Notice: Undefined variable: out in /web/sites/calgaryguide_current/wp-content/themes/cg-new/functions.php on line 570
SEARCH RESULTS FOR: calcium
Nephrotic Syndrome: Pathogenesis and Clinical Findings
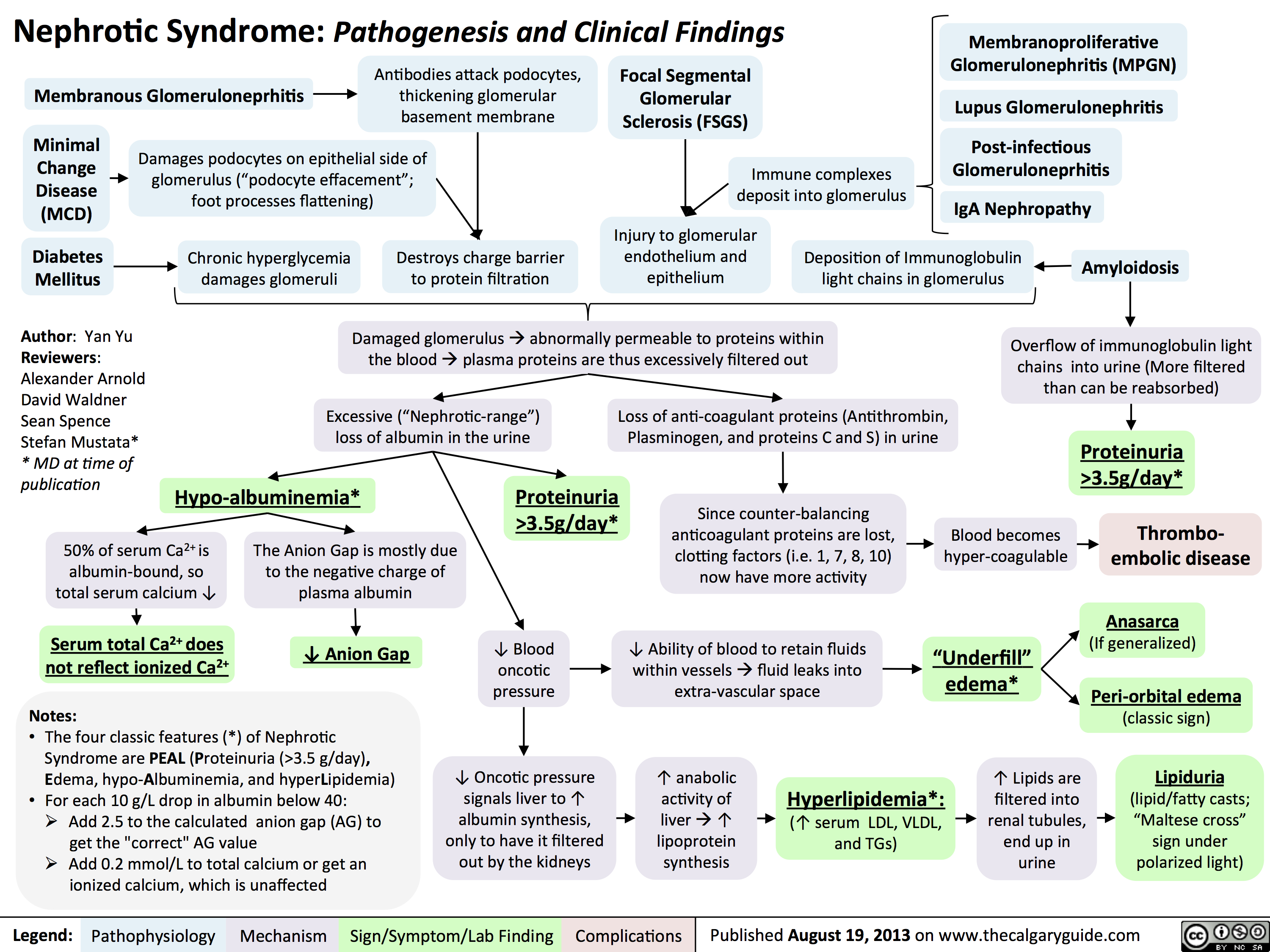 3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" title="Destroys charge barrier to protein filtrationNephrotic Syndrome: Pathogenesis and Clinical FindingsAuthor: Yan YuReviewers:Alexander ArnoldDavid WaldnerSean SpenceStefan Mustata** MD at time of publicationLegend:Published August 19, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsExcessive ("Nephrotic-range") loss of albumin in the urineHypo-albuminemia*Loss of anti-coagulant proteins (Antithrombin, Plasminogen, and proteins C and S) in urineMinimal Change Disease (MCD)"Underfill" edema*Proteinuria >3.5g/day*? Ability of blood to retain fluids within vessels ? fluid leaks into extra-vascular spaceInjury to glomerular endothelium and epitheliumImmune complexes deposit into glomerulusDamaged glomerulus ? abnormally permeable to proteins within the blood ? plasma proteins are thus excessively filtered out? Oncotic pressure signals liver to ? albumin synthesis, only to have it filtered out by the kidneys? anabolic activity of liver ? ? lipoprotein synthesisHyperlipidemia*:(? serum LDL, VLDL, and TGs)Lipiduria(lipid/fatty casts; "Maltese cross" sign under polarized light)Since counter-balancing anticoagulant proteins are lost, clotting factors (i.e. 1, 7, 8, 10) now have more activityThrombo-embolic diseaseBlood becomes hyper-coagulable? Lipids are filtered into renal tubules, end up in urineMembranoproliferative Glomerulonephritis (MPGN)Lupus Glomerulonephritis Post-infectious GlomeruloneprhitisIgA NephropathyDamages podocytes on epithelial side of glomerulus ("podocyte effacement"; foot processes flattening)Diabetes MellitusChronic hyperglycemia damages glomeruliDeposition of Immunoglobulin light chains in glomerulusAmyloidosisAnasarca(If generalized)Peri-orbital edema (classic sign)Focal Segmental Glomerular Sclerosis (FSGS)Membranous GlomeruloneprhitisAntibodies attack podocytes, thickening glomerular basement membraneOverflow of immunoglobulin light chains into urine (More filtered than can be reabsorbed)Proteinuria >3.5g/day*The Anion Gap is mostly due to the negative charge of plasma albumin? Anion GapNotes: The four classic features (*) of Nephrotic Syndrome are PEAL (Proteinuria (>3.5 g/day), Edema, hypo-Albuminemia, and hyperLipidemia)For each 10 g/L drop in albumin below 40:Add 2.5 to the calculated anion gap (AG) to get the "correct" AG valueAdd 0.2 mmol/L to total calcium or get an ionized calcium, which is unaffected50% of serum Ca2+ is albumin-bound, so total serum calcium ? Serum total Ca2+ does not reflect ionized Ca2+ ? Blood oncotic pressure" />
Hypercalcemia - Clinical Findings
![Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be Yu, Yan - Hypercalcemia - Clinical Findings - FINAL.pptx
Hypercalcemia: Clinical FindingsAuthor: Yan YuReviewers:David WaldnerSean SpenceGreg Kline** MD at time of publicationLegend:Published May 7, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsHypercalcemia(serum [Ca2+] > 2.5mmol/L)Na+ channels on neuronal membranes become more resistant to opening (resists Na+ influx)Cognitive dysfunctionIf precipitation occurs in the urinary tract...Fatigue? contractility of GI tract smooth muscle? K+ movement out of TAL epithelial cells into the tubule lumen Alters charge balance across the cell membraneCa2+ precipitates with PO43- throughout the bodyDetected by the Ca-Sensing-Receptor (CaSR) on Thick Ascending Limb (TAL) epithelial cells? neuronal action potential generationSluggish neuronal activity...? appetiteConstipationFlank painInhibit insertion of Renal Outer Medullary K+ (ROMK) channels on TAL's luminal membrane? K+ in TAL lumen to drive Na+/Cl- reabsorption through the Na-K-Cl Cotransporter (NKCC)? Na/Cl in tubule lumen ? osmotically draws water into lumen? drinking (polydipsia)? Urine volume (polyuria)Rationale for the CaSR-pathway: ECF has enough Ca2+, no need for more K+ to be excreted into the tubule lumen to create a more + charge there that drives Ca2+ reabsorptionBehavior compensates to prevent dehydrationKidney stones (nephrolithiasis)Constantly feeling full because of reduced GI motilityCa2+ directly inhibits the insertion of aquaporin channels in the collecting duct membraneLess water reabsorbed into the renal vasculatureMore water remains in the tubule filtrateMuscle Weakness...in central nervous system:...at neuromuscular junction:A rhyme to help you recall the manifestations of one specific cause of hypercalcemia, primary hyperparathyroidism:Bones (Calcium levels are high often due to ? resorption from bones)Stones (? Calcium-containing kidney stones)Groans (GI and skeletal muscle issues) Psychic Moans (Cognitive dysfunction from neuronal disturbances)Note: sick/ICU patients have ? serum albumin, due to ? synthesis from a sick liver. Their lab Ca2+ values can be](http://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Hypercalcemia-Clinical-Findings.jpg)
Chondrocalcinosis Calcium Pyrophosphate Dihydrate Deposition Disease
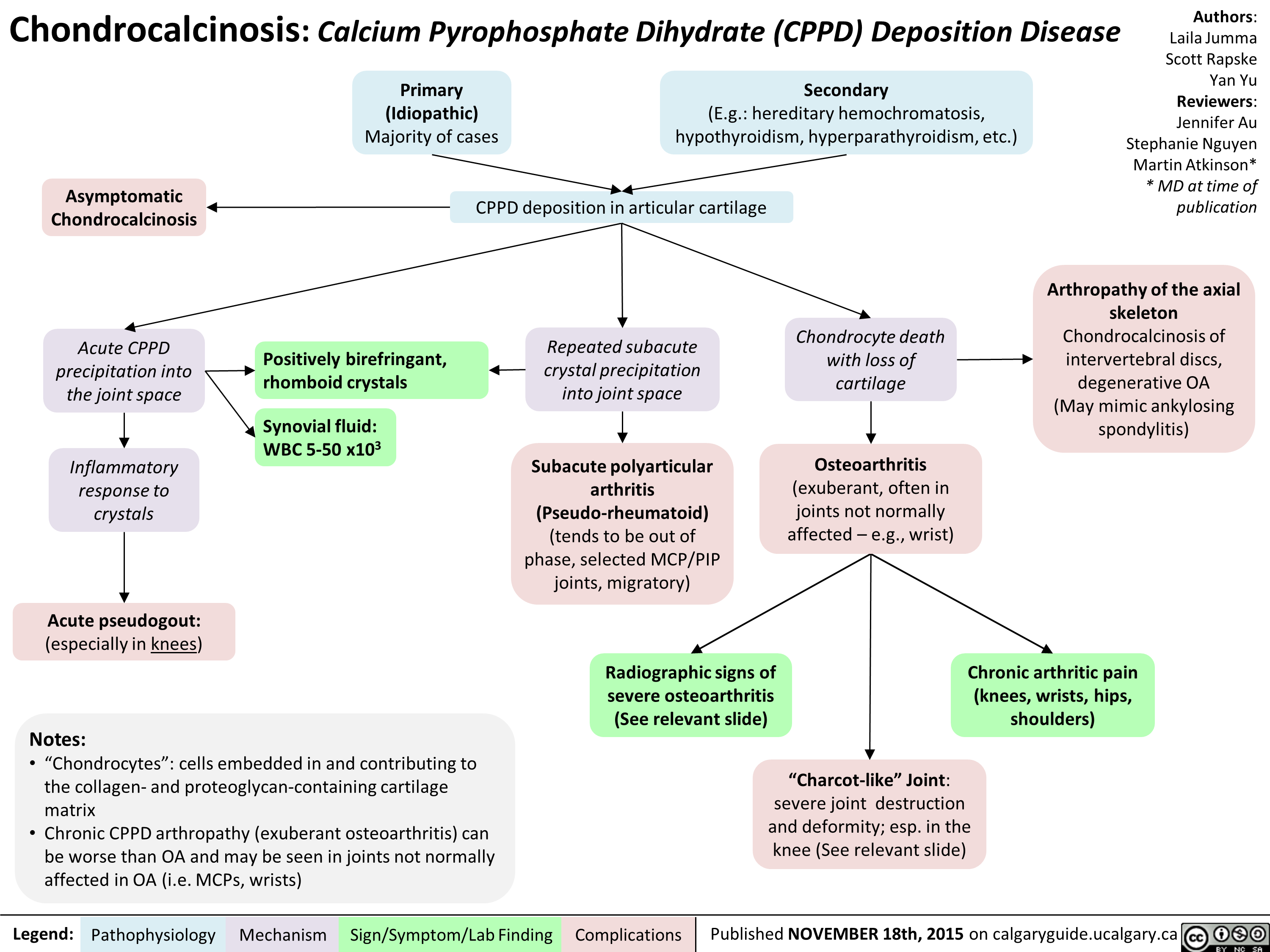
Lung cancer clinical findings and paraneoplastic syndromes
Celiac Disease: Complications
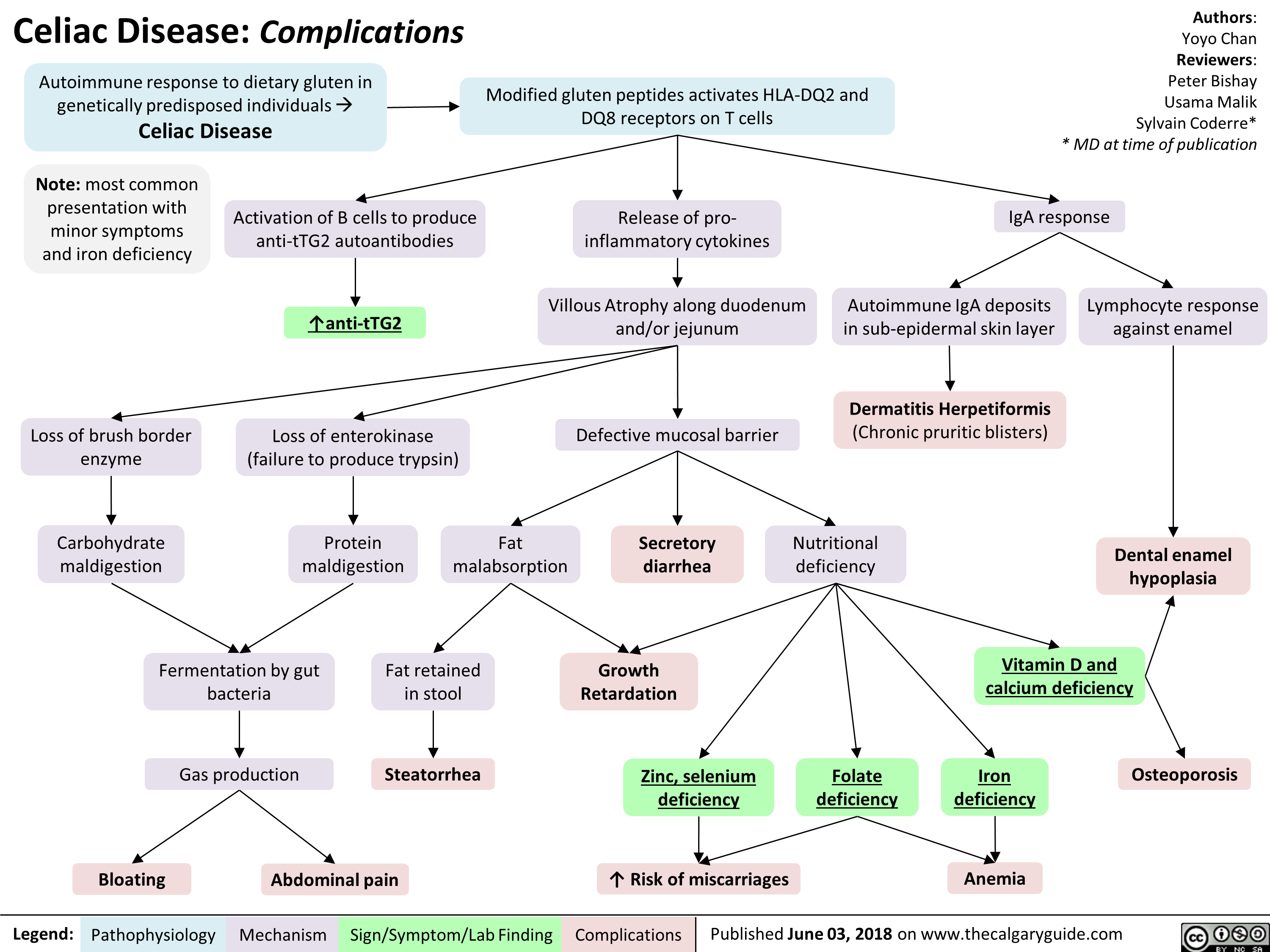
Neuromuscular Junction (NMJ)- Physiology and pharmacology
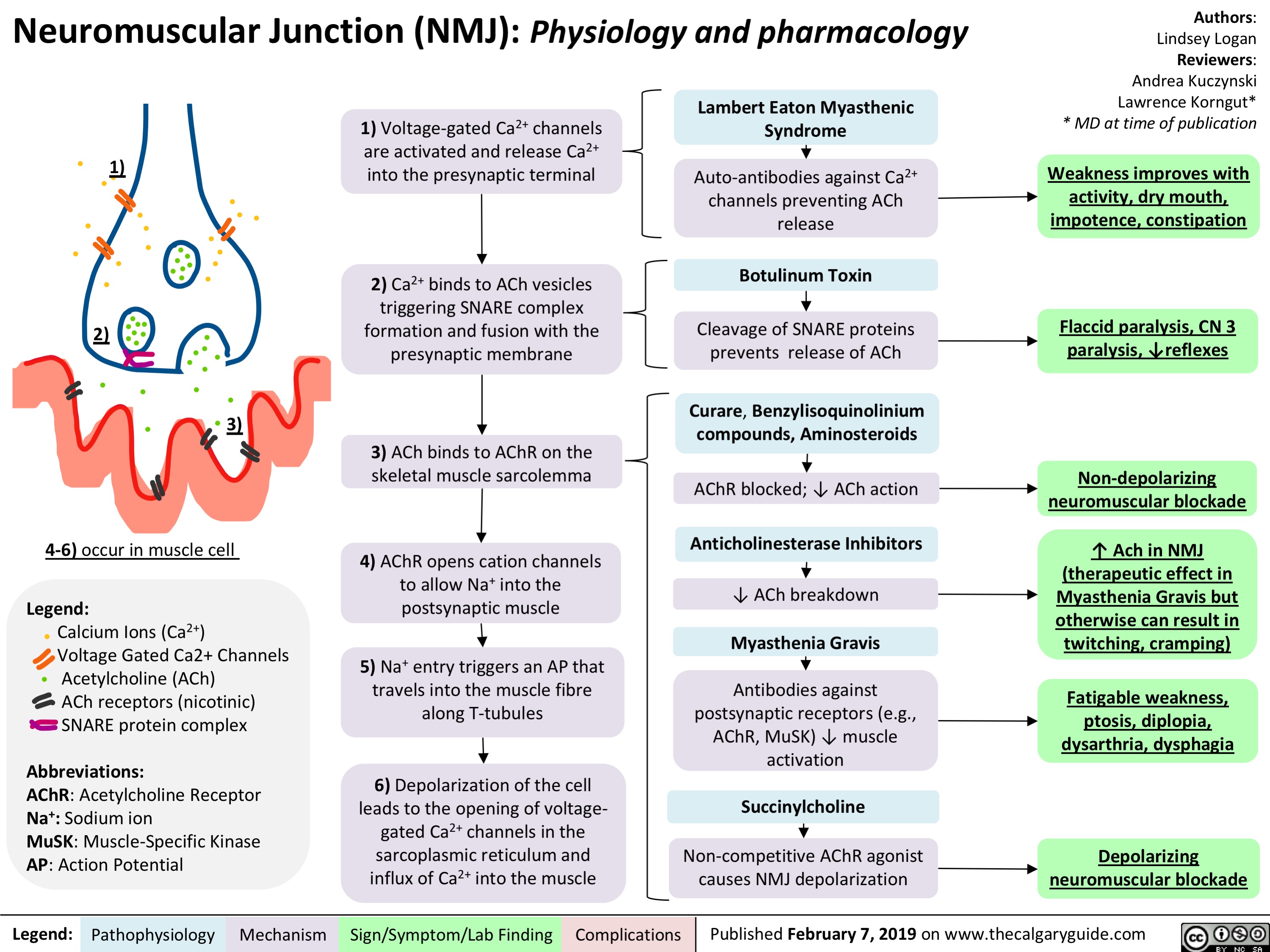
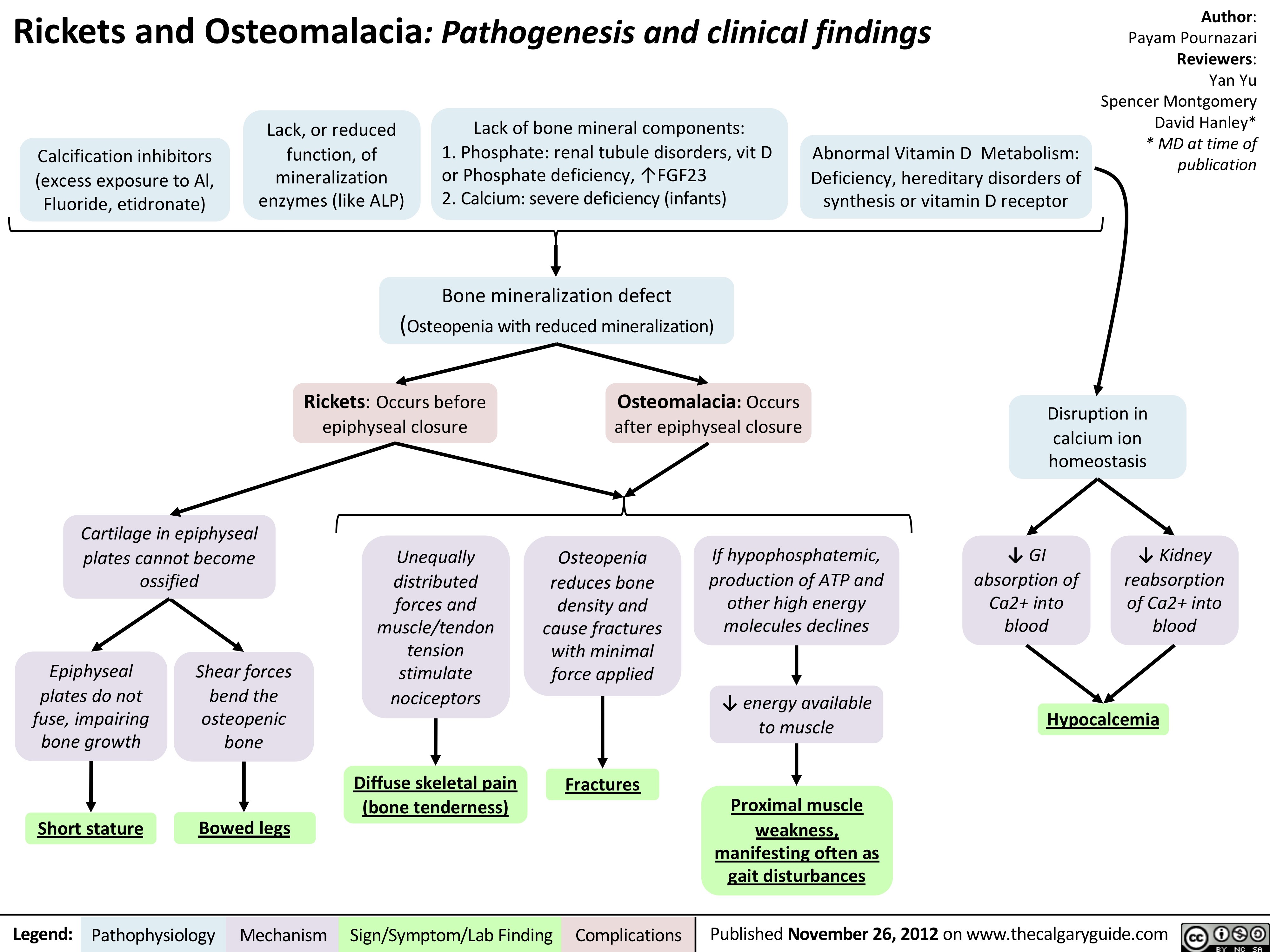
Rickets and Osteomalacia: Pathogenesis and Clinical Findings
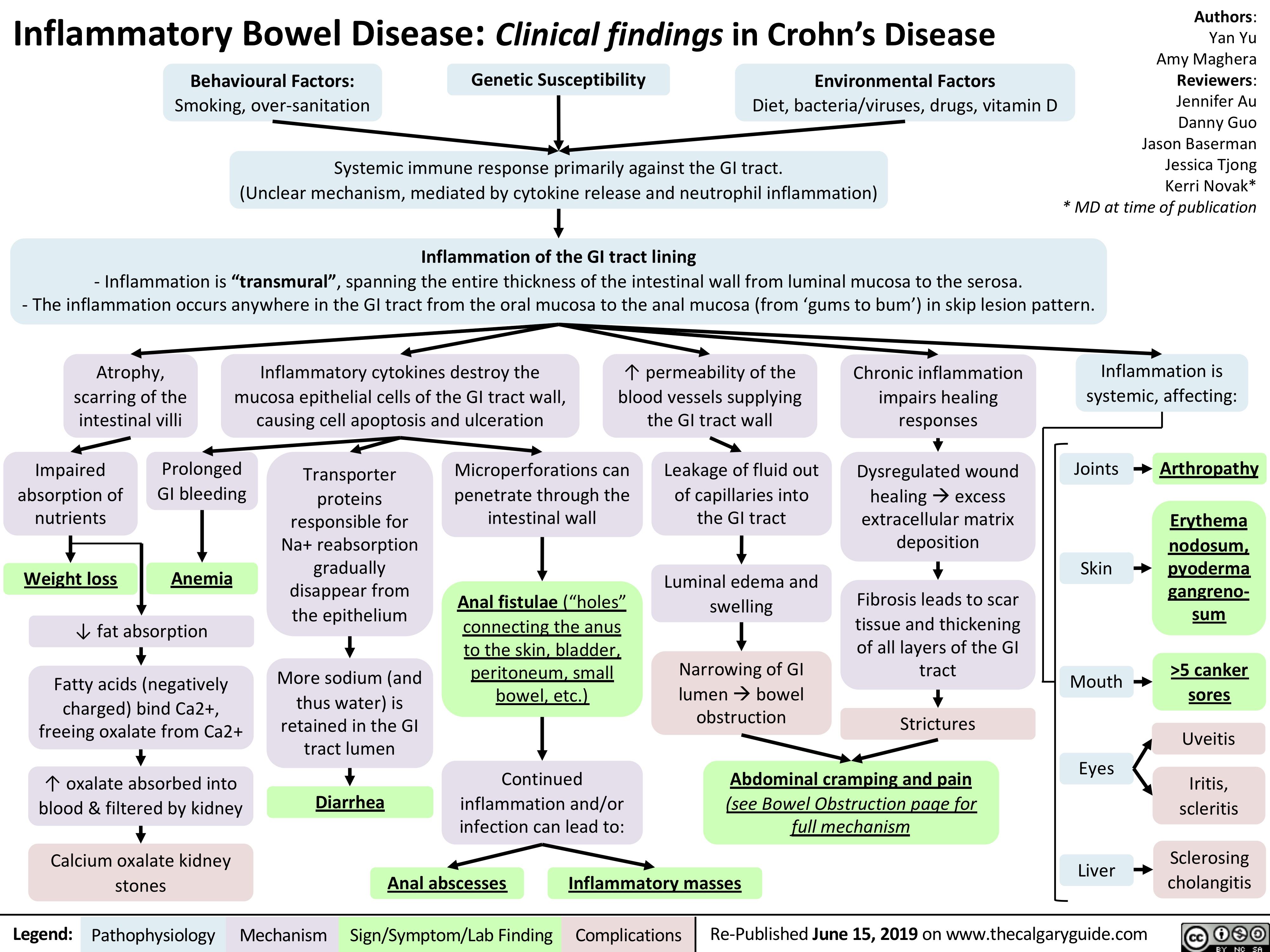
Crohn's Disease
Multiple-Myeloma
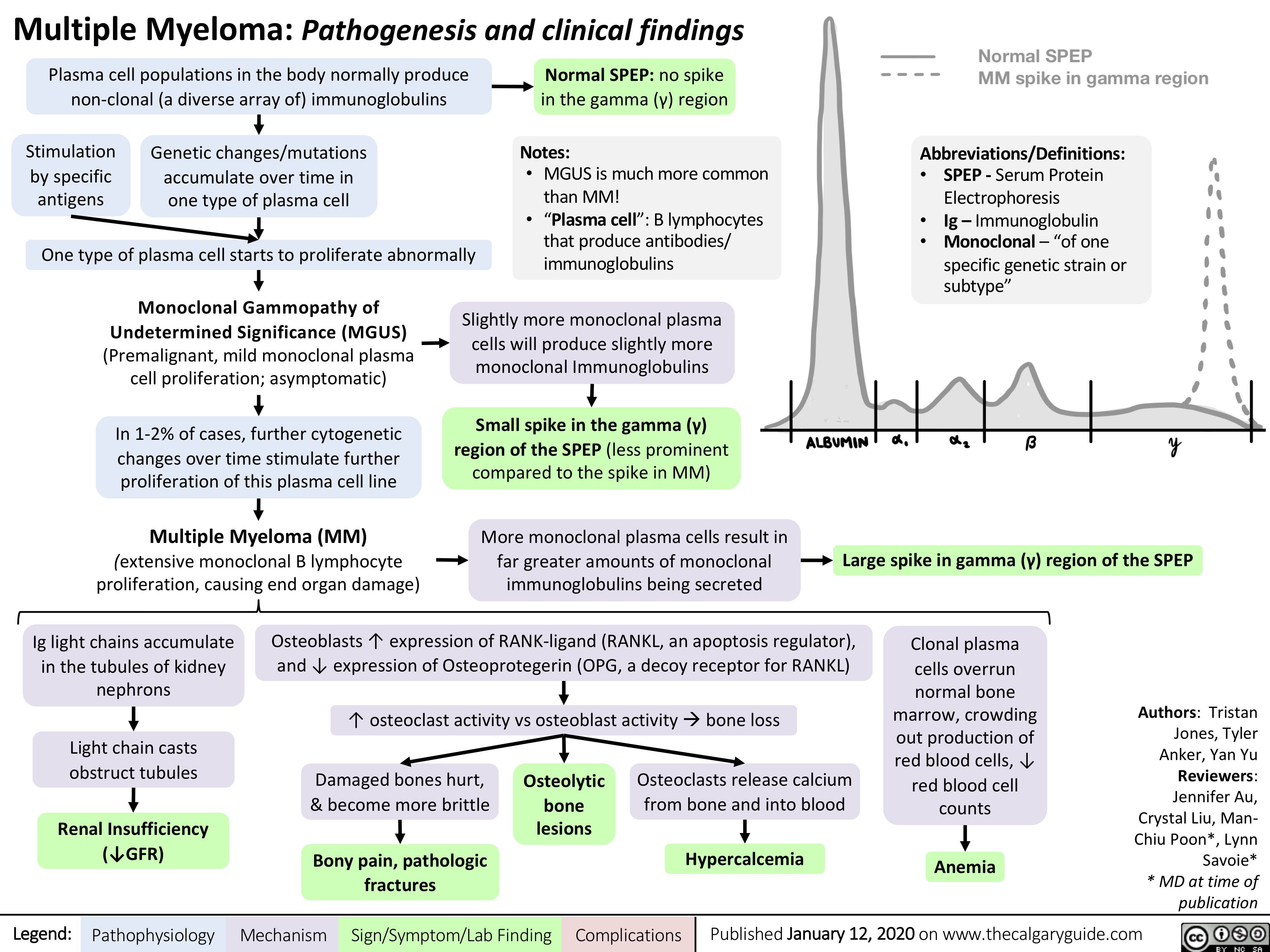
Pseudogout
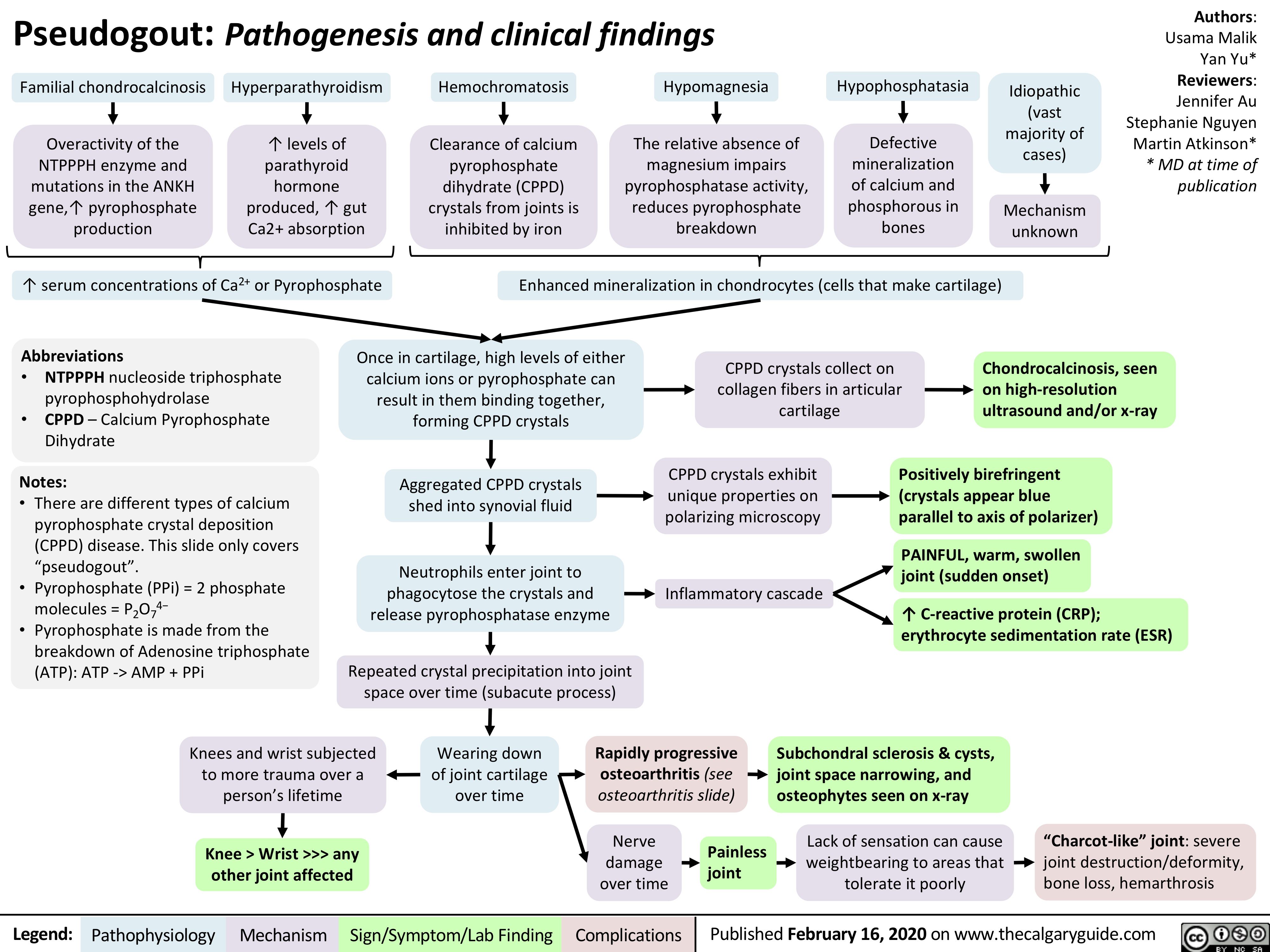
GI-changes-during-pregnancy
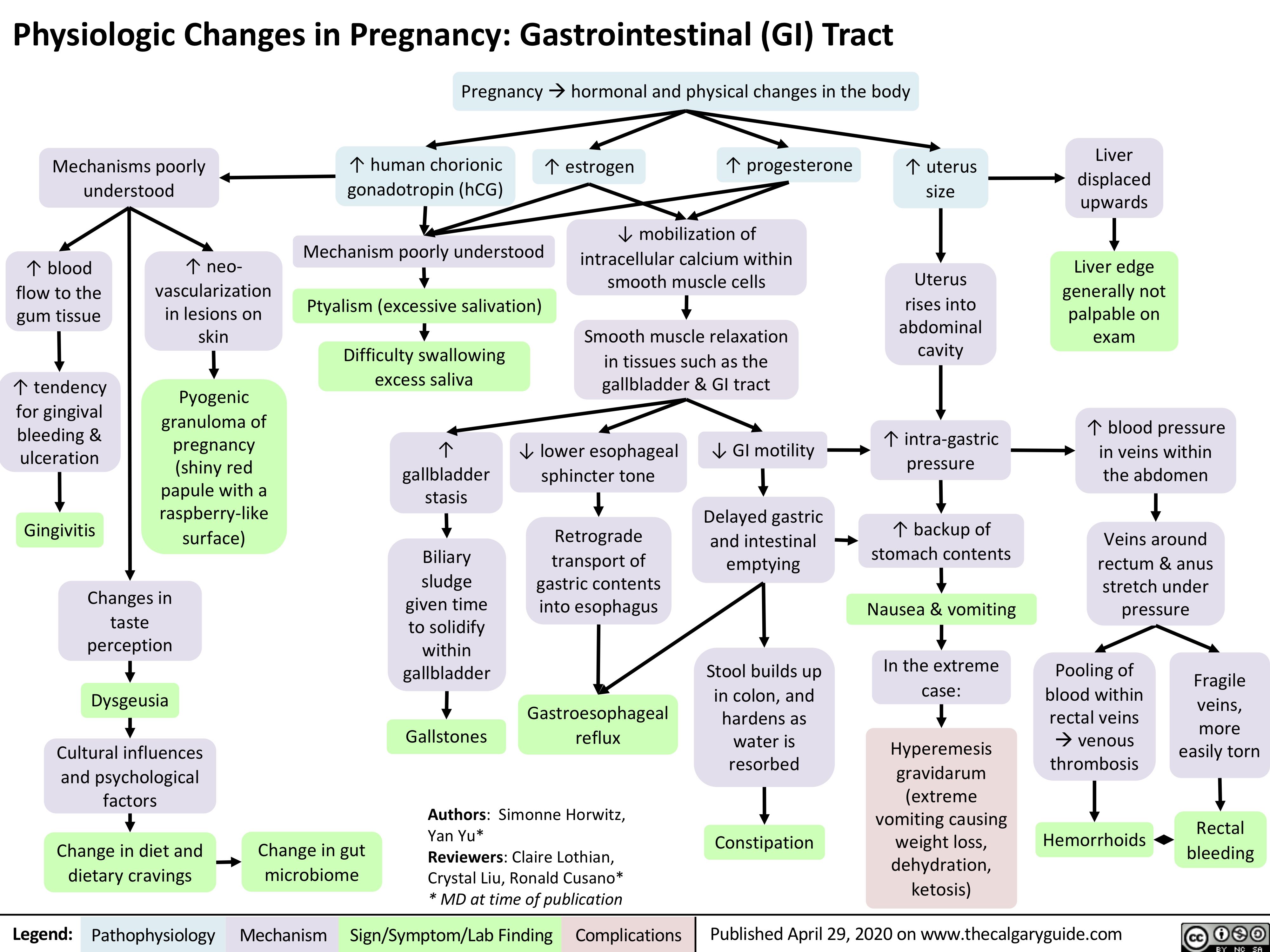
Placenta-Previa
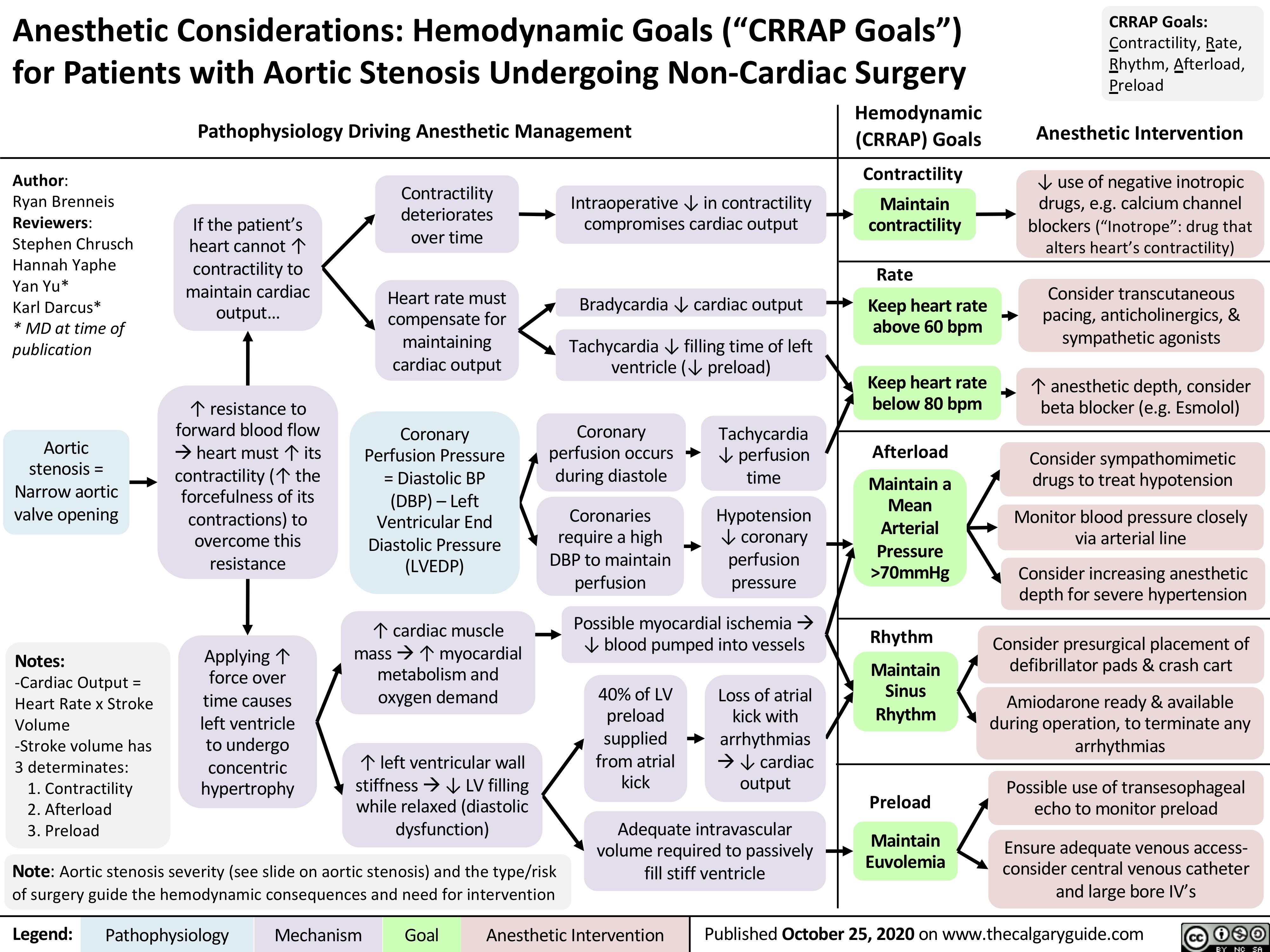
Anesthetic-Considerations-Aortic-Stenosis
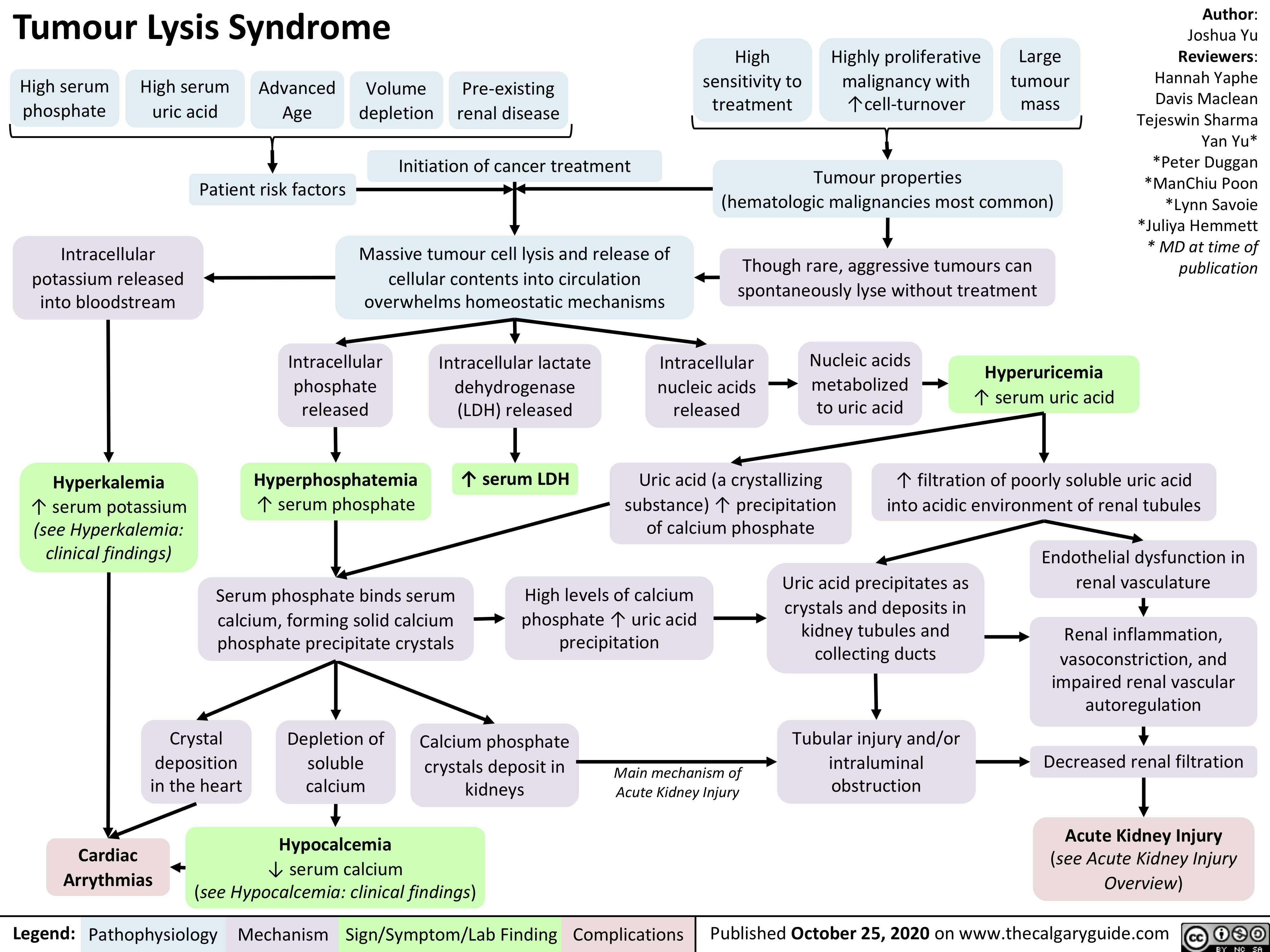
Tumour-Lysis-Syndrome
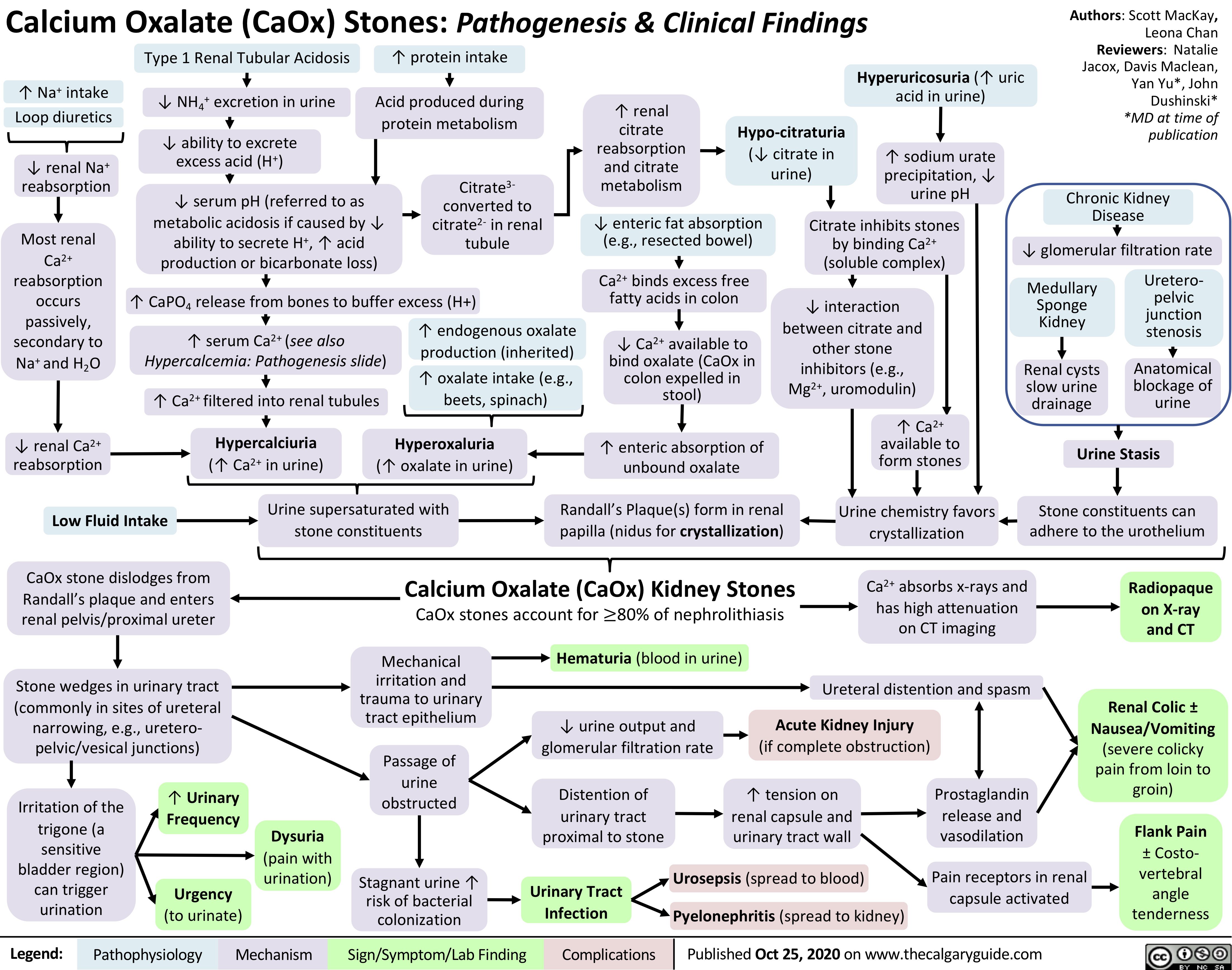
Calcium-Oxalate-Kidney-Stones
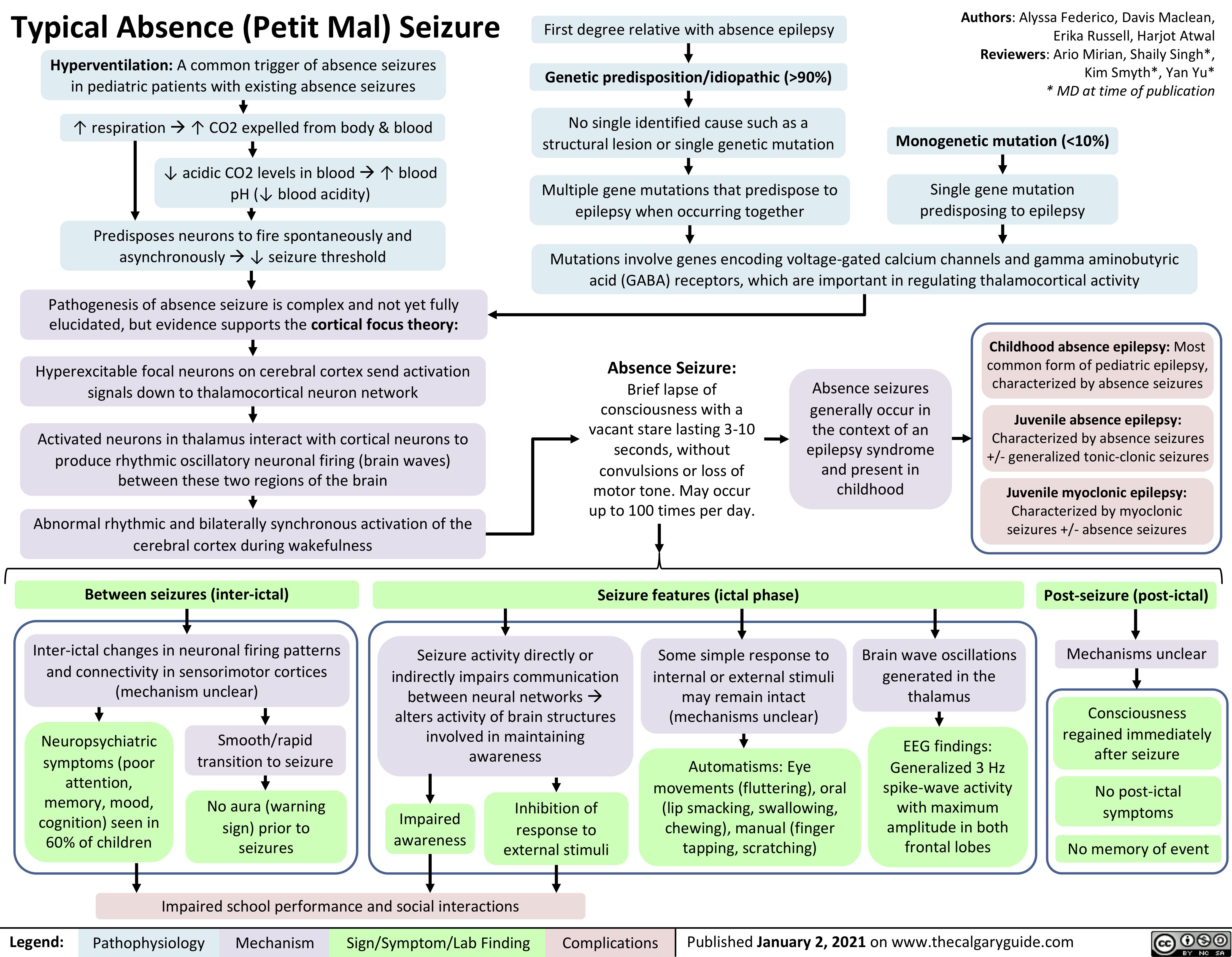
generalized-absence-seizures-petit-mal
Lambert-Eaton-Myasthenic-Syndrome-Pathogenesis-and-Clinical-Findings
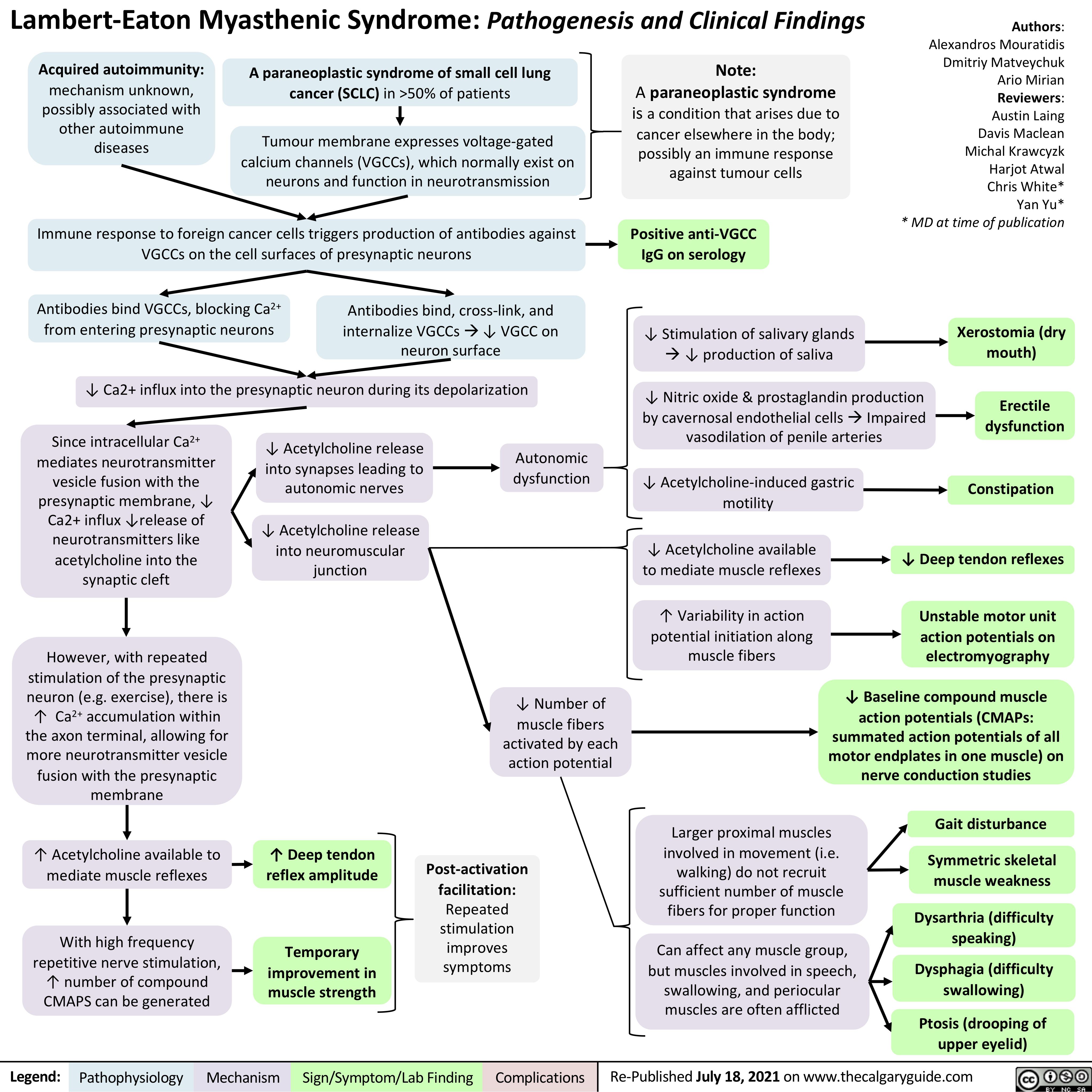
Hypercortisolemia
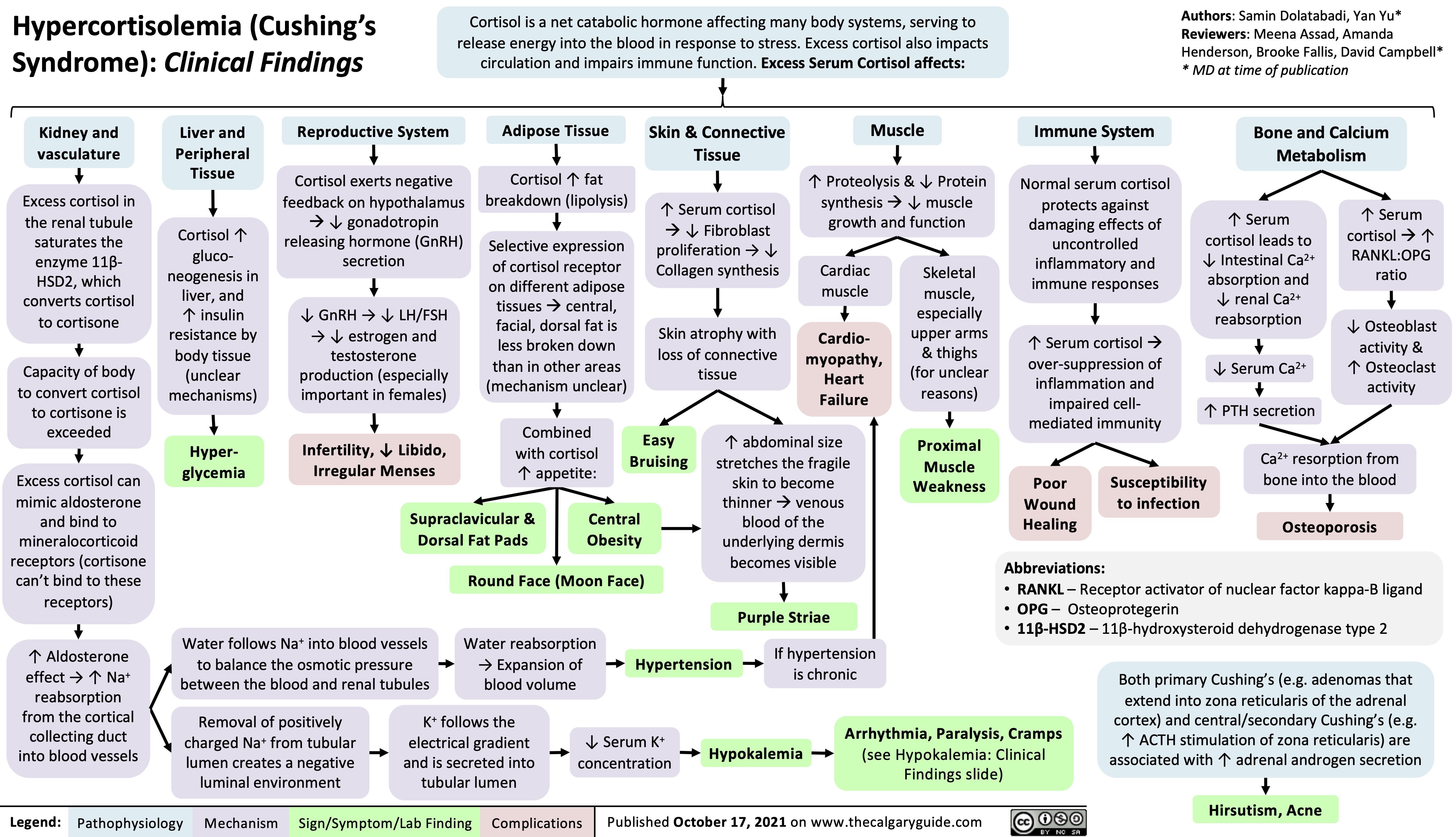
Overview of Calcium Phosphate Vitamin D Physiology
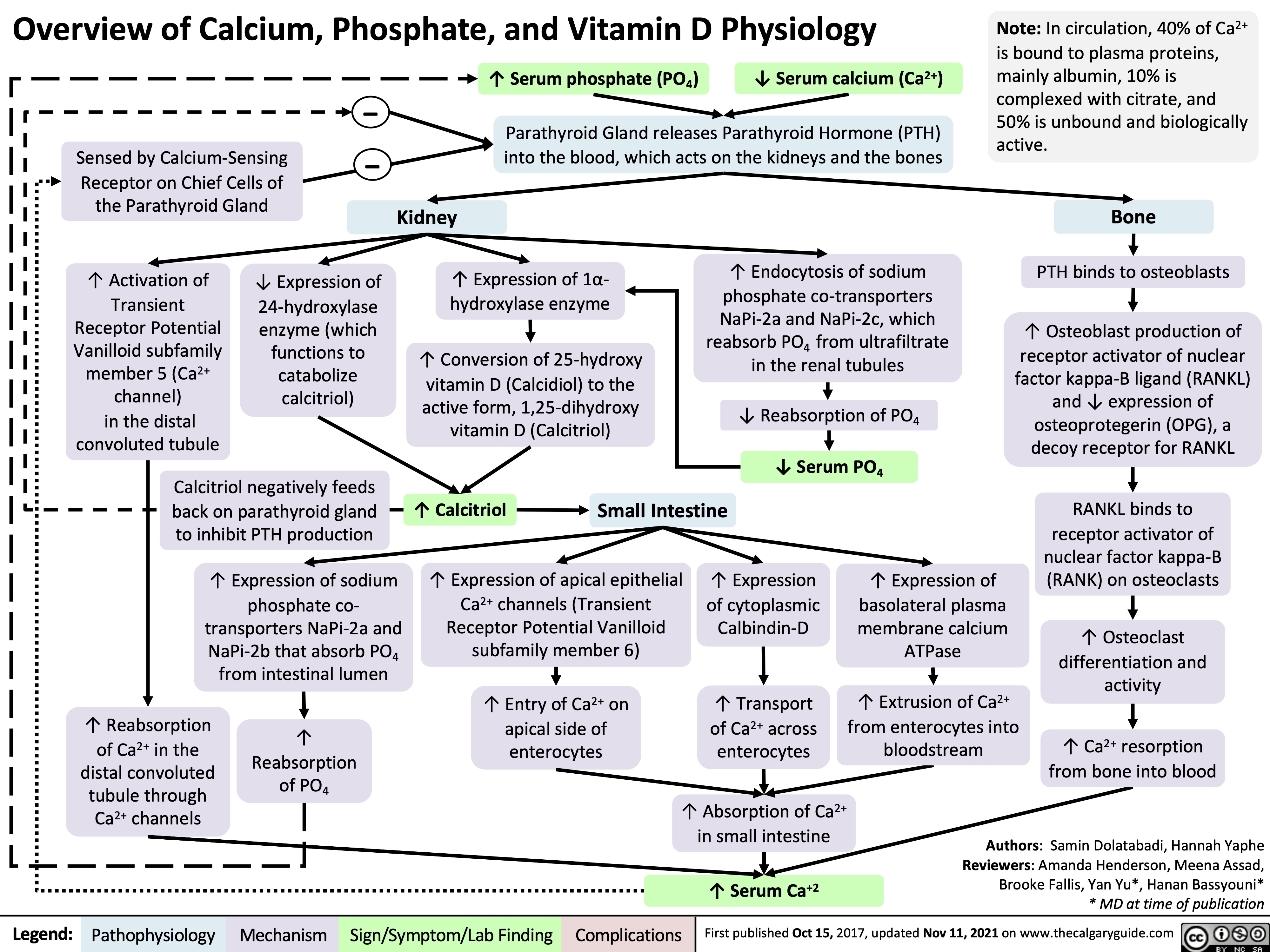
complications-of-chronic-kidney-disease-ckd
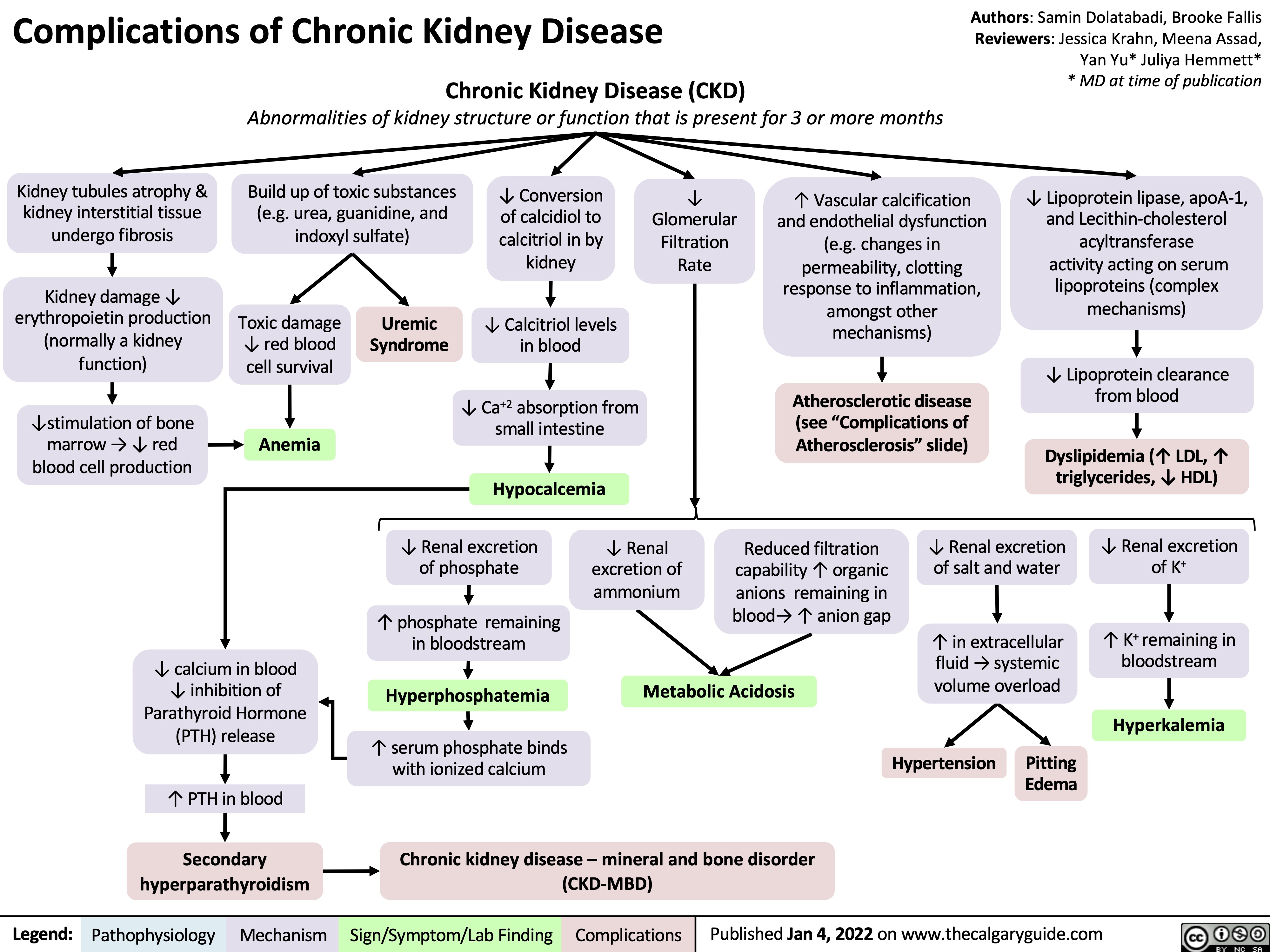
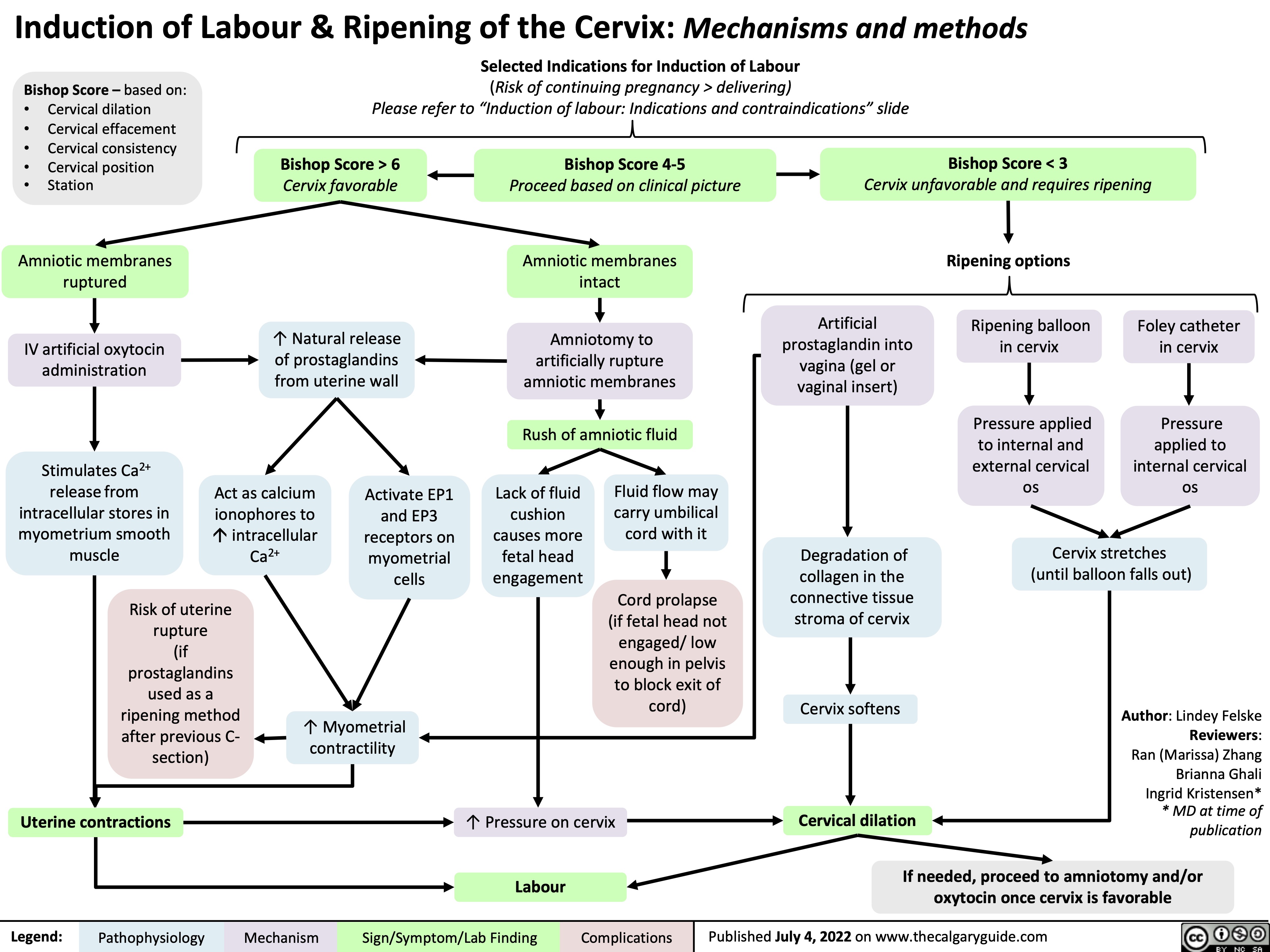
induction-of-labour-ripening-of-the-cervix-mechanisms-and-methods
presentation-of-sah
![Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com
Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/SAH-Clinical-Findings-2022.jpg)
diabetes-insipidus-pathogenesis-and-clinical-findings
![Diabetes Insipidus: Pathogenesis and clinical findings
Hereditary
Autoimmune/ Idiopathic
Auto-antibodies destroy neurons that release antidiuretic hormone (ADH)
Mass Effect/ Tumor Invasion
Mass pressing on hypothalamus or pituitary
Electrolyte Imbalance
(mechanism unclear)
Hereditary
Lithium (Li)
(mechanism unclear)
Li enters principal cells of collecting ducts via ENaCs
Li inhibits GSK3β, reducing adenylyl cyclase activity
↓ cAMP- dependent phosphorylation of aquaporin-2
↑ Serum [Ca2+]
Activation of
CaSR in thick ascending limb of Loop of Henle
↓ NaCl reabsorption in thick ascending limb
↓ Generation of medullary osmotic gradient
↓ Serum [K+]
↑ Degradation of aquaporin-2 channels in collecting duct
↓ Aquaporin- 2 channels transporting water across apical membrane of collecting duct
Mutation of AVPR2 gene on X chromosome
Antidiuretic hormone (ADH) receptor cannot reach basolateral surface of principal cells of collecting duct
Mutation of aquaporin-2 gene on chromosome 12
↓ Fusion of aquaporins with apical membrane of collecting duct
Mutation of WFS1 gene on chromosome 4 (Wolfram syndrome)
↓ Processing of antidiuretic hormone (ADH) precursors and ↓ADH-releasing neurons
Surgery/ Trauma
Injury to hypothalamus or pituitary stalk
Mutation of PCSK1 gene on chromosome 5
Deficiency in PC1/3 (encoded by PCSK1)
↓ Processing of ADH by PC1/3
Aquaporin dysfunction
↓ Kidney response to ADH, which mediates reabsorption of water down its osmotic gradient through aquaporins
↓ Production of ADH by hypothalamus or ↓ secretion from ADH-releasing neurons in posterior pituitary (depending on location of lesion)
Central Diabetes Insipidus
Nephrogenic Diabetes Insipidus
Abbreviations:
AVPR2: arginine vasopressin receptor 2 CaSR: calcium-sensing receptor
ENaC: epithelial sodium channel
GSK3β: glycogen synthase kinase type 3 beta PC1/3: proprotein convertase
Diabetes Insipidus
Decreased ability of kidneys to concentrate urine
↓ Reabsorption of water from collecting duct into vasculature
Author:
Oswald Chen
Reviewers:
Huneza Nadeem,
Ran (Marissa) Zhang,
Yan Yu*
Sam Fineblit*
* MD at time of publication
Urine becomes more dilute
↓ Urine osmolality
↑ Urine output
↓ Blood volume
Blood becomes more concentrated
Occurs during late sleep period
Nocturia
Polyuria
(>3 L/day)
↑ Serum osmolality
Activation of hypothalamic osmoreceptors
Hypernatremia
(Serum [Na+] >145 mEq/L)
Polydipsia
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published September 25, 2022 on www.thecalgaryguide.com
Diabetes Insipidus: Pathogenesis and clinical findings
Hereditary
Autoimmune/ Idiopathic
Auto-antibodies destroy neurons that release antidiuretic hormone (ADH)
Mass Effect/ Tumor Invasion
Mass pressing on hypothalamus or pituitary
Electrolyte Imbalance
(mechanism unclear)
Hereditary
Lithium (Li)
(mechanism unclear)
Li enters principal cells of collecting ducts via ENaCs
Li inhibits GSK3β, reducing adenylyl cyclase activity
↓ cAMP- dependent phosphorylation of aquaporin-2
↑ Serum [Ca2+]
Activation of
CaSR in thick ascending limb of Loop of Henle
↓ NaCl reabsorption in thick ascending limb
↓ Generation of medullary osmotic gradient
↓ Serum [K+]
↑ Degradation of aquaporin-2 channels in collecting duct
↓ Aquaporin- 2 channels transporting water across apical membrane of collecting duct
Mutation of AVPR2 gene on X chromosome
Antidiuretic hormone (ADH) receptor cannot reach basolateral surface of principal cells of collecting duct
Mutation of aquaporin-2 gene on chromosome 12
↓ Fusion of aquaporins with apical membrane of collecting duct
Mutation of WFS1 gene on chromosome 4 (Wolfram syndrome)
↓ Processing of antidiuretic hormone (ADH) precursors and ↓ADH-releasing neurons
Surgery/ Trauma
Injury to hypothalamus or pituitary stalk
Mutation of PCSK1 gene on chromosome 5
Deficiency in PC1/3 (encoded by PCSK1)
↓ Processing of ADH by PC1/3
Aquaporin dysfunction
↓ Kidney response to ADH, which mediates reabsorption of water down its osmotic gradient through aquaporins
↓ Production of ADH by hypothalamus or ↓ secretion from ADH-releasing neurons in posterior pituitary (depending on location of lesion)
Central Diabetes Insipidus
Nephrogenic Diabetes Insipidus
Abbreviations:
AVPR2: arginine vasopressin receptor 2 CaSR: calcium-sensing receptor
ENaC: epithelial sodium channel
GSK3β: glycogen synthase kinase type 3 beta PC1/3: proprotein convertase
Diabetes Insipidus
Decreased ability of kidneys to concentrate urine
↓ Reabsorption of water from collecting duct into vasculature
Author:
Oswald Chen
Reviewers:
Huneza Nadeem,
Ran (Marissa) Zhang,
Yan Yu*
Sam Fineblit*
* MD at time of publication
Urine becomes more dilute
↓ Urine osmolality
↑ Urine output
↓ Blood volume
Blood becomes more concentrated
Occurs during late sleep period
Nocturia
Polyuria
(>3 L/day)
↑ Serum osmolality
Activation of hypothalamic osmoreceptors
Hypernatremia
(Serum [Na+] >145 mEq/L)
Polydipsia
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published September 25, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/09/Diabetes-Insipidus.jpg)
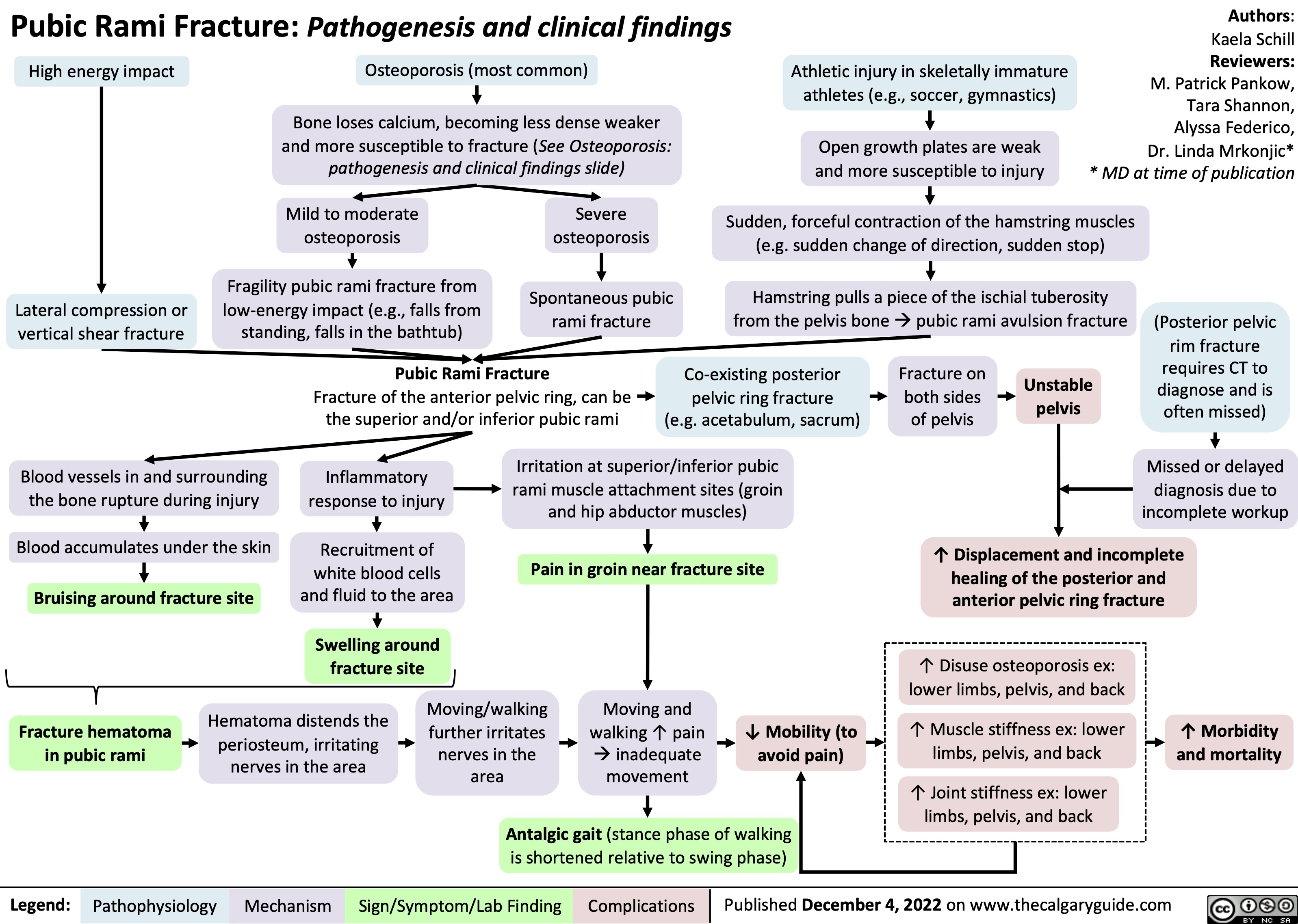
Pubic Rami Fracture: Pathogenesis and clinical findings
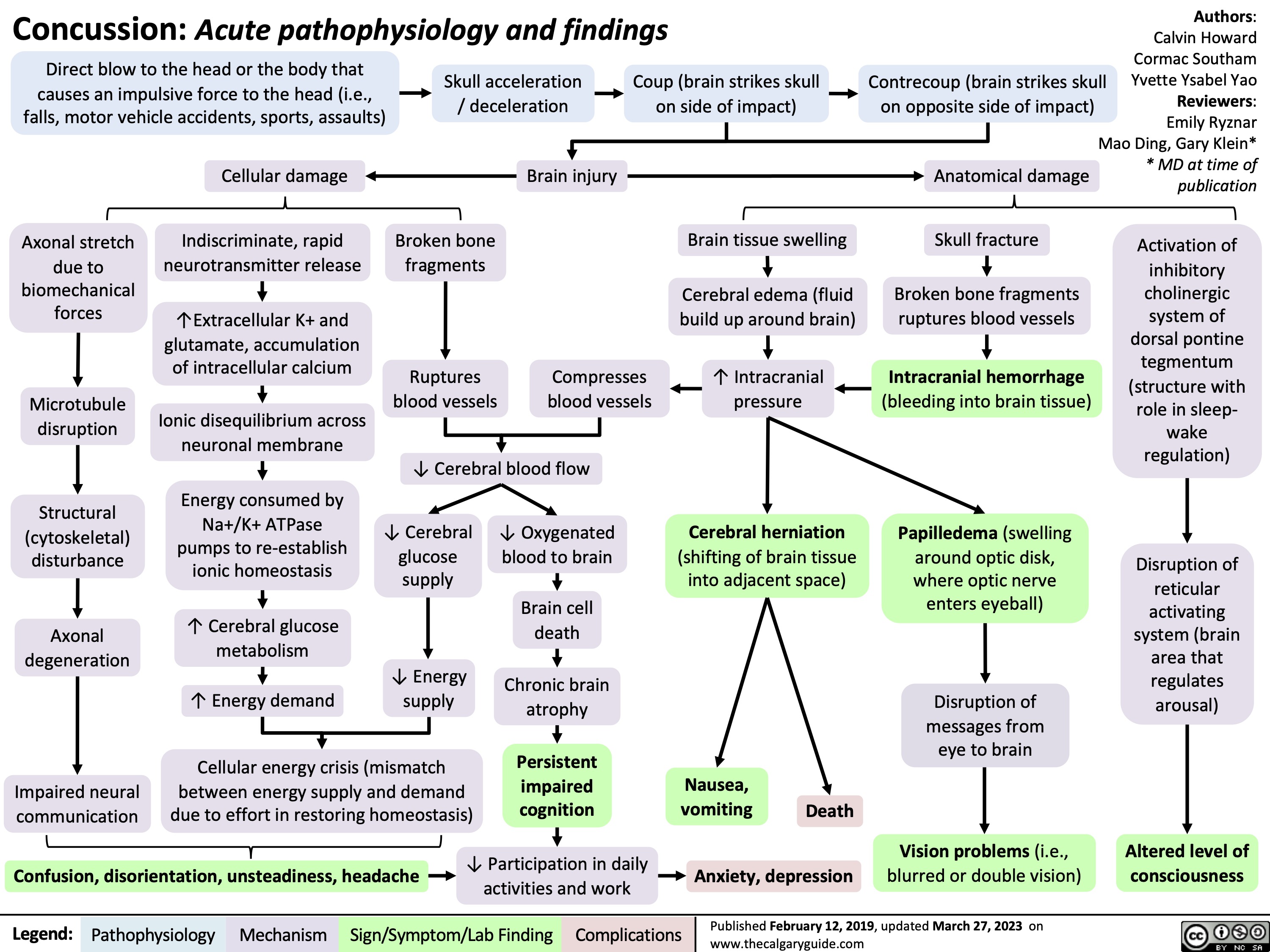
Concussion
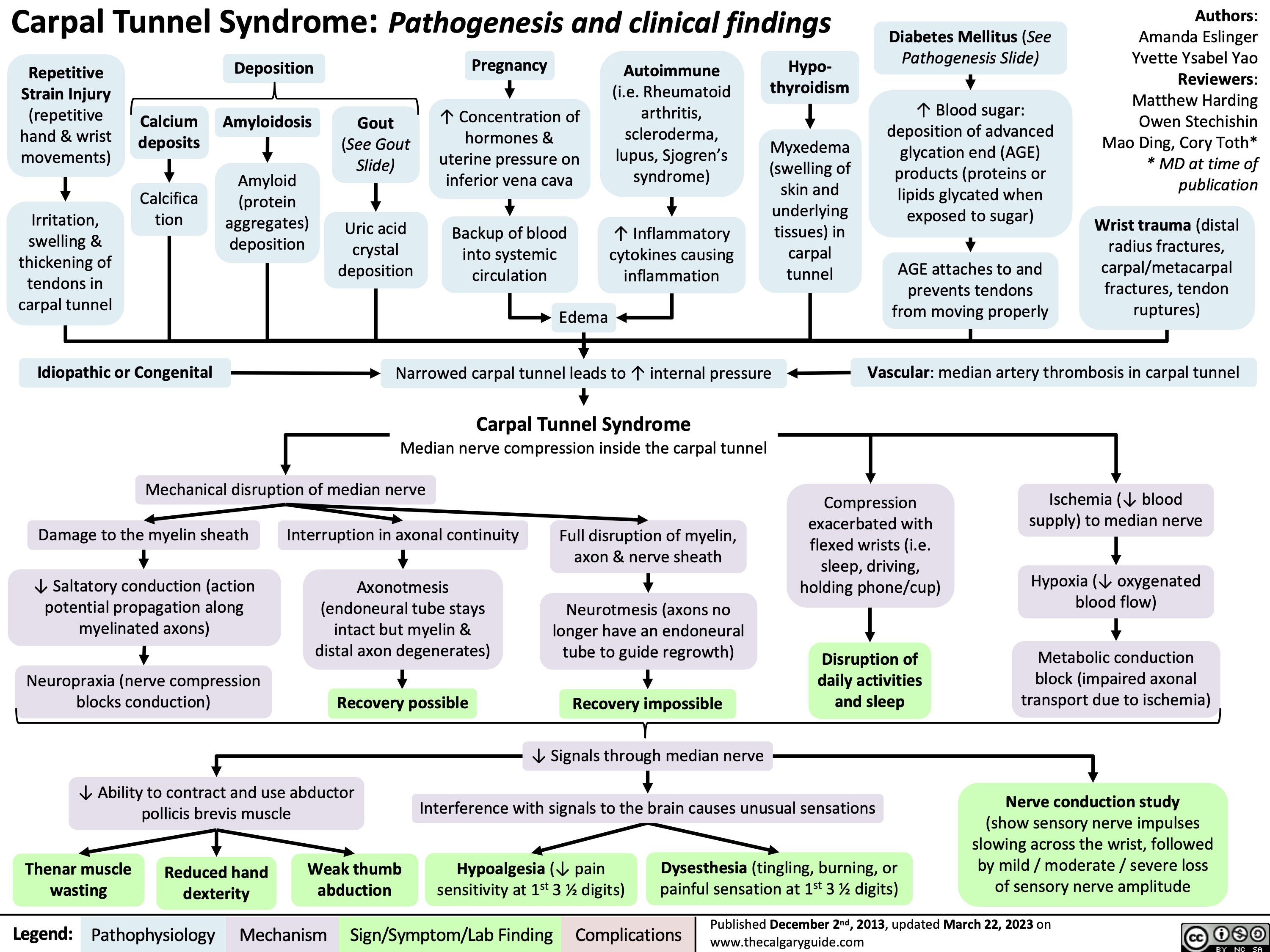
Carpal Tunnel Syndrome
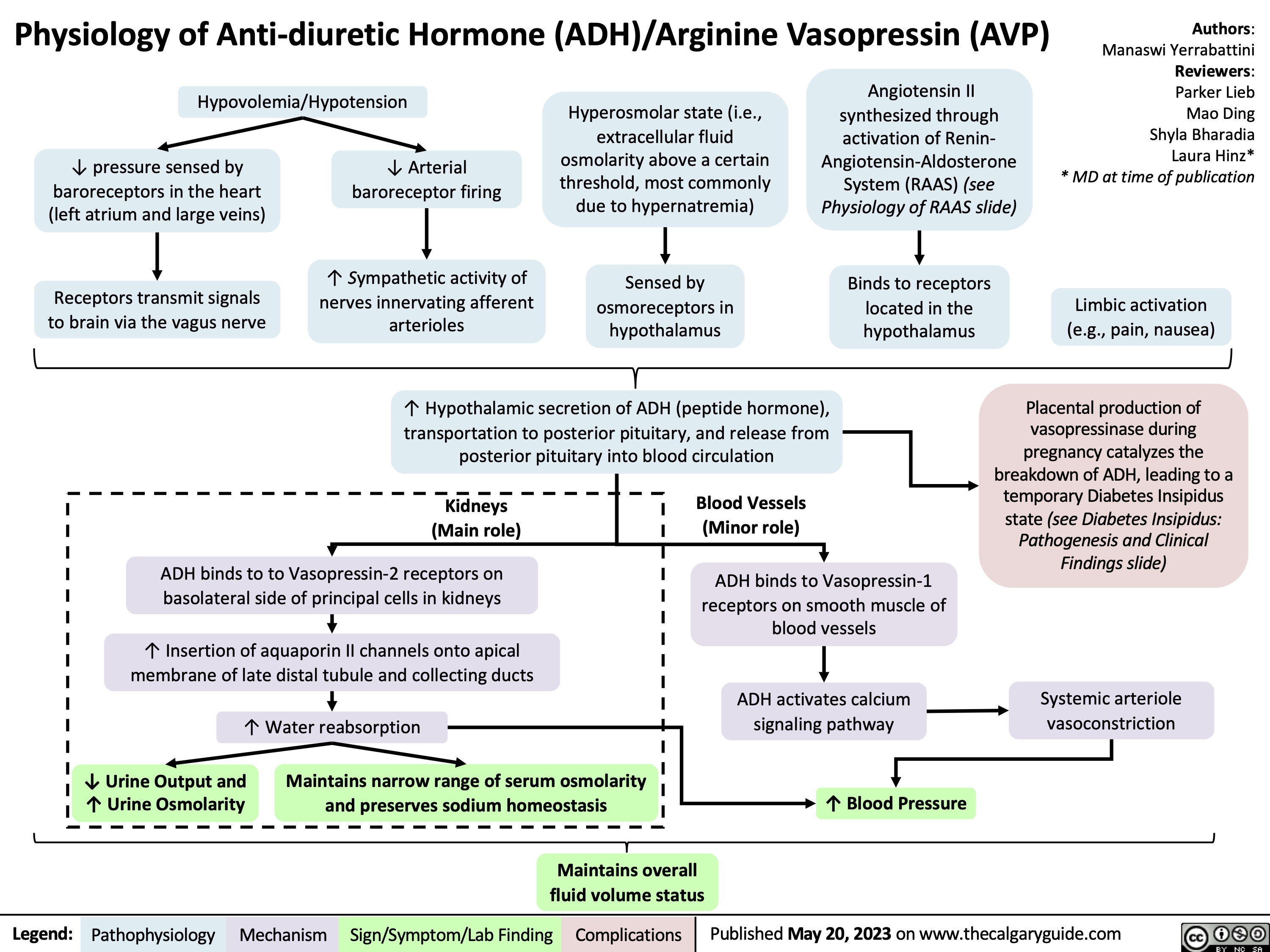
Physiology of Anti-diuretic hormone
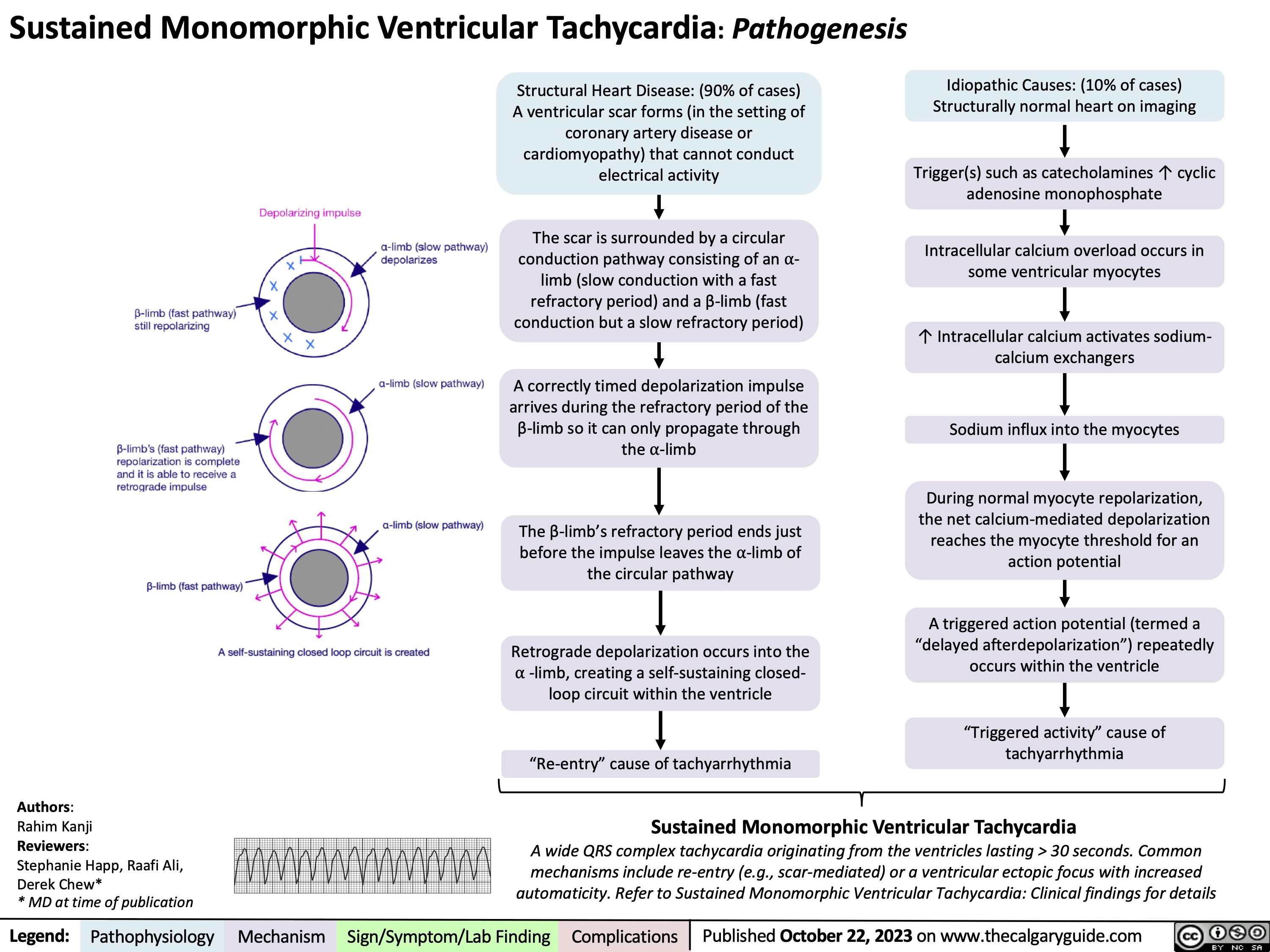
Sustained Monomorphic Ventricular Tachycardia Pathogenesis
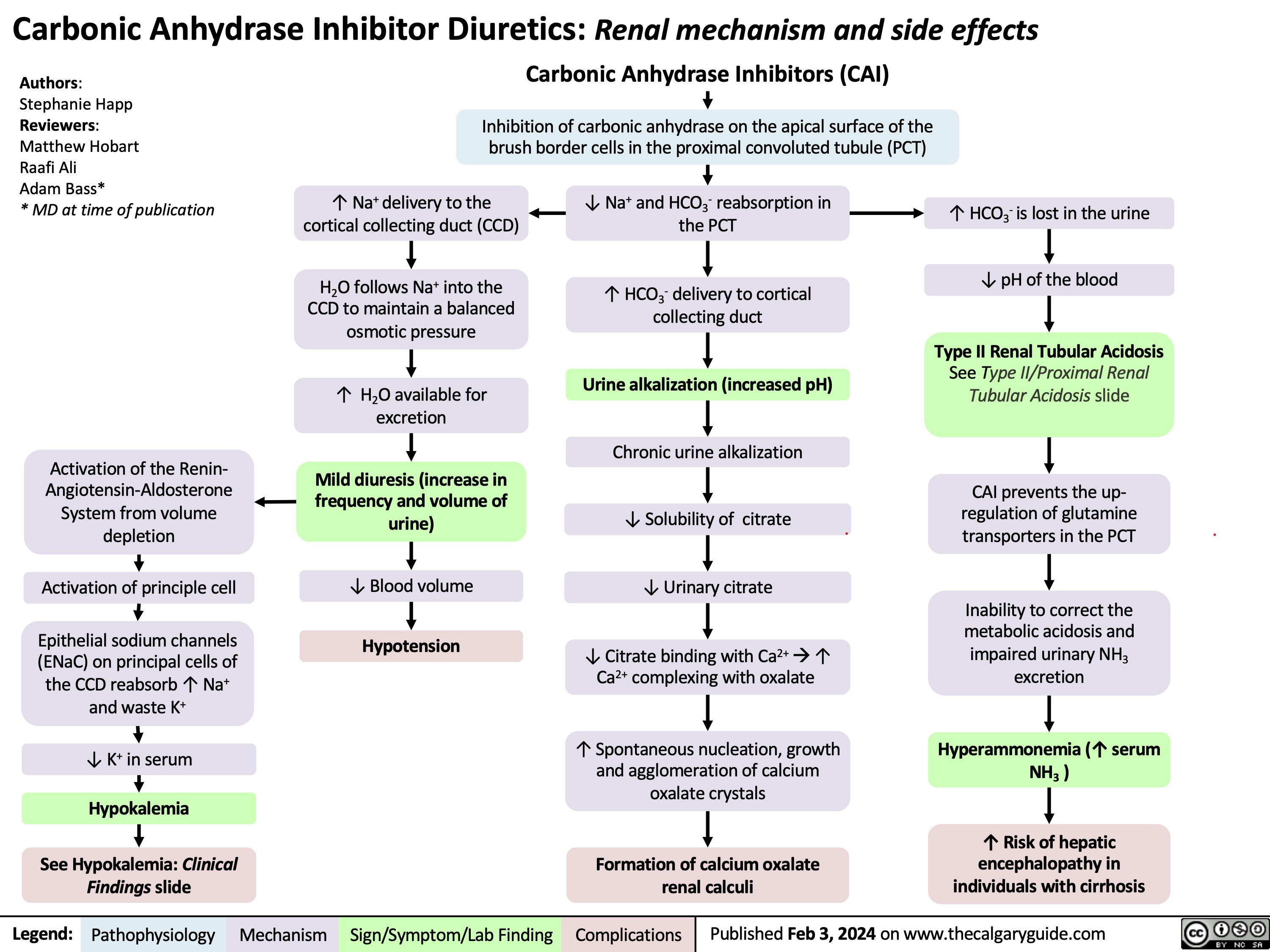
Carbonic Anhydrase Inhibitor Diuretics
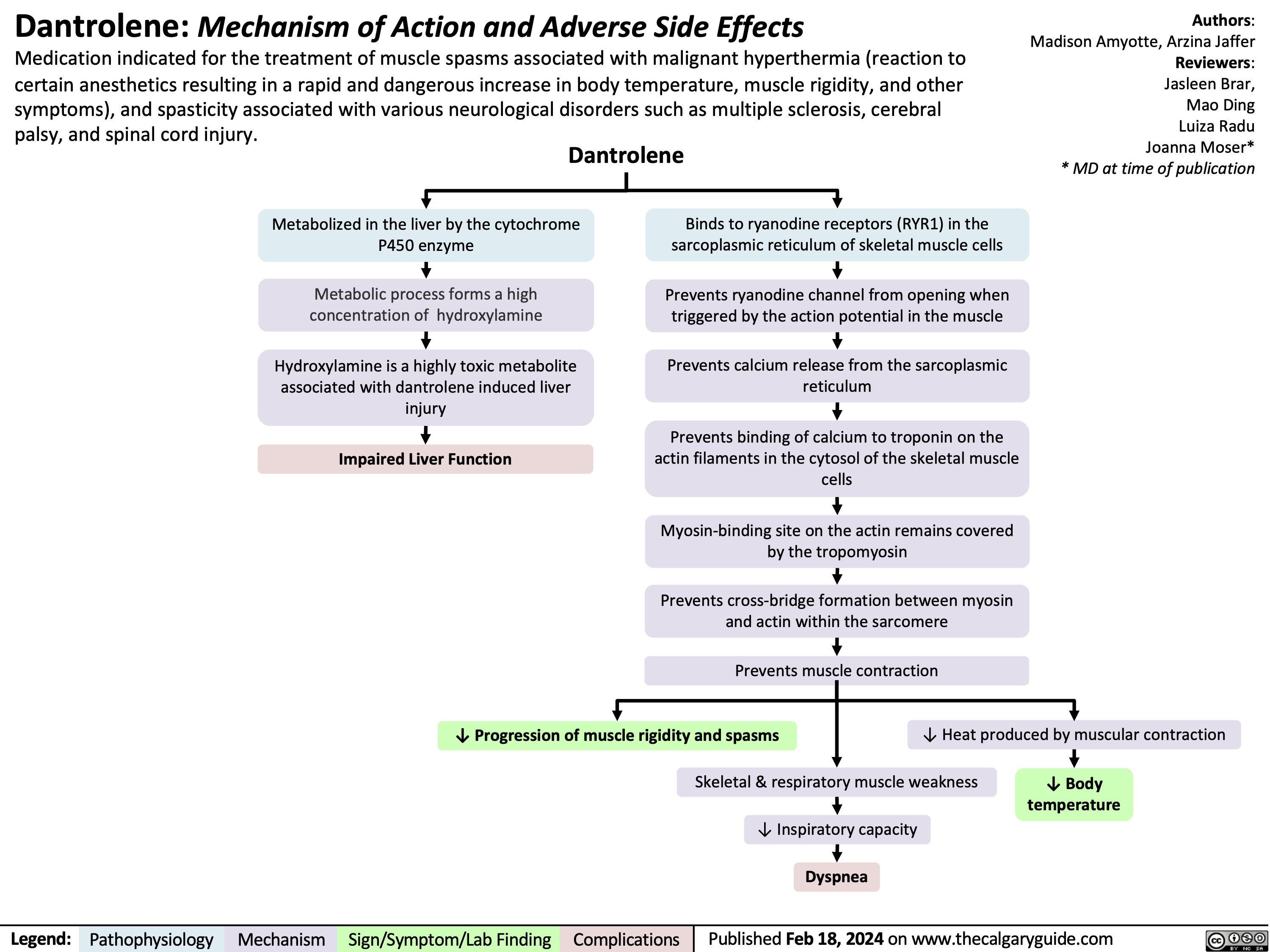
Dantrolene
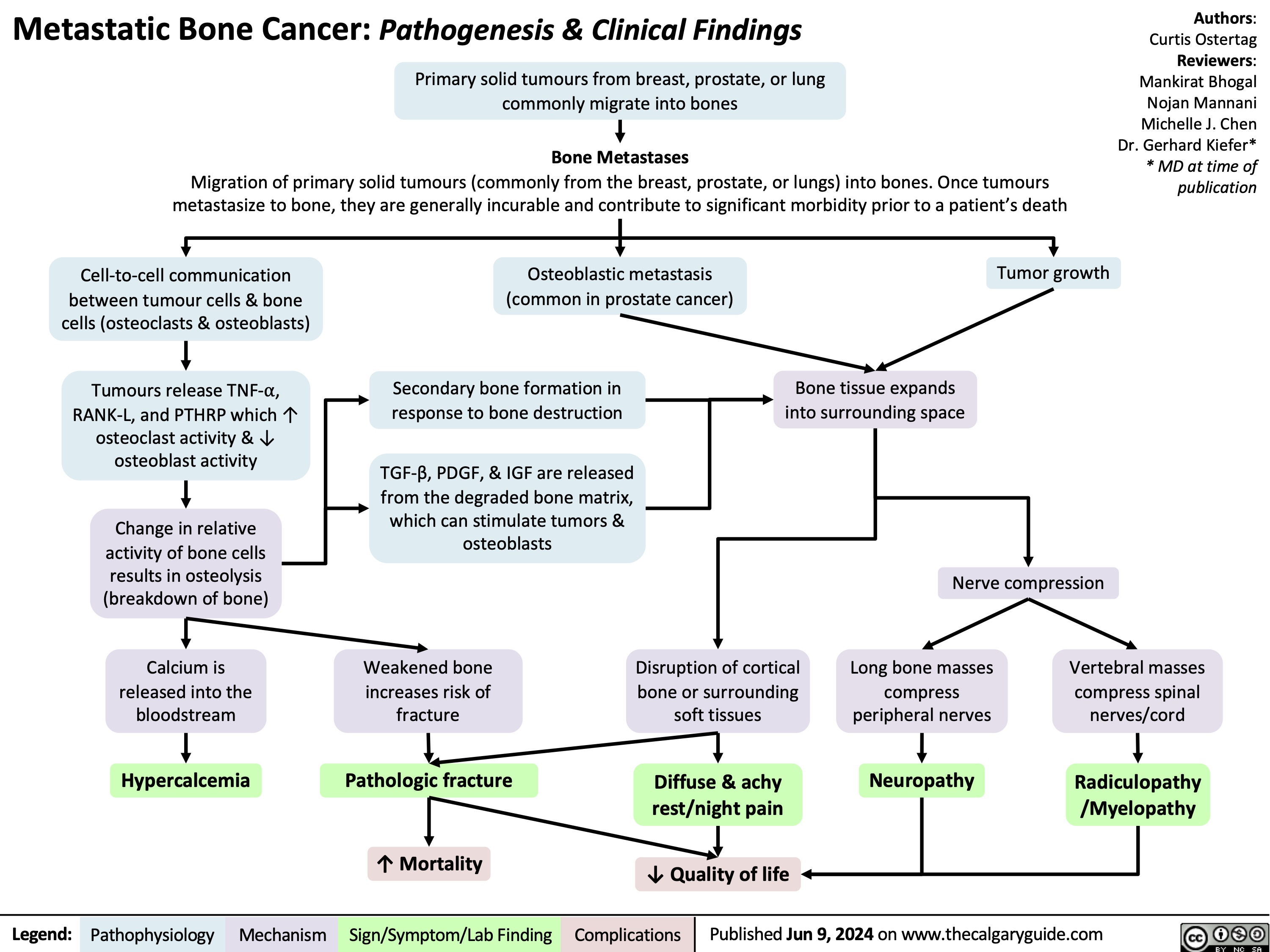
Metastatic Bone Lesions
Massive Transfusion Protocol
![Massive Transfusion Protocol: Considerations and rationale
Massive transfusion protocol (MTP) is a tool used by clinicians when there is a need to rapidly administer a large amount of blood products, including packed red blood cells (pRBCs), fresh frozen plasma (FFP), and platelets. Complications of MTP are commonly referred to as “The Lethal Triad” referring to hypothermia, acidosis and coagulopathy.
Authors: Kayleigh Yang Arzina Jaffer
Reviewers: Jasleen Brar,
Luiza Radu, Karl Darcus*
* MD at time of publication
Intervention
Indications Initial Response Pathophysiology Transfusion Targets
≥ 3 pRBCs unit transfusion requirement in 1 hour
Shock index (heart rate/systolic blood pressure) > 1
Blood volume loss >50% in ≤3 hours
ABC Score ≥ 3 of: 1. Penetrating mechanism of injury 2. Systolic blood pressure < 90 mmHg 3. Heart rate > 120 beats per minute 4. Evidence of hemoperitoneum or hemopericardium on ultrasound (positive FAST U/S exam)
RABT Score ≥ 2 of: 1. Penetrating mechanism of injury 2. Shock index > 1 3. Positive FAST U/S 4. Known or suspected pelvic fracture
Call for help
Activate institution's MTP protocol
Send for STAT type and screen
Establish large-bore intravenous access
Fluid resuscitation
Collect and send STAT bloodwork including hemoglobin, platelet, INR, fibrinogen, electrolytes, creatinine and arterial blood gas (ABG).
Citrate present in blood products to avoid clotting during storage
Stored pRBCs break down and release potassium due to time mediated degeneration
Temporary accumulation of citrate in patient's blood with rapid use of blood products
Citrate chelates calcium
Less negative cell membrane resting potential
Anaerobic metabolism
Promotes hypocalcaemia
Changes in membrane excitability
Lactic acid buildup
Coagulopathy
(see coagulation cascade slide)
Cardiac dysrhythmias (peaked T-waves, atrial block, “sine wave”, asystolic EKG changes)
Metabolic acidosis
End organ damage
Continued blood loss
Volume overload
Avoid hypocalcemia
Avoid hyperkalemia
pH 7.35-7.45
Bleeding source control
Hemoglobin >70-90
Platelets >50 INR <1.5 Fibrinogen >1.5
Avoid dilutional coagulopathy (clotting factor dilution)
Mean Arterial Pressure (MAP) >60mmHg
Temperature >35.0°C
Slow (over 5-10 minutes) IV calcium administration
Inhaled beta agonists
Insulin/Dextrose
EKG monitoring
Sodium bicarbonate
Increase minute ventilation
Fastest control method to prevent further blood loss (i.e., packing wounds)
Early tranexamic acid administration
Administer pRBCs, FFP, and platelets in a 1:1:1 ratio (fibrinogen replacement indicated if <1.5 despite FFP)
Minimize crystalloid use
Administer crystalloids in a 3:1 ratio to estimated blood loss until blood products available
Administer vasopressors to meet target, do not overshoot
Temperature monitoring Fluid warming
↑ [Potassium] in pRBCs solution
Administration of pRBCs ↑ potassium in patient's blood
Blood loss
↓ Hemoglobin
Tissue hypoperfusion
Tissue hypoxia
↑ Diluent volume
↓ Concentration of clotting factors
Tissue death
↓ Coagulation ability
↑ Transfusion requirements
Early fluid resuscitation
Rapid transfusion of cooled or room-temperature blood products/fluids
↑ Blood pressure
Development of hypothermia
↑ Bleeding and clot dislodgement potential
↓ Enzyme activity in the coagulation cascade
↓ Coagulation ability
Legend:
Pathophysiology
Mechanism
Targets
Intervention
Published Sept 5, 2024 on www.thecalgaryguide.com
Massive Transfusion Protocol: Considerations and rationale
Massive transfusion protocol (MTP) is a tool used by clinicians when there is a need to rapidly administer a large amount of blood products, including packed red blood cells (pRBCs), fresh frozen plasma (FFP), and platelets. Complications of MTP are commonly referred to as “The Lethal Triad” referring to hypothermia, acidosis and coagulopathy.
Authors: Kayleigh Yang Arzina Jaffer
Reviewers: Jasleen Brar,
Luiza Radu, Karl Darcus*
* MD at time of publication
Intervention
Indications Initial Response Pathophysiology Transfusion Targets
≥ 3 pRBCs unit transfusion requirement in 1 hour
Shock index (heart rate/systolic blood pressure) > 1
Blood volume loss >50% in ≤3 hours
ABC Score ≥ 3 of: 1. Penetrating mechanism of injury 2. Systolic blood pressure < 90 mmHg 3. Heart rate > 120 beats per minute 4. Evidence of hemoperitoneum or hemopericardium on ultrasound (positive FAST U/S exam)
RABT Score ≥ 2 of: 1. Penetrating mechanism of injury 2. Shock index > 1 3. Positive FAST U/S 4. Known or suspected pelvic fracture
Call for help
Activate institution's MTP protocol
Send for STAT type and screen
Establish large-bore intravenous access
Fluid resuscitation
Collect and send STAT bloodwork including hemoglobin, platelet, INR, fibrinogen, electrolytes, creatinine and arterial blood gas (ABG).
Citrate present in blood products to avoid clotting during storage
Stored pRBCs break down and release potassium due to time mediated degeneration
Temporary accumulation of citrate in patient's blood with rapid use of blood products
Citrate chelates calcium
Less negative cell membrane resting potential
Anaerobic metabolism
Promotes hypocalcaemia
Changes in membrane excitability
Lactic acid buildup
Coagulopathy
(see coagulation cascade slide)
Cardiac dysrhythmias (peaked T-waves, atrial block, “sine wave”, asystolic EKG changes)
Metabolic acidosis
End organ damage
Continued blood loss
Volume overload
Avoid hypocalcemia
Avoid hyperkalemia
pH 7.35-7.45
Bleeding source control
Hemoglobin >70-90
Platelets >50 INR <1.5 Fibrinogen >1.5
Avoid dilutional coagulopathy (clotting factor dilution)
Mean Arterial Pressure (MAP) >60mmHg
Temperature >35.0°C
Slow (over 5-10 minutes) IV calcium administration
Inhaled beta agonists
Insulin/Dextrose
EKG monitoring
Sodium bicarbonate
Increase minute ventilation
Fastest control method to prevent further blood loss (i.e., packing wounds)
Early tranexamic acid administration
Administer pRBCs, FFP, and platelets in a 1:1:1 ratio (fibrinogen replacement indicated if <1.5 despite FFP)
Minimize crystalloid use
Administer crystalloids in a 3:1 ratio to estimated blood loss until blood products available
Administer vasopressors to meet target, do not overshoot
Temperature monitoring Fluid warming
↑ [Potassium] in pRBCs solution
Administration of pRBCs ↑ potassium in patient's blood
Blood loss
↓ Hemoglobin
Tissue hypoperfusion
Tissue hypoxia
↑ Diluent volume
↓ Concentration of clotting factors
Tissue death
↓ Coagulation ability
↑ Transfusion requirements
Early fluid resuscitation
Rapid transfusion of cooled or room-temperature blood products/fluids
↑ Blood pressure
Development of hypothermia
↑ Bleeding and clot dislodgement potential
↓ Enzyme activity in the coagulation cascade
↓ Coagulation ability
Legend:
Pathophysiology
Mechanism
Targets
Intervention
Published Sept 5, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/09/Massive-Transfusion-Protocol.jpg)
Diffuse Axonal Injury

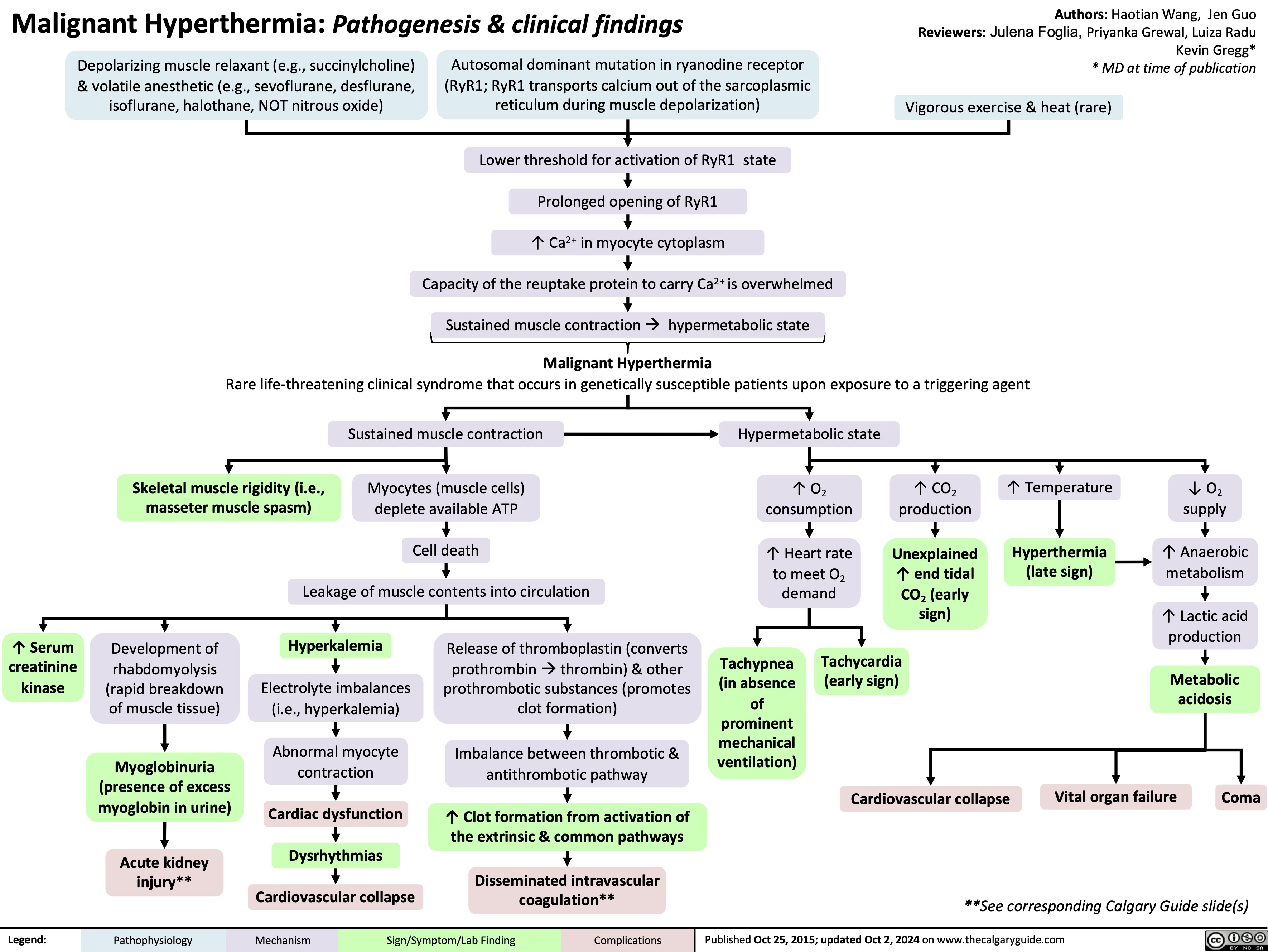
Malignant Hyperthermia
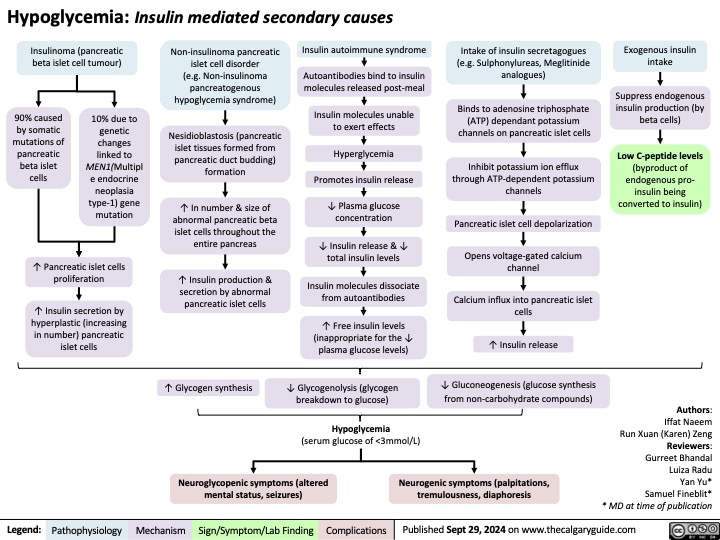
Secondary hypoglycemia Insulin Mediated
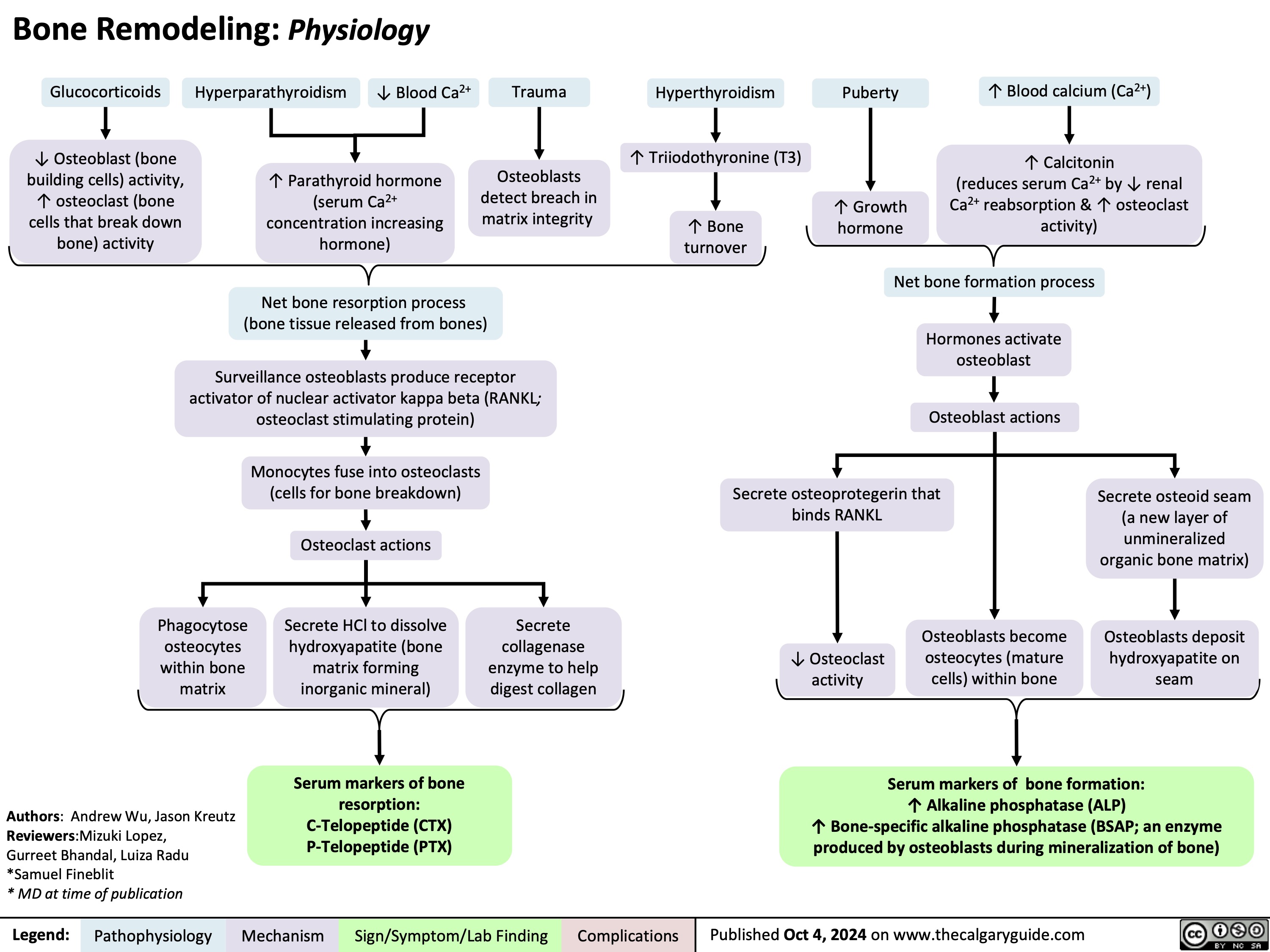
Bone Remodeling Physiology
Calcium Channel Blockers
![Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com
Calcium Channel Blockers: Mechanisms & side effects
Authors:
Caroline Kokorudz Reviewers:
Rafael Sanguinetti Andrew Wu
Luiza Radu
Timothy Pollak*
* MD at time of publication
Calcium channel blocker medications
inhibit Ca2+ channels in smooth muscle
Reduction of Ca2+ influx into smooth muscle cells
Inhibits calcium-dependent aldosterone synthesis reducing Na+ & H2O resorption in renal distal tubules
Negative feedback to pituitary gland causing ↑ ACTH (adrenocorticotropic hormone)
↑ Androgens (testosterone)
Testosterone acts on gingival cells (multiple cell types that support teeth) & connective tissue matrix
Gingival hyperplasia (gum overgrowth)
Non-dihydropyridines:
(Phenylalkylamines [verapamil], Benzothiazepines [diltiazem]) less potent vasodilators & selective for heart muscle
Prevents smooth muscle contraction
Dihydropyridines:
(amlodipine, felodipine, nifedipine) vasodilate vascular smooth muscle
↓ Arterial resistance and blood pressure in coronary & peripheral arteries
Coronary artery vasodilation
↓ Pressure in coronary arteries
↑ Blood flow through coronary arteries
Reduced
ischemia relieves angina
Inhibits L-type Ca2+ channels, preventing rapid nodal depolarization
Reduces excitation of sinoatrial (SA) & atrioventricular (AV) nodal tissues
↓ Conduction speed of electrical impulses
↓ Contractile strength of cardiomyocytes (heart muscle cell)
↓ Systemic vascular resistance & cardiac
afterload (heart pumping resistance)
↑ Blood volume flowing into significantly smaller vessels
↑ Capillary blood pressure
↑ Circulation to face
Flushes (red & warm)
↓ Cardiac output
↓ Tissue perfusion & attempt to ↑ cardiac output
Worsens heart failure
↓ Oxygen demand of heart muscle
More favorable oxygen supply to demand ratio
Relieves angina
↓ Blood pressure
↓ Cerebral perfusion
Syncope (fainting)
Relieves angina
Capillary fluid leak increased to interstitial space
Peripheral edema
↑ Intracranial pressure Compresses nerve endings Headache
↓ Heart rate Bradycardia
Suppresses dysrhythmias (abnormal heart rhythm)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Nov 21, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/11/Calcium-Channel-Blockers.jpg)
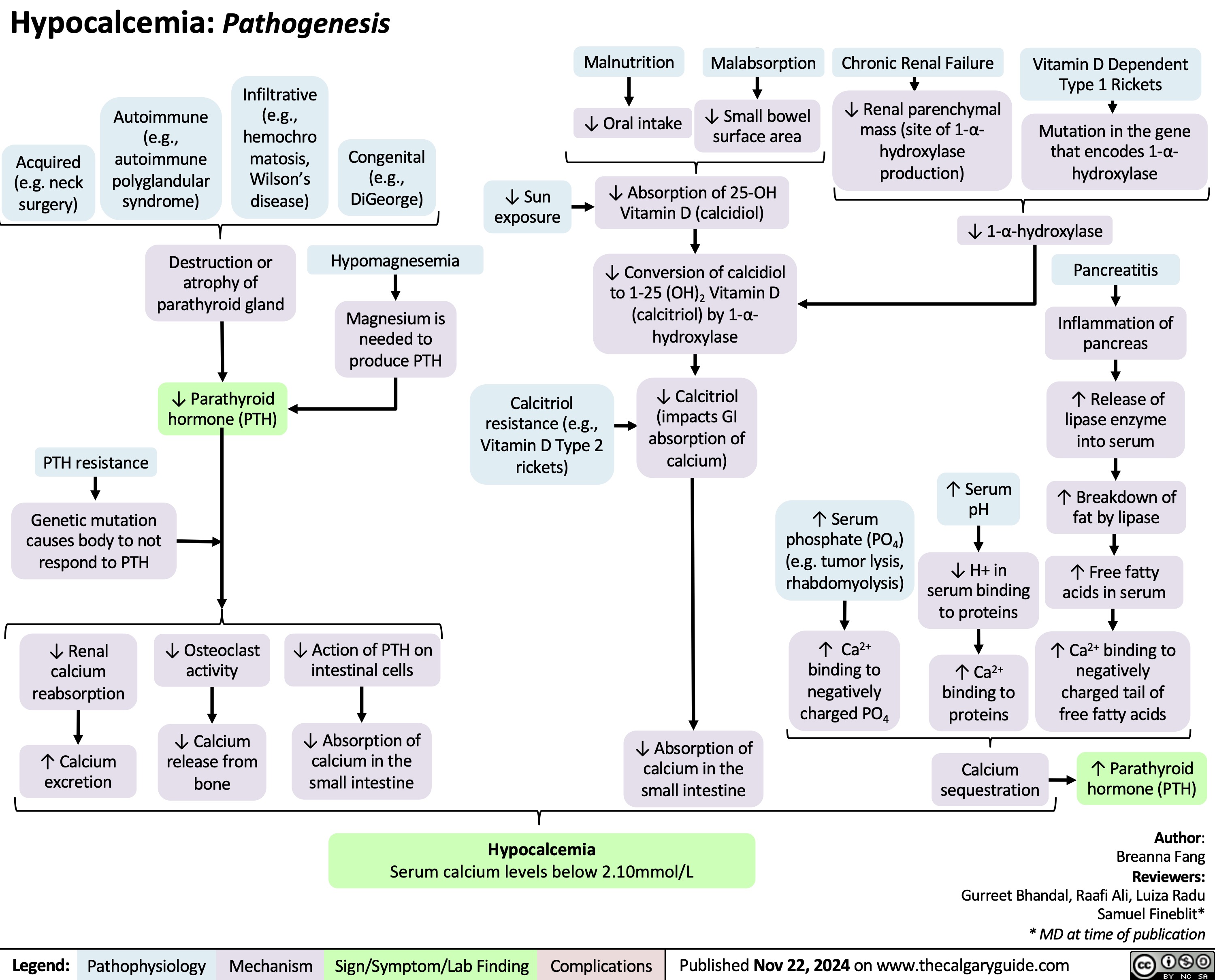
Hypocalcemia Pathogenesis
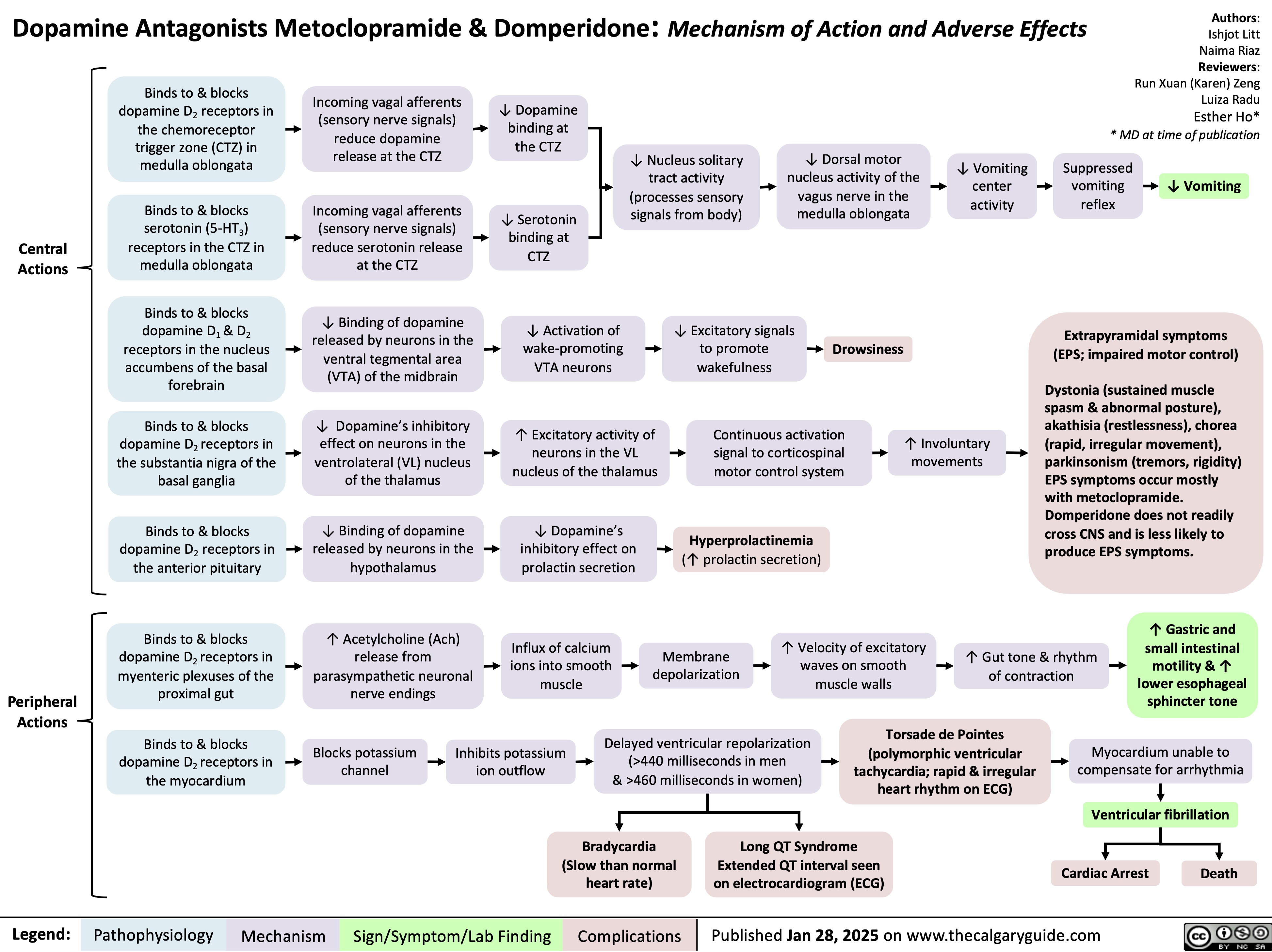
Dopamine Antagonists Metoclopramide & Domperidone
Osgood Schlatter Disease