SEARCH RESULTS FOR: Cirrhosis
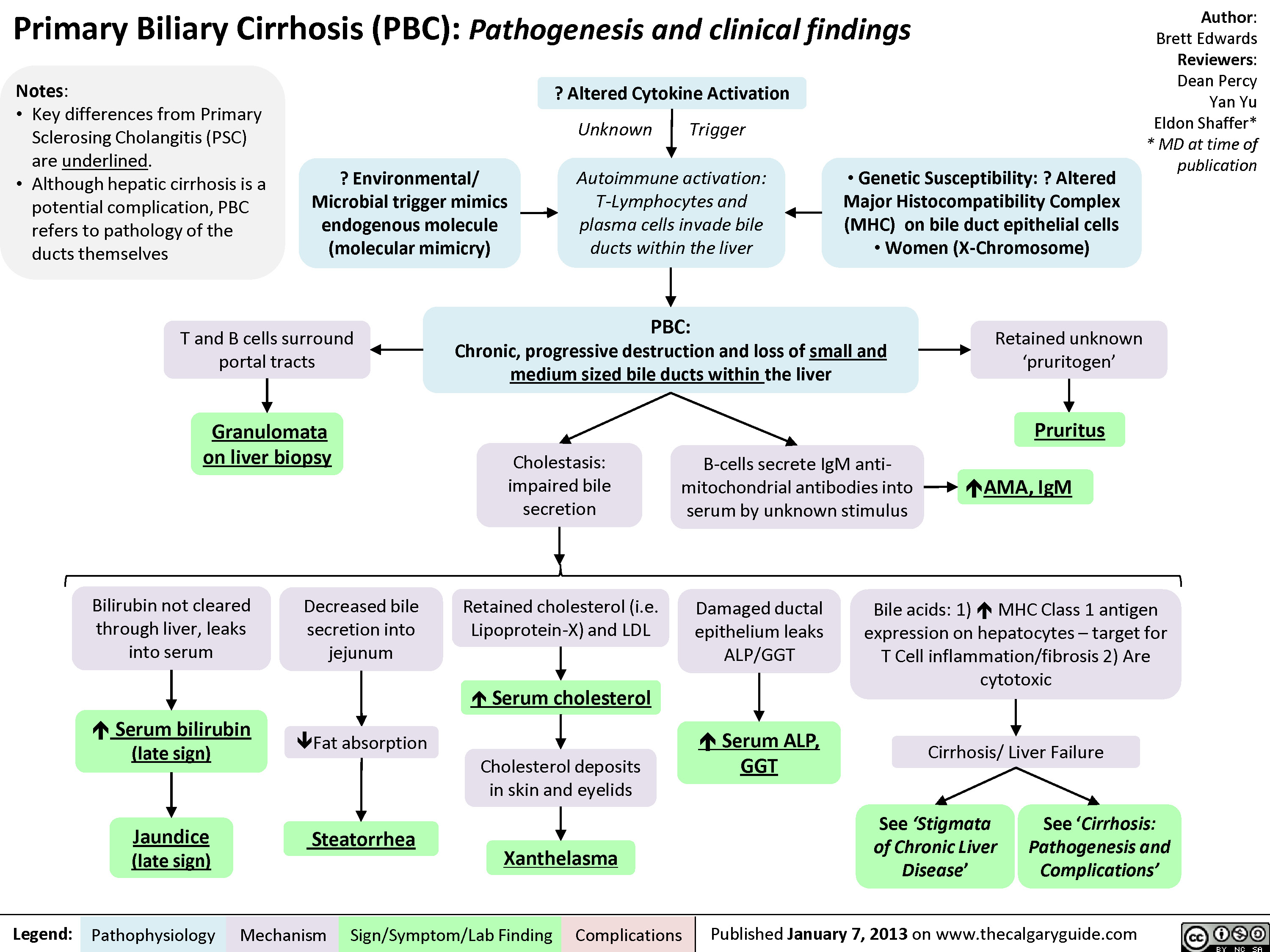
Primary Biliary Cirrhosis (PBC)
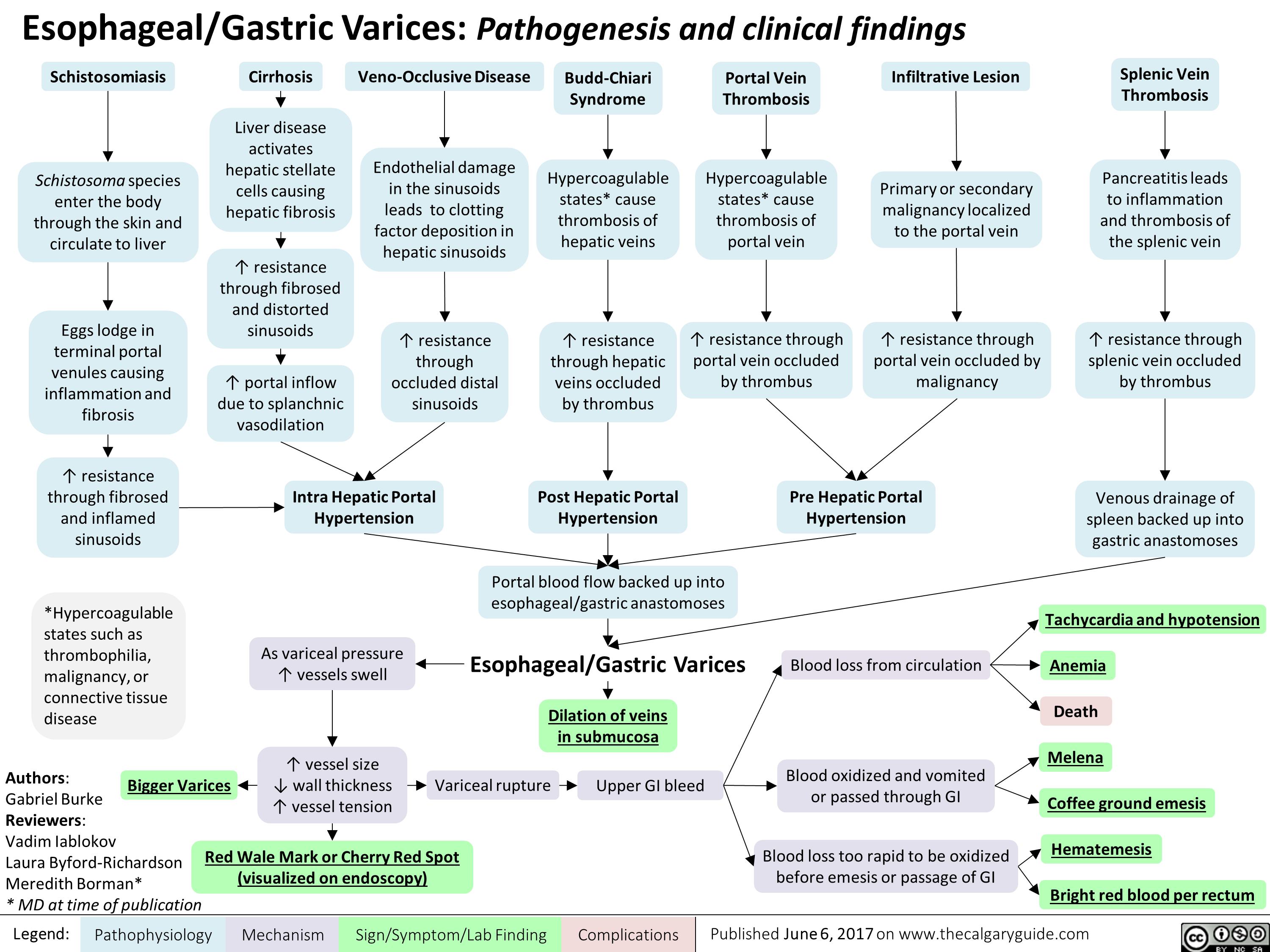
esophageal-gastric-varices
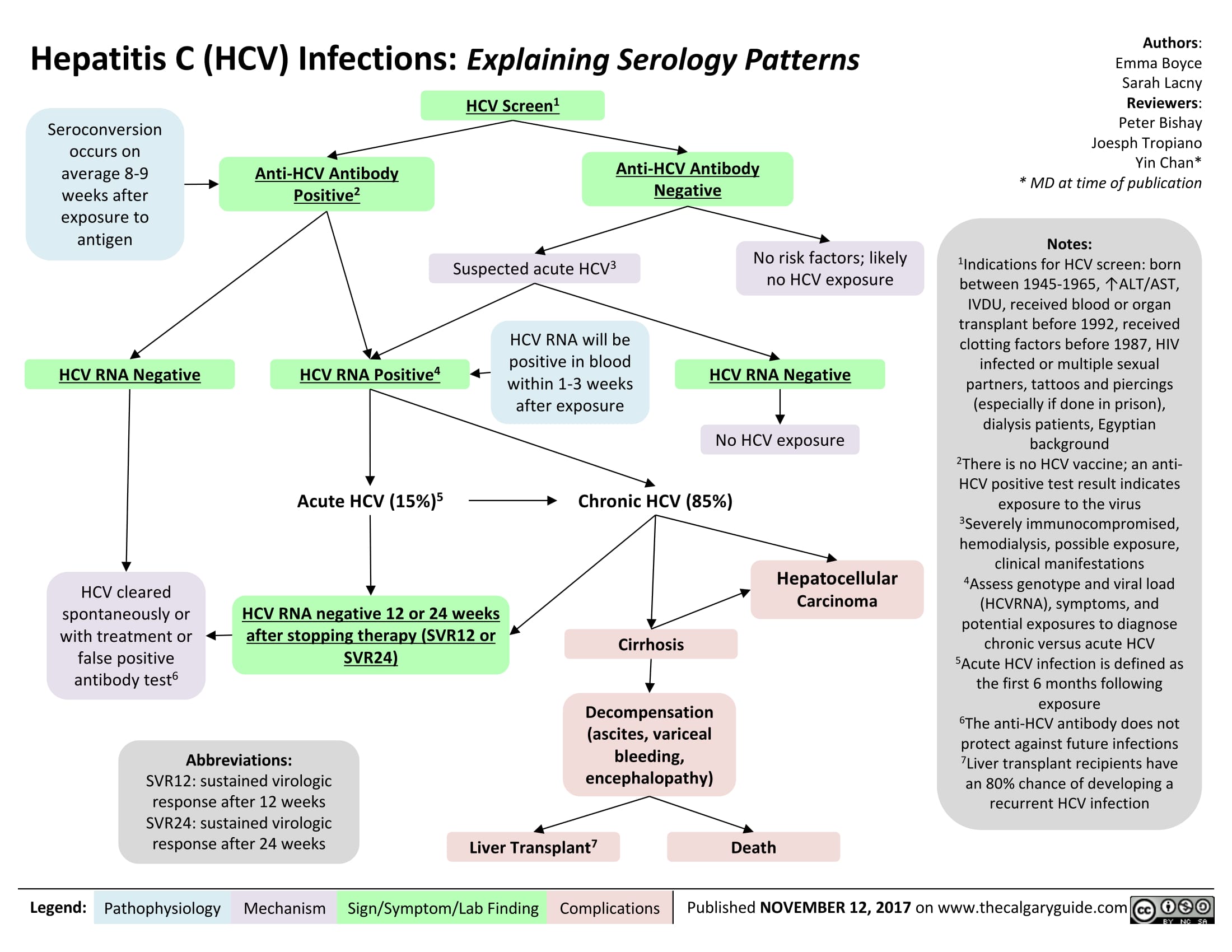
Hepatitis C (HCV) Infections: Explaining Serology Patterns
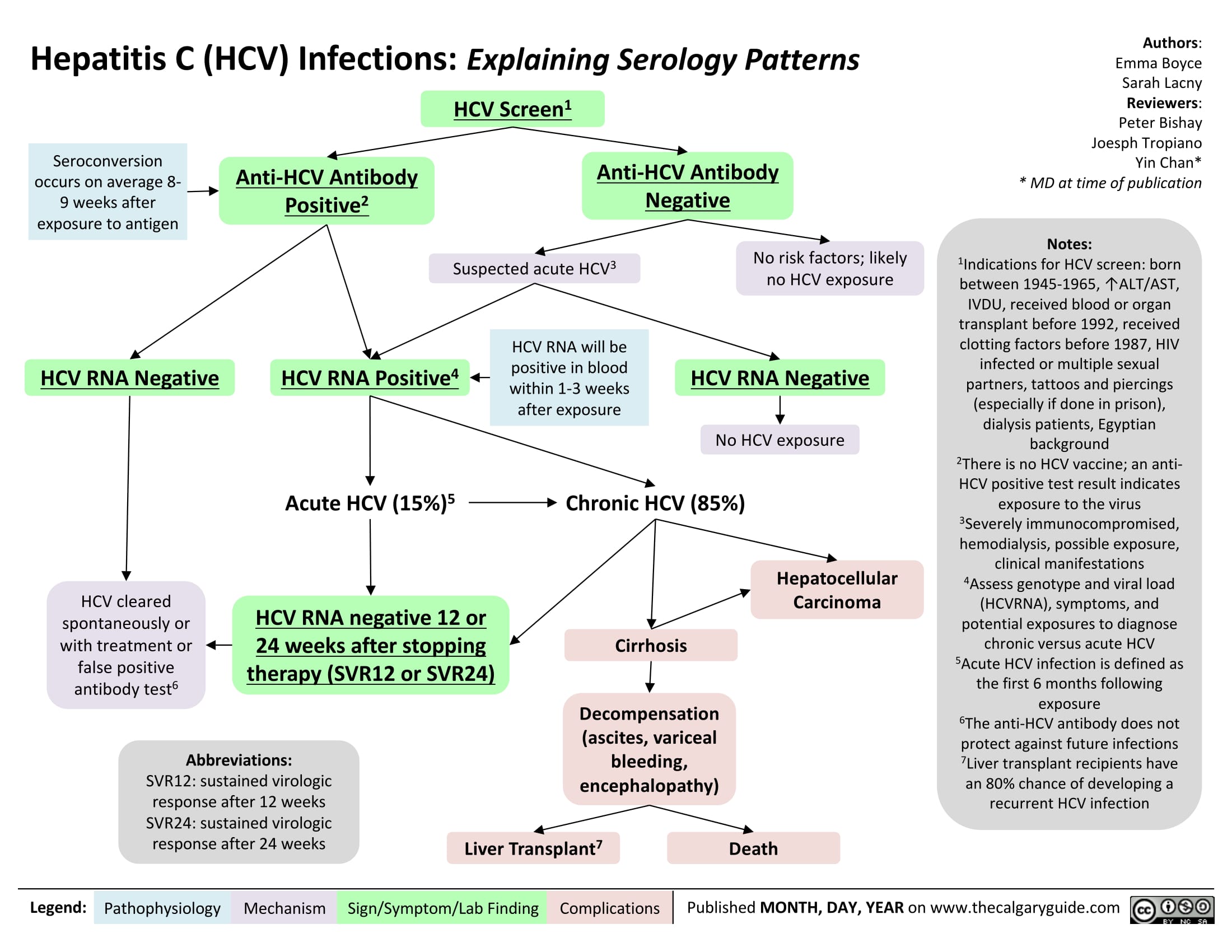
Hepatitis C (HCV) Infection: Explaining Serology Patterns
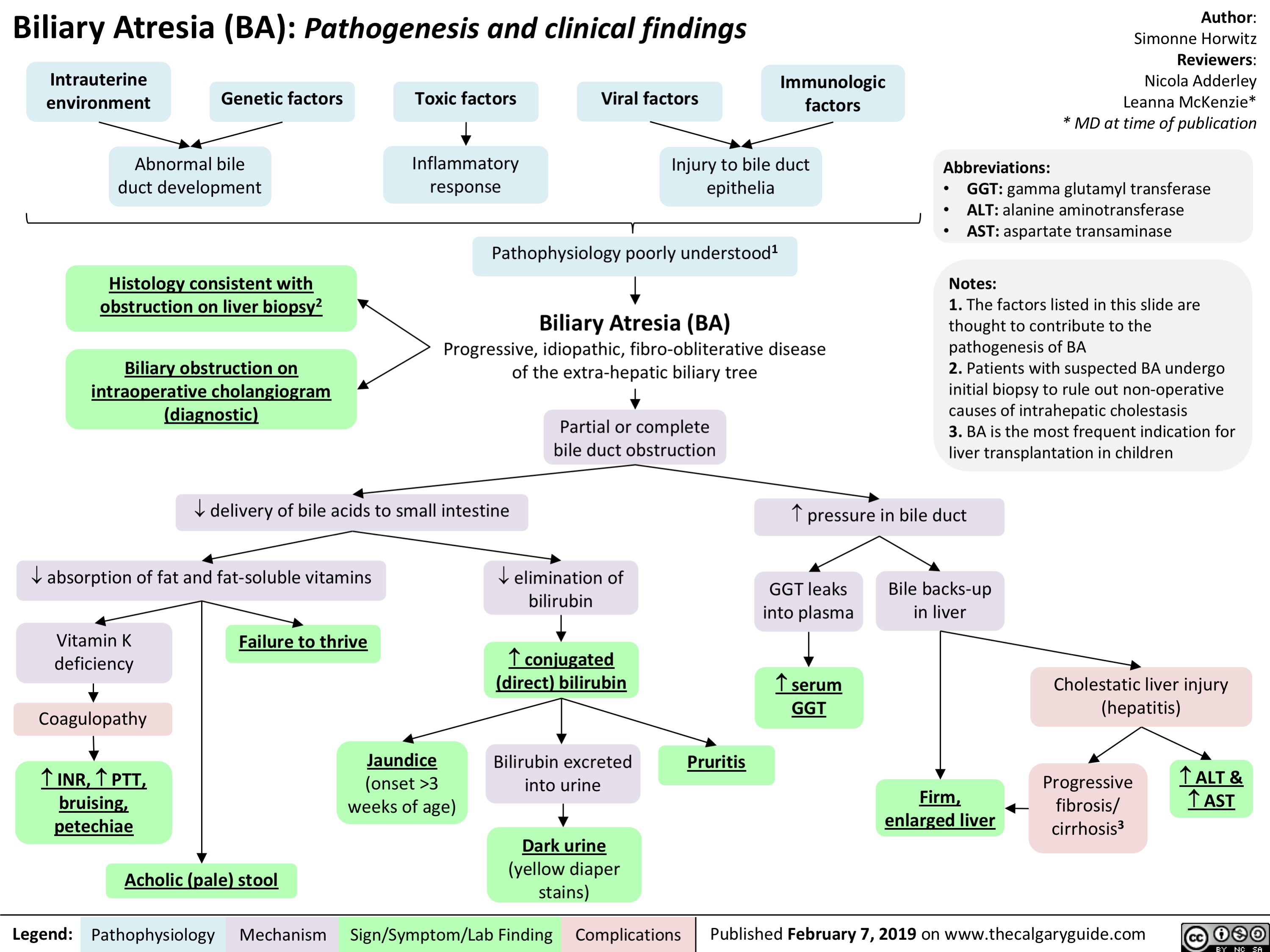
Biliary Atresia (BA)- Pathogenesis and clinical findings
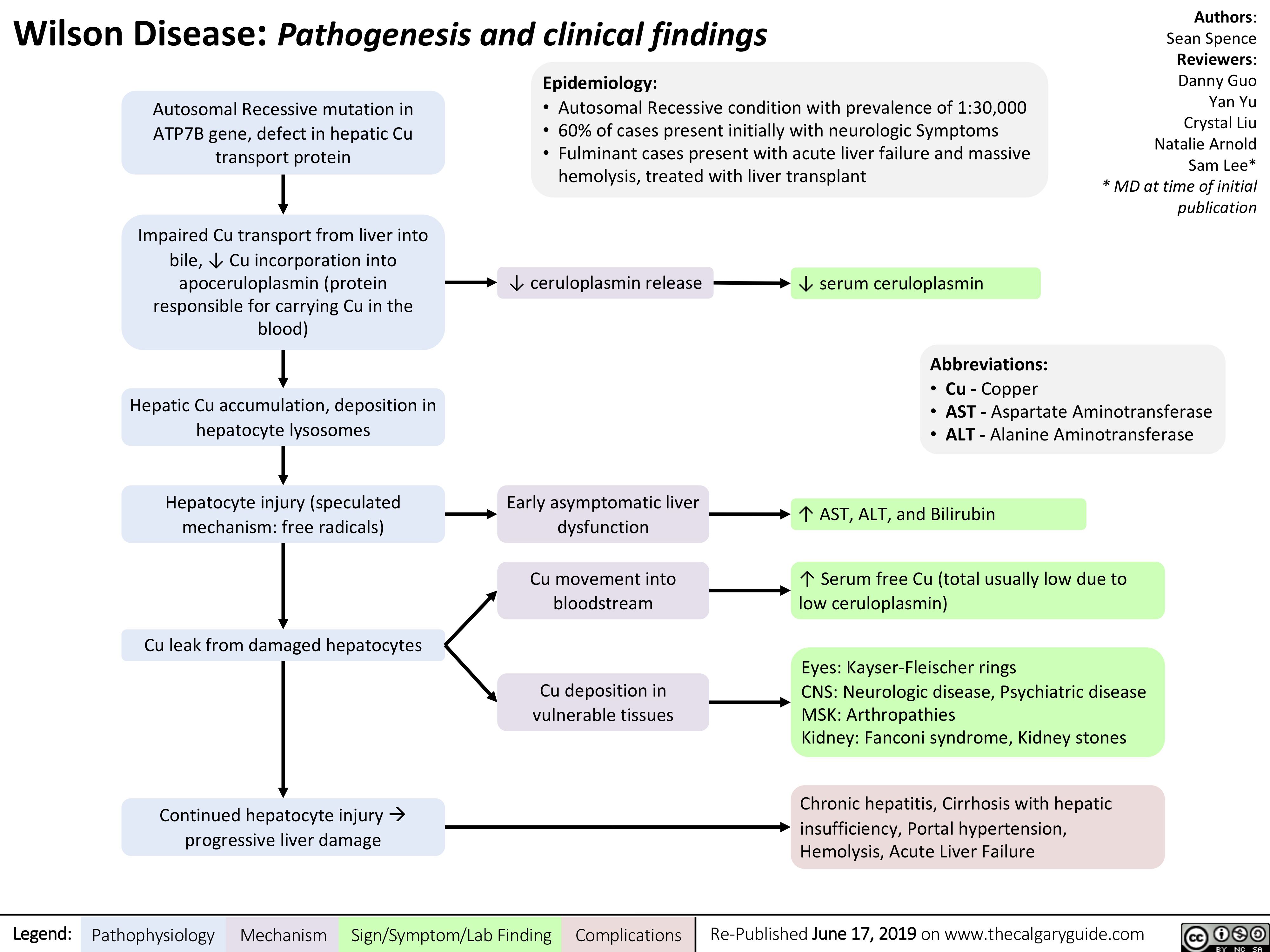
Wilson's Disease
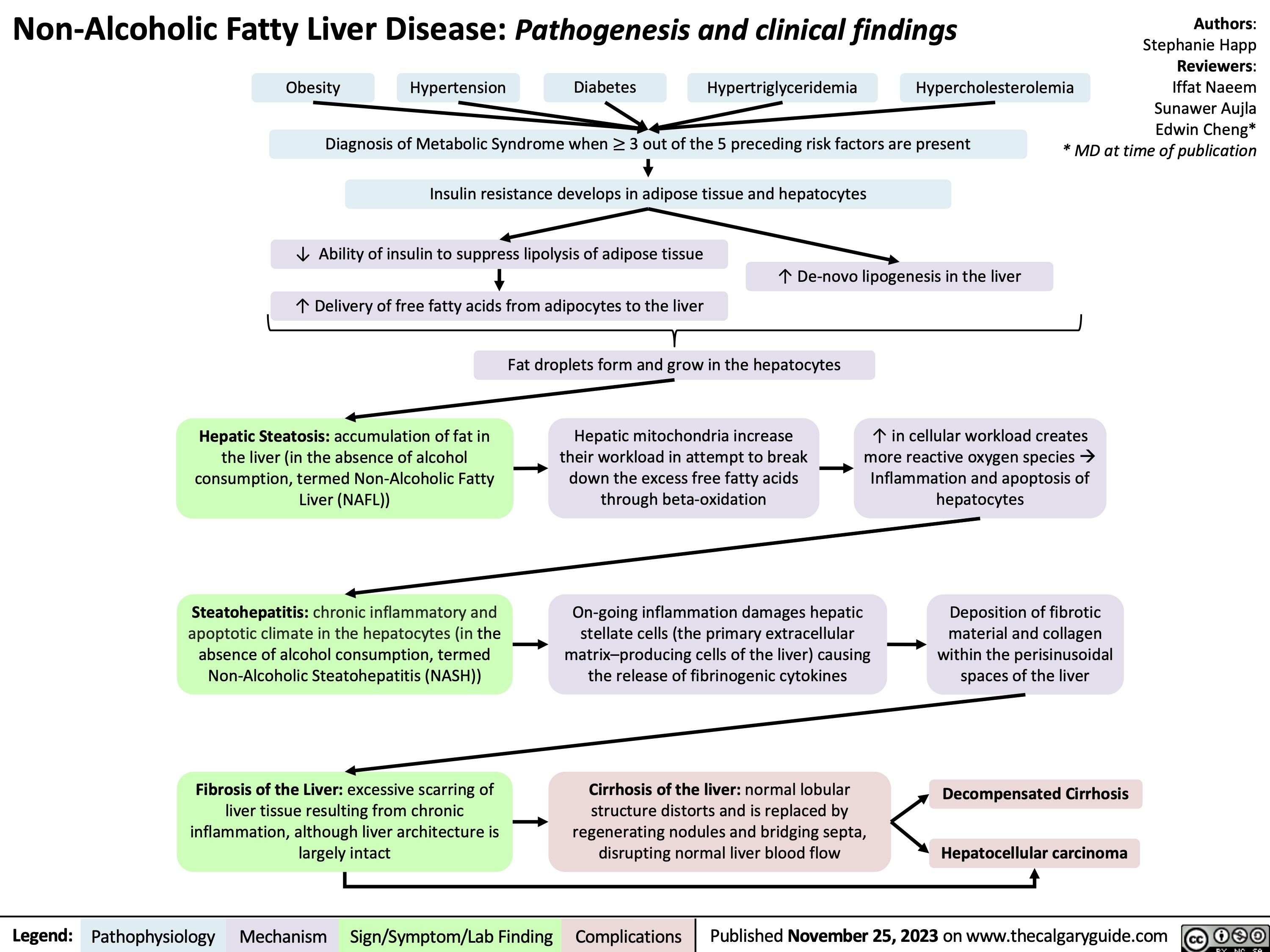
Non-Alcoholic Fatty Liver Disease
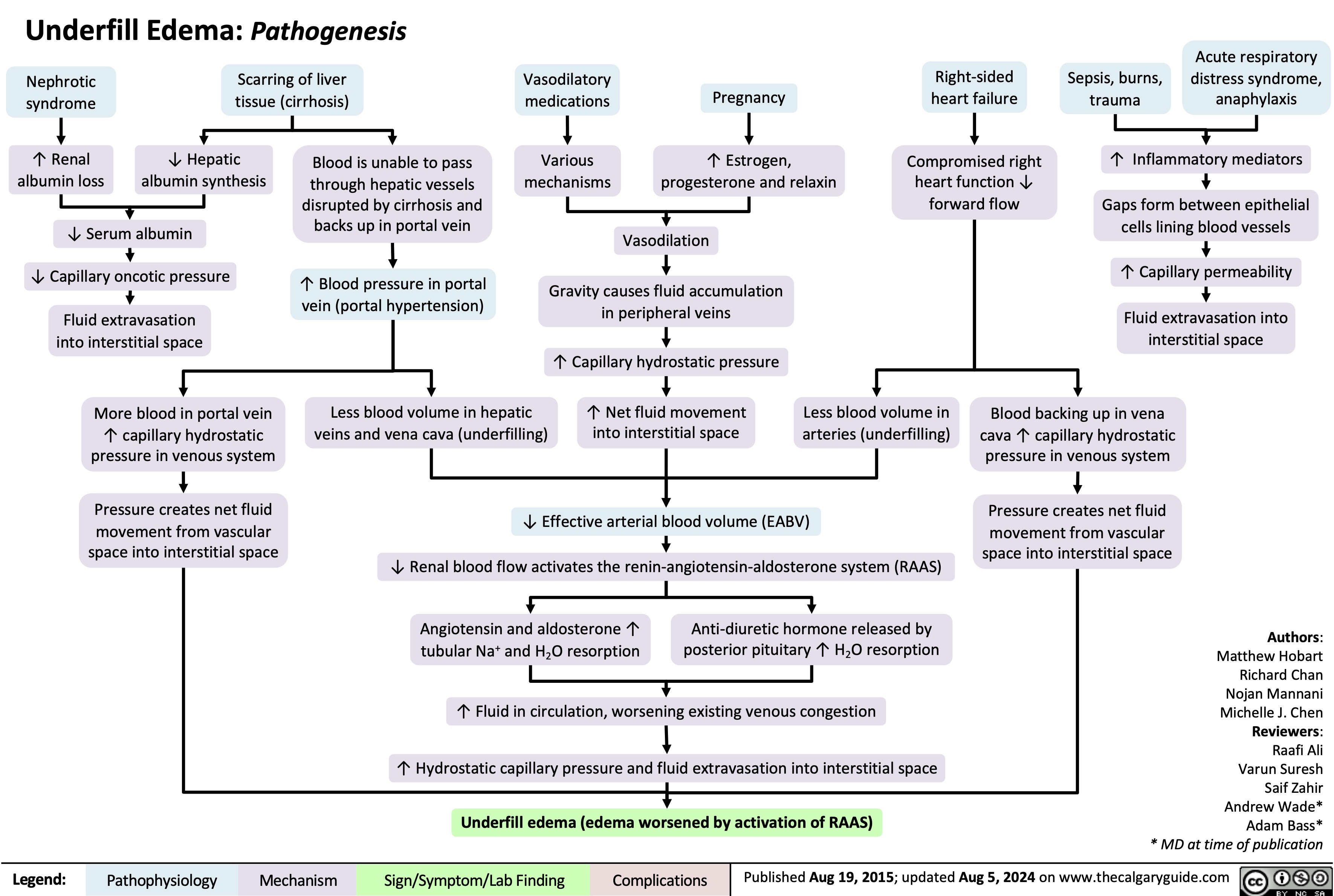
Underfill Edema Pathogenesis
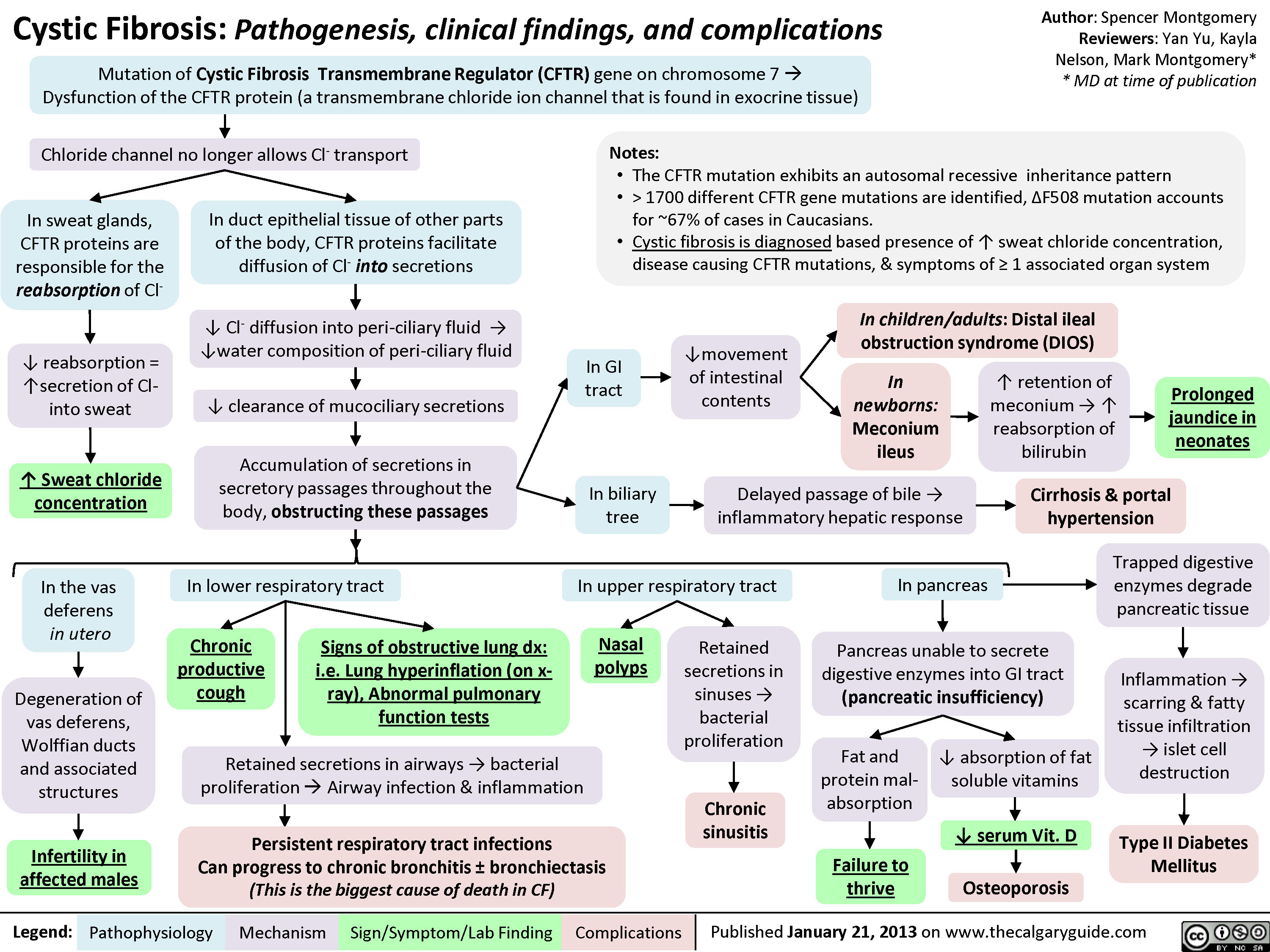
Cystic Fibrosis