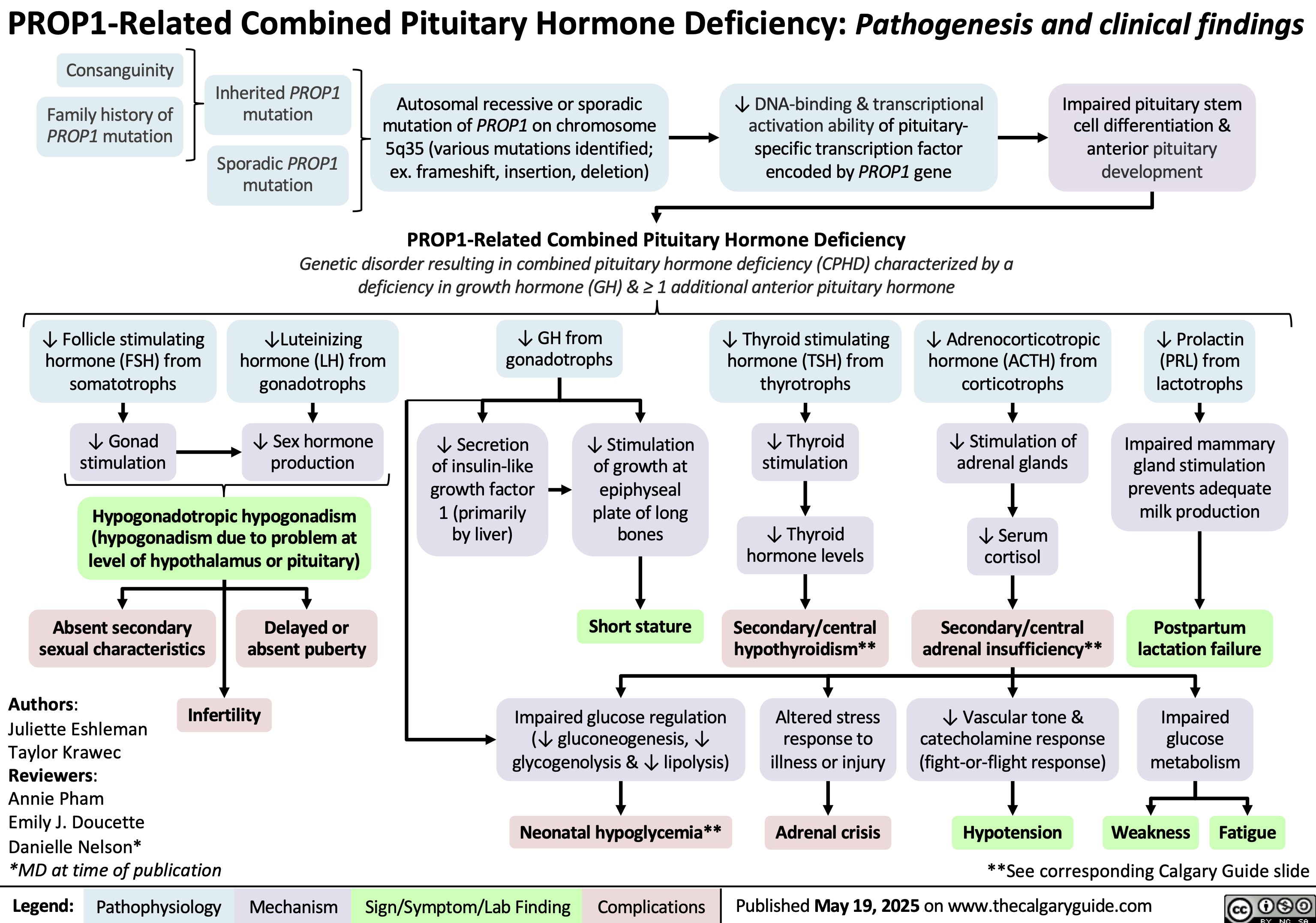
PROP1-Related Combined Pituitary Hormone Deficiency
Obesity Hypoventilation Syndrome
![Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com Obesity Hypoventilation Syndrome: Pathogenesis and clinical findings
Obesity (BMI ≥ 30 kg/m2) risk factors: Poor eating patterns, sedentary lifestyle, genetic predisposition,
hypothyroidism, Cushing’s syndrome, socio-economic factors, age
Sleep-disordered breathing risk factors: Family history, tonsillar or adenoidal
hypertrophy, ↑ neck circumference, type 2 diabetes, HTN
Authors: Mohammad Omer
Mujtaba Siddique
Reviewers:
Ali Babwani
Luiza Radu
Jonathan Liu*
MD at time of publication*
↑ Adipose deposition
in abdomen
Abdominal fat pushes
against diaphragm
↑ Diaphragmatic
displacement
↑ Resistance to chest
wall expansion
↑Leptin resistance
High pressure
Pharyngeal
on upper airway
dilations unable
Secondary depression
↓ Chest wall
↓ Leptins ability to stimulate
↑ lung
to compensate
Narrowing of
(compromised function) of
Poor ventilation to
expansion
ventilation (mechanism unknown)
collapsibility
for weight
upper airways
respiratory system
lower lobes of lungs
↓ Tidal volume (air
that moves in/out of
lungs in a respiratory
cycle)
↑ Respiratory rate
↑ Chest wall thickness ↑ Leptin (a hormone released by
adipose tissues that controls hunger by
signaling fullness)
↑ Adipose
deposition near
upper airways
↑ Buildup of
edema in lower
extremities
↑ Respiratory
workload
↓ Chest wall
compliance (ability to
stretch)
↓ Leptin receptor
expression
↓ Leptin through
blood-brain barrier
↓ Pharyngeal space
Respiratory system is
unable to compensate to ↑
Fluid shifts from
demands
legs to neck during
sleep
Hypoventilation in sleep
↓ Ventilation (air exchange in lungs)
↑ PaCO₂ (partial pressure of arterial carbon
dioxide)
↑ Serum [H+]
↑ Serum [HCO3
-] by renal
reabsorption buffers [H+] rise
↓ PaO₂
(partial pressure of arterial oxygen)
Hypoxia (low
O₂ in tissue)
Higher PaCO₂ required to
reduce pH
↓ O₂ levels in alveoli triggers pulmonary
vessel vasoconstriction
PaCO₂ > 45
mmHG
Respiratory
acidosis
↓ response to CO₂ in central
chemoreceptors in brain
Pulmonary hypertension (high pressure in
pulmonary arteries)
↓ Neural drive
↓ Ventilatory responsiveness
) Right heart pumps against higher
pulmonary pressure leading to
cardiomyocyte hypertrophy
Cor pulmonale
(right-sided heart
failure)
Fatigue
Chronic hypercapnia
(↑ CO2 retention)
Pathophysiology Legend: Mechanism
Sign/Symptom/Lab Finding Complications
Morning headaches
Daytime lethargy
Published Jun 16, 2025 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2025/06/Obesity-Hypoventilation-Syndrome.jpg)
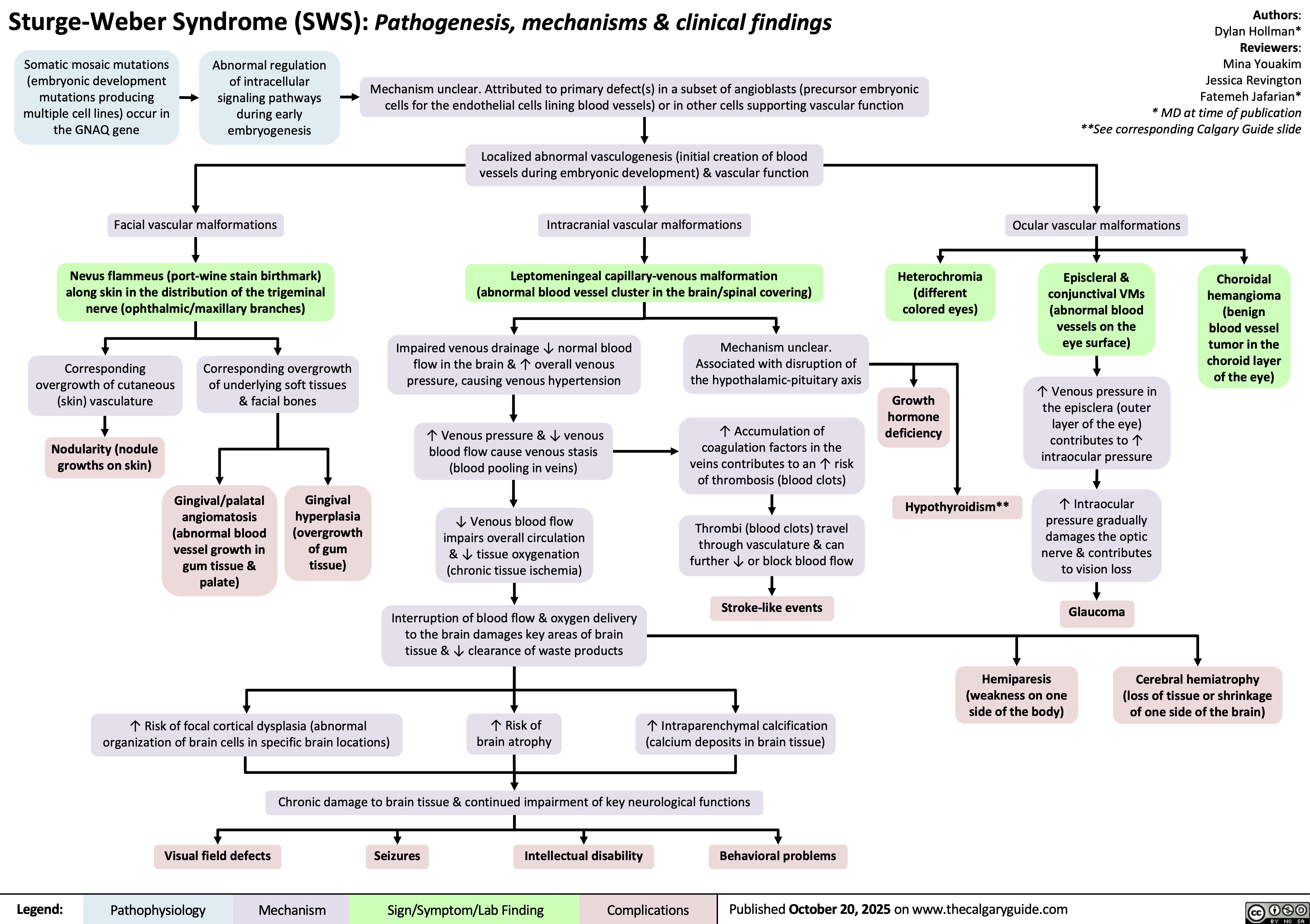
Sturge-Weber Syndrome
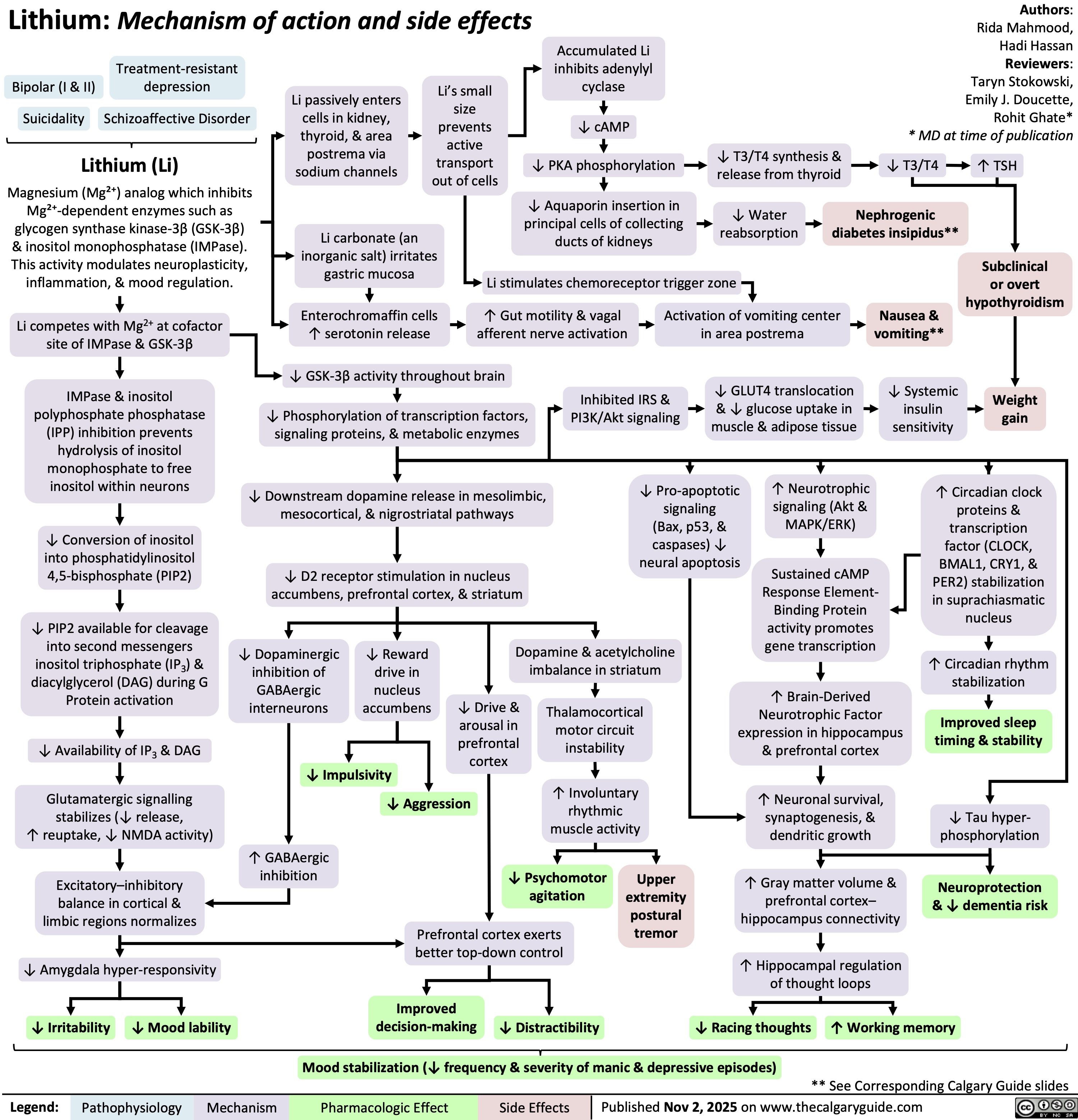
Lithium
Hypothyroidism