SEARCH RESULTS FOR: renal
Nephrotic Syndrome: Pathogenesis and Clinical Findings
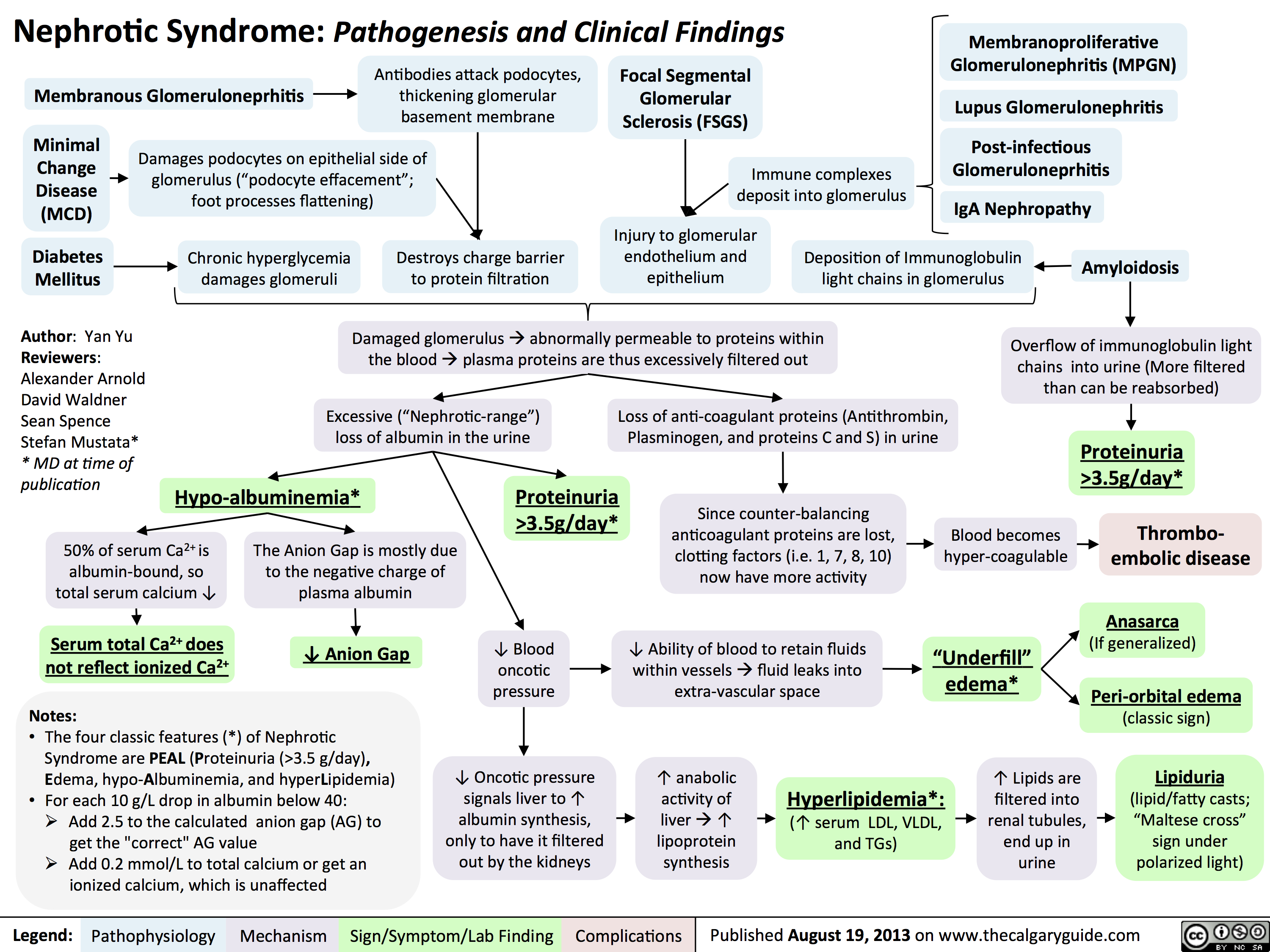 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
Upper Urinary Tract infection (UUTI): Pathogenesis and Clinical Findings
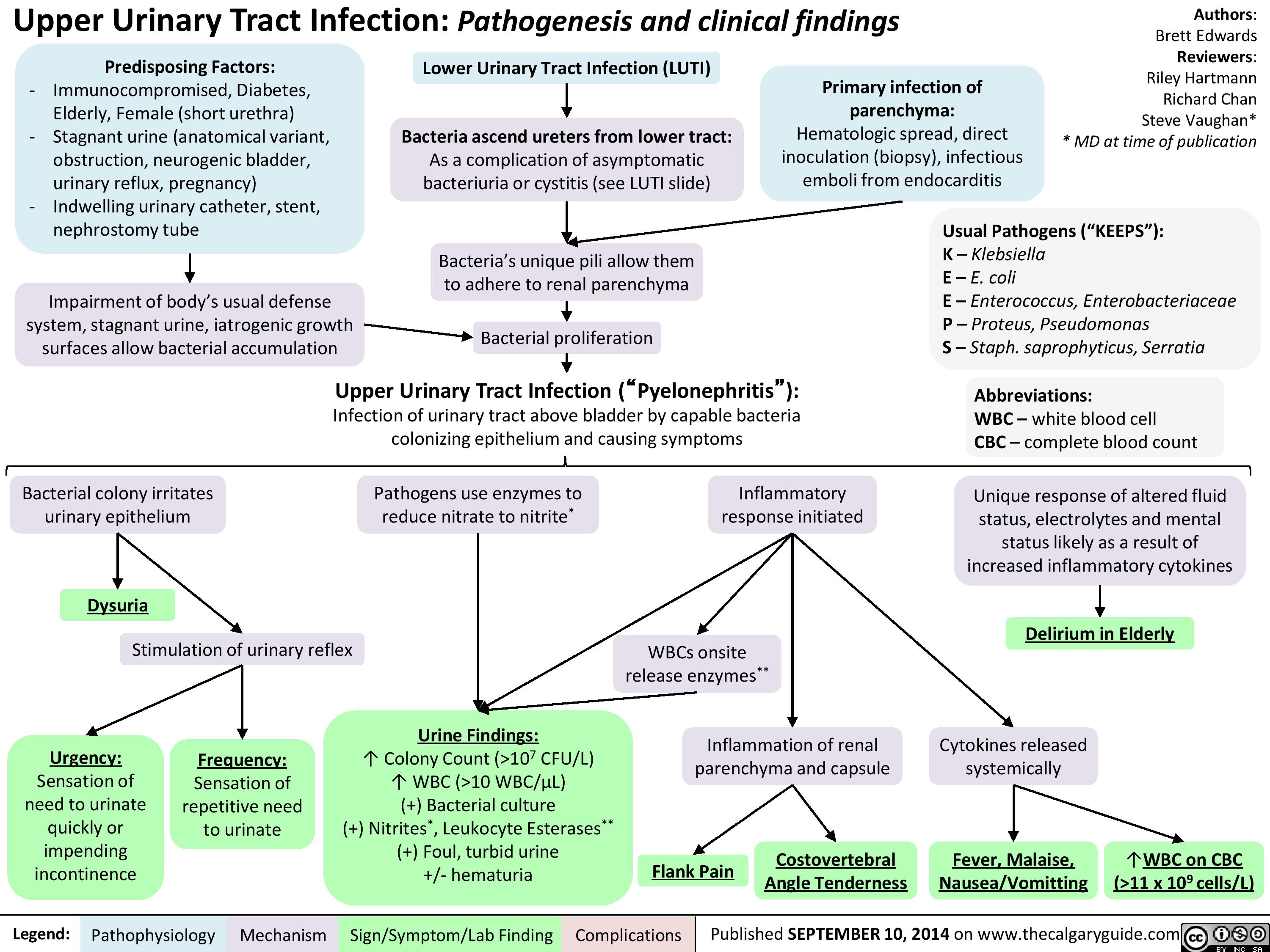
Central Adrenal Insufficiency - Pathogenesis and Clinical Findings
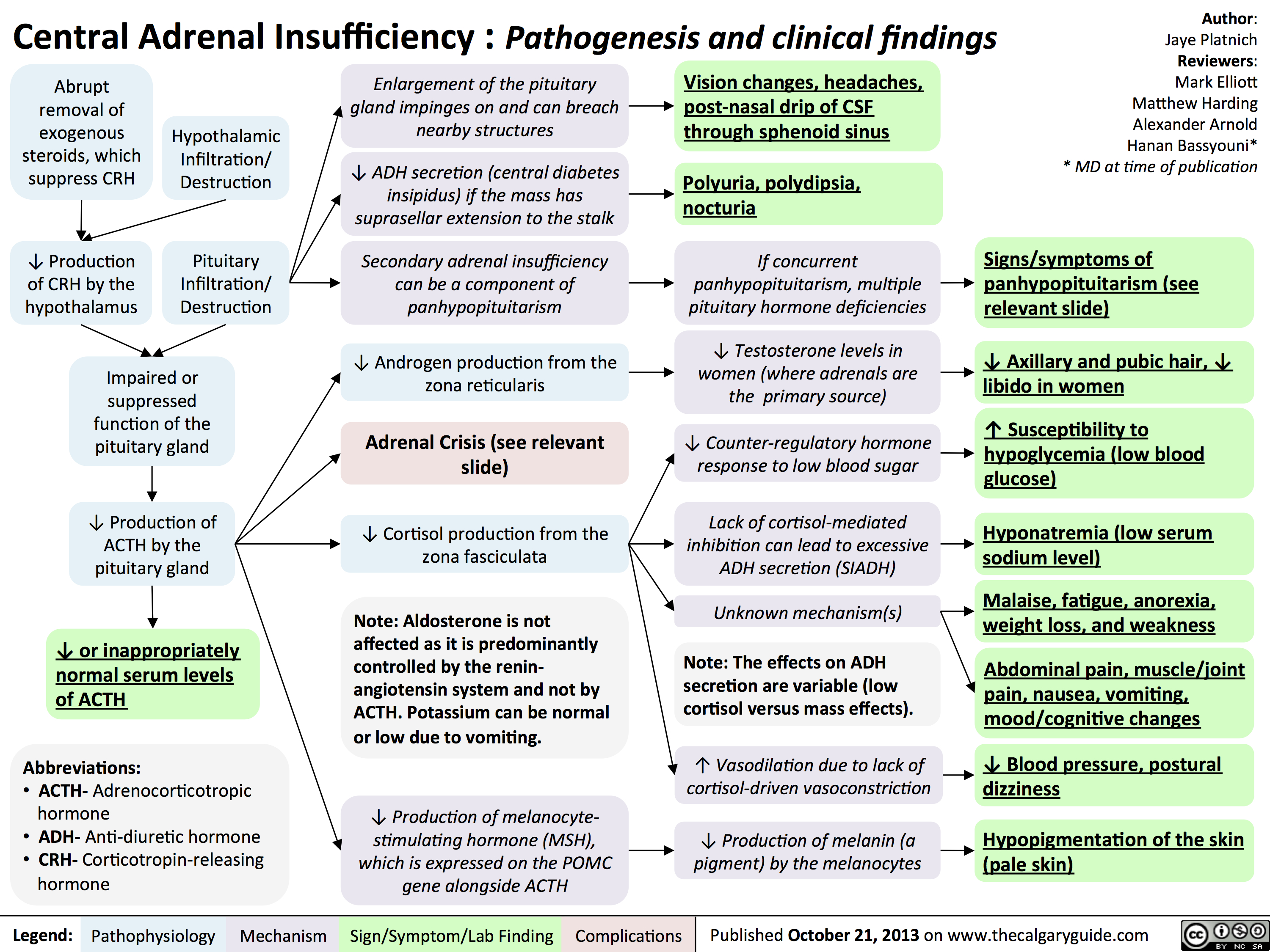
Hypercalcemia - Clinical Findings
![Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hypercalcemia-Clinical-Findings.jpg)
Pathogenesis of Anxiety Disorders

Pre-Renal Acute Kidney Injury Pathogenesis
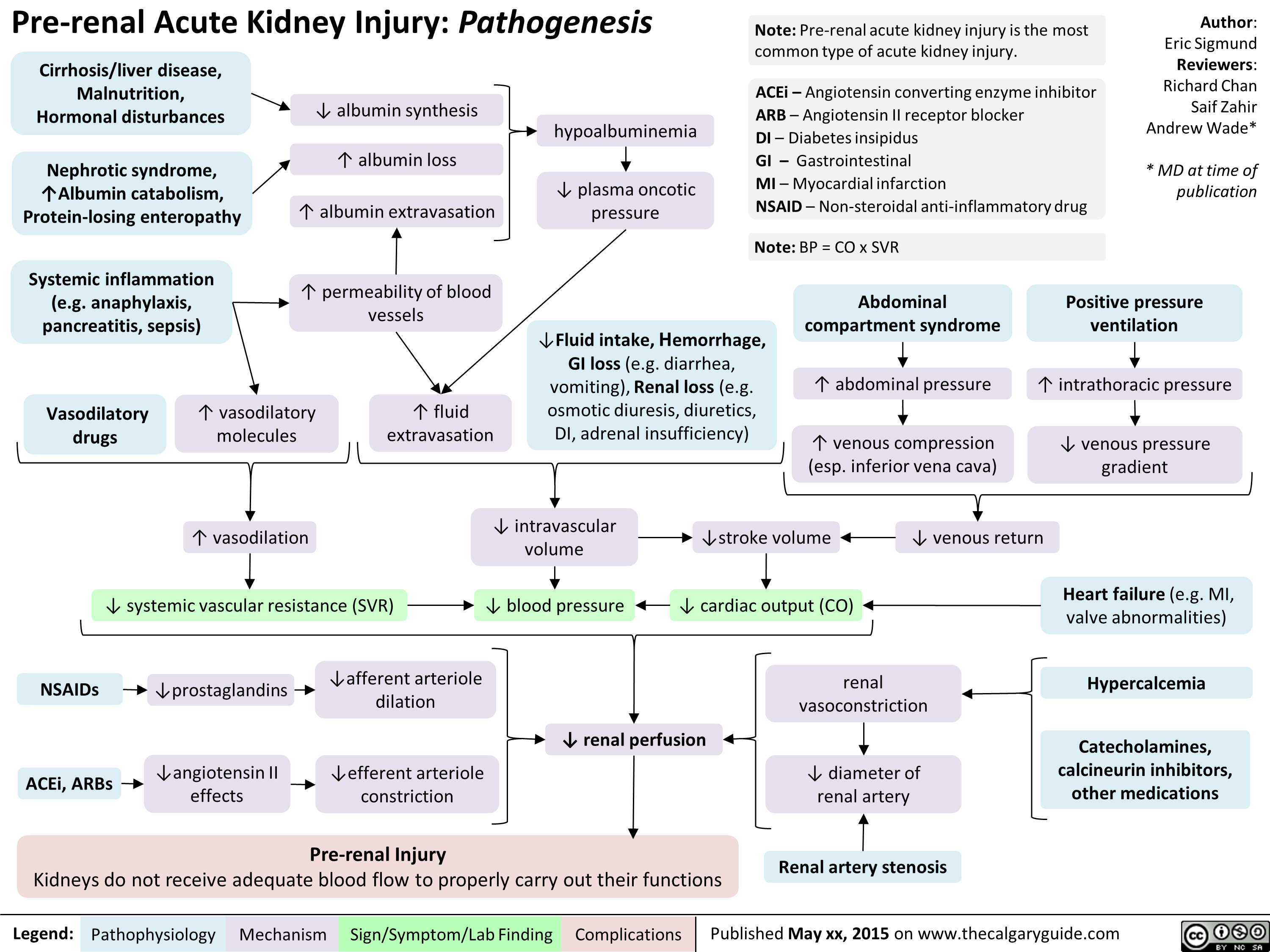
Hyperosmolar Hyperglycemic State
![Hyperosmolar Hyperglycemic State (HHS)
Note: HHS is only seen in Type II DM patients!
Note: In patients with either DKA or HHS, always look for an underlying cause (i.e. an infection)
Author: Yan Yu Reviewers:
Peter Vetere
Gill Goobie
Hanan Bassyouni* * MD at time of publication
Alters total body water & ion osmosis
Inadequate insulin production, insulin resistance, non- adherence to insulin Tx
Relative Insulin deficit
Stresses that ↑ Insulin demand: infections, pneumonia, MI, pancreatitis, etc)
Hyperglycemia
(Very high blood [glucose], higher than in DKA)
When blood [glucose] > 12mmol/L, glucose filtration > reabsorption, ↑ urine [glucose]
Glucosuria
Glucose in filtrate promotes osmotic diuresis: large- volume urine output
Polyuria
Dehydration
(↓ JVP, orthostasis: postural hypotension/ postural tachycardia, ↑ resting HR)
Some insulin still present, but not enoughsome glucose is utilized by muscle/fat cells, some remain in the blood
Cells not “starved”, but still need more energy
↑ release of Catabolic hormones: Glucagon, Epinephrine, Cortisol, GH
Body tries to ↑ blood [glucose], to hopefully ↑ cell glucose absorption
Hypothalamic cells sense low intra-cellular glucose, triggering feelings of hunger
Polyphagia
Note: the presence of some insulin directly inhibits lipolysis; thus, in HHS there is no ketone body production, and no subsequent metabolic acidosis and ketouria (unlike in DKA). If ketones are detected in an HHS patient it’s likely secondary to starvation or other mechanisms.
↓ ECF volume, ↑ ECF osmolarity (i.e. hypernatremia)
↑ Gluconeogenesis ↑ Glycogenolysis (in liver)
↓ Protein synthesis, ↑ proteolysis
(in muscle)
↑ Gluconeogenic substrates for liver If the patient doesn’t drink enough
water to replenish lost blood volume If pt is alert and
Electrolyte imbalance
water is accessible
Water osmotically leaves neurons, shrinking them
Neural damage: delirium, lethargy, seizure, stupor, coma
↓ renal perfusion, ↓ GFR
Renal Failure
(pre-renal cause; see relevant slides)
Polydipsia Note: in HHS, body K+ is lost via osmotic diuresis. But diffusion of K+ out of cells
may cause serum [K+] to be falsely normal/elevated. To prevent hypokalemia, give IV KCl along with IV insulin as soon as serum K+ <5.0mmol/L. But ensure patient has good renal function/urine output first, to avoid iatrogenic hyperkalemia!
Note: Electrolyte imbalances (i.e. hyperkalemia, hypernatremia) are worsened by the acute renal failure commonly coexisting with DKA/HHS
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published November 3, 2016 on www.thecalgaryguide.com
Hyperosmolar Hyperglycemic State (HHS)
Note: HHS is only seen in Type II DM patients!
Note: In patients with either DKA or HHS, always look for an underlying cause (i.e. an infection)
Author: Yan Yu Reviewers:
Peter Vetere
Gill Goobie
Hanan Bassyouni* * MD at time of publication
Alters total body water & ion osmosis
Inadequate insulin production, insulin resistance, non- adherence to insulin Tx
Relative Insulin deficit
Stresses that ↑ Insulin demand: infections, pneumonia, MI, pancreatitis, etc)
Hyperglycemia
(Very high blood [glucose], higher than in DKA)
When blood [glucose] > 12mmol/L, glucose filtration > reabsorption, ↑ urine [glucose]
Glucosuria
Glucose in filtrate promotes osmotic diuresis: large- volume urine output
Polyuria
Dehydration
(↓ JVP, orthostasis: postural hypotension/ postural tachycardia, ↑ resting HR)
Some insulin still present, but not enoughsome glucose is utilized by muscle/fat cells, some remain in the blood
Cells not “starved”, but still need more energy
↑ release of Catabolic hormones: Glucagon, Epinephrine, Cortisol, GH
Body tries to ↑ blood [glucose], to hopefully ↑ cell glucose absorption
Hypothalamic cells sense low intra-cellular glucose, triggering feelings of hunger
Polyphagia
Note: the presence of some insulin directly inhibits lipolysis; thus, in HHS there is no ketone body production, and no subsequent metabolic acidosis and ketouria (unlike in DKA). If ketones are detected in an HHS patient it’s likely secondary to starvation or other mechanisms.
↓ ECF volume, ↑ ECF osmolarity (i.e. hypernatremia)
↑ Gluconeogenesis ↑ Glycogenolysis (in liver)
↓ Protein synthesis, ↑ proteolysis
(in muscle)
↑ Gluconeogenic substrates for liver If the patient doesn’t drink enough
water to replenish lost blood volume If pt is alert and
Electrolyte imbalance
water is accessible
Water osmotically leaves neurons, shrinking them
Neural damage: delirium, lethargy, seizure, stupor, coma
↓ renal perfusion, ↓ GFR
Renal Failure
(pre-renal cause; see relevant slides)
Polydipsia Note: in HHS, body K+ is lost via osmotic diuresis. But diffusion of K+ out of cells
may cause serum [K+] to be falsely normal/elevated. To prevent hypokalemia, give IV KCl along with IV insulin as soon as serum K+ <5.0mmol/L. But ensure patient has good renal function/urine output first, to avoid iatrogenic hyperkalemia!
Note: Electrolyte imbalances (i.e. hyperkalemia, hypernatremia) are worsened by the acute renal failure commonly coexisting with DKA/HHS
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published November 3, 2016 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hyperosmolar-Hyperglycemic-State-HHS.jpg)
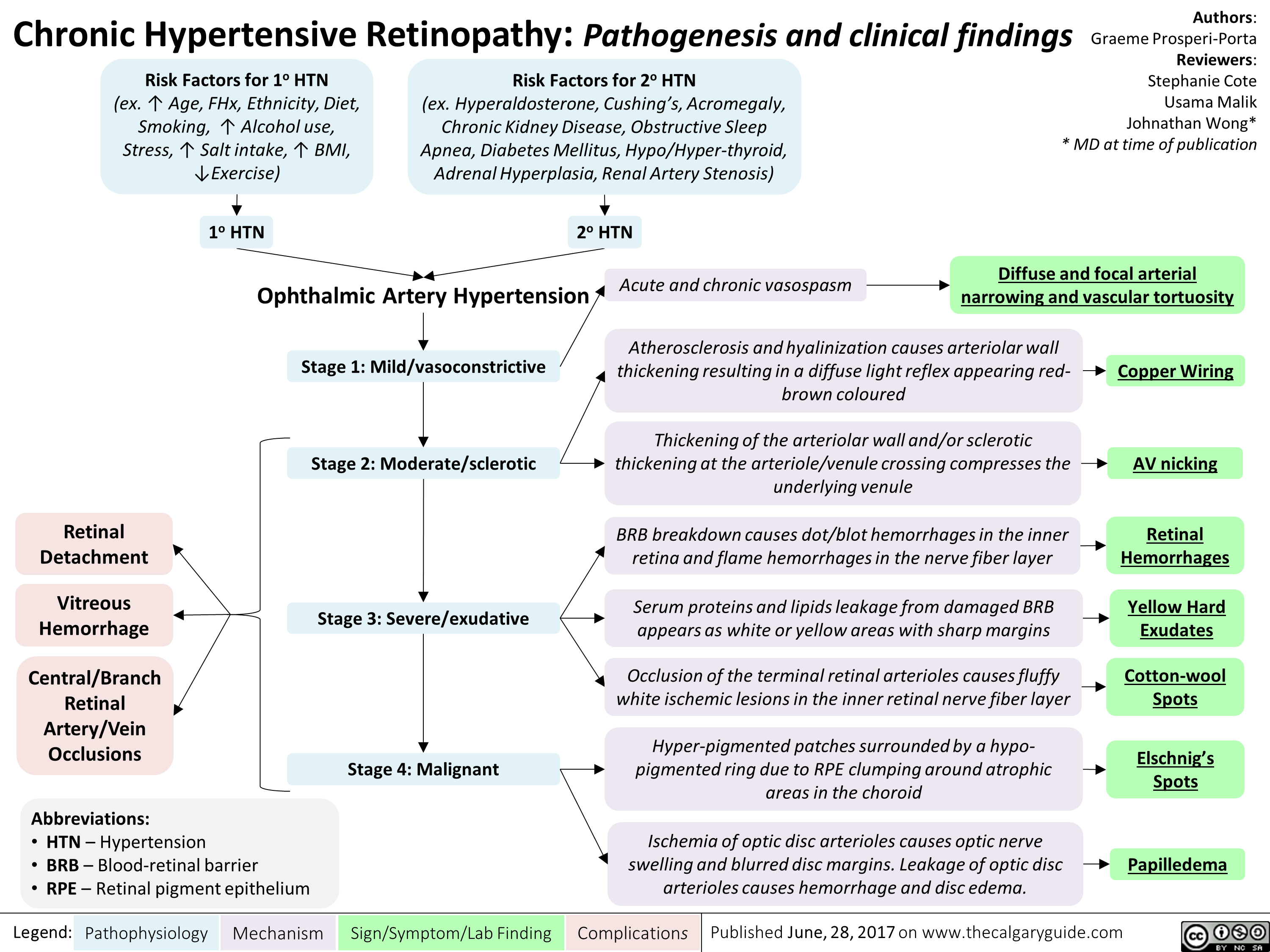
chronic-hypertensive-retinopathy-pathogenesis-and-clinical-findings
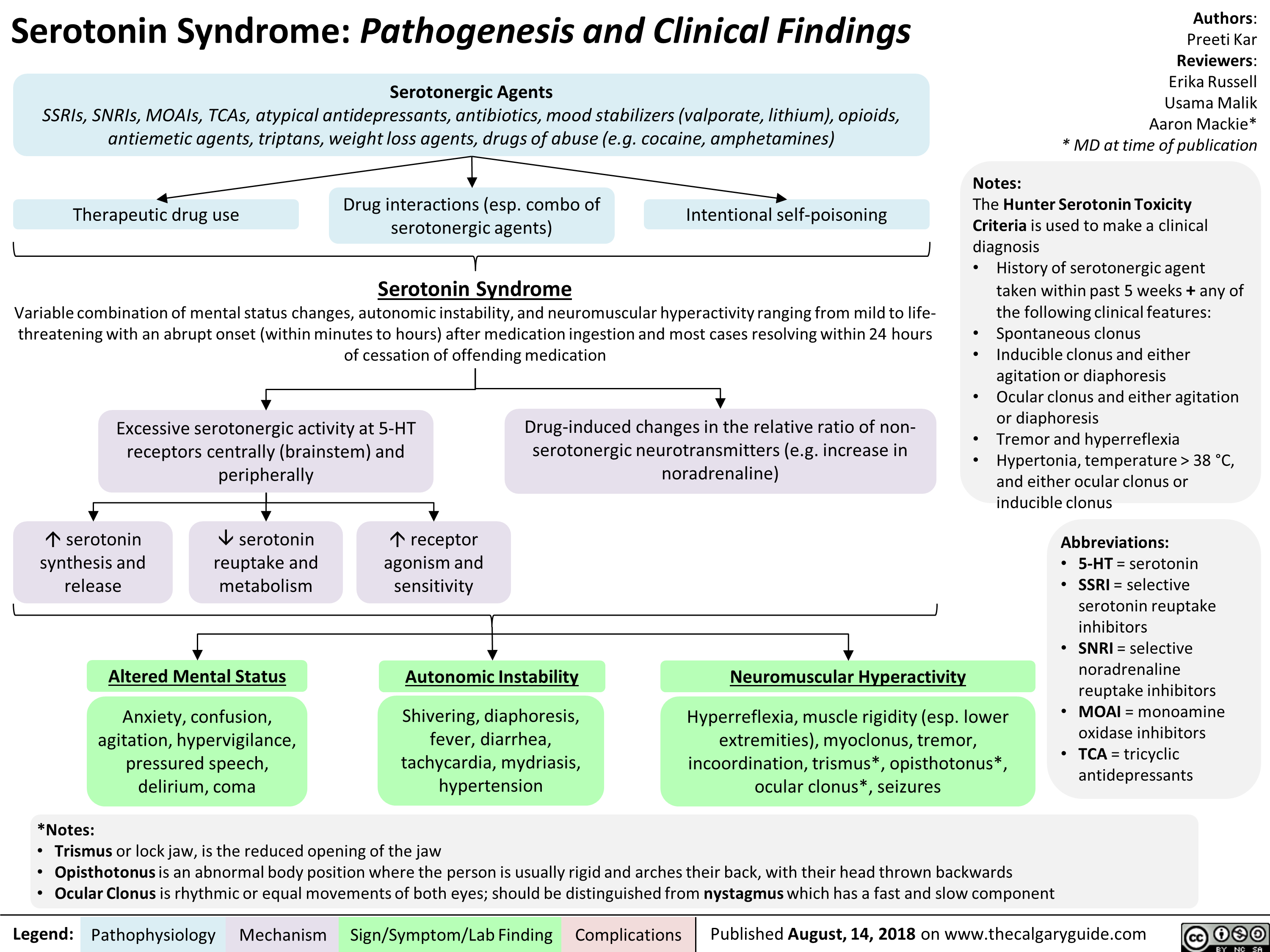
Serotonin Syndrome Pathogenesis and Clinical Findings
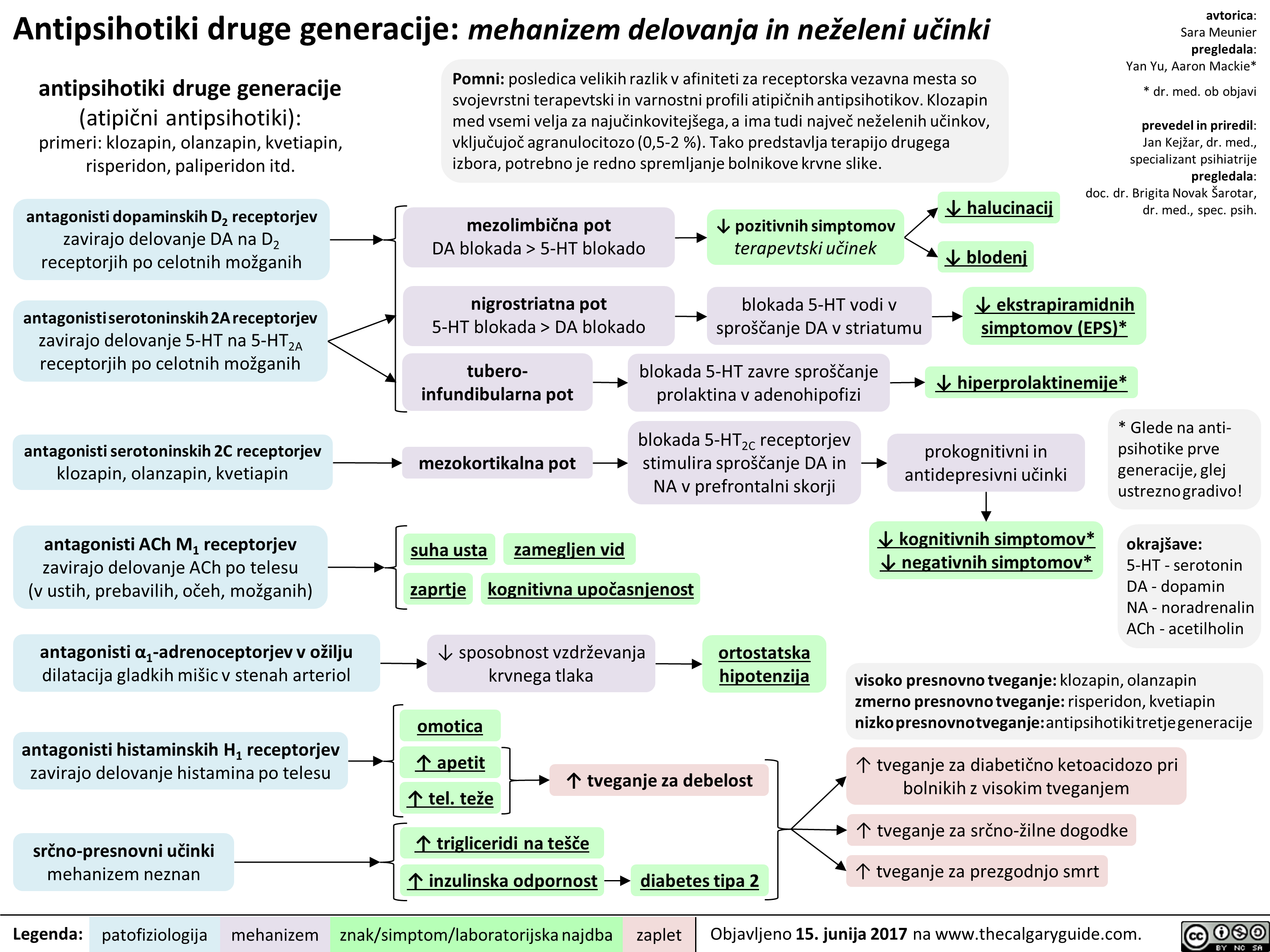
2nd gen antipsychotics (Slovenian translation) - FINAL VERSION
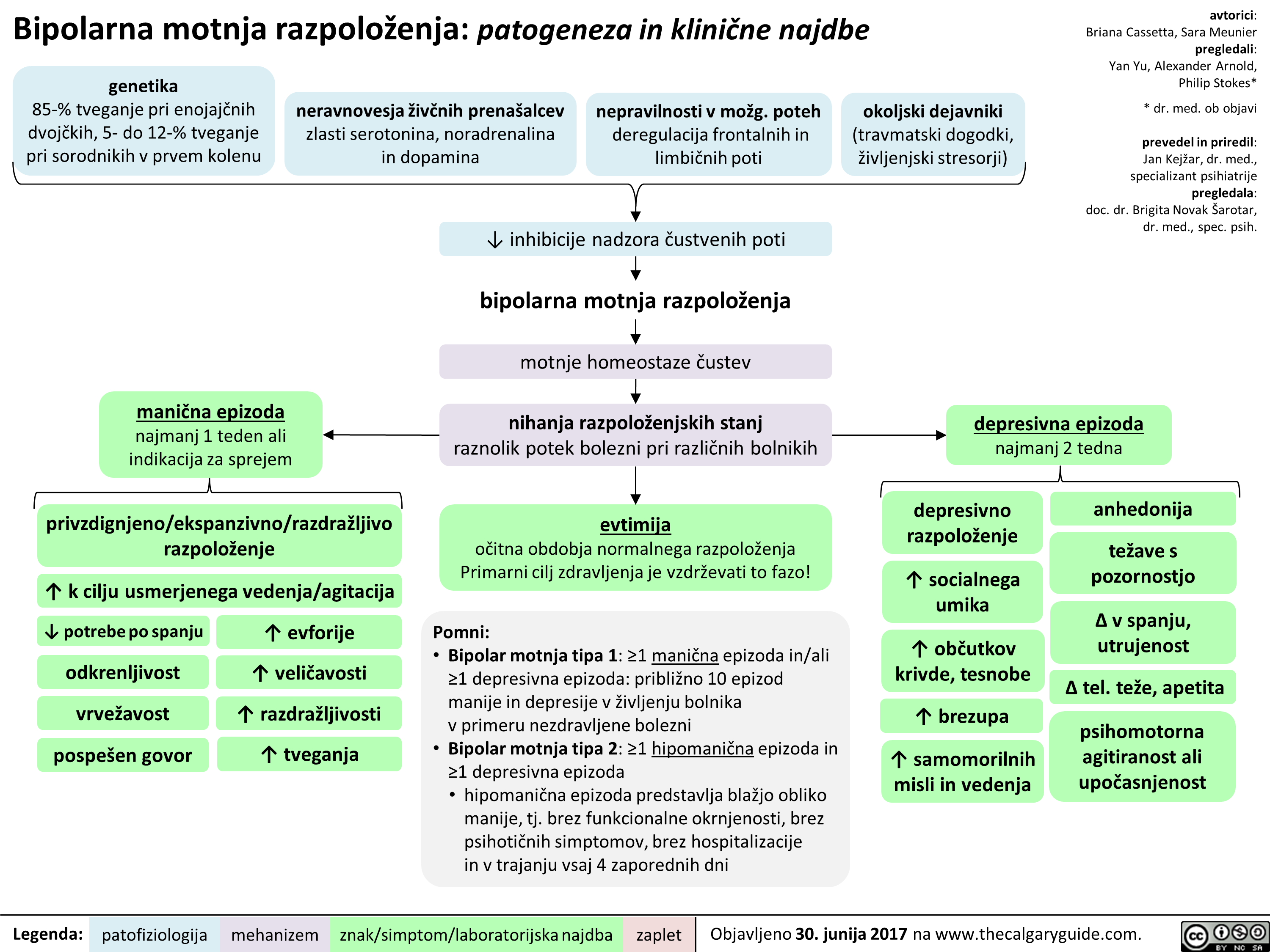
BMR (Slovenian translation) - FINAL VERSION
Bupropion (Slovenian translation) - FINAL VERSION
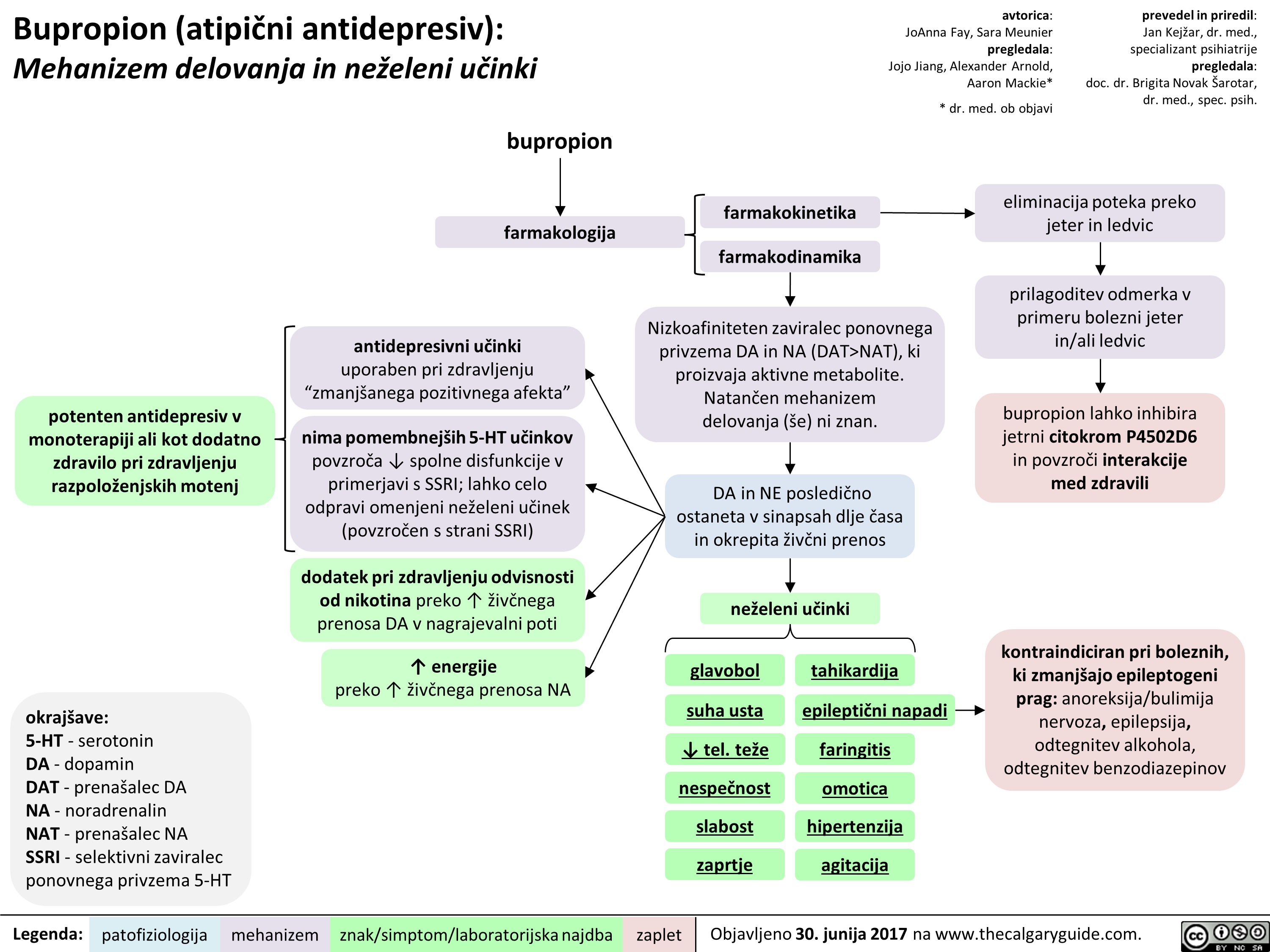 NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" title="Bupropion (atipiEni antidepresiv): Mehanizem delovanja in neieleni utinki
potenten antidepresiv v monoterapiji all kot dodatno zdravilo pri zdravljenju razpoloienjskih motenj
okrajgave: 5-HT - serotonin DA - dopamin DAT - prenagalec DA NA - noradrenalin NAT - prenagalec NA SSRI - selektivni zaviralec ponovnega privzema 5-HT
Legenda:
bupropion
farmakologija
antidepresivni udnki uporaben pri zdravljenju "zmaniganega pozitivnega afekta"
nima pomembnelgih 5-HT udnkov povzraa spolne disfunkcije v primerjavi s SSRI; lahko celo odpravi omenjeni neieleni udnek (povzraen s strani SSRI)
dodatek pri zdravljenju odvisnosti od nikotina preko T iive'nega prenosa DA v nagrajevalni poti
energije preko T 2ivbega prenosa NA
patofiziologija mehanizem
farmakokinetika
farmakodinamika
avtorica: JoAnna Fay, Sara Meunier pregledala: Jojo Jiang, Alexander Arnold, Aaron Mackie*
* dr. med. ob objavi
Nizkoafiniteten zaviralec ponovnega privzema DA in NA (DAT>NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" />
NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" title="Bupropion (atipiEni antidepresiv): Mehanizem delovanja in neieleni utinki
potenten antidepresiv v monoterapiji all kot dodatno zdravilo pri zdravljenju razpoloienjskih motenj
okrajgave: 5-HT - serotonin DA - dopamin DAT - prenagalec DA NA - noradrenalin NAT - prenagalec NA SSRI - selektivni zaviralec ponovnega privzema 5-HT
Legenda:
bupropion
farmakologija
antidepresivni udnki uporaben pri zdravljenju "zmaniganega pozitivnega afekta"
nima pomembnelgih 5-HT udnkov povzraa spolne disfunkcije v primerjavi s SSRI; lahko celo odpravi omenjeni neieleni udnek (povzraen s strani SSRI)
dodatek pri zdravljenju odvisnosti od nikotina preko T iive'nega prenosa DA v nagrajevalni poti
energije preko T 2ivbega prenosa NA
patofiziologija mehanizem
farmakokinetika
farmakodinamika
avtorica: JoAnna Fay, Sara Meunier pregledala: Jojo Jiang, Alexander Arnold, Aaron Mackie*
* dr. med. ob objavi
Nizkoafiniteten zaviralec ponovnega privzema DA in NA (DAT>NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" />
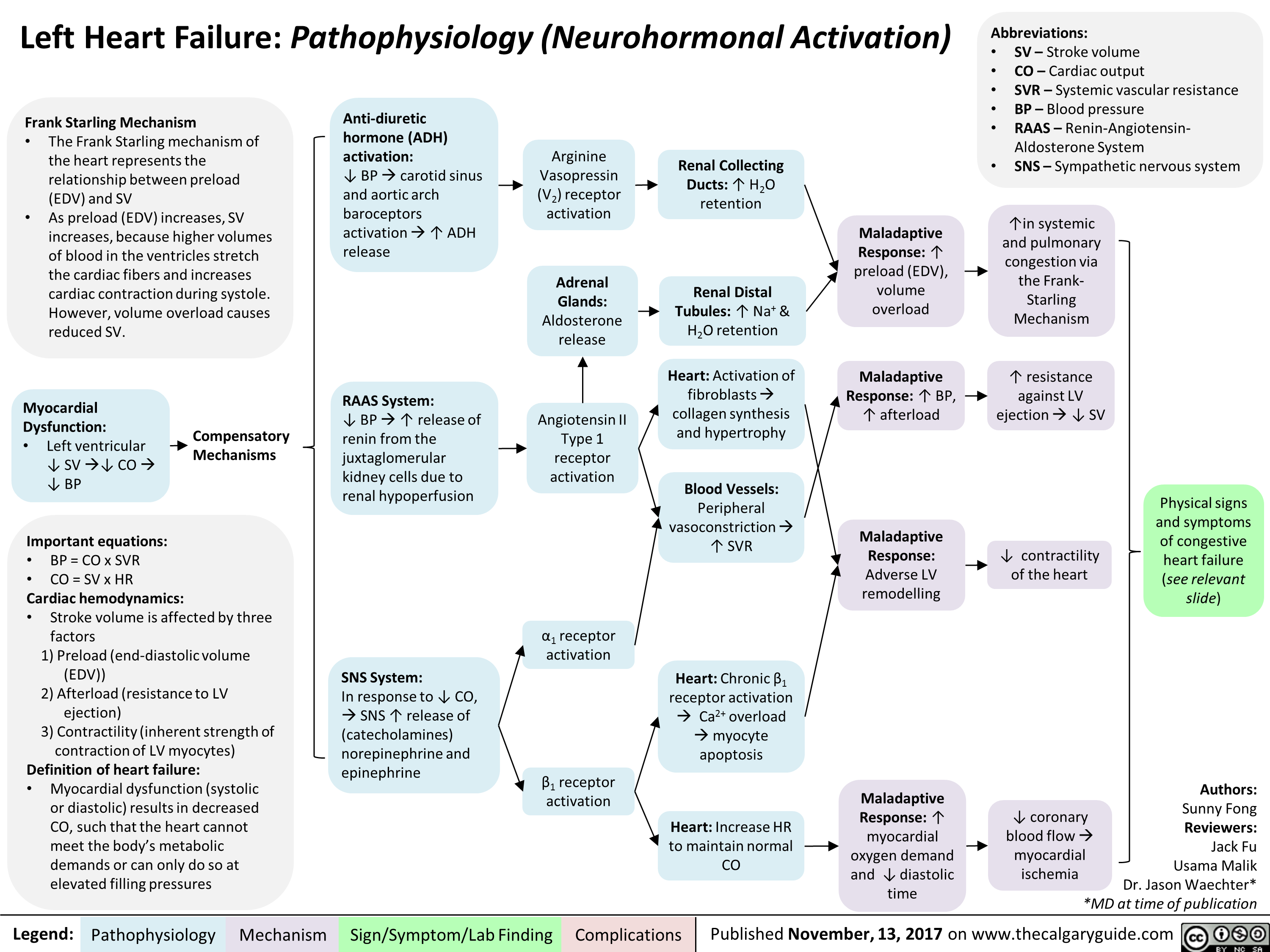
Left Heart Failure: Pathophysiology (Neurohormonal Activation)
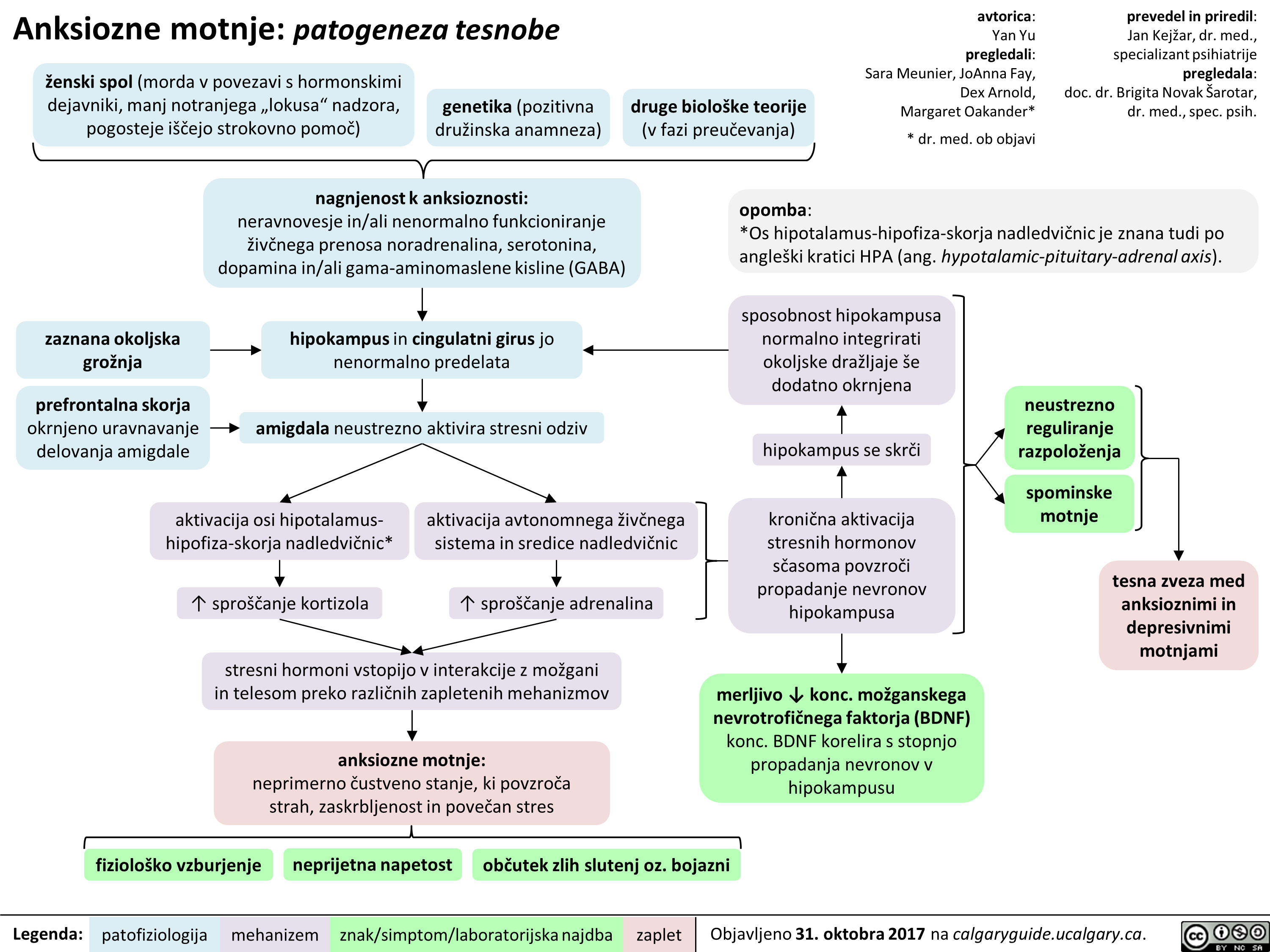
Anksiozne motnje: patogeneza tesnobe
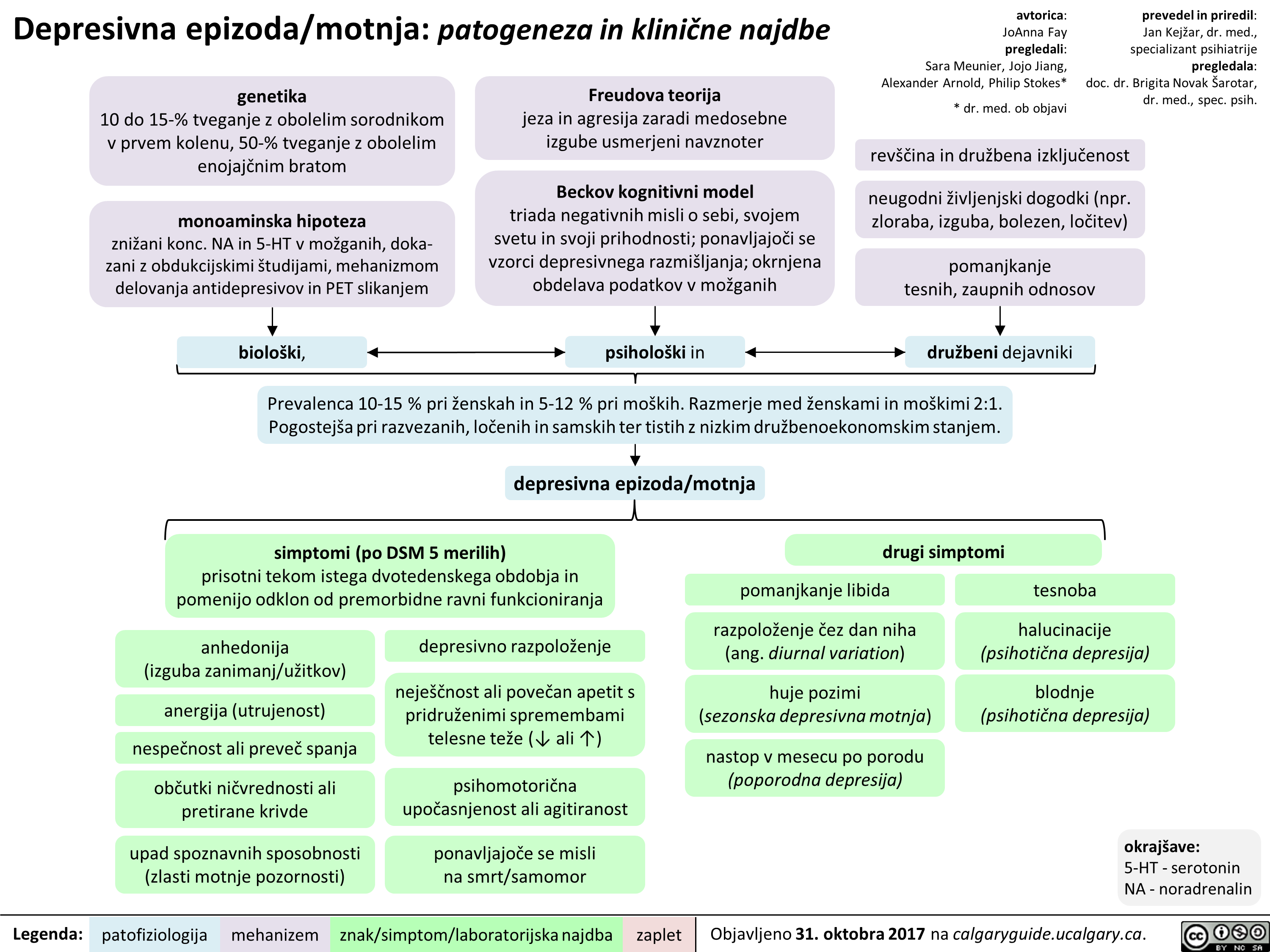
Depresivna epizoda/motnja: patogeneza in klinične najdbe
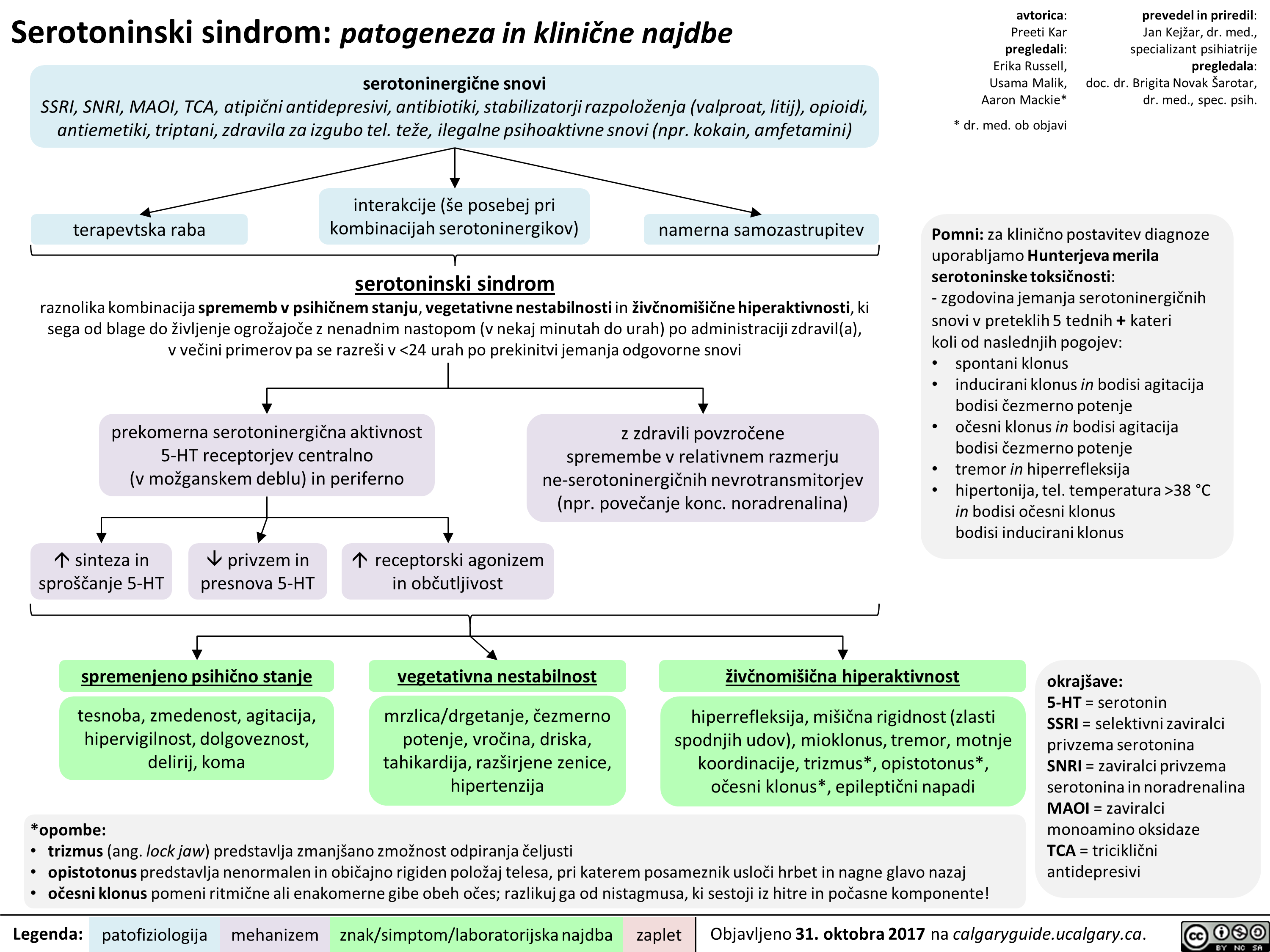
Serotoninski sindrom: patogeneza in klinične najdbe
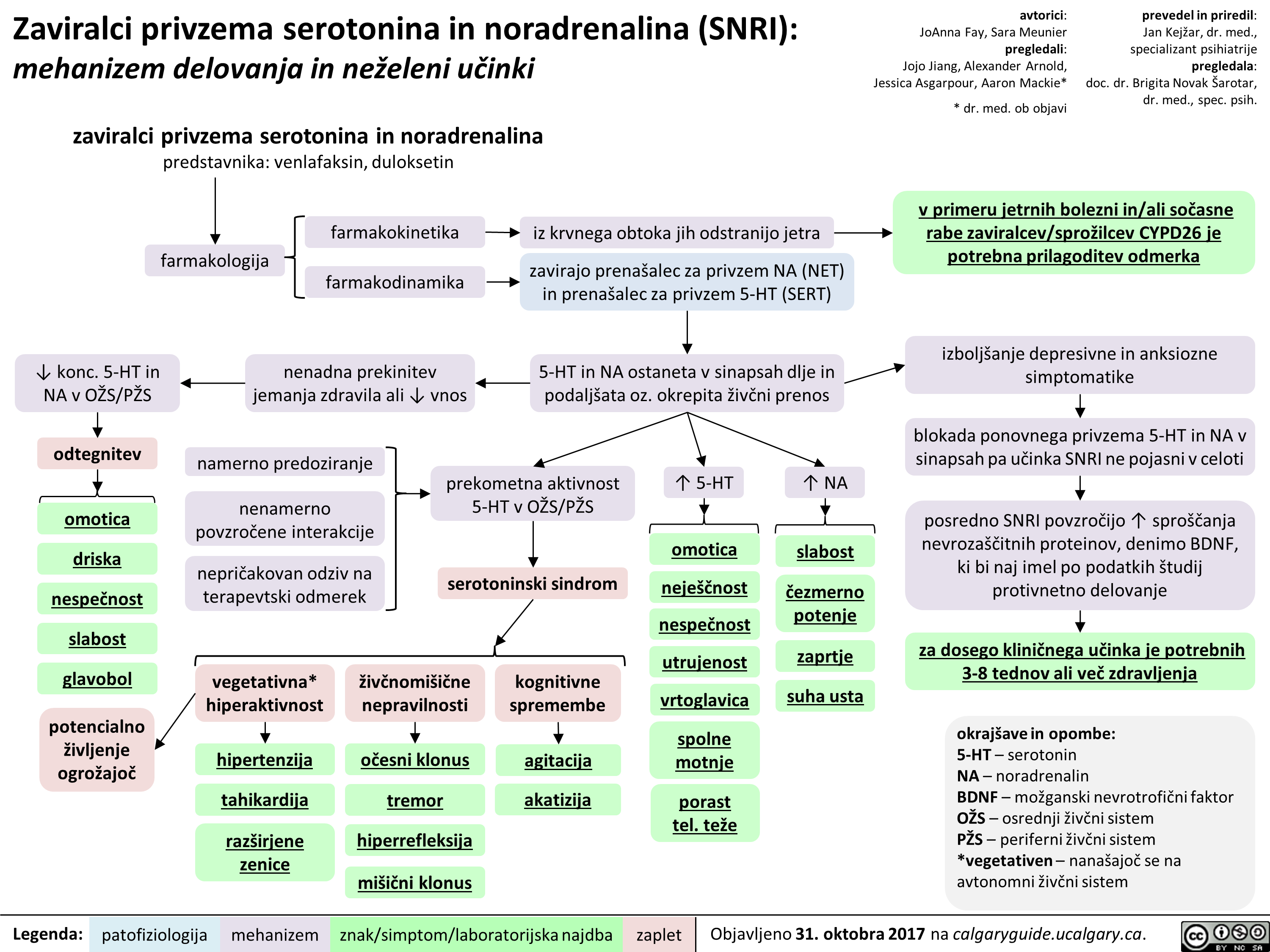
Zaviralci privzema serotonina in noradrenalina (SNRI): mehanizem delovanja in neželeni učinki
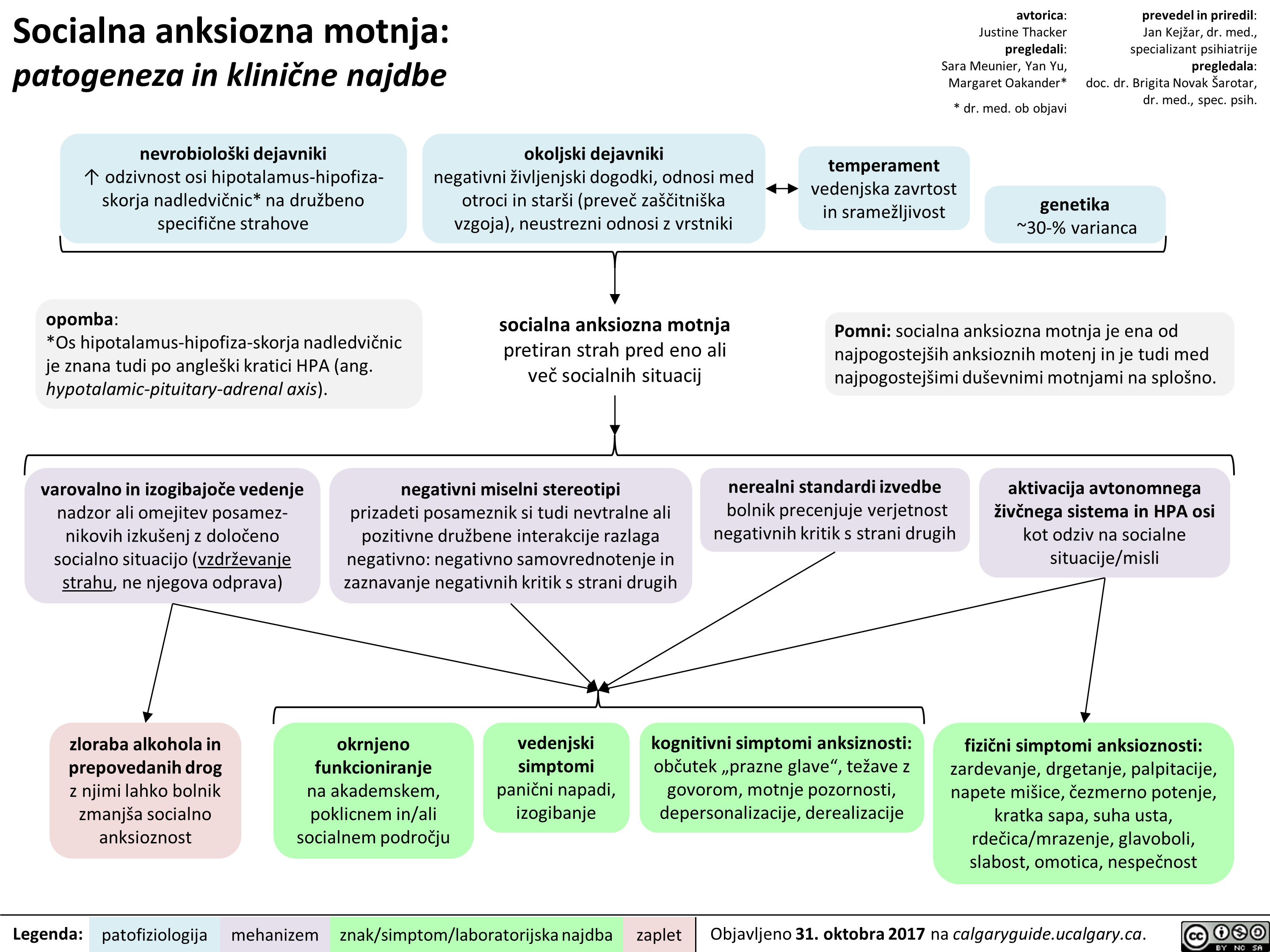
Socialna anksiozna motnja: patogeneza in klinične najdbe
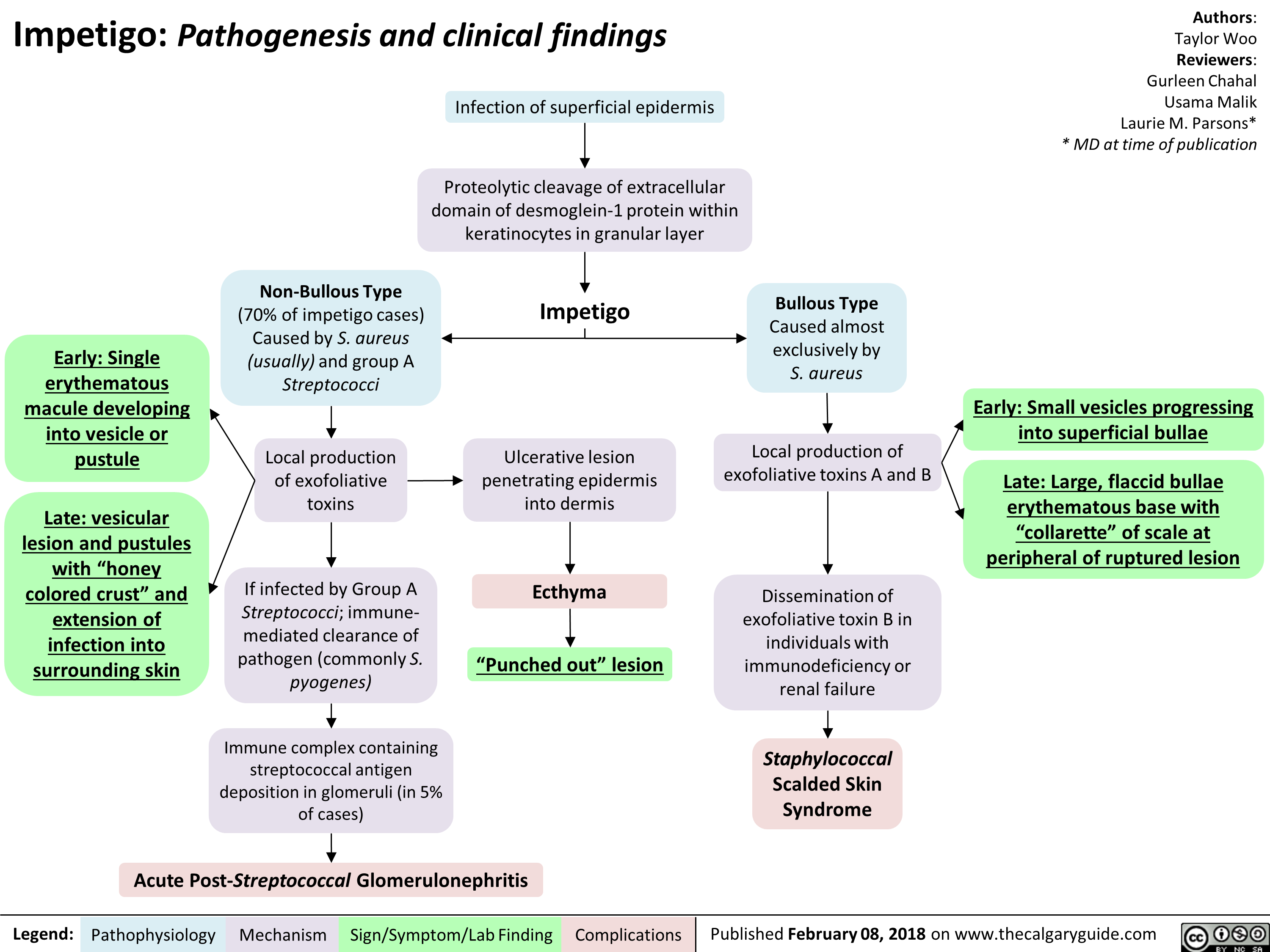
Impetigo Pathogenesis and clinical findings
Fisiologi sistem Renin-Angiotensin-Aldosteron (RAAS)
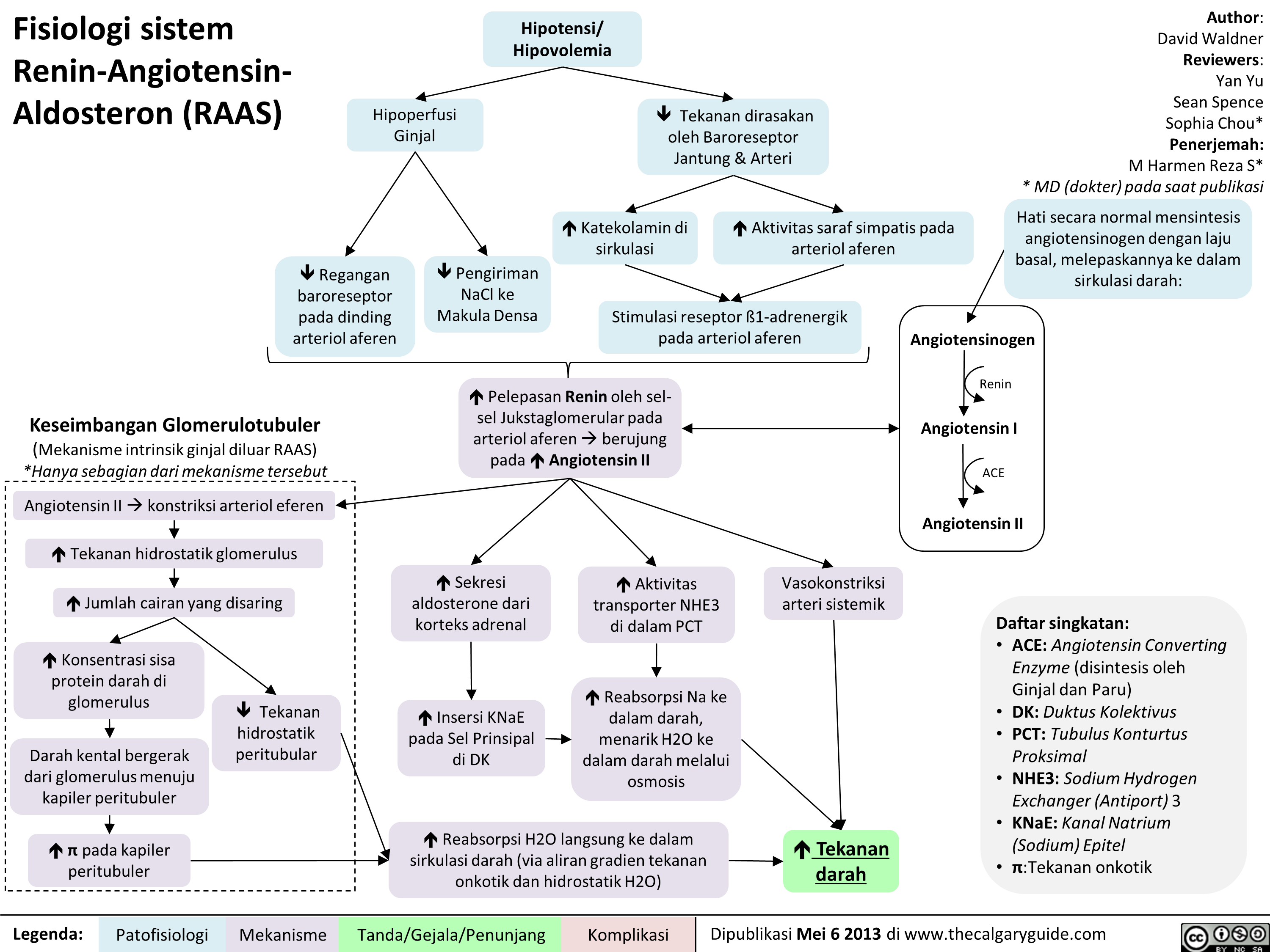
Acetylcholinesterase Inhibitors
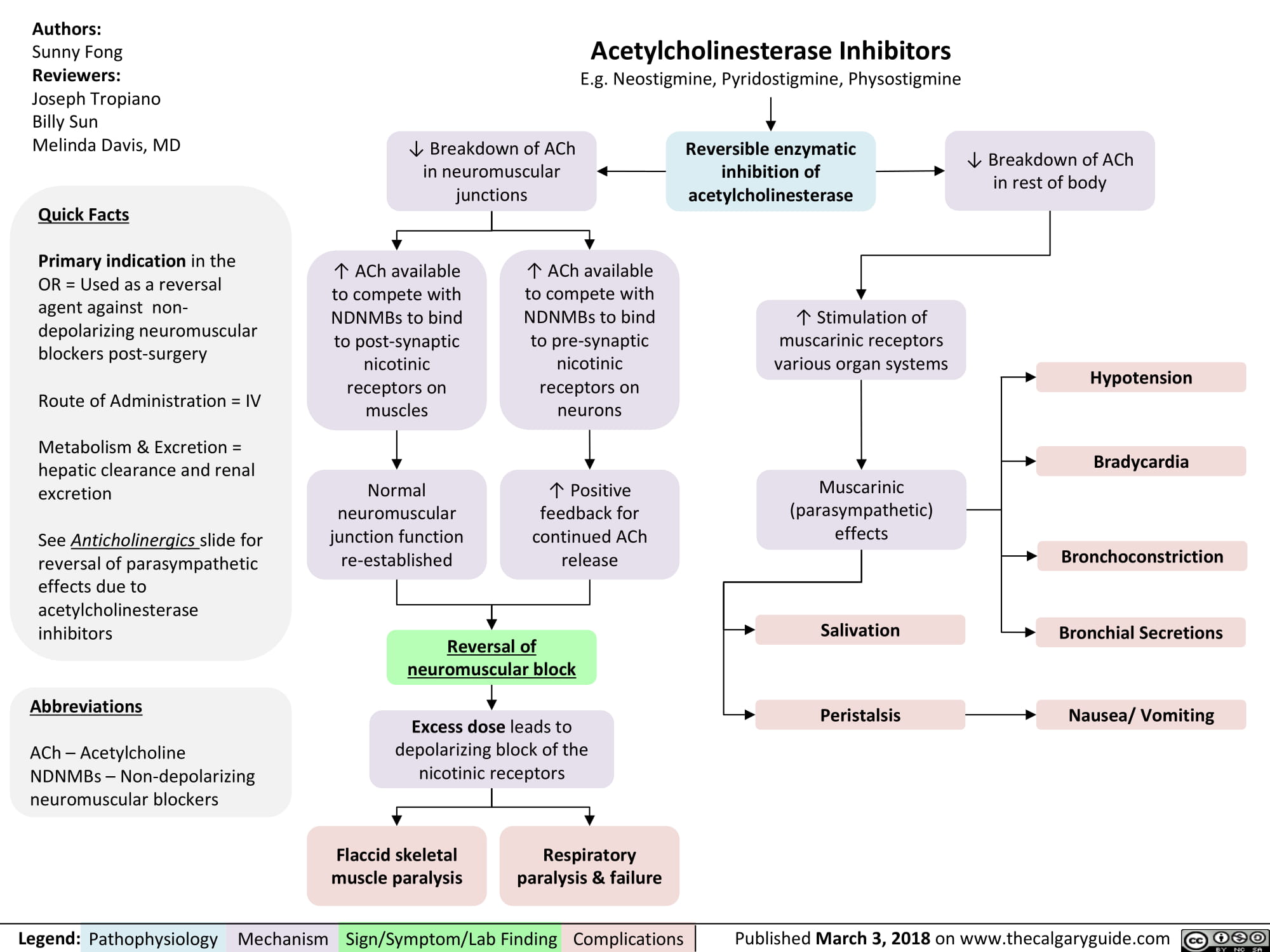
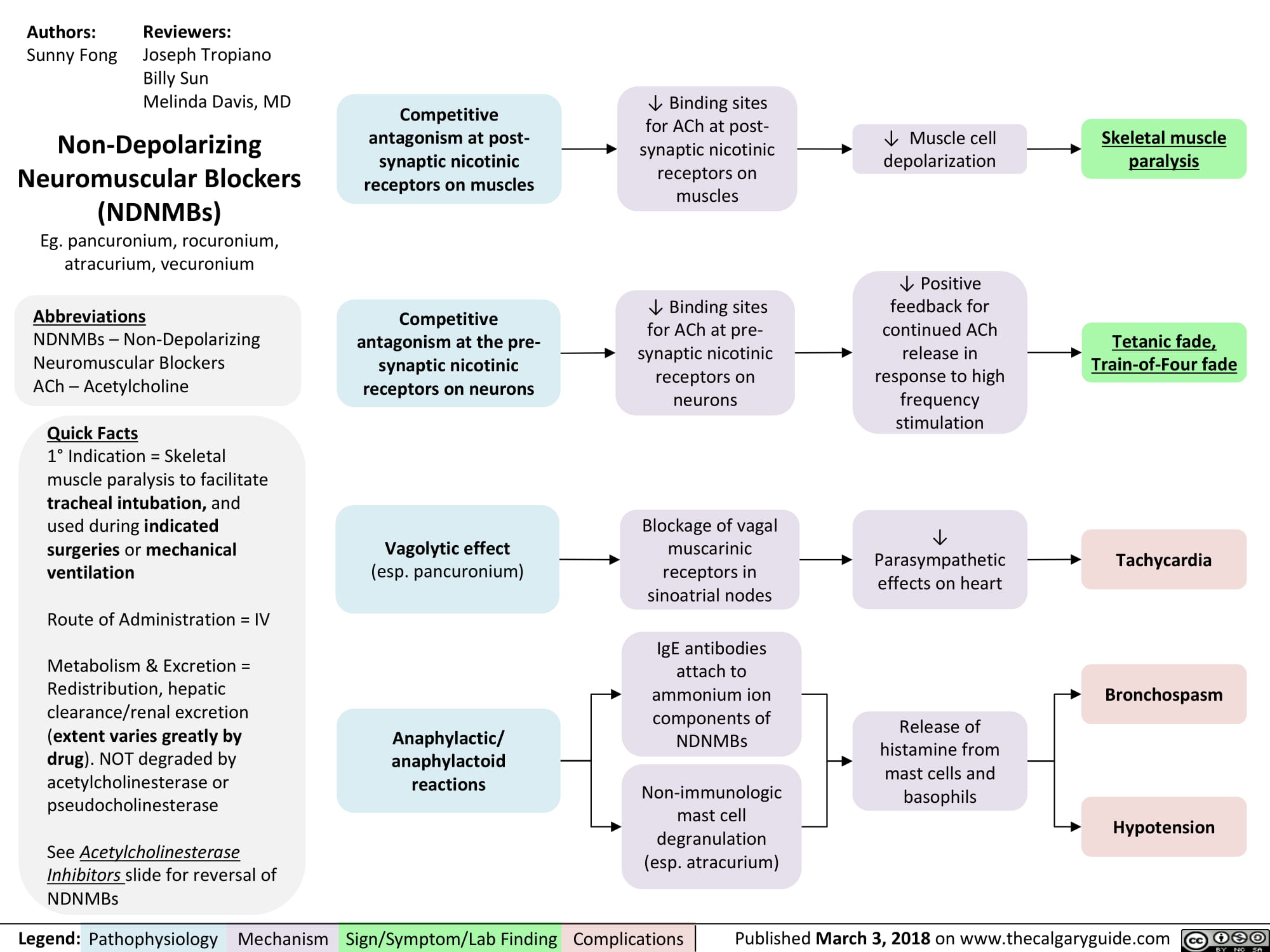
non-depolarizing-neuromuscular-blocks-ndnmbs
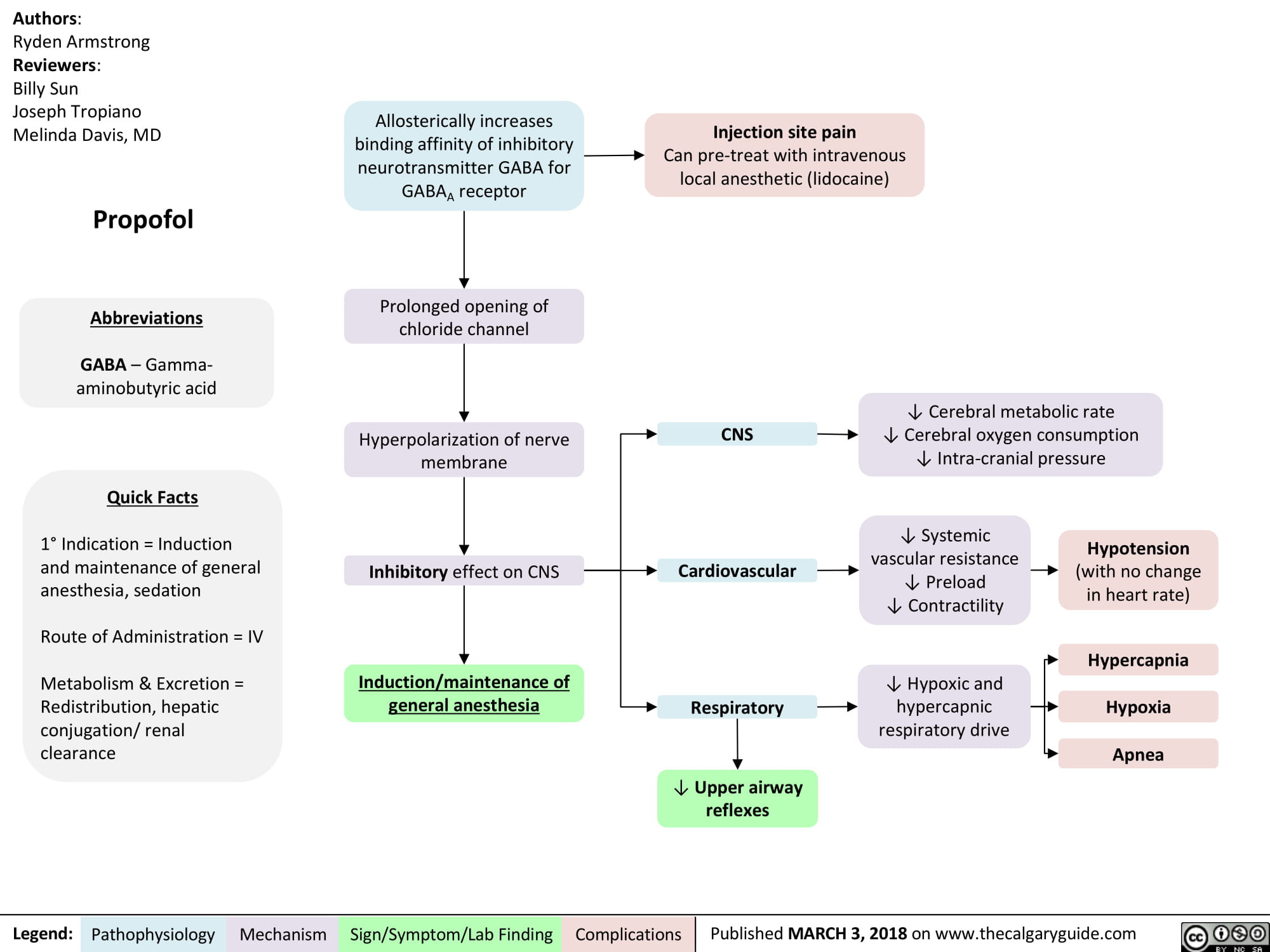
Propofol
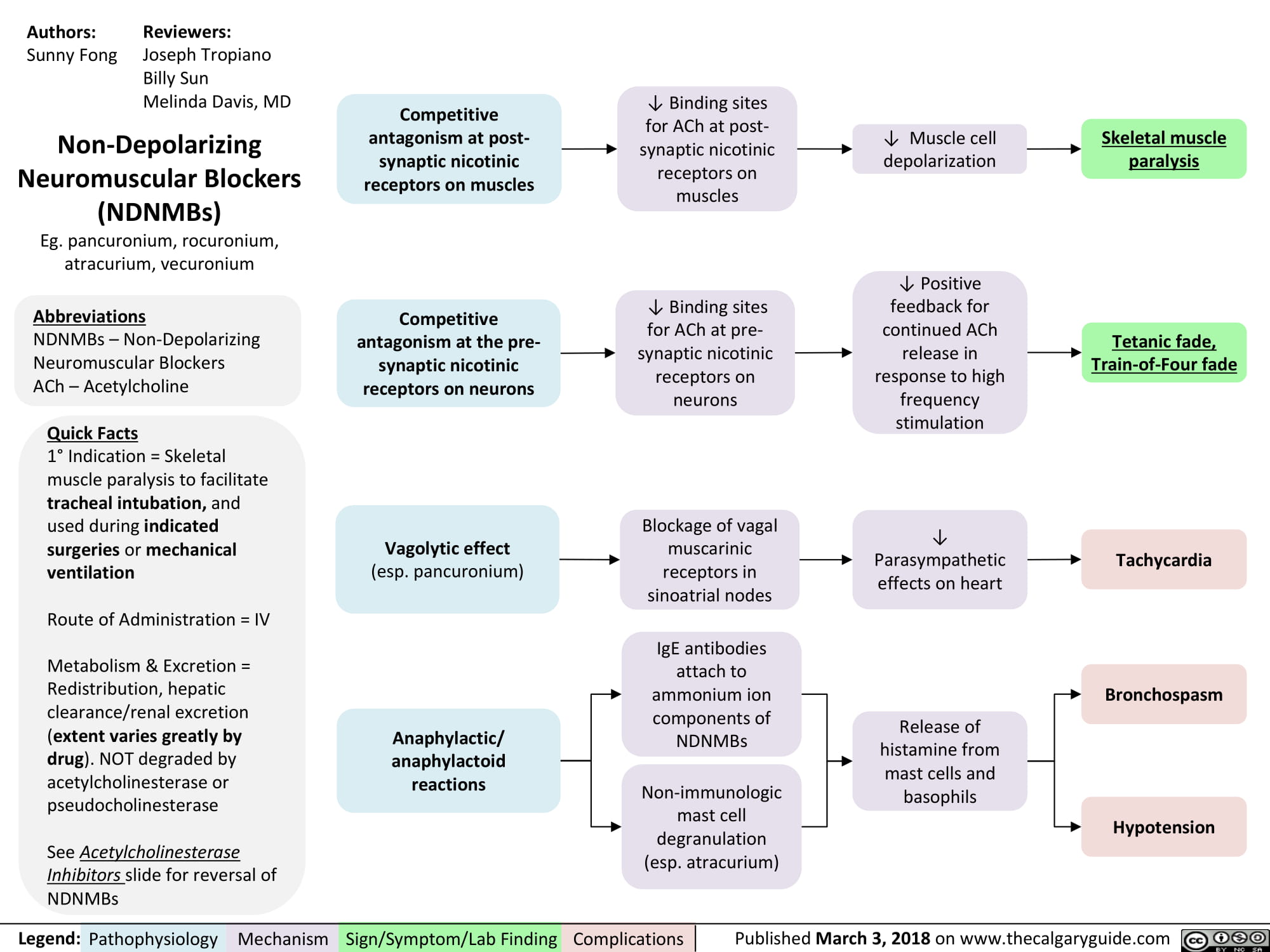
non-depolarizing-neuromuscular-blocks-ndnmbs
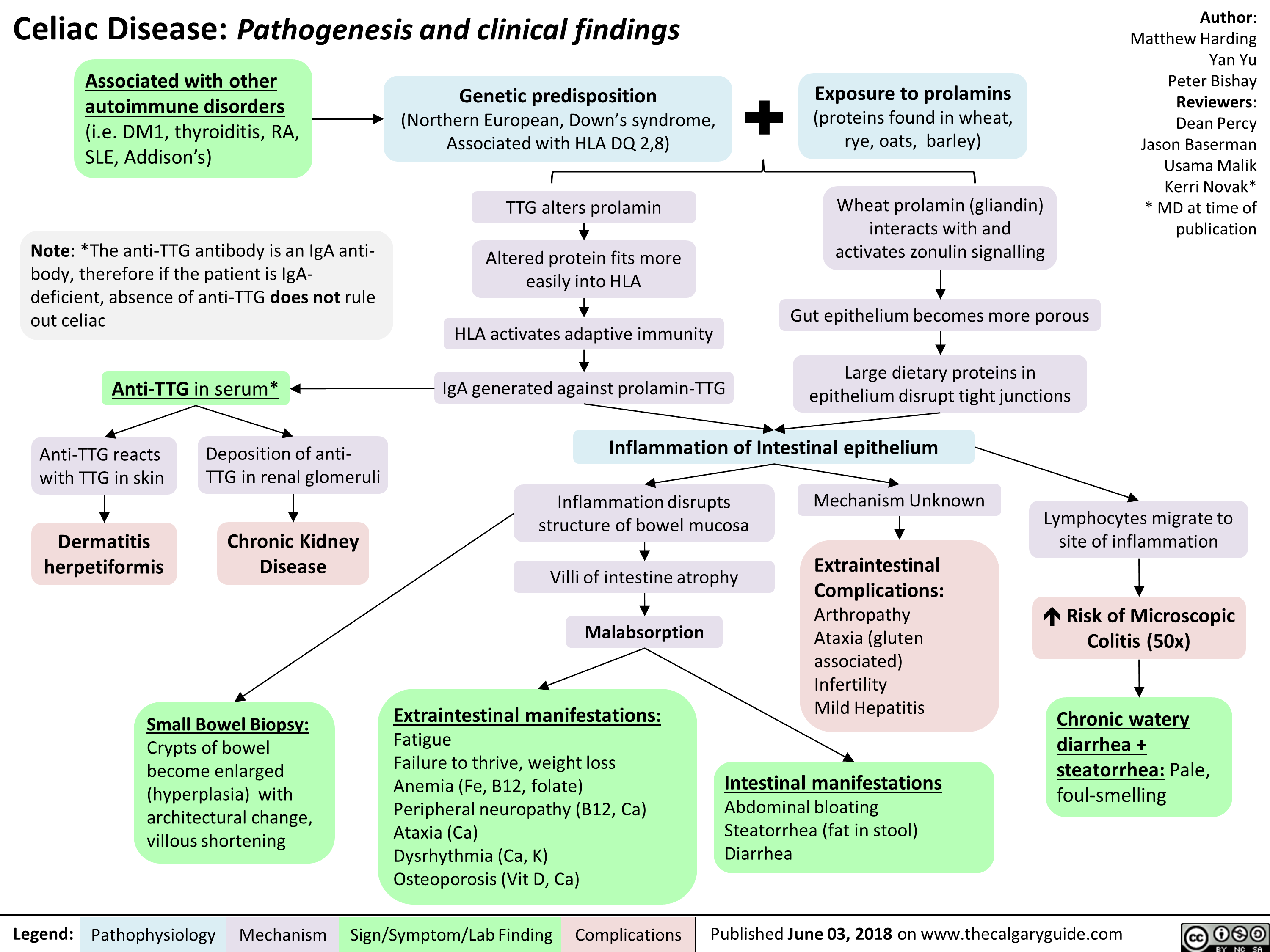
Celiac Disease: Pathogenesis and clinical findings
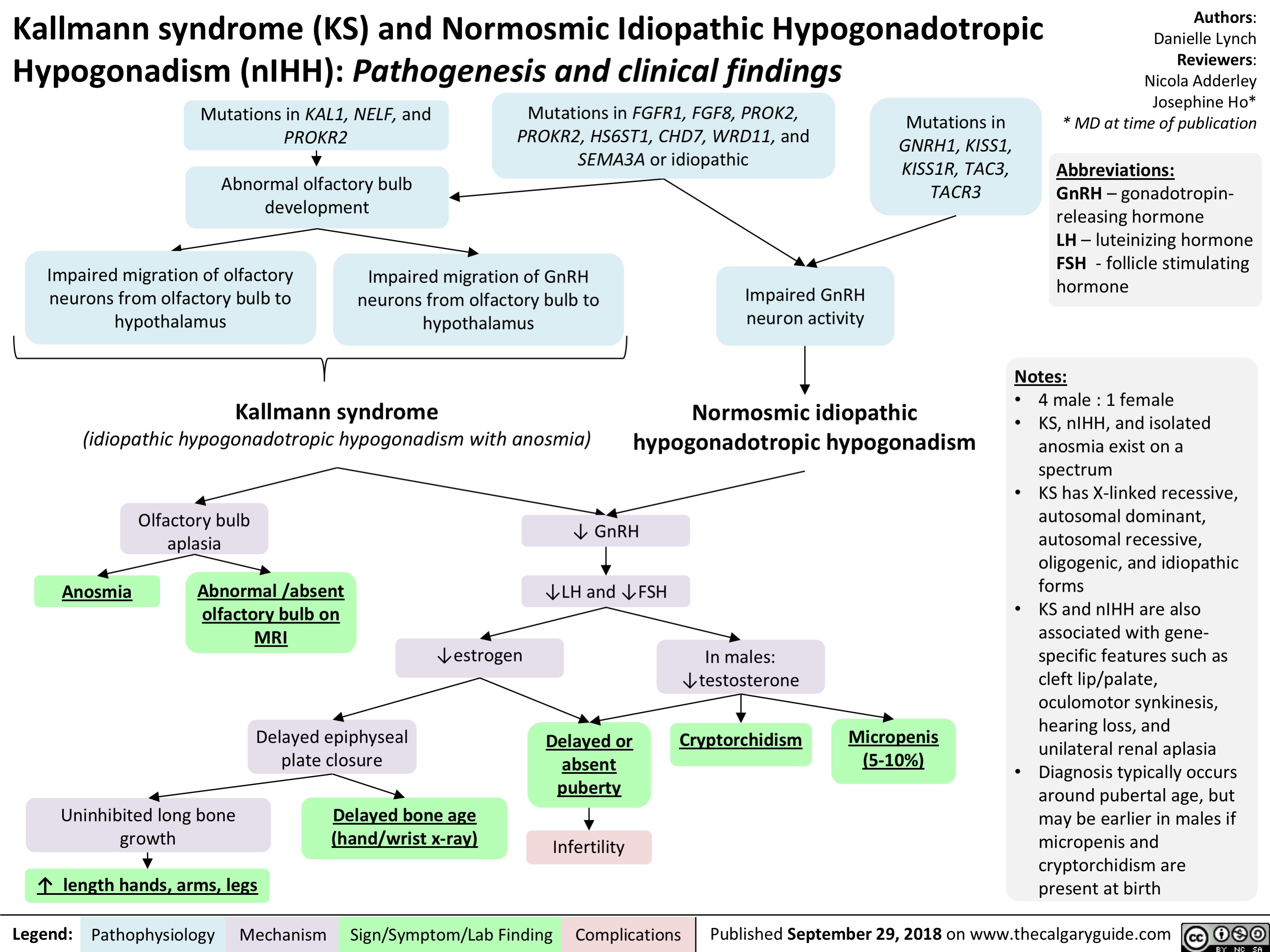
Kallmann Syndrome and Normosmotic Idiopathic Hypogonadotropism: Pathogenesis and Clinical Findings
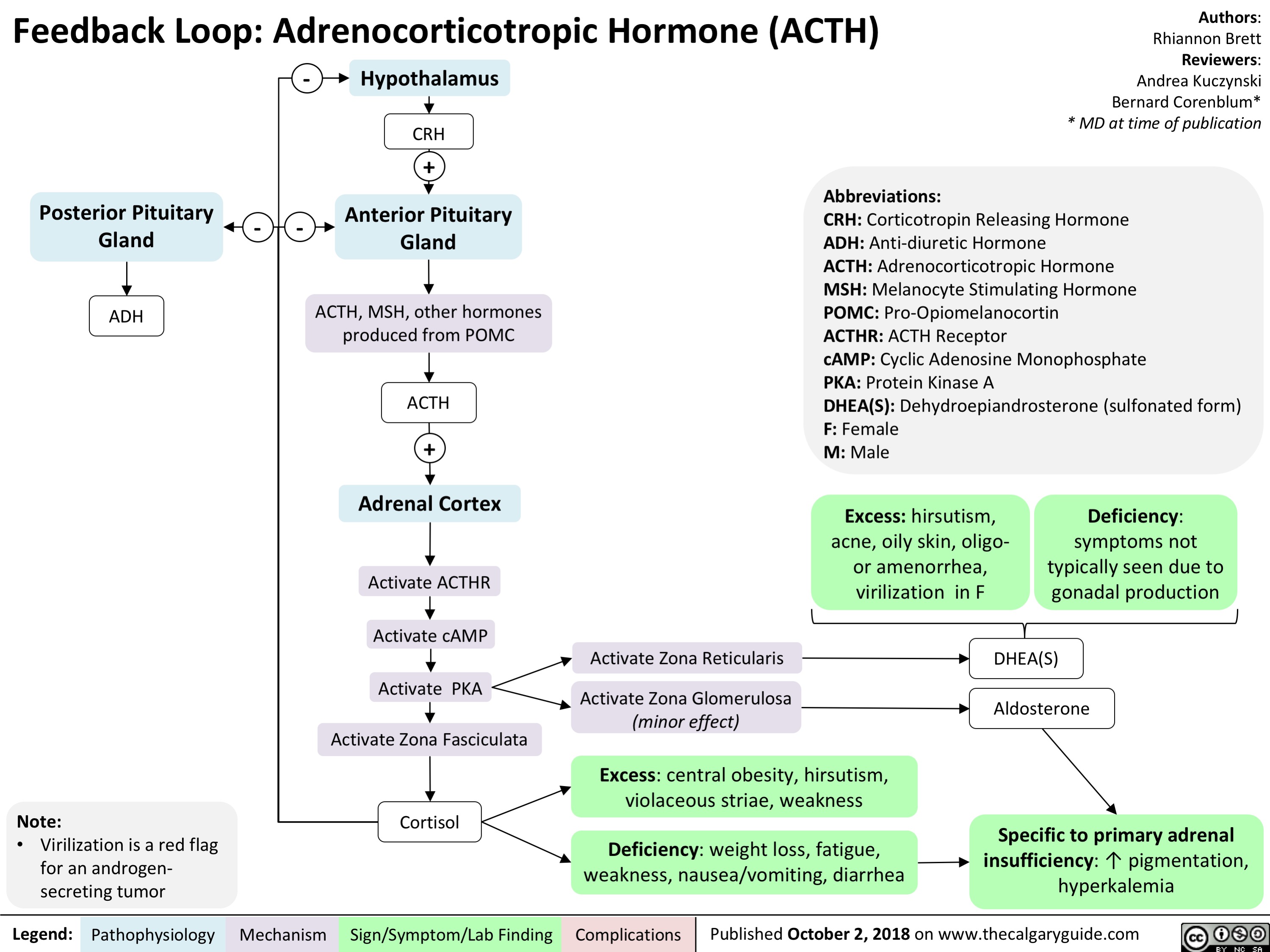
Feedback Loop: Adrenocorticotropic Hormone (ACTH)
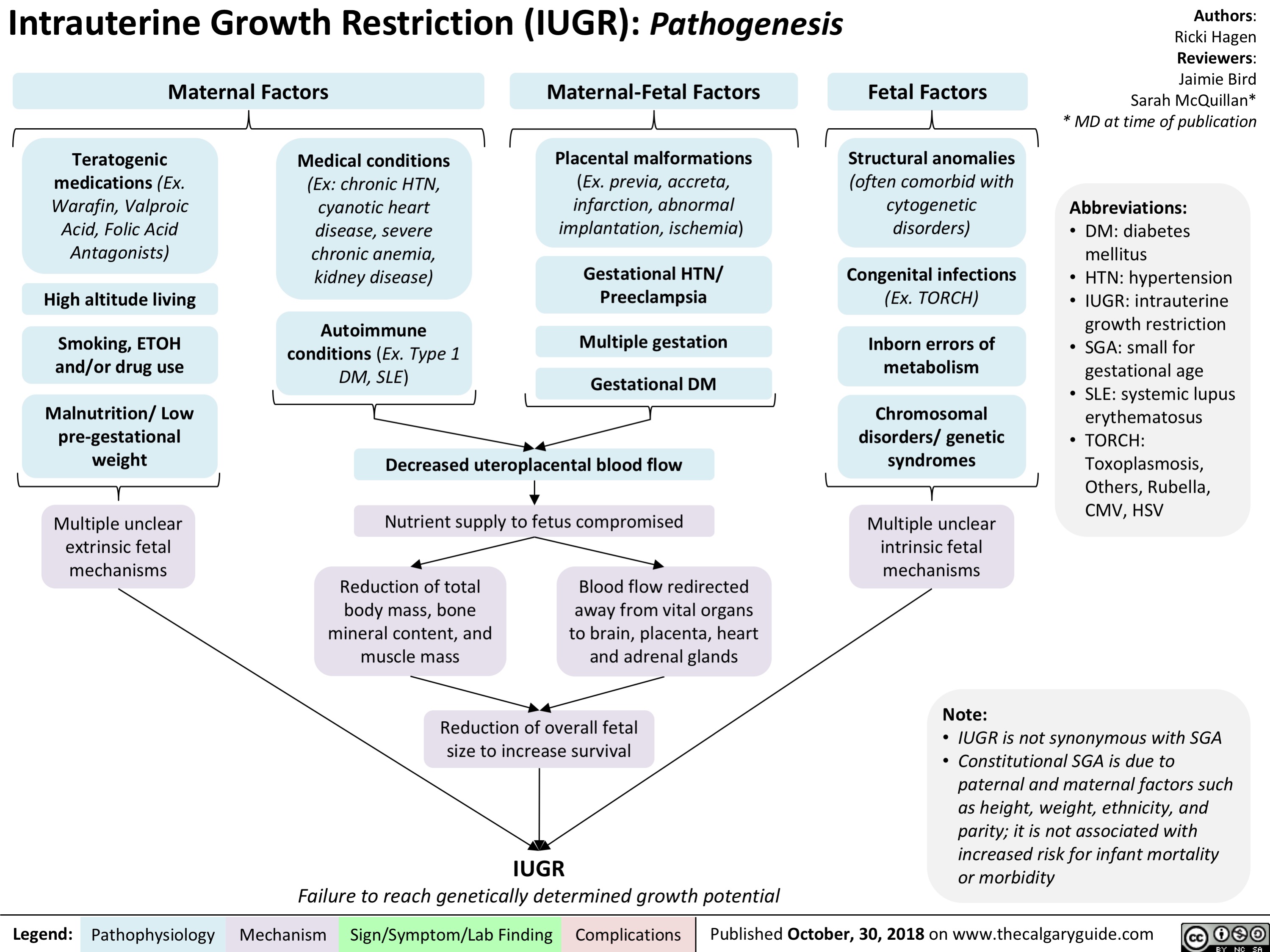
intrauterine-growth-restriction-iugr-pathogenesis
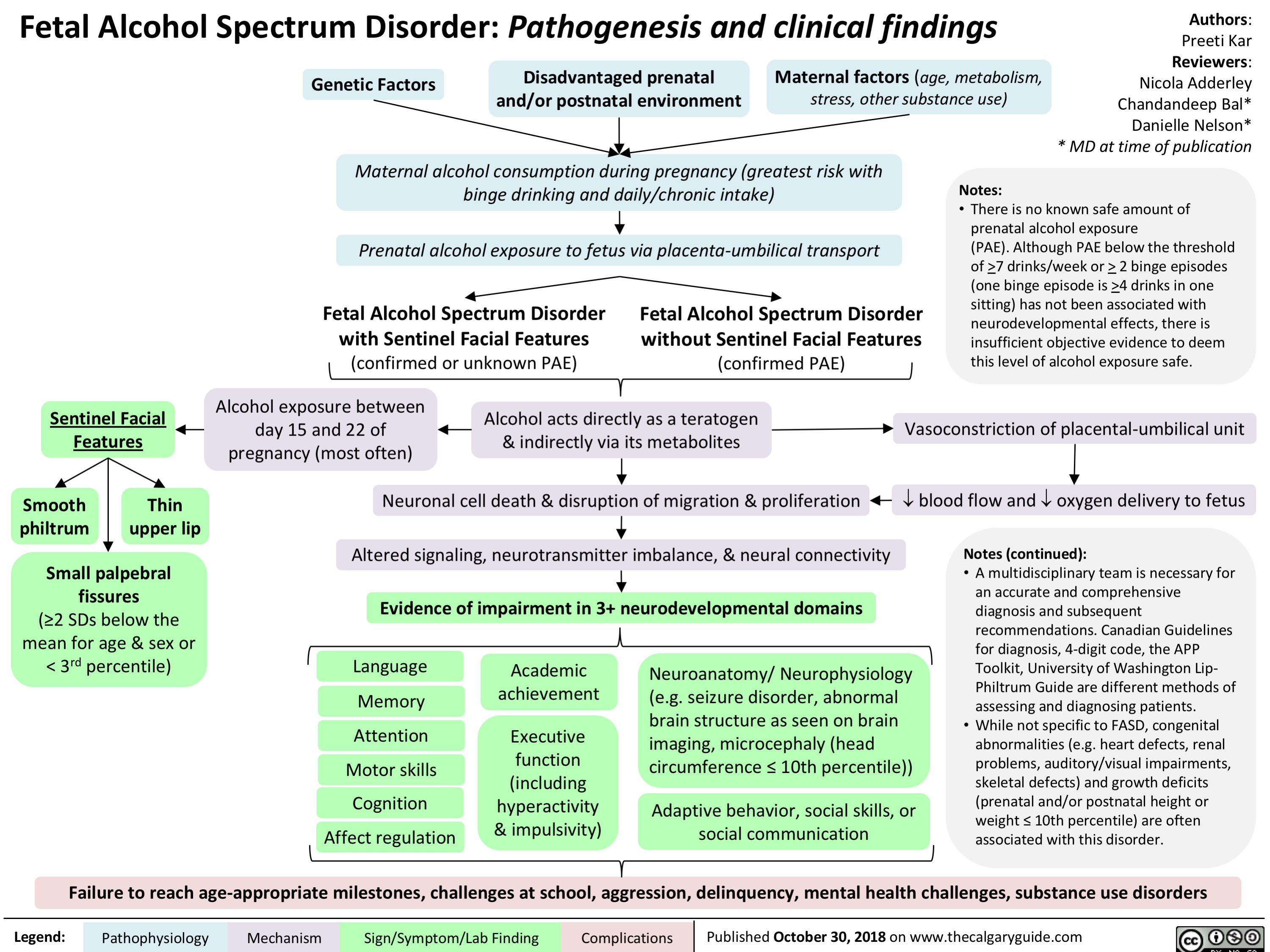
fetal-alcohol-spectrum-disorder-pathogenesis-and-clinical-findings
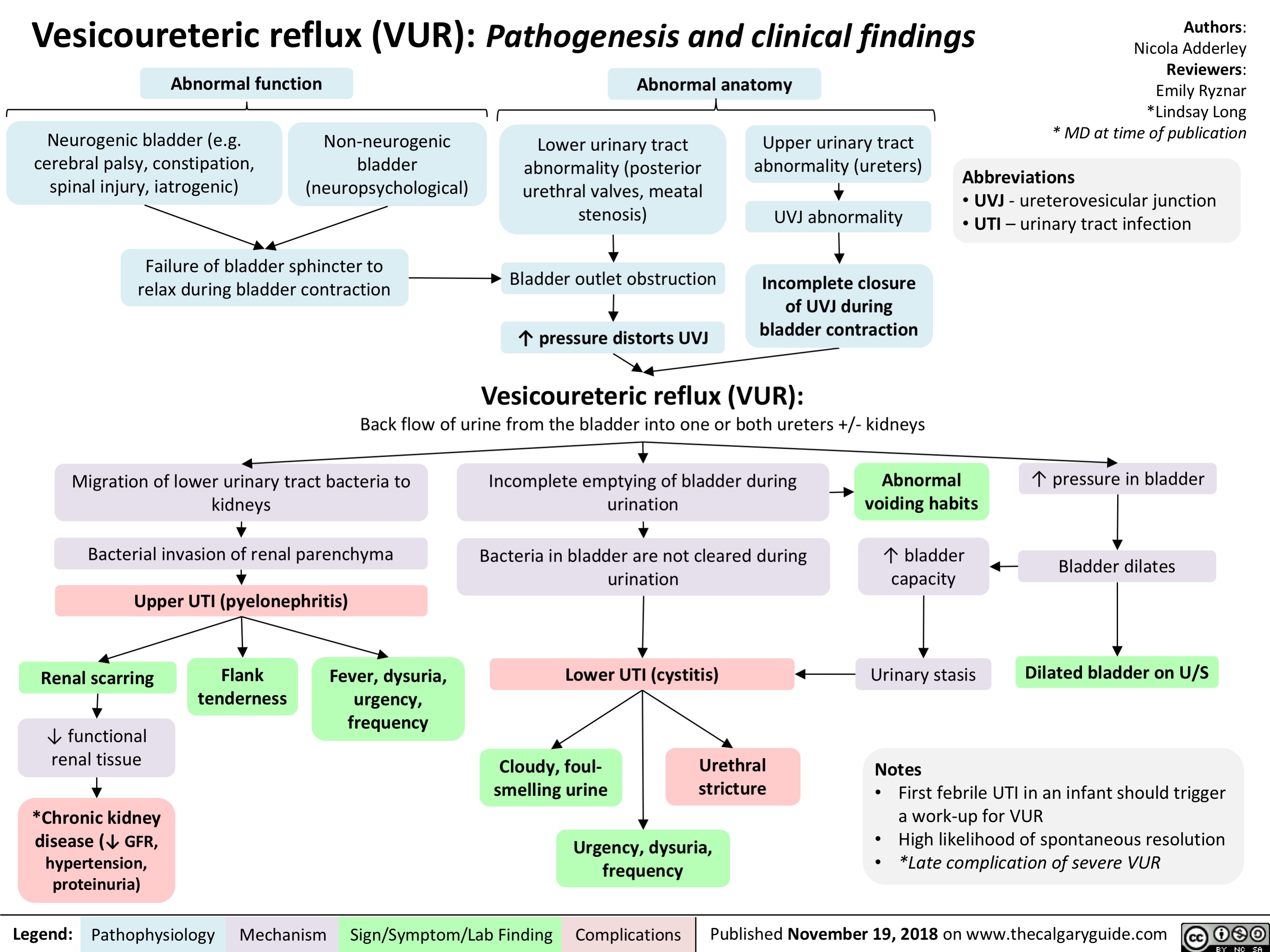
Vesicoureteric reflux (VUR): Pathogenesis and clinical findings
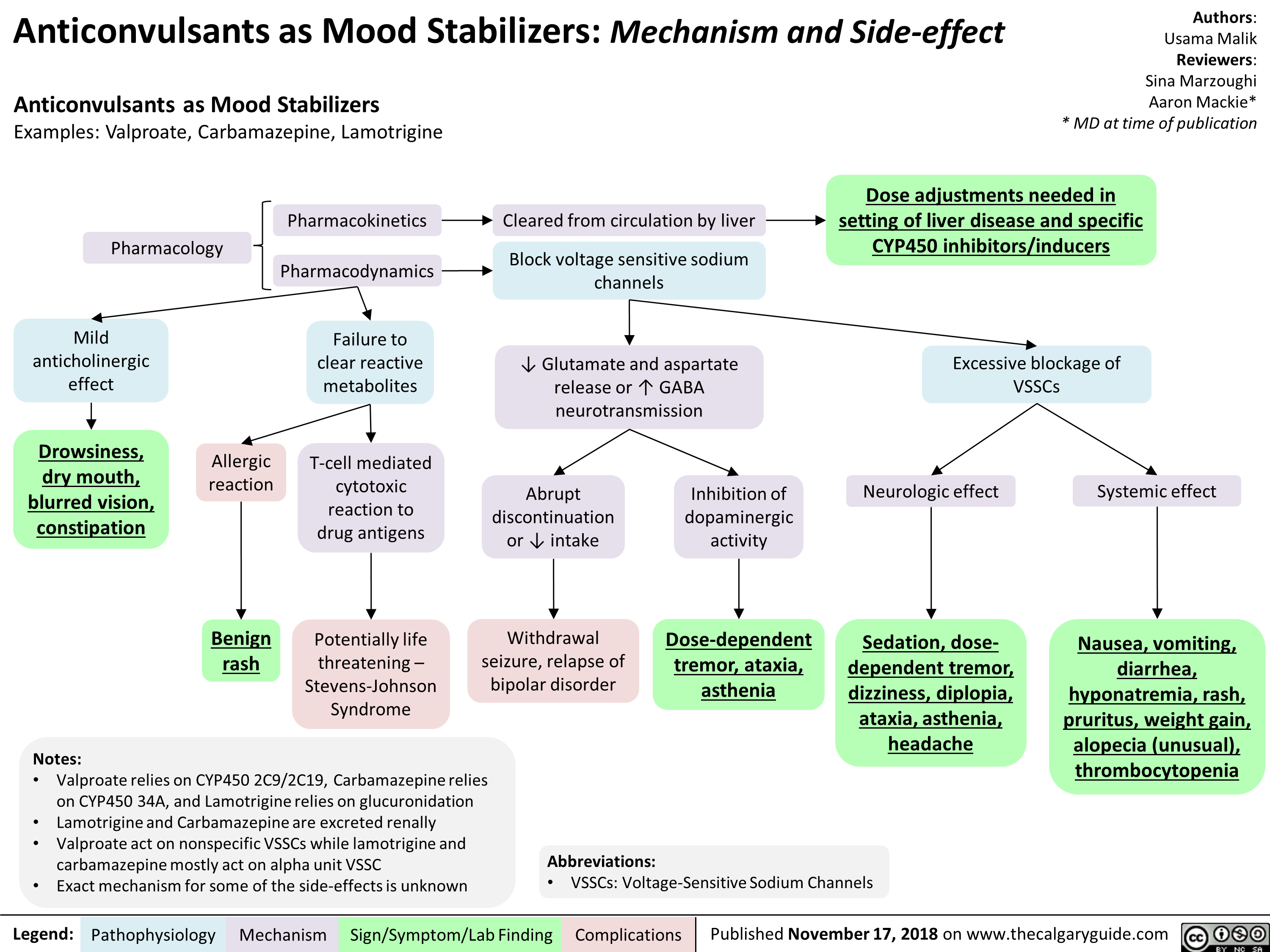
Anticonvulsants as Mood Stabilizers: Mechanism and Side-effect
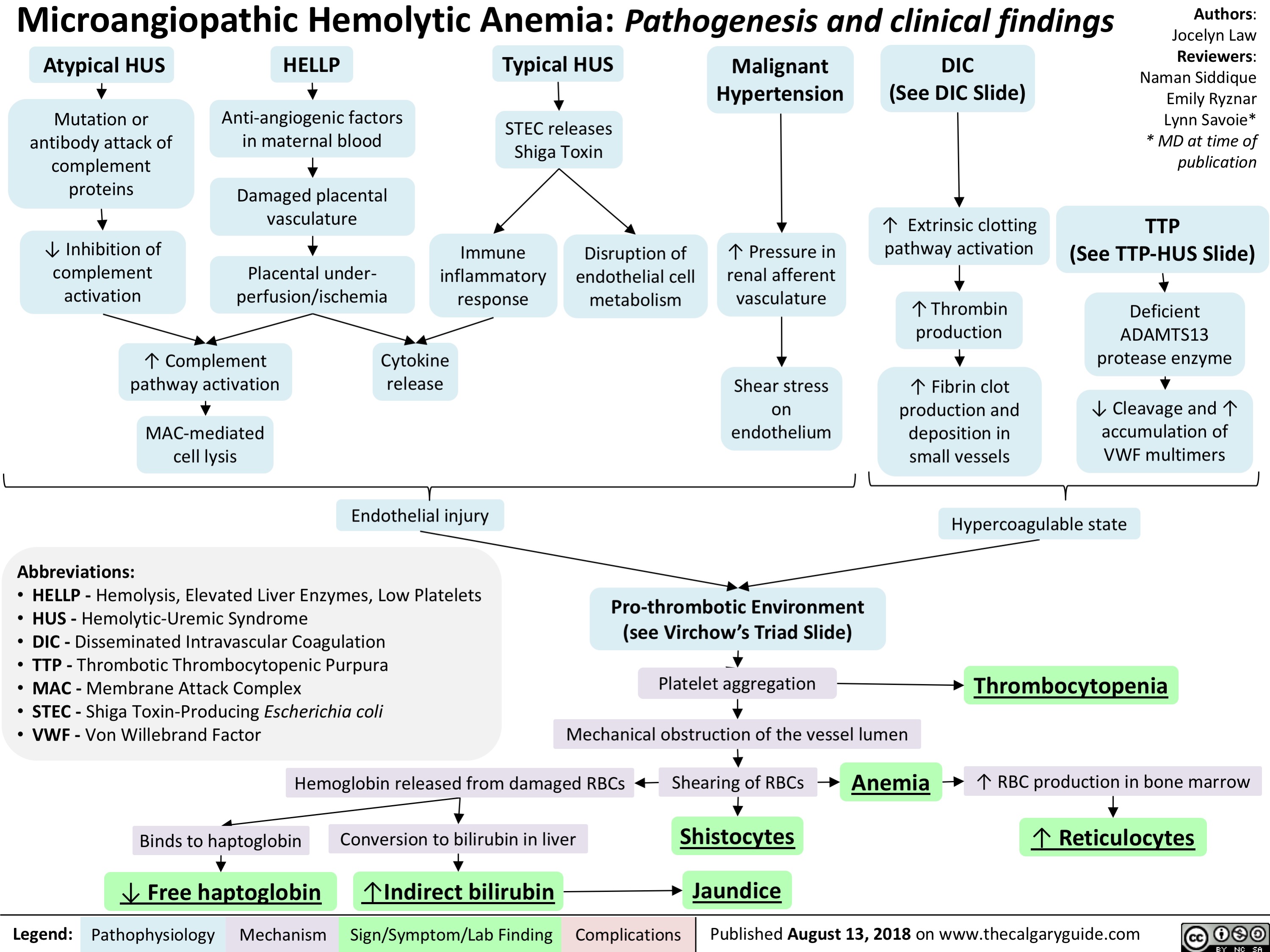
Microangiopathic Hemolytic Anemia: Pathogenesis and clinical findings
Hypernatremia Physiology
![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)
Hyponatremia- Physiology
![Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hyponatremia-Physiology-.jpg)
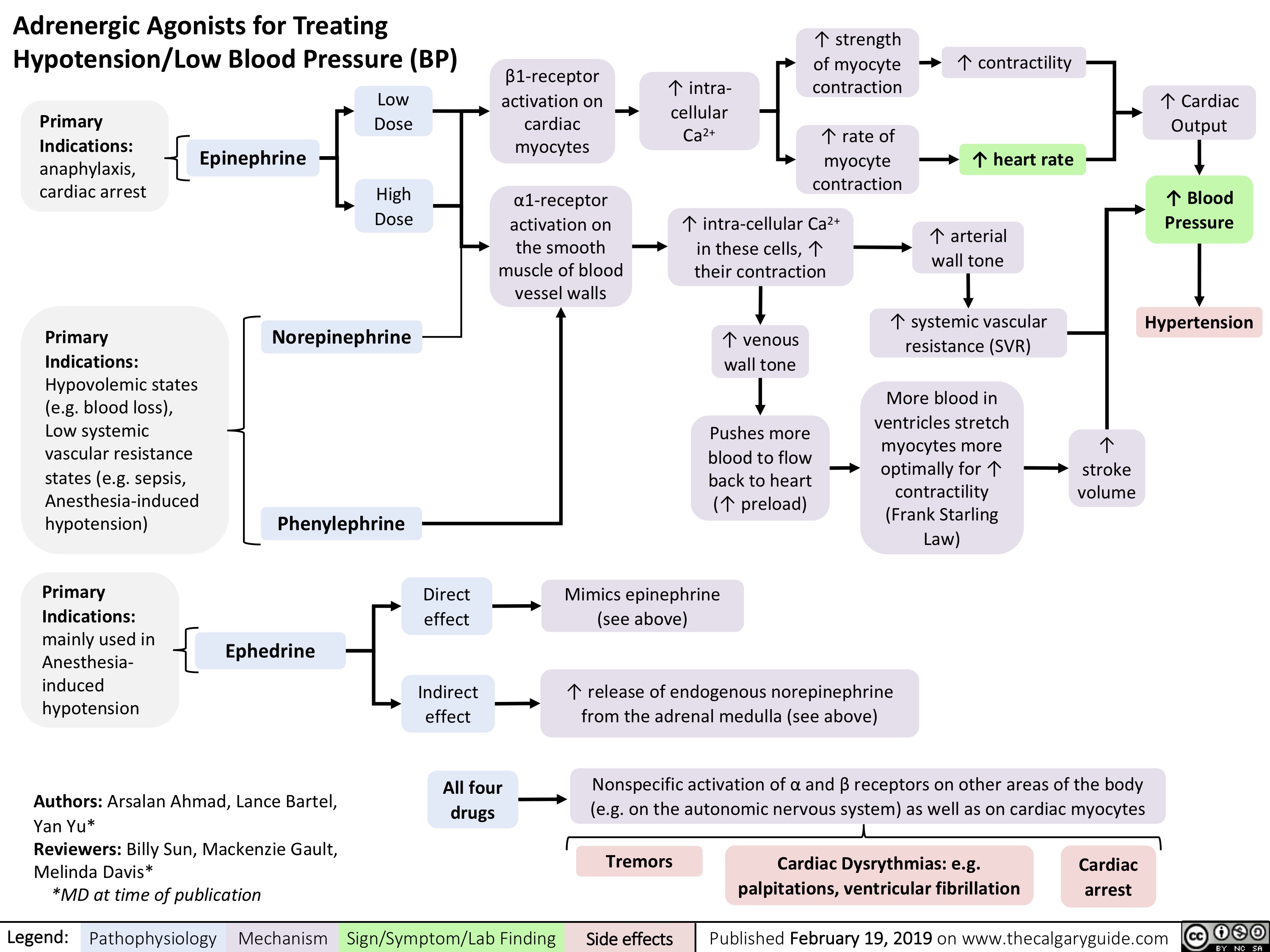
adrenergic-agonists-for-treating-hypotensionlow-blood-pressure
Hyperkalemia- Physiology
![Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hyperkalemia-Physiology-.jpg)
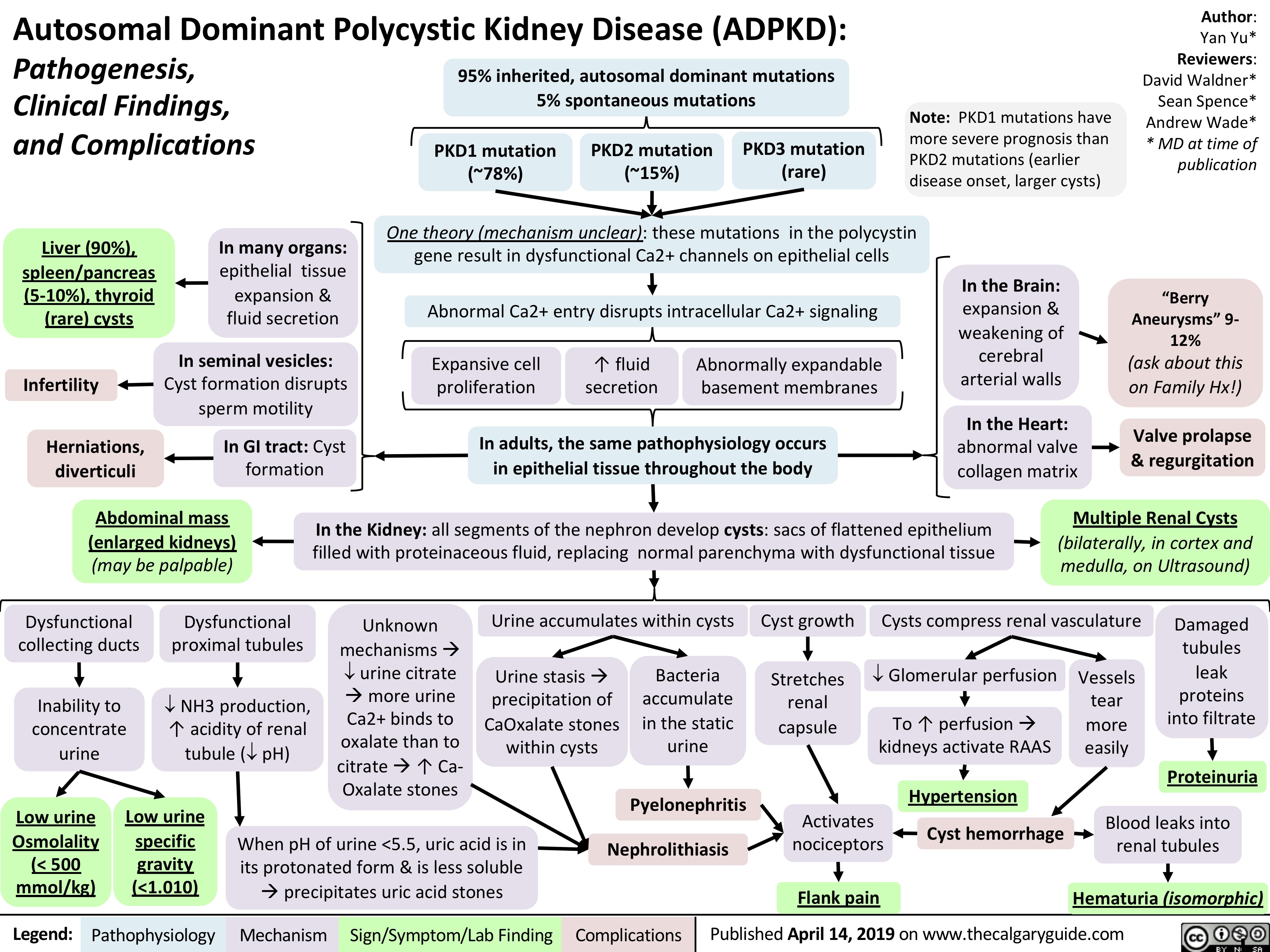
autosomal-dominant-polycystic-kidney-disease-adpkd
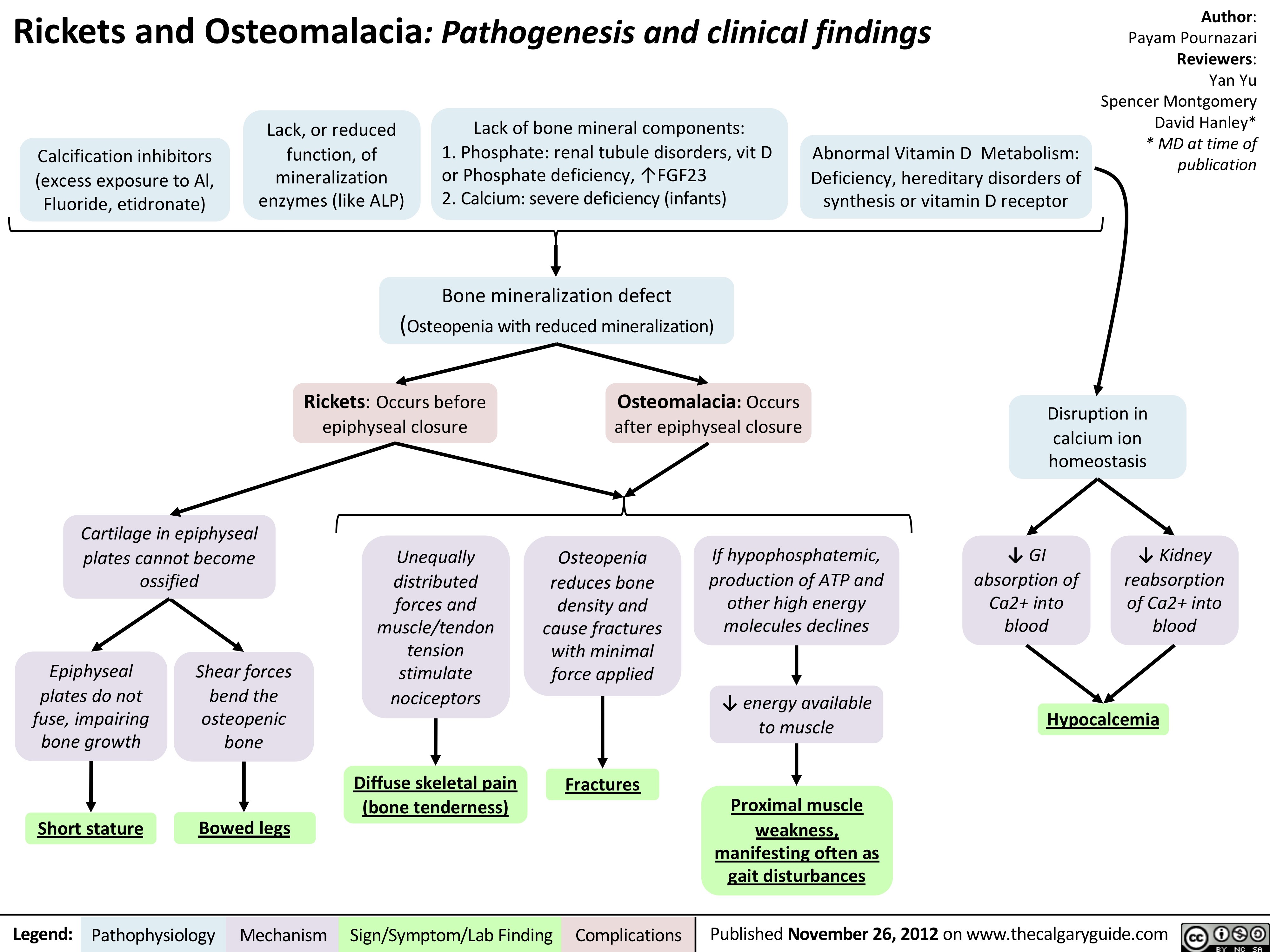
Rickets and Osteomalacia: Pathogenesis and Clinical Findings
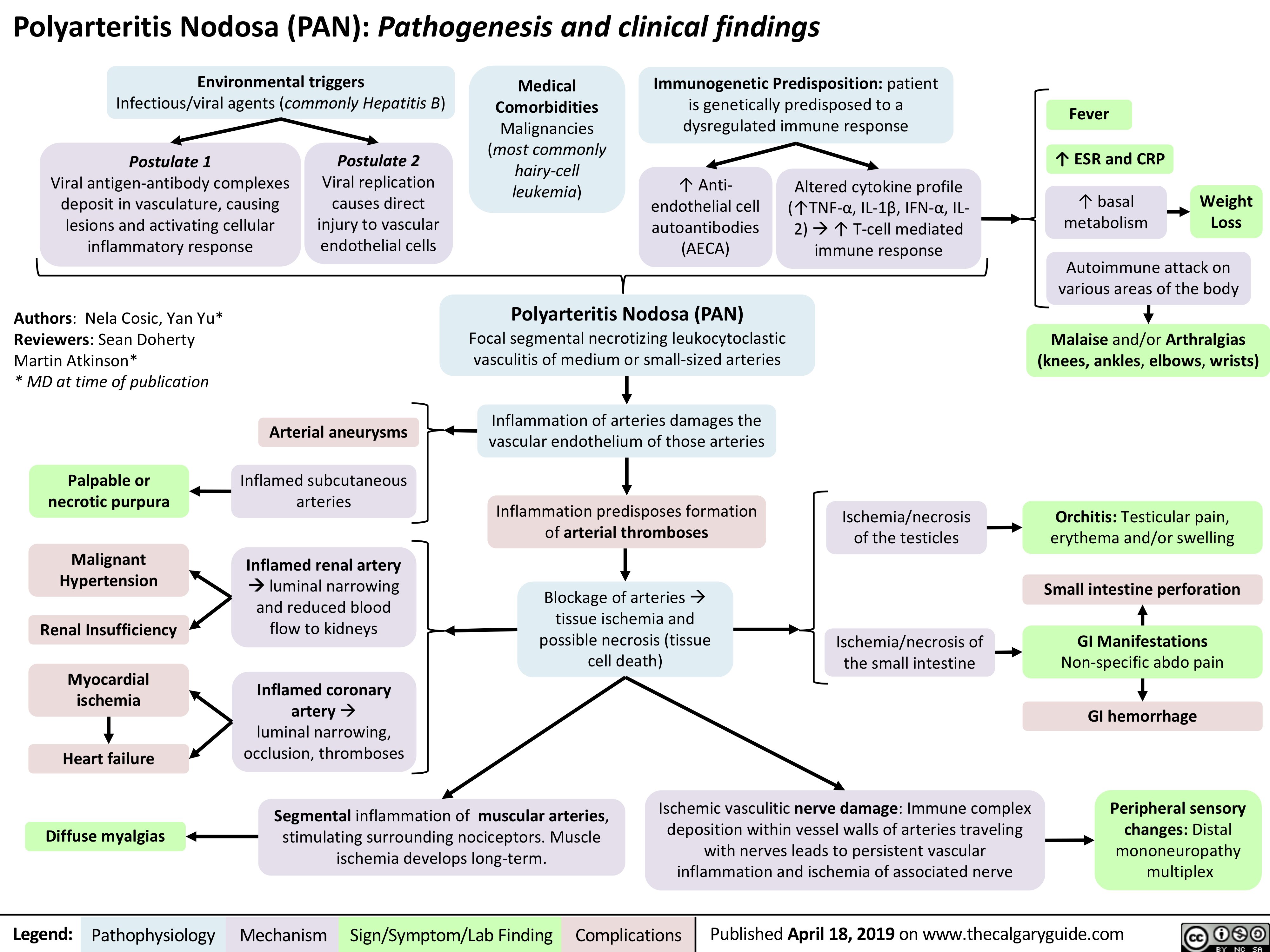
Polyarteritis Nodosa (PAN): Pathogenesis and Clinical Findings
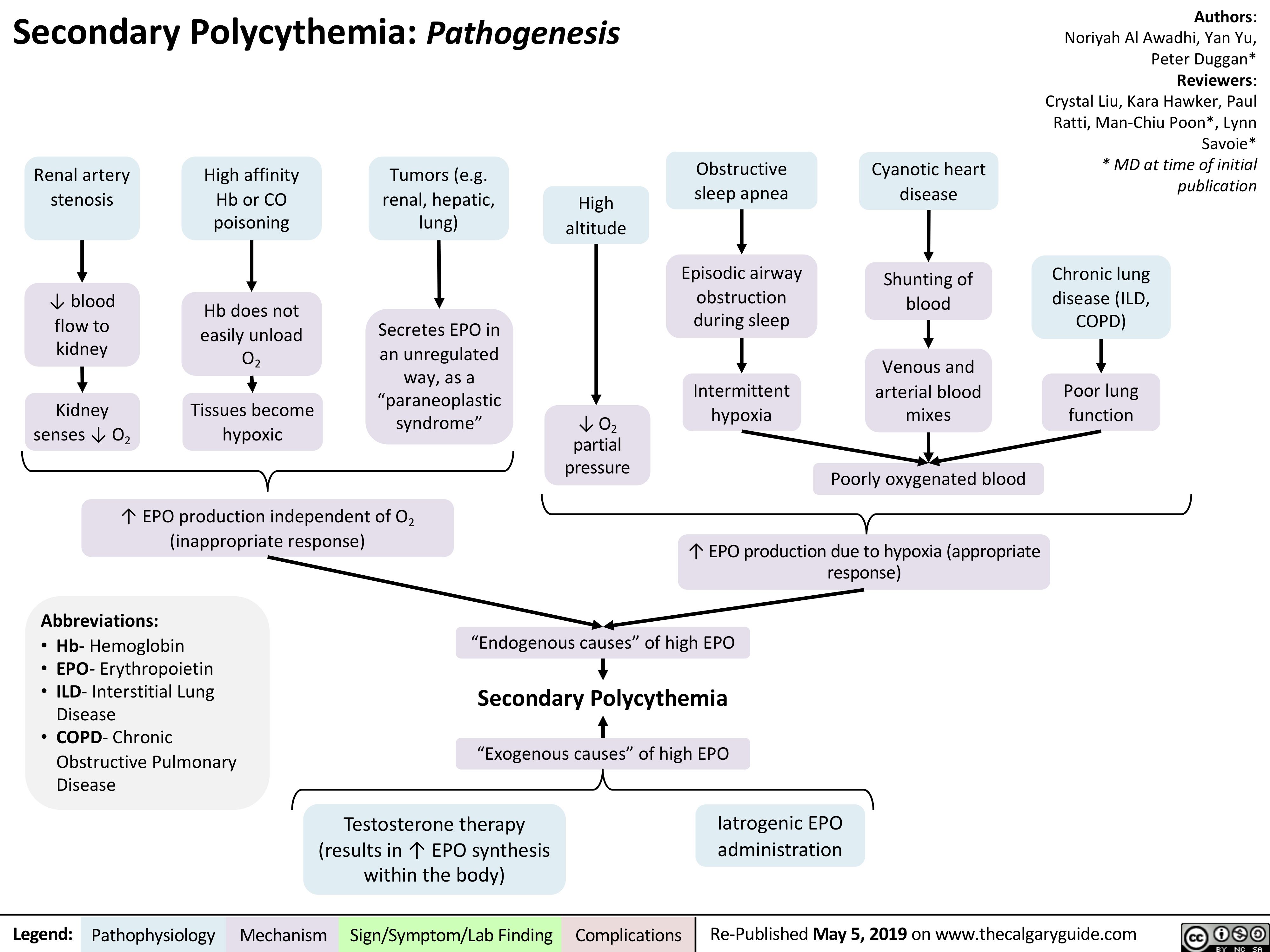
Secondary Polycythemia
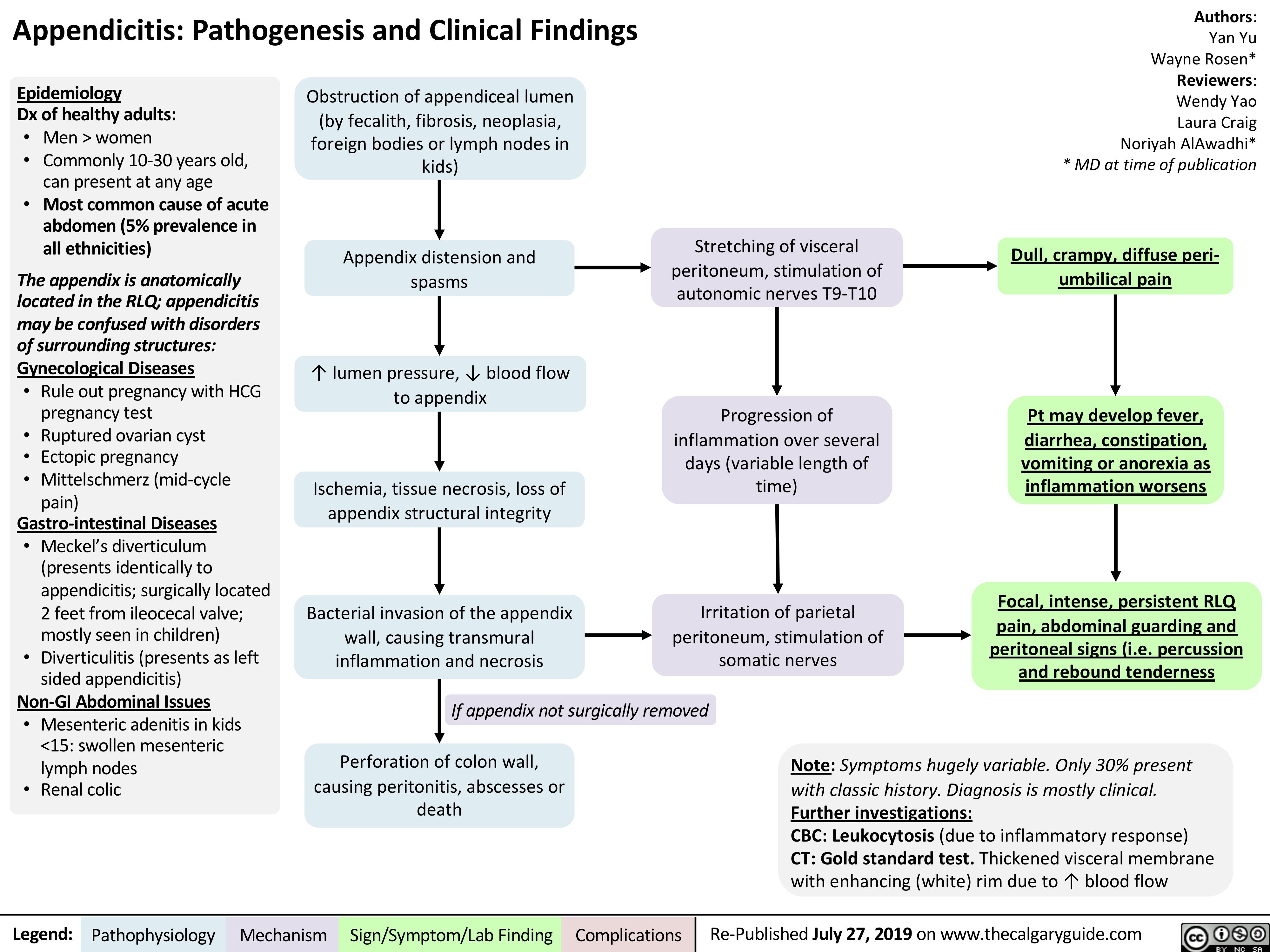
Appendicitis
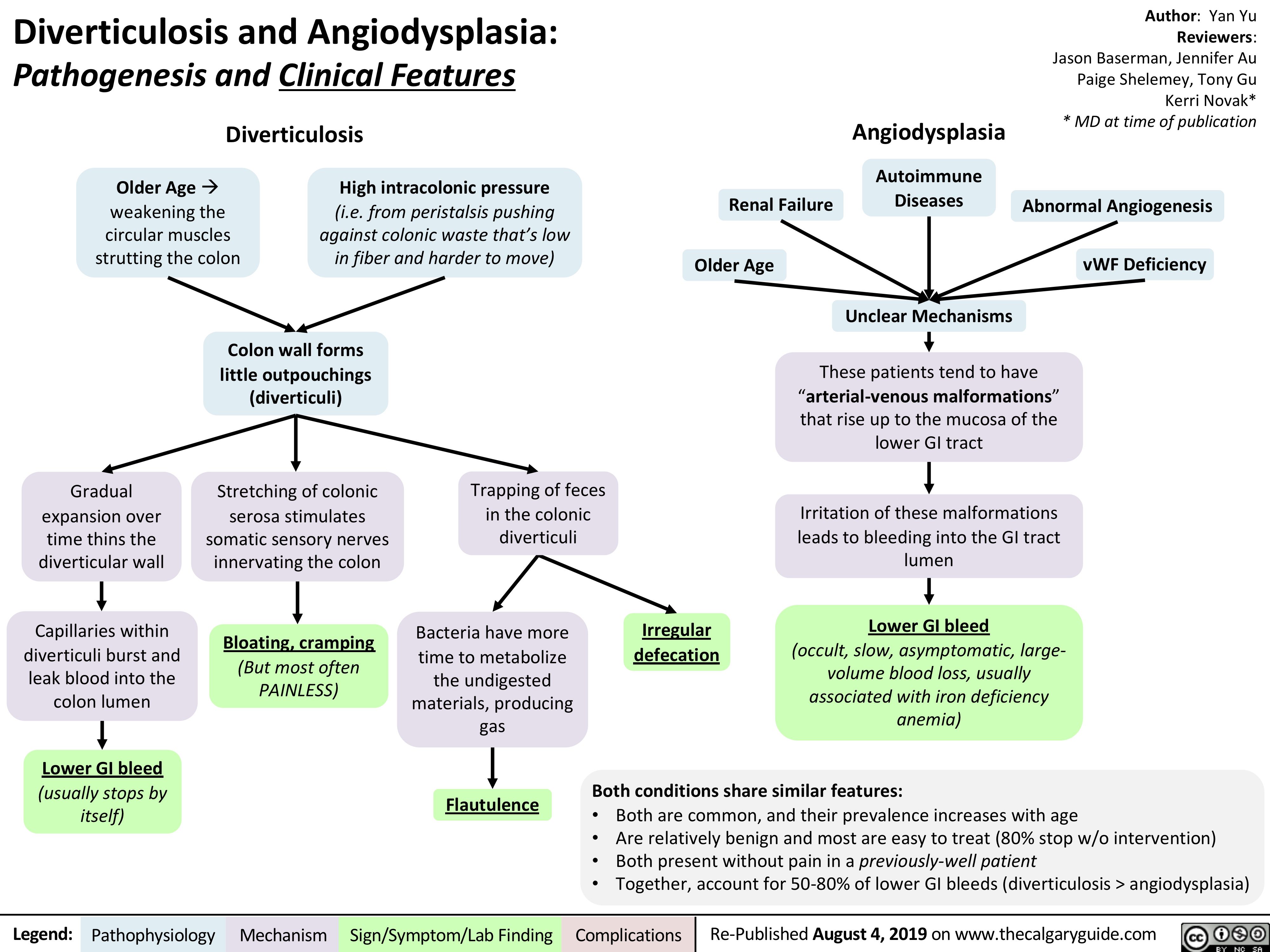
Diverticulosis and Angiodysplasia
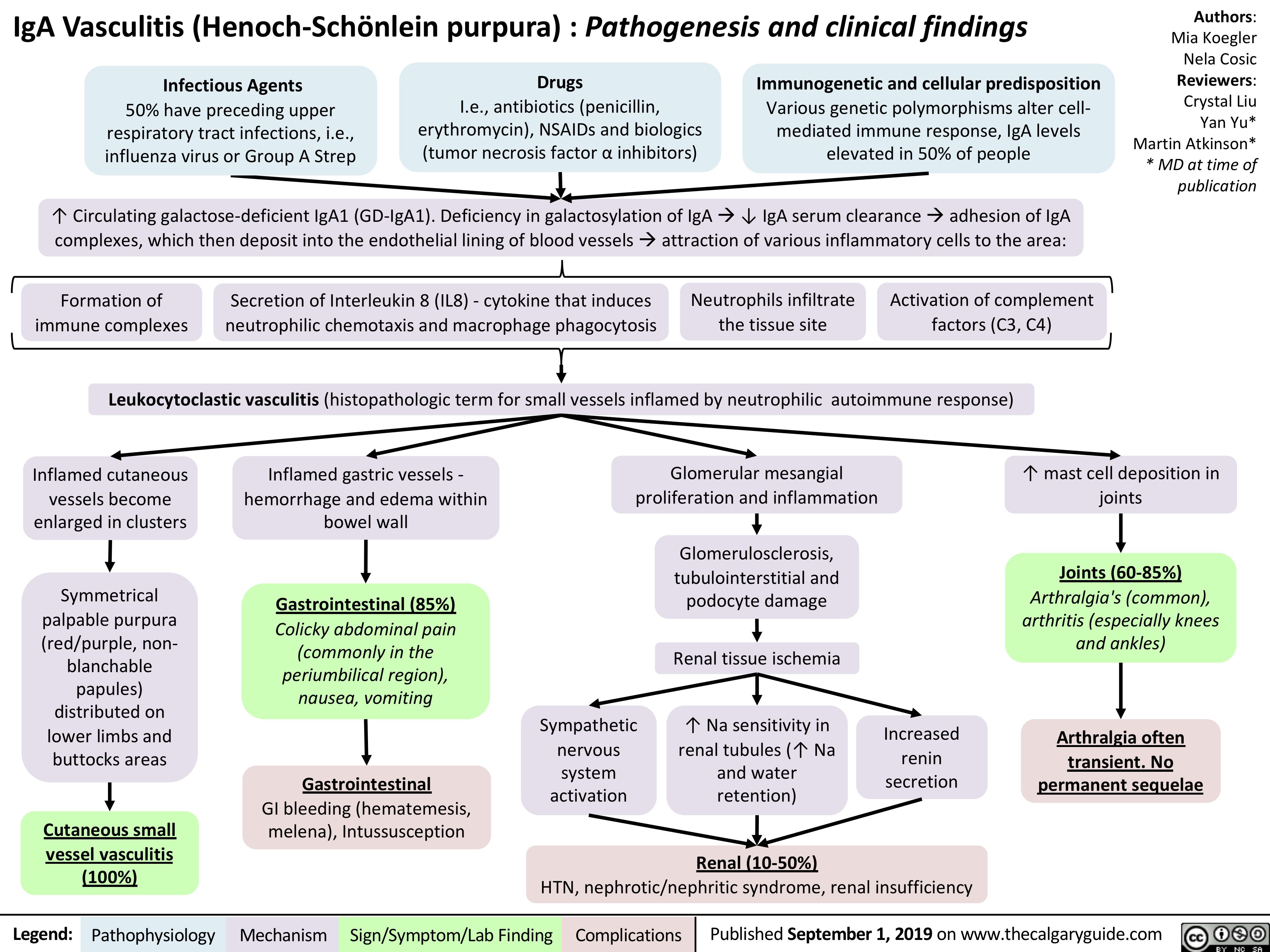
iga-vasculitis-henoch-scholein-purpura-pathogenesis-and-clinical-findings
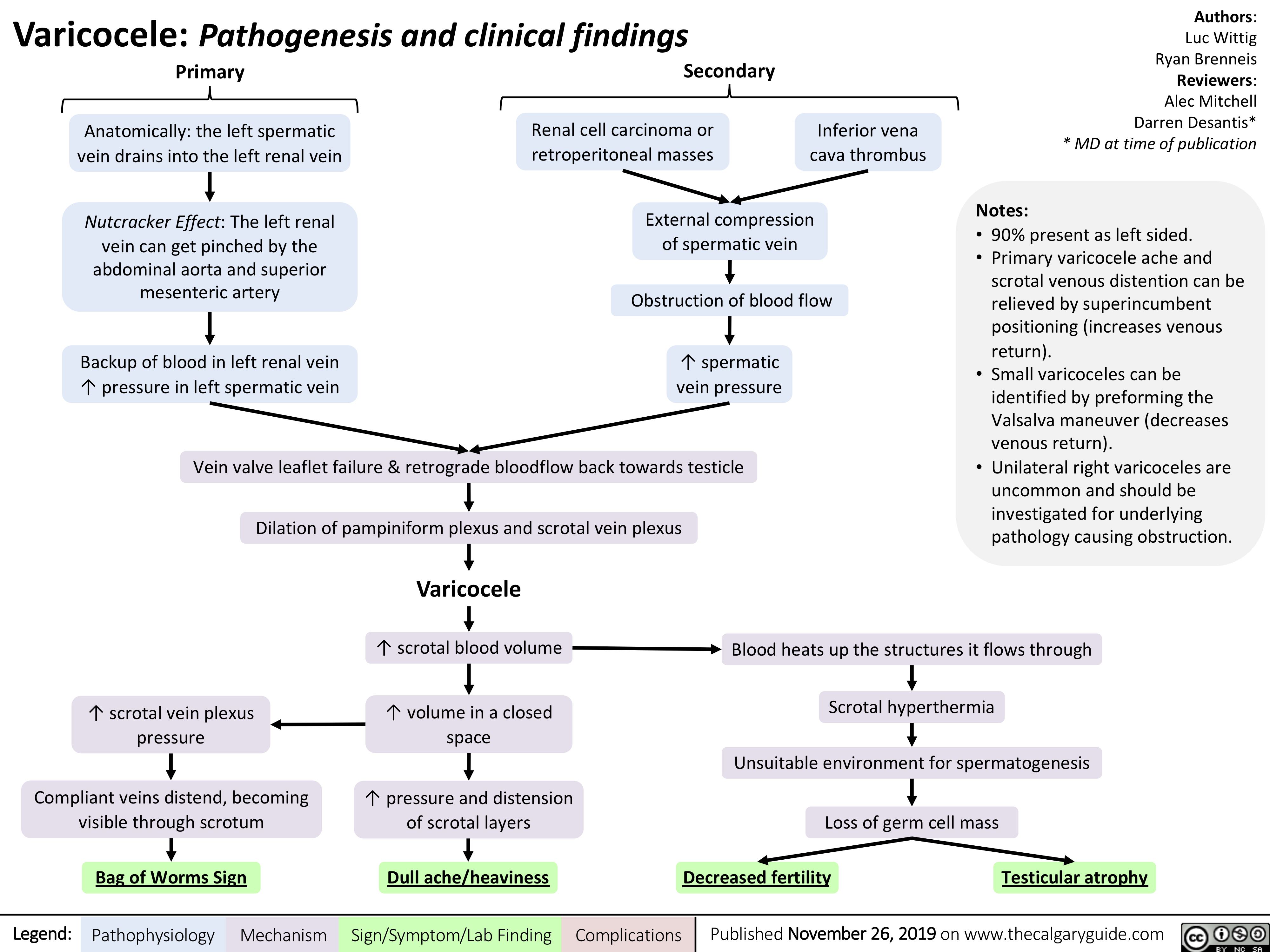
Varicocele
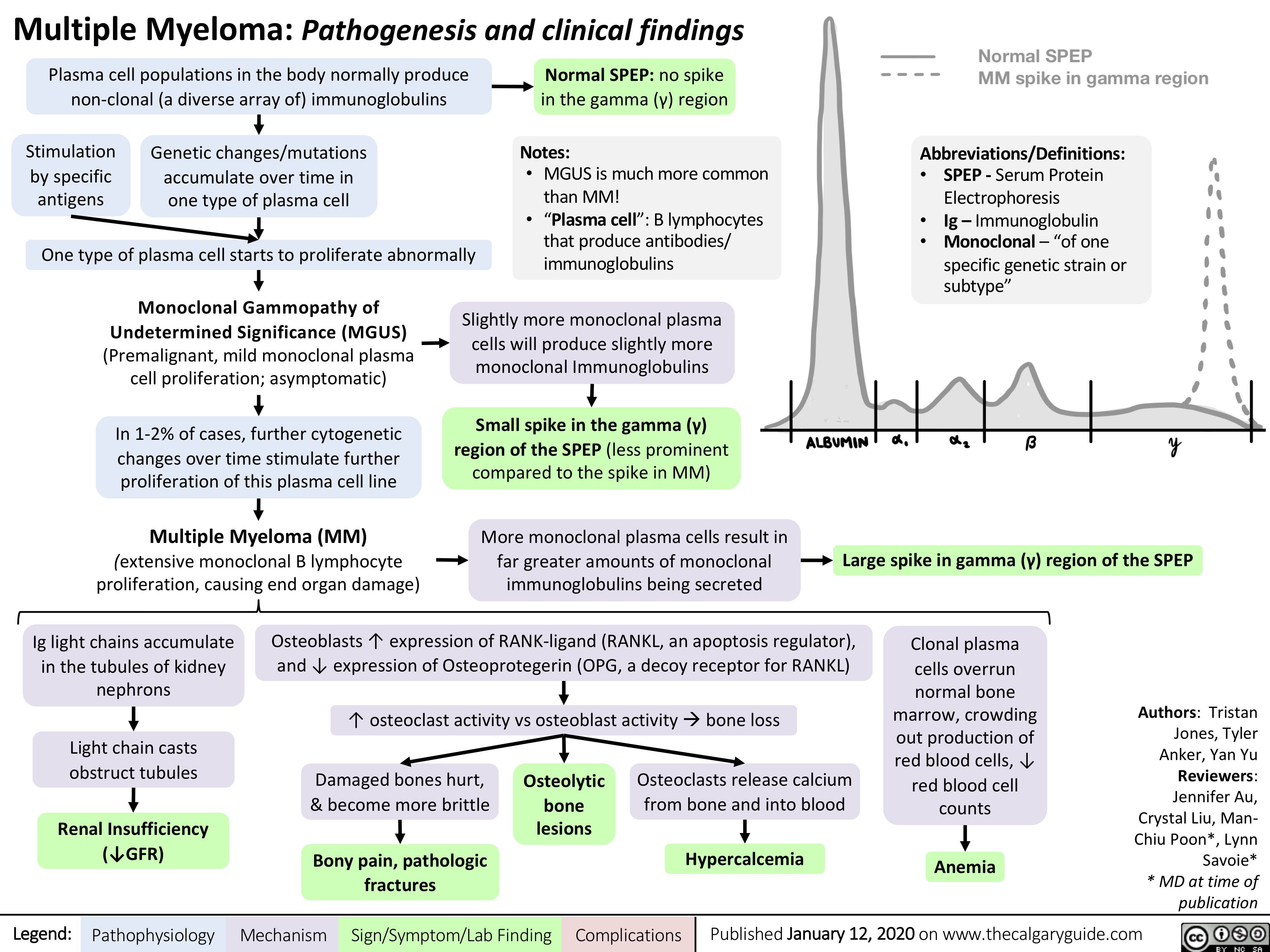
Multiple-Myeloma
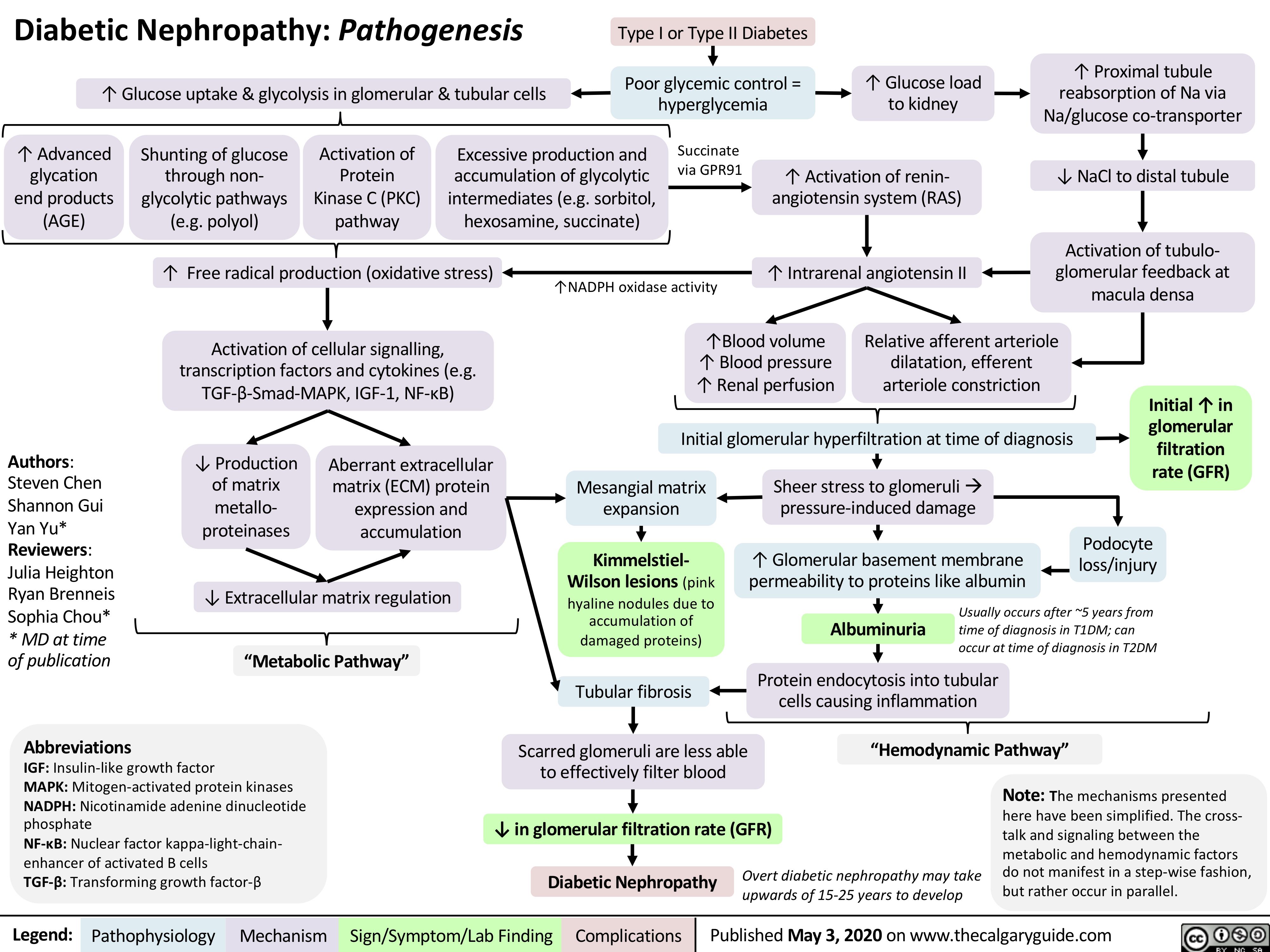
Diabetic-Nephropathy
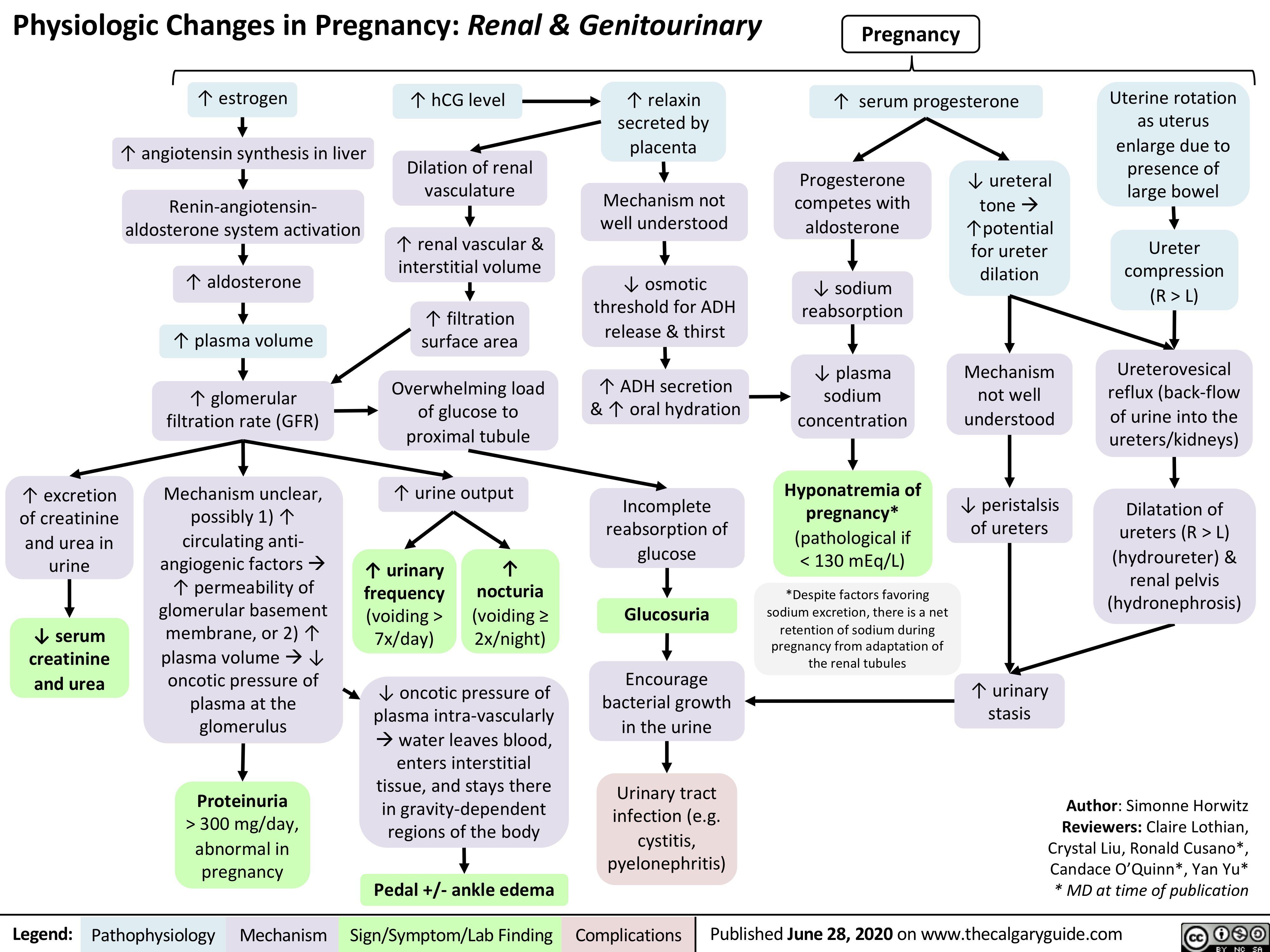
GU-changes-in-pregnancy
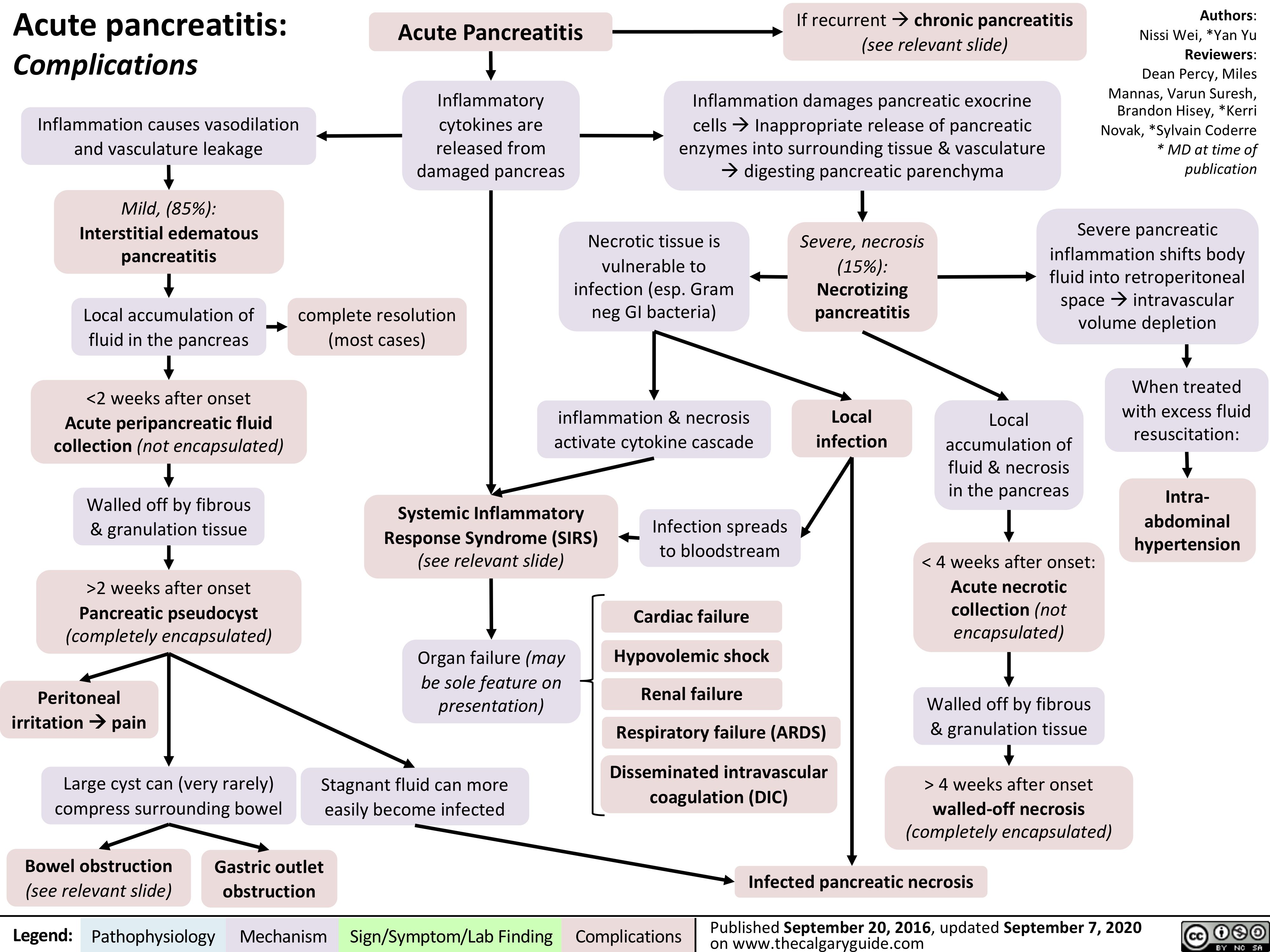
acute-pancreatitis-complications
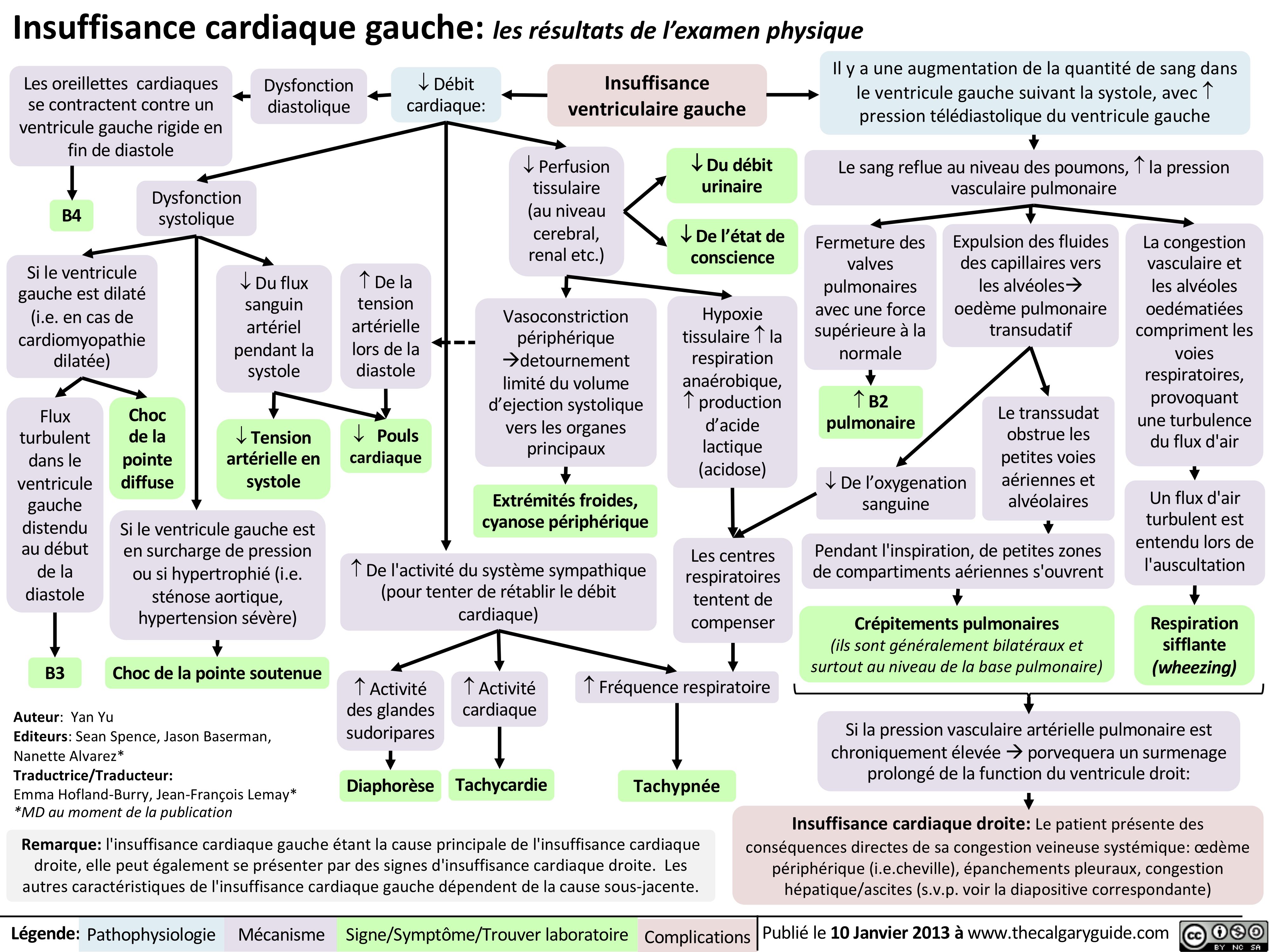
insuffisance-cardiaque-gauche-les-resultats-de-lexamen-physique
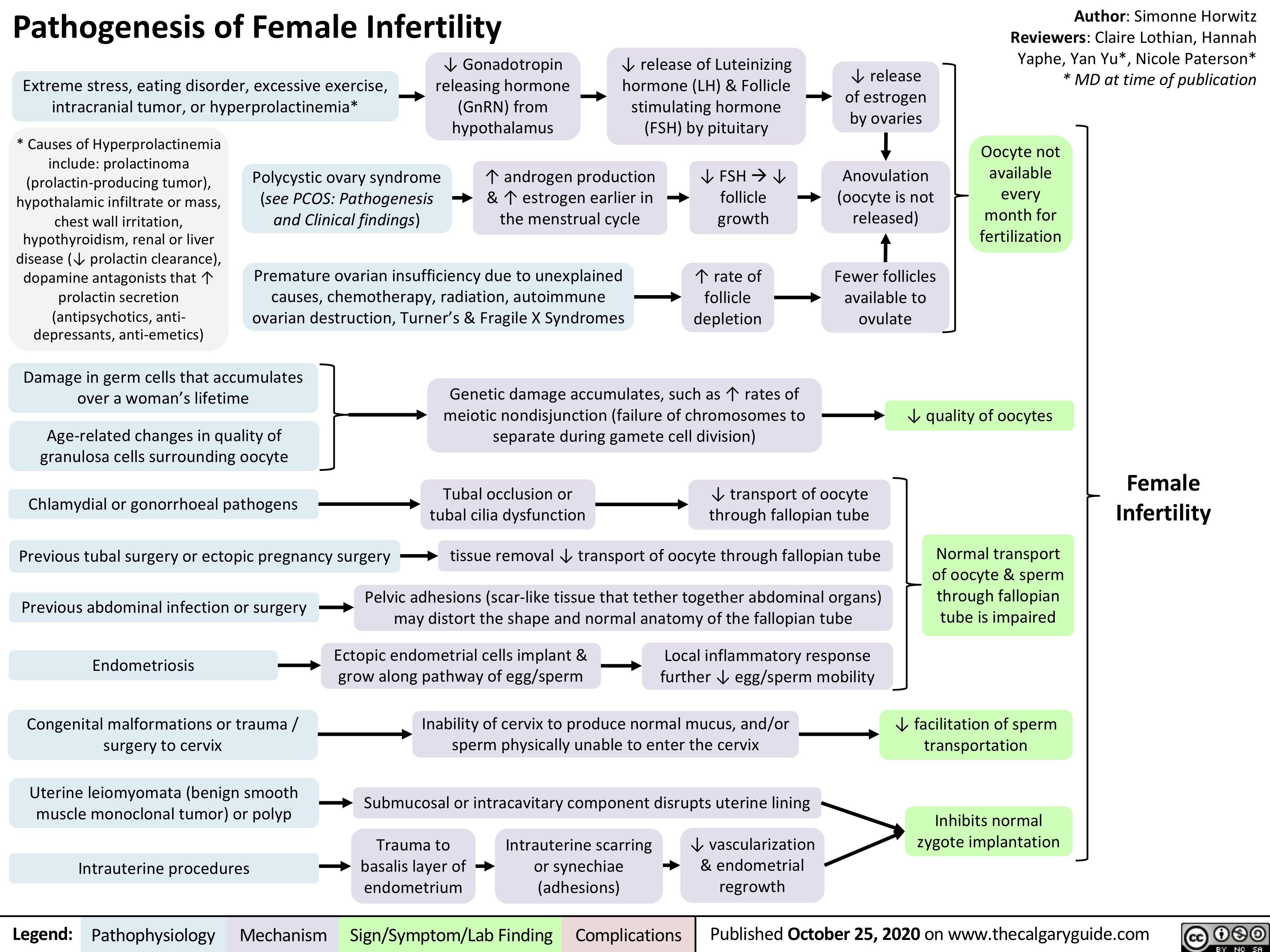
Pathogenesis-of-Female-Infertility
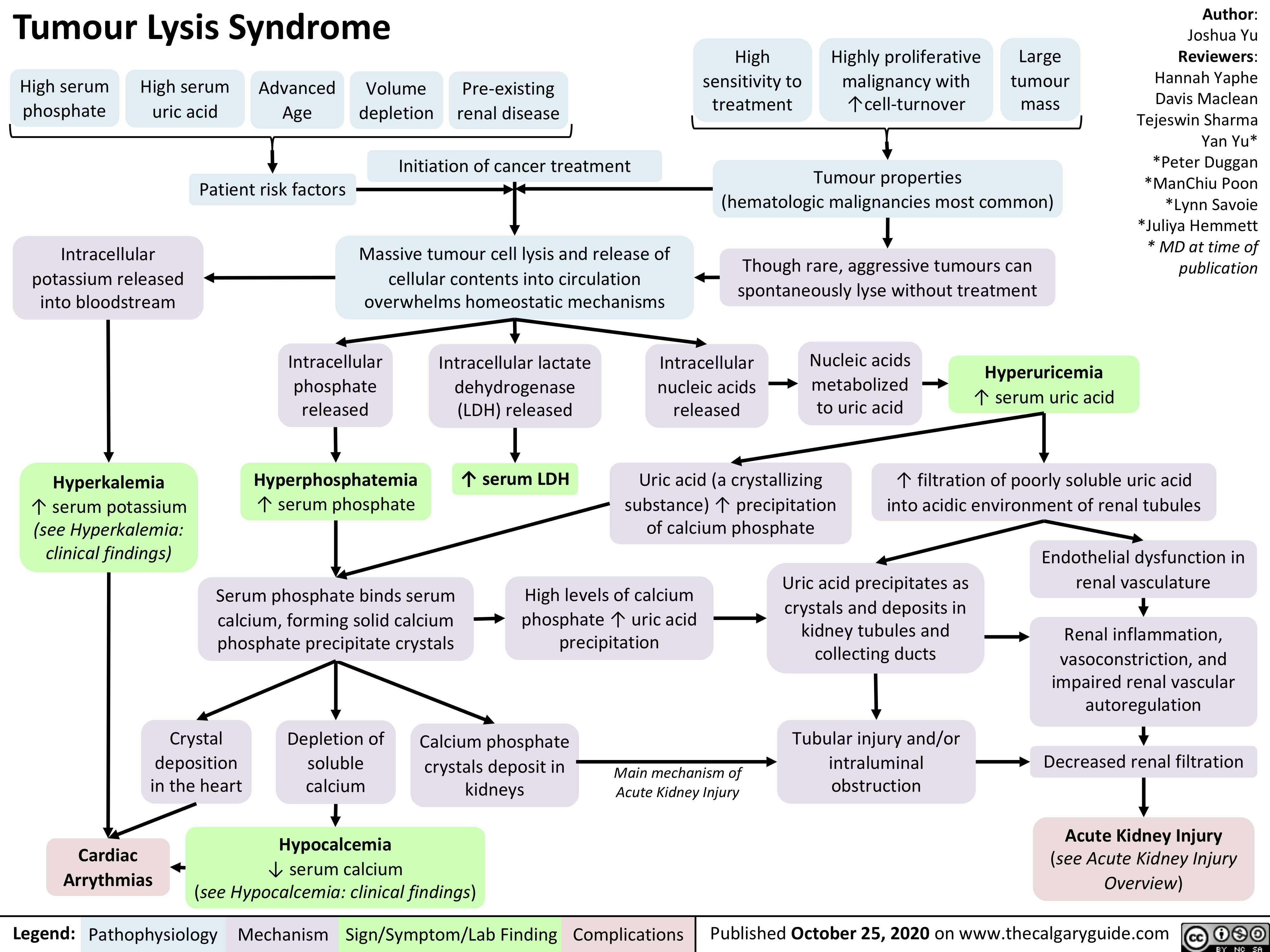
Tumour-Lysis-Syndrome
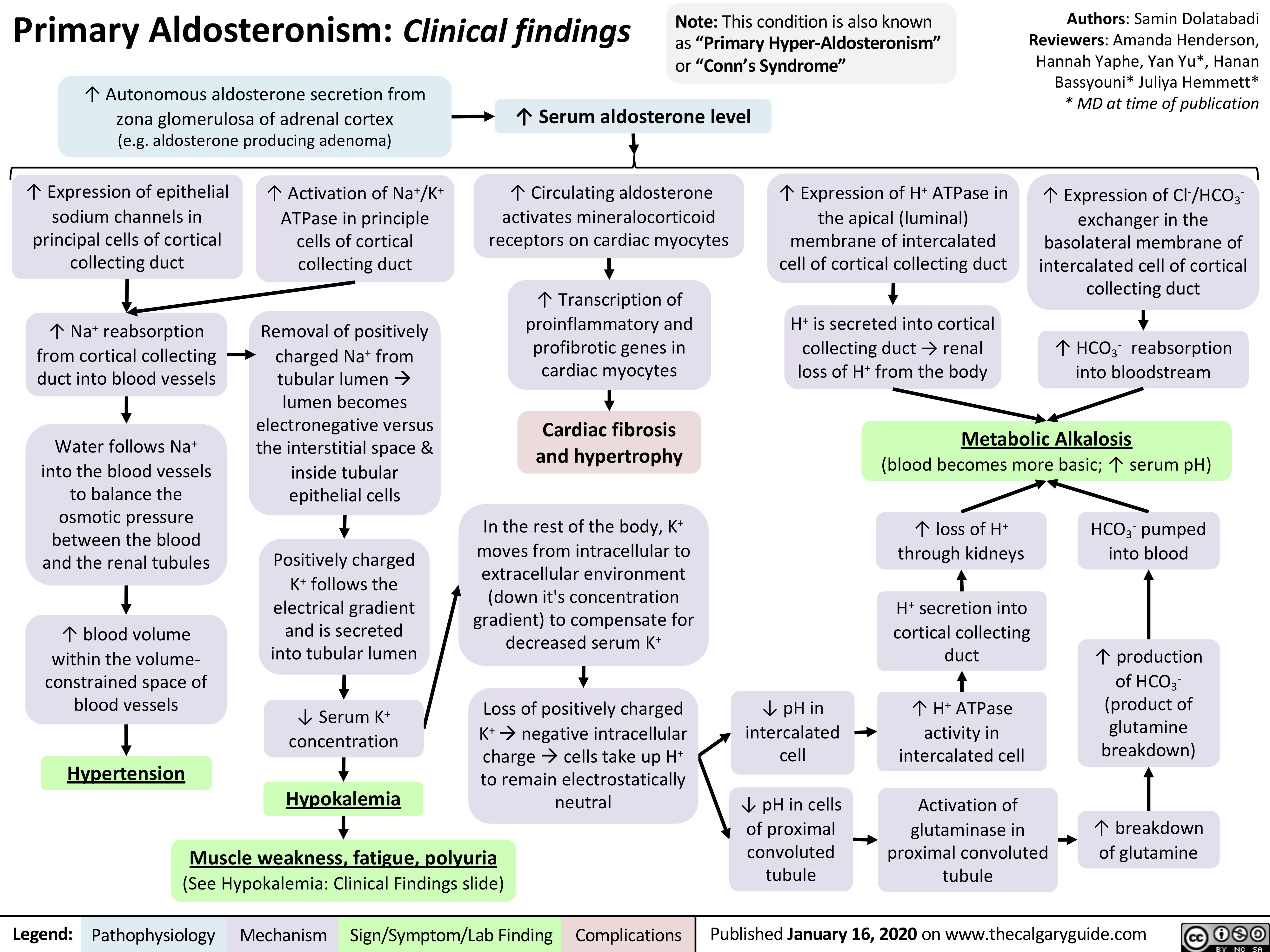
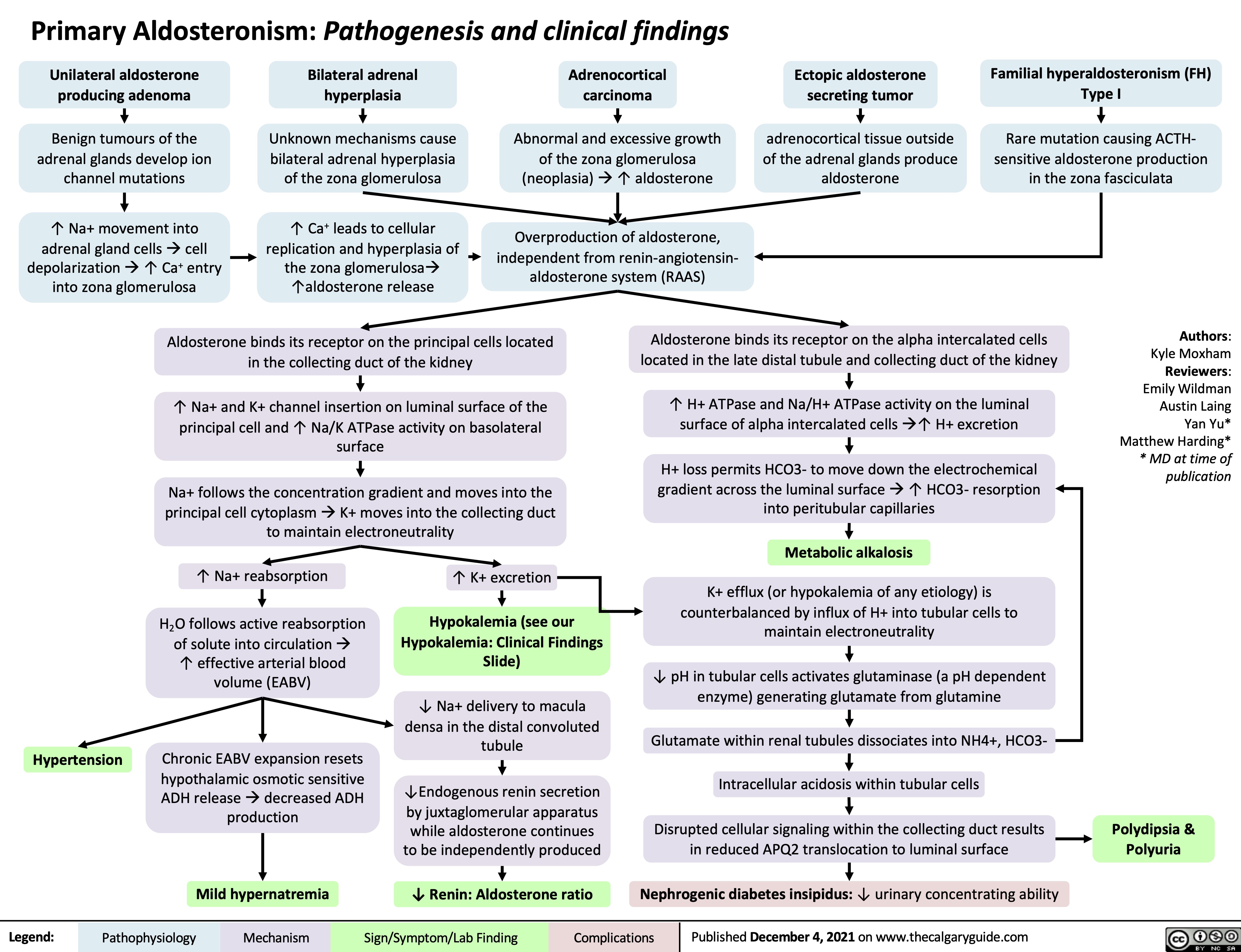
Primary-Aldosteronism
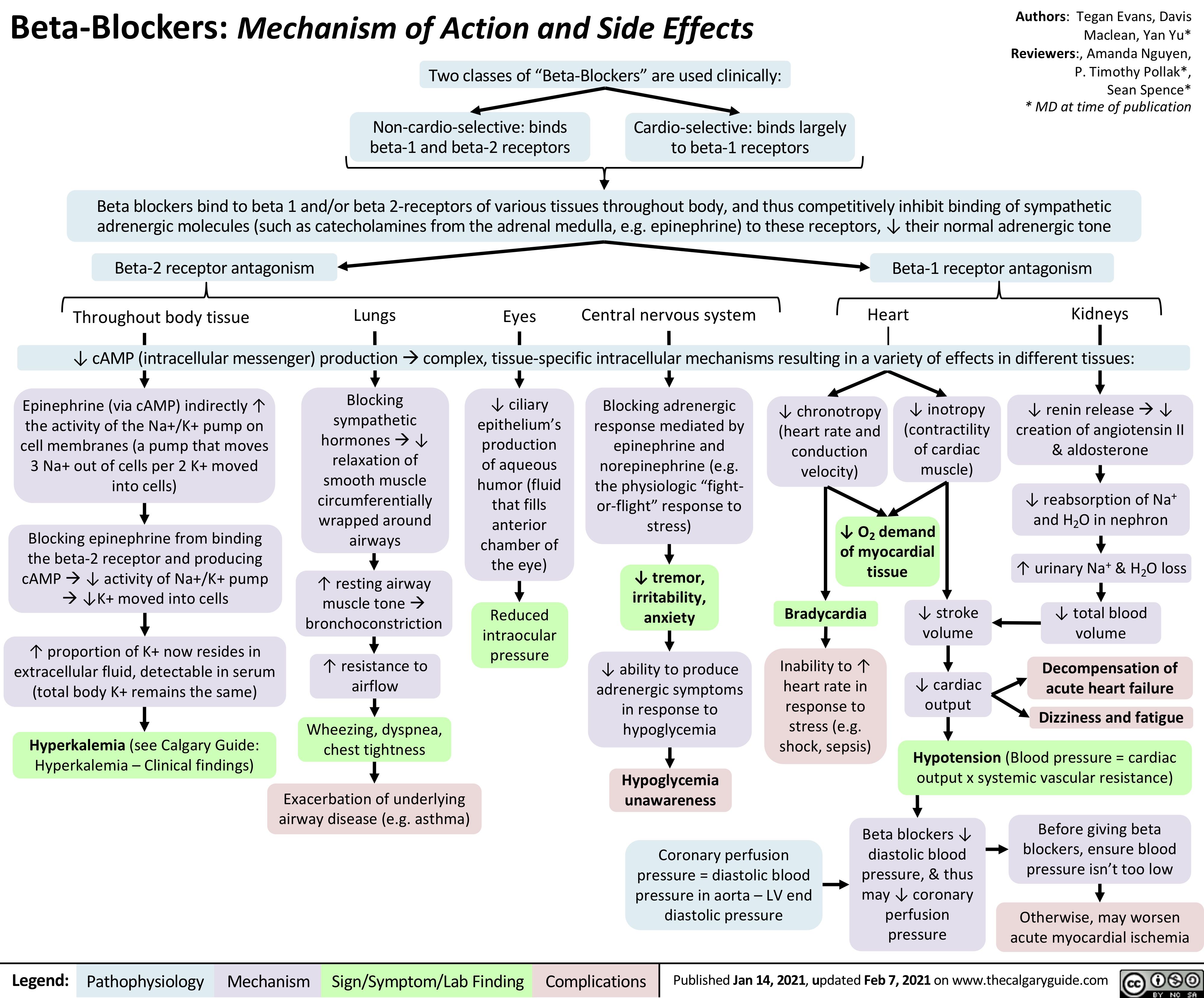
Beta-Blockers-Mechanism-of-Action-and-Side-Effects
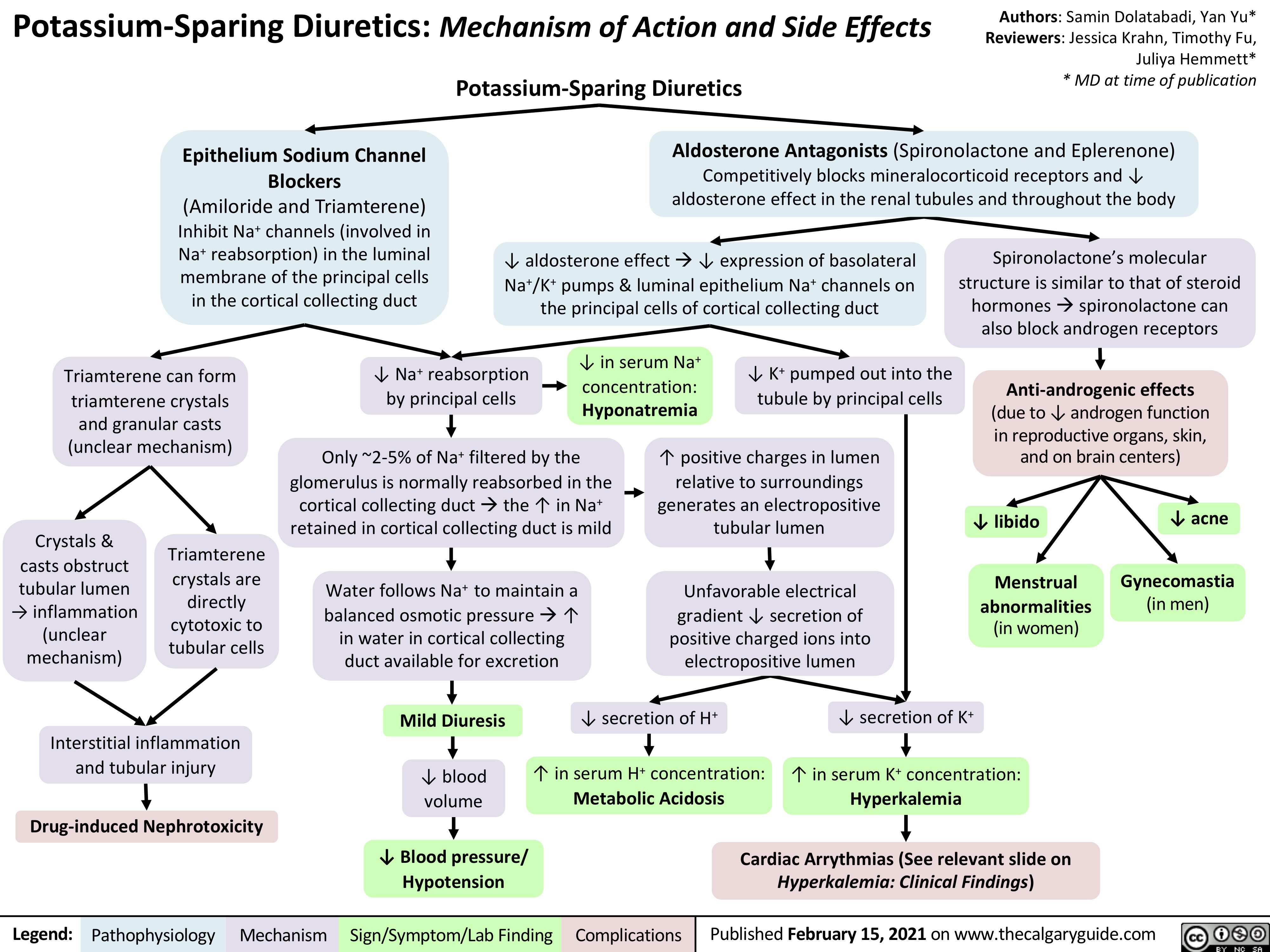
Potassium-Sparing-Diuretics-Mechanism-of-Action-and-Side-Effects
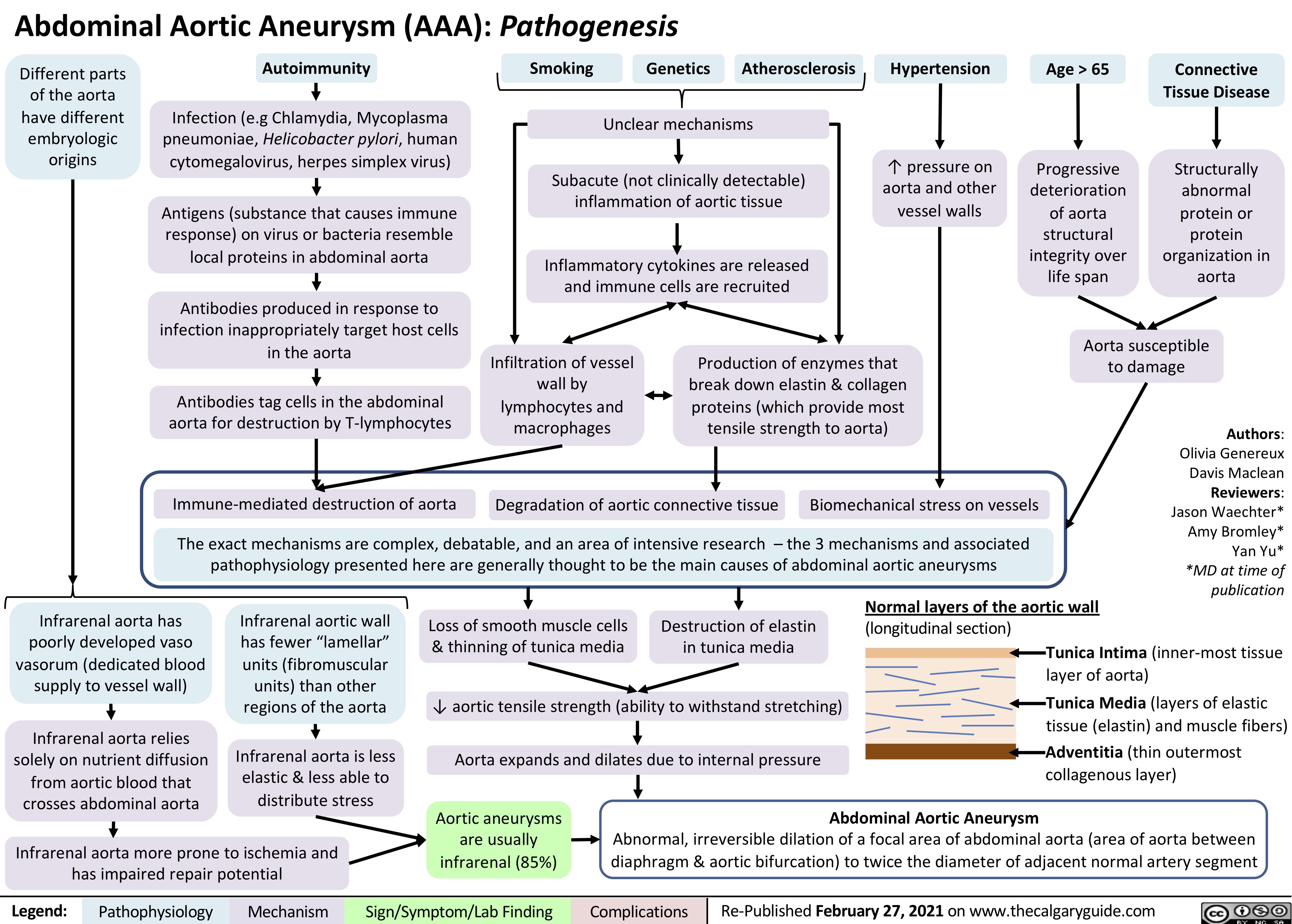
AAA-Pathogenesis
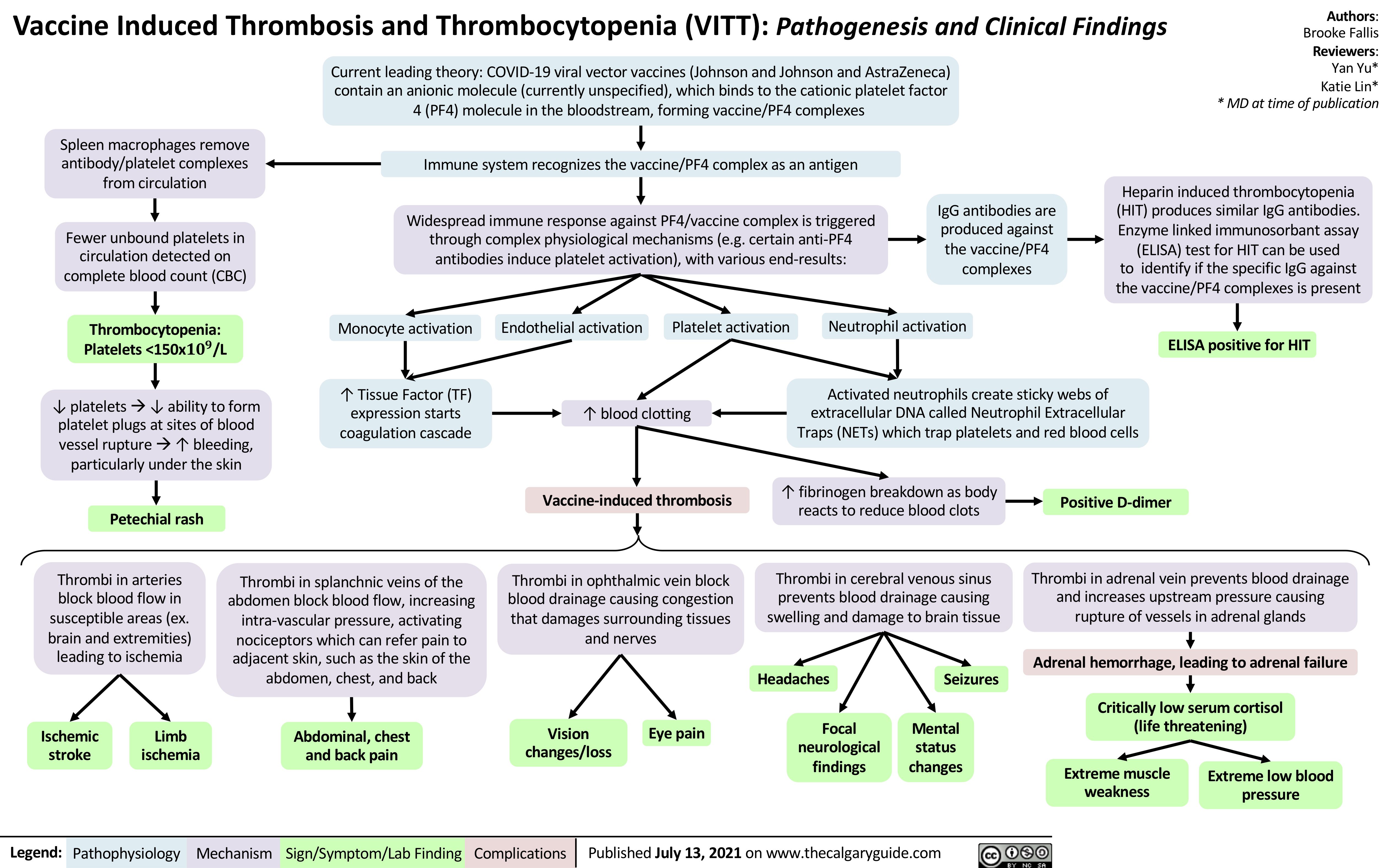
VITT
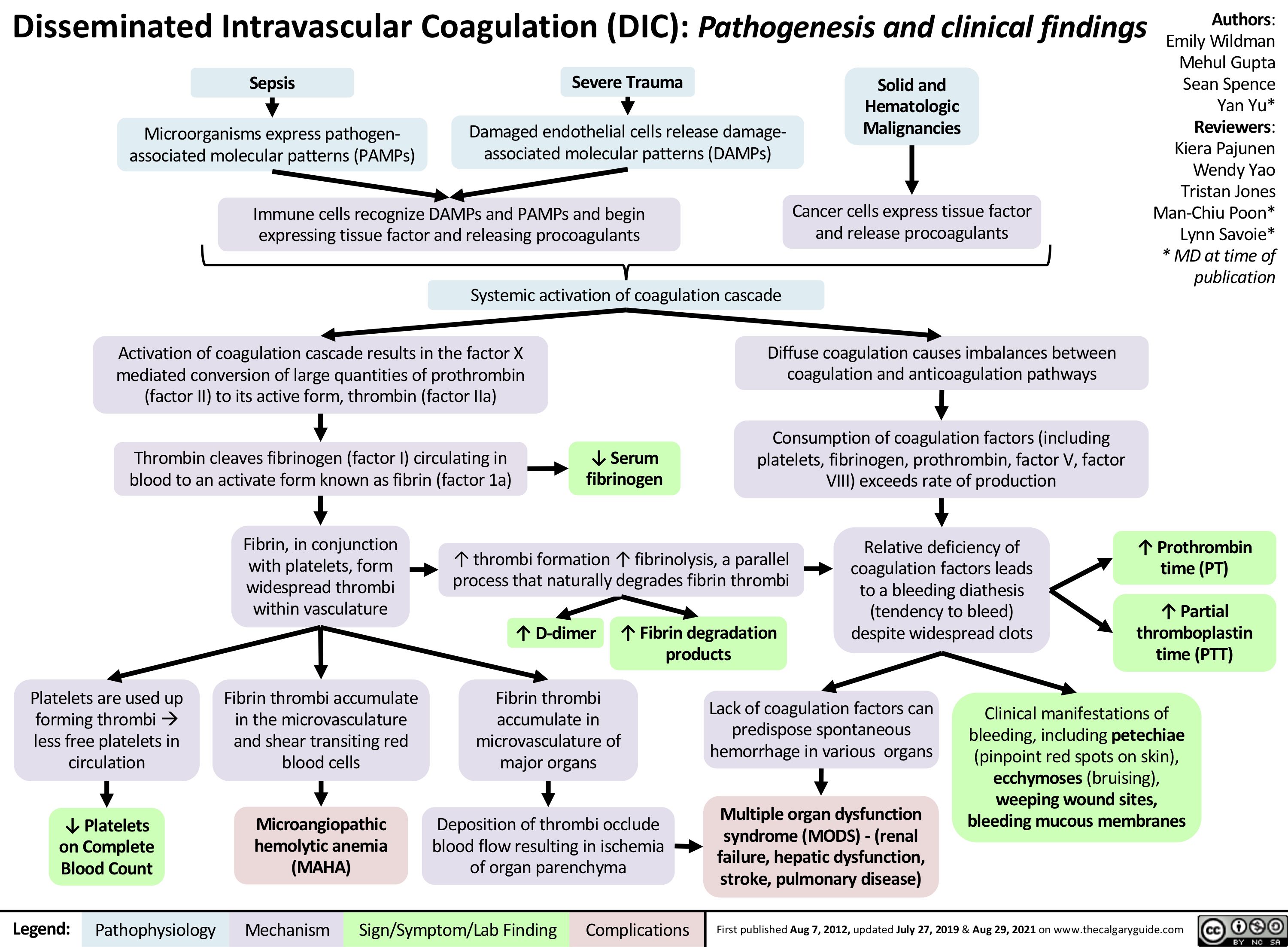
disseminated-intravascular-coagulation
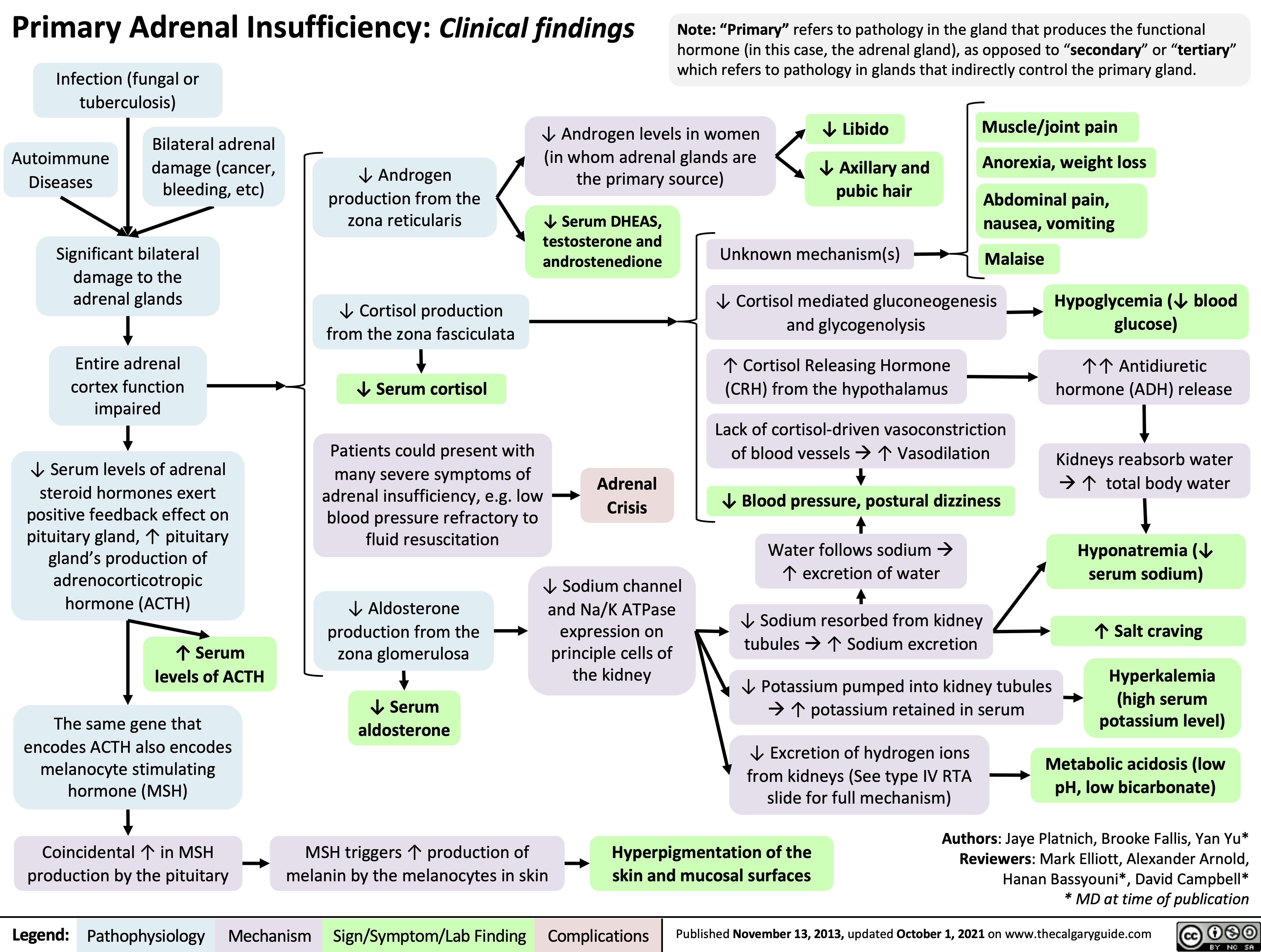
Primary-Adrenal-Insufficiency
Hypercortisolemia
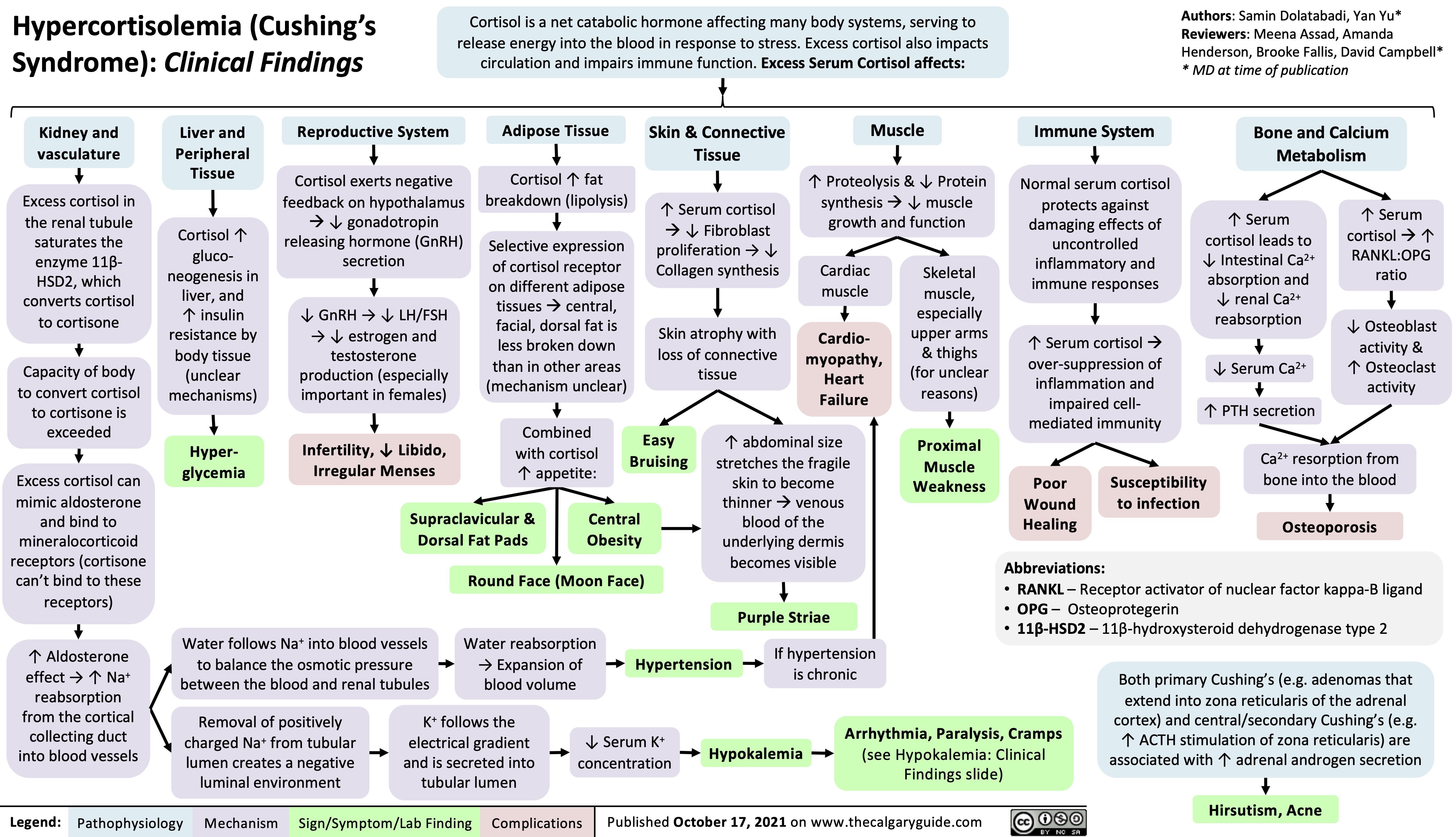
Diabetische Ketoazidose (DKA)
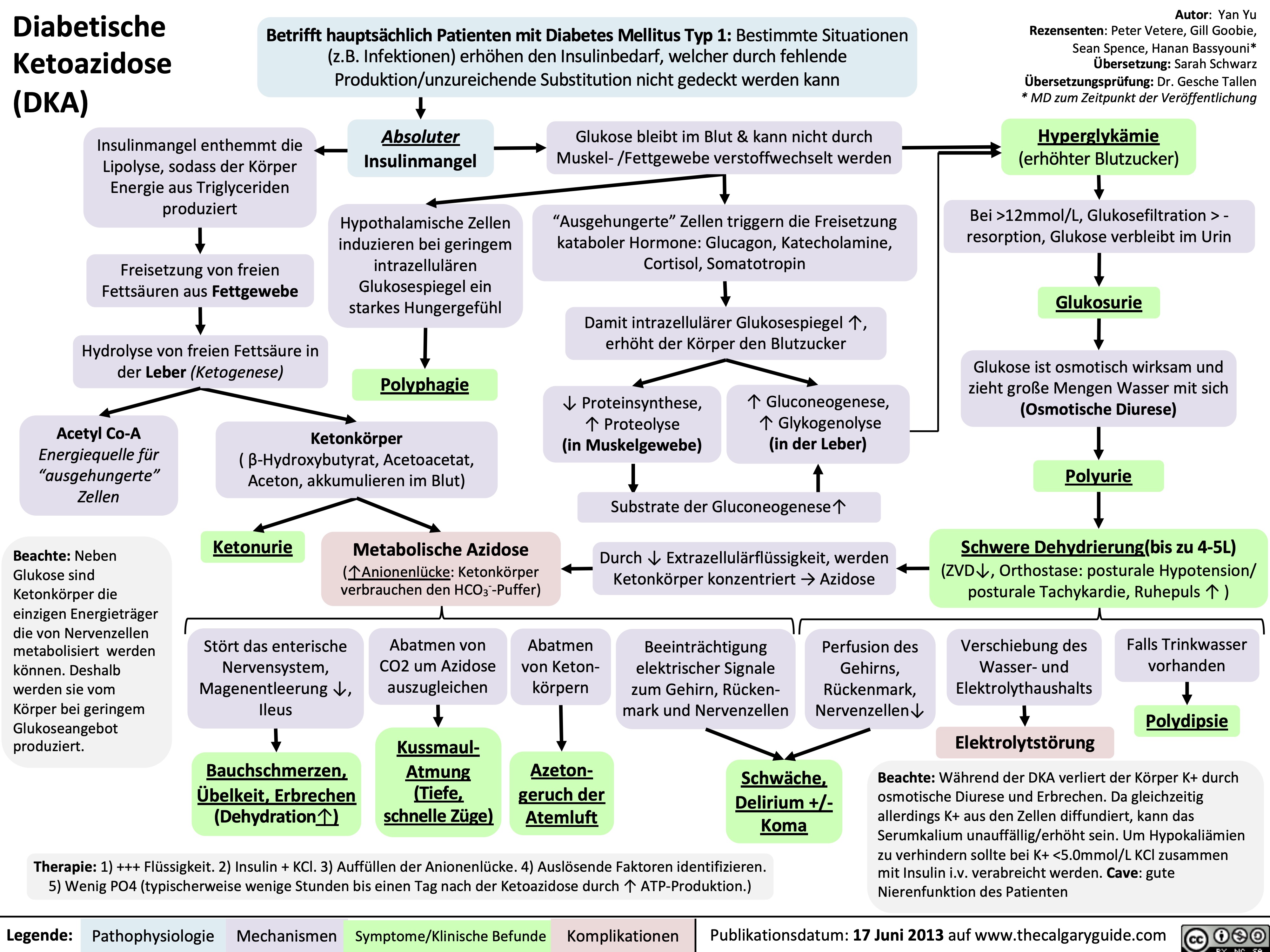
Hyperosmolares/ Hyperglykämisches Koma (HHS)
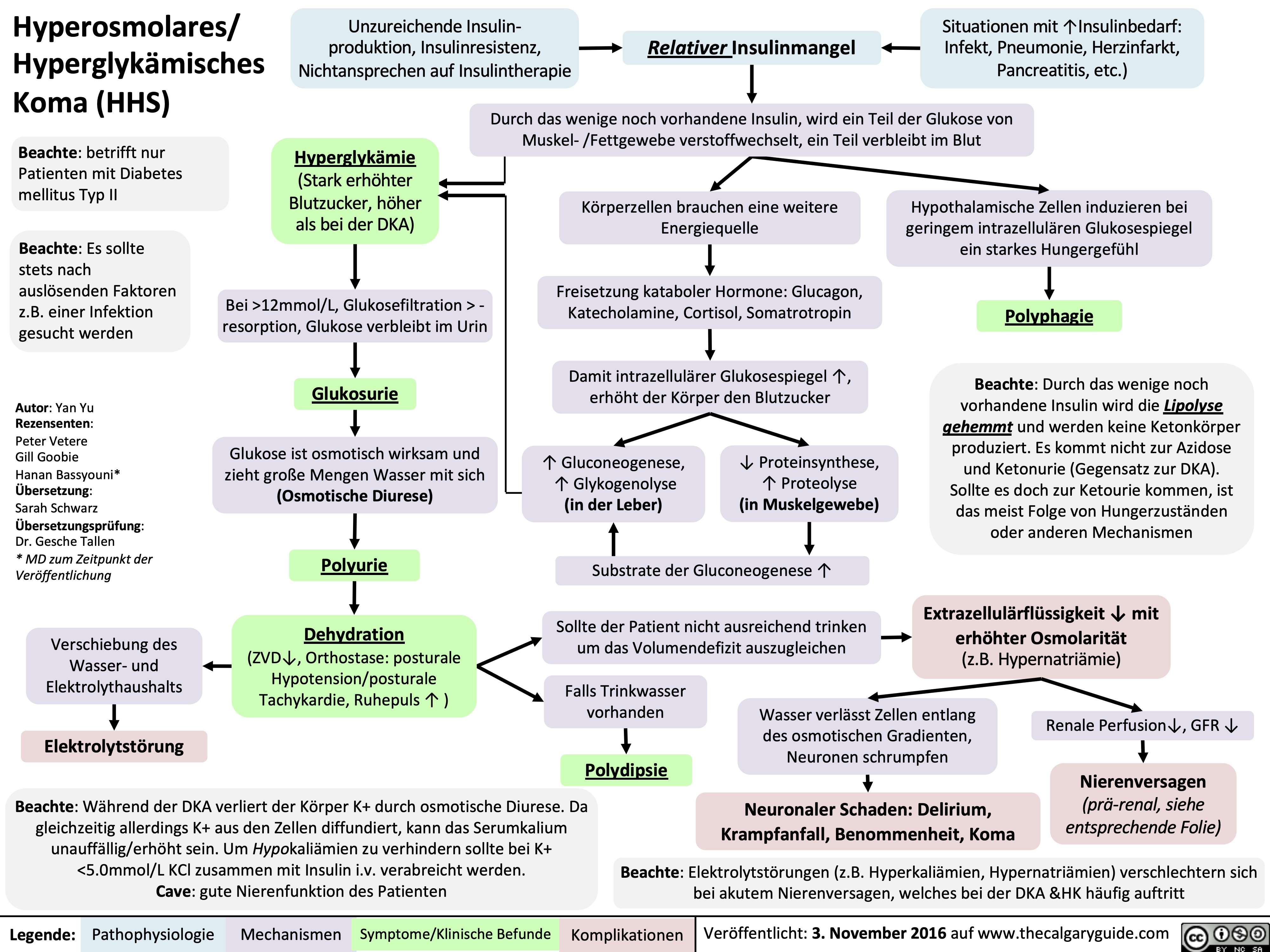
Nephrotisches Syndrom: Pathogenese und klinische Befunde
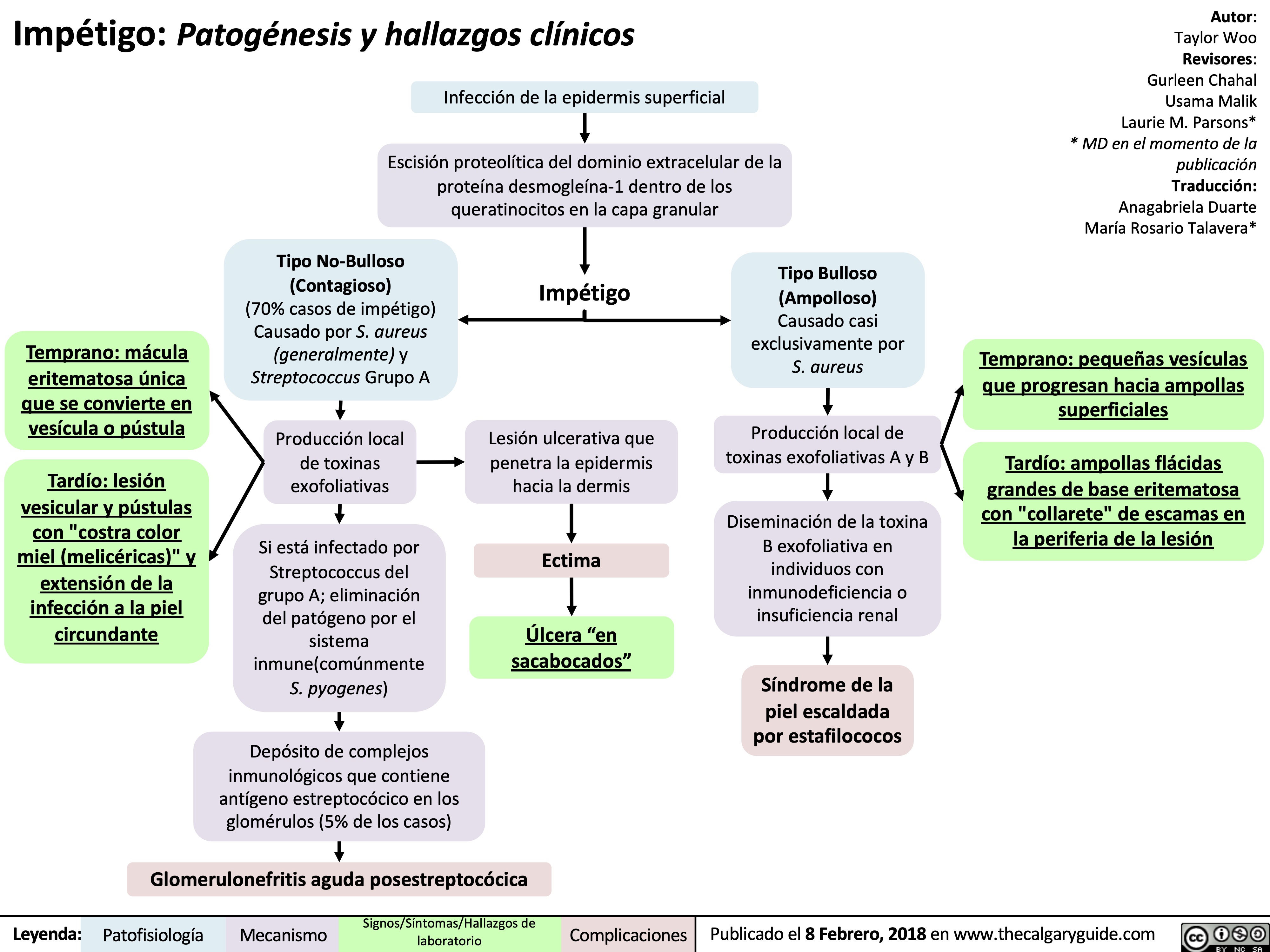
impetigo-patogenesis-y-hallazgos-clinicos
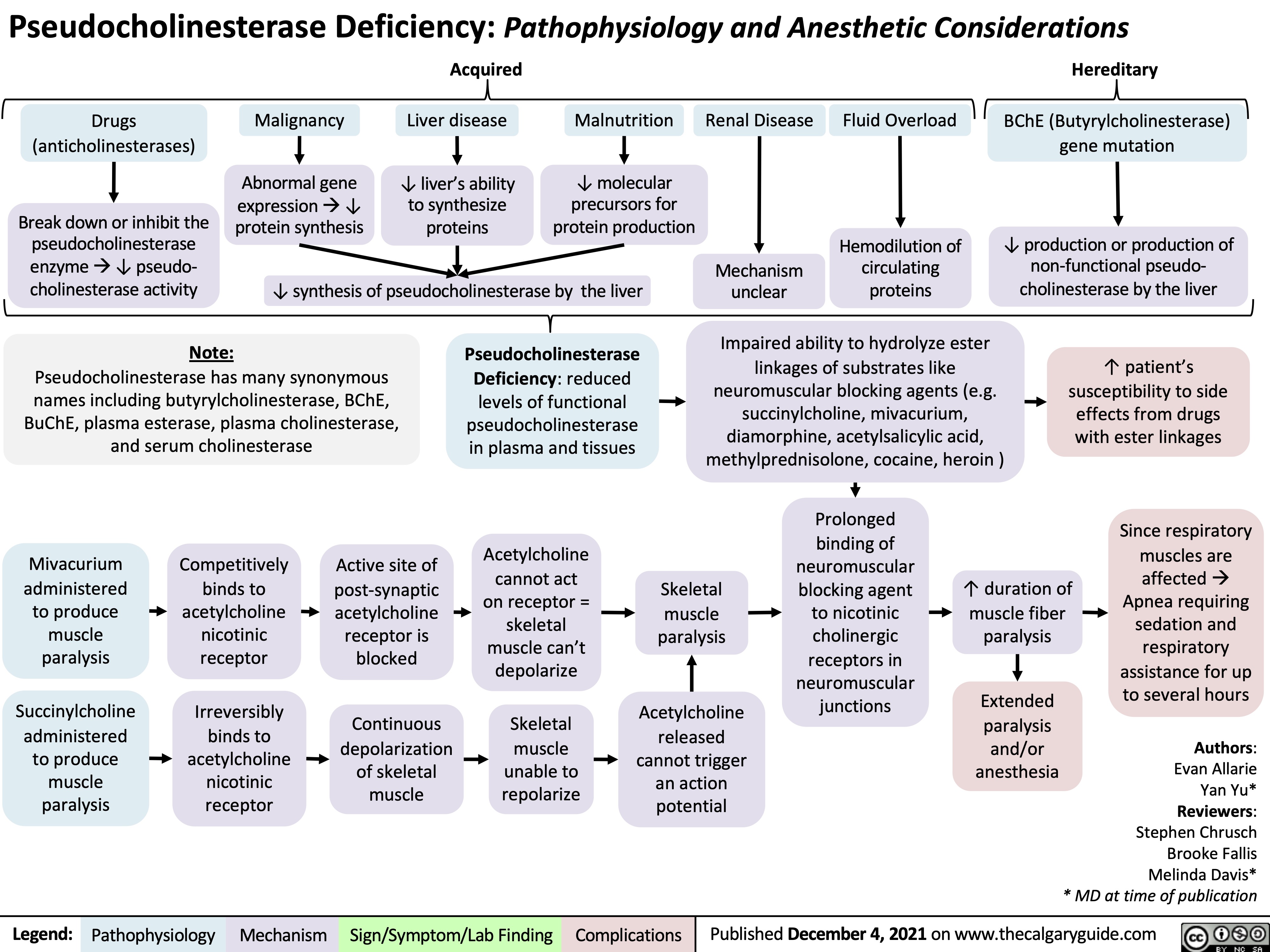
Psuedocholinesterase Deficiency
Primary Aldosteronism Pathogenesis
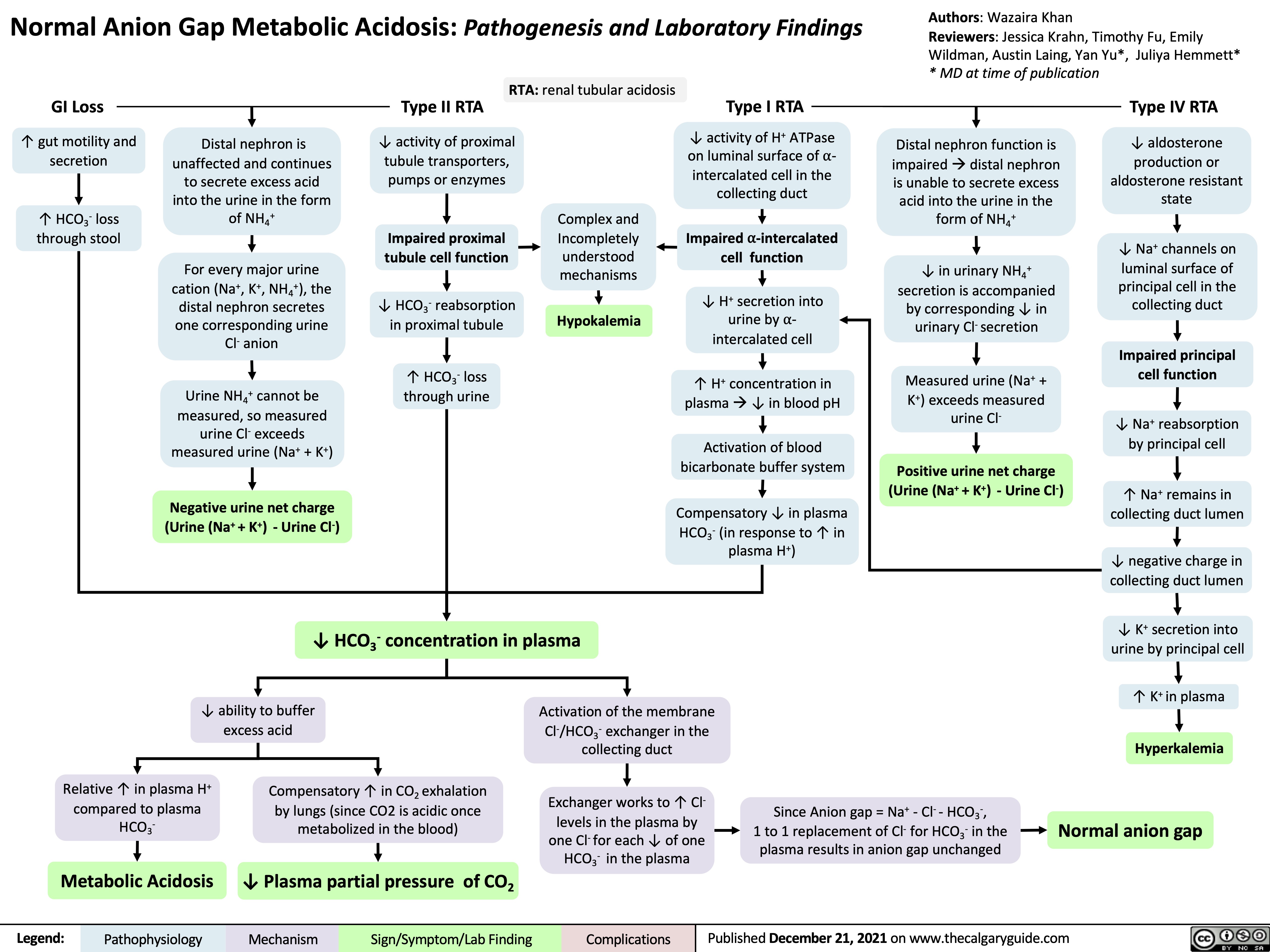
Normal anion gap metabolic acidosis
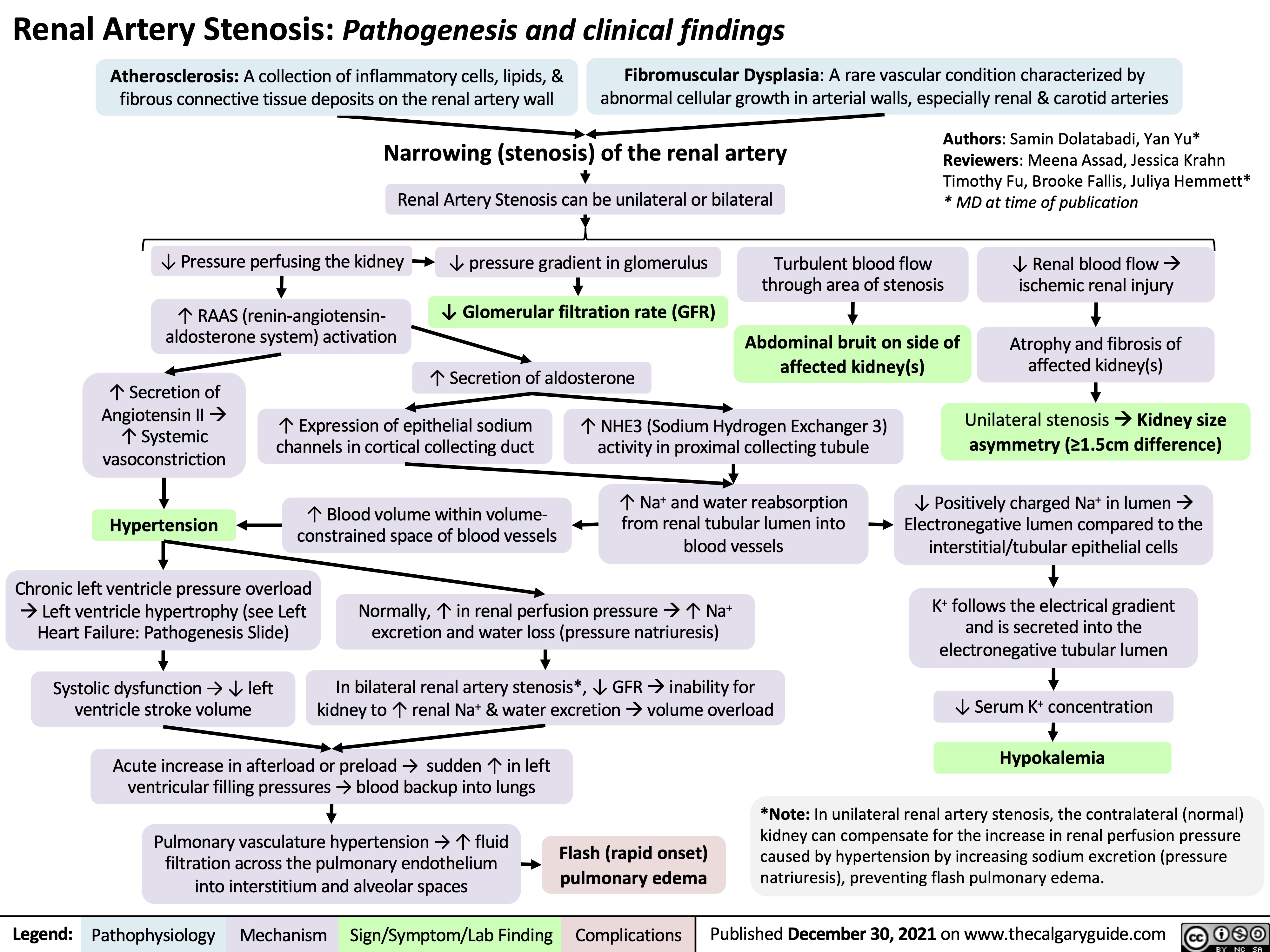
Renal Artery Stenosis
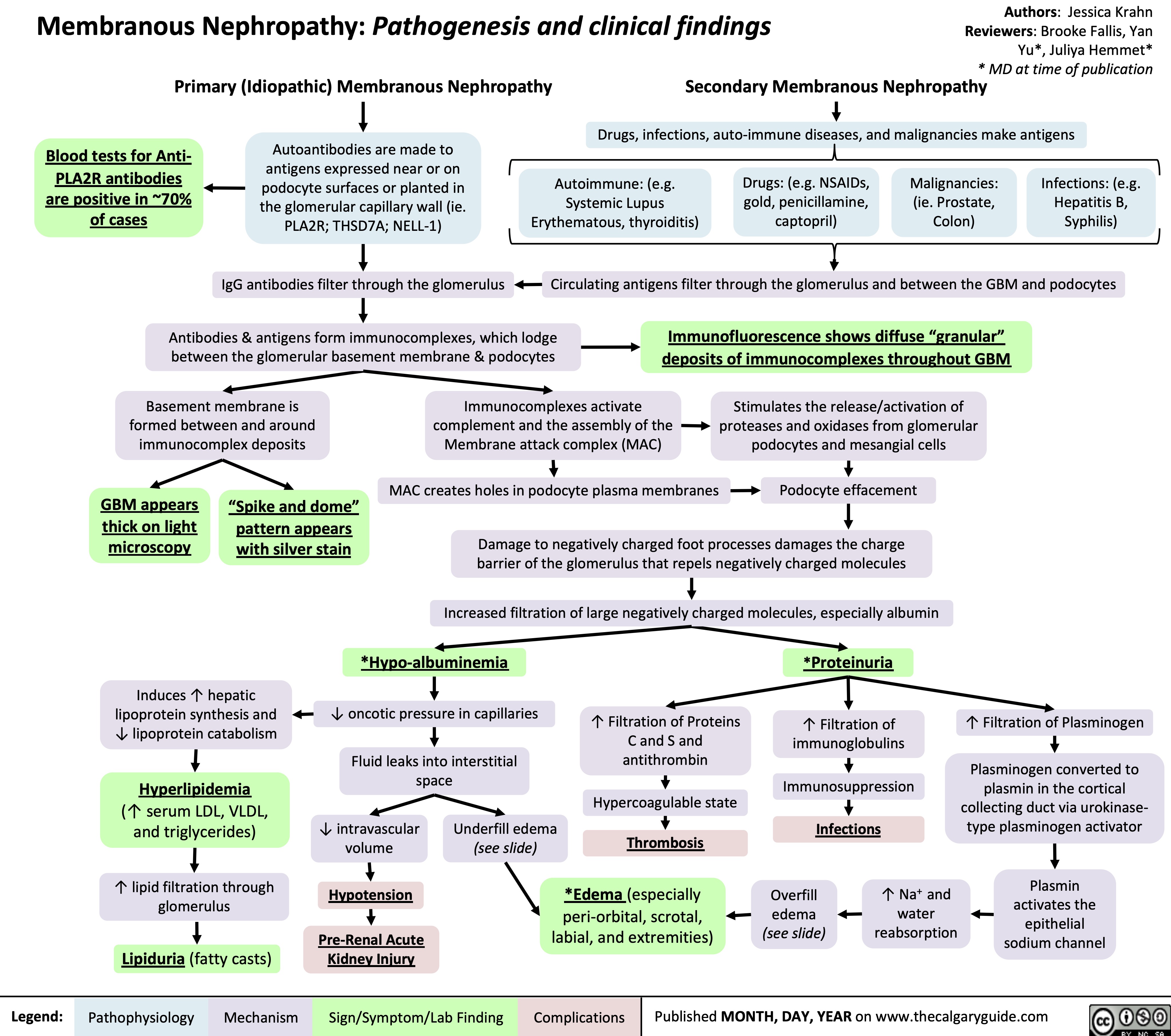
Membranous Nephropathy
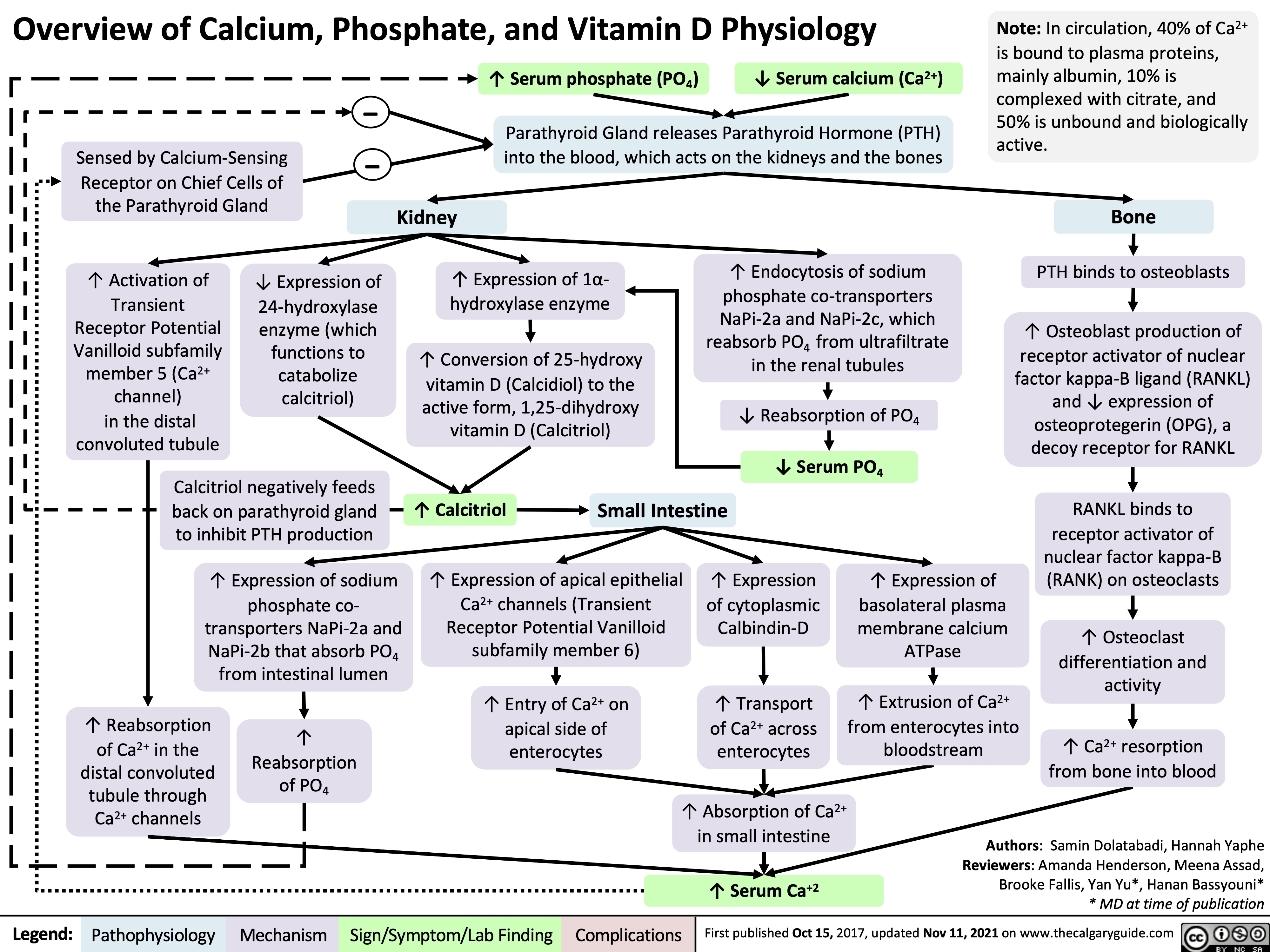
Overview of Calcium Phosphate Vitamin D Physiology
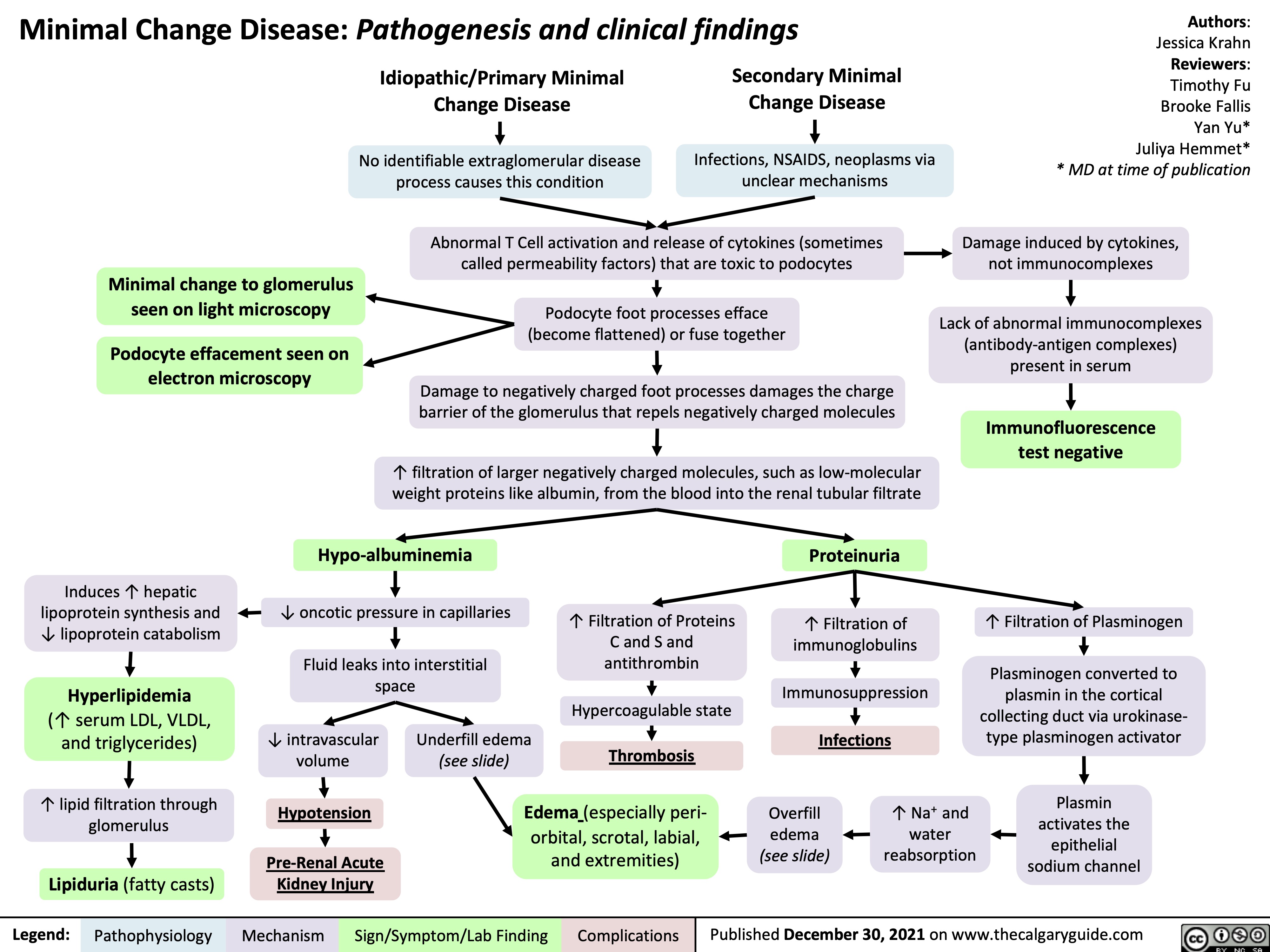
Minimal Change Disease
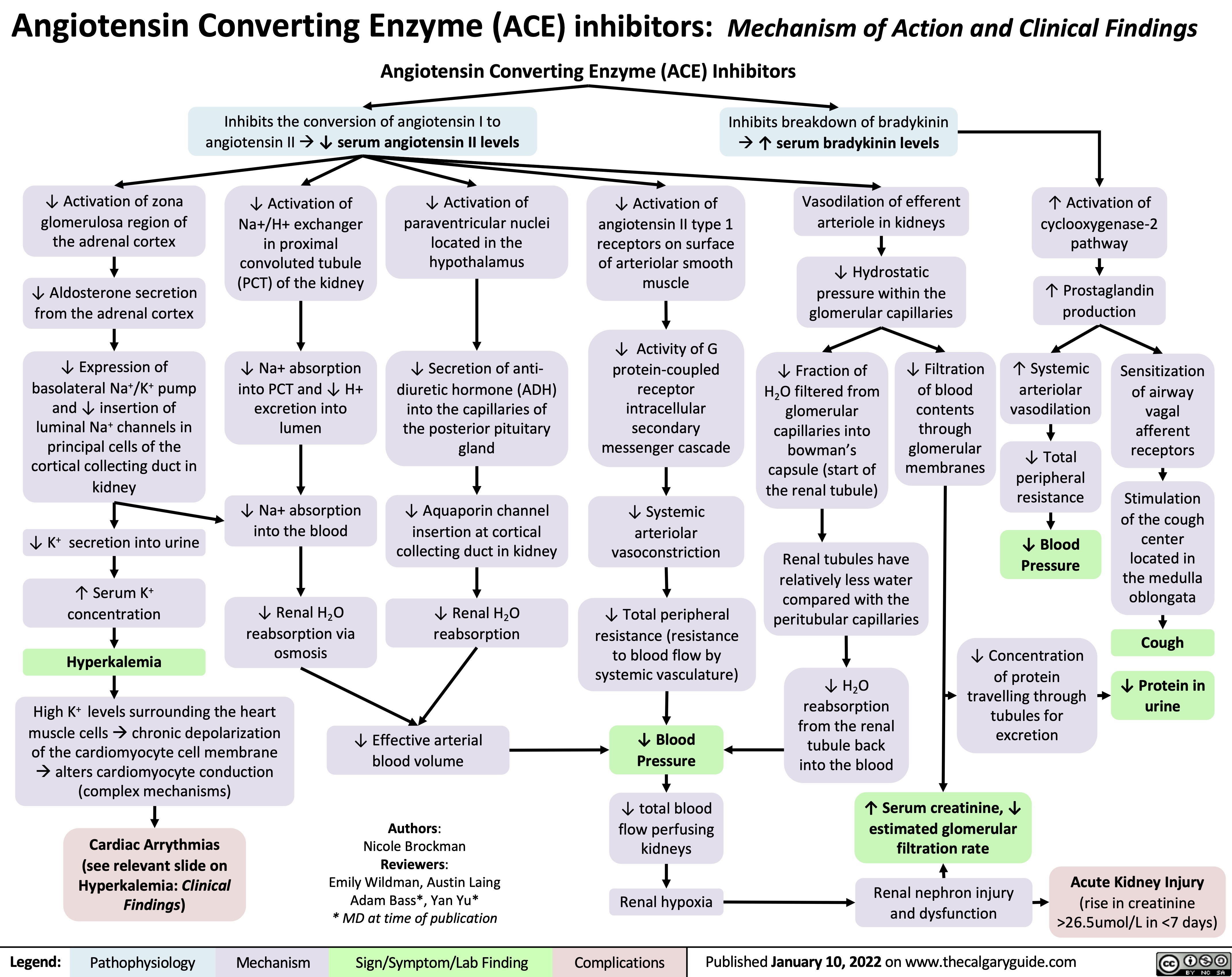
ACE inhibitors
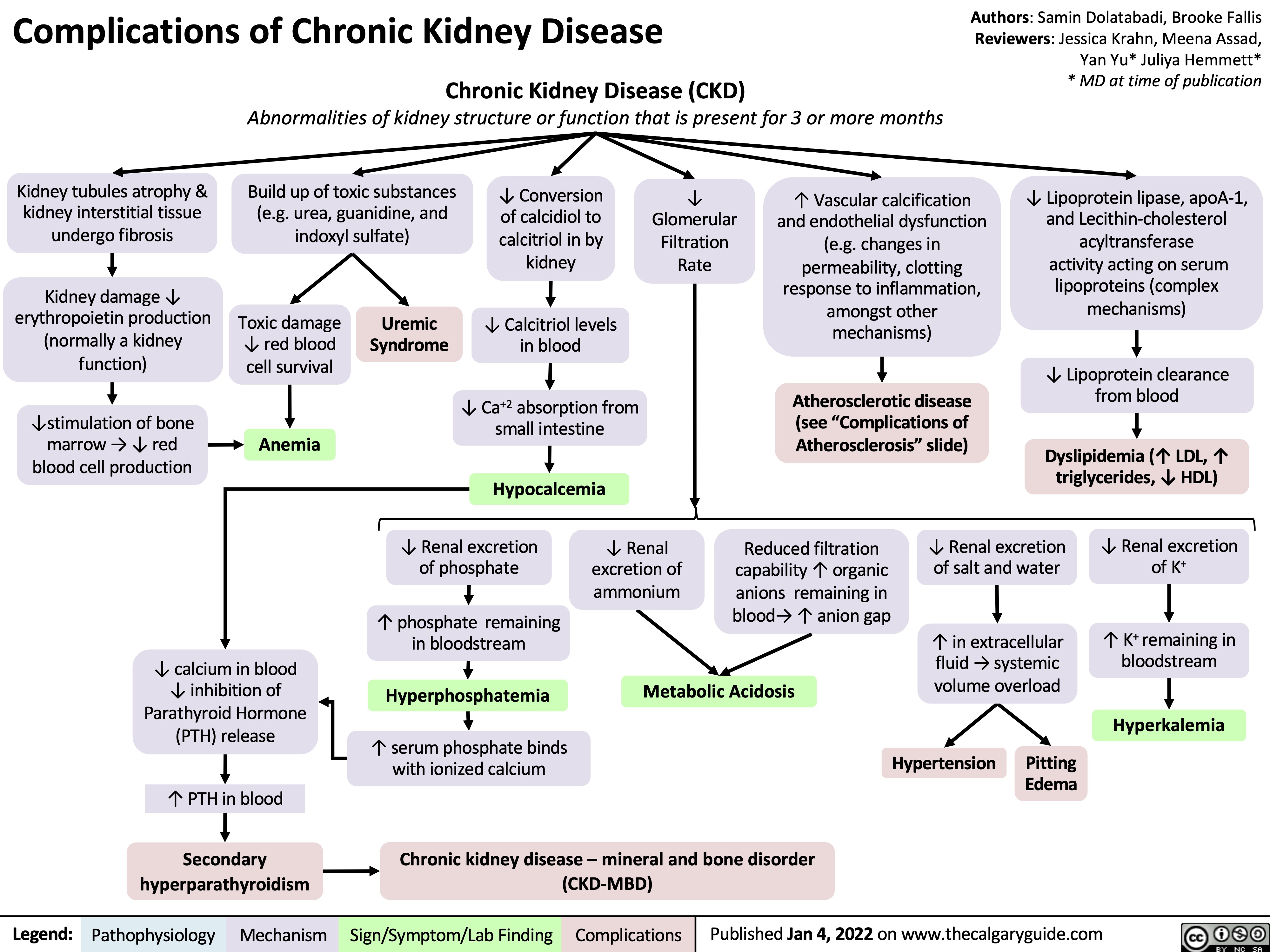
complications-of-chronic-kidney-disease-ckd
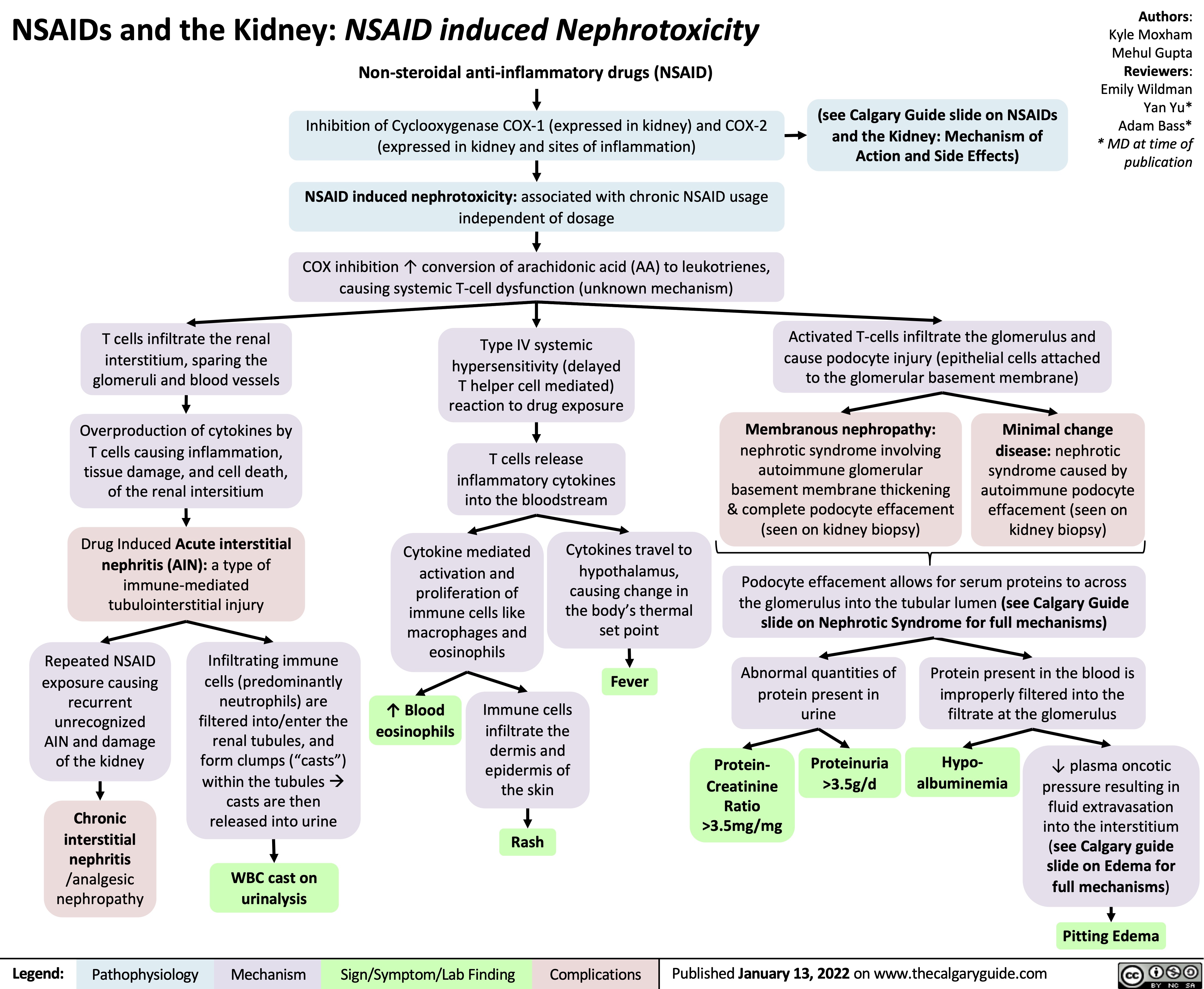
NSAIDs and the Kidney Nephrotoxicity
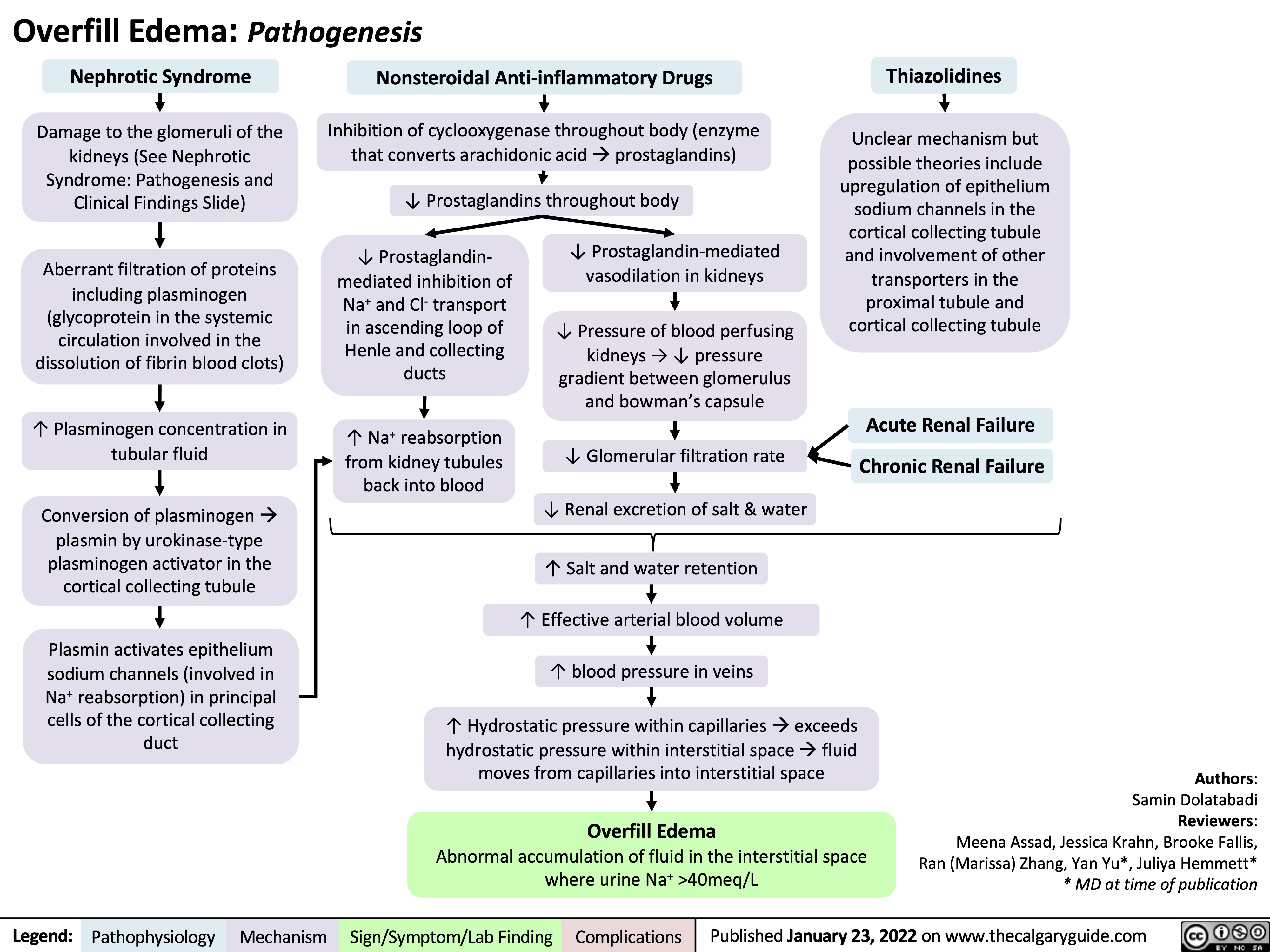
Overfill Edema Pathogenesis
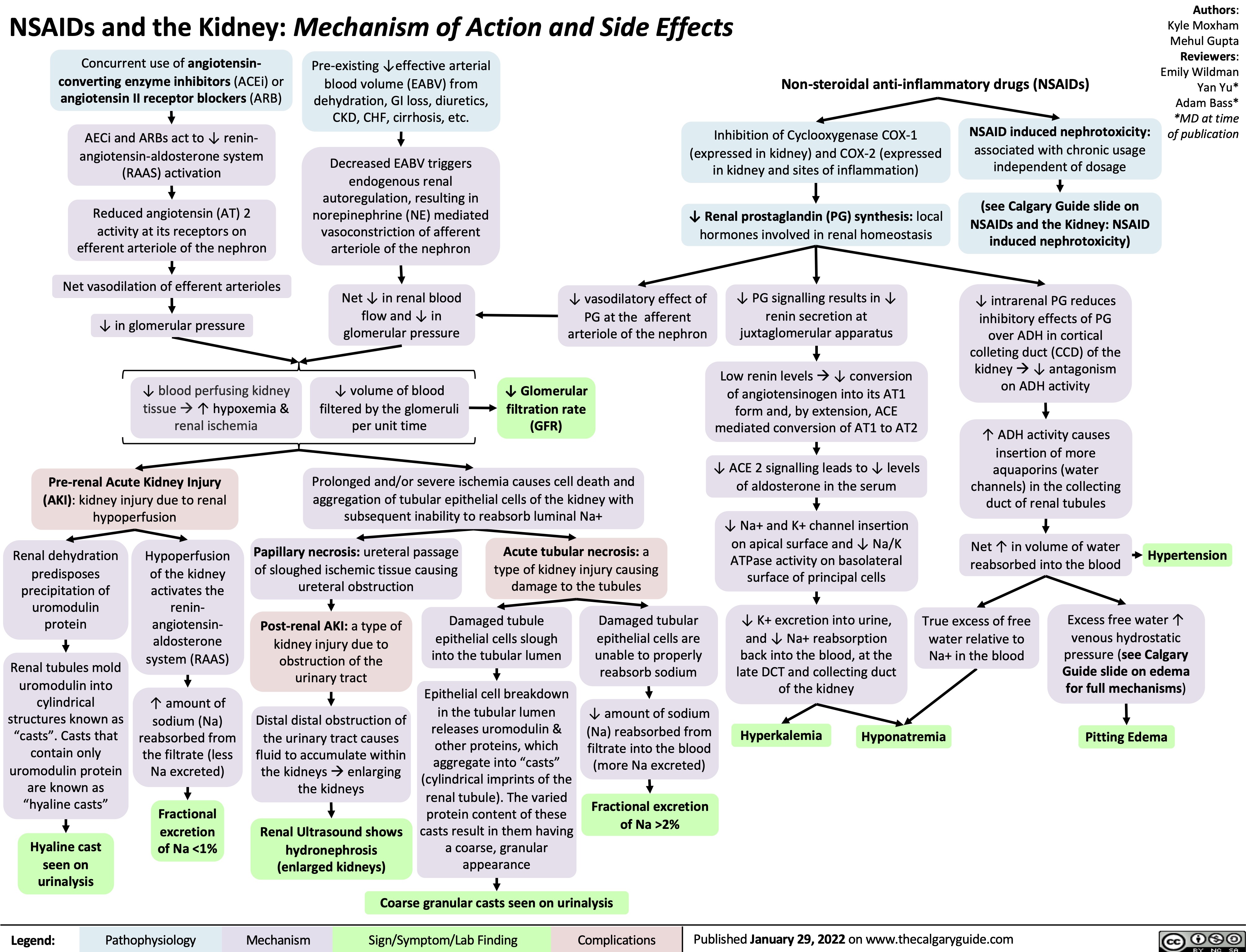
NSAIDs and the Kidney mechanism of action and side effects
Hypokalemia Physiology
![Hypokalemia: Physiology
Authors: Samin Dolatabadi, Ran (Marissa) Zhang, Mannat Dhillon Reviewers: Meena Assad, Yan Yu*, Juliya Hemmett*
Beta-2 receptor stimulation
(e.g. Salbutamol)
↑ Red blood cell production
↑ Na+/K+ ATPase activity in skeletal muscle cells (moves K+ into the cell & Na+ out of cell)
↑ K+ entry into skeletal muscle cells
* MD at time of publication
Abbreviations:
• EABV – Effective Arterial
Blood Volume
• ENaC – Epithelial Sodium
Channel
• HCL – Hydrochloric acid • HCO3- –Bicarbonate ion
↑ K+ uptake by new red blood cells
↑ Intracellular shift of K+ into cells
Refeeding Syndrome Exogenous insulin
↓ K+ dietary intake
(rare cause in isolation)
↑ Insulin in response to carbohydrate load
↑ Na+/K+ ATPase activity in skeletal muscle & hepatic cells
↑ K+ entry into skeletal muscle & hepatic cells
↓ K+ availability for gastrointestinal absorption
Hypokalemia (Serum [K+] < 3.5 mmol/L)
↑ Renal K+ secretion
K+ follows the electrical gradient into tubular lumen
↑ Electronegativity of tubular lumen
↑ Na+ reabsorptionin principal cellsà Cl- left behind in tubular lumen of kidneys
Gastric acid depletionà ↓HCl
Loss of H+àShift in bicarbonate buffer system to ↑ plasma HCO3-
Plasma HCO3- above reabsorptive capacity of the proximal tubule
↑ HCO3- in the distal tubular lumen of kidneys
Vomiting
Diarrhea Laxatives
Renin secreting tumour Hyperaldosteronism Renal artery stenosis Loop and Thiazide
diuretics
Bartter’s and Gittelman’s syndrome
Liddle syndrome
Extracellular fluid volume depletion
↓ EABV ↑ Renin secretion
↓ Afferent arteriole pressure perfusing kidneys
Renin-Angiotensin- Aldosterone System (RAAS) activationà ↑ Aldosterone release from the adrenal cortex
↑ Expression of ENaC (Na+ reabsorption) in principal cells of the cortical collectingduct)
+ ↑Na &
water excretion in kidneys
↓ EABV
Genetic condition leading to inability to degrade ENaC channels in principal cells of the cortical collecting duct
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019, updated Jan 23, 2022 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi Reviewers: Meena Assad Dr. Juliya Hemmett* * MD at time of publication
TTKG > 4 with N/↑ EABV in hypokalemia is inappropriate and a principal cell problem.
β2 Stimulation (e.g., Salbutamol)
↑ Na+/K+ ATPase activity
↑ K+ entry into cell
↑ RBC Production
↑ Cell production
↑ K+ uptake by new cells
↓ Extracellular ↑ Insulin in response to H+
carbohydrate load
↑ Na+/H+ antiporter activity (movement of H+ out of cell and Na+ into cell)
↑ Intracellular Na+
↑ Na+/K+ ATPase activity ↑ K+ entry into cell
↑ Intracellular Shift of K+
Notes:
Refeeding Syndrome
Insulin
Alkalemia
•
Abbreviations:
• CCD – Cortical Collecting Duct
• EABV – Effective Arterial Blood Volume
• RAAS – Renin-Angiotensin-Aldosterone System • TTKG – Trans-tubular Potassium Gradient
• ENaC – Epithelial Sodium Channel
↓ K+ Intake (Rare cause in isolation)
↓ K+ availability
Diarrhea, Vomiting, Laxatives
↑ Gastrointestinal loss of K+
Polyuria
↑ Renal loss of K+ (TTKG < 4 as principal cell is working appropriately but small amount of K+ is lost per urination)
Hypokalemia (Serum [K+] < 3.5 mmol/L)
Liddle Syndrome Hyperaldosteronism Renin Secreting Tumour
Renal artery stenosis
Loop and Thiazide Diuretics
Bartter’s and Gittelman’s Syndrome
Genetic condition leading to inability to degrade ENaC channels ↑ Renin
↑ Renal K+ secretion
K+ follows the electrical gradient
Electronegative lumen
↓ Pressure perfusing the kidney
RAAS activation RAAS activation
↑ Aldosterone
↑ Na+ and water excretion
↓ EABV
↑ Expression of ENaC in principal cells of CCD
↑ Na+ reabsorption
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi, Ran (Marissa) Zhang, Mannat Dhillon Reviewers: Meena Assad, Yan Yu*, Juliya Hemmett*
Beta-2 receptor stimulation
(e.g. Salbutamol)
↑ Red blood cell production
↑ Na+/K+ ATPase activity in skeletal muscle cells (moves K+ into the cell & Na+ out of cell)
↑ K+ entry into skeletal muscle cells
* MD at time of publication
Abbreviations:
• EABV – Effective Arterial
Blood Volume
• ENaC – Epithelial Sodium
Channel
• HCL – Hydrochloric acid • HCO3- –Bicarbonate ion
↑ K+ uptake by new red blood cells
↑ Intracellular shift of K+ into cells
Refeeding Syndrome Exogenous insulin
↓ K+ dietary intake
(rare cause in isolation)
↑ Insulin in response to carbohydrate load
↑ Na+/K+ ATPase activity in skeletal muscle & hepatic cells
↑ K+ entry into skeletal muscle & hepatic cells
↓ K+ availability for gastrointestinal absorption
Hypokalemia (Serum [K+] < 3.5 mmol/L)
↑ Renal K+ secretion
K+ follows the electrical gradient into tubular lumen
↑ Electronegativity of tubular lumen
↑ Na+ reabsorptionin principal cellsà Cl- left behind in tubular lumen of kidneys
Gastric acid depletionà ↓HCl
Loss of H+àShift in bicarbonate buffer system to ↑ plasma HCO3-
Plasma HCO3- above reabsorptive capacity of the proximal tubule
↑ HCO3- in the distal tubular lumen of kidneys
Vomiting
Diarrhea Laxatives
Renin secreting tumour Hyperaldosteronism Renal artery stenosis Loop and Thiazide
diuretics
Bartter’s and Gittelman’s syndrome
Liddle syndrome
Extracellular fluid volume depletion
↓ EABV ↑ Renin secretion
↓ Afferent arteriole pressure perfusing kidneys
Renin-Angiotensin- Aldosterone System (RAAS) activationà ↑ Aldosterone release from the adrenal cortex
↑ Expression of ENaC (Na+ reabsorption) in principal cells of the cortical collectingduct)
+ ↑Na &
water excretion in kidneys
↓ EABV
Genetic condition leading to inability to degrade ENaC channels in principal cells of the cortical collecting duct
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019, updated Jan 23, 2022 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi Reviewers: Meena Assad Dr. Juliya Hemmett* * MD at time of publication
TTKG > 4 with N/↑ EABV in hypokalemia is inappropriate and a principal cell problem.
β2 Stimulation (e.g., Salbutamol)
↑ Na+/K+ ATPase activity
↑ K+ entry into cell
↑ RBC Production
↑ Cell production
↑ K+ uptake by new cells
↓ Extracellular ↑ Insulin in response to H+
carbohydrate load
↑ Na+/H+ antiporter activity (movement of H+ out of cell and Na+ into cell)
↑ Intracellular Na+
↑ Na+/K+ ATPase activity ↑ K+ entry into cell
↑ Intracellular Shift of K+
Notes:
Refeeding Syndrome
Insulin
Alkalemia
•
Abbreviations:
• CCD – Cortical Collecting Duct
• EABV – Effective Arterial Blood Volume
• RAAS – Renin-Angiotensin-Aldosterone System • TTKG – Trans-tubular Potassium Gradient
• ENaC – Epithelial Sodium Channel
↓ K+ Intake (Rare cause in isolation)
↓ K+ availability
Diarrhea, Vomiting, Laxatives
↑ Gastrointestinal loss of K+
Polyuria
↑ Renal loss of K+ (TTKG < 4 as principal cell is working appropriately but small amount of K+ is lost per urination)
Hypokalemia (Serum [K+] < 3.5 mmol/L)
Liddle Syndrome Hyperaldosteronism Renin Secreting Tumour
Renal artery stenosis
Loop and Thiazide Diuretics
Bartter’s and Gittelman’s Syndrome
Genetic condition leading to inability to degrade ENaC channels ↑ Renin
↑ Renal K+ secretion
K+ follows the electrical gradient
Electronegative lumen
↓ Pressure perfusing the kidney
RAAS activation RAAS activation
↑ Aldosterone
↑ Na+ and water excretion
↓ EABV
↑ Expression of ENaC in principal cells of CCD
↑ Na+ reabsorption
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hypokalemia-Physiology.jpg)
metabolic-alkalosis-pathogenesis
![Metabolic Alkalosis: Pathogenesis
Authors: Wazaira Khan Reviewers: Jessica Krahn, Emily Wildman, Austin Laing, Huneza Nadeem, Ran (Marissa) Zhang, Adam Bass* * MD at time of publication
Primary hyperaldosteronism E.g., aldosterone- secreting mass, adrenal hyperplasia
Secondary hyperaldosteronism E.g., renin-secreting mass, renal artery stenosis
Pseudo- hypoaldosteronism E.g., Liddle’s Syndrome
Unregulated aldosterone production in adrenal cortex
Excess aldosterone suppresses renin production
Unregulated renin production by juxtaglomerular cells
Sustained ↑ in mineralocorticoid (aldosterone) activity
Insertion of epithelial sodium channels on principal cells in collecting duct
↑ Water retention by the kidney
↑ Na+ reabsorption by principal cells
↑ EABV
• ↑ Jugular venous pressure • Hypertension
• Urine Na+ > 40 mEq/L
Low renin, high aldosterone state
Activation of renin- angiotensin-aldosterone system (RAAS)
Release of aldosterone from adrenal cortex
High renin, high aldosterone state
↑ Negative charge in collecting duct lumen
K+ leaks into collecting duct lumen by principal cell to maintain electroneutrality
Hypokalemia
Intracellular K+ leaks out of any cell in the body to compensate for low serum K+ levels
Extracellular H+ enters cell to maintain electroneutrality
Intracellular acidosis
Activation of compensatory acid secreting mechanisms in kidney
Impaired tubular function
E.g., loop/thiazide diuretics, Bartter’s/ Gitelman’s Syndrome
Upper GI Loss
E.g., vomiting, nasogastric suction
Unregulated epithelial sodium channel activity in collecting duct mimicking aldosterone function
↑ in Na+ and water retention à RAAS inhibition
↓ Na+ and Cl- reabsorption in thick ascending limb or distal convoluted tubule
Low renin, low aldosterone state
↑ Na+, Cl- and water secretion through kidney
RAAS activation
See “Physiology of RAAS” slide
↓ EABV • • •
Temporary ↑ in mineralocorticoid (aldosterone) activity
↓ Jugular venous pressure Orthostatic hypotension Dry mucous membranes Urine Na+ < 20 mEq/L
•
Loss of fluid through GI tract
↓ HCl delivery to small intestine
↑ NH + secretion and 4
↑ HCO3- reabsorption in proximal tubule
↑ H+ secretion in cortical collecting duct
Loss of gastric contents, including HCl
↓ HCO3- secretion by pancreas, liver and intestines to neutralize HCl
Loss of intrinsic acid to neutralize HCO3- ↑ Plasma [HCO3-]
Metabolic Alkalosis
Arterial blood gas pH > 7.40 Plasma [HCO3-] > 24 mEq/L
Effective Arterial Blood Volume (EABV): component of arterial blood volume that is effectively perfusing organs
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published May 31, 2022 on www.thecalgaryguide.com
Metabolic Alkalosis: Pathogenesis
Authors: Wazaira Khan Reviewers: Jessica Krahn, Emily Wildman, Austin Laing, Huneza Nadeem, Ran (Marissa) Zhang, Adam Bass* * MD at time of publication
Primary hyperaldosteronism E.g., aldosterone- secreting mass, adrenal hyperplasia
Secondary hyperaldosteronism E.g., renin-secreting mass, renal artery stenosis
Pseudo- hypoaldosteronism E.g., Liddle’s Syndrome
Unregulated aldosterone production in adrenal cortex
Excess aldosterone suppresses renin production
Unregulated renin production by juxtaglomerular cells
Sustained ↑ in mineralocorticoid (aldosterone) activity
Insertion of epithelial sodium channels on principal cells in collecting duct
↑ Water retention by the kidney
↑ Na+ reabsorption by principal cells
↑ EABV
• ↑ Jugular venous pressure • Hypertension
• Urine Na+ > 40 mEq/L
Low renin, high aldosterone state
Activation of renin- angiotensin-aldosterone system (RAAS)
Release of aldosterone from adrenal cortex
High renin, high aldosterone state
↑ Negative charge in collecting duct lumen
K+ leaks into collecting duct lumen by principal cell to maintain electroneutrality
Hypokalemia
Intracellular K+ leaks out of any cell in the body to compensate for low serum K+ levels
Extracellular H+ enters cell to maintain electroneutrality
Intracellular acidosis
Activation of compensatory acid secreting mechanisms in kidney
Impaired tubular function
E.g., loop/thiazide diuretics, Bartter’s/ Gitelman’s Syndrome
Upper GI Loss
E.g., vomiting, nasogastric suction
Unregulated epithelial sodium channel activity in collecting duct mimicking aldosterone function
↑ in Na+ and water retention à RAAS inhibition
↓ Na+ and Cl- reabsorption in thick ascending limb or distal convoluted tubule
Low renin, low aldosterone state
↑ Na+, Cl- and water secretion through kidney
RAAS activation
See “Physiology of RAAS” slide
↓ EABV • • •
Temporary ↑ in mineralocorticoid (aldosterone) activity
↓ Jugular venous pressure Orthostatic hypotension Dry mucous membranes Urine Na+ < 20 mEq/L
•
Loss of fluid through GI tract
↓ HCl delivery to small intestine
↑ NH + secretion and 4
↑ HCO3- reabsorption in proximal tubule
↑ H+ secretion in cortical collecting duct
Loss of gastric contents, including HCl
↓ HCO3- secretion by pancreas, liver and intestines to neutralize HCl
Loss of intrinsic acid to neutralize HCO3- ↑ Plasma [HCO3-]
Metabolic Alkalosis
Arterial blood gas pH > 7.40 Plasma [HCO3-] > 24 mEq/L
Effective Arterial Blood Volume (EABV): component of arterial blood volume that is effectively perfusing organs
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published May 31, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/05/Metabolic-Alkalosis-1.jpg)
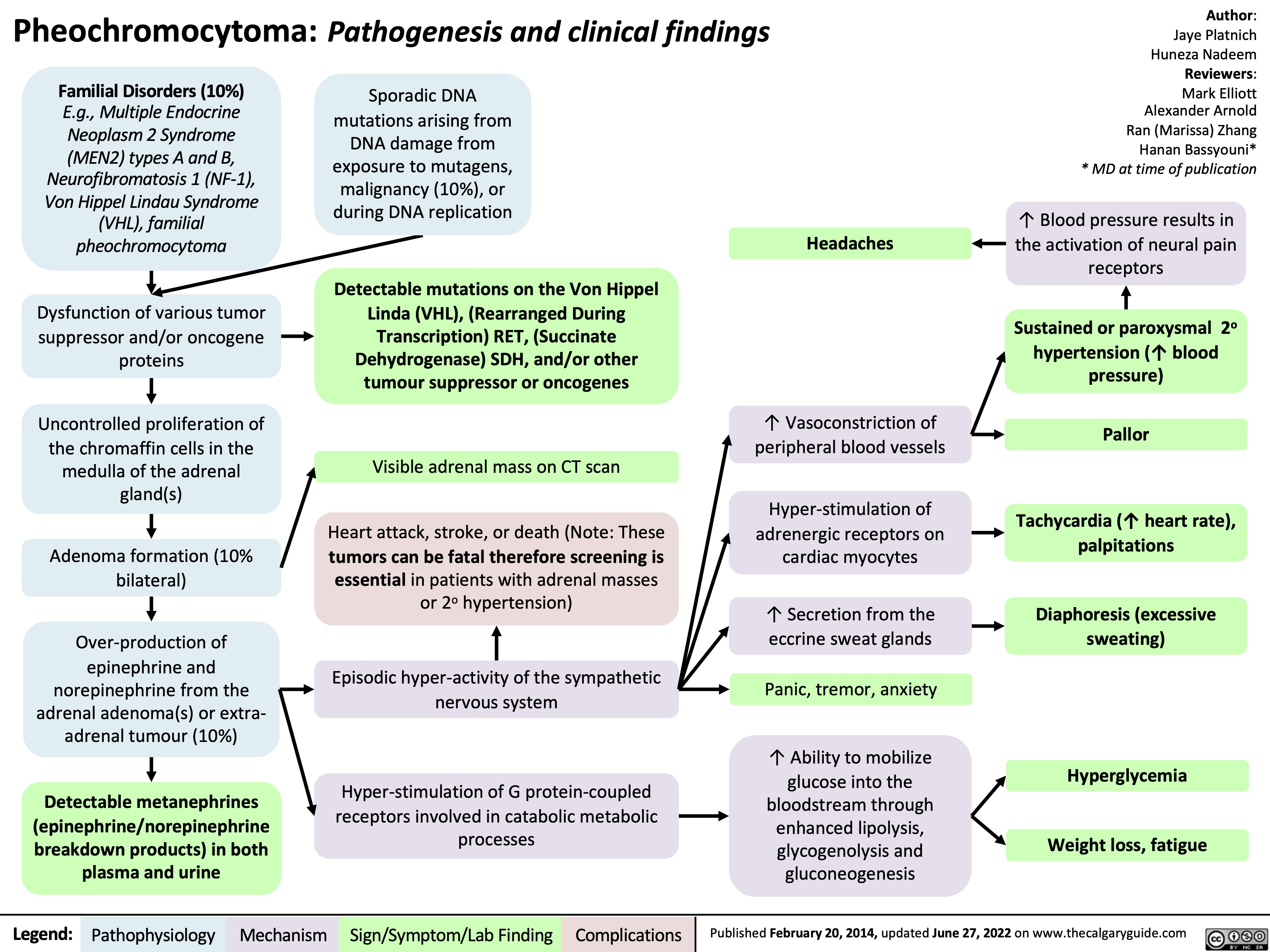
pheochromocytoma-pathogenesis-and-clinical-findings
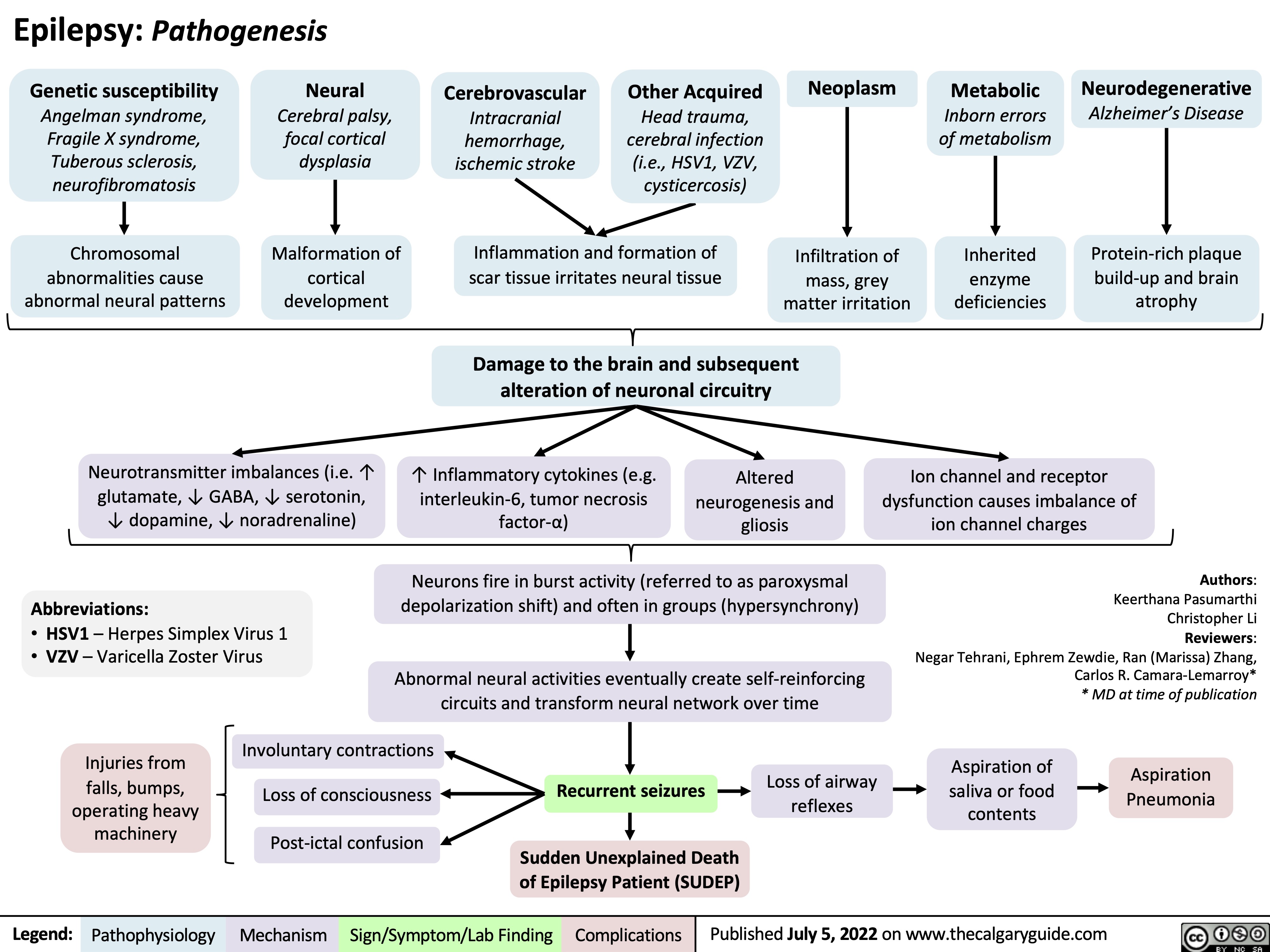
Epilepsy Pathogenesis
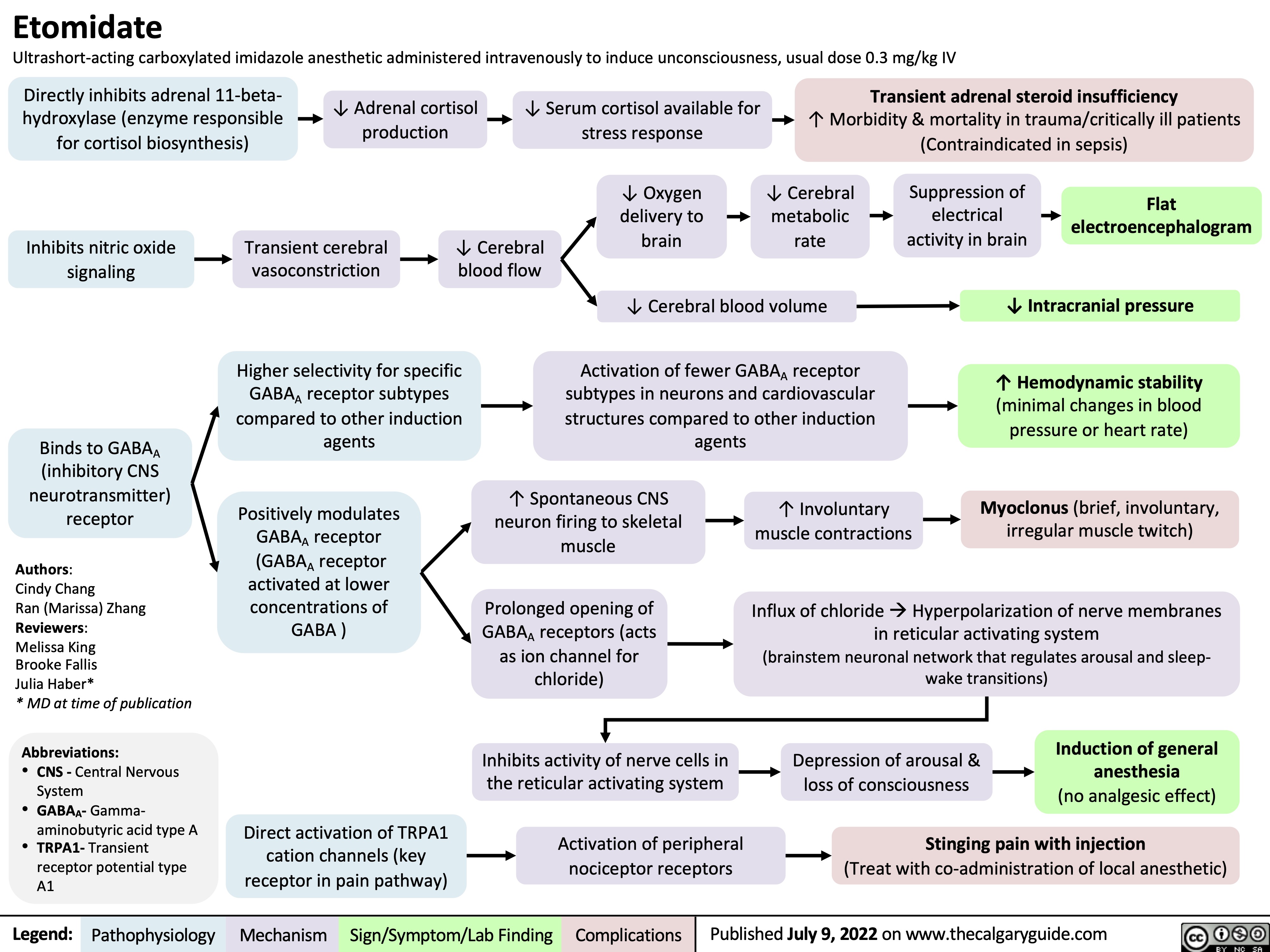
etomidate
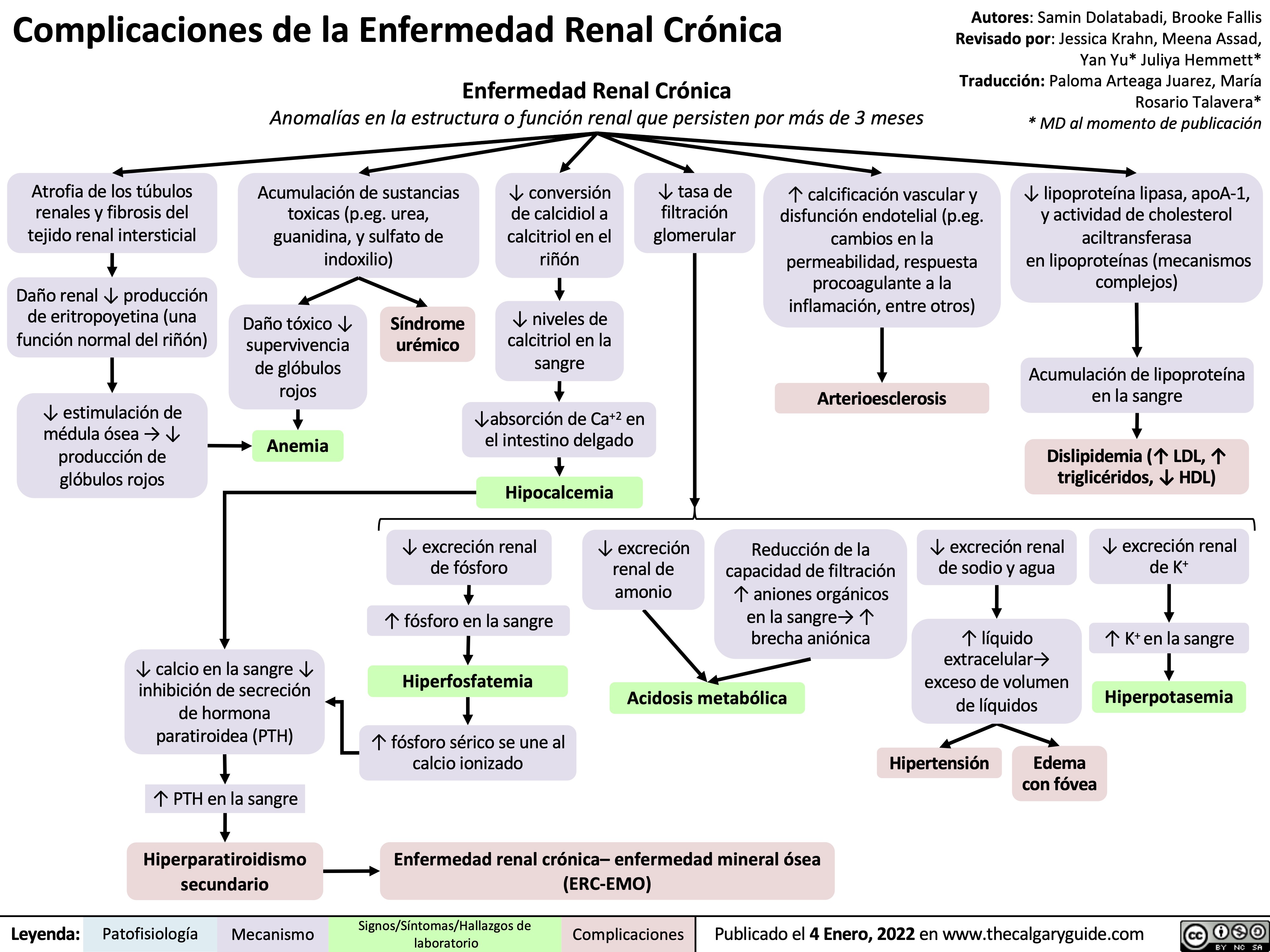
complicaciones-de-la-enfermedad-renal-cronica
hyperkalemia-↓-renal-excretion-pathophysiology
![Hyperkalemia (↓ Renal Excretion): Pathophysiology
Non-steroidal anti- inflammatory drugs (NSAIDs)
Inhibition of prostaglandins which promote renin secretion (see NSAIDs and the Kidney: Mechanism of Action and Side Effects slide)
Diabetic Nephropathy
Acute (AIN)and chronic (CIN) interstitial nephritis
Immune-mediated damage of the kidney tubule and interstitium
Damage to distal tubule leads to aldosterone resistance at principal cell
Aldosterone cannot ↑ EnaC insertion on principal cell of CCD
Epithelial sodium channel (ENaC) blockers
Acute kidney injury and chronic kidney disease
↓ Effective arterial blood volume (volume of blood effectively perfusing tissue)
↓ Oxygen perfusion to renal tissue causing renal ischemia
Autonomic neuropathy ↓ sympathetic drive to produce renin
Chronic juxtaglomerular cell damage ↓ synthesis of renin
Blockage of ENaC on the principal cells of the CCD
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is either not formed (ACEi), or blocked at its receptor (ARBs)
Angiotensin II cannot stimulate the release of aldosterone from the adrenal cortex
Adrenal Insufficiency
Adrenal gland cannot produce sufficient amounts of aldosterone
↓ Renin secretion by the afferent arteriole prevents RAAS activation
↓ Aldosterone release from adrenal cortex
↓ ENaC (Na+ reabsorption channel) expression on principal cells of the cortical collecting duct (CCD)
Damage to kidney causes renal impairment and ↓ glomerular filtration rate
↓ Glomerular filtrate production means ↓ tubular flow rate
↓ Na+ delivery to the distal tubule
↓ Na+ reabsorption by ENaCs at the principal cell in CCD
↓ Na+ and water reabsorption by ENaCs in CCD ↓ Effective arterial blood volume (EABV)
Activates renin-angiotensin-aldosterone system (RAAS) leads to ↑ renin secretion in the afferent arteriole (see Physiology of the renin-angiotensin-aldosterone system (RAAS) slide)
↑ Na+ and water loss in tubular lumen
↑ Positive charge in tubular lumen
↓ Electronegativity gradient in tubular lumen
of CCD
↓ K+ excretion by the principal cell in the CCD as there is less of a electronegative gradient
↑ Accumulation of K+ in blood Hyperkalemia
Serum [K+] > 5.1 mmol/L
See Hyperkalemia: Clinical Findings slide
In the case of diabetic nephropathy and NSAIDS ↓ EABV does not stimulate RAAS and therefore aldosterone production
↓ Renin
↓ Aldosterone
↑ Renin secretion activates an ↑ in aldosterone production but aldosterone action at the principal cell is blocked because of ENaCs or resistance in AIN and CIN
↑ Renin
↑ Aldosterone
In the case of ACEi, ARBs, or adrenal insufficiency ↑ renin secretion does not lead to ↑ aldosterone
↑ Renin
↓ Aldosterone
Note: as described in the above flow chart, measuring serum renin and aldosterone levels can be used to help diagnose the cause of hyperkalemia.
Authors: Mannat Dhillon, Joshua Low, Emily Wildman, Huneza Nadeem Reviewers: Andrea Kuczynski, Marissa (Ran) Zhang, Adam Bass*, Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Aug 2, 2022 on www.thecalgaryguide.com
Hyperkalemia (↓ Renal Excretion): Pathophysiology
Non-steroidal anti- inflammatory drugs (NSAIDs)
Inhibition of prostaglandins which promote renin secretion (see NSAIDs and the Kidney: Mechanism of Action and Side Effects slide)
Diabetic Nephropathy
Acute (AIN)and chronic (CIN) interstitial nephritis
Immune-mediated damage of the kidney tubule and interstitium
Damage to distal tubule leads to aldosterone resistance at principal cell
Aldosterone cannot ↑ EnaC insertion on principal cell of CCD
Epithelial sodium channel (ENaC) blockers
Acute kidney injury and chronic kidney disease
↓ Effective arterial blood volume (volume of blood effectively perfusing tissue)
↓ Oxygen perfusion to renal tissue causing renal ischemia
Autonomic neuropathy ↓ sympathetic drive to produce renin
Chronic juxtaglomerular cell damage ↓ synthesis of renin
Blockage of ENaC on the principal cells of the CCD
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is either not formed (ACEi), or blocked at its receptor (ARBs)
Angiotensin II cannot stimulate the release of aldosterone from the adrenal cortex
Adrenal Insufficiency
Adrenal gland cannot produce sufficient amounts of aldosterone
↓ Renin secretion by the afferent arteriole prevents RAAS activation
↓ Aldosterone release from adrenal cortex
↓ ENaC (Na+ reabsorption channel) expression on principal cells of the cortical collecting duct (CCD)
Damage to kidney causes renal impairment and ↓ glomerular filtration rate
↓ Glomerular filtrate production means ↓ tubular flow rate
↓ Na+ delivery to the distal tubule
↓ Na+ reabsorption by ENaCs at the principal cell in CCD
↓ Na+ and water reabsorption by ENaCs in CCD ↓ Effective arterial blood volume (EABV)
Activates renin-angiotensin-aldosterone system (RAAS) leads to ↑ renin secretion in the afferent arteriole (see Physiology of the renin-angiotensin-aldosterone system (RAAS) slide)
↑ Na+ and water loss in tubular lumen
↑ Positive charge in tubular lumen
↓ Electronegativity gradient in tubular lumen
of CCD
↓ K+ excretion by the principal cell in the CCD as there is less of a electronegative gradient
↑ Accumulation of K+ in blood Hyperkalemia
Serum [K+] > 5.1 mmol/L
See Hyperkalemia: Clinical Findings slide
In the case of diabetic nephropathy and NSAIDS ↓ EABV does not stimulate RAAS and therefore aldosterone production
↓ Renin
↓ Aldosterone
↑ Renin secretion activates an ↑ in aldosterone production but aldosterone action at the principal cell is blocked because of ENaCs or resistance in AIN and CIN
↑ Renin
↑ Aldosterone
In the case of ACEi, ARBs, or adrenal insufficiency ↑ renin secretion does not lead to ↑ aldosterone
↑ Renin
↓ Aldosterone
Note: as described in the above flow chart, measuring serum renin and aldosterone levels can be used to help diagnose the cause of hyperkalemia.
Authors: Mannat Dhillon, Joshua Low, Emily Wildman, Huneza Nadeem Reviewers: Andrea Kuczynski, Marissa (Ran) Zhang, Adam Bass*, Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Aug 2, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/08/Hyperkalemia-renal-excretion.jpg)
presentation-of-sah
![Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com
Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/SAH-Clinical-Findings-2022.jpg)
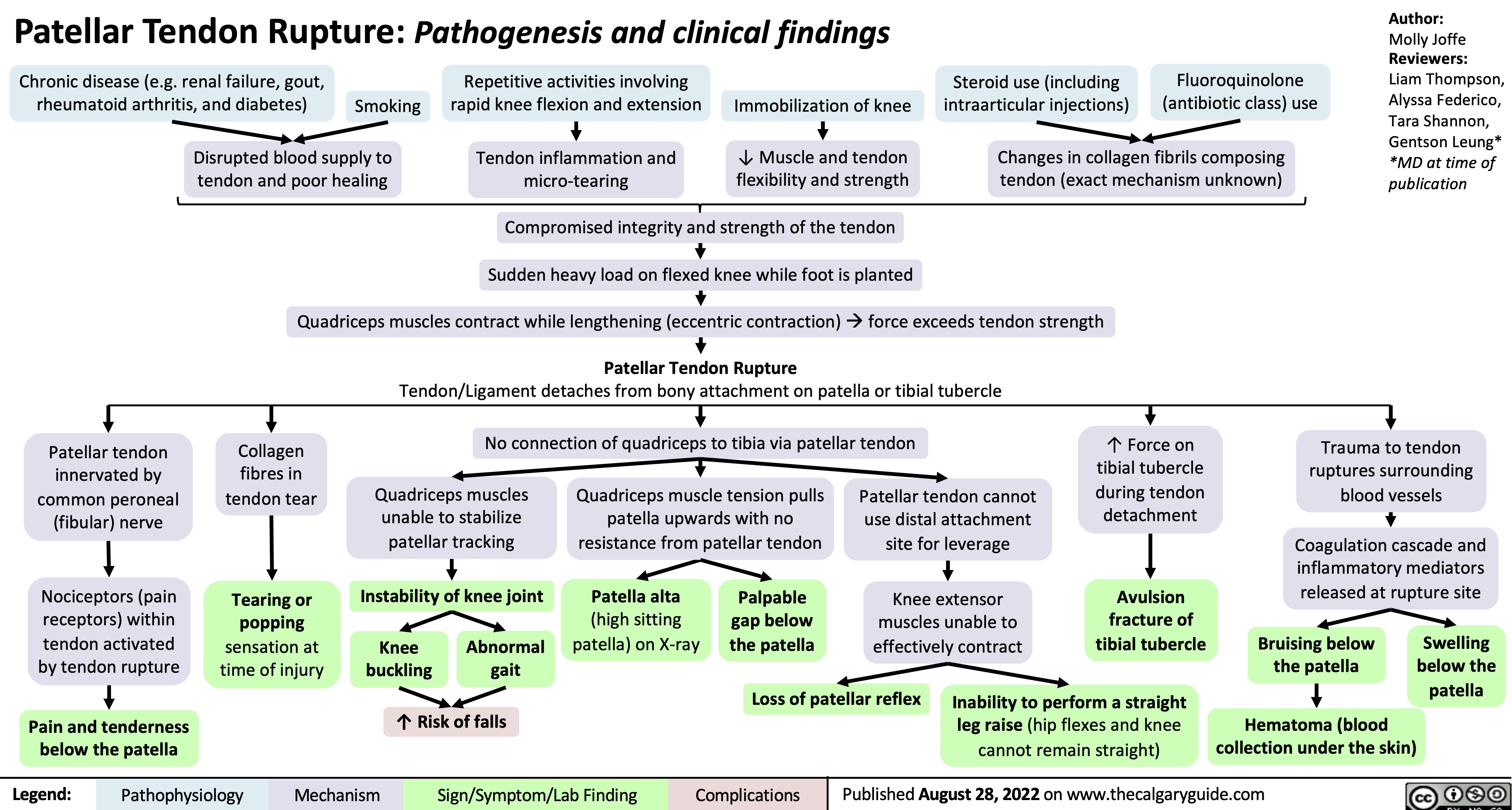
patellar-tendon-rupture-pathogenesis-and-clinical-findings
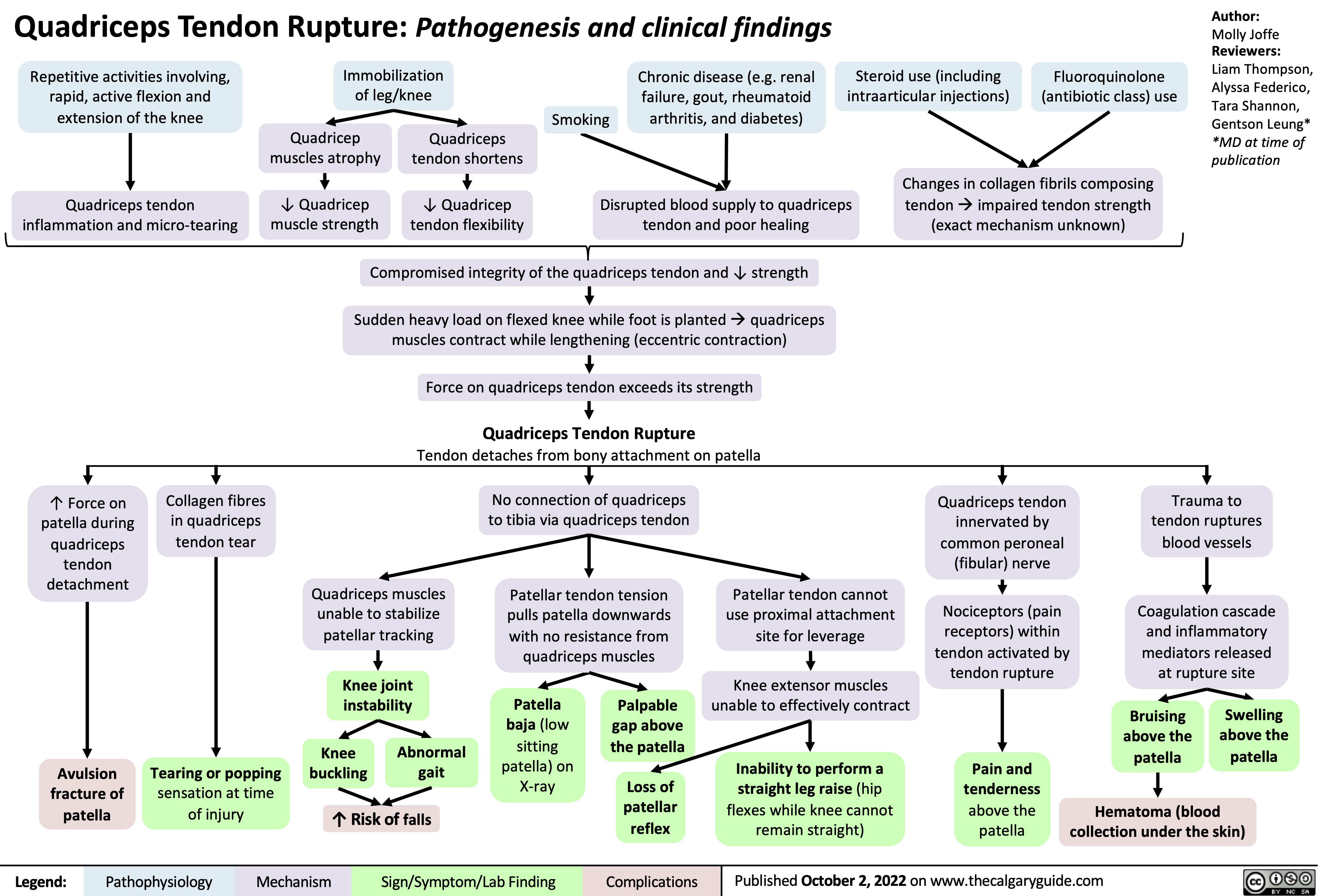
quadriceps-tendon-rupture-pathogenesis-and-clinical-findings
unstable-angina-pathogenesis-and-clinical-findings
![Unstable Angina/Unstable Angina Pectoris: Pathogenesis and clinical findings Primary cause:
Secondary causes:
Coronary artery vasospasm - primary or drug induced (Ex: cocaine, triptans)
Coagulopathy
(Ex: antiphospholipid antibody syndrome)
Vasculitic syndromes (Ex: Takayasu arteritis)
Authors: Marisa Vigna Ryan Wilkie Yan Yu* Reviewers: Julena Foglia Davis Maclean Mehul Gupta Andrew Grant* * MD at time of publication
Atherosclerosis
Fatty plaque accumulates inside the intimal walls of arteries Coronary arterial atherosclerotic plaque rupture or erosion
Plaque disruption exposes subendothelial components of damaged vessel wall to platelets, initiating the coagulation cascade and platelet adhesion
Aggregation of platelets results in the formation of a thrombus Thrombus partially occludes blood flow through a coronary artery âmyocardial blood supply
Congenital anomalies (Ex: myocardial bridge, anomalous coronary)
Spontaneous coronary artery dissection
Increased blood viscosity (Ex: polycythemia, thrombocytopenia)
Factors thatámyocardial (cardiac muscle) oxygen demand (Ex: tachycardia, hypotension, hypertension, anemia, exertion, stress)
Coronary embolism (Ex: A. Fib, endocarditis, prosthetic valve thrombus)
áheart rate, contractility, and/or wall tension ámyocardial oxygen demand
Myocardial ischemia due to imbalance between blood supply and oxygen demand (insufficient blood/oxygen supply)
Unstable Angina/Unstable Angina Pectoris
Can be new onset angina; typically progressive in frequency, severity, or duration; can occur at rest
Subtotal occlusion of a coronary arteryà
reduced, but continued, myocardial blood supply
Maintained perfusion means cardiomyocytes are still alive and thus do not leak troponin into bloodstream
Normal serum troponin
Diaphoresis
(sweating)
Since bloodflow occurs from epicardium to endocardium, myocardial ischemia is more
pronounced in the subendocardium (region furthest away from heart’s external surface)
Sufficient blood flow is maintained in regions superficial to the subendocardium, resulting in non-transmural (partial thickness) heart wall ischemia
Non-inferior wall ischemia triggers a predominantáin sympathetic nervous system activity, given the proximity of cardiac sympathetic nerve innervation
Ischemiaâ cardiomyocyte resting membrane potential andâ action potential duration
Voltage gradient between normal and subendocardial ischemic zones creates injury currents, shifting the ST- vector on ECG
ECG: ST depression
and/or T wave inversion
Cardiac sensory nerve fibres mix with somatic sensory nerve
fibres and enter the spinal cord via the T1-T4 nerve roots
Brain perceives increased cardiac sensory nerve signaling as nerve pain coming from the skin of T1-T4 dermatomes (“Referred Pain”)
Myocardial ischemia causes hypoxic stress on cardiomyocytesàâaerobic (requiring oxygen) metabolism,áanaerobic (not requiring oxygen) metabolism
áanerobic respirationálactic acid production,á[H+], andâcellular pH which impairs cardiomyocyte function
Cardiomyocyte dysfunction impairs myocardial relaxation in diastole and/orâ left ventricular contractility in systole
âleft ventricular cardiac output àbackup of blood in the left ventricle, atrium, and pulmonary vasculature
ápulmonary capillary pressures pushes fluid out of the capillaries into the alveoli in the lungs
Fluid filled alveoliâgas exchange andâ oxygenation, triggering harder and faster breathing in order to compensate
Dyspnea
Activation of sweat glands via acetylcholine release
Hormones bind to cardiac β1 receptors
Tachycardia
(áheart rate)
Epinephrine/ Norepinephrine hormone release from the adrenal medulla
Hormones bind to arterial smooth muscle α1 receptors ávascular tone (vasoconstriction)
Hypertension
The Vagus nerve sits in close physical proximity to the inferior wall of the heart àinferior wall ischemia triggers involuntary Vagus nerve activation
Since the Vagus nerve coordinates parasympathetic activity,áVagus nerve activity leads to a variety of parasympathetic nervous system responses:
Retrosternal discomfort: May present as pain, heaviness, tightness, aching, pressure, burning or squeezing
Pain radiation to T1-T4 dermatomes:
Left shoulder and arm, lower jaw, neck, abdomen, upper back
Syncope
(fainting)
Bradycardia
Nausea Hypotension
(âheart rate)
(âblood pressure)
(áblood pressure)
(shortness of breath)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Findings
Complications
Published Oct 18, 2015, updated Aug 29, 2021 on www.thecalgaryguide.com
Unstable Angina/Unstable Angina Pectoris: Pathogenesis and clinical findings Primary cause:
Secondary causes:
Coronary artery vasospasm - primary or drug induced (Ex: cocaine, triptans)
Coagulopathy
(Ex: antiphospholipid antibody syndrome)
Vasculitic syndromes (Ex: Takayasu arteritis)
Authors: Marisa Vigna Ryan Wilkie Yan Yu* Reviewers: Julena Foglia Davis Maclean Mehul Gupta Andrew Grant* * MD at time of publication
Atherosclerosis
Fatty plaque accumulates inside the intimal walls of arteries Coronary arterial atherosclerotic plaque rupture or erosion
Plaque disruption exposes subendothelial components of damaged vessel wall to platelets, initiating the coagulation cascade and platelet adhesion
Aggregation of platelets results in the formation of a thrombus Thrombus partially occludes blood flow through a coronary artery âmyocardial blood supply
Congenital anomalies (Ex: myocardial bridge, anomalous coronary)
Spontaneous coronary artery dissection
Increased blood viscosity (Ex: polycythemia, thrombocytopenia)
Factors thatámyocardial (cardiac muscle) oxygen demand (Ex: tachycardia, hypotension, hypertension, anemia, exertion, stress)
Coronary embolism (Ex: A. Fib, endocarditis, prosthetic valve thrombus)
áheart rate, contractility, and/or wall tension ámyocardial oxygen demand
Myocardial ischemia due to imbalance between blood supply and oxygen demand (insufficient blood/oxygen supply)
Unstable Angina/Unstable Angina Pectoris
Can be new onset angina; typically progressive in frequency, severity, or duration; can occur at rest
Subtotal occlusion of a coronary arteryà
reduced, but continued, myocardial blood supply
Maintained perfusion means cardiomyocytes are still alive and thus do not leak troponin into bloodstream
Normal serum troponin
Diaphoresis
(sweating)
Since bloodflow occurs from epicardium to endocardium, myocardial ischemia is more
pronounced in the subendocardium (region furthest away from heart’s external surface)
Sufficient blood flow is maintained in regions superficial to the subendocardium, resulting in non-transmural (partial thickness) heart wall ischemia
Non-inferior wall ischemia triggers a predominantáin sympathetic nervous system activity, given the proximity of cardiac sympathetic nerve innervation
Ischemiaâ cardiomyocyte resting membrane potential andâ action potential duration
Voltage gradient between normal and subendocardial ischemic zones creates injury currents, shifting the ST- vector on ECG
ECG: ST depression
and/or T wave inversion
Cardiac sensory nerve fibres mix with somatic sensory nerve
fibres and enter the spinal cord via the T1-T4 nerve roots
Brain perceives increased cardiac sensory nerve signaling as nerve pain coming from the skin of T1-T4 dermatomes (“Referred Pain”)
Myocardial ischemia causes hypoxic stress on cardiomyocytesàâaerobic (requiring oxygen) metabolism,áanaerobic (not requiring oxygen) metabolism
áanerobic respirationálactic acid production,á[H+], andâcellular pH which impairs cardiomyocyte function
Cardiomyocyte dysfunction impairs myocardial relaxation in diastole and/orâ left ventricular contractility in systole
âleft ventricular cardiac output àbackup of blood in the left ventricle, atrium, and pulmonary vasculature
ápulmonary capillary pressures pushes fluid out of the capillaries into the alveoli in the lungs
Fluid filled alveoliâgas exchange andâ oxygenation, triggering harder and faster breathing in order to compensate
Dyspnea
Activation of sweat glands via acetylcholine release
Hormones bind to cardiac β1 receptors
Tachycardia
(áheart rate)
Epinephrine/ Norepinephrine hormone release from the adrenal medulla
Hormones bind to arterial smooth muscle α1 receptors ávascular tone (vasoconstriction)
Hypertension
The Vagus nerve sits in close physical proximity to the inferior wall of the heart àinferior wall ischemia triggers involuntary Vagus nerve activation
Since the Vagus nerve coordinates parasympathetic activity,áVagus nerve activity leads to a variety of parasympathetic nervous system responses:
Retrosternal discomfort: May present as pain, heaviness, tightness, aching, pressure, burning or squeezing
Pain radiation to T1-T4 dermatomes:
Left shoulder and arm, lower jaw, neck, abdomen, upper back
Syncope
(fainting)
Bradycardia
Nausea Hypotension
(âheart rate)
(âblood pressure)
(áblood pressure)
(shortness of breath)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Findings
Complications
Published Oct 18, 2015, updated Aug 29, 2021 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/10/Unstable-Angina-2021.jpg)
type-ii-proximal-renal-tubular-acidosis-pathogenesis-and-laboratory-findings
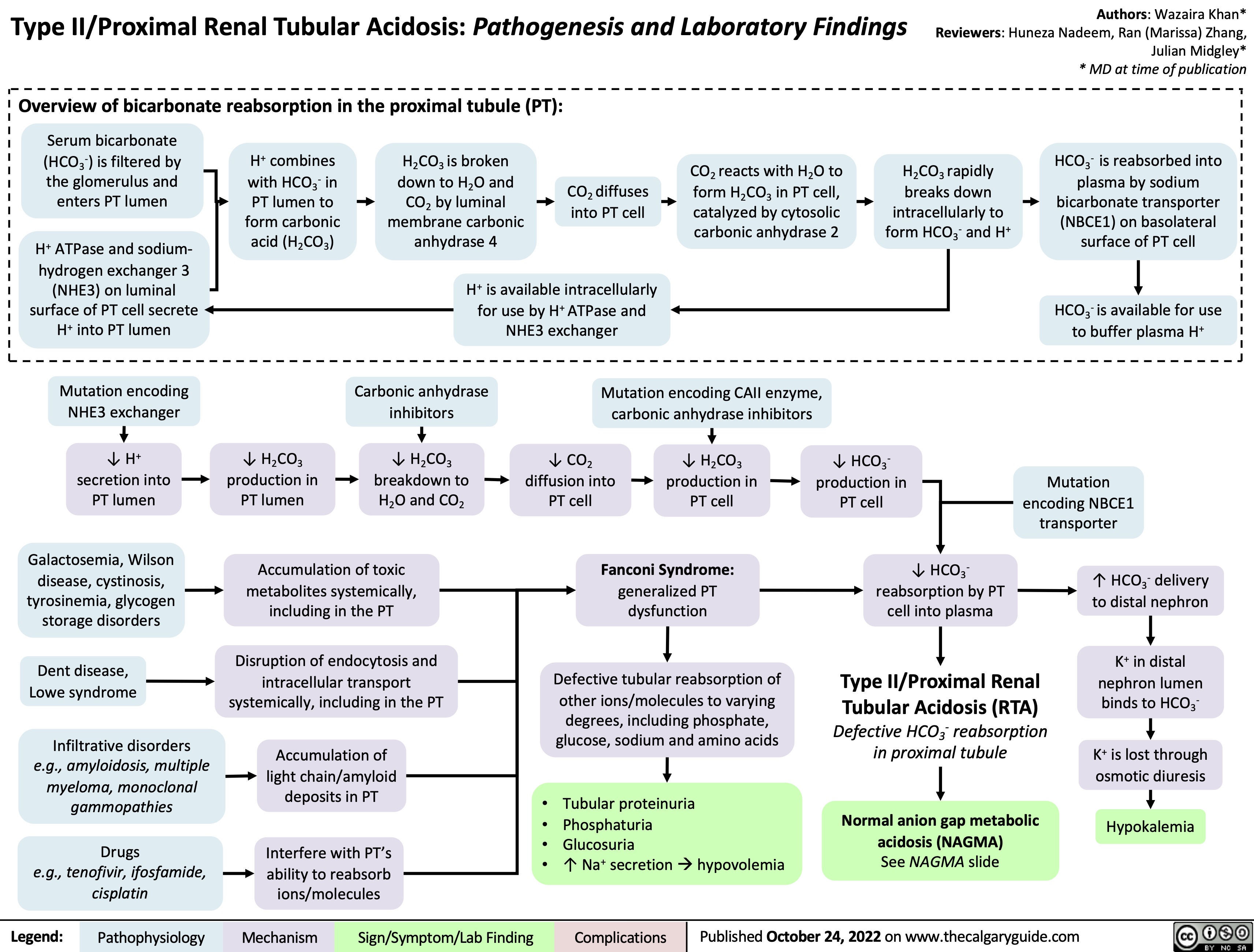
Granulomatosis with Polyangiitis Pathogenesis
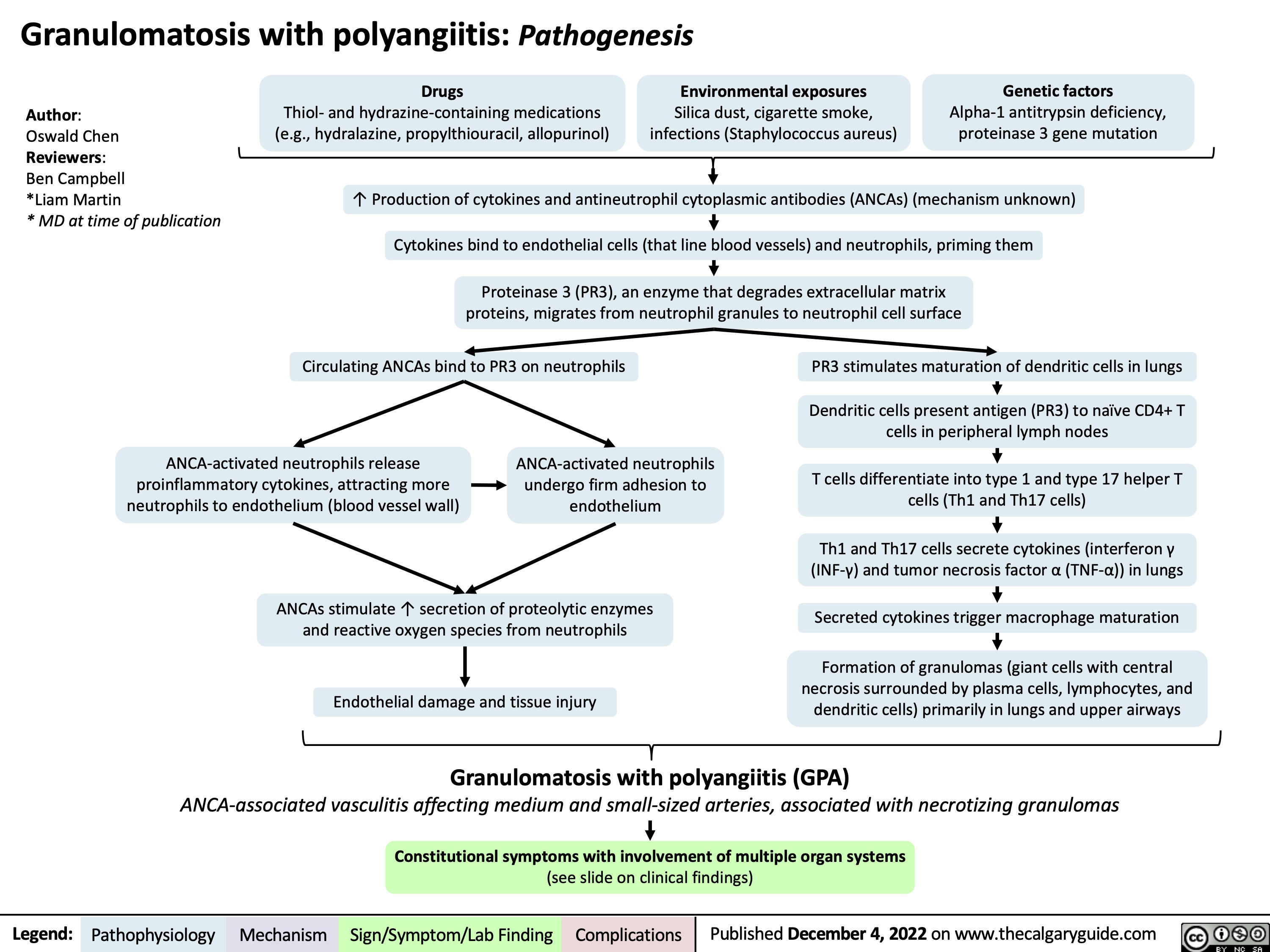
Granulomatosis with polyangiitis: Clinical findings
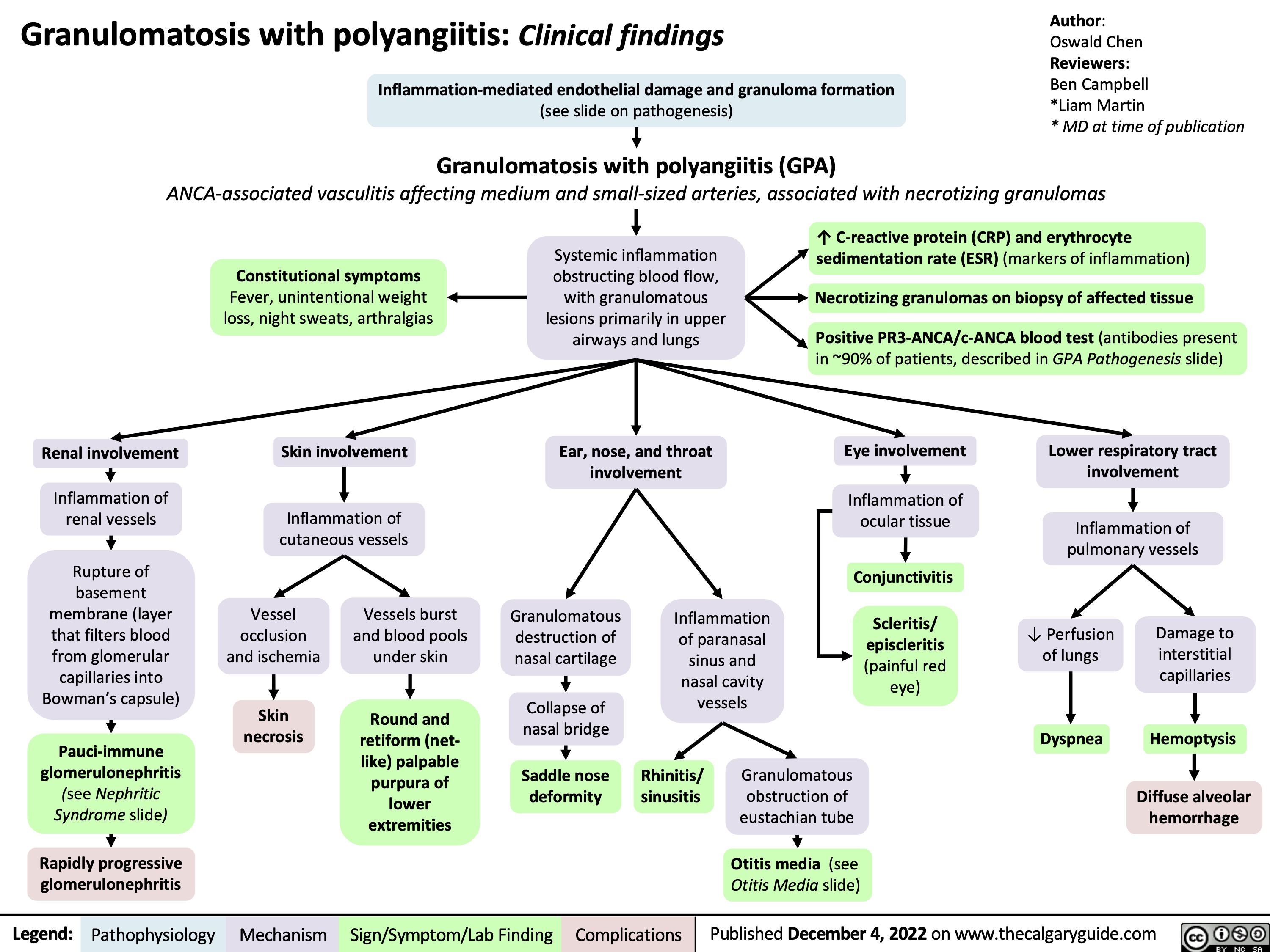
hypovolemic-shock
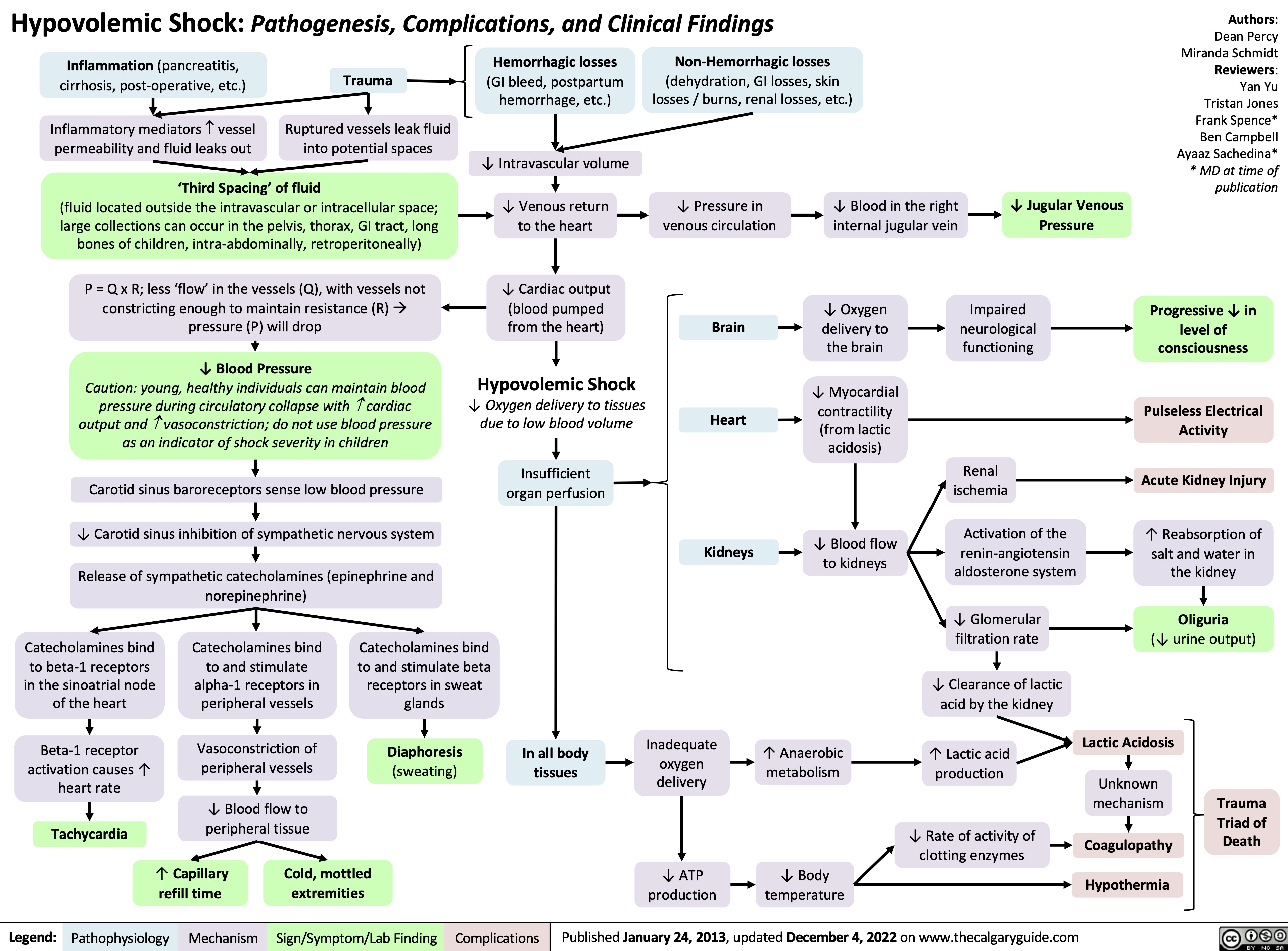
Approach to Arterial Blood Gases ABGs
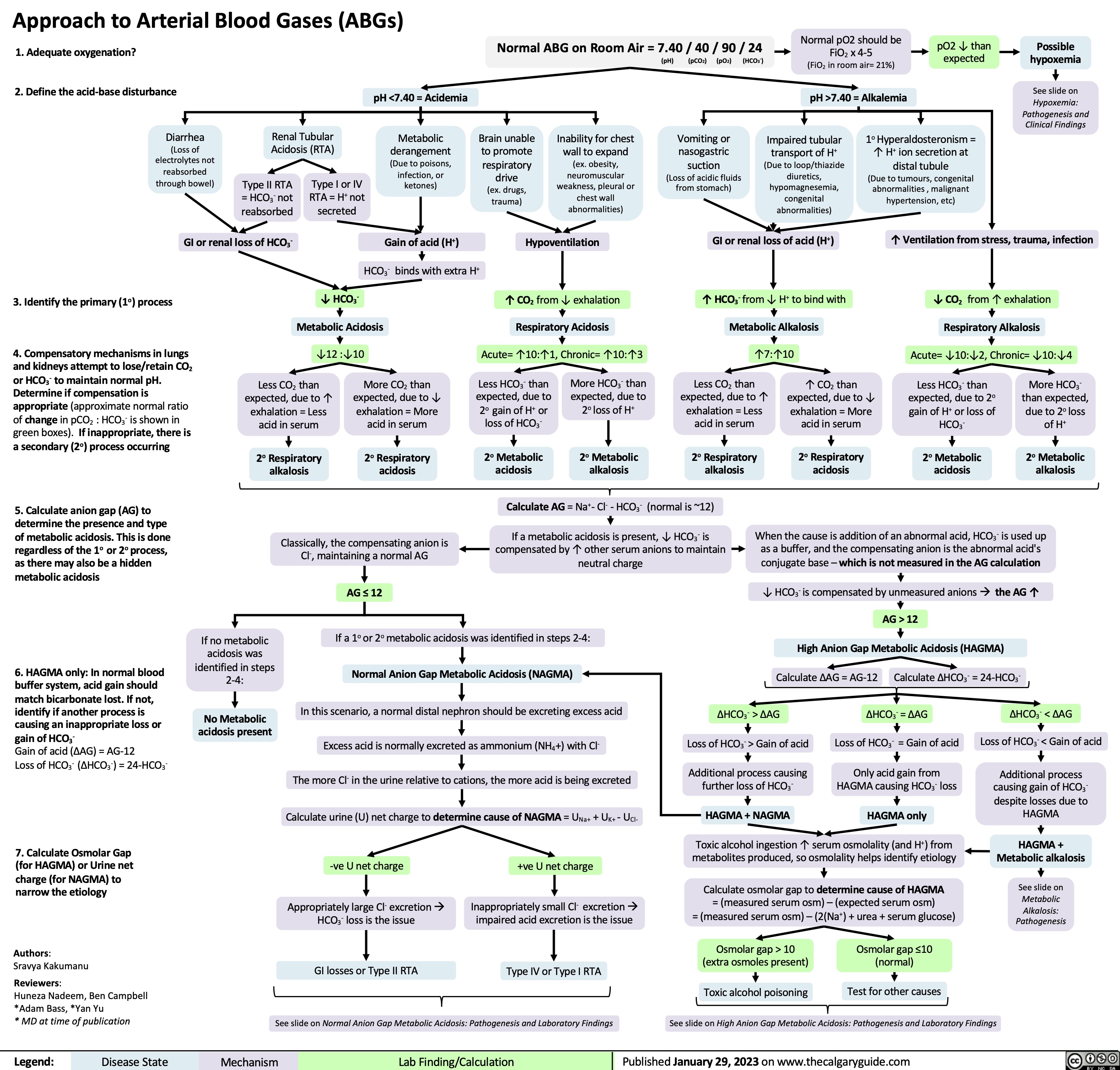
Coronary Artery Bypass Graft CABG Indications
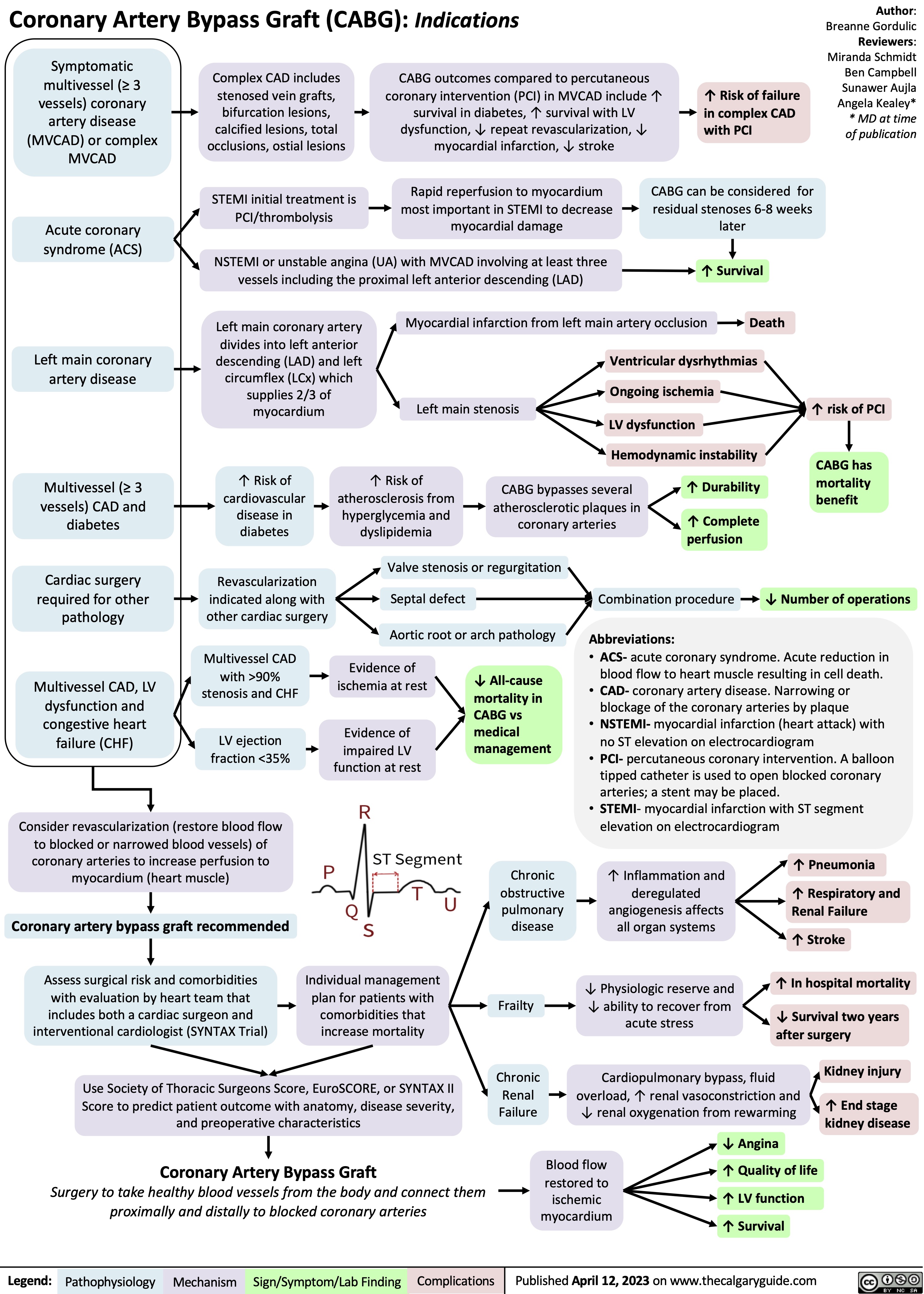
normal neonatal changes pathogenesis and clinical findings
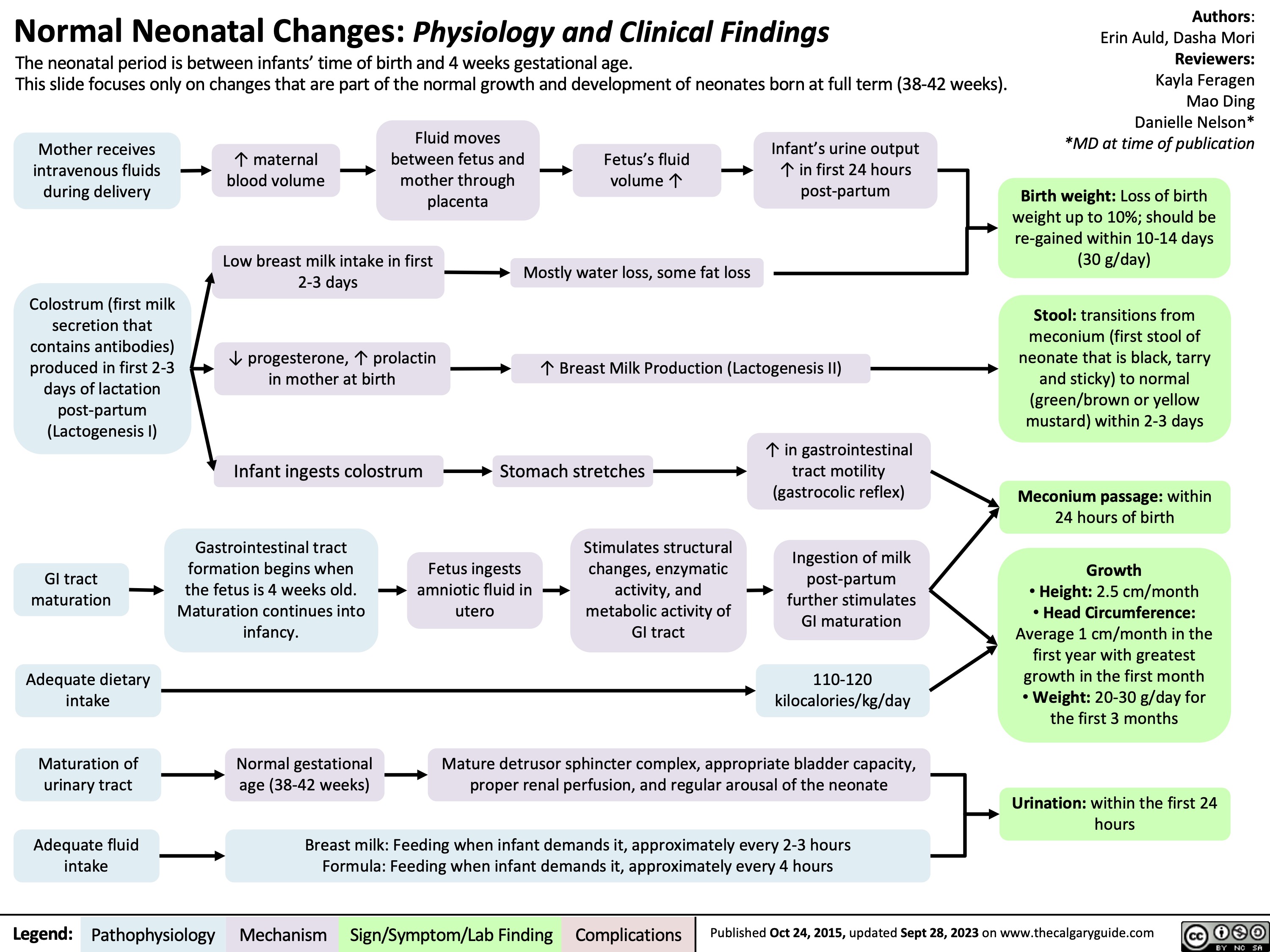
Alcohol Withdrawal Syndrome
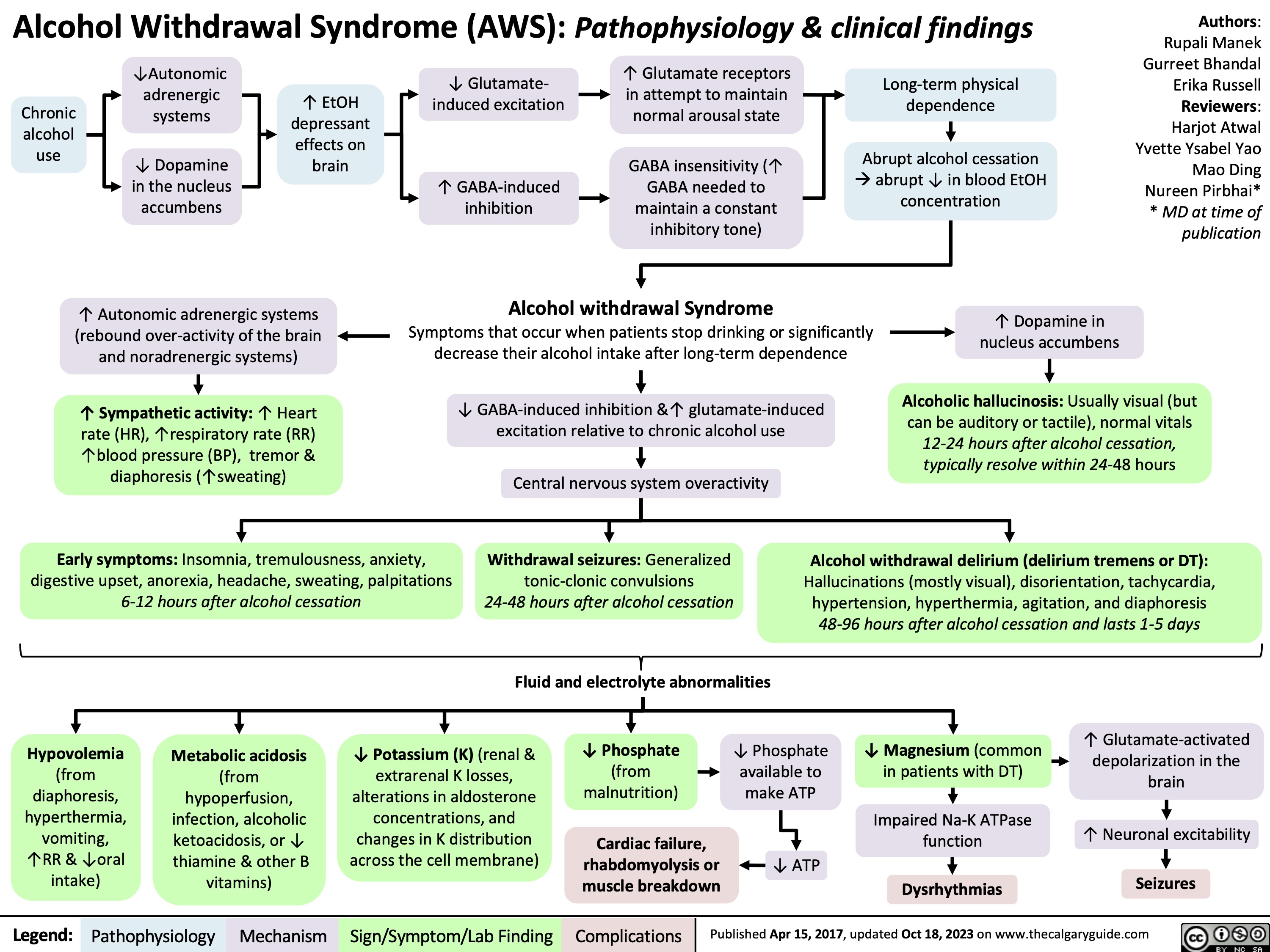
Pharmacotherapy for Dyslipidemia Overview
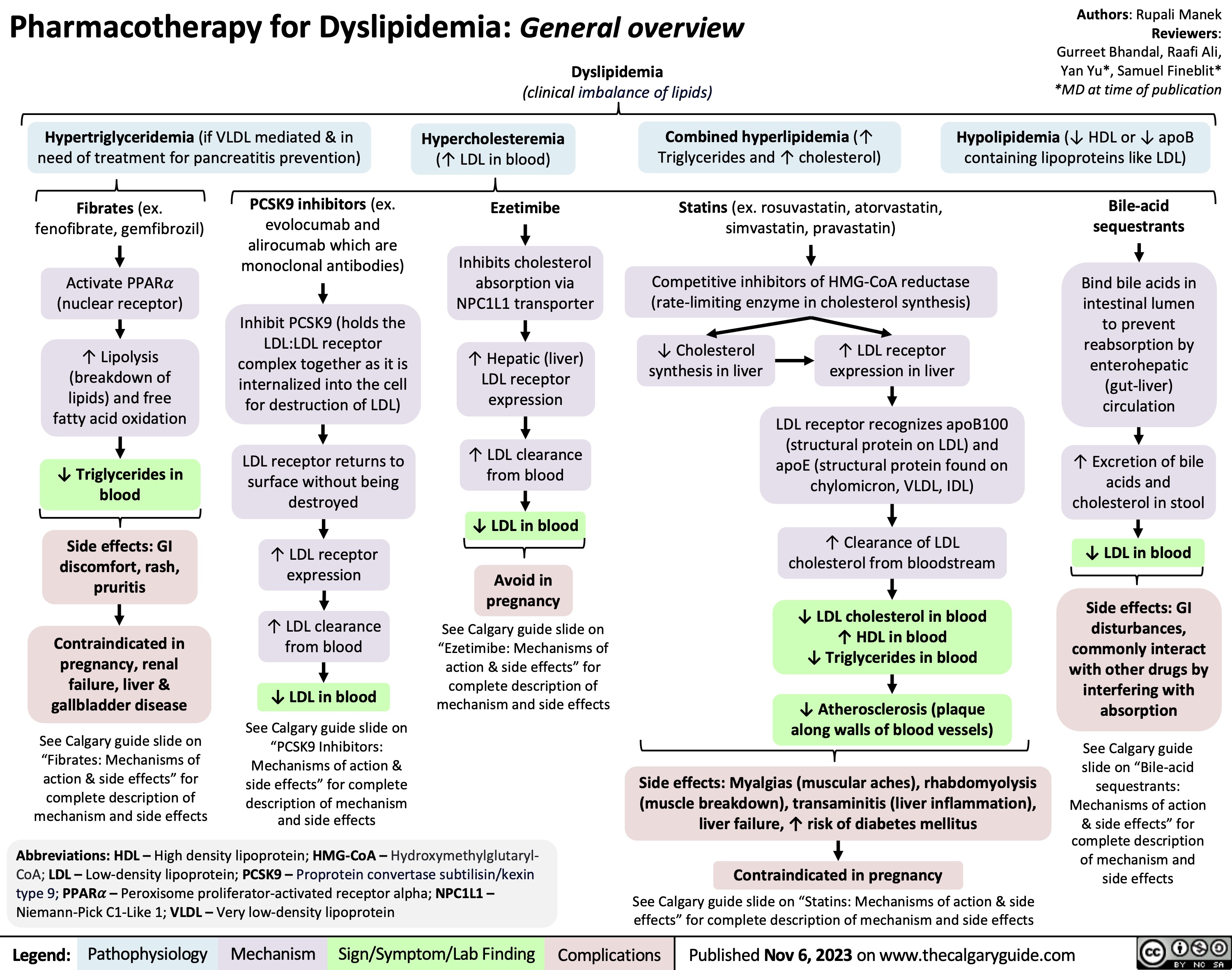
Death Cardiovascular Respiratory and Neurologic Mechanisms
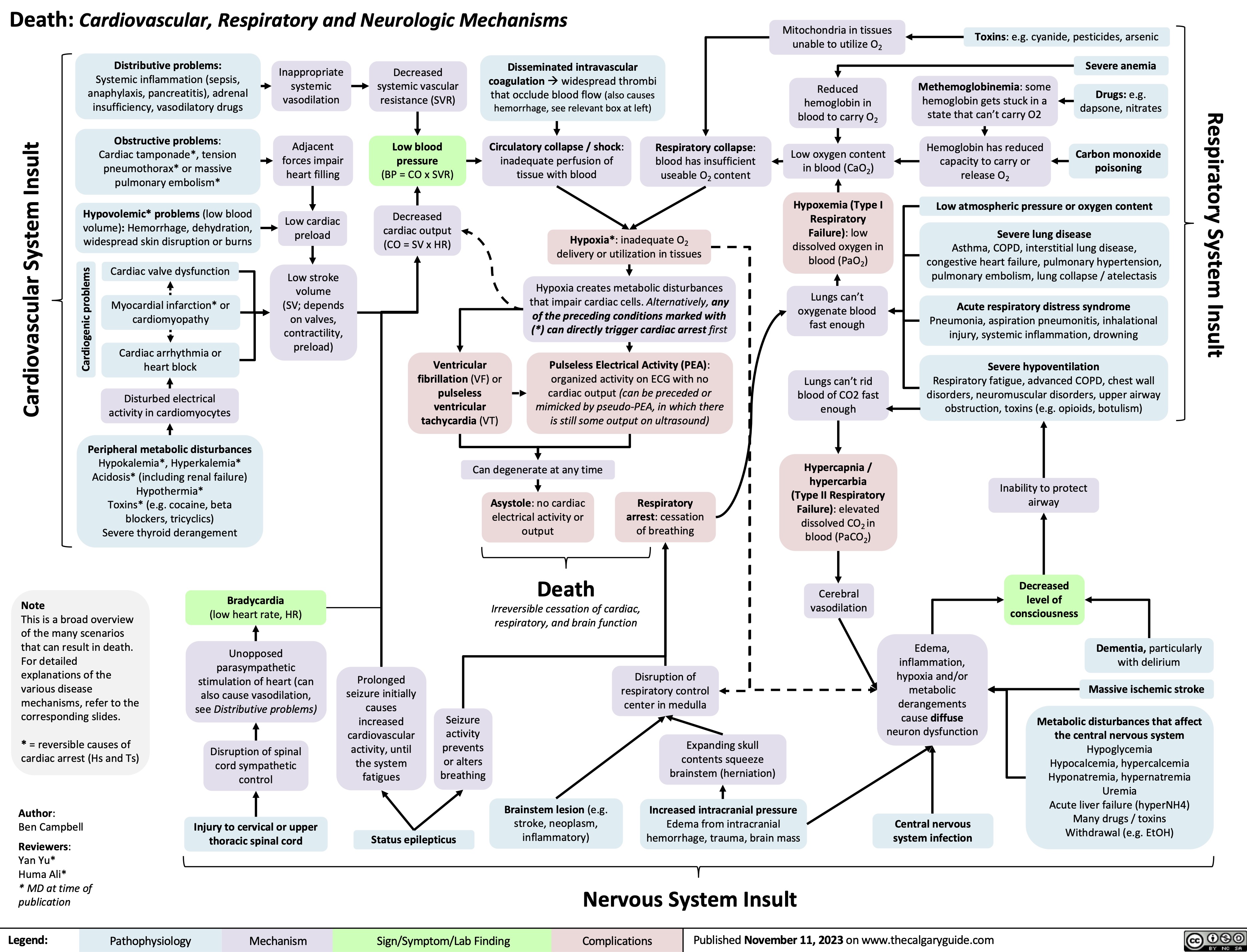
Turner Syndrome Pathogenesis and Clinical Findings
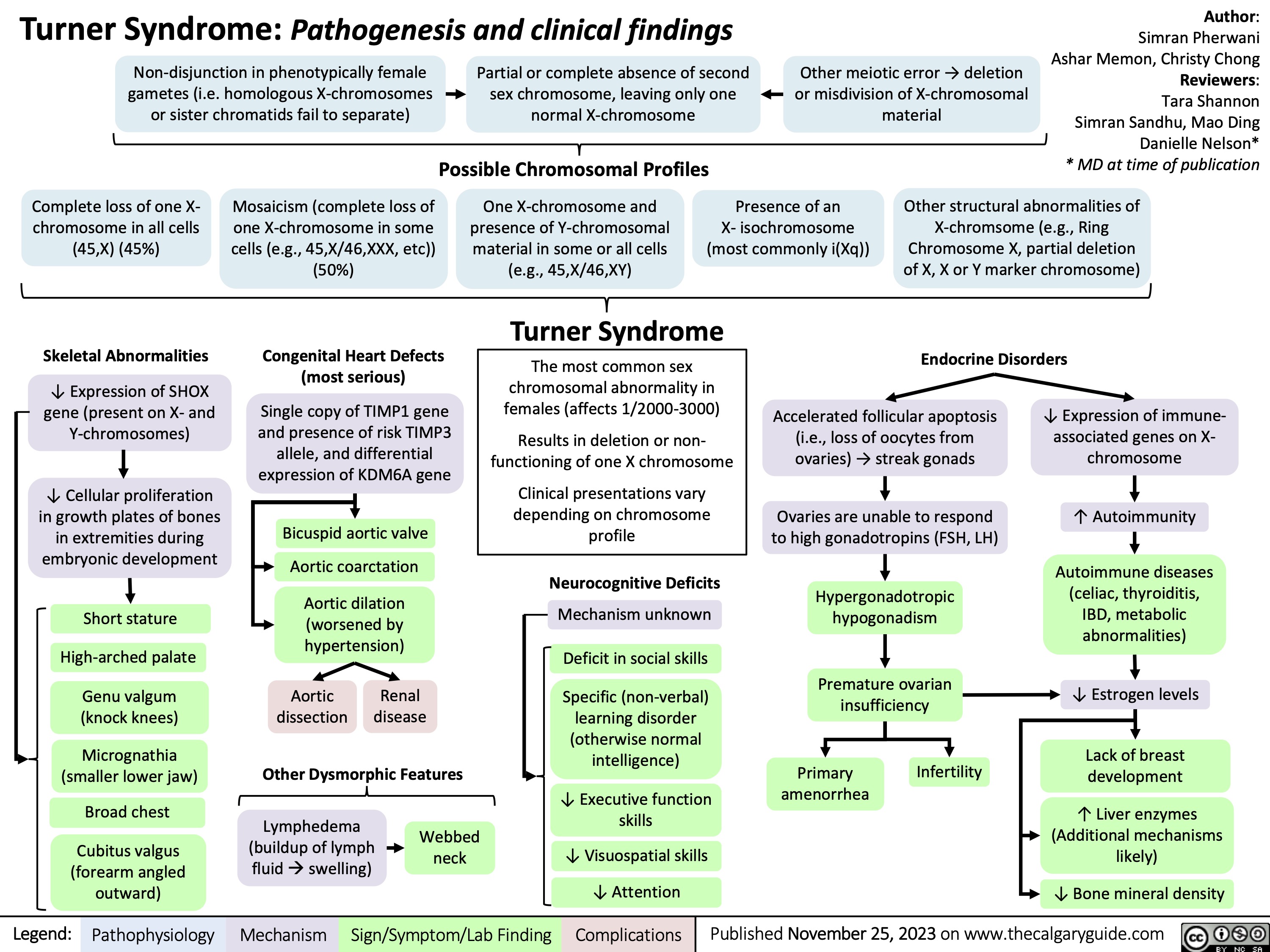
Sugammadex
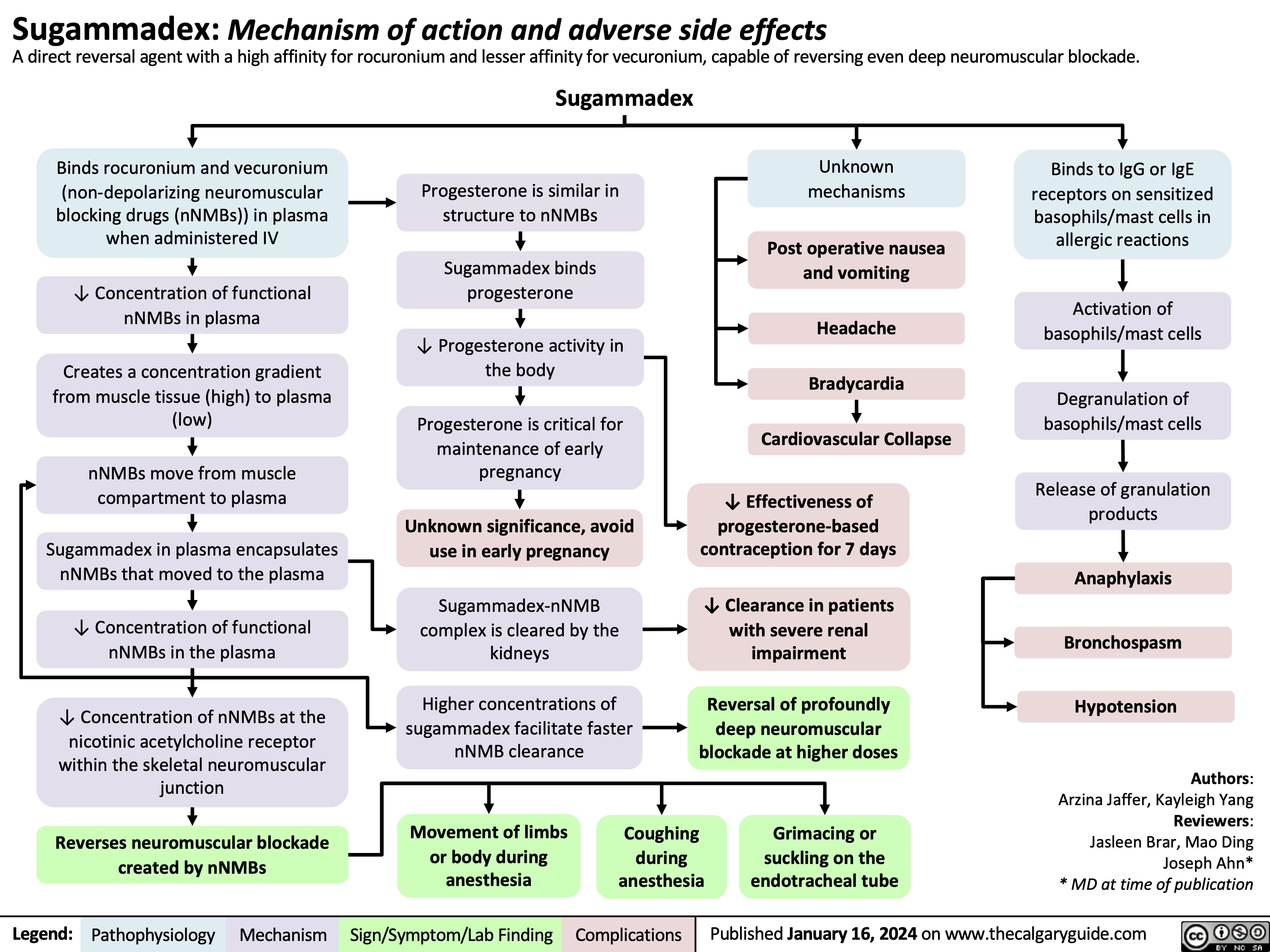
Bacterial Infections from Transfusion
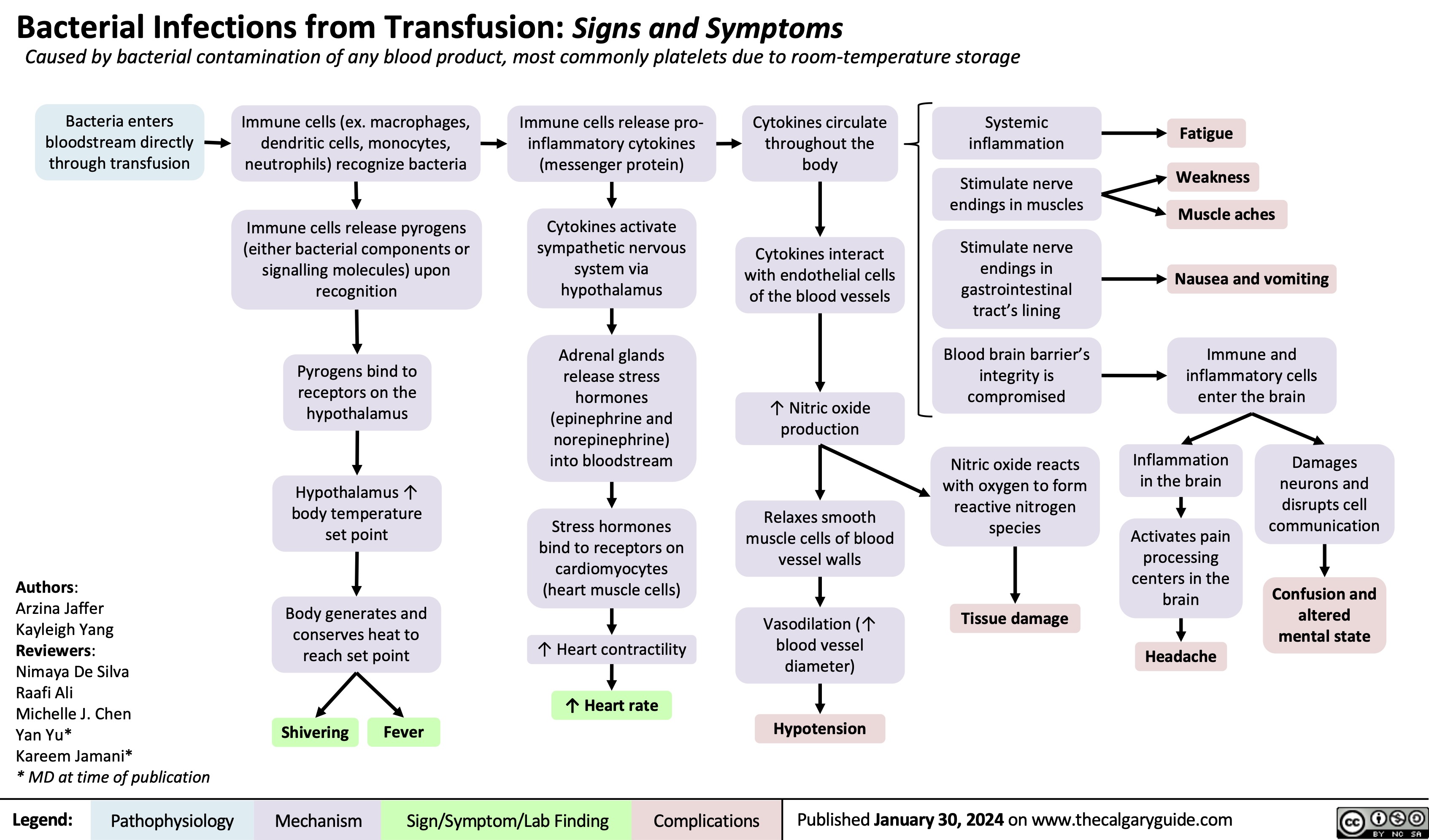
Carbonic Anhydrase Inhibitor Diuretics
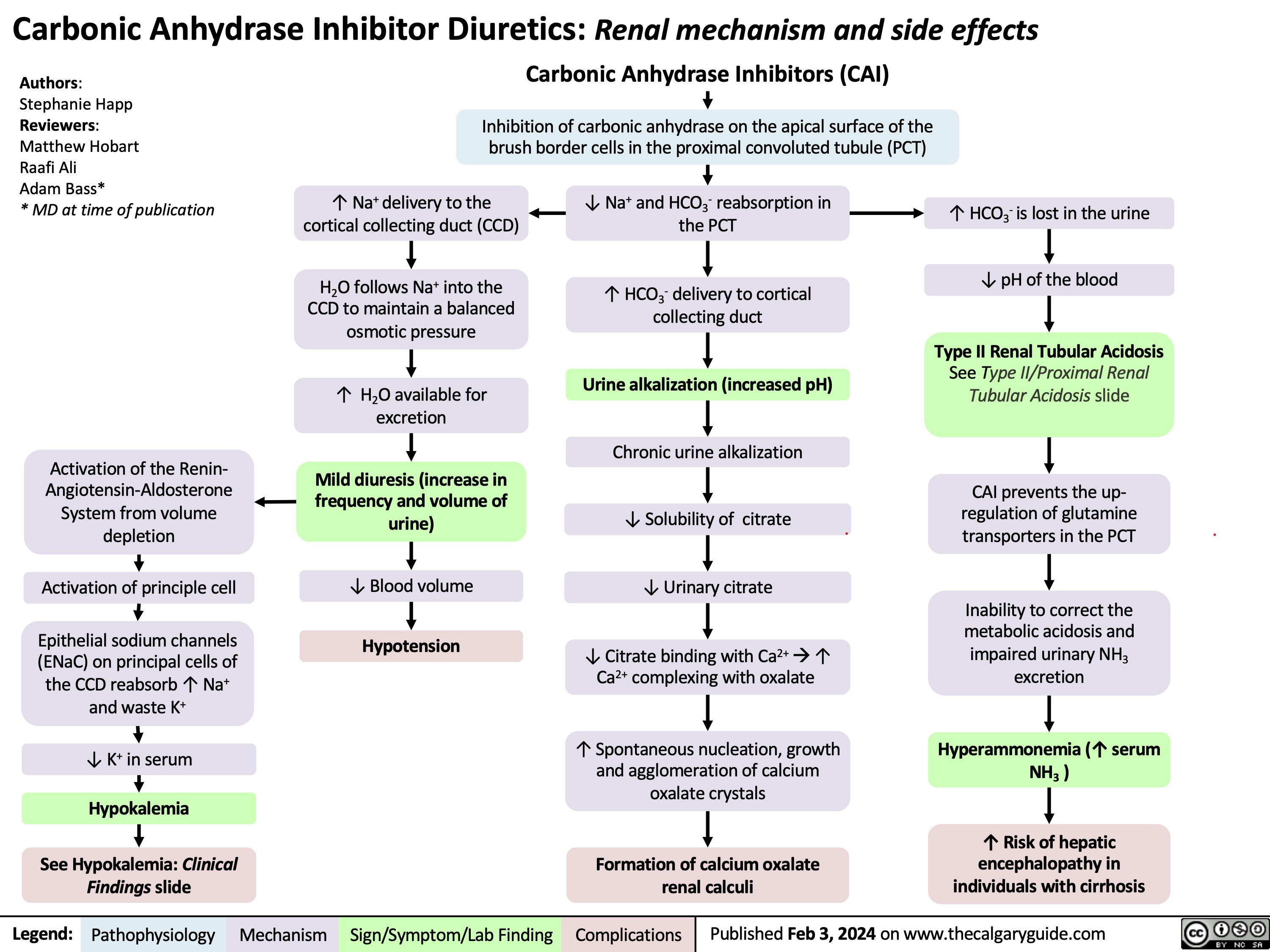
Stable Angina
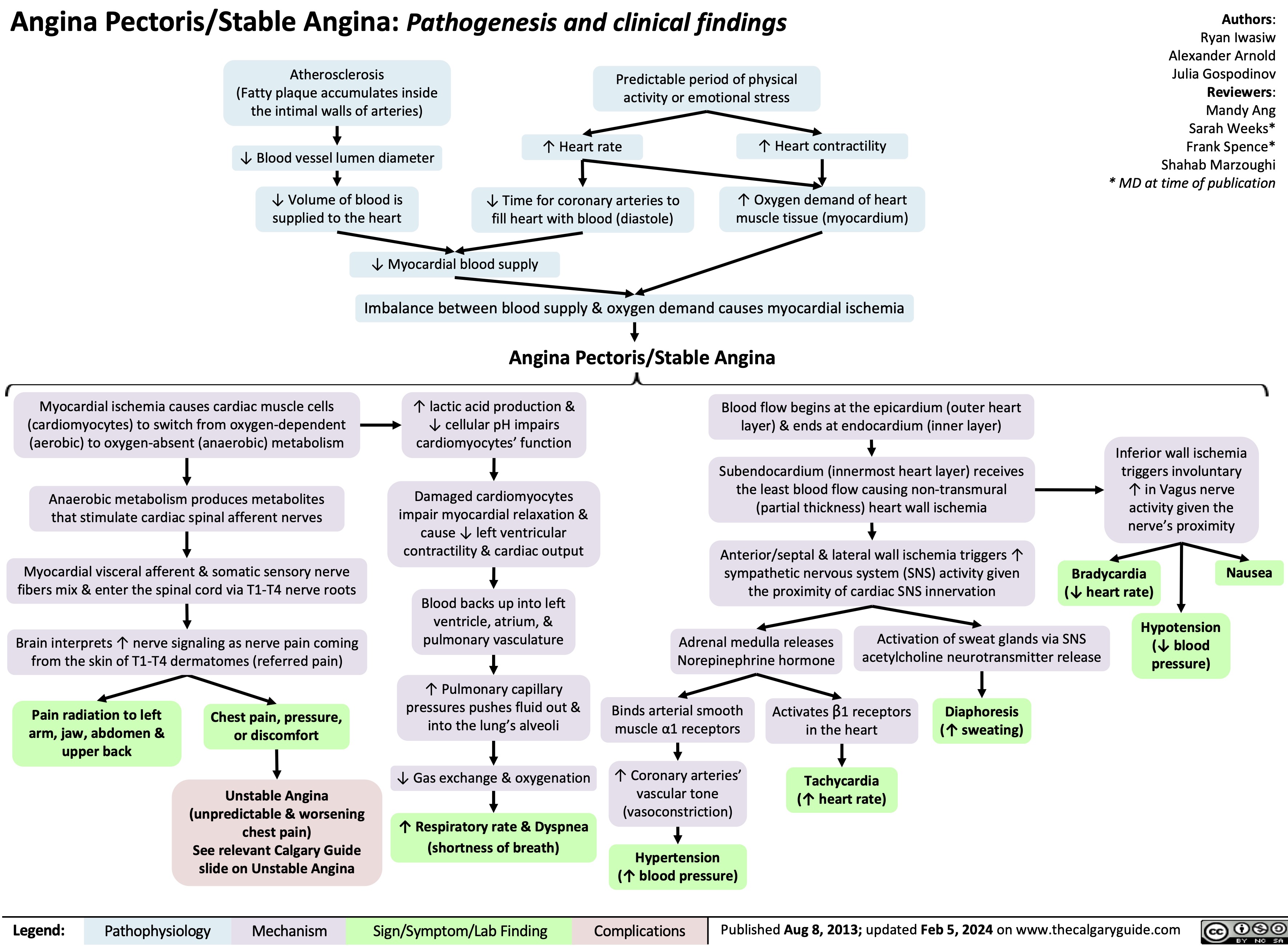
Gestational Diabetes Risk factors and pathogenesis
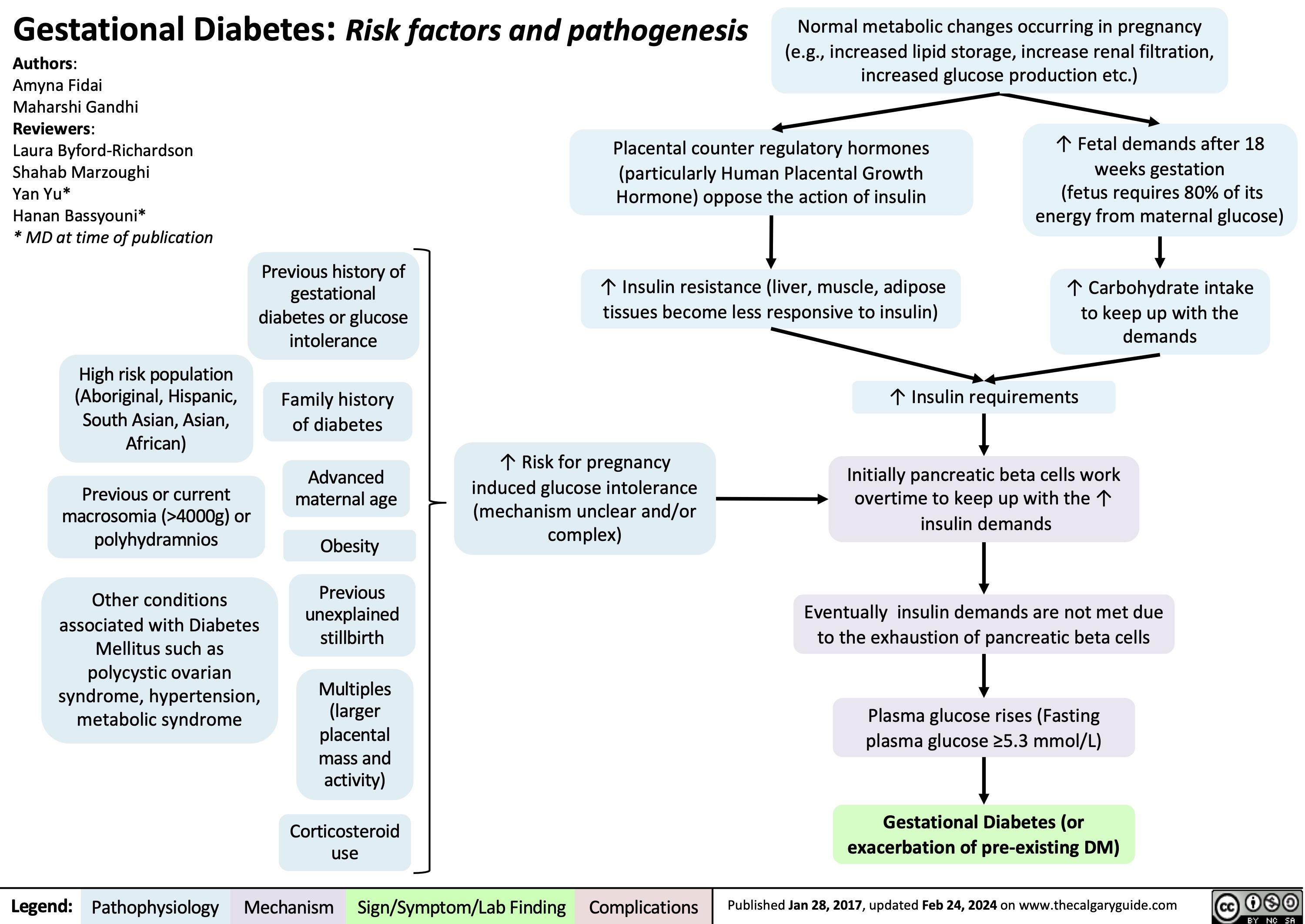
Infectious Small Bowel Diarrhea
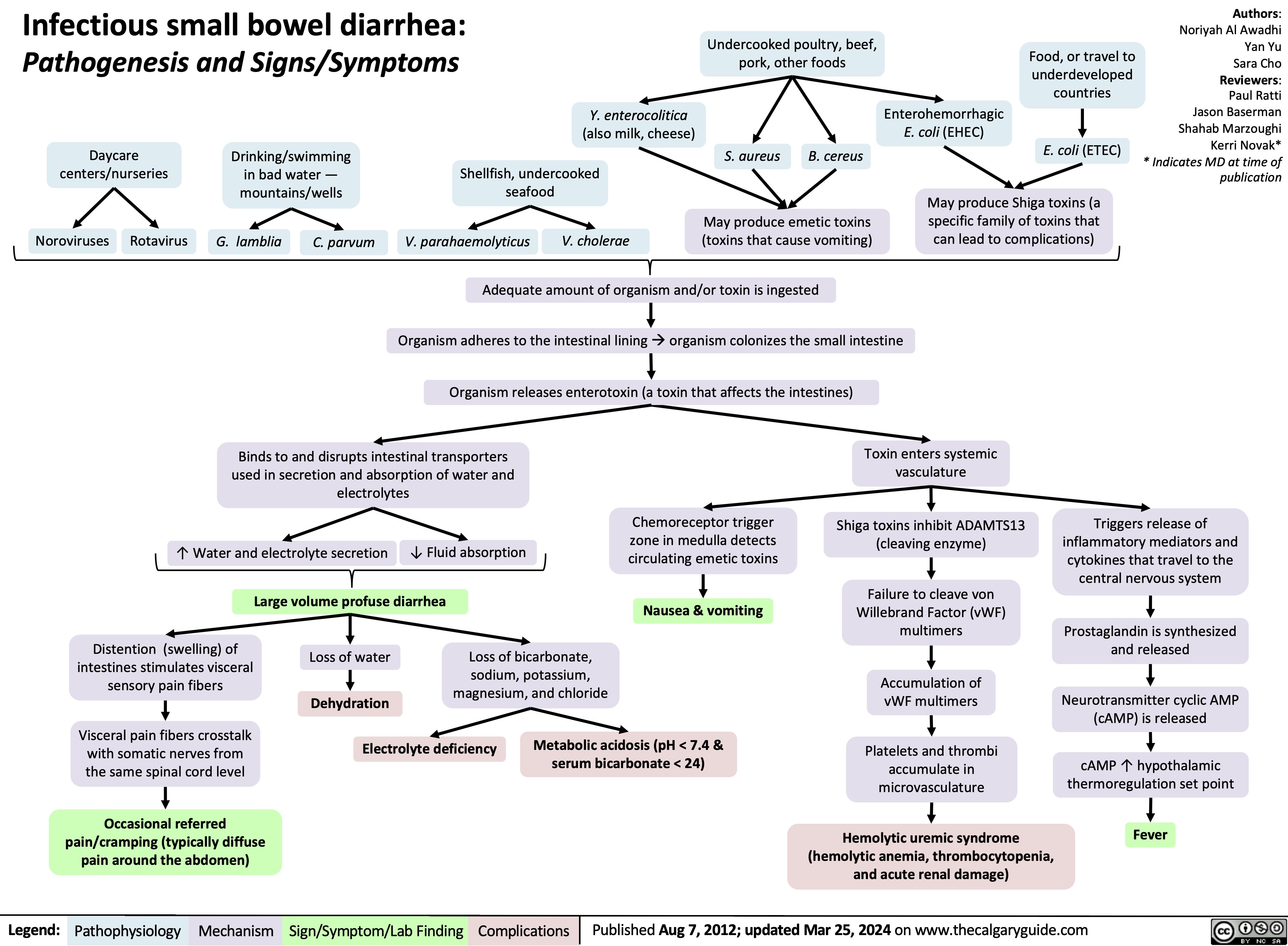
Post-Renal Acute Kidney Injury AKI
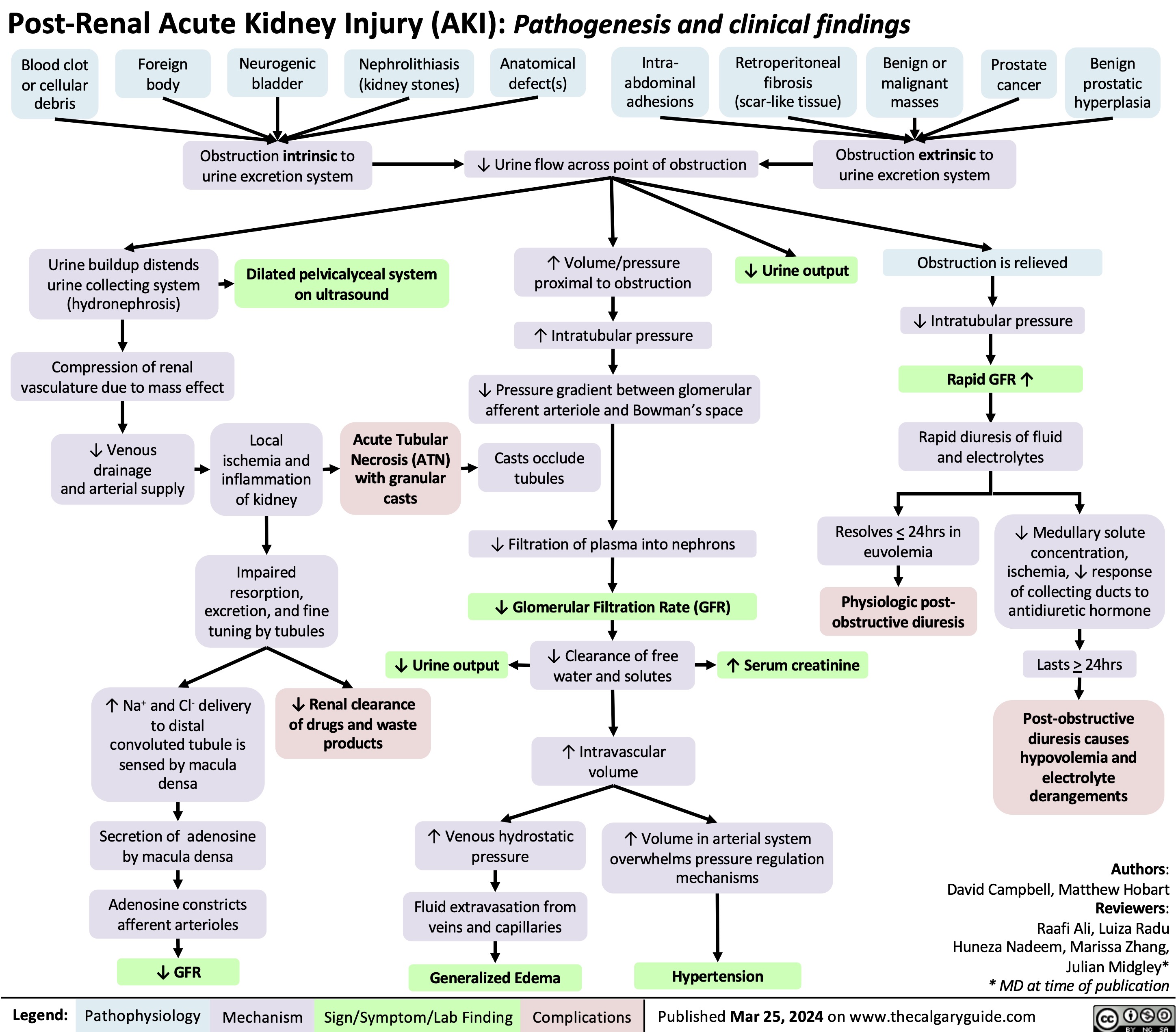
Operative Vaginal Delivery Indications
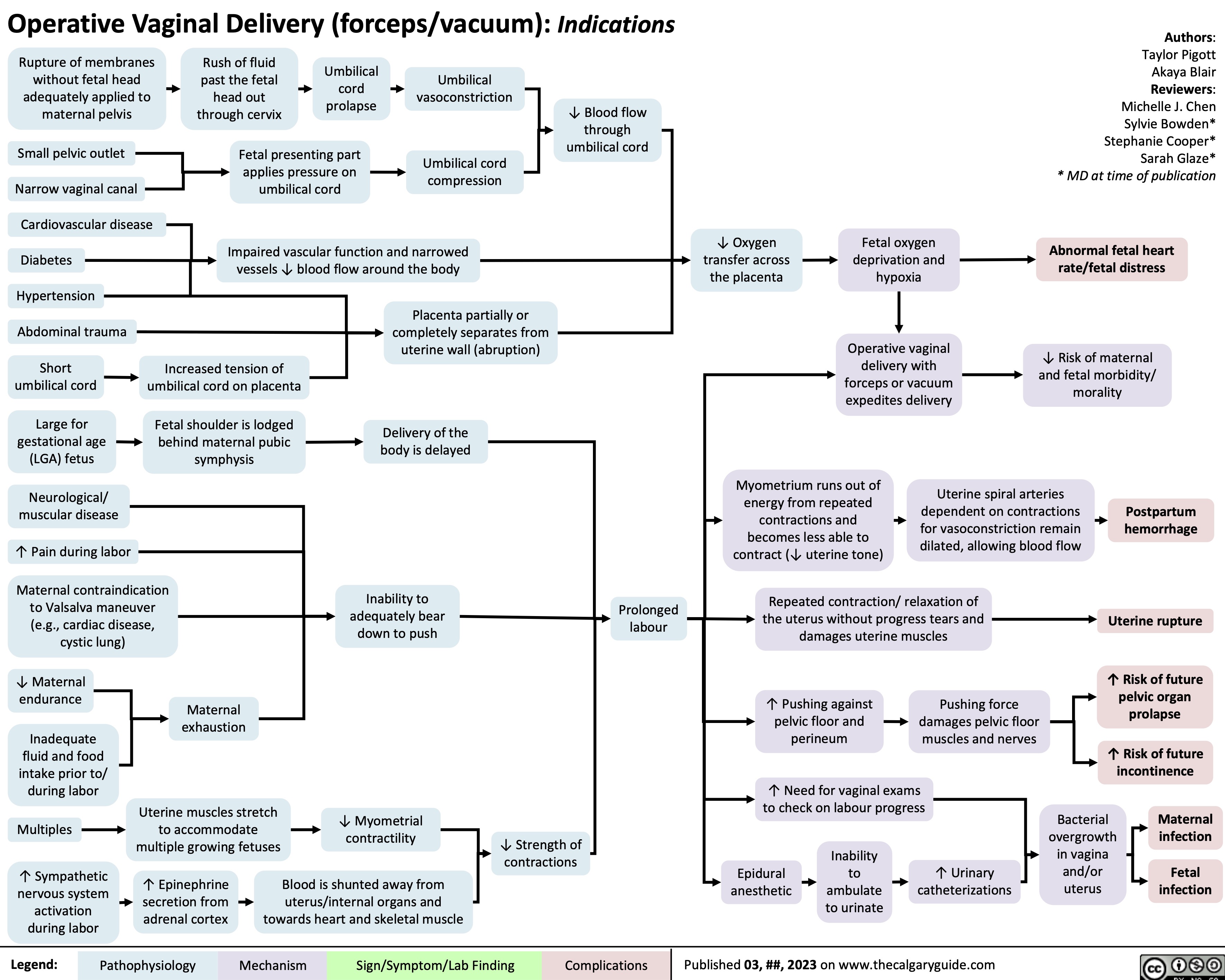
Sickle Cell Disease Pathogenesis Clinical Findings and Complications
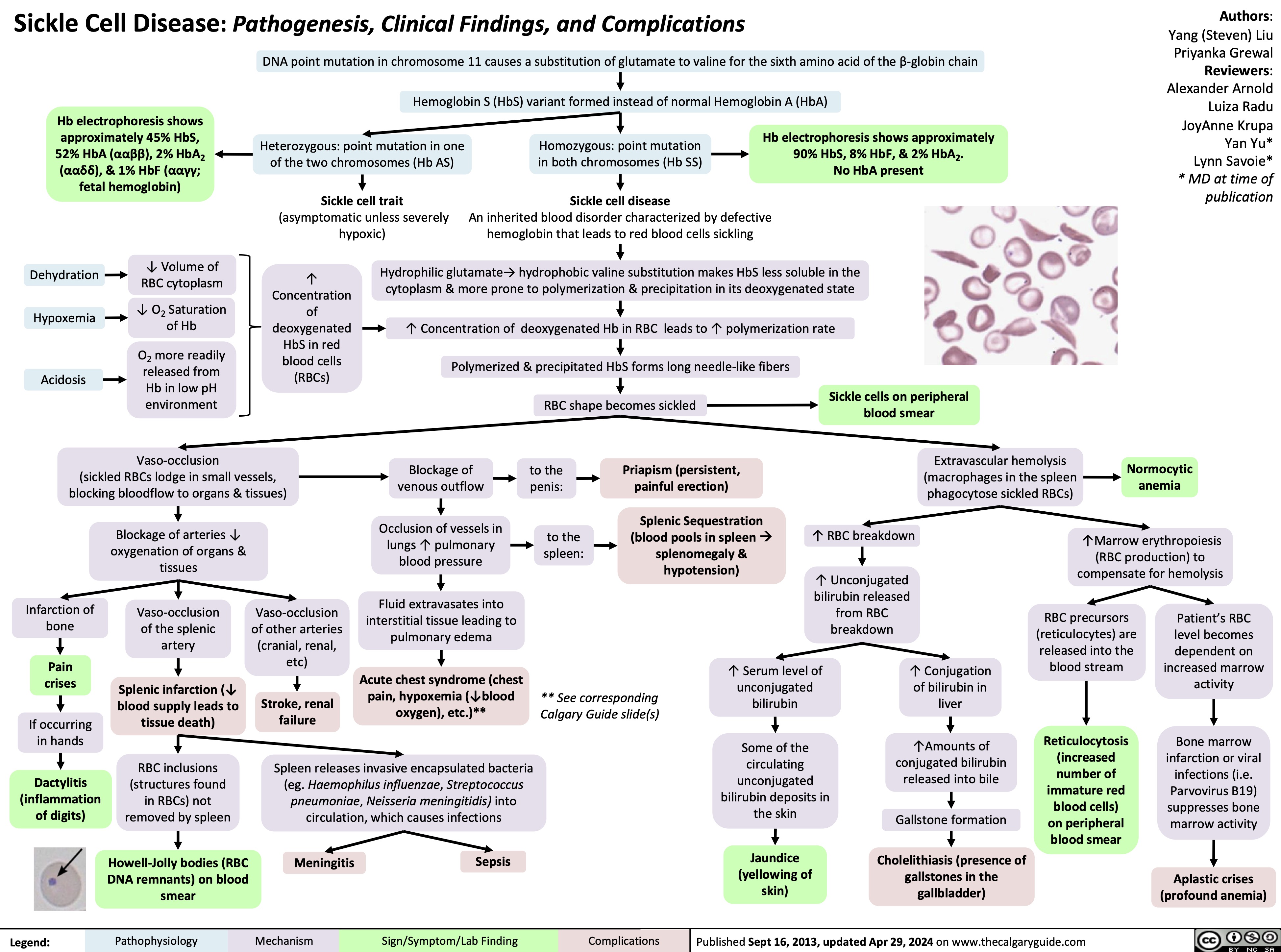
Gynecomastia
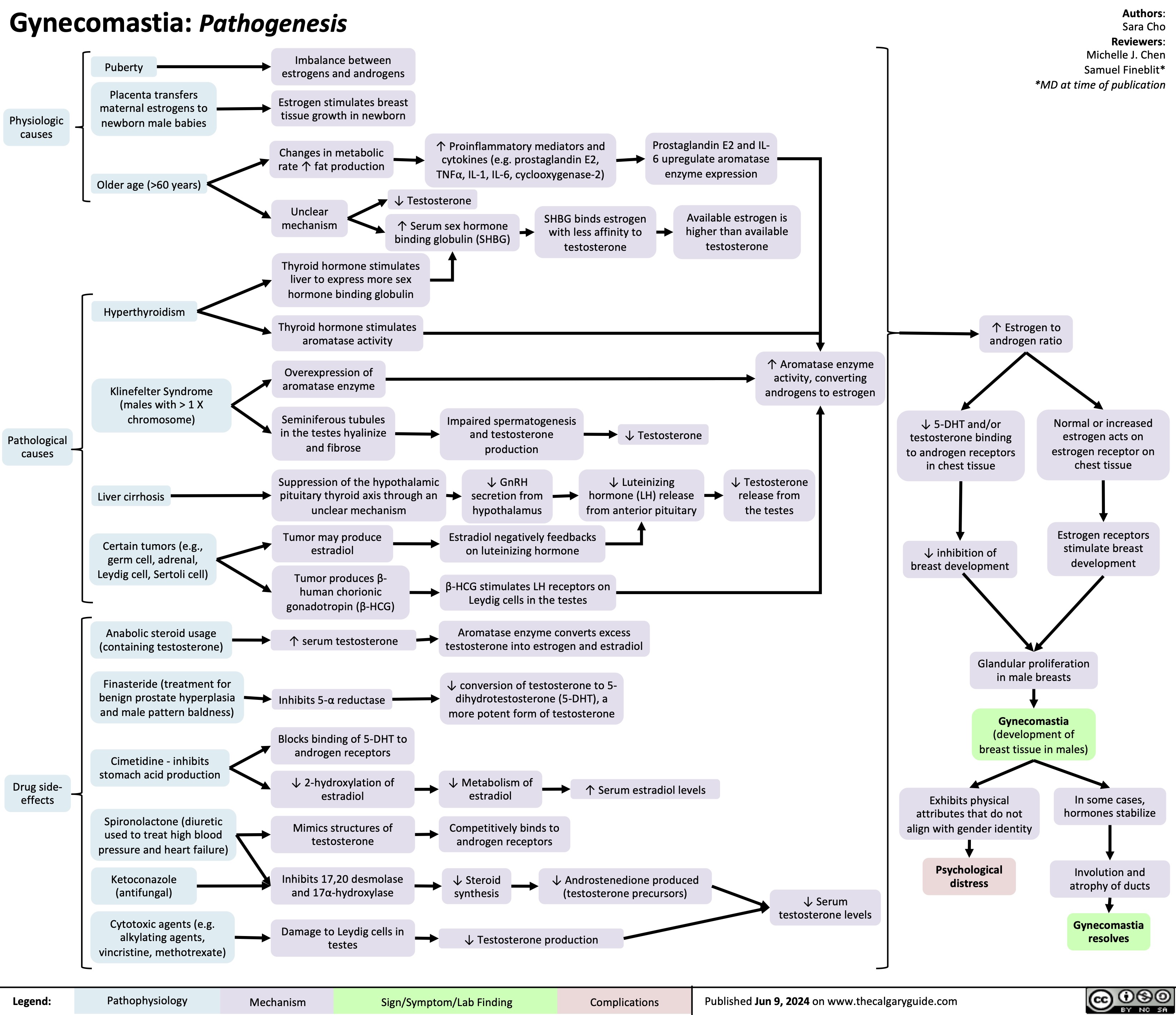
Anesthetic Considerations in Pregnancy
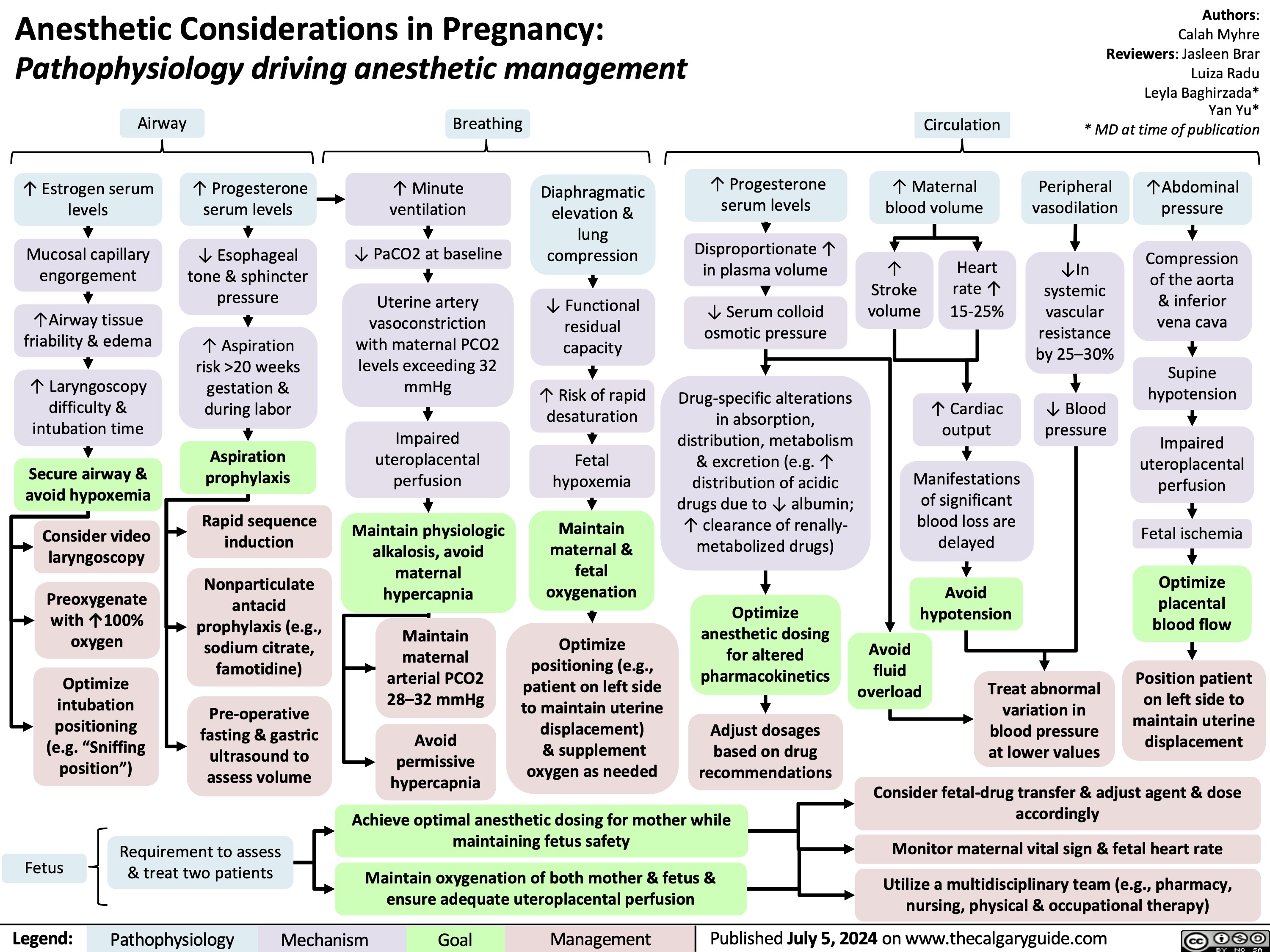
Diabetes Mellitus Pathophysiology Behind Lab Findings
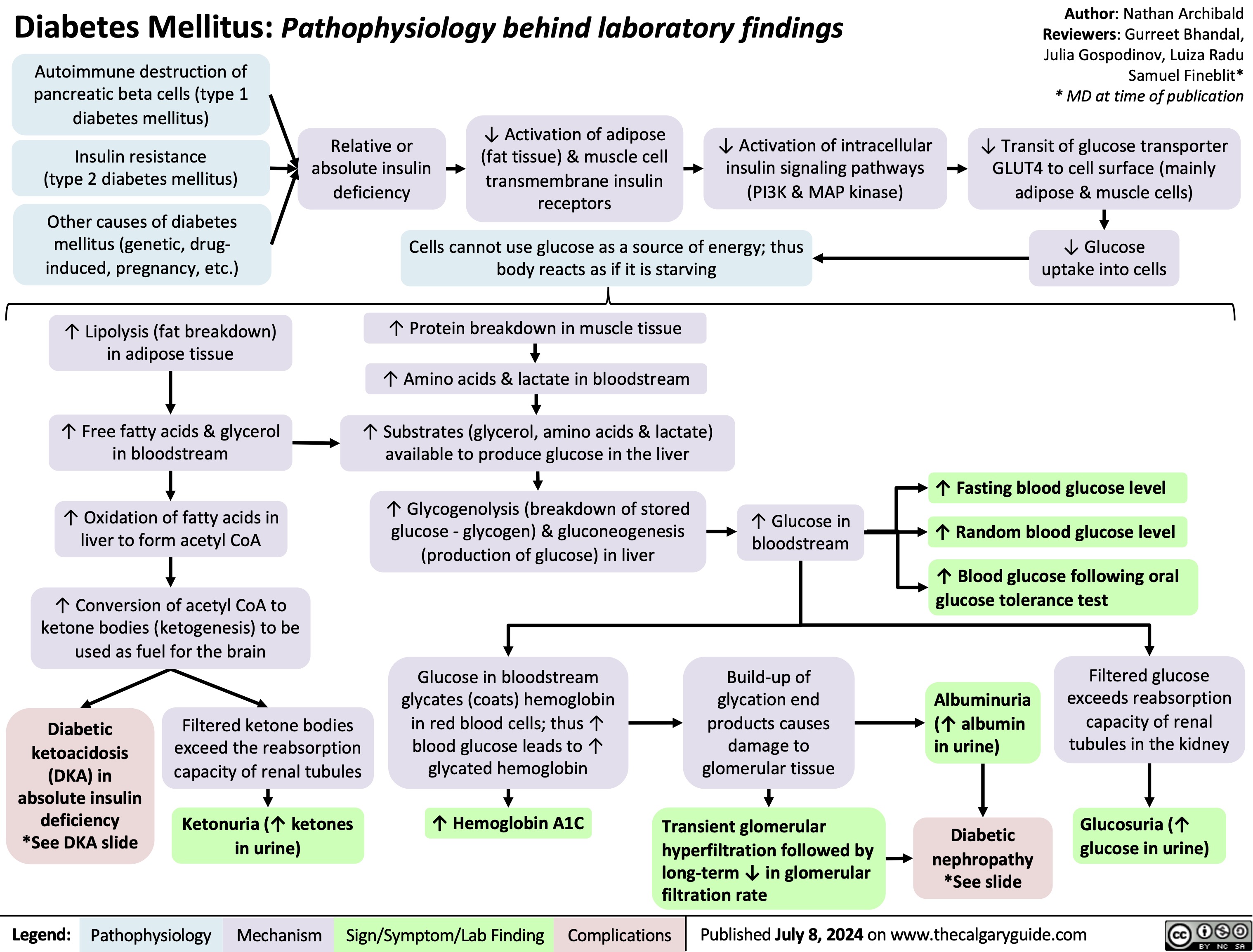
Neonatal Hypoglycemia Pathogenesis
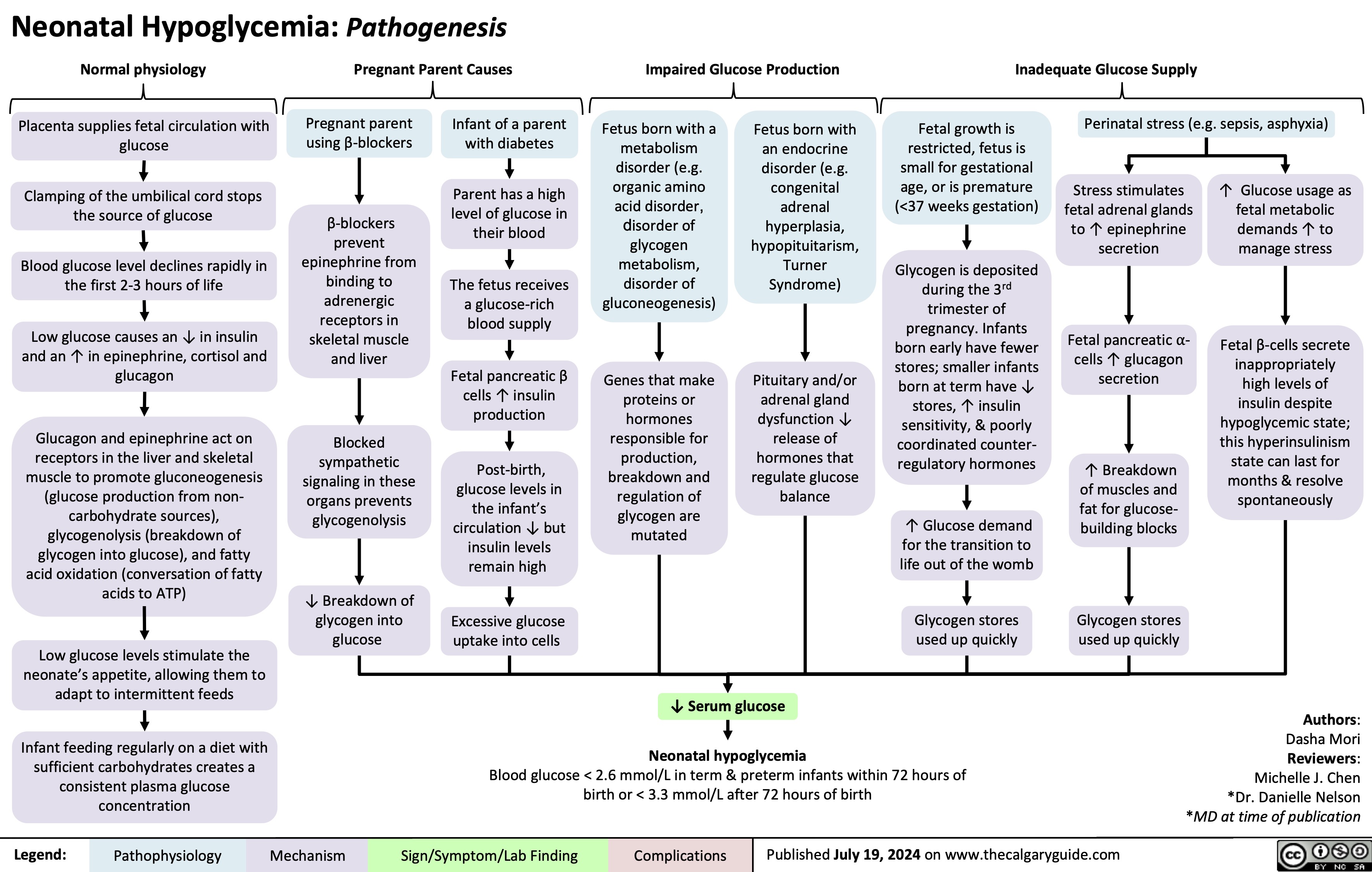
Generalized Anxiety Disorder GAD
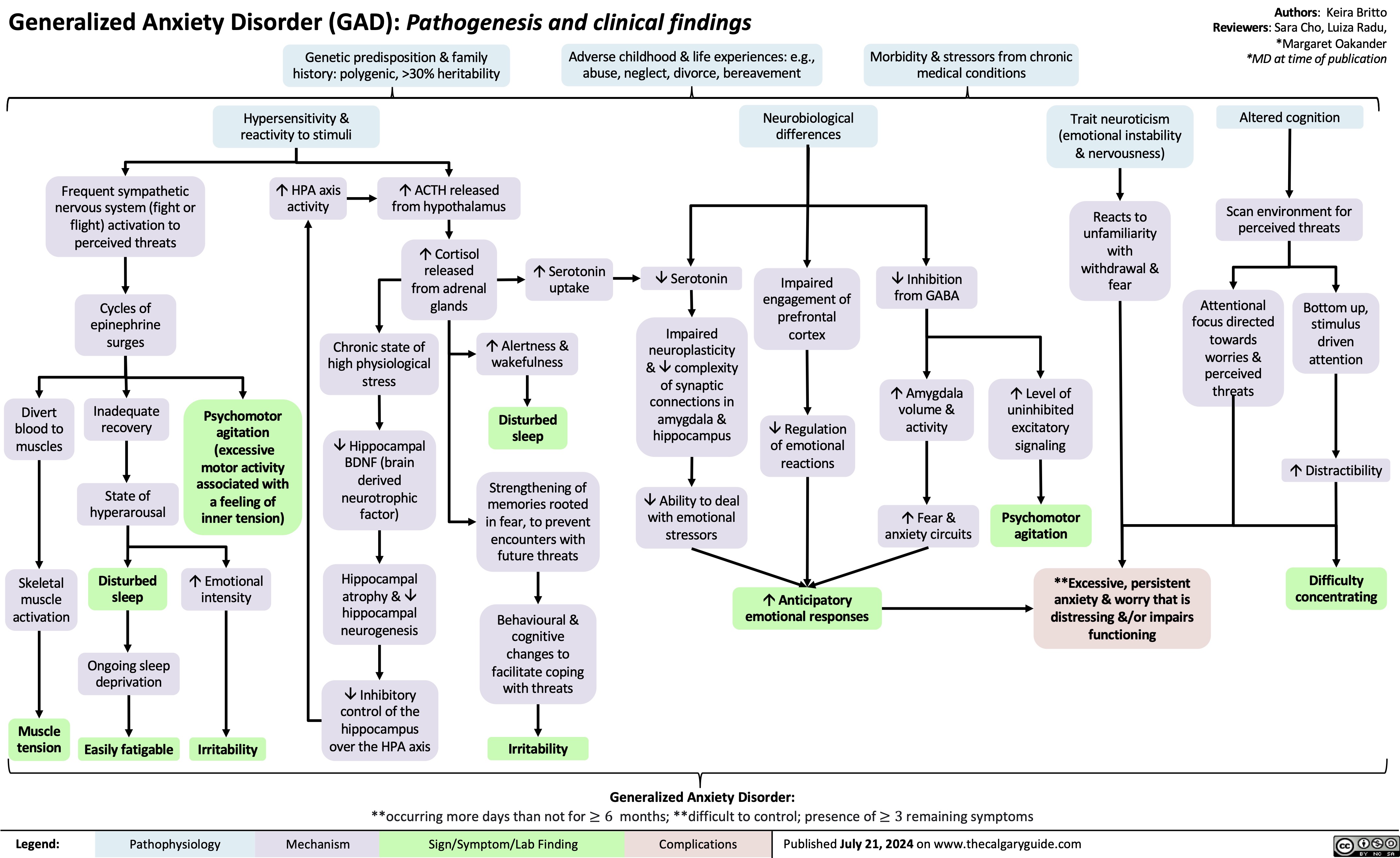
Underfill Edema Pathogenesis
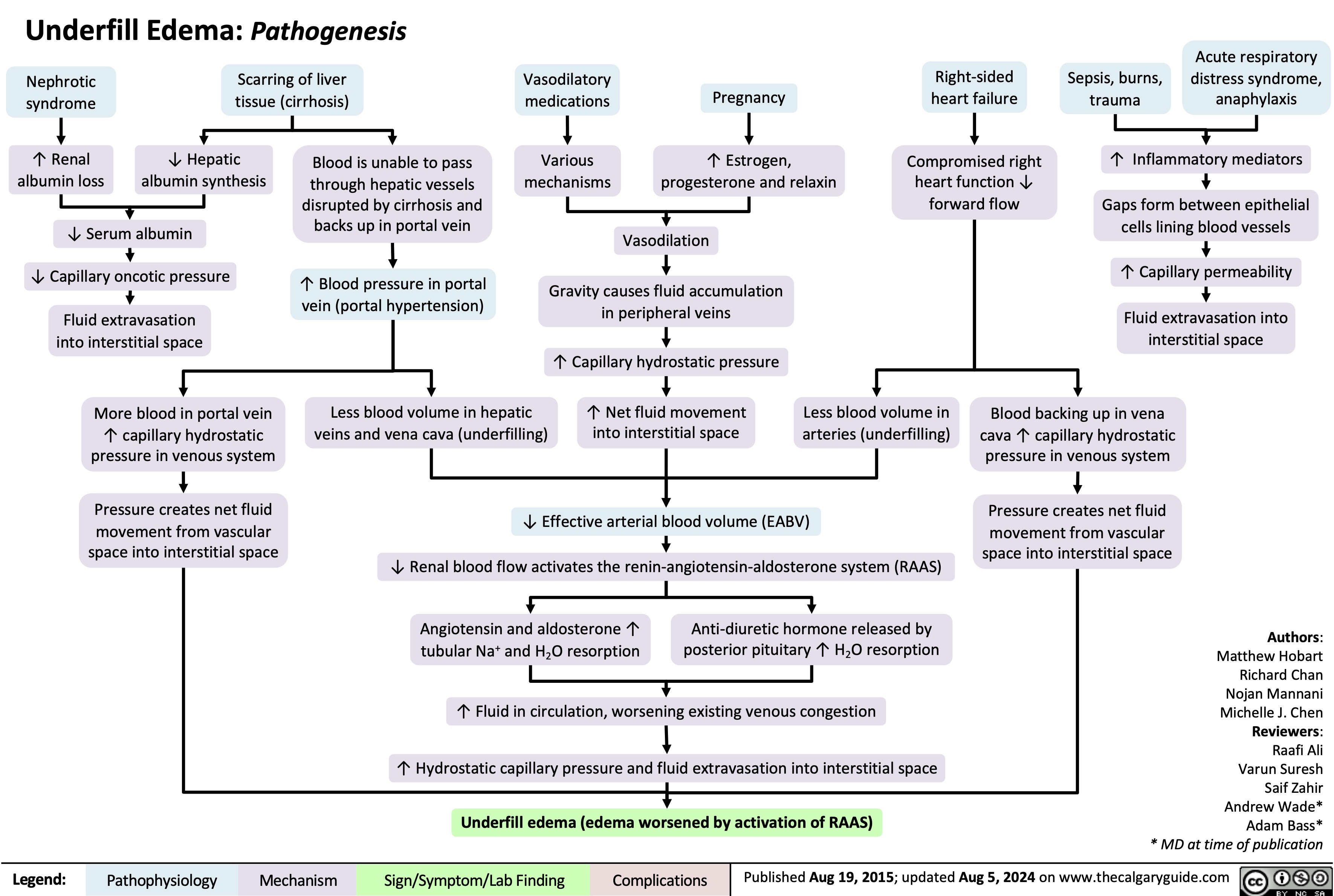
IgA Nephropathy
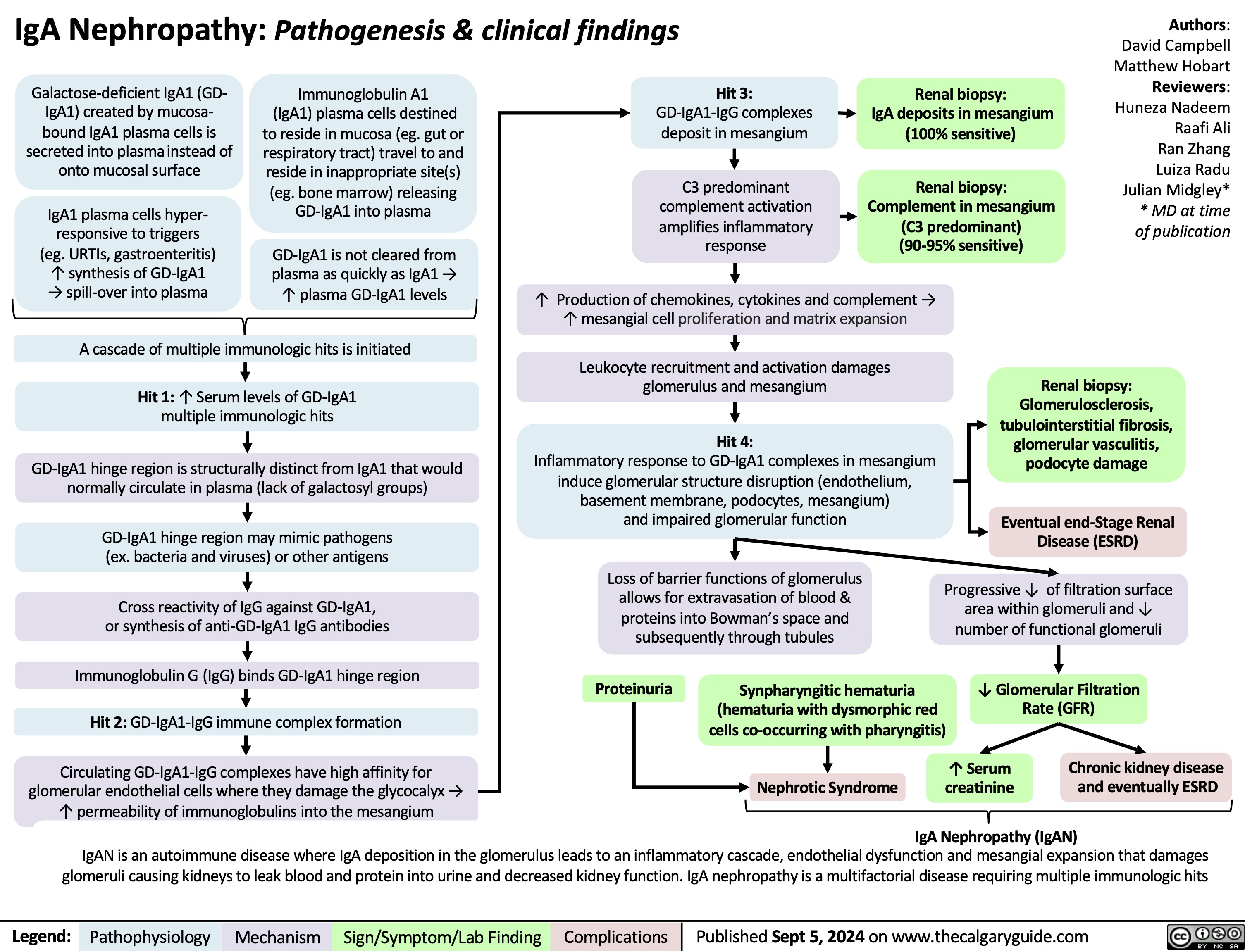
 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />


![Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hypercalcemia-Clinical-Findings.jpg)


![Hyperosmolar Hyperglycemic State (HHS)
Note: HHS is only seen in Type II DM patients!
Note: In patients with either DKA or HHS, always look for an underlying cause (i.e. an infection)
Author: Yan Yu Reviewers:
Peter Vetere
Gill Goobie
Hanan Bassyouni* * MD at time of publication
Alters total body water & ion osmosis
Inadequate insulin production, insulin resistance, non- adherence to insulin Tx
Relative Insulin deficit
Stresses that ↑ Insulin demand: infections, pneumonia, MI, pancreatitis, etc)
Hyperglycemia
(Very high blood [glucose], higher than in DKA)
When blood [glucose] > 12mmol/L, glucose filtration > reabsorption, ↑ urine [glucose]
Glucosuria
Glucose in filtrate promotes osmotic diuresis: large- volume urine output
Polyuria
Dehydration
(↓ JVP, orthostasis: postural hypotension/ postural tachycardia, ↑ resting HR)
Some insulin still present, but not enoughsome glucose is utilized by muscle/fat cells, some remain in the blood
Cells not “starved”, but still need more energy
↑ release of Catabolic hormones: Glucagon, Epinephrine, Cortisol, GH
Body tries to ↑ blood [glucose], to hopefully ↑ cell glucose absorption
Hypothalamic cells sense low intra-cellular glucose, triggering feelings of hunger
Polyphagia
Note: the presence of some insulin directly inhibits lipolysis; thus, in HHS there is no ketone body production, and no subsequent metabolic acidosis and ketouria (unlike in DKA). If ketones are detected in an HHS patient it’s likely secondary to starvation or other mechanisms.
↓ ECF volume, ↑ ECF osmolarity (i.e. hypernatremia)
↑ Gluconeogenesis ↑ Glycogenolysis (in liver)
↓ Protein synthesis, ↑ proteolysis
(in muscle)
↑ Gluconeogenic substrates for liver If the patient doesn’t drink enough
water to replenish lost blood volume If pt is alert and
Electrolyte imbalance
water is accessible
Water osmotically leaves neurons, shrinking them
Neural damage: delirium, lethargy, seizure, stupor, coma
↓ renal perfusion, ↓ GFR
Renal Failure
(pre-renal cause; see relevant slides)
Polydipsia Note: in HHS, body K+ is lost via osmotic diuresis. But diffusion of K+ out of cells
may cause serum [K+] to be falsely normal/elevated. To prevent hypokalemia, give IV KCl along with IV insulin as soon as serum K+ <5.0mmol/L. But ensure patient has good renal function/urine output first, to avoid iatrogenic hyperkalemia!
Note: Electrolyte imbalances (i.e. hyperkalemia, hypernatremia) are worsened by the acute renal failure commonly coexisting with DKA/HHS
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published November 3, 2016 on www.thecalgaryguide.com
Hyperosmolar Hyperglycemic State (HHS)
Note: HHS is only seen in Type II DM patients!
Note: In patients with either DKA or HHS, always look for an underlying cause (i.e. an infection)
Author: Yan Yu Reviewers:
Peter Vetere
Gill Goobie
Hanan Bassyouni* * MD at time of publication
Alters total body water & ion osmosis
Inadequate insulin production, insulin resistance, non- adherence to insulin Tx
Relative Insulin deficit
Stresses that ↑ Insulin demand: infections, pneumonia, MI, pancreatitis, etc)
Hyperglycemia
(Very high blood [glucose], higher than in DKA)
When blood [glucose] > 12mmol/L, glucose filtration > reabsorption, ↑ urine [glucose]
Glucosuria
Glucose in filtrate promotes osmotic diuresis: large- volume urine output
Polyuria
Dehydration
(↓ JVP, orthostasis: postural hypotension/ postural tachycardia, ↑ resting HR)
Some insulin still present, but not enoughsome glucose is utilized by muscle/fat cells, some remain in the blood
Cells not “starved”, but still need more energy
↑ release of Catabolic hormones: Glucagon, Epinephrine, Cortisol, GH
Body tries to ↑ blood [glucose], to hopefully ↑ cell glucose absorption
Hypothalamic cells sense low intra-cellular glucose, triggering feelings of hunger
Polyphagia
Note: the presence of some insulin directly inhibits lipolysis; thus, in HHS there is no ketone body production, and no subsequent metabolic acidosis and ketouria (unlike in DKA). If ketones are detected in an HHS patient it’s likely secondary to starvation or other mechanisms.
↓ ECF volume, ↑ ECF osmolarity (i.e. hypernatremia)
↑ Gluconeogenesis ↑ Glycogenolysis (in liver)
↓ Protein synthesis, ↑ proteolysis
(in muscle)
↑ Gluconeogenic substrates for liver If the patient doesn’t drink enough
water to replenish lost blood volume If pt is alert and
Electrolyte imbalance
water is accessible
Water osmotically leaves neurons, shrinking them
Neural damage: delirium, lethargy, seizure, stupor, coma
↓ renal perfusion, ↓ GFR
Renal Failure
(pre-renal cause; see relevant slides)
Polydipsia Note: in HHS, body K+ is lost via osmotic diuresis. But diffusion of K+ out of cells
may cause serum [K+] to be falsely normal/elevated. To prevent hypokalemia, give IV KCl along with IV insulin as soon as serum K+ <5.0mmol/L. But ensure patient has good renal function/urine output first, to avoid iatrogenic hyperkalemia!
Note: Electrolyte imbalances (i.e. hyperkalemia, hypernatremia) are worsened by the acute renal failure commonly coexisting with DKA/HHS
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published November 3, 2016 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hyperosmolar-Hyperglycemic-State-HHS.jpg)




 NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" title="Bupropion (atipiEni antidepresiv): Mehanizem delovanja in neieleni utinki
potenten antidepresiv v monoterapiji all kot dodatno zdravilo pri zdravljenju razpoloienjskih motenj
okrajgave: 5-HT - serotonin DA - dopamin DAT - prenagalec DA NA - noradrenalin NAT - prenagalec NA SSRI - selektivni zaviralec ponovnega privzema 5-HT
Legenda:
bupropion
farmakologija
antidepresivni udnki uporaben pri zdravljenju "zmaniganega pozitivnega afekta"
nima pomembnelgih 5-HT udnkov povzraa spolne disfunkcije v primerjavi s SSRI; lahko celo odpravi omenjeni neieleni udnek (povzraen s strani SSRI)
dodatek pri zdravljenju odvisnosti od nikotina preko T iive'nega prenosa DA v nagrajevalni poti
energije preko T 2ivbega prenosa NA
patofiziologija mehanizem
farmakokinetika
farmakodinamika
avtorica: JoAnna Fay, Sara Meunier pregledala: Jojo Jiang, Alexander Arnold, Aaron Mackie*
* dr. med. ob objavi
Nizkoafiniteten zaviralec ponovnega privzema DA in NA (DAT>NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" />
NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" title="Bupropion (atipiEni antidepresiv): Mehanizem delovanja in neieleni utinki
potenten antidepresiv v monoterapiji all kot dodatno zdravilo pri zdravljenju razpoloienjskih motenj
okrajgave: 5-HT - serotonin DA - dopamin DAT - prenagalec DA NA - noradrenalin NAT - prenagalec NA SSRI - selektivni zaviralec ponovnega privzema 5-HT
Legenda:
bupropion
farmakologija
antidepresivni udnki uporaben pri zdravljenju "zmaniganega pozitivnega afekta"
nima pomembnelgih 5-HT udnkov povzraa spolne disfunkcije v primerjavi s SSRI; lahko celo odpravi omenjeni neieleni udnek (povzraen s strani SSRI)
dodatek pri zdravljenju odvisnosti od nikotina preko T iive'nega prenosa DA v nagrajevalni poti
energije preko T 2ivbega prenosa NA
patofiziologija mehanizem
farmakokinetika
farmakodinamika
avtorica: JoAnna Fay, Sara Meunier pregledala: Jojo Jiang, Alexander Arnold, Aaron Mackie*
* dr. med. ob objavi
Nizkoafiniteten zaviralec ponovnega privzema DA in NA (DAT>NAT), ki proizvaja aktivne metabolite. Natan'6en mehanizem delovanja (se) ni znan.
znak/simptom/laboratorijska najdba
DA in NE posledi6no ostaneta v sinapsah dlje Casa in okrepita 2iv6ni prenos
neieleni udnki
glavobol
suha usta
4, tel. tee nespeEnost slabost zaprtie
tahikardija
epileptiEni napadi
faringitis
omotica hipertenziia agitacija
prevedel in priredil: Jan Kejiar, dr. med., specializant psihiatrije pregledala: doc. dr. Brigita Novak Sarotar, dr. med., spec. psih.
eliminacija poteka preko jeter in ledvic
prilagoditev odmerka v primeru bolezni jeter in/ali ledvic
bupropion lahko inhibira jetrni citokrom P4502D6 in povzrod interakcije med zdravili
kontraindiciran pri boleznih, ki zmanIgajo epileptogeni prag: anoreksija/bulimija nervoza, epilepsija, odtegnitev alkohola, odtegnitev benzodiazepinov
zaplet Objavljeno 30. junija 2017 na www.thecalgaryguide.com.
" />



















![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)
![Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hyponatremia: Physiology
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Abnormal Renal H2O Handling (hypo-osmolar serum)
AKI/CKD Heart failure
↓ renal blood flow
↓ glomerular filtration
GFR < 25 mL/min, ↓ urine dilution ↑ H2O retention
Note:
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Artifactual hyponatremia can be differentiated by a normal or hyperosmolar serum.
Appropriate ADH secretion
↓ EABV
Hypovolemia: losses via GI, renal, skin, 3rd spacing, bleeding
Hypervolemia: heart failure, cirrhosis
↑ Na+/H2O absorption at PCT
↓ EABV, ↑ H2O retention
Urine [Na+] < 20 mmol/L
Hereditary: tubular disorders
(Bartter, Gitlemann syndromes).
Thiazide diuretics
Inappropriate: SIADH, hypothyroidism, AI
Normal EABV
Anti-diuresis
Primary polydipsia, eating disorder
↑ H2O or ↓ solute intake
↓ Osmoles
Impaired desalination
Block NCC
↑ H2O retention ↑ Na+/K+ excretion
Hyponatremia
Serum [Na+] < 135 mmol/L
Urine osmolality > 100 mmol/L
Urine osmolality < 100 mmol/L
Cerebral edema, ↑ intracranial pressure, vasoconstriction
If hypovolemic: ↓ JVP, ↓ blood pressure
Lethargy, altered mental status
Abbreviations:
AKI: Acute Kidney Injury
CKD: Chronic Kidney Disease
GFR: Glomerular Filtration Rate
H2O: Water
PCT: Proximal Convoluted Tubule
EABV: Effective Arterial Blood Volume
NCC: Na+/Cl- Co-Transporter
SIADH: Syndrome of Inappropriate ADH Secretion AI: Adrenal Insufficiency
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hyponatremia-Physiology-.jpg)

![Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hyperkalemia: Physiology ↓ Renal Excretion
↑ Intake
↓ Intracellular Shift
Acute and chronic kidney disease; CHF
Principal Cell Dysfunction (TTKG < 7)
ACEi/ARB; AI; heparin
Hypovolemia (TTKG > 7)
↓ EABV
↓ distal flow of Na+ and H2O
Urine [Na+] < 20 mmol/L
Cell lysis
↑ osmolarity H2O efflux
Solvent drag
β2 inhibition α1 stimulation
Digoxin ↓ A
NAGMA ↓ insulin
↓ NHE1 activity
Diabetic nephropathy; NSAIDs
↓ A: ↓ R
K+ sparing diuretics; voltage- dependent RTA
↓ Na+/K+ ATPase activity
↓ GFR
↓ A: ↑ R ↑ A: ↑ R
↓ CCD K+ secretion
↑ K+ availability
↑ K+ release
↓ intracellular K+ influx
Chronic: Desensitize voltage- gated Na+ channels and ↓ membrane excitability
ECG: Peaked T-waves, ↑ PR interval, flat/absent P-wave, ↑ QRS, QRST “sine wave”
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Acute: ↑ extracellular [K+] makes the RMP less (-)
Abbreviations:
A: Aldosterone
AI: Adrenal Insufficiency
CCD: Cortical Collecting Duct
CHF: Congestive Heart Failure
EABV: Effective Arterial Blood Volume H+: Hydrogen ion
K+: Potassium ion
Na+: Sodium ion
NAGMA: Normal Anion Gap Metabolic Acidosis
NSAIDs: Non-steroidal anti-inflammatory drugs Note:
Muscle weakness or paralysis, ↓ urinary acid excretion
R: Renin
RTA: Renal Tubular Acidosis
RMP: Resting Membrane Potential TTKG: Transtubular Potassium Gradient
• Pseudohyperkalemia should always be excluded; can be caused by thrombocytosis, leukocytosis or improper blood withdrawal technique.
Authors: Mannat Dhillon Joshua Low Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hyperkalemia-Physiology-.jpg)







































![Hypokalemia: Physiology
Authors: Samin Dolatabadi, Ran (Marissa) Zhang, Mannat Dhillon Reviewers: Meena Assad, Yan Yu*, Juliya Hemmett*
Beta-2 receptor stimulation
(e.g. Salbutamol)
↑ Red blood cell production
↑ Na+/K+ ATPase activity in skeletal muscle cells (moves K+ into the cell & Na+ out of cell)
↑ K+ entry into skeletal muscle cells
* MD at time of publication
Abbreviations:
• EABV – Effective Arterial
Blood Volume
• ENaC – Epithelial Sodium
Channel
• HCL – Hydrochloric acid • HCO3- –Bicarbonate ion
↑ K+ uptake by new red blood cells
↑ Intracellular shift of K+ into cells
Refeeding Syndrome Exogenous insulin
↓ K+ dietary intake
(rare cause in isolation)
↑ Insulin in response to carbohydrate load
↑ Na+/K+ ATPase activity in skeletal muscle & hepatic cells
↑ K+ entry into skeletal muscle & hepatic cells
↓ K+ availability for gastrointestinal absorption
Hypokalemia (Serum [K+] < 3.5 mmol/L)
↑ Renal K+ secretion
K+ follows the electrical gradient into tubular lumen
↑ Electronegativity of tubular lumen
↑ Na+ reabsorptionin principal cellsà Cl- left behind in tubular lumen of kidneys
Gastric acid depletionà ↓HCl
Loss of H+àShift in bicarbonate buffer system to ↑ plasma HCO3-
Plasma HCO3- above reabsorptive capacity of the proximal tubule
↑ HCO3- in the distal tubular lumen of kidneys
Vomiting
Diarrhea Laxatives
Renin secreting tumour Hyperaldosteronism Renal artery stenosis Loop and Thiazide
diuretics
Bartter’s and Gittelman’s syndrome
Liddle syndrome
Extracellular fluid volume depletion
↓ EABV ↑ Renin secretion
↓ Afferent arteriole pressure perfusing kidneys
Renin-Angiotensin- Aldosterone System (RAAS) activationà ↑ Aldosterone release from the adrenal cortex
↑ Expression of ENaC (Na+ reabsorption) in principal cells of the cortical collectingduct)
+ ↑Na &
water excretion in kidneys
↓ EABV
Genetic condition leading to inability to degrade ENaC channels in principal cells of the cortical collecting duct
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019, updated Jan 23, 2022 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi Reviewers: Meena Assad Dr. Juliya Hemmett* * MD at time of publication
TTKG > 4 with N/↑ EABV in hypokalemia is inappropriate and a principal cell problem.
β2 Stimulation (e.g., Salbutamol)
↑ Na+/K+ ATPase activity
↑ K+ entry into cell
↑ RBC Production
↑ Cell production
↑ K+ uptake by new cells
↓ Extracellular ↑ Insulin in response to H+
carbohydrate load
↑ Na+/H+ antiporter activity (movement of H+ out of cell and Na+ into cell)
↑ Intracellular Na+
↑ Na+/K+ ATPase activity ↑ K+ entry into cell
↑ Intracellular Shift of K+
Notes:
Refeeding Syndrome
Insulin
Alkalemia
•
Abbreviations:
• CCD – Cortical Collecting Duct
• EABV – Effective Arterial Blood Volume
• RAAS – Renin-Angiotensin-Aldosterone System • TTKG – Trans-tubular Potassium Gradient
• ENaC – Epithelial Sodium Channel
↓ K+ Intake (Rare cause in isolation)
↓ K+ availability
Diarrhea, Vomiting, Laxatives
↑ Gastrointestinal loss of K+
Polyuria
↑ Renal loss of K+ (TTKG < 4 as principal cell is working appropriately but small amount of K+ is lost per urination)
Hypokalemia (Serum [K+] < 3.5 mmol/L)
Liddle Syndrome Hyperaldosteronism Renin Secreting Tumour
Renal artery stenosis
Loop and Thiazide Diuretics
Bartter’s and Gittelman’s Syndrome
Genetic condition leading to inability to degrade ENaC channels ↑ Renin
↑ Renal K+ secretion
K+ follows the electrical gradient
Electronegative lumen
↓ Pressure perfusing the kidney
RAAS activation RAAS activation
↑ Aldosterone
↑ Na+ and water excretion
↓ EABV
↑ Expression of ENaC in principal cells of CCD
↑ Na+ reabsorption
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi, Ran (Marissa) Zhang, Mannat Dhillon Reviewers: Meena Assad, Yan Yu*, Juliya Hemmett*
Beta-2 receptor stimulation
(e.g. Salbutamol)
↑ Red blood cell production
↑ Na+/K+ ATPase activity in skeletal muscle cells (moves K+ into the cell & Na+ out of cell)
↑ K+ entry into skeletal muscle cells
* MD at time of publication
Abbreviations:
• EABV – Effective Arterial
Blood Volume
• ENaC – Epithelial Sodium
Channel
• HCL – Hydrochloric acid • HCO3- –Bicarbonate ion
↑ K+ uptake by new red blood cells
↑ Intracellular shift of K+ into cells
Refeeding Syndrome Exogenous insulin
↓ K+ dietary intake
(rare cause in isolation)
↑ Insulin in response to carbohydrate load
↑ Na+/K+ ATPase activity in skeletal muscle & hepatic cells
↑ K+ entry into skeletal muscle & hepatic cells
↓ K+ availability for gastrointestinal absorption
Hypokalemia (Serum [K+] < 3.5 mmol/L)
↑ Renal K+ secretion
K+ follows the electrical gradient into tubular lumen
↑ Electronegativity of tubular lumen
↑ Na+ reabsorptionin principal cellsà Cl- left behind in tubular lumen of kidneys
Gastric acid depletionà ↓HCl
Loss of H+àShift in bicarbonate buffer system to ↑ plasma HCO3-
Plasma HCO3- above reabsorptive capacity of the proximal tubule
↑ HCO3- in the distal tubular lumen of kidneys
Vomiting
Diarrhea Laxatives
Renin secreting tumour Hyperaldosteronism Renal artery stenosis Loop and Thiazide
diuretics
Bartter’s and Gittelman’s syndrome
Liddle syndrome
Extracellular fluid volume depletion
↓ EABV ↑ Renin secretion
↓ Afferent arteriole pressure perfusing kidneys
Renin-Angiotensin- Aldosterone System (RAAS) activationà ↑ Aldosterone release from the adrenal cortex
↑ Expression of ENaC (Na+ reabsorption) in principal cells of the cortical collectingduct)
+ ↑Na &
water excretion in kidneys
↓ EABV
Genetic condition leading to inability to degrade ENaC channels in principal cells of the cortical collecting duct
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019, updated Jan 23, 2022 on www.thecalgaryguide.com
Hypokalemia: Physiology
Authors: Samin Dolatabadi Reviewers: Meena Assad Dr. Juliya Hemmett* * MD at time of publication
TTKG > 4 with N/↑ EABV in hypokalemia is inappropriate and a principal cell problem.
β2 Stimulation (e.g., Salbutamol)
↑ Na+/K+ ATPase activity
↑ K+ entry into cell
↑ RBC Production
↑ Cell production
↑ K+ uptake by new cells
↓ Extracellular ↑ Insulin in response to H+
carbohydrate load
↑ Na+/H+ antiporter activity (movement of H+ out of cell and Na+ into cell)
↑ Intracellular Na+
↑ Na+/K+ ATPase activity ↑ K+ entry into cell
↑ Intracellular Shift of K+
Notes:
Refeeding Syndrome
Insulin
Alkalemia
•
Abbreviations:
• CCD – Cortical Collecting Duct
• EABV – Effective Arterial Blood Volume
• RAAS – Renin-Angiotensin-Aldosterone System • TTKG – Trans-tubular Potassium Gradient
• ENaC – Epithelial Sodium Channel
↓ K+ Intake (Rare cause in isolation)
↓ K+ availability
Diarrhea, Vomiting, Laxatives
↑ Gastrointestinal loss of K+
Polyuria
↑ Renal loss of K+ (TTKG < 4 as principal cell is working appropriately but small amount of K+ is lost per urination)
Hypokalemia (Serum [K+] < 3.5 mmol/L)
Liddle Syndrome Hyperaldosteronism Renin Secreting Tumour
Renal artery stenosis
Loop and Thiazide Diuretics
Bartter’s and Gittelman’s Syndrome
Genetic condition leading to inability to degrade ENaC channels ↑ Renin
↑ Renal K+ secretion
K+ follows the electrical gradient
Electronegative lumen
↓ Pressure perfusing the kidney
RAAS activation RAAS activation
↑ Aldosterone
↑ Na+ and water excretion
↓ EABV
↑ Expression of ENaC in principal cells of CCD
↑ Na+ reabsorption
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published March 6, 2019 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2019/03/Hypokalemia-Physiology.jpg)
![Metabolic Alkalosis: Pathogenesis
Authors: Wazaira Khan Reviewers: Jessica Krahn, Emily Wildman, Austin Laing, Huneza Nadeem, Ran (Marissa) Zhang, Adam Bass* * MD at time of publication
Primary hyperaldosteronism E.g., aldosterone- secreting mass, adrenal hyperplasia
Secondary hyperaldosteronism E.g., renin-secreting mass, renal artery stenosis
Pseudo- hypoaldosteronism E.g., Liddle’s Syndrome
Unregulated aldosterone production in adrenal cortex
Excess aldosterone suppresses renin production
Unregulated renin production by juxtaglomerular cells
Sustained ↑ in mineralocorticoid (aldosterone) activity
Insertion of epithelial sodium channels on principal cells in collecting duct
↑ Water retention by the kidney
↑ Na+ reabsorption by principal cells
↑ EABV
• ↑ Jugular venous pressure • Hypertension
• Urine Na+ > 40 mEq/L
Low renin, high aldosterone state
Activation of renin- angiotensin-aldosterone system (RAAS)
Release of aldosterone from adrenal cortex
High renin, high aldosterone state
↑ Negative charge in collecting duct lumen
K+ leaks into collecting duct lumen by principal cell to maintain electroneutrality
Hypokalemia
Intracellular K+ leaks out of any cell in the body to compensate for low serum K+ levels
Extracellular H+ enters cell to maintain electroneutrality
Intracellular acidosis
Activation of compensatory acid secreting mechanisms in kidney
Impaired tubular function
E.g., loop/thiazide diuretics, Bartter’s/ Gitelman’s Syndrome
Upper GI Loss
E.g., vomiting, nasogastric suction
Unregulated epithelial sodium channel activity in collecting duct mimicking aldosterone function
↑ in Na+ and water retention à RAAS inhibition
↓ Na+ and Cl- reabsorption in thick ascending limb or distal convoluted tubule
Low renin, low aldosterone state
↑ Na+, Cl- and water secretion through kidney
RAAS activation
See “Physiology of RAAS” slide
↓ EABV • • •
Temporary ↑ in mineralocorticoid (aldosterone) activity
↓ Jugular venous pressure Orthostatic hypotension Dry mucous membranes Urine Na+ < 20 mEq/L
•
Loss of fluid through GI tract
↓ HCl delivery to small intestine
↑ NH + secretion and 4
↑ HCO3- reabsorption in proximal tubule
↑ H+ secretion in cortical collecting duct
Loss of gastric contents, including HCl
↓ HCO3- secretion by pancreas, liver and intestines to neutralize HCl
Loss of intrinsic acid to neutralize HCO3- ↑ Plasma [HCO3-]
Metabolic Alkalosis
Arterial blood gas pH > 7.40 Plasma [HCO3-] > 24 mEq/L
Effective Arterial Blood Volume (EABV): component of arterial blood volume that is effectively perfusing organs
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published May 31, 2022 on www.thecalgaryguide.com
Metabolic Alkalosis: Pathogenesis
Authors: Wazaira Khan Reviewers: Jessica Krahn, Emily Wildman, Austin Laing, Huneza Nadeem, Ran (Marissa) Zhang, Adam Bass* * MD at time of publication
Primary hyperaldosteronism E.g., aldosterone- secreting mass, adrenal hyperplasia
Secondary hyperaldosteronism E.g., renin-secreting mass, renal artery stenosis
Pseudo- hypoaldosteronism E.g., Liddle’s Syndrome
Unregulated aldosterone production in adrenal cortex
Excess aldosterone suppresses renin production
Unregulated renin production by juxtaglomerular cells
Sustained ↑ in mineralocorticoid (aldosterone) activity
Insertion of epithelial sodium channels on principal cells in collecting duct
↑ Water retention by the kidney
↑ Na+ reabsorption by principal cells
↑ EABV
• ↑ Jugular venous pressure • Hypertension
• Urine Na+ > 40 mEq/L
Low renin, high aldosterone state
Activation of renin- angiotensin-aldosterone system (RAAS)
Release of aldosterone from adrenal cortex
High renin, high aldosterone state
↑ Negative charge in collecting duct lumen
K+ leaks into collecting duct lumen by principal cell to maintain electroneutrality
Hypokalemia
Intracellular K+ leaks out of any cell in the body to compensate for low serum K+ levels
Extracellular H+ enters cell to maintain electroneutrality
Intracellular acidosis
Activation of compensatory acid secreting mechanisms in kidney
Impaired tubular function
E.g., loop/thiazide diuretics, Bartter’s/ Gitelman’s Syndrome
Upper GI Loss
E.g., vomiting, nasogastric suction
Unregulated epithelial sodium channel activity in collecting duct mimicking aldosterone function
↑ in Na+ and water retention à RAAS inhibition
↓ Na+ and Cl- reabsorption in thick ascending limb or distal convoluted tubule
Low renin, low aldosterone state
↑ Na+, Cl- and water secretion through kidney
RAAS activation
See “Physiology of RAAS” slide
↓ EABV • • •
Temporary ↑ in mineralocorticoid (aldosterone) activity
↓ Jugular venous pressure Orthostatic hypotension Dry mucous membranes Urine Na+ < 20 mEq/L
•
Loss of fluid through GI tract
↓ HCl delivery to small intestine
↑ NH + secretion and 4
↑ HCO3- reabsorption in proximal tubule
↑ H+ secretion in cortical collecting duct
Loss of gastric contents, including HCl
↓ HCO3- secretion by pancreas, liver and intestines to neutralize HCl
Loss of intrinsic acid to neutralize HCO3- ↑ Plasma [HCO3-]
Metabolic Alkalosis
Arterial blood gas pH > 7.40 Plasma [HCO3-] > 24 mEq/L
Effective Arterial Blood Volume (EABV): component of arterial blood volume that is effectively perfusing organs
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published May 31, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/05/Metabolic-Alkalosis-1.jpg)




![Hyperkalemia (↓ Renal Excretion): Pathophysiology
Non-steroidal anti- inflammatory drugs (NSAIDs)
Inhibition of prostaglandins which promote renin secretion (see NSAIDs and the Kidney: Mechanism of Action and Side Effects slide)
Diabetic Nephropathy
Acute (AIN)and chronic (CIN) interstitial nephritis
Immune-mediated damage of the kidney tubule and interstitium
Damage to distal tubule leads to aldosterone resistance at principal cell
Aldosterone cannot ↑ EnaC insertion on principal cell of CCD
Epithelial sodium channel (ENaC) blockers
Acute kidney injury and chronic kidney disease
↓ Effective arterial blood volume (volume of blood effectively perfusing tissue)
↓ Oxygen perfusion to renal tissue causing renal ischemia
Autonomic neuropathy ↓ sympathetic drive to produce renin
Chronic juxtaglomerular cell damage ↓ synthesis of renin
Blockage of ENaC on the principal cells of the CCD
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is either not formed (ACEi), or blocked at its receptor (ARBs)
Angiotensin II cannot stimulate the release of aldosterone from the adrenal cortex
Adrenal Insufficiency
Adrenal gland cannot produce sufficient amounts of aldosterone
↓ Renin secretion by the afferent arteriole prevents RAAS activation
↓ Aldosterone release from adrenal cortex
↓ ENaC (Na+ reabsorption channel) expression on principal cells of the cortical collecting duct (CCD)
Damage to kidney causes renal impairment and ↓ glomerular filtration rate
↓ Glomerular filtrate production means ↓ tubular flow rate
↓ Na+ delivery to the distal tubule
↓ Na+ reabsorption by ENaCs at the principal cell in CCD
↓ Na+ and water reabsorption by ENaCs in CCD ↓ Effective arterial blood volume (EABV)
Activates renin-angiotensin-aldosterone system (RAAS) leads to ↑ renin secretion in the afferent arteriole (see Physiology of the renin-angiotensin-aldosterone system (RAAS) slide)
↑ Na+ and water loss in tubular lumen
↑ Positive charge in tubular lumen
↓ Electronegativity gradient in tubular lumen
of CCD
↓ K+ excretion by the principal cell in the CCD as there is less of a electronegative gradient
↑ Accumulation of K+ in blood Hyperkalemia
Serum [K+] > 5.1 mmol/L
See Hyperkalemia: Clinical Findings slide
In the case of diabetic nephropathy and NSAIDS ↓ EABV does not stimulate RAAS and therefore aldosterone production
↓ Renin
↓ Aldosterone
↑ Renin secretion activates an ↑ in aldosterone production but aldosterone action at the principal cell is blocked because of ENaCs or resistance in AIN and CIN
↑ Renin
↑ Aldosterone
In the case of ACEi, ARBs, or adrenal insufficiency ↑ renin secretion does not lead to ↑ aldosterone
↑ Renin
↓ Aldosterone
Note: as described in the above flow chart, measuring serum renin and aldosterone levels can be used to help diagnose the cause of hyperkalemia.
Authors: Mannat Dhillon, Joshua Low, Emily Wildman, Huneza Nadeem Reviewers: Andrea Kuczynski, Marissa (Ran) Zhang, Adam Bass*, Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Aug 2, 2022 on www.thecalgaryguide.com
Hyperkalemia (↓ Renal Excretion): Pathophysiology
Non-steroidal anti- inflammatory drugs (NSAIDs)
Inhibition of prostaglandins which promote renin secretion (see NSAIDs and the Kidney: Mechanism of Action and Side Effects slide)
Diabetic Nephropathy
Acute (AIN)and chronic (CIN) interstitial nephritis
Immune-mediated damage of the kidney tubule and interstitium
Damage to distal tubule leads to aldosterone resistance at principal cell
Aldosterone cannot ↑ EnaC insertion on principal cell of CCD
Epithelial sodium channel (ENaC) blockers
Acute kidney injury and chronic kidney disease
↓ Effective arterial blood volume (volume of blood effectively perfusing tissue)
↓ Oxygen perfusion to renal tissue causing renal ischemia
Autonomic neuropathy ↓ sympathetic drive to produce renin
Chronic juxtaglomerular cell damage ↓ synthesis of renin
Blockage of ENaC on the principal cells of the CCD
Angiotensin converting enzyme inhibitors (ACEi) and angiotensin receptor blockers (ARBs)
Angiotensin II is either not formed (ACEi), or blocked at its receptor (ARBs)
Angiotensin II cannot stimulate the release of aldosterone from the adrenal cortex
Adrenal Insufficiency
Adrenal gland cannot produce sufficient amounts of aldosterone
↓ Renin secretion by the afferent arteriole prevents RAAS activation
↓ Aldosterone release from adrenal cortex
↓ ENaC (Na+ reabsorption channel) expression on principal cells of the cortical collecting duct (CCD)
Damage to kidney causes renal impairment and ↓ glomerular filtration rate
↓ Glomerular filtrate production means ↓ tubular flow rate
↓ Na+ delivery to the distal tubule
↓ Na+ reabsorption by ENaCs at the principal cell in CCD
↓ Na+ and water reabsorption by ENaCs in CCD ↓ Effective arterial blood volume (EABV)
Activates renin-angiotensin-aldosterone system (RAAS) leads to ↑ renin secretion in the afferent arteriole (see Physiology of the renin-angiotensin-aldosterone system (RAAS) slide)
↑ Na+ and water loss in tubular lumen
↑ Positive charge in tubular lumen
↓ Electronegativity gradient in tubular lumen
of CCD
↓ K+ excretion by the principal cell in the CCD as there is less of a electronegative gradient
↑ Accumulation of K+ in blood Hyperkalemia
Serum [K+] > 5.1 mmol/L
See Hyperkalemia: Clinical Findings slide
In the case of diabetic nephropathy and NSAIDS ↓ EABV does not stimulate RAAS and therefore aldosterone production
↓ Renin
↓ Aldosterone
↑ Renin secretion activates an ↑ in aldosterone production but aldosterone action at the principal cell is blocked because of ENaCs or resistance in AIN and CIN
↑ Renin
↑ Aldosterone
In the case of ACEi, ARBs, or adrenal insufficiency ↑ renin secretion does not lead to ↑ aldosterone
↑ Renin
↓ Aldosterone
Note: as described in the above flow chart, measuring serum renin and aldosterone levels can be used to help diagnose the cause of hyperkalemia.
Authors: Mannat Dhillon, Joshua Low, Emily Wildman, Huneza Nadeem Reviewers: Andrea Kuczynski, Marissa (Ran) Zhang, Adam Bass*, Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Aug 2, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/08/Hyperkalemia-renal-excretion.jpg)
![Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com
Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/SAH-Clinical-Findings-2022.jpg)


![Unstable Angina/Unstable Angina Pectoris: Pathogenesis and clinical findings Primary cause:
Secondary causes:
Coronary artery vasospasm - primary or drug induced (Ex: cocaine, triptans)
Coagulopathy
(Ex: antiphospholipid antibody syndrome)
Vasculitic syndromes (Ex: Takayasu arteritis)
Authors: Marisa Vigna Ryan Wilkie Yan Yu* Reviewers: Julena Foglia Davis Maclean Mehul Gupta Andrew Grant* * MD at time of publication
Atherosclerosis
Fatty plaque accumulates inside the intimal walls of arteries Coronary arterial atherosclerotic plaque rupture or erosion
Plaque disruption exposes subendothelial components of damaged vessel wall to platelets, initiating the coagulation cascade and platelet adhesion
Aggregation of platelets results in the formation of a thrombus Thrombus partially occludes blood flow through a coronary artery âmyocardial blood supply
Congenital anomalies (Ex: myocardial bridge, anomalous coronary)
Spontaneous coronary artery dissection
Increased blood viscosity (Ex: polycythemia, thrombocytopenia)
Factors thatámyocardial (cardiac muscle) oxygen demand (Ex: tachycardia, hypotension, hypertension, anemia, exertion, stress)
Coronary embolism (Ex: A. Fib, endocarditis, prosthetic valve thrombus)
áheart rate, contractility, and/or wall tension ámyocardial oxygen demand
Myocardial ischemia due to imbalance between blood supply and oxygen demand (insufficient blood/oxygen supply)
Unstable Angina/Unstable Angina Pectoris
Can be new onset angina; typically progressive in frequency, severity, or duration; can occur at rest
Subtotal occlusion of a coronary arteryà
reduced, but continued, myocardial blood supply
Maintained perfusion means cardiomyocytes are still alive and thus do not leak troponin into bloodstream
Normal serum troponin
Diaphoresis
(sweating)
Since bloodflow occurs from epicardium to endocardium, myocardial ischemia is more
pronounced in the subendocardium (region furthest away from heart’s external surface)
Sufficient blood flow is maintained in regions superficial to the subendocardium, resulting in non-transmural (partial thickness) heart wall ischemia
Non-inferior wall ischemia triggers a predominantáin sympathetic nervous system activity, given the proximity of cardiac sympathetic nerve innervation
Ischemiaâ cardiomyocyte resting membrane potential andâ action potential duration
Voltage gradient between normal and subendocardial ischemic zones creates injury currents, shifting the ST- vector on ECG
ECG: ST depression
and/or T wave inversion
Cardiac sensory nerve fibres mix with somatic sensory nerve
fibres and enter the spinal cord via the T1-T4 nerve roots
Brain perceives increased cardiac sensory nerve signaling as nerve pain coming from the skin of T1-T4 dermatomes (“Referred Pain”)
Myocardial ischemia causes hypoxic stress on cardiomyocytesàâaerobic (requiring oxygen) metabolism,áanaerobic (not requiring oxygen) metabolism
áanerobic respirationálactic acid production,á[H+], andâcellular pH which impairs cardiomyocyte function
Cardiomyocyte dysfunction impairs myocardial relaxation in diastole and/orâ left ventricular contractility in systole
âleft ventricular cardiac output àbackup of blood in the left ventricle, atrium, and pulmonary vasculature
ápulmonary capillary pressures pushes fluid out of the capillaries into the alveoli in the lungs
Fluid filled alveoliâgas exchange andâ oxygenation, triggering harder and faster breathing in order to compensate
Dyspnea
Activation of sweat glands via acetylcholine release
Hormones bind to cardiac β1 receptors
Tachycardia
(áheart rate)
Epinephrine/ Norepinephrine hormone release from the adrenal medulla
Hormones bind to arterial smooth muscle α1 receptors ávascular tone (vasoconstriction)
Hypertension
The Vagus nerve sits in close physical proximity to the inferior wall of the heart àinferior wall ischemia triggers involuntary Vagus nerve activation
Since the Vagus nerve coordinates parasympathetic activity,áVagus nerve activity leads to a variety of parasympathetic nervous system responses:
Retrosternal discomfort: May present as pain, heaviness, tightness, aching, pressure, burning or squeezing
Pain radiation to T1-T4 dermatomes:
Left shoulder and arm, lower jaw, neck, abdomen, upper back
Syncope
(fainting)
Bradycardia
Nausea Hypotension
(âheart rate)
(âblood pressure)
(áblood pressure)
(shortness of breath)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Findings
Complications
Published Oct 18, 2015, updated Aug 29, 2021 on www.thecalgaryguide.com
Unstable Angina/Unstable Angina Pectoris: Pathogenesis and clinical findings Primary cause:
Secondary causes:
Coronary artery vasospasm - primary or drug induced (Ex: cocaine, triptans)
Coagulopathy
(Ex: antiphospholipid antibody syndrome)
Vasculitic syndromes (Ex: Takayasu arteritis)
Authors: Marisa Vigna Ryan Wilkie Yan Yu* Reviewers: Julena Foglia Davis Maclean Mehul Gupta Andrew Grant* * MD at time of publication
Atherosclerosis
Fatty plaque accumulates inside the intimal walls of arteries Coronary arterial atherosclerotic plaque rupture or erosion
Plaque disruption exposes subendothelial components of damaged vessel wall to platelets, initiating the coagulation cascade and platelet adhesion
Aggregation of platelets results in the formation of a thrombus Thrombus partially occludes blood flow through a coronary artery âmyocardial blood supply
Congenital anomalies (Ex: myocardial bridge, anomalous coronary)
Spontaneous coronary artery dissection
Increased blood viscosity (Ex: polycythemia, thrombocytopenia)
Factors thatámyocardial (cardiac muscle) oxygen demand (Ex: tachycardia, hypotension, hypertension, anemia, exertion, stress)
Coronary embolism (Ex: A. Fib, endocarditis, prosthetic valve thrombus)
áheart rate, contractility, and/or wall tension ámyocardial oxygen demand
Myocardial ischemia due to imbalance between blood supply and oxygen demand (insufficient blood/oxygen supply)
Unstable Angina/Unstable Angina Pectoris
Can be new onset angina; typically progressive in frequency, severity, or duration; can occur at rest
Subtotal occlusion of a coronary arteryà
reduced, but continued, myocardial blood supply
Maintained perfusion means cardiomyocytes are still alive and thus do not leak troponin into bloodstream
Normal serum troponin
Diaphoresis
(sweating)
Since bloodflow occurs from epicardium to endocardium, myocardial ischemia is more
pronounced in the subendocardium (region furthest away from heart’s external surface)
Sufficient blood flow is maintained in regions superficial to the subendocardium, resulting in non-transmural (partial thickness) heart wall ischemia
Non-inferior wall ischemia triggers a predominantáin sympathetic nervous system activity, given the proximity of cardiac sympathetic nerve innervation
Ischemiaâ cardiomyocyte resting membrane potential andâ action potential duration
Voltage gradient between normal and subendocardial ischemic zones creates injury currents, shifting the ST- vector on ECG
ECG: ST depression
and/or T wave inversion
Cardiac sensory nerve fibres mix with somatic sensory nerve
fibres and enter the spinal cord via the T1-T4 nerve roots
Brain perceives increased cardiac sensory nerve signaling as nerve pain coming from the skin of T1-T4 dermatomes (“Referred Pain”)
Myocardial ischemia causes hypoxic stress on cardiomyocytesàâaerobic (requiring oxygen) metabolism,áanaerobic (not requiring oxygen) metabolism
áanerobic respirationálactic acid production,á[H+], andâcellular pH which impairs cardiomyocyte function
Cardiomyocyte dysfunction impairs myocardial relaxation in diastole and/orâ left ventricular contractility in systole
âleft ventricular cardiac output àbackup of blood in the left ventricle, atrium, and pulmonary vasculature
ápulmonary capillary pressures pushes fluid out of the capillaries into the alveoli in the lungs
Fluid filled alveoliâgas exchange andâ oxygenation, triggering harder and faster breathing in order to compensate
Dyspnea
Activation of sweat glands via acetylcholine release
Hormones bind to cardiac β1 receptors
Tachycardia
(áheart rate)
Epinephrine/ Norepinephrine hormone release from the adrenal medulla
Hormones bind to arterial smooth muscle α1 receptors ávascular tone (vasoconstriction)
Hypertension
The Vagus nerve sits in close physical proximity to the inferior wall of the heart àinferior wall ischemia triggers involuntary Vagus nerve activation
Since the Vagus nerve coordinates parasympathetic activity,áVagus nerve activity leads to a variety of parasympathetic nervous system responses:
Retrosternal discomfort: May present as pain, heaviness, tightness, aching, pressure, burning or squeezing
Pain radiation to T1-T4 dermatomes:
Left shoulder and arm, lower jaw, neck, abdomen, upper back
Syncope
(fainting)
Bradycardia
Nausea Hypotension
(âheart rate)
(âblood pressure)
(áblood pressure)
(shortness of breath)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Findings
Complications
Published Oct 18, 2015, updated Aug 29, 2021 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/10/Unstable-Angina-2021.jpg)



























