SEARCH RESULTS FOR: diabetes mellitus
Nephrotic Syndrome: Pathogenesis and Clinical Findings
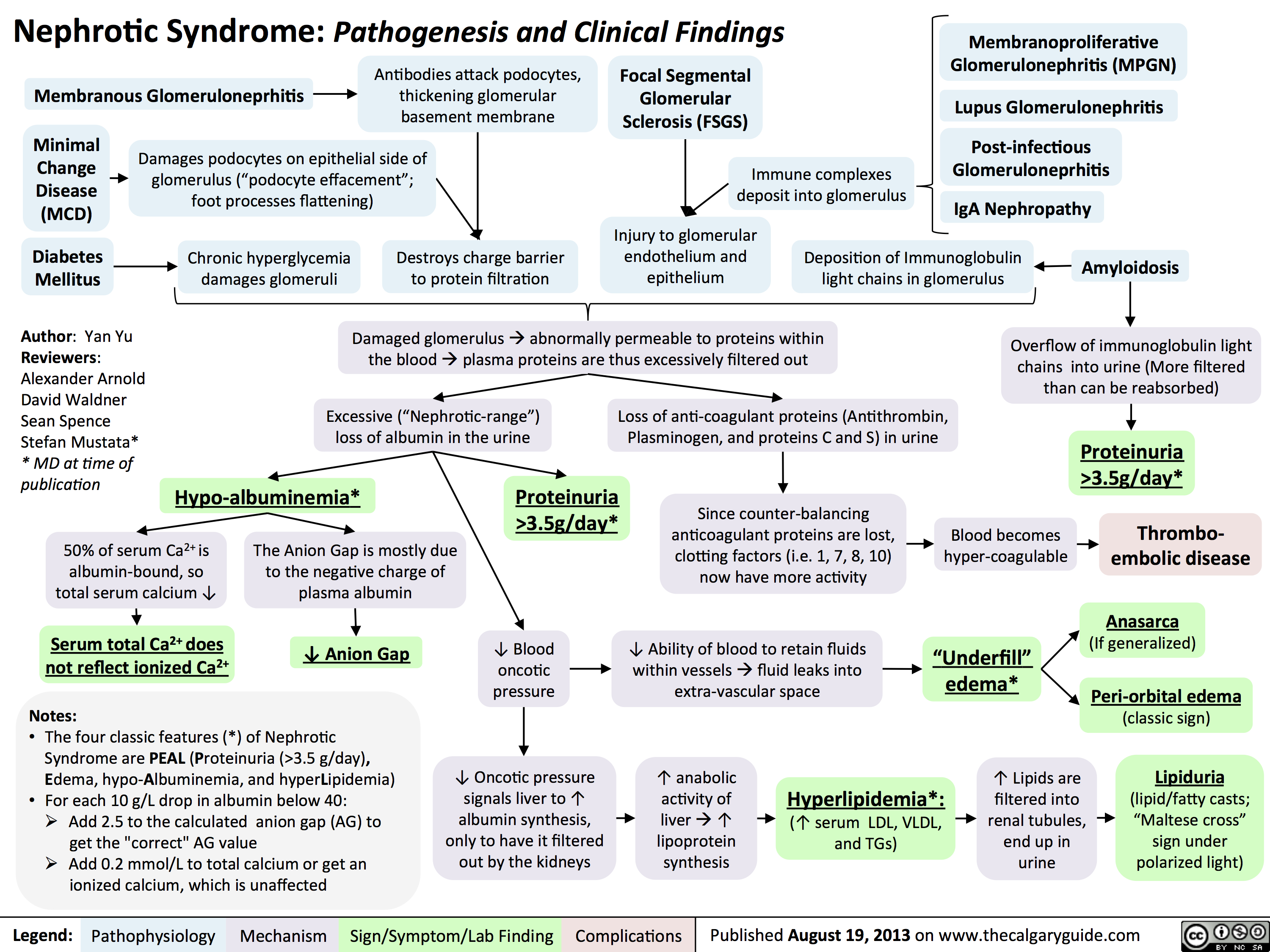 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
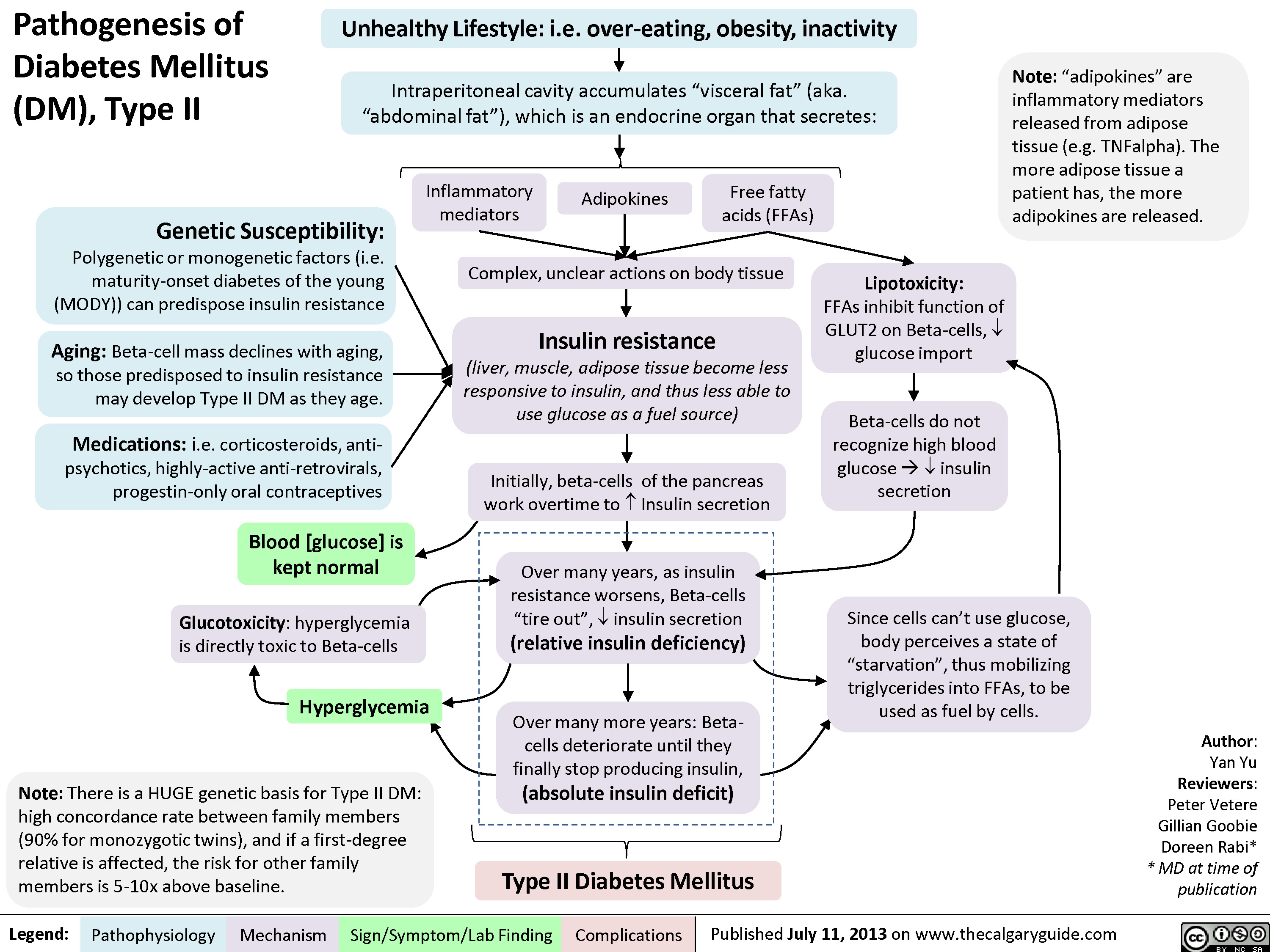
Pathogenesis of Diabetes mellitus DM), Type II
Diabetic Hypoglycemia
![Yu, Yan - Diabetic Hypoglycemia - Clinical Findings - FINAL.pptx
? Epinephrine(Released within seconds as [glucose] falls further) Growth hormone, ? Cortisol (if hypoglycemia persists for minutes)Glucagon should ? when [glucose] falls. But here, glucagon release is inhibited by 1) diabetic auto-immune destruction of Alpha cells & 2) the high insulin.43210Plasma Glucose concentration (mmol/L)Liver should ? glycogenolysis & gluconeogenesisPeripheral vaso-constrictionPlasma [glucose] stays lowActivation of sympathetic (adrenergic) receptors across body, triggering Neurogenic symptomsPlasma [glucose] ?Excess subcutaneous insulin or insulin-secretagogue ?? [insulin] in the bloodOver time: [insulin] in the DM patient depends only on how much was injected or how much secretagogue was consumed; not on the body's physiological state.[Insulin] stays high in excessively-treated DM patientsPlasma [glucose] normally ?, but...High insulin transports plasma glucose into cells!In pts with existing diabetic autonomic neuropathy, epi-nephrine secretion will already be ?Brain does not get enough glucose, ? neuron function ? Neuroglycopenic symptomsTx: glucose intake![Glucose] returns to normalIf no glucose intake:Hypoglycemia-unawareness: No autonomic Sx felt so hypoglycemia not treated early ? pts present later on with more severe hypoglycemia and neuroglycopenic sxBrain cells kept chronically euglycemic due to GLUT1 receptor over-expression (despite rest of body being hypoglycemic)With many hypoglycemic events over time:Brain feels no need to ? glucose, so it ? autonomic epinephrine secretion!This is the normal sequence of hormone responses to ?ing plasma glucose levels.But this normal hormonal response will be blunted over time if there is 1) continued hypoglycemia dampening the sympathetic nervous system, and 2) long-standing diabetic neuropathy! (To be explained later in this flow chart)Abbreviations: [ ] = concentrationTx = TreatmentDM = Diabetes mellitusDiabetic Hypoglycemia: Pathogenesis and Clinical FindingsConfusionCan't concentrateWeaknessSlurred speech? coordination (staggering, etc)SeizuresComa, deathAdrenergic symptoms (epinephrine-mediated):Anxiety, irritability, trembling, pallor (skin vasoconstriction), palpitations, ? systolic BP, tachycardia Cholinergic symptoms(Acetylcholine-mediated):Sweating, hunger, tingling, blurry visionNote: In pts w/out DM, endogenous insulin secretion normally stops when blood [glucose] drops to <4mmol/LAuthor: Yan YuReviewers: Peter Vetere, Gillian Goobie, Hanan Bassyouni** MD at time of publicationLegend:Published June 14, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsMany hypoglycemic events over time blunt epinephrine secretion further.Hypoglycemia unawareness can be reversedIf pt stays hypoglycemia-free for >6 weeks, brain restores its ability to detect low glucose levels? peripheral glucose delivery and uptake (saving more glucose for the brain)Lack of glucagon effect reinforces hypoglycemia
124 kB / 361 words Yu, Yan - Diabetic Hypoglycemia - Clinical Findings - FINAL.pptx
? Epinephrine(Released within seconds as [glucose] falls further) Growth hormone, ? Cortisol (if hypoglycemia persists for minutes)Glucagon should ? when [glucose] falls. But here, glucagon release is inhibited by 1) diabetic auto-immune destruction of Alpha cells & 2) the high insulin.43210Plasma Glucose concentration (mmol/L)Liver should ? glycogenolysis & gluconeogenesisPeripheral vaso-constrictionPlasma [glucose] stays lowActivation of sympathetic (adrenergic) receptors across body, triggering Neurogenic symptomsPlasma [glucose] ?Excess subcutaneous insulin or insulin-secretagogue ?? [insulin] in the bloodOver time: [insulin] in the DM patient depends only on how much was injected or how much secretagogue was consumed; not on the body's physiological state.[Insulin] stays high in excessively-treated DM patientsPlasma [glucose] normally ?, but...High insulin transports plasma glucose into cells!In pts with existing diabetic autonomic neuropathy, epi-nephrine secretion will already be ?Brain does not get enough glucose, ? neuron function ? Neuroglycopenic symptomsTx: glucose intake![Glucose] returns to normalIf no glucose intake:Hypoglycemia-unawareness: No autonomic Sx felt so hypoglycemia not treated early ? pts present later on with more severe hypoglycemia and neuroglycopenic sxBrain cells kept chronically euglycemic due to GLUT1 receptor over-expression (despite rest of body being hypoglycemic)With many hypoglycemic events over time:Brain feels no need to ? glucose, so it ? autonomic epinephrine secretion!This is the normal sequence of hormone responses to ?ing plasma glucose levels.But this normal hormonal response will be blunted over time if there is 1) continued hypoglycemia dampening the sympathetic nervous system, and 2) long-standing diabetic neuropathy! (To be explained later in this flow chart)Abbreviations: [ ] = concentrationTx = TreatmentDM = Diabetes mellitusDiabetic Hypoglycemia: Pathogenesis and Clinical FindingsConfusionCan't concentrateWeaknessSlurred speech? coordination (staggering, etc)SeizuresComa, deathAdrenergic symptoms (epinephrine-mediated):Anxiety, irritability, trembling, pallor (skin vasoconstriction), palpitations, ? systolic BP, tachycardia Cholinergic symptoms(Acetylcholine-mediated):Sweating, hunger, tingling, blurry visionNote: In pts w/out DM, endogenous insulin secretion normally stops when blood [glucose] drops to <4mmol/LAuthor: Yan YuReviewers: Peter Vetere, Gillian Goobie, Hanan Bassyouni** MD at time of publicationLegend:Published June 14, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsMany hypoglycemic events over time blunt epinephrine secretion further.Hypoglycemia unawareness can be reversedIf pt stays hypoglycemia-free for >6 weeks, brain restores its ability to detect low glucose levels? peripheral glucose delivery and uptake (saving more glucose for the brain)Lack of glucagon effect reinforces hypoglycemia
124 kB / 361 words](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Diabetic-Hypoglycemia-Clinical-Findings.jpg)
chronic-hypertensive-retinopathy-pathogenesis-and-clinical-findings
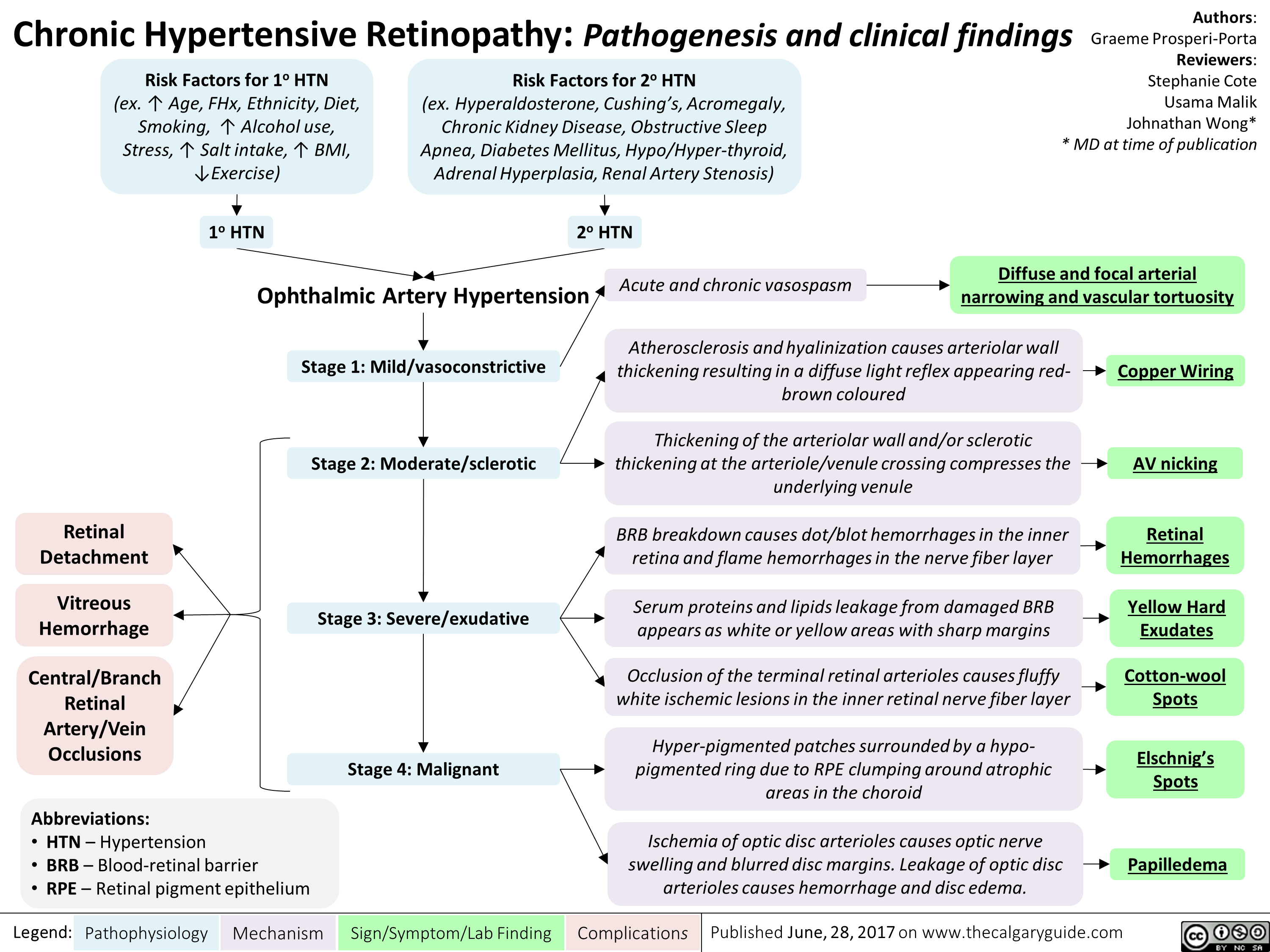
Erectile Dysfunction: Pathogenesis
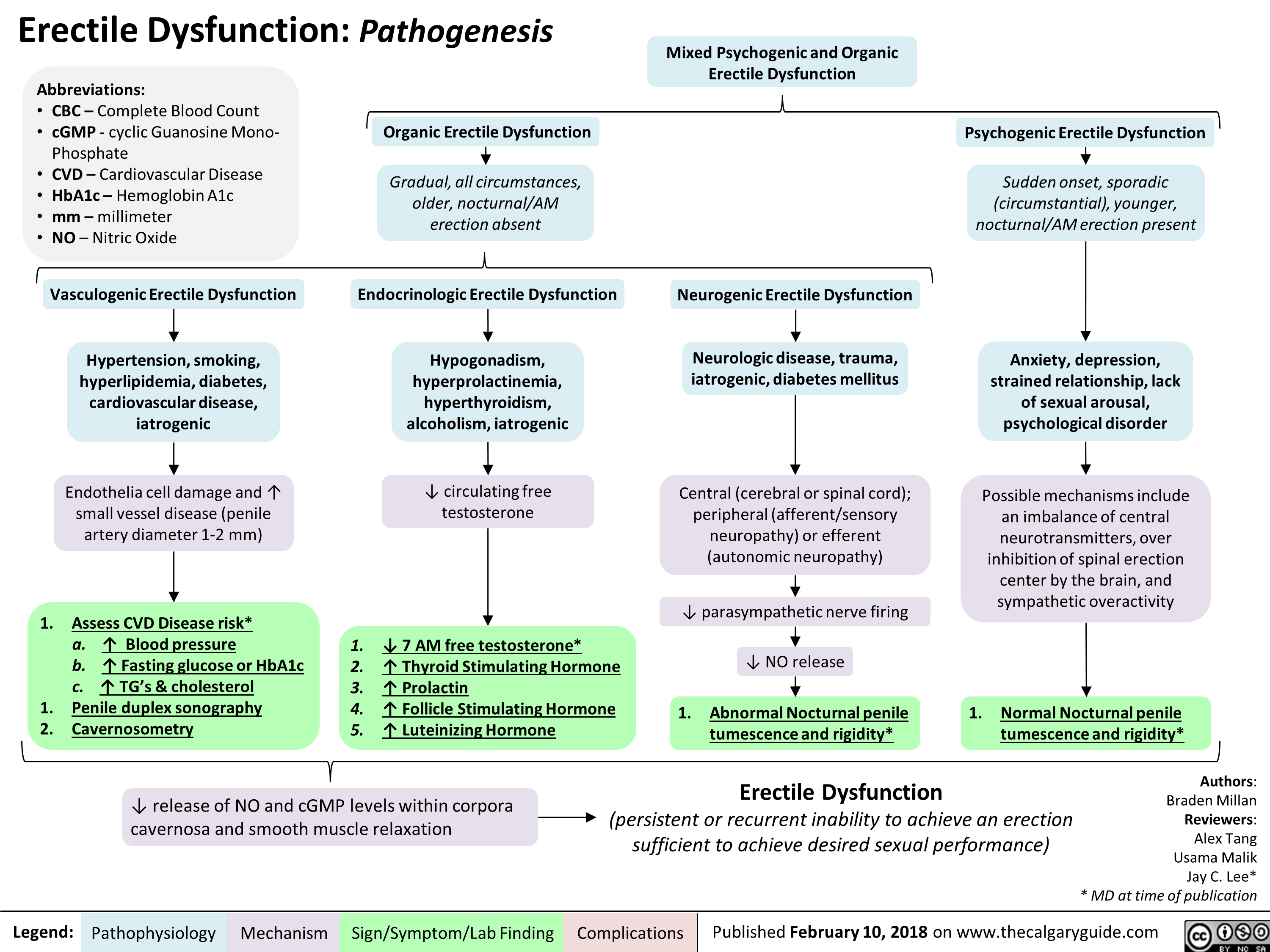
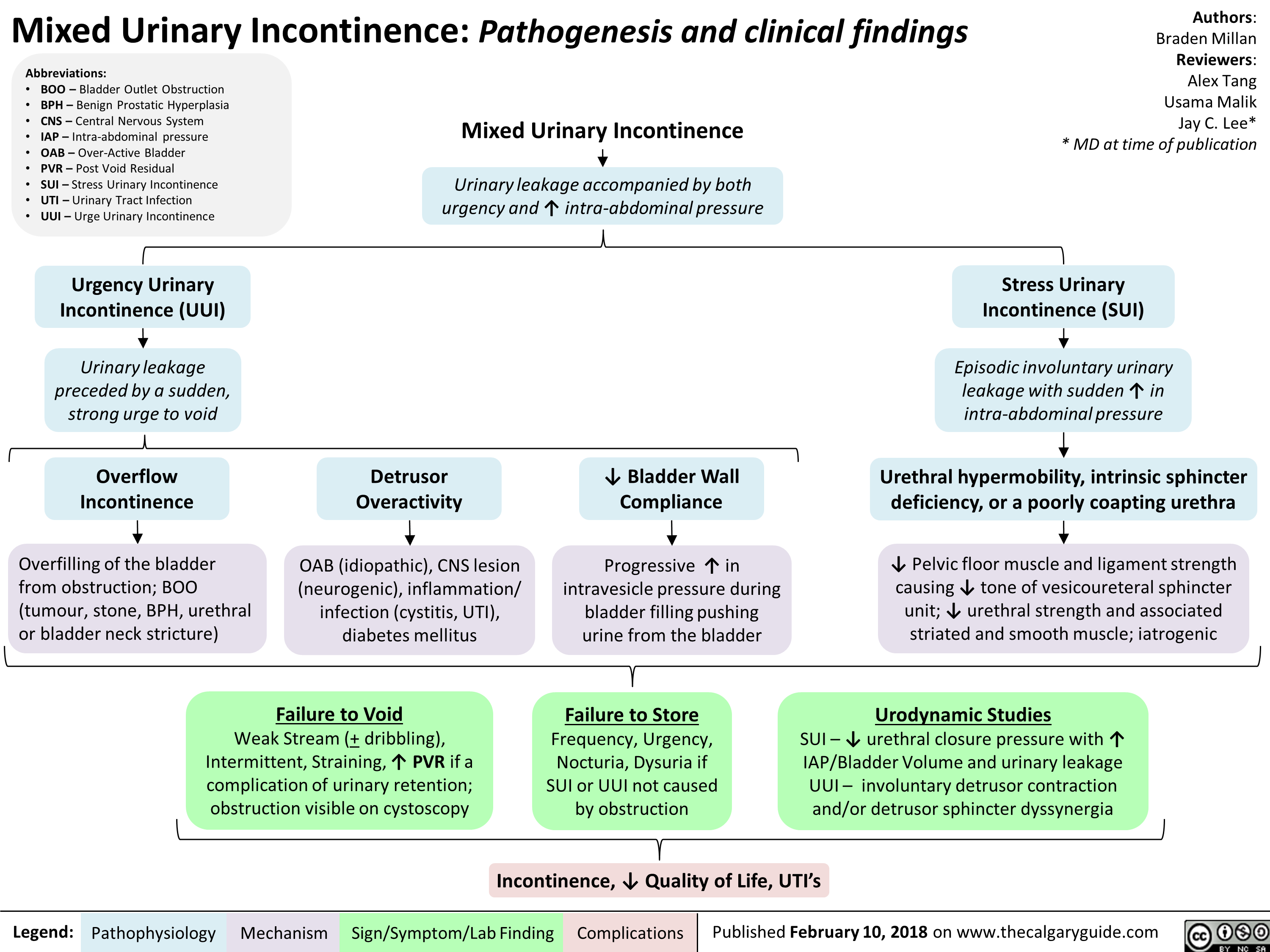
Mixed Urinary Incontinence Pathogenesis and clinical findings
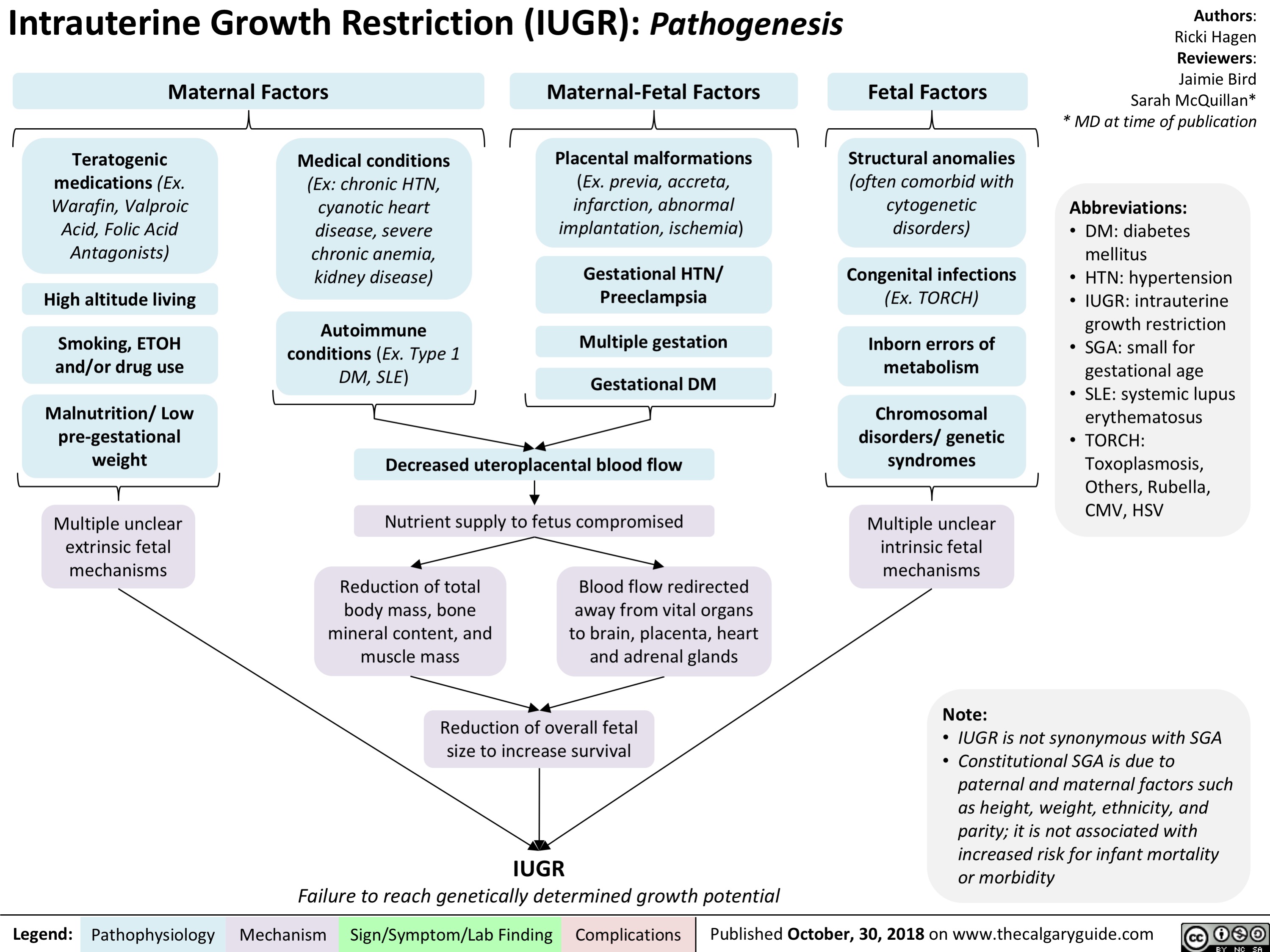
intrauterine-growth-restriction-iugr-pathogenesis
Hypernatremia Physiology
![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)
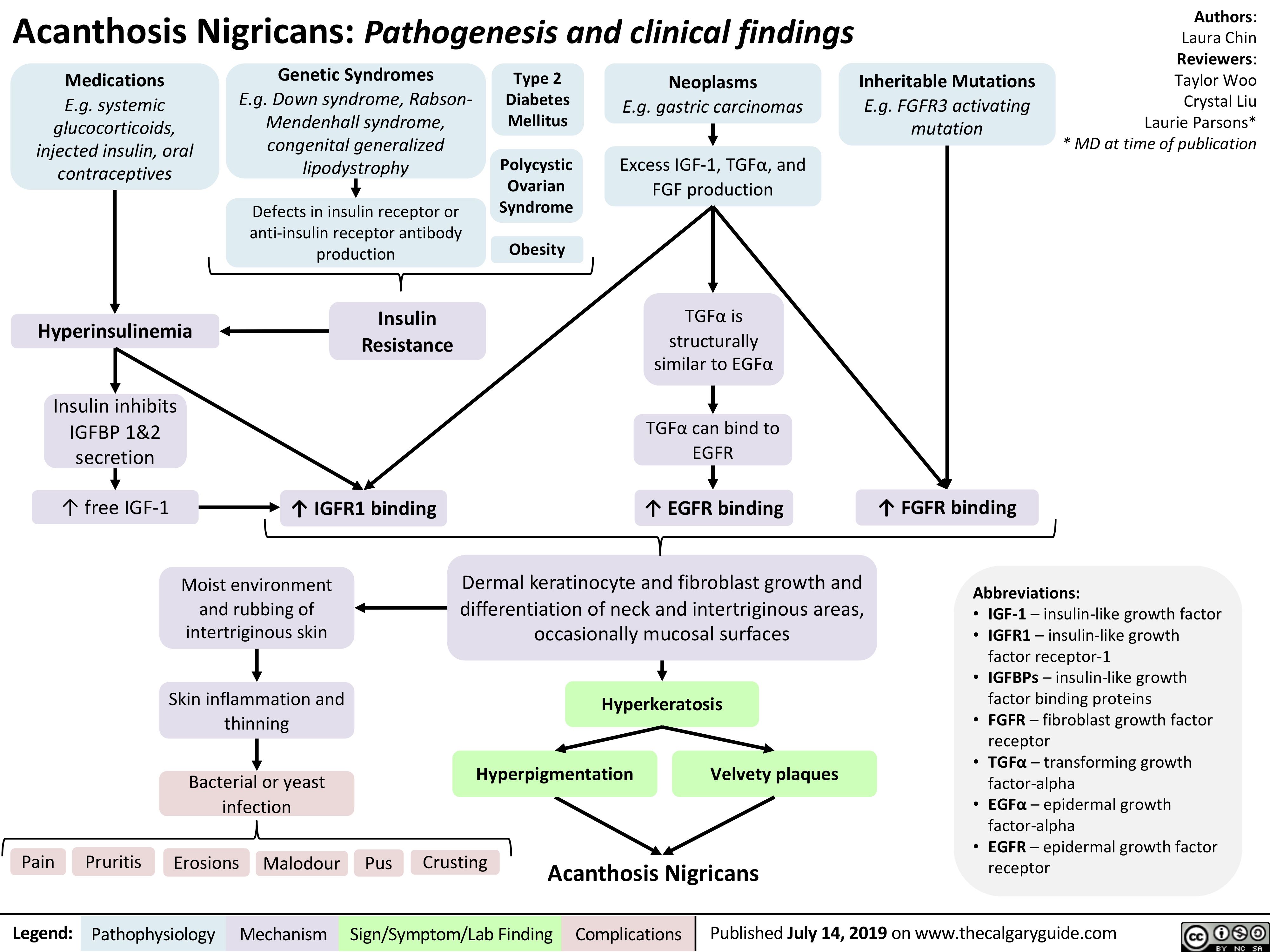
acanthosis-nigricans-pathogenesis-and-clinical-findings
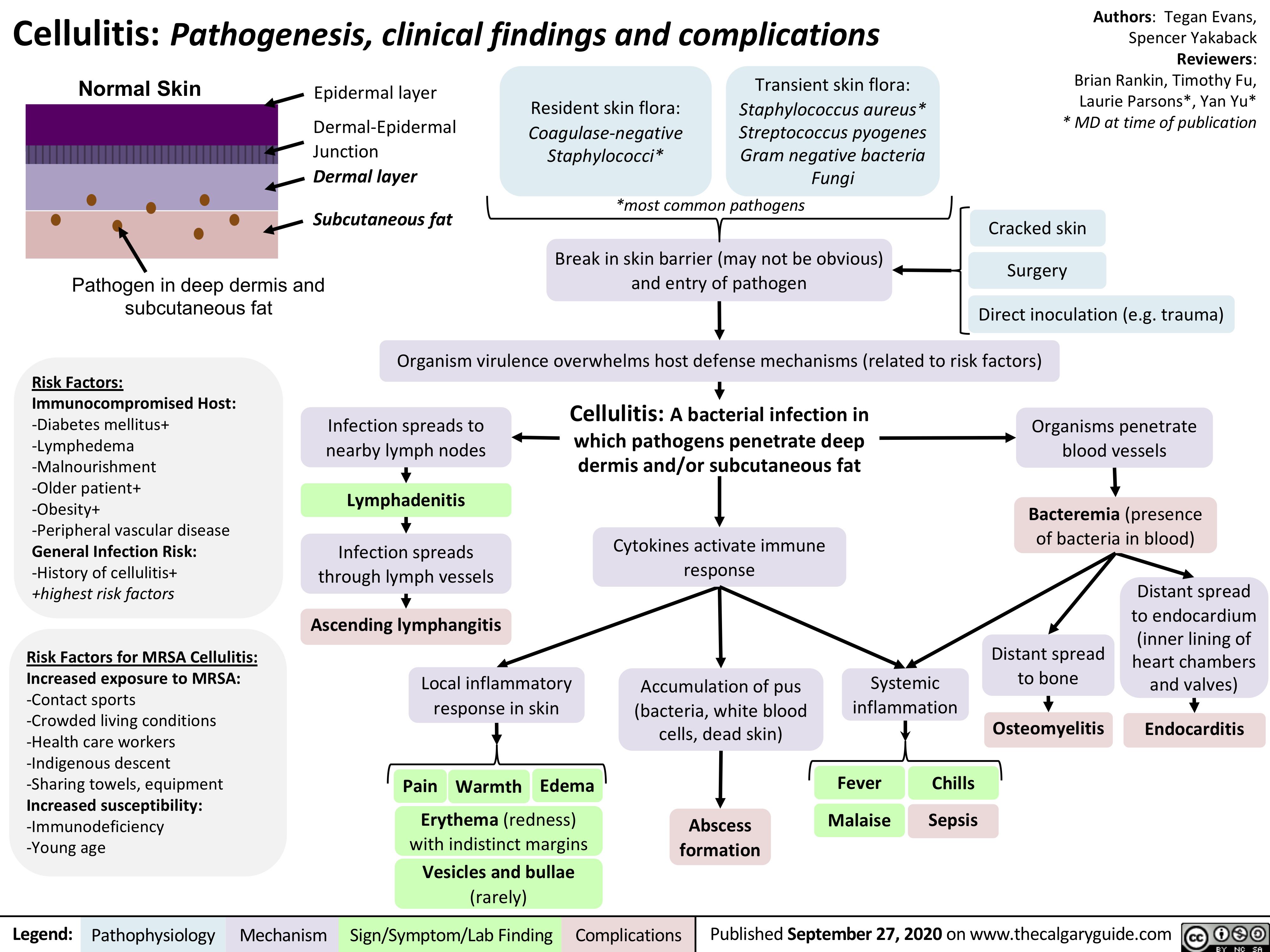
Cellulitis
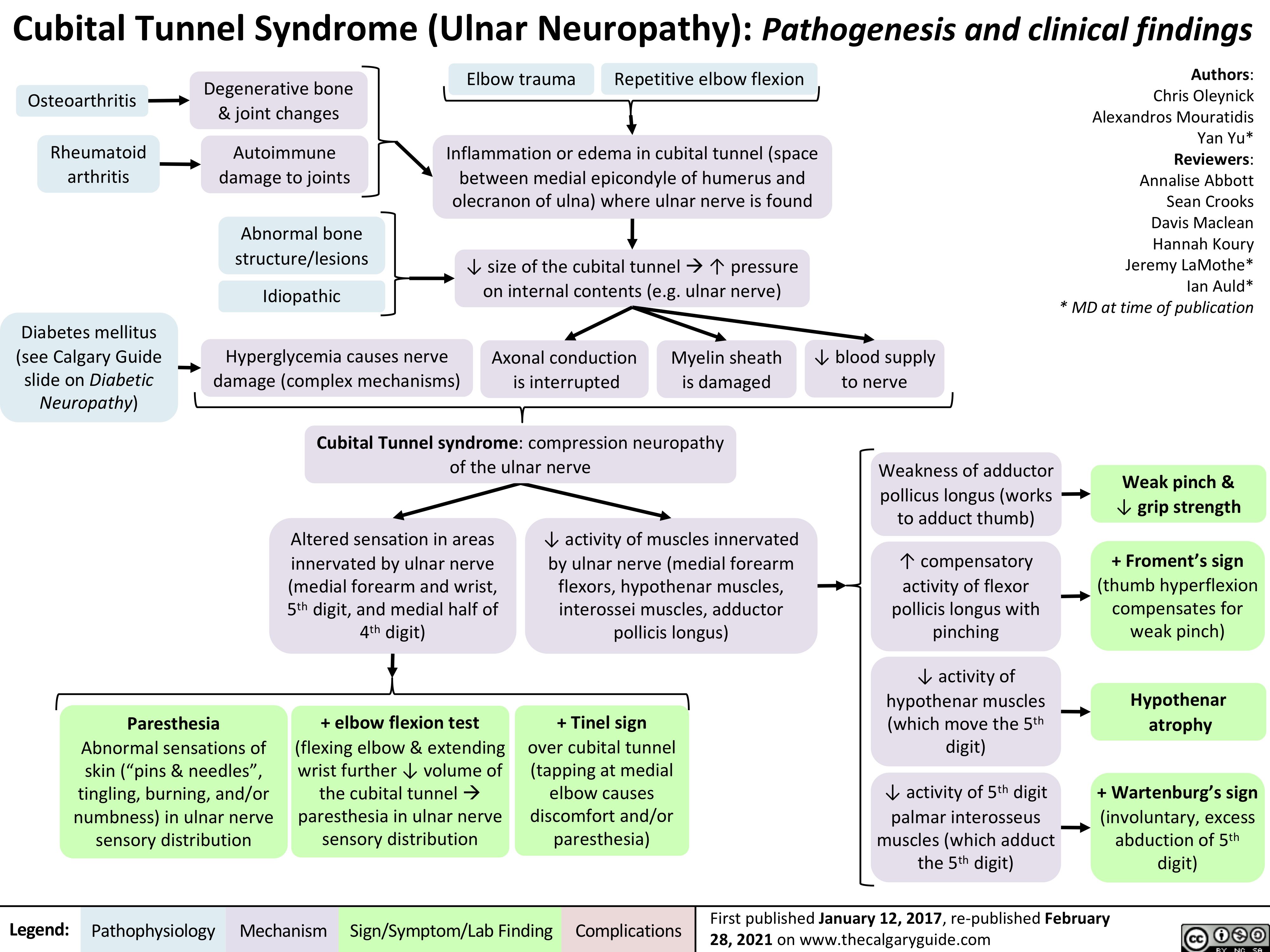
Cubital-Tunnel-Syndrome-Ulnar-Neuropathy
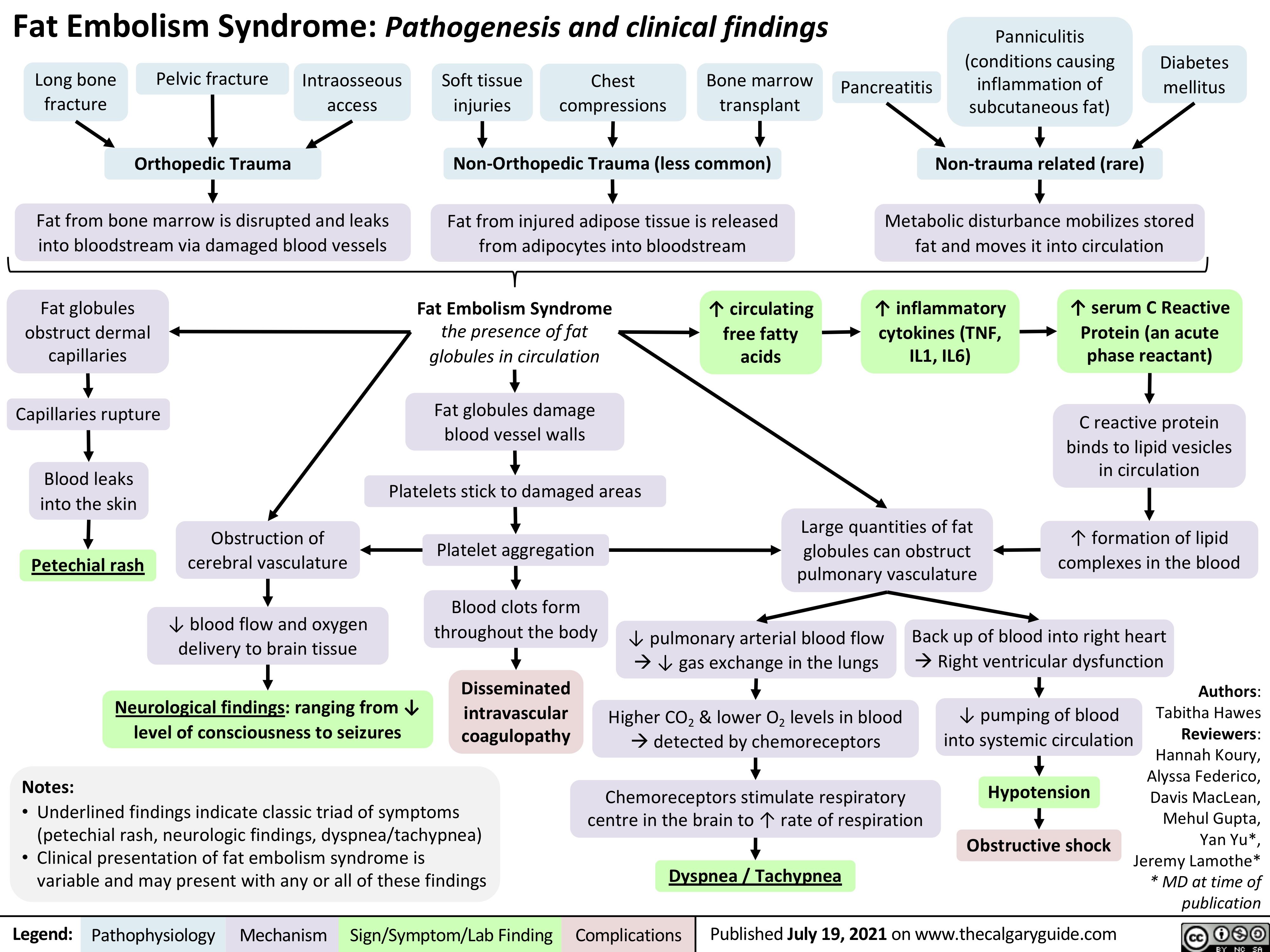
Fat-Embolism-Syndrome
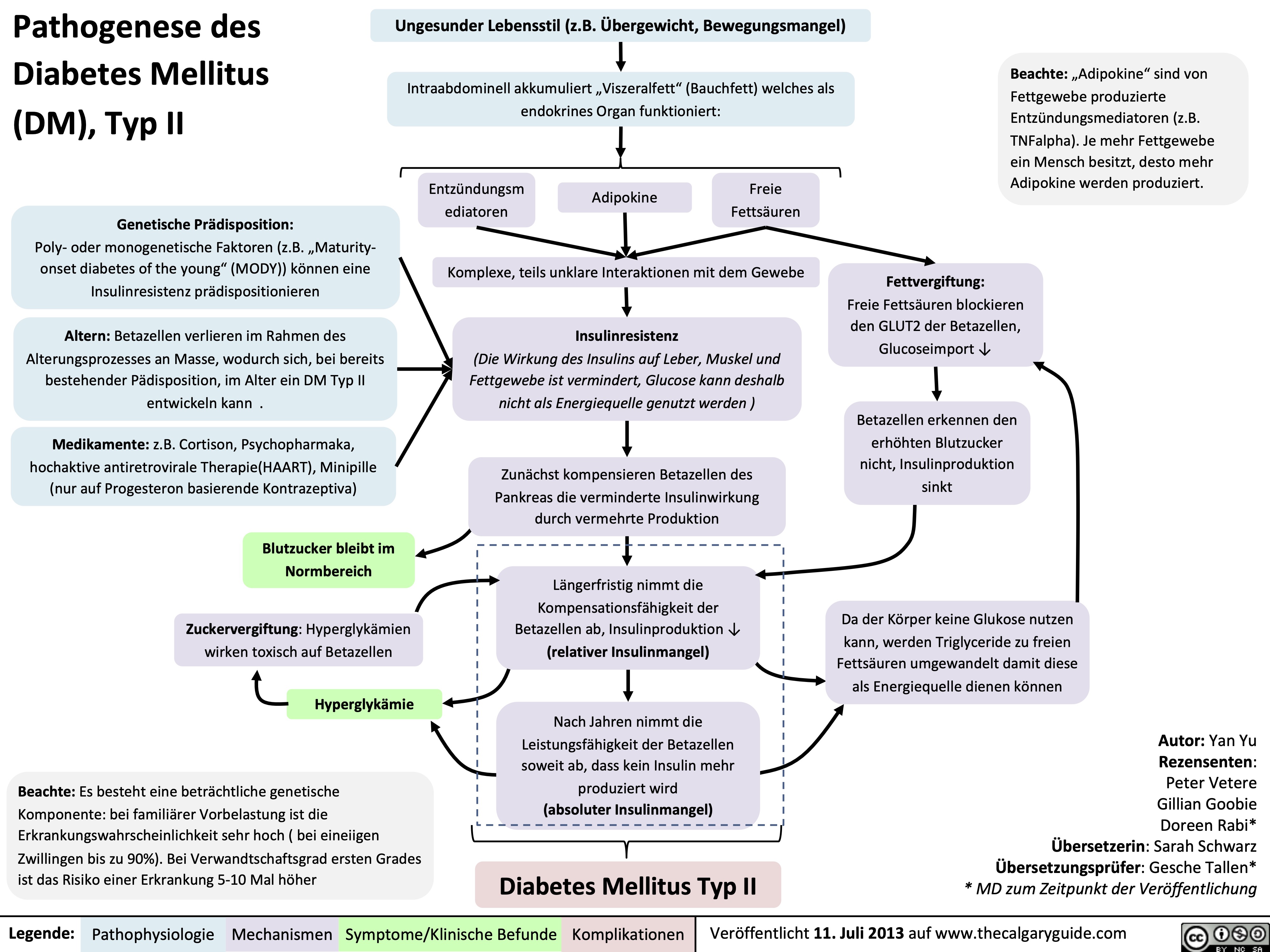
Pathogenese des Diabetes Mellitus (DM), Typ II
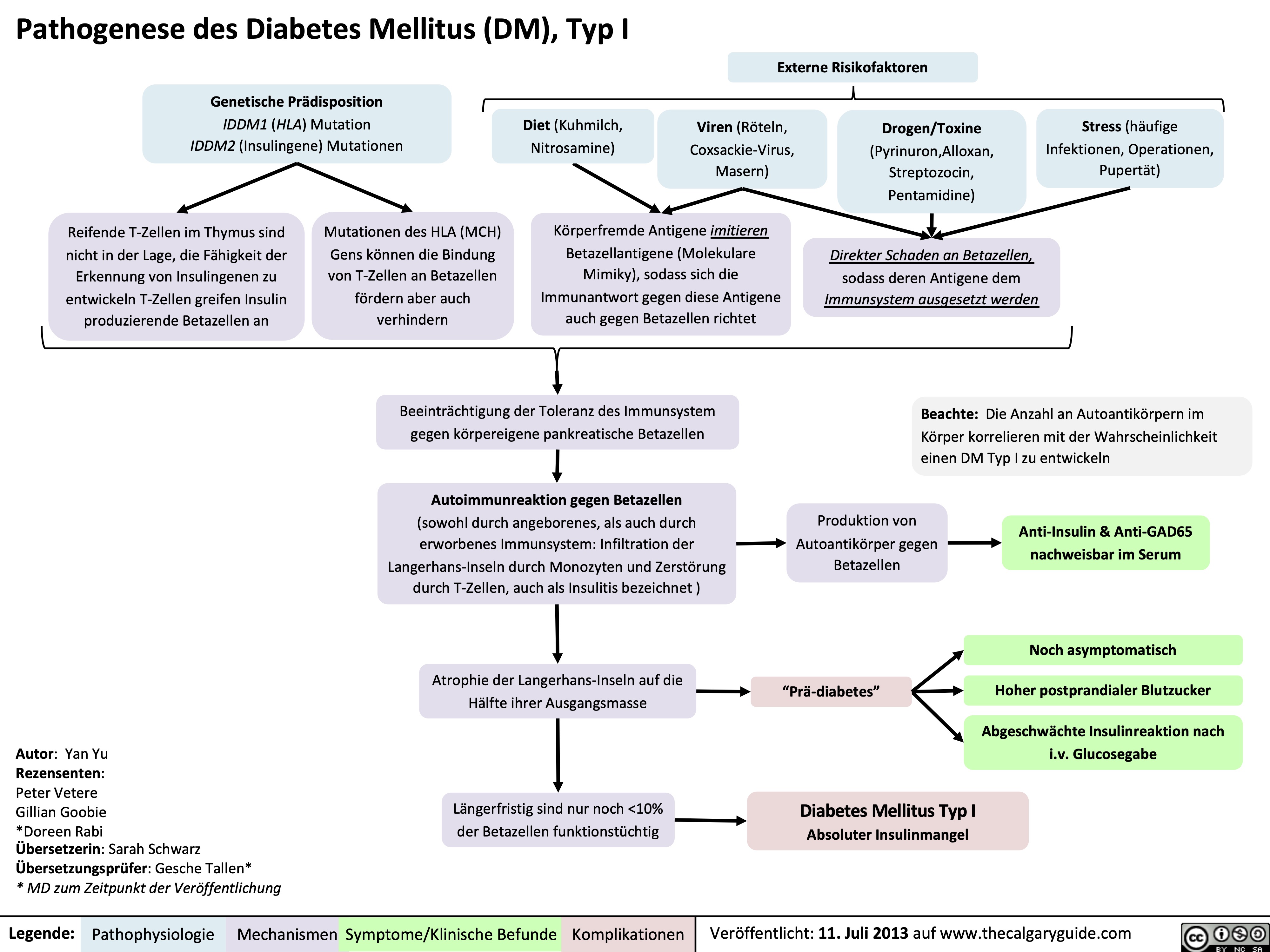
Pathogenese des Diabetes Mellitus (DM), Typ I
Diabetische Ketoazidose (DKA)
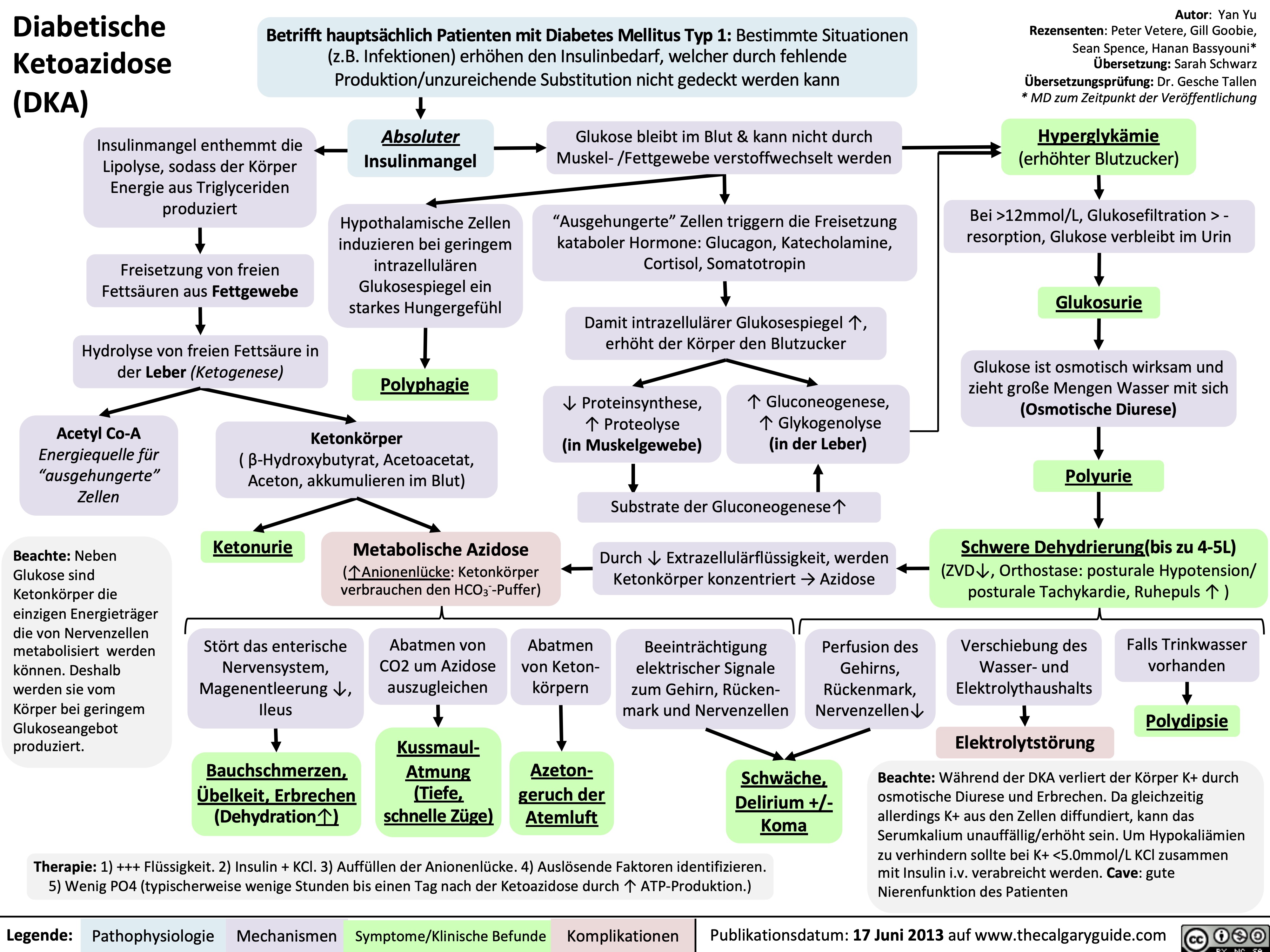
Hyperosmolares/ Hyperglykämisches Koma (HHS)
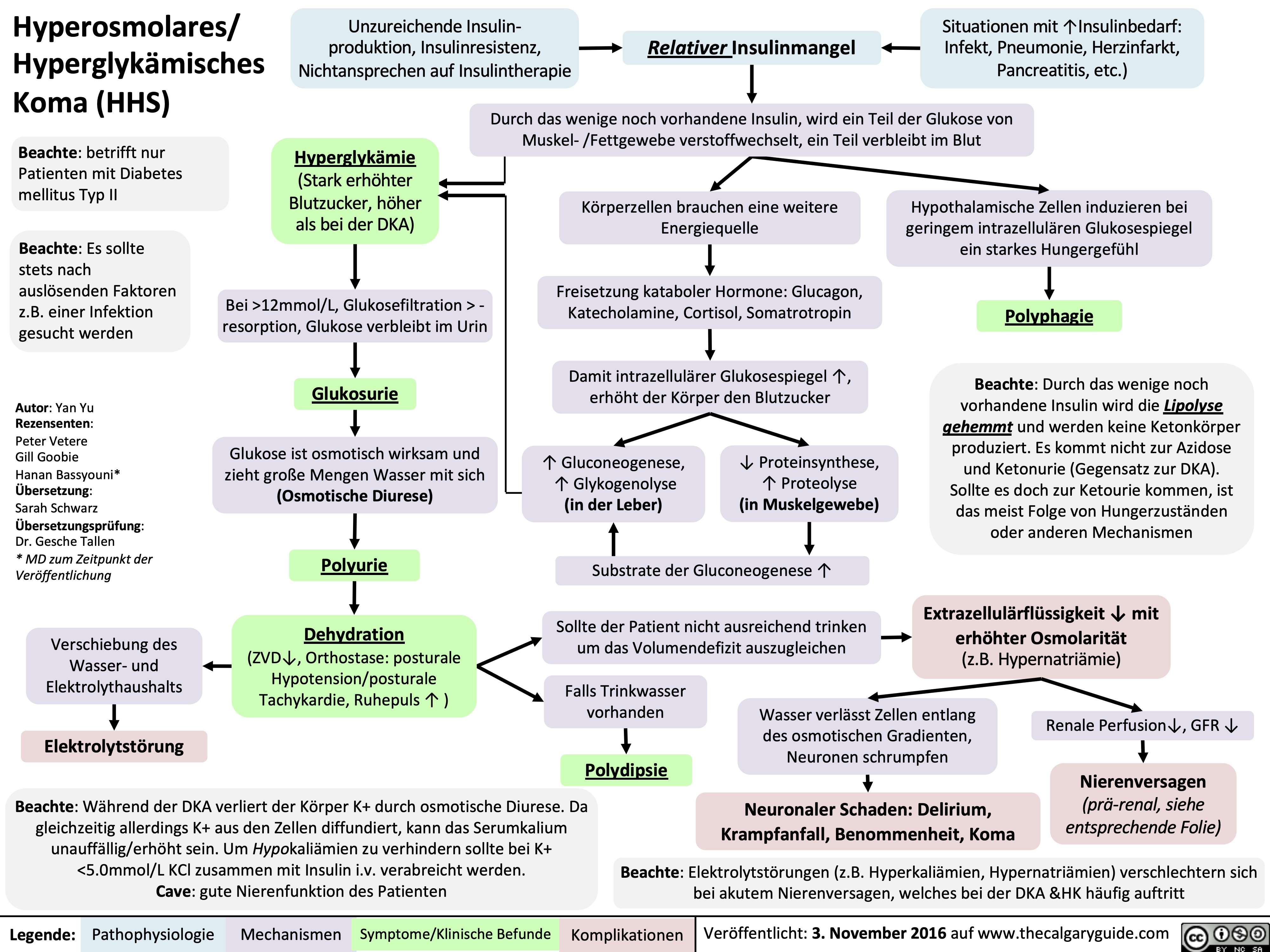
Nephrotisches Syndrom: Pathogenese und klinische Befunde
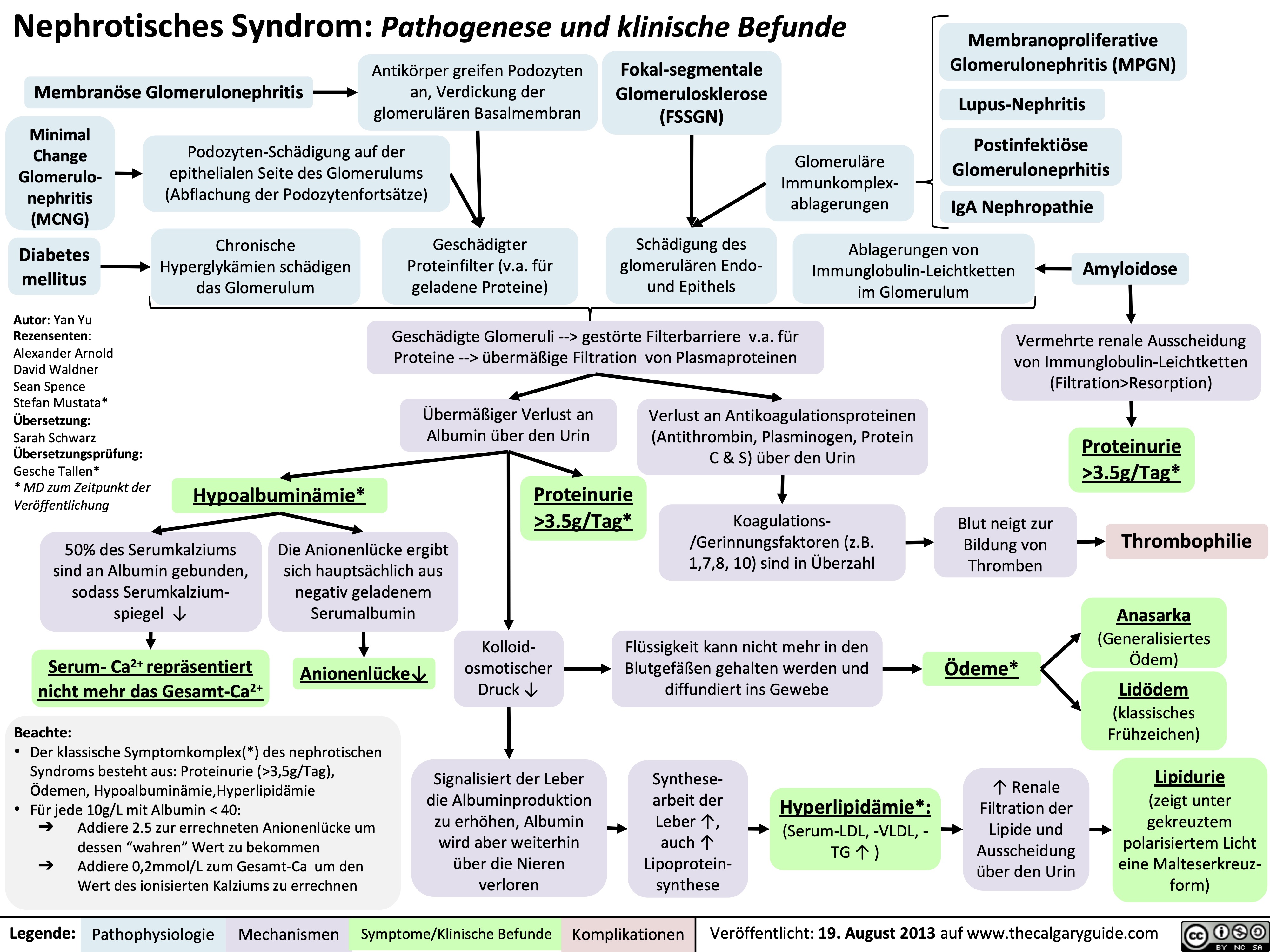
Celulitis
hyperkalemia-pathophysiology-intracellular-shift-and-intake
![Hyperkalemia (intracellular shift and ↑ intake): Pathophysiology
↑ K+ dietary intake (rarely causative)
β2 receptor inhibition (i.e. beta blockers)
Digoxin
α1 receptor stimulation (i.e. epinephrine, norepinephrine)
Insulin deficiency or resistance (i.e. diabetes mellitus)
↓ Stimulation of NHE1 (moves 1 Na+ into cell, 1 H+ out of cell) throughout body
Normal Anion- Gap Metabolic Acidosis (NAGMA)
Excess serum H+ results in ↓ NHE1 activity (See NAGMA: Pathogenesis and Laboratory Findings slide)
↑ Serum osmolarity (i.e. hyperglycemia)
Osmotic movement of water from cells to serum
Cell lysis (i.e. tumour lysis syndrome, rhabdomyolysis, hemolytic anemia)
↑ K+ release from lysed cells into the serum
Na+/K+ ATPase (moves 3K+ into cell, 2 Na+ out) activity inhibited on cells throughout body
↓ Activity of Na+/K+ ATPase on cells throughout the body
↓ Amount of K+ entering cells
↓ NHE1 activity prevents Na+ from entering the cell
Lack of high intracellular [Na+] needed to drive the Na+/K+ ATPase on cells
Loss of water from cells ↑ intracellular [K+]
K+ moves down concentration gradient from cell into serum
↑ K+ available for absorption
Since K+ is dissolved in water, some K+ is carried by water as water osmotically moves through water channels into the serum (phenomenon known as “solvent drag”)
See Hyperkalemia: Clinical Findings slide
Authors:
Mannat Dhillon, Joshua Low, Emily Wildman Reviewers:
Huneza Nadeem, Marissa (Ran) Zhang, Andrea Kuczynski, Yan Yu*, Kevin McLaughlin*, Adam Bass*
* MD at time of publication
↑ K+ in serum
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 17, 2022 on www.thecalgaryguide.com
Hyperkalemia (intracellular shift and ↑ intake): Pathophysiology
↑ K+ dietary intake (rarely causative)
β2 receptor inhibition (i.e. beta blockers)
Digoxin
α1 receptor stimulation (i.e. epinephrine, norepinephrine)
Insulin deficiency or resistance (i.e. diabetes mellitus)
↓ Stimulation of NHE1 (moves 1 Na+ into cell, 1 H+ out of cell) throughout body
Normal Anion- Gap Metabolic Acidosis (NAGMA)
Excess serum H+ results in ↓ NHE1 activity (See NAGMA: Pathogenesis and Laboratory Findings slide)
↑ Serum osmolarity (i.e. hyperglycemia)
Osmotic movement of water from cells to serum
Cell lysis (i.e. tumour lysis syndrome, rhabdomyolysis, hemolytic anemia)
↑ K+ release from lysed cells into the serum
Na+/K+ ATPase (moves 3K+ into cell, 2 Na+ out) activity inhibited on cells throughout body
↓ Activity of Na+/K+ ATPase on cells throughout the body
↓ Amount of K+ entering cells
↓ NHE1 activity prevents Na+ from entering the cell
Lack of high intracellular [Na+] needed to drive the Na+/K+ ATPase on cells
Loss of water from cells ↑ intracellular [K+]
K+ moves down concentration gradient from cell into serum
↑ K+ available for absorption
Since K+ is dissolved in water, some K+ is carried by water as water osmotically moves through water channels into the serum (phenomenon known as “solvent drag”)
See Hyperkalemia: Clinical Findings slide
Authors:
Mannat Dhillon, Joshua Low, Emily Wildman Reviewers:
Huneza Nadeem, Marissa (Ran) Zhang, Andrea Kuczynski, Yan Yu*, Kevin McLaughlin*, Adam Bass*
* MD at time of publication
↑ K+ in serum
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 17, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/07/Hyperkalemia-shift-1.jpg)
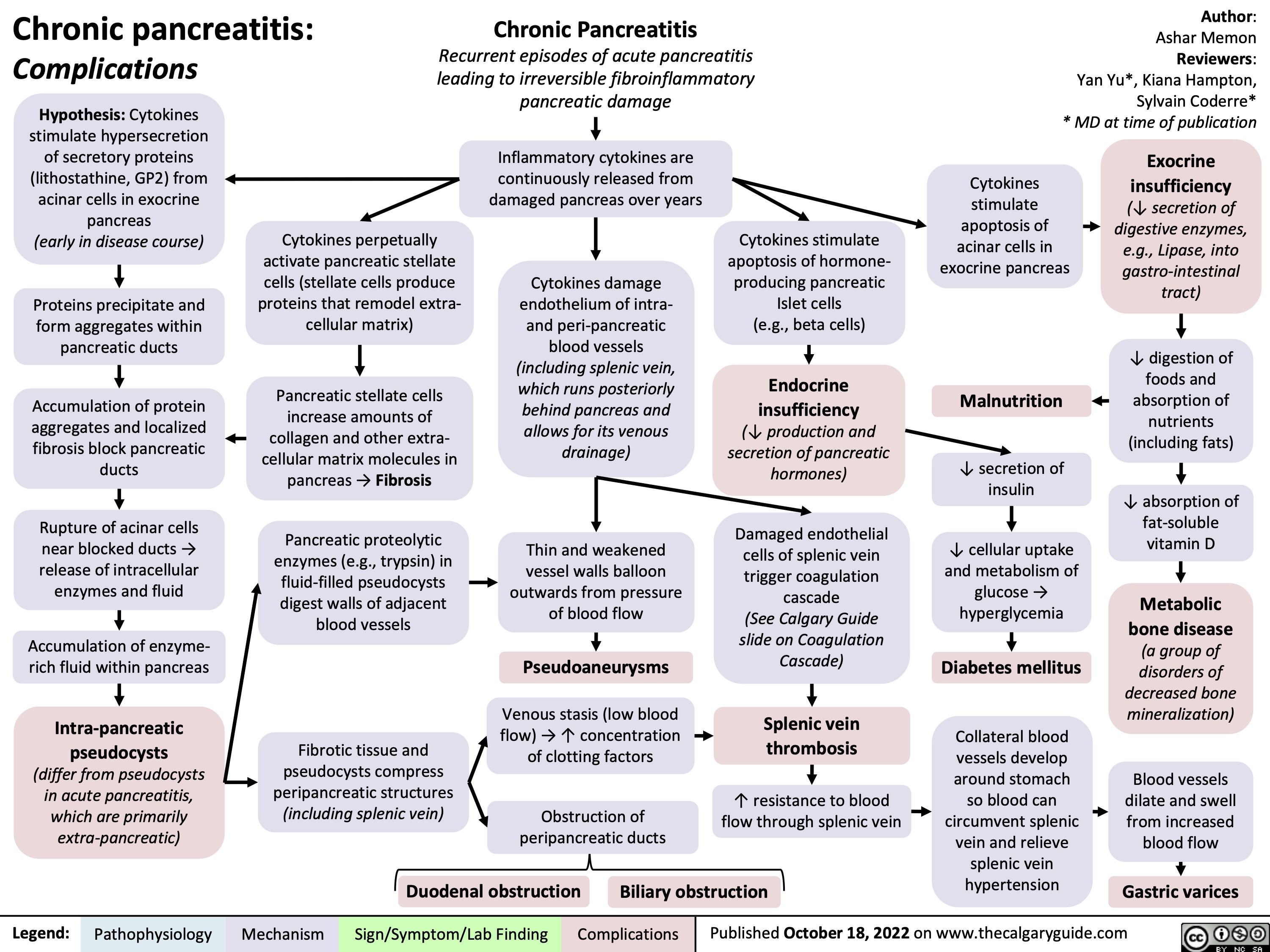
chronic-pancreatitis-complications
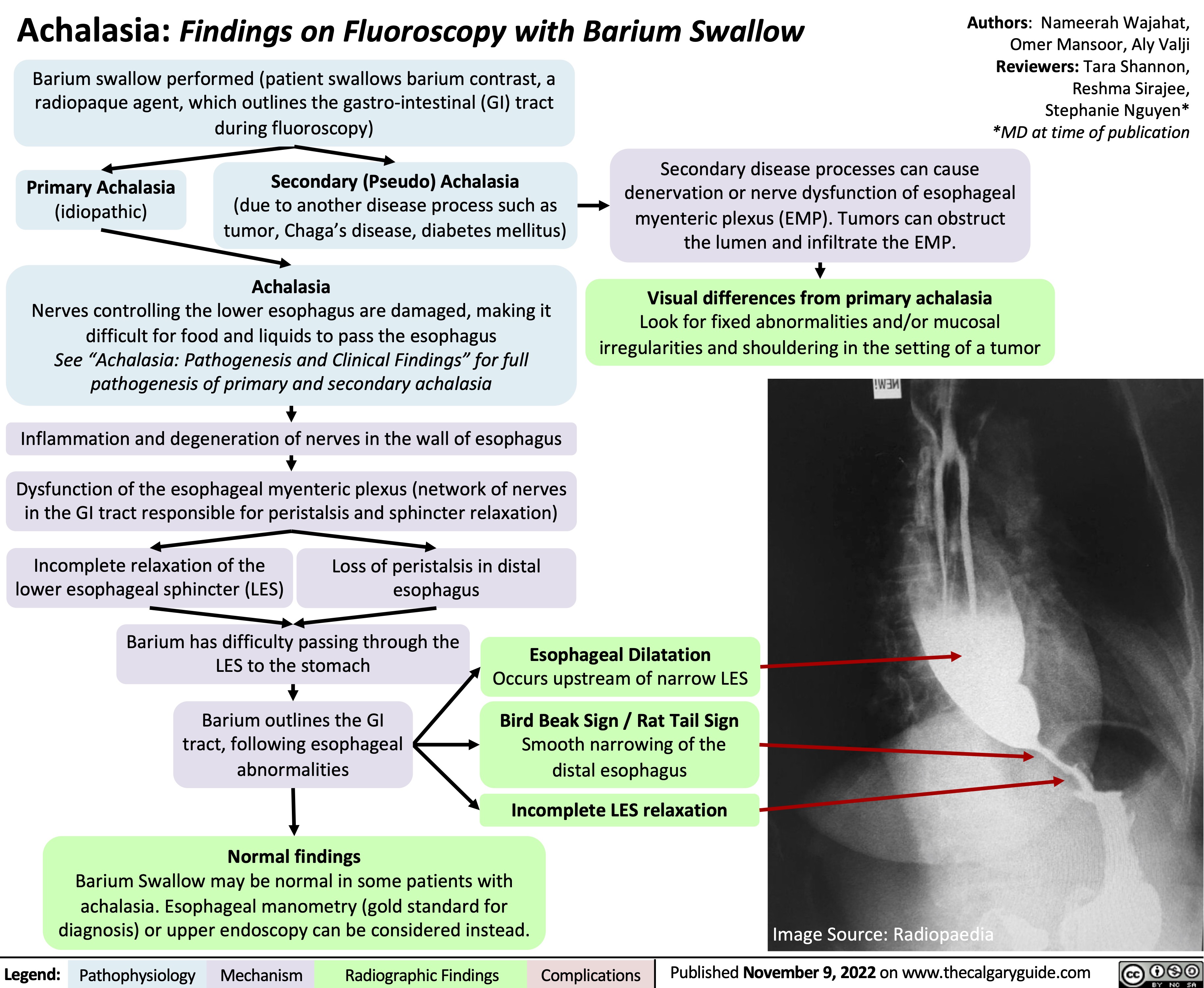
Achalasia: Findings on Fluoroscopy with Barium Swallow
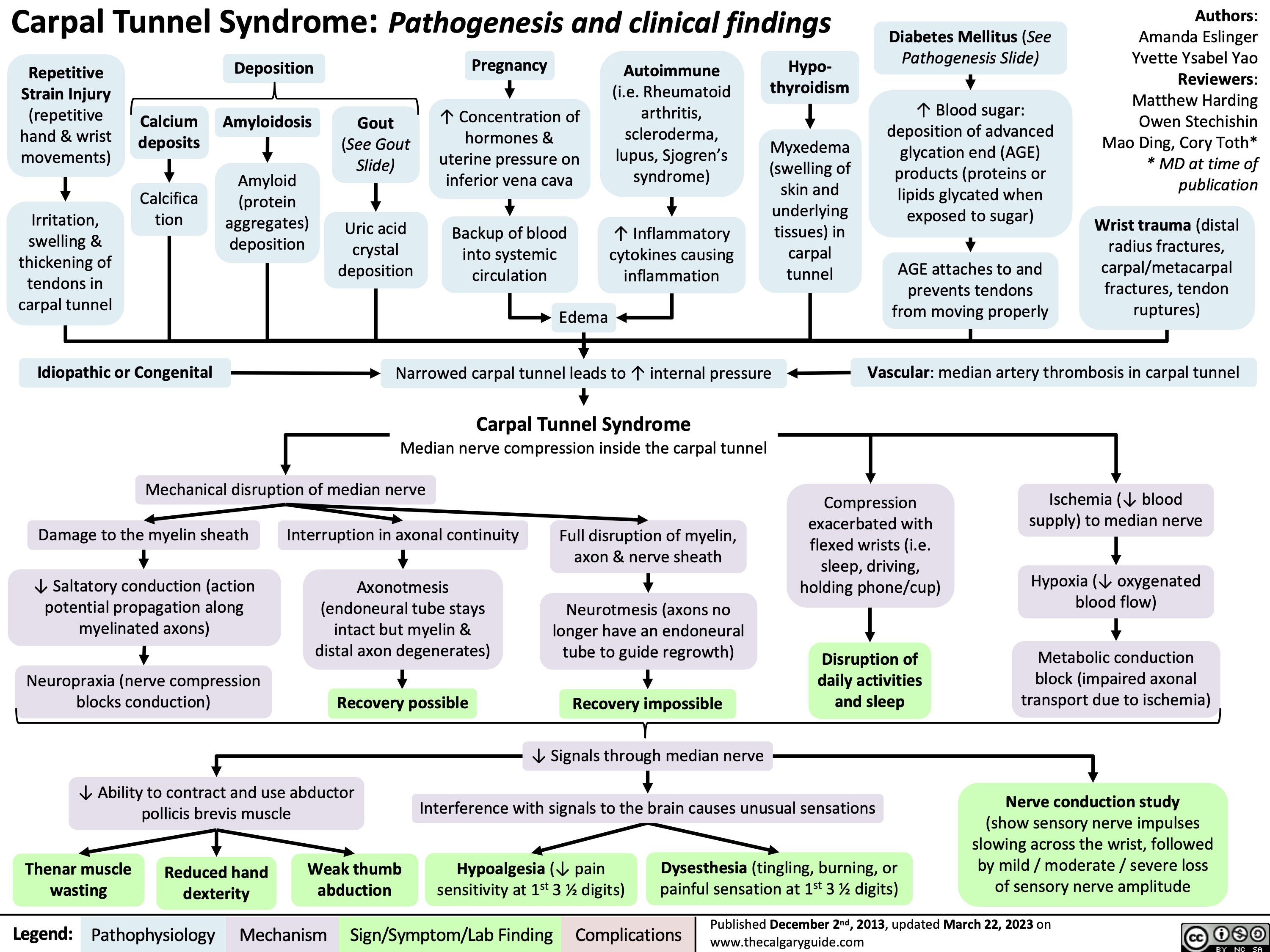
Carpal Tunnel Syndrome
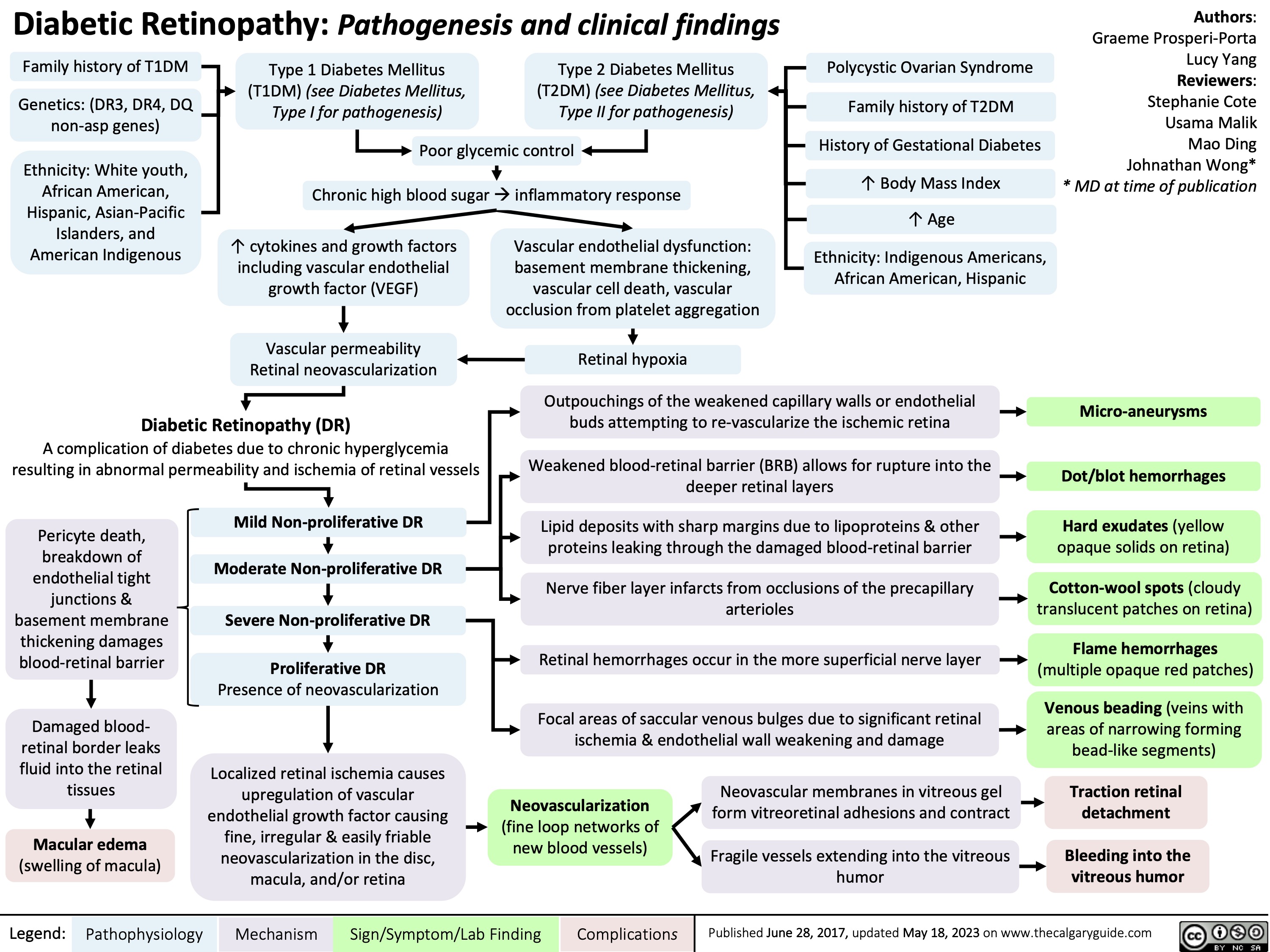
Diabetic Retinopathy
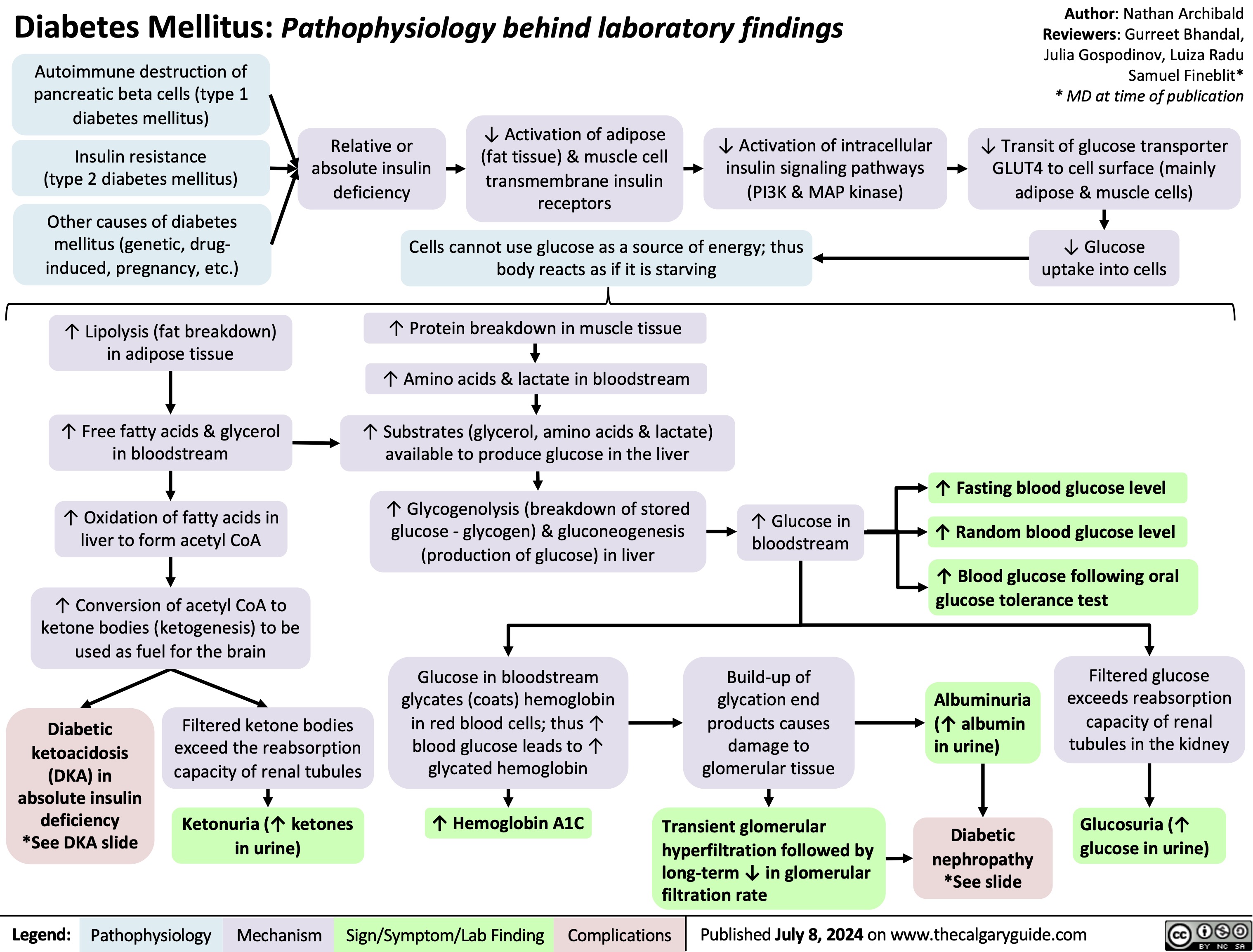
Knee Osteoarthritis
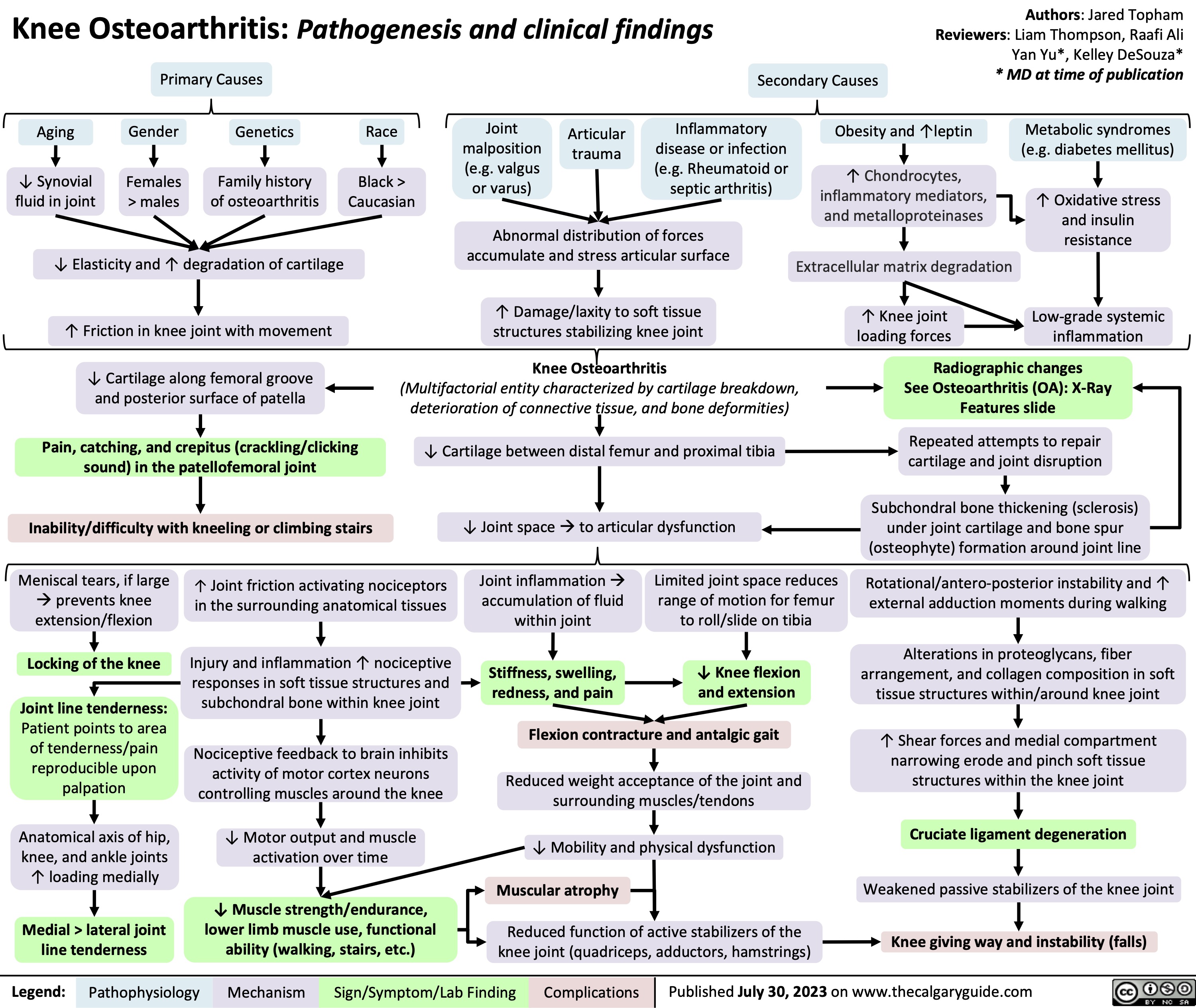
Pharmacotherapy for Dyslipidemia Overview
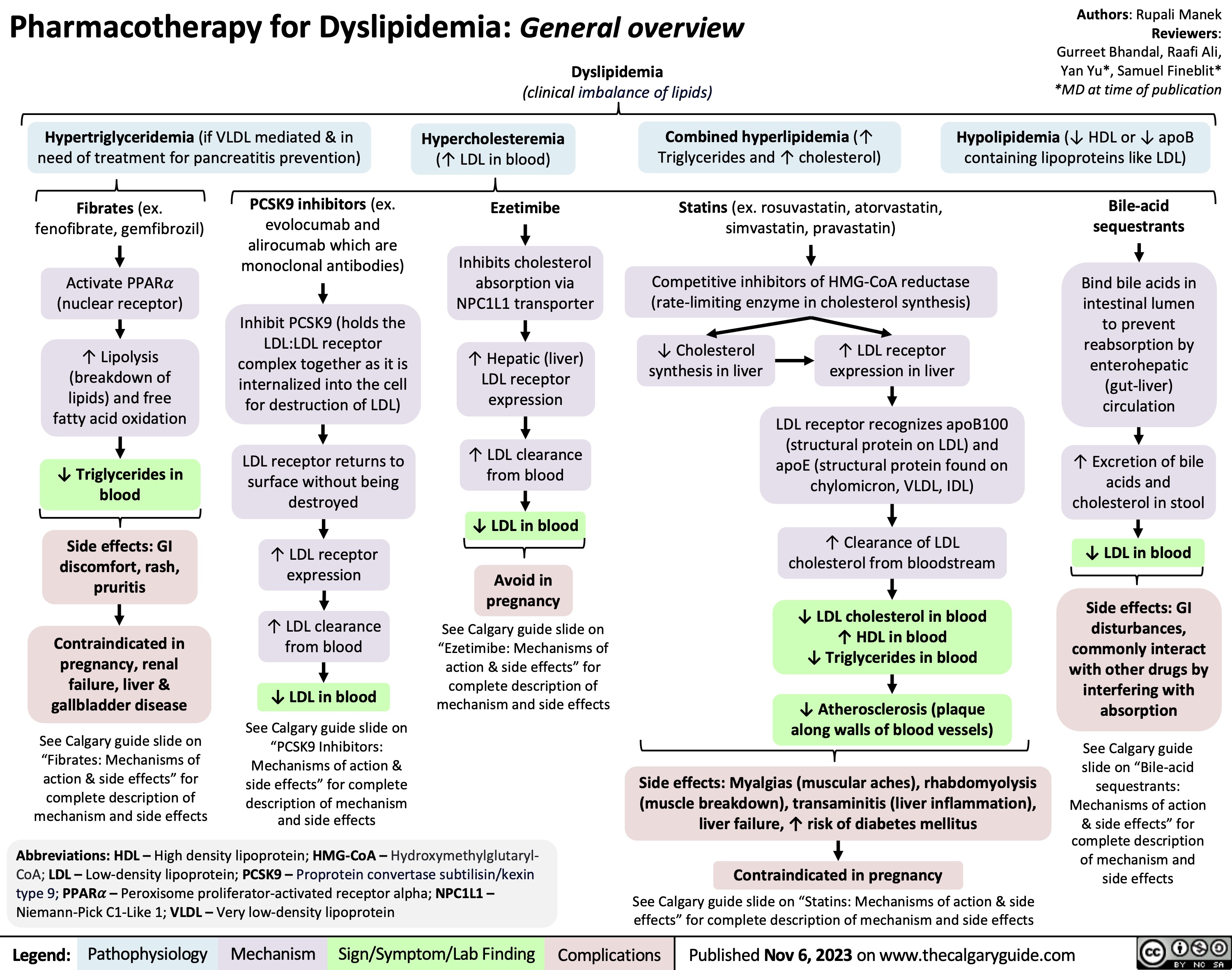
Gestational Diabetes Risk factors and pathogenesis
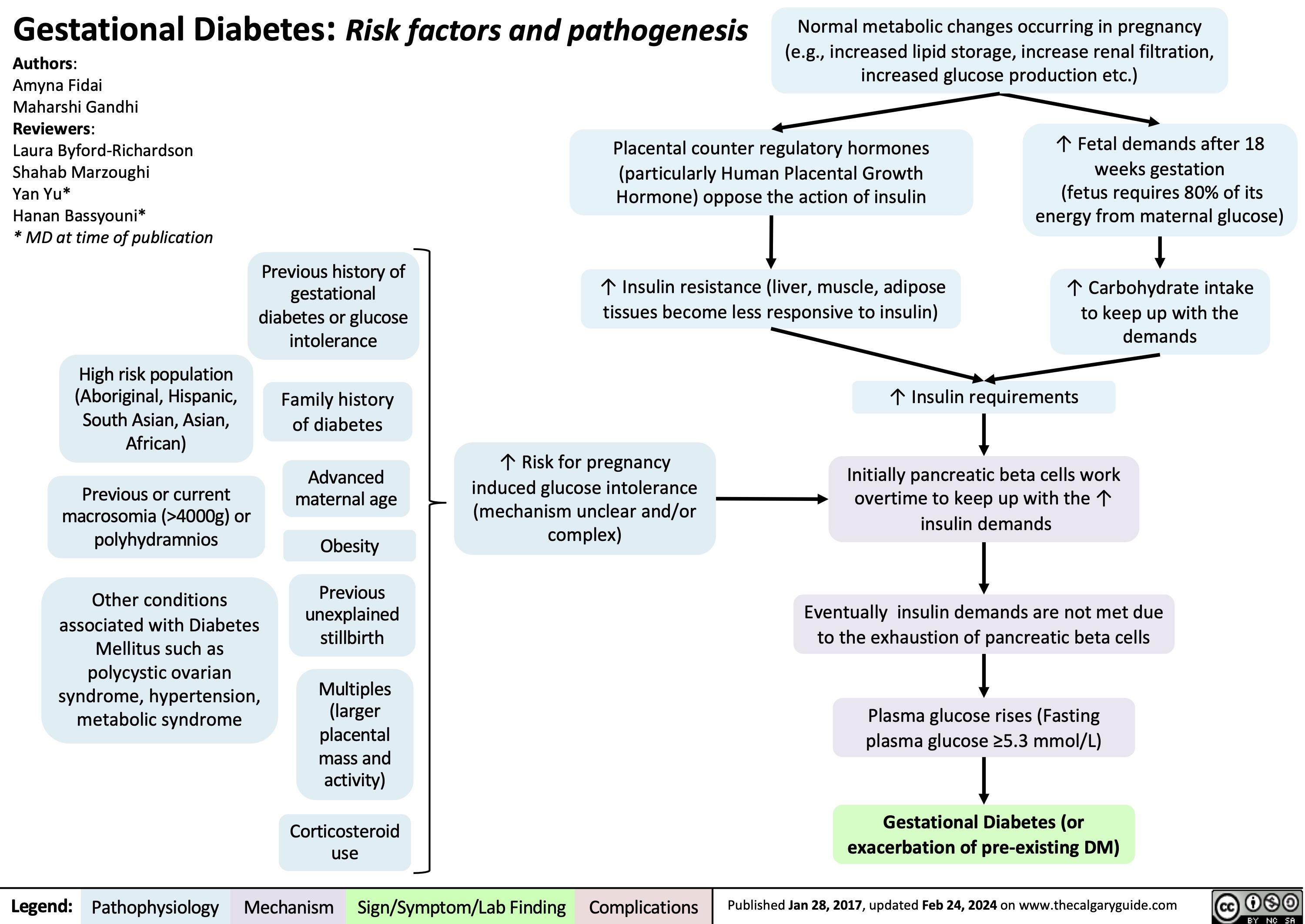
Diabetes Mellitus Pathophysiology Behind Lab Findings
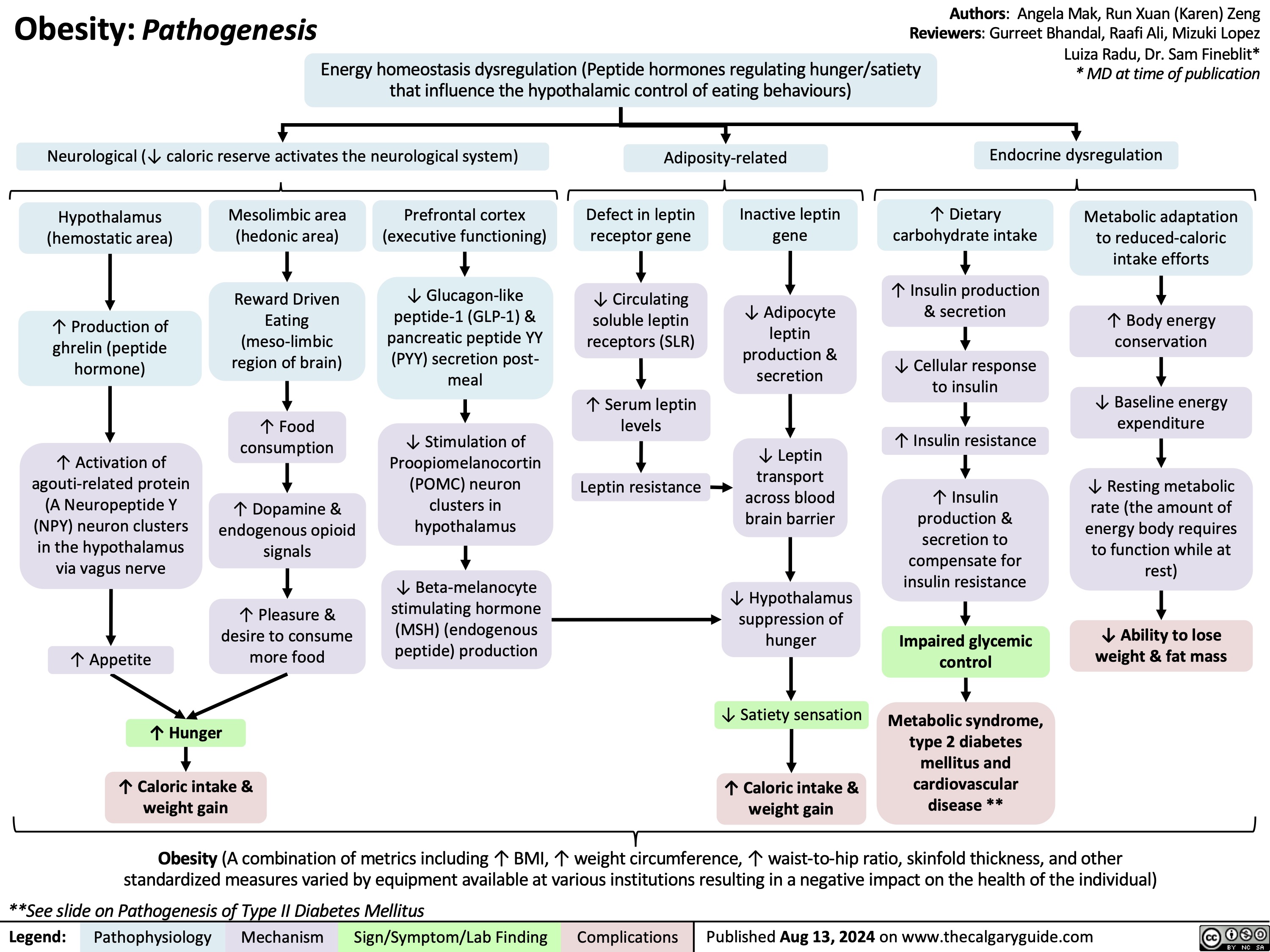
Obesity Pathogenesis
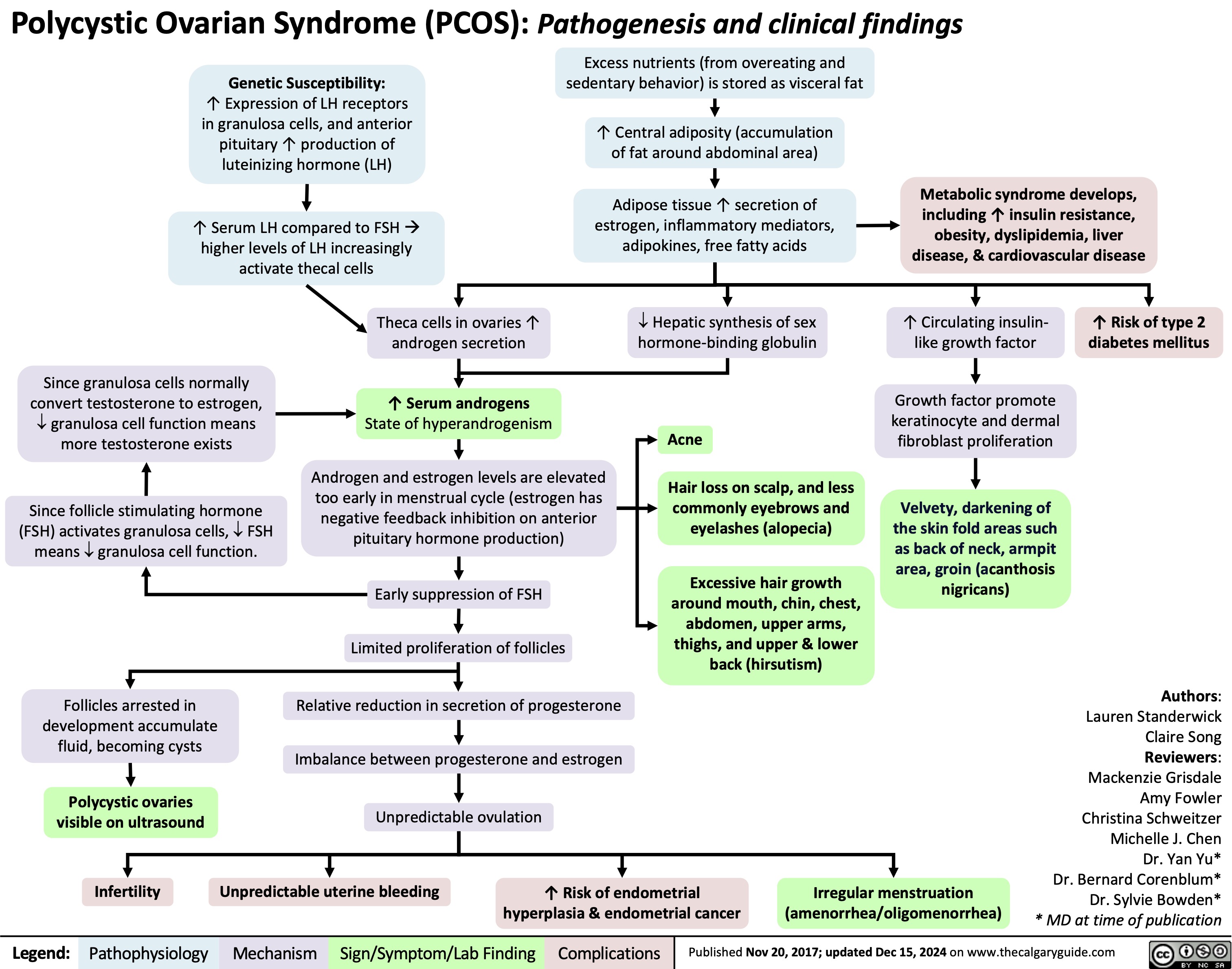
Polycystic Ovarian Syndrome
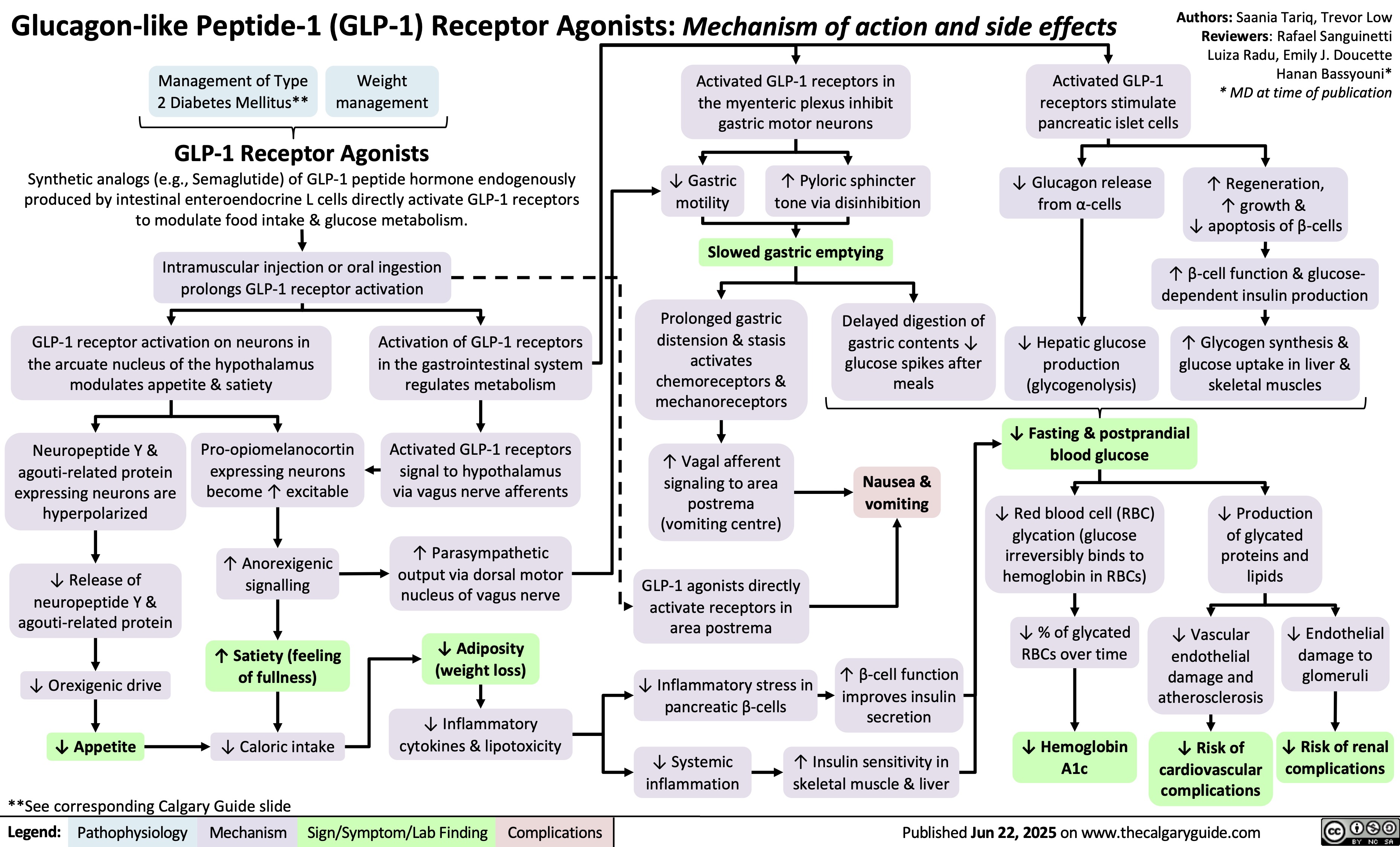
GLP-1 Receptor Agonists
 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />

![Yu, Yan - Diabetic Hypoglycemia - Clinical Findings - FINAL.pptx
? Epinephrine(Released within seconds as [glucose] falls further) Growth hormone, ? Cortisol (if hypoglycemia persists for minutes)Glucagon should ? when [glucose] falls. But here, glucagon release is inhibited by 1) diabetic auto-immune destruction of Alpha cells & 2) the high insulin.43210Plasma Glucose concentration (mmol/L)Liver should ? glycogenolysis & gluconeogenesisPeripheral vaso-constrictionPlasma [glucose] stays lowActivation of sympathetic (adrenergic) receptors across body, triggering Neurogenic symptomsPlasma [glucose] ?Excess subcutaneous insulin or insulin-secretagogue ?? [insulin] in the bloodOver time: [insulin] in the DM patient depends only on how much was injected or how much secretagogue was consumed; not on the body's physiological state.[Insulin] stays high in excessively-treated DM patientsPlasma [glucose] normally ?, but...High insulin transports plasma glucose into cells!In pts with existing diabetic autonomic neuropathy, epi-nephrine secretion will already be ?Brain does not get enough glucose, ? neuron function ? Neuroglycopenic symptomsTx: glucose intake![Glucose] returns to normalIf no glucose intake:Hypoglycemia-unawareness: No autonomic Sx felt so hypoglycemia not treated early ? pts present later on with more severe hypoglycemia and neuroglycopenic sxBrain cells kept chronically euglycemic due to GLUT1 receptor over-expression (despite rest of body being hypoglycemic)With many hypoglycemic events over time:Brain feels no need to ? glucose, so it ? autonomic epinephrine secretion!This is the normal sequence of hormone responses to ?ing plasma glucose levels.But this normal hormonal response will be blunted over time if there is 1) continued hypoglycemia dampening the sympathetic nervous system, and 2) long-standing diabetic neuropathy! (To be explained later in this flow chart)Abbreviations: [ ] = concentrationTx = TreatmentDM = Diabetes mellitusDiabetic Hypoglycemia: Pathogenesis and Clinical FindingsConfusionCan't concentrateWeaknessSlurred speech? coordination (staggering, etc)SeizuresComa, deathAdrenergic symptoms (epinephrine-mediated):Anxiety, irritability, trembling, pallor (skin vasoconstriction), palpitations, ? systolic BP, tachycardia Cholinergic symptoms(Acetylcholine-mediated):Sweating, hunger, tingling, blurry visionNote: In pts w/out DM, endogenous insulin secretion normally stops when blood [glucose] drops to <4mmol/LAuthor: Yan YuReviewers: Peter Vetere, Gillian Goobie, Hanan Bassyouni** MD at time of publicationLegend:Published June 14, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsMany hypoglycemic events over time blunt epinephrine secretion further.Hypoglycemia unawareness can be reversedIf pt stays hypoglycemia-free for >6 weeks, brain restores its ability to detect low glucose levels? peripheral glucose delivery and uptake (saving more glucose for the brain)Lack of glucagon effect reinforces hypoglycemia
124 kB / 361 words Yu, Yan - Diabetic Hypoglycemia - Clinical Findings - FINAL.pptx
? Epinephrine(Released within seconds as [glucose] falls further) Growth hormone, ? Cortisol (if hypoglycemia persists for minutes)Glucagon should ? when [glucose] falls. But here, glucagon release is inhibited by 1) diabetic auto-immune destruction of Alpha cells & 2) the high insulin.43210Plasma Glucose concentration (mmol/L)Liver should ? glycogenolysis & gluconeogenesisPeripheral vaso-constrictionPlasma [glucose] stays lowActivation of sympathetic (adrenergic) receptors across body, triggering Neurogenic symptomsPlasma [glucose] ?Excess subcutaneous insulin or insulin-secretagogue ?? [insulin] in the bloodOver time: [insulin] in the DM patient depends only on how much was injected or how much secretagogue was consumed; not on the body's physiological state.[Insulin] stays high in excessively-treated DM patientsPlasma [glucose] normally ?, but...High insulin transports plasma glucose into cells!In pts with existing diabetic autonomic neuropathy, epi-nephrine secretion will already be ?Brain does not get enough glucose, ? neuron function ? Neuroglycopenic symptomsTx: glucose intake![Glucose] returns to normalIf no glucose intake:Hypoglycemia-unawareness: No autonomic Sx felt so hypoglycemia not treated early ? pts present later on with more severe hypoglycemia and neuroglycopenic sxBrain cells kept chronically euglycemic due to GLUT1 receptor over-expression (despite rest of body being hypoglycemic)With many hypoglycemic events over time:Brain feels no need to ? glucose, so it ? autonomic epinephrine secretion!This is the normal sequence of hormone responses to ?ing plasma glucose levels.But this normal hormonal response will be blunted over time if there is 1) continued hypoglycemia dampening the sympathetic nervous system, and 2) long-standing diabetic neuropathy! (To be explained later in this flow chart)Abbreviations: [ ] = concentrationTx = TreatmentDM = Diabetes mellitusDiabetic Hypoglycemia: Pathogenesis and Clinical FindingsConfusionCan't concentrateWeaknessSlurred speech? coordination (staggering, etc)SeizuresComa, deathAdrenergic symptoms (epinephrine-mediated):Anxiety, irritability, trembling, pallor (skin vasoconstriction), palpitations, ? systolic BP, tachycardia Cholinergic symptoms(Acetylcholine-mediated):Sweating, hunger, tingling, blurry visionNote: In pts w/out DM, endogenous insulin secretion normally stops when blood [glucose] drops to <4mmol/LAuthor: Yan YuReviewers: Peter Vetere, Gillian Goobie, Hanan Bassyouni** MD at time of publicationLegend:Published June 14, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsMany hypoglycemic events over time blunt epinephrine secretion further.Hypoglycemia unawareness can be reversedIf pt stays hypoglycemia-free for >6 weeks, brain restores its ability to detect low glucose levels? peripheral glucose delivery and uptake (saving more glucose for the brain)Lack of glucagon effect reinforces hypoglycemia
124 kB / 361 words](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Diabetic-Hypoglycemia-Clinical-Findings.jpg)




![Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com
Hypernatremia: Physiology Unreplaced H2O loss
Hypodipsia
H2O shift into cells
Severe exercise, electroshock induced seizures
Transient ↑ cell osmolality
Na+ overload
Inappropriate IV hypertonic solution, salt poisoning
Abbreviations:
H2O: Water
GI: Gastrointestinal
DM: Diabetes Mellitus
DI: Diabetes Insipidus
Na+: Sodium ion
IV: Intravenous
ADH: Antidiuretic Hormone LOC: Level of Consciousness
Skin
Sweat, burns
GI
Vomiting, bleeding, osmotic diarrhea
Fluid [Na+] < serum [Na+]
↑ H2O loss compared to Na+ loss
Renal
DM, Mannitol, Diuretics
Absent thirst mechanism
Hypothalamic lesion impairs normal drive for H2O intake
Nephrogenic
↑ renal resistance to ADH
H2O Deprivation Test + no AVP response
↓ access to H2O
DI
Central
↓ ADH secretion
H2O Deprivation Test + AVP response
↑ [Na+] 10- 15 mEq/L within a few minutes
Weakness, irritability, seizures, coma
↑ thirst, ↓ urinary frequency and volume
Note:
Hypernatremia
Serum [Na+] > 145 mmol/L
Intracranial hemorrhage
Headache, vomiting, ↓ LOC
• Plasma [Na+] is regulated by water intake/excretion, not by changes in [Na+].
• Effects on plasma [Na+] of IV fluids or loss of bodily fluids is determined by the tonicity of the fluid, not the osmolality.
Authors: Mannat Dhillon Reviewers: Andrea Kuczynski Kevin McLaughlin* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 11, 2019 on www.thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/01/Hypernatremia-Physiology-.jpg)










![Hyperkalemia (intracellular shift and ↑ intake): Pathophysiology
↑ K+ dietary intake (rarely causative)
β2 receptor inhibition (i.e. beta blockers)
Digoxin
α1 receptor stimulation (i.e. epinephrine, norepinephrine)
Insulin deficiency or resistance (i.e. diabetes mellitus)
↓ Stimulation of NHE1 (moves 1 Na+ into cell, 1 H+ out of cell) throughout body
Normal Anion- Gap Metabolic Acidosis (NAGMA)
Excess serum H+ results in ↓ NHE1 activity (See NAGMA: Pathogenesis and Laboratory Findings slide)
↑ Serum osmolarity (i.e. hyperglycemia)
Osmotic movement of water from cells to serum
Cell lysis (i.e. tumour lysis syndrome, rhabdomyolysis, hemolytic anemia)
↑ K+ release from lysed cells into the serum
Na+/K+ ATPase (moves 3K+ into cell, 2 Na+ out) activity inhibited on cells throughout body
↓ Activity of Na+/K+ ATPase on cells throughout the body
↓ Amount of K+ entering cells
↓ NHE1 activity prevents Na+ from entering the cell
Lack of high intracellular [Na+] needed to drive the Na+/K+ ATPase on cells
Loss of water from cells ↑ intracellular [K+]
K+ moves down concentration gradient from cell into serum
↑ K+ available for absorption
Since K+ is dissolved in water, some K+ is carried by water as water osmotically moves through water channels into the serum (phenomenon known as “solvent drag”)
See Hyperkalemia: Clinical Findings slide
Authors:
Mannat Dhillon, Joshua Low, Emily Wildman Reviewers:
Huneza Nadeem, Marissa (Ran) Zhang, Andrea Kuczynski, Yan Yu*, Kevin McLaughlin*, Adam Bass*
* MD at time of publication
↑ K+ in serum
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 17, 2022 on www.thecalgaryguide.com
Hyperkalemia (intracellular shift and ↑ intake): Pathophysiology
↑ K+ dietary intake (rarely causative)
β2 receptor inhibition (i.e. beta blockers)
Digoxin
α1 receptor stimulation (i.e. epinephrine, norepinephrine)
Insulin deficiency or resistance (i.e. diabetes mellitus)
↓ Stimulation of NHE1 (moves 1 Na+ into cell, 1 H+ out of cell) throughout body
Normal Anion- Gap Metabolic Acidosis (NAGMA)
Excess serum H+ results in ↓ NHE1 activity (See NAGMA: Pathogenesis and Laboratory Findings slide)
↑ Serum osmolarity (i.e. hyperglycemia)
Osmotic movement of water from cells to serum
Cell lysis (i.e. tumour lysis syndrome, rhabdomyolysis, hemolytic anemia)
↑ K+ release from lysed cells into the serum
Na+/K+ ATPase (moves 3K+ into cell, 2 Na+ out) activity inhibited on cells throughout body
↓ Activity of Na+/K+ ATPase on cells throughout the body
↓ Amount of K+ entering cells
↓ NHE1 activity prevents Na+ from entering the cell
Lack of high intracellular [Na+] needed to drive the Na+/K+ ATPase on cells
Loss of water from cells ↑ intracellular [K+]
K+ moves down concentration gradient from cell into serum
↑ K+ available for absorption
Since K+ is dissolved in water, some K+ is carried by water as water osmotically moves through water channels into the serum (phenomenon known as “solvent drag”)
See Hyperkalemia: Clinical Findings slide
Authors:
Mannat Dhillon, Joshua Low, Emily Wildman Reviewers:
Huneza Nadeem, Marissa (Ran) Zhang, Andrea Kuczynski, Yan Yu*, Kevin McLaughlin*, Adam Bass*
* MD at time of publication
↑ K+ in serum
Hyperkalemia
Serum [K+] > 5.1 mmol/L
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 17, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/07/Hyperkalemia-shift-1.jpg)











