SEARCH RESULTS FOR: Inflammation
Myocardial Infarction: Findings on History
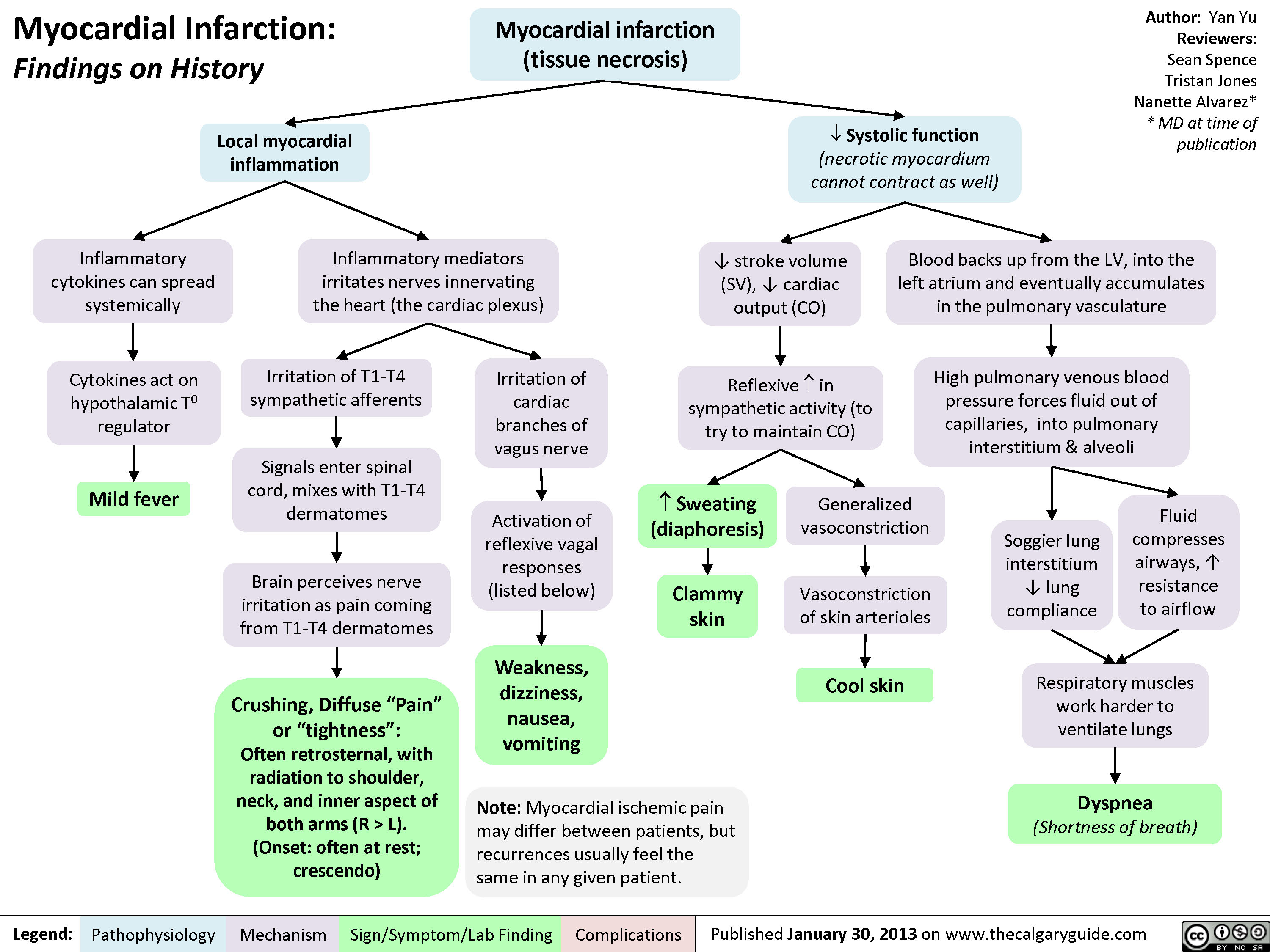 L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" title="Yu Yan - MI Findings on History - FINAL.pptx -
Myocardial Infarction: Findings on HistoryLegend:Published January 30, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsAuthor: Yan YuReviewers:Sean SpenceTristan JonesNanette Alvarez** MD at time of publication Systolic function(necrotic myocardium cannot contract as well)Reflexive ? in sympathetic activity (to try to maintain CO)Clammy skin? stroke volume (SV), ? cardiac output (CO)Myocardial infarction (tissue necrosis)Note: Myocardial ischemic pain may differ between patients, but recurrences usually feel the same in any given patient.Generalized vasoconstrictionVasoconstriction of skin arteriolesCool skinLocal myocardial inflammationIrritation of T1-T4 sympathetic afferentsIrritation of cardiac branches of vagus nerveSignals enter spinal cord, mixes with T1-T4 dermatomesCrushing, Diffuse "Pain" or "tightness": Often retrosternal, with radiation to shoulder, neck, and inner aspect of both arms (R > L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" />
L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" title="Yu Yan - MI Findings on History - FINAL.pptx -
Myocardial Infarction: Findings on HistoryLegend:Published January 30, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsAuthor: Yan YuReviewers:Sean SpenceTristan JonesNanette Alvarez** MD at time of publication Systolic function(necrotic myocardium cannot contract as well)Reflexive ? in sympathetic activity (to try to maintain CO)Clammy skin? stroke volume (SV), ? cardiac output (CO)Myocardial infarction (tissue necrosis)Note: Myocardial ischemic pain may differ between patients, but recurrences usually feel the same in any given patient.Generalized vasoconstrictionVasoconstriction of skin arteriolesCool skinLocal myocardial inflammationIrritation of T1-T4 sympathetic afferentsIrritation of cardiac branches of vagus nerveSignals enter spinal cord, mixes with T1-T4 dermatomesCrushing, Diffuse "Pain" or "tightness": Often retrosternal, with radiation to shoulder, neck, and inner aspect of both arms (R > L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" />
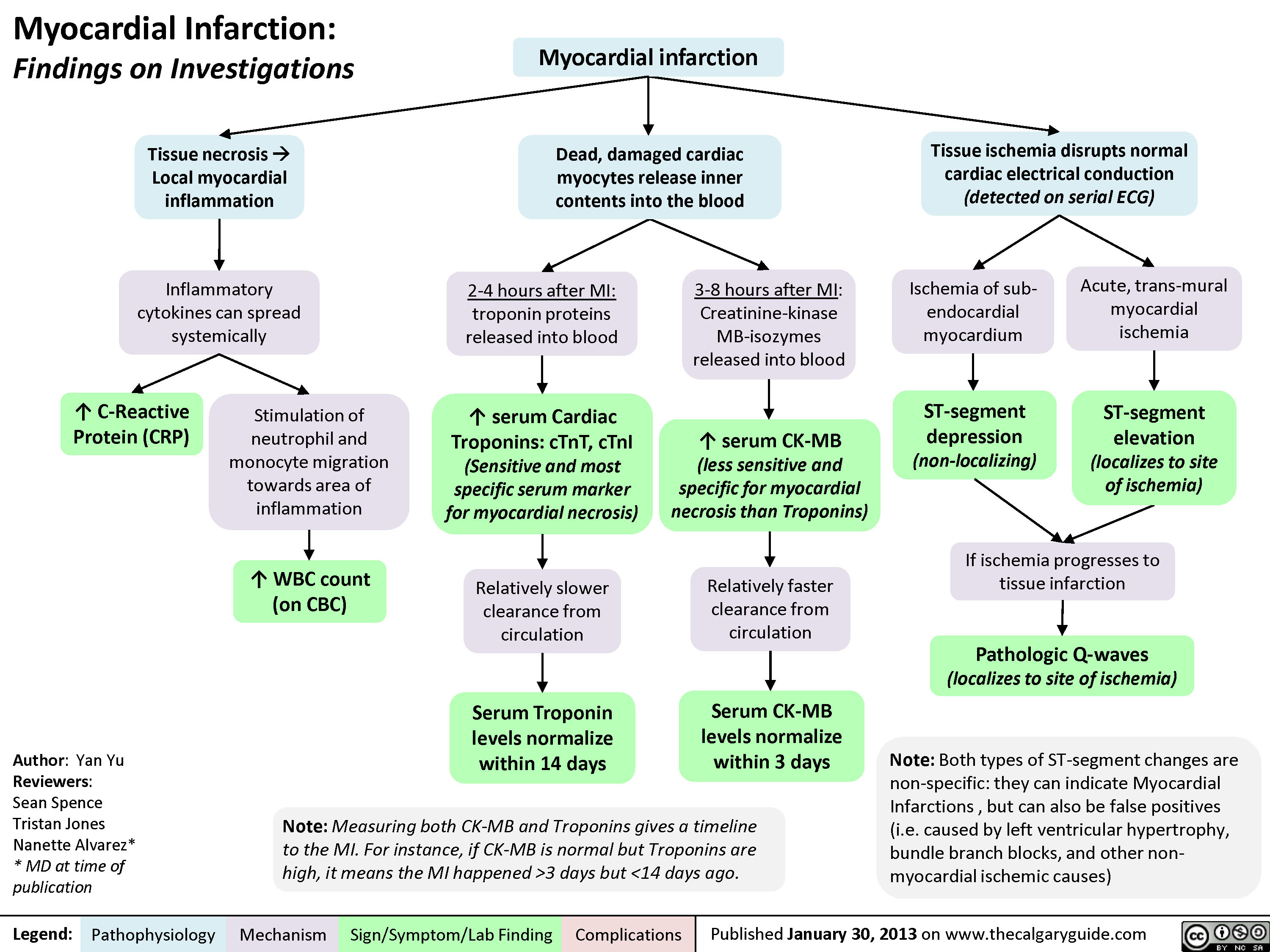
myocardial-infarction-findings-on-investigations
systemic-lupus-erythematosus-gastrointestinal-manifestations

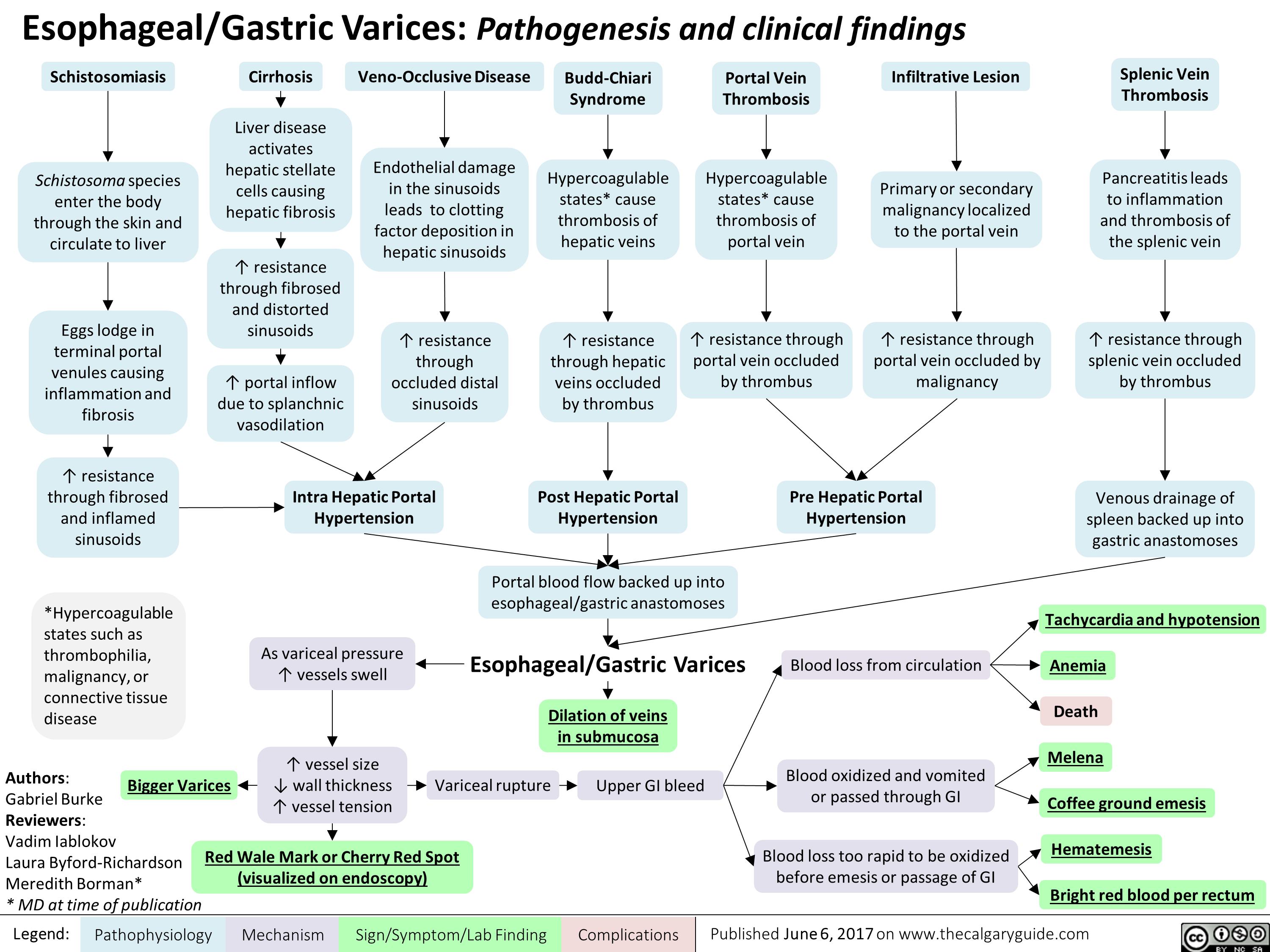
esophageal-gastric-varices
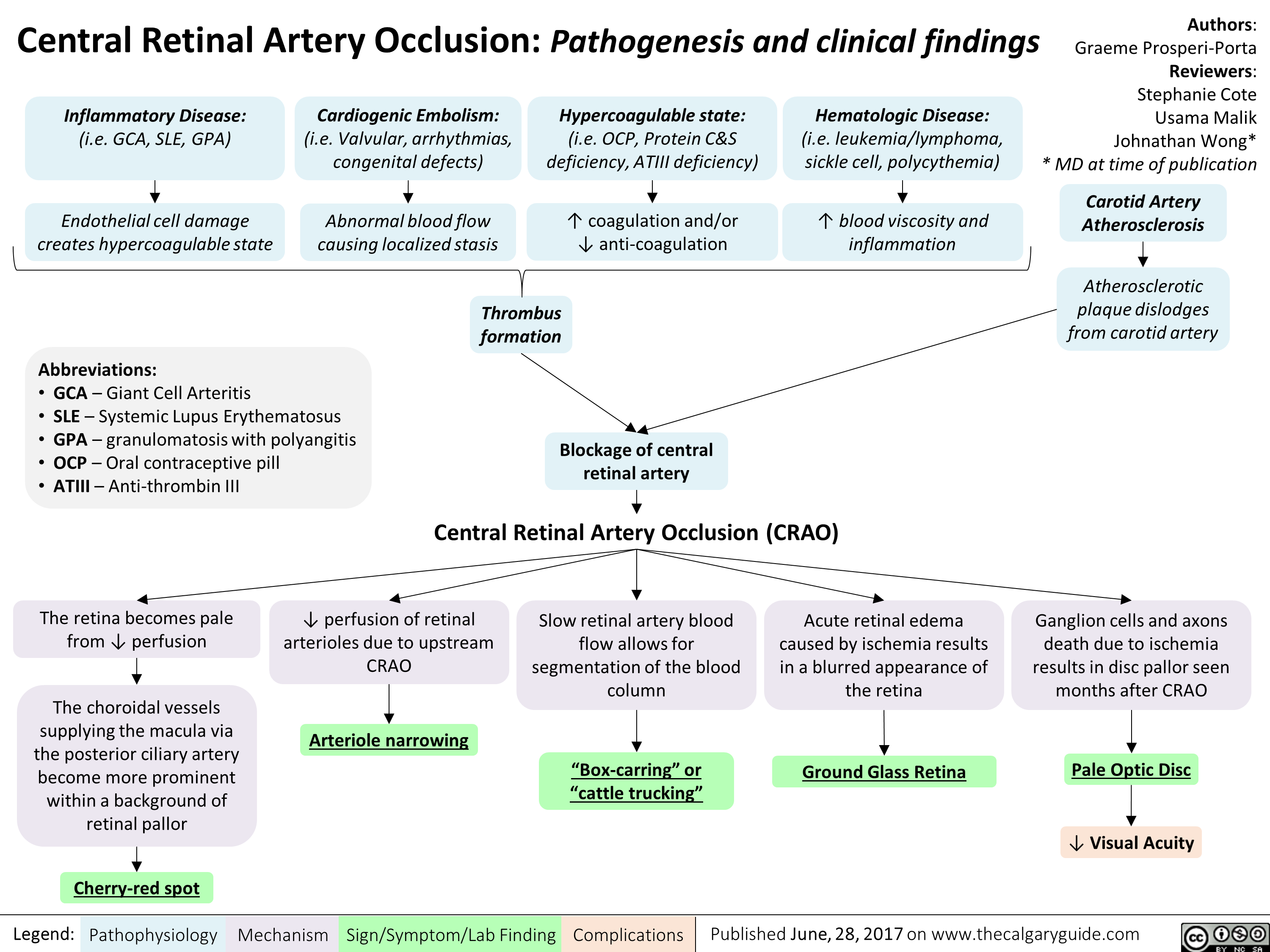
central-retinal-artery-occlusion-pathogenesis-and-clinical-findings
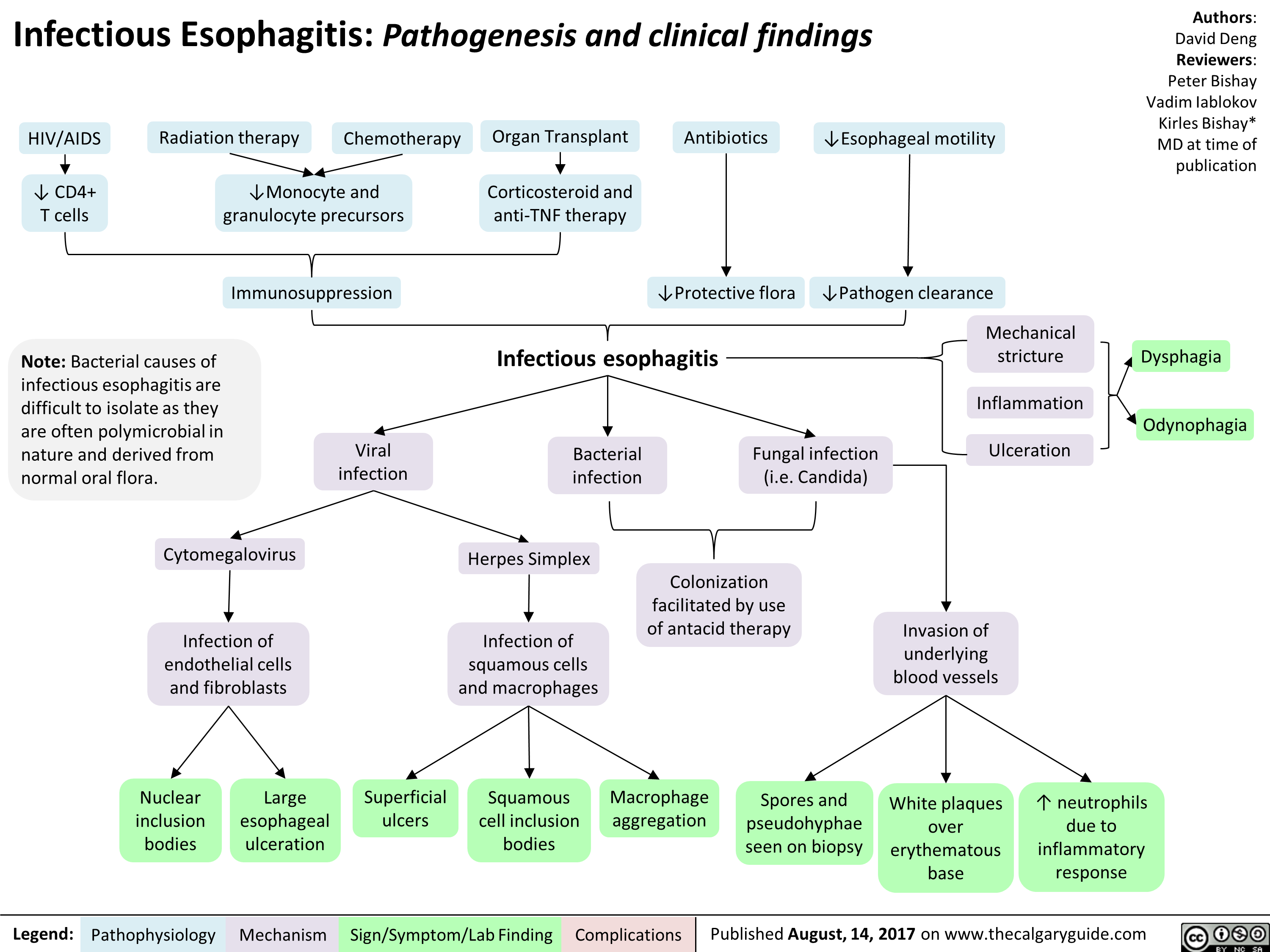
infectious-esophagitis-pathogenesis-and-clinical-findings
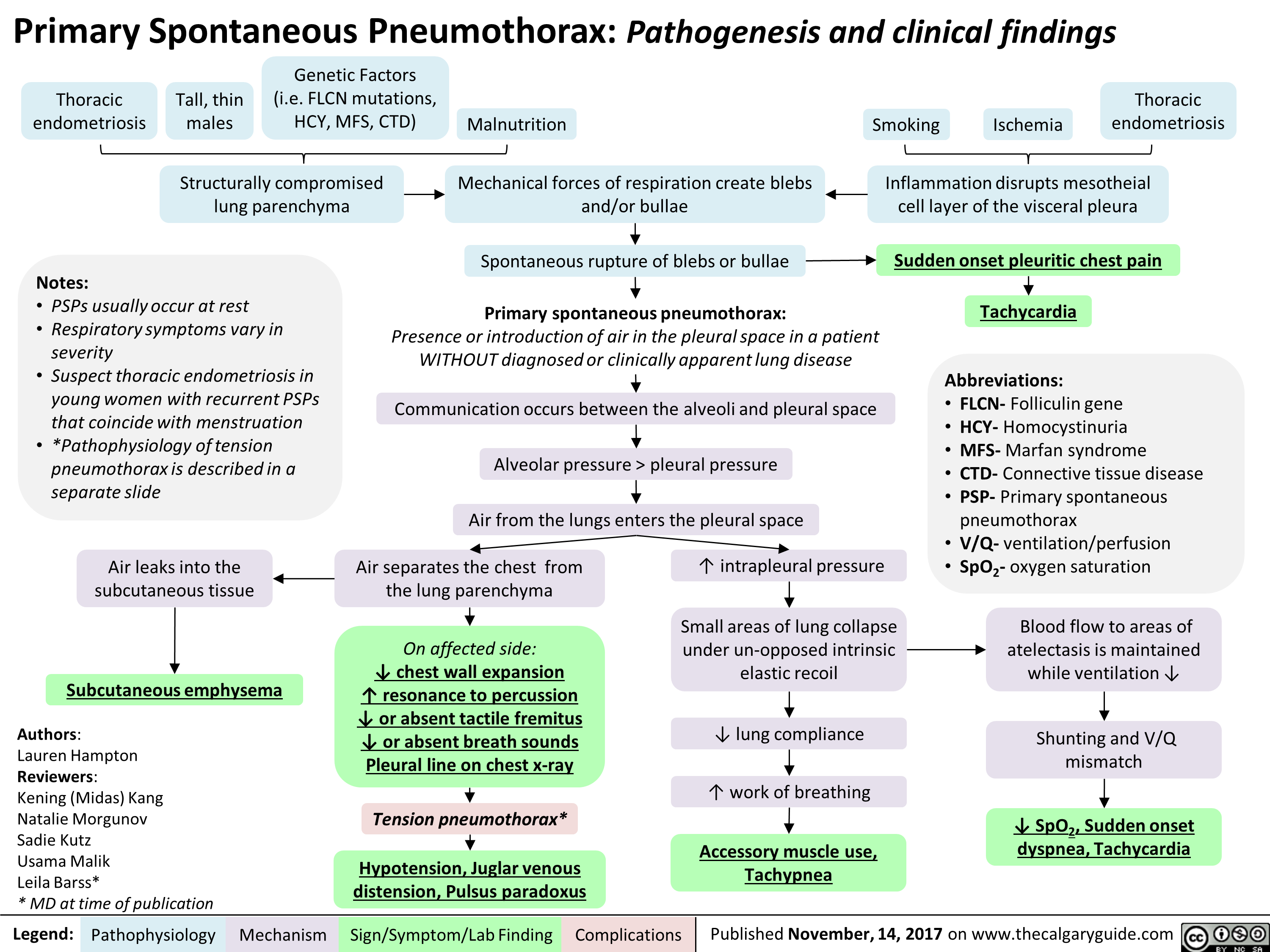
Primary Spontaneous Pneumothorax: Pathogenesis and clinical findings
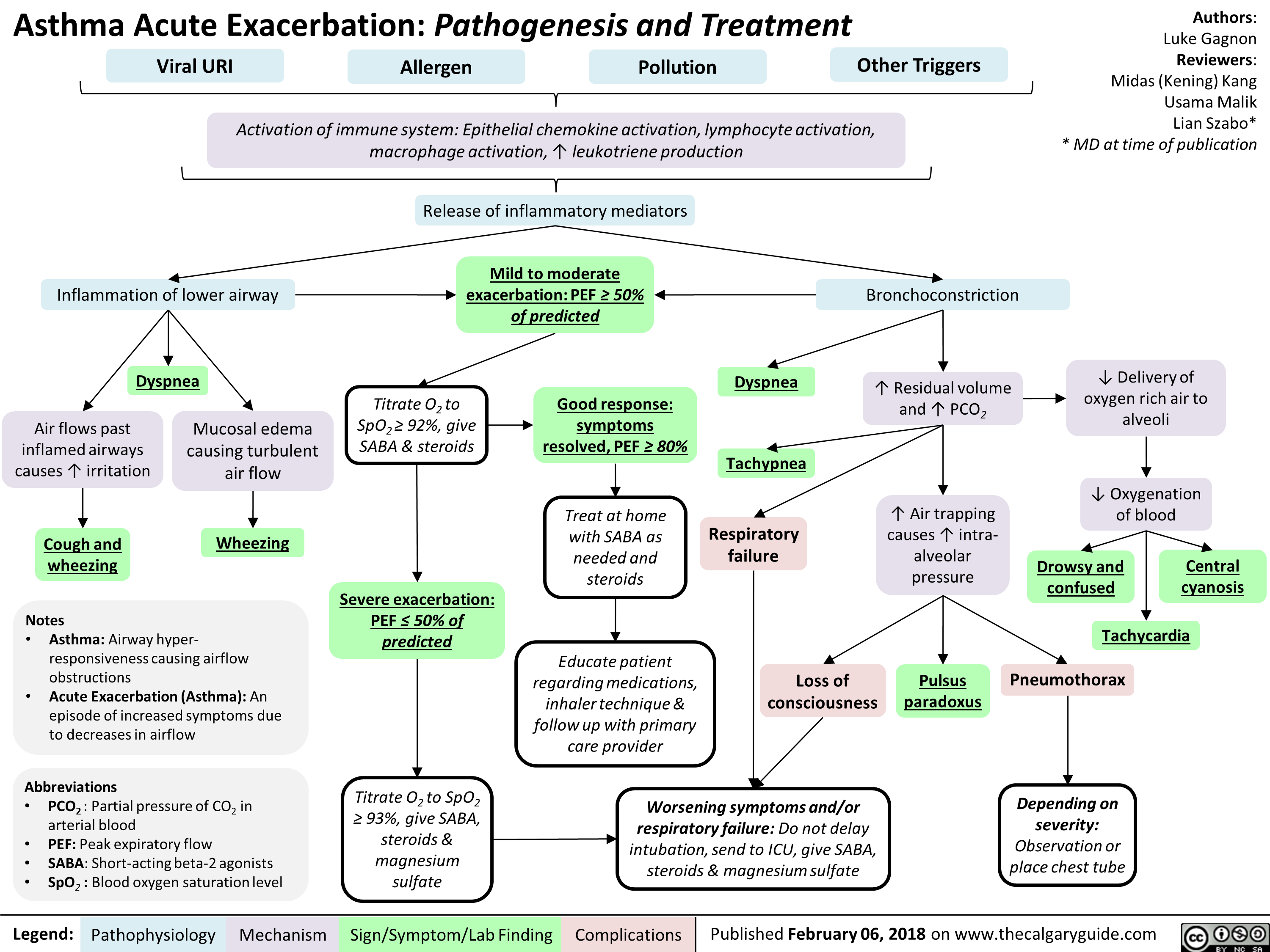
Asthma Acute Exacerbation: Pathogenesis and Treatment
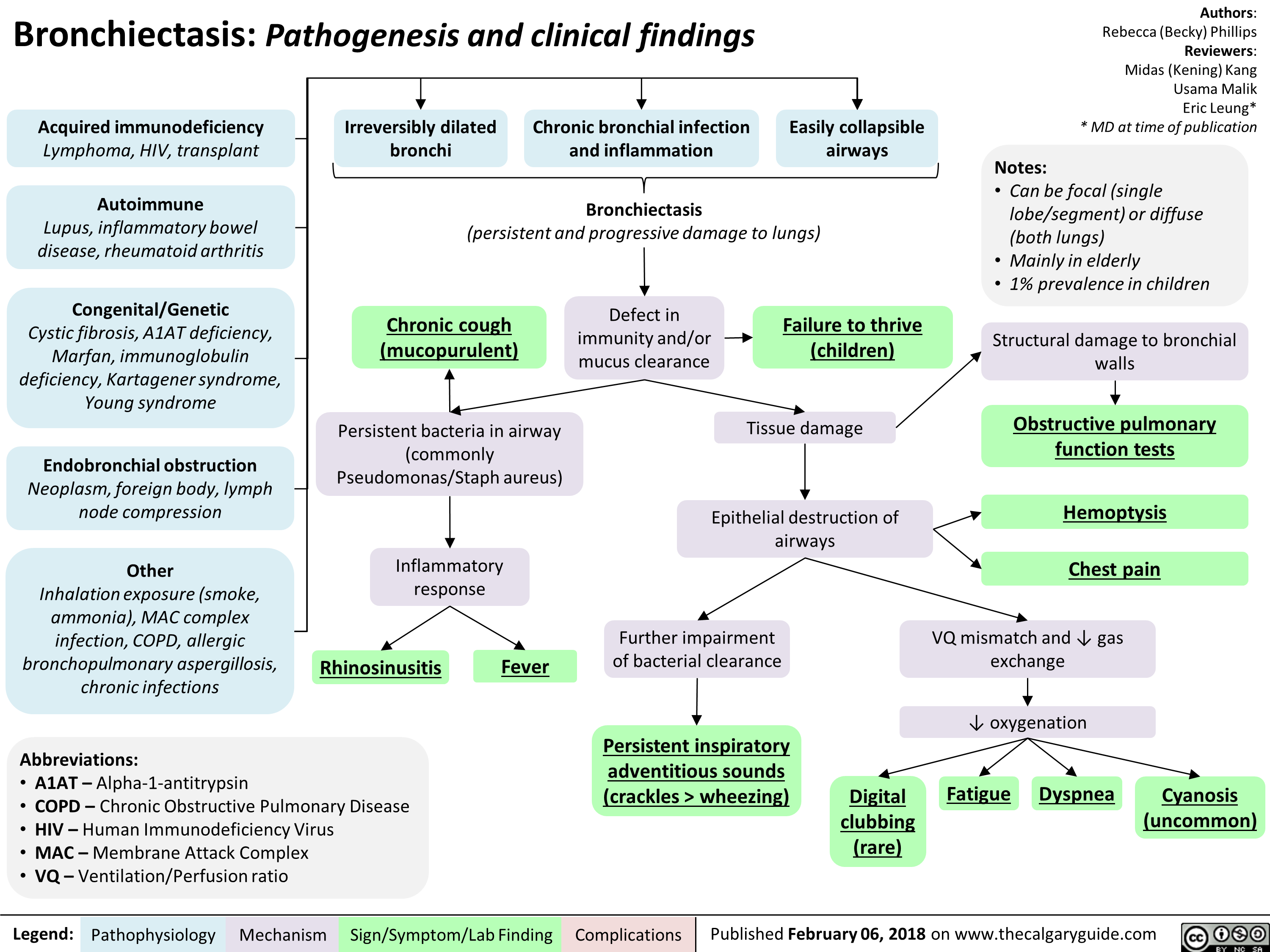
Bronchiectasis Pathogenesis and clinical findings
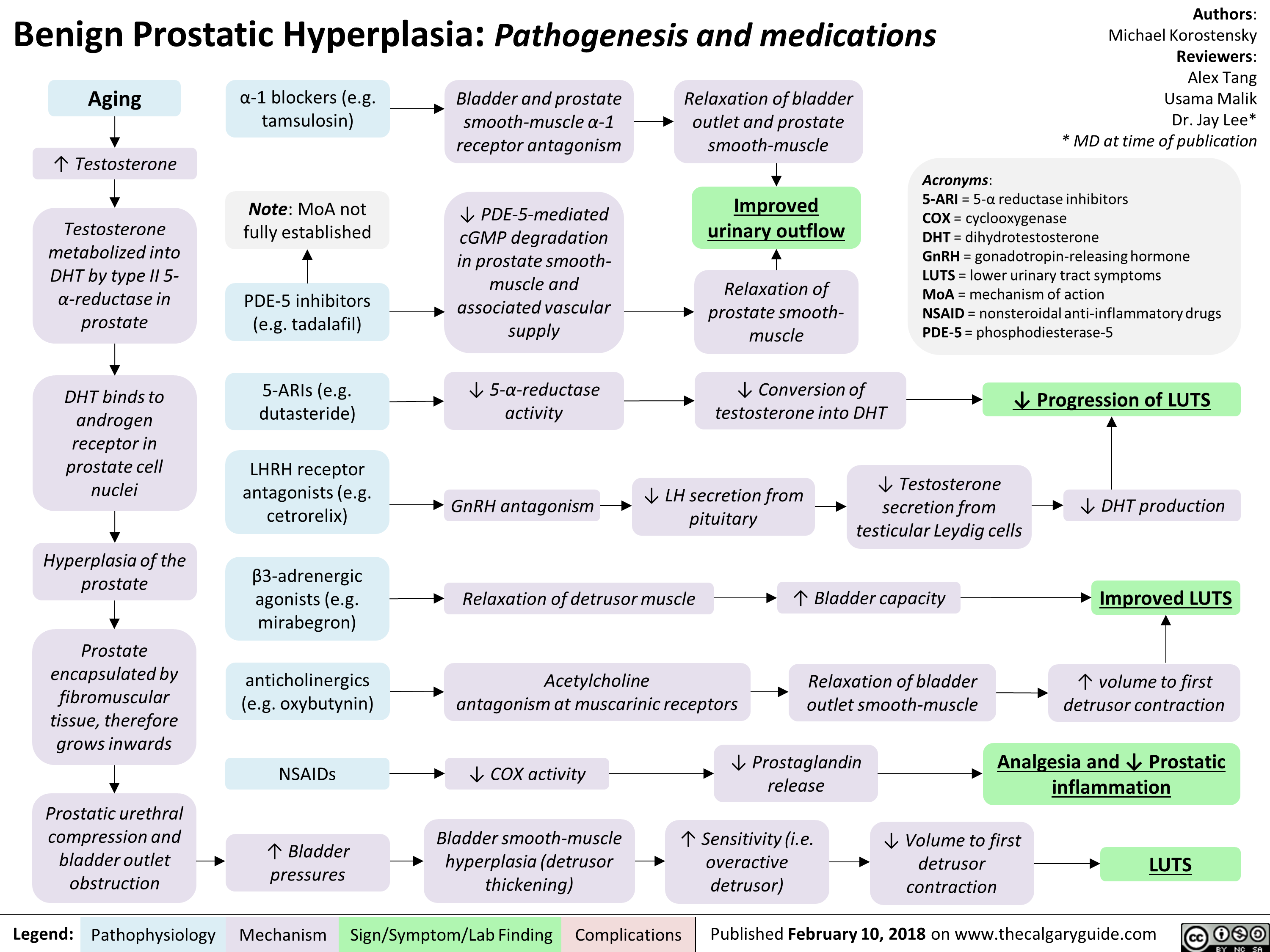
Benign Prostatic Hyperplasia: Pathogenesis and medications
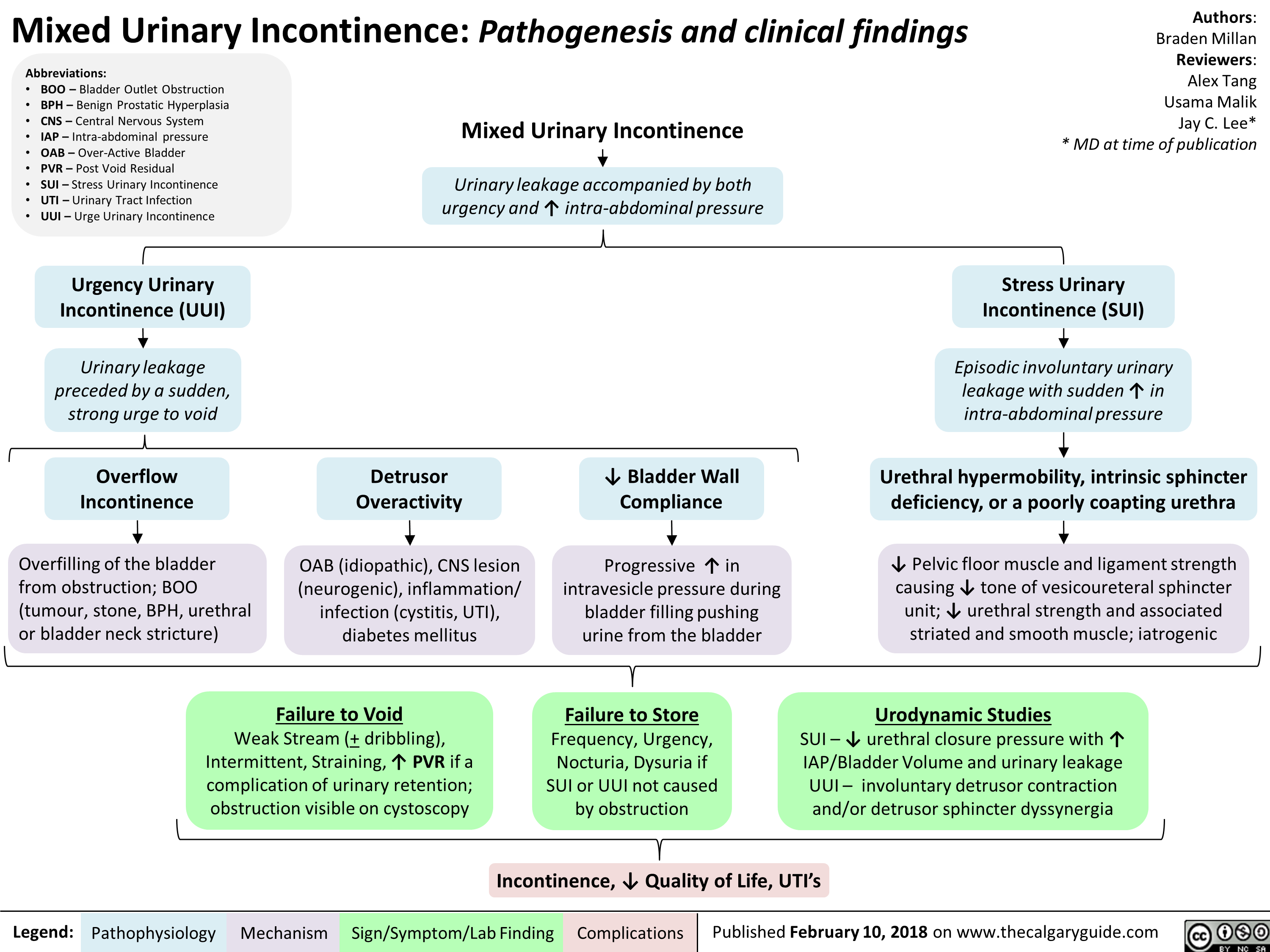
Mixed Urinary Incontinence Pathogenesis and clinical findings
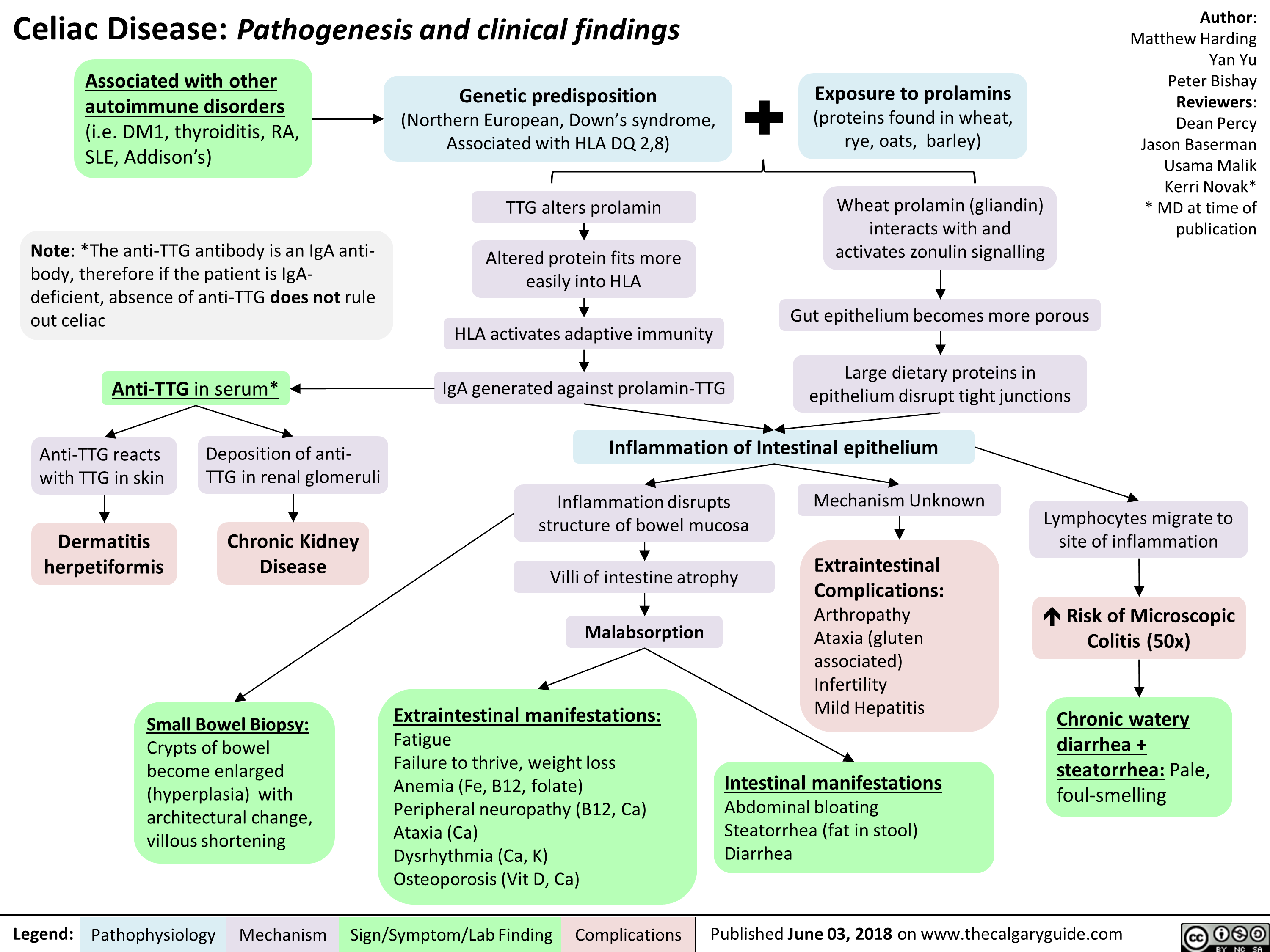
Celiac Disease: Pathogenesis and clinical findings
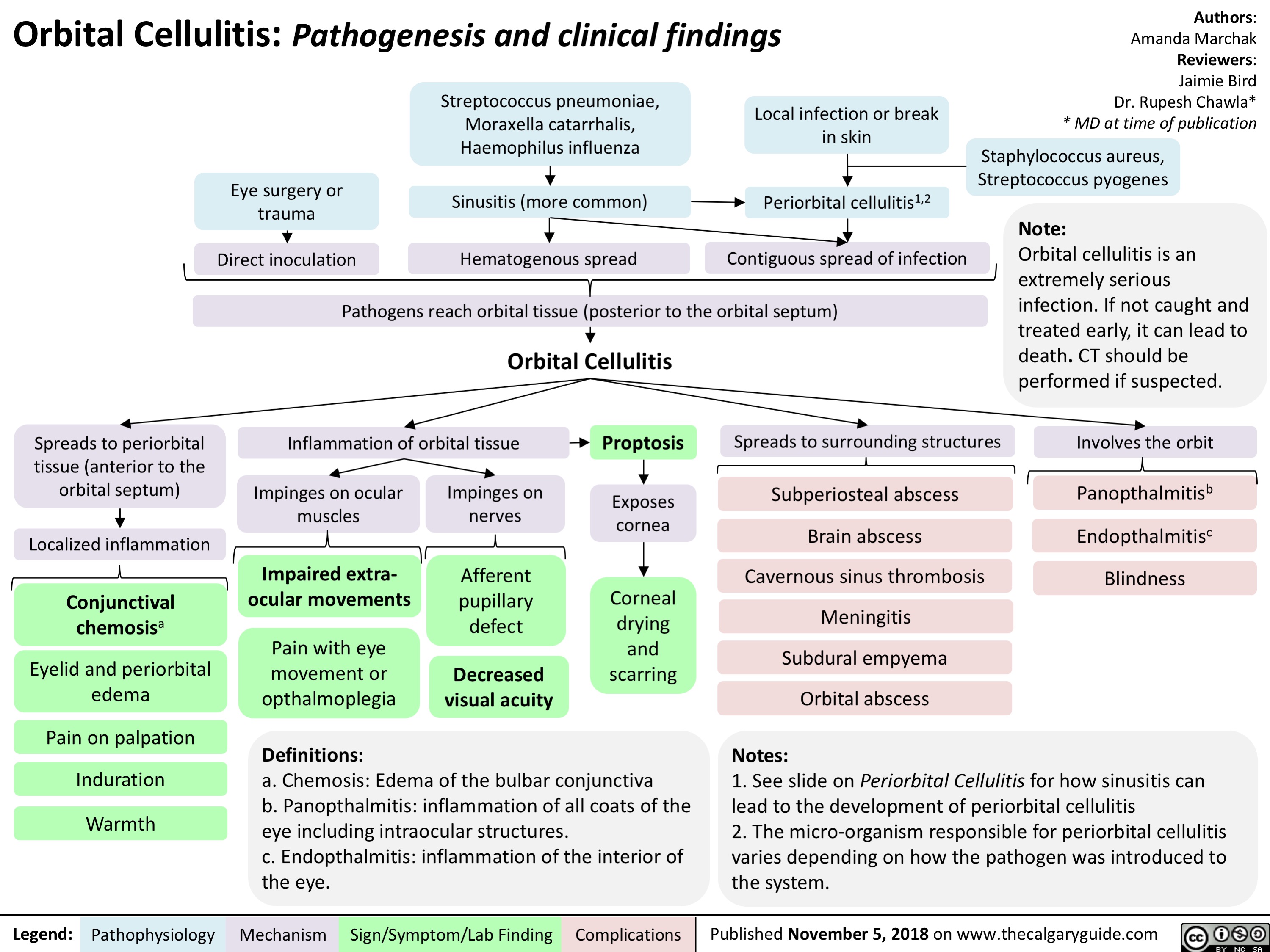
Orbital Cellulitis: Pathogenesis and clinical findings
Periorbital Cellulitis: Pathogenesis and Clinical Findings
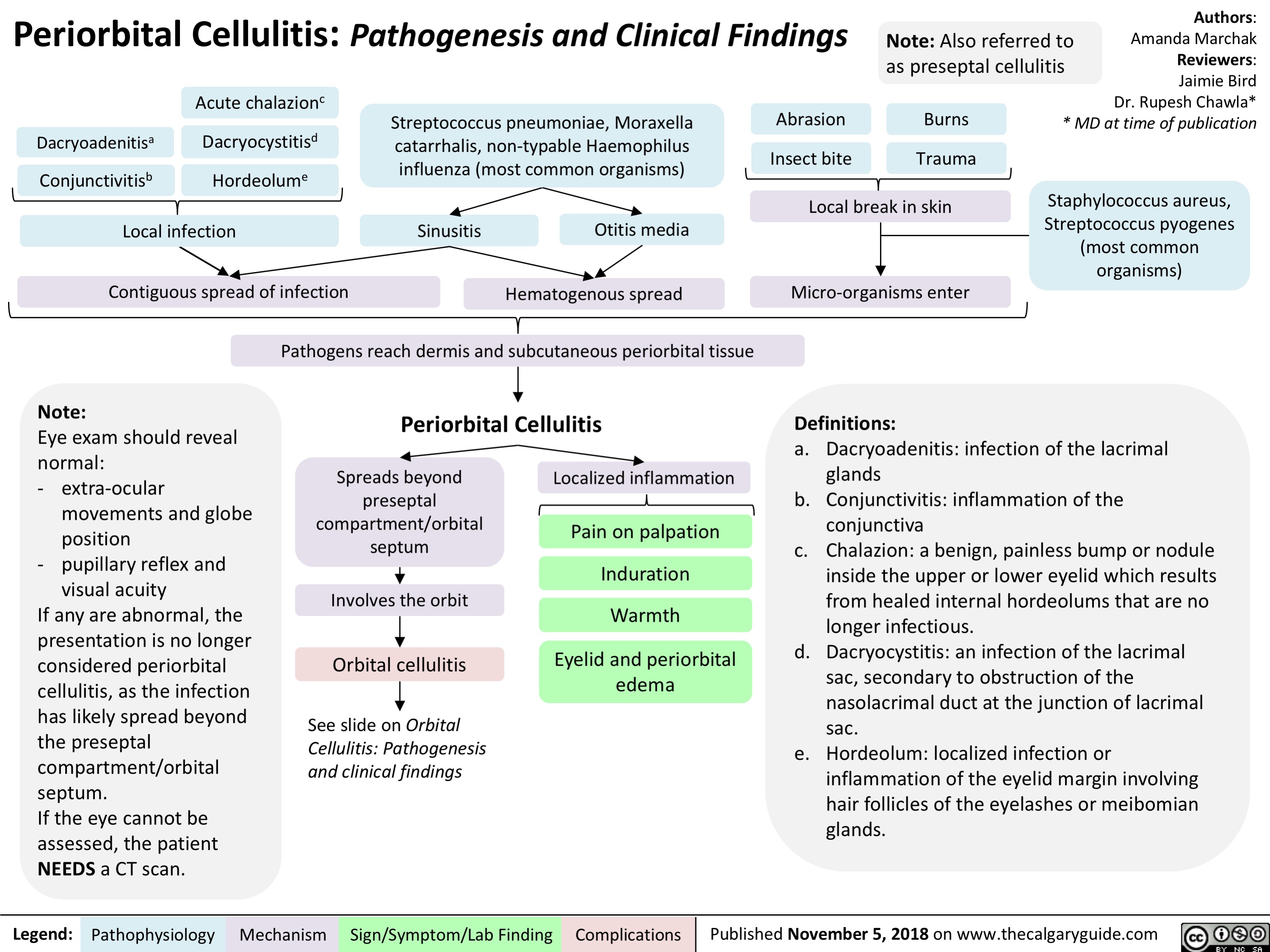
Mastoiditis: Pathogenesis and clinical findings
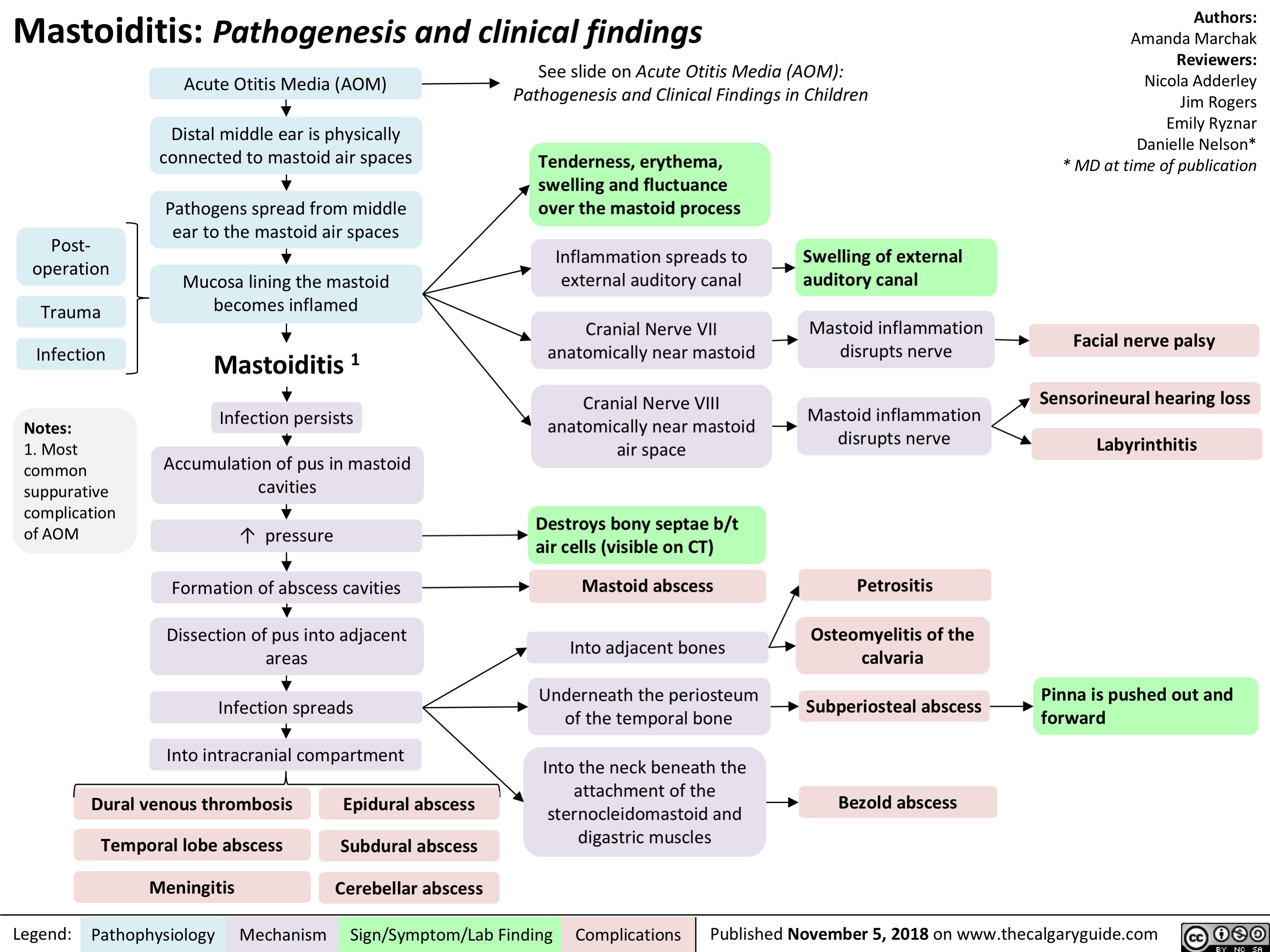
Sinusitis: Pathogenesis and clinical findings
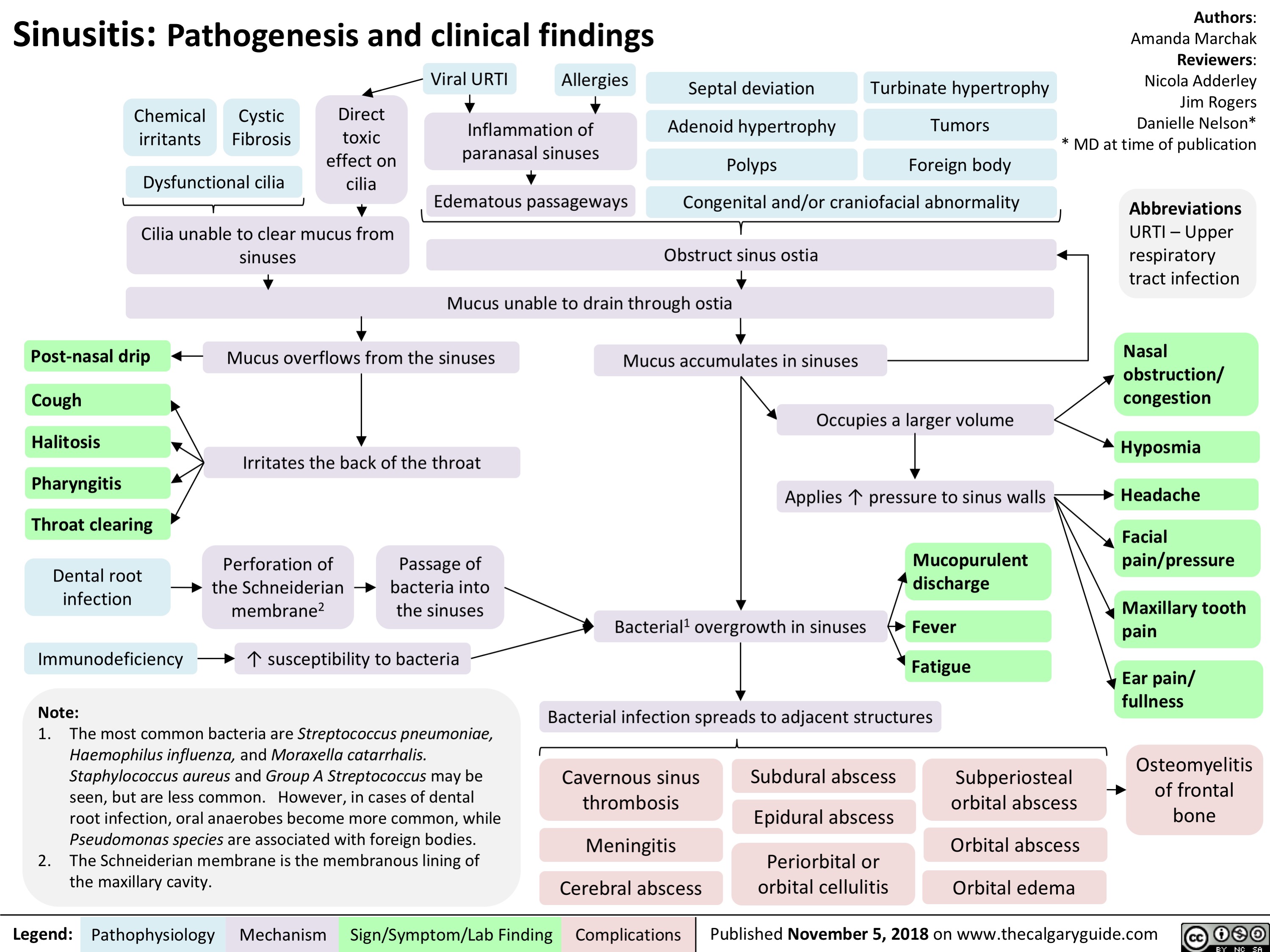
Boutonniere Deformity: Pathogenesis and Complications
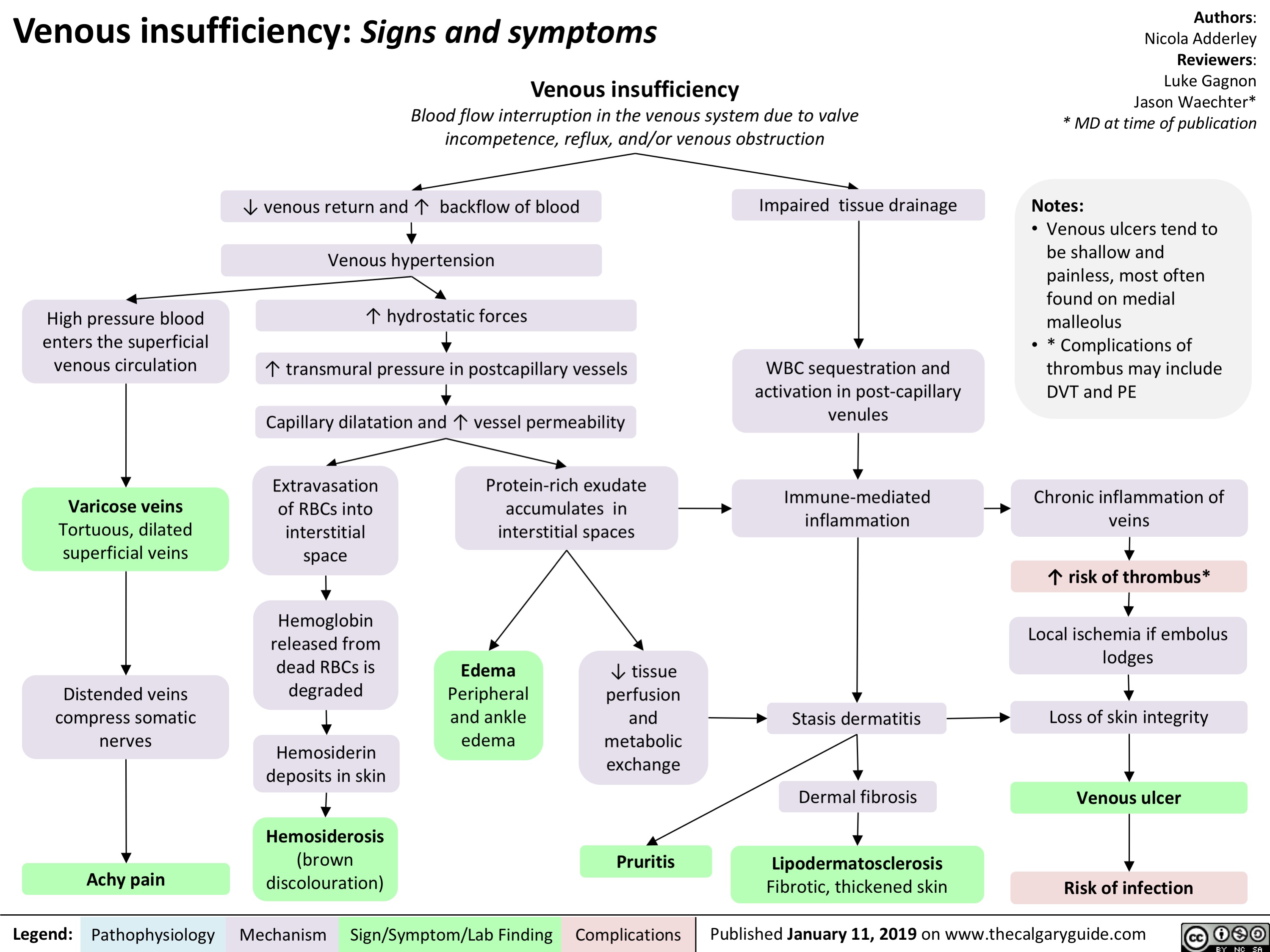
Venous insufficiency- Signs and symptoms
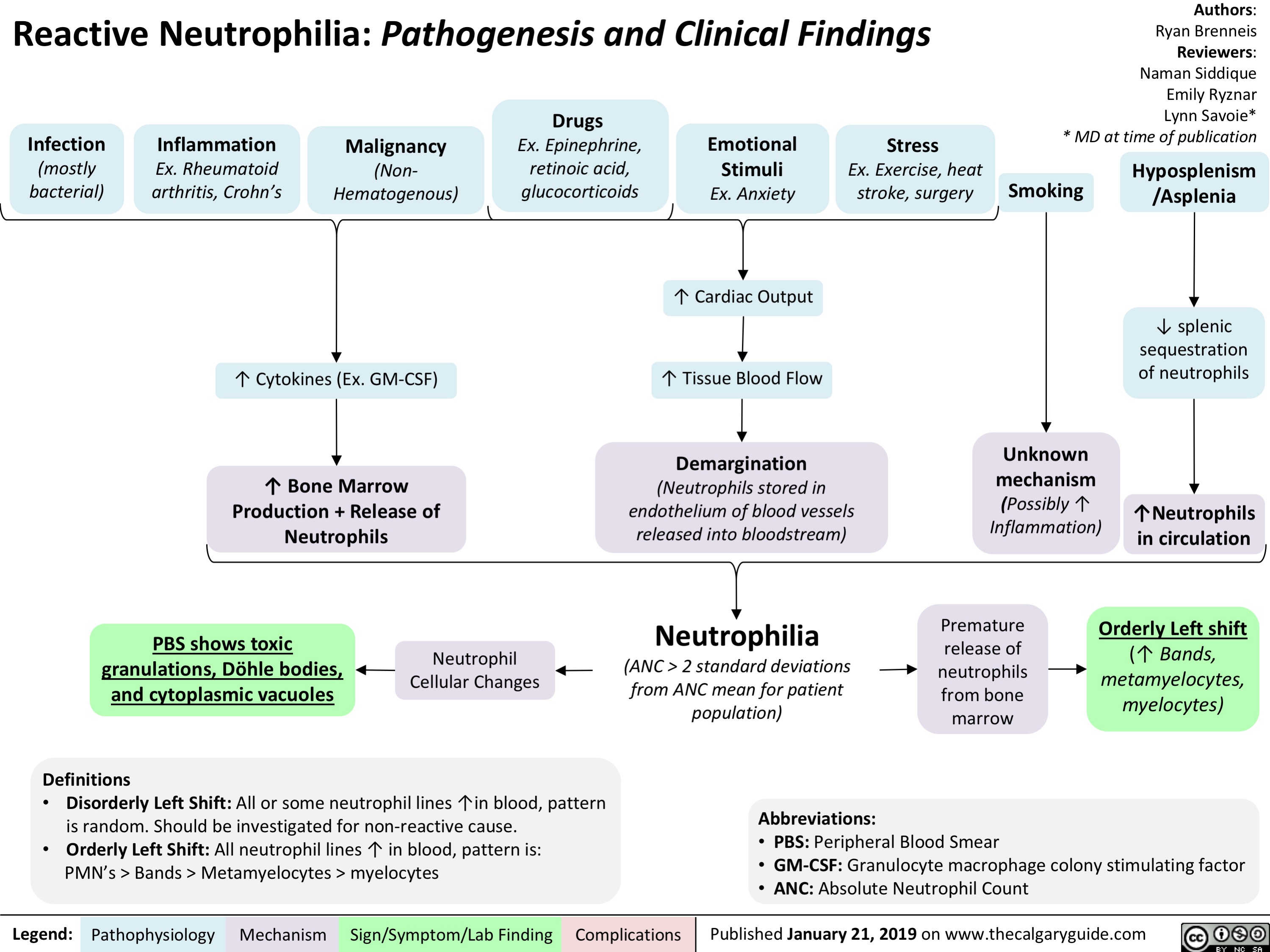
Reactive Neutrophilia- Pathogenesis and Clinical Findings
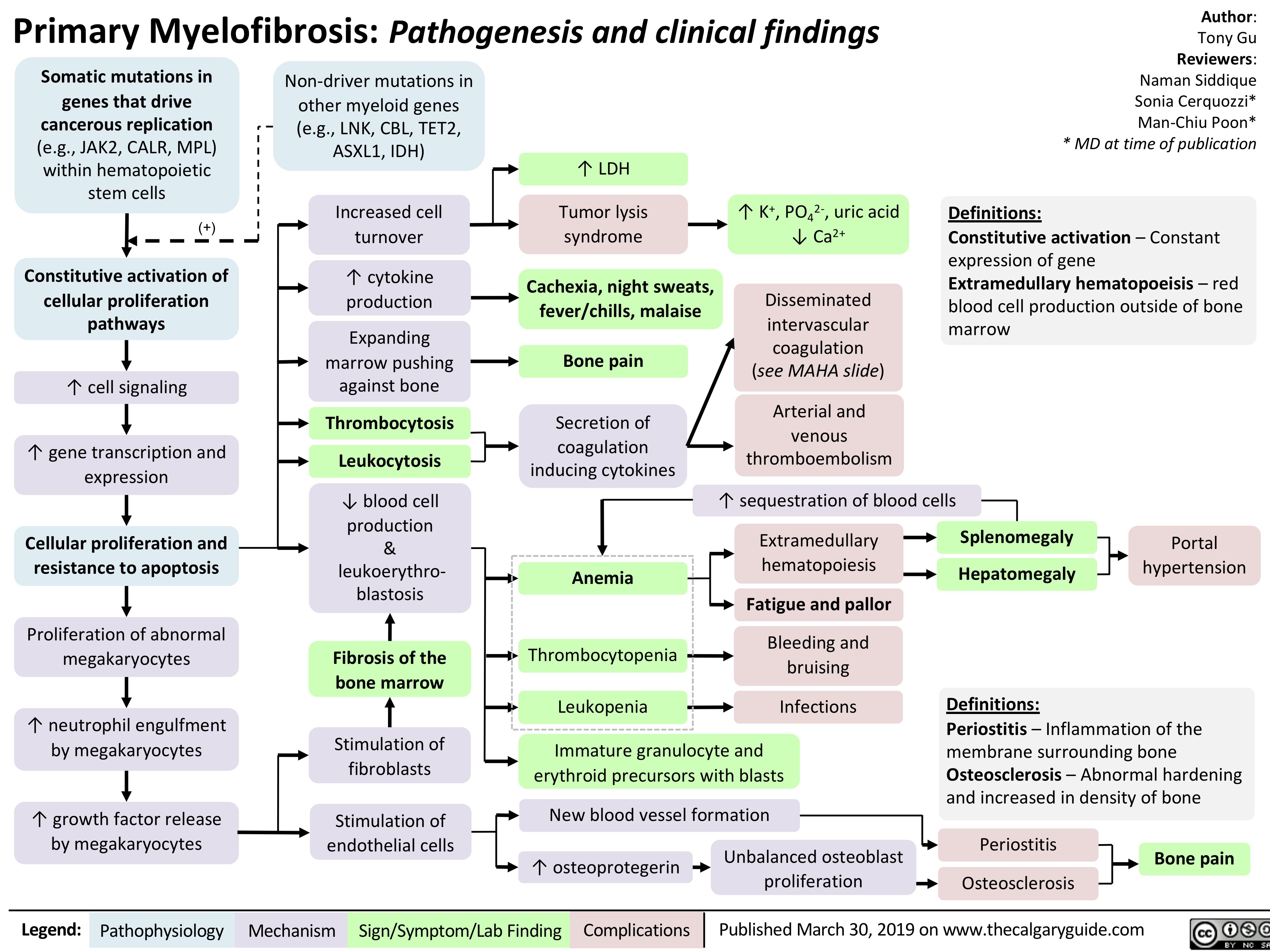
Primary Myelofibrosis pathogenesis and clinical findings
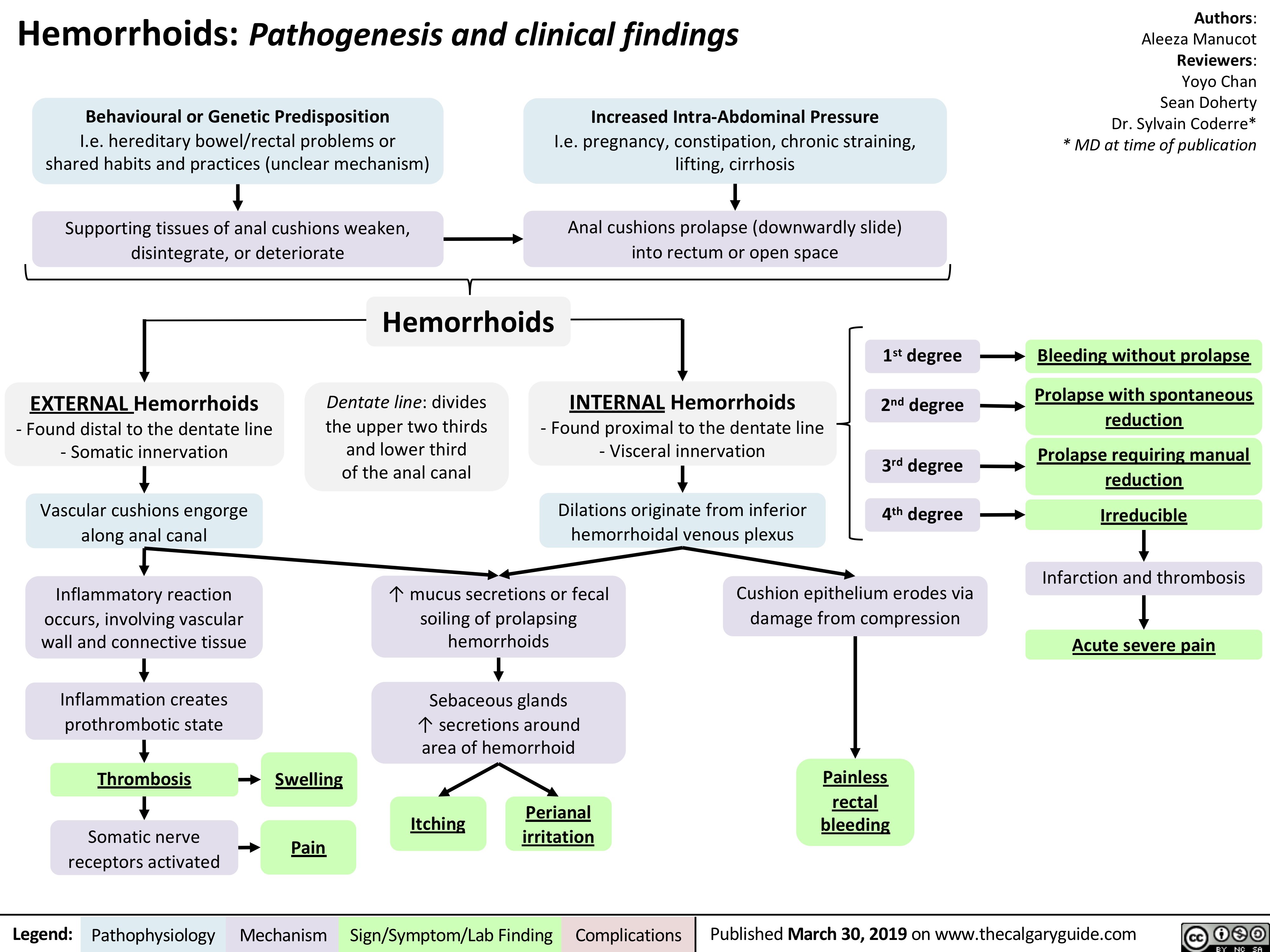
Hemorrhoids - Pathogenesis and Clinical Findings
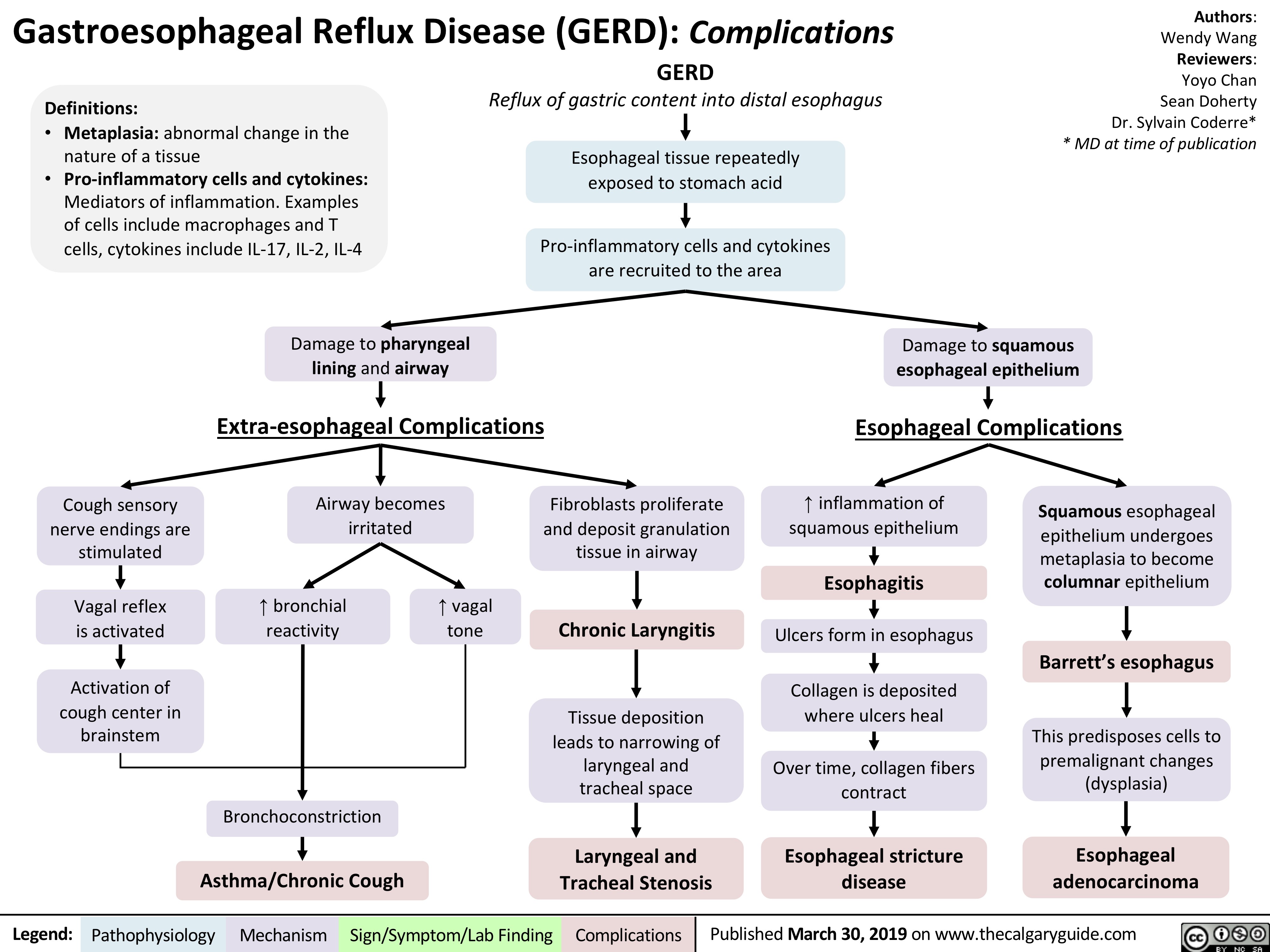
gastroesophageal-reflux-disease-gerd-complications
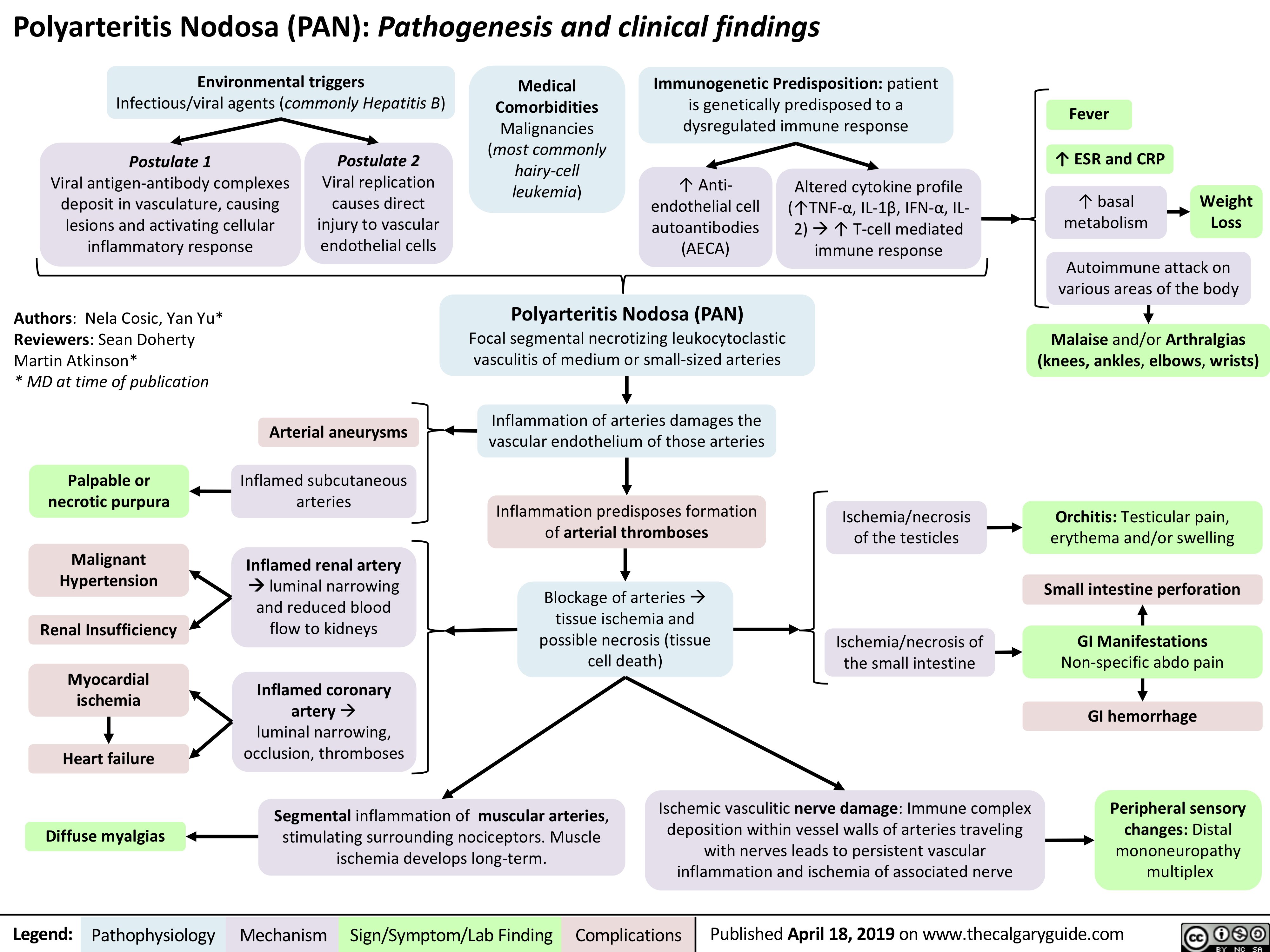
Polyarteritis Nodosa (PAN): Pathogenesis and Clinical Findings
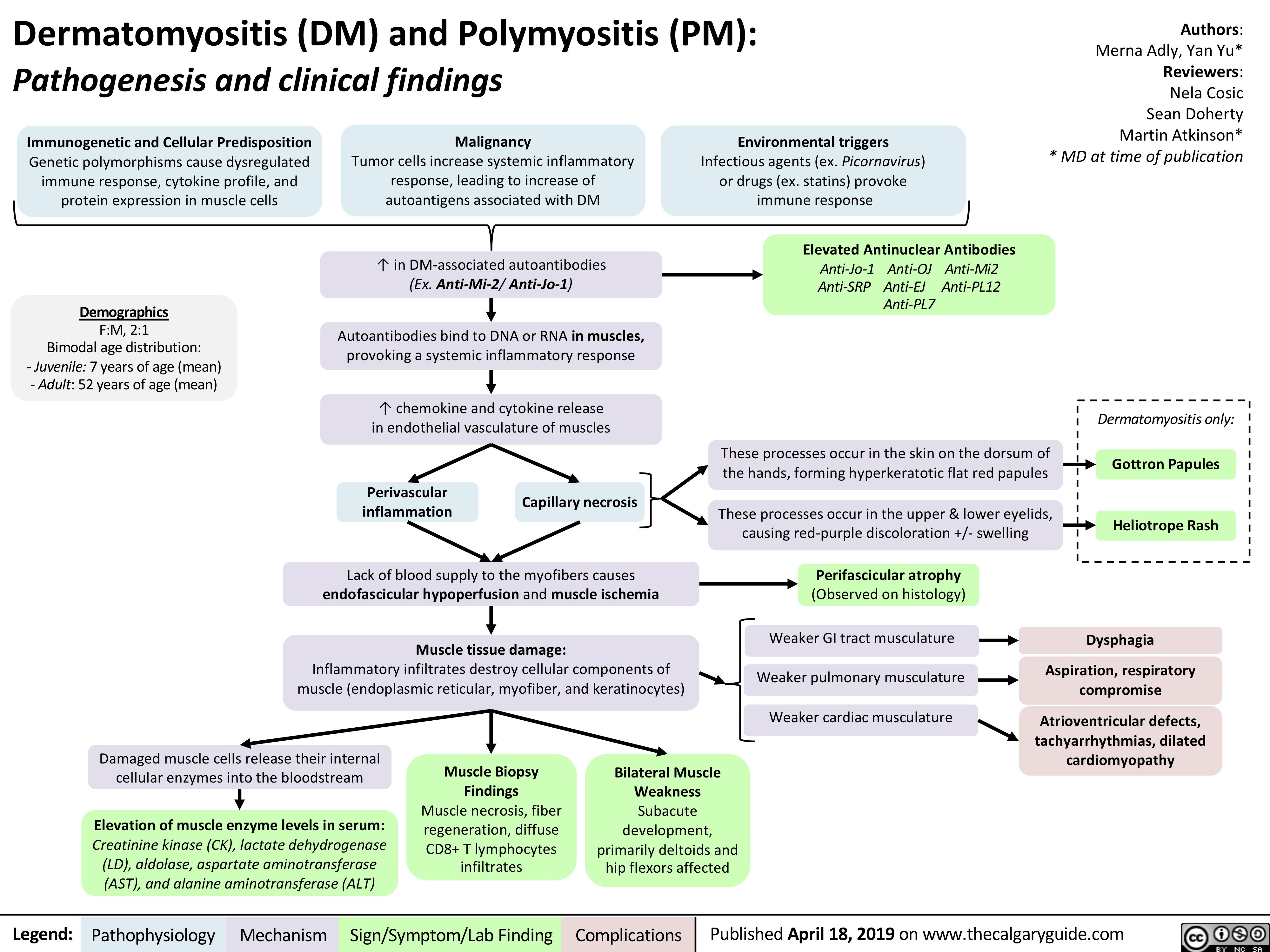
dermatomyositis-dm-and-polymyositis-pm-pathogenesis-and-clinical-findings
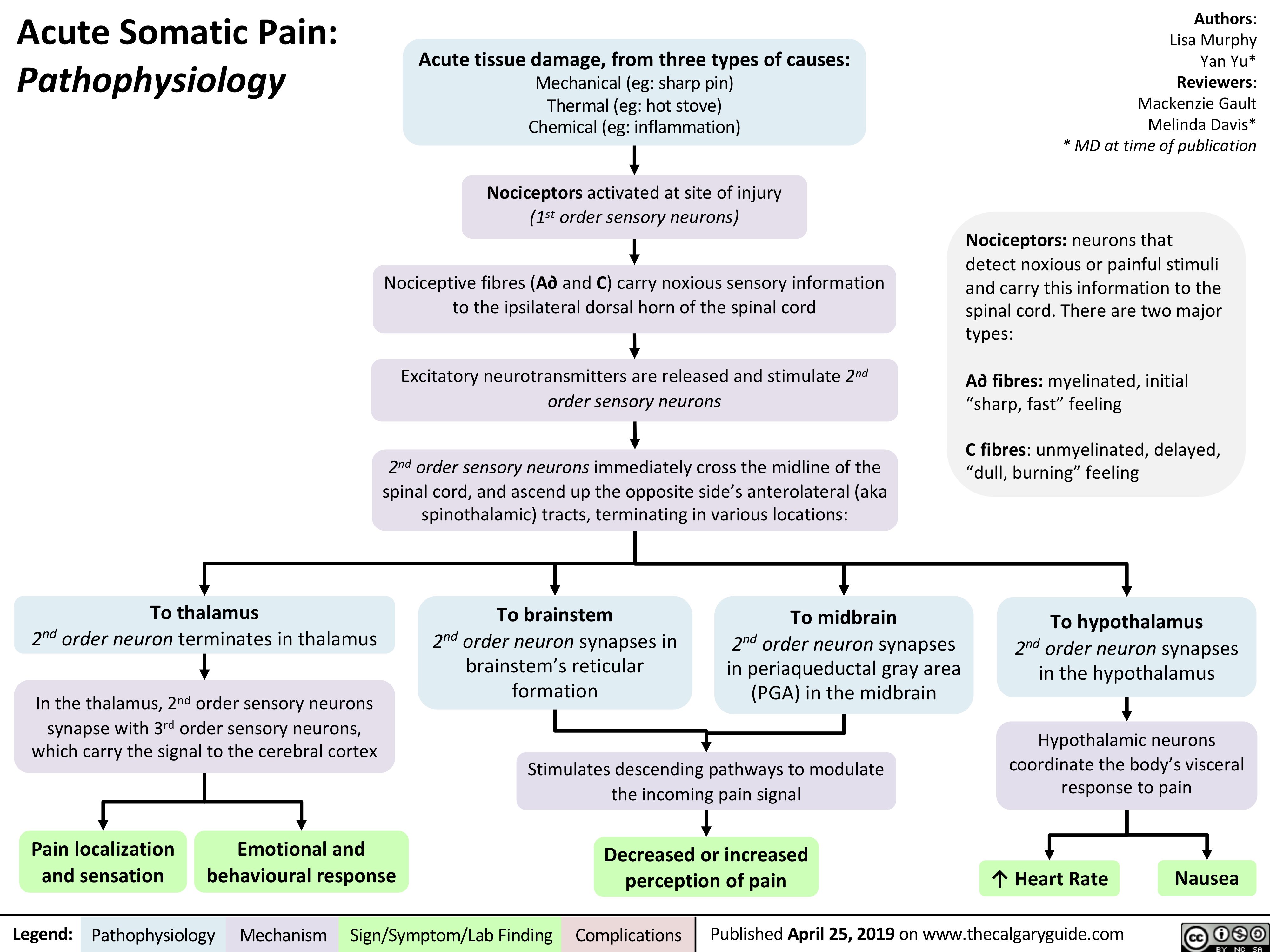
acute-somatic-pain
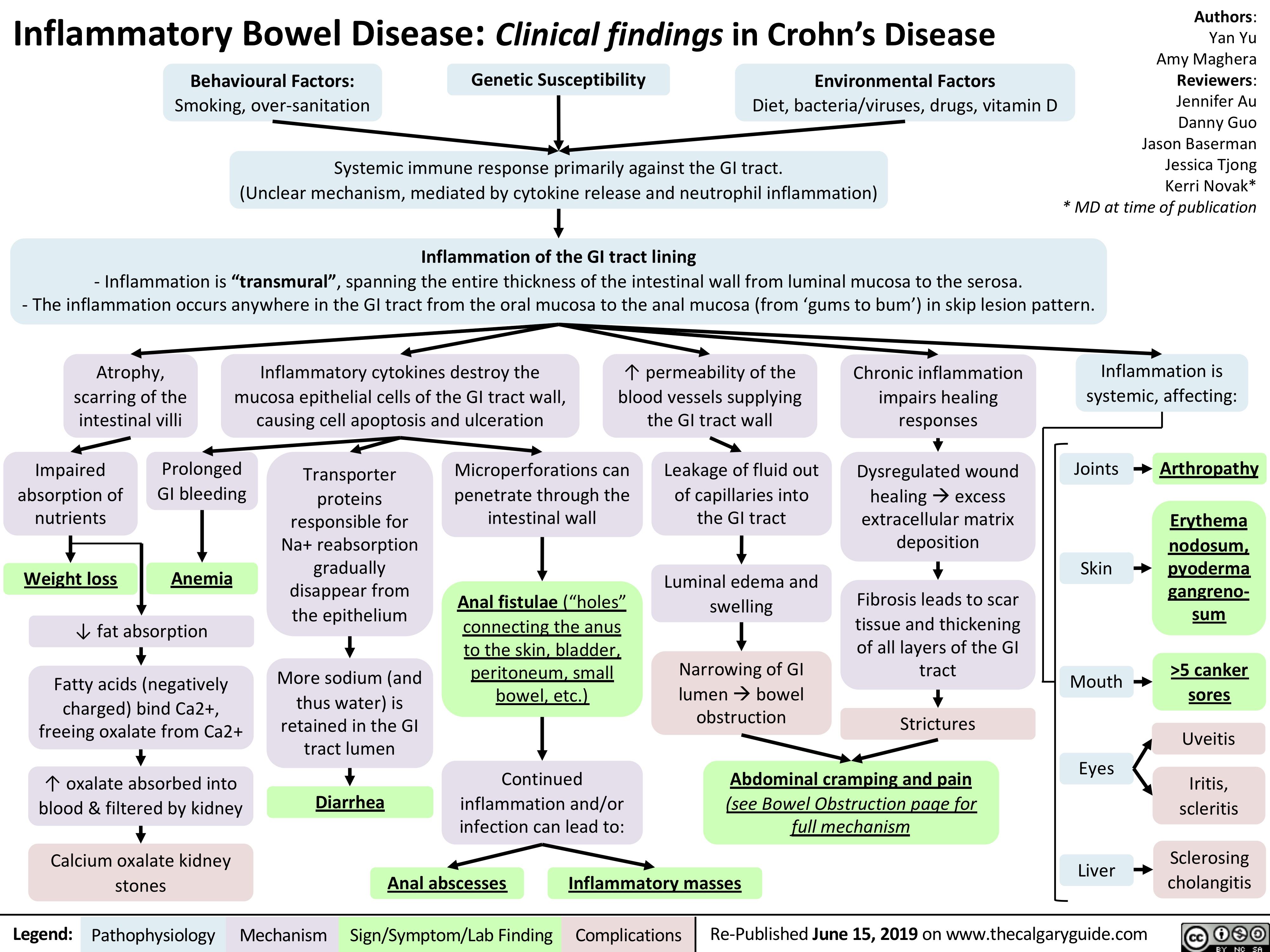
Crohn's Disease
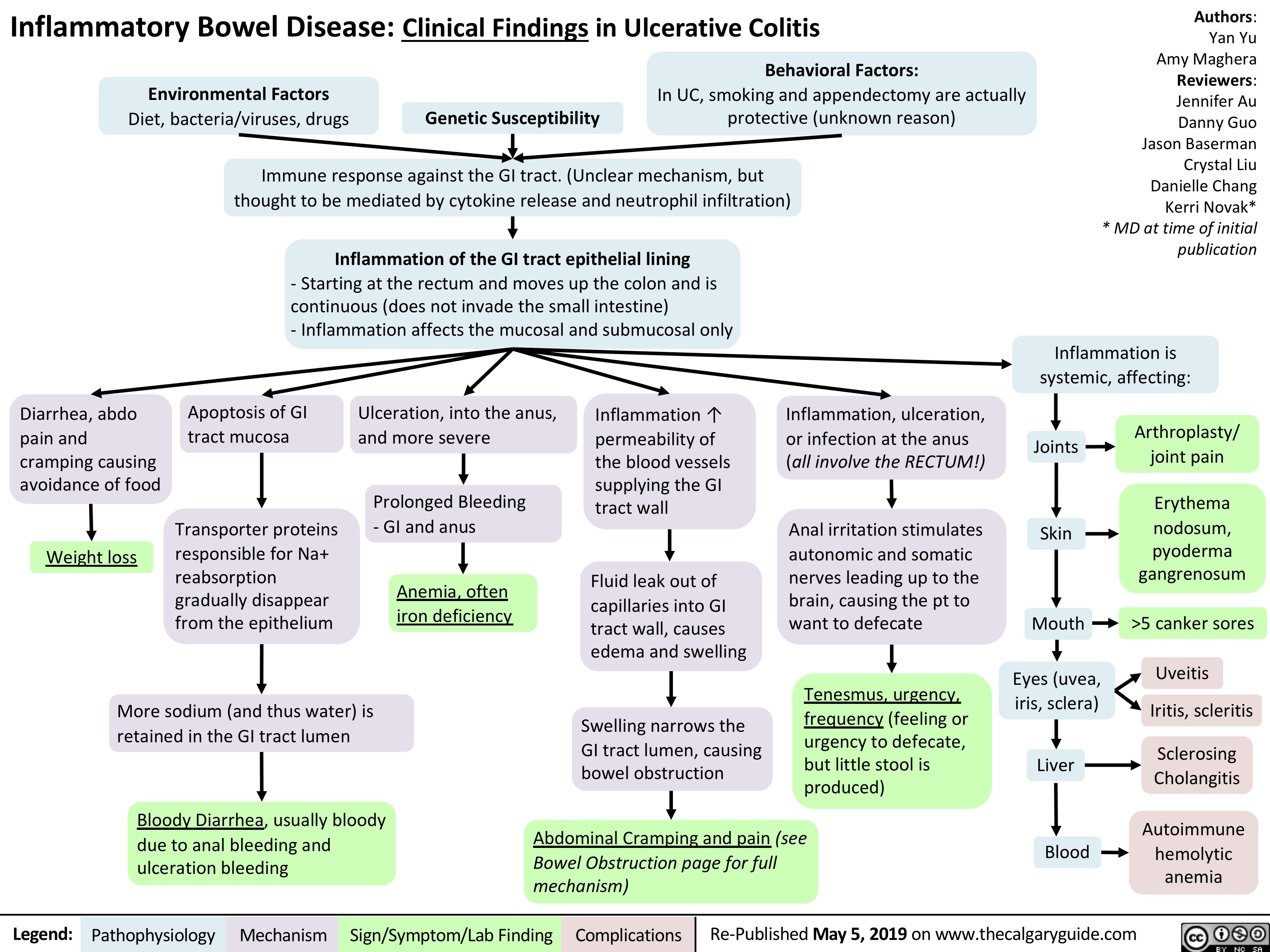
Ulcerative Colitis
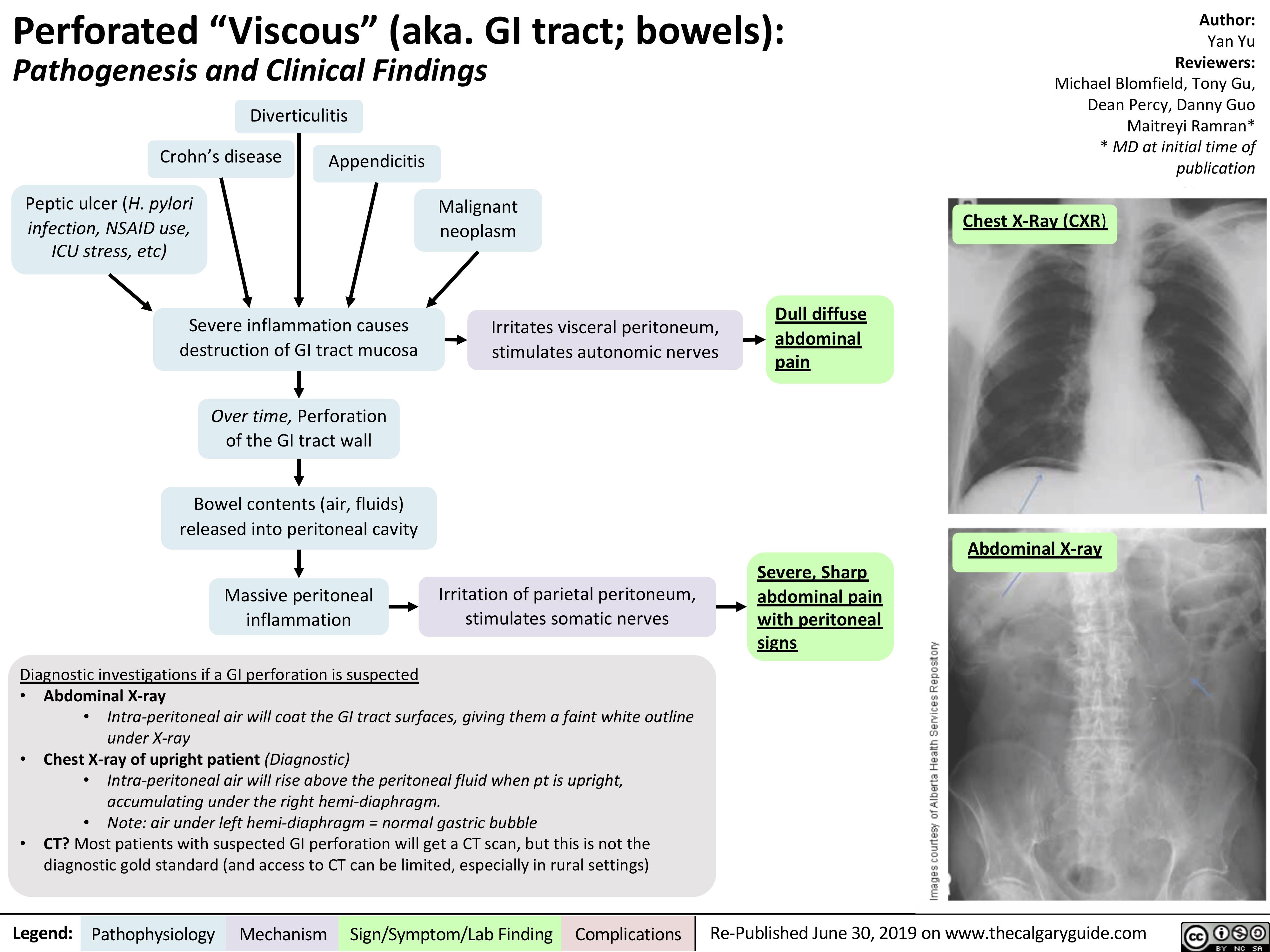
perforated-viscous
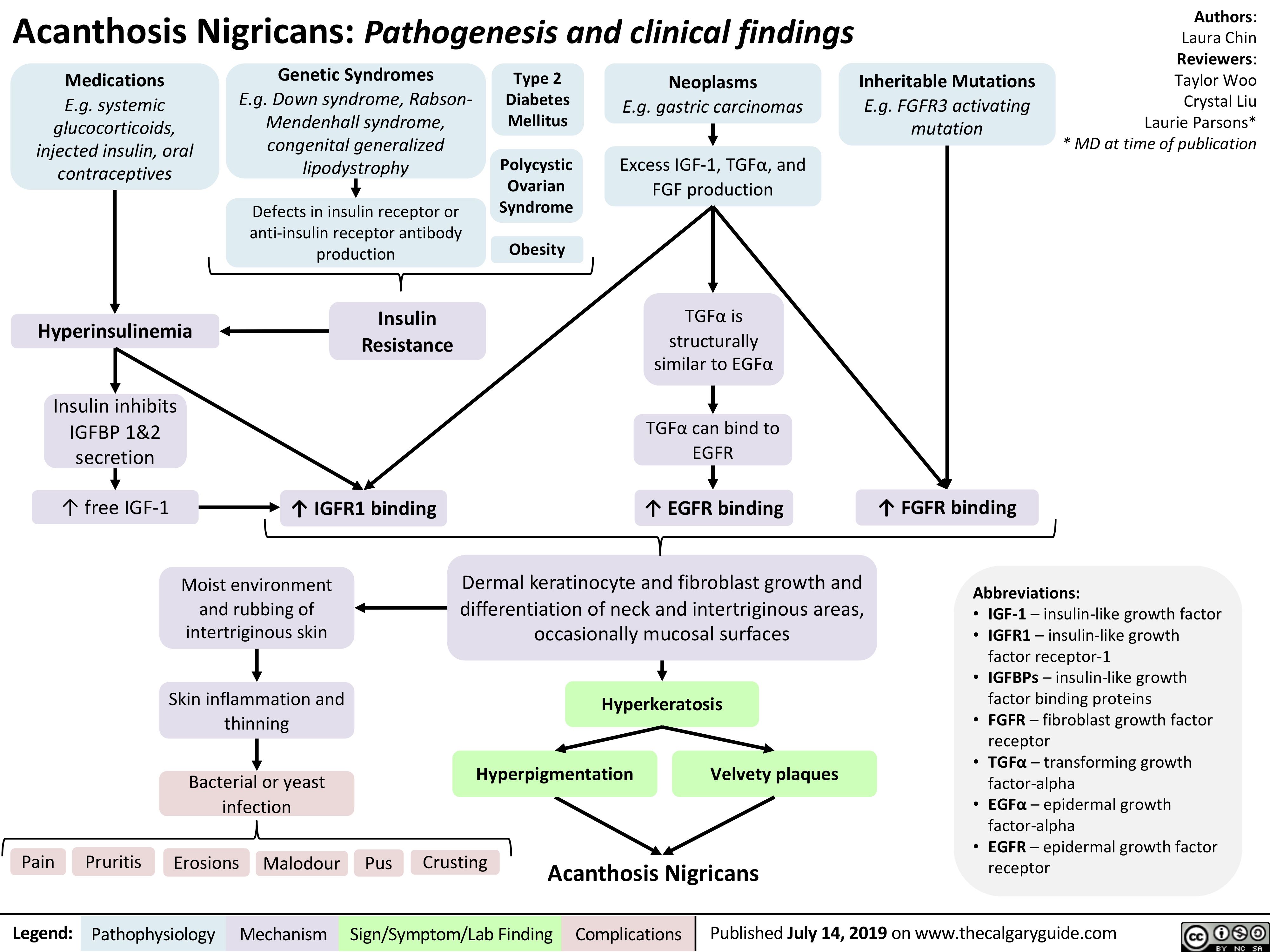
acanthosis-nigricans-pathogenesis-and-clinical-findings
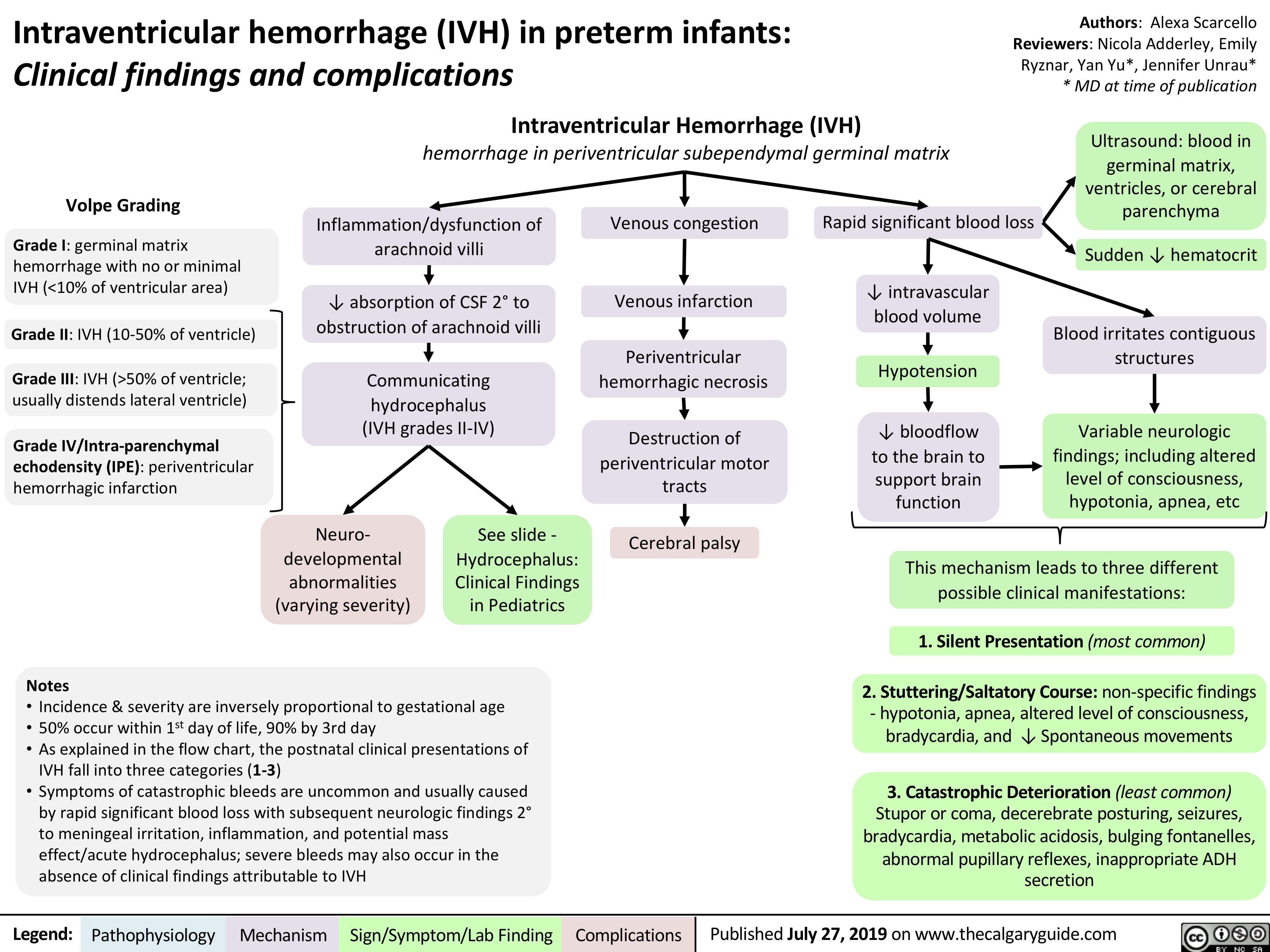
intraventricular-hemorrhage-in-preterm-infants-clinical-findings-and-complications
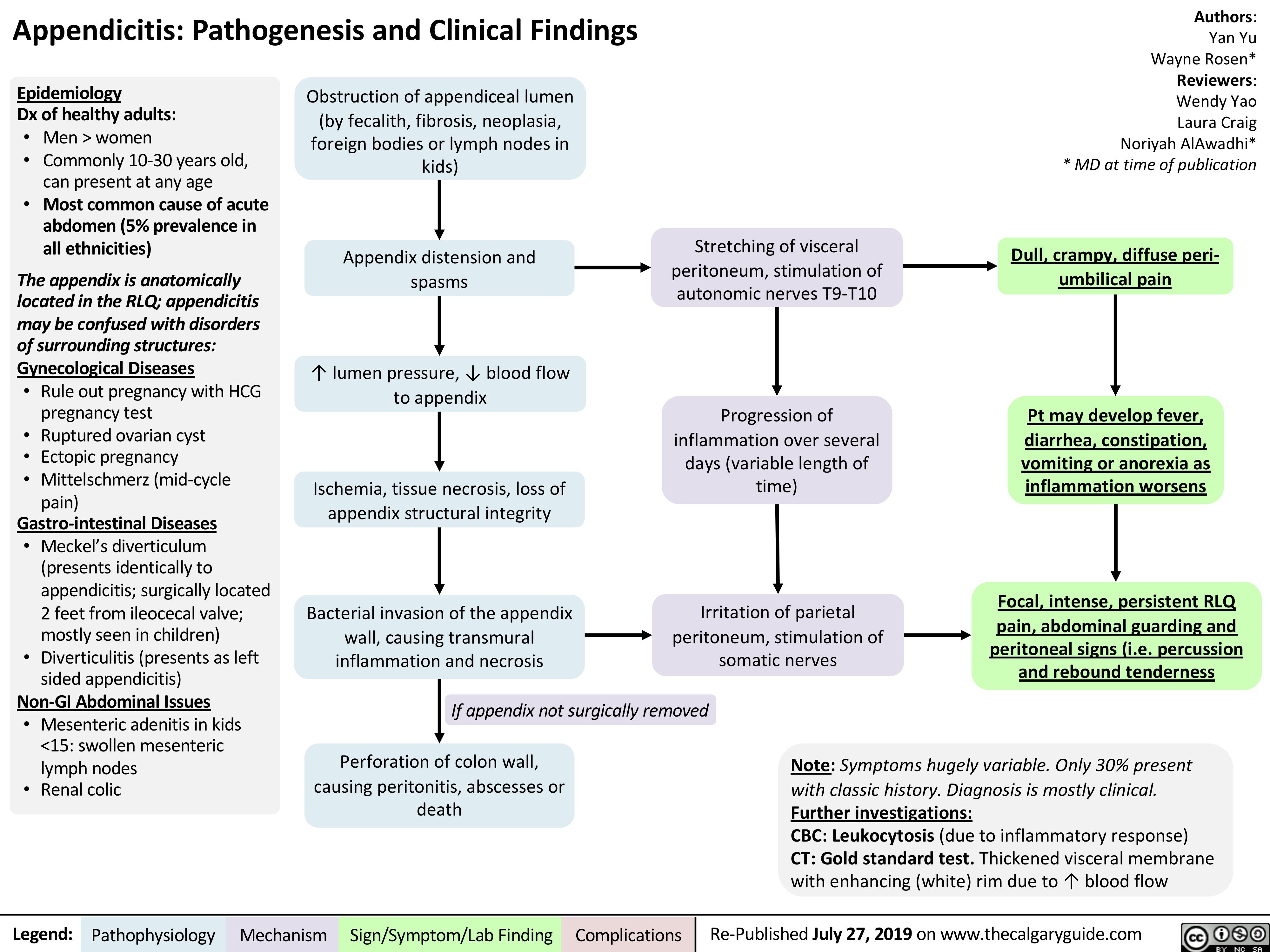
Appendicitis
Acute GI Related Abdominal Pain

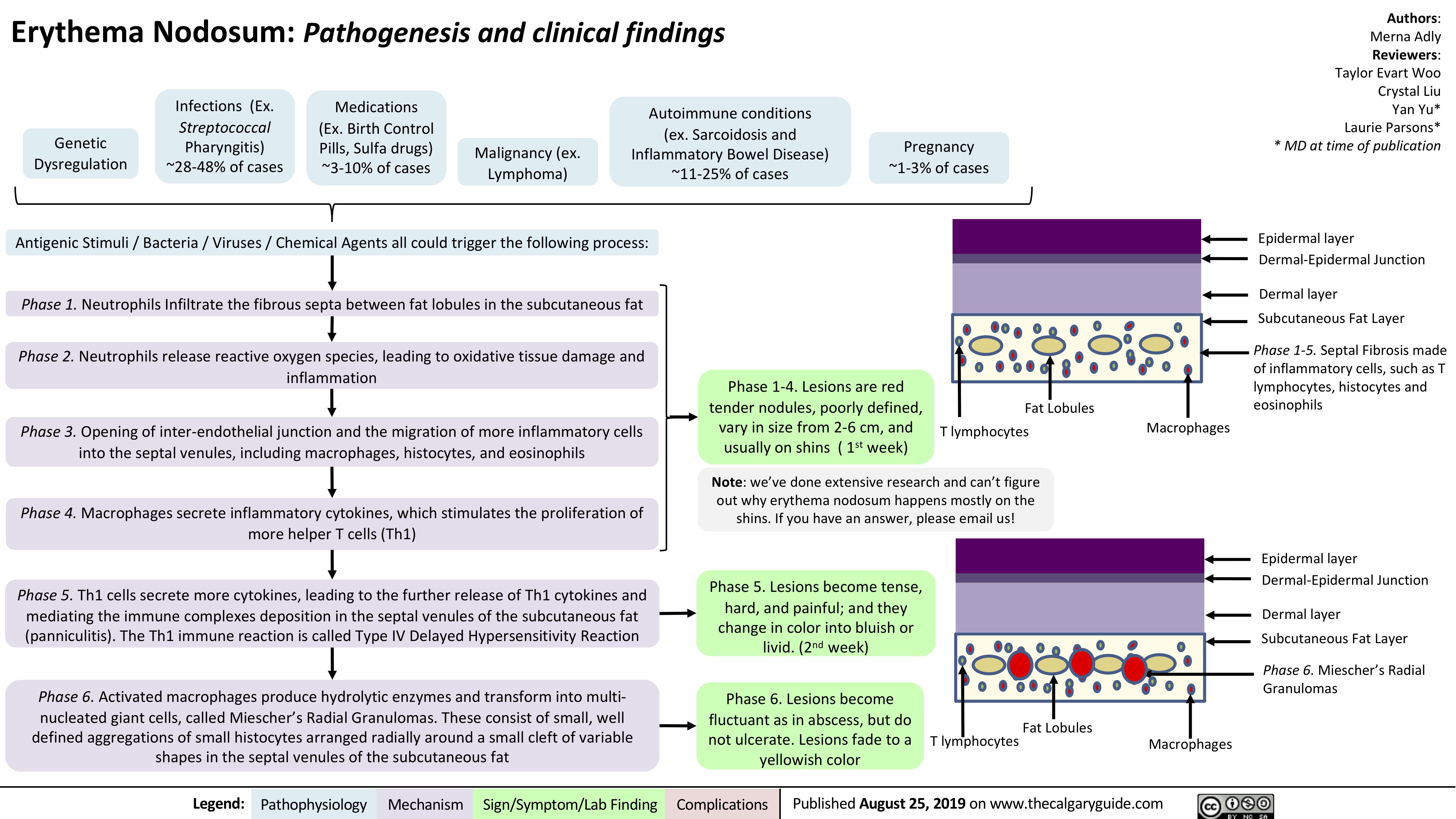
Erythema Nodosum pathogenesis and clinical findings
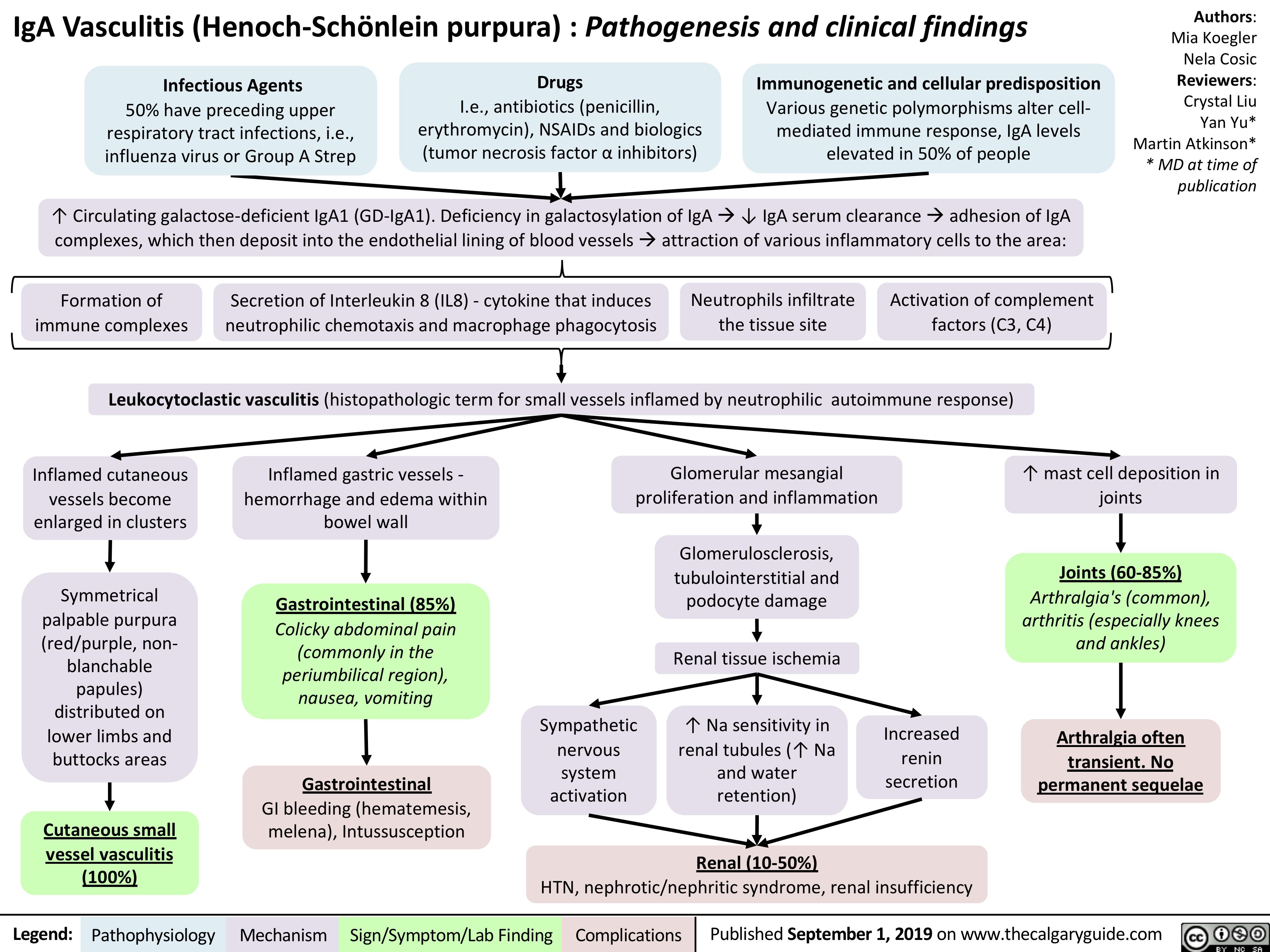
iga-vasculitis-henoch-scholein-purpura-pathogenesis-and-clinical-findings
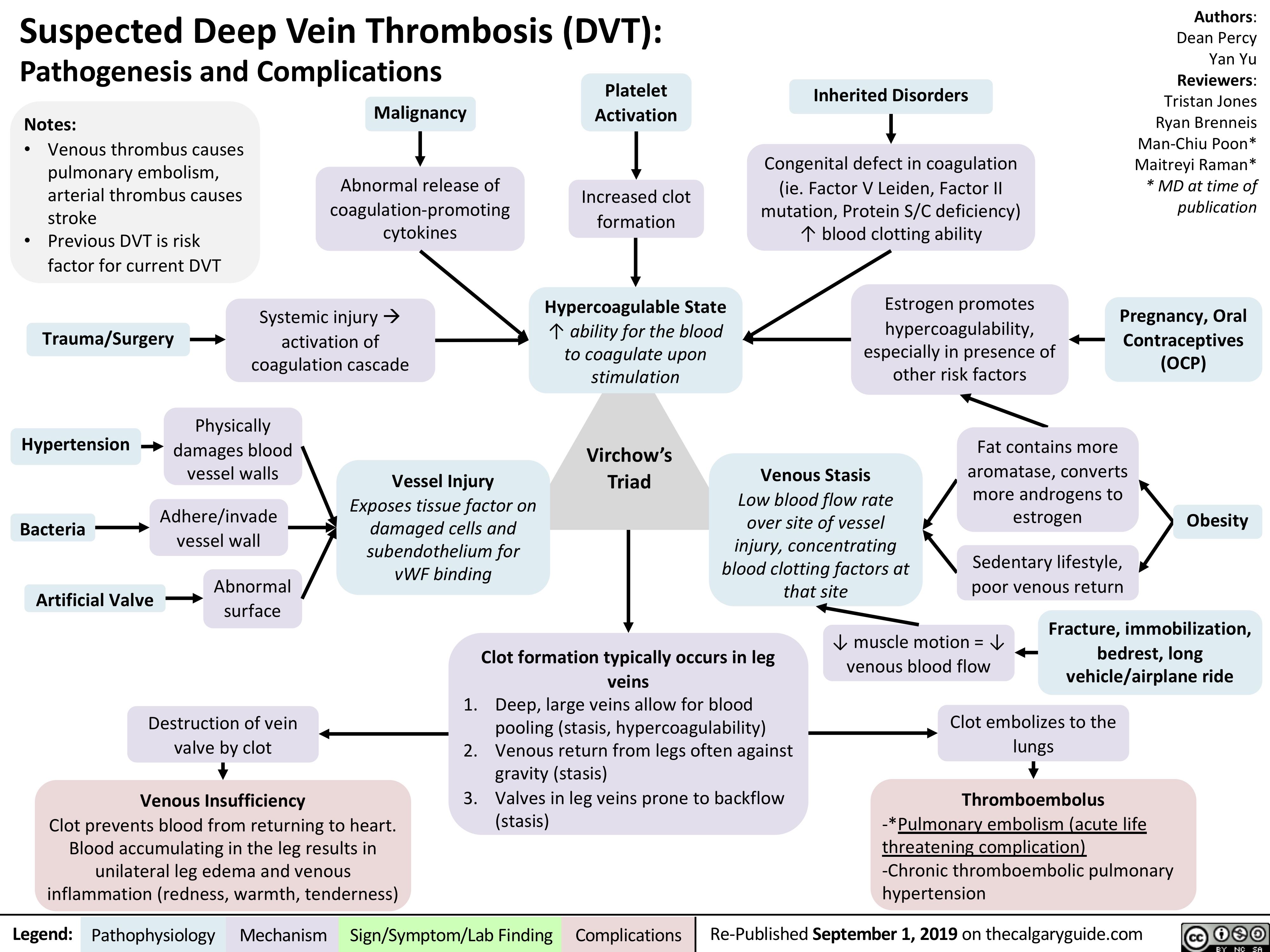
virchows-triad-and-deep-vein-thrombosis-dvt
Acute-Pancreatitis
![Acute Pancreatitis: Pathogenesis and Clinical Findings
Authors: Yan Yu Reviewers: Laura Craig Noriyah AlAwadhi Ryan Brenneis Maitreyi Raman* * MD at time of publication
Associated signs due to intra- abdominal hemorrhage from an unknown mechanism (classically associated with pancreatitis, but happens in <1% of cases):
Note:
It is not enough to just diagnose “acute pancreatitis”. Full management requires determining underlying etiology with further work-up.
Alcohol
↑ Toxic metabolites within pancreas and Spincter of Oddi Spasms
Gallstones
Migration to common bile duct blocks Sphincter of Oddi
Hypertriglyceridemia
Unknown
mechanism (rare)
Idiopathic
Further investigations:
CBC: Cell counts elevated, due to sever hypovolemia
Serum [Lipase]: Gold Standard Diagnostic Test; rupture of pancreatic cells releases lipase into circulation
Pancreatic secretions back up, ↑ pressure within pancreas
Hypercalcemia (Rare; Ca2+ depositions in bile ducts block outflow of pancreatic secretions)
Since pancreas is retroperitoneal, somatic
nerves in the parietal peritoneum are directly stimulated
Inflammation triggers cytokine release
Inflamed pancreas irritates adjacent intestines, causing ileus
Inflamed, more permeable blood vessels leak fluid into pancreas
• •
Cullen’s sign (bruising in peri-umbilical region) Grey-Turner’s sign (bruises along both flanks)
Sudden, severe epigastric pain (with peritoneal signs), radiates to the center of the back
Fever, nausea/vomiting
(general signs of inflammation)
Diminished bowel sounds Profound dehydration
(flat JVP, hypotension, tachycardia, oliguria) – may happen, not always
1. Pressure compresses pancreatic blood vessels, causing tissue ischemia.
2. Activation of inactive proteases (zymogens) digesting pancreatic tissue
Necrosis (death) of pancreatic cells
Inflammation self- perpetuates
Massive systemic inflammatory response
2 main complications, usually detected on CT;
may happen, but not always
1. Pancreatic pseudocyst (enlargement of the
pancreas due to fluid accumulation)
2. Pancreatic necrosis/abscesses (death of a part of the pancreas)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-published September 1, 2019 on thecalgaryguide.com
Acute Pancreatitis: Pathogenesis and Clinical Findings
Authors: Yan Yu Reviewers: Laura Craig Noriyah AlAwadhi Ryan Brenneis Maitreyi Raman* * MD at time of publication
Associated signs due to intra- abdominal hemorrhage from an unknown mechanism (classically associated with pancreatitis, but happens in <1% of cases):
Note:
It is not enough to just diagnose “acute pancreatitis”. Full management requires determining underlying etiology with further work-up.
Alcohol
↑ Toxic metabolites within pancreas and Spincter of Oddi Spasms
Gallstones
Migration to common bile duct blocks Sphincter of Oddi
Hypertriglyceridemia
Unknown
mechanism (rare)
Idiopathic
Further investigations:
CBC: Cell counts elevated, due to sever hypovolemia
Serum [Lipase]: Gold Standard Diagnostic Test; rupture of pancreatic cells releases lipase into circulation
Pancreatic secretions back up, ↑ pressure within pancreas
Hypercalcemia (Rare; Ca2+ depositions in bile ducts block outflow of pancreatic secretions)
Since pancreas is retroperitoneal, somatic
nerves in the parietal peritoneum are directly stimulated
Inflammation triggers cytokine release
Inflamed pancreas irritates adjacent intestines, causing ileus
Inflamed, more permeable blood vessels leak fluid into pancreas
• •
Cullen’s sign (bruising in peri-umbilical region) Grey-Turner’s sign (bruises along both flanks)
Sudden, severe epigastric pain (with peritoneal signs), radiates to the center of the back
Fever, nausea/vomiting
(general signs of inflammation)
Diminished bowel sounds Profound dehydration
(flat JVP, hypotension, tachycardia, oliguria) – may happen, not always
1. Pressure compresses pancreatic blood vessels, causing tissue ischemia.
2. Activation of inactive proteases (zymogens) digesting pancreatic tissue
Necrosis (death) of pancreatic cells
Inflammation self- perpetuates
Massive systemic inflammatory response
2 main complications, usually detected on CT;
may happen, but not always
1. Pancreatic pseudocyst (enlargement of the
pancreas due to fluid accumulation)
2. Pancreatic necrosis/abscesses (death of a part of the pancreas)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-published September 1, 2019 on thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/09/Acute-Pancreatitis.jpg)
Infantile Colic
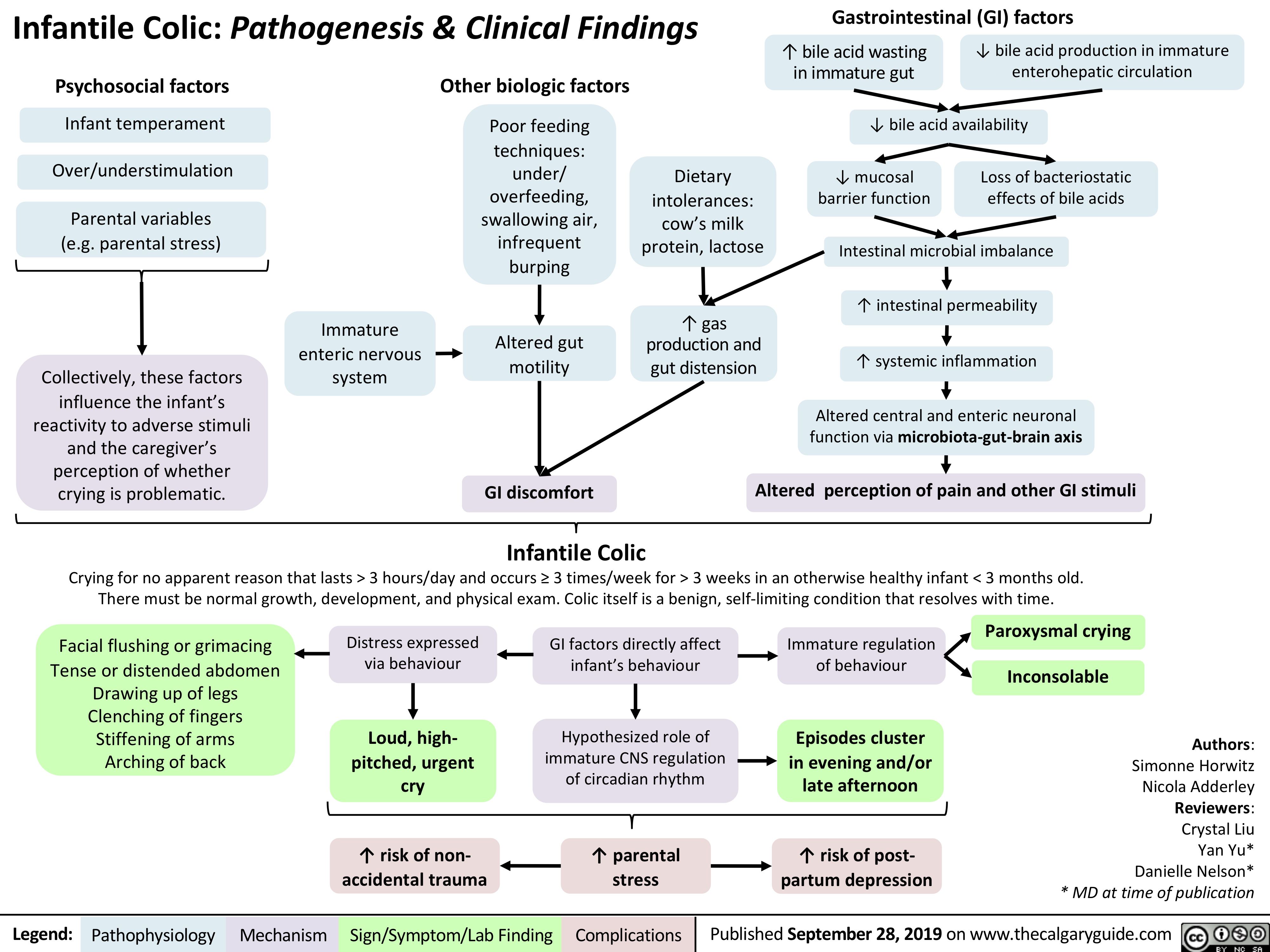
Ischemic Colitis
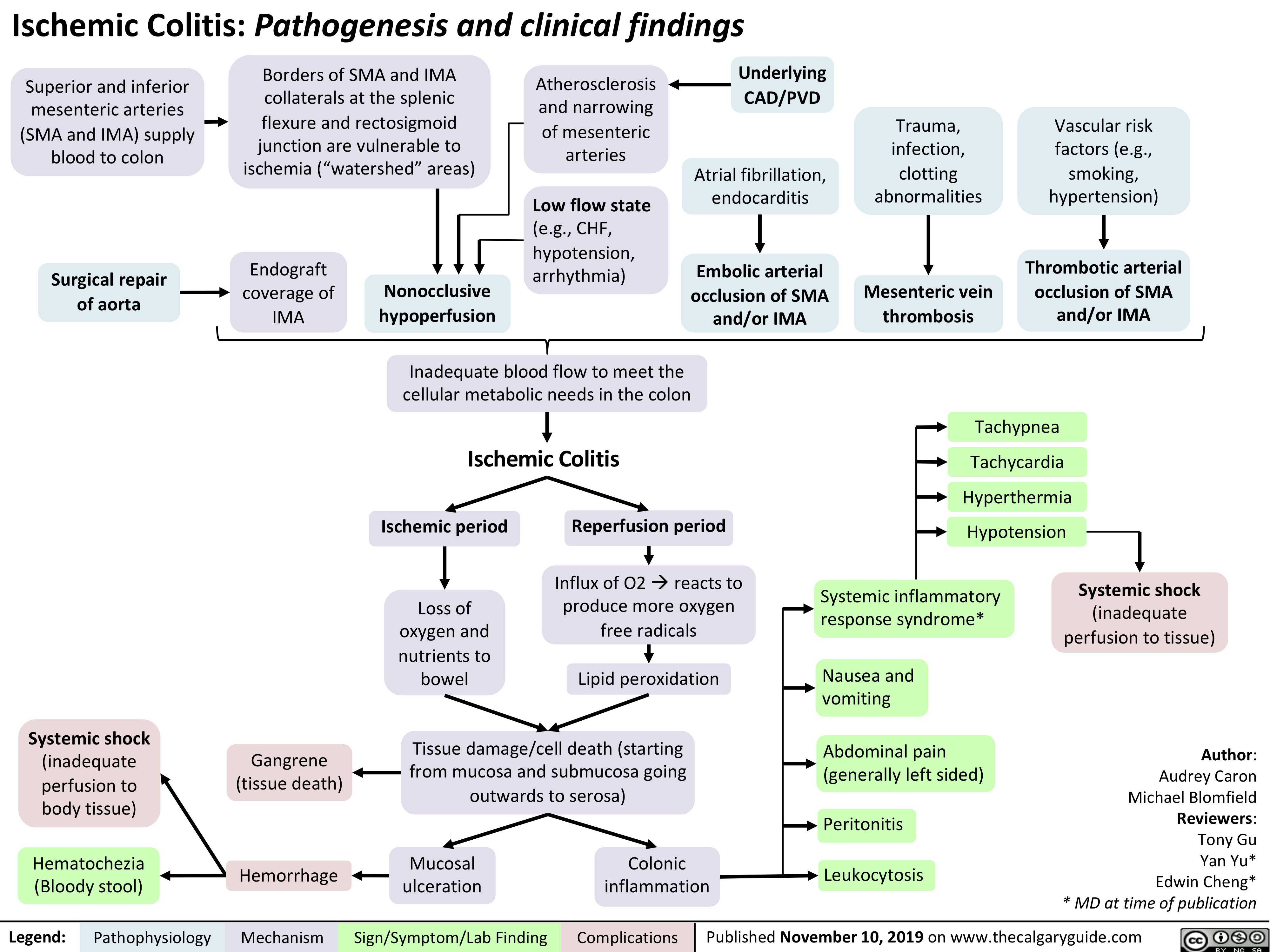
Endometritis
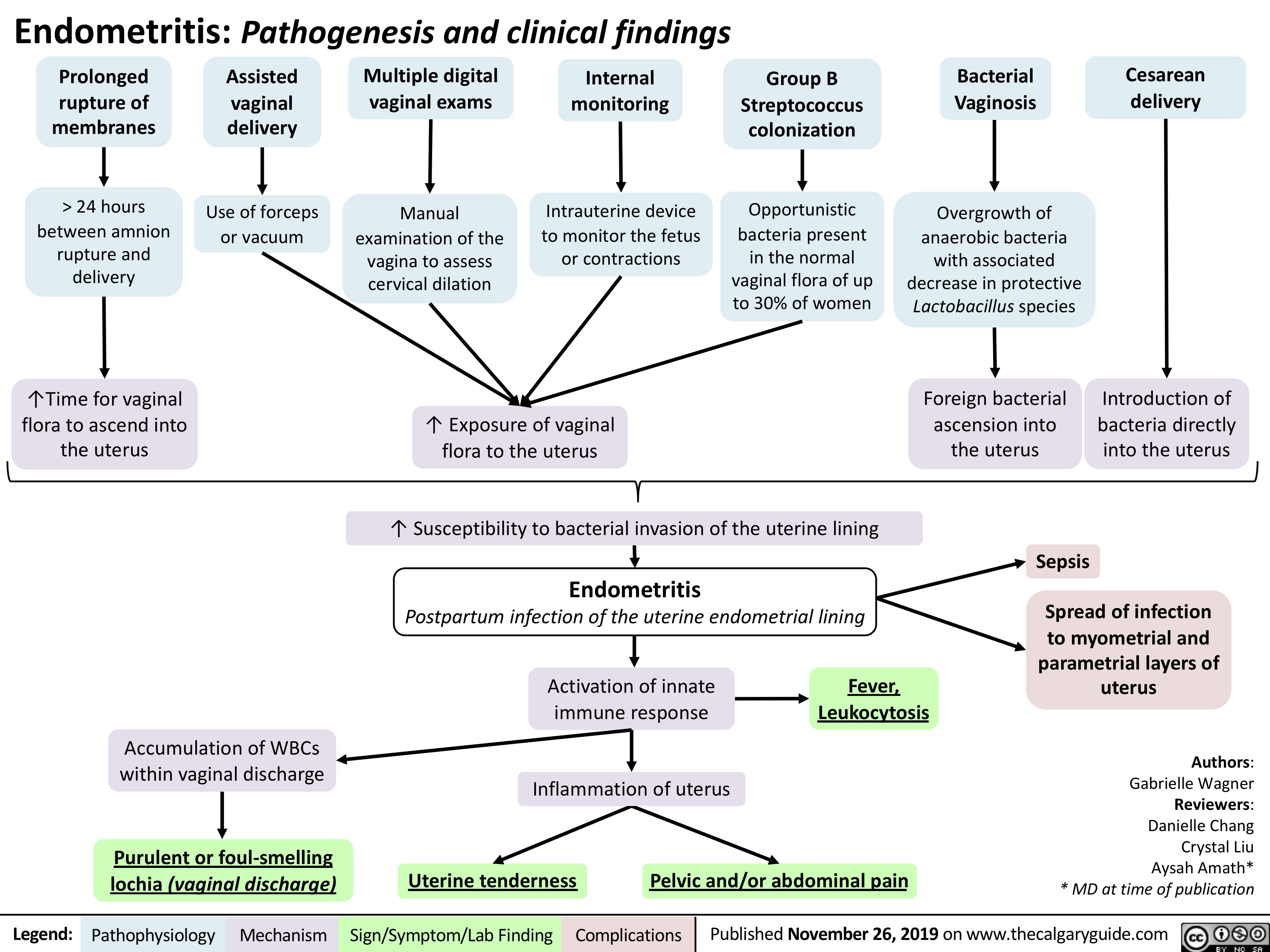
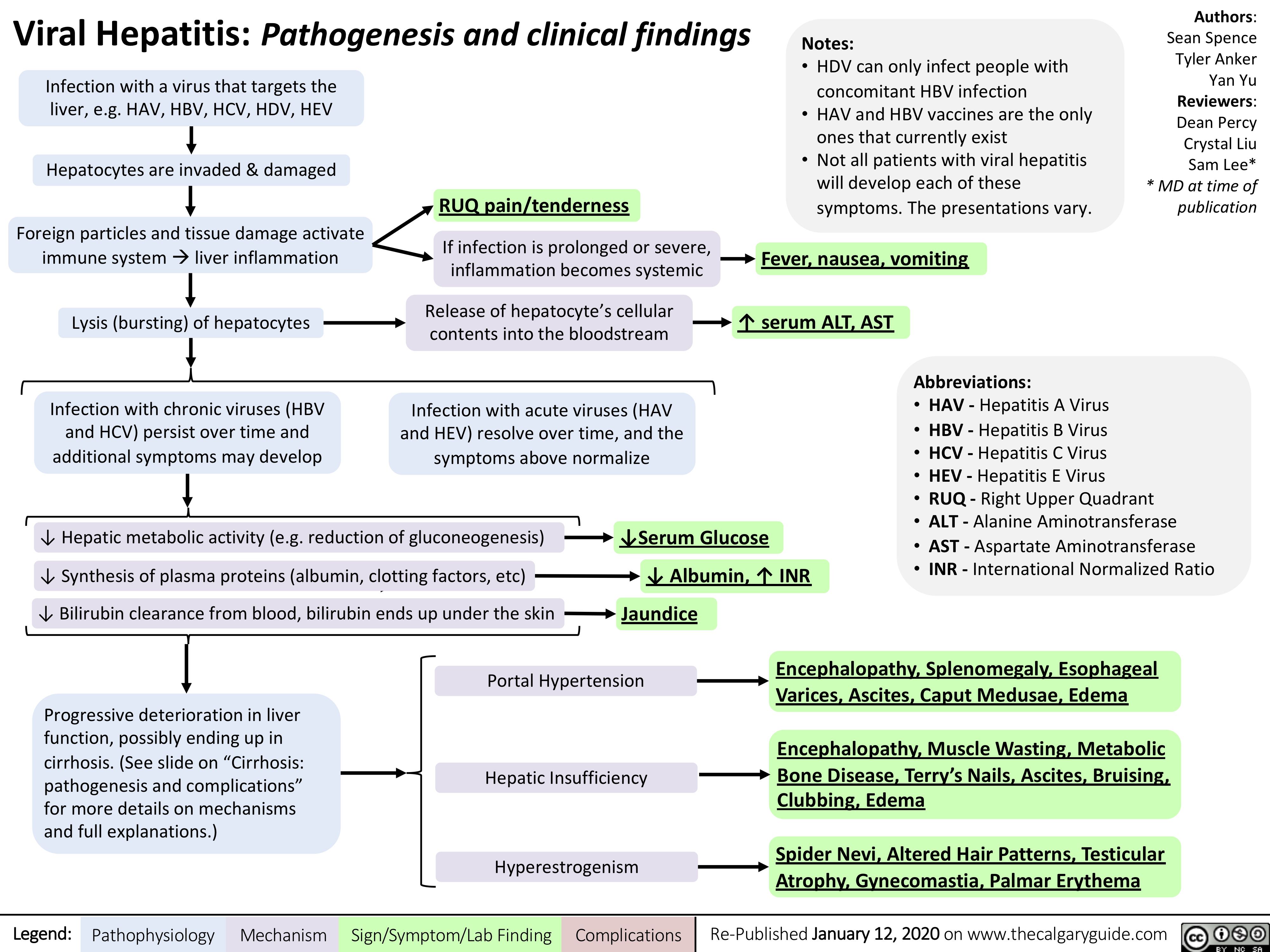
Viral-Hepatitis
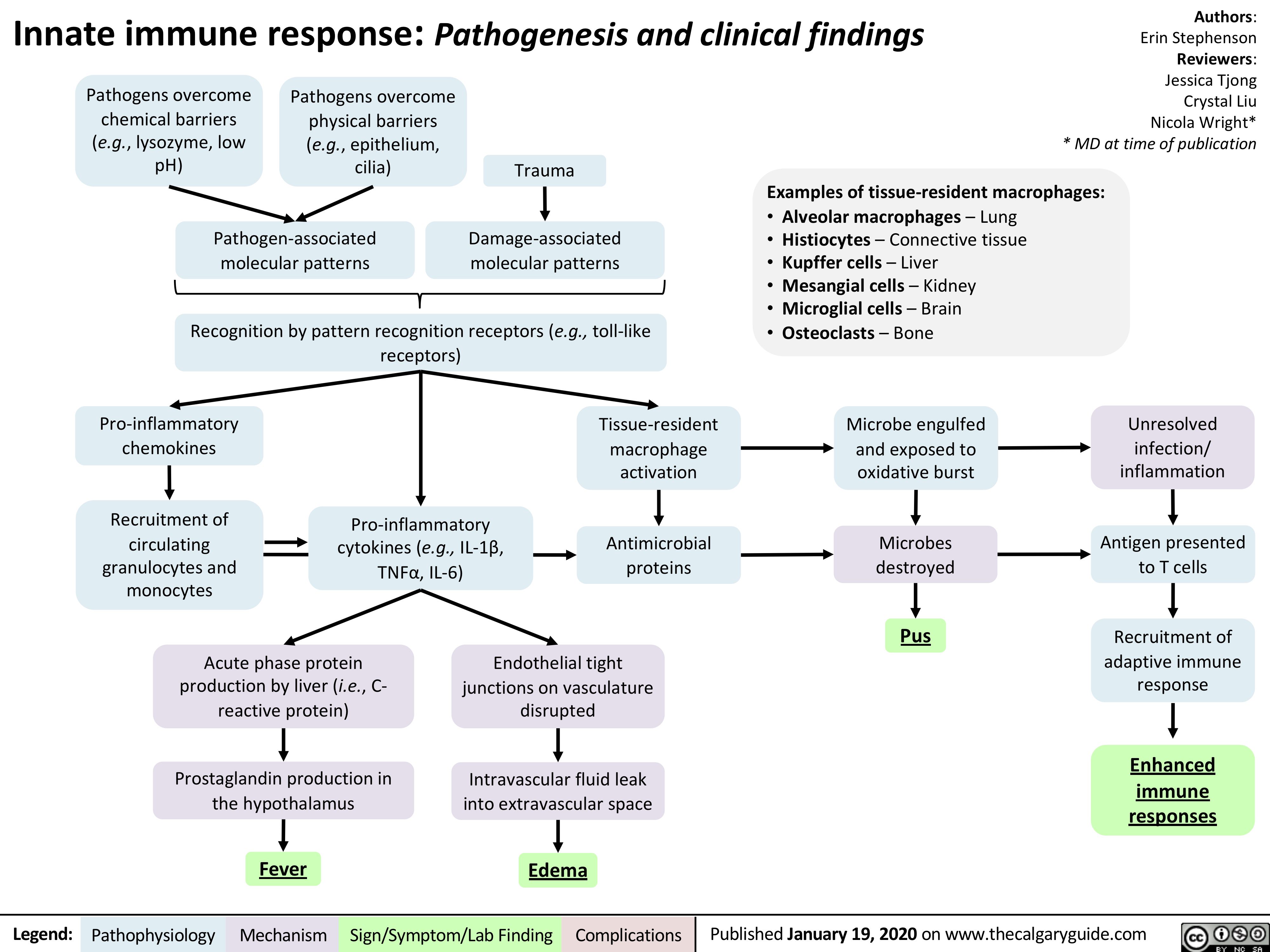
Innate-Immune-Response
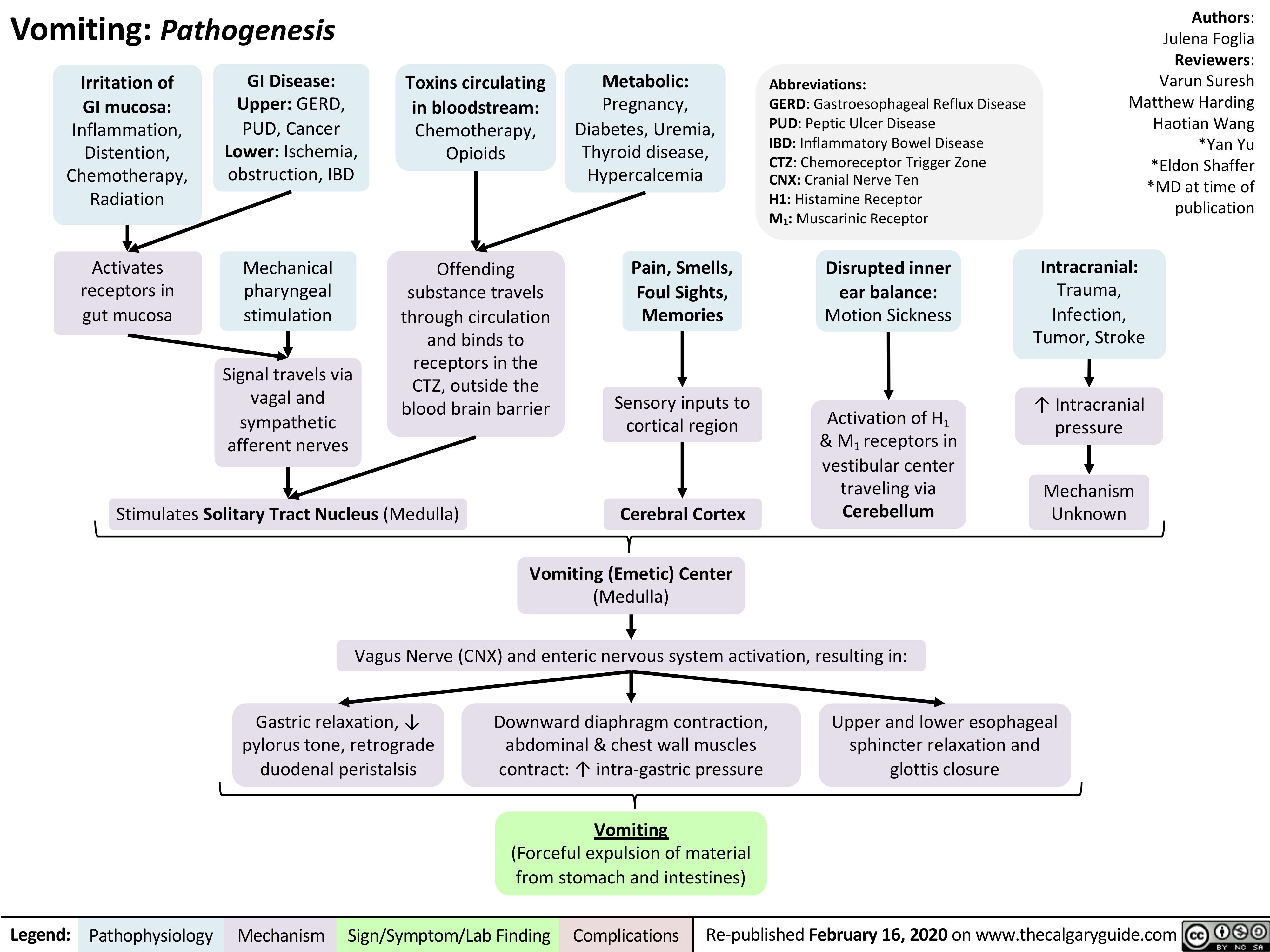
vomiting-pathogenesis
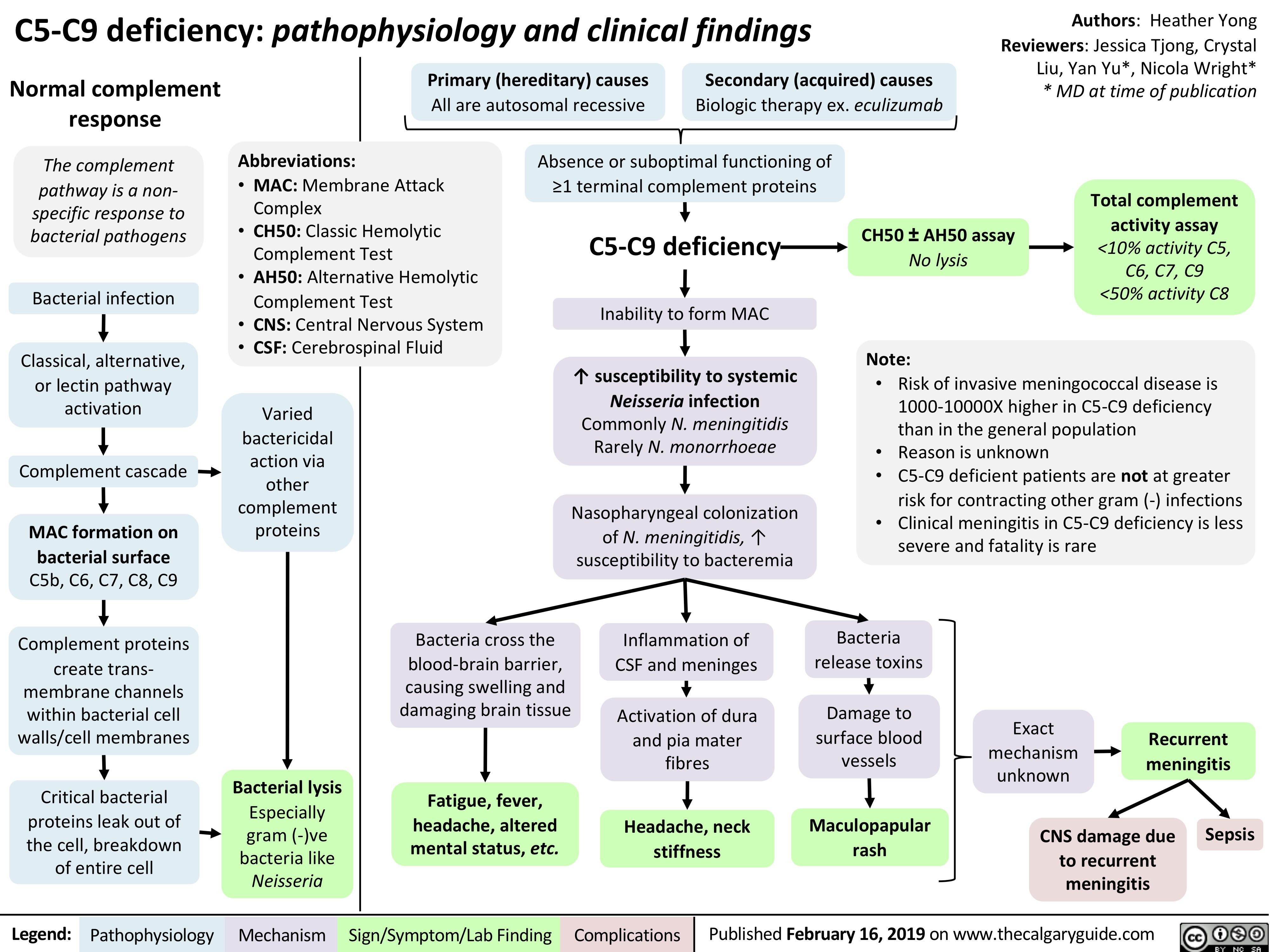
C5-C9-deficiency
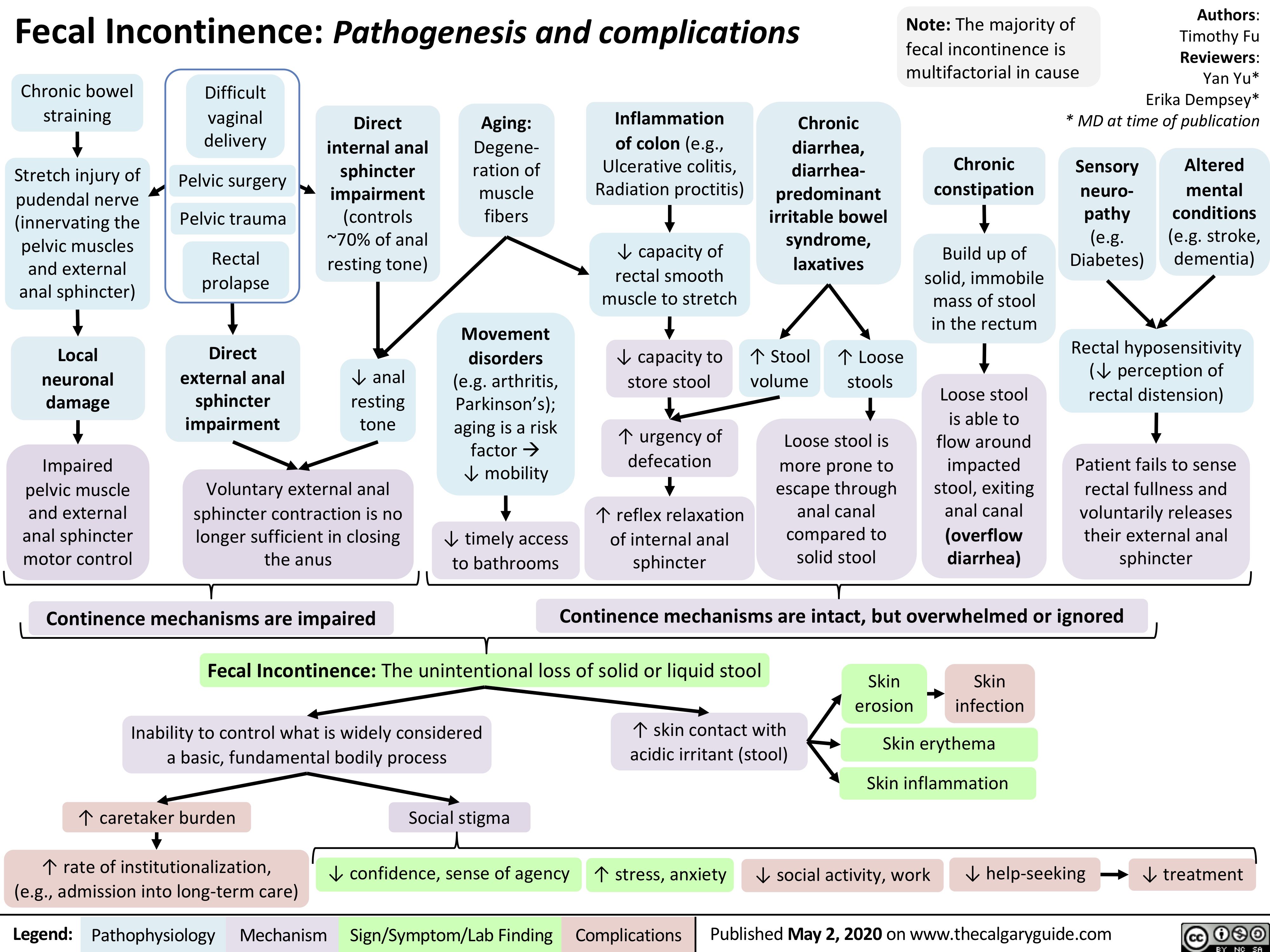
Fecal-Incontinence
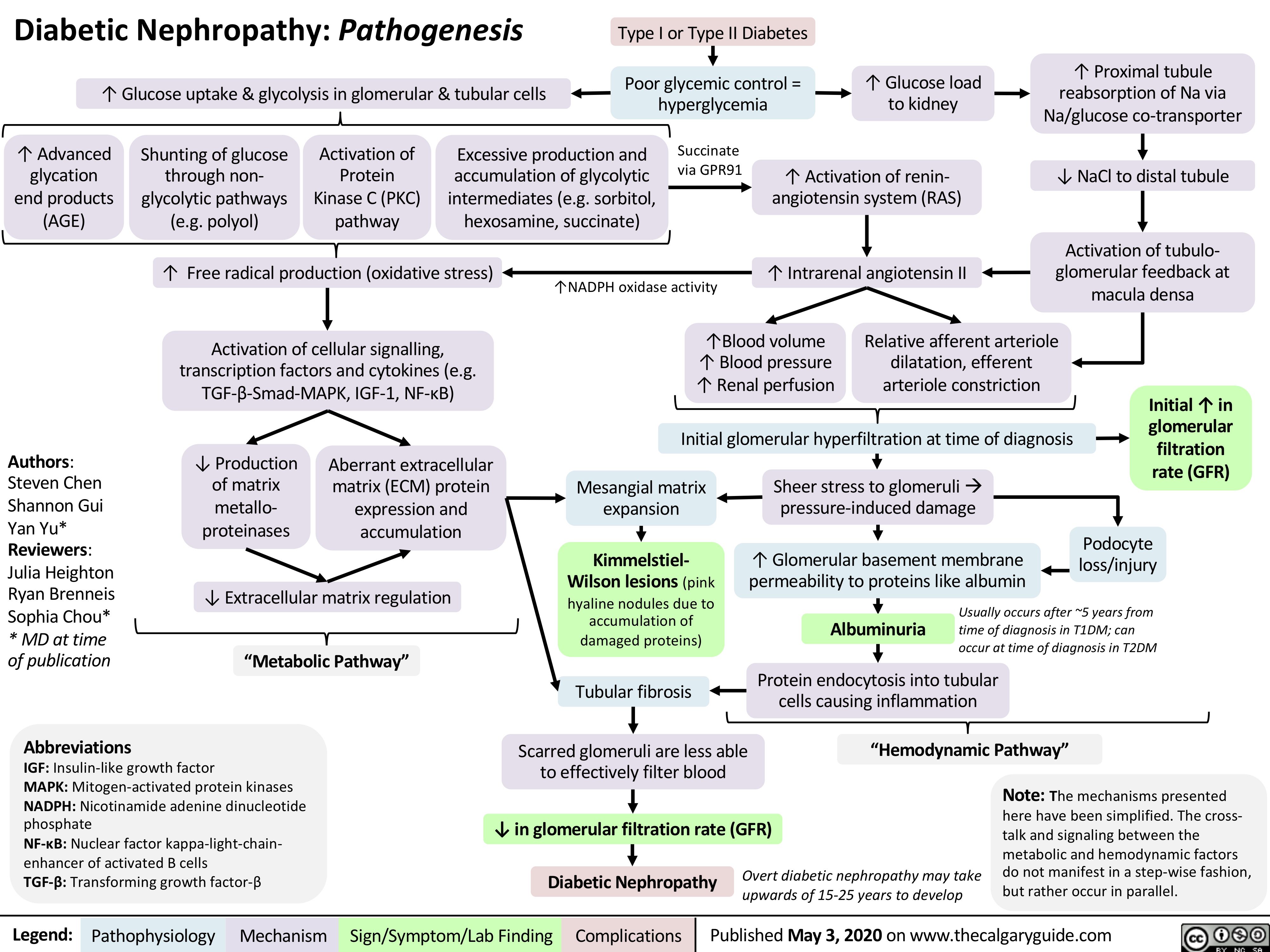
Diabetic-Nephropathy
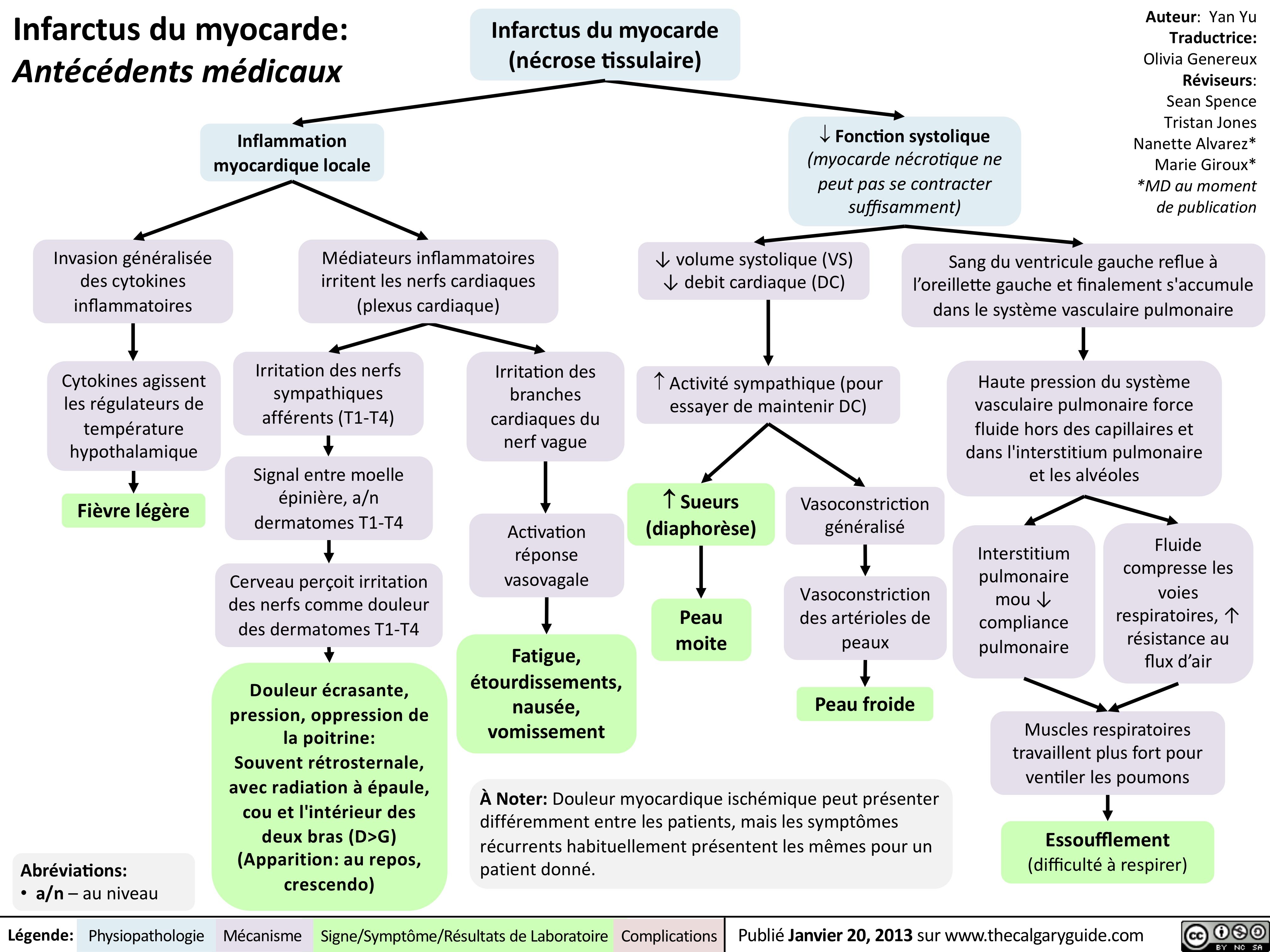
Infarctus du myocarde: Antécédents médicaux
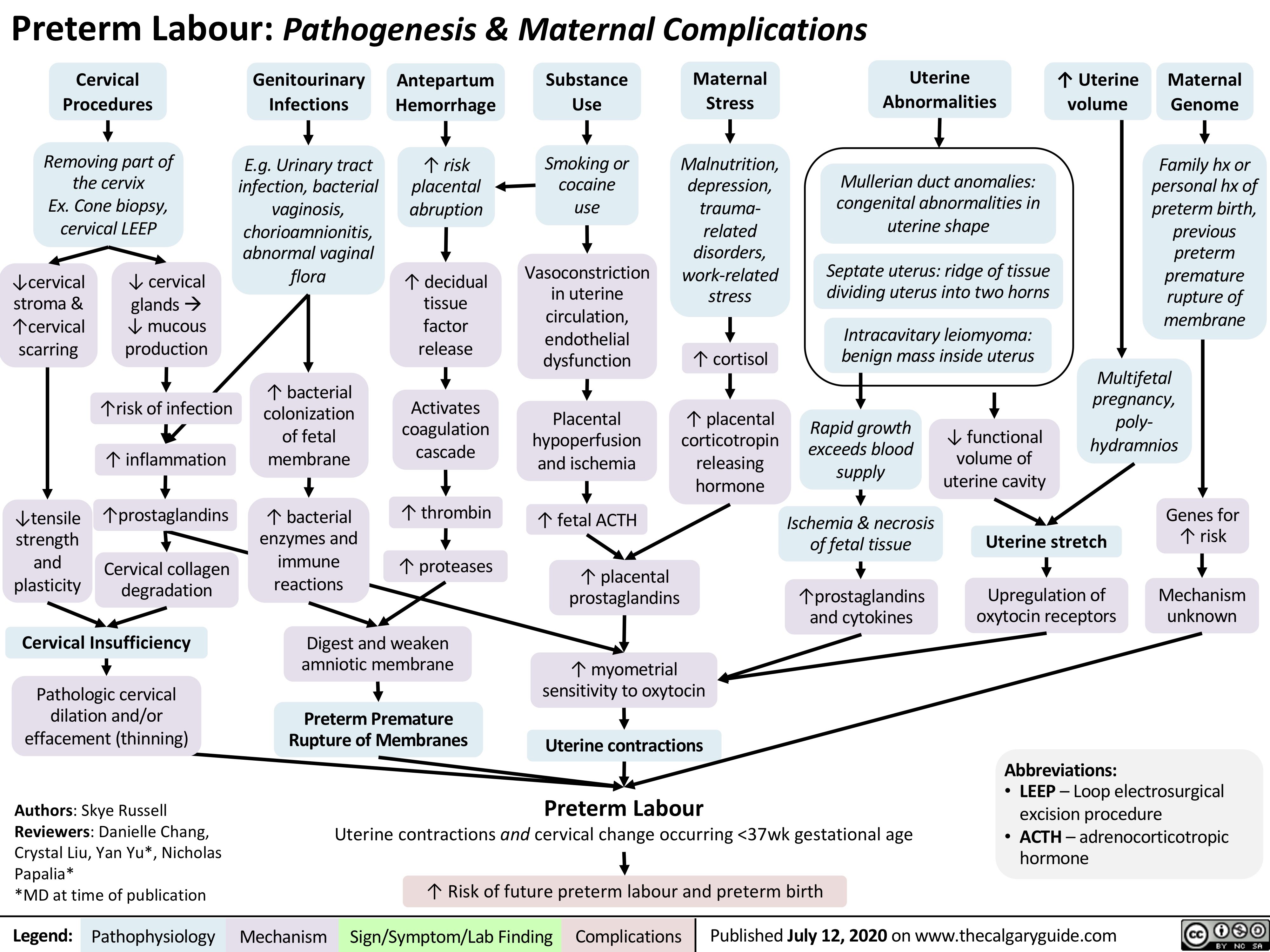
preterm-labour-pathogenesis-maternal-complications
acute-pancreatitis-complications
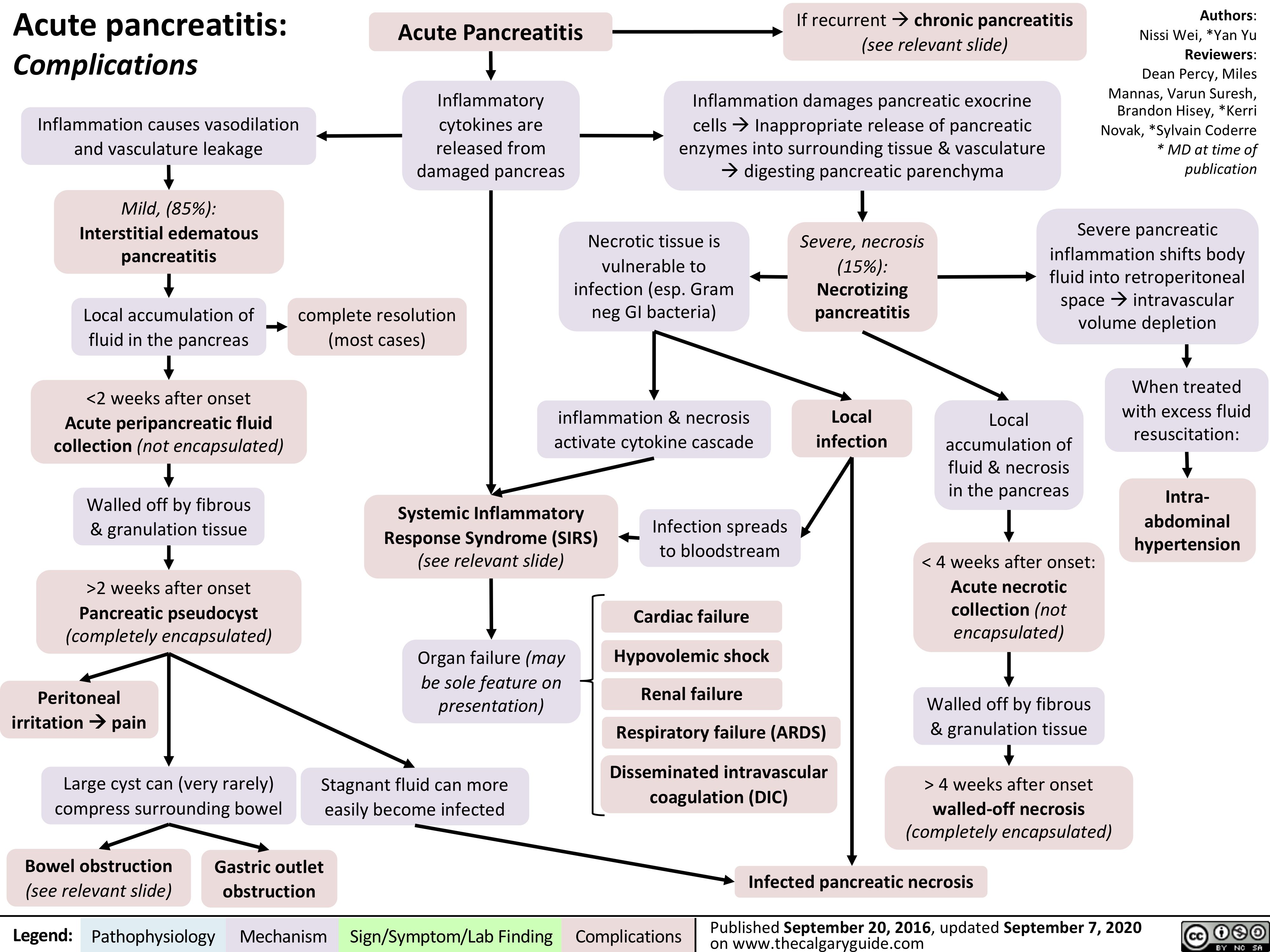
Cellulitis
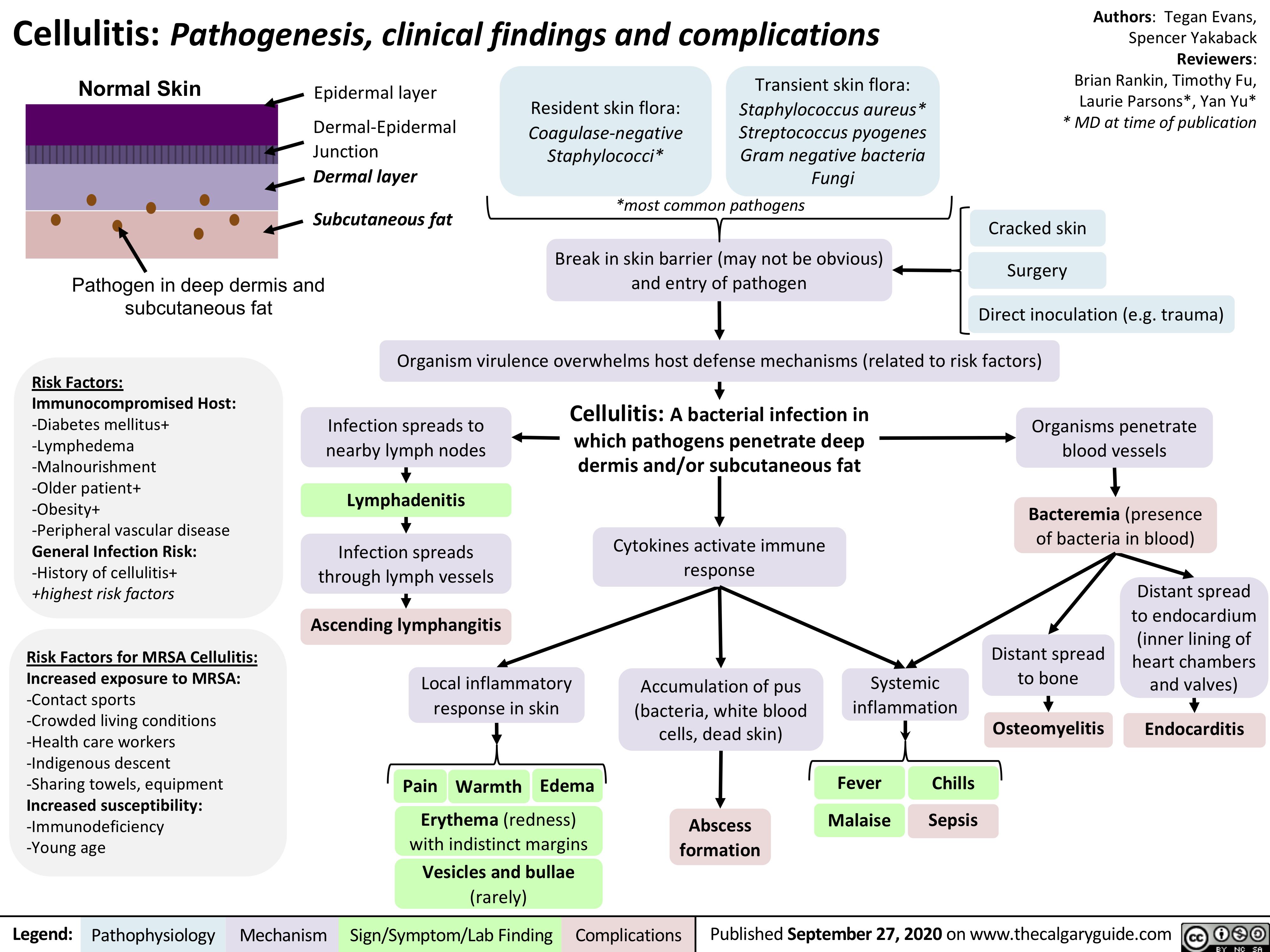
Tumour-Lysis-Syndrome
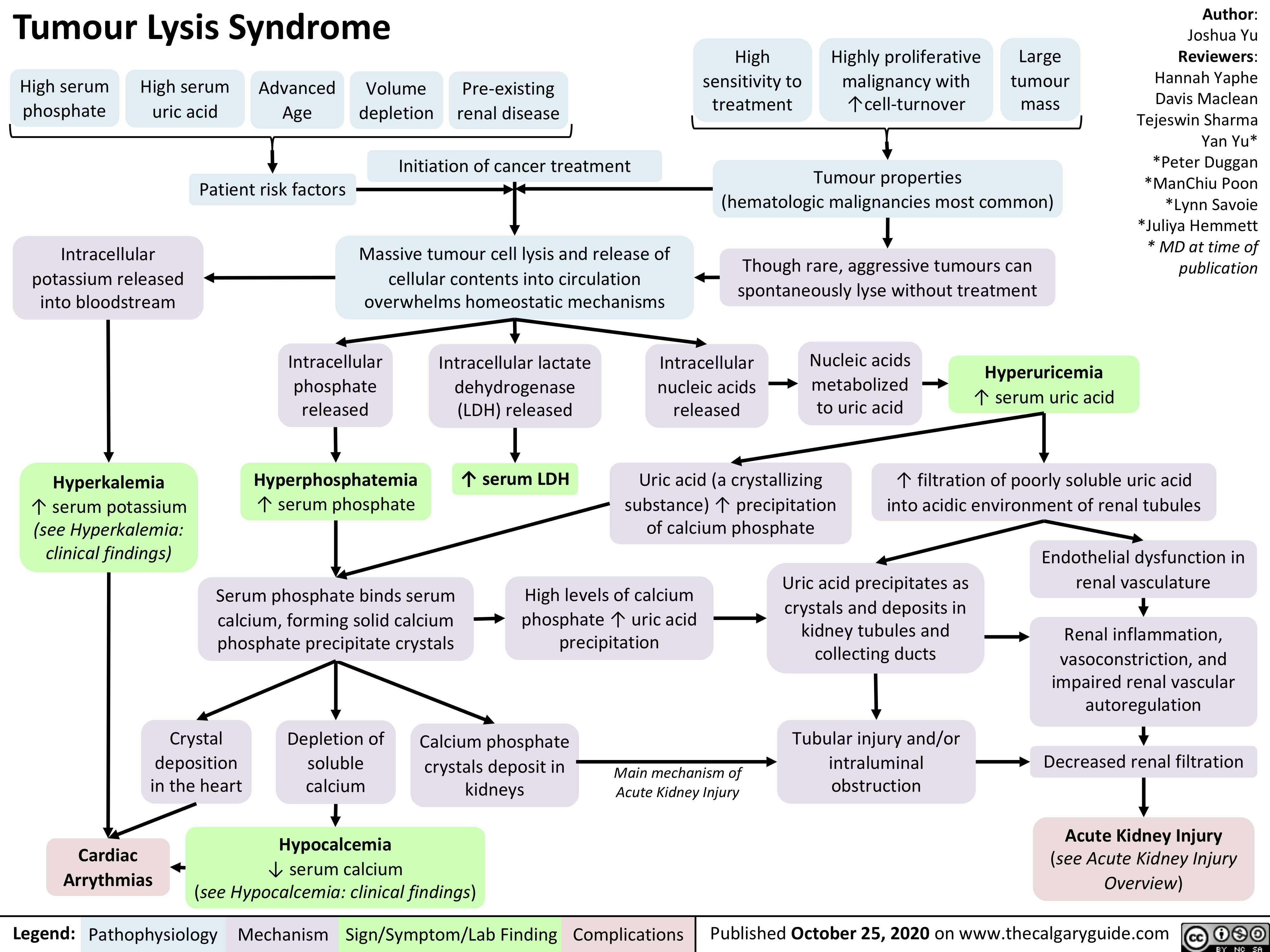
Multiple-Sclerosis-on-Brain-MRI
Bronchiolitis-updated
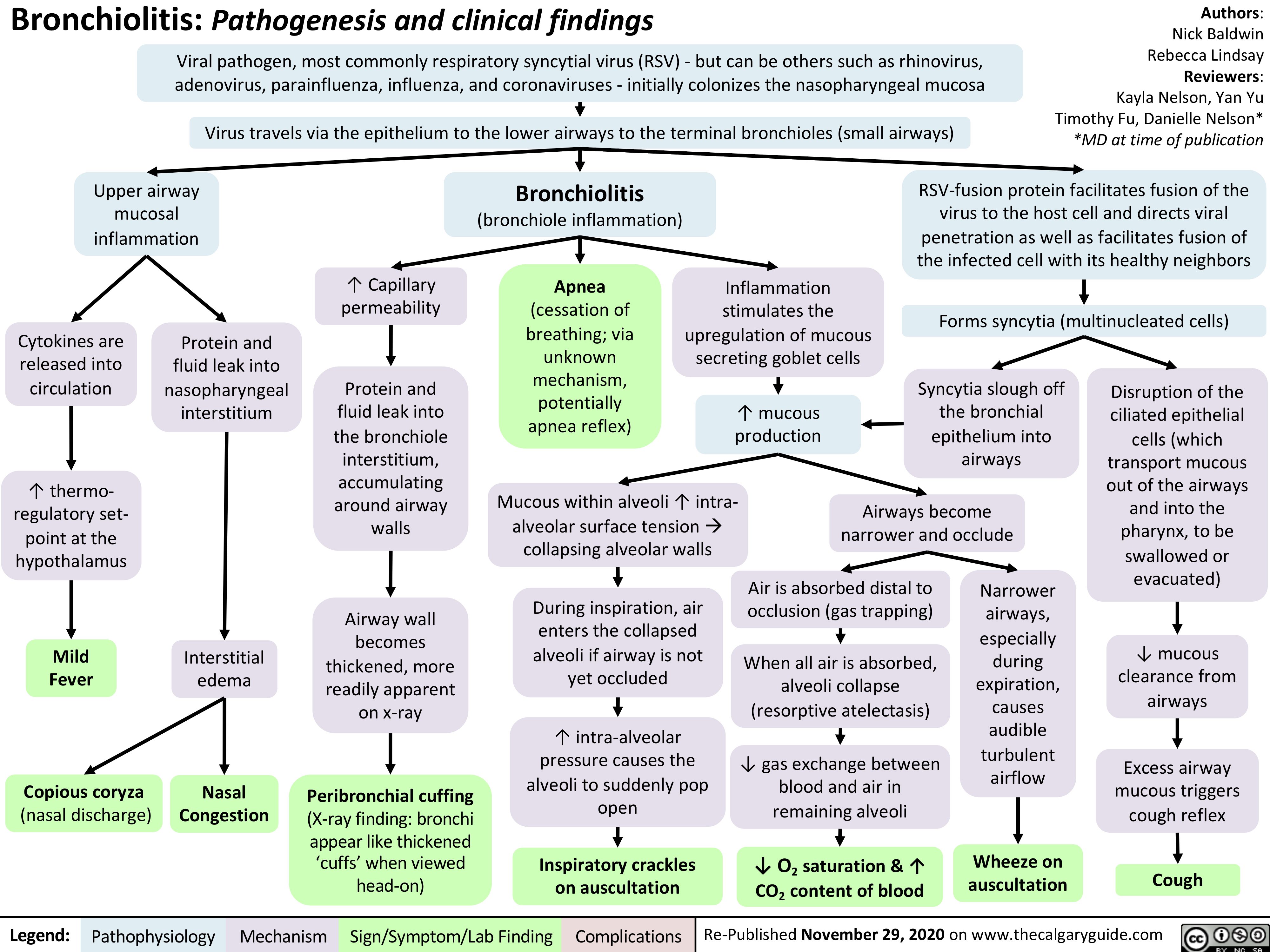
mrna-vaccines-against-coronavirus-disease-2019-covid-19-production-and-mechanism-of-action
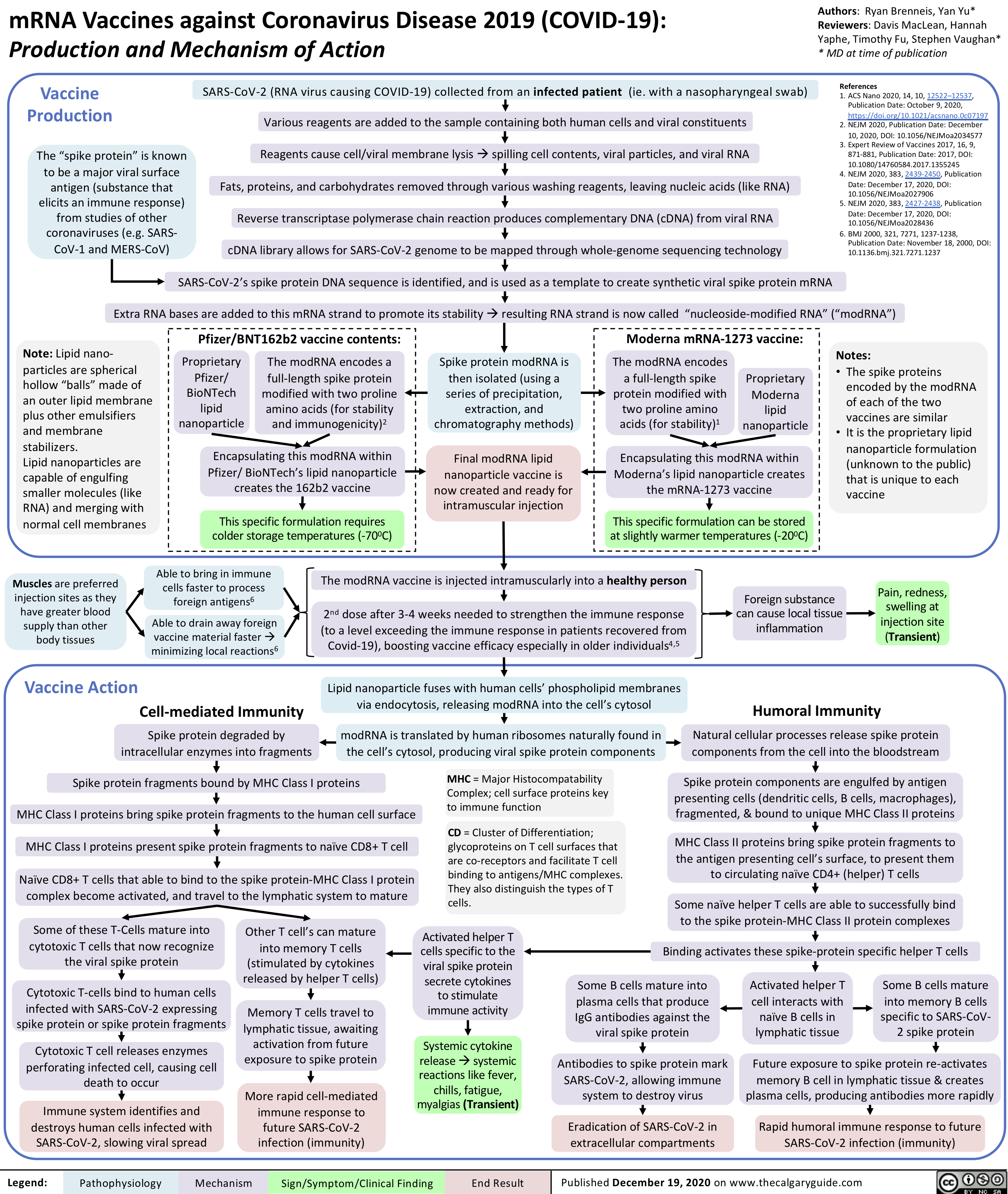
Adenovirus-Vector-Vaccines-Against-COVID19-Production-and-Mechanism-of-Action
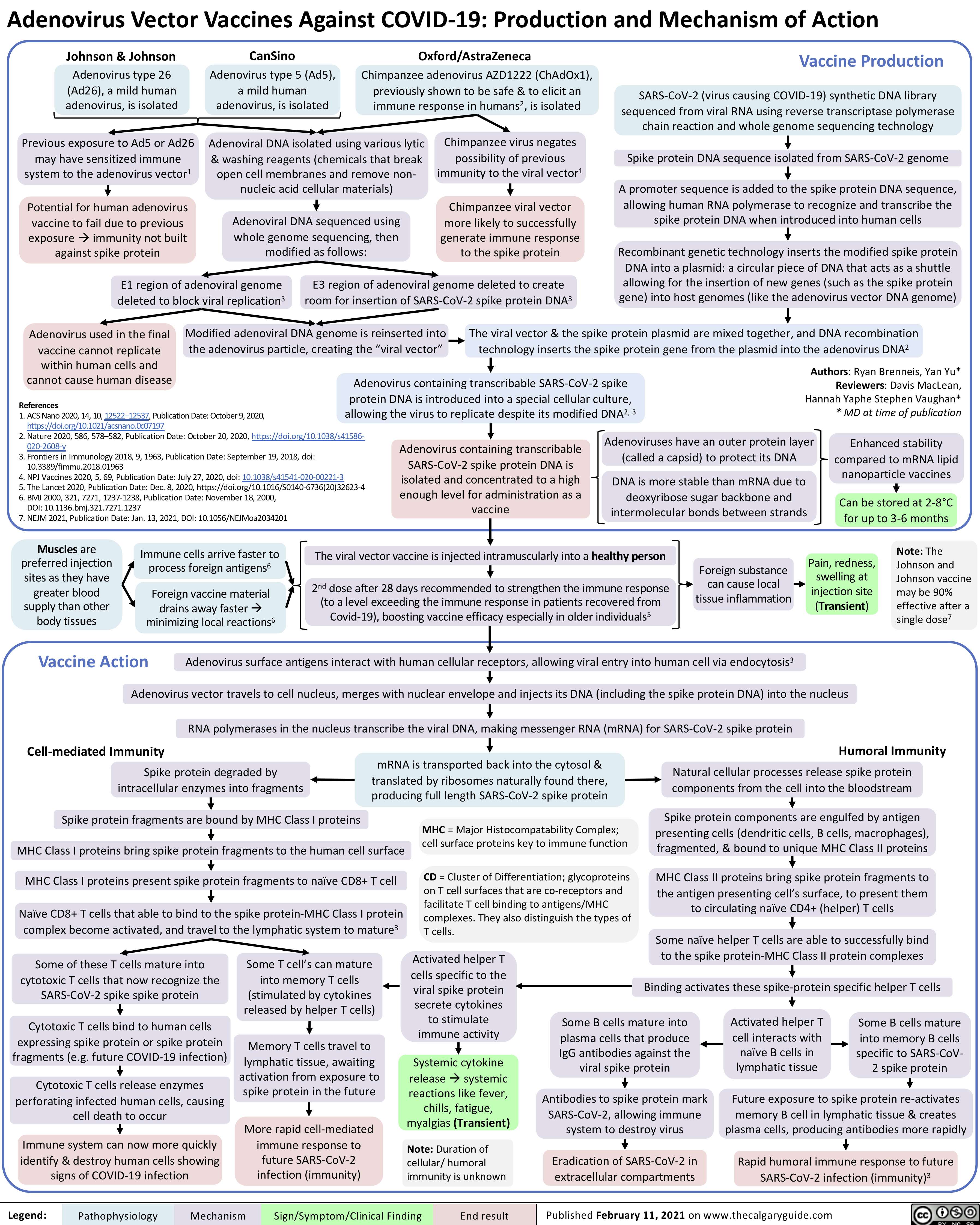
Potassium-Sparing-Diuretics-Mechanism-of-Action-and-Side-Effects
AAA-Pathogenesis
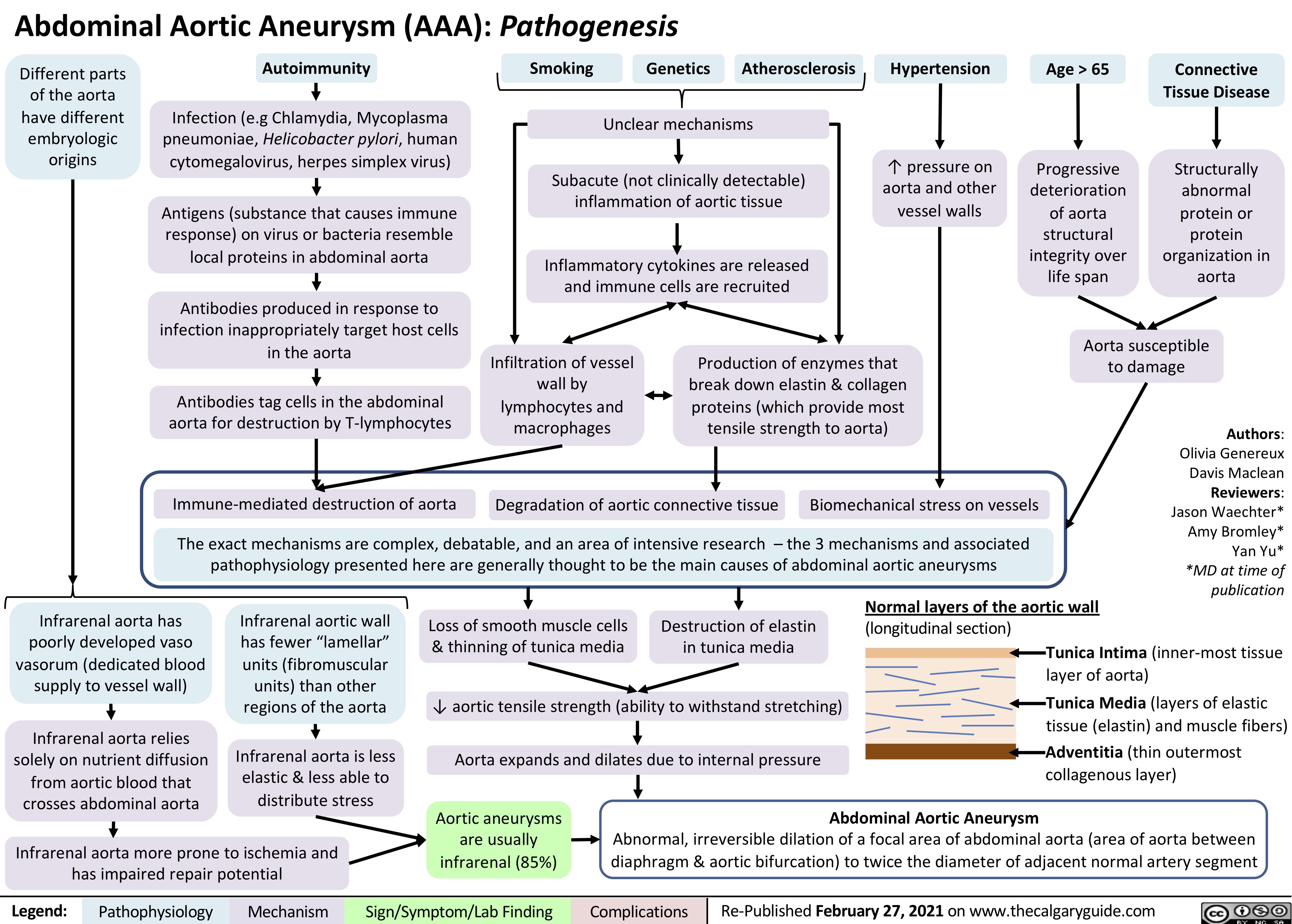
Cubital-Tunnel-Syndrome-Ulnar-Neuropathy
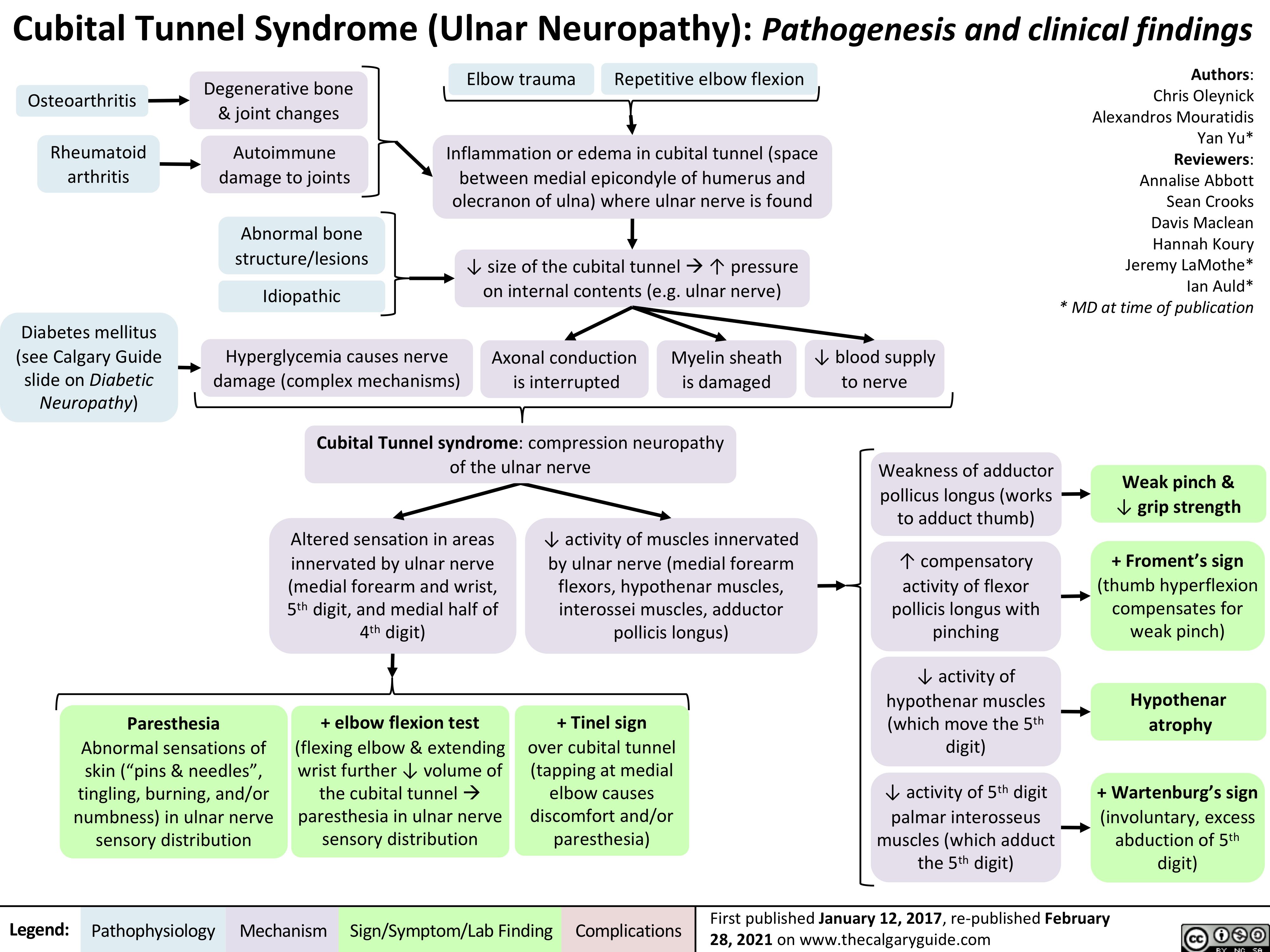
Achilles-Tendon-Rupture
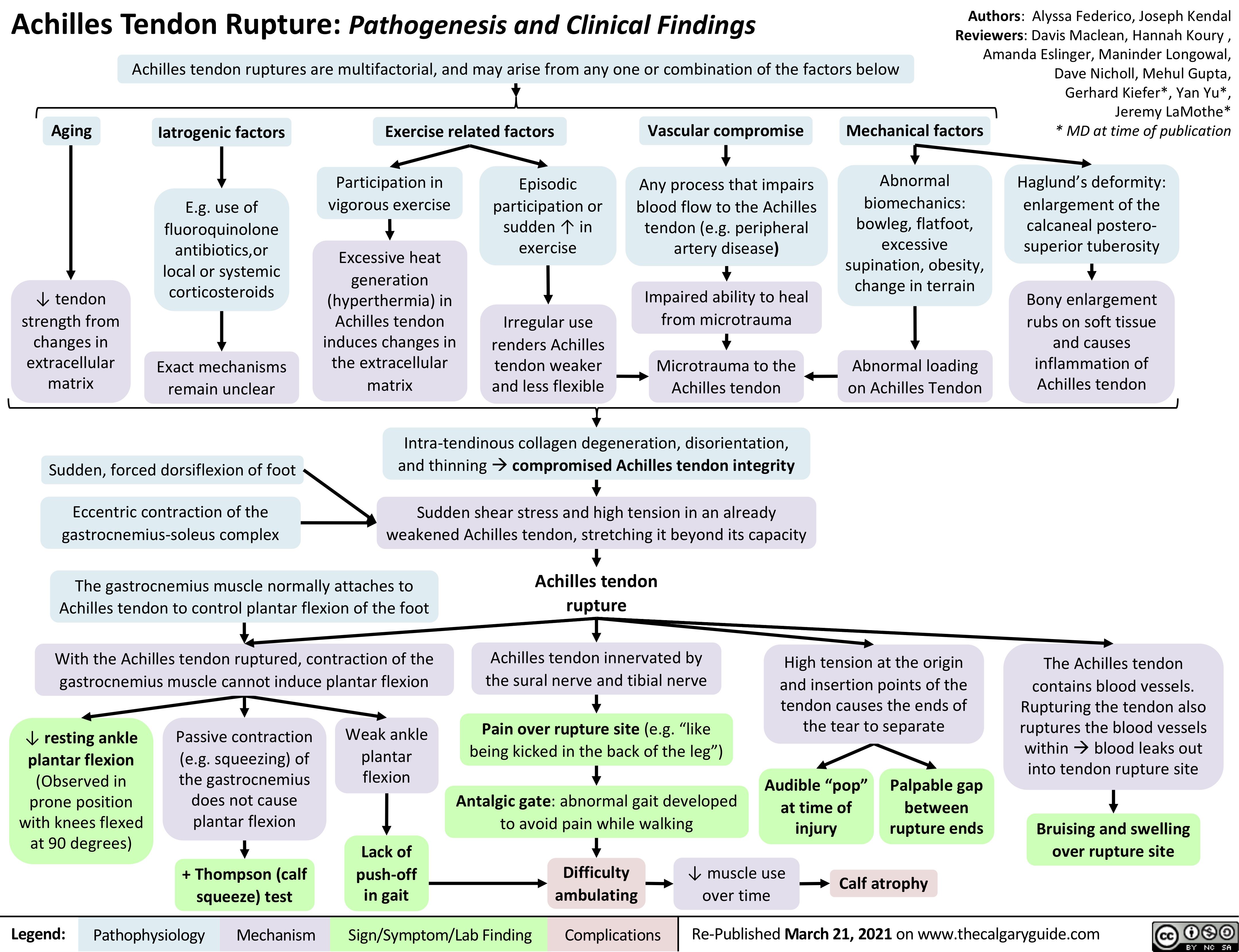
Thyroïdite
Fat-Embolism-Syndrome
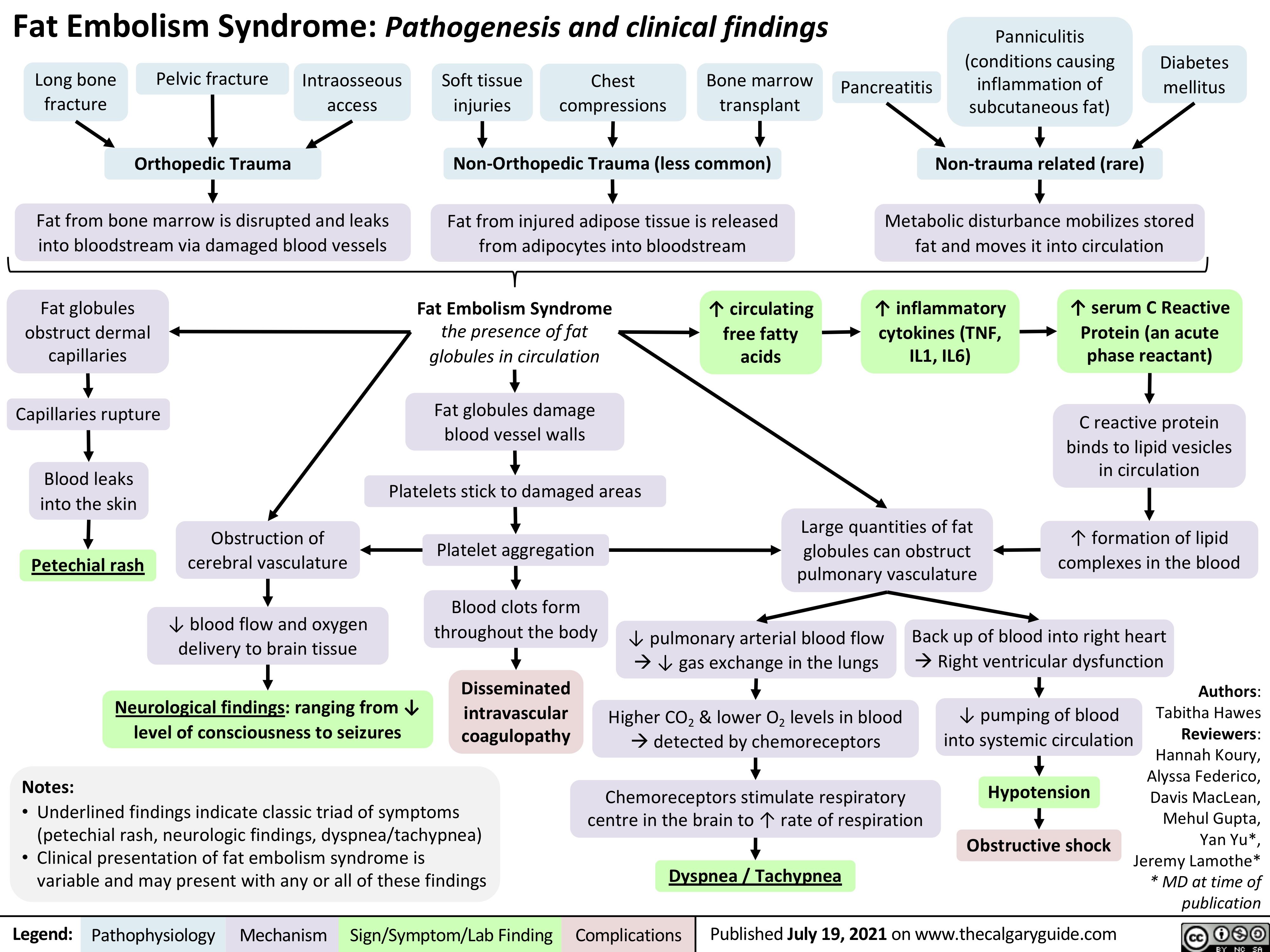
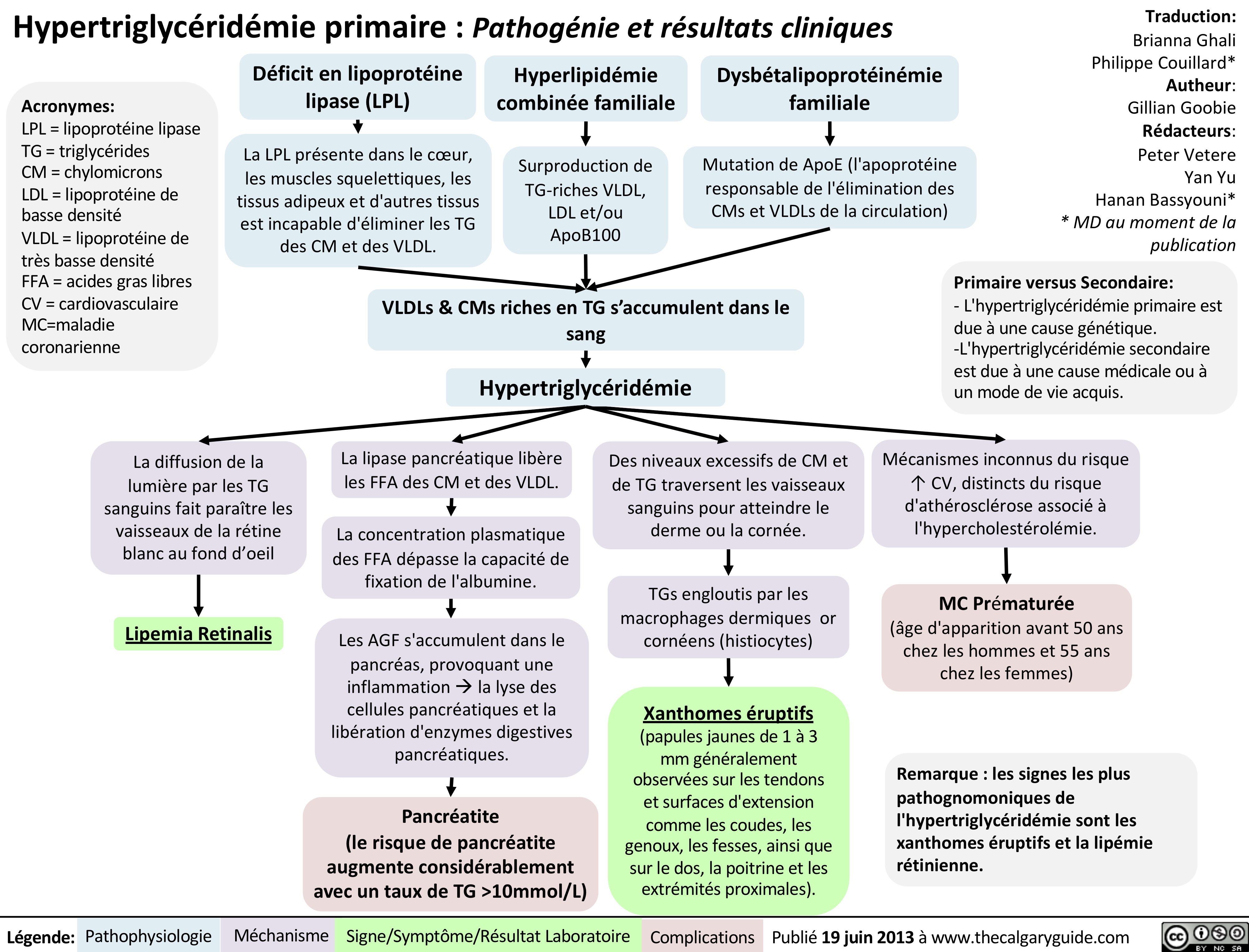
Hypertriglycéridémie primaire
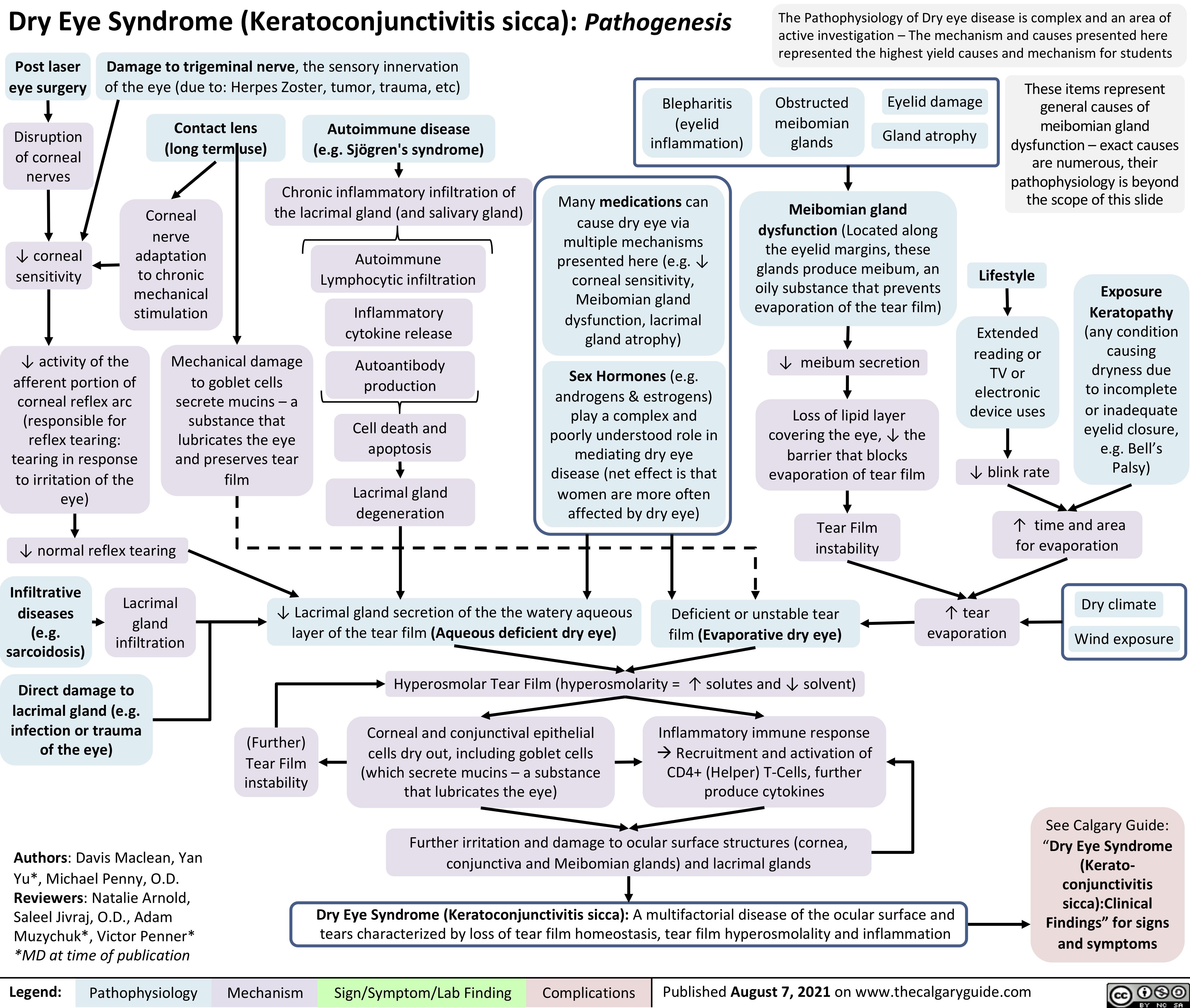
Dry-Eye-Syndrome-Pathogenesis
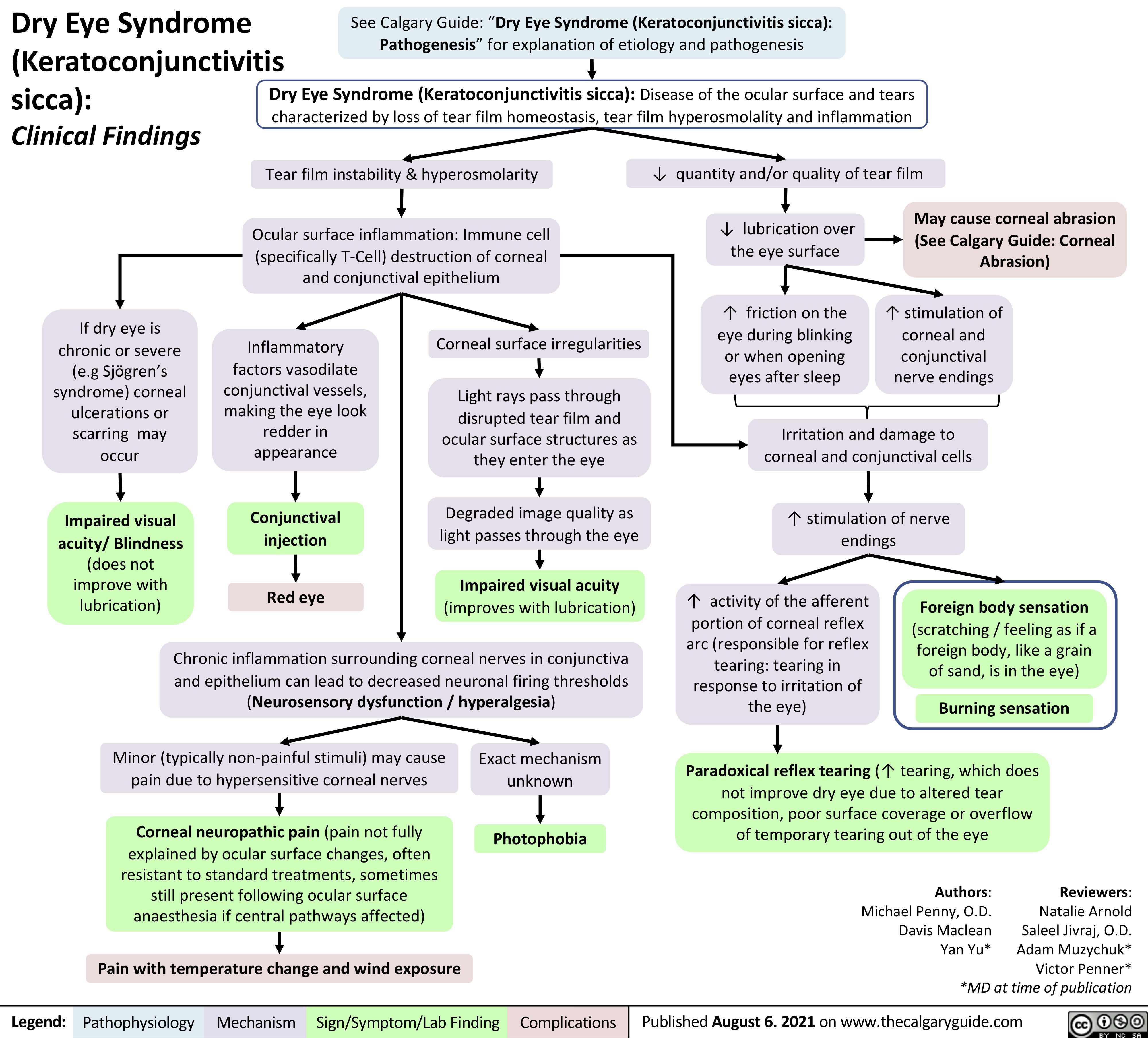
Dry-Eye-Syndrome-Clinical-Findings
epithelial-ovarian-cancer-pathogenesis-and-clinical-findings
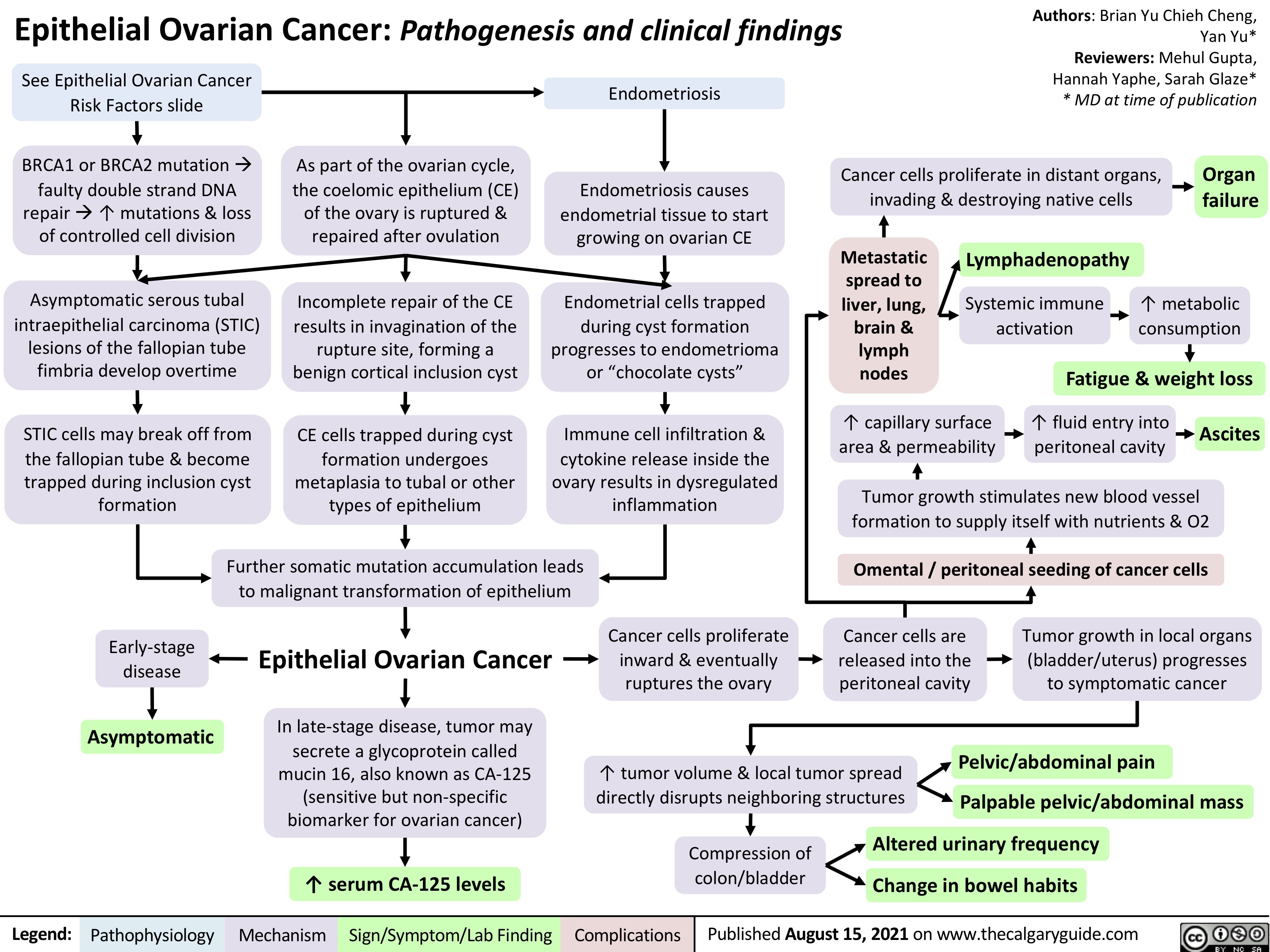
COPD Acute Exacerbations
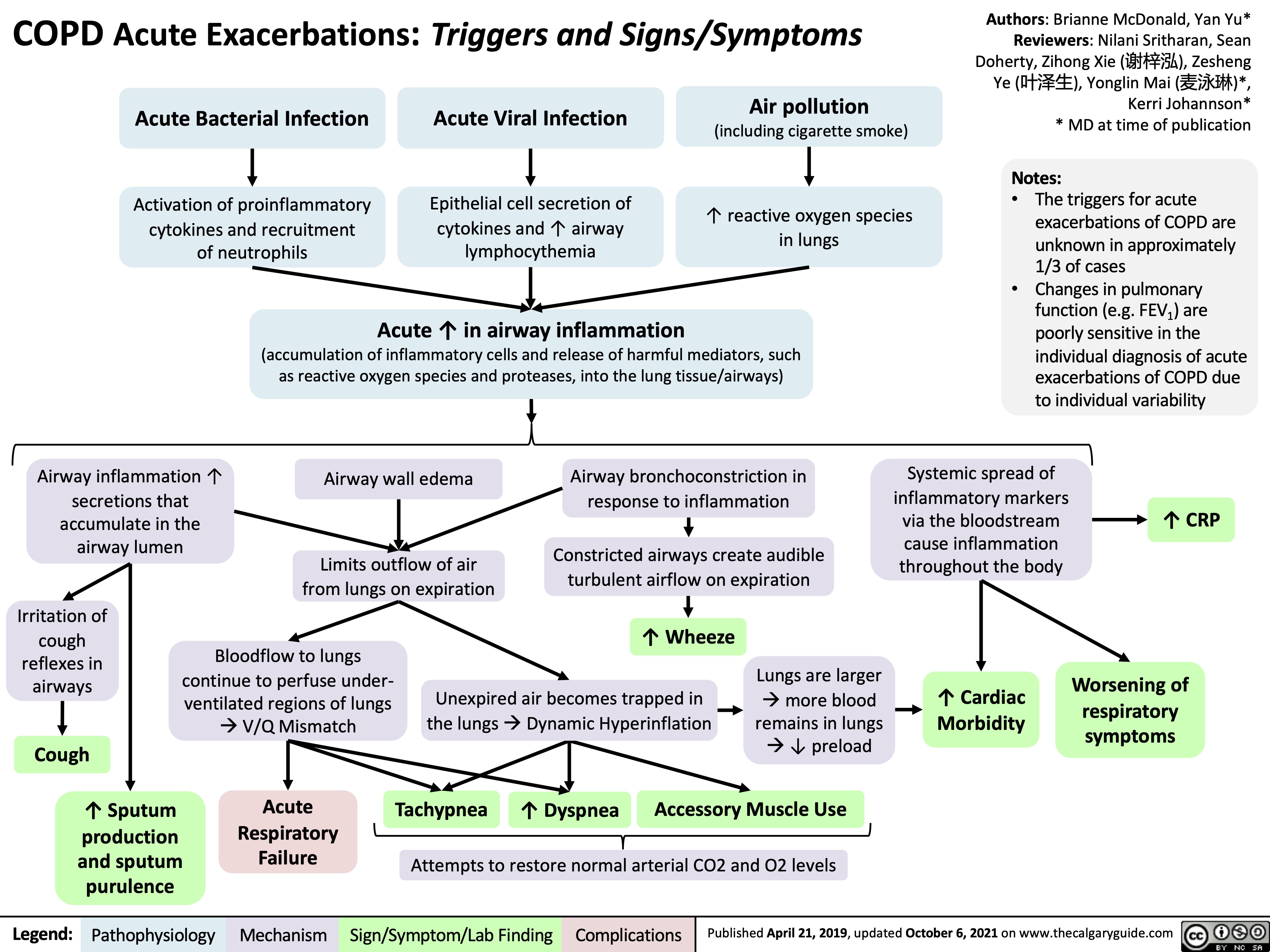
Hypercortisolemia
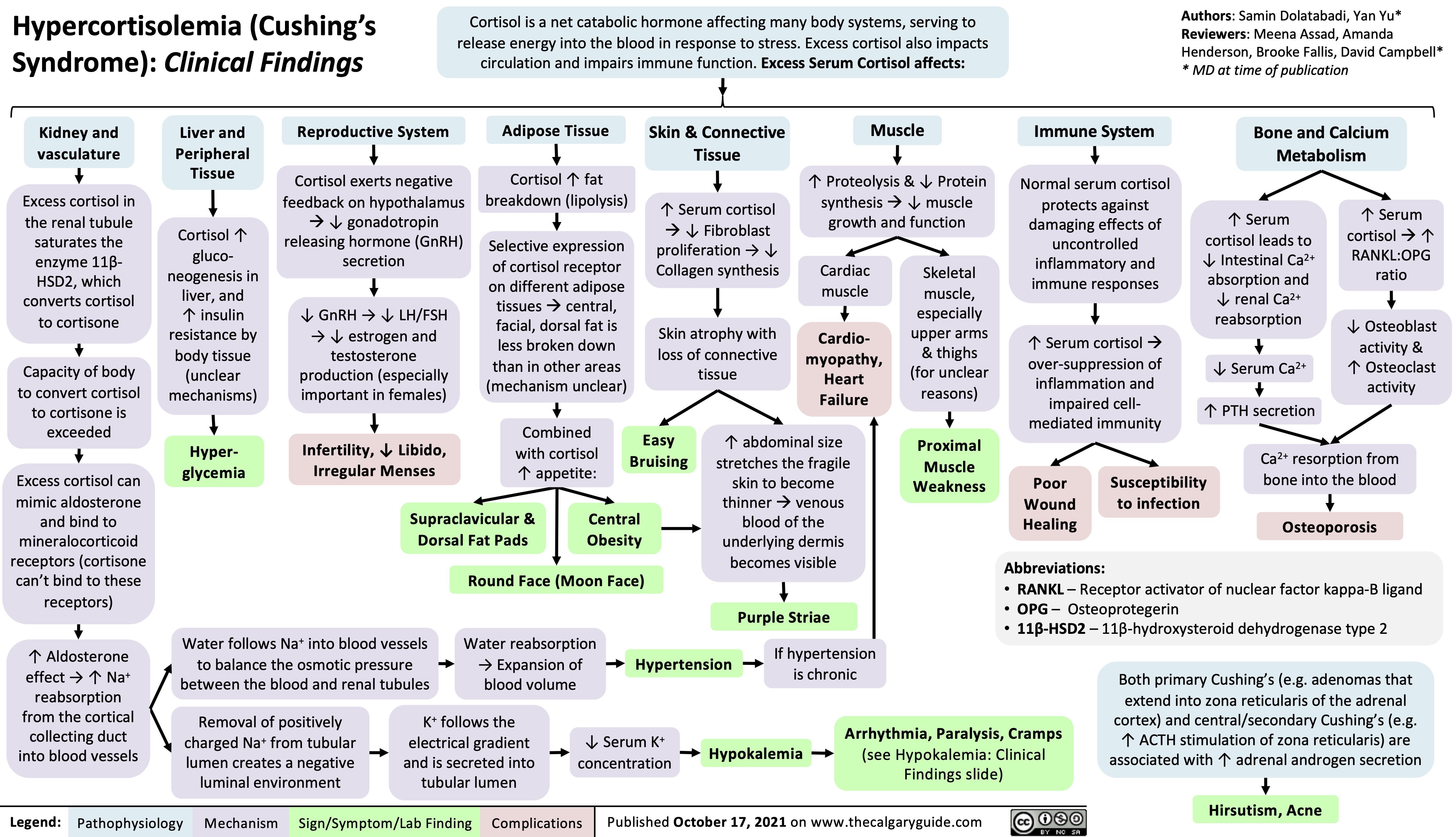
Acne Vulgaris Complications
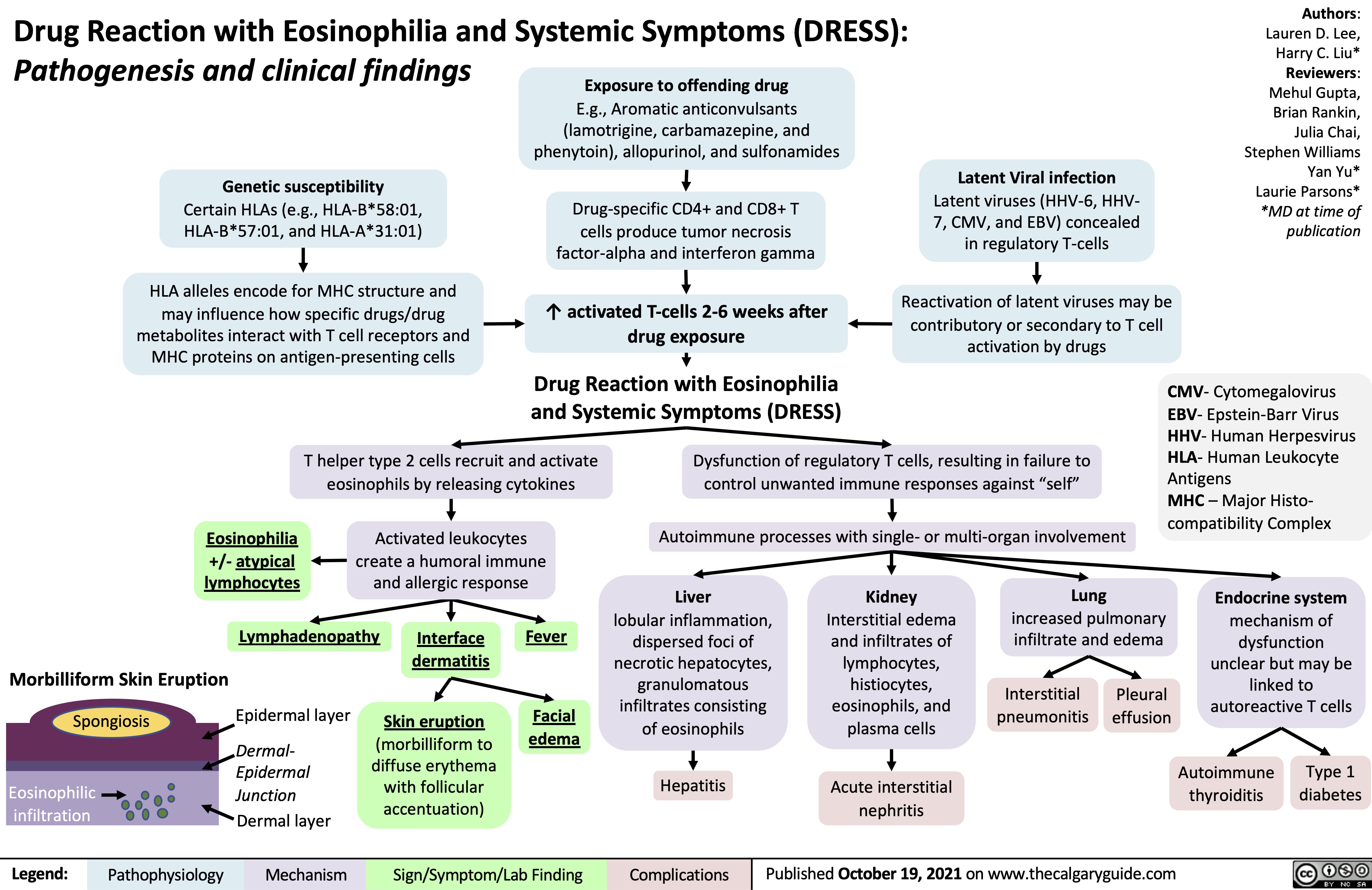
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)
Vestibular Neuritis

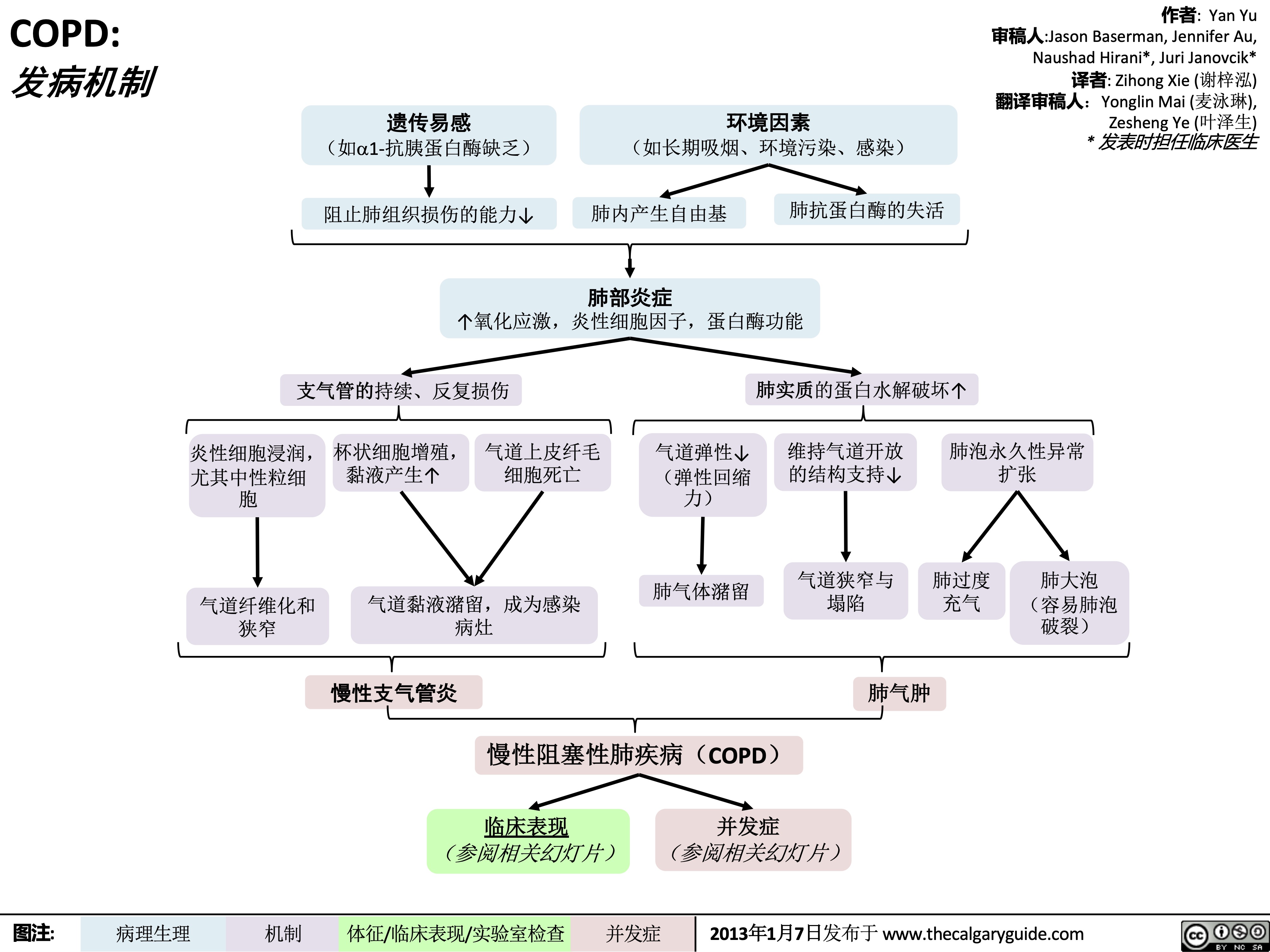
COPD-发病机制
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
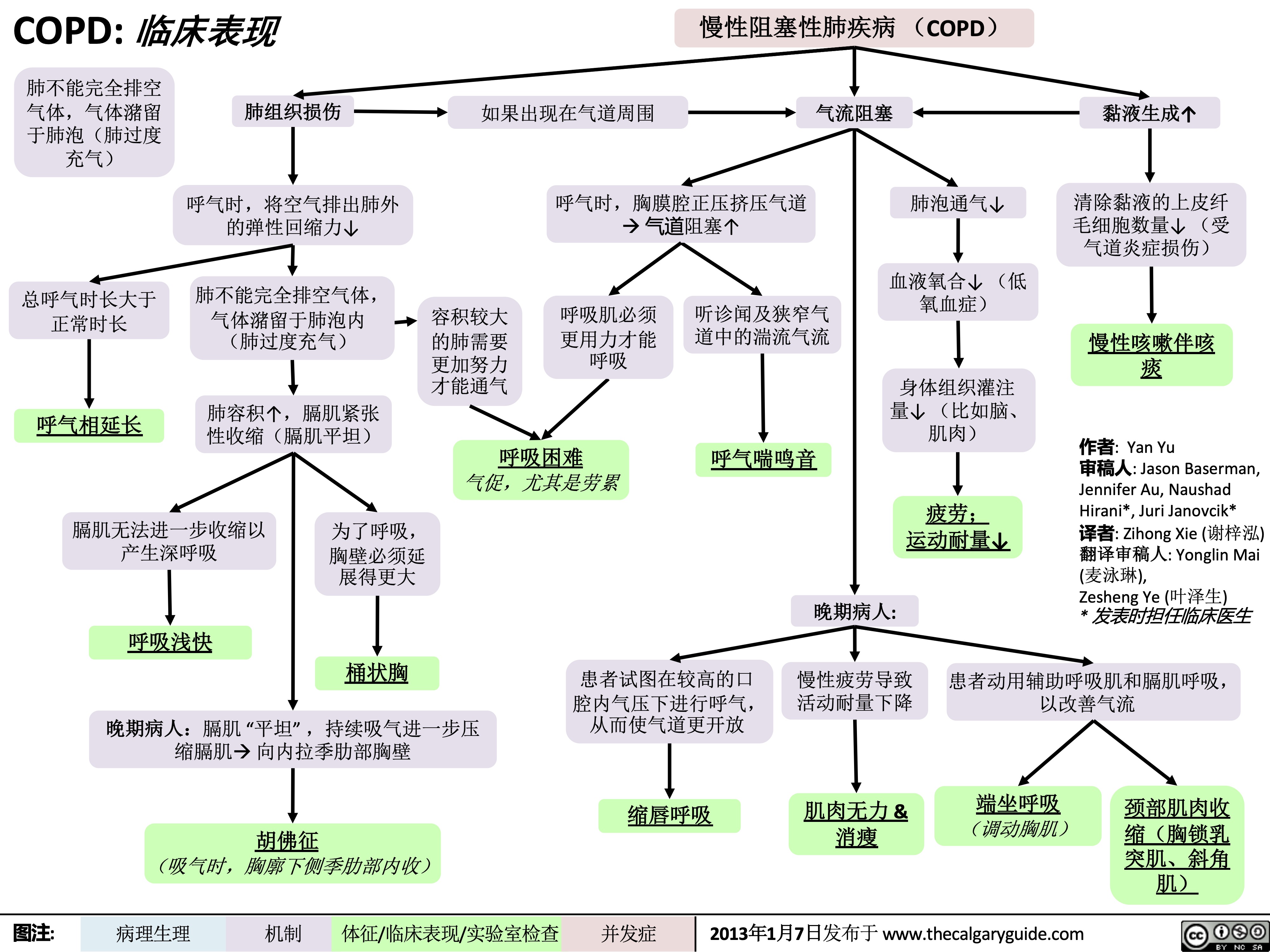
COPD-临床表现
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
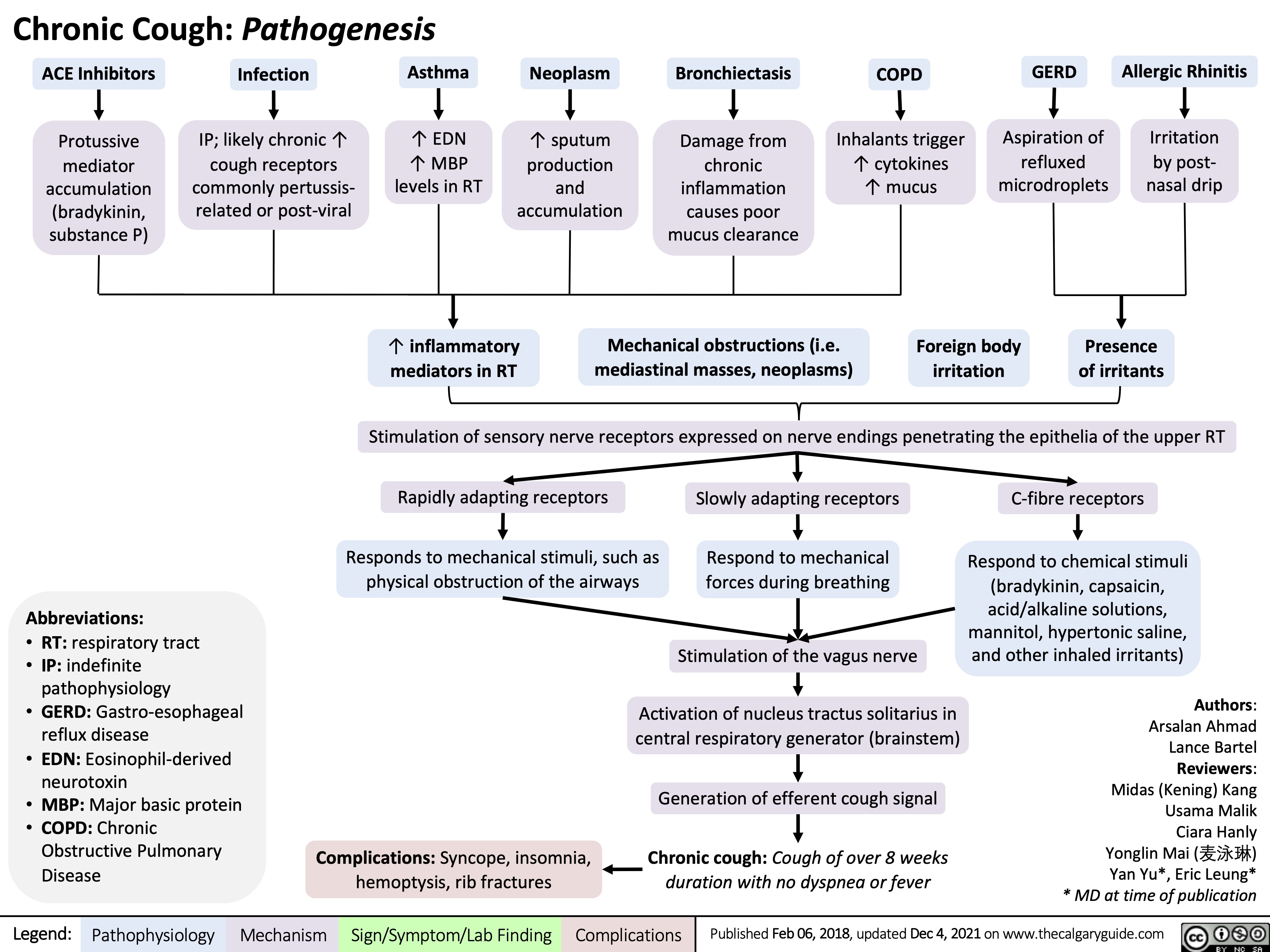
Chronic Cough Pathogenesis_2021
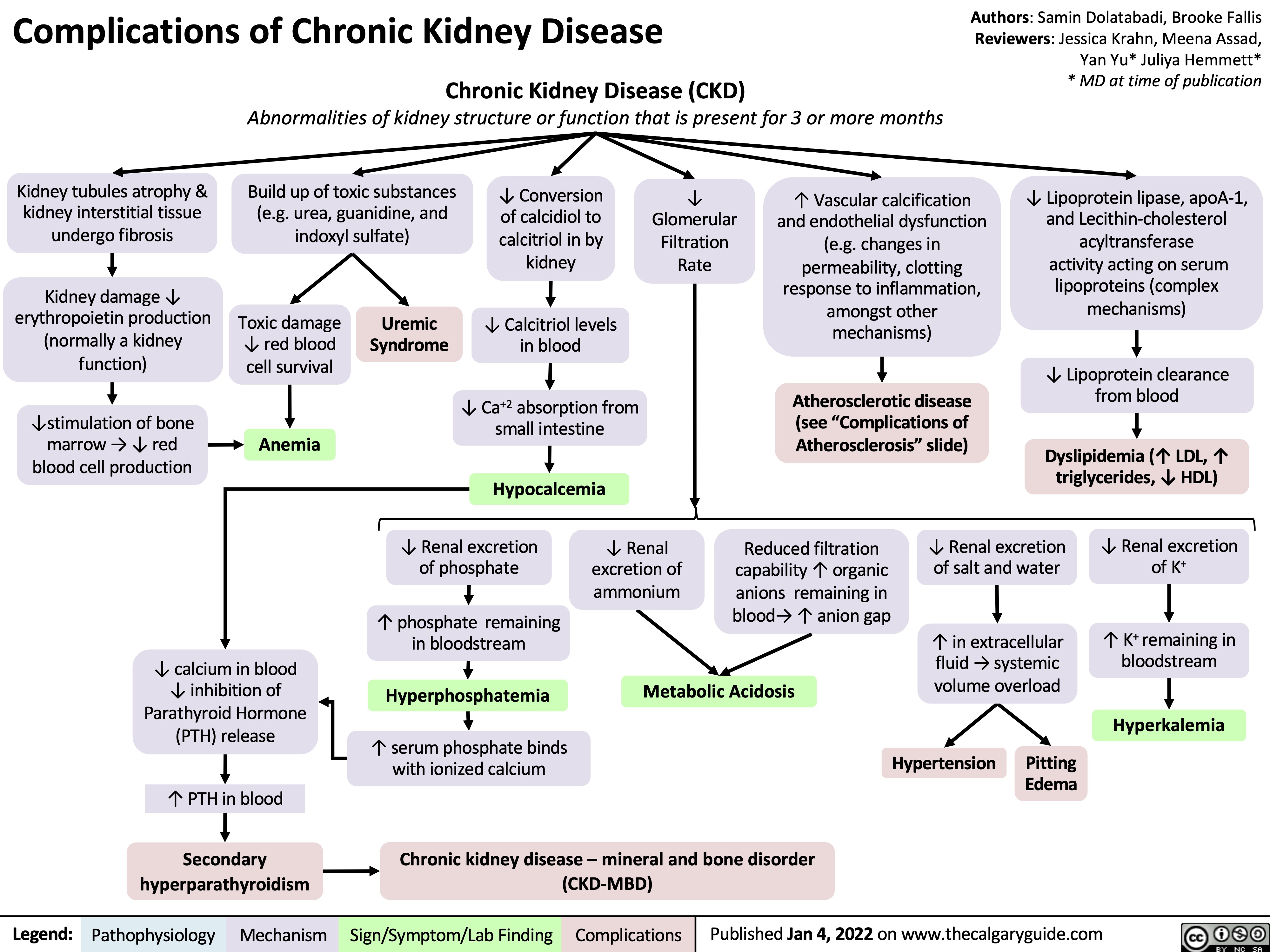
complications-of-chronic-kidney-disease-ckd
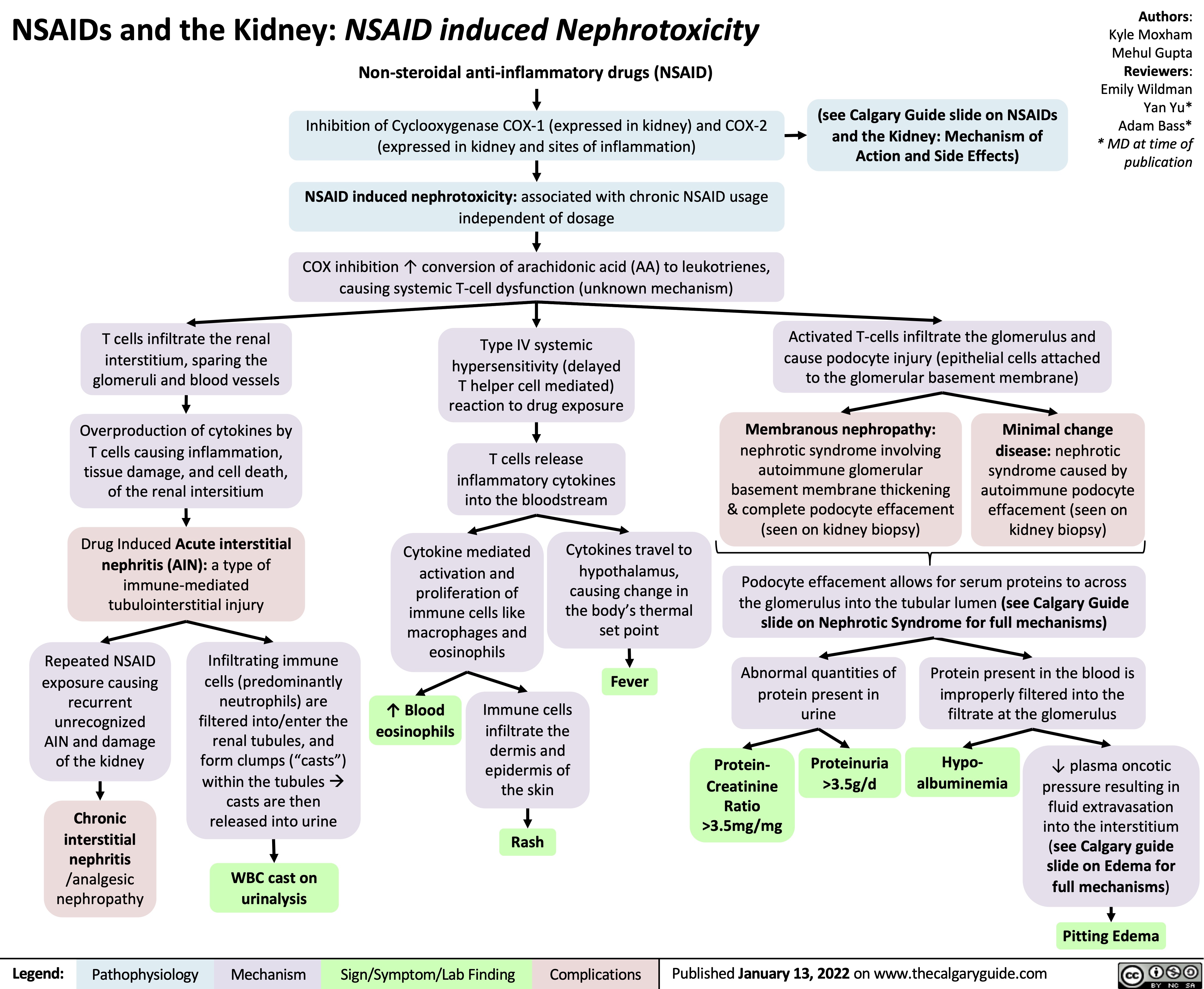
NSAIDs and the Kidney Nephrotoxicity
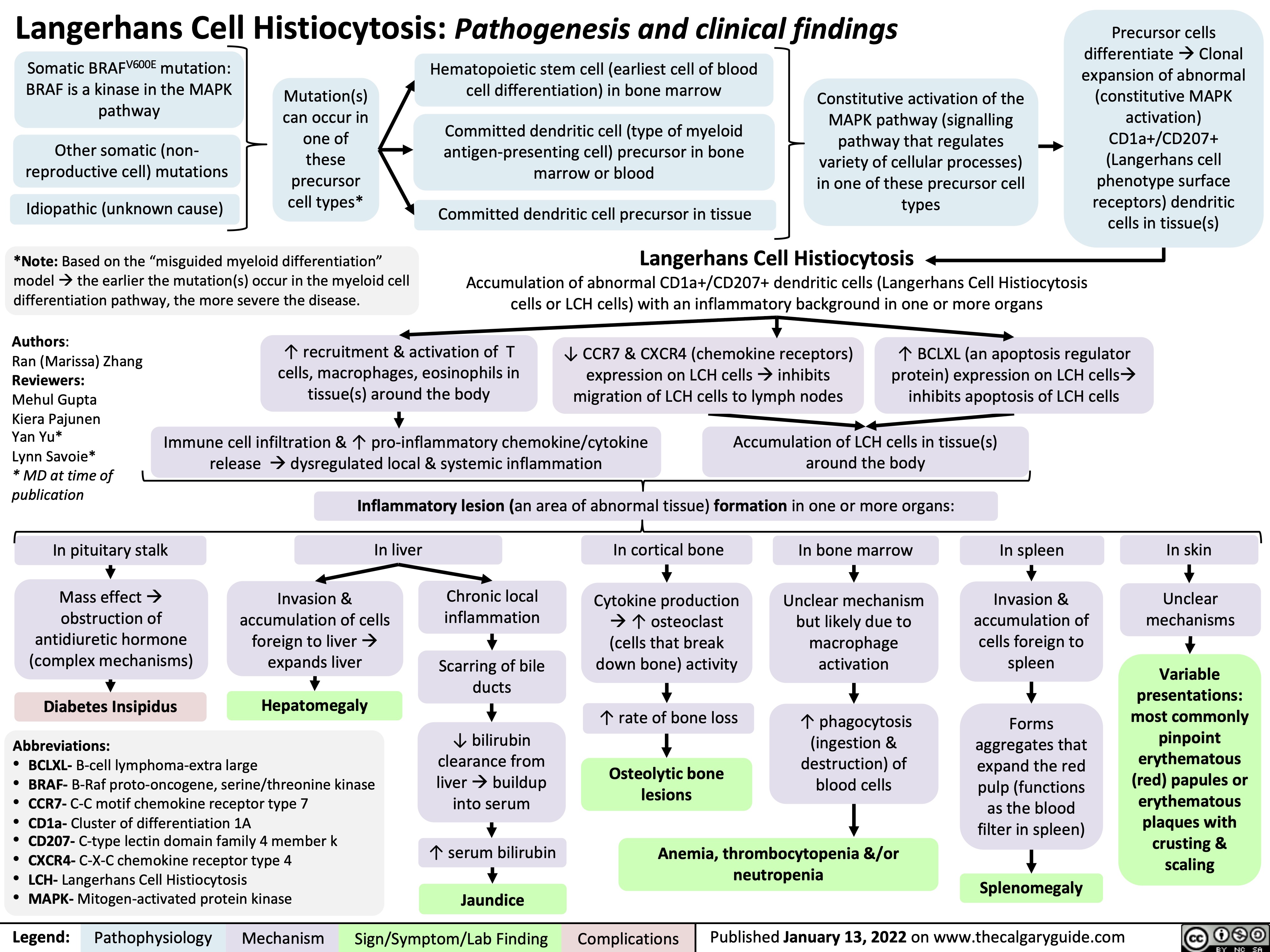
Langerhans Cell Histiocytosis
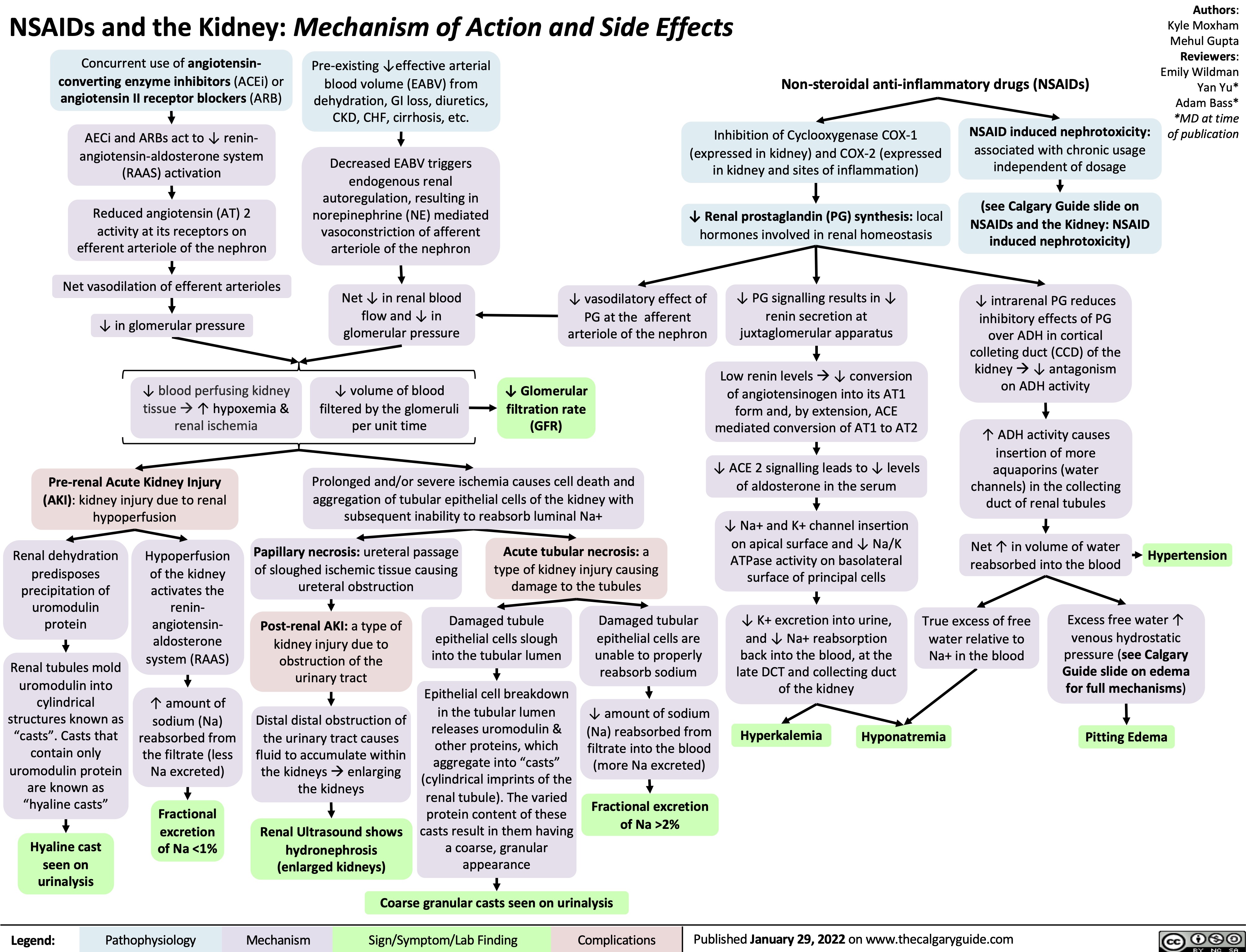
NSAIDs and the Kidney mechanism of action and side effects
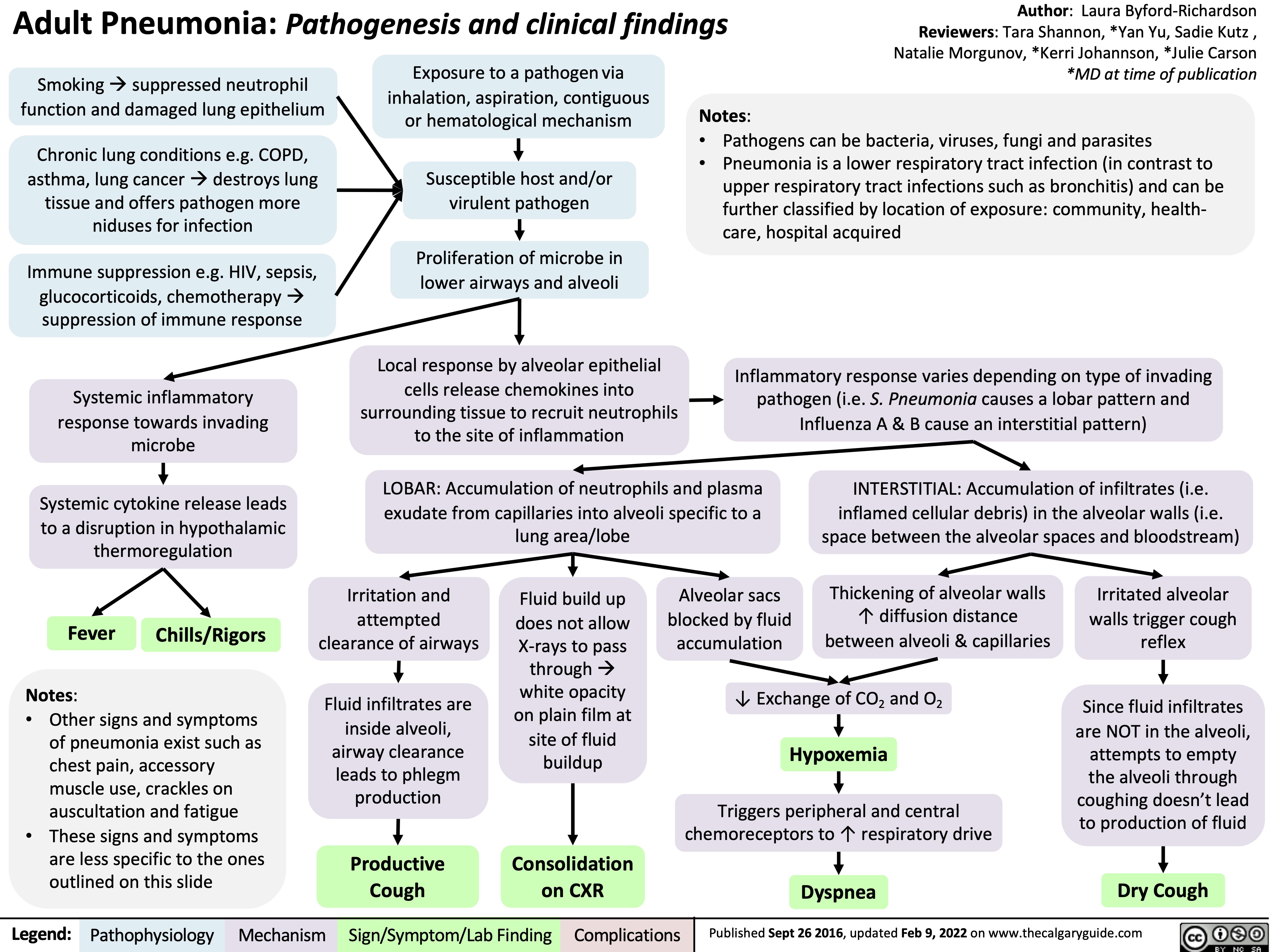
adult-pneumonia-pathogenesis-and-clinical-findings
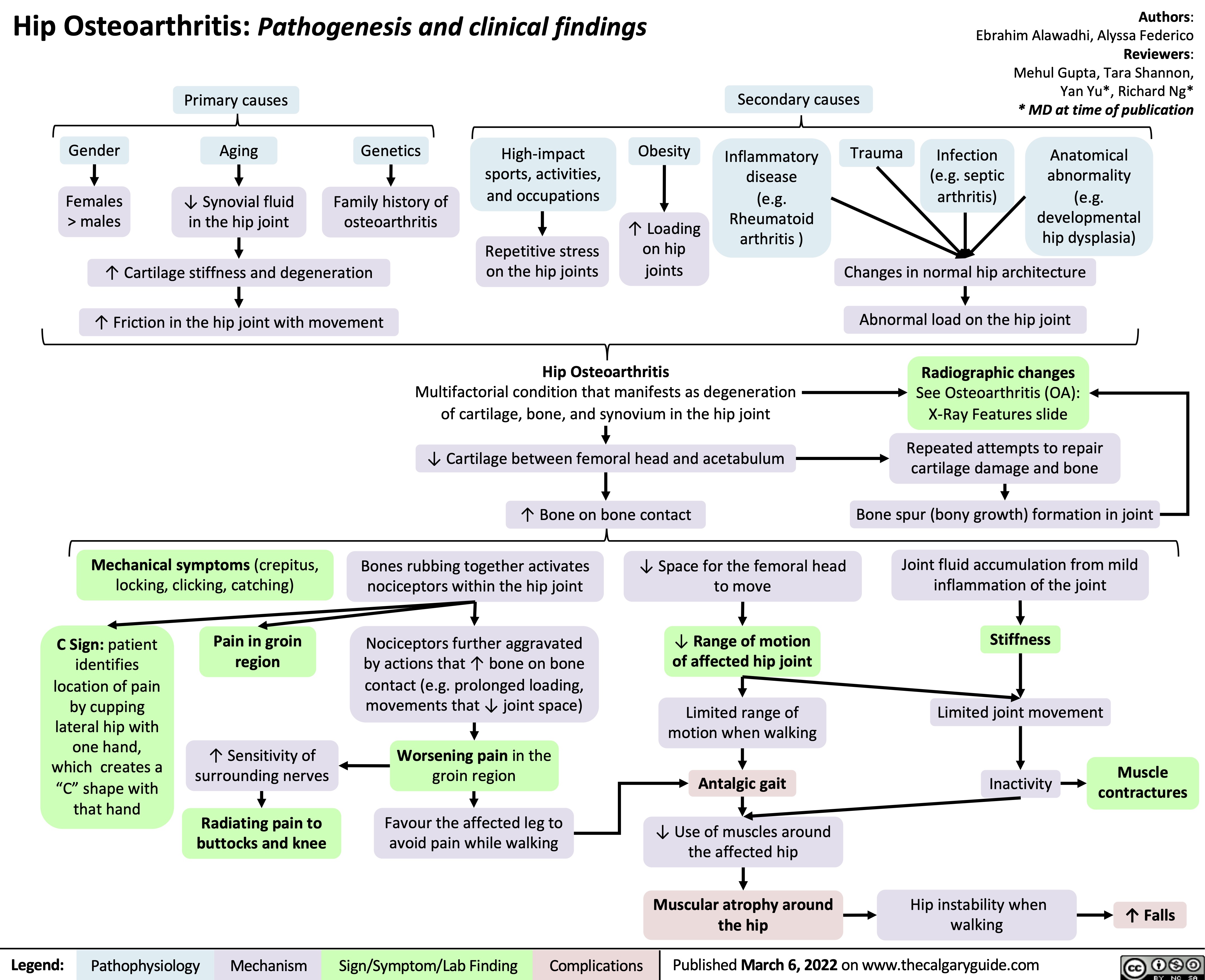
hip-osteoarthritis-pathogenesis-and-clinical-findings
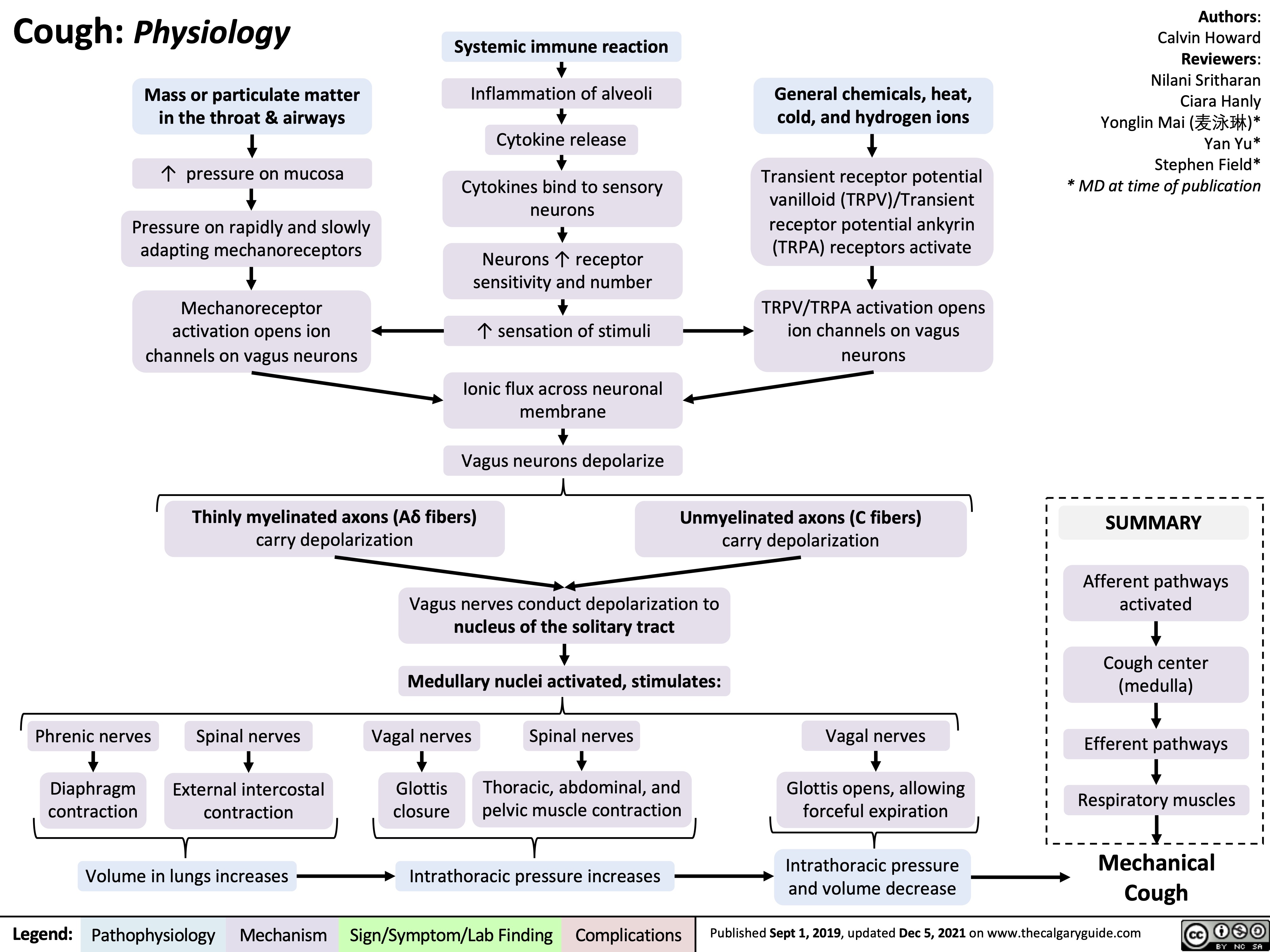
cough-physiology
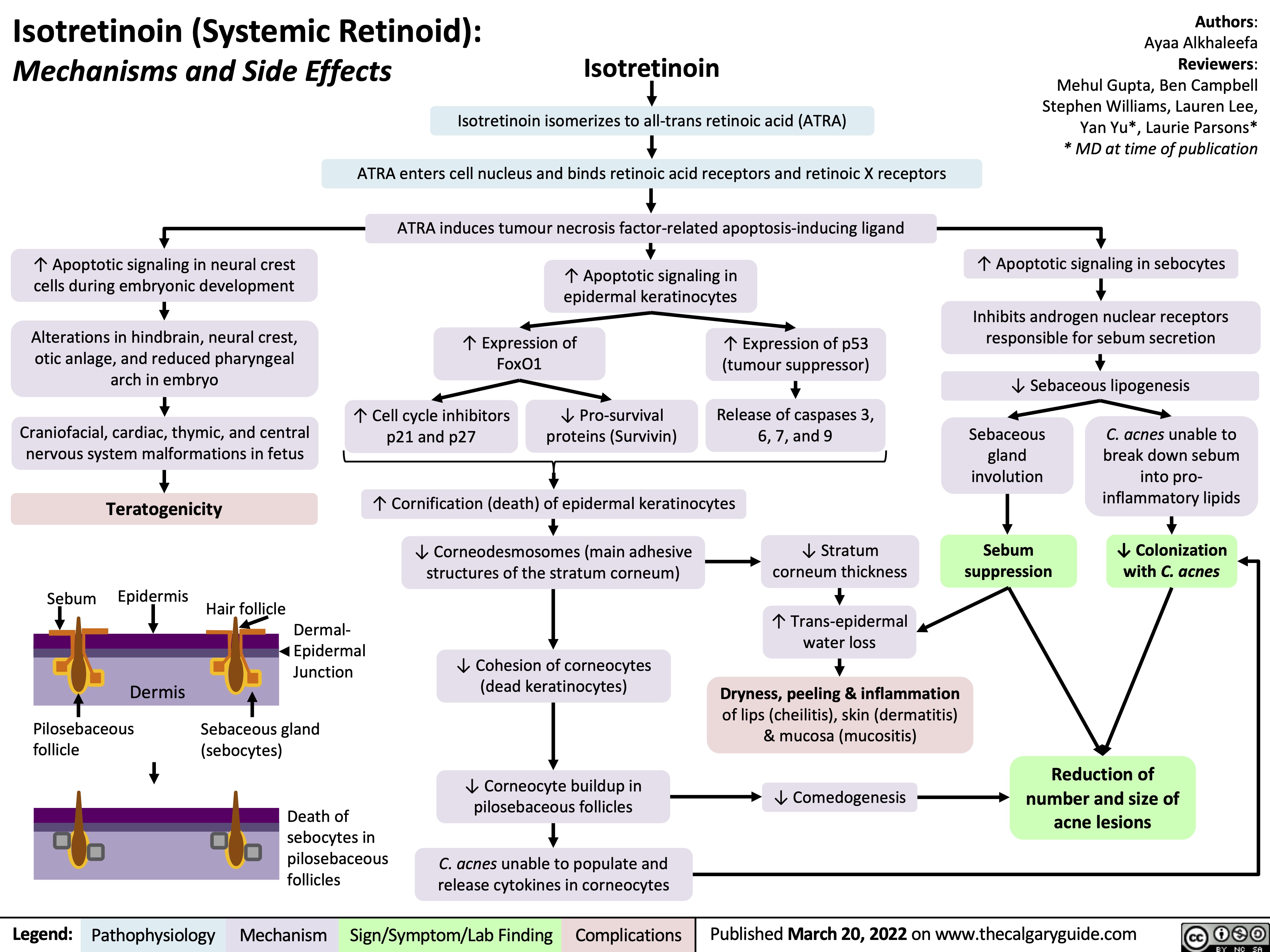
isotretinoin-systemic-retinoid-mechanisms-and-side-effects
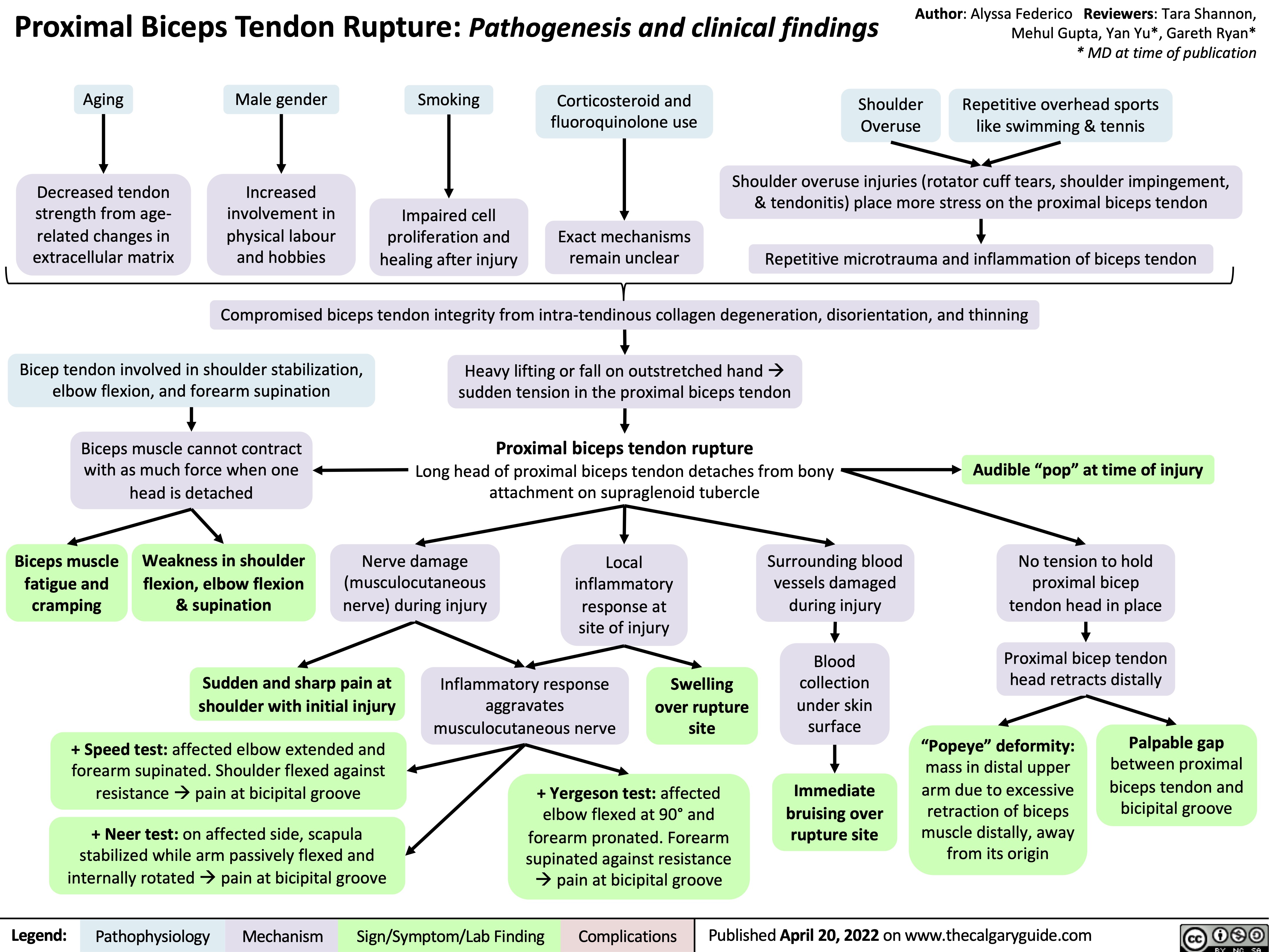
proximal-biceps-tendon-rupture
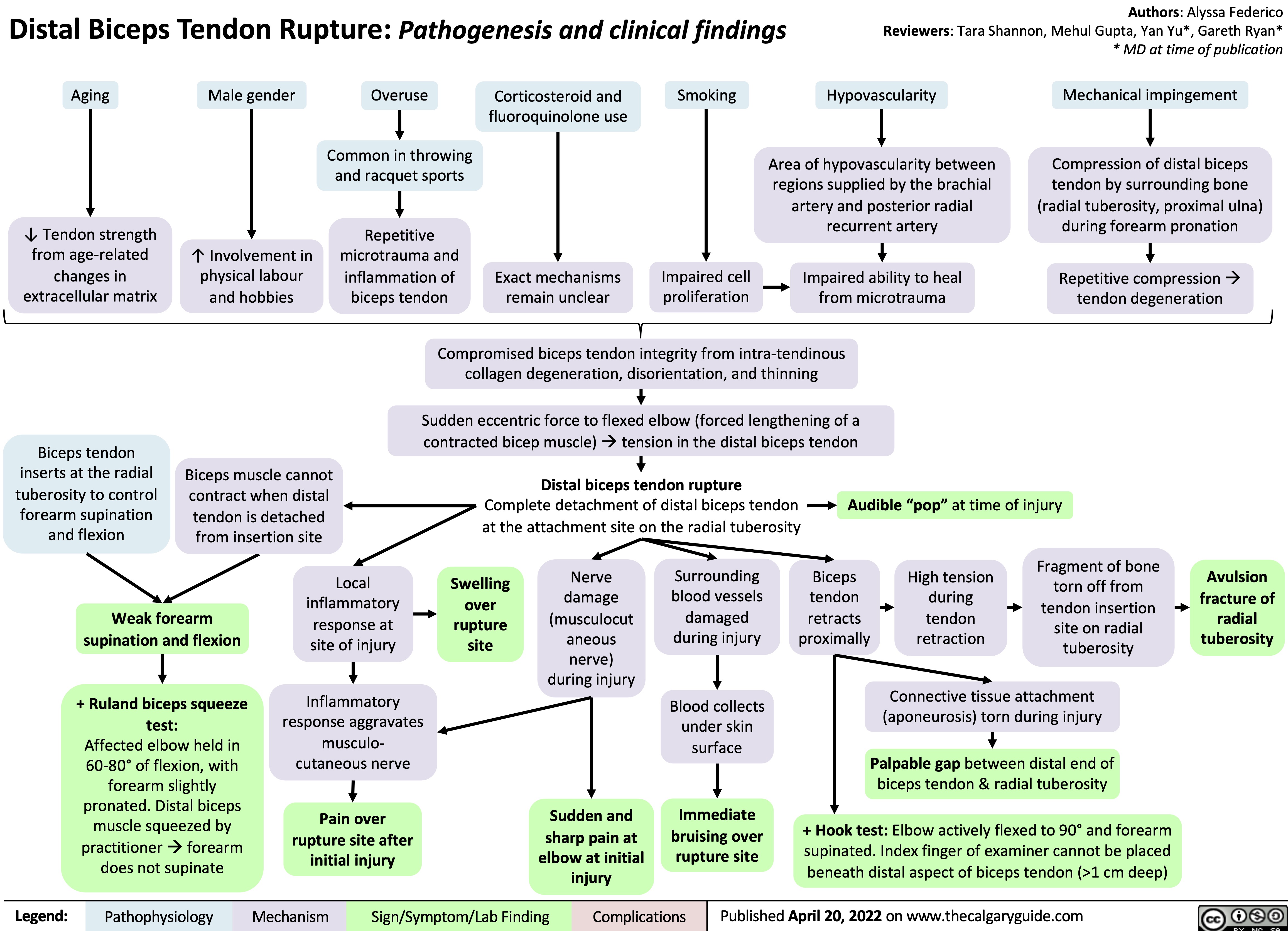
distal-biceps-tendon-rupture
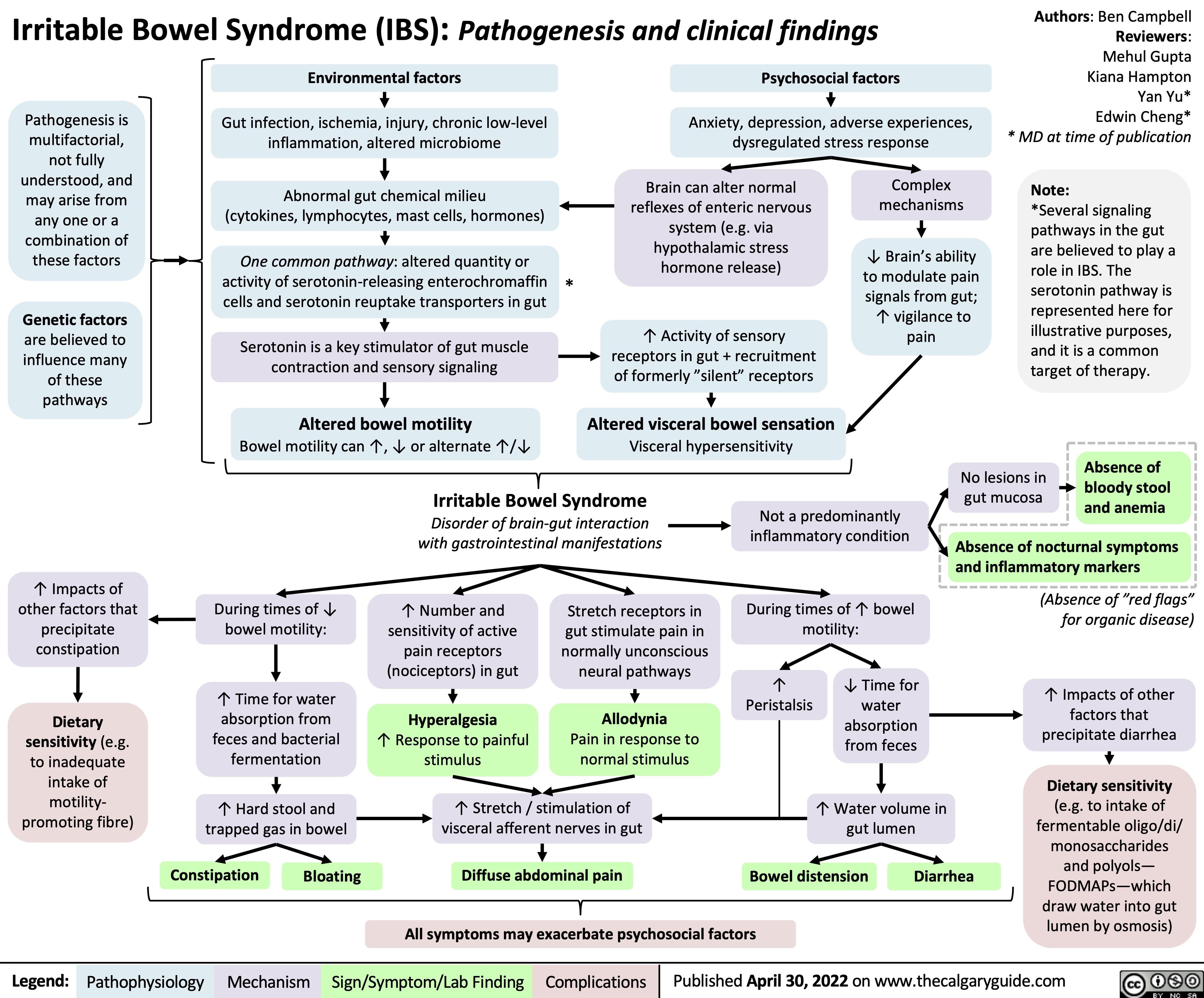
irritable-bowel-syndrome-ibs-pathogenesis-and-clinical-findings
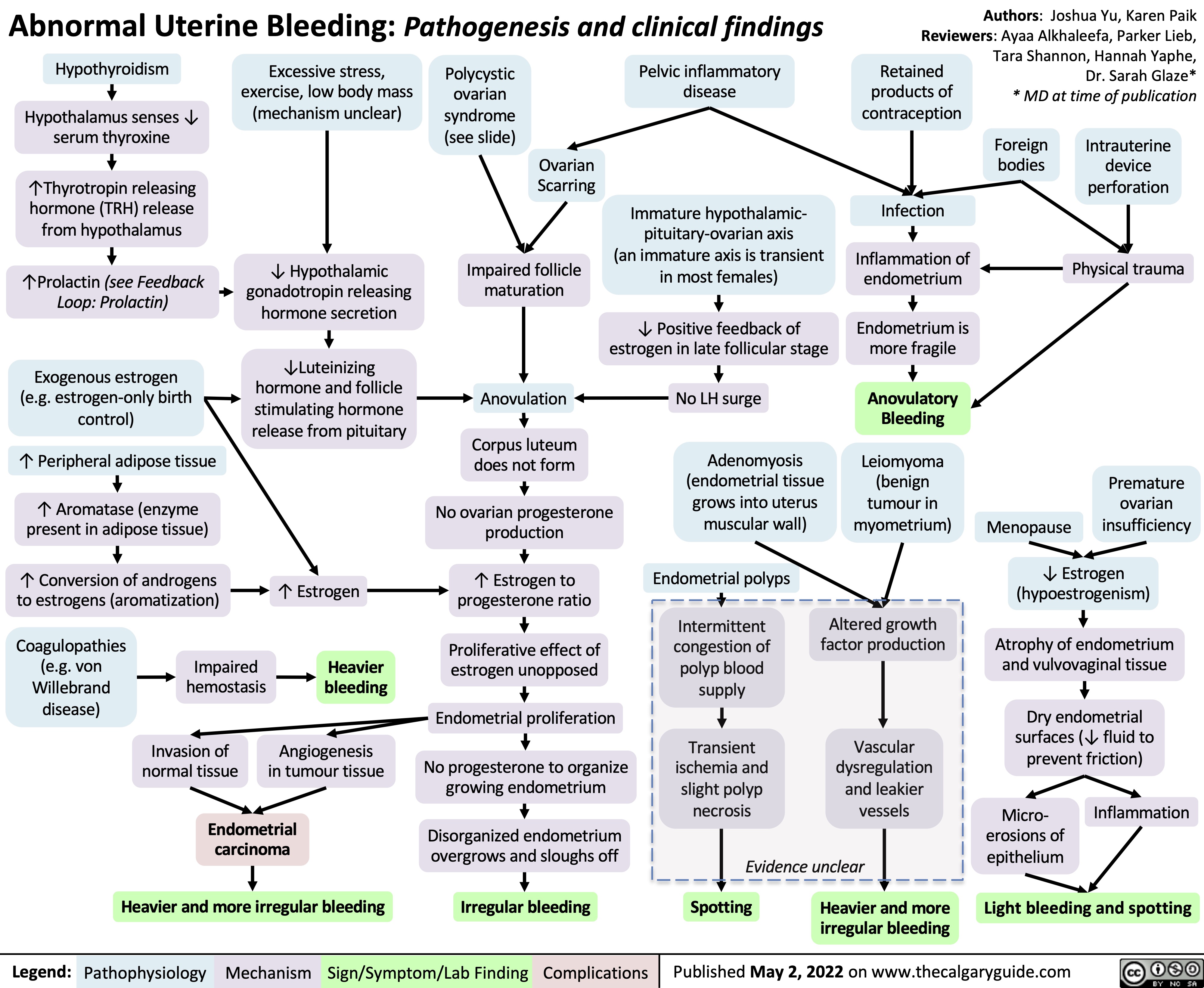
abnormal-uterine-bleeding-aub-pathogenesis-and-clinical-findings
rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
![rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
Psoriatic Arthritis: Pathogenesis and clinical findings
Auteur:
Payam Pournazari
Rédacteurs:
Yan Yu Scott Rapske Liam Martin* Traducteurs: Dianne Ganeswaran Stephen Williams Sylvain Coderre* * MD au moment de publication
Note:
L’arthrite rhumatoïde peut survenir avant ou après le psoriasis (la pathogenèse est la même).
Augmentation de l’activité ostéoclastique
Érosion de l’os sous- chondral mais formation de nouvelle osseuse ailleurs dans l’articulation
Radiographie: la périostite; les syndesmophytes, érosions, déformation du crayon en godets (les articulations IP), ankylose des articulations IP
HLA B27, Cw6 et d’autres sous- types
Antécédents familiaux positifs
Activation des cellules T (mécanisme inconnu)
Infiltration du tissu synovial de l’articulation par les cellules T et B, cellules tueuses naturelles et les macrophages
Production augmentée des molécules inflammatoires (les facteurs de nécrose tumorale [TNFs], les interleukines, etc.) qui agissent de façon systémique (dans tout le corps)
Dans les tendons et le tissu conjonctif
Inflammation des enthèses et du tissu conjonctif provoque le gonflement
Dans la peau
Réaction immunitaire détruisant les structures de la peau et des ongles (Voir diapositive Psoriasis)
Le psoriasis en plaques sur la peau, dépressions punctiformes, onychorrhexie, taches d’huile, onycholyse, décoloration et hyperkératose
Angiogenèse et vascularite dans les vaisseaux sanguins de la membrane synoviale
Dans les articulations
Les cytokines inflammatoires stimulent les nocicepteurs locaux
1. Oligoarthrites - asymétriques
2. Arthrite des articulations IPD
3. Polyarthrite rhumatoïde -
symétrique
4. Atteinte axiale (raison
inconnue, probablement en raison de « biomécanique », « innervation », et
« vascularisation ».
5. Arthrite mutilante (grave et destructrice)
L’enthésite
(Douleur/sensibilité à l’insertion du ligament dans l’os)
La dactylite (Inflammation de tout le doigt – les tissus mous et les articulations sont enflammés
Légende:
Physiopathologie
Mécanisme
Signe/Symptôme/Résultats de Laboratoire
Complications
Publié 10 November 2012 sur www.thecalgaryguide.com
rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
Psoriatic Arthritis: Pathogenesis and clinical findings
Auteur:
Payam Pournazari
Rédacteurs:
Yan Yu Scott Rapske Liam Martin* Traducteurs: Dianne Ganeswaran Stephen Williams Sylvain Coderre* * MD au moment de publication
Note:
L’arthrite rhumatoïde peut survenir avant ou après le psoriasis (la pathogenèse est la même).
Augmentation de l’activité ostéoclastique
Érosion de l’os sous- chondral mais formation de nouvelle osseuse ailleurs dans l’articulation
Radiographie: la périostite; les syndesmophytes, érosions, déformation du crayon en godets (les articulations IP), ankylose des articulations IP
HLA B27, Cw6 et d’autres sous- types
Antécédents familiaux positifs
Activation des cellules T (mécanisme inconnu)
Infiltration du tissu synovial de l’articulation par les cellules T et B, cellules tueuses naturelles et les macrophages
Production augmentée des molécules inflammatoires (les facteurs de nécrose tumorale [TNFs], les interleukines, etc.) qui agissent de façon systémique (dans tout le corps)
Dans les tendons et le tissu conjonctif
Inflammation des enthèses et du tissu conjonctif provoque le gonflement
Dans la peau
Réaction immunitaire détruisant les structures de la peau et des ongles (Voir diapositive Psoriasis)
Le psoriasis en plaques sur la peau, dépressions punctiformes, onychorrhexie, taches d’huile, onycholyse, décoloration et hyperkératose
Angiogenèse et vascularite dans les vaisseaux sanguins de la membrane synoviale
Dans les articulations
Les cytokines inflammatoires stimulent les nocicepteurs locaux
1. Oligoarthrites - asymétriques
2. Arthrite des articulations IPD
3. Polyarthrite rhumatoïde -
symétrique
4. Atteinte axiale (raison
inconnue, probablement en raison de « biomécanique », « innervation », et
« vascularisation ».
5. Arthrite mutilante (grave et destructrice)
L’enthésite
(Douleur/sensibilité à l’insertion du ligament dans l’os)
La dactylite (Inflammation de tout le doigt – les tissus mous et les articulations sont enflammés
Légende:
Physiopathologie
Mécanisme
Signe/Symptôme/Résultats de Laboratoire
Complications
Publié 10 November 2012 sur www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/05/Psoriatic-Arthritis-PsA-Pathclinical.jpg)
acute-cholecystitis
![Acute Cholecystitis: Pathogenesis and clinical findings
Gallstone blocks the cystic duct, backing up bile into the gallbladder
Gallstones causing physical trauma to gallbladder wall
Irritation of adjacent diaphragm, stimulates phrenic nerve (C3-C5)
Activates stretch receptors of visceral peritoneum, stimulates foregut autonomic nerves (T5-T8)
Inflammatory mediator (i.e. prostaglandin) release by gallbladder and systemic inflammatory response
Thickened gallbladder wall on ultrasound (gold standard test)
On inspiration, the diaphragm pushes the gallbladder downward
Irritation of parietal peritoneum, stimulates somatic nerves
↑ Permeability of vessels with systemic inflammation, which leak
fluid from the blood into the interstitial space
Radiating pain to the back and right shoulder
Dull, diffuse abdominal pain referred to the epigastric region
Fever, nausea/vomiting, tachycardia
Positive Murphy’s sign (pain upon palpation of right upper quadrant [RUQ] on inspiration)
Persistent RUQ pain, abdominal guarding and peritoneal signs
Dehydration
Authors: Yan Yu, Vina Fan Reviewers: Dean Percy, Mirna Matta, Crystal Liu, Ben Campbell Maitreyi Raman* * MD at time of publication
Inflammation self-perpetuates
Irritation of inner gallbladder wall/mucosa
↑ Gallbladder lumen pressure
Intraluminal pressure exceeds arterial pressure
↓ Blood flow to gallbladder
Gallbladder ischemia
Local inflammation, loss of gallbladder mucosal integrity
Bacterial invasionàtransmural inflammation of gallbladder
Without treatment, prolonged ischemia and inflammation of the gallbladder
Gallbladder gangrene (20%)
Gallbladder perforation (20%)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 4 2019, updated May 16 2022 on www.thecalgaryguide.com
Acute Cholecystitis: Pathogenesis and clinical findings
Gallstone blocks the cystic duct, backing up bile into the gallbladder
Gallstones causing physical trauma to gallbladder wall
Irritation of adjacent diaphragm, stimulates phrenic nerve (C3-C5)
Activates stretch receptors of visceral peritoneum, stimulates foregut autonomic nerves (T5-T8)
Inflammatory mediator (i.e. prostaglandin) release by gallbladder and systemic inflammatory response
Thickened gallbladder wall on ultrasound (gold standard test)
On inspiration, the diaphragm pushes the gallbladder downward
Irritation of parietal peritoneum, stimulates somatic nerves
↑ Permeability of vessels with systemic inflammation, which leak
fluid from the blood into the interstitial space
Radiating pain to the back and right shoulder
Dull, diffuse abdominal pain referred to the epigastric region
Fever, nausea/vomiting, tachycardia
Positive Murphy’s sign (pain upon palpation of right upper quadrant [RUQ] on inspiration)
Persistent RUQ pain, abdominal guarding and peritoneal signs
Dehydration
Authors: Yan Yu, Vina Fan Reviewers: Dean Percy, Mirna Matta, Crystal Liu, Ben Campbell Maitreyi Raman* * MD at time of publication
Inflammation self-perpetuates
Irritation of inner gallbladder wall/mucosa
↑ Gallbladder lumen pressure
Intraluminal pressure exceeds arterial pressure
↓ Blood flow to gallbladder
Gallbladder ischemia
Local inflammation, loss of gallbladder mucosal integrity
Bacterial invasionàtransmural inflammation of gallbladder
Without treatment, prolonged ischemia and inflammation of the gallbladder
Gallbladder gangrene (20%)
Gallbladder perforation (20%)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 4 2019, updated May 16 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Acute-Cholecystitis-2022.jpg)
clostridium-difficile-infection-pathogenesis-and-clinical-findings
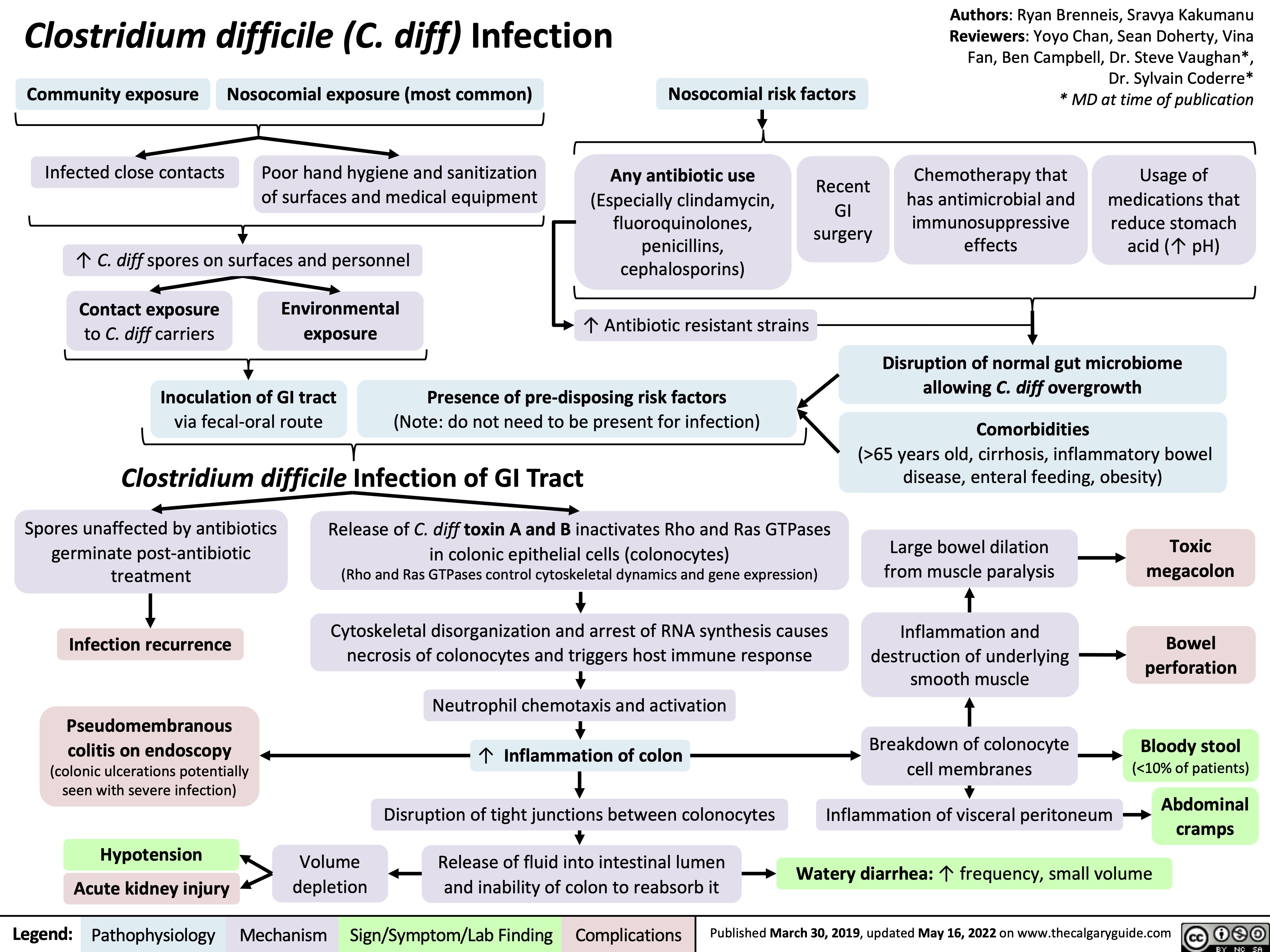
constatations-classiques-de-sclerose-en-plaques-sep-sur-irm-cerebrale
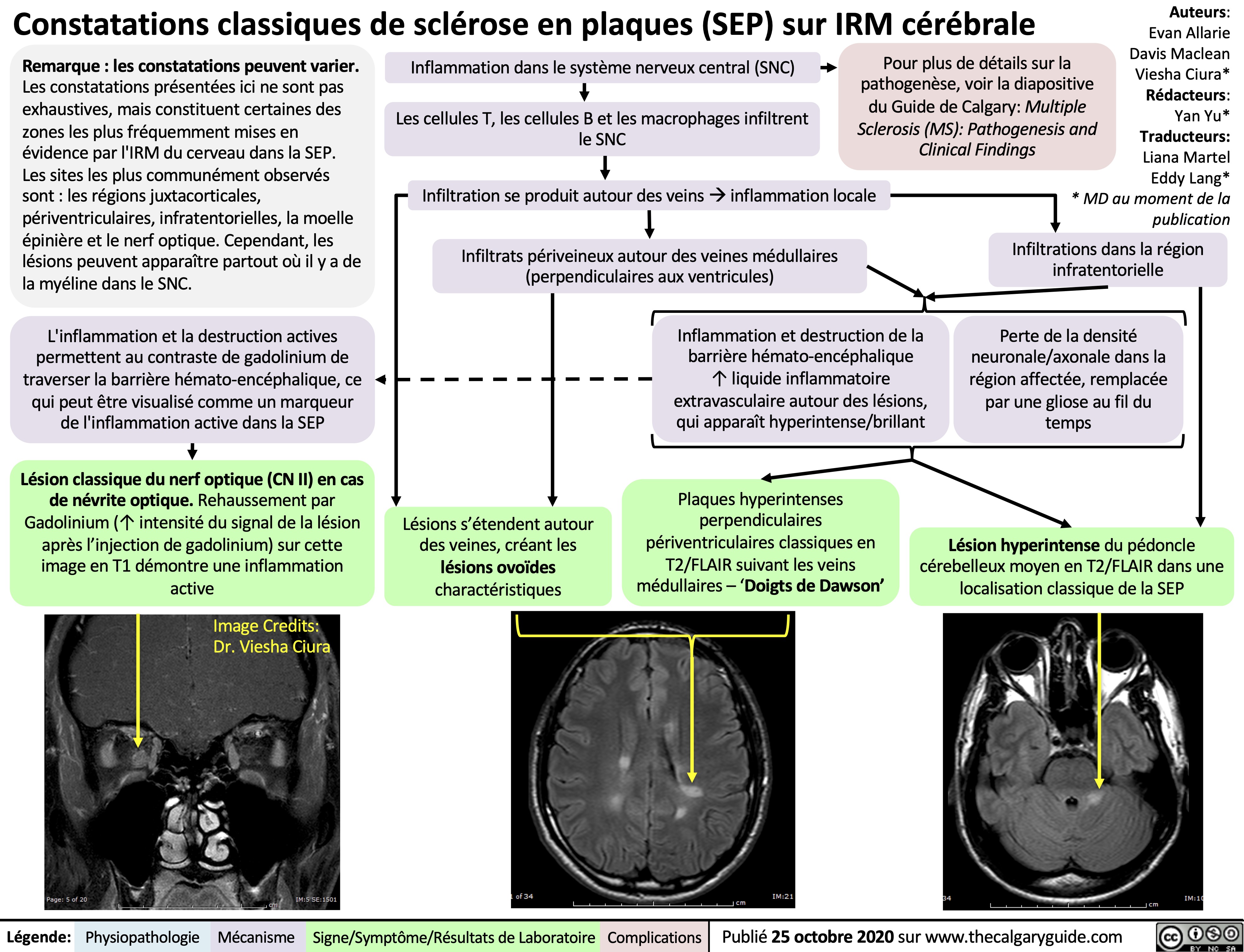
pleural-effusions-pathogenesis-and-anterior-posterior-chest-x-ray-findings
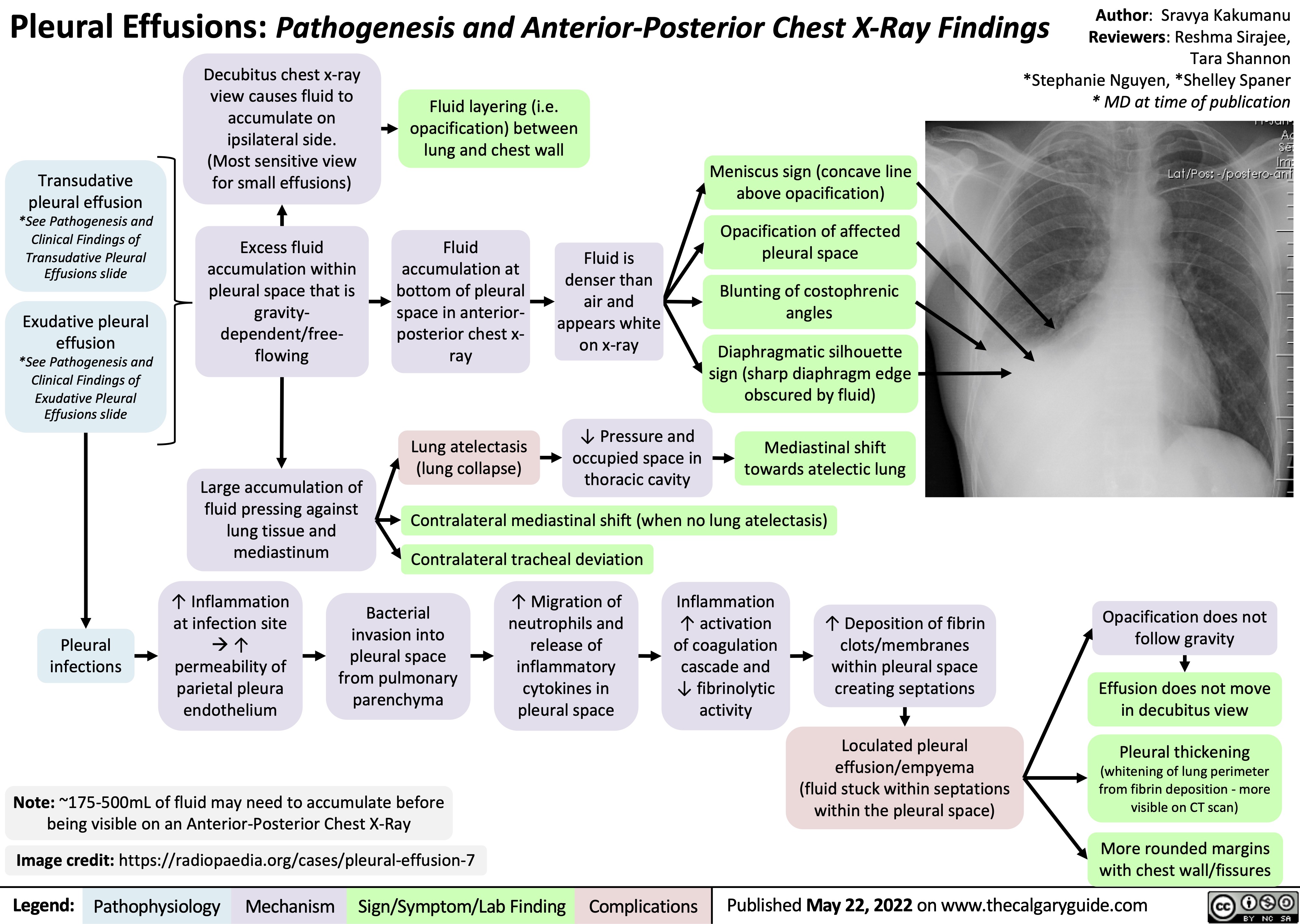
gout-pathogenesis-of-x-ray-findings
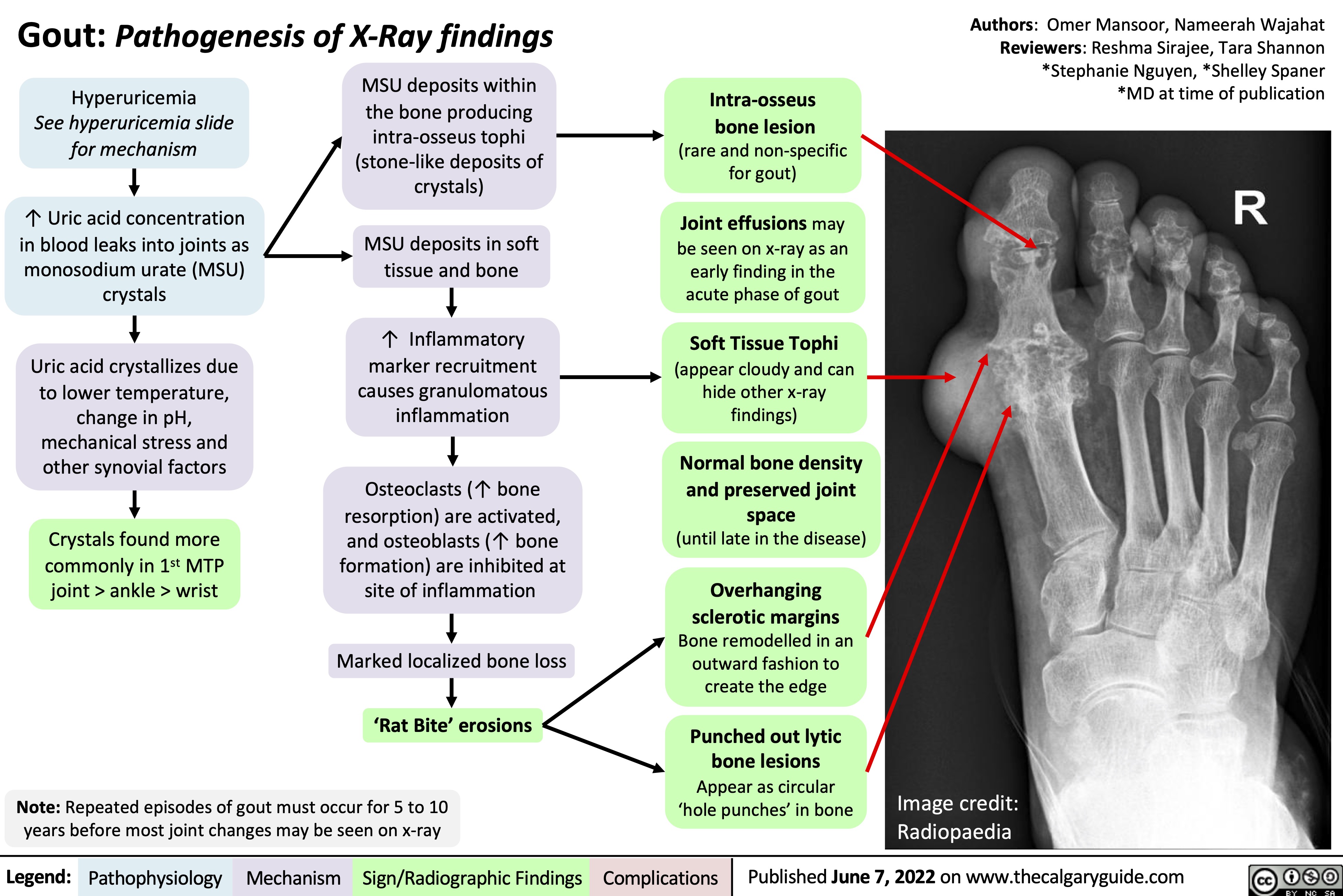
Epilepsy Pathogenesis
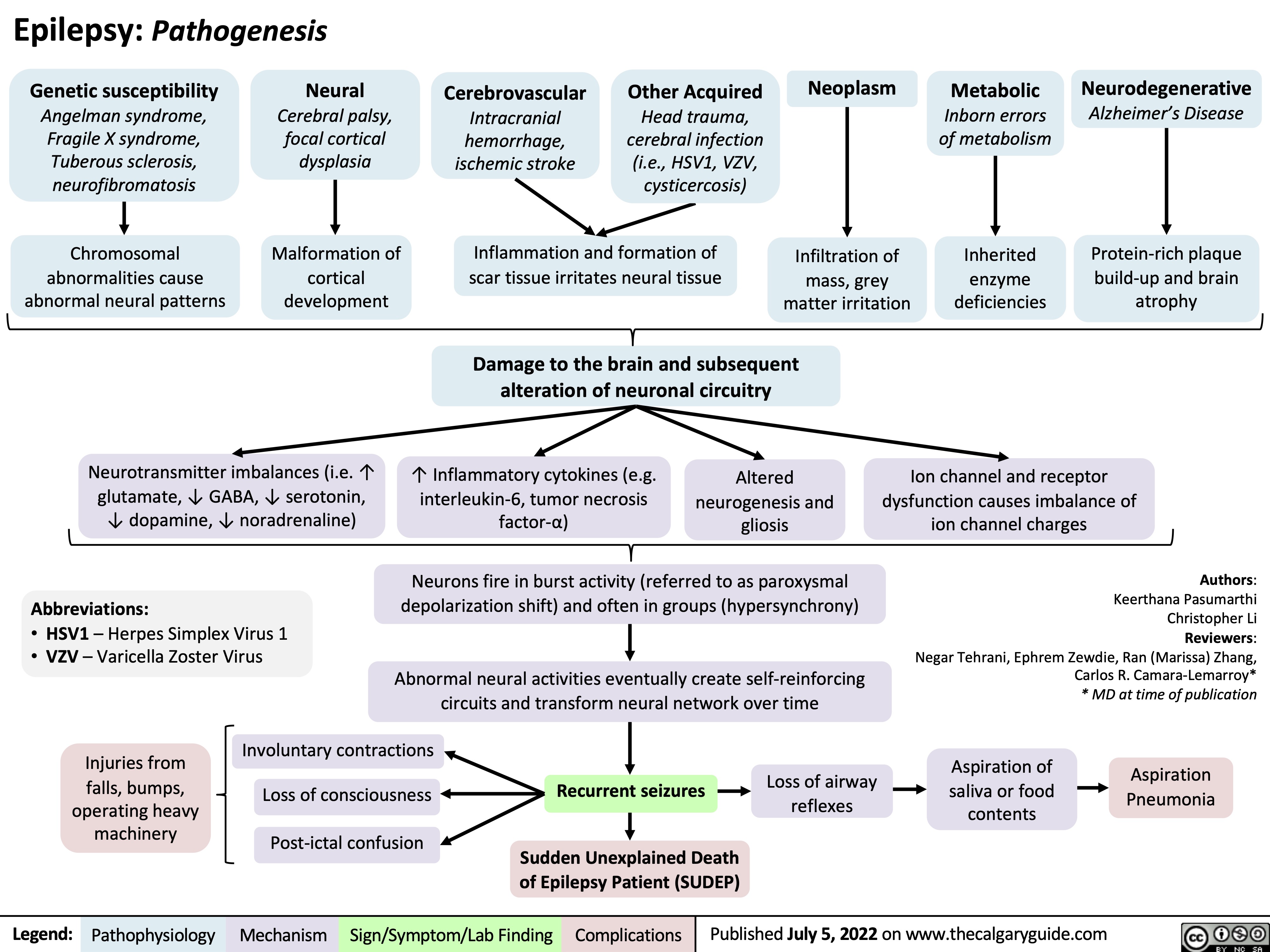
Cervical Insufficiency
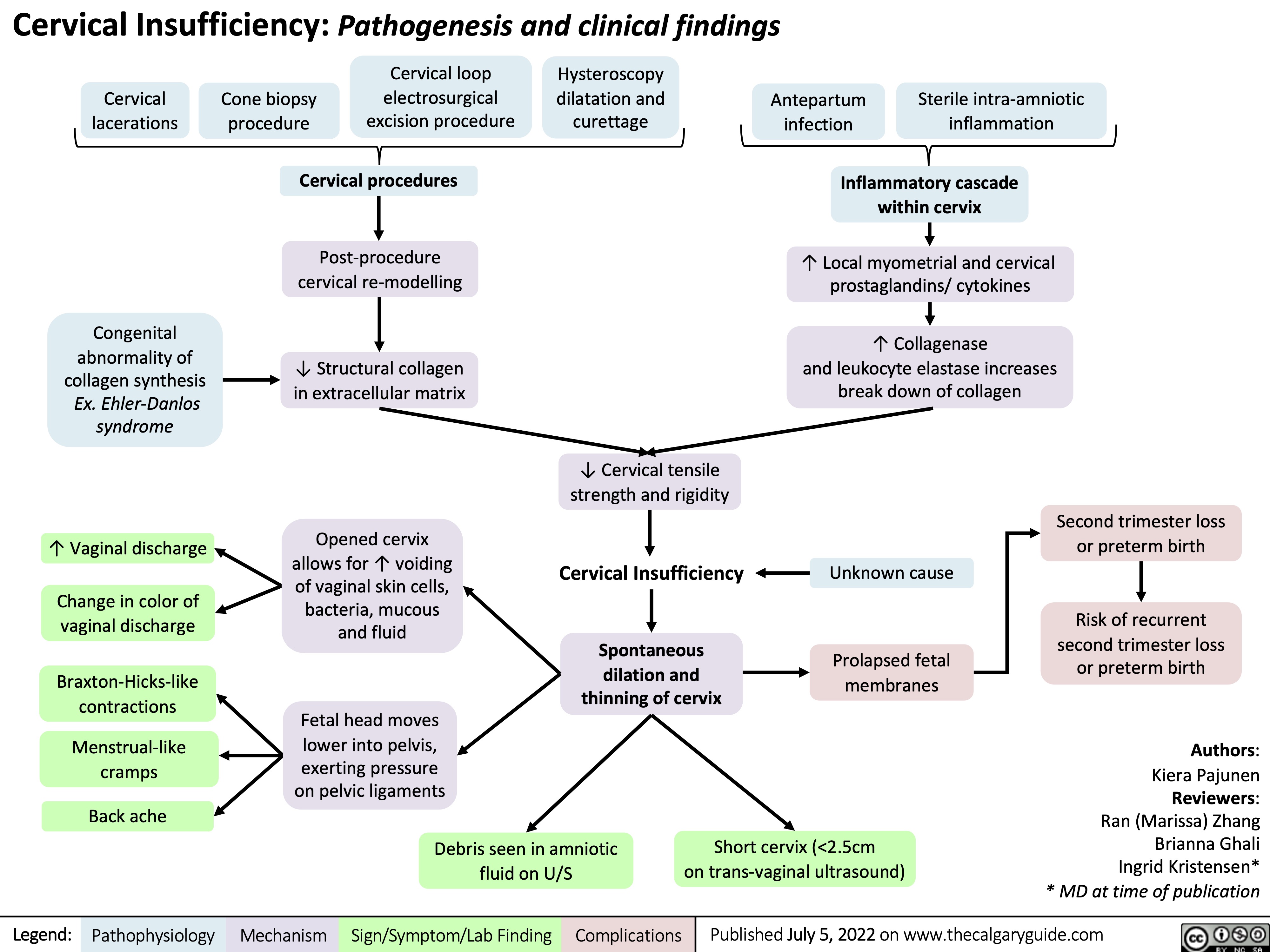
exudative-pleural-effusions-pathogenesis-and-lab-findings
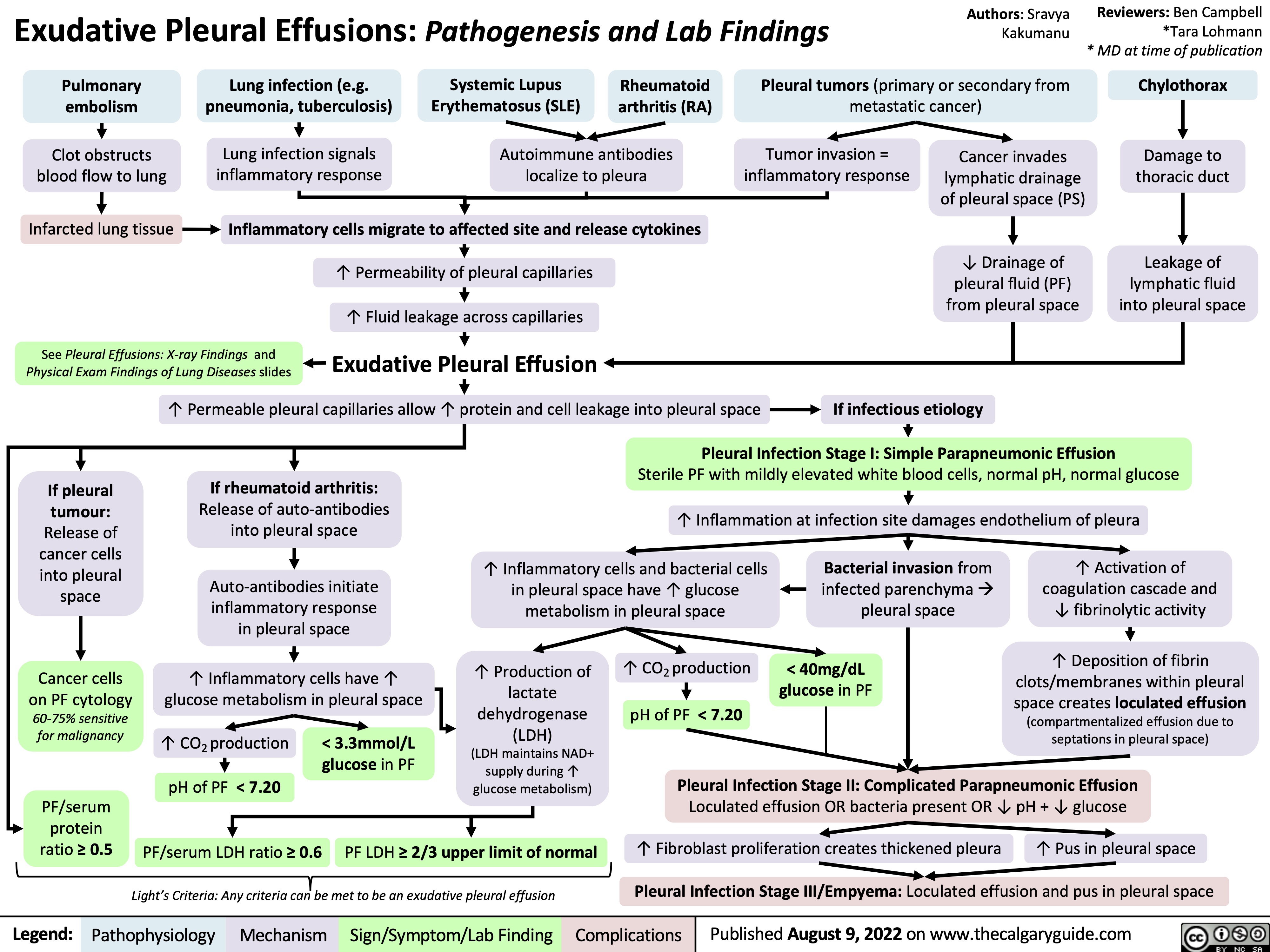
presentation-of-sah
![Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com
Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/SAH-Clinical-Findings-2022.jpg)
thrombose-veineuse-profonde-soupconne-tvp-pathogenese-et-complications
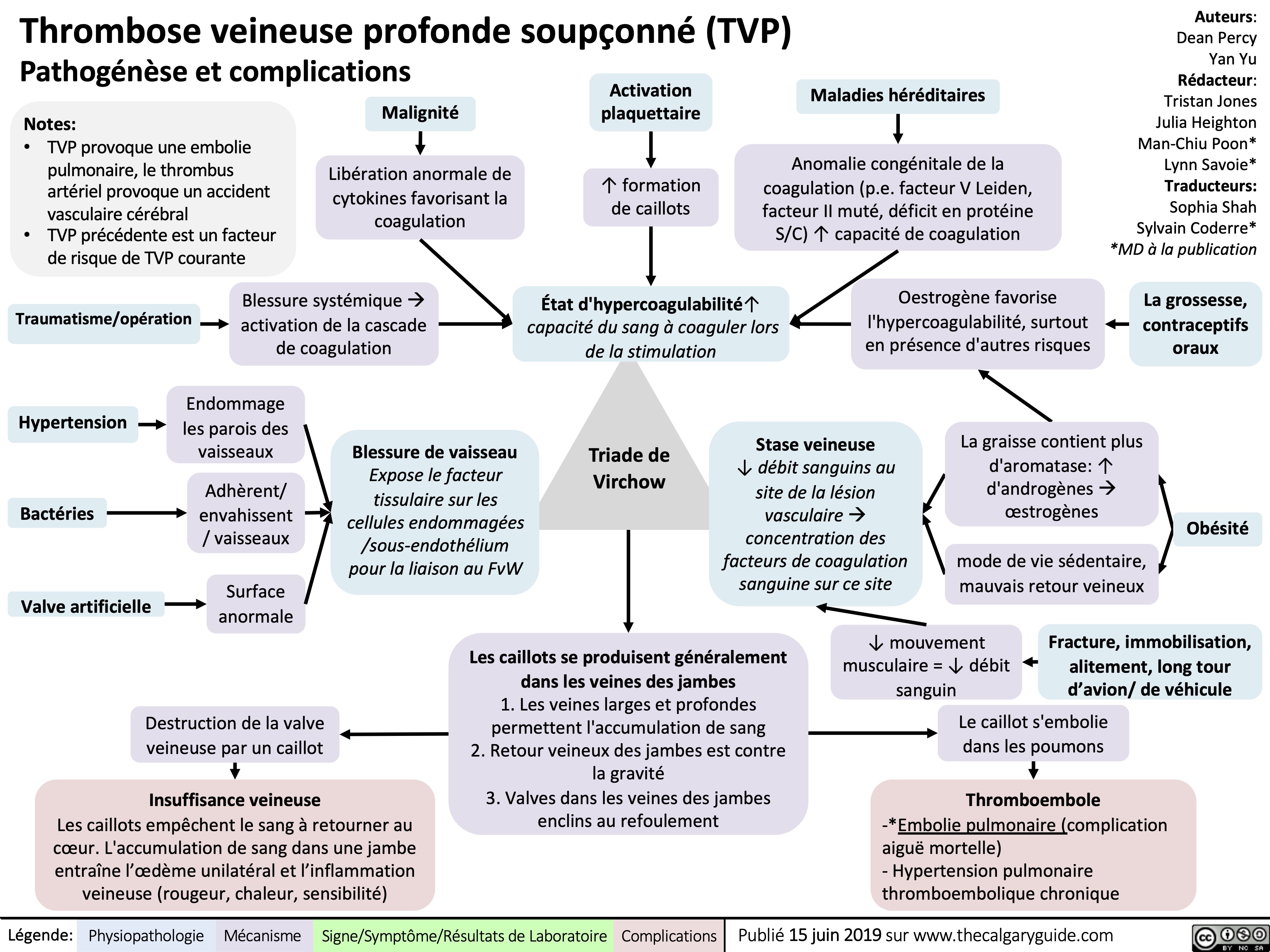
alcoholic-fatty-liver-disease-pathogenesis-and-clinical-findings
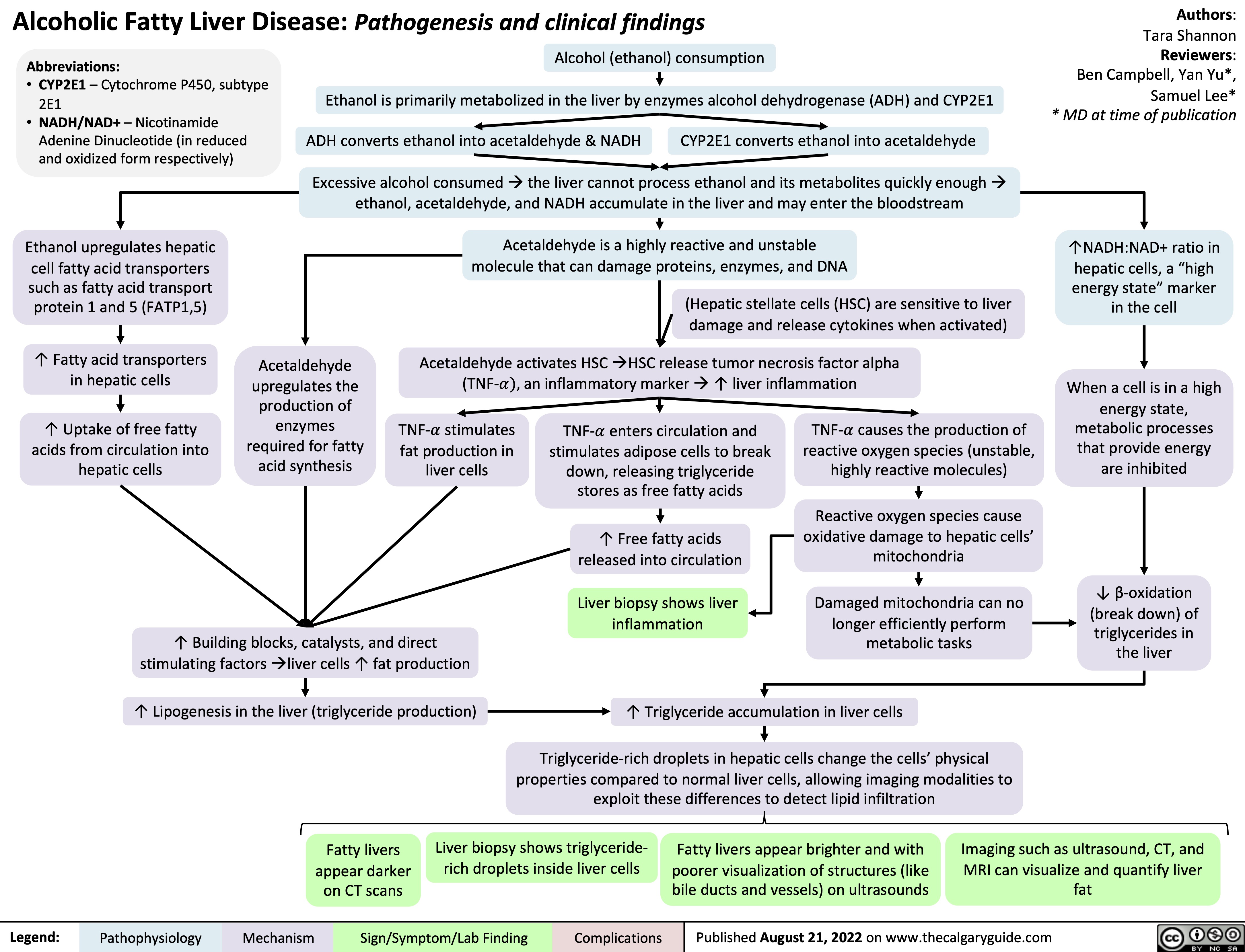
patellar-tendon-rupture-pathogenesis-and-clinical-findings
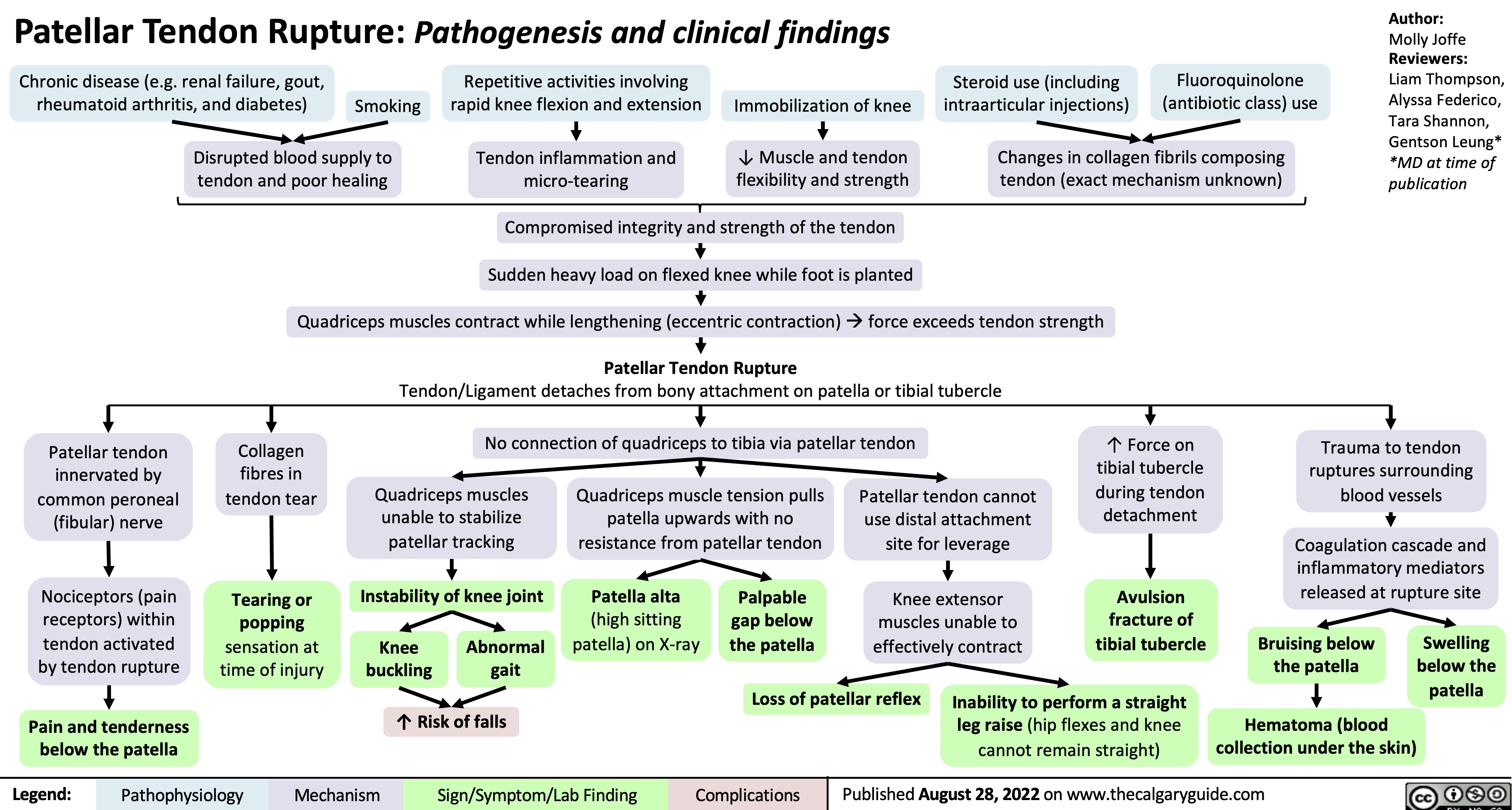
medial-epicondylitis-golfers-elbow-pathogenesis-and-clinical-findings
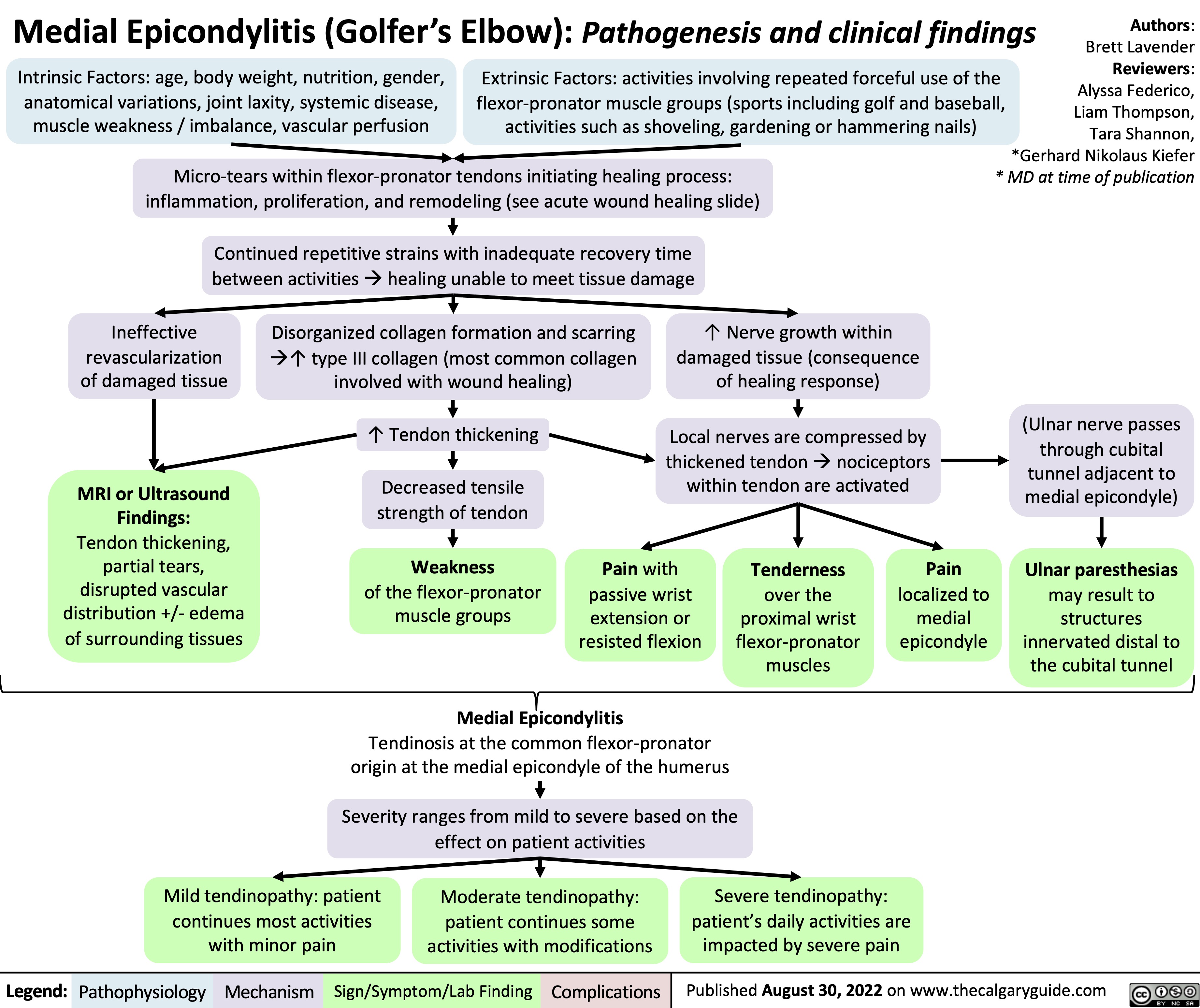
ascending-cholangitis-pathogenesis-clinical-findings
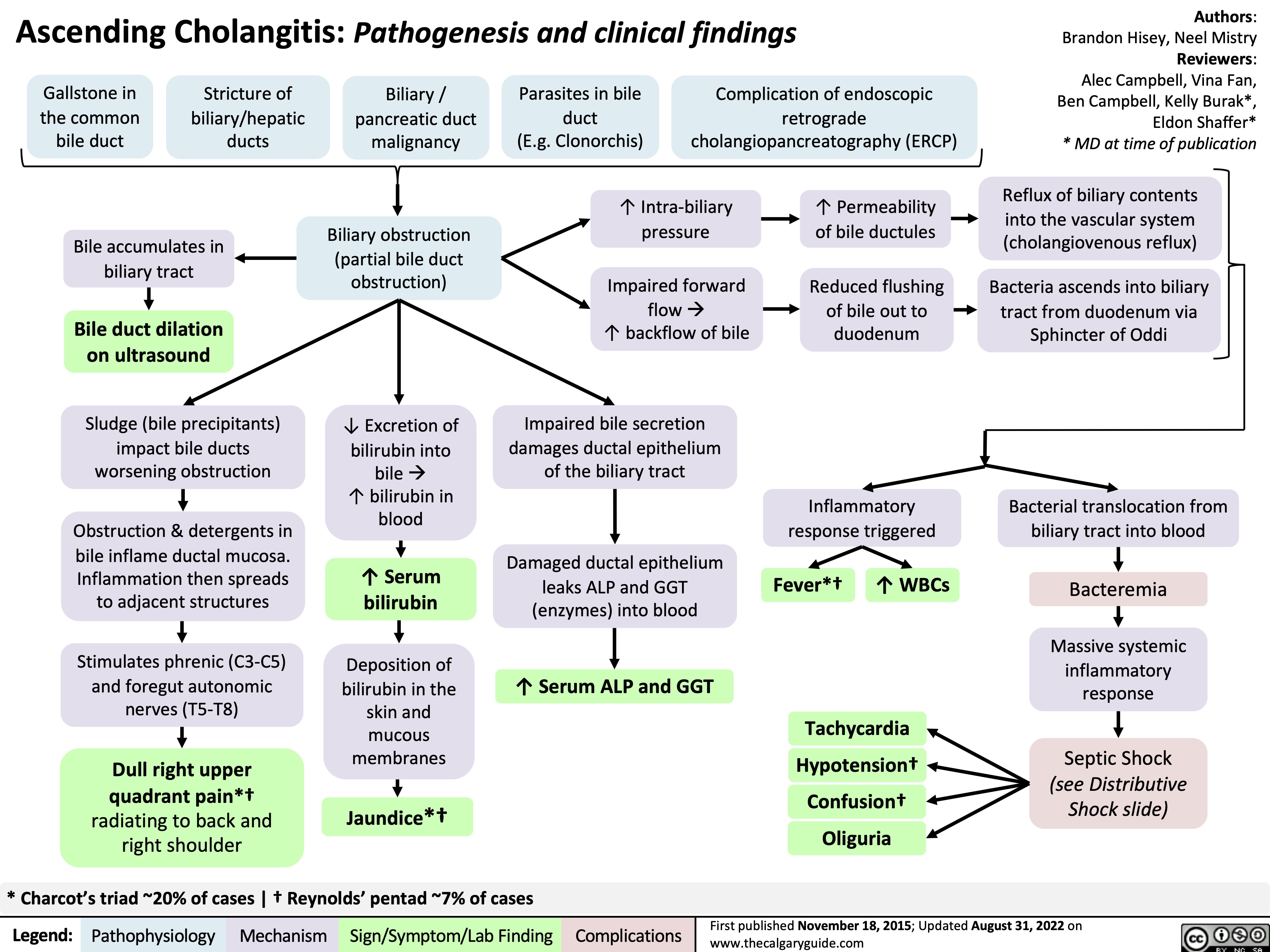
acute-lower-gi-bleeds-pathogenesis-and-clinical-findings
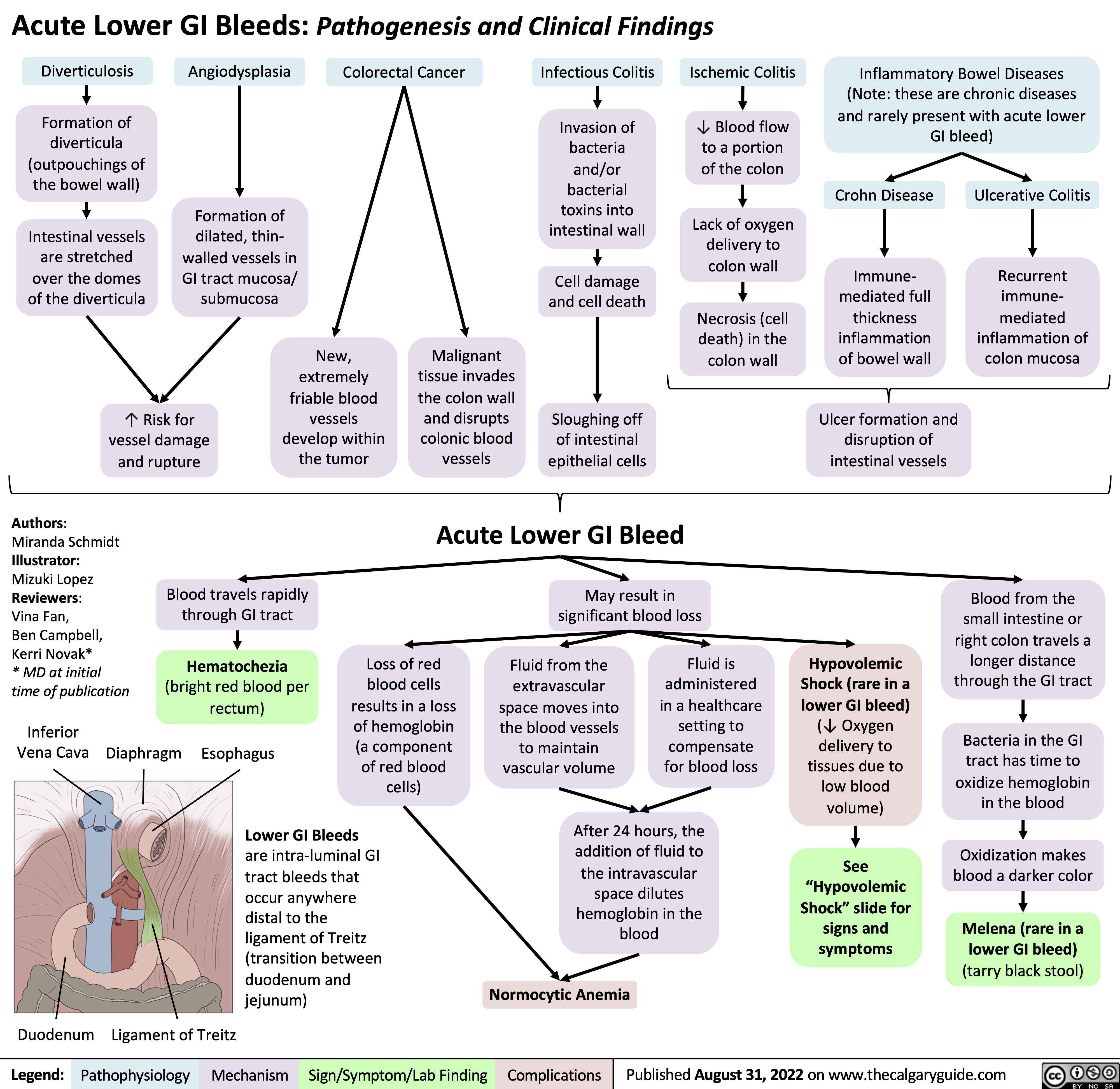
lateral-epicondylitis-tennis-elbow-pathogenesis-and-clinical-findings
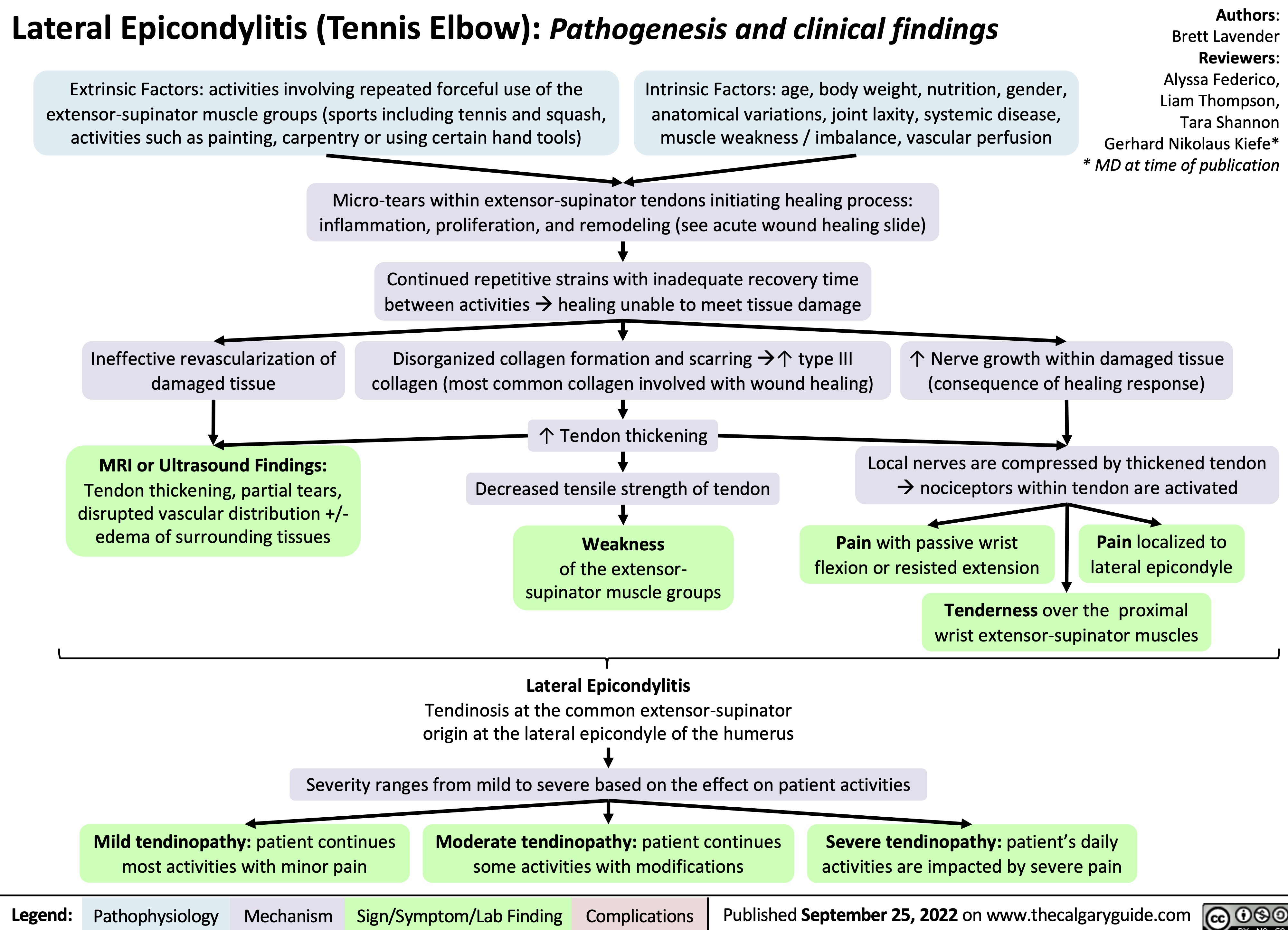
quadriceps-tendon-rupture-pathogenesis-and-clinical-findings
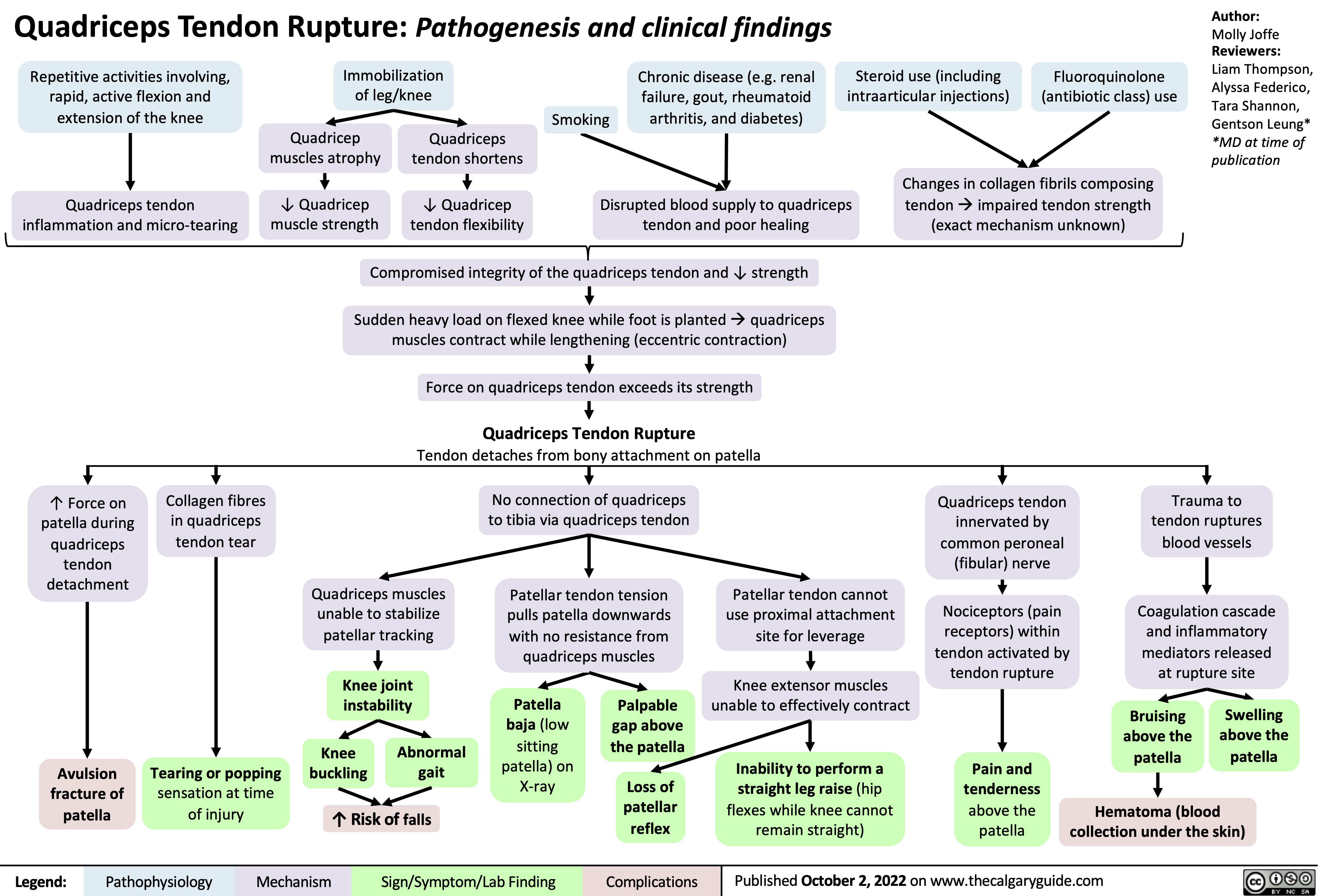
felty-syndrome
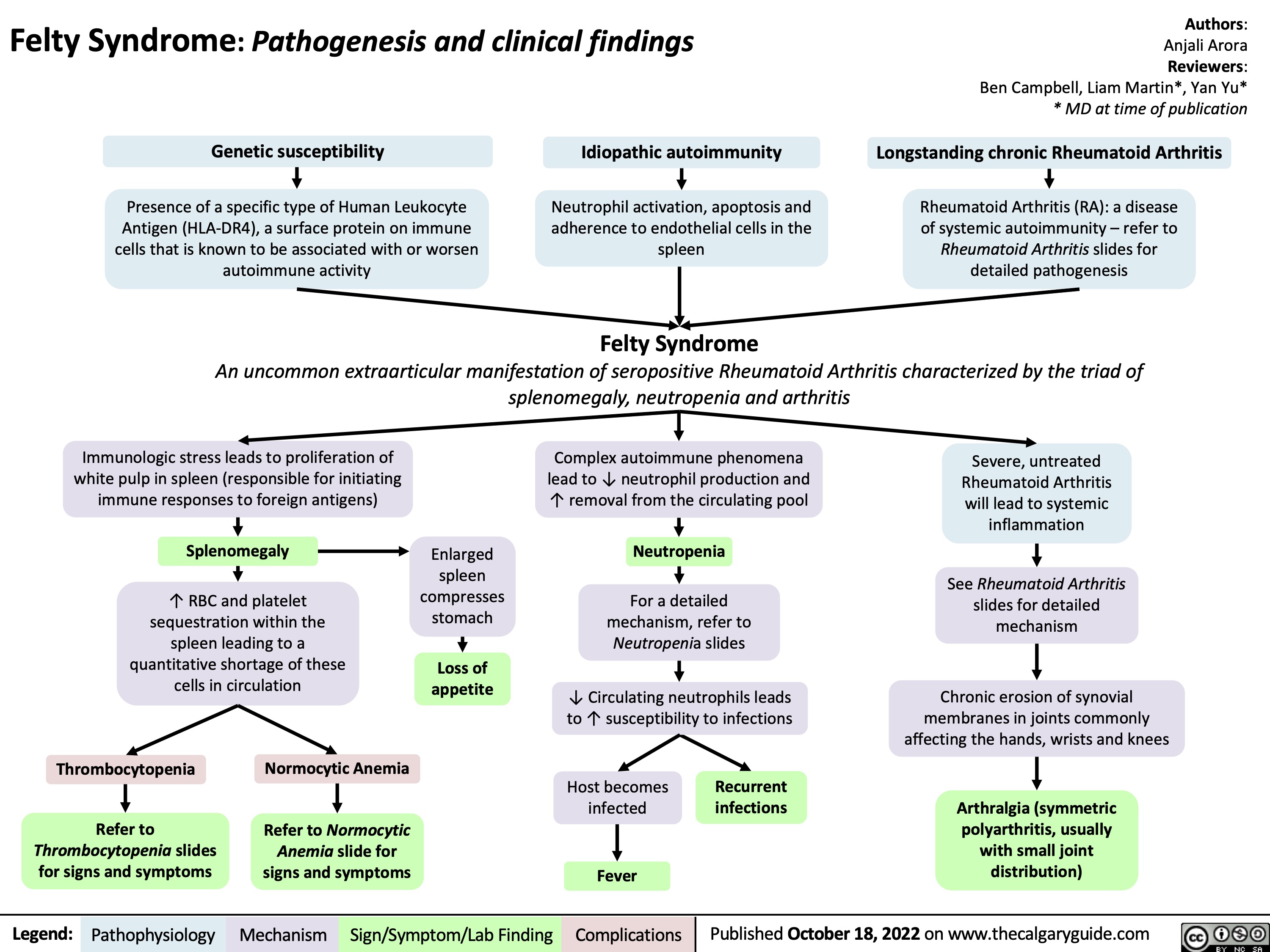
microscopic-colitis-pathogenesis-and-clinical-findings
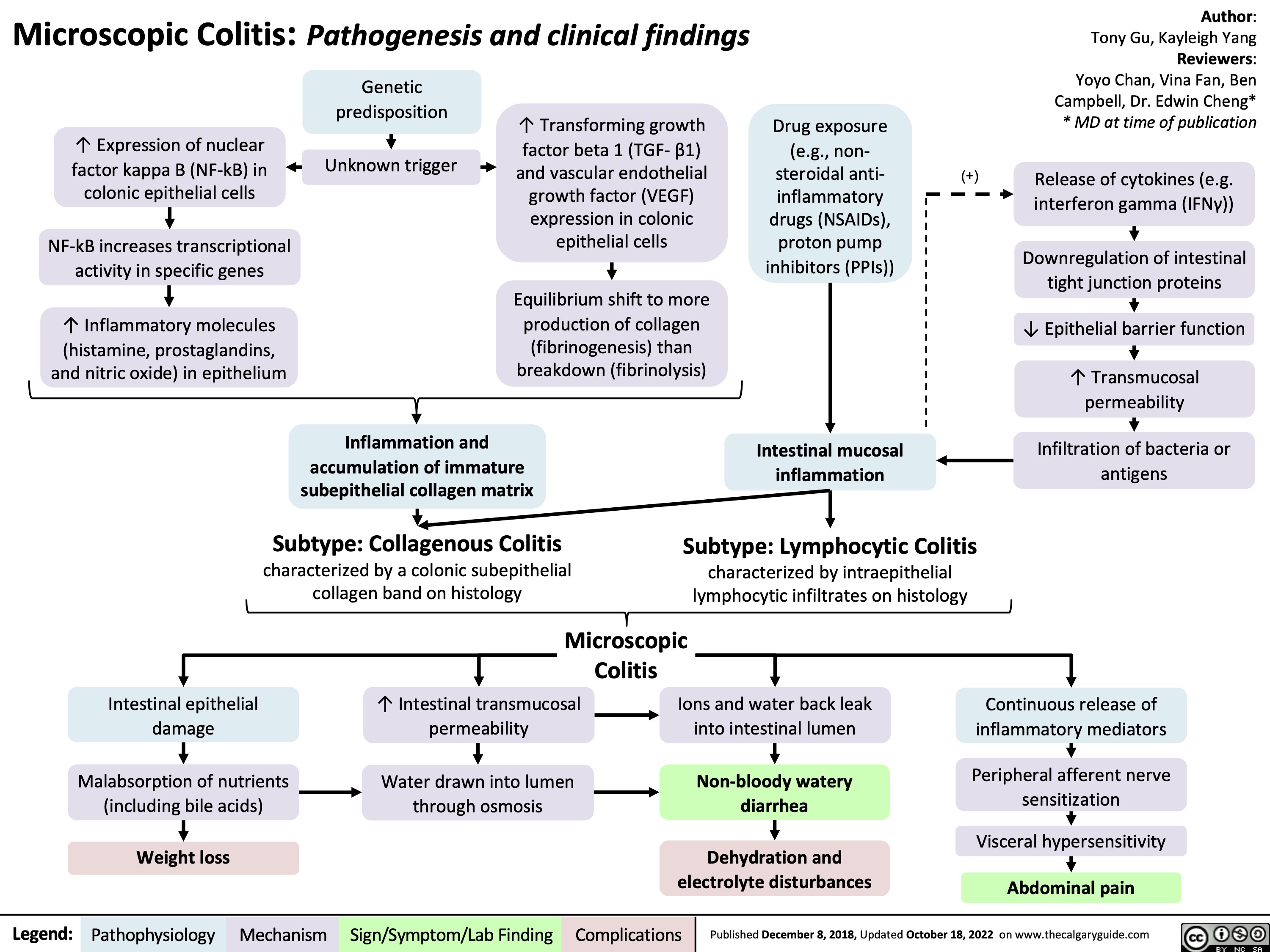
copd-findings-on-posterior-anterior-and-lateral-chest-x-ray-findings
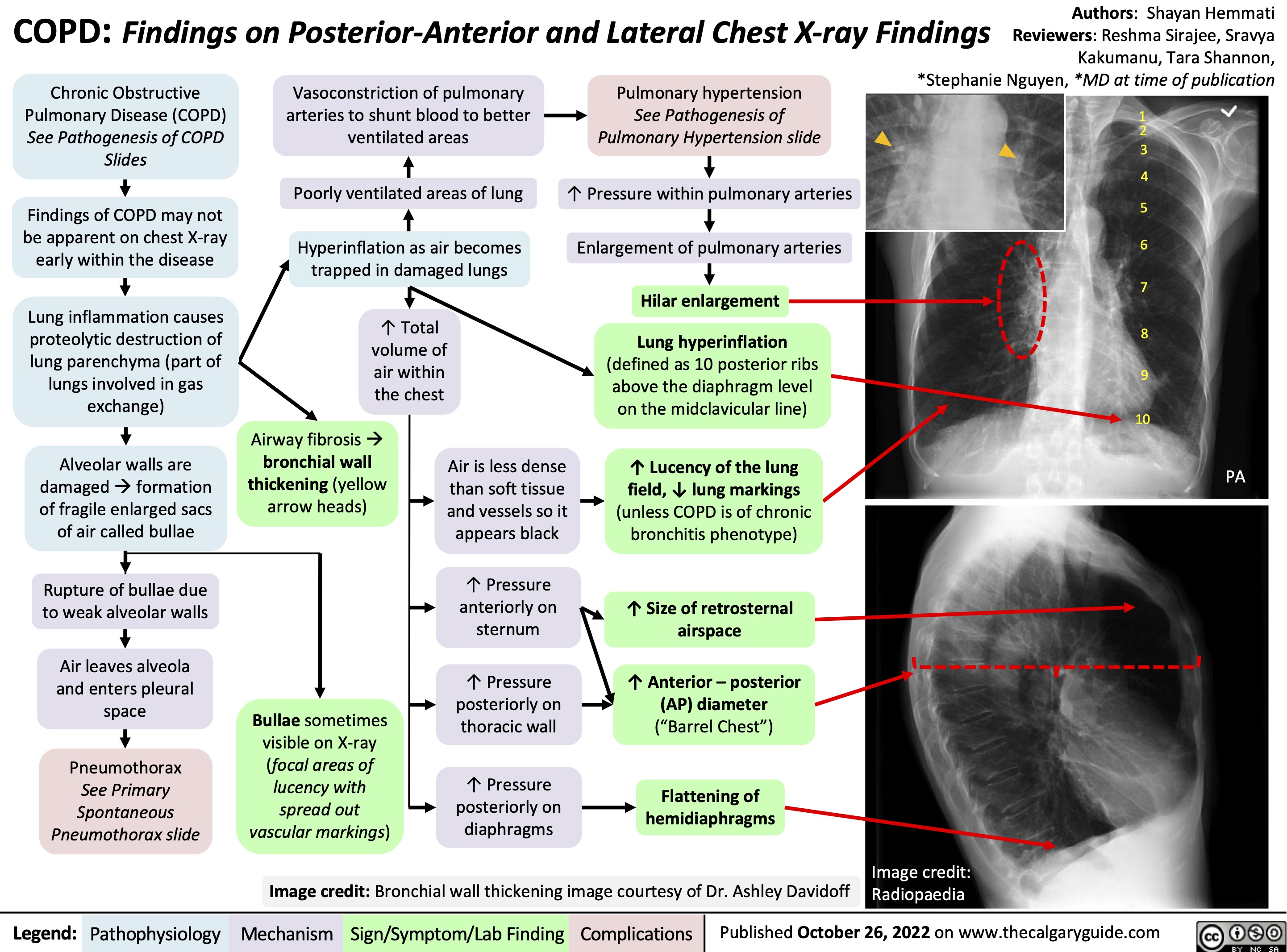
Granulomatosis with Polyangiitis Pathogenesis
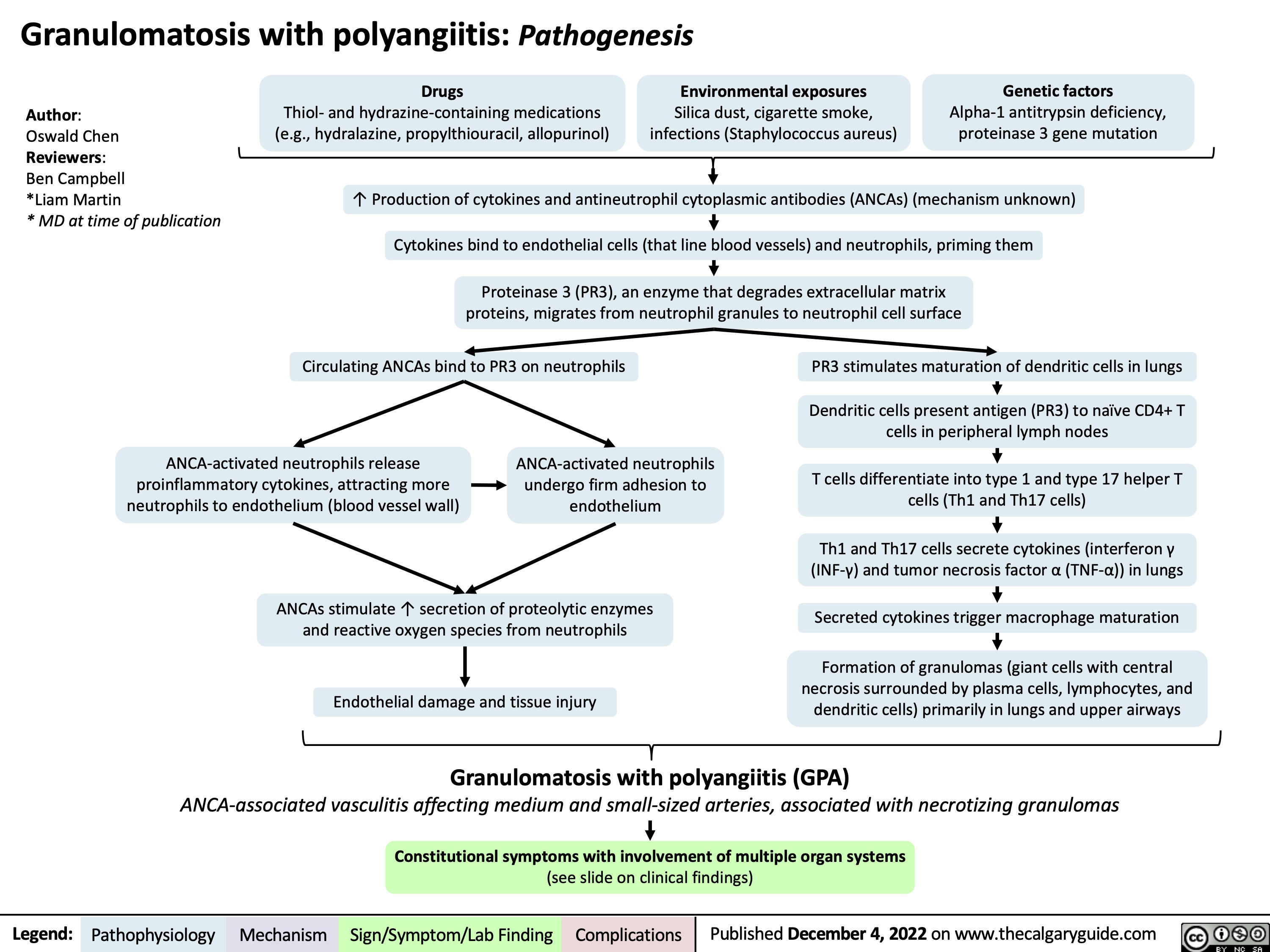
Granulomatosis with polyangiitis: Clinical findings
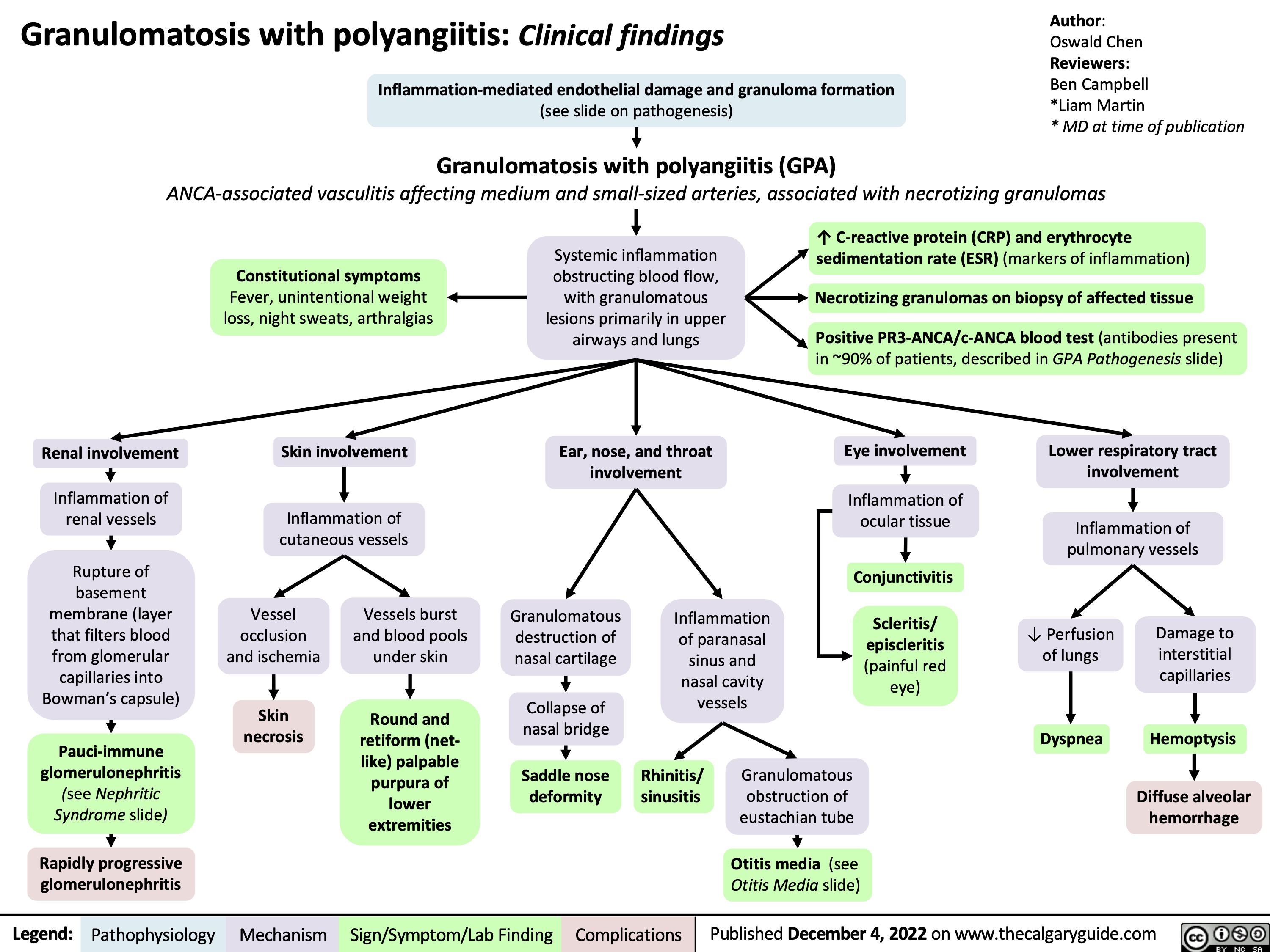
Erythema multiforme (EM): Pathophysiology and clinical findings
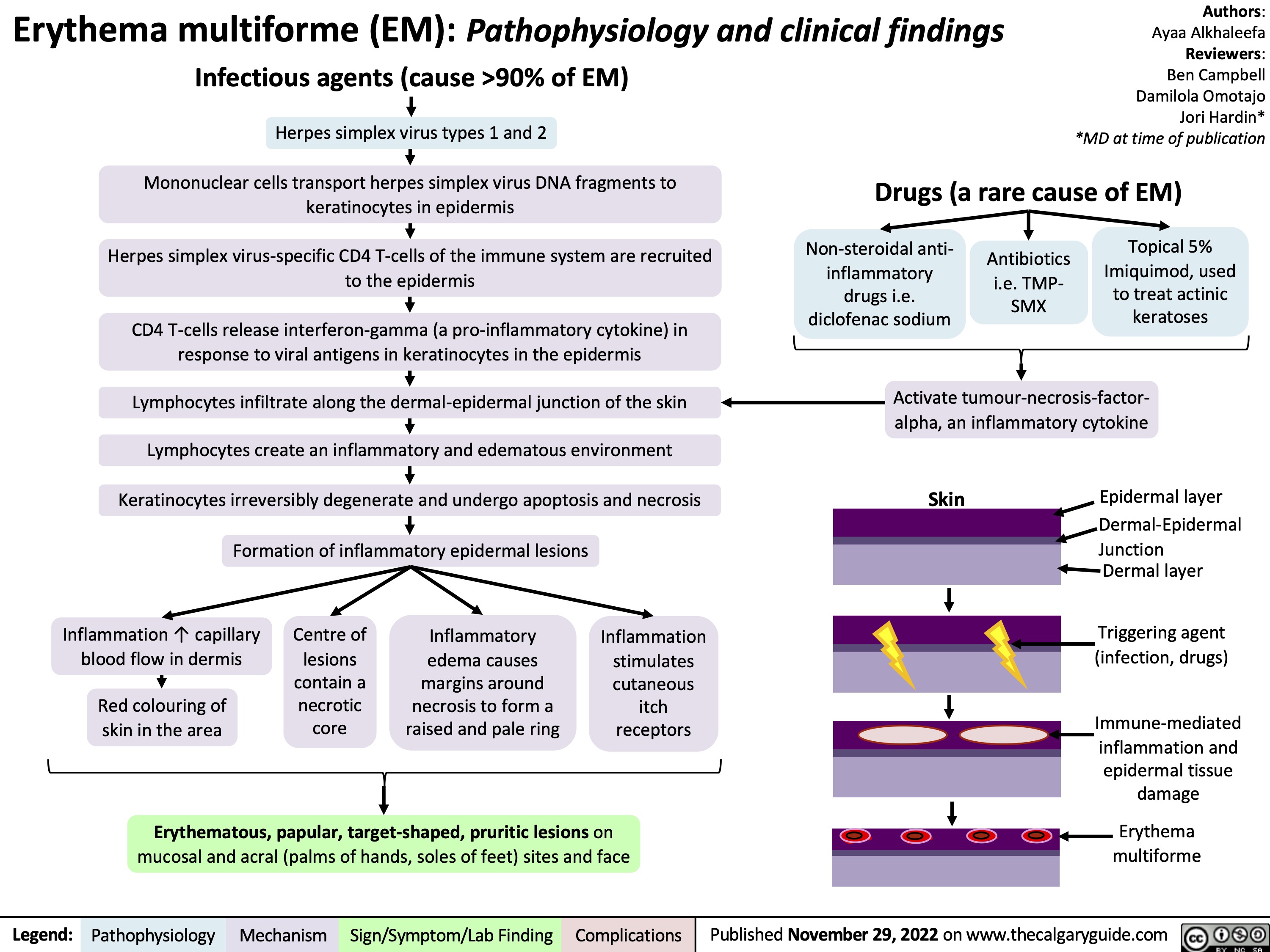
rheumatoid-pneumoconiosis-caplans-syndrome-pathogenesis-and-clinical-findings
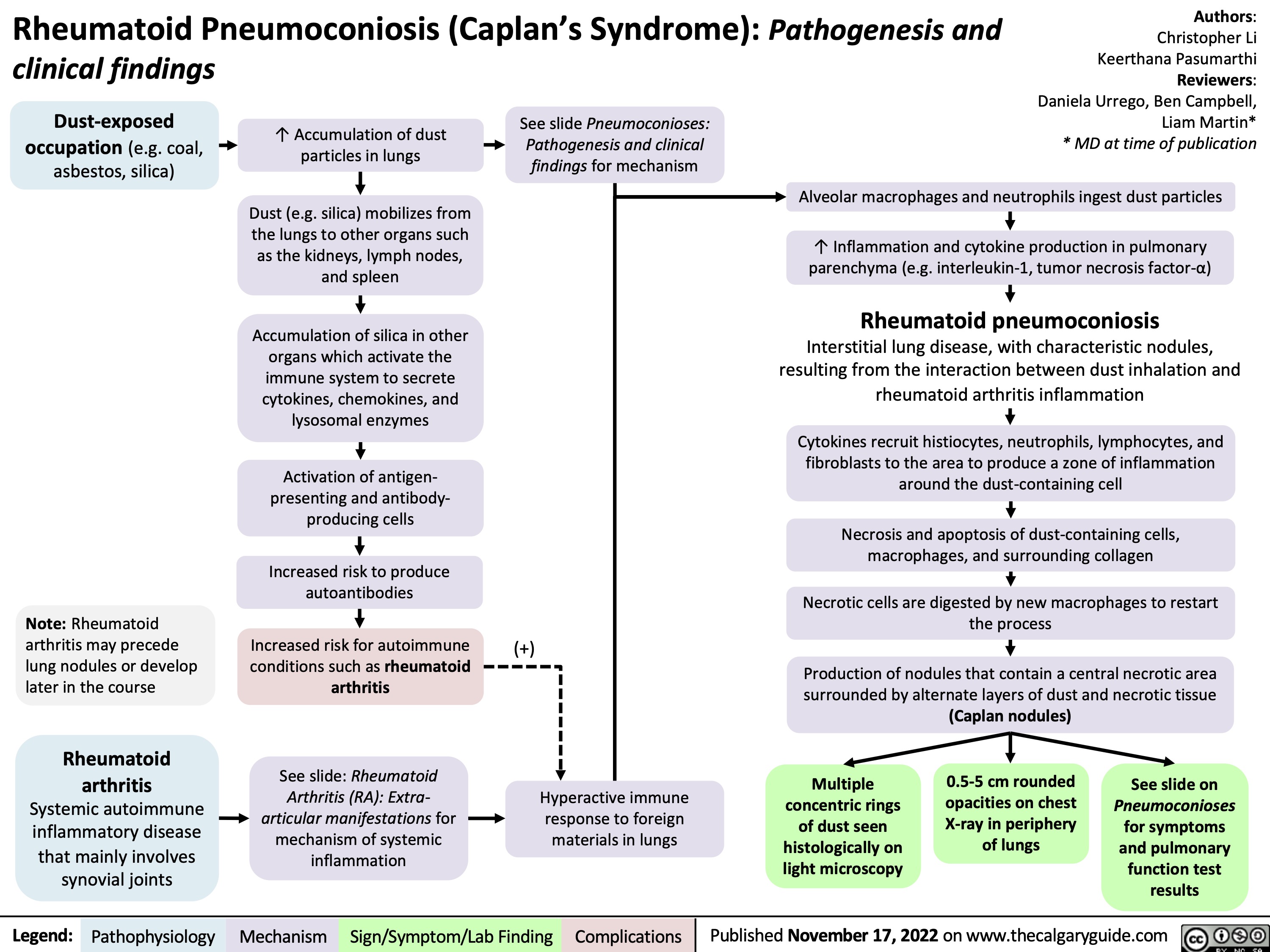
Acute Liver Failure: Pathogenesis and clinical findings
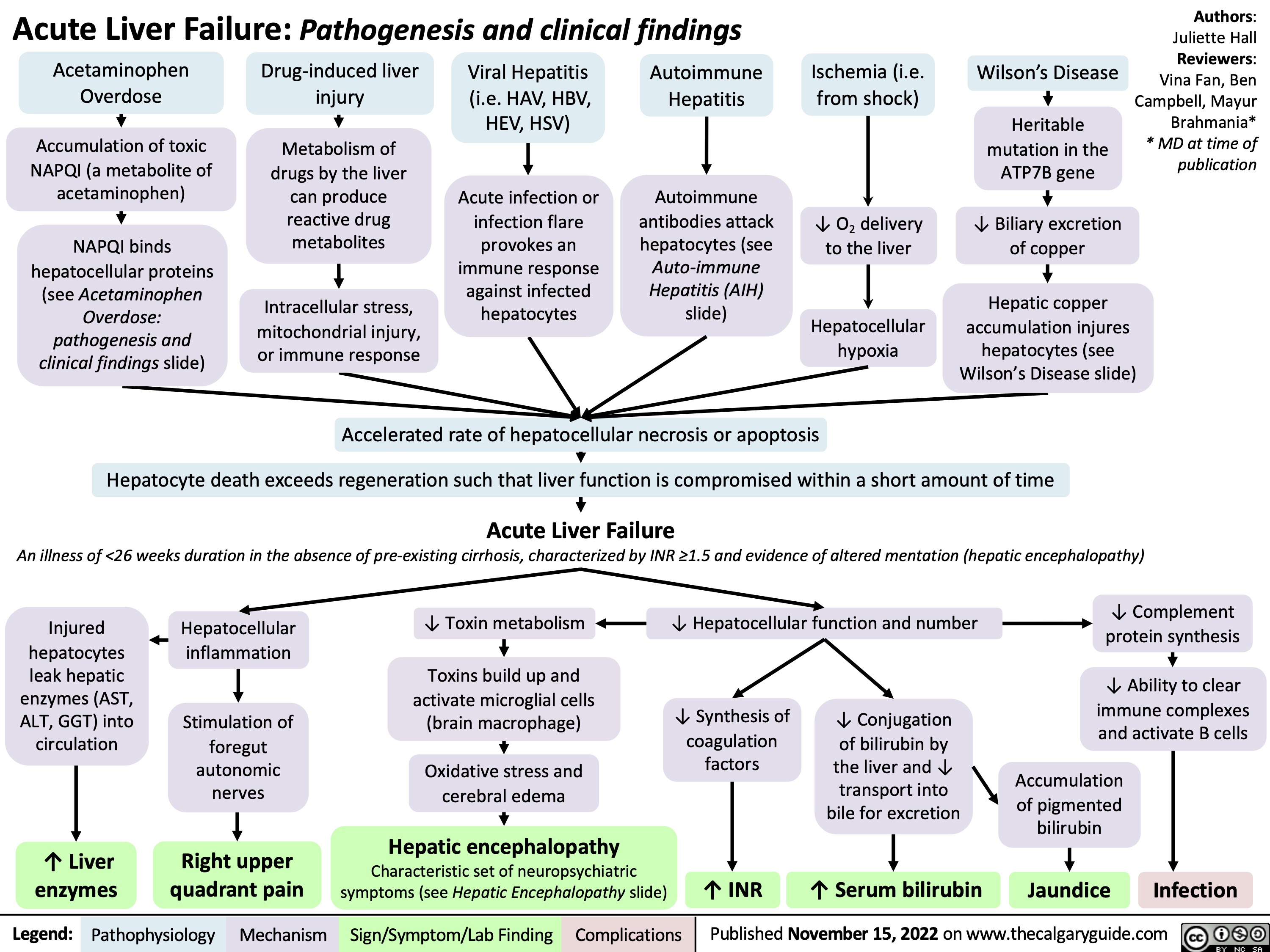
Acute and Chronic Gastritis: Pathogenesis and clinical findings
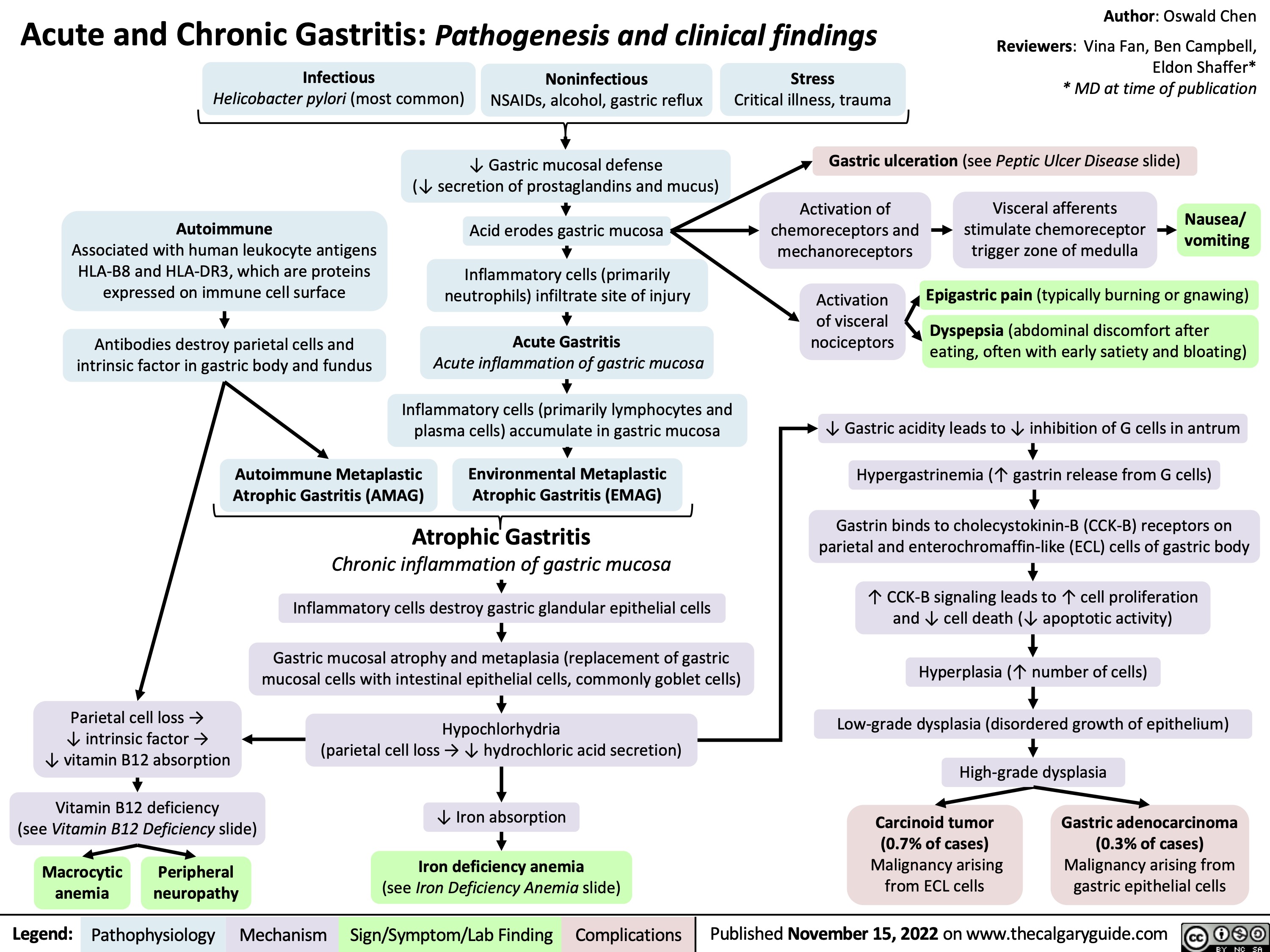
Achalasia: Findings on Fluoroscopy with Barium Swallow
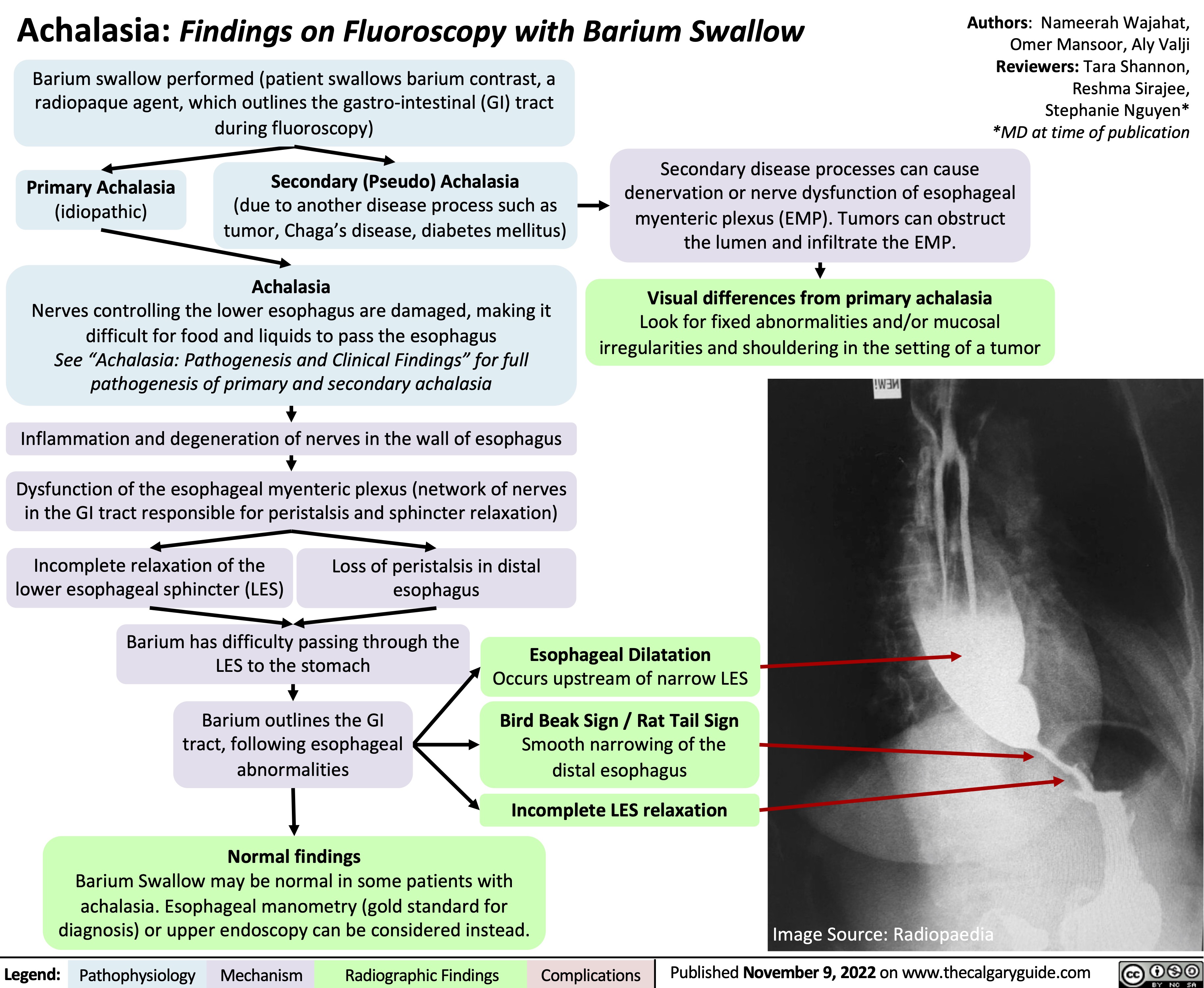
popliteal-bakers-cyst-pathogenesis-and-clinical-findings
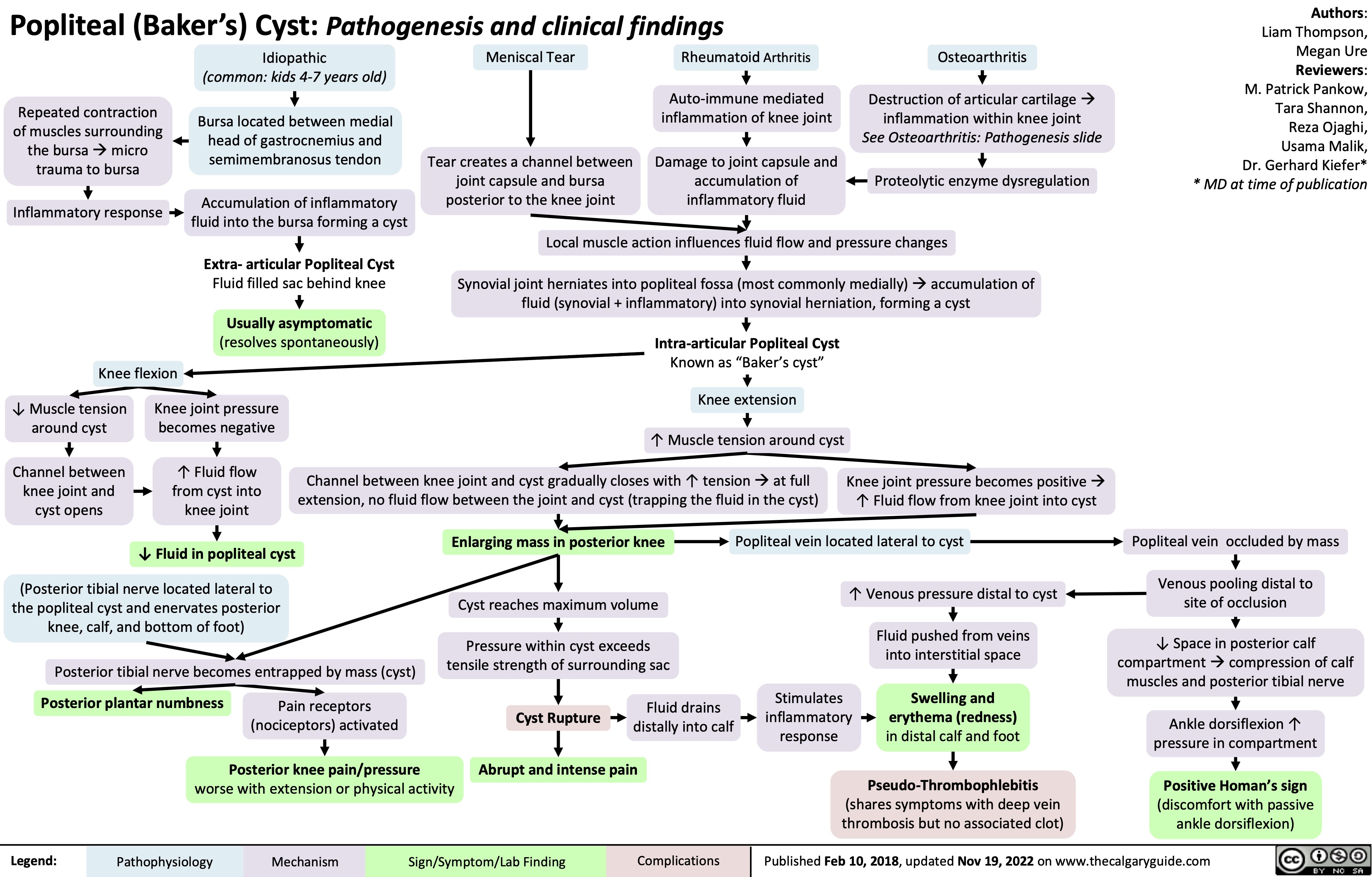
hypovolemic-shock
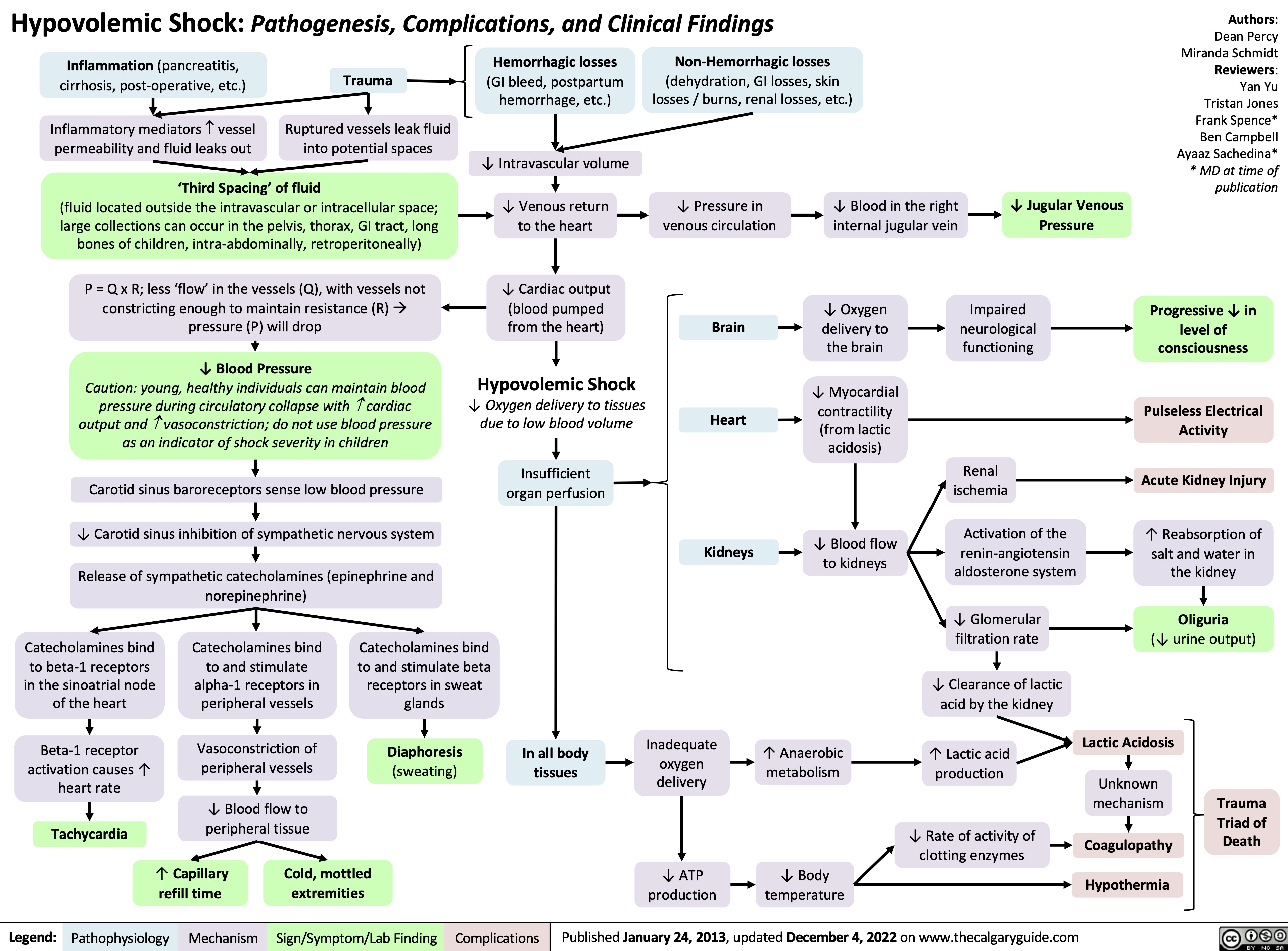
Ischemic Stroke: Pathogenesis
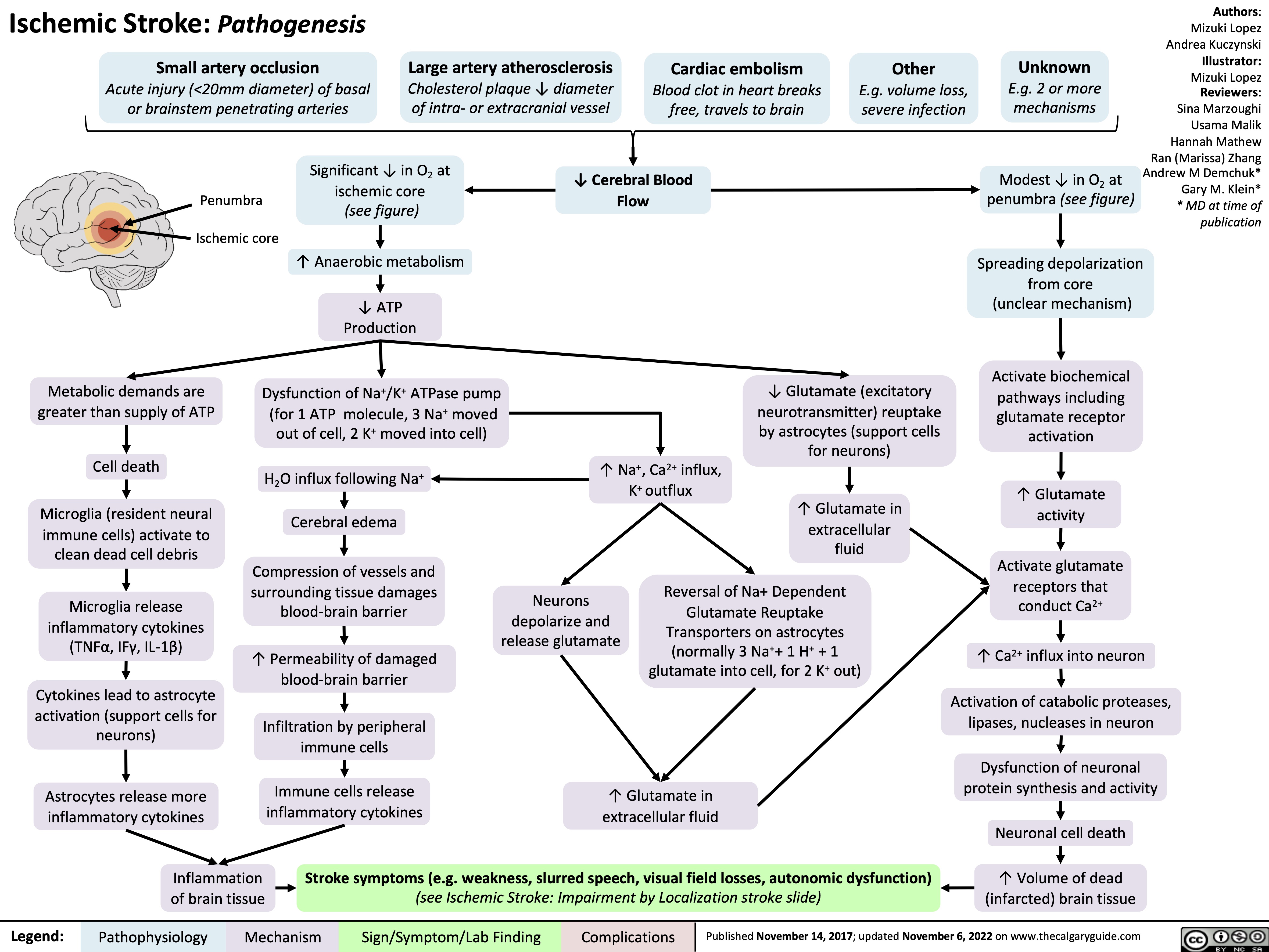
Cirrhosis
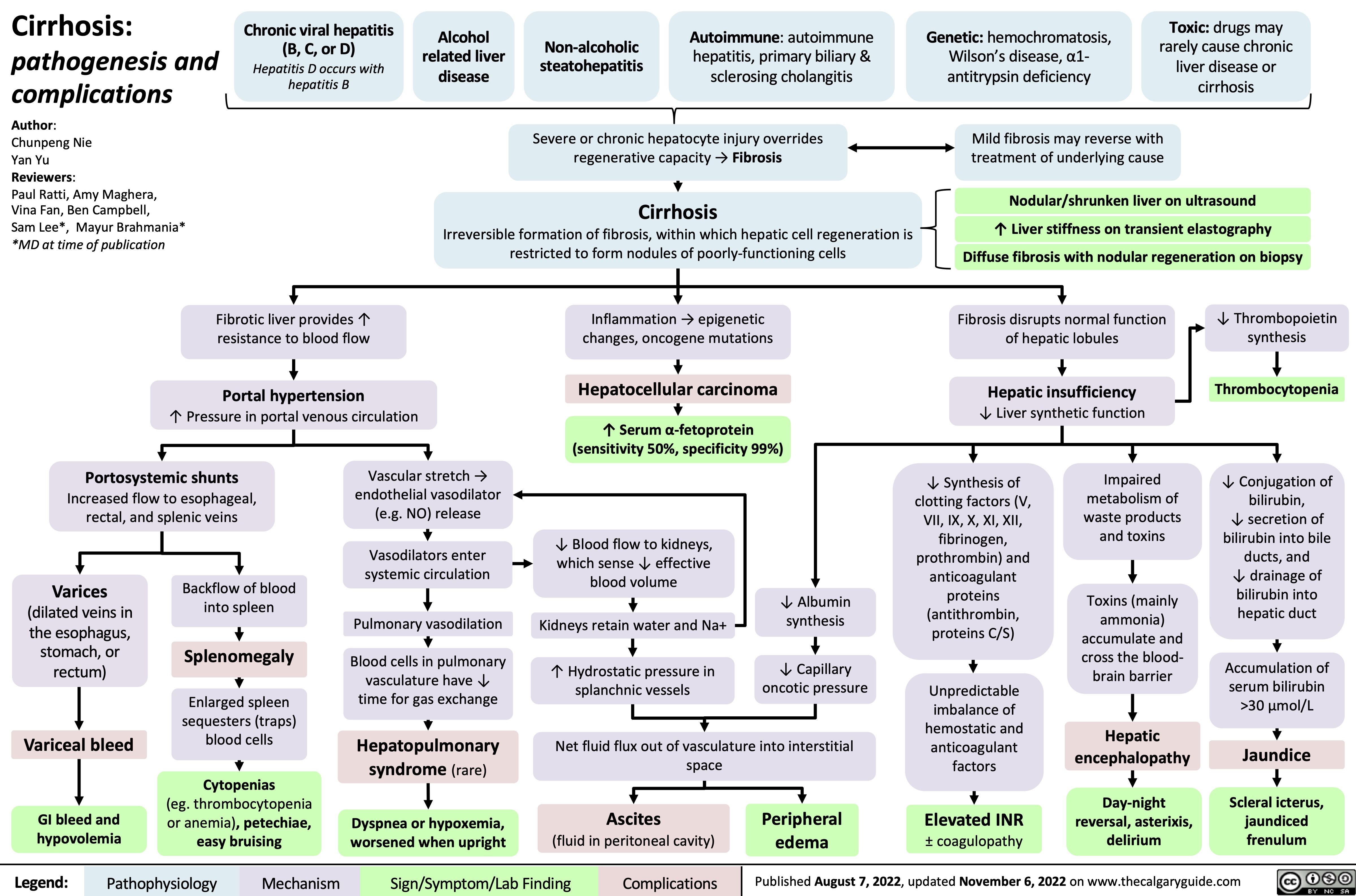
Hidradenitis Suppurativa
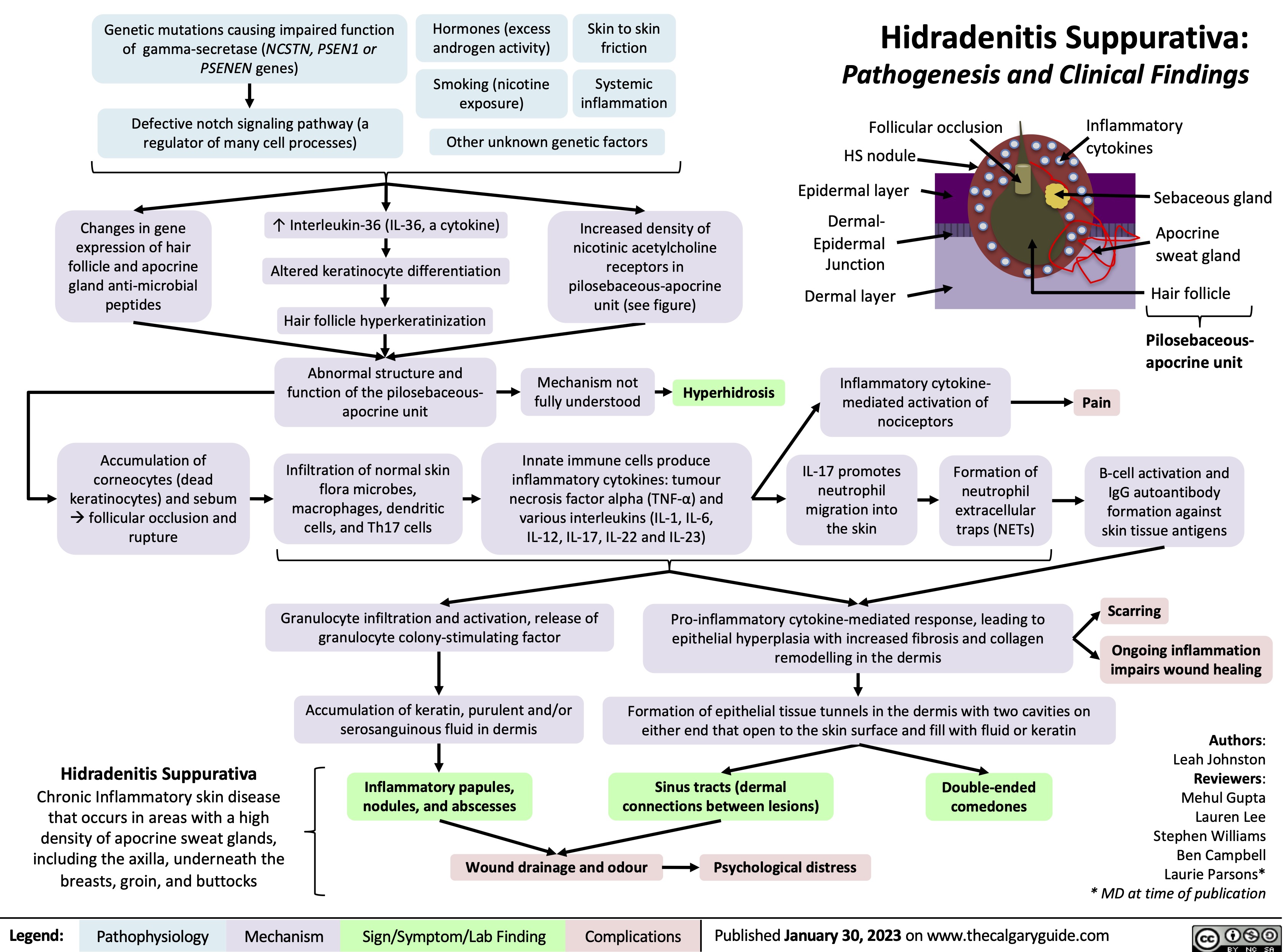
Inhalational Injury
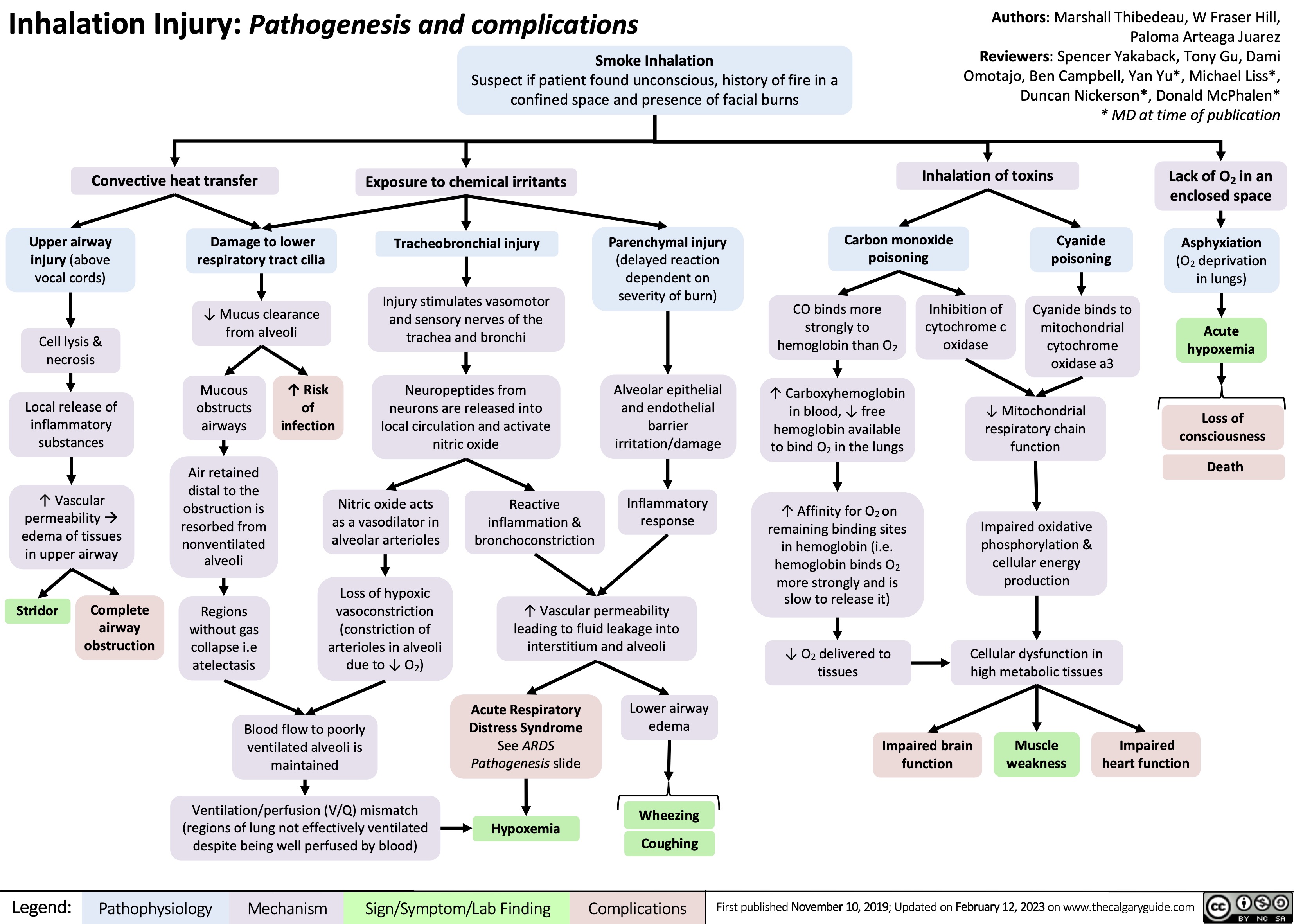
Central Retinal Vein Occlusion
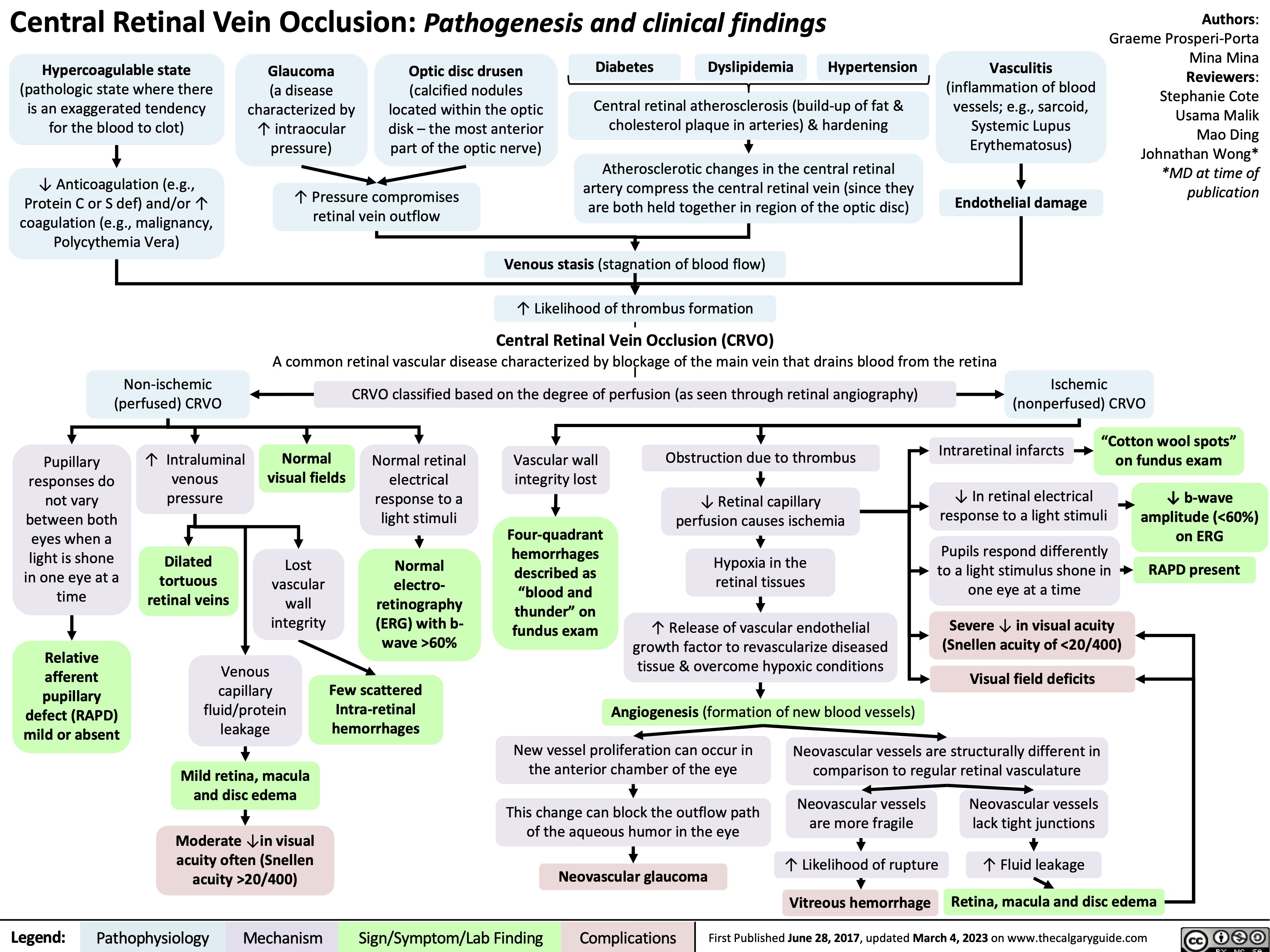
Douleur somatique aigue
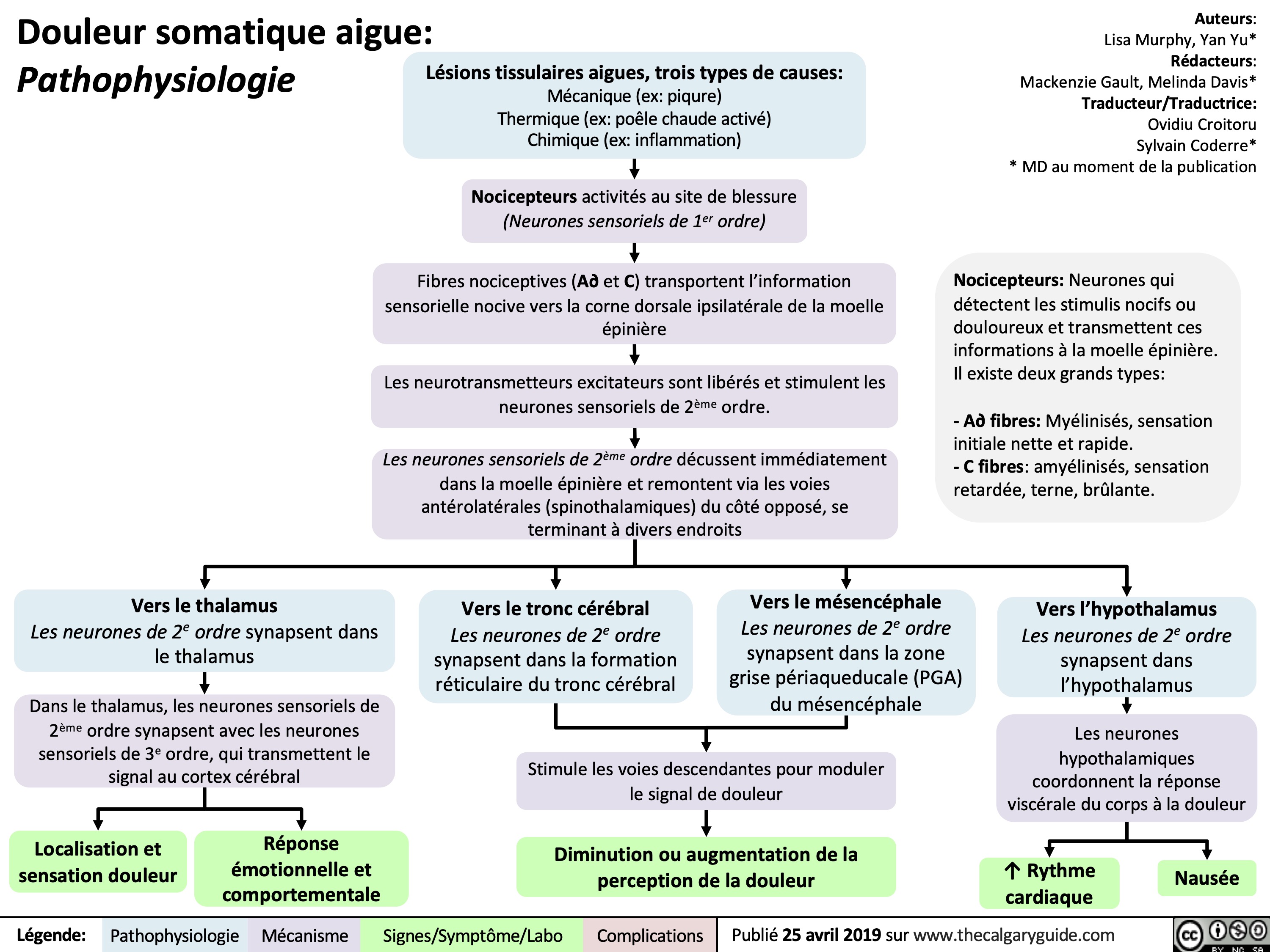
Pyogenic Brain Abscess on MRI
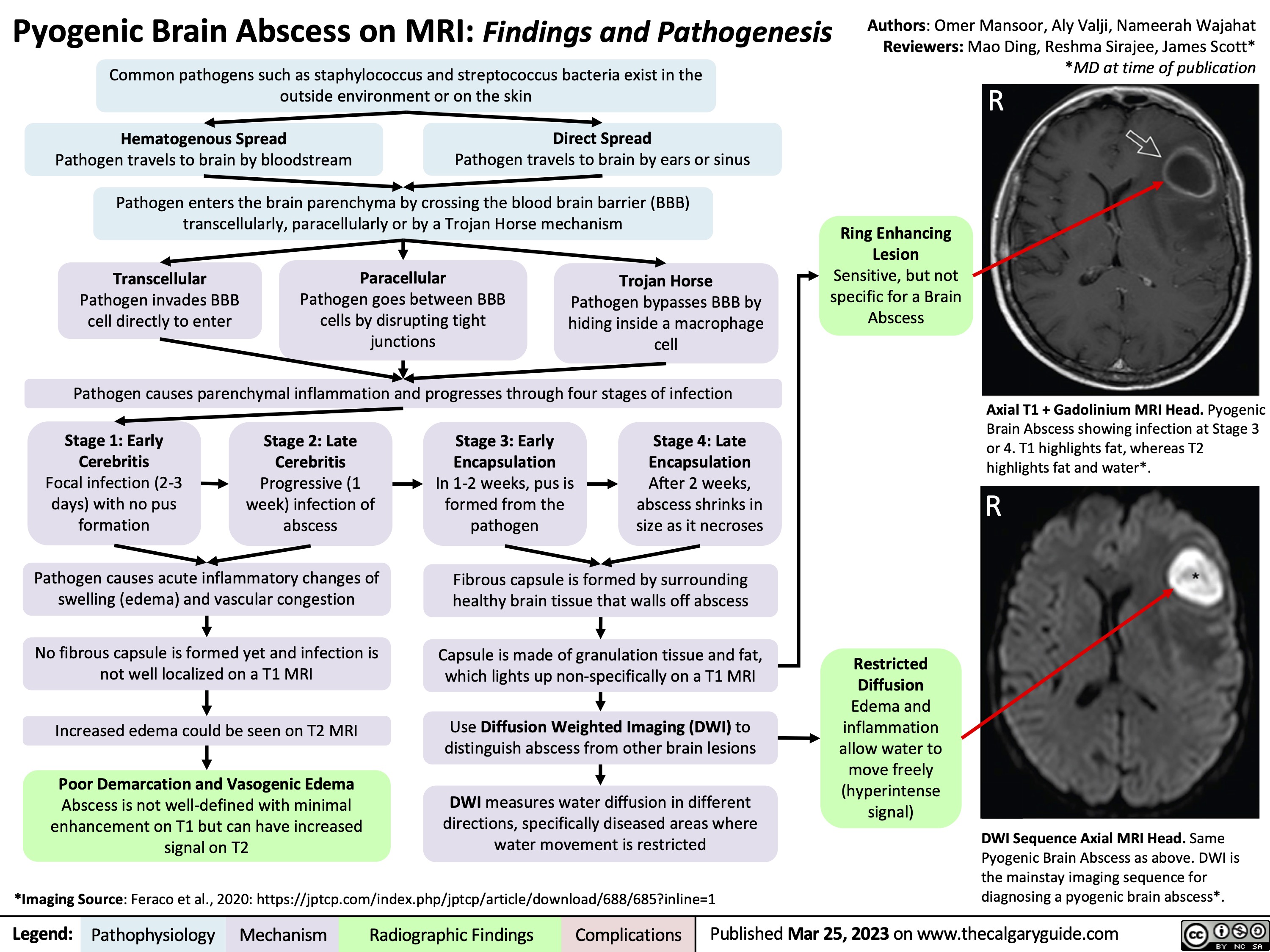
Carpal Tunnel Syndrome
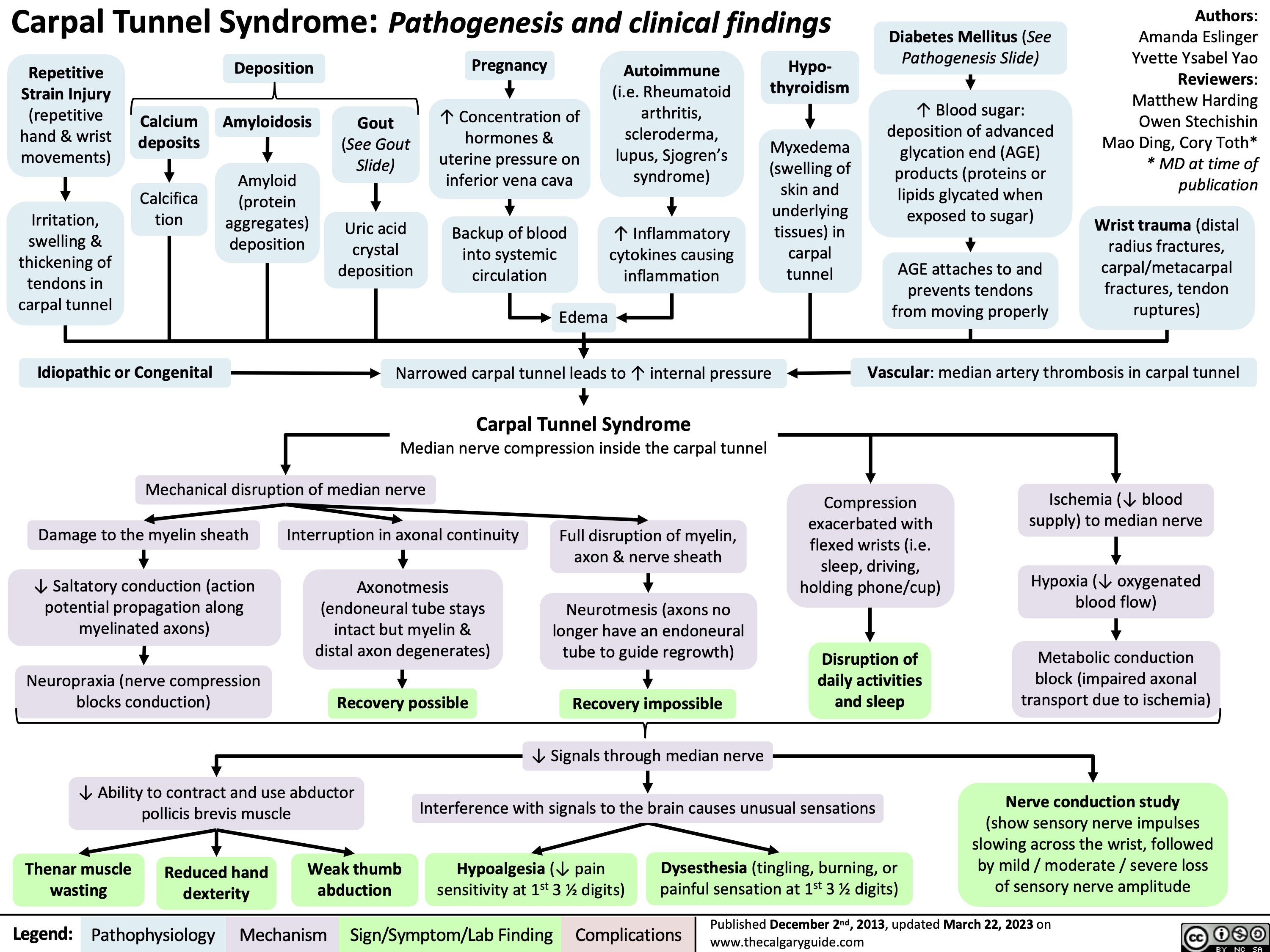
Acute Pulmonary Embolism on CTPA
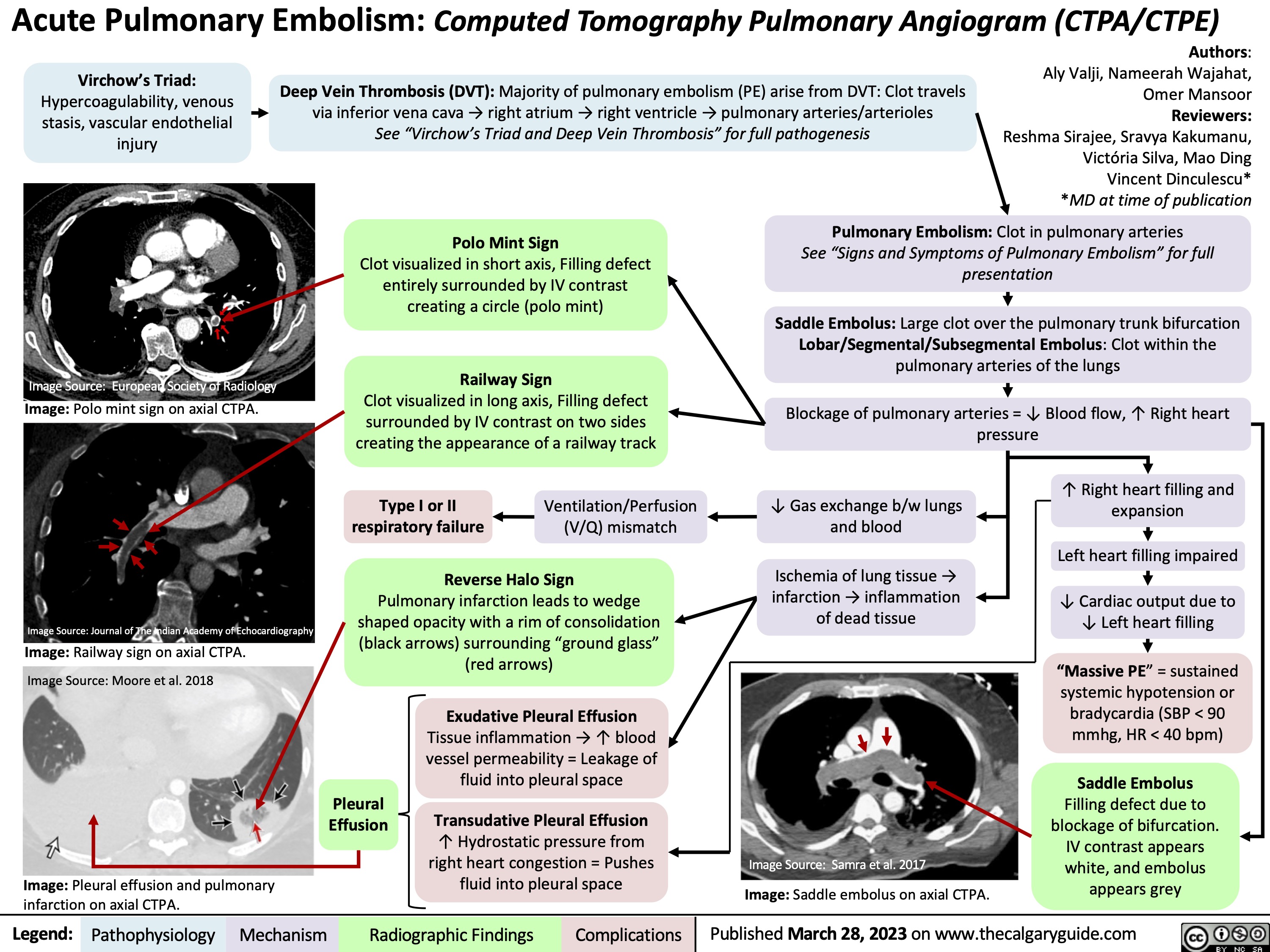
Coronary Artery Bypass Graft CABG Indications
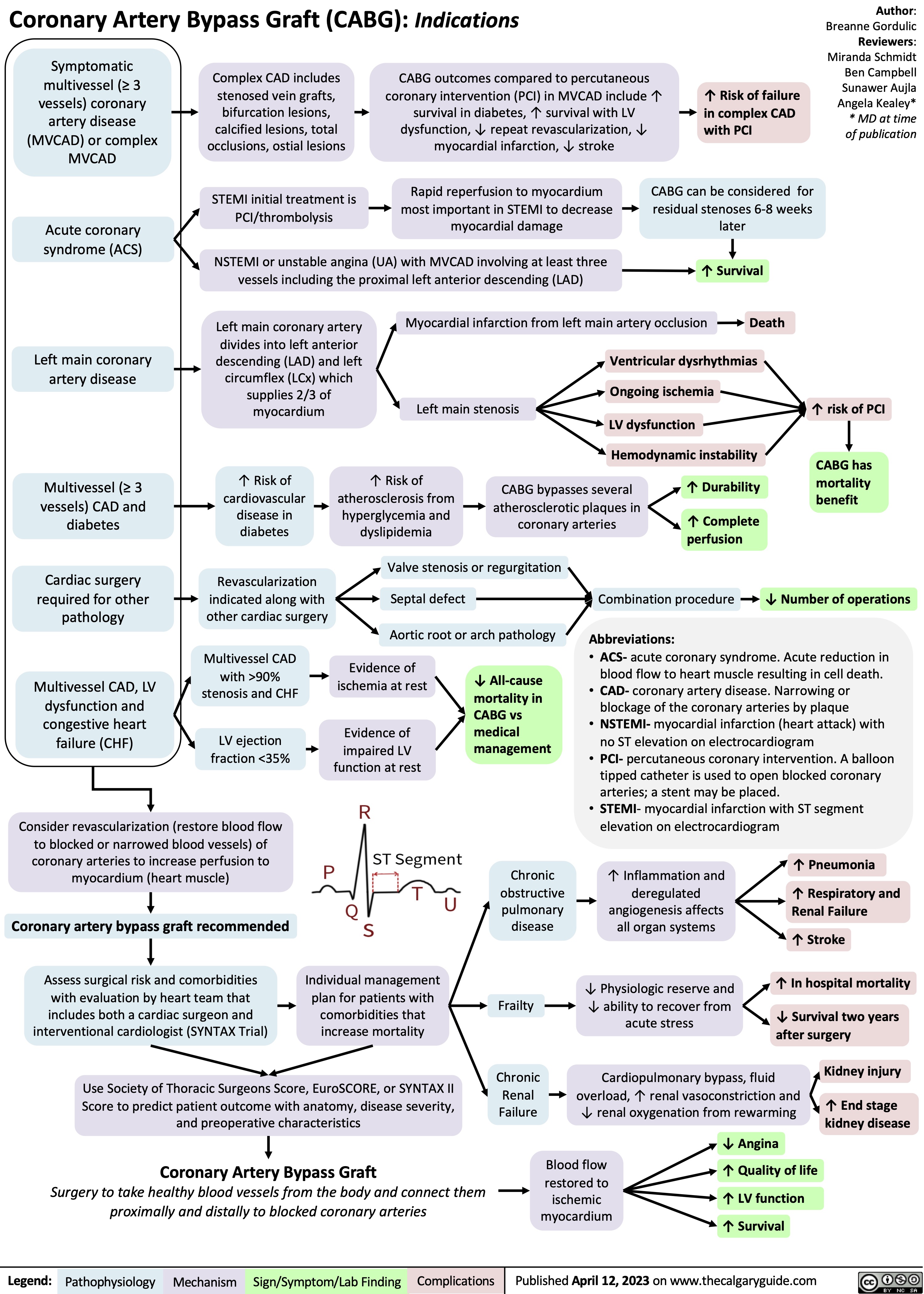
Telogen Effluvium
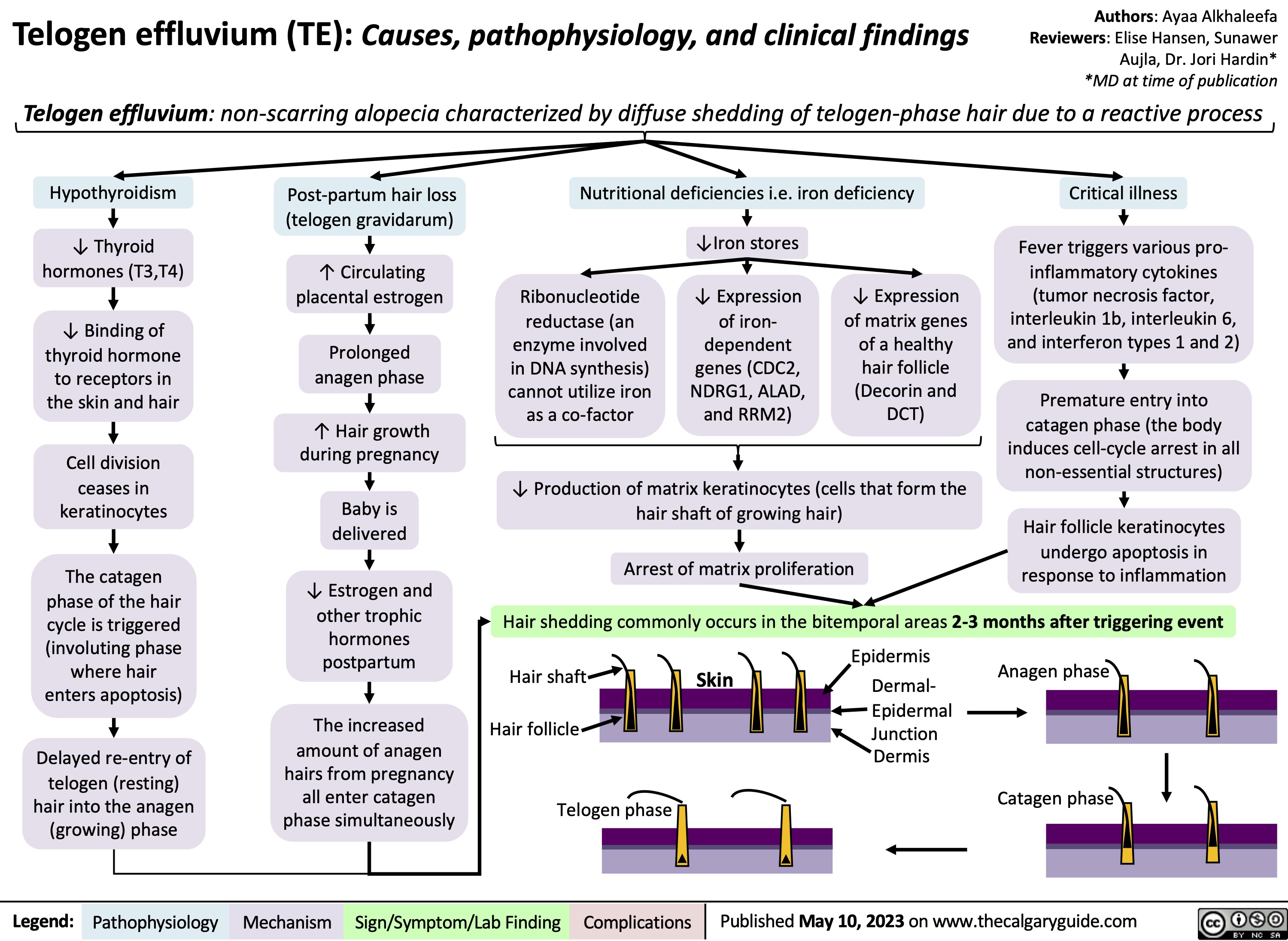
OA Clinical findings
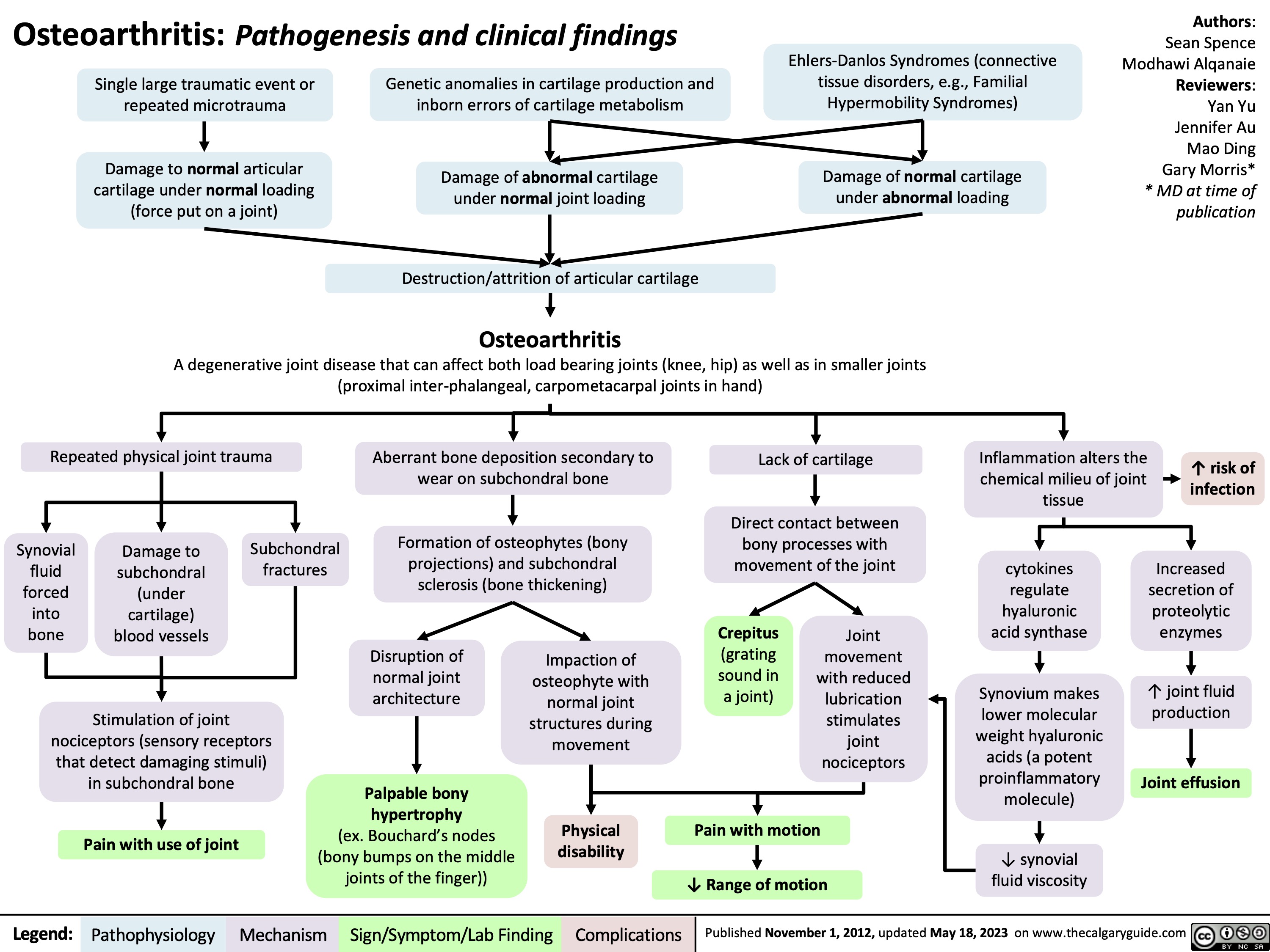
Acute Laryngitis
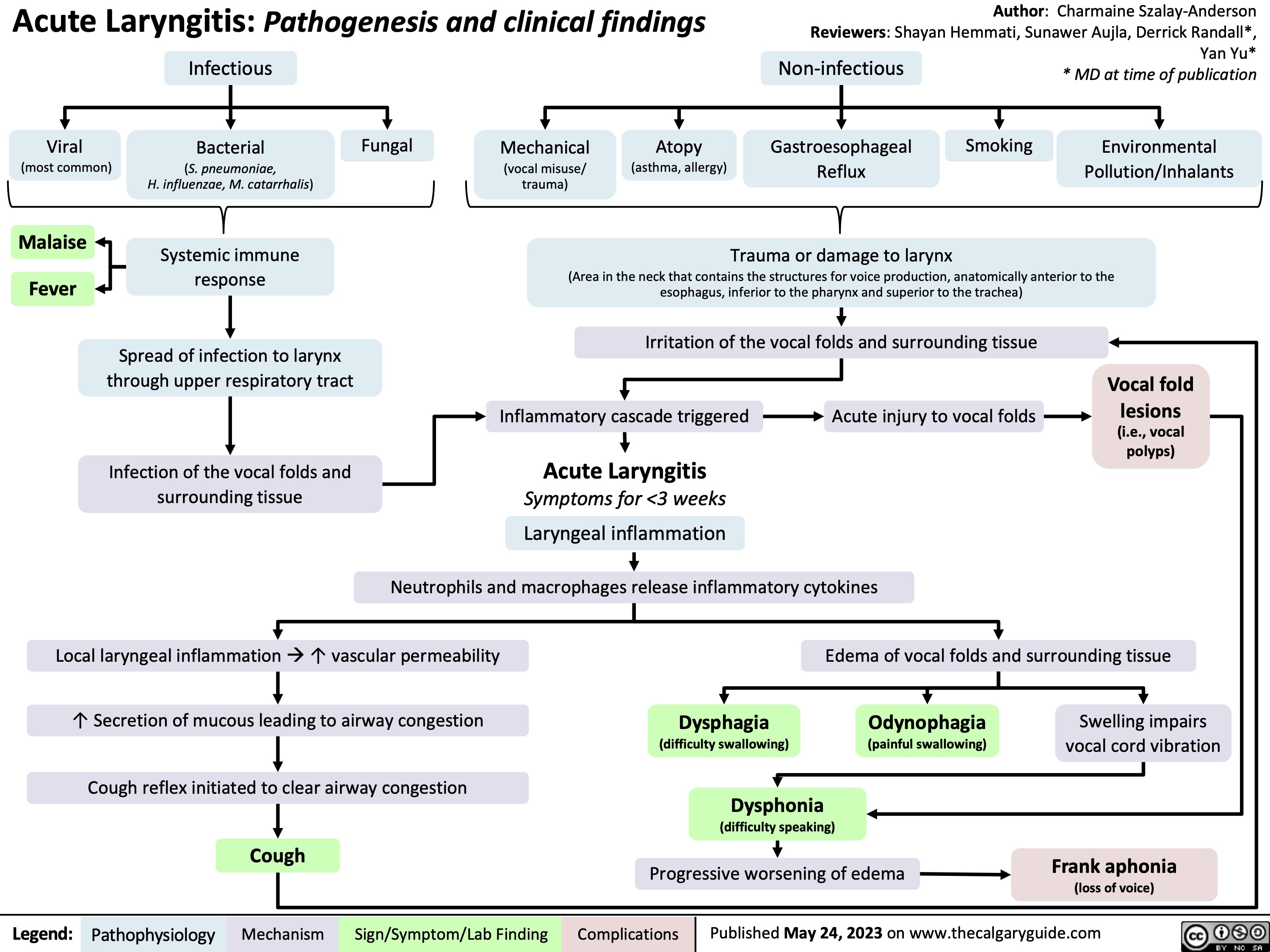
diverticulosis-vs-diverticulitis-distinguishing-features
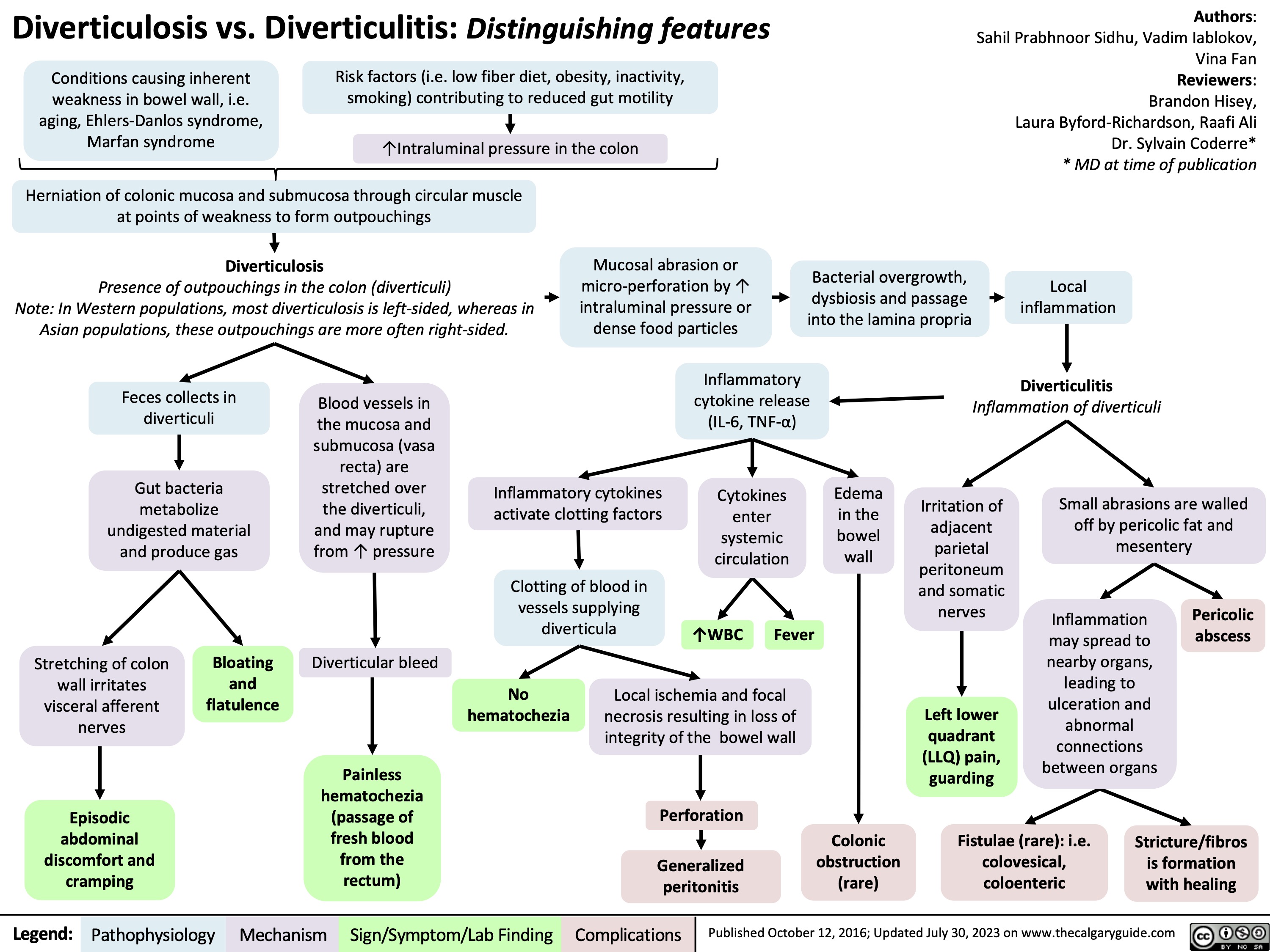
Knee Osteoarthritis
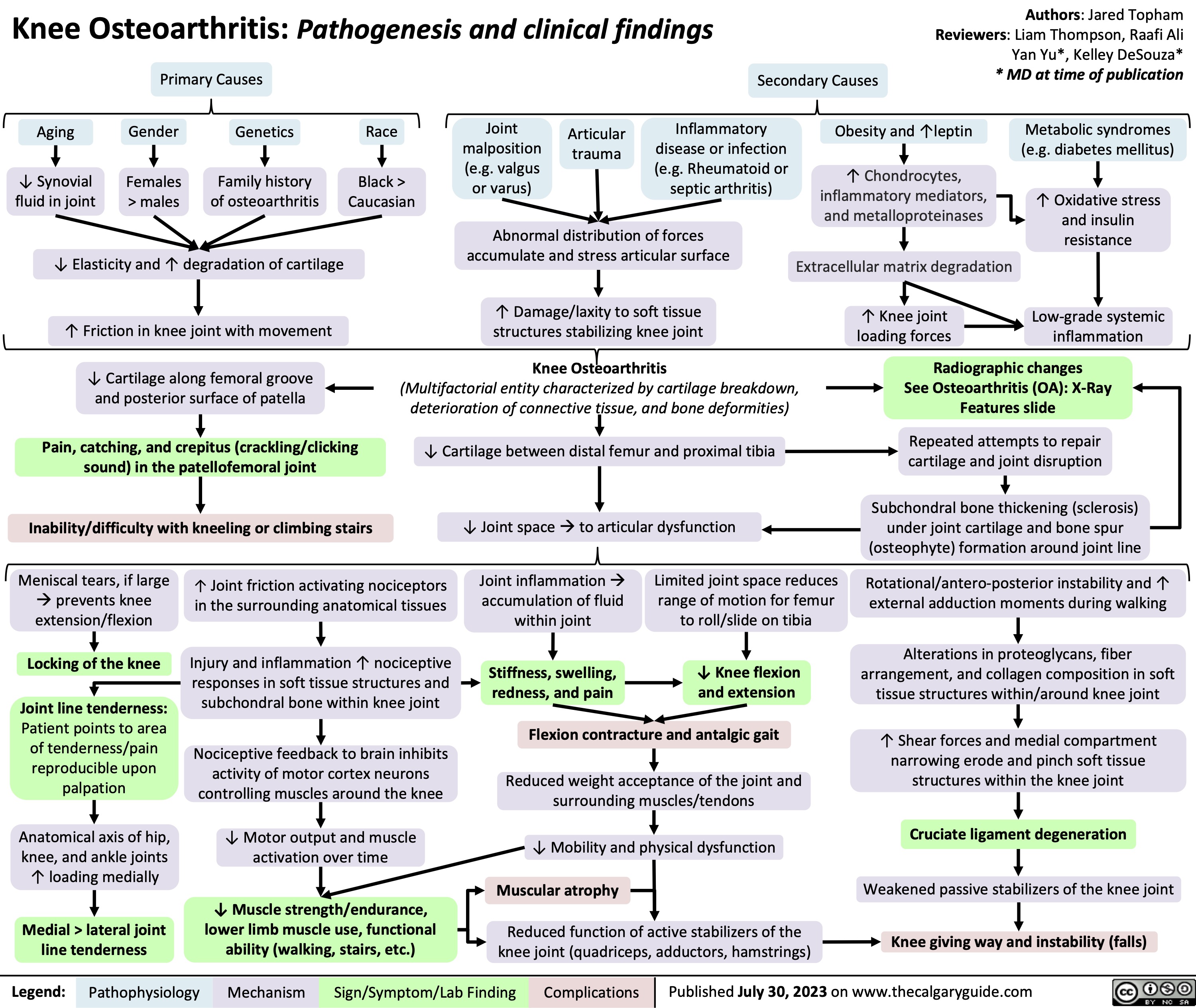
Rotator Cuff Disease
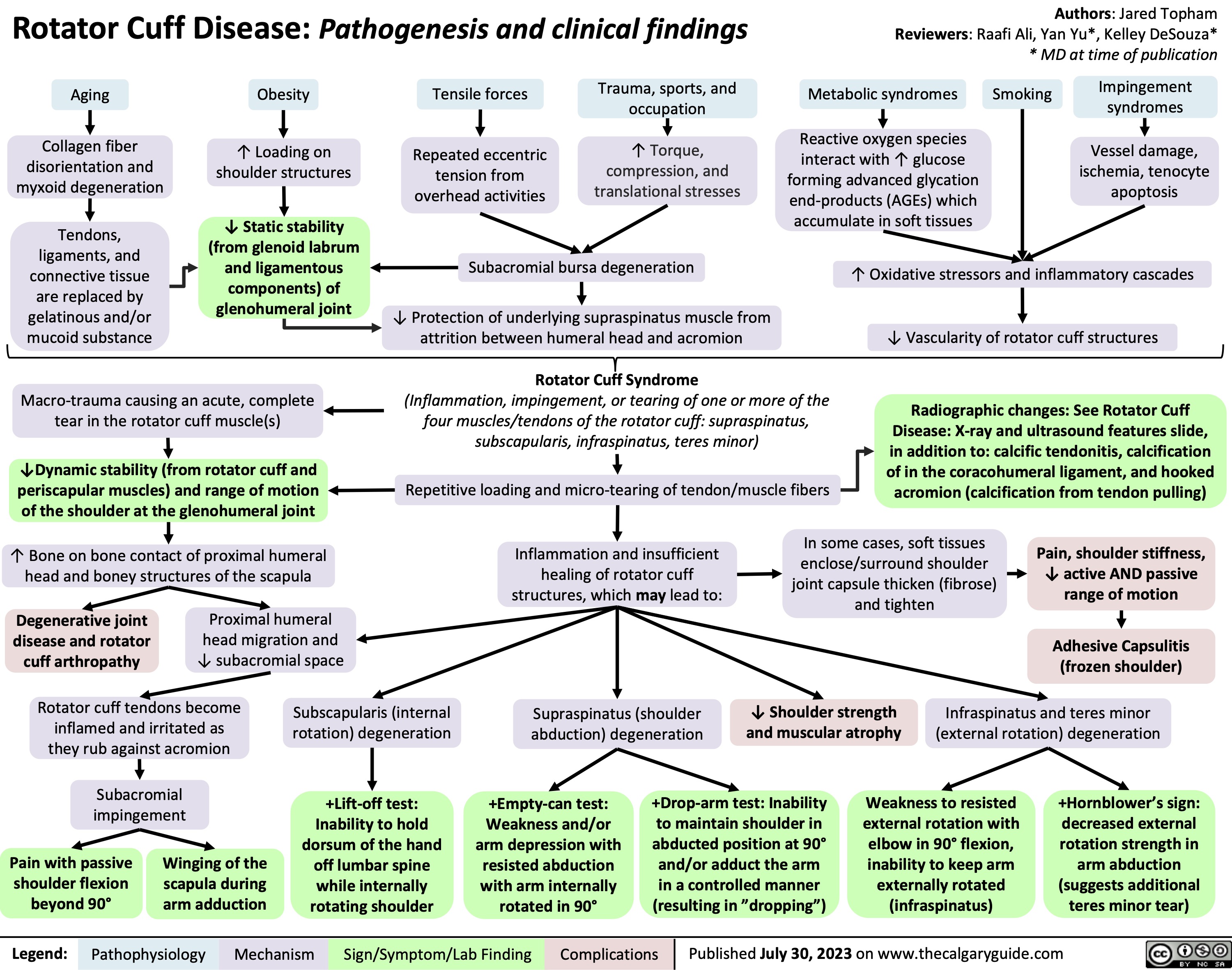
Rotator Cuff Disease Xray and Ultrasound Features
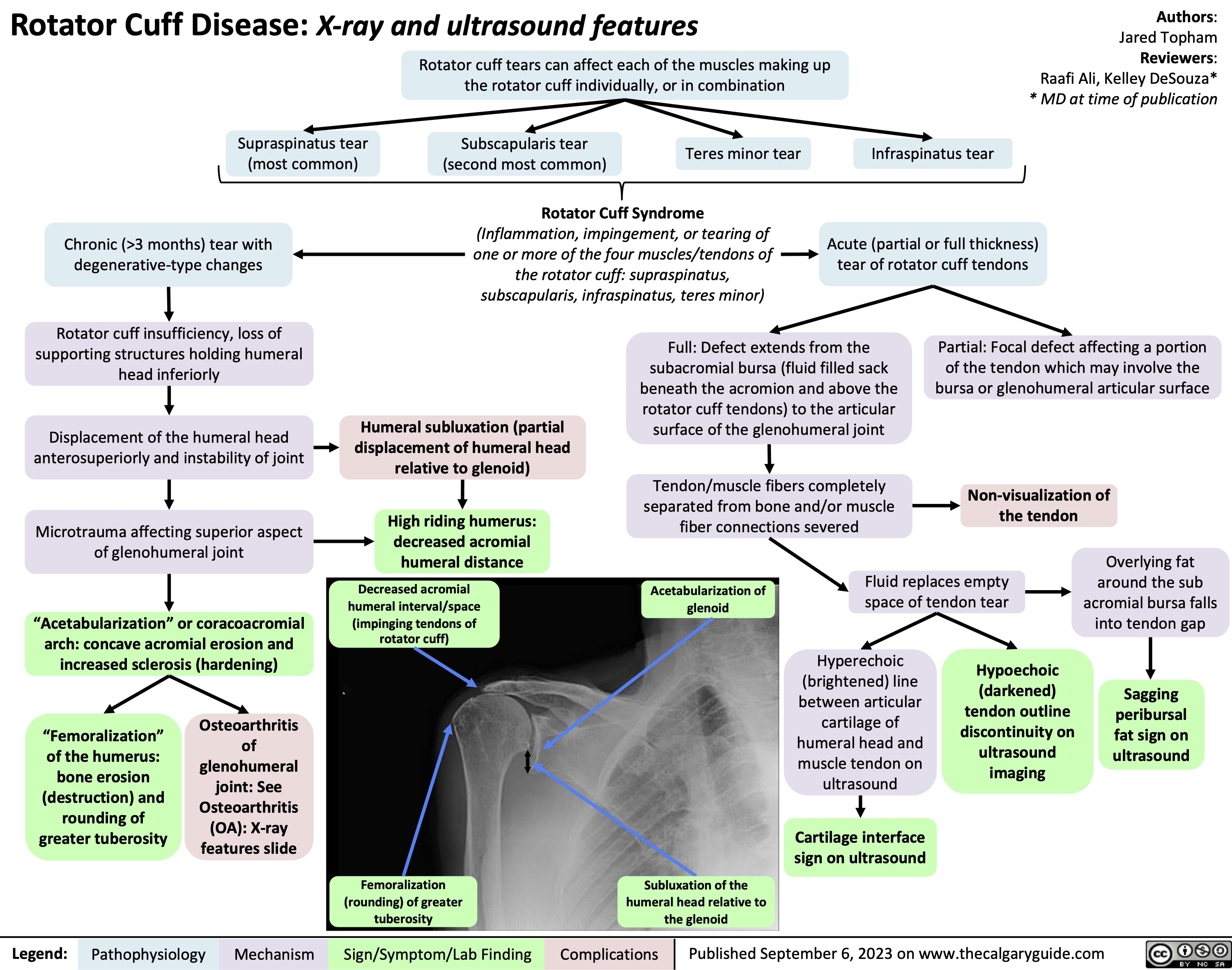
Acute Respiratory Distress Syndrome
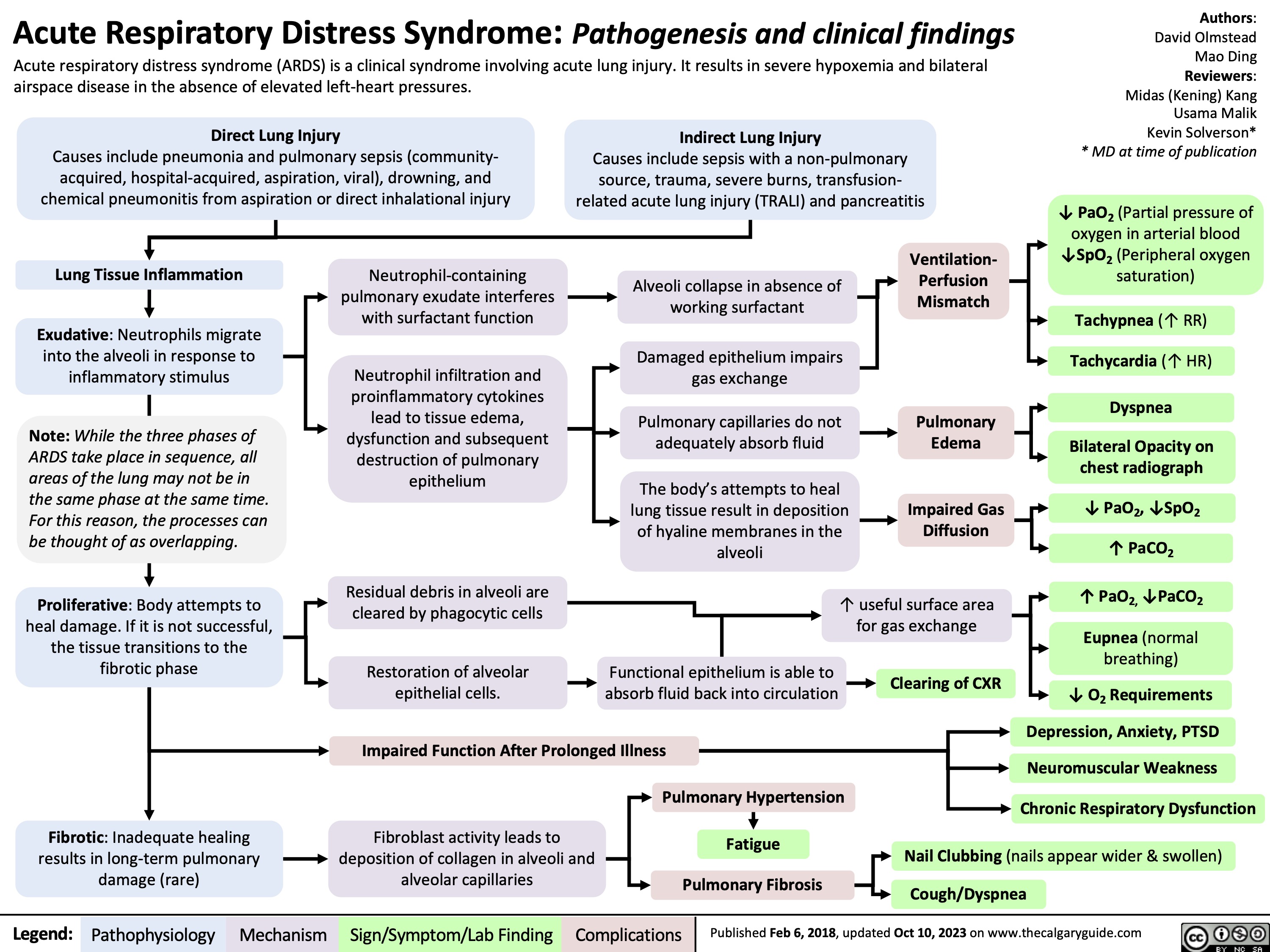
Approach To Dementia
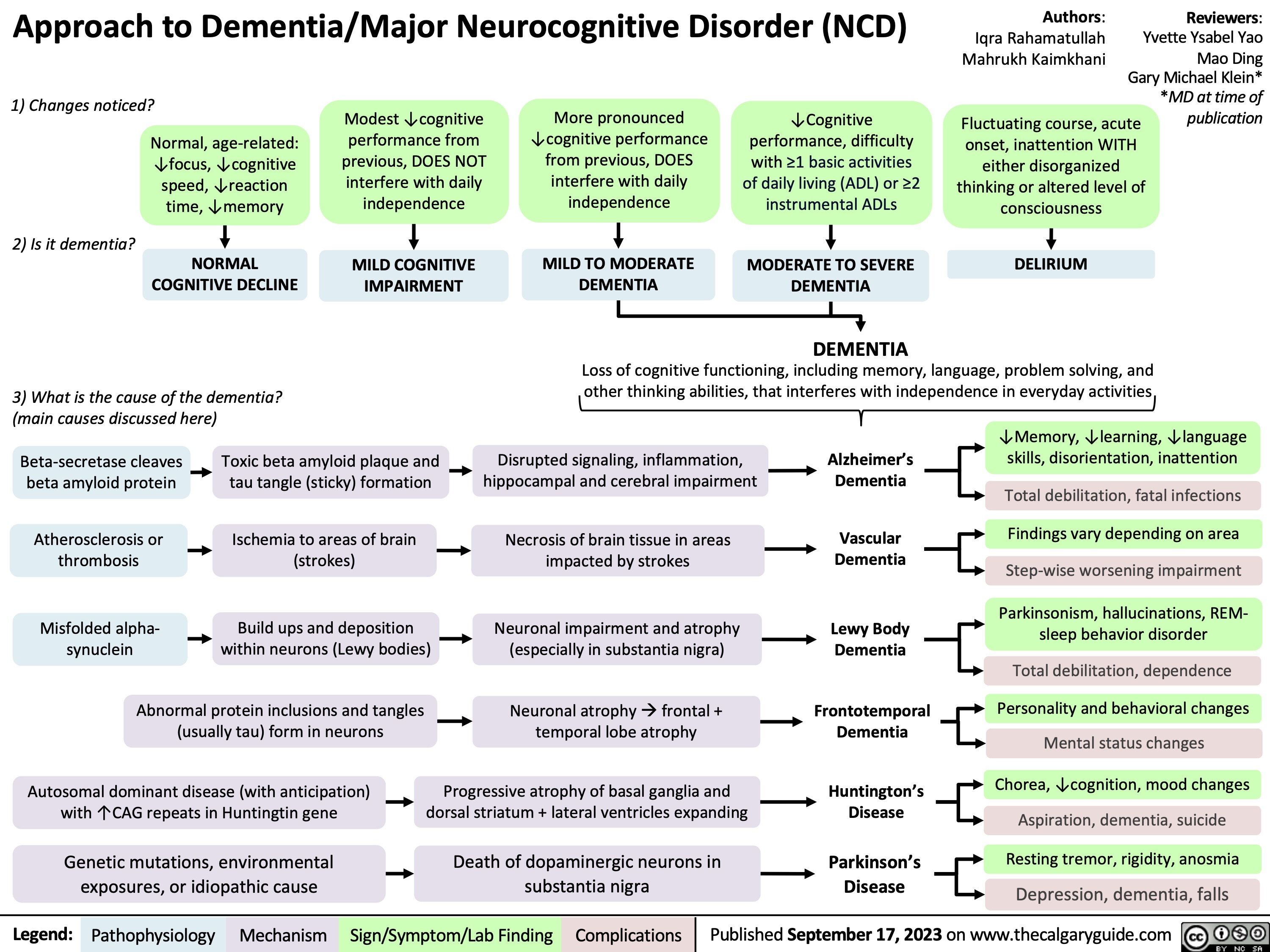
Mitral Regurgitation Pathogenesis and clinical findings
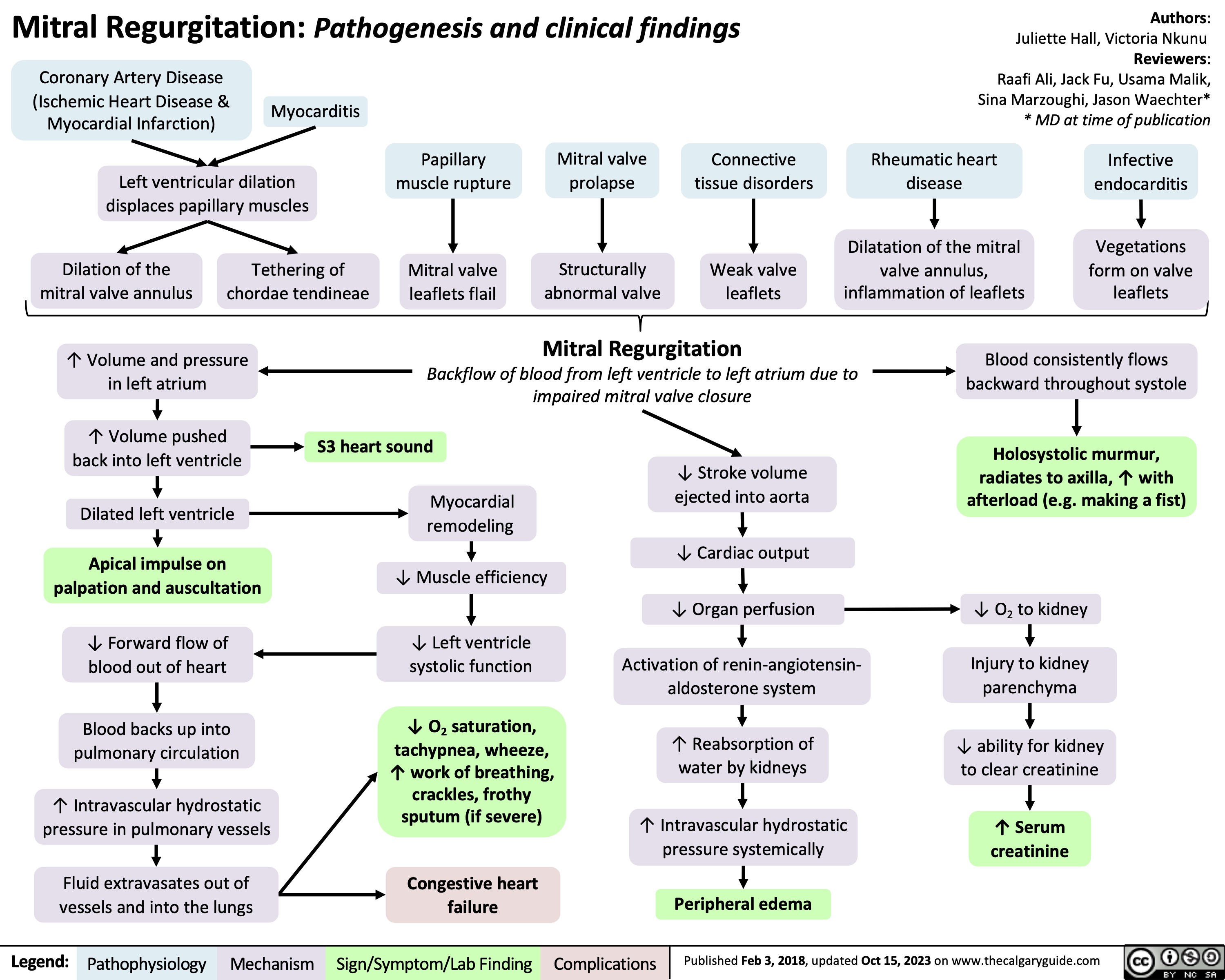
Statins Mechanisms and Side Effects
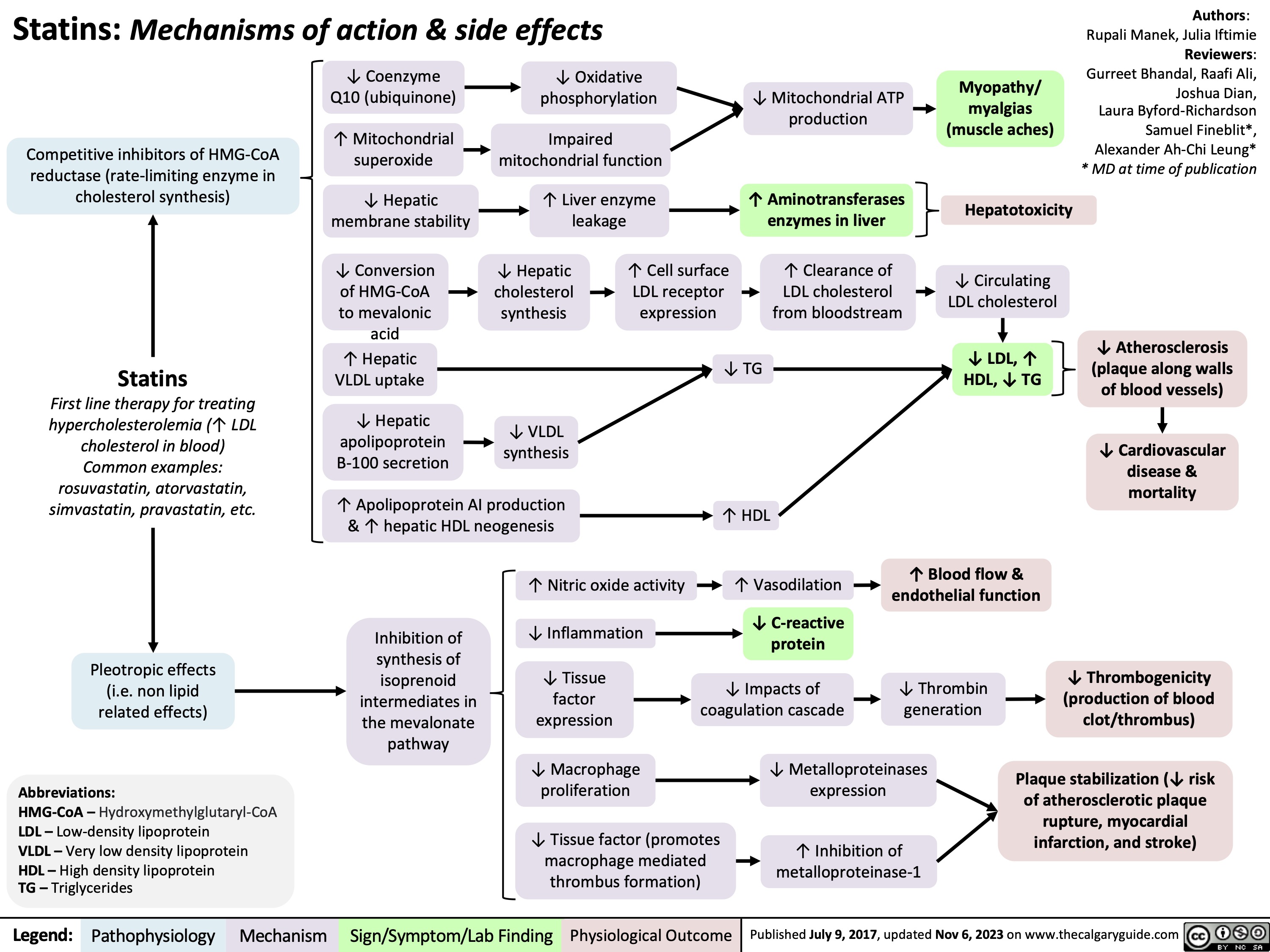
Pharmacotherapy for Dyslipidemia Overview
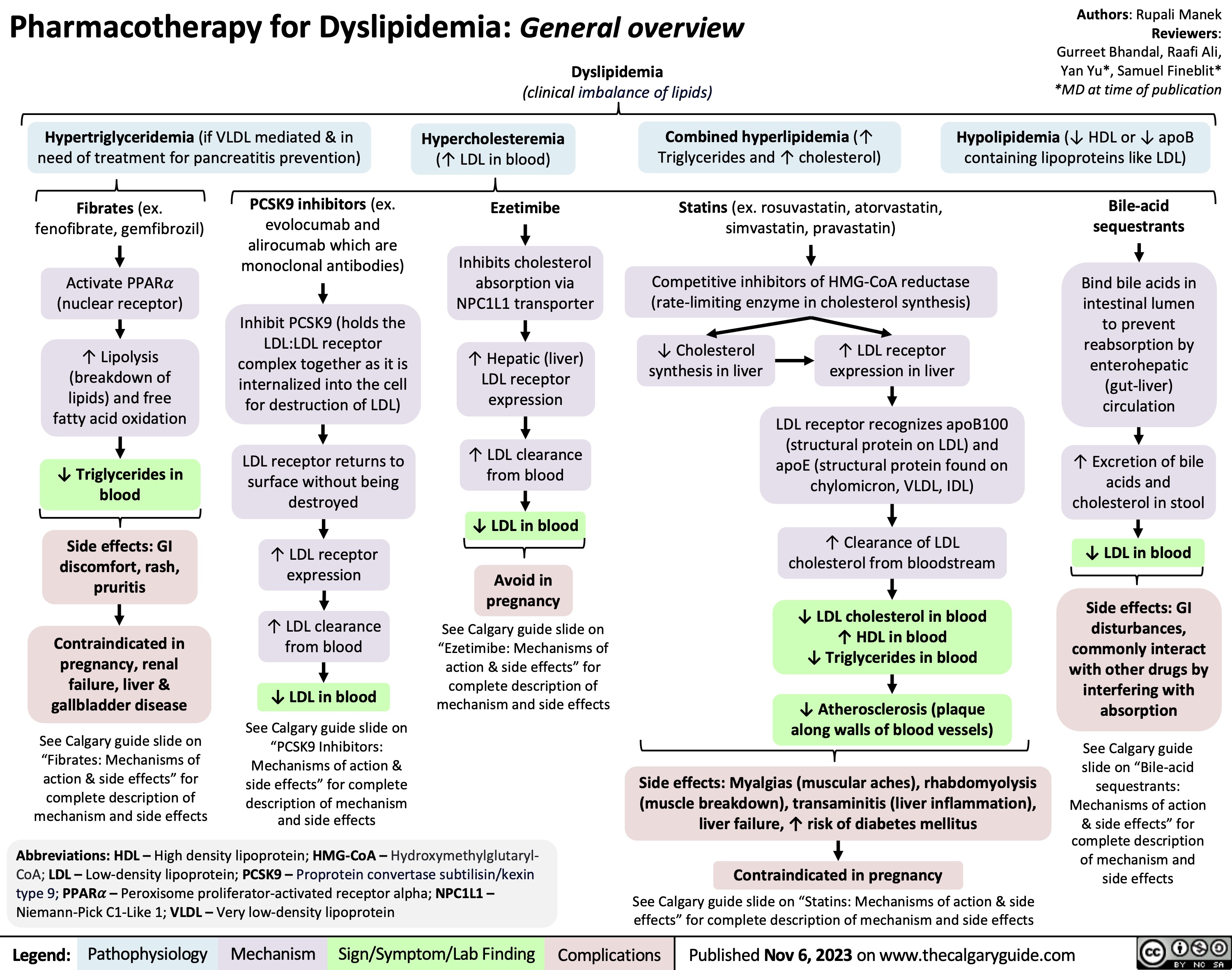
Death Cardiovascular Respiratory and Neurologic Mechanisms
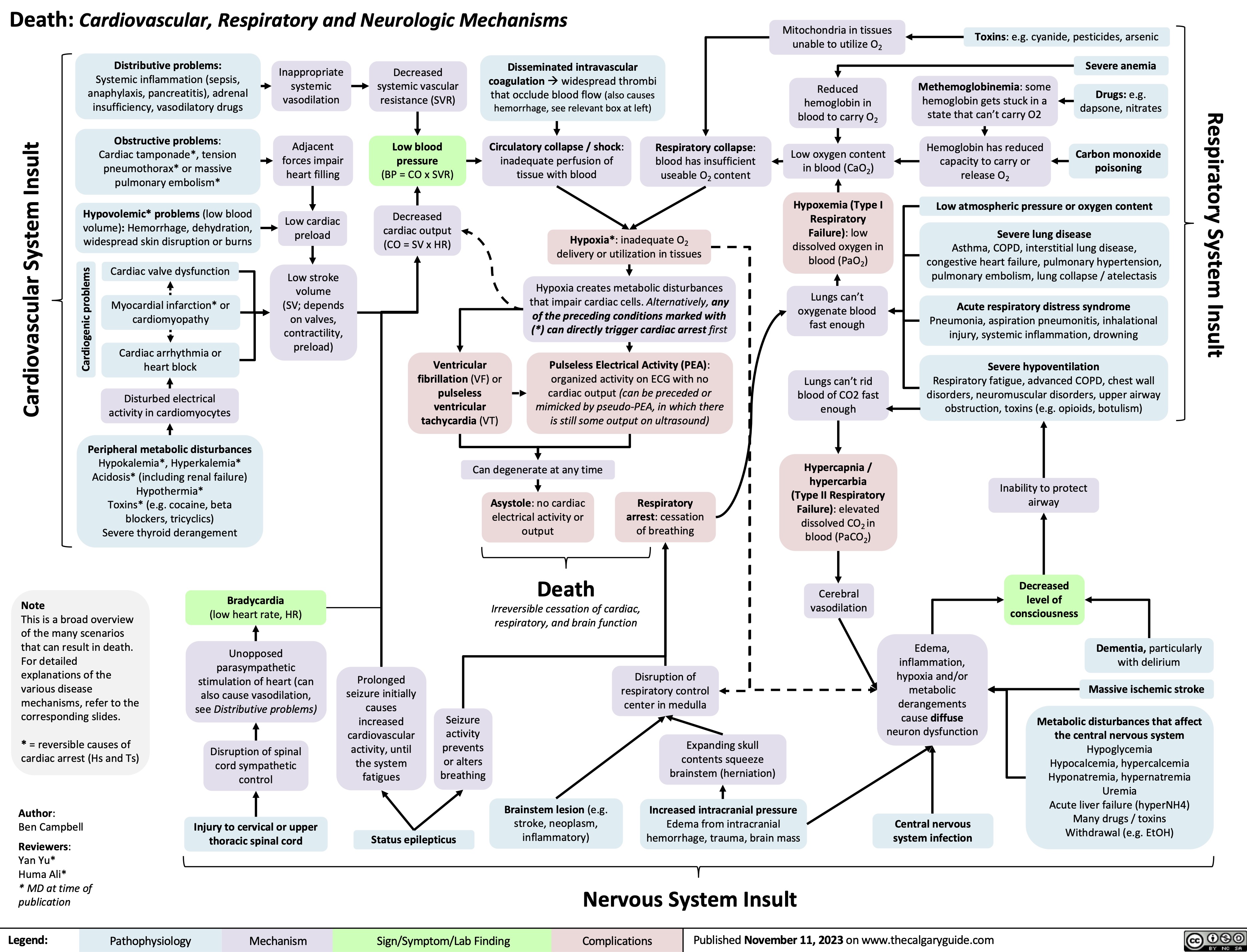
Non-Alcoholic Fatty Liver Disease
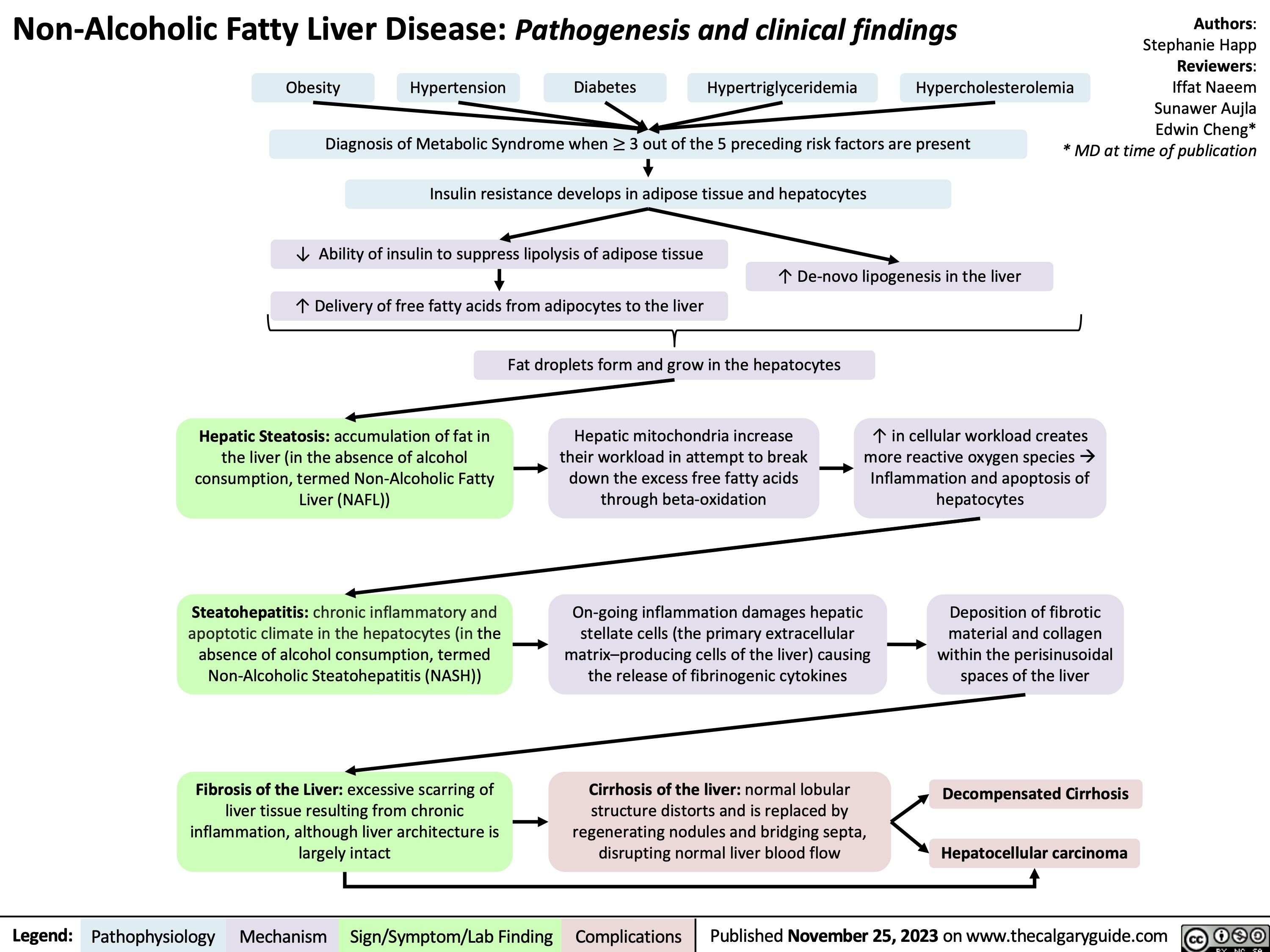
RICE mechanism of action
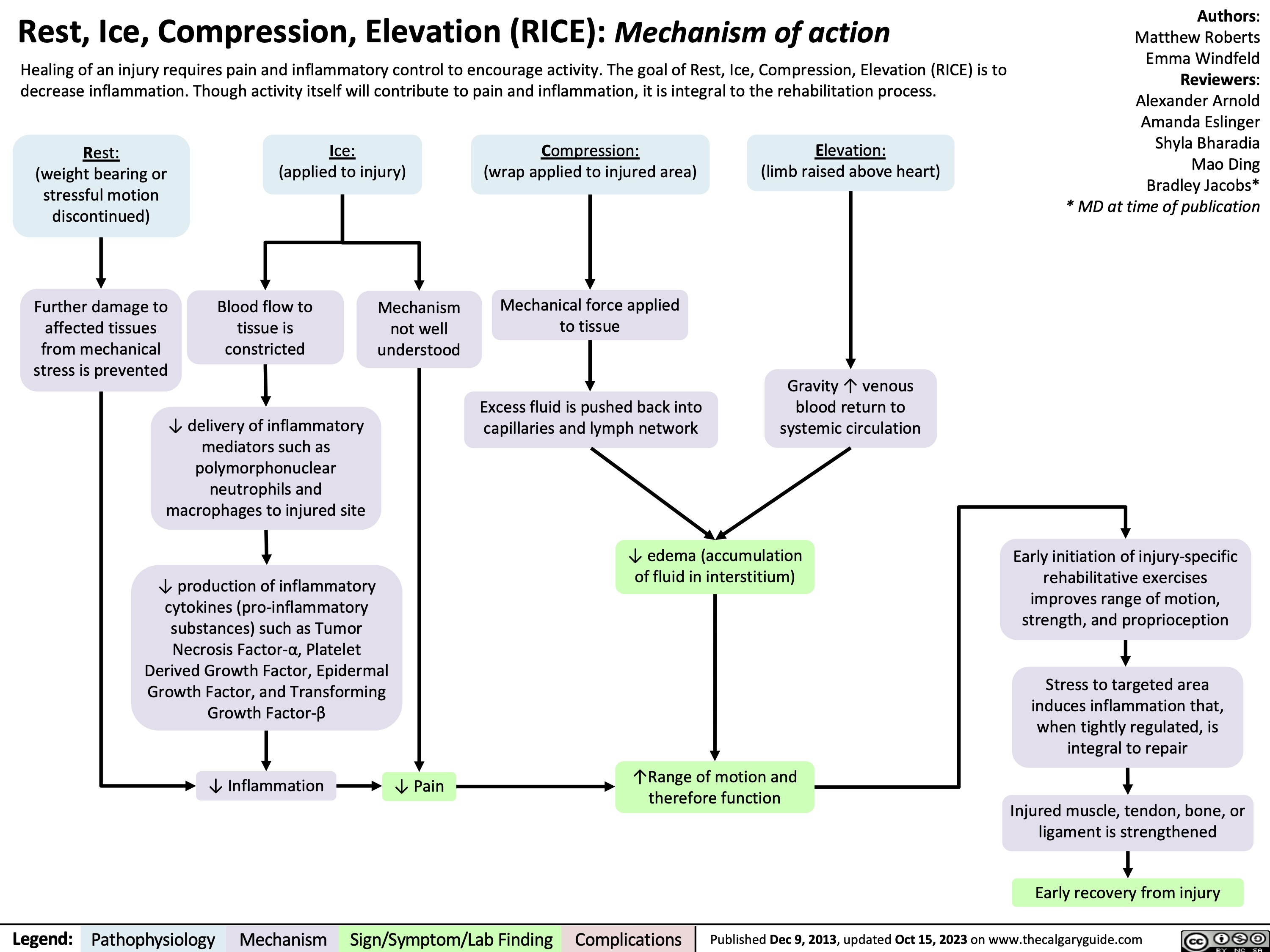
Aspiration Pneumonia
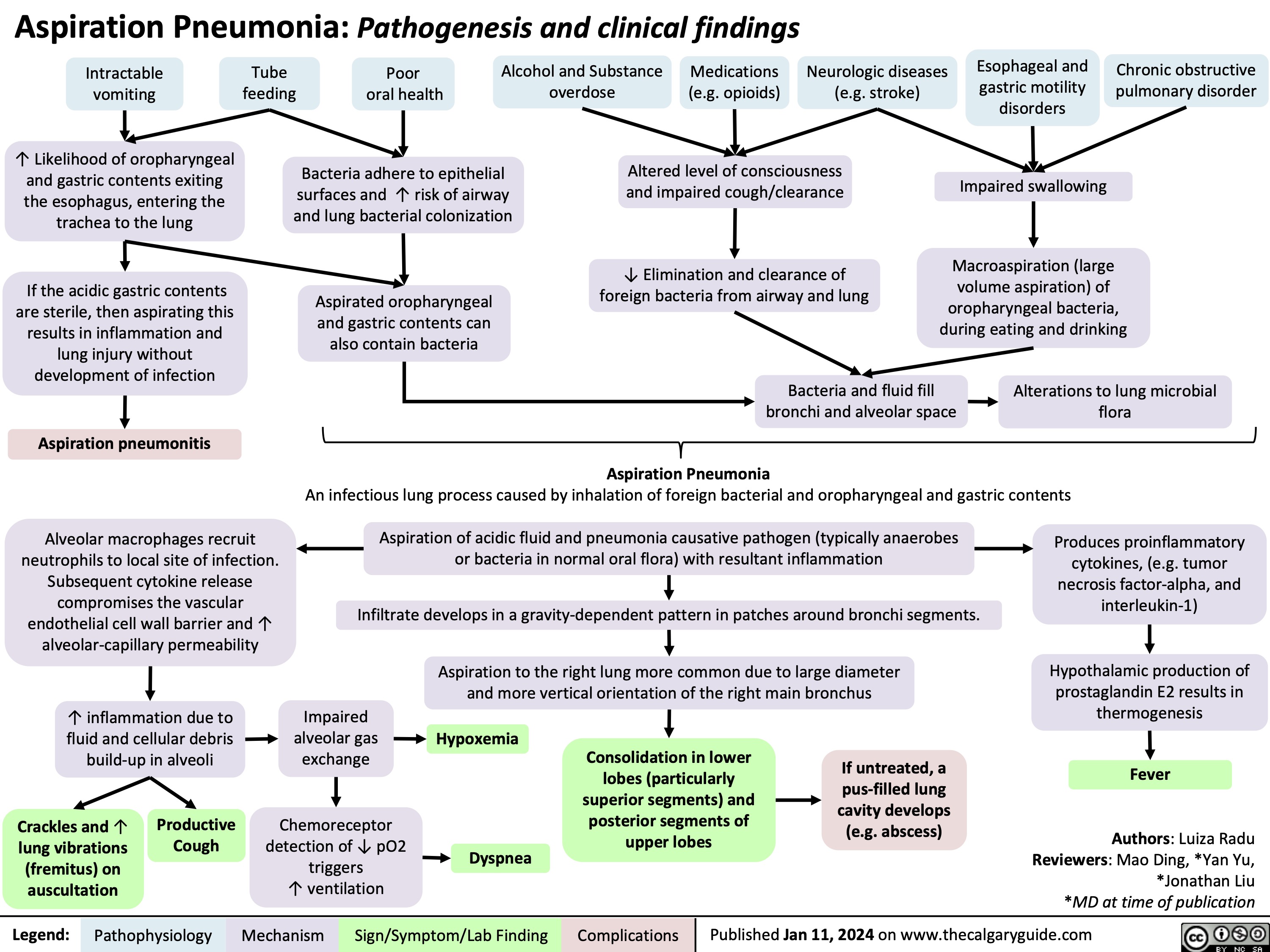
Shoulder Impingement Syndrome
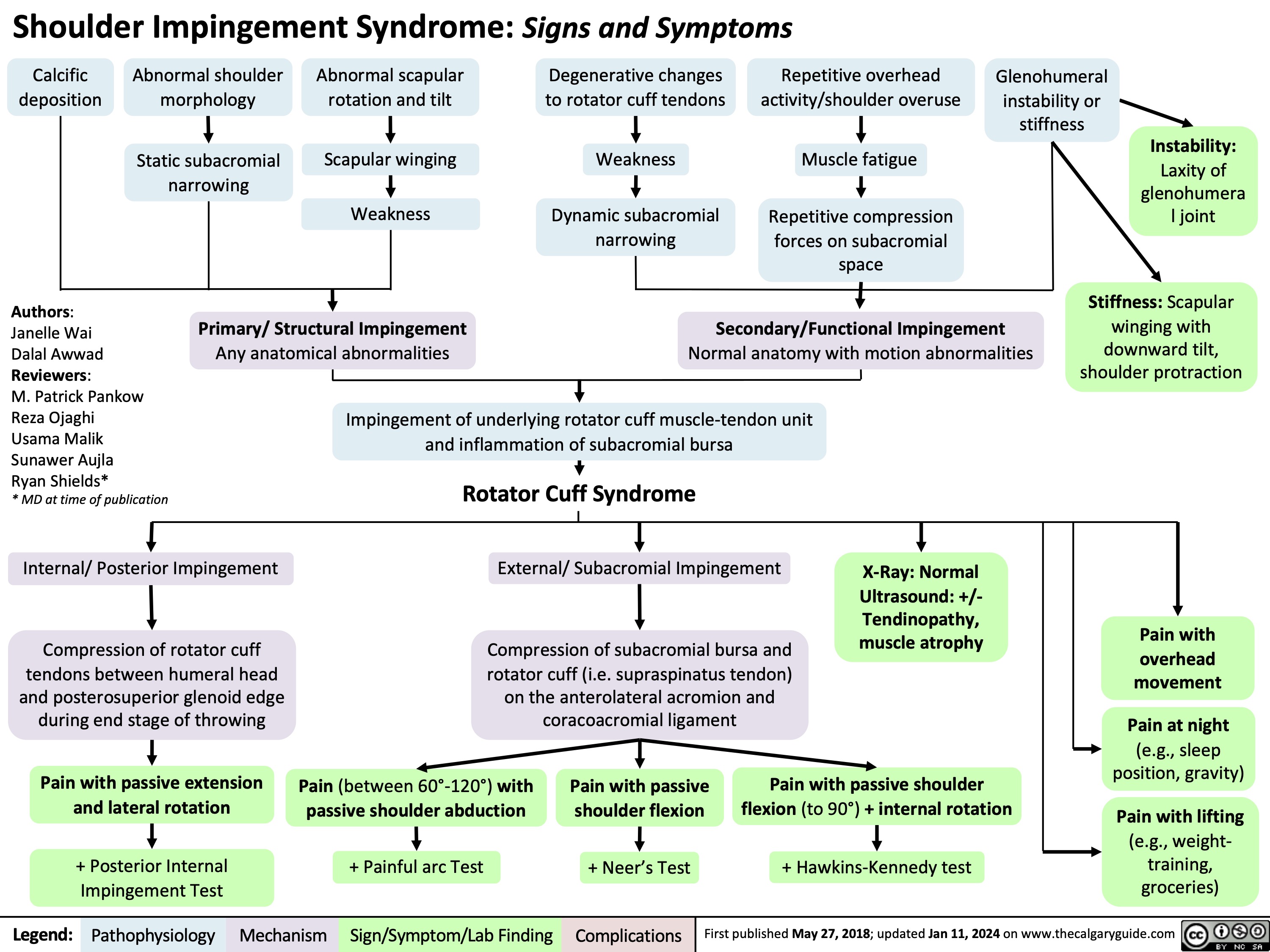
Arachnoid Cysts MRI Findings
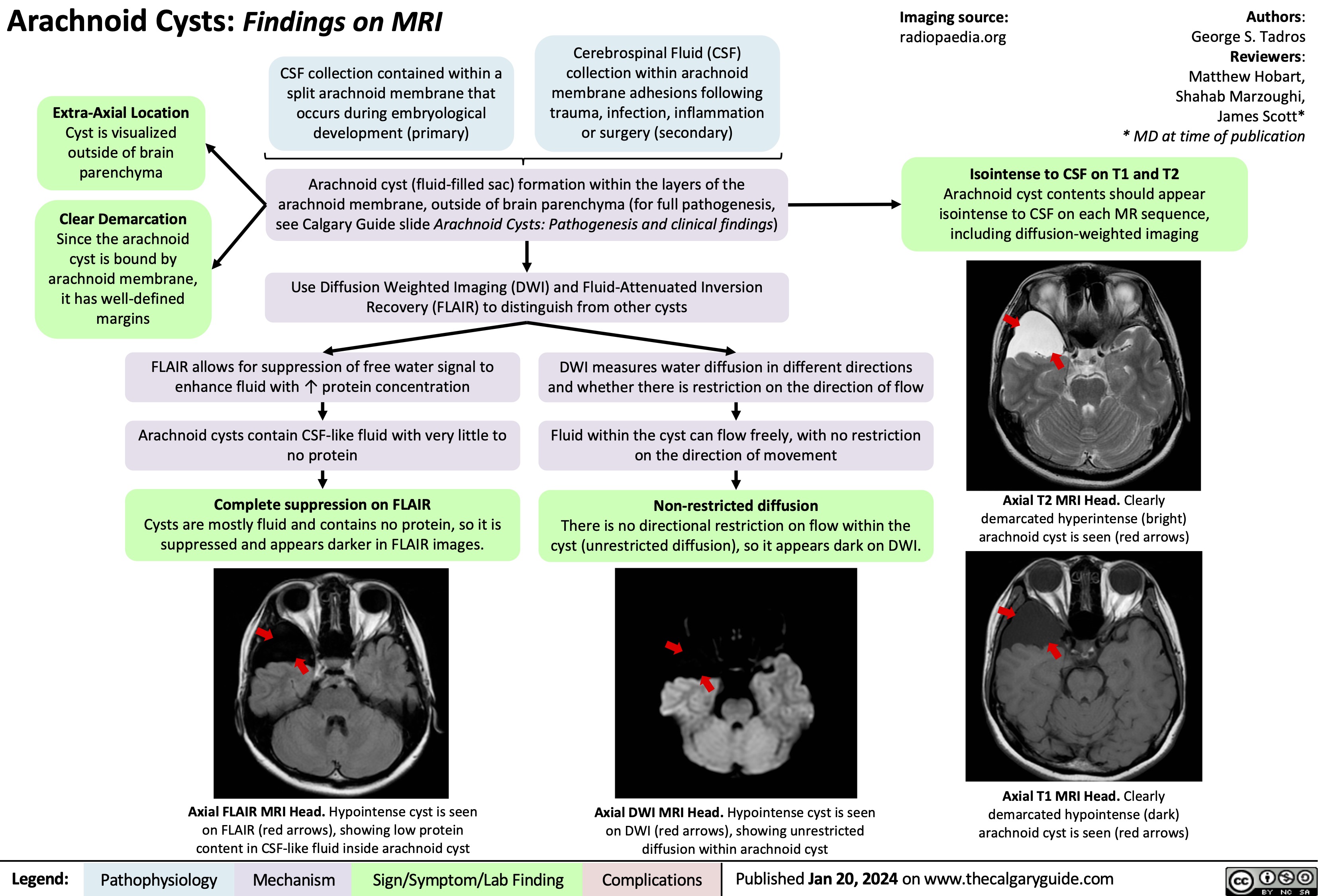
Arachnoid Cysts Pathogenesis and clinical findings
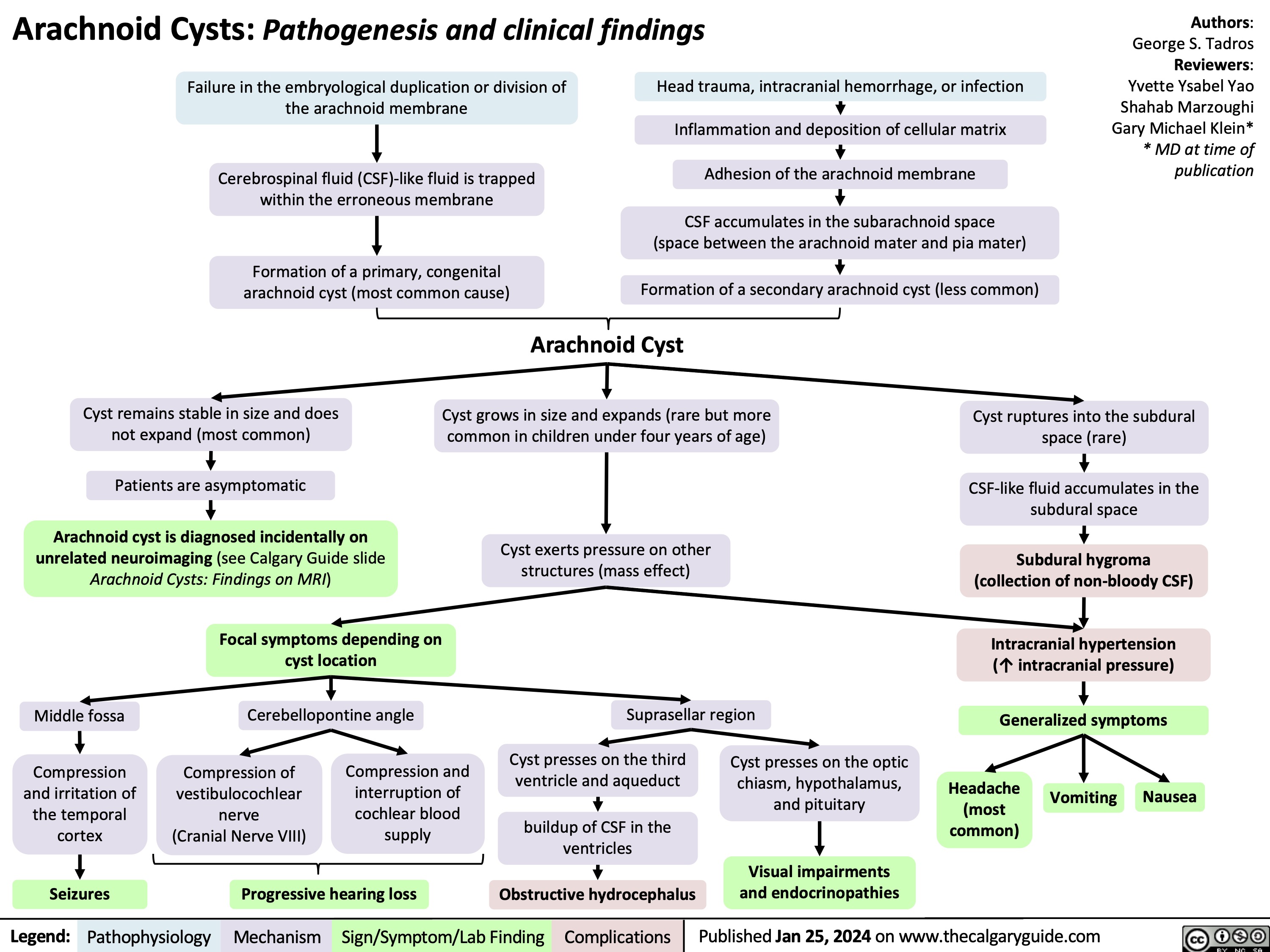
Avascular Necrosis AVN of the Femoral Head Findings on MRI
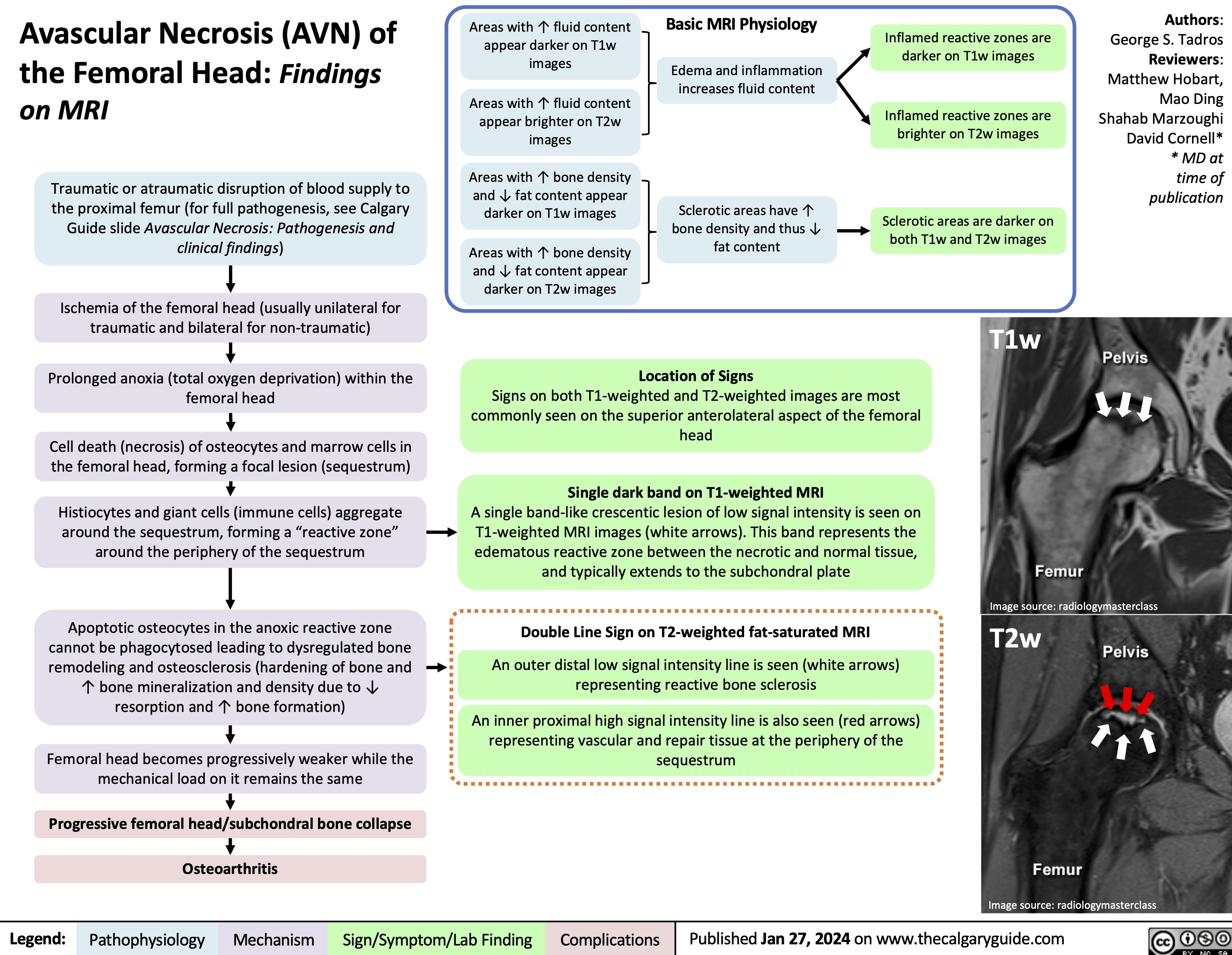
Bacterial Infections from Transfusion
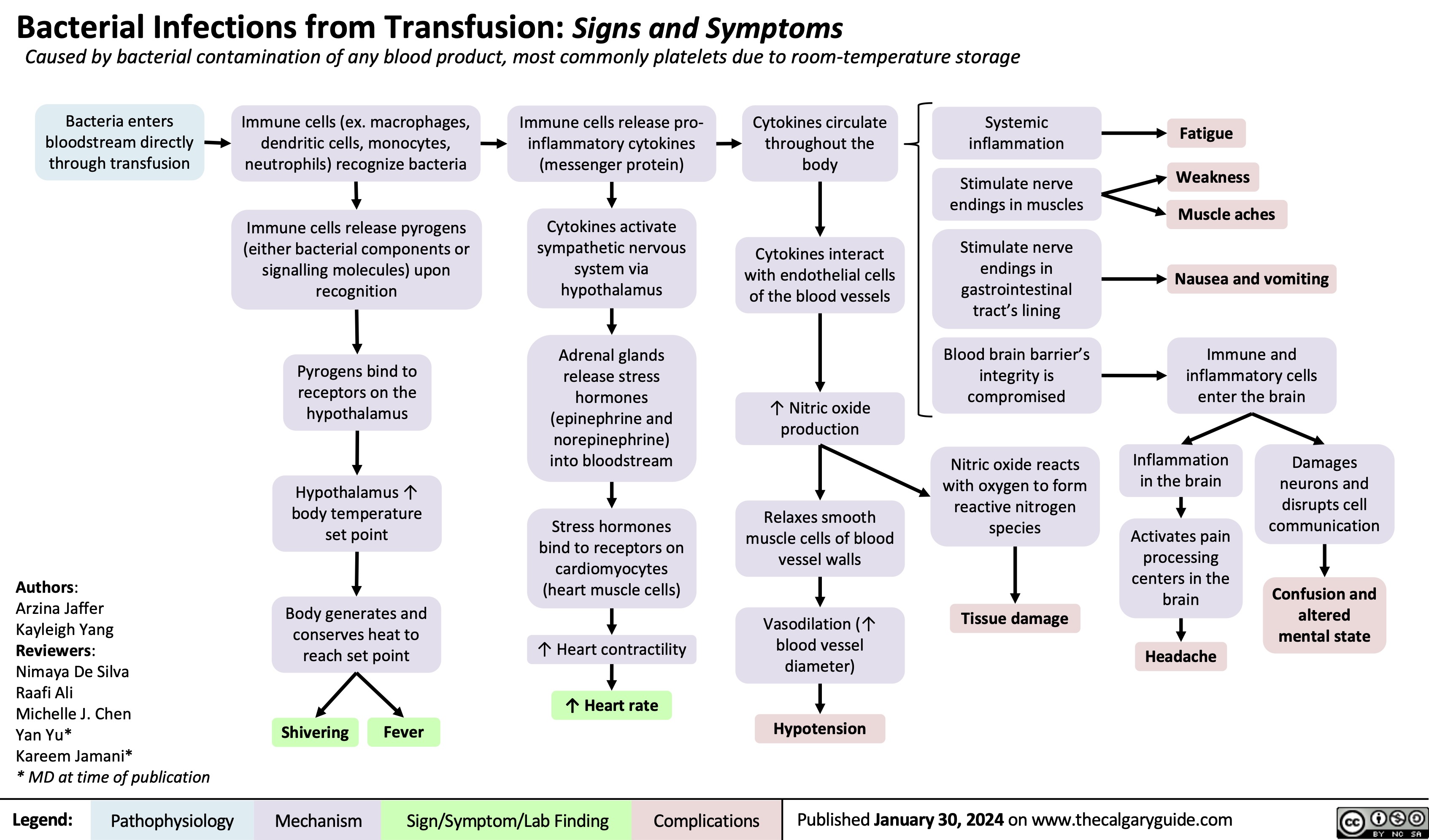
Acute Wound Healing
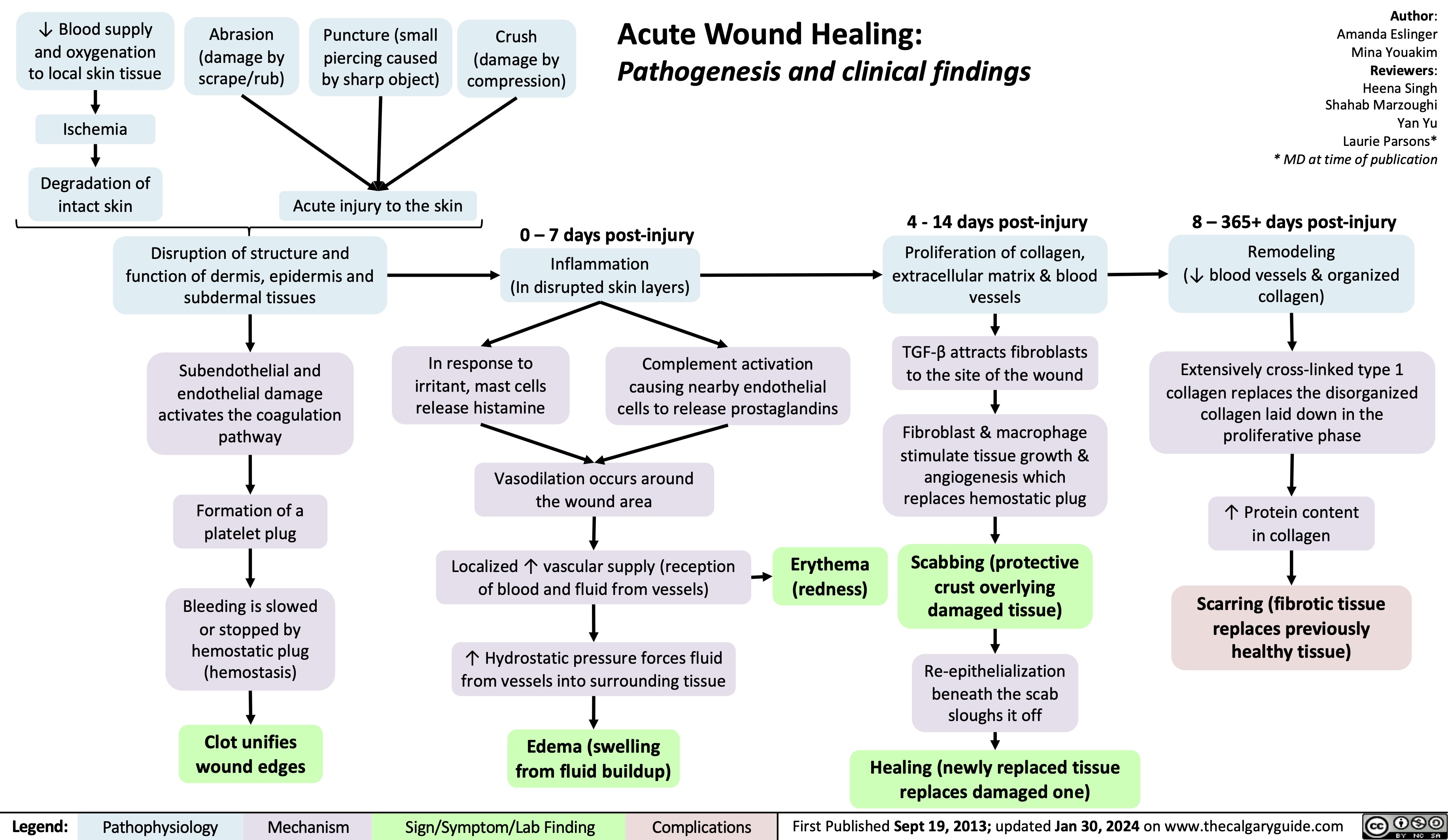
Acute Otitis Externa Complications
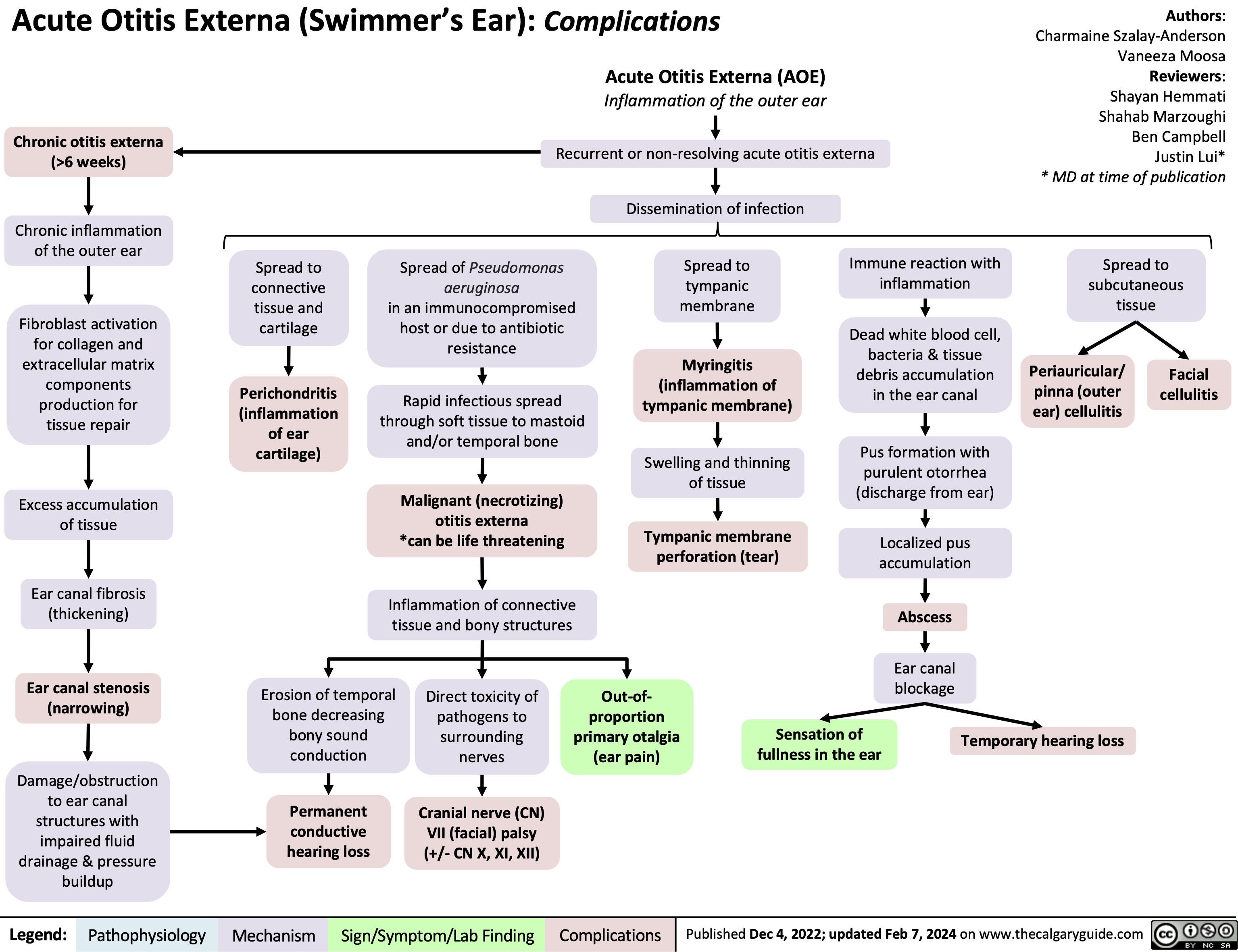
Infective endocarditis
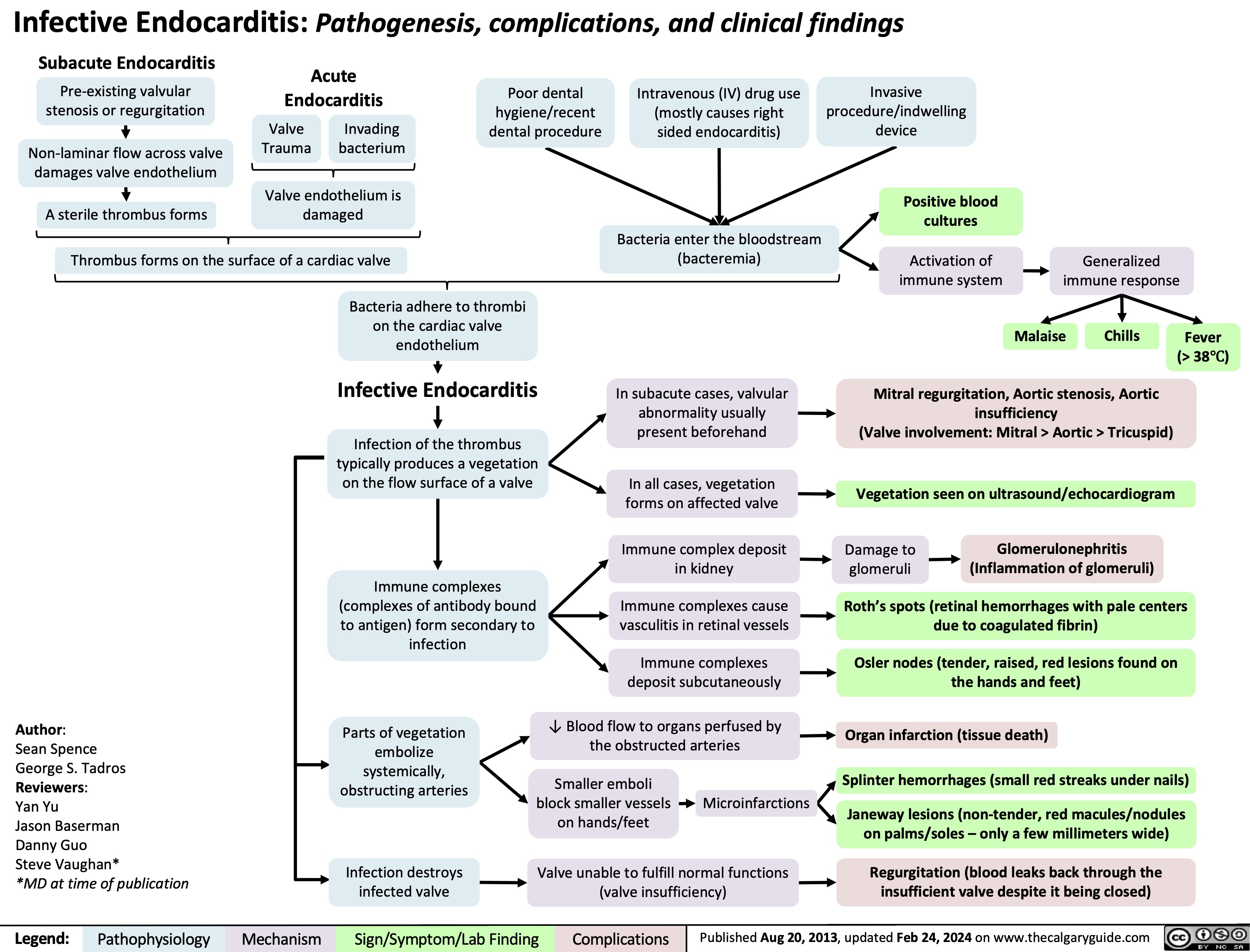
Onychomycosis
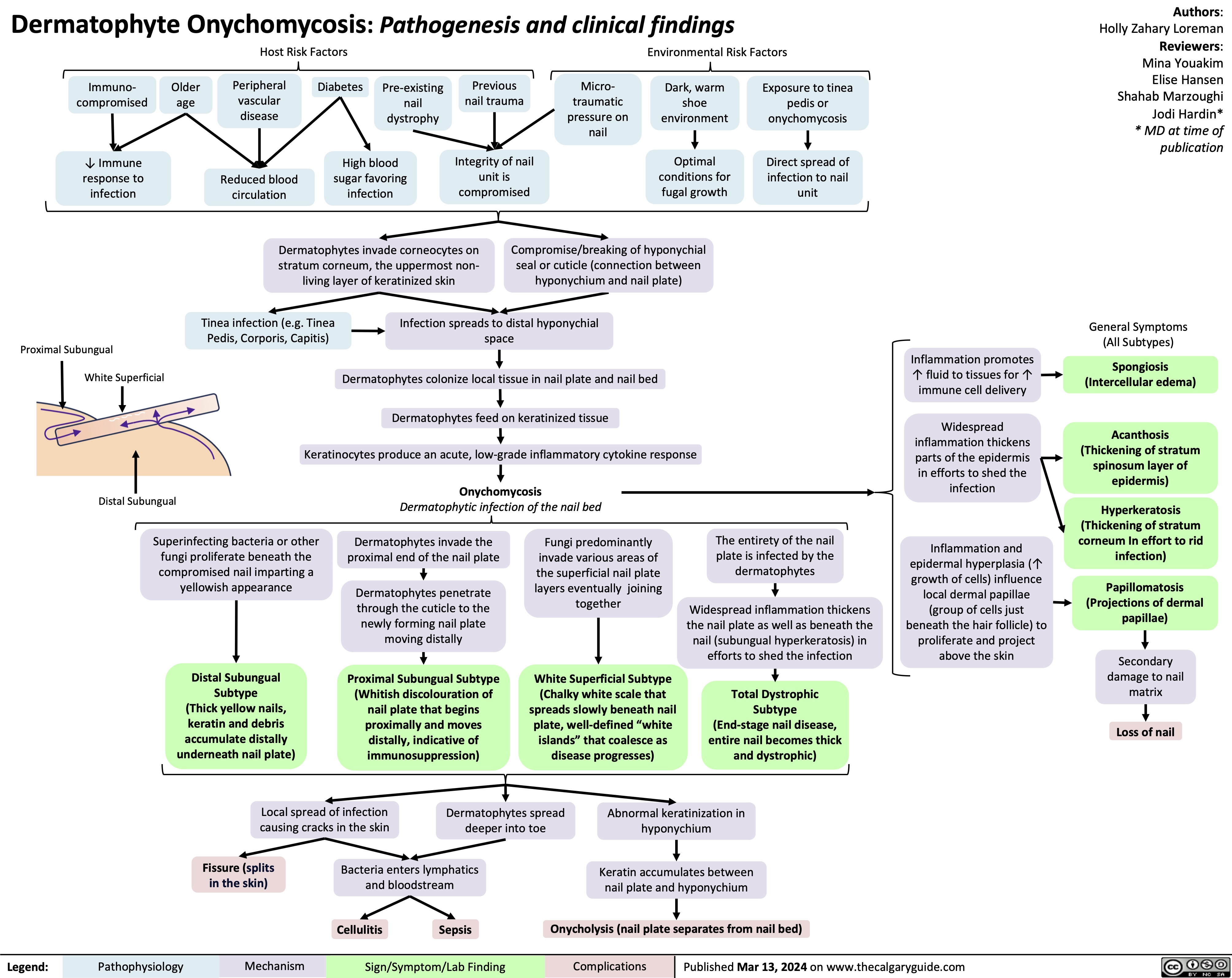
Neonatal Necrotising Enterocolitis in Premature Neonates
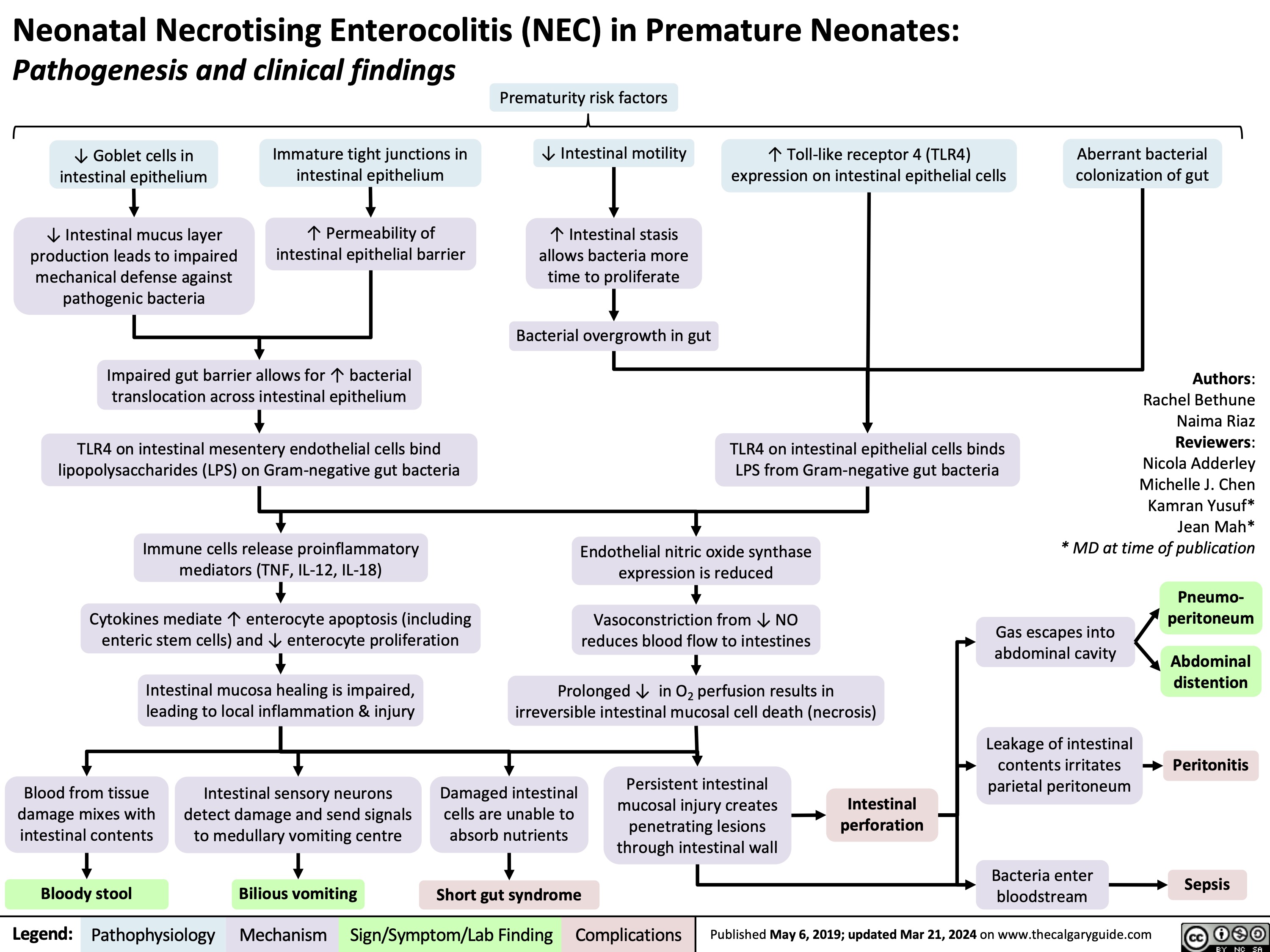
Lichen Sclerosus
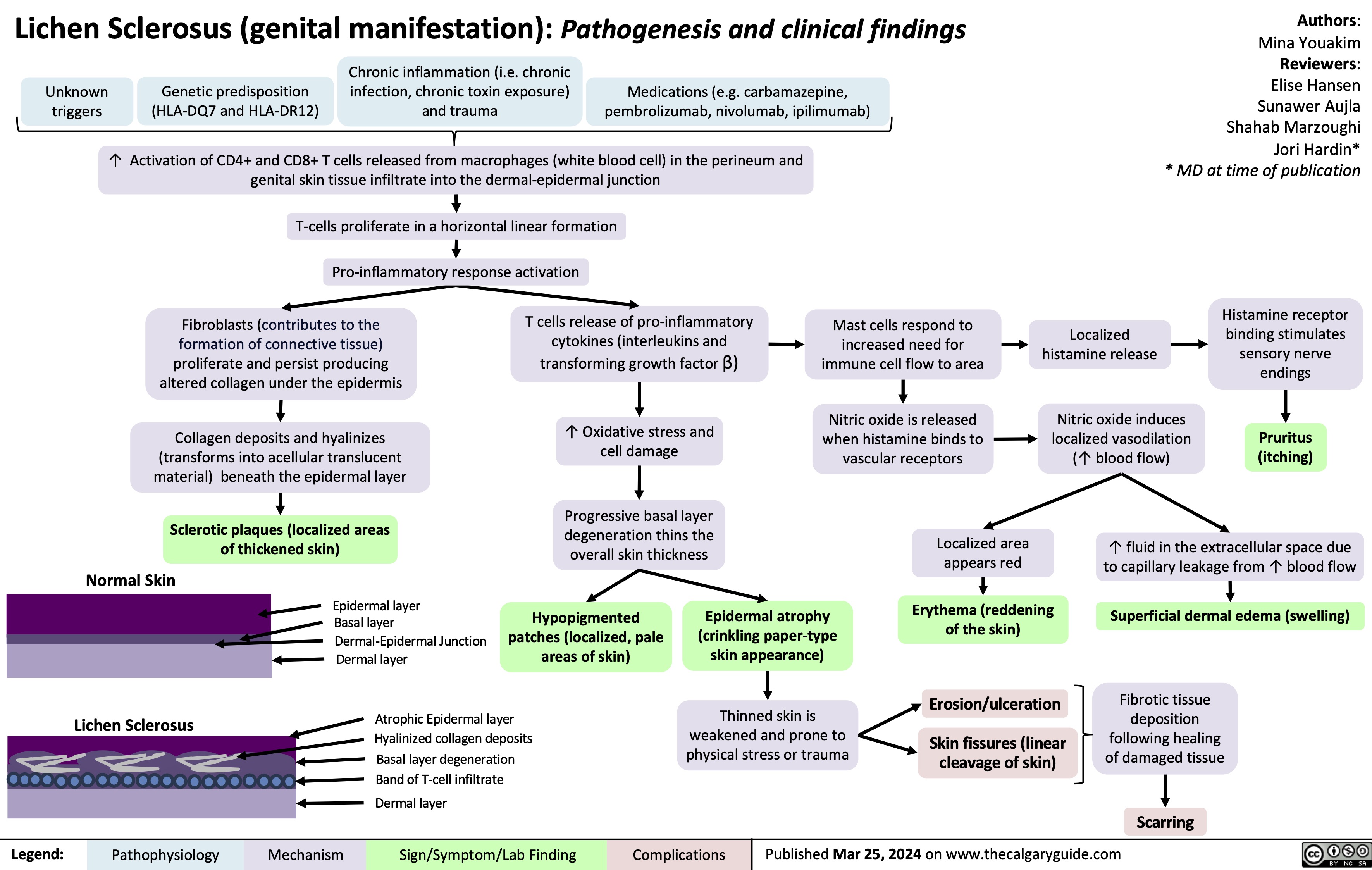
Post-Renal Acute Kidney Injury AKI
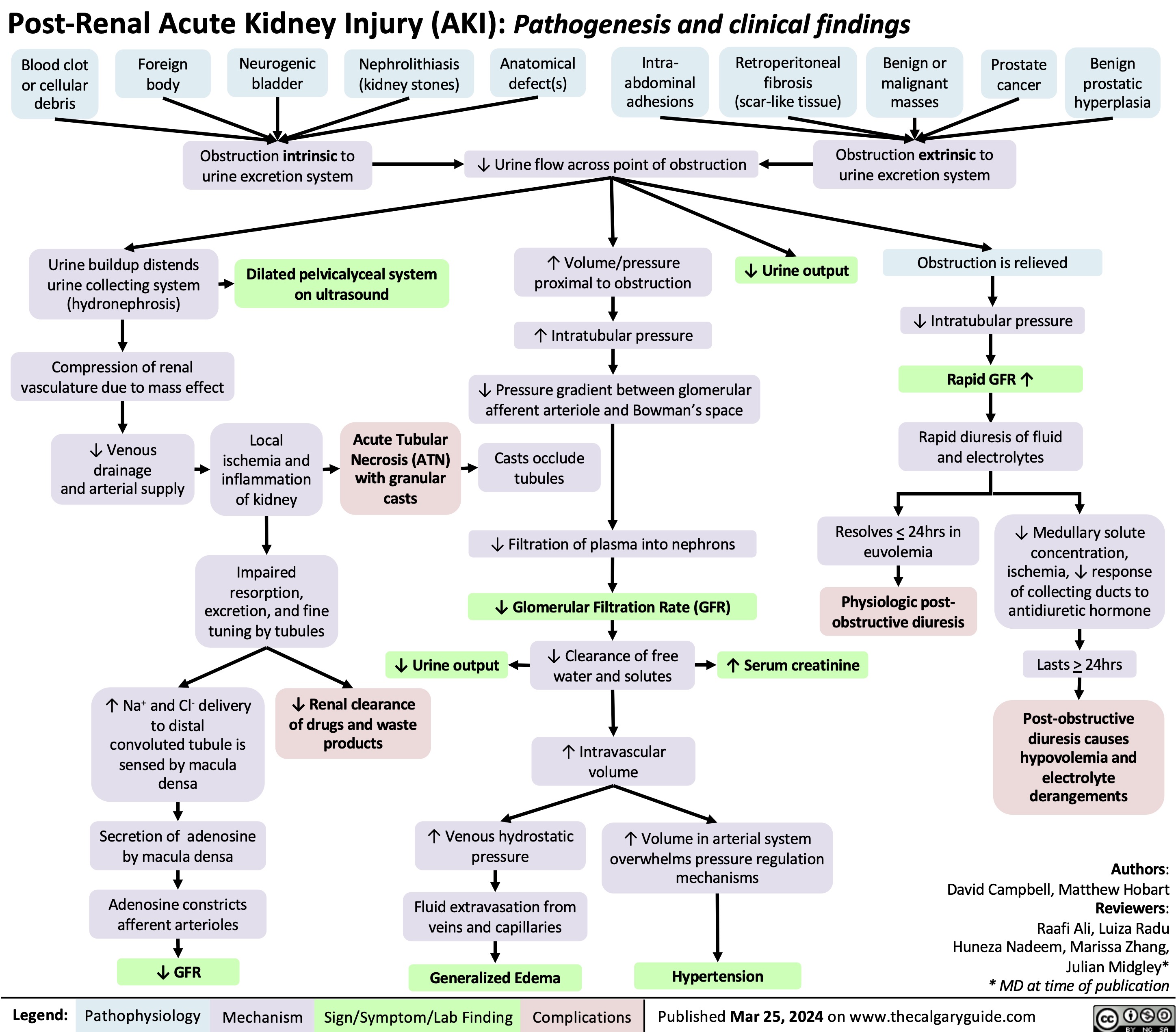
Irritant Contact Dermatitis Pathogenesis and Clinical Findings
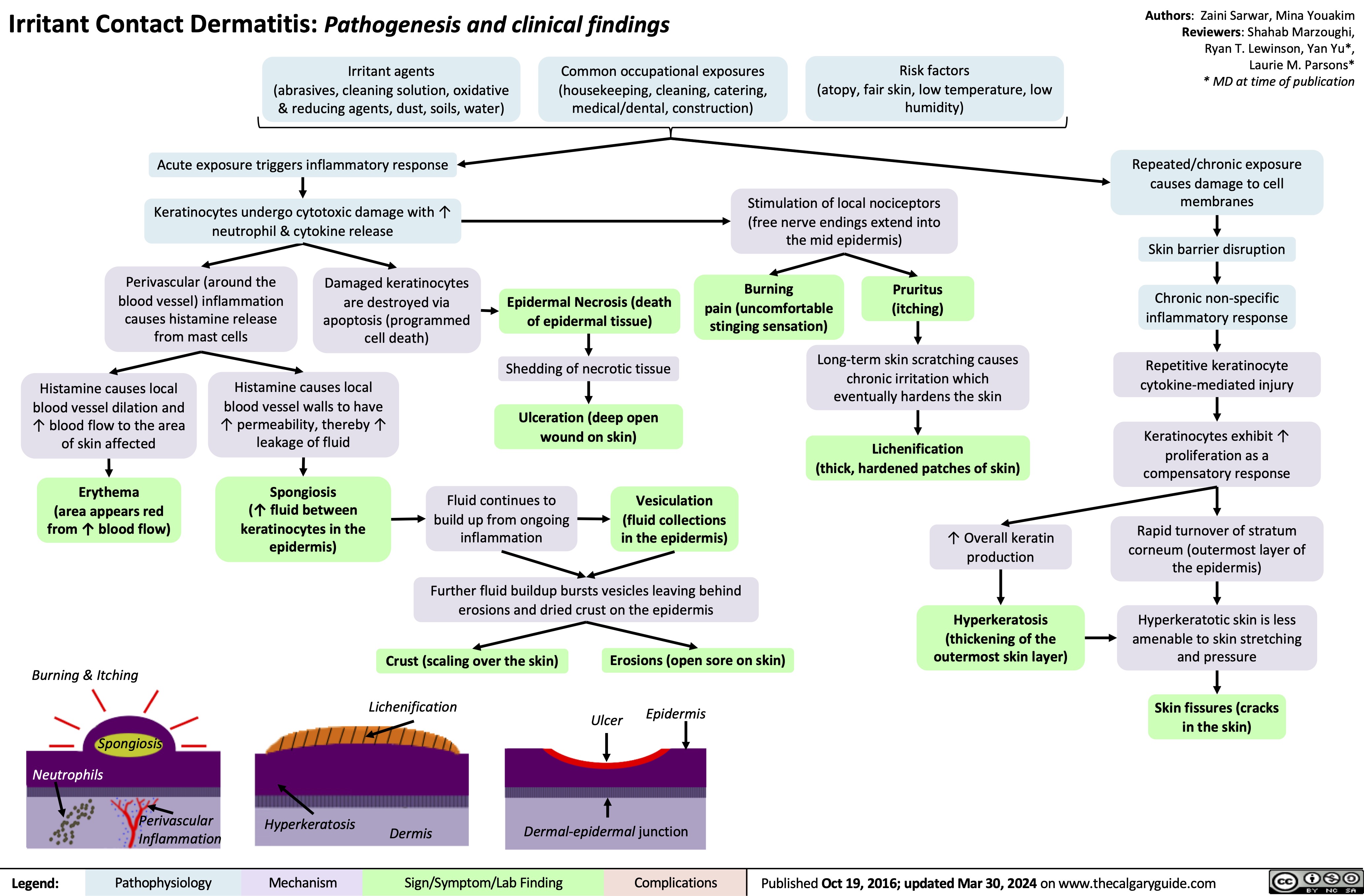
Deep Partial Thickness Burns Pathogenesis and Clinical Findings
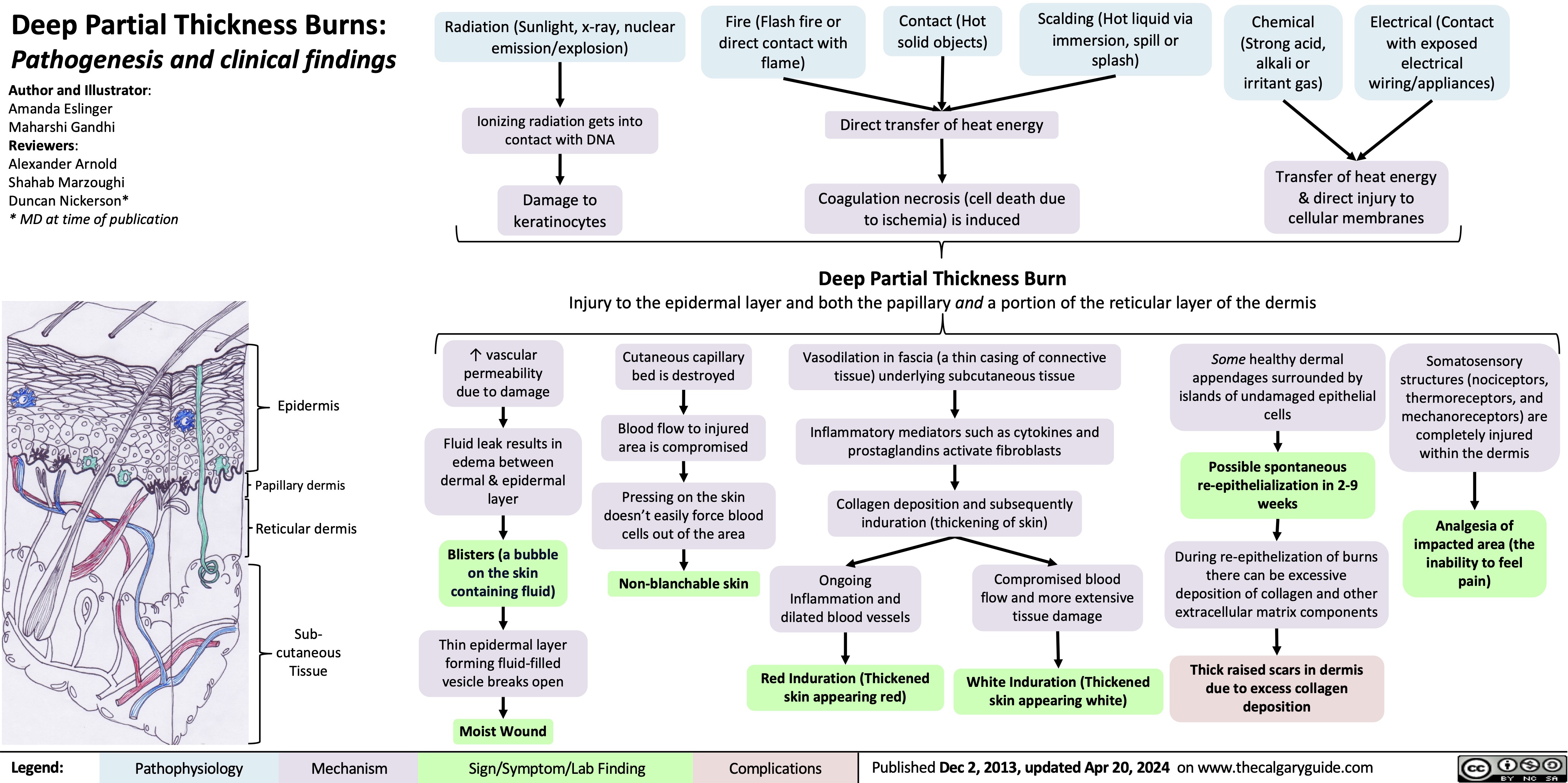
Costochondritis
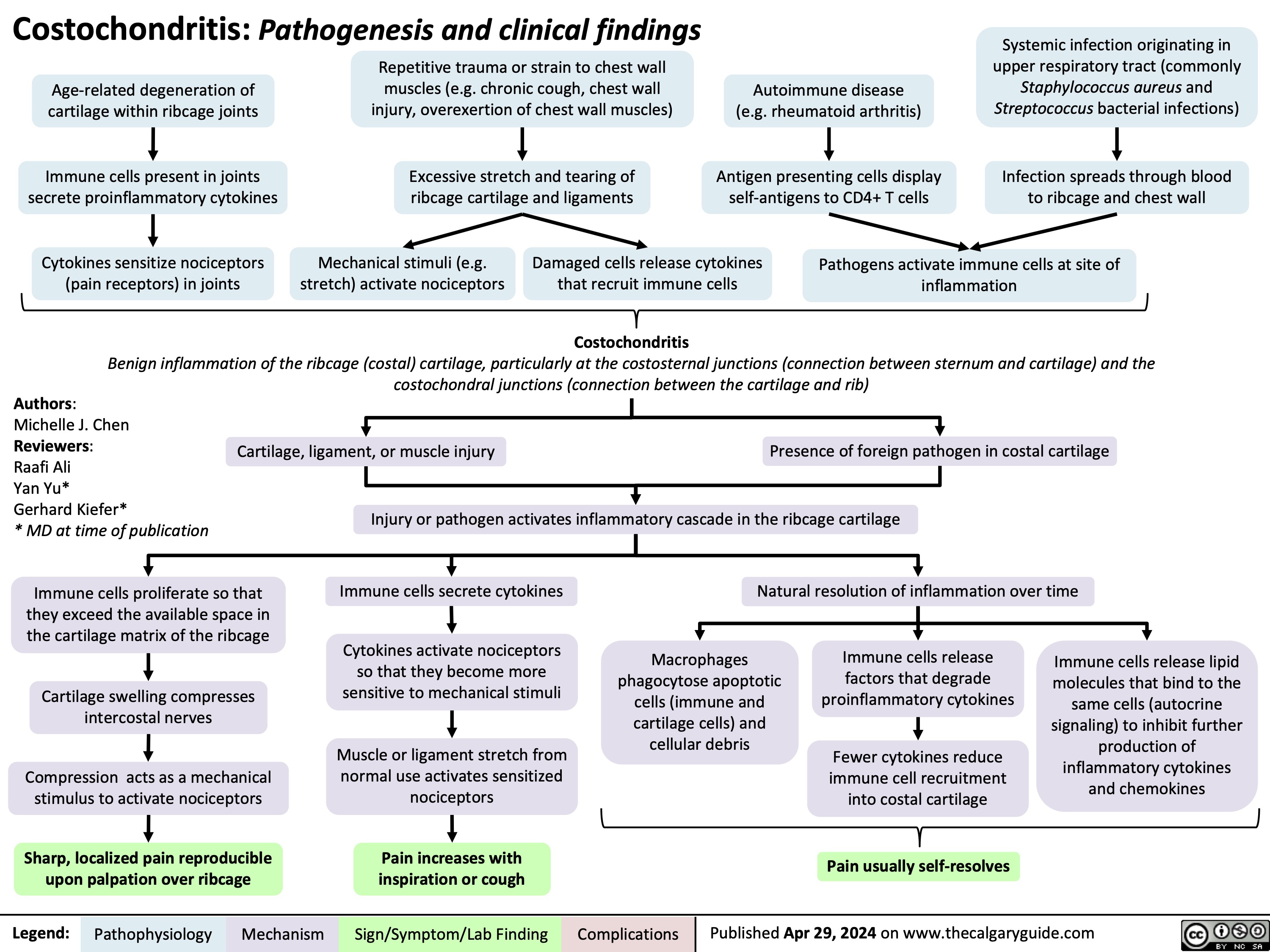
Acute Otitis Media Complications
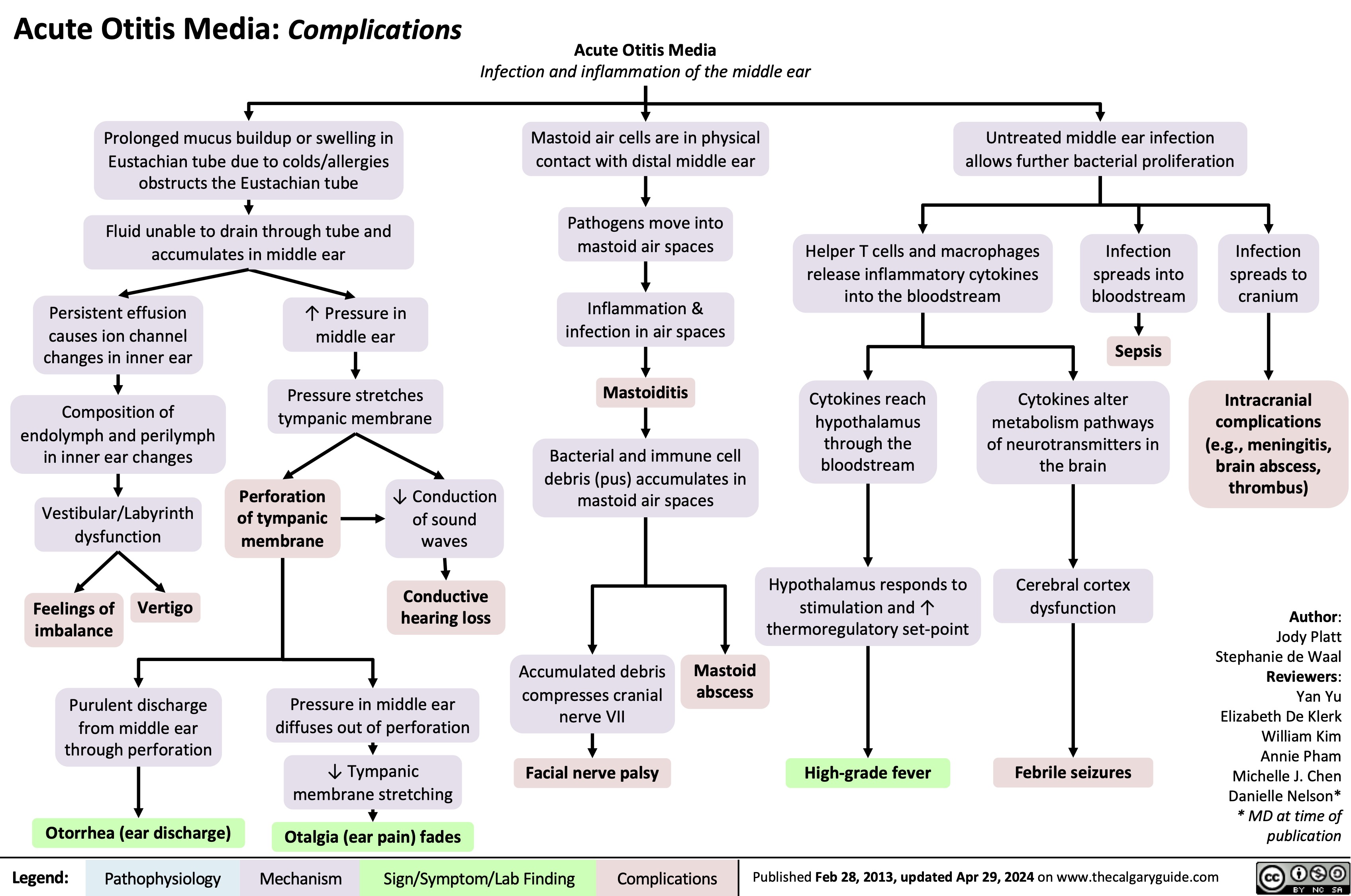
Sickle Cell Disease Pathogenesis Clinical Findings and Complications
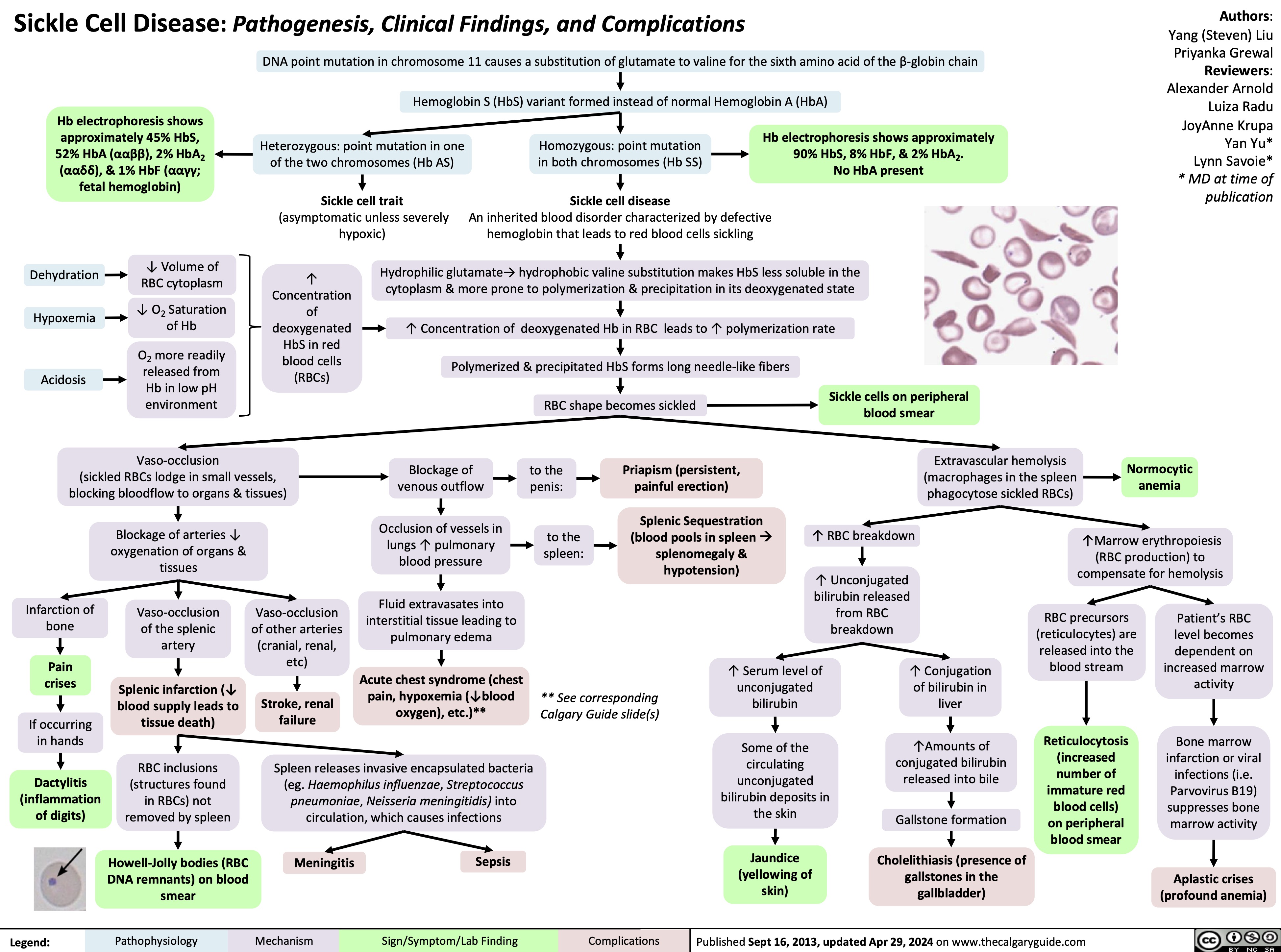
Wiskott-Aldrich Syndrome
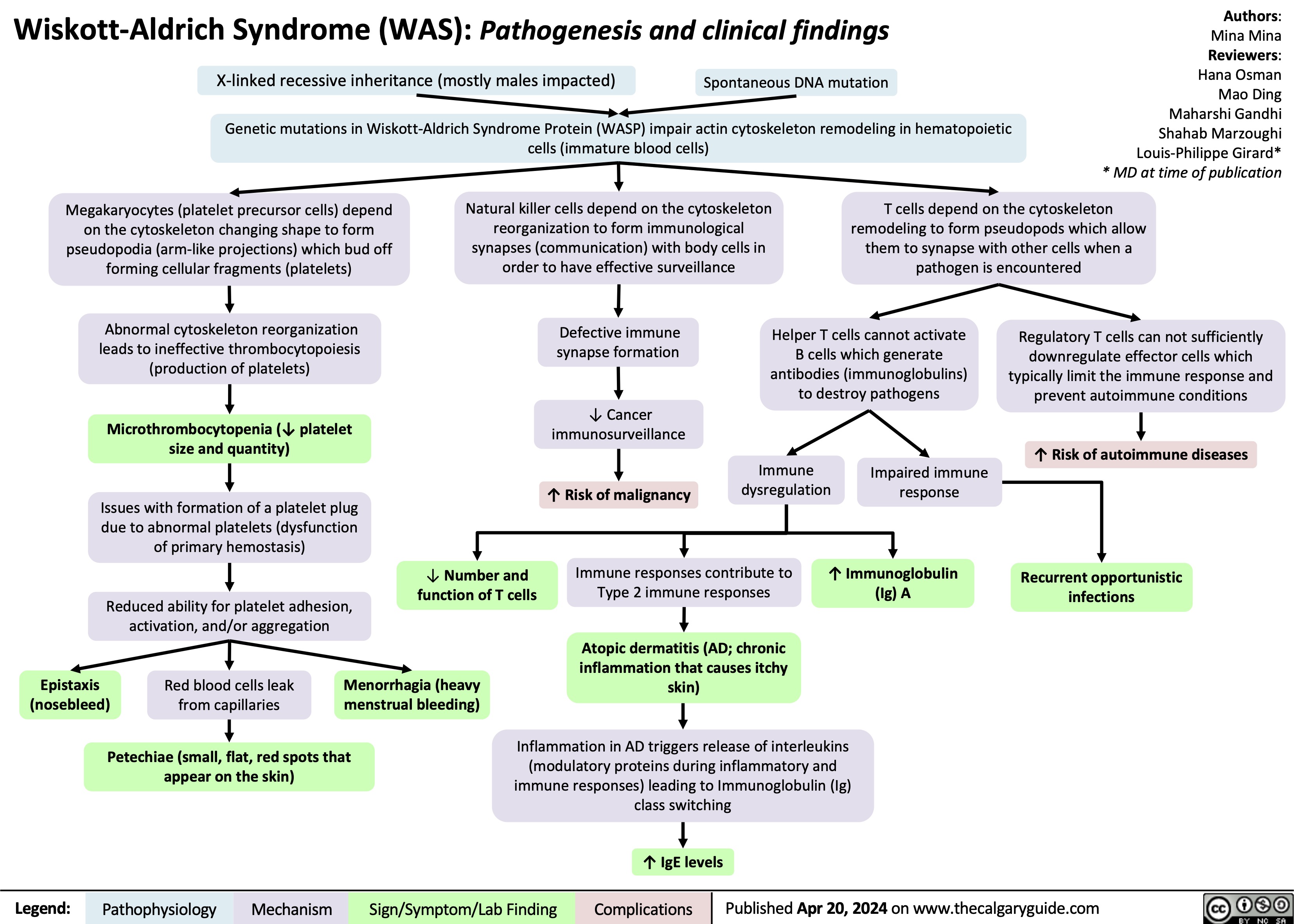
Scabies pathogenesis and clinical findings
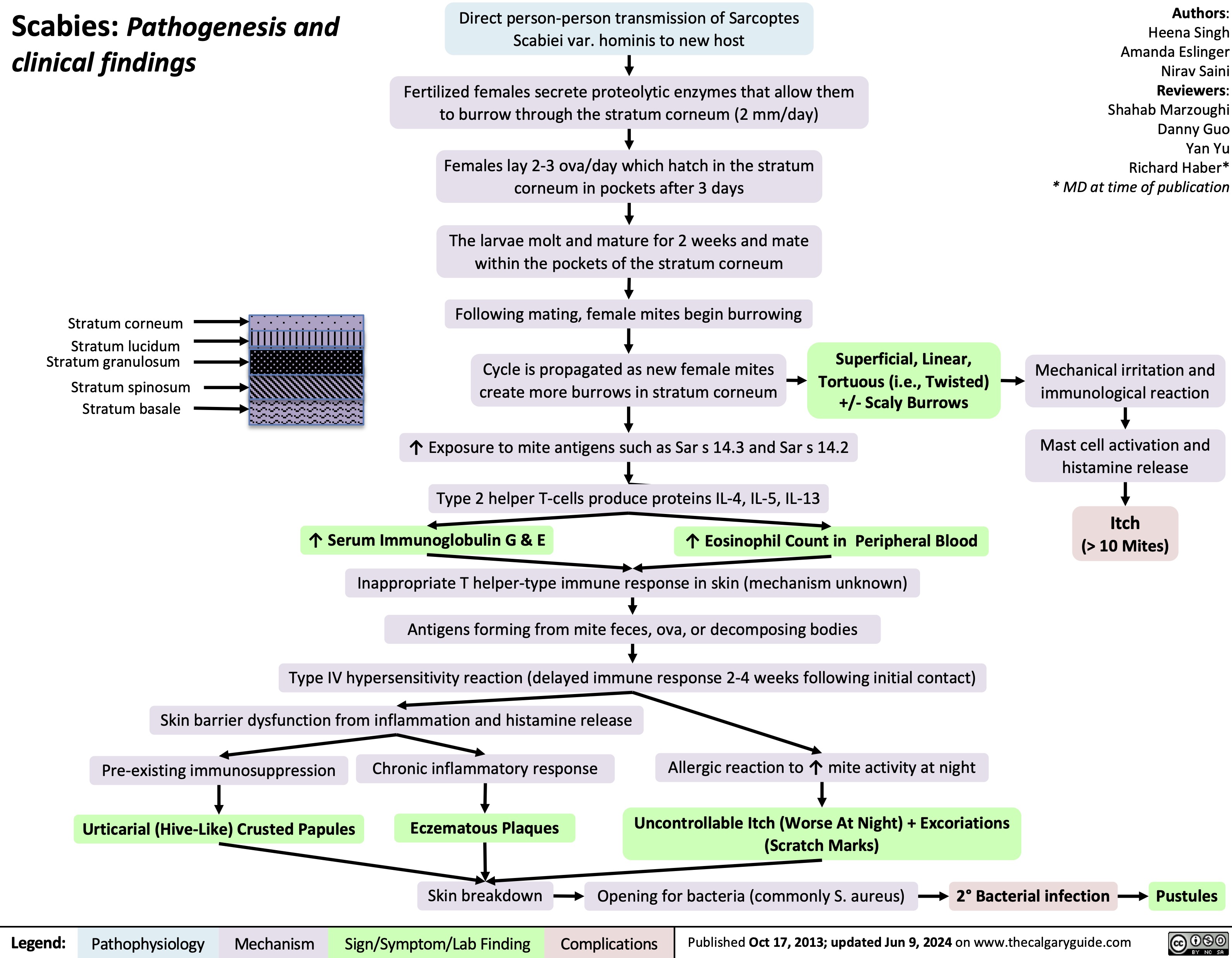
Posterior Cruciate Ligament PCL Injury Pathogenesis and Clinical Findings
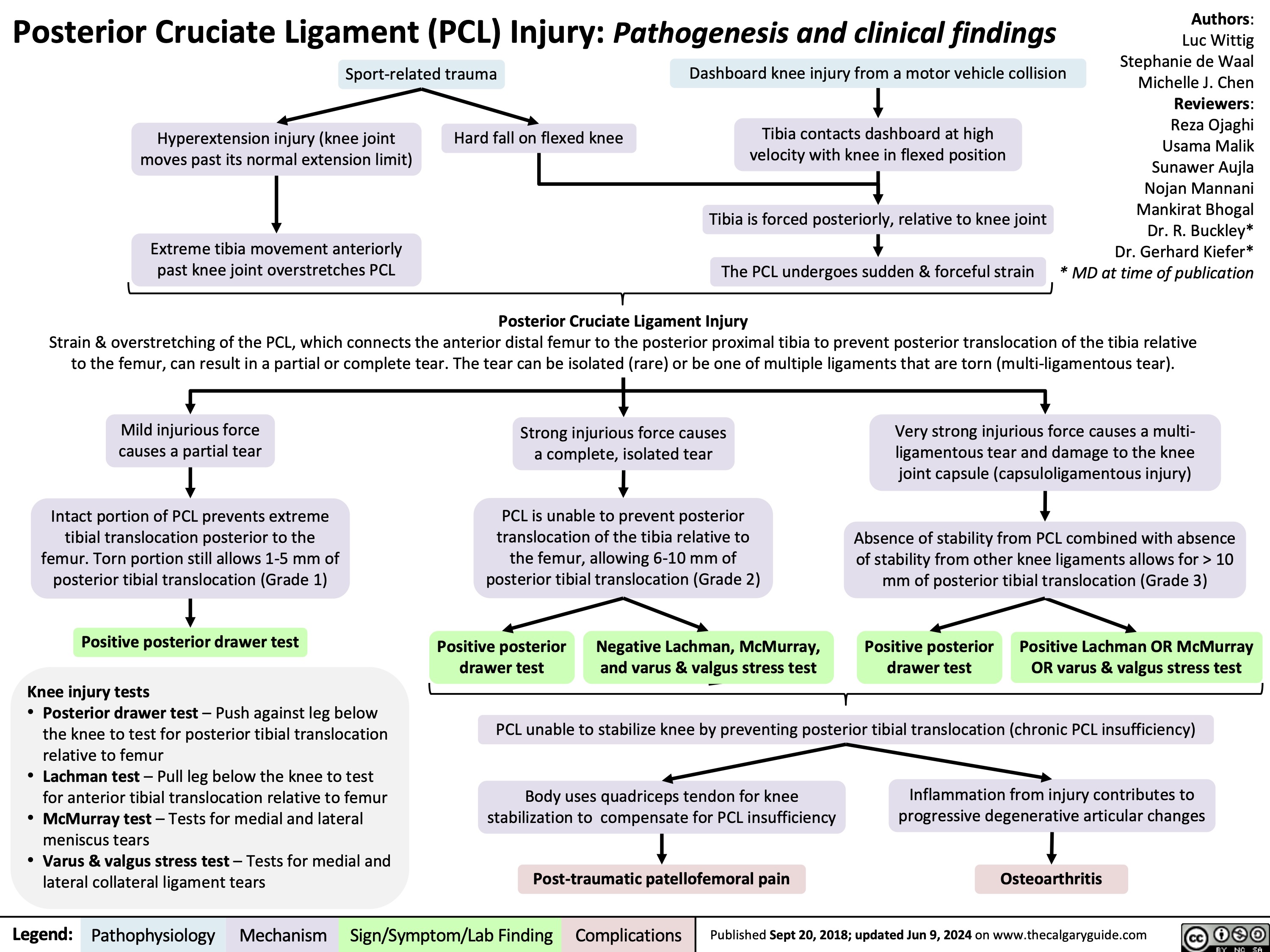
Migraines and auras pathogenesis and clinical findings
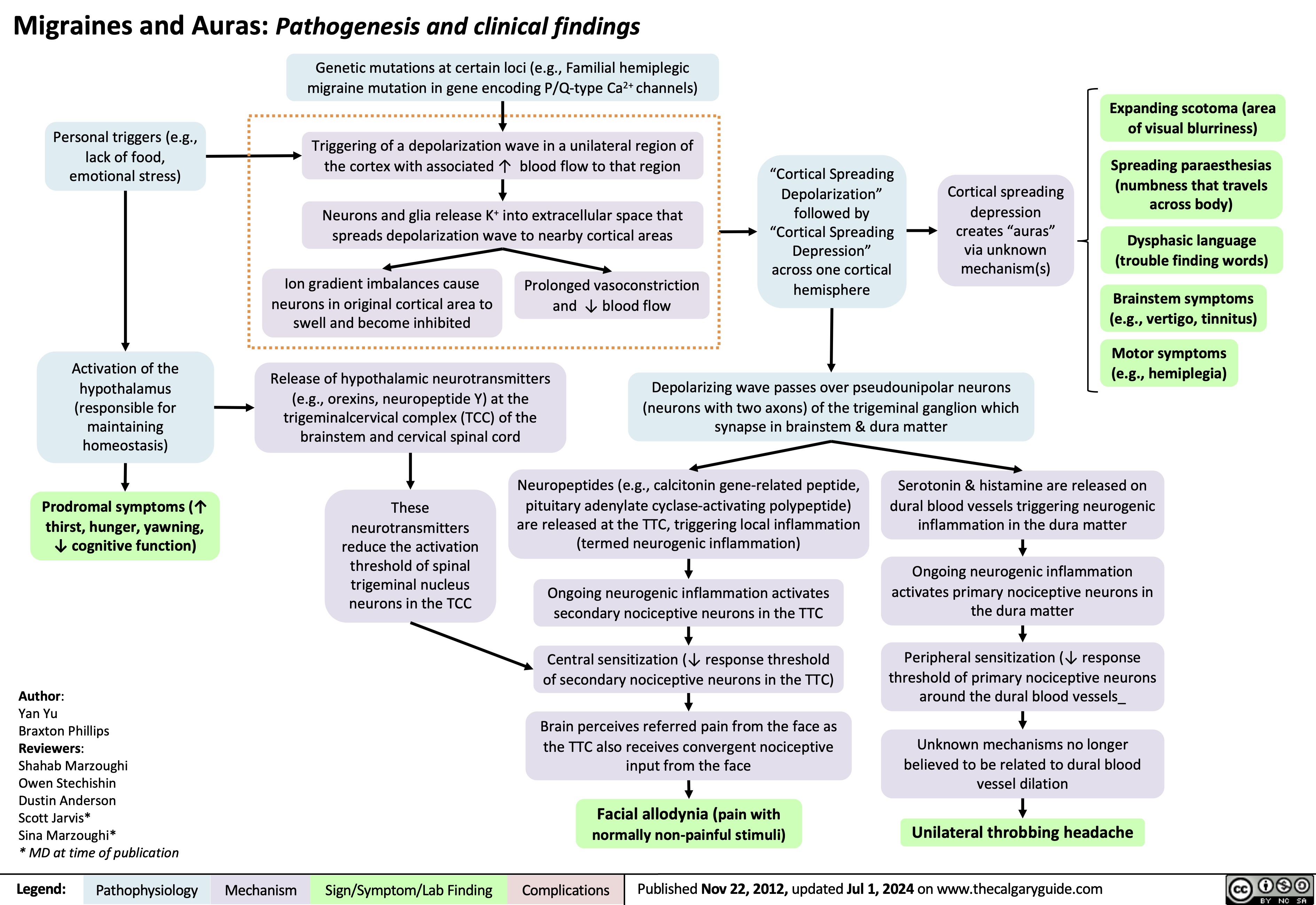
Legg Calve Perthes Disease
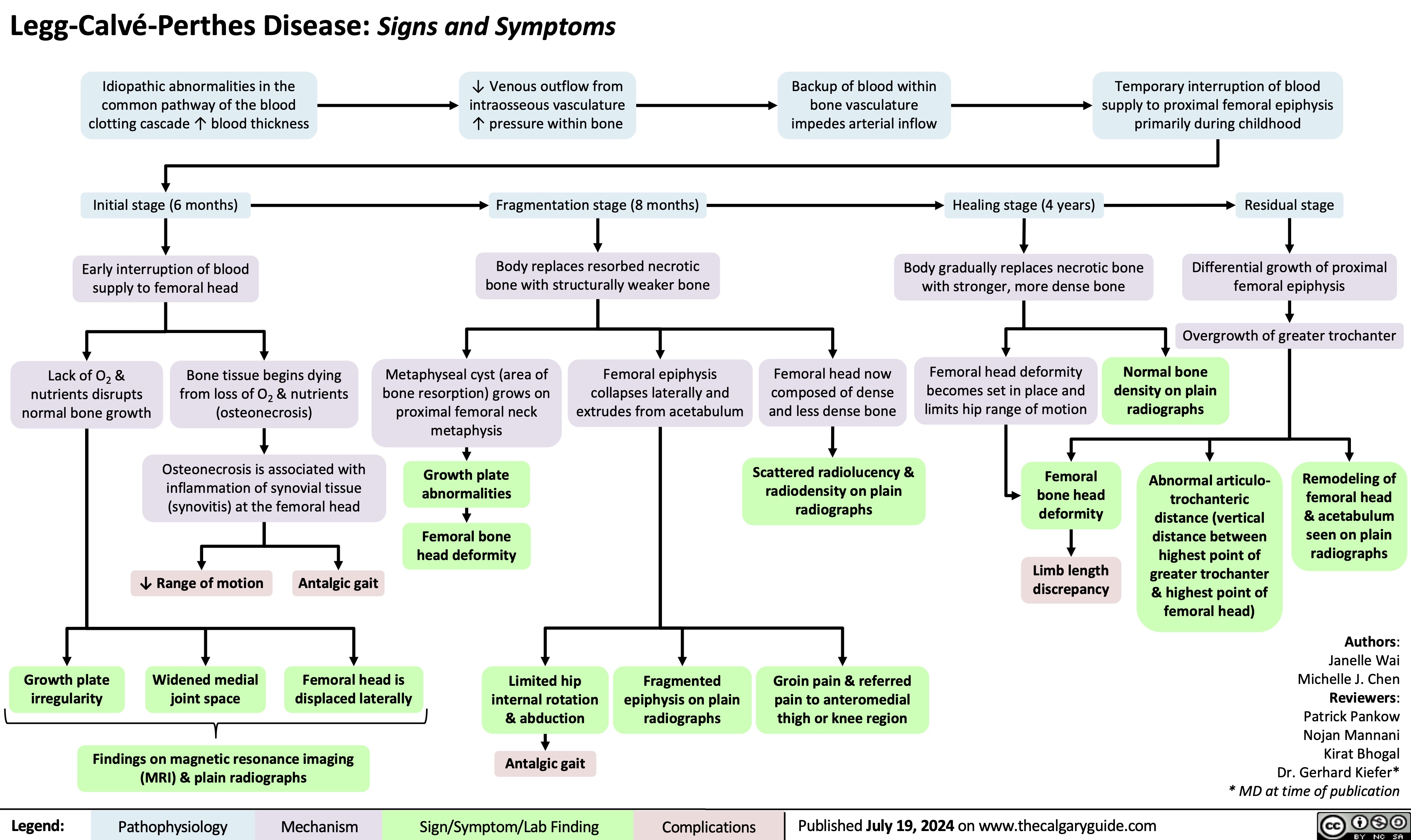
Acute Otitis Media Pathogenesis and Clinical Findings in Children
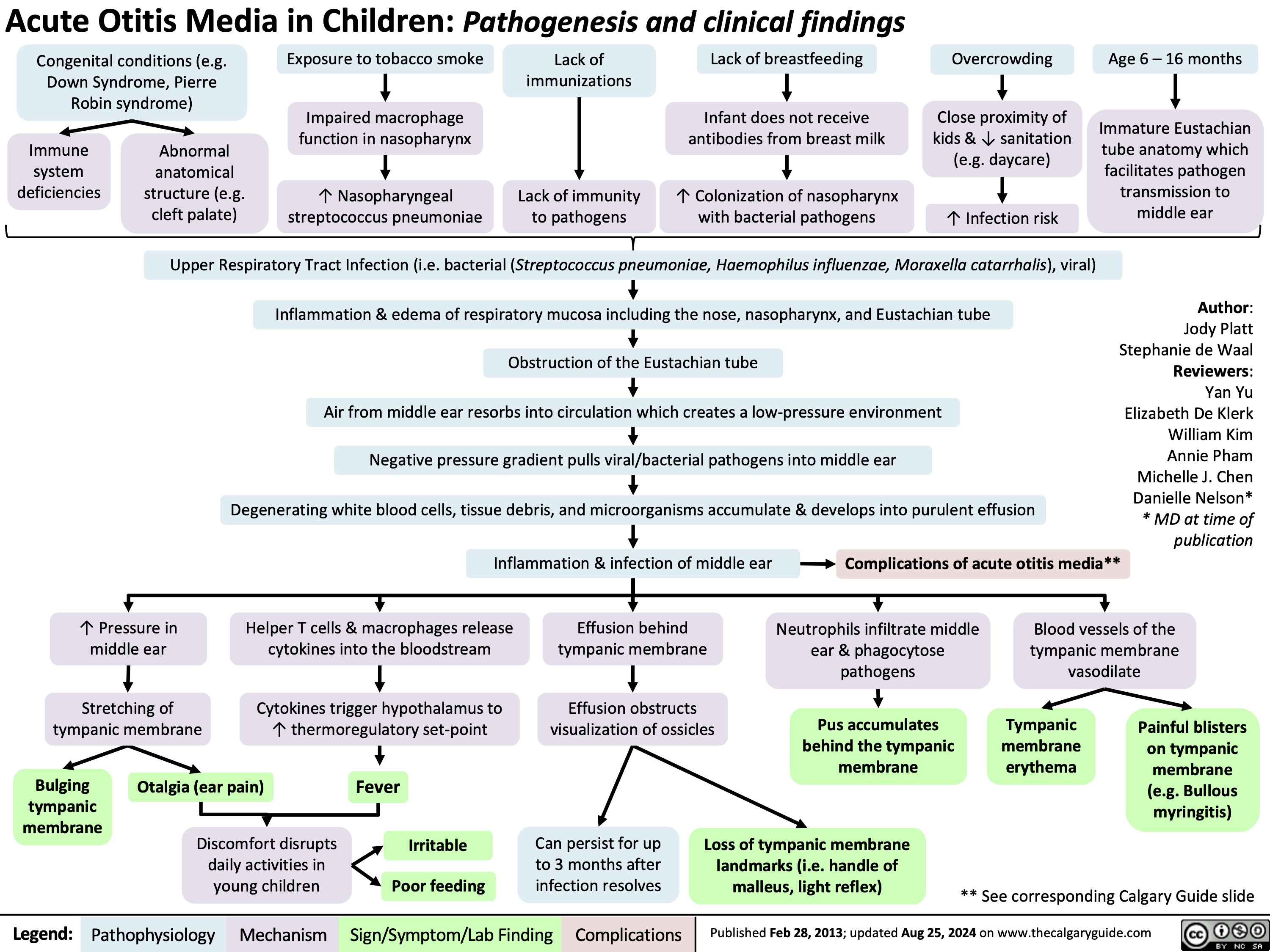
Pediatric Pneumonia Pathogenesis and Clinical Findings
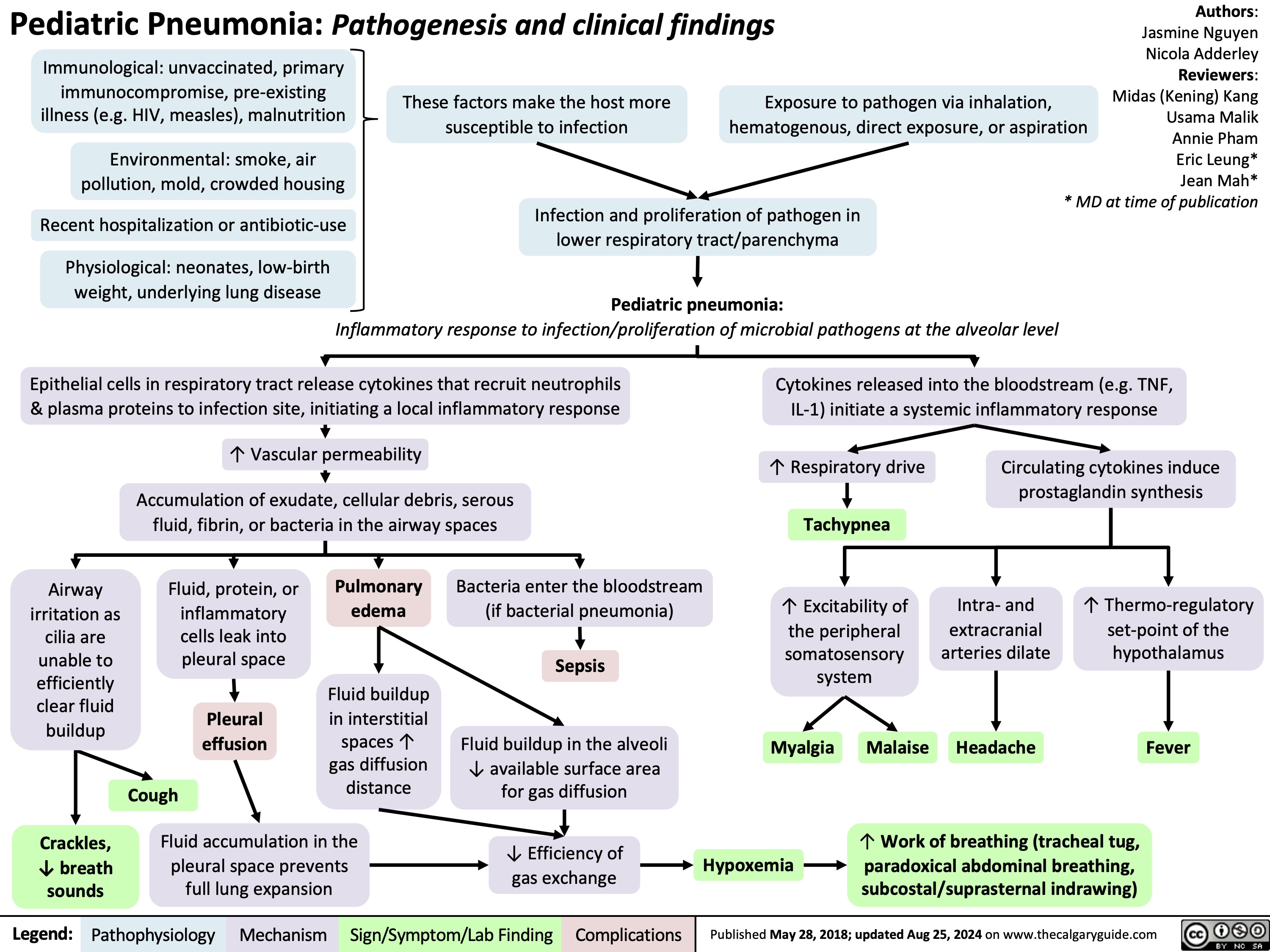
Low Ankle Sprain
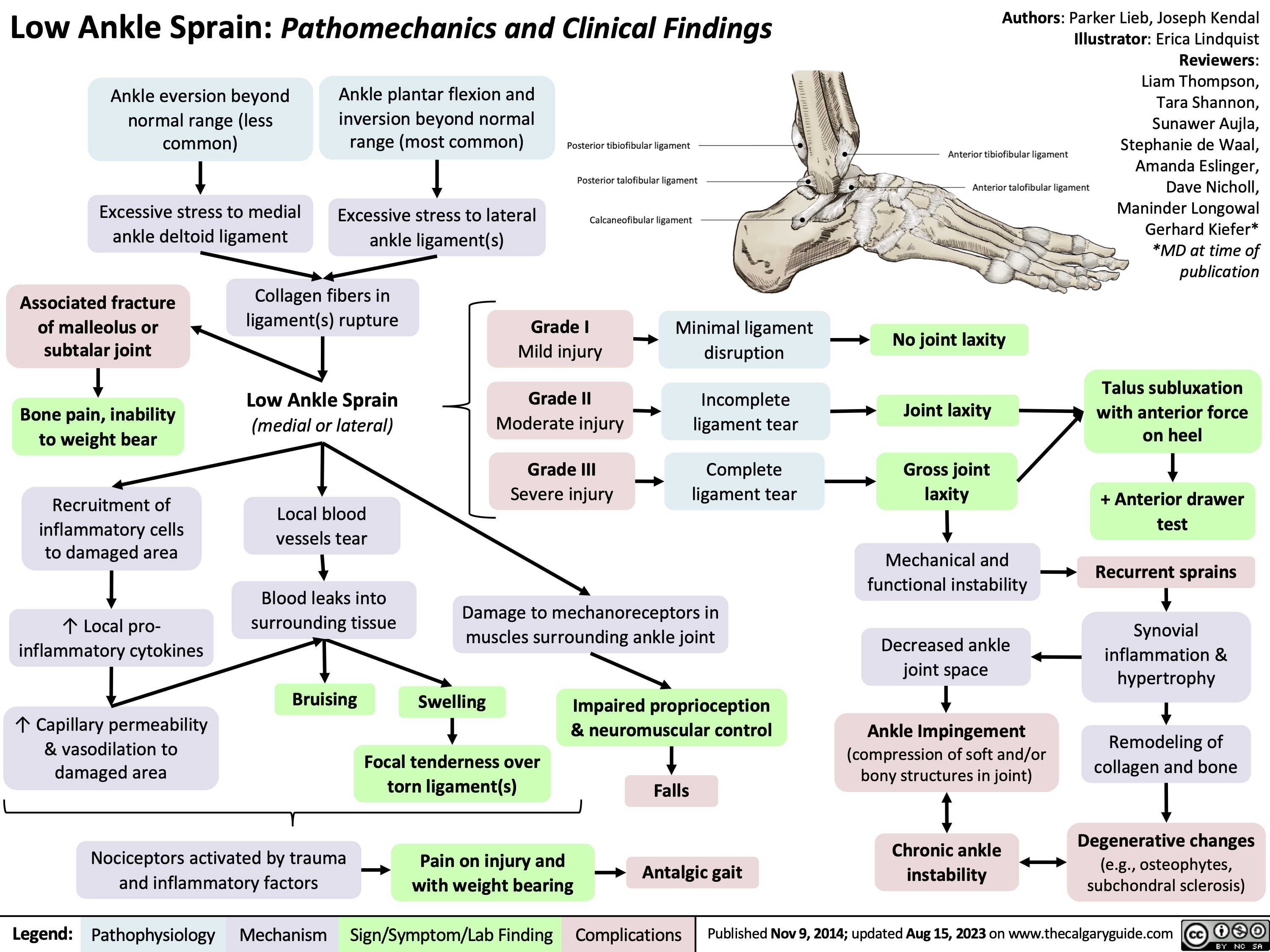
Hyperthyroidism in Pregnancy
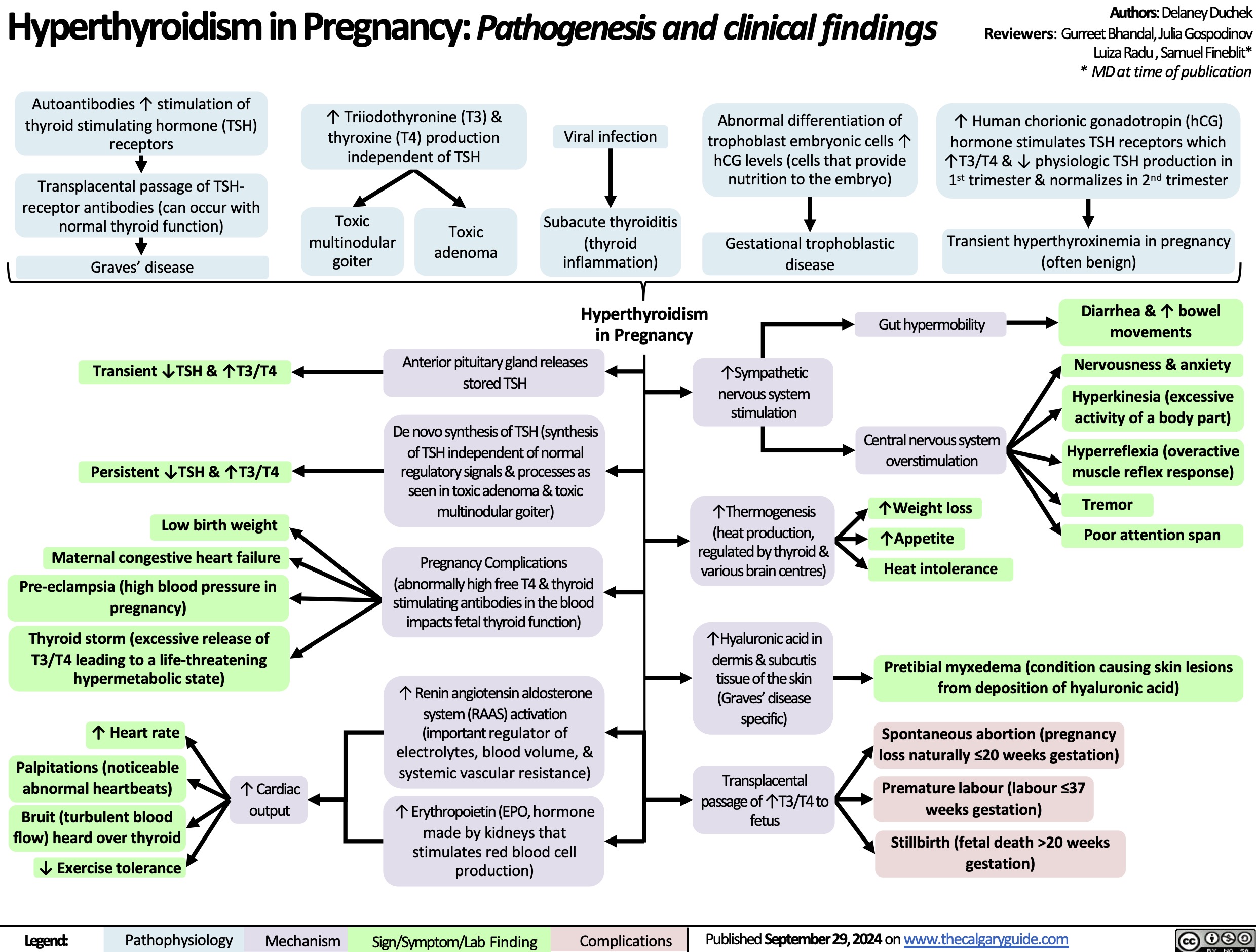
Hypomagnesemia
![Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com
Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/10/Hypomagnesia.jpg)
Spontaneous Rupture of Membranes
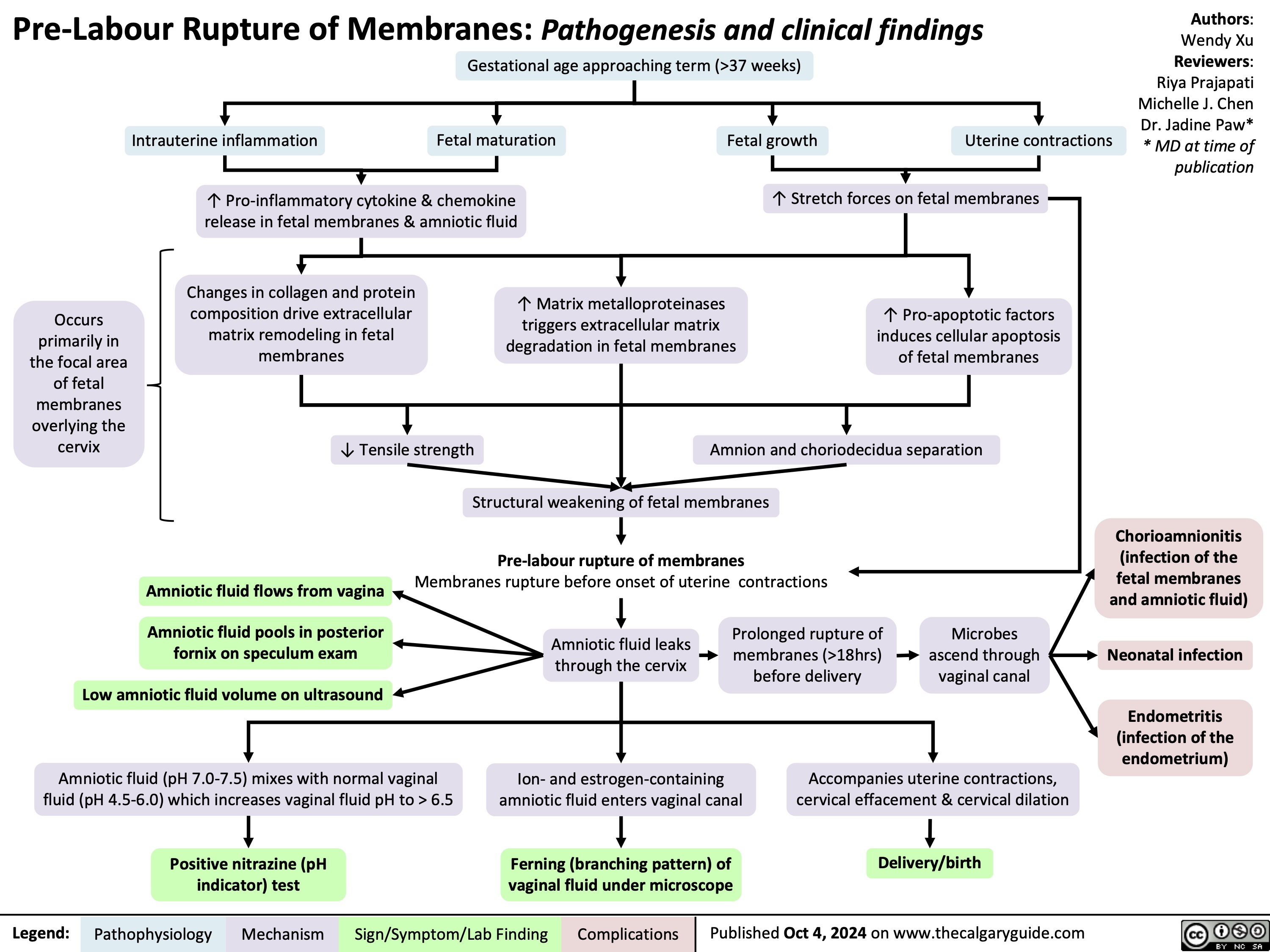
Acute Diverticulitis
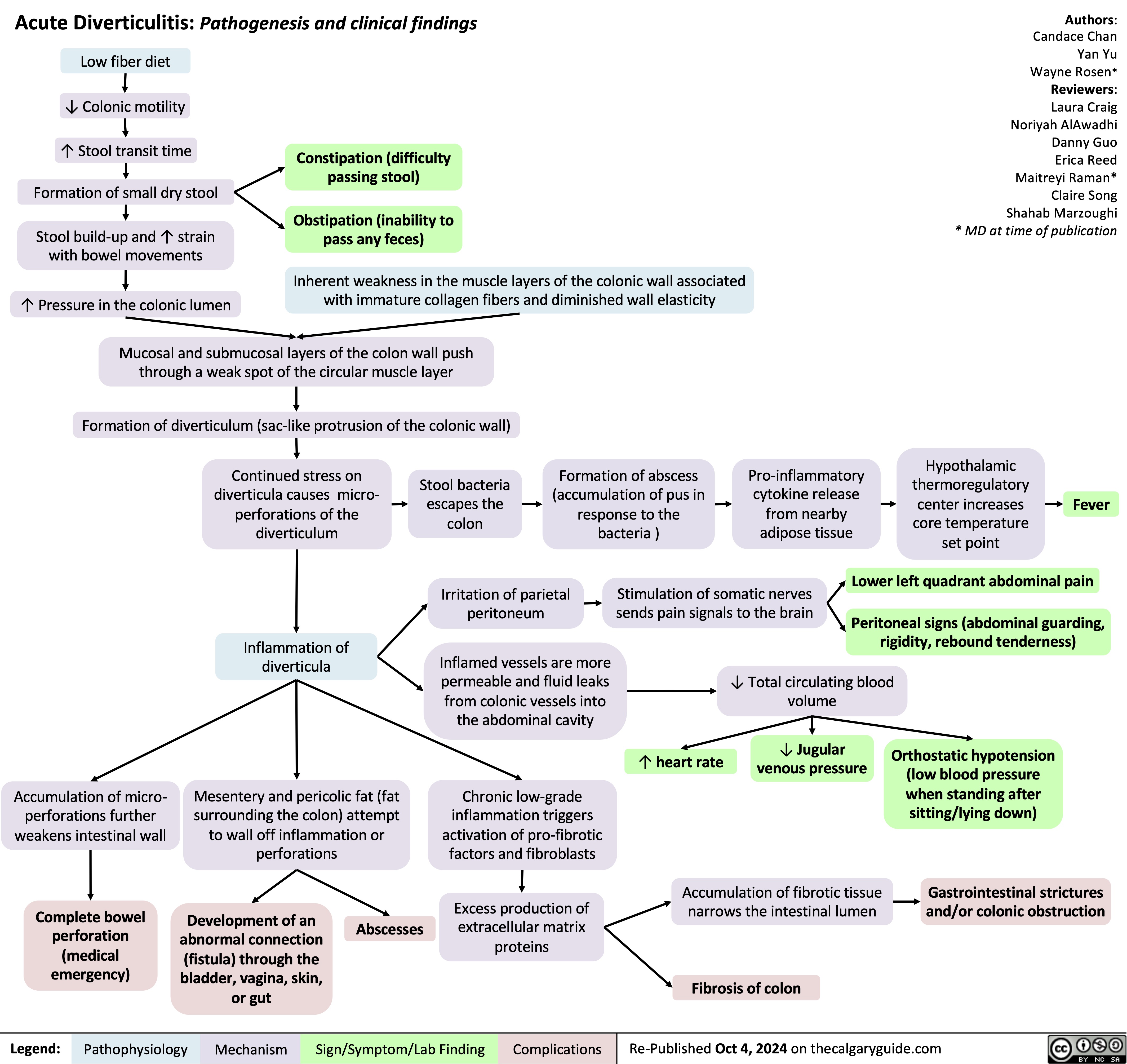
Epilepsy in Older Adults
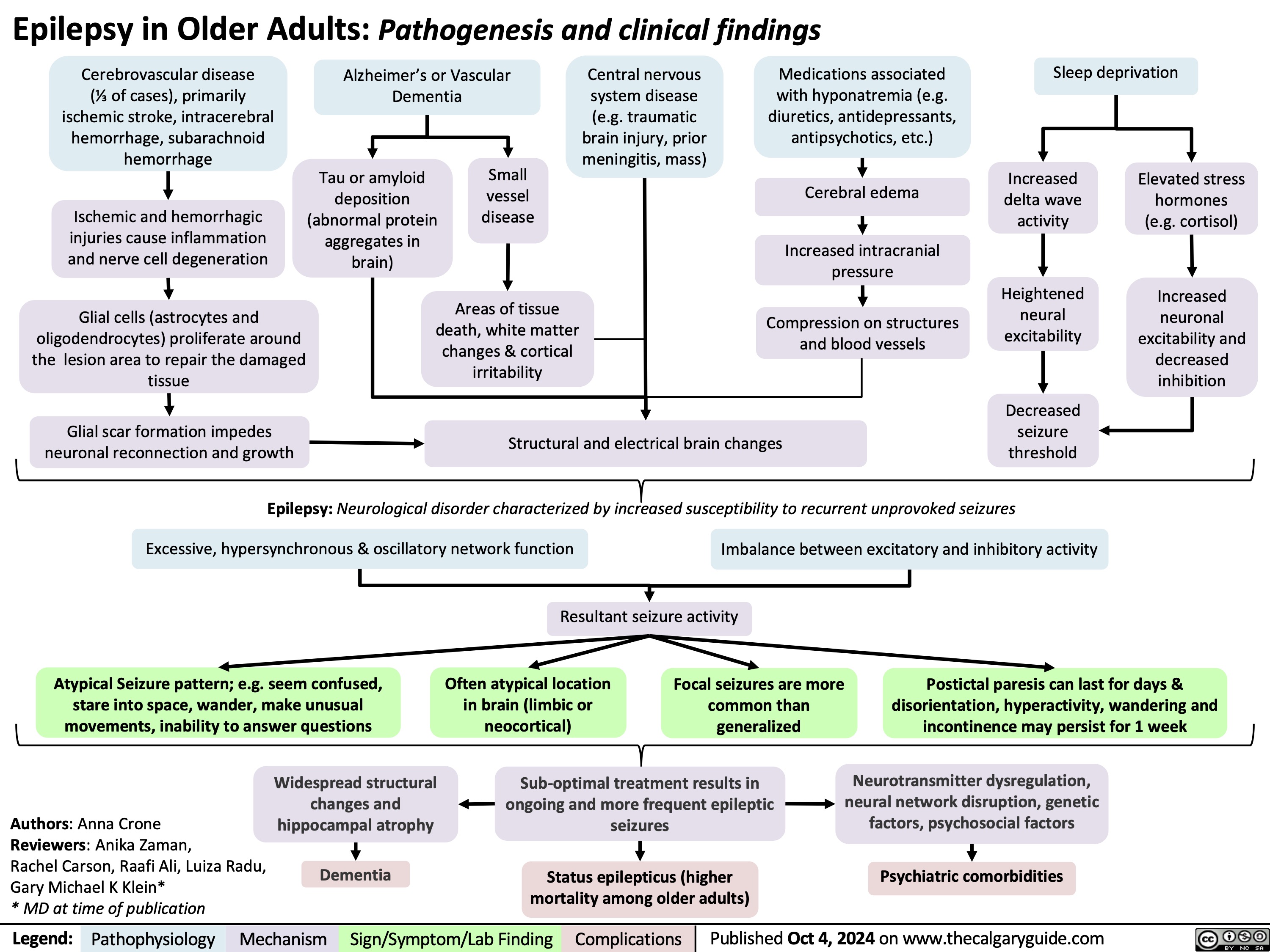
Cholesteatoma of middle ear
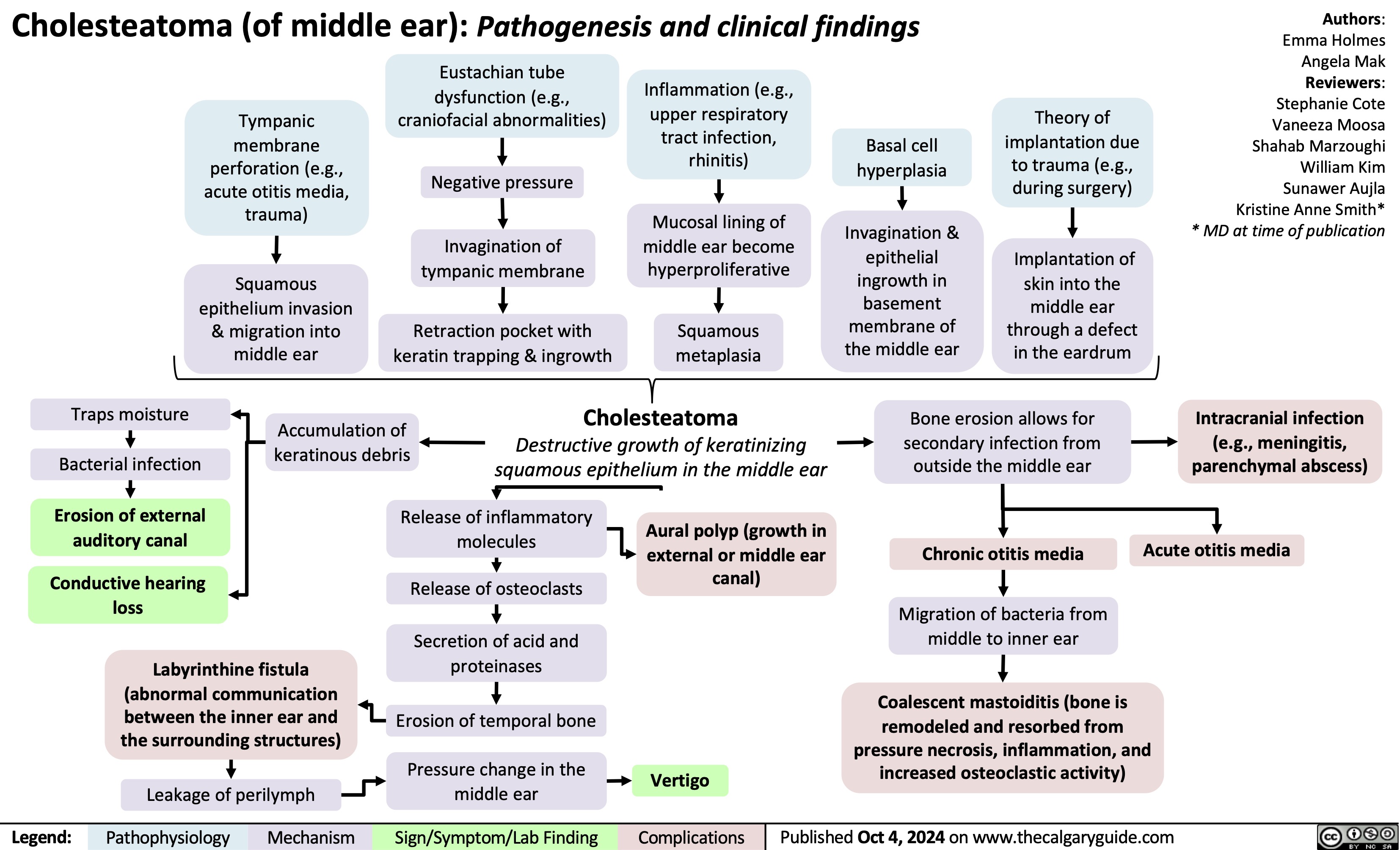
Complex Regional Pain Syndrome
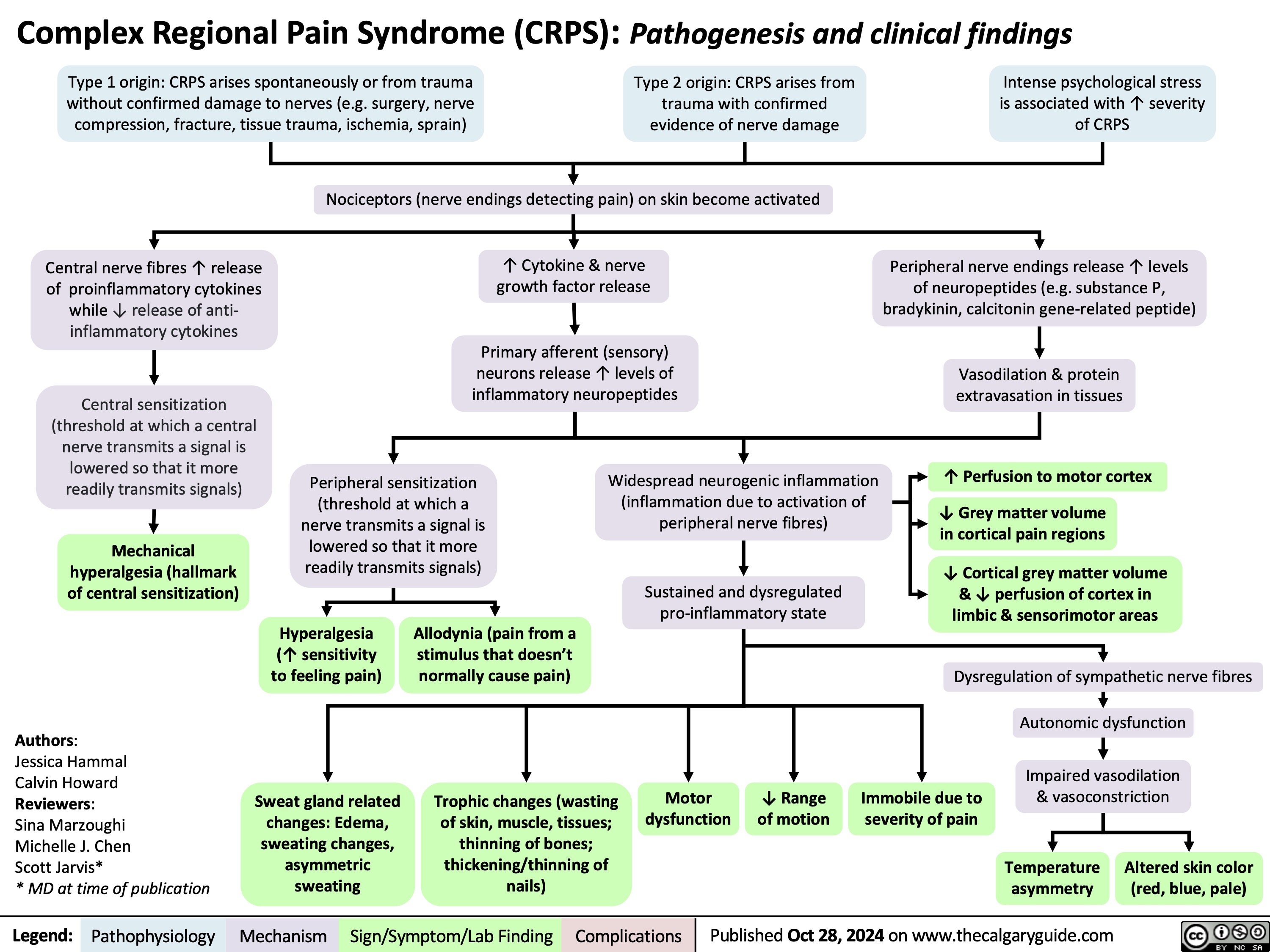
Renal manifestations of SLE
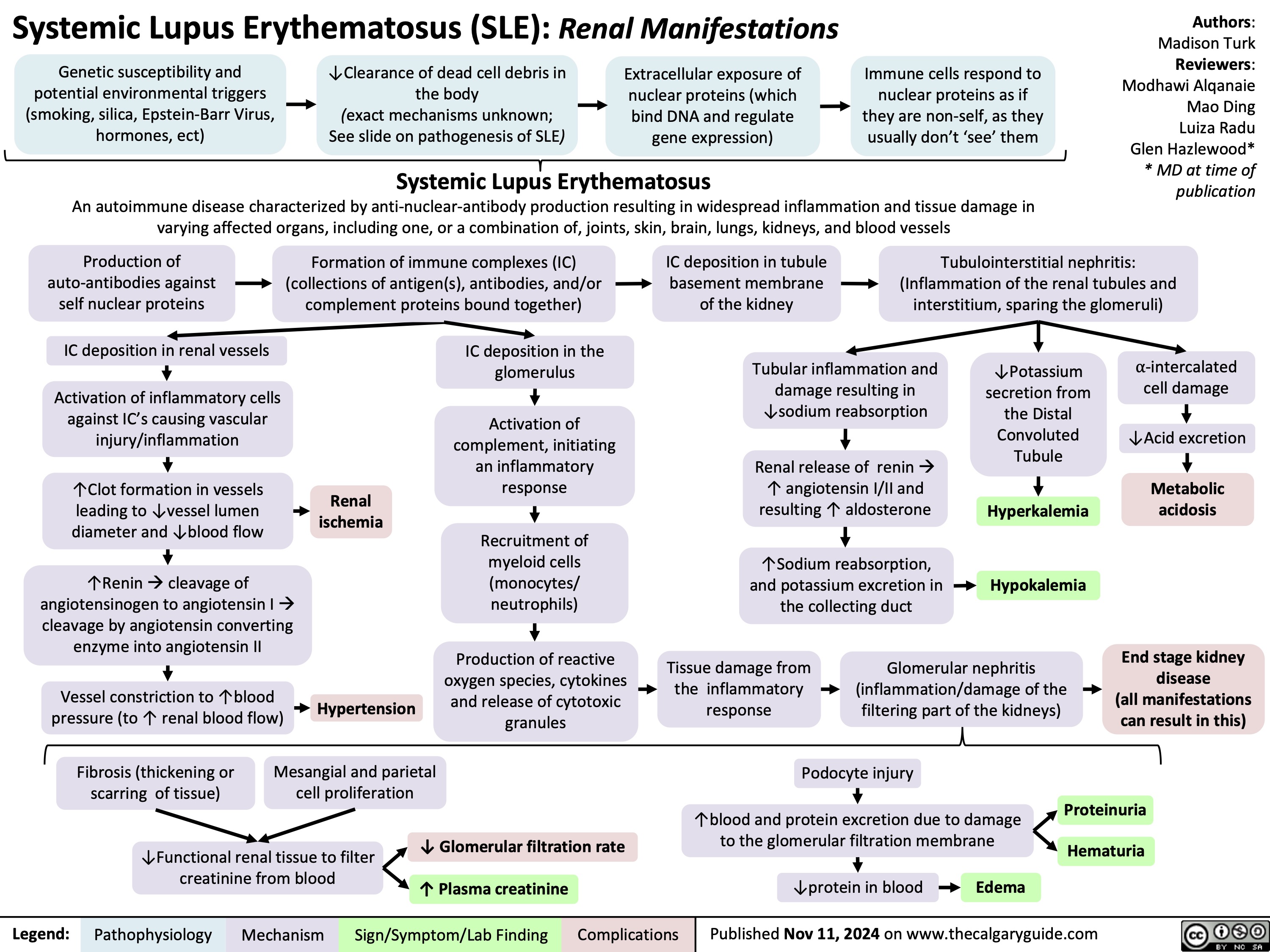
Diabetic Polyneuropathy
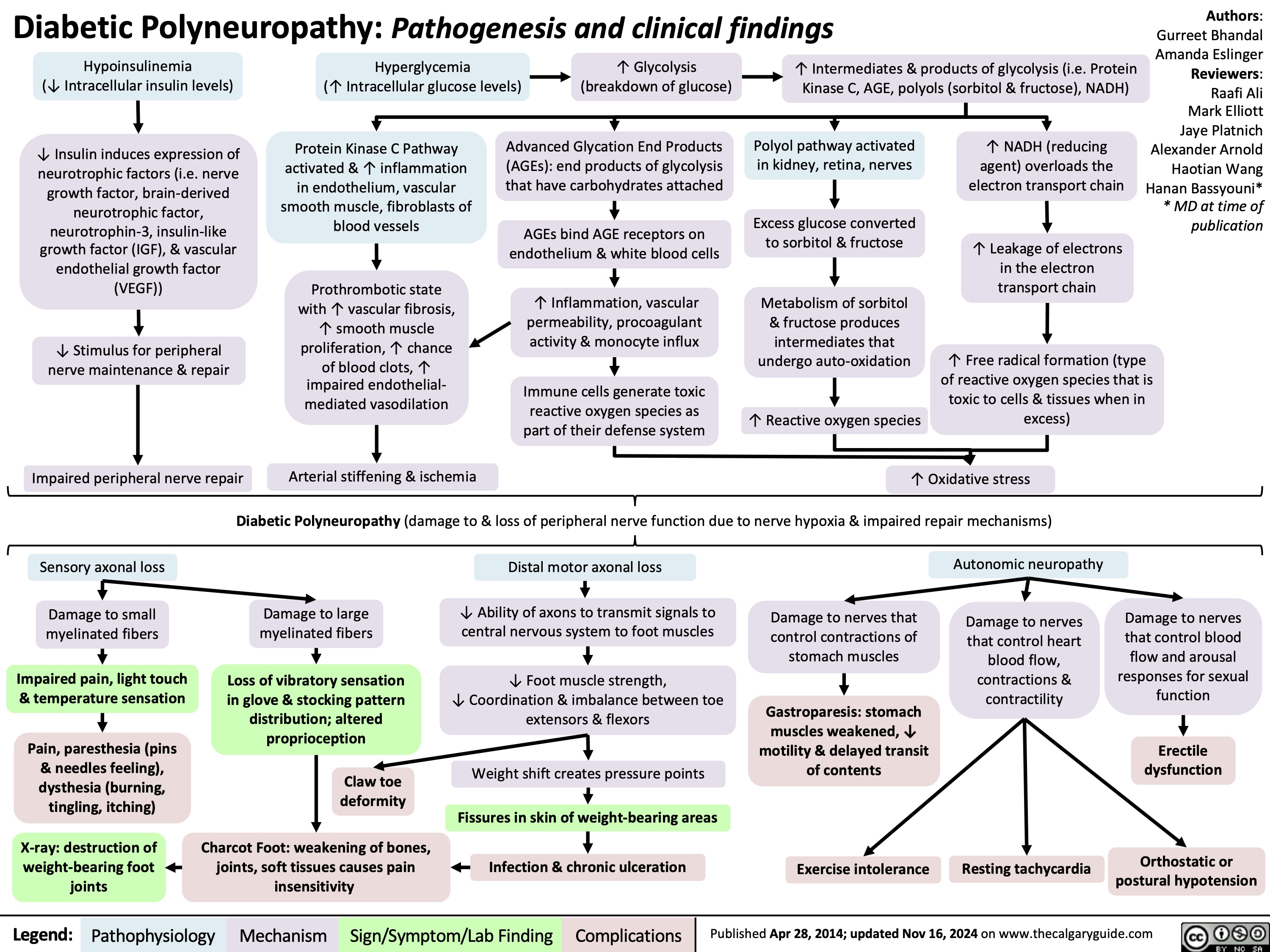
Open Fractures
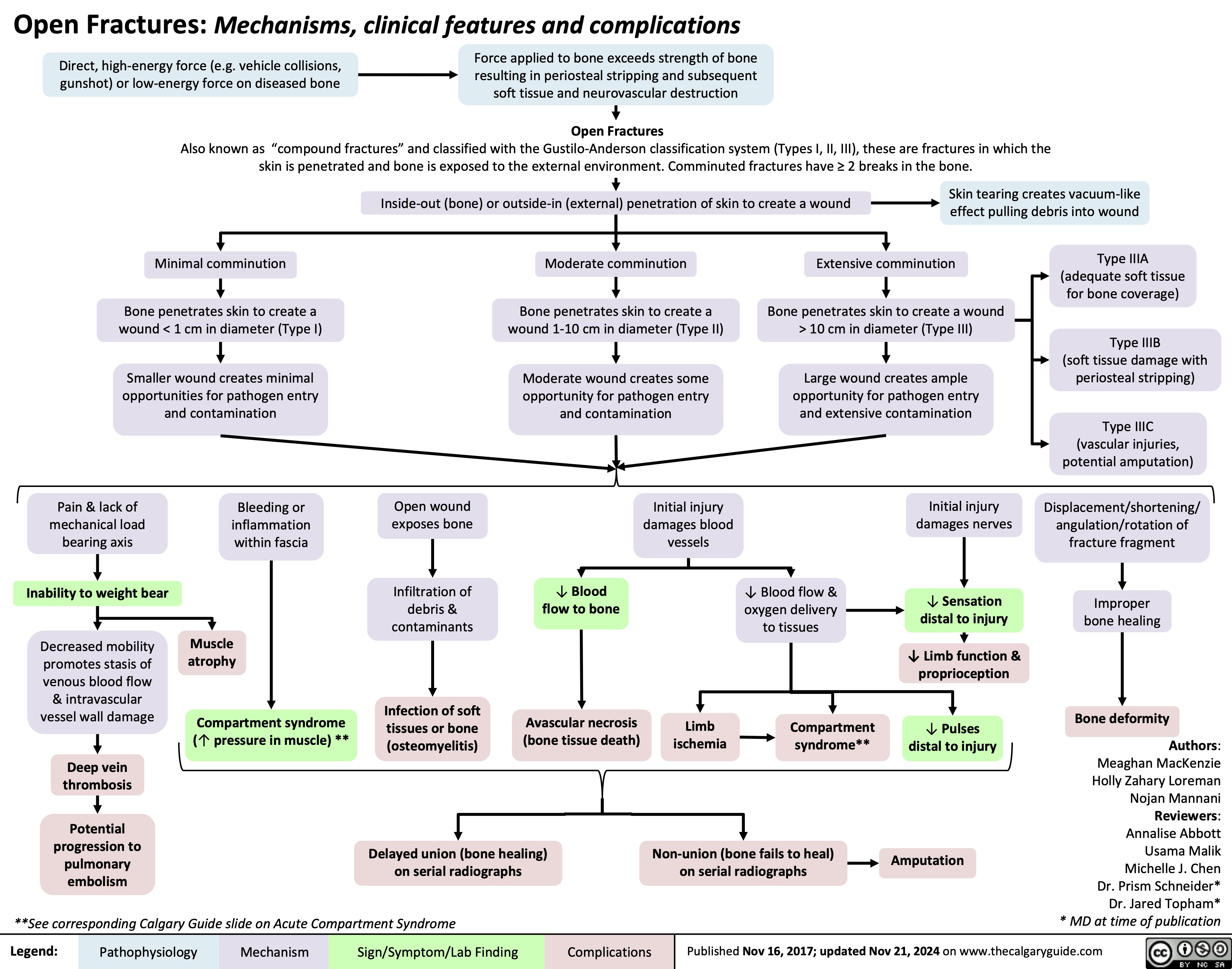
Statins Mechanisms and Side Effects
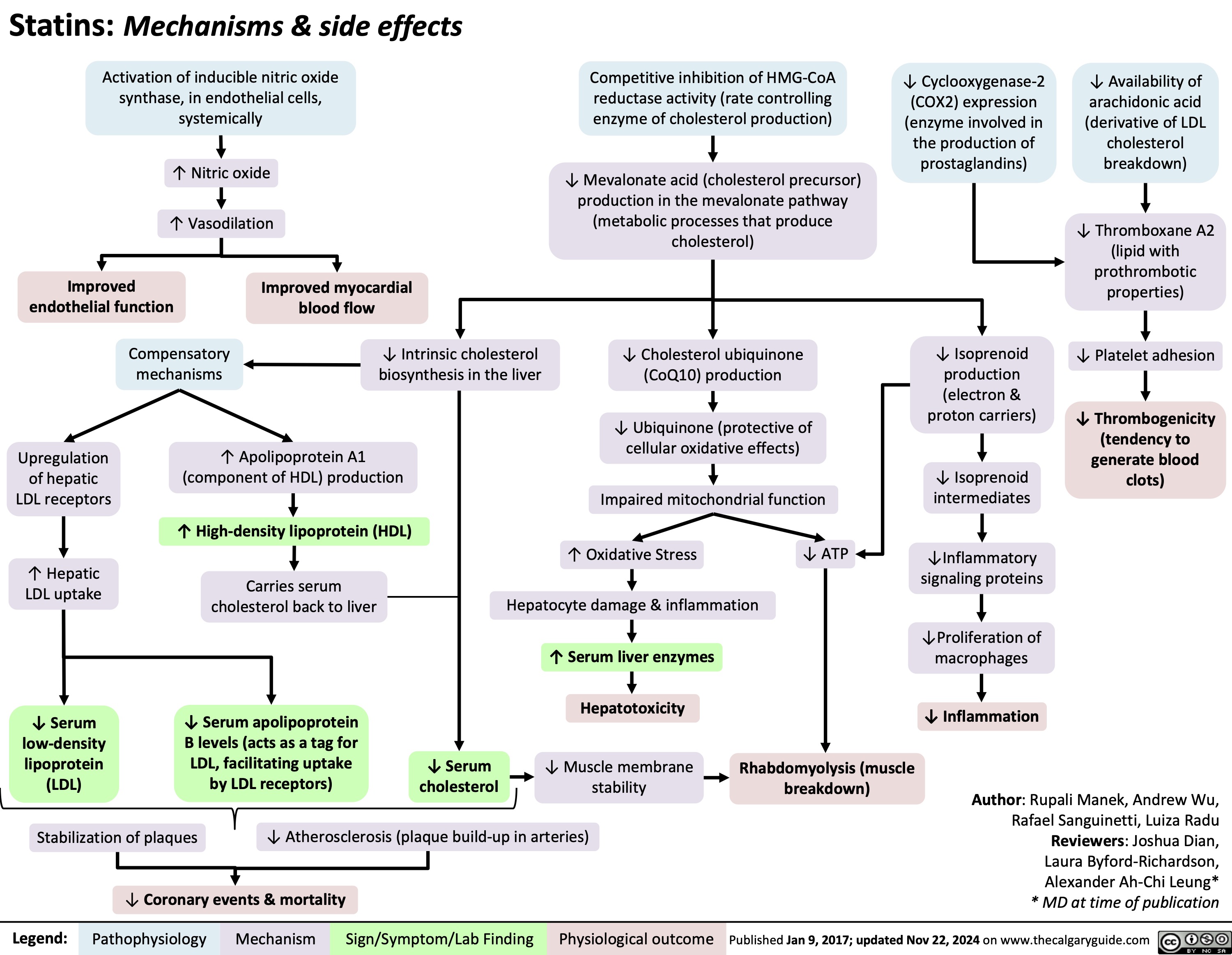
Hypocalcemia Pathogenesis
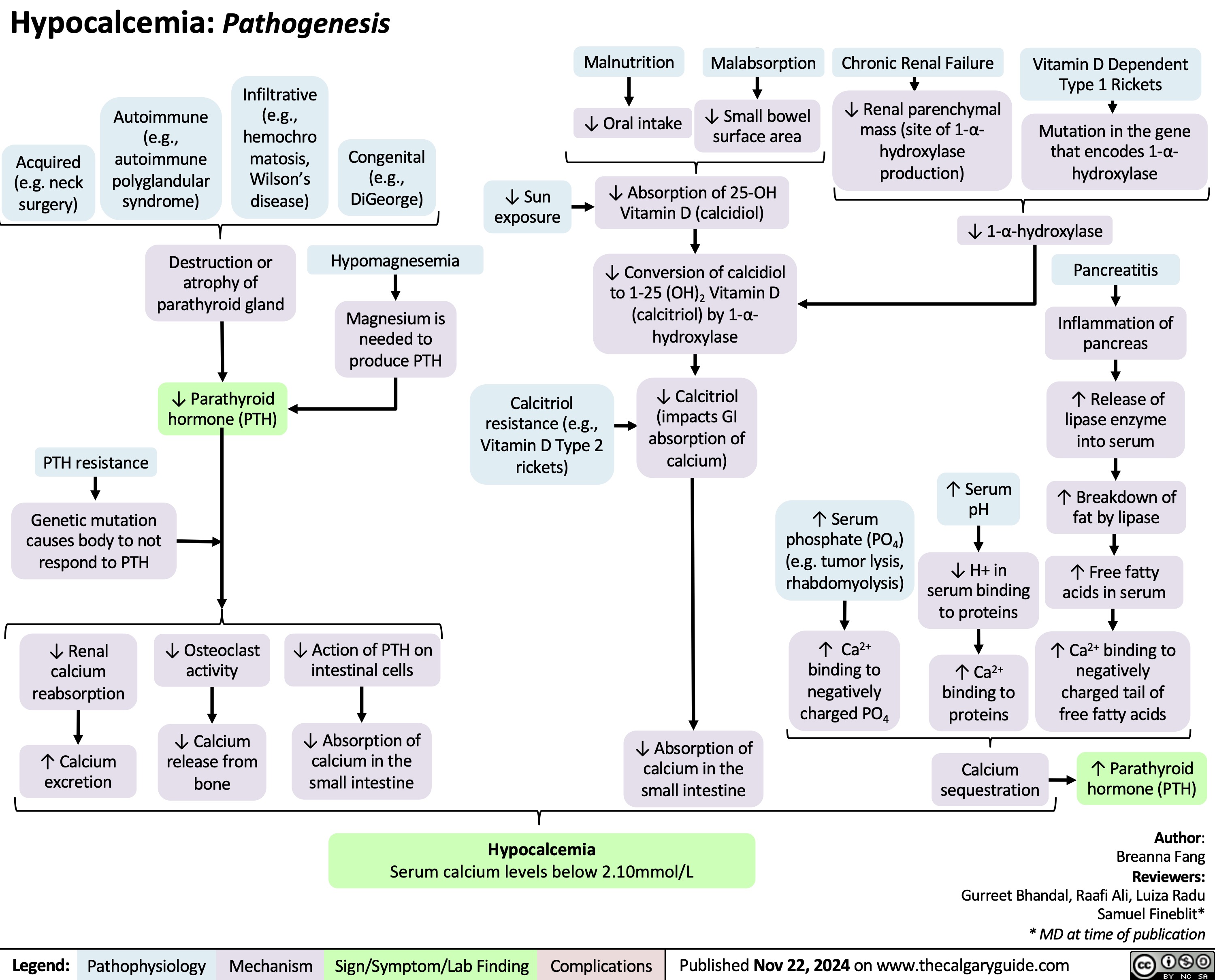
Acquired Inguinal Hernias
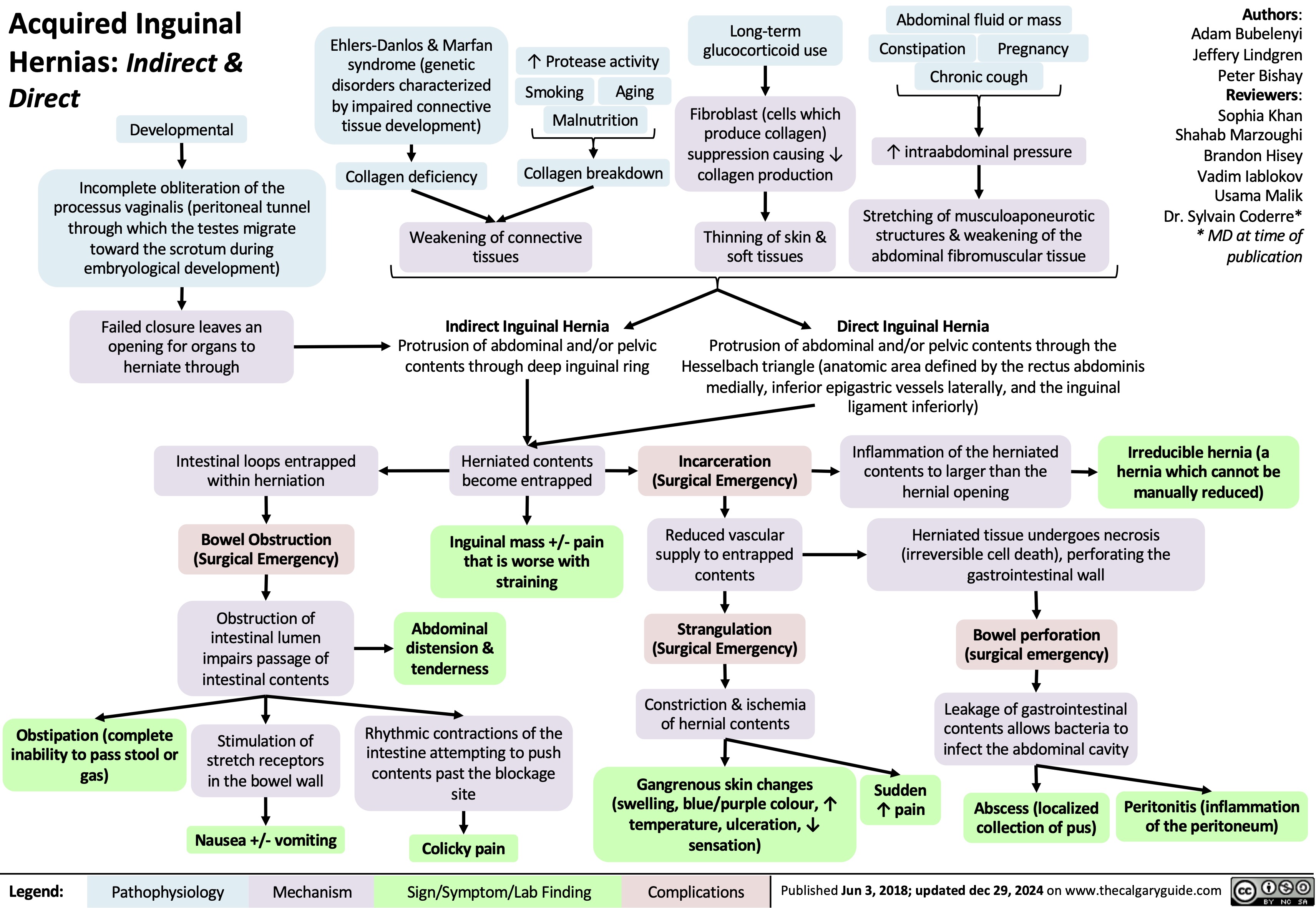
Barretts Esophagus
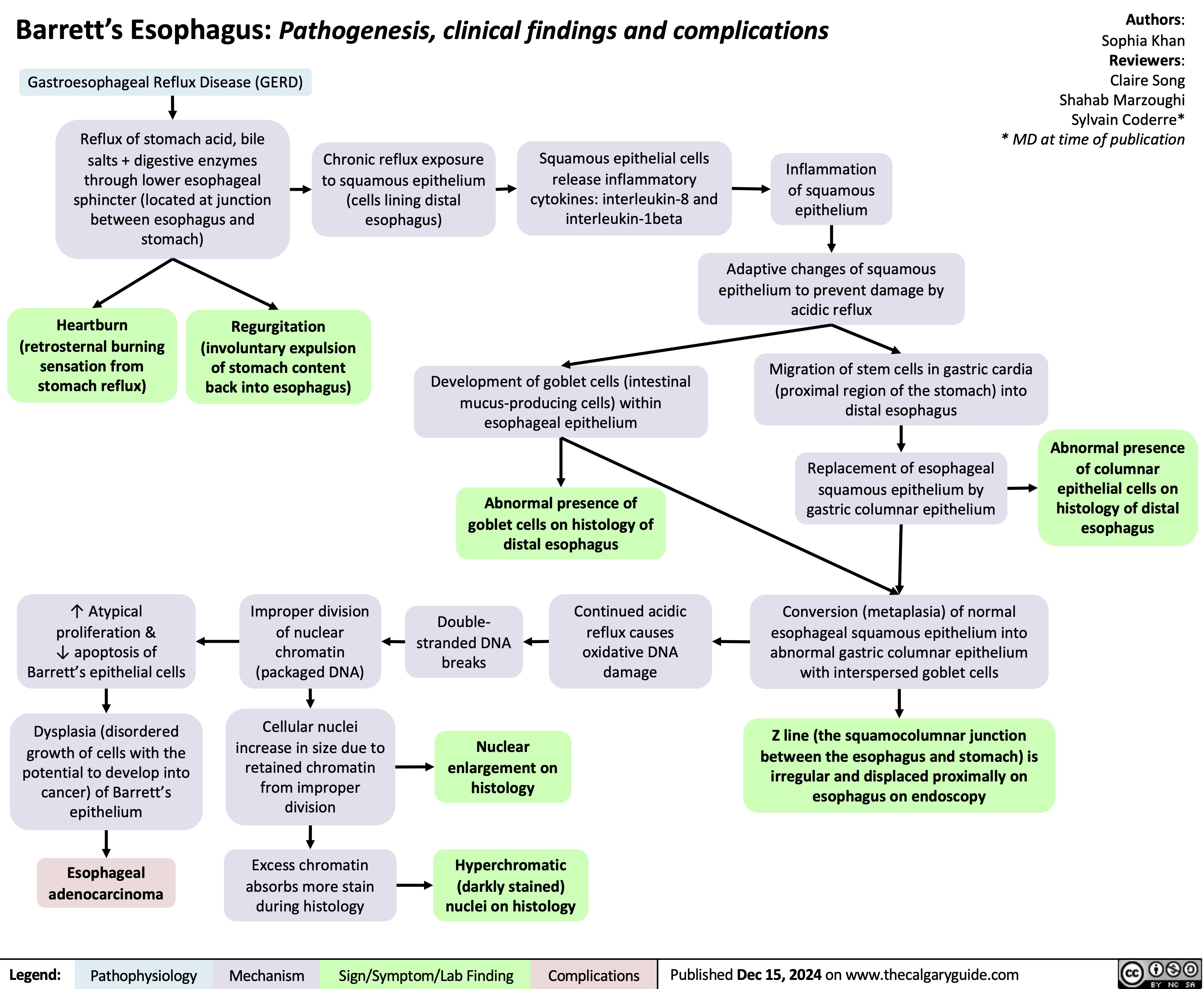
Malignant Esophagitis
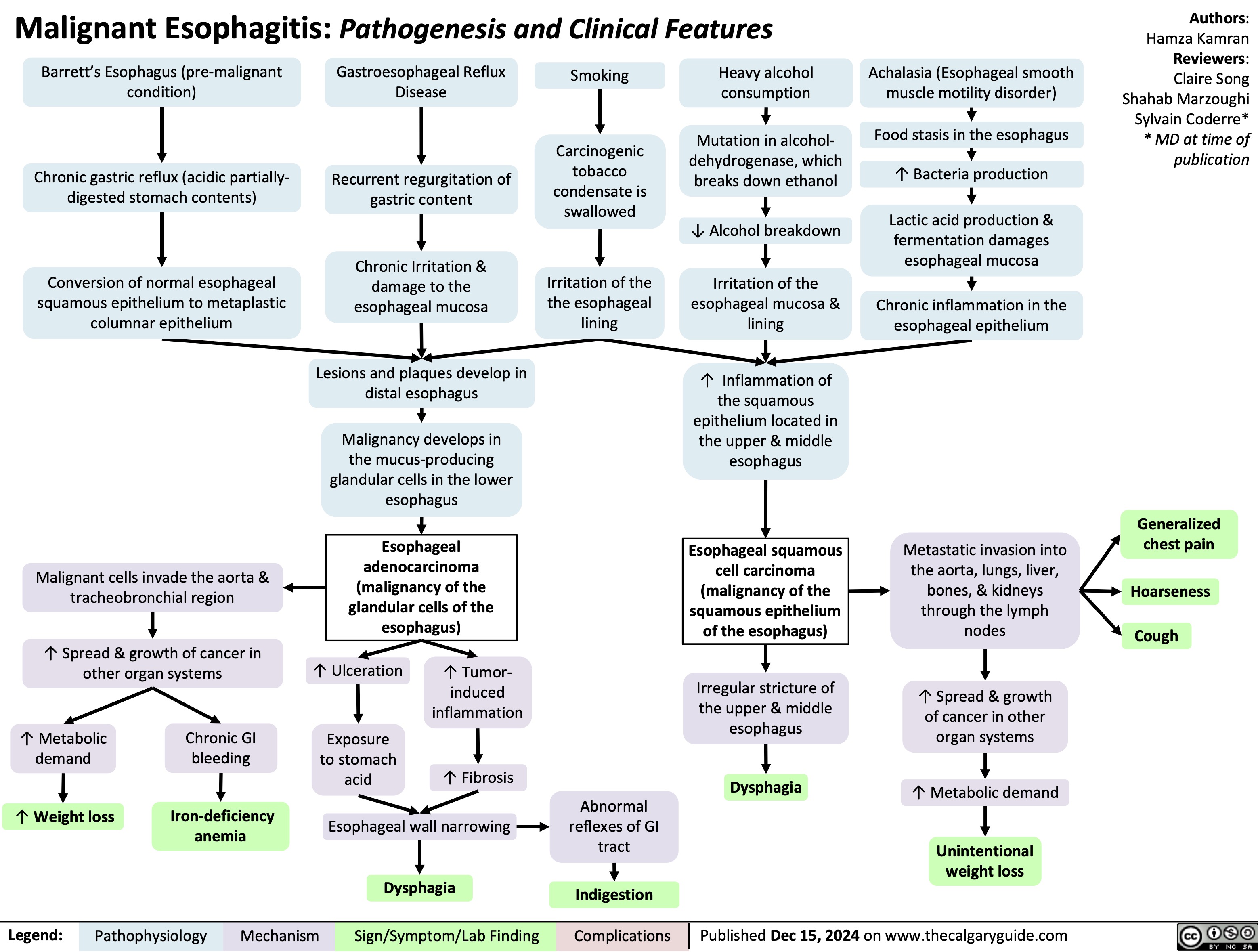
Ewing Sarcoma
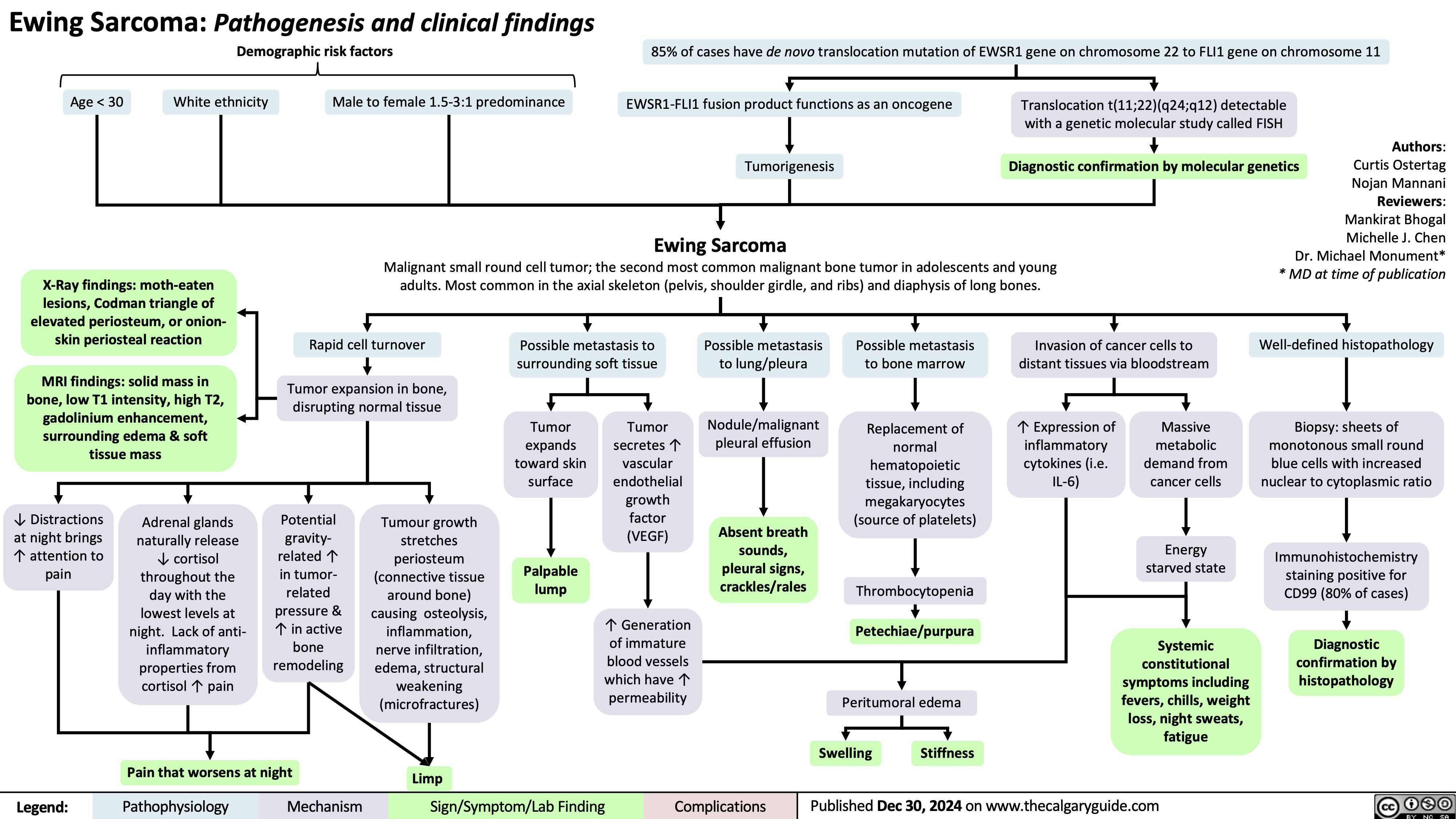
Pituitary Tumour Classification and Clinical Outcomes
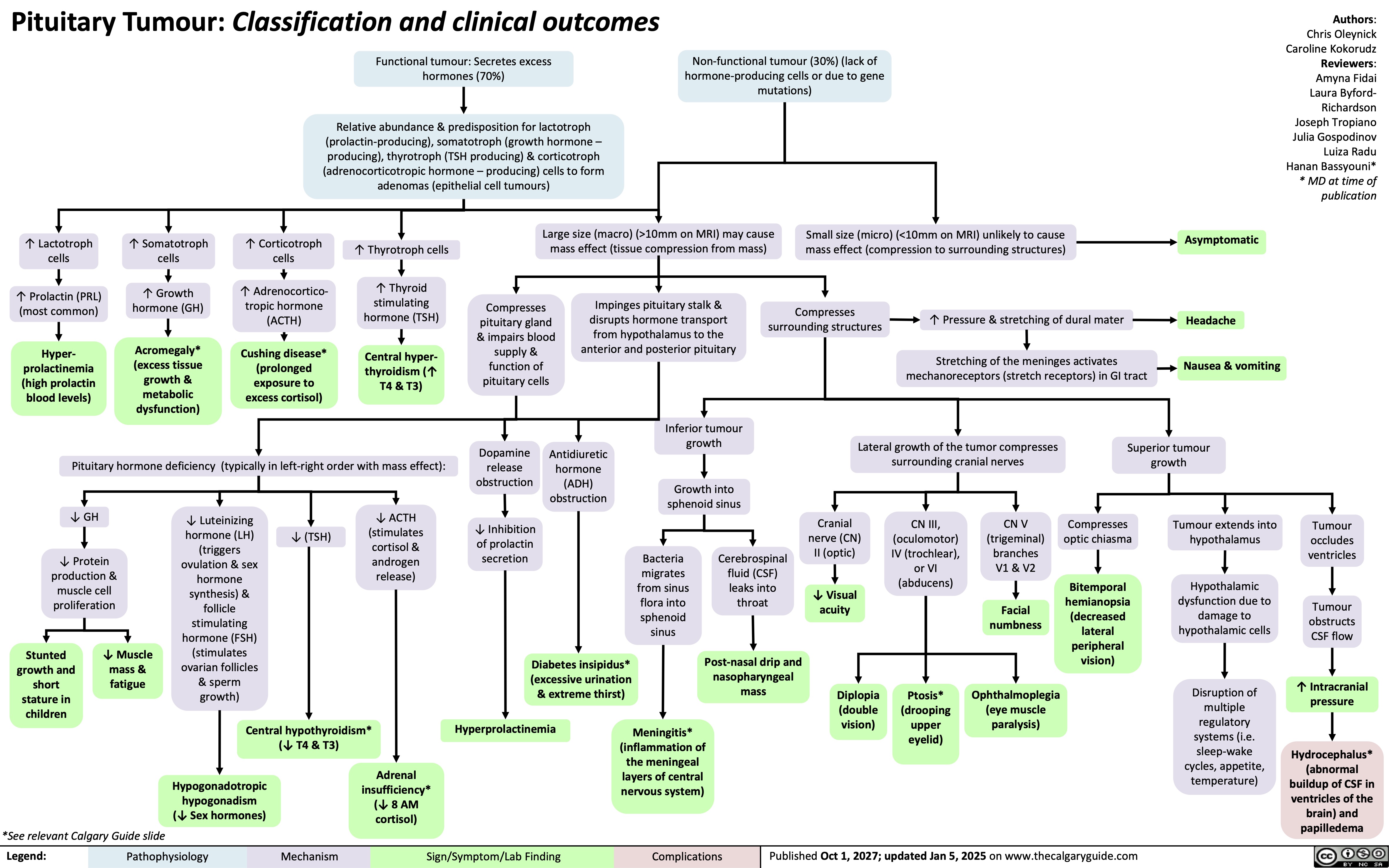
Central Precocious Puberty
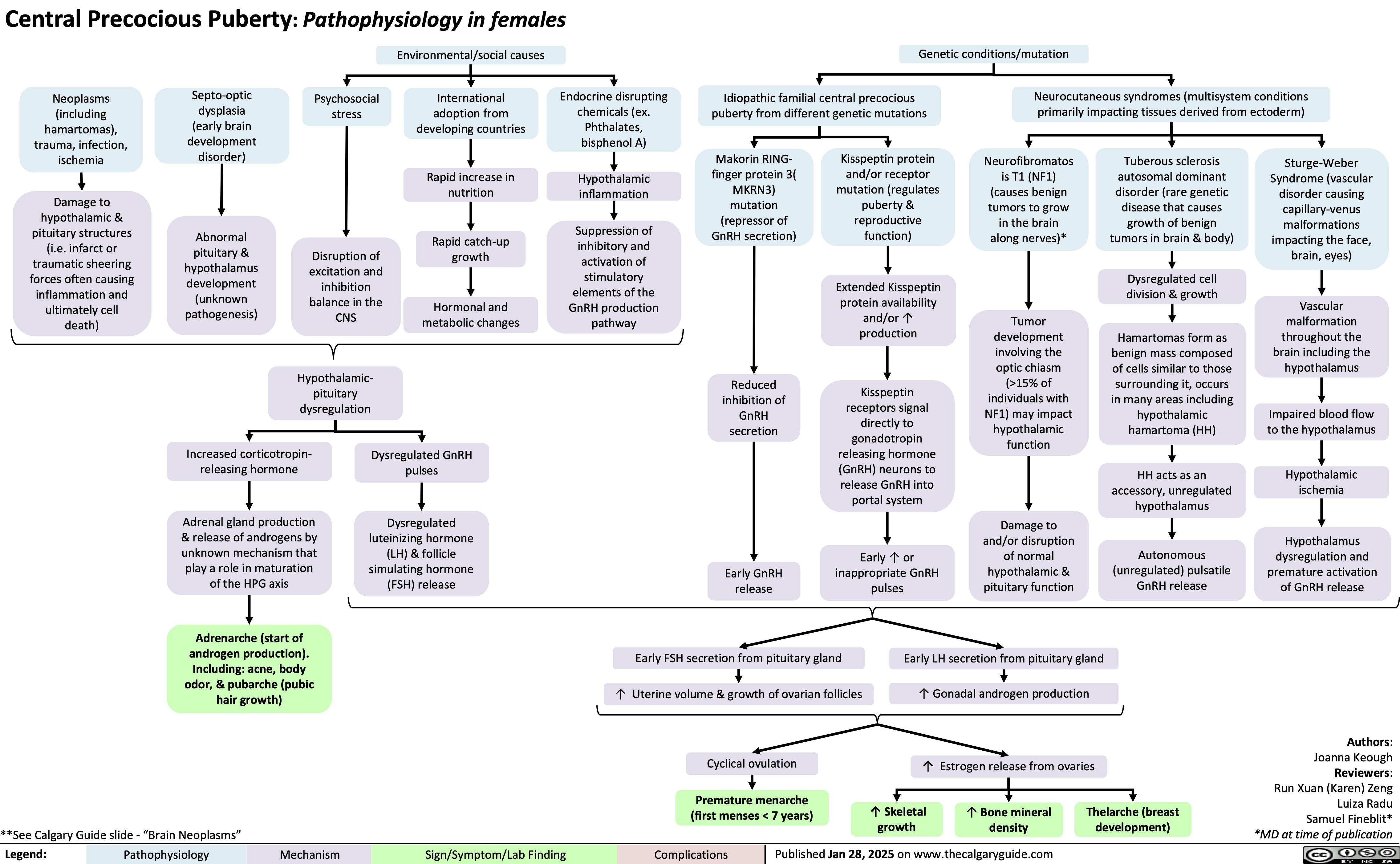
Osgood Schlatter Disease
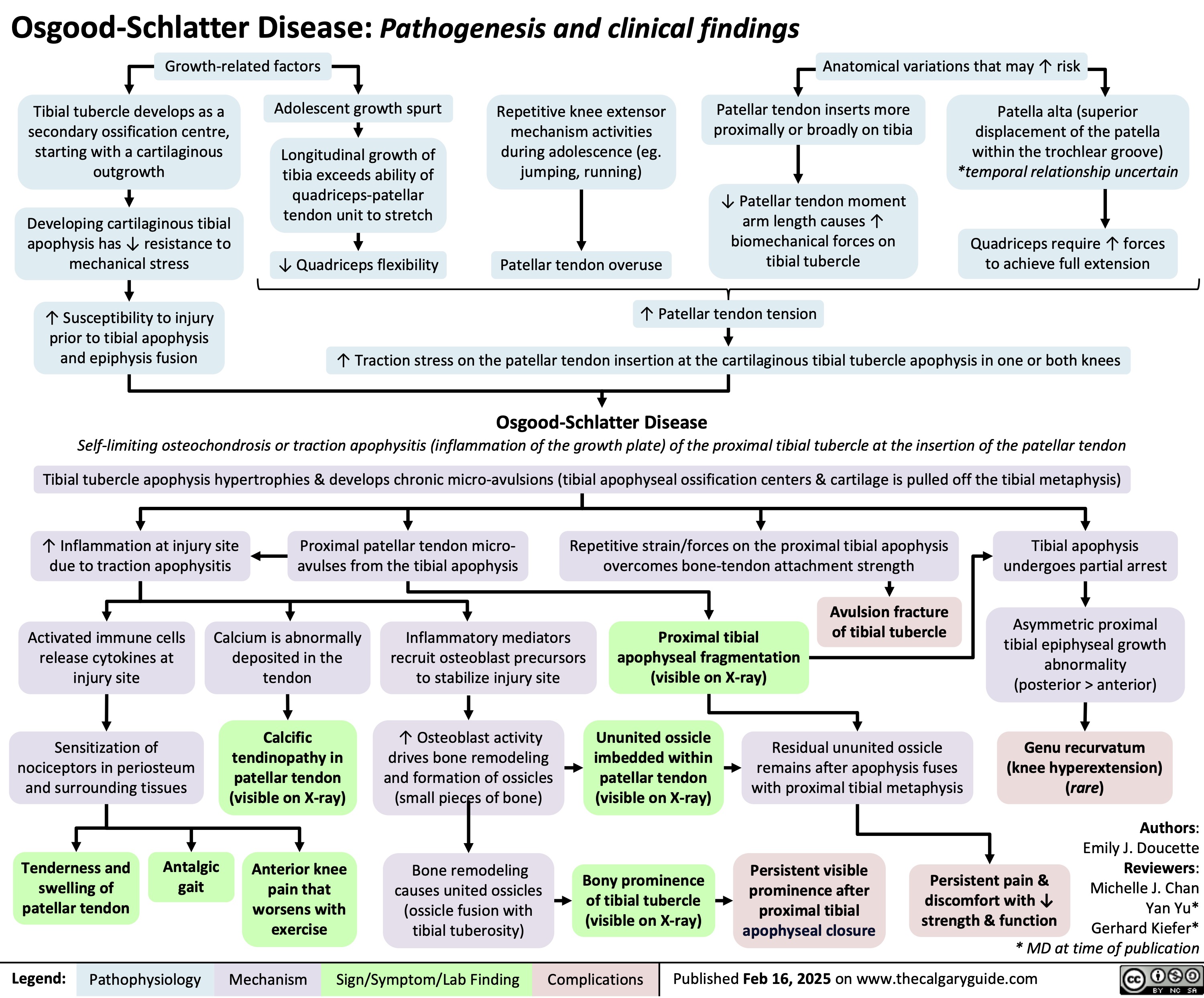
Tonsillitis Pathogenesis and clinical findings
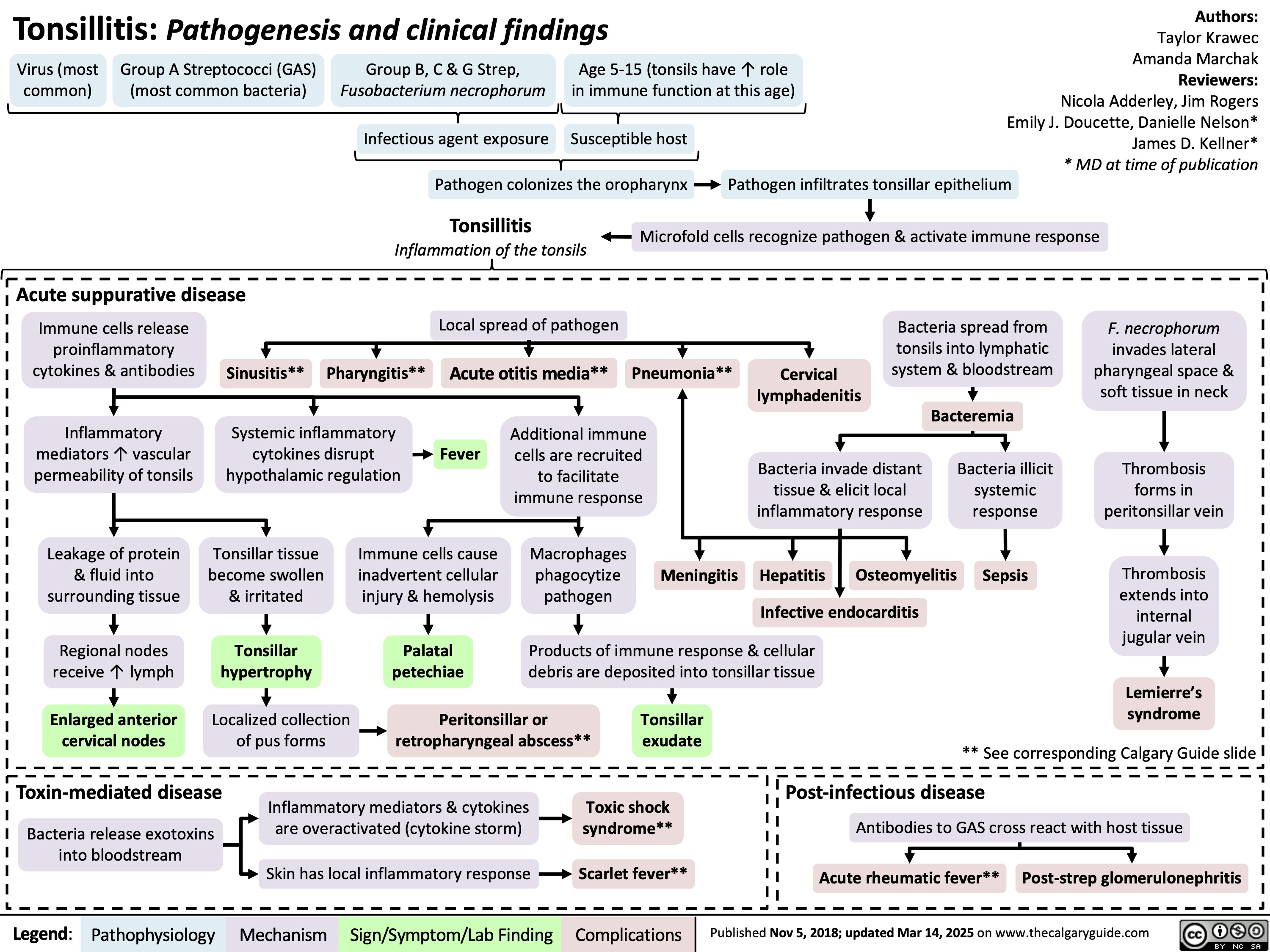
Polymyalgia Rheumatica
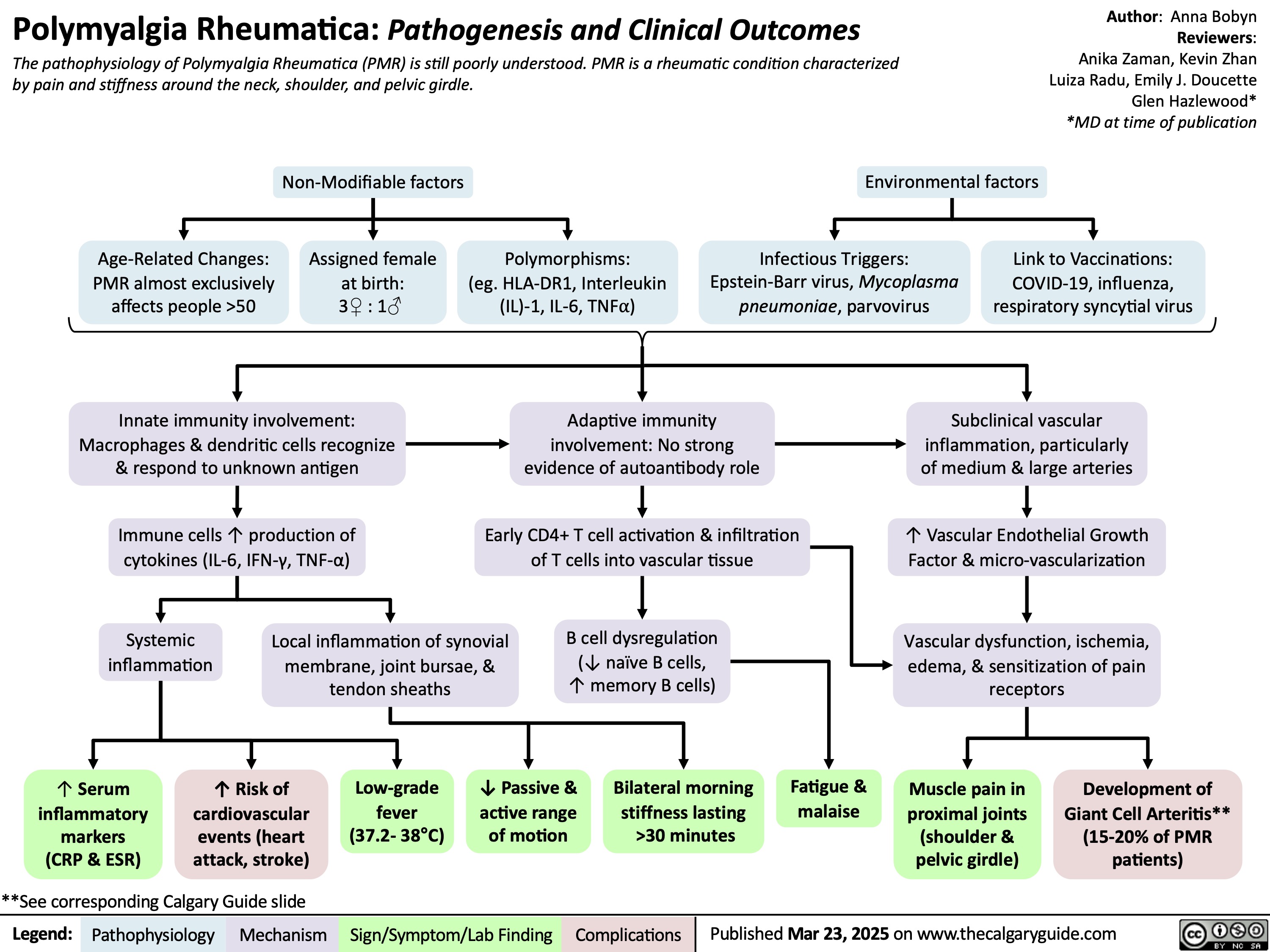
Acute Infectious Mononucleosis
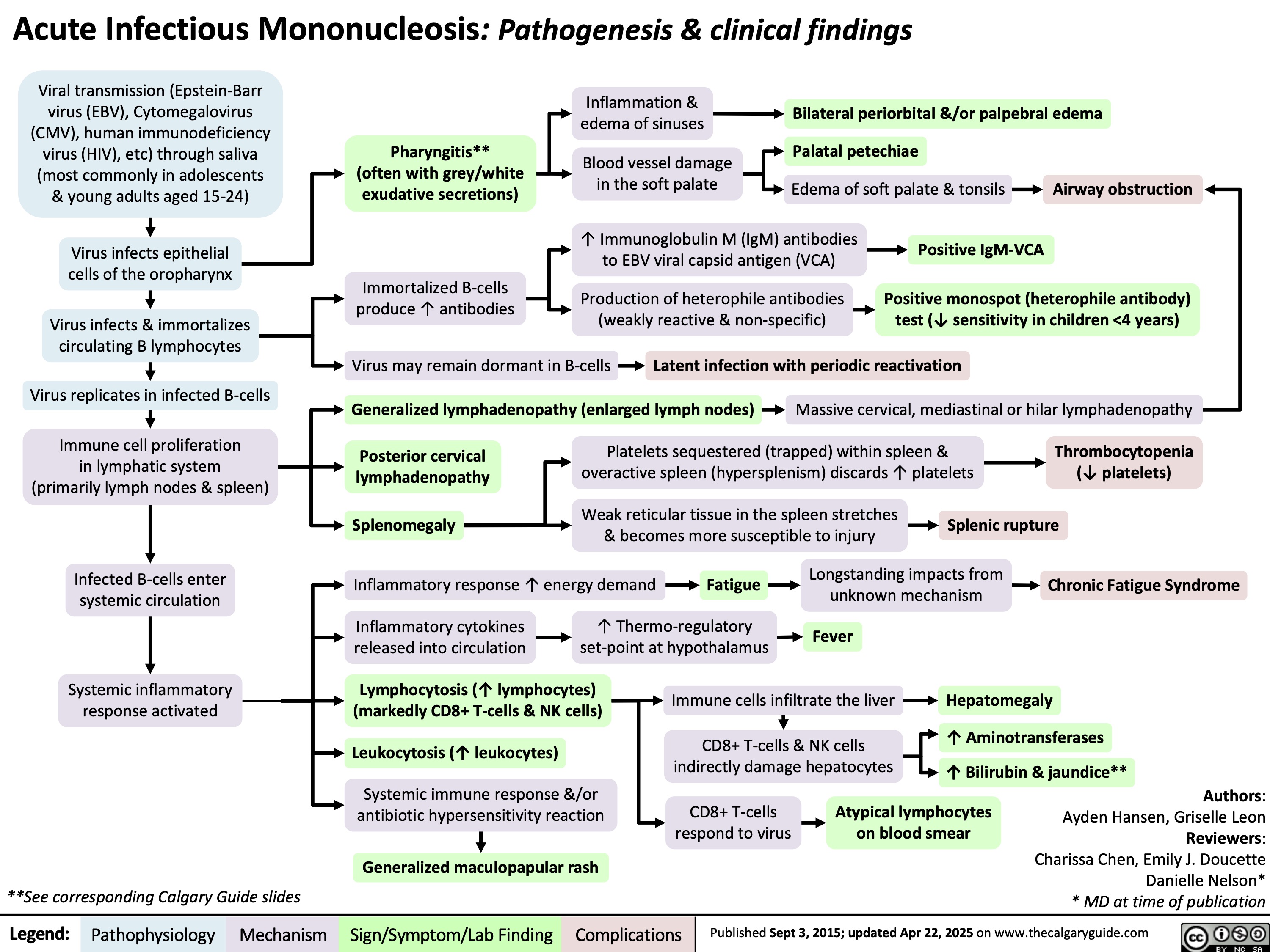
Trachoma
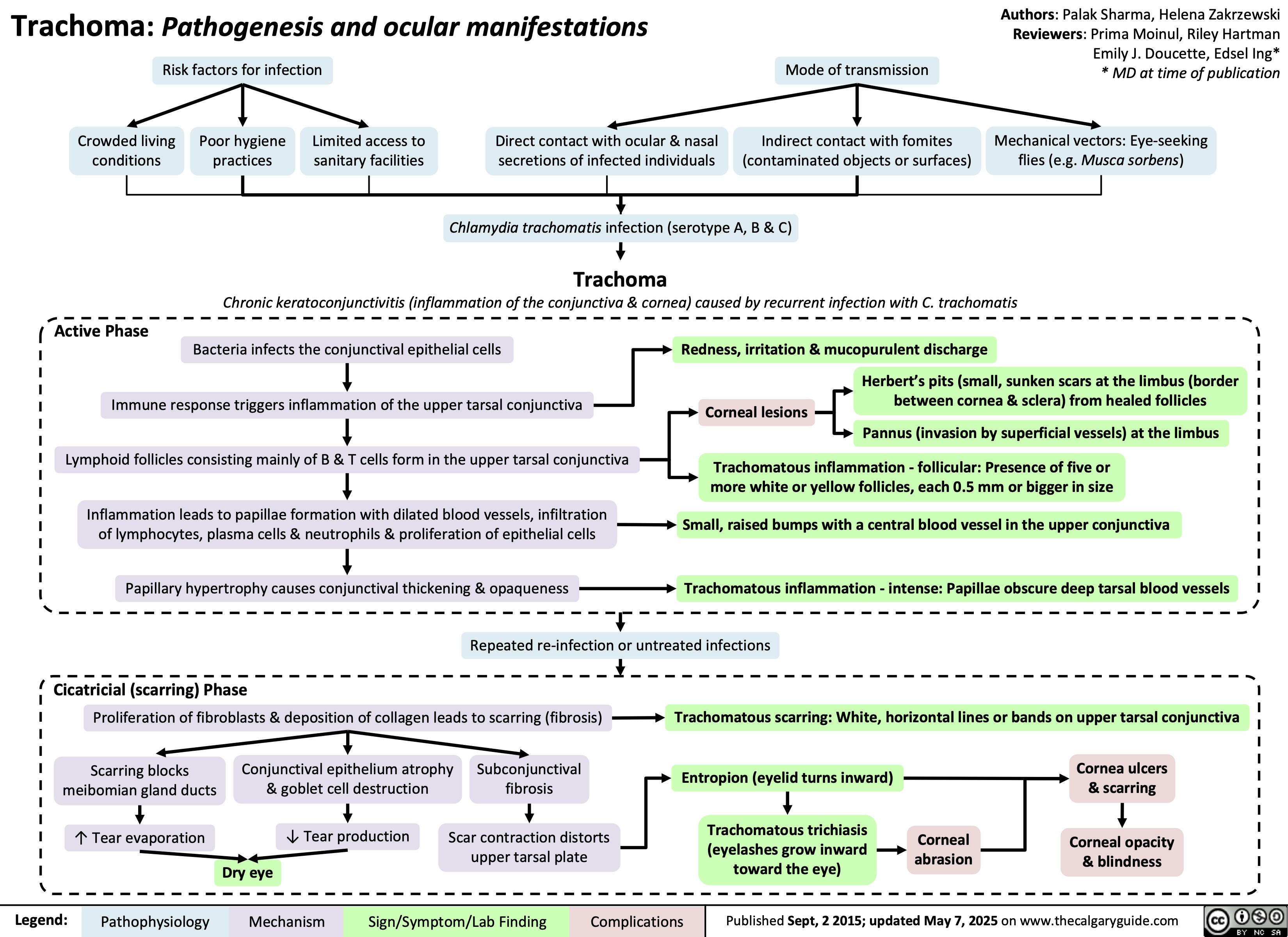
Meckels Diverticulum
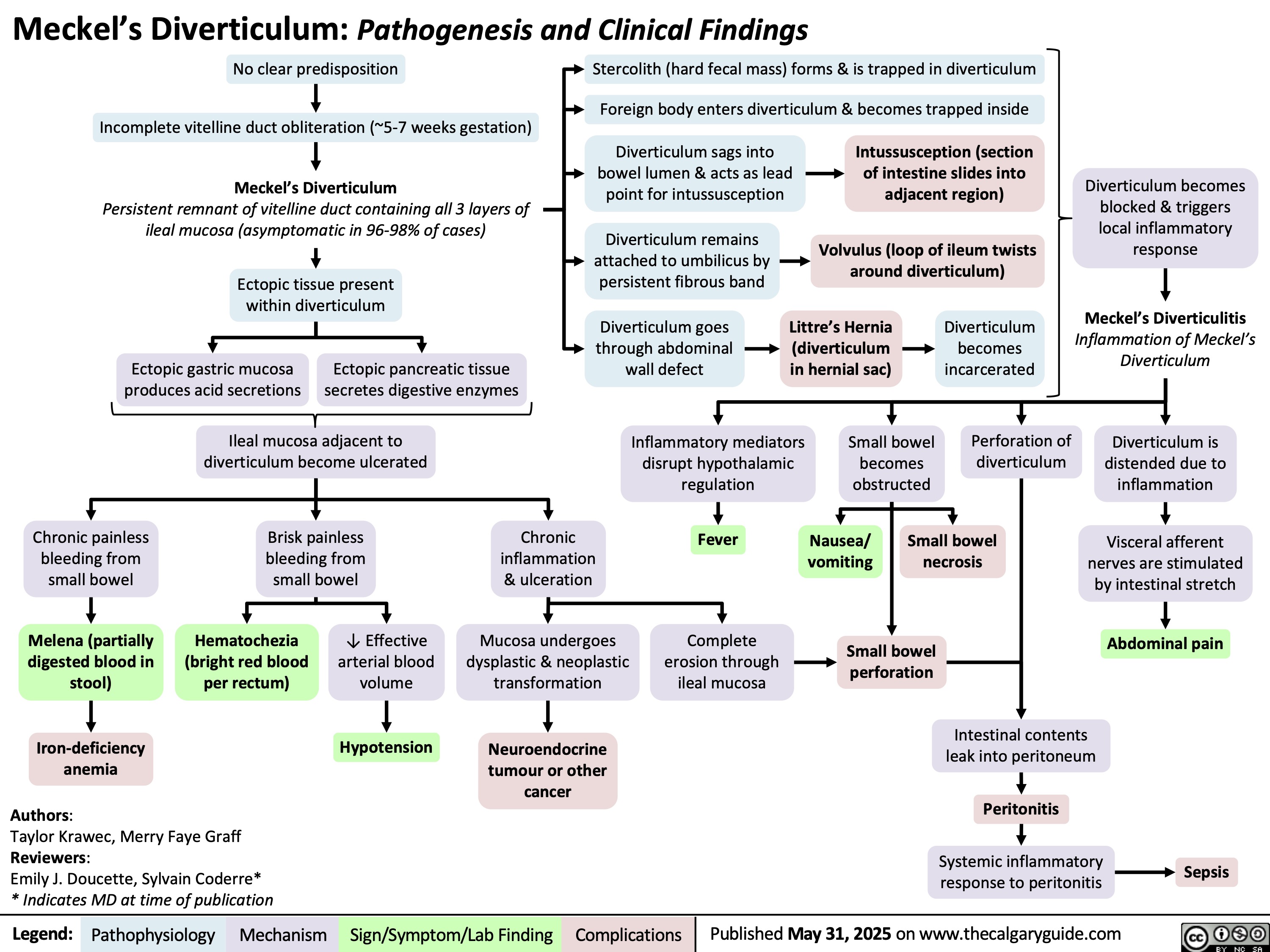
Chronic Mesenteric Ischemia
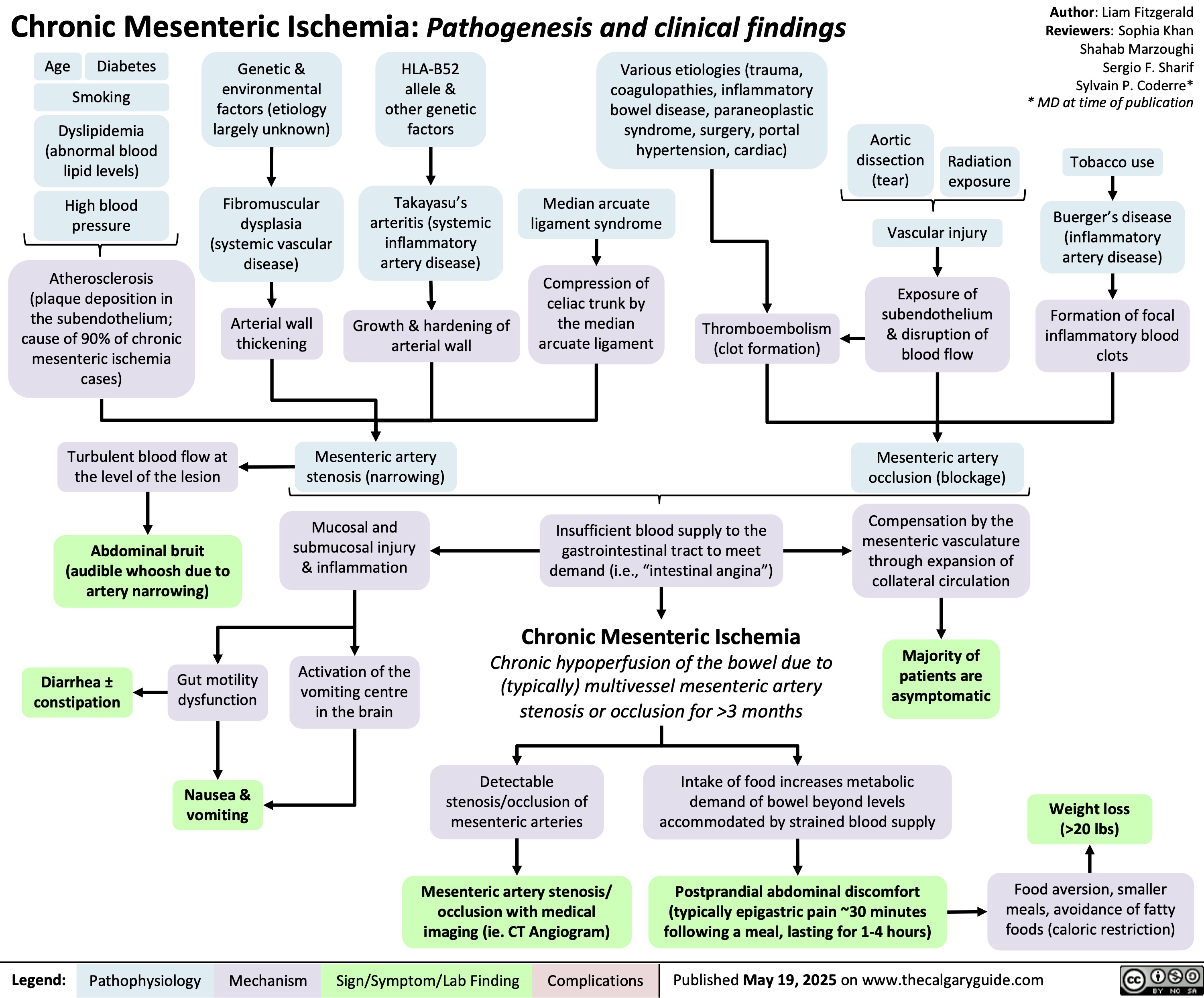
Heparin-Induced Thrombocytopenia
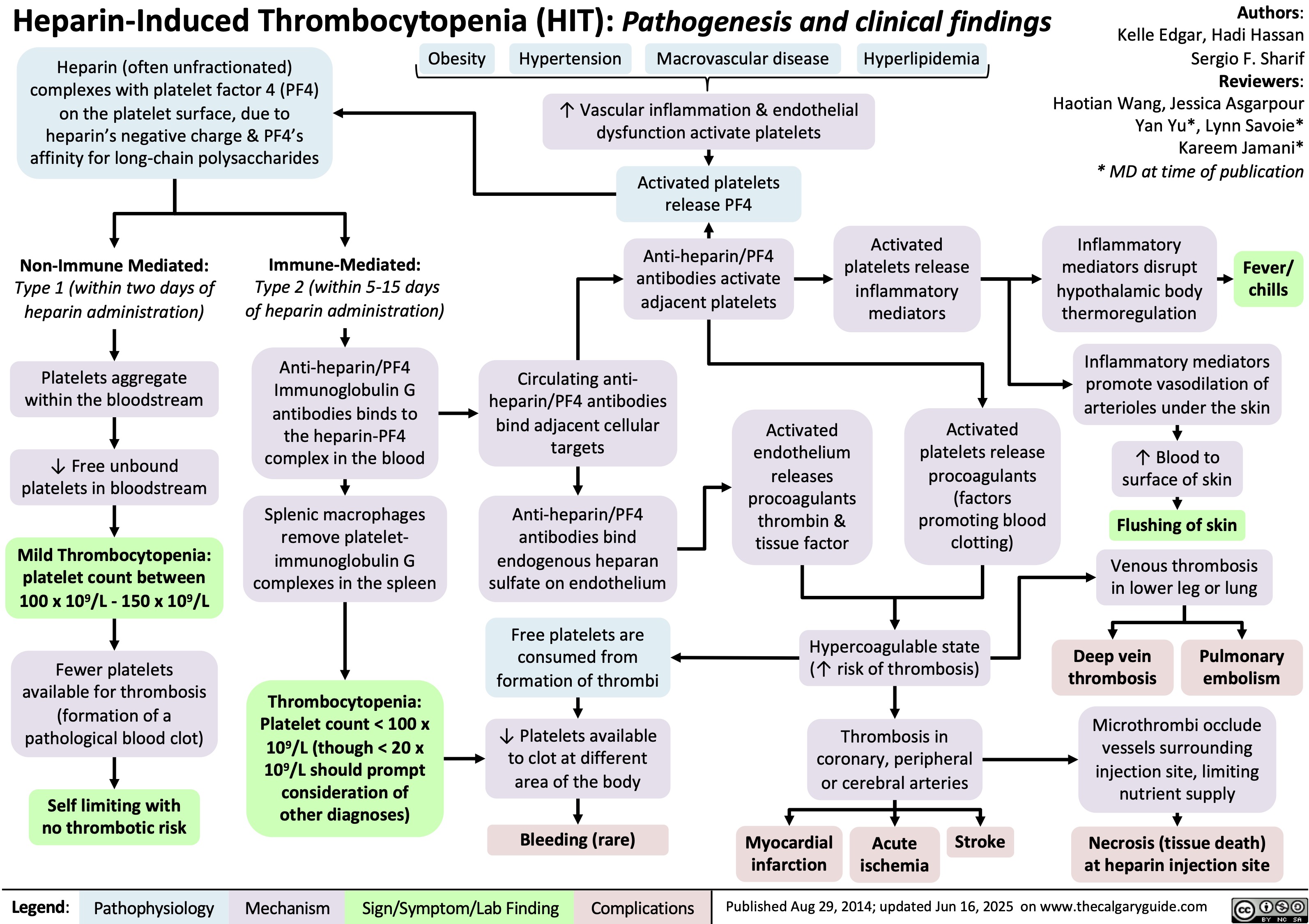
Varicella Zoster Virus
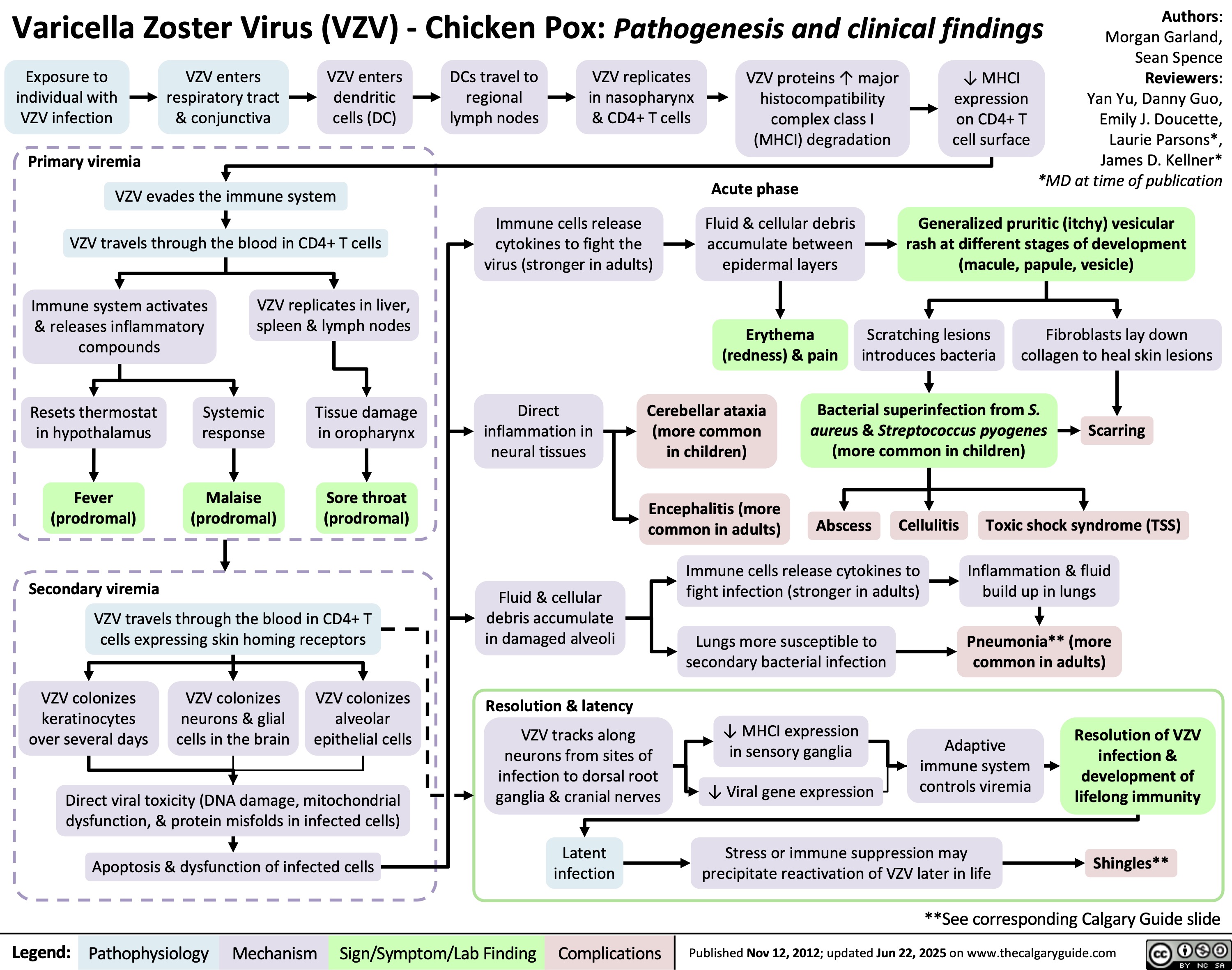
GLP-1 Receptor Agonists
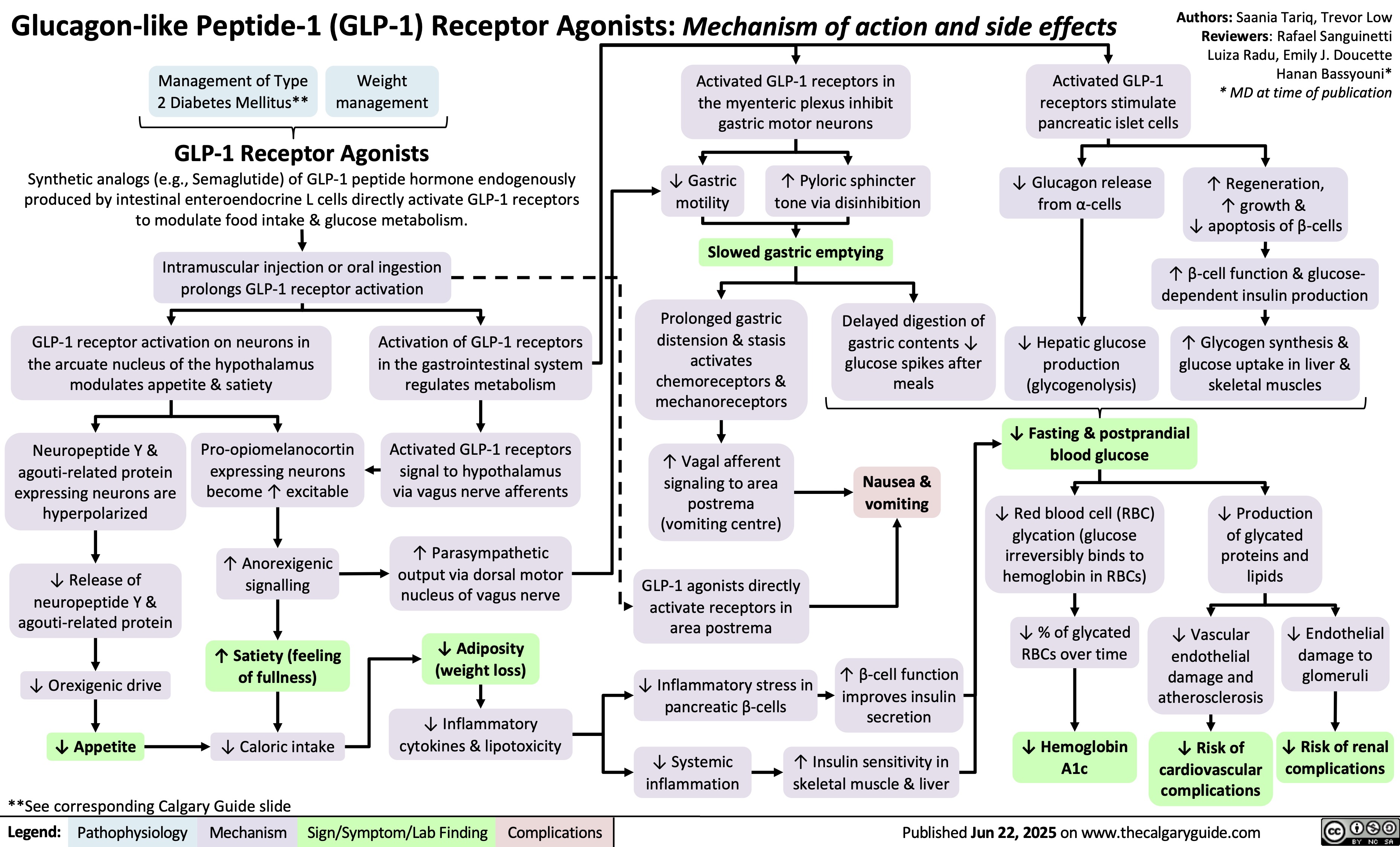
Fluoroquinolones
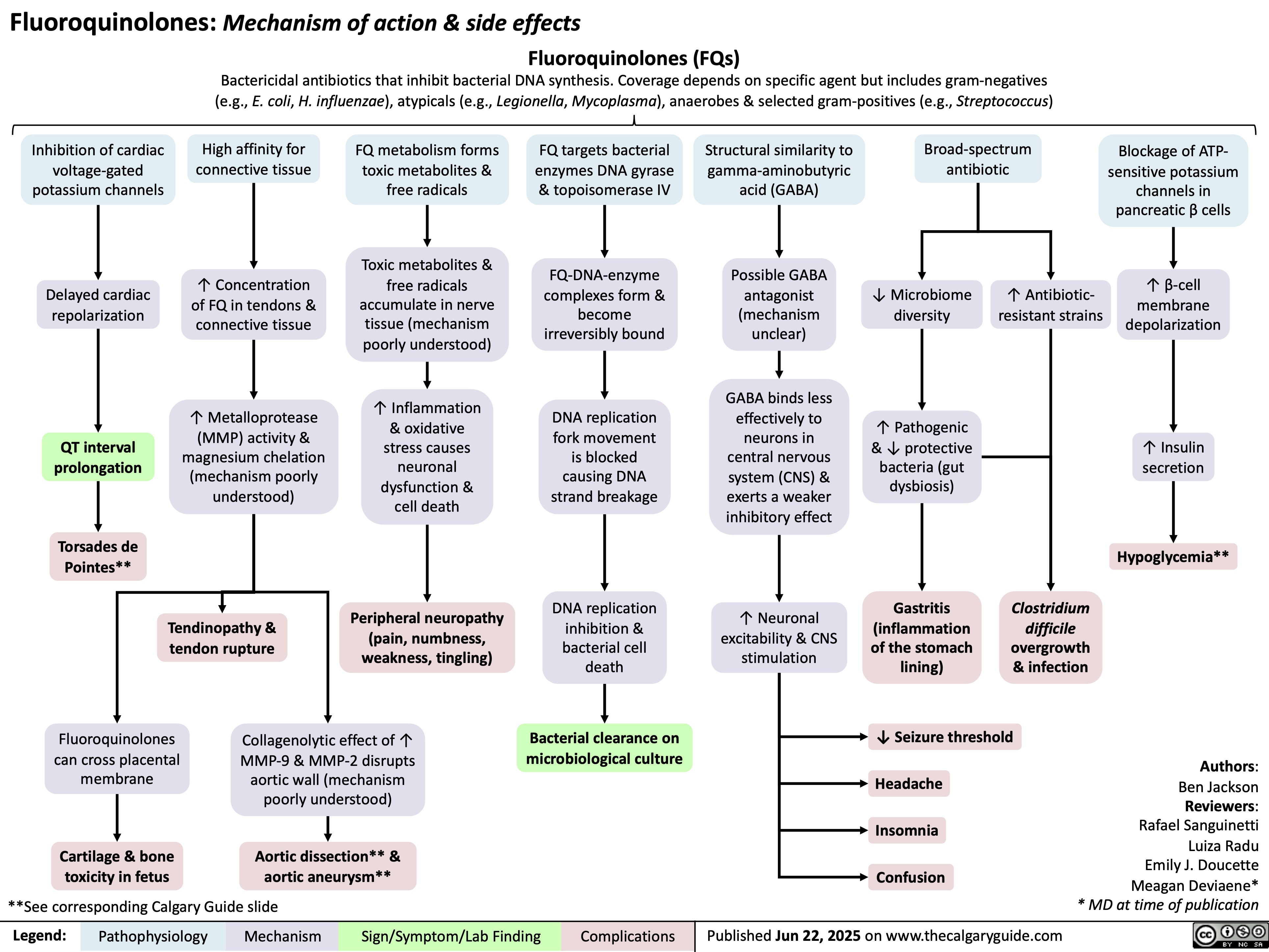
Unconjugated Hyperbilirubinemia
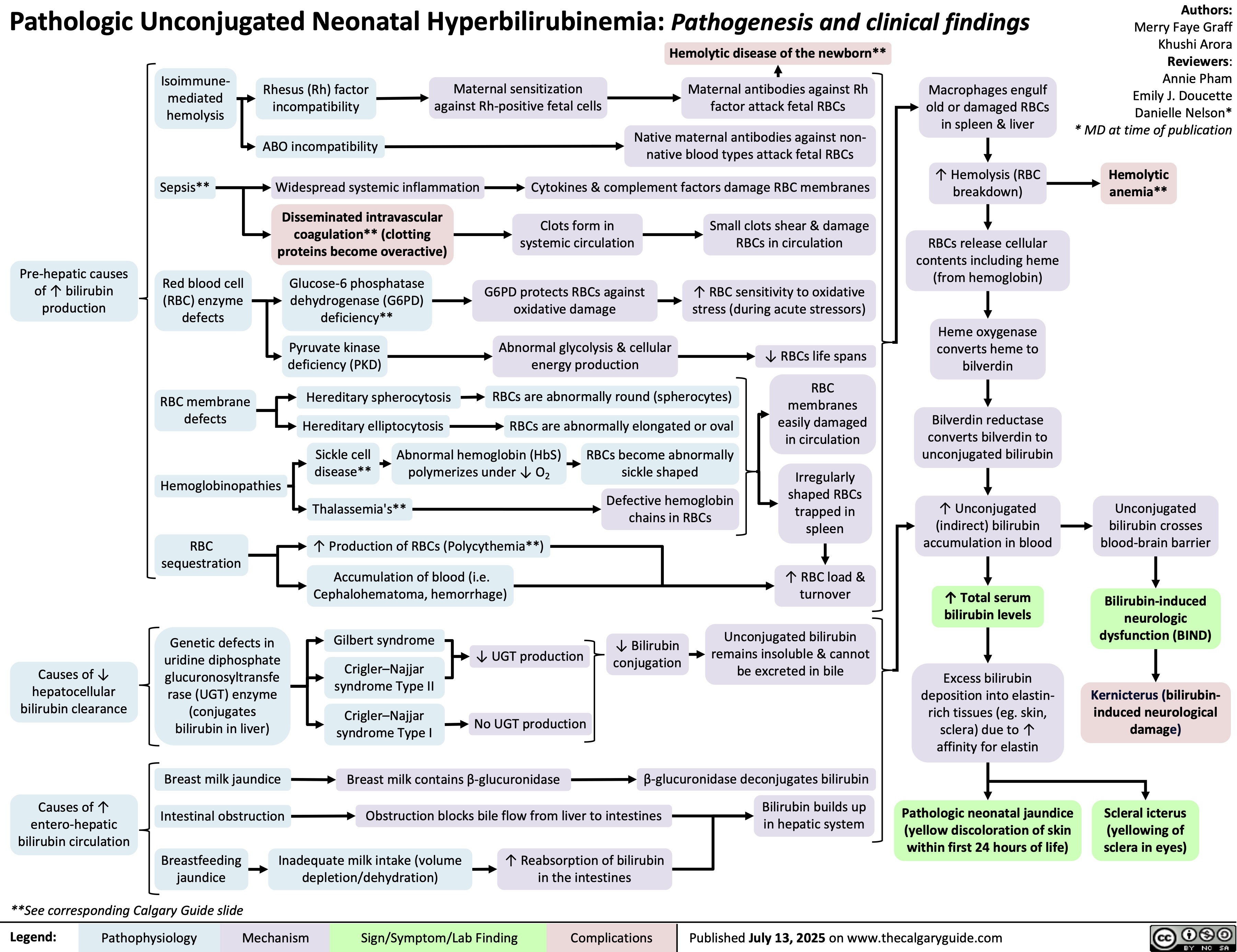
 L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" title="Yu Yan - MI Findings on History - FINAL.pptx -
Myocardial Infarction: Findings on HistoryLegend:Published January 30, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsAuthor: Yan YuReviewers:Sean SpenceTristan JonesNanette Alvarez** MD at time of publication Systolic function(necrotic myocardium cannot contract as well)Reflexive ? in sympathetic activity (to try to maintain CO)Clammy skin? stroke volume (SV), ? cardiac output (CO)Myocardial infarction (tissue necrosis)Note: Myocardial ischemic pain may differ between patients, but recurrences usually feel the same in any given patient.Generalized vasoconstrictionVasoconstriction of skin arteriolesCool skinLocal myocardial inflammationIrritation of T1-T4 sympathetic afferentsIrritation of cardiac branches of vagus nerveSignals enter spinal cord, mixes with T1-T4 dermatomesCrushing, Diffuse "Pain" or "tightness": Often retrosternal, with radiation to shoulder, neck, and inner aspect of both arms (R > L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" />
L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" title="Yu Yan - MI Findings on History - FINAL.pptx -
Myocardial Infarction: Findings on HistoryLegend:Published January 30, 2013 on www.thecalgaryguide.comMechanismPathophysiologySign/Symptom/Lab FindingComplicationsAuthor: Yan YuReviewers:Sean SpenceTristan JonesNanette Alvarez** MD at time of publication Systolic function(necrotic myocardium cannot contract as well)Reflexive ? in sympathetic activity (to try to maintain CO)Clammy skin? stroke volume (SV), ? cardiac output (CO)Myocardial infarction (tissue necrosis)Note: Myocardial ischemic pain may differ between patients, but recurrences usually feel the same in any given patient.Generalized vasoconstrictionVasoconstriction of skin arteriolesCool skinLocal myocardial inflammationIrritation of T1-T4 sympathetic afferentsIrritation of cardiac branches of vagus nerveSignals enter spinal cord, mixes with T1-T4 dermatomesCrushing, Diffuse "Pain" or "tightness": Often retrosternal, with radiation to shoulder, neck, and inner aspect of both arms (R > L).(Onset: often at rest; crescendo)Activation of reflexive vagal responses (listed below)Weakness, dizziness, nausea, vomitingInflammatory mediators irritates nerves innervating the heart (the cardiac plexus)Cytokines act on hypothalamic T0 regulatorMild fever? Sweating (diaphoresis)Inflammatory cytokines can spread systemicallyBrain perceives nerve irritation as pain coming from T1-T4 dermatomesBlood backs up from the LV, into the left atrium and eventually accumulates in the pulmonary vasculatureHigh pulmonary venous blood pressure forces fluid out of capillaries, into pulmonary interstitium & alveoliRespiratory muscles work harder to ventilate lungsSoggier lung interstitium ? lung complianceDyspnea(Shortness of breath)Fluid compresses airways, ? resistance to airflow
102 kB / 204 words" />


































![Acute Pancreatitis: Pathogenesis and Clinical Findings
Authors: Yan Yu Reviewers: Laura Craig Noriyah AlAwadhi Ryan Brenneis Maitreyi Raman* * MD at time of publication
Associated signs due to intra- abdominal hemorrhage from an unknown mechanism (classically associated with pancreatitis, but happens in <1% of cases):
Note:
It is not enough to just diagnose “acute pancreatitis”. Full management requires determining underlying etiology with further work-up.
Alcohol
↑ Toxic metabolites within pancreas and Spincter of Oddi Spasms
Gallstones
Migration to common bile duct blocks Sphincter of Oddi
Hypertriglyceridemia
Unknown
mechanism (rare)
Idiopathic
Further investigations:
CBC: Cell counts elevated, due to sever hypovolemia
Serum [Lipase]: Gold Standard Diagnostic Test; rupture of pancreatic cells releases lipase into circulation
Pancreatic secretions back up, ↑ pressure within pancreas
Hypercalcemia (Rare; Ca2+ depositions in bile ducts block outflow of pancreatic secretions)
Since pancreas is retroperitoneal, somatic
nerves in the parietal peritoneum are directly stimulated
Inflammation triggers cytokine release
Inflamed pancreas irritates adjacent intestines, causing ileus
Inflamed, more permeable blood vessels leak fluid into pancreas
• •
Cullen’s sign (bruising in peri-umbilical region) Grey-Turner’s sign (bruises along both flanks)
Sudden, severe epigastric pain (with peritoneal signs), radiates to the center of the back
Fever, nausea/vomiting
(general signs of inflammation)
Diminished bowel sounds Profound dehydration
(flat JVP, hypotension, tachycardia, oliguria) – may happen, not always
1. Pressure compresses pancreatic blood vessels, causing tissue ischemia.
2. Activation of inactive proteases (zymogens) digesting pancreatic tissue
Necrosis (death) of pancreatic cells
Inflammation self- perpetuates
Massive systemic inflammatory response
2 main complications, usually detected on CT;
may happen, but not always
1. Pancreatic pseudocyst (enlargement of the
pancreas due to fluid accumulation)
2. Pancreatic necrosis/abscesses (death of a part of the pancreas)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-published September 1, 2019 on thecalgaryguide.com
Acute Pancreatitis: Pathogenesis and Clinical Findings
Authors: Yan Yu Reviewers: Laura Craig Noriyah AlAwadhi Ryan Brenneis Maitreyi Raman* * MD at time of publication
Associated signs due to intra- abdominal hemorrhage from an unknown mechanism (classically associated with pancreatitis, but happens in <1% of cases):
Note:
It is not enough to just diagnose “acute pancreatitis”. Full management requires determining underlying etiology with further work-up.
Alcohol
↑ Toxic metabolites within pancreas and Spincter of Oddi Spasms
Gallstones
Migration to common bile duct blocks Sphincter of Oddi
Hypertriglyceridemia
Unknown
mechanism (rare)
Idiopathic
Further investigations:
CBC: Cell counts elevated, due to sever hypovolemia
Serum [Lipase]: Gold Standard Diagnostic Test; rupture of pancreatic cells releases lipase into circulation
Pancreatic secretions back up, ↑ pressure within pancreas
Hypercalcemia (Rare; Ca2+ depositions in bile ducts block outflow of pancreatic secretions)
Since pancreas is retroperitoneal, somatic
nerves in the parietal peritoneum are directly stimulated
Inflammation triggers cytokine release
Inflamed pancreas irritates adjacent intestines, causing ileus
Inflamed, more permeable blood vessels leak fluid into pancreas
• •
Cullen’s sign (bruising in peri-umbilical region) Grey-Turner’s sign (bruises along both flanks)
Sudden, severe epigastric pain (with peritoneal signs), radiates to the center of the back
Fever, nausea/vomiting
(general signs of inflammation)
Diminished bowel sounds Profound dehydration
(flat JVP, hypotension, tachycardia, oliguria) – may happen, not always
1. Pressure compresses pancreatic blood vessels, causing tissue ischemia.
2. Activation of inactive proteases (zymogens) digesting pancreatic tissue
Necrosis (death) of pancreatic cells
Inflammation self- perpetuates
Massive systemic inflammatory response
2 main complications, usually detected on CT;
may happen, but not always
1. Pancreatic pseudocyst (enlargement of the
pancreas due to fluid accumulation)
2. Pancreatic necrosis/abscesses (death of a part of the pancreas)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Re-published September 1, 2019 on thecalgaryguide.com](http://calgaryguide.ucalgary.ca/wp-content/uploads/2019/09/Acute-Pancreatitis.jpg)

































 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 发病机制
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />
45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" title="COPD: 临床表现
作者: Yan Yu 审稿人:Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人:Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
/012
(如a1-抗胰蛋白酶缺乏) 阻止肺组织损伤的能力↓
+,-.
(如长期吸烟、环境污染、感染)
肺内产生自由基
34*5
肺抗蛋白酶的失活
↑氧化应激,炎性细胞因子,蛋白酶功能
支气管的持续、反复损伤
炎性细胞浸润, 杯状细胞增殖, 气道上皮纤毛 尤其中性粒细 黏液产生↑ 细胞死亡
气道弹性↓ (弹性回缩
肺实质的蛋白水解破坏↑ 维持气道开放 肺泡永久性异常
的结构支持↓ 扩张
胞 力)
肺气体潴留 气道狭窄与 肺过度 肺大泡
气道黏液潴留,成为感染 狭窄 病灶
塌陷 充气
肺气肿
(容易肺泡 破裂)
气道纤维化和
%&'()*
慢性阻塞性肺疾病(COPD)
临床表现 并发症 (参阅相关幻灯片) (参阅相关幻灯片)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Clinical Findings Lung tissue
Chronic Obstructive Pulmonary Disease (COPD)
damage
↓ elastic recoil to push air out of lungs on expiration
Lungs don’t fully empty, air is trapped in alveoli (lung hyperinflation)
↑ lung volume means diaphragm is tonically contracted (flatter)
If occurring around airways
Airflow obstruction
↑ mucus production
↓ number of epithelial ciliated cells to clear away the mucus (the cells have been killed by airway inflammation)
Chronic cough with sputum
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction
↓ ventilation of alveoli
↓ oxygenation of blood (hypoxemia)
↓ perfusion of body tissues (i.e. brain, muscle)
Fatigue; ↓ exercise tolerance
Total expiration time takes longer than normal
Prolonged expiration
More effort needed to ventilate larger lungs
Respiratory muscles must work harder to breathe
Turbulent airflow in narrower airways is heard on auscultation
Expiratory Wheeze
Diaphragm can’t flatten much further to generate deep breaths
To breathe, chest wall must expand out more
Dyspnea
Shortness of breath, especially on exertion
Breathes are rapid & shallow
If end-stage:
Chronic fatigue causes deconditioning
Muscle weakness & wasting
Barrel chest
If end-stage: diaphragm will be “flat”. Continued
Patient tries to expire against higher mouth air pressure, forcing airways to open wider
Pursed-lip breathing
Patient breathes with accessory muscles as well as diaphragm to try to improve airflow
inspiratory effort further contracts diaphragmà pull the lower chest wall inwards
Hoover’s sign
(paradoxical shrinking of lower chest during inspiration)
Tripod sitting position (activates pectoral muscles)
Neck (SCM, scalene) muscles contracted
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$
慢性阻塞性肺疾病 (COPD) 如果出现在气道周围 气流阻塞
肺不能完全排空
气体,气体潴留
于肺泡(肺过度
充气)
总呼气时长大于 正常时长
呼气相延长
肺组织损伤
呼气时,将空气排出肺外 的弹性回缩力↓
肺不能完全排空气体,
气体潴留于肺泡内
(肺过度充气)
肺容积↑,膈肌紧张 性收缩(膈肌平坦)
呼气时,胸膜腔正压挤压气道 à 气道阻塞↑
肺泡通气↓ 血液氧合↓ (低
氧血症)
身体组织灌注 量↓ (比如脑、 肌肉)
疲劳; 运动耐量↓
黏液生成↑ 清除黏液的上皮纤
毛细胞数量↓ (受 气道炎症损伤)
慢性咳嗽伴咳 痰
作者: Yan Yu
审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳),
Zesheng Ye (叶泽生)
* 发表时担任临床医生
容积较大 的肺需要
更加努力 才能通气
呼吸肌必须
更用力才能 呼吸
听诊闻及狭窄气
道中的湍流气流
呼气喘鸣音
呼吸困难 气促,尤其是劳累
膈肌无法进一步收缩以
产生深呼吸
呼吸浅快
为了呼吸,
胸壁必须延
展得更大
桶状胸
晚期病人:
患者试图在较高的口 慢性疲劳导致 患者动用辅助呼吸肌和膈肌呼吸,
腔内气压下进行呼气, 活动耐量下降 从而使气道更开放
以改善气流
晚期病人:膈肌 “平坦” ,持续吸气进一步压 缩膈肌à 向内拉季肋部胸壁
胡佛征 (吸气时,胸廓下侧季肋部内收)
缩唇呼吸
肌肉无力 & 消瘦
端坐呼吸 (调动胸肌)
颈部肌肉收
缩(胸锁乳
突肌、斜角
肌)
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Findings on Investigations
Chronic Obstructive Pulmonary Disease (COPD)
Author: Yan Yu Reviewers: Jason Baserman Jennifer Au Naushad Hirani* Juri Janovcik* * MD at time of publication
Airflow obstruction
Lung tissue damage
↓ ventilation of alveoli
Blood perfusing ill- ventilated alveoli does not receive normal amounts of oxygen
During expiration, positive pleural pressure squeezes on airwaysà↑ obstruction)
No elastic recoil to push air out of lungs
Loss of lung parenchyma and vasculature ↓ surface area for gas exchange
↓ diffusion capacity
(on spirometry)
Hypoxemia: PaO2 < 70mmHg (on ABGs)
Abbreviations:
• FEV1: Forced expiratory volume in 1 second
• FVC: Forced vital capacity
• TLC: Total lung capacity
• VC: Vital Capacity
Investigations for COPD :
• Spirometry (Pulmonary function test)
Total expiration time takes longer than normal
FEV1/FEV < 0.7
(on spirometry)
Lungs don’t fully empty
More air trapped within lungs (hyperinflation)
More CO2 remains and diffuses into the blood
Hypercapnia: PaCO2 > 45
(on ABGs)
Ventilation- perfusion mismatch
High A-a gradient
(calculated from ABGs)
Low, flat diaphragm, >10 posterior ribs
(on frontal CXR)
High TLC and VC
(on spirometry)
• •
PaO2: partial pressure of O2 in arterial blood PaCO2: partial pressure of CO2 in arterial blood
• In the setting of fever and productive cough, especially if lung field opacifications are seen on CXR: consider sputum gram stain and culture to rule out pneumonia.
Air does not block X-ray beams, will appear black on X-ray film
Chronic hypercapnia makes breathing centers less sensitive to the high PaCO2 stimulus for breathing, & more reliant on the low PaO2 stimulus
(“CO2 retention”)
Give O2 carefully to these patients (high PaO2 may suppress patients’ hypoxic respiratory drive, ↓ their breathing, & ↑↑↑ PaCO2)
↑ retrosternal air space
(on lateral CXR)
Hyper-lucent
(darker) lung fields, ↓ lung markings (on frontal CXR)
• Arterial Blood Gasses (ABGs)
• Chest X-Ray (CXR): frontal and
lateral
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"#$ 气流阻塞
肺泡通气↓ 呼气时,胸膜腔正压挤压气 道à 阻塞↑
作者: Yan Yu 审稿人: Jason Baserman, Jennifer Au, Naushad Hirani*, Juri Janovcik* 译者:Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
慢性阻塞性肺疾病 (COPD)
肺组织损伤
没有弹性回缩力将
气体排出肺
肺实质与血管分布减少导 致气体交换面积↓
弥散功能↓ (肺功能检查)
更多的CO2残留 并扩散到血液中
高碳酸血症: PaCO2 > 45
(动脉血气)
血流灌注通气不良的肺泡
时无法获得足够的氧气
总呼气时长较正常长
FEV1/FEV < 0.7
(肺功能检查)
肺无法完全排空
更多空气潴留在肺部
(肺过度充气)
低氧血症: PaO2 < 70mmHg
(动脉血气)
通气-灌注不匹配
肺泡-动脉氧分压差↑ (可通过动脉血气分析计算得出)
横膈低平, 下移至第10肋后端 及以下部位 (胸部正位片)
TLC与VC增大 (肺功能检查)
缩写: • • FEV1: 1秒用 •
VC:肺活量
PaO2: 动脉血 力呼气量 氧分压
空气不会阻挡X射线, 在X光片上呈现为黑色
慢性高碳酸血症使呼吸中枢对PaCO2 刺激呼吸的敏感性下降 & 更依赖于低PaO2的刺激 (“二氧化碳潴留”)
给患者吸氧时需注意(高PaO2
可能会抑制患者低氧时对呼吸的 刺激,使呼吸驱动↓ & PaCO2↑↑↑ )
• FVC: 用力肺 • 活量
• TLC:肺总量 慢阻肺相关检查 :
PaCO2: 动脉 血二氧化碳 分压
胸骨后间隙↑
(胸部侧位片) 肺纹理↓
• 肺功能检查
• 动脉血气分析(Arterial Blood Gasses, ABGs)
• 胸部正侧位片
• 当患者发热和湿咳,特别是胸片上见肺野不清晰时:
肺透亮度↑, (胸部正位片)
考虑进行痰革兰氏染色及痰培养以排除肺炎可能
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
COPD: Complications Lung inflammation
Chronic Obstructive Pulmonary Disease (COPD)
Airway obstruction ↓ inhaled air in alveoli and terminal bronchioles
Rupture of emphasematous bullae on surface of lung
Inhaled air leaks into pleural cavity and is trapped there
Pneumothorax
Feeling a loss of control over one’s life, and hopelessness for the future
Goblet cell proliferation, ↑ mucus production
Death of airway
epithelium ciliated cells
↓ oxygenation of the blood passing through the lungs
Chronic hypoxemia
Kidneys compensate by ↑ erythropoietin (EPO) production
↑ Hemoglobin and red blood cell synthesis
Polycythemia (secondary)
Hypoxic alveoli cause the pulmonary arterioles perfusing them to reflexively vasoconstrict
Since most alveoli in the lungs are hypoxic, hypoxic vasoconstriction occurs across entire lung
Vasoconstriction ↑ blood pressure within lung vasculature
Pulmonary hypertension
↑ workload of the right ventricle (to pump against higher pressures)
To compensate, the right ventricle progressively hypertrophies and dilates, but over time its output ↓
Cor pulmonale
(Right heart failure in isolation, not due to Left heart failure)
Mucus trapped in airways, serve as nidus for infection
Acute exacerbation of COPD (AECOPD)
Pneumonia
The chronic, systemic inflammation in COPD is a hyper-metabolic state that consumes calories
Macro-nutrient deficiency
Trouble with respiration lead to inactivity and deconditioning
Wasting, muscle atrophy
More inactivity and deconditioning perpetuates the cycle
Depression
Author: Yan Yu Reviewers: Jason Baserman Naushad Hirani* Juri Janovcik* * MD at time of publication
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published January 7, 2013 on www.thecalgaryguide.com
COPD: !"# 肺部炎症
杯状细胞增殖, 气道上皮纤毛 粘液产生↑ 细胞死亡
黏液潴留呼吸道,成为感
染的病灶
慢性阻塞性肺疾病 (COPD) 气道阻塞à 吸入肺泡和终末细
肺大疱破裂
吸入的空气渗入
并潴留于胸腔
气胸
感觉生活失控,对未
来感到绝望
抑郁
作者: Yan Yu 审稿人: Jason Baserman, Naushad Hirani*, Juri Janovcik* 译者: Zihong Xie (谢梓泓) 翻译审稿人: Yonglin Mai (麦泳琳), Zesheng Ye (叶泽生) * 发表时担任临床医生
支气管的空气 ↓
流经肺的血液进行气 缺氧的肺泡à灌注肺泡的肺小动
慢性阻塞性肺疾 病急性加重期 (AECOPD)
肺炎
体交换↓ 慢性低氧血症
肾脏合成促红细胞 生成素进行代偿↑
血红蛋白与红 细胞合成↑
红细胞增多症 (继发性)
脉发生反射性血管收缩
肺大部分肺泡缺氧à整个肺 都出现缺氧性血管收缩
肺血管收缩 à 肺血管压力↑ 肺动脉高压
↑ 右心室负荷(泵血时对抗高压) 为了代偿,右心室逐渐肥大和扩张,
但随着病程进展,右心室输出量 ↓
肺心病 (单独出现右心衰竭,非左心衰)
COPD所致的慢性全身 呼吸困难导致活 性炎症会使机体处于高 动量减少和活动
代谢状态,消耗能量 耐量降低
宏量营养 素缺乏症
消瘦,肌肉萎缩
运动量下降和活动耐量
的降低造成恶性循环
图注:
病理生理
机制
体征/临床表现/实验室检查
并发症
2013年1月7日发布于 www.thecalgaryguide.com
" />













![rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
Psoriatic Arthritis: Pathogenesis and clinical findings
Auteur:
Payam Pournazari
Rédacteurs:
Yan Yu Scott Rapske Liam Martin* Traducteurs: Dianne Ganeswaran Stephen Williams Sylvain Coderre* * MD au moment de publication
Note:
L’arthrite rhumatoïde peut survenir avant ou après le psoriasis (la pathogenèse est la même).
Augmentation de l’activité ostéoclastique
Érosion de l’os sous- chondral mais formation de nouvelle osseuse ailleurs dans l’articulation
Radiographie: la périostite; les syndesmophytes, érosions, déformation du crayon en godets (les articulations IP), ankylose des articulations IP
HLA B27, Cw6 et d’autres sous- types
Antécédents familiaux positifs
Activation des cellules T (mécanisme inconnu)
Infiltration du tissu synovial de l’articulation par les cellules T et B, cellules tueuses naturelles et les macrophages
Production augmentée des molécules inflammatoires (les facteurs de nécrose tumorale [TNFs], les interleukines, etc.) qui agissent de façon systémique (dans tout le corps)
Dans les tendons et le tissu conjonctif
Inflammation des enthèses et du tissu conjonctif provoque le gonflement
Dans la peau
Réaction immunitaire détruisant les structures de la peau et des ongles (Voir diapositive Psoriasis)
Le psoriasis en plaques sur la peau, dépressions punctiformes, onychorrhexie, taches d’huile, onycholyse, décoloration et hyperkératose
Angiogenèse et vascularite dans les vaisseaux sanguins de la membrane synoviale
Dans les articulations
Les cytokines inflammatoires stimulent les nocicepteurs locaux
1. Oligoarthrites - asymétriques
2. Arthrite des articulations IPD
3. Polyarthrite rhumatoïde -
symétrique
4. Atteinte axiale (raison
inconnue, probablement en raison de « biomécanique », « innervation », et
« vascularisation ».
5. Arthrite mutilante (grave et destructrice)
L’enthésite
(Douleur/sensibilité à l’insertion du ligament dans l’os)
La dactylite (Inflammation de tout le doigt – les tissus mous et les articulations sont enflammés
Légende:
Physiopathologie
Mécanisme
Signe/Symptôme/Résultats de Laboratoire
Complications
Publié 10 November 2012 sur www.thecalgaryguide.com
rhumatisme-psoriasique-pathogenese-et-resultats-cliniques
Psoriatic Arthritis: Pathogenesis and clinical findings
Auteur:
Payam Pournazari
Rédacteurs:
Yan Yu Scott Rapske Liam Martin* Traducteurs: Dianne Ganeswaran Stephen Williams Sylvain Coderre* * MD au moment de publication
Note:
L’arthrite rhumatoïde peut survenir avant ou après le psoriasis (la pathogenèse est la même).
Augmentation de l’activité ostéoclastique
Érosion de l’os sous- chondral mais formation de nouvelle osseuse ailleurs dans l’articulation
Radiographie: la périostite; les syndesmophytes, érosions, déformation du crayon en godets (les articulations IP), ankylose des articulations IP
HLA B27, Cw6 et d’autres sous- types
Antécédents familiaux positifs
Activation des cellules T (mécanisme inconnu)
Infiltration du tissu synovial de l’articulation par les cellules T et B, cellules tueuses naturelles et les macrophages
Production augmentée des molécules inflammatoires (les facteurs de nécrose tumorale [TNFs], les interleukines, etc.) qui agissent de façon systémique (dans tout le corps)
Dans les tendons et le tissu conjonctif
Inflammation des enthèses et du tissu conjonctif provoque le gonflement
Dans la peau
Réaction immunitaire détruisant les structures de la peau et des ongles (Voir diapositive Psoriasis)
Le psoriasis en plaques sur la peau, dépressions punctiformes, onychorrhexie, taches d’huile, onycholyse, décoloration et hyperkératose
Angiogenèse et vascularite dans les vaisseaux sanguins de la membrane synoviale
Dans les articulations
Les cytokines inflammatoires stimulent les nocicepteurs locaux
1. Oligoarthrites - asymétriques
2. Arthrite des articulations IPD
3. Polyarthrite rhumatoïde -
symétrique
4. Atteinte axiale (raison
inconnue, probablement en raison de « biomécanique », « innervation », et
« vascularisation ».
5. Arthrite mutilante (grave et destructrice)
L’enthésite
(Douleur/sensibilité à l’insertion du ligament dans l’os)
La dactylite (Inflammation de tout le doigt – les tissus mous et les articulations sont enflammés
Légende:
Physiopathologie
Mécanisme
Signe/Symptôme/Résultats de Laboratoire
Complications
Publié 10 November 2012 sur www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2022/05/Psoriatic-Arthritis-PsA-Pathclinical.jpg)
![Acute Cholecystitis: Pathogenesis and clinical findings
Gallstone blocks the cystic duct, backing up bile into the gallbladder
Gallstones causing physical trauma to gallbladder wall
Irritation of adjacent diaphragm, stimulates phrenic nerve (C3-C5)
Activates stretch receptors of visceral peritoneum, stimulates foregut autonomic nerves (T5-T8)
Inflammatory mediator (i.e. prostaglandin) release by gallbladder and systemic inflammatory response
Thickened gallbladder wall on ultrasound (gold standard test)
On inspiration, the diaphragm pushes the gallbladder downward
Irritation of parietal peritoneum, stimulates somatic nerves
↑ Permeability of vessels with systemic inflammation, which leak
fluid from the blood into the interstitial space
Radiating pain to the back and right shoulder
Dull, diffuse abdominal pain referred to the epigastric region
Fever, nausea/vomiting, tachycardia
Positive Murphy’s sign (pain upon palpation of right upper quadrant [RUQ] on inspiration)
Persistent RUQ pain, abdominal guarding and peritoneal signs
Dehydration
Authors: Yan Yu, Vina Fan Reviewers: Dean Percy, Mirna Matta, Crystal Liu, Ben Campbell Maitreyi Raman* * MD at time of publication
Inflammation self-perpetuates
Irritation of inner gallbladder wall/mucosa
↑ Gallbladder lumen pressure
Intraluminal pressure exceeds arterial pressure
↓ Blood flow to gallbladder
Gallbladder ischemia
Local inflammation, loss of gallbladder mucosal integrity
Bacterial invasionàtransmural inflammation of gallbladder
Without treatment, prolonged ischemia and inflammation of the gallbladder
Gallbladder gangrene (20%)
Gallbladder perforation (20%)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 4 2019, updated May 16 2022 on www.thecalgaryguide.com
Acute Cholecystitis: Pathogenesis and clinical findings
Gallstone blocks the cystic duct, backing up bile into the gallbladder
Gallstones causing physical trauma to gallbladder wall
Irritation of adjacent diaphragm, stimulates phrenic nerve (C3-C5)
Activates stretch receptors of visceral peritoneum, stimulates foregut autonomic nerves (T5-T8)
Inflammatory mediator (i.e. prostaglandin) release by gallbladder and systemic inflammatory response
Thickened gallbladder wall on ultrasound (gold standard test)
On inspiration, the diaphragm pushes the gallbladder downward
Irritation of parietal peritoneum, stimulates somatic nerves
↑ Permeability of vessels with systemic inflammation, which leak
fluid from the blood into the interstitial space
Radiating pain to the back and right shoulder
Dull, diffuse abdominal pain referred to the epigastric region
Fever, nausea/vomiting, tachycardia
Positive Murphy’s sign (pain upon palpation of right upper quadrant [RUQ] on inspiration)
Persistent RUQ pain, abdominal guarding and peritoneal signs
Dehydration
Authors: Yan Yu, Vina Fan Reviewers: Dean Percy, Mirna Matta, Crystal Liu, Ben Campbell Maitreyi Raman* * MD at time of publication
Inflammation self-perpetuates
Irritation of inner gallbladder wall/mucosa
↑ Gallbladder lumen pressure
Intraluminal pressure exceeds arterial pressure
↓ Blood flow to gallbladder
Gallbladder ischemia
Local inflammation, loss of gallbladder mucosal integrity
Bacterial invasionàtransmural inflammation of gallbladder
Without treatment, prolonged ischemia and inflammation of the gallbladder
Gallbladder gangrene (20%)
Gallbladder perforation (20%)
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published August 4 2019, updated May 16 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/Acute-Cholecystitis-2022.jpg)







![Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com
Subarachnoid Hemorrhage: Clinical Findings
Sudden bleeding into space surrounding the brain (for pathogenesis, see Subarachnoid Hemorrhage: Pathogenesis)
Authors: Jason An, M. Patrick Pankow Reviewers: Owen Stechishin, Dave Nicholl, Haotian Wang, Hannah Mathew, Ran (Marissa) Zhang, Yan Yu*, Cory Toth* * MD at time of publication
Bleed into subarachnoid space
Subarachnoid Hemorrhage (SAH)
Posterior hypothalamus ischemia (↓ Blood flow and oxygen)
Red blood cell lysis from energy depletion or complement activation
Release of spasmogens (spasm inducing agents)
Cerebral vasospasm (narrowing of arteries from persistent contraction) ↓ blood flow
Cerebral ischemia
Release catecholamines (hormones from the adrenal gland; e.g., epinephrine, norepinephrine)
↑ Intracellular calcium
Release of antidiuretic hormone
Antidiuretic hormone acts on the distal convoluted tubule and collecting duct in kidney to reabsorb water
Dilution of serum sodium
Hyponatremia (low blood sodium levels)
Release of epileptogenic (potential seizure causing agents) into cerebral circulation
Seizure
Products from blood breakdown in cerebral spinal fluid
Irritation of meninges (membranes surrounding the brain)
Aseptic meningitis (non-infectious inflammation)
Meningismus
(neck pain + rigidity)
Cerebral infarction (death of tissue)
Obstructs cerebral spinal fluid flow and absorption at subarachnoid granulations
Hydrocephalus (fluid build up in ventricles)
↓ Level of consciousness
Reduced cerebral blood flow
Dilation of cranial vessels to ↑ blood flow
Rapid ↑ internal carotid artery intracranial pressure
Refer to Increased Intracranial Pressure: Clinical Findings slide
Internal carotid artery
Pituitary ischemia
Hypopituitarism
[underactive pituitary gland, failing to produce 1+ pituitary hormone(s)]
Refer to hypopituitarism slides
Myocardial disruption
Left ventricle dysfunction
↑ Pressure in left heart
Blood forced backwards into pulmonary veins
↑ Pulmonary blood pressure
Fluid from blood vessels leaks into lungs
Dysrhythmias (disturbance in rate/rhythm of heart) causing ↓ cardiac output
Syncope
(loss of consciousness due to ↓ blood flow to the brain)
Pulmonary edema
(excess accumulation of fluid in lung)
Cerebral hypoperfusion
Sudden ↑in blood volume
Vessels and meninges suddenly stretch
Thunderclap Headache (worst headache of patient's life)
Shortness of breath
Reactive cerebral hyperemia (excess blood in vessels supplying the brain)
Artery specific findings:
Rapid ↑ internal carotid artery intracranial pressure
Middle cerebral artery
Posterior communicating artery
Compression of outer CN3 Compression of inner CN3
Anterior communicating artery
Nonreactive pupil
Gaze palsy
(eye deviates down and out)
Diplopia
(double vision)
Ptosis
(drooping of upper eyelid)
Frontal lobe ischemia
Avolition
(complete lack of motivation)
Ischemia of motor strip pertaining to the legs
Bilateral leg weakness
Motor strip ischemia
Hemiparesis
(weakness/ inability to move one side of the body)
Ischemia of parietal association areas (brain regions integral for motor control of the eyes, the extremities and spatial cognition)
Aphasia
(impaired ability to speak and/or understand language)/ neglect
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published July 1, 2014, updated August 10, 2022 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2015/05/SAH-Clinical-Findings-2022.jpg)






































































![Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com
Hypomagnesemia: Physiology
Hyperglycemia
An increased amount of glucose enters renal tubules as glomerulus performs blood filtration
↑ [Solute] in renal tubules from ↑ glucose content exerts osmotic force that pulls water & electrolytes, including Mg2+, into renal tubules
↑ Urinary Mg2+ excretion
Lack of insulin
Lack of insulin receptor signaling in distal convoluted tubule (DCT) ↓ glucose uptake from renal tubules
Hypercalcemia
Ca2+ binds to Ca2+ sensing receptors on thick ascending limb (TAL) of loop of Henle, where resorption of Ca2+ & Mg2+ occurs
Receptor activation ↓ Na-K- 2Cl (NKCC) transporter activity which maintains electro- chemical gradient in TAL
Passive paracellular resorption of Ca2+ and Mg2+, dependent on electrochemical gradient, ↓
↑ Extra- cellular fluid
↓ Resorption of Na+ & H2O from renal tubules
Genetic disorders (e.g. Bartter syndrome, familial hypomagnesemia)
Medications (e.g. loop & thiazide diuretics, certain antibiotics, calcineurin inhibitors)
Some metabolic byproducts of these drugs are nephrotoxic
Inability to absorb free fatty acids (FFAs)
Mg2+, which associates with FFAs, is not absorbed through the gut
Steatorrhea (fat in the stool)
Mal- absorption (often due to inflammation or infection) & diarrhea
Acute pancreatitis
↓ Lipase secretion from pancreas ↑ levels of undigested fats in small intestine
↓ Passive Mg resorption from
tubules
Mg saponification in necrotic fat
2+
Varying mechanisms causing defective Mg2+ re-absorption (e.g. impacts to PCT, TAL, DCT disrupting transporters and ion shifting; ↓ gut resorption of Mg2+)
Nutrients & 2+
electrolytes are lost in stool
undergoes
Renal loss of magnesium
Gastrointestinal loss of magnesium
Hypomagnesemia
Serum [Mg2+] < 0.7 mmol/L
Impairs production and release of parathyroid hormone responsible for ↑ blood Ca2+ Hypocalcemia
Muscle cells are unable to activate Mg2+ dependent ATP hydrolysis
Impairs muscle relaxation and reduces the ability to stop muscular contraction
Lack of Ca2+ disrupts neurotransmitter release and neuronal signaling
Impairs rapid depolarization and repolarization during muscle contraction
Neuromuscular excitability (large, rapid change in membrane voltage due to small stimulus)
Delirium
Apathy
QRS widening and peaking of T waves on ECG
Torsade de Pointes
Constant muscle contraction compresses blood vessels
Reduced blood supply to hands, wrists, feet, and ankles
Trousseau sign (carpopedal spasm with inflation of BP cuff)
Authors: Caroline Kokorudz Reviewers: Shyla Bharadia Allesha Eman Michelle J. Chen Dr. Adam Bass* * MD at time of publication
Chvostek sign (facial muscle twitch with cheek touch)
Seizures
Tetany
Weakness
Legend:
Pathophysiology
Mechanism
Sign/Symptom/Lab Finding
Complications
Published Oct 4, 2024 on www.thecalgaryguide.com](https://calgaryguide.ucalgary.ca/wp-content/uploads/2024/10/Hypomagnesia.jpg)




























