SEARCH RESULTS FOR: pharyngitis
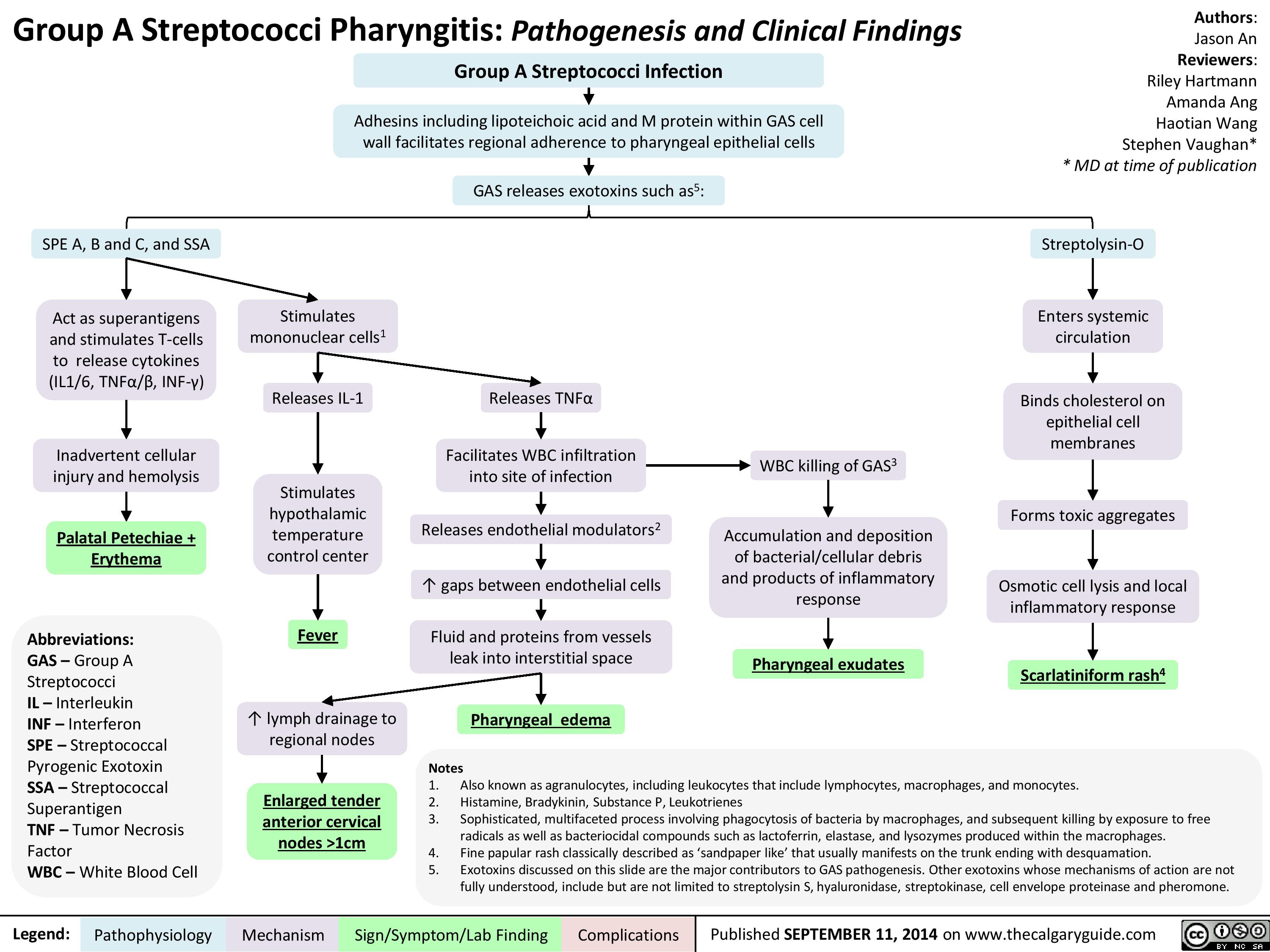
Group A Streptococci Pharyngitis Pathogenesis and Clinical Findings
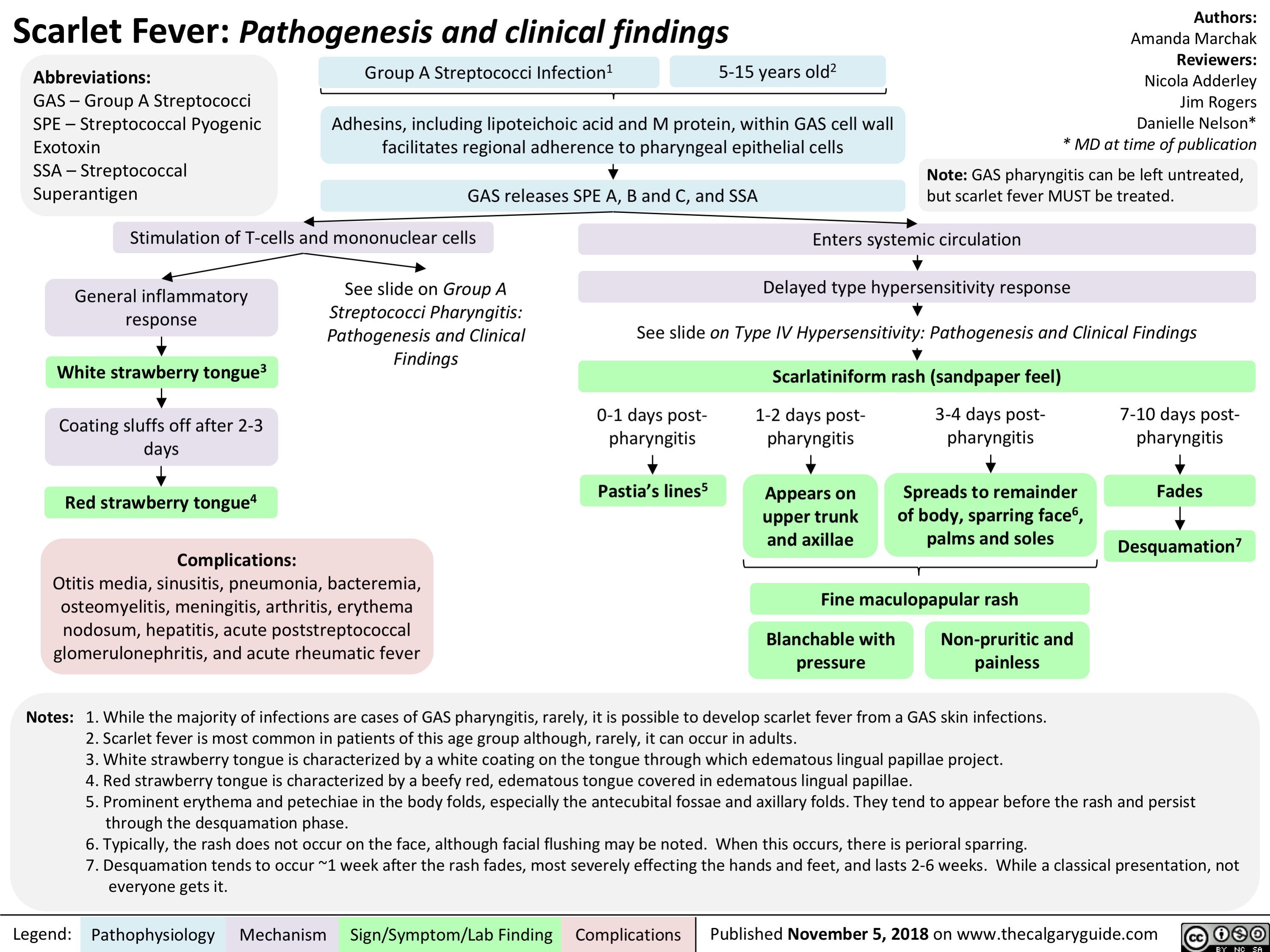
Scarlet Fever: Pathogenesis and clinical findings
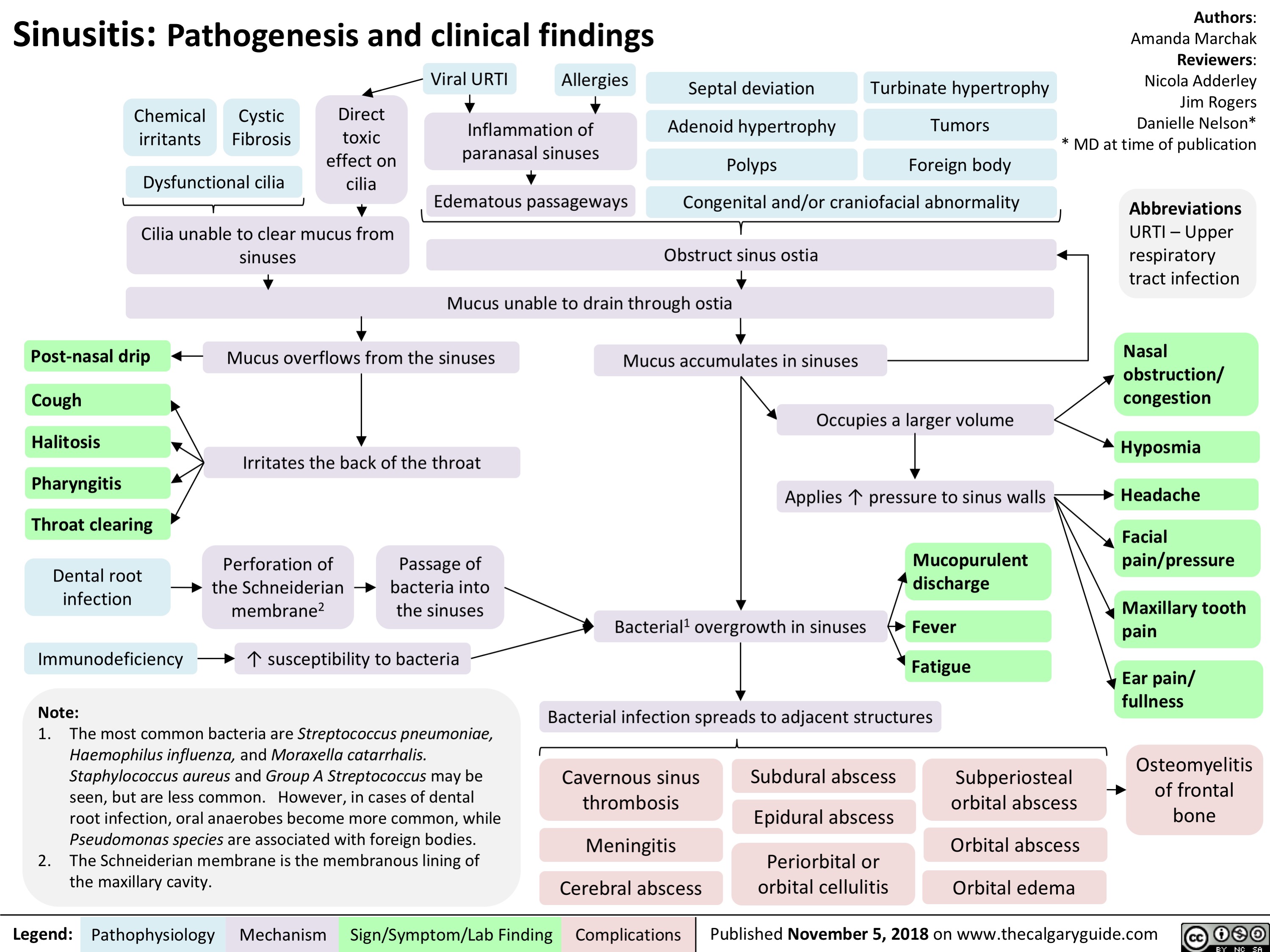
Sinusitis: Pathogenesis and clinical findings
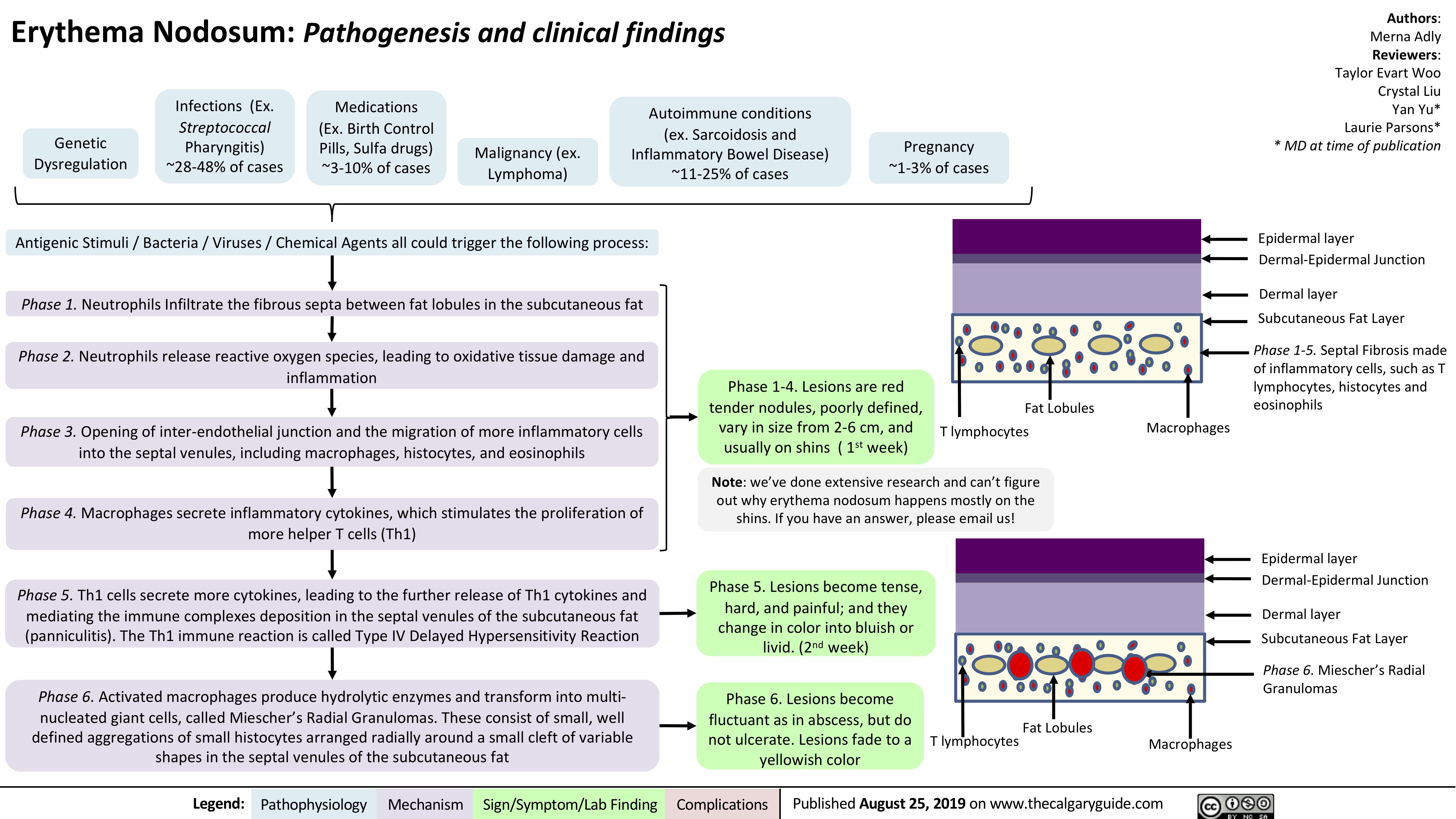
Erythema Nodosum pathogenesis and clinical findings
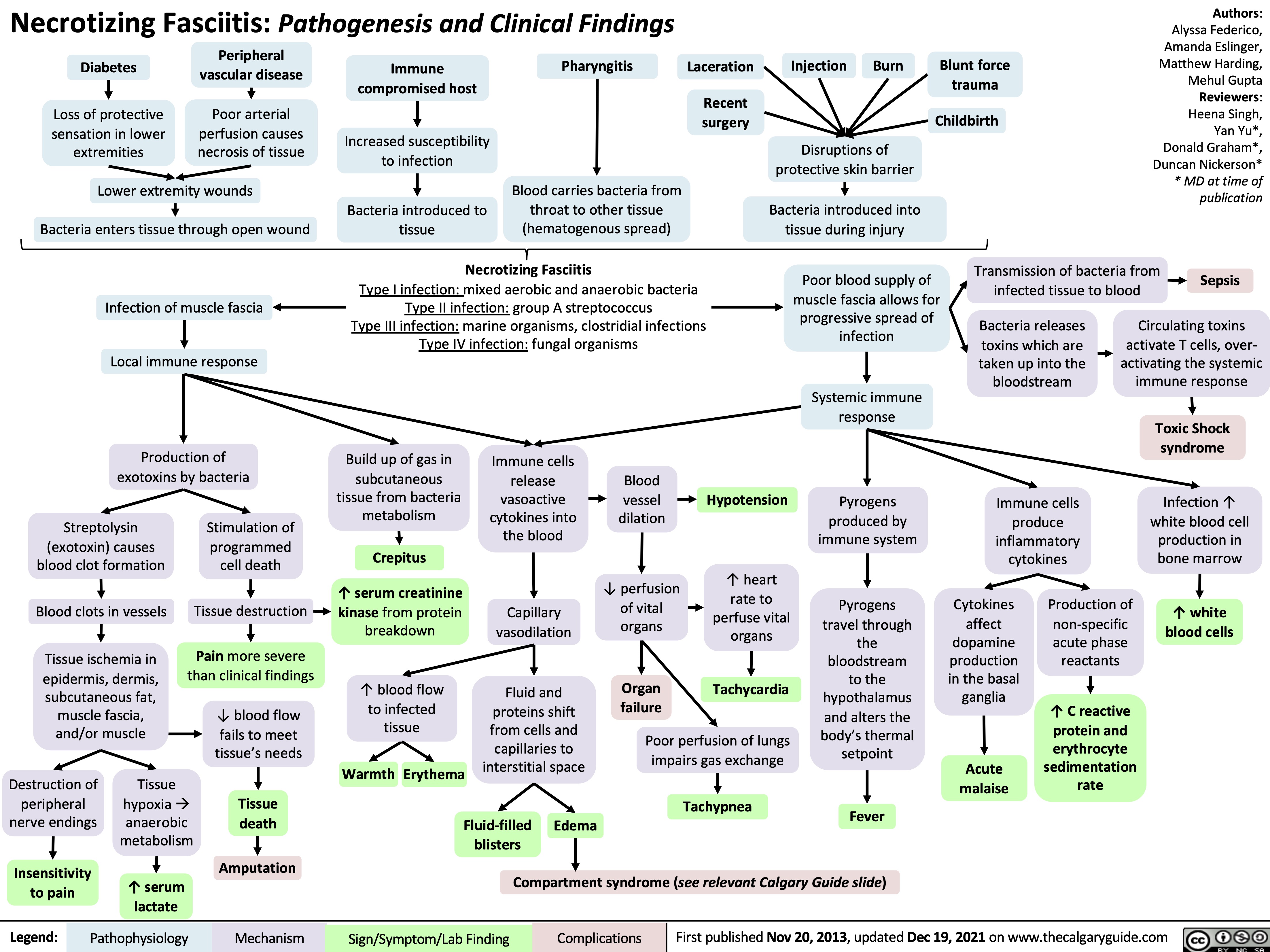
necrotizing fasciitis
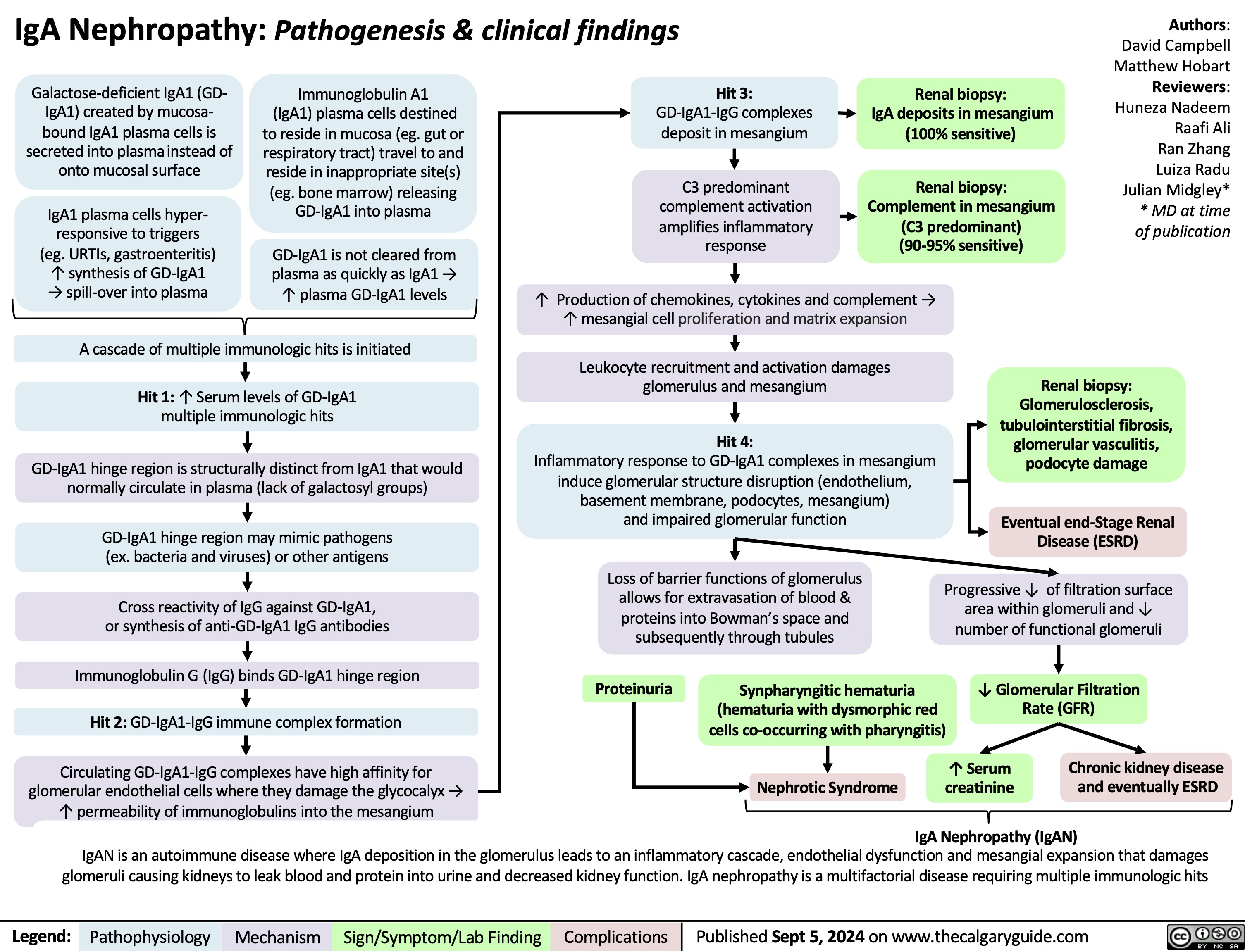
IgA Nephropathy
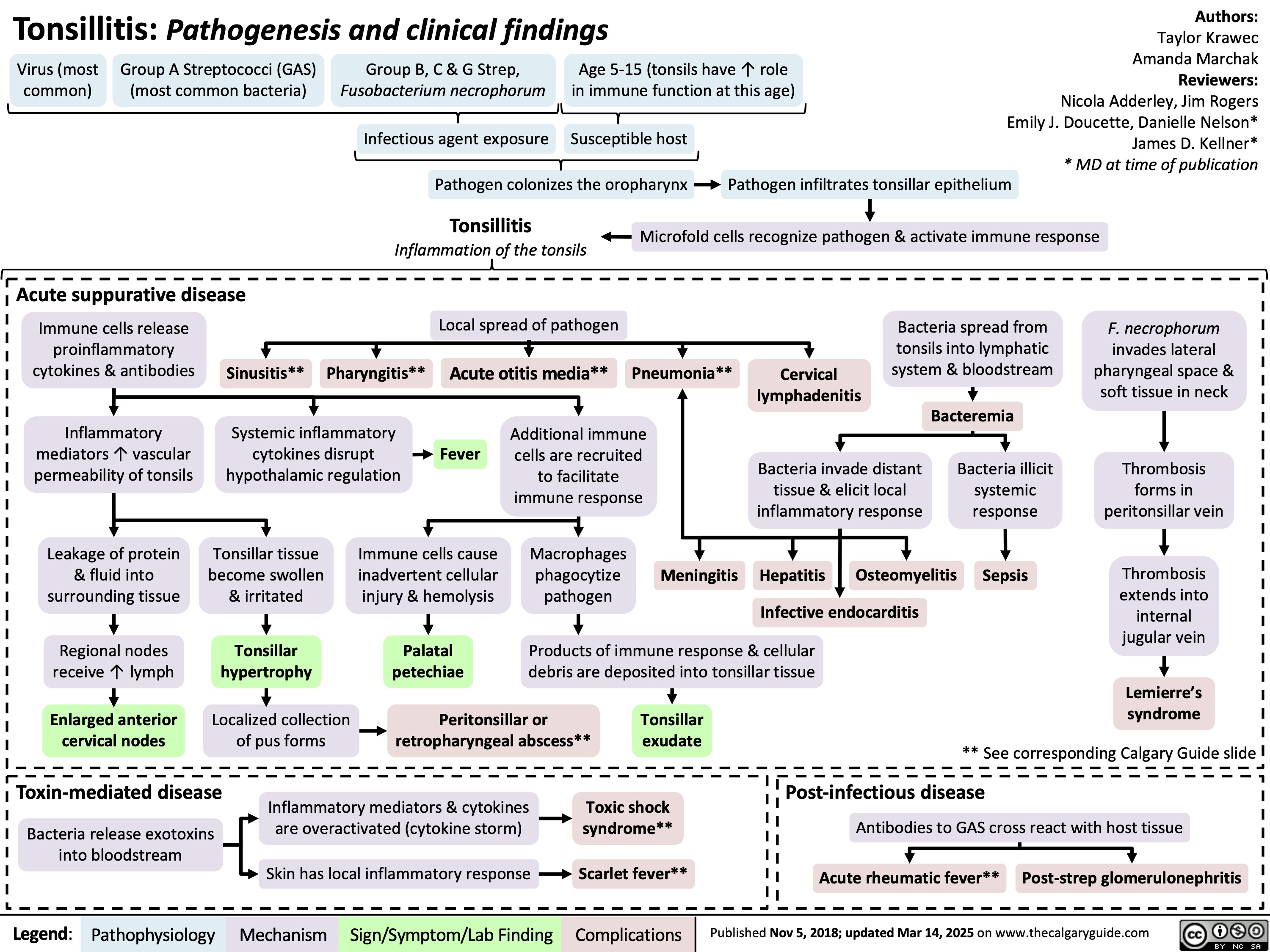
Tonsillitis Pathogenesis and clinical findings
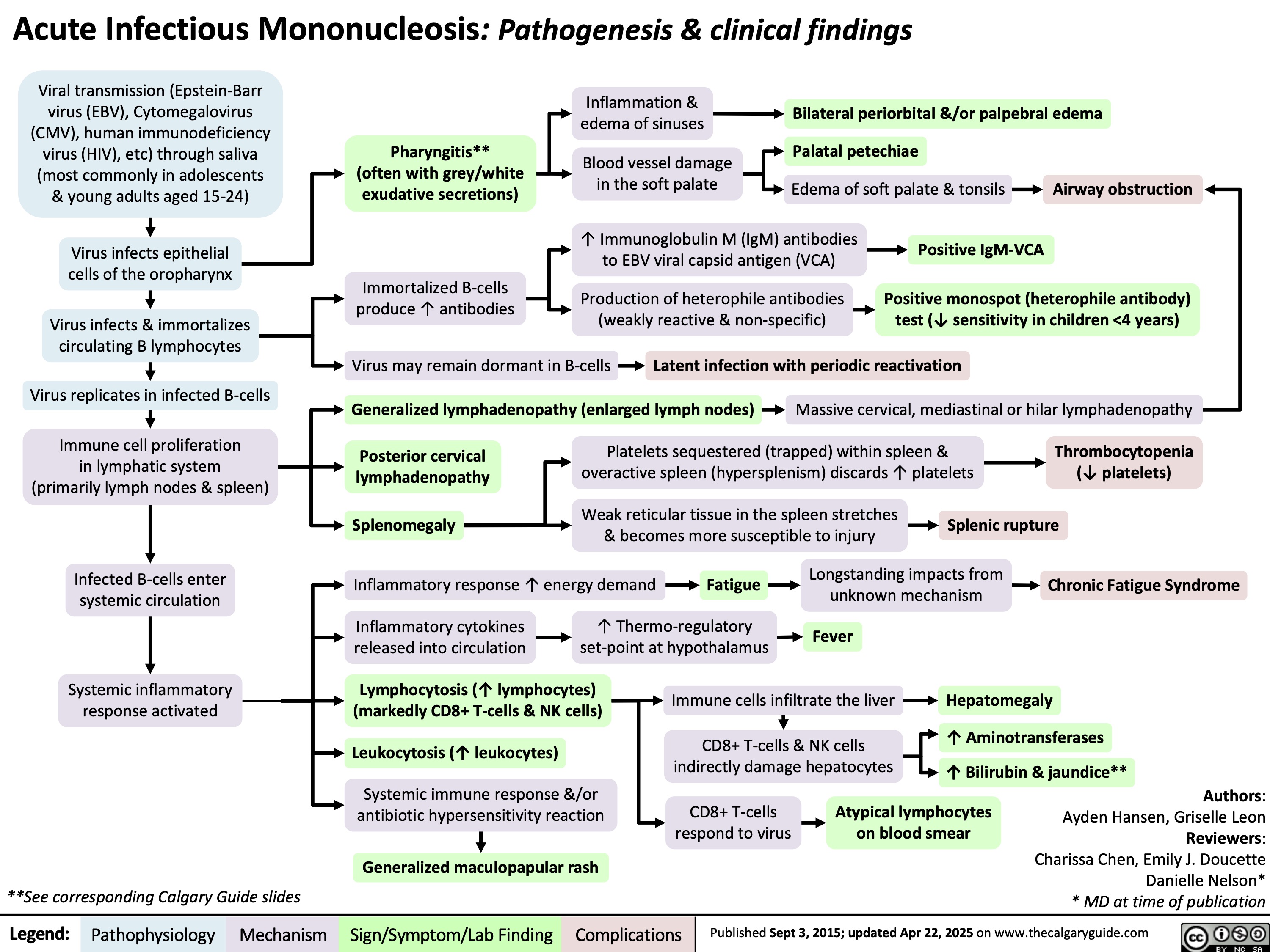
Acute Infectious Mononucleosis
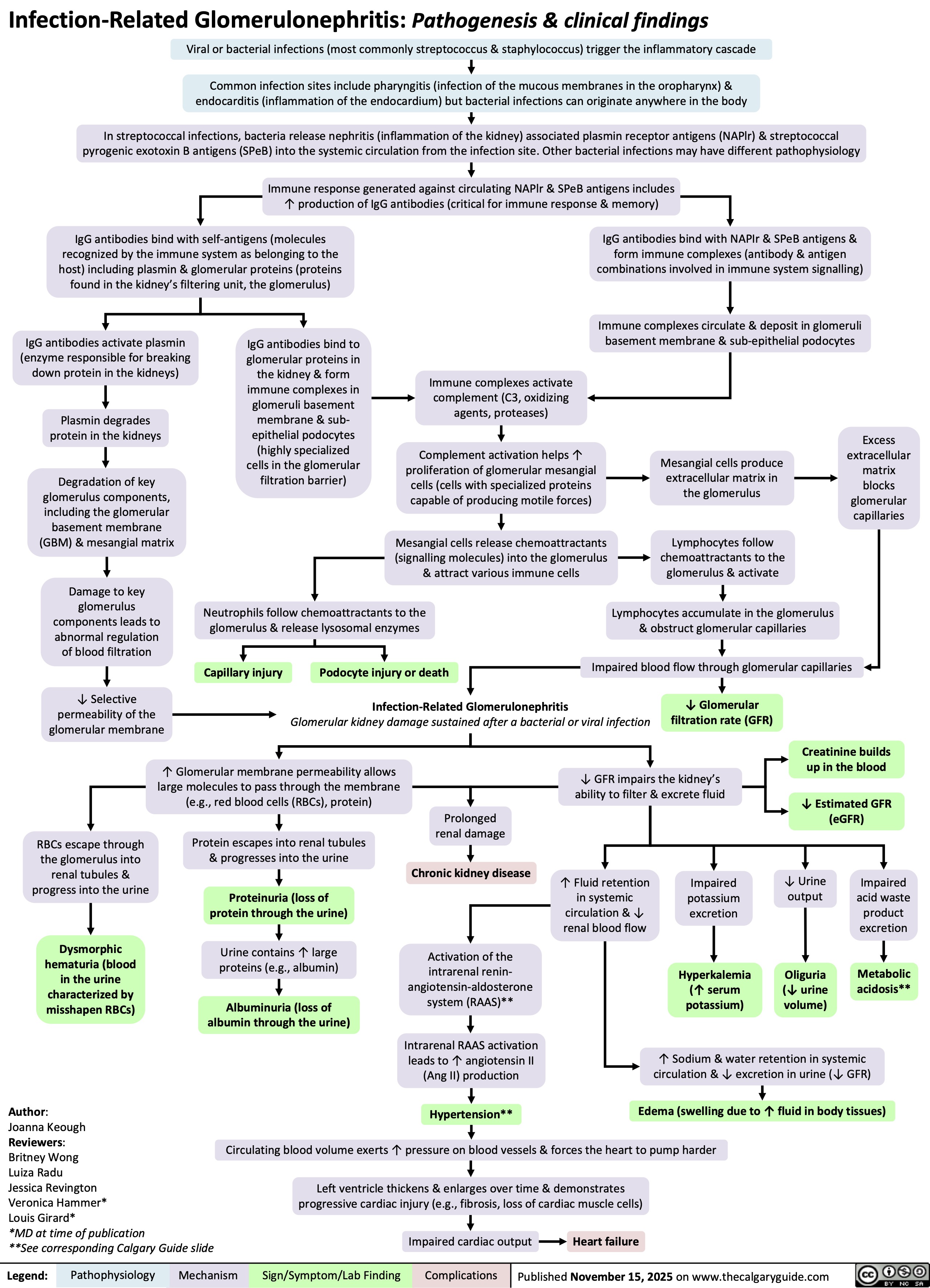
Infection-Related Glomerulonephritis
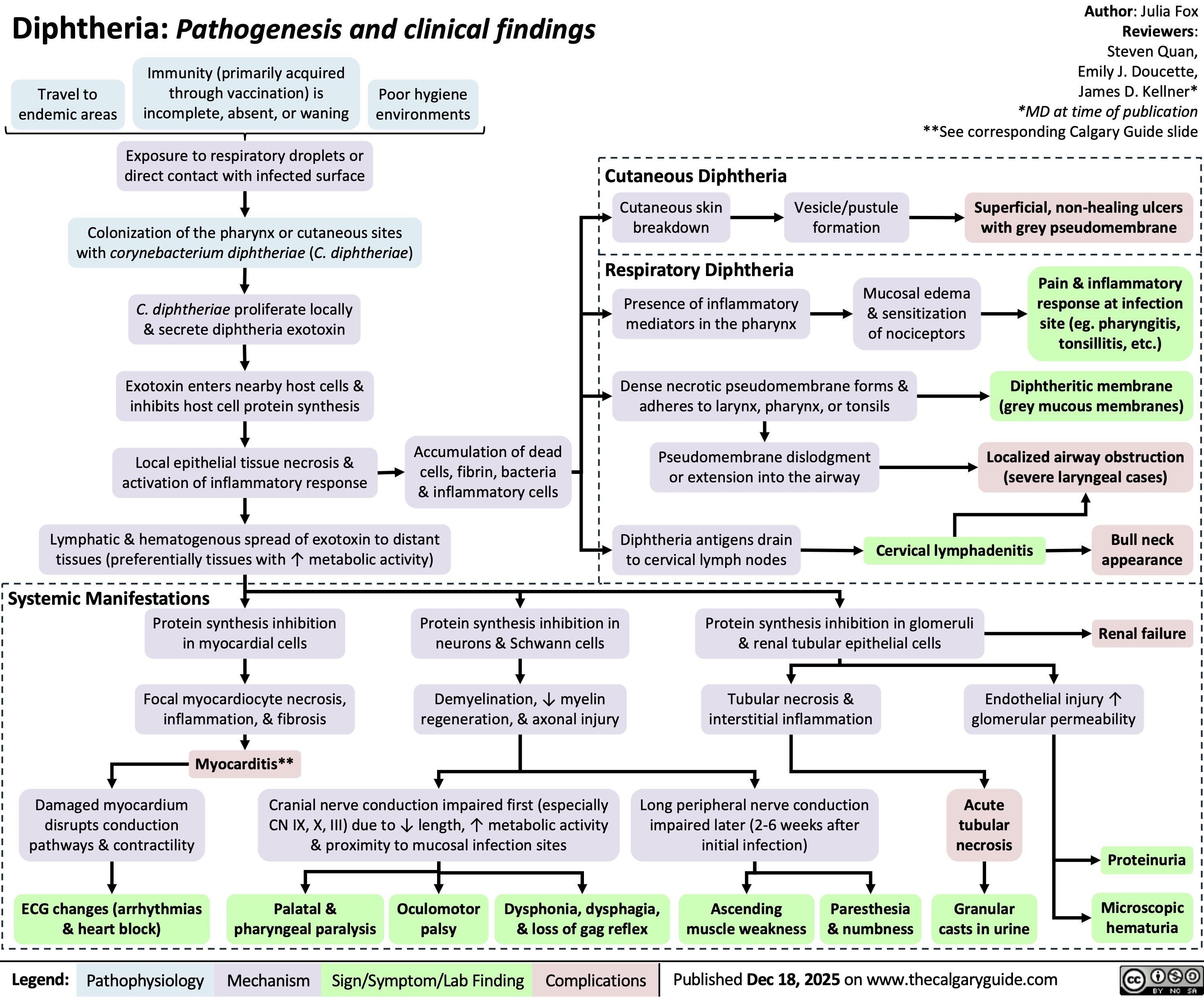
Diphtheria