SEARCH RESULTS FOR: shock
Cardiogenic Shock
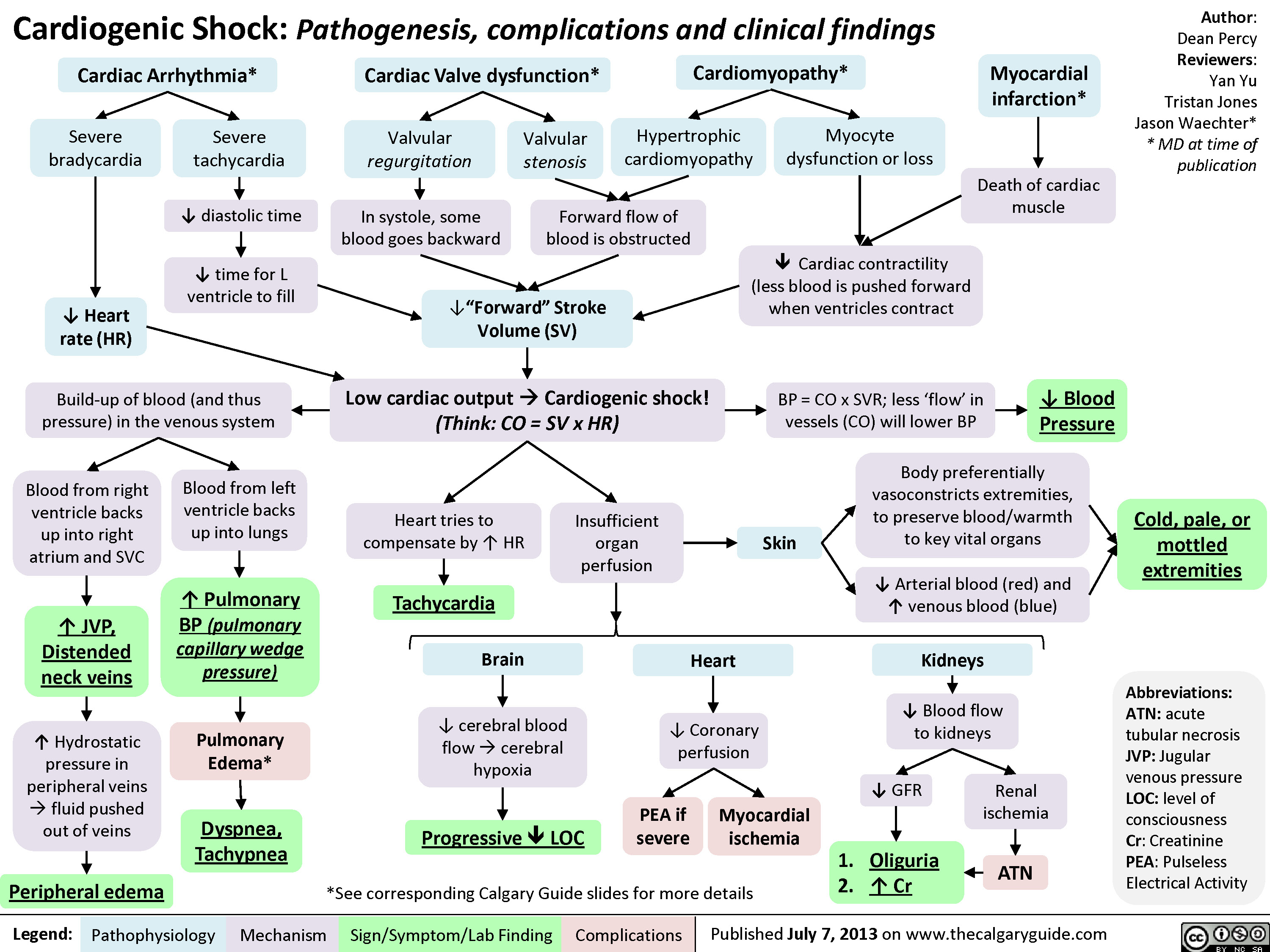
Distributive Shock
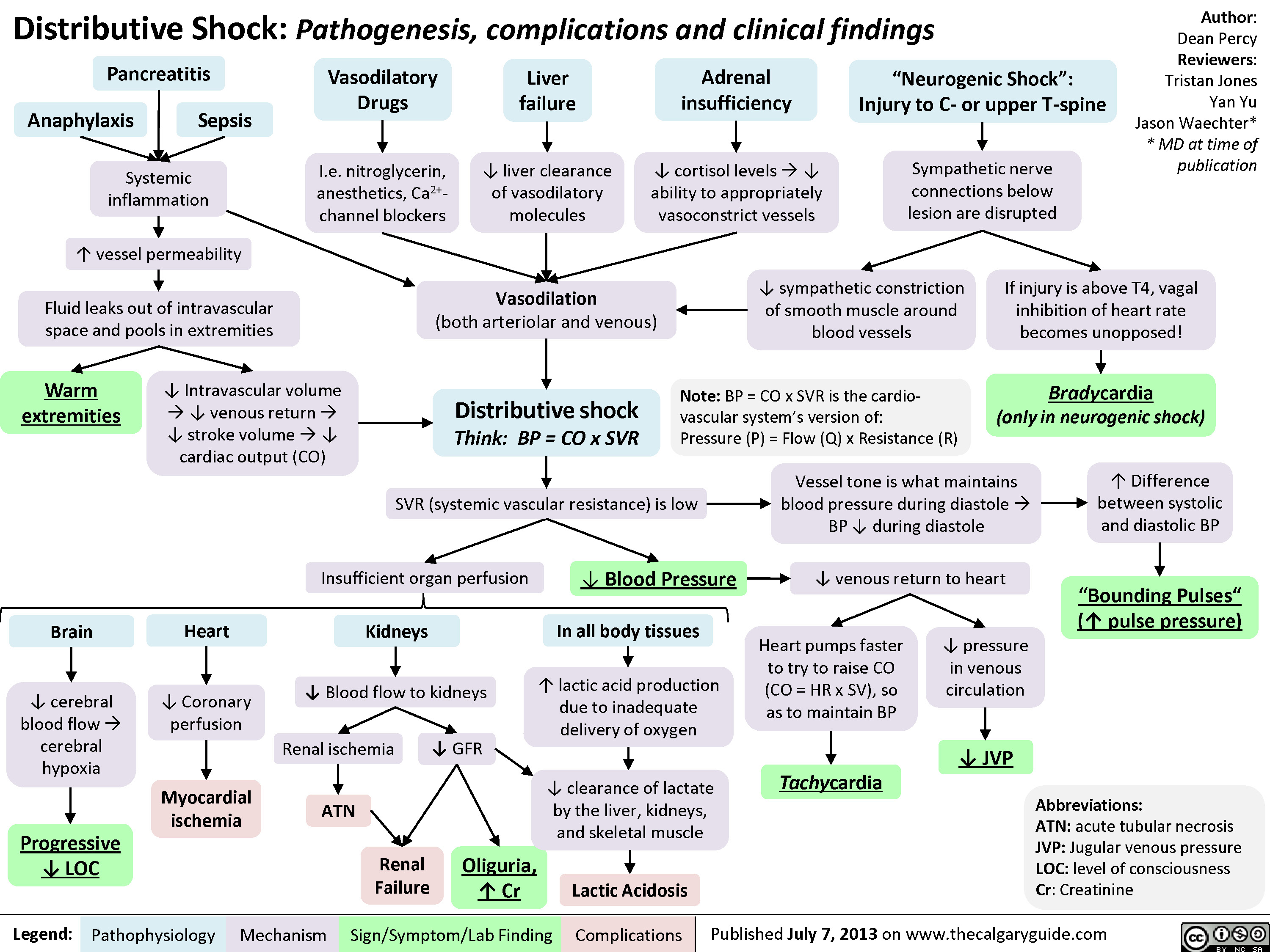
Drugs used to treat shock
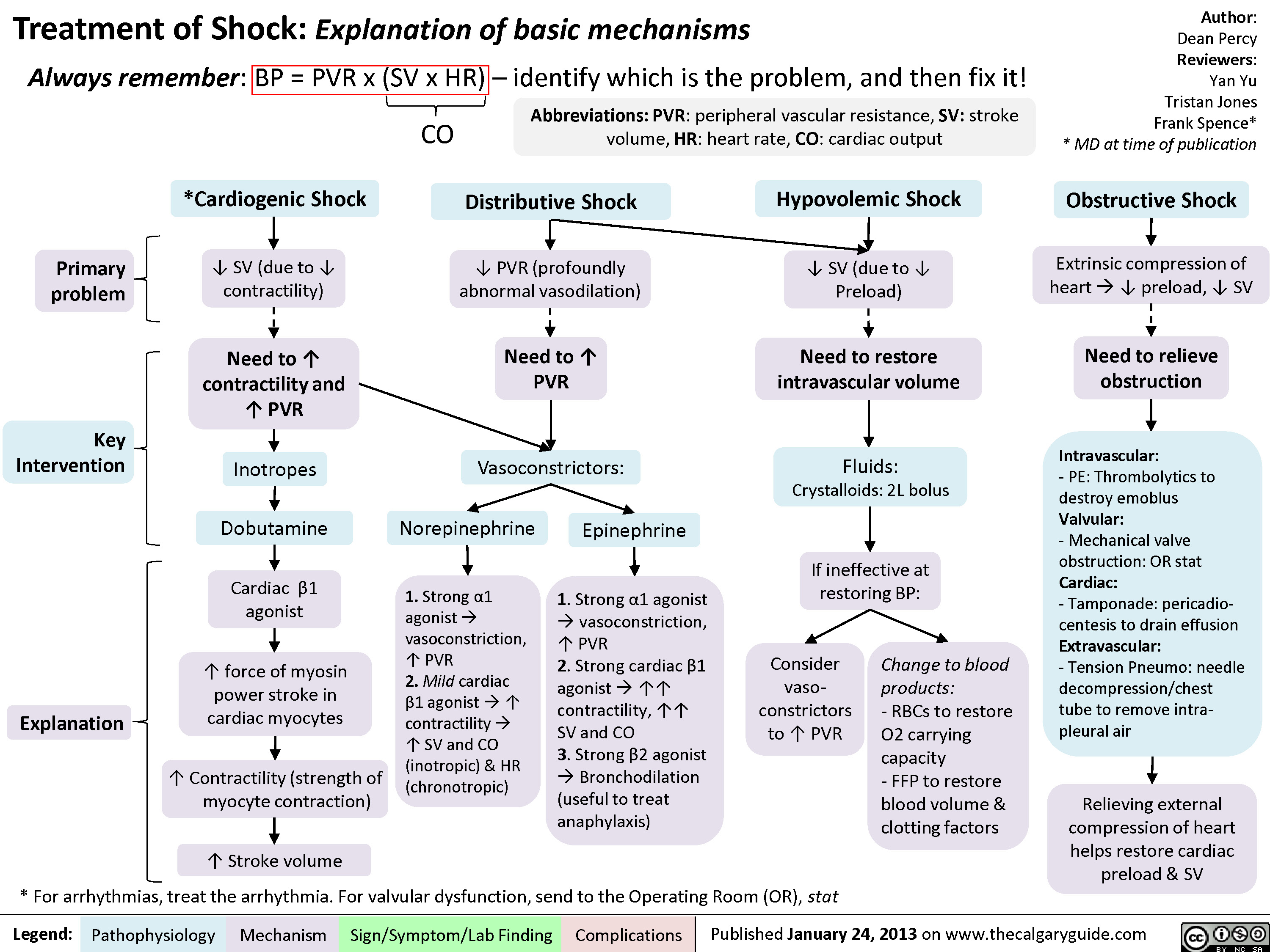
Pediatric Uncompensated Shock: pathogenesis and clinical findings

Hypernatremia Physiology
![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)
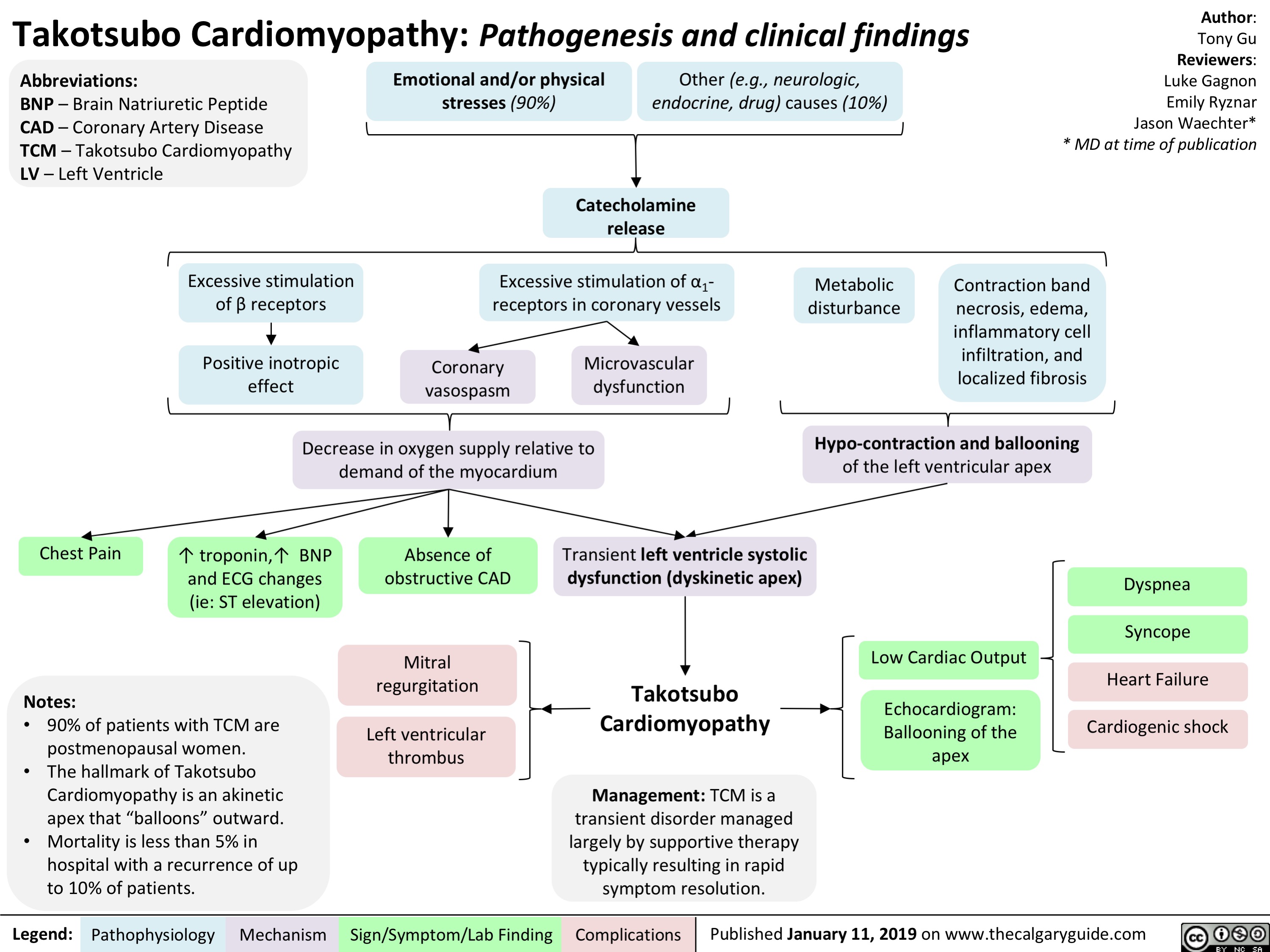
Takotsubo Cardiomyopathy- Pathogenesis and clinical findings
Sepsis, and Septic Shock- Pathogenesis and Clinical Findings
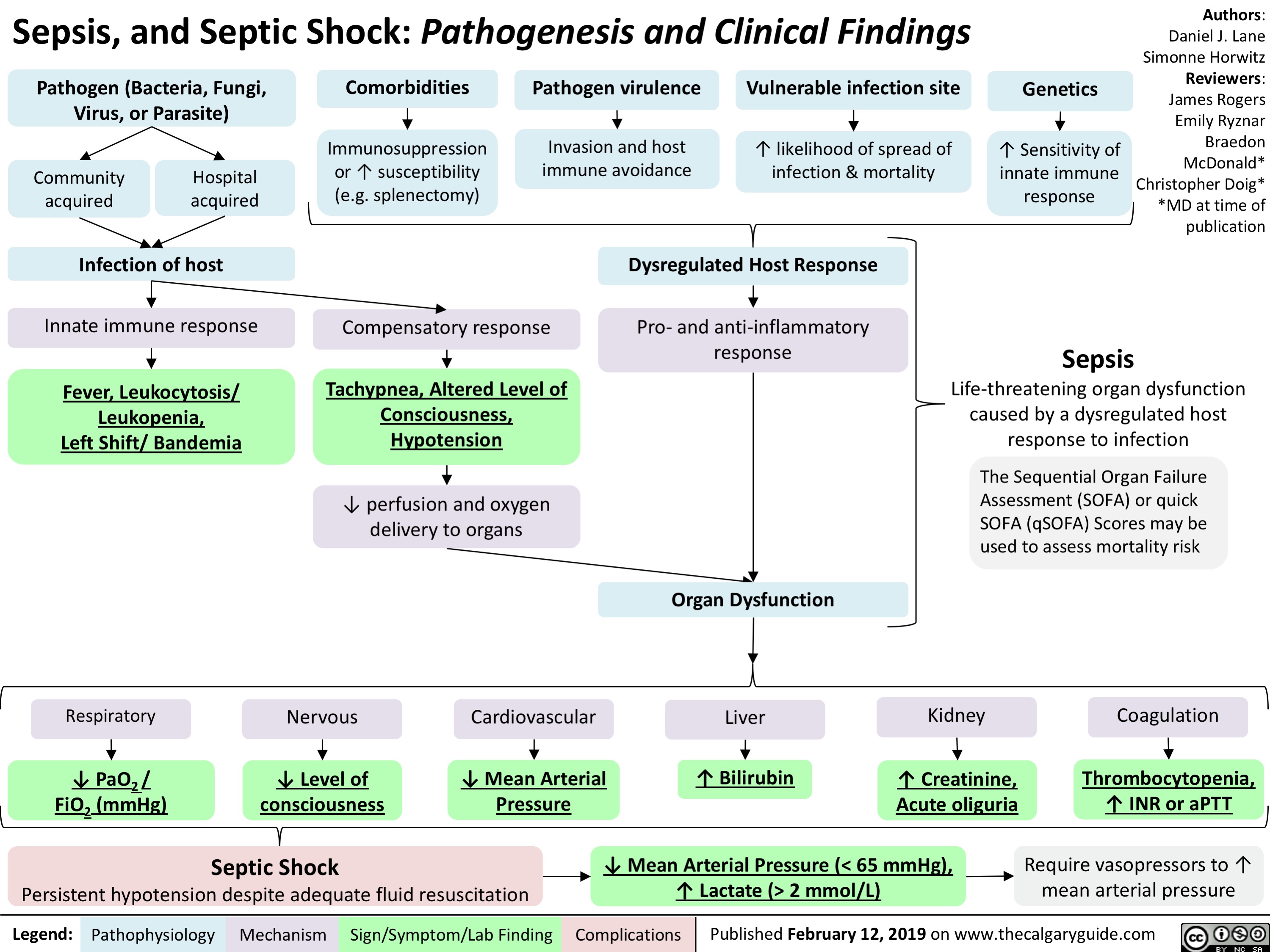
Ischemic Colitis
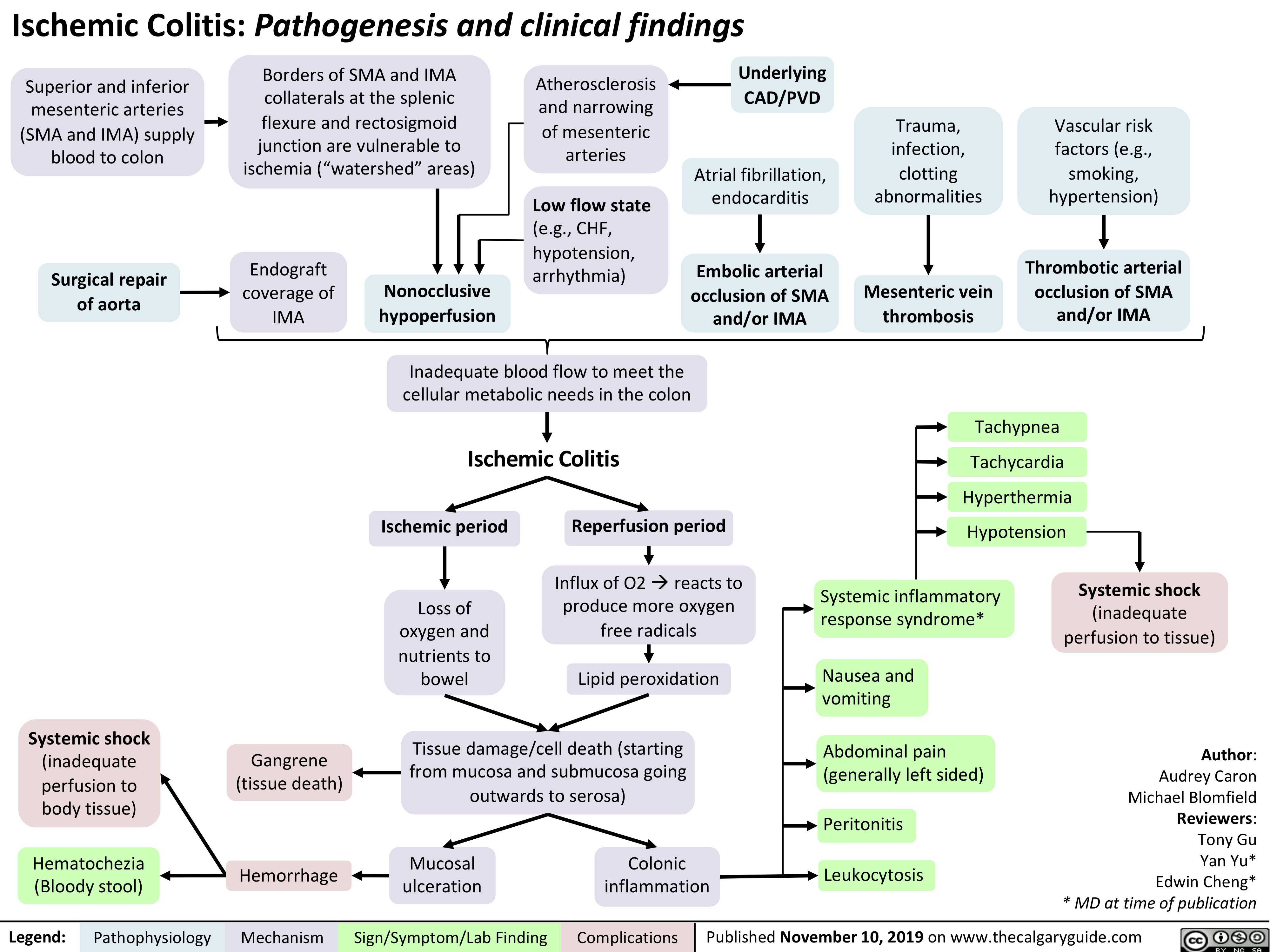
Hereditary Hemorrhagic Telangiectasia (Osler-Weber-Rendu disease)
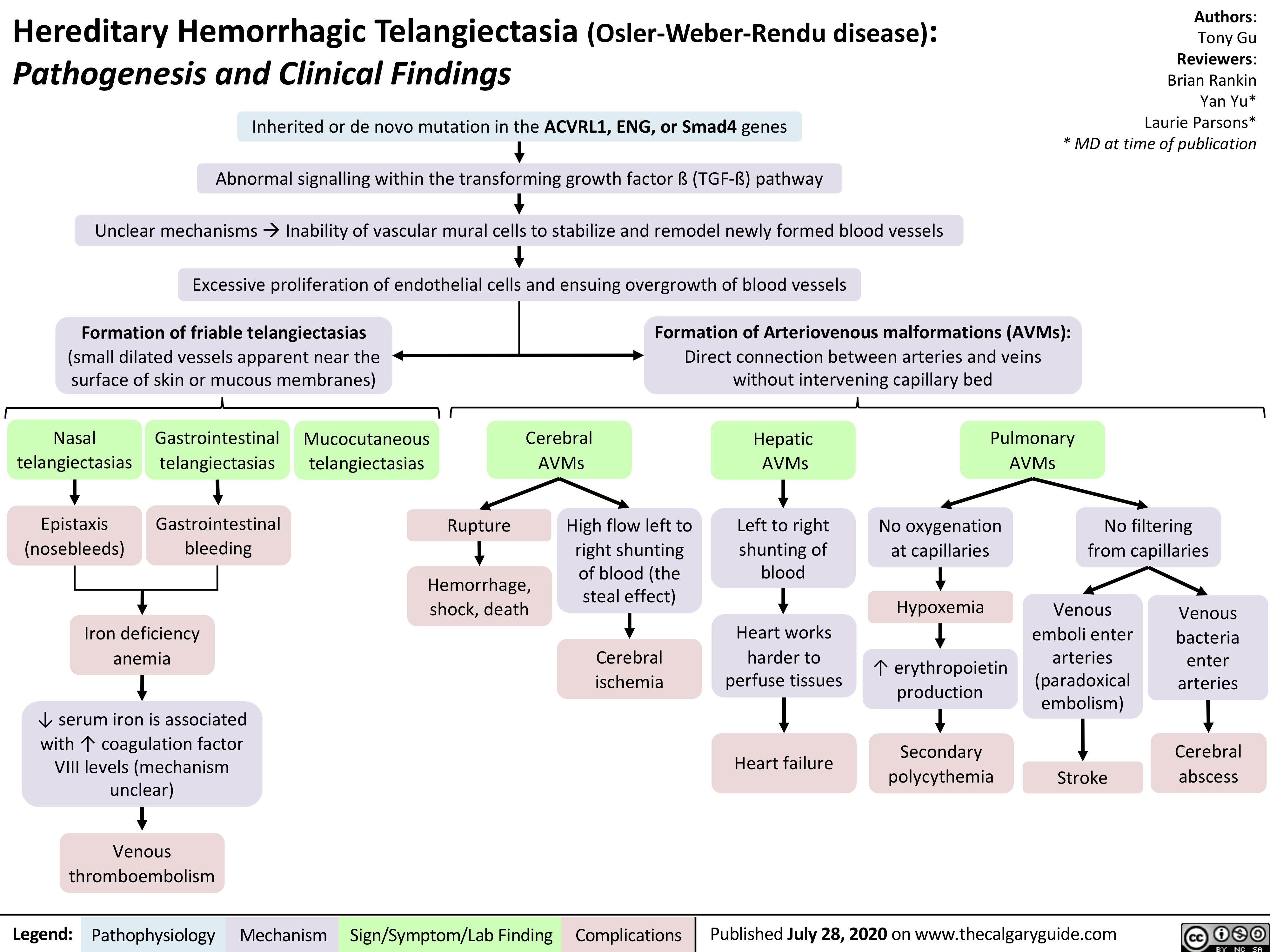
acute-pancreatitis-complications
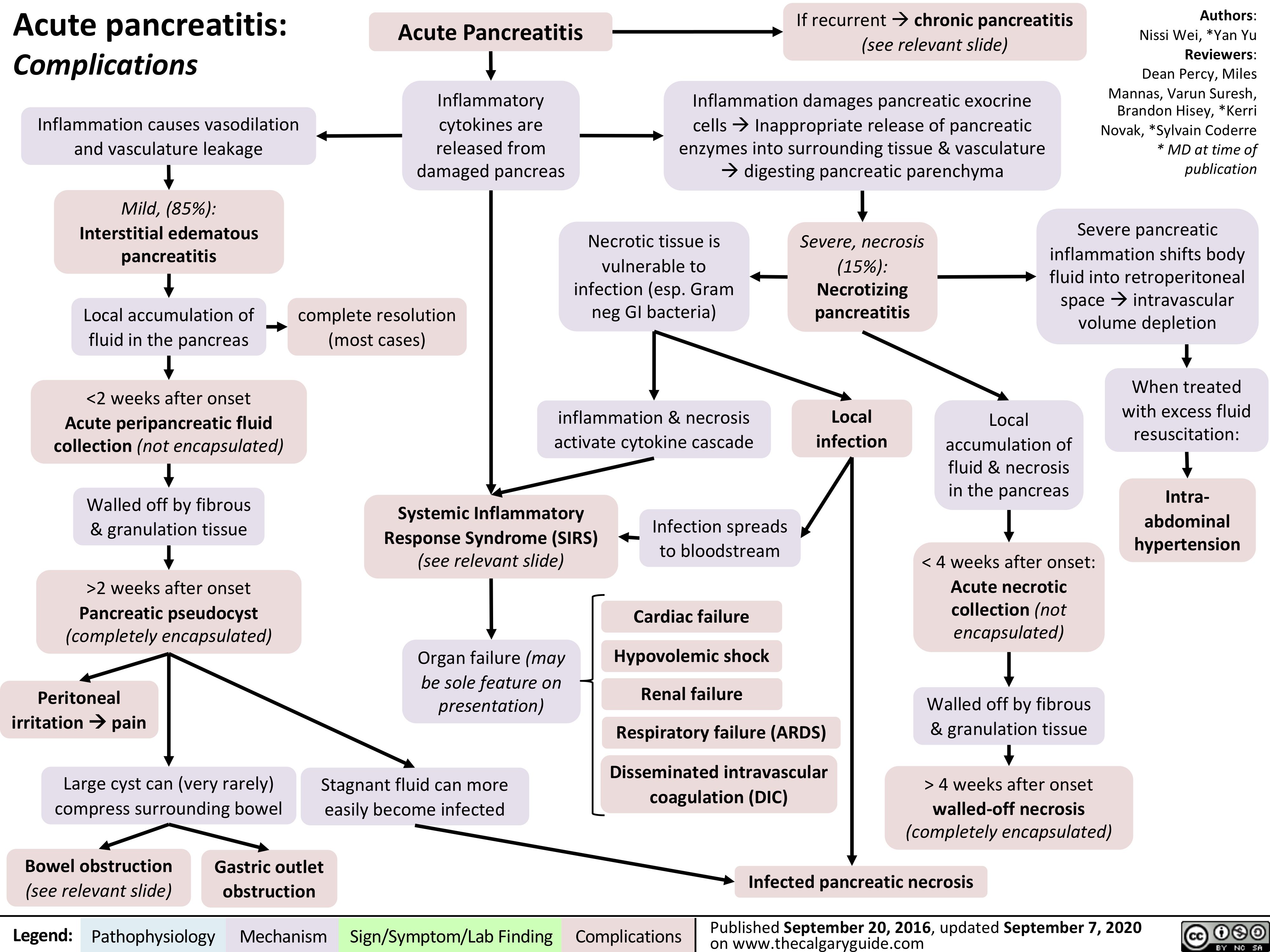
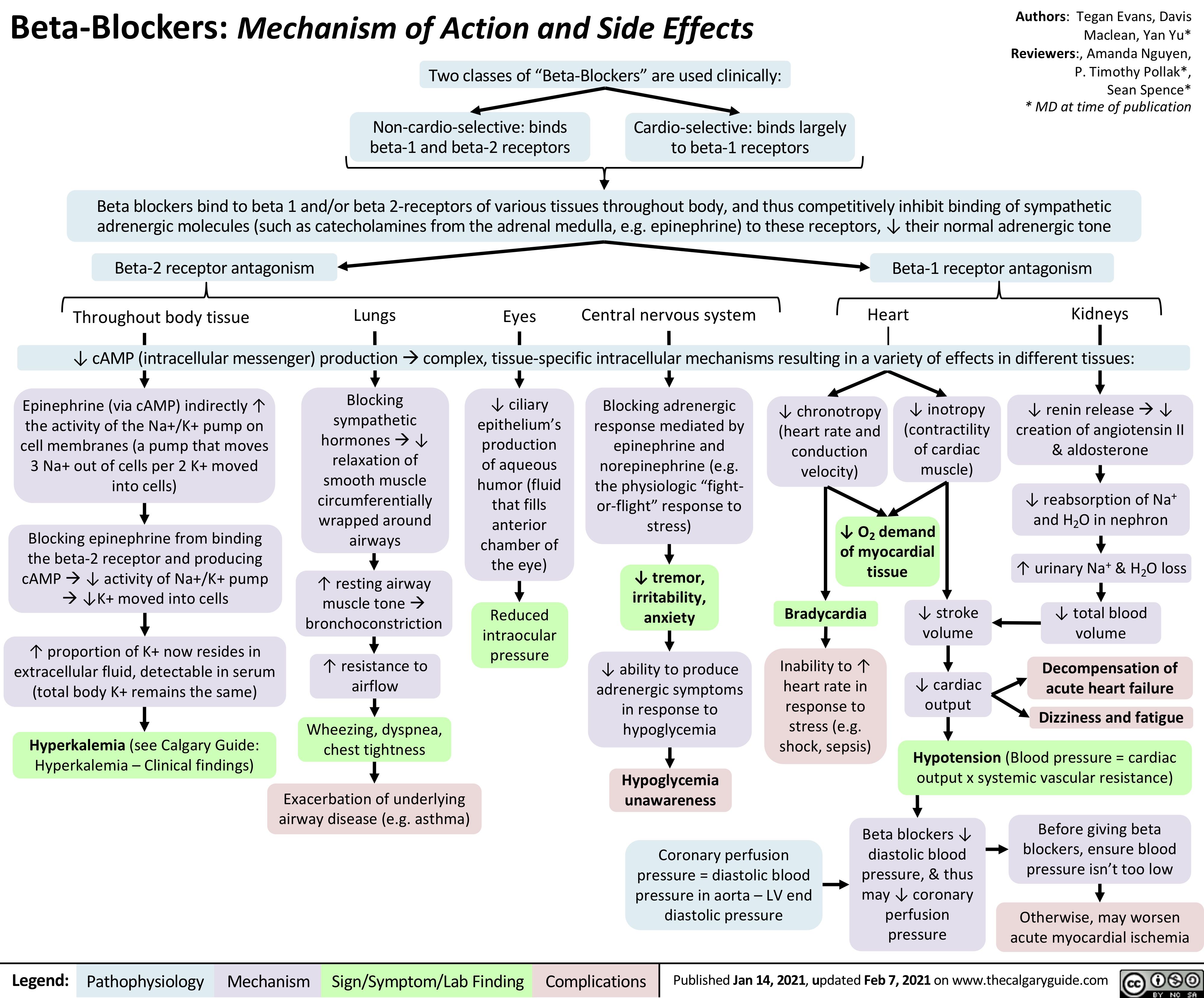
Beta-Blockers-Mechanism-of-Action-and-Side-Effects
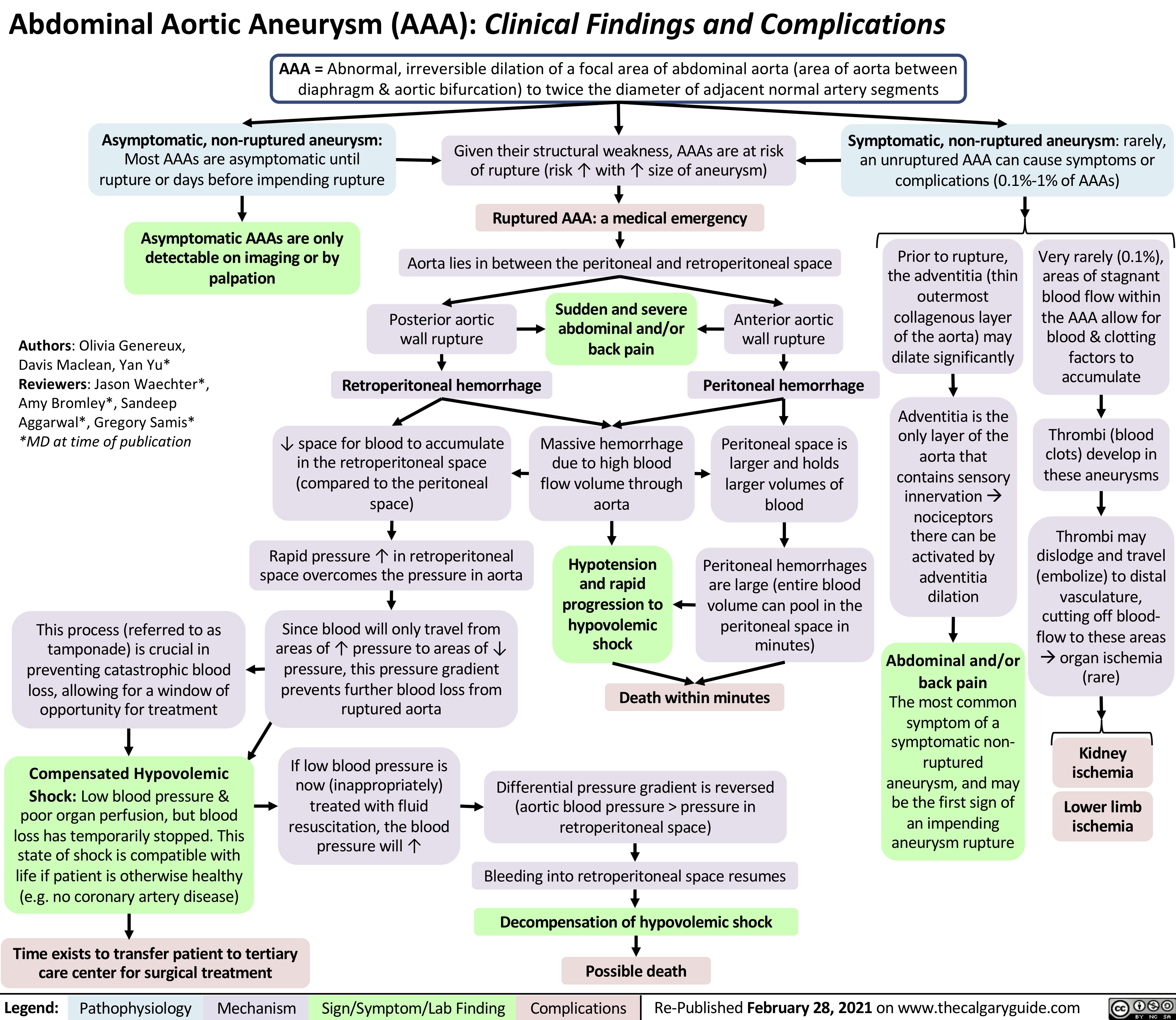
AAA-Clinical-Findings-and-Complications
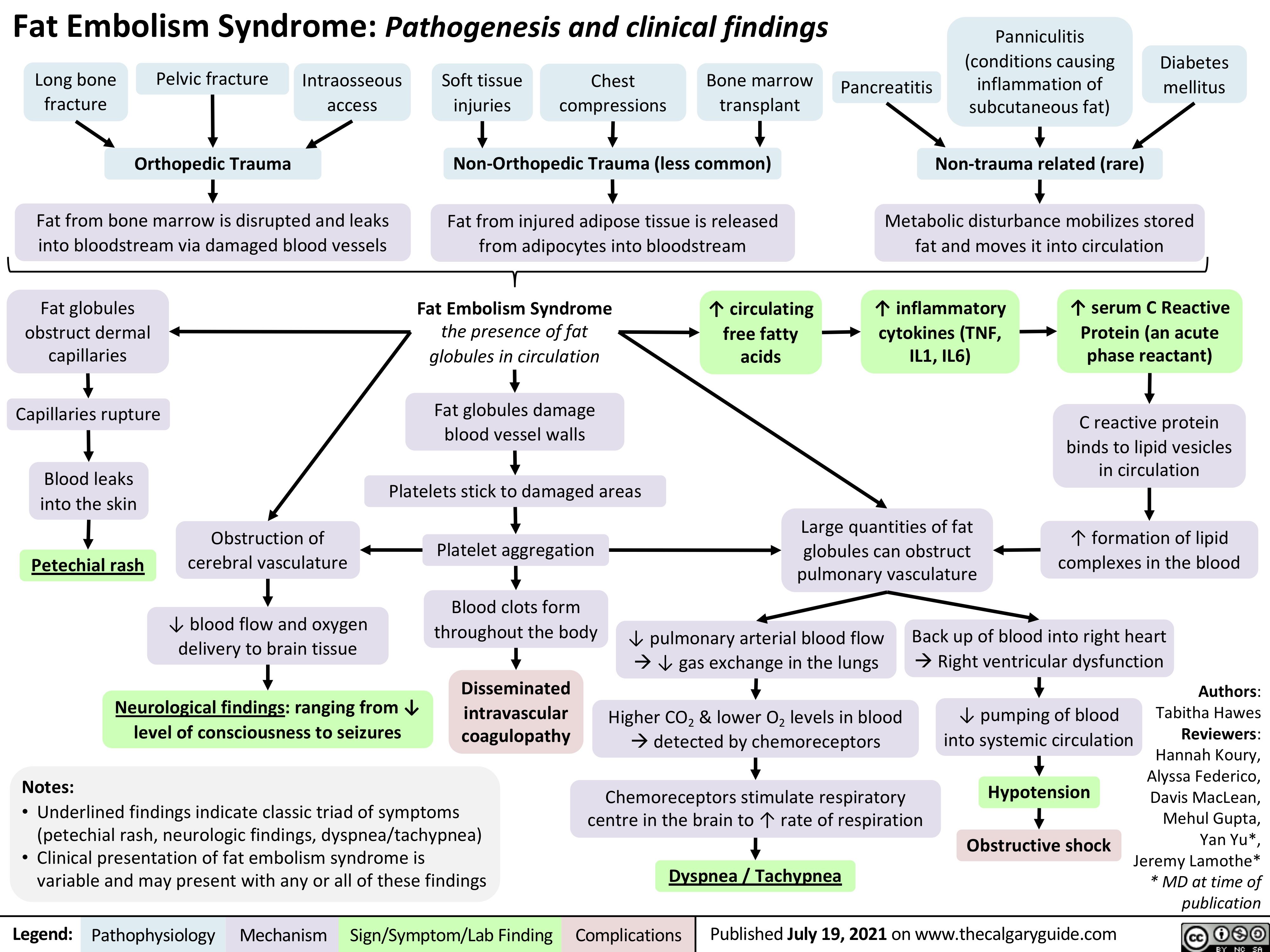
Fat-Embolism-Syndrome
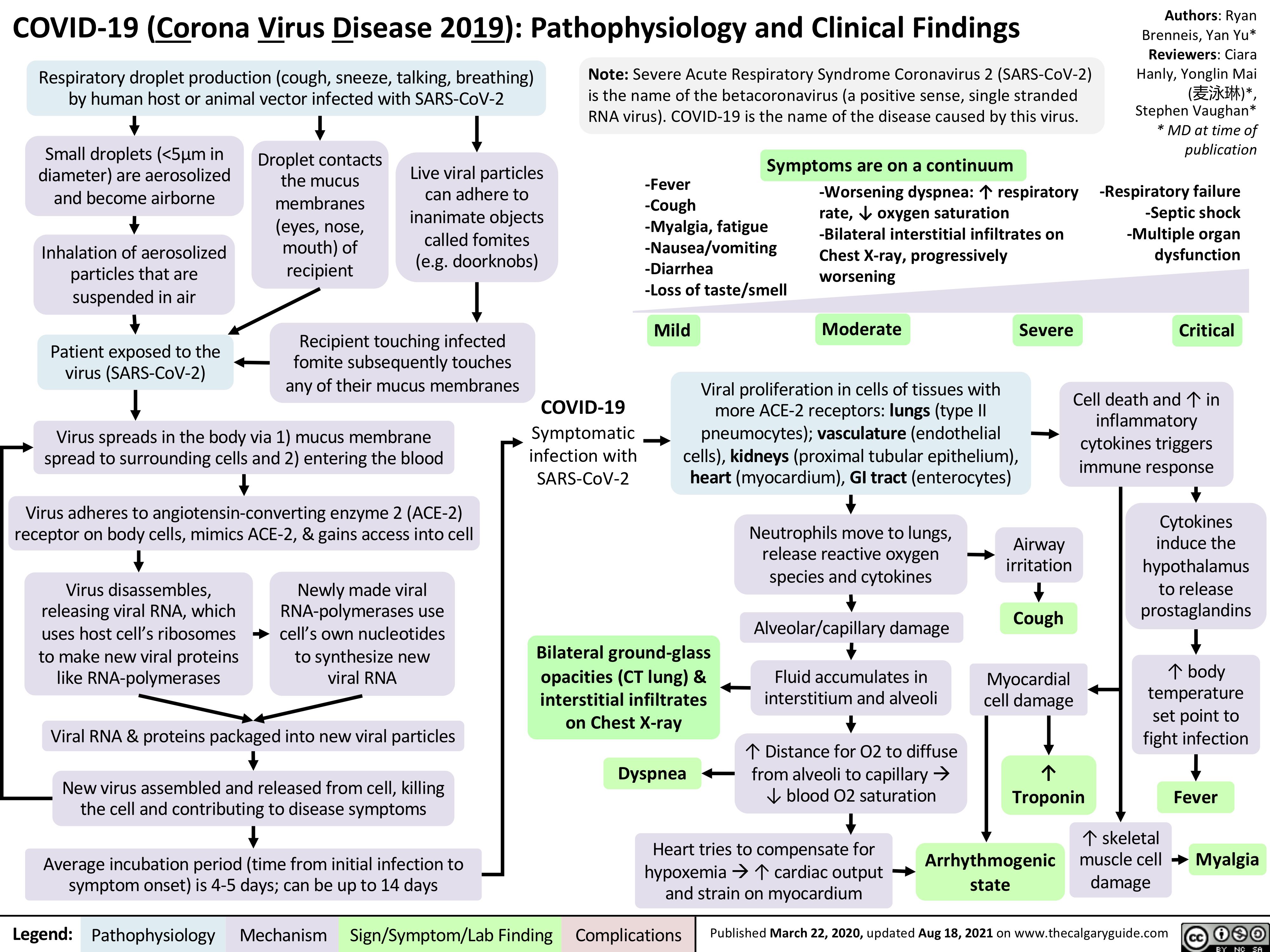
covid-19-pathophysiology-and-clinical-findings
Ectopic Pregnancy
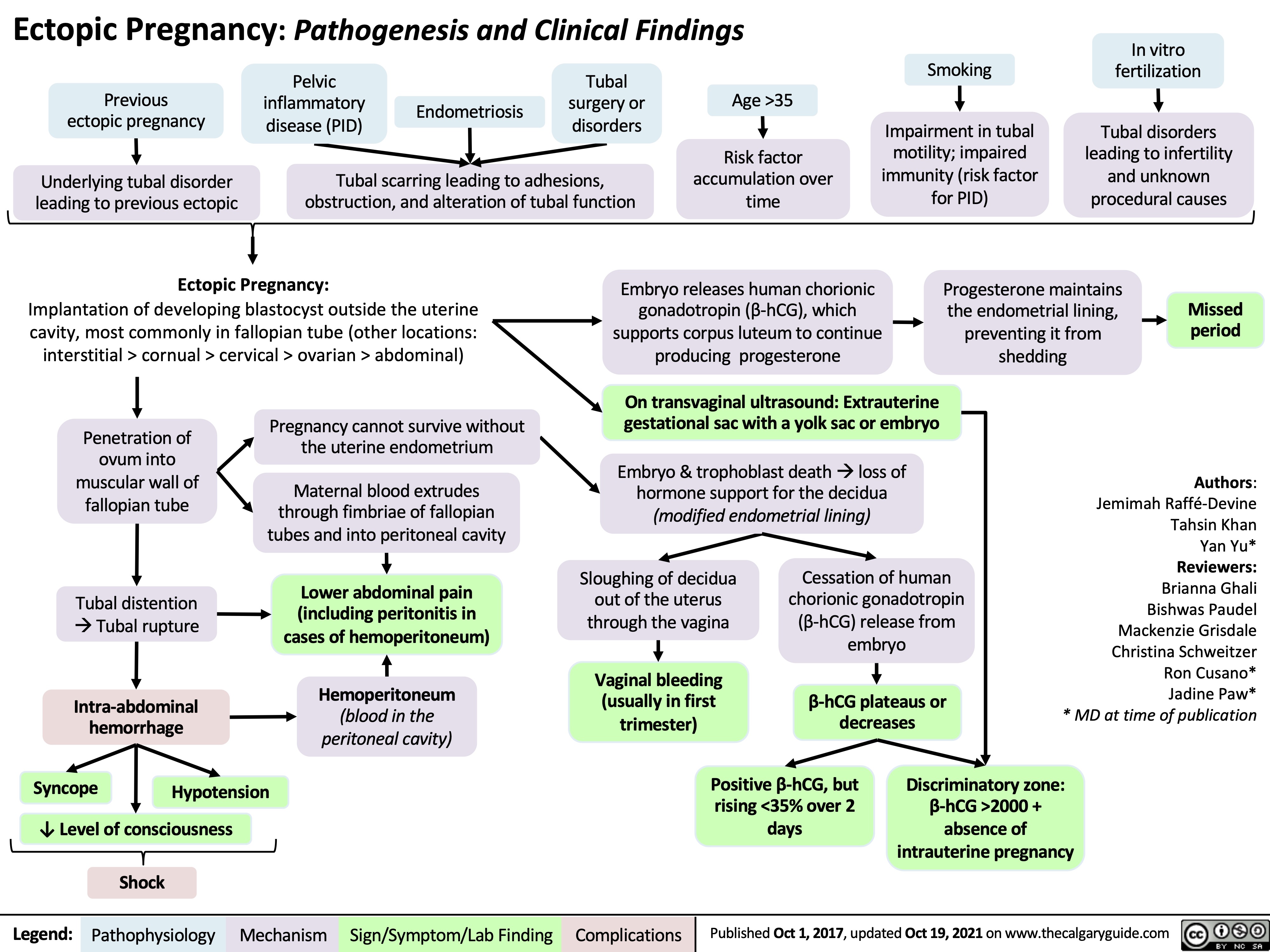
necrotizing fasciitis
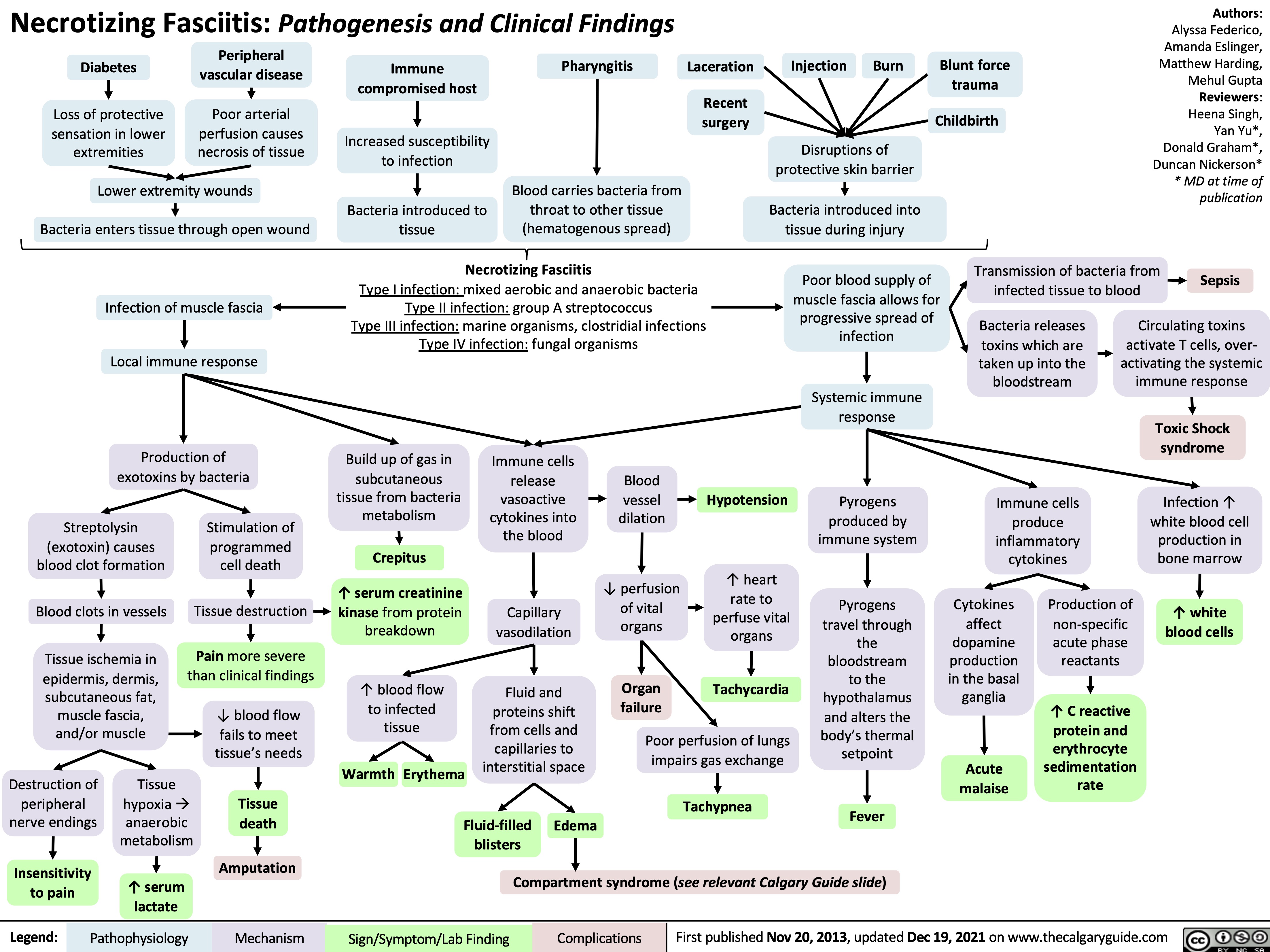
sepsis-y-shock-septico-patogenesis-y-hallazgos-clinicos
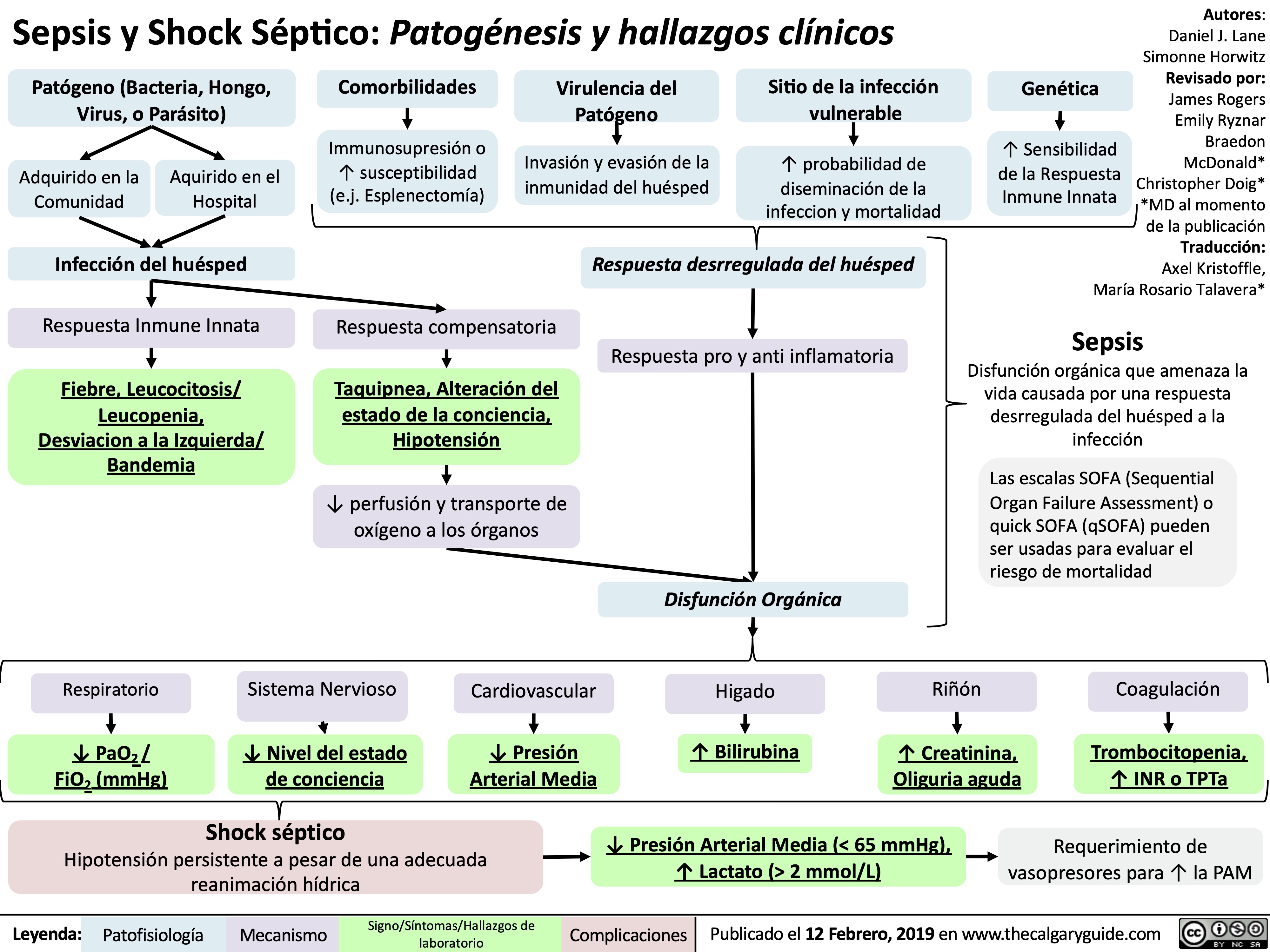
Vitiligo Pathogenesis and Clinical Findings
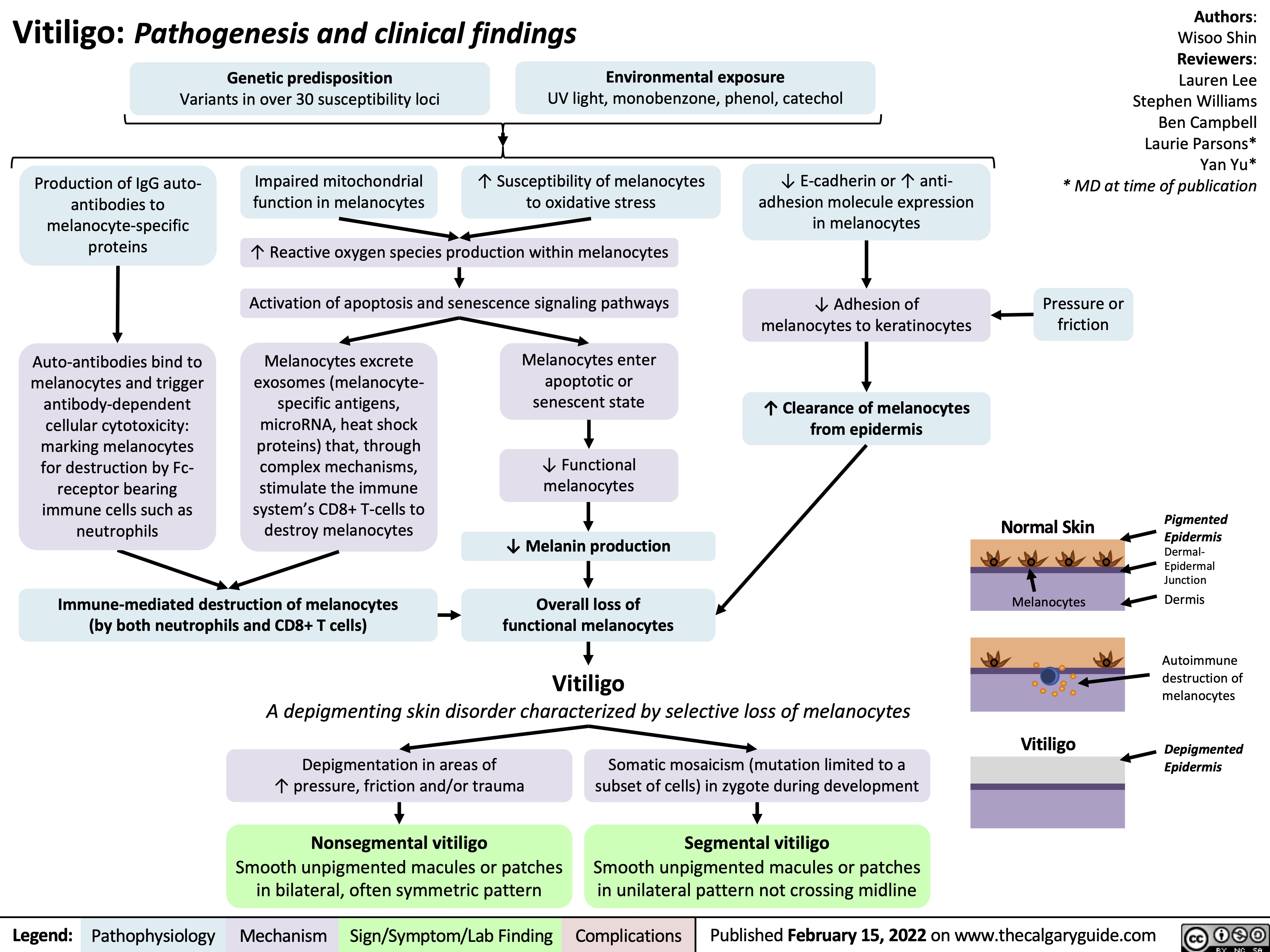
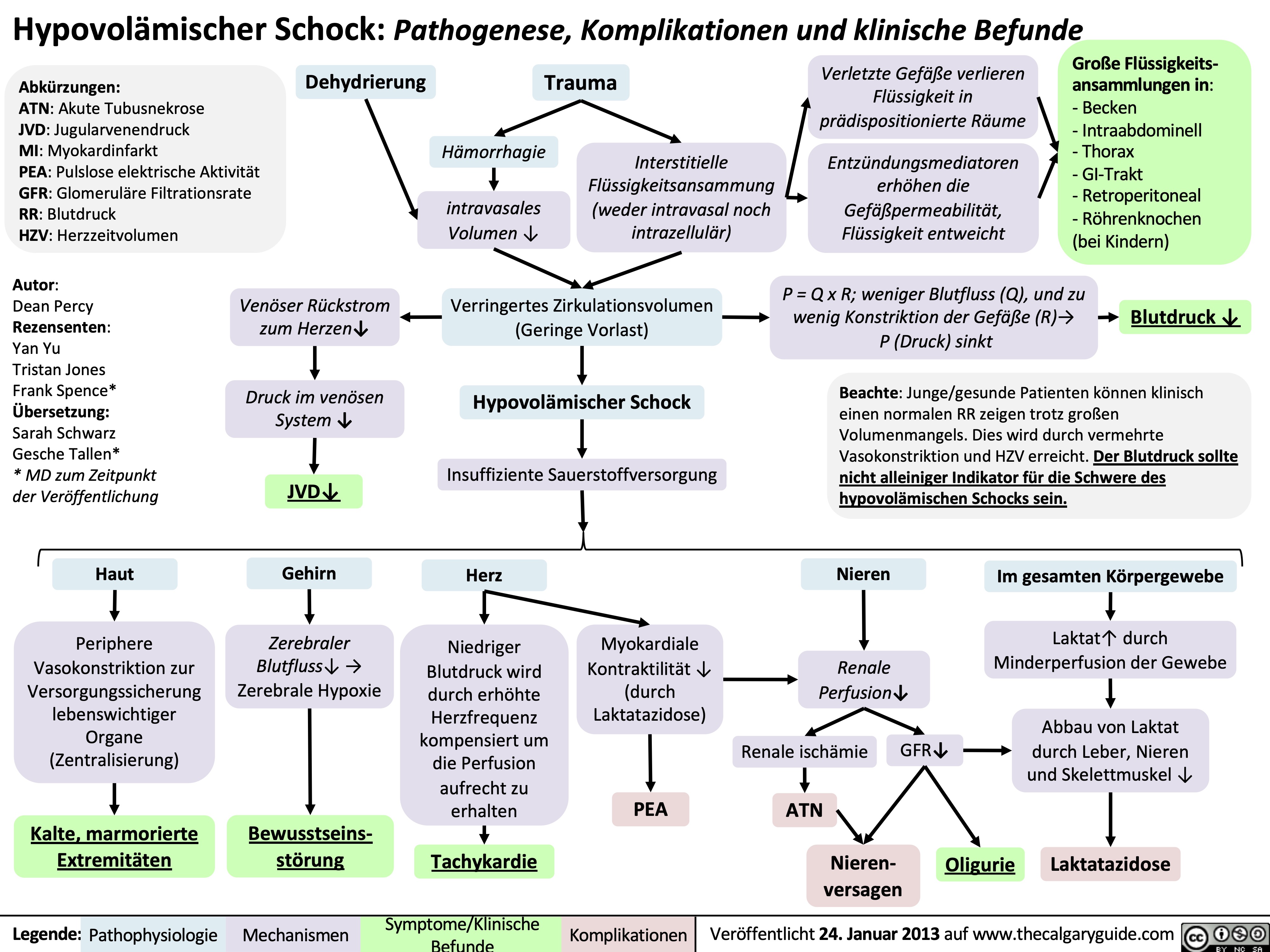
Hypovolämischer Schock: Pathogenese, Komplikationen und klinische Befunde
complications-of-pulmonary-embolism
![Complications of Pulmonary Embolism
Authors:
Sravya Kakumanu, Dean Percy, Yan Yu
Reviewers:
Tristan Jones, Ciara Hanly, Jieling Ma (马杰羚), Ben Campbell, Dr. Man-Chiu Poon*, Dr. Lynn Savoie*, Dr. Tara Lohmann * * MD at time of publication
IF CHRONIC:
Unresolved clot after 2 years leading to fibrosis of pulmonary vasculature
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
(<5% of PE cases)
Venous Stasis Hypercoagulable state
Vessel Injury
Virchow’s Triad (*See Suspected Deep Vein Thrombosis slide)
Deep Vein Thrombosis
Clot migrates from deep limb veins à femoral àiliac veins
ACUTE/MASSIVE PE:
Clot obstructs pulmonary arterial or arteriolar flow
Lung infarction (tissue death) from ischemia
Inflammatory cells migrate to site and release cytokines
↑ Permeability of blood vessels
Permeability-driven (exudate) fluid leakage into pleural space
Pleural Effusion
Clot migratesàinferior vena cava àright atrium (RA) of heartà right ventricle (RV) à gets lodged in pulmonary arteries/arterioles
Pulmonary Embolism (PE)
↑ RV afterload
↑ RV pressure and expansion
Well-ventilated (V) areas of lung do not receive adequate blood supply (Q)
V/Q Mismatch
Leftward shift of ventricular septum
↓ Left ventricle filling in diastole
↓ Cardiac output
Obstructive Shock
Impaired heart filling
Pulseless Electrical Activity
(ECG activity in absence of palpable pulse)
Back up of pressure in systemic venous system
↑ Pressure in capillaries draining parietal pleura
Pressure-driven (transudate) fluid leakage into pleural space
For signs and symptoms, see the Obstructive Shock slide
For signs and symptoms refer to CTEPH slide
Chronic ↑ RV afterload
↑ Stretching of myocytes causing RV hypertrophy and dilation
↓ RV ejection fraction
Right Heart Failure
“Cor Pulmonale”
For signs and symptoms, see the Right Heart Failure slide
Failure to oxygenate blood
Type I Respiratory Failure
Hypoxemic: patient has ↓ blood [O2]
IF MASSIVE PE (less common):
↑ Alveolar dead space
Failure to ventilate
Type II Respiratory Failure Hypercapnic: patient has ↑ blood [CO2]
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 7, 2012, updated Mar 31, 2022 on www.thecalgaryguide.com
Complications of Pulmonary Embolism
Authors:
Sravya Kakumanu, Dean Percy, Yan Yu
Reviewers:
Tristan Jones, Ciara Hanly, Jieling Ma (马杰羚), Ben Campbell, Dr. Man-Chiu Poon*, Dr. Lynn Savoie*, Dr. Tara Lohmann * * MD at time of publication
IF CHRONIC:
Unresolved clot after 2 years leading to fibrosis of pulmonary vasculature
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
(<5% of PE cases)
Venous Stasis Hypercoagulable state
Vessel Injury
Virchow’s Triad (*See Suspected Deep Vein Thrombosis slide)
Deep Vein Thrombosis
Clot migrates from deep limb veins à femoral àiliac veins
ACUTE/MASSIVE PE:
Clot obstructs pulmonary arterial or arteriolar flow
Lung infarction (tissue death) from ischemia
Inflammatory cells migrate to site and release cytokines
↑ Permeability of blood vessels
Permeability-driven (exudate) fluid leakage into pleural space
Pleural Effusion
Clot migratesàinferior vena cava àright atrium (RA) of heartà right ventricle (RV) à gets lodged in pulmonary arteries/arterioles
Pulmonary Embolism (PE)
↑ RV afterload
↑ RV pressure and expansion
Well-ventilated (V) areas of lung do not receive adequate blood supply (Q)
V/Q Mismatch
Leftward shift of ventricular septum
↓ Left ventricle filling in diastole
↓ Cardiac output
Obstructive Shock
Impaired heart filling
Pulseless Electrical Activity
(ECG activity in absence of palpable pulse)
Back up of pressure in systemic venous system
↑ Pressure in capillaries draining parietal pleura
Pressure-driven (transudate) fluid leakage into pleural space
For signs and symptoms, see the Obstructive Shock slide
For signs and symptoms refer to CTEPH slide
Chronic ↑ RV afterload
↑ Stretching of myocytes causing RV hypertrophy and dilation
↓ RV ejection fraction
Right Heart Failure
“Cor Pulmonale”
For signs and symptoms, see the Right Heart Failure slide
Failure to oxygenate blood
Type I Respiratory Failure
Hypoxemic: patient has ↓ blood [O2]
IF MASSIVE PE (less common):
↑ Alveolar dead space
Failure to ventilate
Type II Respiratory Failure Hypercapnic: patient has ↑ blood [CO2]
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 7, 2012, updated Mar 31, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2014/09/Complications-of-Pulmonary-Embolism-2022.jpg)
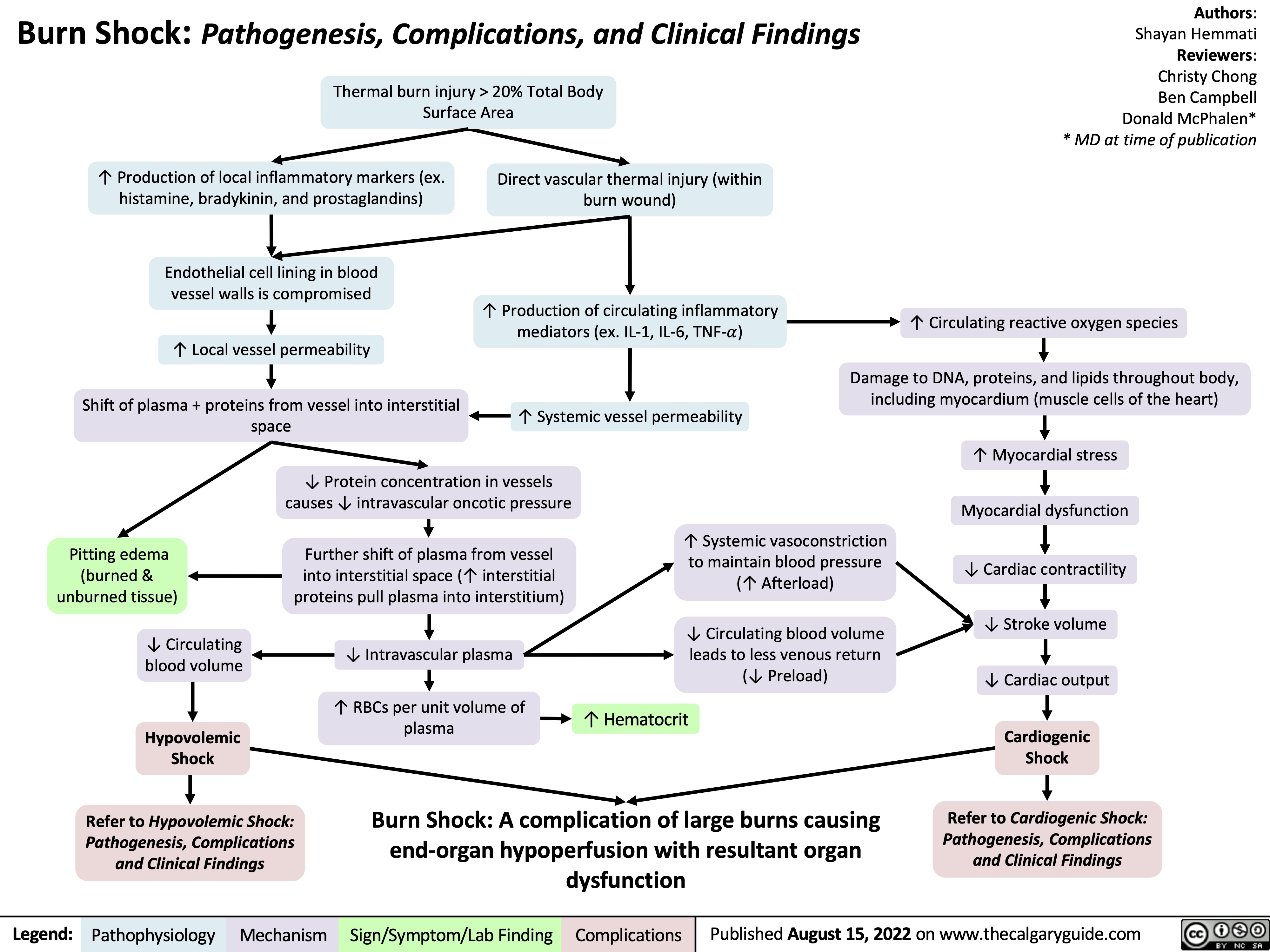
burn-shock-pathogenesis-complications-and-clinical-findings
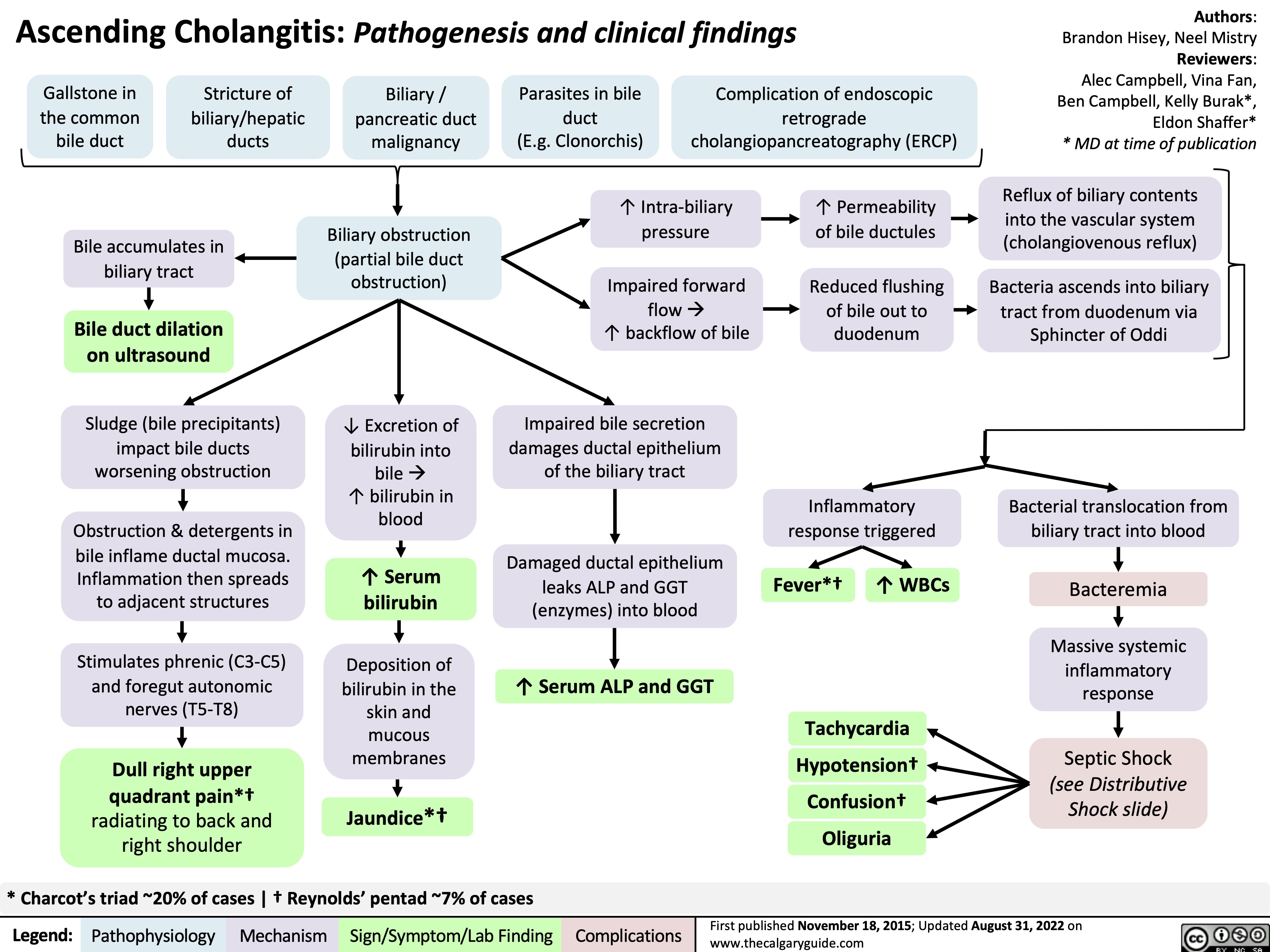
ascending-cholangitis-pathogenesis-clinical-findings
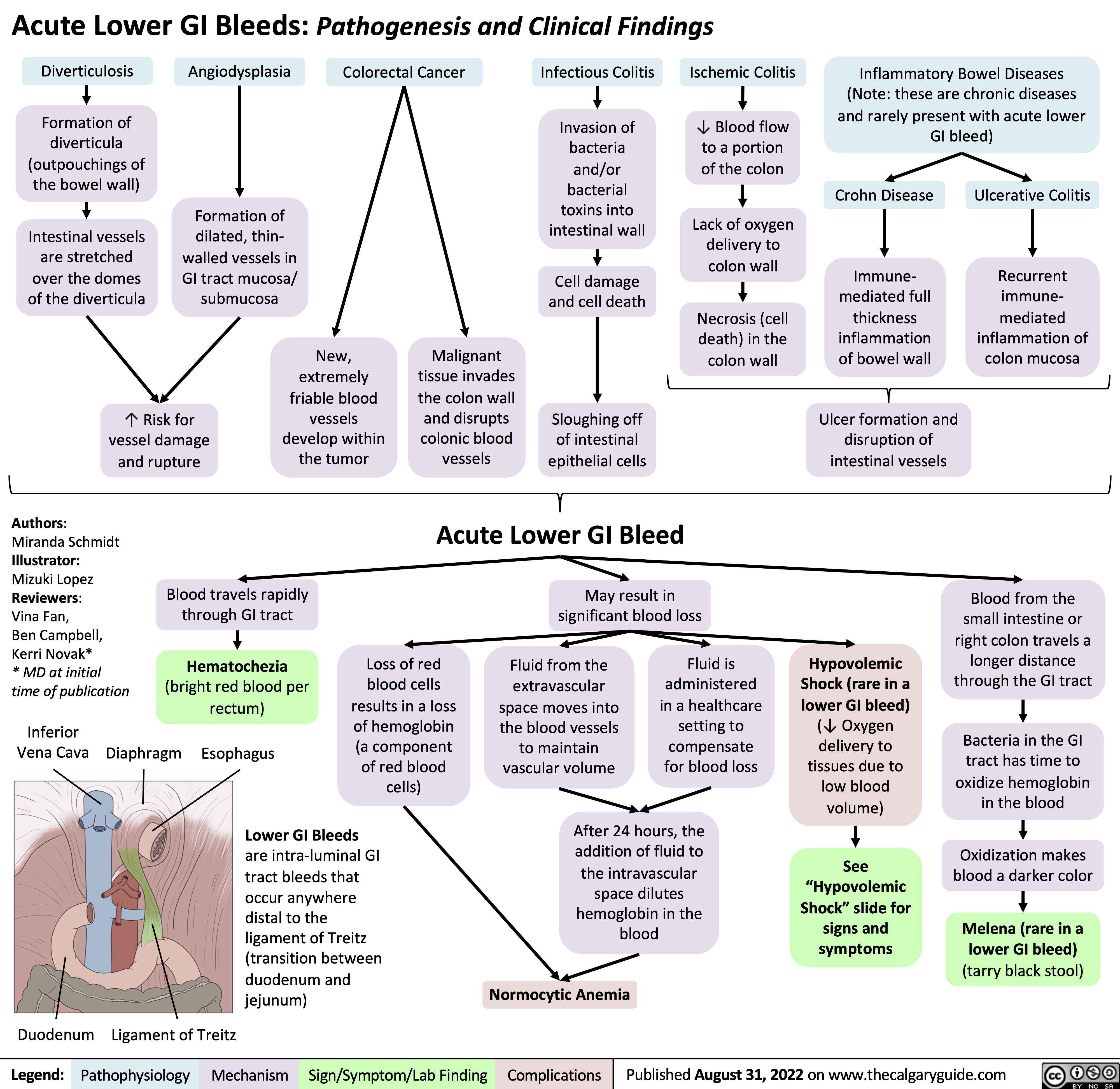
acute-lower-gi-bleeds-pathogenesis-and-clinical-findings
ventilator-associated-pneumonia-pathogenesis-and-clinical-findings
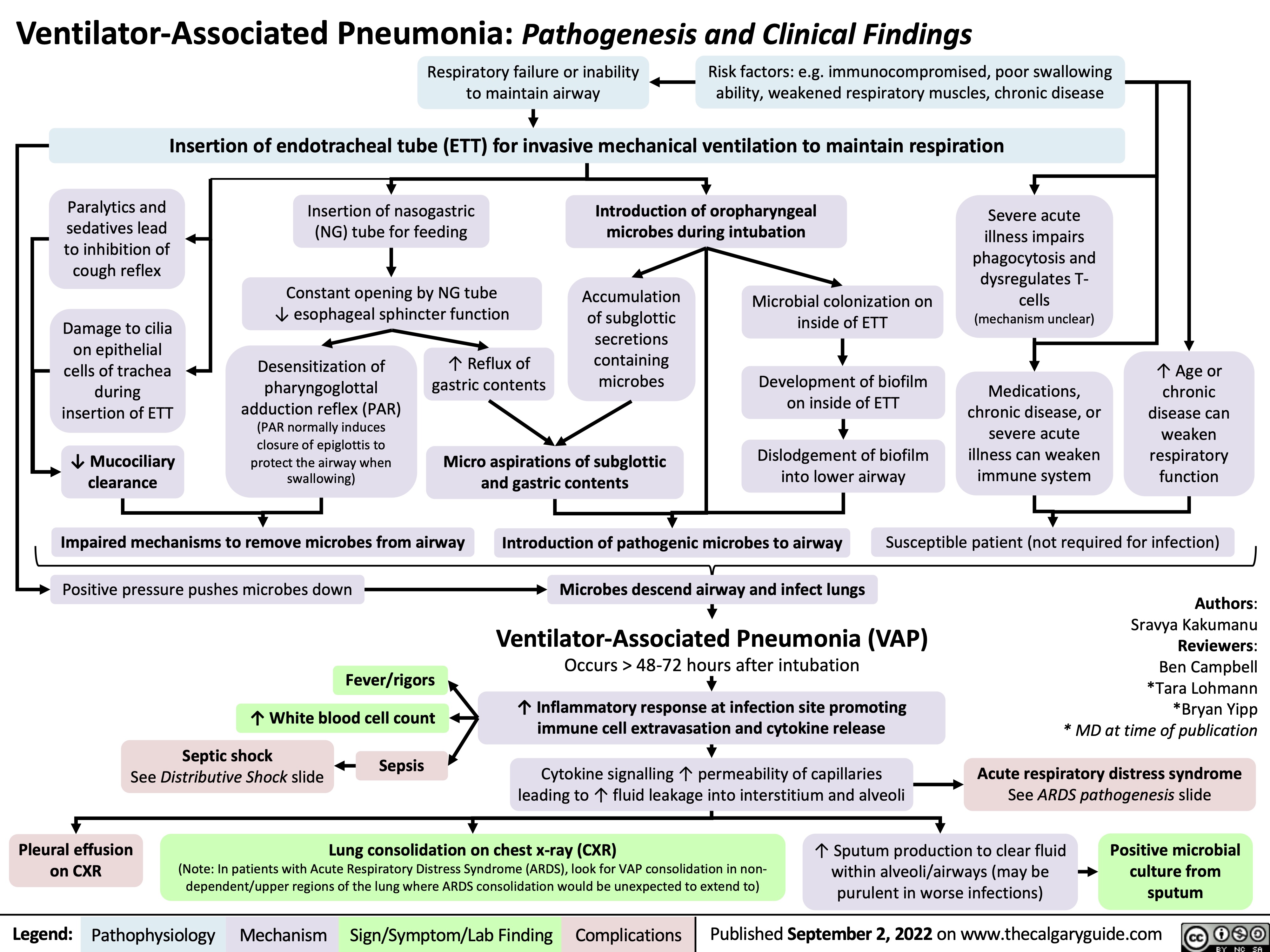
syok-luka-bakar-patogenesis-komplikasi-dan-temuan-klinis
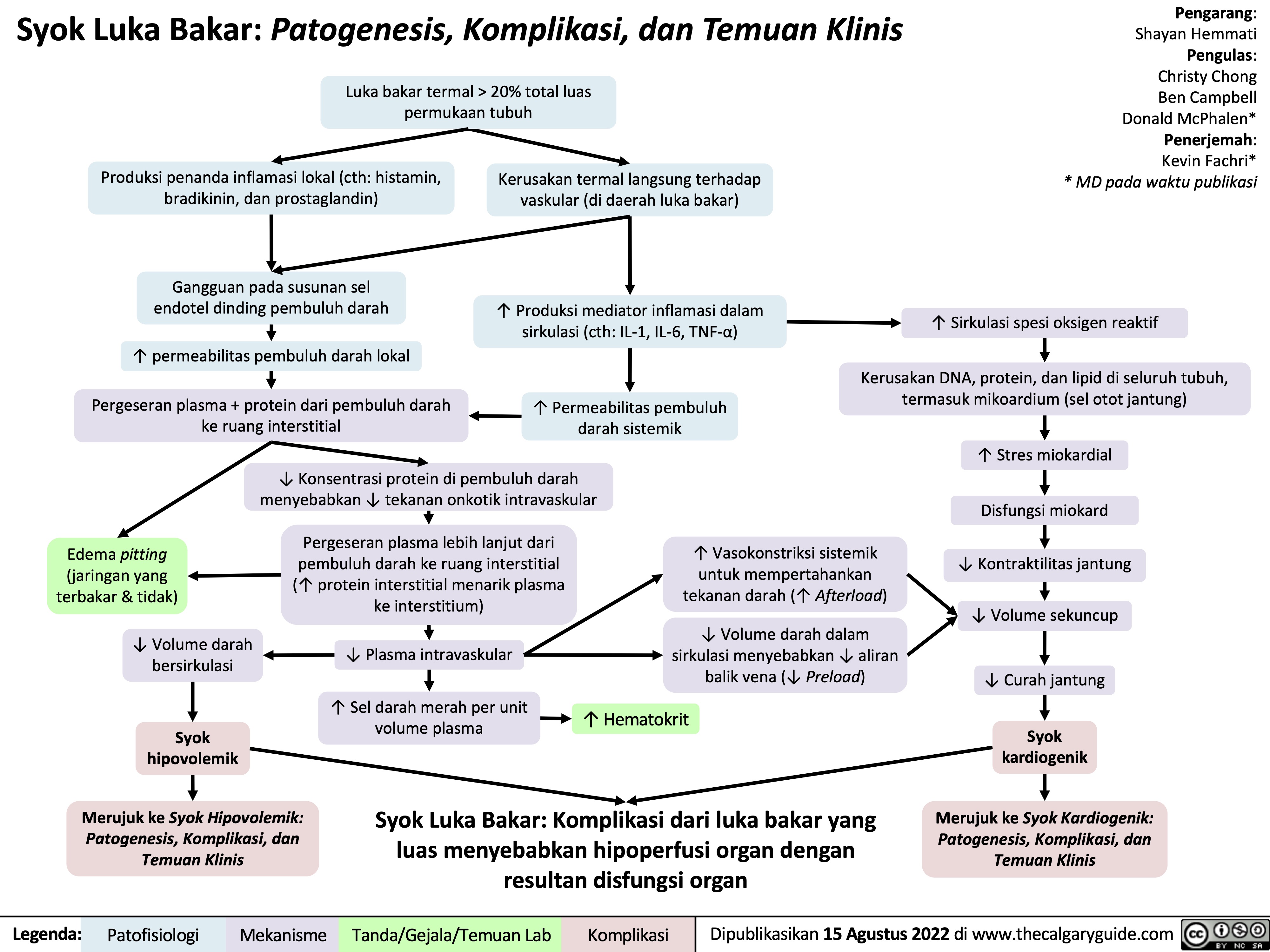
syok-hipovolemik-patogenesis-komplikasi-dan-temuan-klinis
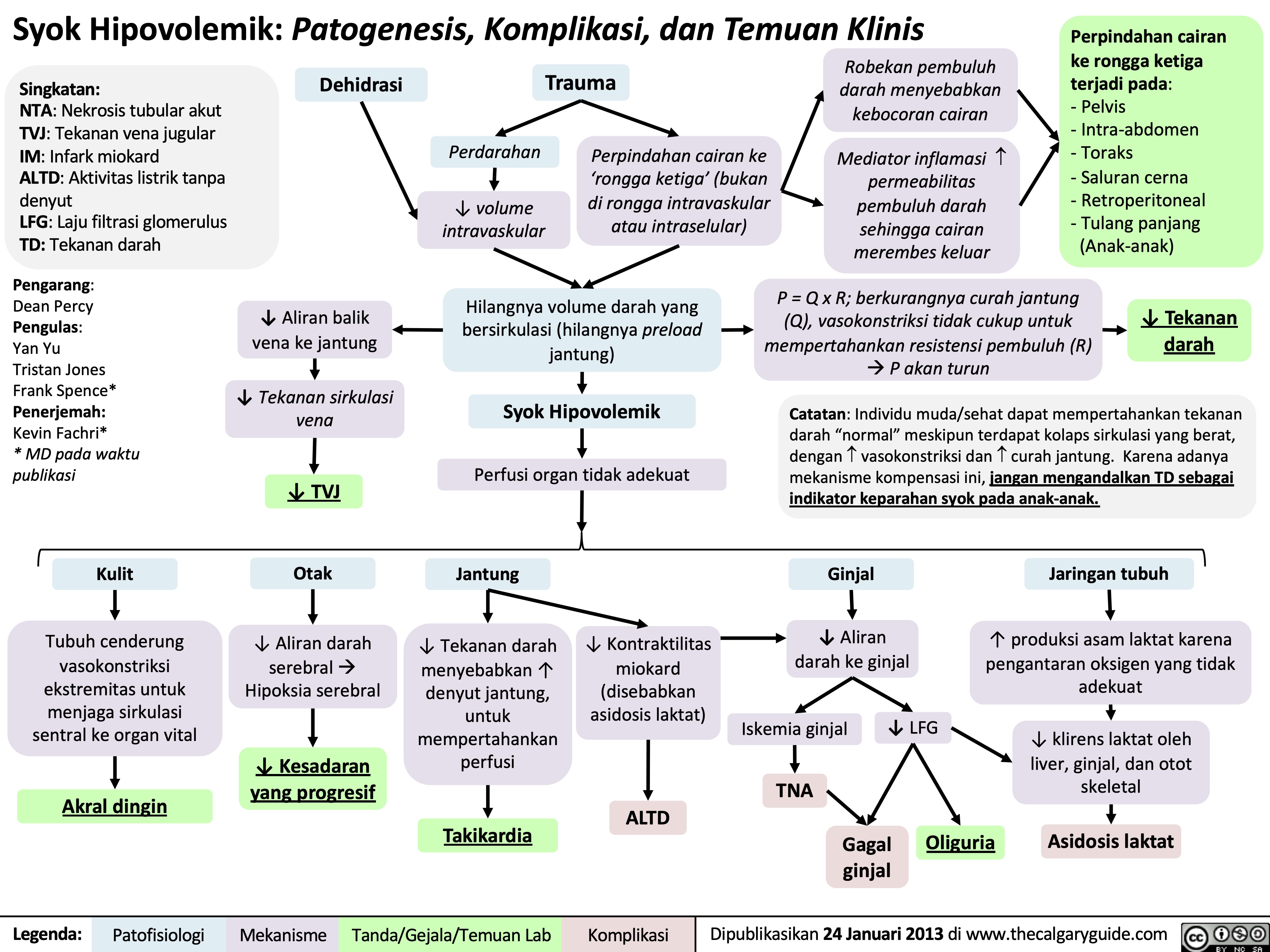
Syok Obstruktif: Patogenesis, komplikasi, dan temuan klinis
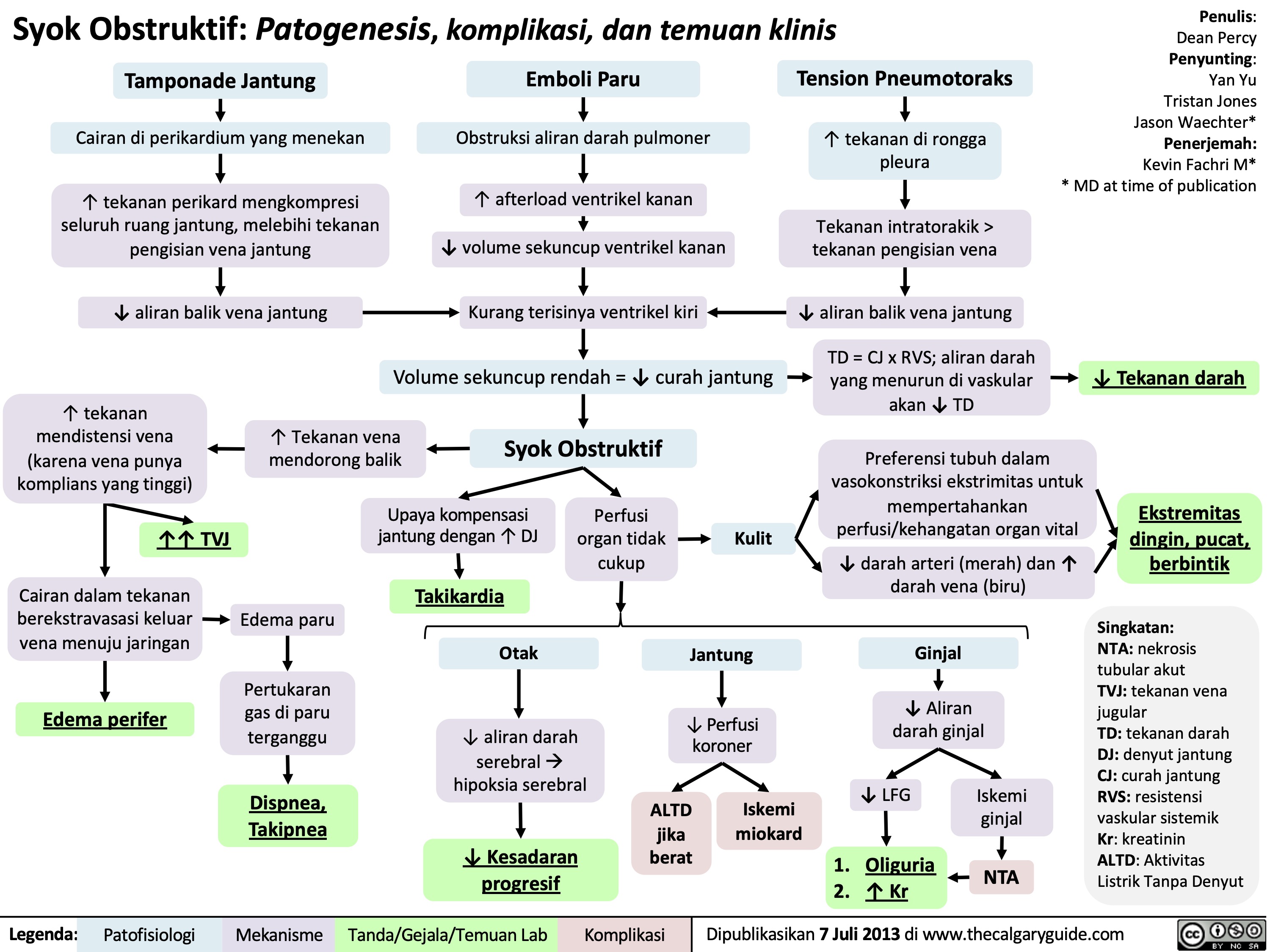
Syok Kardiogenik: Patogenesis, komplikasi, dan temuan klinis
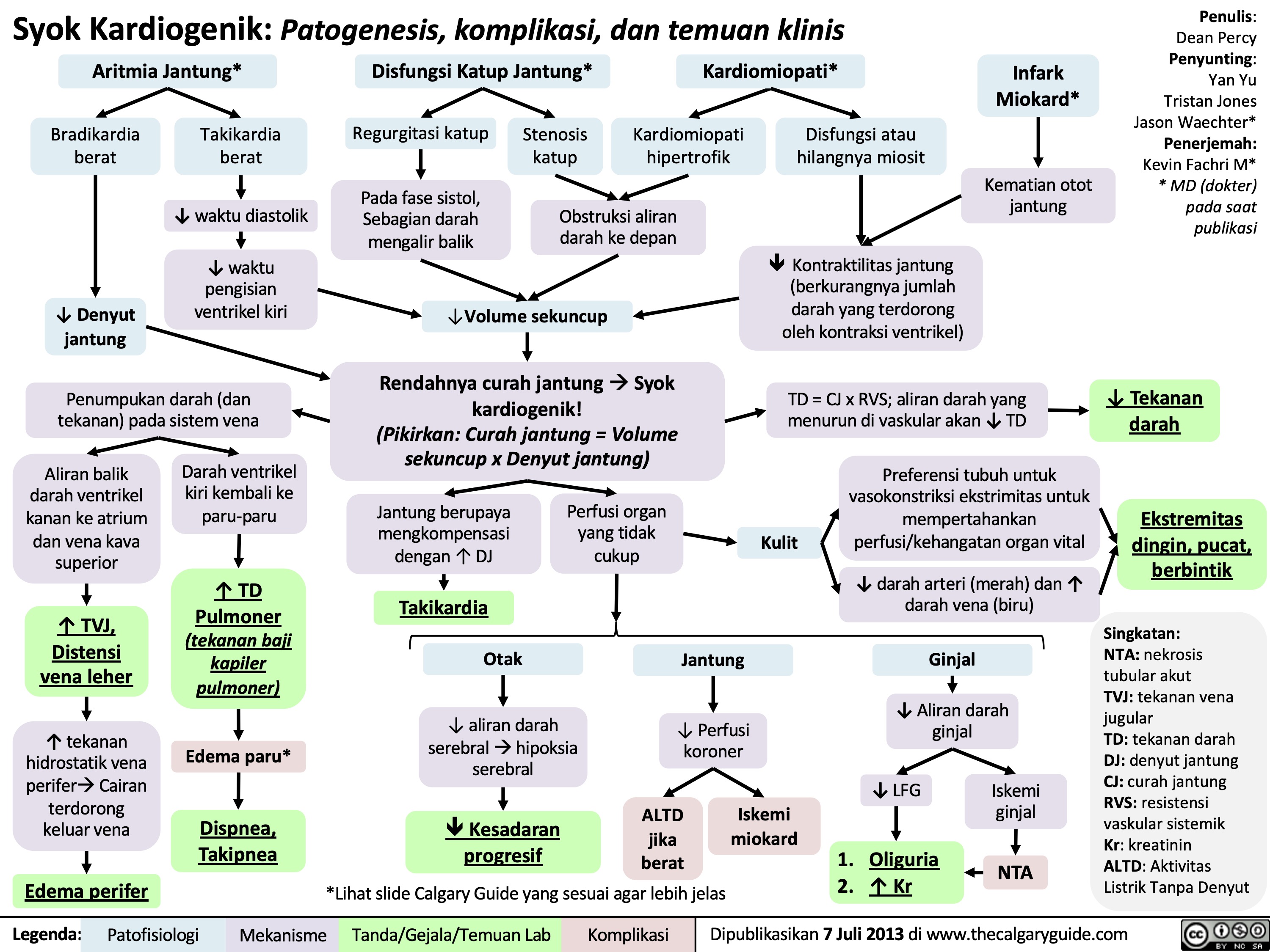
Syok Distributif: Patogenesis, komplikasi, dan temuan klinis
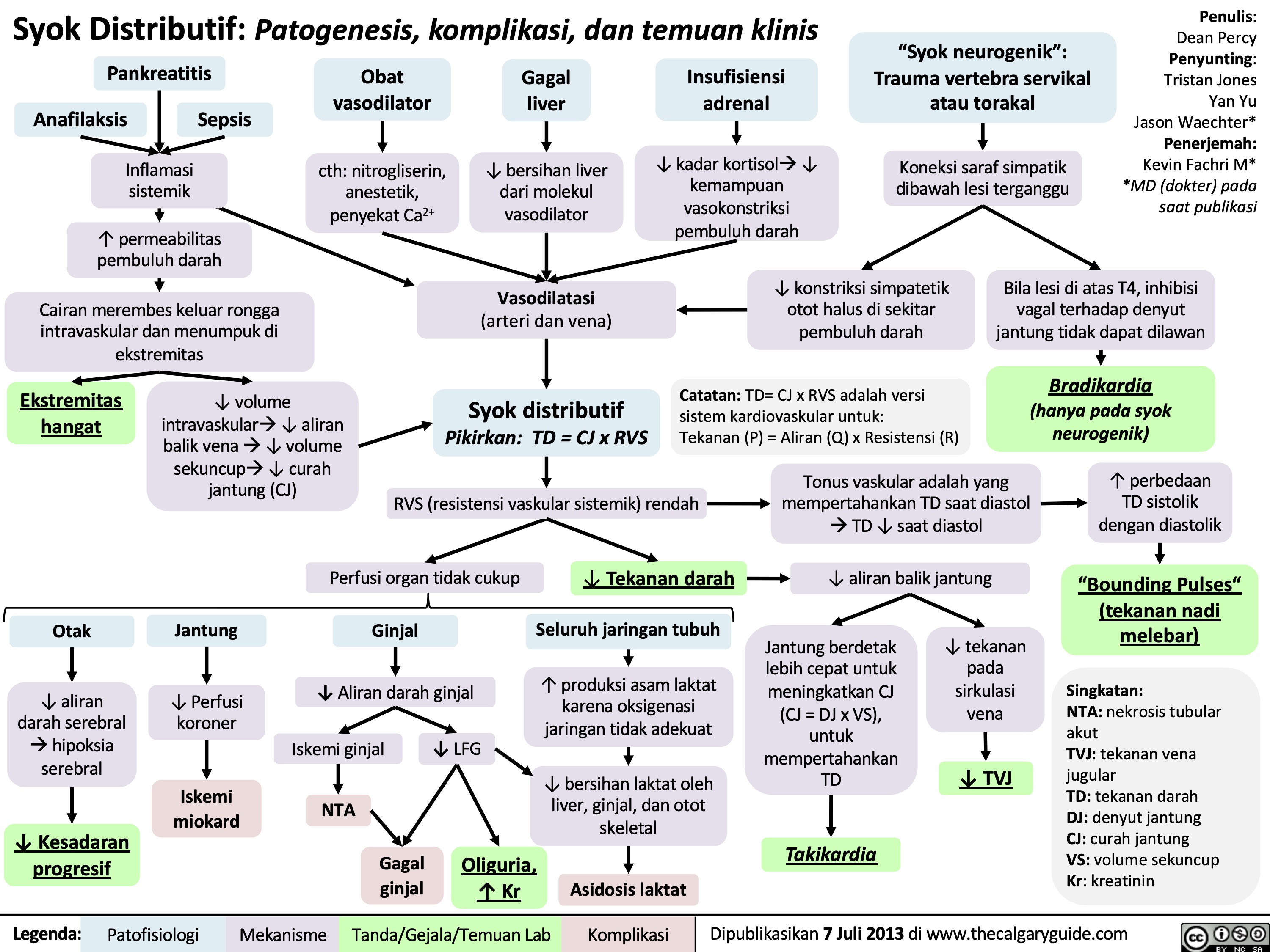
Acute Liver Failure: Pathogenesis and clinical findings
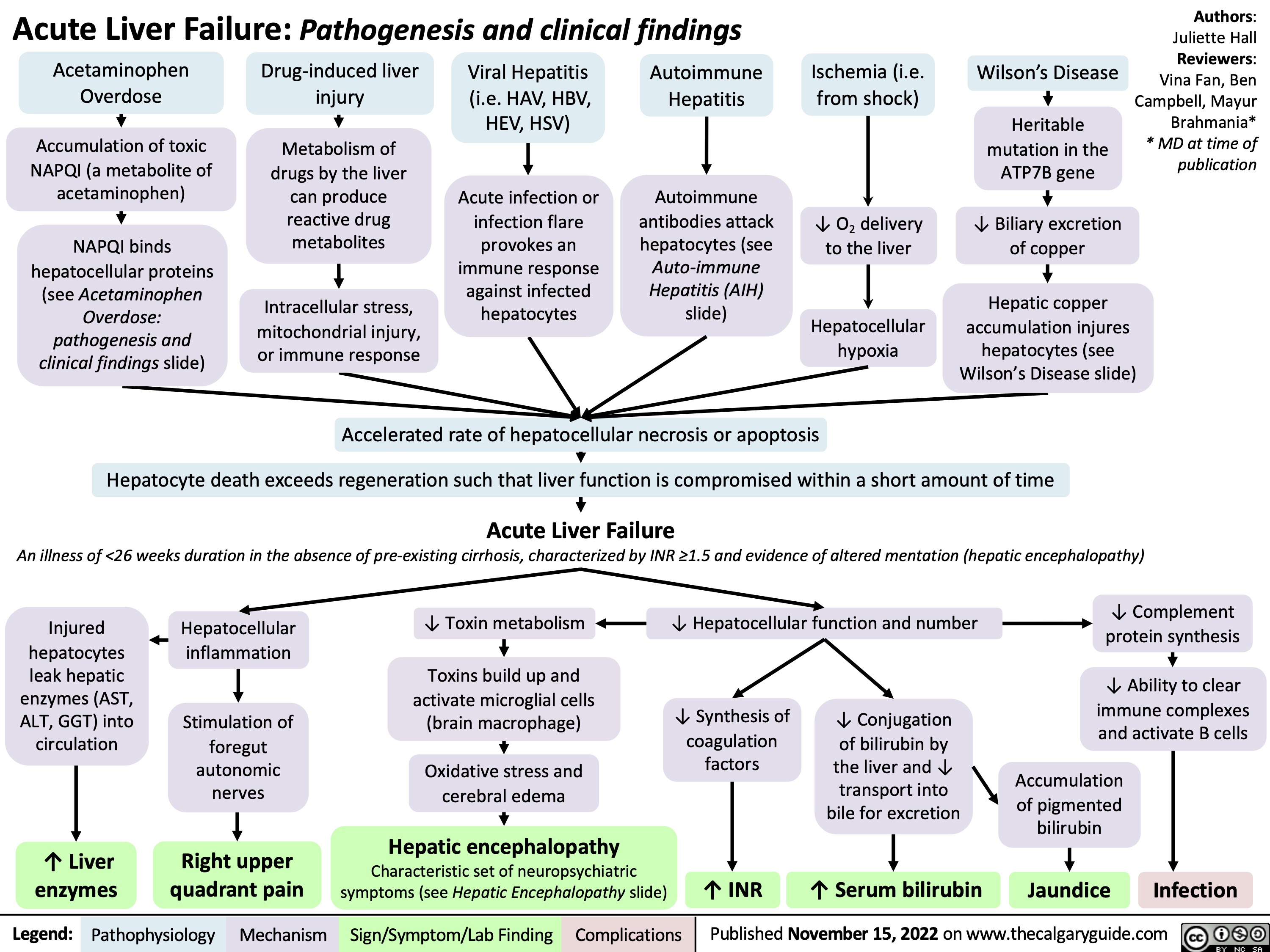
hypovolemic-shock
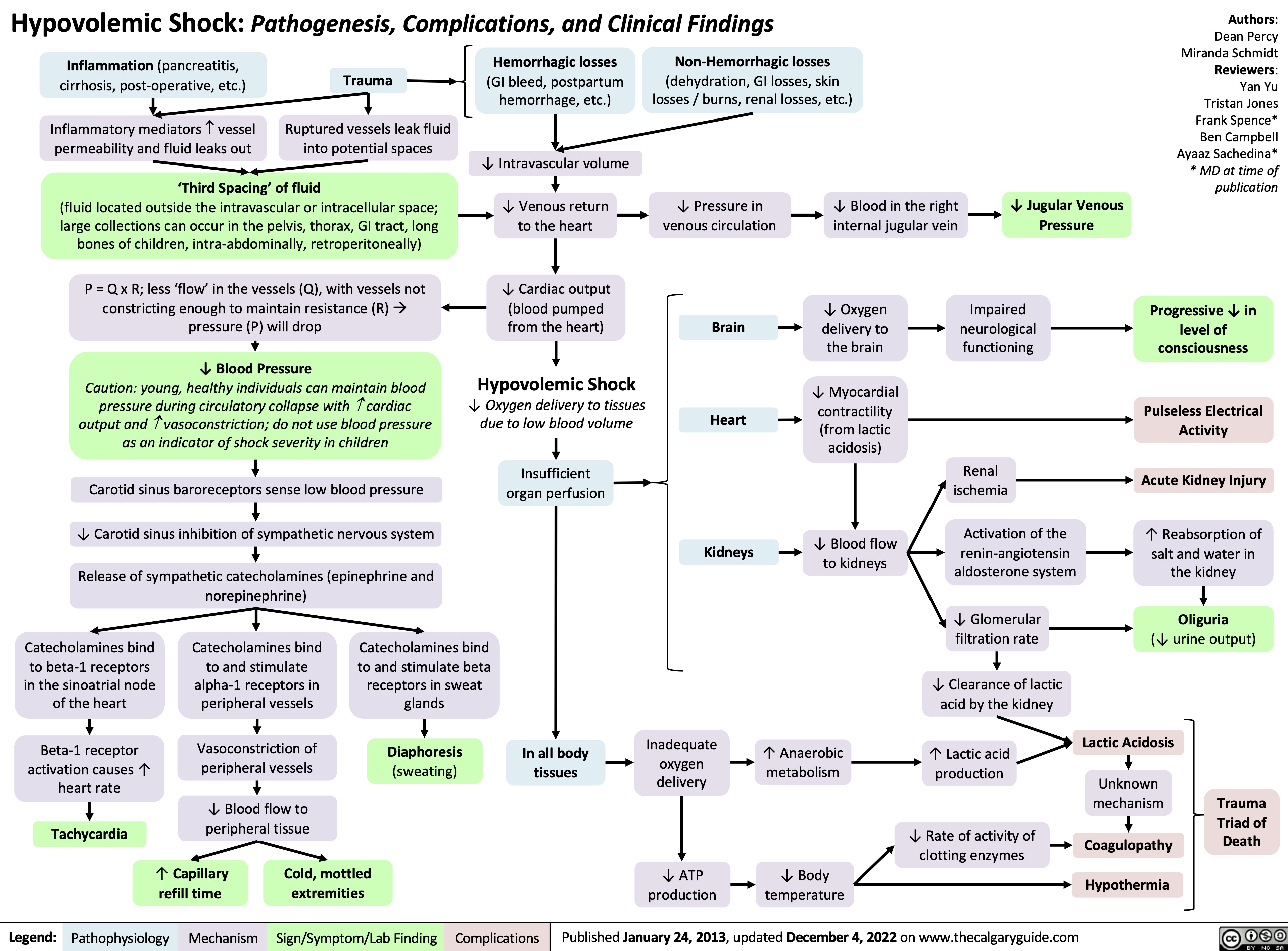
Wstrząs obturacyjny: Patogeneza, powikłania oraz zmiany kliniczne
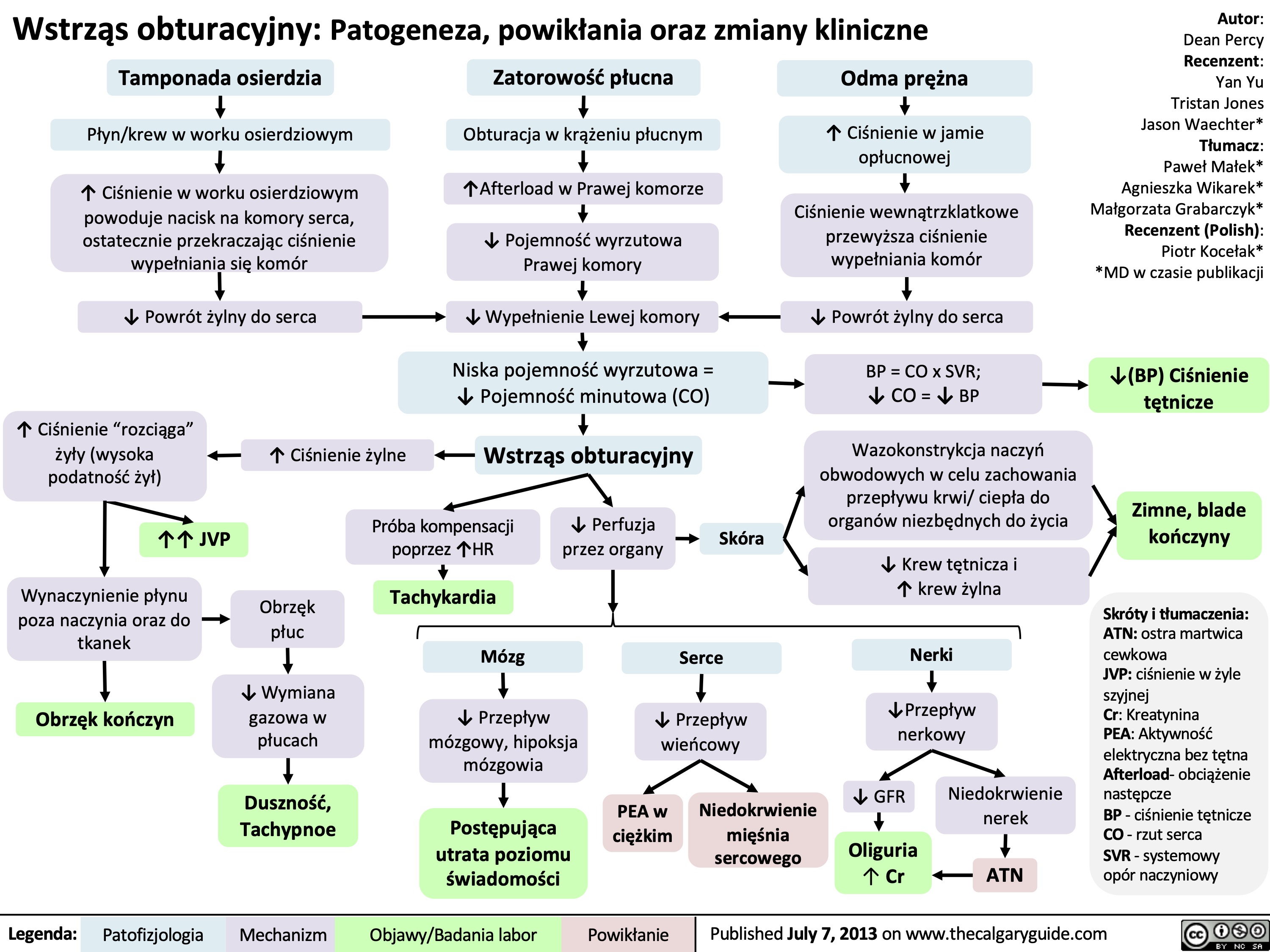
Wstrząs dystrybucyjny: Patogeneza, powikłania oraz zmiany kliniczne
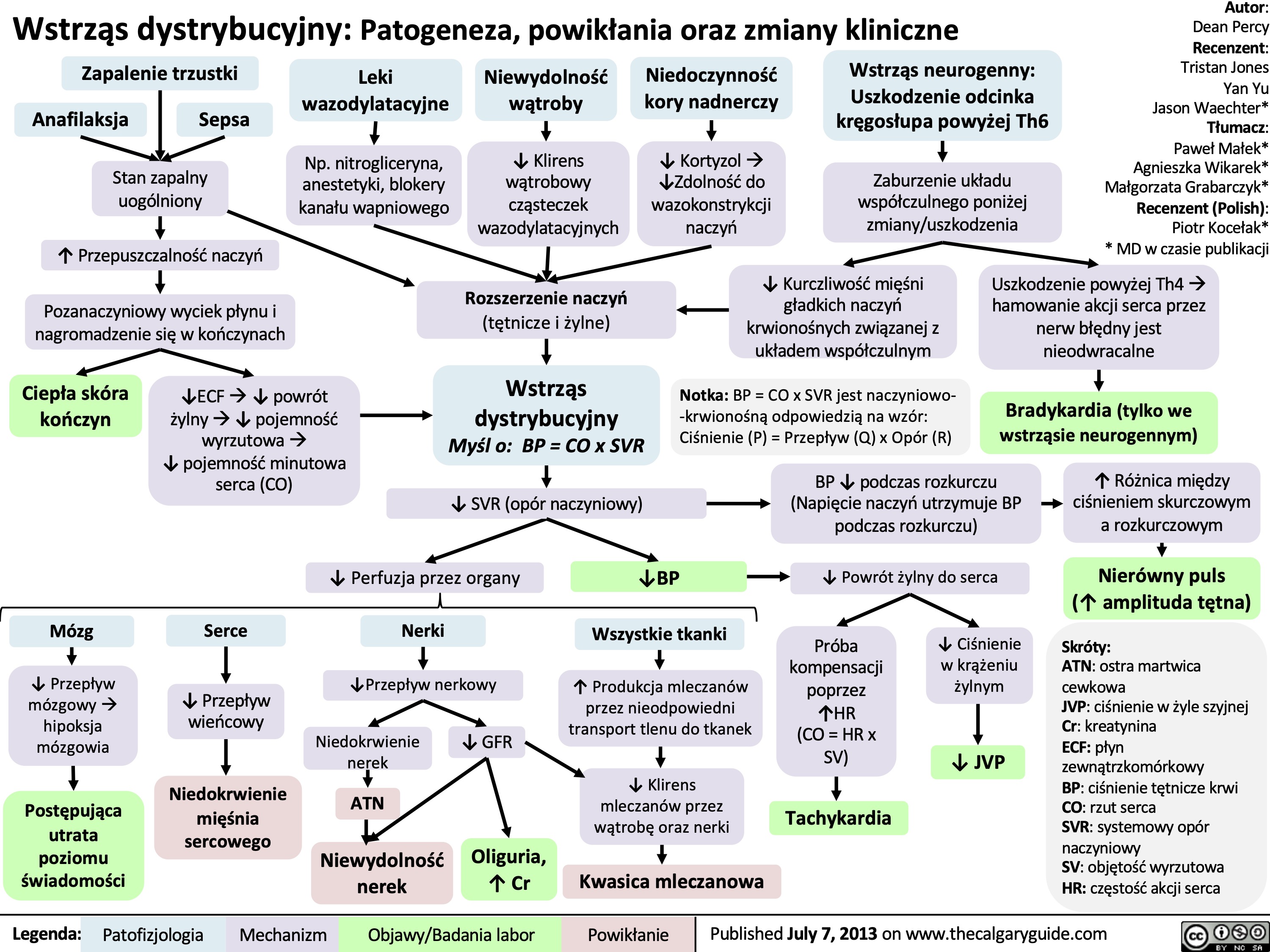
Wstrząs kardiogenny: Patogeneza, powikłania oraz zmiany kliniczne
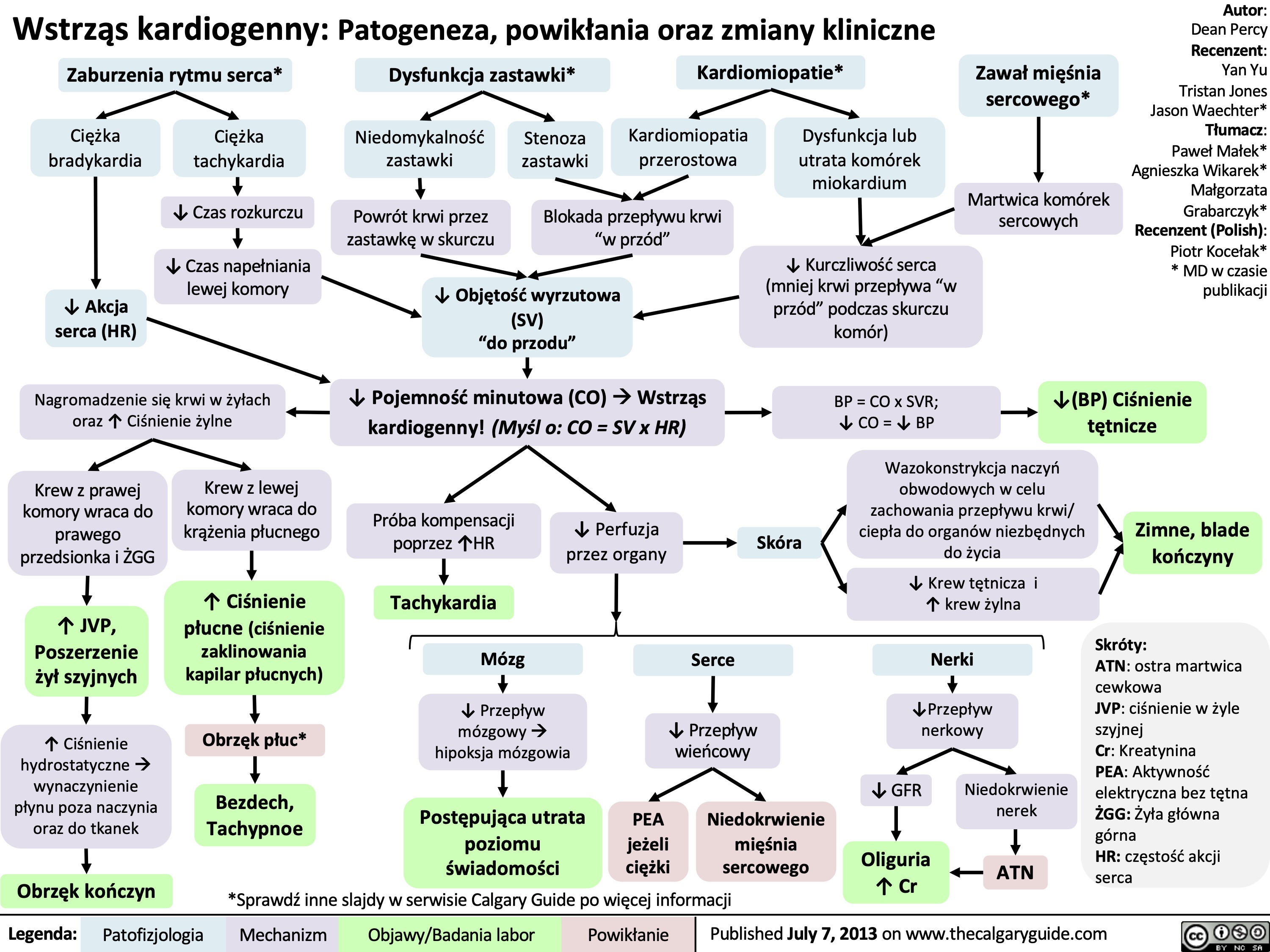
Wstrząs oparzeniowy: patogeneza, powikłania i zmiany kliniczne
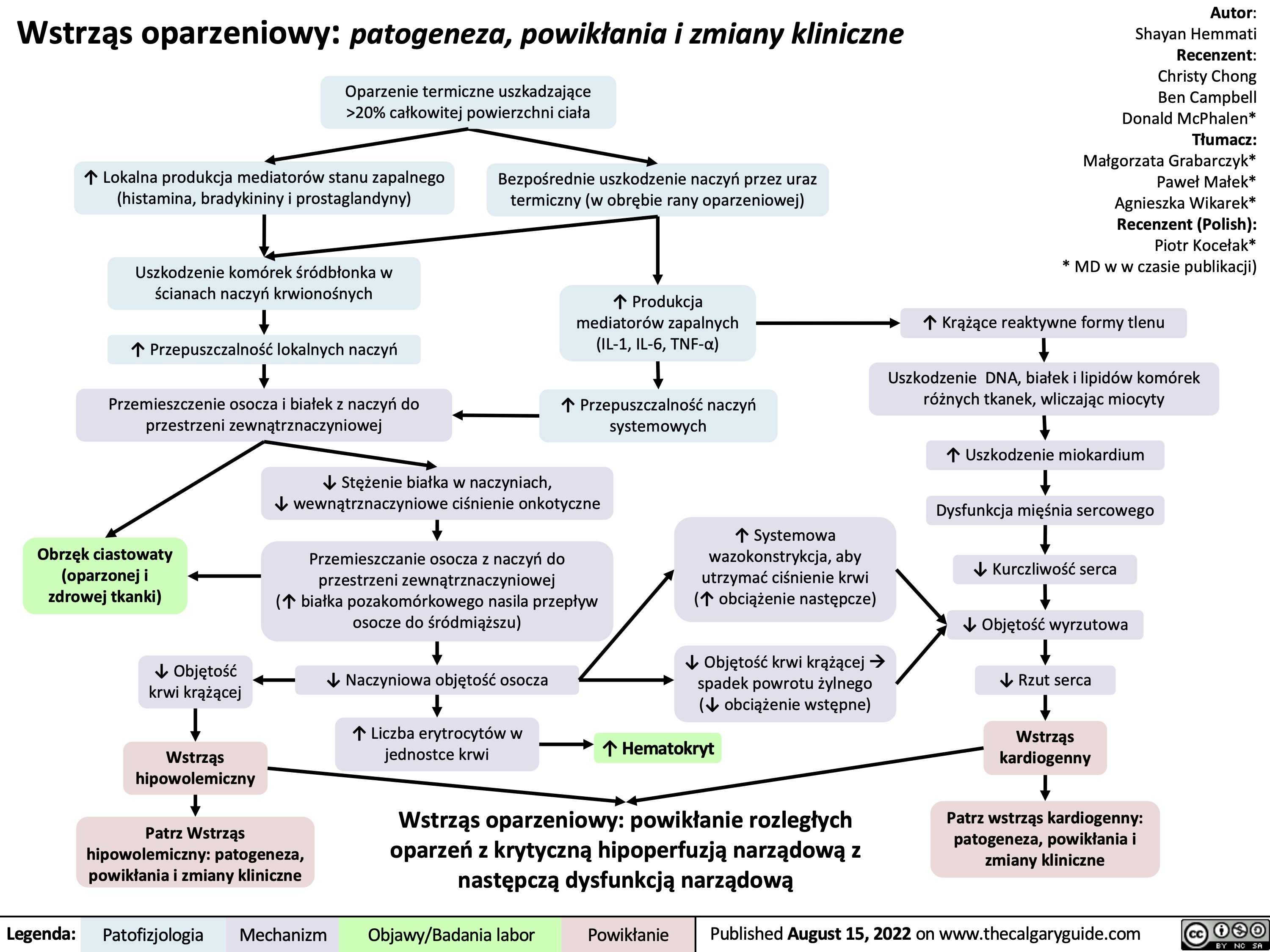
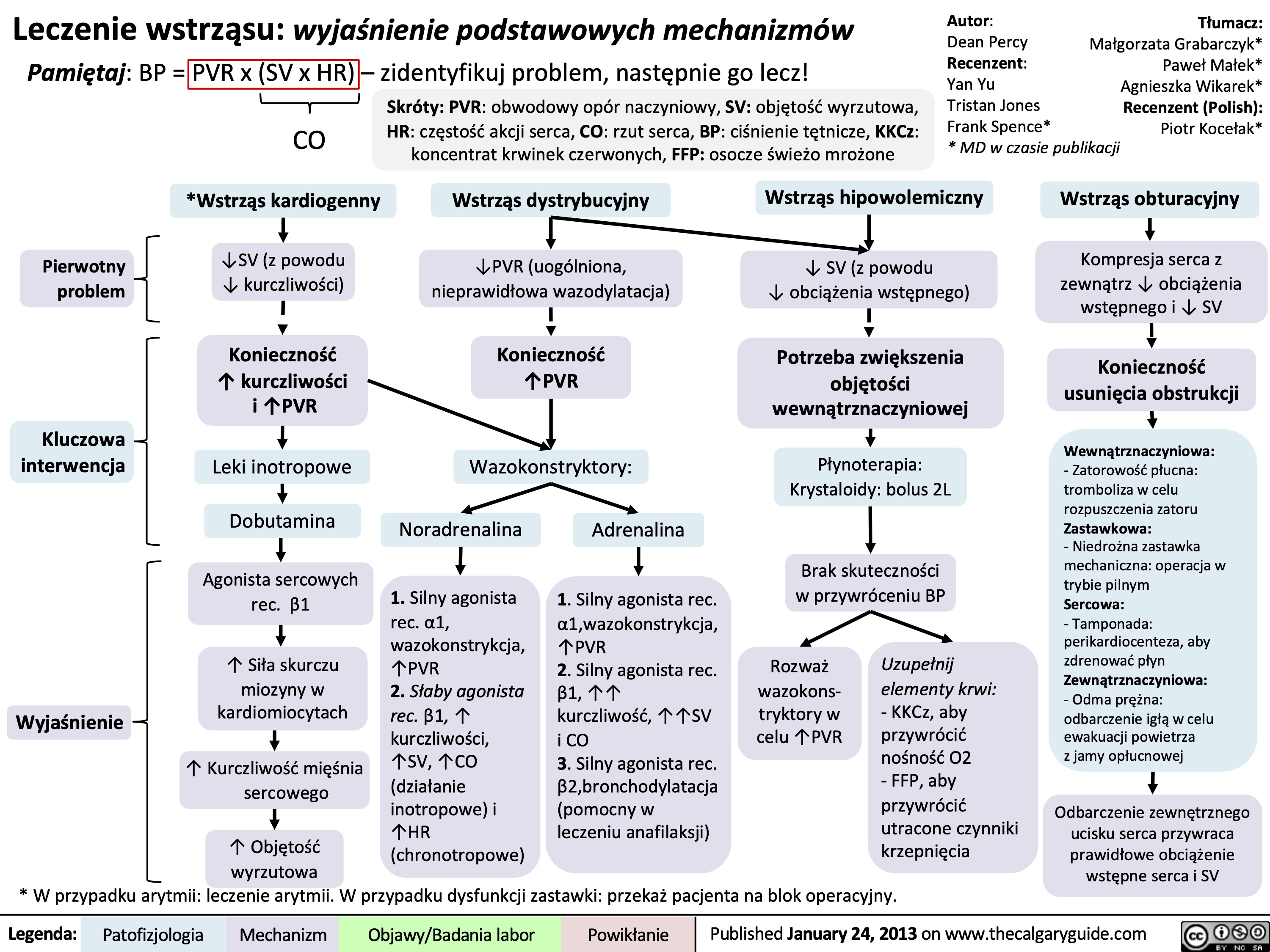
Leczenie wstrząsu: wyjaśnienie podstawowych mechanizmów
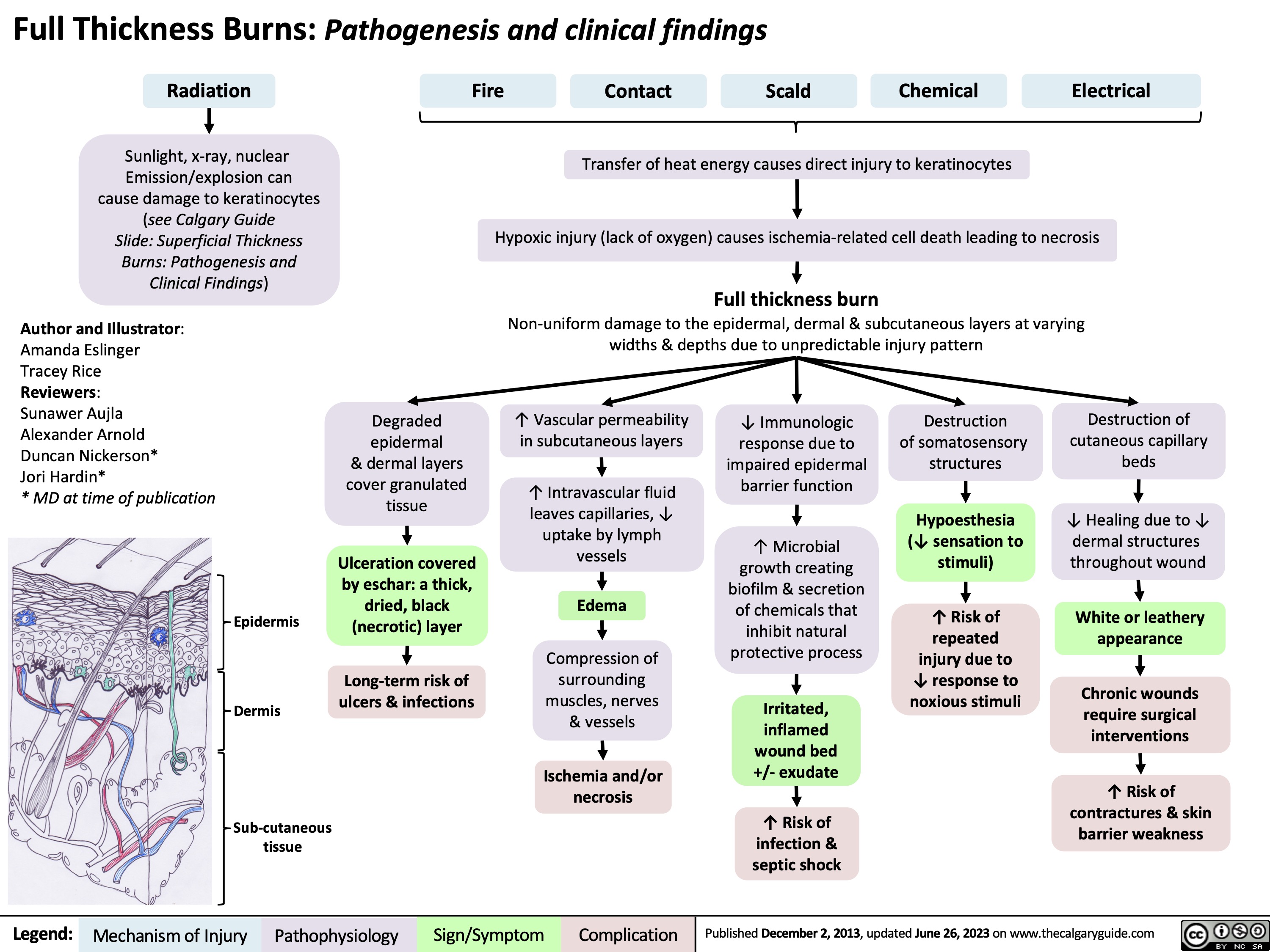
Burns - Full Thickness - Pathogenesis and Clinical Findings 2023
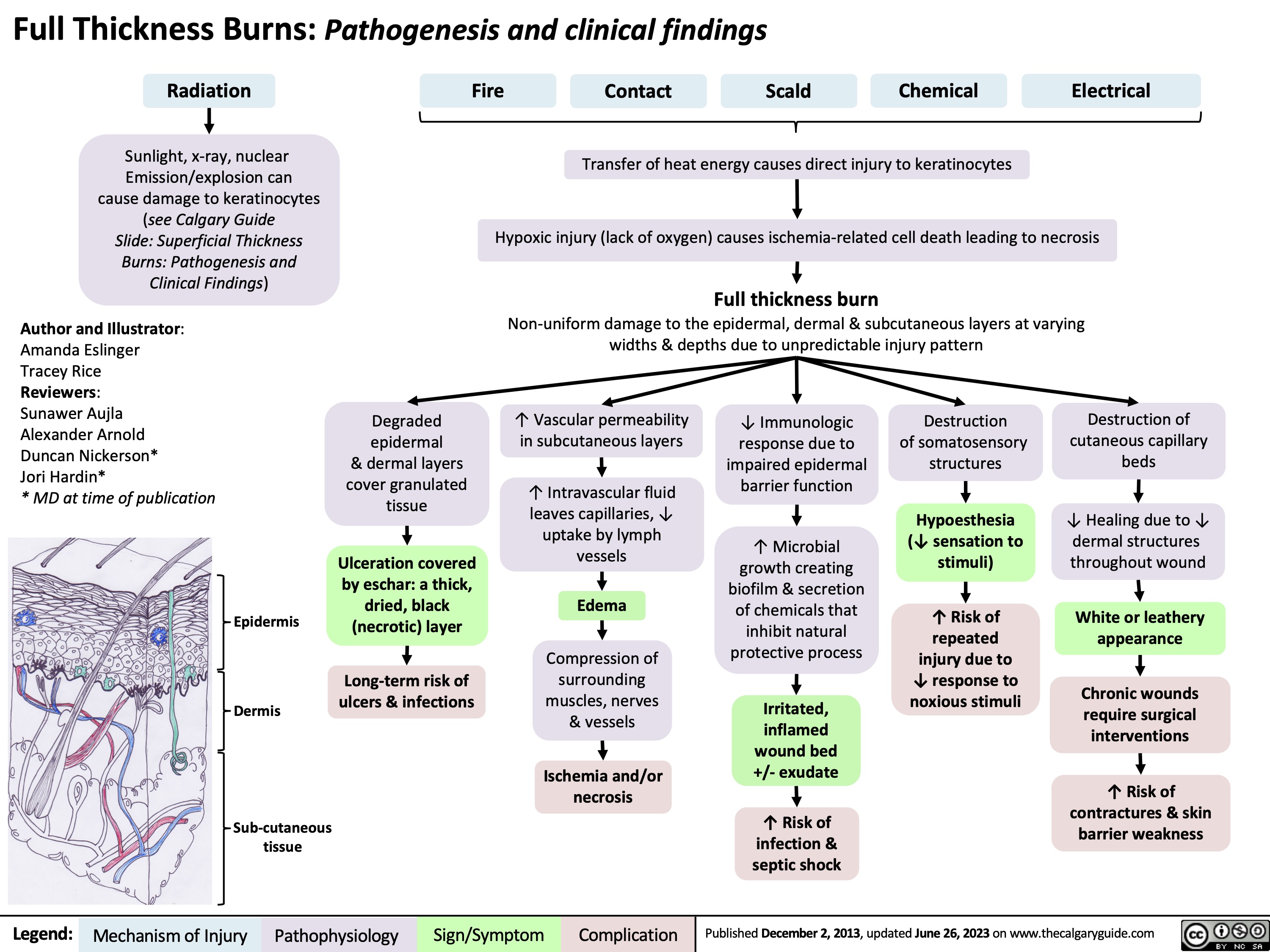
Burns - Full Thickness - Pathogenesis and Clinical Findings
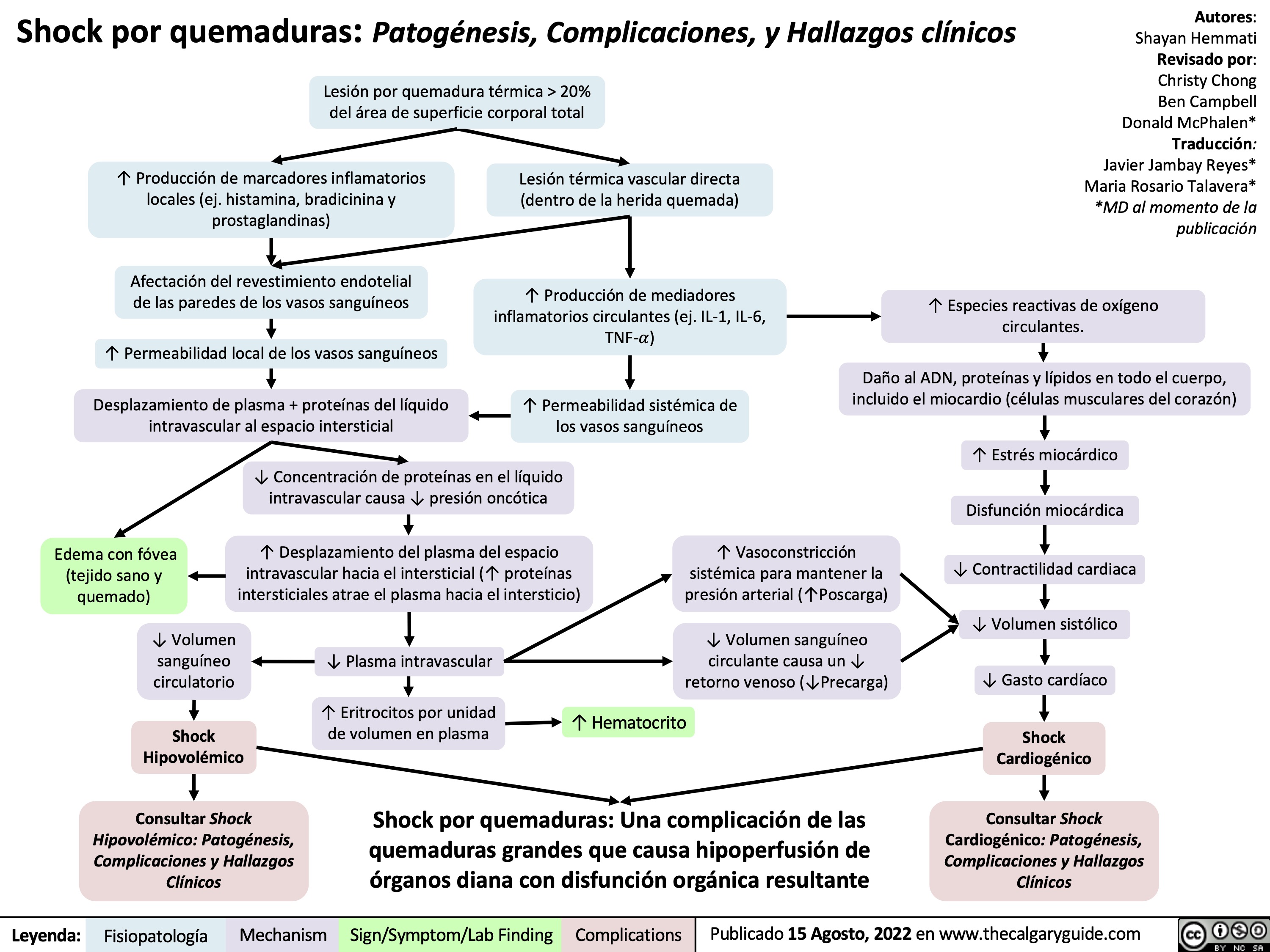
Shock por quemaduras
Tatalaksana Syok Penjelasan dari mekanisme dasar
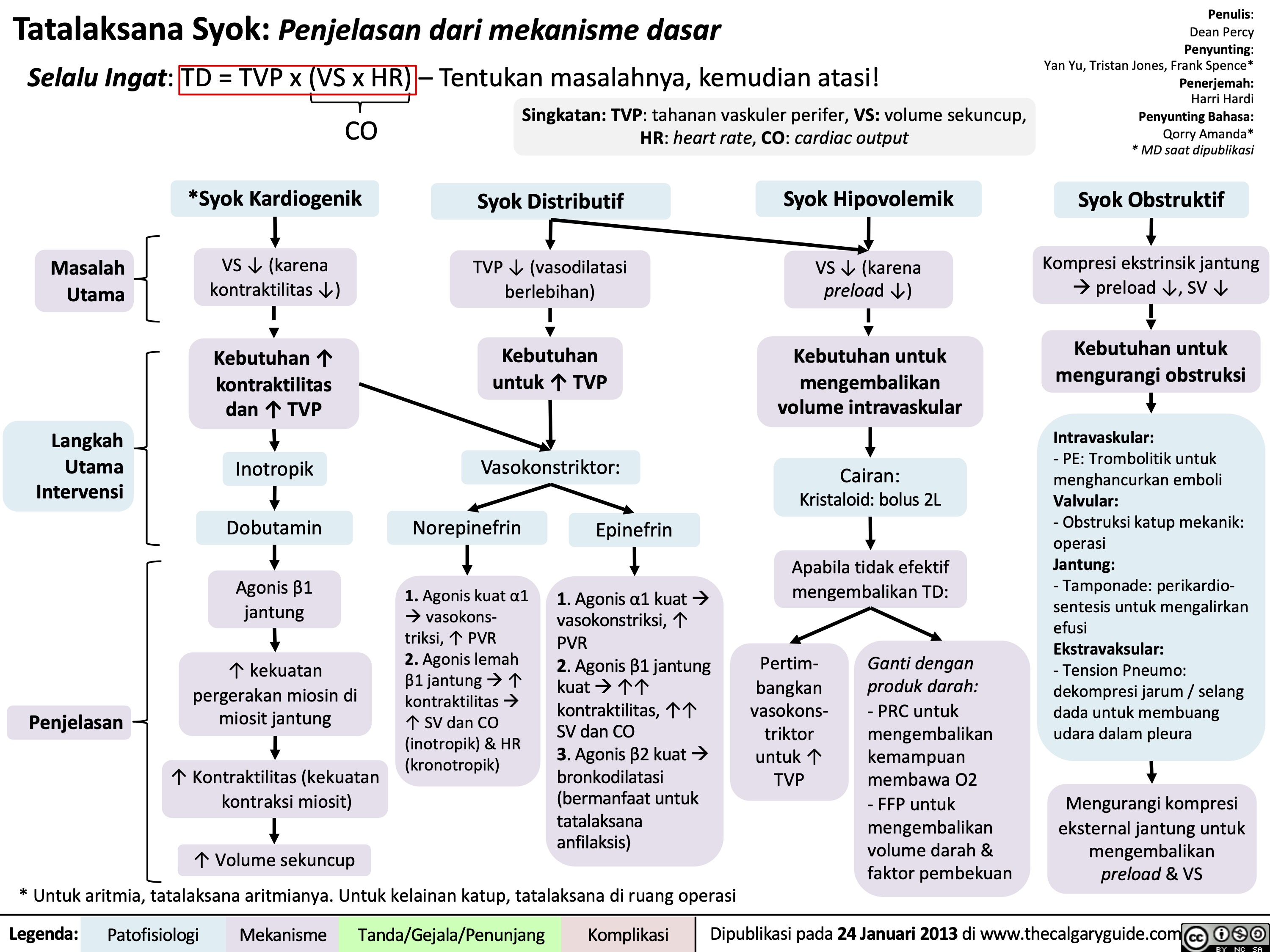
Overview of burns
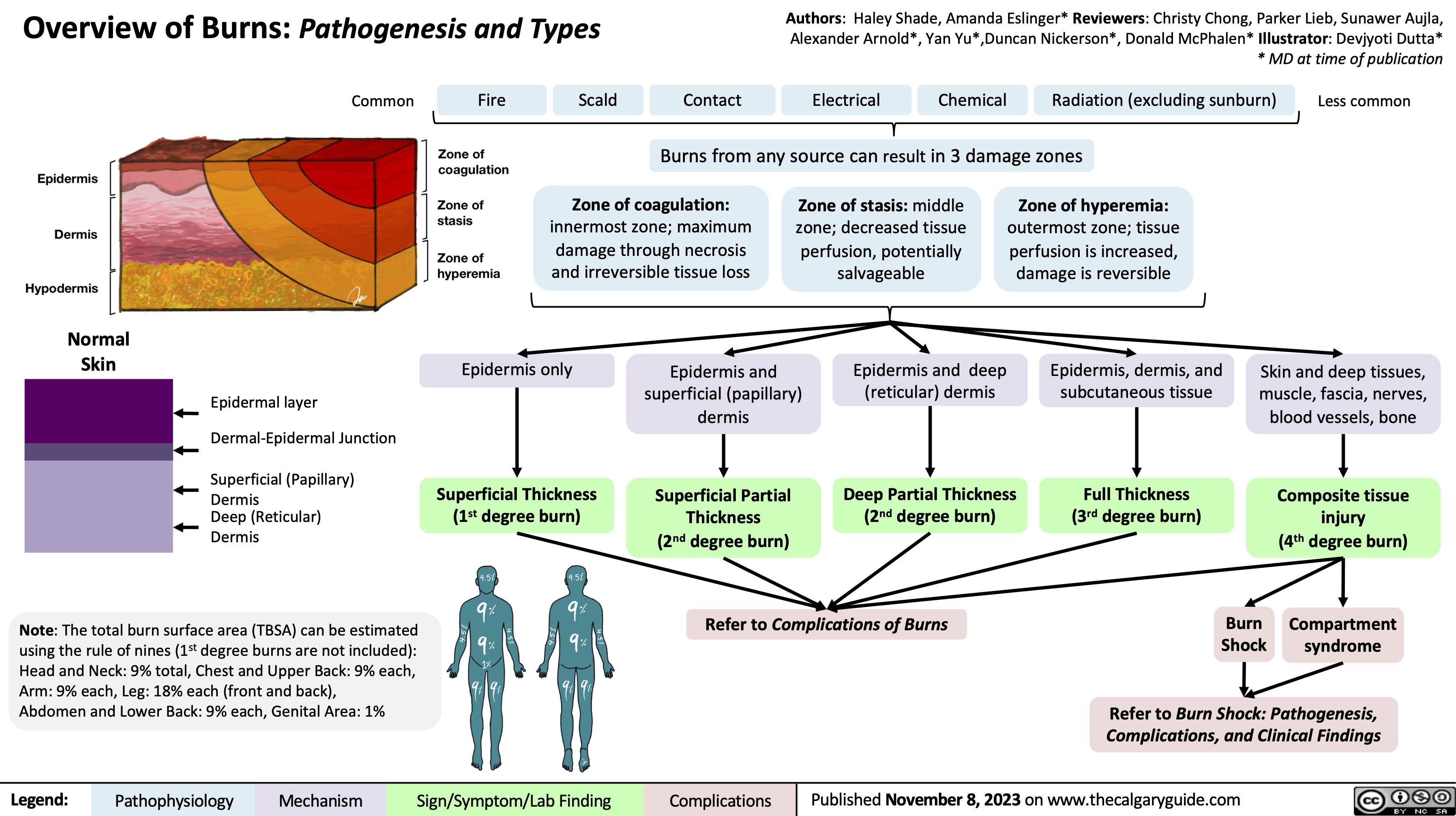
Death Cardiovascular Respiratory and Neurologic Mechanisms
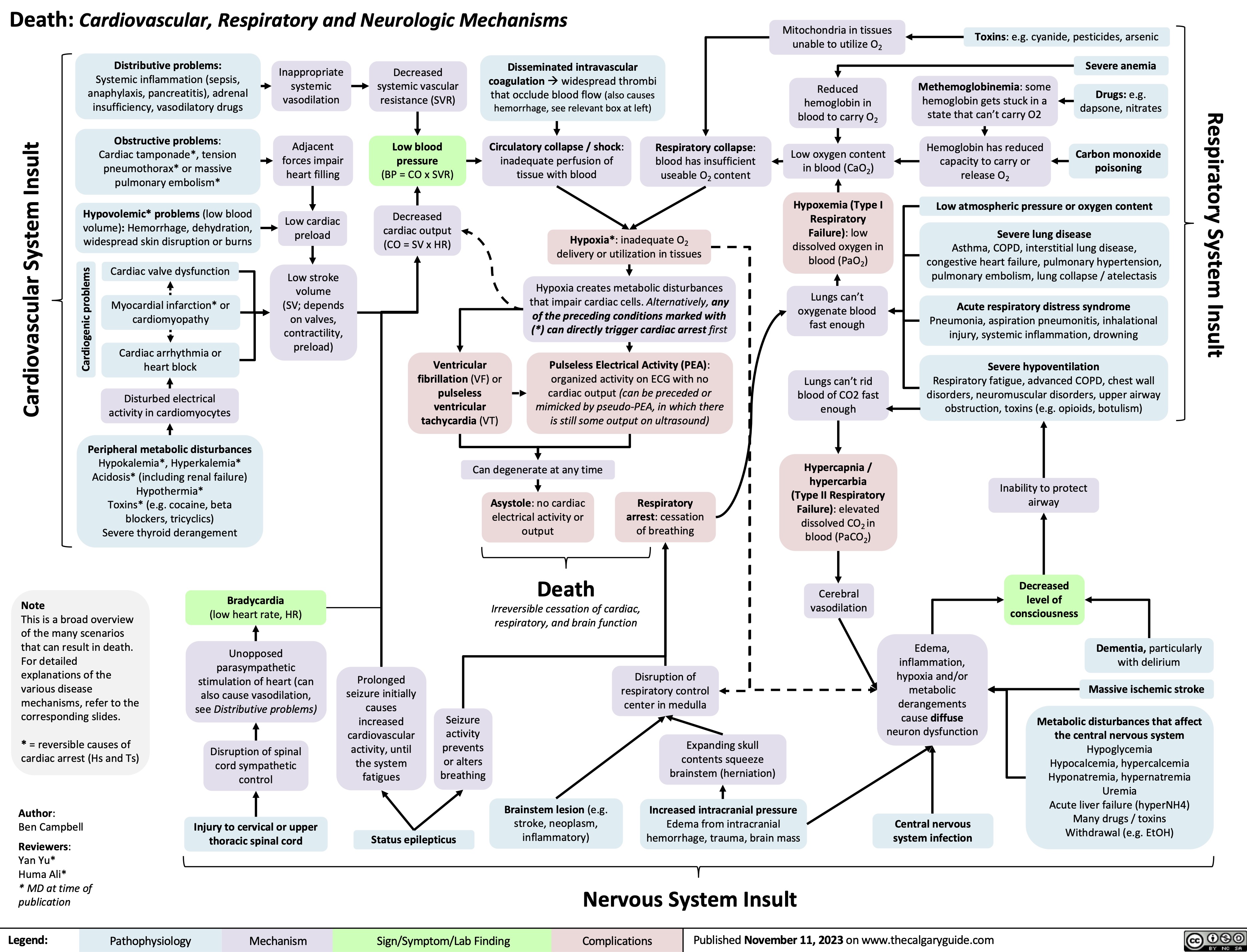
Complication of MI - Acute Mitral Regurgitation
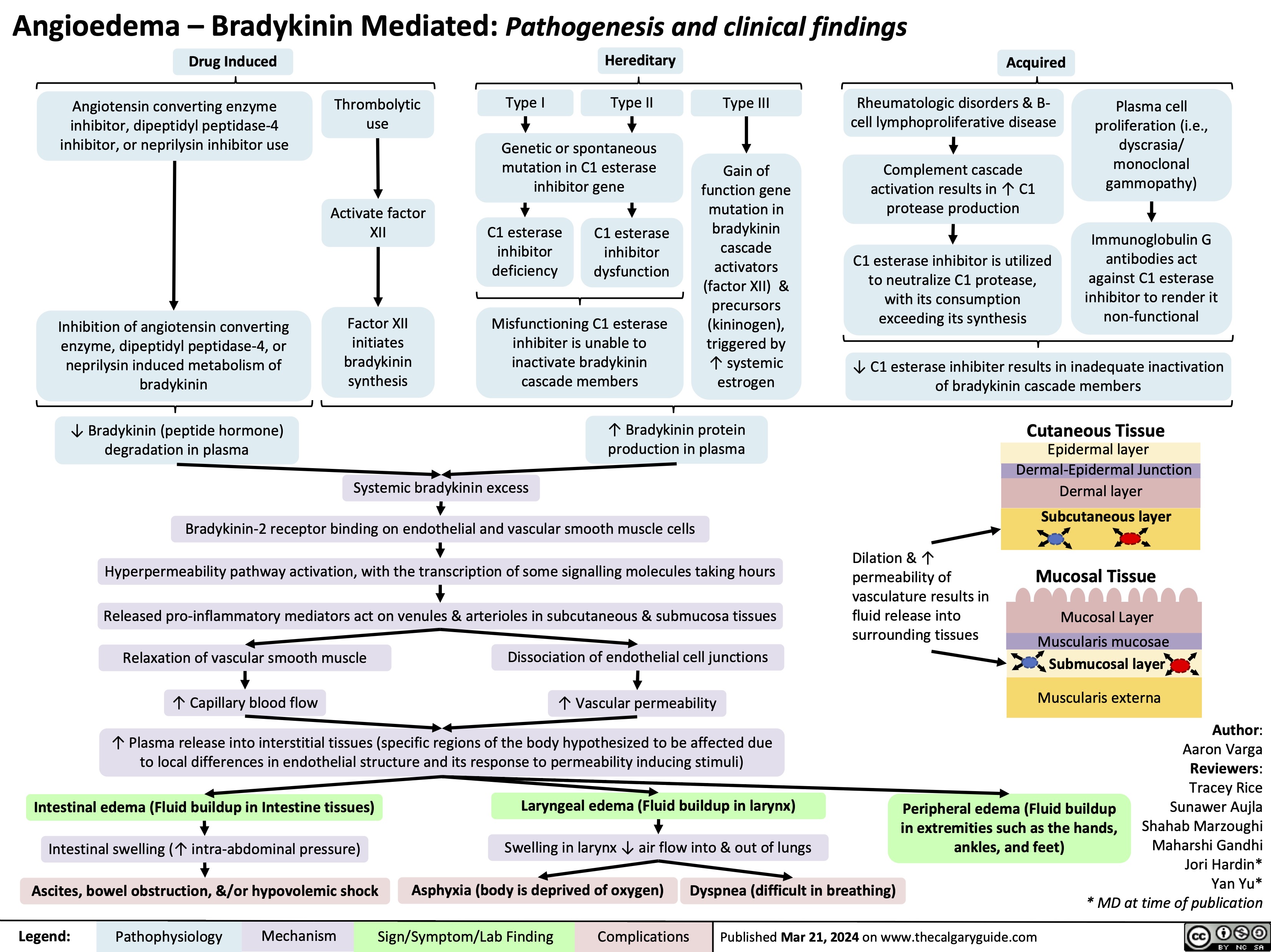
Angioedema Bradykinin Mediated
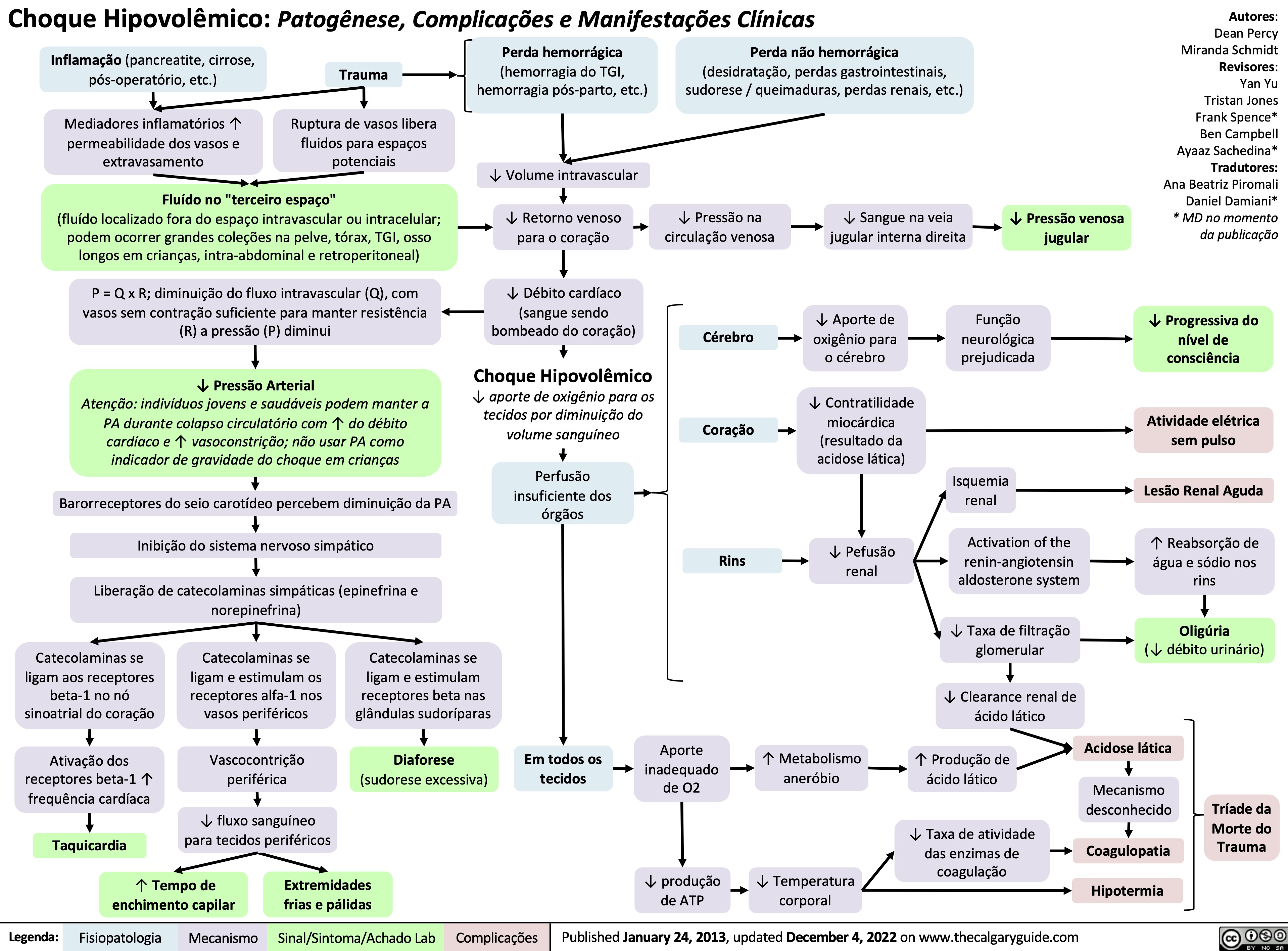
Choque Hipovolemico
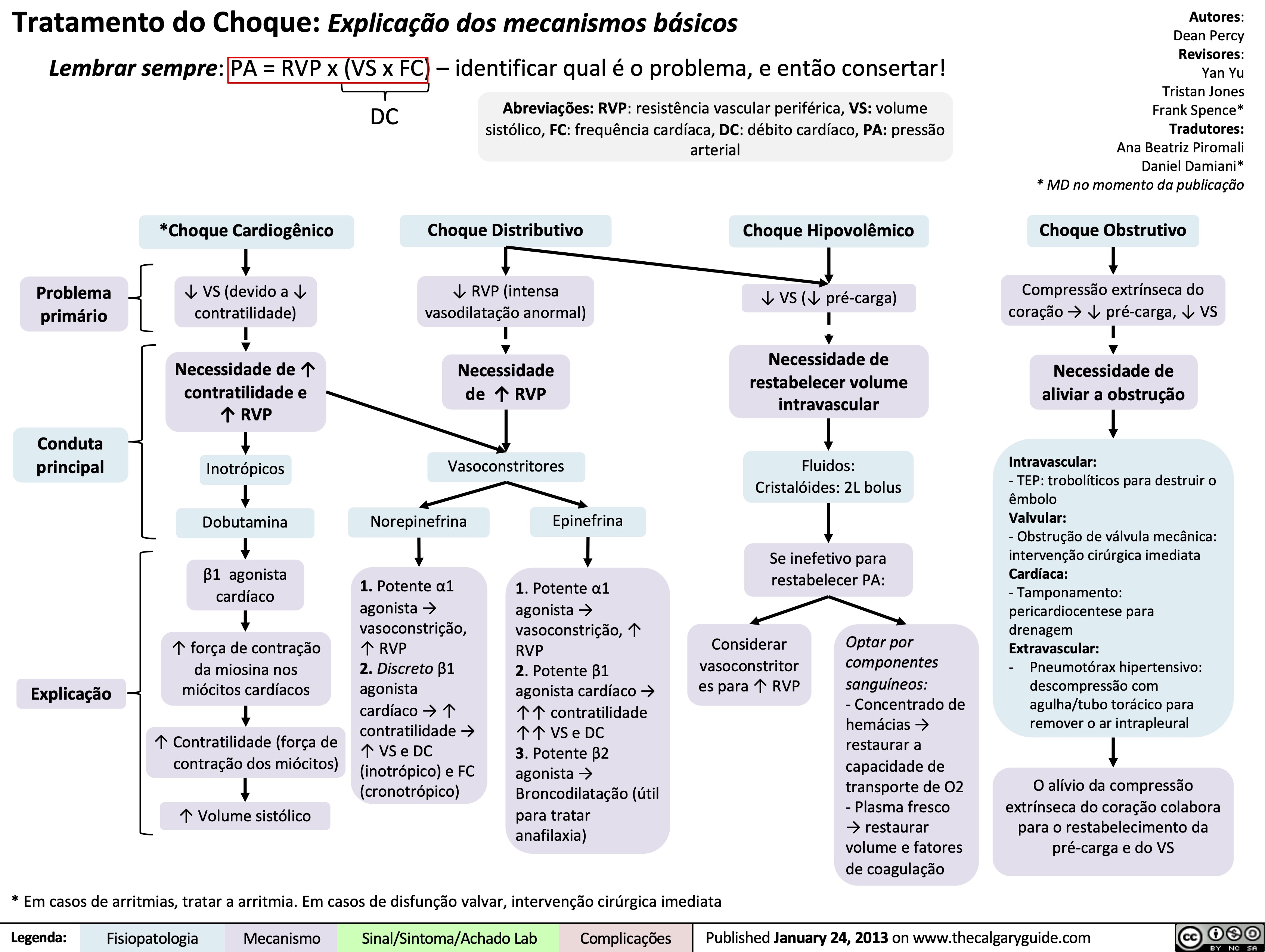
Tratamento do Choque: Explicacao dos mecanismos basicos
Massive Transfusion Protocol
![Massive Transfusion Protocol: Considerations and rationale
Massive transfusion protocol (MTP) is a tool used by clinicians when there is a need to rapidly administer a large amount of blood products, including packed red blood cells (pRBCs), fresh frozen plasma (FFP), and platelets. Complications of MTP are commonly referred to as “The Lethal Triad” referring to hypothermia, acidosis and coagulopathy.
Authors: Kayleigh Yang Arzina Jaffer
Reviewers: Jasleen Brar,
Luiza Radu, Karl Darcus*
* MD at time of publication
Intervention
Indications Initial Response Pathophysiology Transfusion Targets
≥ 3 pRBCs unit transfusion requirement in 1 hour
Shock index (heart rate/systolic blood pressure) > 1
Blood volume loss >50% in ≤3 hours
ABC Score ≥ 3 of: 1. Penetrating mechanism of injury 2. Systolic blood pressure < 90 mmHg 3. Heart rate > 120 beats per minute 4. Evidence of hemoperitoneum or hemopericardium on ultrasound (positive FAST U/S exam)
RABT Score ≥ 2 of: 1. Penetrating mechanism of injury 2. Shock index > 1 3. Positive FAST U/S 4. Known or suspected pelvic fracture
Call for help
Activate institution's MTP protocol
Send for STAT type and screen
Establish large-bore intravenous access
Fluid resuscitation
Collect and send STAT bloodwork including hemoglobin, platelet, INR, fibrinogen, electrolytes, creatinine and arterial blood gas (ABG).
Citrate present in blood products to avoid clotting during storage
Stored pRBCs break down and release potassium due to time mediated degeneration
Temporary accumulation of citrate in patient's blood with rapid use of blood products
Citrate chelates calcium
Less negative cell membrane resting potential
Anaerobic metabolism
Promotes hypocalcaemia
Changes in membrane excitability
Lactic acid buildup
Coagulopathy
(see coagulation cascade slide)
Cardiac dysrhythmias (peaked T-waves, atrial block, “sine wave”, asystolic EKG changes)
Metabolic acidosis
End organ damage
Continued blood loss
Volume overload
Avoid hypocalcemia
Avoid hyperkalemia
pH 7.35-7.45
Bleeding source control
Hemoglobin >70-90
Platelets >50 INR <1.5 Fibrinogen >1.5
Avoid dilutional coagulopathy (clotting factor dilution)
Mean Arterial Pressure (MAP) >60mmHg
Temperature >35.0°C
Slow (over 5-10 minutes) IV calcium administration
Inhaled beta agonists
Insulin/Dextrose
EKG monitoring
Sodium bicarbonate
Increase minute ventilation
Fastest control method to prevent further blood loss (i.e., packing wounds)
Early tranexamic acid administration
Administer pRBCs, FFP, and platelets in a 1:1:1 ratio (fibrinogen replacement indicated if <1.5 despite FFP)
Minimize crystalloid use
Administer crystalloids in a 3:1 ratio to estimated blood loss until blood products available
Administer vasopressors to meet target, do not overshoot
Temperature monitoring Fluid warming
↑ [Potassium] in pRBCs solution
Administration of pRBCs ↑ potassium in patient's blood
Blood loss
↓ Hemoglobin
Tissue hypoperfusion
Tissue hypoxia
↑ Diluent volume
↓ Concentration of clotting factors
Tissue death
↓ Coagulation ability
↑ Transfusion requirements
Early fluid resuscitation
Rapid transfusion of cooled or room-temperature blood products/fluids
↑ Blood pressure
Development of hypothermia
↑ Bleeding and clot dislodgement potential
↓ Enzyme activity in the coagulation cascade
↓ Coagulation ability
Legend:
Pathophysiology
Mechanism
Targets
Intervention
Published Sept 5, 2024 on www.thecalgaryguide.com
Massive Transfusion Protocol: Considerations and rationale
Massive transfusion protocol (MTP) is a tool used by clinicians when there is a need to rapidly administer a large amount of blood products, including packed red blood cells (pRBCs), fresh frozen plasma (FFP), and platelets. Complications of MTP are commonly referred to as “The Lethal Triad” referring to hypothermia, acidosis and coagulopathy.
Authors: Kayleigh Yang Arzina Jaffer
Reviewers: Jasleen Brar,
Luiza Radu, Karl Darcus*
* MD at time of publication
Intervention
Indications Initial Response Pathophysiology Transfusion Targets
≥ 3 pRBCs unit transfusion requirement in 1 hour
Shock index (heart rate/systolic blood pressure) > 1
Blood volume loss >50% in ≤3 hours
ABC Score ≥ 3 of: 1. Penetrating mechanism of injury 2. Systolic blood pressure < 90 mmHg 3. Heart rate > 120 beats per minute 4. Evidence of hemoperitoneum or hemopericardium on ultrasound (positive FAST U/S exam)
RABT Score ≥ 2 of: 1. Penetrating mechanism of injury 2. Shock index > 1 3. Positive FAST U/S 4. Known or suspected pelvic fracture
Call for help
Activate institution's MTP protocol
Send for STAT type and screen
Establish large-bore intravenous access
Fluid resuscitation
Collect and send STAT bloodwork including hemoglobin, platelet, INR, fibrinogen, electrolytes, creatinine and arterial blood gas (ABG).
Citrate present in blood products to avoid clotting during storage
Stored pRBCs break down and release potassium due to time mediated degeneration
Temporary accumulation of citrate in patient's blood with rapid use of blood products
Citrate chelates calcium
Less negative cell membrane resting potential
Anaerobic metabolism
Promotes hypocalcaemia
Changes in membrane excitability
Lactic acid buildup
Coagulopathy
(see coagulation cascade slide)
Cardiac dysrhythmias (peaked T-waves, atrial block, “sine wave”, asystolic EKG changes)
Metabolic acidosis
End organ damage
Continued blood loss
Volume overload
Avoid hypocalcemia
Avoid hyperkalemia
pH 7.35-7.45
Bleeding source control
Hemoglobin >70-90
Platelets >50 INR <1.5 Fibrinogen >1.5
Avoid dilutional coagulopathy (clotting factor dilution)
Mean Arterial Pressure (MAP) >60mmHg
Temperature >35.0°C
Slow (over 5-10 minutes) IV calcium administration
Inhaled beta agonists
Insulin/Dextrose
EKG monitoring
Sodium bicarbonate
Increase minute ventilation
Fastest control method to prevent further blood loss (i.e., packing wounds)
Early tranexamic acid administration
Administer pRBCs, FFP, and platelets in a 1:1:1 ratio (fibrinogen replacement indicated if <1.5 despite FFP)
Minimize crystalloid use
Administer crystalloids in a 3:1 ratio to estimated blood loss until blood products available
Administer vasopressors to meet target, do not overshoot
Temperature monitoring Fluid warming
↑ [Potassium] in pRBCs solution
Administration of pRBCs ↑ potassium in patient's blood
Blood loss
↓ Hemoglobin
Tissue hypoperfusion
Tissue hypoxia
↑ Diluent volume
↓ Concentration of clotting factors
Tissue death
↓ Coagulation ability
↑ Transfusion requirements
Early fluid resuscitation
Rapid transfusion of cooled or room-temperature blood products/fluids
↑ Blood pressure
Development of hypothermia
↑ Bleeding and clot dislodgement potential
↓ Enzyme activity in the coagulation cascade
↓ Coagulation ability
Legend:
Pathophysiology
Mechanism
Targets
Intervention
Published Sept 5, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/09/Massive-Transfusion-Protocol.jpg)
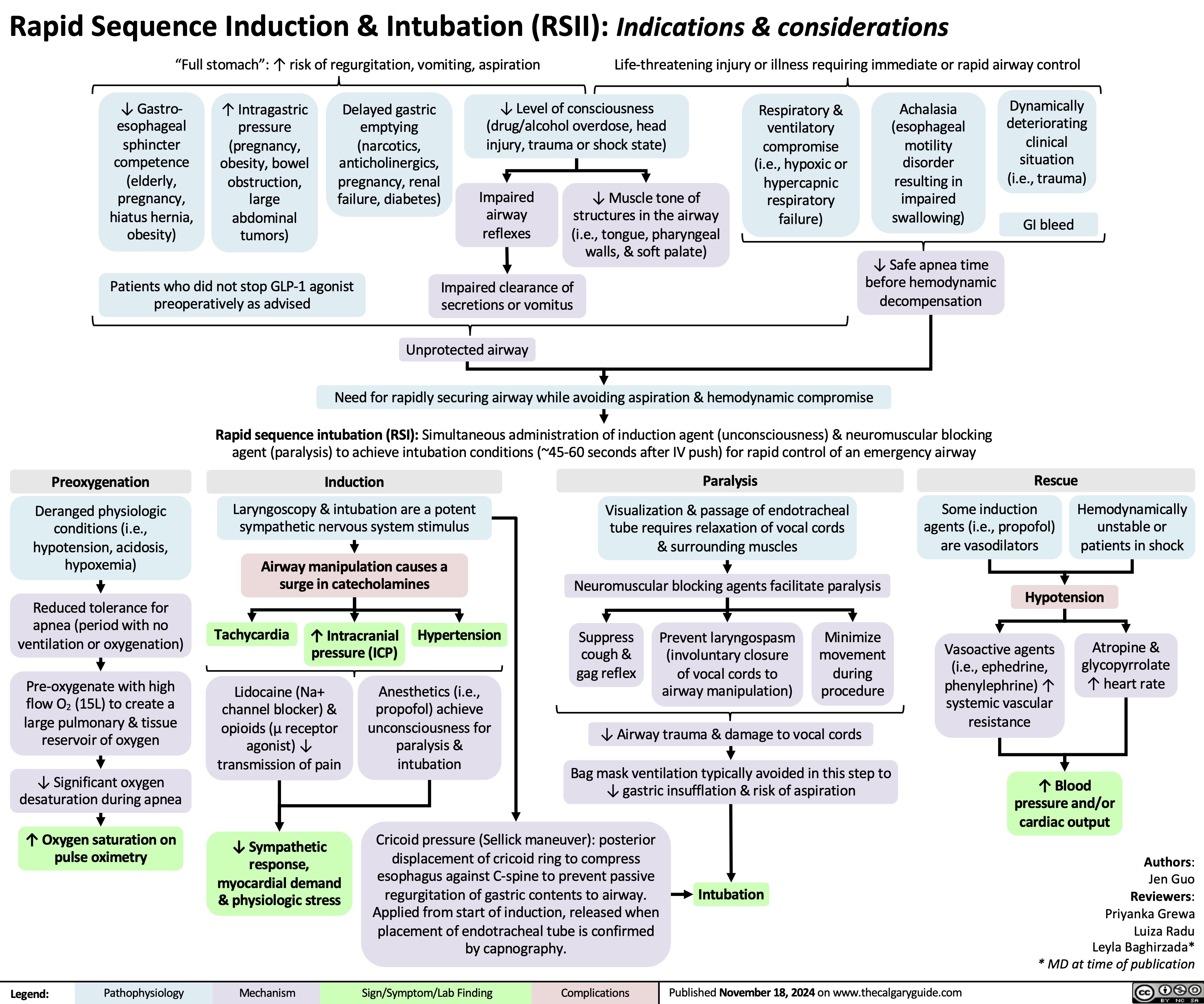
Rapid sequence induction and intubation
Pelvic Ring Fractures
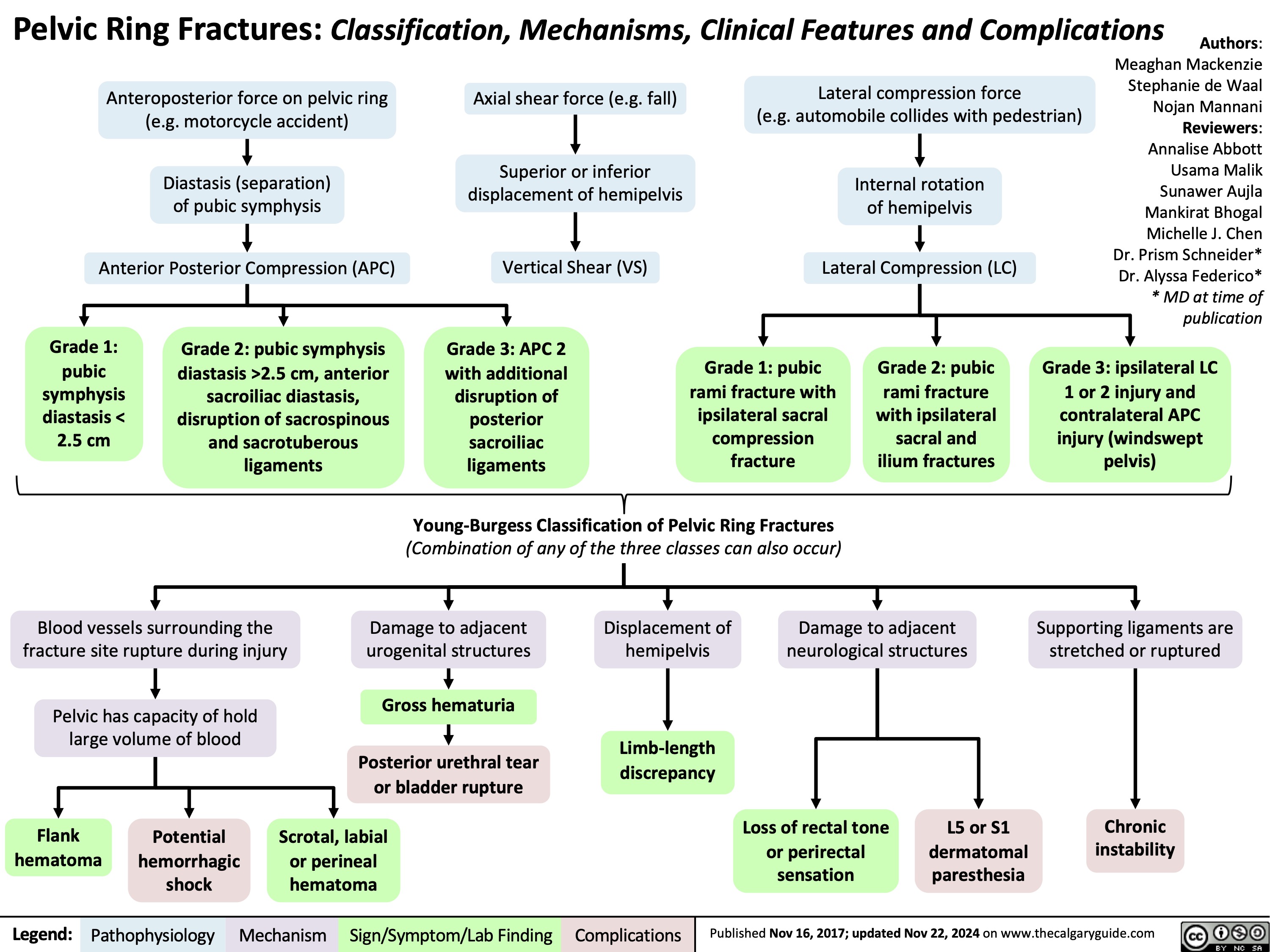
Tonsillitis Pathogenesis and clinical findings
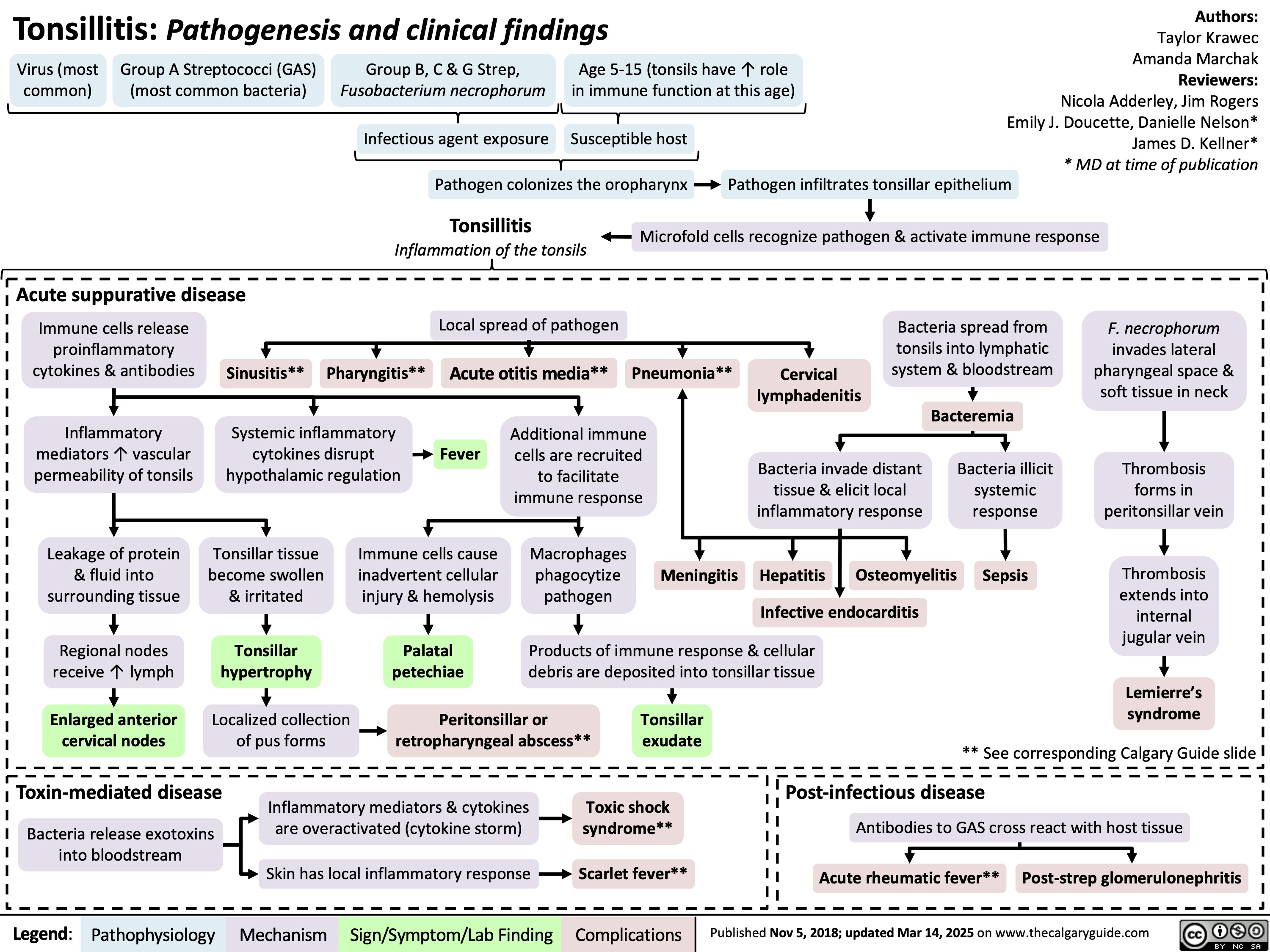
Varicella Zoster Virus
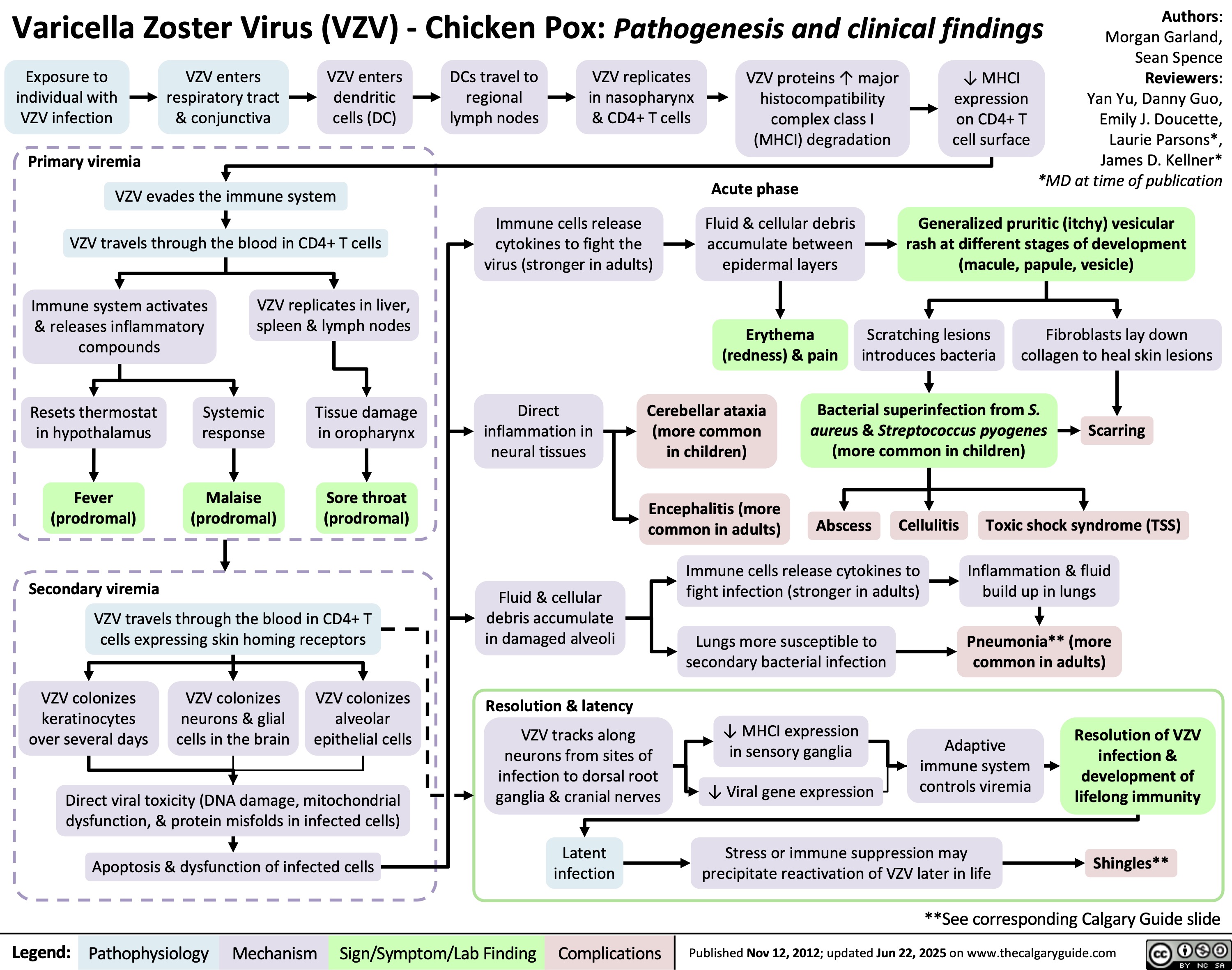
Traitement du choc
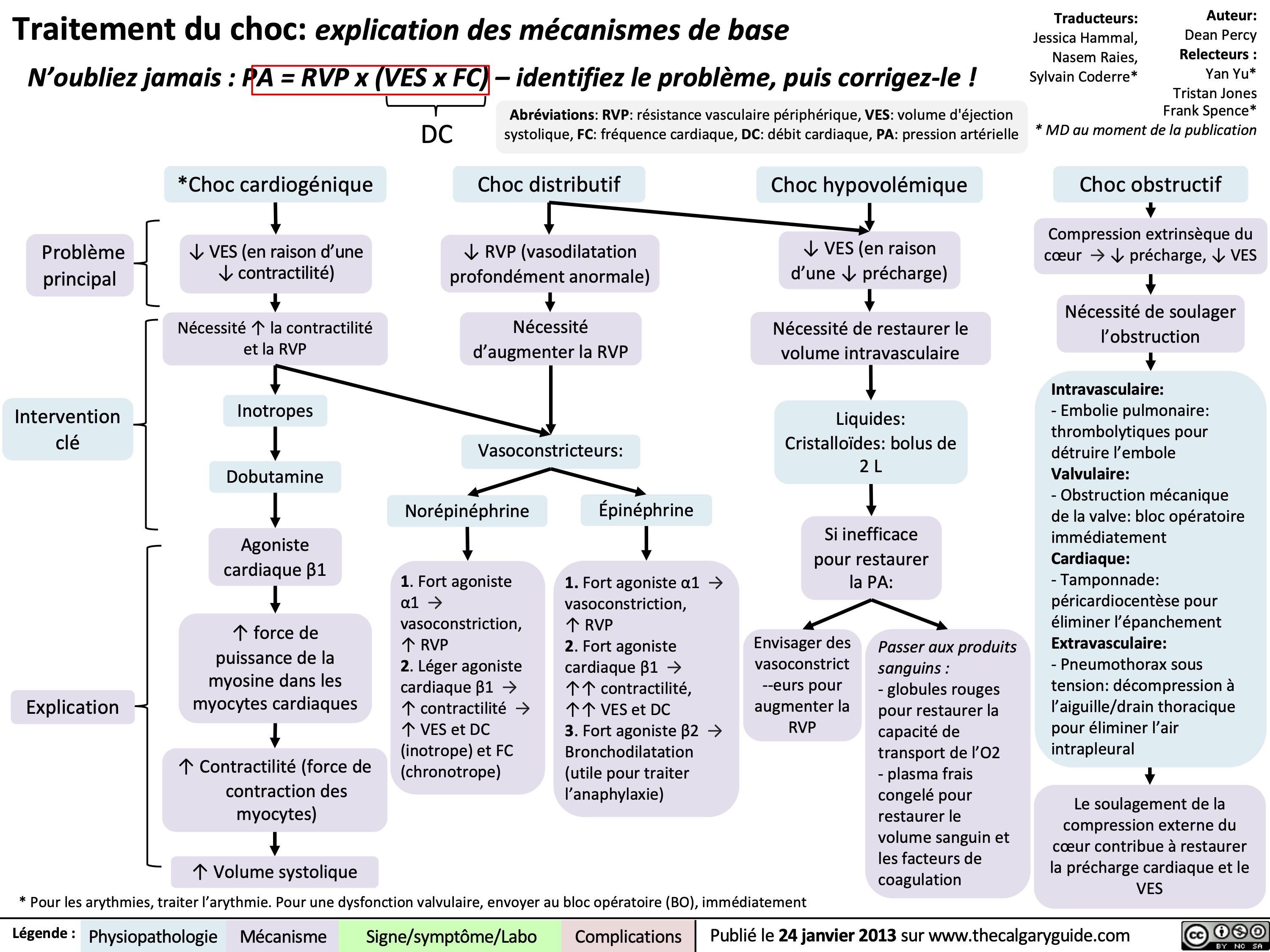
Choc cardiogenique

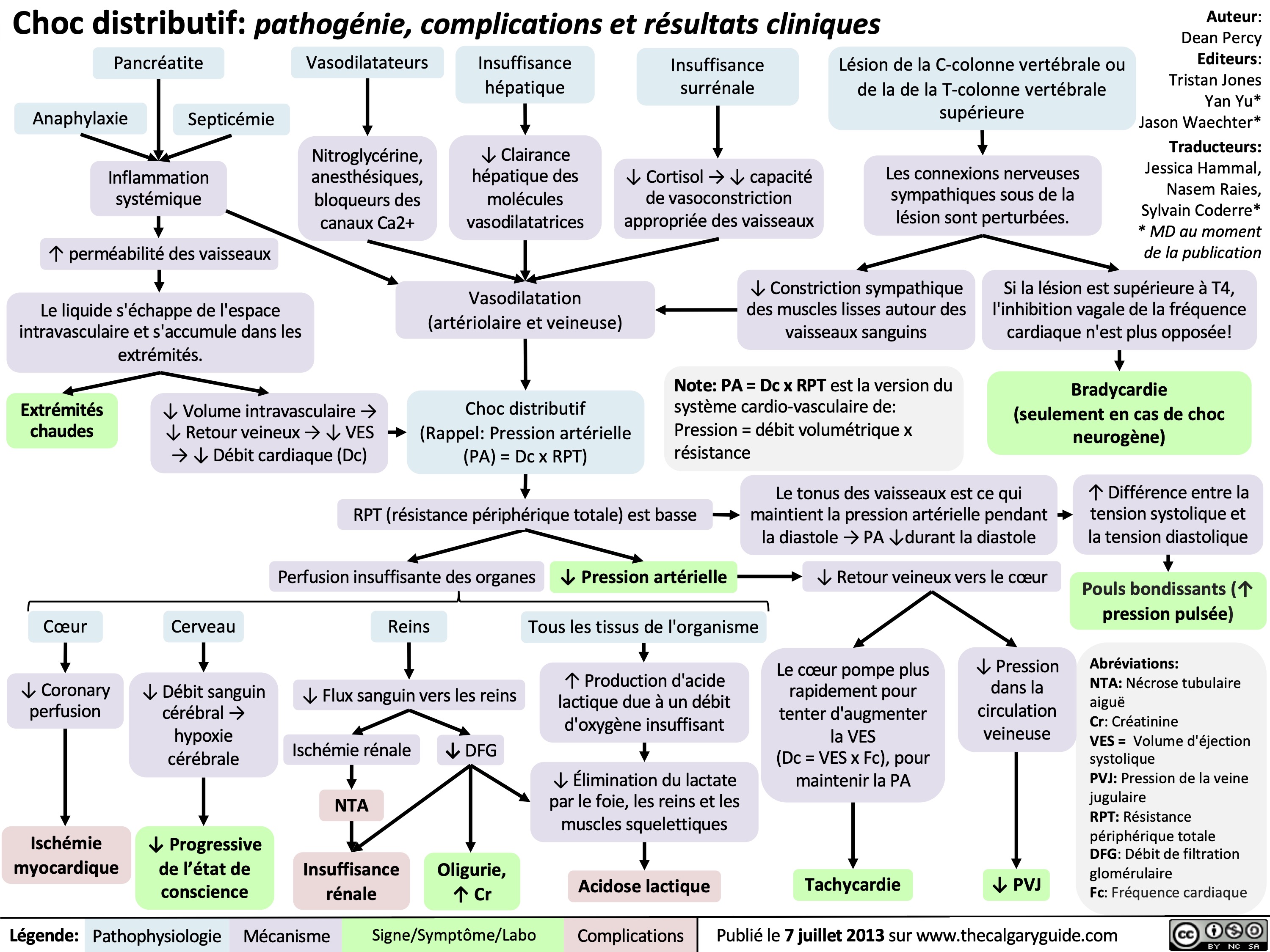
Choc distributif
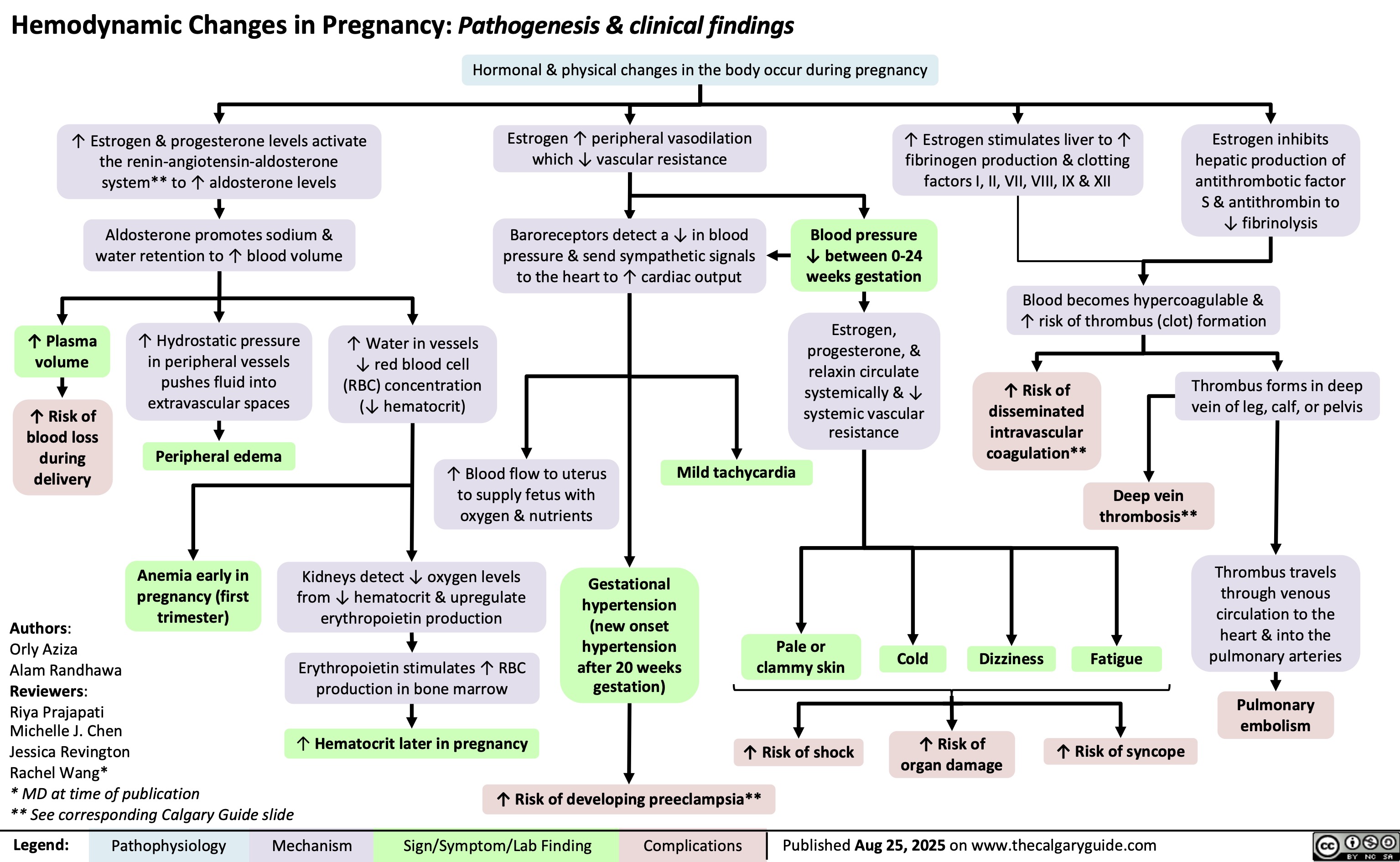
Hemodynamic Changes in Pregnancy
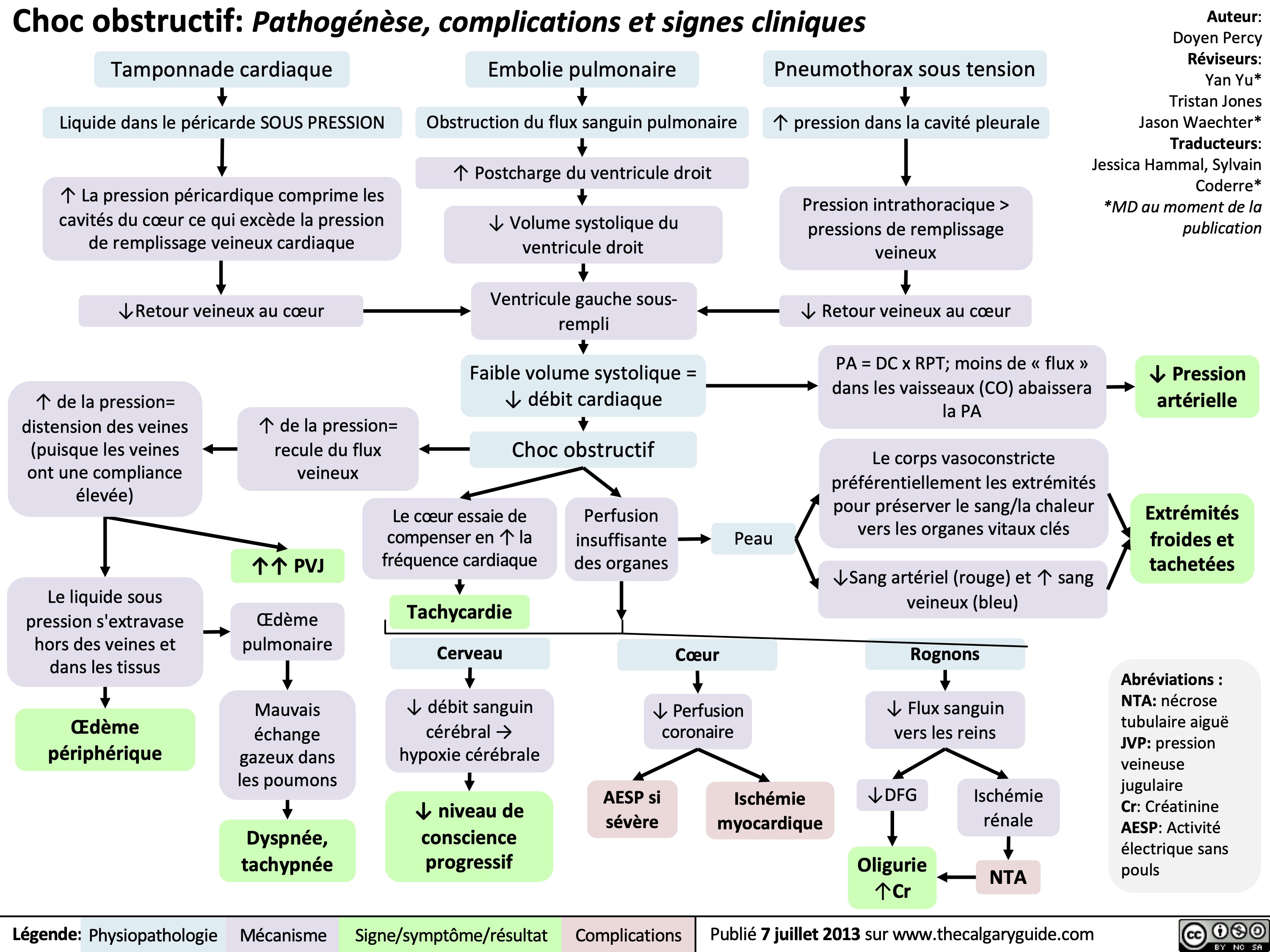
Choc obstructif
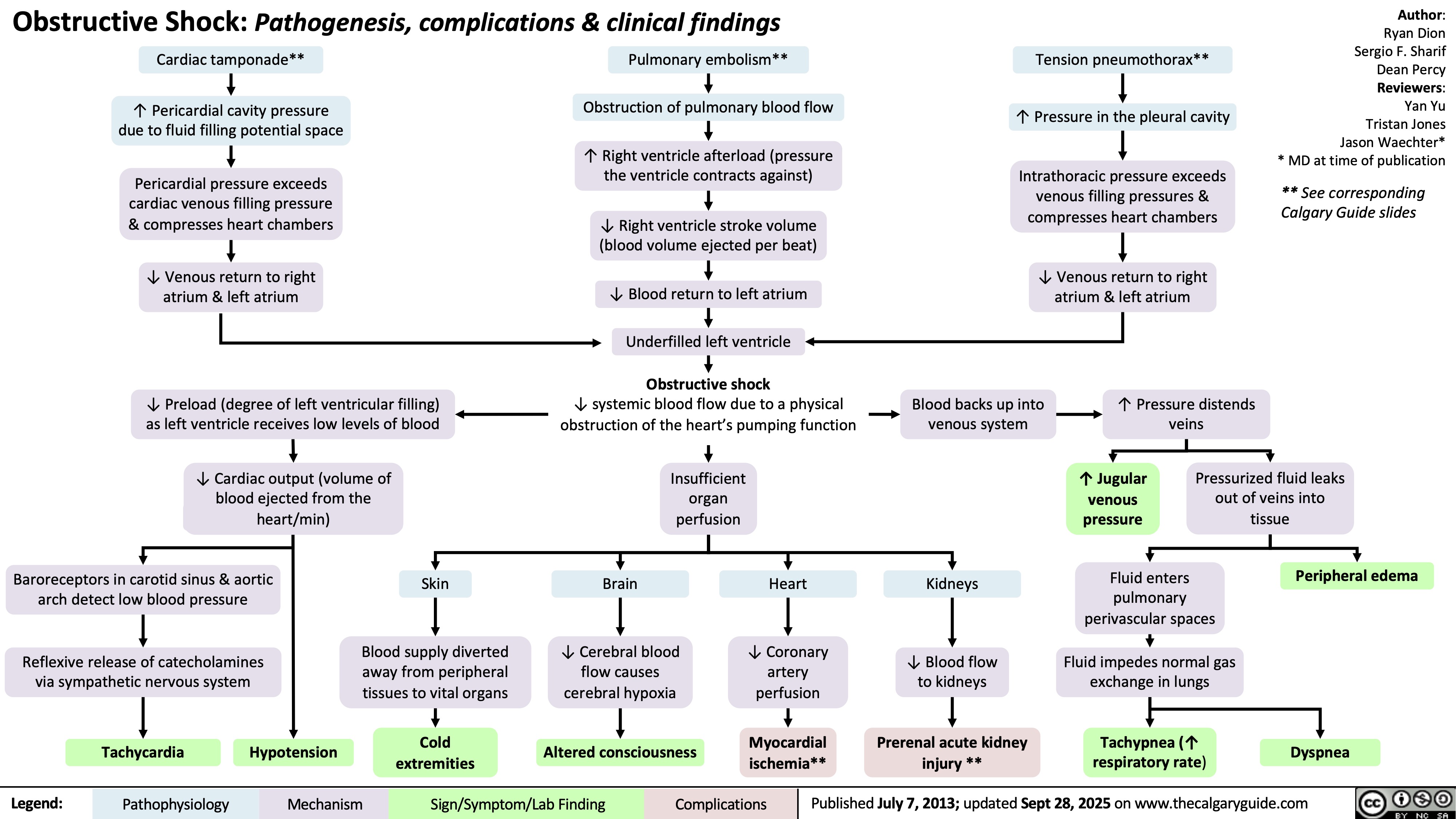
Obstructive Shock
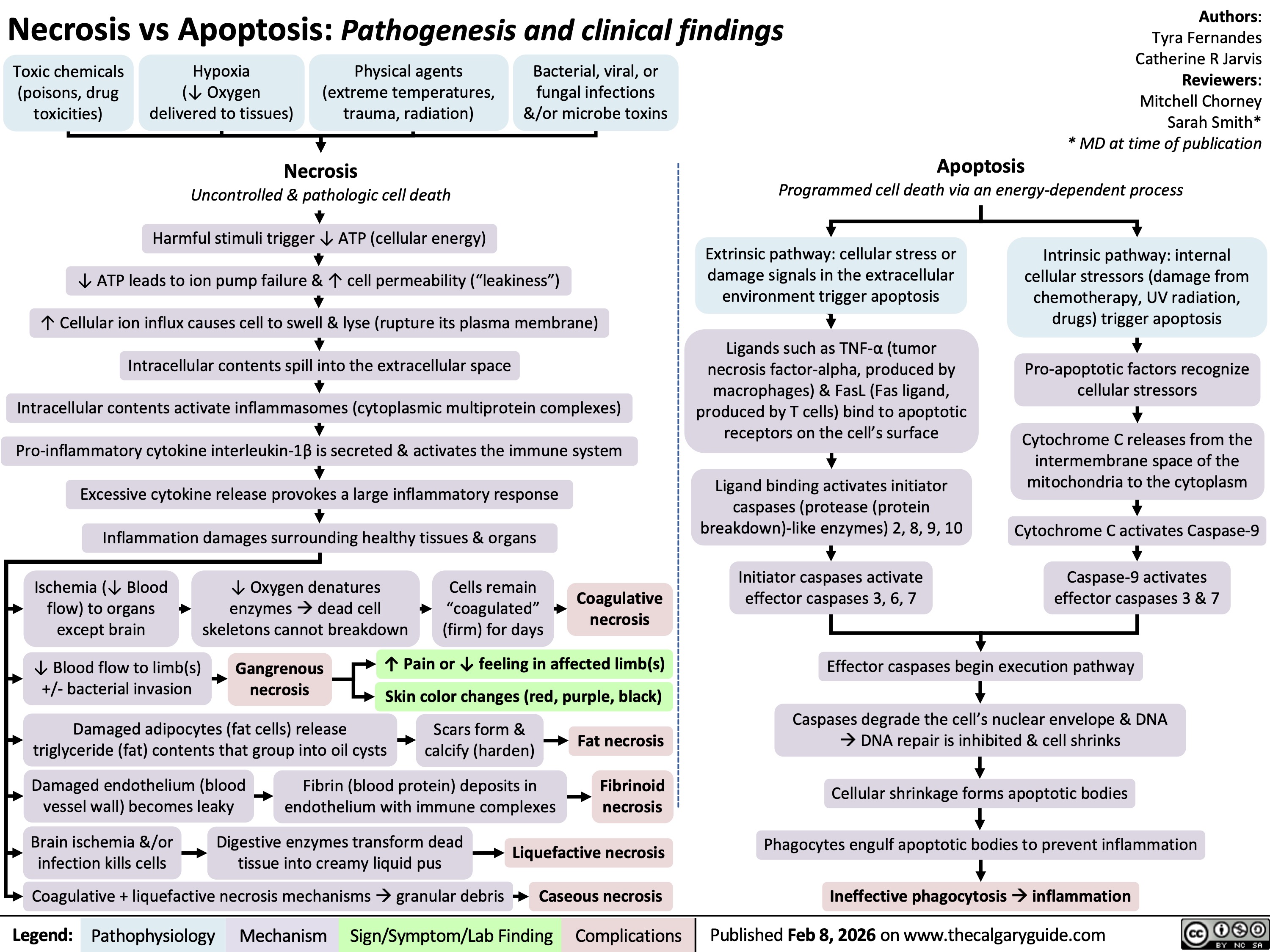
Acute Tubular Necrosis
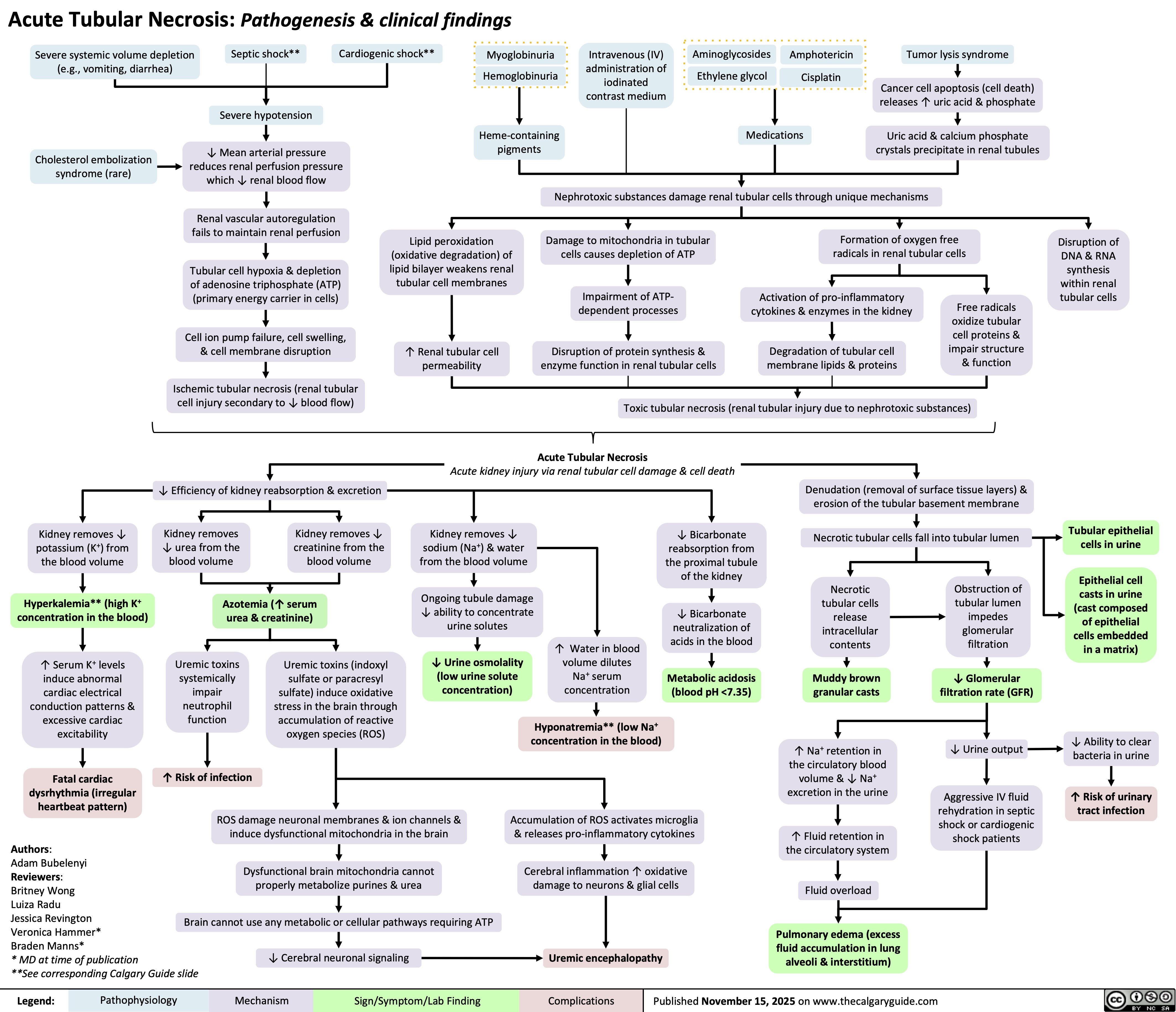
Heart Transplant Indications and Benefits
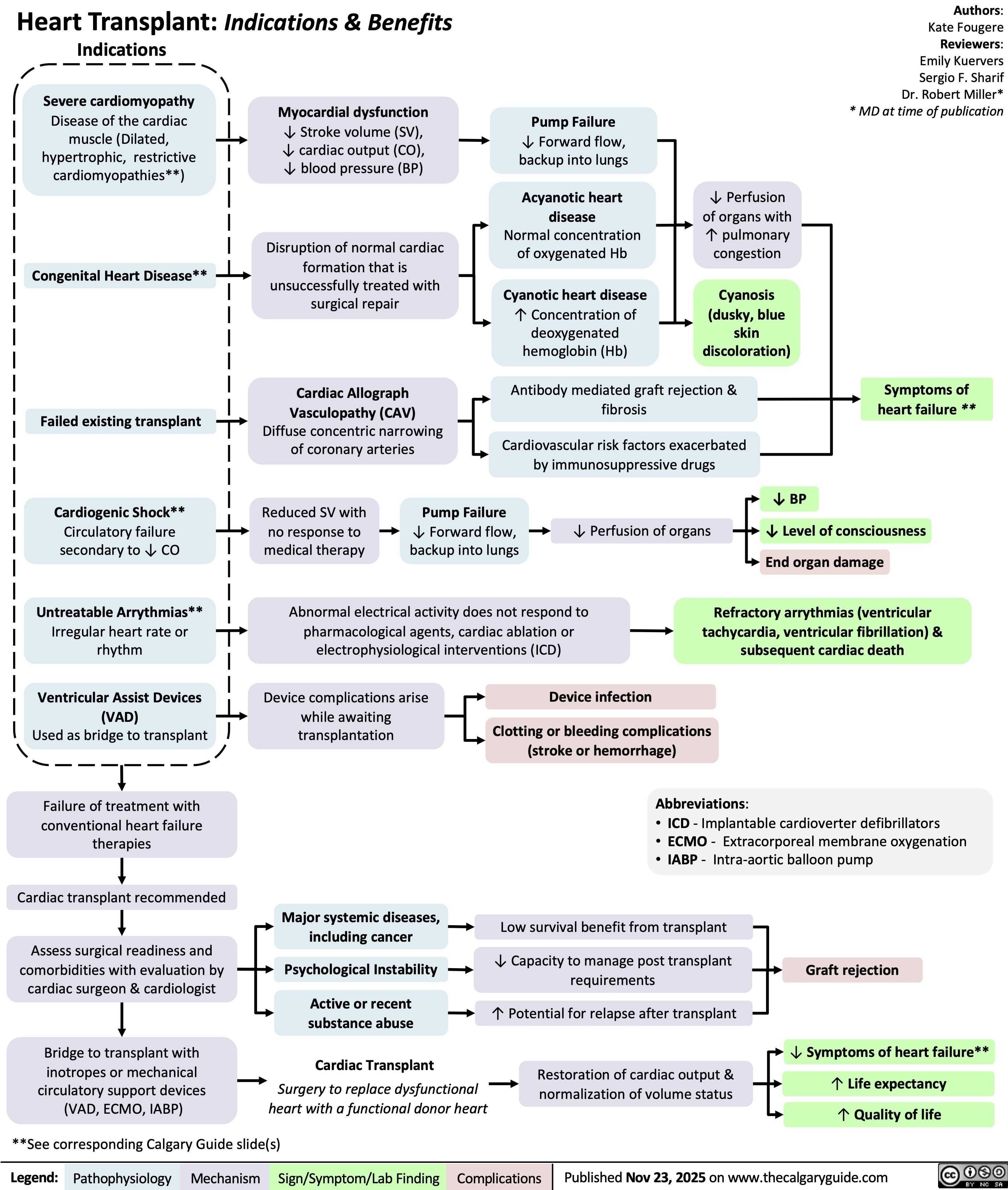
Childhood Immunization Schedule
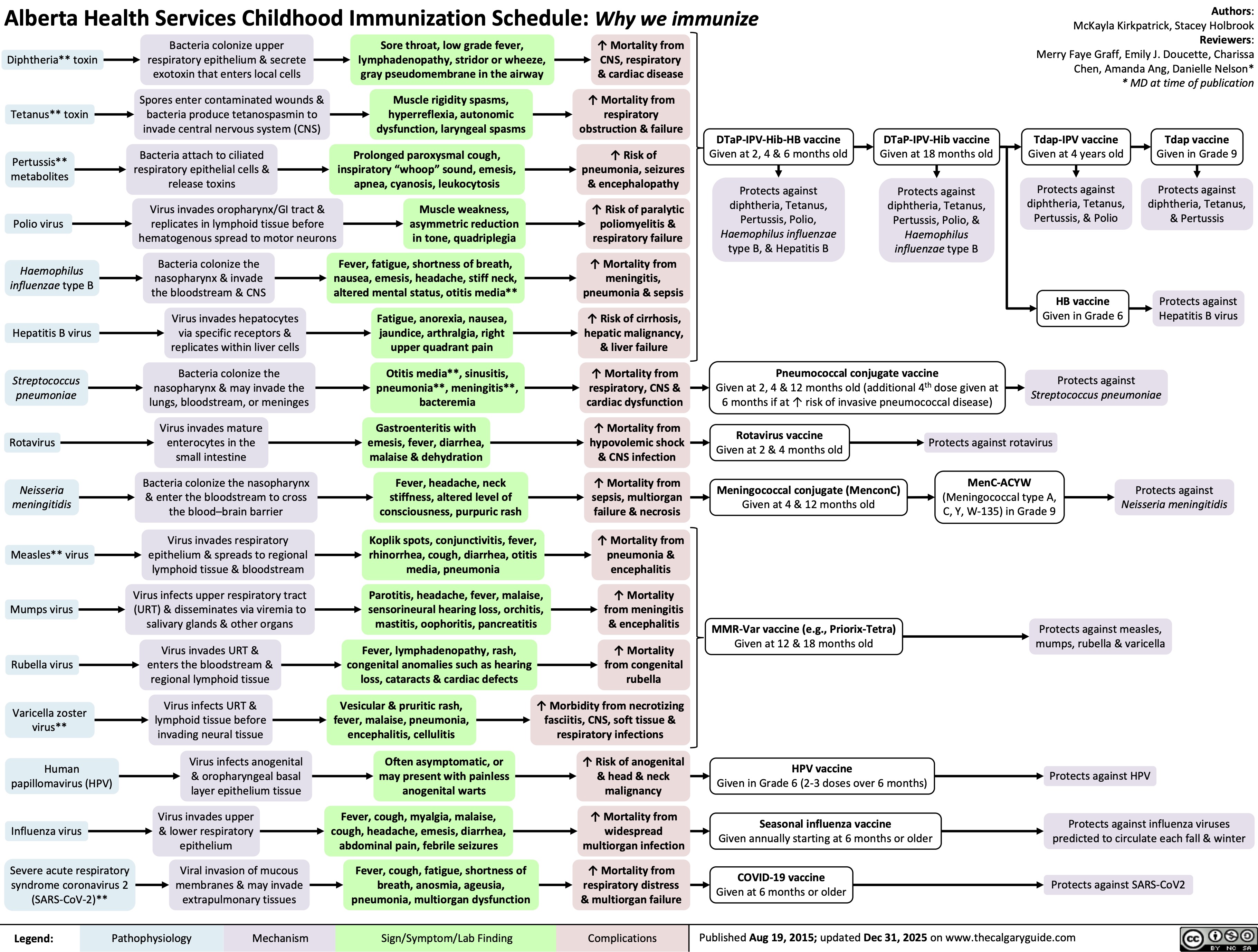
Necrosis versus Apoptosis




![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)














![Complications of Pulmonary Embolism
Authors:
Sravya Kakumanu, Dean Percy, Yan Yu
Reviewers:
Tristan Jones, Ciara Hanly, Jieling Ma (马杰羚), Ben Campbell, Dr. Man-Chiu Poon*, Dr. Lynn Savoie*, Dr. Tara Lohmann * * MD at time of publication
IF CHRONIC:
Unresolved clot after 2 years leading to fibrosis of pulmonary vasculature
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
(<5% of PE cases)
Venous Stasis Hypercoagulable state
Vessel Injury
Virchow’s Triad (*See Suspected Deep Vein Thrombosis slide)
Deep Vein Thrombosis
Clot migrates from deep limb veins à femoral àiliac veins
ACUTE/MASSIVE PE:
Clot obstructs pulmonary arterial or arteriolar flow
Lung infarction (tissue death) from ischemia
Inflammatory cells migrate to site and release cytokines
↑ Permeability of blood vessels
Permeability-driven (exudate) fluid leakage into pleural space
Pleural Effusion
Clot migratesàinferior vena cava àright atrium (RA) of heartà right ventricle (RV) à gets lodged in pulmonary arteries/arterioles
Pulmonary Embolism (PE)
↑ RV afterload
↑ RV pressure and expansion
Well-ventilated (V) areas of lung do not receive adequate blood supply (Q)
V/Q Mismatch
Leftward shift of ventricular septum
↓ Left ventricle filling in diastole
↓ Cardiac output
Obstructive Shock
Impaired heart filling
Pulseless Electrical Activity
(ECG activity in absence of palpable pulse)
Back up of pressure in systemic venous system
↑ Pressure in capillaries draining parietal pleura
Pressure-driven (transudate) fluid leakage into pleural space
For signs and symptoms, see the Obstructive Shock slide
For signs and symptoms refer to CTEPH slide
Chronic ↑ RV afterload
↑ Stretching of myocytes causing RV hypertrophy and dilation
↓ RV ejection fraction
Right Heart Failure
“Cor Pulmonale”
For signs and symptoms, see the Right Heart Failure slide
Failure to oxygenate blood
Type I Respiratory Failure
Hypoxemic: patient has ↓ blood [O2]
IF MASSIVE PE (less common):
↑ Alveolar dead space
Failure to ventilate
Type II Respiratory Failure Hypercapnic: patient has ↑ blood [CO2]
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 7, 2012, updated Mar 31, 2022 on www.thecalgaryguide.com
Complications of Pulmonary Embolism
Authors:
Sravya Kakumanu, Dean Percy, Yan Yu
Reviewers:
Tristan Jones, Ciara Hanly, Jieling Ma (马杰羚), Ben Campbell, Dr. Man-Chiu Poon*, Dr. Lynn Savoie*, Dr. Tara Lohmann * * MD at time of publication
IF CHRONIC:
Unresolved clot after 2 years leading to fibrosis of pulmonary vasculature
Chronic Thromboembolic Pulmonary Hypertension (CTEPH)
(<5% of PE cases)
Venous Stasis Hypercoagulable state
Vessel Injury
Virchow’s Triad (*See Suspected Deep Vein Thrombosis slide)
Deep Vein Thrombosis
Clot migrates from deep limb veins à femoral àiliac veins
ACUTE/MASSIVE PE:
Clot obstructs pulmonary arterial or arteriolar flow
Lung infarction (tissue death) from ischemia
Inflammatory cells migrate to site and release cytokines
↑ Permeability of blood vessels
Permeability-driven (exudate) fluid leakage into pleural space
Pleural Effusion
Clot migratesàinferior vena cava àright atrium (RA) of heartà right ventricle (RV) à gets lodged in pulmonary arteries/arterioles
Pulmonary Embolism (PE)
↑ RV afterload
↑ RV pressure and expansion
Well-ventilated (V) areas of lung do not receive adequate blood supply (Q)
V/Q Mismatch
Leftward shift of ventricular septum
↓ Left ventricle filling in diastole
↓ Cardiac output
Obstructive Shock
Impaired heart filling
Pulseless Electrical Activity
(ECG activity in absence of palpable pulse)
Back up of pressure in systemic venous system
↑ Pressure in capillaries draining parietal pleura
Pressure-driven (transudate) fluid leakage into pleural space
For signs and symptoms, see the Obstructive Shock slide
For signs and symptoms refer to CTEPH slide
Chronic ↑ RV afterload
↑ Stretching of myocytes causing RV hypertrophy and dilation
↓ RV ejection fraction
Right Heart Failure
“Cor Pulmonale”
For signs and symptoms, see the Right Heart Failure slide
Failure to oxygenate blood
Type I Respiratory Failure
Hypoxemic: patient has ↓ blood [O2]
IF MASSIVE PE (less common):
↑ Alveolar dead space
Failure to ventilate
Type II Respiratory Failure Hypercapnic: patient has ↑ blood [CO2]
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 7, 2012, updated Mar 31, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2014/09/Complications-of-Pulmonary-Embolism-2022.jpg)


























![Massive Transfusion Protocol: Considerations and rationale
Massive transfusion protocol (MTP) is a tool used by clinicians when there is a need to rapidly administer a large amount of blood products, including packed red blood cells (pRBCs), fresh frozen plasma (FFP), and platelets. Complications of MTP are commonly referred to as “The Lethal Triad” referring to hypothermia, acidosis and coagulopathy.
Authors: Kayleigh Yang Arzina Jaffer
Reviewers: Jasleen Brar,
Luiza Radu, Karl Darcus*
* MD at time of publication
Intervention
Indications Initial Response Pathophysiology Transfusion Targets
≥ 3 pRBCs unit transfusion requirement in 1 hour
Shock index (heart rate/systolic blood pressure) > 1
Blood volume loss >50% in ≤3 hours
ABC Score ≥ 3 of: 1. Penetrating mechanism of injury 2. Systolic blood pressure < 90 mmHg 3. Heart rate > 120 beats per minute 4. Evidence of hemoperitoneum or hemopericardium on ultrasound (positive FAST U/S exam)
RABT Score ≥ 2 of: 1. Penetrating mechanism of injury 2. Shock index > 1 3. Positive FAST U/S 4. Known or suspected pelvic fracture
Call for help
Activate institution's MTP protocol
Send for STAT type and screen
Establish large-bore intravenous access
Fluid resuscitation
Collect and send STAT bloodwork including hemoglobin, platelet, INR, fibrinogen, electrolytes, creatinine and arterial blood gas (ABG).
Citrate present in blood products to avoid clotting during storage
Stored pRBCs break down and release potassium due to time mediated degeneration
Temporary accumulation of citrate in patient's blood with rapid use of blood products
Citrate chelates calcium
Less negative cell membrane resting potential
Anaerobic metabolism
Promotes hypocalcaemia
Changes in membrane excitability
Lactic acid buildup
Coagulopathy
(see coagulation cascade slide)
Cardiac dysrhythmias (peaked T-waves, atrial block, “sine wave”, asystolic EKG changes)
Metabolic acidosis
End organ damage
Continued blood loss
Volume overload
Avoid hypocalcemia
Avoid hyperkalemia
pH 7.35-7.45
Bleeding source control
Hemoglobin >70-90
Platelets >50 INR <1.5 Fibrinogen >1.5
Avoid dilutional coagulopathy (clotting factor dilution)
Mean Arterial Pressure (MAP) >60mmHg
Temperature >35.0°C
Slow (over 5-10 minutes) IV calcium administration
Inhaled beta agonists
Insulin/Dextrose
EKG monitoring
Sodium bicarbonate
Increase minute ventilation
Fastest control method to prevent further blood loss (i.e., packing wounds)
Early tranexamic acid administration
Administer pRBCs, FFP, and platelets in a 1:1:1 ratio (fibrinogen replacement indicated if <1.5 despite FFP)
Minimize crystalloid use
Administer crystalloids in a 3:1 ratio to estimated blood loss until blood products available
Administer vasopressors to meet target, do not overshoot
Temperature monitoring Fluid warming
↑ [Potassium] in pRBCs solution
Administration of pRBCs ↑ potassium in patient's blood
Blood loss
↓ Hemoglobin
Tissue hypoperfusion
Tissue hypoxia
↑ Diluent volume
↓ Concentration of clotting factors
Tissue death
↓ Coagulation ability
↑ Transfusion requirements
Early fluid resuscitation
Rapid transfusion of cooled or room-temperature blood products/fluids
↑ Blood pressure
Development of hypothermia
↑ Bleeding and clot dislodgement potential
↓ Enzyme activity in the coagulation cascade
↓ Coagulation ability
Legend:
Pathophysiology
Mechanism
Targets
Intervention
Published Sept 5, 2024 on www.thecalgaryguide.com
Massive Transfusion Protocol: Considerations and rationale
Massive transfusion protocol (MTP) is a tool used by clinicians when there is a need to rapidly administer a large amount of blood products, including packed red blood cells (pRBCs), fresh frozen plasma (FFP), and platelets. Complications of MTP are commonly referred to as “The Lethal Triad” referring to hypothermia, acidosis and coagulopathy.
Authors: Kayleigh Yang Arzina Jaffer
Reviewers: Jasleen Brar,
Luiza Radu, Karl Darcus*
* MD at time of publication
Intervention
Indications Initial Response Pathophysiology Transfusion Targets
≥ 3 pRBCs unit transfusion requirement in 1 hour
Shock index (heart rate/systolic blood pressure) > 1
Blood volume loss >50% in ≤3 hours
ABC Score ≥ 3 of: 1. Penetrating mechanism of injury 2. Systolic blood pressure < 90 mmHg 3. Heart rate > 120 beats per minute 4. Evidence of hemoperitoneum or hemopericardium on ultrasound (positive FAST U/S exam)
RABT Score ≥ 2 of: 1. Penetrating mechanism of injury 2. Shock index > 1 3. Positive FAST U/S 4. Known or suspected pelvic fracture
Call for help
Activate institution's MTP protocol
Send for STAT type and screen
Establish large-bore intravenous access
Fluid resuscitation
Collect and send STAT bloodwork including hemoglobin, platelet, INR, fibrinogen, electrolytes, creatinine and arterial blood gas (ABG).
Citrate present in blood products to avoid clotting during storage
Stored pRBCs break down and release potassium due to time mediated degeneration
Temporary accumulation of citrate in patient's blood with rapid use of blood products
Citrate chelates calcium
Less negative cell membrane resting potential
Anaerobic metabolism
Promotes hypocalcaemia
Changes in membrane excitability
Lactic acid buildup
Coagulopathy
(see coagulation cascade slide)
Cardiac dysrhythmias (peaked T-waves, atrial block, “sine wave”, asystolic EKG changes)
Metabolic acidosis
End organ damage
Continued blood loss
Volume overload
Avoid hypocalcemia
Avoid hyperkalemia
pH 7.35-7.45
Bleeding source control
Hemoglobin >70-90
Platelets >50 INR <1.5 Fibrinogen >1.5
Avoid dilutional coagulopathy (clotting factor dilution)
Mean Arterial Pressure (MAP) >60mmHg
Temperature >35.0°C
Slow (over 5-10 minutes) IV calcium administration
Inhaled beta agonists
Insulin/Dextrose
EKG monitoring
Sodium bicarbonate
Increase minute ventilation
Fastest control method to prevent further blood loss (i.e., packing wounds)
Early tranexamic acid administration
Administer pRBCs, FFP, and platelets in a 1:1:1 ratio (fibrinogen replacement indicated if <1.5 despite FFP)
Minimize crystalloid use
Administer crystalloids in a 3:1 ratio to estimated blood loss until blood products available
Administer vasopressors to meet target, do not overshoot
Temperature monitoring Fluid warming
↑ [Potassium] in pRBCs solution
Administration of pRBCs ↑ potassium in patient's blood
Blood loss
↓ Hemoglobin
Tissue hypoperfusion
Tissue hypoxia
↑ Diluent volume
↓ Concentration of clotting factors
Tissue death
↓ Coagulation ability
↑ Transfusion requirements
Early fluid resuscitation
Rapid transfusion of cooled or room-temperature blood products/fluids
↑ Blood pressure
Development of hypothermia
↑ Bleeding and clot dislodgement potential
↓ Enzyme activity in the coagulation cascade
↓ Coagulation ability
Legend:
Pathophysiology
Mechanism
Targets
Intervention
Published Sept 5, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/09/Massive-Transfusion-Protocol.jpg)














